 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
PFAS drinking water treatment trade-offs: comparing the health burden of GAC treatment to the health benefits of reduced PFAS exposure†
Sanne J.
Smith
 *a,
Émile
Sylvestre
a,
Anne Marieke
Motelica-Wagenaar
bc,
Beatrice
Cantoni
d,
Parvathi Suresh
Nair
a and
Mar
Palmeros Parada
a
*a,
Émile
Sylvestre
a,
Anne Marieke
Motelica-Wagenaar
bc,
Beatrice
Cantoni
d,
Parvathi Suresh
Nair
a and
Mar
Palmeros Parada
a
aDelft University of Technology, Department of Water Management, Stevinweg 1, 2628 CN, Delft, The Netherlands. E-mail: s.j.smith@tudelft.nl
bWaternet, Korte Ouderkerkerdijk 7, 1096 AC, Amsterdam, The Netherlands
cRadboud University, Department of Ecology, Radboud Institute for Biological and Environmental Sciences, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands
dPolitecnico Milano, Department of Civil and Environmental Engineering (DICA) – Environmental Section, Piazza Leonardo da Vinci 32, 20133 Milano, Italy
First published on 28th April 2025
Abstract
To protect human health, limits for the concentrations of per- and polyfluoroalkyl substances (PFAS) in drinking water are decreasing in many countries. However, the required treatment to achieve these lower concentrations is more resource and energy intensive than conventional drinking water treatment. Consequently, this intensified water treatment has an indirect negative impact on human health. For example, treatment with granular activated carbon (GAC), commonly used for PFAS removal, can lead to particulate matter emissions and additional global warming. These negative impacts partly off-set the health benefit achieved by lower PFAS exposure via drinking water. In this study, we quantified health impacts of both the increased treatment and the reduced PFAS exposure in disability-adjusted life years (DALYs), to assess whether PFAS removal from drinking water to specified targets with GAC results in a net health benefit. We selected the prospective Dutch drinking water guideline for PFAS of 4.4 ng PFOA-equivalent (PEQ) L−1, as this guideline is amongst the more conservative concentration targets globally. We first conducted a Life Cycle Assessment (LCA) to quantify the health cost associated with the increased reactivation frequency of an existing GAC system in the Netherlands, required to achieve PFAS concentrations below 4.4 ng PEQ L−1. Then, we quantified the health benefit obtained by the corresponding lower PFAS exposure, using pharmacokinetic modelling combined with published dose–response relationships. For the treatment plant investigated in the current study, which uses reactivated wood-based GAC, increasing the reactivation frequency to remove more PFAS was found to result in a net health benefit of 6.9–300 DALYs per 106 persons per year. However, when single-use rather than reactivated GAC would be used for PFAS treatment, the health losses from the GAC production were in the same range as the health benefits from lower PFAS exposure. Overall, the negative health impacts associated with more intensive water treatment should be considered when developing strategies to reduce PFAS exposure.
Environmental significanceTo protect human health, advised concentration limits of per- and polyfluoroalkyl substances (PFAS) in drinking water have become stricter over time. Granular activated carbon (GAC) filtration is a widely implemented water treatment technology, but its effectiveness for PFAS removal depends on the regeneration or replacement frequency. More frequent GAC replacement reduces PFAS concentrations in drinking water, but also incurs human health trade-offs through increased particulate matter emissions and climate-related risks. In this study, we quantified these trade-offs, comparing the benefits of lower PFAS exposure to the negative impacts of the intensified treatment. Our findings provide critical insight for balancing PFAS removal with sustainable treatment, informing regulatory decisions to optimally benefit human health. |
Introduction
Per- and polyfluoroalkyl substances (PFAS) have become ubiquitous in the environment, after decades of widespread use in industry and consumer products.1 PFAS are a large group of organic compounds that contain at least one perfluoroalkyl moiety in their molecular structure.2 Most research on PFAS has focused on a relatively small number of water soluble compounds, the perfluoroalkyl(ether) acids and their precursors.1 These PFAS are highly persistent, or break down to persistent degradation products that are often still PFAS.3 In addition to this persistency, certain PFAS have a high bioaccumulation potential and have been linked to a range of adverse health effects, including immunotoxic effects, kidney cancer and hypothyroidism.4,5The association between PFAS and these health effects has led to the global introduction of limits on their concentration in drinking water. The European drinking water directive currently defines a maximum concentration of the sum of 20 PFAS at 100 ng L−1, and a ‘PFAS total’ limit of 500 ng L−1.6 The UK applies the same guideline of 100 ng L−1, but for the sum of 47 PFAS.7 In Australia, health-based guideline concentrations ranging from 4 to 1000 ng L−1 for four individual PFAS are currently under public consultation, and expected to be finalized in April 2025.8 Canada recently established a concentration objective of 30 ng L−1 for the sum of 25 PFAS.9 In April 2024, the US EPA published maximum contaminant levels for five individual PFAS, ranging from 4 to 10 ng L−1.10 To include mixture effects, four PFAS are also regulated via a hazard index, with health-based limit values between 10 and 2000 ng L−1.
As more information about the health effects of PFAS became available, guidelines for maximum PFAS exposure were lowered over time. For example, the European Food Safety Authority (EFSA) defined a tolerable weekly intake (TWI) of 4.4 ng per kg bodyweight per week for the sum of four PFAS: PFOA, PFOS, PFNA and PFHxS (for full names, see ESI Table 1†).11 Together, these PFAS will hereafter be referred to as ‘∑EFSA4’. This TWI was derived from the association between high serum ∑EFSA4 concentrations and a lower antibody response to vaccination against diphtheria in breastfed one-year-olds.11,12 The value was set to ensure that breastfeeding mothers have sufficiently low PFAS serum concentrations (<6.9 μg L−1) to prevent exceedance of the ‘safe’ PFAS concentration in their children's serum at age 1 (<17.5 μg L−1). Translating this TWI to drinking water guidelines would result in much lower concentration limits than the currently applied 100 ng L−1 for the sum of 20 PFAS. For this reason, some European countries are already introducing more stringent guidelines.
The Netherlands is one such country that introduced PFAS concentration guidelines for drinking water based on the EFSA-TWI, and these PFAS limits will be used as a case study throughout this paper. The National Institute for Public Health and the Environment (RIVM) has translated the EFSA-TWI to drinking water concentrations (CDW), based on an assumed drinking water consumption of 2 L day−1, bodyweight of 70 kg and drinking water contribution of 20% to the total PFAS exposure.13 Where EFSA assumed equipotency in their TWI derivation,11 the RIVM uses relative potency factors (RPFs) to translate concentrations of 23 individual PFAS into ‘PFOA-equivalent’ concentrations (PEQ) and thereby evaluate mixture toxicity.14,15 These RPFs were determined based on benchmark doses for liver toxicity in rats, and range from 0.001 (PFBS) to 10 (PFNA, PFDA).14 Altogether, this has resulted in an advised maximum CDW of 4.4 ng PEQ L−1, which may become legally enforceable in the future.13 RPFs of the 23 PFAS included in this guideline are given in ESI Table 1,† with an example calculation.
Drinking water companies have started preparing their infrastructure to meet this more stringent PFAS guideline. Adsorption onto activated carbon, anion exchange treatment and membrane-based processes, specifically nanofiltration and reverse osmosis, are the treatment technologies for the removal of PFAS from drinking water that have been most demonstrated at relevant scales.16 Of these, adsorption to activated carbon is most used in practice, but reactivation frequencies may need to be increased to meet lowered guideline concentrations. All these technologies are significantly more resource-intensive than conventional drinking water treatments, and are thus also expected to indirectly impact human health via, for example, their global warming potential and fine particulate matter formation. Currently, these impacts are not considered in the cost-benefit analysis of removing PFAS from drinking water.
PFAS have been shown to contribute considerably to disease burden and disability,17–19 so public health and policy interventions are clearly necessary to limit exposure to a tolerable level. However, to adequately quantify the health benefits of intensified drinking water treatment, the indirect health effects associated with the implementation of treatment technologies should be considered as well. Therefore, estimating both types of human health impacts is essential, i.e. the health gained by reduced PFAS exposure via drinking water and the health lost due to the drinking water treatment technologies. By expressing both impacts in disability-adjusted life years (DALYs) and comparing them, we can estimate if installing or upgrading these treatment technologies achieves a net health benefit. DALYs are used by the World Health Organization (WHO) to quantify the overall burden of disease associated with different water-related hazards, where one DALY represents the loss of the equivalent of one year of full health.20 DALYs account for both the severity and the duration of adverse endpoints.
The specific objective of the study was to develop a methodology enabling (i) quantification of the human health ‘gained’ by removing PFAS from drinking water to an advised maximum concentration; (ii) quantification of the human health ‘lost’ due to the impacts of the required treatment technologies; and (iii) a comparison of these ranges to determine whether the concentration guideline leads to a net gain in human health. The paper further serves to start a dialogue about this complex issue and to identify knowledge gaps that can initiate further research. The Leiduin water treatment plant of Waternet, the drinking water production company of the Amsterdam region, was used as a case study. Adsorption to granular activated carbon (GAC) is used at this site, currently with a reactivation frequency sufficient to meet existing PFAS limits. This reactivation frequency may be increased in the future, if PFAS concentration limits are lowered.
Methods
Fig. 1 illustrates the approach followed to enable the quantitative comparison between DALYs gained and DALYs lost by the removal of PFAS from drinking water. The methodology is structured accordingly, with the first subsection explaining the calculation of the DALYs lost due to secondary impacts of the GAC treatment and the second section explaining how the DALYs gained by removing PFAS from drinking water were estimated.Estimation of DALYs from GAC treatment
To estimate the DALY losses associated with an increased GAC reactivation frequency to meet the 4.4 ng PEQ L−1 target, a Life Cycle Assessment (LCA) was conducted following the ISO standards 14040 and 14044. The goal of the assessment was to quantify the difference in human health impacts (DALYs) of water treatment between a status-quo scenario (a) and an increased treatment scenario (b) to meet the target PEQ. The functional unit was defined as one m3 of treated water, which was eventually multiplied with the total production of the Leiduin plant in 2024 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654
654![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3) to get to a unit of DALYs per 106 p per year, since Leiduin supplies drinking water to roughly 106 people. The scope of analysis was centered on the GAC treatment step of the water treatment process, as only the GAC reactivation frequency changes between scenarios. Therefore, while the scope is centered only on the GAC treatment process, a cradle-to-gate analysis was conducted, including GAC production, transportation, and disposal/reactivation (see Fig. 2).
000 m3) to get to a unit of DALYs per 106 p per year, since Leiduin supplies drinking water to roughly 106 people. The scope of analysis was centered on the GAC treatment step of the water treatment process, as only the GAC reactivation frequency changes between scenarios. Therefore, while the scope is centered only on the GAC treatment process, a cradle-to-gate analysis was conducted, including GAC production, transportation, and disposal/reactivation (see Fig. 2).
Impacts were calculated for different GAC options reflecting common practice, namely: single-use and reactivated wood GAC, and single-use and reactivated coal GAC, yielding four variants for each scenario. It is considered that impacts associated to other common water treatment methods will fall within or close to the estimated range, as health impacts from GAC and Ion Exchange (IE) water treatment have been found to be comparable.21 Additionally, the considered GAC alternatives are expected to yield a wide range of impacts, as wood GAC treatment is associated to low health impacts whereas single-use coal GAC is associated to high health impacts when comparing different treatment options.22,23 When included, the reactivation was always modelled as a thermal off-site process, to realistically represent existing practice at the Leiduin site.
To conduct the LCA, the ReCiPe 2016H/H method was applied, as it directly calculates end-point human health impacts in DALYs. The SimaPro software version 9.6.0.1 was used to compile the Life Cycle Inventory (LCI) data and calculate impacts. In the next subsections, more details are presented on the main data and assumptions in the LCI per process step (block in Fig. 2). Since the study investigated human health impacts, only results expressed in DALYs were considered in the data evaluation.
Water treatment – quantify current reactivation frequency
In 2024, Waternet used 931![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg GAC (MGAC), of which 87.5% was reactivated and the rest supplemented with virgin GAC. The used carbon is extruded reactivated pellet wood-based GAC, produced and reactivated by Norit Zaandam. The density of the GAC used was 377 kg m−3 (ρGAC). Waternet uses a two-stage GAC filtration, with twenty filters per stage, each with a volume of 145 m3 (Vf). All new GAC entering the treatment facility enters the second stage filters, for which the replacement frequency (fr) was calculated as per eqn (1). To recalculate this replacement frequency into a maximum stand time of the GAC, it was multiplied by 365 days per year.
000 kg GAC (MGAC), of which 87.5% was reactivated and the rest supplemented with virgin GAC. The used carbon is extruded reactivated pellet wood-based GAC, produced and reactivated by Norit Zaandam. The density of the GAC used was 377 kg m−3 (ρGAC). Waternet uses a two-stage GAC filtration, with twenty filters per stage, each with a volume of 145 m3 (Vf). All new GAC entering the treatment facility enters the second stage filters, for which the replacement frequency (fr) was calculated as per eqn (1). To recalculate this replacement frequency into a maximum stand time of the GAC, it was multiplied by 365 days per year. | (1) |
After the GAC in one of the twenty second stage filters is replaced with new GAC, the GAC from that filter is reused in one of six filters in the first stage. When the GAC from one of these six first stage filters is replaced, it is sent for reactivation and then again enters a second stage filter. The other 14 first stage filters are never renewed. So, the total time the GAC spends in a stage one filter is equal to a factor 6/20 times the time spent in stage two. This process is summarised in ESI Fig. 1.†
Determine required (future) reactivation frequency
To quantify the increase in GAC reactivation frequency needed to achieve the Dutch guideline concentration of 4.4 ng PEQ L−1, we used historical data on the PFAS breakthrough in Waternet's GAC filters. Data from January 2020 to December 2023 (n = 210) was used in a linear regression model that related the operational time of GAC filters (tfilter, days) to PFAS breakthrough from raw water to drinking water (CDW/CRaw) as shown in eqn (2) and ESI Fig. 2.† Here, concentrations of 23 PFAS were expressed in PEQ and summed, with concentrations below the limit of quantification (LOQ) set to zero, according to the Dutch guidelines for determining lower bound PEQ concentrations.24 While breakthrough of individual PFAS is typically non-linear, the breakthrough curve for PEQ fit well to a linear curve (ESI Fig. 2,†R2 = 0.88). | (2) |
Raw water concentration data from January to December 2024 (n = 15) were used to calculate the required removal (i.e., maximum breakthrough) to achieve a mean PFAS CDW below 4.4 ng PEQ L−1. Eqn (2) was then used to relate this maximum breakthrough to a maximum average operational time of the second-stage GAC filters. For simplicity and to include a safety margin, any PFAS removal occurring in the six reactivated filters of the first stage was ignored. The operational time used to determine the required reactivation frequency was twice the operational time found from eqn (2): since multiple GAC filters are operated in parallel, this ensures that the average operational time over all filters is equal to the determined maximum. To quantify a 95% confidence interval over the required reactivation frequency, error propagation including the standard errors over the raw water concentrations and over the regression slope (m, eqn (2)) was used. The data and calculations are presented in ESI methods file 1.†
Eqn (1) was subsequently rewritten to calculate the required amount of GAC from the determined minimum reactivation frequencies (best estimate and 95% upper and lower limits). To verify this calculation method, the PEQ concentration in the drinking water in 2024 was calculated from the known average raw water concentration in 2024 and the known maximum operational time of the filters in 2024 (428 days, see results section). The concentration calculated accordingly was 8.7% lower than the average drinking water concentration measured in 2024, which was deemed acceptable. This calculation is also presented in ESI methods file 1.†
For the prospective scenario, we assumed the same reactivated![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) virgin GAC ratio as in 2024 (87.5% reactivated GAC). We assumed the same yearly amounts for all types of GAC included, while in reality, the PFAS adsorption performance differs for different types of GAC.25 Coal-based GAC typically has a higher removal efficiency than biobased GAC, including wood-based and coconut-based.26,27 It is thus possible that less coal-based GAC would be needed to achieve the required PFAS removal, because of its better performance than wood-based GAC. The estimated human health cost of the coal-based GAC variants may therefore be a minor overestimation. The PFAS removal of virgin versus reactivated GAC is often comparable, both for coal-based and biobased GAC.25–27 The amount of single-use wood-based GAC required is thus probably reasonably close to the estimate based on the reactivated GAC. Because Leiduin uses reactivated wood-based GAC, these results were always taken as the best estimate, and the other GAC types were included to show the approximate range of the potential impact.
virgin GAC ratio as in 2024 (87.5% reactivated GAC). We assumed the same yearly amounts for all types of GAC included, while in reality, the PFAS adsorption performance differs for different types of GAC.25 Coal-based GAC typically has a higher removal efficiency than biobased GAC, including wood-based and coconut-based.26,27 It is thus possible that less coal-based GAC would be needed to achieve the required PFAS removal, because of its better performance than wood-based GAC. The estimated human health cost of the coal-based GAC variants may therefore be a minor overestimation. The PFAS removal of virgin versus reactivated GAC is often comparable, both for coal-based and biobased GAC.25–27 The amount of single-use wood-based GAC required is thus probably reasonably close to the estimate based on the reactivated GAC. Because Leiduin uses reactivated wood-based GAC, these results were always taken as the best estimate, and the other GAC types were included to show the approximate range of the potential impact.
Coal GAC production and reactivation
Coal GAC production requires a carbonization and an activation step. To model these processes, an Ecoinvent database process was used based on the data by Bayer et al. (2005) and Muñoz et al. (2007).28,29 Besides the GAC production process, the database process includes coal extraction and transportation and accounts inputs of electricity, natural gas, water and hard-coal production based on data from European plants. The model includes GAC transport over 35 km to the water treatment plant, which is the real distance between the Leiduin plant and the reactivation process (Norit Zaandam). Spent GAC is transported 20 km to an incineration plant, which is the real distance between the Leiduin plant and the closest waste incineration plant. In the LCA model, spent GAC is incinerated as hazardous waste, as in Ellis et al., 2023.22 The reactivation of coal GAC production was also modelled based on an Ecoinvent dataset for reactivation based on the same studies, and considering a transportation distance of 35 km from the water treatment plant to the reactivation site.Wood GAC production and reactivation
Wood GAC production requires the carbonization of woodchips and the activation of the obtained biochar. To model this process, it was assumed that wood bark chips are commonly available and transported to a central location 50 km away. Activation is modeled based on the data by Gu et al. (2018),30 and following the same assumptions as Vilen et al. (2022) for carbonization.23 That is, the carbonization emissions derive from an Ecoinvent dataset for heat and electricity co-production from wood, with reduced CO2, CO and CH4 emissions by 43%, considering the carbon that remains in the biochar, and are explicitly accounted as biogenic. For details on the LCI, see ESI Tables 2–5.† Wood GAC reactivation was modeled based on the wood-activation process, assuming a decrease in electricity and nitrogen of 24%, and of water of 27% taking as reference the difference in requirements between coal GAC production and activation,23 and considering GAC losses of 12.5% in each reactivation cycle as reported by the Leiduin site. Additionally, emissions to air were assumed to be the same as the emissions from the activation step in wood GAC production (i.e., without carbonization emissions). As with coal GAC reactivation, it is assumed that spent wood GAC is transported 35 km for reactivation.Estimation of ΔDALYs lost from GAC treatment
Finally, eqn (3) was used to estimate the negative health impact (DALYlost) associated with more frequent GAC reactivation to remove PFAS down to a drinking water concentration of 4.4 ng PEQ L−1. Here, DALYGAC, Target is the total human health cost output, in DALYs per 106 p per year, from the LCA model with the targeted (increased) GAC reactivation frequency. Similarly, DALYGAC, Current is the total human health cost output from the LCA model with the current GAC reactivation frequency.| DALYlost [DALYs per 106 people per year] = DALYGAC, Target − DALYGAC, Current | (3) |
Estimation of DALYs from PFAS exposure via drinking water
Current PFAS drinking water concentrations (in 2024, corresponding to the existing GAC reactivation frequency) were obtained from Waternet. Each PFAS was measured in two samples, one from each production line, on 13 dates spread out during 2024 (26 samples total – the Leiduin site has two identical parallel production lines). ‘Target’ PFAS concentrations, corresponding to the increased reactivation frequency, were found from the same linear regression model as described previously (eqn (2)), but now for individual PFAS instead of PEQ. First, the prospective PFOA, PFOS and PFHxS breakthrough values at the increased reactivation frequency were found from their individual linear regression fit. Using the mean concentration in the raw water during 2024 (n = 15), these breakthrough values were then calculated into a ‘target’ CDW of PFOA, PFOS and PFHxS. Since PFNA concentrations in the current drinking water were already always below the LOQ (0.5 ng L−1), PFNA was left out of the entire analysis.These PFAS concentrations in drinking water were then related to PFAS concentrations in plasma, using the same physiologically-based pharmacokinetic (PBPK) model as used by EFSA in their derivation of the 4.4 ng ∑EFSA4 per kg bw per week TWI.11 In this model, EFSA assumed that PFNA behaved identically to PFOA, and PFOS to PFHxS. We adopted the same assumption, so PFOS and PFHxS concentrations were summed and modelled as PFOS. The PBPK model code was obtained from EFSA's 2020 publication11 and rewritten in MATLAB, the full code is available in ESI methods file 2.†
First, EFSA's TWI model of serum concentrations over time was reproduced, to verify our MATLAB code (ESI Fig. 3†). In their model, EFSA assumed a total oral PFOA + PFNA dose of 0.19 ng per kg bw per day, and a total PFOS + PFHxS dose of 0.44 ng per kg bw per day (i.e., a tolerable daily intake (TDI) of 0.63 ng ∑EFSA4 per kg bw per day = 4.4 ng per kg bw per week). Using the RIVM calculation introduced earlier and in eqn (4), relating this to a drinking water concentration limit (Cmax, EFSA,DW) resulted in 1.3 ng L−1 PFOA/PFNA and 3.1 ng L−1 PFOS/PFHxS.
 | (4) |
We used the current and targeted PFAS concentrations in the drinking water supplied by Waternet's Leiduin site (CDW, ng L−1), to calculate the PFOA and PFOS/PFHxS exposure without and with additional drinking water treatment (ExpPFAS, ng per (kg bw day)), as per eqn (5). For the current drinking water concentrations, we modelled the mean PFOA and PFOS/PFHxS concentrations during 2024, and the 95% confidence interval of the mean (assuming a normal distribution, see ESI Fig. 4† for an overview of the current concentrations). For the target concentrations, only the best estimate value was used. The exposure from other sources was kept at 80% of the original EFSA TDI values, as a best case scenario. In reality, however, the total exposure is often above the TDI.13,31 Therefore, we also repeated our analysis using the most recently available data quantifying dietary PFAS exposure in the Dutch population,31 and completed a sensitivity analysis to quantify the effect of the food exposure, these results are included in ESI Fig. 5–7.†
 | (5) |
All these different PFAS exposures were modelled separately using the EFSA PBPK model, to find the influence of different drinking water concentrations on the serum PFAS concentrations over someone's lifetime. The average serum concentrations during the ages relevant for each endpoint were subsequently related to odds ratios for adverse endpoints using dose–response relationships (DRRs, see Table 1). Critical known endpoints associated with PFAS that could be related to a DALY number were included in the study, namely hypertension, kidney cancer, testicular cancer and hypothyroidism in females. Suitable DRRs between serum PFAS concentrations and odds ratios (OR) for these endpoints had already been evaluated and identified in previous literature.17,19 Serum PFOS concentrations resulting from the current CDW were always below the serum PFOS concentrations at the EFSA-recommended CDW (see Fig. 4b). For that reason, the quantification focused on endpoints associated with PFOA. However, for hypothyroidism in females, a DRR with PFOS was included as well. When necessary, PFAS serum and plasma concentrations were assumed to be equal, which is in accordance with measured data.32
| Endpoint | DRR, CPFAS: serum conc. in ng mL−1 | α, β (95% CI) | C ref (ng mL−1) | Original source |
|---|---|---|---|---|
| a C Ref is the reference serum concentration for the corresponding DRR; N/A: not applicable. | ||||
| Hypertension | OR = β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(CPFOA) + α log(CPFOA) + α |
α = −0.0045 (−0.542–0.413) | N/A | 19 and 33 |
| β = 2.412 (1.183–4.035) | ||||
| Kidney cancer | OR = 1 + α(CPFOA − Cref)/10 | α = 0.16 (0.03–0.3) | 0.1 | 34 |
| Testicular cancer | OR = 1 + α(CPFOA − Cref)/10 | α = 0.03 (0.02–0.04) | 0.1 | 34 |
| Hypothyroidism in females | ORPFOA = ln(CPFOA/Cref)α | α = 7.42 (1.14–48.12) | 1 | 35 |
| ORPFOS = ln(CPFOS/Cref)β | β = 3.02 (1.14–8.07) | |||
Eqn (6) was used to relate a DALY number to the odds ratio at the determined serum concentrations. Here, DALYCurrent/Target is the number of DALYs per 106 people per year from a certain endpoint, due to the current or target PFAS exposure, respectively, which is the commonly used unit for comparing health effects in DALYs.36 The OR serves as a proxy for relative risk (RR). Since the prevalence of all endpoints except hypertension is (well) below 10%, this approximation is appropriate.19 For hypertension, the OR was converted to an RR using prevalence data, as described in literature.17I is the incidence rate of the disease in the relevant population (number of cases per year per person), and P is the number of people that fit within that relevant population for a total population of 106. To estimate P, the age and sex distribution of the Dutch population in 2024 was used.37W is the DALY weight, in nr. of DALYs per case. Selected values of I, P and W for each endpoint can be found in ESI Table 6 and ESI methods file 3.†17,19,37–42 We then estimated the change in DALYs gained by installing water treatment to meet the EFSA limit using eqn (7), where DALYPFAS, Target is the number of DALYs lost at the PFAS exposure with the targeted CDW and DALYPFAS, Current is that with the current CDW.
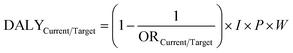 | (6) |
| ΔDALYgained[DALYs per 106 people per year] = DALYPFAS, Current − DALYPFAS, Target | (7) |
In addition to the aforementioned endpoints, infections with Haemophilus influenza type b (Hib), tetanus and diphtheria were included. Even though no dose–response relationship with PFAS serum concentrations could be found for these diseases, the original EFSA TWI was based on a reduced antibody response after vaccination against these diseases. It should be noted that the EFSA TWI approach is based on the precautionary principle, as it does not quantify a disease burden. The original study by Abraham et al. (2020)12 only found an association between PFOA and a reduced antibody response, and it did not find an association with actual infections. Nonetheless, since EFSA concluded that a reduced immune response is the most sensitive endpoint related to PFAS exposure,11 we developed a screening-level assessment to estimate DALYs associated with these infectious diseases in the Netherlands. Using data from the national immunisation programme in the Netherlands,43 the total DALYs from Hib, tetanus and diphtheria per 106 people in 2022 totalled 24 (95% CI: 23–26). Since the lack of a DRR made it impossible to relate this to PFAS, we included the whole range, i.e. we assumed that 0% (low) to 100% (high) of the cases were due to a reduced immune response because of PFAS exposure. We applied a factor 0.2 to correct for the targeted 20% contribution of drinking water to total PFAS exposure (note that this implicitly assumes a linear DRR).
Results and discussion
Estimation of DALYs from GAC treatment
The average operational time of the second stage GAC filters at Waternet's Leiduin site in 2024 was 428 days, corresponding to a reactivation frequency of 0.85 year−1. The linear regression fit relating PFAS (as ∑23PEQ) breakthrough to the operational time of the GAC filters had a slope of 2.05 × 10−3 ± 1.05 × 10−4 per day (R2 = 0.88; eqn (2), see ESI Fig. 2†). The average raw water concentration at Leiduin in 2024 was 22 ± 2.6 ng PEQ L−1, so the maximum breakthrough to stay below 4.4 ng PEQ L−1 was 20%. These combined results led to a maximum time to replacement of 199 ± 26 days, i.e. a replacement frequency of 1.8 per year (95% CI: 1.6–2.1 per year). This reactivation frequency corresponds to a requirement of 2.0 million kg GAC per year (95% CI: 1.8–2.3 million), of which 87.5% is reactivated and 12.5% is virgin GAC.Implementing the currently used (in 2024) amount of GAC in the LCA model resulted in a total DALY cost of 270, 250, 256 and 247 DALYs per 106 p per year for single-use coal, reactivated coal, single-use wood and reactivated wood GAC, respectively. Since the purpose of the LCA was to compare DALY impacts between the reactivation scenarios, the model only considered the GAC treatment step, so these numbers do not represent the full water treatment process. Approximately 60% of these losses were part of the ‘Water Consumption, Human Health’ output for all GAC types, i.e. human health impact due to limited availability of freshwater and its impact on food production and nutrition. However, as the main output of the modelled process is drinking water itself, water withdrawal effects on food production and nutrition are not considered relevant to the analysis. Additionally, because the water use is the same across the different reactivation scenarios, this high DALY estimation did not affect the estimated loss of human health due to increased GAC reactivation. When ignoring the water consumption, the current health impacts were roughly equally related to global warming, particulate matter emissions, and carcinogenic toxicity for all types of GAC, together making up >90% of the total health impact, see ESI Table 7.†
For the increased reactivation frequency scenario, the health impact increased to 298 (292–306), 254 (253–255), 268 (266–271) and 247.9 (247.7–248.2) DALYs per 106 p per year. To facilitate tracking of the calculations, these values are reported with more significant digits than would be justified by the level of certainty. These health impacts again related mostly to water consumption, but excluding that, global warming, particulate matter emissions, and the release of carcinogenic substances each had a contribution of approximately 30% for all GAC types (ESI Table 7†). For all current and prospective scenarios, the human health impact was highest for single-use coal GAC, followed by single-use wood GAC, reactivated coal GAC and reactivated wood GAC. Fig. 3a shows the estimated health loss in DALYs per 106 p per year between the current scenario and the scenario with a higher GAC reactivation frequency, for the different types of GAC. Here, the reactivated wood GAC, corresponding to a health loss of 1.1 (0.9–1.4) DALYs per 106 p per year, represents the current situation best, since this is the type of GAC used at the Leiduin site. This scenario also has the lowest impact on human health compared to the other types of GAC: single-use coal, reactivated coal and single-use wood GAC corresponded to a health loss of 28 (23–36), 4.2 (3.3–5.4) and 12 (10–16) DALYs per 106 p per year, respectively.
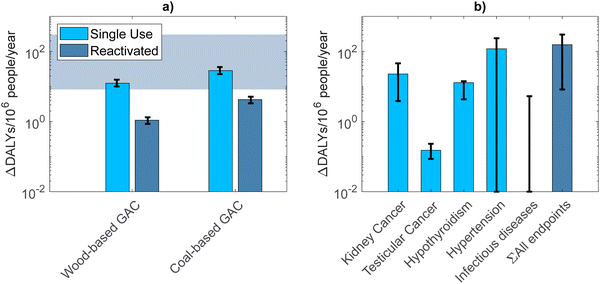 | ||
| Fig. 3 (a) DALYs lost due to the increased GAC reactivation frequency. Error bars represent the 95% CI of the required reactivation frequency. The shaded region represents the estimated range of total (from all endpoints) DALYs gained by lowering the PFAS drinking water concentrations, also shown in (b) DALYs gained by removing PFAS from drinking water down to the EFSA-recommended concentrations. Error bars represent the uncertainty (min–max) due to the variability in the current drinking water concentrations as well as the dose–response relationships used to relate PFAS serum concentration to an OR. Note that when this range goes down to 0, it is represented as 10−2 in the log-scaled plot. For the three infectious diseases,43 the error bar represents the uncertainty due to the assumed relationship with PFAS (0–100% of disease occurrence due to lower immune function from PFAS exposure), and no best estimate is given. | ||
For all scenarios, the total loss of human health because of the increased GAC reactivation frequency was mostly caused by effects related to global warming and to fine particulate matter emissions. Together, these always made up >80% of the total health loss, with roughly equal contributions for both, as shown in Table 2. Potential human health effects caused by the release of toxic substances, both carcinogenic and non-carcinogenic, were responsible for the remainder of the health loss. All these individual contributors to the health losses followed the same trend as the total health losses, i.e. single-use coal > single-use wood > reactivated coal > reactivated wood GAC.
| Cause | Wood-based GAC | Coal-based GAC | ||
|---|---|---|---|---|
| Single-use | Reactivated | Single-use | Reactivated | |
| Global warming | 4.4 (3.4–5.8) | 0.48 (0.38–0.61) | 11 (8.4–14) | 1.8 (1.4–2.3) |
| Fine particulate matter formation | 6.2 (5.3–7.4) | 0.44 (0.35–0.56) | 13 (11–16) | 1.9 (1.5–2.4) |
| Human carcinogenic toxicity | 1.2 (0.9–1.6) | 0.06 (0.05–0.07) | 2.2 (1.7–2.9) | 0.20 (0.16–0.26) |
| Human non-carcinogenic toxicity | 0.48 (0.31–0.71) | 0.10 (0.08–0.13) | 2.5 (1.9–3.3) | 0.39 (0.31–0.50) |
| Total | 12 (10–16) | 1.1 (0.86–1.4) | 28 (23–36) | 4.2 (3.3–5.4) |
For single-use coal and wood GAC, most of the health impacts resulting from an increased replacement frequency are associated with fresh GAC inputs and waste management. Specifically, approximately 70% and 40% of DALY losses are due to GAC inputs for coal- and wood-based GAC, respectively, and 20% and 50% DALY losses are due to waste management. In the case of both wood-based and coal-based reactivated GAC, the major contributors to DALY losses are the inputs of fresh GAC (approximately 60%) and reactivated GAC (approximately 40%). For wood-based GAC, electricity use is the largest contributor (94%) to the total DALY losses associated with the reactivation process. Conversely, transportation of fresh, spent and/or reactivated GAC only has a minimum contribution of <2% in all scenarios. This is consistent with the specific context of the Leiduin plant, where GAC and waste management facilities are located nearby. Nevertheless, other studies that account for much longer transportation distances also conclude that transport has minor contributions.22,23
Estimation of DALYs from PFAS exposure via drinking water
In 2024, the mean PFOA, PFOS and PFHxS concentrations in the Leiduin drinking water were 5.5 ± 0.56, 0.97 ± 0.20 and 1.4 ± 0.17 ng L−1, respectively. PFNA was left out of the analysis, because its concentrations were always <LOQ. PFOS once had a concentration <LOQ (0.2 ng L−1), which was set to the LOQ, because the other sample on that date had a concentration slightly above the LOQ (0.23 ng L−1). Otherwise, all concentration data of these compounds were >LOQ. From the linear regression fits on PFOA, PFOS and PFHxS, a reactivation frequency of 1.8 year−1 would lead to drinking water concentrations of 1.9, 0.28 and 0.40 ng L−1, respectively (see Methods – estimation of DALYs from PFAS exposure via drinking water). These concentrations were thus used as ‘target concentrations’ at the increased reactivation frequency.Fig. 4 shows the projected PFOA and PFOS serum concentrations over someone's lifetime for the different drinking water concentrations (CDW). These adult concentrations are all in the same range as median serum concentrations measured in the populations of various European countries.44 However, a study that measured ∑EFSA4 concentrations in plasma of exclusively breastfed Dutch infants at age 3 months found >2-times lower concentrations than found using the PBPK model at the current drinking water concentrations from Leiduin (ESI Table 8†).45 This might indicate that the PBPK model overestimates the bioaccumulation of PFAS, or that the exposure via food is overestimated. When using the lower bound of the most recently estimated dietary exposure of the Dutch population instead, the measured ∑EFSA4 plasma concentrations fall within the range of the modelled concentrations, indicating that this scenario might be more realistic than that with the EFSA-recommended exposure. Another possible explanation for this discrepancy is that drinking water concentrations in the Amsterdam region are higher than in other regions in the Netherlands that produce drinking water from groundwater instead of dune-infiltrated surface water.13
ESI Table 9† further shows the modelled plasma concentrations at ages relevant for EFSA's derivation of their TWI guideline, indicating that plasma concentrations of ∑EFSA4 remain below or within 1% of the EFSA-recommended values for all scenarios with more frequent GAC reactivation. For PFOA, modelled serum concentrations are similar for the scenarios with the target CDW and with the CDW calculated directly from the EFSA TDI. Conversely, at the current CDW, modelled serum PFOA concentrations are considerably higher and exceed the recommended values. For PFOS, modelled serum concentrations are already below the EFSA-recommended value at the current CDW, but decrease even further at the target concentrations.
Relating these modelled serum concentrations to health impacts from the four endpoints in Table 1 resulted in 146 (min–max: 13.9–156) DALYs lost per 106 people per year for the scenario with the current PFAS concentration in drinking water. This is in the same range as the PFAS-associated DALY cost per 106 p in 2021 found by Plass et al. for Belgium (380; 30–723).19 In comparison, the DALY cost decreased to 33 (min–max 5.2–48) or 35 (min–max 6.2–47) DALYs per 106 p per year for the exposure scenarios with the targeted or the EFSA-recommended CDW, respectively. These relatively high DALY numbers demonstrate the substantial public health risks associated with PFAS.
Fig. 3b shows the estimated change in DALYs per 106 p per year between the current scenario and the scenario with lower PFAS concentrations in drinking water. In absolute numbers, hypertension contributes most to the gain in human health achieved by lower PFAS exposure via drinking water (120 DALYs per 106 p per year, min–max 0–240). However, the uncertainty ranges down to zero and the result depends largely on the exposure via food (see also ESI Fig. 5 and 6†). In comparison, kidney cancer and hypothyroidism have a less uncertain contribution of 23 (min–max 3.8–46) and 13 (min–max 4.3–14) DALYs per 106 p per year, respectively, which is also less dependent on the dietary exposure. Testicular cancer contributes little to the DALY gain (0.15 DALYs per 106 p per year; min–max 0.13–0.18).
The final endpoint, reduced immune response after vaccination against Hib, tetanus and diphtheria, was the basis of the EFSA-derived TDI that was used by the RIVM to calculate the 4.4 ng PEQ L−1 drinking water guideline.11,12 In the Netherlands, infections with Hib, tetanus and diphtheria were associated with 4.9 (95% CI: 4.6–5.3) DALYs per 106 p per year. Note that the only relationship with PFAS included in this number is a 20% contribution of drinking water to total PFAS exposure. Since no relationship between PFAS exposure and infections has been shown,12 the actual loss of DALYs via these endpoints due to PFAS is probably closer to zero. If all occurrence of these infections could be attributed to a lower immune response due to PFAS exposure, the drinking water-associated DALY cost would at most be around 5 DALYs per 106 p per year.
Comparison of DALYs lost from GAC treatment and gained from lower PFAS exposure
Altogether, the lower PFAS concentrations in drinking water lead to a gain in human health of 150 (min–max: 8.3–300) DALYs per 106 p per year via all the endpoints investigated. In comparison, the increased GAC reactivation frequency leads to a loss in human health of 1.1 (0.9–1.4) DALYs per 106 p per year, when using reactivated wood-based GAC. These results are illustrated in Fig. 3. The minimum estimate of the gain due to lower PFAS exposure is over five times higher than the maximum estimate of the health loss associated with GAC reactivation. Since reactivated wood GAC is used at the Leiduin drinking water treatment plant, increasing the GAC reactivation frequency there is thus likely to result in a net health gain of at least 6.9 DALYs per 106 p per year, up to even 300 DALYs per 106 p per year. This conclusion of a significant net health gain is also valid for the use of reactivated coal-based GAC, which is not currently used by the Leiduin drinking water plant of Waternet, but is used at another production plant of Waternet.However, in the hypothetical scenario where single-use GAC is applied for the removal of PFAS, this conclusion changes. For single-use coal-based GAC, the DALY loss of 28 (23–36) DALYs per 106 p per year is already in the same range as the total DALY gain. Since the total DALY gain is dominated by hypertension, which (as described above) is highly dependent on the PFAS exposure via food, the upper limit of this range is probably an overestimation. When excluding hypertension from the total, the estimate becomes 36 (8.3–65) DALYs per 106 p per year, which is similar to the estimated DALY loss. Therefore, these results indicate that applying single-use coal-based GAC for the removal of PFAS from drinking water is unlikely to result in a significant net health gain.
When considering only the hypothetical DALY cost of PFAS in drinking water related to infections from Hib, tetanus, and diphtheria – the basis for the RIVM 4.4 ng PEQ L−1 guideline – it is equally impossible to quantify a net health benefit. When allocating all DALYs associated with these diseases to PFAS, and assuming a 20% contribution of drinking water to total PFAS exposure, the associated health cost is at most 5 DALYs per 106 p per year. This number is probably a significant overestimation: PFAS exposure was only linked to a decreased immune response after vaccination, not to actual infections,11 so allocating all DALYs to PFAS is unrealistic. Therefore, the real health gain is probably closer to or below the 1.1 DALYs per 106 p per year health loss from the treatment impacts. These results indicate that other preventative strategies targeting these specific infectious diseases, such as increasing vaccination frequency, may be more effective to protect human health. Nonetheless, it should be noted that the EFSA TWI and following 4.4 ng PEQ L−1 guideline were established as precaution, to broadly protect against (immune) effects, which has merit given the high uncertainty associated with disease burden estimations.
Identification of uncertainty sources and knowledge gaps for future research
It should be acknowledged that the results presented above are subject to numerous sources of uncertainty. Stochastic uncertainties from the variability in the used data have been included as much as possible in our estimations of the min–max ranges in DALYs. However, key other sources of uncertainty remain, the most notable of which are discussed below (see ESI Table 10† for all identified uncertainties). We classify these uncertainties as either indeterminate (related to uncertainties in future decision-making), epistemic (related to a lack of knowledge) or ambiguous (related to different moral frameworks for interpreting risk).46 These uncertainties should be addressed in further research to establish a greater degree of accuracy in the estimation of human health impacts.An important source of indeterminate uncertainty in the estimation of DALYs lost by increasing the GAC reactivation frequency relates to the determination of the required reactivation frequency to reach the treatment guidelines. Currently, this is based on a linear regression model which assumes that breakthrough only depends on the GAC reactivation frequency, since this is the method actually used at Waternet for these types of determinations. In reality, the required reactivation frequency will also depend on the future raw water PFAS concentrations, which will be affected by the future PFAS discharge into the surface water used for drinking water production. Since PFAS regulations are becoming increasingly strict, raw water concentrations may decrease in the future, leading to different breakthrough curves and different reactivation requirements. On the other hand, potential future drinking water concentration limits for ultra-short chain PFAS, such as trifluoroacetic acid (TFA), would lead to even more resource-intensive reverse osmosis treatment becoming necessary.
Further, our assumption that breakthrough behaviour is identical in all types of GAC is a source of epistemic uncertainty. As explained in the methods section, coal-based GAC may outperform wood-based GAC,26,27 leading to lower reactivation frequencies being necessary to achieve the same PFAS removal. Therefore, the health costs estimated for the coal-based GAC scenarios are probably minor overestimations, because a lower yearly GAC use may suffice. Another source of epistemic uncertainty is the fact that an increased GAC reactivation frequency may result in additional health gains via increased removal of non-PFAS pollutants. Compared to the effect of changes in raw water composition or regulation, we expect these uncertainty sources to be relatively minor.
The main source of uncertainty in the estimation of DALYs gained by lower PFAS exposure via drinking water relates to our choice of endpoints included in the quantification of the health benefits. There is still a lot of scientific debate about which endpoints to include in risk assessment for PFAS, so this uncertainty is partly epistemic and partly ambiguous. While the DRRs included in this study have all been used previously for the purpose of estimating PFAS-associated disease burdens,17,19 kidney cancer, testicular cancer, hypothyroidism, and hypertension were not used by EFSA to derive their TWI. In fact, EFSA concluded in their most recent publications (from 2018 and 2020) that there is insufficient evidence to link hypothyroidism and carcinogenicity to PFAS.11,47 Conversely, the Australian National Health and Medical Research Council (NHMRC) recently (in 2024) derived a PFOA guideline value of 200 ng L−1 in drinking water, based on carcinogenicity.8 Similarly, the NHMRC derived guideline concentrations of 30 ng L−1 and 1000 ng L−1 for PFHxS and PFBS, respectively, both based on thyroid effects. These extreme differences between calculated ‘safe’ concentrations illustrate the significant effect of the choice of endpoint, and also indicate differing moral perspectives on what is ‘safe’. In- or excluding different PFAS-associated endpoints in the calculation of the total DALY gain will thus significantly impact the result. Further research is needed to identify the most relevant PFAS-associated endpoints and to establish reliable DRRs for those endpoints.
Further sources of epistemic uncertainty relate to the used DRRs, the extrapolation of the DRRs to the population from Amsterdam, the allocation of DALY weights to PFAS-related endpoints, and the use of averaged serum concentrations. Exact DRRs between PFAS exposure and specific health outcomes remain uncertain for many PFAS compounds, so reproducing and refining the currently available DRRs is a fruitful area for further work. Variations in baseline disease rates, co-exposures, and health conditions can affect health outcomes across different populations,11,48 so a DRR that is valid in one population may not be valid in another. There is limited epidemiological data on the severity and long-term impact of PFAS-related conditions, and it is currently unknown if or to what extent PFAS-related endpoints differ in severity from the reference endpoint. Finally, we used an average serum concentration in both scenarios, instead of a distribution across the population due to varying dietary exposure, which likely impacted the results. While important, these four sources of uncertainty are expected to have a smaller effect on the determined range of DALYs gained than the choice of which endpoints to include.
Comparing drinking water treatment with other interventions to protect human health
Compared to intensified drinking water treatment, other interventions that limit PFAS exposure may have a higher net health benefit. For example, phasing out all non-essential uses of PFAS is not expected to have any negative secondary health impacts, but will lead to a decreased exposure via multiple routes. Since the phase-out of PFOS and PFOA, concentrations of these chemicals in human blood have declined considerably,49 indicating that phase-out is a successful strategy to prevent human exposure. Source control, to prevent contamination of raw water sources used for drinking water production, is another important strategy. The resource intensity (energy, sorbents, chemicals, etc.) per mass of PFAS removed is lower at high PFAS concentrations,50 so treating PFAS-rich waste streams before they enter the environment is more resource-efficient than treating drinking water.Additionally, for the majority of people, diet is a larger source of PFAS exposure than drinking water.13,31,51,52 For example, it has been estimated that eating one serving of freshwater fish from the USA is equivalent to consuming one month of drinking water containing 48 ng PFOS L−1,53i.e. 96 ng PEQ L−1,15 which is over 20 times higher than the 4.4 ng PEQ L−1 guideline. Therefore, issuing recommendations to limit the consumption of foodstuffs rich in PFAS is also likely to have a higher net benefit than removing PFAS from drinking water that already has relatively low PFAS concentrations. Our results also depended heavily on the intake of PFAS via food, so decreasing this exposure route may also enable more accurate estimations of the effect of lower PFAS concentrations in drinking water on human health.
Finally, it is important to consider that intensified drinking water treatment has a monetary cost as well, in addition to an indirect health cost. Spending this money on other interventions may achieve a higher net health benefit than introducing advanced drinking water treatment to remove PFAS. Such other health interventions can also be unrelated to PFAS, e.g. replacement of lead pipes, improving vaccination schemes, reducing air pollution from traffic, industry and agriculture, and more. Which health intervention has the largest net benefit will probably differ per location and population, and policy makers may need to consider prioritizing the most cost-effective interventions.
Conclusion
The developed methodology enabled a comparison of the health costs and benefits associated with removing PFAS from drinking water using granular activated carbon, which can support the development of future regulations. The results indicate that while the increased reactivation frequency introduces some health trade-offs, the use of reactivated GAC to achieve PFAS concentrations below 4.4 ng PEQ L−1 in drinking water is expected to result in a net positive impact on human health. Conversely, when single-use GAC is used instead, a net benefit is not necessarily achieved. Altogether, the health losses from more frequent GAC reactivation are considerable, and should be taken into account when designing a drinking water treatment system for PFAS. The generalisability of these results is subject to important limitations. We used data from one specific drinking water treatment plant where GAC filtration was already in place, combined with health data specific for the Dutch population. Hence, these conclusions highly depend on the specific PFAS levels in this raw water, the breakthrough behaviour of these PFAS, and population-specific variables. Additionally, as outlined above, key sources of uncertainty remain, which should be addressed in further research.Addressing these identified uncertainties requires a multidisciplinary approach that integrates risk assessment, toxicology and water treatment expertise. Further research should prioritize the identification of which endpoints to include for PFAS risk assessment, and establish reliable DRRs to relate PFAS serum concentrations to the occurrence of those endpoints. However, to tackle indeterminate uncertainties and ambiguity, merely gathering more knowledge will not suffice. In line with responsible innovation, addressing these uncertainties requires engaging with stakeholders, including policymakers, scientists, and the public, to ensure that scientific and policy advances align with societal perspectives, promoting transparency in decision-making and an adaptive regulatory approach.46
Despite the limitations, the methodology developed here may be applied to other scenarios globally, to verify the benefits of PFAS treatment in drinking water production. The 4.4 ng PEQ L−1 limit that was used throughout this study was set to prevent any effect of PFAS from drinking water, without quantifying the severity of the risk. This reasoning is common for chemicals, but complicates the quantification of health impacts. Using pharmacokinetic modelling to translate drinking water concentrations to serum levels, which are linked to adverse endpoints with DRRs, enables risk-based health assessments to guide the determination of drinking water targets. Combining these results with LCA modelling to determine the treatment impact is an important step towards estimating the net health impact of PFAS treatment, as shown in this study.
Altogether, PFAS limits in drinking water may need to be determined on a case by case basis, that considers the current concentration levels in addition to the secondary impact of the required treatment technologies. When drinking water is produced from a highly PFAS-contaminated source, installing advanced treatment technologies will almost certainly result in a net health benefit. On the other hand, if the PFAS concentrations are only slightly above the 4.4 ng PEQ L−1 limit, or equivalent limits in other countries, the impacts of the technology may outweigh the health benefits obtained by removing PFAS. This dilemma also has an ethical dimension, as health gains by removing PFAS are local, whereas health losses due to GAC reactivation are partly global. Additionally, there are other environmental and societal costs related to PFAS exposure and removal that should be considered for decision making.
Data availability
Historical PFAS concentrations in raw water and after GAC filtration, used in the linear regression on GAC breakthrough, are not made available for confidentiality reasons. All other data supporting this article have been included as part of the ESI files.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to express our gratitude to Fred van Schooten (Waternet) for his valuable assistance in determining the required prospective granular activated carbon reactivation frequencies. Images in the TOC graphic were generated using ChatGPT-4.References
- M. G. Evich, M. J. B. Davis, J. P. McCord, B. Acrey, J. A. Awkerman, D. R. U. Knappe, A. B. Lindstrom, T. F. Speth, C. Tebes-Stevens, M. J. Strynar, Z. Wang, E. J. Weber, W. M. Henderson and J. W. Washington, Per- and polyfluoroalkyl substances in the environment, Science, 2022, 375(6580), eabg9065 CrossRef CAS PubMed.
- OECD, Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance, Paris, 2021 Search PubMed.
- I. T. Cousins, J. C. Dewitt, J. Glüge, G. Goldenman, D. Herzke, R. Lohmann, C. A. Ng, M. Scheringer and Z. Wang, The high persistence of PFAS is sufficient for their management as a chemical class, Environ. Sci. Process. Impacts, 2020, 22, 2307–2312 RSC.
- E. M. Sunderland, X. C. Hu, C. Dassuncao, A. K. Tokranov, C. C. Wagner and J. G. Allen, A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects, J. Expo. Sci. Environ. Epidemiol., 2019, 29, 131–147 CrossRef CAS PubMed.
- M. Kirk, K. Smurthwaite, J. Bräunig, S. Trevenar, R. Lucas, A. Lal, R. Korda, A. Clements, J. Mueller and B. P. Armstrong, The PFAS Health Study: Systematic Literature Review, The Australian National University, 2018 Search PubMed.
- Council of the European Union, Review of the drinking water directive, https://ec.europa.eu/environment/water/water-drink/review_en.html, accessed 16 August 2022 Search PubMed.
- Drinking Water Inspectorate, Risk Assessments under Regulation 27 and Associated Reports under Regulation 28 of the Water Supply (Water Quality) Regulations 2016 (2018 in Wales) for Poly and Perfluorinated Alkyl Substances (PFAS), 2022 Search PubMed.
- Australian Government National Health & Medical Research Council, NHMRC Statement: Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water, https://www.nhmrc.gov.au/health-advice/environmental-health/water/PFAS-review/NHMRC-statement-PFAS, accessed 1 November 2024 Search PubMed.
- Health Canada, Objective for Canadian Drinking Water Quality: Per- and Polyfluoroalkyl Substances, EDITIONS UNIVERSITAIRES, 2024 Search PubMed.
- US EPA, Per- and Polyfluoroalkyl Substances (PFAS) Final PFAS National Primary Drinking Water Regulation, https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas, accessed 26 August 2024 Search PubMed.
- D. Schrenk, M. Bignami, L. Bodin, J. K. Chipman, J. del Mazo, B. Grasl-Kraupp, C. Hogstrand, L. Hoogenboom, J. C. Leblanc, C. S. Nebbia, E. Nielsen, E. Ntzani, A. Petersen, S. Sand, C. Vleminckx, H. Wallace, L. Barregård, S. Ceccatelli, J. P. Cravedi, T. I. Halldorsson, L. S. Haug, N. Johansson, H. K. Knutsen, M. Rose, A. C. Roudot, H. Van Loveren, G. Vollmer, K. Mackay, F. Riolo and T. Schwerdtle, Risk to human health related to the presence of perfluoroalkyl substances in food, EFSA J., 2020, 18(9), e06223 CAS.
- K. Abraham, H. Mielke, H. Fromme, W. Völkel, J. Menzel, M. Peiser, F. Zepp, S. N. Willich and C. Weikert, Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: associations between levels of perfluorooctanoic acid (PFOA) and vaccine response, Arch. Toxicol., 2020, 94, 2131–2147 CrossRef CAS PubMed.
- RIVM, Analyse bijdrage drinkwater en voedsel aan blootstelling EFSA-4 PFAS in Nederland en advies drinkwaterrichtwaarde, 2021 Search PubMed.
- W. Bil, M. Zeilmaker, S. Fragki, J. Lijzen, E. Verbruggen and B. Bokkers, Risk Assessment of Per- and Polyfluoroalkyl Substance Mixtures: A Relative Potency Factor Approach, Environ. Toxicol. Chem., 2021, 40, 859–870 CrossRef CAS PubMed.
- M. J. Zeilmaker, Mixture Exposure to PFAS: A Relative Potency Factor Approach, 2018 Search PubMed.
- US EPA, Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) - Technologies for Reducing PFAS in Drinking Water, 2019 Search PubMed.
- V. Obsekov, L. G. Kahn and L. Trasande, Leveraging Systematic Reviews to Explore Disease Burden and Costs of Per- and Polyfluoroalkyl Substance Exposures in the United States, Expo. Health, 2023, 15, 373–394 CrossRef CAS PubMed.
- C. S. Gretta Goldenman, M. Fernandes, M. Holland, T. Tugran, A. Nordin and A. McNeill, The Cost of Inaction - A Socioeconomic Analysis of Environmental and Health Impacts Linked to Exposure to PFAS, 2019 Search PubMed.
- D. Plass, S. Kienzler, J. Bessems, J. Buekers, J. Cops, A. Purece, A. Beloconi, P. Vounatsou and K. Widmer, Estimating the burden of disease due to lead, PFAS, phthalates, Cadmium, Pyrethroids and Bisphenol A Using HBM4EU Data – Test of Feasibility and First Results for Selected Countries, 2023 Search PubMed.
- World Health Organization, Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda, 2022 Search PubMed.
- I. Emery, D. Kempisty, B. Fain and E. Mbonimpa, Evaluation of treatment options for well water contaminated with perfluorinated alkyl substances using life cycle assessment, Int. J. Life Cycle Assess., 2019, 24, 117–128 CrossRef CAS.
- A. C. Ellis, T. H. Boyer, Y. Fang, C. J. Liu and T. J. Strathmann, Life cycle assessment and life cycle cost analysis of anion exchange and granular activated carbon systems for remediation of groundwater contaminated by per- and polyfluoroalkyl substances (PFASs), Water Res., 2023, 243, 120324 CrossRef CAS PubMed.
- A. Vilén, P. Laurell and R. Vahala, Comparative life cycle assessment of activated carbon production from various raw materials, J. Environ. Manage., 2022, 324, 116356 CrossRef PubMed.
- RIVM, Werkwijze toetsing PFAS in drinkwatermonsters, 2023 Search PubMed.
- B. Cantoni, A. Turolla, J. Wellmitz, A. S. Ruhl and M. Antonelli, Perfluoroalkyl substances (PFAS) adsorption in drinking water by granular activated carbon: influence of activated carbon and PFAS characteristics, Sci. Total Environ., 2021, 795, 148821 CrossRef CAS PubMed.
- J. D. McNamara, R. Franco, R. Mimna and L. Zappa, Comparison of Activated Carbons for Removal of Perfluorinated Compounds From Drinking Water, J. AWWA, 2018, 110(1), E2–E14 CrossRef CAS.
- P. Westreich, R. Mimna, J. Brewer and F. Forrester, The removal of short-chain and long-chain perfluoroalkyl acids and sulfonates via granular activated carbons: a comparative column study, Remediation, 2018, 29, 19–26 CrossRef.
- I. Muñoz, J. Peral, J. Antonio Ayllón, S. Malato, M. José Martin, J. Yves Perrot, M. Vincent and X. Domènech, Life-Cycle Assessment of a Coupled Advanced Oxidation-Biological Process for Wastewater Treatment: Comparison with Granular Activated Carbon Adsorption, Environ. Eng. Sci., 2007, 24, 638–651 CrossRef.
- P. Bayer, E. Heuer, U. Karl and M. Finkel, Economical and ecological comparison of granular activated carbon (GAC) adsorber refill strategies, Water Res., 2005, 39, 1719–1728 CrossRef CAS PubMed.
- H. Gu, R. Bergman, N. Anderson and S. Alanya-Rosenbaum, LIFE-CYCLE ASSESSMENT OF ACTIVATED CARBON FROM WOODY BIOMASS, Wood Fiber Sci., 2018, 50, 229–243 CrossRef CAS.
- RIVM, Risk Assessment of Exposure to PFAS through Food and Drinking Water in the Netherlands, 2023 Search PubMed.
- S. Poothong, C. Thomsen, J. A. Padilla-Sanchez, E. Papadopoulou and L. S. Haug, Distribution of Novel and Well-Known Poly- and Perfluoroalkyl Substances (PFASs) in Human Serum, Plasma, and Whole Blood, Environ. Sci. Technol., 2017, 51, 13388–13396 CrossRef CAS PubMed.
- J.-Y. Min, K.-J. Lee, J.-B. Park and K.-B. Min, Perfluorooctanoic acid exposure is associated with elevated homocysteine and hypertension in US adults, Occup. Environ. Med., 2012, 69, 658–662 CrossRef CAS PubMed.
- S. M. Bartell and V. M. Vieira, Critical review on PFOA, kidney cancer, and testicular cancer, J. Air Waste Manage. Assoc., 2021, 71, 663–679 CrossRef CAS PubMed.
- L.-L. Wen, L.-Y. Lin, T.-C. Su, P.-C. Chen and C.-Y. Lin, Association Between Serum Perfluorinated Chemicals and Thyroid Function in U.S. Adults: The National Health and Nutrition Examination Survey 2007–2010, J. Clin. Endocrinol. Metab., 2013, 98, E1456–E1464 CrossRef CAS PubMed.
- T. Gao, X. C. Wang, R. Chen, H. Hao and W. Guo, Disability adjusted life year (DALY): a useful tool for quantitative assessment of environmental pollution, Sci. Total Environ., 2015, 511, 268–287 CrossRef CAS PubMed.
- Centraal Bureau voor de Statistiek, Bevolkingspiramide, https://www.cbs.nl/nl-nl/visualisaties/dashboard-bevolking/bevolkingspiramide, accessed 7 November 2024 Search PubMed.
- S. Safiri, A.-A. Kolahi, M. A. Mansournia, A. Almasi-Hashiani, A. Ashrafi-Asgarabad, M. J. M. Sullman, D. Bettampadi, M. Qorbani, M. Moradi-Lakeh, M. Ardalan, A. Mokdad and C. Fitzmaurice, The burden of kidney cancer and its attributable risk factors in 195 countries and territories, 1990–2017, Sci. Rep., 2020, 10, 13862 CrossRef CAS PubMed.
- F. Pishgar, A. Haj-Mirzaian, H. Ebrahimi, S. Saeedi Moghaddam, B. Mohajer, M. R. Nowroozi, M. Ayati, F. Farzadfar, C. Fitzmaurice and E. Amini, Global, regional and national burden of testicular cancer, 1990–2016: results from the Global Burden of Disease Study 2016, BJU Int., 2019, 124, 386–394 CrossRef PubMed.
- World Health Organization, WHO Methods and Data Sources for Global Burden of Disease Estimates 2000-2019, 2020 Search PubMed.
- Integraal kankercentrum Nederland, NKR Cijfers Incidentie Per Jaar, https://nkr-cijfers.iknl.nl, accessed 7 November 2024 Search PubMed.
- J. Vanhommerig and L. Overbeek, Nivel-cijfers Ziekten op jaarbasis in Nederland - incidentie en prevalentie, prevalentie, https://www.nivel.nl/nl/zorg-en-ziekte-in-cijfers/cijfers-ziekten-op-jaarbasis, accessed 7 November 2024 Search PubMed.
- RIVM, The National Immunisation Programme in the Netherlands - Surveillance and Developments in 2022-2023, 2023 Search PubMed.
- European Human Biomonitoring Initiative, Policy Brief PFAS, 2022 Search PubMed.
- I. A. L. P. van Beijsterveldt, B. D. van Zelst, S. A. A. van den Berg, K. S. de Fluiter, M. van der Steen and A. C. S. Hokken-Koelega, Longitudinal poly- and perfluoroalkyl substances (PFAS) levels in Dutch infants, Environ. Int., 2022, 160, 107068 CrossRef CAS PubMed.
- L. Asveld and D. Stemerding, in New Perspectives on Technology in Society: Experimentation beyond the Laboratory, ed. I. Van de Poel, L. Asveld and D. C. Mehos, Routledge, 1st edn, 2018 Search PubMed.
- H. K. Knutsen, J. Alexander, L. Barregård, M. Bignami, B. Brüschweiler, S. Ceccatelli, B. Cottrill, M. Dinovi, L. Edler, B. Grasl-Kraupp, C. Hogstrand, L. (Ron) Hoogenboom, C. S. Nebbia, I. P. Oswald, A. Petersen, M. Rose, A. Roudot, C. Vleminckx, G. Vollmer, H. Wallace, L. Bodin, J. Cravedi, T. I. Halldorsson, L. S. Haug, N. Johansson, H. van Loveren, P. Gergelova, K. Mackay, S. Levorato, M. van Manen and T. Schwerdtle, Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food, EFSA J., 2018, 16(12), e05194 Search PubMed.
- US EPA, Framework for Cumulative Risk Assessment, Washington, DC, 2003 Search PubMed.
- J. C. Botelho, K. Kato, L.-Y. Wong and A. M. Calafat, Per- and polyfluoroalkyl substances (PFAS) exposure in the U.S. population: NHANES 1999–March 2020, Environ. Res., 2025, 270, 120916 CrossRef CAS PubMed.
- V. Franke, P. McCleaf, K. Lindegren and L. Ahrens, Efficient removal of per- and polyfluoroalkyl substances (PFASs) in drinking water treatment: nanofiltration combined with active carbon or anion exchange, Environ. Sci., 2019, 5, 1836–1843 CAS.
- X. C. Hu, A. K. Tokranov, J. Liddie, X. Zhang, P. Grandjean, J. E. Hart, F. Laden, Q. Sun, L. W. Y. Yeung and E. M. Sunderland, Tap water contributions to plasma concentrations of poly- and perfluoroalkyl substances (PFAS) in a nationwide prospective cohort of U.S. women, Environ. Health Perspect., 2019, 127(6), 67006 CrossRef PubMed.
- A. O. De Silva, J. M. Armitage, T. A. Bruton, C. Dassuncao, W. Heiger-Bernays, X. C. Hu, A. Kärrman, B. Kelly, C. Ng, A. Robuck, M. Sun, T. F. Webster and E. M. Sunderland, PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding, Environ. Toxicol. Chem., 2021, 40, 631–657 CrossRef CAS PubMed.
- N. Barbo, T. Stoiber, O. V. Naidenko and D. Q. Andrews, Locally caught freshwater fish across the United States are likely a significant source of exposure to PFOS and other perfluorinated compounds, Environ. Res., 2023, 220, 115165 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5em00238a |
| This journal is © The Royal Society of Chemistry 2025 |



