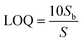Electrochemical investigation of an antipyretic drug in plant extracts and environmental samples at the O-MWCNT/CuO nanostructure modified glassy carbon electrode†
Received
22nd May 2024
, Accepted 6th November 2024
First published on 21st November 2024
Abstract
Opened multiwalled carbon nanotubes (O-MWCNT) were prepared by unzipping MWCNTs using Hummers' method and decorated with CuO to form a nanohybrid (O-MWCNT/CuO) through a simple co-precipitation technique, aimed at developing a novel electrochemical sensor. The O-MWCNT/CuO composite was used to modify a glassy carbon electrode (GCE) for the sensitive detection of the antipyretic drug acetaminophen (ACT) in various matrices. O-MWCNT/CuO was characterized using X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV-visible spectroscopy, cyclic voltammetry (CV), linear sweep voltammetry (LSV) and electrochemical impedance spectroscopy (EIS), which confirmed the successful formation of the nanocomposite as well as its electrical conductivity and catalytic properties. The sensor demonstrates a wide linear detection range (0.005–1450 μM), with a low detection limit (LOD) of 7.2 nM and excellent sensitivity of 0.019 μA cm−2 μM−1. Additionally, the sensor demonstrated good stability (maintaining performance over 65 cycles) and selectivity in various co-interfering compounds. Notably, the electrochemical sensor was applied for the detection of ACT in environmental water samples, pharmaceutical formulations, human biological fluids, and fenugreek plant extracts, achieving good recovery rates (97.37–100.20%) with relative standard deviations (RSD) ranging from 1.0% to 3.3%, using the standard addition method. The novelty of this work lies in the development of a highly sensitive, stable, and selective GCE-modified sensor for ACT detection, with promising applications in real-world sample analysis.
Environmental significance
The widespread use of acetaminophen (ACT) for treating common illnesses has led to its increased presence in various environmental matrices, including human diets, terrestrial food chains, and agroecosystems. This contamination poses significant ecological and health risks, necessitating effective monitoring and remediation strategies. In this study, we present a novel electrochemical sensor based on an unzipped multiwalled carbon nanotube (O-MWCNT) decorated with CuO nanohybrid for the sensitive and selective detection of ACT. By leveraging advanced nanomaterials and electrochemical techniques, our sensor exhibits exceptional performance, including a low detection limit and robust stability, making it a promising tool for environmental monitoring. The successful application of this sensor in real sample analysis underscores its potential to mitigate the impact of pharmaceutical contaminants in plant extracts.
|
1. Introduction
Pharmaceuticals and personal care products (PPCPs) are commonly found in body lotions, illegal drugs, and disinfectants used by humans and animals.1 The human liver is the most vulnerable organ affected by these contaminants.2,3 A significant portion of PPCPs, ranging from 10% to 90% of the dosage, is excreted in their parent form, leading to the contamination of drinking water and agricultural plants. This pollution has adverse effects on fertility, increases hypertension in both genders, and contaminates water and soil, endangering the health of both wildlife and humans.4,5 Consequently, one of the greatest challenges in wastewater treatment is the complete removal of PPCPs.6,7 PPCPs have the potential to contaminate agroecosystems, terrestrial food chains, and ultimately affect human diets and human health.8 In 2014, a total of 19 PPCPs, which are phytotoxic and inhibit plant growth, were detected in the consumable parts of eight popular vegetables irrigated with treated wastewater.9–11 Acetaminophen (ACT) is a widely used generic drug that is more commonly ingested by humans and excreted into the environment, contaminating human diets, terrestrial food chains, and edible agroecosystems.12 The presence of ACT in concentration ranging from ng L−1 to μg L−1 affects aquatic organisms and cell proliferation. It is essential to detect and monitor contamination pathways to control the ACT consumption limit and its contamination. ACT is detected using a variety of techniques such as liquid chromatography,13 spectrophotometry,14 spectro-fluorimetry,15 titrametry,16 chemiluminescence17 and electrochemistry. The electrochemical technique is simple, user-friendly, low cost, portable, sensitive and frequently used in a variety of sensing applications.18,19 The most crucial step is tuning the electrode's conducting and catalytic properties.
Nanostructure transition metal oxides (TMOs) are the subject of significant interest because of their high-performing electronic and catalytic activities. Common TMOs like Co3O4, SnO2, ZnO, Mn2O3, Fe2O3, TiO2, and NiO have been used.20–23 Abundantly available cupric oxide (CuO) nanostructured materials have good mechanical strength, large specific surface area, and excellent chemical stability, are non-toxic and cost-effective and have excellent catalytic characteristics.24,25 Various electrochemical detections have been done by using the nanostructured CuO. For instance, Hwa et al. successfully prepared CuO-nanoflakes using hydrothermal synthesis and ultrasonication for the sensitive detection of the environmental hazardous pollutant, hydroquinone.26 Ramya et al. constructed CuO/MoS2 nanocomposites and employed them for the detection of sulfamethoxazole.27 Nevertheless, the low conductivity (CuO is approximately 10−3 S m−1) consistently impedes its rapid application in electrochemical detection.28,29 Further, carbon materials are widely recognized for their excellent conductivity and strong physical/chemical stability, making them the perfect choice as supports for metal oxides.
The invention of graphene has attracted great interest as an electrode material, however mass production is required for industrial applications due to its excellent electrical, thermal, electrochemical, and mechanical properties. Among the various nano carbon materials, multiwall carbon nanotubes (MWCNTs) demonstrate exceptional sensing characteristics arising from the large surface area and electron flow. Therefore, in-depth research has been done on MWCNT structures for a variety of uses, such as supercapacitors, sensors, and electronics.30–33 However, poor solubility, limited processability, self-aggregation and insolubility in aqueous solvents prevent their electrocatalytic applications.34 Consequently, these restrictions can be addressed by altering the MWCNTs' surface via acid treatment, forming functional groups (–COOH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH),35 or controlled by doping or modifying the surface using other materials. In particular, TMOs have been used as dopants or composited with MWCNTs for the development of electrochemical sensors. Recently, MWCNT structure manipulation has been applied to cut and unzip CNTs, producing shorter pieces and nanoribbons. Unzipping of CNTs is a simple method proposed for mass production of single layer graphene. CNTs can be unzipped through selective etching,36 cryogenic grinding,37 electrochemical preparation,38 hydrothermal,39 Hummers',40 and chemical oxidation methods.41 Studies reveal that open ended MWCNTs are more reactive than close ended ones.42 Combining carbon materials with metal or metal oxide particles enhances conductivity, catalytic, and sensing properties. Recently, Alam et al. used MWCNT and β-cyclodextrin-modified GC electrodes for ACT detection in a linear range of 50 nM–300 μM with an LOD of 11.5 nM.43 Kenarkob et al. developed ZnO/Au monohybrid functionalized MWCNTs for an ACT sensor and obtained a linear sensing range of 0.05–20 μM with an LOD of 9 nM.44 Meanwhile, electrodeposited graphene/ZnO, Fe3O4/graphene, SnO2/f-MWCNT, f-MWCNT and MWCNT/MOF@Au–Ag nanohybrid materials were used for the improvement of ACT sensing.45–48 However, there are very few published literature studies on the usage of nanoribbons made from unzipped MWCNTs and CuO coated with opened MWCNTs for the detection of ACT.
O, –OH),35 or controlled by doping or modifying the surface using other materials. In particular, TMOs have been used as dopants or composited with MWCNTs for the development of electrochemical sensors. Recently, MWCNT structure manipulation has been applied to cut and unzip CNTs, producing shorter pieces and nanoribbons. Unzipping of CNTs is a simple method proposed for mass production of single layer graphene. CNTs can be unzipped through selective etching,36 cryogenic grinding,37 electrochemical preparation,38 hydrothermal,39 Hummers',40 and chemical oxidation methods.41 Studies reveal that open ended MWCNTs are more reactive than close ended ones.42 Combining carbon materials with metal or metal oxide particles enhances conductivity, catalytic, and sensing properties. Recently, Alam et al. used MWCNT and β-cyclodextrin-modified GC electrodes for ACT detection in a linear range of 50 nM–300 μM with an LOD of 11.5 nM.43 Kenarkob et al. developed ZnO/Au monohybrid functionalized MWCNTs for an ACT sensor and obtained a linear sensing range of 0.05–20 μM with an LOD of 9 nM.44 Meanwhile, electrodeposited graphene/ZnO, Fe3O4/graphene, SnO2/f-MWCNT, f-MWCNT and MWCNT/MOF@Au–Ag nanohybrid materials were used for the improvement of ACT sensing.45–48 However, there are very few published literature studies on the usage of nanoribbons made from unzipped MWCNTs and CuO coated with opened MWCNTs for the detection of ACT.
In this study, we used a modified Hummers' method to open MWCNTs, creating graphene nanoribbons and compositing them with CuO nanoparticles. The produced O-MWCNT/CuO nanohybrid was characterized by XRD, FTIR, SEM, TEM, XPS and UV-visible techniques. A selective, sensitive, and repeatable ACT sensor was produced by modifying the samples on a GCE and analysing the electrochemical behaviour using EIS, CV, and LSV techniques. The sensor exhibits a linear range from 5 nM to 1400 μM with an LOD of 7.2 nM. Practical applicability tests were performed using real samples such as human urine, human blood serum, tablets (Vicks Action-500, Paracetamol-650), and ACT-consumed fenugreek plant extracts (root, stem, leaves and ACT contaminated soil).
2. Experimental section
2.1. Chemical reagents
Copper(II) chloride (99%), multiwalled carbon nanotubes (MWCNTs >98% carbon basis, O.D. × L 6–13 nm × 2.5–20 μm), hydrazine hydrate (80% solution in water), acetaminophen (≥99.0%), eugenol (≥98%), chloramphenicol (≥98%), dopamine hydrochloride (≥98% TLC), L-ascorbic acid (99%), D-(+)-glucose (≥99.5% HPLC), uric acid (≥99%), and urea (99.0–100.5%) were purchased from Sigma Aldrich, USA. From Himedia, India, potassium ferrocyanide and potassium ferricyanide (98–102%), monosodium dihydrogen phosphate dihydrate (98%), di-sodium hydrogen phosphate dehydrate (98%), folic acid (95.00–102.00%), and riboflavin (98.00–103.00%) were purchased. During the investigations, Milli-Q water (18.2 MΩ) (MQ) was used. Using ACT (500 mg L−1) tainted water for irrigation, fenugreek plants were grown in a lab. Stem, leaf, and root extracts were collected, processed with a mortar and pestle, and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 RPM. Using Na2HPO4 and NaH2PO4, phosphate buffer solution (PBS, pH 7.0) was prepared for the duration of the experiment, and a Susima, AP-1PLVS (India) was used to monitor pH. The electrochemical measurement and characterization techniques were detailed in the ESI† (Section S1).
000 RPM. Using Na2HPO4 and NaH2PO4, phosphate buffer solution (PBS, pH 7.0) was prepared for the duration of the experiment, and a Susima, AP-1PLVS (India) was used to monitor pH. The electrochemical measurement and characterization techniques were detailed in the ESI† (Section S1).
2.2. Synthesis of O-MWCNT
To unzip MWCNTs, the modified Hummers' technique has been employed.49 Briefly, 2.0 g of MWCNTs was refluxed in 92 mL of concentrated H2SO4 (98%) and 1 g of NaNO3 in an ice bath for 2 h. Then, 6.0 g of KMnO4 was added to the above solution at the reaction temperature below 20 °C. After the ice bath was removed, the mixture was vigorously stirred at 25 °C for 2 h, while adding 92.0 mL of deionized water gradually, and again stirred for 1 h. Finally, 280 mL of deionized water and 20.0 mL of 30% H2O2 were added to the mixture. The mixture was centrifuged several times using Milli-Q water and 10% HCl until the pH of the suspension became neutral. The gray color product (68%) was dried at 80 °C overnight. Then it was treated with 500.0 mg of NaBH4 in 100.0 mL of MQ water and stirred at room temperature for 24 h. Finally, the obtained solid precipitate was centrifuged with ethanol and water several times, after which the precipitate was dried at 60 °C for 12 h.
2.3. Preparation of O-MWCNT/CuO nanocomposites and electrode fabrication
After adding 200.0 mg of O-MWCNT, 0.5 mL of 0.1 M CuCl2 and 0.087 g of SDS in 8.5 mL of MQ water, the mixture was stirred for 1 h in an ice bath. Then, 0.2 mL of 1.0 M NaOH was slowly added to the above mixture and the colour of the solution suddenly changed to blue. Afterwards, 0.8 mL of 0.1 M hydrazine hydrate was added and the colour changed to green. 10.0 mL of MQ water was used to dilute the above mixture and stirred for 1 h in the ice bath for the growth of CuO nanocrystals on the surface of O-MWCNT. Finally, the obtained black precipitate was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 RPM with water and ethanol. The washed precipitate was dried under vacuum at 60 °C overnight to obtain O-MWCNT/CuO. For the control experimentation, pure CuO was synthesised under the same conditions in the absence of O-MWCNT.
000 RPM with water and ethanol. The washed precipitate was dried under vacuum at 60 °C overnight to obtain O-MWCNT/CuO. For the control experimentation, pure CuO was synthesised under the same conditions in the absence of O-MWCNT.
The glassy carbon electrode (GCE) was polished using 0.05 μm alumina powder and rinsed with MQ water. The GCE is potential cycled between −1.5 V and 1.5 V in 0.5 M H2SO4 at a scan rate of 50 mV s−1 for 10 cycles. After the electrodes were washed, 3.0 mg of O-MWCNT/CuO was taken in water and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and 0.1% Nafion and ultra-sonicated for 15 minutes. Finally, 7.0 μL of slurry was coated separately on the GCE and dried at room temperature for 1.5 h (Scheme 1).
2) and 0.1% Nafion and ultra-sonicated for 15 minutes. Finally, 7.0 μL of slurry was coated separately on the GCE and dried at room temperature for 1.5 h (Scheme 1).
 |
| | Scheme 1 Synthesis and electrode fabrication of the O-MWCNT/CuO composite. | |
2.4. Real sample preparation
Feasibility analysis of the sensor was performed on various samples, including human blood serum, urine, Vicks Action 500, Paracetamol 650.0 mg, river water, well water, pond water, and ACT mixed tap water irrigated indoor fenugreek plant root, leaves, stem, and soil extracts. The human blood serum and human urine samples were collected and studied according to the guidelines of Chang Gung Medical Foundation (IRB number: 202201485B0) approved by the Institutional Review Board of Chang-Gung Medical Foundation. Informed consent was attained for the executed study. To evaluate ACT contamination in tiny crops, we collected agricultural soil and fenugreek seeds near the Alagappa University, Karaikudi, India, and allowed them to develop at room temperature with irrigation using acetaminophen-contaminated tap water (500.0 mg L−1). Scheme 2 shows the various stages of plant growth up to the fourteenth day of planting. This shows that adding water containing ACT does not affect the development until day fourteen. Subsequently, the plant irrigated with ACT-spiked water was harvested from the ground and its leaves, roots, stem, and planted soil were separated. 100.0 g of leaves, stem, and roots were chopped in an agate mortar, rinsed with Millipore water, and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 RPM and the solution pH was adjusted to 7.0 before each analysis.
000 RPM and the solution pH was adjusted to 7.0 before each analysis.
 |
| | Scheme 2 Growth mechanism of fenugreek plant at room temperature with spiked contamination (500.0 mg L−1) of ACT. | |
Paracetamol 650.0 mg (Opex P-650 B. No. PSTSS19048) and Vicks Action 500 were purchased from a nearby medical shop. They were finely ground using an agate mortar, used unpurified, and dissolved in PBS (pH 7.0). The water samples were collected from a nearby Alagappa University, Karaikudi, India. To ensure purity, the collected samples underwent filtration using Millipore mixed cellulose ester membrane filters with a pore size of 0.5 μm. Additionally, the pH of the solution was adjusted to 7.0 prior to each analysis. All the pretreated real samples were used to detect ACT.
3. Results and discussion
3.1. Physical characterization
SEM images of CuO, MWCNT, and O-MWCNT/CuO displayed in Fig. 1 confirm the flake-like CuO nanoparticles with a size of 25.0 nm. O-MWCNT in (Fig. 1B) indicates the formation of folded and agglomerated graphene sheets due to the unzipping of the MWCNTs by acid oxidation (con. H2SO4) and reduction (NaBH4) treatment using the modified Hummers' method. The decoration of CuO on O-MWCNT to form the MWCNT/CuO nanohybrid is evident from the SEM (Fig. 1C) and TEM (Fig. 1D) images. It must be recalled here that metals or metal oxides are used to prevent the agglomeration of graphene sheets through their intercalative effects. Fig. 1E and F show the d spacing values (0.25 nm and 0.34 nm) of O-MWCNT/CuO and O-MWCNT from the SAED pattern, respectively. The decreased d spacing value of the MWCNT/CuO nanohybrid confirms the intercalative binding of CuO between the O-MWCNT layers. Further, the EDX spectrum in Fig. S1† confirms the existence of CuO on O-MWCNT with an atomic peak percent of Cu (15.34%), O (17.22%), and C (67.39%).
 |
| | Fig. 1 (A) SEM images of CuO, (B) MWCNT, (C) and O-MWCNT/CuO, (D) TEM image (E) SAED pattern and (F) lattice fringes of O-MWCNT/CuO. | |
The XRD patterns of MWCNT (curve (a)) and O-MWCNT (curve (b)) are given in Fig. 2A. MWCNT exhibited a broad peak at 25–28° which corresponds to the (002) plane. O-MWCNT showed peaks at 25.00° and 43.17° corresponding to the (002) and (110) planes. Fig. 2A curve (c) displays the XRD pattern of pure CuO. The patterns at 32.31°, 35.44°, 38.67°, 48.84°, 53.44°, 57.98°, 61.45°, 66.19°, 67.95°, 72.19°, and 74.97° correspond to the diffraction planes (110), (−111), (111), (−202), (020), (202), (202), (−311), (220), (311), and (−222), respectively, in accordance with reference ICDD no: DB 01-080-1971.50 The XRD peaks obtained after the formation of the O-MWCNT/CuO nanohybrid are shown in Fig. 2A curve (d). The characteristic peaks at 25.6°, 32.31°, 35.44°, 38.67°, 43.17°, 48.84°, 53.44°, 57.98°, 61.45°, 66.19°, 67.95°, 72.19°, and 74.97° correspond to the planes of (002), (110), (−111), (111), (110), (−202), (020), (202), (202), (−311), (220), (311), and (−222). The average crystalline size was obtained using the Scherrer equation:51
| |  | (1) |
From
eqn (1), the obtained
d values for CuO and the O-MWCNT/ CuO nanohybrid are 29.03 nm (CuO) and 25.49 nm (O-MWCNT/CuO), which are quite closer to the
d-spacing values obtained from the TEM image.
Fig. 2B curve (c) shows FTIR broad peaks at 3749 and 2350 cm
−1 corresponding to the asymmetric and symmetric stretching vibrations of the O–H bond from CuO. M–O peaks are observed at 523.98, 539.92 and 600.01 cm
−1 which are due to different bending vibration modes of the Cu–O bond.
52 The FTIR peaks of pure MWCNT are displayed in (
Fig. 2B curve (a)), with the O–H stretching band at 3700 cm
−1 and C–H stretching peaks at 2853 and 2960 cm
−1. The bands at 1785, 1230, 1339, 1480, and 1587 cm
−1 arise from the COOH, C–O, C–OH, and C–O–C functional groups of O-MWCNT,
53,54 as displayed in
Fig. 2B curve (b) with decreased intensities for the O-MWCNT/CuO nanohybrid compared to the pristine CuO and O-MWCNT.
55 Additional bands at 1480 and 1587 cm
−1 correspond to the C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
O stretching, confirming the unzipped MWCNT.
 |
| | Fig. 2 (A) XRD pattern, (B) FTIR, (C) Raman spectra, and (D) UV-vis spectra of (a) MWCNT, (b) O-MWCNT, (c) CuO, and (d) O-MWCNT/CuO nanoparticles. Inset: Tauc plot. | |
Fig. 2C shows the Raman spectra with D and G bands for the pristine MWCNT with a 2D (D mode's overtone) band at 2901 cm−1. The presence of sp2 bonded C in MWCNT is indicated by the increased peak intensity of the G band compared to the D band (Fig. 2C curve (a)). As shown in Fig. 2C curve (b), after the chemical oxidation and reduction of MWCNT for the formation of O-MWCNT, the ID/IG ratio increases from 0.815 to 1.015, confirming the formation of smaller sized sp2 domains by the modified Hummers' method and broken sp3 hybridized carbon for O-MWCNT formation. An increase of D band intensity equal to the G band indicates induced defects, formation of sp2, (Fig. 2C curve (b)), and decreased 2D band for aggregation of single-layered graphene.49 The addition of CuO to O-MWCNT induces very weak bands at 286.87 and 620.30 cm−1 arising from Ag and B2g modes and red shifting of the D and G band positions by 0.7 cm−1, with increased D band intensity compared to the G band and 2D band. Increased 2D band intensity indicates stacked graphene sheets by metal oxide intercalation, (Fig. 2D curve (c)). (Fig. 2D) displays the UV-vis spectra of (a) MWCNT, (b) O-MWCNT, (c) CuO and (d) O-MWCNT/CuO nanoparticles. The pristine MWCNT possess two absorption peaks at 216.18 and 270.35 nm. After unzipping the MWCNT by the Hummers' method, only one peak appears at 253.90 nm due to π–π* transition from the aromatic C–C bond of the graphene sheets. The n–π* transition from the M–O bond is observed at 367.19 nm. The O-MWCNT/CuO nanohybrid has two peaks at 265.54 and 361.44 nm corresponding to the pristine O-MWCNT and CuO nanohybrid. The Tauc plot equation E = (αhν)1/2 is used to calculate the band gap energies of MWCNT, O-MWCNT, CuO, and O-MWCNT/CuO and the obtained values are 1.82, 1.72, 2.09 and 1.57 eV. The combination of O-MWCNT and CuO decreased the band gap compared to the individual O-MWCNT and CuO components.
The survey XPS scan spectrum of the O-MWCNT/CuO nanohybrid is displayed in Fig. 3A with different peaks for the elements Cu, O, and C. Fig. 3B displays peaks at 934.1 and 953.9 eV with a variance of ∼20 eV between Cu 2p3/2 and Cu 2p1/2 spin orbits. Satellite peaks at 942.9 and 962.1 eV exhibit a strong concurrence with the findings of CuO in previous studies.29 The high-resolution deconvoluted C 1s spectra in Fig. 3C show peaks at 288.6, 286.2 and 284.9 eV for the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O and C–C functional groups.32 The high-resolution deconvoluted O1s spectra in Fig. 3D confirm the presence of the metal–oxygen bond at 531.0 eV and the carbonyl functional of O-MWCNT at 528.7 eV, respectively. This confirms the attachment of CuO on the surface of the graphite sheet like O-MWCNT in nanocomposites.
O, C–O and C–C functional groups.32 The high-resolution deconvoluted O1s spectra in Fig. 3D confirm the presence of the metal–oxygen bond at 531.0 eV and the carbonyl functional of O-MWCNT at 528.7 eV, respectively. This confirms the attachment of CuO on the surface of the graphite sheet like O-MWCNT in nanocomposites.
 |
| | Fig. 3 (A) XPS survey scan of the O-MWCNT/CuO nanohybrid and deconvoluted spectra of Cu 2p (B), C1s (C) and O1s (D). | |
3.2. Electrochemical properties of the O-MWCNT/CuO modified electrode
EIS analysis was conducted to comprehend the charge transfer resistance of the modified electrodes. EIS was performed at an applied potential of 0.21 V in the frequency range from 100 kHz to 0.1 MHz in 0.1 M phosphate buffer solution PBS (pH 7.0) containing 1 mM [Fe(CN)6]3−/4−. Fig. 4A shows Nyquist impedance plots of the bare GCE (a), CuO (b), MWCNT (c), O-MWCNT (d), and O-MWCNT/CuO (e). The equivalent circuit RS(QGCdl (RGCW))(QO-MWCNT/CuORQO-MWCNT/CuO) is used for extracting the EIS parameters. Here, RS is the resistance of the solution, QGC dl is the constant phase element representing electrode capacitance, RGC is the resistance of the electrode, W is Warburg's coefficient of diffusion, QO is the double layer capacitance of the O-MWCNT/CuO nanohybrid, and RO-MWCNT/CuO CT is the charge transfer resistance of O-MWCNT/CuO. The bare GCE (curve a) showed a small semi-circle with 543.3 Ω cm2 and CuO (curve b) displays a higher semicircle with an RCT of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 Ω cm2 due to semi-conduction property and higher electron resistivity. On the other hand, O-MWCNT (curve d) showed slightly higher resistance at 1370 Ω cm2. Additionally, the higher resistance value of 3269 Ω cm2 for MWCNT (curve c) than O-MWNCT indicates more defects, which are active towards [Fe(CN)6]3−/4−. Surprisingly, O-MWCNT/CuO (curve e) with the lowest RCT (1043 Ω cm2) depicts the higher conducting behaviour of the composite because of the addition of CuO into its matrix inducing synergistic effects.
540 Ω cm2 due to semi-conduction property and higher electron resistivity. On the other hand, O-MWCNT (curve d) showed slightly higher resistance at 1370 Ω cm2. Additionally, the higher resistance value of 3269 Ω cm2 for MWCNT (curve c) than O-MWNCT indicates more defects, which are active towards [Fe(CN)6]3−/4−. Surprisingly, O-MWCNT/CuO (curve e) with the lowest RCT (1043 Ω cm2) depicts the higher conducting behaviour of the composite because of the addition of CuO into its matrix inducing synergistic effects.
 |
| | Fig. 4 (A) EIS behaviours of the unmodified GCE (a), CuO (b), MWCNT (c), O-MWCNT (d), and O-MWCNT/CuO (e) recorded at an applied potential of 0.210 V and a frequency range of 100 kHz to 0.1 MHz evaluated in the presence of 1.0 mM [Fe(CN)6]3−/4− in PBS buffer (pH 7.0). (B) CV behaviours of the unmodified GCE (a), CuO (b), MWCNT (c), O-MWCNT (d), and O-MWCNT/CuO (e) modified electrodes under similar conditions. (C) Effect of the scan rate and (D) plot of current versus (scan rate)1/2 of the O-MWCNT/CuO modified electrode. | |
Fig. 4B exhibits the CV curves of the bare GCE (a), CuO/GCE (b), MWCNT/GCE (c), O-MWCNT/GCE (d), and O-MWCNT/CuO (e) measured at a scan rate of 50 mV s−1. The bare GCE exhibited a peak-to-peak separation (ΔEP) of 81 mV. Meanwhile the CuO-modified electrode shows ΔEP = 243 mV and pristine MWCNT shows ΔEP = 132 mV due to their poor electron transfer behavior. In addition, O-MWCNT exhibited an improved ΔEP of 113 mV, which confirms its excellent electro catalytic activity. However, the O-MWCNT/CuO modified GCE surface gave ΔEP = 174 mV which is lower than the prepared CuO (243 mV) and higher than the pristine MWCNT (132 mV) and O-MWCNT (113 mV). This clearly confirms the synergetic interaction between O-MWCNT and CuO, improving the electro catalytic activity of CuO while intercalating with O-MWCNT. While comparing the ΔEP values, the current obtained for O-MWCNT was higher (ipa = 19.0 μA) than the bare GCE (18.64 μA), MWCNT (12.56 μA), CuO (12.23 μA) and O-MWCNT/CuO (16.02 μA). In the case of the OMWCNT/CuO composite, the observed ipa was 16.02 μA, and this reduction in current is likely due to the formation of negatively charged ions from CuO on the O-MWCNT/CuO electrode surface, which may create a barrier between the electrode surface and the [Fe(CN)6]3−/4− electrolyte, thus hindering the electron transfer and resulting in a lower current. The study of varying scan rates in the range of 10–100 mV s−1 showed (Fig. 4C) a diffusion-controlled charge transfer reaction, as confirmed in (Fig. 4D) wherein ipa increases linearly with (scan rate)1/2 (ν)1/2 with a correlation coefficient (R2) of 0.9953.
3.3. Electrochemical sensing of ACT
In the CV analysis, five distinct curves (a–e) corresponding to different electrode modifications are observed. CV signals of the GCE (curve a), MWCNT (curve b), O-MWCNT (curve c), CuO (curve d), and O-MWCNT/CuO (curve e) GCE electrodes with ACT (100.0 μM) in 0.1 M PBS at a scan rate of 50 mV s−1 are displayed in Fig. 5A. ACT endures quasi irreversible oxidation at both the bare GCE (curve a) and CuO (curve d) modified electrodes. Irreversibility changes to quasi reversible oxidation at the MWCNT (curve b), O-MWCNT (curve c) and O-MWCNT/CuO (curve e) surfaces in the potential region 0.3–0.5 V. Surprisingly, ipa enhancement was observed for MWCNT/CuO (10.1 μA) compared with MWCNT (5.9 μA), CuO (6.02 μA) and O-MWCNT (8.94 V) due to the synergistic activity of the O-MWCNT/CuO composite. It is also observed that the oxidation peak potential at O-MWCNT/CuO/GCE (0.385 V) is negatively shifted by 83 mV compared with the bare GCE (0.468 V) due to the fast electron transfer, good electrocatalytic behavior and effective interaction of ACT. Based on the aforesaid findings, it can be inferred that the reaction exhibits quasi-reversible behavior with a two-electron transfer mechanism.56 The electrocatalytic mechanism is illustrated in Scheme 3.
 |
| | Fig. 5 (A) Cyclic voltammetry of the bare GCE (curves a), CuO/GCE (curve b), MWCNT/GCE (curve c), O-MWCNT/GCE (curve d) and O-MWCNT/CuO/GCE (curve e) in the presence of 100.0 μM ACT in 0.1 M PBS (pH 7.0) at a scan rate of 50 mV s−1. (B) Effect of pH on the ACT behaviour at the O-MWCNT/CuO/GCE modified electrode. (C) The linear relationship between peak current, potential and solution pH. | |
 |
| | Scheme 3 Electrochemical oxidation mechanism of ACT. | |
Fig. S2† shows the effect of mass loading on the electrochemical performance of the O-MWCNT/CuO-modified GCE, presented as a bar graph. The mass loading of O-MWCNT/CuO on the GCE was varied between 3.0–9.0 μL to evaluate its CV response in the presence of 100 μL of ACT. The results show that the electrochemical response of the O-MWCNT/CuO-modified GCE toward ACT improved as the mass loading increased from 3.0 μL to 7.0 μL. However, beyond this optimal loading (above 7.0 μL), a decline in CV response was observed. This decrease is likely due to the excessive accumulation of nanoparticles, leading to the formation of a thicker film on the electrode surface and it can be blocking the electron transfer between ACT and the O-MWCNT/CuO-modified GCE. Consequently, it reduces the overall sensitivity of the sensor.
3.4. Effect of pH
Optimizing the influence of pH on electrochemical processes is crucial for electrochemical sensors. The effect of pH has been studied in the range of 3.0 to 9.0 on the voltammetric detection of ACT using O-MWCNT/CuO at a scan rate of 50 mV s−1 (Fig. 5B). Upon elevating the pH from 3.0 to 7.0, a discernible increase in the peak current of ACT is observed. Beyond a pH of 7.0, the peak current responses diminish. This decline in ipa under alkaline conditions is attributed to reduced proton interruptions, consequently encouraging a negative shift in Epa. As a result, pH of 7.0 was selected as the optimal working electrolyte pH for further investigations. Fig. 5C depicts that the relationship between pH and Epa obeys a linear regression equation Epa = −0.0519pH − 0.759 with a correlation coefficient R2 = 0.9952. Notably, the obtained slope of −0.0519 mV pH−1 closely to the theoretical value by the Nernst equation (dE/dpH = 0.059χ/αn). This finding confirms the involvement of an equal number of electrons and protons in the ACT reaction at the O-MWCNT/CuO GCE electrode.
3.5. Influence of scan rate and concentration
The CV technique was employed to investigate the influence of scan rate on the electrochemical detection of ACT in 0.1 M PBS at pH 7.0. Fig. 6A shows the diverse scan rates varied from 10 to 100 mV s−1 on O-MWCNT/CuO GCE in 100.0 μM ACT. It was observed that as the scan rate increased within this range, the peak current of ACT showed a consistent rise (see Fig. 6A). The obtained data revealed a linear correlation between the square root of scan rates (mV s−1) and the corresponding anodic peak current (μA), as demonstrated in Fig. 6B. The derived linear equation of ipa = 1.711ν1/2 − 2.106 (R2 = 0.9954) and ipc = 0.7411ν1/2 − 4.277 (R2 = 0.9998), indicating a diffusion-controlled process.57 Furthermore, the calibration plot demonstrated the relationship between the redox peak response versus logarithmic scan rate, revealing linear slopes for Epa and Epc with linear slope values of Epa (V) = 0.03149C (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) + 0.3375 and Epc (V) = −0.0100C (log
v) + 0.3375 and Epc (V) = −0.0100C (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) − 0.3270 (see Fig. S3†). According to Laviron's theory for quasi-reversible electrode reactions:58
v) − 0.3270 (see Fig. S3†). According to Laviron's theory for quasi-reversible electrode reactions:58| |  | (2) |
| |  | (3) |
| |  | (4) |
In these equations, α represents the charge transfer coefficient, n is the number of electrons transferred, ks denotes the heterogeneous electron transfer rate constant, R is the gas constant, T is the temperature in Kelvin, ΔEp is the peak-to-peak separation, and F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 C mol−1). From the slopes of these linear equations, the dynamic parameters α and n were determined using the above equations. The values for α and n were found to be 0.82 and 2.29 (approximated to 2). These results suggest a rapid electron-transfer process for ACT on O-MWCNT/CuO/GCE, indicating a two-electron mechanism consistent with prior studies.59
487 C mol−1). From the slopes of these linear equations, the dynamic parameters α and n were determined using the above equations. The values for α and n were found to be 0.82 and 2.29 (approximated to 2). These results suggest a rapid electron-transfer process for ACT on O-MWCNT/CuO/GCE, indicating a two-electron mechanism consistent with prior studies.59
 |
| | Fig. 6 (A) Effect of different scan rates from 10 to 100 mV s−1 and (B) peak current versus (scan rate)1/2. (C) CVs of different ACT concentrations (from 0 μM to 160.0 μM) in 0.1 M PBS (pH = 7.0) and (D) linear correlation between ipa, ipc and ACT concentration. | |
The effect of ACT concentration on the redox behaviour is studied in concentrations ranging from 0 to 160.0 μM in pH 7.0 (0.1 M PB) at a scan rate of 50 mV s−1 (Fig. 6C). As the concentration of ACT increased, a notable rise in both ipa and ipc was observed. This increase indicates that the electrode effectively promotes enhanced charge transfer processes, allowing for the detection of ACT even at higher concentrations. A linear correlation between ipa and ACT concentration was determined (Fig. 6D), as shown by the regression equation: ipa (μA) = 0.078C [μM] + 2.1475; R2 = 0.9894 and ipc (μA) = 0.034C [μM] + 1.423; R2 = 0.9877, respectively.
3.6. Quantitative detection of ACT on O-MWCNT/CuO
Linear sweep voltammetry (LSV) was employed for the quantitative detection of ACT using O-MWCNT/CuO/GCE under optimal conditions. The experiment involved applying a pulse width = 50 ms, pulse amplitude = 50 mV, and scan rate = 50 mV s−1 in 0.05 M PBS (pH 7.0) electrolyte solution. Fig. 7A displays the LSV response of O-MWCNT/CuO/GCE measured at different ACT doses ranging from 85.0 nM to 1.45 mM and the oxidation current rises linearly with the concentration of ACT. In (Fig. 7B) different linear ranges viz., (i) 60.0 to 128.0 μM; (ii) 132.0 to 200.0 μM and (iii) 200.0 to 1450.0 μM are observed with linear regression equations: (i) ipa (μA) = 0.0137C [μM] + 1.7287 (R2 = 0.9988), (ii) ipa (μA) = 0.01604C [μM] + 2.0424 (R2 = 0.9948) and (iii) ipa (μA) = 0.0104C [μM] + 1.8478 (R2 = 0.9925). Three linear regions are observed due to the charge transfer kinetic limitations at the modified electrode's surface. At lower concentrations, the electrode exhibits a higher number of active sites, involved in the ACT reactions. This results in excellent sensitivity and enhanced ACT reaction as reflected in a steeper slope value. However, the higher linear range of ACT concentration on the O-MWCNT/CuO/GCE surface can lead to sensitivity disturbances due to poor desorption of the adsorbed product.60 The limit of detection (LOD) and limit of quantification (LOQ) are calculated using eqn (5)–(7):61| |  | (5) |
| |  | (6) |
| | 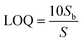 | (7) |
where S is the slope of the calibration curve and Sb is the standard deviation of the blank. The calculated LOD and LOQ of the O-MWCNT/CuO modified GCE are 7.2 nM and 24.8 nM with a sensitivity of 0.019 μA cm−2 μM−1, respectively. Table 1 displays the comparison of the proposed sensor with similar literature in the case of the sensing platform, LOD, sensitivity, and linear range.
 |
| | Fig. 7 (A) LSV signals of ACT oxidation with increasing concentration from 50 nM to 1450 μM measured in PBS (pH 7.0) and (B) linear variation of peak current with concentration. (C) Effect of potential interferences (250.0 μM) on the oxidation of ACT (50.0 μM) at the O-MWCNT/CuO modified electrode using LSV in PBS (pH 7.0) and (D) bar chart of the obtained current with error values. | |
Table 1 Similar literature comparison towards ACT sensing at different modified electrodes
| Modified sensors |
Methods |
Linear range (μM) |
LOD (μM) |
Real samples |
Ref. |
| CuO–graphene |
DPV |
0.025–5.3 |
0.008 |
Blood serum, urine |
52
|
| Fe3O4/N/C@MWCNTs/GCE |
DPV |
0.5–1355 |
0.19 |
Blood serum, urine |
62
|
| CuO–CuFe2O4 |
DPV |
0.01–1.5 |
0.007 |
Blood serum, urine |
63
|
| CuO/rGO |
DPV |
0.1–800 |
0.027 |
ACT tablet, river water |
64
|
| CuO/g-C3N4 |
i–t |
5.0–300 |
0.320 |
— |
65
|
| Cu2O/g-C3N4 |
i–t |
5.0–250 |
0.470 |
— |
65
|
| TFP-DAAQ-COF/WC/NH2–MWCNT/GCE |
DPV |
0.5–200 |
0.120 |
Yellow river water, paracetamol tablet |
66
|
| ZIF-67/MWCNT–COOH/Nafion |
DPV |
0.1–100 |
0.07 |
River water, PCM tablet |
67
|
| Ag–CuO–fCNT |
DPV |
0.02–3.77 and3.77–90.02 |
0.004 |
ACT tablet, urine |
68
|
| La3+–CuO/MWCNTs/GCE |
DPV |
0.5–900 |
0.014 |
Tramadol & ACT tablet, urine |
69
|
| MWCNT/CuO–Au hybrid |
DPV |
0.2–6.0 |
0.016 |
ACT tablets, blood serum |
59
|
| Cu-MOFs/MWCNT–Au@Ag/GCE |
DPV |
1.0–40 and 40–500 |
0.232 |
PCM tablet, blood serum, urine |
70
|
| O-WCNT/CuO/GCE |
LSV |
0.060–1450 |
0.0072 |
Plant exacts, wastewaters, tablets, blood serum, urine |
This work |
3.7. Selectivity investigation of O-MWCNT/CuO/GCE
To evaluate the selectivity of constructed sensors by analyzing their potential interference performance, the LSV signals of ACT in the presence of possible interferents such as folic acid (FA), chloramphenicol (CAP), urea (UA), ascorbic acid (AA), eugenol (EU), riboflavin (RF), glucose (Glu), sucrose (Suc), and dopamine (DA) are depicted in (Fig. 7C). The LSV signals of ACT in the presence of possible metal ion interferents such as lead (Pb2+), cadmium (Cd2+), mercury (Hg2+), sulfate (SO42−), ruthenium (Ru2+), chlorine (Cl−), sodium (Na+), nitrite (NO2−), caffeic acid, catechol and resorcinol are depicted in (Fig. S4†). The results show that O-MWCNT/CuO has a high degree of selectivity towards ACT detection. This interference effect is represented by an error bar in (Fig. 7D) with the corresponding tolerance level. With very minimal signal contribution from interferences, a huge catalytic current was seen for 50.0 μM ACT (5 times lower than the interferents). The findings suggest that the developed sensor remains unaffected by the presence of interferent compounds, demonstrating good selectivity and anti-interference capability for ACT detection.
3.8. Reproducibility, repeatability and stability of the sensor towards ACT
To demonstrate the fabrication reproducibility of the O-MWCNT/CuO sensor for the electrochemical oxidation of 100.0 μM ACT, five different GCE electrodes with similar characteristics are employed for ACT detection (Fig. S5A†). The developed sensor revealed good reproducibility by the observation of a relative standard error (RSD) of 1.5%. Repeatability of sensing activity is monitored by measuring the ipa of 500.0 μM ACT oxidation for 7 repeated measurements (Fig. S5B†), and it showed an excellent reusable capacity. Further, Fig. S5C† illustrates the electrode's storage stability (stored at room temperature) over a duration of 65 days. The results show that the sensing performance of the electrode was stable for the first 40 days and after that (50th day), there is an increase in the current value (1.5%) which may be due to the atmospheric influence. These finding proved its good storage stability.
3.9. Practicability of the sensor
The real sample preparation procedure is given in section 2.4. All the real samples were analyzed using the LSV technique in 0.1 M PBS (pH = 7.0) by the standard addition procedure. In order to test for soil contamination, used soil was combined with MQ water and spiked with PBS, and the results showed that ACT was detected, confirming a high level of soil contamination (Fig. S6†). This example validates the possibility of accurately detecting and disposing of ACTs in the irrigation of edible plants. But when the contaminated soil was again applied for the seeding of fenugreek, interestingly the contaminated soil doesn't show any growth and the seeds got fouled which proves that the soil germination property totally disappeared with the contamination of this pharmaceutical effluent. In addition, various fenugreek plant extract samples including root, leaf, and stem extracts were serially spiked in an electrolyte solution. Every time fenugreek extract is added, there is an increase in the ACT oxidation peak current, which suggests that the plant adsorbs acetaminophen (Fig. S6(B–D)†). The recovery rate and RSD were subsequently determined using the obtained data, as shown in Table 2. The ACT recovery level was determined to be between 96.81% and 100.54% for soil extract and fenugreek plant extract with satisfactory RSD.
Table 2 The practical applicability of ACT detection in plant extracts
| Sample |
Added (μM) |
Found (μM) |
Recovery (%) |
RSD (%) |
| Root extract |
1.0 |
0.98 |
99.80 |
2.61 |
| 3.5 |
3.41 |
97.42 |
1.96 |
| 5.5 |
5.53 |
100.54 |
3.19 |
| 6.0 |
59.6 |
99.33 |
1.65 |
| 8.0 |
7.69 |
96.12 |
2.06 |
| 15 |
14.82 |
98.80 |
1.13 |
| 35 |
34.78 |
99.37 |
2.65 |
| 60 |
60.05 |
100.08 |
1.79 |
| 95 |
93.89 |
98.83 |
2.13 |
| Leaf extract |
2.0 |
1.97 |
98.50 |
2.15 |
| 5.5 |
5.47 |
99.45 |
3.74 |
| 8.0 |
8.0 |
100.00 |
2.44 |
| 9.5 |
9.47 |
99.68 |
1.97 |
| 20 |
19.7 |
98.50 |
2.08 |
| 55 |
53.87 |
97.94 |
2.56 |
| Stem extract |
0.5 |
0.48 |
96.00 |
1.92 |
| 3.5 |
3.54 |
101.14 |
1.49 |
| 6 |
5.98 |
99.66 |
3.04 |
| 7 |
6.9 |
98.52 |
2.67 |
| 8.5 |
8.43 |
99.17 |
2.04 |
| 10 |
9.73 |
97.30 |
2.29 |
| Soil extract |
55 |
53.25 |
96.81 |
2.87 |
| 85 |
84.34 |
99.27 |
1.98 |
| 115 |
113.2 |
98.43 |
3.07 |
| 125 |
122.04 |
97.69 |
2.58 |
The O-MWCNT/CuO sensor was utilized to determine the concentration of ACT in wastewater samples, highlighting its practical application in environmental monitoring. Fig. 8 shows the results obtained from the addition of wastewater samples with varying ACT concentrations using the LSV technique. The recovery rate findings are consolidated in Table S1.† The recovery rate of river water, well water and pond water samples ranged from 97.37% to 100.05%, and RSD was 1.71–3.17%. The findings indicate that the O-MWCNT/CuO sensor demonstrates effectiveness as an electrochemical sensor for detecting ACT in environmental wastewater. To detect ACT in commercial drug samples, specifically Paracetamol 650 mg and Vicks Action 500 were employed as illustrated in (Fig. S7†). A parallel study was conducted to compare pure ACT from a chemical vendor. The sensing signals from the two samples were in closer proximity to one another (Table S2†), suggesting that the sensor designed for pharmaceutical use is very sensitive and efficient. Further, the biological fluids human serum and urine samples were spiked with known concentrations of ACT and detected, (Fig. S8†) and (Table S3†). Notably, O-MWCNT/CuO exhibits an increasing ACT oxidation peak for serial spiking of the ACT contaminated fluids into PBS electrolyte and showed a strong recovery rate (98.58–100.20%) with acceptable RSD values (1.53–3.29%).
 |
| | Fig. 8 LSV measurements of environmental wastewater: (A) river water, (B) well water and (C) pond water by spiking known concentrations of ACT at an applied scan rate of 50 mV s−1 in pH 7.0 (PBS). | |
4. Conclusion
In this study, a straightforward co-precipitation technique and Hummers' method were used to synthesize an O-MWCNT/CuO nanohybrid. Various analytical and spectroscopic methods effectively characterized the formation and structural morphology of the O-MWCNT/CuO nanohybrid. O-MWCNT/CuO has enhanced charge transfer behavior by increased defects caused by the tube opening, which also encourages the formation of sp2 carbon and attachment of metal oxide at these defects. The increased intensity of the 2D band in the presence of CuO on O-MWCNT suggests the intercalative mode of CuO to prevent agglomeration of O-MWCNT to form a layered opened graphitic sheet-like O-MWCNT. The fabricated sensor was highly sensitive to the electrochemical sensing of ACT with an enhanced current response and low detection limit. Additionally, the O-MWCNT/CuO sensor demonstrated outstanding stability, reliability, selectivity, and reproducibility towards ACT. In comparison with previous reports on ACT detection in practicability, this study showcased the significant capabilities of the fabricated O-MWCNT/CuO sensor with satisfactory recovery rates in various real samples including fenugreek plant extracts, environmental wastewater, pharmaceuticals and biological fluids. The fabricated sensor enables on-site detection without the need for sample pre-treatment and is selective, highly stable, and cost-effective, making it suitable for commercial applications.
Data availability
Data will be made available on request.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgements
Y. Allwin Richard and Dr. V. Dharuman acknowledge ICMR (45/09/2022/NAN/BMS: 09.05.2022) for the SRF research fellowship, Alu/RUSA 2.0/EIR/2023, and RUSA 2.0 [F.24-51/2014-U, Policy (TN Multi-Gen), Dept of Edn, Gol] scheme through Alagappa University, Karaikudi. Dr. A.-Y. Lo, Dr. C. Koventhan acknowledges the National Science and Technology Council (NSTC 112-2113-M-027-001-MY3; NSTC 111-2811-E-167-001-MY3; NSTC 111-2221-E-167-008-MY3), Taiwan.
References
- J. O. Osuoha, B. O. Anyanwu and C. Ejileugha, J. Hazard. Mater. Adv., 2023, 9, 100206 CrossRef CAS.
- H. N. P. Vo, G. K. Le, T. M. H. Nguyen, X.-T. Bui, K. H. Nguyen, E. R. Rene, T. D. H. Vo, N.-D. T. Cao and R. Mohan, Chemosphere, 2019, 236, 124391 CrossRef PubMed.
- C. A. Igwegbe, C. O. Aniagor, S. N. Oba, P.-S. Yap, F. U. Iwuchukwu, T. Liu, E. C. de Souza and J. O. Ighalo, J. Ind. Eng. Chem., 2021, 104, 117–135 CrossRef CAS.
- K. Kümmerer, Chemosphere, 2009, 75, 417–434 CrossRef.
- K. Samal, S. Mahapatra and M. H. Ali, Energy Nexus, 2022, 6, 100076 CrossRef CAS.
- Y. Yang, Y. S. Ok, K.-H. Kim, E. E. Kwon and Y. F. Tsang, Sci. Total Environ., 2017, 596, 303–320 CrossRef PubMed.
- Q. Shi, Y. Yuan, Y. Zhou, Y. Yuan, L. Liu, X. Liu, F. Li, C. Leng and H. Wang, Chemosphere, 2023, 341, 140039 CrossRef CAS.
- T. Malchi, Y. Maor, G. Tadmor, M. Shenker and B. Chefetz, Environ. Sci. Technol., 2014, 48, 9325–9333 CrossRef CAS PubMed.
- X. Wu, J. L. Conkle, F. Ernst and J. Gan, Environ. Sci. Technol., 2014, 48, 11286–11293 CrossRef CAS.
- W. Zheng and M. Guo, Curr. Pollut. Rep., 2021, 1–14 Search PubMed.
- M. Bartrons and J. Peñuelas, Trends Plant Sci., 2017, 22, 194–203 CrossRef CAS PubMed.
- T. L. Sorell, AAPS J., 2016, 18, 92–101 CrossRef CAS PubMed.
- C. C. Arıkan, N. Kulabaş and İ. Küçükgüzel, J. Pharm. Biomed. Anal., 2023, 223, 115123 CrossRef.
- E. Souri, S. A. M. Nasab, M. Amanlou and M. B. Tehrani, J. Anal. Chem., 2015, 70, 333–338 CrossRef CAS.
- J. Vilchez, R. Blanc, R. Avidad and A. Navalón, J. Pharm. Biomed. Anal., 1995, 13, 1119–1125 CrossRef CAS PubMed.
- G. Burgot, F. Auffret and J.-L. Burgot, Anal. Chim. Acta, 1997, 343, 125–128 CrossRef CAS.
- Y. Xiao, G. Wang, H. Yi, S. Chen, Q. Wu, S. Zhang, K. Deng, S. Zhang, Z.-Q. Shi and X. Yang, RSC Adv., 2022, 12, 3157–3164 RSC.
- L. Ghasemi, S. Jahani, M. Ghazizadeh and M. M. Foroughi, Mater. Chem. Phys., 2023, 296, 127176 CrossRef CAS.
- X. Cao, L. Luo, Y. Ding, X. Zou and R. Bian, Sens. Actuators, B, 2008, 129, 941–946 CrossRef CAS.
- C. Koventhan, V. Vinothkumar and S. M. Chen, New J. Chem., 2021, 45, 12593–12605 RSC.
- R. Rezaei, M. M. Foroughi, H. Beitollahi and R. Alizadeh, Russ. J. Electrochem., 2018, 54, 860–866 CrossRef CAS.
- C. Koventhan, V. Vinothkumar and S. M. Chen, Microchem. J., 2022, 175, 107082 CrossRef CAS.
- M. M. Foroughi, S. Jahani and S. Rashidi, Microchem. J., 2024, 198, 110156 CrossRef.
- C. Wei, X. Zou, Q. Liu, S. Li, C. Kang and W. Xiang, Electrochim. Acta, 2020, 334, 135630 CrossRef CAS.
- S. John, S. S. Vadla and S. C. Roy, Electrochim. Acta, 2019, 319, 390–399 CrossRef CAS.
- K. Y. Hwa, P. Karuppaiah, N. S. K. Gowthaman, V. Balakumar, S. Shankar and H. N. Lim, Ultrason. Sonochem., 2019, 58, 104649 CrossRef.
- M. Ramya, P. S. Kumar, G. Rangasamy, V. U. Shankar, G. Rajesh and K. Nirmala, Environ. Res., 2023, 216, 114463 CrossRef CAS.
- N. Lu, C. Shao, X. Li, F. Miao, K. Wang and Y. Liu, Ceram. Int., 2016, 42, 11285–11293 CrossRef CAS.
- Z. Zhai, B. Leng, N. Yang, B. Yang, L. Liu, N. Huang and X. Jiang, Small, 2019, 15, 1901527 CrossRef CAS PubMed.
- N. S. Tezerji, M. M. Foroughi, R. R. Bezenjani, N. Jandaghi, E. Rezaeipour and F. Rezvani, Food Chem., 2020, 311, 125747 CrossRef CAS.
- P. N. Manikandan, H. Imran and V. Dharuman, Anal. Methods, 2016, 8, 2691–2697 RSC.
- C. Koventhan, S. M. Babulal, S. M. Chen, A. Y. Lo and C. S. Selvan, Mater. Today Chem., 2024, 35, 101896 CrossRef CAS.
- Y.-C. Chung, A. Julistian, L. Saravanan, P.-R. Chen, B.-C. Xu, P.-J. Xie and A.-Y. Lo, Catalysts, 2021, 12, 23 CrossRef.
- T. Oren and Ü. Anık, New J. Chem., 2017, 41, 11800–11806 RSC.
- S. H. Pisal, N. S. Harale, T. S. Bhat, H. P. Deshmukh and P. S. Patil, IOSR J. Appl. Chem., 2014, 7, 49–52 CrossRef.
- Y. Wang, J. Zhang, J. Zang, E. Ge and H. Huang, Corros. Sci., 2011, 53, 3764–3770 CrossRef CAS.
- C. S. Tiwary, B. Javvaji, C. Kumar, D. R. Mahapatra, S. Ozden, P. M. Ajayan and K. Chattopadhyay, Carbon, 2015, 89, 217–224 CrossRef CAS.
- D. B. Shinde, J. Debgupta, A. Kushwaha, M. Aslam and V. K. Pillai, J. Am. Chem. Soc., 2011, 133, 4168–4171 CrossRef CAS PubMed.
- A. Koutsioukis, K. Spyrou, N. Chalmpes, D. Gournis and V. Georgakilas, Nanomaterials, 2022, 12, 447 CrossRef CAS.
- I. F. Taha, M. El-Ashry and E.-S. M. Duraia, Fullerenes, Nanotubes, Carbon Nanostruct., 2023, 31, 147–156 CrossRef CAS.
- H. Wang, F. Meng, F. Huang, C. Jing, Y. Li, W. Wei and Z. Zhou, ACS Appl. Mater. Interfaces, 2019, 11, 12142–12153 CrossRef CAS PubMed.
- A. Z. Yazdi, K. Chizari, A. S. Jalilov, J. Tour and U. Sundararaj, ACS Nano, 2015, 9, 5833–5845 CrossRef PubMed.
- A. U. Alam, Y. Qin, M. M. R. Howlader, N. X. Hu and M. J. Deen, Sens. Actuators, B, 2018, 254, 896–909 CrossRef CAS.
- M. Kenarkob and Z. Pourghobadi, Microchem. J., 2019, 146, 1019–1025 CrossRef CAS.
- Z. A. Alothman, N. Bukhari, S. M. Wabaidur and S. Haider, Sens. Actuators, B, 2010, 146, 314–320 CrossRef CAS.
- S. Vinoth and S. F. Wang, Environ. Sci. Pollut. Res., 2024, 31, 46484–46497 CrossRef CAS.
- V. N. Palakollu, T. E. Chiwunze, C. Liu and R. Karpoormath, Arabian J. Chem., 2020, 13, 4350–4357 CrossRef CAS.
- L. Jiang, S. Gu, Y. Ding, F. Jiang and Z. Zhang, Nanoscale, 2014, 6, 207–214 RSC.
- D. Long, W. Li, W. Qiao, J. Miyawaki, S.-H. Yoon, I. Mochida and L. Ling, Chem. Commun., 2011, 47, 9429–9431 RSC.
- B. Nahar, S. B. Chaity, M. A. Gafur and M. Z. Hossain, J. Nanomater., 2023, 2023, 1–10 CrossRef.
- C. Koventhan, N. K. R. Kumar, S. M. Chen, K. Pandi and A. Sangili, Colloids Surf., A, 2021, 621, 126625 CrossRef CAS.
- Z. M. Khoshhesab, RSC Adv., 2015, 5, 95140–95148 RSC.
- S. Mallakpour, M. Dinari and V. Behranvand, J. Mater. Sci., 2014, 49, 7004–7013 CrossRef CAS.
- A. Al-Ahmed, A. Sarı, M. A. J. Mazumder, G. Hekimoğlu, F. A. Al-Sulaiman and Inamuddin, Sci. Rep., 2020, 10, 1–16 CrossRef PubMed.
- M. Krishnaveni, A. M. Asiri and S. Anandan, Ultrason. Sonochem., 2020, 66, 105105 CrossRef CAS.
- N. S. Anuar, W. J. Basirun, M. Ladan, M. Shalauddin and M. S. Mehmood, Sens. Actuators, B, 2018, 266, 375–383 CrossRef CAS.
- R.-S. Juang, C.-T. Hsieh, T.-A. Lin, Y.-C. Shao and Y. A. Gandomi, Colloids Surf., A, 2023, 677, 132426 CrossRef CAS.
- E. J. J. Laviron, J. Electroanal. Chem. Interfacial Electrochem., 1979, 101, 19–28 CrossRef CAS.
- P. Shaikshavali, T. M. Reddy, V. N. Palakollu, R. Karpoormath, Y. S. Rao, G. Venkataprasad, T. V. Gopal and P. Gopal, Synth. Met., 2019, 252, 29–39 CrossRef CAS.
- C. Koventhan, S. Pandiyarajan and S. M. Chen, J. Alloys Compd., 2022, 895, 162315 CrossRef CAS.
- C. Koventhan, R. Shanmugam and S. M. Chen, Electrochim. Acta, 2023, 467, 143096 CrossRef CAS.
- S. Yuan, X. Bo and L. Guo, Talanta, 2019, 193, 100–109 CrossRef CAS PubMed.
- F. Hasanpour, M. Taei and S. Tahmasebi, J. Food Drug Anal., 2018, 26, 879–886 CrossRef CAS.
- M. P. Zarandi and H. Beitollahi, Microchem. J., 2022, 181, 107726 CrossRef.
- Y. Chen, Y. Zhu, Y. Zhao, J. Wang and M. Li, Microchem. J., 2021, 163, 105884 CrossRef CAS.
- Z. Lu, H. Guo, X. Wei, L. Sun, Z. Pan, B. Liu, Y. Liu, J. Xu, J. Tian and W. Yang, Microchem. J., 2023, 193, 109075 CrossRef CAS.
- H. Guo, T. Fan, W. Yao, W. Yang, N. Wu, H. Liu, M. Wang and W. Yang, Microchem. J., 2020, 158, 105262 CrossRef CAS.
- D. Balram, K.-Y. Lian and N. Sebastian, Sensors, 2022, 23, 379 CrossRef.
- M. M. Foroughi, S. Jahani and H. H. Nadiki, Sens. Actuators, B, 2019, 285, 562–570 CrossRef CAS.
- W. Yao, H. Guo, H. Liu, Q. Li, R. Xue, N. Wu, L. Li, M. Wang and W. Yang, J. Electrochem. Soc., 2019, 166, B1258 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  cd,
Chelliah
Koventhan
cd,
Chelliah
Koventhan
 *cd,
Venkataraman
Dharuman
*a and
Shakkthivel
Piraman
b
*cd,
Venkataraman
Dharuman
*a and
Shakkthivel
Piraman
b
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH),35 or controlled by doping or modifying the surface using other materials. In particular, TMOs have been used as dopants or composited with MWCNTs for the development of electrochemical sensors. Recently, MWCNT structure manipulation has been applied to cut and unzip CNTs, producing shorter pieces and nanoribbons. Unzipping of CNTs is a simple method proposed for mass production of single layer graphene. CNTs can be unzipped through selective etching,36 cryogenic grinding,37 electrochemical preparation,38 hydrothermal,39 Hummers',40 and chemical oxidation methods.41 Studies reveal that open ended MWCNTs are more reactive than close ended ones.42 Combining carbon materials with metal or metal oxide particles enhances conductivity, catalytic, and sensing properties. Recently, Alam et al. used MWCNT and β-cyclodextrin-modified GC electrodes for ACT detection in a linear range of 50 nM–300 μM with an LOD of 11.5 nM.43 Kenarkob et al. developed ZnO/Au monohybrid functionalized MWCNTs for an ACT sensor and obtained a linear sensing range of 0.05–20 μM with an LOD of 9 nM.44 Meanwhile, electrodeposited graphene/ZnO, Fe3O4/graphene, SnO2/f-MWCNT, f-MWCNT and MWCNT/MOF@Au–Ag nanohybrid materials were used for the improvement of ACT sensing.45–48 However, there are very few published literature studies on the usage of nanoribbons made from unzipped MWCNTs and CuO coated with opened MWCNTs for the detection of ACT.
O, –OH),35 or controlled by doping or modifying the surface using other materials. In particular, TMOs have been used as dopants or composited with MWCNTs for the development of electrochemical sensors. Recently, MWCNT structure manipulation has been applied to cut and unzip CNTs, producing shorter pieces and nanoribbons. Unzipping of CNTs is a simple method proposed for mass production of single layer graphene. CNTs can be unzipped through selective etching,36 cryogenic grinding,37 electrochemical preparation,38 hydrothermal,39 Hummers',40 and chemical oxidation methods.41 Studies reveal that open ended MWCNTs are more reactive than close ended ones.42 Combining carbon materials with metal or metal oxide particles enhances conductivity, catalytic, and sensing properties. Recently, Alam et al. used MWCNT and β-cyclodextrin-modified GC electrodes for ACT detection in a linear range of 50 nM–300 μM with an LOD of 11.5 nM.43 Kenarkob et al. developed ZnO/Au monohybrid functionalized MWCNTs for an ACT sensor and obtained a linear sensing range of 0.05–20 μM with an LOD of 9 nM.44 Meanwhile, electrodeposited graphene/ZnO, Fe3O4/graphene, SnO2/f-MWCNT, f-MWCNT and MWCNT/MOF@Au–Ag nanohybrid materials were used for the improvement of ACT sensing.45–48 However, there are very few published literature studies on the usage of nanoribbons made from unzipped MWCNTs and CuO coated with opened MWCNTs for the detection of ACT.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 RPM. Using Na2HPO4 and NaH2PO4, phosphate buffer solution (PBS, pH 7.0) was prepared for the duration of the experiment, and a Susima, AP-1PLVS (India) was used to monitor pH. The electrochemical measurement and characterization techniques were detailed in the ESI† (Section S1).
000 RPM. Using Na2HPO4 and NaH2PO4, phosphate buffer solution (PBS, pH 7.0) was prepared for the duration of the experiment, and a Susima, AP-1PLVS (India) was used to monitor pH. The electrochemical measurement and characterization techniques were detailed in the ESI† (Section S1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 RPM with water and ethanol. The washed precipitate was dried under vacuum at 60 °C overnight to obtain O-MWCNT/CuO. For the control experimentation, pure CuO was synthesised under the same conditions in the absence of O-MWCNT.
000 RPM with water and ethanol. The washed precipitate was dried under vacuum at 60 °C overnight to obtain O-MWCNT/CuO. For the control experimentation, pure CuO was synthesised under the same conditions in the absence of O-MWCNT.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and 0.1% Nafion and ultra-sonicated for 15 minutes. Finally, 7.0 μL of slurry was coated separately on the GCE and dried at room temperature for 1.5 h (Scheme 1).
2) and 0.1% Nafion and ultra-sonicated for 15 minutes. Finally, 7.0 μL of slurry was coated separately on the GCE and dried at room temperature for 1.5 h (Scheme 1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 RPM and the solution pH was adjusted to 7.0 before each analysis.
000 RPM and the solution pH was adjusted to 7.0 before each analysis.



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, confirming the unzipped MWCNT.
O stretching, confirming the unzipped MWCNT.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O and C–C functional groups.32 The high-resolution deconvoluted O1s spectra in Fig. 3D confirm the presence of the metal–oxygen bond at 531.0 eV and the carbonyl functional of O-MWCNT at 528.7 eV, respectively. This confirms the attachment of CuO on the surface of the graphite sheet like O-MWCNT in nanocomposites.
O, C–O and C–C functional groups.32 The high-resolution deconvoluted O1s spectra in Fig. 3D confirm the presence of the metal–oxygen bond at 531.0 eV and the carbonyl functional of O-MWCNT at 528.7 eV, respectively. This confirms the attachment of CuO on the surface of the graphite sheet like O-MWCNT in nanocomposites.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 Ω cm2 due to semi-conduction property and higher electron resistivity. On the other hand, O-MWCNT (curve d) showed slightly higher resistance at 1370 Ω cm2. Additionally, the higher resistance value of 3269 Ω cm2 for MWCNT (curve c) than O-MWNCT indicates more defects, which are active towards [Fe(CN)6]3−/4−. Surprisingly, O-MWCNT/CuO (curve e) with the lowest RCT (1043 Ω cm2) depicts the higher conducting behaviour of the composite because of the addition of CuO into its matrix inducing synergistic effects.
540 Ω cm2 due to semi-conduction property and higher electron resistivity. On the other hand, O-MWCNT (curve d) showed slightly higher resistance at 1370 Ω cm2. Additionally, the higher resistance value of 3269 Ω cm2 for MWCNT (curve c) than O-MWNCT indicates more defects, which are active towards [Fe(CN)6]3−/4−. Surprisingly, O-MWCNT/CuO (curve e) with the lowest RCT (1043 Ω cm2) depicts the higher conducting behaviour of the composite because of the addition of CuO into its matrix inducing synergistic effects.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) + 0.3375 and Epc (V) = −0.0100C (log
v) + 0.3375 and Epc (V) = −0.0100C (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) − 0.3270 (see Fig. S3†). According to Laviron's theory for quasi-reversible electrode reactions:58
v) − 0.3270 (see Fig. S3†). According to Laviron's theory for quasi-reversible electrode reactions:58


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 C mol−1). From the slopes of these linear equations, the dynamic parameters α and n were determined using the above equations. The values for α and n were found to be 0.82 and 2.29 (approximated to 2). These results suggest a rapid electron-transfer process for ACT on O-MWCNT/CuO/GCE, indicating a two-electron mechanism consistent with prior studies.59
487 C mol−1). From the slopes of these linear equations, the dynamic parameters α and n were determined using the above equations. The values for α and n were found to be 0.82 and 2.29 (approximated to 2). These results suggest a rapid electron-transfer process for ACT on O-MWCNT/CuO/GCE, indicating a two-electron mechanism consistent with prior studies.59