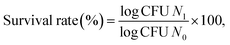Open Access Article
Open Access ArticleDevelopment and characterization of probiotic processed cheese
Deepak Kumara,
Sangita Ganguly *a,
Santosh Tambdea,
Yogesh Khetra
*a,
Santosh Tambdea,
Yogesh Khetra a,
P. Narender Raju
a,
P. Narender Raju a and
Rashmi Hogarehalli Mallappab
a and
Rashmi Hogarehalli Mallappab
aDairy Technology Division, ICAR-National Dairy Research Institute, Karnal, Haryana, India. E-mail: sangitandri@gmail.com; Fax: +91-184-2250042; Tel: +91-184-2259248
bDairy Bacteriology Section, ICAR-National Dairy Research Institute, SRS, Bengaluru, India
First published on 28th November 2025
Abstract
Processed cheese is a popular variety of cheese and is generally devoid of lactic organisms. The incorporation of probiotic organisms into processed cheese will provide functionality and health beneficial properties to the product, along with an increased market demand. Four strains of the probiotic organism Lactiplantibacillus plantarum, namely, Lp1, Lp6, Lp7 and Lp9, were subjected to thermal stability testing, and based on the maximum thermal stability, L. plantarum (Lp9) was selected for further experimentation. Based on the moisture level (46%) and probiotic count, an inoculum of 14% (w/w) was selected for probiotic processed cheese preparation. Probiotic processed cheese was prepared by incorporating an optimized level of probiotic inoculum in normal processed cheese at 60 °C. Probiotic processed cheese had a sensory and compositional quality comparable to that of the control cheese. The product had lower meltability, hardness and cohesiveness than the control cheese. Probiotic processed cheese exhibited improved bio-functional attributes in terms of antioxidant and ACE-inhibitory activity compared with the control cheese. Probiotic processed cheese had an initial probiotic count of 7.66 log CFU g−1, which remained stable in the processed cheese matrix during 35 days of refrigerated storage.
Sustainability spotlightProcessed cheese is a popular variety of cheese and is generally devoid of lactic organisms. The incorporation of probiotic organisms into processed cheese will provide more functionality and health beneficial properties to the product, along with an increased market demand. This study describes a sustainable way to incorporate probiotic organisms into processed cheese for enhanced health beneficial properties for promoting good health and improved lifestyle. This approach contributes to sustainable food innovation by enhancing processed cheese with health promoting properties without compromising shelf stability or processing efficiency. |
1 Introduction
Functional foods are those that provide health benefits beyond basic nutrition, such as preventing diseases and supporting health management,1 and fermented dairy products are a popular type of functional food since they are believed to offer more health benefits than regular milk. The use of fermented dairy products containing probiotic bacteria as functional foods has increased due to new scientific research showing their ability to prevent diseases.Probiotics are live microorganisms that, upon administration in adequate amounts, confer health benefits to the host.2 Probiotic microorganisms offer several health benefits, including immune modulation, cholesterol reduction, and cancer prevention.3,4 Some probiotics commonly used in various fermented products, including cheese, are Lactobacillus and Bifidobacterium spp.5 To provide health benefits to the host, the concentration of probiotic bacteria should be 7–8 log CFU g−1 or mL of the product.6
Cheeses are dairy products having a strong potential to serve as delivery matrices for probiotic microorganisms due to their inherent physical and chemical nature. Cheese has comparatively low acidity, high pH, high buffering capacity and solid consistency. Cheeses are nutrient dense foods with a low oxygen content compared with dahi or yogurt. These inherent properties of cheese provide protection to the probiotic organisms during storage and gastrointestinal transit.7 Hard variety of cheese like Cheddar, may provide certain benefits over yoghurt-type products regarding a delivery medium of viable probiotic, such as the low acidity and the high-fat content as compared to yoghurt environment. Further, the texture of cheese may offer an encapsulation-type protection to the microorganisms during passage through the GI tract.8 Several soft cheeses, like whey Cheese,9 white cheese,10 and cottage cheese, have been used as carriers of live probiotic organisms.11 Processed cheese is a product obtained by blending natural cheese with emulsifying salts and dairy and non-dairy ingredients. This mixture is subjected to a heat treatment and continuous mixing to achieve a uniform consistency suitable for extended storage. Processed cheese differs from conventional cheese since it is not obtained from traditional processes, involving traditional lactic fermentation of milk or direct acidification of milk using food grade organic acids. Processed cheese is widely used in various food applications, including sandwiches, burgers, pizzas, and ready-to-eat meals. Its unique properties, such as high meltability, smoothness, and convenience, make it a popular ingredient in the food industry.12 Some researchers have tried to incorporate beneficial probiotic organisms in ‘requeijao cremoso’ type processed cheese and reported that the fusion stage is the most appropriate for the incorporation of organisms.13 In another study, probiotic processed cheese was prepared by the addition of spores of B. coagulans ATCC7050. The sensory quality of probiotic processed cheese was reduced with an increment in spore count, and the viability of the probiotic organism was maintained at an optimal level during 60 days of storage.
Processed cheese is extremely popular in the Indian market and is mostly used for direct consumption. It is generally devoid of live microorganisms due to its production techniques. However, if probiotic organisms can be incorporated into processed cheese, this could lead to increased consumer acceptability and market demand, leading to categorization of the product as a functional food with added health benefits. To the best of our knowledge, no studies have been conducted in the area of the incorporation of live probiotic organisms into processed cheese. Considering all the aspects, a study is conducted for the development of probiotic processed cheese with the following objectives: (a) to develop probiotic processed cheese, and (b) to characterize the developed probiotic processed cheese, including compositional, functional, rheological, textural, sensory, microbial and bio-functional quality.
2 Materials and methods
2.1 Materials
Raw chilled cow milk (fat −4–4.2% and solid-not-fat −8.2–8.3%) and fresh as well as three-month-old matured cheddar cheese were collected from the Experimental Dairy Plant, National Dairy Research Institute, Karnal, Haryana, India. Skim milk powder (SMP) was obtained from Modern Dairies Ltd, Karnal, Haryana, India. All chemicals used during the investigation were obtained from M/s Sigma Aldrich Ltd, USA. All microbiological media were purchased from M/s Himedia, Mumbai, Maharashtra, India. Probiotic organisms, specifically Lactiplantibacillus plantarum four strains Lp1, Lp6, Lp7 and Lp9, were collected from the Molecular Biology Unit at the National Dairy Research Institute in Karnal, Haryana, India. Alpha Packaging's polypropylene (PP) tubs, manufactured in Surat, Gujarat, India, were procured from a local supplier in Karnal, Haryana, India.2.2 Maintenance, preservation, and propagation of cultures
Freeze-dried cultures were activated and sub-cultured using the methodology described by Sameer et al.14 Lactobacilli stock cultures were stored at −20 °C in De Man, Rogosa and Sharpe (MRS) broth with 20% glycerol. For cheese production, the chosen organism was cultured in milk.where N1 denotes the probiotic count after exposure to thermal treatment and N0 denotes the initial probiotic count.
2.3 Preparation of probiotic processed cheese (PPC)
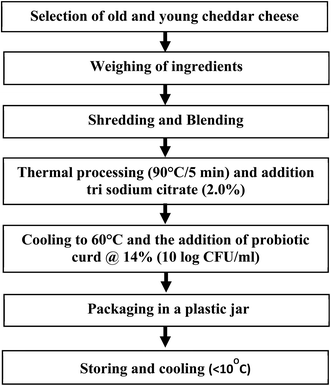
The control and probiotic processed cheeses were prepared in a process cheese kettle with a maximum capacity of 50 kg situated at the experimental dairy plant of ICAR-NDRI, Karnal. In one batch, about 20–25 kg of processed cheese was prepared. The manufacturing process of control processed cheese involves the use of old (i.e., three months old) and young cheddar cheeses. About 25% old cheddar cheese (38.7% moisture) and 75% young cheddar cheese (39.35% moisture) were used for the preparation of the control processed cheese. Shredded cheddar cheese was blended and subjected to thermal processing at 90 °C for 5 minutes and mixed with trisodium citrate at a rate of 2% dissolved in a calculated quantity of water (76.61 ml per kg of processed cheese). Probiotic processed cheese was prepared using old and young cheddar cheese and probiotic curd (inoculum) with 16% total solids. A lower amount of water was required for probiotic processed cheese preparation as the water content of the curd was considered. The probiotic curd was added at a selected rate at 60 °C, followed by thorough mixing using a high speed automated stirrer.2.4 Analysis of probiotic processed cheese
2.5 Statistical analysis
The statistical analyses were performed using IBM SPSS 20 (version 20; IBM Corporation, USA). A two-tailed paired t-test was used to determine the significance of the difference between the two treatments, while an analysis of variance (ANOVA) was employed to examine the significance of the difference between more than two treatments at the 5% level of significance. Three replicates were used for all experimental analyses for probiotic and control processed cheeses.3 Results and discussion
3.1 Screening of probiotic strain
Four probiotic organisms of Lactobacillus spp., Lp1, Lp6, Lp7, and Lp9, were checked for thermal stability at different time–temperature conditions to select the most suitable culture for the development of probiotic processed cheese. Among the four strains, the Lp9 strain showed a higher survival rate of 89.19% and 83.83% when exposed to thermal temperatures of 60 and 65 °C for 5 minutes, respectively (Table 1). When exposed to a temperature of 60 °C for 5 minutes, Lp9 had the highest number of viable probiotic counts (i.e., 9.49 log CFU mL−1), which was significantly greater (p < 0.05) than the other strains. However, when all strains were subjected to a temperature exposure of 70 °C for 5 min, there was a complete absence of live probiotic organisms. Strain Lp9 showed maximum thermal resistance in the experiment; therefore, it was selected for further experiments. The L. plantarum Lp9 is an indigenous strain that displayed a high survival at a low pH and bile and demonstrated health-promoting properties in terms of antioxidative, antibacterial, and cholesterol lowering properties with a potential for exploitation in functional food development.29 De Angelis et al.30 conducted a study on the heat stress tolerance and responsiveness of different strains of L. plantarum. The researchers found that the stationary-phase cells of L. plantarum DPC2739 had decimal reduction times (D value) of 32.9 and 14.7 s at 60 °C and 72 °C, respectively, in sterile milk. Parente et al.31 studied the heat stress behavior of L. plantarum and demonstrated that stationary-phase cells exhibited greater resistance to heat stress compared to cells in the exponential growth phase. De Angelis and Gobbetti32 stated that during the growth phase, cells undergo an adaptive process where certain genes are activated to handle various stress conditions, such as nutrient depletion, acidity, and heat. This adaptive mechanism enables cells to express a broad stress response, resulting in the development of more resilient cells capable of surviving unfavorable growth environments.| Probiotic organisms | Thermal treatments | ||||
|---|---|---|---|---|---|
| Without heat treatment (log CFU mL−1) | 60 °C/5 min | 65 °C/5 min | |||
| Log CFU mL−1 | % Survival | Log CFU mL−1 | % Survival | ||
| a Mean ± S.D (n = 3); means with different superscripts within a row differ significantly (p < 0.05). | |||||
| Lp1 | 10.54a ± 0.01 | 9.30b ± 0.02 | 88.24 | 6.56c ± 0.19 | 62.24 |
| Lp6 | 10.42a ± 0.00 | 9.21b ± 0.00 | 88.39 | 8.62c ± 0.01 | 82.73 |
| Lp7 | 10.58a ± 0.00 | 9.27b ± 0.08 | 87.62 | 6.93c ± 0.02 | 65.50 |
| Lp9 | 10.64a ± 0.05 | 9.49b ± 0.13 | 89.19 | 8.92c ± 0.01 | 83.83 |
| Inoculum level (%) | Moisture of PPC (%) | Probiotic count of PPC (log cfu mL−1) |
|---|---|---|
| a Mean ± S.D (n = 3); means with different superscripts within a row differ significantly (p < 0.05). | ||
| 10 | 44.65d ± 0.06 | 7.54d ± 0.02 |
| 12 | 45.12c ± 0.07 | 7.58c ± 0.02 |
| 14 | 46.09b ± 0.04 | 7.81b ± 0.00 |
| 15 | 47.35a ± 0.25 | 7.91a ± 0.01 |
3.2 Development of probiotic processed cheese
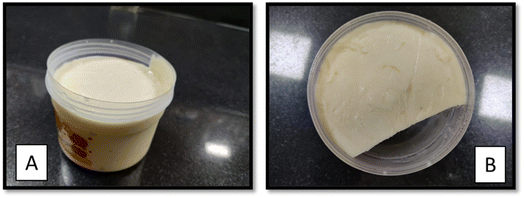
Probiotic processed cheese was prepared as per the given protocol (Fig. 2). The selected L.plantarum (Lp9) was added at a 14% (w/w) level at the final stage of processed cheese preparation at a temperature of 60 °C with thorough mixing. The developed probiotic processed cheese is shown in Fig. 3A and B.3.3 Characterization of probiotic processed cheese
| Parameter | CPC | PPC |
|---|---|---|
| Composition | ||
| a Mean ± S.D (n = 3); means with different superscripts within a row differ significantly (p < 0.05). | ||
| Moisture (%) | 46.00 ± 0.69a | 46.09 ± 0.04a |
| Fat (%) | 26.65 ± 0.86a | 26.33 ± 0.76a |
| Protein (%) | 19.06 ± 0.15a | 19.25 ± 0.03a |
| Lactose (%) | 4.01 ± 0.23a | 4.20 ± 0.14a |
| Ash (%) | 4.38 ± 0.12a | 4.14 ± 0.14a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Physico-chemical properties | ||
| pH | 5.76 ± 0.05a | 5.67 ± 0.04a |
| Titratable acidity (%) LA | 0.97 ± 0.00a | 1.04 ± 0.01a |
| Water activity | 0.94 ± 0.00a | 0.96 ± 0.00a |
| Probiotic count (log CFU g−1) | — | 7.81 ± 0.00 |
3.3.2.1 Meltability. Meltability refers to how easily cheese can flow or spread when heated.34 To evaluate processed cheese quality, meltability, measured as the ratio of melted to unmelted cheese area, is a crucial parameter. In our study, a significant (p < 0.05) difference in meltability was found between control and probiotic processed cheeses, with values of 10.24 and 9.012, respectively (Fig. 4A). The possible reason for the lower meltability of probiotic processed cheese might be due to the addition of curd. Chaudhary et al.35 found that the meltability of processed cheese varied from 10.56 to 15.55 depending on the intact casein content, and our experiment yielded similar meltability values within this range. The incorporation of non-fat-dry milk and whey protein concentrate (WPC) into processed cheese formulations may contribute to decreased meltability in processed cheese.36,37 The reason has been proposed as the heat-induced disulfide interactions, involving the formation of free sulfhydryl groups from β-lactoglobulins, having a marked influence on the melting attributes of the processed cheeses.36 In another study, researchers heated whey protein dispersions to induce disulfide cross-linking between the whey proteins and produced polymerized cross-linked whey proteins, followed by addition into a model processed cheese system. They reported that as the level of polymerized whey proteins increased, there was a decline in the meltability of the process cheese analogs produced.38 Further, an increment in mixing speed during processed cheese manufacturing is associated with a decreased meltability of the final product.39 In our case, probiotic processed cheese manufacturing was associated with extensive stirring and enhanced mixing speed so that probiotic curd could mix uniformly into the processed cheese matrix, leading to a reduced meltability of the probiotic sample compared to the control one.
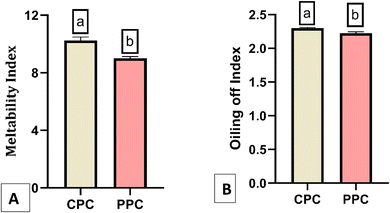 | ||
| Fig. 4 (A) Meltability of the control processed cheese (CPC) and probiotic processed cheese (PPC) and (B) oiling-off of CPC and PPC. | ||
3.3.2.2 Oiling-off. There was a significant (p < 0.05) difference in oiling-off between control and probiotic processed cheeses (Fig. 4B). The control and probiotic processed cheeses had oiling-off values of 2.30 and 2.23, respectively. The higher oiling-off value of the control compared to the probiotic cheese might be attributed to the higher amount of aged cheese in the control compared to the probiotic cheese. Chaudhary et al.35 reported that aged cheeses provided a greater oiling-off compared to young cheddar cheeses due to increased protein breakdown and lipolysis.
Oiling off the properties of processed cheese is contributed by free oil formation and oil separation due to the leakage of fat. This occurred as a result of liquefied fat separation particularly at the surface of the cheese when the cheese is subjected to melting during the heating process.40 Some studies disclosed that the occurrence of free oil formation happens due to the presence of additional proteins in the processed cheese matrix, which decreases the ability of processed cheese to maintain an uniform emulsion of fat inside the protein matrix.41 The stability of processed cheese emulsions can be affected by pH. Process cheese with a higher pH had an open structure and therefore caused a weaker emulsion, resulting in oiling off.12 In our case, the control had a higher pH than the probiotic-processed cheese.
3.4 Textural attributes of control and probiotic processed cheeses
Textural attributes studied for control and probiotic processed cheeses include hardness, adhesiveness, springiness, cohesiveness, gumminess, chewiness, and resilience. The textural attributes of the control and probiotic cheeses are shown in Table 4.| Textural attributes | CPC | PPC |
|---|---|---|
| a Mean ± S.D. (n = 3); means with different superscripts within a row differ significantly (P < 0.05). | ||
| Hardness | 10.19 ± 0.000a | 9.337 ± 0.003b |
| Springiness | 0.37 ± 0.002 a | 0.20 ± 0.000 b |
| Cohesiveness | 0.24 ± 0.002 a | 0.34 ± 0.001 b |
| Gumminess | 2.48 ± 0.021 a | 3.14 ± 0.009 b |
| Chewiness | 0.92 ± 0.001a | 0.63 ± 0.001 b |
The hardness value of probiotic processed cheese was 9.327 N, which was significantly (p < 0.05) lower than that of the control (10.188 N). The higher hardness of the control cheese compared to its probiotic counterpart might be attributed to the addition of curd to the probiotic cheese, as curd typically possesses a weaker structure in comparison to rennet curd. Some researchers have reported that the incorporation of whey proteins in a process cheese formula may lead to an increased firmness.36,37 Similarly, the addition of phytosterols at 3% and 4% levels increased the firmness of processed cheese spreads at a significant (p < 0.05) level compared to the control.42 The increased firmness of the cheese spread might be due to the addition of free phytosterols, which were in powder form and occupied the free space present in the cheese spread. Further, the firmness of all the inulin-incorporated processed cheese spreads was significantly higher than that of the control.43 This might be due to the high water binding capacity and higher molecular weight of inulin.44 Further, the presence of MPC at a higher level in the matrix causes a significant increase in the hardness of processed cheese spread.45 In our case, the addition of curd caused an increment in moisture content, leading to reduced hardness. In a study incorporation of denatured whey proteins in the cheese had resulted higher moisture and lower hardness in processed cheese as compared to UF added processed cheese.46 Similarly, studies have associated the effect of the final pH of process cheese with its firmness.12 It was observed that with the increment of the final pH of the processed cheese from 5.0 to 6.2, its firmness initially improved. In our case, higher pH and hardness were observed in the control cheese. The springiness values for control and probiotic cheese were 0.372 and 0.201, respectively, showing a statistically significant difference (p < 0.05). The control had a chewiness value of 0.923, while the probiotic cheese had a chewiness value of 0.633. The chewiness of the probiotic processed cheese was significantly lower (p < 0.05) compared to the control; this might be due to the lower hardness of the probiotic cheese. Both springiness and chewiness are firmness-dependent parameters, resulting in higher values for control cheese compared to probiotic cheese, which is consistent with findings reported by El-Aidie et al.47 The gumminess values of the control and probiotic processed cheeses were 2.479 and 3.143, respectively. The higher gumminess values of probiotic cheese were owing to the higher cohesiveness values of probiotic cheese compared to control cheese. The cohesiveness values of the control and probiotic processed cheeses were 0.243 and 0.337, respectively. The cohesiveness value of the probiotic cheese was significantly (p < 0.05) higher than that of the control cheese. The disruption of the continuity of the protein matrix due to the addition of curds may lead to a softer texture, which might be the reason for the increased gumminess and cohesiveness of probiotic processed cheese. A similar result was found, in which an increment in gumminess and cohesiveness was observed in the case of the processed whey cheese.48 However, with the addition of phytosterols from 0 to 4%, a sharp, steady and significant (p < 0.05) decrease in the work of adhesion was reported in processed cheese spread.42 The decrease in the work of adhesion was due to the reduction in work needed to overcome the attractive force between the surface of the product and the surface of the probe as a result of the reduction in stickiness of the product and might also be due to weak gel formation as a consequence of the addition of insoluble phytosterols. However, increasing inulin levels caused a marginal decline in the work of adhesion.43
3.5 Rheological attributes of the control and probiotic processed cheeses
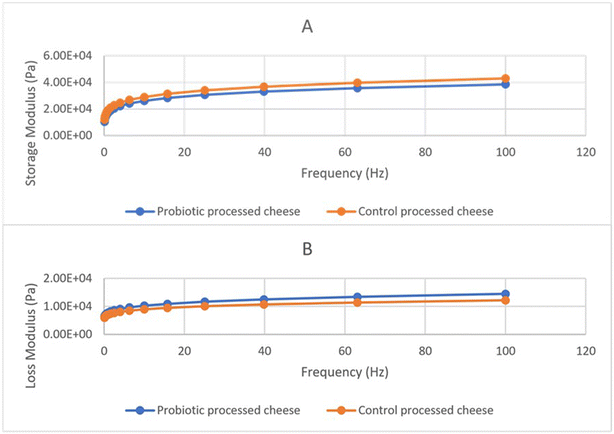 | ||
| Fig. 5 Rheological attributes of the control and probiotic processed cheese: (A) storage modulus and (B) loss modulus. | ||
This behaviour is typically associated with solid viscoelastic materials.49 The values observed for G′ and G″ of the probiotic cheese were lower than those observed for the hard cheese.50 A similar tendency of viscoelasticity was observed for processed cheese.46 Similarly, Lee et al.51 observed that G′ values of unheated processed cheese (at 20 °C) were higher than those of G″ with increasing frequency ranging from 0.01 to 10 Hz. Hence, the investigation of the above experiments matches our results and gives a clear idea of product behaviour. The control and probiotic processed cheeses exhibited a visco-elastic behaviour during the unmelted state at 20 °C. Further, the probiotic processed cheese had a lower elastic modulus (G′) than the control processed cheese, which might be attributed to lower hardness than the control processed cheese, as depicted in Fig. 5.
The probiotic processed cheese had a profile with an increase in G″ and G′ after the unique crossing-point, corresponding to the maximum value of tan δ observed at 65 °C, as shown in Fig. 6a. The viscous component G″ was greater than the elastic component G′. The pattern observed for the viscoelastic changes with temperature for processed cheese is very different from that for the other cheeses.52 This profile shows good meltability of the probiotic processed cheese, which is associated with enough fat emulsification. The control processed cheese had a profile with an increase in G″ and G′ after the unique crossing-point, corresponding to the maximum value of tan δ observed at 61 °C, as illustrated in Fig. 6b. The viscous component G″ was higher than the elastic component G′. The pattern of viscoelastic changes with temperature is the same for both cheeses, i.e., the probiotic processed and control processed cheeses.
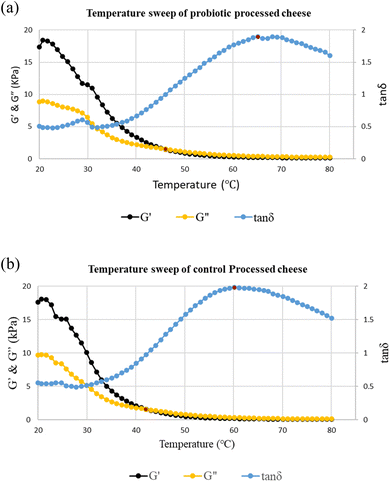 | ||
| Fig. 6 (a): Temperature sweep of probiotic processed cheese. (b): Temperature sweep of the control processed cheese. | ||
In our study, a decline in the G′ was observed with an increment of temperature from 20 to 90 °C for the control and probiotic processed cheeses, indicating the loosening of elasticity of the cheese matrix as the temperature increased. This indicates the breaking of protein–protein interactions inside a casein network as well as the liquefaction of fat together with the distortion of fat globules, which had plasticized in the protein matrix and allowed it to flow.53 The fat completely melted at 40 °C;54 thus, an increment was observed in tan δ in the control and probiotic samples Fig. 6. An increase in tan δ was observed with an increment in temperature after 40 °C, exhibiting a melt-like behaviour. At this temperature, protein softening started in the cheese matrix due to the decline of specific casein–casein interactions in the structure.54,55
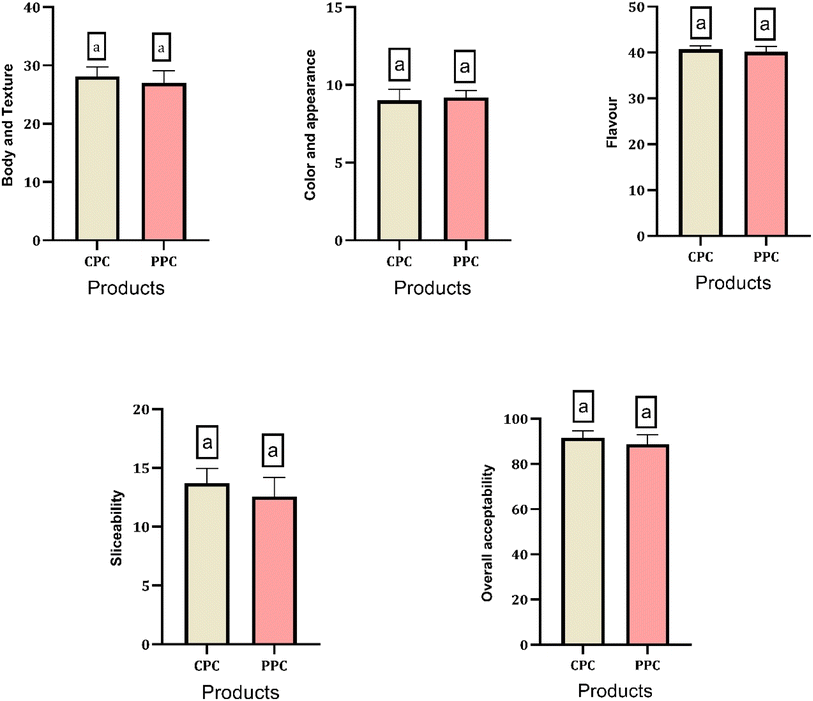
3.6 Sensory quality of products
Sensory evaluation is an important parameter for the acceptability of processed cheese. The body and texture, slicebality and overall acceptability scores were lower for probiotic processed cheese compared to the control. However, the color and flavour scores obtained for probiotic processed cheese were higher than the control. However, there were non-significant differences observed in all sensory parameters between the control processed cheese and the probiotic processed cheese (Fig. 7). Several authors have studied the effects of the incorporation of probiotic organisms into varieties of cheeses. The addition of probiotic Bacillus coagulans spores in the processed cheese non-significantly affected the sensory attributes of the final product.56 Probiotic Minas fresh cheese containing probiotic bacteria L. paracasei subsp. paracasei had a better sensorial quality compared to the control cheese.57 The effect of the addition of whey protein in the process cheese matrix has been extensively studied on its sensory qualities, which exhibited similar or enhanced sensory quality in the final product.36,38,55 In our case, fermented milk had been incorporated into the probiotic processed cheese, which had contributed towards an improved flavour of the probiotic processed cheese. Fermentation of milk is known for the production of several significant aromatic and flavour compounds as metabolites.583.8 Colour profile for control and probiotic processed cheese
Colour is considered an important parameter for the sensory acceptability of a product.59 In colour measurement, “L*” value represents lightness (100) and blackness (0); “a*” represents red (+ve) to green (−ve) hues, while “b*” represents yellow (+ve) to blue (−ve) hues.60 The L* values for control and probiotic processed cheeses were 70.87 and 71.07, respectively, as shown in Table 5. Both cheeses had statistically similar L* values (p > 0.05). The probiotic processed cheese was whiter than the control one, which could be attributed to the incorporation of probiotic curd. The ability of protein particles to scatter light in the visible spectrum can lead to a whitening effect, thereby attributing the product's whiteness to the protein matrix.14 The respective a* values for the control and probiotic cheeses were 1.19 and 1.20, respectively. The a* values of the probiotic and control processed cheeses were not statistically significant from each other (p > 0.05). The b* values for control and probiotic processed cheeses were statistically non-significant (p > 0.05) and 24.91 and 25.83, respectively. The higher yellowness of the probiotic cheese (Table 5) might be attributed to the incorporation of probiotic curd prepared from cow milk.61| Colour parameters | L* | a* | b* | |||
|---|---|---|---|---|---|---|
| Cheeses | CPC | PPC | CPC | PPC | CPC | PPC |
| a Mean ± S.D. (n = 3); means with different superscripts within a row differ significantly (p < 0.05). | ||||||
| Colour values | 70.87 ± 0.33a | 71.07 ± 0.40a | 1.19 ± 0.03a | 1.20 ± 0.06b | 24.91 ± 0.04a | 25.83 ± 0.03a |
3.9 Bio-functional activities of the control and probiotic processed cheeses
Control and probiotic processed cheeses were evaluated for bio-functional activities, viz., in vitro ACE-inhibitory and antioxidant activity.3.10 Viability of probiotic organisms in process cheese
The probiotic count was found to be 7.66 log CFU g−1 in fresh probiotic processed cheese. There was a significant (p < 0.05) increase in probiotic count during refrigerated storage (Fig. 9). The probiotic count was 9 log CFU g−1 on the 20th day of refrigerated storage; thereafter, the count decreased to 8.19 log CFU g−1 at the 35th day of refrigerated storage at 4 °C in PP packaging material. A population of 106–107 CFU g−1 in the final product was reported to be sufficient and effective to consider a product as a probiotic one.71 Several researchers have reported various stabilities of various probiotic organisms in different cheese matrices. An increment in L. plantarum96 was reported in the experimentally manufactured semi-hard cheese with low-cooking curd during a 180-day maturation period, with a final count of 7.39 log CFU g−1.72 The initial count of L. acidophilus La 05 reduced significantly (p < 0.05) from 7.87 log CFU g−1, to 7.6 log CFU g−1 at the end of the 12 days of refrigerated storage in buffalo milk Ricotta cheese.14 Madureira et al.73 and Meira et al.24 observed an increment in probiotic count in Ricotta cheese during refrigerated storage. It was observed that the L. acidophilus Ki strain increased from 7.15 log CFU g−1 to 9.39 log CFU g−1 in the plain whey cheese matrix at the end of 28 days of storage at 7 °C.73 However, the respective organism increased from 7.06 log CFU g−1 to 7.97 log CFU g−1 in the salt-added whey cheese matrix at 28 days of storage at 7 °C.73 The count of L. acidophilus was increased in goat Ricotta cheese matrix from 6.01 ± 0.6 log CFU g−1 to 6.29 ± 0.9 log CFU g−1 at 7 days of refrigerated storage.244 Conclusion
Processed cheese is a popular variety of cheese that is mainly devoid of any live microorganisms due to its production process. The current study attempts to develop probiotic processed cheese through the incorporation of fermented milk containing live lactobacillus spp. in processed cheese emulsion at 60 °C temperature. Probiotic processed cheese exhibited 46% moisture, 28% fat, and 19% protein, with a probiotic count of 7.66 log CFU g−1. The product had acceptable sensory properties that were comparable to those of the control product. The product had lower meltability, hardness and cohesiveness compared to control cheese. Control and probiotic processed cheeses exhibited a visco-elastic behaviour during the unmelted state at 20 °C. However, the probiotic processed cheese had a lower elastic modulus (G′) than the control processed cheese, which might be attributed to the lower hardness of the probiotic processed cheese. The probiotic processed cheese exhibited significantly higher ACE-inhibitory and antioxidant properties as observed through ABTS and DPPH studies. The reason might be that the probiotic fermentation of milk during curd production generated bioactive compounds, which resulted in enhanced bio-activity of probiotic processed cheese. The product had a probiotic count of 7.66 log CFU g−1 in fresh, which increased significantly (p < 0.05) up to the 20th day of refrigerated storage, followed by a decline. Probiotic viability was maintained at more than 8 log CFU g−1 in the processed cheese matrix during 35 days of refrigerated storage.This approach could enhance the functional attributes of processed cheese with enhanced consumer acceptability and market demand. Further, this approach contributes to sustainable food innovation by enhancing processed cheese with health promoting properties without compromising shelf stability or processing efficiency.
Conflicts of interest
The authors declare that they have no conflicts of interest.Data availability
All data supporting the findings of this study are available from the corresponding author upon reasonable request.Acknowledgements
The authors are thankful to the Director, ICAR-NDRI, Karnal, for the funding support for this project.References
- M. Alongi and M. Anese, J. Funct. Foods, 2021, 81, 104466 CrossRef.
- G. Reid, A. A. Gadir and R. Dhir, Front. Microbiol., 2019, 10, 444972 CrossRef PubMed.
- M. Kechagia, D. Basoulis, S. Konstantopoulou, D. Dimitriadi, K. Gyftopoulou, N. Skarmoutsou and E. M. Fakiri, ISRN Nutr., 2013, 2013, 1–7 CrossRef PubMed.
- R. Nagpal, H. Yadav, A. K. Puniya, K. Singh, S. Jain and F. Marotta, Int. J. Probiotics Prebiotics, 2007, 2, 75 Search PubMed.
- J. Alard, V. Peucelle, D. Boutillier, J. Breton, S. Kuylle, B. Pot, S. Holowacz and C. Grangette, Benefic. Microbes, 2018, 9, 317–331 CAS.
- N. K. Ganguly, S. K. Bhattacharya, B. Sesikeran, G. B. Nair, B. S. Ramakrishna, H. P. S. Sachdev and R. Hemalatha, Indian J. Med. Res., 2011, 134, 22–25 Search PubMed.
- R. Karimi, A. M. Mortazavian and A. G. Da Cruz, Dairy Sci. Technol., 2011, 91, 283–308 CrossRef.
- J. M. Castro, M. E. Tornadijo, J. M. Fresno and H. Sandoval, BioMed Res. Int., 2015, 2015, 1–11 CrossRef PubMed.
- A. R. Madureira, M. Amorim, A. M. Gomes, M. E. Pintado and F. X. Malcata, Food Res. Int., 2011, 44, 465–470 CrossRef CAS.
- A. Kasimoǧlu, M. Göncüoǧlu and S. Akgün, Int. Dairy J., 2004, 14, 1067–1073 CrossRef.
- L. Abadía-García, A. Cardador, S. T. Martín del Campo, S. M. Arvízu, E. Castaño-Tostado, C. Regalado-González, B. García-Almendarez and S. L. Amaya-Llano, Int. Dairy J., 2013, 33, 191–197 CrossRef.
- R. Kapoor and L. E. Metzger, Compr. Rev. Food Sci. Food Saf., 2008, 7, 194–214 CrossRef CAS.
- R. Silva, T. C. Pimentel, F. Eustáquio de Matos Junior, E. A. Esmerino, M. Q. Freitas, C. S. Fávaro-Trindade, M. C. Silva and A. G. Cruz, Food Biosci., 2022, 46, 101517 CrossRef CAS.
- B. Sameer, S. Ganguly, Y. Khetra and L. Sabikhi, LWT, 2020, 121, 108944 CrossRef CAS.
- X. Liu, C. P. Champagne, B. H. Lee, J. I. Boye and M. Casgrain, Biotechnol. Res. Int., 2014, 2014, 1–21 CrossRef PubMed.
- Z. Guo, J. Wang, L. Yan, W. Chen, X. ming Liu and H. ping Zhang, LWT–Food Sci. Technol., 2009, 42, 1640–1646 CrossRef CAS.
- FSSAI, FSSAI Annual Report 2020-2021, New Delhi, 2021 Search PubMed.
- IS: SP 18, Part XI, ISI Handbook of Food Analysis. Part XI. Dairy Products, Indian Standards Institution, New Delhi, 1981, p. 43 Search PubMed.
- Association of Official Analytical Chemists (AOAC), Official Methods of Analysis of the Association of Analytical Chemists, 18th edn, AOAC, Gaithersburg, MD, USA, 2005 Search PubMed.
- S. Gunasekaran and M. M. Ak, Cheese Rheology and Texture, CRC Press, 2002 Search PubMed.
- H.-H. Wang and D.-W. Sun, J. Food Eng., 2004, 61, 47–55 CrossRef.
- N. Shirashoji, J. J. Jaeggi and J. A. Lucey, J. Dairy Sci., 2006, 89, 15–28 CrossRef CAS PubMed.
- A. Giri, S. K. Kanawjia and M. P. Singh, J. Food Sci. Technol., 2017, 54, 2443–2451 CrossRef CAS PubMed.
- Q. G. S. Meira, M. Magnani, F. C. de Medeiros Júnior, R. de, C. R. do E. Queiroga, M. S. Madruga, B. Gullón, A. M. P. Gomes, M. M. E. Pintado and E. L. de Souza, Food Res. Int., 2015, 76, 828–838 CrossRef CAS PubMed.
- C. N. Kuchroo and P. F. Fox, Milchwissenschaft, 1982, 37, 331–335 CAS.
- B. Hernández-Ledesma, B. Miralles, L. Amigo, M. Ramos and I. Recio, J. Sci. Food Agric., 2005, 85, 1041–1048 CrossRef.
- B. Hernández-Ledesma, P. J. Martín-Álvarez and E. Pueyo, J. Agric. Food Chem., 2003, 51, 4175–4179 CrossRef PubMed.
- Q. G. S. Meira, M. Magnani, F. C. de Medeiros Júnior, R. de, C. R. do E. Queiroga, M. S. Madruga, B. Gullón, A. M. P. Gomes, M. M. E. Pintado and E. L. de Souza, Food Res. Int., 2015, 76, 828–838 CrossRef CAS PubMed.
- J. K. Kaushik, A. Kumar, R. K. Duary, A. K. Mohanty, S. Grover and V. K. Batish, PLoS One, 2009, 4, e8099 CrossRef PubMed.
- M. De Angelis, R. Di Cagno, C. Huet, C. Crecchio, P. F. Fox and M. Gobbetti, Appl. Environ. Microbiol., 2004, 70, 1336–1346 CrossRef CAS PubMed.
- E. Parente, F. Ciocia, A. Ricciardi, T. Zotta, G. E. Felis and S. Torriani, Int. J. Food Microbiol., 2010, 144, 270–279 CrossRef CAS PubMed.
- M. De Angelis and M. Gobbetti, in Stress Responses of Lactic Acid Bacteria, ed. E. Tsakalidou and K. Papadimitriou, Springer US, New York, 2011, pp. 219–249 Search PubMed.
- S. Ehsannia and M. R. Sanjabi, J. Food Sci. Technol., 2016, 53, 996–1003 CrossRef CAS PubMed.
- K. Muthukumarappan, Y.-C. Wang and S. Gunasekaran, J. Dairy Sci., 1999, 82, 1068–1071 CrossRef CAS.
- D. Chaudhary, C. T. Suresh, Y. Khetra, G. S. Meena and S. Hossain, J. Food Sci. Technol., 2023, 60, 600–608 CrossRef CAS PubMed.
- S. Mleko and E. A. Foegeding, Milchwissenschaft, 2000, 55, 513–516 CAS.
- I. Laye, T. R. Lindstrom, L. Lowry, F. I. Mei, M. Zwolfer, O. Diaz-Castillo, J. Dolande, S. Havlik and V. Rueda, U.S. Patent, 2004126473, 2004 Search PubMed.
- S. Mleko and E. A. Foegeding, Milchwissenschaft, 2001, 56, 612–615 CAS.
- S. K. G. Purna, A. Pollard and L. E. Metzger, J. Dairy Sci., 2006, 89, 2386–2396 CrossRef CAS PubMed.
- E. S. A. Farahat, A. G. Mohamed, M. M. El-Loly and W. A. M. S. Gafour, Food Biosci., 2021, 42, 101128 CrossRef CAS.
- H.-P. Bachmann, Int. Dairy J., 2001, 11, 505–515 CrossRef CAS.
- A. Giri, S. K. Kanawjia and A. Rajoria, Food Chem., 2014, 157, 240–245 CrossRef CAS PubMed.
- A. Giri, S. K. Kanawjia and Y. Khetra, Food Bioprocess Technol., 2014, 7, 1533–1540 CrossRef CAS.
- F. C. A. Buriti, I. A. Castro and S. M. I. Saad, Food Chem., 2010, 123, 1190–1197 CrossRef CAS.
- P. Salunke and L. E. Metzger, Int. Dairy J., 2022, 128, 105324 CrossRef CAS.
- N. S. Joshi, R. P. Jhala, K. Muthukumarappan, M. R. Acharya and V. V. Mistry, Int. J. Food Prop., 2004, 7, 519–530 CrossRef.
- S. A. M. El-Aidie, A. M. Mabrouk, A. R. Abd-Elgawad and H.-E. M. El- Garhi, Biocatal. Agric. Biotechnol., 2023, 51, 102798 CrossRef CAS.
- S. E. Chatziantoniou, A. S. Thomareis and M. G. Kontominas, Eur. Food Res. Technol., 2015, 241, 737–748 CrossRef CAS.
- T. Kahyaoglu and S. Kaya, Int. Dairy J., 2003, 13, 867–875 CrossRef CAS.
- E. B. Muliawan and S. G. Hatzikiriakos, Int. Dairy J., 2007, 17, 1063–1072 CrossRef.
- S. K. Lee, M. Huss, H. Klostermeyer and S. G. Anema, Int. Dairy J., 2013, 32, 79–88 CrossRef CAS.
- J. M. Reparet and Y. Noël, Lait, 2003, 83, 321–333 CrossRef.
- C. Dularia, G. S. Meena, S. Hossain, Y. Khetra and S. Arora, Food Hydrocoll., 2023, 142, 108842 CrossRef CAS.
- C. A. Brickley, M. A. E. Auty, P. Piraino and P. L. H. McSweeney, J. Food Sci., 2007, 72, C483–C490 CrossRef CAS PubMed.
- J. A. Lucey, M. E. Johnson and D. S. Horne, J. Dairy Sci., 2003, 86, 2725–2743 CrossRef CAS PubMed.
- S. Ehsannia and M. R. Sanjabi, J. Food Process. Preserv., 2016, 40, 667–674 CrossRef CAS.
- F. C. A. Buriti, J. S. da Rocha, E. G. Assis and S. M. I. Saad, LWT–Food Sci. Technol., 2005, 38, 173–180 CrossRef CAS.
- W. Engels, J. Siu, S. van Schalkwijk, W. Wesselink, S. Jacobs and H. Bachmann, Foods, 2022, 11, 1005 CrossRef CAS PubMed.
- M. P. Dutra, P. C. Palhares, J. R. O. Silva, I. P. Ezequiel, A. L. S. Ramos, J. R. O. Perez and E. M. Ramos, Small Rumin. Res., 2013, 115, 56–61 CrossRef.
- T. Sanz, A. Salvador, A. Jiménez and S. M. Fiszman, Eur. Food Res. Technol., 2008, 227, 1515–1521 CrossRef CAS.
- E. Šertović, Z. Sarić, M. Barać, I. Barukčić, A. Kostić and R. Božanić, Food Technol. Biotechnol., 2019, 57, 461–471 CrossRef PubMed.
- L. Ong, A. Henriksson and N. P. Shah, Int. Dairy J., 2007, 17, 67–78 CrossRef CAS.
- M. Shakerian, S. H. Razavi, S. A. Ziai, F. Khodaiyan, M. S. Yarmand and A. Moayedi, J. Food Sci. Technol., 2015, 52, 2428–2433 CrossRef CAS PubMed.
- O. N. Donkor, A. Henriksson, T. K. Singh, T. Vasiljevic and N. P. Shah, Int. Dairy J., 2007, 17, 1321–1331 CrossRef CAS.
- T. Virtanen, A. Pihlanto, S. Akkanen and H. Korhonen, J. Appl. Microbiol., 2007, 102, 106–115 CrossRef CAS PubMed.
- A. Gupta, B. Mann, R. Kumar and R. B. Sangwan, Int. J. Dairy Technol., 2009, 62, 339–347 CrossRef CAS.
- Y. Wang, Y. Wu, Y. Wang, H. Xu, X. Mei, D. Yu, Y. Wang and W. Li, Nutrients, 2017, 9, 521 CrossRef PubMed.
- N. Gjorgievski, J. Tomovska, G. Dimitrovska, B. Makarijoski and M. A. Shariati, J. Hyg. Eng. Des., 2014, 8, 88–92 Search PubMed.
- J.-W. Yoon, S.-I. Ahn, J.-W. Jhoo and G.-Y. Kim, Food Sci. Anim. Resour., 2019, 39, 162–176 CrossRef PubMed.
- B. N. P. Sah, T. Vasiljevic, S. McKechnie and O. N. Donkor, Crit. Rev. Food Sci. Nutr., 2018, 58, 726–740 CrossRef CAS PubMed.
- A. Talwalkar, C. W. Miller, K. Kailasapathy and M. H. Nguyen, Int. J. Food Sci. Technol., 2004, 39, 605–611 CrossRef CAS.
- V. Lovayová, E. Dudriková, K. Rimárová and L. Siegfried, J. Food Sci. Technol., 2015, 52, 4697–4702 CrossRef PubMed.
- A. R. Madureira, M. S. Gião, M. E. Pintado, A. M. P. Gomes, A. C. Freitas and F. X. Malcata, J. Food Sci., 2006, 70, M160–M165 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |