Open Access Article
Open Access ArticleValorization of legume by-products in functional formulations: phytochemicals and their simulated gastrointestinal fate
Gulay
Ozkan
 a,
Mustafa Tahsin
Yilmaz
a,
Mustafa Tahsin
Yilmaz
 b,
Elwira
Sieniawska
b,
Elwira
Sieniawska
 c,
Benita
Hryć
c,
Benita
Hryć
 c,
Mehmet Çağlar
Tülbek
c,
Mehmet Çağlar
Tülbek
 d and
Esra
Capanoglu
d and
Esra
Capanoglu
 *a
*a
aDepartment of Food Engineering, Faculty of Chemical and Metallurgical Engineering, Istanbul Technical University, 34469, Maslak, Istanbul, Turkey. E-mail: ozkangula@itu.edu.tr; capanogl@itu.edu.tr
bDepartment of Industrial Engineering, Faculty of Engineering, King Abdulaziz University, 21589, Jeddah, Saudi Arabia. E-mail: mtyilmaz13@gmail.com
cDepartment of Natural Products Chemistry, Medical University of Lublin, Chodźki 1, 20-093 Lublin, Poland. E-mail: esieniawska@pharmacognosy.org; benita.hryc@umlub.pl
dSaskatchewan Food Industry Development Centre, Saskatoon, SK S7M 5V1, Canada. E-mail: mtulbek@foodcentre.sk.ca
First published on 2nd October 2025
Abstract
The high bioactive content of legume by-products enables their utilization in the development of value-added food products. Thus, this study explores the valorization of legume by-products (chickpea hulls (CH), faba bean hulls (FH), and lentil hulls (LH)) through the formulation of novel functional infusions. The effects of in vitro digestion on the phenolic content and antioxidant potential of these infusions were assessed. Aqueous-methanolic (75%) and aqueous extracts of these samples were also evaluated in terms of total phenolic content (TPC), total flavonoid content (TFC), and antioxidant capacity. According to the results, FH and LH showed higher TPC (1002 and 1320 mg GAE/100 g, respectively) and TFC (961 and 986 mg CE/100 g, respectively) values (p < 0.05). Similarly, among the infusions (CHI, FHI, and LHI), which were all prepared from their respective samples, the FHI and LHI exhibited higher levels of TPC, TFC, and antioxidant capacity before and after in vitro digestion (p < 0.05). Additionally, comprehensive LC-ESI-MS/MS phenolic profiling showed the great potential for the retention of individual phenolics in newly formulated infusions. These results suggest that legume by-products have great potential for value-added applications as functional ingredients in infusion formulations, contributing to their sustainable utilization and offering health-promoting properties.
Sustainability spotlightStudies on malnutrition, waste utilization, and sustainability, which have attracted global as well as national attention, are gaining increasing importance day by day. Therefore, in this study, the infusions of legume by-products which have been sporadically used for food applications were investigated to determine their value-added application potential aiming to provide valuable information in terms of complete plant utilization and increased economic values. Our work emphasizes the importance of the following UN Sustainable Development Goals: zero hunger (SDG 2) and good health and well-being (SDG 3). |
1. Introduction
Phenolic compounds represent a broad category of plant secondary metabolites. They are widely accumulated in several higher plant tissues such as vegetables, fruits, condiments, cereals, pulses, and nuts, and involved in several physiological functions including plant characteristics, color, aroma, and stress tolerance.1 Phenolic compounds have recently emerged as promising components due to their antioxidant, antibacterial, anticarcinogenic, and anti-inflammatory properties. In addition it has been shown that phenolic compounds can prevent cardiovascular diseases, diabetes, and oxidative stress-related diseases.1 Legumes, such as chickpea, faba bean, and lentil, which were investigated within this study, contain a variety of phenolic compounds that contribute to their distinct flavor and potential health benefits. These plants can be used as new sources for the production of infusions, which are described as a liquid formulation prepared by pouring boiling water over the plant materials to extract biologically active compounds.2 Infusions have gained popularity due to their ease of preparation and natural origin; however, one key challenge is ensuring sufficient extraction and stability of bioactive compounds, particularly phenolics, during preparation and gastrointestinal digestion. Most commercial herbal infusions are derived from flowers, leaves, or roots, whereas the use of agri-food by-products such as legume hulls remains underexplored. The valorization of legume hulls for infusion production not only addresses sustainability concerns but also offers a low-cost, fiber-rich, and phenolic-rich alternative to conventional herbal materials.Due to the high amount of proteins, dietary fibers, and bioactive compounds in legume by-products, they can be utilized as valuable resources in the formulation of functional food ingredients. Thus, prior studies have investigated the use of legumes and their fractions in functional beverages and extracts. For instance, chickpea-derived phenolic extracts have been shown to exhibit considerable antioxidant activity and proposed for beverage enrichment.3,4 Similarly, faba bean hulls and lentil fractions demonstrated high antioxidant activity and phenolic content, particularly in the hull portion.5,6 Apart from these, by-products of black bean,7 black soybean cooking water powder8 and soybean husk9 were used in plant-based meat, whereas soybean cooking water powder,10 lupine and chickpea mill residue,11 and faba bean husk were utilized in bakery products12 and chickpea husk was added to dairy products.13 However, limited studies have formulated ready-to-drink infusions using these by-products, and even fewer have assessed their phenolic stability and bioaccessibility under simulated digestion. This gap presents a novel opportunity to develop functional infusion formulations from legume by-products. Such infusion formulations offer practical applicability in daily life while aligning with current consumer demand for sustainable, plant-based functional beverages.14 On the other hand, legume by-products are also good sources of dietary fibers, phytochemicals, vitamins, minerals, and residual levels of proteins, making them suitable candidates for reutilization in human nutrition and functional product design.15 According to Johnson and Walsh,16 the chickpea hull by-product typically showed a higher total phenolic content (56–150 mg gallic acid equivalents (GAE)/100 g) and ferric reducing antioxidant power (38–174 mg Trolox equivalents (TE)/100 g) compared to the kernel part (TPC of 65–105 mg GAE/100 g and FRAP of 44–62 mg TE/100 g), depending on the variety of the chickpea. In another study, faba bean hulls and lentil fractions were investigated, and the results indicated that the hulls exhibited high antioxidant activity, measured by the reducing power (RP), antiradical activity (DPPH), or oxygen radical absorbance capacity (ORAC) assays,17 indicating the potential of these by-products.
In order to maximize the health benefits of plants and their infusions, it is essential to understand the bioaccessibility of their phenolic compounds. The first step towards understanding the stability of phenolic compounds in the gastrointestinal tract is to estimate their bioaccessibility, which is defined as the number of bioactive compounds released after gastrointestinal digestion. It is evident that polyphenols may interact with other food constituents, may be metabolized by the body, or degraded during the digestion process.18 Therefore, for improved health outcomes, researchers should develop strategies by understanding which compounds are present in a specific plant and how they are released during digestion. Accordingly, various simulation models for gastrointestinal digestion have been developed to determine the bioaccessibility of bioactive compounds.14,19 Taken together, these points highlight the value of legume by-products in developing sustainable functional infusion formulations while improving the phenolic content of the products. Therefore, this study aimed to explore the potential of chickpea, lentil, and faba bean hulls in the formulation of functional infusions by investigating the effects of different solvent systems on their phenolic content, phenolic profile, and antioxidant activity; evaluating the retention of phenolic compounds during simulated gastrointestinal digestion, thus characterizing the bioactive composition of the infusions using LC-ESI-MS/MS analysis.
2. Materials and methods
2.1. Chemicals
Pepsin (EC 3.4.23.1, from porcine gastric mucosa), pancreatin (EC 232.468.9, from porcine pancreas, contains trypsin, amylase and lipase), bile, Folin–Ciocalteu reagent, gallic acid, catechin, neocuproine, DPPH (1,1-diphenyl-2-picrylhydrazyl), Trolox (6-hydroxy-2,5,7,8 tetramethylchromane-2-carboxylic acid), ABTS (2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt), TPTZ (triphenyltetrazolium), TFA (trifluoroacetic acid), and the other chemicals used to prepare simulated salivary, gastric and intestinal fluids were purchased from Merck Life Science. All other reagents were of HPLC or LC-MS grade and provided by Merck Life Science.2.2. Preparation of plant samples
Legume by-products, including commercial desi chickpea hull (CH), faba bean hull (FH), and lentil hull (LH), were collected after a dehulling and splitting process at AGT Foods R&D Centre (Saskatoon, SK, Canada). Desi chickpea, faba, and lentil hulls were sifted between 14 and 30-mesh screens, and this particular cut/particle size was utilized in the process. Hull samples with 14–30 mesh particle size were washed and rinsed in water. After the washing and rinsing processes, hull samples were roasted and dried at 150 °C for 4 h. Final products were sifted with 30-mesh, and collected as the final product.2.3. Solvent extraction
In order to extract the polyphenols from the legume by-products, they were subjected to aqueous-methanol (75% of methanol) or water treatments. In this respect, the phenolic extraction procedure was adapted from the method used by Ozkan et al.14 with slight modifications. One gram (1 ± 0.01 g) of ground sample was extracted with 10 mL of either 75% aqueous-methanol (75% MeOH + 25% water) or water (W) at ambient temperature. The mixture was vortexed for 10 s and subsequently sonicated for 15 min in an ultrasonic bath (USC900TH; VWR, Radnor, PA, USA). Then, the treated samples were centrifuged at 4500 rpm for 15 min at 4 °C (Universal 32R; Hettich Zentrifugen, Tuttlingen, Germany), and the supernatants were collected. This extraction procedure was repeated twice for the pellet and the supernatants were pooled and made up to a final volume of 20 mL. Prepared extracts were stored at −20 °C until further analysis.2.4. Preparation of the infusions
All infusions, including desi chickpea hull infusion (CHI), faba bean hull infusion (FHI), and lentil hull infusion (LHI), were prepared by grinding the legume by-products followed by hot water treatment, as explained below. First, each sample was separately ground with a common kitchen coffee grinder (Sinbo, Türkiye) and stored at ambient temperature until use. Infusion preparation was adapted from the procedure reported previously.18 This method was developed based on the conclusions of an internal screening test. For each sample, 10 grams of samples were weighed into a beaker, 80 mL of water at 85 °C was added, and then, the mixture was left for 10 min without heating. Thereafter, the mixture was cooled and filtered through Whatman No. 4 paper. All infusions were stored at −20 °C until further analysis.2.5. In vitro simulated gastrointestinal digestion
The stability of phenolic compounds in the infusions during gastrointestinal digestion was determined based on a protocol reported by Minekus et al.20 with minor modifications. This method consists of three sequential simulations of the oral, gastric, and intestinal phases. Simulated salivary fluid contains potassium chloride (KCl), monopotassium phosphate (KH2PO4), sodium bicarbonate (NaHCO3), magnesium chloride hexahydrate (MgCl2(H2O)6), ammonium carbonate ((NH4)2CO3), hydrochloric acid (HCl), and calcium chloride dihydrate (CaCl2(H2O)2). Simulated gastric fluid includes KCl, KH2PO4, NaHCO3, NaCl, MgCl2(H2O)6, (NH4)2CO3, HCl, and CaCl2(H2O)2. Simulated intestinal fluid is composed of KCl, KH2PO4, NaHCO3, NaCl, MgCl2(H2O)6, HCl, and CaCl2(H2O)2. In order to simulate oral digestion, 5 mL of each infusion was mixed with 4 mL of simulated salivary fluid, 25 μL of 0.3 M CaCl2, and 0.975 mL of distilled water to obtain 10 mL of the oral bolus. The mixture was incubated at 37 °C for 2 min in a shaking water bath (Memmert SV1422; Nürnberg, Germany). For the simulation of the gastric digestion stage, 10 mL of oral bolus was mixed with 7.5 mL of simulated gastric fluid, 1.6 mL of pepsin solution (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1), and 5 μL of 0.3 M CaCl2, and the pH was adjusted to 3 with 1 M HCl. Thereafter, the volume of the mixture was made up to 20 mL with the addition of distilled water. The mixture was then incubated at 37 °C for 2 h in a shaking water bath, and 5 mL aliquots were collected from the gastric phase for further analyses. Finally, to simulate the intestinal digestion stage, 15 mL aliquot from the gastric phase, 8.25 mL of simulated intestinal fluid, 3.75 mL of pancreatin (800 U mL−1), 1.875 mL of bile solution (160 mM), and 30 μL of 0.3 M CaCl2 were mixed, and the pH was adjusted to 7 with 1 M NaOH. Then, the volume of gastric chyme was made up to 30 mL with the addition of distilled water. The mixture was incubated at 37 °C for 2 h in a shaking water bath.
000 U mL−1), and 5 μL of 0.3 M CaCl2, and the pH was adjusted to 3 with 1 M HCl. Thereafter, the volume of the mixture was made up to 20 mL with the addition of distilled water. The mixture was then incubated at 37 °C for 2 h in a shaking water bath, and 5 mL aliquots were collected from the gastric phase for further analyses. Finally, to simulate the intestinal digestion stage, 15 mL aliquot from the gastric phase, 8.25 mL of simulated intestinal fluid, 3.75 mL of pancreatin (800 U mL−1), 1.875 mL of bile solution (160 mM), and 30 μL of 0.3 M CaCl2 were mixed, and the pH was adjusted to 7 with 1 M NaOH. Then, the volume of gastric chyme was made up to 30 mL with the addition of distilled water. The mixture was incubated at 37 °C for 2 h in a shaking water bath.
A blank (without the added sample) was incubated under the same conditions to eliminate interferences due to the digestive enzymes and buffers used in the digestion process. All experiments were performed in triplicate. The samples collected from simulated gastric and intestinal phases were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min at 4 °C, and the supernatants were stored at −20 °C until further analysis. The infusions before, during, and after in vitro digestion were grouped as undigested infusions (UD), gastric digested (GD), and intestinal digested (ID) infusions, respectively.
000 rpm for 30 min at 4 °C, and the supernatants were stored at −20 °C until further analysis. The infusions before, during, and after in vitro digestion were grouped as undigested infusions (UD), gastric digested (GD), and intestinal digested (ID) infusions, respectively.
In order to calculate the bioaccessibility, the following equation was used, and the calculated values were expressed as percentage:
| Bioaccessibility (%) = (BCdigested/BCundigested) × 100 |
2.6. Spectrophotometric analyses
Aqueous-methanolic and aqueous extracts of the legume by-products, as well as undigested, gastric digested, and intestinal digested infusion samples, were subjected to spectrophotometric analyses through a UV-visible spectrophotometer (Synergy HT; BioTek Instruments, Winooski, VT, USA). Each measurement was performed at least in triplicate.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17x − 0.0164 and R2 = 0.9983 for CUPRAC; y = 5.9402x + 0.0307 and R2 = 0.9960 for ABTS). All results were reported as Trolox equivalents (TE) per 100 g of sample.
17x − 0.0164 and R2 = 0.9983 for CUPRAC; y = 5.9402x + 0.0307 and R2 = 0.9960 for ABTS). All results were reported as Trolox equivalents (TE) per 100 g of sample.
2.7. Identification and quantification of polyphenols by HPLC-PDA
The method of Ozkan et al.14 was used to quantify polyphenols in the samples. Concisely, the samples were passed through 0.45 μm membrane filters before being injected into a Waters 2695 HPLC system with a PDA detector (Waters, USA). The stationary phase was a Supelcosil LC-18 (25 cm × 4.60 mm, 5 m column, Sigma-Aldrich, Steinheim, Germany). Solvent A: 0.1% TFA in MQ water and Solvent B: 0.1% TFA in acetonitrile were the solvents used for the analysis. Spectral measurements were performed at λ = 280, 312, and 360 nm and had a flow rate of 1 mL min−1 and an injection volume of 10 μL, respectively. A linear gradient was used as follows: at 0 min, 95% solvent A and 5% solvent B; at 45 min, 65% solvent A and 35% solvent B; at 47 min, 25% solvent A and 75% solvent B; and at 54 min, returning to initial conditions. Phenolic compounds were quantified by using their authentic standards. The calibration curves of polyphenol standards showed good linearity (R2 > 0.99) within the established range (0.01–20 mg/100 mL). LOD and LOQ values ranged from 0.01 to 0.03 mg/100 mL and 0.03 to 0.09 mg/100 mL, respectively. All analyses were carried out in triplicate, and the results were expressed as mg/100 g sample.2.8. Identification of phenolic compounds by liquid chromatography-mass spectrometry (LC-MS/MS)
In this study, specific components in infusions subjected to gastrointestinal digestion treatment were targeted. In this respect, the samples were chromatographically separated and spectrally identified according to the conditions described previously.26 Briefly, a C18 Gemini® column (3 μm i.d., with TMS end capping, 110 Å, 100 × 2 mm) connected to a guard column (Phenomenex Inc, Torrance, CA, USA) was used to separate compounds in the following gradient solvent system: 0–60% B for 45 min., next 60–95% B for 1 min, and 95% B for 4 min, at a flow rate of 0.2 mL min−1. Solvent A was water with 0.1% (v/v) formic acid, while solvent B was acetonitrile with 0.1% (v/v) formic acid. Ten μL of the sample was injected into the chromatographic column at 20 °C. The conditions were controlled by using an Agilent 1200 Infinity HPLC coupled to an Agilent 6530B QTOF system (Agilent Technologies, Santa Clara, CA, USA). Mass spectrometry detection was performed in negative ion mode setting with 10 and 30 eV collision energies for every compound. Spectra were acquired in the m/z range from 100 to 1000. Drying gas temperature and flow were 275 °C and 10 L min−1, respectively, while sheath gas temperature and flow were 325 °C and 12 L min−1, respectively. Nebulizer pressure was set at 35 psig. The voltage of the capillary, skimmer, and fragmentor was 4000, 65, and 140, respectively. Compounds were tentatively identified based on their accurate masses and fragmentation patterns, supported by the available databases (PubChem) and literature sources. The volume/concentration changes during the digestion steps were taken into consideration. The observation of changes in the amounts of monitored compounds after digestion was done by comparison of % peak intensities of detected ions from phenolic compounds in plant infusions before, during, and after in vitro digestion. In this respect, the peak intensity values of undigested infusions (UD) were set as 100%, and proportionally compared with the values of gastric digested (GD) and intestinal digested (ID) infusions.2.9. Statistical analysis
All samples were prepared twice and analyzed at least in three replicates. Error bars on the figures show standard deviations. The results were expressed as mean ± standard deviation. Statistical analysis was performed using SPSS software (version 20.0, SPSS Inc., Chicago, IL, USA). Differences were evaluated using one-way analysis of variance (ANOVA), followed by a Tukey post hoc test (p < 0.05). A paired t-test was used to reveal differences between solvent types.3. Results and discussion
3.1. Effect of solvent type on the content of phenolic compounds and antioxidant potential of the plant samples
Fig. 1 shows the effect of solvent type on the phenolic content and antioxidant capacity of the extracts obtained from legume by-products by aqueous-methanolic or aqueous extraction. It is clear from the results that, in general, the TPC, TFC, ABTS, DPPH, and CUPRAC values for the aqueous extracts of the plants were found to be statistically higher (p < 0.05) than those of aqueous-methanolic extracts. It has become evident that a significant number of bioactive compounds can be recovered from legume-based by-products (CH, FH, and LH) simply by extracting with water. In detail, TPC, TFC, ABTS, DPPH, and CUPRAC values of legume-based by-products were in the range of 164–1320 mg GAE/100 g, 156–986 mg RE/100 g, 407–3337 mg TE/100 g, 108–1200 mg TE/100 g, and 265–2486 mg TE/100 g, respectively. For aqueous extracts, faba bean hull and lentil hull possessed the highest TPC, TFC, ABTS, DPPH, and CUPRAC values (p < 0.05). On the other hand, regarding the aqueous-methanolic extracts, TPC, TFC, ABTS, DPPH, and CUPRAC values of lentil hull were statistically higher than those of others (p < 0.05). This trend can be attributed to the inherent differences in the phenolic profiles of the legume species. Faba bean and lentil hulls are known to contain higher levels of condensed tannins, flavonols (such as quercetin and kaempferol derivatives), and hydroxycinnamic acids, which are potent contributors to antioxidant activity.27 Moreover, the denser cellular structure and pigmentation of these hulls may contribute to a higher accumulation of phenolic compounds compared to chickpea hulls. These compositional differences likely underlie their superior radical scavenging and reducing capacity.28Regarding the efficiency of the solvents used for extraction, contradictory results have been reported in the literature. Similar to our results, higher antioxidant activity values or total phenolic contents in water extracts of different plant materials compared to methanolic or ethanolic extracts have been reported for Annona muricata L. (Graviola) leaves29 and Carica papaya L. leaves.30 On the other hand, ethanolic/methanolic extracts were reported to be superior compared to water extracts with respect to antioxidant activities in some plant materials. In the study of Butsat and Siriamornpun,31 the maximum antioxidant activity for Amomum chinense C. leaves was observed with the use of 80% methanol, followed by 80% ethanol, 80% acetone, and distilled water. Some other examples presenting lower values of total phenolics or antioxidant activity in the water extracts include Pinus densiflora S. et Z. bark compared to ethanolic extract,32 and ginger and Convolvulus species compared to their ethanolic and methanolic counterparts.33
The differences between the results may arise from (1) variations in the plant's cellular structure, (2) changes in the compositions and antioxidant activities of the extracts caused by different solvents used in extracting the compounds,34 (3) greater antioxidant capacity of the extract containing phenolic compounds with more hydroxyl groups,35 and (4) difference in antioxidant activities influenced by the extraction method, characteristics of the extraction solvent (i.e. polarity), and extraction parameters including temperature and time.36,37
Apart from spectrophotometric determinations, HPLC analysis was also conducted to identify and quantify the effects of solvent type on the concentration of individual polyphenols in the extracts (Table 1). In aqueous-methanolic and aqueous extracts, the maximum amount (p < 0.05) of epicatechin was detected in FH and LH, rutin in CH, quercetin in LH, and epigallocatechin gallate in LH. The higher epicatechin and quercetin levels in aqueous-methanolic extracts reflect both the solvent's ability to extract mid-polar phenolics and intrinsic compositional differences.38 Faba bean hulls are particularly rich in flavan-3-ols,39 whereas lentil hulls contain flavonols such as quercetin, aligning with their dark pigmented seed coats and reported phenolic profiles.40 From the results obtained, it is evident that some polyphenols can be detected at their highest levels when extracted with aqueous-methanol, while others can be detected in aqueous extracts. These findings highlight the importance of solvent type in the level of polyphenols and provide valuable insights for further research and applications in various industries.41 On the other hand, it is noteworthy to mention that the optimum extraction method and solvent should be determined considering the targeted phenolics for the best results42 due to the fact that not all the phenolics may be extracted with the highest efficiency by using a single method/solvent.
| Phenolics | Sample codes* | Type of solvent | |
|---|---|---|---|
| 75% aqueous methanol | Water | ||
| a–c Within each column, different lowercase superscript letters show differences (p < 0.05) between samples. A,B Within each row, different uppercase superscript letters show differences (p < 0.05) between solvent types. *CH, chickpea hull; FH, faba bean hull; LH, lentil hull. | |||
| Epicatechin (mg/100 g) | CH | ND | ND |
| FH | 30.11 ± 1.28aB | 65.69 ± 0.74bA | |
| LH | 0.88 ± 0.17bB | 128.8 ± 1.0aA | |
| Chlorogenic acid (mg/100 g) | CH | ND | ND |
| FH | 0.28 ± 0.03B | 1.10 ± 0.01A | |
| LH | ND | ND | |
| Rutin (mg/100 g) | CH | 12.40 ± 1.79A | 14.95 ± 3.96A |
| FH | ND | ND | |
| LH | ND | ND | |
| Quercetin (mg/100 g) | CH | 1.33 ± 0.53bA | 1.89 ± 1.10bA |
| FH | 0.18 ± 0.04cB | 0.63 ± 0.01cA | |
| LH | 12.68 ± 1.53aB | 24.18 ± 0.41aA | |
| Syringic acid (mg/100 g) | CH | ND | ND |
| FH | ND | ND | |
| LH | 0.38 ± 0.20B | 0.76 ± 0.07A | |
3.2. Retention of infusion polyphenols and their antioxidant capacities during in vitro gastrointestinal digestion
In order to evaluate how in vitro simulated digestion conditions affected the polyphenol content and antioxidant capacity of infusions, TPC, TFC, ABTS, CUPRAC, and DPPH assays were performed to analyze their bioaccessible fractions. Table 2 illustrates the effects of in vitro gastrointestinal digestion on the phenolic content and antioxidant capacity of infusions prepared from legume by-products (chickpea hull infusion-CHI, faba bean hull infusion-FHI and lentil hull infusion-LHI).| Sample codes* | In vitro digestion** | % loss in activities | % bioaccessibility | ||||
|---|---|---|---|---|---|---|---|
| UD | GD | ID | After GD | After ID | |||
| a–c Within each column, different lowercase superscript letters show differences (p < 0.05) between infusions. A−C Within each row, different uppercase superscript letters show differences (p < 0.05) during digestion. *CHI, chickpea hull infusion; FHI, faba bean hull infusion; LHI, lentil hull infusion. **UD, undigested; GD, gastric digestion; ID, intestinal digestion. | |||||||
| TPC (mg GAE/100 g) | CHI | 586 ± 34cA | 405 ± 37cB | 222 ± 30cC | 30.73 ± 4.03a | 62.03 ± 2.21a | 37.97 ± 2.21b |
| FHI | 973 ± 95bA | 770 ± 64bB | 422 ± 38bC | 20.36 ± 7.81ab | 56.35 ± 4.28a | 43.65 ± 4.28b | |
| LHI | 1318 ± 34aA | 1174 ± 145aA | 706 ± 60aB | 10.89 ± 2.30b | 46.41 ± 1.38b | 53.59 ± 1.38a | |
| TFC (mg RE/100 g) | CHI | 125 ± 3cA | 62 ± 4cB | 44 ± 1bC | 49.99 ± 0.70a | 64.51 ± 0.50a | 35.49 ± 0.50b |
| FHI | 387 ± 15bA | 274 ± 22bB | 166 ± 4aC | 29.13 ± 2.75b | 57.06 ± 1.67b | 42.94 ± 1.67a | |
| LHI | 496 ± 14aA | 436 ± 11aB | 168 ± 13aC | 12.05 ± 2.48c | 66.11 ± 0.96a | 33.89 ± 0.96b | |
| ABTS (mg TE/100 g) | CHI | 742 ± 2cA | 410 ± 44bB | 187 ± 7cC | 44.74 ± 0.15a | 74.80 ± 0.07a | 25.20 ± 0.07c |
| FHI | 985 ± 9bA | 672 ± 5aB | 284 ± 5bC | 31.77 ± 0.62b | 71.17 ± 0.26b | 28.83 ± 0.26b | |
| LHI | 1242 ± 5aA | 690 ± 2aB | 425 ± 1aC | 44.44 ± 0.22a | 65.78 ± 0.14c | 34.22 ± 0.14a | |
| DPPH (mg TE/100 g) | CHI | 183 ± 8bA | 122 ± 13cB | 30 ± 5cC | 33.36 ± 3.09a | 83.61 ± 0.76a | 16.39 ± 0.76c |
| FHI | 294 ± 16aA | 196 ± 11bB | 87 ± 9bC | 33.20 ± 3.64a | 70.35 ± 1.62b | 29.65 ± 1.62b | |
| LHI | 304 ± 4aA | 227 ± 17aB | 143 ± 18aC | 25.32 ± 0.98b | 52.96 ± 0.62c | 47.04 ± 0.62a | |
| CUPRAC (mg TE/100 g) | CHI | 415 ± 41cA | 255 ± 19cB | 225 ± 30cB | 38.15 ± 6.14a | 45.43 ± 5.42a | 54.57 ± 5.42a |
| FHI | 698 ± 56bA | 544 ± 41bB | 376 ± 34bC | 21.73 ± 6.30b | 45.90 ± 4.35a | 54.10 ± 4.35a | |
| LHI | 1029 ± 56aA | 883 ± 50aB | 582 ± 46aC | 14.20 ± 4.69b | 43.33 ± 3.09a | 56.67 ± 3.09a | |
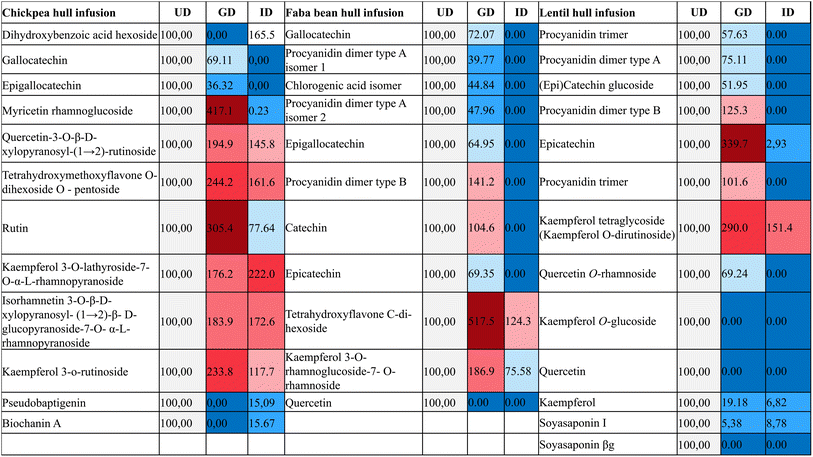
The results show that in vitro digestion significantly (p < 0.05) lowered the phenolic content and antioxidant capacity of some infusions. Accordingly, TPC, TFC, and antioxidant capacity measured by ABTS, DPPH, and CUPRAC methods decreased in the following order: undigested > gastric digestion > intestinal digestion. As a result of gastric digestion, 12.1% to 50.4% of the phenolic content and antioxidant capacity were lost, whereas this loss after intestinal digestion ranged from 43.4% to 83.6% (Table 2). Accordingly, previous studies have provided evidence indicating dramatic decreases in the levels of polyphenols after undergoing in vitro digestion, and this phenomenon has been well-documented and consistently reported in the scientific literature. The loss of phenolics during gastrointestinal digestion can be attributed to various factors.18,43 Polymerization, epimerization, and auto-oxidation are among the key mechanisms identified under intestinal digestion conditions.44,45 Understanding these mechanisms is crucial for elucidating the digestion and absorption of nutrients, as well as their potential impact on health and disease, given that many phenolic compounds in foods can bind to proteins, carbohydrates, and dietary fibers through chemical bonds, thereby modifying their bioavailability for absorption during gastrointestinal digestion. In the context of intestinal digestion, polymerization refers to the joining of monomers to form larger molecules, such as polysaccharides (complex carbohydrates), proteins, or nucleic acids. This process is critical for the breakdown of complex dietary components into simpler forms that can be absorbed by the body. Epimerization may involve the conversion of one form of a molecule, such as a sugar or an amino acid, into another form with a slightly different configuration. This process can impact the bioavailability and metabolism of nutrients. Besides, during the process of gastrointestinal digestion, auto-oxidation may occur when certain dietary components, such as unsaturated fats or antioxidants, come into contact with oxygen in the gut. This process can lead to the generation of oxidative stress and the production of potentially harmful reactive oxygen species (ROS) within the gastrointestinal tract. During in vitro digestion, the oxygen levels are higher compared to those under natural physiological conditions, potentially promoting the epimerization and auto-oxidation of phenolic compounds.46 In addition, increased pH levels, residual dissolved oxygen, and the probable occurrence of reactive oxygen species due to regular digestive processes might trigger several reactions within the intestinal tract, including epimerization and auto-oxidation.47 As a consequence, these processes contribute to the degradation and transformation of phenolic compounds, ultimately leading to their reduced concentration in the digestive system.
Determining the bioaccessibility of bioactive compounds in plant infusions is essential to understanding their digestive fate in order to fully exploit their health benefits. The bioaccessibility values according to TPC and TFC changed from 37.97 to 53.59% and 33.89 to 42.94% (Table 2), respectively. On the other hand, LHI and FHI showed the highest bioaccessibility values by both methods, respectively (p < 0.05). Based on the antioxidant activity methods, ABTS, DPPH, and CUPRAC assays, the bioaccessibility values changed from 25.20 to 34.22%, 16.39 to 47.04%, and 54.10 to 56.67%, respectively (Table 2). Among the methods used to measure the bioaccessibility of the antioxidant potential during digestion, LHI (ABTS and DPPH) exhibited the highest (p < 0.05) antioxidant bioaccessibility value. The decrease in the bioaccessibility values of the infusions can be attributed to several reasons. In general, lentil hull infusion (LHI) and faba bean infusion (FHI) displayed the highest bioaccessibility values because their rich and diverse phenolic profiles, indicating that flavonols and flavan-3-ols are effectively released during gastric digestion and maintain stability under intestinal conditions. These mid-polar compounds show resilience against pH shifts and are less likely to be sequestered by fibers or enzymes.48 In particular, the DPPH assay favors hydrogen-donating flavonols from LHI, while the ABTS assay better captures electron-transfer capabilities of flavan-3-ols from FHI.49 The overall decrease in bioaccessibility observed across all infusions aligns with known influences of pH changes, enzymatic degradation, and phenolic–matrix interactions during gastrointestinal digestion. Despite their relative stability, flavanols are prone to structural transformations, especially epimerization and oxidation at near-neutral gastrointestinal pH (6–7.5), which, alongside enzymatic degradation and matrix interactions, critically shape their evolving bioaccessibility during digestion. Flavanols, a subclass of flavonoids, are known for their high stability. However, they can undergo partial degradation under gastrointestinal (GI) conditions. These changes can result in the epimerization of flavanols when the pH exceeds 6. There are also other factors that influence the bioaccessibility and bioavailability of phenolic compounds, including their chemical structure, interactions with other active compounds in a food matrix, hydrophobicity, absorption in the gastrointestinal tract, digestion of foods, and glucuronic acid level.50
Table 3 shows the effects of in vitro digestion on the individual polyphenols in legume by-product-based infusions. The polyphenols epicatechin, chlorogenic acid, rutin, quercetin, and syringic acid detected in the extracts of the legume by-products were also detected in their infusions. The analysis revealed that the polyphenols could be easily extracted into the liquid phase during the infusion process.
| Phenolics | Sample code* | In vitro digestion** | ||
|---|---|---|---|---|
| UD | GD | ID | ||
| a–c Within each column, different lowercase superscript letters show differences (p < 0.05) between infusions. A−C Within each row, different uppercase superscript letters show differences (p < 0.05) during digestion. *CHI, chickpea hull infusion; FHI, faba bean hull infusion; LHI, lentil hull infusion; ND, not detected. **UD, undigested; GD, gastric digestion; ID, intestinal digestion. | ||||
| Epicatechin (mg/100 g) | CHI | ND | ND | ND |
| FHI | 137.0 ± 19.9aA | 132.7 ± 11.9aA | 54.10 ± 0.22B | |
| LHI | 165.0 ± 22.8aA | 16.13 ± 2.90bB | ND | |
| Chlorogenic acid (mg/100 g) | CHI | ND | ND | ND |
| FHI | 0.31 ± 0.06A | ND | ND | |
| LHI | ND | ND | ND | |
| Rutin (mg/100 g) | CHI | 79.19 ± 2.10A | 4.10 ± 0.18B | 2.29 ± 0.00C |
| FHI | ND | ND | ND | |
| LHI | ND | ND | ND | |
| Quercetin (mg/100 g) | CHI | 6.52 ± 0.18bA | 0.65 ± 0.00bB | ND |
| FHI | 0.69 ± 0.10cB | 0.61 ± 0.00cB | 0.84 ± 0.00bA | |
| LHI | 17.52 ± 1.32aB | 26.79 ± 0.46aA | 8.37 ± 2.43aC | |
| Syringic acid (mg/100 g) | CHI | ND | ND | ND |
| FHI | ND | ND | ND | |
| LHI | 1.39 ± 0.24B | 0.45 ± 0.10C | 20.70 ± 3.96A | |
From Table 3, it is obvious that some polyphenols could be retained in gastric and intestinal digestion stages; in other words, the polyphenols detected in undigested (UD) infusions were also generally detected in gastric digested (GD) and intestinal digested (ID) infusions. However, some polyphenols could not be detected in intestinal digested infusions. For example, chlorogenic acid, which was identified in undigested FHI, was not detected in gastric or intestinal digestion phases. The results of the study demonstrated that these polyphenols were significantly eliminated during the intestinal digestion stage. As a matter of fact, phenolic substances may degrade during digestion because phenolic substances have low stability under alkaline conditions.51 However, it is also important to note that some compounds were able to pass through the gastric digestion stage without much impact. This suggests that while these compounds may pass the initial stages of digestion, they were not able to withstand the harsh conditions of intestinal digestion.
In addition to these, Table 3 shows that a number of polyphenols increased while the others decreased during gastrointestinal digestion. Quercetin in LHI could be given as an example whose amounts increased (p < 0.05) after gastric digestion and decreased after intestinal digestion. In agreement with our results, other investigations on various food samples reported that polyphenol concentrations increased after in vitro gastric digestion and that total phenolic concentrations declined significantly following postdigestion.52,53 The amount of total flavonoids was significantly higher after simulated gastric digestion in raspberry species, as reported by Qin and Wang.54 In summary, our results indicated that the in vitro simulation process had different effects depending on the type, nature, and concentration of the bioactive compounds in infusions, which was consistent with a previous report.14
A significant increase in the contents of quercetin in FHI, and syringic acid in LHI was observed after the intestinal digestion stage, which was in parallel with a previous work.55 Accordingly, epicatechin, quercetin, and syringic acid were assumed to be released during in vitro intestinal digestion and showed an increase in the infusions. This result was consistent with the report of Qin and Wang54 who reported that raspberry samples after intestinal digestion contained the highest levels of total flavonoids, and that intestinal digestion also led to a release of some phenolic compounds. A significant increase in the bioaccessibility of compounds was observed after moving from the acidic stomach environment to a slightly alkaline intestinal environment, which indicates that intestinal conditions enabled the compounds to be released from the plant matrix and remain stable.56
3.3. Liquid chromatography-mass spectrometry (LC-MS/MS) supported identification of individual phenolic compounds in infusions during gastrointestinal digestion
As a part of this study, specific components in infusions after digestion were targeted. Tables 4 and 5 present the MS data of the compounds detected, along with a list of molecular formulae, retention times (tR MS), mass [M–H]−, predicted m/z values, and main fragments (MS2m/z ion fragments) derived from MS/MS analysis. A total of 12 phenolic compounds were present in CHI, 11 in FHI, and 13 in LHI. Percentage peak intensity values of myricetin rhamnoglucoside, quercetin-3-O-β-D-xylopyranosyl-(1→2)-rutinoside, tetrahydroxymethoxy-flavone O-dihexoside O–pentoside, rutin, kaempferol 3-O-lathyroside-7-O-α-L-rhamnopyranoside, isorhamnetin 3-O-β-D-xylopyranosyl-(1→2)-β-D-glucopyranoside-7-O-α-L-rhamnopyranoside, and kaempferol 3-O-rutinoside were observed to increase in CHI during the gastric and intestinal phases. Catechin, tetrahydroxyflavone C-di-hexoside, and kaempferol 3-O-rhamnoglucoside-7-O-rhamnoside in FHI; procyanidin dimer type B, kaempferol tetraglycoside, and Soyasaponin I in LHI were the other phenolic compounds whose % peak intensity value increased during the gastric and intestinal phases. The reason why gastrointestinal digestion increased the amount of these phenolic compounds can be explained by the acidic medium in the gastric phase, which may facilitate the release of polyphenolic compounds in the food matrix by breaking the bonds between bioactive compounds and nutrients, such as fibers, proteins, and carbohydrates,57 allowing them to be easily measured.58 A further explanation could be the improvement in the solubility of certain phenolic compounds, before they are linked or present in a reduced form.59| Phenolic compounds | Molecular formula | t R MS (min) | Negative ion mode | References | ||
|---|---|---|---|---|---|---|
| [M–H]− (m/z), (Δ, ppm) | (m/z) predicted | Fragment ions (m/z) | ||||
| Chickpea hull infusion (CHI) | ||||||
| Dihydroxybenzoic acid hexoside | C13H16O9 | 7.118 | 315.0734 (−3.94) | 315.0722 | 153.0183, 152.0129, 109.0293, 108.0229 | 60 |
| Gallocatechin | C15H14O7 | 8.708 | 305.0670 (−1.06) | 305.0667 | 174.9546, 125.0240, 179.0359, 137.0214, 165.0179, 219.0668, 261.0779 | 61 |
| Epigallocatechin | C15H14O7 | 12.387 | 305.0657 (3.19) | 305.0667 | 125.0240, 174.9560, 179.0370, 137.0239, 219.0673, 165.0175, 261.0747 | Fragmentation |
| Myricetin rhamnoglucoside | C27H30O17 | 21.587 | 625.1406 (0.68) | 625.1410 | 316.0232, 178.9991, 271.0247, 151.0036 | 61 |
| Quercetin-3-O-β-D-xylopyranosyl-(1→2)-rutinoside | C32H38O20 | 22.089 | 741.1882 (0.23) | 741.1884 | 300.0285, 301.0329, 178.9986, 271.0260, 609.1505 | 60 |
| Tetrahydroxymethoxyflavone O-dihexoside O – pentoside | C33H40O21 | 22.590 | 771.1987 (0.30) | 771.1989 | 315.0149, 331.0464, 756.1772, 287.0192, 639.1585 | Fragmentation |
| Rutin | C27H30O16 | 23.343 | 609.1468 (−1.13) | 609.1461 | 300.0286, 301.0362, 271.0244, 151.0043 | 60–62 |
| Kaempferol 3-O-lathyroside-7-O-α-L-rhamnopyranoside | C32H38O19 | 23.427 | 725.1943 (−1.17) | 725.1935 | 284.0327, 255.0281, 575.1360, 593.1615, 227.0297 | 60 and 61 |
| Isorhamnetin 3-O-β-D-xylopyranosyl-(1→2)-β-D-glucopyranoside-7-O-α-L-rhamnopyranoside | C33H40O20 | 23.761 | 755.2065 (−3.28) | 755.2040 | 315.0526, 299.0227, 300.0288, 271.0333, 357.0563, 623.1698 | 60 |
| Kaempferol 3-o-rutinoside | C27H30O15 | 24.932 | 593.1500 (2.01) | 593.1512 | 285.0395, 255.0288, 327.0482 | 60 and 61 |
| Pseudobaptigenin | C16H10O5 | 35.805 | 281.0448 (2.65) | 281.0455 | 253.0498, 223.0418, 135.0094, 91.0185, 208.0525, 195.0443 | 61 and 63 |
| Biochanin A | C16H12O5 | 41.241 | 283.0621 (−3.18) | 283.0612 | 268.0399, 148.9547, 240.0427, 117.0347, 239.0339, 151.0015 | 61–63 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Faba bean hull infusion (FHI) | ||||||
| Gallocatechin | C15H14O7 | 8.620 | 305.0684 (−5.63) | 305.0667 | 125.0244, 174.9551, 165.0204, 179.0342, 167.0367, 219.0667 | 61 and 64 |
| Procyanidin dimer type A isomer 1 | C30H26O13 | 9.707 | 593.1306 (−0.9) | 593.1301 | 407.0732, 177.0170, 289.0688, 125.0232, 245.0791, 290.0709, 305.0659 | 39 and 61 |
| Chlorogenic acid isomer | C16H18O9 | 10.049 | 353.0881 (−0.83) | 353.0878 | 191.0559; 179.0340; 135.0442; 173.0417 | Fragmentation, 61 |
| Procyanidin dimer type A isomer 2 | C30H26O13 | 12.383 | 593.1301 (−0.06) | 593.1301 | 125.0242, 289.0721, 407.0781, 177.0189, 245.0839, 423.0711, 305.0673 | 39 and 61 |
| Epigallocatechin | C15H14O7 | 12.387 | 305.0674 (−2.36) | 305.0667 | 125.0247, 179.0333, 219.0660, 137.0262, 174.9558, 167.0344 | 61, 64 and 65 |
| Procyanidin dimer type B | C30H26O12 | 12.470 | 577.1355 (−0.61) | 577.1351 | 289.0706, 407.0765, 125.0231, 245.0797, 161.0251, 290.0747 | 39, 64 and 66 |
| Catechin | C15H14O6 | 13.136 | 289.0717 (0.21) | 289.0718 | 245.0829, 205.0502, 125.0242, 179.0343, 109.0299, 137.0244, 151.0381, 165.0206 | 64–66 |
| Epicatechin | C15H14O6 | 17.318 | 289.0717 (0.21) | 289.0718 | 245.0827, 205.0509, 125.0245, 179.0357, 109.0290, 137.0232, 165.0190, 151.0393 | 64 and 66 |
| Tetrahydroxyflavone C-di-hexoside | C27H30O15 | 18.990 | 593.1506 (1.00) | 593.1512 | 593.1495, 353.0668, 473.1109, 383.0759, 503.1202, 413.0872 | Fragmentation |
| Kaempferol 3-O-rhamnoglucoside-7- O-rhamnoside | C33H40O19 | 22.503 | 739.2099 (−1.08) | 739.2091 | 284.0322, 285.0366, 255.0306, 575.1430, 593.1481, 227.0358 | 67 and 68 |
| Quercetin | C15H10O7 | 31.284 | 301.0353 (0.25) | 301.0354 | 151.0045, 178.9995, 174.9578, 107.0149, 121.0294, 65.0052 | 61, fragmentation |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Lentil hull infusion (LHI) | ||||||
| Procyanidin trimer | C45H38O20 | 9.123 | 897.1900 (−1.82) | 897.1884 | 711.1278, 303.0487, 289.0692, 177.0169, 125.0223, 407.0762 | 69 and 70 |
| Procyanidin dimer type A | C30H26O13 | 9.708 | 593.1290 (1.79) | 593.1301 | 407.0753, 289.0705, 177.0184, 125.0229, 245.0795, 137.0229 | 70 and 71 |
| (Epi)catechin glucoside | C21H24O11 | 11.632 | 451.1244 (0.41) | 451.1246 | 137.0246, 125.0251, 271.0622, 289.0717, 151.0412, 313.0935 | 71–73 |
| Procyanidin dimer type B | C30H26O12 | 12.468 | 577.1341 (1.82) | 577.1351 | 289.0718, 407.0776, 125.0245, 245.0812, 161.0249, 137.0246 | 69, 70 and 72 |
| Epicatechin | C15H14O6 | 13.054 | 289.0717 (0.21) | 289.0718 | 245.0793, 203.0688, 205.0490, 125.0233, 179.0339, 109.0293 | 69, 71, 72 and 74 |
| Procyanidin trimer | C45H38O18 | 13.221 | 865.1974 (1.31) | 865.1985 | 287.0548, 125.0238, 289.0695, 407.0743, 577.1308, 695.1343 | 69, 70 and 72 |
| Kaempferol tetraglycoside (kaempferol O-dirutinoside) | C39H50O24 | 18.908 | 901.2617 (0.25) | 901.2619 | 755.1982, 756.2009, 284.0302, 285.0374, 430.0871, 575.1342 | 72 and 73 |
| Quercetin O-rhamnoside | C21H20O11 | 25.850 | 447.0933 (−0.03) | 447.0933 | 300.0260, 301.0332, 255.0273, 284.0304, 271.0236, 151.0026 | 72 |
| Kaempferol O-glucoside | C21H20O11 | 26.352 | 447.0931 (0.41) | 447.0933 | 285.0370, 286.0415, 165.0146, 150.9986, 119.0493, 133.0301 | 69 and 72 |
| Quercetin | C15H10O7 | 31.202 | 301.0353 (0.25) | 301.0354 | 151.0023; 174.9565; 178.9964; 121.0324; 130.9661; 179.9942 | 61, fragmentation |
| Kaempferol | C15H10O6 | 31.286 | 285.0400 (1.61) | 285.0405 | 133.0286, 151.0025, 175.0378, 107.0124, 199.0374, 149.0217 | 72 |
| Soyasaponin I | C48H78O18 | 39.314 | 941.5111 (0.47) | 941.5115 | — | 75 |
| Soyasaponin βg | C54H84O21 | 42.493 | 1067.5440 (−0.72) | 1067.5432 | — | 75 |
4. Conclusions
The present study focused on the polyphenols of legume by-products and their infusions as well as the effect of in vitro digestion on antioxidant properties and phenolic compounds of these infusions. It was observed that water extracts of legume by-products had higher TPC, TFC, and antioxidant activity values compared to their methanolic counterparts. FH and LH possessed the highest phenolic contents and the strongest antioxidant capacity. Phenolic content and antioxidant capacity of some infusions were remarkably lowered during in vitro digestion, suggesting the impact of the conditions in the small intestine on the antioxidant activities of the infusions, depending on the type, nature, and concentration of the phenolic compounds present.On the other hand, FHI and LHI were observed to be less affected during the digestion process, and thus their stability and resilience make them valuable components in various industries. By retaining their properties and functionality, FHI and LHI can fulfill their intended purposes and provide the desired benefits to consumers. Based on the results of this study, valuable insights are provided for the potential use of these by-products in infusions and further valorization in different novel applications, including incorporation into functional food formulations.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Acknowledgements
The authors received funding for this work from the Medical University of Lublin: DS 28 (ES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.References
- Y. Zhang, P. Cai, G. Cheng and Y. Zhang, A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity, Nat. Prod. Commun., 2022, 17(1), 1934578X211069721 CrossRef CAS.
- X. Coz-Bolaños, R. Campos-Vega, R. Reynoso-Camacho, M. Ramos-Gómez, G. F. Loarca-Piña and S. Guzmán-Maldonado, Moringa infusion (Moringa oleifera) rich in phenolic compounds and high antioxidant capacity attenuate nitric oxide pro-inflammatory mediator in vitro, Ind. Crops Prod., 2018, 118, 95–101 CrossRef.
- R. Mahbub, N. Francis, C. Blanchard and A. Santhakumar, The anti-inflammatory and antioxidant properties of chickpea hull phenolic extracts, Food Biosci., 2021, 40, 100850 CrossRef CAS.
- N. Sharma, N. Yeasmen and V. Orsat, Green extraction of chickpea (Cicer arietinum)-based functional beverage: Assessment of nutritional quality and storage stability, Food Biosci., 2024, 59, 104237 CrossRef CAS.
- O. Barakat, E. Elsebaie, A. Ammar and K. Elnemr, Utilization of faba bean hulls (seeds coat) as a source to produce antioxidants, J. Food Dairy Sci., 2017, 8(7), 275–278 CrossRef.
- S. D. Siah, S. Agboola, J. A. Wood, I. Konczak and C. L. Blanchard, A comparison study of phenolic contents and in vitro antioxidant activities of Australian grown faba beans (Vicia faba L.) varying in seed coat colours as affected by extraction solvents, Am. J. Anal. Chem., 2019, 10(6), 227–245 CrossRef CAS.
- M. H. Kamani, Y. Luithui and M. Meera, Upgrading black gram by-product to a new texturized vegetable source by extrusion: evaluation of functionality, antinutrients and in vitro digestibility, Waste Biomass Valorization, 2021, 12(8), 4319–4330 CrossRef CAS.
- E. Echeverria-Jaramillo and W.-S. Shin, Black soybean cooking water (aquasoya) powder as a novel clean-label ingredient in plant-based vegan patties, Int. J. Food Sci. Technol., 2023, 58(10), 5121–5133 CrossRef CAS.
- A. P. Araujo-Chapa, V. Urías-Orona, G. Niño-Medina, D. Muy-Rangel, A. L. de la Garza and H. Castro, Dietary fiber from soybean (Glycine max) husk as fat and phosphate replacer in frankfurter sausage: effect on the nutritional, physicochemical and nutraceutical quality, Molecules, 2023, 28(13), 4997 CrossRef CAS PubMed.
- Y.-H. Kim and W.-S. Shin, Evaluation of the physicochemical and functional properties of aquasoya (Glycine max Merr.) powder for vegan muffin preparation, Foods, 2022, 11(4), 591 CrossRef CAS PubMed.
- D. Chatziharalambous, C. Kaloteraki, P. Potsaki, O. Papagianni, K. Giannoutsos and D. I. Koukoumaki, et al., Study of the total phenolic content, total antioxidant activity and in vitro digestibility of novel wheat crackers enriched with cereal, legume and agricultural by-product flours, Oxygen, 2023, 3(2), 256–273 CrossRef CAS.
- S. Chockchaisawasdee, M. C. Mendoza, C. A. Beecroft, A. C. Kerr, C. E. Stathopoulos and A. Fiore, Development of a gluten free bread enriched with faba bean husk as a fibre supplement, LWT, 2023, 173, 114362 CrossRef CAS.
- M. Chakraborty, S. Budhwar and S. Kumar, Development of fermented products with enriched fiber and micronutrients by using underutilized cereal-legume milling by-products as novel food ingredients, Int. J. Gastron. Food Sci., 2022, 27, 100493 CrossRef.
- G. Ozkan, F. B. Sakarya, D. Tas, B. Yurt, S. Ercisli and E. Capanoglu, Effect of In Vitro Digestion on the Phenolic Content of Herbs Collected from Eastern Anatolia, ACS Omega, 2023, 8(14), 12730–12738 CrossRef CAS PubMed.
- X. Orozco-Angelino, J. Espinosa-Ramírez and S. O. Serna-Saldívar, Extrusion as a tool to enhance the nutritional and bioactive potential of cereal and legume by-products, Food Res. Int., 2023, 169, 112889 CrossRef CAS PubMed.
- J. B. Johnson, K. B. Walsh, S. P. Bhattarai and M. Naiker, Partitioning of nutritional and bioactive compounds between the kernel, hull and husk of five new chickpea genotypes grown in Australia, Future Foods, 2021, 4, 100065 CrossRef CAS.
- S. Boudjou, B. D. Oomah, F. Zaidi and F. Hosseinian, Phenolics content and antioxidant and anti-inflammatory activities of legume fractions, Food Chem., 2013, 138(2–3), 1543–1550 CrossRef CAS PubMed.
- G. Ozkan, A. S. Stübler, K. Aganovic, G. Draeger, T. Esatbeyoglu and E. Capanoglu, A comparative study on physicochemical properties and in vitro bioaccessibility of bioactive compounds in rosehip (Rosa canina L.) infusions treated by non-thermal and thermal treatments, J. Food Process. Preserv., 2022, 46(6), e16096 CAS.
- E. Celep, S. Akyuz, Y. İnan and E. Yesilada, Stability of phenolic content of some herbal infusions and their antioxidant activity following in vitro digestion, Turk. J. Biochem., 2017, 42(4), 375–380 CrossRef.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn and C. Bourlieu, et al., A standardised static in vitro digestion method suitable for food–an international consensus, Food Funct., 2014, 5(6), 1113–1124 RSC.
- V. L. Singleton and J. A. Rossi, Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, Am. J. Enol. Vitic., 1965, 16(3), 144–158 CrossRef CAS.
- V. Dewanto, X. Wu, K. K. Adom and R. H. Liu, Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity, J. Agric. Food Chem., 2002, 50(10), 3010–3014 CrossRef CAS.
- P. Molyneux, The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity, Songklanakarin J. Sci. Technol., 2004, 26(2), 211–219 CAS.
- R. Apak, K. Güçlü, M. Özyürek and S. E. Karademir, Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method, J. Agric. Food Chem., 2004, 52(26), 7970–7981 CrossRef CAS PubMed.
- N. J. Miller and C. A. Rice-Evans, Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay, Free Radic. Res., 1997, 26(3), 195–199 CrossRef CAS.
- Ł. Świątek, E. Sieniawska, K. I. Sinan, G. Zengin, A. Boguszewska and B. Hryć, et al., Chemical Characterization of Different Extracts of Justicia secunda Vahl and Determination of Their Anti-Oxidant, Anti-Enzymatic, Anti-Viral, and Cytotoxic Properties, Antioxidants, 2023, 12(2), 509 CrossRef.
- R. Amarowicz and F. Shahidi, Antioxidant activity of faba bean extract and fractions thereof, J. Food Bioact., 2018, 2, 112 CrossRef.
- A. Manco, C. Gerardi, G. Romano, L. D'Amico, A. Blanco and F. Milano, et al., Phenolic profile of whole seeds and seed fractions of lentils and its impact on antioxidant activity, Food Biosci., 2023, 54, 102887 CrossRef CAS.
- Y. Gavamukulya, F. Abou-Elella, F. Wamunyokoli and H. AEl-Shemy, Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola), Asian Pac. J. Tropical Med., 2014, 7, S355–S363 CrossRef CAS.
- Q. V. Vuong, S. Hirun, P. D. Roach, M. C. Bowyer, P. A. Phillips and C. J. Scarlett, Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts, J. Herb. Med., 2013, 3(3), 104–111 CrossRef.
- S. Butsat and S. Siriamornpun, Effect of solvent types and extraction times on phenolic and flavonoid contents and antioxidant activity in leaf extracts of Amomum chinense C, Int. Food Res. J., 2016, 23(1), 180 CAS.
- T. Venkatesan, Y.-W. Choi and Y.-K. Kim, Impact of different extraction solvents on phenolic content and antioxidant potential of Pinus densiflora bark extract, BioMed Res. Int., 2019, 3520675 CAS.
- A. Al-Rifai, A. Aqel, T. Al-Warhi, S. M. Wabaidur, Z. A. Al-Othman and A. Y. Badjah-Hadj-Ahmed, Antibacterial, antioxidant activity of ethanolic plant extracts of some Convolvulus species and their DART-ToF-MS profiling, J. Evid.-based Complement. Altern. Med., 2017, 1, 5694305 CrossRef.
- M. Pinelo, L. Manzocco, M. J. Nuñez and M. C. Nicoli, Interaction among phenols in food fortification: negative synergism on antioxidant capacity, J. Agric. Food Chem., 2004, 52(5), 1177–1180 CrossRef CAS PubMed.
- D. S. Arabshahi, D. V. Devi and A. Urooj, Evaluation of antioxidant activity of some plant extracts and their heat, pH and storage stability, Food Chem., 2007, 100(3), 1100–1105 CrossRef.
- Q. D. Do, A. E. Angkawijaya, P. L. Tran-Nguyen, L. H. Huynh, F. E. Soetaredjo and S. Ismadji, et al., Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica, J. Food Drug Anal., 2014, 22(3), 296–302 CrossRef CAS.
- K. Robards, Strategies for the determination of bioactive phenols in plants, fruit and vegetables, J. Chromatogr., A, 2003, 1000(1–2), 657–691 CrossRef CAS.
- P. Pedrielli, G. F. Pedulli and L. H. Skibsted, Antioxidant mechanism of flavonoids. Solvent effect on rate constant for chain-breaking reaction of quercetin and epicatechin in autoxidation of methyl linoleate, J. Agric. Food Chem., 2001, 49(6), 3034–3040 CrossRef CAS PubMed.
- C. Baginsky, Á. Peña-Neira, A. Cáceres, T. Hernández, I. Estrella and H. Morales, et al., Phenolic compound composition in immature seeds of fava bean (Vicia faba L.) varieties cultivated in Chile, J. Food Compos. Anal., 2013, 31(1), 1–6 CrossRef CAS.
- A. M. Mustafa, D. Abouelenein, L. Acquaticci, L. Alessandroni, S. Angeloni and G. Borsetta, et al., Polyphenols, saponins and phytosterols in lentils and their health benefits: an overview, Pharmaceuticals, 2022, 15(10), 1225 CrossRef CAS.
- S. B. Iloki-Assanga, L. M. Lewis-Luján, C. L. Lara-Espinoza, A. A. Gil-Salido, D. Fernandez-Angulo and J. L. Rubio-Pino, et al., Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum, BMC Res. Notes, 2015, 8(1), 1–14 CrossRef.
- D. Palaiogiannis, T. Chatzimitakos, V. Athanasiadis, E. Bozinou, D. P. Makris and S. I. Lalas, Successive Solvent Extraction of Polyphenols and Flavonoids from Cistus creticus L. Leaves, Oxygen, 2023, 3(3), 274–286 CrossRef CAS.
- M. J. Rodriguez-Roque, M. A. Rojas-Graue, P. Elez-Martinez and O. Martin-Belloso, In vitro bioaccessibility of health-related compounds as affected by the formulation of fruit juice-and milk-based beverages, Food Res. Int., 2014, 62, 771–778 CrossRef CAS.
- W. Qin, S. Ketnawa and Y. Ogawa, Effect of digestive enzymes and pH on variation of bioavailability of green tea during simulated in vitro gastrointestinal digestion, Food Sci. Hum. Wellness, 2022, 11(3), 669–675 CrossRef CAS.
- K. Wojtunik-Kulesza, A. Oniszczuk, T. Oniszczuk, M. Combrzyński, D. Nowakowska and A. Matwijczuk, Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review, Nutrients, 2020, 12(5), 1401 CrossRef CAS.
- M. J. Bae, T. Ishii, K. Minoda, Y. Kawada, T. Ichikawa and T. Mori, et al., Albumin stabilizes (–)-epigallocatechin gallate in human serum: binding capacity and antioxidant property, Mol. Nutr. Food Res., 2009, 53(6), 709–715 CrossRef CAS.
- R. J. Green, A. S. Murphy, B. Schulz, B. A. Watkins and M. G. Ferruzzi, Common tea formulations modulate in vitro digestive recovery of green tea catechins, Mol. Nutr. Food Res., 2007, 51(9), 1152–1162 CrossRef CAS.
- I. Odriozola-Serrano, D. P. Nogueira, I. Esparza, A. A. Vaz, N. Jiménez-Moreno and O. Martín-Belloso, et al., Stability and bioaccessibility of phenolic compounds in rosehip extracts during in vitro digestion, Antioxidants, 2023, 12(5), 1035 CrossRef CAS PubMed.
- Z. Xiao, L. He, X. Hou, J. Wei, X. Ma and Z. Gao, et al., Relationships between structure and antioxidant capacity and activity of glycosylated flavonols, Foods, 2021, 10(4), 849 CrossRef CAS.
- N. Değirmencioğlu, E. Yıldız, Y. Sahan, M. Güldas and O. Gürbüz, Impact of tea leaves types on antioxidant properties and bioaccessibility of kombucha, J. Food Sci. Technol., 2021, 58(6), 2304–2312 CrossRef PubMed.
- G. Ozkan, T. Kostka, G. Dräger, E. Capanoglu and T. Esatbeyoglu, Bioaccessibility and transepithelial transportation of cranberrybush (Viburnum opulus) phenolics: Effects of non-thermal processing and food matrix, Food Chem., 2022, 380, 132036 CrossRef CAS PubMed.
- R. Campos-Vega, K. Vázquez-Sánchez, D. López-Barrera, G. Loarca-Piña, S. Mendoza-Díaz and B. D. Oomah, Simulated gastrointestinal digestion and in vitro colonic fermentation of spent coffee (Coffea arabica L.): Bioaccessibility and intestinal permeability, Food Res. Int., 2015, 77, 156–161 CrossRef CAS.
- I. Seiquer, A. Rueda, M. Olalla and C. Cabrera-Vique, Assessing the bioavailability of polyphenols and antioxidant properties of extra virgin argan oil by simulated digestion and Caco-2 cell assays. Comparative study with extra virgin olive oil, Food Chem., 2015, 188, 496–503 CrossRef CAS PubMed.
- Y. Qin, L. Wang, Y. Liu, Q. Zhang, Y. Li and Z. Wu, Release of phenolics compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bio-activities, J. Funct. Foods, 2018, 46, 57–65 CrossRef CAS.
- G. Zheng, J. Deng, L. Wen, L. You, Z. Zhao and L. Zhou, Release of phenolic compounds and antioxidant capacity of Chinese hawthorn “Crataegus pinnatifida” during in vitro digestion, J. Funct. Foods, 2018, 40, 76–85 CrossRef CAS.
- D. de Paulo Farias, F. F. de Araújo, I. A. Neri-Numa, F. L. Dias-Audibert, J. Delafiori and R. R. Catharino, et al., Effect of in vitro digestion on the bioaccessibility and bioactivity of phenolic compounds in fractions of Eugenia pyriformis fruit, Food Res. Int., 2021, 150, 110767 CrossRef CAS PubMed.
- M. Alminger, A. M. Aura, T. Bohn, C. Dufour, S. El and A. Gomes, et al., In vitro models for studying secondary plant metabolite digestion and bioaccessibility, Compr. Rev. Food Sci. Food Saf., 2014, 13(4), 413–436 CrossRef CAS PubMed.
- S. Ketnawa, J. Suwannachot and Y. Ogawa, In vitro gastrointestinal digestion of crisphead lettuce: Changes in bioactive compounds and antioxidant potential, Food Chem., 2020, 311, 125885 CrossRef CAS PubMed.
- M. S. Gião, S. Gomes, A. R. Madureira, A. Faria, D. Pestana and C. Calhau, et al., Effect of in vitro digestion upon the antioxidant capacity of aqueous extracts of Agrimonia eupatoria, Rubus idaeus, Salvia sp. and Satureja montana, Food Chem., 2012, 131(3), 761–767 CrossRef.
- R. H. Mekky, M. del Mar Contreras, M. R. El-Gindi, A. R. Abdel-Monem, E. Abdel-Sattar and A. Segura-Carretero, Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: A comparative study, RSC Adv., 2015, 5(23), 17751–17767 RSC.
- PubChem, 2023, available from: https://pubchem.ncbi.nlm.nih.gov/, access date: 23.10.2023.
- A. C. de Camargo, A. Concepción Alvarez, M. F. Arias-Santé, J. E. Oyarzún, M. E. Andia and S. Uribe, et al., Soluble free, esterified and insoluble-bound phenolic antioxidants from chickpeas prevent cytotoxicity in human hepatoma HuH-7 cells induced by peroxyl radicals, Antioxidants, 2022, 11(6), 1139 CrossRef CAS PubMed.
- Z. Wu, L. Song, S. Feng, Y. Liu, G. He and Y. Yioe, et al., Germination dramatically increases isoflavonoid content and diversity in chickpea (Cicer arietinum L.) seeds, J. Agric. Food Chem., 2012, 60(35), 8606–8615 CrossRef CAS PubMed.
- S. Yuzuak, J. Ballington and D.-Y. Xie, HPLC-qTOF-MS/MS-based profiling of flavan-3-ols and dimeric proanthocyanidins in berries of two muscadine grape hybrids FLH 13-11 and FLH 17-66, Metabolites, 2018, 8(4), 57 CrossRef.
- D. K. Choudhary, N. Chaturvedi, A. Singh and A. Mishra, Characterization, inhibitory activity and mechanism of polyphenols from faba bean (gallic-acid and catechin) on α-glucosidase: Insights from molecular docking and simulation study, Prep. Biochem. Biotechnol., 2020, 50(2), 123–132 CrossRef CAS.
- I. I. Rockenbach, E. Jungfer, C. Ritter, B. Santiago-Schübel, B. Thiele and R. Fett, et al., Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS, Food Res. Int., 2012, 48(2), 848–855 CrossRef CAS.
- S. Neugart, S. Rohn and M. Schreiner, Identification of complex, naturally occurring flavonoid glycosides in Vicia faba and Pisum sativum leaves by HPLC-DAD-ESI-MSn and the genotypic effect on their flavonoid profile, Food Res. Int., 2015, 76, 114–121 CrossRef CAS.
- S. Zanotto, H. Khazaei, F. M. Elessawy, A. Vandenberg and R. W. Purves, Do faba bean genotypes carrying different zero-tannin genes (zt1 and zt2) differ in phenolic profiles?, J. Agric. Food Chem., 2020, 68(28), 7530–7540 CrossRef CAS.
- M. Mirali, S. J. Ambrose, S. A. Wood, A. Vandenberg and R. W. Purves, Development of a fast extraction method and optimization of liquid chromatography–mass spectrometry for the analysis of phenolic compounds in lentil seed coats, J. Chromatogr. B, 2014, 969, 149–161 CrossRef CAS.
- A. Singh, S. Kumar and B. Kumar, LC-MS Identification of Proanthocyanidins in Bark and Fruit of six Terminalia species, Nat. Prod. Commun., 2018, 13(5), 1934578X1801300511 CrossRef CAS.
- R. Llorach, C. Favari, D. Alonso, M. Garcia-Aloy, C. Andres-Lacueva and M. Urpi-Sarda, Comparative metabolite fingerprinting of legumes using LC-MS-based untargeted metabolomics, Food Res. Int., 2019, 126, 108666 CrossRef CAS PubMed.
- M. Mirali, R. W. Purves and A. Vandenberg, Profiling the phenolic compounds of the four major seed coat types and their relation to color genes in lentil, J. Nat. Prod., 2017, 80(5), 1310–1317 CrossRef CAS PubMed.
- Y. Zou, S. K. Chang, Y. Gu and S. Y. Qian, Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions, J. Agric. Food Chem., 2011, 59(6), 2268–2276 CrossRef CAS PubMed.
- A. L. Jin, J. A. Ozga, D. Lopes-Lutz, A. Schieber and D. M. Reinecke, Characterization of proanthocyanidins in pea (Pisum sativum L.), lentil (Lens culinaris L.), and faba bean (Vicia faba L.) seeds, Food Res. Int., 2012, 46(2), 528–535 CrossRef CAS.
- G. Sagratini, Y. Zuo, G. Caprioli, G. Cristalli, D. Giardina and F. Maggi, et al., Quantification of soyasaponins I and βg in Italian lentil seeds by solid-phase extraction (SPE) and high-performance liquid chromatography− mass spectrometry (HPLC-MS), J. Agric. Food Chem., 2009, 57(23), 11226–11233 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |