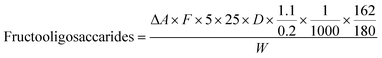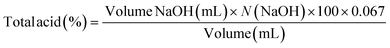Open Access Article
Open Access ArticleEnzymatic bioprocessing of lychee juice for fructooligosaccharide enhancement and sustainable functional jelly formulation
Nattida Pongjuntuka,
Saeid Jafaria,
Sochannet Chheng ab and
Kitipong Assatarakul
ab and
Kitipong Assatarakul *a
*a
aDepartment of Food Technology, Faculty of Science, Chulalongkorn University, Pathumwan, Bangkok, 10330, Thailand. E-mail: Kitipong.A@chula.ac.th; Tel: +66-2218-5515-6
bDepartment of Food Chemical Engineering, Kampong Speu Institute of Technology, Kampong Speu 050601, Cambodia
First published on 3rd November 2025
Abstract
This study presents a sustainable strategy for enhancing the nutritional and functional quality of lychee juice and jelly through enzymatic bioprocessing. Lychee juice was treated with Viscozyme® L under varying incubation conditions (50–60 °C, 0–6 h) to enhance fructooligosaccharide (FOS) content and related bioactive properties. Optimal treatment (55 °C, 2 h) resulted in significant increases in FOS, total phenolic compound (517.53 ± 2.55 mg GAE/L), flavonoids (416.82 ± 0.42 mg QE/L), and antioxidant activity measured by DPPH (714.96 ± 4.02 mg TE/L) and FRAP (562.87 ± 0.67 mg TE/L), compared to untreated juice. Juice yield improved by up to 87.3% along with an increase in total soluble solid. The optimized juice was incorporated into lychee jelly formulations containing 70–80% FOS-enriched juice and 0.5–1.5% kappa-carrageenan. Textural analysis revealed that 1.5% kappa-carrageenan with FOS-enhanced 80% lychee juice produced jellies with superior springiness, cohesiveness, and gumminess, closely resembling commercial standards. These formulations also retained higher phenolics, flavonoids, and antioxidant activity than those with lower FOS-enriched juice content. Sensory evaluation with 50 panelists identified the 80% FOS-enriched juice + 1.5% carrageenan jelly as the most preferred across color, texture, sweetness, and overall liking. During 30 days of refrigerated storage, phenolics, flavonoids, and antioxidant activity declined significantly, accompanied by weakening gel texture, rising water activity, and microbial increases beyond acceptable limits after day 20, although no coliforms or E. coli were detected. This work highlights enzymatic hydrolysis and clean-label gelling as promising tools to develop functional prebiotic jellies that valorize local fruit resources, while also underscoring the need for improved preservation strategies to extend shelf life.
Sustainability spotlightThis study presents a sustainable strategy to enhance the nutritional value of lychee-based products through enzymatic processing, increasing prebiotic fructooligosaccharide (FOS) content and antioxidant activity. By valorizing local fruit resources and minimizing synthetic additives, the research promotes circular food system principles and supports the development of clean-label, health-promoting functional jellies. The approach contributes to sustainable food innovation by combining bioprocessing with the upcycling of agricultural produce for value-added product development. |
Introduction
Consumers are increasingly prioritizing their health-conscious food choices, driving a growing demand for functional products derived from fruits and vegetables. These foods offer significant nutritional benefits and help reduce the risk of non-communicable diseases. Prebiotics, which are indigestible substances in the human digestive system, play a crucial role in stimulating the growth of beneficial probiotic bacteria in the large intestine. Among them, oligosaccharides are widely used due to their function as low-calorie or calorie-free sugar alternatives. Consequently, prebiotics are incorporated into various food products, including health beverages, powdered and fermented dairy products like sour milk and yogurt, as well as specialized nutrition for patients.Lychee (Litchi chinensis Sonn.) is a widely favored fruit among both Thai and international consumers. Thailand is a major global producer of lychee, exporting around 30% of its yield to countries such as China, Singapore, the Philippines, Malaysia, and Australia, while the majority (over 70%) is consumed domestically.1 Lychee is distinguished by its sweet-tart flavor and distinctive aroma. While commonly enjoyed fresh, it is also processed into various products such as canned lychee, lychee jam, and dried lychee to extend its shelf life. However, lychee has a high sugar content (approximately 15 g/100 g) and a short postharvest stability, making it highly perishable. Expanding its range of processed products into more stable, portable formats could enhance its market value and benefit Thailand's agricultural sector. Recent work has demonstrated that optimized packaging and microencapsulation approaches can significantly extend the shelf life and preserve antioxidant stability of plant-based extracts.2 Currently, jelly products are being explored as an alternative lychee-based product due to their growing popularity, particularly among children and adolescents. However, many commercially available jellies are high in sucrose, lack dietary fiber, and contribute to excessive calorie intake, which may increase the risk of obesity and related metabolic disorders.
Jelly products are primarily composed of sugar, typically in the form of sucrose syrup, and are prepared using fruit juice or concentrated fruit extracts such as lychee, mango, pineapple, orange, and strawberry. These products also contain gelling agents like glucomannan, carrageenan, and alginate, along with added flavoring agents. A high-quality jelly should have a soft texture, be non-sticky, and maintain its shape without being runny. With the rising consumer preference for health-oriented foods, jelly formulations are being modified to incorporate functional ingredients such as prebiotics to enhance their nutritional value.3 Similarly, incorporating plant extracts rich in bioactive compounds has shown potential to enhance antioxidant activity, improve texture, and extend the shelf life of jelly products.4 Traditional fruit jellies often contain high amounts of sugar, which can have negative health impacts. To address this issue, enzyme technology is utilized to reduce sugar content in fruit juices before processing into jelly products. Microbial enzymes such as invertase and glucose isomerase are commonly used in food processing; these enzymes convert sucrose into fructooligosaccharides (FOS), a prebiotic that promotes gut health while serving as a lower-calorie sugar alternative.
Recent research has focused on enhancing the nutritional profile of fruit-based products through enzymatic treatments that increase prebiotic content. For example, a study developed a functional prebiotic strawberry preparation by in situ enzymatic conversion of sucrose into fructooligosaccharides (FOS). Using commercial enzymes like Viscozyme® L and Pectinex® Ultra SP-L, the researchers optimized conditions to maximize FOS yield, resulting in a product with over 50% (w/w) prebiotic FOS and an 80% reduction in sucrose content. The FOS demonstrated resistance to gastrointestinal digestion, indicating potential health benefits.5 In another study, the effects of enzymatic degradation on the physicochemical properties and prebiotic activity of Lilium spp. polysaccharides was examined.6 Enzymatic treatment improved antioxidant capacity and prebiotic activity, suggesting its applicability in functional food development.7 Similarly, studies on guava purée by-products treated with cellulase and xylanase enzymes revealed their potential as prebiotic ingredients in yogurt. The enzymatic treatment enhanced the release of fermentable sugars, promoting the growth of beneficial gut bacteria.7 Investigation into prebiotic potential of hydrolyzed pectins has demonstrated that enzymatically treated pectins can modulate gut microbiota by increasing beneficial bacteria and short-chain fatty acid production. These findings underscore the role of enzymatic treatments in developing functional foods with prebiotic properties.8
Despite the growing body of research on enzymatic treatments to enhance prebiotic content in fruit-based products, such as strawberries,5 Lilium spp. polysaccharides,6 guava by-products,7 and hydrolyzed pectins8 which mentioned earlier, there remains a notable gap in the application of these techniques specifically to lychee juice for the production of FOS.
Based on the available evidence, we hypothesized that enzymatic treatment would substantially increase the FOS content in lychee juice, thereby enhancing its antioxidant properties. Furthermore, we anticipated that higher kappa-carrageenan concentrations would produce firmer lychee jellies with improved texture and consumer acceptability.
Therefore, this study aimed to (a) increase FOS content of lychee juice through enzymatic hydrolysis using Viscozyme® L, (b) examine the effect of kappa-carrageenan concentration on the texture, structure, and sensory properties of lychee jelly, and (c) evaluate the physicochemical and microbial stability of lychee jelly during 30 days of refrigerated storage. The findings from this research serve as a scientific foundation for the development of FOS-enriched lychee jelly, offering potential health benefits and paving the way for future advancements in functional fruit-based products.
Materials and methods
Preparation of lychee samples
Frozen lychees were thawed at 4 °C for 24 h, then immersed in a saline solution prepared by dissolving 10 g of salt in 1 L of water for 3–5 min. The fruits were subsequently rinsed with clean water and blended using a Panasonic MX-900M blender for 4 min. The resulting mixture was heated at 90 °C for 1 min9 and then cooled to 60 °C by placing the container in an ice-water bath. Finally, the sample were packaged in a vacuum-sealed aluminum foil bag and stored at −20 °C until further analysis.10Fructooligosaccharide production in lychee using Viscozyme® L
Since lychee naturally has a pH of 4.38, it was adjusted to 5.5 (optimal mildly acidic condition for Viscozyme® L activity) using 0.1 M acetate buffer. The acidity was adjusted using a 0.1 M acetate buffer (pH 5.5) which was prepared using sodium acetate and acetic acid, adjusted with 1 M NaOH. The volume was then adjusted to 1 L with distilled water. To adjust the pH, 1 M NaOH was added until the desired pH of 5.5 was achieved. Next, 1% v/v Viscozyme® L (a mixture of β-glucanases, pectinases, hemicellulases, cellulases, and xylanases) with an activity of 300 AGU/mL was added at 1 mL.10 The experiment varied temperature (50 °C, 55 °C and 60 °C) and incubation time (0, 2, 4 and 6 h) based on our preliminary trials. Afterward, the samples were heated at 90 °C for 5 min in a temperature-controlled water bath to inactivate the enzyme.11 The lychee juice was then centrifuged at 4500×g for 10 min, and the supernatant was separated from the sediment by filtration through Whatman No. 1 filter paper. The supernatant was collected in an amber bottle for further experiments. Control samples were prepared identically to enzyme-treated samples but without Viscozyme® L addition; they underwent the same pH adjustment, heating, centrifugation, and filtration steps.Analysis of fructo-oligosaccharides (FOS) content
Fructooligosaccharides (FOS) content was analyzed using the K-FRUC assay kit (Megazyme International Ireland Ltd, Wicklow, Ireland) following AOAC Method 999.03. Sodium maleate buffer (100 mM, pH 6.5) with 0.5 mg/mL bovine serum albumin and sodium acetate buffer (100 mM, pH 4.5) were prepared. The reagent solution was prepared by mixing Solution A (4-hydroxybenzoic acid hydrazide with HCl) and Solution B (trisodium citrate, calcium chloride, NaOH), combined before use. Additional solutions included 50 mM sodium hydroxide, 10 mg/mL alkaline borohydride, and 200 mM acetic acid. Enzyme Solution A (sucrase, β-amylase) was dissolved in maleate buffer, and Enzyme Solution B (fructanase) in acetate buffer. For analysis, 400 mg sample was extracted with 25 mL of distilled water at 100 °C for 10 min, cooled, and centrifuged. Supernatant (0.2 mL) was treated with Enzyme Solution A at 30 °C for 30 min to remove sucrose and starch, followed by alkaline borohydride at 40 °C for 30 min to convert reducing sugars. 0.2 mL of Solution S (hydrolyzed sample) was divided into three tubes: two with 0.1 mL fructanase (Enzyme Solution B) and one blank with 0.1 mL acetate buffer. All tubes were incubated at 40 °C for 30 min. A D-fructose control and reagent blank were prepared using acetate buffer. After incubation, 5 mL of reagent was added to each tube, and the mixtures were heated at 100 °C for 6 min, cooled, and absorbance was measured at 410 nm. FOS content was calculated based on the difference in absorbance, D-fructose standard factor, sample weight, and dilution factor, adjusted for fructose conversion:where: ΔA = absorbance value of the sample − absorbance value of sample blank, F = convertion factor from absorbance to µg of D-fructose, calculated as (54.5 µg D-fructose)/(absorbance of 54.5 µg D-fructose), 5 = factor used to convert from 0.2 mL to 1 mL, 25 = volume of sample used in the test (mL), D = dilution factor of the sample used for testing, W = weight of sample used for extraction (mg), 1.1/0.2 = correction factor for the D-fructose standard, accounting for 0.2 mL from a total volume of 1.1 mL, 1/1000 = unit conversion from µg to mg, 162/180 = conversion factor between free D-fructose and anhydrofructose equivalents.
Physicochemical properties of the lychee juice
The methodology for evaluating the functional properties of the samples was adapted and optimized from previous studies12,13 with enhanced precision and clarity. Total phenolic compound (TPC) was determined using the Folin–Ciocalteu assay with gallic acid (0–0.5 mg/mL) as the calibration standard. Absorbance was measured at 765 nm using spectrophotometer, and the results were expressed as mg gallic acid equivalents (GAE) per 100 g of dry weight. Total flavonoid content (TFC) was determined using the aluminum chloride colorimetric method, employing a quercetin standard curve (0–1.6 mg/mL). Absorbance was recorded at 415 nm, and the results were expressed as mg quercetin equivalents (QE) per 100 g of dry weight. Antioxidant activity was assessed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, where the absorbance difference between the DPPH solution and the sample was measured at 517 nm. Results were expressed as µmol Trolox equivalents (TE) per 100 g of dry weight. Additionally, the ferric reducing antioxidant power (FRAP) assay was conducted by measuring the absorbance change at 593 nm between the FRAP reagent and the sample, with results reported as µmol Fe2+ equivalents per 100 g of dry weight. All measurements were performed in triplicate to ensure reproducibility, and data were statistically analyzed to confirm accuracy and reliability.Total soluble solids (°Brix) by using digital refractometer (model Hl96801, HANNA Instruments, Italy), and the results were expressed in °Brix.
Percentage of yield (% yield) was determinded according to the method described in ref. 9 using the following formula:
Gelling agent content on quality of lychee jelly with added FOS
The concentration of gelling agent, kappa-carrageenan, was varied at three levels (0.5%, 1%, and 1.5% w/w), and other ingredients were added. The amounts of sucralose and citric acid were kept constant, whereas the lychee juice concentration was varied (70% and 80% w/w) to increase the FOS content for jelly preparation (Table 2), following a modified method from ref. 4. The process involved dissolving kappa-carrageenan and sucralose in water at 80 °C until a clear solution formed, and then adding FOS-enriched lychee juice. The mixture was continuously stirred and pasteurized at 85 °C for 16 sec. After pasteurization, the temperature was reduced to approximately 70 °C, and citric acid and flavorings were added. The mixture was then immediately poured into molds and stored at 4 °C until solidification. The prepared jellies were subsequently analyzed for their quality attributes.Texture analysis was performed using texture analyzer (model TA-XTplus, Stabel Micro System, UK) on lychee jelly samples with a volume of 17.66 cm2, according to the method of ref. 14. Water activity (aw) was determined using water activity analyzer (model Aqualab 4TEV, Meter Group, USA) at 25 °C. Color measurement was carried out using a chroma meter based on the CIE color system, and L*, a* and b* values were recorded. The instrument was calibrated against standard white and black tiles prior to each sample measurement.
Sensory quality testing
The sensory evaluation conducted in this study was approved by the Research Ethics Review Committee for Research Involving Human Research Participants, Group I, Chulalongkorn University, Thailand (Approval No. COA 085/67), and was carried out in compliance with relevant Thai laws and regulations. Informed consent was obtained from all 50 panelists prior to participation. All sensory evaluations were conducted at the Sensory Testing Laboratory, Department of Food Technology, Faculty of Science, Chulalongkorn University. Each panelist performed the tests independently in a designated booth under controlled condidtions (25 ± 2 °C, white light). Each participant spent around 20 min on each test. Product preference ratings for appearance, color, aroma, taste, texture, and overall liking were assessed using a 9-point hedonic scale, where a score of 1 indicated “dislike extremely” and a score of 9 indicated “like extremely”.Quality alterations in lychee jelly products containing FOS during storage
The samples were placed in sterilized polypropylene (PP) containers with a lid that had been sterilized at 100 °C for 10 min, following to the method described in ref. 15. After filling and sealing, the containers were immediately immersed in water for 10 minutes, cooled, and stored at 4 °C. Samples were collected on days 0, 5, 10, 15, 20, 25 and 30 and analyzed for quality parameters as follows:Total titratable acidity, expressed as malic acid, was determined according to the following equation:
Microbiological assessments of lychee jelly samples were conducted in accordance with the FDA Bacteriological Analytical Manual16 guidelines to evaluate microbial quality and safety during 30 days of refrigerated storage. The analyses targeted total viable count (TVC), yeast and mold, coliforms, and Escherichia coli. For TVC, samples were plated on Plate Count Agar (PCA) and incubated at 35 ± 1 °C for 48 ± 2 h. Yeast and mold enumeration was performed using Potato Dextrose Agar (PDA) adjusted to pH 3.5 with tartaric acid, with incubation at 25 ± 1 °C for 5 days. Coliforms were quantified using Violet Red Bile Agar (VRBA), while E. coli was assessed on Eosin Methylene Blue (EMB) Agar; both were incubated at 35 ± 1 °C for 24 ± 2 h.
Sample preparation involved homogenizing 25 g of lychee jelly in 225 mL of sterile Buffered Peptone Water (BPW) using a stomacher for 2 min to ensure uniform dispersion. Serial dilutions (10−1 to 10−6) were prepared in sterile BPW, and 1 mL aliquots from each dilution were plated in duplicate. All microbiological analyses were performed in triplicate to ensure robustness and repeatability. After incubation, colonies were enumerated, and results were expressed as log colony-forming units per gram (log CFU/g). Strict aseptic techniques were employed throughout to prevent contamination, and environmental controls were monitored to validate the incubation conditions.17
Statistical analysis
A completely randomized design (CRD) was performed with 3 replications. The variance of the data was analyzed using Analysis of Variance (ANOVA) at a 95% confidence level. For the sensory evaluation, the experiment was performed by using non-parametric test, analyzing variance by Kruskal–Wallis H test (SPSS Inc., Chicago, IL, USA). The difference in means was compared by Tukey's (HSD) multiple comparison test at P ≤ 0.05 to compare the significant differences using Statistics Package for the Social Sciences (SPSS) version 29.0.1.Results and discussion
Physicochemical properties of fresh lychee juice
Lychee (Litchi chinensis Sonn.) is a subtropical fruit native to southern China, valued commercially for its distinctive fragrant aroma, vibrant red color, delectable taste, and health-promoting bioactive compounds.18 In this study, the key physicochemical properties of the initial fresh lychee juice were systematically evaluated to establish a baseline for subsequent enzymatic treatments (Table 1). The juice exhibited a pH of 4.38 ± 0.01, total soluble solid of 11.3 ± 0.12 °Brix, a yield of 64 ± 0.02%, and total titratable acidity of 0.29 ± 0.31% (expressed as malic acid). Bioactive components included a total phenolic compound of 327.53 ± 2.10 mg GAE/L, total flavonoid content of 336.54 ± 0.83 mg QE/L, antioxidant activity by the DPPH assay of 270.79 ± 1.91 mg TE/L, and by the FRAP assay of 341.53 ± 3.71 mg TE/L.| Properties of lychee juice | Amount |
|---|---|
| pH (acidity-alkaline) | 4.38 ± 0.01 |
| Total soluble solids (°Brix) | 11.3 ± 0.12 |
| Yield (% yield) | 64.02 ± 0.02 |
| Total acid content (% malic acid) | 0.29 ± 0.31 |
| Fructooligosaccharide content (FOS) | Not detected |
| Total phenolic compounds (TPC, mg GAE/L) | 327.53 ± 2.10 |
| Total flavonoid content (TFC, mg QE/L) | 336.54 ± 0.83 |
| Antioxidant activity (DPPH, mg TE/L) | 270.79 ± 1.91 |
| Antioxidant activity (FRAP, mg TE/L) | 341.53 ± 3.71 |
| Ingredient | Proportion of raw materials (% w/w) | |||||
|---|---|---|---|---|---|---|
| Product formula | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Sucralose | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Citric acid | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| κ-Carrageenan | 0.5 | 1 | 1.5 | 0.5 | 1 | 1.5 |
| FOS-enriched lychee juice | 70 | 70 | 70 | 80 | 80 | 80 |
| Water | 28.92 | 28.42 | 27.92 | 18.92 | 18.42 | 17.92 |
| Lychee flavoring agent | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
Impact of enzyme treatment on fructooligosaccharide (FOS) production and quality characteristics in lychee juice
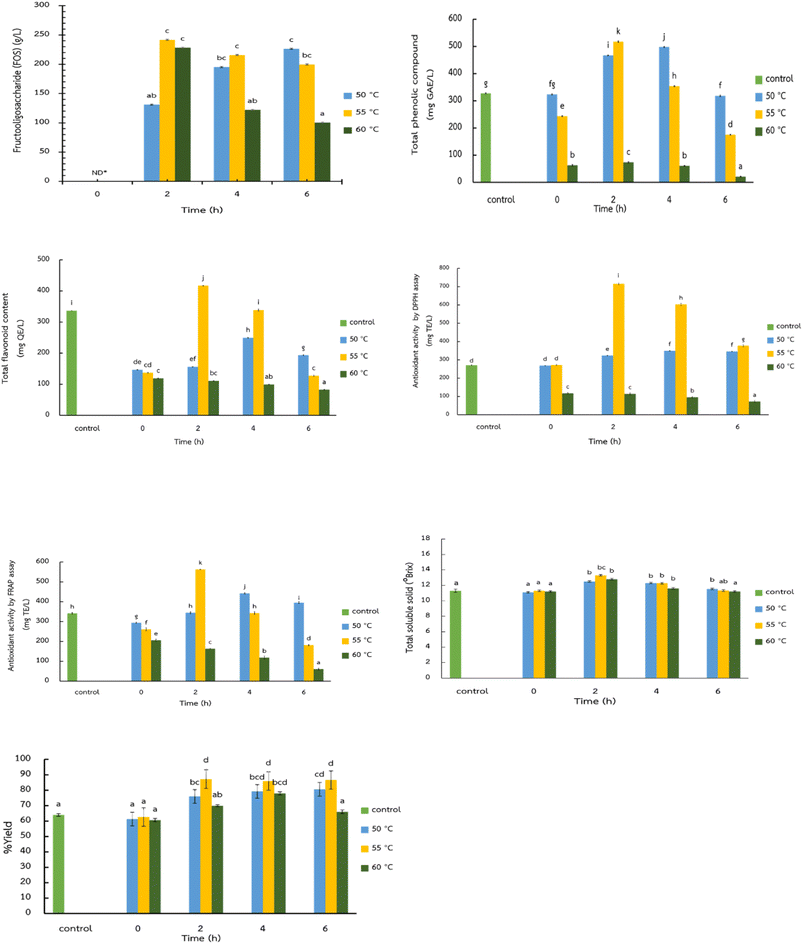
The enzymatic production of FOS in lychee juice using Viscozyme® L provides important insights into the effects of temperature and reaction time on maximizing FOS yield. FOS are valuable prebiotics with well-documented benefits for gastrointestinal health, including promoting the growth of beneficial gut microbiota and enhancing mineral absorption. Moreover, oligosaccharides have been reported to exhibit anticancer properties.19 Their increasing popularity among health-conscious consumers makes the development of natural enzymatically produced FOS beverages particularly appealing. In this study, lychee juice served as a novel substrate for enzymatic transformation due to its high natural sucrose content and appealing sensory properties (Fig. 2). Viscozyme® L is a multi-enzyme complex containing cellulases, hemicellulases, pectinases, and β-glucosidases, which primarily facilitate cell-wall disruption which, enhance juice extraction, and release bound bioactive compounds. While Viscozyme® L does not contain fructosyltransferase (Ftase) as a major activity, certain carbohydrases can exhibit side-activity that contributes to limited transfructosylation reactions, enabling FOS formation under suitable reaction conditions. Consequently, Viscozyme® L in this system supported both cell wall hydrolysis (via carbohydrases) and partial FOS synthesis through auxiliary enzymatic activities.10The data showed that the enzymatic conversion of sucrose to FOS occurred most rapidly within the first 2 h of reaction, particularly at 50 °C and 55 °C (Fig. 1). This rapid early-stage rapid conversion is likely due to the abundant availability of sucrose, which acts as the primary substrate for the transfructosylation reaction. The highest FOS concentration was observed at 55 °C after 2 h, indicating this as the optimal condition among the tested parameters. This temperature likely provides a favorable balance between enzyme activity and stability, enabling efficient transfructosylation while minimizing thermal inactivation of the enzyme complex. Interestingly, while FOS formation also occurred at 60 °C, the reaction rate plateaued after the 2-h mark, similar to the behavior observed at 55 °C. This decline in reaction rate over time could be attributed to substrate depletion (i.e., reduced sucrose availability), enzyme deactivation at elevated temperatures, or potential product inhibition—where the accumulation of FOS or other reaction products might hinder further enzyme activity.
Furthermore, the diminishing reaction rate observed beyond 2 h across all temperatures suggests that extended incubation times may not be economically viable for industrial-scale production. From a practical standpoint, a shorter reaction time at a moderately elevated temperature (e.g., 55 °C for 2 h) not only maximizes FOS yield but also conserves energy and reduces processing costs, making this an attractive condition for the food industry. Moreover, enriching lychee juice with FOS not only improves its functional health properties but may also influence its sweetness profile and overall sensory appeal. Minor differences at 0 h likely reflect initial enzyme substrate interactions before full inactivation, wherease the control samples represent baseline values without enzymatic activity. Future studies could explore the effects of this enzymatic treatment on taste, shelf stability, and the prebiotic efficacy of the final product. Overall, these findings demonstrate the potential for controlled enzymatic modification to transform traditional fruit juices into value-added functional beverages, aligning with current trends in health-oriented food innovation.
Total phenolic compound (TPC)
Phenolic compounds are abundant in vegetables and fruits and are recognized for their biological activity in combating free radicals. Lychee contains various phenolic compounds, including flavonoids and phenolic acids.20 In a study examining the effect of enzyme fermentation on the total phenolic compound (TPC) in lychee juice treated with Viscozyme® L under different incubation temperatures (50 °C, 55 °C and 60 °C) and incubation times (0, 2, 4, and 6 h), TPC values ranged from 21.14 ± 1.73 mg GAE/L to 517.53 ± 2.55 mg GAE/L (Fig. 1). The TPC decreased with increasing temperature and incubation time. At 55 °C for 2 h, the enzymatically treated lychee juice exhibited the highest TPC (517.53 ± 2.55 mg GAE/L), which was significantly higher (P ≤ 0.05) than the untreated lychee juice (327.53 ± 2.10 mg GAE/L). These findings align with Nguyen et al.,11 who reported that enzyme treatment significantly increased TPC in mulberry juice compared to the untreated controls. This enhancement may be attributed to the hydrolysis of pectin in the middle lamella by pectinases, facilitating the release of phenolic antioxidants from the cell cytoplasm.21 Similar enhancements in total phenolic content following cell-wall disruption have also been reported in plant-based systems treated with physical or enzymatic processes, such as ultrasound-assisted extraction of Sesbania javanica flower, where optimized conditions significantly improved phenolic yield and antioxidant activity.22Total flavonoid content (TFC)
Flavonoids are among the most significant polyphenols in plants, being water-soluble and stored in the cell vacuole. This study found that the total flavonoid content (TFC) of lychee juice treated with Viscozyme® L at different temperatures (50 °C, 55 °C and 60 °C) and various incubation times (0, 2, 4 and 6 h) ranged from 82.38 ± 1.25 mg QE/L to 416.82 ± 0.42 mg QE/L (Fig. 1). The TFC decreased as both temperature and incubation time increased. After 2 h of incubation at 55 °C, the treated lychee juice exhibited the highest TFC (416.82 ± 0.42 mg QE/L), which was significantly higher (P ≤ 0.05) than the untreated lychee juice (336.54 ± 0.83 mg QE/L). This increase in TFC may be due to the degradation of pectin by the endogenous pectinase enzyme in the middle lamella of the fruit tissue, leading to enhanced extraction of antioxidant compounds from the cell cytoplasm, a phenomenon also observed for TPC.21Antioxidant activity by DPPH method
The antioxidant activity was tested using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, which is based on the reaction of DPPH, a stable nitrogen radical. When DPPH receives a hydrogen atom from an antioxidant molecule, it undergoes a structural change, rendering it inactive as a free radical.23 In this study, lychee juice treated with Viscozyme® L at different temperatures (50 °C, 55 °C and 60 °C) and incubation times (0, 2, 4 and 6 h) showed DPPH values ranging from 72.46 ± 5.20 mg TE/L to 714.96 ± 4.02 mg TE/L (Fig. 1). Antioxidant activity decreased as temperature and time increased. After 2 h of incubation at 55 °C, lychee juice with enzymatically produced FOS exhibited the highest DPPH activity (714.96 ± 4.02 mg TE/L), which was significantly higher (P ≤ 0.05) than the untreated lychee juice (270.79 ± 1.91 mg TE/L).Antioxidant activity by FRAP method
The antioxidant activity was also measured using the FRAP method, which analyzes the ability of antioxidants to donate electrons and reduce ferric (Fe3+) ions to ferrous (Fe2+) ions, forming a blue-colored complexes.23,24 Lychee juice treated with Viscozyme® L showed FRAP values ranging from 60.64 ± 4.02 mg TE/L to 562.87 ± 0.67 mg TE/L (Fig. 1). Similar to DPPH results, FRAP values decreased with increasing temperature and incubation time. After 2 h of incubation at 55 °C, the enzymatically treated lychee juice exhibited the highest FRAP activity (562.87 ± 0.67 mg TE/L), which was significantly higher (P ≤ 0.05) compared to the untreated lychee juice (341.53 ± 3.71 mg TE/L).The increased antioxidant activity in lychee juice, as measured by both DPPH and FRAP assays, can be attributed to the enzymatic degradation of the middle lamella and primary cell wall, which will facilitate the release of polyphenol compounds stored in the vacuole. These findings are consistent with,25 who investigated the effect of pectinolytic enzyme treatment prior to pressing on the release of polyphenols in blackcurrant juice, and found that enzyme treatment significantly increased the antioxidant activity of the juice compared to the control sample.
Total soluble solid (TSS, °Brix)
It was observed that lychee juice supplemented with FOS exhibited total soluble solid (TSS) values ranging from 11.2 ± 0.12 °Brix to 13.3 ± 0.21 °Brix. At 55 °C for 2 and 4 h, the TSS reached its highest value (13.3 ± 0.21 °Brix), which was significantly higher (P ≤ 0.05) than the untreated lychee juice (11.3 ± 0.21 °Brix) (Fig. 1). Cheng et al.9 noted that the increase in TSS was due to Viscozyme® L enzymes, which consist of cellulolytic and pectinolytic enzyme complexes that catalyze the hydrolysis of cellulose and pectin in fruits, effectively degrading these components in fruit juices. Additionally, the rise in °Brix can result from the hydrolysis of insoluble pectin by pectinases, a long with cellulase-mediated degradation of cellulose into soluble carbohydrates, thereby elevating TSS levels. Our findings are consistent with ref. 11, who observed the quality of mulberry juice fermented with Pectinex Ultra SP-L and Viscozyme® L enzymes. They found that the TSS (°Brix) of mulberry juice increased compared to the initial sample, but over time, the TSS began to decrease due to microbial fermentation of the sugars.Yield percentage (% yield)
Lychee juice supplemented with FOS exhibited yields ranging from 60.67 ± 3.06% to 87.33 ± 5.29%. At 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 2 h, the enzymatically treated lychee juice achieved the highest yield (87.33 ± 5.29%), which was significantly higher (P ≤ 0.05) than the untreated lychee juice (64.00 ± 2.00%) (Fig. 1). This enhancement in yield can be attributed to the enzymatic breakdown of structural carbohydrates in the fruit pulp, such as pectin, hemicellulose, and starch. These carbohydrates have a high water-holding capacity and form a dense network structure. However, when enzymes break down pectin, the water-holding capacity decreases, releasing more free water into the system,26 leading to a higher juice yield. These results align with,27 who reported that enzyme treatment significantly increased juice yield in various fruit juices, including plum, peach and pear.
°C for 2 h, the enzymatically treated lychee juice achieved the highest yield (87.33 ± 5.29%), which was significantly higher (P ≤ 0.05) than the untreated lychee juice (64.00 ± 2.00%) (Fig. 1). This enhancement in yield can be attributed to the enzymatic breakdown of structural carbohydrates in the fruit pulp, such as pectin, hemicellulose, and starch. These carbohydrates have a high water-holding capacity and form a dense network structure. However, when enzymes break down pectin, the water-holding capacity decreases, releasing more free water into the system,26 leading to a higher juice yield. These results align with,27 who reported that enzyme treatment significantly increased juice yield in various fruit juices, including plum, peach and pear.
Impact of gelling agent content on the quality of lychee jelly with enzymatically produced FOS
Jelly is a fruit-based product prepared from fruit or concentrated fruit juice (e.g., pineapple, strawberry, orange, lychee, mango and lemon) combined with sweeteners, flavorings, and gelling agents such as pectin or kappa-carrageenan in suitable proportions. The mixture is heated to dissolve all components and then cooled until it reaches a semi-solid, translucent state. High-quality jelly should have an elastic consistency, a soft texture, and a non-sticky surface.Texture characteristics
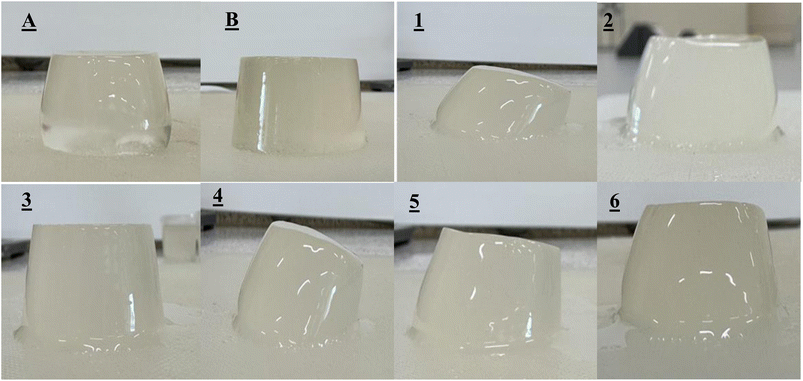
To evaluate the impact of gelling agent concentration on the quality of lychee juice jelly enriched with FOS, enzyme-treated lychee juice (incubated at 55 °C for 2 h) was used as the base. The study involved varying the levels of kappa-carrageenan (0.5%, 1% and 1.5% w/w) and FOS-enhanced lychee juice (70% and 80% w/w), with sucrose incorporated as a sweetener. A total of six jelly formulations were prepared by adjusting both the gelling agent concentration and the proportion of lychee juice. The selected lychee juice levels (70% and 80%) were based on preliminary trials that tested a broader range of concentrations (50%, 60%, 70%, 80% and 90% w/w) to determine the most suitable ratios for jelly production. Jellies containing 50 and 60% w/w FOS-enhanced lychee juice produced a hard and brittle texture, inconsistent with commercial products, whereas the 90% juice formulation developed a yellowish tint that may be undesirable to consumers. In contrast, jellies prepared with 70 and 80% w/w lychee juice had texture and color characteristics similar to those of market products. Therefore, these concentrations were selected for producing 17.66 cm3 of lychee jelly with added FOS, and their texture properties were subsequently analyzed (Fig. 3).The formulation containing 1.5% kappa-carrageenan and 70% FOS-enhanced lychee juice exhibited the highest hardness value among the experimental samples (38.7 ± 4.05 N), which was significantly lower (P ≤ 0.05) than those of two commercial jelly samples, measuring 45.44 ± 4.69 N and 93.68 ± 9.34 N, respectively. In contrast, the formulation with 1.5% kappa-carrageenan and 80% FOS-enhanced lychee juice demonstrated the highest springiness (0.35 ± 0.08 mm), cohesiveness (0.09 ± 0.01), and gumminess (2.94 ± 0.40 N). These values were compared to the commercial samples, which had springiness of 0.41 ± 0.03 mm and 0.22 ± 0.15 mm, cohesiveness of 0.11 ± 0.02 and 0.10 ± 0.01, and gumminess of 4.88 ± 0.57 and 9.48 ± 1.82, respectively.
Hardness values showed an increasing trend with higher kappa-carrageenan concentrations. The natural sugar content also influenced the jelly's texture, as reducing sugars enhanced viscosity by binding water within the matrix. This water-binding effect decreases the amount of free moisture in product.28 These findings align with prior research on star fruit jam, which demonstrated that higher sugar levels result in a firmer gel texture, and an increased kappa-carrageenan concentration enhances jelly firmness. Kappa-carrageenan forms a gel through polymer chains that create a continuous three-dimensional network capable of binding water, thereby reinforcing the structural integrity of the gel. Additionally, its water-binding capability reduces the space between particles, leading to a more compact and solidified gel matrix.29
Total phenolic compound of FOS-enhanced lychee jelly products
The total phenolic content (TPC) of FOS-enhanced lychee jelly and formuated with varying kappa-carrageenan levels ranged from 0.98 ± 0.07 mg GAE/100 g to 5.38 ± 0.06 mg GAE/100 g (Fig. 4). The formulation containing 0.5% kappa-carrageenan and 80% FOS-enhanced lychee juice exhibited the highest TPC (5.38 ± 0.06 mg GAE/100 g), which significantly higher (P ≤ 0.05) than all other formulations. Additionally, formulations with 80% FOS-enhanced lychee juice and all three kappa-carrageenan concentrations had higher TPC values than those with 70% FOS-enhanced lychee juice, aligning with findings by ref. 30. Their study investigated the effect of different ratios of okra and strawberry extracts on TPC, testing five formulations (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 80
0, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 60
30, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 50
40 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) using kappa-carrageenan mixed with glucomannan as a gelling agent and sucralose as a sweetener. The 60
50) using kappa-carrageenan mixed with glucomannan as a gelling agent and sucralose as a sweetener. The 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ratio resulted in the highest TPC (31.17 ± 4.00 mg GAE/100 g). However, compared to the TPC of okra and strawberry extracts before jelly production (228.9 ± 1.75 mg GAE/100 g), the TPC decreased, likely due to thermal exposure and dilution during jelly processing, which can degrade phenolic compounds.
40 ratio resulted in the highest TPC (31.17 ± 4.00 mg GAE/100 g). However, compared to the TPC of okra and strawberry extracts before jelly production (228.9 ± 1.75 mg GAE/100 g), the TPC decreased, likely due to thermal exposure and dilution during jelly processing, which can degrade phenolic compounds.
Total flavonoid content of FOS-enhanced lychee jelly products
The total flavonoid content (TFC) of FOS-enhanced lychee jelly ranged from 1.35 ± 0.06 mg QE/100 g to 3.69 ± 0.15 mg QE/100 g (Fig. 4). The formulation with 1.5% kappa-carrageenan and 80% FOS-enhanced lychee juice exhibited the highest TFC (3.69 ± 0.15 mg QE/100 g), which was significantly higher (P ≤ 0.05) than all other formulations. Additionally, formulations containing 80% FOS-enhanced lychee juice, regardless of the kappa-carrageenan concentration, exhibited higher TFC levels than those with 70% lychee juice. These findings align with the finding of ref. 31, who analyzed the TFC of jelly products prepared from five different types of oranges: blonde maltese, blood orange, grapefruit and bitter orange-reported that blood orange jelly had the highest TFC (9.06 ± 0.26 mg QE/100 g).Antioxidant activity by DPPH and FRAP methods of FOS-enhanced lychee jelly products
The antioxidant activity of FOS-enhanced lychee jelly, evaluated using the DPPH method, ranged from 0.39 ± 0.01 mg TE/100 g to 0.47 ± 0.01 mg TE/100 g (Fig. 4). The formulation containing 0.5% kappa-carrageenan and 80% FOS-enhanced lychee juice exhibited the highest antioxidant activity (0.47 ± 0.01 mg TE/100 g), which was significantly higher (P ≤ 0.05) than all other formulations. Additionally, formulations with 80% FOS-enhanced lychee juice and all three concentrations of kappa-carrageenan demonstrated higher DPPH radical-scavenging capacity than those with 70% FOS-enhanced lychee juice. These findings are consistent with the finding reported by ref. 32, who developed jelly products from pineapple juice supplemented with pineapple and banana pulp, varied kappa-carrageenan concentration from 0.4% to 0.7% (w/w). Their study found that the formulation with 0.5% w/w kappa-carrageenan exhibited the highest antioxidant activity, reaching 51.1% inhibition based on the DPPH assay.The antioxidant activity of FOS-enhanced lychee jelly, as measured by the FRAP method, ranged from 0.11 ± 0.02 mg TE/100 g to 0.65 ± 0.02 mg TE/100 g. The formulation containing 0.5% kappa-carrageenan and 80% FOS-enhanced lychee juice exhibited the highest FRAP value (0.65 ± 0.02 mg TE/100 g), which was significantly higher (P ≤ 0.05) than all other formulations.
Color values by measuring L*, a* and b* values and water activity of FOS-enhanced lychee jelly products
Color is a key indicator of product quality, as it strongly influences consumer perception and acceptance. In the CIE color system, L* represents lightness (0 = black, 100 = white), a* indicates greenness (−a*) to redness (+a*), and b* indicates blueness (−b*) to yellowness (+b*). In this study, the L*, a* and b* values of the FOS-enhanced lychee jelly ranged from 21.08 ± 3.01 to 30.40 ± 3.11, 4.06 ± 0.41 to 4.96 ± 0.30, and 5.09 ± 0.95 to 6.13 ± 0.19, respectively (Table 3). These values were significantly different (P ≤ 0.05) compared to commercial jelly products, which had L*, a*, and b* values ranging from 28.17 ± 2.12 to 30.96 ± 2.48, 0.58 ± 0.29 to 4.47 ± 0.39, and 1.19 ± 0.58 to 4.60 ± 0.26, respectively. These findings align with the finding of Rosida et al.,33 who reported that increasing kappa-carrageenan and green leafy vegetable content led to an increase in a* and b* values, while L* values increased with higher kappa-carrageenan concentrations.| Lychee jelly recipe | Color value | ||
|---|---|---|---|
| L* | a* | b* | |
| a COM1 and COM2 represent two brands of commercially available lychee jelly. Different superscript letters within the same column indicate statistically significant differences (P ≤ 0.05). | |||
| COM1 | 30.96 ± 2.48d | 0.58 ± 0.29a | 1.19 ± 0.58a |
| COM2 | 28.17 ± 2.12c | 4.47 ± 0.39b | 4.60 ± 0.26b |
| 1 | 21.08 ± 3.01a | 4.46 ± 0.10b | 5.09 ± 0.95c |
| 2 | 26.92 ± 0.77b | 4.28 ± 0.33b | 5.45 ± 1.61c |
| 3 | 27.69 ± 1.50bc | 4.54 ± 0.12b | 6.02 ± 0.76d |
| 4 | 21.61 ± 2.11a | 4.06 ± 0.41b | 5.24 ± 1.13c |
| 5 | 26.91 ± 0.62b | 4.65 ± 0.29b | 6.03 ± 0.27d |
| 6 | 30.40 ± 3.11d | 4.96 ± 0.30b | 6.13 ± 0.19d |
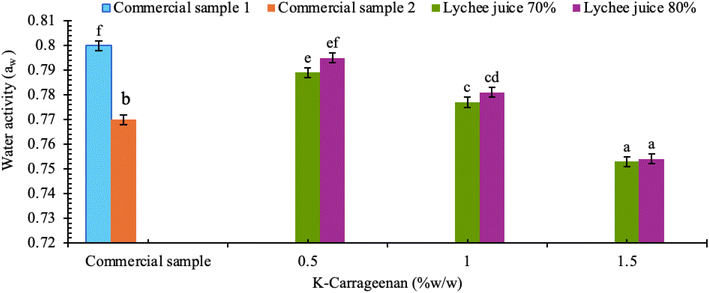
Water activity (aw) is a critical factor affecting food spoilage, as it influences the physical, chemical, biochemical, and microbiological properties of food, ultimately impacting the shelf life.4 It reflects the propotion of free water available for microbial growth.34 In jelly products, the optimal water activity range is between 0.65 and 0.85 (Fig. 5). Since jelly is classified as a semi-solid food, inadequate control of free water content can lead to microbial deterioration if not stored properly.35 This study found that the water activity of the FOS-enhanced lychee jelly increased ranging from 0.75 ± 0.05 to 0.79 ± 0.01, which were significantly different (P ≤ 0.05) compared to commercial jelly samples (0.77 ± 0.05 and 0.80 ± 0.01, respectively). The higher water retention in the jelly may be attributed to kappa-carrageenan, which reduces water evaporation during the drying process and facilitate the formation of a continuous three-dimensional polymer network, that limits the mobility of both free and bound water in the product.36
Sensory evaluation of FOS-enhanced lychee jelly
The sensory evaluation of FOS-enhanced lychee jelly was conducted to determine the influence of varying kappa-carrageenan concentration on the product quality and consumer acceptability. The study examined lychee jelly formulations containing two levels of FOS-enhanced lychee juice (70% and 80% w/w) and three concentrations of kappa-carrageenan (0.5%, 1% and 1.5% w/w), with sucralose used as a sweetener. The formulation containing 80% FOS-enhanced lychee juice and 1.5% kappa-carrageenan received the highest preference scores across multiple attributes, including color, aroma, sweetness, texture, appearance, and overall liking, with values of 8.02 ± 1.00, 7.88 ± 1.25, 7.71 ± 1.07, 8.37 ± 0.89, 8.25 ± 0.90, and 8.33 ± 0.90, respectively (Table 4).| Water content (% lychee juice) | κ-Carrageenan (%) | Color | Aroma | Sweetness | Texture | Appearance | Overall acceptability |
|---|---|---|---|---|---|---|---|
| a Values in the same column with different superscript letters (a–d) are significantly different (P ≤ 0.05). | |||||||
| 70 | 0.5 | 7.23 ± 1.32a | 7.13 ± 1.43b | 6.15 ± 1.46a | 5.60 ± 1.33a | 6.29 ± 1.42a | 5.42 ± 1.33a |
| 70 | 1 | 7.23 ± 1.04a | 6.90 ± 1.40a | 6.23 ± 1.38a | 6.60 ± 1.16b | 6.58 ± 1.11a | 5.71 ± 1.30a |
| 70 | 1.5 | 7.58 ± 1.18ab | 7.23 ± 1.55b | 6.58 ± 1.16a | 7.25 ± 1.31c | 7.23 ± 1.18b | 6.63 ± 1.09b |
| 80 | 0.5 | 7.58 ± 1.00ab | 7.44 ± 1.00b | 6.62 ± 1.29a | 6.31 ± 1.21b | 6.65 ± 1.34a | 6.52 ± 1.32b |
| 80 | 1 | 7.73 ± 1.46ab | 7.37 ± 1.52b | 7.17 ± 1.25b | 7.71 ± 1.13c | 7.69 ± 1.16b | 7.67 ± 1.23c |
| 80 | 1.5 | 8.02 ± 1.00c | 7.88 ± 1.25b | 7.71 ± 1.07b | 8.37 ± 0.89d | 8.25 ± 0.90c | 8.33 ± 0.90d |
Color was identified as the primary factor influencing consumer acceptance. The color preference scores for the six FOS-enhanced lychee jelly formulations ranged from 7.23 ± 1.32 to 8.02 ± 1.00, falling within the “moderately like” to “very like” range. Because the visual differences among samples were minimal, these variations were not statistically significant (P > 0.05). The aroma and sweetness scores ranged from 7.13 ± 1.43 to 7.88 ± 1.25 and 6.15 ± 1.46 to 7.71 ± 1.07, respectively, falling within the “slightly like” to “moderately like” range. Texture, appearance, and overall liking scores ranged from 5.60 ± 1.33 to 8.37 ± 0.89, 6.29 ± 1.42 to 8.25 ± 0.90, and 5.42 ± 1.33 to 8.33 ± 0.90, respectively, spanning from “indifferent” to “very like”.
The formulation containing 70% FOS-enhanced lychee juice and 0.5% kappa-carrageenan received the lowest texture score (5.60 ± 1.33), primarily due to its overly liquid consistency, which was less acceptable to consumers. This finding aligns with the finding of Achariyaphotha et al.,37 which investigated the effects of sucralose and kappa-carrageenan on the quality of ready-to-drink fruit jellies (pineapple, orange, dragon fruit and ginger). Their study, which tested kappa-carrageenan at four levels (1.75, 1.5, 1.25 and 1 g/602 g of jelly), found that the formulation containing 1.25 g of kappa-carrageenan achieved the highest sensory acceptance, attributed to its soft texture, slight water separation, and suitability for scooping or sucking—qualities considered desirable for jelly products.
Based on the combined sensory scores for color, aroma, sweetness, texture, appearance, and overall liking, the formulation containing 80% FOS-enhanced lychee juice and 1.5% kappa-carrageenan was identified as the most preferred. This formulation was therefore selected for further studies on the quality changes of FOS-enhanced lychee jelly during storage.
Quality changes of FOS-enhanced lychee jelly products during cold storage
The phenolic compound content of FOS-enhanced lychee jelly stored at 4 °C for 30 days showed a clear and progressive decline. On day 0, the phenolic compound content was 4.45 ± 0.01 mg GAE/100 g, but by day 30, it had decreased to 0.10 ± 0.04 mg GAE/100 g, with a significant difference observed (P ≤ 0.05) (Fig. 6). A similar decreasing trend was observed for total flavonoid content (TFC), which declined from 3.69 ± 0.02 mg QE/100 g on day 0 to 0.14 ± 0.05 mg QE/100 g by day 30 (P ≤ 0.05). Antioxidant activity also decreased over the storage period. DPPH radical-scavenging activity decreased markedly from 0.46 ± 0.01 mg TE/100 g on day 0 to 0.02 ± 0.01 mg TE/100 g on day 30 (P ≤ 0.05). Similarly, FRAP antioxidant activity declined significantly, from 0.52 ± 0.05 mg TE/100 g on day 0 to 0.03 ± 0.01 mg TE/100 g by day 30 (P ≤ 0.05). These findings indicate substantial degradation of phenolic compounds, flavonoids, and antioxidant activity during refrigerated storage.The observed reduction in phenolic compounds, flavonoids, and antioxidant activity by both DPPH and FRAP methods over time can be attributed to the degradation of these compounds due to environmental factors such as temperature, light exposure, enzyme activity, and oxidative reaction.38 Heat exposure, in particular, may cause the degradation or structural changes of bioactive compounds, disrupt cell integrity, and trigger oxidation processes mediated by oxygen, endogenous enzyme, and light.39
Texture characteristics and water activity (aw) of FOS-enhanced lychee jelly during storage
The texture of FOS-enhanced lychee jelly underwent notable changes during 30 days of refrigerated storage. Hardness, springiness, cohesiveness, and gumminess ranged from 7.05 ± 5.26 to 33.91 ± 3.58 N, 0.08 ± 0.01 to 0.35 ± 0.08 mm, 0.03 ± 0.01 to 0.09 ± 0.01, and 0.24 ± 0.22 to 2.94 ± 0.40 N, respectively (Table 5). These values significantly decreased over the storage period (P ≤ 0.05), indicating progressive weakening of the gel matrix and loss of structural integrity.40| Shelf life (days) | Texture characteristics | Water activity (aw) | |||
|---|---|---|---|---|---|
| Hardness (N) | Springiness (mm) | Cohesiveness | Gumminess (N) | ||
| a Values in the same column with different superscript letters (a–d) differ significantly (P ≤ 0.05). | |||||
| 0 | 33.91 ± 3.58d | 0.35 ± 0.08d | 0.09 ± 0.01c | 2.94 ± 0.40e | 0.75 ± 0.01a |
| 5 | 33.09 ± 6.61d | 0.35 ± 0.01d | 0.09 ± 0.01c | 2.04 ± 0.72d | 0.77 ± 0.02b |
| 10 | 32.92 ± 0.80d | 0.34 ± 0.02d | 0.08 ± 0.01c | 2.00 ± 0.31d | 0.89 ± 0.04c |
| 15 | 26.66 ± 2.70c | 0.31 ± 0.06d | 0.06 ± 0.04b | 1.72 ± 1.69c | 0.89 ± 0.01c |
| 20 | 22.29 ± 2.84c | 0.28 ± 0.01c | 0.05 ± 0.04b | 1.12 ± 0.79c | 0.98 ± 0.01c |
| 25 | 12.64 ± 0.63b | 0.17 ± 0.01b | 0.03 ± 0.01a | 0.33 ± 0.05b | 0.98 ± 0.03c |
| 30 | 7.05 ± 5.62a | 0.08 ± 0.01a | 0.03 ± 0.01a | 0.24 ± 0.22a | 0.99 ± 0.07c |
The water activity (aw) of FOS-enhanced lychee jelly increased progressively from 0.75 ± 0.01 to 0.99 ± 0.07 by day 30. This increase may be associated with syneresis, where contraction of 3D kappa-carrageenan gel network expels water, resulting in greater availability of free water and, consequently, elevated aw values.41
Color analysis revealed that the L* (brightness) value of the FOS-enhanced lychee jelly decreased from 21.61 ± 2.11 on day 0 to 14.40 ± 0.02 on day 30, indicating progressive darkening of the product (Table 6). The a* value (redness) decreased, from 4.06 ± 0.41 to 3.06 ± 0.04, while the b* value (yellowness) increased from 6.03 ± 0.27 to 23.53 ± 0.01 over the same period. These changes were consistent with the trends reported from ref. 4, suggesting that storage-induced physicochemical changes may alter pigment stability and visual quality of the jelly.
| Shelf life (days) | Color value | pH | Total acid content (% malic acid)ns | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| a ns: no significant difference. Values in the same column with different superscript letters (a–d) differ significantly (P ≤ 0.05). | |||||
| 0 | 21.61 ± 2.11d | 4.06 ± 0.41b | 6.03 ± 0.27a | 3.32 ± 0.04b | 0.14 ± 0.01 |
| 5 | 20.18 ± 0.11c | 4.02 ± 0.02b | 6.03 ± 0.13a | 3.32 ± 0.04b | 0.14 ± 0.01 |
| 10 | 20.14 ± 0.01c | 4.02 ± 0.07b | 8.01 ± 0.21b | 3.29 ± 0.04b | 0.14 ± 0.02 |
| 15 | 18.61 ± 0.07b | 3.98 ± 0.01a | 10.66 ± 0.07c | 3.22 ± 0.03b | 0.14 ± 0.01 |
| 20 | 15.47 ± 0.05a | 3.87 ± 0.01a | 17.39 ± 0.03d | 3.02 ± 0.07ab | 0.14 ± 0.01 |
| 25 | 15.38 ± 0.04a | 3.06 ± 0.01a | 23.52 ± 0.01e | 2.66 ± 0.12a | 0.13 ± 0.03 |
| 30 | 14.40 ± 0.02a | 3.06 ± 0.04a | 23.53 ± 0.01e | 2.34 ± 0.04a | 0.13 ± 0.01 |
The pH of the FOS-enhanced lychee jelly decreased significantly (P ≤ 0.05) from 3.32 ± 0.04 on day 0 to 2.34 ± 0.04 on day 30 (Table 6). This reduction in pH may be associated with metabolic activity of surviving microorganism during storage, leading to production of ogranic acids. A similar decline in pH during refrigerated storage has been reported in cucumber juice, where microbial growth contributed to increased activity.42
The total titratable acidity of FOS-enchanced lychee jelly maintained a constant range of 0.14 ± 0.01% to 0.13 ± 0.01% throughout the 30-day storage period, with no significant difference observed (P > 0.05) (Table 6).
This study demonstrated several strengths through its holistic experimental design, spanning from juice optimization to jelly development, where eco-friendly enzymatic processing enabled in situ FOS enrichment. The approach achieved high consumer acceptance without reliance on synthetic additives, while showcasing a sustainable pathway for valorizing Thailand's abundant lychee resources.
Total microorganisms, yeast and mold content, E. coli and coliforms content of FOS-enhanced lychee jelly during storage
Food spoilage typically results in an undesirable sour taste, which is often caused by the growth of microorganisms such as bacteria, molds and yeasts. These microorganisms metabolize carbohydrates, proteins, and lipids into smaller compounds, producing small molecules like carboxylic acids, including lactic and acetic acids.43 According to food safety standards, spoilage criteria specify that the total viable counts and yeast and mold levels in jelly should not exceed 1.00 × 104 and 100 CFU/g, respectively, while coliform counts must remain below 3 MPN/100 g (Table 7).| Storage period (days) | Microbial count (log CFU/g) | E. coli and coliforms (MPN/g) | |
|---|---|---|---|
| TVC | YMC | ||
| a TVC: total viable count; YMC: yeast and mold count; ND: not detected; <3: less than 3 MPN/g. Values in the same column with different superscript letters (a–e) differ significantly (P ≤ 0.05). | |||
| 0 | ND | ND | <3 |
| 5 | ND | ND | <3 |
| 10 | 1.95 ± 0.03a | 1.88 ± 0.05a | <3 |
| 15 | 2.28 ± 0.01b | 2.63 ± 0.01b | <3 |
| 20 | 3.04 ± 0.07c | 3.11 ± 0.09c | <3 |
| 25 | 3.44 ± 0.05c | 3.70 ± 0.03d | <3 |
| 30 | 4.16 ± 0.01d | 4.29 ± 0.01e | <3 |
During 30 days of refrigerated storage, it was observed that the total viable count of FOS-enhanced lychee jelly increased progressively. The count began to rise after 10 days, with values ranging from 1.95 ± 0.03 to 4.16 ± 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU/g. The increase was statistically significant (P ≤ 0.05) and by day 30, the total viable count exceeded the standard limit of 4
log CFU/g. The increase was statistically significant (P ≤ 0.05) and by day 30, the total viable count exceeded the standard limit of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU/g, indicating microbial spoilage at the end of storage.
log CFU/g, indicating microbial spoilage at the end of storage.
Yeast and mold counts also increased progressively duing refrigerated storage. Yeast and mold began to grow on day 10, with a value of 1.88 ± 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU/g, and by day 30, the count had reached 4.29 ± 0.01 log CFU/g, which exceeds the acceptable limit of 2
log CFU/g, and by day 30, the count had reached 4.29 ± 0.01 log CFU/g, which exceeds the acceptable limit of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU/g. In contrast, neither E. coli nor coliforms were detected in any samples during the 30-day period, with both remaining below 3 MPN/g. E. coli and coliforms, which are commonly found in the intestines of humans and animals, are widely used as indicators of hygiene in the food industry.
log CFU/g. In contrast, neither E. coli nor coliforms were detected in any samples during the 30-day period, with both remaining below 3 MPN/g. E. coli and coliforms, which are commonly found in the intestines of humans and animals, are widely used as indicators of hygiene in the food industry.
Based on the total viable count and yeast and mold count, our findings align with those reported in ref. 44 who studied the shelf life of healthy cereal jelly products stored at 8 ± 2 °C. The results showed that after 14 days, the total viable count reaching 1.42 × 104 CFU/g, and the yeast and mold levels increasing to 1.38 × 102 CFU/g, exceeding the standard criteria.
Conclusions
This study demonstrates a sustainable approach to developing a novel fructooligosaccharide (FOS)-enriched lychee jelly by utilizing enzymatic hydrolysis to enhance prebiotic content and employing kappa-carrageenan to optimize textural properties. Enzymatic treatment significantly increased FOS levels and antioxidant activity in lychee juice, supporting the production of a functional jelly formulated with 80% juice and 1.5% kappa-carrageenan that achieved favorable textural attributes and high sensory acceptance. With FOS intake contributing toward the recommended prebiotic level (3–8 g/day), the product aligns with consumer demand for low-calorie, gut-health-supporting, clean-label foods while also supporting the valorization of local fruit resources through eco-efficient enzymatic bioprocessing. Nevertheless, the 30-day shelf life, limited by microbial growth, and the use of untrained sensory panelists present opportunities for further research. Future studies should investigate the bioaccessibility of FOS to confirm its prebiotic functionality, assess natural preservation strategies to extend shelf life without synthetic additives, and explore process scalability. Moreover, evaluating alternative sustainable hydrocolloids and natural sweeteners may further enhance nutritional quality while maintaining consumer appeal, thereby contributing to the development of environmentally responsible functional food.Author contributions
Nattida Pongjuntuk: investigation, formal analysis, data curation and writing – original draft. Saeid Jafari: data curation and writing – original draft. Sochannet Chheng: data curation and writing – original draft. Kitipong Assatarakul: conceptualization, data curation, funding acquisition, project administration, supervision, writing – original draft and writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The data supporting this article have been included within the article.Acknowledgements
This research was supported by the 90th Anniversary of Chulalongkorn University Scholarship under the Ratchadapisek Somphot Endowment Fund (GCUGR1125671048M), Chulalongkorn University. The Second Century Fund (C2F), Chulalongkorn University provided postdoctoral fellowship support to Dr Saeid Jafari. The author would also like to express sincere gratitude to Dr Supaart Sirikantaramas, Department of Biochemistry, Faculty of Science, Chulalongkorn University, for his assistance with the fructooligosaccharide analysis.References
- E. a. M. T. National Institute of Food Technology, Detailed Project Report for Litchi Beverages, Ministry of Food Processing Industries, Government of India, Thanjavur, India, 2020 Search PubMed.
- S. Chheng, M. Fikry, S. Jafari, D. K. Mishra and K. Assatarakul, J. Stored Prod. Res., 2025, 112, 102660 CrossRef CAS.
- S. Hajar-Azhari, M. H. Abd Rahim, S. R. Sarbini, B. J. Muhialdin, L. Olusegun and N. Saari, Food Res. Int., 2021, 149, 110677 CrossRef CAS PubMed.
- S. Chheng, S. Jafari, D. Mishra and K. Assatarakul, Sustainable Food Technol., 2025 10.1039/D5FB00227C.
- D. A. Gonçalves, V. D. Alves, J. A. Teixeira and C. Nobre, Food Res. Int., 2023, 168, 112671 CrossRef PubMed.
- K. Peng, Y. Zhang, Q. Zhang, Y. Wang, Y. Liu and X. Cui, Foods, 2025, 14, 246 CrossRef CAS PubMed.
- C. Y. Hui, K. C. Lee and Y. P. Chang, Plant Foods Hum. Nutr., 2022, 77, 299–306 CrossRef CAS.
- D. P. de Oliveira, S. D. Todorov and J. P. Fabi, Nutrients, 2024, 16, 3689 CrossRef CAS PubMed.
- M. Cywińska-Antonik, Z. Chen, B. Groele and K. Marszałek, Foods, 2023, 12(6), 1181 CrossRef PubMed.
- S. Kamchonemenukool, W. Buasum, M. Weerawatanakorn and T. Thongsook, Foods, 2023, 12, 2895 CrossRef CAS PubMed.
- C. L. Nguyen and H. V. Nguyen, Beverages, 2018, 4, 41 CrossRef CAS.
- S. Jafari, K. Pongsarn, C. Srestasupana, N. Wetchasart and K. Assatarakul, LWT–Food Sci. Technol., 2021, 152, 112309 CrossRef CAS.
- S. Jafari, N. Rungroj, R. W. Worobo and K. Assatarakul, Int. J. Food Microbiol., 2021, 358, 109404 CrossRef CAS PubMed.
- A. A. Masri, F. I. Bakar, M. Abidin and N. H. Malik, Trop. J. Nat. Prod. Res., 2023, 7, p. 3433 Search PubMed.
- M. C. d Cunha, J. S. Silva, H. H. d. S. Elias, E. E. N. Carvalho and E. V. d. B. Vilas Boas, Food Sci. Technol., 2020, 41, 96–104 CrossRef.
- B. A. Manual-BAM, inBacteriological Analytical Manual, Chapter 18: Yeasts, Molds and Mycotoxins, U.S, Food and Drug Administration (FDA), 2001, Online edition, available at: https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam Search PubMed.
- A. Chauhan and T. Jindal, Microbiological methods for food analysis, in Microbiological Methods for Environment, Food and Pharmaceutical Analysis, 2020, pp. 197–302 Search PubMed.
- S. Visuthiwan and K. Assatarakul, Food Control, 2021, 123, 107770 CrossRef CAS.
- J. Yang, X. Ou, X. Zhang, Z. Zhou and L. Ma, J. Food Sci., 2017, 82, 605–612 CrossRef CAS PubMed.
- Z. Wang, G. Wu, B. Shu, F. Huang, L. Dong, R. Zhang and D. Su, RSC Adv., 2020, 10, 6743–6751 RSC.
- J. Oszmiański, A. Wojdyło and J. Kolniak, Food Chem., 2011, 127, 623–631 CrossRef PubMed.
- S. Chheng, M. Fikry, S. Jafari, S. K. Mehta, D. K. Mishra and K. Assatarakul, ES Food Agrofor., 2025, 19, 1434 Search PubMed.
- A. M. Pisoschi and G. P. Negulescu, Biochem. Anal. Biochem., 2011, 1, 106 Search PubMed.
- I. F. Benzie and J. J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed.
- R. Bagger-Jørgensen and A. S. Meyer, Eur. Food Res. Technol., 2004, 219, 620–629 CrossRef.
- M. Gernandt and J. Urlaub, J. Forensic Sci., 1996, 41, 503–504 CrossRef.
- V. Joshi, M. Parmar and N. Rana, Indian J. Nat. Prod. Resour., 2011, 2, 189–197 CAS.
- H.-J. Oh, J.-W. Back, J.-Y. Lee, Y.-J. Oh and S.-B. Lim, Culi. Sci. Hos. Res., 2013, 19, 110–120 Search PubMed.
- F. H. Harahap, N. Harini and R. Anggriani, Food Technol. Halal Sci. J, 2022, 5, 45–61 CrossRef.
- D. R. Nuramalia and E. Damayanthi, IOP Conf. Ser.: Earth Environ. Sci., 2018, 196, 012005 CrossRef.
- I. Ben Rejeb, N. Dhen, S. Kassebi and M. Gargouri, J. Chem., 2020, 2020, 5476872 Search PubMed.
- S. Rittisak, N. Lonuch, S. Buakeeree and S. Yimtoe, Food Res., 2023, 7, 52–59 CAS.
- D. F. Rosida and A. A. Taqwa, Jurnal Agroteknologi, 2019, 13, 62–74 CrossRef.
- N. Chaichana, N. Muneerungsee, Y. Sukpondma and N. Sermwittayawong, LWT–Food Sci. Technol., 2023, 185, 115182 CrossRef CAS.
- M. S. Tapia, S. M. Alzamora and J. Chirife, Water Activity in Foods: Fundamentals and Applications, 2020, pp. 323–355 Search PubMed.
- Y. Wei, S. Lin, W. Lin, Y. Nie, X. Zou, Y. Zheng, B. Li, Y. He, Y. Huang and Y. Huang, Food Sci. Nutr., 2025, 13, e70541 CrossRef CAS PubMed.
- B. Achariyaphotha, J. Ngamsuay, S. Kesorn and N. Waisura, Research Journal Phranakhon Rajabhat: Science and Technology, 2023, 18, 66–82 Search PubMed.
- C. Irawan, I. Dwi Putri and M. Sukiman, J. Phys.: Conf. Ser., 2022, 2239, 012001 CrossRef.
- M. W. Davey, M. V. Montagu, D. Inze, M. Sanmartin, A. Kanellis, N. Smirnoff, I. J. J. Benzie, J. J. Strain, D. Favell and J. Fletcher, J. Sci. Food Agric., 2000, 80, 825–860 CrossRef CAS.
- T. Baydin, O. A. Aarstad, M. J. Dille, M. N. Hattrem and K. I. Draget, Food Hydrocolloids, 2022, 127, 107535 CrossRef CAS.
- E. Jakubczyk, A. Kamińska-Dwórznicka and E. Ostrowska-Ligęza, Gels, 2022, 8, 110 CrossRef CAS PubMed.
- Q. Zhao, Q. Yuan, C. Gao, X. Wang, B. Zhu, J. Wang, X. Sun and T. Ma, Foods, 2021, 10, 1851 CrossRef CAS PubMed.
- V. Dhawan, in Studies on Respiratory Disorders, Springer, 2014, pp. 27–47 Search PubMed.
- K. T. N. Ayutthaya and N. Putduang, J. Food Technol., 2016, 11, 13–20 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |