Open Access Article
Open Access ArticleEffect of consumption of anthocyanin-rich products on NMR lipoprotein subclasses and biomarkers in hypercholesterolemic subjects: a randomized controlled trial (the AppleCOR study)†
A.
Pedret
 ab,
E.
Llauradó
*ab,
L.
Calderón-Pérez
c,
J.
Companys
a,
L.
Pla-Pagà
a,
P.
Salamanca
a,
B. A.
Sandoval-Ramírez
a,
M.
Besora-Moreno
a,
Ú.
Catalán
a,
S.
Fernández-Castillejo
a,
I.
Ludwig‡
ab,
E.
Llauradó
*ab,
L.
Calderón-Pérez
c,
J.
Companys
a,
L.
Pla-Pagà
a,
P.
Salamanca
a,
B. A.
Sandoval-Ramírez
a,
M.
Besora-Moreno
a,
Ú.
Catalán
a,
S.
Fernández-Castillejo
a,
I.
Ludwig‡
 d,
A.
Macià
d,
L.
Rubió-Piqué
d,
A.
Macià
d,
L.
Rubió-Piqué
 d,
M.
Sampson
e,
A. T.
Remaley
ef,
R. M.
Valls
*ab,
M. J.
Motilva§
g and
R.
Solà§
abh
d,
M.
Sampson
e,
A. T.
Remaley
ef,
R. M.
Valls
*ab,
M. J.
Motilva§
g and
R.
Solà§
abh
aUniversitat Rovira i Virgili, Facultat de Medicina i Ciències de la Salut, Functional Nutrition, Oxidation and Cardiovascular Diseases Group (NFOC-Salut), Reus, Spain. E-mail: elisabet.llaurado@urv.cat; rosamaria.valls@urv.cat
bInstitut Investigació Sanitària Pere i Virgili (IISPV), Reus-Tarragona 43204, Spain
cEurecat, Centre Tecnològic de Catalunya, Unitat de Nutrició i Salut, Reus, 43204, Spain
dUniversity of Lleida-Agrotecnio CERCA Center, Av. Alcalde Rovira Roure 191, Lleida, 25198, Spain
eDepartment of Laboratory Medicine, Clinical Center, National Institutes of Health, Bethesda, MD, USA
fLipoprotein Metabolism Section, Cardio-Pulmonary Branch, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, USA
gInstituto de Ciencias de la Vid y del Vino (CSIC, Gobierno de la Rioja, Universidad de La Rioja), Logroño, Spain
hHospital Universitari Sant Joan de Reus, Reus, Spain
First published on 14th February 2025
Abstract
Our aim was to assess the effect of intake of anthocyanin biofortified red-fleshed apples (RFA) versus that of common white apples (WFA) without anthocyanins on the NMR lipoprotein subfraction profile and other NMR metabolites. Additionally, an aronia infusion (AI) arm, matching the anthocyanin content and profile of the RFA, was included. A 6-week, randomized, parallel study was conducted in hypercholesterolemic subjects (n = 121). Anthocyanin-rich products (RFA and AI) decreased LDLc; ApoB; total, large, and small LDL-P; LDL size; TG/HDL ratio; and large TRL, versus WFA. All treatments significantly decreased HDLc, ApoA1, and total HDL-P, with the most significant reductions after RFA treatment. RFA significantly decreased large HDL-P compared to WFA and AI, while medium HDL-P decreased significantly after AI compared to WFA. Anthocyanin-rich products decreased GlycA and alanine and increased acetoacetate versus WFA. WFA and RFA decreased plasma citrate versus AI. Thus, anthocyanin-rich products provided greater protection against CVD risk than WFA.
Introduction
The intake of anthocyanins (ACNs), a flavonoid phenol class, has been inversely associated with the risk of cardiovascular disease (CVD) in both European and US populations.1,2 Data from the NIH-AARP Diet and Health Study in 369![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 827 elderly people showed inverse associations between anthocyanin dietary consumption and total and cardiovascular mortality.3 In recent meta-analyses, ACN-rich foods improved cardiometabolic markers,4 and anthocyanin supplementation improved the cardiovascular lipid profile in healthy and cardiovascular risk individuals.5 In individual studies, anthocyanin consumption has also been shown to improve oxidative status, DNA integrity, and intestinal microbiota composition in healthy individuals; diabetic status in pre- and diabetic individuals; blood pressure in pre- and stage-one-hypertension individuals; and memory discrimination in older adults with cognitive complaints.6
827 elderly people showed inverse associations between anthocyanin dietary consumption and total and cardiovascular mortality.3 In recent meta-analyses, ACN-rich foods improved cardiometabolic markers,4 and anthocyanin supplementation improved the cardiovascular lipid profile in healthy and cardiovascular risk individuals.5 In individual studies, anthocyanin consumption has also been shown to improve oxidative status, DNA integrity, and intestinal microbiota composition in healthy individuals; diabetic status in pre- and diabetic individuals; blood pressure in pre- and stage-one-hypertension individuals; and memory discrimination in older adults with cognitive complaints.6
The diet followed in Western countries does not appear to guarantee an adequate intake of flavonoids.7 Metabolic engineering of plant secondary metabolite pathways for improving human health is a focus of many current plant biotechnology and breeding programs. “Biofortification” is the genetic improvement of food crops to achieve health outcomes.8 Plant cultivars biofortified with specific secondary metabolites have also been produced through traditional breeding programs as an alternative to genetic modification. In this sense, ACNs are the most intensively studied group of secondary plant metabolites. The knowledge of the key genes involved in anthocyanin synthesis has allowed the production of new red cultivars by traditional breeding methods, without genetic modification, and with an enhanced content of ACNs.9
The apple is one of the most widely consumed fruits globally,10 the decrease in LDL cholesterol (LDLc) being the main impact of anthocyanin consumption on CVD markers.5,11 Due to this, our aim was to assess the effect of the intake of ACN biofortified red-fleshed apples (RFA), without genetic modifications versus that of common white-fleshed apples (WFA) without ACN on the NMR lipoprotein subfraction profile and other NMR metabolites. Additionally, we included an aronia infusion (AI) arm, which matched the ACN content and profile of the RFA, to assess the apple matrix effect. We aim to explore the benefits of LDL and other lipoprotein markers beyond LDLc.
Materials and methods
Intervention products
Three intervention products were administered: RFA, WFA, and AI. The RFA variety was the Redlove (a variety biofortified with ACNs in its flesh). The WFA variety was the Granny Smith (ACN-free control). Both apple varieties were provided by NUFRI S.A.T. (Mollerussa, Lleida, Spain). The daily amount of apple snacks provided to participants was 80 g day−1 for RFA and WFA. This amount is equivalent to approximately 640–800 g of fresh apples or roughly three medium-sized apples due to their water removal during freeze-drying. Moreover, for AI, the daily amount was 1 L day−1, taken either in one or multiple doses alongside meals. To ensure shelf stability and preserve the apples’ (poly)phenolic content, a freeze-dried snack format was chosen for the nutritional intervention. RFA and WFA were administered in individual daily seal plastic containers, which were refrigerated (2 °C) until their use for the study. The preparation process of the freeze-dried apple snacks is detailed in our previous study.12Aronia fruit, selected for its high content of cyanidin-3-O-galactoside and cyanidin-O-arabinoside (the main ACNs in RFA), was used in powdered form (Aronia Pulver, BIOJOY, Nuremberg, Germany) to prepare a daily cold-water infusion. Volunteers prepared the infusion by mixing 50 g of aronia fruit powder with 1 L of mineral water (Bezoya mineral water, Calidad Pascual, Aranda de Duero, Burgos, Spain), homogenizing the mixture energetically in a glass bottle for 3 minutes, filtering it with a cloth, and storing the filtered infusion in a light-protected bottle for daily consumption. This preparation provided a daily dose of ACNs equivalent to that found in the daily RFA dose. Daily doses of 80 g of WFA and RFA snacks and 1 L of AI provided 0 mg day−1, 34.5 mg day−1 and 37.4 mg day−1 of total ACNs, respectively. ESI Table 1† shows the phenolic composition present in the products used in the study and the daily dose of macronutrients (g) and other phytochemicals (mg).
Subjects
Subjects from the general population were recruited by means of news in social networks, newspapers, and tableaux advertisements at the Hospital Universitari Sant Joan (HUSJ)-Eurecat, Reus, Spain, between January 2019 and May 2019. Out of the 179 subjects assessed for eligibility, 121 (70 female and 51 male) hypercholesterolemic individuals, according to current guidelines,13 were randomized. Inclusion criteria were being aged ≥18, with LDL-c levels ≥115 mg dL−1 and willingness to provide informed consent before the initial screening visit. Exclusion criteria were: LDL-c levels <115 and ≥190 mg dL−1 or with hyperlipemia treatment (drugs and functional foods); diabetes mellitus type 1 or 2 or with hypoglycaemia treatment; body mass index (BMI) ≥ 35 kg m−2; triglycerides (TG) levels ≥350 mg dL−1; anaemia (haemoglobin ≤13 g dL−1 in men and ≤12 g dL−1 in women); diagnosis of intestinal disorders such as Crohn's disease, ulcerative colitis, coeliac disease and irritable bowel syndrome; fructose and/or sorbitol and/or gluten intolerance; use of antioxidants supplements; to be pregnant or intending to become; to be in a breast-feeding period; chronic alcoholism; smoking; current or past participation in a clinical trial or consumption of a research product in the 30 days prior to inclusion in the study; and failure to follow the study guidelines.Study design
The AppleCOR study was a randomized, controlled, parallel clinical trial. Participants were assigned to one of three intervention groups: WFA, RFA or AI for a duration of 6 weeks. Participants were randomly allocated to intervention groups by a computerized random number generator made by an independent statistician. PROC PLAN (SAS 9.2, Cary, NC: 83 SAS Institute Inc.) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 allocation using random block sizes of 2, 4, and 6 was used. Participants signed informed consent before their participation in the study, which was approved by the Clinical Research Ethical Committee of Institut d'Investigació Sanitària Pere Virgili (S033/04Nov2016), Reus, Spain. The protocol and trial were conducted in accordance with the Helsinki Declaration and Good Clinical Practice Guidelines of the International Conference of Harmonization (GCP ICH) and were reported as CONSORT criteria. The trial was registered at ClinicalTrials.gov Identifier: NCT03795324.
1 allocation using random block sizes of 2, 4, and 6 was used. Participants signed informed consent before their participation in the study, which was approved by the Clinical Research Ethical Committee of Institut d'Investigació Sanitària Pere Virgili (S033/04Nov2016), Reus, Spain. The protocol and trial were conducted in accordance with the Helsinki Declaration and Good Clinical Practice Guidelines of the International Conference of Harmonization (GCP ICH) and were reported as CONSORT criteria. The trial was registered at ClinicalTrials.gov Identifier: NCT03795324.
During the intervention period, subjects were instructed to preserve their lifestyle, physical activity, and dietary habits, to completely refrain from consuming ACN-rich foods (berries, grapefruit, plums, figs, pomegranate, green and red apples, black olives, red and black beans and red wine), and to avoid eating functional foods for reducing cholesterol levels. The adherence of the volunteers to their dietary habits through the study was assessed by a 3-day food record at the baseline and at the end of the study. At each visit, subjects also underwent a physical examination by a general practitioner, completed a Physical Activity Questionnaire Class AF14 and had anthropometric and blood pressure measurements recorded. Plastic and seal containers for RFA and WFA and the daily dose bag of aronia powder were returned after intervention by volunteers to check their compliance. Outcomes were assessed at the beginning of the study (baseline) and at the end (6 weeks) of intervention periods. Primary outcome measures were changes in lipoprotein subclasses and subfractions, and secondary outcomes were other parameters included in the LP4 NMR MetaboProfile™ profile.
Blood samples, collected at the beginning and at the end of the study, were stored at −80 °C in the central laboratory's Biobanc of HUSJ (biobanc.reus@iispv.cat) until required for batch analyses. Serum samples were shipped to the National Heart, Lung and Blood Institute, National Institutes of Health (NIH; Bethesda, MD, USA).
Lipoprotein subclass measurement was performed by nuclear magnetic resonance (NMR) in a Vantera clinical spectrometer, produced by LipoScience (Raleigh, NC, U.S.A.). The NMR LipoProfile test by LipoScience involves measurement of the 400 MHz proton NMR spectrum of samples and uses the characteristic signal amplitude of the lipid methyl group broadcast by every lipoprotein subfraction as the basis for quantification.15 NMR by using the LipoProfile-4 algorithm was performed to quantify the average particle size and concentrations of triglyceride rich lipoproteins (TRL), LDL, and high-density lipoproteins (HDL). NMR LipoProfile spectra use a further-optimized deconvolution algorithm (LP4) to simultaneously measure a novel NMR inflammation biomarker (GlycA). The LP4 deconvolution algorithm also allows the measurement of 7 different HDL particle subspecies, prompted by emerging evidence for the functional and proteomic diversity of different-sized HDL particles.
Biomarkers of dietary adherence
To evaluate the compliance for each intervention, phenol biological metabolites were used as intake biomarkers and analysed in urine and plasma samples at the beginning and at the end of the study, by UPLC-MS/MS, as we have previously described.16 Peonidin-3-O-galactoside was used as an intake biomarker for consumption of ACN-rich products, and phloretin-2′-O-glucuronide as a biomarker of dihydrochalcones (present only in apples).Sample size and power analysis
Assuming a drop-out rate of 10% and a Type I error of 0.05 (2-sided), a sample size of 22 participants per group will allow at least 80% power to detect a statistically significant difference among groups of 0.50 mmol L−1 in LDLc. The population standard deviation of this variable is estimated to be 0.72 mmol L−1.17 The sample size recruited was 40 participants per arm in order to improve the statistical significance.Statistical analyses
The normality of variables was assessed by the Kolmogorov–Smirnov test. Non-parametric variables were log transformed when possible. One-factor analysis of variance (ANOVA) was used to determine differences in baseline characteristics. Analyses were made by intention-to-treat. Multiple imputation was performed by linear regression analysis. Intra- and inter-treatment comparisons for parametric variables were carried out using an ANCOVA model adjusted for age, sex, BMI at the beginning of the study, and baseline values. Linear regression analyses were performed with NMR biomarkers as dependent variables and the phenolic biomarkers of dietary adherence as independent variables. Statistical significance was defined as a P value≤ 0.05 for a 2-sided test. Analyses were performed using SPSS for Windows, version 26 (IBM corp., Armonk, NY, USA).Results
Of the 179 subjects assessed for eligibility, finally, 121 of them (68%, 70 women and 51 men) were allocated to one of three intervention groups: RFA (n = 40), WFA (n = 41), or AI (n = 40). Losses to follow-up or due to receiving allocation intervention were 8, 4, and 2 individuals in RFA, WFA, and AI treatments, respectively. Analyses were made by intention-to-treat (ESI Fig. 1;† flow chart of the study). We could not identify any adverse effects related to the intervention products. Table 1 shows the baseline characteristics of participants. No differences among treatment groups were observed. No differences were observed in physical activity from the beginning to the end of the study (ESI Table 2†). No inter-treatment differences were observed in the daily intake after intervention periods, with the exception of a higher % energy intake from polyunsaturated fat intake in the RFA versus the WFA group, which was not reflected when intakes in grams were compared (ESI Table 3†).| Variable | WFA (n = 41) | AI (n = 40) | RFA (n = 40) | P |
|---|---|---|---|---|
| Data expressed as mean ± standard deviation or percentages. WFA, white-fleshed apple; AI, aronia infusion; RFA, red-fleshed apple; SBP, systolic blood pressure; DBP, diastolic blood pressure; pulse pressure = SBP-DBP; BMI, body mass index (weight/(height in meters)2); pl, plasma; LDL, low density lipoproteins; HDL, high density lipoproteins * median (25th–75th percentiles). AU, arbitrary units: 0–1, inactive; 2–3, very low activity; 4–5, low activity; 6–11, moderately active; > or ≥12, very active. P for ANOVA with logarithmic transformation for triglycerides. A p-value < 0.05 was considered statistically significant. | ||||
| Age, years | 49.8 ± 13.6 | 49.6 ± 13.3 | 46.7 ± 16.3 | 0.566 |
| Females, % | 67.5 | 50 | 55.3 | 0.287 |
| SBP, mm Hg | 127 ± 16.7 | 127 ± 14.4 | 132 ± 16.5 | 0.285 |
| DPB, mm Hg | 76 ± 11.1 | 77 ± 10.1 | 79 ± 9.7 | 0.317 |
| Weight, kg | 68.7 ± 12.4 | 71.8 ± 11.1 | 74.2 ± 11.6 | 0.123 |
| BMI, kg m−2 | 24.6 ± 3.2 | 26.3 ± 4.5 | 26.3 ± 3.8 | 0.078 |
| Waist circumference, cm | 86.9 ± 11.5 | 89.3 ± 9.6 | 90.9 ± 9.1 | 0.253 |
| Waist/height, cm | 0.52 ± 0.06 | 0.54 ± 0.06 | 0.54 ± 0.06 | 0.281 |
| Conicity index | 1.24 ± 0.09 | 1.25 ± 0.07 | 1.26 ± 0.07 | 0.836 |
| Glucose, pl, mg dL−1 | 91 ± 11.3 | 93 ± 6.0 | 92 ± 8.4 | 0.506 |
| Cholesterol, pl, mg dL−1 | ||||
| Total | 220 ± 48 | 223 ± 26 | 213 ± 54 | 0.611 |
| LDL | 145 ± 25.9 | 144 ± 20.3 | 147 ± 19.6 | 0.835 |
| HDL | 60.0 ± 17.1 | 60.0 ± 16.4 | 53.8 ± 16.6 | 0.143 |
| Triglycerides* pl, mg dL−1 | 82 (60–117) | 81 (64–108) | 87 (58–128) | 0.836 |
| Physical activity, AU | 4.34 ± 2.24 | 5.10 ± 1.68 | 4.35 ± 1.95 | 0.146 |
Biomarkers of dietary adherence
Compliance of the volunteers with interventions was reflected in the increase in plasma and urine levels of the ACN peonidin-3-O-galactoside after RFA and AI sustained consumption, with non-detectable values after WFA consumption (Table 2). Phloretin-2′-O-glucuronide, as an intake biomarker of both apple snacks, increased in plasma and urine after RFA and WFA sustained consumption, being non-detectable after AI consumption (Table 2). The level of adherence was acceptable for all products as the consumption was >80%.| Δ Concentration (At the end of intervention (6-weeks) – basal value (day 0); in plasma) | |||
|---|---|---|---|
| WFA snack | RFA snack | AI | |
| Anthocyanins | |||
| Peonidin-3-O-galactoside (nM ± SEM) | n.d. | 0.73 ± 0.03 | 0.63 ± 0.02 |
| Dihydrochalcones | |||
| Phloretin-2′-O-glucuronide (nM ± SEM) | 0.36 ± 0.34 | 8.83 ± 0.37 | n.d. |
| Δ Concentration (At the end of intervention (6-weeks) – basal value (day 0); in 24 h urine excretion) | |||
|---|---|---|---|
| n.d., non-detectable. | |||
| Anthocyanins | |||
| Peonidin-3-O-galactoside (nmols ± SEM) | n.d. | 4.94 ± 0.36 | 18.4 ± 0.22 |
| Dihydrochalcones | |||
| Phloretin-2′-O-glucuronide (μmols ± SEM) | 0.63 ± 0.03 | 1.50 ± 0.03 | −0.06 ± 0.03 |
NMR total cholesterol and LDL measures
Treatment with AI significantly decreased LDLc, apolipoprotein B (ApoB), and total and large LDL particle concentration (LDL-P), with the decreases reaching significance versus changes after WFA treatment (P = 0.025, P = 0.012, P = 0.006, and P < 0.001, respectively), and versus changes after RFA treatment for ApoB (P = 0.047) and large LDL-P (P = 0.003) (Table 3). AI also significantly decreased the LDL size, with this decrease reaching significance versus changes after RFA treatment (P = 0.004). Although no intra-treatment changes were observed for the LDL-P/HDL-P ratio, values after AI treatment were significantly lower than those after RFA (P = 0.009). Treatment with RFA significantly decreased LDL-c, without inter-treatment differences; significantly decreased large LDL-P, with changes being smaller than those observed after AI treatment (P = 0.003); significantly increased medium LDL-P with a borderline significance versus changes after WFA treatment (P = 0.053); and significantly decreased small LDL-P, with the decrease reaching significance versus changes after WFA treatment (P = 0.011) and AI treatment (P = 0.012) (Table 3).| Treatment | Changes among treatments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | WFA (n = 40) | AI (n = 40) | RFA (n = 39) | AI vs. WFA | RFA vs. WFA | RFA vs. AI | ||||||
| Post-int | Change | Post-int | Change | Post-int | Change | Mean (95%CI) | P | Mean (95%CI) | P | Mean (95%CI) | P | |
| Post-int, post-treatment values. Change, change from the baseline. WFA, white-fleshed apple; AI, aronia infusion; RFA, red-fleshed apple; Apo, apolipoproteins; LDL, low density lipoproteins; HDL, high density lipoproteins; TG, triglycerides; TRL, triglyceride rich lipoproteins; TRLC, TRL cholesterol; TRLTG, TRL triglycerides; LDL-P/HDL-P, LDL particles/HDL particles ratio; s-HDL/l-HDL, small HDL/large HDL ratio; HDL-C/HDL-P, HDL cholesterol/HDL particles ratio; TG/HDL, triglycerides/HDL cholesterol ratio; Log, logarithm. Data expressed as mean ± standard deviation or mean (95% confidence interval, CI). * (n = 77). Non-parametric data expressed as median (25th–75th percentile), with Wilcoxon and Mann–Whitney tests for intra- (†P = 0.015) and inter-treatment differences, respectively. Log, logarithm. ANCOVA model adjusted for sex, age, initial body mass index, and baseline values. A p-value < 0.05 was considered statistically significant. | ||||||||||||
| LDL measures | ||||||||||||
| LDL cholesterol, mg dL−1 | 128 ± 23 | −1.86 (−6.0; 2.3) | 117 ± 21 | −8.50 (−12; −4.6) | 119 ± 19 | −4.23 (−8.4; −0.03) | −6.64 (−12; −0.9) | 0.025 | −2.37 (−8.5; 3.7) | 0.441 | 4.27 (−1.4; 10) | 0.142 |
| ApoB, mg dL−1 | 108 ± 20 | −0.043 (−3.5; 3.4) | 99 ± 19 | −6.25 (−9.5; −3.0) | 102 ± 19 | −1.45 (−4.9; 2.0) | −6.20 (−11; −1.4) | 0.012 | −1.41 (−6.5; 3.7) | 0.583 | 4.80 (0.06; 9.5) | 0.047 |
| NMR LDL particle concentration (nmol L−1) | ||||||||||||
| Total | 1469 ± 608 | −9.15 (−68; 50) | 1141 ± 641 | −125 (−181; −69) | 1234 ± 646 | −54.8 (−114; 4.9) | −116 (−199; −33) | 0.006 | −45.6 (−132; 41) | 0.300 | 70.4 (−11; 152) | 0.089 |
| Large | 571 ± 226 | −15.8 (−52; 21) | 444 ± 220 | −141 (−176; −106) | 494 ± 178 | −62 (−99; −24) | −125 (−177; −74) | <0.001 | −45.7 (−99; 7.5) | 0.092 | 79.5 (28; 131) | 0.003 |
| Medium (log) | 2.41 ± 1.27 | −0.070 (−0.2; 0.4) | 2.40 ± 0.95 | 0.187 (−0.1; 0.5) | 2.68 ± 0.25 | 0.496 (0.2–0.8) | 0.117 (−0.29; 0.53) | 0.573 | 0.426 (−0.01–0.9) | 0.053 | 0.309 (−0.10; 0.7) | 0.138 |
| Small | 712 ± 426 | −17.3 (−95; 60) | 734 ± 309 | −24.9 (−100; 50) | 658 ± 430 | −166 (−246; −85) | −7.55 (−116; 101) | 0.891 | −148 (−262; −34) | 0.011 | −141 (−250; −31) | 0.012 |
| Average NMR LDL particle size (nm) | 21.3 ± 0.44 | −0.027 (−0.1; 0.03) | 21.2 ± 0.35 | −0.075 (−0.13; −0.02) | 21.3 ± 0.42 | 0.044 (−0.01; 0.10) | −0.048 (−0.13; 0.03) | 0.233 | 0.071 (−0.12; 0.15) | 0.092 | 0.119 (0.04; 0.20) | 0.004 |
| LDL-P/HDL-P ratio | 80.5 ± 17 | 1.99 (−1.2; 5.2) | 74.4 ± 16 | −1.73 (−4.7; 1.3) | 78.0 ± 15 | 4.21 (0.96; 7.5) | −3.71 (−8.1; 0.73) | 0.100 | 2.22 (−2.5; 6.9) | 0.352 | 5.94 (1.5; 10) | 0.009 |
| HDL measures | ||||||||||||
| HDL cholesterol (mg dL−1) | 59 ± 12 | −2.59 (−4.6; −0.5) | 55 ± 11 | −3.76 (−5.7; −1.8) | 53.5 ± 8.8 | −6.00 (−8.0; −3.9) | −1.17 (−4.0; 1.7) | 0.416 | −3.38 (−6.3; −0.4) | 0.026 | −2.20 (−5.1; 0.6) | 0.129 |
| ApoA1, mg dL−1 | 145 ± 18 | −5.43 (−9.7; −1.1) | 140 ± 19 | −8.29 (−12; −4.1) | 137 ± 17 | −12.3 (−17; −7.9) | −2.85 (−8.9; 3.2) | 0.349 | −6.88 (−13; −0.6) | 0.032 | −4.03 (−10; 2.0) | 0.188 |
| NMR HDL particle concentration (μmol L−1) | ||||||||||||
| Total | 22 ± 2.2 | −0.689 (−1.3; −0.07) | 22 ± 2.4 | −1.20 (−1.80; −0.61) | 22 ± 2.8 | −1.89 (−2.5; −1.3) | −0.515 (−1.4; 0.34) | 0.236 | −1.20 (−2.1; 0.31) | 0.009 | −0.685 (−1.5; 0.18) | 0.119 |
| Large | 2.83 ± 1.7 | −0.243 (−0.43; −0.06) | 2.71 ± 1.3 | −0.112 (−0.29; 0.07) | 2.00 ± 0.9 | −0.731 (−0.9; −0.54) | 0.131 (−0.13; 0.39) | 0.317 | −0.488 (−0.8; −0.2) | <0.001 | −0.619 (−0.9; −0.36) | <0.001 |
| Medium | 5.26 ± 2.2 | 0.017 (−0.44; 0.48) | 4.72 ± 1.7 | −0.692 (−1.1; −0.25) | 5.57 ± 1.7 | 0.140 (−0.33; 0.61) | −0.709 (−1.3; −0.06) | 0.031 | 0.123 (−0.55; 0.8) | 0.718 | 0.831 (0.19; 1.5) | 0.012 |
| Small | 14.4 ± 3.0 | −0.477 (−1.1; 0.15) | 14.4 ± 2.8 | −0.374 (−0.98; 0.23) | 14.0 ± 2.9 | −1.30 (−1.9; −0.67) | 0.103 (−0.77; 0.98) | 0.817 | −0.827 (−1.7; 0.08) | 0.074 | −0.930 (−1.8; −0.05) | 0.039 |
| Average NMR HDL particle size (nm) | 9.1 ± 0.36 | −0.023 (−0.08; 0.03) | 9.0 ± 0.36 | −0.030 (−0.08; 0.02) | 8.9 ± 0.27 | −0.092 (−0.1; −0.04) | −0.007 (−0.08; 0.07) | 0.856 | −0.069 (−0.1; 0.01) | 0.083 | −0.062 (−0.14; 0.01) | 0.104 |
| s-HDL/l-LDL | 7.94 ± 6.6 | 0.007 (−0.30; 032) | 6.65 ± 3.4 | 0.189 (−0.11; 0.49) | 8.39 ± 3.8 | −0.021 (−0.34; 0.29) | −0.505 (−1.3; 0.3) | 0.240 | −0.028 (−0.48; 0.4) | 0.901 | −0.210 (−0.4; 0.22) | 0.337 |
| HDL-C/HDL-P | 2.60 ± 0.43 | −0.046 (−0.11; 0.01) | 2.52 ± 0.37 | −0.044 (−0.10; 0.01) | 2.49 ± 0.33 | −0.038 (−0.10; 0.02) | 0.001 (−0.1; 0.1) | 0.976 | 0.007 (−0.1; 0.1) | 0.868 | 0.006 (−0.1; 0.1) | 0.886 |
| TRL measures | ||||||||||||
| TG (log), mg dL−1 | 2.04 ± 0.18 | 0.021 (−0.01; 0.05) | 2.02 ± 0.14 | −0.009 (−0.03; 0.02) | 2.01 ± 0.16 | −0.005 (−0.02; 0.03) | −0.031 (−0.1; 0.01) | 0.078 | −0.026 (−0.1; 0.01) | 0.152 | 0.004 (−0.03; 0.04) | 0.802 |
| TRLC, mg dL−1 | 29 ± 15 | 1.18 (−0.9; 3.2) | 29 ± 10 | −0.083 (−2.0; 1.9) | 28 ± 11 | −0.057 (−2.1; 2.0) | −1.26 (−4.1; 1.6) | 0.383 | −1.24 (−4.2; 1.8) | 0.417 | 0.026 (−2.8; 2.9) | 0.985 |
| TRLTG (log), mg dL−1 | 1.79 ± 0.29 | 0.053 (0.01; 0.09) | 1.80 ± 0.22 | 0.016 (−0.02; 0.06) | 1.78 ± 0.24 | 0.024 (−0.02; 0.1) | −0.037 (−0.1; 0.2) | 0.205 | −0.030 (−0.1; 0.03) | 0.338 | 0.008 (−0.05; 0.07) | 0.795 |
| NMR TRL particle concentration (nmol L−1) | ||||||||||||
| Total | 164 ± 69 | 0.826 (−11; 13) | 163 ± 49 | −3.15 (−15; 8.3) | 167 ± 56 | 1.42 (−11; 13) | −3.97 (−20; 13) | 0.636 | 0.595 (−17; 18) | 0.946 | 4.57 (−12; 21) | 0.586 |
| Very small | 70 ± 56 | −3.41 (−15; 8.8) | 65 ± 46 | −6.64 (−18; 4.9) | 71 ± 50 | −1.38 (−13; 11) | −3.50 (−20; 13) | 0.679 | 1.76 (−16; 19) | 0.841 | 5.26 (−11; 22) | 0.534 |
| Small | 77 ± 34 | 1.36 (−8.2; 11) | 83 ± 35 | 3.28 (−6.0; 12) | 80 ± 34 | −0.749 (−10; 9) | 1.92 (−11; 15) | 0.777 | −2.11 (−16; 12) | 0.766 | −4.03 (−12; 9.4) | 0.554 |
| Medium | 10.7 (5.5; 19) | 0.014 (−0.29; 0.22) | 10.5 (3.4; 18) | 0.089 (−0.11; 0.29) | 11.8 (7.4; 18) | 0.182 (−0.04; 0.4) | −0.075 (−0.21; 0.36) | 0.606 | 0.167 (−0.14; 0.47) | 0.283 | 0.092 (−0.20; 0.39) | 0.536 |
| Large* | 0.30 (0.0; 3.1) | 0.20† (0.0; 0.6) | 0.10 (0.0; 0.70) | 0.001 (−0.7; 0.1) | 0.35 (−0.02; 4.0) | 0.25 (0.0; 0.6) | −0.190 (−0.7; 0.5) | 0.064 | 0.050 (0.0; 0.1) | 0.847 | 0.240 (0.0; 0.5) | 0.086 |
| Very large* | 0.10 (0.0; 0.1) | 0.10 (0.0; 0.1) | 0.10 (0.0; 0.2) | 0.00 (0.0; 0.1) | 0.10 (0.0; 0.1) | 0.00 (0.0; 0.0) | −0.100 (0.0; 0.1) | 0.909 | −0.100 (0.0; 0.1) | 0.954 | 0.00 (0.0; 0.1) | 0.971 |
| Average NMR TRL particle size (nm) | 40.1 ± 6.9 | 1.09 (−0.07; 2.2) | 38.8 ± 5.6 | −0.305 (−1.4; 0.80) | 39.6 ± 5.8 | 0.963 (−0.21; 2.1) | −1.39 (−3.0; 0.22) | 0.089 | −0.125 (−1.8; 1.6) | 0.884 | 1.27 (−0.34; 2.9) | 0.122 |
| TG/HDL | 2.27 ± 1.48 | 0.295 (−0.13; 0.45) | 2.09 ± 1.07 | 0.026 (−0.13; 0.18) | 2.18 ± 1.12 | 0.157 (−0.01; 0.32) | −0.269 (−0.49; −0.04) | 0.019 | −0.138 (−0.37; 0.10) | 0.253 | 0.131 (−0.10; 0.36) | 0.254 |
NMR HDL measures
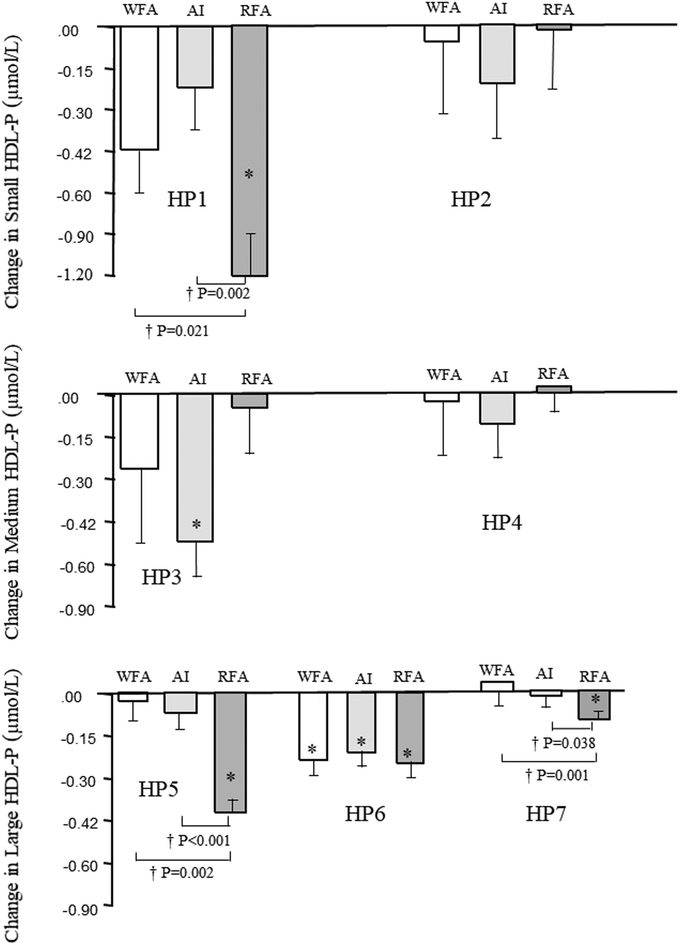
As shown in Table 3, all treatments significantly decreased HDL cholesterol (HDL-c), apolipoprotein A1 (ApoA1), and total HDL particle concentration (HDL-P). The highest decrease in all cases was after RFA treatment, reaching significance versus changes after WFA treatment (P = 0.026, P = 0.032, and P = 0.009, for HDL-c, ApoA1, and HDL-P, respectively). Large HDL-P significantly decreased after WFA and RFA treatments, with the decrease after RFA treatment reaching significance versus changes after WFA and AI treatments (P < 0.001). Medium HDL-P significantly decreased after AI treatment, with the decrease reaching significance versus changes after WFA treatment (P = 0.031) and RFA (P = 0.012). Small HDL-P significantly decreased after RFA treatment, with the decrease reaching significance versus changes after AI treatment (P = 0.039). HDL size significantly decreased after RFA treatment, but without inter-treatment differences. Neither intra- nor inter-treatment changes were observed for small HDL/large HDL or HDL-c/HDL-P ratios.Fig. 1 shows the changes in HDL subspecies after treatments. The significant decrease in HP1, HP5, and HP7 after RFA treatment reached significance versus changes after WFA treatment (P = 0.021, P = 0.002 and P = 0.001 for HP1, HP5, and HP7, respectively) and AI (P = 0.002, P < 0.001, and P = 0.038 for HP1, HP5, and HP7, respectively). HP3 significantly decreased after AI treatment and H6P after all treatments without intertreatment differences. Neither intra- nor inter-treatment changes were observed for H2P or H4P.
NMR triglyceride-rich lipoprotein (TRL) measures
Neither intra- nor inter-treatment changes were observed in TRL measures (Table 3), with the exception of an increase in large TRL particles after WFA treatment (P = 0.015), which reached a borderline significance versus changes after AI treatment (P = 0.064). Although there were no intra-treatment differences, the triglycerides/HDL cholesterol (TG/HDL) ratio decreased after AI treatment versus changes after WFA treatment (P = 0.019) (Table 3).Phenolic biomarkers of dietary adherence and NMR biomarkers
Significant results of the multivariate linear regressions with NMR biomarkers and phenolic biomarkers of dietary adherence are shown in Table 4. Peonidin-3-O-galactoside, a common biomarker for AI and RFA ingestion, shows an inverse relationship with LDLc (β = −5.62, P = 0.011) and total LDL-P (β = −86.4, P = 0.011). Moreover, phloretin-O-xylosyl glucoside and cyanidin-3-O-galactoside compliance biomarkers for RFA and AI intake, respectively, were inversely related to LDL size (β = −0.021, P = 0.028; β = −0.003, P = 0.002, respectively). Also, phloretin-O-xylosyl glucoside was also inversely related to large LDL-P (β = −13.1, P = 0.018), while cyanidin-3-O-galactoside showed a borderline significance (β = −1.22, P = 0.061). Otherwise, peonidin-3-O-galactoside was almost significantly inversely related to the LDL particle/HDL particle ratio (β = −3.23, P = 0.051).| NMR biomarker/compliance biomarker | Compliance biomarker | β | 95%CI | SE | Beta | p |
|---|---|---|---|---|---|---|
| LDLc | ||||||
| Peonidin-3-O-galactoside (plasma), μM | AI and RFA | −5.62 | −9.98 to −1.92 | 2.191 | −0.249 | 0.011 |
| Total LDL particle | ||||||
| Peonidin-3-O-galactoside (plasma), μM | AI and RFA | −86.4 | −152.815 to 20.1 | 33.4 | −0.250 | 0.011 |
| Peonidin-3-O-galactoside (urine), μM | −6.13 | −12.3 to 0.090 | 3.13 | −0.198 | 0.053 | |
| Large LDL particle | ||||||
| Phloretin-O-xylosyl glucoside (urine), mM | RFA | −13.1 | −23.9 to −2.33 | 5.43 | −0.243 | 0.018 |
| Cyanidin-3-O-galactoside (urine), μM | AI | −1.22 | −2.50 to 0.057 | 0.64 | −0.193 | 0.061 |
| LDL particle/HDL particle | ||||||
| Peonidin-3-O-galactoside (plasma), μM | AI and RFA | −3.23 | −6.49 to 18.3 | 1.64 | −0.194 | 0.051 |
| LDL size | ||||||
| Phloretin-O-xylosyl glucoside (urine), mM | RFA | −0.021 | −0.039 to 0.002 | 0.009 | −0.225 | 0.028 |
| Cyanidin-3-O-galactoside (urine), μM | AI | −0.003 | −0.005 to −0.001 | 0.001 | −0.314 | 0.002 |
Changes in other NMR metabolites
WFA and RFA treatments significantly decreased plasma citrate concentrations, with the decreases being significant versus changes after AI treatment (P = 0.048 and P = 0.002, respectively) (Fig. 2). GlycA significantly decreased after AI and RFA treatments, with the decreases reaching significance versus changes after WFA treatment (P = 0.031 and P = 0.030, respectively). The values of alanine significantly decreased after AI and RFA treatments, with the decrease after AI treatment reaching significance versus changes after WFA treatment (P = 0.020) (Fig. 2).In multiple regression analysis, global changes in GlycA were directly associated with medium TRL-P levels in men (R = 0.364, P = 0.016), but not in women, after adjustment for age and BMI values (ESI Fig. 2†). Although no intra-treatment changes were observed in ketone bodies, changes in acetone were significantly higher after AI treatment in comparison with WFA treatment (P = 0.034), whereas the values of β-OH-butyrate and total ketone bodies were significantly lower after RFA treatment versus AI treatment (P = 0.017 and P = 0.033, respectively) (Table 5). No inter-treatment differences were observed for total branched-chain amino acids or the individual ones examined, although significant decreases were obtained in valine values after RFA and AI treatments and in isoleucine after RFA treatment, with a significant increase in leucine after AI treatment (Table 6).
| Treatment | Changes among treatments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | WFA (n = 40) | AI (n = 40) | RFA (n = 39) | AI vs. WFA | RFA vs. WFA | RFA vs. AI | ||||||
| Post-int | Change | Post-int | Change | Post-int | Change | Mean (95%CI) | P | Mean (95%CI) | P | Mean (95%CI) | P | |
| Post-int, post-treatment values. Change, change from the baseline. β-OH-butyrate, beta-OH-butyrate; KedBod, sum of all ketone bodies. Data expressed as mean ± standard deviation or mean (95% confidence interval, CI). Log, logarithm. ANCOVA model adjusted for sex, age, body mass index, and baseline values. | ||||||||||||
| β-OH-butyrate (log), μmol L−1 | 1.87 ± 0.20 | −0.013 (−0.07; 0.05) | 1.95 ± 0.21 | 0.056 (−0.001; 0.11) | 1.84 ± 0.20 | −0.046 (−0.11; 0.01) | 0.069 (−0.01; 0.15) | 0.103 | −0.033 (−0.12; 0.05) | 0.446 | −0.102 (−0.18; −0.02) | 0.017 |
| Acetoacetate (log), μmol L−1 | 1.40 ± 0.37 | −0.036 (−0.12; 0.05) | 1.50 ± 0.26 | 0.045 (−0.04; 0.13) | 1.44 ± 0.22 | −0.043 (−0.13; 0.04) | 0.081 (−0.04; 0.20) | 0.187 | −0.007 (−0.13; 0.12) | 0.917 | −0.088 (−0.21; 0.03) | 0.152 |
| Acetone (log), μmol L−1 | 1.32 ± 0.21 | −0.076 (−0.18; 0.03) | 1.43 ± 0.28 | 0.084 (−0.02; 0.19) | 1.34 ± 0.26 | −0.042 (−0.15; 0.07) | 0.161 (0.01; 0.31) | 0.034 | 0.034 (−0.12; 0.19) | 0.634 | −0.127 (−0.28; 0.02) | 0.095 |
| KetBod (log), μmol L−1 | 2.11 ± 1.80 | 0.008 (−0.04; 0.06) | 2.16 ± 0.19 | 0.045 (−0.05; 0.10) | 2.07 ± 0.15 | −0.035 (−0.09; 0.02) | 0.037 (−0.03; 0.11) | 0.311 | −0.043 (−0.12; 0.03) | 0.270 | −0.080 (−0.15; −0.01) | 0.033 |
| Treatment | Changes among treatments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | WFA (n = 40) | AI (n = 40) | RFA (n = 39) | AI vs. WFA | RFA vs. WFA | RFA vs. AI | ||||||
| Post-int | Change | Post-int | Change | Post-int | Change | Mean (95%CI) | P | Mean (95%CI) | P | Mean (95%CI) | P | |
| Post-int, post-treatment values. Change, change from the baseline. vs., versus. Val, valine; Leu, leucine; Ileu, isoleucine; BCAA, sum of branched amino acids (Val + Leu + Ileu). Data expressed as mean ± standard deviation or mean (95% confidence interval, CI). Log, logarithm. ANCOVA model adjusted for sex, age, body mass index, and baseline values. | ||||||||||||
| Val, μmol L−1 | 232 ± 41 | −7.43 (−16; 1.1) | 236 ± 43 | −10.3 (−18; −2.1) | 239 ± 34 | −10.9 (−20; −2.1) | −2.89 (−15; 9.0) | 0.631 | −3.44 (−16; 9.1) | 0.586 | −0.551 (−12; 11) | 0.928 |
| Leu, μmol L−1 | 148 ± 30 | 7.70 (−0.91; 14) | 154 ± 33 | 7.69 (1.1; 14) | 148 ± 26 | 0.818 (−6.1; 7.7) | −0.013 (−9.5; 9.5) | 0.998 | −6.88 (−17; 3.0) | 0.170 | −6.87 (−16; 2.6) | 0.155 |
| Ileu, μmol L−1 | 57 ± 13 | −0.855 (−4.7; 3.0) | 61 ± 21 | −2.21 (−5.9; 1.5) | 58 ± 14 | −3.89 (−7.8; −0.01) | −1.36 (−6.7; 4.0) | 0.617 | −3.04 (−8.6; 2.5) | 0.282 | −1.68 (−7.0; 3.7) | 0.535 |
| BCAA, μmol L−1 | 436 ± 79 | −0.522 (−18; 16) | 452 ± 96 | −3.65 (−20; 13) | 445 ± 68 | −13.2 (−31; 4.2) | −3.13 (−27; 21) | 0.795 | −12.7 (−37; 12) | 0.316 | −9.52 (−33; 14) | 0.432 |
Discussion
Results of this study showed that RFA and AI, as ACN-rich products, decreased LDLc, ApoB, total, large, and small LDL-P, LDL size, and the TG/HDL ratio, and increased medium LDL-P. All treatments significantly decreased HDLc, ApoA1, and total HDL-P, the greater decreases being after RFA treatment. After RFA intake, there were also significant decreases in large HDL-P (and concomitantly in H5P and H7P subspecies), which were significant versus changes after the other two treatments. Medium HDL-P (and concomitantly H3P subspecies) after AI treatment decreased significantly versus WFA changes. The decrease in small HDL-P after RFA treatment was reflected in the significant decrease of HP1 subspecies versus the other two treatments. ACN-rich products decreased GlycA and alanine, and increased acetoacetate, versus changes after WFA treatment. WFA and RFA treatments decreased plasma citrate concentrations versus changes after AI treatment. Volunteer compliance was high, as indicated by the increase in peonidin-3-O-galactoside after RFA and AI treatments, with undetectable levels after WFA treatment. Phloretin-2′-O-glucuronide as an intake biomarker of apple snacks increased after RFA and WFA treatments, being non-detectable after AI treatment. These findings support the reliability of these results, demonstrating that the observed effects are attributable to the dietary interventions.In agreement with other reports, in our study, the ACN-rich products decreased LDL-c,5,11 as well as other LDL related parameters, which are considered to be better markers for CVD such as LDL-P, particularly when hypertriglyceridemia is involved.18 LDL-c, Apo B, total LDL-P, and the LDL-P/HDL-P ratio have all been shown to be directly associated with the risk of coronary heart disease (CHD).11,19 Concerning LDL-P heterogeneity, although controversial data exist, an abundance of small LDL-P has been associated with a 2–3-fold increase in CHD risk in a primary prevention population and is linked to atherosclerosis in many conditions, such as hyperlipidemia, metabolic syndrome, diabetes, and other disorders.18 The need to monitor the small LDL-P concentration is reflected by many guidelines, such as the 2016 Chinese guidelines and 2019 ESC/EAS guidelines.20 In contrast, in type 2 diabetes mellitus individuals, medium LDL-P has been inversely associated with all-cause mortality, but not with CVD mortality,21 and higher concentrations of large LDL-P have been associated with a lower risk of developing diabetes.22 Although LDL size has previously been inversely related to CHD, this association does not remain after adjustment for LDL-P.23 The atherogenicity of small LDL-P seems to be associated with an increased cellular uptake in the arterial tissue due to a higher affinity for the LDL binding site, as has been shown in experimental studies.24 Thus, overall changes in NMR LDL biomarkers after RFA and AI treatments, as ACN-rich products, improved CVD risk versus changes observed after WFA consumption.
Epidemiologic studies have consistently shown that low serum HDLc is a risk factor for atherosclerotic cardiovascular disease. This concept, however, has failed to be translated into clinical benefits in terms of drug development. Recent studies also suggest that very high HDLc levels can be associated with adverse cardiovascular outcomes and all-cause mortality risk in coronary artery disease individuals.25 HDL comprises a family of lipoproteins whose individual particles differ widely in density, size, charge, protein, and lipid composition, and it is still unclear which HDL specific subclasses or subspecies are more cardioprotective.26,27 In most population studies, small HDL particles are considered to be more strongly associated with an increased CHD risk than the large HDL ones.28,29 Controversial data exist, however, concerning the associations between large and medium HDL-P subclasses and cardiovascular risk.21,30 Therefore, we observed opposite potential effects concerning HDL subclasses in our study. On one hand, the highest decrease in small HDL-P after RFA treatment appears as a protective one; on the other hand, the large and medium HDL-P decreases after the consumption of ACN-rich products versus WFA treatment could exert an opposite effect, although this remains to be elucidated. Decreases in large and medium HDL particles have been observed in the postprandial state after ingestion of black rice fortified with ACNs.31
The controversy surrounding the role of HDL subclasses in CVD has evolved to focus on HDL subspecies differentiation27 for clarifying the role of the different HDL lipoproteins. From our data, the decrease in small HDL-P after RFA treatment is mainly dependent on that of H1P subspecies; the decrease of medium HDL after AI treatment is linked to the H3P ones; and that in large HDL-P after RFA treatment is linked to all H5P, H6P and H7P subspecies; however, significance versus WFA and AI treatments was dependent on the decrease of H5P and H7P subspecies. H7P has been shown to be directly related to interferon gamma, PCSK9 (an HDL proteome component linked to accelerated atherosclerosis),32 and a higher DNA methylation phenotypic age.33 Thus, in this sense, the decrease in H7P after RFA would have a protective character versus CVD risk.
An increase in several proatherogenic subclasses of very low-density lipoproteins (VLDL) after aronia sustained consumption has been recently reported.34 In our study, however, we observed a trend toward a better TRL profile of large particles after AI treatment. All TRL particles have been shown to be directly associated with GlycA, an inflammatory marker,35,36 and TRLs together with GlycA mainly account for myocardial dysfunction in type I diabetes subjects.37 In this study, a decrease in GLycA after the consumption of both ACN-rich products was observed, and changes in GlycA were directly related to the levels of medium TRL particles in men. GlycA is a composite biomarker of systemic inflammation, which reflects the degree of glycosylation of various acute phase proteins. The observed decrease of GlycA after consuming ACN-rich products agrees with our previous data concerning the decrease in inflammatory markers (IL6, CRP and the complement system) observed in the frame of the AppleCOR study.38 Beyond capturing cardiovascular risk, increases in GlycA have been associated with mortality, chronic inflammatory-related severe hospitalization, cancer incidence, and incidence of type 2 diabetes, revealing GlycA as a global marker for cardiometabolic risk.39 In our study, the TG/HDL ratio was lower after AI treatment versus WFA treatment. This ratio has been directly related to hypertension, particularly in women with a low BMI and individuals with type 2 diabetes, and with cardiovascular events and death.39
Concerning other NMR markers, citrate, the first product following acetyl coenzyme A generation from different energy sources, has been directly associated with cardiovascular and all-cause mortality,40 atrial failure,41 and mortality in acute heart failure patients.42 Recently, metabolomic data from the Framingham Study showed an inverse relationship between blood citrate levels and the “ideal cardiovascular health” index.43 In our study, both apples, WFA and RFA, decreased plasma citrate concentrations versus AI treatment. This reinforces the importance of apple pulp consumption for obtaining benefits concerning reduction in citrate levels. Alanine levels decreased after AI and RFA treatments. In previous studies with CVD patients, it was difficult to establish whether the increased levels of alanine observed were predictors or a consequence of the disease.44,45 In a recent cohort study, however, alanine levels were higher in diabetic patients and directly associated with the development of atherosclerotic disease after a 10-year follow-up.46 Increased levels of circulating BCAAs are associated with type 2 diabetes.47 Anthocyanins have been shown to decrease BCAAs in Zucker diabetic fatty rats.48 In our study, we observed intra-treatment decreases in valine and isoleucine after the consumption of anthocyanin-rich products, as well as an increase in leucine after AI treatment. The lack of inter-treatment differences, however, impairs any conclusive results.
Comparison between the two ACN-rich treatments showed that for LDL measures, AI was more effective than RFA in decreasing total and LDL cholesterol, ApoB, total LDL-P and the LDL-P/HDL-P ratio, whereas RFA was more effective in decreasing s-LDL-P and in causing a smaller decrease in large LDL-P. Concerning HDL measures, AI promoted less decrease of HDL-c, ApoA1, and total, large, and medium HDL-P, whereas a higher decrease in the small HDL-P subclass and the H7P subspecies was observed after RFA treatment. AI promoted a better large TRL-P profile, as well as a higher decrease in the TG/HDL-C ratio, than RFA. RFA decreased citrate versus AI, and AI was more effective than RFA in decreasing alanine. RFA decreased beta-OH-butyrate and total ketone bodies compared to AI, but the role of ketone bodies in health and disease remains to be elucidated.49 Thus, both types of ACN-rich products were effective on different lipoprotein particle or other CVD biomarkers, and although AI showed a larger spectrum of benefits than RFA, the latter has benefits on key parameters such as small LDL-P and HDL-P.
Conclusions
In summary, an anthocyanin-rich diet was inversely associated with potentially pro-atherogenic lipoprotein profiles characterized by increased numbers of large TRL and small LDL and HDL lipoprotein particles. The observed decrease in the H7P subfraction can be considered to be protective against an atherogenic phenotype. Also, ACN-rich products decreased GlycA and alanine levels. Our findings also highlight the beneficial role of apple consumption in reducing plasma citrate levels, which has implications for metabolic health. The assessment of volunteers’ compliance throughout intake biomarker analysis reinforces the reliability of these results, demonstrating that the observed effects are attributable to the dietary interventions. Overall, the consumption of ACN-rich products was more protective against CVD risk factors compared to the consumption of WFA. Notably, the comparison between the two ACN-rich products revealed distinct benefits, suggesting that the matrix in which anthocyanins are consumed may influence their metabolic effects and health outcomes. To the best of our knowledge, this is the first report where the association between sustained consumption of ACN-enriched food and NMR lipoprotein particle concentration in humans is described.Author contributions
Conceptualization: M. J. Motilva, R. Solà, R. M. Valls, E. Llauradó, and A. Pedret; formal analysis: A. Pedret, S. Fernández-Castillejo, M. Sampson, and A. T. Remaley; funding acquisition: M. J. Motilva, R. Solà, R. M. Valls, and A. Pedret; methodology: A. Pedret, J. Companys, L. Calderón-Pérez, E. Llauradó, L. Pla-Pagà, P. Salamanca, B. A. Sandoval-Ramírez, Ú. Catalán, S. Fernández-Castillejo, I. Ludwig, A. Macià, M. Besora-Moreno, R. M. Valls, L. Rubió-Piqué, M. Sampson, A. T. Remaley, M. J. Motilva and R. Solà; writing – original draft: A. Pedret, R. Solà, M. Sampson, and A. T. Remaley; review and editing: A. Pedret, E. Llauradó, J. Companys, L. Calderón-Pérez, L. Pla-Pagà, P. Salamanca, B. A. Sandoval-Ramírez, Ú. Catalán, S. Fernández-Castillejo, I. Ludwig, M. Besora-Moreno, A. Macià, R. M. Valls, L. Rubió-Piqué, M. Sampson, A. T. Remaley, M. J. Motilva and R. Solà.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This Applecor study was funded by Ministerio de Economía, Industria y Competitividad, the Agencia Estatal de Investigación (AEI), and the European Regional Development Fund (ERDF) (grant number: AppleCOR Project, AGL2016-76943-C2-2-R). The NFOC-Salut group is a consolidated research group of Generalitat de Catalunya, Spain (2014 SGR 873, 2017 SGR 522 and 2021 SGR 817). We also want to thank the Calidad Pascual company, Bezoya (Aranda de Duero, Burgos, Spain) for providing the mineral water necessary for the preparation of the AI.References
- X. Wang, Y. Ouyang, J. Liu, M. Zhu, G. Zhao, W. Bao and F. B. Hu, Br. Med. J., 2014, 349, g4490 CrossRef PubMed.
- A. Micek, J. Godos, D. Del Rio, F. Galvano and G. Grosso, Mol. Nutr. Food Res., 2021, 65(6), e2001019 CrossRef PubMed.
- Y. Zhao, D. Li and T. Huang, Atherosclerosis, 2023, 365, 1–8 CrossRef CAS PubMed.
- F. Araya-Quintanilla, A. Beatriz-Pizarro, W. Sepúlveda-Loyola, J. Maluf, L. Pavez, J. F. López-Gil and H. Gutiérrez-Espinoza, Eur. J. Nutr., 2023, 62, 1923–1940 CrossRef CAS PubMed.
- H. H. Jang, I. G. Hwang and Y. M. Lee, Front. Nutr., 2023, 15(10), 1207751 CrossRef PubMed.
- A. Merecz-Sadowska, P. Sitarek, T. Kowalczyk, K. Zajdel, M. Jęcek, P. Nowak and R. Zajdel, Nutrients, 2023, 15(13), 3016 CrossRef CAS PubMed.
- A. Vogiatzoglou, A. A. Mulligan, M. A. H. Lentjes, R. N. Luben, J. P. E. Spencer, H. Schroeter, K. T. Khaw and G. G. C. Kuhnle, PLoS One, 2015, 10(5), e0128132 CrossRef PubMed.
- J. E. Mayer, W. H. Pfeiffer and P. Beyer, Curr. Opin. Plant Biol., 2008, 11, 166–170 CrossRef CAS PubMed.
- N. Deacon , Redfleshed apples: their diversity: DIVERSITY website, https://www.suttonelms.org.uk/apple104.html, (accessed 16 April 2024).
- J. Rienks, K. J. Penczynski, S. Schmitting, A. E. Buyken and U. Nöthlings, Br. J. Nutr., 2020, 124, 1198–1206 CrossRef CAS PubMed.
- T. C. Wallace, M. Slavin and C. L. Frankenfeld, Nutrients, 2016, 8(1), 32 CrossRef PubMed.
- S. Yuste, I. A. Ludwig, L. Rubió, M. P. Romero, A. Pedret, R. M. Valls, R. Solà, M. J. Motilva and A. Macià, J. Funct. Foods, 2019, 55, 146–155 CrossRef CAS.
- F. Mach, C. Baigent, A. L. Catapano, K. C. Koskinas, M. Casula, L. Badimon, M. J. Chapman, G. G. De Backer, V. Delgado and B. A. Ference, Eur. Heart J., 2020, 41, 111–188 CrossRef PubMed.
- M. J. Alegre, C. I. Vallbona, C. E. Roure and F. M. Violan, Guía de prescripció d'exercici físic per a la salut (PEFS), 2007 Search PubMed.
- K. Ameta, A. Gupta, D. Ameta, R. Sethi, D. Kumar, I. Ahmad and A. A. Mahdi, Clin. Chim. Acta, 2016, 456, 56–62 CrossRef CAS PubMed.
- A. Macià, M. P. Romero, S. Yuste, I. Ludwig, A. Pedret, R. M. Valls, P. Salamanca, R. Solà, M. José Motilva and L. Rubió, Food Chem., 2022, 384, 132612 CrossRef PubMed.
- A. Pedret, Ú. Catalán, S. Fernández-Castillejo, M. Farràs, R. M. Valls, L. Rubió, N. Canela, G. Aragonés, M. Romeu, O. Castañer, R. De La Torre, M. I. Covas, M. Fitó, M. J. Motilva and R. Solà, PLoS One, 2015, 10(6), e0129160 CrossRef PubMed.
- H. Superko and B. Garrett, Biomedicines, 2022, 10(4), 829 CrossRef CAS PubMed.
- B. T. Steffen, W. Guan, A. T. Remaley, P. Paramsothy, S. R. Heckbert, R. L. McClelland, P. Greenland, E. D. Michos and M. Y. Tsai, Arterioscler., Thromb., Vasc. Biol., 2015, 35, 448–454 CrossRef CAS PubMed.
- S. Ma, M. Xia and X. Gao, Front. Cardiovasc. Med., 2021, 8, 681444 CrossRef CAS PubMed.
- R. Li, J. X. Chen, Q. Lu, T. T. Geng, P. F. Xia, Y. Wang, L. K. Chen, Z. L. Shan, A. Pan and G. Liu, Diabetes, Obes. Metab., 2023, 25, 3259–3267 CrossRef CAS PubMed.
- S. Sokooti, J. L. Flores-Guerrero, H. J. L. Heerspink, M. A. Connelly, S. J. L. Bakker and R. P. F. Dullaart, Cardiovasc. Diabetol., 2021, 20(1), 156 CrossRef CAS PubMed.
- K. El Harchaoui, W. A. van der Steeg, E. S. G. Stroes, J. A. Kuivenhoven, J. D. Otvos, N. J. Wareham, B. A. Hutten, J. J. P. Kastelein, K. T. Khaw and S. M. Boekholdt, J. Am. Coll. Cardiol., 2007, 49, 547–553 CrossRef CAS PubMed.
- N. Galeano, M. Ai-Haideri, F. Keyserman, S. Rumsey and R. Deckelbaum, J. Lipid Res., 1998, 39, 1263–1273 CrossRef CAS.
- C. Liu, D. Dhindsa, Z. Almuwaqqat, Y. A. Ko, A. Mehta, A. A. Alkhoder, Z. Alras, S. R. Desai, K. J. Patel, A. Hooda, M. Wehbe, L. S. Sperling, Y. V. Sun and A. A. Quyyumi, JAMA Cardiol., 2022, 7, 672–680 CrossRef PubMed.
- A. O. Akinkuolie, N. P. Paynter, L. Padmanabhan and S. Mora, Circ. Cardiovasc. Qual. Outcomes, 2014, 7, 55–63 CrossRef PubMed.
- W. S. Davidson, A. L. Cooke, D. K. Swertfeger and A. S. Shah, Curr. Atheroscler. Rep., 2021, 23(6), 23 CrossRef CAS PubMed.
- J. D. Otvos, D. Collins, D. S. Freedman, I. Shalaurova, E. J. Schaefer, J. R. McNamara, H. E. Bloomfield and S. J. Robins, Circulation, 2006, 113, 1556–1563 CrossRef CAS PubMed.
- L. Ahsan, W. Q. Zheng, G. Kaur, A. Kadakuntla, A. T. Remaley, M. Sampson, P. Feustel, A. Nappi, S. Mookherjee and R. Lyubarova, Am. J. Cardiol., 2023, 203, 212–218 CrossRef CAS PubMed.
- R. W. McGarrah, M. Ferencik, S. N. Giamberardino, U. Hoffmann, B. Foldyna, J. Karady, G. S. Ginsburg, W. E. Kraus, P. S. Douglas and S. H. Shah, J. Am. Heart Assoc., 2023, 12(1), e026662 CrossRef PubMed.
- S. J. L. Ou, D. Yang, H. P. Pranata, E. S. Tai and M. H. Liu, npj Sci. Food, 2023, 7(1), 59 CrossRef PubMed.
- M. D. Shapiro, H. Tavori and S. Fazio, Circ. Res., 2018, 122, 1420–1438 CrossRef CAS PubMed.
- L. R. Ortiz-Whittingham, Y. Baumer, A. P. S. Pang, M. Sampson, A. S. Baez, R. R. Rose, S. H. Noonan, J. Mendez-Silva, B. S. Collins, V. M. Mitchell, M. A. Cintron, N. Farmer, A. T. Remaley, M. J. Corley and T. M. Powell-Wiley, Psychoneuroendocrinology, 2023, 157, 106346 CrossRef CAS PubMed.
- S. Lackner, A. Mahnert, C. Moissl-Eichinger, T. Madl, H. Habisch, N. Meier-Allard, C. Kumpitsch, T. Lahousen, A. Kohlhammer-Dohr, S. Mörkl, H. Strobl and S. Holasek, Microbiome, 2024, 12(1), 49 CrossRef CAS PubMed.
- F. Y. Cesena, G. Generoso, R. D. Santos, A. C. Pereira, M. J. Blaha, S. R. Jones, P. P. Toth, P. A. Lotufo, M. S. Bittencourt and I. M. Benseñor, J. Clin. Lipidol., 2023, 17, 261–271 CrossRef PubMed.
- J. Moreno-Vedia, R. Rosales, E. Ozcariz, D. Llop, M. Lahuerta, M. Benavent, R. Rodríguez-Calvo, N. Plana, A. Pedragosa, L. Masana, A. Castro, D. Ibarretxe and J. Girona, Front. Endocrinol., 2022, 12, 775677 CrossRef PubMed.
- C. Puig-Jové, J. Julve, E. Castelblanco, M. T. Julián, N. Amigó, H. U. Andersen, T. S. Ahluwalia, P. Rossing, D. Mauricio, M. T. Jensen and N. Alonso, Cardiovasc. Diabetol., 2022, 21(1), 257 CrossRef PubMed.
- A. Pedret, J. Companys, L. Calderón-Pérez, E. Llauradó, L. Pla-Pagà, P. Salamanca, B.-A. Sandoval-Ramírez, Ú. Catalán, S. Fernández-Castillejo, S. Yuste, A. Macià, L. Gutiérrez-Tordera, M. Bulló, J. Camps, N. Canela, R. M. Valls, L. Rubió-Piqué, M. J. Motilva and R. Solà, Food Funct., 2024, 15(11), 5825–5841 RSC.
- K. A. Riggs, P. H. Joshi, A. Khera, J. D. Otvos, P. Greenland, C. R. Ayers and A. Rohatgi, Am. J. Prev. Cardiol., 2022, 12, 100373 CrossRef PubMed.
- K. Fischer, J. Kettunen, P. Würtz, T. Haller, A. S. Havulinna, A. J. Kangas, P. Soininen, T. Esko, M. L. Tammesoo, R. Mägi, S. Smit, A. Palotie, S. Ripatti, V. Salomaa, M. Ala-Korpela, M. Perola and A. Metspalu, PLoS Med., 2014, 11(2), e1001606 CrossRef PubMed.
- M. Bulló, C. Papandreou, J. García-Gavilán, M. Ruiz-Canela, J. Li, M. Guasch-Ferré, E. Toledo, C. Clish, D. Corella, R. Estruch, E. Ros, M. Fitó, C. H. Lee, K. Pierce, C. Razquin, F. Arós, L. Serra-Majem, L. Liang, M. A. Martínez-González, F. B. Hu and J. Salas-Salvadó, Metabolism, 2021, 125, 154915 CrossRef PubMed.
- S. Stryeck, M. Gastrager, V. Degoricija, M. Trbušić, I. Potočnjak, B. Radulović, G. Pregartner, A. Berghold, T. Madl and S. Frank, Sci. Rep., 2019, 9(1), 6743 CrossRef PubMed.
- Y. Li, A. Gray, L. Xue, M. G. Farb, N. Ayalon, C. Andersson, D. Ko, E. J. Benjamin, D. Levy, R. S. Vasan, M. G. Larson, J. Rong, V. Xanthakis, C. Liu, J. L. Fetterman and D. M. Gopal, J. Am. Heart Assoc., 2023, 12(12), e028022 CrossRef PubMed.
- K. Ameta, A. Gupta, D. Ameta, R. Sethi, D. Kumar, I. Ahmad and A. A. Mahdi, Clin. Chim. Acta, 2016, 456, 56–62 CrossRef CAS PubMed.
- S. Rizza, M. Copetti, C. Rossi, M. A. Cianfarani, M. Zucchelli, A. Luzi, C. Pecchioli, O. Porzio, G. Di Cola, A. Urbani, F. Pellegrini and M. Federici, Atherosclerosis, 2014, 232, 260–264 CrossRef CAS PubMed.
- J. Moreno-Vedia, D. Llop, R. Rodríguez-Calvo, N. Plana, N. Amigó, R. Rosales, Y. Esteban, J. Girona, L. Masana and D. Ibarretxe, Cardiovasc. Diabetol., 2023, 22(1), 249 CrossRef CAS PubMed.
- N. Friedrich, J. Endocrinol., 2012, 215(1), 29–42 CAS.
- K. Chen, X. Wei, J. Zhang, R. Pariyani, J. Jokioja, M. Kortesniemi, K. M. Linderborg, J. Heinonen, T. Sainio, Y. Zhang and B. Yang, J. Agric. Food Chem., 2020, 68(35), 9436–9450 CrossRef CAS PubMed.
- P. Puchalska and P. A. Crawford, Annu. Rev. Nutr., 2021, 41, 49–77 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02949f |
| ‡ Current address: Center for Nutrition Research, University of Navarra, Pamplona, Spain. |
| § Senior authors. |
| This journal is © The Royal Society of Chemistry 2025 |