Open Access Article
Open Access ArticleEnhancing nutritional value through semi-solid fermentation of quinoa and mango–orange juice: a sustainable approach to food processing
Bordoni
Antonella
 a,
Rossetti
Luciana
a,
Rossetti
Luciana
 a,
Rizzo Sergio
Aníbal
a,
Rizzo Sergio
Aníbal
 a,
Dhuique-Mayer
Claudie
a,
Dhuique-Mayer
Claudie
 b,
Bárcena
Nadia
b,
Bárcena
Nadia
 c and
Descalzo Adriana
María
c and
Descalzo Adriana
María
 *a
*a
aInstituto Tecnología de Alimentos (ITA), Instituto Nacional de Tecnología Agropecuaria (INTA), Instituto de Ciencia y Tecnología de Los Sistemas Alimentarios Sustentables (ICyTeSAS) UEDD INTA-CONICET, Hurlingham, Buenos Aires, Argentina. E-mail: adescalzo.inta@yahoo.com
bQualiSud, Univ. Montpellier, CIRAD, Institut Agro, Université D'Avignon, Université de La Réunion, Montpellier, France
cEEA-San Juan, Instituto Nacional de Tecnología Agropecuaria (INTA), Calle 11 y Vidart (5427) Villa Aberastain, San Juan, Argentina
First published on 24th April 2025
Abstract
This research explores the semi-solid fermentation (SSF) of quinoa (Chenopodium quinoa wild) in combination with mango–orange (Mangifera indica–Citrus sinensis) juice as a sustainable and innovative approach to food processing. Quinoa, renowned for its rich nutritional profile, presents challenges due to the presence of antinutrients that can inhibit nutrient absorption. By employing lactic acid bacteria (LAB) from kefir in the fermentation process, this study aims to enhance the bioavailability of essential nutrients while concurrently reducing the levels of antinutritional factors. The results indicate that fermentation leads to a significant increase in protein content, phytosterols, and antioxidant properties, while facilitating the conversion of bound phenolic compounds into more bioavailable forms. The incorporation of mango–orange juice enriched the nutritional profile of the product but lowered the pH, inhibiting microbial growth and necessitating a pH regulator to improve fermentation. Overall, this work contributes to the development of a probiotic-rich, functional beverage that not only supports food security but also aligns with sustainable food practices and promotes the nutritional health of populations where quinoa is a dietary staple. Further investigations will focus on optimizing fermentation conditions and exploring additional quinoa varieties to enhance product quality and health benefits.
Sustainability spotlightSustainable semi-solid fermentation (SSF) of quinoa offers significant environmental advantages, particularly in water-scarce regions, as it requires less water than submerged liquid fermentation. Utilizing non-traditional cereals and their by-products further reduces waste and aligns with circular economy principles. SSF enhances the nutritional profile of cereals by improving nutrient bioavailability, producing vitamins, and lowering antinutritional factors. The process generates natural preservatives like organic acids, extending shelf life and minimizing food waste. Quinoa's resilience and low water requirement contribute to its sustainability as a complete protein source. Incorporating kefir in this fermentation boosts nutrient availability and introduces beneficial probiotics. Ongoing research aims to improve SSF efficiency and scalability, reinforcing its potential as a sustainable food processing method. |
1 Introduction
The search for nutritious and sustainable food alternatives has become a pressing concern in the modern world. Quinoa (Chenopodium quinoa wild), an Andean pseudocereal, has gained significant attention due to its exceptional nutritional profile (proteins, fatty acids, vitamins and minerals) and potential for value-added processing.1The main issue with quinoa is the presence of antinutrients-molecules that interfere with nutrient absorption. Key antinutritional components in quinoa include saponins, phytic acid, oxalates, tannins, and trypsin inhibitors.2 To address this issue, fermentation using Lactic Acid Bacteria (LAB) can help reduce some of these antinutrients. LAB produces phytases, which aid in this process.3 Furthermore, fermentation has been shown to enhance the nutritional value of grains by increasing the levels of essential amino acids such as lysine, methionine, and tryptophan.4
Cereal fermentation has emerged as a promising technique for improving the nutritive value and functional properties of various food and agricultural products for the production of certain metabolites like enzymes, organic acids, and flavours.5
Solid fermentation of cereals has a long history in traditional food preparations, particularly with sorghum and maize using spontaneous fermentation or with the addition of LAB starters, in many African and Asian countries;6,7 reviewed in Ramesh and Montet, 2017.8
For example, fermented maize starch, known as ogi, is used to make Akpan, a traditional yogurt-like food in Benin that provides essential nutrition to its consumers.9 Additionally, an innovative approach to harnessing the benefits of quinoa is through solid fermentation, which can contribute to a more sustainable food system.
The advantage of using quinoa to produce new functional foods through SSF include the use of LAB from kefir. LAB can help improve the nutritional profile of quinoa by increasing the bioavailability of nutrients and producing beneficial metabolites. Additionally, the incorporation of kefir, a symbiotic culture of bacteria and yeast, can enhance the functional properties of the fermented quinoa, leading to the development of novel probiotic-rich food products. The microbial composition of kefir grains comprised 65 to 80% of Lactobacillus sp. and Lactococcus sp. and the remaining portion consisted on yeasts.10
Also the inclusion of pro-vitaminic and antioxidant carotenoids help to improve the nutritional quality of this kind of beverages, as most cereals are not a provitamin A source by excellence, as discussed previously for the development of maize-yogurt type beverage.11
Particularly, mango is an excellent source of cis-beta carotene, which is more soluble in lipid droplets improving its bioaccesibility. However many factors such as time, temperature or occurrence of oxygen can significantly affect β-carotene retention in the food matrix.12 Orange juice provides bioactive compounds as naringin and hesperidin, the main flavonoid with action on microvascular reactivity and cardiovascular health.13 It also contain several carotenoids, primarily β-cryptoxanthin, along with smaller amounts of α-carotene, β-carotene, lutein, and zeaxanthin, which can contribute to preventing chronic diseases such as type 2 diabetes, cardiovascular diseases, and obesity.14
The advantages of semi solid-state fermentation (SSF) using kefir extend beyond the realm of traditional foods, as it can also be applied to the processing of quinoa. However many changes occur to phyto-micronutrients in the course of a fermentation with LAB and kefir. SSF can utilize a broad range of microorganisms, including those found naturally in the raw materials. This can lead to a diverse range of products and potential health benefits. Cereal-based fermented products well fit in this emerging field, since they are, among other, a cheap source of natural prebiotic molecules (e.g. beta-glucans).15,16
The goal of this work is to present the possibility of performing the lactic fermentation of quinoa as a base of functional vegetal yogurt type beverage. The addition of carotenes is also studied as quinoa is not a source of this vitamin, which is critical for the supplementation of the Andean populations were quinoa, is the main staple food.
The combination of quinoa and kefir fermentation creates a nutrient-dense food product that can aid in improving food security, particularly in regions with limited resources. Therefore, the challenge is to use sustainable food processing based on traditional recipes using natural ingredients.
2 Material and methods
2.1. Quinoa samples and preparation of mango–orange powder
The quinoa preparation was divided into two separate batches: batch 1 containing 25% lyophilized mango–orange extract as a source of carotenoids (Lutein and β-carotene respectively). The Batch 2 served as a control without any additives. Both batches were pasteurized at a temperature of 90 °C for 10 min while stirring continuously. After pasteurization, the batches were inoculated with activated kefir (previously diluted in MRS broth and maintained at 37 °C for 2 h, to use it as inoculum). Finally, the inoculated product was transferred into 175 mL flasks and were incubated under stirring at 37 °C for an additional 24 h to allow fermentation to occur properly. For each sampling time, triplicate flasks were used. Bacterial count, pH and lactic acid were determined at each sampling time in the fresh sample. The rest of the products were kept frozen and in darkness at −20 °C for the determination of micronutrients.
• Q0: samples without pasteurization.
• Q1: pasteurized samples.
• F0: samples inoculated before fermentation (for initial CFU count).
• F24: samples fermented for the time indicated by the number.
Q: quinoa control QMO: quinoa with added mango–orange juice QMONaOH: quinoa with added mango–orange juice and pH adjusted. Samples were taken at different stages and fermentation times for the determination of:
• Macronutrients (Q0; Q1; F24; Table 1).
| Treatment | Protein (g kg−1) | Fat (g kg−1) | L-Lactic acid (g kg−1) |
|---|---|---|---|
| a Nd: not detected. Different letters within the same column indicates differences at the 0.05 level determined by Tukey test. | |||
| Q 0 | 11.86 ± 0.32 b | 3.5 ± 0.03 a | nd |
| Q 1 | 11.53 ± 0.24 b | 3.5 ± 0.02 a | nd |
| F 24 | 13.61 ± 0.71 a | 3.5 ± 0.04 a | 9.04 ± 0.11 |
• Micronutrients in the conventional quinoa beverage (Q0, Q1, F: 0; 4; 8; 16; 20; 22; 24, Table 2).
| Step | βδ-toc (mg kg−1) | γ-toc (mg per kg) | α-toc (mg per kg) | Lutein (mg kg−1) | Stigmasterol (mg kg−1) | β-Sitosterol (mg kg−1) | Total phenols (mg GA/L) | FRAP (trolox eq. L−1) |
|---|---|---|---|---|---|---|---|---|
| a Different letters within the same column indicates differences at the 0.05 level determined by Tukey test. | ||||||||
| Q 0 | 0.207 ± 0.011 a | 3.69 ± 0.88 a | 2.31 ± 0.58 a | 0.13 ± 0.03 abc | 2.94 ± 0.24 cd | 17.55 ± 6.32 c | 23.55 ± 0.11 ef | 367.2 ± 24.2 abc |
| Q 1 | 0.139 ± 0.021 a | 3.54 ± 0.04 a | 0.91 ± 0.04 b | 0.14 ± 0.00 abc | 5.99 ± 0.91 ab | 31.48 ± 5.47 abc | 24.66 ± 0.31 de | 334.7 ± 6.9 abc |
| F 0 | 0.123 ± 0.018 a | 3.59 ± 0.58 a | 1.71 ± 0.02 ab | 1.82 ± 0.02 a | 7.09 ± 0.50 a | 41.36 ± 5.15 a | 22.96 ± 0.42 ef | 369.1 ± 25.2 ab |
| F 4 | 0.124 ± 0.018 a | 1.42 ± 0.62 b | 0.43 ± 0.01 b | 0.07 ± 0.00 c | 4.0 ± 0.65 cd | 19.22 ± 0.64 bc | 22.95 ± 0.21 f | 289.9 ± 1.9 bc |
| F 8 | 0.100 ± 0.009 a | 1.92 ± 0.15 b | 0.34 ± 0.04 b | 0.09 ± 0.00 bc | 3.98 ± 0.03 bcd | 25.25 ± 2.65 bc | 23.68 ± 0.31 ef | 276.9 ± 10.8 c |
| F 16 | 0.204 ± 0.015 a | 2.74 ± 0.08 b | 0.61 ± 0.03 b | 0.12 ± 0.06 abc | 5.83 ± 0.25 abc | 36.47 ± 2.76 ab | 27.99 ± 0.52 a | 376.6 ± 10.1 ab |
| F 18 | 0.132 ± 0.002 a | 1.47 ± 0.03 b | 0.28 ± 0.00 b | 0.08 ± 0.02 bc | 4.21 ± 0.67 bcd | 30.15 ± 1.35 abc | 27.02 ± 0.42 ab | 300.53 ± 11.3 bc |
| F 20 | 0.106 ± 0.001 a | 1.08 ± 0.01 b | 0.36 ± 0.00 b | 0.06 ± 0.01 c | 2.56 ± 1.65 d | 24.93 ± 0.22 bc | 25.62 ± 0.21 bcd | 328.2 ± 4.2 abc |
| F 22 | 0.108 ± 0.055 a | 1.80 ± 0.09 b | 0.32 ± 0.05 b | 0.07 ± 0.00 bc | 4.07 ± 0.57 bcd | 27.07 ± 2.72 abc | 26.36 ± 0.42 bc | 341.9 ± 16.3 abc |
| F 24 | 0.199 ± 0.007 a | 1.55 ± 0.28 b | 0.55 ± 0.05 b | 0.12 ± 0.00 bc | 5.12 ± 0.13 abcd | 32.65 ± 0.75 abc | 25.32 ± 0.31 cd | 386.9 ± 6.92 a |
• Micronutrients in quinoa plus orange mango juice (before pasteurization; before fermentation; after 24 h fermentation).
• Growth curves and pH (from 0 to 24 h; F: 0; 2; 4; 6; 8; 14; 16; 18; 20; 22; 24 h).
2.2. Kefir grains preparation
Traditional kefir was propagated in one household for three years. For sub-culturing, grains (10% w/v) were inoculated into UHT-sterilized cow milk (1.5% fat, La Serenísima®, Argentina) followed by incubation at 25 °C for 24 h. Kefir preparation was propagated twice and 2 L of the preparation were lyophilized and conserved at −20 °C to be used as starters. Cell viability was determined regularly by platting in adequate media.2.3. Protein content
Protein content was determined using the Kjeldahl method. For digestion, 1 g of the sample, 7 g of potassium sulphate, 0.7 g of copper sulphate, and 12 mL of concentrated sulphuric acid were weighed. A reaction blank (without sample) and a nitrogen standard (weighing 0.2 g of acetanilide plus the aforementioned reagents) were prepared in parallel. The mixture was heated in a combustion furnace at 420 °C for 1 h (until a clear emerald green solution was obtained). After digestion, the samples, blank, and the standard were cooled to room temperature and distilled by steam distillation, collecting the distillate in a 4% boric acid solution. The resulting solution was titrated with 0.1 N hydrochloric acid (previously standardized), using a mixed indicator of methyl red and bromocresol green until obtaining a pink solution. The percentage of nitrogen in the sample was calculated using the following formula, employing a factor of 5.7 (for cereals) to convert to total protein percentage. This percentage was corrected based on the value obtained for the standard.| % N = (VHCl − Vo) × NHCl × PeqN × 100)/m (g) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 007 g per eq.). VHCl: volume of hydrochloric acid used in the titulation. Vo: initial volume of the sample before the addition of acid.
007 g per eq.). VHCl: volume of hydrochloric acid used in the titulation. Vo: initial volume of the sample before the addition of acid.
2.4. Determination of lipid percentage
The Soxhlet method was used. A 3 g sample was weighed and placed in pre-weighed metal cups with 50 mL of analytical-grade hexane. The extraction process was carried out in a SOXTEC SYSTEM HT 1043 extraction unit for approximately 4 h. Subsequently, the metal cups were heated to 100 °C for 4 h, and the remaining fat was weighed. The lipid percentage was calculated gravimetrically.2.5. Determination of lactic acid content
L-Lactic acid was determined at different stages of the fermentation process (Megazyme ®, Argentina) using UV detection at 340 nm. Results are expressed mg L−1.2.6. Tocopherols, lutein β-carotene and phytosterols determination
1 g of quinoa flour was homogenized with 4 mL of 0.05 M pH 7.7 phosphate buffer using an Ultra-Turrax for 1 min. A 1 g aliquot of the homogenate was then mixed with 2 mL of 1% pyrogallol in ethanol and vortexed for 30 s. To saponify the mixture, 0.3 mL of 12 N KOH was added, and the sample was incubated at 70 °C for 30 min (1 h for phytosterols) with vortexing every 10 min. After cooling on ice, 1 mL of distilled water was added, and the lipids were extracted twice with 5 mL of HPLC-grade hexane. The combined organic phases were evaporated to dryness under a nitrogen stream. The residue was reconstituted in 0.5 mL of methanol and analyzed by HPLC using a mobile phase of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 ethanol
60 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol at a flow rate of 1 mL min−1. Alpha, beta, delta, and gamma tocopherols (vitamin E) were quantified using a fluorescence detector (excitation 296 nm, emission 330 nm), while beta-sitosterol and stigmasterol were detected at 210 nm and carotenes at 445 nm using a diode array detector. The methodology was adapted from the determination previously used for pecans.18
methanol at a flow rate of 1 mL min−1. Alpha, beta, delta, and gamma tocopherols (vitamin E) were quantified using a fluorescence detector (excitation 296 nm, emission 330 nm), while beta-sitosterol and stigmasterol were detected at 210 nm and carotenes at 445 nm using a diode array detector. The methodology was adapted from the determination previously used for pecans.18
2.7. Total phenolic content
The total phenolic content was determined using the Folin–Ciocalteu method and adapted from Lingua et al., 2022.19 2 g of quinoa flour were extracted with a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 methanol–water solution for 3 h. A portion of the extract was mixed with the Folin–Ciocalteu reagent, allowed to react for 5 min, and then sodium carbonate was added. After a 2 h incubation at 23 °C, the absorbance was measured at 750 nm using a UV-vis spectrophotometer. A standard curve was constructed using gallic acid at concentrations ranging from 0 to 100 mg L−1 to quantify the total phenolic content.
30 methanol–water solution for 3 h. A portion of the extract was mixed with the Folin–Ciocalteu reagent, allowed to react for 5 min, and then sodium carbonate was added. After a 2 h incubation at 23 °C, the absorbance was measured at 750 nm using a UV-vis spectrophotometer. A standard curve was constructed using gallic acid at concentrations ranging from 0 to 100 mg L−1 to quantify the total phenolic content.
2.8. Total antioxidant determination
The FRAP (Ferric Reducing Antioxidant Potential) assay was employed to determine the antioxidant capacity. 1 g of quinoa beverage was mixed with 5 mL of 0.05 M phosphate buffer (pH 7.7) and vortexed for 2 min. After centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 30 min, the supernatant was collected. The FRAP reagent was prepared by combining a 300 mM acetate buffer, a 10 mM TPTZ (2,4,6-tri(2-pyridyl)-s-triazine, Sigma-Aldrich, Argentina) solution in 40 mM HCl, and a 20 mM FeCl3·6H2O solution in a ratio of 10
000×g for 30 min, the supernatant was collected. The FRAP reagent was prepared by combining a 300 mM acetate buffer, a 10 mM TPTZ (2,4,6-tri(2-pyridyl)-s-triazine, Sigma-Aldrich, Argentina) solution in 40 mM HCl, and a 20 mM FeCl3·6H2O solution in a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Eighty-three microliters of the supernatant was mixed with 2.5 mL of the FRAP reagent and incubated at 37 °C for 20 min. Following a 10 minute cooling period on ice, the absorbance was measured at 593 nm using a UV-vis spectrophotometer. A standard curve was generated using Trolox at concentrations ranging from 0 to 1000 μmol L−1. The assay was adapted from Lingua et al., 2002, previously applied to maize samples.19
1. Eighty-three microliters of the supernatant was mixed with 2.5 mL of the FRAP reagent and incubated at 37 °C for 20 min. Following a 10 minute cooling period on ice, the absorbance was measured at 593 nm using a UV-vis spectrophotometer. A standard curve was generated using Trolox at concentrations ranging from 0 to 1000 μmol L−1. The assay was adapted from Lingua et al., 2002, previously applied to maize samples.19
2.9. Microbial growth
UFC per mL were determined in Man-Rogosa-Sharp (MRS) agar and PCA agar in order to follow the microbial growth. Food samples were diluted in peptone–water, and plated on the respective media.For microbial growth the Baranyi and Roberts model was applied
| dy/dt = μmax × q(t) × y(t) |
q(t) is defined as:
| q(t) = 1 − exp(−ν × t)/[1 + exp(−ν × t) × (exp(y0 − ymax) − 1)] |
The model was fitted using the USDA software https://combasebrowser.errc.ars.usda.gov/DMFit.aspx used to fit the better curves and describe the microbial growth parameters.
2.10. Statistical analysis
A one-way ANOVA was used contrasting treatments for the different parameters. Means were contrasted using the Tukey test at 0.05. The software used was XLSTAT 2023.3 Results & discussion
3.1 Effect of processing steps on macronutrients present in quinoa beverages
The initial phase of the investigation involved determining whether pasteurization and fermentation affected the macronutrients in the product. It was determined by using an untreated slurry (Q0), pasteurized slurry (Q1, heated during 10 min at 90 °C), and fermented slurry using milk kefir for 24 h at 37 °C (F24).As presented in Table 1, the protein content exhibited a moderate increase following fermentation with kefir, while the lipid content remained unaffected. The protein content of the beverage was twofold higher than that observed in a soy whey fermented with kefir20 and quinoa beverage fermented with kefir21 and exceeded that of the majority (12 of 17) of commercial plant-based milk substitutes previously analysed in the United States.22 The fat content was comparable to other plant-based beverages, such as a chickpea-coconut beverage23 and commercial oat milk,22 and lower than most commercial milk products in the United States.22 This discrepancy is likely attributable to the addition of vegetable fats as sensory enhancers in commercial preparations 24.
The lactic acid content was approximately 1 g per kg product. This result is comparable to the lactic acid content in soy whey fermented with water kefir for 48 h, achieving a pH of approximately 3.5.20 In the present study, a pH of 3.7 was attained after 24 h of fermentation (Fig. 3). This finding is consistent with the high concentration of LAB exceeding 109 CFU per g present in the kefir utilized (Table 3).
| Initial value a (log10 CFU) | Lag/shoulderb (h) | Maximum rate μc (h−1) | N max (log10 CFU) | G (h) | R-squaref | SE of fitf | |
|---|---|---|---|---|---|---|---|
| a Initial value: refers to the microbial population. b Lag/shoulder quantifies the duration of the lag phase. c μ: the maximum specific growth rate during the exponential growth phase. d N max: maximum population density that the environment can support. e G: generation time is the time it takes for a microbial population to double in size during the exponential growth phase. It can be calculated from the maximum specific growth rate (μ) using the formula: G = ln(2)/μ. f R-square and SE: represent the proportion of the variance in the dependent variable (microbial population) that is predictable from the independent variable (time) and its standard error. | |||||||
| Q | 4.901 ± 0.057 | 4.226 ± 0.378 | 0.393 ± 0.021 | 9.509 ± 0.047 | 1.763 | 0.996 | 0.124 |
| QMO | 5.082 ± 0.083 | 5.637 ± 0.528 | 0.640 ± 0.152 | 8.294 ± 0.065 | 1.081 | 0.978 | 0.226 |
| QMONaOH | 4.844 ± 0.148 | 0.401 ± 1.028 | 0.290 ± 0.023 | 9.901 ± 0.151 | 2.390 | 0.978 | 0.304 |
3.2 Impact of processing steps on the micronutrients and antioxidants in quinoa beverages
The quinoa beverage is a starch-rich slurry resulting from wet grinding and sieving and with the addition of lyophilized fruits. Therefore, it is important to address the effect of processing steps on the quality of the micronutrients during the semi-solid fermentation (SSF) processing of the product. Following the inoculation, samples were collected at various intervals from t = 0 h up to 24 h (samples designated F0 to 24) at 37 °C, where LAB achieved the maximum growth, to assess how fermentation affected the quality of micronutrients compared to the unfermented drink. The impact of fermentation on the levels of tocopherol isomers in the processed product is presented in Table 2. The data indicate that the highest concentration of tocopherol in the quinoa product was in the gamma isomer, followed by alpha, with beta-tocopherol being present in very low amounts. Fermentation resulted in a significant decrease (p < 0.05) in the content of alpha and gamma tocopherol isomers over time. The alpha isomer was particularly susceptible to the pasteurization step, indicating that the heat treatment had a detrimental effect on this molecule. Although the gamma isomer was not affected by pasteurization, its levels did decrease during fermentation.The reduction in tocopherols during fermentation can be attributed to the incubation temperature and the presence of dissolved oxygen caused by stirring in the fermentation medium. Previous studies have reported similar declines in tocopherol levels during fermentation.25 The effect of processing on lutein mirrored that observed for gamma-tocopherol; while pasteurization did not change lutein concentration, levels decreased during fermentation.
The concentration of phytosterols was comparable to those found in maize fermented with Lactobacillus casei and increased during fermentation, likely due to the release of free sterols.11 Additionally, the total phenols and antioxidants in the SSF medium increased during fermentation. Previous findings show the transformation of bound phenolic compounds transform by the metabolic activity of fermenting microorganisms from their linked or conjugated forms into free forms. These released phenolic compounds may then enhance antioxidant activity.26
To be labeled as containing vitamins, a food product must provide at least 0.2 mg of alpha-tocopherol per serving, a minimum of 0.1 mg of lutein per serving, and 0.65 g of phytosterols per serving.27 In our case, the obtained product is far to accomplish these requirements.28 This argument supports the idea of supplementing SSF with natural extracts to enhance micronutrient levels. Adding fruit extracts to quinoa beverages can positively influence the growth of LAB29 as they usually contain sugars, vitamins, and phytochemicals that act as additional nutrients or substrates. Orange and mango juices provide monosaccharides as fructose, complex sugars as pectic oligosaccharides (POS) and polyphenols that can be fermented by LAB as Lactobacillus sp. and Bifidobactirum sp.30,31 This can result in increased growth rates and overall biomass of the bacteria. However, the inclusion of fruit extracts in SSF media can alter the pH, and hence the nutrient availability, and overall composition of the fermentation medium, which subsequently affects the growth kinetics of LAB. It is important to note that acidic extracts can lower the pH, which may negatively affect the growth of some LAB, particularly since the initial pH in SSF media is a crucial factor.32
3.3 Growth parameters of kefir lactic acid bacteria in the quinoa preparations
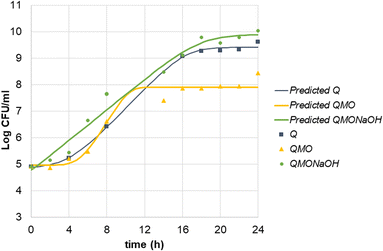
Milk kefir was activated in peptone broth for 2 h at 37 °C prior to inoculating the beverages in order to dilute and minimize the presence of lactose as a substrate. As illustrated in Fig. 2, the kefir cultivated in the quinoa SSF (Q) achieved a microbial count of up to 9.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log in MRS agar. Coinciding with this growth, the pH decreased from 6.2 to 3.67 throughout the fermentation period (Fig. 3).
log in MRS agar. Coinciding with this growth, the pH decreased from 6.2 to 3.67 throughout the fermentation period (Fig. 3).
The addition of juice typically results in a lower initial pH. In the QMO treatment, the initial pH was reported to drop to approximately 3.75 (Fig. 3). Lower pH levels can extend the lag phase of LAB growth, as some strains may require more time to adapt to the acidic environment. As indicated in Table 3, the lag phase for the QMO treatment was longer and the final LAB count lower compared to the control sample, suggesting that the acidic conditions created by the juice adversely affected the initiation of bacterial growth.
To enable a fair comparison of microbial growth under similar acidic conditions, the pH of the mango–orange beverage was adjusted using NaOH (QMONaOH). This adjustment aimed to evaluate growth parameters and assess the impact of incorporating a carotenoid-rich source into the beverage without considering the effect of pH.
By adjusting the pH of the quinoa beverage with juice, similar growth curves and pH curves were obtained for Q and QMONaOH treatments (Fig. 2 and 3).
Microbial growth parameters are detailed in Table 3. All three samples exhibited high R-squared values ranging from 0.978 to 0.996, indicating a strong fit of the growth model to the data. The three substrates had similar initial counts of LAB, ranging from 4.8 to 5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log. However, differences were found in the length of the lag phase. The QMO sample displayed a significantly longer lag phase of 5.637 h compared to the Q sample, which had a lag phase of 4.226 h, and particularly in comparison to the pH-adjusted sample, which only had a lag phase of 0.401 h. This longer lag phase suggests a delay in growth initiation, likely due to the addition of juice and the consequent initial low pH of 3.75, extending the lag phase by a factor of ten. Nevertheless, LAB showed an efficient use of QMO substrate. Despite the longer lag phase, the growth ratio (μmax) was higher than in the other substrates Q and QMONaOH (0.640 h−1vs. 0.393 h−1 and 0.290 h−1) reducing the generation time (G). However, this occurred at expenses of a lower CFU counts at the end of the growth period. This can be a product of the appearance of inhibitory molecules accumulation or a rapid depletion of essential nutrients.
log. However, differences were found in the length of the lag phase. The QMO sample displayed a significantly longer lag phase of 5.637 h compared to the Q sample, which had a lag phase of 4.226 h, and particularly in comparison to the pH-adjusted sample, which only had a lag phase of 0.401 h. This longer lag phase suggests a delay in growth initiation, likely due to the addition of juice and the consequent initial low pH of 3.75, extending the lag phase by a factor of ten. Nevertheless, LAB showed an efficient use of QMO substrate. Despite the longer lag phase, the growth ratio (μmax) was higher than in the other substrates Q and QMONaOH (0.640 h−1vs. 0.393 h−1 and 0.290 h−1) reducing the generation time (G). However, this occurred at expenses of a lower CFU counts at the end of the growth period. This can be a product of the appearance of inhibitory molecules accumulation or a rapid depletion of essential nutrients.
The maximum cell density corresponded to QMONaOH treatment with a value of 9.901![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log. Q Control reached a high cell density of 9.509
log. Q Control reached a high cell density of 9.509![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log. The QMO sample, although lower, still reached a significant maximum cell density of 8.294
log. The QMO sample, although lower, still reached a significant maximum cell density of 8.294![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log. This result is in line with previous findings that demonstrate that LAB can grow in low pH conditions using grape juice33 or with the addition of mango residues.29
log. This result is in line with previous findings that demonstrate that LAB can grow in low pH conditions using grape juice33 or with the addition of mango residues.29
Present results indicate that milk kefir can be effectively adapted for growth in quinoa-based SSF, and that LAB can thrive in acidic environments. However, optimal growth is achieved when the pH is favourable. Considering the trade-off, the final cell count is critical for a potential probiotic effect. For industrial use, a lower μmax and a higher final CFU count might be more efficient than a rapid metabolic activity. The use of a pH regulator34 should be further assayed in order to maximize the growth performance and the maximal CFU counts in complex substrates.
3.4 Presence of carotenoids, tocopherols, and phytosterols in uncooked, pasteurized, and fermented products
According to Burlinghame, 2010 (FAO) “Sustainable Diets are those diets with low environmental impacts that contribute to food and nutrition security and to healthy life for present and future generations. Sustainable diets are protective and respectful of biodiversity and ecosystems, culturally acceptable, accessible, economically fair and affordable; nutritionally adequate, safe and healthy; while optimizing natural and human resources”.35In the present work, we agree with this statement. Using quinoa that is a plant presenting a great biodiversity, which grow in very aggressive environments and require less water than other cereals. At the same time, their seeds are rich in high quality protein and bioactive compounds, mainly polyphenols.36
The addition of mango and orange fruit extracts from the subtropical regions of Yuto and Concordia respectively in Argentina can be a solution for the use of perishable fruits that enhanced the levels of carotenoids, tocopherols, and phytosterols in the products.
Generally, adjusting the pH contributed to higher concentrations of these phyto-micronutrients (see Table 4). Sodium hydroxide (NaOH), a strong base, promotes saponification and acid hydrolysis, which facilitate the release of sterols. These methods are effective for profiling total sterols; however, there is a lack of information on the structure of phytosterol conjugates.37
| Treatment | Stigmasterol (mg kg−1) | β-Sitosterol (mg kg−1) | Lutein (mg k−1) | β-Carotene (mg kg−1) | β + δ-Tocopherol (mg kg−1) | γ-Tocopherol (mg kg−1) | α-Tocopherol (mg kg−1) |
|---|---|---|---|---|---|---|---|
| a Nd: not detected. Different letters within the same column indicates differences at the 0.05 level determined by Tukey test. | |||||||
| Q-0 | 2.89 ± 0.98d | 17.25 ± 6.34 | 0.13 ± 0.03 ef | nd | 0.10 ± 0.02 e | 3.63 ± 0.901 def | 2.27 ± 0.57 cd |
| Q-1 | 5.61 ± 0.53 cd | 29.42 ± 2.87 d | 0.12 ± 0.02 f | nd | 0.12 ± 0.02 e | 2.36 ± 1.25 f | 0.57 ± 0.37 d |
| Q-24 | 7.00 ± 1.05 c | 45.18 ± 8.79 d | 0.14 ± 0.02 ef | nd | 0.17 ± 0.03 de | 3.57 ± 0.55 ef | 0.71 ± 0.08 d |
| QMO-0 | 5.65 ± 0.83 cd | 192.19 ± 14.67 a | 0.50 ± 0.05 ab | 0.83 ± 0.20 b | 0.65 ± 0.01 a | 7.82 ± 0.58 abc | 18.51 ± 0.94 a |
| QMO-1 | 5.39 ± 1.01 cd | 181.74 ± 6.83 ab | 0.41 ± 0.04 bc | 0.73 ± 0.07 b | 0.59 ± 0.13 ab | 7.85 ± 0.10 abc | 19.10 ± 0.21 a |
| QMO-24 | 5.44 ± 0.76 cd | 170.49 ± 9.52 ab | 0.38 ± 0.03 bcd | 0.66 ± 0.06 b | 0.56 ± 0.05 ab | 7.75 ± 0.26 ab | 18.70 ± 0.79 a |
| QMONaOH-0 | 7.83 ± 2.15 bc | 120.69 ± 13.18 c | 0.43 ± 0.09 bc | 0.67 ± 0.07 b | 0.31 ± 0.06 cd | 5.28 ± 0.83 cde | 10.13 ± 2.25 b |
| QMONaOH-1 | 11.27 ± 0.72 ab | 145.74 ± 2.43 bc | 0.48 ± 0.02 ab | 0.91 ± 0.34 ab | 0.30 ± 0.03 cd | 6.03 ± 0.10 bcd | 13.23 ± 0.03 b |
| QMONaOH-24 | 13.69 ± 1.48 a | 189.89 ± 24.29 a | 0.61 ± 0.07 a | 1.22 ± 0.167 a | 0.29 ± 0.05 cd | 7.56 ± 0.93 abc | 16.85 ± 0.16 a |
| QNaOH-0 | 6.47 ± 0.71 cd | 40.198 ± 0.76 d | 0.28 ± 0.02 cd | nd | 0.38 ± 0.02 c | 7.99 ± 0.35 ab | 4.67 ± 0.11 c |
| QNaOH-1 | 7.38 ± 0.89 bc | 55.91 ± 2.78 d | 0.29 ± 0.01 cd | nd | 0.41 ± 0.02 bc | 8.84 ± 0.04 a | 3.27 ± 0.02 cd |
| QNaOH-24 | 8.38 ± 2.78 bc | 49.76 ± 1.41 d | 0.25 ± 0.01 de | nd | 0.37 ± 0.09 c | 7.10 ± 0.26 abc | 1.83 ± 0.16 cd |
Carotene and other provitamin A carotenoids serve as sources of vitamin A, helping to prevent vitamin A deficiency. However, some specific functions beyond this role have not been fully identified. No Dietary Reference Intakes (DRIs) have been established for any carotenoids, including those without provitamin A activity.†
The current product, boosted with mango–orange concentrate, can be regarded as a source of vitamin E; phytosterols, beta-carotene, and lutein (see Table 5). On average, it is recommended to consume two servings to obtain the necessary micronutrients. Additionally, the product demonstrates potential probiotic activity, containing more than 109 CFU mL−1 per serving.
| RDIa | AIb | Source ofc | g of product to be considered source of (15% RDI) | |
|---|---|---|---|---|
| a RDI: recommended daily intake. b AI: adequate intake. c Average quantity to be considered source of a nutrient. d The RDI for protein for adults is 0.8 g of protein per kg of body weight. This is the minimum amount needed to prevent deficiency. e Probiotics are now defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (expert panel FAO/WHO). | ||||
| Vitamin E (α-tocopherol) | 15 mg | 6 mg | 2.25 g | 125 g QMO |
| Phytosterols | 0.5 g (2–3 g for hipercholesterolemic patients) | 0.2–0.3 g | 0.075 g | 375 g QMO |
| β-Carotene | 1.8 mg | 0.27 mg | 245 g QMO | |
| Lutein | 0.4 mg | 0.06 mg | 109 g QMO | |
| Protein | 50–80 gd | 7.5 g | 500 g Q; QMO and QMONaOH | |
| Probiotice | nd | 108–109 CFU g−1 product | nd | 100 g Q; QMO and QMONaOH |
The recommended daily intake (RDI) is the level that is sufficient to meet the nutrient requirements of nearly all (97–98%) healthy individuals in a particular life stage and gender group and aim to prevent nutrient deficiencies. On the other hand, the average intake (AI) is the recommended nutrient intake based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people. In this concern, quinoa boasts a well-balanced amino acid profile, with relatively high levels of lysine, an amino acid often lacking in plant-based proteins.38
Concerning probiotics, a source of living microorganisms containing 108 to 109 CFU mL−1 of food is recommended.39
The designed product has not been tested for health benefits, so its probiotic action cannot be labeled. However, scientific studies support the health benefits of milk kefir, which is recognized as a safe and affordable probiotic drink that is easy to make at home. Regular consumption of kefir may improve digestion and lactose tolerance, provide antibacterial effects, reduce cholesterol, control plasma glucose, and offer anti-hypertensive, anti-inflammatory, antioxidant, anti-carcinogenic, and anti-allergic benefits. Most of these studies were conducted in vitro or in vivo models40
4 Conclusions
The study demonstrates the potential of quinoa-based products to be sources of vitamin E, phytosterols, beta-carotene, and lutein, also with potential probiotic activity, recommending two servings to obtain necessary micronutrients.Further research could explore different quinoa varieties, fermentation conditions, and starter cultures, as well as investigating nutrient bioavailability and the beverage's effects on gut microbiota.
Optimizing the production process for industrial-scale production, including cost-effective and scalable methods for fermentation and sustainable downstream processing, is crucial.
In addition, future research should explore the sensory attributes of the beverage and assess consumer preferences or if it could be used as a base for a variety of other food products, such as yogurt, smoothies, and baked goods. Other aspect is optimizing the production process for industrial-scale production, including the development of cost-effective and scalable methods for fermentation and downstream sustainable processing.
Data availability
Data available at: https://osf.io/pwjqv/?view_only=dd724260a5964434978fb64720bd3a0f.Author contributions
Dr Descalzo is responsible for conceptualization, data curation, formal analysis, supervision, validation and writing the original draft, PhD student Bordoni is responsible for formal analysis, data curation and writing as part of the doctoral thesis, Dr Rossetti is responsible for project administration, resources and formal analysis of vitamins, Dr Rizzo is responsible for the formal analysis of biochemical parameters and data curation, Dr Dhuique-Mayer is responsible for validation as expert in nutrition and carotenoids, Dr Bárcena is responsible for formal analysis, macronutrients, phenological determinations and collection of quinoas for the assay.Conflicts of interest
The authors state that there are no conflicts to declare.Acknowledgements
The authors want to thank Dr Adriana Pazos for her contribution in protein measurement and Dr Sebastian Cunzolo for aiding with lipid fractions. This work is part of the PICT-2018- 04254 “Tecnologías Combinadas de Fermentación Sólida y Deshidratación Para la Mejora de la Bioaccesibilidad de Carotenoides y Tocoferoles Naturales en Alimentos y PAI (Productos Alimentarios Intermedios) obtenidos a base de maíz y frutas.“. The fellowship of the University of Montpellier, MUSE EXPLORE#5 and the 2024 COHORT program hosted by the Montpellier Advanced Knowledge Institute on Transitions (MAK'IT) that supported Adriana Descalzo as visiting scientist at the UMR-Qualisud, CIRAD. Also, the resources that corresponded to the INTA Project 2023-PD-L04-I121 “Functional Foods”. The authors acknowledge the use of Grammarly® and Gemini® for proofreading and enhancing the grammatical accuracy of this manuscript.Notes and references
- R. Kumar, S. Bhardwaj, M. Sikarwar, A. Kumar, B. R. Singh, M. Gupta and R. Shukla, J. Food Compos. Anal., 2025, 139, 107133 CrossRef CAS.
- S. Pathan and R. A. Siddiqui, Nutrients, 2022, 14, 558 CrossRef CAS PubMed.
- D. Cizeikiene, G. Juodeikiene, E. Bartkiene, J. Damasius and A. Paskevicius, Int. J. Food Sci. Nutr., 2015, 66, 736–742 CrossRef CAS PubMed.
- M. Manzoor, D. Singh, G. K. Aseri, J. S. Sohal, S. Vij and D. Sharma, J. Appl. Biol. Biotechnol., 2021, 9, 7–16 CrossRef CAS.
- S. R. Couto and M. Á. Sanromán, J. Food Eng., 2006, 76, 291–302 CrossRef CAS.
- J. Misihairabgwi and A. Cheikhyoussef, J. Ethn. Foods., 2017, 4, 145–153 CrossRef.
- L. Ewuoso, O. Animashaun and A. Adejumo, Am. J. Microbiol. Res., 2020, 8, 63–72 Search PubMed.
- S. S. Mishra, R. C. Ray, S. K. Panda and D. Montet, in Fermented Foods, Part II, ed. Ray and Montet, CRC Press, Florida, USA, 1st edn, 2017, ch. 2, pp. 21–45 Search PubMed.
- A. K. Carole Sanya, Y. E. Madode, S. E. Schoustra, E. J. Smid and A. R. Linnemann, Food Res. Int., 2023, 170, 113038 CrossRef CAS PubMed.
- D. D. Rosa, M. M. S. Dias, Ł. M. Grześkowiak, S. A. Reis, L. L. Conceição and M. do C. G. Peluzio, Nutr. Res. Rev., 2017, 30, 82–96 CrossRef CAS PubMed.
- A. M. Descalzo, S. A. Rizzo, A. Servent, L. Rossetti, M. Lebrun, C. D. Pérez, R. Boulanger, C. Mestres, D. Pallet and C. Dhuique-Mayer, J. Food Sci. Technol., 2018, 55, 1859–1869 CrossRef CAS PubMed.
- C. Pénicaud, N. Achir, C. Dhuique-Mayer, M. Dornier and P. Bohuon, Fruits, 2011, 66, 417–440 CrossRef.
- C. Morand, C. Dubray, D. Milenkovic, D. Lioger, J. F. Martin, A. Scalbert and A. Mazur, Am. J. Clin. Nutr., 2011, 93, 73–80 CrossRef CAS PubMed.
- C. Dhuique-Mayer and A. Servent, J. Food Sci., 2025, 90, e17576 CrossRef CAS PubMed.
- N. Luana, C. Rossana, J. A. Curiel, P. Kaisa, G. Marco and C. G. Rizzello, Int. J. Food Microbiol., 2014, 185, 17–26 CrossRef CAS PubMed.
- P. Russo, M. L. V. de Chiara, V. Capozzi, M. P. Arena, M. L. Amodio, A. Rascón, M. T. Dueñas, P. López and G. Spano, LWT–Food Sci. Technol., 2016, 68, 288–294 CrossRef CAS.
- M. Gies, A. M. Descalzo, A. Servent and C. Dhuique-Mayer, LWT, 2019, 111, 105–110 CrossRef CAS.
- A. M. Descalzo, A. Biolatto, S. A. Rizzo, C. D. Pérez, E. A. Frusso, F. Carduza and L. Rossetti, Postharvest Biol. Technol., 2021, 179, 111591 CrossRef CAS.
- M. S. Lingua, M. Gies, A. M. Descalzo, A. Servent, R. B. Páez, M. V. Baroni, J. E. Blajman and C. Dhuique-Mayer, Food Chem., 2022, 370, 130993 CrossRef CAS PubMed.
- C. Tu, F. Azi, J. Huang, X. Xu, G. Xing and M. Dong, LWT, 2019, 113, 108258 CrossRef CAS.
- A. Pugliese, M. Ulzurrun, F. C. Leskow, G. D. Antoni and E. Kakisu, J. Food Nutr. Res., 2023, 62, 363–373 CAS.
- S. Jeske, E. Zannini and E. K. Arendt, Plant Foods Hum. Nutr., 2017, 72, 26–33 CrossRef CAS PubMed.
- L. Rincon, R. Braz Assunção Botelho and E. R. de Alencar, LWT, 2020, 128, 109479 CrossRef CAS.
- U. Fadillah, A. Dirpan and A. Syarifuddin, Future Foods, 2024, 10, 100490 CrossRef CAS.
- D. Jafarpour and S. M. B. Hashemi, Fermentation, 2023, 9, 80 CrossRef CAS.
- F. Melini and V. Melini, Fermentation, 2021, 7, 20 CrossRef CAS.
- S. S. AbuMweis, R. Barake and P. J. H. Jones, Food Nutr. Res., 2008, 52 DOI:10.3402/fnr.v52i0.1811.
- Institute of Medicine, Panel on Dietary Antioxidants and Related Compounds, in Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids, The National Academies Press, Washington DC, 2000 Search PubMed.
- M. W. Kuria, J. W. Matofari and J. M. Nduko, J. Agric. Food Res., 2021, 6, 100206 CAS.
- A. O. Ademosun, O. F. Ajeigbe, M. T. Ademosun, O. O. Ogunruku and G. Oboh, Hum. Nutr. Metab., 2025, 39, 200295 CrossRef CAS.
- M. Wongkaew, P. Tangjaidee, N. Leksawasdi, K. Jantanasakulwong, P. Rachtanapun, P. Seesuriyachan, Y. Phimolsiripol, T. Chaiyaso, W. Ruksiriwanich, P. Jantrawut and S. R. Sommano, Front. Nutr., 2022, 9 DOI:10.3389/fnut.2022.798543.
- K. Śliżewska and A. Chlebicz-Wójcik, Biology, 2020, 9, 423 CrossRef PubMed.
- S. Echresh, B. Alizadeh Behbahani, F. Falah, M. Noshad and S. A. Ibrahim, Appl. Food Res., 2025, 5, 100748 CrossRef CAS.
- E. Abedi and S. M. B. Hashemi, Heliyon, 2020, 6, e04974 CrossRef CAS PubMed.
- B. Burlingame and S. Dernini, Sustainable diets and biodiversity: Directions and solutions for policy, research and action, In Proceedings of the International Scientific Symposium Biodiversity and sustainable diets united against hunger, FAO Headquarters, Rome 3–5, 2010 Search PubMed.
- S. Schlag, S. Götz, F. Rüttler, S. M. Schmöckel and W. Vetter, J. Agric. Food Chem., 2022, 70, 9856–9864 CrossRef CAS PubMed.
- D. D. Evtyugin, D. V. Evtuguin, S. Casal and M. R. Domingues, Molecules, 2023, 28, 6526 CrossRef CAS PubMed.
- V. Angeli, P. Miguel Silva, D. Crispim Massuela, M. W. Khan, A. Hamar, F. Khajehei, S. Graeff-Hönninger and C. Piatti, Foods, 2020, 9, 216 CrossRef CAS PubMed.
- G. Vinderola, J. Reinheimer and S. Salminen, Int. Dairy J., 2019, 96, 58–65 CrossRef CAS.
- J. M. R. Tingirikari, A. Sharma and H.-J. Lee, J. Ethn. Foods., 2024, 11, 35 CrossRef.
Footnote |
| † https://www.ncbi.nlm.nih.gov/books/NBK225478/#ddd0000022. |
| This journal is © The Royal Society of Chemistry 2025 |