Open Access Article
Open Access ArticleFunctional and structural characterization of legume protein derivatives for advanced plant-based food applications
Harsha Varayil and
Jayeeta Mitra *
*
Agricultural and Food Engineering Department, Indian Institute of Technology Kharagpur, Kharagpur, 721302, West Bengal, India. E-mail: jayeeta.mitra@agfe.iitkgp.ac.in
First published on 15th December 2025
Abstract
The global shift toward plant-based (PB) diets is driven by its health benefits and sustainability, with plant proteins offering potential to address malnutrition. This study aims to assess the protein secondary structural characteristics, functional attributes, thermal stability, and quality characteristics of various commercial legume protein derivatives: pea protein (PP), cowpea protein (CP), mung bean protein (MP), lentil protein (LP), and fava bean protein (FBP). Isolates exhibited superior functional properties, followed by concentrates and protein powder. Methionine and cystine are the most limiting AAs in all derivatives (0.09–1.01 g/100 g). The in vitro protein digestibility (IVPD) varied between 64% and 84%, with PP exhibiting the highest and FBP the lowest. PP serves as a potential source to meet the dietary needs of adults over 18 years (amino acid score > 100) with the exception of methionine and cystine. Structural and thermal analyses indicated a positive correlation with the ordered structure (β-sheet, α-helix, and β-turns) of protein derivatives. The proportion of the α-helix was higher in the PP isolate (33.30%), followed by the MP concentrate (31.10%), which reflects its thermal stability (MP: 99.3 °C). The higher thermal stability and secondary structure configuration are responsible for the formation of the fibrous structure during the structuring process. Multivariate analysis demonstrated a strong correlation between functional and structural properties and protein purity. The study successfully analysed the functionality of commercial plant proteins and categorized them into various derivatives for potential application in PB products.
Sustainability spotlightGlobally, the production of plant-based foods is increasing rapidly due to their sustainable approach, addressing environmental impact, health benefits, and ethical concerns. Legumes are rich sources of protein with high nutritional profiles, presenting a sustainable alternative source to animal proteins. To mimic the nutritional and functional attributes of animal proteins, it is essential to choose a strategic combination of various plant proteins. The study explored the structural and functional characteristics of different commercially available legume protein derivatives to understand their potential for plant-based alternative applications. The detailed analysis of these characteristics facilitates the identification of protein blends that contribute to the development of nutritionally balanced and sustainable food innovations. |
1. Introduction
The global population is expected to reach more than 9 billion by 2050, posing challenges to feed the growing population.1 It is important to adopt a sustainable approach to food production. The production of animal protein raises a serious concern due to its substantial demand for land and water resources. The concerns of environmental sustainability, health issues, and ethical considerations have driven interest among consumers towards PB products. PB products mimic the sensory and nutritional characteristics of animal-based products, which has led to increased utilization of plant proteins in the dairy, meat, and poultry industries. Various sources, including cereals, oilseeds, pulses, millets, vegetables, and fungi, have been explored for their potential. The industrial exploration of meat analogues mainly involves pea (Pisum sativum L.) and soy (Glycine max) proteins; however, novel protein sources such as legume proteins (lentils (Lens culinaris L.), cowpea (Vigna unguiculata), fava beans (Vicia faba L.), mung bean (Vigna radiata L.)), microbial proteins (spirulina and mycoprotein), hemp seed, chia seed, quinoa, insect proteins and many more are also gaining attention for the production of PB products.2,3 Legume proteins include cowpea (22–30%), lentils (24–30%), fava bean (24–35%), and mung bean (20–25%), which have slightly less protein than soybean (30–40%) and about the same as that of yellow pea (20–32%).3–5 The composition of pulse proteins primarily consists of globulins and albumin, with glutelin, gliadin, and prolamin present in smaller quantities.6,7 The comparative evaluation of plant protein quality and the AA profile in relation to animal proteins has been a subject of considerable interest and investigation. It has been observed that plant proteins do not have all the essential AAs compared to animal proteins, making them a less preferred choice in terms of nutritional requirements.8,9The nutritional indices, including the IVPD, AAS, PDCAAS, PER, and BV, are used to assess the quality of proteins, which indicates the digestibility, adequacy, and biological absorption.10 Typically, plant proteins are not a complete protein source except for soy, quinoa, and hemp seed proteins.7 Therefore, incorporating various combinations of plant proteins into the diet can supply balanced AAs to meet dietary needs, comparable to that of animal protein.11 Mung bean protein shows a comparable AA profile to eggs, with fewer sulphur-containing AAs.12 Similarly, fava beans exhibit a balanced AA profile in which a good amount of essential AAs are present.13 Soybeans possess a significant proportion of AAs found in animal proteins, particularly those containing sulphur, such as methionine, lysine, and cysteine.14 The allergenic characteristics of soy protein prompt the investigation of alternative pulse proteins over soy protein. The preferences of consumers are shifting towards soy-free and gluten-free food products by considering allergies, intolerances, hormonal concerns, and taste preferences.
Commercially, legume proteins are widely produced from pea, fava bean, chickpea, lentils, and mung bean. These proteins can be used as the ingredients for egg, poultry, dairy, and meat alternatives.15 However, their suitability is largely influenced by their structural and functional properties (solubility, emulsification, gelation, and water holding capacity (WHC)), which play a crucial role in determining the texture, flavour, and overall sensory experience of the final product.7,11,16 Despite these advantages, these proteins have several challenges that limit their wide applications. Variations in processing methods, such as alkaline extraction, isoelectric precipitation, or air classification, often lead to significant differences in protein purity, structural integrity, and functional behaviour. Based on the extraction and protein source, its interactions, structural configuration, etc., differ.17,18 Additionally, plant proteins have low digestibility due to the presence of antinutritional factors such as tannic acid, lecithin, saponin, phytic acid, trypsin, and protease inhibitors, which influence the digestive process.19
Currently, the majority of cereals and legume-based protein derivatives are available in the market. However, the functionality of the commercially available proteins is often inferior compared to lab-isolated proteins.20 Most of the studies focus on the extraction and characterization of proteins at a lab scale, highlighting the evident advantages of achieving superior properties due to the controlled conditions during the process. There is a critical need to assess the quality of commercially available PB proteins for potential applications in the alternative food sector.
The present study has been formulated with two specific objectives: (1) to understand the functional and structural properties of commercially available legume protein derivatives and (2) to explore the correlations among the studied properties using multivariate analysis techniques. Specifically, the legume protein derivatives chosen for analysis include yellow pea protein (PP), mungbean protein (MB), lentil protein (LP), cowpea protein (CP), and fava bean protein (FBP) powder. These legume protein derivatives were selected based on their market prevalence and prospective use in food product development.
2. Materials and methods
2.1 Materials
Different commercially available legume protein powders (PP, FBP, LP, MP, and CP) were procured from Ambe NS Agro Products Pvt. Ltd (Uttar Pradesh, India) and Devigere Biosolutions Pvt. Ltd (Karnataka, India). All reagents and chemicals utilized (sodium dodecyl sulphate (SDS), hexane, NaOH, H2SO4, CuSO4, K2SO4, methyl red, bromocresol green, Na2HPO4·2H2O, and NaH2PO4·2H2O), pepsin, and pancreatin were of analytical grade and purchased from Merck India.2.2 Proximate analysis
The proximate compositions of the legume proteins were analysed by AOAC official methods.21 The crude protein content and fat were determined using the Kjeldahl method (Gerhardt Kjeldatherm, Germany; %N × 6.25) and a hexane extraction system (Soxtherm, Gerhardt, Germany), respectively.2.3 Amino acid analysisb
The sample preparation for liquid chromatography-mass spectrometry (LC-MS, Nexera with LCMS-8045, Shimadzu Corporation, Kyoto, Japan) analysis was performed based on the method outlined by Nimbalkar et al. (2012)22 with slight modifications. 1 g of protein derivative powder was dispersed in 10 mL of Milli-Q water utilizing a magnetic stirrer for 3 h. The solutions were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C. The resulting supernatant was collected and subjected to filtration through a 0.45 µm nylon membrane filter. The quantification of AAs was carried out using a Shim-pack GISS C18 column (150 × 2.1 mm, 1.9 µm; Shimadzu). The mobile phase consisted of water and formic acid in a ratio of 100
000 rpm for 15 min at 4 °C. The resulting supernatant was collected and subjected to filtration through a 0.45 µm nylon membrane filter. The quantification of AAs was carried out using a Shim-pack GISS C18 column (150 × 2.1 mm, 1.9 µm; Shimadzu). The mobile phase consisted of water and formic acid in a ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 for solvent A, while solvent B was methanol at 100% concentration. The injection volume utilised for the experimental procedure was 10 µL. The data acquisition and analysis in this study were conducted using the LC Lab Solutions software.
0.1 for solvent A, while solvent B was methanol at 100% concentration. The injection volume utilised for the experimental procedure was 10 µL. The data acquisition and analysis in this study were conducted using the LC Lab Solutions software.
2.4 In vitro protein digestibility (IVPD)
The IVPD of protein derivatives was evaluated based on the methodology explained by Terefe et al. (2021)23 with slight modifications. 0.5 mg of each sample was mixed with 1.5 mg of pepsin in 0.1 N HCl and incubated at 37 °C for 3 hours. Following incubation, 7.5 mL of 0.2 M NaOH was added to neutralize the sample. 4 mg of pancreatin in 7.5 mL of phosphate buffer (pH 8.0) was added to the sample and incubated at 37 °C for 24 hours to initiate the pancreatin digestion. Furthermore, to cease the reaction, 5 mg of trichloroacetic acid was added and centrifuged at 5000g for 10 min. The supernatant was collected, and protein content was measured by the Kjeldahl method as described in Section 1.2. The values of IVPD were calculated by using the following eqn (1).
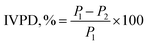 | (1) |
2.5 Amino acid score (AAS), protein digestibility corrected amino acid score (PDCAAS), protein efficiency ratio (PER), and biological value (BV)
The AAS was determined by dividing the individual AA values by their corresponding reference value, considering the daily AA requirements for adults and infants.10 The PDCAAS was then calculated by multiplying the lowest (most limiting) AAS by the protein digestibility. The PER (ref. 24) and BV (ref. 25) of proteins were calculated numerically using the predictive equations based on the amino acid composition.| PER1 = −0.684 + 0.456 × leusine − 0.047 × proline | (2) |
| PER2 = −0.468 + 0.454 × leusine − 0.105 × tyrosine | (3) |
| PER3 = −1.86 + 0.435 × methionine + 0.78 × leusine + 0.211 × hystidine − 0.944 × tyrosine | (4) |
| BV = 102.15 × lysine0.41 × (phenylalanine + tyrosine)0.60 × (methionine + cystine)0.77 × threonine2.4 × tryptophan0.21 | (5) |
2.6 Determination of functional properties
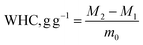 | (6) |
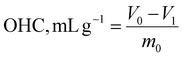 | (7) |
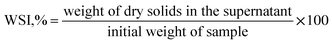 | (8) |
 | (9) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. The values of FC and FS were estimated employing the given eqn (10) and (11).
000 rpm for 1 min. The values of FC and FS were estimated employing the given eqn (10) and (11).
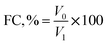 | (10) |
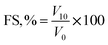 | (11) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min. The emulsion was collected immediately (0 min) and after 10 min of homogenization, and diluted with 0.1% sodium dodecyl sulfate (SDS) solution with 100 as the dilution factor. The absorbance value for both solutions (0 min and 10 min) was noted at 500 nm (optical path is 0.01 m) using a UV-visible spectrophotometer (Labman Scientific Instruments, India). The calculations are performed using eqn (12) and (13).
000 rpm for 2 min. The emulsion was collected immediately (0 min) and after 10 min of homogenization, and diluted with 0.1% sodium dodecyl sulfate (SDS) solution with 100 as the dilution factor. The absorbance value for both solutions (0 min and 10 min) was noted at 500 nm (optical path is 0.01 m) using a UV-visible spectrophotometer (Labman Scientific Instruments, India). The calculations are performed using eqn (12) and (13).
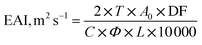 | (12) |
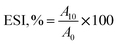 | (13) |
2.7 Determination of thermal properties
A differential scanning calorimeter (DSC) was used to examine the thermal characteristics of legume proteins (NETZSCH DSC300 Classic, Germany) following the method described in Shen et al. (2022)31 with slight modifications. A 5 mg powder sample was placed on an aluminium pan, and the properties were measured at temperatures ranging from 10–250 °C using a heating rate of 10 °C × min−1.2.8 Determination of molecular weight
Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) was used to determine the molecular weight (MW) of legume proteins (Ultraflextreme, Bruker Daltonik GmbH, Germany) following the method explained by Chang et al. (2021).32 The sample (2 µL of 1 mg mL−1) was prepared by mixing the protein dispersion and matrix solution (sinapinic acid) dissolved in 0.01 M sodium chloride (2 µL), which was spotted on an MTP 384 ground steel plate (Bruker Daltonik) and left to dry at room temperature. The spectrum was recorded from mass (m/z) 100 to 2000 Da in reflector mode.2.9 Determination of structural properties
The secondary structural details of legume proteins are investigated with Fourier-transform infrared (FTIR) spectroscopy and circular dichroism (CD). In FTIR, both soluble and insoluble protein fractions were analyzed, whereas in CD, only soluble fractions of proteins were analyzed.
 | (14) |
2.10 Statistical analysis
Statistical evaluations for all experiments were conducted utilizing one-way ANOVA, followed by the ‘Tukey post hoc test’ (IBM SPSS Statistics 20). The test aimed to ascertain any significant difference (p < 0.05) between the mean values. All experiments were conducted in triplicate, with results shown as mean ± standard deviation. Additionally, multivariate analysis, including Pearson correlation, hierarchical cluster analysis (HCA), and principal component analysis (PCA), was executed using Origin Pro 2025 (Origin Lab Corporation, United States).3. Results and discussion
3.1 Proximate analysis
The proximate analysis of each protein sample is given in Table 1. As per the protein content, the samples can be categorized as protein isolate (protein content > 75%), concentrates (40–75%), and powder (below 40%). According to protein content, PP was considered as protein isolate (75.01 ± 1.56%), MP (58.00 ± 0.85%), LP (51.04 ± 0.52%), and CP (61.08 ± 0.46%) as protein concentrates, and FBP as protein powder (20.62 ± 1.32%). The source and purity (processing conditions) of protein derivatives have a significant impact on their structural and functional characteristics.35 Plant proteins with higher protein content and amino acid profiles are taken into consideration in order to replicate the nutritional profile in comparison to animal proteins. Furthermore, the proximate contents are crucial for achieving improved functionality. PP isolate was observed to have an ash content of 4.10 ± 0.23% while concentrates had ash contents ranging from 3.41 to 5.92%. Similar observations are reported for concentrates and isolates of chickpea, pea, wheat, fava bean, and soy.36 Fat content was observed to be higher at MPC (4.58 ± 0.20%), and this could be due to the presence of more quantity of lipids even after the isolation process.37 The fat content of legume derivatives varies significantly (p < 0.05).| Parameters | CP | FBP | PP | MB | LP |
|---|---|---|---|---|---|
| a The data are presented as mean ± SD. Different letters within each row indicate significant differences (p < 0.05) based on the Tukey test. FBP = fava bean protein, PP = pea protein, MP = mung bean protein, LP = lentil protein. | |||||
| Moisture content (%) | 6.37 ± 0.04b | 5.01 ± 0.07d | 5.57 ± 0.04c | 3.58 ± 0.08e | 6.59 ± 0.06a |
| Ash (%) | 5.46 ± 0.29a | 3.93 ± 0.25b | 4.10 ± 0.23b | 5.92 ± 0.53a | 3.41 ± 0.31b |
| Protein (%) | 61.08 ± 0.46b | 20.62 ± 1.32e | 75.01 ± 1.56a | 58.00 ± 0.85c | 51.04 ± 0.52d |
| Fat (%) | 1.18 ± 0.32d | 1.61 ± 0.03cd | 1.72 ± 0.12c | 4.58 ± 0.20a | 2.32 ± 0.13b |
3.2 Amino acid analysis
The AA composition of the protein is crucial for both nutritional and functional purposes. It significantly contributes to providing essential AAs for nutrition and determining the protein's functionality based on the presence of polar and non-polar AAs.20 Animal products such as meat, egg, fish, dairy, and poultry products, and plant products like soybean, quinoa, and buckwheat are considered complete sources of protein because they contain all nine essential AAs in appropriate proportions required for human health.38 Essential AAs cannot be synthesized by the human body and, therefore, need to be acquired from food sources. When a protein source lacks one or more essential AAs, the body cannot synthesize them, resulting in a deficiency.19 Meat has a superior essential and non-essential AA composition compared to legume proteins.The AA profiles of all the samples were determined by LC-MS/MS, and the results are presented in Table 2. In general, legumes contain glutamic acid (12.62–15.64 g/100 g) as a major constituent, followed by aspartic acid (10.12–13.58 g/100 g). Sulfur-containing AAs such as methionine and cystine are the major flavour-giving compounds in meat, which are very low in studied legume proteins (<0.8 g/100 g methionine and <0.21 g/100 g cystine). Nosworthy et al. (2017)39 also observed similar results in kidney beans, lentils, green peas, and black beans.
| Amino acid g/100g | CP | FBP | PP | MP | LP | |
|---|---|---|---|---|---|---|
| a FBP = fava bean protein, PP = pea protein, MP = mung bean protein, LP = lentil protein. | ||||||
| Essential amino acids | Histidine | 1.41 | 0.62 | 1.9 | 1.66 | 1.02 |
| Isoleucine | 1.19 | 0.45 | 3.7 | 1.23 | 3.13 | |
| Leucine | 3.39 | 2.10 | 6.4 | 3.45 | 6.03 | |
| Lysine | 3.73 | 2.41 | 5.7 | 3.57 | 3.25 | |
| Methionine | 0.62 | 0.02 | 0.8 | 0.57 | 0.20 | |
| Phenylalanine | 2.16 | 1.12 | 4.2 | 2.52 | 2.16 | |
| Threonine | 1.32 | 2.13 | 2.8 | 1.74 | 1.38 | |
| Tryptophan | 0.42 | 0.14 | 0.7 | 0.33 | 0.23 | |
| Valine | 1.12 | 1.20 | 4.0 | 1.05 | 2.64 | |
| Alanine | 2.77 | 1.32 | 3.6 | 2.92 | 2.12 | |
| Non-essential amino acids | Aspartic acid | 13.04 | 10.12 | 13.12 | 13.58 | 12.68 |
| Cystine | 0.14 | 0.07 | 0.21 | 0.14 | 0.09 | |
| Glutamic acid | 14.25 | 12.62 | 15.64 | 14.48 | 13.89 | |
| Glycine | 2.55 | 1.12 | 3.25 | 2.81 | 1.98 | |
| Proline | 3.26 | 2.65 | 3.78 | 3.21 | 3.06 | |
| Serine | 2.94 | 2.12 | 4.2 | 3.2 | 3.46 | |
| Tyrosine | 1.72 | 1.32 | 1.90 | 1.49 | 1.63 | |
| Arginine | 3.52 | 6.21 | 5.12 | 3.54 | 4.31 | |
The total essential AAs of the PP isolate was 30.2 g/100 g, which meets the recommended essential AA requirement of adults as per WHO/FAO/UNU,40 whereas those of other derivatives (CP-15.36 g/100 g; MP-16.12 g/100 g; LP-20.04 g/100 g; and FBP-10.19 g/100 g) are below the recommended level. The guideline is established based on the suggested daily protein intake of 0.66 g per kilogram of body weight for adults. Thus, it is advised to include a variety of plant protein sources in the diet. Among concentrates, MP has higher essential AAs (histidine, isoleucine, leucine, phenylalanine, and threonine) than CP and LP concentrates. LP has higher aspartic acid (13.58 g/100 g) and lower tyrosine (1.49 g/100 g) and valine (1.05 g/100 g) than other samples.
The PP isolate exhibits the highest total non-essential AA content (50.82 g/100 g), followed by MP (45.37 g/100 g), CP (44.19 g/100 g), and LP (43.22 g/100 g) concentrates, and FBP (37.55 g/100 g). The functional properties, such as WHC and OHC, are highly related to the polar and nonpolar AA composition. The higher the polar AAs, the greater the WHC and the lower the OHC, and vice versa. The polar AAs and non-polar AAs were observed to be thigher in the PP isolate (50.59 g/100 g; 30.43 g/100 g), while the lowest was in FBP (37.62 g/100 g; 10.12 g/100 g). Overall, the results confirm that increasing processing purity influences the AA compositions. A minor difference was observed among the concentrates, whereas major differences were observed when comparing protein powder, concentrates, and isolates. And to achieve a better nutritional profile, a mixture of protein sources can be used for different food product formulations.41
3.3 In vitro protein digestibility (IVPD)
IVPD of legume derivatives are presented in Table 3 The digestibility of a protein reflects the extent to which it is broken down by digestive enzymes and its subsequent bioavailability.42 Plant proteins encompass a range of antinutritional components, such as tannic acid, lecithin, saponin, phytic acid, trypsin, and protease inhibitors, which influence the digestive process. Among the derivatives, the PP isolate exhibited a higher IVPD (84.39 ± 0.5%), while FBP showed the lowest (64.34 ± 0.32%). There is no significant change in the IVPD of concentrates of LP (77.57 ± 0.61%) and MP (80.25 ± 0.30%), whereas a slight decrease in CP (76.21 ± 0.41%) was observed. The difference could be due to the variations in the processing conditions. During the process of extraction, samples undergo various chemical (alkali and acid) and thermal treatments, which reduce the antinutritional factors and thereby enhance the digestibility. Pastor-Cavada et al. (2010)43 reported that isolates of L. clymenum have higher digestibility (95 ± 0.9%) than their flour (80.30 ± 1.53). The protein isolation process results in a reduction of antinutritional factors by approximately 60–70%.44 The study, which compared the IVPD of various pulse flours and concentrates, found similar results. The concentrates of FBP, LP, chickpea, and red gram protein concentrates showed higher digestibility (80–85%) than their protein flours (71–77%). Protein flour contains more antinutritional factors that hinder the digestibility process compared to isolates and concentrates.| Quality parameters | PP | CP | MP | LP | FBP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 year | Adult (18+) | 0.5 year | Adult (18+) | 0.5 year | Adult (18+) | 0.5 year | Adult (18+) | 0.5 year | Adult (18+) | ||
| a FBP = fava bean protein, PP = pea protein, MP = mung bean protein, LP = lentil protein, AAS = amino acid score, PDCAAS = protein digestibility corrected amino acid score, IVPD = in vitro protein digestibility, PER = protein efficiency ratio, BV = biological value, His = histidine, Iso = isoleucine, Leu = leucine, Thr = Threonine, Try = Tryptophan, Val = valine, Met = methionine, Cys = cysteine, Phe = phenylalanine, Tyr = Tyrosine. | |||||||||||
| AAS | His | 95.0 | 126.7 | 70.5 | 94.0 | 83.0 | 110.7 | 51.0 | 68.0 | 31.0 | 41.3 |
| Iso | 115.6 | 123.3 | 37.2 | 39.7 | 38.4 | 41.0 | 97.8 | 104.3 | 14.1 | 15.0 | |
| Leu | 97.0 | 108.5 | 51.4 | 57.5 | 52.3 | 58.5 | 91.4 | 102.2 | 31.8 | 35.6 | |
| Lys | 86.4 | 126.7 | 56.5 | 82.9 | 54.1 | 79.3 | 49.2 | 72.2 | 36.5 | 53.6 | |
| Thr | 42.4 | 121.7 | 20.0 | 57.4 | 26.4 | 75.7 | 20.9 | 60.0 | 32.3 | 92.6 | |
| Try | 10.6 | 116.7 | 6.4 | 70.0 | 5.0 | 55.0 | 3.5 | 38.3 | 2.1 | 23.3 | |
| Val | 60.6 | 102.6 | 17.0 | 28.7 | 15.9 | 26.9 | 40.0 | 67.7 | 18.2 | 30.8 | |
| Met + Cys | 15.3 | 45.9 | 11.5 | 34.5 | 10.8 | 32.3 | 4.4 | 13.2 | 1.4 | 4.1 | |
| Phe + Tyr | 92.4 | 160.5 | 58.8 | 102.1 | 60.8 | 105.5 | 57.4 | 99.7 | 37.0 | 64.2 | |
| — | PDCAAS | 12.9 | 38.74 | 8.90 | 26.80 | 8.60 | 25.90 | 3.30 | 10.05 | 0.90 | 2.63 |
| IVPD (%) | 84.39 ± 0.5 | 77.57 ± 0.61 | 80.25 ± 0.3 | 76.21 ± 0.41 | 64.34 ± 0.32 | ||||||
| PER1 | 2.05 | 0.70 | 0.73 | 1.9 | 0.15 | ||||||
| PER2 | 1.92 | 1.04 | 1.01 | 0.83635 | 0.48 | ||||||
| PER3 | 3.26 | 1.02 | 1.16 | 3.01 | 0.17 | ||||||
| BV | 45.27 | 39.42 | 37.20 | 40.61 | 30.01 | ||||||
3.4 Amino acid score (AAS), protein digestibility corrected amino acid score (PDCAAS), protein efficiency ratio (PER), and biological value (BV)
The quality parameters of proteins, such as the AAS, PDCAAS, predicted PER, and predicted BV based on the numerical model equation, are presented in Table 3. The AAS indicates the essential AAs relative to the reference AA diet requirement, based on the FAO/WHO pattern for adults (>18 years) and infants (0.5 years). The score determines whether the protein could fulfill the dietary requirement. PP showed above 100 score for all the essential AAs except for methionine and cystine, which indicates that it is almost fulfilling the requirement for adults. In the case of CP and MP, isoleucine, valine, methionine, and cystine are limited, and all others showed more than 50 AAS, which indicates that the protein is partially providing the requirement for adults. Similar to PP, LP concentrates exhibited limited sulphur-containing AAs (13.2). Concentrates and protein powders didn't fulfill the complete dietary requirement. Therefore, combining different sources of proteins is essential to meet the complete protein requirement. The AAS varies based on age group, physical condition, and activity.40 Infants require a higher AAS and protein quality than adults for their growth and development.40,45 Most of the essential AAs are not fulfilling the requirement for infants, as the AAS is less than 100. Galves et al. (2025)46 studied different genotypes of PP and found that their AAS can vary from 32 to 88. Stone et al. (2015)47 revealed that sulfur amino acids such as methionine and cystine are limited in legume proteins.The calculation of the PDCAAS was based on the limiting AAS, which was methionine + cystine. The amount of these two AAs was the lowest, and the sum was varied from 0.09–1.01 g/100 g of protein among all the AAs. Consequently, the value of the PDCAAS was very low for all the derivatives. The PP isolate exhibited 38.72%, while CP and MP had 26.79% and 25.89% respectively, for adults. The values of LP (10.05%) and FBP (2.63%) were the lowest among the derivatives. In legume proteins, sulfur-containing amino acids are very low, and for FBP isolates, they varied between 0.6 and 3.0%.48 Similar results were reported by Shrestha et al., (2023)20 in which the sum ranged from 0.3 to 1.1 g/100 g of protein. In such a case, the value of PDCAAS is comparable with that in the present study. However, a study on L. clymenum and L. annuus protein flour and isolate showed PDCAAS values of 45–52% and 20% respectively.43
The PER and BV were calculated using the numerical predictive model equation based on the amino acid content. Three theoretical PER values were computed based on leucine (PER1), leucine and tyrosine (PER2), and methionine, leucine, histidine, and tryptophan (PER3). The PER values of PP ranged between 1.92 and 3.26, with concentrates falling within the range of 0.99 to 2.97, whereas FBP showed values from 0.17 to 0.48. A similar method was employed for computing the PER of FBP flour and isolate, and it was observed that the value ranged between 2.5 and 2.8 for flour and 2.6 and 2.9 for isolate.48 Chavan et al. (2001)49 reported the PER value of the alkaline extracted PP isolate to be 2.75–2.81 based on predicted model equations.
The BV predicted from the model equation is presented in Table 3. Among the derivatives, the PP isolate exhibited the highest value (45.27), followed by concentrates of LP (40.61), CP (39.42), and MP (37.20), whereas FBP powder (30.01) showed the lowest. Comparable results were observed for the alkaline-extracted PP isolate, which showed a BV of 36.5.49 In contrast, the sunflower protein isolate exhibited a much lower BV (17.80) when assessed based on a similar numerical method.43 Furthermore, Vioque et al. (2012)48 reported the BV of FB flour as 40.2 and 47.9 for the corresponding isolate.
3.5 Determination of functional properties
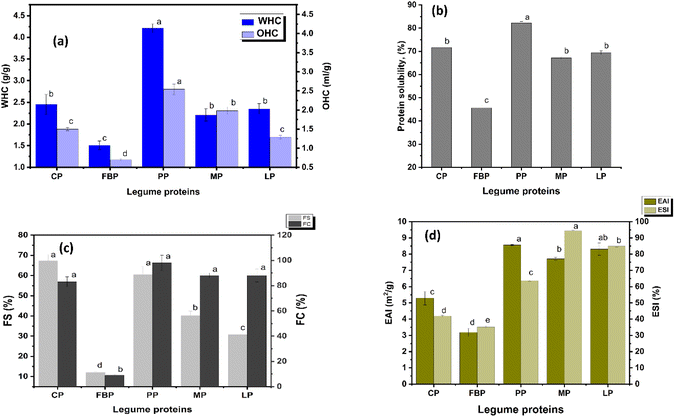
The PP isolate showed higher WHC and OHC (4.21 ± 0.029 g g−1 and 2.54 ± 0.14 mL g−1, respectively) compared to protein concentrates (CP, MP, and LP) and protein powders (FBP) (Fig. 1a). According to Ma et al., (2022)51 WHC of the PP isolate was 5.14 ± 0.27 g g−1, exceeding the value obtained from the present investigation. The higher value can be attributed to the controlled lab extraction process and greater protein content of the PP isolate used in their work (85%). The functional properties of proteins are influenced by various factors, including protein concentration, pH, ionic strength, the ratio of polar and non-polar AAs, and the balance of other AA groups.51 Specifically, the charged AA side chains have a strong potential to bind water molecules. The total polar amino acid in the PP isolate was 50.59 g/100 g, which directly correlated with its WHC. In contrast, there was no significant difference in the WHC among MP, LP, and CP concentrates, whose polar amino acid contents (MP – 43.4 g/100 g, LP – 41.71 g/100 g, and CP – 44.19 g/100 g) also showed an insignificant effect. Similarly, the lower WHC of FBP was observed, as both its protein content (20.62 ± 1.32%) and polar amino acid constituents (37.62 g/100 g) were comparatively low. This suggests that higher polar amino acids can form stronger H-bonds.52,53 Soy flour has higher WHC compared to CP and MP flour due to its higher polar amino acids. A study reported that higher WHC of pulse protein concentrates compared to protein powders was due to their high protein content.54 The finding of L. de Paiva Gouvêa et al. (2023)55 indicated that WHC of isolate pulse protein (soy protein and PP isolates) procured from a commercial market exceeded 4 g g−1, which aligned with the present study. Similar observations were reported by Megha & Grant (1986),56 who found that concentrates of PP had higher WHC and OHC than their flour.
Compared to the WHC of all the proteins, OHC was lower due to the higher amount of polar AAs and the hydrophilic nature of proteins. Similar observations were reported by Sosulski & McCurdy (1987).57 In line with WHC, the PP isolate exhibited higher OHC, which was attributed to its non-polar AAs (30.43 g/100 g), and could benefit oil-rich food formulations. CP, MP, and LP showed a non-significant difference in OHC, which directly correlated with their non-polar AA contents. The OHC of legume derivatives, including soy, PP, FBP, and common bean, varied from 1.22 to 2.84 mL g−1.55 The present study revealed that the values ranged from 1.29–2.54 mL g−1, with the exception of FBP powder (0.7 mL g−1). and this is evident from their lower non-polar AA content (10.12 g/100 g).
The functional properties of proteins are essential in product formulation and processing, significantly influencing the quality and consumer acceptability of the final product. Proteins with high WHC and OHC can effectively retain moisture and oil. This directly impacts the textural attributes of the product, such as juiciness, tenderness, flavour retention, and mouthfeel,58 qualities that are highly preferred by consumers, especially in meat products.59,60 Considering the PB meat analogue, replicating the sensory characteristics of traditional meat products is essential for consumer satisfaction. Juiciness and tenderness are two of the most important textural qualities consumers associate with meat. To mimic these attributes in PB formulations, it is crucial to select and utilize protein derivatives with comparatively high WHC. PB nuggets formulated with the PP isolate (72.3 ± 0.7 g/100 g protein and 26.17 ± 2.2 g per g WHC), resulted in improvement in textural properties.61 Similarly, PB sausages made with 30% CP curd showed comparable results, indicating their potential as a suitable alternative to processed meat.62
MP and LP concentrates exhibited lower FS (40.1 ± 2.3% and 30.84 ± 2.5%, respectively) compared to the pea variety. Various studies reported that higher fat content in the protein derivatives produces unstable forms, due to the interference of lipids at the interface, which hinders the adsorption of protein onto the air–water interface. The fat content of MP and LP was 4.58 ± 0.20% and 2.32 ± 0.13% respectively, which contributed to their low stability. A study reported that the FS of commercial chickpea concentrates varied between 34 and 40%, while for PP it varied between 76 and 79%, and they correlated the results with total protein content.75
FC and FS are crucial in both egg and milk alternative products, significantly affecting the texture and sensory attributes. FBP isolate microgels were used as an ingredient for making whipping cream, and they could form a stable gel-like structure.76 In general, casein and whey protein are the key ingredients for whipping cream. Recent studies reorted that MP, soybean protein, and kidney bean protein can also be used as alternatives to animal protein to develop whipping cream.71,77,78 Also, in the dairy industry, foaming properties help in incorporating air into the mixture, which affects the overrun (the amount of air incorporated) and creaminess of the final product.79 PB yogurt was developed from PP and MP by the fermentation of proteins. Their superior foaming properties and hydrophobic interactions resulted in the formation of stable gels.80 The application of MP as an ingredient for an egg alternative was investigated, and it was observed that pH shifting and calcium addition enhance the foaming stability.81 Isolates and concentrates of derivatives showed superior foaming properties, which can form stable gel-like structures and provide further scope in the development of dairy and egg alternative products.
3.6 Determination of thermal properties
The protein thermal stability was analyzed by DSC (Fig. 2). The peak temperature represents the denaturation temperature, and it is associated with the higher thermal stability of globular proteins.85 One endothermic peak was observed in all the samples. The derivatives exhibited a single endothermic peak, likely corresponding to the denaturation of 7S or 8S vicilin.86 According to Kudre et al. (2013),85 protein thermal stability is strongly linked to their conformations, secondary structures, and AA composition.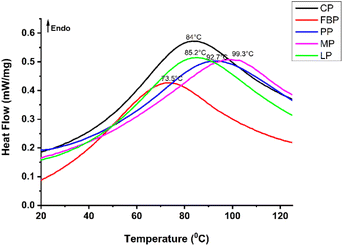 | ||
| Fig. 2 Differential scanning calorimetry (DSC) curve for legume proteins. CP = cowpea protein, FBP = fava bean protein, PP = pea protein, MP = mung bean protein, LP = lentil protein. | ||
The MB concentrate demonstrated a higher peak value at 99.3 °C, indicating a higher proportion of β-sheet conformations. This supports the positive correlation between the denaturation peak temperature and proportion of β-sheets. Higher peak temperatures are associated with the amount of more ordered structure of the α-helix, β-sheets, and β-turns.31 Comparable findings were reported for the kidney bean isolate, which exhibited higher thermal stability compared with the field PP isolate, which is attributed to its respective β-sheet structure.87 The data showed that thermal stability decreased in the order of MP, PP, LP, CP, and FBP (Table 4). A less ordered structure of α-helix, β-sheets, and β-turns was observed in FBP, which shows its lower thermal stability (73.5 °C). A study compared the thermal stability of lab-extracted and commercial protein isolates. They reported the presence of two denaturation peaks in the lab-extracted protein isolate, which indicated its higher thermal stability compared to the commercially available proteins.20 Shand et al. (2007)73 reported that the denaturation peak for the PP isolate and soy protein isolate was observed at 85.07 °C and 92.71 °C, respectively. Furthermore, Tang observed comparable findings to the present study, where legume protein isolates including MB, red, and kidney bean, exhibited denaturation temperatures between 87.12 and 94.9 °C.86 The enthalpy (ΔH) value represents the extent of the ordered structure. FBP showed a lower ΔH (172.5 J g−1) compared to other legume derivatives (varied between 190.6 and 213.8 J g−1), and it is correlated with their secondary structure analysis results. The reduced thermal stability of commercial proteins might be due to severe processing conditions, including high pH and temperature.73
| CP | FBP | PP | MP | LP | |
|---|---|---|---|---|---|
| a FBP = fava bean protein, PP = pea protein, MP = mung bean protein, LP = lentil protein. | |||||
| Peak temperature (tp) °C | 84.0 | 73.5 | 92.7 | 99.3 | 85.2 |
| Onset °C | 45.5 | 35.2 | 49.0 | 54.5 | 43.5 |
| Inflection °C | 59.5 | 49.8 | 68.9 | 108.4 | 60.4 |
| End °C | 77.7 | 60.6 | 80.2 | 90.1 | 79.6 |
| Enthalpy (ΔH) J g−1 | 200.2 | 172.5 | 196.7 | 190.6 | 213.8 |
3.7 Determination of molecular weight
The molecular weights of soluble fractions of protein are obtained from spectral analysis (Fig. SI1). The MW observed are, the PP isolate has MW < 918.260 Da, MP concentrate has MW < 986.250 Da, CP concentrate has MW < 986.249 Da, LP concentrate has MW < 559.639 Da, and FBP has MW < 986.249 Da. Comparable findings for the PP isolate were noted by Chang et al. (2021).32 The MW data were further used for calculating the percentage of the secondary structure of legume protein derivatives by CD.3.8 Determination of structural properties
Variations in secondary structures were observed among pulse protein sources, influencing their functional properties, thermal stability, and digestibility.88 In general, the protein bands in FTIR spectra are observed in the amide I (1700–1600 cm−1), amide II (1580–1500 cm−1), and amide III (1400–1200 cm−1) regions.89 The amide-I region (1700–1600 cm−1) of the IR spectra of proteins is highly sensitive to the secondary structure (β-sheet, α-helix, random coils, and β-turns). Therefore, the region was analysed to estimate the distribution of secondary structures in legume proteins. The specific absorption bands were used to represent the secondary structures: 1640–1610 cm−1 for β-sheets, 1660–1650 cm−1 for α-helices, 1700–1660 cm−1 for β-turns, and 1650–1640 cm−1 for random coils.87 The FTIR spectra of all proteins are presented in Fig. 3, and the content of each secondary structure is summarized in Table 5. The secondary structures were estimated to be 12–33% α-helix 10–27% c, 8–12% β-turns, and 22–40% random coil structures. In contrast, concentrates of PP, jack bean protein, and soy protein isolate showed a much higher percentage of β-sheet structure (53–64%) than in the present study.69 FBP showed decreased α-helix and β-structures and increased random coils; the simple reason for this is the lower thermal stability due to the thermal process.90 The DSC result of FBP supports the same. The proportion of α-helix (33.30%) was higher in the PP isolate, followed by MP (31.10%), CP (30.02%), and LP (27.10%) concentrates. Similar observations were reported by Shrestha et al. (2023)20 for the commercial PP isolate and soy protein isolate (α-helix-33.89%, β-turns-12.1%, β sheet-24.96% and random coil-29.04%; α-helix-19.5%, β-turns-14.91%, β sheet-18.87% and random coil-46.71%, respectively).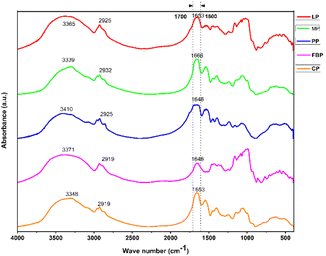 | ||
| Fig. 3 Fourier transform infrared (FTIR) spectroscopy of legume proteins. CP = cowpea protein, FBP = fava bean protein, PP = pea protein, MP = mung bean protein, LP = lentil protein. | ||
| Proteins | β-Turn (%) | α-Helix (%) | Random coil (%) | β-Sheet (%) | |
|---|---|---|---|---|---|
| a FBP = fava bean protein, PP = pea protein, MP = mung bean protein, LP = lentil protein. | |||||
| FTIR | CP | 12.10 | 30.02 | 22.09 | 23.63 |
| FBP | 8.12 | 12.30 | 40.10 | 10.37 | |
| PP | 12.24 | 33.30 | 23.16 | 24.61 | |
| MP | 12.15 | 31.10 | 26.72 | 26.87 | |
| LP | 10.21 | 27.10 | 24.23 | 20.15 | |
| CD | CP | 14.10 | 6.20 | 42.20 | 37.50 |
| FBP | 14.40 | 3.50 | 43.10 | 39.10 | |
| PP | 14.60 | 4.40 | 43.30 | 38.00 | |
| MP | 14.80 | 10.20 | 41.50 | 33.90 | |
| LP | 14.40 | 3.50 | 43.10 | 39.00 | |
The soluble protein fractions of derivatives are analysed from CD spectra. The region in the UV spectra 190–260 nm where the peptide bond dominates helps identify the secondary structure composition.91 In the CD spectra, CP and MP concentrates exhibited a strong positive peak at 190–198 nm and a broad negative peak at 205–230 nm (Fig. 4). A CD spectrum of α-helix rich proteins shows a positive (190–190 nm) and two broad negative peaks (208 nm and 222 nm range). The observed negative peak at 222 nm is associated with the ‘hydrogen-bonding’ pattern specific to the α-helical structure and is generally consistent regardless of helix length. In general, proteins show a negative band within the 210–220 nm range, along with a positive peak near 195–200 nm. In contrast, the unfolded or random coil conformation exhibits a strong negative peak close to 200 nm, reflecting its disordered nature.91–93 Meanwhile, the PP isolate had minimum negative ellipticity at 195–215 nm. Except for CP and MP, no other protein samples showed a positive peak at 190–198 nm. This might be due to the protein solubility and extraction techniques. MB (10.2%) exhibited a higher proportion of α-helix among the derivatives, followed by CP, PP, LP, and lastly FBP. The proportion of random coil was higher for FBP (40.10%), indicating its reduced thermal stability. This observation was further supported by the DSC curve obtained, which reflected the secondary structure characteristics. Shrestha et al. (2023)20 noted the secondary structure through CD spectral analysis, estimating that the α-helix content ranged from 10.3 to 14.5% for lab-extracted legume protein isolates, whereas for commercial samples, it was between 7.8 and 7.6%. Additionally, it was noted that there are no significant difference in β turns, β sheets, and random coils between lab-extracted and commercially available derivatives.
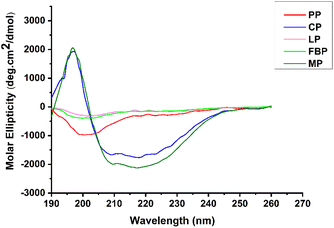 | ||
| Fig. 4 Circular dichroism (CD) spectra of legume proteins. CP = cowpea protein, FBP = fava bean protein, PP = pea protein, MP = mung bean protein, LP = lentil protein. | ||
3.9 Correlation and multivariate analysis
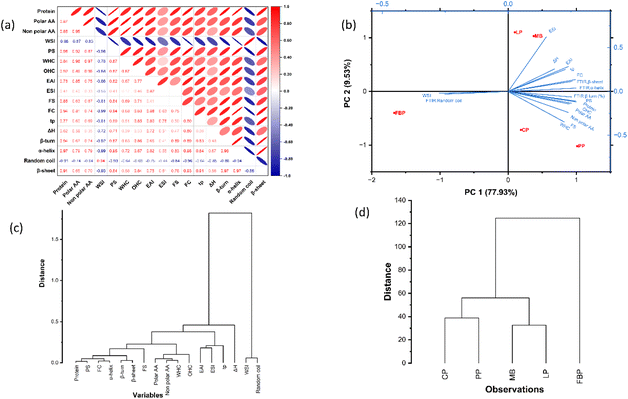
The study helps to illustrate the relationship between two or more variables. Furthermore, it evaluates the ‘extent’ or ‘strength’ of association between the variables, along with its direction indicated by the correlation coefficient (r), which ranges from −1 to +1.94 Pearson's correlation was employed to study the relationships among the variables, with a higher absolute value representing the strong correction between them.Fig. 5a illustrates Pearson's correlation matrix of various characteristics of legume derivatives. The strength and direction of associations are depicted by colour and shape. Positive and negative correlations are indicated by red and blue, respectively, with opposite orientations. In addition to that, more intense colour and a thinner ellipse illustrate stronger association between the variables.
Protein content showed a strong correlation with functional properties (r value varies from 0.84 to 0.98) and ordered structure of proteins (β-sheet, r = 0.91, α-helix, r = 0.97 and β-turns, r = 0.94). A study indicated that the increased WHC of pulse protein concentrates in comparison to protein powders can be attributed to their higher protein content.54 Protein isolates generally exhibit enhanced functional and structural properties in comparison to flour, owing to their purity.35 The purity of protein ensures the availability of functional groups, exposing binding sites, structural unfolding, and their interactions, resulting in superior characteristics, whereas in flour, the inclusion of other components such as carbohydrates, lipids, and fats impairs the same.95
The significant correlation of WHC with polar AAs (r = 0.96) and OHC with non-polar AAs (r = 0.96) confirms the increased WHC and OHC in the PP isolate compared to other legume derivatives. The β-sheet secondary structure was positively correlated with denaturation temperature, tp (r = 0.91). β-Sheet secondary structures are more stable than an α-helix; therefore, the protein with a higher fraction of β-sheets exhibits high denaturation temperatures. The WSI is negatively correlated with other functional properties of the protein (foaming, emulsification, and protein solubility); the inverse relationship may be explained by non-protein constituents that hinder the functionality.
PCA of the dataset describes the association and interactions among the variables. The database can be represented in either a two or three-dimensional format, where the axis corresponds to factors (principal component, PC). Every PC is a linear combination of the responses and are orthogonal to each other.
The analysis involved two principal components – PC1 and PC2, which together explained 87.46% of the overall variability, with PC1 contributing 77.93% while PC2 contributed 9.53% of the variance. Fig. 5b depicts the bi-plot between PC1 and PC2, illustrating the positive correlation of the responses, which includes ESI, EAI, FC, FS, β-sheet, β-turns, α-helix, polar AA, nonpolar AA, WHC, OHC, PS, denaturation temperature and enthalpy with PC1 and negative correlation of the WSI and random coil with PC1. The findings align closely with Pearson correlation analysis (Fig. 5 a).
The blue colour lines represent the variable vector and the length of those indicates how well the variables influence the PCs. The extended length of the vector originating from the origin reflects the strong influence of that principal component, while a shorter vector indicates a weaker contribution. Furthermore, the angle formed by the vectors provides insight into the relationship between the variables. A small angle between the vectors suggests a stronger positive correlation, while a larger angle indicates a negative correlation.96 In this regard, the biplot clearly demonstrates that the length of the ESI vector is greater than that of the other variables, which are nearly equal, suggesting its significant influence on the principal component. Furthermore, there is a favourable correlation between β-sheet, β-turn, and α-helix structures with functional characteristics and denaturation temperature. The relationship between protein purity (protein content) and the WSI is quite broad, suggesting a strong negative correlation or a lack of association altogether. The biplot clearly illustrates a strong positive correlation between the amino acid profile and both WHC and OHC, which aligns with the previously discussed correlation matrix.
The experimental data set was categorized using HCA based on their association, with the results presented as a dendrogram (Fig. 5c and d). The approach effectively visualizes the similarities among variables, generating a hierarchical/tree-like structure where Euclidean distance indicates the proximity among the variables. Fig. 5c illustrates the dendrogram of dependent variables; two distinct clusters are observed, in which cluster 1 grouped all the variables except the WSI and random coil, assembled in cluster 2. The HC for legume derivatives (Fig. 5d) also showed two clusters, isolates and concentrates grouped in cluster 1 and FBP in a separate cluster. This was evident from the statistical analysis conducted. Protein derivatives within the same clusters are more comparable than those in different clusters. The solubility, emulsification, and foaming properties are closely related, and this is evident from the small groups within cluster 1. The merging of these small subgroups identifies how functional properties are interconnected with structural and thermal properties.
Thus, it can be inferred that the type of protein and its purity are key factors influencing the functional and structural properties of plant proteins. Improved protein solubility can lead to greater functionality. Thermal and structural stability is important for specific applications, and appropriate processing conditions must be adopted.
4. Conclusions
The study highlights significant differences in the quality, and structural, functional, and thermal properties between commercially available legume proteins. When compared with lab-extracted proteins, commercially available proteins have notable differences in their structural, functional, and thermal properties due to the controlled processing conditions. The protein content and quality have a significant impact on the functionality of protein sources. In addition, the composition and arrangement of proteins greatly affect their properties. The PP isolate (protein content 75.01 ± 1.56%) exhibited superior functional properties compared to other derivatives. Isolates and concentrates of plant protein have superior functionalities, including emulsifying and foaming ability. Hence, selecting an appropriate derivative is essential to attain the desired attributes in the final product. However, the quality indices revealed that most of the derivatives do not fulfill the dietary requirements of humans. The presence of anti-nutritional factors reduces the digestibility. To minimize the effect, various pre-treatments should be employed. CD analysis revealed a notable presence of α-helical structures in the soluble-protein fractions of MB and CP concentrates. Investigating the potential of novel plant proteins in new product development brings benefits to both human health and the food industry. Through the exploration of different combinations of plant proteins, it is possible to achieve results that are comparable to animal proteins, thus creating alternatives to animal products. Recent studies have revealed exciting progress in the field of PB alternatives, providing consumers with a healthier and more sustainable choice. This understanding of the correlation between protein composition and functionality holds great promise for advancing the use of legume proteins in meat alternatives. Future research can further explore the application of these plant proteins in developing not only meat substitutes but also alternatives for eggs, dairy, fish, and other animal-based products, adding scope for a sustainable future. The good emulsification properties of MP and the foaming ability of CP can be explored for egg replacement in the bakery and plant egg areas. The superior characteristics of the PP isolate can be further explored in a meat alternative through extrusion and a shear cell structuring device. Adding a suitable gelling or stabilizing agent along with the plant protein improves the texture of the final product. By leveraging their functional properties, these proteins can contribute to the creation of high-quality, sustainable, and nutritious alternatives, addressing both consumer demands and industry needs.Author contributions
Harsha Varayil: conceptualization, formal analysis, investigation, methodology, writing – original draft. Jayeeta Mitra: investigation, data curation, validation, supervision, review & editing.Conflicts of interest
The authors have found no conflict of interest to declare.Data availability
The data that support the findings of this study are available upon request.Supplementary information (SI): spectral data of matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF). See DOI: https://doi.org/10.1039/d5fb00307e.
Acknowledgements
The authors express their gratitude for the financial support provided by the All India Coordinated Research Projects on Post Harvest Engineering & Technology, New Delhi, India.References
- FAO, How to Feed the World in 2050, Insights from an Expert Meeting at FAO, 2009, pp. , pp. 1–35, http://www.fao.org/wsfs/forum2050/wsfs-forum/en/ Search PubMed.
- I. Jain, R. Kaur, A. Kumar, M. Paul and N. Singh, Emerging protein sources and novel extraction techniques: a systematic review on sustainable approaches, Int. J. Food Sci. Technol., 2024, 6797–6820 CrossRef CAS.
- H. Khazaei and A. Vandenberg, Seed mineral composition and protein content of faba beans (Vicia faba L.) with contrasting tannin contents, Agronomy, 2020, 10(4), 511 CrossRef CAS.
- R. S. Bhatty and G. I. Christison, Composition and nutritional quality of peas (Pisum sativum L.), faba bean (Vicia faba L. spp. minor) and lentil (Lens culinaris Medik.) meals, protein concentrates and isolates, Plant Foods Hum. Nutr., 1984, 34, 41–51 CrossRef CAS.
- V. V. Garcia and C. W. Coffmann, Functional properties and amino acid content of a protein isolate from mung bean flour, Int. J. Food Sci. Technol., 1977, 12(76), 473–484 Search PubMed.
- R. Gupta and S. Dhillon, Characterization of seed storage proteins of Lentil (Lens culinaris M.), Ann. Biol., 1993, 9(1), 71–78 Search PubMed.
- M. Wang, L. Jiang, Y. Li, Q. Liu, S. Wang and X. Sui, Optimization of extraction process of protein isolate from mung bean, Procedia Eng., 2011, 15, 5250–5258, DOI:10.1016/j.proeng.2011.08.973.
- R. T. Ahnen, S. S. Jonnalagadda and J. L. Slavin, Role of plant protein in nutrition, wellness, and health, Nutr. Rev., 2019, 77(11), 735–747 CrossRef PubMed.
- L. Day, J. A. Cakebread and S. M. Loveday, Food proteins from animals and plants: Differences in the nutritional and functional properties, Trends Food Sci. Technol., 2022, 119, 428–442, DOI:10.1016/j.tifs.2021.12.020.
- Consultation FAOE, Dietary protein quality evaluation in human nutrition. Report of an FAQ Expert Consultation, FAO Food Nutr. Pap., 2013, 92, 1–66 Search PubMed.
- M. Tan, M. A. Nawaz and R. Buckow, Functional and food application of plant proteins–a review, Food Rev. Int., 2023, 39(5), 2428–2456, DOI:10.1080/87559129.2021.1955918.
- C. Coffmann and V. Garciaj, Functional properties and amino acid content of a protein isolate from mung bean flour, Int. J. Food Sci. Technol., 1977, 12, 473–484, DOI:10.1111/j.1365-2621.1977.tb00132.x.
- K. A. Millar, E. Gallagher, R. Burke, S. McCarthy and C. Barry-Ryan, Proximate composition and anti-nutritional factors of fava-bean (Vicia faba), green-pea and yellow-pea (Pisum sativum) flour, J. Food Compos. Anal., 2019, 82, 103233, DOI:10.1016/j.jfca.2019.103233.
- W. Kudełka, M. Kowalska and M. Popis, Quality of soybean products in terms of essential amino acids composition, Molecules, 2021, 26(16), 1–9 CrossRef PubMed.
- M. Appiani, C. Cattaneo and M. Laureati, Sensory properties and consumer acceptance of plant-based meat, dairy, fish and eggs analogs: a systematic review, Front. Sustain. Food Syst., 2023, 7, 1268068 CrossRef.
- J. I. Boye, F. Zare and A. Pletch, Pulse proteins: Processing, characterization, functional properties and applications in food and feed, Food Res. Int., 2010, 43(2), 414–431, DOI:10.1016/j.foodres.2009.09.003.
- R. Zolqadri and Y. Li, A Comprehensive Review of Cowpea Proteins: Chemistry, Extraction, Techno-Functionality, Modification, and Food Applications, Compr. Rev. Food Sci. Food Saf., 2025, 24(4), e70218 CrossRef CAS PubMed.
- D. De Angelis, V. Latrofa, G. Squeo, A. Pasqualone and C. Summo, Techno-functional, rheological, and chemical properties of plant-based protein ingredients obtained with dry fractionation and wet extraction, Curr. Res. Food Sci., 2024, 9, 100906, DOI:10.1016/j.crfs.2024.100906.
- L. Day, J. A. Cakebread and S. M. Loveday, Trends in Food Science & Technology Food proteins from animals and plants : Differences in the nutritional and functional properties, Trends Food Sci. Technol., 2022, 119, 428–442, DOI:10.1016/j.tifs.2021.12.020.
- S. Shrestha, L. V. Hag, V. Haritos and S. Dhital, Comparative study on molecular and higher-order structures of legume seed protein isolates: lentil, mungbean and yellow pea, Food Chem., 2023, 411, 135464, DOI:10.1016/j.foodchem.2023.135464.
- AOAC, Official Methods of Analysis, Association of Official Analytical Chemists, Washington (DC), 18th edn, 2005 Search PubMed.
- M. S. Nimbalkar, S. R. Pai, N. V. Pawar, D. Oulkar and G. B. Dixit, Free amino acid profiling in grain Amaranth using LC-MS/MS, Food Chem., 2012, 134(4), 2565–2569, DOI:10.1016/j.foodchem.2012.04.057.
- Z. K. Terefe, M. N. Omwamba and J. M. Nduko, Effect of solid state fermentation on proximate composition, antinutritional factors and in vitro protein digestibility of maize flour, Food Sci. Nutr., 2021, 9(11), 6343–6352 CrossRef CAS PubMed.
- R. H. Alsmeyer and A. E. Cunningham MLH, Analysis, Equations predict PER from amino acid, Food Technol., 1974,(28), 34–38 CAS.
- I. L. K. Mørup and E. S. Olesen, New method for prediction of protein value from essential amino acid pattern, Nutr. Rep. Int., 1976, 13, 355–365 Search PubMed.
- M. J. Amaresh and M. Kaushal, Influence of incorporation of peanut protein isolate on pasting, rheological and textural properties of rice starch, J. Food Eng., 2023, 34, 111312, DOI:10.1016/j.jfoodeng.2022.111312.
- V. Stojceska, P. Ainsworth, A. Plunkett and Ş. Ibanoǧlu, The effect of extrusion cooking using different water feed rates on the quality of ready-to-eat snacks made from food by-products, Food Chem., 2009, 114(1), 226–232 CrossRef CAS.
- K. Tsumura, T. Saito, K. Tsuge, H. Ashida, W. Kugimiya and K. Inouye, Functional properties of soy protein hydrolysates obtained by selective proteolysis, LWT–Food Sci. Technol., 2005, 38(3), 255–261 CrossRef CAS.
- F. F. Liu, Y. Q. Li, C. Y. Wang, X. Z. Zhao, Y. Liang and J. X. He, et al., Impact of pH on the physicochemical and rheological properties of mung bean (Vigna radiata L.) protein, Process Biochem., 2021, 111(P1), 274–284, DOI:10.1016/j.procbio.2021.10.008.
- S. Jiang, M. Altaf hussain, J. Cheng, Z. Jiang, H. Geng and Y. Sun, et al., Effect of heat treatment on physicochemical and emulsifying properties of polymerized whey protein concentrate and polymerized whey protein isolate, LWT–Food Sci. Technol., 2018, 98, 134–140, DOI:10.1016/j.lwt.2018.08.028.
- Y. Shen, Z. Du, X. Wu and Y. Li, Modulating molecular interactions in pea protein to improve its functional properties, J. Agric. Food Res., 2022, 8, 100313, DOI:10.1016/j.jafr.2022.100313.
- C.-Y. Chang, J.-D. Jin, H.-L. Chang, K.-C. Huang, Y.-F. Chiang and M. Ali, Antioxidative Activity of Soy, Wheat and Pea Protein Isolates Characterized by Multi-Enzyme Hydrolysis, Nanomaterials, 2021, 11(6), 1509 CrossRef CAS PubMed.
- Y. Premjit and J. Mitra, Synthesis, characterization, and in vitro digestion of electrosprayed and freeze-dried probiotics encapsulated in soy protein isolate-sunflower oil emulsions, Food Biosci., 2023, 53, 102532, DOI:10.1016/j.fbio.2023.102532.
- N. J. Greenfield, Using circular dichroism spectra to estimate protein secondary structure, Nat. Protoc., 2007, 1(6), 2876–2890 CrossRef PubMed.
- K. K. Ma, L. Grossmann, A. A. Nolden, D. J. McClements and A. J. Kinchla, Functional and physical properties of commercial pulse proteins compared to soy derived protein, Future Foods, 2022, 6, 7 CrossRef.
- K. Jakobson, A. Kaleda, K. Adra, M.-L. Tammik, H. Vaikma, T. Kriščiunaite and R. Vilu, Techno-functional and sensory characterization of commercial plant protein powders, Foods, 2023, 12(14), 2805 CrossRef CAS PubMed.
- M. Kaur and N. Singh, Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars, Food Chem., 2007, 102(1), 366–374 CrossRef CAS.
- S. Akhtar, T. Ismail, A. Layla, M. Hussain and M. Qamar, An Overview of Plant-Based Protein Rich Products, in Plant Protein Foods, ed. A. Manickavasagan, L. T. Lim and A. Ali, Springer International Publishing, Cham, 2022, pp. 27–60, DOI:10.1007/978-3-030-91206-2_2.
- M. G. Nosworthy, J. Neufeld, P. Frohlich, G. Young, L. Malcolmson and J. D. House, Determination of the protein quality of cooked Canadian pulses, Food Sci. Nutr., 2017, 896–903 CrossRef CAS PubMed.
- WHO/FAO/UNU, WHO/FAO/UNU Expert Consultation Protein and Amino Acid Requirements in Human Nutrition, W. H. O. Tech. Rep. Ser., 2007, 935, 1–284 Search PubMed.
- A. G. A. Sá, Y. M. F. Moreno and B. A. M. Carciofi, Food processing for the improvement of plant proteins digestibility, Crit. Rev. Food Sci. Nutr., 2020, 60(20), 3367–3386, DOI:10.1080/10408398.2019.1688249.
- Z. F. Bhat, J. D. Morton, A. E. D. A. Bekhit, S. Kumar and H. F. Bhat, Non-thermal processing has an impact on the digestibility of the muscle proteins, Crit. Rev. Food Sci. Nutr., 2022, 62(28), 7773–7800, DOI:10.1080/10408398.2021.1918629.
- E. Pastor-Cavada, R. Juan, J. E. Pastor, M. Alaiz and J. Vioque, Protein isolates from two Mediterranean legumes: Lathyrus clymenum and Lathyrus annuus. Chemical composition, functional properties and protein characterisation, Food Chem., 2010, 122(3), 533–538, DOI:10.1016/j.foodchem.2010.03.002.
- M. Samtiya, R. E. Aluko and T. Dhewa, Plant food anti-nutritional factors and their reduction strategies: an overview, Food Prod., Process. Nutr., 2020, 2(1), 1–14 CrossRef.
- D. J. Millward, Amino acid scoring patterns for protein quality assessment, Br. J. Nutr., 2012, 108(S2), 31–42 CrossRef PubMed.
- C. Galves, K. K. Gali, T. D. Warkentin, J. House and M. Nickerson, High- and Low-Protein Pea Genotypes: a Comparative Study of the Physicochemical, Functional, and Quality Properties of Protein Isolates, Cereal Chem., 2025, 102(4), 697–710 CrossRef CAS.
- A. K. Stone, N. A. Avarmenko, T. D. Warkentin and M. T. Nickerson, Functional properties of protein isolates from different pea cultivars, Food Sci. Biotechnol., 2015, 24(3), 827–833 CrossRef CAS.
- J. Vioque, M. Alaiz and J. Girón-Calle, Nutritional and functional properties of Vicia faba protein isolates and related fractions, Food Chem., 2012, 132(1), 67–72, DOI:10.1016/j.foodchem.2011.10.033.
- U. D. Chavan, D. B. McKenzie and F. Shahidi, Functional properties of protein isolates from beach pea (Lathyrus maritimus L.), Food Chem., 2001, 74(2), 177–187 CrossRef CAS.
- S. Samard and G. H. Ryu, A comparison of physicochemical characteristics, texture, and structure of meat analogue and meats, J. Sci. Food Agric., 2019, 99(6), 2708–2715 CrossRef CAS PubMed.
- K. K. Ma, L. Grossmann, A. A. Nolden, D. J. McClements and A. J. Kinchla, Functional and physical properties of commercial pulse proteins compared to soy derived protein, Future Foods, 2022, 6, 7 CrossRef.
- S. Scheiner, T. Kar and Y. Gu, Strength of the CαH··O Hydrogen Bond of Amino Acid Residues, J. Biol. Chem., 2001, 276(13), 9832–9837, DOI:10.1074/jbc.M010770200.
- L. Menon, S. D. Majumdar and U. Ravi, Development and analysis of composite flour bread, J. Food Sci. Technol., 2015, 52(7), 4156–4165 CrossRef PubMed.
- S. Ruckmangathan, H. Ganapathyswamy, A. Sundararajan, U. Thiyagamoorthy, R. Green and T. Subramani, Physico-chemical, structural, and functional properties of protein concentrate from selected pulses: A comparative study, J. Food Process. Preserv., 2022, 46(12), 1–12 Search PubMed.
- L. de Paiva Gouvêa, R. Caldeira, T. de Lima Azevedo, M. C. Galdeano, I. Felberg and J. R. Lima, et al., Physical and techno-functional properties of a common bean protein concentrate compared to commercial legume ingredients for the plant-based market, Food Hydrocolloids, 2023, 137, 108351 CrossRef.
- A. V. Megha and D. R. Grant, Effect of Heat on the Functional Properties of Pea Flour and Pea Protein Concentrate, Can. Inst. Food Sci. Technol. J., 1986, 19(4), 174–180, DOI:10.1016/S0315-5463(86)71627-1.
- F. W. Sosulski and A. McCurdy, Functionality of Flours, Protein Fractions and Isolates from Field Peas and Faba Bean, J. Food Sci., 1987, 52(4), 1010–1014 CrossRef.
- M. Kumar, M. Tomar, J. Potkule, P. S. Reetu and J. Dhakane-Lad, et al., Functional characterization of plant-based protein to determine its quality for food applications, Food Hydrocolloids, 2022, 123, 106986, DOI:10.1016/j.foodhyd.2021.106986.
- R. J. Polkinghorne and J. M. Thompson, Meat standards and grading. A world view, Meat Sci., 2010, 86(1), 227–235, DOI:10.1016/j.meatsci.2010.05.010.
- L. B. Acebrón and D. C. Dopico, The importance of intrinsic and extrinsic cues to expected and experienced quality: an empirical application for beef, Food Qual. Prefer., 2000, 11(3), 229–238 CrossRef.
- H. G. Zhu, H. Q. Tang, Y. Q. Cheng, Z. G. Li and L. T. Tong, Potential of preparing meat analogue by functional dry and wet pea (Pisum sativum) protein isolate, LWT–Food Sci. Technol., 2021, 148, 111702, DOI:10.1016/j.lwt.2021.111702.
- D. F. Rosida, D. Elianarni and U. Sarofa, Optimation 1,2 formulation of meat analog from cowpea (Vigna unguiculata L Walp) protein curds and cocoyams (Xanthosoma sagittifolium) modification starch as filler, Int. J. Food Sci. Technol., 2022, 42, 1–8 Search PubMed.
- A. Kaleda, K. Talvistu, H. Vaikma, M. L. Tammik, S. Rosenvald and R. Vilu, Physicochemical, textural, and sensorial properties of fibrous meat analogs from oat-pea protein blends extruded at different moistures, temperatures, and screw speeds, Future Foods, 2021, 4, 100092, DOI:10.1016/j.fufo.2021.100092.
- M. A. Mune Mune, S. R. Minka and I. L. Mbome, Optimising functional properties during preparation of cowpea protein concentrate, Food Chem., 2014, 154, 32–37, DOI:10.1016/j.foodchem.2013.12.108.
- N. J. E. de Mesa, S. Alavi, N. Singh, Y. C. Shi, H. Dogan and Y. Sang, Soy protein-fortified expanded extrudates: Baseline study using normal corn starch, J. Food Eng., 2009, 90(2), 262–270 CrossRef CAS.
- K. Gao, J. Rao and B. Chen, Plant protein solubility: a challenge or insurmountable obstacle, Adv. Colloid Interface Sci., 2024, 324, 103074, DOI:10.1016/j.cis.2023.103074.
- D. De Angelis, V. Latrofa, G. Squeo, A. Pasqualone and C. Summo, Techno-functional, rheological, and chemical properties of plant-based protein ingredients obtained with dry fractionation and wet extraction, Curr. Res. Food Sci., 2024, 9, 100906 CrossRef CAS PubMed.
- N. A. Hardt, A. J. van der Goot and R. M. Boom, Influence of high solid concentrations on enzymatic wheat gluten hydrolysis and resulting functional properties, J. Cereal Sci., 2013, 57(3), 531–536, DOI:10.1016/j.jcs.2013.03.006.
- J. E. G. Costa, J. D. S. Matos, P. Z. Azevedo, F. D. C. D. A. Souza, S. Rodrigues and F. A. N. Fernandes, et al., Techno-functionality of jack bean (Canavalia ensiformis) protein concentrate: a comparative study with soy and pea proteins, J. Sci. Food Agric., 2025, 105(6), 3439–3452 CrossRef CAS PubMed.
- T. G. Burger, I. Singh, C. Mayfield, J. L. Baumert and Y. Zhang, Comparison of physicochemical and emulsifying properties of commercial pea protein powders, J. Sci. Food Agric., 2022, 102(6), 2506–2514 CrossRef CAS PubMed.
- S. Wu, Z. Zhang, C. Liu and T. Ma, Effect of pH-shifting and sonication-assisted treatment on properties and stability of vegetable oil-based whipped cream stabilized by kidney bean protein aggregates, Food Hydrocolloids, 2023, 141(11), 108736, DOI:10.1016/j.foodhyd.2023.108736.
- J. Ge, C. X. Sun, A. Mata, H. Corke, R. Y. Gan and Y. Fang, Physicochemical and pH-dependent functional properties of proteins isolated from eight traditional Chinese beans, Food Hydrocolloids, 2021, 112, 106288, DOI:10.1016/j.foodhyd.2020.106288.
- P. J. Shand, H. Ya, Z. Pietrasik and P. K. J. P. D. Wanasundara, Physicochemical and textural properties of heat-induced pea protein isolate gels, Food Chem., 2007, 102(4), 1119–1130 CrossRef CAS.
- D. Chao and R. E. Aluko, Modification of the structural, emulsifying, and foaming properties of an isolated pea protein by thermal pretreatment, Cienc. Tecnol. Aliment., 2018, 16(1), 357–366, DOI:10.1080/19476337.2017.1406536.
- A. K. Stone, A. Karalash, R. T. Tyler, T. D. Warkentin and M. T. Nickerson, Functional attributes of pea protein isolates prepared using different extraction methods and cultivars, Food Res. Int., 2015, 76(P1), 31–38, DOI:10.1016/j.foodres.2014.11.017.
- J. Zhang, R. Zhu and Z. Meng, Whipped cream stabilized by faba bean protein isolate microgel particles substituted for sodium caseinate: whipping performance and foam stabilization analysis, Food Hydrocolloids, 2024, 157, 110388, DOI:10.1016/j.foodhyd.2024.110388.
- L. Fu, Z. He, M. Zeng, F. Qin and J. Chen, Effects of soy protein composition in recombined soy-based cream on the stability and physical properties of whipping cream, J. Sci. Food Agric., 2020, 100(6), 2732–2741 CrossRef CAS PubMed.
- L. Wei, K. Song, D. Shao, C. Dong, L. Wang and J. Jiang, The effect of endogenous phenol and gallic acid on structure and functionalities of mung bean protein in aerated emulsion system, Food Hydrocolloids, 2023, 143, 108942, DOI:10.1016/j.foodhyd.2023.108942.
- M. K. Hossain, M. Petrov, O. Hensel and M. Diakité, Microstructure and physicochemical properties of light ice cream: effects of extruded microparticulated whey proteins and process design, Foods, 2021, 10(6), 1433 CrossRef CAS PubMed.
- M. Yang, N. Li, L. Tong, B. Fan, L. Wang and F. Wang, et al., Comparison of physicochemical properties and volatile flavor compounds of pea protein and mung bean protein-based yogurt, LWT–Food Sci. Technol., 2021, 152, 112390, DOI:10.1016/j.lwt.2021.112390.
- Y. Wang, J. Zhao, S. Zhang, X. Zhao, Y. Liu and J. Jiang, et al., Structural and rheological properties of mung bean protein emulsion as a liquid egg substitute: The effect of pH shifting and calcium, Food Hydrocolloids, 2022, 126, 107485, DOI:10.1016/j.foodhyd.2022.107485.
- E. Dickinson, Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets, Food Hydrocolloids, 2012, 28(1), 224–241, DOI:10.1016/j.foodhyd.2011.12.017.
- K. K. Ma, M. Greis, J. Lu, A. A. Nolden, D. J. Mcclements and A. J. Kinchla, Functional Performance of Plant Proteins, 2022, 1–23.
- Z. Yi-Shen, S. Shuai and R. Fitzgerald, Mung bean proteins and peptides: Nutritional, functional and bioactive properties, Food Nutr. Res., 2018, 62, 1–11 Search PubMed.
- T. G. Kudre, S. Benjakul and H. Kishimura, Comparative study on chemical compositions and properties of protein isolates from mung bean, black bean and bambara groundnut, J. Sci. Food Agric., 2013, 93(10), 2429–2436 CrossRef CAS PubMed.
- C. H. Tang, Thermal denaturation and gelation of vicilin-rich protein isolates from three Phaseolus legumes: a comparative study, LWT–Food Sci. Technol., 2008, 41(8), 1380–1388 CrossRef CAS.
- K. Shevkani, N. Singh, A. Kaur and J. C. Rana, Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study, Food Hydrocolloids, 2015, 43, 679–689, DOI:10.1016/j.foodhyd.2014.07.024.
- K. Shevkani, N. Singh, Y. Chen and A. Kaur, Pulse proteins: secondary structure, functionality and applications, J. Food Sci. Technol., 2019, 56(6), 2787–2798, DOI:10.1007/s13197-019-03723-8.
- M. Du, J. Xie, B. Gong, X. Xu, W. Tang and X. Li, et al., Extraction, physicochemical characteristics and functional properties of Mung bean protein, Food Hydrocolloids, 2018, 76, 131–140, DOI:10.1016/j.foodhyd.2017.01.003.
- M. Carbonaro and A. Nucara, Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region, Amino Acids, 2010, 679–690 CrossRef CAS PubMed.
- P. Gupta, A. Islam, F. Ahmad and M. I. Hassan, Applications of Circular Dichroism Spectroscopy in Studying Protein Folding, Stability, and Interaction, in Protein Folding Dynamics and Stability: Experimental and Computational Methods, ed. P. Saudagar and T. Tripathi, Springer, Singapore, 2023, pp. 1–23 Search PubMed.
- L. A. Linhares and C. H. I. Ramos, Unlocking Insights into Folding , Structure, and Function of Proteins through Circular Dichroism Spectroscopy—A Short Review, Applied Biosciences, 2023, 639–655 CrossRef.
- A. Haque, P. Kaur, A. Islam and I. Hassan, Application of circular dichroism spectroscopy in studying protein folding, stability, and interaction, Advances in Protein Molecular and Structural Biology Methods, Elsevier Inc., 2022, pp. 213–224, DOI:10.1016/B978-0-323-90264-9.00014-3.
- N. J. Gogtay and U. M. Thatte, Principles of correlation analysis, J. Assoc. Physicians India, 2017, 65, 78–81 Search PubMed.
- J. E. Kinsella, Functional properties of soy proteins, J. Am. Oil Chem. Soc., 1979, 56(3), 242–258 CrossRef CAS.
- T. J. Joshi and P. Srinivasa Rao, Characterization and multivariate analysis of decortication-induced changes in pearl millet, J. Food Compos. Anal., 2024, 125, 105788 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |