 Open Access Article
Open Access ArticleSpiers Memorial Lecture: A retrospective view on the non-classical features revealed by advanced imaging of biominerals
Laurie
Gower
 *
*
Department of Materials Science & Engineering, University of Florida, Gainesville, FL, USA. E-mail: lgower@mse.ufl.edu
First published on 14th May 2025
Abstract
Biominerals have unique morphologies and complex hierarchical microstructures, so the study of biomineralization has benefited greatly from the development of advanced microscopy and characterization tools. In my career, I witnessed a revolutionary change in the theories relating to biomineral formation mechanisms. While much of this was due to the advancements in imaging techniques, I present an argument to suggest that in vitro model systems played an important role in steering the biomineral community toward resolving the non-classical crystallization processes that are now understood to lie at the foundation of biological calcification processes. This retrospective review will discuss two case studies that are classic examples of biominerals, mollusk nacre for the invertebrates, and bone for the vertebrates. It will therefore be biased given my group's discovery of the Polymer-Induced Liquid-Precursor (PILP) process, which serendipitously emulated the morphologies and textures of these (and other) biominerals. The goal, however, is not to repeat that body of literature, but rather to demonstrate how the use of model systems has helped decipher mineralization mechanisms, and to propose new ideas that could be explored to further advance the field.
1 Introduction
When I first entered the biomineralization field as a graduate student in 1990, I must admit, I was mainly attracted to the visually appealing microscopy aspect of this field. Having grown up in Florida with a hobby of collecting shells, it was especially fascinating to learn that shells and marine invertebrates have beautiful internal microstructures as well. And as I became a materials scientist, discovering that those microstructures serve a functional purpose was just icing on the cake. This of course relates to the Materials Science & Engineering (MSE) paradigm: processing determines structure → structure determines properties → properties determine performance → performance determines application. From a biologist's perspective, performance underlies potential evolutionary success, so they also have great interest in understanding how these complex structures are processed. Thus, a primary goal when developing advanced imaging techniques is to build an understanding of the biological processing that occurs during the complex interplay between matrix formation and mineral deposition. But there are inherent limitations of microscopy in studying dynamic biological processes because one typically has to resort to a set of snapshots throughout the process. This is no means a comprehensive historical review, and I apologize to those who's valuable papers I didn't cover. I am simply recalling the papers that stood out the most in my memory, and that means this review will be from a biased perspective.Much of the early biomineral literature was focused on demonstrating the fascinating morphologies and microstructures of biologically-controlled mineralizations, and as such, advancements in microscopy played a key role in illustrating the various enigmatic features of biominerals. For example, how do mollusks make the aragonite in nacre in the form of flat tablets and films, yet synthetic aragonite forms spherulitic bundles of needles? Or why is high Mg-bearing calcite commonly found in the shells of marine invertebrates (e.g. 30% in red coralline algae),1 yet calcium carbonate that is precipitated in the beaker switches over to the aragonite phase above around 8% Mg?2,3 The enigma in vertebrates was how do nanocrystals of hydroxyapatite (HAp) end up INSIDE collagen fibrils in bone,4–6 yet HAp formed in the presence of collagen in vitro simply generated a spherulitic crust on the surface of the matrix? But the thing that stands out the most in my memory, and which was often referred to as the hallmark of biominerals, was how do invertebrates create such complex non-equilibrium morphologies? By this I mean, how are single crystals of calcite created with smoothly curved surfaces, which should be highly energetically unfavorable, rather than a faceted crystal habit? For example, in Weiner & Addadi's 1987 paper, they describe the skeletal elements of the sea urchin as follows: “Their natural surfaces display a glassy conchoidal fracture rather than the normal smooth (104) cleavage of synthetic calcite.” When I joined the field as a grad student in the early 90s, the most widely accepted hypothesis was that organisms might use a set of species-specific proteins that bind to stereospecific crystal faces during crystal formation, thereby altering the growth rates of the concomitant crystallographic directions, leading to the altered crystal “habit”.7–9 After all, crystal additives are commonly used in industrial processing for that purpose, and proteins, given their highly-specific molecular recognition sites, could presumably do so in an even more complex fashion.
An important finding back in the day was that many biomineral proteins are highly enriched with acidic amino acid residues,10 and given their intimate association with the biomineral (even being occluded within),11 these intracrystalline soluble acidic proteins were considered to play a key role in modulating biomineral formation. It therefore became popular to extract these intracrystalline proteins to examine their interactions with crystals grown in vitro, to try and correlate with biological features.11,12 Indeed, that was how my career started in the field, where the plan was to use tailor-made peptides designed to bind to specific crystallographic faces. But that project became side-tracked when I noticed some unusual features (helices and films)13 produced by the negative control reaction, which used the simple additive of polyaspartic acid (Na-salt) to mimic the Asp-rich proteins found associated with biominerals. In situ observations using a polarized light microscope ultimately led to the discovery of what I called the polymer-induced liquid-precursor (PILP) process.14 Although I do not intend for PILP to be the focus of this paper, it certainly did alter my perception of the micrographs being shown in the biomineral literature. In fact, all those enigmatic features that the biomineral literature had been pondering started to make perfect sense from this new perspective. This will be illustrated with two case studies of the most popularly studied biominerals: (1) nacre, being carefully studied because of its remarkable fracture toughness,15 was nicely amenable to the application of the continually evolving tools for advanced microscopy; and (2) bone, being heavily studied because of its importance to human health, required a different set of microscopy skills because of its complex hierarchical structure. Some of this may be familiar to those who have read my group's papers, but these case studies are presented differently, more of an exposition of how the tools led, or misled, the field of biomineralization. In addition, I offer many suggestions for experiments that I think would be valuable to move the field forward. I no longer have a lab to perform such experiments since I semi-retired in 2021, but I am happy to assist others in a consulting capacity.
2 Case studies
2.1 A case study in invertebrates: the nacre story
Much of the early biomineral imaging was focused on mollusk nacre because of its fascinating “brick-n-mortar” microstructure. Quite a few of the outstanding microscopists studying nacre were from Japan (names like K. Wada,16 Hiroshi Nakahara,17 Normitsu Watabe,18 come to mind), which I guess was because of its importance to the pearl industry, and the nationalistic pride in various aspects of marine biology. Scientists began developing clever ways to stop the biomineral reactions midway in order to examine formation mechanisms. The papers I started collecting in my grad school days showed scanning electron micrographs (SEM) of nacreous tablets which curiously expressed a variety of species-specific morphologies during the formation stage, ranging from perfectly round disks to well-faceted rhomboids and pseudohexagons (aragonite is orthorhombic) (Fig. 1).18–22 When fully formed, the crystals all end up as flat “tablets”, unlike the needle-like morphology expected for aragonite, or rhombic shapes expected for calcite (which is found in bryozoan semi-nacre). In those earlier days, the flat tabular morphology was thought to arise from disrupted growth along the c-axis from protein adsorption on the basal planes (a version of the selective adsorption hypothesis). But as images of forming nacre started showing continuous interlamellar organic sheets, the concept shifted toward deposition of sheets/membranes that confined crystals to planar growth. | ||
| Fig. 1 Scanning electron microscopy has been particularly useful for demonstrating the various morphologies of forming nacre tablets. (A) The pseudo-hexagonal shape is a commonly observed morphology. In this example of sheet nacre, a couple of “screw dislocations” can be seen. However, these presumed dislocations are clearly far more than a molecular layer in thickness. (B) Circular tablets are not uncommon. (C) A top-down image of columnar nacre shows somewhat irregular tablets at the growing surface which become well-faceted tablets below. (D) Tablets sometimes have rhombic-shaped outlines but are confined to two-dimensions. (E) These rounded tablets exhibit layers with an interesting central mound. (F) These rhomboid tablets with truncated corners are beginning to merge into a continuous sheet. (A) Reproduced from ref. 19, Copyright 1971, with permission from Trans. Am. Microsc. Soc. (B) Reproduced from ref. 18, Copyright © 1974, Springer Nature Limited. (C) Reproduced from ref. 20, Copyright 2010, with permission of Springer Nature. (D) and (E) Reproduced from ref. 21. Copyright 2012, MDPI. (F) Reproduced from ref. 22, Copyright © 2008 Elsevier Inc. All rights reserved. | ||
Atomic force microscopy (AFM) had been gaining momentum in the 90s, and in vitro crystallization studies were now resolving nanoscale crystal growth steps and the influence of molecular and protein additives.23–26 In 1997, Schaffer et al.27 employed AFM on biomineral tissues by capitalizing on the clever “flat pearl” approach developed earlier by Fritz et al. (1994),28,29 which provided flat nacre surfaces accessible to AFM. They found a porous network within the interlamellar organic sheets and proposed the concept of mineral bridges providing interconnectivity of crystal orientation within columns of tablets (Fig. 2),27 as opposed to the prevailing view of hetero-epitaxial nucleation (a loosely applied definition of epitaxy based on charge matching between protein, or chitin, and specific crystallographic planes).30 With the advent of focused ion beam (FIB) tomography, Checa et al. studied nacre with the slice-and-view method to determine the 3D arrangement of pores and interconnectivity.31 Their study also supported the mineral bridge hypothesis, although they argued that the interconnectivity occurs at larger holes (150–200 nm) that appeared to be ruptures in the interlamellar membrane, which they hypothesized might arise due to “differences in osmotic pressure across it when the interlamellar space below becomes reduced at an advanced stage of calcification.”
 | ||
| Fig. 2 As questioned by Schaffer et al.,27 “Does abalone nacre form by heteroepitaxial nucleation or by growth through mineral bridges?” (A) This SEM image of forming columnar nacre shows nicely preserved interlamellar organic sheets, which have generally been considered as the basis of membranous compartments that restrict mineral growth to two-dimensional tablets. (B) In a nice use of AFM, nanopores were demonstrated within the fibrous interlamellar membrane. (C) Buds of newly forming tablets appear to provide “bridges” to the next mineral layer. Note – the organic membrane appears to be “draped” over the underlying layers which contain the pyramid of newly forming tablets. (A) Reproduced from ref. 27, Copyright 1997, with permission from ACS Publications. | ||
As exemplified in these papers, it was generally accepted that the tabular morphology of nacre is caused by constrained growth within compartments created by the interlamellar sheets. For example, Schaffer et al.27 described the overall bridging process as follows: “each newly nucleated tablet grows vertically toward the mantle, until it hits the next interlamellar sheet, where vertical growth is terminated… once the growing tablet hits a pore in the next interlamellar sheet above the tablet (mantle side), it grows through that pore as a mineral bridge, to nucleate a new tablet.”
The concept of nacre formation being constrained to thin tablets within membrane bound compartments was/is a reasonable hypothesis based on “snapshots” of forming nacre. But when the PILP model system serendipitously produced very similar tablets (Fig. 3), some rounded, some hexagonal (Fig. 3F and H),32–34 but all with a thickness (≈500 nm) similar to nacre/seminacre, it prompted me to take a second look at this literature, but from a new perspective based on this two-step precursor process I had observed in vitro.14 It made sense (to me) that this similar thickness in both nacre (from mollusks) and seminacre (from bryozoan organisms), as well as the PILP system, was not simply coincidence, but that the amount of amorphous precursor generated would depend on the given supersaturation one can achieve in a CaCO3 solution, and thus the amount of ions that can be sequestered by a given amount of polymer/protein within a given small volume of solution would naturally lead to a similar thickness. Others were not convinced, I think because this model system was too simplistic, especially since it only used a polypeptide and not an actual biomineral protein. Therefore, I was tickled to see Belcher et al.’s study using extracted nacre proteins,35 which in vitro led to thin film morphologies practically identical to the PILP system (Fig. 4). Most people's attention, however, was focused on the crystal phase switching, and not similarity to PILP. Nevertheless, these random splotches of CaCO3 films, produced by Belcher's proteins as well as PILP, obviously lack the organization that would presumably be provided by the organic matrices in biomineralization. This brings to mind the concept of compartmentalization, but that will be addressed a little later.
 | ||
| Fig. 3 A comparison of the internal features found in nacre versus PILP-formed tablets. Both are ≈ half a micron thick, appear to be single-crystalline, and yet have a variety of internal substructures. (A) This is a calcareous sponge spicule (not nacre), but I wanted to include Sethmann's image of nanogranular texture since it was the first time I saw such a thing. (B) Rousseau et al.'s52 AFM image is of a single-crystalline nacre tablet, and the phase contrast nicely highlights the organic matter intercalated between nanograins. (C) Our group also found a nanogranular texture in PILP formed tablets and films. Such a texture seems to indicate that PILP does not stay pure liquid for long; yet such coalescence would not be expected for solid nanoparticles, thus implying the PILP phase becomes gel-like. (D) Rousseau's52 high magnification SEM image revealed nanoscale sub-layers within a nacreous tablet. (E) We used AFM to reveal nanoscale sub-layers in a PILP formed tablet. (F) Polarized light microscopy (with gypsum 1st-order red retardation plate) of a pseudo-hexagonal “tablet” forming within an ACC film, shows a uniform extinction direction, indicating it is single crystalline. The SAED spot pattern further confirmed its single-crystalline nature, and that it was the aragonite phase. (G) Seminacre (from Bryozoans) was frequently found to exhibit a preferential etching pattern of alternate sectors within the hexagonal tablets of calcite. Nacre tablets also show preferential etching, but along orthorhombic crystallographic sectors (bottom illustration). (H) A similar pattern of alternate sectors was revealed with optical microscopy of forming tablets (not from etching). In the central hexagonal tablet, one has to extrapolate that “wrinkly” transition bars, which are created by excluded polymer (demonstrated with fluorescently tagged polymer), would lead to more defective sectors, and thus might be preferentially etched under dissolution conditions in old shells. (I) Li et al.57 used in situ dynamic atomic force microscope observations of stressed nacre tablets and found partial rotation and deformation of the nanograins. (J) As nicely illustrated in a review by Sun et al.,56 this has important implications for mechanical properties of nacre, which seems to benefit from a multitude of hierarchical structural features. (A) Reproduced from ref. 51, Copyright © 2008 Elsevier Ltd. All rights reserved. (B) and (D) Reproduced from ref. 52, Copyright © 2005 Elsevier Ltd. All rights reserved. (C) Reproduced from ref. 33, Copyright 2007, American Chemical Society. (E) and (F) Reproduced from ref. 32. Copyright 2007, with permission from American Chemical Society. (G) Reproduced from ref. 54. Copyright 1995, Courtesy of JSTOR from this site: https://www.jstor.org/stable/pdf/1542305.pdf. (H) Reproduced from ref. 34, Copyright 2008, with permission from American Chemical Society. (I) Reproduced from ref. 57, Copyright © 2006, American Chemical Society. (J) Reproduced from ref. 56, Copyright 2012, with permission from the Royal Society of Chemistry. | ||
 | ||
| Fig. 4 Comparison between CaCO3 films produced by the PILP process using polyaspartate (Na-salt) additive (A) and (B) versus CaCO3 films produced using proteins extracted from nacre (C) and (D). (A) and (B) The PILP CaCO3 films are typically about a half a micron in thickness, which is an ideal thickness for examining with polarized light microscopy. The color gradients arise from interference colors across variations in thickness, but upon rotation, the whole film patch exhibited a single-crystalline extinction direction. (C) and (D) Belcher's paper on “Control of crystal phase switching and orientation by soluble mollusc-shell proteins”35 was primarily focused on how the proteins caused a shift from calcite to aragonite phase. However, it is notable that these extracted proteins, when used as additives with CaCO3 precipitated in vitro, led to single crystalline patches of calcite film with non-equilibrium morphologies, very similar to the PILP model system (A) vs. (C). The polycrystalline patches of film contained a centralized 3D aggregate, which appeared to somehow modulate the orientation of the underlying single-crystalline “tablets” into a radiating pattern (B) vs. (D). (A) and (B) Reproduced from ref. 34, Copyright © 2008, American Chemical Society. (C) and (D) Reproduced from ref. 35, Copyright © 1996, Springer Nature Limited. | ||
About 5 years after I published the first PILP paper, the concept of “non-classical crystallization” processes was introduced to the biomineral community.36 I wish I had thought to use that terminology, but I think it came about from Helmut Cölfen's discovery of mesocrystal assembly.37,38 Soon thereafter, I discovered the work by Peter Vekilov, who had been examining non-classical two-step crystallization processes that occurred for some organic molecules and proteins, where the first step entailed liquid–liquid phase separation.39–44 Such a crystallization seemed analogous to the PILP process, which also entailed a first step of liquid–liquid phase separation; however, there was a key difference in that his organic crystals nucleated and grew within the precursor phase, rather than retaining the shape of the precursor, as occurs for the inorganics in the PILP reaction.
Cölfen's studies on mesocrystal assembly were soon followed by the extremely popular concept of oriented attachment.36,45–48 While those studies presented an interesting nanoscale phenomenon, it seemed unlikely (to me) to lead to large scale structures, so I wasn't convinced that it had anything to do with biomineralization. And especially since, over the years, my group had been finding a variety of internal features of PILP formed tablets/films that serendipitously matched internal features of nacre tablets (Fig. 3A–H). In 2005, I was excited to see the Sethmann et al.49 paper that described a nanotexture in biominerals that appeared to result from nano-clustered calcite growth (Fig. 3A).49–51 We had started using AFM and sure enough, our smooth single-crystalline PILP films had a nanocluster texture as well (Fig. 3C).33 We even observed nanoscale sublayers within the single-crystalline aragonite tablets (Fig. 3E),32 similar to those Rousseau et al.52 had resolved in nacre tablets (Fig. 3D). The “transition bars” we discovered from in situ observations of the amorphous-to-crystalline transformation (see Fig. 5)14,53 matched the natural etching patterns seen in alternate sectors of nacre and seminacre54 (one of those enigmatic features) (Fig. 3G vs. H).53 I felt that such distinct nanoscale textures even more strongly supported my hypothesis that nacre tablets are formed by a similar precursor process because, as any materials scientist knows, defect textures in crystalline materials are related to their formation mechanism. Therefore, I started referring to these various textures as “mineralogical signatures” because such features could potentially be used for identifying the various non-classical crystallization processes.34,55 In addition to understanding formation mechanisms, nanotextures, like microstructures, are undoubtedly important with respect to mechanical properties. Sun et al.56 gave an excellent review on this, which highlighted the remarkable study by Li et al.,57 who used in situ dynamic atomic force microscopy to demonstrate partial rotation and deformation of nanograins during stress applied to isolated nacre tablets (Fig. 3H–J).
Interesting biomineral textures had been observed for some time, such as in the naturally etched sectors of nacre (Fig. 3G),54 or the deliberate etching techniques designed to reveal the substructure of nacre tablets (Fig. 5A–F).58 An impressive study by Aizenberg et al.,59 back in 1995, used synchrotron radiation to measure crystal textures (coherence lengths and angular spreads) in calcitic sponge spicules whose morphologies do not reflect the hexagonal symmetry of calcite. They argued that because the reconstructed shapes of the reduced symmetry domains matched the spicule specific morphologies, that this supported the prevailing hypothesis of that time of physical or stereochemically driven adsorption of proteins to specific crystal planes. But when my group discovered the phenomenon of transition bars in the PILP system (Fig. 5G–J), I offered an alternative explanation for the crystallographic location of the intercalated proteins. I suggested it could be due to exclusion of proteinaceous impurities from the crystallization front, which naturally occurs along specific crystallographic planes,14 or in concentric bands with radial spherulitic growth, as we demonstrated with fluorescently tagged polymer (Fig. 5J).53 Of course proteins might selectively bind to specific crystal faces (or step edges, as was later shown with AFM24,26), but this would presumably have to occur as they are being excluded since crystal faces are not even present during deposition of the amorphous precursor phase. This is just one example that illustrates the value of an in vitro model system towards understanding the relationship of developmental processing → structural features.
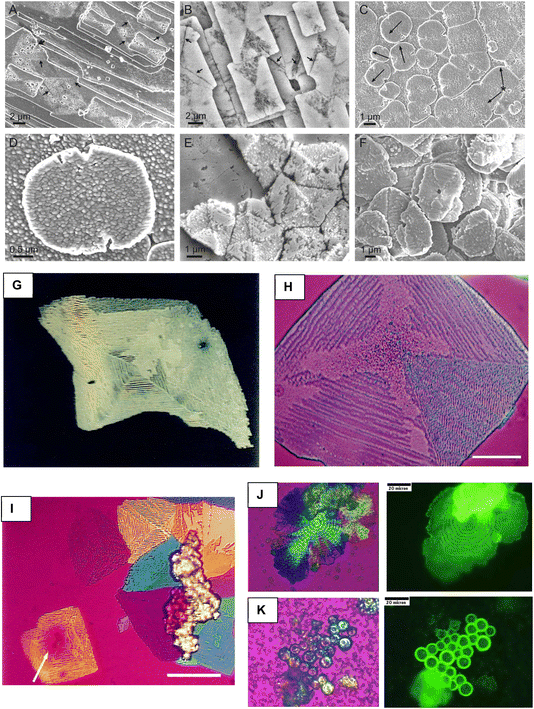 | ||
Fig. 5 Correlations between transition bars and crystallographic features. (A)–(F) Checa et al.58 developed some useful etching techniques to reveal the substructure of nacre tablets by differential removing of material with variable solubility. (A)–(D) Nacre tablets treated with protease reveals alternate sector patterns in the rectangular tablets of Pinna nobilis ((A) and (B); SE vs. BE), and vermiculations in the tablets of Pteria hirundo ((C) and (D)). Given the etchant was protease, this indicates there was more protein in the preferentially etched sectors. The vermiculations were described as being parallel to the crystallographic a-axis of aragonite, while the triangles were aligned with the b-axis, corresponding to the advance of the {010} faces during the growth of the tablet. (E) and (F) In contrast, pronounced relief etching was created using sodium hypochlorite and Mutvei's solution (a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of glutaraldehyde and 1% acetic acid to which alcian blue is added), which revealed strong sectorization patterns in tablets from Mytilus edulis (E) and Gibbula umbilicalis ((F); Mutvei etch only). Linear striations could also be seen in these (which somewhat resemble the pattern of transition bars). (G) We used polarized light microscopy to examine the PILP reactions in situ, where this example shows the rhombic symmetry (in a 2D film) expected for calcite. The transition bars eventually crystallized to yield a single-crystalline “tablet” of calcite. (H)–(K) A gypsum 1st-order red retardation λ-plate was used to examine the amorphous-to-crystalline transformation. Isotropic regions, including the glass background, appear magenta color (1st order red). (H) This tablet is magenta because it is oriented along calcite's isotropic c-axis, but the wavy blue texture in the wrinkled sector on the bottom right indicates a wavering orientation, highlighting an interesting defect texture that we speculated was caused by shrinkage compressive stresses because it always occurs in narrower sectors. (I) This example shows a prevalence of sectorization, where the sectors are clearly related to the crystallography of the transforming calcite crystals. (J) Spherulitic growth in 2D films leads to concentric laminations rather than sectorization, which is clearly related to the radial crystal growth direction. The fluorescence image of the same region (right) revealed the fluorescently tagged polymer was being excluded into the transition bar regions. This then provides a reason as to why crystallization is delayed in the bar regions. (K) In 3D globules, the polymer is heavily enriched in the outer shell, and presumably concentrated there during the exclusion of impurities during crystallization. Therefore, it was speculated that the spacing between bars (or concentric laminations) may be diffusion limited, and thus might relate to the viscosity of the precursor phase. If that is the case, many pathological deposits, which have concentric laminations at the nanoscale, might be more viscous, implying phase separation of a more gelatinous phase. (A)–(F) Reproduced from ref. 58, Copyright © 2013 Elsevier Inc. All rights reserved. (G)–(I) Reproduced from ref. 14, Copyright © 2000 Elsevier Science B.V. All rights reserved. (J)–(K) Reproduced from ref. 53, Copyright © 2008, American Chemical Society. 1 mixture of glutaraldehyde and 1% acetic acid to which alcian blue is added), which revealed strong sectorization patterns in tablets from Mytilus edulis (E) and Gibbula umbilicalis ((F); Mutvei etch only). Linear striations could also be seen in these (which somewhat resemble the pattern of transition bars). (G) We used polarized light microscopy to examine the PILP reactions in situ, where this example shows the rhombic symmetry (in a 2D film) expected for calcite. The transition bars eventually crystallized to yield a single-crystalline “tablet” of calcite. (H)–(K) A gypsum 1st-order red retardation λ-plate was used to examine the amorphous-to-crystalline transformation. Isotropic regions, including the glass background, appear magenta color (1st order red). (H) This tablet is magenta because it is oriented along calcite's isotropic c-axis, but the wavy blue texture in the wrinkled sector on the bottom right indicates a wavering orientation, highlighting an interesting defect texture that we speculated was caused by shrinkage compressive stresses because it always occurs in narrower sectors. (I) This example shows a prevalence of sectorization, where the sectors are clearly related to the crystallography of the transforming calcite crystals. (J) Spherulitic growth in 2D films leads to concentric laminations rather than sectorization, which is clearly related to the radial crystal growth direction. The fluorescence image of the same region (right) revealed the fluorescently tagged polymer was being excluded into the transition bar regions. This then provides a reason as to why crystallization is delayed in the bar regions. (K) In 3D globules, the polymer is heavily enriched in the outer shell, and presumably concentrated there during the exclusion of impurities during crystallization. Therefore, it was speculated that the spacing between bars (or concentric laminations) may be diffusion limited, and thus might relate to the viscosity of the precursor phase. If that is the case, many pathological deposits, which have concentric laminations at the nanoscale, might be more viscous, implying phase separation of a more gelatinous phase. (A)–(F) Reproduced from ref. 58, Copyright © 2013 Elsevier Inc. All rights reserved. (G)–(I) Reproduced from ref. 14, Copyright © 2000 Elsevier Science B.V. All rights reserved. (J)–(K) Reproduced from ref. 53, Copyright © 2008, American Chemical Society. | ||
As a side note (unrelated to nacre), spherulitic growth led to exclusion of impurities into concentric rings (Fig. 5J and K);53,60 notably, concentric layering is a common feature exhibited by many pathological biomineral deposits (e.g., Randall's plaque61 and kidney stones62).63 Thus, this model system offers an alternative hypothesis to the often prevailing view that concentrically laminated ultrastructures are caused by deposition of daily growth rings.
At some point in my career, given that similar external tabular morphologies and internal defect textures both resulted from the precursor process, it seemed likely (to me) that the key modulatory role of the infamous, soluble acidic proteins was as a process-directing agent.34 By this I mean that the inhibitory activity of the charged polymer disrupted the classical nucleation and growth process to yield a non-classical two-step precursor process. A few years after the PILP discovery, it started to become recognized that proteins with a large amount of charged residues tend to be intrinsically disordered proteins (IDPs).64,65 Indeed, many of the soluble biomineral proteins, which are notoriously known for being highly charged, have now been shown to be IDPs, or at least have domains of disorder.66–69 So it became more reasonable to accept that a simple polypeptide of polyaspartate, and even non-biological polymers such as polyacrylic acid, could actually serve as a reasonable mimic to these IDPs involved in biomineralization, because now we are talking about interactions between a flexible polyelectrolyte and ion clusters, rather than a molecular recognition “lock-n-key” mechanism found in globular proteins. The latter, being a historical pillar of molecular biology, made the PILP process a difficult concept for the biomineral community to accept as being potentially relevant to biological processing.
As it became apparent that the PILP system was able to emulate so many of the enigmatic biomineral features,34 beyond what could be coincidence, people started to take notice. Biomineral microscopists started searching for the elusive amorphous phase (first in bone, see Case Study 2). For nacre, this took some advanced tools given that ACC does not stay amorphous for long. In 2005, Nassif et al.70 used high resolution TEM to find ACC in the region surrounding a tablet (Fig. 6A and B). People were not fully convinced due to the problems with applying TEM to beam sensitive materials, which is the case for ACC, and especially biogenic ACC. Ten years later, Gilbert's group made direct observation of ACC precursors in nacre using synchrotron spectromicroscopy (Fig. 6C–H).71 Her group's development of advanced spectroscopic tools, such as the photoemission electron spectromicroscopy (PEEM) and X-ray absorption near-edge structure (XANES) spectroscopy, have been revolutionary to the biomineral field. In addition to mapping phases, these tools have provided a means to identify proto organization of precursor phases. Surprisingly, in the nacre case,71 PEEM did not indicate a proto-aragonite precursor, as one would expect for nacre. They also found the ACC to be short-lived, and suggest this is because the ACC crystallizes via iso-epitaxy on the underlying mineral bridge. This was a fantastic study, but I would have liked to have seen a control ACC spectrum prepared by a PILP method, rather than ACC formed by some non-physiological conditions. One has to wonder how the ordering within these proto phases would be affected by polymer/protein sequestered ion clusters. After all, Belcher et al. had already shown in 1996 that the soluble polyanionic proteins extracted from abalone shell could cause crystal phase switching.35 So combining these advanced tools with a controllable model system might provide more mechanistic insight.
 | ||
| Fig. 6 Evidence that nacre forms from an amorphous phase. (A) and (B) Nassif et al.70 used high resolution TEM to find ACC at the outer rim of forming nacre tablets, which seems to suggest that ACC is a short-lived phase under the nacre forming conditions. (C) and (D) The XPEEM study by DeVol et al.71 illustrated the composition of various intermediate phases during nacre development. As described by the authors, this is “a component map of a nearby fractured cross-section obtained from stacks of PEEM images acquired across the Ca L-edge, after embedding, polishing, and coating.” The bottom row shows the four spectral components used for component analysis. (A) and (B) Reproduced from ref. 70, Copyright 2005, National Academy of Sciences. (C) and (D) Reproduced from ref. 71, Copyright 2015, with permission from American Chemical Society. https://pubs.acs.org/doi/10.1021/jacs.5b07931. Further permissions related to the material excerpted should be directed to the ACS. | ||
A couple years later, in 2017, Macías-Sánchez did a very detailed study on the nanoglobular structures within immature tablets of gastropod nacre (Fig. 7).72 Surprisingly, their Fast Fourier Transform (FFT) analysis demonstrated “complex digitiform shapes” of the nanoglobules (Fig. 7A–C). While the individual fingers constituted the crystalline cores of nanogranules, they were found to be largely co-oriented, suggesting that they must connect in the third dimension. Based on the compositional changes going from the amorphous to crystalline phase (Fig. 7D–I), they concluded that “the final nanogranular structure observed is produced during the transformation of ACC into aragonite.” The authors argued that their observations did not support the oriented attachment mechanism proposed by Zhang and Xu in 2013,73 who had found similarly misoriented nanodomains in the nacre of a bivalve. While I agree about the unlikelihood of oriented attachment, I don't fully agree with their description of the amorphous-to-crystalline transformation, where they state the following: “This is only possible through the interface-coupled dissolution–precipitation process, which implies the existence of a fluid phase (partly resulting from H2O released during the transformation of ACC into aragonite). In this way, reshaping of the overall nanogranular structure of grains takes place by regrowth of the crystalline phase (which can proceed via a classic layer-by-layer mechanism), until the nanograins acquire their final shapes, sizes (up to one order of magnitude bigger than the precursor ACC nanoparticles) and arrangements. This mechanism leads to pseudomorphs, thereby preserving the nanogranular structure imprinted during the early stages of nacre.”72 I understand why one would conclude that water must be necessary to enable molecular motions, but we have observed that PILP films, which were still amorphous when removed from solution, went ahead and crystallized after air drying. So just the small amount of water within the precursor film (produced at 4 °C) seemed sufficient to enable a pseudomorphic transformation. It's possible this fits with the author's description (the caveat that mentions water in the amorphous phase), but I've seen too many people arguing in favor of dissolution and recrystallization in bulk solution, so it would be nice if there was more precise wording that could discriminate between the various mechanisms, given that my description of a pseudomorphic transformation14 was apparently not sufficient. In addition, I do have to wonder how the interfaces, which as the authors describe become coated with proteins during their exclusion from crystallization, will have enough surface exposure to be available for dissolution and recrystallization. At the very least, it's hard to imagine it being uniform. Here is yet another example of where an in vitro model system, which leads to very similar outcomes and textures as the biomineral, and which can provide control over select variables, could be used to obtain a better understanding of the A-to-C transformation.
 | ||
| Fig. 7 Highlights from an in depth analysis of immature tablets forming in gastropod nacre. (A) TEM of an agglomerate comprised of nanocrystal cores embedded within amorphous phase. (B) FFT analysis shows small arcs, indicating slight misalignments between nanodomains. (C) An RGB color model built on planar reflections revealed the “complex digitiform shapes” of the nanoglobules. The individual fingers constituted the crystalline cores of nanogranules, which were found to be largely co-oriented. (D) HAADF image shows bright nanoglobules along a dark line across multiple forming tablets, as can be seen by the interlamellar membrane (ILM) separating them. (E) SE imaging shows protruding bumps, referred to as hillocks. (F) AFM phase imaging demonstrates a more adhesive material overlies the crystals. (G) In SE SEM imaging, the steep bumps appear as white dots. (H) and (I) STEM-EELS line scan showed distinct and progressive changes in the C K-edge from crystalline to amorphous areas. Reproduced from ref. 72. Copyright 2017, The Authors. | ||
While I was happy to see an amorphous precursor finally revealed in nacre, this was only half the story in my mind as I am particularly interested in the fluidity of the precursor. Therefore, I want to return to the initial discussion on compartmentalization. If my explanation for the thickness of the mineral layers being related to the given supersaturation and sequestration by polymer was correct, then I wondered – is it even necessary for those interlamellar sheets to form a compartment? Of course organic layers may provide biomechanical benefits, and I agree that the porosity and mineral bridging seems to enable interconnectivity of crystals across the columnar layers (as does tessellation in the layering of sheet nacre); but I was still not convinced the matrix is preformed, as suggested by the Schaffer et al. paper,27 and further supported by Gehrke et al.'s clever experiment of retrosynthesis of tablets within a mineral depleted nacre matrix.74 Those were certainly excellent studies; but there were some things that didn't quite add up. For one thing, I couldn't imagine how the network of “screw dislocations” found in seminacre (Fig. 8A and B) could be formed within a set of layered preformed compartments. Secondly, when Yao et al. resolved the large network of tessellations in abalone nacre (Fig. 8C–F),75 that would seemingly throw a wrench into the idea of preformed compartments as well. Thirdly, and most convincingly (to me), was the fabulous set of micrographs in an old 1979 paper by Nakahara (Fig. 9).76 This paper is hard to find as an electronic copy, but it's so valuable, I keep sharing images I had acquired years ago.
 | ||
| Fig. 8 Spiral growth of thin tablets and layers would seemingly throw into question the hypothesis of organic compartments constraining growth to create the thin tabular morphology. (A) and (B) The spiral steps caused by “screw dislocations” in seminacre create disorganized layers, as shown here at low and high magnification. (C) Nacre also has “screw dislocations” that can lead to a spiral “motion”, referred to here as tesselation. Yao et al.75 used electron microscopy, ion microscopy, and an in situ nano-manipulator to determine that there are ∼106 screw dislocations per square centimeter in abalone (columnar) nacre. (D) The layers between I to II can be clearly seen as arising from a gradual incline. Again, it is hard to imagine how a compartment could be involved, at least not over long range. (E) Bright-field TEM shows dark bands which are platelets of the same crystal orientation (reversed contrast), where the different widths indicate that the centers of those platelets are not vertically coaxial. (F) FIB milling was used to track through a screw dislocation core. This side view nicely shows the gradual incline of the layer. The authors suggest that the lamellar layers of aragonite propagate via a large number of continuous spiral growth domains as the “stacks of coins” become confluent. Again, such a spiral motion seems counterintuitive to the concept of compartments controlling tablet morphology. (G) In some PILP tablets/films, there was a gradual thickening that created microlevel steps, which can be nicely visualized in PLM as higher order interference color (white patches). Unfortunately, we didn't do SEM on those steps, so one has to use their imagination to see how the gradual thickness increase might be similar to the gradual thickness increase seen in nacre layers (D) & (F). (H) The tight spiral “screw dislocations”, as seen in (A) and (B), were not seen in the PILP system, but we did wonder if the large 3D helical growths were caused by spiral steps in the membrane overlying the spherulites, causing them to twist into helices. (A) and (B) Reproduced from ref. 54. Copyright 1995, Courtesy of JSTOR from this site: https://www.jstor.org/stable/pdf/1542305.pdf. (C–F) Reproduced from ref. 75, Copyright © 2006, The Materials Research Society. (G) Reproduced from ref. 34. Copyright © 2008, American Chemical Society. (H) Reproduced from ref. 13. Copyright 1998, with permission from Elsevier Science B.V. All rights reserved. | ||
 | ||
| Fig. 9 From Nakahara's paper “An electron microscope study of the growing surface of nacre in two gastropod species, Turbo cornutus and Tegula pfeifferi”.76 (A) SEM of gastropod columnar nacre with preserved interlamellar sheets. In a typical SEM view, it appears that tablets are formed within compartments. (B) TEM cross-section that still contains some pyramids of dark mineral tablets. Note the stack of organic sheets at the top. The pyramid on the left has a forming tablet that has separated one additional sheet layer ahead of the pyramid on the right. (C) Different magnifications of the preserved organic matrix only, again showing the stacks of organic sheets at the uppermost surface. If the image had been taken at the red box, it would appear that tablets were forming within preformed compartments. But the images below, that are zoomed in on the top of a couple of pyramidal stacks, show irregular mineral starting to push up and separate the bottom sheet of the stack. (D) Zoomed in at high magnification, one can see bits of mineral (the remnant holes) that appear to have “infiltrated” several sheets while still building up mineral tablets below. (A) Reproduced from ref. 76, Copyright 1979, with permission from Springer Nature. | ||
Nakahara describes the process as follows,77 “In bivalve nacre, each sheet is formed independently within the extrapallial fluid, while in the gastropod a relatively thick surface sheet forms as a first deposit of organic structure, and then thin, ordinary sheets separate one by one from the surface sheet to form regularly spaced compartments.” As can be seen in his TEM cross-sectional images of forming nacre, the stacks of organic sheets do not start out as preformed compartments of tabular thickness (Fig. 9A and B),76 but instead, each sheet appears to get separated from the stack as it is pushed upward by small bits of mineral until it reaches its final thickness (Fig. 9C and D). Meanwhile, the mineral is simultaneously expanding laterally until running into the neighboring column's tablet to form a mineral filled lamellar “compartment”. Note – a zoomed in region (such as the red box added to Fig. 9C-top) would seemingly appear to show sheets organized as preformed compartments (as often shown in the literature); but one needs to step back to lower magnification to see that each sheet is separated only up to the level of the uppermost sheet being infiltrated with mineral, beyond which the organic sheets are still present within the stacked sheets (Fig. 9C-bottom). This may seem a trivial point, but my reason for bringing this up (beyond highlighting the importance of taking representative images) relates to this “infiltration” mechanism. These images are perhaps suggestive of infiltration via capillary action of a liquid-like precursor, where the precursor could be drawn through the pores into the narrow spaces between stacked sheets, where it would expand the “compartment” until it reaches the natural tabular thickness (∼500 nm, as found in the PILP model system). Perhaps there are analogies to intrafibrillar collagen mineralization in bone (see next Case study).
Let's consider the alternative hypothesis based on ion diffusion. Ions would have to diffuse from the extrapallial fluid and across the porous interlamellar sheets, given that there are presumably no ion pumping channels (like one might expect in a cell membrane). The extrapallial space below the mantel epithelium is very small (Fig. 10), estimated by Checa et al. (from TEM images of bivalve sheet nacre) as being on the order of 100 nm thick.31 This brings a memory to mind, back when I was a graduate student presenting my initial PILP observations in a poster at the 1996 Gordon conference on Biomineralization. A highly esteemed biomineral expert came to my poster and said he liked the PILP concept because he had always wondered how enough ions can be provided to a growing biomineral formed within a membrane-bound compartment (such as urchin spicules, coccoliths, etc.) given that there is usually very little space, and thus solution volume, and thus ion content, between the forming mineral and surrounding membrane. He liked the idea that a highly ion-enriched PILP phase could be slathered onto a growing biomineral for rapid growth. In the case of nacre, I wonder if some theoretical calculations could determine the rate at which ions would have to be flowing from the cells into the limited extrapallial volume, without going above crashing out supersaturation, relative to the mineral deposition rate of forming nacre. This could be compared to the addition of an inhibitory polymer that stabilizes the solution against classical nucleation, while sequestering ions into an ion rich phase for rapid mineral deposition.
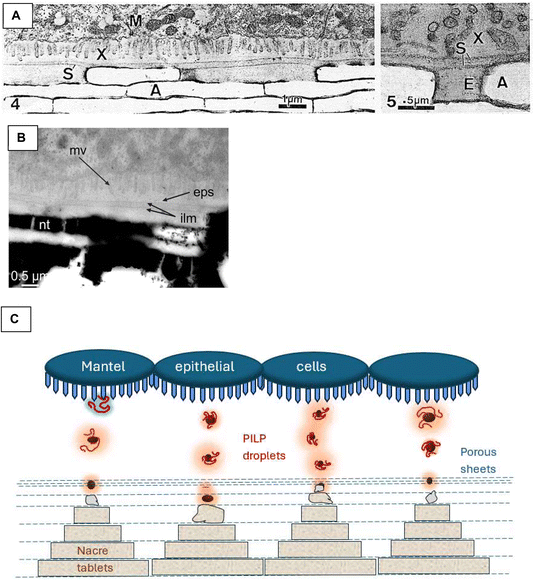 | ||
| Fig. 10 The extrapallial space and compartmentalization concepts. These images are from bivalves sheet nacre formation; I haven't been able to find similar micrographs of gastropod (columnar) nacre. (A) Nakahara used a uranyl acetate–lead citrate double stain, which dissolves away the aragonite crystals, A, but shows nice detail of the cellular and organic constituents.76 The extrapallial space, X, is below the mantel, M, in which the microvilli of the epithelial cells descend into the extrapallial fluid. In the right image, an “envelope” surrounding the forming tablets is highlighted. (B) Checa's31 TEM of bivalve nacre has retained the nacre tablets, nt. Again, the extrapallial space, eps, is still very small, as seen between the microvilli, mv, and interlamellar membrane, ilm. (C) A schematic to illustrate my alternate hypothesis on nacre formation: the overlying mantel epithelial cells secrete proteins into the narrow extrapallial space; the charged IDPs sequester ion clusters to form PILP-like droplets; strands of precursor phase infiltrate via the porous membrane (perhaps by capillary action) to fill the interlamellar “compartment” with amorphous precursor; lateral growth ensues while the precursor phase rapidly solidifies and crystallizes, yielding a variety of 2D morphologies until they merge with the neighboring tablets to form continuous lamellae of mineral; exclusion of impurities leads to organics between tablets, as well as transition bar occlusions within the nacre crystals; solidification of precursor phase around a variety of organics (fibers, sheets) produces a “fuzzy” interface that provides enhanced mechanical properties. (A) Reproduced from ref. 76, Copyright 1979, with permission from Springer Nature. (B) Reproduced from ref. 31, Copyright 2018, Open Access from Frontiers. | ||
The porosity of the interlamellar membranes was described by Schaffer et al.27 as a statistical random network, so one might ask – what would create the relatively uniform lateral spacing between the columns of tablets? I suspect this arises from the spacing between the overlying cells in the mantel epithelium, from which the proteins are secreted into the extrapallial space. In the PILP model system, we have observed whitish streaks in the solution from vertical “strands” of connected precursor droplets sinking from below their point of formation at the air/water interface near the ammonium carbonate diffusion membrane.78 So it is not hard to imagine that vertical strands of precursor droplets might deposit directly below each epithelial cell as the secreted proteins induce the aggregating precursor colloids (Fig. 10C). I think the cellular dimensions are commensurate with the spacing between nacre columns, but I would love for a microscopist to see if this hypothesized cell/column alignment is found in the biological system. Direct proof of a PILP type mechanism would be difficult if not impossible to obtain in vivo, but the value of the in vitro model system becomes clear; direct observations could be obtained, which then offer new hypotheses of potential mechanisms.
Thus far, I've not talked about sheet nacre (in bivalves), which forms quite differently than columnar nacre (in gastropods). Rousseau et al. used a novel approach to examine forming sheet nacre from both the mantle side and nacre side by using a methacrylate fixative to fracture the two sides.79 Their focus was on the Voronio model of tablet tiling, but I was fascinated to see the rounded mineral tablets which appear as whitened patches that gradually materialize within the sheet (Fig. 11A and B). Notably, the Raman peaks are the same for the brightened patches and whatever goo is surrounding them (Fig. 11C and D), suggesting a similar composition. So perhaps the goo is composed of some densifying ACC/organic matrix, with excluded organics ultimately leading to the inter-tabular and -lamellar membranes. The authors concluded that “a viscous fluid is necessary to shape the compartment and define its thickness”. In light of what is known from the PILP model, it isn't hard to envision that such a sheet could be a PILP type film (but containing a large amount of biogenic matrix), within which the densification would emerge during an amorphous to crystalline transformation. I would love to see a more in-depth compositional study, which could provide information about this unique mineralizing goo. According to a 1996 paper on sheet nacre, the organic matrix was described as containing a silk-like protein, along with chitin, and an assemblage of hydrophilic glycoproteins that are rich in aspartic acid.80 I'm not sure why silk fibroin or chitin, neither of which are soluble in water, would form a hydrogel, so I assume the Asp rich glycoproteins were responsible. In addition, Mary Marsh had a series of papers back in the 80s that discussed the prevalence of phosphoproteins in the extrapallial fluid.81,82 Notably, we found that phosphorylated peptides were highly effective at promoting the PILP process, even at low molecular weight (unpublished results). Therefore, it wouldn't be surprising that these Asp- and phospho-rich glycoproteins could induce a PILP-like amorphous precursor. These intriguing sheet nacre observations lead to an interesting question – what would happen to a PILP-like precursor that is deposited onto a relatively hydrophobic matrix?
 | ||
| Fig. 11 Rousseau et al.79 examined the formation of sheet nacre using a unique approach, where they separated the shell side from the mantle side, which had been hardened with methacrylate, to reveal features of the deposited sheets. (A) On the mantle side of the fracture surface, a stairs-like growth front is seen, which as the authors describe, results from fractures occurring along lines where the layers contained more mineralized tablets and thus were becoming rigid. Bright round patches are seen to be gradually evolving from within a preformed sheet, where the brightness is caused by the higher Z-contrast of mineral. (B) On the shell side of the fracture surface, the tablets have thickened into cylinders. At this maturity level, much of the organic matrix remained attached in the region surrounding the tablets. A fibrous texture is present, presumed to be chitin based. (C) and (D) Raman spectra taken of the starred regions seem to show the same peaks, but with more background noise on the surrounding film, along with what appears to be a broad peak next to the 705.3 peak. Surprisingly, the peaks seem the same in these two different regions. Perhaps the same mineral peak is just due to the depth of penetration reaching into the underlying mineral layer; but I still would have expected a more dominant set of peaks from organics. (A) Reproduced from ref. 79, Copyright © 2004 Elsevier Inc. All rights reserved. | ||
Hovden et al.83 employed a wedge-polishing technique adapted from the semiconductor industry to prepare a large electron-transparent region that encompassed the whole prismatic-to-nacre transition zone in Pinna nobilis, which is a bivalve that forms sheet nacre. They observed aggregation of nanoparticles (50–80 nm) within an organic matrix that arranges in fibre-like polycrystalline configurations before particle accretion, as seen in early stage nacre (Fig. 12). I found this presentation to be confusing, because the authors conclude that “the particle number increases successively and, when critical packing is reached, they merge into early-nacre platelets.” But I don't think one can infer that a transition zone in space is the same as a transition in time. Even though it is the direction of the nacre formation front, this is an interface where the cell secretions are switching over to produce two very different compositions and phases, so amorphous nanoparticles, while not yet in the zone to be incorporated into a tablet, will appear polycrystalline at this later timepoint after sample preparation. An alternative interpretation of these images could be that there is “condensation” or nucleation of some mineral phase on the fibrous matrix that is still lingering from the prismatic zone (the authors even suggest it is the same organic matrix). Given that those fibrous paths end in the regions of disordered tablets (Fig. 12C), it’s possible that this matrix is actually hindering the ability for these mineral precursors to fully densify into the single-crystalline tablets, before the zone is finally reached where only the proper nacre proteins are secreted.
 | ||
| Fig. 12 Hovden et al.83 used a wedge polishing technique to prepare large-area specimens that cover the prismatic to nacre transition zone. (A) A “nanoscale assembly process” is described, being driven by aggregation of nanoparticles (50–80 nm) within an organic matrix, which arrange in “fibre-like polycrystalline configurations”. (B) This series of images give the impression of polycrystalline nanoparticles coalescing into nacre's single-crystalline tablets. (C) It should be remembered that these images were not taken over time, so they actually show a disordered transitioning interface, where the epithelial cells presumably start changing over to secrete a different set of proteins to go from modulating prismatic calcite to aragonitic nacre. It appears (to me) to be “condensation” of some mineral “phase” on the fibrous matrix. Given that those fibrous paths end in the regions of disordered tablets makes me think that this remnant prismatic matrix is hindering the ability for the mineral precursors to fully densify into the single-crystalline tablets that eventually develop. Reproduced from ref. 83, Copyright © 2015, The Author(s). | ||
In any case, if we accept that sheet nacre does form by a particle accretion mechanism (whether amorphous or crystalline), as indicated by the many papers that now show nacre's nanogranular texture, then the accretion and densification would seemingly be responsible for the whitening patches observed in Rousseau's study (Fig. 11A).79 The melding of nanoparticles into single-crystalline tablets suggests the precursor must have, at the very least, gel-like properties. I proposed this option of PILP and biominerals forming from a viscoelastic hydrogel in my Colloid Assembly & Transformation paper,84 with the intent of putting the focus more on the coalescence behavior of a mineral precursor, rather than on if, or how long, it remains a liquid. How such a precursor phase would behave when formed within such biogenic matrices would be a very interesting system to study. This could readily be done by combining the PILP model system with biogenic matrices.
The nacre case study highlights the inherent limitations of microscopy in studying the dynamic processes that occur in biological tissues, and especially when one is operating with the wrong set of assumptions (e.g., based on classical crystallization theory). However, it also highlights the value of in vitro model systems, which can be studied in situ, and with manipulation of variables of interest, to enhance our understanding of the physicochemical reactions that take place in potentially related non-classical crystallization process. Notably, it was a plain old fashioned polarized light microscope that enabled the discovery of the PILP model system,14 which to my knowledge was the first non-classical mineralization process to be suggested for invertebrate biomineralization (except amorphous phases described in the iron oxide biominerals of chitin and limpet teeth). Obviously, many advanced microscopy tools have played an important role in elucidating these intriguing features of nacre, but I believe, if we truly want to understand biomineralization mechanisms, we need to employ a combination of tools and model systems which enable a comparative analysis between “mineralogical signatures” that can help decipher formation mechanisms.
2.2 A case study in vertebrates: the bone story
The vertebrates do not straightaway present the beautiful microscopy images one finds for the elaborate non-equilibrium morphologies of the invertebrates, because bone is organized into various hierarchical levels of structure (Fig. 13).85 Nevertheless, there were various enigmatic features discussed in the earlier literature on bone, but these features were found mostly at the nanoscale, where, for example, one couldn't readily see the hydroxyapatite (HAp) crystals in SEM because they are intimately intercalated within the collagen matrix. High levels of expertise were required to image the interesting features of bone, and even now, more levels of bone's hierarchical structure keep being resolved.86 Back in the early days of my career, I learned a great deal about bone's hierarchical structure from the exceptional works of Weiner, Wagner and Traub.87,88 These earlier studies was focused on the relationship between mineral and collagen matrix, such as resolving whether the nanocrystals were intrafibrillar or interfibrillar. Another debate was if bone's tiny nanocrystals were platelets rather than needles, the latter of which was commonly perceived due to the appearance of dark thin streaks within collagen fibrils. | ||
| Fig. 13 Representation of bone's hierarchical levels of structure and their associated size scales. I wanted to use my original favorite (Weiner & Wagner, The material bone: Structure mechanical function relations),87 because it was so valuable to me when I first started learning about bone, but the journal is now charging for permission. (1) The first level is the individual constituents, tropolcollagen ‘molecules’ assembled into a fibril, and hydroxyapatite nanocrystals. (2) The mineralized collagen fibril was one of the primary enigmas of bone formation with respect to how the crystals end up inside the fibril. (3) Collagen fibrils assemble into dense-packed arrays in lamellar bone, often with a twisted plywood organization. (4) The lamellae may be found in the trabeculae of cancellous bone, or (5) the concentric layers of osteons within cortical bone. (6) Whole bones have different regions, such as compact cortical bone, with internal trabeculae of cancellous bone. Reproduced from ref. 85, Copyright © 2014, Sage Publications. | ||
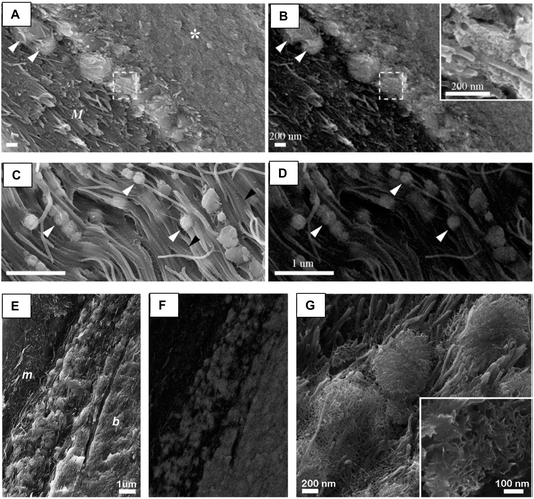
In the first conference on bone I attended, the hot debate of the day was on the role of the matrix vesicles found in the extracellular matrix of bone osteoid – were they transporting ions or crystals to the collagen fibrils (Fig. 14)?89 I didn't think the latter hypothesis made much sense given that it didn't seem possible for crystals to be transported into the interior of collagen fibrils. So I figured the streaks of crystals were caused by beam damage. However, even more recent images have been rather convincing that crystals sometimes formed in the vesicles before they reach the fibrils, and still mineralize the neighboring collagen as well (Fig. 14C–E).90 It should be noted, however, that these images always show a low density collagen matrix, with an appearance similar to primary bone formation, which is a rapid process of cartilage calcification. In cartilage calcification, one sees many matrix vesicles that form into large calcification nodules between the more disorganized and lower density collagen matrix (which is comprised of mainly type 2 collagen), so this always seemed very different from the parallel-aligned high-density collagen in secondary bone (e.g., the cortical bone region in Fig. 14A). It was generally thought that primary and secondary bone form by very different mechanisms. Interestingly, when we mineralized a low-density extracellular matrix (from kidney tissue), our TEM images showed similar globules with crystallites growing within them (Fig. 14F and G).91 Even though crystallites were already forming, dark streaks of collagen mineralization were also emerging, which appear remarkably similar to that shown in Fig. 14C–E.90 Perhaps things proceed differently in these low density extracellular matrices, with a mixture of intrafibrillar nanocrystals and extrafibrillar nodules. I recall having a difficult time deciphering that older literature as to92 whether cartilage calcification leads to intrafibrillar mineral, because it was generally thought that type 2 collagen doesn't mineralize in that way. And even now, it can be confusing comparing the different bone types (Fig. 14A MB vs. CB). In any case, it is worth noting that, even in the very early literature (before my time), an amorphous phase was frequently mentioned as being present in these matrix vesicles; however, I would say its significance wasn't realized. The focus was usually on potential epitaxial relationships between HAp and collagen, or non-collagenous proteins bound to collagen.93,94
 | ||
| Fig. 14 Matrix vesicles in bone formation. (A) and (B) Bonucci and Gherardi (1975)89 examined medullary bone from pigeons because medullary bone is formed (via osteoblasts) and resorbed (via osteoclasts) in a similar fashion to other bone types, but much more rapidly, making it a useful model system. As noted by the authors, medullary bone has more non-collagenous proteins and proteoglycans, and with only half the content of collagen fibrils, it is easier to image the matrix vesicles. (A) This low magnification image shows tissue stained with a Periodic Acid–Schiff (PAS) method and counterstained with hematoxylin. Note the medullary bone, MB, which is much more reactive than cortical bone, CB, stained more darkly. (B) High magnification of the calcification front shows “calcifying globules” (black arrows), some of which were surrounded by a membrane, and some of which contained clusters of small crystals (white arrowhead). These appear to lead to the calcifying nodules, which as seen here, contain a radiating assembly of crystals. (C)–(E) In a review by Hasegawa90 many years later (2018), much more detailed information on enzymatic involvement, inorganic pyrophosphates, etc., was available. The TEM images appear to confirm crystal formation within matrix vesicles, and even goes further to show close contact between the calcifying nodules (CN) and early-stage collagen mineralization. Although crystallite texture is apparent in the calcifying nodules in image (C), the dark CN nodules in (D) and (E) appear stippled. The fibrils in this region of bone (near cartilage deposition) are not tightly packed, similar to the medullar bone above. (F) and (G) PILP mineralization of decellularized porcine kidney. (F) Although this was not a bone-related experiment, there is a striking similarity between the crystallites forming in PILP drops and the matrix vesicles shown above. (G) The elongated dark patches are where PILP phase has started to infiltrate collagen fibrils. (A) and (B) Reproduced from ref. 89. Copyright 1975, with permission from Springer Nature. (C)–(E) Reproduced from ref. 90, Copyright 2018 with permission from Springer-Verlag GmbH Germany. (F) and (G) Reproduced from ref. 91, Copyright 2019 with permission from Springer Nature. | ||
One of the enigmatic features of bone back in the day was – how does it form such a high degree of mineralization, roughly 60 wt%? In bone, this high degree of mineralization arises from the intercalation of nanocrystals within and around the collagen fibrils, so this intrafibrillar mineralization was really the enigmatic feature, given that most attempts to mineralize collagen in the beaker at that time led to an extrafibrillar crust of platy spherulites. I recall one clever approach to achieving this high degree of mineralization in vitro was by soaking collagen matrices in alternating mineral salt solutions.95 While they did reach a high degree of mineralization, comparable to bone, the nanostructure did not resemble bone (Fig. 15). Again, the simple PILP model system came to the rescue. First, in 2003, we tested calcium carbonate since we knew how to make a CaCO3 PILP phase. It did seem to infiltrate collagen fibrils, but there were these odd disks of mineral orthogonal to the fibril axis as well.96,97 But once the conditions for calcium phosphate were realized in 2007, it naturally formed nanocrystals of hydroxyapatite aligned parallel to the fibril, producing an interpenetrating composite that closely emulated bone's nanostructure (Fig. 16).98 Serendipity again!
 | ||
| Fig. 15 Góes et al.95 achieved a high degree of mineralization by alternate soaking of mineral salt solutions. More mineral was formed in anionic collagen matrices prepared with an increased concentration of carboxylic groups (A and C) versus the native collagen matrices (B and D). They were able to fabricate a bone-like composition of up to 60 wt% mineral after 100 soak cycles, as determined by thermogravimetric analysis. This method did manage to form HAp on the interior of the porous collagen scaffold (C and D), but it does appear to be intrafibrillar. Reproduced from ref. 95, Copyright 2007 with permission from Elsevier. | ||
 | ||
| Fig. 16 A synopsis of the ability to achieve intrafibrillar mineralization of collagen using the PILP model system. (A) A typical collagen matrix prior to mineralization. (B) Mineralization without polymer process-directing agent leads to an extrafibrillar crust comprised of spherulitic clusters of HAp, typical of what was shown in most literature prior to the PILP discovery. (C) Collagen matrix mineralized with polyAsp additive. The mineral is not readily apparent, other than fibrils have become thicker; but EDS inset shows large Ca and P peaks (greater than the C peak from matrix) from the underlying mineral. Thermogravimetric analysis found this scaffold had 65 wt% mineral, indicating the mineral is not visible because it is intrafibrillar. These fibrils appear to be devoid of any extrafibrillar mineral, as is the case for the DF-TEM image in (E). (D) TEM of non-stained fibril has sufficient Z-contrast from the infiltrated mineral. Some adsorbed droplets were found on this fibril. They were quite large compared to later studies by the Sommerdijk group. (E) Dark-field TEM was used with the corresponding SAED pattern to illuminate the numerous [001] oriented nanocrystals within this fibril. (F) Schematic representation of our proposed mechanism of intrafibrillar mineralization of collagen. (a) Infiltration of PILP precursor phase into gap zones of collagen fibrils via capillary action. (b) After precursor becomes intercalated throughout the interstices of the fibril, it solidifies into amorphous calcium phosphate (ACP), and (c) ACP crystallizes, creating a fibril embedded with aligned nanocrystals of HAp. (A) Reproduced from ref. 101, Copyright 2010, with permission from Elsevier. (B), (D) and (F) Reproduced from ref. 98, Copyright © 2007 Elsevier B.V. All rights reserved. (C) Reproduced from ref. 102, Copyright 2022, Open Access. (E) Reproduced from ref. 107, Copyright 2009, with permission from Elsevier B.V. All rights reserved. | ||
Long before our discovery, in 1993 Landis et al. had done some fabulous tomographic imaging of mineral forming within turkey tendon (a valuable biological model system), and described how ‘‘a crystal is not confined by the length of either the collagen hole or overlap zone” (Fig. 17).99 I considered this important because it was providing a clue that, once again, the gap zones were not little compartments that crystals grew within, but that something was driving infiltration of mineral throughout the whole of the collagen interstices (between collagen microfibrils,100 as collagen structure became more resolved). Therefore, we proposed a mechanism of capillary action being responsible for the infiltration of nanodroplets of mineral precursor.98 However, I can't say that we directly observed a liquid phase of calcium phosphate, like we had for calcium carbonate, because the CaP precursor “droplets” were incredibly small (13–16 nm),101 except for some phase that accumulated in the matrix of a decellularized porcine kidney (Fig. 14F and G). Instead, we were operating on the assumption that polymer interactions with CaP were probably similar to those in CaCO3. And the wobbly particle shapes seen in nanoparticle tracking analysis was supportive of the concept of liquid-like precursor droplets.102
 | ||
| Fig. 17 Landis et al.99 studied early-stage mineralization of collagen within turkey tendon, which is a nice model system because the gastrocnemius tendon naturally mineralizes. As far as I am aware, Landis was the first in the biomineral community that used tomographic imaging of biominerals. (A) A uranyl acetate and lead citrate stain were used, which nicely marks the periodic banding of the fibrils. Note – the aligned fibrils within tendon are much easier to image than the collagen in bone matrix, making this a particularly useful model system. (B) One of a set of tomographic images of the region marked with an arrowhead in (A). The crystallographic orientation is evident in the dark streaks that run parallel to the fibril axes. (C) A series of shaded surface renderings of the mineral components with a calcifying region, with each view rotated 10°. From this work, the authors confirmed the presence of irregularly shaped mineral platelets, which were described as having variable widths but uniform thickness (∼4–6 nm). They also indicated that the crystals apparently fuse in coplanar alignment to form larger platelets, and that c-axial growth is unhindered by collagen hole zone dimensions. Reproduced from ref. 99, Copyright 1993, with permission from Elsevier. | ||
I like to think that our success in emulating bone's intrafibrillar nanostructure prompted the biomineral community to revisit the issue of an amorphous precursor in bone. In 2008, Mahamid et al. provided strong evidence of an amorphous phase in bone by examining the continuously growing fin bony rays of zebrafish (Fig. 18).103 In their 2010 follow-up paper, they mapped the ACP delivery from cells to collagen matrix (Fig. 19), revealing how “mineral bearing globules appear to be fusing into the collagen fibrils within the growth zone.”104 Although the authors hypothesized that nanoparticles were released into the fibrils, I think the “whitening” along the length of the fibrils (highlighted with backscattered imaging, Fig. 19C and D) seems unlikely to be nanoparticle transport along the circuitous path within a fibril. The visual appearance seems more like something that might be expected for capillary infiltration. This group then did another beautiful cryo-EM study of developing mouse calvaria and long bones, showing osteoblasts actively produce disordered mineral “packets” within intracellular vesicles which are then transported for mineralization of the extracellular matrix (Fig. 19E–G).105 Interestingly, they determined these packets are initially enriched in phosphate, and gradually sequester calcium within the vesicles.
 | ||
| Fig. 18 Images of bone formation in the continuously elongating TL caudal fin of zebrafish. (A) Polarized light microscopy can show the entire skeletal elements. Note the increasing birefringence as one moves away from the newly forming bone at the distal end and the rays gradually becomes more crystalline. (B) SEM of the fin ray segments after critical point drying. (C) Intensity plots of birefringence (orange) and mineral density (gray) signals generated from the polarized-light micrograph and micro-CT image, respectively. Reproduced from ref. 103, Copyright 2008, with permission © 2008 by The National Academy of Sciences. | ||
 | ||
| Fig. 19 An in-depth study on mineralizing matrix of the zebrafish fin bones (A)–(D) and on mouse trabecular bone (E)–(G). (A) and (B) SEM and BSE mode imaging of osteoid matrix that is becoming mineralized by “large, mineral-bearing globules”. They appear to meld into the matrix. (C) and (D) This region is described by the authors as “Mineral-bearing globules infusing the collagen fibrils within the growth zone.” (D) In BSE mode, one can see the whitening along the length of the fibrils. The authors suggest there might be a release of nanoparticles. None are apparent on the surface, but they could have been washed away in sample preparation. But it certainly gives the impression that mineralization traversed internally down the length of the fibrils away from the globules. (E)–(G) In the mouse trabecular bone, matrix infusion appeared somewhat similar. However, in (G), the zoomed in inset of the mineral globules shows they are comprised of irregular platelets. So these may be similar to the calcification nodules described earlier in Fig. 14, which I think was also trabecular bone. (A)–(D) Reproduced from ref. 104, Copyright 2010, with permission from Proc. Natl. Acad. Sci. U. S. A. (E)–(G) Reproduced from ref. 105, Copyright © 2011 Elsevier Inc. All rights reserved. | ||
In 2010, Nudelman et al. skillfully made good use of our in vitro model system in their studies using in situ cryo-TEM to examine collagen fibrils becoming mineralized in the presence of polyanionic additives (Fig. 20).106 They produced some amazing images showing tiny amorphous nanoparticles lined up at the a-bands where they seem to infiltrate into the gap zones of the fibril (Fig. 20B–D), and then spread amorphous material toward the interior of the fibril, from which the dark streaks of nanocrystals emerge (Fig. 20D). Amazingly, their images looked just like our previous schematic of the proposed PILP infiltration mechanism, with tiny nanodroplets lined up at the gap zones (Fig. 20E).107 Lucky guess? Not really. We surmised that the precursor phase had to be pulled inside the fibril somehow given that we often saw fibrils with purely intrafibrillar mineral and no external crust (Fig. 16C and E).102,108
 | ||
| Fig. 20 Nudelman et al.106 employed cryogenic TEM and tomography to examine the infiltration of amorphous precursor “particles”, created with polyanionic process-directing agents, into collagen fibrils. (A) Deformation of the fibril from infiltration of mineral precursor can be seen at 48 h. I was fascinated to see this internal view because we often saw (with SEM) lumpy fibrils midway in the mineralization (see Fig. 7C in Olszta, 2007).98 One can also see here (and other images in the paper) that the fibril appears to mineralize from the inside to outside. (B) and (C) The authors carefully discerned the sub-band location within the hole zones where the amorphous precursor appears to enter the fibrils. (D) This image beautifully illustrates all the stages, going from particle entry at the periphery, to diffuse amorphous substance within the fibril, to the aligned dark streaks of crystalline HAp. Note prevalence of mineral toward the interior. (E) Schematic by our group representing what we thought was happening during intrafibrillar mineralization, which was impressively close to what was later observed in Nudelman's image (D). (A)–(D) Reproduced from ref. 106, Copyright © 2010, Springer Nature Limited. (E) Reproduced from ref. 107, Copyright © 2009 Elsevier B.V. All rights reserved. | ||
Nudelman et al.'s106 cryo study beautifully revealed what was happening in our model system, but they proposed a different infiltration mechanism based on particle diffusion. It wasn't clear to me why diffusion would lead to more mineral in the interior of the fibril, figuring that particle adsorption would occur all along the diffusion path before even reaching the interior. That riddle seemed to be solved by Niu et al. in 2016,109 who proposed an alternative hypothesis based on a Gibbs–Donnan effect (which I am embarrassed to say, I had never heard of). Their hypothesis is something of an expansion of the Price et al. hypothesis of “mineralization by inhibitor exclusion”,110 which additionally needs to balance electroneutrality with osmotic equilibrium (Fig. 21). Niu et al.109 proposed that stress relaxation of the viscoelastic fibrils provides the driving mechanism for the influx of the polyelectrolyte-stabilized mineralization precursors. In fact, they examined both polycationic (poly(allylamine) hydrochloride, PAH) and polyanionic (polyaspartic acid, PAsp) process-directing agents to stabilize the amorphous precursors. While it makes sense that the Gibbs–Donnan equilibrium would provide the driving force for infiltration, there still are observations that are hard to reconcile with that mechanism. Namely, why do other bivalent ions (Mg, Sr, Ba) dramatically disrupt the process (unpublished results)? And also, studies on bone formation have always shown the involvement of particles, matrix vesicles, which seemingly wouldn't be necessary by that mechanism alone.
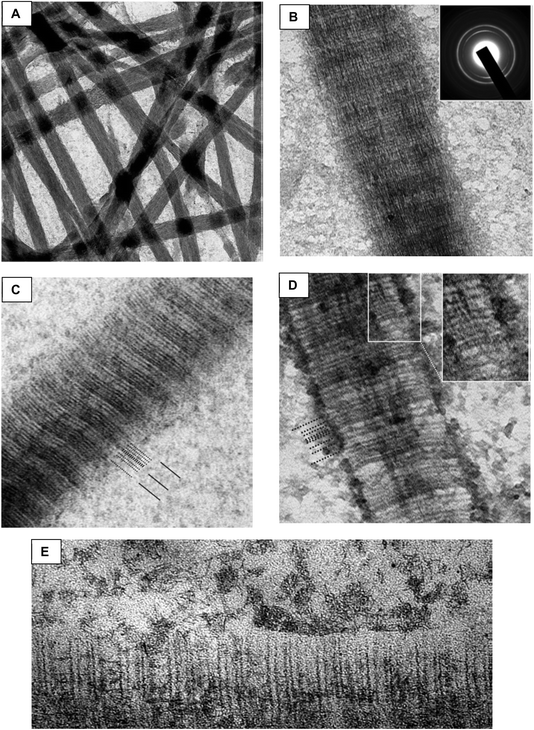 | ||
| Fig. 21 Niu et al.'s109 elegant study on intrafibrillar mineralization supporting the concept of Gibbs–Donnan equilibrium. Arrows and labels did not copy over, so please refer to original paper for full markup. (A) Conventional TEM without staining shows strong contrast from the heavily mineralized fibrils using PAH-stabilized calcium phosphate complexes. (B) Uranyl-acetate stain highlights the collagen banding, with inset showing the proper [001] orientation of HAp, which can be seen as the dark striations in the fibril. (C) At 12 h, ACP is seen to still be infiltrating the fibril (collagen is stained). The authors indicate there was no predilection of sub-band entry location (as seen in the Nudelman paper using polyanionic additives). (D) This stained fibril shows dark CaP complexes at the fibril's periphery. The authors state that “communication of CaP complexes from the fibril's surface with ACP in the intrafibrillar spaces supports the fluidic nature of mineralization precursors (highlighted in inset).” (E) At 18 h, crystallization has commenced. The authors note there are filamentous structures in the surrounding ACP similar to those observed in their cryogenic TEM images. Reproduced from ref. 109, Copyright © 2016, Springer Nature Limited. | ||
I should mention, there was an alternative hypothesis on bone formation presented by Wang et al. in 2012,111 that I fully disagree with. They argued that collagen alone, without non-collagenous proteins, could lead to intrafibrillar mineralization. It was true that intrafibrillar mineral was created in vitro using their method; but the implications for relevance to bone formation were misleading. Their method used a very high, non-physiological, ion concentration, such that amorphous mineral became entrapped within the fibrils as they were forming. Although not discussed, this presence of an amorphous phase was indicated by the 31P MAS NMR spectrum (in their Supplementary Fig. S10). In light of the Mahamid et al. papers103–105 on forming bone (as well as most of the bone literature), it is clear that the collagen matrix is laid down prior to the addition of amorphous phase, which is then transported to the matrix in matrix vesicles (as described in the older literature) or amorphous mineral-bearing globules (in the more recent papers). Thus, while Wang's fabrication of a bone-like nanostructure may have valuable biomedical applications, their approach doesn't seem relevant to providing an understanding of bone formation (as was directly implied in the paper).
Another long-standing enigmatic feature of bone had been the [001] crystallographic orientation of the hydroxyapatite nanocrystals. In the old days, it was assumed to be due to some epitaxial type relationship between crystals and collagen. Indeed, my attempt to publish our exciting results in Science was rejected because a reviewer said “Less than a year ago, the crystal structure of osteocalcin was published by Nature, suggesting very strongly that epitaxial growth in bone is likely to be one of the mechanisms by which the magnificent structure of bone is formed.” This paper that was cited (Hoang 2003)93 didn't generate any bone-like structures; it just modelled which crystal face of HAp might osteocalcin bind to. Our results did, however, produce the proper bone-like crystal orientation; but that apparently was not enough to overcome the bias of that reviewer.
When we discovered that the PILP mineralization naturally led to the [001] orientation,98 using only polyaspartate as the process-directing agent, it became apparent that a specifically nucleating non-collagenous protein was not needed to control HAp orientation. Therefore, we proposed that “the uniaxial orientation of the crystals may simply result from a uniaxial constraint of the common spherulitic growth pattern of HA within the collagen fibril, rather than any specific epitaxial type interaction”. This concept of physical confinement was nicely demonstrated by Cantaert et al., who designed a clever experiment using nanoporous track-etch membranes to demonstrate that nanoscale confinement leads to [001] oriented growth of HAp due to the more rapid growth direction becoming dominant (Fig. 22).112 I loved the experiment, but did find their discussion odd, where they state: “A striking feature of mineralization of collagen fibrils, which we have not been able to investigate herein, is the ability of CaP to infiltrate so effectively into the 2–6 nm gaps in collagen fibrils. It is hard to imagine that this does not derive from the specific chemical structures, and indeed interplay between the collagen matrix and non-collagenous proteins, as is fully consistent with the literature.” Consistent with what literature? Literature using in vitro models had already shown that CaP precursors could infiltrate collagen interstices, and therewith lead to oriented growth (as they demonstrated), without the use non-collagenous proteins (NCPs). So here again is that bias that biologists expect protein interactions to involve specific molecular recognition. When we later demonstrated that the same intrafibrillar mineralization process could be induced with osteopontin,113 and much more rapidly,102 this garnered more attention from the biology-minded community.
 | ||
| Fig. 22 Cantaert et al.112 designed a clever experiment to determine the role of confinement on crystal orientation. (A) Schematic representation of the nanoporous track-etch anodized alumina membrane, which enabled double-diffusion of ions to grow HAp within the elongated pores. (B) Schematic representation of how ACP transforms into HAp with preferential orientation created by the confinement effects. This occurs because there is a strong anisotropy in the HAP lattice, and the [001] axis becomes dominant as it is the most rapid growth direction. (C)–(E) SAED images with corresponding insets, showing strong orientation for the 50 nm pores, and moderate to no orientation for the 200 nm pores (D) and (E). In (C), the SAED pattern looks remarkably like bone's, which was surprising to me as I thought the twisting collagen microfibrils were responsible for the slight tilt of crystals in collagen. In this experiment, it seemingly arises from offset tilted crystals allowed by the channel width. Intrafibrillar channels in collagen, however, are much narrower. Reproduced from ref. 112, Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Xu et al.114 then extended the physical confinement concept further by using X-ray diffraction (XRD) to determine that the voids in collagen fibrils contain cylindrical pores with diameters of ∼2 nm. They also examined collagen mineralization with HAp, CaCO3 and γ-FeOOH, and concluded that “confinement within these pores, together with the anisotropic growth of HAp, dictates the orientation of HAp crystals within the collagen fibril” (Fig. 23). The γ-FeOOH also led to aligned needles within fibrils, but the mineral didn't reach the interior of the fibrils as effectively (Fig. 23A–D). Again, this was a wonderful expansion of our in vitro model system. However, I might have interpreted things a little differently in looking at some of their data. For example, the Supplementary Movie 5 shows filaments of precursor phase, which appear as chains of stippled dots, seemingly enter into the collagen fibril, and remain connected along circuitous paths (this can be seen if one moves the video forward and back) (Fig. 24). The visualization of these filaments staying connected even as they traverse into fibrils along the interior channels could be supportive of the concept of capillary infiltration. However, one would not expect a liquid phase to appear as stippled dots. Yet an advanced imaging paper by Sommerdijk's group has shown a stippled appearance to the PILP precursor for CaCO3,115 and stippled amorphous and early crystalline phase of calcium phosphates is seen in several papers (e.g. the early crystallites in matrix vesicles in Fig. 14D and E).90 This may suggest that the mineral precursor's fluidity is caused by the flexible polyelectrolytes that hold the collection of nanoclusters together. If this is correct, it seems like quite an interesting form of matter.
 | ||
| Fig. 23 Xu et al.114 studied the effect of confinement on crystal growth in collagen by using XRD to determine the voids in collagen are cylindrical pores with diameters of around 2 nm. (A) and (B) In addition to HAp (not shown here), they also demonstrated the concept of confinement with lepidocrocite, γ-FeOOH, which also has an anisotropic lattice structure (orthorhombic). (C) and (D) Tomographic reconstruction with an averaging of ten y- and z-slices. The orientations of some γ-FeOOH platelets are highlighted by yellow arrows in (C). (E)–(G) They found that CaCO3 produced a mix of calcite and vaterite (not shown here), and with a sustained granular texture, they showed no preferential orientation. Interestingly, their intrafibrillar calcite produced orthogonal bulges similar to what we had observed in our first CaCO3 collagen study. It would be interesting if someone could produce intrafibrillar aragonite, because it normally grows as anisotropic needles. Reproduced from ref. 114, Copyright © 2020, The Authors. | ||
 | ||
| Fig. 24 A series of still frames captured from Xu et al.'s Supplementary Movie 5.114 The reader is encouraged to look at the video for a better realization of the connectivity between the filaments and channels within the collagen fibril. It's quite amazing to see that the stippled dots always appear to remain together in a connected filament, even after entering into the interior of the fibril. Notably, at various rotations, one can see the interior of the fibril is more enriched with dark mineral phase than the exterior, as noted in the earlier study by the Sommerdijk group (Nudelman et al.).106 (A) & (B) At ∼5 s, the bottom filament takes a sharp upward turn after it enters into the fibril, and then it splits into two channels, as seen with these two still frames that are a split second apart. (C) At ∼9 s, a mild bend occurs after entry of the bumpy filament into the fibril. (D) At ∼10 s, a relatively sharp bend in the filament. The streaks of mineral highlight the collagen twist in this orientation. (E) At ∼8 s, a curvy filament, which upon entry into fibril, seems to dip behind and then connect to the northward filament above. (F) At ∼13 s, a split filament entering fibrils. Still frames captured from Supplementary Movie 5, from ref. 114, Copyright © 2020, The Authors. | ||
As I brought up earlier, which is further exemplified in the Xu et al. Movie S5,114 there appears to be more mineral at the interior of the fibril. So once again, it is hard to envision this as being diffusion of a stream of nanoparticles along these circuitous paths. In addition, I think it should be emphasized that the confining channels within which the crystals grow are in reality channels of amorphous phase, not voids in the collagen, as implied by the paper. It's possible that the amorphous phase seeps its way throughout the interstices, creating its own channels, with the driving force being capillarity and/or Gibbs–Donnan. In other words, I don't think a pre-existing compartment is necessary, and in fact, capillarity will be stronger in narrower channels. If the amorphous precursor had infiltrated all around the quasihexagonally arranged microfibrils, it solidification and crystallization would result in “platelets” with a curving irregular morphology.
The work by Xu et al.114 nicely bolstered the earlier studies by Landis et al. (1993),99 by once again demonstrating that the crystals are not confined to the dimensions of the gap zones, nor are they actually flat platelets, as mentioned above. I bring this up because it seems many computational simulations of bone mechanics often show little isolated flat platelets residing in the gap zones. I believe it was Rosen et al. (2002)116 who first showed (as others did later)98,117 that the high degree of mineralization in bone would lead to an interpenetrating composite; yet the overly simplistic view of nanocrystals in gap zones often prevails. In my opinion, the concept of an interpenetrating composite is incredibly important because once the percolation threshold is surpassed, one gets a very brittle composite. This is something we learned from our in vitro model system, where we had spent so many years optimizing the conditions to get a high degree of mineralization, we actually overreacted the mineral content, and our composites became flaky and brittle. In fact, once when we failed to get a high degree of mineral infiltration, we made a nice tough mineralized collagen scaffold that behaved more like antlers (unpublished results). This is another area that could benefit from more study.
With respect to mechanical properties, we came to realize that isolated fibrils just get brittle when mineralized. Thus, another important feature of compact bone is the densely-packed quasi-parallel arrays of collagen fibrils. We began a collaboration with collagen expert Jeff Ruberti, who was able to make highly organized dense arrays of collagen using the ‘molecular crowding’ approach.118 This basically capitalizes on the liquid-crystalline properties of the highly anisotropic collagen molecules. This was a controversial issue back in the day, when biologists preferred to believe that the cells were solely responsible, but Marie-Madeleine Giraud-Guille did a remarkable job of demonstrating the similarities between extracellular matrices and liquid-crystalline textures (Fig. 25A–C).119 In fact, if one looks through all the schematics in Carter's 1990 book on “Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends”,120 you can see that many biomineral textures resemble liquid-crystalline textures, particularly the textures containing splay. Perhaps this points to an interesting form of matter, as mentioned above. Using Ruberti's dense collagen arrays, our team was able to expand our bone mimetic structure to the third level of the hierarchy represented in Fig. 13, with nanoscale organization based on intrafibrillar mineralization, to microscale organization through the use of densely-packed collagen arrays (Fig. 25D–F).121,122
 | ||
| Fig. 25 Toward emulating bone's hierarchical structure by capitalizing on the liquid-crystalline nature of collagen. (A) Giraud-Guille119 resolved the liquid-crystalline texture found in a variety of extracellular matrices, such as the ‘twisted plywood’ structure observed in lamellar bone. This schematic represents how thin slices taken along different orientations will appear in microscopy thin sections. (B) This TEM of a slice of lamellar bone shows the arcs expected for slice c of figure (A). (C) This twisting structure is from a crab carapace, which is comprised of a chitin extracellular matrix that becomes mineralized with CaCO3. Notably, liquid-crystalline textures appear in a variety of biomineralized matrices. (D) Using Ruberti's ‘molecular crowding’ approach118 to densify a solution of collagen leads to the cholesteric (or twisted nematic) phase, which upon fibrillogenesis provides collagen matrices that emulate the twisted plywood organization of lamellar bone's extracellular matrix. (E) Mineralization of these dense collagen matrices using the PILP process led to a hierarchical structure comprised of the twisted plywood microscale structure, built upon the intrafibrillarly-mineralized collagen nanostructure. (F) SEM of a fracture surface of the biomimetic bone revealed the twisting layers collagen within (note the splay of fibrils), somewhat resembling the crab carapace shown in (C). (A)–(C) Reproduced from ref. 119, Copyright © 1986 Published by Elsevier Ltd. (D) and (E) Reproduced from ref. 121, (2018), Open Access. (F) Reproduced from ref. 122, Copyright © 2016 Elsevier B.V. All rights reserved. | ||
Over the years, with more and more advanced tools being developed, further refinements have been made in resolving bone's complex 3D structure (Fig. 26). Reznikov et al. now describe XII levels of bone's hierarchical structure in their schematic.123 So apparently, we still have a ways to go in fully emulating bone's hierarchical structure, which became painfully clear when the mechanical properties of our biomimetic bone did not fully emulate those of bone. I think the cross-fibrillar architecture demonstrated by Reznikov et al.123 (Fig. 26D) may be an important aspect that deserves further study. I would be happy to consult on such a venture, because I still believe that by emulating bone's composition and hierarchical structure, this approach will one day enable the fabrication of load-bearing, bioresorbable biomimetic bone substitutes.
 | ||
| Fig. 26 As I mentioned earlier, the parallel fibrils in turkey tendon provide more readily interpreted mineral–collagen relationships than bone, which has a far more complex hierarchical structure. This is effectively illustrated by Reznikov et al.,123 who now describe XII levels of bone's hierarchical structure. (A) In these three projections of bone, one can see how different the structural organization will appear in the planar view, with the top slice showing the dark streaks typical of crystals aligned with collagen fibrils; the middle slice showing a lacy pattern with curved crystals around voids; the bottom slice showing nested rosettes. The schematics to the right show models based on tomograms reconstructed from the STEM tilt series. (B) and (C) A labeling algorithm highlights the lacy (blue) and filamentous (pink) patterns in the tomogram slices. (D) This is a reconstructed volume of demineralized and stained collagen fibrils in bone, where 100 different color labels show the distribution of segment lengths. These cross-over segments are clearly different from the long, parallel-aligned collagen in tendon, and most likely play an important role in bone's mechanical properties. Reproduced from ref. 123 Copyright © 2018, The American Association for the Advancement of Science. | ||
In conclusion, the bone case study also demonstrates the value of a good in vitro model system. Firstly, there was such a good correlation between the mineralogical signatures found in vitro to those in real bone, I think this pushed the biomineral microscopy community to re-examine bone formation, which had, years earlier, abandoned the amorphous precursor theory. But with modern tools, scientists were finally able to find much more convincing evidence of the amorphous precursor, and more importantly, its relevance. Secondly, the PILP model system was capitalized on by several groups for studying in vitro how intrafibrillar mineralization of collagen can be achieved. There are still issues of debate, so I hope this model system can be further utilized to help fully resolve mechanisms. In the case of bone, understanding of how intrafibrillar mineralization is achieved is extremely important, not just for understanding bone biology, but also for emulating bone's processing to fabricate the next generation biomimetic bone substitutes.
3 Evolution and serendipity
Returning to the topic of defect textures, the invertebrate biomineral story became particularly interesting when it was shown that the nanogranular/nanocolloidal texture present in the PILP formed tablets was ultimately found to occur in nearly all CaCO3 biominerals (that I am aware of), supporting the premise that a Colloid Assembly and Transformation (CAT) process arose early in the evolution of biominerals. Indeed, Gilbert's group has beautifully demonstrated that early calcifiers also used a “crystallization by particle attachment” mechanism to form their biominerals, and suggest that convergent evolution may have been dictated by the same thermodynamics and kinetics observed today.124 I used to get a chuckle when I'd hear people talk about how nacre was designed to give it remarkable mechanical properties. Those mollusks don't have a brain to design anything; it's evolution capitalizing on serendipitous mutations that led to some improvement of a property that improved the likelihood of reproduction.In retrospect, the use of an amorphous precursor should not be surprising given that the earliest forms of biominerals must have arisen from the need for cells to control calcium levels (I would like to cite this idea, but I don't know where I have seen it in the literature). It is easy to imagine that relatively simple, charged proteins could have sequestered ion clusters off into a less harmful solid amorphous phase, and then when such ions were needed for cellular activity, they could be more readily retrieved from the stored amorphous phase. One could understand how these relatively simple “primitive” IDPs could easily evolve into the process-directing agents that modulate biologically-controlled biomineralization, where mineral features that used to seem very different (fibers vs. tablets, etc.) are, in actuality, more related to cellular control over matrix or compartment, which mold and shape the precursor phase into an infinite array of morphologies.
With respect to vertebrate evolution, bone's metabolic function seems to rely on maintaining a poorly-crystalline nanoapatite phase. The old literature referred to this as paracrystalline,125,126 which might actually be a better descriptor as it seems likely that nanocrystals of this extremely small size (which are smaller than synthetically produced hydroxyapatite nanocrystals) would not be stable if it were not for them being embedded within and protected by the collagen fibrils. Biologically, it's then a relatively simple task for the collagen to be enzymatically removed, exposing the paracrystalline mineral for dissolution under mildly acidic conditions, thereby enabling calcium transport and/or bone remodeling.
The PILP process was apparently a difficult concept for the biomineral community to grasp given the entrenchment of protein tertiary structure in the biologist's education (i.e., globular protein's lock-n-key interactions). So quite a few biomineral enigmas needed to be demonstrated with the PILP model system to finally gather interest. This included mineral tablets and films,13,14 mineral fibers,97,127–129 2D templated films,33,130 3D molded mineral with curved surfaces,131 high-Mg calcite,132 transition bars53 and concentrically laminated spherulites,55,60 mineral fusion,14 tessellated layering, nanogranular texture.33,129 These have all been reported on, so there is no need to repeat that here except to point out that many of these reproduced features were not being deliberately tested, but rather serendipitous discoveries as the PILP system was further explored by some devoted students. This model system has also been useful for developing methods to remineralize dentin caries,133,134 fabricate hard-tissue engineering scaffolds,135,136 and for studying pathological mineral deposition.55,60,91,137,138
The serendipitous nature of the PILP discoveries is an appealing aspect with respect to considering the evolutionary aspects of biomineralization, given that evolution relies on serendipity. Rather than considering the complicated nature of trying to design molecular recognition between protein–crystal faces (as was required for pseudo-epitaxial or stereospecific mechanisms), and which differs for each species of a given organism, etc., it seems much easier to envision that a broad class of charged proteins could provide the inhibitory features needed to block undesired crystal nucleation, while simultaneously serving as a process-directing agent that enables the molding and shaping of the amorphous precursor phase without the surface energetic constraints of faceting. Thus, the control of mineralization by IDPs is mainly through kinetics, while cells provide the spatiotemporal control needed for creating a confined reaction space, such as a biomineral compartment or matrix, and cells had already evolved these abilities. I suspect if someone with expertise in this area were to examine the Cambrian explosion, the “burst of biomineralization” might involve the development of IDPs that induce something like a PILP/CAT mechanism.
3.1 Addendum: infiltration across semipermeable membranes
The day after I submitted this paper, a new paper came out with experiments that seem to address the issue of mineralization across a semipermeable membrane.150 Dispersed nanosheets of graphene oxide (GO) were spin coated to form a membrane, and as mineralizing solution infiltrated across the membrane, it lifted off the substrate to create a compartment, within which a self-organizing multilayered structure was formed that resembles nacre. This was a nice set of experiments with fascinating data, but I don't agree with their interpretation of the mechanism. Notably, their mineralization system also included polyaspartate, and under the exact conditions that lead to PILP films, yet PILP was never mentioned. It was evident in their negative control reaction, without GO membrane (their Fig. S2), that the thin film morphology was a result of the PILP process. Therefore, it is not clear to me how measurements of ion diffusivity across the membrane are relevant given that many of the ions will be incorporated within PILP droplets throughout the solution. Plus, it is possible that the PILP phase might infiltrate across the nanoporous membrane via capillary action, rather than ion diffusion. So while this nice experiment was close to what I was wishing for regarding the Nakahara discussion on Fig. 9 (except I was envisioning PILP infiltration across a stack of multiple nanoporous sheets),76 unfortunately the authors didn't even consider this possible infiltration mechanism.While the authors did not consider the PILP process in their solution, they did propose a liquid–liquid phase separation scenario as occurring WITHIN the compartment. They suggest the liquid–liquid phase separation between a CaCO3-rich and a PAsp-rich phase leads to the self-organizing multilayered structure. Given that their mineral film is obviously a PILP formed film, it is possible that the periodic pAsp interlamellar layers could be related to the PILP phenomenon we described as transition bars. Consider for example our nanolaminated tablet shown in Fig. 3E; although we didn't perform PFIR characterization as they did, one could easily imagine there might be excluded polymer between those regular sublayers.
More generally, their discussion on how the polymer inhibits crystallization outside the compartment brings to mind the inhibitor exclusion hypothesis proposed in the earlier literature for collagen intrafibrillar mineralization. And given that polymer exclusion is at the foundation of the Gibbs–Donnan equilibrium, it makes me wonder if that is actually the driving force for this mineral infiltration across their GO membrane that creates the compartment. And this made me wonder – is the Gibbs–Donnan effect involved in nacre formation, across those interlamellar sheets identified by Nakahara? I find this an interesting thing to contemplate, that perhaps a similar infiltration mechanism is occurring for very different biomineral systems, from invertebrates to vertebrates, who make different semipermeable membranes to create their various expanding mineralization “compartments”.
4 A (biased) view on the future of biomineral research
4.1 Will machine learning and AI play a role in biomineral research?
Nowadays, it seems most people think artificial intelligence (AI, or machine learning) will make huge contributions to future research. Maybe I'm too short-sighted to recognize how, but in the field of biomineralization, I think a word of caution is needed. In my day, it was all about computational modeling. Of course that has contributed greatly to many scientific disciplines, including materials science, but in the field of biomineralization, that is debatable. In fact, I would argue that computational studies have often been misleading. I already mentioned above my experience with the reviewer for our paper submitted to Science, who discounted our hypothesis on intrafibrillar mineralization of collagen by claiming that this couldn't be how bone is formed because a computational model supported the possibility of a potential epitaxial relationship between a face of hydroxyapatite and osteocalcin. The problem was, computational simulations had been operating under the wrong set of assumptions for many years because they were based on classical crystallization mechanisms. So, in the early years, they were modeling epitaxy or stereospecific interactions between amino acid residues and crystallographic faces. At least later it was realized that organics likely interact with crystal step edges. Nevertheless, given that crystal faces are not present when the amorphous phase is deposited (when the morphology is actually being molded), those studies were irrelevant. Things improved a little when it was discovered that prenucleation clusters and/or liquid condensed phases might be the reactive species during mineralization reactions. But these often modeled interactions with carbonate, without including bicarbonate, even though it is the dominant species at neutral pH. It likely plays a role in stabilizing the amorphous phase. But overall, from my perspective, the most relevant system to model would need to include liquid–liquid phase separation, while including water, polymer, ions and ion clusters, which as far as I am aware, is beyond the scope of computational simulations. I'm sure there must have been some useful computational studies, but I don't know since I stopped reading that literature years ago when I had already realized that biomineralization did not follow classical crystallization processes.This discussion leads to another word of caution, because it's not just computational studies that seem to magically show what they expect to find; microscopists are prone to the same problem. And even more broadly, I would caution against hypothesis driven research. Scientists often “see” the data that fits their hypothesis and inadvertently overlook the data that does not. It's not usually deliberate; it's simply human nature to want and expect your hypothesis to be correct. With imaging, this can be a severe problem, and especially when one is choosing to show the most representative image. I would prefer to see all 100 images that were taken, but of course that is not feasible in the literature (but it is with one's graduate student).
Back to AI. AI is based on prior literature (or web pages, yikes), so if much of the prior biomineral literature is incorrect, being based on the wrong assumptions relating to classical crystallization theory, how will AI be able to discriminate that? Having said that, I do see where AI might be useful for microscopy, being able to screen hundreds of images to search for things, but I just hope it is searching for the correct thing.
4.2 In vitro model systems of biomineralization
As much fun as it is to discover the cool secrets hidden within these biomineral gems, I'm not sure how much more useful information can be obtained from the advanced imaging tools that are being developed, at least not from a materials processing perspective. There may be more things to learn with respect to biomechanical design, or biological evolution, etc., but I think there are better ways to resolve the mechanistic aspects. These will still involve development of advanced imaging and analytical tools, but I would argue that they should also be applied to other model systems to determine if there are correlative features, which I like to call “mineralogical signatures” of biomineralization.A particularly striking example of mineralogical signatures is the nanocolloidal texture found in PILP formed films. Given that a nanocolloidal texture has now been found in nearly all biologically-controlled biominerals (and pathological as well139), I proposed that a PILP-like process,34 or more broadly, a Colloid Assembly and Transformation (CAT) process,84 may lie at the foundation of nearly all biomineralization processes. Yes, that was a grandiose claim. But as further advancements in microscopy ensued over the years of my career, it seemed that nearly all literature examples kept lending support to this hypothesis. People kept discovering things that I had witnessed in vitro and known all along. For example, lattice strain or shifting orientation in single crystals, crystallographic splay and spherulitic textures along gradients, etc. I don't make these grandiose claims as a narcissistic play to gain attention for myself, but as a desire for more attention to be paid to a model system that I believe can help answer questions and resolve mechanisms that could never be ascertained from snapshots of forming biominerals.
Contrary to my perceived zealousness, I don't believe PILP is the whole story; it's actually just the beginning of the story. There are many unanswered questions and so much more that could be learned from this model system, so I hope others will continue using it to better understand protein/polymer interactions in modulating non-classical mineralization reactions. I may have retired from teaching, but I am not running short on ideas for research projects, so I am happy to consult and assist others with writing proposals and consulting on grants.
Some unanswered questions that still need to be addressed:
(1) What is the influence of protein primary structure (AA sequence) on sequestration of ion clusters, stabilization of amorphous phase(s), fluidic or viscoelastic properties of the amorphous phase, and transformation of the amorphous phase to the crystalline phase?
(a) Influence of AA composition versus sequence, charge density and type, and overall protein length versus ID domains.
(b) Influence of # and type of post-translational modifications.
(i) Phosphorylation and glycosylation functional groups and concentration.
(2) What is the influence of substrates on nucleation of the crystalline phase within the amorphous precursor?
(a) How substrate/matrix dictates transformation kinetics, crystal texture (single vs. polycrystalline, defects).
(b) How substrate dictates crystal location (and thus size), crystal orientation and phase.
(3) What is the mechanism of infiltration (i.e. transport) of mineral precursor into organic matrices (such as collagen and chitin).
(a) Capillary action vs. Gibbs–Donnan, or both, or other?
(b) Why/how does temperature influence collagen structure and/or infiltration.
(4) What is the influence of temperature?
(a) Lower temperature promotes CaCO3 PILP formation, but reduces transformation rate.
(b) Collagen infiltration is degraded at temperatures lower than 37 °C.
(i) Would marine animal collagen be more stable?
(5) What is the influence of inorganic impurities/additives?
(a) Why does Mg-ion strongly promote CaCO3 PILP films but inhibit CaP collagen intrafibrillar mineralization (as does Sr and Ba).
(6) What is the influence of organic impurities/additives?
(a) Citrate influence on collagen mineralization.
(b) Collagen crosslinking influence on mineralization.
(c) How to control the degree of collagen mineralization to avoid embrittlement.
(d) How do glycosaminoglycans (GAGs) influence collagen assembly and mineralization?
(i) How do advanced glycation end-products (AGE) affect collagen and mineralization?
(7) Are similar mechanisms involved in pathological biomineralizations?
(a) Ubiquitous nature of concentrically laminated spherules.
(i) Initiated with amorphous particles/droplets?
(ii) Exclusion of impurities leading to layers?
(8) Is the liquid–liquid phase separation in cellular processes similar to LL phase separation in PILP and biomineralization?
(9) What other inorganic systems could this process be applied to?
(a) Can we make advanced functional materials?
(i) Types of polymer/protein interactions with inorganic species.
(b) Is thin layering required to enable solidification? (Protein and water exclusion?)
(c) Can we control the spatiotemporal aspects that are accomplished by cellular processing in biology?
(i) Is enzymatic cleavage of proteins a means for modulating inhibitory activity, and thus process-directing agent activity is dynamically altered throughout biomineralization?
4.3 Development of an in vivo model system for studying biomineralization in situ
Some of the most valuable biomineral discoveries have been based on in situ examination of biomineralization occurring within a living organism. I consider the work by Beniash et al. on sea urchin larval spicule formation as being revolutionary.140,141 Notably, much of the information from these studies was also obtained with optical microscopy, because it provides the distinct advantage of enabling in situ observations. With the development of a plethora of labeling tools, there's no doubt that such tools have proven incredibly valuable to the biological realm overall. But from a biomineralization perspective, there are only so many organisms small enough to be examined in situ (such as urchin embryos and coccolithophores). Therefore, we have formed a multidisciplinary team at the University of Florida to take advantage of the unique facilities and expertise at the Whitney Laboratory for Marine Biosciences (located along Florida's northeast coast in the town of Marineland). The goal of this team is to develop a platform that will enable us (and others in the biomineral community) to experimentally study some of the questions posed above. To do this, we are creating our own small organismal model system, but one that can be designed and manipulated to study some of these questions.The first phase of our project is focused on the question relating to the role of post-translational modifications (PTMs) found in the IDPs regulating biomineral formation. It has long been known that many of these highly charged proteins also contain extensive glycosylation and phosphorylation. In the latter case, there have been studies to suggest that the phosphorylated residues play an important role in modulating biomineral formation, but precisely what that role is remains debatable. For example, the phosphorylated proteins have been shown in vitro to be highly inhibitory to classical crystal nucleation.142–144 But we need to move past the old school perspective that considered proteins as either “inhibitors” or “promoters” of mineral formation, because we now know that it is precisely this inhibitory activity that can transform a crystallization reaction to a non-classical reaction, namely the two-step amorphous precursor reaction pathway. In other words, the IDPs “function” is probably not just as an inhibitor, but as a process-directing agent.
With respect to glycosylation, this has mostly been ignored by the biomineral community (with a few exceptions),145–147 but not without reason; it is a nightmare to characterize glycosylation and there are relatively few experts in the biomineral field with this training. Another hurdle has been the fact that recombinant methods for producing proteins to study in vitro usually use the E. coli expression system, but bacteria do not produce PTMs, so studies using these proteins could totally miss the true activity of the protein. Therefore, to overcome this hurdle, our team wanted to work with an animal model system that could potentially provide the necessary enzymatic machinery to produce PTMs.
We chose the starlet sea anemone (Nematostella vectensis) because it is a small marine animal of size amenable to in vivo microscopic analysis. Anemones are in the animal kingdom and thus can produce PTMs, and being closely related to coral, we expect it to have similar enzymatic machinery that can yield similar PTMs. Because anemones do not naturally biomineralize, this system provides a blank slate for studying what might happen if we engineer it to secrete biomineral associated proteins. Martindale's group at the Whitney Laboratory has worked extensively with Nematostella, thereby providing this team with the opportunity to genetically engineer the anemone to produce such non-native proteins.148 A diverse set of biomineralizing IDP type proteins was chosen for our preliminary studies to determine the robustness of this system as a platform for studying PTMs, starting with coral CARP proteins (being a closely related organism from same phylogenetic branch of the Cnidarian family of Non-Actiniarian Anthozoans), to sea urchin spicule matrix protein SM-30 (prevalent in developing spicules), and human enamel ameloblastin (a vertebrate protein that modulates calcium phosphate deposition).
Our current efforts have been devoted to purification and analysis of these secreted proteins, to firstly determine if the anemone produces PTMs, and how those PTMs compare to the native organism, and then how such PTMs influence mineral formation. Mineral modulation with these secreted proteins is currently being examined in vitro, but we are also developing “cellular masonry” techniques149 that will ultimately enable the team to build cellular compartments to study the influence of such secreted proteins within a tailor-made biomineral compartment containing designer matrices.
Given that this paper is supposed to be on advanced microscopy tools, I don't want to go into too much detail. I just want to give a brief overview because it is our hopes that others in the biomineral community with complementary skills and advanced microscopy expertise might wish to collaborate with us, or more generally in the future, utilize this genetically-engineered animal model system for their own biomineral studies.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by NSF grant # IOS 2314456. I thank all the graduate students and postdoctoral associates who diligently performed all these studies on the PILP process. I am eternally grateful.References
- J. W. Morse and M. L. Bender, Partition coefficients in calcite: Examination of factors influencing the validity of experimental results and their application to natural systems, Chem. Geol., 1990, 82, 265–277 CrossRef CAS.
- G. D. Rosenberg, The “vital effect” on skeletal trace element content as exemplified by magnesium, in Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends, ed. J. G. Carter, Van Nostrand Reinhold, 1990, pp. 567–577 Search PubMed.
- S. Weiner and P. M. Dove, An overview of biomineralization processes and the problem of the vital effect, Biomineralization, 2003, 54, 1–29 CAS.
- W. J. Landis, K. J. Hodgens, J. Arena, M. J. Song and B. F. McEwen, Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography, J. Electron Microsc. Tech., 1996, 33, 192–202 CAS.
- J. H. Kinney, S. Habelitz, S. J. Marshall and G. W. Marshall, The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin, J. Dent. Res., 2003, 82(12), 957–961 CrossRef CAS PubMed.
- A. L. Arsenault, A comparative electron microscopic study of apatite crystals in collagen fibrils of rat bone, dentin and calcified turkey leg tendons, Bone Miner., 1989, 6(2), 165–177 CrossRef CAS PubMed.
- L. Addadi and S. Weiner, Stereochemical and structural relations between macromolecules and crystals in biomineralization, in Biomineralization - Chemical and Biochemical Perspectives, ed. S. Mannet al., VCH Pub, 1989, pp. 133–156 Search PubMed.
- L. Addadi, J. Moradian-Oldak and S. Weiner, Macromolecule-crystal recognition in biomineralization, in Surface Reactive Peptides and Polymers- Discovery and Commercialization, ed. C. S. Sikes and A. P. Wheeler, ACS Symp. Ser., 1991, vol. 444, pp. 13–27 Search PubMed.
- L. Addadi, Z. Berkovitch-Yellin, I. Weissbuch, J. v. Mil, L. J. W. Shimon, M. Lahav and L. Leiserowitz, Growth and dissolution of organic crystals with tailor-made inhibitors - Implications in stereochemistry and materials science, Angew. Chem., Int. Ed. Engl., 1985, 24, 466–485 CrossRef.
- S. Weiner and L. Addadi, Acidic macromolecules of mineralized tissues: the controllers of crystal formation, Trends Biochem. Sci., 1991, 16(7), 252–256 CrossRef CAS PubMed.
- S. Albeck, L. Addadi and S. Weiner, Regulation of calcite crystal morphology by intracrystalline acidic proteins and glycoproteins, Connect. Tissue Res., 1996, 35(1–4), 419–424 Search PubMed.
- S. Albeck, J. Aizenberg, L. Addadi and S. Weiner, Interactions of various skeletal intracrystalline components with calcite crystals, J. Am. Chem. Soc., 1993, 115(25), 11691–11697 CrossRef CAS.
- L. A. Gower and D. A. Tirrell, Calcium carbonate films and helices grown in solutions of poly(aspartate), J. Cryst. Growth, 1998, 191(1–2), 153–160 CrossRef CAS.
- L. B. Gower and D. J. Odom, Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process, J. Cryst. Growth, 2000, 210(4), 719–734 CrossRef CAS.
- A. P. Jackson, J. F. V. Vincent and R. M. Turner, The mechanical design of nacre, Proc. R. Soc. London, 1988, 234(1277), 415–440 Search PubMed.
- K. Wada, Spiral growth of nacre, Nature, 1966, 211(5056), 1427 CrossRef.
- G. Bevelander and H. Nakahara, An electron microscope study of the formation of the nacreous layer in the shell of certain bivalve molluscs, Calcif. Tissue Res., 1969, 3(1), 84–92 CrossRef CAS PubMed.
- H. K. Erben and N. Watabe, Crystal formation and growth in bivalve nacre, Nature, 1974, 248(5444), 128–130 CrossRef.
- S. W. Wise and J. deVilliers, Scanning electron microscopy of molluscan shell ultrastructures: screw dislocations in pelecypod nacre, Trans. Am. Microsc. Soc., 1971, 90, 376–380 CrossRef.
- H. Mutvei and E. Dunca, Crystalline structure, orientation and nucleation of the nacreous tablets in the cephalopod Nautilus, Paläont. Z., 2010, 84, 457–465 CrossRef.
- M. Rousseau and C. Rollion-Bard, Influence of the Depth on the Shape and Thickness of Nacre Tablets of Pinctada margaritifera PearlOyster, and on Oxygen Isotopic Composition, Minerals, 2012, 2(1), 55–64 CrossRef CAS.
- F. Nudelman, E. Shimoni, E. Klein, M. Rousseau, X. Bourrat, E. Lopez, L. Addadi and S. Weiner, Forming nacreous layer of the shells of the bivalves Atrina rigida and Pinctada margaritifera: an environmental- and cryo-scanning electron microscopy study, J. Struct. Biol., 2008, 162(2), 290–300 CrossRef CAS PubMed.
- H. H. Teng, P. M. Dove and J. J. DeYoreo, Reversed calcite morphologies induced by microscopic growth kinetics: Insight into biomineralization, Geochim. Cosmochim. Acta, 1999, 63(17), 2507–2512 CrossRef CAS.
- C. A. Orme, A. Noy, A. Wierzbicki, M. T. McBride, M. Grantham, H. H. Teng, P. M. Dove and J. J. DeYoreo, Formation of chiral morphologies through selective binding of amino acids to calcite surface steps, Nature, 2001, 411, 775–779 CrossRef CAS PubMed.
- P. M. Dove, F. Michael and J. Hochella, Calcite precipitation mechanisms and inhibition by orthophosphate: In situ observations by scanning force microscopy, Geochim. Cosmochim. Acta, 1993, 57, 705–714 CrossRef CAS.
- J. J. De Yoreo, A. Wierzbicki and P. M. Dove, New insights into mechanisms of biomolecular control on growth of inorganic crystals, CrystEngComm, 2007, 9(12), 1144–1152 RSC.
- T. E. Schaffer, C. Ionescu-Zanetti, R. Proksch, M. Fritz, D. A. Walters, N. Almqvist, C. M. Zaremba, A. M. Belcher, B. L. Smith, G. D. Stucky, D. E. Morse and P. K. Hansma, Does abalone nacre form by heteroepitaxial nucleation or by growth through mineral bridges?, Chem. Mater., 1997, 9(8), 1731–1740 CrossRef.
- M. Fritz, A. M. Belcher, M. Radmacher, D. A. Walters, P. K. Hansma, G. D. Stucky, D. E. Morse and S. Mann, Flat pearls from biofabrication of organized composites on inorganic substrates, Nature, 1994, 371, 49–51 CrossRef CAS.
- C. M. Zaremba, A. M. Belcher, M. Fritz, Y. Li, S. Mann, P. K. Hansma, D. E. Morse, J. S. Speck and G. D. Stucky, Critical transitions in the biofabrication of abalone shells and flat pearls, Chem. Mater., 1996, 8, 679–690 CrossRef CAS.
- S. Weiner, Y. Talmon and W. Traub, Electron-diffraction of mollusk shell organic matrices and their relationship to the mineral phase, Int. J. Biol. Macromol., 1983, 5(6), 325–328 CrossRef CAS.
- A. G. Checa, Physical and Biological Determinants of the Fabrication of Molluscan Shell Microstructures, Front. Mar. Sci., 2018, 5, 353 CrossRef.
- F. F. Amos, D. M. Sharbaugh, D. R. Talham and L. B. Gower, Formation of single-crystalline aragonite tablets/films via an amorphous precursor, Langmuir, 2007, 23(4), 1988–1994 CrossRef CAS PubMed.
- Y.-Y. Kim, E. P. Douglas and L. B. Gower, Patterning inorganic (CaCO3) thin films via a polymer-induced liquid-precursor process, Langmuir, 2007, 23(9), 4862–4870 CrossRef CAS PubMed.
- L. B. Gower, Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization, Chem. Rev., 2008, 108(11), 4551–4627 CrossRef CAS PubMed.
- A. M. Belcher, X. H. Wu, R. J. Christensen, P. K. Hansma, G. D. Stucky and D. E. Morse, Control of crystal phase switching and orientation by soluble mollusc-shell proteins, Nature, 1996, 381(6577), 56–58 CrossRef CAS.
- M. Niederberger and H. Cölfen, Oriented attachment and mesocrystals: Non-classical crystallization mechanisms based on nanoparticle assembly, Phys. Chem. Chem. Phys., 2006, 8(28), 3271–3287 RSC.
- H. Cölfen and M. Antonietti, Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment, Angew. Chem., Int. Ed., 2005, 44(35), 5576–5591 CrossRef PubMed.
- T. X. Wang, H. Cölfen and M. Antonietti, Nonclassical crystallization: Mesocrystals and morphology change of CaCO3 crystals in the presence of a polyelectrolyte additive, J. Am. Chem. Soc., 2005, 127(10), 3246–3247 CrossRef CAS PubMed.
- O. Galkin, K. Chen, R. L. Nagel, R. E. Hirsch and P. G. Vekilov, Liquid-liquid separation in solutions of normal and sickle cell hemoglobin, Proc. Natl. Acad. Sci. U. S. A., 2002, 99(13), 8479–8483 CrossRef CAS PubMed.
- M. Shah, O. Galkin and P. G. Vekilov, Smooth transition from metastability to instability in phase separating protein solutions, J. Chem. Phys., 2004, 121(15), 7505–7512 CrossRef CAS PubMed.
- P. G. Vekilov, Dense liquid precursor for the nucleation of ordered solid phases from solution, Cryst. Growth Des., 2004, 4(4), 671–685 CrossRef CAS.
- L. F. Filobelo, O. Galkin and P. G. Vekilov, Spinodal for the solution-to-crystal phase transformation, J. Chem. Phys., 2005, 123(1), 014904 CrossRef PubMed.
- D. Kashchiev, P. G. Vekilov and A. B. Kolomeisky, Kinetics of two-step nucleation of crystals, J. Chem. Phys., 2005, 122(24), 244706 CrossRef PubMed.
- P. G. Vekilov, Two-step mechanism for the nucleation of crystals from solution, J. Cryst. Growth, 2005, 275(1–2), 65–76 CrossRef CAS.
- D. Li, M. H. Nielsen, J. R. I. Lee, C. Frandsen, J. F. Banfield and J. J. De Yoreo, Direction-Specific Interactions Control Crystal Growth by Oriented Attachment, Science, 2012, 336(6084), 1014–1018 CrossRef CAS PubMed.
- L. Bahrig, S. G. Hickey and A. Eychmüller, Mesocrystalline materials and the involvement of oriented attachment – a review, CrystEngComm, 2014, 16(40), 9408–9424 RSC.
- M. P. Boneschanscher, W. H. Evers, J. J. Geuchies, T. Altantzis, B. Goris, F. T. Rabouw, S. A. P. van Rossum, H. S. J. van der Zant, L. D. A. Siebbeles, G. Van Tendeloo, I. Swart, J. Hilhorst, A. V. Petukhov, S. Bals and D. Vanmaekelbergh, Long-range orientation and atomic attachment of nanocrystals in 2D honeycomb superlattices, Science, 2014, 344(6190), 1377–1380 CrossRef CAS PubMed.
- N. Gehrke, H. Cölfen, N. Pinna, M. Antonietti and N. Nassif, Superstructures of calcium carbonate crystals by oriented attachment, Cryst. Growth Des., 2005, 5(4), 1317–1319 CrossRef CAS.
- I. Sethmann, A. Putnis, O. Grassmann and P. Lobmann, Observation of nano-clustered calcite growth via a transient phase mediated by organic polyanions: A close match for biomineralization, Am. Mineral., 2005, 90, 1213–1217 CrossRef CAS.
- I. Sethmann, R. Hinrichs, G. Wörheide and A. Putnis, Nano-cluster composite structure of calcitic sponge spicules—A case study of basic characteristics of biominerals, J. Inorg. Biochem., 2006, 100, 88–96 CrossRef CAS PubMed.
- I. Sethmann and G. Wörheide, Structure and composition of calcareous sponge spicules: A review and comparison to structurally related biominerals, Micron, 2008, 39(3), 209–228 CrossRef CAS PubMed.
- M. Rousseau, E. Lopez, P. Stempfle, M. Brendle, L. Franke, A. Guette, R. Naslain and X. Bourrat, Multiscale structure of sheet nacre, Biomaterials, 2005, 26(31), 6254–6262 CrossRef CAS PubMed.
- L. Dai, X. Cheng and L. B. Gower, Transition Bars during Transformation of an Amorphous Calcium Carbonate Precursor, Chem. Mater., 2008, 20(22), 6917–6928 CrossRef CAS.
- M. J. Weedon and P. D. Taylor, Calcitic nacreous ultrastructures in bryozoans: Implications for comparative biomineralization of lophophorates and molluscs, Biol. Bull., 1995, 188(3), 281–292 CrossRef CAS PubMed.
- L. B. Gower, F. F. Amos and S. R. Khan, Mineralogical Signatures of Stone Formation Mechanisms, Urol. Res., 2010, 38(4), 281–292 CrossRef CAS PubMed.
- J. Sun and B. Bhushan, Hierarchical structure and mechanical properties of nacre: a review, RSC Adv., 2012, 2, 7617–7632 RSC.
- X. Li, Z.-H. Xu and R. Wang, In situ observation of nanograin rotation and deformation in nacre, Nano Lett., 2006, 6(10), 2301–2304 CrossRef CAS PubMed.
- A. G. Checa, H. Mutvei, A. J. Osuna-Mascaró, J. T. Bonarski, M. Faryna, K. Berent, C. M. Pina, M. Rousseau and E. Macías-Sánchez, Crystallographic control on the substructure of nacre tablets, J. Struct. Biol., 2013, 183(3), 368–376 CrossRef CAS PubMed.
- J. Aizenberg, J. Hanson, T. F. Koetzle, L. Leiserowitz, S. Weiner and L. Addadi, Biologically induced reduction in symmetry - a study of crystal texture of calcitic sponge spicules, Chem.–Eur. J., 1995, 1(7), 414–422 CrossRef CAS.
- F. F. Amos, L. Dai, R. Kumar, S. R. Khan and L. B. Gower, Mechanism of formation of concentrically laminated spherules: implication to Randall's plaque and stone formation, Urol. Res., 2009, 37(1), 11–17 CrossRef PubMed.
- A. P. Evan, F. L. Coe, S. R. Rittling, S. M. Bledsoe, Y. Shao, J. E. Lingeman and E. M. Worcester, Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: Osteopontin localization, Kidney Int., 2005, 68(1), 145–154 CrossRef CAS PubMed.
- S. R. Khan, Calcium Phosphate/Calcium Oxalate Crystal Association in Urinary Stones: Implications for Heterogeneous Nucleation of Calcium Oxalate, J. Urol., 1997, 157(1), 376–383 CrossRef CAS PubMed.
- R. L. Ryall, The future of stone research: rummagings in the attic, Randall's plaque, nanobacteria, and lessons from phylogeny, Urol. Res., 2008, 36(2), 77–97, DOI:10.1007/s00240-007-0131-3.
- J. S. Evans, ‘Apples' and ‘oranges': comparing the structural aspects of biomineral- and ice-interaction proteins, Curr. Opin. Colloid Interface Sci., 2003, 8(1), 48–54 CrossRef CAS.
- N. L. Huq, K. J. Cross, M. Ung and E. C. Reynolds, A review of protein structure and gene organisation for proteins associated with mineralised tissue and calcium phosphate stabilisation encoded on human chromosome 4, Arch. Oral Biol., 2005, 50(7), 599–609 CrossRef CAS PubMed.
- G. K. Hunter, J. O'Young, B. Grohe, M. Karttunen and H. A. Goldberg, The flexible polyelectrolyte hypothesis of protein-biomineral interaction, Langmuir, 2010, 26(24), 18639–18646 CrossRef CAS PubMed.
- M. Wojtas, P. Dobryszycki and A. Ożyhar, Intrinsically Disordered Proteins in Biomineralization, in Advanced Topics in Biomineralization, ed. J. Seto, 2012 Search PubMed.
- V. N. Uversky, Unreported intrinsic disorder in proteins: Building connections to the literature on IDPs, Intrinsically Disord. Proteins, 2014, 2(1), e970499 CrossRef PubMed.
- A. L. Boskey and E. Villarreal-Ramirez, Intrinsically disordered proteins and biomineralization, Matrix Biol., 2016, 52–54, 43–59 CrossRef CAS PubMed.
- N. Nassif, N. Pinna, N. Gehrke, M. Antonietti, C. Jager and H. Cölfen, Amorphous layer around aragonite platelets in nacre, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(36), 12653–12655 CrossRef CAS PubMed.
- R. T. DeVol, C.-Y. Sun, M. A. Marcus, S. N. Coppersmith, S. C. B. Myneni and P. U. P. A. Gilbert, Nanoscale Transforming Mineral Phases in Fresh Nacre, J. Am. Chem. Soc., 2015, 137, 13325–13333 CrossRef CAS PubMed.
- E. Macías-Sánchez, M. G. Willinger, C. M. Pina and A. G. Checa, Transformation of ACC into aragonite and the origin of the nanogranular structure of nacre, Sci. Rep., 2017, 7(1), 12728 CrossRef PubMed.
- G. Zhang and J. Xu, From colloidal nanoparticles to a single crystal: New insights into the formation of nacre's aragonite tablets, J. Struct. Biol., 2013, 182, 36–43 CrossRef CAS PubMed.
- N. Gehrke, N. Nassif, N. Pinna, M. Antonietti, H. S. Gupta and H. Cölfen, Retrosynthesis of nacre via amorphous precursor particles, Chem. Mater., 2005, 17(26), 6514–6516 CrossRef CAS.
- N. Yao, A. Epstein and A. Akey, Crystal growth via spiral motion in abalone shell nacre, J. Mater. Res., 2006, 21(6), 1939–1946 CrossRef CAS.
- H. Nakahara, An electron microscope study of the growing surface of nacre in two gastropod species, Turbo cornutus and Tegula pfeifferi, Venus, 1979, 38, 205–211 Search PubMed.
- H. Nakahara, Nacre formation in bivalve and gastropod molluscs, in Mechanisms and Phylogeny of Mineralization in Biological Systems, ed. S. Suga and H. Nakahara, Springer-Verlag, 1991, pp. 344–350 Search PubMed.
- E. DiMasi, T. Liu, M. J. Olszta and L. B. Gower, Laser light scattering studies of a polymer-induced liquid-precursor (PILP) process for mineralization, in Biological and Bio-Inspired Materials and Devices, ed. K. H. Sandhageet al., Mat. Res. Society Proceedings, 2005, pp. 177–183 Search PubMed.
- M. Rousseau, E. Lopez, A. Couté, G. Mascarel, D. C. Smith, R. Naslain and X. Bourrat, Sheet nacre growth mechanism: a Voronoi model, J. Struct. Biol., 2005, 149(2), 149–157 CrossRef PubMed.
- G. Falini, S. Albeck, S. Weiner and L. Addadi, Control of aragonite or calcite polymorphism by mollusk shell macromolecules, Science, 1996, 271, 67–69 CrossRef.
- M. E. Marsh and R. L. Sass, Calcium-Binding Phosphoprotein Particles in the Extrapallial Fluid and Innermost Shell Lamella of Clams, J. Exp. Zool., 1983, 226, 193–203 CrossRef CAS.
- M. E. Marsh, Biomineralization in the Presence of Calcium-Binding Phosphoprotein Particles, J. Exp. Biol., 1986, 239, 207–220 CrossRef CAS PubMed.
- R. Hovden, S. E. Wolf, M. E. Holtz, F. Marin, D. A. Muller and L. A. Estroff, Nanoscale assembly processes revealed in the nacroprismatic transition zone of Pinna nobilis mollusc shells, Nat. Commun., 2015, 6, 10097 CrossRef CAS PubMed.
- L. Gower and J. Elias, Colloid assembly and transformation (CAT): The relationship of PILP to biomineralization, J. Struct. Biol.:X, 2022, 6, 100059 CAS.
- A. Ural and D. Vashishth, Hierarchical perspective of bone toughness – from molecules to fracture, Int. Mater. Rev., 2014, 59(5), 245–263 CrossRef CAS.
- D. J. Buss, R. Kröger, M. D. McKee and N. Reznikov, Hierarchical organization of bone in three dimensions: A twist of twists, J. Struct. Biol.:X, 2022, 6, 100057 CAS.
- S. Weiner and H. D. Wagner, THE MATERIAL BONE: Structure-Mechanical Function Relations, Annu. Rev. Mater. Sci., 1998, 28(1), 271–298 CrossRef CAS.
- S. Weiner, W. Traub and H. Wagner, Lamellar Bone: structure-function relations, J. Struct. Biol., 1999, 126(3), 241–256 CrossRef CAS PubMed.
- E. Bonucci and G. Gherardi, Histochemical and electron microscope investigations on medullary bone, Cell Tissue Res., 1975, 163(1), 81–97 CrossRef CAS PubMed.
- T. Hasegawa, Ultrastructure and biological function of matrix vesicles in bone mineralization, Histochem. Cell Biol., 2018, 149(4), 289–304 CrossRef CAS PubMed.
- A. C. Lovett, S. R. Khan and L. B. Gower, Development of a two-stage in vitro model system to investigate the mineralization mechanisms involved in idiopathic stone formation: stage 1—biomimetic Randall's plaque using decellularized porcine kidneys, Urolithiasis, 2019, 47(4), 321–334 CrossRef PubMed.
- W. Traub, A. Jodaikin, T. Arad, A. Veis and B. Sabsay, Dentin phosphophoryn binding to collagen fibrils, Matrix, 1992, 12(3), 197–201 CrossRef CAS PubMed.
- Q. Q. Hoang, F. Sicheri, A. J. Howard and D. S. C. Yang, Bone recognition mechanism of porcine osteocalcin from crystal structure, Nature, 2003, 425(6961), 977–980 CrossRef CAS PubMed.
- A. Veis, Mineral-matrix interactions in bone and dentin, J. Bone Miner. Res., 1993, 8(S2), S493 CrossRef PubMed.
- J. C. Góes, S. D. Figueiró, A. M. Oliveira, C. C. Silva, N. M. P. S. Ricardo and A. S. B. Sombra, Apatite coating on anionic and native collagen films by an alternate soaking process, Acta Biomater., 2007, 3, 773–778 CrossRef PubMed.
- M. J. Olszta, E. P. Douglas and L. B. Gower, Scanning electron microscopic analysis of the mineralization of type I collagen via a polymer-induced liquid-precursor (PILP) process, Calcif. Tissue Int., 2003, 72(5), 583–591 CrossRef CAS PubMed.
- M. J. Olszta, D. J. Odom, E. P. Douglas and L. B. Gower, A new paradigm for biomineral formation: Mineralization via an amorphous liquid-phase precursor, Connect. Tissue Res., 2003, 44, 326–334 CrossRef CAS PubMed.
- M. J. Olszta, X. Cheng, S. S. Jee, R. Kumar, Y.-Y. Kim, M. J. Kaufman, E. P. Douglas and L. B. Gower, Bone structure and formation: A new perspective, Mater. Sci. Eng., R, 2007, 58(3–5), 77–116 CrossRef.
- W. J. Landis, M. J. Song, A. Leith, L. McEwen and B. F. McEwen, Mineral and Organic Matrix Interaction in Normally Calcifying Tendon Visualized in Three Dimensions by High-Voltage Electron Microscopic Tomography and Graphic Image Reconstruction, J. Struct. Biol., 1993, 110, 39–54 CrossRef CAS PubMed.
- J. Orgel, T. C. Irving, A. Miller and T. J. Wess, Microfibrillar structure of type I collagen in situ, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(24), 9001–9005 CrossRef CAS PubMed.
- S.-S. Jee, T. T. Thula and L. B. Gower, Development of bone-like composites via the polymer-induced liquid-precursor (PILP) process. Part 1: Influence of polymer molecular weight, Acta Biomater., 2010, 6(9), 3676–3686 CrossRef CAS PubMed.
- N. Saxena, J. Mizels, M. A. Cremer, V. Guarnizo, D. E. Rodriguez and L. B. Gower, Comparison of Synthetic vs. Biogenic Polymeric Process-Directing Agents for Intrafibrillar Mineralization of Collagen, Polymers, 2022, 14(4), 775 CrossRef CAS PubMed.
- J. Mahamid, A. Sharir, L. Addadi and S. Weiner, Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(35), 12748–12753 CrossRef CAS PubMed.
- J. Mahamid, B. Aichmayer, E. Shimoni, R. Ziblat, C. H. Li, S. Siegel, O. Paris, P. Fratzl, S. Weiner and L. Addadi, Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(14), 6316–6321 CrossRef CAS PubMed.
- J. Mahamid, A. Sharir, D. Gur, E. Zelzer, L. Addadi and S. Weiner, Bone mineralization proceeds through intracellular calcium phosphate loaded vesicles: A cryo-electron microscopy study, J. Struct. Biol., 2011, 174(3), 527–535 CrossRef CAS PubMed.
- F. Nudelman, K. Pieterse, A. George, P. H. H. Bomans, H. Friedrich, L. J. Brylka, P. A. J. Hilbers, G. de With and N. A. J. M. Sommerdijk, The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors, Nat. Mater., 2010, 9(12), 1004–1009 CrossRef CAS PubMed.
- S. S. Jee, L. Culver, Y. Li, E. P. Douglas and L. B. Gower, Biomimetic mineralization of collagen via an enzyme-aided PILP process, J. Cryst. Growth, 2010, 312, 1249–1256 CrossRef CAS.
- T. T. Thula, S.-S. Jee, Y. Li and L. B. Gower, Collagen-Hydroxyapatite Composites Mimicking Bone Nano-Architecture: Cell Adhesion and Proliferation Study, in 25th Southern Biomedical Engineering Conference 2009, ed. A. McGoronet al., Springer, Miami, Florida, USA, 2009 Search PubMed.
- L.-n. Niu, S. E. Jee, K. Jiao, L. Tonggu, M. Li, L. Wang, Y.-d. Yang, J.-h. Bian, L. Breschi, S. S. Jang, J.-h. Chen, D. H. Pashley and F. R. Tay, Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality, Nat. Mater., 2017, 16(3), 370–378 CrossRef CAS PubMed.
- P. A. Price, D. Toroian and J. E. Lim, Mineralization by Inhibitor Exclusion - The calcification of collagen with fetuin, J. Biol. Chem., 2009, 284(25), 17092–17101 CrossRef CAS PubMed.
- Y. Wang, T. Azais, M. Robin, A. Vallee, C. Catania, P. Legriel, G. Pehau-Arnaudet, F. Babonneau, M.-M. Giraud-Guille and N. Nassif, The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite, Nat. Mater., 2012, 11(8), 724–733 CrossRef CAS PubMed.
- B. Cantaert, E. Beniash and F. C. Meldrum, Nanoscale Confinement Controls the Crystallization of Calcium Phosphate: Relevance to Bone Formation, Chem.–Eur. J., 2013, 19(44), 14918–14924 CrossRef CAS PubMed.
- D. E. Rodriguez, T. Thula-Mata, E. J. Toro, Y.-W. Yeh, C. Holt, L. S. Holliday and L. B. Gower, Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts, Acta Biomater., 2014, 10(1), 494–507 CrossRef CAS PubMed.
- Y. Xu, F. Nudelman, E. D. Eren, M. J. M. Wirix, B. Cantaert, W. H. Nijhuis, D. Hermida-Merino, G. Portale, P. H. H. Bomans, C. Ottmann, H. Friedrich, W. Bras, A. Akiva, J. P. R. O. Orgel, F. C. Meldrum and N. Sommerdijk, Intermolecular channels direct crystal orientation in mineralized collagen, Nat. Commun., 2020, 11(1), 5068 CrossRef CAS PubMed.
- Y. Xu, K. C. H. Tijssen, P. H. H. Bomans, A. Akiva, H. Friedrich, A. P. M. Kentgens and N. A. J. M. Sommerdijk, Microscopic structure of the polymer-induced liquid precursor for calcium carbonate, Nat. Commun., 2018, 9, 2582 CrossRef PubMed.
- V. B. Rosen, L. W. Hobbs and M. Spector, The ultrastructure of anorganic bovine bone and selected synthetic hydroxyapatites used as bone graft substitute materials, Biomaterials, 2002, 23, 921–928 CrossRef PubMed.
- P.-Y. Chen, D. Toroian, P. A. Price and J. McKittrick, Minerals Form a Continuum Phase in Mature Cancellous Bone, Calcif. Tissue Int., 2011, 88(5), 351–361 CrossRef CAS PubMed.
- N. Saeidi, K. P. Karmelek, J. A. Paten, R. Zareian, E. DiMasi and J. W. Ruberti, Molecular crowding of collagen: A pathway to produce highly-organized collagenous structures, Biomaterials, 2012, 33(30), 7366–7374 CrossRef CAS PubMed.
- M.-M. Giraud-Guille, Direct visualization of microtomy artefacts in sections of twisted fibrous extracellular matrices, Tissue Cell, 1986, 18(4), 603–620 CrossRef CAS PubMed.
- Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends, ed. J. G. Carter, Van Nostrand Reinhold, 1990 Search PubMed.
- B. Wingender, Y. Ni, Y. Zhang, C. Taylor and L. Gower, Hierarchical Characterization and Nanomechanical Assessment of Biomimetic Scaffolds Mimicking Lamellar Bone via Atomic Force Microscopy Cantilever-Based Nanoindentation, Materials, 2018, 11(7), 1257 CrossRef PubMed.
- B. Wingender, P. Bradley, N. Saxena, J. W. Ruberti and L. Gower, Biomimetic organization of collagen matrices to template bone-like microstructures, Matrix Biol., 2016, 52–54, 384–396 CrossRef CAS PubMed.
- N. Reznikov, M. Bilton, L. Lari, M. M. Stevens and R. Kröger, Fractal-like hierarchical organization of bone begins at the nanoscale, Science, 2018, 360, 6388 CrossRef PubMed.
- P. U. P. A. Gilbert, S. M. Porter, C.-Y. Sun, S. Xiao, B. M. Gibson, N. Shenkar and A. H. Knoll, Biomineralization by particle attachment in early animals, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(36), 17659–17665 CrossRef CAS PubMed.
- E. J. Wheeler and D. Lewis, An X-ray study of the paracrystalline nature of bone apatite, Calcif. Tissue Int., 1977, 24(1), 243–248 CrossRef CAS PubMed.
- D. G. A. Nelson, J. D. B. Featherstone, J. F. Duncan and T. W. Cutress, Paracrystalline disorder of biological and synthetic carbonate-substituted apatites, J. Dent. Res., 1982, 61(11), 1274–1281 CrossRef CAS PubMed.
- M. J. Olszta, S. Gajjeraman, M. M. Kaufman and L. B. Gower, Nanofibrous calcite synthesized via a solution-precursor-solid mechanism, Chem. Mater., 2004, 16(12), 2355–2362 CrossRef CAS.
- S. J. Homeijer, M. J. Olszta, R. A. Barrett and L. B. Gower, Growth of nanofibrous barium carbonate on calcium carbonate seeds, J. Cryst. Growth, 2008, 310(11), 2938–2945 CrossRef CAS.
- S. J. Homeijer, R. A. Barrett and L. B. Gower, Polymer-Induced Liquid-Precursor (PILP) Process in the Non-Calcium Based Systems of Barium and Strontium Carbonate, Cryst. Growth Des., 2010, 10(3), 1040–1052 CrossRef CAS.
- Y.-y. Kim and L. Gower, Biomimetic Patterning of Ceramic Thin Films, in 10th International Ceramics Congress- Part B- Non Conventional Routes to Ceramics, CIMTEC ed, 2002 Search PubMed.
- X. Cheng and L. B. Gower, Molding Mineral within Microporous Hydrogels by a Polymer-Induced Liquid-Precursor (PILP) Process, Biotechnol. Prog., 2006, 22(1), 141–149 CrossRef CAS PubMed.
- X. Cheng, P. L. Varona, M. J. Olszta and L. B. Gower, Biomimetic synthesis of calcite films by a polymer-induced liquid-precursor (PILP) process 1. Influence and incorporation of magnesium, J. Cryst. Growth, 2007, 307(2), 395–404 CrossRef CAS.
- T. Thula-Mata, A. Burwell, L. B. Gower, S. Habelitz and G. Marshall, Remineralization of Artificial Dentin Lesions via the Polymer-Induced Liquid-Precursor (PILP) Process, in Biological Hybrid Materials for Life Sciences, ed. L. Stanciuet al., Mater. Res. Soc. Symp. Proc. JJ-, 2011, pp. 1–9 Search PubMed.
- A. K. Burwell, T. Thula-Mata, L. B. Gower, S. Habeliz, M. Kurylo, S. P. Ho, Y.-C. Chien, J. Cheng, N. F. Cheng, S. A. Gansky, S. J. Marshall and G. W. Marshall, Functional Remineralization of Dentin Lesions Using Polymer-Induced Liquid-Precursor Process, PLoS One, 2012, 7(6), e38852 CrossRef CAS PubMed.
- C. M. França, G. Thrivikraman, A. Athirasala, A. Tahayeri, L. B. Gower and L. E. Bertassoni, The influence of osteopontin-guided collagen intrafibrillar mineralization on pericyte differentiation and vascularization of engineered bone scaffolds, J. Biomed. Mater. Res., Part B, 2019, 107(5), 1522–1532 CrossRef PubMed.
- G. Thrivikraman, A. Athirasala, R. Gordon, L. Zhang, R. Bergan, D. R. Keene, J. M. Jones, H. Xie, Z. Chen, J. Tao, B. Wingender, L. Gower, J. L. Ferracane and L. E. Bertassoni, Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization, Nat. Commun., 2019, 10, 3520 CrossRef PubMed.
- A. Chidambaram, D. Rodriguez, S. Khan and L. Gower, Biomimetic Randall's plaque as an in vitro model system for studying the role of acidic biopolymers in idiopathic stone formation, Urolithiasis, 2015, 43(1), 77–92 CrossRef CAS PubMed.
- A. L. O'Kell, A. C. Lovett, B. K. Canales, L. B. Gower and S. R. Khan, Development of a two-stage model system to investigate the mineralization mechanisms involved in idiopathic stone formation: stage 2 in vivo studies of stone growth on biomimetic Randall's plaque, Urolithiasis, 2019, 47(4), 335–346 CrossRef PubMed.
- S. E. Wolf, J. Harris, A. Lovett and L. Gower, Non-classical crystallization processes:Potential relevance to stone formation, in Kidney Stones: Medical and Surgical Management, ed. F. L. Coeet al., Jaypee Brothers, Medical Publishers Pvt. Limited, 2nd revised edn, 2017, p. 900 Search PubMed.
- E. Beniash, J. Aizenberg, L. Addadi and S. Weiner, Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth, Proc. R. Soc. London, Ser. B, 1997, 264, 461–465 CAS.
- E. Beniash, L. Addadi and S. Weiner, Cellular Control Over Spicule Formation in Sea Urchin Embryos: A Structural Approach, J. Struct. Biol., 1999, 125(1), 50–62 CrossRef CAS PubMed.
- A. Gericke, C. Qin, L. Spevak, Y. Fujimoto, W. T. Butler, E. S. Sorensen and A. L. Boskey, Importance of phosphorylation for osteopontin regulation of biomineralization, Calcif. Tissue Int., 2005, 77(1), 45–54 CrossRef CAS PubMed.
- A. L. Boskey, B. Christensen, H. Taleb and E. S. Sørensen, Post-translational modification of osteopontin: Effects on in vitro hydroxyapatite formation and growth, Biochem. Biophys. Res. Commun., 2012, 419, 333–338 CrossRef CAS PubMed.
- E. Villarreal-Ramirez, R. Garduño-Juarez, A. Gericke and A. Boskey, The role of phosphorylation in dentin phosphoprotein peptide absorption to hydroxyapatite surfaces: a molecular dynamics study, Connect. Tissue Res., 2014, 55(S1), 134–137 CrossRef CAS PubMed.
- C. Qin, O. Baba and W. T. Butler, Post-translational Modifications of SIBLING Proteins and Their Roles in Osteogenesis and Dentinogenesis, Crit. Rev. Oral Biol. Med., 2004, 15(3), 126–136 CrossRef CAS PubMed.
- B. Christensen, T. E. Petersen and E. S. Sørensen, Post-translational modification and proteolytic processing of urinary osteopontin, Biochem. J., 2008, 411, 53–61 CrossRef CAS PubMed.
- J. S. Evans, Glycosylation: A “Last Word” in the Protein-Mediated Biomineralization Process, Crystals, 2020, 10(9), 818 CrossRef CAS.
- B. Foster, F. Hugosson, F. Scucchia, C. Enjolras, L. S. Babonis, W. Hoaen and M. Q. Martindale, A novel in vivo system to study coral biomineralization in the starlet sea anemone, Nematostella vectensis, iScience, 2024, 27(3), 109131 CrossRef CAS PubMed.
- C. S. O'Bryan, C. P. Kabb, B. S. Sumerlin and T. E. Angelini, Jammed Polyelectrolyte Microgels for 3D Cell Culture Applications: Rheological Behavior with Added Salts, ACS Appl. Bio Mater., 2019, 2(4), 1509–1517 CrossRef PubMed.
- Y. Lu, et al., Tailoring Self-Organized Growth of Biomimetic Inorganic–Organic Multilayers with a Permeable Microcompartment, Small, 2025, 2503097 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
