Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of new lipid ingredients during pregnancy and lactation on rat offspring brain gene expression†
Valentina
Origüela
 ab,
Antonio
Gázquez
ab,
Antonio
Gázquez
 ab,
María José
López-Andreo
c,
Pilar
Bueno-Vargas
ab,
María José
López-Andreo
c,
Pilar
Bueno-Vargas
 d,
Mustafa
Vurma
e,
José M.
López-Pedrosa
d,
Brian J.
Leyshon
e,
Matthew
Kuchan
e,
Jia Pei
Chan
e and
Elvira
Larqué
d,
Mustafa
Vurma
e,
José M.
López-Pedrosa
d,
Brian J.
Leyshon
e,
Matthew
Kuchan
e,
Jia Pei
Chan
e and
Elvira
Larqué
 *ab
*ab
aDepartment of Physiology, Faculty of Biology, University of Murcia, Campus of Espinardo, 30100 Murcia, Spain. E-mail: elvirada@um.es
bBiomedical Research Institute of Murcia (IMIB-Arrixaca), 30120 Murcia, Spain
cMolecular Biology Section, Scientific and Technical Research Area (ACTI), University of Murcia, 30100 Murcia, Spain
dResearch and Development Department, Abbott Nutrition, 18004 Granada, Spain
eResearch and Development Department, Abbott Nutrition, Columbus, 43215 Ohio, USA
First published on 11th December 2024
Abstract
Maternal dietary fat intake during pregnancy and lactation may influence the bioavailability of essential lipophilic nutrients, such as docosahexaenoic acid (DHA), that are important for both the mother and her child's development. This study aimed to evaluate the effects of different maternal fat diets on fat absorption and pup brain development by analyzing gene expression. Rats were fed diets with different lipid matrices during pregnancy and lactation: diet A, mono and diglycerides (MDG) + soy lecithin phospholipids (PL); diet B, MDG + soy lecithin PL + milk-derived PL; and a control diet. All diets contained the same amount of DHA. We determined maternal dietary fat absorption, as well as the offspring fatty acid (FA) profile in both plasma and brain samples at birth and in pups at 14 days post-natal. In addition, microarray analysis was performed to characterize the pup brain gene expression. Maternal dietary fat and DHA apparent absorption was enhanced only with diet B. However, we observed higher plasma DHA and total FA concentrations in lactating pups from the experimental groups A and B compared to the control. Both brain DHA and total FA concentrations were also higher in fetuses and 14-day-old pups from group A with respect to the control, with diet B following the same trend. Offspring brain gene expression was affected by both diets A and B, with changes observed in synaptic and developmental processes in the fetuses, and the detoxification process in 14-day-old pups. Incorporating MDG and PL-rich lipid matrices into maternal diets during pregnancy and lactation may be highly beneficial for ensuring proper neurodevelopment of the fetus and newborn.
Introduction
The human brain is a lipid-rich organ1 that undergoes significant growth from the 12th week of gestation through the first 3 years of life.2 During this period, polyunsaturated fatty acids (PUFA), especially docosahexaenoic acid (DHA, 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 omega-3), accumulate in the infant's brain.3 This fatty acid (FA) can be synthesized in the liver and brain, among other tissues, from alpha-linolenic acid (ALA, C18:3 omega-3) through desaturation (desaturases delta-5 and delta-6) and elongation (elongases 2 and 5).4 Although this conversion is a relevant biochemical process for the organism, it can be limited by various factors, such as hepatic steatosis or oxidative stress.5 In addition, during the fetal stage, this conversion is not sufficient to ensure adequate fetal DHA accretion, making the maternal contributions during gestation and lactation crucial.3 Currently, many women in Western countries do not meet the recommended dietary intake of omega-3 FA,6 requiring nutritional supplements to ensure adequate fetal supply. In this context, any dietary strategy that enhances bioavailability of DHA during pregnancy would be highly beneficial.
6 omega-3), accumulate in the infant's brain.3 This fatty acid (FA) can be synthesized in the liver and brain, among other tissues, from alpha-linolenic acid (ALA, C18:3 omega-3) through desaturation (desaturases delta-5 and delta-6) and elongation (elongases 2 and 5).4 Although this conversion is a relevant biochemical process for the organism, it can be limited by various factors, such as hepatic steatosis or oxidative stress.5 In addition, during the fetal stage, this conversion is not sufficient to ensure adequate fetal DHA accretion, making the maternal contributions during gestation and lactation crucial.3 Currently, many women in Western countries do not meet the recommended dietary intake of omega-3 FA,6 requiring nutritional supplements to ensure adequate fetal supply. In this context, any dietary strategy that enhances bioavailability of DHA during pregnancy would be highly beneficial.
It is known that the bioavailability of lipophilic nutrients in the gastrointestinal tract can be modified by their emulsion structure.7 The addition of specific lipid species to the maternal diet may improve fat absorption in the small intestine due to their emulsifying properties.7–10 Mono and diglycerides (MDG) are major intermediates in intestinal fat uptake, and together with phospholipids (PL), they are known to promote the formation of smaller fat droplets that are more readily accessible to digestive enzymes.9,11 Thus, it is interesting to understand their roles in the absorption of compounds that are important for neurodevelopment, such as DHA.
DHA is the major structural component of the brain and is mostly concentrated at the sn-2 position of PL in cell membranes, playing a key role in neurogenesis and synaptogenesis.12 High plasma levels of DHA in the mother and breast milk have been associated with better growth and development of the brain and visual system in children.13 Greater intestinal bioavailability of PL and DHA during pregnancy and lactation could improve the cognitive development of the fetus/newborn. Certain PL, such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and sphingomyelin (SM), play critical roles in the nervous system.14–16 Their availability during development is essential for the membrane structure and function of nerve cells.17,18 In fact, the milk fat globule membrane (MFGM), rich in these PL, has been associated with better cognitive development in children,19,20 and its inclusion in formula-fed infants is relevant.21
The main objective of this study was to determine if adding specific lipids, such as MDG and PL, to the maternal diet may improve fat absorption and DHA content during gestation and lactation. Additionally, microarray analyses were conducted in the offspring brain to identify potential benefits of these lipid matrices on neurodevelopment.
Materials and methods
Animals and study design
68 female adult Sprague–Dawley rats (8 weeks of age) were supplied by the Animal Laboratory Service of the University of Murcia. Animals were housed in groups (4 animals per cage) with ad libitum access to food and water in a humidity and temperature-controlled (22 ± 1 °C) room under a 12 h light/dark cycle. Animal weight was recorded every week throughout the experiment. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Murcia (Murcia, Spain) (A13221102, December 14, 2021) and conformed to the ARRIVE guidelines for animal research.22 Animals received humane treatment in accordance with the European Union guidelines for the care and use of laboratory animals.Female rats were randomized into three groups that received different experimental diets during the study: a control diet (n = 22), without lipid matrices; diet A (n = 22), with MDG and soy lecithin PL; and diet B (n = 24), with the same composition as diet A plus an extra source of PL (whey protein PL concentrated, WPPC). The compositions of MDG (Radiamuls MG 2152K, Oleon, Belgium) and WPPC (ISO Chill® 6000, Agropur, Minnesota, USA) are included in Table 1. All diets conformed to the standardized rodent AIN-93G diet requirements of vitamins and minerals,23 and contained the same amount of DHA in the form of triglycerides (Table 1).
| Diet | Control (g kg−1 diet) | A (g kg−1 diet) | B (g kg−1 diet) |
|---|---|---|---|
| DHA, docosahexaenoic acid; MDG, mono and diglycerides; PL, phospholipids; WPPC, whey protein phospholipid concentrated. MDG is a commercial oil with 42% monoglycerides, 33% diglycerides, and 25% triglycerides. WPPC composition per gram of product: 22.18 mg of phosphatidylcholine, 18.83 mg of phosphatidylethanolamine, 18.28 mg of sphingomyelin, 8.07 mg of phosphatidylserine, and 3.32 mg of phosphatidylinositol. | |||
| Corn starch | 317.97 | 317.97 | 317.97 |
| Maltodextrin | 100.41 | 100.01 | 85.04 |
| Sucrose | 91.60 | 91.57 | 93.06 |
| Lactose | 0.31 | 0.31 | 2.96 |
| Glucose | 2.79 | 2.78 | 2.36 |
| Casein | 175.02 | 175.02 | 150.71 |
| Soy oil | 65.37 | 62.10 | 51.66 |
| DHA | 1.00 | 1.00 | 1.00 |
| MDG | 0 | 2.90 | 2.90 |
| Soy lecithin | 0 | 0.60 | 0.60 |
| WPPC | 0 | 0 | 40 |
| Fiber | 45.80 | 45.80 | 45.80 |
| Choline | 1.27 | 1.29 | 1.29 |
| L-Cystine | 3.48 | 3.48 | 3.92 |
| Mineral mix | 32.80 | 32.80 | 32.80 |
| Vitamin mix | 9.40 | 9.40 | 9.40 |
After a one-week period of adaptation to the cage and the diets, rats were placed in individual metabolic cages for four days for an apparent fat digestibility assay of the diets. This allowed measurement of diet intake and fecal collection prior to pregnancy to study dietary fat absorption. Then, male rats were placed into the female cages for mating (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and, once mating had taken place (indicated by sperm presence in a vaginal smear under a microscope), they were removed. Female pregnant rats were allocated to appropriate cages (4 animals per cage) and fed their assigned test diets throughout the pregnancy period.
2) and, once mating had taken place (indicated by sperm presence in a vaginal smear under a microscope), they were removed. Female pregnant rats were allocated to appropriate cages (4 animals per cage) and fed their assigned test diets throughout the pregnancy period.
At days 20–21 of gestation, before delivery, some pregnant rats in each group (17 control, 17 group A, and 19 group B) were sacrificed, and samples from both mothers and fetuses were taken. The remainder (n = 5 per group) were allowed to give birth to their pups in individual cages (4 offspring per mother, 2 males and 2 females), where they were maintained until day 14 of life, when all the remaining animals were sacrificed.
Sample collection
At days 20–21 of gestation, animals were first anesthetized by inhalation of 5% isoflurane, followed by an intramuscular injection of 5 mg ketamine hydrochloride and 0.2 mg xylazine hydrochloride per 100 g animal weight. Maternal blood was extracted by heart puncture using EDTA-coated tubes. After separation of the fetuses, female rats were killed by an intracardiac injection of 20 mg of sodium pentobarbital per 100 g of animal weight. In the case of the fetuses, they were sacrificed by decapitation, and blood and brain samples were collected. The plasma and brain tissues from all fetuses from the same mother were pooled. All sacrifices were made at 10 am after overnight fasting. At day 14 post-partum, lactating animals were fasted for only 4 hours and anesthetized with the above-mentioned protocol, and blood and brain samples were collected.Blood samples were centrifuged at 1400g for 10 min at 4 °C to obtain the plasma. Brains were frozen in liquid nitrogen and stored at −80 °C until analysis.
Dietary fat and DHA absorption
Fat apparent absorption was calculated as the ratio between total fat content in feces and total fat intake. The total fat content in feces was determined gravimetrically; 5 g of lyophilized feces was treated with hydrochloric acid 37.5% (v/v) at 100 °C for 1 h and then total fat was extracted with diethyl ether under reflux (Tecator Soxtec System HT 1043, FOSS, Barcelona, Spain), dried, and weighed.24 Regarding total fat intake, it was calculated based on the dry weight of the diet consumed during the digestibility assay and its fat percentage. The moisture of the diets was determined gravimetrically after 24 h by heating at 105 ± 1 °C until constant weight (JP 200 SELECTA, Barcelona, Spain).In addition, in the same way as the fat absorption, we estimated dietary DHA apparent absorption considering DHA intake and DHA in the feces (according to the method given by Folch et al.,25 see the section ‘Fatty acid analyses’ in the ‘Materials and methods’).
Fatty acid analyses
Total lipids were extracted from 200 mg of feces, 100 μL of plasma, and 50 mg of brain tissue into chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2
methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) according to the method given by Folch et al.25 The feces and brain were first homogenized in chloroform
1 v/v) according to the method given by Folch et al.25 The feces and brain were first homogenized in chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2
methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) with butylated hydroxytoluene (BHT) using a metal-blade homogenizer. Prior to extraction, 0.05 mg of pentadecanoic acid was added to the samples as the internal standard. FA methyl esters were produced according to Stoffel et al.26 by adding 1 mL of 3 N methanolic HCl (Supelco, Sigma-Aldrich, Massachusetts, USA) and heating at 90 °C for 1 h. The derivatives were extracted into hexane and stored at −20 °C until gas chromatographic analysis.
1 v/v) with butylated hydroxytoluene (BHT) using a metal-blade homogenizer. Prior to extraction, 0.05 mg of pentadecanoic acid was added to the samples as the internal standard. FA methyl esters were produced according to Stoffel et al.26 by adding 1 mL of 3 N methanolic HCl (Supelco, Sigma-Aldrich, Massachusetts, USA) and heating at 90 °C for 1 h. The derivatives were extracted into hexane and stored at −20 °C until gas chromatographic analysis.
FA methyl esters were analyzed by gas chromatography using an SP-2560 capillary column (100 m × 0.25 mm i.d. × 20 μm) (Supelco, Sigma-Aldrich, Massachusetts, USA) in a Hewlett-Packard 6890 gas chromatograph (Agilent Technologies, Madrid, Spain) equipped with a flame ionization detector.27 The temperature of the detector and the injector was 240 °C. The oven temperature was programmed at 175 °C for 30 min, increased at 2 °C min−1 to 230 °C and held at this temperature for 17 min. Helium was used as the carrier gas at a pressure of 45 psi. Peaks were identified by comparison of their retention times with appropriate FA methyl ester standards (Sigma-Aldrich, Massachusetts, USA) and the FA concentrations were determined in relation to the peak area of the internal standard. The FA data are represented as the concentration (g L−1 or mg g−1) and percentage of total FA (g per 100 g of total FA).
Microarray analysis
RNA extraction was carried out using 30 mg of brain samples (from fetuses and 14-day-old pups) using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The amount and quality of the RNA were verified by using a Nanodrop 2000 (Thermo Fisher Scientific, Massachusetts, USA) and a Bioanalyzer 2100 (Agilent Technologies, California, USA). Single-stranded cDNAs (ss-cDNAs) were synthesized from 100 ng of the aforementioned RNA, using the Clariom S Assay Rat (Thermo Fisher Scientific, Massachusetts, USA; 902935). According to the protocol supplied by the manufacturer, 2.3 μg of fragmented and biotinylated ss-cDNA were included in the hybridization mix, using the GeneChip Hybridization, Wash, and Stain Kit (Thermo Fisher Scientific, Massachusetts, USA; 900720) and then hybridized to the Clariom S Array Rat (Thermo Fisher Scientific, Massachusetts, USA; 902935). This type of array is designed to provide extensive coverage of all known well-annotated genes including more than 231800 total probes to define the level of expression of more than 22900 genes of the rat transcriptome.After scanning, microarray data were processed using the Affymetrix Expression Command Console (Affymetrix; Thermo Fisher Scientific, California, USA). Raw data analysis was then performed using the robust multiarray average (RMA) method which allows background correction, log2 transformation, and quantile normalization to obtain the individual intensity values for each probe set.
Statistical analysis
Statistical analysis of animal weight, dietary intake, and FA variables was conducted using the SPSS 28.0 software package (IBM Corp., New York, USA). The normal distribution of continuous variables was checked using the Kolmogorov–Smirnov normality test. Differences between the experimental groups were assessed using one-way ANOVA, followed by a post hoc Bonferroni test. The results were expressed as mean ± standard error of the mean (SEM), considering statistical significances at P ≤ 0.05.Regarding the microarray statistical analysis, we used Partek Genomics Suite and Partek Pathways software (Partek Incorporated, Missouri, USA), and performed a one-way ANOVA test with a restrictive threshold at P ≤ 0.05 and |fold-change| ≥ 1.5. The molecular interaction, reaction, and relation networks that showed differentially expressed genes were finally analyzed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and the Gene Ontology (GO) classification. These pathways and processes were classified according to their respective enrichment scores.
Results
Dietary fat digestibility and fatty acid status
Maternal weight was similar among groups at day 20 of pregnancy and day 14 after delivery (Table 2). Placental weight tended to be higher in group B compared to the control group. Fetal weight and the number of fetuses presented no differences among the groups; however, offspring at 14 days of life were smaller in group B compared to the other two groups (Table 2).| Diet | Control | A | B | P |
|---|---|---|---|---|
| Data are expressed as mean ± SEM. Data not sharing the same superscript letters indicate statistically significant differences between the groups (P ≤ 0.05). DHA, docosahexaenoic acid; FA, fatty acid. | ||||
| Mothers | n = 17 | n = 17 | n = 19 | |
| Diet intake during digestibility assay (g day−1) (dry matter) | 11.69 ± 0.34a | 11.37 ± 0.33a | 13.69 ± 0.90b | 0.013 |
| Fat intake during digestibility assay (g) | 2.45 ± 0.07a | 2.39 ± 0.07a | 3.07 ± 0.20b | <0.001 |
| Fat in feces during digestibility assay (mg) | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.384 |
| DHA in feces during digestibility assay (μg g−1) | 16.73 ± 0.88 | 17.45 ± 0.73 | 12.63 ± 0.76 | 0.076 |
| Maternal weight at delivery – d20 (g) | 367.37 ± 7.34 | 351.38 ± 7.96 | 359.40 ± 8.45 | 0.388 |
| Maternal plasma DHA at delivery (%) | 9.49 ± 0.30 | 9.14 ± 0.38 | 9.02 ± 0.39 | 0.641 |
| Maternal plasma DHA at delivery (g L−1) | 0.83 ± 0.05 | 0.71 ± 0.04 | 0.68 ± 0.06 | 0.105 |
| Maternal plasma total FA at delivery (g L−1) | 8.95 ± 0.75 | 7.95 ± 0.60 | 7.62 ± 0.66 | 0.355 |
| Placental weight (g) | 0.45 ± 0.01 | 0.47 ± 0.01 | 0.50 ± 0.02 | 0.052 |
| Maternal weight at d14 (g) (n = 5 per group) | 265.30 ± 4.95 | 258.94 ± 7.11 | 281.76 ± 11.31 | 0.171 |
| Fetuses | n = 17 | n = 17 | n = 19 | |
| Number of fetuses | 15.29 ± 0.38 | 13.88 ± 0.83 | 12.58 ± 1.23 | 0.118 |
| Fetal weight at delivery – d20 (g) | 3.45 ± 0.16 | 3.66 ± 0.16 | 3.88 ± 0.17 | 0.192 |
| Fetal plasma DHA (%) | 10.74 ± 0.27 | 11.08 ± 0.29 | 10.18 ± 0.53 | 0.275 |
| Fetal plasma DHA (g L−1) | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.225 |
| Fetal plasma total FA (g L−1) | 1.96 ± 0.06 | 1.80 ± 0.04 | 1.86 ± 0.04 | 0.081 |
| Fetal brain DHA (%) | 14.10 ± 0.29 | 14.51 ± 0.19 | 14.18 ± 0.22 | 0.441 |
| Fetal brain DHA (mg g−1) | 1.73 ± 0.06a | 1.99 ± 0.05b | 1.82 ± 0.06ab | 0.023 |
| Fetal brain total FA (mg g−1) | 12.03 ± 0.23a | 13.71 ± 0.29b | 12.79 ± 0.39ab | 0.010 |
| Offspring (14 days old) | n = 20 | n = 20 | n = 20 | |
| Offspring sex (male/female) | 10/10 | 10/10 | 10/10 | 1.000 |
| Offspring weight at d14 (g) | 40.46 ± 0.51a | 42.10 ± 0.35a | 38.09 ± 0.63b | <0.001 |
| Offspring plasma DHA at d14 (%) | 13.32 ± 0.21 | 12.81 ± 0.19 | 13.35 ± 0.20 | 0.105 |
| Offspring plasma total FA at d14 (g L−1) | 4.48 ± 0.12a | 5.60 ± 0.14b | 5.17 ± 0.12b | <0.001 |
| Offspring brain DHA at d14 (%) | 18.38 ± 0.11a | 18.81 ± 0.14b | 18.45 ± 0.08ab | 0.010 |
| Offspring brain total FA at d14 (mg g−1) | 22.46 ± 0.89a | 25.22 ± 0.43b | 23.83 ± 0.40ab | 0.023 |
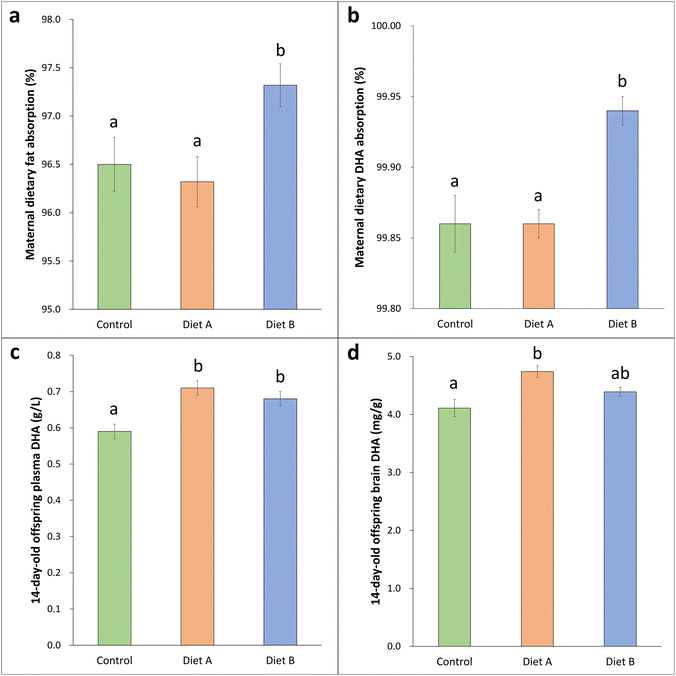
During the digestibility assay of diets before gestation, we found that group B had higher diet intake and fat intake compared to the other groups (Table 2). Nevertheless, the fecal fat content was similar among the groups (Table 2), resulting in higher dietary fat apparent absorption in group B compared to group A and the control group (Fig. 1a). In addition, the feces of mothers from group B tended to show a lower DHA concentration (Table 2) and the estimated dietary DHA apparent absorption was significantly higher in these mothers with respect to the other two groups (Fig. 1b).
No significant differences were observed in the plasma content of DHA and total FA, for either mothers or fetuses at delivery (Table 2). However, lactating offspring from groups A and B presented higher plasma DHA (Fig. 1b) and total FA concentrations (Table 2) compared to the control group. Regarding brain analyses, group A fetuses and lactating offspring presented higher total FA and DHA concentrations, as well as DHA percentage compared to the control group (Table 2 and Fig. 1d). Diet B followed the same line. These results could indicate an improvement in the offspring DHA status by the administered maternal dietary lipid matrices.
Gene expression in the fetal brain
Gene expression in the fetal brain was impacted by maternal diet. The shared GO processes altered by both experimental diets A and B compared to the control were the multicellular organismal process, the developmental process, biological regulation, behavior, and locomotion, among others (Fig. 2a). Diet B had higher enrichment scores than diet A in most of the affected processes (Fig. 2a). All the GO processes impacted in the fetal brain are shown in the ESI (Annex 1†). Concerning KEGG pathways, serotonergic synapse and tryptophan metabolism were the shared pathways affected in groups A and B compared to the control (ESI, Annex 2†). | ||
| Fig. 2 Brain GO processes affected in diets A and B compared to the control, classified by the enrichment score. (a) Fetuses. (b) 14-day-old offspring. GO, gene ontology. | ||
The fetal brain from group A presented 10 differentially expressed genes compared to the control group: 1 of them was down-regulated (Dlx6) and 9 were up-regulated (Prph, Dbh, Tph2, Hoxb5, Hoxc4, Lamp5, Hoxa2, Pax2, and Slc6a5) (Table 3). On the other hand, the fetal brain tissue from group B had 16 differentially expressed genes compared to the control: 12 of them were down-regulated (Tiam2, Satb2, Tbr1, Neurod6, Sla, Fezf2, Foxg1, Neurod2, Mpped1, Dlx2, Chrm1, and Zfp238) and the remaining 4 were up-regulated (Tph2, Pax2, Slc6a5, and Hoxa2) (Table 3). These 4 up-regulated genes were the only ones shared by both experimental diets and are related to neurological development: Tph2 (tryptophan hydroxylase 2) is required for the serotonin biosynthesis, Pax2 (paired box 2) is involved in the development of the central nervous system, Slc6a5 (solute carrier family 6, member 5) is related to the maintenance of the presynaptic pool of neurotransmitters, and Hoxa2 (homeo box A2) is related to the embryonic development of the face.
| Gene symbol | Encoded protein | Biological functiona | Gene ID | P | Fold change |
|---|---|---|---|---|---|
| Only genes with P ≤ 0.05 and |fold-change| ≥ 1.5 are listed.a Biological functions extracted from GeneCards: the human gene database. | |||||
| Diet A | |||||
| Down-regulated | |||||
| Dlx6 | Distal-less homeo box 6 | Forebrain and craniofacial development | ENSRNOT00000014468 | 0.003 | −1.524 |
| Up-regulated | |||||
| Prph | Peripherin | Cytoskeletal protein in neurons of the peripheral nervous system | NM_012633 | 0.032 | 1.508 |
| Dbh | Dopamine beta-hydroxylase | Conversion of dopamine to norepinephrine | NM_013158 | 0.004 | 1.520 |
| Tph2 | Tryptophan hydroxylase 2 | Biosynthesis of serotonin | NM_173839 | 0.041 | 1.576 |
| Hoxb5 | Homeo box B5 | Developmental regulatory system | NM_001191925 | 0.007 | 1.685 |
| Hoxc4 | Homeo box C4 | Morphogenesis in all multicellular organisms | NM_001109884 | 0.019 | 1.829 |
| Lamp5 | Lysosomal-associated membrane protein family, member 5 | Short-term synaptic plasticity in a subset of GABAergic neurons | NM_001014183 | 0.004 | 1.896 |
| Hoxa2 | Homeo box A2 | Embryonic development | ENSRNOT00000008023 | 0.005 | 1.927 |
| Pax2 | Paired box 2 | Eyes and central nervous system development | NM_001106361 | 0.018 | 1.931 |
| Slc6a5 | Solute carrier family 6, member 5 | Glycinergic synapse | NM_203334 | 0.018 | 1.947 |
| Diet B | |||||
| Down-regulated | |||||
| Tiam2 | T-cell lymphoma invasion and metastasis 2 | Neural cell development (GDP–GTP exchange activity) | ENSRNOT00000065386 | 0.012 | −1.829 |
| Satb2 | SATB homeo box 2 | Transcription regulation and chromatin remodeling | NM_001109306 | 0.029 | −1.803 |
| Tbr1 | T-box, brain, 1 | Cortical development | NM_001191070 | 0.021 | −1.773 |
| Neurod6 | Neuronal differentiation 6 | Nervous system development and differentiation | NM_001109237 | 0.014 | −1.644 |
| Sla | Src-like adaptor | Cell differentiation | NM_178097 | 0.024 | −1.638 |
| Fezf2 | Fez family zinc finger 2 | Nervous system development and neuron differentiation | NM_001107251 | 0.031 | −1.602 |
| Foxg1 | Forkhead box G1 | Establishment of regional subdivision of the developing brain | NM_012560 | 0.021 | −1.586 |
| Neurod2 | Neuronal differentiation 2 | Neuronal differentiation | NM_019326 | 0.017 | −1.575 |
| Mpped1 | Metallophosphoesterase domain containing 1 | Predicted to enable hydrolase activity | NM_001130569 | 0.046 | −1.541 |
| Dlx2 | Distal-less homeo box 2 | Forebrain and craniofacial development | NM_001191746 | 0.009 | −1.533 |
| Chrm1 | Cholinergic receptor muscarinic 1 | Influences the effects of acetylcholine on the central and peripheral nervous systems | NM_080773 | 0.048 | −1.516 |
| Zfp238 | Zinc finger protein 238 | Neuronal development | NM_022678 | 0.016 | −1.508 |
| Up-regulated | |||||
| Tph2 | Tryptophan hydroxylase 2 | Biosynthesis of serotonin | NM_173839 | 0.040 | 1.534 |
| Pax2 | Paired box 2 | Eyes and central nervous system development | NM_001106361 | 0.045 | 1.686 |
| Slc6a5 | Solute carrier family 6, member 5 | Glycinergic synapse | NM_203334 | 0.048 | 1.716 |
| Hoxa2 | Homeo box A2 | Embryonic development | NM_012581 | 0.046 | 1.790 |
Gene expression in 14-day-old pups’ brain samples
At 14 days of age, different sets of genes were impacted by maternal diets compared to what we observed in the fetal brains. The GO processes affected by both maternal diets A and B included detoxification, the multi-organism process, response to stimulus, localization, and the developmental process, among others. Similar to what was observed in the fetal brain, diet B presented a greater effect than diet A on these GO processes, according to higher enrichment scores (Fig. 2b). All the GO processes affected in the 14-day-old pups’ brain samples are also shown in the ESI (Annex 3†). Regarding KEGG pathways, mineral absorption was the shared pathway affected by both experimental diets A and B compared to the control (ESI, Annex 4†).In the pups’ brain samples from group A, 13 genes were differentially expressed compared to the control group, all of them down-regulated (Mt1M, Mt2A, Myeov2, Slc1a6, Ppp1r17, Pcp2, Rps29, Atp5e, Nhlh1, Cbln1, Dbp, Mfap4, and Ifi27) (Table 4). In group B, only 2 genes were differentially expressed compared to the control, and both were down-regulated (Hba-a1 and Mt2A) (Table 4). Mt2A (metallothionein 2A) was the only gene affected – shared by both diet groups. It is involved in detoxification by regulating the intracellular levels of heavy metals.
| Gene symbol | Encoded protein | Biological functiona | Gene ID | P | Fold change |
|---|---|---|---|---|---|
| Only genes with P ≤ 0.05 and |fold-change| ≥ 1.5 are listed.a Biological functions extracted from GeneCards: the human gene database. | |||||
| Diet A | |||||
| Down-regulated | |||||
| Mt1M | Metallothionein 1M | Detoxification | ENSRNOT00000047663 | 0.001 | −1.746 |
| Mt2A | Metallothionein 2A | Detoxification | NM_001137564 | 0.002 | −1.705 |
| Myeov2 | Myeloma overexpressed 2 | Pseudogene | NM_001109044 | 0.015 | −1.688 |
| Slc1a6 | Solute carrier family 1, member 6 | Glutamate uptake | NM_032065 | 0.021 | −1.670 |
| Ppp1r17 | Protein phosphatase 1, regulatory subunit 17 | Protein phosphatase inhibitor in cerebellar Purkinje cells | NM_153467 | 0.037 | −1.669 |
| Pcp2 | Purkinje cell protein 2 | Catalytic activity in neuronal cell body | NM_001107116 | 0.009 | −1.603 |
| Rps29 | Ribosomal protein S29 | Component of the small ribosomal subunit | NM_012876 | 0.017 | −1.569 |
| Atp5e | ATP synthase subunit epsilon, mitochondrial | Subunit of mitochondrial ATP synthase | NM_139099 | 0.021 | −1.561 |
| Nhlh1 | Nescient helix loop helix 1 | Cell-type determination in the developing nervous system | NM_001105970 | 0.015 | −1.553 |
| Cbln1 | Cerebellin 1 precursor | Synapse integrity and synaptic plasticity | NM_001109127 | 0.049 | −1.529 |
| Dbp | D site of albumin promoter (albumin D-box) binding protein | Circadian period and sleep regulation | NM_001289982 | 0.011 | −1.517 |
| Mfap4 | Microfibrillar-associated protein 4 | Extracellular matrix protein | NM_001034124_2 | 0.028 | −1.514 |
| Ifi27 | Interferon alpha-inducible protein 27 | Cellular protein metabolic process | NM_203410 | 0.001 | −1.514 |
| Diet B | |||||
| Down-regulated | |||||
| Hba-a1 | Hemoglobin alpha adult chain 1 | Oxygen transport | NM_001013853 | 0.010 | −1.593 |
| Mt2A | Metallothionein 2A | Detoxification | NM_001137564 | 0.027 | −1.517 |
Discussion
In this study, we fed female rats during pregnancy and lactation with diets enriched with different lipid species, all of them containing the same amount of DHA: diet A was fortified with MDG and soy lecithin PL; diet B had the same fortification as diet A with an extra source of milk PL (WPPC); and the control diet was without these fortifications. Maternal dietary fat and DHA absorption was higher with diet B, although lactating pups from both experimental groups A and B showed higher DHA and total FA concentrations in plasma compared to the control. Regarding the brain results, fetuses and 14-day-old pups from diet A contained higher total fat and DHA content with respect to the control, with diet B following the same trend. Both diets A and B up-regulated the fetal brain expression of genes involved in synaptic and developmental processes – Tph2, Pax2, Slc6a5, and Hoxa2 – compared to the control. In contrast, Mt2A, a detoxification related gene, was down-regulated in both groups compared to the control during lactation. These lipid matrices affected the brain DHA composition in lactating pups, as well as that for its transcriptome.Dietary fat digestibility was significantly enhanced by diet B compared to diet A and the control diet. The impact of MDG and PL on intestinal fat absorption has been extensively studied due to their emulsifying capacity.7–10 In fact, it has been proposed that they could synergistically be employed to improve the intestinal absorption of lipophilic nutrients.28 In this study, the inclusion of MDG and soy lecithin PL (diet A) was not enough to improve maternal fat apparent absorption. However, the addition of WPPC (similar PL composition to MFGM29) as an extra source of PL (diet B) did increase intestinal fat and DHA absorption. Similarly, studies both in vitro and in vivo have suggested that PL from milk are absorbed more efficiently that those from soybean.30–32 This is associated with the lower stability of soybean PL liposomes31 and with their lower production of free FA.32 All of this suggests that the source of dietary PL is crucial to determine the intestinal absorption efficiency.
Regarding DHA and total FA status in plasma at delivery, no differences were observed in the mothers, and consequently, not in the fetuses either. The DHA statuses of the mother and the newborn are highly correlated at the time of birth.13 Nevertheless, in 14-day-old offspring from groups A and B, we observed a higher concentration of DHA and total FA in the plasma compared to the control. Valenzuela et al., in a study in rat with different sources of DHA supplementation during pregnancy, reported higher maternal tissue DHA accretion and higher milk DHA content after its dietary supplementation as PL to the mother, despite no differences being observed in maternal plasma DHA levels.33 In fact, DHA is primarily transported in blood lipoproteins in the form of PC.34 Whether a higher availability of PL structures in maternal tissues might lead to increased DHA secretion and/or milk fat digestibility for the pups needs further investigation. Nevertheless, the higher plasma DHA content observed in 14-day-old pups from groups A and B in the present study would be highly beneficial, since it has been reported that newborns with a high DHA status exhibit more mature encephalogram patterns and better attention capacity.35
The brain samples from both fetuses and pups showed higher fat content and DHA concentration and percentage. These differences were statistically significant in terms of diet A with respect to the control, with diet B following the same line, and this could have affected the brain transcriptome. In fact, in terms of the gene expression in the fetal brain, both diets A and B up-regulated 4 genes related to synaptic and developmental processes (Tph2, Pax2, Slc6a5, and Hoxa2) compared to the control. The Tph2 gene encodes tryptophan hydroxylase 2, which catalyzes the initial and rate limiting steps in serotonin biosynthesis,36 and its deletion in mice has been linked to delayed growth and persistent thinness.37 In fact, low levels of serotonin have been associated with autism.38 The Pax2 gene encodes a highly conserved transcription factor with an important role in the development of the central nervous system.39 It is involved in the differentiation of GABA precursor neurons40 and its deletion in mice has resulted in altered GABA levels and impaired synaptic processes, constituting a potential cause of anxiety-like behaviors.41,42 The Slc6a5 gene encodes a Na/Cl-dependent glycine transporter (GlyT2), responsible for glycine reuptake at presynaptic terminals.43 The activity of GlyT2 is essential for proper motor function, and its alterations have been associated with neuromotor deficiencies such as human hyperekplexia.44,45 Finally, Hoxa2 is a highly conserved transcription factor that plays a critical role in the developing brain by regulating the formation and differentiation of neural structures.46 Taking all this together, our results indicate changes in synaptic and developmental processes in the fetal brains of groups A and B compared to the control. The shared source of PL in both experimental diets was soy lecithin, which mainly contains PC and represents a source of choline. This could favor the synthesis of acetylcholine in the brain,47 essential for the growing nervous system.48 In addition, PC is important for neuronal membrane fluidity and interneuronal communication, as it is one of the most abundant PL in neuronal membranes.17
The related GO processes modified by diets A and B in the fetal brain compared to the control were development, behavior, and locomotion, among others. However, according to the higher enrichment scores, diet B affected these functions to a greater extent. Diet B contained WPPC as an additional source of PL like PC, PE, PS, and SM, including others. Early supplementation of these PL has been reported to enhance cognitive development in neonatal piglets.14 In particular, PE and PS are abundant PL in nerve cell membranes and usually contain DHA esterified in the sn-2 position.12,49 PS has been reported to support cognitive functions, including memory, learning, concentration, communication, and locomotion.15 In fact, a decline of PL in the neuronal membrane, particularly PS, has been associated with memory impairment and deficits in mental cognitive abilities.15 On the other hand, SM plays an important role in myelin integrity and function, as well as in axonal maturation.50 SM constitutes a relevant lipid during brain development from mid-gestation to the end of the first postnatal year, when central nervous system myelin dramatically increases.16 In infant formulas, PL prepared from milk are considered to be closer to human milk than soy lecithin because they contain SM.30 Thus, the supplementation of women's diet during pregnancy and lactation with these lipid matrices may improve both maternal fat and DHA bioavailability, which might influence neurodevelopment and growth during the perinatal period.
Regarding the 14-day-old pup brain gene expression, both experimental diets down-regulated the Mt2A gene which encodes metallothionein 2A, a metal-binding protein generally classified as a stress responder.51 Exposure to heavy metals or oxidative stress causes elevated gene expression of metallothioneins52 and this could be relevant in the brain since it presents a high level of oxygen consumption and is highly susceptible to oxidative stress.51 The addition of WPPC in diet B again affected GO processes to a greater extent, particularly detoxification, showing higher enrichment scores than diet A. Considering all this, Mt2A gene down-regulation in the pup brain samples of groups A and B could indicate a lower oxidative stress status in these animals. This is in line with the higher plasma and brain concentrations of DHA observed in the lactating pups of groups A and B, since omega-3 FA, especially eicosapentaenoic acid and DHA, exert antioxidant properties.53 In addition, despite the limited literature available, some studies in animal models and human neurons cultures suggest a possible antioxidant effect of PC and PS, promoting significant reductions of reactive oxygen species production.54,55 However, further studies are needed to better understand the possible antioxidant effect of MDG/PL lipid matrices in fetuses/newborns.
The main limitation of our study is that we analyzed the whole brain rather than specific regions. In addition, due to the complexity of the brain's transcriptome and its boundless molecular networks, it is challenging to determine the ultimate biological effect resulting from diets with lipid matrices. However, our study also has some strengths. We evaluated for the first time the whole-genome gene expression in the brains of fetuses and lactating pups after supplementation with different lipid matrices to the mothers.
Conclusions
In conclusion, maternal supplementation with lipid matrices containing MDG and PL affected offspring brain gene expression, related to processes like synaptic function, development, and detoxification. Increased plasma DHA and total FA concentrations were observed during lactation in 14-day-old pups in the experimental groups receiving MDG/PL supplements. The brain DHA content was also higher in both fetuses and 14-day-old pups. Thus, the inclusion of MDG/PL-rich lipid matrices in maternal supplements during pregnancy and lactation could be of great interest to ensure proper neurodevelopment of fetuses/newborns.Author contributions
VO: methodology, formal analysis, and writing – original draft. AG: methodology, formal analysis, and writing – review and editing. MJLP: methodology and writing – review and editing. PBV: dietary preparation and writing – review and editing. MV, JMLP, and BJL: writing – review and editing. MK and JPC: conceptualization and writing – review and editing. EL: conceptualization, resources, funding acquisition, supervision, project administration, and writing – review and editing.Data availability
The data that support the findings of this study are not publicly available due to funding agreements. However, the data can be provided under reasonable request.Conflicts of interest
PBV, MV, JMLP, BJL, MK, and JPC are employees at Abbott Nutrition S.L. The remaining authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.Acknowledgements
This research was funded by Abbott Nutrition S. L. (NaturLipids project). The funder was not involved in the study design, collection, analysis, and interpretation of data.References
- S. Salvati, L. Attorri, C. Avellino, A. Di Biase and M. Sanchez, Diet, lipids and brain development, Dev Neurosci, 2000, 22, 481–487 CrossRef CAS PubMed.
- B. L. G. Morgan, Nutritional requirements for normative development of the brain and behavior, Ann. N. Y. Acad. Sci., 1990, 602, 127–132 CrossRef CAS PubMed.
- L. Lauritzen, P. Brambilla, A. Mazzocchi, L. B. S. Harsløf, V. Ciappolino and C. Agostoni, DHA effects in brain development and function, Nutrients, 2016, 4, 6 CrossRef.
- R. Valenzuela, A. H. Metherel, G. Cisbani, M. E. Smith, R. Chouinard-Watkins, B. J. Klievik, L. A. Videla and R. P. Bazinet, Protein concentrations and activities of fatty acid desaturase and elongase enzymes in liver, brain, testicle, and kidney from mice: Substrate dependency, BioFactors, 2024, 50, 89–100 CrossRef CAS.
- L. A. Videla, M. C. Hernandez-Rodas, A. H. Metherel and R. Valenzuela, Influence of the nutritional status and oxidative stress in the desaturation and elongation of n-3 and n-6 polyunsaturated fatty acids: Impact on non-alcoholic fatty liver disease, Prostaglandins Leukot. Essent. Fatty Acids, 2022, 181, 102441 CrossRef CAS.
- R. A. Murphy, P. P. Devarshi, S. Ekimura, K. Marshall and S. H. Mitmesser, Long-chain omega-3 fatty acid serum concentrations across life stages in the USA: An analysis of NHANES 2011–2012, BMJ Open, 2021, 11, e043301 CrossRef PubMed.
- Y. Zhang, Y. Yang, Y. Mao, Y. Zhao, X. Li, J. Hu and Y. Li, Effects of mono- and di-glycerides/phospholipids (MDG/PL) on the bioaccessibility of lipophilic nutrients in a protein-based emulsion system, Food Funct., 2022, 13, 8168–8178 RSC.
- A. Gázquez, I. Hernández-Albaladejo and E. Larqué, Docosahexaenoic acid supplementation during pregnancy as phospholipids did not improve the incorporation of this fatty acid into rat fetal brain compared with the triglyceride form, Nutr. Res., 2017, 37, 78–86 CrossRef.
- E. Boyle and J. B. German, Monoglycerides in membrane systems, Crit. Rev. Food Sci. Nutr., 1996, 36, 785–805 CrossRef CAS.
- L. Rydhag and I. Wilton, The function of phospholipids of soybean lecithin in emulsions, J. Am. Oil Chem. Soc., 1981, 58, 830–837 CrossRef CAS.
- H. J. Kayden, J. R. Senior and F. H. Mattson, The monoglyceride pathway of fat absorption in man, J. Clin. Invest., 1967, 46, 1695–1703 CrossRef CAS.
- P. M. Kidd, Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids, Altern. Med. Rev., 2007, 12, 207–227 Search PubMed.
- L. Lauritzen and S. E. Carlson, Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status, Matern Child Nutr, 2011, 7, 41 CrossRef PubMed.
- H. Liu, E. C. Radlowski, M. S. Conrad, Y. Li, R. N. Dilger and R. W. Johnson, Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets, J. Nutr., 2014, 144, 1903 CrossRef CAS PubMed.
- M. J. Glade and K. Smith, Phosphatidylserine and the human brain, Nutrition, 2015, 31, 781–786 CrossRef CAS.
- N. Schneider, J. Hauser, M. Oliveira, E. Cazaubon, S. C. Mottaz, B. V. O’Neill, P. Steiner and S. C. L. Deoni, Sphingomyelin in brain and cognitive development: Preliminary data, eNeuro, 2019, 6(4), ENEURO.0421-18 CrossRef.
- M. Almasieh, H. Faris and L. A. Levin, Pivotal roles for membrane phospholipids in axonal degeneration, Int. J. Biochem. Cell Biol., 2022, 150, 106264 CrossRef CAS.
- S. Alashmali, C. Walchuk, C. Cadonic, B. C. Albensi, M. Aliani and M. Suh, The effect of choline availability from gestation to early development on brain and retina functions and phospholipid composition in a male mouse model, Nutr. Neurosci., 2022, 25, 1594–1608 CrossRef CAS PubMed.
- L. R. Brink and B. Lönnerdal, Milk fat globule membrane: The role of its various components in infant health and development, J. Nutr. Biochem., 2020, 85, 108465 CrossRef CAS PubMed.
- D. Yao, C. S. Ranadheera, C. Shen, W. Wei and L. Z. Cheong, Milk fat globule membrane: Composition, production and its potential as encapsulant for bioactives and probiotics, Crit. Rev. Food Sci. Nutr., 2024, 64(33), 12336–12351 CrossRef CAS PubMed.
- G. S. Raza, K. H. Herzig and J. Leppäluoto, Invited review: Milk fat globule membrane—A possible panacea for neurodevelopment, infections, cardiometabolic diseases, and frailty, J. Dairy Sci., 2021, 104, 7345–7363 CrossRef CAS PubMed.
- C. Kilkenny, W. Browne, I. C. Cuthill, M. Emerson and D. G. Altman, Animal research: Reporting in vivo experiments: The ARRIVE guidelines, Br. J. Pharmacol., 2010, 160, 1577–1579 CrossRef CAS PubMed.
- P. G. Reeves, F. H. Nielsen and G. C. Fahey, AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet, J. Nutr., 1993, 123, 1939–1951 CrossRef CAS.
- W. Stoldt, Vorschlag zur Vereinheitlichung der Fettbestimmung in Lebensmitteln, Fette und Seifen, 1952, 54, 206–207 CrossRef CAS.
- J. Folch, M. Lees and G. H. Sloane Stanley, A simple method for the isolation and purification of total lipides from animal tissues., J. Biol. Chem., 1957, 226, 497–509 CrossRef CAS.
- W. Stoffel, F. Chu and E. H. Ahrens, Analysis of long-chain fatty acids by gas–liquid chromatography, Anal. Chem., 1959, 31, 307–308 CrossRef CAS.
- E. Larqué, P. A. García-Ruiz, F. Perez-Llamas, S. Zamora and A. Gil, Dietary trans fatty acids alter the compositions of microsomes and mitochondria and the activities of microsome delta6-fatty acid desaturase and glucose-6-phosphatase in livers of pregnant rats, J. Nutr., 2003, 133, 2526–2531 CrossRef.
- Y. Zhang, Y. Yang, Y. Mao, Y. Zhao, X. Li, J. Hu and Y. Li, Effects of mono- and di-glycerides/phospholipids (MDG/PL) on the bioaccessibility of lipophilic nutrients in a protein-based emulsion system, Food Funct., 2022, 13, 8168–8178 RSC.
- M. A. Levin, K. J. Burrington and R. W. Hartel, Composition and functionality of whey protein phospholipid concentrate and delactosed permeate, J. Dairy Sci., 2016, 99, 6937–6947 CrossRef CAS PubMed.
- J. Shen, Y. Wu, T. Wei, Y. He, X. Liu, Z. Deng and J. Li, The digestion and absorption characteristics of human milk phospholipid analogs: A combination study between in vitro and in vivo, Food Funct., 2023, 14, 10617–10627 RSC.
- W. Liu, A. Ye, C. Liu, W. Liu and H. Singh, Structure and integrity of liposomes prepared from milk- or soybean-derived phospholipids during in vitro digestion, Food Res. Int., 2012, 48, 499–506 CrossRef CAS.
- X. Zhu, A. Ye, T. Verrier and H. Singh, Free fatty acid profiles of emulsified lipids during in vitro digestion with pancreatic lipase, Food Chem., 2013, 139, 398–404 CrossRef CAS.
- A. Valenzuela, S. Nieto, J. Sanhueza, M. J. Nuñez and C. Ferrer, Tissue accretion and milk content of docosahexaenoic acid in female rats after supplementation with different docosahexaenoic acid sources, Ann. Nutr. Metab., 2005, 49, 325–332 CrossRef CAS PubMed.
- W. Bernhard, C. Maas, A. Shunova, M. Mathes, K. Böckmann, C. Bleeker, J. Vek, C. F. Poets, E. Schleicher and A. R. Franz, Transport of long-chain polyunsaturated fatty acids in preterm infant plasma is dominated by phosphatidylcholine, Eur. J. Nutr., 2018, 57, 2105–2112 CrossRef CAS PubMed.
- J. Colombo, K. N. Kannass, D. J. Shaddy, S. Kundurthi, J. M. Maikranz, C. J. Anderson, O. M. Blaga and S. E. Carlson, Maternal DHA and the development of attention in infancy and toddlerhood, Child Dev, 2004, 75, 1254–1267 CrossRef.
- R. P. Patrick and B. N. Ames, Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism, FASEB J., 2014, 28, 2398–2413 CrossRef CAS.
- L. Gutknecht, N. Araragi, S. Merker, J. Waider, F. M. J. Sommerlandt, B. Mlinar, G. Baccini, U. Mayer, F. Proft, M. Hamon, A. G. Schmitt, R. Corradetti, L. Lanfumey and K. P. Lesch, Impacts of brain serotonin deficiency following Tph2 inactivation on development and raphe neuron serotonergic specification, PLoS One, 2012, 7, e43157 CrossRef CAS PubMed.
- R. P. Patrick and B. N. Ames, Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism, FASEB J., 2014, 28, 2398–2413 CrossRef CAS PubMed.
- A. Namm, A. Arend and M. Aunapuu, Expression of Pax2 protein during the formation of the central nervous system in human embryos, Folia Morphol., 2014, 73, 272–278 CrossRef CAS PubMed.
- T. Fauquier, E. Romero, F. Picou, F. Chatonnet, X. N. Nguyen, L. Quignodon and F. Flamant, Severe impairment of cerebellum development in mice expressing a dominant-negative mutation inactivating thyroid hormone receptor alpha1 isoform, Dev. Biol., 2011, 356, 350–358 CrossRef CAS.
- H. Wei, M. Wang, N. Lv, H. Yang, M. Zhao, B. Huang and R. Li, Increased repetitive self-grooming occurs in Pax2 mutant mice generated using CRISPR/Cas9, Behav. Brain Res., 2020, 393, 112803 CrossRef CAS.
- N. Lv, Y. Wang, M. Zhao, L. Dong and H. Wei, The role of PAX2 in neurodevelopment and disease, Neuropsychiatr. Dis. Treat., 2021, 17, 3559 CrossRef.
- V. Eulenburg, W. Armsen, H. Betz and J. Gomeza, Glycine transporters: Essential regulators of neurotransmission, Trends Biochem. Sci., 2005, 30, 325–333 CrossRef CAS.
- J. Gomeza, K. Ohno, S. Hülsmann, W. Armsen, V. Eulenburg, D. W. Richter, B. Laube and H. Betz, Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality, Neuron, 2003, 40, 797–806 CrossRef CAS.
- F. Zafra, I. Ibáñez and C. Giménez, Glycinergic transmission: Glycine transporter GlyT2 in neuronal pathologies, Neuronal Signaling, 2016, 1(1), NS20160009 CrossRef PubMed.
- Y. Zhao and H. Westphal, Homeobox genes and human genetic disorders, Curr. Mol. Med., 2002, 2, 13–23 CrossRef CAS.
- J. K. Blusztajn, M. Liscovitch and U. I. Richardson, Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 5474–5477 CrossRef CAS.
- S. H. Zeisel, Choline: essential for brain development and function, Adv Pediatr, 1997, 44, 263–295 CrossRef CAS.
- S. Basak, R. Mallick and A. K. Duttaroy, Maternal docosahexaenoic acid status during pregnancy and its impact on infant neurodevelopment, Nutrients, 2020, 12, 1–25 Search PubMed.
- N. Schneider, J. Hauser, M. Oliveira, E. Cazaubon, S. C. Mottaz, B. V. O’Neill, P. Steiner and S. C. L. Deoni, Sphingomyelin in brain and cognitive development: Preliminary data, eNeuro, 2019, 6(4), ENEURO.0421-18 CrossRef.
- D. Juárez-Rebollar, C. Rios, C. Nava-Ruíz and M. Méndez-Armenta, Metallothionein in brain disorders, Oxid. Med. Cell. Longevity, 2017, 5828056 CrossRef PubMed.
- E. A. Albrecht, S. M. Dhanasekaran and S. Tomlins, Immediate early inflammatory gene responses of human umbilical vein endothelial cells to hemorrhagic venom, Inflammation Res., 2011, 60, 213–217 CrossRef CAS PubMed.
- G. Li, Y. Li, B. Xiao, D. Cui, Y. Lin, J. Zeng, J. Li, M. J. Cao and J. Liu, Antioxidant activity of docosahexaenoic acid (DHA) and its regulatory roles in mitochondria, J. Agric. Food Chem., 2021, 69, 1647–1655 CrossRef CAS.
- A. F. Khafaga, Exogenous phosphatidylcholine supplementation retrieve aluminum-induced toxicity in male albino rats, Environ. Sci. Pollut. Res. Int., 2017, 24, 15589–15598 CrossRef CAS PubMed.
- H. C. Chaung, C. D. Chang, P. H. Chen, C. J. Chang, S. H. Liu and C. C. Chen, Docosahexaenoic acid and phosphatidylserine improves the antioxidant activities in vitro and in vivo and cognitive functions of the developing brain, Food Chem., 2013, 138, 342–347 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo04425h |
| This journal is © The Royal Society of Chemistry 2025 |