Extraction of pectin from Assam lemon (Citrus limon) peel and its use in preparation of low-fat mayonnaise
Received
11th November 2024
, Accepted 25th April 2025
First published on 25th April 2025
Abstract
This study mainly focused on the formulation of low-fat mayonnaise using lemon peel-based pectin gel as a fat replacer. Assam lemon peel powder was prepared and was used as a sustainable source for the development of pectin using the ultrasound-assisted extraction method. The physicochemical properties of lemon peel powder were studied on the basis of moisture content, bulk density, tapped density, and pH. The yield of pectin was found to be 12.84 ± 0.14%. Furthermore, the extracted pectin was characterized by XRD spectroscopy and FTIR spectroscopy. In XRD spectroscopy, the diffraction peak at 2θ = 18.9° indicates the characteristic peak of pectin. Furthermore, the extracted pectin was utilized to prepare pectin gel with soy protein and sodium tripolyphosphate and was stabilized using sodium alginate. The prepared pectin gel was incorporated for formulating low-fat mayonnaise formulations such as low-veg-fat mayonnaise (LVM) and low-egg-fat mayonnaise (LEM) to improve the texture, stability, and nutritional profile. Furthermore, these two formulations were compared with the commercial mayonnaise. Among all, low-veg-fat mayonnaise (LVM) exhibited the lowest fat content of 44.89%. However, LVM and LEM had a viscosity of 284 Pa s−1 and 316 Pa s−1, respectively. In terms of firmness and stickiness, variations were observed among the mayonnaise samples, with LVM showing the lowest firmness of 0.86 ± 0.07 N and stickiness of 0.54 ± 0.05 N. Thus, this research contributes to the exploration of sustainable alternatives in food ingredient sourcing and product development, with implications for both the food industry and consumer health.
Sustainability spotlight
Assam lemon is well known for its excellent flavour, aroma, health benefits, and visual appearance. From the lemon-based beverage industry, a huge amount of lemon peels has been generated as a waste material. These peels are valued for various components including essential oils, pectin, flavonoids, etc. The pectin obtained from lemon peel can be used as a thickening, gelling, emulsifying and fat replacing agent for the formulation of various food products. This study has mainly focused on the formulation of low-fat mayonnaise using lemon peel-based pectin gel as a fat replacer. Here, lemon peel powder has been used as a sustainable source for the development of pectin using the ultrasound-assisted extraction method. This method is considered one of the most efficient and environmentally friendly methods as compared to other chemical extraction methods. Thus, by minimizing the chemical waste generation, this approach of extracting pectin from orange peel to develop low fat mayonnaise promotes formulation of more sustainable and healthier products.
|
Introduction
Lemon (Citrus lemon), a significant fruit crop of the Northeast, belongs to the Rutaceae family. It stands as the 3rd most important citrus species, trailing only behind oranges and mandarins in terms of production.1 Among the different varieties of lemon, Assam lemon is one of the important varieties of lemon. The absence of seeds is the key characteristic of Assam lemon, which differentiates it from other lemon varieties. However, the availability of seeds in Assam lemon may vary by district. Assam lemon is famous for its excellent flavour, aroma, and visual appearance due to which it has got the recognition in both the local and global markets. Moreover, lemon contains several important components such as pectin, citric acid, and flavonoids, which provide excellent antioxidant, and medicinal effects.2 Due to its various beneficial properties, lemon is widely used in the food, beverage, pharmaceutical and cosmetic industries. When it comes to the beverage industry, a huge amount of lemon peels has been generated as a waste material, which is rich in high valued components including essential oil, pectin, and flavonoids.3 This peel mainly comprises two layers such as flavedo (outer layer) and albedo (inner layer). The flavedo layer is the external pigmented layer having a green to yellow hue and imparts fragrance to a product.4 The albedo layer is the inner white layer, which is mainly utilized for the extraction of pectin.5 On a dry basis, lemon peel contains approximately 19–23% of pectin, making it a valuable byproduct for industries that require pectin for food processing or other industrial applications.6
Pectin is a type of polysaccharide found in the cell wall of fruits, mostly in citrus peels, apples, and berries.7,8 Pectin consists of D-galacturonic acid residues that are linked through α-(1,4) glycosidic linkage. It is known for its excellent properties, making it a valuable component in a variety of sectors. One of the major qualities of pectin is its gel forming ability due to the presence of pectinic acids.9 Therefore, it is considered an excellent gelling and thickening agent for various food products. Pectin has the ability to improve the stability and smoothness of jam, jelly, and fruit preserves.10 It also enhances the stability of the pulp that has been used in the beverage industry.11 Apart from this, pectin is considered an excellent emulsifier. In the preparation of mayonnaise, especially for low fat mayonnaise, pectin is used as an emulsifying agent.12 It is used as a fat replacer in many food products because it has a texture and mouthfeel similar to fat. Additionally, pectin and carrageenan were also mixed to develop low-fat sausage. In addition, a low-fat Manchego cheese was developed with a commercial fat replacer derived from low methoxyl pectin. Furthermore, pectin from apple pomace has been used as a fat replacer in different types of cookies.13
Mayonnaise, a widely available condiment with a rich and creamy texture, has become a staple in culinary traditions worldwide.14 Mayonnaise is a semi-solid oil-in-water emulsion, prepared from egg yolks, oil, vinegar or lemon juice, and seasonings such as mustard.15 The stability and consistency of traditional mayonnaise largely depend on the amount of oil and egg yolk used in the formulation.16 However, the use of large amounts of oil and egg yolk may cause many health issues due to their high fat and cholesterol content. This concern has led to the development of low-fat mayonnaise, which contains significantly less oil typically between 20% and 40%.17 Such formulations have gained significant attention as they offer a healthier alternative while maintaining desirable sensory attributes. Traditionally, fat replacers include protein- or carbohydrate-based alternatives, but their functional properties often limit their effectiveness in replicating the mouthfeel and stability of conventional mayonnaise.
Therefore, this study explores the potential of pectin derived from Assam lemon peel as a natural fat mimetic and emulsifier, offering a sustainable and functional alternative for low-fat mayonnaise formulation. The adoption of ultrasound-assisted extraction (UAE) not only enhances pectin yield but also preserves its functional properties, making the extraction process more efficient and environmentally friendly compared to conventional methods. Unlike prior research, which predominantly relies on commercial pectin, this study utilizes naturally extracted pectin gel, thereby providing a cleaner-label alternative that retains the desired texture and stability of traditional mayonnaise.
A novel aspect of this work is the incorporation of pectin gel stabilized with sodium alginate and soy protein, which enhances the emulsifying properties and structural integrity of the low-fat mayonnaise. This approach leverages the synergistic interaction between pectin and stabilizers to improve viscosity and minimize phase separation, addressing key challenges in fat-reduced formulations. Furthermore, by valorizing citrus peel, an abundant agro-industrial byproduct, this study not only advances the development of functional food products but also aligns with sustainable food processing practices by repurposing waste into high-value ingredients.
Thus, the primary objective of this research is to formulate and evaluate pectin-based fat mimetics for low-fat mayonnaise, specifically low-veg-fat mayonnaise (LVM) and low-egg-fat mayonnaise (LEM). By partially substituting egg yolk and utilizing pectin as an emulsifier, these formulations seek to replicate the sensory and textural attributes of conventional mayonnaise while offering a healthier alternative. To assess their performance, LVM and LEM are systematically compared with commercial veg-mayonnaise (CVM) and commercial egg-mayonnaise (CEM), providing a comprehensive analysis of their structural and functional properties.
Experimental section
Materials collection
Fresh and mature Assam lemon (Citrus lemon) fruit and other ingredients required for the formulation of mayonnaise were purchased from the local market of Tezpur University campus, Napaam, Assam, India. For the study, sodium hydroxide and selenium dioxide were procured from Thermo Fisher Scientific India Pvt. Ltd Mumbai. Copper sulphate, potassium sulphate, and sulfuric acid were obtained from Avantor Performance Materials India Ltd, Maharashtra. Soya protein isolate was acquired from Medizen Labs Pvt. Ltd, Bengaluru. Petroleum benzine was received from Merck Life Science Private Ltd Mumbai. All the other chemicals used in this study were of analytical grade and high purity.
Extraction of pectin
For preparing the lemon peel powder, peels were collected, washed thoroughly and then dried in a hot air oven at 45 °C until the peel dried. After the drying process, the dried peels were ground and sieved using a 1 mm sieve to obtain the powder. The powder was packed in a polyethylene bag and stored for further analysis. Furthermore, pectin was extracted using ultrasound assisted extraction method. Initially, lemon peel powder (10–15 g) was mixed with a fixed quantity of citric acid solution. The mixture was then ultrasonicated for 10, 20, and 30 min at 100% power. After ultrasonication, the mixture was kept at room temperature for filtration using Whatman No. 1 filter paper. The filtrate, containing pectin, was cooled down and then centrifuged at 6000 rpm for 30 min. In this extraction, propanol was used to precipitate the supernatant and left undisturbed for an hour to allow the pectin to float. The floating pectin was then separated by filtration followed by drying at 45 °C. The resulting dried pectin was ground to form powdered pectin and stored for further analysis. The yield of pectin was determined using eqn (1) as mentioned below:| |  | (1) |
Preparation of pectin gel
For the preparation of pectin gel, several steps have been followed. Firstly, pectin powder was dissolved in distilled water to form a pectin solution for 30 min with a variation in the temperature between 70 °C and 90 °C to ensure uniform dispersion. After that, soy protein isolate (1%) and sodium alginate (2%) were added to the solution and mixed. Furthermore, sodium tripolyphosphate (0.5%) was added gradually to the mixture to facilitate the cross-linking between pectin molecules. The solution was kept in a magnetic stirrer for another 6 h at 80 °C to allow the gel to achieve a proper structure and good strength. Finally, the resulting gel mixture was allowed to set at room temperature for 24 h.
Formulation of pectin gel incorporated low-fat mayonnaise.
Low-fat mayonnaise samples i.e. LVM and LEM were formulated using the composition of the ingredients specified in Table 1. Initially, pectin gel was thoroughly blended with egg yolk/milk according to the type along with salt and sugar. After that, vinegar was added to the blend, followed by further mixing of the ingredients. Subsequently, soyabean oil was slowly added to the mixture and subjected to agitation to ensure even distribution of all the ingredients. This process results in the formulation of low-fat mayonnaise with the desired texture and flavour.
Table 1 Composition of ingredients for the formulation of mayonnaisea
| Ingredients |
LVM |
LEM |
|
LVM: low-veg-mayonnaise; LEM: low egg mayonnaise.
|
| Sunflower oil |
45% |
40% |
| Egg yolk |
— |
40% |
| Vinegar solution |
5% |
5% |
| Salt |
1% |
1% |
| Garlic |
— |
1% |
| Pectin gel |
5% |
5% |
| Lemon juice |
2% |
2% |
| Sugar |
1% |
1% |
| Milk |
40% |
— |
Proximate analysis
Moisture content.
The moisture content indicates the quantity of water present in a substance or material. Approximately 3 g of each sample was dried for 3–4 h in a hot air oven at 105 °C until a constant weight was obtained. The moisture content was calculated by using eqn (2) as mentioned below.18| |  | (2) |
Protein content.
The crude protein content was determined using the Kjeldahl method. Approximately, 5 g sample of mayonnaise was digested in a digestion tube with sulfuric acid. Then, the samples were loaded into a digestion set (Make #Borosil Model #LabQuest KC010 Serial No. #2001794771). The digested sample was then mixed with 40 (w/w) % sodium hydroxide (NaOH) and titrated with 0.1 N hydrochloric acid (HCl). The protein content in the mayonnaise was estimated by multiplying the nitrogen content by a factor of 6.25.19| | 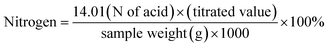 | (3) |
Fat content.
The fat content was determined using the semi-continuous solvent extraction method (AOAC 2005).20 A homogenized sample (1–3 g) was hydrolyzed in an automatic hydrolyzing unit (Pelican Equipment's Model #SCS6). The hydrolyzed sample was then extracted with petroleum ether at 40–60 °C using an automated fat extraction system (Gerhardt, Germany) for 2–3 h. The extracted fat was dried at 105 °C for 3 h followed by cooling and then weighed to determine the fat content.| |  | (4) |
Ash content.
The ash content of the sample was determined by the weight difference method/gravimetric method (IP SELECTA, UK).21 Approximately, 2 g of sample was taken into a crucible. The crucible along with the sample was placed in a muffle furnace at 600 °C for 4 h. After heating, the final weight of the crucible along with the sample was taken.2| |  | (5) |
Physicochemical properties
pH test.
The pH value of low-fat mayonnaise was determined at room temperature using a pH meter. For each sample, three replications were done.
Bulk density.
To measure the bulk density, approximately 3 g of sample was placed in a 10 ml graduated cylinder. Furthermore, the sample was settled without compacting the cylinder and the volume occupied by the sample was recorded.22| |  | (6) |
Tapped density.
To measure the tapped density, a glass rod was used to tap a graduated cylinder until a consistent volume was achieved. Furthermore, the volume of the sample was recorded.22| |  | (7) |
Angle of repose.
To determine the angle of repose, the sample was poured into a funnel and the funnel was gradually moved upward until the sample took the shape of a cone. After that, the circular area covered by the sample was marked and the diameter (d) of the marked area was determined. Furthermore, the value of ‘θ’ was calculated by using eqn (8) as mentioned below.| |  | (8) |
In the above formula, h is height (cm) and d is the diameter (cm).
Hydration properties
Water holding capacity.
The water holding capacity (WHC) was measured using the method followed by Wang et al., 2021 and Moczkowska et al., 2019 with minor modifications.23,24 Approximately 0.5 g of sample (W1) was dissolved in a known amount (20 ml) of distilled water and equilibrated for 24 h at ambient temperature. After that, centrifugation was done for 15 min at 6700 rpm and 25 °C using a Remi Elektrotechnik Ltd centrifuge (Model # R-8C PLUS, Serial No. # ZILN-46961). The residual weight (W2) was determined immediately after removing the water. The WHC was calculated using eqn (9) as mentioned below:| |  | (9) |
where W2 = final weight of water content and W1 = initial weight of water content.
Oil holding capacity.
The oil holding capacity (OHC) was measured using the procedure followed by Zhang et al. (2017)25 with minor modifications. Initially, 0.5 g sample (W1) was poured into 5 ml of refined soybean oil and was kept for 1 h at 5 °C without any disturbance. Furthermore, the mixture was centrifuged at 6700 rpm for 15 min at 25 °C. The residual weight (W2) was measured immediately after the centrifugation. The OHC was calculated using eqn (10) as mentioned below:| |  | (10) |
where O2 = final weight of oil and O1 = initial weight of oil.
X-ray diffraction (XRD).
The developed materials were characterized via X-ray diffraction (XRD) using an X-ray diffractometer (AXS SMART APEX-I; BRUKER AXS, Germany, and Rigaku Corporation, Japan) equipped with Cu Kα radiation (λ = 1.5406 Å). The selected scanning range for the samples was between 5° and 80°.
Viscometer analysis.
The viscometer analysis was conducted to determine the exact viscosity of the four samples (LVM, CVM, LEM and CEM). This measurement reflects the extent of resistance experienced from the sample.
Textural analysis.
The firmness, stickiness (texture), and spreadability of the mayonnaise were measured using a TAXT plus C Texture Analyzer (Stable Micro Systems, Godalming, UK) equipped with a 30 kg load cell at room temperature. For the analysis, chilled mayonnaise samples were filled into a tube with an inner diameter of 26 mm and a height of 35 mm. A plastic cylinder probe with a diameter of 12.7 mm (P/0.5–1/2′′ Dia Cylinder Delrin, Stable Micro Systems) was used. The probe entered the sample at a speed of 1 mm s−1 for a distance of 10 mm and then returned to the starting position at the same speed.
Color measurement.
The color of the mayonnaise sample was measured using Hunter color Lab (Ultra-Scan VIS, Hunter Lab, USA). At first, the mayonnaise sample container was placed in the color measurement area, ensuring uniform coverage of the sample. Furthermore, the measurement process was initiated and readings for CIE L*, a*, and b* values were obtained. The values were analysed to interpret differences in lightness, redness-greenness, and yellowness-blueness among the samples. This facilitated a comparison of color parameters to assess variations in intensity and hue.
Sensory evaluation.
The sensory analysis of LVM, CVM, LEM and CEM were carried out after storing them for a complete one day at room temperature. Sensory analysis was conducted on the basis of appearance, color, odor, texture, taste, and overall acceptance. These parameters were judged by a panel of 10 members aged between 22 and 40 years. A 9-point hedonic scale was used, with 1 representing the lowest score and 9 representing the highest score. Labels of mayonnaise samples were also obtained with different codes. The order of the sample presentation was fully randomized. However, water was supplied between samples to cleanse the palate and as mouthwash.
Results and discussion
Physicochemical properties of lemon peel powder
The physicochemical and hydration properties of lemon peel powder are presented in Table 2. The low moisture content (11.49 ± 0.08%) suggested better shelf stability, as lower moisture reduces microbial growth and enzymatic degradation.26 The obtained bulk density was 0.70 ± 0.01 g ml−1 (Bakshi & Ananthanarayan 2022 and Rawat & Ghosh 2025),27,28 while the tapped density showed a higher value of 0.82 ± 0.03 g ml−1, suggesting that the powder exhibited good compressibility under tapping or vibration, as previously reported by Rafiq et al., 2018.29 The increase in bulk density with decreasing particle size can be attributed to the higher packing efficiency of smaller particles, reducing interstitial voids.30 However, the angle of repose of powder was found to be 33.80 ± 0.69°, which indicates that the powder particles exhibited moderate flowability and cohesion. This may be due to the fibrous nature of lemon peel, which can create interparticle friction, limiting free flow. Apart from this, lemon peel powder showed slight acidic behavior with a pH of 3.53 ± 0.05 mainly due to the presence of organic acids such as citric and ascorbic acids, which contribute to the powder's potential as an acidulant in food formulations. Furthermore, the hydration properties further demonstrated the functional potential of lemon peel powder. The high water-holding capacity (WHC: 7.97 ± 0.55 g g−1) suggests strong hydrophilic interactions, likely due to pectin, cellulose, and hemicellulose content, which form hydrogen bonds with water molecules. However, the oil-holding capacity (OHC: 2.12 ± 0.01 g g−1) was influenced by the surface characteristics of fiber and protein content, allowing oil entrapment through capillary action.
Table 2 Physicochemical and hydration properties of lemon peel powder
| Properties |
Contenta |
|
The results are represented as mean ± std.
|
| Moisture content (%) |
11.49 ± 0.08 |
| Bulk density (g ml−1) |
0.70 ± 0.01 |
| Tapped density (g ml−1) |
0.82 ± 0.03 |
| Angle of repose (°) |
33.80 ± 0.69 |
| pH |
3.53 ± 0.05 |
| WHC (g g−1) |
7.97 ± 0.55 |
| OHC (g g−1) |
2.12 ± 0.01 |
Extraction of pectin using ultrasound assisted extraction.
From the ultrasound assisted extraction (UAE), the average yield of the pectin was found to be 12.84 ± 0.14%. This result was similar to the result observed by Kurita et al., 2008,31 where the yield of pectin from lemon was found to be 11%. However, when studied for orange peel the yield was reduced to 8% (Kurita et al., 2008).31 Therefore, the study concluded that lemon peel has a higher pectin content as compared to other citrus peels. The high yield of pectin from lemon peel powder underscores its potential as a valuable raw material for the production of pectin-based products in various industries.
Fourier transform infrared spectroscopy (FT-IR).
FTIR analysis was conducted to examine the functional properties of pectin extracted from lemon peel and to compare them with those of commercial pectin, as illustrated in Fig. 1A. The primary chain of galacturonic acid contains intramolecular bonds, which contribute to O–H stretching, as shown by the absorption peak observed between 3490 and 3250 cm−1.32 Furthermore, a long chain linear aliphatic molecule was illustrated by an absorption band at 2930 cm−1, which was due to C–H stretching vibration of the CH3 group linked to the O-acetyl groups.33 Another absorption peak at 1738 cm−1 was attributed to ester carbonyl (COO-R) stretching, and a peak at 1648 cm−1 indicated the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching and vibration of the carboxylate ion. The presence of the α-D-mannopyranose ring was confirmed by the peak at 827 cm−1, whereas the peak at 1222 cm−1 was associated with the C–C bond in the ring structure of pectin.24,25,34
O) stretching and vibration of the carboxylate ion. The presence of the α-D-mannopyranose ring was confirmed by the peak at 827 cm−1, whereas the peak at 1222 cm−1 was associated with the C–C bond in the ring structure of pectin.24,25,34
 |
| | Fig. 1 Extracted pectin functional properties, (a) FTIR spectroscopy and (b) XRD spectroscopy. | |
X-ray diffraction (XRD).
In the XRD diffractogram of lemon peel-derived pectin, a distinct sharp-intensity crystalline peak was observed at a 2θ value of 18.9°. The specified diffraction peak defines the crystalline nature of pectin as shown in Fig. 1B. The aim of using an X-ray diffractometer is to confirm the development of pectin from lemon peel. The figure depicts the X-ray diffraction curve angle of extracted pectin. The peak obtained at 18.9° reveals a slight change in the crystallinity of pectin after treatment. The broad peak is identified as being due to the amorphous portion, and the sharp peak is identified because of having a crystalline structure. There may be a difference in the crystallite size of the material after the ultrasound treatment.35 Furthermore, a recent study by Singhal et al. (2024) analyzed the XRD pattern of pectin extracted from Assam lemon peel, identifying well-defined crystalline regions. The observed diffraction peaks, which reflect the crystalline characteristics of the material, were observed at 2 θ = 11.9°, 12.60°, 13.89°, 14.29°, 20.49°, 20.79°, 21.49° and 21.89°.32
Proximate analysis of mayonnaise.
The proximate analysis of the four different mayonnaise samples showed significant variations in their key nutritional components, as mentioned in Table 3. The fat content of CEM (Commercial Egg Mayonnaise) was 65.13%; which was higher than that of all samples. LVM (Low Fat Veg Mayonnaise) has low fat content (44.89%) compared to the other samples. The protein content of CVM was significantly lower (0.46%) than that of the other samples. Generally, commercial mayonnaise has a low protein content compared to fat content, as mentioned by Amin et al., 2014.36
Table 3 Proximate analysis of mayonnaise samples
| Sample |
LVMa |
CVMa |
LEMa |
CEMa |
|
Mean = 3 ± SD. CEM = commercial egg mayonnaise, CVM = commercial veg mayonnaise, LVM = low fat veg mayonnaise, and LEM = low fat egg mayonnaise.
|
| Moisture content (%) |
34.76 ± 0.33 |
32.57 ± 0.18 |
32.54 ± 0.18 |
20.88 ± 0.49 |
| Protein (%) |
1.03 ± 0.15 |
0.46 ± 0.09 |
3.53 ± 0.05 |
2.41 ± 0.07 |
| Fat (%) |
44.89 ± 0.02 |
52.33 ± 0.08 |
46.14 ± 0.12 |
65.13 ± 0.25 |
| Ash content (%) |
1.14 ± 0.07 |
1.21 ± 0.02 |
0.89 ± 0.45 |
0.83 ± 0.01 |
pH analysis of mayonnaise.
The pH test of the four different mayonnaise samples explained their proximity to the pH values of commercial mayonnaise, as shown in Table 4. All the samples exhibited pH values in the range of 3.8–4.2 as specified by the FSSAI regulations. In comparison to the commercial variants, low-fat samples showed slightly higher pH of 4.1 and 4.2. Although these values remained within the permissible limits, they could be further reduced through the incorporation of vinegar, lemon extract, or acidic salts. Notably, the pH values of the commercial samples were slightly lower, typically falling below 3.8 and 3.9, consistent with the findings reported by Amin et al., 2014, and Satriawan et al., 2022.36,37
Table 4 pH analysis of mayonnaise
| Sample |
pH |
| LVM |
4.1 |
| CVM |
3.8 |
| LEM |
4.2 |
| CEM |
3.9 |
Viscometer analysis.
The viscosity of the four different mayonnaise samples, expressed in pascal-seconds (Pa s), was measured using a viscometer. Among the samples, CVM exhibited the highest viscosity at 355 ± 0.50 Pa s, followed by LEM at 316 ± 0.40 Pa s. Furthermore, for LVM and CEM, the viscosity value was recorded as 284 ± 0.30 Pa s and 288 ± 0.30 Pa s, respectively. The flow behavior of a mixture is influenced by multiple factors, including its composition, size, shape, and charge of its constituent molecules.38 These findings highlighted the variations in the rheological properties of the mayonnaise samples, which can be attributed to differences in ingredient composition, processing conditions, and emulsification techniques. A higher viscosity, as observed in the formulated mayonnaise, may enhance spreadability and stability, whereas lower viscosity, as seen in CEM, may be more suitable for applications requiring easier pouring or dispensing.39 Moreover, a three-dimensional network of aggregated droplets may form due to their close proximity, facilitating intermolecular interactions. When subjected to increasing shear rates, these aggregates undergo deformation and eventual disruption due to hydrodynamic forces, leading to a reduction in viscosity (Table 5).40
Table 5 Viscosity analysis of mayonnaise
| Sample |
Viscosity (Pa s) |
| LVM |
284 ± 0.30 |
| CVM |
355 ± 0.50 |
| LEM |
316 ± 0.40 |
| CEM |
288 ± 0.30 |
Color measurement.
The CIELAB coordinates of luminosity (L*) and chromaticity (a* and b*) were used to assess the color of developed mayonnaise. The acquired findings are shown in Table 6. The four mayonnaise samples were subjected to color measurement analysis using CIELAB coordinates. Variations in L* and chromaticity values were recorded for the samples. The luminosity values of the samples LVM and CEM were found to be marginally lower as compared to the other formulations. In terms of chromaticity, sample LEM showed the highest a* value at 1.67 ± 0.07, indicating a slightly reddish hue, while sample LEM had the highest b* value at 19.76 ± 0.59, suggesting a more yellowish tone. Conversely, LVM and CVM exhibited lower a* and b* values compared to LEM and CEM, indicating differences in hue and intensity among the mayonnaise samples.
Table 6 Color analysis of mayonnaise
| Properties |
L* |
a* |
b* |
| LVM |
88.20 ± 0.67 |
0.62 ± 0.51 |
12.23 ± 0.56 |
| CVM |
91.73 ± 1.27 |
0.57 ± 0.09 |
9.74 ± 0.20 |
| LEM |
93.87 ± 0.19 |
1.67 ± 0.07 |
19.76 ± 0.59 |
| CEM |
88.25 ± 0.011 |
3.13 ± 0.002 |
16.64 ± 0.006 |
Texture analysis.
Notable variations were observed among the four mayonnaise samples in terms of textural properties. The CVM sample exhibited the highest firmness value, followed by the LEM sample, which recorded a firmness value of 1.13 ± 0.19 N. In contrast, the hardness values for the CEM and LVM samples were comparatively lower, measuring 0.88 ± 0.05 N and 0.86 ± 0.07 N, respectively. In terms of stickiness, the CVM sample demonstrated the highest negative force, measuring −0.94 ± 0.04 N, followed by the LEM sample with a value of −0.77 ± 0.10 N, as presented in Table 7. As reported by Liu et al. (2007), the stickiness values of the LVM and CEM samples were comparatively lower, measuring −0.54 ± 0.05 N and −0.56 ± 0.03 N, respectively.16 The high contact surface area between the oil droplets in mayonnaise creates significant friction force, which impedes the free movement of the emulsion under shear stress, thereby increasing its viscosity. Furthermore, a reduction in the oil droplet diameter enhanced the interfacial contact area, further contributing to an increase in viscosity due to stronger droplet–droplet interactions within the emulsion system.39
Table 7 Texture analysis of mayonnaise
| Samples |
Firmness (N) |
Stickiness (N) |
| LVM |
0.86 ± 0.07 |
0.54 ± 0.05 |
| CVM |
1.55 ± 0.08 |
0.94 ± 0.04 |
| LEM |
1.13 ± 0.19 |
0.77 ± 0.10 |
| CEM |
0.88 ± 0.05 |
0.56 ± 0.03 |
Sensory analysis of mayonnaise.
The sensory analysis of mayonnaise samples revealed variations in perceived attributes among the four variations tested: low-fat egg mayonnaise (LEM), commercial egg mayonnaise (CEM), low-fat vegetable mayonnaise (LVM), and commercial vegetable mayonnaise (CVM) which are presented in Table 8. Overall, the results indicated that the developed mayonnaise formulations received comparable ratings across key sensory parameters, including color flavor, appearance, sourness, taste, and overall acceptability. The results indicated that LVM exhibited a higher rating for color (8.0 ± 0.73) compared to CVM (7.5 ± 0.52) and LEM (7.6 ± 0.41), while CEM received the highest score (8.2 ± 0.82). Similarly, the flavor profile of LVM (8.0 ± 0.68) was rated slightly higher than that of CVM (7.6 ± 0.44), whereas LEM (7.8 ± 0.23) and CEM (8.0 ± 0.71) demonstrated comparable acceptability. In terms of appearance, LVM scored the highest (8.5 ± 0.59), followed by CEM (8.2 ± 0.83), while LEM (7.8 ± 0.25) and CVM (7.7 ± 0.80) received slightly lower ratings. The sourness remained consistent for LVM, CVM, and LEM (8.0 ± 0.75, 8.0 ± 0.70, and 8.0 ± 0.36, respectively), whereas CEM exhibited a slightly lower rating (7.6 ± 0.63). Furthermore, taste acceptability was found to be highest in CEM and LVM (8.2 ± 0.76 and 8.2 ± 0.43, respectively), followed by LEM (8.0 ± 0.39) and CVM (7.7 ± 0.53). Overall acceptability scores indicated that CEM was the most preferred (8.3 ± 0.64), while LVM (8.2 ± 0.47) and LEM (7.8 ± 0.24) also received favorable ratings. In contrast, CVM exhibited the lowest overall acceptability (7.5 ± 0.64).
Table 8 Sensory analysis of mayonnaise
|
|
LVM |
CVM |
LEM |
CEM |
| Colour |
8.0 ± 0.73 |
7.5 ± 0.52 |
7.6 ± 0.41 |
8.2 ± 0.82 |
| Flavour |
8.0 ± 0.68 |
7.6 ± 0.44 |
7.8 ± 0.23 |
8.0 ± 0.71 |
| Appearance |
8.5 ± 0.59 |
7.7 ± 0.80 |
7.8 ± 0.25 |
8.2 ± 0.83 |
| Sourness |
8.0 ± 0.75 |
8.0 ± 0.70 |
8.0 ± 0.36 |
7.6 ± 0.63 |
| Taste |
8.2 ± 0.43 |
7.7 ± 0.53 |
8.0 ± 0.39 |
8.2 ± 0.76 |
| Overall acceptability |
8.2 ± 0.47 |
7.5 ± 0.64 |
7.8 ± 0.24 |
8.3 ± 0.64 |
Conclusions
In conclusion, this work demonstrates the successful utilization of lemon peel powder as a sustainable source for pectin extraction, employing techniques such as ultrasound-assisted extraction. The extracted pectin was further utilized to develop a pectin gel enriched with soy protein and sodium tripolyphosphate, offering enhanced textural properties and stability for incorporation into low-fat mayonnaise formulations. Proximate analysis revealed notable differences in the nutritional composition of the final product compared to traditional mayonnaise formulations, indicating the potential for utilizing pectin as a functional ingredient in low-fat food formulations. Overall, this research contributes to the exploration of sustainable alternatives in food ingredient sourcing and product development, with implications for improving both the nutritional quality and sustainability of food products in industry. Low-fat mayonnaise was successfully developed using pectin gel extracted from lemon peel powder. Significant reduction in fat content was observed compared to traditional mayonnaise variants, with proximate analysis indicating 44% for vegetable and 46% for egg mayonnaise. The sensory evaluation results showed favorable attributes, including color, flavor, appearance, sourness, taste, and overall acceptability, comparable to its commercial counterparts.
Data availability
Data can be obtained by request to the authors.
Author contributions
Nithish S: experimentation, original draft writing, review and editing. Ram Prasanna Kumar: experimentation, original draft writing, review and editing. Laxmikant Rawat: original draft writing, review and editing. Nurin Afzia: original draft writing, review and editing. Tabli Ghosh: conceptualization, supervision, validation, final review and editing.
Conflicts of interest
The authors declare no conflict of interest related to this research.
References
- J. Akhtar, M. G. Abrha, P. K. Omre and G. G. Gebru, Res. J. Chem. Environ. Sci., 2020, 8, 25–37 Search PubMed.
- S. Akhtar, R. Ahmed, K. Begum, A. Das, S. Saikia, R. A. Laskar and S. Banu, Sci. Rep., 2024, 1, 3886 Search PubMed.
- H. Jiang, W. Zhang, Y. Xu, L. Chen, J. Cao and W. Jiang, Trends Food Sci. Technol., 2022, 124, 219–236 Search PubMed.
- S. Multari, C. Licciardello, M. Caruso, A. Anesi and S. Martens, J. Food Meas. Charact., 2021, 15, 1754–1762 Search PubMed.
- D. Demir, S. Ceylan, D. Gokturk and N. Bolgen, Polym. Bull., 2021, 78, 2211–2226 CrossRef CAS.
- N. Afzia, N. Shill, B. J. Kalita and N. Sit, Food Meas., 2024, 14, 100166 CrossRef.
- A. K. Sista Kameshwar and W. Qin, Bioresour. Bioprocess., 2018, 5(1), 1–16 CrossRef.
- A. Noreen, J. Akram, I. Rasul, A. Mansha, N. Yaqoob, R. Iqbal and K. M. Zia, Int. J. Biol. Macromol., 2017, 101, 254–272 CrossRef CAS PubMed.
-
S. H. Christensen, Food Hydrocoll, CRC Press, 2020, pp. 205–230 Search PubMed.
- V. Chandel, D. Biswas, S. Roy, D. Vaidya, A. Verma and A. Gupta, Foods, 2022, 17, 2683 CrossRef PubMed.
- A. Assifaoui, G. Hayrapetyan, C. Gallery and G. Agoda-Tandjawa, Carbohydr. Polym. Tech., 2024, 100496 Search PubMed.
-
T. Vanitha and M. Khan, Pectins-extraction, Purification, Characterization and Applications, 2019, vol. 10 Search PubMed.
- J. Lim, S. Ko and S. Lee, Food Sci. Biotechnol., 2014, 23, 1837–1841 CrossRef CAS.
- M. Mirzanajafi-Zanjani, M. Yousefi and A. Ehsani, Int. J. Food Sci. Nutr., 2019, 8, 2471–2484 Search PubMed.
- I. M. K. Al-Aubadi, Syst. Rev. Pharm., 2021, 1, 1142–1150 Search PubMed.
- H. Liu, X. M. Xu and S. D. Guo, LWT-Food Sci. Tech., 2007, 6, 946–954 CrossRef.
- J. Metri-Ojeda, M. Ramírez-Rodrigues, L. Rosas-Ordoñez and D. Baigts-Allende, Appl. Sci., 2022, 15, 7456 CrossRef.
- R. Samuelsson, J. Burvall and R. Jirjis, Biomass Bioenergy, 2006, 11, 929–934 CrossRef.
- B. Beljkas, J. Matic, I. Milovanovic, P. Jovanov, A. Misan and L. Saric, Accredit. Qual. Assur., 2010, 15, 555–561 CrossRef CAS.
-
W. Horwitz and G. W. Latimer, Official Methods of Analysis of AOAC International, AOAC International: Gaithersburg, MD, USA, 2005 Search PubMed.
-
S. S. Nielsen and B. P. Ismail, Food Analysis Laboratory Manual, 2017, pp. 117–119 Search PubMed.
- N. Savlak, B. Türker and N. Yeşilkanat, Food Chem., 2016, 213, 180–186 CrossRef CAS PubMed.
- K. Wang, M. Li, Y. Wang, Z. Liu and Y. Ni, Food Hydrocolloids, 2021, 110, 106162 CrossRef CAS.
- M. Moczkowska, S. Karp, Y. Niu and M. A. Kurek, Food Hydrocolloids, 2019, 90, 105–112 CrossRef CAS.
- L. Zhang, L. J. Wang, W. Jiang and J. Y. Qian, LWT-Food Sci. Technol., 2017, 84, 73–81 CrossRef CAS.
- A. S. Abdelwahab and A. Abouelyazeed, J. Food Sci. Technol., 2018, 1, 44–67 CrossRef.
- G. Bakshi and L. Ananthanarayan, J. Food Sci. Technol., 2022, 59(7), 2535–2544 CrossRef CAS PubMed.
- L. K. Rawat and T. Ghosh, Sustainable Food Technol., 2025, 1, 204–214 RSC.
-
S. Rafiq, Doctoral dissertation, SKUAST Jammu, 2018.
- J. Ahmed, H. Al-Attar and Y. A. Arfat, Food Hydrocolloids, 2016, 52, 888–895 CrossRef CAS.
- O. Kurita, T. Fujiwara and E. Yamazaki, Carbohydr. Polym., 2008, 3, 725–730 CrossRef.
- S. Singhal, S. C. Deka, A. Koidis and N. R. S. Hulle, Biomass Convers. Biorefin., 2024, 1–12 Search PubMed.
- D. Trujillo-Ramírez, C. Lobato-Calleros, A. Román-Guerrero, L. Hernández-Rodríguez, J. Alvarez-Ramirez and E. J. Vernon-Carter, React. Funct. Polym., 2018, 123, 61–69 CrossRef.
- J. Gan, Z. Huang, Q. Yu, G. Peng, Y. Chen, J. Xie and M. Xie, Food Hydrocolloids, 2020, 101, 105549 CrossRef CAS.
- D. Panwar, P. S. Panesar and H. K. Chopra, Biomass Convers. Biorefin., 2022, 1–13 Search PubMed.
- M. H. H. Amin, A. E. Elbeltagy, M. Mustafa and A. H. Khalil, J. Agroaliment. Processes Technol., 2014, 1, 54–63 Search PubMed.
-
T. U. Satriawan, H. Evanuarini and I. Thohari, in E3S Web of Conferences, EDP Sciences, 2022, 335, p. 00021 Search PubMed.
- C. Sun, R. Liu, B. Liang, T. Wu, W. Sui and M. Zhang, Food Res. Int., 2018, 108, 151–160 CrossRef CAS PubMed.
- A. S. Thomareisa and S. Chatziantoniou, Procedia Food Sci., 2011, 1, 1997–2002 CrossRef.
- H. Liu, X. M. Xu and S. D. Guo, LWT-Food Sci. Technol., 2007, 40, 946–954 CrossRef CAS.
Footnote |
| † The authors have contributed equally. |
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*









![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching and vibration of the carboxylate ion. The presence of the α-D-mannopyranose ring was confirmed by the peak at 827 cm−1, whereas the peak at 1222 cm−1 was associated with the C–C bond in the ring structure of pectin.24,25,34
O) stretching and vibration of the carboxylate ion. The presence of the α-D-mannopyranose ring was confirmed by the peak at 827 cm−1, whereas the peak at 1222 cm−1 was associated with the C–C bond in the ring structure of pectin.24,25,34

