Promoting the growth of rice and reducing the accumulation of Cd in rice by pig bedding derived carbon dots (PBCDs) under Cd stress†
Tianlian
He
a,
Xingyu
Hao
a,
Ying
Chen
a,
Zhenguo
Li
a,
Xinyu
Zheng
 *b,
Mingwei
Yang
b,
YuLin
Wang
b,
Chengzhen
Gu
b,
Jianghua
Ye
c and
Haibin
Wang
c
*b,
Mingwei
Yang
b,
YuLin
Wang
b,
Chengzhen
Gu
b,
Jianghua
Ye
c and
Haibin
Wang
c
aCollege of JunCao Science and Ecology, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China
bCollege of Life Science, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China. E-mail: zxy0621@fafu.edu.cn
cCollege of Tea and Food Science, Wuyi University, Wuyi, Fujian 354300, China
First published on 14th November 2024
Abstract
Cadmium (Cd) causes significant disruption to plant growth and poses a threat to human health, necessitating urgent and effective measures to mitigate its absorption and translocation in rice. This study employed a co-treatment of carbon dots (PBCDs) with Cd. The potential mechanisms underlying the alleviation of Cd toxicity in rice by PBCDs were investigated by observing changes in photosynthesis, the antioxidant system, and the content of other divalent metals in rice. The results showed that under Cd stress, PBCDs mitigated the interference of Cd in photosynthesis. Notably, treatments with 100 and 250 mg L−1 PBCDs significantly increased the rice fresh weight by 32.45% and 31.54%, and reduced Cd concentrations in rice leaves by 53.82% and 45.81%, respectively. Moreover, PBCDs effectively reduced the shoot-to-leaf translocation factor (TF) of Cd by up to 45.76%, likely due to enhanced Zn concentrations in shoots. Furthermore, PBCDs enhanced the activity of antioxidant enzymes (SOD, POD, CAT) in rice, resulting in decreased levels of MDA induced by Cd stress. In conclusion, PBCDs enhanced rice antioxidant enzyme activity, photosynthetic efficiency, and biomass while mitigating cellular damage and reducing Cd concentrations in various tissues. These findings provide theoretical guidance and data support for the study of novel nanomaterials to promote crop growth under Cd stress conditions and alleviate Cd accumulation in plants.
Environmental significanceCarbon dots (CDs), which are carbon nanomaterials, have been greatly developed for several applications including fluorescent sensors for antioxidant activity and plant growth promotion due to their excellent photophysical properties and non-toxicity. Cadmium (Cd), a highly toxic and water-soluble element, poses a significant threat to human health as it's absorbed by plants and enters the food chain. Urgent measures are necessary to reduce Cd levels in rice for public health safety. Therefore, this research focused on the synthesis of multifunctional carbon dots from pig padding and used a co-treatment of PBCDs and Cd. The potential mechanisms of PBCDs in alleviating Cd toxicity in rice seedlings were investigated by monitoring physiological parameters like photosynthesis and antioxidants. |
Introduction
Cadmium (Cd) is a highly toxic and water-soluble element with no biological function in plants. It is readily absorbed by plants, enters the food chain, and poses risks of acute or chronic toxicity to humans.1,2 As China's primary staple crop, rice has a pronounced ability to absorb and accumulate Cd. Excessive Cd accumulation in rice inhibits growth, disrupts normal physiological processes, and results in decreased yields and compromised food quality.3,4 Surveys indicate that approximately 33.54% of China's farmland is affected by Cd pollution, leading to an increase in Cd-contaminated rice.5 Therefore, it is crucial to promptly implement effective strategies to mitigate Cd absorption and transportation in rice, which is essential for ensuring food security and protecting public health.Excessive Cd accumulation in plants disrupts cellular metabolism, hinders photosynthesis, reduces antioxidant enzyme activity, and induces oxidative stress, all of which adversely affect overall plant growth.6,7 In recent years, nanomaterials have gained significant interest for mitigating heavy metals (HMs) stress in plants due to their unique properties.8 Their small size allows them to be internalized by plants through endocytosis and phagocytosis, facilitating transport via both apoplastic and symplastic pathways.8 Nanomaterials enhance the plant's internal environment through various mechanisms, such as alleviating cellular oxidative damage,9 enhancing nutrient uptake,10 and promoting CO2 assimilation for improved photosynthetic efficiency.11 Additionally, they regulate specific molecular mechanisms to reduce the accumulation of toxic ions in plant cells, thus preventing ion stress. In summary, nanomaterials effectively alleviate HM-induced plant toxicity and contribute to enhancing the plant's tolerance to non-biological stress.12 Among various nanomaterials, carbon dots (CDs) have attracted attention due to their abundant surface functional groups and photoluminescence properties.13 Li et al. found that the spraying of CDs on rice seedlings increases photosynthetic efficiency.14 To further support their findings, Li et al. utilized biomass CDs synthesized by a one-step hydrothermal method and demonstrated that CDs can eliminate reactive oxygen species (ROS) in plant cells and effectively alleviate oxidative damage in lettuce under salt stress.15 Additionally, others' research showed that applying CDs to rice not only promotes growth but also enhances disease resistance.16 Therefore, CDs have substantial potential to alleviate plant stress by promoting plant growth and reducing excessive ROS accumulation.
Plant roots serve as the first line of defense against Cd stress, playing a crucial role in plant resistance.17 When confronted with various environmental adversities, plants primarily employ avoidance strategies, one of which involves synthesizing organic acids to chelate and sequester HMs.18 Studies have shown that under Cd stress, plants secrete organic acids from their roots, which chelate toxic elements, thereby reducing Cd absorption, affecting the bioavailability of HMs, and influencing the absorption of essential ions by plants.19 The interaction between divalent metal ions and Cd is particularly significant due to their chemical similarity, leading to competition for absorption and shared transport proteins.20 However, excessive Cd accumulation can lead to an outburst of ROS, disrupting cellular homeostasis.21 To counteract this, plants rely on antioxidant enzymes to maintain a balance between ROS production and scavenging.22 Additionally, phenolic compounds, secondary metabolites in plants, effectively protect them from oxidative damage caused by HMs stress.23 Numerous studies have shown that increased levels of phenolic compounds in plant roots represent a unique response to abiotic stress.24–26 Furthermore, plants can alleviate HM stress by regulating the levels of endogenous hormones that control their growth and metabolism.27,28
Padding is an important component of the fermentation bedding used during breeding, primarily composed of rice husks, distillers' grains, shredded straw, and dried cattle manure, making up 90% of the total composition. The remaining 10% consists of soil and a small amount of coarse salt. In livestock and poultry farms, bedding is typically spread on the ground to facilitate manure decomposition. Interestingly, converting bedding into biochar can spontaneously incorporate beneficial mineral elements such as nitrogen (N), silicon (Si), magnesium (Mg), and potassium (K), which support plant growth.29,30 Recent research has highlighted the significant role of CDs in alleviating Cd stress in plants. Panahirad et al. found that CDs can mitigate the chlorophyll disruption caused by Cd in grapevines.31 Building on prior research, Zhu et al.32 demonstrated that CDs derived from Salvia miltiorrhiza can reduce Cd absorption in wheat by generating excess Fe signals, which inhibit the expression of genes related to HM uptake. Similarly, Li et al.33 found that CDs enhance the expression of glutathione synthesis genes in grapefruits, improving their physiological characteristics and mitigating the harmful effects of Cd. Inspired by these findings, we proposed using pig bedding as a raw material to produce CDs. The resulting CDs were then uniformly dispersed in various concentrations to create spraying solutions for application on rice leaves. By monitoring the physiological responses, Cd distribution, and Cd transport characteristics in rice, we aimed to gain a deeper understanding of the effects of pig bedding-derived CDs on rice under Cd stress. Finally, we explored the mechanisms by which these CDs regulate Cd accumulation in rice. This study provides valuable insights and technology support for utilizing discarded biomass materials to control and mitigate HM absorption and accumulation in crops.
Materials and methods
PBCD synthesis
The pig padding, sourced from a breeding farm in Fuqing City, Fujian Province, was subjected to pre-carbonization at 400 °C for 2 hours. Biochar (2.00 g) was placed in a 250 mL round-bottom flask, followed by the addition of 30 mL of 15% H2O2. The mixture was then refluxed at 130 °C for 3 h using a heating magnetic stirrer. After cooling, the solution was filtered through a 0.22 μm membrane and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes, yielding a clear, pale yellow transparent solution. The CDs were purified by dialyzing in a 1000 Da dialysis bag for 24 h, resulting in the final product, PBCDs.
000 rpm for 10 minutes, yielding a clear, pale yellow transparent solution. The CDs were purified by dialyzing in a 1000 Da dialysis bag for 24 h, resulting in the final product, PBCDs.
The size and morphology of PBCDs were observed using transmission electron microscopy (JEOL JEM-2100F, Japan). The infrared spectrum of PBCDs was analyzed using a Fourier-transform infrared spectrometer (AVATAR 360 FTIR, USA). The fluorescence spectrum of PBCDs was investigated using a fluorescence spectrophotometer (FS-5-STM, USA), and the elemental composition of PBCDs was confirmed through energy-dispersive X-ray analysis (FEI Talos F200x, USA).
The superoxide dismutase (SOD) mimetic activity and hydroxyl radical scavenging activity of PBCDs were evaluated using the NBT photoreduction method and the Fenton reaction, respectively.15
Plant cultivation
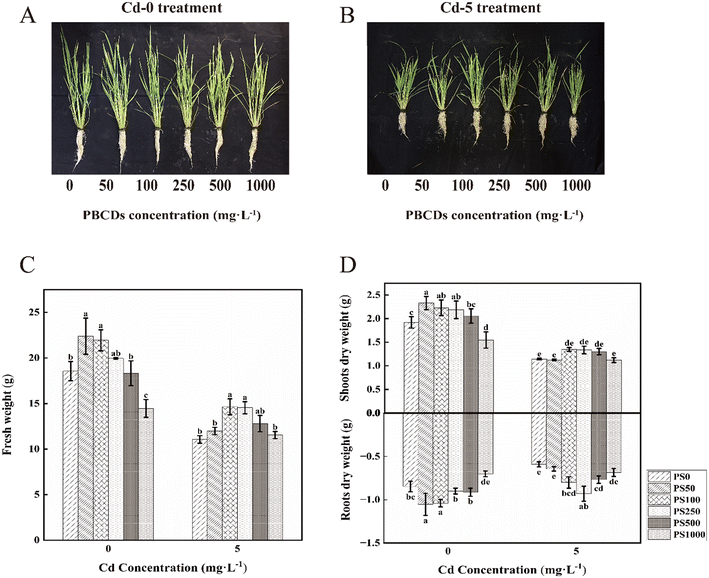
Rice seeds of “Baixiang 139” were selected and disinfected by soaking in 0.5% NaClO. After disinfection and thorough rinsing, the seeds were placed in a constant-temperature incubator set to 30 °C for germination. Once the rice seedlings reached approximately 5 cm in height, they were transplanted under controlled environmental conditions. The light intensity was set to 200 μmol m−2 s−1), with a 12 hour light/12 hour dark photoperiod. The temperature was maintained at 30 °C during the light and dark period, with relative humidity kept at 50–60%. After one week, they were moved to 1/2-Hoagland and cultured for 7 days. On the 8th day, they were transplanted into 5 L cultivation pots at a density of six seedlings per pot. One day before Cd stress, the rice leaves were sprayed with PBCDs at concentrations of 0 (PS0), 50 (PS50), 100 (PS100), 250 (PS250), 500 (PS500), and 1000 (PS1000) mg L−1, with 30 mL per pot. The control group was sprayed with an equal amount of purified water on the same day, ensuring uniform wetting of all leaves without dripping water as the standard. Cd stress treatments, 0 mg L−1 (Cd-0) and 5 mg L−1 (Cd-5), were applied the day after the pre-spraying of PBCDs. Throughout the experiment, no artificial controls were applied to regulate humidity, temperature, or photoperiod. The experiment included 12 treatments, each repeated three times. The nutrient solution was changed every 5 days during cultivation, and spraying was conducted every 4 days. After 15 days, the fresh weight and dry weight of the rice seedlings were measured and recorded.Photosynthetic rate and chloroplast ATPase activity
The photosynthetic rate among different treatments was measured using a portable photosynthesis system (TPS-2, USA). Three uniform seedlings were selected for each treatment, and three mature leaves from each selected seedling were used for the measurements.The Mg2+-ATP and Ca2+-ATP activities in rice leaves were determined using an ATPase assay kit supplied by Nanjing Jiancheng Bioengineering Institute.
Soluble carbohydrate content
Fresh rice leaves (0.10 g) were added to 5 mL of distilled water and heated in boiling water for 30 min. The resulting extract was then filtered and diluted to a final volume of 25 mL, serving as the soluble sugar extraction solution. Following the methodology of Moya et al.,34 the absorbance values among different treatments were measured at a wavelength of 485 nm, enabling the calculation of soluble sugar content in the samples.Metal concentration determination
Dry rice roots, stems, and leaves (0.2 g) were digested using a mixture of HNO3–HClO4 (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).35 The Cd concentration was analyzed using ASS (WFX-220A, China), and the concentration of Fe, Zn, Cu, Mn, K, Ca, and Mg was determined by ICP-MS (Agilent-7700, USA).
1).35 The Cd concentration was analyzed using ASS (WFX-220A, China), and the concentration of Fe, Zn, Cu, Mn, K, Ca, and Mg was determined by ICP-MS (Agilent-7700, USA).
The Cd translocation factor (TF) is the ratio of Cd content in roots to Cd content in tissues aboveground,36 and the formula is as follows:
| TFroot–stem = Cdstem/Cdroot |
| TFroot–leaf = Cdleaf/Cdroot |
| TFroot–shoot = Cdshoot/Cdroot |
Antioxidant enzyme activity and malondialdehyde content
The activities of peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) were determined using the guaiacol method, the nitroblue tetrazolium photochemical reduction method, and the hydrogen peroxide method, respectively. The malondialdehyde (MDA) content was measured using the thiobarbituric acid method. Fresh samples (0.20 g) were ground and filtered in 2 mL phosphate buffer (50 mmol L−1, pH 7.8) for antioxidant enzyme determination. Additionally, fresh leaf samples (0.20 g) were ground with 2 mL TCA, and the supernatant obtained after centrifugation at 4000 rpm for 10 min was used for MDA content determination.37Determination of organic acids and phenolic acids
The determination of organic acids was conducted with reference to established protocols,38 while the phenolic acid content followed specific standardized procedures39 and was detected using an HPLC system (Agilent 1260, USA) and an Eclipse XDB-C18 chromatographic column (4.6 mm × 250 mm, 5 μm).Statistical analysis
All data were statistically analyzed using SPSS 22 and expressed as the mean ± standard deviation (SD). Analysis of variance (one-way ANOVA, Duncan's test) was employed for statistical analysis of differences among different treatments, with comparisons conducted at a significance level of P < 0.05. All experiments were conducted with a minimum of three repetitions.Results
Characterization and antioxidant properties of PBCDs
Transmission electron microscopy (TEM) revealed the morphological characteristics of PBCDs, showing a nearly circular shape with a uniform size of 2.5 ± 0.5 nm (based on measurements of 215 particles) (Fig. 1A). The fluorescence spectrum (Fig. 1C) confirmed the fluorescence properties of PBCDs, with excitation/emission (EX/Em) at 347 nm/458 nm. Fourier-transform infrared (FTIR) spectroscopy was used to analyze the surface functional groups of PBCDs (Fig. 1B). The wavelength band at 3412 cm−1 was assigned to the vibrational peak of active hydrogen functional groups such as –OH and –NH. The band at 1594 cm−1 was associated with the deformation vibration of –N–H or –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.40 The wavelength band observed at 1121 cm−1 was assigned to Si–O–Si bonds,41 while those at 762 cm−1 and 465 cm−1 represented the symmetric stretching vibrations of the –Si–O– bond. In summary, the FTIR results revealed the presence of active hydrogen and silicon–oxygen-based functional groups on the surface of PBCDs. Energy-dispersive X-ray (EXD) spectroscopy (Fig. 1D) further indicated a high proportion of oxygen and Si elements within PBCDs.
O.40 The wavelength band observed at 1121 cm−1 was assigned to Si–O–Si bonds,41 while those at 762 cm−1 and 465 cm−1 represented the symmetric stretching vibrations of the –Si–O– bond. In summary, the FTIR results revealed the presence of active hydrogen and silicon–oxygen-based functional groups on the surface of PBCDs. Energy-dispersive X-ray (EXD) spectroscopy (Fig. 1D) further indicated a high proportion of oxygen and Si elements within PBCDs.
Based on the ROS in vitro simulation experiments conducted in this study, PBCDs exhibited superoxide SOD-mimetic activity. As shown in Fig. 1E, the absorbance of the reaction solution significantly decreased in the presence of PBCDs, indicating their SOD-like activity. Furthermore, at a concentration of 500 mg L−1, PBCDs achieved a scavenging efficiency of 91.71%, demonstrating their excellent SOD activity (Fig. 1F). Hydroxyl radicals (·OH) represent another critical ROS. As illustrated in Fig. 1G, a noticeable absorption peak was observed when PBCDs coexisted with Fe2+ and H2O2, and this peak diminished with increasing PBCDs concentration, suggesting effective ·OH scavenging by PBCDs. Fig. 1H shows a dose-dependent ·OH scavenging effect by PBCDs, with an elimination efficiency of 29.64% at a concentration of 1000 mg L−1. Overall, PBCDs exhibit outstanding intrinsic antioxidant properties, effectively neutralizing both superoxide anions (·O2−) and ·OH.
Effects of PBCDs on rice growth
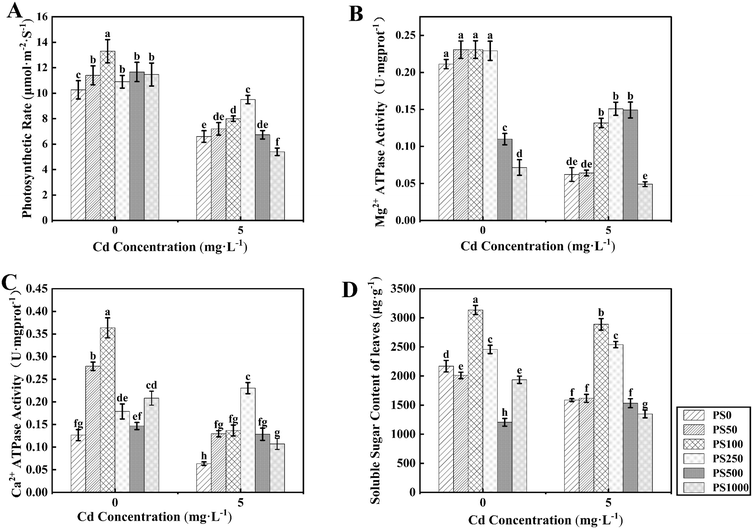
Biomass is frequently utilized as an indicator of the plant growth status under abiotic stress conditions. As illustrated in Fig. 2C (right), Cd typically inhibits growth, resulting in a significant reduction in plant fresh weight of 40.29%. In this study, the application of PBCDs on rice under normal growth conditions demonstrated a dose-dependent effect; low to moderate concentrations promoted growth, while a high concentration (1000 mg L−1) inhibited it (Fig. 2C, left). Specifically, when PBCDs were applied at concentrations ranging from 50 to 500 mg L−1, the rice fresh weight increased by 7.53– 20.68%, the shoot dry weight by 7.12–21.35%, and the root dry weight by 7.87–24.41%. Under Cd stress, the application of PBCDs markedly alleviated the growth inhibition caused by Cd. Treatments with PS100 and PS250 resulted in increases in rice fresh weight of 32.45% and 31.54%, respectively. Additionally, the shoot dry weight increased by 17.87%, 16.85%, and 13.79% under PS100, PS250, and PS500 treatments, respectively, while the root dry weight increased by 35.59%, 57.63%, and 30.08%. Overall, PBCDs mitigated the growth inhibition of rice under Cd stress, with the most significant effects observed at concentrations of 100 mg L−1 and 250 mg L−1.The photosynthetic coefficient reflects the impact of PBCDs on plant photosynthesis. Under Cd-0 treatment, PBCDs enhanced the photosynthetic rate of plants (Fig. 3A). Among them, the PS100 treatment exhibited the most significant increase, with a 29.55% enhancement compared to PS0. It is well known that the photosynthetic efficiency of plants decreases significantly under Cd stress. In this study, Cd reduced the photosynthetic rate of rice by 35.71%. However, the application of PBCDs mitigated the impact of Cd stress on plant photosynthesis, increasing the photosynthetic rate by 2.02–43.94% at concentrations of 50 to 500 mg L−1. The PS100 and PS250 treatments yielded the best results, increasing the photosynthetic rate by 21.21% and 43.94%, respectively.
ATPases play a crucial role in the energy conversion of photosynthesis. By measuring the activities of Mg2+-ATPase and Ca2+-ATPase, we further investigated the effects of various concentrations of PBCDs on the rice photosynthesis. Compared to PS0, the Mg2+-ATPase activity (Fig. 3B) showed no significant changes under PS50, PS100, and PS250 treatments. However, under PS500 and PS1000 treatments, there was a marked decrease, with a more pronounced reduction at higher concentrations. Under Cd stress, the Mg2+-ATPase activity significantly increased by 112.03–142.91% at PBCD concentrations of 100–500 mg L−1, but no significant change was observed under PS50 treatment. For Ca2+-ATPase (Fig. 3C), the PBCDs application increased its activity by 68.46–262.48% under Cd-0 treatment. Under Cd stress, PBCDs significantly enhanced Ca2+-ATPase activity. Compared to PS0, the activities under PS50, PS100, PS250, PS500, and PS1000 treatments increased by 103.79%, 115.13%, 262.47%, 102.22%, and 68.46%, respectively. In summary, the effects of PBCDs on the Mg2-ATPase and Ca2-ATPase activity (Fig. 3B and C) suggest a role in enhancing plant photosynthesis under both normal and Cd stress conditions.
Soluble sugar, as a product of photosynthesis, serves as an important indicator for evaluating the beneficial effects of PBCDs on plant health and stress resistance. Under Cd-0 treatment, the soluble sugar content in leaves varies under different concentrations of PBCDs (Fig. 3D). Under Cd stress, there was no significant difference in the soluble sugar content of leaves under PS50 treatment. At the concentrations of PBCDs of 100 and 250 mg L−1, the soluble sugar content increased by 82.04% and 60.05%, respectively. However, at higher concentrations (PS500 and PS1000), the soluble sugar content decreased, possibly due to a balance between stress resistance and photosynthesis.
Effect of PBCDs on the antioxidative system
Plants enhance their tolerance to stress by modulating their antioxidant mechanisms, with the MDA content serving as an indirect indicator of cellular damage. Under Cd-0 treatment, the MDA content decreased by 2.07–14.79% at concentrations of PBCDs from 50 to 500 mg L−1, demonstrating their non-toxicity to rice growth and beneficial effects in reducing oxidative damage to plant cells. This inherent ROS scavenging capability of PBCDs effectively helps eliminate ·OH and ·O2− from plant tissues. Consequently, the SOD activity in rice decreased by 14.85–19.17% at PBCDs concentrations of 50–250 mg L−1. Similarly, the POD activity decreased by 8.51% and 9.66% at PBCDs concentrations of 250 and 500 mg L−1, respectively. However, CAT, one of the enzymes capable of decomposing H2O2, showed a significant increase in activity compared to PS0 following PBCDs treatment (Fig. 4C), with PS50 exhibiting the most notable increase of 34.19%.Under Cd-5 treatment, excessive accumulation of ROS in plants leads to oxidative stress. Enhanced activity of antioxidant enzymes is crucial for alleviating this stress. Compared to the PS0 control, SOD activities increased by 22.83%, 59.09%, 26.91%, and 11.67% under PS100, PS250, PS500, and PS1000 treatments, respectively. This suggests an enhanced ability to convert ·O2− into H2O2 and molecular oxygen (O2), thereby reducing oxidative stress in plant tissues. Similarly, the POD activity increased by 2.08–25.27% at PBCDs concentrations of 100–1000 mg L−1 (Fig. 4B). The CAT activity increased by 7.16–43.77% at PBCDs concentrations of 50–500 mg L−1 (Fig. 4C). Compared to the control group, PBCDs resulted in a decrease in MDA content by 7.73% to 29.94% (Fig. 4D). Overall, the application of PBCDs under high Cd stress conditions reduces the MDA content, enhances the activities of antioxidant enzymes (SOD, POD, CAT), and ultimately eliminates excessive ROS. This collective effect contributes to mitigating oxidative stress in rice plants.
Effect of PBCDs on Cd accumulation in rice
Different concentrations of PBCDs significantly altered the Cd concentration in various rice tissues (Fig. 5A). The application of PBCDs significantly reduced Cd concentrations in roots by 5.44–18.75% at concentrations of 50, 100, 250, and 1000 mg L−1, except for the PS500 treatment, where no significant change was observed. In stems, Cd concentrations decreased by 4.99–14.72% at PBCDs concentrations of 50–1000 mg L−1. In leaves, Cd concentrations decreased by 28.67–53.82% at the same concentrations. Additionally, as shown in Fig. 5B, PBCDs treatments significantly altered the Cd content in different tissues. Due to biomass influences, the total Cd content in roots showed no significant change under PS50, PS100, and PS1000 treatments, but increased by 37.84% and 34.15% under PS250 and PS500 treatments, respectively. In stems, the Cd content increased by 6.82% under the PS250 treatment but decreased by 1.56–19.92% at PBCDs concentrations of 50, 100, 500, and 1000 mg L−1. In leaves, the Cd content decreased by 23.76–44.15% at PBCDs concentrations of 50–1000 mg L−1. Overall, PBCDs treatments significantly reduced Cd concentrations in various rice tissues, with the most pronounced effect in leaves. This was achieved by increasing Cd accumulation in the roots, thereby reducing Cd translocation to the stems and leaves. Among all treatments, PBCDs concentrations of 100 and 250 mg L−1 were relatively the most effective.The Cd TF values between different parts of rice were calculated, as shown in Table 1. Compared to PS0, the TF from roots to stems decreased by 11.05%, 12.76%, 7.19%, and 14.55% under the PS50, PS100, PS250, and PS500 treatments, respectively. Similarly, under PBCDs concentrations ranging from 50 to 1000 mg L−1, the TF from stems to leaves decreased by 35.61%, 45.76%, 38.11%, 21.83%, and 32.01%, respectively. Furthermore, the TF considering the total Cd movement between shoots and roots decreased by 11.95% to 24.71% under PBCDs concentrations of 50–500 mg L−1. Overall, PBCDs induce changes in the Cd content across various tissues of rice, effectively reducing the internal translocation of Cd within the plant.
| PBCD concentration (mg L−1) | ||||||
|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 250 | 500 | 1000 | |
| Note: TF1 (leaf/stem); TF2 (stem/root); TF3 (shoot/root). Bars represent the standard deviation (± SD) of the means (n = 3). Treatments marked with different letters are significantly different at P < 0.05. | ||||||
| TF1 | 0.22 ± 0.03a | 0.14 ± 0.01b | 0.12 ± 0.03b | 0.13 ± 0.02b | 0.17 ± 0.03ab | 0.15 ± 0.03b |
| TF2 | 0.24 ± 0.01ab | 0.22 ± 0.03b | 0.21 ± 0.03b | 0.22 ± 0.02b | 0.21 ± 0.01b | 0.27 ± 0.02a |
| TF3 | 0.14 ± 0.01a | 0.12 ± 0.02ab | 0.10 ± 0.01b | 0.12 ± 0.01ab | 0.11 ± 0.01ab | 0.14 ± 0.01a |
Effect of PBCDs on the content of organic acids and phenolic acids
Organic acids are crucial metal chelators in the tricarboxylic acid (TCA) cycle, playing a vital role in regulating plants' ability to adapt to various stress environments. In rice roots, seven organic acids were identified, including oxalic, tartaric, citric, succinic, fumaric, acetic, and propionic acids. Among these, oxalic, tartaric, and citric acids were found at higher concentrations. Our study revealed that the PS100 treatment significantly increased the content of oxalic, fumaric, and acetic acids in rice roots by 30.20%, 103.16%, and 164.05%, respectively. However, under the PS250 treatment, propionic acid decreased by 42.00% (Table S4†). Overall, the content of organic acids in rice roots correlated with root Cd levels. Oxalic, tartaric, and citric acids showed (r = 0.91, 0.89, and 0.63, respectively), while succinic acid exhibited a significant negative correlation (r = −0.66) (P < 0.05) (Fig. 6A).Phenolic acids, as secondary metabolites in plants, play an essential role in plant growth and environmental adaptation. Typically, the synthesis of phenolic compounds in plants increases as their adaptability to the environment enhances. In this study, nine phenolic acids were identified in rice roots. Under Cd stress, the concentrations of neochlorogenic acid, p-coumaric acid, ferulic acid, and cinnamic acid increased significantly (Table S6†). Specifically, at PS100 and PS250 treatments, neochlorogenic acid in rice roots increased by 70.16% and 32.71%, vanillic acid by 52.14% and 41.75%, and cinnamic acid by 36.59% and 30.70%, respectively. Furthermore, root Cd levels exhibited positive correlations with neochlorogenic acid, vanillic acid, p-coumaric acid, and cinnamic acid (r = 0.94, 0.73, 0.93, and 0.93, respectively), but a negative correlation with benzoic acid (r = −0.80) (P < 0.01) (Fig. 6B). In conclusion, organic acids and phenolic acids play a key regulatory role in rice adaptation to Cd stress.
PBCDs altered the content of other divalent metals in rice tissues
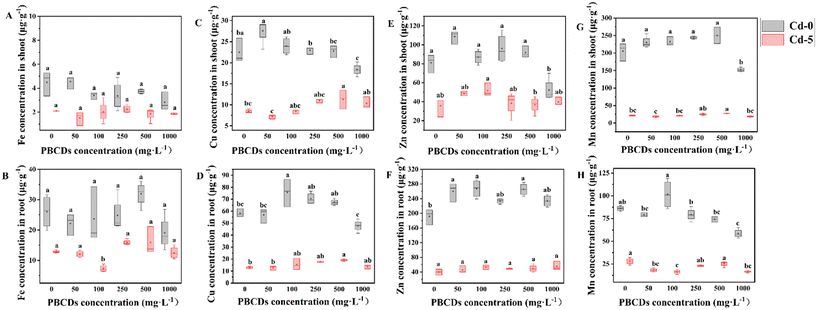
This study investigated the effects of PBCDs on the concentrations of other metals in different tissues under Cd stress. Under the Cd-5 treatment, PS100 and PS250 treatments resulted in an increase in Fe content in the shoots by 41.43% and 20.44%, respectively, while the Cu content increased by 3.90% and 21.79%, and the Zn content increased by 43.44% and 2.57%. Conversely, in the roots, the PS100 treatment caused a decrease in Fe and Mn content by 43.19% and 42.52%, respectively (Fig. 7). Furthermore, under the Cd-0 treatment, PS100 and PS250 treatments reduced the Fe content in the roots by 16.94% to 36.92%. Notably, PBCDs increased the concentrations of essential macronutrients (Mg, Ca, and K) in both shoots and roots, with higher concentrations showing a greater effect. Under Cd stress, PS100 and PS250 treatments increased the Mg content in the shoots by 15.19% and 20.09%, and in the roots by 24.62% and 64.96%; the Ca content in the shoots by 17.76% and 63.20%, and in the roots by 63.12% and 86.31%; and the K content in the shoots by 9.07% and 13.13%, and in the roots by 12.06% and 62.06% (Fig. 8). These findings suggest that PBCDs may alter Cd uptake in rice by modulating the expression of divalent metal transporters and promote rice growth by enhancing the accumulation of essential macronutrients.Discussion
Characteristics of PBCDs
Biomass materials derived from any part of plants or animals can be utilized as carbon sources for synthesizing CDs, offering potentially lower toxicity and production costs compared to nanobiochar.42 Moreover, CDs derived from biomass exhibit smaller sizes, enhancing their versatility in agricultural applications.43 In contrast to traditional remediation methods, nanomaterials provide the advantage of immobilizing and degrading pollutants without the need for additional chemical agents.44 Specifically, waste biomass is considered an ideal precursor for producing nanomaterials for environmental remediation, ensuring higher safety standards from the outset.45 As shown in Fig. 2, PBCDs exhibited a positive promoting effect on the growth of rice, correlated with the inherent properties of PBCDs. The size of PBCDs ranged from 1.0 to 6.4 nm (Fig. 1A), implying that after the application of PBCDs to rice leaves, they can enter the plant through stomata and the cuticle layer on the leaf surface, exerting their effects inside the plant.46 The fluorescence spectrum of PBCDs showed an Ex 345 nm and an Em 458 nm. Under natural light conditions, PBCDs can absorb this excitation wavelength, and the emitted wavelength generated from 345 nm excitation can be used by chloroplasts for photosynthesis. Hence, PBCDs can promote photosynthesis in plants, a phenomenon observed in studies on CDs promoting plant photosynthesis.14,47Moreover, the functional groups present on PBCDs (Fig. 1C), such as –OH and –NH, can undergo complexation reactions with Cd2+. The Si–O–Si groups also participate in the adsorption process of Cd2+, forming stable compounds by binding to the surface functional groups of CDs.48 These compounds are transported and stored in vacuoles, reducing Cd2+ toxicity and decreasing Cd accumulation in rice.49 Additionally, the active hydrogen groups on the CDs' surface can cleave into CO2 and compounds similar to plant hormones, promoting rice plant growth.18 In this study, PBCDs facilitated the increase of GA3, 6-BA, and IAA levels and the decrease of ABA levels in the Cd-0 treatment (Fig. S1†). Conversely, an increase in ABA was observed in the Cd-5 treatment (Fig. S1D†). ABA plays a crucial role in Cd absorption and transport in plants.50 Furthermore, PBCDs demonstrated excellent antioxidant properties, effectively scavenging ·O2− and ·OH, suggesting that PBCDs can mitigate oxidative damage by removing ROS within plant cells.15 Due to their low toxicity, cost-effective production, and inherent photoluminescence and antioxidant properties, PBCDs hold promising applications in the field of botany.
Effects of PBCDs on the growth of rice
After entering the plant system, PBCDs significantly promote rice growth through their extensive range of physicochemical properties.51 As illustrated in Fig. 2, rice plants treated with PBCDs showed enhanced photosynthesis, reduced ROS, and substantial increases in both fresh and dry weights. Exposure to Cd stress in rice resulted in a noticeable decrease in the photosynthetic rate.52–54 However, PBCDs treatment markedly increased the photosynthetic rate (Fig. 3A), as well as the activities of auxiliary photosynthetic enzymes Mg2+-ATPase and Ca2+-ATPase (Fig. 3B and C). Additionally, the soluble sugar content, a product of photosynthesis, also changed significantly (Fig. 3D). Under the Cd-5 treatment, PBCDs significantly mitigated the adverse effects of Cd on photosynthesis, thereby alleviating growth inhibition in rice. Similar studies have also demonstrated that CDs promote plant growth. For example, Ali et al.55 reported that CDs enhanced the photosynthetic efficiency of Lactuca sativa L., and Chen et al.56 found that N-CDs significantly alleviated stress. Overall, PBCDs promote photosynthesis in rice through their inherent fluorescence emissions upon excitation, contributing to improved plant growth.In plant cells, ROS act as crucial signaling molecules, rapidly responding to various stimuli and playing a vital role in the activation of responses, particularly under non-biological stress conditions.57 Existing studies indicate that CDs possess ROS-scavenging properties. Ji et al. demonstrate that the foliar application of CDs can eliminate the accumulation of ROS in soybean leaves under drought stress.58 Another study by Li et al. suggests that CDs exhibit excellent ROS-clearing effects in plant cells.15 In this experiment, as illustrated in Fig. 1E–H, PBCDs demonstrated the ability to scavenge ·O2− and ·OH, with removal efficiencies of 105.79% and 29.64%, respectively. This indicates that PBCDs can effectively eliminate ROS within plant cells, mitigating oxidative damage caused by various abiotic stresses. Even at low concentrations, Cd, a highly toxic pollutant, induces ROS production in plant cells, leading to enzyme activity disruption and affecting plant growth.59–61 Plants activate the oxidative defense system, involving antioxidant enzymes such as SOD, POD, and CAT, as the primary strategy to alleviate HMs stress.62–64 In this study, spraying PBCDs in the Cd-5 treatment significantly decreased the activities of SOD and POD in the plant, with the most pronounced effect observed at the PS250 treatment (Fig. 4A and B). Under Cd stress, rice leaves showed a significant increase in MDA content (Fig. 4D), a prevalent indicator of membrane lipid peroxidation that directly reflects the degree of stress and cellular damage in plants.33 Treatments with PBCDs through foliar spraying on Cd-stressed rice led to a significant increase in the activities of SOD, POD, and CAT enzymes (Fig. 4A–C), resulting in reduced MDA content (Fig. 4D). This enzymatic activity led to a reduction in MDA content, thereby alleviating growth inhibition in rice.
Phenolic compounds, acting as non-enzyme antioxidants, counteract the negative effects of ROS, H2O2, and free radicals under stressful conditions, typically by scavenging or neutralizing them.65,66 In this study, it was observed that the content of phenolic acids in the roots significantly increases under Cd stress, which might be related to the neutralization of ROS.67 Among the nine phenolic acids detected in rice roots (Tables S5 and S6†), neochlorogenic acid, vanillin acid, and p-coumaric acid exhibited strong antioxidant activity.68–70 Additionally, cinnamic acid, another phenolic acid with strong antioxidant activity, exhibited a positive correlation with the root Cd content. It has been suggested that cinnamic acid can influence Cd absorption by altering the bioavailability of Cd and Zn.71,72 This study found a significant negative correlation between roots Cd content and Zn content (Table 2). Overall, PBCDs treatments under Cd stress increased the phenolic acid content in roots, particularly when the root Cd content was relatively low. Interestingly, at a spraying concentration of 250 mg L−1, the root phenolic acid content significantly decreased despite the relatively low root Cd content observed at this concentration (Fig. 5). This unexpected decline might be due to the transformation of root phenolic acids into Cd chelates. By chelating with Cd, these phenolic acids could immobilize a higher proportion of Cd in the rice roots, preventing its upward translocation to the shoots.
| Mg | Ca | K | Fe | Mn | Zn | Cu | |
|---|---|---|---|---|---|---|---|
| Note: ‘*’ indicates a significant correlation between the factors at a 0.05 level; ‘**’ indicates a very significant correlation between the factors at a 0.01 level. | |||||||
| Shoot Cd | −0.11 | −0.29 | 0.09 | 0.28 | 0.23 | −0.53* | 0.02 |
| Root Cd | 0.92** | 0.68** | 0.61** | 0.56** | 0.45* | −0.09 | 0.78** |
Effects of PBCDs on Cd accumulation in rice
Changes in biomass can reflect the extent of damage inflicted on plants by HMs stress. In this study, under the Cd-5 treatment, the treatments of PS100 and PS250 resulted in the highest observed biomass. Analysis of variations in Cd concentrations across different rice tissues subjected to various PBCDs treatments (Fig. 5A) revealed a significant reduction in concentration in leaves. Additionally, considering the biomass factor, PBCDs treatments resulted in diverse degrees of Cd content in roots, while the Cd content in stems and leaves generally showed a decreasing trend (Fig. 5A). The observed reduction in Cd content might be attributed to PBCDs influencing the transport channels of other divalent ions, thereby altering Cd absorption and translocation within the rice plant.73 This was further supported by the effects of PBCDs on the distribution and absorption of other divalent trace elements in rice tissues, as shown in Fig. 7 and 8. Notably, root Fe, Mn, and Cu concentrations were significantly positively correlated with the root Cd content (Table 2), and the fluctuations in these divalent cations may relate to the expression of specific transport proteins, as demonstrated in previous studies.74,75 In rice shoots, the Zn content showed a significant negative correlation with the Cd content, with studies indicating that Zn can enhance antioxidant activity in leaves, thereby reducing leaf Cd levels.76Interestingly, our study also observed alterations in the concentrations of macro-metallic nutrients such as Mg, Ca, and K in rice shoots and roots (Fig. 8). These macro-elements play crucial roles in plant metabolism and growth, contributing to the enhanced growth of rice observed following PBCDs treatment.77,78 It was observed that Mg, Ca, and K contents in the shoots did not change significantly, whereas the contents of these elements in the roots increased with rising Cd levels (Table 2), which might explain the substantial changes in root dry weight (Fig. 2D). In summary, we hypothesize that PBCDs may reduce trace metal elements in co-treated plants by inhibiting metal transport mechanisms. Conversely, PBCDs might also promote plant growth by facilitating the accumulation of essential trace elements in the roots.
Furthermore, in this experiment, the detected oxalic acid, tartaric acid, and citric acid in the roots exhibited a significant positive correlation with the root Cd content (Fig. 6A), consistent with previous findings that increased tartaric acid content enhances plant Cd uptake.79 Citric acid and oxalic acid possess a stronger ligand affinity for metal chelation, effectively mitigating the toxicity of free Cd ions and thereby limiting the upward transport of Cd.80 Therefore, PBCDs might influence plant adaptation to stress environments by modulating the TCA cycle in the roots. This modulation could regulate Cd uptake and prevent its upward transport within rice plants, influencing overall Cd distribution. However, at PS100 and PS250 treatments, the increase in root Cd content might be attributed to two factors. First, the increased root biomass could contribute to the higher total Cd content. Second, organic acids in the roots might be used for chelation and sequestration of HMs, leading to higher Cd accumulation.18 The presence of Si in PBCDs might also contribute to the reduction in Cd content in rice shoots. Si has been shown to improve rice yields and significantly inhibit Cd accumulation.81 It can alleviate Cd toxicity by forming complexes with Cd or by regulating gene expression to suppress HMs transport in rice.82,83 Therefore, it is reasonable to speculate that the complexation between the Si–O–Si groups from PBCDs and Cd induces the increment of the root Cd content. Due to the specific size of PBCDs, Cd is chelated and accumulated in the roots after transport within the plant, thereby increasing the root Cd content and preventing further upward transport. However, further experiments are necessary to confirm this hypothesis.
The basic mechanism of PBCDs promoting rice growth and reducing Cd accumulation
As a non-essential element for plant growth, Cd negatively impacts photosynthesis and reduces antioxidant enzyme activity when accumulated in excess within plant tissues. This triggers oxidative stress reactions that impact plant growth.6,7 Unlike other mitigation strategies, the innovative approach of utilizing biomass materials for CDs synthesis circumvents the high costs associated with hormonal treatments84,85 and mitigates the risks of metal accumulation by application of nutrient antagonism,76 achieving similar Cd sequestration effects. In this study, the fundamental mechanisms by which PBCDs promote rice growth and reduce Cd transport are elucidated as follows: firstly, PBCDs, due to their small size and compatibility with plants cells, enhance rice photosynthesis through fluorescence emission upon excitation. Furthermore, PBCDs exhibit ROS scavenging activity, boosting antioxidant enzymes (SOD, POD, and CAT) and raising antioxidant compound levels within the plant. This dual action reduces MDA levels, limiting oxidative damage caused by Cd stress and alleviating growth inhibition to increased rice biomass.PBCDs also influence Cd concentrations across different rice tissues by modulating divalent cation channels and organic acid levels. This promotes the uptake of essential nutrients (Mg, Ca, and K) and mitigates Cd-induced growth inhibition. Under the Cd-5 treatment, the PS100 and PS250 treatments were notably effective, particularly at the PS250 treatment, in reducing root Cd levels. However, accounting for biomass, the total Cd content in the roots increased due to the combined effects of PBCD concentration and biomass growth. Concentration-dependent effects on Cd accumulation have been observed in studies on other nanomaterials. For instance, titanium dioxide nanoparticles at low concentrations (10 mg L−1) do not affect rice Cd levels under Cd stress, but high concentrations (1000 mg L−1) significantly influence Cd accumulation.86 Similarly, graphene oxide alters the capacity of rice plants to accumulate Cd. Low concentrations (5–200 mg L−1) have no effect, whereas high concentrations (400 mg L−1) inhibit Cd accumulation.87 In summary, the research results suggest that PBCDs can be recognized as an outstanding and promising stress mitigator and growth promoter towards Cd stress.
Conclusion
This study highlights the effects of the foliar application of PBCDs on rice growth under Cd stress. The results demonstrate that PBCDs significantly reduce the Cd content in rice leaves and limit its movement between tissues. Analysis of root organic acids and phenolic acids reveals that PBCDs modulate root organic acids, particularly oxalic, tartaric, and citric acids, thereby promoting Cd sequestration in the roots and hindering its translocation. Additionally, PBCDs regulate Cd uptake in rice by altering the absorption of divalent cations such as Fe, Mn, Zn, and Cu, with optimal effects observed within the concentration range of 100–250 mg L−1. The reduction in Cd content in leaves and stems translates into a significant improvement in rice growth. Enhanced activities of antioxidant enzymes (SOD, POD, and CAT), increased levels of the antioxidant catechin, and elevated concentrations of essential trace elements (Ca, Mg, and K) all contribute to this improvement, alongside a decrease in MDA content. Additionally, PBCDs effectively mitigate the inhibitory effect of Cd on the photosynthetic system, as reflected in the increased soluble sugar content in rice leaves, as well as the enhanced fresh weight and dry weight of the plants. Overall, the foliar application of PBCDs positively influences rice growth, Cd accumulation, and Cd sequestration under Cd stress. The study provides a physiological and biochemical explanation for the mechanisms by which PBCDs promote rice growth and reduce Cd accumulation in rice under Cd stress. However, the specific molecular mechanisms through which PBCDs regulate the absorption and distribution of Cd in rice require further investigation. Additionally, as an exogenous biomass material, excessive use of PBCDs may disrupt the ecological balance, necessitating cautious and appropriate application.Data availability
The data supporting the findings of this study are available within the article and its ESI.† Additional raw data and materials related to this study can be made available upon reasonable request from the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the funding support from the Special Fund for Science and Technology Innovation of Fujian Agriculture and Forestry University (KFB23079 and KFB23065), the Natural Science Foundation of Fujian Province (2021J01095), and the Distinguished Youth Talent Program of Fujian Agriculture and Forestry University (XJQ201922).References
- W. E. Song, S. B. Chen, J. F. Liu, C. Li, N. N. Song, L. Ning and B. Liu, Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution, J. Integr. Agric., 2015, 14, 1845–1854 CrossRef CAS.
- S. H. Yu, S. Li, H. P. Mao, X. S. Huang, L. S. Lin and Y. Y. Li, Physiological response of Conyza Canadensis to cadmium stress monitored by Fourier transform infrared spectroscopy and cadmium accumulation, Spectrochim. Acta, Part A, 2020, 229, 118007 CrossRef CAS.
- A. Sharma and A. K. Nagpal, Contamination of vegetables with heavy metals across the globe: hampering food security goal, J. Food Sci. Technol., 2020, 57, 391–403 CrossRef PubMed.
- V. Verma, B. Vishal, A. Kohli and P. P. Kumar, Systems-based rice improvement approaches for sustainable food and nutritional security, Plant Cell Rep., 2021, 40, 2021–2036 CrossRef CAS.
- X. H. Yuan, N. D. Xue and Z. G. Han, A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years, J. Environ. Sci., 2021, 101, 217–226 CrossRef CAS.
- M. Rizwan, S. Ali, M. Adrees, H. Rizvi, M. Zia-Ur-Rehman, F. Hannan, M. F. Qayyum, F. Hafeez and Y. S. Ok, Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review, Environ. Sci. Pollut. Res., 2016, 23, 17859–17879 CrossRef CAS PubMed.
- M. Abtahi, Y. Fakhri, C. G. Oliveri, H. Keramati, Y. Zandsaalimi, Z. Bahmani, R. H. Pouya, M. Sarkhosh, B. Moradi, N. Amanidaz and S. M. Ghasemi, Heavy metals (As, Cr, Pb, Cd and Ni) concentrations in rice (Oryza sativa) from Iran and associated risk assessment: a systematic review, Toxin Rev., 2017, 36, 331–341 CrossRef CAS.
- N. Manzoor, L. Ali, T. Ahmed, M. Noman, M. Adrees, M. S. Shahid, S. O. Ogunyemi, K. S. A. Radwan, G. Wang and H. E. M. Zaki, Recent Advancements and Development in Nano-Enabled Agriculture for Improving Abiotic Stress Tolerance in Plants, Front. Plant Sci., 2022, 13, 951752 CrossRef.
- G. Zhao, Y. Y. Zhao, W. Lou, J. C. Su, S. Q. Wei, X. M. Yang, R. Wang, R. Z. Guan, H. M. Pu and W. B. Shen, Nitrate reductase-dependent nitric oxide is crucial for multi-walled carbon nanotube-induced plant tolerance against salinity, Nanoscale, 2019, 11, 10511–10523 RSC.
- P. Wang, E. Lombi, F. J. Zhao and P. M. Kopittke, Nanotechnology: A New Opportunity in Plant Sciences, Trends Plant Sci., 2016, 21, 699–712 CrossRef CAS PubMed.
- M. Horie, K. Nishio, H. Kato, K. Fujita, S. Endoh, A. Nakamura, A. Miyauchi, S. Kinugasa, K. Yamamoto, E. Niki, Y. Yoshida, Y. Hagihara and H. Iwahashi, Cellular responses induced by cerium oxide nanoparticles: induction of intracellular calcium level and oxidative stress on culture cells, J. Biochem., 2011, 150, 461–471 CrossRef CAS.
- F. Ghouri, M. J. Shahid, J. Liu, M. Y. Lai, L. X. Sun, J. W. Wu, X. D. Liu, S. Ali and M. Q. Shahid, Polyploidy and zinc oxide nanoparticles alleviated Cd toxicity in rice by modulating oxidative stress and expression levels of sucrose and metal-transporter genes, J. Hazard. Mater., 2023, 448, 130991 CrossRef CAS.
- Z. Hallaji, Z. Bagheri, Z. Tavassoli and B. Ranjbar, Fluorescent carbon dot as an optical amplifier in modern agriculture, Sustainable Mater. Technol., 2022, 34, e00493 CrossRef CAS.
- Y. D. Li, X. Q. Pan, X. K. Xu, Y. Wu, J. L. Zhuang, X. J. Zhang, H. R. Zhang, B. F. Lei, C. F. Hu and Y. L. Liu, Carbon dots as light converter for plant photosynthesis: Augmenting light coverage and quantum yield effect, J. Hazard. Mater., 2020, 410, 124534 CrossRef PubMed.
- Y. J. Li, W. Li, X. Yang, Y. Y. Kang, H. R. Zhang, Y. Y. Liu and B. F. Lei, Salvia Miltiorrhiza-derived carbon dots as scavengers of reactive oxygen species for reducing oxidative damage of plants, ACS Appl. Nano Mater., 2020, 4, 113–120 CrossRef CAS.
- H. Li, J. Huang, F. Lu, Y. Liu, Y. Song, Y. Sun, J. Zhong, H. Huang, Y. Wang, S. Li, Y. Lifshitz, S. T. Lee and Z. Kang, Impacts of Carbon Dots on Rice Plants: Boosting the Growth and Improving the Disease Resistance, ACS Appl. Bio Mater., 2018, 1, 663–672 CrossRef CAS.
- Y. Huili, R. X. Liu, Z. M. Peng, H. Zhang, S. G. Hao, G. Wang, B. H. Wang, W. Wang, Y. Yu, H. Zhang, T. H. Qian, W. X. Xu, M. Mi and Z. Y. He, A phytoexclusion strategy for reducing contamination risk of rice based on low-Cd natural variations pyramid of root transporters, J. Hazard. Mater., 2023, 458, 131865 CrossRef.
- Y. L. Zhang, S. R. He, Z. Zhang, H. J. Xu, J. J. Wang, H. Y. Chen, Y. L. Liu, W. Xue and Y. T. Li, Glycine transformation induces repartition of cadmium and lead in soil constituents, Environ. Pollut., 2019, 251, 930–937 CrossRef CAS PubMed.
- Z. Niu, X. Li and M. Mahamood, Accumulation Potential Cadmium and Lead by Sunflower (Helianthus annuus L.) under Citric and Glutaric Acid-Assisted Phytoextraction, Int. J. Environ. Res. Public Health, 2023, 20, 4107 CrossRef CAS PubMed.
- Y. M. Cai, W. B. Xu, M. Wang, W. P. Chen, X. Z. Li, Y. H. Li and Y. H. Cai, Mechanisms and uncertainties of Zn supply on regulating rice Cd uptake, Environ. Pollut., 2019, 253, 959–965 CrossRef CAS.
- M. A. Bari, S. A. Prity, U. Das, M. S. Akther, S. A. Sajib, M. A. Reza and A. H. Kabir, Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice, Plant Biol., 2020, 22, 472–479 CrossRef CAS PubMed.
- A. Iqbal, Z. W. Mo, S. G. Pan, J. Y. Qi, T. Hua, M. Imran, M. Duan, Q. Gu, X. B. Yao and X. G. Tang, Exogenous TiO2 Nanoparticles Alleviate Cd Toxicity by Reducing Cd Uptake and Regulating Plant Physiological Activity and Antioxidant Defense Systems in Rice (Oryza sativa L.), Metabolites, 2023, 13, 765 CrossRef CAS PubMed.
- M. Jańczak-Pieniążek, J. Cichoński, P. Michalik and G. Chrzanowski, Effect of Heavy Metal Stress on Phenolic Compounds Accumulation in Winter Wheat Plants, Molecules, 2022, 28, 241 CrossRef PubMed.
- J. Nicolas-Espinosa, P. Garcia-Ibañez, A. Lopez-Zaplana, L. Yepes-Molina, L. Albaladejo-Marico and M. Carvajal, Confronting Secondary Metabolites with Water Uptake and Transport in Plants under Abiotic Stress, Int. J. Mol. Sci., 2023, 24, 2826 CrossRef CAS PubMed.
- S. Shalaby and B. A. Horwitz, Plant phenolic compounds and oxidative stress: integrated signals in fungal-plant interactions, Curr. Genet., 2015, 61, 347–357 CrossRef CAS PubMed.
- S. Chen, Mechanism of Zn alleviates Cd toxicity in mangrove plants (Kandelia obovata), Front. Plant Sci., 2022, 13, 1035836 CrossRef PubMed.
- S. Gangwar, V. P. Singh, D. K. Tripathi, D. K. Chauhan, S. M. Prasad and J. N. Maurya, Plant responses to metal stress: the emerging role of plant growth hormones in toxicity alleviation, Emerg. Technol. Manag. Crop Stress Toler., 2014, vol. 2, pp. 215–248 Search PubMed.
- R. Akula and G. A. Ravishankar, Influence of abiotic stress signals on secondary metabolites in plants, Plant Signaling Behav., 2011, 6, 1720–1731 CrossRef.
- J. De Vrieze, G. Colica, C. Pintucci, J. Sarli, C. Pedizzi, G. Willeghems, A. Bral, S. Varga, D. Prat, L. Peng and M. Spiller, Resource recovery from pig manure via an integrated approach: A technical and economic assessment for full-scale applications, Bioresour. Technol., 2019, 272, 582–593 CrossRef CAS.
- C. Rong, W. Li, Z. G. Li, R. Jing, X. Y. Zheng and B. Huang, Preparation and simultaneous detection of dopamine and uric acid by beddingbiomaterial activated carbon modified electrode, Fenxi Shiyanshi, 2023, 42, 1461–1467 CAS.
- S. Panahirad, M. Dadpour, G. Gohari, A. Akbari, G. Mahdavinia, H. Jafari, M. Kulak, R. Alcázar and V. Fotopoulos, Putrescine-functionalized carbon quantum dot (put-CQD) nanoparticle: A promising stress-protecting agent against cadmium stress in grapevine (Vitis vinifera cv. Sultana), Plant Physiol. Biochem., 2023, 197, 107653 CrossRef CAS PubMed.
- Y. Zhu, Q. Zhang, Y. Li, Z. Pan, C. Liu, D. Lin, J. Gao, Z. Tang, Z. Li, R. Wang and J. Sun, Role of Soil and Foliar-Applied Carbon Dots in Plant Iron Biofortification and Cadmium Mitigation by Triggering Opposite Iron Signaling in Roots, Small, 2023, 19, e2301137 CrossRef.
- J. L. Li, L. Xiao, Y. C. Cheng, Y. X. Cheng, Y. Q. Wang, X. L. Wang and L. Y. Ding, Applications of carbon quantum dots to alleviate Cd2+ phytotoxicity in Citrus maxima seedlings, Chemosphere, 2019, 236, 124385 CrossRef PubMed.
- J. L. Moya, R. Ros and I. Picazo, Influence of cadmium and nickel on growth, net photosynthesis and carbohydrate distribution in rice plants, Photosynth. Res., 1993, 36, 75–80 CrossRef CAS PubMed.
- Z. G. Li, X. Y. Hao, T. L. He, Y. Chen, M. W. Yang, C. Rong, C. Z. Gu, Q. T. Xiao, R. Y. Lin and X. Y. Zheng, Bamboo vinegar regulates the phytoremediation efficiency of Perilla frutescens (L.) Britt. by reducing membrane lipid damage and increasing cadmium retention, J. Hazard. Mater., 2024, 476, 135155 CrossRef CAS.
- Y. Li, S. Zhang, Q. Bao, Y. Chu, H. Sun and Y. Huang, Jasmonic acid alleviates cadmium toxicity through regulating the antioxidant response and enhancing the chelation of cadmium in rice (Oryza sativa L.), Environ. Pollut., 2022, 304, 119178 CrossRef CAS PubMed.
- S. Huang, G. Rao, U. Ashraf, L. He, Z. Zhang, H. Zhang, Z. Mo, S. Pan and X. Tang, Application of inorganic passivators reduced Cd contents in brown rice in oilseed rape-rice rotation under Cd contaminated soil, Chemosphere, 2020, 259, 127404 CrossRef CAS PubMed.
- S. Chen, Y. Hong, X. S. He, Y. M. Huang, J. Peng, J. J. Zhang, Q. T. Xiao, X. Y. Zheng and R. Y. Lin, Changes of low molecular weight organic acids and cadmium in Perilla frutescens under cadmium stress, Journal of Fujian Agriculture and Forestry University, 2018, 47, 7 Search PubMed.
- X. Y. Zheng, R. J. Ye, Y. B. He, X. C. Zhou, R. Y. Lin and W. X. Lin, Determination of 10 phenolic acid in root exudates of rice using solid-phase extraction and high performance liquid chromatography, Yunnan Daxue Xuebao, Ziran Kexueban, 2013, 35, 219–224 CAS.
- R. K. Xu, S. C. Xiao, J. H. Yuan and A. Z. Zhao, Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues, Bioresour. Technol., 2011, 102, 10293–10298 CrossRef CAS PubMed.
- R. Ellerbrock, M. Stein and J. Schaller, Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR, Sci. Rep., 2022, 12, 11708 CrossRef CAS.
- B. Cheng, Z. Yang, F. Chen, L. Yue, X. Cao, J. Li, H. L. Qian, X. P. Yan, C. Wang and Z. Wang, Biomass-derived carbon dots with light conversion and nutrient provisioning capabilities facilitate plant photosynthesis, Sci. Total Environ., 2023, 901, 165973 CrossRef CAS.
- T. C. Wareing, P. Gentile and A. N. Phan, Biomass-based carbon dots: current development and future perspectives, ACS Nano, 2021, 15, 15471–15501 CrossRef CAS PubMed.
- L. Marcon, J. Oliveras and V. F. Puntes, In situ nanoremediation of soils and groundwaters from the nanoparticle's standpoint: A review, Sci. Total Environ., 2021, 791, 148324 CrossRef CAS.
- A. Barhoum, J. Jeevanandam, A. Rastogi, P. Samyn, Y. Boluk, A. Dufresne, M. K. Danquah and M. Bechelany, Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials, Nanoscale, 2020, 12, 22845–22890 RSC.
- B. Guo, G. Liu, W. Li, C. Hu, B. Lei, J. Zhuang, M. Zheng and Y. Liu, The role of carbon dots in the life cycle of crops, Ind. Crops Prod., 2022, 187, 115427 CrossRef CAS.
- W. Li, S. Wu, H. Zhang, X. Zhang, J. Zhuang, C. Hu, Y. Liu, B. Lei, L. Ma and X. Wang, Enhanced biological photosynthetic efficiency using light-harvesting engineering with dual-emissive carbon dots, Adv. Funct. Mater., 2018, 28, 1804004 CrossRef.
- L. Guo, A. Chen, C. Li, Y. Wang, D. Yang, N. He and M. Liu, Solution chemistry mechanisms of exogenous silicon influencing the speciation and bioavailability of cadmium in alkaline paddy soil, J. Hazard. Mater., 2022, 438, 129526 CrossRef CAS PubMed.
- J. Li, L. Xiao, Y. Cheng, Y. Cheng, Y. Wang, X. Wang and L. Ding, Applications of carbon quantum dots to alleviate Cd2+ phytotoxicity in Citrus maxima seedlings, Chemosphere, 2019, 236, 124385 CrossRef CAS PubMed.
- C. Shen, Y. M. Yang, Y. F. Sun, M. Zhang, X. J. Chen and Y. Y. Huang, The regulatory role of abscisic acid on cadmium uptake, accumulation and translocation in plants, Front. Plant Sci., 2022, 13, 953717 CrossRef PubMed.
- A. Guirguis, W. Yang, X. A. Conlan, L. Kong, D. M. Cahill and Y. Wang, Boosting plant photosynthesis with carbon dots: a critical review of performance and prospects, Small, 2023, 19, 2300671 CrossRef CAS.
- M. F. Adil, S. Sehar, Z. Han, J. L. W. Lwalaba, G. Jilani, F. Zeng, Z. H. Chen and I. H. Shamsi, Zinc alleviates cadmium toxicity by modulating photosynthesis, ROS homeostasis, and cation flux kinetics in rice, Environ. Pollut., 2020, 265, 114979 CrossRef CAS PubMed.
- Y. P. Huang, Y. Xi, L. Gan, D. Johnson, Y. Wu, D. Ren and H. Liu, Effects of lead and cadmium on photosynthesis in Amaranthus spinosus and assessment of phytoremediation potential, Int. J. Phytorem., 2019, 21, 1041–1049 CrossRef CAS PubMed.
- C. Wang, T. Cheng, H. Liu, F. Zhou, J. Zhang, M. Zhang, X. Liu, W. Shi and T. Cao, Nano-selenium controlled cadmium accumulation and improved photosynthesis in indica rice cultivated in lead and cadmium combined paddy soils, J. Environ. Sci., 2021, 103, 336–346 CrossRef CAS PubMed.
- E. Ali, A. Maodzeka, N. Hussain, I. H. Shamsi and L. Jiang, The alleviation of cadmium toxicity in oilseed rape (Brassica napus) by the application of salicylic acid, Plant Growth Regul., 2015, 75, 641–655 CrossRef CAS.
- Q. Chen, B. Liu, H. Man, L. Chen, X. Wang, J. Tu, Z. Guo, G. Jin, J. Lou and L. Ci, Enhanced bioaccumulation efficiency and tolerance for Cd (II) in Arabidopsis thaliana by amphoteric nitrogen-doped carbon dots, Ecotoxicol. Environ. Saf., 2020, 190, 110108 CrossRef CAS PubMed.
- R. Mittler, S. I. Zandalinas, Y. Fichman and F. Van Breusegem, Reactive oxygen species signalling in plant stress responses, Nat. Rev. Mol. Cell Biol., 2022, 23, 663–679 CrossRef CAS.
- Y. Ji, L. Yue, X. Cao, F. Chen, J. Li, J. Zhang, C. Wang, Z. Wang and B. Xing, Carbon dots promoted soybean photosynthesis and amino acid biosynthesis under drought stress: Reactive oxygen species scavenging and nitrogen metabolism, Sci. Total Environ., 2023, 856, 159125 CrossRef CAS PubMed.
- K. Khanna, S. K. Kohli, P. Ohri, R. Bhardwaj and P. Ahmad, Agroecotoxicological Aspect of Cd in Soil-Plant System: Uptake, Translocation and Amelioration Strategies, Environ. Sci. Pollut. Res., 2022, 29, 30908–30934 CrossRef CAS PubMed.
- R. K. Srivastava, P. Pandey, R. Rajpoot, A. Rani and R. S. Dubey, Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings, Protoplasma, 2014, 251, 1047–1065 CrossRef CAS PubMed.
- F. M. Yu, K. H. Liu, M. S. Li, Z. M. Zhou, H. Deng and B. Chen, Effects of cadmium on enzymatic and non-enzymatic antioxidative defences of rice (Oryza sativa L.), Int. J. Phytorem., 2013, 15, 513–521 CrossRef CAS PubMed.
- H. Wu, J. Tong, X. Jiang, J. Wang, H. Zhang, Y. Luo, J. Pang and J. Shi, More effective than direct contact: Nano hydroxyapatite pre-treatment regulates the growth and Cd uptake of rice (Oryza sativa L.) seedlings, J. Hazard. Mater., 2023, 463, 132889 CrossRef.
- N. Liu, Z. Lin, L. Guan, G. Gaughan and G. Lin, Antioxidant enzymes regulate reactive oxygen species during pod elongation in Pisum sativum and Brassica chinensis, PLoS One, 2014, 9, e87588 CrossRef PubMed.
- F. B. Wang, J. C. Liu, L. J. Zhou, G. Pan, Z. W. Li, S. H. R. Zaidi and F. M. Cheng, Senescence-specific change in ROS scavenging enzyme activities and regulation of various SOD isozymes to ROS levels in psf mutant rice leaves, Plant Physiol. Biochem., 2016, 109, 248–261 CrossRef CAS.
- A. S. Ganjavi, M. Oraei, G. Gohari, A. Akbari and A. Faramarzi, Glycine betaine functionalized graphene oxide as a new engineering nanoparticle lessens salt stress impacts in sweet basil (Ocimum basilicum L.), Plant Physiol. Biochem., 2021, 162, 14–26 CrossRef CAS PubMed.
- G. Gohari, E. Zareei, H. Rostami, S. Panahirad, M. Kulak, H. Farhadi, M. Amini, M. Del Carmen Martinez-Ballesta and V. Fotopoulos, Protective effects of cerium oxide nanoparticles in grapevine (Vitis vinifera L.) cv. Flame Seedless under salt stress conditions, Ecotoxicol. Environ. Saf., 2021, 220, 112402 CrossRef CAS PubMed.
- F. Azimi, M. Oraei, G. Gohari, S. Panahirad and A. Farmarzi, Chitosan-selenium nanoparticles (Cs-Se NPs) modulate the photosynthesis parameters, antioxidant enzymes activities and essential oils in Dracocephalum moldavica L. under cadmium toxicity stress, Plant Physiol. Biochem., 2021, 167, 257–268 CrossRef CAS PubMed.
- S. Kurita, T. Kashiwagi, T. Ebisu, T. Shimamura and H. Ukeda, Identification of neochlorogenic acid as the predominant antioxidant in Polygonum cuspidatum leaves, Ital. J. Food Sci., 2016, 28, 25–31 CAS.
- H. R. A. Ghareib, M. S. Abdelhamed and O. H. Ibrahim, Antioxidative effects of the acetone fraction and vanillic acid from Chenopodium murale on tomato plants, Weed Biol. Manage., 2010, 10, 64–72 CrossRef.
- H. Boz, p-Coumaric acid in cereals: presence, antioxidant and antimicrobial effects, Int. J. Food Sci. Technol., 2015, 50, 2323–2328 CrossRef CAS.
- S. Chen, R. Lin, H. Lu, Q. Wang, J. Yang, J. Liu and C. Yan, Effects of phenolic acids on free radical scavenging and heavy metal bioavailability in kandelia obovata under cadmium and zinc stress, Chemosphere, 2020, 249, 126341 CrossRef CAS PubMed.
- E. M. Al Olayan, A. S. Aloufi, O. D. AlAmri, H. Ola and A. E. A. Moneim, Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: Role of oxidative stress, inflammation and apoptosis, Sci. Total Environ., 2020, 723, 137969 CrossRef CAS.
- Y. Zhu, Q. Zhang, Y. Li, Z. Pan, C. Liu, D. Lin, J. Gao, Z. Tang, Z. Li, R. Wang and J. Sun, Role of Soil and Foliar-Applied Carbon Dots in Plant Iron Biofortification and Cadmium Mitigation by Triggering Opposite Iron Signaling in Roots, Small, 2023, 19, e2301137 CrossRef.
- Y. You, L. Liu, Y. Wang, J. Li, Z. Ying, Z. Hou, H. Liu and S. Du, Graphene oxide decreases Cd concentration in rice seedlings but intensifies growth restriction, J. Hazard. Mater., 2021, 417, 125958 CrossRef CAS.
- J. Liu, K. Li, J. Xu, J. Liang, X. Lu, J. Yang and Q. Zhu, Interaction of Cd and five mineral nutrients for uptake and accumulation in different rice cultivars and genotypes, Field Crops Res., 2003, 83, 271–281 CrossRef.
- S. Zhen, H. Shuai, C. Xu, G. Lv, X. Zhu, Q. Zhang, Q. Zhu, A. Núñez-Delgado, M. Conde-Cid, Y. Zhou and D. Huang, Foliar application of Zn reduces Cd accumulation in grains of late rice by regulating the antioxidant system, enhancing Cd chelation onto cell wall of leaves, and inhibiting Cd translocation in rice, Sci. Total Environ., 2021, 770, 145302 CrossRef CAS PubMed.
- R. Johnson, K. Vishwakarma, M. S. Hossen, V. Kumar, A. M. Shackira, J. T. Puthur, G. Abdi, M. Sarraf and M. Hasanuzzaman, Potassium in plants: Growth regulation, signaling, and environmental stress tolerance, Plant Physiol. Biochem., 2022, 172, 56–69 CrossRef CAS PubMed.
- Z. Wang, M. U. Hassan, F. Nadeem, L. Wu, F. Zhang and X. Li, Magnesium fertilization improves crop yield in most production systems: a meta-analysis, Front. Plant Sci., 2020, 10, 495191 Search PubMed.
- Q. Tao, J. Zhao, J. Li, Y. Liu, J. Luo, S. Yuan, B. Li, Q. Li, Q. Xu and X. Yu, Unique root exudate tartaric acid enhanced cadmium mobilization and uptake in Cd-hyperaccumulator Sedum alfredii, J. Hazard. Mater., 2020, 383, 121177 CrossRef CAS PubMed.
- H. Li, Y. Liu, G. Zeng, L. Zhou, X. Wang, Y. Wang, C. Wang, X. Hu and W. Xu, Enhanced efficiency of cadmium removal by Boehmeria nivea (L.) Gaud. in the presence of exogenous citric and oxalic acids, J. Environ. Sci., 2014, 26, 2508–2516 CrossRef.
- H. Lu, S. Qin, J. Zhao, P. Pan, F. Wang, S. Tang, L. Chen, K. Akhtar and B. He, Silicon inhibits the upward transport of Cd in the first internode of different rice varieties in a Cd stressed farm land, J. Hazard. Mater., 2023, 458, 131860 CrossRef CAS.
- L. Guo, A. Chen, C. Li, Y. Wang, D. Yang, N. He and M. Liu, Solution chemistry mechanisms of exogenous silicon influencing the speciation and bioavailability of cadmium in alkaline paddy soil, J. Hazard. Mater., 2022, 438, 129526 CrossRef CAS.
- D. Chen, D. Chen, R. Xue, J. Long, X. Lin, Y. Lin, L. Jia, R. Zeng and Y. Song, Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants, J. Hazard. Mater., 2019, 367, 447–455 CrossRef CAS.
- F. Wang, H. Tan, L. Huang, C. Cai, Y. Ding, H. Bao, Z. Chen and C. Zhu, Application of exogenous salicylic acid reduces Cd toxicity and Cd accumulation in rice, Ecotoxicol. Environ. Saf., 2021, 207, 111198 CrossRef CAS PubMed.
- I. Singh and K. Shah, Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings, Phytochemistry, 2014, 108, 57–66 CrossRef CAS.
- Y. Ji, Y. Zhou, C. Ma, Y. Feng, Y. Hao, Y. Rui, W. Wu, X. Gui, Y. Han, Y. Wang and B. Xing, Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake, Plant Physiol. Biochem., 2017, 110, 82–93 CrossRef CAS.
- Y. You, L. Liu, Y. Wang, J. Li, Z. Ying, Z. Hou, H. Liu and S. Du, Graphene oxide decreases Cd concentration in rice seedlings but intensifies growth restriction, J. Hazard. Mater., 2021, 417, 125958 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00682h |
| This journal is © The Royal Society of Chemistry 2025 |