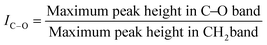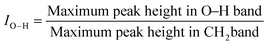Developing environmentally relevant micro- and nanoplastics to assess removal efficiencies in wastewater treatment processes†
Martín
Benzo
 *,
María Eugenia
Pérez Barthaburu
,
Andrés
Pérez-Parada
*,
María Eugenia
Pérez Barthaburu
,
Andrés
Pérez-Parada
 ,
Álvaro
Olivera
,
Álvaro
Olivera
 and
Laura
Fornaro
and
Laura
Fornaro
Grupo de Desarrollo de Materiales y Estudios Ambientales, Departamento de Desarrollo Tecnológico, Centro Universitario Regional del Este, Universidad de la República, Ruta 9 y Ruta 15, Rocha, Uruguay. E-mail: martin.benzo@cure.edu.uy
First published on 29th October 2024
Abstract
Micro and nanoplastics (MNP) pollution has become an increasingly concerning environmental issue. Wastewater treatment plants represent a significant source of MNP pollution, as the treatments involved do not completely remove them. Studies on their removal from water and wastewater are of current interest. However, suitable reference materials are necessary to conduct these studies accurately and to calibrate and validate analytical techniques capable of determining their concentration in water and wastewater. This work provides new insights into developing such materials. By a simple, straightforward, and cost-effective method, we produced MNP of target commodity polymers: polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC) and polyethylene terephthalate (PET) in sizes ranging from 20 to 3500 nm through non-solvent precipitation. The MNP obtained were aged by exposure to UV/O3 to simulate natural plastic weathering. We assessed the dispersibility of the particles in various media and conducted a series of coagulation/flocculation tests using both aged and non-aged particles in different aqueous media. The results of these tests suggest that an eco-corona was formed, which strongly influences the colloidal behavior of MNP. The MNP obtained in this work proved to be suitable for assessing MNP removal efficiency in coagulation/flocculation processes, provided that an adequate medium with a chemical composition resembling that of wastewater is used. This research not only contributes to the development of representative reference materials but also provides new insights into the colloidal behavior of MNP in wastewater, which could help optimize removal efficiencies in wastewater treatment processes.
Environmental significanceWastewater treatment plants are a significant source of micro and nanoplastic pollution, as conventional treatments are not designed to specifically remove them. Hence, studies focusing on optimizing the removal of micro and nanoplastics from wastewaters are of great interest. For accurate design and performance of these studies, suitable reference materials are needed. In this work, we explored the production of reference micro and nanoplastics that resemble those found in wastewater in various characteristics: shape, range of sizes, superficial chemical composition, and the formation of an ‘eco-corona’. |
Introduction
In recent decades, micro and nanoplastic (MNP) pollution has become an increasingly concerning environmental issue. MNP have been found in practically all environmental compartments,1–6 and it is becoming clear that they pose a risk to environmental, animal and human health.7–12 While their full environmental impact is still under examination, they are known to disrupt cellular functions in organisms7,13 and have the propensity to accumulate in marine life. This accumulation raises concerns about human health risks, mainly through the consumption of seafood, given the increasing prevalence of these particles in marine environments. They also are efficient carriers of harmful contaminants like heavy metals and persistent organic pollutants.14,15The 2023 ISO standard defines microplastics (MP) as plastic particles ranging in size from 1 μm to 1 mm, and categorizes them into several size classes: 1–5 μm, 5–10 μm, 10–50 μm, 50–100 μm, 100–500 μm, and 500–1000 μm.16 Some studies similarly classify MP as large, small, and very small, though the boundaries of these categories differ. The threshold between small and very small microplastics has been identified as 50 μm,17 10 μm,18 or even 5 μm,19,20 depending on the author. Although nanoplastics (NP) are not covered in the ISO standard, they are commonly defined as plastic particles exhibiting colloidal behavior, typically within the size range of 1 to 1000 nm.21,22
The occurrence of MNP in wastewater treatment plants (WWTP) has been documented, with these being detected in highly variable concentrations (up to 680 μg L−1 in influents, and up to 24 μg L−1 in effluents). There is evidence that in conventional WWTP, a portion of the MNP is removed. However, the removal is not complete, and due to the large quantities of effluents discharged worldwide, without specific treatment to remove MNP or adaptations to existing processes to optimize their removal, WWTP constitute a significant source of pollution.23–25
Standardized analytical techniques are essential as fundamental tools for reliably monitoring MNP in the environment and other matrices, such as wastewater and drinking water. In recent years, various procedures have been applied to isolate, identify, quantify and characterize MNP with varying levels of reliability and reproducibility,3,4,26,27 but a standardized methodology has not yet been proposed. One reason is the lack of reference materials that mimic the characteristics of MNP found in environmental samples. Developing such materials is challenging because MNP are not homogeneous; they exhibit diverse shapes, sizes, chemical compositions, and degrees of surface degradation.
Most toxicological and environmental studies use polystyrene (PS) micro and nanospheres28,29 as they are readily available commercial options. However, it is well documented that polyethylene (PE) and polypropylene (PP) are the most common polymers found in MNP in the environment.30 In recent years, efforts have been made to produce reference MNP of various polymers in different shapes and sizes using physical and chemical methods. Physical methods include laser ablation,31 cryogenic milling32,33 and ultrasonic treatment.20,34 These methods offer the advantage of producing MNP with characteristics similar to those isolated from environmental samples, such as irregular shapes, a wide range of sizes (from 100 nm to 200 μm depending on the method), and degraded surfaces. However, they have the drawbacks of requiring expensive equipment (as in the case of cryogenic milling and laser ablation) or producing MNP in limited quantities (as with laser ablation and ultrasonic treatment).
MNP can be chemically synthesized in the micro or nanoscale using the emulsion-polymerization method, where monomers serve as the starting material, and polymerization is induced in aqueous medium with surfactants.35–37 However, this process requires high pressures, such as 100 bar for synthesizing PE nanoparticles,35 and the resulting dispersions need purification to remove residual monomers, initiators and surfactants. Another chemical alternative for producing MNP involves self-assembling polymeric materials to create micro or nanoparticles, which two methods can achieve. The first method consists of dissolving the desired polymer in an appropriate organic solvent and then creating an emulsion of the solution in water. Once the emulsion forms, the organic solvent is evaporated, resulting in an aqueous dispersion of MNP. The key to success in this method is achieving a high-quality emulsion, homogenized to an ultra-fine level, typically by high energy mixing (ultrasound) and adding surfactants to stabilize the emulsion. Solvent evaporation must be carried out at low temperatures to prevent polymer re-dissolution.29,38 The second method, known as non-solvent precipitation or nanoprecipitation, primarily produces NP. It involves precipitating them from the polymer solution by adding a miscible non-solvent with the initial organic solvent. Non-solvent precipitation has found widespread applications in pharmaceutical research for nanoencapsulation of drugs,39,40 as well as in food engineering for producing nanoparticles of natural polymers like starch.41,42 Only a few studies have applied this method to produce reference MNP. Rodríguez-Hernández et al.,43 dissolved ground polyethylene terephthalate (PET) in concentrated trifluoroacetic acid (TFA) and precipitated it by adding a dilute solution of TFA in water without the addition of a surfactant. After separating the particles from the solvent/non-solvent mixture, they obtained a stable dispersion of PET nanoparticles with diameters ranging from 50 to 200 nm, using a surfactant. Tanaka et al. produced nanoparticles of PE, PS, polypropylene (PP) and polyvinyl chloride (PVC) by dissolving PE and PP in xylene at 120 °C, PS in toluene at 50 °C and PVC in cyclohexanone at 60 °C. They precipitated the polymers by adding non-solvents: dimethyl sulfoxide (PE, PP), dimethyl sulfoxide/distilled water (PVC), and distilled water/ethanol (PS). The resulting NP had spherical shapes with diameters in the range of 150 to 500 nm.44
The present work is embedded in a project focused on studying the removal efficiencies of micro- and nanoplastics in both conventional and emerging wastewater treatment processes. In this context, and based on the background information provided earlier, the primary objective was to obtain environmentally relevant MNP that would allow us to study the removal of these particles from water and wastewater. We focused on obtaining particles within a size range where suspensions exhibited colloidal or near-colloidal behavior, since these types of particles are more challenging to remove by conventional wastewater treatment processes.45 To achieve this goal, we adopted a bottom-up approach.
First we developed a simple, straightforward, and cost-effective synthesis procedure based on non-solvent precipitation. This method allowed us to obtain very small microplastics (considered in this case as those smaller than 5 μm) and nanoplastics. From this point forward, both, very small microplastics and nanoplastics will be referred to collectively as vMNP. With the aim of obtaining particles in a wide range of sizes, we investigated the impact of synthesis parameters on the sizes of the produced vMNP. Previous reports have indicated that key factors governing particle size in non-solvent precipitation include the choice of non-solvent, the solvent-to-non-solvent ratio, the incorporation of a surfactant, and the polymer concentration. Among these parameters, the polymer concentration emerges as a key factor influencing the resultant particle sizes.41,42
Secondly, to better replicate the surface characteristics of MNP found in real-world samples, we also investigated artificial aging processes. A few previous studies have explored artificial aging of MNP using various approaches, including UV irradiation,46–48 advanced oxidation processes involving Fe2+/H2O2 and K2S2O8,49 and ozone treatment.48,50 In all cases, short-term treatments (3–96 h, depending on the method) increased the presence of polar groups (such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–H and C–O) on the surface of the MNP, comparable to several months or even years of natural environmental exposure.47,49 In our study, we attempted to produce vMNP with degraded surfaces through two methods: 1) exposing vMNP synthesized from virgin polymers to UV/O3 and 2) synthesizing vMNP through non-solvent precipitation using naturally weathered plastic waste as the starting material.
O, O–H and C–O) on the surface of the MNP, comparable to several months or even years of natural environmental exposure.47,49 In our study, we attempted to produce vMNP with degraded surfaces through two methods: 1) exposing vMNP synthesized from virgin polymers to UV/O3 and 2) synthesizing vMNP through non-solvent precipitation using naturally weathered plastic waste as the starting material.
It has been reported that in natural environments, MNP tend to accumulate a layer of proteins, polysaccharides, and other organic matter, known as an eco-corona. This coating alters the surface properties of the MNPs, preventing their aggregation and enhancing stability by promoting steric and electrostatic repulsion.1,13 To assess this phenomenon, we examined how the formation of an eco-corona impacts the generation of stable aqueous vMNP dispersions by exposing them to different media.
Finally, to assess the suitability of the produced vMNP as model particles for evaluating their removal from water and wastewater, we conducted coagulation/flocculation tests using vMNP dispersions. Coagulation/flocculation was specifically selected for this phase of our research because it serves as a fundamental process in water and wastewater treatment plants. Its primary aim is to improve the separation of particulate matter in subsequent treatment stages like sedimentation and filtration. By agglomerating colloidal particles and finely divided substances, these processes create larger particles that can be efficiently removed.51 Given their extensive use in water and wastewater treatment, it offers a significant potential for enhancement, particularly in optimizing the removal of MNP.
The findings of this study contribute to the development of more accurate reference materials and provide valuable insights into vMNP behavior during coagulation/flocculation, a widely used wastewater treatment process. These results set the foundation for future evaluations and optimization of removal efficiency in wastewater treatment plants.
Experimental section
vMNP synthesis
The synthesis procedure involved dissolving the polymer in the solvent at the temperatures and concentrations shown in Table 1. Subsequently, the solutions were cooled down, if necessary, to the synthesis temperature. Under magnetic stirring, the vMNP were precipitated by rapidly adding the non-solvent (pre-heated to the synthesis temperature if necessary) to the polymer solution in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. The synthesis temperature for PE and PP vMNP was set at 70 °C, because, based on experimental verification, both polymers precipitate from the solution at temperatures between 60–65 °C. In contrast, for PS, PVC, and PET, this precipitation did not occur; therefore, vMNP syntheses were conducted at room temperature (20 °C).
1 volume ratio. The synthesis temperature for PE and PP vMNP was set at 70 °C, because, based on experimental verification, both polymers precipitate from the solution at temperatures between 60–65 °C. In contrast, for PS, PVC, and PET, this precipitation did not occur; therefore, vMNP syntheses were conducted at room temperature (20 °C).
| Exp. | Polymer conc. (mg mL−1) | Tween 60 conc. (% w/v) | Starting material | Solvent (T dissolution) | Non-solvent (non-solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent ratio) solvent ratio) |
T synthesis (°C) |
|---|---|---|---|---|---|---|
| PE1 | 1.5 | 0 | Virgin PE | Toluene (90 °C) | Ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 |
| PE2 | 1.5 | 2 | Virgin PE | Toluene (90 °C) | Ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 |
| PE3 | 15 | 0 | Virgin PE | Toluene (90 °C) | Ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 |
| PE4 | 15 | 2 | Virgin PE | Toluene (90 °C) | Ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 |
| wPE1 | 1.5 | 0 | Naturally weathered PE | Toluene (90 °C) | Ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 |
| wPP1 | 1.5 | 0 | Naturally weathered PP | o-Xylene (120 °C) | Ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 |
| PP1 | 1.5 | 0 | Virgin PP | o-Xylene (120 °C) | Ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 |
| PS1 | 1.5 | 0 | Virgin PS | Tetrahydrofuran (60 °C) | Ethanol (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20 |
| PVC1 | 1.5 | 0 | Virgin PVC | Tetrahydrofuran (60 °C) | Ethanol (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20 |
| PET1 | 1.5 | 0 | Virgin PET | Trifluoroacetic acid (50 °C) | Ethanol (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20 |
The reaction mix (solvent + non-solvent + vMNP) was centrifuged at 4000 rpm for 3 minutes to remove the solvent. The supernatant was discarded, and the solids were re-dispersed in ethanol. This procedure was repeated, and the resulting solids were stored in ethanol.
Four experiments were carried out for polyethylene (PE) to study the influence of two synthesis parameters in the obtained particle size distribution: polymer concentration and the presence of surfactant (Tween 60) in the reaction mix. The conditions of each experiment are summarised in Table 1.
Additionally, to test whether the synthesis applied to other polymers, experiments were conducted with PP, PS, PVC and PET, under the conditions described in Table 1. In the PS, PVC and PET experiments, higher non-solvent to solvent ratios were used because it was observed that at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the particles collapsed after centrifugation and aggregated, forming macroscopic films and fibres.
1 ratio, the particles collapsed after centrifugation and aggregated, forming macroscopic films and fibres.
PE (pellet, low density, melt index 25 g/10 min), PVC (powder, average Mw 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, average Mn 35
000, average Mn 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), PET (granular), Tween 60 (for synthesis), and trifluoroacetic acid (for synthesis) were purchased from Sigma Aldrich (Merck). PP (pellets, melt index 1.5 g/10 min) was acquired from Braskem S.A., while PS (pellets, melt index 18.16 g/10 min) was obtained from Videolar-Innova S.A. As for the solvents, toluene (ACS) was sourced from Macron Fine Chemicals, o-xylene was purchased from Fischer Scientific (Fischer Scientific UK Limited), ethanol (absolute, ACS, ISO, Reag Ph Eur) from Merck Millipore (Merck KGaA), and tetrahydrofuran (ACS, ISO, Reag Ph Eur) from Supelco (Merck KGaA).
000), PET (granular), Tween 60 (for synthesis), and trifluoroacetic acid (for synthesis) were purchased from Sigma Aldrich (Merck). PP (pellets, melt index 1.5 g/10 min) was acquired from Braskem S.A., while PS (pellets, melt index 18.16 g/10 min) was obtained from Videolar-Innova S.A. As for the solvents, toluene (ACS) was sourced from Macron Fine Chemicals, o-xylene was purchased from Fischer Scientific (Fischer Scientific UK Limited), ethanol (absolute, ACS, ISO, Reag Ph Eur) from Merck Millipore (Merck KGaA), and tetrahydrofuran (ACS, ISO, Reag Ph Eur) from Supelco (Merck KGaA).
To determine whether vMNP with oxidized surfaces could be produced, PE and PP vMNP were synthesized using macroplastic residues weathered by environmental exposure as the starting material (experiments wPE1 and wPP1). PE and PP were selected because they are among the two most widely produced polymers globally52 and are frequently found among the top three polymers in MNP detected in wastewater samples.25,53
Naturally weathered PE and PP waste samples were collected from a local beach in the Department of Rocha, Uruguay. Various pieces of plastic, mainly fragments of bottles and caps, were selected based on their brittleness, indicating prolonged environmental exposure. The plastics were subsequently analyzed by FTIR to confirm their chemical identity and to detect indicators of surface oxidation, such as –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –C–O, and –O–H peaks.
O, –C–O, and –O–H peaks.
Artificial aging process
vMNP obtained in the PE1 and PP1 experiments were dried overnight in an oven at 60 °C. In each experiment, 10 mg of vMNP were weighed in a Petri dish and exposed to UV light (15 mW cm−2 at 185 nm) and O3 in a UV/O3 cleaner (L2002A2, Ossila Ltd.). The tested exposure times were 30, 60, 120 and 180 min.Immediately after each exposure time, a small sample was analyzed in an FTIR spectrophotometer (Perkin Elmer Frontier with ATR accessory), and the spectrum between 750 and 4000 cm−1 was recorded. Three replicates were conducted for each exposure time.
Carbonyl, hydroxyl, and carbon–oxygen indexes were calculated using the following equations:47,49
where the C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band corresponds to 1550–1810 cm−1 (C
O band corresponds to 1550–1810 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), the C–O band to 1210–1124 cm−1 (C–O stretching) and the O–H band to 3200–3550 cm−1 (O–H stretching). The methylene band (3000–2800 cm−1) was used as the reference for the calculation of the three indexes.47,49
O stretching), the C–O band to 1210–1124 cm−1 (C–O stretching) and the O–H band to 3200–3550 cm−1 (O–H stretching). The methylene band (3000–2800 cm−1) was used as the reference for the calculation of the three indexes.47,49
Characterization of vMNP
Size distribution was measured by Dynamic Light Scattering (DLS) using a particle size analyzer (Zeta-Plus, Brookhaven Instruments Corporation, Holtsville, NY) at 635 nm and a dispersion angle of 90°. Samples were dispersed in deionized water with 0.05% Tween 60 and ultrasonicated for 10 min at 40 kHz (Branson Ultrasonics). The measurement cuvette was filled with deionized water (previously filtered with a 0.22 μm syringe filter) and two drops of the vMNP dispersion were added. The cuvette was homogenised by shaking it manually.Morphology of the vMNP was observed in a transmission electron microscope (TEM, JEOL JEM 2100) at 200 kV voltage acceleration. Size distribution was obtained by image analysis (FIJI/Image J software54) and compared with the DLS size distribution. To obtain the size distribution, Feret's diameter—representing the longest distance across the particle in the image plane—was measured in a selection of between 700 and 1000 particles for each sample. Log-normal distribution functions were fitted, deriving mean diameters and standard deviations from these fitted functions.55
Dispersibility of the vMNP
To evaluate the aging process's success in the vMNP's dispersibility, 7 mg of dried PE or PP vMNP (non-aged and aged) were suspended in 200 mL of deionized water using an Ultra Turrax (for 30 seconds) and an ultrasound bath (30 minutes at 40 kHz, Branson Ultrasonics). The turbidity of the resulting dispersions was measured with a TB100 turbidimeter (Bante Instruments). Every dispersion experiment was carried out independently three times, and the final turbidity was measured in triplicate.We conducted a similar procedure using filtered wastewater as the medium for both PE and PP vMNP (non-aged and aged). This experiment aimed to evaluate the dispersion of vMNP in an actual wastewater sample.
Untreated municipal wastewater was collected from a local wastewater treatment plant (WWTP) in the City of Maldonado, Uruguay, where the treatment process involves coagulation/flocculation. To be able to measure the turbidity caused by the dispersion of the vMNP, we eliminated most of the suspended solids. The wastewater was subsequently filtered using cellulose membrane filters (0.45 μm pore size) to serve as a medium for the coagulation/flocculation tests. After filtering the wastewater the initial turbidity was 0.75 ± 0.18 NTU, and the pH was 8.10.
Coagulation/flocculation tests
Coagulation tests were conducted using PE and PP vMNP, non-aged and aged, in deionized water and filtered wastewater. Aluminum sulfate (alum, technical grade, Droguería Industrial Uruguaya) was used as both the coagulant and flocculant. To minimize variables in the tests, we did not use additional polymers to enhance coagulation.For each coagulation/flocculation test, 7 mg of non-aged or aged PE vMNP were dispersed in a 100 mL beaker containing deionized water or filtered wastewater. This dispersion was achieved using an Ultra Turrax for 30 seconds and an ultrasound bath for 30 minutes at 40 kHz (Branson Ultrasonics). The initial turbidity was standardized to an identical value for all tests by diluting the dispersions with the respective medium. Turbidity was subsequently measured using a turbidimeter (TB100, Bante Instruments).
The alum dosage, mixing velocities, and settling times were configured to replicate the typical process conditions of the wastewater treatment plant from which the wastewater sample was taken. Alum was added at a 120 mg L−1 dose and the beakers were stirred in a magnetic stirrer with multiple positions (RT 15, IKA-Werke GmbH & CO) at 250 rpm for 15 minutes (fast mixing) and 50 rpm for 1 minute (slow mixing). After the mixing stage, the content of the beakers was allowed to settle for 30 minutes, and final turbidity was measured. Each test was repeated three times, with turbidity and pH measurements taken in triplicate for each repetition. Turbidity-based removal efficiencies (ηR) were calculated as:
In a subsequent phase, additional tests were conducted to differentiate the effects of the eco-corona and ionic strength on flocculation. Non-aged PE and PP were dispersed in two media: one containing only protein and the other containing protein with adjusted ionic strength. The media compositions were as follows:
• 10 g L−1 peptone solution in deionized water
• 10 g L−1 peptone solution in 0.1 M NaCl
Peptone (Oxoid) was selected as the protein source because it is a key component in synthetic wastewater formulations.56
Statistical analyses
To evaluate potential significant differences in the vMNP dispersions in different media and in the efficiencies obtained in the dispersion and coagulation/flocculation tests, mean values of three replicates were compared using a paired t-test (α = 0.05) via InfoStat software.57Results and discussion
Production of vMNP using a bottom-up approach
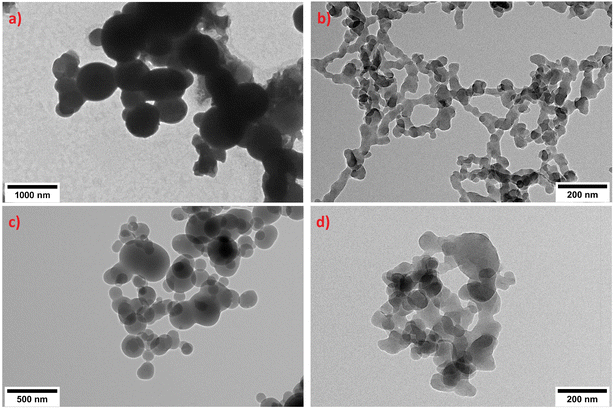
Both TEM micrographs and DLS measurements depict particles within the low micrometre and sub-micrometre ranges, exhibiting granular shape in experiments PE1 to PE4 (Fig. 1 and Material S1,† SM1). As indicated in the size distributions shown in Figure SM1, the observed particle diameters, determined via TEM analysis, ranged from a minimum of 50 nm to a maximum of 3500 nm. Statistical parameters derived from the distribution functions fitted to the data are detailed in Table 2.| Exp | PE conc. (mg mL−1) | Tween 60 conc. (%) | DLS | TEM | ||
|---|---|---|---|---|---|---|
| D mean (nm) | SD (nm) | D mean (nm) | SD (nm) | |||
| PE1 | 1.5 | 2 | 888 | 146 | 599 | 208 |
| PE2 | 1.5 | 0 | 567 | 173 | 316 | 93 |
| PE3 | 15 | 2 | 1354 | 268 | 1132 | 464 |
| PE4 | 15 | 0 | 982 | 172 | 776 | 360 |
The difference between the mean diameters obtained by TEM and DLS is attributed to the fact that DLS measures the hydrodynamic diameter, which includes the layer of surfactant molecules attached to the particle surface and the electric double layer that moves along with the particle, whereas TEM measures the actual particle diameter.
Fig. 2 shows the graph of the mean diameter obtained by TEM versus the mean diameter obtained by DLS. The graph shows a linear relationship with an approximate slope of 1 and a y-intercept of 284 nm. The slope of approximately 1 indicates that, on average, the difference between the diameters measured by both techniques is constant and does not depend on the particle size. The y-intercept (284 nm) represents the value of this constant difference, which accounts for the combined thickness of surfactant and electrical double layers. Both DLS and TEM size distributions show a tendency for the mean particle diameter to increase with both polymer and surfactant concentrations.
There are contradicting reports on the influence of these factors in the resulting particle sizes. Lebouille et al. developed a theoretical model for the precipitation of polymer nanoparticles with a non-solvent,39 and found that for fast mixing times, particle diameter is independent of the polymer concentration, but when mixing times are slow, it is proportional to polymer concentration to the 1/3 power. Others found that particle diameters decrease when polymer concentration increases within certain ranges.41 When a non-solvent is introduced into a solution, it induces supersaturation and initiates nucleation. The rate of nucleation increases under higher supersaturation conditions caused by elevated solute concentrations. This implies that more nuclei are generated, resulting in smaller particles, as the same amount of solute is distributed among a greater number of nuclei. Once the solute concentration is reduced below the critical supersaturation concentration, the formation of nuclei stops, and the formed particles start growing through the addition of solute molecules to the particle surface, and the adhesion of two or more particles. Particle growth rates depend on the diffusion speed of the solute and the collision frequency between particles. These last two factors increase at higher polymer concentrations.42 In summary, increasing polymer concentration has two opposing effects: it increases both, nucleation (favouring the formation of more and smaller particles) and growth rates (favouring the formation of larger particles). In the concentration ranges used in this study (1.5–15 mg mL−1), the increase in growth rates prevails, resulting in larger particles at higher polymer concentrations.
The effects of adding surfactant in the type of synthesis used in our work are unclear. Some authors report that adding them results in smaller particle sizes58 and attribute it to the presence of surfactant micelles compartmentalizing the system and inhibiting particle coalescence.39 Others observe the opposite and attribute the size increase to the formation of complexes with the polymer.41 In our study, syntheses with the addition of 2% Tween 60 resulted in significantly larger particles compared to experiments conducted under the same conditions but without the surfactant. One possible explanation for this observation is that the applied surfactant concentrations were too high. According to Joye and McClements, the presence of surfactants should increase the nucleation rate, primarily due to the reduction of surface tension, resulting in the formation of smaller particles. However, when surfactants are present in high concentrations, they can promote particle growth and coalescence, leading to the opposite effect.42
The findings from this stage of the research highlight the potential for adjusting the final size of vMNP by manipulating polymer and surfactant concentrations. This strategy allows for covering a wide range of sizes within the categories of very-small microplastics and nanoplastics.
Applying the same synthesis procedure under the conditions described in Table 1 for experiments PP1, PVC1, PS1 and PET1, granular-shaped vMNP were obtained in all cases (Fig. 3). Figure SM2 illustrates the size distributions obtained by TEM for each experiment, while the statistical parameters derived from the fitted log-normal distributions are shown in Table 3. DLS measurements were not conducted for these experiments because our previous work with PE demonstrated that DLS diameters are proportional to TEM diameters, with the latter providing a more accurate reflection of the actual particle size. These results demonstrate that the synthesis procedure is also applicable to other polymers, as long as the appropriate solvent and non-solvent are chosen.
| Exp | Polymer conc. (mg mL−1) | Tween 60 conc. (%) | TEM | |
|---|---|---|---|---|
| Mean diameter (nm) | SD (nm) | |||
| PP1 | 1.5 | 0 | 790 | 337 |
| PS1 | 1.5 | 0 | 231 | 136 |
| PVC1 | 1.5 | 0 | 53 | 12 |
| PET1 | 1.5 | 0 | 82 | 26 |
The considerable differences between the mean diameters obtained with the different polymers can be attributed to other factors that were not constant through all the experiments, such as the synthesis temperature, the difference in polarity between solvent and non-solvent and the chemical structure of the polymer itself.
Development of environmentally relevant vMNP: artificial surface weathering
When exposed PE and PP vMNP to UV/O3, their FTIR spectrum showed a band at approximately 1715 cm−1. This band is associated with the stretching vibration of carbonyl groups (ν-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O),47,48,50 and its intensity increased with exposure time (Fig. 4 and 5).
O),47,48,50 and its intensity increased with exposure time (Fig. 4 and 5).
In the FTIR spectrum for PE vMNP (Fig. 4), the band at 1300–850 cm−1 also showed an increase in absorbance with exposure time. This band displays three main peaks, with maxima at 1177 cm−1, 1063 cm−1, and 942 cm−1. In particular, the peak at 1177 cm−1 is associated with the stretching vibration of the C–O bond (ν-C–O) characteristic of the ester group,47,59 and it was selected for the calculation of the C–O index. The same behavior is observed in PP vMNP spectra (Fig. 5). Still, the spectrum for non-aged PP shows a peak at 1167 cm−1 (one of the characteristic peaks of isotactic PP folding in helical structures60), which interferes with the C–O peak at 1177 cm−1. To correct this interference, in calculating the C–O index in PP vMNP, the absorbance at 1177 cm−1 at t = 0 (non-aged PP) was subtracted for each exposure time.
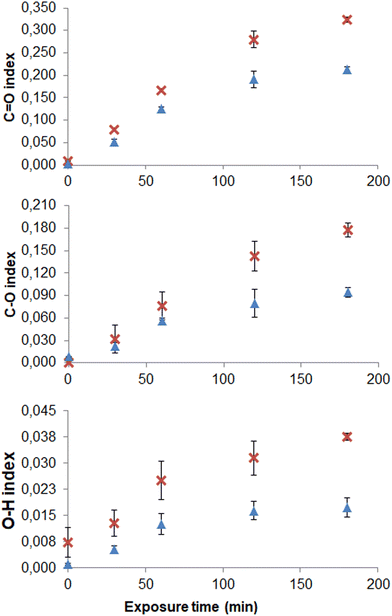
The evolution of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–H, and C–O indices with exposure time for PE and PP vMNP is presented in Fig. 6. In all cases, an increase in all three indices is observed with exposure time, tending to stabilize after approximately 180 minutes. Aging by UV/O3 results in an FTIR spectrum similar to those of naturally weathered macroplastics (Fig. 7). This aligns with findings reported in other studies.47,61
O, O–H, and C–O indices with exposure time for PE and PP vMNP is presented in Fig. 6. In all cases, an increase in all three indices is observed with exposure time, tending to stabilize after approximately 180 minutes. Aging by UV/O3 results in an FTIR spectrum similar to those of naturally weathered macroplastics (Fig. 7). This aligns with findings reported in other studies.47,61
Regarding the synthesis of vMNP from naturally weathered PE and PP macroplastics, as seen in Fig. 7 and 8, the FTIR spectra of the obtained vMNP are similar to those of non-aged vMNP. The oxidation of macroplastics occurs on the surface, forming a layer of oxidized polymer, beneath which the virgin polymer remains intact. This observation could be explained by the polymer chains forming the oxidized layer exhibiting a stronger affinity for the non-solvent, likely due to the presence of polar functional groups within them. As a result, these chains stay dissolved in the solvent/non-solvent mixture, while the inner chains lacking these polar functional groups precipitate as vMNP.
The effects of aging in the colloidal behaviour of vMNP were assessed in the dispersibility studies. The resulting turbidity of dispersing both PE and PP vMNP in deionized water (DW) and filtered wastewater (FWW) are shown in Table 4.
| Medium | Turbidity (NTU) | |||
|---|---|---|---|---|
| Non-aged vMNP | Aged vMNP (180 min) | vMNP from naturally weathered plastics | ||
| DW = deionized water, FWW = filtered wastewater, PE = polyethylene, PP = polypropylene. | ||||
| PE | DW | 9.0 (2.3)a | 53.9 (4.1)b | 8.1 (1.2)a |
| FWW | 62.2 (3.9)b | 60.5 (2.2)b | — | |
| PP | DW | 13.9 (1.9)c | 151.3 (1.7)d | 15.9 (2.6)c |
| FWW | 148.8 (10.3)d | 149.5 (7.5)d | — | |
Few particles were dispersed when both non-aged vMNP and vMNP from naturally weathered plastics were exposed to deionized water and ultrasound. As a result, the vMNP powder remained agglomerated at the water surface, leading to lower turbidity measurements. This was due to only a small portion of the particles achieving dispersion. This observation is consistent with the spectra presented in Fig. 8 and 9, which show identical peak profiles for both non-aged vMNP and vMNP from naturally weathered plastics, for both PE and PP.
 | ||
| Fig. 9 Dimensionless turbidity of a monodisperse system (extracted from Melik and Fogler62). τ·λ/φ – dimensionless turbidity, λ – turbidimeter wavelength, φ – particle concentration expressed as volumetric fraction (mass concentration/polymer density), α = 2πr/λ – dimensionless particle radius, r – particle radius, m – ratio between particle and medium refraction indexes. The estimated coordinates for our vMNP are α = 1.154 and m = 1.125 (PE), and α = 2.886 and m = 1.103 (PP). The negligible difference in ϕ between PE and PP vMNP dispersions, due to similar densities, does not account for the significant disparity in dimensionless turbidity. | ||
On the other hand, successful dispersion was achieved for aged vMNP in deionized water (DW), and for both non-aged and aged vMNP in filtered wastewater (FWW). Visually, the dispersions appeared homogeneous, with no agglomerated particles visible to the naked eye. The resulting turbidities for these three dispersions of the same polymer were not statistically different (see means with the same letter in Table 4).
The higher turbidity observed when dispersing aged vMNP in DW compared to non-aged vMNP suggest that UV/O3 aging enhances the affinity of the vMNP for the aqueous phase. When dry non-aged vMNP, consisting for both PE and PP on –C–H– chains, are brought into contact with deionized water, they fail to form polar interactions with water molecules, which are crucial for breaking the Van der Waals interactions holding the particle agglomerates together.63 Consequently, only a small amount of the particles disperses, leading to a lower turbidity.
On the other hand, aged vMNP develop polar groups on their surface (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –O–H, and –C–O–), enabling the formation of hydrogen bonds and polar Van der Waals interactions between water and polymer molecules on the vMNP surface. These interactions aid in disrupting the forces between the particles when they come into contact with water, enabling the particles to disperse in water without the need for surfactants.
O, –O–H, and –C–O–), enabling the formation of hydrogen bonds and polar Van der Waals interactions between water and polymer molecules on the vMNP surface. These interactions aid in disrupting the forces between the particles when they come into contact with water, enabling the particles to disperse in water without the need for surfactants.
Our studies revealed that higher turbidities were obtained for PP vMNP compared to PE vMNP. Given that the mass concentration is constant for both polymers and that PP vMNP particles are larger (790 nm mean diameter for PP vs. 316 nm for PE), the particle concentration (number of particles per liter) is expected to be higher for PE vMNP than for PP vMNP, which would typically suggest lower turbidity for PP. However, Melik and Fogler62 conducted studies on turbidity at the nanoscale and found that while turbidity generally decreases with particle size, within a certain range, the trend reverses, and an increase in turbidity is observed as particle size increases. This behavior is depicted in Fig. 9, where the turbidity (τ) of a monodisperse system is theoretically modeled. Considering the particle diameters shown in Tables 2 and 3 and using a turbidimeter wavelength of λ = 860 nm, and refractive indices of 1.50 for PE, 1.47 for PP and 1.33 for water, the corresponding points for estimating dimensionless turbidities for our PE and PP vMNP dispersions are marked in Fig. 9 in red and green, respectively. These findings confirm that significantly higher turbidities are indeed expected for PP vMNP dispersions.
An important characteristic that likely significantly affects colloidal behavior is surface morphology (roughness). Veclin et al. found that surface morphology influences the zeta potential of nanoplastics and consequently, their colloidal behavior. However, the same study concluded that surface morphology is not the determining factor in homoaggregation, with particle shape having the greatest influence. Spherical particles tend to be more stable in high ionic strength media, while anisotropic particles—more representative of MNP found in environmental samples—are prone to aggregate more easily.64 Furthermore, the study does not account for the influence of the eco-corona, which is likely to form around vMNP in environmental conditions. The presence of this organic coating could mask the particle's surface roughness, diminishing its effect on colloidal behavior. This masking could play a significant role in modifying how anisotropic particles aggregate and disperse in real-world environments.
The vMNP synthesized in our study exhibit anisotropic characteristics, which can complicate their dispersion in water, particularly in the absence of organic matter. A more detailed study on the effects of different shapes and surface morphologies on dispersibility would be of great interest, though it exceeds the scope of this current work.
Production of environmentally relevant vMNP: eco-corona
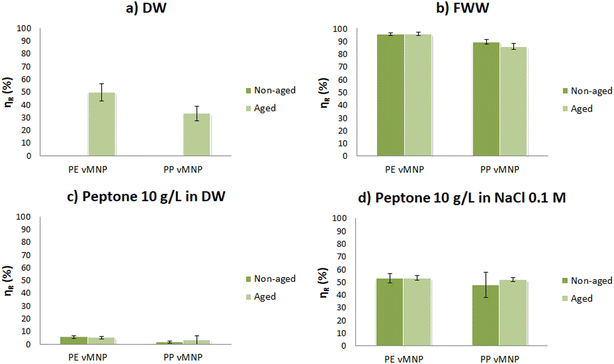
The results from the dispersibility assays show that both aged and non-aged vMNP fully disperse in a medium containing organic matter (FWW). However, in a medium without organic matter (DW), only aged vMNP achieve full dispersion. Additionally, we found no significant difference in turbidity between non-aged and aged vMNP dispersions in FWW (see Table 4). These findings provide the first evidence that particle surface chemistry may not be the primary factor influencing the colloidal behavior of vMNP in this medium. A possible explanation is the formation of an eco-corona, a layer of macromolecules that adhere to the particle surface. The eco-corona might function similarly to a surfactant, obscuring the particle surface, and the polar groups within these macromolecules could enhance the interaction between the particles and the surrounding water molecules.Coagulation and flocculation tests provide further insight into this subject. The results of these tests are detailed in Table SM2† and illustrated in Fig. 10a and b. Tests conducted with non-aged vMNP in DW were excluded from the results, as dispersion was minimal and the target initial turbidity (50–60 NTU) could not be reached. We observed that, regardless of the polymer and aging, using filtered FWW as the medium enhances agglomeration, and higher removal efficiencies are achieved. Within each polymer, the efficiencies obtained in the FWW tests were not statistically different for both aged and non-aged vMNP.
Agglomeration also occurs when aged vMNP are used with DW as the medium, following the addition of the coagulant (see Fig. 10a). However, in this case, the flocs do not group together and settle. Instead, vMNP form larger agglomerates that remain suspended and are visible to the naked eye. The removal efficiencies observed in these tests are significantly lower compared to those achieved with the same polymer and aging, but using FWW as the medium.
These observations could be explained by two hypotheses:
1) An eco-corona was formed, in both cases (aged and non-aged vMNP) when using FWW as the medium. As the particle is covered by the eco-corona, its surface chemical composition would not affect the interactions with water molecules.
2) The higher ionic strength in FWW in comparison to DW. Wastewaters contain dissolved salts, which increase the ionic strength of the solution. Ionic strength affects the repulsion distance between charged particles by compressing the electrical double layer surrounding each particle. Increasing ionic strength decreases the Debye length—the distance over which electrostatic interactions are significant—thereby reducing the interaction distance between particles and promoting agglomeration.65 The higher ionic strength of FWW, combined with the addition of a coagulant (which also increases ionic strength), could explain the increased agglomeration observed in FWW during coagulation/flocculation tests, leading to higher removal efficiencies.
However, the ionic strength hypothesis seems to contradict the results of our dispersibility assays, which consistently showed greater stability of dispersions in a medium with higher ionic strength (FWW). To address this discrepancy, we conducted additional coagulation/flocculation tests using non-aged and aged PE and PP vMNP dispersed in a medium containing only protein (10 g L−1 peptone in DW), as well as the same medium with adjusted ionic strength (10 g L−1 peptone in 0.1 M NaCl). The results, shown in Table SM2† and Fig. 10c and d, suggest that both eco-corona formation and ionic strength effects are at play.
Both non-aged PE and PP were successfully dispersed in both media, supporting the eco-corona hypothesis. The successful dispersion of vMNP from both polymers in the peptone in DW medium and FWW, but not in DW alone, suggests that peptone, along with proteins and other macromolecules present in FWW, likely attaches to the vMNP surface enabling the particles to remain suspended.
Another observation that suggests the formation of an eco-corona is the lack of significant differences in removal efficiencies when organic matter was present (FWW, peptone in DW, and peptone in 0.1 M NaCl), regardless of the aging state of the vMNP surface (see Table SM2†). This is consistent with findings by Farraj et al., who observed that for PS nanoplastics incubated in synthetic wastewater, removal efficiency during coagulation/flocculation correlated with the removal of total suspended solids, irrespective of the nanoplastics' size, concentration, or surface chemistry. This was attributed to the eco-corona, which masked the nanoplastic surface due to dissolved organic substances in the wastewater matrix.66,67
The ionic strength hypothesis is supported by the higher removal efficiencies observed in experiments using peptone in 0.1 M NaCl compared to peptone in DW. This suggests that increased initial ionic strength promotes flocculation, aids particle sedimentation, and results in better removal efficiencies. To further confirm this, we tested flocculation at higher alum doses in the peptone in DW experiments. Initially, almost no turbidity removal was observed, but as we increased the alum dose to 360 mg L−1, flocculation occurred (see Table SM2†). This confirms that the initial ionic strength of the medium has an important influence on the coagulant dose required to achieve effective flocculation.
In summary, both the eco-corona and ionic strength hypotheses appear to be correct, with their effects being oppositional: the eco-corona facilitates dispersion, while increased ionic strength promotes agglomeration. In the tested media, prior to the addition of the coagulant, the stabilization provided by the eco-corona likely outweighs the agglomeration tendency caused by ionic strength, allowing the particles to remain suspended. When alum is added, the ionic strength increases significantly due to the high charge of the Al3+ cations. It is hypothesized that in media with existing ionic strength (like FWW and peptone in 0.1 M NaCl medium), this further increase destabilizes the dispersion, leading to flocculation. In contrast, when no initial ionic strength is present (as in the peptone in DW medium), the ionic strength from alum is insufficient to cause destabilization, preventing flocculation, as suggested by the limited removal efficiency in these experiments.
The effect of organic matter on vMNP removal efficiencies in coagulation/flocculation processes has been explored in a limited number of studies. It has been observed that incubating PE and PS microplastics (45–53 μm) in real wastewater, where an initial ionic strength is present, enhances removal efficiency during coagulation/flocculation.68 Conversely, when PS nanoplastics are treated in deionized water with added humic acid (but without initial ionic strength), removal efficiency has been found to decrease. This reduction is likely due to steric stabilization and electrostatic repulsion caused by the adsorption of humic acid onto the nanoplastic surface.69 These findings are in line with our observations, suggesting that both ionic strength and the nature of organic matter play a critical role in determining the effectiveness of MNP removal during coagulation/flocculation.
Conclusions
This study developed a cost-effective procedure for synthesizing vMNP from five major polymers (PE, PP, PVC, PS, and PET) using a non-solvent precipitation method. The obtained vMNP exhibit granular shapes and sizes within the low micrometric and nanometric ranges, with controllable particle size based on polymer and surfactant concentrations during synthesis. Aging the vMNP with UV/O3 exposure modifies their surface chemistry, introducing polar functional groups that enable suspension in water, which is critical for wastewater removal studies.Evidence suggests the formation of an eco-corona in the presence of organic matter, which masks the surface chemistry of the particles. This masking effect enables dispersion when the vMNP surface is not oxidized and equalizes coagulation/flocculation performance, regardless of whether the surface is aged or not.
The results also emphasize the importance of ionic strength in coagulation/flocculation processes. The significantly lower removal efficiencies observed in deionized water (compared to filtered wastewater), and in peptone in deionized water (compared with peptone in 0.1 M NaCl), highlight the necessity of initial ionic strength for achieving effective flocculation at coagulant doses similar to those used in wastewater treatment plants. These findings suggest that tests performed in deionized or low-strength media may underestimate vMNP removal efficiency or require higher non-representative coagulant doses.
This study highlights the necessity of considering both particle synthesis and medium composition when developing reference materials for wastewater treatment tests. Media that mimic environmental conditions, such as filtered wastewater or synthetic wastewater, are expected to provide more accurate results. Additionally, the synthesis procedure's applicability to other polymers like PS, PVC, and PET, and further exploration of aging and dispersibility, will be addressed in future research.
Overall, the feasibility of producing environmentally relevant PE and PP vMNP has been demonstrated, showing promise as reference materials for wastewater treatment, especially in coagulation/flocculation assays. The suitability of these materials as reference standards will be confirmed after evaluating their removal efficiencies under real wastewater treatment plant conditions in the later stages of the research.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Martín Benzo: methodology, investigation, project administration, funding acquisition, writing-original draft. María Eugenia Pérez-Barthaburu: methodology, writing-review & editing, supervision. Andrés Pérez-Parada: methodology, writing-review & editing, supervision. Álvaro Olivera: Investigation (obtention of TEM images). Laura Fornaro: conceptualization, resources, writing-review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding for this research was provided by Agencia Nacional de Investigación e Innovación (FMV_3_2022_1_172436), Comisión Académica de Posgrados at Universidad de la República, Uruguay and Programa de Desarrollo de Ciencias Básicas (PEDECIBA). We extend our gratitude to Ph.D. Eduardo Méndez from Facultad de Ciencias at Universidad de la República for his collaboration in conducting the DLS measurements. Additionally, we appreciate the collaboration of M.Sc. David Berger from CIEMSA and Eng. Fabián Gómez from OSE for their assistance in the wastewater sampling process.References
- L. Wang, W.-M. Wu, N. S. Bolan, D. C. W. Tsang, Y. Li and M. Qin, et al., Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives, J. Hazard. Mater., 2021, 401, 123415 CrossRef CAS PubMed.
- D. Eerkes-Medrano, R. C. Thompson and D. C. Aldridge, Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs, Water Res., 2015, 75, 63–82 CrossRef CAS PubMed.
- H. Cai, E. G. Xu, F. Du, R. Li, J. Liu and H. Shi, Analysis of environmental nanoplastics: Progress and challenges, Chem. Eng. J., 2021, 410, 128208 CrossRef CAS.
- W. Fu, J. Min, W. Jiang, Y. Li and W. Zhang, Separation, characterization and identification of microplastics and nanoplastics in the environment, Sci. Total Environ., 2020, 721, 137561 CrossRef CAS PubMed.
- J. Wang, X. Zhao, F. Wu, L. Niu, Z. Tang and W. Liang, et al., Characterization, occurrence, environmental behaviors, and risks of nanoplastics in the aquatic environment: Current status and future perspectives, Fundam. Res., 2021, 1(3), 317–328 CrossRef CAS.
- A. Ter Halle, L. Jeanneau, M. Martignac, E. Jardé, B. Pedrono and L. Brach, et al., Nanoplastic in the North Atlantic Subtropical Gyre, Environ. Sci. Technol., 2017, 51(23), 13689–13697 CrossRef CAS PubMed.
- S. Abnumani and P. Kakkar, Ecotoxicological effects of microplastics on biota: a review, Environ. Sci. Pollut. Res., 2018, 25, 14373–14396 CrossRef PubMed.
- B. Jovanović, Ingestion of microplastics by fish and its potential consequences from a physical perspective, Integr. Environ. Assess. Manage., 2017, 13(3), 510–515 CrossRef.
- C. Barría, I. Brandts, L. Tort, M. Oliveira and M. Teles, Effect of nanoplastics on fish health and performance: A review, Mar. Pollut. Bull., 2020, 151, 110791 CrossRef.
- C. Yong, S. Valiyaveettil and B. Tang, Toxicity of Microplastics and Nanoplastics in Mammalian Systems, Int. J. Environ. Res. Public Health, 2020, 17(5), 1509 CrossRef CAS.
- A. Banerjee and W. L. Shelver, Micro- and nanoplastic induced cellular toxicity in mammals: A review, Sci. Total Environ., 2021, 755, 142518 CrossRef CAS.
- K. Blackburn and D. Green, The potential effects of microplastics on human health: What is known and what is unknown, Ambio, 2022, 51(3), 518–530 CrossRef PubMed.
- S. Gangadoo, S. Owen, P. Rajapaksha, K. Plaisted, S. Cheeseman and H. Haddara, et al., Nano-plastics and their analytical characterisation and fate in the marine environment: From source to sea, Sci. Total Environ., 2020, 732, 138792 CrossRef CAS PubMed.
- M. Smith, D. C. Love, C. M. Rochman and R. A. Neff, Microplastics in Seafood and the Implications for Human Health, Curr. Environ. Health Rep., 2018, 5(3), 375–386 CrossRef CAS.
- D. W. Skaf, V. L. Punzi, J. T. Rolle and K. A. Kleinberg, Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation, Chem. Eng. J., 2020, 386, 123807 CrossRef CAS.
- ISO 24187:2023, Principles for the analysis of microplastics present in the environment, Geneva, Switzerland, 2023.
- Z. Huang and H. Wang, Study on the impact of photoaging on the generation of very small microplastics (MPs) and nanoplastics (NPs) and the wettability of plastic surface, Environ. Sci. Pollut. Res., 2023, 30(40), 92963–92982 CrossRef CAS PubMed.
- C. Schwaferts, P. Schwaferts, E. von der Esch, M. Elsner and N. P. Ivleva, Which particles to select, and if yes, how many?, Anal. Bioanal. Chem., 2021, 413(14), 3625–3641 CrossRef CAS PubMed.
- W. Hu, R. Tang, S. Yuan, M. Gong, P. Shi and W. Wang, et al., Modification of fluorescence staining method for small-sized microplastic quantification: Focus on the interference exclusion and exposure time optimization, Environ. Sci. Pollut. Res., 2023, 30(19), 56330–56342 CrossRef CAS.
- J. R. Peller, S. P. Mezyk, S. Shidler, J. Castleman, S. Kaiser and R. F. Faulkner, et al., Facile nanoplastics formation from macro and microplastics in aqueous media, Environ. Pollut., 2022, 313, 120171 CrossRef CAS.
- J. Gigault, A. Halle, M. Baudrimont, P.-Y. Pascal, F. Gauffre and T.-L. Phi, et al., Current opinion: What is a nanoplastic?, Environ. Pollut., 2018, 235, 1030–1034 CrossRef CAS.
- C. Schwaferts, R. Niessner, M. Elsner and N. P. Ivleva, Methods for the analysis of submicrometer- and nanoplastic particles in the environment, TrAC, Trends Anal. Chem., 2019, 112, 52–65 CrossRef CAS.
- I. Ali, T. Ding, C. Peng, I. Naz, H. Sun and J. Li, et al., Micro- and nanoplastics in wastewater treatment plants: Occurrence, removal, fate, impacts and remediation technologies – A critical review, Chem. Eng. J., 2021, 423, 130205 CrossRef CAS.
- L. Tian, E. Skoczynska, R.-J. van Putten, H. A. Leslie and G.-J. M. Gruter, Quantification of polyethylene terephthalate micro- and nanoplastics in domestic wastewater using a simple three-step method, Sci. Total Environ., 2023, 857, 159209 CrossRef CAS.
- Y. Xu, Q. Ou, X. Wang, F. Hou, P. Li and J. P. van der Hoek, et al., Assessing the Mass Concentration of Microplastics and Nanoplastics in Wastewater Treatment Plants by Pyrolysis Gas Chromatography-Mass Spectrometry, Environ. Sci. Technol., 2023, 57(8), 3114–3123 CrossRef CAS.
- S. Mariano, S. Tacconi, M. Fidaleo, M. Rossi and L. Dini, Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques, Front. Toxicol., 2021, 3, 1–17 Search PubMed.
- B. Nguyen, D. Claveau-Mallet, L. M. Hernandez, E. G. Xu, J. M. Farner and N. Tufenkji, Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples, Acc. Chem. Res., 2019, 52(4), 858–866 CrossRef CAS.
- N. N. Phuong, A. Zalouk-Vergnoux, L. Poirier, A. Kamari, A. Châtel and C. Mouneyrac, et al., Is there any consistency between the microplastics found in the field and those used in laboratory experiments?, Environ. Pollut., 2016, 211, 111–123 CrossRef CAS PubMed.
- G. Balakrishnan, M. Déniel, T. Nicolai, C. Chassenieux and F. Lagarde, Towards more realistic reference microplastics and nanoplastics: preparation of polyethylene micro/nanoparticles with a biosurfactant, Environ. Sci.: Nano, 2019, 6(1), 315–324 RSC.
- K. Enders, R. Lenz, C. A. Stedmon and T. G. Nielsen, Abundance, size and polymer composition of marine microplastics ≥10 μm in the Atlantic Ocean and their modelled vertical distribution, Mar. Pollut. Bull., 2015, 100(1), 70–81 CrossRef CAS PubMed.
- D. Magrì, P. Sánchez-Moreno, G. Caputo, F. Gatto, M. Veronesi and G. Bardi, et al., Laser ablation as a versatile tool to mimic polyethylene terephthalate nanoplastic pollutants: Characterization and toxicology assessment, ACS Nano, 2018, 12(8), 7690–7700 CrossRef.
- L. Eitzen, S. Paul, U. Braun, K. Altmann, M. Jekel and A. S. Ruhl, The challenge in preparing particle suspensions for aquatic microplastic research, Environ. Res., 2019, 168, 490–495 CrossRef CAS PubMed.
- J. Seghers, E. A. Stefaniak, R. La Spina, C. Cella, D. Mehn and D. Gilliland, et al., Preparation of a reference material for microplastics in water—evaluation of homogeneity, Anal. Bioanal. Chem., 2022, 414(1), 385–397 CrossRef CAS.
- E. von der Esch, M. Lanzinger, A. J. Kohles, C. Schwaferts, J. Weisser and T. Hofmann, et al., Simple Generation of Suspensible Secondary Microplastic Reference Particles via Ultrasound Treatment, Front. Chem., 2020, 8, 1–15 CrossRef.
- F. M. Bauers, R. Thomann and S. Mecking, Submicron polyethylene particles from catalytic emulsion polymerization, J. Am. Chem. Soc., 2003, 125(29), 8838–8840 CrossRef CAS.
- G. Billuart, E. Bourgeat-Lami, M. Lansalot and V. Monteil, Free Radical Emulsion Polymerization of Ethylene, Macromolecules, 2014, 47(19), 6591–6600 CrossRef CAS.
- B. Korthals, I. Göttker-Schnetmann and S. Mecking, Nickel(II)−Methyl Complexes with Water-Soluble Ligands L [(salicylaldiminato-κ 2 N , O )NiMe(L)] and Their Catalytic Properties in Disperse Aqueous Systems, Organometallics, 2007, 26(6), 1311–1316 CrossRef CAS.
- S. Hornig, T. Heinze, C. R. Becer and U. S. Schubert, Synthetic polymeric nanoparticles by nanoprecipitation, J. Mater. Chem., 2009, 19(23), 3838 RSC.
- J. G. J. L. Lebouille, R. Stepanyan, J. J. M. Slot, M. A. Cohen Stuart and R. Tuinier, Nanoprecipitation of polymers in a bad solvent, Colloids Surf., A, 2014, 460, 225–235 CrossRef CAS.
- T. Pulingam, P. Foroozandeh, J.-A. Chuah and K. Sudesh, Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles, Nanomaterials, 2022, 12(3), 576 CrossRef CAS PubMed.
- H. Saari, C. Fuentes, M. Sjöö, M. Rayner and M. Wahlgren, Production of starch nanoparticles by dissolution and non-solvent precipitation for use in food-grade Pickering emulsions, Carbohydr. Polym., 2017, 157, 558–566 CrossRef CAS.
- I. J. Joye and D. J. McClements, Production of nanoparticles by anti-solvent precipitation for use in food systems, Trends Food Sci. Technol., 2013, 34(2), 109–123 CrossRef CAS.
- A. G. Rodríguez-Hernández, J. A. Muñoz-Tabares, J. C. Aguilar-Guzmán and R. Vazquez-Duhalt, A novel and simple method for polyethylene terephthalate (PET) nanoparticle production, Environ. Sci.: Nano, 2019, 6(7), 2031–2036 RSC.
- K. Tanaka, Y. Takahashi, H. Kuramochi, M. Osako, S. Tanaka and G. Suzuki, Preparation of Nanoscale Particles of Five Major Polymers as Potential Standards for the Study of Nanoplastics, Small, 2021, 17(49), 2105781 CrossRef CAS.
- Y. Zhang, A. Diehl, A. Lewandowski, K. Gopalakrishnan and T. Baker, Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment, Sci. Total Environ., 2020, 720, 137383 CrossRef CAS.
- G. Liu, Z. Zhu, Y. Yang, Y. Sun, F. Yu and J. Ma, Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater, Environ. Pollut., 2019, 246, 26–33 CrossRef CAS.
- C. Sorasan, C. Edo, M. González-Pleiter, F. Fernández-Piñas, F. Leganés and A. Rodríguez, et al., Generation of nanoplastics during the photoageing of low-density polyethylene, Environ. Pollut., 2021, 289, 117919 CrossRef CAS PubMed.
- J. Liu, T. Zhang, L. Tian, X. Liu, Z. Qi and Y. Ma, et al., Aging Significantly Affects Mobility and Contaminant-Mobilizing Ability of Nanoplastics in Saturated Loamy Sand, Environ. Sci. Technol., 2019, 53(10), 5805–5815 CrossRef CAS PubMed.
- P. Liu, L. Qian, H. Wang, X. Zhan, K. Lu and C. Gu, et al., New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes, Environ. Sci. Technol., 2019, 53(7), 3579–3588 CrossRef CAS PubMed.
- R. Zafar, S. Y. Park and C. G. Kim, Surface modification of polyethylene microplastic particles during the aqueous-phase ozonation process, Environ. Eng. Res., 2020, 26(5), 200412 CrossRef.
- N. K. Shammas, Coagulation and Flocculation Physicochemical Treatment Processes, Physicochem Treat Process, 2005, vol. 3, pp. 103–139 Search PubMed.
- Plastics Europe, Plastics – the Facts 2022 Plastic Europe, 2022 Search PubMed.
- E. D. Okoffo and K. V. Thomas, Mass quantification of nanoplastics at wastewater treatment plants by pyrolysis–gas chromatography–mass spectrometry, Water Res., 2024, 254(1), 121397 CrossRef CAS PubMed.
- J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair and T. Pietzsch, et al., Fiji: an open-source platform for biological-image analysis, Nat. Methods, 2012, 9(7), 676–682 CrossRef CAS.
- R. Keuchkerian, L. Suescun, C. Crisci, I. Aguiar, W. Martínez-López and M. E. Pérez Barthaburu, et al., Optimization of solvothermal experimental parameters to control the size of KMgF3 nanoparticles for photodynamic therapy, Appl. Nanosci., 2023, 13(9), 6271–6279 CrossRef CAS.
- OCED, Simulation test - aerobic sewage treatment 303A, OCED Publ Paris, 2001, pp. 1–4 Search PubMed.
- J. Di Rienzo, F. Casanoves, M. Balzarini, L. Gonzalez, M. Tablada and C. Robledo, InfoStat versión 2011, Grup InfoStat, FCA, Univ Nac Córdoba, Argentina, 2011, http://www.infostat.com.ar Search PubMed.
- S. Banerjee, R. Premchandran, M. Tata, V. T. John, G. L. McPherson and J. Akkara, et al., Polymer Precipitation Using a Micellar Nonsolvent: The Role of Surfactant−Polymer Interactions and the Development of a Microencapsulation Technique, Ind. Eng. Chem. Res., 1996, 35(9), 3100–3107 CrossRef CAS.
- M. Hamzah, M. Khenfouch, A. Rjeb, S. Sayouri, D. S. Houssaini and M. Darhouri, et al., Surface chemistry changes and microstructure evaluation of low density nanocluster polyethylene under natural weathering: A spectroscopic investigation, J. Phys.: Conf. Ser., 2018, 984(1), 012010 CrossRef.
- J. Kang, J. Chen, Y. Cao and H. Li, Effects of ultrasound on the conformation and crystallization behavior of isotactic polypropylene and β-isotactic polypropylene, Polymer, 2010, 51(1), 249–256 CrossRef CAS.
- M. N. Miranda, M. J. Sampaio, P. B. Tavares, A. M. T. Silva and M. F. R. Pereira, Aging assessment of microplastics (LDPE, PET and uPVC) under urban environment stressors, Sci. Total Environ., 2021, 796, 148914 CrossRef CAS PubMed.
- D. Melik and H. Fogler, Turbidimetric determination of particle size distributions of colloidal systems, J. Colloid Interface Sci., 1983, 92(1), 161–180 CrossRef CAS.
- V. P. Zhdanov, Nanoparticles without and with protein corona: van der Waals and hydration interaction, J. Biol. Phys., 2019, 45(3), 307–316 CrossRef CAS PubMed.
- C. Veclin, C. Desmet, A. Pradel, A. Valsesia, J. Ponti and H. El Hadri, et al., Effect of the Surface Hydrophobicity–Morphology–Functionality of Nanoplastics on Their Homoaggregation in Seawater, ACS ES&T Water, 2022, 2(1), 88–95 Search PubMed.
- C. Burns, W. Spendel, S. Puckett and G. Pacey, Solution ionic strength effect on gold nanoparticle solution color transition, Talanta, 2006, 69(4), 873–876 CrossRef CAS PubMed.
- S. Abi Farraj, M. Lapointe, R. S. Kurusu, Z. Liu, B. Barbeau and N. Tufenkji, Targeting nanoplastic and microplastic removal in treated wastewater with a simple indicator, Nat. Water., 2024, 2(1), 72–83 CrossRef.
- K. E. Wheeler, A. J. Chetwynd, K. M. Fahy, B. S. Hong, J. A. Tochihuitl and L. A. Foster, et al., Environmental dimensions of the protein corona, Nat. Nanotechnol., 2021, 16(6), 617–629 CrossRef CAS.
- Y. Zhang, A. Diehl, A. Lewandowski, K. Gopalakrishnan and T. Baker, Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment, Sci. Total Environ., 2020, 720, 137383 CrossRef CAS.
- Y. Zhang, X. Wang, Y. Li, H. Wang, Y. Shi and Y. Li, et al., Improving nanoplastic removal by coagulation: Impact mechanism of particle size and water chemical conditions, J. Hazard. Mater., 2022, 425, 127962 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00250d |
| This journal is © The Royal Society of Chemistry 2025 |