Open Access Article
Open Access ArticleMulti-omics analysis reveals the anti-fatigue mechanism of BCAA-enriched egg white peptides: the role of the gut–muscle axis†
Shengrao
Li
ab,
Jingbo
Liu
ab,
Qi
Yang
 ab,
Siwen
Lyu
ab,
Siwen
Lyu
 ab,
Qingwen
Han
ab,
Menghan
Fu
ab,
Zhiyang
Du
ab,
Qingwen
Han
ab,
Menghan
Fu
ab,
Zhiyang
Du
 ab,
Xuanting
Liu
ab,
Xuanting
Liu
 ab and
Ting
Zhang
ab and
Ting
Zhang
 *ab
*ab
aJilin Provincial Key Laboratory of Nutrition and Functional Food, Jilin University, Changchun, 130062, People's Republic of China. E-mail: tingzhang413@hotmail.com
bCollege of Food Science and Engineering, Jilin University, Changchun, 130062, People's Republic of China
First published on 21st January 2025
Abstract
Bioactive peptides rich in branched-chain amino acids (BCAAs) are an effective way to alleviate fatigue conditions, but the deep mechanism remains unclear. This study investigated the anti-fatigue effect of branched-chain amino acid-enriched egg white peptides (BEWPs) through the gut–muscle axis by gut bacteria and untargeted metabolomic analyses. The results demonstrated that BEWPs enhanced exercise endurance and strength by also promoting gastrocnemius development in mice. Furthermore, there was a reduction in oxidative stress, inflammatory response, and the accumulation of unexpected metabolites generated under fatigue conditions. The intake of BEWPs increased the abundances of Lactobacillus, Akkermansia, and unclassified_f_Lachnospiraceae, while decreasing the abundance of Bacteroides. BEWPs also regulated the levels of key metabolites in mouse muscles, including L-glutamic acid by arginine biosynthesis and bile secretion pathways. Notably, Spearman's correlation analysis indicated that there was a significant correlation between these altered metabolites, microbial populations, and indicators of fatigue. In summary, our research demonstrated that BEWPs alleviated fatigue through the gut–muscle axis, which provided new insights into fatigue management and prevention.
1. Introduction
Fatigue is a common and complex physiological response that occurs following intense physical activity. Its onset involves various complex factors, including oxidative stress, inflammatory response and metabolite accumulation in vivo.1,2 Fatigue influences excitation–contraction coupling at the neuromuscular junction, leading to muscle fibrosis, impaired muscle contraction, and reduced power output.3,4 As a result, there is reduced strength and diminished exercise endurance.5 Additionally, chronic fatigue may cause physical and mental discomfort, endocrine imbalances, and other organic diseases, which present a significant threat to overall health.6,7 Therefore, finding effective ways to alleviate fatigue has become the current research focus. Nowadays, nutritional interventions are regarded as a promising approach for reducing fatigue because of their safety and low side effects.Bioactive peptides are small fragments with low molecular weights obtained from proteins through hydrolysis or fermentation.8 Previous research found that bioactive peptides, especially peptides that are rich in BCAAs, could restore muscle function and provide anti-fatigue effects.9–11 The physiological mechanism underlying fatigue induction lies in the fact that continuous physical exertion prompts an upsurge in tryptophan levels within the body.12 BCAAs provide anti-fatigue effects by competing with free tryptophan for receptors and thereby preventing serotonin production.13,14 Moreover, BCAAs also activate and stimulate the mammalian target of rapamycin (mTOR) signaling pathway to promote muscle protein synthesis and improve muscle function.15,16 Our previous studies have indicated that egg white is one of the raw materials for preparing bioactive peptides and possess favorable functions.17–19 However, the low content of BCAAs in EWPs limits its anti-fatigue potential and sports nutrition application.20 Therefore, increasing the content of BCAAs in EWPs and verifying their role in fatigue recovery are of great significance for promoting the application of EWPs in sports beverage and related functional foods.
With growing attention to disease prevention, the crucial role of gut bacteria in safeguarding human health has been emphasized.21 Bioactive peptides can enhance the diversity of gut bacteria and the growth of beneficial bacterial microbiota.22 Muscle protein, atrophy markers, and gene expression related to muscle growth and mitochondria are impacted by gut bacteria and their metabolites.23,24 These effects contribute to improving muscle conditions and relieving fatigue. Therefore, the gut bacteria may serve as the mediator of the gut–muscle axis. Interestingly, Daily et al. also found the interaction between gut bacteria and skeletal muscles, suggesting that the gut–muscle axis may represent a new approach for alleviating fatigue.25 However, the relevant mechanisms of branched-chain amino acid-enriched egg white peptides (BEWPs) alleviating fatigue through the gut–muscle axis are still unknown.
In this study, we used gut bacteria analysis and untargeted metabolomic techniques to investigate the mechanisms by which BEWPs exert anti-fatigue effects through the gut–muscle axis. The research would extend the use of egg white peptides as a premium nutritional supplement in sports nutrition products.
2. Materials and methods
2.1 Materials and chemicals
Eggs were purchased from Xingguo Farm (Changchun, China). Alcalase (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1) and flavorzyme (15
000 U g−1) and flavorzyme (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1) were purchased from Solarbio Technology Co., Ltd (Beijing, China). Activated carbon (100 mesh) was purchased from Green Source Co., Ltd (Henan, China). Whey peptides (98% purity) were purchased from Kanghong Biology Co., Ltd (Shanxi, China). The ELISA kits for glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) were supplied by Shanghai Enzyme-Link Bio-Technology Co., Ltd. Lactic acid (LA), lactate dehydrogenase (LDH) and blood urea nitrogen (BUN) kits were also provided by the same company. All other chemicals were of analytical grade.
000 U g−1) were purchased from Solarbio Technology Co., Ltd (Beijing, China). Activated carbon (100 mesh) was purchased from Green Source Co., Ltd (Henan, China). Whey peptides (98% purity) were purchased from Kanghong Biology Co., Ltd (Shanxi, China). The ELISA kits for glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) were supplied by Shanghai Enzyme-Link Bio-Technology Co., Ltd. Lactic acid (LA), lactate dehydrogenase (LDH) and blood urea nitrogen (BUN) kits were also provided by the same company. All other chemicals were of analytical grade.
2.2 BEWP preparation
Distilled water was mixed with egg white to prepare a 5% protein solution. The solution was denatured by stirring at 90 °C for 10 min and cooled to 45 °C and then the pH of the solution was adjusted to 11.0 with 1 mol L−1 NaOH. Subsequently, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1 alkaline protease was added for the first enzymatic hydrolysis. After 90 min, the solution temperature was increased to 55 °C, and 15
000 U g−1 alkaline protease was added for the first enzymatic hydrolysis. After 90 min, the solution temperature was increased to 55 °C, and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1 flavor protease was added. The enzymatic reaction was halted by heating the solution for 10 min to inactivate the enzymes. The hydrolysate was separated by centrifugation (4 °C, 10
000 U g−1 flavor protease was added. The enzymatic reaction was halted by heating the solution for 10 min to inactivate the enzymes. The hydrolysate was separated by centrifugation (4 °C, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 10 min. At this stage, the egg white peptides that have not been enriched with BCAAs were referred to as EWPs. Then the pH of the supernatant was adjusted to 3.0 with 1 mol L−1 HCl mixed with activated carbon at a solid-to-liquid ratio of 1
000 rpm) for 10 min. At this stage, the egg white peptides that have not been enriched with BCAAs were referred to as EWPs. Then the pH of the supernatant was adjusted to 3.0 with 1 mol L−1 HCl mixed with activated carbon at a solid-to-liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and stirred at 25 °C for 150 min. After adsorption, the solution was passed through a 0.22 μm membrane to remove the activated carbon. The pH was adjusted to 7.0, and then desalting was performed using a 150–300 Da ultrafiltration membrane. The final product was spray-dried and stored at −20 °C.
10, and stirred at 25 °C for 150 min. After adsorption, the solution was passed through a 0.22 μm membrane to remove the activated carbon. The pH was adjusted to 7.0, and then desalting was performed using a 150–300 Da ultrafiltration membrane. The final product was spray-dried and stored at −20 °C.
2.3 Determination of amino acid content and sequence analysis of BEWPs
EWPs and BEWPs were dissolved in 6 mol L−1 HCl and nitrogen was blown for 15 min using a nitrogen evaporator, and then the tube was sealed. The solution was hydrolyzed in a 110 °C oil bath for 22 h. Subsequently, the solution was diluted to 50 mL and concentrated by rotary evaporation. 1 mL of the sample solution was filtered and analyzed using an amino acid analyzer.BEWP sequence determination methods were referred to Pan et al.26 Briefly, the samples were first re-dissolved in 0.2% formic acid, spiked onto a pre-column and separated on a capillary column with a laser-towed nebulizer, both columns being packed with a 4 μm C18 material. Gradient elution was performed under the following conditions: A phase as 0.1% formic acid in water, and B phase as 0.1% formic acid in acetonitrile solution: 5–35% B for 0–60 min; 35–75% B for 60–64 min; 75% B for 64–74 min, with a flow rate of 300 nL min−1. The eluted peptides were sprayed into an Orbitrap Elite mass spectrometer equipped with a nano-ESI ion source. Peptide sequences were obtained through software analysis and comparison with protein databases.
2.4 Animal and experimental design
Healthy male ICR mice (7 weeks old, SPF) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd (SYXK 2019-0012). The animals were kept at the Animal Center of the First Hospital of Jilin University at 23 ± 1 °C and 50% humidity under a 12-hour light/dark cycle. After 5 days of adaptation, the mice were randomly assigned to five groups (n = 6): control check (CK), model (M), whey protein peptides (WP), low-dose BEWPs (LBEWP) and high-dose BEWPs (HBEWP). The CK and M groups received regular water. The WP group which was set as the positive control received 200 mg kg−1 whey protein peptides. Additionally, the LBEWP group and the HBEWP group were administered with 100 mg kg−1 and 200 mg kg−1 BEWPs, respectively. The intervention lasted 31 days. On the final day, all mice except those in the CK group underwent an exhaustive swimming test. Blood was collected, and the mice were euthanized after 30 min of rest (Fig. 1A). Gastrocnemius, liver, and other tissues were collected and fixed in 4% paraformaldehyde for histological analysis, and the rest were stored at −80 °C for further use.2.5 Exhaustion swimming time measurement
On day 31, following 30 min of feeding in their respective groups, mice from the M, WP, LBEWP, and HBEWP groups were put to an intensive swimming test. The procedure was adapted from Zhu et al., and involved attaching a weight equal to 5% of each mouse's body weight to its tail with an iron wire.27 Mice were then placed in a pool with 30 cm of water at 25 ± 1 °C. The water was stirred to force the mice to swim. Exhaustion was defined as the time when a mouse sank to the bottom and did not surface within 10 seconds, and this duration was recorded.2.6 Grip force measurement
The forelimbs of the mouse were placed on the metal sensor of the grip strength meter. The experimenter gently and steadily pulled the mouse by the tail, recording the maximum grip strength displayed. To avoid measurement mistakes, care was taken to ensure that the mouse's hind limbs did not come into contact with the sensor.2.7 Fatigue indicator measurement
After collecting the mouse blood, it was centrifuged at 3000 rpm for 15 min at 4 °C to extract the serum. Oxidative stress indicators (GSH, SOD and MDA) and metabolites (LA, LDH and BUN) in the serum were measured using ELISA kits. The cytokines (TNF-α, IL-1β and IL-6) were measured using the gastrocnemius homogenate to indicate the inflammation levels.2.8 Histological analysis
After fixation in formalin, liver and gastrocnemius tissues were prepared through dehydration, embedding, and sectioning. Hematoxylin and eosin (H&E) were used to stain the sections, and an optical microscope was used to analyze them.2.9 Gut bacteria analysis
Cecal contents from the mice were collected to investigate the changes in gut bacteria. Microbial DNA was extracted using the E.Z.N.A. DNA Kit (Omega Bio-tek, Norcross, GA, USA), and its concentration was measured. The bacterial 16S rRNA gene was then amplified using primers 338F and 806R, followed by PCR. The amplified products were sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, USA), and the sequencing data were analyzed using the Majorbio Cloud Platform.2.10 Untargeted metabolomic analysis of muscles
Fifty milligrams of gastrocnemius were accurately weighed and mixed with 80% methanol. Initially, low-temperature ultrasonic treatment (5 °C, 40 kHz, 6 min) was used to extract the metabolites. After being kept at −20 °C for half an hour, the samples were centrifuged (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 063 rpm, 4 °C, 15 min) to extract the supernatant for quality assurance. The supernatant was analyzed using LC-MS/MS in both positive and negative ion modes, and the results were compared with biochemical databases to identify the metabolite types. Significant metabolites between the experimental groups were selected based on the criteria of P < 0.05 and VIP (Variable Importance in Projection) > 1.
063 rpm, 4 °C, 15 min) to extract the supernatant for quality assurance. The supernatant was analyzed using LC-MS/MS in both positive and negative ion modes, and the results were compared with biochemical databases to identify the metabolite types. Significant metabolites between the experimental groups were selected based on the criteria of P < 0.05 and VIP (Variable Importance in Projection) > 1.
2.11 Statistical analysis
All data were expressed as means ± standard deviations (SD). Statistical analyses were conducted using SPSS software; P < 0.05 was considered statistically significant.3. Results
3.1 BEWP amino acid content and sequence
The amino acid composition of BEWPs enriched with BCAAs and EWPs produced by enzymatic hydrolysis is shown in Table S1.† BCAAs accounted for 19.01% and 23.61% in EWPs and BEWPs, respectively. This represented an increase of nearly 24.20% in BEWPs compared to EWPs. Additionally, the content of aromatic amino acids (AAAs) in BEWPs was only 3.94%, a decrease of 71.03% compared to EWPs. UPLC-MS/MS was used to identify the peptide sequences in the BEWPs, and the results are shown in Table S2.†3.2 BEWPs enhanced exercise endurance
Exercise endurance is a critical measure of anti-fatigue capacity. To evaluate the effects of BEWPs on exercise endurance, we established a mice exhaustion swimming model and monitored the physiological changes in the mice throughout the feeding period. The results indicated that WPs and BEWPs resulted in normal weight gain in mice, which was consistent with the trends observed in the other experimental groups (Fig. 1B). Liver tissues from mice in different experimental groups were observed by H&E staining. We found that the hepatocytes of mice in all groups were well organized, with clear cell borders, uniform cytoplasm and no lipid accumulation (Fig. 1G). The findings showed that WPs and BEWPs were non-toxic and effectively maintained normal physiological conditions in mice. Furthermore, there was no significant increase in the exhaustion swimming time of mice in the LBEWP group compared to the M group. In contrast, the HBEWP group mice exhibited an extended exhaustion swimming time of 9.65 min, reflecting a 19.73% increase over the M group. The WP group also demonstrated a 14.64% increase in the exhaustion swimming time (Fig. 1C). These findings indicated that a high dose of BEWPs significantly enhanced exercise endurance in mice.3.3 BEWPs improved the condition of gastrocnemius
Organismic fatigue can directly result in the deterioration of the quality and functionality of skeletal muscles.28 The gastrocnemius, being a significant component of skeletal musculature, has been evidenced to play a cardinal role in anti-fatigue.29,30 Therefore, to ascertain the ameliorative effect of BEWPs on the gastrocnemius muscle, we evaluated the gastrocnemius status in mice through grip strength, coefficient calculations, and histopathological analysis. During the feeding period, grip strength increased in all groups (Fig. 1D). The CK and M groups displayed similar trends, suggesting that age-related factors contributed to the rise in grip strength. However, mice that received WP and BEWP interventions showed a significant increase in grip strength. To further investigate the effect of BEWPs, the forelimb grip strength of mice on the final day was measured (Fig. 1E). The data showed no significance in grip strength between the CK and M groups (P > 0.05). However, grip strength significantly increased in the WP, LBEWP, and HBEWP groups (P < 0.0001). Specifically, WP intervention increased grip strength from 1.33 N to 1.44 N, while HBEWP intervention increased it to 1.52 N. Additionally, WPs and HBEWPs significantly increased the gastrocnemius coefficient in mice (P < 0.05) (Fig. 1F) and reversed exercise-induced muscle fiber fragmentation and muscle cell shrinkage (Fig. 1H).3.4 BEWPs modulated the fatigue markers
Fatigue is commonly associated with inflammatory and oxidative stress responses. Compared to the CK group, the levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 in the gastrocnemius of mice significantly increased after exhaustive swimming (P < 0.05). These inflammatory cytokines can impair muscle structure and function, contributing to fatigue. As presented in Fig. 2A–C, the interventions with WPs, LBEWPs and HBEWPs all led to a decline in the levels of these three inflammatory factors. However, among the three, LBEWPs demonstrated the least effective alleviation. Fig. 2D–F indicated that, in contrast to the M group, mice in the WP group showed a decrease MDA levels in serum from 6.85 nmol mL−1 to 5.31 nmol mL−1, while GSH and SOD levels increased by 2.10 ng mL−1 and 104.44 U mL−1, respectively. Furthermore, interventions with different concentrations of BEWPs effectively increased the contents of GSH and SOD and decreased the MDA concentration, showing a concentration-dependent effect.The changes in the content of serum metabolites also constitute an important indicator for evaluating anti-fatigue capacity. Fatigue led to a significant increase in the contents of BUN, LA, and LDH in the serum of mice (P < 0.05), and this situation was reversed after peptide intervention (Fig. 2G-I). In the LBEWP group, the concentrations of the three metabolites, namely BUN, LA, and LDH, in mice were 18.03%, 15.66%, and 16.07%, lower than those in the M group, respectively. Significantly, the concentrations of these metabolites in the HBEWP group were found to be even lower, suggesting a negative correlation between the concentrations of the aforesaid three metabolites and the dose of BEWPs.
In conclusion, the findings showed that BEWPs were capable of relieving inflammation and oxidative stress reactions and lowering the levels of metabolites in the serum, thereby exhibiting favorable anti-fatigue capabilities. It is worth noting that combining the previous results, the LBEWP group performed considerably worse than the WP and HBEWP groups and did not show an excellent anti-fatigue effect. Therefore, we did not think about including the LBEWP group into the following analyses.
3.5 BEWPs altered gut bacteria in fatigued mice
We analyzed the composition of the gut bacteria in mice by 16S rRNA gene sequencing. And we obtained a total of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671
671![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 245 sequences from 24 samples. After quality control, 5429 operational taxonomic units (OTUs) were identified at a 97% sequence similarity level. Pan/core species analysis indicated that both curves approached a plateau, confirming the reliability of the sequencing data and the adequacy of the sampling (Fig. 3A and B). Fig. 3C presents the NMDS clustering analysis based on the Euclidean algorithm, reflecting the β-diversity of the gut bacteria. Notably, the groups intervened with HBEWPs and WPs were distinctly separated from the model group, suggesting that the peptides altered the gut bacteria. Venn analysis revealed unique OTUs for each group, with counts of 749, 831, 437, and 523 for the respective experimental groups (Fig. 3D). This result provided more evidence that HBEWP intervention significantly altered the gut bacteria.
245 sequences from 24 samples. After quality control, 5429 operational taxonomic units (OTUs) were identified at a 97% sequence similarity level. Pan/core species analysis indicated that both curves approached a plateau, confirming the reliability of the sequencing data and the adequacy of the sampling (Fig. 3A and B). Fig. 3C presents the NMDS clustering analysis based on the Euclidean algorithm, reflecting the β-diversity of the gut bacteria. Notably, the groups intervened with HBEWPs and WPs were distinctly separated from the model group, suggesting that the peptides altered the gut bacteria. Venn analysis revealed unique OTUs for each group, with counts of 749, 831, 437, and 523 for the respective experimental groups (Fig. 3D). This result provided more evidence that HBEWP intervention significantly altered the gut bacteria.
To investigate the impact of HBEWPs on the gut bacteria composition, we conducted community abundance analyses at various taxonomic levels. At the phylum level, the microbial community was primarily composed of Firmicutes, Bacteroidota, Actinobacteriota, Patescibacteria, Verrucomicrobiota, and Deferribacterota (Fig. 3E). However, the abundance changed after WP and HBEWP interventions, mainly in the form of an increase in Firmicutes and a decrease in Bacteroidota. At the genus level, the top ten most abundant bacteria were determined and are shown in Fig. 3F, including Lachnospiraceae_NK4A136_group, Lactobacillus, and unclassified_f_Lachnospiraceae. Fig. 3G further shows the changes at the microbial level. In the HBEWP group, the abundances of Lactobacillus, unclassified_f_Lachnospiraceae, Akkermansia, Lachnospiraceae_UCG-006, Ruminococcus, and Enterorhabdus were higher compared to the M group. Additionally, HBEWP treatment reduced the abundances of norank_f_Muribaculaceae, Lachnospiraceae_NK4A136_group, Alistipes, and Bacteroides.
3.6 BEWPs exerted an influence on muscle metabolism in fatigued mice
To better reveal the important role of the gut–muscle axis in fatigue recovery, we analyzed the regulatory effects of HBEWPs on muscle metabolites using untargeted metabolomic techniques. Both positive and negative ion mode analyses revealed distinct separations between the CK group and the other experimental groups, with notable differences between the HBEWP and M groups (Fig. 4A and B). These results suggested that exercise and peptide intervention influenced the metabolite profile of the gastrocnemius. Then, we identified and constructed differential metabolite sets (P < 0.05 and VIP > 1) for further analysis. After the consumption of WPs and HBEWPs, the abundance of lipids, peptides and organic acids increased, while the abundance of steroids, nucleic acids, hormones and transmitters and carbohydrates decreased (Fig. 4C). Besides, Fig. 4D illustrates the identification of 87, 54, and 200 differential metabolites in the CK vs. M, WP vs. M, and HBEWP vs. M sets, respectively. Additionally, 72, 24, and 166 unique metabolites were detected in each group. The comparison with the KEGG database revealed that HBEWPs increased the levels of lipids, peptides, and carbohydrates in the gastrocnemius. Simultaneously, they decreased the levels of steroids, nucleic acids, and organic acids (Fig. 4E). The gastrocnemius of mice receiving WPs exhibited similar metabolic changes. The differential metabolites identified were predominantly involved in pathways related to metabolism, biological systems, and human diseases. Furthermore, in the CK vs. M set, 34 metabolites were upregulated, while 53 were downregulated (Fig. 4F). In the WP vs. M set, 40 metabolites showed upregulation, while 14 were downregulated (Fig. 4G). Compared with the M group, 68 metabolites were upregulated and 132 were downregulated in the HBEWP group (Fig. 4H).3.7 BEWPs altered muscle metabolites and metabolic pathways
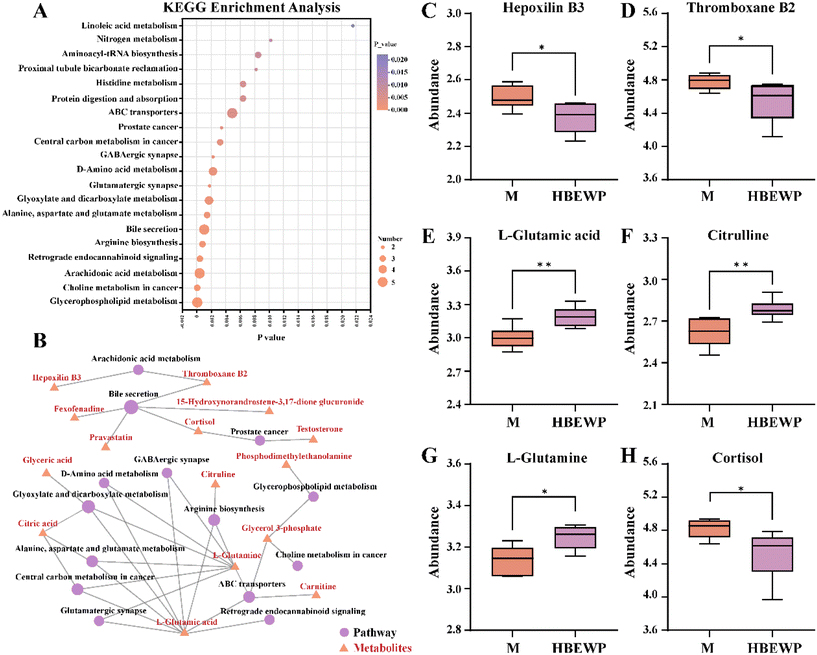
KEGG pathway enrichment analysis was performed on the significantly altered metabolites to elucidate the mechanisms by which HBEWPs mitigate exercise-induced fatigue in mice. The analysis revealed that HBEWPs modulated the following key pathways in fatigued mice, including arachidonic acid metabolism, bile secretion, arginine biosynthesis, and retrograde endocannabinoid signaling (Fig. 5A).The network between metabolites and metabolic pathways was constructed and is shown in Fig. 5B. Hepoxilin B3 and thromboxane B2 were involved in arachidonic acid metabolism, while thromboxane B2 and cortisol participated in bile secretion pathways. L-Glutamic acid and L-glutamine were associated with multiple metabolic pathways, including arginine biosynthesis, retrograde endocannabinoid signaling, and ABC transporters. In addition, arginine biosynthesis was linked to citrulline. Subsequent validation of these key metabolites revealed that HBEWPs significantly reduced the abundances of hepoxilin B3, thromboxane B2, and cortisol, while increasing the levels of L-glutamic acid, citrulline, and L-glutamine (Fig. 5C–H). These results indicated that HBEWPs modulated metabolite abundance through key metabolic pathways such as bile secretion and arginine biosynthesis.
3.8 Correlation between fatigue, gut bacteria, and muscle metabolites
Spearman's correlation analysis was performed to explore the relationships between fatigue, gut bacteria, and muscle metabolites (Fig. 6). The analysis indicated that in the HBEWP group, an increase in Lactobacillus abundance positively correlated with both grip strength and the gastrocnemius coefficient. Conversely, unclassified_f_Lachnospiraceae and Akkermansia showed negative correlations with the levels of inflammatory cytokines in the gastrocnemius and serum metabolites, but positive correlations with L-glutamic acid, citrulline, and L-glutamine. Cortisol was negatively correlated with Enterorhabdus, grip strength, and the gastrocnemius coefficient, while it was positively correlated with Bacteroides and inflammatory cytokines. Additionally, grip strength negatively correlated with both the gastrocnemius coefficient and inflammatory cytokines. | ||
| Fig. 6 Heatmap of Spearman's correlation analysis. ***P < 0.001 indicates significant correlations between the indicators, and numbers represent the correlation coefficients. | ||
4. Discussion
Recently, the gut–muscle axis has been regarded as a new approach to anti-fatigue.31 Gut bacteria, along with their metabolites, are capable of targeting fatigue states which are associated with oxidative stress and inflammation.32 They can regulate muscle-related gene and protein expression, ultimately affecting muscle conditions.23 Therefore, in this study, we prepared BEWPs and validated their anti-fatigue ability mediated by the gut–muscle axis.During exercise, the elevation of reactive oxygen species (ROS) and free radicals in the mitochondria occurs.33 This phenomenon impedes vasodilation and decelerates blood flow, subsequently delaying the delivery of oxygen and nutrients to organs and accelerating the onset of fatigue.34,35 Meanwhile, intense exercise triggers the accumulation of pro-inflammatory cytokines, which induces mitochondrial dysfunction.36 The resultant dysfunction further amplifies ROS production, thereby creating a self-perpetuating and progressively worsening vicious cycle. The intake of BEWPs augmented the antioxidant capacity and efficiently counteracted the elevation of inflammatory cytokines in the gastrocnemius muscle induced by exercise (P < 0.05).
Furthermore, high-intensity exercise disrupts homeostasis, generating more unexpected metabolites.37 These accumulate in muscles, impeding neurotransmitter transmission and ATP synthesis, leading to insufficient energy and fatigue.38 Previous research indicated that BCAAs can not only promote muscle growth and strength but also relieve the accumulation of adverse metabolites like LA, BUN, and LDH.39 This is consistent with our research findings. Exhaustive swimming raised metabolite levels in mice, but BEWP intervention reversed this. Fatigue detrimentally affects muscle structure and function, manifesting as diminished exercise endurance and muscular weakness.40 We observed that BEWPs boosted the swimming endurance, grip strength, and gastrocnemius coefficient of the mice. Zhu et al. also reported similar results; they observed that an anti-fatigue maca aqueous extract greatly improved grip strength and exercise endurance in fatigued mice.41 In conclusion, the above results demonstrate that BEWPs possess excellent anti-fatigue capabilities.
The role of HBEWPs in the regulation of muscle metabolism was explored by us using untargeted metabolomic techniques. KEGG pathway enrichment analysis revealed that HBEWPs modulate metabolites through the pathways of arginine biosynthesis, bile secretion, and retrograde endogenous cannabinoid signaling. Citrulline involved in this pathway is a precursor for the synthesis of arginine.42 Arginine boosts nitric oxide (NO) production, which stimulates mitochondrial function, and improves oxygen distribution in muscles, thereby positively impacting muscle performance.43,44 Early studies have indicated that bioactive substances can mitigate fatigue by promoting the biosynthesis of arginine, aligning with our findings.45 Increased abundance of L-glutamine can reduce fatigue by enhancing glycogen synthase activity, boosting ATP production, and protecting the intestinal barrier.46,47 Meanwhile, L-glutamic acid interacts with pre- and postsynaptic sites at neuromuscular junctions to improve exercise performance.48 Bile acid secretion is a crucial pathway linking the gut–muscle axis. Early research demonstrated that bacteria-mediated bile acids protect the intestinal barrier and affect muscle function.49–51 However, HBEWPs reduced cortisol contents in this metabolic pathway, which is known to impair muscle mass.52,53 Therefore, BEWPs may alleviate fatigue by regulating metabolites through the arginine biosynthesis and bile secretion pathways in fatigued mice.
To further explore the potential mechanism by which HBEWPs alleviate fatigue through the gut–muscle axis, an analysis of the gut bacteria of mice was performed. It has been reported that fatigue leads to decreased abundances of Lactobacillus and Akkermansia.54 Notably, HBEWPs function to safeguard the mice by modulating the abundances of these bacteria. Lactobacillus has been demonstrated to contribute to muscle fiber synthesis and exhibits remarkable anti-inflammatory and antioxidant capacities.55Akkermansia, which is a dominant bacterium in the gut, demonstrates anti-fatigue properties. It produces acetate via the acetyl-CoA pathway and exploits mucin as its distinctive nitrogen and carbon sources to bolster host metabolism.56 In addition, the gut bacteria supply energy to the host by decomposing nutrients, and SCFAs produced by them cross the intestinal barrier to act on muscles.57 The results showed an increase in the abundances of unclassified_f_Lachnospiraceae, Lachnospiraceae_UCG-006 and Enterorhabdus that produce butyric acid in the gut. Butyric acid protects the gut by preventing harmful microorganisms from entering the bloodstream and peripheral organs, and it can enhance muscle conditions by modulating Akt/mTOR/Foxo3a and Fbox32/Trim63.58–60 HBEWPs significantly reduced Bacteroides abundance. Li et al. found that Bacteroides abundance was negatively correlated with butyric acid content, implying that it might be harmful.61 Based on the theoretical analysis, the gut bacteria regulated by HBEWPs correlate with fatigue markers and muscle conditions. To verify this, Spearman's correlation analysis was used to show the correlations among these indicators, suggesting that BEWPs may alleviate fatigue via the gut–muscle axis.
5. Conclusion
In conclusion, this study highlighted the critical role of the gut–muscle axis in alleviating fatigue modulated by BEWPs. BEWP intervention was proved to positively influence arginine biosynthesis and bile secretion pathways by increasing the abundances of Lactobacillus, Akkermansia, unclassified_f_Lachnospiraceae, Lachnospiraceae_UCG-006, and Enterorhabdus, while decreasing Bacteroides abundance. This alteration improved gastrocnemius quality and function, diminished oxidative stress and inflammatory response, and reduced the unexpected metabolites in fatigue conditions. These findings offer new insights into the potential of egg white bioactive peptides as functional foods and sports supplements. However, further research is needed to clarify the specific metabolic pathways and key proteins involved in the anti-fatigue effects of BEWPs.Author contributions
Shengrao Li: methodology, investigation, formal analysis and writing. Jingbo Liu: investigation and editing. Qi Yang: methodology and writing. Siwen Lyu: formal analysis and validation. Qingwen Han: methodology and formal analysis. Menghan Fu: conceptualization and editing. Zhiyang Du: methodology and validation. Xuanting Liu: conceptualization and supervision. Ting Zhang: conceptualization, writing, editing, and supervision.Ethical consideration statement
All the animal experimental procedures in this study followed the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publications, no. 8023, revised 1978). Additionally, the present experiment was approved by the Welfare and Ethics Committee of The First Hospital Laboratory Animal Center of Jilin University (no. IACUC-2020-0436).Data availability
The data that support the findings of this study are available from the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Key R&D Program of China (2022YFD2101002).References
- H. J. Zhang, R. Kang, T. C. Song, F. Y. Ren, J. Liu and J. Wang, Advances in relieving exercise fatigue for curcumin: Molecular targets, bioavailability, and potential mechanism, J. Food Sci., 2024, 89, 4604–4619 CrossRef PubMed.
- S. Liu, F. Meng, D. Zhang, D. Shi, J. Zhou, S. Guo and X. Chang, Lonicera caerulea Berry Polyphenols Extract Alleviates Exercise Fatigue in Mice by Reducing Oxidative Stress, Inflammation, Skeletal Muscle Cell Apoptosis, and by Increasing Cell Proliferation, Front. Nutr., 2022, 9, 853225 CrossRef PubMed.
- J. G. Vieira, A. V. Sardeli, M. R. Dias, J. Elias, Y. Campos, L. Sant'Ana, L. Leitao, V. Reis, M. Wilk, J. Novaes and J. Vianna, Effects of Resistance Training to Muscle Failure on Acute Fatigue: A Systematic Review and Meta-Analysis, Sports Med., 2022, 52, 1103–1125 CrossRef PubMed.
- D. Constantin-Teodosiu and D. Constantin, Molecular Mechanisms of Muscle Fatigue, Int. J. Mol. Sci., 2021, 22, 11587 CrossRef PubMed.
- X. Y. Li, C. L. Jiang, C. Zheng, C. Z. Hong, L. H. Pan, Q. M. Li, J. P. Luo and X. Q. Zha, Polygonatum cyrtonema Hua Polysaccharide Alleviates Fatigue by Modulating Osteocalcin-Mediated Crosstalk between Bones and Muscles, J. Agric. Food Chem., 2023, 71, 6468–6479 CrossRef PubMed.
- X. Y. Li, C. L. Jiang, C. Zheng, C. Z. Hong, L. H. Pan, Q. M. Li, J. P. Luo and X. Q. Zha, Hua Polysaccharide Alleviates Fatigue by Modulating Osteocalcin-Mediated Crosstalk between Bones and Muscles, J. Agric. Food Chem., 2023, 71, 6468–6479 CrossRef CAS PubMed.
- X. L. Chen, D. H. Liang, Z. Q. Huang, G. Jia, H. Zhao and G. M. Liu, Anti-fatigue effect of quercetin on enhancing muscle function and antioxidant capacity, J. Food Biochem., 2021, 45, e13968 CAS.
- M. W. Chen, F. Zhang, Y. J. Su, C. H. Chang, J. H. Li, L. P. Gu and Y. J. Yang, Immunomodulatory effects of egg white peptides on immunosuppressed mice and sequence identification of immunomodulatory peptides, Food Biosci., 2022, 49, 101873 CrossRef CAS.
- L. Fang, R. X. Zhang, Y. Wei, K. Ling, L. Lu, J. Wang, X. C. Pan and M. Y. Cai, Anti-fatigue effects of fermented soybean protein peptides in mice, J. Sci. Food Agric., 2022, 102, 2693–2703 CrossRef CAS PubMed.
- X. T. Lu, M. Wang, H. Yue, X. X. Feng, Y. Y. Tian, C. H. Xue, T. T. Zhang and Y. M. Wang, Novel peptides from sea cucumber intestines hydrolyzed by neutral protease alleviate exercise-induced fatigue via upregulating the glutaminemediated Ca2+/Calcineurin signaling pathway in mice, J. Food Sci., 2024, 89, 1727–1738 CrossRef CAS PubMed.
- C. Zhao, Y. Gong, L. Zheng and M. Zhao, The Degree of Hydrolysis and Peptide Profile Affect the Anti-Fatigue Activities of Whey Protein Hydrolysates in Promoting Energy Metabolism in Exercise Mice, J. Agric. Food Chem., 2023, 71, 3010–3021 CrossRef CAS PubMed.
- L. Lanser, P. Kink, E. M. Egger, W. Willenbacher, D. Fuchs, G. Weiss and K. Kurz, Inflammation-Induced Tryptophan Breakdown is Related With Anemia, Fatigue, and Depression in Cancer, Front. Immunol., 2020, 11, 249 CrossRef CAS PubMed.
- R. Hormoznejad, A. Z. Javid and A. Mansoori, Effect of BCAA supplementation on central fatigue, energy metabolism substrate and muscle damage to the exercise: a systematic review with meta-analysis, Sports Sci. Health, 2019, 15, 265–279 CrossRef.
- Y. B. Chang, K. B. Hong, M. G. Kim, H. J. Suh and K. Jo, Effect of the protein hydrolysate of rice syrup meal on the endurance exercise performance of BALB/c mice, Food Funct., 2021, 12, 1338–1348 RSC.
- D. G. Le Couteur, S. M. Solon-Biet, V. C. Cogger, R. Ribeiro, R. de Cabo, D. Raubenheimer, G. J. Cooney and S. J. Simpson, Branched chain amino acids, aging and age-related health, Ageing Res. Rev., 2020, 64, 101198 CrossRef CAS PubMed.
- M. S. Kaspy, S. J. Hannaian, Z. W. Bell and T. A. Churchward-Venne, The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: an update, Nutr. Res. Rev., 2023, 37, 273–286 CrossRef PubMed.
- Q. Yang, S. Lyu, M. Xu, S. Li, Z. Du, X. Liu, X. Shang, Z. Yu, J. Liu and T. Zhang, Potential Benefits of Egg White Proteins and Their Derived Peptides in the Regulation of the Intestinal Barrier and Gut Microbiota: A Comprehensive Review, J. Agric. Food Chem., 2023, 71, 13168–13180 CrossRef CAS PubMed.
- H. Ge, Z. Cai, J. Chai, J. Liu, B. Liu, Y. Yu, J. Liu and T. Zhang, Egg white peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition, Food Chem., 2021, 360, 129981 CrossRef CAS PubMed.
- Q. Y. Xu, L. Zheng, M. T. Huang and M. M. Zhao, Collagen derived Gly-Pro-type DPP-IV inhibitory peptides: Structure-activity relationship, inhibition kinetics and inhibition mechanism, Food Chem., 2024, 441, 138370 CrossRef CAS PubMed.
- H. F. Ge, B. Y. Zhang, T. Li, Q. Yang, Y. H. Tang, J. B. Liu and T. Zhang, In vivo and in silico studies on the mechanisms of egg white peptides in relieving acute colitis symptoms, Food Funct., 2021, 12, 12774–12787 RSC.
- Z. Feng, Y. Wei, Y. Xu, R. Zhang, M. Li, H. Qin, R. Gu and M. Cai, The anti-fatigue activity of corn peptides and their effect on gut bacteria, J. Sci. Food Agric., 2022, 102, 3456–3466 CrossRef CAS PubMed.
- T. X. Zhi, D. Hong, Z. J. Zhang, S. T. Li, J. X. Xia, C. Wang, Y. L. Wu, Y. M. Jia and A. J. Ma, Anti-inflammatory and gut microbiota regulatory effects of walnut protein derived peptide LPF, Food Res. Int., 2022, 152, 110875 CrossRef CAS PubMed.
- L. Mancin, G. D. Wu and A. Paoli, Gut microbiota-bile acid-skeletal muscle axis, Trends Microbiol., 2023, 31, 254–269 CrossRef CAS PubMed.
- J. M. Cai, L. J. Xing, W. A. Zhang, J. Zhang, L. Zhou and Z. X. Wang, Effect of Yeast-Derived Peptides on Skeletal Muscle Function and Exercise-Induced Fatigue in C2C12 Myotube Cells and ICR Mice, J. Agric. Food Chem., 2023, 71, 15522–15537 CrossRef CAS PubMed.
- J. W. Daily and S. Park, Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle, Cells, 2022, 11, 338 CrossRef CAS PubMed.
- F. Pan, Z. Cai, H. Ge, S. Ma, Y. Yu, J. Liu and T. Zhang, Transcriptome analysis reveals the hepatoprotective mechanism of soybean meal peptides against alcohol-induced acute liver injury mice, Food Chem. Toxicol., 2021, 154, 112353 CrossRef CAS PubMed.
- J. Zhu, J. Yi, Q. Kang, J. Huang, Y. Cui, G. Zhang, Z. Wang, L. Zhang, Z. Zheng, J. Lu and L. Hao, Anti-fatigue activity of hemp leaves water extract and the related biochemical changes in mice, Food Chem. Toxicol., 2021, 150, 112054 CrossRef CAS PubMed.
- K. Ibeas, L. Herrero, P. Mera and D. Serra, Hypothalamus-skeletal muscle crosstalk during exercise and its role in metabolism modulation, Biochem. Pharmacol., 2021, 190, 114640 CrossRef CAS PubMed.
- C. L. Yin, X. Q. Fu, J. Y. Chou, J. K. Li, Y. J. Chen, J. X. Bai, J. Y. Wu, Y. Wu, X. Q. Wang and Z. L. Yu, A proprietary herbal drug Young Yum Pill ameliorates chronic fatigue syndrome in mice, Phytomedicine, 2021, 88, 153602 CrossRef PubMed.
- D. E. Cho, G. M. Choi, Y. S. Lee, J. P. Hong, M. Yeom, B. Lee and D. H. Hahm, Long-term administration of red ginseng non-saponin fraction rescues the loss of skeletal muscle mass and strength associated with aging in mice, J. Ginseng Res., 2022, 46, 657–665 CrossRef PubMed.
- H. K. Zhu, H. T. Zhao, H. Qian and C. Liu, Urolithin A Ameliorates Athletic Ability and Intestinal Microbiota in Sleep Deprivation from the Perspective of the Gut-Muscle Axis, Mol. Nutr. Food Res., 2024, 68, 2300599 CrossRef PubMed.
- Y. P. Zhou, Z. X. Chu, Y. Luo, F. Y. Yang, F. L. Cao, F. J. Luo and Q. L. Lin, Dietary Polysaccharides Exert Anti-Fatigue Functions via the Gut-Muscle Axis: Advances and Prospectives, Foods, 2023, 12, 83 Search PubMed.
- S. Acevedo-Juárez, D. Guajardo-Flores, E. Heredia-Olea and M. Antunes-Ricardo, Bioactive peptides from nuts: A review, Int. J. Food Sci. Technol., 2022, 57, 2226–2234 CrossRef.
- R. Lv, Y. Dong, Z. Bao, S. Zhang, S. Lin and N. Sun, Advances in the activity evaluation and cellular regulation pathways of food-derived antioxidant peptides, Trends Food Sci. Technol., 2022, 122, 171–186 CrossRef.
- M. D. V. Mishima, H. S. D. Martino, T. S. Meneguelli and E. Tako, Effect of food derived bioactive peptides on gut health and inflammatory mediators in vivo: a systematic review, Crit. Rev. Food Sci. Nutr., 2023, 8, 1–11 Search PubMed.
- X. Kang, J. Qian, Y. X. Shi, X. T. Bian, L. D. Zhang, G. M. Li, L. T. Wang, J. Zhao, Z. Y. Dong, M. M. Yang, Y. J. N. Chen, K. L. Tang and H. M. Miao, Exercise-induced Musclin determines the fate of fibro-adipogenic progenitors to control muscle homeostasis, Cell Stem Cell, 2024, 31(2), 212–226.e7 CrossRef PubMed.
- M. Cai, H. Zhu, L. Xu, J. Wang, J. Xu, Z. H. Li, K. Yang, J. Y. Wu and P. L. Sun, Structure, anti-fatigue activity and regulation on gut microflora in vivo of ethanol-fractional polysaccharides from Dendrobium officinale, Int. J. Biol. Macromol., 2023, 234, 123572 CrossRef PubMed.
- F. Li, X. Xie, X. Xu and X. Zou, Water-soluble biopolymers calcium polymalate derived from fermentation broth of Aureobasidium pullulans markedly alleviates osteoporosis and fatigue, Int. J. Biol. Macromol., 2024, 268, 132013 CrossRef PubMed.
- A. Salem, K. Ben Maaoui, H. Jahrami, M. A. AlMarzooqi, O. Boukhris, B. Messai, C. C. T. Clark, J. M. Glenn, H. A. Ghazzaoui, N. L. Bragazzi, A. Ammar, K. Trabelsi and H. Chtourou, Attenuating Muscle Damage Biomarkers and Muscle Soreness After an Exercise-Induced Muscle Damage with Branched-Chain Amino Acid (BCAA) Supplementation: A Systematic Review and Meta-analysis with Meta-regression, Sports Med. Open, 2024, 10, 42 CrossRef PubMed.
- S. M. Lee, Y. H. Kim, Y. R. Kim, B. R. Lee, S. Shin, J. Y. Kim, I. C. Jung and M. Y. Lee, Anti-fatigue potential of Pinus koraiensis leaf extract in an acute exercise-treated mouse model, Biomed. Pharmacother., 2022, 153, 113501 CrossRef PubMed.
- H. K. Zhu, W. Q. Xu, N. Wang, W. H. Jiang, Y. L. Cheng, Y. H. Guo, W. R. Yao, B. Hu, P. Du and H. Qian, Anti-fatigue effect of Lepidium meyenii Walp. (Maca) on preventing mitochondria-mediated muscle damage and oxidative stress in vivo and vitro, Food Funct., 2021, 12, 3132–3141 RSC.
- H. C. Rhim, S. J. Kim, J. Park and K. M. Jang, Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis, J. Sports Health Sci., 2020, 9, 553–561 CrossRef PubMed.
- S. Duttagupta, N. K. Roy and G. Dey, Efficacy of amino acids in sports nutrition- review of clinical evidences, Food Res. Int., 2024, 187, 114311 CrossRef PubMed.
- C. L. K. Copetti, F. Diefenthaeler, F. Hansen, F. G. K. Vieira and P. F. Di Pietro, Fruit-Derived Anthocyanins: Effects on Cycling-Induced Responses and Cycling Performance, Antioxidants, 2022, 11, 11020387 CrossRef PubMed.
- Y. Zhang, Y. He, L. Y. Yuan, J. C. Shi, J. L. Zhao, C. P. Tan, Y. F. Liu and Y. J. Xu, Multi-omics revealed anti-fatigue property of polyphenol from areca nut, Phytomedicine, 2024, 132, 155838 CrossRef PubMed.
- A. Y. Coqueiro, M. M. Rogero and J. Tirapegui, Glutamine as an Anti-Fatigue Amino Acid in Sports Nutrition, Nutrients, 2019, 11, 11040863 CrossRef PubMed.
- B. J. Deters and M. Saleem, The role of glutamine in supporting gut health and neuropsychiatric factors, Food Sci. Hum. Wellness, 2021, 10, 149–154 CrossRef.
- M. N. Colombo and M. Francolini, Glutamate at the Vertebrate Neuromuscular Junction: From Modulation to Neurotransmission, Cells, 2019, 8, 8090996 CrossRef PubMed.
- Q. Yang, J. B. Liu, T. Li, S. Lyu, X. T. Liu, Z. Y. Du, X. M. Shang and T. Zhang, Integrated Microbiome and Metabolomic Analysis Reveal the Repair Mechanisms of Ovalbumin on the Intestine Barrier of Colitis Mice, J. Agric. Food Chem., 2023, 71, 8894–8905 CrossRef PubMed.
- D. Sun, C. Xie, Y. Zhao, J. Liao, S. Li, Y. Zhang, D. Wang, K. Hua, Y. Gu, J. Du, G. Huang and J. Huang, The gut microbiota-bile acid axis in cholestatic liver disease, Mol. Med., 2024, 30, 104 Search PubMed.
- M. Chen, W. Wei, Y. Li, S. L. Ge, J. M. Shen, J. Y. Guo, Y. Zhang, X. Huang, X. Y. Sun, D. L. Cheng, H. Y. Zheng, F. F. Chang, J. Y. Chen, J. Liu, Q. X. Zhang, T. J. K. Zhou, K. Yu and P. F. Tang, Cholestyramine alleviates bone and muscle loss in irritable bowel syndrome via regulating bile acid metabolism, Cell Proliferation, 2024, 57, E13638 CrossRef PubMed.
- S. Katsuhara, M. Yokomoto-Umakoshi, H. Umakoshi, Y. Matsuda, N. Iwahashi, H. Kaneko, M. Ogata, T. Fukumoto, E. Terada, R. Sakamoto and Y. Ogawa, Impact of Cortisol on Reduction in Muscle Strength and Mass: A Mendelian Randomization Study, J. Clin. Endocrinol. Metab., 2022, 107, 1477–1487 Search PubMed.
- A. McArdle, S. Pringle, J. Kumiscia, K. Hemmings, D. Bartolini, A. Migni, M. Venturelli, J. Vina, F. Galli and M. Jackson, Role of altered cortisol production in the development of sarcopenia, Physiology, 2023, 38, 5735268 Search PubMed.
- T. Y. Guan, S. S. Li, Q. J. Guan, J. S. Shi, Z. M. Lu, Z. H. Xu and Y. Geng, Spore Powder of Food and Chemical ToxicologyShapes Gut Microbiota to Relieve Exercise-Induced Fatigue in Mice, Nutrients, 2022, 14, 2973 CrossRef CAS PubMed.
- H. Jeon, K. Lee, J. Y. Kim, J. J. Shim and J. L. Lee, Effect of HY7602-Fermented Antler on Sarcopenia in Mice, Fermentation, 2023, 9, 429 CrossRef CAS.
- Y. S. Jing, M. S. Li, Y. Q. Li, T. Ma, Y. Qu, B. B. Hu, Y. H. Xie and Z. W. Li, Structural characterization and anti-fatigue mechanism based on the gut-muscle axis of a polysaccharide from, Int. J. Biol. Macromol., 2024, 283, 137621 CrossRef PubMed.
- C. Greenhill, Exercise affects gut microbiota and bone, Nat. Rev. Endocrinol., 2018, 14, 322 CrossRef PubMed.
- L. Wang and J. Y. Liu, Localized butyrate restores gut homeostasis, Nat. Biomed. Eng., 2023, 7, 3–5 CrossRef CAS PubMed.
- X. Q. He, D. Liu, H. Y. Liu, D. T. Wu, H. B. Li, X. S. Zhang and R. Y. Gan, Prevention of Ulcerative Colitis in Mice by Sweet Tea via the Regulation of Gut Microbiota and Butyric-Acid-Mediated Anti-Inflammatory Signaling, Nutrients, 2022, 14, 2208 CrossRef CAS PubMed.
- H. Liu, Q. L. Xi, S. J. Tan, Y. D. Qu, Q. Y. Meng, Y. N. Zhang, Y. X. Cheng and G. H. Wu, The metabolite butyrate produced by gut microbiota inhibits cachexia-associated skeletal muscle atrophy by regulating intestinal barrier function and macrophage polarization, Int. Immunopharmacol., 2023, 124, 111001 CrossRef CAS PubMed.
- H. H. Li, Z. Y. Shang, X. J. Liu, Y. Y. Qiao, K. W. Wang and J. Y. Qiao, Clostridium butyricum Alleviates Enterotoxigenic Escherichia coli K88-Induced Oxidative Damage Through Regulating the p62-Keap1-Nrf2 Signaling Pathway and Remodeling the Cecal Microbial Community, Front. Immunol., 2021, 12, 771826 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo04220d |
| This journal is © The Royal Society of Chemistry 2025 |