Open Access Article
Open Access ArticleUpdated global warming potentials of inhaled halogenated anesthetics, isoflurane and sevoflurane from new temperature dependent OH-kinetics†
Sara
Espinosa
ab,
Francisco
Martínez
b,
María
Antiñolo
 ab,
Ole J.
Nielsen
ab,
Ole J.
Nielsen
 c and
Elena
Jiménez
c and
Elena
Jiménez
 *ab
*ab
aUniversidad de Castilla-La Mancha (UCLM), Facultad de Ciencias y Tecnologías Químicas, Departamento de Química Física, Avda. Camilo José Cela, 1B, 13071 Ciudad Real, Spain. E-mail: Elena.Jimenez@uclm.es
bInstituto de Investigación en Combustión y Contaminación Atmosférica (ICCA), UCLM, Camino de Moledores, s/n, 13071 Ciudad Real, Spain
cCopenhagen Centre for Atmospheric Research, Department of Chemistry, University of Copenhagen, Copenhagen, Denmark
First published on 13th June 2025
Abstract
Despite the use of scavenging systems in anesthesia machines, inhaled halogenated anesthetic gases (HAGs), such as isoflurane (CF3CHClOCHF2) and sevoflurane ((CF3)2CHOCH2F), are still emitted directly into the atmosphere. In 2014, their atmospheric concentrations were 0.097 ppt (isoflurane) and 0.13 pptv (sevoflurane). As halogenated species, their impact on global warming has to be known. Notably, the global warming potential at a time horizon of 100 years (GWP100 years) for sevoflurane differs between IPCC and WMO sources, creating regulatory uncertainty. For that reason, in this work GWP100 years for isoflurane and sevoflurane was reevaluated from the atmospheric chemical lifetimes, τOHHAG, derived from the kinetic study of the gas-phase reactions of hydroxyl (OH) radicals with the HAGs and the radiative efficiencies (REs) derived from the (IR) absorption cross sections in the atmospheric window (1500–500 cm−1). The temperature dependence of the OH-rate coefficients (k1(T) for isoflurane and k2(T) for sevoflurane) between 263 and 353 K was determined at 100 Torr by using the pulsed laser photolysis/laser-induced fluorescence technique. The obtained Arrhenius expressions are k1(T) = (1.1 ± 0.5) × 10−13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−(1234 ± 144)/T} and k2(T) = (1.6 ± 0.7) × 10−12
exp{−(1234 ± 144)/T} and k2(T) = (1.6 ± 0.7) × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−(1065 ± 138)/T} cm3 molecule−1 s−1. At 272 K, a τOHHAG of 3.0 years for isoflurane and 1.2 years for sevoflurane were estimated relative to CH3CCl3 from k1 and k2. Moreover, the ultraviolet (UV) absorption cross sections were determined between 190 and 400 nm at 298 K, and the absorption was found to be negligible above 290 nm, indicating minimal photolysis by sunlight. In contrast, the IR absorption in the atmospheric window is significant and the IR absorption cross sections (4000–500 cm−1) were determined by Fourier Transform infrared spectroscopy. The lifetime-corrected radiative efficiencies (REs) were 0.44 and 0.30 W m−2 ppbv−1 for isoflurane and sevoflurane, respectively. From lifetime-corrected REs and τOHHAG, GWP100 years was estimated to be 508 for isoflurane (5% lower than IPCC/WMO values) and 125 for sevoflurane (36% lower than IPCC and 11% lower than WMO). These findings confirm isoflurane to be a high-GWP gas (above 150) according to the EU 2024 regulation, while sevoflurane does not meet the high-GWP threshold. A reassessment of the IPCC and WMO values is recommended.
exp{−(1065 ± 138)/T} cm3 molecule−1 s−1. At 272 K, a τOHHAG of 3.0 years for isoflurane and 1.2 years for sevoflurane were estimated relative to CH3CCl3 from k1 and k2. Moreover, the ultraviolet (UV) absorption cross sections were determined between 190 and 400 nm at 298 K, and the absorption was found to be negligible above 290 nm, indicating minimal photolysis by sunlight. In contrast, the IR absorption in the atmospheric window is significant and the IR absorption cross sections (4000–500 cm−1) were determined by Fourier Transform infrared spectroscopy. The lifetime-corrected radiative efficiencies (REs) were 0.44 and 0.30 W m−2 ppbv−1 for isoflurane and sevoflurane, respectively. From lifetime-corrected REs and τOHHAG, GWP100 years was estimated to be 508 for isoflurane (5% lower than IPCC/WMO values) and 125 for sevoflurane (36% lower than IPCC and 11% lower than WMO). These findings confirm isoflurane to be a high-GWP gas (above 150) according to the EU 2024 regulation, while sevoflurane does not meet the high-GWP threshold. A reassessment of the IPCC and WMO values is recommended.
Environmental significanceThe healthcare sector must reduce its carbon footprint, including emissions from anesthetic gases like isoflurane and sevoflurane, which are released directly into the atmosphere (∼0.1 ppt). To prioritize mitigation efforts, accurate global warming potentials of these gases are essential. This study evaluates their primary degradation pathway in the troposphere—reaction with OH radicals (estimation of atmospheric lifetimes)—and their radiative efficiencies through the experimental IR absorption cross sections in the atmospheric window. Although photolysis in the troposphere is unlikely, UV absorption cross sections (190–400 nm) were analyzed to address discrepancies in existing data. These findings are crucial for understanding the environmental impact of these anesthetics and guiding strategies to minimize their contribution to global warming over 100 years. |
1. Introduction
Inhaled anesthetics are used worldwide for clinical interventions, either in humans or animals, and most of them are eliminated by exhalation (>70%) without being metabolized (0.2–5%).1 In the 1980s, halogenated anesthetic gases (HAGs) replaced nitrous oxide (N2O), used since the XIX century as an anesthetic gas. Its declining role in general anesthesia is driven by a mix of environmental concerns, clinical risks, and the availability of better anesthetics. As the world population keeps growing and modern anesthesia becomes available in more regions of the world, the global use and emissions of HAGs are expected to grow.2 In 2022, more than 3.3 million surgical interventions were performed in Spain according to data released by the Spanish National Health System in 2023.3 Despite the introduction of scavenging systems (based on silica zeolites, for example) in anesthesia machines, most HAGs are still released directly into the atmosphere.2 For example, isoflurane (CF3CHClOCHF2, HCFE-235da2) and sevoflurane ((CF3)2CHOCH2F, HFE-347mmz1) (Fig. 1) presented atmospheric abundances in 2014 of around 0.097 and 0.13 ppt, respectively.1 In 2019, inhaled anesthetics were responsible for around 5% of the healthcare-related climate footprint in England.4,5 | ||
| Fig. 1 Chemical structures of (A) isoflurane (CF3CHClOCHF2, HCFE-235da2) and (B) sevoflurane ((CF3)2CHOCH2F, HFE-347mmz1). | ||
Even though the use of fluorinated gases (F-gases) in the European Union (EU) declined by 37% in metric tons and by 47% in terms of tons of CO2 equivalent from 2015 to 2019, the EU commission presented a new regulation on emission prevention measures of F-gases, including isoflurane in 2024.6 This EU regulation defines a high global warming potential (GWP) relative to CO2 to values above 150. For isoflurane, the International Panel on Climate Change (IPCC)7 and the World Meteorological Organization (WMO)8 recommend a GWP at a horizon time of 100 years (GWP100years) of 539 and 536, respectively. So, according to the EU regulation isoflurane is a potent greenhouse gas with higher GWP100 years than N2O (GWP100 years = 273).7 However, N2O remains a major contributor to climate change due to its widespread use.9 In contrast, the recommended GWP100 years for sevoflurane varies depending on the source (GWP100 years = 195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and GWP100 years = 140
7 and GWP100 years = 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) and it falls under the scope of the EU regulation or not.
8) and it falls under the scope of the EU regulation or not.
For long-lived species, the atmospheric lifetime (τ) is often estimated from the OH radical (τOH) reactivity relative to that of methyl chloroform (CH3CCl3) at a mean tropospheric temperature of 272 K. As the main atmospheric fate of these HAGs is the reaction with hydroxyl (OH) radicals, τOHHAG of isoflurane and sevoflurane directly impacts the GWP calculation. Sulbaek Andersen et al.10 first used an estimation of the rate coefficients for the OH-reactions for isoflurane (reaction (R1)) and sevoflurane (reaction (R2)) at 272 K to estimate τOHHAG. These authors derived k1(273 K) and k2(273 K) from calculated Arrhenius parameters using the method described by DeMore.11
| OH + CF3CHClOCHF2 → products k1(T) | (R1) |
| OH + (CF3)2CHOCH2F → products k2(T) | (R2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and k2(T) in the range of 241–298 K,13 GWP100 years was recalculated by Sulbaek Andersen et al.13 For isoflurane, no change in GWP100 years (=510) was reported. However, these authors recommended in 2023
12 and k2(T) in the range of 241–298 K,13 GWP100 years was recalculated by Sulbaek Andersen et al.13 For isoflurane, no change in GWP100 years (=510) was reported. However, these authors recommended in 2023![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 a GWP100 years of 539 as reported by the IPCC (2023).7 For sevoflurane, the recalculated GWP100 years changed from 210 to 130 (∼40% reduction).13 Nevertheless, a new correction of GWP100 years was reported by Sulbaek Andersen et al.15 based on the current JPL16 recommendation and the updated GWP calculation method,17 changing from 130 to 144 (∼10% increase). The variability of GWP100 years in these studies is mainly due to the different methods used for calculating REs and τOHHAG, estimated from the rate coefficients. For isoflurane and sevoflurane, lifetime-corrected REs do not differ greatly. Therefore, the difference in reported GWPs may be due to τOHHAG estimated from the rate coefficients, highlighting the importance of measuring them. Additionally, for sevoflurane, it is crucial to determine whether it falls within the scope of the EU regulation,6 which imposes some limitations on F-gases with a GWP100 years > 150.
14 a GWP100 years of 539 as reported by the IPCC (2023).7 For sevoflurane, the recalculated GWP100 years changed from 210 to 130 (∼40% reduction).13 Nevertheless, a new correction of GWP100 years was reported by Sulbaek Andersen et al.15 based on the current JPL16 recommendation and the updated GWP calculation method,17 changing from 130 to 144 (∼10% increase). The variability of GWP100 years in these studies is mainly due to the different methods used for calculating REs and τOHHAG, estimated from the rate coefficients. For isoflurane and sevoflurane, lifetime-corrected REs do not differ greatly. Therefore, the difference in reported GWPs may be due to τOHHAG estimated from the rate coefficients, highlighting the importance of measuring them. Additionally, for sevoflurane, it is crucial to determine whether it falls within the scope of the EU regulation,6 which imposes some limitations on F-gases with a GWP100 years > 150.
In the literature, several kinetic studies on reactions (R1) and (R2) have been found, especially at around room temperature (293–300 K). Particularly, the kinetics of reaction (R1) has been widely investigated by absolute13,18–20 and relative kinetic techniques,12,19,21,22 determining k1(298 K) at low pressures (2.0–6.3 Torr) using the discharge flow-resonance fluorescence (DF-RF) technique. At higher total pressures (50 mbar ≡ 37.5 Torr), Langbein et al.20 used the laser long-path absorption (LLPA) technique to monitor OH radicals and, more recently, Sulbaek Andersen et al.13 employed the pulsed laser photolysis-laser induced fluorescence (PLP-LIF) technique to determine k1(298 K) at 111 Torr. At atmospheric pressure (760 Torr), Nolan et al.21 determined k1(298 K) using a relative method using gas chromatograph coupled to a flame ionization detector (GC/FID) and Fourier Transform Infrared (FTIR) spectroscopy as detection techniques of the anesthetics and the reference compound. Between 2.0 and 760 Torr, no pressure dependence of k1(298 K) was observed.
Regarding the temperature dependence of k1(T), there are two absolute kinetic studies in the literature. Tokuhashi et al.12 combined DF and photolysis methods coupled to RF detection of OH radicals to determine k1(T) between 250 and 430 K in the 5–60 Torr interval, whereas Beach et al.22 measured k1(T) above 293 K up to 393 K. The observed temperature dependencies of k1(T) by these two groups are not in agreement. Activation energies (Ea) and pre-exponential factors (A) obtained by Beach et al.22 (Ea = 7.8 ± 0.8) kJ mol−1 and A = (4.5 ± 1.3) × 10−12 cm3 molecule−1 s−1 and Tokuhashi et al.12 (Ea = 10.6 ± 0.8) kJ mol−1 and A = (1.12 ± 0.18) × 10−12 cm3 molecule−1 s−1 differ by more than 25% and 75%, respectively. The Arrhenius parameters Ea and A are essential to accurately derive the rate coefficient at 272 K, an appropriate temperature to estimate the atmospheric lifetime of long-lived species, such as the HAGs. Therefore, an additional kinetic study on the temperature dependence of k1(T) is needed.
The rate coefficient for the reaction (R2) has been widely investigated at room temperature, k2(298 K), by absolute methods.13,18–20 No agreement is found among the results of the four previous kinetic studies for reaction (R2). The reported k2(298 K) range from 2.7 to 7.3 × 10−14 cm3 molecule−1 s−1, so it is needed to perform an additional study to elucidate which k2(T) is correct. The only comprehensive kinetic study of k2(T) as a function of temperature was performed by Sulbaek Andersen et al.13 by PLP-LIF between 298 and 241 K at 111 Torr and at 243 K and 300 Torr. The temperature dependence study of k2(T) is recommended to be extended to higher temperatures also to gain insight into the kinetic behavior of sevoflurane. For that reason, in this work, we revisited the temperature dependencies of k1(T) and k2(T) between 253 and 423 K at 100 Torr of helium using the PLP-LIF technique.
Ultraviolet (UV) photolysis of hydrofluoroethers, like isoflurane and sevoflurane, in the solar actinic region (λ > 290 nm) is not expected to occur. However, these species strongly absorb in the vacuum UV (VUV) region and the UV spectra of isoflurane and sevoflurane have been reported at 298 K between 115 and 350 nm.20,23 Particularly, Langbein et al.20 recorded the UV spectra of HAGs in the wavelength range of 200–350 nm, providing the absorption cross sections (in base 10) between 200 and 230 nm. These authors found that isoflurane and sevoflurane do not absorb at wavelengths longer than 215 nm and 200 nm, respectively. More recently, Lange et al.23 recorded the high-resolution (0.1 nm) VUV spectra using synchrotron radiation over the 115–248 nm range, corresponding to photon energies between 5.0 and 10.8 eV. These authors reported the VUV absorption cross sections in megabarn, 1 Mb ≡ 10−18 cm2, and they were compiled (in base e) between 115 and 340 nm in the MPI-Mainz UV/vis spectral atlas.24 However, after scaling the reported UV absorption cross sections to the same base (base e) there is a disagreement, especially for sevoflurane, between the studies of Langbein et al.20 and Lange et al.23 above 200 nm. Therefore, in this work, the absolute UV absorption cross sections were also determined between 190 and 400 nm at 298 K. Langbein et al.20 and Lange et al.23 also calculated the photochemical atmospheric lifetimes of isoflurane and sevoflurane up to 36 km and up to 50 km, respectively, assuming a photolysis quantum yield of 1. These authors concluded that these HAGs were not degraded in the troposphere and stratosphere by photolysis.
As stated above, in addition to the OH-kinetics, the infrared (IR) spectra of isoflurane and sevoflurane are crucial to properly assess the GWP calculation of these HAGs. Currently, there is no agreement between the studies of Brown et al.18,19 and Sulbaek Andersen et al.10 Brown et al.18 recorded the IR spectra of isoflurane and sevoflurane between 1200 and 600 cm−1 at a resolution of 2.40 cm−1. The IR absorption cross sections are systematically lower than those reported by Sulbaek Andersen et al.10 who reported them between 2000 and 650 cm−1 at a much higher resolution, 0.25 cm−1. Therefore, the present work may elucidate this discrepancy by determining the absolute IR absorption cross sections of these HAGs between 500 and 4000 cm−1 at a resolution of 0.5 cm−1. In addition, integrated IR absorption cross sections in the range of 800–1200 cm−1 and 650–1500 cm−1 are reported here for both HAGs for comparing with those from Sulbaek Andersen et al.10 and Brown et al.19
2. Experimental section
2.1. Absolute gas-phase kinetics of OH-reactions
The experimental system used in this work to determine the second-order rate coefficient, ki(T) (i = 1 or 2), was described elsewhere.25–27 Briefly, the OH radicals were generated in situ in a jacketed Pyrex® reactor (V∼200 cm3) by PLP at 248 nm of a OH-precursor, either hydrogen peroxide (H2O2) or nitric acid (HNO3). UV radiation was provided by using a KrF excimer laser (Coherent, mod. ExciStar 200). The OH radicals were excited at 282 nm by the second harmonic of a rhodamine-6G dye laser (LiopTech, mod. LiopStar) pumped by the second harmonic of an Nd-YAG laser (InnoLas, mod. SpitLight 1200). Laser induced fluorescence from excited OH radicals was collected at around 310 nm as a function of the reaction time.All gases were introduced into the reactor by calibrated mass flow controllers (MFCs). As the investigated reactions are very slow, large mixing ratios of the HAG in helium (f = pHAG/750 Torr, where pHAG = 70–80 Torr) were needed (f = (9.2 × 10−2 − 1.2 × 10−1), as shown in Tables S1 and S2 of the ESI.† As the HAG/He mixture had no similar properties (density, thermal conductivity and heat capacity) compared to the gases used in the factory calibration (He among others), the MFC had to be calibrated for each HAG/He mixture used in the kinetic experiments. Examples of the calibration of the mass flow rate of HAG/He mixtures are shown in Fig. S2.† Tables S1 and S2† also summarize the mass flow rates employed in the experiments at the investigated temperature (253–423 K) for HAG/He (FHAG/He), OH-precursor/He (FPrec) and He (FHe). By changing FR and the total flow rate, [HAG] in the reactor was varied ([isoflurane] = (0.18 − 6.91) × 1016 molecules cm−3 and [sevoflurane] = (0.17 − 6.88) × 1016 molecules cm−3). In addition to the mass flow controller calibration, the mixing ratio of isoflurane and sevoflurane was checked off-line by recording IR spectra as a function of the HAG mass flow rate and using the IR absorption cross sections determined in this work (more details in the ESI†).
Under pseudo-first order conditions (i.e. HAG in large excess with respect to OH radicals), the LIF signal (ILIF) follows a single exponential function. Some examples of the linearized temporal evolution plots of ILIF at several temperatures are shown in Fig. S4.† For a given HAG concentration, [HAG], and temperature, the pseudo-first order coefficient (k′) is obtained from the slope of the corresponding linearized ILIF decay. The total pressure was set to 100 Torr of helium. Under these conditions, there is a linear relationship between k′ and [HAG], as shown in eqn (E1).
 | (E1) |
 ). The individual second-order rate coefficients, ki(T) were determined from
). The individual second-order rate coefficients, ki(T) were determined from  vs. [HAG] plots. Fig. S5† shows examples of plots of eqn (E1) for the OH-reaction at room temperature and at the minimum and maximum temperatures investigated in this work, 253 K and 423 K and at a total pressure of 100 Torr.
vs. [HAG] plots. Fig. S5† shows examples of plots of eqn (E1) for the OH-reaction at room temperature and at the minimum and maximum temperatures investigated in this work, 253 K and 423 K and at a total pressure of 100 Torr.
The presence of reactive impurities may affect the measured rate coefficient. In the present work, the purity of the HAG samples was investigated by GC (Shimadzu, GC-2010 Plus) coupled to a time-of-flight mass spectrometer (ToF-MS, Jeol, AccuTOF GCv) for peak identification. The GC used is equipped with an Equity-1701 column (30 m × 0.32 mm I.D., 1 μm) and the gaseous sample (1 μL) was injected into the GC with a temperature ramp set as 35 °C for 2 min, then 5 °C min−1 to 100 °C, 10 °C min−1 to 200 °C, and 25 °C min−1 to 250 °C. In both HAG samples, the chromatogram did not show other peaks. A purity of >99.8% for both HAGs was estimated from the ratio of the peak intensity and the baseline of the chromatogram.
2.2. IR and UV absorption spectroscopy in the gas-phase
The experimental set-ups have been already described in previous studies.27–29 The IR absorption spectra of the HAGs were recorded between 4000 and 500 cm−1 using a FTIR spectrometer (Bruker, Tensor 27). The instrumental resolution of the FTIR spectrometer was set to 0.5 cm−1 corresponding with a data spacing of around 0.24 cm−1. A single path stainless steel cell was used to measure the absorbance at each wavenumber (A
was used to measure the absorbance at each wavenumber (A![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) under static conditions. The absorption cell was filled with pure isoflurane (0.8–2.8 Torr) or sevoflurane (1.4–3.3 Torr) at room temperature, yielding concentration ranges of (2.46 − 9.20) × 1016 and (0.46 − 1.08) × 1017 molecules cm−3, respectively.
) under static conditions. The absorption cell was filled with pure isoflurane (0.8–2.8 Torr) or sevoflurane (1.4–3.3 Torr) at room temperature, yielding concentration ranges of (2.46 − 9.20) × 1016 and (0.46 − 1.08) × 1017 molecules cm−3, respectively.
The UV absorption spectroscopy system consists of a UV jacketed Pyrex® cell  . The UV spectra of isoflurane and sevoflurane between 190 and 400 nm were recorded under static conditions at room temperature. An instrumental resolution of 3 nm yields a data spacing of 0.5 nm. The experiments were carried out by introducing into the UV cell a certain pressure of the HAG ((5.5–35.0 Torr) of isoflurane and (6.0–46.9 Torr) of sevoflurane), yielding concentration ranges of [isoflurane] = (0.18 − 1.14) × 1018 molecules cm−3 and [sevoflurane] = (0.19 − 1.53) × 1018 molecules cm−3.
. The UV spectra of isoflurane and sevoflurane between 190 and 400 nm were recorded under static conditions at room temperature. An instrumental resolution of 3 nm yields a data spacing of 0.5 nm. The experiments were carried out by introducing into the UV cell a certain pressure of the HAG ((5.5–35.0 Torr) of isoflurane and (6.0–46.9 Torr) of sevoflurane), yielding concentration ranges of [isoflurane] = (0.18 − 1.14) × 1018 molecules cm−3 and [sevoflurane] = (0.19 − 1.53) × 1018 molecules cm−3.
From the slope of the Beer–Lambert's plot eqn (E2) at each wavenumber, ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) , the IR absorption cross sections (in base e), σ
, the IR absorption cross sections (in base e), σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) , were derived.
, were derived.
 | (E2) |
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) vs. [HAG] plots are shown for selected wavenumbers, all presenting good linearity in the entire concentration range. In addition to the absolute σ
vs. [HAG] plots are shown for selected wavenumbers, all presenting good linearity in the entire concentration range. In addition to the absolute σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) , the integrated IR absorption cross sections, Sint(
, the integrated IR absorption cross sections, Sint(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 1 −
1 − ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 2) given by eqn (E3) were determined in certain wavenumber ranges by applying Beer–Lambert's law, expressed in terms of the integrated absorbance, Aint (Fig. S6† – panels B and D).
2) given by eqn (E3) were determined in certain wavenumber ranges by applying Beer–Lambert's law, expressed in terms of the integrated absorbance, Aint (Fig. S6† – panels B and D).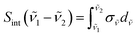 | (E3) |
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) and Sint(
and Sint(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 1 −
1 − ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 2).
2).
In a similar way, the UV absorption cross sections for isoflurane, at a wavelength λ, σλ (in base e), were determined by applying eqn (E2) in terms of λ, i.e. σλ is obtained from the slope of the Aλvs. [HAG] plot (Fig. S7†). In contrast, no absorption was observed for sevoflurane in the 190–400 nm wavelength range.
2.3. Chemicals
Helium (99.999%, Nippon Gases) and an aqueous solution of HNO3 (65% w/w, Scharlab) were used as supplied. The aqueous solution of H2O2 (>50% v/v, Scharlab) was pre-concentrated as described in Albaladejo et al.25 Liquid CF3CHClOCHF2 (95%, Chemspace) and (CF3)2CHOCH2F (95%, Chemspace) were used after degasification by several freeze–pump–thaw cycles.3. Results and discussion
3.1. Temperature dependence of k1(T) and k2(T)
The individual k1(298 K) and k2(298 K) together with the experimental conditions used in the kinetic study are listed as a function of temperature in Tables S1 and S2.† The rate coefficients at a single temperature provided in Table 1 were determined from the average of the rate coefficients obtained from vs. [HAG] plots (Fig. S5†). Over the temperature range investigated, the obtained rate coefficients are relatively low. k1(T) was on the order of 10−15–10−14 cm3 molecule−1 s−1 and k2(T) was about one order of magnitude larger (10−14–10−13 cm3 molecule−1 s−1). At room temperature, for example, k1(298 K) = (1.96 ± 0.41) × 10−14 cm3 molecule−1 s−1 and k2(298 K) = (4.25 ± 0.86) × 10−14 cm3 molecule−1 s−1. Both k1(T) and k2(T) increase with increasing temperature. The difference in ki(253 K) with respect to ki(423 K) is 86% for isoflurane and 81% for sevoflurane.
vs. [HAG] plots (Fig. S5†). Over the temperature range investigated, the obtained rate coefficients are relatively low. k1(T) was on the order of 10−15–10−14 cm3 molecule−1 s−1 and k2(T) was about one order of magnitude larger (10−14–10−13 cm3 molecule−1 s−1). At room temperature, for example, k1(298 K) = (1.96 ± 0.41) × 10−14 cm3 molecule−1 s−1 and k2(298 K) = (4.25 ± 0.86) × 10−14 cm3 molecule−1 s−1. Both k1(T) and k2(T) increase with increasing temperature. The difference in ki(253 K) with respect to ki(423 K) is 86% for isoflurane and 81% for sevoflurane.
| T/K | k 1(T)/10−14 cm3 molecule−1 s−1 | k 2(T)/10−14 cm3 molecule−1 s−1 |
|---|---|---|
| 253 | 0.80 ± 0.17 | 2.53 ± 0.51 |
| 263 | 1.02 ± 0.22 | 2.76 ± 0.59 |
| 273 | 1.17 ± 0.23 | 2.89 ± 0.68 |
| 283 | 1.39 ± 0.28 | 3.54 ± 0.73 |
| 298 | 1.96 ± 0.41 | 4.25 ± 0.86 |
| 323 | 2.84 ± 0.61 | 5.78 ± 1.33 |
| 353 | 3.49 ± 0.77 | 7.85 ± 1.75 |
| 393 | 4.45 ± 1.18 | 10.1 ± 2.0 |
| 423 | 5.47 ± 1.59 | 13.3 ± 3.1 |
A weighted fit of the rate coefficients to the Arrhenius expressions yields eqn (E4) and (E5):
k1(T) = (1.1 ± 0.5) × 10−13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−(1234 ± 144)/T} cm3 molecule−1 s−1 exp{−(1234 ± 144)/T} cm3 molecule−1 s−1 | (E4) |
k2(T) = (1.6 ± 0.7) × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−(1065 ± 138)/T} cm3 molecule−1 s−1 exp{−(1065 ± 138)/T} cm3 molecule−1 s−1 | (E5) |
There are two previous studies on the temperature dependence of k1(T). Tokuhashi et al.12 (eqn (E6), blue line in Fig. 2A) and Beach et al.22 (eqn (E7), red line in Fig. 2A) reported the following Arrhenius expressions (uncertainties ±2σ).
k1(T = 250 − 430 K) = (1.12 ± 0.36) × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−(1280 ± 100)/T} cm3 molecule−1 s−1 exp{−(1280 ± 100)/T} cm3 molecule−1 s−1 | (E6) |
k1(T = 293 − 393 K) = (4.5 ± 1.3) × 10−13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−(940 ± 100)/T} cm3 molecule−1 s−1 exp{−(940 ± 100)/T} cm3 molecule−1 s−1 | (E7) |
k2(T = 302, 423 K) = 1.53 × 10−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−(900 ± 500)/T} cm3 molecule−1 s−1 exp{−(900 ± 500)/T} cm3 molecule−1 s−1 | (E8) |
k2(T = 241 − 298 K) = (9.98 ± 3.24) × 10−13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−(969 ± 82)/T} cm3 molecule−1 s−1 exp{−(969 ± 82)/T} cm3 molecule−1 s−1 | (E9) |
Table 2 shows a comparison of the Arrhenius parameters obtained in this work with those from the literature. If uncertainties in reported A and Ea for reactions (R1) and (R2) are considered, the results reported in this work are in agreement with those determined by Beach et al.22 and Tokuhashi et al.12 for (R1) and from Brown et al.19 and Sulbaek Andersen et al.13 for reaction (R2). Current JPL16 recommendations (brown line in Fig. 2A) for the Arrhenius parameters for reaction (R1) are A = 1.1 × 10−12 cm3 molecule−1 s−1 and Ea/R = 1275 K in the 250–430 K range (with parameters f(298 K) = 1.07 and g = 50 that can be used to calculate an estimated rate constant uncertainty at any given temperature). The recommended value for Ea/R is derived from the fit to the two temperature dependent data sets of Tokuhashi et al.12 For the OH + sevoflurane reaction, the Arrhenius expression recommended by JPL (brown line in Fig. 2B) is based on the absolute rate coefficients from Sulbaek Andersen et al.13 The recommended Arrhenius parameters were Ea/R = 960 K and A = 8.77 × 10−13 cm3 molecule−1 s−1 in the 241–422 K range (f(298 K) = 1.15; g = 100).
| Anesthetic | T/K | P/Torr | k i (298 K)/10−14 cm3 molecule−1 s−1 | A/10−12 cm3 molecule−1 s−1 | (Ea/R)/K | E a/kJ mol−1 | Techniqueb | Reference |
|---|---|---|---|---|---|---|---|---|
| a k 1(293 K). b PLP-LIF: pulsed laser photolysis-laser induced fluorescence; DF-RF: discharge flow resonance fluorescence; FP: flash photolysis; RR: relative rate; GC-FID: gas chromatography-flame ionization detection; FTIR: fourier transform infrared spectroscopy; LLPA: laser long-path absorption. | ||||||||
| Isoflurane | 253–423 | 100 | 1.96 ± 0.41 | 1.1 ± 0.5 | 1234 ± 144 | 10.3 ± 1.2 | PLP-LIF | This work |
| 295 ± 4 | 111 | 1.5 ± 0.2 | PLP-LIF | Sulbaek Andersen et al.13 | ||||
| 293–393 | 2.0–3.4 | 1.9 ± 0.2a | 4.5 ± 1.3 | 940 ± 100 | 7.8 ± 0.8 | DF-RF | Beach et al.22 | |
| 250–430 | 20–40 | 1.48 ± 0.12 | 1.12 ± 0.36 | 1280 ± 100 | 10.6 ± 1.6 | FP-LIF | Tokuhashi et al.12 | |
| 20–40 | 1.48 ± 0.14 | PLP-LIF | Tokuhashi et al.12 | |||||
| 5–6 | 1.63 ± 0.16 | DF-LIF | Tokuhashi et al.12 | |||||
| 760 | 2.3 ± 0.2 | RR | Nolan et al.21 | |||||
| GC-FID and FTIR | ||||||||
| 298 | 37.5 | 1.7 ± 0.3 | PLP-LLPA | Langbein et al.20 | ||||
| 298 | 3–6.3 | 2.1 ± 0.7 | DF-RF | Brown et al.19 | ||||
| Sevoflurane | 253–423 | 100 | 4.25 ± 0.86 | 1.6 ± 0.7 | 1065 ± 138 | 8.9 ± 1.2 | PLP-LIF | This work |
| 241–298 | 111 | 3.9 ± 0.3 | 0.998 ± 0.324 | 969 ± 82 | 8.1 ± 0.7 | PLP-LIF | Sulbaek Andersen et al.13 | |
| 295 ± 4 | 700 | 3.5 ± 0.7 | RR/FTIR | Sulbaek Andersen et al.13 | ||||
| 298 | 37.5 | 2.7 ± 0.5 | PLP-LLPA | Langbein et al.20 | ||||
| 302, 423 | 1.9 | 7.3 ± 2.2 | 1.53 | 900 ± 500 | 7.5 ± 4.2 | DF-RF | Brown et al.19 | |
For isoflurane, no pressure dependence of k1(298 K) is observed, within the stated uncertainties, independent of the kinetic technique. k1(298 K) is in reasonable agreement with previously reported k1(298 K), especially considering that measuring very low rate coefficients, such as k1, is absolutely challenging. k1(298 K) reported in this work is in good agreement, within the error limits, with k1(298 K) reported by Brown et al.,19 Beach et al.,22 and Langbein et al.20k1(298 K) determined in this work is slightly lower than the relative k1(298 K) reported by Nolan et al.,21 whereas it is higher than absolute k1(298 K) determined by Tokuhashi et al.12 and Sulbaek Andersen et al.13
For sevoflurane, there is a good agreement between k2(298 K) determined in this work and that reported by Sulbaek Andersen et al.13 by PLP-LIF (111 Torr) and RR/FTIR (700 Torr). In contrast, k2(298 K) determined in this work is much higher than that reported by Langbein et al.20 The opposite is observed when compared with Brown et al.'s 19 work, and k2(298 K) reported in this work is lower. This was also observed by Sulbaek Andersen et al.13 and was attributed to the presence of reactive impurities.
As stated in the Experimental section, in the present work an upper limit of 0.2% for the concentration of a potential impurity in the reactor is considered. The contribution of the OH-reaction with the potential impurity has been estimated considering two scenarios. When an OH-rate coefficient for the potential impurity is assumed to be on the order of 10−11 cm3 molecule−1 s−1, as Sulbaek Andersen et al.13 assumed in their work, to obtain k1(298 K) and k2(298 K) consistent with the experimental ones, these rate coefficients would be more than one order of magnitude lower than those reported here and by other authors.13,19,20,22 So, the impurity, if any, is not reacting that fast with OH. When the OH-rate coefficient for the potential impurity is assumed to be below 10−12 cm3 molecule−1 s−1, calculated k1(298 K) and k2(298 K) are within the experimental uncertainties and no effect would be noticeable on the measured k1(298 K) and k2(298 K). Therefore, we are confident in the results presented in Table 1.
The current JPL16 recommendation for k1(298 K) is an average of the results from Langbein et al.,20 Sulbaek Andersen et al.,13 and Tokuhashi et al.12 (1.5 × 10−14 cm3 molecule−1 s−1). The obtained k1(298 K) is somehow higher, as the recommended value is essentially that reported by Sulbaek Andersen et al.13 For sevoflurane, the JPL16 recommended value for k2(298 K) = 3.5 × 10−14 cm3 molecule−1 s−1 is an average of the values from the relative rate and absolute measurements of Sulbaek Andersen et al.,13 with which this work is in agreement.
3.2. Infrared and ultraviolet absorption cross sections
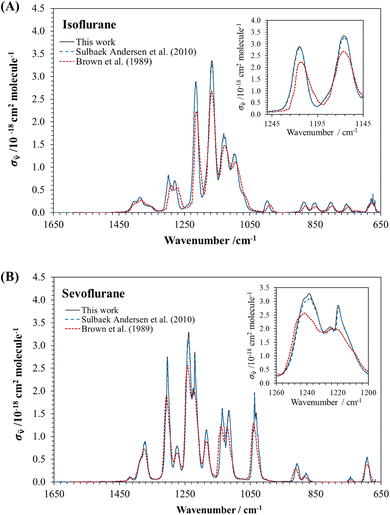
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) values for isoflurane and sevoflurane at 1 cm−1 intervals between 500 and 4000 cm−1. The IR spectra obtained between 650 and 1650 cm−1 for the investigated HAGs are depicted as black lines in Fig. 3, while IR spectra previously reported in the literature are plotted as dashed lines. As Brown et al.18 did not list absolute σ
values for isoflurane and sevoflurane at 1 cm−1 intervals between 500 and 4000 cm−1. The IR spectra obtained between 650 and 1650 cm−1 for the investigated HAGs are depicted as black lines in Fig. 3, while IR spectra previously reported in the literature are plotted as dashed lines. As Brown et al.18 did not list absolute σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) for isoflurane and sevoflurane, the IR spectra reported in Fig. 1 in ref. 18 have been digitalized and converted into σ
for isoflurane and sevoflurane, the IR spectra reported in Fig. 1 in ref. 18 have been digitalized and converted into σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (in base e, as in this work) for comparison purposes. Above 2950 cm−1, isoflurane and sevoflurane present negligible absorption and a very weak band in the 2950–3100 cm−1 range. Hence, this portion of the spectra is not shown in Fig. 3.
(in base e, as in this work) for comparison purposes. Above 2950 cm−1, isoflurane and sevoflurane present negligible absorption and a very weak band in the 2950–3100 cm−1 range. Hence, this portion of the spectra is not shown in Fig. 3.
In Table S3,† the position and value of the maximum absorption cross section, σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ,max, are listed together with those reported in the literature. The maximum absorption peak for isoflurane and sevoflurane is located at 1166.3 cm−1 and 1238.7 cm−1, respectively, which is in agreement with those reported in the literature. In general, we observe a good agreement with the IR spectra reported by Sulbaek Andersen et al.10 in the entire wavenumber range. The largest difference was observed in σ
,max, are listed together with those reported in the literature. The maximum absorption peak for isoflurane and sevoflurane is located at 1166.3 cm−1 and 1238.7 cm−1, respectively, which is in agreement with those reported in the literature. In general, we observe a good agreement with the IR spectra reported by Sulbaek Andersen et al.10 in the entire wavenumber range. The largest difference was observed in σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ,max. Our reported values of σ
,max. Our reported values of σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ,max are ca. 3% higher than those reported by Sulbaek Andersen et al.10 for isoflurane and ca. 5% higher for sevoflurane. However, this difference remains within the experimental uncertainties. With respect to σ
,max are ca. 3% higher than those reported by Sulbaek Andersen et al.10 for isoflurane and ca. 5% higher for sevoflurane. However, this difference remains within the experimental uncertainties. With respect to σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ,max reported by Brown et al.,18 there is a difference of ca. 20% when the value obtained in this work is compared. However, this difference in σ
,max reported by Brown et al.,18 there is a difference of ca. 20% when the value obtained in this work is compared. However, this difference in σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) values is observed in the entire wavenumber range. This discrepancy can be due to the lower instrumental resolution used by Brown et al.18 since the digitalization process of the IR spectra may account for less than 2% uncertainty in σ
values is observed in the entire wavenumber range. This discrepancy can be due to the lower instrumental resolution used by Brown et al.18 since the digitalization process of the IR spectra may account for less than 2% uncertainty in σ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) values. This was confirmed by digitalizing our IR spectra from Fig. 3. No change in the position of the bands was observed either.
values. This was confirmed by digitalizing our IR spectra from Fig. 3. No change in the position of the bands was observed either.
Table S4† summarizes Sint(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 1 −
1 − ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 2) over two wavenumber ranges to compare with previous studies. For isoflurane, Sint(800–1200 cm−1) obtained here is higher than that reported by Brown et al.,18 while it is in agreement with that calculated by Sulbaek Andersen et al.,10 within our experimental uncertainties. Similarly, Sint(650–1500 cm−1) from Sulbaek Andersen et al.10 is within the error limits of the present work, since these authors reported an uncertainty of 5%. For sevoflurane both Sint(800–1200 cm−1) and Sint(650–1500 cm−1) reported in the present study are in excellent agreement with those reported by Brown et al.18 and Sulbaek Andersen et al.10
2) over two wavenumber ranges to compare with previous studies. For isoflurane, Sint(800–1200 cm−1) obtained here is higher than that reported by Brown et al.,18 while it is in agreement with that calculated by Sulbaek Andersen et al.,10 within our experimental uncertainties. Similarly, Sint(650–1500 cm−1) from Sulbaek Andersen et al.10 is within the error limits of the present work, since these authors reported an uncertainty of 5%. For sevoflurane both Sint(800–1200 cm−1) and Sint(650–1500 cm−1) reported in the present study are in excellent agreement with those reported by Brown et al.18 and Sulbaek Andersen et al.10
4. Atmospheric implications
4.1. Lifetime calculations
Chemical pathways such as the gas-phase reaction with atmospheric oxidants, like OH radicals and Cl atoms, and UV photolysis may govern the removal of isoflurane and sevoflurane in the atmosphere. The contribution of these processes to the global removal of these HAGs is given by the corresponding individual lifetime (τ).For gases with lifetimes greater than few months, the atmospheric lifetime due to the reaction with OH is usually estimated relative to CH3CCl3 at the tropospheric mean temperature, 272 K.30 For the investigated HAGs, τOHHAG was calculated according to eqn (E10).
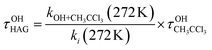 | (E10) |
 is the atmospheric lifetime of CH3CCl3 due to reaction with OH (5.99 years) and kOH+CH3CCl3(272 K) is 6.03 × 10−15 cm3 molecule−1 s−1.31,32 The rate coefficients k1(272 K) = (1.19 ± 0.43) × 10−14 cm3 molecule−1 s−1 and k2(272 K) = (3.11 ± 1.05) × 10−14 cm3 molecule−1 s−1 were derived from eqn (E4) and (E5) determined in the present work. Therefore, the estimated τOHHAG is 3.0 years and 1.2 years for isoflurane and sevoflurane, respectively. As shown in Table 3, current recommendations by WMO8 and IPCC7 (3.7 and 1.5 years, respectively) are higher than τOHHAG estimated in this work and those from Sulbaek Andersen et al.13
is the atmospheric lifetime of CH3CCl3 due to reaction with OH (5.99 years) and kOH+CH3CCl3(272 K) is 6.03 × 10−15 cm3 molecule−1 s−1.31,32 The rate coefficients k1(272 K) = (1.19 ± 0.43) × 10−14 cm3 molecule−1 s−1 and k2(272 K) = (3.11 ± 1.05) × 10−14 cm3 molecule−1 s−1 were derived from eqn (E4) and (E5) determined in the present work. Therefore, the estimated τOHHAG is 3.0 years and 1.2 years for isoflurane and sevoflurane, respectively. As shown in Table 3, current recommendations by WMO8 and IPCC7 (3.7 and 1.5 years, respectively) are higher than τOHHAG estimated in this work and those from Sulbaek Andersen et al.13
| Anesthetic | τ OHHAG/years | REi/W m−2 ppbv−1 | Lifetime-corrected REi/W m−2 ppbv−1 | GWP100 years | Reference |
|---|---|---|---|---|---|
| a Taken from Hodnebrog et al.17 b Taken from Hodnebrog et al.37 c Taken from Sulbaek Andersen et al.10 d See text. | |||||
| Isoflurane | 3.0 | 0.50 | 0.44 | 508 | This work |
| 3.7 | n. r. | 0.43a | 539 | IPCC7 | |
| 3.7 | n. r. | 0.43a | 536 | WMO8 | |
| 3.7 | 0.47b | 0.43 | 565 | Hodnebrog et al.17 | |
| 3.2 | 0.45c | 0.40 | 489 | Sulbaek Andersen et al.13 | |
| 5.1 | n. r. | — | 625 | Langbein et al.20 | |
| 2.0 | n. r. | — | 311 | Brown et al.19 | |
| Sevoflurane | 1.2 | 0.39 | 0.30 | 125 | This work |
| 1.5 | n. r. | 0.31a | 195 | IPCC7 | |
| 1.5 | n. r. | 0.31a | 140 | WMO8 | |
| 1.4 | 127/144d | Sulbaek Andersen et al.15 | |||
| 1.9 | 0.37b | 0.31 | 205 | Hodnebrog et al.17 | |
| 1.1 | 0.35c | 0.27 | 102 | Sulbaek Andersen et al.13 | |
| 4.0 | n. r. | — | 250 | Langbein et al.20 | |
| 0.9 | n. r. | — | 62 | Brown et al.19 | |
Most of the values of τOHHAG presented in Table 3 were estimated using ki(T) at the tropospheric average temperature (272–277 K) (see Table S5† for details). For both HAGs, Brown et al.19 estimated τOHHAG relative to CH3CCl3, while Sulbaek Andersen et al.13 used an average OH concentration of 106 radicals per cm3, in very good agreement with those obtained in our work. These τOHHAG estimated from ki(272–277 K) varies from 2.0 to 3.2 years for isoflurane and from 0.9 to 1.2 years for sevoflurane. As shown in Table 2, Langbein et al.20 reported much longer τOHHAG. This difference is not only attributable to a smaller rate coefficient. In fact, if τOHHAG are estimated relative to CH3CCl3, as was done in our work, and assuming their ki(298 K), the values are not as different as those reported in our work and in the bibliography (τOHHAG = 2.12 years for isoflurane; τOHHAG = 1.33 years for sevoflurane). Therefore, the main difference seems to be the use of the one-dimensional photochemical transport model. In this model, the concentration profile of OH with altitude between 0 and 36 km was considered and a constant value of ki(298 K) was assumed through the troposphere and stratosphere.
The lifetime of isoflurane and sevoflurane due to Cl-reaction (τClHAG) can be estimated from eqn (11), using a 24-h average Cl concentration, [Cl]24h, of 103 cm−3 (ref. 33) and the rate coefficient for the Cl-reaction at room temperature: (4.5 ± 0.8) × 10−15 cm3 molecule−1 s−1 for isoflurane and (1.1 ± 0.1) × 10−13 cm3 molecule−1 s−1 for sevoflurane. 13
 | (E11) |
As shown in Fig. S8,†σλ for isoflurane is very low in the UV solar actinic region (λ > 290 nm) and presents large uncertainties (grey lines) due to this very weak absorption. Sevoflurane presents even lower absorption. Considering the long lifetime of these HAGs, they are likely to be transported to the stratosphere. Overlapping the spectral actinic flux (Fλ) at two altitudes (0 km – troposphere- and 36 km – stratosphere) with the UV spectra of these HAGs, it is expected that photolysis is not an important removal process in the atmosphere, as concluded by Langbein et al.20 These authors averaged the photolysis rate (J) of isoflurane over 24 h and integrated it between 0 and 36 km altitude (z) at a geographical latitude of 50°N in the equinox. To confirm this, the upper limit of J between 0 and 36 km was also estimated here from Fλ taken from the NCAR ACOM TUV model34 for a summer solstice day in a medium latitude city in Spain (39°N). Assuming a photolysis quantum yield of 1 for isoflurane, as Langbein et al.20 did, and considering the uncertainties in σλ, an upper limit of J would be (1.42 ± 10.1) × 10−7 s−1 which yields a lower limit for the lifetime due to photolysis (τPhotoHAG) of (81 ± 578) days. Langbein et al.20 considered σλ between 200 and 230 nm in their calculations reporting a τPhotoHAG of 3130 years for isoflurane. Given the extremely high uncertainties in J and τPhotoHAG, it cannot be conclusively stated that UV photolysis of isoflurane in the troposphere and stratosphere constitutes a significant removal pathway. For sevoflurane, since σλ determined in this work is negligible, within the extremely large uncertainties in the evaluated λ range, it is inappropriate to estimate τPhotoHAG. Langbein et al.20 did not report τPhotoHAG of sevoflurane due to its negligible UV absorption.
4.2. Radiative efficiencies and GWPs
REs (in W m−2 ppbv−1) and GWP100 years of HAGs were calculated using the same methodology as in our previous studies.26 The instantaneous RE (in W m−2 ppbv−1) for isoflurane and sevoflurane was calculated for a 0–1 ppbv increase in mixing ratio from the integrated IR absorption cross sections in 1 cm−1 between 500 and 3000 cm−1 and using the radiative forcing per integrated absorption cross section parameterized in 1-cm−1 intervals, Fσ(in W m−2 (cm−1)−1 (cm2 molecule−1)−1), from Hodnebrog et al.17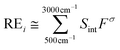 | (E12) |
The obtained instantaneous REs for isoflurane were 0.50 W m−2 ppbv−1 and 0.39 W m−2 ppbv−1 for sevoflurane. These values were corrected with the fractional correction factor (fτ) defined in eqn (E13).35
 | (E13) |
In this work, the lifetime-corrected REs were used to calculate GWP100 years of HAGs using eqn (E14).
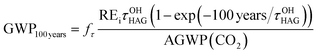 | (E14) |
The values reported by Langbein et al.20 were relative to CFC-12 (CF2Cl2) and those reported by Brown et al.19 were relative to CFC-11 (CFCl3). Therefore, we expressed these GWP100 years relative to CO2 by using GWP100 years for CFC-11 and CFC-12 taken from the IPCC7 (6230 and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, respectively). All GWP100 years listed in Table 3 are then relative to CO2. Although Brown et al.19 reported the infrared integrated absorption cross sections for isoflurane and sevoflurane between 800 and 1200 cm−1, and used them to calculate GWP100 years (311 for isoflurane and 62 for sevoflurane), no RE values were reported and GWP100 years is not lifetime-corrected. Moreover, our GWP100 years for isoflurane and sevoflurane are 63% and 102% higher than that of Brown et al.19 and are much lower than those in the rest of studies due to smaller atmospheric lifetimes (see Table 3). Similarly, Langbein et al.20 calculated GWP100 years using the IR integrated absorption cross section provided by Brown et al.18 and showed larger lifetimes than Brown et al.19 As a consequence, GWP100 years was higher than previously reported by Brown and coworkers (625 for isoflurane and 250 for sevoflurane). If our results are compared with theirs, the reported GWP100 years for isoflurane is 19% lower, while it is half for sevoflurane. This large difference is due to the non-correction of REs with the HAG lifetime. If this lifetime correction is applied to REs reported by Sulbaek Andersen et al.,13 our GWP100 years is 4% lower than theirs for isoflurane (GWP100 years = 489) and 22% higher for sevoflurane (GWP100 years = 102). For sevoflurane, Sulbaek Andersen et al.15 updated GWP100 years calculations considering an atmospheric lifetime of 1.4 years based on the JPL recommendation.16 They determined a GWP100 years of 127 when using AGWP(CO2) from IPCC 2013
500, respectively). All GWP100 years listed in Table 3 are then relative to CO2. Although Brown et al.19 reported the infrared integrated absorption cross sections for isoflurane and sevoflurane between 800 and 1200 cm−1, and used them to calculate GWP100 years (311 for isoflurane and 62 for sevoflurane), no RE values were reported and GWP100 years is not lifetime-corrected. Moreover, our GWP100 years for isoflurane and sevoflurane are 63% and 102% higher than that of Brown et al.19 and are much lower than those in the rest of studies due to smaller atmospheric lifetimes (see Table 3). Similarly, Langbein et al.20 calculated GWP100 years using the IR integrated absorption cross section provided by Brown et al.18 and showed larger lifetimes than Brown et al.19 As a consequence, GWP100 years was higher than previously reported by Brown and coworkers (625 for isoflurane and 250 for sevoflurane). If our results are compared with theirs, the reported GWP100 years for isoflurane is 19% lower, while it is half for sevoflurane. This large difference is due to the non-correction of REs with the HAG lifetime. If this lifetime correction is applied to REs reported by Sulbaek Andersen et al.,13 our GWP100 years is 4% lower than theirs for isoflurane (GWP100 years = 489) and 22% higher for sevoflurane (GWP100 years = 102). For sevoflurane, Sulbaek Andersen et al.15 updated GWP100 years calculations considering an atmospheric lifetime of 1.4 years based on the JPL recommendation.16 They determined a GWP100 years of 127 when using AGWP(CO2) from IPCC 2013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 and 144 when using the updated AGWP(CO2) from Hodnebrog et al.17 GWP100 years recommended in this work agrees with their first updated calculation and is 13% lower than the last one.
36 and 144 when using the updated AGWP(CO2) from Hodnebrog et al.17 GWP100 years recommended in this work agrees with their first updated calculation and is 13% lower than the last one.
GWP100 years for sevoflurane reported here and from the study of Sulbaek Andersen et al.15 is notably lower than current recommendations of GWP100 years by the WMO31 and IPCC.2 Sulbaek Andersen et al.15 provided further evidence that these recommendations need to be modified.
5. Conclusions
A comprehensive study on temperature dependence of the absolute OH-rate coefficients for isoflurane and sevoflurane has been carried out in this work at temperatures above and below 298 K. Our results confirm the observed T-dependencies of k1(T) and k2(T) recommended by JPL,16 expanding the T range of the Sulbaek Andersen et al.13 study on which the recommendation is based. Both reactions present positive low activation energies of around 9 kJ mol−1. The knowledge of the OH-rate coefficients at the average atmospheric temperature (272 K) is essential to accurately calculate the atmospheric chemical lifetime of these inhaled anesthetics. In this work, we report k1(272 K) = 1.31 × 10−14 cm3 molecule−1 s−1 and k2(272 K) = 3.04 × 10−14 cm3 molecule−1 s−1 and used them to derive τOHHAG, 3.0 years for isoflurane and 1.2 years for sevoflurane. These τOHHAG are lower than those currently recommended by the WMO8 and IPCC.7 This directly impacts the resulting lifetime-corrected radiative efficiencies and, therefore, global warming potentials, GWP100 years = 457 and 125 respectively. The obtained values of GWP100 years are also lower than current recommendations. As the calculated lifetime-corrected radiative efficiencies for isoflurane and sevoflurane are in excellent accordance with previous studies, the decrease in τOHHAG led to differences in GWP100 years with respect to the recommended ones by the WMO8 and IPCC.7 For isoflurane, this difference is 5%; however, for sevoflurane, the difference in GWP100 years is 11% with respect to the recommended value by the WMO8 and 36% with respect to the IPCC.7In summary, this work updates the current recommendations of τOHHAG and GWP100 years for isoflurane and sevoflurane, confirming that, according to the EU 2024 regulation,6 isoflurane is a high-GWP gas (GWP100 years > 150), while sevoflurane does not meet the high-GWP threshold. A reassessment of the IPCC and WMO values is recommended.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
S. Espinosa: experiments, formal analysis, writing the original draft, review and editing. F. Martínez: experiments, formal analysis, writing the original draft, writing, review and editing. M. Antiñolo: methodology, formal analysis, review and editing. Ole J. Nielsen: conceptualization, methodology, formal analysis. E. Jiménez: conceptualization, supervision, funding acquisition, formal analysis, review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the INTERESFERA project (Ref. SBPLY/23/180225/000054) funded by the regional government of Castilla-La Mancha (JCCM), the European Regional Development Fund (FEDER) and by the HALOGAS project (Ref. PID2023-146369OB-I00) awarded by the Spanish Ministry of Science, Innovation and Universities (MICIU). This research has also been partially supported by the University of Castilla-La Mancha – UCLM through the Ayudas para la financiación de actividades de investigación dirigidas a grupos (Ref. 2022-GRIN-34143). S. Espinosa also acknowledges the CHEMLIFE project (Ref. PID2020-113936GB-I00 awarded by the Spanish MCIN/AEI) for funding her contract during the performance of this investigation. F. Martínez acknowledges the INVESTIGO program of UCLM funded by JCCM through the European Social Funds Plan of Castilla-La Mancha 2021–2027. We thank Prof. Pilar Martín for helping in the GC/MS analysis of the HAG samples.References
- M. K. Vollmer, T. S. Rhee, M. Rigby, D. Hofstetter, M. Hill, F. Schoenenberger and S. Reimann, Abrupt reversal in emissions and atmospheric abundance of HCFC-133a (CF3CH2Cl), Geophys. Res. Lett., 2015, 42, 1606–1611 CrossRef CAS
.
- Y. Ishizawa, General anesthetic gases and the global environment, Anesth. Analg., 2011, 112, 213–217 CrossRef PubMed
.
-
Ministry of Health, Annual Report of the National Health System 2022 Reports, Studies and Research, 2023 Search PubMed
.
- O. J. Nielsen and M. P. Sulbaek Andersen, Inhalational volatile anaesthetic agents: the atmospheric scientists' viewpoint, Anaesthesia, 2024, 79, 246–251 CrossRef CAS PubMed
.
- I. Tennison, S. Roschnik, B. Ashby, R. Boyd, I. Hamilton, T. Oreszczyn, A. Owen, M. Romanello, P. Ruyssevelt, J. D. Sherman, A. Z. P. Smith, K. Steele, N. Watts and M. J. Eckelman, Health care's response to climate change: a carbon footprint assessment of the NHS in England, Lancet Planet. Health, 2021, 5, 84–92 CrossRef PubMed
.
- Regulation 2024/573, Regulation (EU) 2024/573 of the European Parliament and of the Council of 7 February 2024 on fluorinated greenhouse gases, amending Directive (EU) 2019/1937 and repealing Regulation (EU) No 517/2014 Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species, http://data.europa.eu/eli/reg/2024/573/oj.
- Intergovernmental Panel on Climate Change (IPCC), Climate Change Summary for Policymakers, in Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. Core Writing Team, H. Lee and J. Romero, IPCC, Geneva, Switzerland, pp. , pp. 1–34 Search PubMed.
-
World Meteorological Organization (WMO), Scientific Assessment of Ozone Depletion: 2022: Executive Summary, 2022, p. 56, G. R. No. 278, W. G. S Search PubMed
.
- H. Gadani and A. Vyas, Anesthetic gases and global warming: Potentials, prevention and future of anesthesia, Anesth. Essays Res., 2011, 5, 5–10 CrossRef PubMed
.
- M. P. Sulbaek Andersen, S. P. Sander, O. J. Nielsen, D. S. Wagner, T. J. Sanford and T. J. Wallington, Inhalation anaesthetics and climate change, Br. J. Anaesth., 2010, 105, 760–766 CrossRef CAS PubMed
.
- W. B. DeMore, Regularities in Arrhenius parameters for rate constants of abstraction reactions of hydroxyl radical with C–H bonds, J. Photochem. Photobiol., A, 2005, 176, 129–135 CrossRef CAS
.
- K. Tokuhashi, A. Takahashi, M. Kaise and S. Kondo, Rate constants for the reactions of OH radicals with CH3OCF2CHFCl, CHF2OCF2CHFCl, CHF2OCHClCF3, and CH3CH2OCF2CHF2, J. Geophys. Res. Atmos., 1999, 104, 18681–18688 CrossRef CAS
.
- M. P. Sulbaek Andersen, O. J. Nielsen, B. Karpichev, T. J. Wallington and S. P. Sander, Atmospheric chemistry of isoflurane, desflurane, and sevoflurane: kinetics and mechanisms of reactions with chlorine atoms and OH radicals and global warming potentials, J. Phys. Chem. A, 2012, 116, 5806–5820 CrossRef CAS PubMed
.
- M. P. Sulbaek Andersen, O. J. Nielsen and J. D. Sherman, Assessing the potential climate impact of anaesthetic gases, Lancet Planet. Health, 2023, 7, e622–e629 CrossRef PubMed
.
- M. P. Sulbaek Andersen, O. J. Nielsen and J. D. Sherman, The Global Warming Potentials for Anesthetic Gas Sevoflurane Need Significant Corrections, Environ. Sci. Technol., 2021, 55, 10189–10191 CrossRef PubMed
.
-
J. B. Burkholder, S. P. Sander, J. Abbatt, J. R. Barker, C. Cappa, J. D. Crounse, T. S. Dibble, R. E. Huie, C. E. Kolb, M. J. Kurylo, V. L. Orkin, C. J. Percival, D. M. Wilmouth, and P. H. Wine, Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 19, JPL Publication 19-5, Jet Propulsion Laboratory, Pasadena, 2019, http://jpldataeval.jpl.nasa.gov Search PubMed
.
- Ø. Hodnebrog, B. Aamaas, J. S. Fuglestvedt, G. Marston, G. Myhre, C. J. Nielsen, M. Sandstad, K. P. Shine and T. J. Wallington, Updated Global Warming Potentials and Radiative Efficiencies of Halocarbons and Other Weak Atmospheric Absorbers, Rev. Geophys., 2020, 58, e2019RG000691 CrossRef PubMed
.
- A. C. Brown, C. E. Canosa-Mas, A. D. Parr, J. M. T. Pierce and R. P. Wayne, Tropospheric lifetimes of halogenated anaesthetics, Nature, 1989, 341, 635–637 CrossRef CAS PubMed
.
- A. C. Brown, C. E. Canosa-Mas, A. D. Parr and R. P. Wayne, Laboratory studies of some halogenated ethanes and ethers: Measurements of rates of reaction with OH and of infrared absorption cross-sections, Atmos. Environ., 1990, 24, 2499–2511 CrossRef
.
- T. Langbein, H. Sonntag, D. Trapp, A. Hoffmann, W. Malms, E.-P. Röth, V. Mörs and R. Zellner, Volatile anaesthetics and the atmosphere, Br. J. Anaesth., 1999, 82, 66–73 CrossRef CAS PubMed
.
- S. Nolan, N. O'Sullivan, J. Wenger, H. Sidebottom and J. Treacy, Kinetics and Mechanisms of the OH Radical Initiated Degradation of a Series of Hydrofluoroethers, Trans. Ecol. Environ., 1999, 28, 120–123 Search PubMed
.
- S. D. Beach, K. M. Hickson, I. W. M. Smith and R. P. Tuckett, Rate constants and Arrhenius parameters for the reactions of OH radicals and Cl atoms with CF3CH2OCHF2, CF3CHClOCHF2 and CF3CH2OCClF2, using the discharge-flow/resonance fluorescence method, Phys. Chem. Chem. Phys., 2001, 3, 3064–3069 RSC
.
- E. Lange, F. Ferreira Da Silva, N. C. Jones, S. V Hoffmann, D. Duflot and P. Limão-Vieira, The lowest-lying electronic states of isoflurane and sevoflurane in the 5.0–10.8 eV energy range investigated by experimental and theoretical methods, Chem. Phys. Lett., 2019, 716, 42–48 CrossRef CAS
.
- H. Keller-Rudek, G. K. Moortgat, R. Sander, and R. Sorensen, The MPI-Mainz UV/VIS Spectral Atlas of Gaseous Molecules of Atmospheric Interest, https://essd.copernicus.org/articles/5/365/2013/.
- J. Albaladejo, B. Ballesteros, E. Jiménez, P. Martín and E. Martínez, A PLP-LIF Kinetic Study of the Atmospheric Reactivity of a Series of C4-C7 Saturated and Unsaturated Aliphatic Aldehydes with OH, Atmos. Environ., 2002, 36, 3231–3239 CrossRef CAS
.
-
(a) S. Blázquez, S. Espinosa, M. Antiñolo, J. Albaladejo and E. Jiménez, Kinetics of CF3CH2OCH3 (HFE-263fb2), CHF2CF2CH2OCH3 (HFE-374pcf), and CF3CF2CH2OCH3 (HFE-365mcf3) with OH radicals, IR absorption cross sections, and global warming potentials, Phys. Chem. Chem. Phys., 2022, 24, 14354–14364 RSC
; (b) S. Espinosa, M. Asensio, M. Antiñolo, J. Albaladejo and E. Jiménez, Atmospheric chemistry of CF3CHFCF2OCH3 (HFE-356mec3) and CHF2CHFOCF3 (HFE-236ea1) initiated by OH and Cl and their contribution to global warming, Environ. Sci. Pollut. Res., 2024, 31, 50347–50358 CrossRef CAS PubMed
.
- E. Jiménez, B. Lanza, A. Garzón, B. Ballesteros and J. Albaladejo, Atmospheric Degradation of 2-Butanol, 2-Methyl-2-butanol, and 2,3-Dimethyl-2-butanol: OH Kinetics and UV Absorption Cross Sections, J. Phys. Chem. A, 2005, 109, 10903–10909 CrossRef PubMed
.
- M. Antiñolo, E. Jiménez, A. Notario, E. Martínez and J. Albaladejo, Evaluation of the daytime tropospheric loss of 2-methylbutanal, Atmos. Chem. Phys., 2010, 10, 1911–1922 CrossRef
.
- S. Blázquez, D. González, E. M. Neeman, B. Ballesteros, M. Agúndez, A. Canosa, J. Albaladejo, J. Cernicharo and E. Jiménez, Gas-phase kinetics of CH3CHO with OH radicals between 11.7 and 177.5 K, Phys. Chem. Chem. Phys., 2020, 22, 20562–20572 RSC
.
- C. M. Spivakovsky, J. A. Logan, S. A. Montzka, Y. J. Balkanski, M. Foreman-Fowler, D. B. A. Jones, L. W. Horowitz, A. C. Fusco, C. A. M. Brenninkmeijer, M. J. Prather, S. C. Wofsy and M. B. McElroy, Three-dimensional climatological distribution of tropospheric OH: Update and evaluation, J. Geophys. Res. Atmos., 2000, 105, 8931–8980 CrossRef CAS
.
- M. J. Kurylo and V. L. Orkin, Determination of Atmospheric Lifetimes via the Measurement of OH Radical Kinetics, Chem. Rev., 2003, 102, 5049–5076 CrossRef PubMed
.
- R. G. Prinn, J. Huang, R. F. Weiss, D. M. Cunnold, P. J. Fraser, P. G. Simmonds, A. McCulloch, C. Harth, P. Salameh, S. O'Doherty, R. H. J. Wang, L. Porter and B. R. Miller, Evidence for substantial variations of atmospheric hydroxyl radicals in the past two decades, Science, 2001, 292, 1882–1888 CrossRef CAS PubMed
.
- H. B. Singh, A. N. Thakur, Y. E. Chen and M. Kanakidou, Tetrachloroethylene as an Indicator of low Cl Atom Concentrations in the Troposphere, Geophys. Res. Lett., 1996, 23, 1529–1532 CrossRef CAS
.
-
S. Madronich and S. Flocke, The Role of Solar Radiation in Atmospheric Chemistry, in Environmental Photochemistry, 1999, pp. 1–26 Search PubMed
.
- K. P. Shine, G. Myhre, K. P. Shine and G. Myhre, The spectral nature of stratospheric temperature adjustment and its application to halocarbon radiative forcing, J. Adv. Model. Earth Syst., 2020, 2020(12), e2019MS001951 CrossRef
.
-
T. F. Stocker, G.-K. D. Qin, M. Plattner, S. K. Tignor, J. Allen, A. Boschung, et al., Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental, Cambridge University Press, IPCC, 2013, Cambridge, United Kingdom and New York, NY Search PubMed
.
- Ø. Hodnebrog, M. Etminan, J. S. Fuglestvedt, G. Marston, G. Myhre, C. J. Nielsen, K. P. Shine and T. J. Wallington, Global warming potentials and radiative efficiencies of halocarbons and related compounds: A comprehensive review, Rev. Geophys., 2013, 51, 300–378 CrossRef
.
Footnote |
† Electronic supplementary information (ESI) available: Tables S1 and S2 present the experimental conditions and individual rate coefficients for the OH + CF3CHClOCHF2 (Isoflurane) and OH + (CF3)2CHOCH2F (Sevoflurane) reactions. Tables S3 and S4 show a comparison with literature data of the IR absorption peaks and integrated absorption cross sections for the investigated HAGs. Table S5 presents a summary of the atmospheric lifetimes of HAGs due to the gas-phase reaction with OH radicals estimated in this work and in the literature. Fig. S1 shows a schematic of the introduction of gases in a jacketed reaction cell. Fig. S2 shows examples of the calibration plots of the mass flow controllers used for diluted mixtures of isoflurane and sevoflurane. Fig. S3 presents some examples of the comparison of HAG concentration from flow measurements and from IR spectroscopy. Fig. S4 presents several examples of the temporal evolution of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ILIF in the presence of a similar concentration of the halogenated anesthetics at several temperatures. Fig. S5 shows some examples of the bimolecular plots at 253 K, 298 K, and 423 K at a similar [HAG] (∼1.2 × 1016 molecules cm−3) for (A) isoflurane and (B) sevoflurane. Fig. S6 and S7 present examples of Beer–Lambert's plots at several IR wavenumbers for both HAGs and several UV wavelengths for isoflurane. In Fig. S8 the UV absorption spectrum for isoflurane and the spectral actinic flux are overlapped. See DOI: https://doi.org/10.1039/d5em00061k ILIF in the presence of a similar concentration of the halogenated anesthetics at several temperatures. Fig. S5 shows some examples of the bimolecular plots at 253 K, 298 K, and 423 K at a similar [HAG] (∼1.2 × 1016 molecules cm−3) for (A) isoflurane and (B) sevoflurane. Fig. S6 and S7 present examples of Beer–Lambert's plots at several IR wavenumbers for both HAGs and several UV wavelengths for isoflurane. In Fig. S8 the UV absorption spectrum for isoflurane and the spectral actinic flux are overlapped. See DOI: https://doi.org/10.1039/d5em00061k |
| This journal is © The Royal Society of Chemistry 2025 |