Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of daily avocado consumption on gut microbiota in adults with abdominal obesity: an ancillary study of HAT, a randomized controlled trial†
Jieping
Yang
 a,
On Kei
Lei
ai,
Shrikant
Bhute
bc,
Penny M.
Kris-Etherton
d,
Alice H.
Lichtenstein
e,
Nirupa R.
Matthan
e,
Kristina S.
Petersen
a,
On Kei
Lei
ai,
Shrikant
Bhute
bc,
Penny M.
Kris-Etherton
d,
Alice H.
Lichtenstein
e,
Nirupa R.
Matthan
e,
Kristina S.
Petersen
 d,
Joan
Sabaté
f,
David M.
Reboussin
g,
Laura
Lovato
g,
Mara Z.
Vitolins
g,
Sujatha
Rajaram
f,
Jonathan P.
Jacobs
bch,
Jianjun
Huang
a,
Meileen
Taw
a,
Scarlet
Yang
a and
Zhaoping
Li
*ah
d,
Joan
Sabaté
f,
David M.
Reboussin
g,
Laura
Lovato
g,
Mara Z.
Vitolins
g,
Sujatha
Rajaram
f,
Jonathan P.
Jacobs
bch,
Jianjun
Huang
a,
Meileen
Taw
a,
Scarlet
Yang
a and
Zhaoping
Li
*ah
aCenter for Human Nutrition, Department of Medicine, David Geffen School of Medicine, Los Angeles, CA 90095, USA. E-mail: zli@mednet.ucla.edu
bDavid Geffen School of Medicine, Department of Medicine, Los Angeles, CA 90095, USA
cGoodman-Luskin Microbiome Center, University of California Los Angeles, Los Angeles, CA 90095, USA
dDepartment of Nutritional Sciences, The Pennsylvania State University, University Park, PA 16802, USA
eCardiovascular Nutrition Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA 02111, USA
fCenter for Nutrition, Healthy Lifestyle and Disease Prevention, School of Public Health, Loma Linda University, CA 92354, USA
gDepartment of Biostatistics, Wake Forest University School of Medicine, Winston-Salem, NC 27101, USA
hDepartment of Medicine VA Greater Los Angeles HealthCare System, Los Angeles, CA 90073, USA
iFaculty of Education, University of Macau, Macao, China
First published on 6th December 2024
Abstract
Objectives: This study aimed to investigate short-term and long-term impact of avocado consumption without caloric restriction on the gut microbiota of free-living adults with abdominal obesity. Methods: The Habitual Diet and Avocado Trial (HAT) was a 26-week, multi-center, randomized, controlled trial involving 1008 individuals with abdominal obesity. Participants were randomly assigned to the Avocado Supplemented Diet Group (AVO), receiving one avocado per day, or the Habitual Diet group (HAB), maintaining their usual dietary habits. Fecal samples were collected at baseline, week 4 and week 26 from a subset of participants recruited at a University of California Los Angeles site (n = 230). Fecal microbiota was assessed with shotgun metagenomics sequencing. Alpha diversity was assessed using the Chao1 and Shannon indices; beta diversity was assessed using Bray–Curtis dissimilarity with significance determined by repeated measures permutational multivariat analysis of variance. Potential association of intervention at week 4 and 26 with alpha diversity, species and metabolic pathways was examined using linear mixed effect models. Results: Compared to the HAB group, the AVO group had higher alpha diversity by 4 weeks, which persisted through the 26-week study period. Exploratory analysis based on healthy eating index-2015 (HEI-2015) indicated that participants with a low HEI score at baseline (≤52.7), had an increase in alpha diversity in the AVO group vs. HAB group. The AVO group had a significant change in beta diversity at week 26 compared to the HAB group. At the species level, the AVO group had significantly increased Faecalibacterium prausnitzii and Bacterium AF16_15 at week 26 compared to the HAB group. Functional analysis showed no significant difference in metabolic pathways between the HAB and AVO groups. Conclusions: Our findings document a potentially favorable effect of avocados on gut microbiota diversity. The prebiotic potential of avocados is more pronounced in individuals with a low diet quality score. This trial is registered at clinicaltrials.gov as NCT03528031 (https://clinicaltrials.gov/study/NCT03528031).
Introduction
Avocados are a nutrient dense food that are high in monounsaturated fats, dietary fibers, and a variety of phytochemicals, such as lutein, vitamins C, E, K, and B6, niacin, folate, and phytosterols.1 Other clinical studies as well as our prior assessment of the impact of avocado consumption in cardiovascular health have shown positive effects on blood lipid profile.1,2 The Habitual Diet and Avocado Trial (HAT) was a multi-center, randomized, controlled trial designed to investigate the effect of providing one avocado per day for six months, compared to a habitual diet (HAB) low in avocados, in a diverse cohort of 1008 individuals with an elevated waist circumference.2 Many clinical outcome measures, related to metabolic status were collected. These included anthropometric data, visceral and hepatic fat, blood glucose and insulin levels, blood pressure, inflammation markers and blood lipids. The Healthy Eating Index-2015 (HEI-2015) was derived from 24 hour recall data. The Avocado Supplemented Diet (AVO) group exhibited modest yet statistically significant reductions in both total and low-density lipoprotein cholesterol, and an increase in the HEI-2015 compared to the HAB group.2Diet exerts a profound influence on the composition of the intestinal microbiome, acting as a central regulator of host metabolism.3,4 The significant impact of dietary fiber on the gut microbiome is well-documented.5 Additionally, phytochemicals, including phytosterols, alter the gut microbiome.6,7 A recent animal study elucidated that the modulation of gut microbiome serves as the underlying mechanism for phytosterol-induced changes in cholesterol levels.7 Studies have indicated a correlation between dietary lipid levels and the composition of the gut microbiota.8 The monounsaturated fats in avocados, after digestion, could be utilized as metabolic substrates by gut bacteria.8,9 We and others have shown that avocado consumption for 12 weeks regulates the composition and metabolic function of gut microbiota in participants with overweight and obesity.10,11 In these two studies, avocado was included either as part of a hypocaloric diet or an isocaloric diet for 12 weeks. In contrast, the focus of the HAT trial was on evaluating the effects of consumption of one avocado per day for six months compared to a habitual diet without additional dietary intervention in participants with abdominal obesity (defined as increased waist circumstance), which is positively associated with a risk of metabolic syndrome and cardiovascular disease.
The study aimed to explore the impact of daily avocado consumption, without caloric restriction, on the gut microbiota in free-living adults with abdominal obesity over 4 weeks and 26 weeks. Our hypothesis was that incorporating one avocado a day into participants’ habitual diet without additional dietary consultation will lead to a metabolically healthier gut microbiome, characterized by an increase in alpha diversity and shift in predominant species.
Materials and methods
Study design
HAT was a 4-center, randomized, controlled trial designed to investigate the health effect of consuming one avocado per day over six months, compared to a habitual diet low in avocados in a diverse cohort of 1008 individuals with an elevated waist circumference.2 The trial is registered at clinicaltrials.gov as NCT03528031. In this trial, individuals aged 25 or older, with a waist circumference of 35 inches or more for women and 40 inches or more for men, and who regularly consumed two or fewer avocados per month, were randomly assigned to either the HAB or the AVO.2 Individuals in the AVO group were instructed to maintain their usual diet and lifestyle while being regularly supplied with fresh Hass avocados, enabling them to consume one avocado daily for 6 months. No further dietary counseling or guidance was offered. Individuals in the HAB group were directed to adhere to their typical diet and lifestyle, with the specific instruction to restrict their avocado intake to two or fewer avocados per month for the entire 6 month study period. At the University of California Los Angeles site, 254 participants were randomly assigned to either the AVO group or the HAB group. There were 8 participants lost to follow-up, with 3 from the AVO group and 5 from the HAB group. A total of 246 participants completed the study, with 124 in the AVO group and 122 in the HAB group. In addition to study measures collected at all four clinical sites,2 we were able to collect fecal samples at baseline from 115 out of 124 participants in the AVO group and 115 out of 122 participants in the HAB group. At week 4, we collected fecal samples from 113 participants in both the HAB and AVO groups. At week 26, fecal samples were collected from 73 participants in the HAB group and 72 participants in the AVO group (ESI Fig. 1†). We had complete stool sample collections including baseline, week 4 and week 26 for 70 subjects in the AVO group and 71 subjects in the HAB group. Clinical outcome data from this trial have been previously published.2Dietary information
A detail avocado intake information has been previously reported.12 Four 24 hour dietary recalls were conducted for each study participant during the course of the study. Recalls were collected by phone prior to randomization, and then within a 1 to 2 weeks timeframe around baseline, week 8, 16 and 26. The recalls were used to calculate the Healthy Eating Index (HEI) 2015.13 24 hour dietary recalls at week 0 and week 26 were missing from 6 participants (HAB_week 0: n = 113; HAB_week 26: n = 71; AVO_week 0: n = 111; AVO_week 26: n = 69).Clinical and biochemical measures
Demographic, anthropometric, HEI-2015, MRI and biochemical measures, including glucose, insulin, high-sensitivity C-reactive protein (hsCRP), total cholesterol (TC), triglycerides (Trig), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), very low density lipoprotein-cholesterol (VLDL-C), visceral fat (VAT) and hepatic fat fraction (HFF) were determined as previously described.2 All anthropometric and biochemical measures, with the exception of systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse rate, were assessed at baseline and week 26. SBP, DBP, and pulse rate were additionally measured at week 4.2Fecal sample collection and DNA extraction
Participants received stool specimen collection kits during the screening visit, intended for baseline, 4 week, and 26 week sample collection. They were instructed to collect stool specimens within a 24 hour period before each scheduled sampling visit. Participants then brought the specimens in an insulated cooler with a pre-frozen ice pack to the clinic staff during their visit. Around 100–250 mg of each fecal sample was utilized for extracting microbial DNA using DNeasy PowerSoil Pro Kits (Qiagen, Germantown, MD), following the manufacturer's instruction.Shotgun sequencing and data analysis
The resulting DNA was fragmented and barcoded using the Illumina DNA Prep kit (San Diego, CA) following the manufacturer's instructions.14 The barcoded shotgun libraries were sequenced on an Illumina NovaSeq 6000 system (Illumina, San Diego, CA) using S4 flowcells and a 2 × 150 base pair (bp) sequencing configuration. Sequencing was performed to achieve target depth of 10 million paired-end sequences per sample, corresponding to approximately 3 gigabases of data per sample. The raw sequence reads were processed using KneadData for quality filtering and removal of human-derived reads. The filtered reads were then analyzed using the following bioinformatics tools for taxonomic composition and functional assessments.15 First, MetaPhlAn4 was used to identify the bacterial species present in the samples and determine the compositional profiles of the microbial communities.16 Next, HUMAnN3 was employed to annotate bacterial genes and determine their functional roles and metabolic pathways.15 The bacterial gene abundances were aggregated into metabolic pathways based on the MetaCyc pathway classifications.17 Alpha diversity indices (Chao1 and Shannon) were estimated with data, rarefied using with phyloseq,18 ggplot2,19 vegan20 packages as previously described.21Statistical analysis
This analysis was limited to participants with collected stool samples. Baseline characteristics were presented as mean (SD). The Student t test and Fisher's exact test were used to analyze differences in baseline characteristics between groups and calculated using IBM SPSS version 29 (IBM SPSS Inc.). For HEI 2015 scores, change from baseline was calculated by subtracting end-of-study values from baseline values. Independent Welch's t tests were used to test whether mean change in the variables of interest differed significantly between AVO and HAB groups. To detect difference in changes in clinical, biochemical measures and alpha diversity indices between HAB and AVO groups over time, we built linear mixed models that included fixed effects of intervention, time, intervention–time interaction and subjects as random effects using the “lmertest” package.22Post hoc Tukey tests were conducted to identify specific significant differences while accounting for multiple comparisons. Prespecified subgroups defined using baseline HEI-2015 (median split) from the original cohort of 1008 participants. Participants with HEI ≤ 52.7 were classified as HEI_low, and those with HEI > 52.7 were classified as HEI_high.2 Prespecified subgroup analyses, models with a 3-way interaction between intervention–time-subgroup were fit. The significance of the interaction term adjusted for multiple comparisons was used to test for subgroup effects. P values < 0.05 were considered statistically significant. Beta diversity was calculated using Bray–Curtis dissimilarity and visualized by Principal Coordinates Analysis (PCoA). The significance of beta diversity differences across groups were determined by permutational multivariate analysis of variance (PERMANOVA) (‘vegan’ package, v2.6.4), using Vegan in R.23Differential abundance analysis at the species and genus level was conducted using per-feature testing in MaAsLin2 (multivariate association with linear models), implemented in the R package.24 Linear mixed-effects models were employed to explore potential associations between species/genera and intervention, time, and the interaction between intervention and time, as previously described.25 These models accounted for within-individual correlation arising from the study's repeated sampling design. All identified associations were adjusted for subjects as a random effect, as well as other fixed-effects metadata including age, gender, race (with white as the reference level), intervention (with HAB as the reference level), time (with W0 as the reference level), and the interaction between intervention and time. The current analysis was performed after filtering at a minimum abundance level of 0.00001 and a minimum prevalence of 0.05. Relative abundances were log-transformed. Only significant associations with a q-value ≤ 0.25, following false discovery rate (FDR) correction, were included. Differential abundance analysis was also performed separately for participants in the HEI_low and HEI_high groups.
Mediation analysis was used to determine the extent to which the association between avocado intake and microbial changes was due to differences in HEI component scores “mediated” the association. The analysis was performed in Mediation implemented in the R package.26 Avocado intake (against habitual diet) was regarded as the primary exposure and (1) HEI components (energy intake, total fruits, whole fruits, and fatty acids, the HEI-2015 total score) as the mediators, and microbial alpha diversity and fecal AF16_15 and Faecalibacterium prausnitzii as the outcomes. Each mediator was tested separately in the analysis, while including the age, gender and race as covariates and adjusted for subjects as a random effect.
Results
Anthropometric and biochemical measures at baseline and at the end of the 26 weeks intervention in participants at the UCLA site
There were no significant differences in the baseline characteristics between HAB and AVO groups, including age, gender, race, weight, body mass index (BMI), waist circumference, HEI 2015 total score, SBP, DBP, insulin, hsCRP, glucose, TC, Trig, HDL-C, LDL-C, VLDL-C, VAT and HFF (Table 1). All anthropometric and biochemical measures remained unchanged with the AVO group over 26 weeks (Table 2).| HAB (n = 115) | AVO (n = 115) | P value | |
|---|---|---|---|
| BMI: body mass index; HEI-2015: healthy eating index 2015; DBP: diastolic blood pressure; SBP: systolic blood pressure; hsCRP: high-sensitivity C-reactive protein; TC: total cholesterol; Trig: triglycerides; HDL-C: high density lipoprotein-cholesterol; LDL-C low density lipoprotein-cholesterol; VLDL-C: very low density lipoprotein-cholesterol; VAT: visceral fat; and HFF: hepatic fat fraction. Student t tests test and Fisher exact test were used to analyze differences in baseline characteristics between groups. | |||
| Age | 45.42 (13.06) | 47.05 (14.39) | 0.37 |
| Gender (F%) | 84.2% | 77.2% | 0.17 |
| Race (Asian/black/mixed/other/unknown/white)% | (8/18/0/4/25/46) | (6/22/1/8/18/46) | 0.472 |
| Weight (kg) | 89.88 (18) | 89.97 (19.75) | 0.972 |
| BMI (kg m−2) | 32.74 (5.92) | 32.63 (5.87) | 0.803 |
| Waist circumference (cm) | 107.47 (11.9) | 107.45 (13.21) | 0.990 |
| HEI-2015 | 54.62 (15.83) | 54.38 (14.8) | 0.905 |
| DBP (mmHg) | 76.71 (10.51) | 77.51 (10.43) | 0.565 |
| SBP (mmHg) | 119.69 (15.75) | 121.38 (16.99) | 0.440 |
| Pulse | 69.9 (9.83) | 70.17 (10.31) | 0.842 |
| Insulin (μIU mL−1) | 15.6 (16.54) | 18.37 (27.19) | 0.358 |
| hsCRP (mg L−1) | 5.85 (5.86) | 6.22 (7.18) | 0.677 |
| Glucose (mg dL−1) | 95.24 (18.13) | 98.92 (28.96) | 0.255 |
| TC (mg dL−1) | 184.42 (39.36) | 174.64 (40.56) | 0.069 |
| Trig (mg dL−1) | 109.81 (58.14) | 118.87 (86.92) | 0.244 |
| HDL-C (mg dL−1) | 55.66 (12.74) | 53.55 (14.19) | 0.359 |
| VLDL-C (mg dL−1) | 21.96 (11.63) | 23.77 (17.38) | 0.057 |
| LDL-C (mg dL−1) | 106.8 (31.89) | 98.47 (33.4) | 0.261 |
| VAT (L) | 2.77 (1.36) | 2.98 (1.4) | 0.996 |
| HFF % | 0.1 (0.11) | 0.1 (0.11) | 0.244 |
| AVO | HAB | P wk4 | P wk26 | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 26 | Baseline | Week 4 | Week 26 | |||
| BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; hsCRP: high-sensitivity C-reactive protein; TC: total cholesterol; Trig: triglycerides; HDL-C: high density lipoprotein-cholesterol; LDL-C low density lipoprotein-cholesterol; VLDL-C: very low density lipoprotein-cholesterol; VAT: visceral fat; and HFF: hepatic fat fraction. Values are presented as mean (SD). No significance (NS). | ||||||||
| N | 115 | 113 | 72 | 115 | 113 | 73 | ||
| BMI (kg m−2) | 32.72 (5.83) | — | 33.48 (6.01) | 32.87 (5.94) | — | 32.81 (5.61) | NS | |
| VAT (L) | 2.98 (1.4) | — | 3.07 (1.42) | 2.77 (1.36) | — | 2.83 (1.43) | NS | |
| HFF (%) | 0.1 (0.11) | — | 0.09 (0.1) | 0.1 (0.11) | — | 0.1 (0.12) | NS | |
| DBP (mmHg) | 78 (10) | 76 (9) | 76 (7) | 77 (11) | 77 (9) | 77 (8) | NS | NS |
| SBP (mmHg) | 121 (17) | 120 (15) | 121 (13) | 120 (16) | 120 (13) | 122 (17) | NS | NS |
| Pulse | 70.17 (10.31) | 74.3 (9.75) | 71.36 (9.03) | 69.9 (9.83) | 75.67 (22.91) | 69.23 (10.63) | NS | NS |
| Insulin (μIU mL−1) | 18.37 (27.19) | — | 15.88 (13.34) | 15.6 (16.54) | — | 14.58 (11.05) | NS | |
| hsCRP (mg L−1) | 6.22 (7.18) | — | 6.6 (7.09) | 5.85 (5.86) | — | 6.31 (5.92) | NS | |
| Glucose (mg dL−1) | 98.92 (28.96) | — | 104.83 (25) | 95.24 (18.13) | — | 104.13 (27.55) | NS | |
| TC (mg dL−1) | 175 (41) | — | 176 (41) | 184 (39) | — | 191 (42) | NS | |
| Trig (mg dL−1) | 119 (87) | — | 118 (83) | 110 (58) | — | 113 (52) | NS | |
| HDL-C (mg dL−1) | 54 (14) | — | 54 (14) | 56 (13) | — | 53 (12) | NS | |
| VLDL-C (mg dL−1) | 24 (17) | — | 24 (17) | 22 (12) | — | 23 (10) | NS | |
| LDL-C (mg dL−1) | 98 (33) | — | 99 (37) | 107 (32) | — | 115 (34) | NS | |
The impact of avocado intake on dietary quality and total and lipoprotein cholesterol varies according to participants’ baseline dietary quality scores
Table 3 shows the changes of the HEI-2015 total score as well as component scores that compose the total score across 26 week in both ABO and HAB groups. There were no significant between-group differences in change from baseline for total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, sodium, refined grains, added sugars and saturated fats. Changes in energy intake (p = 0.046), total fruits (p = 0.000), whole fruits (p = 0.000), fatty acid (p = 0.021), and HEI-2015 total score (p = 0.028) were significantly different between the AVO and the HAB groups (Table 3).| HEI component scores | AVO | HAB | Effect | 95% CI | P value |
|---|---|---|---|---|---|
| Values represent change from baseline to end of study. Welch's t tests were used to assess between–group differences in absolute change across 26 weeks. Values in bold are statistically significant (p < 0.05). No significance (NS). | |||||
| Energy intake (kcal) | 266.31 | 9.78 | 0.34 | 0.01 to 0.67 | 0.046 |
| Total vegetables | −0.53 | −0.09 | −0.2 | −0.53 to 0.13 | NS |
| Greens and beans | −0.2 | −0.14 | −0.02 | −0.35 to 0.31 | 0.897 |
| Total fruits | 1.92 | 0.08 | 0.78 | 0.44 to 1.13 | 0.000 |
| Whole fruits | 2.06 | 0.06 | 0.76 | 0.42 to 1.1 | 0.000 |
| Whole grains | 0.18 | 0.27 | −0.02 | −0.35 to 0.31 | 0.922 |
| Dairy | −0.37 | −0.24 | −0.03 | −0.36 to 0.3 | 0.866 |
| Total protein foods | −0.11 | −0.03 | −0.06 | −0.39 to 0.27 | 0.725 |
| Seafood and plant proteins | 0.13 | 0.18 | −0.02 | −0.35 to 0.31 | 0.914 |
| Fatty acids | 2.1 | 0.18 | 0.39 | 0.06 to 0.73 | 0.021 |
| Sodium | 1.91 | 0.4 | 0.28 | −0.05 to 0.61 | NS |
| Refined grains | 0.55 | −0.37 | 0.18 | −0.15 to 0.52 | NS |
| Added sugars | 0.31 | 0.92 | −0.19 | −0.52 to 0.14 | NS |
| Saturated fats | −0.09 | 0.43 | −0.12 | −0.45 to 0.21 | NS |
| HEI-2015 total score | 7.86 | 1.65 | 0.38 | 0.04 to 0.71 | 0.028 |
Interestingly, improvement of HEI-2015 total score (p = 0.001) and HDL-C (p = 0.0008) were limited to participants with a low HEI (HEI ≤ 52.7; HEI_low) at baseline (Table 4), but not in participants with a high HEI (HEI > 52.7; HEI_high) at baseline (ESI Table 1†). Among HEI_low participants, at week 26, HEI-2015 total score increased by 41% from baseline in the AVO group, compared to 19% in the HAB group (Table 4). HDL-C remained unchanged (0.09%) in the AVO group and decreased by 5.5% in the HAB group (Table 4).
| AVO | HAB | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 26 | Baseline | Week 4 | Week 26 | P wk4 | P wk26 | |
| Values are presented as mean (SD); P ≥ 0.05, no significance (NS). Values in bold are statistically significant (p < 0.05). | ||||||||
| N | 48 | 48 | 32 | 57 | 56 | 34 | ||
| BMI (kg m−2) | 32.44 (5.92) | — | 32.91 (5.92) | 33.47 (6.26) | — | 33.07 (6.23) | NS | |
| HEI-2015 | 40.48 (7.79) | — | 57.07 (13.5) | 42.21 (8.11) | — | 50.43 (14.99) | 0.001 | |
| VAT (L) | 2.93 (1.38) | — | 2.95 (1.4) | 2.73 (1.48) | — | 2.8 (1.64) | NS | |
| HFF (%) | 0.1 (0.12) | — | 0.08 (0.09) | 0.1 (0.11) | — | 0.09 (0.12) | NS | |
| DBP (mmHg) | 78 (9) | 78 (8) | 78 (8) | 75 (10) | 76 (10) | 76 (9) | NS | NS |
| SBP (mmHg) | 122 (14) | 120 (13) | 121 (15) | 118 (15) | 120 (13) | 120 (17) | NS | NS |
| Pulse | 72.15 (11.69) | 74.59 (10.16) | 73.19 (9.44) | 70.98 (9.73) | 77.23 (30.76) | 68.53 (11.19) | NS | NS |
| Insulin (μIU mL−1) | 20.24 (36.14) | — | 17.55 (17.14) | 16.49 (21.35) | — | 12.88 (10.32) | NS | |
| hsCRP (mg L−1) | 6.02 (4.18) | — | 5.59 (5.42) | 6.44 (6.08) | — | 6.59 (5.63) | NS | |
| Glucose (mg dL−1) | 99.38 (23.6) | — | 108.22 (28.31) | 94.58 (19.72) | — | 102.91 (29.26) | NS | |
| TC (mg dL−1) | 178 (47) | — | 175 (50) | 178 (34) | — | 183 (38) | NS | |
| Trig (mg dL−1) | 110 (64) | — | 116 (80) | 98 (58) | — | 104 (45) | NS | |
| HDL-C (mg dL −1 ) | 54 (17) | — | 54 (17) | 54 (12) | — | 51 (12) | 0.0008 | |
| VLDL-C (mg dL−1) | 22 (13) | — | 23 (16) | 20 (12) | — | 21 (9) | NS | |
| LDL-C (mg dL−1) | 103 (37) | — | 99 (45) | 105 (29) | — | 111 (31) | NS | |
Effects of avocado intake on gut microbiome
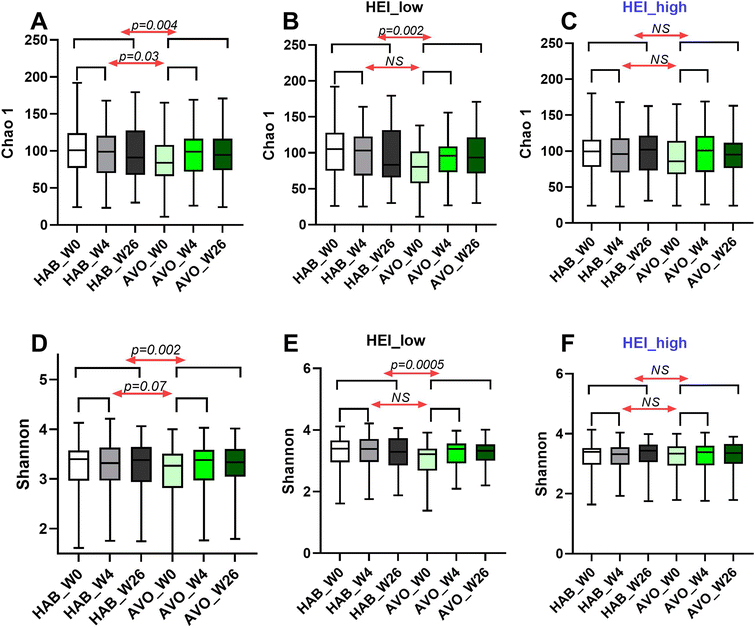
Two alpha diversity indices, Chao1 and Shannon, were calculated. Compared to HAB group, we observed a significant increase of species richness (Chao1) associated with avocado intake by week 4, which persisted through week 26 (week 4: p = 0.03; week 26: p = 0.004) (Fig. 1A). A significant increase in species richness and evenness, as indicated by Shannon index, was only observed at week 26 (week 4: p = 0.07; week 26: p = 0.02) (Fig. 1D) in AVO group compared to HAB group.Consistent with the subgroup analysis results related to HEI-2015 total score and HDL–cholesterol, we observed a significant increase in alpha diversity indices Chao1 (week 4: p = 0.37; week 26: p = 0.01) and Shannon (week 4: p = 0.04; week 26: p = 0.0005) associated with avocado consumption in participants with low HEI scores at baseline (Fig. 1B and E), but not high HEI scores (Fig. 1C and F). Beta diversity assessed by calculating the Bray–Curtis dissimilarity distances demonstrated significant differences in microbial composition between the AVO and HAB groups at week 26 (R2 = 0.002, P = 0.02, Fig. 2). Similar to alpha diversity, significant differences in microbial composition between AVO and HAB groups at week 26 were only detected in participant with low baseline HEI scores (R2 = 0.002, p = 0.04) but not high baseline HEI scores (R2 = 0.002, p = 0.60).
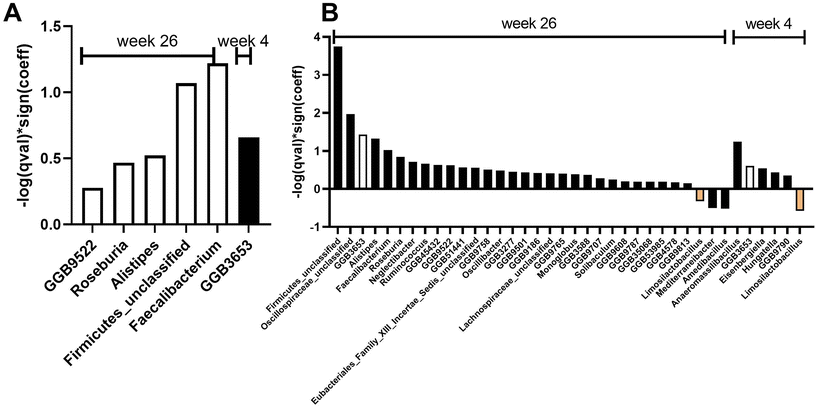
Differential abundance analysis at the species and genus level revealed eight and six significant features, respectively, when considering treatment over time and controlling for covariates (age, gender, race) (Fig. 3 and 5). Specifically, at week 26, there was a significant increase in four species (q < 0.25): Bacterium AF16_15 (family Firmicutes_unclassified), Faecalibacterium prausnitzii (family Oscillospiraceae), Clostridium leptum (family Oscillospiraceae), and GGB9522 SGB14921 (family Lactobacillaceae) in the AVO group compared to the HAB group (Fig. 3A–E). At week 4, relative abundance of GGB3653 SGB4964 (family Lachnospiraceae, F), Ruminococcus bicirculans (family Oscillospiraceae, G), Eisenbergiella tayi (family Lachnospiraceae, H) and Marseille Q4145 (family Clostridiaceae, I) were significantly different between AVO group and HAB group. At the genus level, relative abundance of Faecalibacterium (family Oscillospiraceae), Firmicutes_unclassified, GGB9522 (family Lactobacillaceae), Roseburia (family Lachnospiraceae) and Alistipes (family Rikenellaceae) significantly increased in AVO group compared to HAB group at week 26; GGB3653 was significantly increased at week 4 (Fig. 5A). At the phylum level, no significant association was identified. Differential abundance analysis of microbial metabolic pathways and functions revealed 24 nominally significant features between the AVO group and HAB group when considering treatment effects and controlling for covariates. Specifically, 12 significant features were observed at week 4 and another 12 at week 26. However, after adjusting for multiple comparisons using a q-value threshold of ≤0.25, no significant differences remained (ESI Fig. 2†).
Given the more pronounced increase in alpha diversity observed in the AVO group with HEI_low participants compared to HEI_high participants, we performed an exploratory investigation into differential abundance analysis at the species level within both subgroups. In HEI_low participants, we observed more significant associations with avocado intake (Fig. 4: 9 species at week 4 and 32 species at week 26) than were identified in previous analyses conducted on all participants (Fig. 3: 4 species at week 4 and 4 species at week 26). At week 4, 9 species exhibited significant associations, and this increased to 32 species by week 26 (Fig. 4 and ESI Table 2†). Notably, Streptococcus australis (family Streptococcaceae); Bacteroides ovatus (family Bacteroidaceae), and GGB3653 SGB4964 (family Lachnospiraceae) consistently showed significant associations with avocado intake during intervention period (orange, green and white bars, Fig. 4). At the genus level, 6 genera at week 4 and 32 genera at week 26 exhibited significant associations with avocado intake. Notably, Limosilactobacillus and GGB3653 consistently demonstrated associations from week 4 to week 26 (white and orange bars, Fig. 5B). At the phylum level, Bacteroidetes consistently showed a positive association with avocado intake from week 4 to week 26, while Euryarchaeota only appeared at week 26 (data not shown). In HEI_high participants, no significant associations were detected. In addition, 45 and 33 microbial metabolic pathways and functions were nominally significantly associated with avocado intake in HEI_low participants (ESI Fig. 3A†) and HEI_high participants (ESI Fig. 3B†), respectively. In HEI_low participants, the four metabolic pathways, including anaerobic energy metabolism invertebrates cytosol, gluconeogenesis III, superpathway of geranylgeranyl diphosphate biosynthesis II via MEP, and colanic acid building blocks biosynthesis consistently exhibited positive associations with avocado intake throughout the intervention period, from week 4 to week 26 (ESI Fig. 3A†). However, after adjusting for multiple comparisons using a q-value threshold of ≤0.25, no significant differences remained.
Dietary patterns and gut microbiota interaction
There were significant associations between avocado intake, HEI components (energy intake, total fruits, whole fruits, fatty acids, and the HEI-2015 total score), alpha diversity indexes (Chao1 and Shannon), and fecal bacterium_AF16_15 and Faecalibacterium prausnitzii. We performed mediation analyses to investigate whether HEI-2015 components (energy intake, total fruits, whole fruits, and fatty acids, the HEI-2015 total score) might mediate the observed avocado associated changes in alpha diversity and AF16_15 and Faecalibacterium prausnitzii. None of the HEI component explained the relationship between avocado intake and microbial changes (alpha diversity, Bacterium AF16_15 and Faecalibacterium prausnitzii). HEI-2015 total score likely contributed to 11% Chao1 changes with P = 0.068 (ESI Table 3†).Discussion
Nutrition-related clinical trials can investigate causal relationships between diet and various risk factors associated with non-communicable and chronic diseases. They are important for establishing dietary requirements and developing nutrition guidance for health promotion and disease prevention. One challenging issue encountered when conducting nutrition-related clinical trials is addressing participant's nutritional status including their habitual dietary pattern. In the HAT multi-center clinical trial, we aimed to investigate the effects of incorporating one avocado per day in free living participants without changing their usual diet and lifestyle.2 Among a wide range of clinical and biochemical outcomes evaluated, only very modest but nominal significant reduction in total and LDL-C was observed.2 The significant reduction in total cholesterol (−2.9 mg dL−1 by AVO vs. HAB) and LDL-cholesterol (−2.5 mg dL−1 by AVO vs. HAB) levels previously observed in 1008 participants was not significant in the subset of participants at the UCLA site (n = 230).2 Potential factors such as lower statistical power due to smaller sample size, demographics, lifestyle, food quality, and dietary habits of the study population may contribute to this disparity, warranting further investigation. However, the improvement in HEI scores (8.4 by avocado) and a trend in SBP changes were consistent with previous findings in the larger cohort.2 A noteworthy increase in HEI-2015 is consistent with findings from the original cohort of 1008 participants.In a subset of participants from HAT, limited to participants from UCLA only, we investigated the effects of incorporating one avocado per day on the gut microbial composition and function, anthropometric and biochemical measures, as well as association with HEI scores relative to habitual diet. The major finding of this study was that avocado consumption altered gut microbial composition, including increased microbial alpha diversity, elicited changes in beta diversity, and alterations in relative abundance of specific species and genera. Long-term treatment (26 weeks) showed a more profound impact on microbiome composition compared to short-term treatment (4 weeks), particularly in those participants with lower baseline diet quality, assessed using HEI scores. We observed avocado intake induced significant microbial compition changes, however, participant factors (R2 = 0.65, p = 0.007) emerged as more significant than treatment × time interaction, indicating that individual variability was the primary driver of differences in community composition rather than the intervention.
Although the retention rate was high (97%), there was a significant loss of fecal samples collected in this ancillary study due to the extraordinary challenges associated with the COVID-19 pandemic (baseline: 7% in the AVO group and 6% in the HAB group; week 4: 9% in the AVO group and 7% in the HAB group; week 26: 41% in both the AVO group and the HAB group). To assess the impact of this attrition and robustness of our findings, we performed sensitivity analyses restricted to cases who completed the entire 26 week intervention period. This included 141 complete cases, with 70 in the AVO group and 71 in the HAB group. The results indicated similar findings to the entire cohort. There were increases in alpha diversity indices at week 26 were observed in the restricted compared to entire AVO group (ESI Fig. 4A and D†). Increases in Chao 1 and Shannon indices at week 26 were significant only in participants with low HEI scores at baseline (ESI Fig. 4B and E†), but not high HEI scores (ESI Fig. 4C and F†). Beta diversity analysis of these complete cases showed no significant difference in microbial composition between the AVO and HAB groups at week 4 and week 26 (week 4: R2 = 0.002, P = 0.34; week 26: R2 = 0.002, P = 0.10) (ESI Fig. 5A†). However, in participants with low HEI scores, significant differences between the AVO and HAB groups were detected at week 26 (week 4: R2 = 0.003, P = 0.46; week 26: R2 = 0.008, P = 0.01), and participant factors were no longer more significant than the treatment*time interaction (ESI Fig. 5B†). No significant difference was detected in participants with high HEI scores (week 4: R2 = 0.002, P = 0.79; week 26: R2 = 0.002, P = 0.63) (ESI Fig. 5C†). In addition, we performed differential abundance analysis at the species level, restricted to participants who completed the entire 26 week intervention period. Differential abundance analysis identified 33 significant species at week 26 and 18 significant species at week 4 when time, treatment, time × treatment interaction and controlling for covariates were included in the model (ESI Table 4†). However, post-adjustment for multiple comparisons yielded only one significant species, Bacterium AF16_15 (Firmicutes unclassified), which was significantly increased in the AVO group compared to the HAB group at week 26 (q = 0.17, ESI Table 4†). In HEI_low participants, 12 species at week 4 and 58 species at week 26 species exhibited significant association with avocado intake (ESI Table 5†). However, post-adjustment for multiple comparisons yielded only one significant species, Bacterium AF16_15 (Firmicutes unclassified), that was significantly increased in the AVO group compared to the HAB group at week 26 (q = 0.02, ESI Table 5†). No significant species difference was detected in participants with high HEI scores.
The gut microbiome plays a critical role in human health and could be one of the underlying mechanisms behind the observed diverse responses associated with participants’ initial dietary quality. In this study, participants with a waist circumference of 35 inches or more for women and 40 inches or more for men, were considered to have visceral obesity. Both gut microbiota and diet have been demonstrated to significantly influence visceral fat mass, which is a major risk factor for cardiometabolic disorders.27 A recent study investigating the complex relationship between the gut microbiome, host metabolism, and habitual diet suggests that microbial biomarkers can predict many cardiometabolic markers.3 Specifically, the study found a significant association between microbial alpha diversity and indicators of cardiometabolic health.3 Previous study suggested that visceral fat was more closely correlated with the gut microbiome composition compared with BMI,28 suggesting an intrinsic connection between the gut microbiome and visceral fat and its related metabolic disorders. Although the primary outcomes of the HAT study demonstrated that avocado intake did not change visceral fat or other obesity markers such as BMI,2 we observed significant increase of alpha diversity indices with avocado intake, particular in participants with low HEI scores. The effects of avocado on alpha diversity index Chao1 might be week and need further investigation as the main effect of interaction of Chao1 is not significant (p = 0.10), but post hoc analysis at showed significance associated with avocado consumption at week 26 in HEI_low group. In addition, we observed some lean-associated microbial changes induced by avocado intake, such as enrichment of Faecalibacterium and Alistipes. Plant-enriched diets have been shown to selectively promote the proliferation of specific butyrate-producing bacteria, such as Roseburia hominis.29 In addition to Roseburia, the relative abundance of some well-known butyrate producers like Clostridium leptum and Faecalibacterium prausnitzii were increased with the addition of one avocado per day to participants’ usual diet.30Faecalibacterium prausnitzii, comprising approximately 5% of fecal bacteria, is one of the predominant anaerobic bacteria in the human gut microbiome. Reduction of Faecalibacterium prausnitzii has been associated with many diseases, including IBD, colorectal cancer and diabetes.31 Dysbiosis in patients with ulcerative colitis is characterized by a reduction in the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii.32 This finding is in the line with the anti-inflammatory properties of avocado in IBD.33 We also found that avocado intake increased the relative abundance of Ruminococcus. Interestingly, Ruminococcus was previously identified as an obesity-associated genus in Western studies, but it seems to exhibit a lean-associated profile in Eastern populations.27,34 This divergence underscores the contextual nature of microbial associations, highlighting the influence of cultural, race and dietary factors on microbial dynamics.
The interaction between dietary patterns and gut microbiota is crucial. Given that macronutrients (carbohydrates, fats, proteins, dietary fibers) can significantly impact gut microbiota composition, we performed mediation analysis to investigate whether the components of the Healthy Eating Index (HEI) and the total HEI-2015 score could explain the most profound microbial changes we identified (alpha diversity, Bacterium_AF16_15, and Faecalibacterium prausnitzii). Our analysis indicates that HEI components do not significantly contribute to the observed changes associated with avocado consumption. Only the total HEI-2015 score appears promising, explaining 11% of the changes in Chao1. This suggests that while specific macronutrients may not be potent enough to drive changes in gut microbiota, the overall dietary quality, as reflected by the total HEI-2015 score, plays a more substantial role. In addition, we did not detect any avocado-induced changes in sleep quality and physical activity. Therefore, it is unlikely that the observed microbial changes are related to sleep quality and physical activity (ESI 1†). In addition, we performed a mediation analysis to determine if microbial diversity (Chao1 and Shannon indices) mediated the observed avocado-associated changes in HDL among HEI_low participants. None of the alpha diversity indices explained the relationship between avocado intake and HDL changes. It is noteworthy that only a limited number of metabolic markers were evaluated in this clinical trial. Further investigation is required to determine whether changes in the gut microbiome lead to improvements in other clinical markers. Further research is needed to explore the complex interactions between diet and gut microbiota, considering other potential mediators and confounding factors. This could help in developing more targeted dietary interventions to modulate gut microbiota for better health outcomes. In summary, we observed significant differences in HEI, HDL-C levels, and microbial composition and diversity in the UCLA subgroup of the HAT cohort with poor baseline dietary habits (HEI_low) who consumed avocados, as compared to the HAB group. This finding highlights the importance of enhancing counseling efforts to improve diet quality in individuals with poor dietary quality. Using HEI to identify potential diet responders could be a new personalized nutrition approach targeted to both individuals and populations that would be expected to benefit from improved gut microbial composition and diversity.
Abbreviations
| AVO | Avocado group |
| DBP | Diastolic blood pressure |
| FDR | False discovery rate |
| HAB | Habitual diet group |
| HAT | Habitual diet and avocado trial |
| HDL-C | High density lipoprotein-cholesterol |
| HEI | Healthy eating index |
| HFF | Hepatic fat fraction |
| LDL-C | Low density lipoprotein-cholesterol |
| SBP | Systolic blood pressure |
| TC | Total cholesterol |
| Trig | Triglycerides |
| Vat | Visceral fat |
| VLDL-C | Very low density lipoprotein-cholesterol |
Author contributions
ZL, PK, AL, NM, KP, JS, DR, LL, MV and SR designed research and reviewed the paper; JY, LK, SB, JH, MT and SY conducted research; JY, OL, SB, MT and JJ analyzed data; JY and ZL wrote the paper. ZL had primary responsibility for final content. All authors read and approved the final manuscript.Data availability
Data described in the manuscript will be made available upon request.Conflicts of interest
David M. Reboussin reports a relationship with Hass Avocado Board that includes: consulting or advisory. This study was supported by the Avocado Nutrition Center. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was supported by the Avocado Nutrition Center. Declaration of generative AI and AI-assisted technologies in the writing process: During the preparation of this work the author(s) used Microsoft Copilt in order to edit English. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.References
- M. L. Dreher and A. J. Davenport, Hass avocado composition and potential health effects, Crit. Rev. Food Sci. Nutr., 2013, 53, 738–750 CrossRef CAS PubMed.
- A. H. Lichtenstein, P. M. Kris-Etherton, K. S. Petersen, N. R. Matthan, S. Barnes, M. Z. Vitolins, Z. P. Li, J. Sabaté, S. Rajaram, S. Chowdhury, K. M. Davis, J. Galluccio, C. H. Gilhooly, R. S. Legro, J. Li, L. Lovato, L. H. Perdue, G. Petty, A. M. Rasmussen, G. Segovia-Siapco, R. Sirirat, A. Sun and D. M. Reboussin, Effect of Incorporating 1 Avocado Per Day Versus Habitual Diet on Visceral Adiposity: A Randomized Trial, J. Am. Heart Assoc., 2022, 11, 1–21 Search PubMed.
- F. Asnicar, S. E. Berry, A. M. Valdes, L. H. Nguyen, G. Piccinno, D. A. Drew, E. Leeming, R. Gibson, C. Le Roy, H. A. Khatib, L. Francis, M. Mazidi, O. Mompeo, M. Valles-Colomer, A. Tett, F. Beghini, L. Dubois, D. Bazzani, A. M. Thomas, C. Mirzayi, A. Khleborodova, S. Oh, R. Hine, C. Bonnett, J. Capdevila, S. Danzanvilliers, F. Giordano, L. Geistlinger, L. Waldron, R. Davies, G. Hadjigeorgiou, J. Wolf, J. M. Ordovas, C. Gardner, P. W. Franks, A. T. Chan, C. Huttenhower, T. D. Spector and N. Segata, Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals, Nat. Med., 2021, 27, 321–332 CrossRef CAS PubMed.
- K. D. Corbin, E. A. Carnero, B. Dirks, D. Igudesman, F. Yi, A. Marcus, T. L. Davis, R. E. Pratley, B. E. Rittmann, R. Krajmalnik-Brown and S. R. Smith, Host-diet-gut microbiome interactions influence human energy balance: a randomized clinical trial, Nat. Commun., 2023, 14, 3161 CrossRef CAS.
- P. Cronin, S. A. Joyce, P. W. O’Toole and E. M. O’Connor, Dietary Fibre Modulates the Gut Microbiota, Nutrients, 2021, 13, 1–22 CrossRef PubMed.
- R. Yin, H. C. Kuo, R. Hudlikar, D. Sargsyan, S. Li, L. Wang, R. Wu and A. N. Kong, Gut microbiota, dietary phytochemicals and benefits to human health, Curr. Pharmacol. Rep., 2019, 5, 332–344 CrossRef PubMed.
- W. J. Lv, J. Y. Huang, J. Lin, Y. M. Ma, S. Q. He, Y. W. Zhang, T. Z. Wang, K. Cheng, Y. Xiong, F. G. Sun, Z. C. Pan, J. B. Sun, W. Mao and S. N. Guo, Phytosterols Alleviate Hyperlipidemia by Regulating Gut Microbiota and Cholesterol Metabolism in Mice, Oxid. Med. Cell. Longevity, 2023, 2023, 6409385 Search PubMed.
- M. Schoeler and R. Caesar, Dietary lipids, gut microbiota and lipid metabolism, Rev. Endocr. Metab. Disord., 2019, 20, 461–472 CrossRef CAS PubMed.
- C. Yue, Y. Tang, M. Chang, Y. Wang, H. Peng, X. Wang, Z. Wang, X. Zang, H. Ben and G. Yu, Dietary supplementation with short- and long-chain structured lipids alleviates obesity via regulating hepatic lipid metabolism, inflammation and gut microbiota in high-fat-diet-induced obese mice, J. Sci. Food Agric., 2024, 104(9), 5089–5103 CrossRef CAS PubMed.
- S. V. Thompson, M. A. Bailey, A. M. Taylor, J. L. Kaczmarek, A. R. Mysonhimer, C. G. Edwards, G. E. Reeser, N. A. Burd, N. A. Khan and H. D. Holscher, Avocado Consumption Alters Gastrointestinal Bacteria Abundance and Microbial Metabolite Concentrations among Adults with Overweight or Obesity: A Randomized Controlled Trial, J. Nutr., 2021, 151, 753–762 CrossRef CAS PubMed.
- S. M. Henning, J. P. Yang, S. L. Woo, R. P. Lee, J. J. Huang, A. Rasmusen, C. L. Carpenter, G. Thames, I. Gilbuena, C. H. Tseng, D. Heber and Z. P. Li, Hass Avocado Inclusion in a Weight-Loss Diet Supported Weight Loss and Altered Gut Microbiota: A 12-Week Randomized, Parallel-Controlled Trial, Curr. Dev. Nutr., 2019, 3(8), nzz068 CrossRef PubMed.
- A. H. Lichtenstein, P. M. Kris-Etherton, K. S. Petersen, N. R. Matthan, S. Barnes, M. Z. Vitolins, Z. Li, J. Sabate, S. Rajaram, S. Chowdhury, K. M. Davis, J. Galluccio, C. H. Gilhooly, R. S. Legro, J. Li, L. Lovato, L. H. Perdue, G. Petty, A. M. Rasmussen, G. Segovia-Siapco, R. Sirirat, A. Sun and D. M. Reboussin, Effect of Incorporating 1 Avocado Per Day Versus Habitual Diet on Visceral Adiposity: A Randomized Trial, J. Am. Heart Assoc., 2022, 11, e025657 CrossRef PubMed.
- N. C. I. D. o. C. C. a. P. Sciences, ASA24 resources related to the Healthy Eating Index (HEI), DOI: Available at: https://epi.grants.cancer.gov/hei/sas-code.html.
- M. P. Sato, Y. Ogura, K. Nakamura, R. Nishida, Y. Gotoh, M. Hayashi, J. Hisatsune, M. Sugai, I. Takehiko and T. Hayashi, Comparison of the sequencing bias of currently available library preparation kits for Illumina sequencing of bacterial genomes and metagenomes, DNA Res., 2019, 26, 391–398 CrossRef CAS PubMed.
- F. Beghini, L. J. McIver, A. Blanco-Miguez, L. Dubois, F. Asnicar, S. Maharjan, A. Mailyan, P. Manghi, M. Scholz, A. M. Thomas, M. Valles-Colomer, G. Weingart, Y. Zhang, M. Zolfo, C. Huttenhower, E. A. Franzosa and N. Segata, Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3, eLife, 2021, 10, 1–42 CrossRef PubMed.
- A. Blanco-Miguez, F. Beghini, F. Cumbo, L. J. McIver, K. N. Thompson, M. Zolfo, P. Manghi, L. Dubois, K. D. Huang, A. M. Thomas, W. A. Nickols, G. Piccinno, E. Piperni, M. Puncochar, M. Valles-Colomer, A. Tett, F. Giordano, R. Davies, J. Wolf, S. E. Berry, T. D. Spector, E. A. Franzosa, E. Pasolli, F. Asnicar, C. Huttenhower and N. Segata, Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4, Nat. Biotechnol., 2023, 41, 1633–1644 CrossRef CAS.
- R. Caspi, T. Altman, R. Billington, K. Dreher, H. Foerster, C. A. Fulcher, T. A. Holland, I. M. Keseler, A. Kothari, A. Kubo, M. Krummenacker, M. Latendresse, L. A. Mueller, Q. Ong, S. Paley, P. Subhraveti, D. S. Weaver, D. Weerasinghe, P. Zhang and P. D. Karp, The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases, Nucleic Acids Res., 2014, 42, D459–D471 CrossRef CAS PubMed.
- P. J. McMurdie and S. Holmes, phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data, PLoS One, 2013, 8, e61217 CrossRef CAS.
- H. Wickham, ggplot2: Elegant Graphics for Data Analysis, Springer-Verlag, New York, 2016 Search PubMed.
- J. Oksanen, F. G. Blanchet, R. Kindt, P. Leg-endre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens and H. Wagner, vegan: Community Ecology Package. R package version 2.5-4, 2019 Search PubMed.
- Q. Y. Lu, A. M. Rasmussen, J. P. Yang, R. P. Lee, J. J. Huang, P. Shao, C. L. Carpenter, I. Gilbuena, G. Thames, S. M. Henning, D. Heber and Z. P. Li, Mixed Spices at Culinary Doses Have Prebiotic Effects in Healthy Adults: A Pilot Study, Nutrients, 2019, 11, 1–14 Search PubMed.
- A. Kuznetsova, P. B. Brockhoff and R. H. B. Christensen, lmerTest Package: Tests in Linear Mixed Effects Models, J. Stat. Software, 2017, 82, 1–26 CrossRef.
- H. M. P. R. N. C. Integrative, The Integrative Human Microbiome Project, Nature, 2019, 569, 641–648 CrossRef.
- H. Mallick, A. Rahnavard, L. J. McIver, S. Y. Ma, Y. C. Zhang, L. H. Nguyen, T. L. Tickle, G. Weingart, B. Y. Ren, E. H. Schwager, S. Chatterjee, K. N. Thompson, J. E. Wilkinson, A. Subramanian, Y. R. Lu, L. Waldron, J. N. Paulson, E. A. Franzosa, H. C. Bravo and C. Huttenhower, Multivariable association discovery in population-scale meta-omics studies, PLoS Comput. Biol., 2021, 17, 1–27 CrossRef PubMed.
- J. P. Yang, R. P. Lee, Z. Schulz, A. L. Hsu, J. A. T. Pai, S. R. Yang, S. M. Henning, J. J. Huang, J. P. Jacobs, D. Heber and Z. P. Li, Mixed Nuts as Healthy Snacks: Effect on Tryptophan Metabolism and Cardiovascular Risk Factors, Nutrients, 2023, 15, 1–12 Search PubMed.
- D. Tingley, T. Yamamoto, K. Hirose, L. Keele and K. Imai, mediation: R Package for Causal Mediation Analysis, J. Stat. Software, 2014, 59, 1–38 Search PubMed.
- C. I. Le Roy, R. C. E. Bowyer, J. E. Castillo-Fernandez, T. Pallister, C. Menni, C. J. Steves, S. E. Berry, T. D. Spector and J. T. Bell, Dissecting the role of the gut microbiota and diet on visceral fat mass accumulation, Sci. Rep., 2019, 9, 9758 CrossRef PubMed.
- H. Yan, Q. Qin, J. Chen, S. Yan, T. Li, X. Gao, Y. Yang, A. Li and S. Ding, Gut Microbiome Alterations in Patients With Visceral Obesity Based on Quantitative Computed Tomography, Front. Cell. Infect. Microbiol., 2021, 11, 823262 CrossRef CAS PubMed.
- C. Haro, M. Montes-Borrego, O. A. Rangel-Zuniga, J. F. Alcala-Diaz, F. Gomez-Delgado, P. Perez-Martinez, J. Delgado-Lista, G. M. Quintana-Navarro, F. J. Tinahones, B. B. Landa, J. Lopez-Miranda, A. Camargo and F. Perez-Jimenez, Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population, J. Clin. Endocrinol. Metab., 2016, 101, 233–242 CrossRef CAS PubMed.
- J. Kabeerdoss, V. Sankaran, S. Pugazhendhi and B. S. Ramakrishna, Clostridium leptum group bacteria abundance and diversity in the fecal microbiota of patients with inflammatory bowel disease: a case-control study in India, BMC Gastroenterol., 2013, 13, 20 CrossRef CAS.
- M. Parsaei, N. Sarafraz, S. Y. Moaddab and H. Ebrahimzadeh Leylabadlo, The importance of Faecalibacterium prausnitzii in human health and diseases, New Microbes New Infect., 2021, 43, 100928 CrossRef CAS.
- K. Machiels, M. Joossens, J. Sabino, V. De Preter, I. Arijs, V. Eeckhaut, V. Ballet, K. Claes, F. Van Immerseel, K. Verbeke, M. Ferrante, J. Verhaegen, P. Rutgeerts and S. Vermeire, A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis, Gut, 2014, 63, 1275–1283 CrossRef CAS PubMed.
- E. C. S. de Oliveira, L. M. Dalmau, C. A. R. de Almeida Costa, L. D. de Almeida Junior, C. R. Ballard, M. R. Marostica Junior, M. A. Stahl, R. Grimaldi, A. Witaicenis and L. C. Di Stasi, Dietary intervention with avocado (Persea americana Mill.) ameliorates intestinal inflammation induced by TNBS in rats, Inflammopharmacology, 2023, 31, 485–498 CrossRef CAS PubMed.
- M. Pinart, A. Dotsch, K. Schlicht, M. Laudes, J. Bouwman, S. K. Forslund, T. Pischon and K. Nimptsch, Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis, Nutrients, 2021, 14, 1–41 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo03806a |
| This journal is © The Royal Society of Chemistry 2025 |