Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Compositional variability in roller-mill processed streams: distribution of macro- and micronutrients, phytochemicals, and contaminants (heavy metals and anti-nutrients)
Veeranna Hitlamani a,
Swamy Gowda M. R.†
b,
Salony Azam Sheikh†cd,
Nandini P. Shettybd,
Sridevi Annapurna Singhc and
Aashitosh Ashok Inamdar
a,
Swamy Gowda M. R.†
b,
Salony Azam Sheikh†cd,
Nandini P. Shettybd,
Sridevi Annapurna Singhc and
Aashitosh Ashok Inamdar *ad
*ad
aDepartment of Flour Milling, Baking and Confectionery Technology, CSIR-Central Food Technological Research Institute, Mysuru 570020, India. E-mail: aainamdar@cftri.res.in
bDepartment of Plant Cell Biotechnology, CSIR-Central Food Technological Research Institute, Mysuru 570020, India
cDepartment of Traditional Foods & Applied Nutrition, CSIR-Central Food Technological Research Institute, Mysuru 570020, India
dAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, 201002, India
First published on 26th November 2025
Abstract
This study investigated the micro- and macronutrient compositions, phytochemicals, heavy metals, and antinutritional factors of industrial wheat milling streams, including straight run flour (SRF), breaks (B1–B4), reductions (C1–C5), and by-products (fine bran, coarse bran, and germ). Specific streams such as B2, C1, C2F1, and C2F2 exhibited higher brightness, while the proximate composition varied significantly (p <0.05) across streams. Endosperm-rich flours were dominated by proline (6.07–14.19%) and glutamic acid (22.97–41.40%), whereas germ was richer in lysine (6.28%), arginine (7.13%), and threonine (4.27%). Break flours contained significantly (p <0.05) lower mineral levels but higher gluten strength (10.07–15.47%), while by-products were abundant in phenolics (127.16–138.80 mg GAE/100 g), carotenoids (0.115–0.141 mg/100 g), and antioxidant capacity (FRAP: 36.08–69.19 µmol Fe2+ per g). The particle size distribution (PSD) (<50 µm: 0.86–38.38%) influenced the functionality, with finer fractions showing significantly reduced phytochemical content. Heavy metals and anti-nutrients (tannins: 0.59–1.397 mg g−1; phytic acid: 0.157–0.167%) were concentrated in bran fractions. Principal component analysis (PCA) revealed substantial variability, with PC1 and PC2 explaining 53.5% and 12.52% of the variation, respectively. Overall, these findings emphasize the potential of precision milling and strategic stream recombination to develop tailored ingredients, refined flours optimized for functionality, and by-products enriched for nutritional fortification. Additionally, understanding the distribution of acrylamide formation precursors (amino acids and reducing sugars) provides opportunities to minimize acrylamide formation in end products.
Sustainability spotlightThis study highlights the untapped sustainability potential of industrial wheat milling by showing how different flour streams and by-products can be strategically valorized. Endosperm-rich fractions, such as B2, C1, C2F1, and C2F2, provided refined flours with desirable brightness, gluten strength, and functional suitability for baked goods. In contrast, bran and germ fractions, often relegated to by-products, were shown to be nutrient-dense reservoirs rich in essential amino acids, phenolics, carotenoids, and antioxidant capacity. By mapping micro- and macronutrients, bioactive compounds, heavy metals, and antinutritional factors across streams, the study underscores the value of precision milling as a tool for circular food systems. Rather than viewing bran, germ, and coarse fractions as waste, they can be repositioned as functional fortifiers in nutrition-focused formulations. Importantly, insights into particle size distribution and acrylamide precursors (amino acids and reducing sugars) offer pathways for reducing food-processing contaminants, aligning with sustainable production and safer consumption goals (SDG 12). Together, these findings show that targeted recombination of streams can reduce post-harvest losses, promote whole-grain utilization, and expand the nutritional portfolio of wheat-based foods. Its emphasis on by-product valorization and flour blending optimization for better nutrition and safety highlights its importance for sustainable milling practices and food system innovation. |
1. Introduction
Wheat milling is one of the world's oldest and most widespread food industries, evolving significantly in technology, equipment, automation, and management. Key drivers of this change include globalization and food safety demands.1 While globalization has expanded the market for milling products, a significant challenge remains to adhere to regional standards when exporting locally processed raw materials using domestic machinery. The valorization of roller-mill flour streams presents a significant opportunity to enhance flour-based products' nutritional quality and functional properties. These streams, consisting of various fractions obtained during wheat milling, exhibit diverse distributions of essential nutrients, including proteins, vitamins, amino acids, minerals, sugars, bioactive compounds, heavy metals, and anti-nutrients. Understanding their nutritional profile is crucial for optimizing their utilization in creating specialty flour/choosing the right flour streams for food fortification and functional food development.2The endosperm is first separated from the bran layers and then crushed into many milling stream flours during the mechanical progressive reduction of milling wheat.3 The quality of grain and milling process flow determines the number and quality of the milling fractions produced.4 Roller mills are the most popular mills for making wheat flour used in the bakery and cereal-based industries. This procedure involves successively grinding cleaned and conditioned wheat with corrugated and smooth rolls, sifting, and purification. A certain amount of flour is created in each phase, removed, and blended in appropriate ratios to make the required flour.5
Proteins (10–15%), lipids (1.5–2%), and accessible and unavailable carbohydrates (65–70%) constitute the majority of the chemical components of wheat. Because of the uneven distribution of these elements throughout the wheat kernel, separate streams have varied compositions and functional features.6 The composition of various anatomical sections and the endosperm zone from which they originate determines the functional qualities of the flour fractions acquired at different technological stages. The type of millstream affects the granularity, presence of damaged starch, protein content, ash level, fat content, and the degree of enzymatic activity and amount of fibers,4 as well as the presence of amino acids, minerals, phytic acid, and bioactive compounds. With more breaks and reductions, protein and ash content increased.7 The streams created during the later reduction passes include more lipids. Many experimental studies8–11 have reported the distribution of hydrolytic (amylases, proteases, etc.) and oxidation–reduction (oxygenase, polyphenol oxidase, peroxidase, etc.) enzymes in different mill streams, typically about the dough and breadmaking. Every et al.10,11 also investigated the breadmaking characteristics of wheat milling fractions with glutathione and oxidized glutathione concentrations.
Knowing different flour streams' composition, function, and quality characteristics is crucial for choosing the right ones for different end products.4 Breaking, size splitting, and reduction are three milling operations that produce flour mill streams that vary in composition, rheological characteristics, and overall technical usefulness. As a result, flour mill streams may be used to determine how effectively milling processes work and how to blend flour mill streams in the right proportions to produce tail or end-milled flour. Such flours have specific end-use characteristics that satisfy consumer preferences.12 The quality of the wheat grain dictates the type and amount of flour produced at each break, and the reduction rollers are located next to the sizing (sifter and purifier). A miller carefully combined the resultant mill streams to create a variety of flours with various qualities and levels of fineness for different end purposes. One may regulate the flour's baking qualities by carefully selecting the flour streams to connect.13,14
Numerous studies have shown that understanding the variety of physicochemical and rheological characteristics of mill streams is essential to maximize the quality of the end product.15,16 It is important to note that straight-grade flour was created during milling by combining all flour streams from each roll. Specialty flour, on the other hand, is created by combining flour mill streams to obtain the appropriate quality for final goods such as bread, cookies, biscuits, and cakes.14,17
Many prior investigations on wheat milling streams were narrow in scope, focusing on a limited set of components such as basic macronutrients or isolated compound classes. However, few studies have comprehensively examined the co-distribution of essential nutrients, phytochemicals, and potential contaminants across roller-milled wheat streams. To bridge this gap, our study provides a multi-parameter analysis by exploring the compositional differences across industrial roller-milled streams (break, reduction, and by-products), simultaneously assessing macro- and micronutrients, amino acids, sugars, heavy metals, anti-nutrients, and bioactive compounds.
This integrated approach aims to identify high-value streams that can be leveraged to improve dietary intake, promote health benefits, and maximize the potential of wheat milling by-products, thereby reducing waste.
2. Materials and methods
2.1. Raw materials
Medium hard wheat (Triticum aestivum) was procured from a local market in Mysuru, Karnataka, India. They were cleaned using a Labofix laboratory cleaner (Brabender, Germany).2.2. Wheat milling process
The wheat milling process began with controlled conditioning, where cleaned wheat kernels were hydrated to 15% moisture content using an automated moisture control system (MYFC-MOZF, Buhler AG, Switzerland) and tempered at 24 ± 2 °C for 24 hours. Milling was conducted using an industrial-scale Buhler roller mill with programmable logic control at CFTRI's facility in Mysuru, India. The mill configuration consisted of four break rolls (B1–B4), five reduction rolls (C1–C5), two purifiers (S1–S2), and bran finishers that separated three by-product streams: fine bran (FB), coarse bran (CB), and germ (GE). Operating at 20 metric tons per day capacity, the mill achieved a 72% flour extraction rate with 25–26% bran yield. Critical to the process was the break stream configuration, where adjustable roller gaps (0.1–2.5 mm) were precisely set according to kernel size distribution to optimize endosperm release, with the first two breaks (B1–B2) showing the most significant operational variability due to their initial kernel disintegration role. Two flour types were collected for analytical purposes: progressive-milled fractions from individual streams and straight-run flour as a composite blend. All milling parameters followed the AACC Method 26-21.02 standards, maintaining roller speed differentials of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for break rolls and 1.25
1 for break rolls and 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for reduction rolls to ensure consistent particle size reduction and separation efficiency.
1 for reduction rolls to ensure consistent particle size reduction and separation efficiency.
2.3. Particle size analysis of wheat flour mill streams
The PSD of flour streams obtained from the roller mill was measured using a Microtrac S3500 particle size analyzer (Montgomeryville, PA). This instrument employs three red laser diodes for optimal alignment, ensuring accurate particle size characterization. Data analysis was performed using the integrated Flex software within the Microtrac system. To ensure reliability, triplicate measurements were taken for each sample, and the mean value was reported as the final result.2.4. Color analysis of wheat flour mill streams
The stream color was assessed using a Hunter Lab Color Measuring System (LabScan XE, Reston, VA). Before measurement, the instrument was calibrated with a barium sulfate standard whiteboard, ensuring 100% reflectance. Samples were loaded into the holder, and reflectance values were recorded across a 360–800 nm wavelength range. The L* value indicated lightness (0 = pure black; 100 = pure white), while a* values represented red (positive) or green (negative) tones, and b* values denoted yellow (positive) or blue (negative) hues. Total color difference (ΔE) was calculated using the standard formula.| ΔL = L2 − L1, Δa = a2 − a1, Δb = b2 − b1 |
2.5. Nutritional analysis of milled streams
All streams were comprehensively analyzed for key quality parameters using approved AACC International methods.18 Oven drying determined moisture content (Method 44-15.02), while protein content was measured via the Kjeldahl nitrogen procedure (Method 46-10.01) with a conversion factor of 5.7. Total fat content was quantified by ether extraction (Method 30-10.01), and ash content was assessed by incineration at 550 °C (Method 08-01.01). By hand washing, gluten quality was evaluated through dry gluten determination (Method 38-10.02). The wheat milled streams' reducing and total sugar levels were evaluated following the AOAC method 939.03.19 Reducing sugars were reported as glucose equivalents in grams per 100 g of flour. Each analysis was conducted in triplicate, and the results were expressed as mean ± standard deviation (SD).2.6. Amino acid analysis of milled streams
The amino acid composition of roller-milled stream samples was determined using cation-exchange chromatography following acid hydrolysis. Samples (100 mg) underwent hydrolysis with 6 M hydrochloric acid (1 mL containing 0.01% phenol) at 110 °C for 24 h in sealed tubes under a nitrogen atmosphere to prevent oxidative degradation. After evaporation of the hydrolysate, the residue was dissolved in 0.01 M HCl (1 mL) and neutralized with 0.01 M NaOH.The analysis was performed on an S433 amino acid analyzer (Sykam GmbH, Germany) equipped with a sodium-based cation-exchange column (NACA K07/Li; 150 × 4.6 mm; 7 µm particle size). Before injection, samples were filtered through 0.2 µm membrane filters to remove particulate matter. The system employed post-column ninhydrin derivatization with dual-wavelength detection at 440 nm (proline) and 570 nm (primary amino acids). The method was adapted from an established protocol20 with modification21 to optimize separation conditions for wheat protein hydrolysates.
2.7. Heavy metal analysis
Approximately 0.5 g of each stream sample was weighed into Teflon tubes. Then, 6–7 mL of concentrated HNO3 and 500 µL of H2O2 were added for digestion. Additionally, 500 µL of 10 ppm yttrium (Y) was added as an internal standard. Before sealing the tubes, the samples were allowed to stand for 10–15 minutes for pre-digestion. After complete digestion, the solutions were diluted to a final volume of 50 mL with deionized water. A PerkinElmer ICP-MS (operated in KED mode) was used for analysis. The instrument was calibrated using a minimum of five linearity points, followed by a blank run and subsequent sample measurements. The concentrations of heavy metals (Pb, As, Cd, Hg, Cu, and Sn) were determined and reported in ppm (mg kg−1) on a sample weight basis.182.8. Phytochemical assays
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether (1
petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) mixture, which were taken in 15 mL tubes and mixed by rotating (30 rpm, 1 h, and 25 ± 2 °C) the tubes. After centrifugation (3000×g, 7 min, and 20 °C), the supernatant was mixed with 5 mL of water, vortexed (15 s), and the petroleum ether layer was extracted. Absorbance (450 nm) was recorded (Shimadzu UV-1800) against a petroleum ether blank. Content was calculated via a β-carotene standard curve (0–10 µg mL−1; R2 >0.99) as µg β-carotene equivalents per g. The method, adapted from Giordano et al.,22 included bran-specific optimizations for matrix effects.
1 v/v) mixture, which were taken in 15 mL tubes and mixed by rotating (30 rpm, 1 h, and 25 ± 2 °C) the tubes. After centrifugation (3000×g, 7 min, and 20 °C), the supernatant was mixed with 5 mL of water, vortexed (15 s), and the petroleum ether layer was extracted. Absorbance (450 nm) was recorded (Shimadzu UV-1800) against a petroleum ether blank. Content was calculated via a β-carotene standard curve (0–10 µg mL−1; R2 >0.99) as µg β-carotene equivalents per g. The method, adapted from Giordano et al.,22 included bran-specific optimizations for matrix effects.2.9. Statistical analysis
Statistical calculations were performed using Microsoft Excel 2016 (Microsoft Corporation, USA), with results expressed as mean ± standard deviation (SD). Data analysis was conducted using SPSS 16.0 (IBM, USA), where analysis of variance (ANOVA) was applied to determine the significance. Mean differences were adjusted using the Tukey multiple range test, with statistical significance set at p <0.05.Multivariate analysis: principal component analysis (PCA) was applied to evaluate the chemical compositions of roller-milled fractions. The multivariate analysis of flour fractions was carried out using OriginPro 2025 (Learning edition) statistical software (version 10.2, OriginLab).
3. Results and discussion
The roller milling process produced SRF with 72% extraction rate, demonstrating efficient separation of wheat kernel components. Two flour types were collected for analytical purposes: progressive-milled streams and SRF as a composite blend. Our analysis revealed significant variations (p ≤0.05) in physicochemical and nutritional characteristics across milling streams, including PSD, color parameters, phytochemicals/bioactive compounds, amino acid profiles, and heavy metal content. These components showed distinct partitioning patterns among the three main mill stream categories, break flours (B1–B4), reduction flours (C1–C5), and by-products (FB, CB, and GE). The GE contained 20–30% CB contamination, reflecting the practical challenges of achieving complete germ purity in our-scale milling operations. The comprehensive distribution patterns of these chemical compositions and phytochemical components across milling fractions are presented and discussed in detail in subsequent sections, highlighting opportunities for targeted stream utilization based on their unique compositional profiles.3.1. Particle size distribution
Wheat flour fractions from the break (B1–B4) and reduction (C1–C5) streams showed significantly different (p ≤0.05) patterns in their PSD (Table 1), indicative of their different compositions and milling sources. B1 and B2 displayed comparable distributions in the break streams, with B1 having a relatively uniform, medium-fine texture typical of early break flours, with 51.38% of its particles falling between 50 and 100 µm and 19.97% finer than 50 µm.3 B2 contained a significantly greater percentage of particles between 100 and 150 µm (35.69%), indicating coarser fragmentation, whereas B3 maintained a balance between intermediate (28.67% at 100–150 µm) and finer (49.55% at 50–100 µm) particles. Among break streams, B4 was notable for having the highest percentage of particles <50 µm (38.38%) and the lowest fraction of particles >150 µm (3.52%), which reflected its finer, more refined nature as a result of progressive endosperm purification.24| Fractions | Particle size distribution | Color values | ||||||
|---|---|---|---|---|---|---|---|---|
| <50 µm | 50–100 µm | 100–150 µm | >150 µm | L* | a* | b* | DE | |
| a Measurements with different letters (a, b, and c) are significantly different from each other (p <0.05). Measurements that share the same letter are not significantly different.b Breaks – B1, B2, B3, & B4; reductions – C1F1/C1F2, C2F1, C2F2, C3, C4, C5; FB – fine bran; CB – coarse bran; GE – germ; SRF – straight run flour. | ||||||||
| B1 | 19.97 | 51.38 | 20.84 | 7.81 | 87.37 ± 0.01c | 0.62 ± 00g | 8.98 ± 0.02f,g | 8.28 ± 0.05f |
| B2 | 12.48 | 44.52 | 35.69 | 7.31 | 87.58 ± 0.06c | 0.56 ± 0.01h | 9.12 ± 0.01f | 8.23 ± 0.02f |
| B3 | 15.67 | 49.55 | 28.67 | 6.11 | 87.22 ± 0.01c | 0.64 ± 00g | 9.65 ± 0.03f | 8.87 ± 0.06f |
| B4 | 38.38 | 45.89 | 12.12 | 3.52 | 85.83 ± 0.07d | 0.79 ± 00f | 9.77 ± 0.00f | 9.88 ± 0.05e |
| C1F1/C1F2 | 24.59 | 41.24 | 26.64 | 7.53 | 89.55 ± 0.02a | 0.25 ± 0.01j | 8.21 ± 0.01h | 6.35 ± 00h |
| C2A | 21.66 | 40.84 | 33.68 | 3.82 | 90.11 ± 0.01a | 0.23 ± 00j | 8.3 ± 0.01h | 6.84 ± 1.13h |
| C2B | 6.73 | 26.74 | 51.61 | 14.92 | 88.86 ± 0.02b | 0.44 ± 0.01i | 9.14 ± 0.02f | 7.52 ± 0.04g |
| C3 | 0.86 | 8.79 | 57.27 | 33.15 | 84.36 ± 0.02e | 1.15 ± 0.02d | 11.17 ± 0.01d | 11.94 ± 0.06d |
| C4 | 24.30 | 39.94 | 25.1 | 10.66 | 84.68 ± 0.02e | 1.06 ± 0.01e | 10.73 ± 0.06e | 11.4 ± 0.15d |
| C5 | 3.93 | 9.37 | 34.84 | 51.85 | 81.06 ± 0.01f | 1.8 ± 0.01c | 11.61 ± 0.02d | 14.77 ± 0.05c |
| FB | — | — | — | — | 60.86 ± 0.03g | 5.72 ± 0.01b | 15.91 ± 0.01c | 34.98 ± 0.08b |
| CB | — | — | — | — | 56.79 ± 0.03i | 6.59 ± 0.01a | 16.33 ± 0.02b | 39.03 ± 0.01a |
| GE | — | — | — | — | 58.25 ± 0.01h | 6.21 ± 0.01a | 17.05 ± 0.06a | 37.86 ± 0.09a |
| SRF | 21.52 | 38.85 | 32.97 | 6.66 | 87.75 ± 0.15c | 0.42 ± 0.005i | 8.45 ± 0.01g | 7.6 ± 0.09g |
However, the reduction streams C1 and C2A showed no significant changes when compared to those of early break flours; according to Doblado-Maldonado et al.,25 C1 had 41.24% particles in the 50–100 µm range and 24.59% particles of <50 µm size. The greater percentage of 100–150 µm particles (33.68%) in C2A was probably produced by leftover bran fragments. However, there were significant (p ≤0.05) changes in C2B and C3, with C2B having 51.61% particles in the 100–150 µm range and C3 having 57.27% particles in this fraction and 33.15% of >150 µm size. This suggests that the material was coarser and less refined from later reduction stages. C5 was the coarsest stream, with 51.85% of particles of >150 µm size, indicating considerable bran contamination, whereas C4 returned to a finer profile (39.94% of 50–100 µm size).
With 38.85% particles in the 50–100 µm range and 32.97% particles in the 100–150 µm range, SRF flour showed a balanced distribution that closely resembled C1 and B1, suggesting that it was composite from early break and reduction streams. According to Posner and Hibbs,26 the <50 µm fraction (21.52%) satisfies the requirements for premium flour in baking applications. For superior baking, break flours (B1–B3), which are finer and more consistent, were used. the later reduction streams (C3 and C5) are coarser, they might need to be blended or reprocessed. Similar trends were observed in the study by Mahajan et al.27 and Hitlamani and Inamdar.21 The intermediate particle size of straight-run flour contributes to its adaptability in industrial settings. As detailed in the following discussion, the PSD among the milled fractions directly affects their color, chemical composition, and bioactive content.
3.2. Color characteristics of fractions
Color parameters (L*, a*, b*, and ΔE) revealed significant differences (p ≤0.05) among milling streams, reflecting varying bran contamination and compositional features (Table 1). Break flours (B1–B3) maintained high brightness (L*: 85.83–87.58), indicating pure endosperm material, while B4 showed slightly lower L* (85.83) due to increased number of bran particles.4 Yellowness (b*) progressively increased from B1 (8.98) to B4 (9.77), suggesting carotenoid accumulation in peripheral layers.28Reduction streams exhibited more pronounced variations, with early fractions (C1–C2A) displaying the highest purity (L*: 89.55–90.11; a*: 0.23–0.25; b*: 8.21–8.30). Later reduction streams (C3–C5) showed significantly increased bran content (L*: 81.06–84.68; a*: 1.06–1.80; b*: 10.73–11.61). The total color difference (ΔE) ranged from 6.35 (C1) to 14.77 (C5), demonstrating progressive color change from pure white flour.
By-products displayed distinct color profiles, with GE showing maximum yellowness (b*: 17.05) and CB exhibiting the darkest color (L*: 56.79) and highest redness (a*: 6.59). These color variations have important quality implications, bright white flours (C1–C2A) suit refined products, while more colored fractions (B3–B4 and C3–C5) may serve whole grain applications. SRF showed premium quality characteristics (L*: 89.10; ΔE: 6.95), confirming effective bran separation.21 The results demonstrate how milling progressively segregates kernel components by color attributes, enabling targeted stream utilization based on end-use requirements.26
3.3. Nutritional composition
The nutritional composition of milled fractions varied significantly (p <0.05) across different streams, as presented in Table 2 (on a dry weight basis). This distribution reflects the heterogeneous nature of wheat kernel components, with distinct nutritional profiles emerging from the break, reduction, and by-product fractions. The comprehensive quantitative data in Table 2 demonstrate how modern roller milling effectively segregates these nutritional components into specific flour streams, due to the PSD in flour streams.21 Similar trends were observed in the study of Mahajan et al.27 The detailed variation has been discussed as follows.| Proximate (%) | SRF | Break | Reduction | By-products | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | C1F1/C1F2 | C2F1 | C2F2 | C3 | C4 | C5 | FB | CB | Germ | ||
| a The results are expressed on a dry weight basis. Measurements with different letters are significantly different from each other (p <0.05). Measurements that share the same letter are not significantly different.b SD is zero. | ||||||||||||||
| Moisture | 11.26 ± 0.02c | 12.12 ± 0.01a | 12.05 ± 0.04a | 12.01 ± 0.00a | 10.03 ± 0.01 | 12.84 ± 0.02a | 12.50 ± 0.01a | 12.57 ± 0.03a | 12.43 ± 0.05a | 11.92 ± 0.02b | 9.02 ± 0.05e | 10.56 ± 0.04d | 8.00 ± 0.08f | 11.83 ± 0.06b |
| Ash | 0.60 ± 0.00g | 0.61 ± 0.01g | 0.66 ± 0.01g | 0.78 ± 0.02f | 1.29 ± 0.01d | 0.71 ± 0.03f | 0.54 ± 0.01h | 0.72 ± 0.03f | 0.93 ± 0.01e | 1.37 ± 0.00c | 1.23 ± 0.02d | 4.57 ± 0.01b | 4.93 ± 0.03a | 4.60 ± 0.01b |
| AIAb | 0.01 | 0.09 | 0.07 | 0.08 | 0.10 | 0.05 | 0.03 | 0.01 | 0.02 | 0.03 | 0.21 | 0.22 | 0.19 | 0.23 |
| Fat | 1.67 ± 0.01g | 1.14 ± 0.05i | 1.15 ± 0.05i | 1.81 ± 0.07f | 2.61 ± 0.03d | 1.48 ± 0.01h | 1.53 ± 0.05h | 1.92 ± 0.05e | 4.13 ± 0.07c | 4.18 ± 0.16c | 4.21 ± 0.09b | 4.12 ± 0.02c | 4.78 ± 0.07a | 4.31 ± 0.17b |
| Protein | 9.63 ± 0.02f | 9.70 ± 0.05f | 11.11 ± 0.03e | 12.22 ± 0.01d | 10.95 ± 0.05e | 8.31 ± 0.01g | 8.60 ± 0.05g | 8.31 ± 0.05g | 9.42 ± 0.00f | 11.18 ± 0.03e | 9.37 ± 0.02f | 17.34 ± 0.14b | 16.79 ± 0.11c | 24.32 ± 0.19a |
| Wet gluten | 33.44 ± 1.5c | 28.69 ± 1.21g | 36.85 ± 1.9b | 43.79 ± 2.1a | 31.82 ± 2.34e | 33.30 ± 1.42c | 32.24 ± 0.5d | 30.74 ± 0.87f | 29.16 ± 0.19f,g | 27.17 ± 1.31h | 26.05 ± 1.81i | — | — | — |
| Dry gluten | 11.46 ± 0.21d | 10.07 ± 0.17f | 13.03 ± 0.21b | 15.47 ± 0.20a | 12.05 ± 0.19c | 11.32 ± 0.11d | 10.92 ± 0.09e | 10.49 ± 0.14e | 10.10 ± 0.21e | 10.25 ± 0.089e | 9.12 ± 0.24g | — | — | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| Minerals (mg/100g) | ||||||||||||||
| Sodium | 4.81 ± 0.00g | 2.08 ± 0.01i | 9.07 ± 0.02e | 1.50 ± 0.00j | 4.95 ± 0.01g | 22.63 ± 0.02b | 21.91 ± 0.01c | 27.84 ± 0.01a | 3.23 ± 0.07h | 6.16 ± 0.03f | 12.63 ± 0.09d | 22.56 ± 0.05b | 28.02 ± 0.05a | 9.42 ± 0.09e |
| Magnesium | 3.64 ± 0.01k | 2.10 ± 0.01l | 5.99 ± 0.01i | 2.11 ± 0.01l | 4.62 ± 0.02j | 16.13 ± 0.06d | 15.17 ± 0.03e | 20.90 ± 0.01c | 7.49 ± 0.03h | 9.45 ± 0.02g | 11.33 ± 0.08f | 27.90 ± 0.022b | 34.82 ± 0.06a | 19.20 ± 0.11c |
| Phosphorus | 3.10 ± 0.02e | 2.78 ± 0.01f | 2.39 ± 0.00f | 3.04 ± 0.02e | 3.17 ± 0.00e | 3.72 ± 0.01d | 3.11 ± 0.01 | 2.58 ± 0.00 | 4.92 ± 0.05c | 7.00 ± 0.01b | 7.29 ± 0.03b | 24.83 ± 0.08a | 24.85 ± 0.07 a | 23.75 ± 0.06a |
| Zinc | 0.10 ± 0.03g | 0.07 ± 0.00h | 0.12 ± 0.00f | 0.09 ± 0.00h | 0.16 ± 0.00f | 0.54 ± 0.01c | 0.43 ± 0.01d | 0.77 ± 0.01b | 0.25 ± 0.01e | 0.21 ± 0.01e | 0.47 ± 0.01d | 1.16 ± 0.01a | 0.54 ± 0.03c | 0.43 ± 0.01d |
| Potassium | 3.52 ± 0.00j | 3.21 ± 0.01j | 2.95 ± 0.01k | 3.66 ± 0.05j | 3.91 ± 0.01i | 4.84 ± 0.00h | 4.18 ± 0.00h | 6.37 ± 0.05f | 5.66 ± 0.01g | 7.16 ± 0.02e | 8.46 ± 0.02d | 35.83 ± 0.03a | 32.51 ± 0.05c | 33.41 ± 0.05b |
| Calcium | 8.00 ± 0.01k | 3.43 ± 0.01m | 12.74 ± 0.01i | 4.06 ± 0.07l | 9.81 ± 0.05j | 36.12 ± 0.00c | 34.13 ± 0.01d | 43.90 ± 0.097b | 13.19 ± 0.01i | 15.38 ± 0.09h | 22.35 ± 0.07f | 32.81 ± 0.07e | 54.38 ± 0.03a | 18.27 ± 0.00g |
| Iron | 0.41 ± 0.01f | 0.37 ± 0.03g | 0.48 ± 0.07f | 0.34 ± 0.02g | 0.29 ± 0.00h | 0.89 ± 0.011d | 0.86 ± 0.00d | 1.43 ± 0.01b | 1.14 ± 0.01b | 0.45 ± 0.05f | 5.38 ± 0.03a | 0.94 ± 0.04c | 0.98 ± 0.09c | 0.61 ± 0.001e |
The protein composition of each stream directly dictates these functional properties. For instance, the germ's abundant non-gluten proteins, while detrimental to gluten network formation and loaf volume in conventional baking, provide significant benefits, such as improved water-binding, emulsification, and lysine fortification, for plant-based and gluten-free formulations.21 Conversely, the insoluble structural proteins in bran typically impair dough strength and crumb texture by disrupting the gluten matrix. However, their inherent association with dietary fibers makes them a valuable ingredient for enhancing water retention and nutritional density in high-fiber and low-carbohydrate food systems.
Break flours showed distinct mineral partitioning: B2 contained elevated calcium (12.74 mg/100 g) and sodium (9.07 mg/100 g) (p <0.05), while B4 was richer in potassium (3.91 mg/100 g) and zinc (0.16 mg/100 g). In the reduction streams, C2F2 and C5 stood out with significantly higher iron (1.43 and 5.38 mg/100 g, respectively) and sodium (27.84 mg/100 g in C2F2) (p <0.01) (Table 2), indicating progressive mineral retention during later milling stages. These distribution patterns highlight how modern milling effectively concentrates minerals in bran and germ fractions while producing relatively mineral-depleted endosperm flours,5 creating a nutritional trade-off between refined and whole grain products.
| ppm kg−1 | SRF | Breaks | Reductions | By-products | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | C1 | C2F1 | CF2F2 | C3 | C4 | C5 | FB | CB | GE | ||
| a The results are expressed on a dry weight basis. Measurements with different letters are significantly different from each other (p <0.0001). Measurements that share the same letter are not significantly different. | ||||||||||||||
| Pb | 0.0063049 | 0.011844 | 0.008265 | 0.01244 | 0.074 | 0.00513 | 0.00528 | 0.00124 | 0.01212 | 0.03019 | 0.0291 | 0.0788 | 0.01437 | 0.02587 |
| As | 0.0101955 | 0.011610 | 0.010263 | 0.01262 | 0.032 | 0.02546 | 0.00748 | 0.01627 | 0.01452 | 0.01135 | 0.00658 | 0.0645 | 0.04904 | 0.04601 |
| Cd | 0.0165131 | 0.014947 | 0.015458 | 0.00777 | 0.030 | 0.01263 | 0.01343 | 0.00773 | 0.00877 | 0.0216 | 0.01271 | 0.05956 | 0.05514 | 0.0464 |
| Hg | −0.0198884 | 0.000435 | 0.000323 | 0.00115 | −0.013 | −0.0242 | 0.00054 | −0.0226 | −0.0008 | −0.0006 | 0.00013 | 0.00121 | −0.0226 | 0.00294 |
| Cu | 1.6324004 | 1.590507 | 1.406273 | 1.75318 | 2.995 | 1.43328 | 1.27883 | 1.45525 | 2.28801 | 3.16998 | 2.55566 | 8.85727 | 8.61579 | 8.15012 |
| Sn | 0.0100306 | 0.118370 | 0.054934 | 0.03734 | 0.100 | 0.0308 | 0.02446 | 0.00545 | 0.0436 | 0.14092 | 0.0617 | 0.1119 | 0.00672 | 0.05798 |
| Cr | 0.022149 | 0.082613 | 0.046571 | 0.0399 | 0.114 | 0.05893 | 0.03549 | 0.02027 | 0.02009 | 0.0417 | 0.03401 | 0.07269 | 0.0469 | 0.07515 |
| Ni | 0.047152 | 0.069315 | 0.045985 | 0.03737 | 0.06345 | 0.03071 | 0.03066 | 0.03043 | 0.05162 | 0.06358 | 0.06003 | 0.28821 | 0.30214 | 0.24786 |
When it comes to food safety, the concurrent concentration of both beneficial minerals and hazardous metals in bran fractions presents a challenge for food applications. While by-products are potent natural mineral fortifiers, their heavy metal loads necessitate rigorous quality control. The selective enrichment of iron in C5 and other nutritionally valuable minerals in specific streams supports tailored flour blending for nutritional interventions. However, the elevated metal content in bran underscores the need for mitigation strategies such as pre-milling grain washing to reduce soil-borne contaminants, selective bran removal for high-risk populations, and sourcing wheat from low-pollution regions.36 Regular monitoring of minerals and heavy metals is essential to balance nutritional enhancement with food safety, particularly for whole grain products that retain the metal-rich bran and germ fractions.
| Break | Reduction | By-products | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | C1F1/C1F2 | C2A | C2B | C3 | C4 | C5 | CB | FB | Germ | |
| a Measurements with different letters are significantly different from each other (p <0.001). Measurements that share the same letter are not significantly different. | |||||||||||||
| Non-essential amino acids | |||||||||||||
| Aspartic acid | 5.58 ± 0.18e,f | 3.58 ± 0.02i | 3.85 ± 0.08i | 5.41 ± 0.05g | 5.53 ± 0.11f | 5.40 ± 0.19g | 4.81 ± 0.49h | 5.90 ± 0.03e | 6.13 ± 0.05d,e | 6.56 ± 0.24d | 10.53 ± 0.14b | 7.07 ± 0.08c | 11.37 ± 0.17a |
| Serine | 4.32 ± 0.00b | 4.39 ± 0.02b | 3.94 ± 0.01d | 3.98 ± 0.00d | 4.44 ± 0.05b | 4.27 ± 0.01b,c | 4.12 ± 0.05c | 4.12 ± 0.01c | 4.22 ± 0.16b,c | 4.59 ± 0.79b | 4.63 ± 0.02a | 4.62 ± 0.21a | 4.78 ± 0.08a |
| Glutamic acid | 39.47 ± 0.10b | 40.15 ± 0.23b | 41.40 ± 0.18a | 36.87 ± 0.06c,d | 37.53 ± 0.46c | 39.18 ± 0.14b | 39.23 ± 0.37b | 37.34 ± 0.07c | 36.28 ± 0.09c,d | 34.75 ± 2.60d | 22.97 ± 0.15f | 33.68 ± 1.56e | 23.63 ± 0.13f |
| Glycine | 4.20 ± 0.00e | 4.39 ± 0.03e | 4.04 ± 0.05 | 4.71 ± 0.00d | 4.17 ± 0.04e | 4.29 ± 0.04e | 4.00 ± 0.01f | 4.15 ± 0.03e,f | 5.14 ± 0.07c | 6.15 ± 2.31b | 6.86 ± 0.04a | 4.56 ± 0.17d | 6.97 ± 0.02a |
| Alanine | 3.51 ± 0.04f | 3.36 ± 0.04f,g | 3.11 ± 0.11g | 3.80 ± 0.03e | 3.65 ± 0.04f | 3.44 ± 0.08f | 3.19 ± 0.05g | 3.74 ± 0.12e | 4.26 ± 0.26d | 4.98 ± 1.28c | 7.34 ± 0.24b | 4.31 ± 0.11d | 8.12 ± 0.11a |
| Cysteine | 0.26 ± 0.02b | 0.24 ± 0.09b | 0.51 ± 0.30a | 0.17 ± 0.00d | 0.23 ± 0.14b | 0.14 ± 0.01g | 0.14 ± 0.02g | 0.16 ± 0.00e | 0.16 ± 0.02e | 0.18 ± 0.06c | 0.15 ± 0.01f | 0.21 ± 0.01b | 0.18 ± 0.01c |
| Tyrosine | 2.40 ± 0.13c | 2.42 ± 0.01c | 2.09 ± 0.00g | 2.35 ± 0.01d | 2.54 ± 0.05b | 2.21 ± 0.05f | 2.43 ± 0.00c | 2.46 ± 0.15c | 2.27 ± 0.00e | 1.99 ± 0.40h | 2.58 ± 0.01b | 2.27 ± 0.24e | 2.71 ± 0.04a |
| Arginine | 2.18 ± 0.04g | 2.67 ± 0.11f | 2.91 ± 0.11e | 3.18 ± 0.06d | 2.70 ± 0.18e | 2.58 ± 0.15f | 2.78 ± 0.03e | 3.21 ± 0.27d | 3.62 ± 0.11c | 2.18 ± 0.48g | 6.86 ± 0.05b | 3.55 ± 0.15c | 7.13 ± 0.07a |
| Proline | 12.74 ± 0.81c | 13.16 ± 0.55b | 11.92 ± 0.38d | 12.56 ± 0.21c | 12.65 ± 0.41c | 12.96 ± 0.60c | 12.54 ± 0.00c | 11.67 ± 0.33 | 11.38 ± 0.82 | 12.67 ± 0.35c | 6.78 ± 0.07e | 14.19 ± 4.22a | 6.07 ± 0.29 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||||
| Essential amino acids | |||||||||||||
| Threonine | 2.67 ± 0.01e | 2.65 ± 0.01e | 2.53 ± 0.00f | 2.66 ± 0.01e | 2.78 ± 0.03d | 2.65 ± 0.01e | 2.65 ± 0.03e | 2.75 ± 0.01d | 2.79 ± 0.07d | 2.85 ± 0.21d | 3.89 ± 0.02b | 3.00 ± 0.13c | 4.27 ± 0.06a |
| Valine | 3.10 ± 0.16f | 2.96 ± 0.03g | 3.62 ± 0.01c | 3.14 ± 0.03f | 3.40 ± 0.02d | 3.07 ± 0.03f | 3.44 ± 0.04d | 3.65 ± 0.04c | 3.14 ± 0.00f | 3.58 ± 0.22c | 3.77 ± 0.08b | 3.30 ± 0.13e | 4.68 ± 0.03a |
| Methionine | 1.01 ± 0.01f | 1.13 ± 0.02e | 1.23 ± 0.05d | 1.43 ± 0.01c | 1.04 ± 0.02e | 1.12 ± 0.00e | 1.30 ± 0.07d | 1.24 ± 0.06d | 1.14 ± 0.02e | 0.99 ± 0.07f | 1.64 ± 0.06b | 0.88 ± 0.05g | 1.97 ± 0.00a |
| Isoleucine | 2.28 ± 0.03e | 2.23 ± 0.01e | 2.77 ± 0.08a | 2.38 ± 0.01d | 2.54 ± 0.00c | 2.33 ± 0.03d | 2.64 ± 0.03b | 2.72 ± 0.00a | 2.14 ± 0.04f | 2.40 ± 0.01d | 2.40 ± 0.03d | 2.29 ± 0.11e | 2.58 ± 0.17c |
| Leucine* | 6.83 ± 0.06 | 6.77 ± 0.03 | 6.76 ± 0.23 | 6.93 ± 0.06 | 7.13 ± 0.03 | 6.87 ± 0.13 | 7.11 ± 0.07 | 7.09 ± 0.00 | 6.68 ± 0.00 | 6.92 ± 0.04 | 6.49 ± 0.10 | 6.38 ± 0.43 | 6.52 ± 0.02 |
| Phenylalanine | 4.83 ± 0.34b | 5.05 ± 0.03a | 4.75 ± 0.01b | 4.94 ± 0.00b | 5.19 ± 0.07a | 4.79 ± 0.28b | 4.97 ± 0.08b | 4.68 ± 0.42c | 4.81 ± 0.02b | 4.37 ± 0.74c | 3.78 ± 0.04d | 4.10 ± 0.58c | 3.69 ± 0.01d |
| Histidine | 2.86 ± 0.15c | 2.86 ± 0.10c | 2.53 ± 0.03f | 3.02 ± 0.03b | 2.51 ± 0.29f | 2.64 ± 0.10e | 2.60 ± 0.07e | 2.72 ± 0.05d | 3.06 ± 0.35b | 2.70 ± 0.31d | 3.59 ± 0.11a | 2.88 ± 0.12c | 3.76 ± 0.11a |
| Lysine | 1.77 ± 0.00f | 1.99 ± 0.03 | 2.05 ± 0.01e | 2.45 ± 0.06d | 1.97 ± 0.05e,f | 2.08 ± 0.01e | 2.05 ± 0.02e | 2.41 ± 0.00d | 2.77 ± 0.01c | 2.15 ± 0.21d | 5.73 ± 0.04b | 2.71 ± 0.15c | 6.28 ± 0.19a |
Due to their predominance in gluten-forming storage proteins, the break streams (B1–B4) had noticeably greater quantities of non-essential amino acids (NEAAs), especially glutamic acid (36.87–41.40%) and proline (11.92–13.16%). On the other hand, the reduction streams (C1–C5) showed a progressive rise in aspartic acid, glycine, alanine, and essential amino acids (lysine, arginine, threonine), an amino acid signature indicative of structural and metabolic proteins rather than the storage proteins predominant in the subaleurone layer.25 As a result of wheat's evolutionary adaptation for protein storage, these NEAAs together made up between 70 and 80% of the total amino acids in endosperm-derived fractions.
The generation of acrylamide during high-temperature processing (such as baking or frying) is affected by this distribution.1 The higher percentage of NEAAs in break flours may theoretically raise the risk of acrylamide since the Maillard reaction between reducing sugars and free asparagine, a NEAA, is the primary mechanism by which acrylamide is formed.21 However, this worry is lessened by wheat's low asparagine content (not quantified explicitly in this study, but usually less than 1–0.5% in flour) compared to other cereals.40 Instead, if processed at high temperatures without optimization, the reduction streams (C3–C5) may present a marginally increased risk of acrylamide due to their higher protein diversity and possible residual sugars from damaged starch.21
Despite having a high quantity of essential amino acids, the germ and bran fractions include more free sugars and asparagine, which may increase acrylamide synthesis if utilized in products that have undergone thermal processing.41 Therefore, break flours have a moderate risk of acrylamide despite their higher baking functionality, whereas by-products (if added to baked products) might need mitigation techniques (asparaginase treatment or optimal baking conditions). This emphasizes how tailored stream blending can help wheat flour products strike the ideal balance between nutritional value and technological performance.
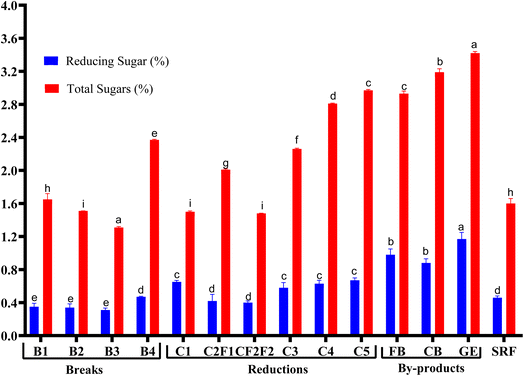
The reduction streams, responsible for further refining endosperm particles, exhibited a rising trend in sugar content from C1 to C5. C1 (0.65% reducing sugar and 1.50% total sugar) had moderate levels, likely due to residual bran particles. C2A (0.42%) and C2B (0.40%) showed lower reducing sugars, possibly due to purer endosperm extraction. C3–C5 exhibited a sharp increase in reducing sugar (0.58–0.67%) and total sugars (2.26–2.97%), suggesting greater aleurone and germ incorporation in finer streams.25
On the other hand, the SRF exhibited 0.46% of reducing sugar and 1.60% of total sugar values between early break and reduction flours, reflecting a blend of multiple milling streams. Its sugar content is lower than bran-rich fractions but higher than purified patent flours, consistent with commercial milling practices.26
As expected, bran and germ fractions contained the significantly highest (p <0.05) sugar levels (Fig. 1). The FB, CB, and GE exhibited 0.98%, 0.88%, and 1.17% of reducing sugars and 2.93%, 3.19%, and 3.42% of total sugars, respectively. This confirms that non-endosperm components are richer in free sugars, particularly reducing sugars like glucose and fructose, which are more in the germ and aleurone layer.21 The germ is metabolically active and contains high soluble sugars for seedling development, explaining its peak values. These findings highlight how milling fractionation affects sugar distribution,16 with bran and germ being the richest sources, while refined flours (early breaks, C2) contain minimal sugars. These sugar variations in the streams will directly affect acrylamide formation in the end products. So, optimizing flour selection based on sugar content can improve product quality and safety.
3.4. Phytochemical distributions
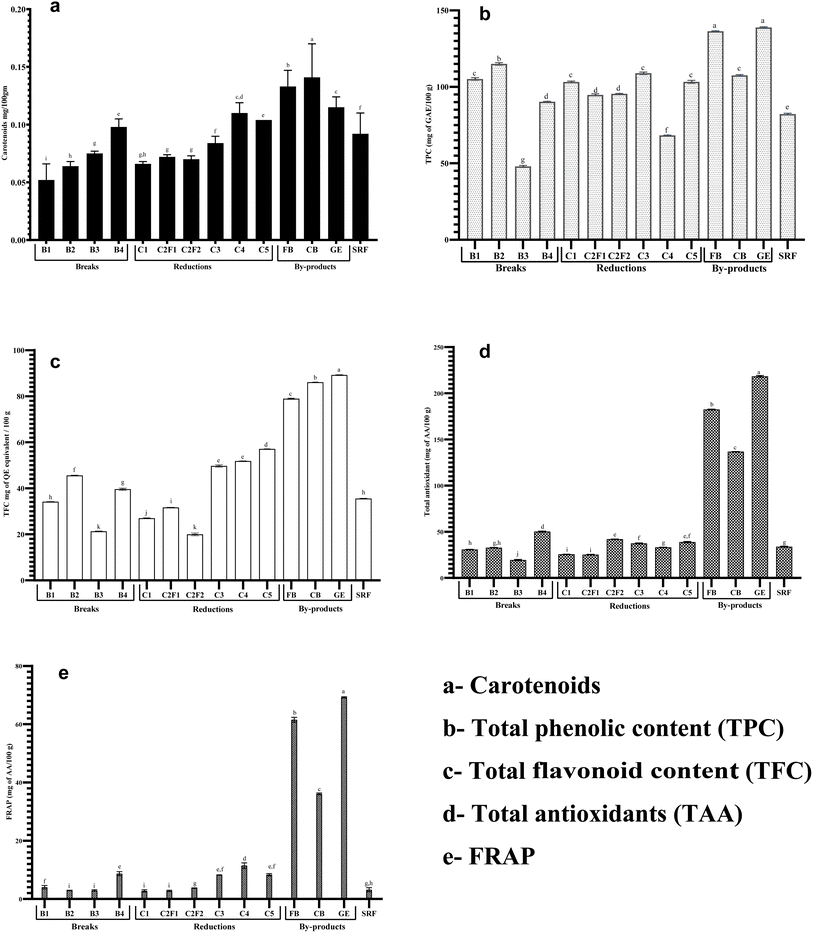
The distribution of phytochemicals varied significantly (p <0.05) across all milling streams (Fig. 2), with distinct compositional profiles observed for carotenoids, phenolic content, flavonoids, and antioxidant activity. Notably, FB, CB, and GE fractions exhibited the highest concentrations of these bioactive compounds, consistent with their role as primary reservoirs of phytochemicals in wheat kernels.22,42,43 However, select flour streams B4, C4, and C5 also contained significantly higher (p <0.05) levels of bioactive compounds than other breaks and reductions, suggesting partial retention of nutrient-rich aleurone and subaleurone layers during milling.15 The individual phytochemical components displayed unique distribution patterns. These compounds are directly associated with the color values of flour fractions.The by-products with the most significant (p <0.05) levels of carotenoid content were the GE (0.115 mg/100 g), FB (0.133 mg/100 g), and CB (0.141 mg/100 g), indicating that these fractions are essential sources of lutein and other xanthophylls in wheat.42 According to Liu et al.,15 the bran fractions' higher carotenoid content is consistent with their function in shielding the kernel from oxidative stress. In keeping with its blended nature, SRF (0.092 mg/100 g), a mixture of several streams, showed intermediate carotenoid levels. Break and reduction flours retain modest carotenoid levels. However, due to bran contamination, B4, C4, and C5 streams offer enhanced nutritional value for whole-grain applications.
With GE (138.80 mg GAE/100 g), FB (136.31 mg GAE/100 g), and CB (127.16 mg GAE/100 g) exhibiting 1.5–3 times greater TPC than typical flour streams, by-products had significantly higher (p <0.05) phenolic contents. These results are consistent with earlier studies that found that the bran and germ of wheat are the primary sources of phenolic acids, especially ferulic acid and other phenolics attached to cell walls.42,43 The intermediate values displayed by the SRF (82.19 mg GAE/100 g) aligned with its composition as a combination of many streams.
These findings point to significant results for product development and milling practices. Compared to more refined streams like B3, the higher phenolic content in early break (B1–B2) and select reduction (C3, C5) streams indicates that these fractions may offer more nutritional value. According to Beta et al.,45 the by-products' extraordinarily high TPC highlights their potential as beneficial dietary additives for phenolic fortification. The variance between streams highlights the planned use of milling fractionation to create flours with the desired phenolic content for particular nutritional uses.
By-products demonstrated the highest TFC values, with GE (89.20 mg CE/100 g) and CB (86.11 mg CE/100 g) containing 2.5–4.2 times more flavonoids than most flour streams (Fig. 2c). These findings align with previous studies showing that wheat bran and germ are primary reservoirs of flavonoid glycosides, particularly apigenin and luteolin derivatives.44 FB (78.93 mg CE/100 g) also showed elevated TFC, though marginally lower than CB and GE, likely due to partial loss of flavonoid-rich aleurone layers during milling.39 SRF (35.53 mg CE/100 g) showed intermediate TFC, consistent with its composition as a blend of multiple streams. These results demonstrate how milling fractionation can strategically separate flavonoid-rich fractions while maintaining flour functionality, supporting the development of value-added wheat ingredients.
C4 had the highest FRAP (11.45 µmol Fe2+ per g) in the reduction system, presumably as a result of leftover germ and aleurone components, while C2F2 (42.03 µmol TE per g) and C5 (38.82 µmol TE per g) showed increased antioxidant activity. The highest antioxidant capacity was demonstrated by the by-products, which confirmed their richness in phenolic acids, flavonoids, and other redox-active compounds GE (218.28 µmol TE per g and 69.19 µmol Fe2+ per g), FB (182.37 µmol TE per g and 61.54 µmol Fe2+ per g), and CB (136.69 µmol TE per g and 36.08 µmol Fe2+ per g).45 Sovrani et al.43 and Giordano et al.22 observed similar trends. In keeping with its composite nature, SRF had moderate values of TAA and FRAP (33.84 µmol TE per g and 3.18 µmol Fe2+ per g, respectively).
3.5. Anti-nutrient distribution
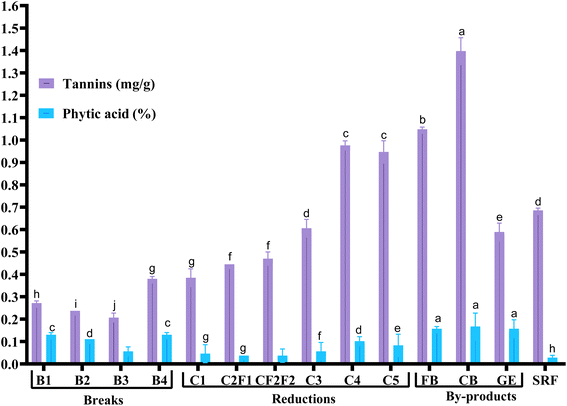
Anti-nutrient distribution across roller-milled wheat flour fractions varied significantly (p <0.001) (Fig. 3), reflecting their concentration in the outer grain layers.The sharp decline in phytic acid from bran fractions (>0.15%) to refined flours (<0.1%) demonstrates milling's effectiveness in reducing anti-nutrients. However, tannins persist in reduction streams (0.385–0.976 mg g−1) due to satisfactory bran adhesion. Blending high-anti-nutrient streams (bran and germ) with others may deteriorate mineral chelation, requiring interventions like fermentation.48 Our results align with studies reporting bran tannins at 0.3–0.8% (ref. 48) and phytic acid at 2–6% in aleurone.44 The low phytic acid content in SRF (<0.03%) matches with refined flour data, supporting its nutritional advantage. Tannins and phytic acid are concentrated in bran and germ; milling reduces the challenges of eliminating them. For fortified bakery products, low-extraction flours minimize anti-nutrient effects, while bran-rich blends may need phytase activation (e.g., fermentation) to improve mineral absorption.
4. Principal component analysis interpretation
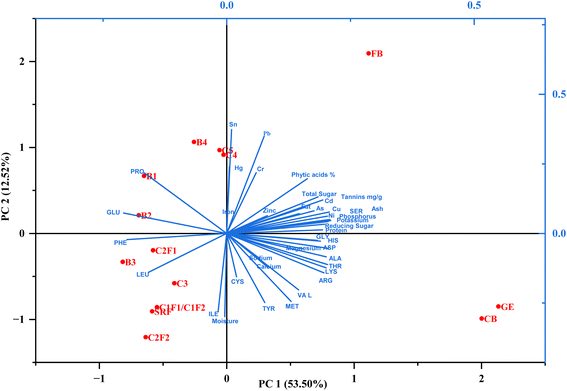
Principal component analysis (PCA) of the chemical compositions of milled streams (Fig. 4) reveals distinct clustering based on compositional differences. The first two principal components (PC1: 53.50%; PC2: 12.52%) collectively explain 66.02% of the total variance. The PCA biplot clearly separates bran streams and break/reduction flours, indicating significant compositional differences. Bran streams are positioned at the far right along PC1 and are associated with higher levels of non-essential amino acids (NEAAs), heavy metals, anti-nutrients, sugars, and ash. In contrast, the break flour (B1–B4) cluster in the upper-left quadrant is closely aligned with C4 and C5, suggesting shared intermediate characteristics. The reduction flour (C1–C3) group in the lower-left quadrant, near SRF, reflects their refined endosperm-dominated composition.Bran streams correlate strongly with ash, sugars, phenolics, and anti-nutrients. C4 and C5 show transitional properties, positioned near FB; however, they have lower bioactive content. Pure endosperm fractions (B1–B3 and SRF) cluster separately, indicating minimal bran contamination and higher gluten quality. This PCA highlights a nutritional functionality divide: bran streams are rich in micronutrients, but they are also anti-nutrients and heavy metals, while break/reduction flours offer superior baking functionality.
5. Conclusion
This study comprehensively maps the nutritional and compositional gradients in industrial wheat milling streams. A key finding is the inherent trade-off: refinement enhances technological functionality but reduces nutrients and bioactives. Significant variations were observed, with bran and germ streams identified as rich sources of lysine (6.28%), antioxidants (FRAP: 36–69 µmol Fe2+ per g), and minerals, albeit with concurrent higher levels of anti-nutrients and heavy metals. Break flours (B1–B4) demonstrated superior baking potential due to their high gluten-forming protein content, while later streams (B4, C4, and C5) offered greater nutrient density, making them ideal for whole-grain applications.The observed 5–10-fold variation in essential nutrients underscores the potential of precision milling. Strategic applications include the targeted recombination of streams to create nutritionally enhanced flours and the specialized use of streams and break flours for premium baking, reduction streams for balanced blends, and by-products as functional fortifiers. These findings establish a groundwork for developing specialty flours with improved amino acid profiles, sustained baking quality, and potential for acrylamide mitigation in end products.
However, this study has certain limitations. The compositional analysis is primarily investigative; it does not include functional validation of the proposed stream recombination through baking tests or rheological studies. Furthermore, the chance of anti-nutrients during processing and their impact on nutrient bioavailability were not investigated. Future work should therefore focus on optimizing specific blending ratios and conducting comprehensive functional evaluations to balance nutritional enhancement and acrylamide mitigation with end-product performance.
Author contributions
Veeranna H: conceptualization, writing – original draft, investigation, methodology, formal analysis, data curation, graphics, statistics, visualization, validation. Swamy Gowda M. R. & Salony Azam Sheikh: formal analysis, data curation. Nandini P. Shetty & Sridevi Annapurna Singh: resource, supervision. A. A. Inamdar: conceptualization, funding acquisition, writing – review & editing, project administration, supervision, resources, validation.Conflicts of interest
The authors declare no competing interests.Data availability
Data will be made available on request.Consent for publication: all authors agreed to the publication of the manuscript.
Acknowledgements
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The author Mr Veeranna H. is grateful to the Indian Council of Medical Research (ICMR), Government of India, for awarding a Senior Research Fellowship [Award No. 3/1/2/240/2021-Nut (https://www.sciencedirect.com/science/article/pii/S0956713524001543)].References
- V. Hitlamani and A. A. Inamdar, Technology processing strategies to reduce the Acrylamide formation in wheat-based bakery products and future prospects: A review, Food Control, 2024, 162, 110437, DOI:10.1016/j.foodcont.2024.110437.
- M. Abo-Dief, T. Abo-Bakr, M. Youssef and A. Moustafa, Physicochemical and rheological properties of Australian and Russian wheat flour mill streams, Cereal Chem., 2022, 99(2), 421–431, DOI:10.1002/cche.10508.
- G. M. Campbell, Roller milling of wheat, Handbook of Powder Technology, 2007, vol. 12, pp. 383–419, DOI:10.1016/S0167-3785(07)12010-8.
- P. Prabhasankar, M. L. Sudha and P. H. Rao, Quality characteristics of wheat flour milled streams, Food Res. Int., 2000, 33(5), 381–386, DOI:10.1016/S0963-9969(00)00059-4.
- Y. Pomeranz, Wheat Chemistry and Technology (vols 1 and 2), American Association of Cereal Chemists, St. Paul, MN, 1988 Search PubMed.
- K. Dewettinck, F. Van Bockstaele, B. Kühne, D. Van de Walle, T. M. Courtens and X. Gellynck, Nutritional value of bread: Influence of processing, food interaction and consumer perception, J. Cereal Sci., 2008, 48(2), 243–257, DOI:10.1016/j.jcs.2008.01.003.
- E. Dornez, K. Gebruers, S. Wiame, J. A. Delcour and C. M. Courtin, Insight into the distribution of arabinoxylans, endoxylanases, and endoxylanase inhibitors in industrial wheat roller mill streams, J. Agric. Food Chem., 2006, 54(22), 8521–8529, DOI:10.1021/jf061728n.
- D. W. Hatcher and J. E. Kruger, Distribution of polyphenol oxidase in flour millstreams of Canadian common wheat classes milled to three extraction rates, Cereal Chem., 1993, 70, 51 CAS.
- K. U. Rani, U. P. Rao, K. Leelavathi and P. H. Rao, Distribution of enzymes in wheat flour mill streams, J. Cereal Sci., 2001, 34(3), 233–242, DOI:10.1006/jcrs.2000.0393.
- D. Every, S. C. Morrison, L. D. Simmons and M. P. Ross, Distribution of glutathione in millstreams and relationships to chemical and baking properties of flour, Cereal Chem., 2006, 83(1), 57–61, DOI:10.1094/CC-83-0057.
- D. Every, L. D. Simmons and M. P. Ross, Distribution of redox enzymes in millstreams and relationships to chemical and baking properties of flour, Cereal Chem., 2006, 83(1), 62–68, DOI:10.1094/CC-83-0062.
- E. Yahata, W. Maruyama-Funatsuki, Z. Nishio, Y. Yamamoto, A. Hanaoka, H. Sugiyama, M. Tanida and H. Saruyama, Relationship between the dough quality and content of specific glutenin proteins in wheat mill streams, and its application to making flour suitable for instant Chinese noodles, Biosci., Biotechnol., Biochem., 2006, 70(4), 788–797, DOI:10.1271/bbb.70.788.
- B. Iuliana, S. Georgeta, I. Violeta and A. Iuliana, Physicochemical and rheological analysis of flour mill streams, Cereal Chem., 2010, 87(2), 112–117, DOI:10.1094/CCHEM-87-2-0112.
- S. D. Sakhare, A. A. Inamdar, D. Indrani, M. H. Madhu Kiran and G. V. Rao, Physicochemical and microstructure analysis of flour mill streams and milled products, J. Food Sci. Technol., 2015, 52(1), 407–414, DOI:10.1007/s13197-013-1029-4.
- C. Liu, L. Liu, L. Li, C. Hao, X. Zheng, K. E. Bian, J. Zhang and X. Wang, Effects of different milling processes on whole wheat flour quality and performance in steamed bread making, LWT–Food Science and Technology, 2015, 62(1), 310–318, DOI:10.1016/j.lwt.2014.08.030.
- D. D. Ramseyer, A. D. Bettge and C. F. Morris, Flour mill stream blending affects sugar snap cookie and Japanese sponge cake quality and oxidative cross-linking potential of soft white wheat, J. Food Sci., 2011, 76(9), C1300–C1306, DOI:10.1111/j.1750.3841.2011.02404.x.
- P. Lewko, A. Wójtowicz and M. Gancarz, Distribution of arabinoxylans and their relationship with physiochemical and rheological properties in wheat flour mill streams as an effective way to predict flour functionality, Appl. Sci., 2023, 13(9), 5458, DOI:10.3390/app13095458.
- A. A. C. C. American Association of Cereal Chemists International, Approved Methods of Analysis, Methods 44-51, 54030, 56-81B, and 76-30A. Available online only, AACC International, St. Paul, MN, 11th edn, 2000 Search PubMed.
- The Association of Official Analytical Chemists International, Approved Methods of the AOACI, The Association, 2000 Search PubMed.
- S. A. Sheikh, T. Tamilselvan, J. Ramesh, A. Rangi, Y. C. Radhalakshmi, P. Prabhasankar and S. A. Singh, Leveraging insect-derived mulberry sericin for high-protein white bread: a case study on dough optimization, techno-functional properties and sensory acceptability, J. Food Sci. Technol., 2024, 13, 1–3, DOI:10.1007/s13197-024-06155-1.
- V. Hitlamani and A. A. Inamdar, Effect of milling methods on acrylamide levels in chapatti and poori, Food Chem., 2025, 16, 145195, DOI:10.1016/j.foodchem.2025.145195.
- D. Giordano, M. Locatelli, F. Travaglia, M. Bordiga, A. Reyneri, J. D. Coïsson and M. Blandino, Bioactive compound and antioxidant activity distribution in roller-milled and pearled fractions of conventional and pigmented wheat varieties, Food Chem., 2017, 233, 483–491, DOI:10.1016/j.foodchem.2017.04.065.
- N. Okarter, C. S. Liu, M. E. Sorrells and R. H. Liu, Phytochemical content and antioxidant activity of six diverse varieties of whole wheat, Food Chem., 2010, 119(1), 249–257, DOI:10.1016/j.foodchem.2009.06.021.
- C. Fang and G. M. Campbell, On predicting roller milling performance V: effect of moisture content on the particle size distribution from first break milling of wheat, J. Cereal Sci., 2003, 37(1), 31–41, DOI:10.1006/jcrs.2002.0476.
- A. F. Doblado-Maldonado, O. A. Pike, J. C. Sweley and D. J. Rose, Key issues and challenges in whole wheat flour milling and storage, J. Cereal Sci., 2012, 56(2), 119–126, DOI:10.1016/j.jcs.2012.02.015.
- E. S. Posner, and A. N. Hibbs, Wheat Flour Milling, American Association of Cereal Chemists. Inc., St. Paul, MN, USA, 2nd. edn, 2005, vol. 489, pp. 445–453 Search PubMed.
- S. Mahajan, A. Kumar, J. Rajiv, A. A. Inamdar and S. D. Sakhare, Evaluation and utilization of commercial processed flour mill streams for development of specialty flour for pizza base, J. Food Sci. Technol., 2025, 26, 1–0, DOI:10.1007/s13197-025-06335-7.
- A. Hidalgo and A. Brandolini, Protein, ash, lutein and tocols distribution in einkorn (Triticum monococcum L. subsp. monococcum) seed fractions, Food Chem., 2008, 107(1), 444–448, DOI:10.1016/j.foodchem.2007.08.009.
- V. Greffeuille, J. Abecassis, M. Rousset, F. X. Oury, A. Faye, C. B. L'Helgouac'h and V. Lullien-Pellerin, Grain characterization and milling behaviour of near-isogenic lines differing by hardness, Theor. Appl. Genet., 2006, 114(1), 1–2, DOI:10.1007/s00122-006-0403-2.
- L. Brütsch, I. Huggler, S. Kuster and E. J. Windhab, Industrial roller milling process characterisation for targeted bread quality optimization, Food Bioprocess Technol., 2017, 10(4), 710–719, DOI:10.1007/s11947-016-1856-1.
- C. Antoine, S. Peyron, V. Lullien-Pellerin, J. Abecassis and X. Rouau, Wheat bran tissue fractionation using biochemical markers, J. Cereal Sci., 2004, 39(3), 387–393, DOI:10.1016/j.jcs.2004.02.001.
- H. Wieser, Chemistry of gluten proteins, Food Microbiol., 2007, 24(2), 115–119, DOI:10.1016/j.fm.2006.07.004.
- N. D. Brier, S. V. Gomand, E. Donner, D. Paterson, J. A. Delcour, E. Lombi and E. Smolders, Distribution of minerals in wheat grains (Triticum aestivum L.) and in roller milling fractions affected by pearling, J. Agric. Food Chem., 2015, 63(4), 1276–1285, DOI:10.1021/jf5055485.
- M. F. Wahab and D. M. Jamil, Determination of some heavy metals in different wheat flour brands in Sulaimani, Kurdistan Region-Iraq, Czech J. Food Sci., 2023, 41(6), 455–561, DOI:10.17221/85/2023-CJFS.
- S. Y. Lee, Y. Y. Lee and K. S. Cho, Inoculation effect of heavy metal tolerant and plant growth promoting rhizobacteria for rhizoremediation, Int. J. Environ. Sci. Technol., 2024, 21(2), 1419–1434, DOI:10.1007/s13762-023-05078-2.
- W. H. O., Guidelines for Heavy Metals in Food, Geneva, World Health Organization, 2023 Search PubMed.
- P. R. Shewry, V. Piironen, A. M. Lampi, L. Nystrom, L. Li, M. Rakszegi, A. Fras, D. Boros, K. Gebruers, C. M. Courtin and J. A. Delcour, Phytochemical and fiber components in oat varieties in the HEALTHGRAIN diversity screen, J. Agric. Food Chem., 2008, 56(21), 9777–9784, DOI:10.1021/jf801880d.
- Y. Liu, J. B. Ohm, G. Hareland, J. Wiersma and D. Kaiser, Sulfur, protein size distribution, and free amino acids in flour mill streams and their relationship to dough rheology and breadmaking traits, Cereal Chem., 2011, 88(2), 109–116, DOI:10.1094/CCHEM-06-10-0086.
- Y. Hemery, X. Rouau, V. Lullien-Pellerin, C. Barron and J. Abecassis, Dry processes to develop wheat fractions and products with enhanced nutritional quality, J. Cereal Sci., 2007, 46(3), 327–347, DOI:10.1016/j.jcs.2007.09.008.
- N. G. Halford, T. Y. Curtis, Z. Chen and J. Huang, Effects of abiotic stress and crop management on cereal grain composition: implications for food quality and safety, J. Exp. Bot., 2015, 66(5), 1145–1156, DOI:10.1093/jxb/eru473.
- N. Muttucumaru, N. G. Halford, J. S. Elmore, A. T. Dodson, M. Parry, P. R. Shewry and D. S. Mottram, Formation of high levels of acrylamide during the processing of flour derived from sulfate-deprived wheat, J. Agric. Food Chem., 2006, 54(23), 8951–8955, DOI:10.1021/jf0623081.
- K. K. Adom and R. H. Liu, Antioxidant activity of grains, J. Agric. Food Chem., 2002, 50(21), 6182–6187, DOI:10.1021/jf0205099.
- V. Sovrani, M. Blandino, V. Scarpino, A. Reyneri, J. D. Coïsson, F. Travaglia, M. Locatelli, M. Bordiga, R. Montella and M. Arlorio, Bioactive compound content, antioxidant activity, deoxynivalenol and heavy metal contamination of pearled wheat fractions, Food Chem., 2012, 135(1), 39–46, DOI:10.1016/j.foodchem.2012.04.045.
- C. Liu, R. Zhang, B. Liu and X. Zheng, Effect of steam explosion treatment on phenolic acid composition of wheat bran and its antioxidant capacity, Trans. Chin. Soc. Agric. Eng., 2016, 32(6), 308–314 CrossRef PubMed.
- T. Beta, S. Nam, J. E. Dexter and H. D. H. D. Sapirstein, Phenolic content and antioxidant activity of pearled wheat and roller-milled fractions, Cereal Chem., 2005, 82(4), 390–393, DOI:10.1094/CC-82-0390.
- J. Venegas, M. J. Guttieri, J. D. Boehm Jr, R. Graybosch, G. Bai, P. C. St. Amand, N. Palmer, W. Hussain, S. Blecha and P. S. Baenziger, Genetic architecture of the high-inorganic phosphate phenotype derived from a low-phytate mutant in winter wheat, Crop Sci., 2022, 62(3), 1228–1241, DOI:10.1002/csc2.20738.
- L. Dykes and L. W. Rooney, Phenolic compounds in cereal grains and their health benefits, Cereal Foods World, 2007, 52(3), 105–111 CAS.
- C. Badia-Olmos, J. Sánchez-García, L. Laguna, E. Zúñiga, C. M. Haros, A. M. Andrés and A. Tarrega, Flours from fermented lentil and quinoa grains as ingredients with new techno-functional properties, Food Res. Int., 2024, 177, 113915, DOI:10.1016/j.foodres.2023.113915.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |