Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The influence of food matrices on the bioavailability of curcuminoids from a dried colloidal turmeric suspension: a randomized, crossover, clinical trial†
Katja A.
Schönenberger
 a,
Cristina
Ranzini
a,
Cristina
Ranzini
 b,
Julie
Laval
b,
Julie
Laval
 b,
Pascale
Bellenger
b,
Mathieu
Tenon
b,
Pascale
Bellenger
b,
Mathieu
Tenon
 b and
Pascale
Fança-Berthon
b and
Pascale
Fança-Berthon
 *b
*b
aGivaudan International SA, Kemptthal, Switzerland
bGivaudan France Naturals, Avignon, France. E-mail: pascale.fanca-berthon@givaudan.com
First published on 10th January 2025
Abstract
Curcuminoid absorption can be influenced by the presence of additional compounds, but there has been no study investigating this in a robust manner. The aim of this clinical trial was to assess the effect of the type of food matrix on the absorption of curcuminoids from a highly bioavailable turmeric formulation. Participants consumed the turmeric formulation in the form of capsules, a ready-to-drink fruit nectar, a sports nutrition bar, a dairy analogue (oat milk), pectin gummies, and a probiotic drink in a randomized, crossover study. Plasma samples were collected over a 24-hour period to assess the pharmacokinetics of curcuminoids. The relative bioavailability of total curcuminoids was increased in all the food matrices compared to that in the capsule formulation. The dairy analogue showed the highest increase in dose-normalized AUC24 h (+76%, p < 0.0001) and Cmax (+105%, p < 0.0001). The sports nutrition bar resulted in increased dose-normalized AUC24 h (+40%, p = 0.0112) and Cmax (+74%, p < 0.0001). The probiotic drink showed increased dose-normalized AUC24 h (+35%, p = 0.0318) and Cmax (+52%, p < 0.0001). The ready-to-drink and gummy formulations were bioequivalent to the capsules. The distribution of curcuminoid metabolites was similar in all the matrices. In conclusion, there was no negative food matrix effect; on the contrary, the bioavailability of curcuminoids can be improved when administered via food matrices, particularly those containing lipids in a suspended form or polar lipids.
Introduction
Curcumin, the primary bioactive component of the rhizome of Curcuma longa (turmeric), has gained significant attention owing to its potential health effects and benefits. Alongside curcumin, other bioactive components known as curcuminoids have been identified. They are natural yellow-orange pigments and hydrophobic polyphenols derived from turmeric. Notably, turmeric extracts generally contain approximately 75%–80% of curcumin, with, its two demethoxy compounds, demethoxycurcumin (DMC) comprising 15%–20% and bisdemethoxycurcumin (BDMC) accounting for 0%–10%.1Curcumin and curcuminoids have been extensively studied for their antioxidant and anti-inflammatory properties. These properties have sparked interest in their potential efficacy in modulating various health conditions.2,3
Upon oral ingestion, the majority of curcumin, along with potentially DMC and BDMC, is excreted in an unmetabolized form through faeces.4 However, the absorbed portion undergoes phase I and phase II metabolism. In phase I metabolism, curcumin, DMC, and BDMC undergo successive reduction to their dihydro-, tetrahydro-, hexahydro-, and octahydro-metabolites in the liver and intestinal mucosa. Both curcumin and these metabolites are then conjugated with glucuronic acid and sulphate, forming phase II metabolites. Reduction and conjugation are general metabolic pathways of curcuminoids, occurring in hepatic and intestinal tissues.5 Additionally, curcumin can be metabolized by intestinal microorganisms through a two-step reduction process, resulting in the formation of dihydrocurcumin and tetrahydrocurcumin (THC).6
Curcuminoids exhibit higher solubility in organic solvents than in water.7 Consequently, they have low aqueous solubility and poor gastrointestinal absorption.7–9 Studies have documented that curcumin and curcuminoids exhibit low absorption from the gut, rapid metabolism, and rapid systemic elimination.9–11 These factors contribute to the limited bioavailability of curcuminoids, thereby restricting their use in general healthcare and as an adjunct in managing various health conditions. Serum levels of curcumin and tissue distribution are found to be low, regardless of the route of administration.10,12,13
To enhance the bioavailability of curcumin and curcuminoids, several approaches have been explored. These include the use of adjuvants such as piperine, which interferes with glucuronidation, as well as liposomal curcumin, nanoparticles, phospholipid complexes, and structural analogues of curcumin.10 One solution to improve the absorption of curcuminoids is their delivery as a dried colloidal suspension. A previous study has demonstrated that a low dose (300 mg) of this turmeric formulation (TF) resulted in high absorption of unconjugated and conjugated curcuminoids, with significant differences compared to a high dose (1500 mg) of the standard extract.14 Building upon this study, our objective was to investigate whether the absorption of curcuminoids from TF can be influenced by additional factors, particularly their inclusion in different food matrices. Therefore, the aim of this clinical trial was to evaluate the bioavailability of curcuminoids from TF in various food matrix formulations.
Materials & methods
Ethics
This clinical trial received approval from the Committee of Protection of Persons (Comité de protection des personnes Sud-Ouest et Outre-mer I; reference number 1-21-061) and the French National Agency for Medicines and Health Products Safety (Agence nationale de sécurité du médicament et des produits de santé; reference number 2021-A00317-34). The trial was performed in accordance with the principles stated in the Declaration of Helsinki adopted by the 18th World Medical Association General Assembly in 1964 and amended by the 64th World Medical Association General Assembly in 2013 and registered with ClinicalTrials.gov (https://clinicaltrials.gov/study/NCT06300021). Prior to study inclusion, all participants provided written informed consent.Study design
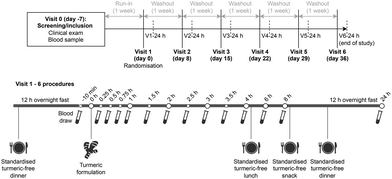
The study followed a randomized, cross-over, and open-label design. The procedures are depicted in Fig. 1. On six different days, with wash-out periods of at least one week in between, participants consumed either a 300 mg TF capsule or 300 mg of TF in one of five different food matrices in a randomized order. Participants arrived at each experimental visit in a fasting state with standard meals (ESI Table 1†) provided for dinner the previous evening as well as for lunch, mid-afternoon snack, and dinner during the experimental session. As per the U.S. Food and Drug Administration (FDA) guidance,15 pharmacokinetic (PK) blood samples were collected at baseline (10 minutes before TF consumption) and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, and 24 hours after consumption.Investigational products
The TF used in this trial was a dried colloidal suspension of curcuminoids composed of standard turmeric extract, quillaja extract, sunflower oil, and acacia gum. It contains a minimum of 30% curcuminoids and is commercially available as Turmipure Gold® (Givaudan France Naturals, Avignon, France). This TF has been clinically proven to enhance the bioavailability of curcuminoids.14 In addition to the capsules (Caps, reference format), the highly bioavailable TF was investigated in five different food matrices selected to assess the effect of acidity, protein/sugar/fibre interaction, protein/fat interaction, gelification, and dairy protein/dairy fat interaction on its absorption. The specific food matrices were obtained from commercially available products.• Ready to drink (RTD): 300 mg of TF dispersed in 60 mL of mango fruit nectar, followed by the consumption of 150 mL of water
• Sports nutrition bar (SBar): 32 g sports nutrition bar containing 300 mg of TF, followed by the consumption of 150 mL of water
• Dairy analogue (DA): 300 mg of TF dispersed in 240 mL of oat milk
• Gummies (Gum): 10 g of pectin gummies containing 300 mg of TF, followed by the consumption of 150 mL of water
• Probiotic drink (Prob): 300 mg of TF dispersed in 100 g of plain Actimel® (Danone, Paris, France), followed by the consumption of 150 mL of water
The composition of SBar and Gum is detailed in the ESI,† along with the nutritional values of all investigational products in ESI Table 2.† The measured content of individual curcuminoids in different products is provided in Table 1, and the analytical methodology for quantifying curcuminoids in food matrices is described in the ESI.†
| Parameter | Caps (n = 10) | RTD (n = 10) | SBar (n = 8) | DA (n = 9) | Gum (n = 10) | Prob (n = 9) |
|---|---|---|---|---|---|---|
| Results are presented as mean (SD) of product samples tested. BDMC, bisdemethoxycurcumin; Caps, capsule; DA, dairy analogue; DMC, demethoxycurcumin; Gum, gummies; Prob, probiotic drink; RTD, ready to drink; SBar, sports nutrition bar. | ||||||
| Total curcuminoids, mg | 101.19 (2.16) | 99.98 (0.75) | 79.88 (3.83) | 100.22 (1.44) | 93.70 (10.30) | 99.15 (1.43) |
| Curcumin, mg | 86.56 (1.83) | 85.35 (0.64) | 68.61 (3.33) | 85.55 (1.22) | 79.97 (8.81) | 84.64 (1.21) |
| DMC, mg | 13.53 (0.30) | 13.44 (0.10) | 10.42 (0.47) | 13.48 (0.20) | 12.65 (1.37) | 13.33 (0.20) |
| BDMC, mg | 1.10 (0.03) | 1.19 (0.01) | 0.85 (0.04) | 1.19 (0.02) | 1.07 (0.12) | 1.18 (0.02) |
Study population
The study included participants who met the following criteria: between 18 and 45 years of age, body mass index between 18.5 and 24.9 kg m−2, and maintained a stable weight within three kilograms in the last three months. Their routine blood chemistry values had to be within the normal range. Women either had to use the same reliable contraception for at least three cycles or be menopausal for at least three months. Participants had to be either non-smokers or have a tobacco consumption of no more than five cigarettes per day and agree not to smoke during the study. They also had to have good general and mental health, with no clinically significant abnormalities in their medical history or physical examination. The exclusion criteria comprised various medical conditions such as metabolic or endocrine disorders, chronic diseases, liver diseases, and gastrointestinal disorders. They also covered specific medical histories, recent illnesses, allergies or intolerances, pregnancy or lactation, certain medication use, recent supplementation, changes in food habits or physical activity, eating disorders, excessive alcohol consumption or drug dependence, incompatible lifestyle, recent participation in other clinical trials, legal protection or deprivation of rights, incapability to sign informed consent, inability to be contacted in emergencies, specific dietary habits (including having consumed curcumin-containing foods or supplements at least 3 times per week and for 2 weeks prior to testing), recent blood donation, and clinically significant abnormalities in control records. For a comprehensive list of inclusion and exclusion criteria, please refer to ClinicalTrials.gov, identifier https://clinicaltrials.gov/study/NCT06300021.Sample size
Various guidelines for determining an appropriate sample size for a pilot study have been published,16–21 suggesting a range of 10 to 40 participants per group. In consideration of an anticipated dropout rate between 10% and 30%, we intended to include 40 participants in this study.Sample collection
Blood samples were collected using a catheter from an antecubital vein. A total of 5 mL of blood was collected in tubes containing sodium citrate 3.8% (Greiner bio-one). The tubes were then centrifuged at 2200g for 15 minutes at 4 °C, and the resulting plasma was divided into microtubes. These plasma samples were stored at −80 °C until further analysis.Plasma and curcuminoid analysis
Endpoints
The primary endpoint of the study was the dose-normalized area under the plasma concentration–time curve (AUC) over 24 hours (AUC24 h) of total curcuminoids. Secondary endpoints included dose-normalized (except total curcuminoids, which is the primary endpoint) and non-normalized AUC24 h, dose-normalized and non-normalized AUC8 h and AUC∞ (plasma concentration extrapolated to infinite time), relative bioavailability compared to capsule formulation over 24 hours (Frel 24 h) and 8 hours (Frel 8 h), calculated as the ratio of the corresponding dose-normalized AUCs, dose-normalized and non-normalized peak plasma concentration (Cmax), time to reach Cmax (Tmax), and half-life (T1/2) of all curcuminoid components (Table 2). Safety and tolerability were assessed through the follow-up of any treatment-emergent adverse events (TEAE) and measurement of vital signs.| Individual curcuminoids | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grouped components | Curcumin | DMC | BDMC | THC | HHC | Curcumin sulphate | DMC sulphate | BDMC sulphate | THC sulphate | HHC sulphate | Curcumin glucuronide | DMC glucuronide | BDMC glucuronide | THC glucuronide | HHC glucuronide |
| Components (rows) were calculated as the sum of the concentrations of individual curcuminoids indicated with x. BDMC, bisdemethoxycurcumin; DMC, demethoxycurcumin; HHC, hexahydrocurcumin; THC, tetrahydrocurcumin. | |||||||||||||||
| Total curcuminoids | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| Parent compounds | x | x | x | ||||||||||||
| Sulphate metabolites | x | x | x | x | x | ||||||||||
| Glucuronide metabolites | x | x | x | x | x | ||||||||||
| Parent compounds and their sulphate and glucuronide metabolites | x | x | x | x | x | x | x | x | x | ||||||
| Curcumin and its sulphate and glucuronide metabolites | x | x | x | ||||||||||||
| DMC and its sulphate and glucuronide metabolites | x | x | x | ||||||||||||
| BDMC and its sulphate and glucuronide metabolites | x | x | x | ||||||||||||
| Curcumin and all its metabolites | x | x | x | x | x | x | x | x | x | ||||||
Statistical analysis
PK parameters, including AUC, Cmax, Tmax, and T1/2, were calculated using non-compartmental analysis22 with R software version 4.0.223 using the ncappc package version 0.3.0.24 Concentration-related PK parameters (AUC and Cmax) were additionally normalized to the amount of ingested curcuminoids (Table 1). The AUC values were normalized to parent curcuminoid intake by dividing the observed AUC (expressed in ng h mL−1) by the corresponding parent curcuminoid dosage of each matrix (expressed in mg). Likewise, dose-normalized Cmax values correspond to the peak plasma concentration (expressed in ng mL−1) divided by the corresponding parent curcuminoid dosage of each matrix (expressed in mg). Therefore, the dose-normalized AUC and Cmax values are expressed in ng h mL−1 mg−1 and ng mL−1 mg−1, respectively.Statistical analyses were conducted using SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA). A significance level of 0.05 was used for all statistical tests.
The primary analysis was the log-transformed AUC24 h of total curcuminoids using a mixed model for repeated measures (SAS® PROC MIXED, statistical model no. 1). The model included the sequence, product (capsule or one of the five food matrices), visit (one of the six experimental visits), and baseline value as fixed effects, and the participant as a random effect. If a significant period or sequence effect was observed, potential explanations were explored, and a secondary analysis was conducted on the first visit only. The five different food matrix formulations containing TF were compared to the capsule formulation (five comparisons), and a Dunnett correction was applied to account for multiple comparisons.
All secondary endpoints except Frel were likewise analysed using model no. 1. The percentage difference in PK parameters between the capsule and food matrix formulations was reported based on the geometric mean ratio obtained in model no. 1. Frel was analysed using a mixed model for repeated measures (SAS® PROC MIXED, statistical model no. 2). Model no. 2 included the sequence, product (one of the five food matrices), and visit (one of the five visits with food matrices) as fixed effects, and the participant as a random effect. A post-hoc analysis compared the estimated means of Frel between the five food matrices (ten comparisons) applying a Tukey's correction to adjust for multiple comparisons.
Assumptions of normality and homoscedasticity for linear models were assessed through graphical representations of residuals generated by the statistical models. PK parameters derived from concentration measures were logarithmically transformed prior to analysis.
All analyses were conducted on the full analysis set, which included all randomized participants who consumed at least one dose of the study product and completed at least one study treatment period. A per protocol set was also analysed, excluding participants or visits with major deviations from the protocol.
Results
A total of 35 participants were enrolled in the trial between 14 September 2021 and 08 April 2022. The study flow is presented in ESI Fig. 1,† and the baseline characteristics of the study participants are summarized in Table 3. None of the conditions or prior health issues reported as part of the participants’ medical histories were determined to have a meaningful impact on the study's outcomes or the safety of the participants. All participants were included in the per protocol population, although some experimental sessions were excluded from the analysis (4 deviations due to adverse events and an elevated HHC sulphate level). In addition, major deviations related to incorrect kinetic intervals for blood sampling occurred in five distinct subjects during five different experimental sessions. These biological values (V5, T180; V3, T15; V2, T15; V2, T15; V2, T45) were set to missing at the timepoint in question and computed using the copyMean method.| Baseline parameter | All participants (n = 35) |
|---|---|
| BMI, body mass index. | |
| Female sex, n (%) | 29 (82.9) |
| Age, mean (SD) | 33.5 (6.5) |
| Smoker, n (%) | 7 (20.0) |
| Alcohol consumption, n (%) | |
| No alcohol consumption | 10 (28.6) |
| <1 drink per day or <7 drinks per week | 24 (68.6) |
| 3 drinks per day or 21 drinks per week | 1 (2.9) |
| BMI, kg m−2, mean (SD) | 22.2 (1.8) |
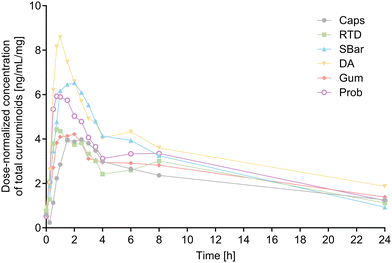
The observed kinetics of dose-normalized total curcuminoids plasma concentration over 24 hours are depicted in Fig. 2, with corresponding non-normalized results and SD listed in ESI Table 5.† Descriptive statistics for all PK parameters of total curcuminoids are presented in Table 4.
| Caps | RTD | SBar | DA | Gum | Prob | |
|---|---|---|---|---|---|---|
| a Primary endpoint. Descriptive statistics displayed as mean (SD). AUC, area under the concentration–time curve from 0 to 24 hours, 0 to 8 hours, and total area from 0 to extrapolated infinite time; Caps, capsules; Cmax, peak concentration; DA, dairy analogue; Frel, relative bioavailability to capsule from 0 to 24 hours and 0 to 8 hours (ratio of does-normalized areas under the concentration–time curve); Gum, gummies; Norm, dose-normalized; N/A, not applicable; Prob, probiotic drink; RTD, ready to drink; SBar, sports nutrition bar; T1/2, half-life; Tmax, time to reach peak concentration. | ||||||
Norm AUC24 h, ng h mL−1 mg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
49.67 (29.47) | 53.26 (29.87) | 62.83 (24.94) | 76.34 (19.96) | 54.98 (23.96) | 62.43 (23.84) |
| Norm AUC8 h, ng h mL−1 mg−1 | 23.30 (11.52) | 24.09 (9.08) | 35.74 (10.44) | 39.27 (14.74) | 24.88 (11.80) | 30.90 (10.93) |
| Norm AUC∞, ng h mL−1 mg−1 | 71.44 (51.17) | 90.98 (110.44) | 65.73 (20.87) | 141.02 (158.84) | 120.53 (185.09) | 97.87 (118.22) |
| AUC24 h, ng h mL−1 | 5026.09 (2982.46) | 5325.13 (2986.59) | 5018.87 (1991.94) | 7651.00 (2000.28) | 5152.02 (2244.62) | 6190.13 (2363.26) |
| AUC8 h, ng h mL−1 | 2357.32 (1166.04) | 2408.25 (907.65) | 2855.27 (833.70) | 3935.30 (1476.79) | 2330.90 (1105.64) | 3063.58 (1083.96) |
| AUC∞, ng h mL−1 | 7228.81 (5177.76) | 9095.72 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 041.59) 041.59) |
5250.76 (1667.21) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133.03 (15 133.03 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 918.73) 918.73) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293.70 (17 293.70 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343.12) 343.12) |
9703.91 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 721.96) 721.96) |
| Norm Cmax, ng mL−1 mg−1 | 5.31 (2.69) | 5.60 (2.46) | 8.27 (3.00) | 10.06 (3.94) | 5.91 (2.27) | 7.46 (3.15) |
| C max, ng mL−1 | 536.83 (272.13) | 560.34 (245.76) | 660.80 (239.50) | 1008.08 (394.86) | 553.46 (212.89) | 739.58 (312.47) |
| T max, minutes | 152.44 (116.14) | 106.59 (101.56) | 110.47 (58.64) | 166.92 (376.28) | 211.45 (274.78) | 81.89 (65.65) |
| T 1/2, minutes | 852.32 (781.59) | 1124.71 (1999.14) | 366.75 (254.36) | 1156.55 (2055.57) | 1762.41 (5034.62) | 805.25 (1061.87) |
| F rel 24 h | N/A | 1.30 (0.90) | 1.58 (1.14) | 2.12 (1.78) | 1.26 (0.71) | 1.66 (1.32) |
| F rel 0–8 | N/A | 1.19 (0.68) | 1.92 (1.18) | 2.06 (1.31) | 1.13 (0.54) | 1.61 (0.93) |
Fig. 3 illustrates the dose-normalized AUC24 h for total curcuminoids. The statistical model comparing the dose-normalized AUC24 h for total curcuminoids (primary endpoint) revealed a significant product effect (p < 0.0001), and significant differences were found between the reference matrix Caps and SBar (+40%; p = 0.0112), DA (+76%; p < 0.0001) and Prob (+35%; p = 0.0318). Since a significant visit effect was detected (p = 0.0310), a post-hoc sensitivity analysis was conducted on data from all visits except visit 3, which was identified as responsible for the visit effect. A significant product effect was confirmed (p < 0.0001) and similar significant differences with the reference matrix Caps were found, with no visit or sequence effect. These effects were reflected in the relative bioavailability of total curcuminoids in food matrices compared to Caps (Table 4). All mean Frel values were greater than one, indicating higher bioavailability of curcuminoids in food matrices compared to Caps. Significantly higher Frel 24 h of total curcuminoids was observed for DA versus RTD (+56%; p = 0.0017) and versus Gum (+36%; p = 0.0455).
The percentage differences in all PK parameters of food matrices to Caps, including the statistical significance, are presented in Fig. 4. There were no notable significant decreases in dose-normalized and non-normalized AUC24 h, AUC8 h, AUC∞, and Cmax. On the contrary, SBar, DA, and Prob showed significant increases for all or some of these parameters for total curcuminoids, sulphate metabolites, glucuronide metabolites, and parent compounds and their sulphate and glucuronide metabolites. A notable number of models for Tmax and T1/2 had doubtful assumptions and were thus considered invalid. For the parameters with valid models for Tmax and T1/2, a reduction was generally observed, with a significant reduction of −46% of Tmax for total curcuminoids in Prob compared to Caps (p = 0.0177) and a nearly significant −41% reduction in DA compared to Caps (p = 0.0543).
The distribution of circulating curcuminoids over 24 hours is presented in Table 5. The proportions did not vary remarkably between matrices. Glucuronide metabolites constituted approximately three-quarters of all curcuminoids, and sulphate metabolites accounted for the remaining quarter, with parent compounds and unconjugated curcumin virtually absent (representing less than 1% of the quantified metabolites). Curcumin and all its metabolites constituted about 90% of all curcuminoids, with minor proportions of DMC, BDMC, and their metabolites.
| Caps | RTD | SBar | DA | Gum | Prob | |
|---|---|---|---|---|---|---|
| All proportions (expressed as percentage of total curcuminoids) were calculated as the ratio of the mean area under the concentration–time curve from 0 to 24 hours of the curcuminoid(s) of interest divided by the mean area under the concentration–time curve from 0 to 24 hours of total curcuminoids multiplied by 100. If the percentages do not add up exactly to 100%, this is due to inter-individual variability of participants. BDMC, bisdemethoxycurcumin; Caps, capsules; DA, dairy analogue; DMC, demethoxycurcumin; Gum, gummies; HHC, hexahydrocurcumin; Prob, probiotic drink; RTD, ready to drink; SBar, sports nutrition bar; THC, tetrahydrocurcumin. | ||||||
| Curcumin | 0.1 | 0.2 | 0.4 | 0.2 | 0.7 | 0.5 |
| DMC | 0.2 | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 |
| BDMC | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
| THC | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| HHC | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Curcumin sulphate | 6.9 | 6.7 | 7.1 | 5.3 | 6.1 | 6.3 |
| DMC sulphate | 2.2 | 1.8 | 1.8 | 1.2 | 2.4 | 1.7 |
| BDMC sulphate | 2.9 | 3.1 | 2.2 | 2.1 | 2.2 | 2.2 |
| THC sulphate | 0.5 | 1.8 | 1.8 | 1.4 | 2.3 | 1.5 |
| HHC sulphate | 15.2 | 15.5 | 17.1 | 15.5 | 14.2 | 14.4 |
| Curcumin glucuronide | 12.9 | 12.2 | 11.4 | 8.9 | 11.3 | 10.5 |
| DMC glucuronide | 5.3 | 5.4 | 4.8 | 3.8 | 4.8 | 4.2 |
| BDMC glucuronide | 1.0 | 0.7 | 0.8 | 0.4 | 1.1 | 0.9 |
| THC glucuronide | 31.9 | 32.9 | 29.9 | 34.8 | 33.0 | 32.0 |
| HHC glucuronide | 25.2 | 25.3 | 31.8 | 32.7 | 25.7 | 32.4 |
| Parent compounds | 0.3 | 0.3 | 0.5 | 0.2 | 0.9 | 0.8 |
| Sulphate metabolites | 27.4 | 27.7 | 28.5 | 25.0 | 27.0 | 25.3 |
| Glucuronide metabolites | 73.7 | 73.5 | 75.5 | 77.3 | 73.5 | 76.9 |
| Parent compounds and their sulphate and glucuronide metabolites | 30.9 | 29.8 | 27.8 | 21.3 | 28.3 | 26.0 |
| Curcumin and its sulphate and glucuronide metabolites | 19.7 | 19.1 | 18.7 | 14.2 | 18.1 | 17.2 |
| DMC and its sulphate and glucuronide metabolites | 7.7 | 7.3 | 6.6 | 5.1 | 7.3 | 6.1 |
| BDMC and its sulphate and glucuronide metabolites | 4.0 | 3.8 | 3.1 | 2.5 | 3.3 | 3.1 |
| Curcumin and all its metabolites | 88.7 | 89.6 | 91.5 | 93.2 | 90.0 | 91.1 |
| Total curcuminoids | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
A total of 54 TEAEs were reported during the study. None of them were serious. One reported TEAE (headache and vomiting) was severe and occurred on the day of an experimental session after ingestion of SBar. This TEAE was classified as not related to the study product. All other reported TEAEs were mild or moderate. Despite the markedly increased bioavailability of TF in some food matrices, the maximal AUC24 h was not increased accordingly. The ratio of maximal AUC24 h in food matrices and Caps ranged from 0.7 (Gum/Caps) to 1.2 (RTD/Caps). The observed ranges of PK parameters related to curcuminoid exposure are shown in ESI Table 6.†
Discussion
The results showed that the bioavailability of total curcuminoids over 24 hours, as assessed by the dose-normalized AUC24 h, varied between the different matrices. Significant increases were found between the reference matrix Caps and the food matrices containing lipids in a suspended form or polar lipids, specifically +76% for DA, +40% for SBar, and +35% for Prob. Notably, the curcuminoid doses of DA and Prob were nearly equivalent to that of Caps, indicating that dose-normalization had virtually no effect on these results. The Tmax of total curcuminoids was shorter for all matrices except Gum, with marked decreases for the liquid food matrices (−46% for Prob and a −41% for DA). Despite the gelification process involved in the preparation of the pectin-based gummies, which could potentially affect bioavailability and absorption rate, Gum and Caps demonstrated comparable bioavailability and Tmax, indicating their bioequivalence.Curcuminoids have poor solubility in water, which limits their bioavailability. However, trapping them in small particles such as micelles, liposomes, emulsions, and solid lipid particles can enhance their intestinal absorption.25 Additionally, lipids increase the secretion of bile acids, which may further support the emulsification of curcuminoids.26 Therefore, the presence of lipids in DA, Prob, and SBar may explain the increased bioavailability of curcuminoids in these food matrices. Previous studies have also observed improved curcuminoid bioavailability when administered with lipid-based formulations. For example, a clinical study comparing solid lipid curcumin particle formulation to unformulated curcumin extract demonstrated a marked improvement in the bioavailability of free curcumin after oral administration with a lipid vehicle.27 Pre-clinical data have shown only modest increases in bioavailability with simple mixtures of curcumin and lipids or complexation with phospholipids.28–30 Furthermore, the polar lipids, amphiphilic lipids with a hydrophilic head and a lipophilic tail, present in the DA, Prob, and SBar may facilitate absorption. Acting as surfactants, phospholipids have been suggested to improve the absorption of poorly water-soluble phytochemicals, including curcumin.31 Clinical studies have shown that curcuminoid mixtures with lecithin or phosphatidylcholine (a principal component of lecithin) can increase the bioavailability of curcuminoids.32,33 Fu and colleagues34 investigated the in vitro bioavailability of curcuminoids in buttermilk and yogurt and found that curcuminoids delivered in yogurt were 15-fold more bioaccessible than curcuminoids in aqueous dispersion.34
The distribution of metabolites remains consistent across different matrices, indicating that the matrix effect does not alter the metabolism of parent compounds. Our results show that curcumin metabolites account for approximately 90% of total curcuminoids, while DMC and BDMC account for approximately 7% and 3%, respectively. This distribution is similar to the proportions of curcumin, DMC, and BDMC ingested (85%, 13%, and 1%, respectively). Additionally, there are minimal amounts of parent compounds detected in the plasma over 24 hours (<1%), while the rates of sulphation and glucuronidation remain constant across matrices, accounting for approximately 25% and 75%, respectively, of total curcuminoids. In contrast, Asher and colleagues33 observed marked differences in curcumin metabolites between standard and phosphatidylcholine curcumin extracts. Plasma DMC and BDMC conjugates were drastically reduced in the phosphatidylcholine extract compared to the standard extract, while HHC was increased by a factor of twenty. However, there was a ten-fold difference in curcumin dose between the two formulations, which limits the interpretability of the results.35
It is worth mentioning that the main PK parameters of total curcuminoids and the proportion of metabolites obtained after a single intake of 300 mg of TF in capsule format in our study are very similar to those obtained in the previous PK study.14 Despite being conducted on different populations of participants and with 14 data points compared to 11 in the previous study, the level of absorption and bioavailability of curcuminoids in the plasma after oral consumption of the investigated TF in capsule format has been confirmed. Consistent with the previous study, unconjugated curcumin represents less than 1% of the metabolites found in the plasma, further supporting the notion that unconjugated curcumin is not a major compound when considering curcuminoid bioavailability.
The safety profiles of all products tested in this study did not show any serious adverse events related to the investigational product. Despite the higher exposure to curcuminoids with certain food matrices, the range and maximal concentrations in the plasma were not increased to the same extent. Only SBar exhibited a higher maximal AUC24 h value compared to Caps, with a maximal value 1.2 times that of Caps. Furthermore, the safety and tolerance of the investigated TF have been previously established in a toxicological study36 and a clinical safety trial, which demonstrated the safety at a high dosage of 1000 mg d−1 for up to 5 weeks of supplementation (https://clinicaltrials.gov/study/NCT03945149, unpublished results, 2021).
The strengths of this study include its cross-over design, which eliminates inherent between-group differences in curcuminoid metabolism. Additionally, the curcuminoid dose in capsules and food matrices was similar (ranging from 80 mg in SBar to 101 mg in Caps), allowing for valid comparisons of the food matrices through dose-normalized and non-normalized results. It is important to note that pronounced differences in curcuminoid doses can limit the interpretability of the results due to the non-linear relationship between the dose of native curcuminoids and PK parameters of native and metabolized curcuminoids.35 The blood sampling schedule was planned according to the FDA guidance.15 However, a limitation of the study is the number of evaluated outcomes, which increases the risk of a type I error. To mitigate this risk, the significances were adjusted by adequate corrections for multiple comparisons. Moreover, there are more reduced metabolites of curcumin, as well as mixed and complex conjugates,37 than the fifteen curcuminoids we quantified. The contribution of these reduced metabolites is minimal. Regarding mixed and complex conjugates, since sulphatase hydrolyses both glucuronide and sulphate conjugates, disulphate, mixed sulphate-glucuronide, and sulphate–diglucuronide conjugates counted as sulphate compounds, while diglucuronide compounds would fall under glucuronide compounds. This may lead to a slight overestimation of sulphate compounds but, at the same time, this enhances the accuracy of the total curcuminoids measurement. Importantly, these factors do not affect the findings of this study as the imprecision is consistent across all food matrices and the main conclusions are based on total curcuminoids. Another inherent limitation of this study is the inability to blind the participants to the received food matrix. However, the effect of participants’ awareness of the food matrix on absorption and metabolism is presumably negligible. Finally, recruitment resulted in a high proportion of female participants to be included in the study. Our previous study found no significant formulation–sex interaction for the AUC24 h of total curcuminoids or the individually quantified metabolites.14 Moreover, since the aim of this study was to compare the bioavailability of the same TF within different food matrices in a cross-over design rather than interpreting absolute bioavailability, we are confident that the high proportion of women in the study does not jeopardize the study's findings.
Conclusions
This study is, to the best of our knowledge, the first clinical investigation of the bioavailability of curcuminoids in different food matrices. The absorption and bioavailability of curcuminoids are increased when administered in food matrices, particularly if they contain lipids in a suspended form or polar lipids. Among the PK parameters, significant improvements were obtained with DA, SBar and Prob, as per the AUC24 h, AUC8 h, Cmax, Frel 24 h and Frel 8 h. When comparing the relative bioavailability (Frel 24 h) of food matrices, DA showed significantly higher values than RTD and Gum, and Prob tended to be higher than RTD. The Cmax of total curcuminoids was significantly increased with Prob. Importantly, there is no indication of impaired safety of TF caused by the increased bioavailability with food matrices as the maximal values of relevant pharmacokinetic parameters remain similar to Caps.Abbreviations
| AUC | Area under the plasma concentration–time curve |
| BDMC | Bisdemethoxycurcumin |
| Caps | Capsules |
| C max | Peak plasma concentration |
| DA | Dairy analogue |
| DMC | Demethoxycurcumin |
| F rel | Relative bioavailability compared to capsule formulation |
| Gum | Gummies |
| HHC | Hexahydrocurcumin |
| Prob | Probiotic drink |
| RTD | Ready to drink |
| SBar | Sports nutrition bar |
| T 1/2 | Half-life time |
| TEAE | Treatment-emergent adverse events |
| TF | Turmeric formulation |
| THC | Tetrahydrocurcumin |
| T max | Time to reach peak plasma concentration |
| UHPLC | ultra-high-performance liquid chromatography |
Author contributions
KAS: visualization; writing – original draft. CR: conceptualization; project administration; writing – review and editing. JL: writing – review and editing. PB: investigation; methodology; writing – review and editing. MT: investigation; methodology; writing – review and editing. PFB: conceptualization; writing – review and editing.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
This research was funded by Givaudan France Naturals. KAS is an employee of Givaudan International SA. CR, JL, PB, MT, and PFB are employees of Givaudan France Naturals.References
- S. K. Sandur, M. K. Pandey, B. Sung, K. S. Ahn, A. Murakami, G. Sethi, P. Limtrakul, V. Badmaev and B. B. Aggarwal, Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism, Carcinogenesis, 2007, 28(8), 1765–1773, DOI:10.1093/carcin/bgm123.
- B. B. Aggarwal and K. B. Harikumar, Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases, Int. J. Biochem. Cell Biol., 2009, 41(1), 40–59, DOI:10.1016/j.biocel.2008.06.010.
- S. J. Hewlings and D. S. Kalman, Curcumin: A Review of Its Effects on Human Health, Foods, 2017, 6(10), 92, DOI:10.3390/foods6100092.
- G. M. Holder, J. L. Plummer and A. J. Ryan, The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat, Xenobiotica, 1978, 8(12), 761–768, DOI:10.3109/00498257809069589.
- C. R. Ireson, D. J. Jones, S. Orr, M. W. Coughtrie, D. J. Boocock, M. L. Williams, P. B. Farmer, W. P. Steward and A. J. Gescher, Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine, Cancer Epidemiol. Biomarkers Prev., 2002, 11(1), 105–111 CAS.
- A. Hassaninasab, Y. Hashimoto, K. Tomita-Yokotani and M. Kobayashi, Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(16), 6615–6620, DOI:10.1073/pnas.1016217108.
- S. Gopi, J. Jacob, K. Varma, S. Jude, A. Amalraj, C. A. Arundhathy, R. George, T. R. Sreeraj, C. Divya, A. B. Kunnumakkara and S. J. Stohs, Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study, Phytother. Res., 2017, 31(12), 1883–1891, DOI:10.1002/ptr.5931.
- Y. He, Y. Yue, X. Zheng, K. Zhang, S. Chen and Z. Du, Curcumin, inflammation, and chronic diseases: how are they linked?, Molecules, 2015, 20(5), 9183–9213, DOI:10.3390/molecules20059183.
- S. C. Gupta, B. Sung, J. H. Kim, S. Prasad, S. Li and B. B. Aggarwal, Multitargeting by turmeric, the golden spice: From kitchen to clinic, Mol. Nutr. Food Res., 2013, 57(9), 1510–1528, DOI:10.1002/mnfr.201100741.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Bioavailability of curcumin: problems and promises, Mol. Pharm., 2007, 4(6), 807–818, DOI:10.1021/mp700113r.
- M. Pulido-Moran, J. Moreno-Fernandez, C. Ramirez-Tortosa and M. Ramirez-Tortosa, Curcumin and Health, Molecules, 2016, 21(3), 264, DOI:10.3390/molecules21030264.
- S. Prasad, S. C. Gupta, A. K. Tyagi and B. B. Aggarwal, Curcumin, a component of golden spice: from bedside to bench and back, Biotechnol. Adv., 2014, 32(6), 1053–1064, DOI:10.1016/j.biotechadv.2014.04.004.
- S. Prasad, A. K. Tyagi and B. B. Aggarwal, Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice, Cancer Res. Treat., 2014, 46(1), 2–18, DOI:10.4143/crt.2014.46.1.2.
- P. Fança-Berthon, M. Tenon, S. L. Bouter-Banon, A. Manfre, C. Maudet, A. Dion, H. Chevallier, J. Laval and R. B. van Breemen, Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study, J. Nutr., 2021, 151(7), 1802–1816, DOI:10.1093/jn/nxab087.
- U.S. Department of Health and Human Services, Food and Drug Administration and Center for Drug Evaluation and Research, Bioavailability Studies Submitted in NDAs or INDs—General Considerations: Guidance for Industry, 2022 Search PubMed.
- M. A. Birkett and S. J. Day, Internal pilot studies for estimating sample size, Stat. Med., 1994, 13(23–24), 2455–2463, DOI:10.1002/sim.4780132309.
- R. H. Browne, On the use of a pilot sample for sample size determination, Stat. Med., 1995, 14(17), 1933–1940, DOI:10.1002/sim.4780141709.
- M. A. Hertzog, Considerations in determining sample size for pilot studies, Res. Nurs. Health, 2008, 31(2), 180–191, DOI:10.1002/nur.20247.
- S. A. Julious, Sample size of 12 per group rule of thumb for a pilot study, Pharm. Stat., 2005, 4(4), 287–291, DOI:10.1002/pst.185.
- J. Sim and M. Lewis, The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency, J. Clin. Epidemiol., 2012, 65(3), 301–308, DOI:10.1016/j.jclinepi.2011.07.011.
- M. D. Teare, M. Dimairo, N. Shephard, A. Hayman, A. Whitehead and S. J. Walters, Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study, Trials, 2014, 15, 264, DOI:10.1186/1745-6215-15-264.
- H. Y. Barnett, H. Geys, T. Jacobs and T. Jaki, Methods for Non-Compartmental Pharmacokinetic Analysis With Observations Below the Limit of Quantification, Stat. Biopharm. Res., 2020, 13(1), 59–70, DOI:10.1080/19466315.2019.1701546.
- R Core Team, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org, 2020 Search PubMed.
- C. Acharya, A. C. Hooker, G. Y. Turkyilmaz, S. Jonsson and M. O. Karlsson, A diagnostic tool for population models using non-compartmental analysis: The ncappc package for R, Comput. Methods Programs Biomed., 2016, 127, 83–93, DOI:10.1016/j.cmpb.2016.01.013.
- B. Zheng and D. J. McClements, Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability, Molecules, 2020, 25(12), 2791, DOI:10.3390/molecules25122791.
- Q. Ye, S. Kwon, Z. Gu and C. Selomulya, Stable nanoemulsions for poorly soluble curcumin: From production to digestion response in vitro, J. Mol. Liq., 2024, 394, 123720, DOI:10.1016/j.molliq.2023.123720.
- V. S. Gota, G. B. Maru, T. G. Soni, T. R. Gandhi, N. Kochar and M. G. Agarwal, Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers, J. Agric. Food Chem., 2010, 58(4), 2095–2099, DOI:10.1021/jf9024807.
- K. Maiti, K. Mukherjee, A. Gantait, B. P. Saha and P. K. Mukherjee, Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats, Int. J. Pharm., 2007, 330(1–2), 155–163, DOI:10.1016/j.ijpharm.2006.09.025.
- T. H. Marczylo, R. D. Verschoyle, D. N. Cooke, P. Morazzoni, W. P. Steward and A. J. Gescher, Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine, Cancer Chemother. Pharmacol., 2007, 60(2), 171–177, DOI:10.1007/s00280-006-0355-x.
- A. Liu, H. Lou, L. Zhao and P. Fan, Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin, J. Pharm. Biomed. Anal., 2006, 40(3), 720–727, DOI:10.1016/j.jpba.2005.09.032.
- P. M. Kidd, Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts, Altern. Med. Rev., 2009, 14(3), 226–246 Search PubMed.
- J. Cuomo, G. Appendino, A. S. Dern, E. Schneider, T. P. McKinnon, M. J. Brown, S. Togni and B. M. Dixon, Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation, J. Nat. Prod., 2011, 74(4), 664–669, DOI:10.1021/np1007262.
- G. N. Asher, Y. Xie, R. Moaddel, M. Sanghvi, K. S. Dossou, A. D. Kashuba, R. S. Sandler and R. L. Hawke, Randomized Pharmacokinetic Crossover Study Comparing 2 Curcumin Preparations in Plasma and Rectal Tissue of Healthy Human Volunteers, J. Clin. Pharmacol., 2017, 57(2), 185–193, DOI:10.1002/jcph.806.
- S. Fu, M. A. Augustin, L. Sanguansri, Z. Shen, K. Ng and S. Ajlouni, Enhanced Bioaccessibility of Curcuminoids in Buttermilk Yogurt in Comparison to Curcuminoids in Aqueous Dispersions, J. Food Sci., 2016, 81(3), H769–H776, DOI:10.1111/1750-3841.13235.
- S. Flory, N. Sus, K. Haas, S. Jehle, E. Kienhofer, R. Waehler, G. Adler, S. Venturelli and J. Frank, Increasing Post-Digestive Solubility of Curcumin Is the Most Successful Strategy to Improve its Oral Bioavailability: A Randomized Cross-Over Trial in Healthy Adults and In Vitro Bioaccessibility Experiments, Mol. Nutr. Food Res., 2021, 65(24), e2100613, DOI:10.1002/mnfr.202100613.
- K. R. Phipps, N. Quesnot, K. Privat, N. J. Baldwin, E. Ahlborn and P. Fanca-Berthon, Toxicological safety evaluation of a novel highly bioavailable turmeric extract formulation, J. Appl. Toxicol., 2020, 40(2), 285–299, DOI:10.1002/jat.3903.
- P. B. Luis, A. G. Kunihiro, J. L. Funk and C. Schneider, Incomplete Hydrolysis of Curcumin Conjugates by beta-Glucuronidase: Detection of Complex Conjugates in Plasma, Mol. Nutr. Food Res., 2020, 64(6), 1901037, DOI:10.1002/mnfr.201901037.
Footnote |
| † Electronic supplementary information (ESI) available: Nutritional information, analytical materials & methods, study flowchart, plasma concentration of curucmionids over 24 hours, range of curcuminoid exposure. See DOI: https://doi.org/10.1039/d4fo03414g |
| This journal is © The Royal Society of Chemistry 2025 |