Open Access Article
Open Access ArticleDetermination of pentacyclic triterpenes and polyphenols from table olives in colon and plasma and their chemopreventive effects on 1,2-dimethylhydrazine-induced preneoplastic lesions in rat colon
Rocío
Moreno-González
 ,
M. Emília
Juan
,
M. Emília
Juan
 * and
Joana M.
Planas
* and
Joana M.
Planas
 *
*
Grup de Fisiologia i Nutrició Experimental (FINEX), Departament de Bioquímica i Fisiologia, Facultat de Farmàcia i Ciències de l'Alimentació and Institut de Recerca en Nutrició i Seguretat Alimentària (INSA-UB, María de Maeztu Unit of Excellence), Universitat de Barcelona (UB), Av. Joan XXIII 27-31, 08028-Barcelona, Spain. E-mail: mejuan@ub.edu; jmplanas@ub.edu; Tel: +34 93 402 45 05
First published on 4th February 2025
Abstract
Table olives are a rich dietary source of pentacyclic triterpenes (PT) and polyphenols (P), many of which have demonstrated significant antiproliferative and proapoptotic activities. This study aimed to evaluate the effect of this food on the early stages of colon carcinogenesis induced by 1,2-dimethylhydrazine (DMH) at 20 mg kg−1. Male Sprague-Dawley rats were administered either water or a suspension of Arbequina table olives (OA; 3.85 g kg−1) by gavage at 10 mL kg−1 for 49 days. Each group was then divided into two subgroups that received subcutaneous injections of the carcinogen (DMH+/Olives- and DMH+/Olives+) or the solvent (DMH−/Olives− and DMH−/Olives+) on days 8, 15, and 22. Analysis by LC-MS of AO enabled us to calculate the administered doses of PT (12.38 mg kg−1) and P (4.02 g kg−1) as well as the colon content of these compounds. At the end of the intervention, we found 5.1% of PT and 0.2% of P of the administered dose in the colonic content of the DMH+/Olives+ group. The highest concentrations were for maslinic and oleanolic acids (321 ± 67 and 84.8 ± 14.3 nmol g−1, respectively) followed by hydroxytyrosol (3.31 ± 0.24 nmol g−1). The supplementation with AO reduced aberrant crypt foci by 54.1%, and mucin depleted foci by 35.7% compared to the control group. The daily consumption of table olives exerts chemopreventive activities by reducing preneoplastic intestinal lesions, which might be explained, at least in part, by the significant concentrations of PT and P remaining in the colon.
1. Introduction
Foods derived from Olea europaea L. have attracted considerable scientific interest due to their distinctive profile of bioactive compounds, which have been found to confer significant health benefits.1 Olive oil has been extensively investigated and has been shown to have cardioprotective, anti-inflammatory and antitumour properties, among others.1 In contrast, table olives have received relatively little attention, despite sharing the same origin as olive oil and even having a higher phytochemical content.2 Consequently, our recent research has focused on how dietary supplementation with table olives can elicit health benefits by providing an adequate supply of bioactive compounds.3–5 Arbequina table olives (AO) are rich in pentacyclic triterpenes (PT, approximately 3 g kg−1) and polyphenols (P, around 1 g kg−1).6 Among them, maslinic acid and hydroxytyrosol stand out for their high concentrations and significant antitumoral activities.7,8 These chemopreventive effects have been described in human colon cancer cell lines for PT,9,10 as well as for P.11,12 The antitumor effects against colorectal cancer have also been observed in vivo for PT13–15 as well as for P.16,17 Furthermore, it is widely established that adherence to a “healthy” dietary pattern is associated with a lower risk of developing colorectal cancer.18 Notably, table olives are one of the common foods in the Mediterranean diet that are characterised by a high content of PT and P. Therefore, we set out to study whether regular intake of the right amount of AO could achieve protective effects on colon cancer.Consequently, an in vivo model of colon cancer was established using Sprague-Dawley rats in which preneoplastic lesions are induced by 1,2-dimethylhydrazine (DMH). This carcinogen leads to the formation of aberrant crypt foci (ACF), and mucin-depleted foci (MDF) that are dysplastic lesions exhibiting characteristics comparable to those observed in the most common form of intestinal neoplasia, sporadic non-familial colorectal cancer.19,20 Using this model, we investigated the effect of AO at a dose of 3.85 g of destoned olives per kg body weight which is the equivalent to human consumption of 30 AO and double the serving size recommended by the Mediterranean diet pyramid.21 To this aim, the concentrations of PT and P were determined in AO as well as in plasma and colon content. Finally, we studied the effect of the regular intake of AO on the number of preneoplastic lesions in the colon of rats. The reductions of these carcinogenic markers could provide a scientific basis for recommending table olive supplementation as a dietary source rich in bioactive compounds with chemopreventive properties.
2. Materials and methods
2.1. Chemicals and reagents
Hydroxytyrosol and hydroxytyrosol acetate were supplied by Seprox Biotech S.L. (Madrid, Spain). Caffeic acid, catechol, o-coumaric acid, p-coumaric acid, 2-(3-hydroxyphenyl) ethanol (Internal Standard (IS) of P), erythrodiol, maslinic acid, oleuropein, (+)-pinoresinol, quercetin, rutin, salidroside, vanillic acid, and verbascoside were supplied from Sigma-Aldrich (Tres Cantos, Spain). Apigenin, betulinic acid (IS of PT), luteolin, luteolin-7-O-glucoside, oleanolic acid, tyrosol, ursolic acid, and uvaol were purchased from Extrasynthèse (Genay, France). L-Ascorbic acid, 10% buffered formalin pH 7.4, phosphate-buffered solution pH 7.4 (PBS) were provided by Sigma-Aldrich. Acetone, acetonitrile, isopropanol, methanol, and tetrahydrofuran were from Panreac Química S.L.U. (Castellar del Vallès, Spain). Ethyl acetate and ethanol were acquired from J. T. Baker (Deventer, Holland) and glacial acetic acid was from Merck (Darmstadt, Germany). All chemicals were of analytical grade, and the solvents were of liquid chromatography-mass spectrometry (LC-MS) grade. Ultrapure water was employed in all experiments (Millipore, Madrid, Spain).2.2. Animals and diets
Adult male rats of the Sprague-Dawley strain (7 weeks old) were supplied from the Animal House Facility of the Facultat de Farmàcia i Ciències de l'Alimentació (Universitat de Barcelona, UB). Animals were housed in cages (n = 2 per cage) and kept under controlled conditions of temperature (22 ± 2 °C), relative humidity (50 ± 10%), and a dark-light cycle of 12 h. Rats were maintained on a 2014 Teklad Global 14% Protein Rodent Maintenance diet (Envigo RMS Spain S.L., Barcelona, Spain) and water ad libitum. The commercial diet contained (g kg−1): 150.0 protein, 40.0 fiber, 480.0 carbohydrate and 40.0 lipid, with a total metabolizable energy content of 290 kcal per 100 g of feed. All rat manipulations were performed in the morning to avoid the effects of any circadian rhythm. All animal experiments complied with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. Handling and killing were in full accordance with the ethical requirements established by the Guide for the Care and Use of Laboratory Animals. The experimental protocols were approved by the Ethics Committee of Animal Experimentation of the UB (ref. 106/17) and the Generalitat de Catalunya (ref. 9468).2.3. Arbequina table olives and dose preparation
The variety of table olives used was the Arbequina from the 2016/2017 harvest (Cooperativa del Camp, Foment Maialenc SCCL, Maials, Spain). The trees of Olea europaea L. were cultivated in Ribera d'Ebre (Tarragona, Spain) in orchards submitted to drip irrigation. Olives were picked in the green-yellow stage of maturation and were processed as natural olives in brine following the Greek style. The composition of AO consisted of (g kg−1 of olives): 210 lipids, 16 proteins, 72 fiber, and 41.3 salt. The total metabolizable energy content per 100 g of olives was of 211 kcal.AO were administered orally as a homogenous suspension at a dose of 3.85 g of destoned olives per kg of rat body weight every day for 49 days. The animal dose is equivalent to the human intake of 30 small-size Arbequina olives, calculated following the body surface area normalization method described by Reagan-Shaw et al.22 Hence, Milli-Q water was added to the edible part of the olive before being carefully ground with 6 short pulses of 30 s using a Polytron homogenizer (PT 20 TS rotor, setting 5; Kinematica AG, Lucerne, Switzerland). The olive suspension was prepared every two days and kept at 4 °C protected from light.
2.4. Determination of pentacyclic triterpenes and polyphenols in Arbequina table olives
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) that contained betulinic acid (IS of PT) and 2-(3-hydroxyphenyl) ethanol (IS of P). The mixture was vigorously vortex-mixed for 5 min and centrifuged at 3345g at 4 °C for 30 min (Megafuge 1.0R, Heraeus Instruments GmbH, Hanau, Germany). After removing the supernatant, the pellet was submitted to two additional extractions with 3 mL of methanol–ethanol (1
1; v/v) that contained betulinic acid (IS of PT) and 2-(3-hydroxyphenyl) ethanol (IS of P). The mixture was vigorously vortex-mixed for 5 min and centrifuged at 3345g at 4 °C for 30 min (Megafuge 1.0R, Heraeus Instruments GmbH, Hanau, Germany). After removing the supernatant, the pellet was submitted to two additional extractions with 3 mL of methanol–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v). The pooled supernatants were centrifuged at 25
1; v/v). The pooled supernatants were centrifuged at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min at 2 °C (Centrifuge 5417R, Eppendorf Ibérica S.L., San Sebastián de los Reyes, Spain) and filtered with a 0.45 μm PTFE syringe filter. Prior to LC-MS analysis, the filtrate was submitted to a 1
000g for 30 min at 2 °C (Centrifuge 5417R, Eppendorf Ibérica S.L., San Sebastián de los Reyes, Spain) and filtered with a 0.45 μm PTFE syringe filter. Prior to LC-MS analysis, the filtrate was submitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 dilution with methanol 80% to quantify maslinic acid, oleanolic acid, hydroxytyrosol, verbascoside, and luteolin. The other polyphenols were measured in a 1
50 dilution with methanol 80% to quantify maslinic acid, oleanolic acid, hydroxytyrosol, verbascoside, and luteolin. The other polyphenols were measured in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dilution. Finally, erythrodiol, ursolic acid, and uvaol were assessed in a non-diluted sample.
4 dilution. Finally, erythrodiol, ursolic acid, and uvaol were assessed in a non-diluted sample.
| Analyte | m/z | Arbequina table olives (mg kg−1) | Plasma (nM) | Colon content (nmol g−1) | ||
|---|---|---|---|---|---|---|
| DMH−/Olives+ | DMH+/Olives+ | DMH−/Olives+ | DMH+/Olives+ | |||
Results are expressed as means ± SEM in the analysis of table olives (n = 6) as well as the samples of plasma and colon content from DMH−/Olives + (n = 3) and DMH+/Olives + (n = 6) groups. Pentacyclic triterpenes and polyphenols in plasma and colon content were compared by Student's unpaired t test. Different from DMH− rats: *p < 0.05; φ, not detected; LOD, limit of detection (signal-to-noise ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); LOQ, limit of quantification (signal-to-noise ratio of 5 1); LOQ, limit of quantification (signal-to-noise ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). |
||||||
| Pentacyclic triterpenes | ||||||
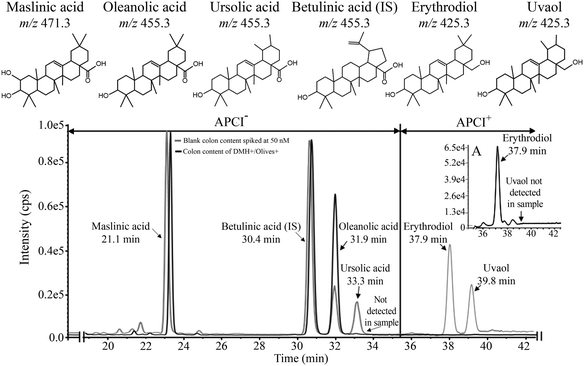
| Maslinic acid | 471.3 | 2342 ± 82.2 | 3.64 ± 0.27 | 10.1 ± 2.74* | 55.5 ± 5.22 | 321 ± 67.0* |
| Oleanolic acid | 455.3 | 862 ± 44.0 | 2.25 ± 0.17 | 3.19 ± 0.58 | 22.8 ± 1.30 | 84.8 ± 14.3* |
| Ursolic acid | 455.3 | <LOD | φ | φ | φ | φ |
| Erythrodiol | 425.3 | 10.4 ± 0.11 | φ | φ | 0.13 ± 0.01 | 1.19 ± 0.27* |
| Uvaol | 425.3 | <LOD | φ | φ | φ | φ |
| Total | 3214 ± 123 | 5.89 ± 0.10 | 13.2 ± 3.32 | 78.4 ± 6.52 | 407 ± 81.2 | |
| Phenolic compounds | ||||||
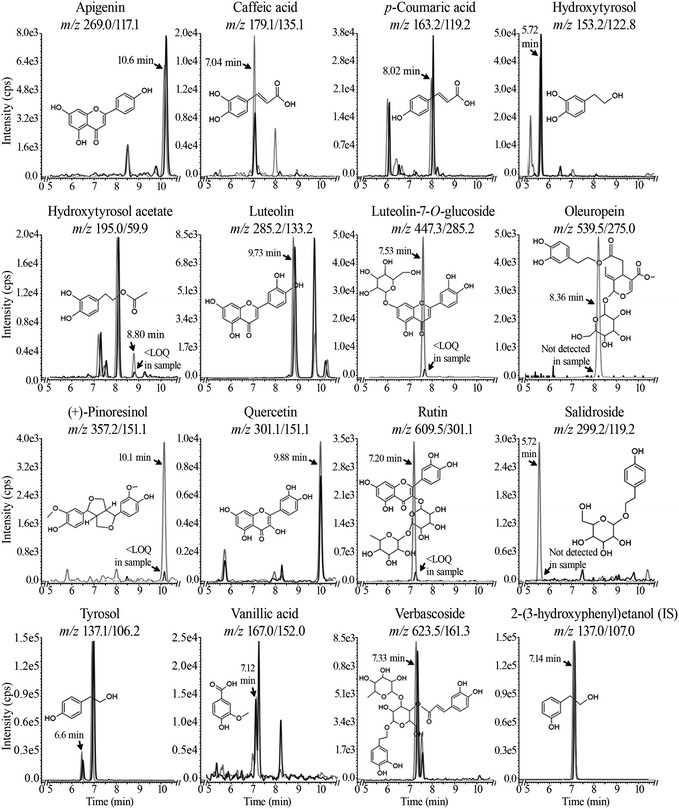
| Apigenin | 269.0/117.1 | 4.52 ± 0.17 | φ | φ | 0.47 ± 0.07 | 0.37 ± 0.02 |
| Caffeic acid | 179.1/135.1 | 4.64 ± 0.14 | φ | φ | 0.76 ± 0.23 | 0.73 ± 0.19 |
| Catechol | 108.8/91.0 | φ | φ | φ | φ | φ |
| o-Coumaric acid | 163.2/119.2 | <LOD | φ | φ | φ | φ |
| p-Coumaric acid | 163.2/119.2 | 5.65 ± 0.10 | φ | φ | 0.92 ± 0.20 | 1.83 ± 0.67 |
| Hydroxytyrosol | 153.2/122.8 | 475 ± 11.8 | 2.11 ± 0.02 | 2.18 ± 0.54 | 1.36 ± 0.30 | 3.31 ± 0.24* |
| Hydroxytyrosol acetate | 195.0/59.0 | 26.9 ± 0.71 | φ | φ | <LOQ | <LOQ |
| Luteolin | 285.2/133.2 | 89.6 ± 2.97 | φ | φ | 0.49 ± 0.19 | 0.43 ± 0.26 |
| Luteolin-7-O-glucoside | 447.3/285.2 | 11.1 ± 1.74 | φ | φ | <LOQ | <LOQ |
| Oleuropein | 539.5/275.0 | 12.6 ± 0.24 | φ | φ | φ | φ |
| (+)-Pinoresinol | 357.2/151.1 | 3.08 ± 0.22 | φ | φ | <LOQ | <LOQ |
| Quercetin | 301.1/151.1 | 6.49 ± 0.16 | φ | φ | 0.23 ± 0.03 | 0.23 ± 0.07 |
| Rutin | 609.5/301.0 | 26.0 ± 3.14 | φ | φ | <LOQ | <LOQ |
| Salidroside | 299.2/119.2 | 17.4 ± 0.98 | φ | φ | φ | φ |
| Tyrosol | 137.1/106.2 | 23.1 ± 0.58 | φ | φ | 1.17 ± 0.10 | 1.46 ± 0.19 |
| Vanillic acid | 167.0/152.0 | 3.56 ± 0.06 | φ | φ | 1.00 ± 0.13 | 1.12 ± 0.11 |
| Verbascoside | 623.5/161.3 | 334 ± 30.8 | φ | φ | 0.16 ± 0.002 | 0.20 ± 0.01 |
| Total | 1043 ± 46.9 | 2.11 ± 0.02 | 2.18 ± 0.54 | 6.56 ± 0.08 | 9.68 ± 0.77 | |
Quantification was performed using the standard addition method. Therefore, the working solutions were spiked into the filtered olive supernatant diluted either at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 or 1
50 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to match the samples. Then, calibration standards were at concentrations of 0; 0.5; 1.0; 1.5; 2.0; and 3.0 μM. Both working solutions and calibration standards were freshly prepared before each analysis. In every analytical run, a complete set of standards, including a reagent blank, was injected. Results were expressed in milligrams per kilogram of destoned olives (mg kg−1).
4 to match the samples. Then, calibration standards were at concentrations of 0; 0.5; 1.0; 1.5; 2.0; and 3.0 μM. Both working solutions and calibration standards were freshly prepared before each analysis. In every analytical run, a complete set of standards, including a reagent blank, was injected. Results were expressed in milligrams per kilogram of destoned olives (mg kg−1).
Calibration standards were used to validate the process following the recommendations of the FDA23 for accuracy, precision, linearity, and carry-over. Matrix effect was determined as described by Matuszewski24 in terms of relative standard line slope.
2.5. Experimental design
Rats were stratified by body weight and distributed into 4 groups to avoid differences in the mean initial weight. The following experimental groups were established: DMH−/Olives− (negative control: no carcinogen/no test agent; n = 3), DMH−/Olives+ (no carcinogen/Arbequina table olives; n = 3), DMH+/Olives− (positive control: DMH/no test agent; n = 6) and DMH+/Olives+ (DMH/Arbequina table olives; n = 6). Animals in the Olives+ groups were orally administered 3.85 g of destoned olives per kg rat body weight through a stainless-steel animal feeding tube (18 gauge × 76 mm, ref. FTSS-18S-76, Instech Laboratories, Inc., PA, USA) at 10 mL kg−1 every day for 49 days, whereas the ones assigned to the Olives− groups only received water. The DMH+ groups were given a subcutaneous injection of the carcinogen (20 mg DMH per kg dissolved in EDTA 1 mmol L−1, pH 6.5; 1 mL kg−1) on days 8, 15, and 22 of the experimental period. The rats in the DMH− groups were injected subcutaneously with the solvent (EDTA 1 mmol L−1, pH 6.5; 1 mL kg−1). The carcinogen was freshly prepared immediately before each administration.Throughout the study, the body weight was registered daily, whereas food and water consumption were checked once per week. Food consumption was monitored by weighing the pellets before being placed on the cage grill, and any remaining food was weighed again after one week. Additionally, the cages were inspected daily, and any residual food that had fallen to the bottom was collected and weighed. This approach allowed us to accurately account for any potential food spillage and ensure reliable calculations of total food intake. Food conversion efficiency (FCE) was determined as a percentage by dividing the weekly body weight gain by the weekly food consumption.
2.6. Sample collection
After 49 days of supplementation with AO, samples were collected between 17–19 h following the last oral administration. Hence, overnight fasted rats were anesthetized with 90 mg kg−1 of ketamine (Imalgene 100 mg mL−1, Merial Laboratorios S.A., Barcelona, Spain) and 10 mg kg−1 of xylacine (Rompun 20 mg mL−1, Bayer Hispania S.L., Barcelona, Spain). Blood samples were obtained by cardiac puncture and collected into 4 mL EDTA-K3 tubes (Sarstedt, Nümbrecht, Germany). The samples were then centrifuged at 3345g for 15 min at 4 °C (Megafuge 1.0R) to separate the plasma, which was kept at −80 °C until analysis by LC-MS.Immediately following blood collection, a gross necropsy was performed. The liver, brain, lungs, kidneys, testicles, spleen, heart, and thymus were removed, separated from any adherent mesenteric tissue, and their wet weights were immediately recorded. Results are expressed as organ weight relative to 100 g of body weight (%).
Then, the colon was excised, and the intestinal lumen was flushed with 1 mL of cold PBS (pH 7.4) to obtain the intestinal content that was stored at −80 °C until analysis by LC-MS. Subsequently, the colonic tissue was washed with PBS, trimmed from mesenteric tissue, and divided into three segments of similar length: proximal (close to the cecum), medial, and distal (close to the rectum).
2.7. Determination of pentacyclic triterpenes and polyphenols in plasma and colon content
PT and P were quantified in both DMH−/Olives+ and DMH+/Olives+ groups using the validated methods established for plasma by Kundisová et al.25 and colon content by Lozano-Mena et al.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; v/v) with betulinic acid (IS of PT) and 2-(3-hydroxyphenyl) ethanol (IS of P). Samples were homogenized using 2 pulses of 30 seconds each in a Polytron homogenizer (PT 10 TS rotor, setting 6). The tip was rinsed four times with 1 mL of 80% methanol to collect the remaining solid particles and to obtain a final pooled volume of 8 mL. Then, samples were shaken in a vortex (5 min), sonicated (10 min), and centrifuged (16400g, 30 min, 2 °C; Centrifuge 5417R). Before LC-MS analysis, the clear supernatant was filtered with a 0.45 μm PTFE syringe. For the analysis of maslinic acid and oleanolic acid, the filtered supernatant was diluted 1
20; v/v) with betulinic acid (IS of PT) and 2-(3-hydroxyphenyl) ethanol (IS of P). Samples were homogenized using 2 pulses of 30 seconds each in a Polytron homogenizer (PT 10 TS rotor, setting 6). The tip was rinsed four times with 1 mL of 80% methanol to collect the remaining solid particles and to obtain a final pooled volume of 8 mL. Then, samples were shaken in a vortex (5 min), sonicated (10 min), and centrifuged (16400g, 30 min, 2 °C; Centrifuge 5417R). Before LC-MS analysis, the clear supernatant was filtered with a 0.45 μm PTFE syringe. For the analysis of maslinic acid and oleanolic acid, the filtered supernatant was diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. The non-diluted filtered supernatant was used for the determination of the other compounds.
25. The non-diluted filtered supernatant was used for the determination of the other compounds.
| Analyte | m/z | Plasma (nM) | Colon content (nmol g−1) | ||||
|---|---|---|---|---|---|---|---|
| RT (min) | DMH−/Olives+ | DMH+/Olives+ | RT (min) | DMH−/Olives+ | DMH+/Olives+ | ||
| Results are expressed as means ± SEM in the analysis of the samples of plasma and colon content from DMH−/Olives+ (n = 3) and DMH+/olives+ (n = 6) groups. Data were analysed by Student's unpaired t test. Different from DMH− rats: *p < 0.05; φ, not detected. | |||||||
| Metabolites of maslinic acid | |||||||
| Monohydroxylation | |||||||
| MA-M1a | 487.3 | 13.8 | 2.01 ± 0.65 | 3.71 ± 1.33 | 14.0 | 17.2 ± 1.48 | 35.1 ± 2.38* |
| MA-M1b | — | φ | φ | 15.0 | 12.1 ± 0.31 | 18.3 ± 1.05* | |
| Monohydroxylation and dehydrogenation | |||||||
| MA-M2 | 485.3 | — | φ | φ | 16.7 | 14.0 ± 2.05 | 15.6 ± 0.63 |
| Dihydroxylation | |||||||
| MA-M3 | 503.3 | — | φ | φ | 10.8 | 10.6 ± 0.22 | 11.1 ± 0.08 |
| Dihydroxylation and dehydrogenation | |||||||
| MA-M4 | 501.3 | — | φ | φ | 13.8 | 10.6 ± 0.32 | 11.1 ± 0.16 |
| Metabolites of oleanolic acid | |||||||
| Monohydroxylation | |||||||
| OA-M1 | 471.5 | 17.0 | φ | 3.56 ± 0.78 | — | φ | φ |
| Monohydroxylation and dehydrogenation | |||||||
| OA-M2 | 469.5 | 13.8 | φ | 0.37 ± 0.05 | 14.0 | 5.54 ± 0.32 | 7.32 ± 0.09 |
| Sulfation | |||||||
| OA-M3a | 535.5 | — | φ | φ | 13.6 | 4.40 ± 0.01 | 4.55 ± 0.04 |
| OA-M3b | — | φ | φ | 14.3 | 4.43 ± 0.04 | 4.53 ± 0.04 | |
| Metabolites of hydroxytyrosol | |||||||
| Sulfation | |||||||
| Hty-M1a | 233.0/153.2 | 5.50 | 1.55 ± 0.1 | 2.15 ± 0.66 | 5.40 | 0.22 ± 0.05 | 0.33 ± 0.04 |
| Hty-M1b | 5.65 | 3.17 ± 0.2 | 5.84 ± 2.46 | — | φ | φ | |
| Glucuronidation | |||||||
| Hty-M2 | 329.0/153.2 | — | φ | φ | 6.64 | 0.06 ± 0.001 | 0.06 ± 0.005 |
| Metabolites of luteolin | |||||||
| Sulfation | |||||||
| Lu-M1 | 365.0/285.2 | — | φ | φ | 10.8 | 0.04 ± 0.02 | 0.05 ± 0.01 |
| Glucuronidation | |||||||
| Lu-M2 | 461.2/285.2 | — | φ | φ | — | 0.007 ± 0.001 | 0.009 ± 0.001 |
2.8. Aberrant crypt foci in colon
Aberrant crypt foci (ACF) were assessed following the method described by Bird.27 Proximal, medial, and distal colon segments were opened along the longitudinal median, pinned flat onto a polystyrene board and fixed in 10% buffered formalin (pH 7.4) for at least 24 h. Fixed segments were washed out with PBS, then stained with methylene blue 0.2% for 8 min (proximal) or 10 min (medial and distal). Excess dye was removed by rinsing the tissues with PBS. For analysis, segments were placed mucosa-side up on slides and observed under a light microscope at 10× magnification (BX41, Olympus Corporation, Tokyo, Japan). ACF were identified based on the criteria described by Bird.27 Lesions are characterized by a thickened epithelial layer that displays a more intense stain, distorted, slit-like luminal openings, and larger size (2–3 times that of normal surrounding crypts). Aberrant crypts (AC) were determined as a single altered crypt or as a cluster of altered crypts that form a focus termed ACF. The number of ACF was counted in the proximal, medial, and distal segments of each rat. Crypt multiplicity was assessed as the number of AC per focus. The scores were checked by two independent observers who were blinded to the treatments. Images of the mucosal surface were taken with an XC 50-UTV1X-2 camera (Olympus Corporation, Tokyo, Japan).2.9. Mucin depleted foci in colon
After counting ACF, colon segments were kept in PBS at 4 °C until being dyed with the high-iron diamine/alcian blue/neutral red staining (HID-AB) to assess mucin production.28 The segments were first washed with PBS and stained with high-iron diamine for 18–24 h at room temperature, protected from the light. Then, tissues were rinsed in PBS and stained with alcian blue 1% in acetic acid 3% for 5 min. Subsequently, tissues were washed again, and finally stained for 2 min in neutral red 0.1% with acetic acid 0.002%. Then, the stained segments were placed in slides with the mucosal side up and examined under a light microscope at 20× magnification. Mucin-depleted foci (MDF) were identified by their lack of mucins, distorted lumen openings, and elevated lesions above the colon surface.28 The number of MDF and the multiplicity expressed as mucin-depleted aberrant crypts (MDAC) per aberrant focus were determined. Scores were evaluated by two independent, blinded observers.2.10. Statistical analysis
Results are presented as means ± standard error of the means (SEMs). Outliers were rejected using Chauvenet's criterion. Data evaluation, statistical analysis, and elaboration of graphs were done using Prism version 6 (GraphPad Software Inc., San Diego, CA, USA). Normality was assessed with the Kolmogorov–Smirnov test and based on the results, a parametric or non-parametric analysis was performed. Statistical analysis was performed by two-way ANOVA followed by Tukey's multiple comparison test. When, body weight, water intake, food consumption, and FCE were the dependent variables, the two-way ANOVA analysis used experimental model (DMH− or DMH+) and treatment (water or AO) as the independent variables. For ACF and MDF counts as dependent variables, the analysis incorporated the intestinal segments (proximal, medial or distal) and treatment (water or AO) as independent variables. For multiplicity of ACF and MDF, the analysed considered the number of crypts (1, 2, 3 or ≥4) and treatment (water or AO) as independent variables. Significant differences of ACF and MDF in total colon, as well as AC and MDAC were compared using Student's unpaired t test. Differences in the concentration of PT and P and their metabolites in plasma and colon content were established by Student's unpaired t test. For all tests three levels were considered as significant: *p < 0.05; ** p < 0.01; and ***p < 0.001.3. Results
3.1. Determination of pentacyclic triterpenes and polyphenols in Arbequina table olives
The total PT in AO were 3214 ± 123 mg kg−1, exceeding the total P content of 1043 ± 46.9 mg kg−1. Analysis of the PT revealed three compounds: maslinic acid accounting for 72.9%, oleanolic acid which represented a 26.8% and erythrodiol constituting 0.3% of the total PT content (Table 1). Regarding the total P content, 15 compounds were identified. The most abundant was hydroxytyrosol accounting for 45.5% of the total of P, followed by verbascoside comprising 32%, and luteolin representing an 8.6%. Twelve additional phenolic compounds were identified, collectively accounting for 13.9% of total P (Table 1).
3.2. Body weight, food and water consumption, and food conversion efficiency
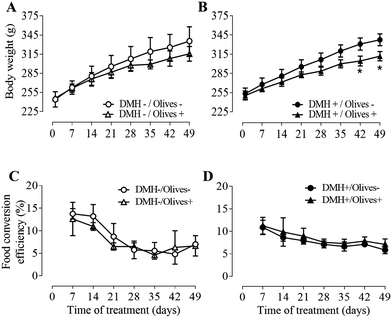
Animals in the DMH−/Olives− group showed an initial body weight of 244 ± 12 g which increased to 335 ± 22 g after 7 weeks, representing a weight gain of 91.0 ± 0.5 g (Fig. 1A). In contrast, the DMH−/Olives+ group had an initial body weight of 246 ± 2 g that rose to 315 ± 12 g by week 7. The DMH-/Olives+ rats gained 69.0 ± 9.5 g, which was 24% lower (p < 0.05) than the weight gain observed in the DMH−/Olives− group.A similar trend was observed in the groups treated with DMH (Fig. 1A). The DMH+/Olives− rats had an initial mean body weight of 253 ± 9 g that increased to 337 ± 9 g by day 49. Over the same period, the body weight of the DMH+/Olives+ group rose from 250 ± 7 g to 311 ± 7 g. Hence, the DMH+/Olives− rats gained 84.2 ± 2.6 g while the DMH+/Olives+ rats increased 61.3 ± 6.9 g, which was 27% lower (p < 0.05) than the positive control group.
Concerning the food consumption or water intake, no significant differences were found between the control and the treated groups. Daily energy consumption was monitored throughout the study period. In the olive-treated groups, the total energy intake comprised both dietary food consumption and energy provided by olive gavage. Daily energy intake was consistent across all experimental groups, with mean values of 54.2 ± 0.9 kcal in DMH−/Olives− rats, 50.6 ± 0.8 kcal in DMH−/Olives + rats, 53.0 ± 0.9 kcal in DMH+/Olives− rats, and 49.7 ± 1.2 kcal in DMH+/Olives+ rats.
FCE was highest during the first week, decreased during the second and third weeks, and remained constant until the end of the experiment (Fig. 1B). No significant differences were observed for FCE in both DMH− and DMH+ groups.
3.3. General observation and gross necropsy
All rats were routinely monitored throughout the study, and no adverse effects or mortality were found. Stool consistency was pelleted and firm, with no visible differences between groups. At the end of the experiment, animals were submitted to a thorough post-mortem examination of the internal organs. No macroscopic changes in size, colour, or texture were observed in any of the groups, upon examination of the liver, brain, lungs, kidneys, testicles, spleen, heart, and thymus. The supplementation with AO at a dose of 3.85 g kg−1 did not significantly affect the final relative weight (g per 100 g body weight) of the examined organs (p > 0.05).3.4. Determination of pentacyclic triterpenes, polyphenols, and their metabolites in rat plasma
Oleanolic acid was detected at 2.25 ± 0.17 nM in DMH+/Olives− rats and no metabolites were found (Table 1). In contrast, in DMH+/Olives+ rats, the parent compound (3.19 ± 0.58 nM) was accompanied by two Phase I metabolites: a monohydroxylated derivative (50.0%) and a monohydroxylated and dehydrogenated derivative (5.24%) (Table 2).
The plasma concentrations of hydroxytyrosol were around 2 nM in both groups (Table 1). This compound underwent extensive Phase II metabolism, mainly forming two sulfate (Hty-M1) isomers (Table 2). A similar pattern was observed in DMH−/Olives+ and DMH+/Olives− groups, with the parent compound representing around 25%, with the two sulfate isomers representing approximately 22% and 52% respectively.
3.5. Determination of pentacyclic triterpenes, polyphenols, and their metabolites in colon content
Maslinic acid was the predominant bioactive compound in AO and in the colon content, found at 55.5 ± 5.22 nmol g−1 in the DMH−/Olives+ group and 321 ± 67.0 nmol g−1 in the DMH+/Olives+ rats (Table 1). Metabolite screening identified 5 compounds, all from Phase I reactions (Table 2). Despite searching for Phase II derivatives, none of the most common conjugates were found. The identified metabolites were categorized into four groups: monohydroxylated (MA-M1, including two isomers), monohydroxylated and dehydrogenated (MA-M2), dihydroxylated (MA-M3), and dihydroxylated and dehydrogenated (MA-M4). In the DMH−/Olives+ group, maslinic acid constituted 46.2% of the total, with metabolites making up 53.8%. In contrast, in the DMH+/Olives+ group, maslinic acid represented 78.0%, and metabolites accounted for only 22.0% (Table 2).
Oleanolic acid, the second most prevalent bioactive compound in AO, was also the second most abundant in the colon content, at concentrations of 22.8 ± 1.30 nmol g−1 in the DMH−/Olives+ rats, and 84.8 ± 14.3 nmol g−1 in the DMH+/Olives+ animals. Three metabolites were identified: one Phase I (monohydroxylated and dehydrogenated, OA-M2) and two Phase II sulfate conjugates (OA-M3). The parent compound was predominant in both groups, with higher relative abundance in DMH+/Olives+ rats (61.3%) compared to DMH−/Olives+ rats (83.8%).
Hydroxytyrosol was the third most abundant compound in colon content with concentrations of 1.36 ± 0.30 nmol g−1 in the DMH−/Olives+ rats and 3.31 ± 0.24 nmol g−1 in the DMH+/Olives+ animals, respectively (Table 1). This phenolic alcohol underwent Phase II biotransformation, resulting in two sulfate conjugates (Hty-M1) and one glucuronide conjugate (Hty-M2). In both groups, the sulfate derivative comprised 12%, the glucuronide 3%, and hydroxytyrosol 85% of the total.
Interestingly, although p-coumaric acid is present in olives in minor quantities, it was the fourth most abundant compound in the colon, with concentrations of 0.92 ± 0.20 nmol g−1 in DMH−/Olives+ rats and 1.83 ± 0.67 nmol g−1 in DMH+/Olives+ rats. Other bioactive compounds were present at similar concentrations across both groups, ranging from 1.46 ± 0.19 nmol g−1 for tyrosol to 0.16 ± 0.002 nmol g−1 for verbascoside (Table 1).
3.6. Aberrant crypt foci in colon
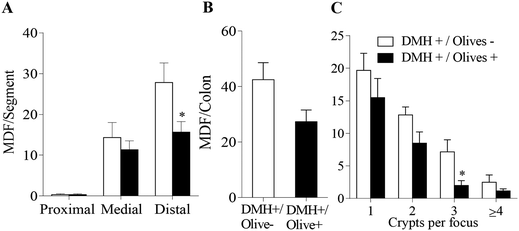
No preneoplastic ACF were observed in the DMH−/Olives− and DMH−/Olives+ groups, so the results focus on the DMH-treated groups only (Fig. 4).ACF exhibited a regional distribution pattern along the colon in DMH-treated rats, with fewer lesions in the proximal colon that progressively increased towards the distal colon (Fig. 4A). This distribution pattern was consistent across all groups. Oral administration of table olives at 3.85 g kg−1 for 49 days reduced ACF numbers by 43.5% (p < 0.05), 45.9% (p < 0.05), and 60.0% (p < 0.01) in the proximal, medial, and distal colon segments, respectively (Fig. 4A). Overall, ACF numbers in the total colon decreased by 54.1% (p < 0.01), from 114 ± 14 crypts in the DMH+/Olives− group to 52 ± 5 crypts in the DMH+/Olives+ group (Fig. 4B).
Crypt multiplicity analysis revealed that most ACF in both DMH-treated groups, consisted of single crypts, with fewer foci containing 2, 3, or ≥4 crypts (Fig. 4C). AO supplementation reduced ACF with 1, 2, 3, and 4 crypts in the total colon by 52.0% (p < 0.001), 56.1% (p < 0.01), 63.0% (p < 0.01), and 38.5% (p > 0.05), respectively. Total aberrant crypts in the colon dropped by 55.3% from 174 ± 23.2 to 78 ± 5.8 crypts (p < 0.01) with AO supplementation.
3.7. Mucin depleted foci in colon
MDF showed a distribution pattern along the colon similar to ACF but in lower numbers (Fig. 5). The consumption of AOs reduced MDF by 20.9% (p > 0.05) and 43.7% (p < 0.05) in the medial and distal colon segments, respectively (Fig. 5A), with a total MDF decrease of 35.7% (p > 0.05) (Fig. 5B).Crypt multiplicity was also analysed for MDAC (Fig. 5C). AO reduced MDAC with 1 crypt by 21.2% (p > 0.05); of 2 crypts by 33.8% (p > 0.05), of 3 crypts by 72.1% (p < 0.05), and ≥4 crypts by 53.3% (p > 0.05). Furthermore, the total number of MDAC was reduced in a 43.9% (p < 0.05) after the supplementation with AO.
4. Discussion
Colorectal cancer is one of the most prevalent cancers worldwide. While genetics play a role in individual risk, the incidence is largely influenced by modifiable environmental factors, particularly lifestyle and dietary habits.18 Key preventive measures include adopting a diet rich in fruits and vegetables, which provide essential micronutrients, dietary fiber, and phytochemicals.18 In this context, table olives could be incorporated into a dietary pattern aimed at decreasing the risk of developing colorectal cancer, due to their unique profile of beneficial compounds, including PT and P.2 Therefore, this study aims to evaluate the effect of AO on the development of early markers of colon carcinogenesis in DMH-treated rats. Moreover, to better understand the potential role of bioactive compounds from AO in colorectal cancer prevention, we analysed PT and P by LC-MS. First, the determination was performed in the food itself to quantify their concentration in the administered dose. Subsequently, we measured them in plasma and colon content, to assess how much of PT and P reached the target organ.The analysis of AO revealed distinct differences in PT and P content and align with previous studies on Greek-style processed table olives across various cultivars. Our observed PT values (around 3 g kg−1) fall within the previously reported range (2.10 to 3.28 g kg−1) for cultivars such as Empeltre, Manzanilla, Cellina di Nardò, Hojiblanca, Conservolea, and Kalamata.6,29,30 Similarly, our P content (around 1 g kg−1) is consistent with the range (0.35 to 1.20 g kg−1) reported for cultivars including Marfil, Empeltre, Cellina de Nardò, Bella di Cerignola, Termite di Bitetto, and Manzanilla.6,31–33 The supplementation was equivalent to the human consumption of 30 AO, which doubles the recommended serving size according to the Mediterranean diet pyramid.21 Consequently, rats received a dose of 3.85 g AO per kg, supplying 12.38 mg of PT per kg body weight and 4.02 mg of P kg−1. Among them, the most abundant was maslinic acid (9.02 mg kg−1), followed by oleanolic acid (3.32 mg kg−1), hydroxytyrosol (1.83 mg kg−1), verbascoside (1.29 mg kg−1) and luteolin (0.34 mg kg−1). Hence, AO provided substantial amounts of PT and P with demonstrated colon cancer chemopreventive activities in vivo.13–17
After administration, the analysis of PT and P in plasma yielded low concentrations of only three parent compounds (Table 1): maslinic acid, oleanolic acid and hydroxytyrosol at the end of the 7-week intervention and 17–19 hours after the final dose of AO. In our targeted metabolomics approach, we searched for both Phase I and Phase II metabolites of the main pentacyclic triterpenes and polyphenols. Phase I metabolism primarily involves oxidation, reduction, and hydrolysis reactions, whereas Phase II metabolism includes conjugation reactions.34 These metabolic processes occur predominantly in the liver, although other organs can also contribute to metabolize different compounds from the diet. Analysis of plasma samples revealed that 73% of maslinic acid remained unaltered, while 27% was present as a monohydroxylated metabolite, consistent with previous studies showing limited Phase I biotransformation of this compound.35 Conversely, oleanolic acid and hydroxytyrosol underwent significant biotransformation upon absorption and only approximately 45% of oleanolic acid and a mere 21% of hydroxytyrosol were detected in their unaltered forms in plasma. Oleanolic acid yielded two Phase I metabolites whereas hydroxytyrosol underwent Phase II transformation, resulting in two sulfate conjugates. These findings agree with those obtained in rats after the administration of pure compounds.5,36,37 Our results in plasma confirm that the bioactive compounds from Arbequina table olives are effectively absorbed, reaching the blood.
Although previous studies have reported on the plasma concentrations of PT and P following table olive consumption,5,25,38 there is a lack of data regarding the disposition of these compounds in colon content after oral administration of this food. To address this, we employed an analytical method previously developed for the analysis of maslinic acid in intestinal content.26 The validation of the procedure indicated a lack of matrix effect, as well as adequate sensitivity, linearity, precision and accuracy thus demonstrating its suitability for the measurement of PT and P in colon content. The application of the analytical method showed that three PT and nine P from those determined in AO, were detected in the colon content at higher amounts than in plasma. Previous research by Cameron et al.39 reported that rats injected with DMH exhibited significantly prolonged transit times of 36.4 ± 3.8 hours compared to 25.4 ± 4.2 hours in negative control animals not exposed to DMH. This delayed transit in the DMH-treated group could allow for an augmented exposure duration of bioactive compounds in the intestinal lumen before excretion occurs. To get insight into the bioavailability and potential efficacy of these compounds at the target site for colon cancer prevention, we calculated the percentage of PT and P reaching the colon relative to the amount supplemented to the rats. The most abundant compound in the colonic content in DMH+/olives+ was maslinic acid that was found at 321 nmol g−1, which represented a 5.6% of the administered dose. In the colonic content, 78% of maslinic acid remained unaltered, while five distinct Phase I metabolites represented 22% of the total maslinic acid detected. The second compound in abundance was oleanolic acid (84.8 nmol g−1) that was found at 3.9% from the amounts ingested in the olives. This PT was detected intact in an 84%, whereas a 16% underwent transformation, yielding one Phase I and two Phase II metabolites. Concerning PT, erythrodiol that was found in Arbequina table olives at very low amounts was detected in the colonic content at 1.19 nmol g−1 which constitutes a 4.3% of the dose administered. Unlike the PT, where all three compounds present in table olives were detected in the colon content, P did not exhibit the same pattern, and only a 0.2% of the administered dose were found in the colon. The most abundant P in Arbequina table olives were not the ones with the higher concentrations in the colon. The compounds detected in the colon included hydroxytyrosol, p-coumaric acid, tyrosol, vanillic acid, caffeic acid, luteolin, apigenin, quercetin, and verbascoside. Notably, the results for p-coumaric acid, vanillic acid, caffeic acid, and apigenin in the colon content are particularly significant, given that these compounds were present in AO at very low concentrations. Additionally, we did not detect hydroxytyrosol acetate, rutin, salidroside, oleuropein or luteolin-7-O-glucoside. Our results agree with studies on the effects of the gastrointestinal digestion on table olives through a simulated in vitro gastrointestinal digestion, which describe a progressive decrease in the concentrations of P along the gut, reaching the lowest amounts in the large intestine.40,41 Furthermore, our findings are in accordance with D'Antuono et al.40 who reported the breakdown of oleuropein into hydroxytyrosol, verbascoside into hydroxytyrosol and caffeic acid, salidroside into tyrosol or comselogoside into p-coumaric acid, among others. These transformations, may explain, in part, our results where various P appeared in colonic content that were originally present in AO at very low concentrations, and the absence of other P initially present in the food.
The cancer chemopreventive activities of AO was investigated using an experimental design previously established in our laboratory consisting in three subcutaneous injections of DMH, followed by an observation period of 4 weeks.42 This protocol led to the formation of preneoplastic lesions, ACF and MDF, in a number that was consistent with previous studies in our group15,41 as well as for other authors.13,14,16,17 No signs of toxicity were observed during the experimental period, and neither food nor water intake was affected by either AO or the DMH treatment. Body weight remained unaffected by DMH administration. However, supplementation with AO induced a small decrease in total body weight of 24% (DMH−/olives+) and 27% (DMH+/olives+) compared to their respective controls. These findings agree with the weight reducing effects of different olive oils enriched in pentacyclic triterpenes and polyphenols elicited in various experimental models.43–45
The administration of AO over a 49-day period exerted a chemopreventive effect, as evidenced by a 54.1% reduction in the total number of ACF and a 35.7% decrease in MDF in the colon. Furthermore, AO influenced the multiplicity (number of crypts per focus) of ACF and MDF, with a decrease in the number of foci containing 1, 2, 3, and ≥4 aberrant crypts. We assessed the development of ACF in the DMH−/olives+ group to confirm that table olive supplementation alone did not promote the formation of these preneoplastic lesions. Previous research has demonstrated that caloric restriction, which leads to reduced body weight, significantly inhibits the formation of new ACF and delays the progression of existing ACF in the AOM-induced colorectal cancer model in rats.46 Consistent with these findings, the reduced body weight observed in rats supplemented with AO may have contributed, in part, to the decreased ACF number and multiplicity found in our study. Moreover, the significant reduction in ACF coincides with the presence of bioactive compounds in the colon content, suggesting a potential relationship between their concentration and chemopreventive effects. Maslinic acid was the bioactive compound that we have found in higher concentration in the colon (321 nmol g−1) after the supplemented by gavage of AO that contained a dose of 9 mg kg−1 of this compound and resulted in a reduction of ACF of 54%. A previous study from our group, addressing the effect of maslinic acid in the same animal model, revealed that when administered by gavage at 10 mg kg−1, the concentration achieved in the colon was lower (90 nmol g−1) and the reduction of ACF was only of a 18%.15 The higher reduction achieved with AO compared to the pure compound may be attributed to the greater concentration of bioactive compounds in the colon, correlating with in vitro findings in HT29 and Caco-2 colon cancer cell lines.9,10 These studies showed a dose-dependent relationship, with increasingly pronounced antiproliferative and pro-apoptotic effects at higher concentrations of maslinic acid. However, in evaluating the results, we cannot rule out the effect of the other compounds from this food, since in addition to PT and P, AO are rich in unsaturated fatty acids, essential amino acids, minerals, vitamins and fiber.2
There is no previous data on the effects of regular table olive intake on colon cancer in vivo, although this murine model has been used to evaluate the chemopreventive properties of olive oil.47–49 While Femia et al.47 did not find any effect of extra virgin olive oil (EVOO) on colon carcinogenesis, regardless of its P content, França et al.48 observed a reduction in the number of ACF. This finding was corroborated by Nanda et al.,49 who reported that EVOO lowered tumour incidence and inhibited tumour development. Although the reduction of colonic tumorigenesis has been associated with the high content of monounsaturated fatty acids, particularly oleic acid, the presence of other minor compounds cannot be disregarded.48,49 In this regard, the effects on DMH-induced colon carcinogenesis in rats have been evaluated for various PT, including maslinic acid15 and oleanolic acid13,14 as well as P like p-coumaric acid17 and luteolin.16 The research on the chemopreventive properties of AO reveals a complex interplay of multiple bioactive compounds that act synergistically, producing a more potent effect than individual components alone, thus highlighting the importance of studying whole foods.
The chemopreventive activity of AO could be associated not only with the anti-tumour properties but also with their positive influence on gut microbiota. Studies have shown that supplementation with table olives can increase beneficial bacterial genera and probiotic strains,4 contributing to their chemopreventive effects by reshaping the gut microbiota, which is crucial since gut dysbiosis is associated with colorectal cancer development.50 Moreover, the anti-inflammatory properties described for PT and P could also play a role.8,51 These bioactive compounds have also been reported to suppress the pro-inflammatory NF-κB pathway implicated in colon cancer progression, and by downregulating iNOS and COX-2 expression, they can target the local inflammation detected in early mucin-depleted foci stages.8,51 Moreover, the inflammation and Wnt/β-catenin pathway activation in the colonic mucosa leads to increased transcription of proliferative genes like c-Myc, c-Jun, cyclin-D1 as well as anti-apoptotic Bcl-2, Bcl-xl, p53 that could be modulated by PT and P8,51 contributing to the antitumoral activity induced by OA.
5. Conclusion
Our findings demonstrate that bioactive compounds from AO exhibit differential bioavailability and colonic exposure. Table olives appear to be a particularly effective vehicle for delivering bioactive compounds to the colon, thus 5.1% of the PT and 0.2% of P from the administered dose arrived unmetabolized to this region. This localized exposure holds significance as these phytochemicals may contribute to the cancer chemopreventive activities exerted by AO that diminished early preneoplastic lesions, both ACF and MDF in rat colon. Thus, these results suggest that the daily consumption of table olives may confer health benefits, particularly concerning colon cancer prevention.Author contributions
Rocío Moreno-González: investigation, formal analysis, data curation, writing – original draft, writing – review and editing, visualization. M. Emília Juan: investigation, methodology, formal analysis, data curation, validation, writing – original draft, writing – review and editing, visualization, supervision. Joana M. Planas: conceptualization, methodology, formal analysis, data curation, validation, writing – original draft, writing – review and editing, visualization, supervision, resources, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.Data availability
Data for this paper, including body weight, food and water consumption, organ weight, ACF, MDF and LC-MS data of pentacyclic triterpenes and polyphenols in Arbequina table olives, plasma and colon content are available at the Science Data Bank at https://doi.org/10.57760/sciencedb.12841.Conflicts of interest
The authors declared no conflict of interest.Acknowledgements
This work was funded by grants AGL2013-41188 from Ministerio de Economía y Competitividad and 2017SGR945 and 2021SGR300 from Generalitat de Catalunya, Spain. Institut de Recerca en Nutrició i Seguretat Alimentària (INSA-UB) is María de Maeztu Unit of Excellence (grant CEX2021-001234-M) funded by MICIN/AEI/FEDER. R.M-G. was a recipient of Ayuda para Contratos Predoctorales para la Formación de Doctores (BES-2014-06945) (MINECO). Arbequina table olives were kindle supplied by Cooperativa del Camp Foment Maialenc SCCL (Maials, Lleida). The authors thank Dr Isidre Casals, Olga Jáuregui and Alberto Adeva from CCiTUB for technical assistance and advice.References
- A. Isaakidis, J. E. Maghariki, S. Carvalho-Barros, A. M. Gomes and M. Correia, Is there more to olive oil than healthy lipids?, Nutrients, 2023, 15, 3625 CrossRef CAS PubMed.
- D. Boskou, Table olives: a vehicle for the delivery of bioactive compounds, J. Exp. Food Chem., 2017, 3, 1 Search PubMed.
- T. Franco-Ávila, R. Moreno-González, M. E. Juan and J. M. Planas, Table olive elicits antihypertensive activity in spontaneously hypertensive rats, J. Sci. Food Agric., 2023, 103, 64–72 CrossRef PubMed.
- A. Gómez-Contreras, T. Franco-Ávila, L. Miró, M. E. Juan, M. Moreto and J. M. Planas, Dietary intake of table olives exerts antihypertensive effects in association with changes in gut microbiota in spontaneously hypertensive rats, Food Funct., 2023, 14, 2793–2806 RSC.
- I. Kundisová, H. Colom, M. E. Juan and J. M. Planas, Pharmacokinetics of hydroxytyrosol and its sulfate and glucuronide metabolites after the oral administration of table olives to Sprague-Dawley rats, J. Agric. Food Chem., 2024, 72, 2154–2164 CrossRef PubMed.
- R. Moreno-González, M. E. Juan and J. M. Planas, Profiling of pentacyclic triterpenes and polyphenols by LC-MS in Arbequina and Empeltre table olives, LWT – Food Sci. Technol., 2020, 126, 109310 CrossRef.
- G. Lozano-Mena, M. Sánchez-González, M. E. Juan and J. M. Planas, Maslinic acid, a natural phytoalexin-type triterpene from olives-a promising nutraceutical?, Molecules, 2014, 19, 11538–11559 CrossRef PubMed.
- A. Sain, S. Sahu and D. Naskar, Potential of olive oil and its phenolic compounds as therapeutic intervention against colorectal cancer: a comprehensive review, Br. J. Nutr., 2022, 128, 1257–1273 CrossRef CAS PubMed.
- M. E. Juan, J. M. Planas, V. Ruiz-Gutierrez, H. Daniel and U. Wenzel, Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells, Br. J. Nutr., 2008, 100, 36–43 CrossRef CAS PubMed.
- F. J. Reyes-Zurita, E. E. Rufino-Palomares, L. García-Salguero, J. Peragón, P. P. Medina, A. Parra, M. Cascante and J. A. Lupiáñez, Maslinic acid, a natural triterpene, induces a death receptor-mediated apoptotic mechanism in Caco-2 p53-deficient colon adenocarcinoma cells, PLoS One, 2016, 11, e0146178 CrossRef PubMed.
- M. E. Juan, U. Wenzel, H. Daniel and J. M. Planas, Cancer chemopreventive activity of hydroxytyrosol: a natural antioxidant from olives and olive oil, in Olives and Olive Oil in Health and Disease Prevention, ed. V. R. Preedy and R. R. Watson, Academic Press, 2010, pp. 1295–1300 Search PubMed.
- R. Mateos, G. Pereira-Caro, J. R. Bacon, R. Bongaerts, B. Sarriá, L. Bravo and P. A. Kroon, Anticancer activity of olive oil hydroxytyrosyl acetate in human adenocarcinoma Caco-2 cells, J. Agric. Food Chem., 2013, 61, 3264–3269 CrossRef CAS PubMed.
- T. Kawamori, T. Tanaka, A. Hara, J. Yamahara and H. Mori, Modifying effects of naturally occurring products on the development of colonic aberrant crypt foci induced by azoxymethane in F344 rats, Cancer Res., 1995, 55, 1277–1282 CAS.
- N. B. Janakiram, C. Indranie, S. V. Malisetty, P. Jagan, V. E. Steele and C. V. Rao, Chemoprevention of colon carcinogenesis by oleanolic acid and its analog in male F344 rats and modulation of COX-2 and apoptosis in human colon HT-29 cancer cells, Pharm. Res., 2008, 25, 2151–2157 CrossRef CAS PubMed.
- M. E. Juan, G. Lozano-Mena, M. Sánchez-González and J. M. Planas, Reduction of preneoplastic lesions induced by 1,2-dimethylhydrazine in rat colon by maslinic acid, a pentacyclic triterpene from Olea europaea L., Molecules, 2019, 24, 1266 CrossRef CAS PubMed.
- V. Manju and N. Nalini, Protective role of luteolin in 1,2-dimethylhydrazine induced experimental colon carcinogenesis, Cell Biochem. Funct., 2007, 25, 189–194 CrossRef CAS PubMed.
- S. H. Sharma, D. R. Chellappan, P. Chinnaswamy and S. Nagarajan, Protective effect of p-coumaric acid against 1,2-dimethylhydrazine induced colonic preneoplastic lesions in experimental rats, Biomed. Pharmacother., 2017, 94, 577–588 CrossRef CAS PubMed.
- N. Keum and E. Giovannucci, Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies, Nat. Rev. Gastroenterol. Hepatol., 2019, 16, 713–732 CrossRef PubMed.
- A. P. Femia, J. E. Paulsen, P. Dolara, J. Alexander and G. Caderni, Correspondence between flat aberrant crypt foci and mucin-depleted foci in rodent colon carcinogenesis, Anticancer Res., 2008, 28, 3771–3776 Search PubMed.
- M. Perše and A. Cerar, Morphological and molecular alterations in 1,2-dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats, J. Biomed. Biotechnol., 2011, e473964 Search PubMed.
- L. Serra-Majem, L. Tomaino, S. Dernini, E. M. Berry, D. Lairon, J. Ngo de la Cruz, A. Bach-Faig, L. M. Donini, F. X. Medina, R. Belahsen, S. Piscopo, R. Capone, J. Aranceta-Bartrina, C. La Vecchia and A. Trichopoulou, Updating the Mediterranean diet pyramid towards sustainability: focus on environmental concerns, Int. J. Environ. Res. Public Health, 2020, 17, 8758 CrossRef CAS PubMed.
- S. Reagan-Shaw, M. Nihal and N. Ahmad, Dose translation from animal to human studies revisited, FASEB J., 2008, 22, 659–661 CrossRef CAS PubMed.
- U. S. Department of Health and Human Services, Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Guidance for industry, bioanalytical method validation, Rockville, MD, 2018 Search PubMed.
- B. K. Matuszewski, Standard line slopes as a measure of a relative matrix effect in quantitative HPLC-MS bioanalysis, J. Chromatogr. B:Anal. Technol. Biomed. Life Sci., 2006, 830, 293–300 CrossRef CAS PubMed.
- I. Kundisová, M. E. Juan and J. M. Planas, Simultaneous determination of phenolic compounds in plasma by LC-ESI-MS/MS and their bioavailability after the ingestion of table olives, J. Agric. Food Chem., 2020, 68, 10213–10222 CrossRef PubMed.
- G. Lozano-Mena, M. Sánchez-González, A. Parra, M. E. Juan and J. M. Planas, Identification of gut-derived metabolites of maslinic acid, a bioactive compound from Olea europaea L., Mol. Nutr. Food Res., 2016, 60, 2053–2064 CrossRef CAS PubMed.
- R. P. Bird, Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings, Cancer Lett., 1987, 37, 147–151 CrossRef CAS PubMed.
- G. Caderni, A. P. Femia, A. Giannini, A. Favuzza, C. Luceri, M. Salvadori and P. Dolara, Identification of mucin-depleted foci in the unsectioned colon of azoxymethane-treated rats: correlation with carcinogenesis, Cancer Res., 2003, 63, 2388–2392 CAS.
- M. Durante, M. Tufariello, L. Tommasi, M. S. Lenucci, G. Bleve and G. Mita, Evaluation of bioactive compounds in black table olives fermented with selected microbial starters, J. Sci. Food Agric., 2018, 98, 96–103 CrossRef CAS PubMed.
- P. García, C. Romero and M. Brenes, Bioactive substances in black ripe olives produced in Spain and the USA, J. Food Compos. Anal., 2018, 66, 193–198 CrossRef.
- C. Romero, M. Brenes, K. Yousfi, P. García, A. García and A. Garrido, Effect of cultivar and processing method on the contents of polyphenols in table olives, J. Agric. Food Chem., 2004, 52, 479–484 CrossRef CAS.
- I. D'Antuono, A. Bruno, V. Linsalata, F. Minervini, A. Garbetta, M. Tufariello, G. Mita, A. F. Logrieco, G. Bleve and A. Cardinali, Fermented Apulian table olives: effect of selected microbial starters on polyphenols composition, antioxidant activities, and bioaccessibility, Food Chem., 2018, 248, 137–145 CrossRef.
- R. Moreno-González, M. E. Juan and J. M. Planas, Table olive polyphenols: a simultaneous determination by liquid chromatography-mass spectrometry, J. Chromatogr. A, 2020, 1609, 460434 CrossRef PubMed.
- L. A. Stanley, Chapter 26 - Drug Metabolism, in Pharmacognosy, ed. S. Badal McCreath and Y. N. Clement, Academic Press, London, 2nd ed., 2024, pp. 597–624 Search PubMed.
- M. Sánchez-González, G. Lozano-Mena, A. Parra, M. E. Juan and J. M. Planas, Identification in rat plasma and urine by linear trap quadrupole-orbitrap mass spectrometry of the metabolites of maslinic acid, a triterpene from olives, J. Agric. Food Chem., 2015, 63, 1126–1132 CrossRef PubMed.
- D. W. Jeong, Y. H. Kim, H. H. Kim, H. Y. Ji, S. D. Yoo, W. R. Choi, S. M. Lee, C. K. Han and H. S. Lee, Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats, Biopharm. Drug Dispos., 2007, 28, 51–57 CrossRef CAS PubMed.
- M. C. López de las Hazas, L. Rubió, A. Kotronoulas, R. de la Torre, R. Solà and M. J. Motilva, Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats, Mol. Nutr. Food Res., 2015, 59, 1395–1399 CrossRef PubMed.
- E. Giménez, M. E. Juan, S. Calvo-Melià and J. M. Planas, A sensitive liquid chromatography-mass spectrometry method for the simultaneous determination in plasma of pentacyclic triterpenes of Olea europaea L., Food Chem., 2017, 229, 534–541 CrossRef PubMed.
- I. L. Cameron, W. E. Hardman and D. W. Heitman, The nonfermentable dietary fiber lignin alters putative colon cancer risk factors but does not protect against DMH-induced colon cancer in rats, Nutr. Cancer, 1997, 28, 170–176 CrossRef CAS PubMed.
- I. D'Antuono, A. Garbetta, B. Ciasca, V. Linsalata, F. Minervini, V. M. T. Lattanzio, A. F. Logrieco and A. Cardinali, Biophenols from table olive cv Bella di Cerignola: chemical characterization, bioaccessibility, and intestinal absorption, J. Agric. Food Chem., 2016, 64, 5671–5678 CrossRef.
- M. P. Fernández-Poyatos, A. Ruiz-Medina and E. J. Llorent-Martínez, Phytochemical profile, mineral content, and antioxidant activity of Olea europaea L. cv. Cornezuelo table olives. Influence of in vitro simulated gastrointestinal digestion, Food Chem., 2019, 297, 124933 CrossRef PubMed.
- I. Alfaras, M. E. Juan and J. M. Planas, trans-Resveratrol reduces precancerous colonic lesions in dimethylhydrazine-treated rats, J. Agric. Food Chem., 2010, 58, 8104–8109 CrossRef CAS PubMed.
- C. M. Claro-Cala, J. C. Quintela, M. Pérez-Montero, J. Miñano, M. A. de Sotomayor, M. D. Herrera and A. R. Rodríguez-Rodríguez, Pomace olive oil concentrated in triterpenic acids restores vascular function, glucose tolerance and obesity progression in mice, Nutrients, 2020, 12, 323 CrossRef CAS PubMed.
- A. Vazquez, E. Sanchez-Rodriguez, F. Vargas, S. Montoro-Molina, M. Romero, J. A. Espejo-Calvo, P. Vilchez, S. Jaramillo, L. Olmo-García, A. Carrasco-Pancorbo, R. de laTorre, M. Fito, M. I. Covas, E. Martínez de Victoria and M. D. Mesa, Cardioprotective effect of a virgin olive oil enriched with bioactive compounds in spontaneously hypertensive rats, Nutrients, 2019, 11, 1728 CrossRef CAS PubMed.
- L. Álvarez-Amor, A. L. Sierra, A. Cárdenas, L. López-Bermudo, J. López-Beas, E. Andújar, M. Pérez-Alegre, R. Gallego-Durán, L. M. Varela, A. Martin-Montalvo, G. Berná, A. Rojas, M. J. Robles-Frías, A. Hmadcha, M. Romero-Gómez, R. Kleemann and F. Martín, Extra virgin olive oil improved body weight and insulin sensitivity in high fat diet-induced obese LDLr-/-.Leiden mice without attenuation of steatohepatitis, Sci. Rep., 2021, 11, 8250 CrossRef PubMed.
- C. M. Lasko and R. P. Bird, Modulation of aberrant crypt foci by dietary fat and caloric restriction: the effects of delayed intervention, Cancer Epidemiol., Biomarkers Prev., 1995, 4, 49–55 CAS.
- A. P. Femia, P. Dolara, M. Servili, S. Esposto, A. Taticchi, S. Urbani, A. Giannini, M. Salvadori and G. Caderni, No effects of olive oils with different phenolic content compared to corn oil on 1,2-dimethylhydrazine-induced colon carcinogenesis in rats, Eur. J. Nutr., 2008, 47, 329–334 CrossRef CAS PubMed.
- F. da S. França, C. Bodack, A. Costa, F. C. Deschamps and F. Martinello, The effect of dietary oils on the development of aberrant crypt foci, bifidobacteria, and fecal pH, In Vivo, 2014, 28, 197–203 Search PubMed.
- N. Nanda, S. Mahmood, A. Bhatia, A. Mahmood and D. K. Dhawan, Chemopreventive role of olive oil in colon carcinogenesis by targeting noncoding RNAs and methylation machinery, Int. J. Cancer, 2019, 144, 1180–1194 CrossRef CAS PubMed.
- H. Pandey, D. W. T. Tang, S. H. Wong and D. Lal, Gut microbiota in colorectal cancer: biological role and therapeutic opportunities, Cancers, 2023, 15, 866 CrossRef CAS PubMed.
- S. H. Sharma, S. Thulasingam and S. Nagarajan, Terpenoids as anti-colon cancer agents - a comprehensive review on its mechanistic perspectives, Eur. J. Pharmacol., 2017, 795, 169–178 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |