 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sustainable development of dairy-based functional beverages enriched with bee pollen: a circular economy approach
Eliara Acipreste
Hudson
ab,
Herlândia Cotrim
Santos
a,
Laís Fernanda
Batista
a,
Jaqueline de Paula
Rezende
c,
Kely de Paula
Correa
d,
Izabela Maria Montezano
de Carvalho
e,
Márcia Cristina Teixeira Ribeiro
Vidigal
a and
Ana Clarissa dos Santos
Pires
 *a
*a
aApplied Molecular Thermodynamic Group (THERMA), Food Technology Department, Federal University of Viçosa, Av. PH Rolfs, s/n, 36570-900, Viçosa, MG, Brazil. E-mail: ana.pires@ufv.br
bFood Science and Technology Department, Federal Institute of Southeast of Minas Gerais, 36180-000, Rio Pomba, MG, Brazil
cFood Science Department, Federal University of Lavras, Trevo Rotatório Professor Edmir Sá Santos, s/n, 37203-202, Lavras, MG, Brazil
dCândido Tostes Dairy Institute, Agricultural Company of Minas Gerais (EPAMIG), Lieutenant Luiz de Freitas, 116, 36045-560, Juiz de Fora, MG, Brazil
eLaboratory of Food Analysis, Department of Nutrition and Health, Federal University of Viçosa, Av. PH Rolfs, s/n, 36570-900, Vicosa, MG, Brazil
First published on 3rd October 2025
Abstract
The growing demand for sustainable and functional foods has driven the development of novel formulations that both valorize dairy co-products and incorporate functional ingredients. Six fermented dairy beverages were developed by partially replacing skim milk (0–75%, w/w) with buttermilk and/or sweet cheese whey. The formulations were enriched with 1% bee pollen, banana, and honey, fermented using a commercial yogurt culture, and stored at 4 °C. We evaluated the proximate composition, pH and titratable acidity, water-holding capacity (WHC) and syneresis, antioxidant activity, colloidal stability (Turbiscan Stability Index – TSI), rheological behavior, texture, and sensory properties. All formulations exhibited similar acidification profile during storage (pH change from 4.45 to 4.15 and acidity from 0.82 to 0.92% lactic acid over 30 days), indicating unaltered lactose fermentation despite milk replacement. Formulations containing 37.5% of buttermilk or whey achieved higher WHC (>60%) and lower syneresis (∼9–10%), correlating with low TSI values (<0.9) and pseudoplastic rheology. Textural analyses showed that the same formulations achieved a balance between gel strength and cohesiveness, without compromising adhesiveness or elasticity. Antioxidant assays revealed that 75% buttermilk samples exhibited the greatest radical-scavenging activity (DPPH: 72.5 ± 2.1%; ABTS: 63.8 ± 1.9%), underscoring the influence of polar lipids on functionality. Sensory tests pointed to the high acceptance of moderate-replacement samples, linked to creamy, sweet, yogurt-like attributes, whereas 75% whey samples scored lower due to bitterness. Overall, moderate substitution with buttermilk or whey, combined with bee pollen enrichment, produces stable, antioxidant-rich, and consumer-acceptable beverages, supporting functional food development and the circular economy in the dairy sector.
Sustainability spotlightThe dairy milk sector plays a key role in providing nutritious food worldwide; however, it is also one of the major contributors to environmental impact. Therefore, it is strategic to ensure that the natural resources used in milk and dairy production are utilized to their fullest potential. In developed countries, whey and buttermilk are commonly reused as food ingredients; however, in developing countries, large amounts of whey and, particularly, buttermilk are underutilized and discarded. This work proposes a simple and sustainable strategy to enhance the valorization of these dairy co-products without compromising product quality. Using a constrained mixture design approach, we partially replaced milk with whey, buttermilk, or their combination to produce a functional fermented dairy beverage enriched with bee pollen. Our results demonstrated that up to 37.5% of milk substitution improved the antioxidant activity and physicochemical properties (water holding capacity, syneresis, and colloidal stability), while rheology, texture, and sensory characteristics remained comparable to the control beverage made exclusively with milk. This approach supports the following UN sustainable development goals: zero hunger (SDG 2); good health and well-being (SDG 3); industry, innovation, and infrastructure (SDG 9); responsible consumption and production (SDG 12); and climate action (SDG 13). |
1 Introduction
The growing global demand for sustainable food systems has driven the development of innovative technologies focused on safety, nutritional functionality, and resource efficiency.1 In this scenario, the valorization of agro-industrial by-products, particularly their reintegration into new food products, emerges as a key strategy aligned with circular economy principles.2 Among these by-products, those derived from the dairy industry, such as whey and buttermilk, stand out due to their underutilization, particularly in developing countries, despite their high nutritional and techno-functional values.Previous studies have investigated the application of fluid whey or buttermilk in fermented dairy beverages. A study evaluating strawberry-flavored yogurts and whey-based beverages found that fermented whey beverages were also well accepted by consumers. Fermented samples were preferred over nonfermented ones, which were perceived as less acidic, less viscous, and overly sweet.3 In a study conducted by Santos et al.,4 low-fat fermented formulations such as “Greek-type yogurt” containing up to 50% buttermilk exhibited reduced syneresis, enhanced water-holding capacity, and acceptable sensory properties compared to formulations using 100% skim milk. However, higher buttermilk ratios (>50%) adversely affect the flavor and aroma of the beverages.
Although both whey and buttermilk are nutritious by-products, they differ in composition and techno-functional properties. Whey is rich in lactose, minerals, and whey protein, providing excellent solubility and gelation characteristics. In contrast, buttermilk contains significant amounts of phospholipids (up to 15 times more than whole milk), which contribute to its superior emulsifying and stabilizing properties.5 Despite their complementary functionalities, the combined use of whey and buttermilk remains scarcely explored, yet it holds promise for enhancing the functional and technological quality of dairy formulations.
De Bassi et al.6 developed fermented beverages by blending whey (30%) and buttermilk (30%) with milk (70%), followed by the addition of strawberry puree after fermentation. All formulations achieved comparable pH, acidity, viscosity, and consumer acceptance scores. Although the study demonstrated that combining whey and buttermilk in a fermented matrix is both feasible and well accepted, it focused solely on basic physicochemical parameters (pH, acidity, and viscosity), microbial viability, and overall liking. It did not explore the impact of milk replacement by co-products on key properties of dairy-fermented beverages, such as rheology, texture, and stability.
Being rich in proteins, bioactive peptides, lactose, lipids, and micronutrients, whey and buttermilk represent promising raw materials for the development of functional beverages.7,8 To further enhance their nutritional profile, these dairy by-products can be combined with other bioactive-rich ingredients. Bee pollen is a nutrient-dense natural ingredient rich in phenolic compounds, proteins, essential amino acids, vitamins, and minerals, which contribute to its recognized antioxidant and immunomodulatory properties.9,10 Its incorporation into fermented dairy beverages based on whey and buttermilk represents a promising strategy to enhance the nutritional and functional values of these formulations, aligning with current demands for health-promoting and sustainable food products. In the hive, bees naturally transform pollen into bee bread through a lactic acid fermentation process. This conversion increases nutrient bioavailability and enriches the product with additional bioactive metabolites, making bee bread even more biologically active than raw pollen.11,12 Studies have demonstrated its antioxidant, immunomodulatory, and anti-inflammatory properties, highlighting its relevance in nutraceutical applications.13 While the nutraceutical relevance of bee bread is growing, the present study focused exclusively on bee pollen, which is more widely available, standardized, and already applied in food formulations, making it a suitable candidate for the development of dairy-based functional beverages.
However, despite its bioactive potential, the addition of bee pollen to dairy beverages may present some challenges, such as undesirable sensory impacts (e.g., residual taste or texture alterations) and possible changes in the physicochemical properties and stability of the product, which require further investigation. Therefore, this study aimed to develop a sustainable and functional dairy beverage by fermenting a mixture of milk, whey, and buttermilk enriched with bee pollen. The investigation included the characterization of physicochemical and rheologic properties, evaluation of antioxidant capacity of the beverages, and a detailed sensory analysis, enabling comprehensive product characterization. This approach supports the valorization of dairy by-products and bioactive ingredients for the development of innovative and sustainable functional foods.
2 Materials and methods
2.1. Materials
The fermented dairy beverages were prepared using UHT skimmed milk, skimmed milk powder, sucrose, buttermilk, sweet cheese whey, honey, banana, and dehydrated bee pollen. Buttermilk and cheese whey were kindly provided by the Dairy School “Produtos Viçosa” (Viçosa, Minas Gerais, Brazil). The dehydrated multifloral bee pollen and honey were acquired from ApisFlora (São Paulo, SP, Brazil). All other ingredients, including UHT skimmed milk, skimmed milk powder, bananas, and sucrose, were purchased locally. The UHT skimmed milk contained 4.8% lactose, with a pH of 6.7 and titratable acidity of 0.15 g lactic acid/100 g. The buttermilk presented 4.5% lactose, with a pH of 7.02 and titratable acidity of 0.05 g lactic acid/100 g, while sweet cheese whey contained 5.0% lactose, with a pH of 6.17 and titratable acidity of 0.12% g lactic acid/100 g. A commercial freeze-dried direct vat set (DVS) starter culture (YoFlex® YF-L903, Chr. Hansen Indústria e Comércio Ltda., Brazil), containing Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus, was used to initiate fermentation.Sodium hydroxide and potassium persulfate were of analytical grade (Vetec, Brazil); 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) reagents and methanol and ethanol solvents were purchased from Sigma-Aldrich.
2.2. Experimental design and formulation development
The fermented dairy beverage formulations were developed using a constrained mixture design approach based on established methods,14,15 with component boundaries defined as follows: skimmed milk (M), 25–100%; buttermilk (B), 0–75%; and whey (W) 0–75%. To capture the effects of varying dairy component proportions, six representative formulations were designed: one pure skimmed milk control (M100), two extreme binary vertices (MB75 and MW75), two intermediate binary mixtures (MB37.5 and MW37.5), and one modified ternary centroid (MBW). This design enabled comprehensive evaluation of both individual and synergistic effects of dairy component substitution while respecting technological feasibility limits observed in preliminary trials. The coding system reflects each formulation's milk-replacement strategy, where the numerical values represent the percentage of the ingredient replacing milk (e.g., MB37.5 = 37.5% buttermilk replacing skimmed milk, with the remaining 62.5% being skimmed milk). MBW refers to the constrained ternary blend (25% skimmed milk + 37.5% buttermilk + 37.5% whey). The complete mixture proportions for all experimental formulations are presented in Table 1.| Formulation code | Skimmed milk (%) | Buttermilk (%) | Whey (%) |
|---|---|---|---|
| MB37.5 | 62.5 | 37.5 | 0.0 |
| MW37.5 | 62.5 | 0.0 | 37.5 |
| MW75 | 25.0 | 0.0 | 75.0 |
| MBW | 25.0 | 37.5 | 37.5 |
| M100 | 100.0 | 0.0 | 0.0 |
| MB75 | 25.0 | 75.0 | 0.0 |
The protein and fat contents varied significantly across dairy ingredients. Skimmed milk had the highest protein level (3.0% w/w) with 0.5% (w/w) fat. In contrast, buttermilk and whey contained 0.6% (w/w) fat each but differed in protein content (1.2% (w/w) and 0.7% (w/w), respectively). All dairy base blends (skimmed milk, buttermilk, and whey in varying proportions) were combined with honey and skimmed milk powder to standardize the protein concentration (5% w/w) at all formulations. The mixture was then pasteurized (72 °C/15 s), cooled to 42 °C, and inoculated with 0.2% freeze-dried starter culture (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). Fermentation was carried out at 42 °C until the pH reached 4.6 ± 0.1.4 Each formulation was then supplemented with 1% (w/w) bee pollen flour (ground to <500 μm), 10% (w/w) honey, and 20% (w/w) banana purée. The mixtures were homogenized using a commercial blender at high speed for 3 minutes to ensure complete integration of all ingredients.
1 ratio). Fermentation was carried out at 42 °C until the pH reached 4.6 ± 0.1.4 Each formulation was then supplemented with 1% (w/w) bee pollen flour (ground to <500 μm), 10% (w/w) honey, and 20% (w/w) banana purée. The mixtures were homogenized using a commercial blender at high speed for 3 minutes to ensure complete integration of all ingredients.
Since bee pollen can influence the growth and metabolism of lactic acid bacteria,16 it was added after the fermentation process to minimize potential effects on fermentation kinetics, proteolysis, and EPS production by lactic acid bacteria.
Preliminary sensory testing showed that 1% was the highest concentration of bee pollen that remained acceptable to consumers while still offering functional benefits previously reported in dairy systems.17,18 Additionally, banana purée was added to enhance the aroma and help mask the strong taste of the bee pollen.
2.3. Centesimal composition of the fermented dairy beverages
The centesimal composition of the developed dairy beverages was determined using standard proximate analysis methods. Moisture content was measured by drying the samples at 105 °C until a constant weight was reached (AOAC 925.10). Protein content was determined using the Kjeldahl method with a nitrogen conversion factor of 6.38 applied to milk proteins (AOAC 991.20). Fat content was determined by the Soxhlet method (AOAC 989.05), while carbohydrate content was calculated by difference, subtracting the sum of moisture, protein, fat, and ash from 100%. Ash content was determined by incinerating the samples at 550 °C to constant weight (AOAC 923.03). All analyses were performed in triplicate to ensure reliability, and the results were expressed as percentage values on a dry weight basis.2.4. pH and titratable acidy determination
The pH of the dairy beverage samples was measured using a digital pH meter (Bell Engineering, model PHS3BW, UK), calibrated with standard pH 4.0 and 7.0 buffer solutions (AOAC 981.12). Measurements were conducted in triplicate under gentle stirring to ensure homogeneity, and results were recorded in logarithmic units.Titratable acidity was assessed via acid–base titration, following standard procedures (AOAC 947.05). Briefly, 10 mL of the homogenized sample was diluted with 10 mL of distilled water in an Erlenmeyer flask, and 3 drops of 1% phenolphthalein indicator were added. The solution was titrated with 0.1 N sodium hydroxide (NaOH) until a faint pink endpoint persisted for 30 seconds. Acidity was expressed as percent lactic acid (% w/v), calculated as eqn (1):
 | (1) |
2.5. Determination of water-holding capacity and syneresis
The stability of the fermented dairy beverages during refrigerated storage (5 °C) was monitored over 15 days by assessing their water-holding capacity (WHC) and syneresis on days 0, 3, 6, 9, 12, and 15. The procedures were adapted from Ge et al.19 and Mizuta et al.20 To evaluate the WHC, approximately 20 g of each sample were placed in centrifuge tubes and spun at 4500×g for 10 minutes at 4 °C using a refrigerated centrifuge (Heraeus Fresco 21, Thermo Fisher Scientific, Germany). After centrifugation, the amount of liquid released from the matrix was measured. The WHC was expressed as the percentage of retained moisture relative to the original mass of the sample, with higher values indicating greater stability.For syneresis analysis, 10 g of each sample were subjected to centrifugation at 500×g for 20 minutes at 4 °C. The volume of liquid separated from the gel structure was collected and weighed. The results were expressed as the percentage of liquid separated, with higher values indicating greater instability. All measurements were performed in triplicate, and results were reported as mean values with standard deviations.
2.6. Antioxidant activity assays
To assess the antioxidant capacity of dairy beverages, two complementary methods, DPPH and ABTS, were employed to evaluate distinct mechanisms of action associated with the bioactive compounds present in the matrix.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (w/v) and centrifuged at 4000×g for 20 minutes at 4 °C. The resulting clear supernatant was used for the assay. A fresh 0.1 mM DPPH solution in methanol was prepared daily. For the reaction, 0.5 mL of the sample supernatant was mixed with 1.5 mL of methanol and 1.0 mL of the DPPH solution. After a 30-minute incubation in the dark at 25 °C, absorbance was measured at 517 nm. Antioxidant activity was calculated using the standard equation (eqn (2)), and all measurements were performed in triplicate.
2 ratio (w/v) and centrifuged at 4000×g for 20 minutes at 4 °C. The resulting clear supernatant was used for the assay. A fresh 0.1 mM DPPH solution in methanol was prepared daily. For the reaction, 0.5 mL of the sample supernatant was mixed with 1.5 mL of methanol and 1.0 mL of the DPPH solution. After a 30-minute incubation in the dark at 25 °C, absorbance was measured at 517 nm. Antioxidant activity was calculated using the standard equation (eqn (2)), and all measurements were performed in triplicate.| Inhibition (%) = ((A0 − As)/A0) × 100 | (2) |
2.7. Kinetics of destabilization
Sample stability was monitored through dynamic near-infrared (NIR) backscatter measurements. Formulations were loaded into 15 mL borosilicate tubes and subjected to controlled storage (4 °C ± 0.5 °C). A Turbiscan MA 2000 dispersion analyzer (Formulation Smart Scientific Analysis, France) equipped with a pulsed 880 nm diode laser acquired backscatter profiles for 60 min, with data collected at 15-min intervals (T0.5, T15, T30, T45, and T60). The Turbiscan Stability Index (TSI) served as a composite metric for system instability, derived from the integrated absolute difference between the baseline and time-point scans (eqn (3)).23 | (3) |
2.8. Texture profile analysis (TPA)
Measurements were performed using a texture analyzer (Instron Corporation, Norwood, MA, USA) equipped with a 250 N load cell and a cylindrical probe of 15 mm in diameter. Samples (approximately 40 mm high) were conditioned in standardized cylindrical containers (50 mm in diameter) and analyzed at 5 °C. Each sample underwent a double compression cycle with a deformation of 60% of its original height at a constant speed of 1 mm s−1. The following textural parameters were determined: gel strength (N), cohesiveness (dimensionless), adhesiveness (mJ), and elasticity (dimensionless, as the ratio of the distance recovered during decompression).24 All measurements were performed in triplicate.2.9. Rheological analysis
Rheological measurements were performed using an Anton Paar rheometer (model MCR102e) with MCR 102e Standard software (SN 84225085), equipped with a temperature control system. The flow curve was obtained using concentric cylinder geometries (inner radius = 17 mm; outer radius = 18.44 mm; and 5 mm gap). The sample was transferred to the rheometer geometry and remained at rest for 1 min before starting the measurements to stabilize the temperature at 25 °C. Three curves were obtained: 0.01 to 200 s−1, 200 to 0.01 s−1, and 0.01 s−1 with 2 minutes each.The cone-plate geometry (49 mm and 0.103 mm gap) was used to determine viscoelasticity. A strain sweep (0.001–1%) was performed at a constant frequency of 1 Hz to determine the linear viscoelastic region (LVR). A frequency sweep from 0.01 to 10 Hz was then performed at a continuous strain, as determined by the LVR. Then, the changes in the storage (G′) and loss (G′′) moduli were checked with respect to the frequency changes.25 All analyses were performed in triplicate, and a new sample was used for each repetition.
2.10. Sensory evaluation
The sensory analysis was conducted with 100 habitual consumers of fermented dairy beverages (58 females and 42 males, aged between 18 and 61 years), following approval from the Ethics Committee on Human Research at the Federal University of Viçosa (approval number: 5470985). The evaluations were carried out in individual booths under controlled lighting and temperature conditions. Each participant received approximately 30 mL of each sample, served at 5 °C in 50 mL transparent plastic cups. Samples were presented monadically in randomized order and coded with three-digit numbers to ensure blinding. The six formulations were assessed for appearance, aroma, texture, flavor, and overall impression using a 9-point hedonic scale, where 1 = “dislike extremely” and 9 = “like extremely.” Purchase intention was also measured on a 5-point scale ranging from 1 = “certainly would not buy” to 5 = “certainly would buy.” In addition to the affective tests, a Rate-All-That-Apply (RATA) descriptive sensory method was applied to explore specific sensory attributes.4,26 Twenty-one sensory terms commonly used to describe fermented dairy products and possibly related to bee pollen were selected based on literature reports. Panelists were asked to indicate all applicable attributes and rate their intensity on a 4-point scale: 0 = “none,” 1 = “low,” 2 = “medium,” and 3 = “high.” RATA responses were treated as continuous data for statistical analysis.2.11. Volatile compound analysis
The volatile compounds of fermented beverages were separated and analyzed using a gas chromatograph coupled to a mass spectrometer (Shimadzu, GCMS-QP2010 Plus; Agilent Technologies, Inc., CA, USA) equipped with an autosampler (AOC-5000, Shimadzu) with headspace injection mode with solid phase microextraction (HS-SPME). The analysis was performed according to Bao et al. with some modifications. Initially, 5 ± 0.002 g of sample in headspace vials were incubated at 40 °C for 10 min for equilibration. Then, the fiber assembly (30 μm (CAR/PDMS layer) and 50 μm (DVB layer), Sigma-Aldrich) was exposed to the headspace for sampling at 40 °C for 40 min by the HS-SPME method, followed by thermal desorption in the injection port at 250 °C for 3 min. Before and after the extraction process, the fiber was conditioned according to the manufacturer's recommendations for use. The injection mode was split-less, and the column temperature program was as follows: initial temperature of 40 °C for 3 min, increased to 120 °C at a rate of 4 °C min−1 and held for 8 min, followed by increasing to 200 °C at 5 °C min−1 and held for 2 min, and increased to 250 °C at 10 °C min−1 and held for 3 min. Ultrahigh purity helium was used as the carrier gas (1.0 mL min−1 at constant pressure), and a DB-WAX capillary column (30 m × 0.25 mm i.d.; 0.25 μm film thickness; Agilent, USA) was used for the separation process. Mass spectral data were collected in the scan mode, monitoring masses from 35 to 350 m/z. The volatile compounds were identified by matching the mass spectra obtained with those of the Wiley 8 and FFNSC 1.2 Libraries database, and the calculated Kovats index of each compound (RIcal) was compared with the Kovats index reported in the literature (RIref). The RIcal was obtained according to eqn (4),27,28 based on the data obtained from the n-alkane series (C8–C24) subjected to the same chromatographic conditions described above.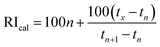 | (4) |
2.12. Statistical analysis
All data were subjected to one-way analysis of variance (ANOVA). Significant differences between means were determined by Tukey's test. A confidence level of 95% (p <0.05) was used. At least two individual productions of each formulation and treatment were performed. All analyses were replicated three times.3 Results and discussion
3.1. Compositional analysis of the fermented dairy-based beverages
The proximate composition of the fermented dairy beverages provides insights into the nutritional quality and balance of the formulations (Table 2).| Formulation | Moisture (% w/w) | Ash (% w/w) | Protein (% w/w) | Fat (% w/w) | Carbohydrates (% w/w) |
|---|---|---|---|---|---|
| MB37.5 | 79.42 ± 0.40 | 0.67 ± 0.02 | 2.81 ± 0.00 | 0.41 ± 0.02 | 16.70 ± 0.41 |
| MW37.5 | 80.62 ± 0.66 | 0.56 ± 0.01 | 2.34 ± 0.06 | 0.43 ± 0.04 | 16.05 ± 0.67 |
| MW75 | 79.80 ± 0.15 | 0.50 ± 0.03 | 2.47 ± 0.02 | 0.44 ± 0.05 | 16.78 ± 0.15 |
| MBW | 79.36 ± 0.07 | 0.61 ± 0.04 | 2.76 ± 0.02 | 0.41 ± 0.04 | 16.85 ± 0.19 |
| M100 | 79.38 ± 0.24 | 0.58 ± 0.02 | 2.80 ± 0.02 | 0.36 ± 0.05 | 16.87 ± 0.24 |
| MB75 | 81.30 ± 0.42 | 0.57 ± 0.02 | 2.53 ± 0.02 | 0.34 ± 0.00 | 15.26 ± 0.37 |
Despite the distinct milk, buttermilk, and whey ratios, all formulations presented similar macronutrient profiles. Moisture content ranged from 79.36% to 81.30%, protein content from 2.34% to 2.81%, lipid content from 0.3450% to 0.4406%, ash content from 0.5051% to 0.6691%, and carbohydrate content from 15.26% to 16.87%, with all values falling within typical ranges for dairy-based fermented products. All fermented dairy beverages were classified as low-fat foods, since they presented fat content inferior to 0.5% (w/w). Results indicate that the partial replacement of milk (up to 75%) with buttermilk and/or whey does not substantially alter the nutritional composition of the beverages. This supports the use of these dairy co-products in beverage development without compromising nutritional value, which is relevant for product formulation strategies aligned with circular economy and sustainability goals.
3.2. pH and titratable acidity
The analysis of pH and titratable acidity is essential for monitoring the quality, safety, and sensory characteristics of fermented dairy beverages, especially when varying proportions of dairy ingredients are used. These parameters were monitored over 30 days of refrigerated storage. At each time point, the pH values among formulations were statistically similar (p >0.05), allowing the use of overall means: 4.45 at day 0, 4.31 at day 14, and 4.15 at day 30. A gradual decline in pH was observed over time. In parallel, titratable acidity showed a slight increase, from 0.82% to 0.92% lactic acid, indicating continued post-fermentation acidification during storage. The results indicate that the partial replacement of milk with buttermilk and/or whey did not significantly affect lactose fermentation by lactic acid bacteria, as all formulations exhibited a similar acidification pattern over time. The pH values and titratable acidity levels observed across the 30-day storage period are consistent with those typically reported for fermented dairy beverages such as yogurt, with pH generally in the range of 4.0 to 4.6 and 0.7 to 1.0% lactic acid.303.3. Water-holding capacity and syneresis of formulations during storage
Monitoring the water-holding capacity (WHC) and syneresis is critical for assessing the physical stability and sensory quality of fermented dairy beverages during refrigerated storage. In this study, WHC and syneresis were evaluated over 15 days of storage at 4 °C (days 0, 3, 6, 9, 12, and 15), revealing distinct trends across formulations (Fig. 1). | ||
| Fig. 1 Water-holding capacity (WHC) and syneresis of fermented dairy beverages during 15 days of refrigerated storage. | ||
On day 0, the control (M100) exhibited a WHC of 51% and syneresis of 14%. Partial replacement of milk with 37.5% of either whey (MW37.5) or buttermilk (MB37.5) significantly improved stability, leading to higher WHC (≈57–58%) and reduced syneresis (≈11–12%). These results reflect the potential of both whey and buttermilk to enhance the physical properties of fermented dairy beverages when used at moderate levels. The improved water retention observed for MB37.5 is primarily attributed to the presence of free phospholipids in buttermilk. During butter manufacture, churning disrupts the native milk fat globule membrane, releasing its components, including phospholipids, into the aqueous phase. These liberated phospholipids, in conjunction with caseins also present in buttermilk, may enhance protein–water interactions and contribute to a more cohesive gel structure with reduced whey separation.5,31 The MW37.5 formulation also demonstrated high physical stability, which may be attributed to the excellent hydration capacity of whey proteins. These proteins are known for their ability to bind and immobilize water within gel networks, contributing to a cohesive and stable gel matrix.32
In contrast, MB75 and MBW presented lower WHC and higher syneresis at day 0. It is well known that whey proteins form a weaker acid gel compared to caseins.33 The excess of phospholipids (in MB75) or their combination with whey protein (in MBW) interfered with the formation of a casein network, resulting in a weaker gel structure.34 A similar effect was reported by Santos et al.,4 where a formulation containing 25% buttermilk and 75% milk exhibited the highest WHC and the lowest syneresis during 16 days of refrigerated storage. However, when the proportion of buttermilk was increased, no direct correlation was observed between buttermilk concentration and improvements in syneresis reduction or WHC enhancement.
Throughout 15 days of refrigerated storage, all samples, except MBW and MB75, showed slight increases in WHC and gradual reductions in syneresis. By day 15, MB37.5 and MW37.5 maintained their superior performance, reaching WHC values of ≈70–72% and syneresis levels of ≈6–7%. This behavior can be explained due to some structural changes occurring within the gel matrix over time, e.g., rearrangements in the protein network, since proteins continue interacting leading to the formation of a denser and more cohesive three-dimensional network, which retains more water.35 In contrast, MBW and MB75 remained the least stable, with WHC ≈46% and syneresis ≈17%, confirming that the excess phospholipids and their combination with whey protein compromised the casein network throughout the storage. These findings indicate that both whey and buttermilk can positively influence water retention and reduce serum release when used strategically.
3.4. Kinetics of destabilization
Ensuring colloidal stability throughout storage is a critical technological requirement for the quality and shelf-life of dairy beverages. Turbiscan Stability Index (TSI) reflects the cumulative effect of all destabilizing phenomena detected within the measurement cell; consequently, higher TSI readings correspond to greater instability in fermented beverages.36Fig. 2 displays the TSI histograms at different times. | ||
| Fig. 2 Turbiscan Stability Index (TSI) histograms of dairy formulations at different times, measured at 4 °C. | ||
At 30 seconds, all samples showed excellent stability, with TSI values ≤0.1 (Category A+), meaning no detectable destabilization occurred immediately after preparation. By 15 minutes, however, MW75, MBW, and MB75 began to show early signs of micro-instability (TSI: 0.6–0.7, Category A), suggesting slight protein flocculation or initial particle sedimentation, including that of pollen, although no visible phase separation was observed yet. In contrast, M100, MW37.5, and MB37.5 maintained their A+ stability (TSI <0.5), proving more resistant to early destabilization. This higher stability can be attributed to the balanced casein concentration maintained through the low substitution of milk with buttermilk or whey, which preserves the integrity of the casein network. Additionally, the presence of phospholipids and whey proteins, acting as natural emulsifiers, enhances protein–water and interfacial interactions. This cohesive colloidal structure increases the viscosity of the continuous phase and reinforces the interfacial film around oil droplets and hydrophobic pollen particles, effectively delaying sedimentation and coalescence and contributing to the overall physical stability of the beverage.37,38
By 30 minutes, MBW and MB75 crossed the TSI = 1.0 threshold (Category B), indicating the onset of sedimentation or coalescence, while MW75 neared this limit. The 37.5% co-product formulations (MW37.5 and MB37.5) remained in Category A, showing only moderate changes.
At 45 minutes, MBW, MB75, and MW75 further destabilized (TSI: 1.1–1.3, Category B), whereas MW37.5 and MB37.5 stayed more stable (TSI: 0.6–0.8, Category A). Finally, after 60 minutes (T60), MBW and MB75 reached the highest TSI values (1.5–1.6), which, although not indicative of severe destabilization (TSI <2), are consistent with literature reports associating values ≥1.5 with measurable sedimentation and early phase separation in dairy and cream systems.39 M100 and MB75 followed closely (TSI: 1.3, Category B), while MW37.5 and MB37.5 remained the most stable (TSI ≤0.9, Category A), demonstrating their superior resistance to destabilization over time. The results are consistent with those of the WHC and syneresis analysis.
3.5. Texture profile analysis – effects of dairy matrix composition
The texture of fermented dairy beverages reflects the structural arrangement of proteins, lipids, and water within the gel matrix and is highly dependent on the composition and interactions of the dairy base. To evaluate the impact of incorporating buttermilk, whey, or their combinations on the mechanical properties of the formulations, a Texture Profile Analysis (TPA) was conducted at 5 °C, measuring the gel strength, cohesiveness, adhesiveness, and elasticity of samples (Table 3).| Formulation | Hardness max. (N) | Cohesiveness | Adhesiveness (mJ) | Elasticity |
|---|---|---|---|---|
| a There is no statistical difference between the means indicated with the same letter in each column (p >0.05). | ||||
| MB37.5 | 0.31 ± 0.02a | 0.51 ± 0.02c | 0.81 ± 0.04a | 1.09 ± 0.05d |
| MW37.5 | 0.23 ± 0.01b | 0.53 ± 0.03bc | 0.81 ± 0.04a | 1.17 ± 0.06b |
| MW75 | 0.20 ± 0.01bc | 0.56 ± 0.03ab | 0.52 ± 0.03b | 1.19 ± 0.06ab |
| MBW | 0.25 ± 0.01ab | 0.55 ± 0.03ab | 0.82 ± 0.04a | 1.13 ± 0.05c |
| M100 | 0.25 ± 0.01ab | 0.54 ± 0.03bc | 0.82 ± 0.04a | 1.13 ± 0.05c |
| MB75 | 0.16 ± 0.00c | 0.63 ± 0.03a | 0.31 ± 0.02c | 1.22 ± 0.06a |
Partial or total replacement of skim milk with buttermilk or whey markedly altered the texture by reshaping the protein network. The hardness values ranged from 0.163 to 0.314 N, which are compatible with drinkable fermented dairy beverages.40,41 Pure milk (M100) and the milk–buttermilk–whey mix (MBW) showed intermediate textural properties, with hardness values around 0.248–0.253 N and elasticity near 1.130. MB37.5 (62.5% milk + 37.5% buttermilk) combined the highest hardness (0.314 N) with the lowest elasticity (1.096), indicating a rigid, tightly cross-linked gel. Conversely, MB75 (75% buttermilk) had the weakest hardness (0.163 N) yet the greatest elasticity (1.216), reflecting a softer, more deformable matrix. Whey blends (MW37.5 and MW75) occupied the mid-range for both strength (∼0.200–0.229 N) and elasticity (∼1.174–1.190), consistent with whey proteins favoring elasticity over firmness. Thus, moderate buttermilk addition, via MFGM proteins and phospholipid cross-linking, best enhances hardness, while higher coproduct levels shift the network toward elasticity.
Cohesiveness values ranged from 0.509 to 0.634. Interestingly, while MB37.5 demonstrated superior strength, it exhibited the lowest cohesiveness (0.509) among the modified blends.
Most formulations, including MB37.5, MW37.5, MBW, and pure milk (M100), showed remarkably consistent adhesiveness (0.8 mJ), indicating that moderate coproduct substitution had little impact on stickiness to the oral surface or utensils. However, we observed significant reductions only at the highest coproduct concentrations: MW75 (0.5 mJ) and MB75 (0.3 mJ). Since fat content varies minimally across samples, these dramatic decreases in adhesiveness probably reflect changes in water-binding behavior or increased serum viscosity rather than differences in lipid composition.42
Compared to the pure milk formulation (M100), moderate co-product inclusion resulted in distinct but balanced textural attributes. For instance, MB37.5 exhibited higher hardness and lower elasticity while maintaining similar adhesiveness. In contrast, excessive substitution (MB75 and MW75) led to marked reductions in hardness and adhesiveness, indicating disruption of the protein network.
3.6. Rheology analysis
Rheological properties provide important insights into the structural behavior and flow characteristics of dairy beverages. Understanding how different formulations respond to shear stress helps to reveal the underlying protein network and its stability, which are key factors influencing texture and consumer perception.43Fig. 3 presents the flow curves (shear stress versus shear rate) of various dairy formulations studied at 25 °C.The shear stress versus shear rate curves showed that all the dairy beverage formulations behaved as non-Newtonian, shear-thinning (pseudoplastic) fluids, which are typical for protein-based structured systems. This rheological behavior is consistent with that reported for other fermented dairy beverages.4,44 Among the samples, MB37.5 (62.5% milk and 37.5% buttermilk) had the highest shear stress values across the entire range, reaching about 35 Pa at 200 s−1. This greater resistance to flow matches the highest hardness value measured in the texture analysis (0.314 N), indicating a rigid and tightly linked network likely formed by synergistic interactions between milk proteins and membrane components from the buttermilk. On the other hand, MB75 (75% buttermilk) showed the lowest shear stress (29.9 Pa at 200 s−1), which aligns with its lowest strength (0.163 N) and highest elasticity (1.216) in the texture tests. This suggests that an excessive amount of buttermilk may weaken the protein matrix, resulting in a more flexible and elastic system but with reduced mechanical strength and lower flow resistance.31
The whey-rich formulations (MW37.5 and MW75) exhibited intermediate shear stress profiles, consistent with their moderate strength and relatively high elasticity seen in texture measurements. These findings support the notion that whey proteins promote more flexible structures, which tolerate deformation but form fewer strong cross-links. Interestingly, MBW (25% milk, 37.5% buttermilk, and 37.5% whey) showed rheological behavior close to that of pure milk (M100), reaching about 32.3 Pa at 200 s−1. This suggests that combining both coproducts may compensate for each other's structural limitations, resulting in satisfactory flow resistance while maintaining acceptable texture (strength around 0.248 N; elasticity, 1.130). Overall, the rheological results corroborate and expand the findings observed in the texture analysis. A moderate proportion of buttermilk enhances the firmness and flow resistance; however, excessive amounts of either buttermilk or whey, may result in weaker, more fluid-like systems.
To better understand the internal structure and viscoelastic properties of the fermented dairy beverages, dynamic rheological measurements were conducted, focusing on the storage modulus (G′), loss modulus (G′′), and the loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = G′′/G′) (Table 4). These parameters reflect the elastic (solid-like) and viscous (liquid-like) behavior of the matrix. A predominant G′ over G′′ throughout the shear strain range indicates a gel-like, semi-solid structure, which was observed in all formulations.45 In all cases, the storage modulus (G′) was higher than the loss modulus (G′′), and tan
δ = G′′/G′) (Table 4). These parameters reflect the elastic (solid-like) and viscous (liquid-like) behavior of the matrix. A predominant G′ over G′′ throughout the shear strain range indicates a gel-like, semi-solid structure, which was observed in all formulations.45 In all cases, the storage modulus (G′) was higher than the loss modulus (G′′), and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ remained below 1, confirming a viscoelastic, gel-like structure across samples.
δ remained below 1, confirming a viscoelastic, gel-like structure across samples.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) of dairy beverage formulationsa
δ) of dairy beverage formulationsa
| Formulation | G′ (Pa) | G′′ (Pa) | tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ δ |
|---|---|---|---|
| a There is no statistical difference between the means indicated with the same letter in each column (p >0.05). | |||
| MB37.5 | 8800 ± 440a | 1900 ± 95a | 0.22 ± 0.01ab |
| M100 | 7900 ± 395a | 1600 ± 80a | 0.20 ± 0.01a |
| MBW | 7600 ± 353a | 1850 ± 92.5a | 0.24 ± 0.01ab |
| MW37.5 | 4100 ± 205b | 930 ± 47b | 0.23 ± 0.01ab |
| MW75 | 2600 ± 130b | 540 ± 27c | 0.21 ± 0.01a |
| MB75 | 55 ± 3c | 17 ± 1d | 0.31 ± 0.01b |
Among the formulations, MB37.5 exhibited the highest values for both G′ (8800 Pa) and G′′ (1900 Pa), significantly different from most other samples (p <0.05), suggesting a highly structured, elastic network. This result aligns well with its superior hardness value (0.314 N) and highest flow resistance in shear curves, reflecting a dense and cohesive protein matrix reinforced by buttermilk-derived MFGM components.
M100 (100% milk) and MBW (with both buttermilk and whey) showed slightly lower G′ and G′′ values than MB37.5, but the differences were not statistically significant (G′ ≈ 7600–7900 Pa; G′′ ≈ 1600–1850 Pa). These formulations also maintained low tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values (∼0.20–0.24), supporting the presence of elastic-dominant structures. MBW's behavior notably resembled that of M100, reinforcing earlier observations that combining whey and buttermilk can compensate for individual weaknesses and yield a balanced texture.
δ values (∼0.20–0.24), supporting the presence of elastic-dominant structures. MBW's behavior notably resembled that of M100, reinforcing earlier observations that combining whey and buttermilk can compensate for individual weaknesses and yield a balanced texture.
In contrast, MW37.5 and MW75, both rich in whey, had significantly lower G′ and G′′ values than the milk- and buttermilk-dominant blends (G′ ∼4100 and 2600 Pa, respectively). Nevertheless, their tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values remained below 0.25, indicating a continued prevalence of elastic behavior. These outcomes support prior findings from texture analysis, where whey-based systems showed intermediate strength and high elasticity, attributed to the flexible but less cross-linked protein structures formed by whey proteins.
δ values remained below 0.25, indicating a continued prevalence of elastic behavior. These outcomes support prior findings from texture analysis, where whey-based systems showed intermediate strength and high elasticity, attributed to the flexible but less cross-linked protein structures formed by whey proteins.
MB75, containing 75% buttermilk, demonstrated the most distinct rheological profile, with drastically reduced moduli (G′ = 55 Pa; G′′ = 17 Pa), significantly different from all other groups. Its higher tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ value (0.31) further indicates a more viscous and deformable system. These results are fully consistent with texture data showing low gel hardness but high elasticity, as well as with flow curves suggesting diminished structural resistance. Thus, excessive buttermilk appears to compromise the protein network integrity, probably by diluting casein–casein interactions or oversaturating the system with emulsifying components.
δ value (0.31) further indicates a more viscous and deformable system. These results are fully consistent with texture data showing low gel hardness but high elasticity, as well as with flow curves suggesting diminished structural resistance. Thus, excessive buttermilk appears to compromise the protein network integrity, probably by diluting casein–casein interactions or oversaturating the system with emulsifying components.
Together, the rheological analysis reinforces a clear trend: moderate coproduct incorporation, especially up to 37.5%, optimally balances firmness and flexibility, enhancing both viscoelastic structure and functional performance. Exceeding this threshold, particularly with buttermilk, results in significant structural weakening, corroborating the trade-off observed in texture and flow behavior. In addition, polyphenols from bee pollen, particularly flavonoids and phenolic acids, are known to interact with milk proteins through hydrophobic interactions and hydrogen bonding, which may have contributed to the antioxidant protection and gel stability observed in the present formulations.46,47 It should be emphasized that although specific studies addressing the effect of fibrous components of bee pollen on the rheological and textural properties of food gels are not yet available, there is consistent evidence from related systems that insoluble fibers can modulate gel networks.48,49 However, in the present study, only 1% (w/v) of bee pollen was incorporated into the beverages, which makes it unlikely that the fibrous exine and intine layers of the pollen grains exerted a strong reinforcing effect on the gel matrix. Thus, while it is plausible that pollen particles might contribute marginally to the viscoelastic response as fillers within the protein–phospholipid network, their influence is expected to be limited at this concentration. More relevant contributions of bee pollen in this system are probably related to its bioactive compounds rather than to structural reinforcement of the gel.
Moreover, despite these molecular interactions, the presence of bee pollen at the low concentration used in this study (1% w/v) is unlikely to induce the formation of layered or lamellar structures in the protein gel matrix. The formation of layered or lamellar structures in protein-based gels is generally associated with phase separation processes or with the presence of relatively high concentrations of insoluble fillers. Such microstructural arrangements have not been reported in systems containing bee pollen, and given the low level used in this study (1% w/v), the occurrence of lamellar organization in the milk–buttermilk matrix appears unlikely. Instead, pollen grains are expected to remain as dispersed inclusions, interacting locally with proteins and phospholipids without promoting stratified structures. This interpretation is consistent with observations in other protein–polysaccharide systems, such as myofibrillar protein gels with ≤1% konjac glucomannan, where the addition of an insoluble material enhanced network compactness but did not induce layered microstructures.50
3.7. Antioxidant activity of the beverages
The antioxidant potential of the formulated dairy beverages was assessed using DPPH and ABTS radical-scavenging assays, which are widely employed to evaluate the ability of food matrices to neutralize free radicals. These assays provide complementary insights into the antioxidant mechanisms of complex food systems and their different reaction kinetics and sensitivities to various antioxidant compounds.51 The results, expressed as a percentage of radical inhibition, reflect the combined effects of bee pollen addition and the intrinsic antioxidant properties of the dairy bases (milk, buttermilk, and whey) (Fig. 4). | ||
| Fig. 4 Antioxidant activity of fermented dairy beverages determined by DPPH and ABTS assays. Bars represent the mean values, and error bars indicate standard deviations. | ||
Bee pollen is recognized as a rich natural source of antioxidant compounds, including flavonoids, phenolic acids, and carotenoids, which contribute to its high radical-scavenging capacity.17 Despite all formulations having received the same concentration of bee pollen (1%), significant differences (p <0.05) were observed in their antioxidant activities, as measured by DPPH and ABTS assays. These differences are attributed to the varying proportions of milk, buttermilk, and whey, which possess distinct intrinsic antioxidant potentials.
Among the samples, the MB75 formulation (72.5 ± 2.1% DPPH inhibition; 63.8 ± 1.9% ABTS inhibition) showed the highest antioxidant activity, significantly outperforming all other formulations (p <0.05). This superior performance can be explained by the high proportion of buttermilk (75%), known to be rich in polar lipids such as phosphatidylcholine and sphingomyelin and enzymes such as xanthine oxidase. These compounds have well-documented radical-scavenging properties and protective effects against lipid peroxidation, contributing to enhanced antioxidant capacity.52,53
The MBW formulation (65.3 ± 3.8% DPPH; 56.1 ± 3.2% ABTS) presented intermediate antioxidant activity, significantly higher than MW75 (60.7 ± 2.5% DPPH; 52.4 ± 2.3% ABTS) and MB37.5 (58.9 ± 3.1% DPPH; 50.2 ± 2.7% ABTS) (p <0.05), but still lower than MB75. This result suggests a beneficial effect from the combined presence of buttermilk (37.5%) and whey (37.5%), potentially due to a synergistic interaction between polar lipids from buttermilk and antioxidant proteins or peptides from whey. Bioactive peptides generated from whey proteins, especially following thermal processing, have been shown to enhance antioxidant capacity by donating electrons or hydrogen atoms to neutralize free radicals, as well as by chelating pro-oxidative metals.54 Furthermore, the partial denaturation of whey proteins may expose free sulfhydryl and other redox-active groups, increasing their capacity to scavenge reactive oxygen species.55
MW75 and MB37.5 did not differ significantly from each other (p >0.05) in either assay, suggesting that, at these substitution levels, the individual contributions of whey and buttermilk were comparable in promoting antioxidant activity. Although whey is rich in antioxidant proteins such as β-lactoglobulin and lactoferrin,56 its impact appears less pronounced when compared to the higher buttermilk concentration found in MB75.
The MW37.5 formulation (53.2 ± 2.8% DPPH; 45.6 ± 2.1% ABTS) and M100 formulation (48.3 ± 1.5% DPPH; 41.7 ± 1.3% ABTS) exhibited the lowest antioxidant activities, significantly inferior to all other samples (p <0.05). This finding is consistent with the known lower antioxidant potential of milk alone, especially after thermal processing, which can degrade thermolabile bioactive compounds.57 Overall, the results show that, even with identical bee pollen supplementation, the composition of the dairy base critically influenced the antioxidant properties of the beverages. Formulations enriched with buttermilk and whey enhanced functionality owing to their distinct bioactive profiles while simultaneously promoting by-product valorization and supporting the development of sustainable and functional dairy-based foods.
3.8. Sensory evaluation
Understanding sensory perception is fundamental for determining consumer acceptance of fermented dairy beverages, especially when formulating with milk substitutes such as dairy by-products.4 For the food industry, it is particularly important to assess how such substitutions influence consumer experience. Table 5 presents the outcomes of the sensory tests, including both acceptance ratings and purchase intent across the different formulations.| Formulation | Appearance | Aroma | Texture | Flavor | Overall impression | Purchase intention |
|---|---|---|---|---|---|---|
| a There is no statistical difference between the means indicated with the same letter in each column (p >0.05). | ||||||
| MB37.5 | 7.52 ± 0.32a | 6.92 ± 0.27a | 7.10 ± 0.28a | 6.53 ± 0.27a | 6.90 ± 0.27a | 3.30 ± 0.13a |
| AI (%) | 83.4 | 76.8 | 78.8 | 72.3 | 76.8 | |
| MW37.5 | 7.29 ± 0.31ab | 6.89 ± 0.27a | 7.02 ± 0.28a | 6.75 ± 0.27a | 6.80 ± 0.27a | 3.25 ± 0.13a |
| AI (%) | 80.9 | 76.2 | 78.2 | 75.5 | 75.7 | |
| MW75 | 6.73 ± 0.27b | 6.26 ± 0.25b | 6.10 ± 0.24b | 5.34 ± 0.21b | 5.86 ± 0.23b | 2.62 ± 0.10b |
| AI (%) | 74.6 | 69.4 | 68.0 | 59.8 | 65.5 | |
| MBW | 7.35 ± 0.31ab | 6.94 ± 0.27a | 6.90 ± 0.27ac | 6.61 ± 0.27a | 6.83 ± 0.27a | 3.36 ± 0.13a |
| AI (%) | 81.5 | 76.9 | 77.2 | 73.3 | 75.7 | |
| M100 | 7.40 ± 0.31a | 6.87 ± 0.27ab | 7.36 ± 0.31a | 6.61 ± 0.27a | 6.61 ± 0.27a | 3.30 ± 0.13a |
| AI (%) | 82.5 | 76.1 | 82.6 | 74.1 | 76.1 | |
| MB75 | 7.32 ± 0.31ab | 6.71 ± 0.27ab | 6.66 ± 0.27ab | 6.37 ± 0.25a | 6.47 ± 0.26ab | 3.03 ± 0.12ab |
| AI (%) | 81.1 | 74.3 | 74.5 | 70.1 | 71.6 | |
The consumer evaluation results (Table 5) identified three clear groups of sensory acceptance among the tested formulations. While the MW75 formulation consistently scored lowest (“b” group) across all five attributes – appearance, aroma, texture, flavor, and overall impression – four formulations (MB37.5, MW37.5, MBW, and M100) maintained positions in the highest acceptance group (“a”). The MB75 formulation showed intermediate performance, frequently appearing in the “ab” group. Notably, MB37.5 and M100 achieved the highest appearance scores, with MW37.5, MBW and MB75 showing no significant difference from these leaders, while MW75 scored significantly lower. This pattern repeated for aroma, where MB37.5, MW37.5, and MBW led the group, followed closely by M100 and MB75, with MW75 again scoring the lowest. Texture evaluation identified M100, MB37.5, and MW37.5 as top performers, MBW and MB75 as intermediate, and MW75 as significantly inferior.
For flavor perception, all formulations except MW75 (5.34) achieved statistically equivalent high scores (6.53–6.75). The overall impression scores corroborate these trends, with MB37.5, MW37.5, MBW, and M100 (6.80–6.89) comprising the top tier, MB75 (6.47) occupying the intermediate level, and MW75 (5.86) ranking the lowest.
When converted to Acceptability Index (IA) values using a 70% threshold on the 9-point scale, MW75 emerged as the only formulation failing to meet minimum acceptability standards, particularly for flavor (59.3% IA) and overall impression (66.7% IA). All other formulations comfortably exceeded the threshold (71.6–82.6% IA), supported by purchase intention scores ≥3.03, indicating strong consumer acceptance. These results clearly demonstrate that milk replacement levels up to 37.5% with either buttermilk (MB37.5), whey (MW37.5), or their combination (MBW) maintain sensory quality equivalent to whole milk (M100), corroborating with previous studies.4,6 The superior performance of these formulations likely stems from effective synergy between casein micelles and natural emulsifiers (whey proteins or buttermilk phospholipids), which appear to preserve desirable textural and flavor characteristics. In contrast, the poor performance of the 75% whey formulation (MW75) suggests that high whey concentrations may expose inherent astringency while providing insufficient lipid-based masking or interfacial stabilization. Wang et al.58 found that high whey concentrations may expose inherent astringency, as acidic whey-protein beverages (pH ∼3.0–3.5) are known to provoke mouth-drying and puckering sensations. These effects are attributed to protein–saliva interactions and aggregate formation and tend to be more pronounced when lipid-based masking agents are scarce. Therefore, for such high-whey systems to achieve commercial viability, additional formulation strategies such as fat content optimization, flavor masking agents, or hydrocolloid stabilizers would likely be necessary to improve sensory performance to acceptable levels.
To better understand the sensory factors influencing consumer acceptance and purchase intent, we conducted a descriptive analysis using the Rate-All-That-Apply (RATA) method. While affective testing quantified overall liking, RATA allowed us to pinpoint the specific attributes – flavor, aroma, texture, and appearance – that drove consumer preferences. The Principal Component Analysis (PCA) plot (Fig. 5) illustrates how each formulation relates to these key sensory descriptors.
The first principal component (PC1) explained 80.63% of the total variation between the formulations and the second component (PC2) 9.14%. Together, these two components explained over 70.00% of the total variation in the data, proving adequate for distinguishing the formulations concerning the overall acceptability. The PCA revealed clear sensory groupings that aligned perfectly with acceptance test results. On the right side of PC1 – the “sweet spot” containing positive drivers like sweet taste, creaminess, and characteristic yogurt flavor/aroma – we find M100, MB37.5, MW37.5, and MBW. This clustering explains their top-tier acceptance scores. At the opposite extreme, MW75 anchors the negative end of PC1, strongly associated with bitter taste, sourness, and astringency, matching its poor acceptance performance. MB75 occupies a middle ground, showing some buttery and floral notes (PC2) while drifting toward the creamy-sweet quadrant, consistent with its intermediate “ab” acceptance rating. Therefore, the RATA results corroborated our acceptance test findings: formulations closest to sweet/creamy/yogurt attributes were most preferred, while those linked to bitter or off-flavors were least liked.
3.9. Composition of volatile compounds of fermented dairy beverages
The determination of volatile compounds of formulations is essential to understand the impact of bee pollen and different proportions of milk, buttermilk, and whey on the sensory profile of fermented dairy beverages. Table 6 presents the volatile compounds identified with greater abundance in the different formulations of fermented beverages.| Compounds | Flavor descriptiona | CAS | MB37.5 | MW37.5 | MW75 | MBW | M100 | MB75 |
|---|---|---|---|---|---|---|---|---|
| a Flavor description was checked from the specifications for flavourings presented by the JECFA (Joint FAO/WHO Expert Committee on Food Additives) (https://www.fao.org/food-safety/scientific-advice/jecfa/en/) and Flavor Substances website (https://www.flavornet.org/); “-” indicates information not available. Table S1 in the SI presents all volatile compounds identified in different samples. The chromatograms are presented in Fig. S1 in the SI. | ||||||||
| Ethyl acetate | Pineapple | 141-78-6 | 1.56 | 4.25 | 1.79 | 3.55 | 1.68 | 4.76 |
| 2-Pentyl acetate | Herbaceous | 626-38-0 | 3.76 | 8.73 | 5.72 | 8.53 | 9.11 | 10.32 |
| Isoamyl acetate | Banana | 123-92-2 | 4.51 | 14.41 | 9.55 | 10.66 | 6.21 | 13.68 |
| Isobutyl butyrate | Sweet, fruity apple, and pineapple | 539-90-2 | 3.65 | 2.68 | 3.66 | 3.07 | 4.21 | 1.88 |
| Propanoic acid, 2-methyl-, and 1-methylbutyl ester | — | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340-93-1 340-93-1 |
0.47 | 2.55 | 3.86 | 3.71 | 9.23 | 2.42 |
| Isoamyl butyrate | Fruity banana | 106-27-4 | 25.70 | 24.11 | 25.35 | 23.82 | 27.36 | 16.97 |
| Isoamyl isovalerate | Fruity apple | 659-70-1 | 22.96 | 2.96 | 17.67 | 3.04 | 2.68 | 2.19 |
| (E)-Hex-2-enal | Apple and green vegetables | 6728-26-3 | 4.77 | 9.68 | 9.55 | 12.08 | 15.79 | 12.31 |
A total of thirty-five volatile compounds were identified in the fermented beverage samples, including twenty-one esters, two ketones, seven alcohols, two aldehydes, one ether, and two acids. In general, the volatile compound profile of the six formulations was similar, that is, most of the identified compounds were present in all samples. The group formed by the compounds isoamyl butyrate, isoamyl acetate, (E)-Hex-2-enal, isoamyl isovalerate, and 2-pentyl acetate was predominant in all formulations, representing 55.5% (MB75) to 67.8% (MW75) of the volatiles identified in the gas phase. It is worth mentioning that the compounds ethyl butyrate, 2-pentanol, isobutyl isovalerate, ethyl hexanoate, butyl isovalerate, hexyl acetate, isoamyl 2-methylbutyrate, prenyl isobutyrate, and methyl octanoate were not identified in all samples; however, they were not present in a significant way, with relative quantities ranging from 0.09 to 0.95%. In all samples, volatile compounds related to banana aroma (isobutyl acetate, isoamyl butyrate, and isoamyl acetate) and fruity aroma (isobutyl butyrate, isoamyl isobutyrate, (E)-Hex-2-enal, and isoamyl butyrate) were identified and reported by the evaluators in the descriptive sensory analysis – RATA.
The presence of many volatile compounds identified in fermented beverage formulations has previously been reported in fermented dairy products. Zhang et al.28 related the presence of ethanol (product of microbial spoilage), ethyl acetate, and 2-pentanone (an oxidation product of unsaturated fatty acids) to the development of off-flavor in yogurt after 10 days of storage. They also identified ethyl butyrate, isoamyl acetate, 2-pentanol, 2-heptanone, hexanol, and hexanoic acid, whose presence was attributed to the yogurt fermentation process.28 In another evaluation of yogurt volatiles, several compounds similar to those found in beverage formulations were identified, including esters (ethyl acetate, methyl butanoate, ethyl butyrate, ethyl hexanoate, and hexyl acetate), ketones (2-pentanone and 2-heptanone), acids (hexanoic acid and octanoic acid), aldehydes (hexanal), and alcohols (ethanol, isobutanol, butanol, 3-methyl-1-butanol, and 1-hexanol).59
Adding bee pollen to fermented dairy beverages also enriched their aroma and flavor profiles, introducing volatile organic compounds such as acids, esters, and alcohols, contributing to a more fruity and floral sensory experience. The fatty acids hexanoic and octanoic acid, along with the esters methyl octanoate and ethyl hexanoate, present in the beverage formulations, are reported as frequent constituents of the primary volatiles of bee pollen.60,61 In addition to these two esters, other volatiles such as ethyl acetate, methyl butanoate, ethyl butyrate, hexanol, 2-hexenal, (E)- and hexanal, associated with fruity, green and herbal aromas, are also found in bee pollen.62
Although the MW75 formulation presented inferior performance in the sensory tests, including for the aroma attribute, the volatile profile obtained for it did not reveal any discrepancy in comparison with other samples that could be associated with sensory perception. This behavior shows that the food matrix itself (which has different proportions of milk, whey, and buttermilk) can influence the retention and release of aromatic compounds due to the different interactions they have with the proteins, carbohydrates, and lipids that structure the matrix. Furthermore, it is known that some physicochemical properties of foods, such as viscosity, pH, ionic strength, fat content, water content, and protein interactions, can modulate the volatility, solubility, and partitioning of aromatic compounds in the mouth and nose.59,63,64 In addition, given the high phospholipid content of buttermilk, membrane lipids may interact with volatile compounds from bee pollen, enhancing their retention and potentially contributing to the aroma stability.65 Phospholipids spontaneously arrange into amphiphilic layers and vesicular structures that create unique microenvironments. These structures selectively trap volatile compounds, which significantly slows their evaporation and shields them from oxidative degradation. Furthermore, interactions with bioactive compounds, particularly phenolic acids and flavonoids, can enhance this protective effect by modifying the microenvironment's properties. This synergy not only improves stability but also facilitates a more sustained release of aroma molecules. This mechanism is well supported by research in dairy science and liposomal encapsulation, demonstrating that phospholipid-rich matrices are highly effective at stabilizing a wide range of volatiles, whether derived from fermentation or from botanical sources like pollen.66–68 This means that different food matrices can alter the intensity of the aroma perception without altering the actual profile of volatiles detected by gas chromatography.
4 Conclusions
This study demonstrates that partially replacing skim milk with buttermilk and cheese whey and enriching with bee pollen yield fermented dairy beverages that combine sustainability, functionality, and consumer appeal. Using a constrained mixture design, we showed that formulations with up to 37.5% buttermilk (MB37.5) or whey (MW37.5), and their 37.5/37.5 blend (MBW), exhibited enhanced water-holding capacity, reduced syneresis, and superior colloidal stability. Rheological and texture profile analyses further revealed balanced gel strength, cohesiveness, and elasticity, while sensory evaluation highlighted their creamy, sweet, and yogurt-like attributes with strong overall liking.In contrast, the 75% whey formulation (MW75) suffered from increased bitterness, lower stability, and reduced acceptability, indicating that extensive whey replacement may require hydrocolloid stabilizers or fat optimization to mask off-flavors and improve texture.
Bee pollen enrichment significantly enhanced antioxidant capacity across all formulations, with MB75 (75% buttermilk) achieving the highest DPPH and ABTS inhibitions, underscoring the synergistic role of polar lipids and phenolic compounds. Moreover, bee pollen inclusion at 1% w/w did not negatively affect sensory acceptance in moderate-replacement samples, supporting its use as a natural bioactive additive.
Altogether, these findings support a circular economy approach to valorize dairy co-products and apicultural ingredients in the development of functional beverages and provide a basis for scaling up production while balancing sustainability, health functionality, and market preferences.
It is important to note that the shelf life of the developed dairy-based functional beverages was not assessed in the present study. Future research should focus on evaluating the microbiological, physicochemical, and sensory stability of these formulations during storage, assessing consumer acceptance in larger populations and investigating the scale-up potential for industrial production, in order to ensure commercial viability, broader applicability, and consumer safety.
Author contributions
EAH: methodology, writing – original draft, investigation, data curation and formal analysis. HCS: data curation and formal analysis. LFB: data curation and formal analysis. JPR: data curation, formal analysis, and writing – original draft. KPC: data curation and formal analysis. IMMC: data curation and formal analysis. MCTRV: data curation, formal analysis, and writing – original draft. ACSP: writing – review & editing, conceptualization, supervision, and project administration.Conflicts of interest
The authors declare that they have no competing interests.Data availability
All data supporting the findings of this study are included in the article.Supplementary information: chromatographic (GC-MS) profiles and an additional table. See DOI: https://doi.org/10.1039/d5fb00337g.
Acknowledgements
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP), and Fundação de Apoio à Pesquisa de Minas Gerais (FAPEMIG). The authors would like to thank the Central of Analysis and Chemical Prospecting (CAPQ) of the Federal University of Lavras for supplying the equipment and providing technical support for experiments involving chromatographic analyses.References
- M. I. Malliaroudaki, N. J. Watson and R. Ferrari, et al., Energy management for a net zero dairy supply chain under climate change, Trends Food Sci. Technol., 2022, 126, 153–167, DOI:10.1016/J.TIFS.2022.01.015.
- T. Ahmad, F. Esposito and T. Cirillo, Valorization of agro-food by-products: Advancing sustainability and sustainable development goals 2030 through functional compounds recovery, Food Biosci., 2024, 62, 105194, DOI:10.1016/J.FBIO.2024.105194.
- D. R. Janiaski, T. C. Pimentel and A. G. Cruz, et al., Strawberry-flavored yogurts and whey beverages: What is the sensory profile of the ideal product?, J. Dairy Sci., 2016, 99(7), 5273–5283, DOI:10.3168/jds.2015-10097.
- H. C. Santos, G. V. F. Leonel and L. C. da S. Ramos, et al., Enhancing dairy sustainability: Rheological, sensory, and physical-chemical properties of low-fat fermented beverages incorporating buttermilk, J. Cleaner Prod., 2024, 443, 141159, DOI:10.1016/J.JCLEPRO.2024.141159.
- A. Ubeyitogullari and S. S. H. Rizvi, Production of high-purity phospholipid concentrate from buttermilk powder using ethanol-modified supercritical carbon dioxide, J. Dairy Sci., 2020, 103(10), 8796–8807, DOI:10.3168/jds.2020-18697.
- L. G. De Bassi, G. C. C. Ferreira and A. S. Da Silva, et al., Evaluation of physicochemical, microbiological and sensorial characteristics of fermented milk beverages with buttermilk addition, Int. J. Dairy Technol., 2012, 65(2), 282–286, DOI:10.1111/J.1471-0307.2011.00764.X.
- C. C. Kuo, D. Chen and R. Jiménez-Flores, et al., Valorization of byproducts from meat and dairy industries through fermentation to produce peptides, Sustainable Food Technol., 2024, 2(5), 1469–1475, 10.1039/D4FB00058G.
- Z. Usmani, M. Sharma and J. Gaffey, et al., Valorization of dairy waste and by-products through microbial bioprocesses, Bioresour. Technol., 2022, 346, 126444, DOI:10.1016/J.BIORTECH.2021.126444.
- J. Qiao, Y. Zhang and E. Haubruge, et al., New insights into bee pollen: Nutrients, phytochemicals, functions and wall-disruption, Food Res. Int., 2024, 178, 113934, DOI:10.1016/J.FOODRES.2024.113934.
- A. El Ghouizi, M. Bakour and H. Laaroussi, et al., Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties, Antioxidants, 2023, 12(3), 557, DOI:10.3390/ANTIOX12030557.
- M. Kieliszek, K. Piwowarek and A. M. Kot, et al., Pollen and bee bread as new health-oriented products: A review, Trends Food Sci. Technol., 2018, 71, 170–180, DOI:10.1016/J.TIFS.2017.10.021.
- S. A. M. Khalifa, M. Elashal and M. Kieliszek, et al., Recent insights into chemical and pharmacological studies of bee bread, Trends Food Sci. Technol., 2020, 97, 300–316, DOI:10.1016/J.TIFS.2019.08.021.
- M. Kieliszek, K. Piwowarek and A. M. Kot, et al., Recent advances and opportunities related to the use of bee products in food processing, Food Sci. Nutr., 2023, 11(8), 4372–4397, DOI:10.1002/FSN3.3411.
- J. A. Cornell. Experiments with Mixtures : Designs, Models, and the Analysis of Mixture Data, Wiley, New York, 3rd edn, 2002 Search PubMed.
- S. M'hir, M. Ziadi and A. Mejri, et al., Mixture of whey-milk and palm sap for novel kefir beverage using simplex-centroid mixture design, Kuwait J. Sci., 2023, 50(4), 690–696, DOI:10.1016/J.KJS.2023.04.008.
- W. I. Mora-Adames, C. A. Fuenmayor and M. A. Benavides-Martín, et al., Bee pollen as a novel substrate in pilot-scale probiotic-mediated lactic fermentation processes, LWT–Food Sci. Technol., 2021, 141, 110868, DOI:10.1016/J.LWT.2021.110868.
- A. M. G. Darwish, A. A. Abd El-Wahed and M. G. Shehata, et al., Chemical Profiling and Nutritional Evaluation of Bee Pollen, Bee Bread, and Royal Jelly and Their Role in Functional Fermented Dairy Products, Molecules, 2022, 28(1), 227, DOI:10.3390/MOLECULES28010227.
- I. K. Karabagias, V. K. Karabagias and I. Gatzias, et al., Bio-Functional Properties of Bee Pollen: The Case of “Bee Pollen Yoghurt.”, Coatings, 2018, 8(12), 423, DOI:10.3390/COATINGS8120423.
- Z. Ge, D. Yin and Z. Li, et al., Effects of Commercial Polysaccharides Stabilizers with Different Charges on Textural, Rheological, and Microstructural Characteristics of Set Yoghurts, Foods, 2022, 11(12), 1764, DOI:10.3390/FOODS11121764.
- A. G. Mizuta, E. da S. Alves and J. F. Silva, et al., Improving Probiotic Strawberry Dairy Beverages with High-Intensity Ultrasound: Syneresis, Fatty Acids, and Sensory Insights, Foods, 2025, 14(4), 616, DOI:10.3390/FOODS14040616.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, Use of a free radical method to evaluate antioxidant activity, LWT–Food Sci. Technol., 1995, 28(1), 25–30, DOI:10.1016/S0023-6438(95)80008-5.
- R. Re, N. Pellegrini and A. Proteggente, et al., Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radical Biol. Med., 1999, 26(9–10), 1231–1237, DOI:10.1016/S0891-5849(98)00315-3.
- K. Wang, G. Li and B. Zhang, Opposite results of emulsion stability evaluated by the TSI and the phase separation proportion, Colloids Surf., A, 2018, 558, 402–409, DOI:10.1016/J.COLSURFA.2018.08.084.
- L. F. Batista, C. S. Marques and A. C. dos S. Pires, et al., Artificial neural networks modeling of non-fat yogurt texture properties: effect of process conditions and food composition, Food Bioprod. Process., 2021, 126, 164–174, DOI:10.1016/J.FBP.2021.01.002.
- M. J. Povey and D. I. Hefft, Characterising the mechanical properties of soft solids through acoustics and rheology, exemplified by anhydrous milk fat, Soft Matter, 2023, 19(43), 8349–8359, 10.1039/D3SM01097J.
- A. de C. C. Soares, M. B. F. B. Tavares and E. de P. M. Ortega, et al., Rheological and sensorial evaluation of fruit nectar with chia mucilage, Int. J. Gastron. Food Sci., 2024, 35, 100849, DOI:10.1016/J.IJGFS.2023.100849.
- C. Bao, M. Yan and M. Diao, et al., Effect of Ganoderma lucidum water extract on flavor volatiles and quality characteristics of set-type yogurt, Food Chem., 2025, 464, 141687, DOI:10.1016/j.foodchem.2024.141687.
- Y. C. Zhang, Q. B. Lin and H. N. Zhong, et al., Identification and source analysis of volatile flavor compounds in paper packaged yogurt by headspace solid-phase microextraction-gas chromatography-mass spectrometry, Food Packag. Shelf Life, 2022, 34, 100947, DOI:10.1016/j.fpsl.2022.100947.
- Y. Biçer, G. Turkal and G. Sönmez, et al., Production of yoghurt from kefir beverage: Analysis of fermentation kinetics, volatile organic compounds, texture, and microbial characteristics, Int. Dairy J., 2024, 158, 106039, DOI:10.1016/j.idairyj.2024.106039.
- I. M. Ramos, S. Seseña and J. M. Poveda, et al., Screening of Lactic Acid Bacteria Strains to Improve the Properties of Non-fat Set Yogurt by in situ EPS Production, Food Bioprocess Technol., 2023, 16(11), 2541–2558, DOI:10.1007/S11947-023-03080-7/METRICS.
- A. Crespo-Villanueva, B. Gumí-Audenis and F. Sanz, et al., Casein interaction with lipid membranes: Are the phase state or charge density of the phospholipids affecting protein adsorption?, Biochim. Biophys. Acta, Biomembr., 2018, 1860(12), 2588–2598, DOI:10.1016/j.bbamem.2018.09.016.
- M. Henriques, D. Gomes and C. Pereira, Liquid Whey Protein Concentrates Produced by Ultrafiltration as Primary Raw Materials for Thermal Dairy Gels, Food Technol. Biotechnol., 2017, 55(4), 454, DOI:10.17113/FTB.55.04.17.5248.
- Y. T. P. Cubides, P. R. Eklund and E. A. Foegeding, Casein as a Modifier of Whey Protein Isolate Gel: Sensory Texture and Rheological Properties, J. Food Sci., 2019, 84(12), 3399–3410, DOI:10.1111/1750-3841.14933.
- N. Ahn, J. H. Park and C. Chai, et al., The interaction of milk sphingomyelin and proteins on stability and microstructure of dairy emulsions, J. Dairy Sci., 2022, 105(5), 3832–3845, DOI:10.3168/JDS.2021-21253/ASSET/5B9173FA-B5D4-4A6D-A37F-E3739CFCA3A2/MAIN.ASSETS/GR5.JPG.
- S. Sadighbathi, S. Amiri and P. E. J. Saris, et al., Development and properties of functional yoghurt enriched with postbiotic produced by yoghurt cultures using cheese whey and skim milk, Front. Microbiol., 2023, 14, 1276268, DOI:10.3389/FMICB.2023.1276268/PDF.
- C. S. Wu, J. H. Guo and M. J. Lin, Stability Evaluation of pH-Adjusted Goat Milk for Developing Ricotta Cheese with a Mixture of Cow Cheese Whey and Goat Milk, Foods, 2020, 9(3), 366, DOI:10.3390/FOODS9030366.
- C. C. Loi, G. T. Eyres and E. J. Birch, Effect of milk protein composition on physicochemical properties, creaming stability and volatile profile of a protein-stabilised oil-in-water emulsion, Food Res. Int., 2019, 120, 83–91, DOI:10.1016/j.foodres.2019.02.026.
- A. Jukkola, R. Partanen and W. Xiang, et al., Food emulsifiers based on milk fat globule membranes and their interactions with calcium and casein phosphoproteins, Food Hydrocolloids, 2019, 94, 30–37, DOI:10.1016/J.FOODHYD.2019.03.005.
- D. Mańko-Jurkowska and E. Domian, The Effect of Heat- and Salt Treatment on the Stability and Rheological Properties of Chickpea Protein-Stabilized Emulsions, Appl. Sci., 2024, 14(7), 2698, DOI:10.3390/APP14072698/S1.
- M. Ziarno, D. Zaręba and E. Kowalska, et al., A Study into the Effects of Chosen Lactic Acid Bacteria Cultures on the Quality Characteristics of Fermented Dairy, Dairy–Oat, and Oat Beverages, Appl. Sci., 2025, 15(7), 3714, DOI:10.3390/APP15073714.
- K. Skryplonek, I. Dmytrów and A. Mituniewicz-Małek, Probiotic fermented beverages based on acid whey, J. Dairy Sci., 2019, 102(9), 7773–7780, DOI:10.3168/jds.2019-16385.
- K. Kusio, J. O. Szafrańska and W. Radzki, et al., Effect of Whey Protein Concentrate on Physicochemical, Sensory and Antioxidative Properties of High-Protein Fat-Free Dairy Desserts, Appl. Sci., 2020, 10(20), 7064, DOI:10.3390/APP10207064.
- A. Mehta, L. Kumar and L. Serventi, et al., Exploring the textural dynamics of dairy and plant-based yoghurts: A comprehensive study, Food Res. Int., 2023, 171, 113058, DOI:10.1016/j.foodres.2023.113058.
- D. V. Vukić, V. R. Vukić and S. D. Milanović, et al., Modeling of rheological characteristics of the fermented dairy products obtained by novel and traditional starter cultures, J. Food Sci. Technol., 2018, 55(6), 2180–2188, DOI:10.1007/S13197-018-3135-9/FIGURES/3.
- C. W. Seo and N. S. Oh, Rheological, Physicochemical, Microbiological, and Aroma Characteristics of Sour Creams Supplemented with Milk Protein Concentrate, Food Sci. Anim. Resour., 2023, 43(3), 540, DOI:10.5851/KOSFA.2023.E16.
- A. Kostić, D. D. Milinčić and N. S. Stanisavljević, et al., Polyphenol bioaccessibility and antioxidant properties of in vitro digested spray-dried thermally-treated skimmed goat milk enriched with pollen, Food Chem., 2021, 351, 129310, DOI:10.1016/j.foodchem.2021.129310.
- T. Mao, P. Wescombe and M. S. Mohan, Predominance of non-covalent interactions of polyphenols with milk proteins and their health promoting properties, Crit. Rev. Food Sci. Nutr., 2024, 64(32), 11871–11893, DOI:10.1080/10408398.2023.2245037.
- Y. Zhao, G. Zhou and W. Zhang, Effects of regenerated cellulose fiber on the characteristics of myofibrillar protein gels, Carbohydr. Polym., 2019, 209, 276–281, DOI:10.1016/J.CARBPOL.2019.01.042.
- X. Zhuang, M. Han and X. Jiang, et al., The effects of insoluble dietary fiber on myofibrillar protein gelation: Microstructure and molecular conformations, Food Chem., 2019, 275, 770–777, DOI:10.1016/J.FOODCHEM.2018.09.141.
- X. Zhuang, L. Wang and X. Jiang, et al., Insight into the mechanism of myofibrillar protein gel influenced by konjac glucomannan: Moisture stability and phase separation behavior, Food Chem., 2021, 339, 127941, DOI:10.1016/J.FOODCHEM.2020.127941.
- N. B. Sadeer, D. Montesano and S. Albrizio, et al., The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations, Antioxidants, 2020, 9(8), 709, DOI:10.3390/ANTIOX9080709.
- P. Y. Y. Wong and D. D. Kitts, Chemistry of buttermilk solid antioxidant activity, J. Dairy Sci., 2003, 86(5), 1541–1547, DOI:10.3168/JDS.S0022-0302(03)73739-4.
- C. Bourlieu, D. Cheillan and M. Blot, et al., Polar lipid composition of bioactive dairy co-products buttermilk and butterserum: Emphasis on sphingolipid and ceramide isoforms, Food Chem., 2018, 240, 67–74, DOI:10.1016/j.foodchem.2017.07.091.
- A. R. Madureira, T. Tavares and A. M. P. Gomes, et al., Invited review: Physiological properties of bioactive peptides obtained from whey proteins, J. Dairy Sci., 2010, 93(2), 437–455, DOI:10.3168/JDS.2009-2566.
- L. Zhang, R. Zhou and J. Zhang, et al., Heat-induced denaturation and bioactivity changes of whey proteins, Int. Dairy J., 2021, 123, 105175, DOI:10.1016/J.IDAIRYJ.2021.105175.
- M. Stobiecka, J. Król and A. Brodziak, Antioxidant Activity of Milk and Dairy Products, Animals, 2022, 12(3), 245, DOI:10.3390/ANI12030245.
- P. C. Pereira, Milk nutritional composition and its role in human health, Nutrition, 2014, 30(6), 619–627, DOI:10.1016/J.NUT.2013.10.011.
- T. Wang, S. Y. Tan and W. Mutilangi, et al., Application of infrared portable sensor technology for predicting perceived astringency of acidic whey protein beverages, J. Dairy Sci., 2016, 99(12), 9461–9470, DOI:10.3168/JDS.2016-11411.
- C. Liu, P. Yang and H. Wang, et al., Identification of odor compounds and odor-active compounds of yogurt using DHS, SPME, SAFE, and SBSE/GC-O-MS, LWT–Food Sci. Technol., 2022, 154, 112689, DOI:10.1016/J.LWT.2021.112689.
- M. Csóka, R. Végh and L. Sipos, Volatile Profile of Bee Pollens: Optimization of Sampling Conditions for Aroma Analysis, Identification of Potential Floral Markers, and Establishment of the Flavor Wheel, Food Sci. Nutr., 2024, 13, e4707, DOI:10.1002/FSN3.4707.
- S. Capparelli, Y. Pieracci and S. Sagona, et al., The volatile and sensory profiles of Tuscan bee pollens stored at different temperatures, Nat. Prod. Res., 2024, 3, 1–8, DOI:10.1080/14786419.2024.2389312.
- S. Prdun, L. Svečnjak and M. Valentić, et al., Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles, Foods, 2021, 10, 2103, DOI:10.3390/FOODS10092103.
- M. V. Beret, I. V. Wolf and S. Rebechi, et al., Microparticulated and concentrated whey proteins as structure and flavour enhancers in semi-skim high-protein yoghurts, Int. Dairy J., 2024, 157, 106008, DOI:10.1016/J.IDAIRYJ.2024.106008.
- L. Zhang, E. Gionfriddo and V. Acquaro, et al., Direct immersion solid-phase microextraction analysis of multi-class contaminants in edible seaweeds by gas chromatography-mass spectrometry, Anal. Chim. Acta, 2018, 1031, 83–97, DOI:10.1016/J.ACA.2018.05.066.
- L. Zhao, R. Feng and F. Ren, et al., Addition of buttermilk improves the flavor and volatile compound profiles of low-fat yogurt, LWT–Food Sci. Technol., 2018, 98, 9–17, DOI:10.1016/J.LWT.2018.08.029.
- Z. Zhang, M. Zang and K. Zhang, et al., Effects of phospholipids and reheating treatment on volatile compounds in phospholipid-xylose-cysteine reaction systems, Food Res. Int., 2021, 139, 109918, DOI:10.1016/J.FOODRES.2020.109918.
- M. Rudzińska, A. Grygier and G. Knight, et al., Liposomes as Carriers of Bioactive Compounds in Human Nutrition, Foods, 2024, 13(12), 1814, DOI:10.3390/FOODS13121814.
- A. A. Jovanović, B. Balanč and P. M. Petrović, et al., Liposome-Based Encapsulation of Extract from Wild Thyme (Thymus serpyllum L.) Tea Processing Residues for Delivery of Polyphenols, Foods, 2025, 14(15), 2626, DOI:10.3390/FOODS14152626/S1.
| This journal is © The Royal Society of Chemistry 2025 |


