 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Energy recovery from organic matter in municipal wastewater using a two-stage system with high-rate contact stabilization and activated sludge processes under seasonal water temperature variations
Kensuke
Sakurai
 *,
Yuji
Okayasu†
and
Chika
Abe
*,
Yuji
Okayasu†
and
Chika
Abe
Innovative Materials and Resources Research Center, Public Works Research Institute, 1-6, Minamihara, Tsukuba, Ibaraki 305-8516, Japan. E-mail: recycle-imarrc21@pwri.go.jp
First published on 19th March 2025
Abstract
To maximize energy recovery in municipal wastewater treatment plants, the high-rate contact stabilization and activated sludge (HiCS–AS) process—consisting of a two-stage sequencing batch reactor—represents a promising technology for the efficient recovery of organic matter from wastewater as sludge that can be readily converted to methane. The HiCS–AS process was studied under practical conditions using actual wastewater to determine the effect of seasonal water temperature fluctuations (15.9–26.5 °C) in the reaction tank on the methane gas production of sludge recovered from the entire system, compared with the simple activated sludge (SAS) process. The methane recovery rates were in the ranges of 0.13–0.17 g COD CH4 per g COD (produced methane as g COD per g COD of influent) for the HiCS–AS process and 0.08–0.15 g COD CH4 per g COD for the SAS process across all periods, with the HiCS–AS process consistently having higher methane recovery rates. Methane production from HiCS sludge ranged from 0.41 to 0.45 NL CH4 per g volatile solid (VS), surpassing the range of 0.27–0.28 NL CH4 per g VS for the SAS sludge across all periods. Furthermore, the quality of the effluent was verified, and the concentration of residual organic matter in the effluent of the HiCS–AS process was equivalent to that of the SAS process.
Water impactReducing substantial energy consumption for sustainability is an urgent issue in the field of urban wastewater management. In this study, a new technology using a high-rate contact stabilization process for wastewater treatment demonstrated superior energy recovery compared to existing processes, even while taking seasonal variations into account. The results provide essential knowledge for the practical application of the technology. |
Introduction
Sewerage systems are crucial for improving public health by facilitating the drainage of domestic wastewater and stormwater and preserving the quality of public waters. As of 2014, the electricity consumption associated with wastewater drainage and treatment accounted for 1% of global electricity usage, a figure expected to rise in the future.1 Consequently, reducing net energy consumption in the wastewater sector is a pressing necessity to combat global warming. At wastewater treatment plants (WWTPs), organic matter collected through sewage networks is processed, with a portion being diverted to excess sludge. This sludge then generates methane gas via anaerobic digestion, enabling WWTPs to offset their energy consumption by producing electricity.2,3 However, the widely used activated sludge (AS) process often falls short in maximizing organic matter recovery as excess sludge, as much of the organic matter is oxidized while remaining in the system. To enhance the sludge recovery rate—the proportion of organic matter recovered as excess sludge in influent organics—the high-rate AS (HRAS) process employs a relatively short solids retention time (SRT) of less than two days in full-scale WWTPs.4An innovative approach to improving sludge recovery is the high-rate contact stabilization (HiCS) process, an advancement over the HRAS process.5 The HiCS process operates with sequencing batch reactor (SBR) and continuous flow reactor configurations, incorporating stabilization, contact, and sedimentation stages. In the stabilization stage, returned sludge is aerated, and this aerated sludge subsequently interacts with the influent in the contact stage.
Carbon redirection and carbon harvesting are two important mechanisms for the recovery of organic matter.6 The HiCS process achieves high sludge redirection rates even in primary treated wastewater with low particulate organic matter content, such as the effluent from primary sedimentation tanks (PSTs) and chemically enhanced primary treatment (CEPT), owing to enhanced biological flocculation.7,8 Although PSTs require a large site area, their use remains a relatively energy-efficient process that minimally oxidizes organics while discharging them as primary sludge.9 Implementing the HiCS process subsequent to PSTs has been calculated to be more energy efficient than the HRAS process.10
In general, high sludge recovery rates require high carbon harvesting rates. The carbon harvesting rate represents the proportion of the recovered excess sludge in the sludge discharged from the HiCS process. Poor sedimentation is a key factor inhibiting sludge recovery.6 Given the importance of efficient solid–liquid separation for achieving high carbon harvest rates, the SBR configuration was adopted; this is because, in contrast to continuous flow systems, SBR configurations avoid inflow and outflow, which can interfere with sedimentation.11,12
In temperate regions such as Japan, California in the U.S., Greece, and Jordan, influent wastewater temperatures in WWTPs typically fluctuate between 15 °C and 25 °C.13 The impact of seasonal water temperature fluctuations on organic matter recovery in the HiCS process is an area where knowledge remains insufficient, presenting a significant challenge for practical application. Similarly, the HRAS process encounters these challenges, as noted by the review of Guthi et al.4
Due to the relatively high concentration of residual organic matter in the effluent from the HiCS process, additional post-treatment stages may be required to achieve water quality comparable to that of a conventional wastewater treatment process. In this study, the AS process was utilized as a later-stage treatment in a two-stage SBR configuration, referred to as the HiCS–AS process, assuming that it would be implemented in a WWTP without any discharge standards for nutrients.
In WWTPs, the methane production rate of recovered sludge is as crucial as the sludge recovery rate for evaluating energy recovery efficiency. Nevertheless, research reports on this topic remain scarce. Meerburg et al.5 observed that the methane production rate of HiCS sludge was significantly higher than that of AS sludge. Song et al.14 reported enhanced methane production rates in HiCS sludge treated with synthetic wastewater compared to HRAS and AS sludge. However, these studies primarily focused on untreated wastewater, not primary effluent. Since the sludge from the HRAS process fed with untreated wastewater contains a substantial amount of settleable organic matter equivalent to primary sludge, the methane generation rates are likely to differ from those in sludge produced from the HiCS process fed with primary effluent. Methane production rates in the HiCS process for primary effluent remain poorly understood, highlighting a gap that could be explored for future applications in full-scale WWTPs.
Reportedly, applying the SBR-based HiCS process to PST effluent and coupling it with an SBR-based AS process for post-treatment appear promising to maximize energy recovery while simultaneously achieving optimal effluent quality. However, aside from the limited understanding of the sludge recovery and methane conversion rates of the HiCS unit processes, there is a paucity of knowledge regarding methane recovery rates and treated water quality by the HiCS–AS process as a whole. Moreover, the effect of seasonal water temperature changes on methane recovery, crucial for the full-scale implementation of this process, remains understudied. This study aims to assess the efficacy of the HiCS–AS process concerning overall energy recovery and the effluent quality, as well as the impact of water temperature fluctuations, in comparison with the simple AS (SAS) process in a practical setting utilizing actual wastewater. This investigation involved operating a bench-scale pilot plant installed at a full-scale municipal WWTP and measuring the methane recovery rate, quantified by the sludge recovery rate and the methane conversion rate, and the effluent quality, during three different periods throughout the year and with different reactor water temperatures.
Materials and methods
Test apparatus description
In this study, a two-stage system comprising a HiCS process and an AS process, referred to as the HiCS–AS process, was examined. Each process was operated using an SBR configuration. For comparative analysis, a SAS process, defined as a one-stage AS process in an SBR configuration, was concurrently operated in parallel with the HiCS–AS process as shown in Fig. 1. The test apparatus, consisting of the HiCS, AS, and SAS processes, was housed within a municipal WWTP. The grit chamber effluent from the municipal WWTP was continuously treated by a PST at a surface overflow rate of 25 m3 m−2 d−1, slightly below the typical rate of 30–50 m3 m−2 d−1.13 The operating cycles for each process are detailed later in this section. During the lowest water temperature period, the reactor was placed in a 16 °C water bath to minimize heat loss from the water in the reactor due to the large temperature difference between the air and water. The flow rates of influent, wasted sludge and effluent, and the reaction volume for each process are provided in Table 1. All processes maintained a volume exchange ratio of 50%. In the HiCS process, sludge was not initially inoculated. The initial sludge inoculum for the AS and SAS processes was collected from the other lab-scale bioreactor treating the same municipal wastewater.| Process | Influent flow (L d−1) | Reactor volume (L) | HRT (h) | Sludge wasting (L d−1) | Effluent flow (L d−1) |
|---|---|---|---|---|---|
| Note: HiCS and AS processes are part of a two-stage HiCS–AS process.a In period A, sludge wasting and effluent flow in the HiCS process was 18 and 162 L d−1. | |||||
| HiCS | 180 | 30 | 4 | 24a | 156a |
| AS | 72 | 24 | 8 | 1.8 | 70.2 |
| SAS | 72 | 24 | 8 | 1.8 | 70.2 |
Analytical measurements
The operational period was divided into three phases: A, B, and C based on water temperature. Samples were collected four times over a 2-week period, following a preliminary run that lasted three times longer than the SRT to acclimate the sludge to the target temperature. This sequence of operations was repeated from periods A to C. These collected samples were refrigerated and analyzed within 24 h. Total chemical oxygen demand (tCOD) and soluble COD (sCOD) were measured using COD reaction vials and a VIS spectrophotometer (HACH Company, Loveland, CO). sCOD was determined by measuring the COD of the filtrate obtained using glass microfiber filters (Whatman Grade 934-AH). Particulate COD (pCOD) was determined by subtracting the sCOD from the tCOD. Dissolved oxygen levels in the reaction tank were monitored using a dissolved oxygen meter (HQ40D, HACH Company, Loveland, CO). Water temperatures of the influent and reaction tank were recorded every 20 min using a temperature logger (TR-71wb; T and D Corporation, Japan), with daily averages calculated. Total suspended solids (TSS) in the reaction tank were quantified according to the standard methods.15Methane production test
Following each operational period, sludge was sampled from each reactor as a substrate, gravity thickened, refrigerated, and tested within 48 h. Methane production tests were conducted in triplicate for each type of sludge using an automated methane potential test system (AMPTS2; Bioprocess Control AB, Sweden). The inoculum, digested sludge from the full-scale WWTP, was pre-incubated at 35 °C for at least one week to minimize endogenous methane production. The COD and volatile solids (VS) of the substrates and the inoculum were measured. For each test, 400 mL of inoculum was added to each glass vessel. The substrates were diluted with boiled tap water to a final volume of 100 mL, ensuring that the VS weight was 10–20% of the inoculum's VS weight to avoid excessive substrate input, and then transferred to the test vials. Each vial was maintained in a water bath at 35 °C and agitated continuously. The produced biogas was passed through 3 M sodium hydroxide to remove carbon dioxide and hydrogen sulfide. Following the protocol of the test system, the volume of the released methane gas was determined with a wet gas flow measuring device using liquid displacement and buoyancy and normalized to standard conditions. The methane production rate was calculated by dividing the normal volume of methane gas produced by the end of a 28 day test period, by the VS weight of the substrate. Net methane volumes were determined by subtracting the methane output of the blank assay from the total measured volumes. In the control assay, glucose was used as a substrate to ensure that the methane production was at least 85% of the theoretical value as expected. The pH of the substrate–inoculum mixture was measured at the end of the test, confirming a pH value of above 6.8.16 The pH of the digested sludge was measured using a pH meter (HM-40P; DKK-TOA Corporation, Japan).Calculation
SRT (d) was calculated using eqn (1): | (1) |
 | (2) |
Additionally, the sludge recovery rate (g COD g COD−1) is calculated as the product of the carbon redirection rate (g COD g COD−1) and the carbon harvesting rate (g COD g COD−1). Following the methodology of Rahman et al.,7 the carbon redirection rate (R) and the carbon harvesting rate (H) were computed using eqn (3) and (4), respectively:
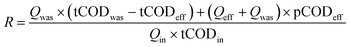 | (3) |
 | (4) |
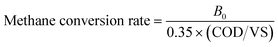 | (5) |
The methane recovery rate (g COD CH4 per g COD), representing the proportion of methane gas generated from recovered sludge relative to the organic matter in the influent wastewater in terms of COD, was computed using eqn (6):
| Methane recovery rate = Sludge recovery rate × Methane conversion rate | (6) |
To evaluate substantial differences in the methane generation rates among sludge produced by the HiCS, AS, and SAS processes during each period, multiple comparisons were conducted using Tukey's test with BellCurve for Excel 4.05 (Social Survey Research Information Co., Ltd., Tokyo, Japan). Moreover, two-sample Student's t-test was conducted to assess differences between the mean methane recovery rates of the HiCS–AS and SAS processes for each period using Microsoft Excel (Microsoft Corporation, Redmond, Washington, U.S.).
Results and discussion
Reactor operation and effluent quality
The COD of influent and effluents from each process is shown in Fig. 2. COD residuals in the effluent from the HiCS–AS process were comparable to those of the effluent from the SAS process. The SRT of the HiCS process during all periods was 0.39–0.49 days. The water temperature in the reactor tank, ranging from 15.9–26.5 °C, was consistently a few degrees below that of the influent throughout the period. The operational overview is also shown in Table 2.| Process | Period A | Period B | Period C | |
|---|---|---|---|---|
| Month of sampling | All | Sept.–Oct. | Oct.–Nov. | Jan.–Feb. |
| Note: HiCS and AS processes are part of a two-stage HiCS–AS process. The influent was PST effluent and was fed to HiCS and SAS processes. | ||||
| Water temperature in the reactor (°C) | Influent | 27.1 ± 1.2 | 23.9 ± 0.8 | 17.8 ± 0.7 |
| HiCS | 26.5 ± 2.0 | 22.1 ± 1.4 | 16.0 ± 0.3 | |
| AS | 25.9 ± 2.5 | 20.7 ± 1.7 | 16.0 ± 0.2 | |
| SAS | 25.8 ± 2.3 | 20.7 ± 1.6 | 15.9 ± 0.1 | |
| SRT (d) | HiCS | 0.49 ± 0.05 | 0.39 ± 0.04 | 0.42 ± 0.04 |
| AS | 4.8 ± 0.6 | 6.3 ± 0.2 | 5.9 ± 0.3 | |
| SAS | 5.3 ± 1.0 | 6.0 ± 0.4 | 6.2 ± 0.2 | |
| TSS in the stabilization or aeration phase (mg L−1) | HiCS | 250 ± 20 | 230 ± 10 | 170 ± 40 |
| AS | 470 ± 210 | 1460 ± 210 | 1300 ± 320 | |
| SAS | 2280 ± 1130 | 2310 ± 990 | 3010 ± 310 | |
Redirection pathways of influent organic matter
The redirection pathways of the influent organics for the HiCS–AS and SAS processes are illustrated in Fig. 3. The average sludge recovery rates for the HiCS–AS and SAS processes were 0.22 ± 0.06 and 0.21 ± 0.09 g COD g COD−1, respectively, across all periods (the value after the plus–minus sign indicates the standard deviation, hereinafter). The HiCS–AS value is the sum of those of the HiCS and AS processes. The sludge recovery rates for the HiCS–AS and SAS processes were higher at lower water temperatures. Regarding the individual unit processes, the sludge recovery rate for the HiCS process alone averaged 0.12 ± 0.03 g COD g COD−1 across all periods, showing little variation with water temperature. This stability is consistent with the findings of Shao et al.,17 who observed a similar trend of sludge recovery in the HRAS process at a SRT of 3 days. In contrast with the findings of Sakurai and Okayasu,18 which indicated differences in sludge recovery rates for the HiCS process between periods, this study did not identify any such differences between periods. The observed sludge recovery rate is lower than the 0.40 g COD g COD−1 average recovery rate for the HiCS process reported by Meerburg et al.5 This discrepancy could be attributed to the higher tCOD (757 mg L−1) and pCOD (268 mg L−1) of the synthetic wastewater used by Meerburg et al., which originally contained more settleable organic matter. Rahman et al.7 and Van Winckel et al.19 reported sludge recovery rates of 0.32 and 0.35 g COD g COD−1, respectively, from CEPT effluents under similar experimental conditions, which are significantly higher than those observed in our study. In contrast to the HiCS process, the AS and SAS processes exhibited variable sludge recovery in response to fluctuations in water temperature, with higher sludge recovery rates during the lower temperature periods. | ||
| Fig. 3 Redirection pathways of influent organic matter for the HiCS–AS and SAS processes, with standard deviation indicated by error bars. | ||
Notably, during period A, the average sludge recovery rates of the HiCS and SAS processes were nearly 0.12 g COD g COD−1, demonstrating close agreement. However, owing to the high methane conversion rate of sludge generated by the HiCS process (as described later), the methane recovery rate of the HiCS process during period A was considerably higher than that of the SAS process. In addition, methane gas recovered from AS sludge further enhances the energy recovery efficiency of the HiCS–AS process.
The proportions of residual organics in the effluents for the HiCS–AS process averaged 0.14 ± 0.04 g COD g COD−1 over the entire period, which is comparable to that of the SAS process (0.16 ± 0.05). For the HiCS process alone, the residual rate was 0.65 ± 0.08 g COD g COD−1. This is higher than the 0.45 g COD g COD−1 residual rate in effluent for the HiCS process treating CEPT effluent reported by Rahman et al.7 The proportions of organic matter oxidized in the HiCS–AS and SAS processes were similar, with values of 0.64 ± 0.07 and 0.63 ± 0.11 g COD g COD−1, respectively. For the HiCS process alone, the oxidation rate was 0.23 ± 0.07 g COD g COD−1, which aligns with the 0.23 g COD g COD−1 reported by Rahman et al.7 for the HiCS process treating CEPT effluent.
Carbon redirection and harvesting rates
The carbon redirection rate (R) and carbon harvesting rate (H) are calculated by eqn (3) and (4), and are presented in Table 3. The carbon redirection rate for the HiCS process was slightly lower than the pCOD to tCOD ratio in the influent, which ranged from 0.38 to 0.42 g COD g COD−1. This observation aligns with the studies by Rahman et al.7 and Van Winckel et al.,19 which examined the HiCS process treating CEPT effluents containing minimal settleable suspended organics. Throughout all periods, the carbon redirection rates for the AS and SAS processes were consistently lower than those observed for the HiCS process, attributable to the longer SRT and increased degradation. The carbon redirection rates were influenced by the conversion of organic matter in the influent to sludge through adsorption and assimilation as well as by its reduction via endogenous respiration and hydrolysis.4 Regarding interperiod differences, the lower carbon redirection rates were observed during warmer periods for the AS and SAS processes. This suggests that, during the warmer period, the extent of organic matter reduction through endogenous respiration and hydrolysis exceeds the extent of its conversion to sludge via adsorption and assimilation. The influence of water temperature on organic matter reduction through endogenous respiration and hydrolysis may be the primary factor for interperiod differences in the carbon conversion rates in the AS and SAS processes. Unlike AS and SAS sludge, HiCS sludge remains in the system for a shorter duration, resulting in minimal degradation effects on the sludge redirection rate. This reduced susceptibility of the carbon redirection rate to degradation in the HiCS process is likely a key factor for its considerably higher value than that in the AS and SAS processes during high-temperature periods.| Process | Period A | Period B | Period C | |
|---|---|---|---|---|
| Note: HiCS and AS processes are part of a two-stage HiCS–AS process. The influent was PST effluent and was fed to HiCS and SAS processes. | ||||
| pCOD in tCOD (g COD g COD−1) | Influent | 0.40 ± 0.06 | 0.42 ± 0.04 | 0.38 ± 0.13 |
| Carbon redirection rate (g COD g COD−1) | HiCS | 0.39 ± 0.05 | 0.37 ± 0.03 | 0.35 ± 0.05 |
| AS | 0.16 ± 0.09 | 0.17 ± 0.03 | 0.25 ± 0.09 | |
| HiCS–AS | 0.49 ± 0.02 | 0.48 ± 0.04 | 0.53 ± 0.07 | |
| SAS | 0.17 ± 0.06 | 0.21 ± 0.09 | 0.32 ± 0.04 | |
| Carbon harvesting rate (g COD g COD−1) | HiCS | 0.32 ± 0.04 | 0.32 ± 0.04 | 0.35 ± 0.11 |
| AS | 0.54 ± 0.12 | 0.88 ± 0.07 | 0.78 ± 0.10 | |
| HiCS–AS | 0.37 ± 0.06 | 0.45 ± 0.03 | 0.49 ± 0.12 | |
| SAS | 0.82 ± 0.05 | 0.90 ± 0.09 | 0.91 ± 0.04 | |
As shown in Table 3, the carbon harvesting rates for the HiCS process ranged from 0.32 to 0.35 g COD g COD−1, with no apparent effect of varying water temperatures observed. The carbon harvesting rates for the SAS process were consistently high across all periods, ranging from 0.82 to 0.91 g COD g COD−1, with higher rates typically observed at lower temperatures. For the AS process, the rates varied between 0.54 and 0.88 g COD g COD−1.
One factor limiting the carbon harvesting rate is the settling characteristics of the sludge.6 According to Ekama and Wentzel,20 when the sludge age is less than 4 days, the non-sedimentation component of the AS floc increases because of limited predatory activity of protozoa. Accordingly, because the carbon harvesting rate is fundamentally low in the HiCS process, efforts to increase the carbon harvesting rate could be effective for sludge recovery. In addition, the higher the carbon harvesting rate, the more the sludge that settles and is brought into the next cycle. Therefore, increasing the carbon harvesting rate would also improve the carbon redirection rate. Bisogni and Lawrence21 conducted a study on sludge with SRTs ranging from 0.25 to 12 days and found that sludge settled better when the SRT was 1 day or longer. Consequently, extending the SRT expectedly improves sludge sedimentation rates. However, because increasing the SRT will decrease the observed biomass yield by increasing the endogenous respiration and the hydrolysis as well known, it is beneficial to identify and maintain an optimal SRT that allows for high sludge sedimentation and low sludge degradation. In the study by Sakurai and Okayasu,18 the HiCS process operated at a longer SRT had superior sludge recovery at low water temperatures, with a maximum carbon harvesting rate of 0.48 g COD g COD−1. In contrast, herein, the carbon harvesting rate of the HiCS process during the low-water-temperature period was relatively low (0.35 g COD g COD−1). During the low-water-temperature period, extending the SRT to enhance sludge settleability can benefit sludge recovery as lower temperatures suppress endogenous respiration and hydrolysis of the sludge.22
In the studies by Rahman et al.7 and Van Winckel et al.,19 the carbon harvesting rates for the HiCS process treating CEPT effluents were 0.58 and 0.68 g COD g COD−1, respectively. In contrast, the carbon harvesting rate of the HiCS process in our study ranged from 0.32 to 0.35 g COD g COD−1, which is lower than those reported in these studies. As reported by Checa-Fernández et al.,23 iron ions added during the coagulation process may remain in the CEPT effluent. Residual iron ions are known to improve the settling of sludge in biological treatment after CEPT.24,25 The absence of CEPT treatment in our study likely contributed to the relatively low carbon harvesting rate observed. Further research is necessary, as there are few instances of the HiCS process being applied to PST effluent.
Van Winckel et al.19 compared sludge from the HiCS and HRAS processes treating CEPT effluents and found that HiCS sludge exhibited superior bioflocculation and organic capture, albeit with a lower settling velocity that increased sensitivity to the surface overflow rate in the sedimentation tank. This underscores the significance of the sedimentation process in enhancing the carbon harvesting rate in the HiCS process. Additionally, a study by Rey-Martínez et al.26 indicated that the SBR configuration resulted in better sludge settling compared to a continuous flow setup. The implementation of the SBR configuration in our HiCS process likely played a role in achieving a relatively higher carbon harvesting rate.
Considering the overall carbon conversion and carbon harvesting rates, the combined HiCS–AS process exhibited a higher carbon redirection rate but a lower carbon harvesting rate across all periods than the SAS process as shown in Table 3. In particular, during period A, the carbon harvesting rate of the HiCS–AS process was slightly lower than half that of the SAS process. However, as previously mentioned, the high carbon redirection rate of the HiCS process led to the high carbon redirection rate of the HiCS–AS process, which was approximately three times higher than that of the SAS process.
Methane recovery rates
The methane production rates are shown in Fig. 4. The methane production rate of HiCS sludge ranged from 0.41 to 0.45 NL CH4 per g VS, surpassing the rates observed for SAS sludge, which were between 0.27 and 0.28 NL CH4 per g VS across all periods. Through multiple comparisons, the methane production rate of HiCS sludge was observed to be statistically significantly higher than those of the AS and SAS sludge at the 5% significance level across all periods. The high methane production rate represents a substantial advantage concerning energy recovery within the HiCS process. Noike27 reported methane production rates for primary and excess sludge at four full-scale WWTPs in Japan, with values ranging from 0.24 to 0.48 and from 0.18 to 0.31 NL CH4 per g VS, respectively. The methane production rate for HiCS sludge in our study was higher than that of the excess sludge and within the range of the primary sludge in his study.27 Sakurai and Okayasu18 observed that the methane production rate from sludge in the HiCS process treating low-strength wastewater was 0.38 NL CH4 per g VS. Except for their study, no studies were identified that specifically examined the methane production rates from HiCS sludge treating PST effluent, making our findings a valuable contribution to the field.Generally, sludge from the HRAS process fed with non-primary treated wastewater has similar properties to primary sludge due to its high organic content, suggesting that its methane production rate should approximate that of primary sludge. Previous studies on the HRAS process treating wastewater without primary treatment reported methane production rates ranging between 0.30 and 0.46 NL CH4 per g VS.14,28,29 Despite containing less settleable organic matter, the HiCS sludge treated with PST effluent exhibited a methane production rate comparable to that of primary sludge. This phenomenon can be attributed to the fact that HiCS sludge, unlike conventional AS sludge, contains a greater proportion of easily degradable organic matter that has not been completely utilized by the microorganisms owing to the short residence time, facilitating methanogenesis, as noted by Trzcinski et al.29 Methane production rates for sludge from the AS process, the subsequent stage of the HiCS process, varied from 0.27 to 0.38 NL CH4 per g VS, occasionally exceeding those of the SAS sludge, though the underlying reasons remain unclear.
The COD/VS of the recovered sludge was instrumental in calculating the methane conversion rates, presented in Fig. 4. The COD/VS of HiCS sludge ranged from 1.53 to 1.80, higher than that of AS and SAS sludge during the same periods. Ahnert et al.30 reported that typical primary sludge and excess sludge have COD/VS ratios of 1.77 ± 0.29 and 1.53 ± 0.22, respectively, indicative of their lipid, protein, and carbohydrate composition ratios. The COD/VS values of HiCS sludge in our study were close to those of primary and excess sludge as described by Ahnert et al.,30 indicating that this sludge may possess similar compositions. The methane conversion rate of HiCS sludge varied from 0.65 to 0.82 g COD CH4 per g COD, reflecting differences in COD/VS across different periods.
The methane recovery rates for the HiCS–AS and SAS processes for each period, calculated using eqn (6), are depicted in Fig. 5. The methane recovery rate for the HiCS–AS process is the combined total of the methane recovery rates from the HiCS and AS processes. Across all periods, the methane recovery rates for the HiCS–AS and SAS processes ranged from 0.13 to 0.17 g COD CH4 per g COD and from 0.08 to 0.15 g COD CH4 per g COD, respectively, with the HiCS–AS process consistently having higher methane recovery rates. Compared with the SAS process, the HiCS–AS process requires a HiCS tank with a volume equivalent to 50% of the SAS tank volume. The addition of this HiCS tank to the WWTP itself could be an attractive option. When comparing the methane recovery rates of the HiCS–AS and SAS processes across periods, period A exhibited a significant difference at the 5% significance level. This difference is primarily attributed to the considerably higher methane conversion rate of HiCS sludge than that of the SAS sludge across all periods. Moreover, the greater recovery of the HiCS sludge, even during warmer periods when the SAS sludge is prone to degradation, contributes to the above difference. The methane recovery rate of HiCS–AS was 0.05 g COD CH4 per g COD higher than that of SAS in period A. This difference in the methane recovery rate is equivalent to a difference of 19 W h m−3 in power production, assuming an influent COD of 271 mg L−1, a methane calorific value of 13.9 kJ g−1 COD CH4,31 and an electricity generation efficiency of 35%.32 The implementation of the HiCS–AS process is expected to result in a notable increase in power production during the warmer season, considering that the average power production of full-scale WWTPs in Japan with anaerobic digesters and power generation facilities is 103 W h m−3.33 In contrast, no significant differences were observed in the methane recovery rates between the HiCS–AS and SAS processes during periods B and C. This study indicates that the implementation of the SBR-based HiCS–AS process results in higher methane recovery rates during warmer seasons compared to a simple SBR-based AS process.
Conclusions
This research evaluated the HiCS–AS and SAS processes, both fed with PST effluent, at varying water temperatures (15.9–26.5 °C) to determine their impact on energy recovery. The sludge recovery rates for the HiCS–AS and SAS processes varied because of water temperature fluctuations, and averaged 0.22 ± 0.06 g COD g COD−1 and 0.21 ± 0.09 g COD g COD−1, respectively, across all periods. The sludge recovery rate for the HiCS process alone was consistently 0.12 ± 0.03 g COD g COD−1, showing minimal variation between periods. The AS and SAS processes exhibited higher sludge recovery rates during lower temperature periods. The methane production rate from the HiCS sludge ranged from 0.41 to 0.45 NL CH4 per g VS, exceeding the range from 0.27 to 0.28 NL CH4 per g VS observed for the SAS sludge in all periods. The high methane production rate represents a substantial advantage concerning energy recovery within the context of the HiCS process. The organic residuals in the effluent from the HiCS–AS process were comparable to those of the effluent from the SAS process. Consequently, the methane recovery rates for the HiCS–AS and SAS processes, derived from the sludge recovery and methane conversion rates for each process, ranged from 0.13 to 0.17 g COD CH4 per g COD and from 0.08 to 0.15 g COD CH4 per g COD, respectively, across all periods, with the HiCS–AS process consistently having higher methane recovery rates. Notably, during the highest water temperature period, the methane recovery rate of the HiCS–AS process statistically significantly outperformed that of the SAS process (Student's t-test, p < 0.05), largely due to the higher sludge recovery rate and enhanced methane production from the HiCS sludge. This study supports the implementation of the SBR-based HiCS–AS process, particularly during warmer seasons, as it yields higher methane recovery rates compared to the SBR-based simple AS process.Data availability
Data will be made available on reasonable request.Conflicts of interest
The authors declare no competing interest.Acknowledgements
The authors thank the staff of the local government and the wastewater treatment plant for their kind support.References
- IEA (International Energy Agency), Water energy nexus (Excerpt from the world energy outlook), 2016, https://www.iea.org/reports/water-energy-nexus, (Accessed 22th May 2024) Search PubMed.
- V. Bohra, K. U. Ahamad, A. Kela, G. Vaghela, A. Sharma and B. J. Deka, Chapter 2 - Energy and resources recovery from wastewater treatment systems, in Clean Energy and Resource Recovery, ed. A. An, V. Tyagi, M. Kumar and Z. Cetecioglu, Elsevier Inc., 2022, pp. 17–36 Search PubMed.
- S. Periyasamy, T. Temesgen, V. Karthik, I. J. Beula, S. Kavitha, B. J. Rajesh and P. Sivashanmugam, Chapter 17 - Wastewater to biogas recovery, in Clean Energy and Resource Recovery, ed. A. An, V. Tyagi, M. Kumar and Z. Cetecioglu, Elsevier Inc., 2022, pp. 301–314 Search PubMed.
- R. S. Guthi, K. Tondera, S. Gillot, P. Buffiere, M. Boillot and F. Chazarenc, A-Stage process - Challenges and drawbacks from lab to full scale studies: A review, Water Res., 2022, 226, 119044 CrossRef CAS PubMed.
- F. A. Meerburg, N. Boon, T. Van Winckel, J. A. R. Vercamer, I. Nopens and S. E. Vlaeminck, Toward energy-neutral wastewater treatment: a high-rate contact stabilization process to maximally recover sewage organics, Bioresour. Technol., 2015, 179, 373–381 CrossRef CAS PubMed.
- J. Canals, A. Cabrera-Codony, O. Carbo, J. Toran, M. Martin, M. Baldi, B. Gutierrez, M. Poch, A. Ordonez and H. Monclus, High-rate activated sludge at very short SRT: Key factors for process stability and performance of COD fractions removal, Water Res., 2023, 231, 119610 CrossRef CAS PubMed.
- A. Rahman, F. A. Meerburg, S. Ravadagundhi, B. Wett, J. Jimenez, C. Bott, A. Al-Omari, R. Riffat, S. Murthy and H. De Clippeleir, Bioflocculation management through high-rate contact-stabilization: A promising technology to recover organic carbon from low-strength wastewater, Water Res., 2016, 104, 485–496 CrossRef CAS PubMed.
- A. Rahman, M. Hasan, F. Meerburg, J. A. Jimenez, M. W. Miller, C. B. Bott, A. Al-Omari, S. Murthy, A. Shaw, H. De Clippeleir and R. Riffat, Moving forward with A-stage and high-rate contact-stabilization for energy efficient water resource recovery facility: Mechanisms, factors, practical approach, and guidelines, J. Water Process Eng., 2020, 36, 101329 CrossRef.
- R. Gori, F. Giaccherini, L. M. Jiang, R. Sobhani and D. Rosso, Role of primary sedimentation on plant-wide energy recovery and carbon footprint, Water Sci. Technol., 2013, 68, 870–878 CrossRef CAS PubMed.
- A. Rahman, H. De Clippeleir, W. Thomas, J. A. Jimenez, B. Wett, A. Al-Omari, S. Murthy, R. Riffat and C. Bott, A-stage and high-rate contact-stabilization performance comparison for carbon and nutrient redirection from high-strength municipal wastewater, Chem. Eng. J., 2019, 357, 737–749 CrossRef CAS.
- U.S. EPA (U.S. Environmental Protection Agency), Wastewater technology fact sheet; Sequencing Batch Reactors, 1999, https://www3.epa.gov/npdes/pubs/sbr_new.pdf, (Accessed 22th May 2024) Search PubMed.
- M. H. Gerardi, SBR phases, in Troubleshooting the Sequencing Batch Reactor, John Wiley & Sons, Inc., Hoboken, New Jersey, 2010, pp. 17–28 Search PubMed.
- Metcalf & Eddy Inc., Wastewater engineering: Treatment and resource recovery, McGraw-Hill Education, New York, NY, 5th edn, 2014 Search PubMed.
- M. Song, M. Chun, J. Park, S. Jeong, T. Lee, Y. Shin, J. Kim and H. Bae, Effects of contact/stabilization time ratio on organic carbon-methane conversion in high-rate contact stabilization system, J. Water Process Eng., 2024, 60, 105247 CrossRef.
- APHA (American Public Health Association), AWWA (American Water Works Association) and WEF (Water Environment Federation), Standard methods for the examination of water and wastewater, American Public Health Association, Washington DC, 2017 Search PubMed.
- VDI 4630, Fermentation of organic materials - Characterization of the substrate, sampling, collection of material data, fermentation tests, Engl. VDI-Gesellschaft Energie und Umwelt, 2016 Search PubMed.
- Y. Shao, Y. Wang, H. Wang, G. Liu, L. Qi, X. Xu, J. Zhang, S. Liu and W. Sun, Effect of operating temperature on the efficiency of ultra-short-sludge retention time activated sludge systems, Environ. Sci. Pollut. Res., 2021, 28, 39257–39267 CrossRef CAS PubMed.
- K. Sakurai and Y. Okayasu, Improvement of organic carbon recovery from wastewater by changing water temperature in high-rate contact stabilization process, Int. J. Environ. Sci. Technol., 2025, 22, 2127–2136 CrossRef CAS.
- T. Van Winckel, N. Ngo, B. Sturm, A. Al-Omari, B. Wett, C. Bott, S. E. Vlaeminck and H. De Clippeleir, Enhancing bioflocculation in high-rate activated sludge improves effluent quality yet increases sensitivity to surface overflow rate, Chemosphere, 2022, 308, 136294 CrossRef CAS PubMed.
- G. A. Ekama and M. C. Wentzel, Organic matter removal, in Biological wastewater treatment: Principles, modelling and design, ed. G. Chen, M. C. M. van Loosdrecht, G. A. Ekama and D. Brdjanovic, IWA Publishing, London, 2nd edn, 2023, pp. 111–160 Search PubMed.
- J. J. Bisogni and A. W. Lawrence, Relationships between biological solids retention time and settling characteristics of activated sludge, Water Res., 1971, 5(9), 753–763 CrossRef CAS.
- M. Henze, W. Gujer, T. Mino and M. van Loosedrecht, Activated Sludge Models ASM1, ASM2, ASM2d and ASM3, IWA Publishing, London, 2006 Search PubMed.
- A. Checa-Fernández, L. M. Ruiz, J. M. Torre-Marín, A. Muñoz-Ubina, J. I. Pérez and M. A. Gómez, Direct application of chemically enhanced primary treatment in a municipal wastewater treatment plant: A case study, Chem. Eng. Res. Des., 2024, 204, 183–192 Search PubMed.
- E. J. Lees, B. Noble, R. Hewitt and S. A. Parsons, The impact of residual coagulant on downstream treatment processes, Environ. Technol., 2001, 22, 113–122 Search PubMed.
- E. J. Lees, B. Noble, R. Hewitt and S. A. Parsons, The impact of residual coagulant on the respiration rate and sludge characteristics of an activated microbial biomass, Process Saf. Environ. Prot., 2001, 79, 283–290 CrossRef CAS.
- N. Rey-Martínez, A. Barreiro-López, A. Guisasola and J. A. Baeza, Comparing continuous and batch operation for high-rate treatment of urban wastewater, Biomass Bioenergy, 2021, 149, 106077 CrossRef.
- T. Noike, Methane fermentation, Gihodo shuppan Co., Ltd., Tokyo, Japan, 2009 Search PubMed.
- A. Taboada-Santos, E. Rivadulla, L. Paredes, M. Carballa, J. Romalde and J. M. Lema, Comprehensive comparison of chemically enhanced primary treatment and high-rate activated sludge in novel wastewater treatment plant configurations, Water Res., 2020, 169, 115258 Search PubMed.
- A. P. Trzcinski, L. Ganda, C. Kunacheva, D. Q. Zhang, L. L. Lin, G. Tao, Y. Lee and W. J. Ng, Characterization and biodegradability of sludge from a high rate A-stage contact tank and B-stage membrane bioreactor of a pilot-scale AB system treating municipal wastewaters, Water Sci. Technol., 2016, 74, 1716–1725 CrossRef CAS PubMed.
- M. Ahnert, T. Schalk, H. Bruckner, J. Effenberger, V. Kuehn and P. Krebs, Organic matter parameters in WWTP - a critical review and recommendations for application in activated sludge modelling, Water Sci. Technol., 2021, 84, 2093–2112 CrossRef CAS PubMed.
- E. S. Heidrich, T. P. Curtis and J. Dolfing, Determination of the internal chemical energy of wastewater, Environ. Sci. Technol., 2011, 45, 827–832 CrossRef CAS PubMed.
- P. L. McCarty, J. Bae and J. Kim, Domestic wastewater treatment as a net energy producer–Can this be achieved?, Environ. Sci. Technol., 2011, 45, 7100–7106 CrossRef CAS PubMed.
- JSWA (Japan Sewage Works Agency), Electricity energy self-sufficiency in sewage treatment plants (in Japanese), 2020, https://www.jswa.go.jp/g/g5/g5m/mb/pdf04/222-1.pdf, (accessed 20th December 2023) Search PubMed.
Footnote |
| † Present address: Water Environment Research Group, Public Works Research Institute, 1-6, Minamihara, Tsukuba, Ibaraki 305-8516, Japan. |
| This journal is © The Royal Society of Chemistry 2025 |




