Open Access Article
Open Access ArticleHydro-treatment of wild bitter apricot kernel oil: a cheap and cost-effective alternative for reducing toxicity and enhancing quality
Swati Joia,
Himanshi Rao,
Raashid Ahmad Siddiqi and
Tajendra Pal Singh *
*
Department of Food Technology, Eternal University, Baru Sahib, Himachal Pradesh, India 173101. E-mail: swatijoia2611@gmail.com; himanshirao404@gmail.com; siddiqi.raashid786@gmail.com; tajendra@eternaluniversity.edu.in
First published on 25th November 2025
Abstract
Wild bitter apricot kernel oil (AKO) is an underutilized resource due to the presence of cyanogenic glycosides (specifically amygdalin), which can pose toxicity during its direct utilization. This study explores the potential of hydro-treatment to detoxify AKO while preserving its physicochemical and nutritional parameters. The results revealed that non-treated apricot kernel oil possesses a yellow appearance, 1.468 refractive index and 0.92 specific gravity, and treated apricot kernel oil exhibits a pale-yellow appearance, 1.468 refractive index and 0.93 specific gravity. Notably, quantitative analysis revealed that the total phenolic content was higher in non-treated AKO (27.28 µg GAE per g) compared to treated AKO (13.92 µg GAE per g). AKO exhibited a comparable composition of prominent (especially oleic and linoleic acids) fatty acids. Both variants showed good antioxidant properties. Importantly, amygdalin was undetectable in the hydro-treated AKO, confirming its safety for utilization. Hydro-treatment did not significantly affect the yield, refractive index, specific gravity, fatty acid profile, acid value, iodine value, and peroxide and saponification values, with all parameters complying with the prescribed range of FAO/WHO and PFA specifications for almond oil. In conclusion, hydro-treatment is an effective approach to detoxify AKO, ensuring its safety and quality for potential applications in the food industry.
Sustainability spotlightThe sustainable hydro-treatment was applied to wild bitter apricot kernel oil. It converts a not fully tapped resource into a safe and functional food input. It minimizes harmful amygdalin while retaining the most critical physicochemical and nutritional characteristics. This approach is an example of a reasonable and responsible application of natural resources. It goes beyond conforming to international safety standards to increasing the prospect of utilising wild bitter apricot kernel oil as a sustainable food source, thereby advancing environmental and nutritional security. |
1 Introduction
Fruit waste, which accounts for approximately 25–30% of global fruit commodities, is a rich source of bioactive compounds and nutrients.1 Fruit waste, i.e. skin, peel, pomace, shells, seeds and kernels, contains various phytochemicals (flavonoids, polyphenols, and carotenoids), dietary fibres, vitamins, minerals, and enzymes. These compounds exhibit various biological activities, including antibacterial, antiviral, anti-tumoral, anti-mutagenic and cardio-protective actions.2,3Nowadays, the interest of researchers has been garnered in the utilisation of underutilised seeds. Apricot (Prunus armeniaca L.) is a deciduous stone fruit belonging to the genus Prunus, subfamily Prunoideae, family Rosaceae, and order Rosales. This species is extensively cultivated in temperate climates worldwide, particularly in regions with suitable chill hours and moderate winters. In India, Prunus armeniaca L. is primarily cultivated in the Himalayan foothills, specifically in the states of Himachal Pradesh, Jammu and Kashmir, and Uttarakhand. Notably, the genus Armeniaca is also grown in mountainous regions, although it is considered a synonym of Prunus.4,5
Apricot kernels can be grouped into two main categories based on their taste and amygdalin content, i.e. sweet kernel (Nyarmo) and bitter kernel (Khante).6 Phytochemically, Prunus species seeds, including apricot kernels (bitter variety), are characterised by their high amygdalin content, ranging from 0.5 to 5.0%. Apricot kernels exhibit considerable variation in amygdalin content, influenced by several factors, including kernel type (sweet or bitter), geographical origin, and growing conditions. Sweet apricot kernels contain relatively low levels of amygdalin (less than 5 mg kg−1), with respect to bitter kernels, having very high concentrations (more than 200 mg/100 g).7 This variability in amygdalin content is significant for the potential applications as well as the safety of apricot kernels. In addition, the seeds of multiple Prunus spp, such as P. dulcis var. amara (bitter almond), P. armeniaca (apricot), P. avium (cherry), P. avium var. sativa (sweet cherry), P. persica (peach), P. domestica (plum), P. persica var. nectarina (nectarine) and Malus domestica (apple) contain a high amount of amygdalin.8 When the seed is ruptured, the amygdalin is enzymatically hydrolysed into glucose, benzaldehyde and hydrogen cyanide (HCN), which causes cyanide poisoning. The toxicological threshold of cyanide in humans is around 1.5 mg kg−1.9
Apricot kernel serves as a valuable source of high-quality oil and protein, along with essential minerals, vitamins and phenolic compounds.10 Due to the high content of mono- and polyunsaturated fatty acids and natural antioxidants such as polyphenols and tocopherols, apricot kernel oil is used as edible oil in salad dressing, confectionery and gourmet oils. It is also used as an ingredient in medicinal supplements and pharmaceutical products due to its cardioprotective, antioxidant, and anti-inflammatory properties. Due to the similar fatty acid profile to almond oil, apricot kernel oil is also used as a substitute in food and cosmetic product formulations.11
Despite the high oil content, bitter apricot kernels remain underutilised, primarily due to the presence of toxic cyanogenic glycosides, among which amygdalin is the most prevalent. During oil extraction, a considerable amount of amygdalin is transferred from bitter apricot kernels to its oil. According to Meng and Zhang (2013),12 a concentration of amygdalin below 5 mg kg−1 is considered safe. Therefore, to make bitter apricot oil safe, environmentally friendly and sustainable, the amygdalin content needs to be lowered to a safe level. Various treatments have been reported to be effective in reducing the amygdalin content of bitter apricot kernels, thereby enhancing their usability in the pharmaceutical, cosmetic, and food industries. Zhang et al., (2020)13 revealed that ultrasonic treatment (700 W for 50 minutes) is an effective alternative method for reducing cyanide levels to as low as 4.65 mg kg−1. Further research is needed to develop a cost-effective, efficient and environment-friendly method to effectively lower the amygdalin content in apricot kernel oil to make it safe, expand its application in food and non–food industries, and make it a top alternative source of oil.
The current study aimed to investigate the efficacy of the hydro-treatment process in reducing the amygdalin content. The study was further extended to study the effect of hydro-treatment on the yield, physicochemical properties, total phenolic content, antioxidant activity and fatty acid composition of bitter apricot kernel oil.
2 Materials and methods
2.1. Materials
In this study, wild bitter apricot kernels were procured from the upper Himalayan region (with coordinates 33.86°N 75.29°E and an altitude of 2000 meters above sea level) of Jammu & Kashmir, India.14 Wild bitter apricots were harvested and depulped. Their kernels were dried and stored for up to 3 months at 4 °C. Apricot kernels were manually cracked with a hammer, separated from shells, stored in airtight containers at 4 °C and processed within a week. Apricot kernels were soaked in boiled water (∼100 °C) for 2–3 min to remove the skin of kernels followed by drying at 50 °C for 2 h. The dried peeled apricot kernels were ground to a fine powder for oil extraction. All the chemicals used were of analytical grade.2.2. Extraction of apricot kernel oil
Apricot kernel powder was mixed with hexane in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and shaken for 1 h at 150 rpm at 35–40 °C. This process was repeated twice. Hexane was removed from the miscella by rotary evaporation under reduced pressure at 40–45 °C, followed by a short nitrogen purge for 10 min, to recover an oil termed control apricot kernel oil (AKOC).
10 and shaken for 1 h at 150 rpm at 35–40 °C. This process was repeated twice. Hexane was removed from the miscella by rotary evaporation under reduced pressure at 40–45 °C, followed by a short nitrogen purge for 10 min, to recover an oil termed control apricot kernel oil (AKOC).
2.3. Hydrotreatment to remove amygdalin
Amygdalin was removed from bitter apricot kernel oil using a detoxification method, conceptually adapted from Mirzaei and Rezaei (2019).15 Briefly, miscella was mixed with distilled water in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and shaken vigorously/vortexed for 15 min. Furthermore, the mixture was centrifuged at 6000g for 15 min followed by careful decanting of water. This process was repeated thrice. Hexane was removed from the treated miscella to obtain the oil, which was referred to as treated apricot kernel oil (AKOT).
2 and shaken vigorously/vortexed for 15 min. Furthermore, the mixture was centrifuged at 6000g for 15 min followed by careful decanting of water. This process was repeated thrice. Hexane was removed from the treated miscella to obtain the oil, which was referred to as treated apricot kernel oil (AKOT).
The treated (AKOT) and control (AKOC) apricot kernel oils were stored at room temperature for further analysis.
2.4. Physicochemical properties
where 56.1 is the equivalent weight of KOH.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glacial acetic acid 1
glacial acetic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180) (25 mL) and kept in the dark for 30 min. After the reaction, 10% potassium iodide solution (15 mL) and distilled water (30 mL) were added to the reaction mixture with vigorous shaking. Then, the mixture was titrated with 0.1 N sodium thiosulfate solution using the starch indicator (3–4 drops) until the blue colour disappeared. Finally, the IV was calculated as follows:
180) (25 mL) and kept in the dark for 30 min. After the reaction, 10% potassium iodide solution (15 mL) and distilled water (30 mL) were added to the reaction mixture with vigorous shaking. Then, the mixture was titrated with 0.1 N sodium thiosulfate solution using the starch indicator (3–4 drops) until the blue colour disappeared. Finally, the IV was calculated as follows:where BV and TV are the titre value of the blank and sample, respectively. N = normality of NaS2O3, 12.69 = molar mass of iodine.
where BV and TV are the titre value of the blank and sample, respectively, and 56.1 is the equivalent weight of KOH.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform mixture 3
chloroform mixture 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 1 mL saturated potassium iodide solution and 30 mL distilled water were added to the mixture, followed by shaking for 1 min, and then titrated with 0.01 N sodium thiosulfate solution using the starch indicator until the blue colour disappeared. The peroxide value was measured using the following formula:
2). 1 mL saturated potassium iodide solution and 30 mL distilled water were added to the mixture, followed by shaking for 1 min, and then titrated with 0.01 N sodium thiosulfate solution using the starch indicator until the blue colour disappeared. The peroxide value was measured using the following formula:where TV and BV = titre value of the sample and blank, respectively, N = normality of NaS2O3
2.4.7.1 DPPH radical scavenging activity. DPPH scavenging activity was determined according to the method of Olszowy and Dawidowicz (2016),17 with slight modifications. Briefly, 0.4 mL of the methanolic oil extract (0.7–3.5 mg mL−1) was mixed with 2.8 mL of 90 mM DPPH solution (prepared in 100% methanol). The mixture was vortexed for 1–2 min and kept in the dark for 30 min. The absorbance of the resulted mixture was measured at 515 nm using a UV-VIS spectrophotometer (PerkinElmer, Korea). 100% methanol was used as a blank. The IC50 (Inhibitory concentration) value was calculated based on the minimum sample concentration needed to inhibit 50% of the DPPH activity using the calibration curve.
where C = absorbance of the control and S = absorbance of the sample.
2.4.7.2 Ferric reducing antioxidant activity (FRAP). The ferric-reducing activity of AKOs was assessed following the method of Chen et al., (2016)18 with slight modifications. Briefly, 2.5 mL of the methanolic oil extract (1–5 mg mL−1) was mixed with an equal volume of 0.2 M phosphate buffer (pH 6.6) and 1 mL of 1% potassium ferricyanide, followed by incubation at 50 °C for 30 min. To stop the reaction, 2.5 mL of 10% trichloroacetic acid was added, followed by centrifugation at 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Then, 2.5 mL of the upper layer was mixed with an equal amount of distilled water and 0.1% ferric chloride solution. The absorbance of the mixture was measured at 700 nm using a spectrophotometer (PerkinElmer, Korea), with 100% methanol as the blank. The EC50 (inhibitory concentration) value of AKOs was determined by calculating the effective concentration needed to exhibit 50% of the FRAP activity using the calibration curve.
000 rpm for 10 min. Then, 2.5 mL of the upper layer was mixed with an equal amount of distilled water and 0.1% ferric chloride solution. The absorbance of the mixture was measured at 700 nm using a spectrophotometer (PerkinElmer, Korea), with 100% methanol as the blank. The EC50 (inhibitory concentration) value of AKOs was determined by calculating the effective concentration needed to exhibit 50% of the FRAP activity using the calibration curve.
2.5. Amygdalin content
The amygdalin content of control and treated AKOs was measured using HPLC (Agilent 1260 Infinity II, G7115 1260 DAD WR), according to the method of Mirzaei and Rezaei (2019).15 To extract amygdalin from AKOs, the sample (1 mL) was mixed with hexane (1 mL) and distilled water (2 mL) and vortexed for 30 min, followed by centrifugation at 5000g for 10 min. The upper layer of water was filtered using a 0.22 µm pore–size filter and transferred into an HPLC vial. Amygdalin was separated on an Agilent Column (C18, 4.6 × 100 mm, 4 µm) at 30 °C and detected at a UV wavelength of 214 nm. The mixture of water and acetonitrile (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v) was used as a mobile phase. A 10 µL sample was injected and the flow rate was set at 0.7 mL min−1. The limit of detection (LOD) for amygdalin standard was 0.1 ppm, allowing reliable quantification of trace amounts of amygdalin in AKO.
30, v/v) was used as a mobile phase. A 10 µL sample was injected and the flow rate was set at 0.7 mL min−1. The limit of detection (LOD) for amygdalin standard was 0.1 ppm, allowing reliable quantification of trace amounts of amygdalin in AKO.
2.6. Fatty acid composition analysis
The fatty acid profile of AKOs was determined by preparing fatty acid methyl esters (FAMEs) using the method described by Joia et al., (2025).14 About 80 mg of AKOs was mixed with 3 mL of 6% methanolic HCL and heated in a water bath at 75–80 °C for methylation. After cooling, 2 mL of hexane was added to the mixture, followed by 1 min of mixing and 10 min of centrifugation at 5000g. Then, the upper layer was carefully collected and used for FAME analysis using GC-MS/MS (Agilent, 8890 GC, USA).FAMEs were analysed using an Agilent 8890 GC system (Agilent Company, US) equipped with a capillary column (100 m × 0.25 mm × 0.25 µm id), a flame–ionization detector (FID, temperature 275 °C) and a splitless injector (temperature 220 °C). The initial oven temperature was 80 °C with a holding time of 1 min, which increased at a rate of 5 °C min−1 to 250 °C with a holding time of 5 min. The carrier gas used was helium (He) with a flow rate and split flow of 1 mL min−1 and 50 mL min−1, respectively. The makeup flow and airflow of He were 25 mL min−1 and 300 mL m−1, respectively. FAMEs were identified by matching their retention times with the standard of FAMEs under the same conditions. The calculation of fatty acids was done by measuring their relative area percentage.
2.7. FTIR spectroscopy
The infrared spectra of AKOs were recorded using ATR-FTIR (PerkinElmer, UATR Two, UK). A small quantity of oil sample (200 µL) was directly placed on the ATR crystal area. Scans were performed at an interval of 16 periodically, with a resolution of 8 cm−1, covering a 400–4000 cm−1 spectral range. The ATR crystal was cleaned with isopropyl alcohol before each measurement.2.8. Statistical analysis
All the experiments were performed in replicates. All the data were reported as mean ± standard deviation. Data were statistically analysed using a t–test (p ≤ 0.05) using Minitab-17 (Minitab Inc., State College, USA).3 Results and discussion
Oil is the most significant factor affecting the overall commercial viability of seeds. The yield of apricot kernel oil (AKOs) ranged from 49 to 51%, with no significant (p > 0.05) difference between AKOC (50.2%) and AKOT (49.8%). The consistency in oil yield after the hydrotreatment process indicated that the subsequent washing-centrifugation steps effectively separated the aqueous and oil phases, without miscella/oil loss. The high recovery of AKOT was attributed to the lipophilic nature of triacylglycerols, which resist aqueous partitioning.19 No oil-in-water emulsion was observed, likely due to the low phospholipid content in apricot kernel, which reduces the interfacial stability.20 Wang (2013)9 also reported apricot kernel oil ranging from 44.0% to 55.17%. Matthäus and Özcan (2015)21 and Chu et al., (2013)22 reported higher oil yields using Soxhlet extraction, with 53.4% in sweet apricot kernels and 58.78% in Amygdalus pedunculata, respectively. These variations among the studies might be due to differences in apricot variety, kernel maturity, extraction method and also, to some extent, growing environmental conditions.3.1. Physicochemical analysis
| Parameters | Untreated AKO (AKOC) | Treated AKO (AKOT) | FAO/WHO standard25 | |
|---|---|---|---|---|
| Values are expressed as mean ± standard deviation. Values with different superscript letters in the same row are significantly different (p ≤ 0.05). | ||||
| Yield, (%) | 49 ± 1.17a | 51 ± 1.76a | — | |
| Visual appearance | Yellow | Pale yellow | — | |
| Color values | L* | 49.64 ± 0.039a | 49.53 ± 0.210a | |
| a* | −1.83 ± 0.130a | −2.14 ± 0.076b | ||
| b* | 13.74 ± 0.075a | 13.70 ± 0.717a | ||
| Chroma (C*) | 4.88 ± 0.012a | 4.81 ± 0.134a | ||
| Hue (h°, degrees) | 97.49 ± 0.493a | 98.87 ± 0.174b | ||
| Refractive index | 1.468 ± 0.001a | 1.468 ± 0.001a | 1.46–1.47 | |
| Specific gravity | 0.92 ± 0.006a | 0.93 ± 0.006a | 0.90–1.16 | |
| Acid value (mg KOH per g) | 2.43 ± 0.32a | 2.43 ± 0.32a | 4.0 | |
| Iodine value (I2 g/100g) | 93.14 ± 0.25a | 92.64 ± 0.67a | 80–106 | |
| Peroxide value (meq O2 per kg) | 1.50 ± 0.71a | 1.67 ± 0.58a | <10 | |
| Saponification value (mg KOH per g) | 192.50 ± 7.78a | 187.00 ± 11.0a | 181.40 ± 2.60 | |
| Total phenolic content (µg GAE per g) | 27.28 ± 1.58a | 13.92 ± 2.85b | — | |
| Antioxidant activity | ||||
| DPPH (IC50, mg g−1) | 6.84 ± 0.76a | 6.69 ± 0.89a | ||
| FRAP (EC50, mg g−1) | 4.16 ± 0.37a | 4.26 ± 1.01a | ||
3.2. Total phenolic content and antioxidant activity
Phenolic compounds play a significant role in enhancing the antioxidant capacity of oils. The total phenolic content (TPC) and antioxidant activity of AKOs are shown in Table 1. The results showed that AKOC had significantly (p < 0.05) higher TPC (27.28 µg GAE per g) as compared to AKOT (13.92 µg GAE per g). The lower TPC in AKOT can be attributed to the polarity-based migration of hydrophilic phenolic compounds into the aqueous phase19 during hydro-treatment. Zhang et al., (2022)19 also reported a notable loss of TPC and phytosterol contents in rapeseed oil after washing, indicating that washing results in the partial loss of polar bioactive compounds. Uluata (2016)24 also reported similar TPC values (24.9 to 26.3 µg GAE per g) for apricot kernel oil.As shown in Table 1, the DPPH scavenging activity (IC50) and reducing power (FRAP, EC50) of AKOs varied from 6.69 to 6.84 mg g−1 and 4.16 to 4.26 mg g−1, respectively. There was no significant difference (p > 0.05) in the values of these activities between AKOC and AKOT, indicating that the hydro-treatment did not alter the antioxidant ability of AKO. Despite a reduction in TPC, non-phenolic lipid-soluble antioxidants remained unaffected mid-treatment, contributing to the stable antioxidant activity of AKO. In contrast, Szydłowska-Czerniak et al., (2011)36 reported a reduction in TPC and antioxidant activities of palm oil after aqueous washing (at 90–95 °C). Tavakoli et al., (2019)37 reported that the DPPH scavenging activity (IC50) of pistachio kernel oils varied from 12.7 to 15.9 mg mL−1. However, Fratianni et al., (2021)38 reported the higher antioxidant activity in cold-pressed seed apricot oil (DPPH IC50, 10.01 µg mL−1 and FRAP EC50, 0.128 mg QE per g). Lower IC50 and EC50 values indicate higher antioxidant activity, suggesting that AKO has a relatively strong antioxidant potential. Variations among studies might be due to differences in cultivar and extraction techniques.
3.3. Amygdalin content
The amygdalin content in apricot kernel oils (AKOC and AKOT) is presented in Fig. 1. The amygdalin content in AKOC was 17.27 mg/100 g, whereas AKOT was amygdalin free. The amygdalin content in oil must be less than 5 mg kg−1 to ensure safety.5 Mirzaei and Rezaei (2019)15 reported that hexane-extracted wild almond oil contained about 26 to 49 mg of amygdalin per 100 mL oil. Pavlović et al., (2018)33 revealed that the supercritical CO2 extraction method was more effective in reducing amygdalin content (20 mg/100 g) compared with the cold-pressed method (40 mg/100 g). These findings suggest that the selected extraction method can effectively reduce the amygdalin content, enhancing the oil's safety and expanding its potential uses. Mirzaei and Rezaei (2019)15 observed no differences in the amygdalin content between hot-pressed and cold-pressed wild almond oil. Zhang et al., (2020)13 reported that the ultrasonic treatment (700 W, 50 min) was the most effective method to reduce the cyanide content (4.65 mg kg−1) in bitter apricot kernel oil compared with microwave heating (8.33 mg kg−1) and water boiling (5.32 mg kg−1).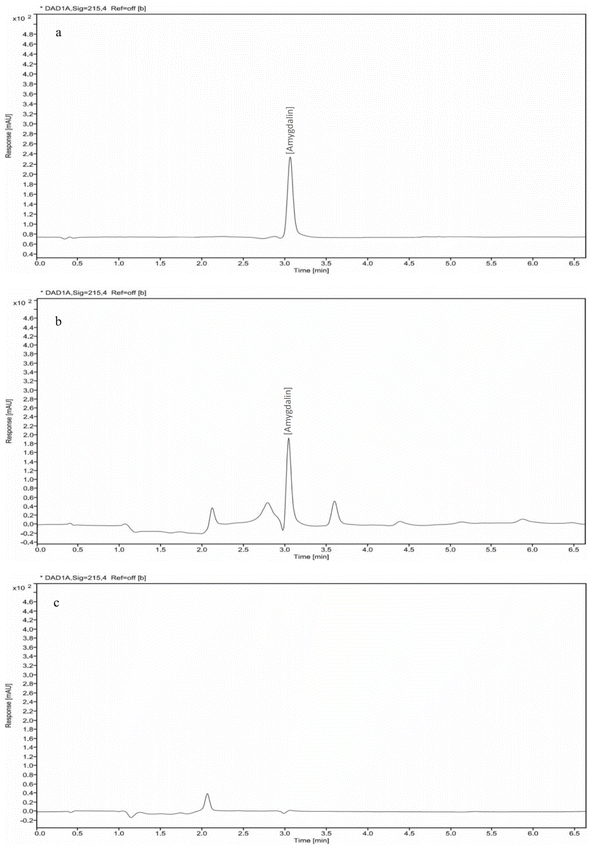 | ||
| Fig. 1 HPLC chromatogram of Amygdalin standard (a), untreated (b) and treated (c) apricot kernel oil. | ||
Amygdalin is a polar and hydrophilic compound with limited solubility in non-polar solvents like hexane.15 During oil extraction, a portion of the amygdalin in apricot kernels is transferred to the oil.13,15,33 However, during miscella washing with water, amygdalin migrates into the aqueous phase due to the polarity difference. This polarity-driven separation efficiently removes amygdalin, resulting in amygdalin–free AKOT.
3.4. Fatty acid composition
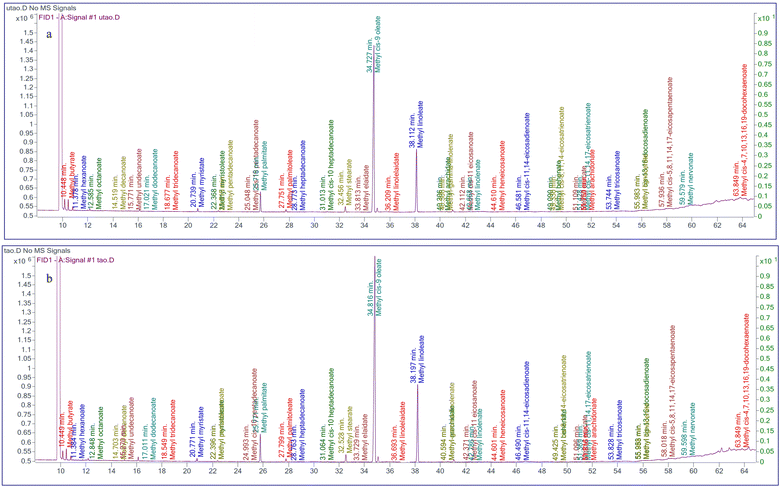
The effects of hydro–treatment on the fatty acid profile of AKO are shown in Table 2 and Fig. 2. The results showed a non-significant (p ≥ 0.05) difference in the fatty acid composition between AKOC and AKOT, indicating that hydro-treatment did not alter the fatty acid profile of AKO. The results indicated that the hydro-treatment process targets only polar impurities without affecting the non-polar matrix. This stability confirmed that hydro-treatment can safely detoxify the oil without affecting its nutritional properties. Zhang et al., (2022)19 also reported that the different aqueous treatments did not alter the fatty acid profile of rape seed oil. In AKOs, unsaturated and saturated fatty acids varied from 91.18–92.18% and 7.48–7.57%, respectively. Furthermore, the unsaturated fraction of AKO consisted of monounsaturated fatty acids (MUFAs) (74.83–75.05%) and polyunsaturated fatty acids (PUFAs) (24.93–25.17%) with oleic and linoleic acid as the major fatty acids. AKO also contained omega-3 fatty acids (DHA: 0.93–1.14% and EPA: 0.17–0.18%). The high levels of oleic acid (67.60–68.55%) and linoleic acid (21.68–21.84%) impart both oxidative stability and essential fatty acid benefits, making AKO suitable for application purposes. Palmitic acid (5.24–5.36% of AKO) was prominent in saturated fatty acids, followed by stearic acid (1.64–1.67% of AKO). All these fatty acids comprised more than 98% of the total fatty acid content. Therefore, other fatty acids were detected in minor quantities, less than 1–2%. The fatty acid composition of AKO is consistent with earlier reports for almond oil and wild plum seed oil (Prunus spinosa) reported by Roncero et al., (2021)39 and Atik et al., (2022).40 Similarly, Manzoor et al., (2012)41 and Gupta et al., (2012)31 also reported that apricot kernel oil contains oleic (62.07–80.97%) and linoleic (13.13–30.33%) acids as the primary fatty acids, followed by palmitic acids (3.35–7.79%). Moreover, Zhou et al., (2016)10 and Jin et al., (2018)5 also reported a similar fatty acid composition in Longwangmo and Bitter (Armeniaca sibirica L.) apricot kernel oil, with oleic acid ranging from 70.29–72.40%, linoleic acid from 21.80–23.0% and palmitic acid from 4.45–4.87%.| Fatty acid (%) | Untreated apricot kernel oil | Treated apricot kernel oil |
|---|---|---|
| Values are expressed as mean ± standard deviation. Values with different superscript letters in the same row are significantly different (p ≤ 0.05). | ||
| Saturated fatty acid | ||
| Palmitic acid | 5.24 ± 0.23a | 5.36 ± 0.21a |
| Stearic acid | 1.67 ± 0.06a | 1.64 ± 0.04a |
| Myristic acid | 0.57 ± 0.14a | 0.57 ± 0.06a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Monounsaturated fatty acid | ||
| Oleic acid | 68.55 ± 1.48a | 67.60 ± 0.81a |
| Palmitoleic acid | 0.64 ± 0.02a | 0.63 ± 0.01a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Polyunsaturated fatty acid | ||
| Linoleic acid | 21.68 ± 0.49a | 21.84 ± 0.38a |
| DHA (Docosahexaenoic acid) | 1.14 ± 0.30a | 0.93 ± 0.06a |
| EPA (Eicosapentaenoic acid) | 0.17 ± 0.00a | 0.18 ± 0.02a |
3.5. FTIR spectra of oil
The FTIR spectra of apricot kernel oils are shown in Fig. 3. No noticeable change in the FTIR spectra of AKO after hydro–treatment was observed, indicating that hydro-treatment is a mild process that preserves the chemical structure and quality of the oil. The wavenumber regions and corresponding functional groups are summarised in Table 3. The characteristic band at 3005 cm−1 corresponds to the cis double bond (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) stretching, confirming the presence of unsaturated fatty acids, particularly oleic acid.42 No shift/change in the intensity of this band indicated no alteration in IV or the degree of saturation of oil after hydro-treatment.43 Strong absorption bands at 2922 and 2858 cm−1 were due to asymmetric and symmetric stretching of C–H aliphatic groups CH2, respectively. No changes in the region of 3050–2800 cm−1 confirmed the unchanged PV of AKO after hydro-treatment.44 A sharp peak at 1742 cm−1 corresponds to the ester carbonyl (C
C–H) stretching, confirming the presence of unsaturated fatty acids, particularly oleic acid.42 No shift/change in the intensity of this band indicated no alteration in IV or the degree of saturation of oil after hydro-treatment.43 Strong absorption bands at 2922 and 2858 cm−1 were due to asymmetric and symmetric stretching of C–H aliphatic groups CH2, respectively. No changes in the region of 3050–2800 cm−1 confirmed the unchanged PV of AKO after hydro-treatment.44 A sharp peak at 1742 cm−1 corresponds to the ester carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bond stretching. The consistent intensity and position of this band confirmed intact ester linkages, indicating no hydrolysis and oxidation of oil after hydro-treatment.43,45 The additional peaks at 1460 cm−1 and 1414 cm−1 were due to the CH2/CH3 bending and olefins rocking vibrations, respectively, whereas, the peak at 1376 cm−1 was due to the bending symmetric vibrations of CH2 groups. The C–O stretching region (1238–1095 cm−1) and 1742 cm−1 ester band confirmed the unchanged SV via preserved glycerol ester content.43,45 The peak at 722 cm−1 was due to CH2 bending vibrations, characteristics of long fatty acids.43,46 The FTIR spectra of AKOs exhibited a similar pattern to the previously reported FTIR spectra of extra virgin oil46 and mustard oil.47 Overall, the FTIR spectra of AKO showed no changes in AV, IV, PV, and SV of the oil, validating hydro-treatment as a safe, non-destructive detoxification method.
O) bond stretching. The consistent intensity and position of this band confirmed intact ester linkages, indicating no hydrolysis and oxidation of oil after hydro-treatment.43,45 The additional peaks at 1460 cm−1 and 1414 cm−1 were due to the CH2/CH3 bending and olefins rocking vibrations, respectively, whereas, the peak at 1376 cm−1 was due to the bending symmetric vibrations of CH2 groups. The C–O stretching region (1238–1095 cm−1) and 1742 cm−1 ester band confirmed the unchanged SV via preserved glycerol ester content.43,45 The peak at 722 cm−1 was due to CH2 bending vibrations, characteristics of long fatty acids.43,46 The FTIR spectra of AKOs exhibited a similar pattern to the previously reported FTIR spectra of extra virgin oil46 and mustard oil.47 Overall, the FTIR spectra of AKO showed no changes in AV, IV, PV, and SV of the oil, validating hydro-treatment as a safe, non-destructive detoxification method.
 | ||
| Fig. 3 ATR-FTIR spectra of untreated (a) and treated (b) apricot kernel oils in the spectral region (4000–400 cm−1). | ||
| Wavenumber (cm−1) | Characteristic group |
|---|---|
| 3005 | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching (cis double bonds) C–H stretching (cis double bonds) |
| 2922 | Asymmetric stretching of aliphatic groups (CH2 and CH3) |
| 2858 | Symmetric stretching of aliphatic groups (CH2 and CH3) |
| 1742 | Stretching of ester carbonyl group (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
| 1460 | –C–H (CH2 and CH3) bending |
| 1414 | Rocking vibrations of aliphatic groups (CH2 and CH3) |
| 1376 | Bending vibrations of CH2 groups |
| 1238 | Stretching vibration of –C–O groups |
| 1163 | Stretching vibration of –C–O groups |
| 1120 | Stretching vibration of –C–O group |
| 1095 | Stretching vibration of –C–O group |
| 722 | CH2 bending (rocking) vibrations and –HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– (cis) group of di-substituted olefins CH– (cis) group of di-substituted olefins |
4 Conclusion
Bitter apricot kernel oil is generally undervalued due to the presence of cyanogenic glycosides (amygdalin), which can be effectively detoxified by hydrotreatment. Amygdalin is effectively removed using this process without affecting the yield or essential quality attributes (acid, saponification, iodine and peroxide values) in accordance with the FAO/WHO specifications. The oil still retains its fatty acid profile with considerable amounts of monounsaturated fatty acids. Notably, the post-treatment analysis confirms the complete removal of amygdalin, demonstrating the efficacy of the process in bitter apricot kernel oil and its safety for human consumption. As a result, hydro-treatment is an environmentally friendly and cost-effective approach for the utility of bitter apricot kernel oil in the food industry.Author contributions
Swati Joia: methodology, original draft preparation, investigation; Himashi Rao: investigation; Raashid Ahmad Siddiqui: review and editing; Tajendra Pal Singh: visualisation, supervision, conceptualisation, review and editing.Conflicts of interest
The authors have no conflicts of interest.Data availability
Data supporting this study are available from the corresponding author upon reasonable request.References
- Food and Agriculture Organization of the United Nations (FAO), FAOSTAT Statistical Database, https://www.fao.org/faostat/en/#data, accessed December 2022..
- V. K. Joshi, Int. J. Food Ferment. Technol., 2020, 10, 67–94 Search PubMed.
- Z. Ali, N. Gupta, J. Singh, A. Bhat, M. Sood, J. D. Bandral and M. Reshi, Chem. Sci. Rev. Lett., 2023, 12, 49–54 Search PubMed.
- B. Gayas, G. Kaur and K. Gul, J. Food Process Eng., 2017, 40, e12439 CrossRef.
- F. Jin, J. Wang, J. M. Regenstein and F. Wang, J. Oleo Sci., 2018, 67, 813–822 CrossRef.
- K. Targais, T. Stobdan, A. Yadav and S. B. Singh, Indian J. Tradit. Knowl., 2011, 10, 304–306 Search PubMed.
- F. A. Yildirim and M. A. Askin, Afr. J. Biotechnol., 2010, 9, 6522–6524 CAS.
- R. H. Salama, A. E. R. G. Ramadan, T. A. Alsanory, M. O. Herdan, O. M. Fathallah and A. A. Alsanory, Int. J. Biochem. Pharmacol., 2019, 1, 21–26 CrossRef.
- L. Wang, Ind. Crops Prod., 2013, 50, 838–843 CrossRef CAS.
- B. Zhou, Y. Wang, J. Kang, H. Zhong and P. D. Prenzler, Eur. J. Lipid Sci. Technol., 2016, 118, 236–243 CrossRef CAS.
- K. R. Pawar and P. K. Nema, Food Science and Biotechnology, 2023, 32, 249–263 CrossRef PubMed.
- F. X. Meng and H. J. Zhang, Food Res. Dev., 2013, 34, 100–102 CAS.
- Q. A. Zhang, F. F. Shi, J. L. Yao and N. Zhang, RSC Adv., 2020, 10, 10624–10633 RSC.
- S. Joia, R. A. Siddiqi and T. P. Singh, Waste Biomass Valori, 2025, 1–12 Search PubMed.
- H. Mirzaei and K. Rezaei, J. Am. Oil Chem. Soc., 2019, 96, 1163–1171 CrossRef CAS.
- AOAC, Official Methods of Analysis of AOAC International, AOAC International, Gaithersburg, MD, USA, 18th edn, 2006 Search PubMed.
- M. Olszowy and A. L. Dawidowicz, Monatsh. fur Chem., 2016, 147, 2083–2091 CrossRef CAS.
- Y. Chen, R. Zhang, C. Liu, X. Zheng and B. Liu, J. Food Sci. Technol., 2016, 53, 3028–3034 CrossRef CAS PubMed.
- L. Zhang, S. Akhymetkan, J. Chen, Y. Dong, Y. Gao and X. Yu, LWT, 2022, 155, 112947 CrossRef CAS.
- S. Latif and F. Anwar, J. Am. Oil Chem. Soc., 2009, 86, 393–400 CrossRef CAS.
- B. Matthäus and M. M. Özcan, Antioxid, 2015, 4, 124–133 CrossRef.
- J. Chu, X. Xu and Y. Zhang, Bioresour. Technol., 2013, 134, 374–376 CrossRef CAS PubMed.
- G. Durmaz, I. Karabulut, A. Topçu, M. Asiltürk and T. Kutlu, J. Am. Oil Chem. Soc., 2010, 87, 401–409 CrossRef CAS.
- S. Uluata, Akademik Gıda, 2016, 14, 333–340 Search PubMed.
- A. A. Adegbe, R. A. Larayetan and T. J. Omojuwa, Am. J. Chem., 2016, 6, 23–28 Search PubMed.
- R. K. Bachheti, I. Rai, A. Joshi and V. Rana, Int. Food Res. J., 2012, 19, 577–581 CAS.
- M. Alpaslan and M. Hayta, J. Am. Oil Chem. Soc., 2006, 83, 469–471 CrossRef CAS.
- A. Sharma, D. Vaidya, A. Gupta and M. Kaushal, J. Pharmacogn. Phytochem., 2019, 8, 1017–1021 CAS.
- T. Wang, in Vegetable Oils in Food Technology: Composition, Properties and Uses, ed. F. D. Gunstone, Wiley-Blackwell, 2nd edn., 2011, pp. 18–58 Search PubMed.
- M. M. Meador and W. Xinping, Final-Hygienic Standard for Edible Vegetable Oils (GB2716-2005): Unofficial Translation, USDA Foreign Agricultural Service, Beijing, China, 2013, GAIN Report CH13002 Search PubMed.
- A. Gupta, P. C. Sharma, B. M. K. S. Tilakratne and A. K. Verma, Indian J. Nat. Prod. Resour., 2012, 3, 366–370 Search PubMed.
- T. S. Bisht, S. K. Sharma, R. C. Sati, V. K. Rao, V. K. Yadav, A. K. Dixit, A. K. Sharma and C. S. Chopra, J. Food Sci. Technol., 2015, 52, 1543–1551 CrossRef CAS PubMed.
- N. Pavlović, S. Vidović, J. Vladić, L. Popović, T. Moslavac, S. Jakobović and S. Jokić, Eur. J. Lipid Sci. Technol., 2018, 120(11), 1800043 CrossRef.
- N. Shariatifar, I. M. Pourfard, G. J. Khaniki, R. Nabizadeh, A. Akbarzadeh and A. S. M. Nejad, Asian J. Chem., 2017, 29, 2011–2015 CrossRef CAS.
- J. Yang, Z. Pan, G. Takeoka, B. Mackey, G. Bingol, M. T. Brandl, K. Garcin, T. H. McHugh and H. Wang, Food Chem., 2013, 138, 671–678 CrossRef CAS PubMed.
- A. Szydłowska-Czerniak, K. Trokowski, G. Karlovits and E. Szłyk, Food Chem., 2011, 129, 1187–1192 CrossRef PubMed.
- J. Tavakoli, K. Hajpour Soq, A. Yousefi, P. Estakhr, M. Dalvi and A. Mousavi Khaneghah, J. Food Sci. Technol., 2019, 56, 5336–5345 CrossRef CAS PubMed.
- F. Fratianni, A. d’Acierno, M. N. Ombra, G. Amato, V. De Feo, J. F. Ayala-Zavala, R. Coppola and F. Nazzaro, Front. Nutr., 2021, 8, 775751 CrossRef PubMed.
- J. M. Roncero, M. Álvarez-Ortí, A. Pardo-Giménez, A. Rabadán and J. E. Pardo, Food, 2021, 10, 1049 CrossRef CAS PubMed.
- I. Atik, S. Karasu and R. Sevik, Riv. Ital. Sostanze Gr., 2022, 99, 13–20 Search PubMed.
- M. Manzoor, F. Anwar, M. Ashraf and K. M. Alkharfy, G.A., 2012, 63, 193–201 CAS.
- N. Mashodi, N. Y. Rahim, N. Muhammad and S. Asman, M. J. Fund. Appl. Sci., 2020, 5, 35–44 Search PubMed.
- M. D. Guillen and N. Cabo, J. Am. Oil Chem. Soc., 1997, 74, 1281–1286 CrossRef CAS.
- N. Vlachos, Y. Skopelitis, M. Psaroudaki, V. Konstantinidou, A. Chatzilazarou and E. Tegou, Anal. Chim. Acta, 2006, 573, 459–465 CrossRef.
- A. Rohman, Appl. Spectrosc. Rev., 2017, 52, 589–604 CrossRef CAS.
- M. A. Poiana, E. Alexa, M. F. Munteanu, R. Gligor, D. Moigradean and C. Mateescu, Open Chem., 2015, 13, 689–698 CAS.
- R. Jamwal, S. Kumari, S. Sharma, S. Kelly, A. Cannavan and D. K. Singh, Vib. Spectrosc., 2021, 113, 103222 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |