Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Properties of steam exploded orange peel fiber as compared to commercial citrus fiber and peel powders†
Christina
Dorado
 *,
Wei
Zhao
*,
Wei
Zhao
 ,
Dave
Wood
and
Anne
Plotto
,
Dave
Wood
and
Anne
Plotto
United States Department of Agriculture, Agricultural Research Service, U.S. Horticultural Research Laboratory, Ft. Pierce, FL, USA. E-mail: christina.dorado@usda.gov
First published on 28th February 2025
Abstract
Citrus is a major fruit crop in the state of Florida. Most of which, is processed into juice. The remaining peel and membrane are converted to byproducts with little value. However, the properties of citrus peel and membrane make it an excellent candidate as a high value, gluten free fiber. Steam explosion (STEX) has been used as a treatment method on citrus peel and membrane in the past, but the properties of the resulting fiber have not been studied. In this research, we compared STEX citrus fiber to a commercial citrus fiber and powders. A novel STEX process was used to produce twelve steam dietary fibers from orange juice processing side streams using a combination of temperature (130, 150, 170 degrees Celsius) and hold time (1, 2, 4, 8 minutes). The STEX orange fibers were characterized and compared to commercial citrus fiber and peel powder products. Desirable properties obtained by steam treatment did not lie with a single temperature and hold time combination. STEX produced fibers with far greater water retention and water swelling capacities as compared to the commercial fiber and peel powder products. STEX fibers with median sample diameters and insoluble, soluble, and total dietary fiber values similar to those of the commercial fiber product were possible. This work shows that STEX can serve as a scalable method for converting orange juice processing side streams into fiber products with properties similar to commercial citrus fiber products.
Sustainability spotlightAccording to the Food and Agricultural Organization of the United Nations (UN), 20 million metric tons of citrus were used for processing worldwide. After processing, half of the fruit mass remains mostly in the form of peel and membrane. Steam explosion for the production of gluten free fibers from the remnants of citrus juice processing is explored in this study as a method for converting this material to value-added products in an effort to protect the environment from the effects of its disposal and to increase revenue for processors. This aligns with the UN's Sustainable Development Goal 12: ensure sustainable consumption and production patterns. |
1 Introduction
Global markets for gluten-free products were valued at 5.6 billion US dollars (USD) in 2020 and are expected to grow at a compound annual growth rate of 8.1% to a value of 8.3 billion USD by 2025.1 Citrus-derived fibers are gluten-free and possess properties, including water retention, gelation, thickening, and fat replacement, that are beneficial for food production. Citrus fibers can also be used as stabilizers and emulsifiers. These properties allow for the replacement of more expensive food production ingredients such as gums, dairy products, and eggs which reduces the overall production cost and is driving the growth of the citrus fiber global market. Citrus fiber is being used in meat, dairy, and bakery products with the bakery industry implicated in the projected economic value growth.2,3 Another factor driving this growth is the addition to the Food and Drug Administration's list of dietary fibers and their ability to replace carrageenan, a gum derived from red seaweed used for thickening, gelling, stabilizing, and glazing in foods, which is now listed as an unacceptable food ingredient as a result of the Food Safety and Modernization Act.3,4In 2023 oranges were the top agricultural citrus crop in the state of Florida with a value of approximately 223 million dollars and 87% of that value came from oranges processed into juice.5 Most seasons, 90% of orange juice produced in the U.S. is from Florida.6 By the end of the 2019–2020 Florida citrus season, approximately 3.3 million tons of citrus had been cultivated.7 This includes oranges, grapefruit, tangerines, and tangelos. The majority, however, is made up of oranges that are processed mostly into juice. After the fruit is processed into juice, about half of the fruit mass is left behind in the form of peel, juice sacs, membrane and seeds.8 That equates to 1.3 million tons of orange juice processing remnants for the 2019–2020 season alone. Approximately 60–88 kg of citrus fibers can be isolated from a metric ton of the wet citrus juice processing side stream9,10 and citrus fiber can sell for as much as 100 USD per kilogram11 depending on the quality of the final product.
Steam explosion (STEX) is a chemical and physical hydrothermal process that incorporates the use of steam under pressure and at high temperature for a specified time followed by rapid decompression which results in hydrolysis of hemicellulose and the disruption of the lignin, cellulose, pectin and hemicellulose fibril structure12–14 STEX has been studied extensively as a method for the pretreatment of biomass for enzymatic hydrolysis13–15 and the production of fibers for composite materials16,17 and other non-food applications.18,19
Recently, STEX has been applied to various types of biomass including okara,20 Mongolian oak,21 wheat bran,22 bamboo leaves,23 cactus racket,24 garlic skin,25 rice straw,26 banana flowers,27 apple pomace,28 sweet potato residue,29 secondary date varieties,30 olive cake, olive stones,31 corn straw, wheat straw,32 and potato waste residue33 for the production of dietary fibers. What these feedstocks have in common is that they are not primary sources of food for human consumption and are in many cases the side stream of food processing.
To date, there have been two publications34,35 and several U.S. patents36–59 on the use of STEX for the preparation of dietary fibers from citrus peel. Processing conditions of polysaccharide mixtures affect their rheological and sensory properties.60 The properties, such as water retention and cellulose crystallinity, of the resultant STEX material are directly related to the conditions they are exposed to also known as the severity factor.61 The Gusek, Hansen, Sample, Staunstrup, and Weibel patents include information on the properties of the fiber resulting from their processes36–41,49–53,59 as well as the Wang and Yuan publications.34,35 The Sample patented processes37–39 do not discuss the properties of the fiber they are able to produce. The remaining patented processes utilize alcohol and or acid as a pre-treatment to citrus plant material for fiber production via STEX.36,40–59 Finally, only the Gusek and Sample patents discuss peel oil collection as part of their processes.37–39,59
While our STEX process has been studied in the past as a pretreatment method for ethanol production and for the recovery of pectin, sugars, phenolics, flavonoids and peel oil,62–67 it has not been studied for the production of fibers. Our process differs enough from what is found in the existing literature and patents that it needs to be investigated further as a method for fiber production from citrus peel (Table S1†). Therefore, the objective of this study was to compare citrus peel fibers using our batch STEX system with commercial citrus/powder products, and to identify the STEX conditions that can produce a product similar to what is commercially available. Raw and wet Citrus sinensis juice processing side streams of the varieties Hamlin and Valencia were subjected to STEX using various temperatures and hold times. These two varieties make up most of the oranges cultivated in the state of Florida and consumed as juice in the US. STEX fiber samples as well as commercial fiber/powder were analyzed for their food application properties including compositional and soluble sugars, galacturonic acid content, particle size, color, fiber, water retention capacity, water swelling capacity, oil retention capacity, and peel oil content.
2 Materials and methods
2.1 Citrus fruit
The citrus juice processing season begins in October/November and continues into April/May. Fruit is juiced based on when it matures with Hamlin maturing during the first half of the season and Valencia maturing in the second half of the season. The chemical composition of citrus fruits can differ based on the part of the fruit that is being analyzed, from variety to variety, and over the course of a season. Remnants from juice processing of Citrus sinensis (L.) Osbeck of the varieties Hamlin and/or Valencia were obtained from a local processor based on seasonal availability from December 2020 to March 2021. Remnants consisted of mostly peel and membrane. Remnants were size reduced by running material twice through a Fitz-Mill Model D-S6 (W. J. Fitzpatrick Company, Chicago, USA). Approximately 600 grams of size reduced sample was then placed in resealable bags and refrigerated at 4 °C until they were ready to use.2.2 Steam treatment
Approximately 600 grams of size reduced citrus juice processing remnants were loaded into the vertical pipe of a static STEX system10 and exposed to saturated steam at various temperature (130, 150, and 170 °C) and hold time combinations (1, 2, 4, and 8 min). The 130 °C temperature is the minimum temperature required for the material to move from the reaction pipe into the catch pot when the pressure is released. The 170 °C temperature is the maximum temperature that can be reached on the boiler that is being used. The hold times were chosen based on previous work.68 At the end of each experiment, a manual pressure relief valve was opened leading to rapid decompression and projection of fragmented material into a stainless steel catch pot (Fig. S1†). This was repeated at least two more times for each temperature and hold time combination. The replicates were pooled in resealable bags and were stored at 4 °C followed by storage at −20 °C until analysis could be completed.2.3 Citrus fiber production
After STEX, the sample was collected and allowed to cool at 4 °C for at least 60 minutes. The entire cooled sample was weighed and transferred into a 5 gallon bucket. Ethanol (200 Proof, CAS# 64-17-5, Greenfield Global USA Inc., Shelbyville, KY) was added in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass of sample in the bucket to volume ethanol. The ethanol and steam exploded sample were mixed using a long spatula for approximately 5 minutes and was covered and allowed to sit at 4 °C overnight. The following day the ethanol and steam exploded citrus peel slurry were removed from refrigeration and mixed again using a long spatula for approximately 5 minutes. A volume of the slurry was transferred using a 1000 mL plastic beaker into a stainless steel tabletop fruit press (1.25 gallon, Pleasant Hill Grain, Hampton, NE) lined with a mesh bag until it was half full or less. The mesh bag top was twisted and folded over and the press was placed on top. The slurry was then pressed until most of the liquid had been expelled. The remaining solid was emptied from the mesh bag into a clean and dry 5 gallon bucket and covered. This was repeated until all the ethanol and steam exploded citrus peel slurry was pressed. The pressed solid was broken up by hand in the bucket until a uniform damp meal was obtained. The damp meal was then transferred to trays covered with non-stick aluminum foil (Reynolds Wrap, Reynolds Consumer Products, Lake Forest, IL or Kingsford Heavy Duty, The Clorox Company, Oakland, CA). Several trays were stacked upon one another and separated by wood blocks in between to permit air flow in the hood for 3–6 days. Samples where then transferred to a 30 °C oven for further drying for 2–5 days.
1 mass of sample in the bucket to volume ethanol. The ethanol and steam exploded sample were mixed using a long spatula for approximately 5 minutes and was covered and allowed to sit at 4 °C overnight. The following day the ethanol and steam exploded citrus peel slurry were removed from refrigeration and mixed again using a long spatula for approximately 5 minutes. A volume of the slurry was transferred using a 1000 mL plastic beaker into a stainless steel tabletop fruit press (1.25 gallon, Pleasant Hill Grain, Hampton, NE) lined with a mesh bag until it was half full or less. The mesh bag top was twisted and folded over and the press was placed on top. The slurry was then pressed until most of the liquid had been expelled. The remaining solid was emptied from the mesh bag into a clean and dry 5 gallon bucket and covered. This was repeated until all the ethanol and steam exploded citrus peel slurry was pressed. The pressed solid was broken up by hand in the bucket until a uniform damp meal was obtained. The damp meal was then transferred to trays covered with non-stick aluminum foil (Reynolds Wrap, Reynolds Consumer Products, Lake Forest, IL or Kingsford Heavy Duty, The Clorox Company, Oakland, CA). Several trays were stacked upon one another and separated by wood blocks in between to permit air flow in the hood for 3–6 days. Samples where then transferred to a 30 °C oven for further drying for 2–5 days.
2.4 Size reduction
STEX, untreated citrus peel, and a commercial citrus peel product (CitraFiber, Citrus Extracts, LLC, Ft. Pierce, FL) were subjected to size reduction using a Robot-Coupe R2, 3 Quart (Robot-Coupe, Ridgeland, MS) for approximately 3–5 minutes. In some cases, a coffee grinder was used to further size reduce the samples (Cuisinart DCG-12BC coffee grinder, Stamford, CT, USA). The size reduced material was then passed through a 100 mesh sieve (Advantech No. 100, W.S. Tyler Co., Mentor, OH) so that only particles of 100 mesh or smaller were collected for further analysis. This was repeated until the desired amount of <100 mesh fiber was collected.2.5 Dry weight determination
Total dry weight was determined gravimetrically by oven-drying samples at 70 °C for a minimum of 24 h, followed by 1 h at 70–75 °C under a vacuum on single samples. Dry weights were completed in triplicate.2.6 Compositional sugar and galacturonic acid analysis
Compositional sugar analysis was conducted as before69 with some modifications. Specifically, dry <100 mesh citrus fiber was rehydrated to 2.5 g based on initial sample dry weight. That was then further diluted using 8.75 mL of enzyme solution (enzymes and DI) and 1.25 mL sodium acetate buffer (50 mmol L−1, pH 4.8). The enzymes used in solution were 500 μL each of pectinase (pectinase from Aspergillus aculeatus, aqueous solution, ≥3800 polygalacturonic units per mL, Product# P2611, Sigma-Aldrich, St. Louis, MO) and cellulase (cellulase, enzyme blend, ≥1000 hydrolysis units per gram, Product# SAE020, Sigma-Aldrich, St. Louis, MO). Cycloheximide (5 mg mL−1 stock) and chloramphenicol (10 mg mL−1 stock) were added in a volume of 37 μL each to prevent microbial growth. Samples were then vortexed (Fisher Mini Shaker Model 58, Fisher Scientific Co., Waltham, MA, USA) and rotated (New Brunswick Model TC-8, New Brunswick Scientific Co., Inc., Edison, NJ, USA) for 24 hours at 45 °C. Samples were then passed through a 0.45 μm GD/X Nylon syringe filter (Whatman) to remove insoluble solids prior to analysis for sugars (rhamnose, arabinose, galactose, glucose, xylose, fructose, sucrose) and galacturonic acid.2.7 Soluble sugar and galacturonic acid analysis
Soluble sugar analysis was conducted as before69 with some modifications. Specifically, dry <100 mesh citrus fiber was rehydrated to 2.5 g based on initial sample dry weight. That was then further diluted using 7.5 mL of deionized water to a final total volume of 10 mL. Insoluble solids were removed by filtration, using a 0.45 μm GD/X Nylon syringe filter (Whatman). Samples were analyzed for sugars (rhamnose, arabinose, galactose, glucose, xylose, fructose, sucrose) and galacturonic acid.2.8 Particle size
Particle size distribution was measured by laser diffraction technology using a CILAS 990 Particle Size Analyzer70 (CILAS S.A., Orléans, France), ranging from 0.20 to 500 μm, using water as a dispersing medium (refractive index 1.33) and ultrasound for dispersion of clusters. A dispersion of 0.025% of <100 mesh orange peel fiber in water was made by adding 0.2 g peel powder into 800 mL distilled water, then stirring on a magnetic stirrer for 30 min at room temperature. The dispersion was sonicated for 60 s (the sonication was conducted also by CILAS 990 Particle Size Analyzer) before measurement. The measurement was conducted at room temperature (25 °C). Distilled water was used as a blank for the measurement.2.9 Color
Color was measured with a chroma meter (model CR-400, Konica Minolta, Tokyo, Japan) used as per the manufacturer's instructions. Three L*, a*, and b* measurements were recorded per sample on evenly distributed fiber powder in a 60 × 15 mm plastic culture dish that was shrouded from ambient light. The instrument measured the sample in an open Petri dish to avoid light refraction. Color data software CM-S100w SpectraMagic™ NX (Professional/Lite, Ver. 3.2, Konica Minolta, Inc., Tokyo, Japan)71,72 was used to visualize the data by generating the target sample data as the origin on the axis and d as the “distance” other sample measurements are from the target. Hue angle was calculated from a* and b* values [h_ = arctan(b*/a*)].732.10 Water retention capacity (WRC)
Water retention capacity (WRC) of citrus fiber samples was measured according to the method by Robertson et al. with some modification.74 Specifically, 1.000 g of each citrus fiber sample was weighed (Adventurer Pro AV114C, Ohaus Corporation, Parsippany, NJ) into a dry 50 mL centrifuge tube (Nalgene ® Oak Ridge Style 3119-0050, Thermofisher Scientific, Waltham, MA). This was done in triplicate for each sample type. To prevent microbial growth, 30 mL of 0.02% lithium azide solution (0.02% made using lithium azide 20% by weight in water, 480![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525, Millipore Sigma, St. Louis, MO) was added to each tube. Tubes were capped and gently vortexed (Fisher mini shaker model 58, Thermofisher Scientific, Waltham, MA) to dislodge and disperse the sample from the bottom of the tube. Samples were then left at room temperature (∼22 °C) for 18 hours.
525, Millipore Sigma, St. Louis, MO) was added to each tube. Tubes were capped and gently vortexed (Fisher mini shaker model 58, Thermofisher Scientific, Waltham, MA) to dislodge and disperse the sample from the bottom of the tube. Samples were then left at room temperature (∼22 °C) for 18 hours.
After 18 hours, samples were centrifuged at 3000×g, for 20 minutes at 20 °C (Beckman Coulter J-E, rotor JA-20, Beckman Coulter Life Sciences, Indianapolis, IN). Supernatant was decanted and tubes inverted for a few seconds. Leaving tubes inverted for more than a few seconds caused the pellet to start to slide resulting in possible loss of sample.
After inversion, weight of wet pellet and tube was recorded. Wet pellets and tubes were placed in a 70 °C oven (Binder ED115, Binder GmbH, Tuttlingen, Germany) for 5–7 days to ensure complete drying. Weight of dry pellet and tube was recorded. Tare weight of tube was subtracted from both total wet weight and total dry weight giving wet pellet weight and dry pellet weight, respectively. Weights of wet pellet and dry pellet were used in eqn (1).
 | (1) |
2.11 Water swelling capacity (WSC)
Water swelling capacity (WSC) of citrus fiber samples was measured according to the method by Robertson et al. with some modification.74 To prevent microbial growth, 5 mL of 0.02% lithium azide solution (0.02% made using lithium azide 20% by weight in water, 480![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525, Millipore Sigma, St. Louis, MO) was added to a dry glass 10 mL volumetric cylinder. Then, 100 mg of citrus fiber was weighed (Adventurer Pro AV114C, Ohaus Corporation, Parsippany, NJ and/or Spectrum Chemicals, SCA-314.C, New Brunswick, NJ) and added to the 10 mL volumetric cylinder. Finally, another 5 mL of the 0.02% lithium azide solution was added to the volumetric cylinder. The sample was gently dispersed to rehydrate the sample. The volumetric cylinder was covered and allowed to sit overnight (approximately 18 hours) at room temperature. After sitting overnight, the mixture was gently swirled to even out the bed volume, if needed. The volume occupied by the sample (bed volume) was recorded. This was done in triplicate for each sample type.
525, Millipore Sigma, St. Louis, MO) was added to a dry glass 10 mL volumetric cylinder. Then, 100 mg of citrus fiber was weighed (Adventurer Pro AV114C, Ohaus Corporation, Parsippany, NJ and/or Spectrum Chemicals, SCA-314.C, New Brunswick, NJ) and added to the 10 mL volumetric cylinder. Finally, another 5 mL of the 0.02% lithium azide solution was added to the volumetric cylinder. The sample was gently dispersed to rehydrate the sample. The volumetric cylinder was covered and allowed to sit overnight (approximately 18 hours) at room temperature. After sitting overnight, the mixture was gently swirled to even out the bed volume, if needed. The volume occupied by the sample (bed volume) was recorded. This was done in triplicate for each sample type.
The volume occupied by the sample after sitting overnight and the original sample dry weight were used in eqn (2).
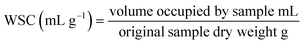 | (2) |
2.12 Oil retention capacity (ORC)
Oil Retention capacity (ORC) of citrus fiber samples was measured according to the method by Wang et al. with some modification.34 Specifically, 1.000 g of each citrus fiber sample was weighed (Spectrum Chemicals, SCA-314.C, New Brunswick, NJ and/or Adventurer Pro AV114C, Ohaus Corporation, Parsippany, NJ) into a dry 50 mL centrifuge tube (Nalgene ® Oak Ridge Style 3119-0050, Thermofisher Scientific, Waltham, MA). Then 10 mL of neutral oil (Wesson Vegetable Oil, Richardson, Memphis, TN) was added to the sample in the centrifuge tube. The sample and oil were mixed until the sample was rehydrated. The oil and sample mixture were allowed to sit for 1 hour at 4 °C and was then centrifuged (Beckman Coulter J-E, rotor JA-20, Beckman Coulter Life Sciences, Indianapolis, IN) at 7000×g for 15 minutes at 4 °C. The supernatant was decanted, and tubes inverted for a few seconds. Leaving tubes inverted for more than a few seconds caused the pellet to start to slide resulting in possible loss of sample.After inversion, weight of the hydrated pellet and tube was recorded. Tare weight of tube was subtracted from this value and both values were used in eqn (3). This was done in triplicate for each sample type. The average of all three measurements and their standard errors are reported.
 | (3) |
2.13 Fiber content
Total dietary fiber (TDF), soluble dietary fiber (SDF), and insoluble dietary fiber (IDF) were determined gravimetrically using the AOAC 991.43 method with some modifications by Medallion Labs (General Mills, Inc., Minneapolis, MN). Citrus fiber samples (<100 mesh) were digested with three enzymes (alpha-amylase, protease and amyloglucosidase) to remove starch and protein. Any solid particles not in solution after digestion are considered IDF and are filtered off and measured gravimetrically. The filtrate from this first filtration is collected and ethanol is added to precipitate the SDF. The soluble fraction is then filtered and measured gravimetrically. All residues are corrected for ash and protein content. TDF is calculated as the sum of the IDF and SDF. Results are reported in % dry weight.2.14 Peel oil
Peel oil was determined as described previously69 with some modifications. Briefly, approximately 2 grams of 100 mesh sample were weighed into a 250 mL round bottom flask. Samples were prepared in duplicate. Deionized water was added until 25 mL of liquid was achieved. An additional 25 mL deionized water and 25 mL of 2-propanol were then added. Then the sample was distilled as previously described.2.15 Statistical analyses
Statistical analysis was performed on compositional sugars (CS), compositional galacturonic acid (CGalA), soluble sugars (SS), Water Holding Capacity (WHC), Water Swelling Capacity (WSC), Oil Retention Capacity (ORC), soluble dietary fiber (SDF), insoluble dietary fiber (IDF), and total dietary fiber (TDF). One-way analysis of variance (ANOVA) was performed using XLSTAT (Addinsoft 1995–2024, New York). Normality distribution of the data was verified, and when not normal, ANOVA was performed using the log-transformed data. Means separation was performed using the Tukey HSD test with a confidence interval of 95%. Non-transformed data are presented in graphs and tables.A Principal Components Analysis (PCA) was also performed on the mean data. In a first analysis, particle size (PS) explained 96% of the variation on PC1 preventing visualization of the data in the remaining variable space. PS was therefore excluded from a second analysis, and those data are presented herein.
3 Results and discussion
While there are several patents on the use of steam treatment for the preparation of fiber from citrus peel, most do not report the effects on the physiochemical properties of the resulting fiber.36–59 There are only a couple of publications on the impact of STEX on the properties of the resulting dietary fiber from citrus peel.34,35 We did not come across any publications that directly compared the properties of a commercial citrus fiber product to citrus fiber produced by STEX. In STEX, raw material is placed in a confined space at high temperature and pressure. Saturated steam is introduced and fills the cell tissues of the raw material. After a specified amount of time, the pressure is released causing the steam filled tissue cells to burst leaving behind a surface structure of microporous cell walls. This allows the release of small molecular weight substances.753.1 Total compositional and soluble sugars
The sum of the average of triplicate measurements of rhamnose, arabinose, galactose, glucose, xylose, fructose, sucrose, and cellobiose are reported in Fig. 1. The total amount of compositional sugars are generally greater in the steam treated samples with higher temperatures and longer hold times, with the maximum amount of compositional sugars obtained at the 170 °C and 4 min hold time conditions followed by the 170 °C and 4 min hold time. This is because STEX destroys the structure of the feedstock and breaks down cellulose and hemicellulose75 that are then available for enzymatic hydrolysis. The opposing trend is observed for soluble sugars, with total soluble sugar content decreasing with increasing temperature and hold time (Fig. 2). The lowest soluble sugar content observed in STEX samples was in samples subjected to temperatures and time greater than 170 °C and 2 min, respectively. This is because the greater pressure, and therefore the greater temperature, is correlated with greater degradation in STEX.76 And so STEX disrupts the lignocellulosic complex exposing more of the hemicellulose and cellulose for enzymatic conversion to sugars, and soluble sugars already present in the material are degraded further. The commercial citrus fiber had the lowest soluble sugars content (Fig. 2).3.2 Compositional galacturonic acid
No galacturonic acid was found in any of the samples prepared for soluble sugar analysis (see ESI†). The maximum amount of compositional galacturonic acid was found in 130 °C 8 min hold time steam treated sample (34% dw) and was similar to that found in the commercial citrus fiber (33% dw) (Fig. 3). A lower galacturonic acid content comparable to the commercial lemon peel powder was observed in samples treated at 150 °C for 4 and 8 min, and samples treated at 170 °C for 1 and 2 min. There was a decrease in galacturonic acid in samples treated with higher temperatures (150 and 170 °C) and longer holding time (4 and 8 min). This is most likely due to thermal breakdown of galacturonic acid in these samples at higher temperatures and longer hold times which has been observed in our previous work.68 The galacturonic acid was significantly lower in all untreated samples as compared to samples treated at 130 °C, 150 °C and 170 °C for 1 and 2 minutes hold times. This is because STEX breaks down the cellulose and hemicellulose structure75 which allows for components such as pectin to be released. STEX samples treated at 170 °C for 4 and 8 minutes also had the lowest galacturonic acid.3.3 Particle size
The median sample diameter of the commercial citrus fiber and peel powders ranged between 33 and 79 μm (Fig. 4). A similar range of particle sizes were observed for all samples that were treated at 150 °C and 170 °C, as well as the 130 °C 8 min hold time. The steam treated sample with the smallest median sample diameter was the 150 °C 4 min hold time (38 μm) but this was still slightly greater than that of the commercial citrus fiber (33 μm). The untreated samples as well as samples treated at 130 °C for 1–4 min had median sample diameters above 100 μm. A reduction in particle size was observed for steam exploded pomelo peel and attributed to the degradation of cellulose or hemicellulose.35Earlier season untreated samples (December 2020–January 2021) has a much smaller particle size (100–150 μm) as compared to later season untreated samples (February–March 2021). These three untreated samples had the greatest particle sizes, above 300 μm.
3.4 Color
The commercial citrus fiber was established as the target for hue angle (da*) and lightness (dL*) measurements in Fig. 5 and is identified as the green dot with a pink outline at the center of the axis for both color properties. The sample treated with steam at 130 °C for 2 min exhibited the closest Hue angle to the commercial citrus fiber (Fig. 5A). Conversely, the sample treated at 170 °C for 4 min was the furthest in hue angle from the commercial citrus fiber sample (Fig. 5B). This color change is primarily due to reactions at higher temperatures that lead to browning. For example, steam-exploded okra seed flour was explored for developing GF cookies.77 It was observed that STEX led to the production of more browning compounds, like pigments and reducing sugars as a result of the Maillard reaction.77 In pomelo peel, the brown color is attributed to the caramelization of hemicellulose.35 As a result, the untreated sample HV-UNT-2-19-21, was the sample closest in lightness to the commercial citrus fiber (Fig. 5C). The sample treated with steam at 170 °C for 8 min was the darkest (Fig. 5D).3.5 Fiber content
The percent insoluble dietary fiber (%IDF), percent soluble dietary fiber (%SDF), percent total dietary fiber (%TDF), and the soluble dietary fiber to insoluble dietary fiber ratio (SDF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IDF) are reported in Table 1. SDF includes pectin, while IDF includes cellulose, hemicellulose and lignin.35 Steam treatment resulted in an increase in %SDF and %TDF. However, %SDF began to decrease and %IDF began to increase in steam treated samples at 150 °C for 2–8 min and 170 °C for 1–8 min. Despite this, %SDF remained higher at the highest temperature and longest hold time compared to untreated samples. This same phenomenon is observed in steam treatment of pomelo peel35 and in grain and oil processing products and has been shown to be attributed to the pressure and independent of hold time.78 STEX destroys the structure of the feedstock and breaks down cellulose and hemicellulose which promotes SDF liberation.75 Samples treated at 150 °C for 4–8 min as well as 170 °C for 1–2 min produced samples with %SDF similar to that of the commercial citrus fiber sample. The maximum %TDF was observed for the sample treated at 170 °C for 8 min and was similar to that of the commercial citrus fiber. The sample that was most similar in %IDF, %SDF, and %TDF to the commercial citrus fiber was the sample treated with steam at 170 °C for 4 min. However, the SDF
IDF) are reported in Table 1. SDF includes pectin, while IDF includes cellulose, hemicellulose and lignin.35 Steam treatment resulted in an increase in %SDF and %TDF. However, %SDF began to decrease and %IDF began to increase in steam treated samples at 150 °C for 2–8 min and 170 °C for 1–8 min. Despite this, %SDF remained higher at the highest temperature and longest hold time compared to untreated samples. This same phenomenon is observed in steam treatment of pomelo peel35 and in grain and oil processing products and has been shown to be attributed to the pressure and independent of hold time.78 STEX destroys the structure of the feedstock and breaks down cellulose and hemicellulose which promotes SDF liberation.75 Samples treated at 150 °C for 4–8 min as well as 170 °C for 1–2 min produced samples with %SDF similar to that of the commercial citrus fiber sample. The maximum %TDF was observed for the sample treated at 170 °C for 8 min and was similar to that of the commercial citrus fiber. The sample that was most similar in %IDF, %SDF, and %TDF to the commercial citrus fiber was the sample treated with steam at 170 °C for 4 min. However, the SDF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IDF was less (0.32) than that of commercial citrus fiber (0.48). An SDF
IDF was less (0.32) than that of commercial citrus fiber (0.48). An SDF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IDF of 0.33 to 0.50 is what distinguishes a product as a good source of fiber.79 Of the samples in this study, only the commercial citrus fiber and lemon peel powder fell within that range.
IDF of 0.33 to 0.50 is what distinguishes a product as a good source of fiber.79 Of the samples in this study, only the commercial citrus fiber and lemon peel powder fell within that range.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IDF Ratio) of commercial (COM) citrus fiber, orange peel powder, and lemon peel powder as well as untreated (UNT) and steam exploded (STEX) Hamlin (H) and Valencia (V) citrus juice processing remnants. STEX samples were treated at 130, 150, and 170 °C for 1, 2, 4, and 8 minutes. The conditions for each STEX experiment are given in parenthesis followed by the date. UNT and STEX samples were obtained from a local processor from December 2020 to March 2021
IDF Ratio) of commercial (COM) citrus fiber, orange peel powder, and lemon peel powder as well as untreated (UNT) and steam exploded (STEX) Hamlin (H) and Valencia (V) citrus juice processing remnants. STEX samples were treated at 130, 150, and 170 °C for 1, 2, 4, and 8 minutes. The conditions for each STEX experiment are given in parenthesis followed by the date. UNT and STEX samples were obtained from a local processor from December 2020 to March 2021
| Sample | % IDF | % SDF | % TDF | SDF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IDF ratio IDF ratio |
|---|---|---|---|---|
| H-UNT-12-11-20 | 42.40 | 9.10 | 51.50 | 0.21 |
| H-UNT-1-12-21 | 43.10 | 8.20 | 51.30 | 0.19 |
| H-UNT-1-22-21 | 39.50 | 7.10 | 46.60 | 0.18 |
| H-UNT-2-3-21 | 40.00 | 8.30 | 48.30 | 0.21 |
| HV-UNT-2-19-21 | 45.10 | 7.10 | 52.20 | 0.16 |
| V-UNT-3-5-21 | 40.20 | 8.90 | 49.10 | 0.22 |
| H-STEX_130-1_2-3-21 | 41.10 | 34.20 | 75.30 | 0.83 |
| H-STEX_130-2_1-22-21 | 43.30 | 33.40 | 76.70 | 0.77 |
| H-STEX_130-4_2-3-21 | 38.40 | 36.20 | 74.60 | 0.94 |
| V-STEX_130-8_3-5-21 | 39.70 | 36.30 | 76.00 | 0.91 |
| V-STEX_150-1_3-5-21 | 38.70 | 37.30 | 76.00 | 0.96 |
| H-STEX_150-2_1-12-21 | 41.00 | 34.70 | 75.70 | 0.85 |
| H-STEX_150-4_12-11-20 | 48.10 | 26.00 | 74.10 | 0.54 |
| HV-STEX_150-8_2-19-21 | 47.00 | 26.30 | 73.30 | 0.56 |
| H-STEX_170-1_12-11-20 | 46.00 | 26.80 | 72.80 | 0.58 |
| HV-STEX_170-2_2-19-21 | 46.40 | 26.60 | 73.00 | 0.57 |
| H-STEX_170-4_1-22-21 | 55.60 | 17.60 | 73.20 | 0.32 |
| H-STEX_170-8_1-12-21 | 68.90 | 12.10 | 81.00 | 0.18 |
| COM-citrus fiber | 54.30 | 26.10 | 80.40 | 0.48 |
| COM-orange peel powder | 41.00 | 10.60 | 51.60 | 0.26 |
| COM-lemon peel powder | 39.70 | 18.10 | 57.80 | 0.46 |
3.6 Water retention, water swelling, and oil retention capacities
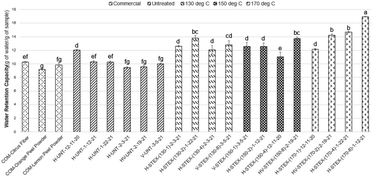
Fig. 6 illustrates that the water retention capacity of steam treated samples at high temperatures was improved compared to untreated and commercial samples. Water retention capacity increased as temperature and hold time increased with a maximum of 16.9 g g−1 observed in the steam treated sample at 170 °C for 8 min. Water swelling capacity was above 20 mL g−1 for steam treated samples at 130 °C for 1–4 min (Fig. 7), whereas commercial and untreated samples were between 10 and 15 mL g−1. While water swelling capacity decreased at 130 °C for 8 min and for all 150 °C treatments, the opposing trend was observed once samples were treated at 170 °C, with water swelling capacity increasing with increasing hold time. Untreated and steam treated samples at 130 °C for 1–2 min and 170 °C for 2–8 min had a greater oil retention capacity as compared to the commercial fiber and powders, with untreated samples exhibiting the greatest oil retention capacity among the samples in this study (Fig. 8).STEX produces a porous and honeycomb-like structure and impacts the physiochemical properties, especially water retention and swelling capacities.78 In steam exploded pomelo peel, the internal structure became looser and hydrophilic groups were exposed by high-speed shear force resulting in enhanced hydration properties.35 In STEX and acid treated sweet orange peel, a large surface area was observed which allowed for more space for holding water molecules and hydrogen bonding and/or dipole formation.34 However, excessive STEX has been observed to reduce the oil-holding capacity in grape pomace.75
3.7 Principal components analysis (PCA)
A PCA of carbohydrate and fiber data showed a clear separation of all untreated peels and commercial powders (orange peel and lemon peel), located on the negative side of PC1 (explaining 61.85% of the variation) and away from all STEX-treated samples and commercial orange fiber all located on the positive side of PC1 (Fig. 9). PC1 was explained by SDF and TDF, with eigenvector values greater than 0.5 (data not shown). SDF and TDF were strongly negatively correlated with SS (r = −0.629 and −0.824, respectively). This is because SS corresponds to free monosaccharides present in the samples that are readily soluble in water. SDF and TDF correspond to polysaccharides that do not breakdown to their monomers, specifically monosaccharides, in the presence of water alone. PC 2, explaining 22.92% of the variation, was driven by IDF and CS on the positive side, and CGal and SDF on the negative side. CGalA and SDF had a strong positive correlation (r = 0.822), but CS and IDF did not (r = 0.411). The correlation between CGal and SDF is explained by the fact that CGalA is directly related to pectin content as GalA is a building block of pectin, which is a soluble dietary fiber. While CS and IDF appear to be correlated in Fig. 9, this is because the PCA is two-dimensional, and CS has a high value in the third dimension. CS was not strongly correlated with IDF, SDF, or TDF. This is notable because CS is obtained from the hydrolysis of cellulose, hemicellulose, and pectin to their corresponding monosaccharides using enzymes. This indicates that there is most likely a type of fiber present that is not hydrolyzed by the enzyme mixtures used in this study. STEX treated samples at 170 °C for 4 and 8 min had the highest IDF and CS. Samples treated at 170 and 150 °C for 1 and 2, and 4 and 8 min, respectively, had fiber content (TDF) closest to the commercial citrus fiber. WRC, WSC and ORC contributed little to the overall variation, with only relevant eigenvector values in PC6, PC7 and PC8.3.8 Oil
The oil collected from citrus fruit comes from the peel and is made up of mostly D-limonene and is typically extracted before, during, and/or after juicing.80 The amount of oil in orange juice remnants, like those used in this study, is dependent on the process used to remove the oil as well as the juicing process. Specifically, the force used to squeeze the oranges to extract their juice will affect how much oil is released from the peel. Table 2 shows the oil content of the treated and untreated orange juice remnants from this study as well as the commercial citrus fiber and orange and lemon peel powders. The samples have been grouped based on the collection date and variety. The untreated samples (highlighted in gray) are relatively consistent at about 0.19% v/dw except for late season Hamlin (H-UNT-2-3-21) and early season Valencia (V-UNT-3-5-21). We can also see that the untreated samples have a higher concentration of oil than the samples treated with steam. This is because volatilization and release of the oil are inherent to the STEX process. On a larger scale, these vapors can be condensed and collected. Since the oil and water don't mix, the oil can be separated from condensed steam.| % v/dw oil | |
|---|---|
| H-UNT-12-11-20 | 0.0192 ± 0.000271 |
| H-STEX-(150-4)-12-11-20 | 0.0144 ± 0.000102 |
| H-STEX-(170-1)-12-11-20 | 0.0144 ± 0 |
| H-UNT-1-12-21 | 0.0193 ± 0 |
| H-STEX-(150-2)-1-12-21 | 0.0082 ± 0.0000583 |
| H-STEX-(170-8)-1-12-21 | 0.0144 ± 0 |
| H-UNT-1-22-21 | 0.0192 ± 0 |
| H-STEX-(130-2)-1-22-21 | 0.0204 ± 0.00177 |
| H-STEX-(170-4)-1-22-21 | 0.0134 ± 0 |
| H-UNT-2-3-21 | 0.0347 ± 0.000371 |
| H-STEX-(130-1)-2-3-21 | 0.0297 ± 0 |
| H-STEX-(130-4)-2-3-21 | 0.0179 ± 0.00169 |
| HV-UNT-2-19-21 | 0.0191 ± 0.000201 |
| HV-STEX-(150-8)-2-19-21 | 0.0166 ± 0.003184 |
| HV-STEX-(170-2)-2-19-21 | 0.0150 ± 0 |
| V-UNT-3-5-21 | 0.0286 ± 0.000300 |
| V-STEX-(130-8)-3-5-21 | 0.0160 ± 0.000223 |
| V-STEX-(150-1)-3-5-21 | 0.0160 ± 0.00157 |
| COM-citrus fiber | 0.0263 ± 0 |
| COM-orange peel powder | 0.0576 ± 0.000204 |
| COM-lemon peel powder | 0.0228 ± 0.00164 |
3.9 Potential value
Most orange juice processing plants convert their by-products into value-added products like animal feed pellets, silage, flake, molasses, and peel oil, primarily composed of D-limonene. Table 3 shows the typical values of these products from a Florida orange juice feed mill as well as the range of values for a citrus fiber product produced in Florida. Citrus fiber is 74–86% more valuable than all the traditional feed mill products on a US$ per kg basis combined. Given that both use similar processes and equipment, it is clear that commercial citrus fiber offers a better return on investment. STEX can be easily integrated into current citrus juice feed mill operations, allowing for control over processing conditions and the properties of the final peel fiber product. Finally, the highest value product from the orange juice processing feed mill is oil. By using STEX to vaporize, condense, and isolate any remaining oil in the peel, plants can extract another high-value product alongside the fiber.| US$ per kg | |
|---|---|
| Pellets/silage/flake | 0.18–0.30 |
| Molasses | 0.22–0.28 |
| Oil | 2.76–3.86 |
| Total feed mill | 3.16–4.44 |
| Commercial citrus fiber | 5.51–8.27 |
4 Conclusions
In this work we compared the properties of citrus fiber produced using a novel steam explosion process (STEX) to that of commercial citrus fiber and powder products. Samples treated at 150 °C for 4 and 8 min and at 170 °C for 1 and 2 min had similar %TDF to that of the commercial citrus fiber. Increasing the hold time (4 and 8 min) for samples treated at 170 °C increased their compositional sugars and insoluble dietary fiber, while decreasing temperature (150 °C for 1 and 2 min) or 130 °C increased soluble dietary fiber and galacturonic acid. Ultimately, desirable fiber properties did not lie with a single hold time and temperature treatment. The rheological properties of the commercial citrus fiber and powder products or the STEX citrus fibers were not investigated. This is another important property of food fibers that needs to be studied to properly determine final application and value. Future studies on the rheological properties of STEX citrus fibers and commercial citrus fiber and powders need to be completed. An estimate on the economic value of producing citrus fiber instead of current feed mill products show that there is potential for a greater profit to be obtained by processors.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Christina Dorado: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing – original draft, writing – review & editing. Wei Zhao: data curation, formal analysis, investigation, methodology, validation, writing – review & editing. Dave Wood: data curation, formal analysis, visualization, writing – review & editing. Anne Plotto: data curation, formal analysis, validation, writing – review & editing.Conflicts of interest
There are no conflicts to declare. The findings and conclusions in this publication are those of the author(s) and should not be construed to represent any official USDA or U.S. Government determination or policy. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.Acknowledgements
This research was supported by the U.S. Department of Agriculture, Agricultural Research Service under project number 6034-41000-018-00D. The authors would like to thank Sandra Matlack and PeiLing Li for the technical support and Briseida Juarez for conducting a patent search.In accordance with Federal civil rights law and U.S. Department of Agriculture (USDA) civil rights regulations and policies, the USDA, its Agencies, offices, and employees, and institutions participating in or administering USDA programs are prohibited from discriminating based on race, color, national origin, religion, sex, disability, age, marital status, family/parental status, income derived from a public assistance program, political beliefs, or reprisal or retaliation for prior civil rights activity, in any program or activity conducted or funded by USDA (not all bases apply to all programs). Remedies and complaint filing deadlines vary by program or incident. Persons with disabilities who require alternative means of communication for program information (e.g., Braille, large print, audiotape, American Sign Language, etc.) should contact the responsible Agency or USDA's TARGET Center at (202) 720-2600 (voice and TTY) or contact USDA through the Federal Relay Service at (800) 877-8339. Additionally, program information may be made available in languages other than English. To file a program discrimination complaint, complete the USDA Program Discrimination Complaint Form, AD-3027, found online at How to File a Program Discrimination Complaint and at any USDA office or write a letter addressed to USDA and provide in the letter all of the information requested in the form. To request a copy of the complaint form, call (866) 632-9992. Submit your completed form or letter to USDA by: (1) mail: U.S. Department of Agriculture, Office of the Assistant Secretary for Civil Rights, 1400 Independence Avenue, SW, Washington, D.C. 20250-9410; (2) fax: (202) 690-7442; or (3) email: E-mail: program.intake@usda.gov. USDA is an equal opportunity provider, employer, and lender.
References
- Markets and Markets, Gluten-free Products Market by Type, 2021, https://www.marketsandmarkets.com/Market-Reports/gluten-free-products-market-738.html, accessed 21 December 2021.
- Globe Newswire, Citrus Fiber Valued at US$92 Million in 2018, Bakery Industry Driving Growth, Fact.MR., 2018, https://www.globenewswire.com/news-release/2018/11/09/1648943/0/en/Citrus-Fiber-Valued-at-US-92-Million-in-2018-Bakery-Industry-Driving-Growth.html, accessed 16 April 2019.
- Fact.MR, Citrus Fiber Market Forecast, Trend Analysis & Competition Tracking – Global Market Insights 2018-2028, 2018, https://www.factmr.com/report/2305/citrus-fiber-market, accessed 16 April 2019.
- R. Clemens and P. Pressman, Nutr. Today, 2017, 5(1), 258–260 CrossRef.
- National Agricultural Statistics Service, 2023 State Agriculture Overview, 2025, https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=FLORIDA, accessed January 28, 2025.
- Visit Florida, Facts About Florida Oranges & Citrus, 2021, https://www.visitflorida.com/travel-ideas/articles/eat-drink-facts-about-florida-citrus-oranges/, accessed December 22, 2021.
- Florida Department of Agriculture and Consumer Services & National Agricultural Statistics Service, Florida Citrus Statistics 2019-2020, 2021, https://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Citrus/Citrus_Statistics/index.php, accessed December 22, 2021.
- R. J. Braddock, Handbook of Citrus By-Products and Processing Technology, John Wiley & Sons, New York, 1999 Search PubMed.
- K. Grohmann, R. G. Cameron and B. S. Buslig, Bioresour. Technol., 1995, 54, 129–141, DOI:10.1016/0960-8524(95)00121-2.
- W. Widmer, W. Zhou and K. Grohmann, Bioresour. Technol., 2010, 101, 5242–5249, DOI:10.1016/j.biortech.2009.12.038.
- Alibaba, Citrus Dietary Fiber, 2021, https://www.alibaba.com/showroom/citrus-dietary-fiber_1.html accessed 21 December 2021.
- H. Chen, Gas Explosion Technology and Biomass Refinery, Springer & Chemical Industry Press, Beijing, 2015 Search PubMed.
- N. Jacquet, G. Maniet, C. Canderghem, F. Delvigne and A. Richel, Ind. Eng. Chem. Res., 2015, 54, 2593–2598, DOI:10.1021/ie503151g.
- G. Brodeur, E. Yau, K. Badal, J. Collier, K. B. Ramachandran and S. Ramakrishnan, Enzyme Res., 2011, 17, DOI:10.4061/2011/787532.
- J. Singh, M. Suhag and A. Dhaka, Carbohydr. Polym., 2015, 117, 624–631, DOI:10.1016/j.carbpol.2014.10.012.
- I. Kellersztein, U. Shani, I. Zilber and A. Dotan, Polym. Compos., 2019, 40, E53–E61, DOI:10.1002/pc.24472.
- B. Deepa, E. Abraham, B. M. Cherian, A. Bismarck, J. J. Blaker, L. A. Pothan, A. L. Leao, S. F. de Souza and M. Kottaisamy, Bioresour. Technol., 2011, 102(2), 1988–1997, DOI:10.1016/j.biortech.2010.09.030.
- X. Zhang, G. Han, W. Jiang, Y. Zhang, X. Li and M. Li, BioResources, 2016, 11(3), 6590–6599, DOI:10.15376/BIORES.11.3.6590-6599.
- Z. Dong, X. Hou, F. Sun, L. Zhang and Y. Yang, Cellulose, 2014, 21(5), 3851–3860, DOI:10.1007/s10570-014-0401-5.
- B. Li, W. Yang, Y. Nie, F. Kang, H. D. Goff and W. F. Cui, Food Hydrocoll., 2019, 94, 48–56, DOI:10.1016/j.foodhyd.2019.02.042.
- J. Y. Jung, J. M. Heo and J.-K. Yang, BioResources, 2019, 14(1), 1512–1524, DOI:10.15376/biores.14.1.1512-1524.
- W. Sui, X. Xie, R. Liu, T. Wu and M. Zhang, Food Hydrocoll., 2018, 84, 571–580, DOI:10.1016/j.foodhyd.2018.06.027.
- J. Yan, J. Hu, R. Yang and W. Zhao, Int. J. Food Sci. Technol., 2018, 53(10), 2394–2404, DOI:10.1111/ijfs.13832.
- M. Cheikh Rouhou, S. Abdelmoumen, S. Thomas, H. Attia and D. Ghorbel, Int. J. Biol. Macromol., 2018, 116, 901–910, DOI:10.1016/j.ijbiomac.2018.05.090.
- W. Liu, H. Ma, J. Luo and H. Yao, J. Chin. Inst. Food Sci. Technol., 2018, 18(5), 58–67, DOI:10.16429/j.1009-7848.2018.05.008.
- J. Yan, J. Hu, R. Yang, Z. Zhang and W. Zhao, ACS Sustainable Chem. Eng., 2018, 6(3), 3482–3492, DOI:10.1021/acssuschemeng.7b03765.
- Y. Hu, L. Zheng, B. Ai, X. Zheng, J. Yang and Z. Sheng, J. Chin. Inst. Food Sci. Technol., 2018, 18(2), 134–140 Search PubMed.
- X. Liang, J. Ran, J. Sun, T. Wang, Z. Jiao, H. He and M. Zhu, CyTA–J. Food, 2018, 1, 20–26, DOI:10.1080/19476337.2017.1333158.
- T. Wang, X. Liang, J. Ran, J. Sun, Z. Jiao and H. Mo, Int. J. Food Sci. Technol., 2017, 52(3), 741–747, DOI:10.1111/ijfs.13329.
- A. Mrabet, G. Rodríguez-Gutiérrez, R. Guillén-Bejarano, R. Rodríguez-Arcos, A. Ferchichi, M. Sindic and A. Jiménez-Araujo, LWT–Food Sci. Technol., 2015, 60(1), 518–524, DOI:10.1016/j.lwt.2014.09.055.
- G. Rodríguez-Gutiérrez, F. Rubio-Senent, A. Lama-Muñoz, A. García and J. Fernández-Bolaños, J. Agric. Food Chem., 2014, 62(36), 8973–8981, DOI:10.1021/jf502062b.
- L. W. He, Q. X. Meng, D. Y. Li, Y. W. Zhang and L. P. Ren, Br. Poult. Sci., 2015, 56(1), 88–93, DOI:10.1080/00071668.2014.981503.
- J.-S. Lu, C.-M. Wei, J.-M. Xu, H. Zhu and Z. K. Xie, Chin. J. Anal. Chem., 2007, 35(3), 443–446 CAS.
- L. Wang, H. Xu, F. Yuan, R. Fan and Y. Gao, Food Chem., 2015, 185, 90–98, DOI:10.1016/j.foodchem.2015.03.112.
- Z. Yuan, J. Yan, Q. Zhang, J. Zheng and A. Hu, Int. J. Food Eng., 2023, 19(10), 457–465, DOI:10.1515/ijfe-2023-0099.
- M. K. Weibel, Parenchymal cell cellulose and related materials, US Pat., 4831127, 1989 Search PubMed.
- E. W. Sample, System and method for continuous steam injected citrus peel cellular expansion, US Pat., 8017171, 2011 Search PubMed.
- E. W. Sample, System and method for continuous citrus peel cellular expansion. US Pat., 8383186, 2013 Search PubMed.
- E. W. Sample, System and method for continuous citrus peel cellular expansion, US Pat., 9192182, 2015 Search PubMed.
- J. H. Hansen, W. D. Henrickson, H. L. Pedersen, T. E. Pedersen and J. A. Staunstrup, Activated Pectin-Containing Biomass Compositions, Products, and Methods of Producing, US Pat., 20200046008A1, 2020 Search PubMed.
- J. H. Hansen, W. D. Henrickson, H. L. Pedersen, T. E. Pedersen and J. A. Staunstrup, Activated Pectin-Containing Biomass Compositions, Products, Methods Of Producing, US Pat., 20230085193A1, 2023 Search PubMed.
- J. G. E. Van Leeuwen and L. E. Bevers, Polypeptides Having Cellulolytic Enhancing Activity And Uses Thereof, US Pat., 20190345461 A1, 2019 Search PubMed.
- J. G. E. Van Leeuwen and L. E. Bevers, Polypeptides Having Cellulolytic Enhancing Activity And Uses Thereof. US Pat., 10913938B2, 2021 Search PubMed.
- J. G. E. Van Leeuwen and L. E. Bevers, Polypeptides Having Cellulolytic Enhancing Activity And Uses Thereof, US Pat., 20210123030 A1, 2021 Search PubMed.
- H. J. Wijma, D. B. Janssen and J. G. E. Van Leeuwen, Beta-Glucosidase And Uses Thereof, US Pat., 20190002860A1, 2019 Search PubMed.
- J. G. E. Van Leeuwen, Beta-Glucosidase And Uses Thereof, US Pat., 20180362945A1, 2018 Search PubMed.
- J. G. E. Van Leeuwen, Beta-Glucosidase And Uses Thereof, US Pat., 10808235B2, 2020 Search PubMed.
- J. G. E. Van Leeuwen, Beta-Glucosidase And Uses Thereof, US Pat., 20200392475A1, 2020 Search PubMed.
- J. A. Staunstrup, C. Klit, J. E. Trudsø, T. E. Pedersen and D. F. Hiscock, Activated Pectin-Containing Biomass Compositions, Products, And Methods Of Producing, US Pat., 20180230242A1, 2018 Search PubMed.
- J. A. Staunstrup, C. Klit, J. E. Turdsø, T. E. Pedersen and D. F. Hiscock, Activated Pectin-Containing Biomass Compositions, Products, and Methods Of Producing, US Pat., 20190112396A1, 2019 Search PubMed.
- J. A. Staunstrup, C. Klit, J. E. Turdsø, T. E. Pedersen and D. F. Hiscock, Methods Of Producing Activated Pectin-containing Biomass Compositions, US Pat., 10287366B2, 2019 Search PubMed.
- J. A. Staunstrup, C. Klit, J. E. Turdsø, T. E. Pedersen and D. F. Hiscock, Activated Pectin-containing Biomass Compositions And Products, US Pat., 11008407B2, 2021 Search PubMed.
- J. A. Staunstrup, C. Klit, J. E. Turdsø, T. E. Pedersen and D. F. Hiscock, Activated Pectin-Containing Biomass Compositions, Products, And Methods Of Producing, US Pat., 20210189016A1, 2021 Search PubMed.
- J. A. Staunstrup, C. Klit, J. E. Turdsø, T. E. Pedersen and D. F. Hiscock, Activated Pectin-Containing Biomass Compositions, Products, And Methods Of Producing, US Pat., 20230183387A1, 2023 Search PubMed.
- A. V. Gusakov, P. J. Punt, J. C. Verdoes, J. Van der Meij, A. P. Sinitsyn, E. Vlasenko, S. W. A. Hinz, M. Gosink and Z. Jiang, Novel Fungal Eneymes, US Pat., 20090280105A1, 2009 Search PubMed.
- A. V. Gusakov, P. J. Punt, J. C. Verdoes, J. Van der Meij, A. P. Sinitsyn, E. Vlasenko, S. W. A. Hinz, M. Gosink and Z. Jiang, Fungal Enzymes, US Pat., 7923236B2, 2011 Search PubMed.
- A. V. Gusakov, P. J. Punt, J. C. Verdoes, J. Van der Meij, A. P. Sinitsyn, E. Vlasenko, S. W. A. Hinz, M. Gosink and Z. Jiang, Novel Fungal Enzymes, US Pat., 20120030838A1, 2012 Search PubMed.
- A. V. Gusakov, P. J. Punt, J. C. Verdoes, J. Van der Meij, A. P. Sinitsyn, E. Vlasenko, S. W. A. Hinz, M. Gosink and Z. Jiang, Novel Fungal Enzymes, US Pat., 20120036599A1, 2012 Search PubMed.
- T. W. Gusek, L. Zullo and A. M. Eyal, Process For Obtaining Pectin, US Pat., 20090110798A1, 2009 Search PubMed.
- D. Agarwal, L. Hewson and T. J. Foster, Food Funct., 2018, 9, 1144–1151, 10.1039/C7FO01495C.
- R. Luque and J. H. Clark JH, Sustainable Chem. Processes, 2013, 1, 10, DOI:10.1186/2043-7129-1-10.
- R. G. Cameron, H. K. Chau and J. A. Manthey, J. Chem. Technol. Biotechnol., 2016, 91, 2597–2606, DOI:10.1002/jctb.4854.
- W. W. Widmer, J. A. Narciso, K. Grohmann and M. R. Wilkins, Biol. Eng. Trans., 2009, 2(1), 17–29, DOI:10.13031/2013.29174.
- R. G. Cameron, H. K. Chau, A. T. Hotchkiss and J. A. Manthey, Food Hydrocoll., 2017, 72, 52–61, DOI:10.1016/j.foodhyd.2017.05.025.
- R. G. Cameron, H. K. Chau, A. T. Hotchkiss and J. A. Manthey, J. Sci. Food Agric., 2017, 13, 4467–4475, DOI:10.1002/jsfa.8310.
- W. Zhou, W. Widmer and J. A. Manthey, Proc. Fla. State Hortic. Soc., 2008, 121, 307–310 Search PubMed.
- M. Wilkins, W. W. Widmer and K. Grohmann, Process Biochem., 2007, 42, 1614–1619, DOI:10.1016/j.procbio.2007.09.006.
- C. Dorado, R. G. Cameron, J. A. Manthey and K. F. Ferguson, Bench scale batch steam explosion of Florida red and white grapefruit juice processing residues, Future Foods, 2021, 3, 100020, DOI:10.1016/j.fufo.2021.100020.
- C. Dorado, R. G. Cameron, J. A. Manthey, J. Bai and K. L. Ferguson, Front. Nutr., 2021, 8, 691663, DOI:10.3389/fnut.2021.691663.
- R. G. Frizzo, A. Zaccaron, V. de Souza Nandi and A. M. Bernardin, Pyroplasticity on porcelain tiles of the albite-potassium feldspar-kaolin system: A mixture design analysis, J. Build. Eng., 2020, 31, 101432, DOI:10.1016/j.jobe.2020.101432.
- Color Data Software CM-S100w, SpectraMagic™ NX, Professional/Lite Ver. 2.8, Instruction Manual, Konica Minolta, Inc., 2011–2017 Search PubMed.
- Precise Color Communication, Color Control from Perception to Instrumentation, Konica Minolta Sensing, Inc., 2007 Search PubMed.
- F. M. Clydesdale, Colorimetry–methodology and applications, CRC Crit. Rev. Food Sci. Nutr., 1978, 10(3), 243–301, DOI:10.1080/10408397809527252.
- J. A. Robertson, F. D. de Monredon, P. Dysseler, F. Guillon, R. Amado and J.-F. Thibault, Hydration Properties of Dietary Fibre and Resistant Starch: A European Collaborative Study, LWT–Food Sci. Technol., 2000, 33(2), 72–79, DOI:10.1006/fstl.1999.0595.
- C. Wang, M. Lin, Q. Yang, C. Fu and Z. Guo, Foods, 2023, 12(17), 3307, DOI:10.3390/foods12173307.
- W. Chen, S. Zhang, Y. Li, H. Wu, Q. Meng and Z. Zhou, J. Anim. Sci., 2019, 97(6), 2414–2423, DOI:10.1093/jas/skz127.
- L. Hu, J. Guo, X. Zhu, R. Liu, T. Wu, W. Sui and M. Zhang, Food Nutr. Sci., 2020, 8(8), 4409–4421, DOI:10.1002/fsn3.1739.
- X.-Y. Tian, J.-F. Liu, N.-N. Wu and B. Tan, Cereal Chem., 2024, 101, 437–449, DOI:10.1002/cche.10746.
- I. J. Iwassa, M. A. dos Santos Ribeiro, E. C. Meurer, L. Cardozo-Filho L, B. C. Bolanho and C. da Silva, J. Food Process. Eng., 2019, 42, e13060, DOI:10.1111/jfpe.13060.
- The orange book, Tetra Pak Processing Systems AB, ed. Ringbloom U., Lund, Sweden, 1st edn, 2004 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00286e |
| This journal is © The Royal Society of Chemistry 2025 |