Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Well exposed: exploring the chemical and microbial composition in well and municipal drinking waters in Iowa†
Jemima E.
Ohwobete
 a,
Drew E.
Latta
a,
Drew E.
Latta
 bc,
Adam R.
Hoffman
d,
Darrin A.
Thompson
bc,
Adam R.
Hoffman
d,
Darrin A.
Thompson
 ce,
Daniel W.
Gilles
g,
David M.
Cwiertny
ce,
Daniel W.
Gilles
g,
David M.
Cwiertny
 bcf and
Sarah
Haig
bcf and
Sarah
Haig
 *ah
*ah
aDepartment of Civil and Environmental Engineering, University of Pittsburgh, 707 Benedum Hall, 3700 OHara Street, Pittsburgh, PA 15261, USA. E-mail: sjhaig@pitt.edu
bDepartment of Civil & Environmental Engineering, University of Iowa, Iowa City, IA, USA
cCenter for Health Effects of Environmental Contamination, University of Iowa, Iowa City, IA, USA
dDepartment of Natural and Applied Sciences, University of Dubuque, Dubuque, IA, USA
eDepartment of Occupational and Environmental Health, University of Iowa, Iowa City, IA, USA
fDepartment of Chemistry, University of Iowa, Iowa City, IA, USA
gDepartment of Hydroscience and Engineering, University of Iowa, Iowa City, IA, USA
hDepartment of Environmental and Occupational Health, University of Pittsburgh, Pittsburgh, PA, USA
First published on 26th June 2025
Abstract
The Safe Drinking Water Act (SDWA) regulates water quality in public drinking water systems, leaving most individuals who obtain their drinking water from private wells unprotected by this legislation. Given that 15% of the U.S. population relies on unregulated, privately owned wells for their drinking water (well drinking water; WDW), there is an urgent need to assess whether WDW contains elevated levels of water quality constituents that could detrimentally affect human health. Additionally, the SDWA does not regulate many emerging microbial contaminants, including drinking water-associated pathogens that can infect immunocompromised individuals (DWPIs), which are part of the broader group of microbial contaminants estimated by the CDC to cause 7.15 million waterborne illnesses annually in the U.S. This study compared concentrations of 33 chemical parameters and the absolute abundance of two DWPIs in 20 well and 20 municipal drinking water (MDW) samples in northeast Iowa. Differences in microbial community structure were also assessed using 16S rRNA amplicon sequencing. Samples were collected from 11 municipal systems, and WDW samples were selected based on proximity to municipal service areas. WDW samples, on average, contained higher concentrations of most chemical contaminants and DWPIs, and exhibited twice the species richness of MDW samples. Among regulated chemicals, only nitrate exceeded the SDWA limit, and only in one WDW sample. At the microbiome level, WDW and MDW samples had distinct community compositions, with the specific aquifer supplying the water explaining the greatest variance in structure. These findings provide new insights into potential exposures among private well users.
Water impactThis study uncovered notable microbiome differences in rural Iowa drinking water, revealing that well water harbored twice the microbial diversity of municipal water. One well exceeded EPA nitrate thresholds, while others complied. Additionally, well water contained higher levels of most chemical contaminants, highlighting the need for improved monitoring and regulatory oversight of private wells to ensure safe drinking water. |
1. Introduction
In the United States, 45 million people—approximately 15% of the population—obtain their drinking water from privately owned, self-supplied wells,1,2 while the majority rely on public water systems regulated under the Safe Drinking Water Act (SDWA). These public systems include not only municipal utilities, but also systems operated by schools, businesses, and other facilities serving 25 or more people. In this study, all public water systems tested were municipally operated and are hereafter referred to as municipal drinking water (MDW). Unlike MDW, which undergoes physical and chemical treatment prior to consumption, well drinking water (WDW) is not regulated under the SDWA. This places a disproportionate responsibility on the rural, low-income, immigrant, and minority communities who typically rely on WDW.3,4 Many in these communities may not have access to the education or resources necessary to maintain water sanitation measures, making it an unexpected and costly burden to ensure that their well water source is potable.3,5 For example, studies examining well water quality in eastern Iowa revealed that 73% of participants lacked any water treatment system for their well water.3 Further analyses of these wells underscore their vulnerability, with contaminants such as fecal coliforms, nitrates, pesticides, neonicotinoids, and PFAS frequently detected, demonstrating the diverse inorganic, organic, and microbial pollutants that pose significant health risks to private well users.3,6,7The lack of treatment for most WDW raises significant concerns as aquifers, the primary water source for wells, can be negatively impacted by changes in land use, agricultural practices, malfunctioning septic systems, and the nearby operations of fracking and mining industries.4 Additionally, climate change-induced natural disasters, such as floods in the Midwest, pose a risk to these private wells by introducing a wide range of biological and chemical constituents8–10 that could negatively impact human health. Meanwhile, municipal drinking water systems, although regulated and treated, are increasingly burdened by aging infrastructure,11 lack of investment, and outdated regulations, as evidenced by the C-grade assigned to the U.S. drinking water infrastructure by the American Society of Civil Engineers.12 While the challenges differ—unregulated exposure in wells versus infrastructural failings in municipal systems—both pose significant risks to public health. This combination of insufficient infrastructure and a lack of updated regulatory oversight means that the full spectrum of contaminants potentially posing risks to public health in both municipal and well water are not being adequately addressed. Such research should not only encompass regulated chemical contaminants and fecal pathogens,13 but also address new and emerging contaminants that have been largely unaccounted for in previous studies.14–16
Considering these potential interactions between chemicals and the overall microbial community (microbiome) in DW, there is a notable lack of studies that comparatively assess these dynamics in MDW and WDW within the same geographical area.17,18 Additionally, there is a significant lack of regulation and knowledge regarding Drinking Water Pathogens that predominantly cause infection in Immunocompromised individuals (DWPIs). DWPIs, such as Legionella pneumophila and nontuberculous mycobacteria (NTM),16,19–24 contribute significantly to waterborne disease morbidity and mortality in the U.S., and result in annual direct healthcare costs of $3.3 billion1–3,25 which significantly outpaces the traditionally monitored fecal-borne pathogens.19–21,26 Literature has found that MDW systems and building plumbing supplied by these MDW plants are significant reservoirs of DWPIs.27–31 However, only one New Jersey study32 has compared the abundance of NTM in biofilm swabs taken from plumbing supplied by either WDW or MDW sources within the same geographic area. Despite the importance of this paper,32 it remains unknown if differences in water chemistry, DWPIs, and the wider microbiome exist in the water consumed by individuals supplied by WDW and MDW. Additionally, numerous studies have established connections between DWPIs and chemical water quality parameters,30,33–38 underscoring the importance of further understanding these relationships. Given these factors, there is an urgent need to explore the well water exposome—including both its chemical and microbial components—to improve public health decision-making through the provision of actionable information.
Although no governmental body has set specific regulatory limits on these DWPIs, the European Union (EU) recently added Legionella pneumophila and other emerging pathogens and contaminants of concern to their Drinking Water Directive.39 This mandates that all water systems in the EU, including those that serve well water, conduct routine monitoring, risk assessments, and implement control measures across member states.39 In the U.S., the Environmental Protection Agency (EPA) has added these DWPIs and emerging contaminants, such as lithium, to the 5th Unregulated Contaminant Monitoring Rule40,41 (UCMR 5). This rule requires monitoring across a representative number of distribution systems, primarily those serving more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people, as well as selected smaller municipalities. However, unlike the EU directive, the UCMR's scope does not extend to privately owned well water systems, leaving these users outside the regulatory framework. While the UCMR is a positive step toward addressing contaminants of concern, the current approach of the EU highlights ongoing gaps in U.S. regulations. These gaps, particularly regarding private well water users, underscore the need for comprehensive updates to the SDWA to ensure that all water sources, both municipal and private, are adequately monitored and protected.
000 people, as well as selected smaller municipalities. However, unlike the EU directive, the UCMR's scope does not extend to privately owned well water systems, leaving these users outside the regulatory framework. While the UCMR is a positive step toward addressing contaminants of concern, the current approach of the EU highlights ongoing gaps in U.S. regulations. These gaps, particularly regarding private well water users, underscore the need for comprehensive updates to the SDWA to ensure that all water sources, both municipal and private, are adequately monitored and protected.
Understanding the full exposome, which includes all the environmental exposures related to WDW consumed by American populations, is essential for informing public health decisions and policy.
This study addresses a critical gap by documenting biogeochemical interactions—specifically the relationships between chemical composition, microbial community structure, and the presence of DWPIs—across MDW and WDW sources within the same geographical area. By analyzing 20 samples from each source in Dubuque County, Iowa, this study characterizes how the absence of standard treatment in WDW may contribute to divergent microbial and chemical profiles. Aquifer information was also incorporated to capture the potential influence of underlying hydrogeology. We hypothesized that WDW would contain higher levels of target chemicals and DWPIs than MDW and that each water source would exhibit a distinct microbial community structure.
2. Methodology
2.1 Participant selection and sample collection
To assess disparities in water quality between municipal and well water sources, samples were collected from Dubuque County, Iowa, an area characterized by a mix of municipal water systems and a substantial number of private wells. This study focused on comparing water quality in wells located near, but outside of, municipal service areas—regions where extending distribution lines can be costly and often leaves rural residents reliant on self-supplied water. By selecting well and municipal samples in close geographic proximity, this study aimed to provide insight into how water quality may vary due to a home's location relative to city limits. Following IRB guidelines, participants were informed of their individual results if any chemical concentration in their water sample exceeded the SDWA standards.Drinking water samples were collected using standard protocols,42,43 which included the use of sterile gloves, collection in acid-washed bottles, and immediate placement on ice for transport. Samples were collected from faucets in 40 homes (Fig. 1), twenty of which received their drinking water from 11 different Iowa municipalities (MDW), and another twenty that used private wells (WDW). Participants were identified through local networks, referrals, and known well users in the region to ensure access to both municipal and private well households across a range of geographies. Prior to sampling, residents completed a brief survey on their water use practices, such as filtration methods, bottled water consumption, and tap water usage during cooking. Samples were collected between August 2021 and October 2021. Briefly, 1 liter of cold water was collected in a sterile Nalgene bottle after flushing the tap for 1 to 2 minutes. Two 100 mL aliquots were removed for water chemical quality and coliform analyses. The remaining 800 mL sample were filtered through 0.2 μm polycarbonate filters and shipped to the University of Pittsburgh on ice. The filters were then stored at −20 °C until further analysis. Appropriate field and filtration controls were also processed.
Source aquifer information was readily available for all MDW sources through either the Iowa Department of Natural Resources or the websites of each water provider. For private wells, we used publicly available information in the Iowa Department of Natural Resources Private Well Tracking System, with assistance from personnel at the Iowa Geological Survey, to make assignments of the aquifers from which WDW was sourced. Details of how aquifer assignments were made have been previously described.44 Across all sampling locations, MDW and WDW were sourced from the Cambrian–Ordovician, Silurian, Galena, or alluvial aquifers, with many municipal samples drawing water from a combination of two or more of these aquifers (Table S1†). The primary difference between the water sources is that MDW samples undergo treatment at a drinking water plant before consumption (Table S2†).
2.2 Consumer survey
All study participants were asked to complete an IRB-approved survey regarding their use of household water treatment. Surveys included questions about (1) the use of a water treatment system within the home, and if so, which type; (2) whether a water softener was installed, and if so, how frequently it was maintained; (3) the use of filters either attached to faucets or within pitchers, and if so, how frequently filters were changed; (4) use of bottled water instead of tap water; and (5) use of tap water in cooking and food preparation. Surveys were collected for all 40 study participants, 20 municipal and 20 well homes.2.3 Water quality analysis
Thirty-three water quality parameters were analyzed at the University of Iowa using standard methods45 (Table S3†). On-site data for temperature and pH were collected using a YSI multiparameter sonde (Yellow Spring Instruments, Yellow Springs, OH, USA). Although temperature and pH were measured in the field, these data were not consistently available across all samples and are therefore not reported in Table S3† to avoid presenting incomplete results.Total metal concentrations were evaluated using inductively coupled plasma mass spectrometry (NexION 300x, PerkinElmer, Waltham, MA for arsenic or Agilent 7900, Agilent Technologies, Santa Clara, CA for the remaining) after digestion in 2% nitric acid. Nitrate plus nitrite, total coliform, and E. coli analysis were performed at the State Hygienic Laboratory of Iowa. Nitrate plus nitrite (as N) was measured using EPA standard method 353.2, whereas total coliform and E. coli bacteria were determined using standard method 9223 B.
2.4 Digital droplet PCR and sequencing
DNA was extracted from the stored filters using the FastDNA Spin kit (MP Biomedicals, Solon, OH) following the procedure documented in Spencer-Williams et al.,38 and the resulting DNA was stored at −20 °C. The density of total bacteria, and drinking water-associated pathogens (DWPIs), including L. pneumophila and non-tuberculous mycobacteria (NTM), were determined using droplet digital PCR (ddPCR), targeting the 16S rRNA gene, the mip gene, and the atpE gene, respectively.46 ddPCR reactions were performed for all DNA samples, alongside negative controls (ddPCR negative controls, filtration controls, and extraction controls) and positive controls (gBlocks of the target amplicons, Integrated DNA Technologies, Inc., Coralville, IA, USA). Droplets were generated to a 20 μL reaction volume in a 96-well plate that was heat-sealed. PCR was then performed on the C1000 Touch™ Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) within 15 minutes of droplet generation. Plates were run on the droplet reader within 1 hour of PCR completion, and thresholds were set for each ddPCR assay using Quantasoft v1.0.596 (Table S3†).16S rRNA gene amplicon library preparation and sequencing were performed on all samples at Argonne National Laboratory following the Illumina Earth Microbiome Protocol.47 Samples were sequenced on an Illumina HiSeq2500, generating a total of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 301
301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 721 raw reads. Microbiome analysis was conducted using QIIME2, with quality filtering based on the method described by Bolyen et al.48 Reads were assigned to operational taxonomic units (OTUs) using a 97% cutoff and the closed reference OTU-picking protocol in QIIME2 (version 2020.2), utilizing both the Silva (version 132.5) and Greengenes (version 13.5) databases. Due to low sequencing read counts, nine samples were removed from the dataset, resulting in 16 WDW samples and 15 MDW samples.
721 raw reads. Microbiome analysis was conducted using QIIME2, with quality filtering based on the method described by Bolyen et al.48 Reads were assigned to operational taxonomic units (OTUs) using a 97% cutoff and the closed reference OTU-picking protocol in QIIME2 (version 2020.2), utilizing both the Silva (version 132.5) and Greengenes (version 13.5) databases. Due to low sequencing read counts, nine samples were removed from the dataset, resulting in 16 WDW samples and 15 MDW samples.
2.5 Functional analyses
To assess potential functional differences in the microbiome of MDW and WDW, operational taxonomic unit (OTU) matrices were processed using the online BugBase database for phenotype prediction.49 BugBase can predict the presence of various phenotypic traits in microbial communities by analyzing OTU data. The default BugBase analysis evaluates common traits for most prokaryotic organisms, including aerobic and anaerobic respiration, Gram-negative and Gram-positive delineation, pathogenic presence, and stress tolerance. Given the rural and highly agricultural nature of the sample locations, additional traits related to phosphorus, nitrogen, and sulfur metabolism were chosen from a BugBase-compatible Kyoto Encyclopedia of Genes and Genomes (KEGG) list and analyzed (Table S4†).2.6 Statistical analyses
Hellinger transformation was performed on the OTU tables generated to mitigate the effects of the zeros and low/rare taxa in the dataset prior to microbiome analysis.49 The transformed OTU data were then used to calculate pairwise dissimilarities between samples based on the Bray–Curtis dissimilarity index, with the resulting matrices examined for temporal and spatial patterns in the bacterial community structure by NMDS. Significant differences in the microbial community compositions were analyzed by both aquifer source and treatment type (MDW or WDW) by using nonparametric multivariate analysis of variance (MANOVA) using Adonis.Shannon diversity index, Chao's richness, Pielou's evenness, and rarefaction curves were calculated on rarefied samples at a 3% genetic distance. To examine the relationships between environmental parameters and bacterial community composition, canonical correspondence analysis (CCA) was performed, with overall model significance and individual term significance assessed using permutation-based analysis of variance (ANOVA) tests (n = 999). Collinear variables were first removed using variance inflation factor (VIF) analysis. The final set of environmental predictors was selected using the ordiR2step() function in the vegan package, which performs forward and backward stepwise selection based on adjusted R2. Variables were retained in the final model if they meaningfully increased the model's R2 and were significant at p < 0.05 in permutation testing.
To examine localized differences, pairwise analysis was conducted on 23 geographically matched MDW–WDW sample pairs. These pairs were defined based on spatial proximity, with each municipal sample located within 3 miles of a corresponding well sample. In cases where multiple MDW samples clustered around a single well (Fig. 1), each formed a unique geographic pairing. Statistical differences across these spatial pairs were assessed using paired Wilcoxon signed-rank tests.
To evaluate broader differences between all MDW and WDW samples, non-parametric Mann–Whitney U tests (Wilcoxon rank-sum tests) were used to compare absolute total bacterial abundance, DWPI abundances, and chemical parameters. This conservative approach was selected to accommodate the modest sample size (n = 20 per group) and the non-normal distribution of several variables, thereby enhancing the robustness and interpretability of the findings. The functional relationships between water quality parameters and bacterial groups were analyzed using stepwise multivariate forward/reverse regression analysis. All statistical analyses were performed in R (v4.0.2),50 with significance set at P < 0.05.
3. Results and discussion
3.1 Survey-reported household water treatment and usage
The survey results revealed notable differences between municipal and well water users. Whole-house filtration systems were used by one municipal participant and five well users. Water softeners were installed in 50% of municipal households, but seven lacked an established cleaning routine, with one reporting they never cleaned their system, one cleaning annually, and one cleaning biweekly. Among the 95% of well households with softeners, 13 had no cleaning routine, while the remainder reported varying frequencies ranging from monthly to biweekly maintenance. Point-of-use filters in kitchens were absent in all municipal households but present in 15% of well households, with one participant having no filter replacement routine, and the others replacing filters every three months or another annually. Bottled water usage was reported by 30% of well households but by no municipal participants. Despite these differences, all households reported using tap water for cooking and food preparation.3.2 Comparative evaluation of chemical constituents
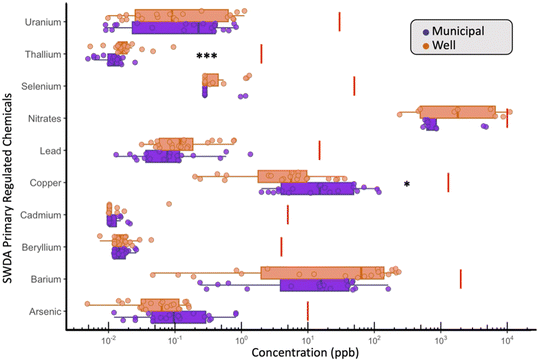
Across all 33 measured water quality parameters, the U.S. EPA's primary regulated contaminants—antimony, arsenic, barium, beryllium, cadmium, copper, lead, nitrate, selenium, thallium, and uranium—were evaluated in every sample (Table S4†). Nitrate was the only contaminant to exceed its Safe Drinking Water Act (SDWA) regulatory threshold, with one WDW sample containing 11 mg L−1 as N, surpassing the EPA's maximum contaminant level (MCL) of 10 mg L−1 as N for nitrate in MDW (Fig. 2). Additionally, three other well samples contained nitrate concentrations above 5 mg L−1, a level increasingly linked to adverse health outcomes, such as colorectal cancer and thyroid disease, as discussed in recent reviews of nitrate-associated health risks.51,52 These findings underscore potential limitations of the current US EPA MCL in protecting against chronic health effects. On average, nitrate levels in WDW samples were 2.78 times higher than in MDW (WDW: 4.054 ppm vs. MDW: 1.46 ppm, p = 0.3911). Given that wells near septic tanks or in agricultural regions are particularly susceptible to nitrate contamination, this finding underscores the burden on residents to manage and treat their water, reinforcing the health risks associated with self-supplied sources in vulnerable areas. In Iowa specifically, nitrate and nitrite MCL violations account for 44% of health-based standard violations,53 highlighting the prevalent risks in these communities.The risks posed by nitrate contamination in well water are not unique to the U.S. but have been addressed more proactively in other regions. In contrast to the SDWA, Europe has tackled this issue through the Nitrates Directive54 (Council Directive 91/676/EEC), a policy implemented in 1991 to reduce agricultural nitrate pollution in both surface and groundwater. This directive designates nitrate vulnerable zones (NVZs) and requires action programs that limit fertilizer use, improve manure management, and encourage sustainable farming practices. Since its implementation, monitored groundwater nitrate levels have declined by 20% in several regions,52,54 demonstrating its effectiveness. The directive's success in reducing contamination highlights the importance of proactive agricultural policies in protecting water resources and provides a potential model for similar initiatives in the United States. Adopting stricter measures like those in Europe could help address nitrate pollution and mitigate associated health risks, particularly in rural areas reliant on self-supplied water sources.
In WDW, thallium (Tl) was found to have concentrations 1.78 times (Tl avg. – WDW: 0.022 ppb vs. MDW: 0.01 ppb; p = 0.0019) higher when compared to MDW (Fig. 2), while copper (Cu) concentrations in MDW were 3.3 times higher (Cu avg. – MDW: 29.52 vs. WDW: 8.911 ppb; p = 0.034) than those found in WDW (Fig. 2). Although it is difficult to explain the increased (but small) concentration of Tl in WDW, Tl is a common trace metal enriched in sulfide-hosted copper, lead, and zinc ores,55,56 and the higher abundance could be due to differences in the presence of sediment sulfides. The significantly higher Cu concentrations in MDW can likely be explained by copper and/or brass corrosion in pipes, faucets, and fixtures due to the presence of free and total chlorine.57–59
In addition to the primary drinking water standards, the SDWA also includes secondary drinking water standards, which set action limits on chemicals that primarily affect the aesthetic qualities of water (e.g., taste, odor). These chemicals include aluminum, iron, manganese, and zinc (Fig. 3).
Comparisons between WDW and MDW samples (Table S5†) revealed that iron (Fe), manganese (Mn) and zinc (Zn) were significantly higher in WDW, with concentrations 4.39, 10.25 and 1.87 times higher, respectively (Fe avg. – WDW: 159.489 vs. MDW: 36.32 ppb, p = 0.036) (Mn avg. – WDW: 10.252 vs. MDW: 1.00 ppm, p = 0.006) (Zn avg. – WDW: 13.69 vs. MDW: 7.28 ppb, p = 0.034). In contrast, aluminum (Al) was detected slightly more frequently in MDW (Al avg. – MDW: 9.88 vs. 3.37, p = 0.014). The presence of higher Fe and Mn concentrations in WDW relative to MDW is expected; all the MDW supplies are chlorinated for disinfection (Table S2†) and/or conduct aeration treatment to remove Fe and/or Mn, likely removing dissolved Mn by oxidation to insoluble Mn(III/IV) oxides during treatment. Increased Zn in WDW could be due to multiple factors including differences in plumbing materials, such as the presence of galvanized steel drop pipe in wells or galvanized pipe in the well supply line or in the home, use of brass fittings—as well as the naturally occurring background concentration of Zn in Iowa groundwater, as previously reported by the Iowa Geological Survey (TIS-57).1,60
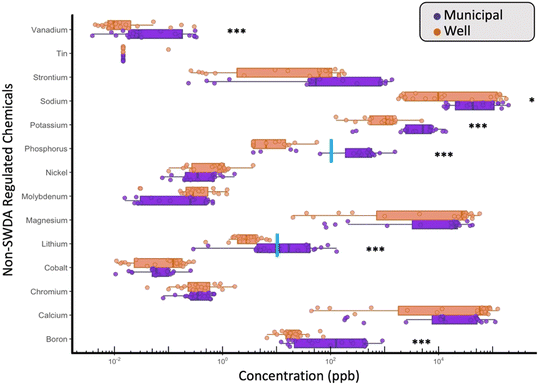
Finally, although the SWDA has not set regulations on the contaminants listed in Table S6† (cobalt, lithium, nickel, magnesium, phosphorus), there are several peer reviewed studies that set recommended advisory limits for these chemicals.61–63 For example, although no regulatory health standard exists for phosphorus in drinking water, a U.S. Geological Survey (USGS) nutrient criterion of 0.1 ppm (100 ppb)64 is commonly used to assess the potential for eutrophication in freshwater systems. While none of the WDW samples exceeded this environmental benchmark, 75% of the MDW samples did (Fig. 4). On average, phosphorus concentrations were significantly higher in MDW samples (avg. 439.2 ppb) compared to WDW (avg. 11.8 ppb; p < 0.001; Table S6†). This difference is expected, as phosphate is commonly added to municipal water systems as a corrosion inhibitor to reduce the leaching of lead and copper from household plumbing,65,66 a practice deployed by the MDW treatment plants in this study (Table S2†).
Although no governmental body has placed regulatory limits on lithium (Li) in drinking water, a recent study by the researchers at the USGS and EPA suggested a non-regulatory health-based screening level of 10 ppb. It is noteworthy that 50% of the MDW samples from our study were above this level and that they contained, on average, 9.56 times higher concentrations than WDW samples, with values ranging from 0.28 to 129.6 ppb (Fig. 4). Li is a naturally occurring alkali metal and has been identified by the USGS as a common contaminant in groundwater sources – this suggests trace amounts of Li would be expected in both WDW and MDW.61 Importantly, the study area in northeast Iowa encompasses a recognized transition in geology of the Cambrian–Ordovician aquifer from unconfined (in the northwest portions of the study area) to confined (by the Maquoketa shale) toward the west and southwest portions of the study area.67,68 As the Cambrian–Ordovician aquifer changes from being unconfined to confined, the quality of the water in the aquifer changes significantly. Specifically, the amount of total dissolved solids, including Na, K, Ca, and Mg as well as other soluble trace metals like Li, Sr, and Ba, are higher in the confined portion of the aquifer, in part, due to increased groundwater age.68,69 In general, a significant correlation between K and Li (Spearman's rank correlation coefficient (rho) = 0.906, p < 0.001) was observed in the Cambrian–Ordovician aquifer consistent with that of the regionally observed correlation (rho = 0.91) between K and Li in USGS studies.68 In addition, K and Li were significantly correlated in all samples (rho = 0.729, p < 0.001). Accordingly, the higher Li concentrations in MDW observed relative to WDW likely reflect differences in source aquifers employed.
3.3 Absence of DWPI detection in WDW and MDW
Total microbial densities in WDW were, on average, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log higher (p < 0.001) compared to MDW, which was expected due to the lack of exposure to chemical disinfectants (Table 1). This increase in microbial density aligns with the observed presence of total coliforms and E. coli: total coliforms were detected in 1 of 20 MDW samples and 7 of 20 WDW samples, while E. coli was found in 1 WDW sample and was absent in all MDW samples. Unlike total coliforms, which are a general indicator of water quality and potential system contamination, E. coli specifically indicates fecal contamination and suggests a higher likelihood of harmful pathogens. The well containing E. coli also exhibited elevated nitrate levels (8.7 mg L−1 as N) and was among the 7 wells with total coliform bacteria, suggesting a sanitary defect and either septic or agricultural pollution as a potential source of contamination.
log higher (p < 0.001) compared to MDW, which was expected due to the lack of exposure to chemical disinfectants (Table 1). This increase in microbial density aligns with the observed presence of total coliforms and E. coli: total coliforms were detected in 1 of 20 MDW samples and 7 of 20 WDW samples, while E. coli was found in 1 WDW sample and was absent in all MDW samples. Unlike total coliforms, which are a general indicator of water quality and potential system contamination, E. coli specifically indicates fecal contamination and suggests a higher likelihood of harmful pathogens. The well containing E. coli also exhibited elevated nitrate levels (8.7 mg L−1 as N) and was among the 7 wells with total coliform bacteria, suggesting a sanitary defect and either septic or agricultural pollution as a potential source of contamination.
| Microbial contaminants | Average density ± standard deviation (p-value) | Detection frequency | ||
|---|---|---|---|---|
| Municipal | Well | Municipal | Well | |
| L. pneumophila (gene copies per L) | 4.8 × 105 ± 2.16 × 105 | 5.3 × 104 ± 1.63 × 105 | 80% | 75% |
| (p-Value: 0.757) | ||||
| NTM (gene copies per L) | 7.06 × 106 ± 3.13 × 107 | 1.09 × 106 ± 3.36 × 106 | 45% | 60% |
| (p-Value: 0.126) | ||||
| Total bacteria (gene copies per L) | 1.13 × 107 ± 4.09 × 106 | 1.2 × 108 ± 1.63 × 105 | 100% | 100% |
| (p-Value: <0.001) | ||||
As for the DWPIs, L. pneumophila was detected at a greater frequency in MDW than WDW samples, aligning with studies in which its abundance within the distribution system and premise plumbing70 have been established. Conversely, NTM, which has been identified throughout the water transect,35,71,72 was detected more frequently in WDW compared to MDW. Similar to findings in the New Jersey study,32 the detection frequency for NTM remained low, with both MDW and WDW exhibiting detection rates below 60%. The higher detection in WDW may be due to more favorable hydrological and mineral conditions for NTM survival73 but the overall low detection frequency can be attributed to the difficulties in extracting DNA from the NTM cellular envelope, which is notoriously hard to open.74
When compared to a study on 113 private well water samples in Louisiana,75 the detection frequencies for Legionella and NTM were slightly higher in the Louisiana study, with 86.7% and 68.1%, respectively, compared to our findings of 75% and 60%. Additionally, the Louisiana study reported broader ranges in gene concentrations for both pathogens, ranging from 0.60 to 5.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 GC/100 mL for Legionella and 0.67 to 5.95
log10 GC/100 mL for Legionella and 0.67 to 5.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 GC/100 mL for NTM. This difference in detection is likely attributed to differences in the locations and source waters of the samples. Nevertheless, one thing is clear: these DWPIs are ubiquitous in all potable water sources.
log10 GC/100 mL for NTM. This difference in detection is likely attributed to differences in the locations and source waters of the samples. Nevertheless, one thing is clear: these DWPIs are ubiquitous in all potable water sources.
3.4 Pairwise analysis on 23 well and municipal geographic sample pairs
Paired Wilcoxon tests were performed on 23 geographically defined MDW–WDW sample pairs, in which each municipal sample was located within 3 miles of its paired well. This spatial pairing revealed localized chemical and microbiological differences that were not fully captured in the aggregate dataset analysis. Microbial assessments indicated that total microbial densities were higher in well water, as expected, with significantly elevated levels of specific microbes such as NTM and L. pneumophila. NTM concentrations were notably higher in WDW (mdn. = 2.04 × 105 gene copies per L) compared to MDW (mdn. = 0 gene copies per L; p = 0.0015), suggesting a greater exposure risk for WDW users. Similarly, L. pneumophila concentrations were elevated in WDW (mdn. = 6.08 × 103 gene copies per L) relative to MDW (mdn. = 3.07 × 103 gene copies per L; p = 0.0005). In practical terms, this means that a single 8 oz glass of tap water from WDW could contain approximately 4.8 × 104 NTM gene copies and 1.44 × 103L. pneumophila gene copies, compared to MDW's lower exposure levels of 0 NTM and around 728 L. pneumophila gene copies. It is important to note that exposure does not directly translate to infection, as these are opportunistic pathogenic organisms, with the greatest risk posed to individuals with chronic illness, respiratory issues, or otherwise compromised immune systems. These findings emphasize the higher microbial burden in WDW, likely due to a lack of disinfection in WDW sources.In addition to the significant differences in the chemical parameters aluminum, boron, copper, iron, lithium, manganese, potassium, thallium found in the total dataset, the pairwise sample analysis revealed further significant differences in lead and strontium. Lead levels were greater in WDW (median = 0.2 ppb) compared to MDW (median = 0.1 ppb; p = 0.002), although both WDW and MDW concentrations remained well below the maximum contaminant levels specified by the SWDA. Strontium showed the reverse trend, with MDW displaying higher concentrations (mdn. = 509.2 ppb) than WDW (mdn. = 52 ppb; p = 0.002).
These microbial and chemical disparities in paired well and municipal samples underscore the heightened exposure risks faced by well users, particularly in areas just beyond municipal service boundaries. Municipal samples primarily drew water from the deeper Cambrian–Ordovician aquifer, often mixed with water from the shallower Silurian aquifer. In contrast, well samples generally relied on shallower sources, such as the Galena or Silurian aquifers, which are more susceptible to contamination due to their proximity to surface activities. Despite some overlap in source water, the lack of treatment for WDW contributed to significantly higher microbial and chemical exposure risks for well users. This highlights the disproportionate burden placed on well users to protect and monitor their water sources, further emphasizing the potential public health benefits of expanding centralized water systems to rural and semi-rural populations.
3.5 Diversity indices highlight importance of water source and treatment
Analyses of the microbial alpha diversity indices found that WDW samples had marginally higher Chao's species richness than municipal counterparts (Fig. 5a). The chemical and physical treatment received by MDW samples may explain this result. When examining the differences in aquifer alpha diversity, there was only a significant difference between the Cambrian–Ordovician and Galena aquifers (Fig. 5b). This is consistent with our observation that the Galena aquifer is the main source for WDW in the study area, while the Cambrian–Ordovician aquifer primarily supplies MDW. Previous studies have shown that species diversity tends to decrease as water undergoes treatment.76 The lower species diversity observed in MDW compared to WDW samples (Fig. 5a) may be largely driven by selective pressures from the treatment process, particularly in Cambrian–Ordovician aquifer samples. These findings suggest that reduced species diversity may reflect water treatment practices rather than inherent aquifer differences. However, the small sample size limits our conclusions, emphasizing the need for larger sampling campaigns with true paired samples from each aquifer to confirm these results and clarify potential aquifer-specific biological impacts.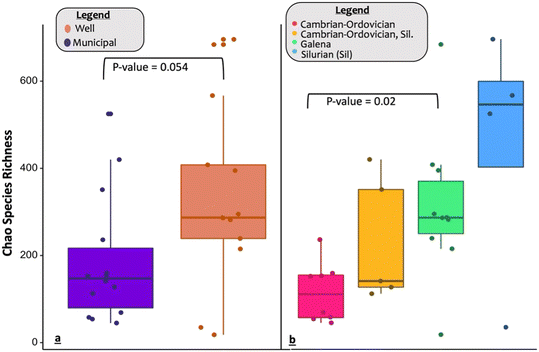 | ||
| Fig. 5 (a and b) Chao species richness plots (a) separated by type: municipal and well (b) and separated by aquifer combinations. | ||
3.6 Unraveling aquifer-specific microbiota in well and municipal waters
The NMDS and permutational multivariate analysis of variance at the 97% sequence dissimilarity showed distinct separations in the microbial community when sorted by water type (Fig. 6a). Similarly, distinct clustering was observed in community composition based on the aquifer supplying the WDW or MDW (Fig. 6b), except for the Silurian aquifer in which sample size was constrained. The influence and importance of water source in explaining the microbial community composition has been identified previously, however these studies focused on comparing the microbiomes of ground and surface water supplied MDW plants.77,78 This is the first study, to the authors' knowledge, that has found that the specific aquifer supplying drinking water in the same geographical region impacts the DW microbiome.The dominant phyla in both WDW and MDW samples were Proteobacteria, which is consistent with other drinking water studies.79 In the municipal samples, the biggest phyla (after Proteobacteria), are Actinobacteria and Cyanobacteria. Actinobacteria had been thoroughly studied and found to be responsible for taste and odor problems within MDW samples.80,81 Additionally, the DWPI genera Mycobacterium and Pseudomonas were amongst the top 10 known municipal genera (Fig. 7b). This is consistent with previous work as DWPIs, formally known as opportunistic pathogens, have been identified as prevalent bacteria within the water distribution system.30,33,82,83
As for Cyanobacteria, MDW reads were significantly higher than WDW samples—1659% greater, with 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 529 vs. 3214 reads (p = 0.021). Notably, Dubuque Water Works, which sources from a combination of the Cambrian–Ordovician, Silurian, and the shallower Alluvial aquifer, showed 749% more reads than all other municipal sources (p = 0.002). In contrast, the other MDW samples drew from the deeper Cambrian–Ordovician and Silurian formations, lacking Alluvial inputs. The elevated Cyanobacteria reads in the Dubuque Water Works municipality compared to other sources may be related to connectivity between the Mississippi River and the alluvial aquifer in the source water of this system. However, one cannot rule out the possibility that tap samples were contaminated by Cyanobacteria growth on plumbing fixtures or due to the presence of Cyanobacteria in deep groundwater sources.84
529 vs. 3214 reads (p = 0.021). Notably, Dubuque Water Works, which sources from a combination of the Cambrian–Ordovician, Silurian, and the shallower Alluvial aquifer, showed 749% more reads than all other municipal sources (p = 0.002). In contrast, the other MDW samples drew from the deeper Cambrian–Ordovician and Silurian formations, lacking Alluvial inputs. The elevated Cyanobacteria reads in the Dubuque Water Works municipality compared to other sources may be related to connectivity between the Mississippi River and the alluvial aquifer in the source water of this system. However, one cannot rule out the possibility that tap samples were contaminated by Cyanobacteria growth on plumbing fixtures or due to the presence of Cyanobacteria in deep groundwater sources.84
The top three dominant microbial phyla (after Proteobacteria) observed in WDW samples were Bacteroidetes, Nitrospirae, and Thaumarchaeota (Fig. 7a). Nitrospirae and Thaumarchaeota are associated with nitrification processes.85–87 The significant presence of Nitrospirae in well samples is an indicator of nitrogen leaching, likely a byproduct of extensive agricultural activities in Dubuque County. For example, the 2022 USDA Census of Agricultural indicates that Dubuque County (population ∼99,000) has nearly 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 acres in row crop production (corn for grain and soybeans for beans), while also serving as home to over 120
000 acres in row crop production (corn for grain and soybeans for beans), while also serving as home to over 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cattle and calves and 110
000 cattle and calves and 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 hogs and pigs.88 This microbial profile suggests potential public health implications for WDW users, as nitrogen compounds introduced via land application of both anhydrous ammonia fertilizer and manure can contribute to water contamination. These findings underscore the need for tailored water quality regulations for WDW, particularly in agricultural regions, to address unique contamination risks and protect vulnerable communities relying on self-supplied water sources.
700 hogs and pigs.88 This microbial profile suggests potential public health implications for WDW users, as nitrogen compounds introduced via land application of both anhydrous ammonia fertilizer and manure can contribute to water contamination. These findings underscore the need for tailored water quality regulations for WDW, particularly in agricultural regions, to address unique contamination risks and protect vulnerable communities relying on self-supplied water sources.
In both the well and municipal samples, a notable known genus is Sulfuricurvum was observed,suggesting high levels of sulfur may be found in aquifer sediment,89 while the category labeled “other”—which includes uncultured or unclassified bacteria—had the highest overall relative abundance. The Galena aquifer,90 which serves as the primary aquifer for many wells in this study, is characterized by the presence of the mineral galena (PbS), a lead sulfide. The weathering of PbS can release reduced sulfur species into the groundwater,91 creating an environment conducive to the proliferation of sulfur-oxidizing bacteria such as Sulfuricurvum.92 It is important to note that the top 10 known genera are displayed (Fig. 7b) and that there are many uncultured bacteria that dominate the well water microbiota. The number of unknown/uncultured bacteria illustrates the importance of this study, and highlights the need for more research, to close research gaps surrounding the well water, its microbiome, and the associated exposome.
3.7 Multivariate models show relation between metal cofactors and MDW and WDW bacterial densities
To determine which abiotic factors explained the microbial community composition, linear mixed-effect models were developed for MDW and WDW samples (Table 2). The model containing all samples revealed that water type (well or municipal) explained 6.08% of the variance in microbial community composition, with municipal water being associated with lower microbial diversity than well water. Nitrate levels also emerged as an important variable, directly correlating with microbial activity across all models. Notably, nitrate levels in the samples ranged widely, with most falling either below 1 mg L−1 as N or exceeding 11 mg L−1 as N, highlighting the variability in exposure levels across water sources.| Sample type | Model components | Overall model | |
|---|---|---|---|
| Explained (%) | Most influential abiotic component | ||
| Total samples | −Aquifer17.26% − Type6.08% + Nitrates5.12% + Phosphorus4.05% + Vanadium4.04% − Boron4.01% − Molybdenum3.83% | 44.37 | Aquifer |
| Municipal samples | Aquifer25.45% + Copper9.27% − Boron8.58% + Nitrates8.20% + Phosphorus8.04% + Zinc7.36% | 66.89 | Aquifer |
| Well samples | Nitrates12.7% + Aquifer12% + Vanadium10.96% − Molybdenum8.09% − Calcium8.06% − Thallium8.02% | 59.86 | Nitrates |
Interestingly, in the individual models for MDW, WDW, and the combined model using all samples, aquifer source was a significant variable, explaining 24.45%, 12%, and 17.26%, respectively, of the microbial community composition. These results indicate that both water source and treatment significantly influence microbial community composition, consistent with findings from similar studies.93,94 Together, these findings emphasize the role of both chemical factors, such as nitrate levels, and physical factors, like aquifer source, in shaping microbial diversity in drinking water systems. It is important to note that interactions between environmental variables and their effects on microbial community composition were not evaluated in this study due to the modest sample size, which limited the statistical power needed to detect such effects. Future research should investigate the presence and significance of these interactions to provide deeper ecological insight into the factors shaping microbial communities.
It is unsurprising that zinc and copper were influential in the municipal model. As aforementioned, copper is commonly used in service lines, premise plumbing, and brass fittings and fixtures, and zinc is common in galvanized coatings for iron and present as an alloying metal in brass.95,96 Zinc coatings are intended to act as corrosion control agents, mitigating leaching issues and extending pipe viability within the distribution system. However, as these coatings degrade, they often further contribute to water quality issues years after their installation.97 Zinc is also commonly added to phosphate corrosion control agents, although its value in preventing lead corrosion is questioned.98 Unfortunately, zinc-phosphate and phosphate-alone corrosion control agents are not separately defined in the publicly available source and treatment data provided by the Iowa Department of Natural Resources, so we are unable to assess whether zinc is added at municipal water systems within the study area. As these metals are also common micronutrients needed for cellular enzyme function,99 their increased availability from compromised pipes can significantly influence microbial activity and community composition in water systems, impacting overall water quality.100
The well model showed that the metals molybdenum (Mo), thallium (Tl), and vanadium (V) collectively account for more than 45% of the explained variation in microbial community composition. Interestingly, V is positively correlated with microbial growth, whereas Mo shows an inverse relationship. Both V and Mo are recognized enzymatic cofactors for nitrogen fixation in bacteria, with Mo being the more efficient metal. Mo is also essential for sulfur, carbon, and nitrogen metabolism,101,102 but its effectiveness is optimal within a narrow concentration range of 0.005–0.2 ppb.103 In this study, some Mo concentrations in both WDW and MDW exceed this ideal range, with well water samples ranging from 0.0296–1.2182 ppb and municipal water samples ranging from 0.0155–0.661 ppb. These elevated concentrations may explain the observed negative correlation between Mo levels and microbial activity. At higher concentrations, Mo can exceed thresholds that support microbial nitrogen fixation, potentially causing toxicity or disrupting key metabolic processes.103,104 Furthermore, excessive Mo levels might selectively favor specific microbial taxa, thereby altering community composition and reducing the overall activity or diversity of nitrogen-fixing microbes. These findings align with prior research, which shows that nitrogen-fixing organisms, such as Nostoc and Anabaena, experience Mo limitation at concentrations below 1–5 nM but thrive only within narrow, optimal ranges.105,106
As mentioned earlier, Tl concentrations were found to be significantly higher in well samples, although still lower than the EPA's maximum contaminant level goal of 0.5 ppb. The low levels of Tl and its correlation with bacterial community composition may reflect variation in a geochemical or geologic parameter not measured in this study. The effects of Tl on microbial processes have not been extensively explored, but is known that bacteria are capable of oxidizing Tl to Tl2O3, further promoting bioaccumulation of Tl within the environment.107
3.8 Analyzing microbial physiological predictive features in well and municipal water sources
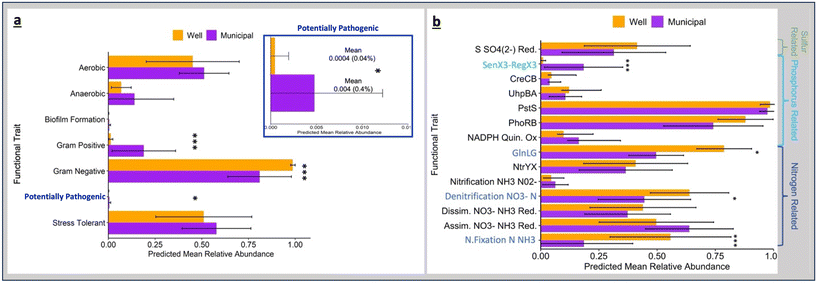
Among the BugBase default predictive phenotypes, Gram-positive bacteria were statistically higher in MDW (Fig. 8a), while Gram-negative bacteria were higher in WDW. Potentially pathogenic bacteria were statistically higher in MDW, likely due to the presence of the Mycobacterium and Pseudomonas genera, which were among the top 10 known bacterial genera in MDW samples. Furthermore, the prevalence of predicted traits related to nitrogen fixation and denitrification being higher in WDW samples (Fig. 8b) is not surprising given the abundance of nitrogen-fixing bacteria within WDW samples (Fig. 7a and b).In addition, to difference in pathogenicity and nitrogen metabolism, differences in sulfur reduction and phosphorus metabolism, specifically phosphate starvation response, were observed between MDW and WDW (Fig. 8b). Furthermore, the predictive trait for phosphate starvation response, a two-component signal transduction system (SenX3–RegX3) that allows bacteria to respond to environmental changes and modify their gene expression,108,109 was significantly higher in MDW samples. SenX3–RegX3 is ubiquitous in Mycobacterium species and its higher abundance in MDW samples is likely due to the higher relative abundance of Mycobacterium in MDW samples compared to WDW (Fig. 7b).109
4. Conclusion
This study highlights significant differences in the chemical and microbial composition of WDW and MDW in close geographic proximity, underscoring the need for comprehensive and robust regulatory measures tailored to each water source. Concentrations of iron, manganese, nitrate, thallium, and zinc were statistically higher in WDW samples, while aluminum, boron, copper, lithium, phosphorus, potassium, sodium, and vanadium were statistically higher in MDW samples. Notably, one WDW sample exceeded the SDWA limit for nitrate, highlighting a critical instance of regulatory non-compliance. Although more extensive sampling is needed to establish causality for these contaminants, these preliminary findings suggest a comparable burden of potential health risks associated with both water sources.Paired sample analysis further emphasized microbial disparities MDW and WDW, revealing that well water users within a 3-mile distance from a municipal connection may experience a notably higher exposure to certain pathogens. Specifically, the average concentration of NTM in WDW was significantly higher than in MDW, with WDW consumers being potentially exposed to approximately 4.8 × 104 NTM gene copies per 8 oz glass of water. Similarly, Legionella pneumophila levels were also elevated in WDW, with WDW consumers exposed to around 1.44 × 103 gene copies per glass, while MDW users had a reduced exposure of approximately 728 gene copies. The higher frequency and concentration of these microbes in WDW underscore the elevated microbial burden and associated health risks for individuals relying on well water, particularly in areas not served by centralized treatment. These findings highlight the public health implications of well water use in agricultural areas, suggesting a critical need for targeted interventions and enhanced water quality monitoring for well water systems.
Despite sourcing from aquifers in the same region, well and municipal water microbiomes showed significant differences, indicating that municipal treatment protocols substantially alter the microbial landscape. This study provides a novel perspective on the unique chemical and microbial characteristics of well water in the U.S. and emphasizes the importance of understanding these distinct microbial communities and their public health implications. Future research should explore a broader range of geographical areas, land-use types (e.g., farming, mining), climatic conditions, and seasonal variations to more fully assess water quality disparities affecting well water users. Additionally, collecting true paired samples from wells and municipal sources at the same depth and geographic location, as well as investigating water chemistry and plumbing materials (such as corrosion potential), could deepen our understanding of the specific health risks associated with reliance on well water.
The critical need for stronger regulation and monitoring of drinking water quality is evident. Current gaps in regulatory oversight, particularly for well water, highlight the importance of updating the SDWA to address emerging chemical and microbial contaminants and additional efforts to protect vulnerable communities, especially those that currently fall outside of protections afforded by the SDWA. By providing actionable information, this research aims to arm public health decision-makers with the data necessary to ensure that all populations have access to safe drinking water. Future initiatives should include widespread sampling and assessment, building on the foundation laid by this study. The goal is to move towards equitable water quality for all, ensuring that all regulatory frameworks are robust enough to address the diverse challenges posed by different water sources. This study underscores the significance of continued and expanded research to protect public health and inform policy, ultimately ensuring safe and clean drinking water for every community.
Data availability
The environmental and sequencing data that support the findings of this study are openly available in Zenodo at https://zenodo.org/records/14969168, reference number https://doi.org/10.5281/zenodo.14969168.Author contributions
Please note that author contributions are formatted in alphabetical order using the CRediT110,111 format. Dr. David M. Cwiertny – conceptualization, data curation, funding acquisition, resources, and writing – reviewing & editing. Mr. Daniel W. Gilles – visualization. Dr. Sarah Haig – conceptualization, methodology, project administration, resources, supervision, validation, and writing – review and editing. Dr. Adam R. Hoffman – investigation, resources, and writing – review & editing. Dr. Drew E. Latta – data curation, formal analysis, investigation, validation, and writing – review and editing. Ms. Jemima E. Ohwobete – data curation, formal analysis, investigation, methodology, software, validation, visualization, writing – original draft, and writing – review & editing. Dr. Darrin A. Thompson – data curation, investigation, project administration, and writing – review & editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank the Center for Health Effects of Environmental Contamination (CHEEC), University of Iowa (Grant number: S03189-01) for funding this study. Funding for CHEEC is made available through the State of Iowa and the Iowa Department of Natural Resources. We also thank the University of Pittsburgh High-Throughput Cluster Computational Services for computational processing of our sequencing data. JEO is supported by a Pitt NSF-STRIVE fellowship (Grant number 1434012) from the University of Pittsburgh. The authors would also like to acknowledge Rick Langel of the Iowa Geological Survey for his assistance with private well source aquifer assignments and Dr. Isaiah Spencer-Williams for his guidance in processing the sequencing and BugBase analysis in this paper.References
- Domestic (Private) Supply Wells|U.S. Geological Survey, https://www.usgs.gov/mission-areas/water-resources/science/domestic-private-supply-wells, (accessed 19 May 2023).
- Y. Zheng and S. V. Flanagan, The Case for Universal Screening of Private Well Water Quality in the U.S. and Testing Requirements to Achieve It: Evidence from Arsenic, Environ. Health Perspect., 2017, 125, 085002 CrossRef.
- P. M. Bradley, D. W. Kolpin, D. A. Thompson, K. M. Romanok, K. L. Smalling, S. E. Breitmeyer, M. C. Cardon, D. M. Cwiertny, N. Evans, R. W. Field, M. J. Focazio, L. E. Beane Freeman, C. E. Givens, J. L. Gray, G. L. Hager, M. L. Hladik, J. N. Hofmann, R. R. Jones, L. K. Kanagy, R. F. Lane, R. B. McCleskey, D. Medgyesi, E. K. Medlock-Kakaley, S. M. Meppelink, M. T. Meyer, D. A. Stavreva and M. H. Ward, Juxtaposition of intensive agriculture, vulnerable aquifers, and mixed chemical/microbial exposures in private-well tapwater in northeast Iowa, Sci. Total Environ., 2023, 868, 161672 CrossRef CAS.
- J. VanDerslice, Drinking Water Infrastructure and Environmental Disparities: Evidence and Methodological Considerations, Am. J. Public Health, 2011, 101, S109–S114 CrossRef.
- C. Pace, C. Balazs, K. Bangia, N. Depsky, A. Renteria, R. Morello-Frosch and L. J. Cushing, Inequities in Drinking Water Quality Among Domestic Well Communities and Community Water Systems, California, 2011–2019, Am. J. Public Health, 2022, 112, 88–97 CrossRef.
- D. A. Thompson, D. W. Kolpin, M. L. Hladik, H.-J. Lehmler, S. M. Meppelink, M. C. Poch, J. D. Vargo, V. A. Soupene, N. M. Irfan, M. Robinson, K. Kannan, L. E. Beane Freeman, J. N. Hofmann, D. M. Cwiertny and R. W. Field, Prevalence of neonicotinoid insecticides in paired private-well tap water and human urine samples in a region of intense agriculture overlying vulnerable aquifers in eastern Iowa, Chemosphere, 2023, 319, 137904 CrossRef CAS.
- K. L. Smalling, K. M. Romanok, P. M. Bradley, M. C. Morriss, J. L. Gray, L. K. Kanagy, S. E. Gordon, B. M. Williams, S. E. Breitmeyer, D. K. Jones, L. A. DeCicco, C. A. Eagles-Smith and T. Wagner, Per- and polyfluoroalkyl substances (PFAS) in United States tapwater: Comparison of underserved private-well and public-supply exposures and associated health implications, Environ. Int., 2023, 178, 108033 CrossRef CAS.
- K. M. Eccles, S. Checkley, D. Sjogren, H. W. Barkema and S. Bertazzon, Lessons learned from the 2013 Calgary flood: Assessing risk of drinking water well contamination, Appl. Geogr., 2017, 80, 78–85 CrossRef.
- K. R. Drewry, C. N. Jones, W. Hayes, R. E. Beighley, Q. Wang, J. Hochard, W. Mize, J. Fowlkes, C. Goforth and K. J. Pieper, Using Inundation Extents to Predict Microbial Contamination in Private Wells after Flooding Events, Environ. Sci. Technol., 2024, 58, 5220–5228 CrossRef CAS.
- O. US EPA, Potential Well Water Contaminants and Their Impacts, https://www.epa.gov/privatewells/potential-well-water-contaminants-and-their-impacts, (accessed 4 June 2024).
- Water Insecurity And Population Health, https://www.healthaffairs.org/do/10.1377/hpb20230921.68748/full/, (accessed 17 May 2024).
- Drinking Water, https://infrastructurereportcard.org/cat-item/drinking-water-infrastructure/, (accessed 17 May 2024).
- J. W. A. Charrois, Private drinking water supplies: challenges for public health, CMAJ, 2010, 182, 1061–1064 Search PubMed.
- R. P. Richards, D. B. Baker, N. L. Creamer, J. W. Kramer, D. E. Ewing, B. J. Merryfield and L. K. Wallrabenstein, Well Water Quality, Well Vulnerability, and Agricultural Contamination in the Midwestern United States, J. Environ. Qual., 1996, 25, 389–402 CrossRef CAS.
- S. K. Alawattegama, T. Kondratyuk, R. Krynock, M. Bricker, J. K. Rutter, D. J. Bain and J. F. Stolz, Well water contamination in a rural community in southwestern Pennsylvania near unconventional shale gas extraction, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2015, 50, 516–528 CrossRef CAS.
- M. J. Eggers, J. T. Doyle, M. J. Lefthand, S. L. Young, A. L. Moore-Nall, L. Kindness, R. O. Medicine, T. E. Ford, E. Dietrich, A. E. Parker, J. H. Hoover and A. K. Camper, Community Engaged Cumulative Risk Assessment of Exposure to Inorganic Well Water Contaminants, Crow Reservation, Montana, Int. J. Environ. Res. Public Health, 2018 DOI:10.3390/ijerph15010076.
- R. P. Richards, D. B. Baker, N. L. Creamer, J. W. Kramer, D. E. Ewing, B. J. Merryfield and L. K. Wallrabenstein, Well Water Quality, Well Vulnerability, and Agricultural Contamination in the Midwestern United States, J. Environ. Qual., 1996, 25, 389–402 CrossRef CAS.
- M. J. Eggers, J. T. Doyle, M. J. Lefthand, S. L. Young, A. L. Moore-Nall, L. Kindness, R. Other Medicine, T. E. Ford, E. Dietrich, A. E. Parker, J. H. Hoover and A. K. Camper, Community Engaged Cumulative Risk Assessment of Exposure to Inorganic Well Water Contaminants, Crow Reservation, Montana, Int. J. Environ. Res. Public Health, 2018, 15, 76 CrossRef.
- J. Adjemian, T. B. Frankland, Y. G. Daida, J. R. Honda, K. N. Olivier, A. Zelazny, S. Honda and D. R. Prevots, Epidemiology of Nontuberculous Mycobacterial Lung Disease and Tuberculosis, Hawaii, USA, Emerging Infect. Dis., 2017, 23, 439–447 CrossRef.
- K. M. Benedict, Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water — United States, 2013–2014, Morb. Mortal. Wkly. Rep., 2014, 66(44), 1216–1221, DOI:10.15585/mmwr.mm6644a3.
- B. A. Cunha, A. Burillo and E. Bouza, Legionnaires' disease, Lancet, 2016, 387, 376–385 CrossRef.
- R. Thomson, C. Tolson, R. Carter, C. Coulter, F. Huygens and M. Hargreaves, Isolation of Nontuberculous Mycobacteria (NTM) from Household Water and Shower Aerosols in Patients with Pulmonary Disease Caused by NTM, J. Clin. Microbiol., 2013, 51, 3006–3011 CrossRef.
- L. E. Garrison, J. M. Kunz, L. A. Cooley, M. R. Moore, C. Lucas, S. Schrag, J. Sarisky and C. G. Whitney, Vital Signs: Deficiencies in Environmental Control Identified in Outbreaks of Legionnaires' Disease — North America, 2000–2014, Morb. Mortal. Wkly. Rep., 2016, 65, 576–584 CrossRef.
- D. O. Schwake, E. Garner, O. R. Strom, A. Pruden and M. A. Edwards, Legionella DNA Markers in Tap Water Coincident with a Spike in Legionnaires' Disease in Flint, MI, Environ. Sci. Technol. Lett., 2016, 3, 311–315 CrossRef.
- J. M. Kunz, Surveillance of Waterborne Disease Outbreaks Associated with Drinking Water — United States, 2015–2020, MMWR Surveill Summ, DOI:10.15585/mmwr.ss7301a1.
- L. M. Feazel, L. K. Baumgartner, K. L. Peterson, D. N. Frank, J. K. Harris and N. R. Pace, Opportunistic pathogens enriched in showerhead biofilms, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 16393–16399 CrossRef CAS.
- C. Proctor, E. Garner, K. A. Hamilton, N. J. Ashbolt, L. J. Caverly, J. O. Falkinham, C. N. Haas, M. Prevost, D. R. Prevots, A. Pruden, L. Raskin, J. Stout and S.-J. Haig, Tenets of a holistic approach to drinking water-associated pathogen research, management, and communication, Water Res., 2022, 211, 117997 CrossRef CAS.
- S.-J. Haig, S. Cahalan, L. Kalikin, L. Caverly, T. Spilker, L. Raskin and J. LiPuma, in Pediatric Pulmonology, 2018, vol. 53, p. 268 Search PubMed.
- K. S. Dowdell, H. D. Greenwald, S. Joshi, M. Grimard-Conea, S. Pitell, Y. Song, C. Ley, L. C. Kennedy, S. Vosloo, L. Huo, S.-J. Haig, K. A. Hamilton, K. L. Nelson, A. Pinto, M. Prévost, C. R. Proctor, L. M. Raskin, A. J. Whelton, E. Garner, K. J. Pieper and W. J. Rhoads, Legionella pneumophila occurrence in reduced-occupancy buildings in 11 cities during the COVID-19 pandemic, medRxiv, 2022, preprint, DOI:10.1101/2022.06.28.22277022.
- S.-J. Haig, N. Kotlarz, J. J. LiPuma and L. Raskin, A High-Throughput Approach for Identification of Nontuberculous Mycobacteria in Drinking Water Reveals Relationship between Water Age and Mycobacterium avium, mBio, 2018, 9(1) DOI:10.1128/mBio.02354-17.
- S.-J. Haig, N. Kotlarz, L. M. Kalikin, T. Chen, S. Guikema, J. J. LiPuma and L. Raskin, Emerging investigator series: bacterial opportunistic pathogen gene markers in municipal drinking water are associated with distribution system and household plumbing characteristics, Environ. Sci.: Water Res. Technol., 2020, 6, 3032–3043 RSC.
- S. M. Blanc, D. Pender, C. Vinnard, M. L. Gennaro and N. L. Fahrenfeld, Nontuberculous Mycobacteria in the Biofilm Microbiome of Private Well and Premise Plumbing, Environ. Eng. Sci., 2021, 38, 607–625 CrossRef CAS.
- S.-J. Haig, N. Kotlarz, L. M. Kalikin, T. Chen, S. Guikema, J. J. LiPuma and L. Raskin, Emerging investigator series: bacterial opportunistic pathogen gene markers in municipal drinking water are associated with distribution system and household plumbing characteristics, Environ. Sci.: Water Res. Technol., 2020, 6, 3032–3043 RSC.
- S.-J. Haig, N. Kotlarz, L. M. Kalikin, T. Chen, S. Guikema, J. J. LiPuma and L. Raskin, Emerging investigator series: bacterial opportunistic pathogen gene markers in municipal drinking water are associated with distribution system and household plumbing characteristics, Environ. Sci.: Water Res. Technol., 2020, 6, 3032–3043 RSC.
- C. Proctor, E. Garner, K. A. Hamilton, N. J. Ashbolt, L. J. Caverly, J. O. Falkinham III, C. N. Haas, M. Prevost, D. R. Prevots and A. Pruden, Tenets of a holistic approach to drinking water-associated pathogen research, management, and communication, Water Res., 2022, 211, 117997 CrossRef CAS.
- K. S. Dowdell, H. Greenwald Healy, S. Joshi, M. Grimard-Conea, S. Pitell, Y. Song, C. Ley, L. C. Kennedy, S. Vosloo, L. Huo, S.-J. Haig, K. A. Hamilton, K. L. Nelson, A. Pinto, M. Prévost, C. R. Proctor, L. Raskin, A. J. Whelton, E. Garner, K. J. Pieper and W. J. Rhoads, Legionella pneumophila occurrence in reduced-occupancy buildings in 11 cities during the COVID-19 pandemic, Environ. Sci.: Water Res. Technol., 2023, 9, 2847–2865 RSC.
- S. Pitell and S.-J. Haig, Assessing the impact of anti-microbial showerheads on the prevalence and abundance of opportunistic pathogens in shower water and shower water-associated aerosols, Front. Microbiomes, 2023, 2 DOI:10.3389/frmbi.2023.1292571.
- I. Spencer-Williams, A. Balangoda, R. Dabundo, E. Elliott and S.-J. Haig, Exploring the Impacts of Full-Scale Distribution System Orthophosphate Corrosion Control Implementation on the Microbial Ecology of Hydrologically Connected Urban Streams, Microbiol. Spectrum, 2022, 10 DOI:10.1128/spectrum.02158-22.
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast) (Text with EEA relevance), 2020, vol. 435.
- O. US EPA, Contaminant Candidate List 5 - CCL 5, https://www.epa.gov/ccl/contaminant-candidate-list-5-ccl-5, (accessed 23 July 2024).
- O. US EPA, Fifth Unregulated Contaminant Monitoring Rule, https://www.epa.gov/dwucmr/fifth-unregulated-contaminant-monitoring-rule, (accessed 23 July 2024).
- S.-J. Haig, N. Kotlarz, L. M. Kalikin, T. Chen, S. Guikema, J. J. LiPuma and L. Raskin, Emerging investigator series: bacterial opportunistic pathogen gene markers in municipal drinking water are associated with distribution system and household plumbing characteristics, Environ. Sci.: Water Res. Technol., 2020, 6, 3032–3043 RSC.
- L. S. Clesceri, A. E. Greenberg and A. D. Eaton, APHA-AWWA-WPCF (1998a) Method 4500-NO3-I, Standard Methods For the Examination of Water and Wastewater, 1998, pp. 4–121 Search PubMed.
- M. E. Carolan, R. J. Langel, D. May, A. DeSalvo, H. E. Gonzalez-Ribot, A. J. Mattson, M. D. Schueller, D. A. Thompson, D. M. Cwiertny and T. Z. Forbes, Survey of Ra/Ra and inorganic constituents in Iowa private drinking water wells, AWWA Water Sci., 2022, 4, e1311 CrossRef CAS.
- Drinking Water and Public Health in the United States, https://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2020/01/13/drinking-water-and-public-health-in-the-united-states, (accessed 23 April 2023).
- S.-J. Haig, N. Kotlarz, L. M. Kalikin, T. Chen, S. Guikema, J. J. LiPuma and L. Raskin, Emerging investigator series: bacterial opportunistic pathogen gene markers in municipal drinking water are associated with distribution system and household plumbing characteristics, Environ. Sci.: Water Res. Technol., 2020, 6, 3032–3043 RSC.
- J. G. Caporaso, G. Ackermann, A. Apprill, M. Bauer, D. Berg-Lyons, J. Betley, N. Fierer, L. Fraser, J. A. Fuhrman, J. A. Gilbert, N. Gormley, G. Humphrey, J. Huntley, J. K. Jansson, R. Knight, C. L. Lauber, C. A. Lozupone, S. McNally, D. M. Needham, S. M. Owens, A. E. Parada, R. Parsons, G. Smith, L. R. Thompson, L. Thompson, P. J. Turnbaugh, W. A. Walters and L. Weber, EMP 16S Illumina Amplicon Protocol.
- E. Bolyen, J. R. Rideout, M. R. Dillon, N. A. Bokulich, C. C. Abnet, G. A. Al-Ghalith, H. Alexander, E. J. Alm, M. Arumugam, F. Asnicar, Y. Bai, J. E. Bisanz, K. Bittinger, A. Brejnrod, C. J. Brislawn, C. T. Brown, B. J. Callahan, A. M. Caraballo-Rodríguez, J. Chase, E. K. Cope, R. Da Silva, C. Diener, P. C. Dorrestein, G. M. Douglas, D. M. Durall, C. Duvallet, C. F. Edwardson, M. Ernst, M. Estaki, J. Fouquier, J. M. Gauglitz, S. M. Gibbons, D. L. Gibson, A. Gonzalez, K. Gorlick, J. Guo, B. Hillmann, S. Holmes, H. Holste, C. Huttenhower, G. A. Huttley, S. Janssen, A. K. Jarmusch, L. Jiang, B. D. Kaehler, K. B. Kang, C. R. Keefe, P. Keim, S. T. Kelley, D. Knights, I. Koester, T. Kosciolek, J. Kreps, M. G. I. Langille, J. Lee, R. Ley, Y.-X. Liu, E. Loftfield, C. Lozupone, M. Maher, C. Marotz, B. D. Martin, D. McDonald, L. J. McIver, A. V. Melnik, J. L. Metcalf, S. C. Morgan, J. T. Morton, A. T. Naimey, J. A. Navas-Molina, L. F. Nothias, S. B. Orchanian, T. Pearson, S. L. Peoples, D. Petras, M. L. Preuss, E. Pruesse, L. B. Rasmussen, A. Rivers, M. S. Robeson, P. Rosenthal, N. Segata, M. Shaffer, A. Shiffer, R. Sinha, S. J. Song, J. R. Spear, A. D. Swafford, L. R. Thompson, P. J. Torres, P. Trinh, A. Tripathi, P. J. Turnbaugh, S. Ul-Hasan, J. J. J. van der Hooft, F. Vargas, Y. Vázquez-Baeza, E. Vogtmann, M. von Hippel, W. Walters, Y. Wan, M. Wang, J. Warren, K. C. Weber, C. H. D. Williamson, A. D. Willis, Z. Z. Xu, J. R. Zaneveld, Y. Zhang, Q. Zhu, R. Knight and J. G. Caporaso, Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2, Nat. Biotechnol., 2019, 37, 852–857 CrossRef CAS.
- T. Ward, J. Larson, J. Meulemans, B. Hillmann, J. Lynch, D. Sidiropoulos, J. R. Spear, G. Caporaso, R. Blekhman, R. Knight, R. Fink and D. Knights, BugBase predicts organism-level microbiome phenotypes, bioRxiv, 2017, preprint, DOI:10.1101/133462.
- R Core Team, R: A Language for Data Analysis and Graphics, J. Comput. Graph. Stat., 1996, 5, 299–314 CrossRef.
- M. H. Ward, R. R. Jones, J. D. Brender, T. M. De Kok, P. J. Weyer, B. T. Nolan, C. M. Villanueva and S. G. Van Breda, Drinking Water Nitrate and Human Health: An Updated Review, Int. J. Environ. Res. Public Health, 2018, 15, 1557 CrossRef.
- W. T. Clements, S.-R. Lee and R. J. Bloomer, Nitrate Ingestion: A Review of the Health and Physical Performance Effects, Nutrients, 2014, 6, 5224–5264 CrossRef.
- State of Iowa Public Drinking Water Program Annual Compliance Report - 2023.
- Directive - 91/676 - EN - EUR-Lex, https://eur-lex.europa.eu/eli/dir/1991/676/oj/eng, (accessed 27 January 2025).
- Thallium Statistics and Information|U.S. Geological Survey, https://www.usgs.gov/centers/national-minerals-information-center/thallium-statistics-and-information, (accessed 17 May 2024).
- A. L. J. Peter and T. Viraraghavan, Thallium: a review of public health and environmental concerns, Environ. Int., 2005, 31, 493–501 CrossRef CAS.
- I. T. Vargas, D. A. Fischer, M. A. Alsina, J. P. Pavissich, P. A. Pastén and G. E. Pizarro, Copper Corrosion and Biocorrosion Events in Premise Plumbing, Materials, 2017, 10, 1036 CrossRef.
- N. Boulay and M. Edwards, Role of temperature, chlorine, and organic matter in copper corrosion by-product release in soft water, Water Res., 2001, 35, 683–690 CrossRef CAS.
- X. Zhang, S. O. Pehkonen, N. Kocherginsky and G. Andrew Ellis, Copper corrosion in mildly alkaline water with the disinfectant monochloramine, Corros. Sci., 2002, 44, 2507–2528 CrossRef CAS.
- C. E. Hruby, R. D. Libra, N. Hall, M. D. Schueller, P. J. Weyer, C. L. Fields, D. W. Kolpin, L. E. Hubbard, M. R. Borchardt, S. K. Spencer, M. D. Wichman and E. T. Furlong, 2013 Survey of Iowa groundwater and evaluation of public well vulnerability classifications for contaminants of emerging concern, Iowa Department of Natural Resources, Iowa City, Iowa, USA, 2015 Search PubMed.
- B. D. Lindsey, K. Belitz, C. A. Cravotta, P. L. Toccalino and N. M. Dubrovsky, Lithium in groundwater used for drinking-water supply in the United States, Sci. Total Environ., 2021, 767, 144691 CrossRef CAS.
- J. Shen, X. Li, X. Shi, W. Wang, H. Zhou, J. Wu, X. Wang and J. Li, The toxicity of lithium to human cardiomyocytes, Environ. Sci. Eur., 2020, 32, 59 CrossRef CAS.
- O. US EPA, Drinking Water Regulations and Contaminants, https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants, (accessed 21 May 2023).
- USGS WRI99-4007 Review of Phosphorus Control Measures in the United States and Their Effects on Water Quality, https://pubs.usgs.gov/wri/wri994007/, (accessed 21 May 2023).
- I. Spencer-Williams, M. Meyer, W. DePas, E. Elliott and S.-J. Haig, Assessing the Impacts of Lead Corrosion Control on the Microbial Ecology and Abundance of Drinking-Water-Associated Pathogens in a Full-Scale Drinking Water Distribution System, Environ. Sci. Technol., 2023, 57, 20360–20369 CrossRef CAS.
- E. Rosales, G. Del Olmo, C. Calero Preciado and I. Douterelo, Phosphate Dosing in Drinking Water Distribution Systems Promotes Changes in Biofilm Structure and Functional Genetic Diversity, Front. Microbiol., 2020, 11 DOI:10.3389/fmicb.2020.599091.
- H. L. Young, Summary of ground-water hydrology of the Cambrian-Ordovician aquifer system in the northern Midwest, United States, U.S. Geological Survey, 1992 Search PubMed.
- Scientific Investigations Report, 2012.
- HA 730-J Cambrian-Ordovician aquifer system text, https://pubs.usgs.gov/ha/ha730/ch_j/J-text8.html, (accessed 21 May 2023).
- A. C. Cullom, R. L. Martin, Y. Song, K. Williams, A. Williams, A. Pruden and M. A. Edwards, Critical Review: Propensity of Premise Plumbing Pipe Materials to Enhance or Diminish Growth of Legionella and Other Opportunistic Pathogens, Pathogens, 2020, 9, 957 CrossRef CAS.
- J. O. Falkinham, Nontuberculous Mycobacteria from Household Plumbing of Patients with Nontuberculous Mycobacteria Disease, Emerging Infect. Dis., 2011, 17, 419–424 CrossRef.
- I. Rahmatika, D. Simazaki, F. Kurisu, H. Furumai and I. Kasuga, Occurrence and diversity of nontuberculous mycobacteria affected by water stagnation in building plumbing, Water Supply, 2023, 23, 5017–5028 CrossRef CAS.
- S. M. Blanc, D. Robinson and N. L. Fahrenfeld, Potential for nontuberculous mycobacteria proliferation in natural and engineered water systems due to climate change: A literature review, City Environ. Interact., 2021, 11, 100070 CrossRef.
- L. J. Caverly, L. A. Carmody, S.-J. Haig, N. Kotlarz, L. M. Kalikin, L. Raskin and J. J. LiPuma, Culture-Independent Identification of Nontuberculous Mycobacteria in Cystic Fibrosis Respiratory Samples, PLoS One, 2016, 11, e0153876 CrossRef.
- J. Xue, B. Zhang, J. Lamori, K. Shah, J. Zabaleta, J. Garai, C. M. Taylor and S. P. Sherchan, Molecular detection of opportunistic pathogens and insights into microbial diversity in private well water and premise plumbing, J. Water Health, 2020, 18, 820–834 CrossRef.
- C. Thom, C. J. Smith, G. Moore, P. Weir and U. Z. Ijaz, Microbiomes in drinking water treatment and distribution: A meta-analysis from source to tap, Water Res., 2022, 212, 118106 CrossRef CAS.
- S. Potgieter, Z. Dai, M. Havenga, S. Vosloo, M. Sigudu, A. Pinto and S. Venter, Reproducible Microbial Community Dynamics of Two Drinking Water Systems Treating Similar Source Waters, ACS ES&T Water, 2021, 1, 1617–1627 Search PubMed.
- W. Lin, Z. Yu, H. Zhang and I. P. Thompson, Diversity and dynamics of microbial communities at each step of treatment plant for potable water generation, Water Res., 2014, 52, 218–230 CrossRef CAS.
- G. Rizzatti, L. R. Lopetuso, G. Gibiino, C. Binda and A. Gasbarrini, Proteobacteria: A Common Factor in Human Diseases, BioMed Res. Int., 2017, 2017, 9351507 CAS.
- H. Zhang, M. Ma, T. Huang, Y. Miao, H. Li, K. Liu, W. Yang and B. Ma, Spatial and temporal dynamics of actinobacteria in drinking water reservoirs: Novel insights into abundance, community structure, and co-existence model, Sci. Total Environ., 2022, 814, 152804 CrossRef CAS.
- H. Zhang, D. Zhao, M. Ma, T. Huang, H. Li, T. Ni, X. Liu, B. Ma, Y. Zhang, X. Li, X. Lei and Y. Jin, Actinobacteria produce taste and odor in drinking water reservoir: Community composition dynamics, co-occurrence and inactivation models, J. Hazard. Mater., 2023, 453, 131429 CrossRef CAS.
- J. O. Falkinham, E. D. Hilborn, M. J. Arduino, A. Pruden and M. A. Edwards, Epidemiology and Ecology of Opportunistic Premise Plumbing Pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa, Environ. Health Perspect., 2015, 123, 749–758 CrossRef CAS.
- M. M. Moritz, H.-C. Flemming and J. Wingender, Integration of Pseudomonas aeruginosa and Legionella pneumophila in drinking water biofilms grown on domestic plumbing materials, Int. J. Hyg. Environ. Health, 2010, 213, 190–197 CrossRef CAS.
- F. Puente-Sánchez, A. Arce-Rodríguez, M. Oggerin, M. García-Villadangos, M. Moreno-Paz, Y. Blanco, N. Rodríguez, L. Bird, S. A. Lincoln, F. Tornos, O. Prieto-Ballesteros, K. H. Freeman, D. H. Pieper, K. N. Timmis, R. Amils and V. Parro, Viable cyanobacteria in the deep continental subsurface, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 10702–10707 CrossRef.
- M. Pester, C. Schleper and M. Wagner, The Thaumarchaeota: an emerging view of their phylogeny and ecophysiology, Curr. Opin. Microbiol., 2011, 14, 300–306 CrossRef CAS.
- K. Kitzinger, C. C. Padilla, H. K. Marchant, P. F. Hach, C. W. Herbold, A. T. Kidane, M. Könneke, S. Littmann, M. Mooshammer, J. Niggemann, S. Petrov, A. Richter, F. J. Stewart, M. Wagner, M. M. M. Kuypers and L. A. Bristow, Cyanate and urea are substrates for nitrification by Thaumarchaeota in the marine environment, Nat. Microbiol., 2019, 4, 234–243 CrossRef CAS.
- H. Daims, E. V. Lebedeva, P. Pjevac, P. Han, C. Herbold, M. Albertsen, N. Jehmlich, M. Palatinszky, J. Vierheilig, A. Bulaev, R. H. Kirkegaard, M. von Bergen, T. Rattei, B. Bendinger, P. H. Nielsen and M. Wagner, Complete nitrification by Nitrospira bacteria, Nature, 2015, 528, 504–509 CrossRef CAS.
- U.S. Department of Agriculture, 2022 Census of Agriculture: Dubuque County Profile, https://data.nass.usda.gov/Publications/AgCensus/2022/Online_Resources/County_Profiles/Iowa/cp19061.pdf, (accessed 5 November 2024).
- W. Cheng, J. Zhang, Z. Wang, M. Wang and S. Xie, Bacterial communities in sediments of a drinking water reservoir, Ann. Microbiol., 2014, 64, 875–878 CrossRef CAS.
- J. C. Prior, et al., Iowa's Groundwater Basics, 1st edn, 2003 Search PubMed.
- Z. Bao, T. Al, M. Couillard, G. Poirier, J. Bain, H. K. Shrimpton, Y. Z. Finfrock, A. Lanzirotti, D. Paktunc, E. Saurette, Y. Hu, C. J. Ptacek and D. W. Blowes, A cross scale investigation of galena oxidation and controls on mobilization of lead in mine waste rock, J. Hazard. Mater., 2021, 412, 125130 CrossRef CAS.
- B. Cron, P. Henri, C. S. Chan, J. L. Macalady and J. Cosmidis, Elemental Sulfur Formation by Sulfuricurvum kujiense Is Mediated by Extracellular Organic Compounds, Front. Microbiol., 2019, 10, 2710 CrossRef.
- Y. Zhou, C. Kellermann and C. Griebler, Spatio-temporal patterns of microbial communities in a hydrologically dynamic pristine aquifer, FEMS Microbiol. Ecol., 2012, 81, 230–242 CrossRef CAS.
- S. Potgieter, Z. Dai, M. Havenga, S. Vosloo, M. Sigudu, A. Pinto and S. Venter, Reproducible Microbial Community Dynamics of Two Drinking Water Systems Treating Similar Source Waters, ACS ES&T Water, 2021, 1, 1617–1627 Search PubMed.
- O. US EPA, Lead and Copper Rule, https://www.epa.gov/dwreginfo/lead-and-copper-rule, (accessed 24 May 2023).
- B. Pawłowski, D. Tyrala and M. Pilch, Metallographic Investigations of the Premature Corrosion Failure of Steel Seam-Welded Galvanized Cold Water Pipes, J. Fail. Anal. Prev., 2020, 20, 9–14 CrossRef.
- S. Gonzalez, J. Appels and J. Cortina, Presence of metals in drinking water distribution networks due to pipe material leaching: A review, Toxicol. Environ. Chem., 2013, 95(6), 870–889 CrossRef CAS.
- O. D. Schneider, M. W. Lechevallier, H. F. Reed and M. J. Corson, A comparison of zinc and nonzinc orthophosphate-based corrosion control, J. AWWA, 2007, 99, 103–113 CrossRef CAS.
- J. S. Klein and O. Lewinson, Bacterial ATP-driven transporters of transition metals: physiological roles, mechanisms of action, and roles in bacterial virulence†, Metallomics, 2011, 3, 1098–1108 CrossRef CAS.
- D. Lee, G. Calendo, K. Kopec, R. Henry, S. Coutts, D. McCarthy and H. M. Murphy, The Impact of Pipe Material on the Diversity of Microbial Communities in Drinking Water Distribution Systems, Front. Microbiol., 2021, 12 DOI:10.3389/fmicb.2021.779016.
- L. Demtröder, F. Narberhaus and B. Masepohl, Coordinated regulation of nitrogen fixation and molybdate transport by molybdenum, Mol. Microbiol., 2019, 111, 17–30 CrossRef.
- C. Iobbi-Nivol and S. Leimkühler, Molybdenum enzymes, their maturation and molybdenum cofactor biosynthesis in Escherichia coli, Biochim. Biophys. Acta, 2013, 1827, 1086–1101 CrossRef CAS.
- J. B. Glass, R. P. Axler, S. Chandra and C. R. Goldman, Molybdenum limitation of microbial nitrogen assimilation in aquatic ecosystems and pure cultures, Front. Microbiol., 2012, 3 DOI:10.3389/fmicb.2012.00331.
- E. M. Attridge and P. Rowell, Growth, heterocyst differentiation and nitrogenase activity in the cyanobacteria Anabaena variabilis and Anabaena cylindrica in response to molybdenum and vanadium, New Phytol., 1997, 135, 517–526 CrossRef CAS.
- P. Fay and L. de Vasconcelos, Nitrogen metabolism and ultrastructure in Anabaena cylindrica. II. The effect of molybdenum and vanadium, Arch. Microbiol., 1974, 99, 221–230 CrossRef CAS.
- A. L. Zerkle, C. H. House, R. P. Cox and D. E. Canfield, Metal limitation of cyanobacterial N2 fixation and implications for the Precambrian nitrogen cycle, Geobiology, 2006, 4, 285–297 CrossRef CAS.
- J. Sun, X. Zou, T. Xiao, Y. Jia, Z. Ning, M. Sun, Y. Liu and T. Jiang, Biosorption and bioaccumulation of thallium by thallium-tolerant fungal isolates, Environ. Sci. Pollut. Res., 2015, 22, 16742–16748 CrossRef CAS.
- G. Baruzzo, A. Serafini, F. Finotello, T. Sanavia, L. Cioetto-Mazzabò, F. Boldrin, E. Lavezzo, L. Barzon, S. Toppo, R. Provvedi, R. Manganelli and B. Di Camillo, Role of the Extracytoplasmic Function Sigma Factor SigE in the Stringent Response of Mycobacterium tuberculosis, Microbiol. Spectrum, 2023, 11(2) DOI:10.1128/spectrum.02944-22.
- S. Himpens, C. Locht and P. Supply, Molecular characterization of the mycobacterial SenX3–RegX3 two-component system: evidence for autoregulation, Microbiology, 2000, 146, 3091–3098 CrossRef CAS.
- A. Brand, L. Allen, M. Altman, M. Hlava and J. Scott, Beyond authorship: attribution, contribution, collaboration, and credit, Learn. Publ., 2015, 28, 151–155 CrossRef.
- CRediT, https://credit.niso.org/, (accessed 5 June 2025).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ew00216h |
| This journal is © The Royal Society of Chemistry 2025 |