Open Access Article
Open Access ArticleTransforming waste into a bioactive resource: phenolic profile, antioxidant, and antimutagenic properties of pineapple (Ananas comosus) crown infusion
Talita Braga de Brito Nogueira a,
Lays Souza da Silvab,
Carlos Fernando Araujo Limabc,
Mateus Grilo de Oliveira Carvalhoa,
Mônica Regina da Costa Marquesd,
Israel Felzenszwalbb,
Juliano Lemos Bicase,
Mariana Simões Larraz Ferreira
a,
Lays Souza da Silvab,
Carlos Fernando Araujo Limabc,
Mateus Grilo de Oliveira Carvalhoa,
Mônica Regina da Costa Marquesd,
Israel Felzenszwalbb,
Juliano Lemos Bicase,
Mariana Simões Larraz Ferreira *af and
Ana Elizabeth Cavalcante Fai
*af and
Ana Elizabeth Cavalcante Fai *ag
*ag
aFood and Nutrition Graduate Program (PPGAN), Federal University of State of Rio de Janeiro (UNIRIO), Rio de Janeiro, RJ, Brazil. E-mail: mariana.ferreira@unirio.br
bEnvironmental Mutagenesis Laboratory, Department of Biophysics and Biometry, Rio de Janeiro State University (UERJ), Rio de Janeiro, RJ, Brazi
cPharmaceutical and Technological Innovation Laboratory, Federal University of the State of Rio de Janeiro (UNIRIO), Rio de Janeiro, RJ, Brazil
dFernanda Coutinho Analytical Center, Graduate Program in Chemistry, Rio de Janeiro State University (UERJ), Rio de Janeiro, RJ, Brazil
eDepartment of Food Science and Nutrition, School of Food Engineering, University of Campinas (UNICAMP), Campinas, SP, Brazil
fLaboratory of Protein Biochemistry, Center of Innovation in Mass Spectrometry (UNIRIO), Rio de Janeiro, RJ, Brazil
gLaboratory of Multidisciplinary Practices for Sustainability (LAMPS), Nutrition Institute, Rio de Janeiro State University (UERJ), Rio de Janeiro, RJ, Brazil. E-mail: ana.fai@uerj.br
First published on 10th December 2025
Abstract
The pineapple crown accounts for 30% of the waste from pineapple processing. This study investigated the potential of pineapple crown, an upcycled agro-industrial by-product, for the development of infusions with health-promoting properties. The pineapple crown was processed into flour (PCF) to extend its shelf life as a food ingredient. It had a low moisture content (6.30), low pH (4.7) and low water activity (0.36). ICP-MS analysis confirmed the presence of essential minerals, including potassium, calcium and magnesium. The color of the PCF infusion was lighter and less reddish than that of the flour, but both samples were yellowish. HPLC/DAD analysis identified five phenolic compounds in the infusion, with pyrogallol, catechin and p-coumaric acid being the most abundant. The 15-minute infusion showed the highest total reducing capacity (TRC) (10.25 ± 0.29 mg GAE per 100 mL), total flavonoid (TF) (1.92 ± 0.055 mg CE per 100 mL), and antioxidant capacity. No significant differences were observed compared to the 10-minute infusion for TRC (9.90 ± 0.49 mg GAE per 100 mL), DPPH (192.48 ± 38.59 µg TE per 100 mL), and FRAP (320.26 ± 7.90 µg TE per 100 mL). A 10-minute infusion was determined to be the optimal preparation time. Toxicological screening showed that PCF was not mutagenic in the Ames test and had no cytotoxic effects on normal liver cells (HepG2 and FC3H). Notably, PCF showed dose-dependent cytotoxicity on gastric adenocarcinoma cells (HGC-27). PCF infusions offer a health-oriented beverage alternative that strengthens the role of upcycled agro-industrial material in food innovation and contributes to circular economy strategies in the food and pharmaceutical sectors.
Sustainability spotlightThis study supports the sustainable valorization of pineapple crown, which accounts for 30% of pineapple processing waste, by upcycling it into a bioactive infusion with antioxidant and chemopreventive potential. By transforming an agro-industrial by-product into a health-promoting ingredient, the research promotes food innovation that is in line with the principles of circular economy and waste reduction. The results contribute directly to the United Nations Sustainable Development Goals, in particular SDG 3 (Good Health and Well-being), SDG 9 (Industry, Innovation and Infrastructure) and SDG 12 (Responsible Consumption and Production). The safe, functional use of pineapple crown flour underlines the importance of integrating sustainability and bioeconomy in the development of value-added food and pharmaceutical products. |
1. Introduction
The pineapple (Ananas comosus), a tropical fruit of the bromeliad family (Bromeliaceae), includes varieties cultivated worldwide such as Smooth Cayenne, Queen and Spanish, with red or green morphological variations.1 The fruit consists of pulp (46–53%), crown (2–24%), peel (12–28%) and seed (7–17%).2–5 After minimal processing of pineapple, approximately 50% (w/w) becomes by-products (core, peel, and crown).6 Crowns account for about 2–24% of fruit mass, with major cultivars such as ‘Smooth Cayenne’ (13.6%) and ‘Pérola’ (10%) at the higher end.2–4,6,7 In a 20-day survey of two fruit and vegetable markets in Rio de Janeiro, 123.52 kg of minimally processed pineapple generated 61.76 kg of residues (50% w/w) (Brito et al.,10 2020). Assuming a constant rate, this corresponds to 2.25 t processed and 1.13 t of residues per year across the two markets (linear extrapolation).Accordingly, the pineapple crown is rich in insoluble fibers, including about 60% cellulose, 20% hemicellulose and 5% lignin.8,9 Previous work with pineapple crown flour (PCF) confirmed the high content of total (67.22%), soluble (58.51%) and insoluble (8.71%) fiber,10 combined with a remarkable antioxidant potential due to phenolic compounds bound to the lignocellulosic matrix.11,12 Mass spectrometric analyses revealed the presence of phenolic acids (e.g. ferulic acid, p-coumaric acid, caffeic acid, vanillic acid, benzoic acid and 5-caffeoylquinic acid) and flavonoids such as catechins, epicatechins and quercetin-3-O-rutinoside.12,13 These compounds contribute to the antioxidant properties of the methanolic and ethanolic extracts of the crown12 and are responsible for a wide range of bioactivities, including antimicrobial, anti-inflammatory and anticancer effects, as well as technological functionalities relevant to food, pharmaceutical and textile applications.12,14–16
Herbal infusions, traditionally consumed for their aromatic and medicinal properties, offer a practical and cost-effective method for extracting bioactives from plant matrices.17 Many studies have focused on infusions of fruits and herbs with therapeutic potential. For example, raspberry infusions showed higher phenolic content than black tea and comparable levels to green tea, with high antioxidant capacity due to compounds such as gallic acid, ellagic acid, catechins and flavonoids.18–20 Green tea (Camellia sinensis) is often studied for its anti-cancer and anti-inflammatory effects which are attributed to its phytochemical profile.21–23 In the study by Kaur et al.,24 dehydrated pineapple peels were infused both individually and in combination with green tea. The infusion of green tea with pineapple peel significantly increased these parameters, suggesting that, despite their lower intrinsic antioxidant capacity, pineapple peel – and potentially pineapple crown residues – may serve as valuable additives to enhance the nutritional and bioactive properties of commonly consumed beverages. Infusions prepared from pineapple crowns fermented with Aspergillus tubingensis exhibited high levels of phenolic compounds, along with pronounced antioxidant and antimicrobial activities, and demonstrated probiotic potential due to the presence of xylooligosaccharides formed during fermentation.25
Despite centuries of traditional use, the biological efficacy and safety of herbal infusions require further scientific confirmation. Some natural substances have shown therapeutic effects, while others have mutagenic or cytotoxic properties, emphasizing the importance of rigorous toxicological evaluation.26–28
The upcycling of agro-industrial by-products such as pineapple crowns is in line with the United Nations 2030 Agenda and the Sustainable Development Goals (SDGs), in particular SDG 9 (Industry, Innovation and Infrastructure), SDG 12 (Responsible Consumption and Production) and SDG 13 (Climate Action).29 These actions are also in line with the principles of the circular economy, which promote the prevention of waste, the conservation of materials in use and the regeneration of natural systems.30,31
One of the key targets defined under Sustainable Development Goal (SDG) 12.3 seeks to reduce global per capita food waste by 50% by 2030. This target encompasses the reduction of losses occurring across the entire production and supply chain, as well as reduction of waste at the retail and consumer levels.32 The valorization of pineapple waste, particularly the crown, can contribute to achieving SDG target 12.3, as pineapple crowns can be recovered at multiple stages along the production, distribution, and commercialization chain.
Against this background, this study hypothesizes that pineapple crown, an agro-industrial by-product, is a valuable resource to produce bioactive infusions with high antioxidant capacity, without mutagenic or cytotoxic effects in healthy cells and with potential selective antitumor activity. Therefore, the aim of this work was to evaluate the potential of pineapple crown for the development of infusions with health-promoting properties.
2. Materials and methods
2.1. Acquisition of samples and obtaining conventional and organic pineapple crown flour
Conventional pineapple crowns (Ananas comosus cv. Pérola) were collected on three nonconsecutive days from a fruit and vegetable market in Rio de Janeiro, Brazil. The pineapple crowns were washed, defoliated, fractionated and dried in a ventilated oven (Marconi, MA 035) at 60 °C for 12 h and then at 90 °C for 1 h as described by Brito et al.102.2. Obtaining the infusion of pineapple crown flour
The infusion was obtained according to Moraes et al.33 with modifications. Two grams of PCF were added to 70 mL of boiling distilled water. Then, the infusion was filtered through filter paper after times of 5, 10 and 15 min and transferred to a volumetric flask and made up to 100 mL and frozen (−20 °C) until the analyses were carried out.2.3. Physicochemical characterization
2.4. Mineral content
Mineral analysis of PCF and PCF infusion was performed using an Inductively Coupled Plasma Mass Spectrometer (ICP MS, Aurora M90, Bruker). Samples were prepared using conventional microwaves. The range of the calibration curve for each mineral was 100–1000 ppb.37,382.5. Antioxidant capacity
| % InhibitionDPPH = [(AbsDPPH − Abssample)/AbsDPPH] × 100 |
2.6. Phenolic compound profile of the PCF infusion by HPLC
Initially, the infusion was centrifuged (2000×g; 5 min) and filtered through a 0.45 µm PTFE membrane filter (Millex; Millipore, Germany). Phenolic compounds were analyzed on an HPLC–DAD system (PerkinElmer, Flexar, USA) using a quaternary pump. The injection of the samples and the standard solution was done through a Rheodyne valve with a 20 µL loop. A C18 reversed-phase column (5 µm × 150 mm × 4.6 mm; Kromasil; AkzoNobel, Sweden) was used. The flow rate of the mobile phase [0.3% aqueous formic acid (eluent A), methanol (eluent B) and acetonitrile (eluent C)] was 0.8 mL min−1. The mobile phase gradient was: 0 min, 14.3% B and 0.7% C; 7 min, 29.5% B and 1.5% C; 14 min, 44.6% B and 2.4% C; 21 min, 59.8% B and 3.2% C; and 25 min, 90.3% B and 4.7% C. Column elution was monitored at 260, 280 and 320 nm and the identification of peaks of phenolic compounds was conducted by comparing relative retention times and PDA spectra (λ from 230 to 350 nm) of commercial standards. To obtain and analyze the data, the Chromera Data system 2012 software (PerkinElmer, USA) was used. Quantitative analysis was performed according to the external calibration curve with the pool of standards (SI Table 1). Analyses were performed in triplicate, and phenolic compound concentrations were expressed in µg mL−1.452.7. In vitro toxicity screening
The cells were seeded in a 96 well-plate at 1 × 104 cells per well and incubated for 24 h at 37 °C and 5% CO2. The cells received the same treatment as in the WST assay. After the treatment period, the supernatant was moved to a new 96-well plate and the LDH reagent was added. The plate was read at 492 nm (Polaris Microplate Reader, Celer, Brazil) after 30 min of incubation at room temperature protected from light.27 The percentage of cytotoxicity was calculated according to the kit protocols. The lethal concentration (LC50) for 50% of cultured cells was determined by comparison based on this reference level. The results for LC50 represent the average ± SD of three independent experiments for each cell viability assay. To evaluate the possible impact of PCF self-fluorescence in both assays, a background check was carried out with the medium and the sample (data not shown). No difference in the absorbance profiles was observed, indicating no interference in the optical density measurements.
2.8. Statistical analysis
Data on physicochemical and antioxidant parameters were subjected to analysis of variance (one-way ANOVA) and the means were compared using the post-hoc Tukey test (95% confidence level) in the XLSTAT statistical software (Addinsoft, 2023.1.3).49 Concerning the biological models, both in bacterial and eukaryotic cell assays, the nonlinear regression fit of the dose–response curves was calculated using GraphPad Prism software (version 8.0.2). The same software was applied to determine statistical differences between groups, using one-way ANOVA with Tukey's post hoc test. Experiments were repeated at least twice in the bacterial model and three times in the mammal cell model, and in triplicate for each exposure setting.503. Results and discussion
3.1. Physicochemical characterization of the pineapple crown flour infusion
Table 1 shows the values of the physical–chemical characteristics of the PCF and the infusion. The PCF showed low humidity (6.30 ± 0.21) and water activity (0.36 ± 0.01), which are important for the microbiological stability of the flour, inhibiting the growth of microorganisms during the storage period. PCF moisture is similar to that of green tea and commercial black tea of different brands found in the study by Das et al.,51 with values between 7.61 and 8.46% for green tea and 6.13–7.18% for black tea. The pH of the PCF and the infusion was similar (4.75–4.84) and the titratable acidity value increased, characterizing a decrease in acidity when the infusion was prepared (2.88–5.26).| PCF infusion | ||
|---|---|---|
| a PCF: pineapple crown flour.b Titratable acidity: % citric acid. | ||
| pH | 4.84 ± 0.02 | |
| Titratable acidity (%)b | 5.26 ± 0.26 | |
| Soluble solids (%) | 1.0 ± 0.00 | |
| Total solids (%) | 1.08 ± 0.03 | |
| Total sugar content (%) | 7.86 ± 0.05 | |
| Reducing sugar content (%) | 6.11 ± 0.38 | |
| Sucrose (%) | 1.66 ± 0.37 | |
| Colorimetric parameters | L* | 91.61 ± 0.52 |
| a* | −1.46 ± 0.05 | |
| b* | 22.20 ± 1.76 | |
The infusions prepared from fresh and autoclaved pineapple crowns (121 °C for 15 minutes) exhibited similar pH values, ranging from 5.18 to 4.75. However, the titratable acidity of the pineapple crown flour (PCF) infusion was significantly higher than that of both the fresh (0.0084%) and sterilized (0.014%) crown infusions.25 In the infusion, low values of soluble and total solids and low sugar content can be observed. However, the total solid content in the PCF infusion was approximately ten times greater than that in the other two preparations, while the soluble solid content was also slightly higher, ranging from 0.6% to 0.8%.25
Regarding colorimetric properties, the PCF infusion was lighter and exhibited lower red intensity compared to the flour, although both samples presented a yellowish hue.10 Fig. 1 displays the colorimetric parameters of the pineapple crown flour (PCF) (a) and its corresponding infusion (b). Compared to the PCF infusion, the infusion prepared from fresh pineapple crowns was significantly lighter (L* = 94.38 ± 0.20), with lower green (a* = 0.69 ± 0.02) and yellow (b* = 5.36 ± 0.11) chromaticity values.25 In contrast, the infusion obtained from autoclaved pineapple crowns exhibited colorimetric values (L* = 92.64 ± 0.06; a* = 1.35 ± 0.03; b* = 11.67 ± 0.10) comparable to those of the PCF infusion.25 This similarity is likely attributable to thermal processing, which may have promoted chlorophyll degradation and induced Maillard reactions, leading to increased yellowness and a slight darkening of the infusion.52
3.2. Mineral content
The mineral values found in the PCF and in the PCF infusion are described in Table 2. Cadmium and lead were not found in PCF and the infusion. Nickel and copper were present in the flour sample but in the infusion these minerals were not found. The other minerals (Mn, Cu, Zn, Na, Mg, K, Ca and Fe) were present in amounts similar to those found in previous studies with PCF analyzed by flame atomic absorption spectrometry (FAAS). However, potassium was approximately 5 times lower in this study and manganese was 9 times lower, while sodium content was almost 5 times higher.10 Regarding the infusion, the values of Na and Ca were higher than those found in the infusion of lemon balm (0.3 ± 0.04 and 1.2 ± 0.05 mg per 100 mL, respectively) and the same value of Mg (3.6 ± 0 0.02 mg per 100 mL). As for Mn, Zn and K, the values found in the infusion of PCF were lower than those in the infusion of lemon balm.53 The PCF infusion, with a mineral content similar to herbal infusions and widespread herbal residues, presents implications that are worth contemplating. Infusions of hibiscus, horsetail, linden, sage, white mulberry leaf, and yerba mate, for instance, have Ca values similar to PCF (∼3.5 mg per 100 mL). Similarly, the K content is akin to chamomile, linden, purga herb, St. John's wort, and white mulberry leaf infusions (∼4.7 mg per 100 mL). Other minerals evaluated, such as Mg and Na, in these and other infusions showed significantly lower values than PCF.54 Among the trace elements (Mn, Zn, and Fe), PCF revealed higher Fe contents than all infusions in the comparative study and lower Mn and Zn contents. These findings underscore the implications of PCF infusion research, which can be compared to teas and infusions popularly consumed worldwide, known for their medicinal and bioactive properties.| Minerals | mg per 100 g | mg per 100 mL |
|---|---|---|
| C | 0.00526 ± 0.0005 | ND |
| Mn | 7.5 ± 0.5 | 0.000298 ± 0.000004 |
| Ni | 0.0062 ± 0.0004 | ND |
| Cu | 0.556 ± 0.009 | ND |
| Zn | 1.46 ± 0.06 | 0.00019 ± 0.00001 |
| Cd | ND | ND |
| Pb | ND | ND |
| Na | 51.0 ± 3.0 | 10.35 ± 0.07 |
| Mg | 222.0 ± 4.0 | 3.62 ± 0.03 |
| K | 480.0 ± 40.0 | 4.80 ± 0.02 |
| Ca | 354.0 ± 7.0 | 4.04 ± 0.03 |
| Fe | 3.8 ± 0.3 | 0.0781 ± 0.0004 |
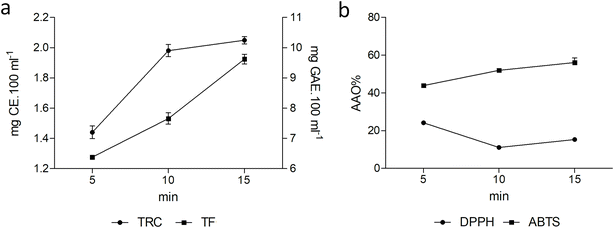
3.3. Antioxidant capacity
The total reducing capacity, flavonoid content and antioxidant capacity of the PCF infusion were evaluated at three different infusion times. Fig. 2 and Table 3 show the values at times of 5, 10 and 15 minutes. The sample infused for 15 minutes showed the highest Folin-reducing capacity (10.25 ± 0.29 mg GAE per 100 mL) and the greatest flavonoid content (1.92 ± 0.055 mg CE per 100 mL). Nevertheless, no significant differences were observed between the 15- and 10-minute infusion times for TRC (9.90 ± 0.49 mg GAE per 100 mL), DPPH (192.48 ± 38.59 µg TE per 100 mL), and FRAP (320.26 ± 7.90 µg TE per 100 mL) assays. The 5-minute infusion time showed lower antioxidant capacity in general, but no difference was verified with the 15-minute time in the DPPH and ORAC analyses. In general, commercial teas suggest a preparation time of between 3 and 10 minutes, so a time of 10 minutes can be considered as the recommended time, as it is more effective for extracting phenolic compounds and increasing antioxidant capacity in the infusion. Previous studies have reported high antioxidant capacity and significant phenolic compound content in pineapple crown flour (PCF), in both methanolic and ethanolic extracts, including free and bound phenolic fractions.12 The total reducing capacity (TRC) ranged from 542.30 to 579.21 mg GAE per 100 g in the free extract and from 953.82 to 1325.70 mg GAE per 100 g in the bound extract.12 The release of these bound compounds further promoted a 3- or 4-fold increase in PCF antioxidant capacity.| 5 min | 10 min | 15 min | |
|---|---|---|---|
| a Values are expressed as the mean ± standard deviation. After a two-way analysis of variance (ANOVA) a Tukey test (p < 0.01) was applied. Different lowercase letters on a line indicate significant differences between teas with different infusion times. | |||
| TRC (mg GAE per 100 mL) | 7.20 ± 0.51b | 9.90 ± 0.49a | 10.25 ± 0.29a |
| TF (mg CE per 100 mL) | 1.28 ± 0.01c | 1.53 ± 0.07b | 1.92 ± 0.06a |
| DPPH (µg TE per 100 mL) | 209.04 ± 30.72a | 192.48 ± 38.59a | 190.47 ± 6.55a |
| FRAP (µg TE per 100 mL) | 203.17 ± 1.37b | 320.26 ± 7.90a | 348.01 ± 7.80a |
| ABTS (µg TE per 100 mL) | 249.12 ± 2.69c | 336.79 ± 5.83b | 369.22 ± 14.40a |
| ORAC (µg TE per 100 mL) | 386.83 ± 69.46 ab | 296.67 ± 17.76b | 428.77 ± 5.63a |
Although the extraction of phenolic compounds by the infusion method does not promote the release of these bound compounds, it can be observed that contact with water for a longer time can release more compounds and increase the antioxidant capacity of the sample. In a study with the infusion of dried tangerine peel at different temperatures, similar values of TRC were found between 10.84 and 11.75 mg GAE per 100 mL.55 Pineapple pulp and pineapple peel infusions are popularly consumed but have been little studied. Widowati et al.56 evaluated the antioxidant capacity of Telang infusion (Clitoria ternatea) with dehydrated pineapple infusion and found an interesting antioxidant capacity (%) of mixed infusions (45.12 ± 1.09) and pineapple infusion (81.79 ± 6.56) by the DPPH method. However, the pineapple infusion showed the lowest content of total phenolics and flavonoids (0.82 µg GAE per 100% and 0.17 µg QE per 100%) and the lowest antioxidant capacity by FRAP (19.56 ± 2.82) and ABTS (22.77 ± 1.08). Fig. 1 shows the antioxidant capacity calculated by the DPPH and ABTS methods of the infusions prepared at times 5, 10 and 15 min. It is observed that by ABTS the AAO of the infusion increased significantly (p < 0.01) with its preparation time, reaching about 56% in the time of 15 min. As for the DPPH, the AAO was not linear where the time of 10 min was the one that presented the lowest AAO (11%) and the time of 5 min the highest AAO (24%). In the study by Veljkovic et al.,20 which analyzed 26 commercial infusions, including 11 fruit infusions, the pineapple infusion (45.84 ± 3.82 and 25.35 ± 0.57 g kg−1) had the highest content of phenolic compounds and flavonoids compared to chamomile (36.39 ± 1.94 and 11.79 ± 0.14 g per kg). However, among the fruit infusions analyzed (cherry, strawberry, raspberry, forest fruits, apricot, sweet cherry, blueberry, apple, pomegranate and exotic fruits), the pineapple infusion had the lowest CRT and TFC value. In another study, the pineapple peel infusion exhibited lower levels of phenolic compounds (49.33 ± 0.04 mg TAE per g) and antioxidant capacity (15.93 ± 0.04 mg AAE per g) compared with green tea. However, when pineapple peel was infused together with green tea, these values increased to 63.46 ± 0.05 mg TAE per g and 18.93 ± 0.05 mg AAE per g, respectively.24
In a recent study, infusions were prepared from pineapple crowns fermented for 7, 12, and 15 days with Aspergillus tubingensis.25 The control infusion, obtained from sterilized (autoclaved) crowns, exhibited lower TRC (2.72 ± 0.22 mg GAE per 100 mL) and TF (2.63 ± 0.62 mg CE per 100 mL) values compared with the fermented samples. The infusion produced from crowns fermented for 12 days showed the highest TRC (4.10 ± 0.17 mg GAE per 100 mL), TF (5.34 ± 0.23 mg CE per 100 mL), and antioxidant capacity, as determined by the DPPH (13.22 ± 0.49 mg TE per 100 mL), FRAP (5.31 ± 0.11 mg TE per 100 mL), and ABTS (21.23 ± 1.22 mg TE per 100 mL) assays. The authors suggested that enzymes produced during fermentation promoted the release of phenolic compounds previously bound to the lignocellulosic matrix of the pineapple crown.25 Although fermentation markedly enhanced the TRC, TF, and antioxidant capacity of the crown infusions, the PCF infusion presented a TRC value nearly five times higher than that of the control and twice that of the 12-day fermented crown infusion, possibly due to the particle size and drying process applied to the PCF, which concentrates bioactive compounds.55
3.4. Phenolic compound profile of the PCF infusion by HPLC
In the HPLC analysis, 5 phenolic compounds were identified, as shown in Table 4 and SI Fig. 1 and 2. Pyrogallol was the compound present in the largest amount in the sample (17.91 ± 1.72 µg mL−1) followed by catechin (11.07 ± 2.33 µg mL−1) and p-coumaric acid (4.59 ± 0.04 33 µg mL−1). Given pyrogallol's irritation potential57 and the absence of an EFSA ADI, EFSA's TTC Cramer Class III value (1.5 µg kg−1 day−1) was used as a conservative screening threshold. Therefore, the phenolic profile data are interpreted in a screening-level context rather than as a definitive risk assessment.58 An isomer of chlorogenic acid was found but could not be identified. The CGA isomer assignment by HPLC-DAD is tentative. Confirmation would require LC-HRMS/MS and, if feasible, NMR in future studies. In the previous study with PCF, 177 phenolic compounds were tentatively identified by UPLC-MSE in hydroalcoholic extracts containing free and bound compounds.12 Among them, only pyrogallol and p-coumaric acid were identified in free and bound extracts, but the chlorogenic acid isomer, catechin and vanillinic acid were not found. In the pineapple infusion analyzed by HPLC20 interesting amounts of gallic acid (0.73 ± 0.05), caffeic acid (0.26 ± 0.01), (+)-catechin (0.028 ± 0.001), (−)-epicatechin (0.039 ± 0.002), quercetin (0.018 ± 0.001) and protocatechuic acid (0.028 ± 0.001) were observed. The catechins present in tea are related to several health benefits such as anti-inflammatory, anticarcinogenic, antiviral, and cardioprotective effects, and regulation of carbohydrate metabolism, among others.59 Pyrogallol present in Awa tea demonstrated anti-allergic activity helping to alleviate nasal symptoms by suppressing NFAT-mediated IL-9 gene expression through inhibition of NFAT dephosphorylation.60| µg mL−1 | Class | Subclass | |
|---|---|---|---|
| Pyrogallol | 17.91 ± 1.72 | Other polyphenols | Other polyphenols |
| Vanillinic acid | 0.30 ± 0.07 | Other polyphenols | Hydroxybenzaldehydes |
| Catechin | 11.07 ± 2.33 | Flavonoids | Flavanols |
| Chlorogenic acid isomer | 2.34 ± 0.07 | Phenolic acid | Hydroxycinnamic acids |
| p-Coumaric acid | 4.59 ± 0.04 | Phenolic acid | Hydroxycinnamic acids |
3.5. In vitro toxicity screening
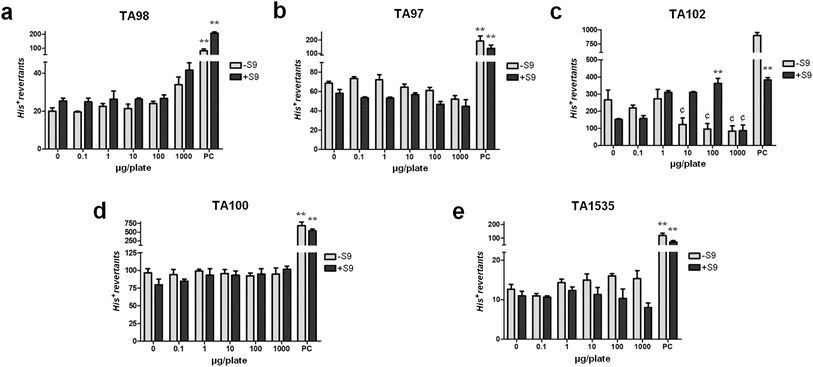
This study was designed as a screening study to determine concentration–response and LC50 values; mechanistic assays were not conducted and are identified as priorities for future studies. The results of the bacterial reverse mutation assay in a microsuspension (Fig. 3) point to the absence of mutagenicity of PCF against Salmonella enterica Typhimurium strains TA 98 (Fig. 3a), TA97 (Fig. 3b), TA 102 (Fig. 3c), TA 100 (Fig. 3d) and TA 1535 (Fig. 3e), in both the absence and presence of metabolic activation with the S9 mix. It is possible to observe that there is no significant increase in the number of revertant colonies of TA97, TA98, TA100 and TA1535 in any of the tested conditions and a slight reduction of bacterial populations in exposures to the highest concentrations of the extract. Concerning TA102, it is possible to observe a clear dose–response curve of His+ revertant colonies, with a statistically significant result at the 100 µg per plate exposure dose, followed by a cytotoxic response at the highest dose of PCF in +S9 incubation. These results suggest a cytotoxic effect mainly after metabolic activation, and this is consistent with the profile of plant extracts with antimicrobial activity, as PCF possibly is, and may be considered a limiting factor in the bacterial model for predicting their mutagenicity.61 Since TA102 displays distinct sensitivity compared to other tester strains in mutagenicity screening due to its susceptibility to chemical oxidants,62 and considering the intricate nature of phenolic compounds and flavonoids within PCF, which can exhibit an antioxidant/pro-oxidant duality contingent upon the cellular context, experimental conditions (particularly in the presence of metabolic activation that enhances oxidative events), concentration variations, and synergistic combinations,63 become pivotal in comprehending the mutagenic characteristics of the aforementioned extract in TA102. Considering the cytotoxicity in mammalian cell lineages (SI Table 2), PCF did not induce any cytotoxic effects in liver cell lineages (HepG2 and FC3H). However, PCF was able to induce cell death in HGC-27 gastric cells, in a dose and time-dependent manner. After 24 h of exposure, the LC50 was 3289 ± 33 µg mL−1, after 48 h of exposure, the LC50 was 2813 ± 41 µg mL−1 and after 72 h of exposure, the LC50 was 2184 ± 44 µg mL−1. Bearing in mind that this lineage is composed of cancer cells (gastric adenocarcinoma), these results are encouraging in the context of antitumor potential of this sample and merits further studies.Cytotoxicity screening is an essential stage of preclinical toxicology assessment of herbal extracts.64 In the Salmonella/microsome assay, PCF did not induce mutagenic effects at the analyzed concentrations; likewise, it did not induce cytotoxicity in HepG2 and FC3H cells, despite presenting phenolic compounds and flavonoids that can be considered mutagenic when incubated separately, such as quercetin and m-coumaric acid.65 The genotoxicity of these flavonoids may be inhibited by accompanying compounds with chemopreventive effects, such as hesperidin,66 possibly acting as antigenotoxic agents in healthy cells, through the modulation of redox status, scavenging of free radicals and activation of antioxidant defenses in eukaryotic organisms.67–69 The same components and synergistic interactions exhibit cytotoxic effects when applied to cells derived from gastric cancer, HGC-27. These results resonate well with each other, as the redox metabolism of these cells is known to be altered70 making them specific targets for the cytotoxicity of these extracts when the same bioactive compounds that can act as antigenotoxic agents under normal conditions may trigger redox imbalance and cell death in cancerous cells as seen in other studies involving natural products and gastric cancer cells.71
4. Conclusion
PCF proved to be a stable food ingredient due to its physical–chemical properties and had a rich profile of bioactive phenolic compounds, including pyrogallol, p-coumaric acid and catechin, as well as essential minerals such as potassium, calcium and magnesium. The preparation of the infusion had a significant impact on bioactivity, with a 10-minute extraction maximizing phenolic content, flavonoids and antioxidant capacity, reaching levels comparable to those of commercial teas. Sensory analysis was not conducted in this screening study and is planned for future work. Toxicological tests confirmed the absence of mutagenicity in most Salmonella enterica serovar Typhimurium strains, with the exception of oxidation-sensitive TA102, and no cytotoxicity in normal liver (HepG2) or fibroblast (FC3H) cells. Conversely, PCF infusions showed dose-dependent cytotoxicity against gastric adenocarcinoma cells (HGC-27). These results point to the antioxidant/pro-oxidant duality characteristic of complex phytochemical matrices, emphasizing the need for further mechanistic studies. By transforming a high-volume agro-industrial by-product into a bioactive, health-promoting infusion, this research is in line with the principles of the circular economy and the United Nations 2030 Agenda for sustainable production and waste utilization. In addition to its use as a bioactive ingredient in food applications, PCF could be explored for nutraceutical and pharmaceutical uses, pending mechanistic confirmation, in vivo studies, and regulatory evaluation.Conflicts of interest
There are no conflicts to declare.Data availability
Data are available upon request to the authors.Acknowledgements
This work was supported by UNIRIO, UERJ, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (401053/2019-9). Dr Araujo Lima, C. F. received grant funding from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (cod E-26/010.100956/2018). Dr Felzenszwalb, I. received grant funding from FAPERJ (codes E-26/202.759/2017; E-26/210.096/2023) and CNPq (code 302345/2017-5). Dra. Ferreira, M.S.L. received grant funding from FAPERJ (codes 26/010.000171/2016; 26/010.100988/2018; 26/202/709-2018; 26/201.317/2022) and CNPq (314100/2023-7). Dra. Fai, A. E. C. received grant funding from FAPERJ (codes E- 26/201.428/2022 and E-26/210.326/2022).References
- J. D. L. C. Medina and H. S. García, Pineapple: Post-harvest Operations FAO, 2005, p. 38 Search PubMed.
- Z. Nor, L. Ramchandran, M. Duke and T. Vasiljevic, Characteristic properties of crude pineapple waste extract for bromelain purification by membrane processing, J. Food Sci. Technol., 2015, 52, 7103–7112 CrossRef.
- G. G. Granada, R. C. Zambiazi and C. R. B. MendonÇA, Abacaxi: Produção, Mercado E Subprodutos, Bol. do Cent. Pesqui. Process. Aliment., 2004, 22(2), 405–422 Search PubMed.
- S. Ketnawa, P. Chaiwut and S. Rawdkuen, Pineapple wastes: A potential source for bromelain extraction, Food Bioprod. Process., 2012, 90(3), 385–391 CrossRef CAS.
- O. V. Omotoyinbo and D. M. Sanni, Characterization of Bromelain from Parts of Three Different Pineapple Varieties in Nigeria, Am. J. Biosci., 2017, 5, 35 CrossRef CAS.
- T. B. N. Brito, T. P. M. Silva, D. A. Luiz, C. J. Andrade, L. M. Andrade, M. S. L. Ferreira and A. E. C. Fai, Fruits and vegetable-processing waste: a case study in two markets at Rio de Janeiro, RJ, Brazil, Environ. Sci. Pollut. Res., 2020, 27, 18530–18540 CrossRef PubMed.
- T. B. N. Brito, M. S. L. Ferreira and A. E. C. Fai, Utilization of Agricultural By-products: Bioactive Properties and Technological Applications, Food Rev. Int., 2020, 1–25 Search PubMed.
- V. Johny, A. Kuriakose Mani, S. Palanisamy, V. K. Rajan, M. Palaniappan and C. Santulli, Extraction and Physico-Chemical Characterization of Pineapple Crown Leaf Fibers (PCLF), Fibers, 2023, 11(1), 5 CrossRef CAS.
- R. Roy, D. Debnath and S. Ray, Comprehensive Assessment of Various Lignocellulosic Biomasses for Energy Recovery in a Hybrid Energy System, Arabian J. Sci. Eng., 2022, 47(5), 5935–5948 CrossRef CAS.
- T. B. N. Brito, A. P. A. Pereira, G. M. Pastore, R. F. A. Moreira, M. S. L. Ferreira and A. E. C. Fai, Chemical composition and physicochemical characterization for cabbage and pineapple by-products flour valorization, LWT, 2020, 124, 109028 CrossRef CAS.
- B. A. Acosta-Estrada, J. A. Gutiérrez-Uribe and S. O. Serna-Saldívar, Bound phenolics in foods, a review, Food Chem., 2014, 152, 46–55 CrossRef CAS PubMed.
- T. B. N. Brito, L. R. S. Lima, M. C. B. Santos, R. F. A. Moreira, L. C. Cameron, A. E. C. Fai and M. S. L. Ferreira, Antimicrobial, Antioxidant, Volatile And Phenolic Profiles Of Cabbage-Stalk And Pineapple-Crown Flour Revealed By GC-MS And UPLC-MSE, Food Chem., 2021, 339, 127882 CrossRef CAS PubMed.
- B. Moreira, E. Pereira, T. C. Finimundy, J. Pinela, R. C. Calhelha, M. Carocho, D. Stojković, M. Sokovic, I. C. F. R. Ferreira, C. Caleja and L. Barros, Pineapple by-products as a source of bioactive compounds with potential for industrial food application, Food Funct., 2022, 13(19), 9959–9972 RSC.
- Z. I. M. Arshad, A. Amid, F. Yusof, I. Jaswir, K. Ahmad and S. P. Loke, Bromelain: an overview of industrial application and purification strategies, Appl. Microbiol. Biotechnol., 2014, 98(17), 7283–7297 CrossRef CAS PubMed.
- A. Masyita, R. Mustika Sari, A. Dwi Astuti, B. Yasir, N. Rahma Rumata, T. B. Emran, F. Nainu and J. Simal-Gandara, Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives, Food Chem.:X, 2022, 13, 100217 CAS.
- F. Fitriani, S. Aprilia, N. Arahman, M. R. Bilad, H. Suhaimi and N. Huda, Properties of biocomposite film based on whey protein isolate filled with nanocrystalline cellulose from pineapple crown leaf, Polymers, 2021, 13(24), 4278 CrossRef CAS PubMed.
- Q. P. Dou, Tea in Health and Disease, Nutrients, 2019, 11(4), 929 CrossRef CAS PubMed.
- C.-N. Zhao, G.-Y. Tang, S.-Y. Cao, X.-Y. Xu, R.-Y. Gan, Q. Liu, Q.-Q. Mao, A. Shang and H.-B. Li, Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas, Antioxidants, 2019, 8(7), 215 CrossRef CAS PubMed.
- C. Caleja, T. C. Finimundy, C. Pereira, L. Barros, R. C. Calhelha, M. Sokovic, M. Ivanov, A. M. Carvalho, E. Rosa and I. C. F. R. Ferreira, Challenges of traditional herbal teas: plant infusions and their mixtures with bioactive properties, Food Funct., 2019, 10(9), 5939–5951 RSC.
- J. N. Veljkovic, A. N. Pavlovic, S. S. Mitic, S. B. Tosic, G. S. Stojanovic, B. M. Kalicanin, D. M. Stankovic, M. B. Stojkovic, M. N. Mitic and J. M. Brcanovic, Evaluation of individual phenolic compounds and antioxidant properties of black, green, herbal and fruit tea infusions consumed in Serbia: spectrophotometrical and electrochemical approaches, J. Food Nutr. Res., 2013, 52(1), 12–24 CAS.
- T. Zhao, C. Li, S. Wang and X. Song, Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology, Molecules, 2022, 27(12), 3909 CrossRef CAS PubMed.
- C. Musial, A. Kuban-Jankowska and M. Gorska-Ponikowska, Beneficial Properties of Green Tea Catechins, Int. J. Mol. Sci., 2020, 21(5), 1744 CrossRef CAS PubMed.
- Y. Shirakami and M. Shimizu, Possible Mechanisms of Green Tea and Its Constituents against Cancer, Molecules, 2018, 23(9), 2284 CrossRef PubMed.
- A. Kaur, Y. Jain, K. Mittal and N. Mittal, Synergistic Effect Of Pineapple Peels & Green Tea On Antioxidant Levels Of Pineapple Peels Infused Kangra Green Tea, Global J. Biotechnol. Biochem., 2018, 7(3), 392 Search PubMed.
- T. B. N. Brito, R. C. Moreira, M. G. O. Carvalho, H. S. Arruda, I. A. Neri-Numa, G. M. Pastore, M. S. L. Ferreira, A. E. C. Fai and J. L. Bicas, From pineapple crown to multi-targeting bioactive compounds by solid-state fermentation using Aspergillus tubingensis, Food Res. Int., 2025, 220, 117060 CrossRef CAS PubMed.
- C. F. Araujo-Lima, A. S. Fernandes, E. M. Gomes, L. L. Oliveira, A. F. Macedo, R. Antoniassi, A. E. Wilhelm, C. A. F. Aiub and I. Felzenszwalb, Antioxidant Activity and Genotoxic Assessment of Crabwood (Andiroba, Carapa guianensis Aublet) Seed Oils, Oxid. Med. Cell. Longev., 2018, 2018, 3246719 CrossRef PubMed.
- B. V. D. Galvão, C. F. Araujo-Lima, M. C. P. d. Santos, M. P. Seljan, E. K. Carrão-Dantas, C. A. F. Aiub, L. C. Cameron, M. S. L. Ferreira, É. C. B. d. Andrade Gonçalves and I. Felzenszwalb, Plinia cauliflora (Mart.) Kausel (Jaboticaba) leaf extract: In vitro anti-Trypanosoma cruzi activity, toxicity assessment and phenolic-targeted UPLC-MSE metabolomic analysis, J. Ethnopharmacol., 2021, 277, 114217 CrossRef PubMed.
- D. C. Vodnar, L. F. Călinoiu, F. V. Dulf, B. E. Ştefănescu, G. Crişan and C. Socaciu, Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste, Food Chem., 2017, 231, 131–140 CrossRef CAS PubMed.
- ONU, Transforming Our World: the 2030 Agenda for Sustainable Development, Department of Economic and Social Affairs, United Nations, 2015, pp. 1–35 Search PubMed.
- A. Murray, K. Skene and K. Haynes, The Circular Economy: An Interdisciplinary Exploration of the Concept and Application in a Global Context, J. Bus. Ethics, 2017, 140(3), 369–380 CrossRef.
- K. R. Skene, The Circular Economy: A Critique of the Concept, in Towards a Circular Economy: Transdisciplinary Approach for Business, Alvarez-Risco, A., Rosen, M. A., and Del-Aguila-Arcentales, S., ed. Springer International Publishing, Cham, 2022, pp. 99–116 Search PubMed.
- U. Nations, Transforming Our World: The 2030 Agenda for Sustainable Development, Goal 12: Ensure Sustainable Consumption and Production Patterns, Target 12.3, https://sdgs.un.org/goals/goal12 Search PubMed.
- T. V. Moraes, J. P. G. Ferreira, M. R. A. Souza and R. F. A. Moreira, Atividade antioxidante e conteúdo de compostos fenólicos do chá do caule da Pereskia aculeataMiller fresco e armazenadosob congelamento, Res., Soc. Dev., 2020, 9(5), e34953140 CrossRef.
- T. B. Brito, J. F. Carrajola, E. C. B. A. Gonçalves, M. Martelli-Tosi and M. S. L. Ferreira, Fruit and vegetable residues flours with different granulometry range as raw material for pectin-enriched biodegradable film preparation, Food Res. Int., 2019, 121, 412–421 CrossRef CAS PubMed.
- I. A. Lutz, Métodos Físico-Químicos para Análise de Alimentos, Instituto Adolfo Lutz, São Paulo, 1 edn, 2008, p. 1020 Search PubMed.
- J. H. Lane and L. Eynon, Method for determination of reducing and non-reducing sugars, J. Soc. Chem. Ind., 1923, 42(2), 32–37 CrossRef CAS.
- APHA, AWWA, and WPCF, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, D. C., 19th edn, 1995 Search PubMed.
- AOAC, Official Methods of Analysis of Official Analytical Chemist International, Associtation of Official Analytical Chemistry, Maryland, USA, 2002 Search PubMed.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventós, Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent, Methods Enzymol., 1999, 299, 152–178 CAS.
- Z. Zhou, X. Chen, M. Zhang and C. Blanchard, Phenolics, flavonoids, proanthocyanidin and antioxidant activity of brown rice with different pericarp colors following storage, J. Stored Prod. Res., 2014, 59, 120–125 CrossRef.
- J. Pires, P. B. Torres, D. Y. A. C. Santos, and F. Chow, Ensaio em Microplaca do Potential Antioxidante Através do Método de Sequestro do Radical Livre Dpph Para Extratos de Algas, Instituto de Biociências, Universidade de São Paulo, 2017 Search PubMed.
- V. Urrea-Victoria, J. Santos, P. Torres, F. Chow, and D. Santos, Ensaio Antioxidante em Microplaca do Poder de Redução do Ferro (Frap) Para Extratos de Algas. 2016 Search PubMed.
- P. B. Torres, J. S. Pires, D. Y. A. C. Santos, and F. Chow, Ensaio do Potential Antioxidante de Extratos de Algas Através do Sequestro do Abts•+ em Microplaca, Instituto de Biociências, Universidade de São Paulo, São Paulo, 2017 Search PubMed.
- A. Zulueta, M. J. Esteve and A. Frígola, ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products, Food Chem., 2009, 114, 310–316 CrossRef CAS.
- S. Gomes and A. G. Torres, Optimized extraction of polyphenolic antioxidant compounds from Brazil nut (Bertholletia excelsa) cake and evaluation of the polyphenol profile by HPLC, J. Sci. Food Agric., 2016, 96(8), 2805–2814 CrossRef CAS PubMed.
- N. Y. Kado, G. N. Guirguis, C. P. Flessel, R. C. Chan, K. I. Chang and J. J. Wesolowski, Mutagenicity of fine (less than 2.5 microns) airborne particles: diurnal variation in community air determined by a Salmonella micro preincubation (microsuspension) procedure, Environ. Mutagen., 1986, 8(1), 53–66 CrossRef CAS PubMed.
- D. M. Maron and B. N. Ames, Revised methods for the Salmonella mutagenicity test, Mutat. Res., 1983, 113(3–4), 173–215 CAS.
- A. D. C. Goldstein, C. F. Araujo-Lima, A. D. S. Fernandes, R. Santos-Oliveira and I. Felzenszwalb, In vitro genotoxicity assessment of graphene quantum dots nanoparticles: A metabolism-dependent response, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2023, 885, 503563 CrossRef CAS PubMed.
- Lumivero, XLSTAT Statistical and Data Analysis Solution, New York, USA, 2024 Search PubMed.
- E. K. Carrão Dantas, C. F. Araújo-Lima, C. L. S. Ferreira, A. d. C. Goldstein, C. A. F. Aiub, M. G. P. Coelho and I. Felzenszwalb, Toxicogenetic assessment of a pre-workout supplement: In vitro mutagenicity, cytotoxicity, genotoxicity and glutathione determination in liver cell lines and in silico ADMET approaches, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2022, 879–880, 503517 CrossRef PubMed.
- S. Das, A. Giri and G. Chatterjee, Assessment of Proximate Composition and Antioxidant Potential of Different Commercially Packaged Tea Samples in West Bengal, Asian J. Dairy Food Res., 2023, 42(2), 204–209 Search PubMed.
- C. H. Tan, C. L. Hii, C. Borompichaichartkul, P. Phumsombat, I. Kong and L. P. Pui, Valorization of fruits, vegetables, and their by-products: Drying and bio-drying, Dry. Technol., 2022, 40(8), 1514–1538 CrossRef.
- R. G. Newman, Y. Moon, J. C. Tou, T. McManus and N. L. Waterland, Harvest Stage and Brewing Conditions Impact Mineral Content, Phenolic Compounds, and Antioxidant Capacity of Lemon Balm (Melissa officinalis L.) Herbal Tea, Plant Foods Hum. Nutr., 2023, 78(2), 336–341 CrossRef CAS PubMed.
- M. Długaszek and M. Kaszczuk, Assessment of the nutritional value of various teas infusions in terms of the macro- and trace elements content, J. Trace Elem. Med. Biol., 2020, 59, 126428 CrossRef PubMed.
- J.-H. Han, D. Kim and J.-Y. Chun, Effect of Thickness and Drying Method on Properties of Dried Tangerine Tea, J. Korean Soc. Food Sci. Nutr., 2022, 51(3), 245–253 CrossRef CAS.
- W. Widowati, T. L. Wargasetia, M. Marthania, T. S. Hanifa, T. M. Zakaria, M. S. Gunadi, N. Halim and S. Santiadi, Antioxidant Properties of TeNan Herbal Tea Formulation “Telang (Clitoria ternatea) and Pineapple (Ananas comosus)”, Jurnal Kedokteran Brawijaya, 2017, 520, 676–685 Search PubMed.
- J. I. Martinez-Costa and R. Leyva-Ramos, Effect of surfactant loading and type upon the sorption capacity of organobentonite towards pyrogallol, Colloids Surf., A, 2017, 520, 676–685 CrossRef CAS.
- S. C. Efsa, S. J. More, V. Bampidis, D. Benford, C. Bragard, T. I. Halldorsson, A. F. Hernández-Jerez, S. Hougaard Bennekou, K. P. Koutsoumanis, K. Machera, H. Naegeli, S. S. Nielsen, J. R. Schlatter, D. Schrenk, V. Silano, D. Turck, M. Younes, U. Gundert-Remy, G. E. N. Kass, J. Kleiner, A. M. Rossi, R. Serafimova, L. Reilly and H. M. Wallace, Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment, EFSA J., 2019, 17(6), e05708 Search PubMed.
- J. Kochman, K. Jakubczyk, J. Antoniewicz, H. Mruk and K. Janda, Health Benefits and Chemical Composition of Matcha Green Tea: A Review, Molecules, 2021, 26(1), 85 CrossRef CAS PubMed.
- T. Nakano, M. Ikeda, T. Wakugawa, Y. Kashiwada, O. Kaminuma, N. Kitamura, M. Yabumoto, H. Fujino, Y. Kitamura, H. Fukui, N. Takeda and H. Mizuguchi, Identification of pyrogallol from Awa-tea as an anti-allergic compound that suppresses nasal symptoms and IL-9 gene expression, J. Med. Investig., 2020, 67(3.4), 289–297 CrossRef PubMed.
- E. Zeiger and K. Mortelmans, The Salmonella (Ames) test for mutagenicity, in Curr Protoc Toxicol, chapter 3, unit 3.1, 2001 Search PubMed.
- D. E. Levin, M. Hollstein, M. F. Christman and B. N. Ames, Detection of oxidative mutagens with a new Salmonella tester strain (TA102), Methods Enzymol., 1984, 105, 249–254 CAS.
- R. Castañeda-Arriaga, A. Pérez-González, M. Reina, J. R. Alvarez-Idaboy and A. Galano, Comprehensive Investigation of the Antioxidant and Pro-oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress?, J. Phys. Chem. B, 2018, 122(23), 6198–6214 CrossRef PubMed.
- G. Sponchiado, M. L. Adam, C. D. Silva, B. Silva Soley, C. de Mello-Sampayo, D. A. Cabrini, C. J. Correr and M. F. Otuki, Quantitative genotoxicity assays for analysis of medicinal plants: A systematic review, J. Ethnopharmacol., 2016, 178, 289–296 CrossRef PubMed.
- F. A. Resende, W. Vilegas, L. C. Dos Santos and E. A. Varanda, Mutagenicity of Flavonoids Assayed by Bacterial Reverse Mutation (Ames) Test, Molecules, 2012, 5255–5268 CrossRef CAS PubMed.
- A. Ahmadi and A. Shadboorestan, Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent, Nutr. Cancer, 2016, 68(1), 29–39 CrossRef CAS PubMed.
- C. F. Araujo-Lima, A. S. Fernandes, E. M. Gomes, L. L. Oliveira, A. F. Macedo, R. Antoniassi, A. E. Wilhelm, C. A. F. Aiub and I. Felzenszwalb, Antioxidant Activity and Genotoxic Assessment of Crabwood (Andiroba, Carapa guianensis Aublet) Seed Oils, Oxid. Med. Cell. Longev., 2018, 2018, 11 Search PubMed.
- K.-Z. Peng, S.-Y. Zhang and H.-L. Zhou, Toxicological evaluation of the flavonoid-rich extract from Maydis stigma: Subchronic toxicity and genotoxicity studies in mice, J. Ethnopharmacol., 2016, 192, 161–169 CrossRef CAS PubMed.
- Â. G. Batista, A. S. Ferrari, D. C. da Cunha, J. K. da Silva, C. B. B. Cazarin, L. C. Correa, M. A. Prado, L. B. d. Carvalho-Silva, E. A. Esteves and M. R. Maróstica Júnior, Polyphenols, antioxidants, and antimutagenic effects of Copaifera langsdorffii fruit, Food Chem., 2016, 197, 1153–1159 CrossRef PubMed.
- S. Zhang, T. Li, L. Zhang, X. Wang, H. Dong, L. Li, D. Fu, Y. Li, X. Zi, H.-M. Liu, Y. Zhang, H. Xu and C.-Y. Jin, A novel chalcone derivative S17 induces apoptosis through ROS dependent DR5 up-regulation in gastric cancer cells, Sci. Rep., 2017, 7(1), 9873 CrossRef PubMed.
- Z. Gao, G. Deng, Y. Li, H. Huang, X. Sun, H. Shi, X. Yao, L. Gao, Y. Ju and M. Luo, Actinidia chinensis Planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression, Biomed. Pharmacother., 2020, 126, 110092 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |