 Open Access Article
Open Access ArticlePer- and poly-fluoroalkyl substances (PFAS) contamination of surface waters by historic landfills via groundwater plumes: ecosystem exposure and downstream mass loading†
J. W.
Roy
 *a,
V. R.
Propp
b,
T.
Hua
b,
S. J.
Brown
a,
C.
Brinovcar
a,
J. E.
Smith
b and
A. O.
De Silva
*a,
V. R.
Propp
b,
T.
Hua
b,
S. J.
Brown
a,
C.
Brinovcar
a,
J. E.
Smith
b and
A. O.
De Silva
 a
a
aWater Science and Technology Directorate, Environment And Climate Change Canada, Canada. E-mail: jim.roy@ec.gc.ca
bSchool of Earth, Environment and Society, McMaster University, Canada
First published on 18th March 2025
Abstract
Many historic landfill sites have groundwater plumes that discharge to nearby surface waters. Recent research indicates that leachate of historic landfills can contain elevated concentrations of per- and polyfluoroalkylated substances (PFAS), but there is limited data on resulting PFAS inputs to aquatic ecosystems as might inform on this potential environmental threat. The objective of this study was to evaluate PFAS exposure in three ecological zones and PFAS mass loading downstream, over 1 year, at two historic landfill sites where landfill plumes discharge to nearby surface waters (1 pond with outlet stream, called HB site; 1 urban stream, called DC site). The three zones experienced different magnitudes and patterns of PFAS concentration exposure (i.e., contaminant presence in the zone). The endobenthic zone of the sediments receiving the landfill plumes experienced the highest concentrations (∑PFAS >4000 ng L−1 (HB) and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 (DC)), often year-round and over a substantial area at each site. Dilution of landfill PFAS in surface waters was observed though concentrations were still elevated (∑PFAS: >120 ng L−1 (HB) and >60 ng L−1 (DC)), with evidence of year-round pelagic zone exposure. PFAS concentrations in the epibenthic zones could vary between that of the endobenthic and pelagic zones, sometimes with daily, event-based, and longer-term patterns. Together these findings suggest historic landfill plumes can lead to substantial PFAS exposure to a variety of aquatic life. Downstream PFAS mass loadings during base flows were relatively small individually (15 (HB) and 36 (DC) g per year (∑PFAS)); however, collective loadings from the numerous historic landfills in a watershed could contribute to increasing PFAS concentrations of connected water bodies, with implications for ecological health, drinking water sources, and fisheries.
000 ng L−1 (DC)), often year-round and over a substantial area at each site. Dilution of landfill PFAS in surface waters was observed though concentrations were still elevated (∑PFAS: >120 ng L−1 (HB) and >60 ng L−1 (DC)), with evidence of year-round pelagic zone exposure. PFAS concentrations in the epibenthic zones could vary between that of the endobenthic and pelagic zones, sometimes with daily, event-based, and longer-term patterns. Together these findings suggest historic landfill plumes can lead to substantial PFAS exposure to a variety of aquatic life. Downstream PFAS mass loadings during base flows were relatively small individually (15 (HB) and 36 (DC) g per year (∑PFAS)); however, collective loadings from the numerous historic landfills in a watershed could contribute to increasing PFAS concentrations of connected water bodies, with implications for ecological health, drinking water sources, and fisheries.
Environmental significanceThis work demonstrates that historic landfills can be a threat of per- and polyfluoroalkylated substances (PFAS) contamination to surface waters through leachate-impacted groundwater plumes. It considers commonly-measured PFAS (i.e., short-and long-chain perfluoroalkylated acids), but also several rarely-measured ultra-short-chain PFAS, adding to limited information on these at landfill sites. The study further illustrates, assesses, and compares the magnitudes and spatiotemporal variations in concentrations that may impact three different ecological zones (endobenthic, epibenthic, pelagic) for lentic and lotic systems. It also provides estimates of PFAS mass loading downstream and how the cumulative loadings from historic landfills compares to some other measured sources. These findings should inform PFAS management of historic landfills. |
1. Introduction
Per- and polyfluoroalkylated substances (PFAS; a group of thousands of fluorinated chemicals) have become a major concern for human health and the environment over the past few decades (see reviews (ref. 1 and 2)), with widespread occurrence in human blood serum (e.g., (ref. 3 and 4)) and in wildlife (e.g., (ref. 5)). Many PFAS are mobile in the environment and have been detected routinely in precipitation, surface waters, groundwaters, and drinking water, particularly near point sources.6 Furthermore, many of the PFAS are highly recalcitrant or (if PFAS-precursors themselves) will degrade to other such PFAS under natural conditions.7 Many PFAS have also been shown to bioaccumulate in fish and other organisms.2 As a result of these concerns, the use of several PFAS has been phased out or restricted in many countries over the past two decades.8Municipal landfill leachate is a proven source of PFAS (reviewed in ref. 9), with potential contributions from many household and office materials (e.g., cleaning products, personal care products, non-stick cookware, carpet, upholstery, treated fabrics and paper, etc.;10) and biosolids from wastewater treatment facilities.11 The use of PFAS-containing firefighting foam at landfill fires is another potential source. Some municipal landfills may have also received PFAS from hazardous sources; this may be especially applicable to historic landfills (here defined as closed >25 years) given lax landfilling regulations in the past. Several studies over the past decade have shown that historic landfills can be a source of PFAS to surrounding groundwater (e.g., ref. 12–17), though elevated concentrations were only found for landfills that closed after the 1950s by Propp et al.15 This follows the years when PFAS was first introduced commercially, possibly sometime in the 1940s.16 Landfill-impacted groundwater plumes may extend at least 3 km,17 while PFAS contamination in drinking water wellfields has been linked to a source >10 km away.18 Such plumes thus pose a threat of contamination to nearby surface waters and those further afield through mass loading and subsequent broader circulation and downstream transport.
Arguably, the threat to surface waters from contaminated groundwater is greater for historic than modern landfills, for several reasons. While leachate containment infrastructure of modern landfills can fail and allow groundwater contamination to occur, historic landfills are especially prone to this problem as these often lack engineered liners and leachate collection systems. The plumes from historic landfills will typically have had more time to travel, allowing them to reach greater distances. Historic landfills are also ubiquitous across the urban and rural landscape in many countries (e.g., comprise the majority of >100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 landfills in the U.S.;19), and are often located close to surface waters.20 Furthermore, some small, informal, or very old landfills may be unmanaged, allowing unrestricted transport off-site. However, even regulation-standard management of historic landfills may be ineffective for preventing PFAS transport to nearby surface waters, given that PFAS are rarely monitored at historic landfill sites.
000 landfills in the U.S.;19), and are often located close to surface waters.20 Furthermore, some small, informal, or very old landfills may be unmanaged, allowing unrestricted transport off-site. However, even regulation-standard management of historic landfills may be ineffective for preventing PFAS transport to nearby surface waters, given that PFAS are rarely monitored at historic landfill sites.
Currently, there is little field data demonstrating or assessing the threat posed by groundwater plumes from historic landfills for PFAS loading to nearby surface waters and the resulting exposure to their aquatic ecosystems. Here, even just the presence of PFAS within specific aquatic ecological zones (e.g., endobenthic, epibenthic, pelagic) is considered as evidence of exposure, noting no studies have organism-based data of exposure. This limited information includes several porewater samples collected at the edge of a surface water body (reflecting discharging groundwater) from a few of the 20 historic landfill sites reported by Propp et al.15 Subsequently, Quan et al.21 and Walsh and Woods22 detected elevated PFAS concentrations in stream waters that are adjacent landfill sites (predominantly active landfills), but with no groundwater measurements. And just recently, McFarlan and Lemke17 published a study of an historic landfill plume, delineated through groundwater wells and numerical modeling, impacting several ephemeral ponds used to rear fish. Sampling found PFAS in the pond waters (up to 60 ng L−1) and in several adjacent streams. Additionally, to the best of our knowledge, only four published studies have reported field measurements of groundwater transport of PFAS to surface waters for other (non-landfill) PFAS sources.23–26 None of these eight studies (landfill or other sources) focused on PFAS exposures occurring to multiple aquatic zones, but for that of Tokranov et al.,24 who measured porewater (15–100 cm depth; endobenthic zone, at least in part) and surface water (20 cm above the pond bottom; pelagic zone) for flow through lakes affected by PFAS sourced from a former fire fighter training facility and associated wastewater storage basins.
The objective of this study was to assess the threat posed from PFAS plumes of historic landfills to aquatic ecosystems of nearby surface waters, considering the exposure to elevated PFAS concentrations from three distinct aquatic zones (endobenthic, epibenthic, pelagic) and the potential level of mass loading off-site. This objective was addressed through ∼1-year field studies at two sites of historic landfills with known plume impact on a nearby surface water body; one is a stream (Dyment's Creek – DC site) and one is a pond (called HB site). Both sites had samples with relatively high PFAS concentrations (∑17PFAS >1.5 μg L−1) within the leachate survey of 20 historic landfills by Propp et al.15 Both landfills are close to the receptor surface water body, making them extreme but not uncommon examples in this regard. Details on the groundwater – surface water interactions and landfill plume discharge characteristics have been documented by Propp et al.27 for the DC site and by Hua et al.28 for the HB site. This current study explored PFAS concentrations (i) in discharging groundwater collected from the shallow sediments to address benthic zone exposure, (ii) in the epibenthic zone above the sediment based on continual measurements of specific conductance as a proxy, and (iii) in the receiving surface water for benthic and pelagic exposure on site and downstream. The sample data and measured stream discharge for streams exiting each site (DC stream or HB pond outlet stream) were used for calculating PFAS mass discharge downstream. The focus throughout is on maximum concentrations/mass discharge and providing insight into potential variability of these in space and time, as the available data is too limited to determine broadly-applicable average values.
Most studies of landfill leachate or leachate-impacted groundwater have primarily reported concentrations of short-chain (SC, C4–C7) and long-chain (LC, C8–C16) PFAA (perfluoroalkyl acids; see Table 1 for examples and compound nomenclature), including the classes of PFCA (perfluoroalkyl carboxylic acids; such as PFOA) and PFSA (perfluoroalkylsulfonic acids; such as PFOS). However, landfill leachate can contain many other PFAS, particularly fluorotelomer carboxylic acids (FTCAs).29 Recently, Björnsdotter et al.30 reported high concentrations of USC (C1 to C3) PFCA and PFSA in landfill leachate. In the present study, targeted PFAS analysis included a suite of 34 compounds combining commonly measured SC and LC PFAA, sulfonamides and various other PFAS, with a subset of samples also analyzed for three USC PFAS (Table 1). It must be noted that although some studies indicate PFCA and PFSA groups (most measured here) tend to be the dominant PFAS in landfill leachate, likely because they are terminal degradation products,31,32 this targeted PFAS analysis may miss a substantial amount of PFAS, a common issue outlined by Wang et al.33 Finally, PFAS analysis was only performed on a small portion of the total samples measured at both sites (as reported (ref. 27 and 28)). Thus, results for the artificial sweetener saccharin, a proven leachate tracer for historic landfills,27,28,34 ammonium, chloride, and specific conductance, are sometimes presented to extrapolate or interpolate the PFAS findings.
| PFAS name | C-chain | SC/LC (Propp15) | SC/LC (an. 1) | SC/LC (an. 2) | USC |
|---|---|---|---|---|---|
| Ultra short chain (USC) PFAA | |||||
| Trifluoromethanesulfonic acid | 1 | TFMS | |||
| Perfluoropropionic acid | 3 | PFPrA | |||
| Perfluoropropanesulfonate | 3 | PFPrS | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Short chain (SC) PFCA | |||||
| Perfluorobutanoic acid | 4 | PFBA | PFBA | PFBA | |
| Perfluoropentanoic acid | 5 | PFPeA | PFPeA | PFPeA | |
| Perfluorohexanoic acid | 6 | PFHxA | PFHxA | PFHxA | |
| Perfluoroheptanoic acid | 7 | PFHpA | PFHpA | PFHpA | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Long chain (LC) PFCA | |||||
| Perfluorooctanoic acid | 8 | PFOA | PFOA | PFOA | |
| Perfluorononanoic acid | 9 | PFNA | PFNA | PFNA | |
| Perfluorodecanoic acid | 10 | PFDA | PFDA | PFDA | |
| Perfluoroundecanoic acid | 11 | PFUnA | PFUnDA | PFUnDA | |
| Perfluorododecanoic acid | 12 | PFDoDA | PFDoDA | PFDoDA | |
| Perfluorotridecanoic acid | 13 | PFTriDA | PFTrDA | PFTrDA | |
| Perfluorotetradecanoic acid | 14 | PFTeDA | PFTeDA | PFTeDA | |
| Perfluorohexadecanoic acid | 16 | PFHxDA | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Short chain (SC) PFSA | |||||
| Perfluorobutanesulfonate | 4 | PFBS | PFBS | PFBS | |
| Perfluoropentanesulfonate | 5 | PFPeS | PFPeS | ||
| Perfluorohexanesulfonate | 6 | PFHxS | PFHxS | PFHxS | |
| Perfluoroheptanesulfonate | 7 | PFHpS | PFHpS | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Long chain (LC) PFSA | |||||
| Perfluorooctanesulfonate | 8 | PFOS | PFOS | PFOS | |
| Perfluoroethylcyclohexanesulfonate | 8 | PFECHS | PFECHS | PFECHS | |
| Perfluorononanesulfonate | 9 | PFNS | |||
| Perfluorodecanesulfonate | 10 | PFDS | PFDS | PFDS | |
| Perfluorododecanesulfonate | 12 | PFDoDS | PFDoDS | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Sulfonamide PFAS | |||||
| Perfluorobutylsulfonamide | 4 | FBSA | FBSA | ||
| Perfluorohexanesulfonamide | 6 | FHxSA | |||
| Perfluorooctanesulfonamide | 8 | FOSA | FOSA | FOSA | |
| Perfluorodecanesulfonamide | 10 | FDSA | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Alternate PFAS | |||||
| 3H-Perfluoro-3-(3-methoxypropoxy) propanoic acid | 7 | ADONA | ADONA | ||
| Hexafluoropropylene oxide dimer acid (GenX) | 6 | HFPO-DA | HFPO-DA | ||
| Sodium 8-chloroperfluoro-1-octanesulfonate | 8 | 8Cl-PFOS | 8Cl-PFOS | ||
| 6:2 Chlorinated polyfluoroalkyl ether sulfonate (component of F53B) | 8 | 6:2 Cl-PFAES | 6:2 Cl-PFAES | ||
| 8:2 Chlorinated polyfluoroalkyl ether sulfonate (component of F53B) | 10 | 8:2 Cl-PFAES | 8:2 Cl-PFAES | ||
2. Methods
2.1 Site descriptions
The landfill study sites are in southern Ontario, Canada, with the HB site (pond28) in a rural setting with adjacent agricultural fields and natural woodland, and the DC site (Dyment's Creek27) in the downtown area of the City of Barrie. There is much uncertainty over the types of waste received for both landfill sites. Neither site has a landfill liner or leachate collection or plume capture system in place.The HB site (Fig. 1a) contains a mounded sanitation landfill that operated from 1970 to 1986, which is about 480 m long (N–S) by 280 m wide, and with fill up to 10 m thick. An engineered pond, which is 200 m (N–S) by 80 m (E–W) and typically <1.2 m deep, is situated ∼40 m west of the landfill. The pond bottom is covered by fine sediment, with aquatic plants covering much of its area. A small ephemeral stream, which flows during winter and spring, enters from the west. The pond empties at its southern tip through a drainage gate connected to a culvert, which then issues to the pond outlet stream that feeds a small perennial south-flowing stream. Details on groundwater – surface water interactions and contaminant patterns for the site are provided by previously published data,28 which includes numerous methods (i.e., shallow groundwater sampling transects, geophysics – electromagnetic and electrical resistivity, areal and vertical pond-sediment temperature and specific conductance measures, vertical hydraulic gradient measurements). The findings indicate the pond is receiving groundwater year-round over the majority of its area, but perhaps for the southern end, with spatially-variable discharge rates. Also, a leachate-impacted groundwater plume is travelling through the shallow sand aquifer to the pond and discharging along its east shore, with a discharging plume footprint extending 150 m N–S and about 25 m out into the pond.
 | ||
| Fig. 1 Map of the (a) HB site, showing the single large landfill and the receiving pond to the west, with sampling of discharging groundwater at two transects (Transect N–S, ∼135 m long, N end at +50 m mark and S end at −85 m mark; Transect E–W, ∼72 m long, east edge at 0 m mark and crosses Transect N–S at its +30 m mark (sample locations shown in Fig. S8†)) and of surface water at the outlet stream; and (b) DC site with its three landfills (A, B, C) situated along Dyment's Creek (flowing west), with sampling of discharging groundwater at five locations along one streambank at two stretches: B (20 m long) and C (40 m long) (as shown in Fig. S9†), and surface water sampling at up-, middle-, and down-stream locations (DC-U, DC-M, and DC-D, respectively). Satellite photos of the sites provided in ESI (Fig. S1 and S2).† | ||
The DC site (Fig. 1b) has three historical municipal landfills that were sequentially operational for ∼1 year each from 1960–1963 and are adjacent to each other along 0.5 km of Dyment's Creek. The landfills are generally only a few m thick, with fill material occasionally visible along the stream bank. Dyment's Creek is 3–5 m wide, with a typical depth <0.3 m during base flows, over this reach. Past observations indicate that the streambed and nearby aquifer sediments in the area are predominantly sand (fine sand to some gravel).27,35,36 Previously published data (methods similar to those noted above for the HB site but without geophysics) suggest that landfill contaminants discharge primarily near the streambank closest the landfill materials, with less input to the middle of the stream, but this pattern is also influenced by hyporheic flow patterns.27
2.2 Field measurements
At the HB site (Fig. 1a), sampling of discharging groundwater occurred along the east edge of the pond, with five sample locations along Transect N–S and at eleven locations across the northern portion of the pond along Transect E–W (detailed view in Fig. S8; picture in Fig. S7†). At the DC site (Fig. 1b), discharging groundwater samples were collected from five locations each at a 20 m long straight section called Stretch B (Fig. S5;† 2 or 5 m sample spacing along S bank) and a 40 m long meandering section called Stretch C (Fig. S6;† 10 m sample spacing along N bank) (detailed plan in Fig. S9†). Samples of the shallow sediment porewater (i.e., discharging groundwater) were collected using a mini-profiler system37 driven to depths of 0.1–0.2 m below the pond sediment surface at the HB site and using mini-piezometers installed 0.15 m below the streambed27 at the DC site. For both sampler types, the sampling tips and incorporated screen mesh were made of stainless-steel and were attached to 1/4′′ polyethylene tubing. Groundwater was removed using a peristaltic pump, with sample collection commencing after values stabilized for temperature, electrical conductivity (EC), pH, and dissolved oxygen (DO), measured using hand-held meters (Pro Plus, Pro ODO; YSI). Samples of stream water were collected routinely from the pond outlet stream (where it exits the culvert) at the HB site (Fig. 1a), and from Dyment's Creek (DC site; Fig. 1b) at upstream (DC-U; Fig. S3†), midstream (DC-M; less frequently), and downstream (DC-D; Fig. S4†) locations. The DC-U and DC-D locations are ∼300 m apart. These grab samples were collected using a plastic syringe or directly with the sample bottle opened and closed below the water surface. PFAS samples were collected unfiltered in pre-rinsed 500 mL HDPE bottles and were stored on ice during transport and refrigerated prior to analysis; details on filtering and preservation for other chemical suites are provided in Table S2 (ESI).†Leachate exposure to the epibenthic zone was based on measurements of specific conductance (SpC; standard temperature of 25 °C), calculated from EC and temperature measurements according to Hayashi,38 taken at ∼1 cm above the sediment interface. For the HB site, continual (15 min) SpC readings were made from August 2, 2019 to February 10, 2020 (subsequent data were lost due to covid travel restrictions) using HOBO saltwater conductivity/salinity data loggers deployed at three locations in the pond, approximately 10 m north of Transect E–W (Fig. 1a) at approximate distances of 10, 20, and 40 m from the east shore (EC-E, EC-M, and EC-W, respectively; not shown). At the DC site, synoptic SpC measurements were made at multiple locations at Stretches B and C using a hand-held probe (YSI).
Stream discharge was determined by stream gauging using the midpoint method,39 with vertically averaged velocity at each point measured using a flow meter (Global Water Model FP101; range 0.4–4.5 m s−1, accuracy 0.03 m s−1). Gauging was performed at the drain culvert for the HB site pond's outlet stream and at the DC-U and DC-D locations of Dyment's Creek (Fig. 1, S3 and S4†). Contaminant mass loadings off-site were calculated as the product of stream discharge measurements and co-located sample concentrations.
2.3 Chemical analyses
All chemical analyses were performed in Environment and Climate Change Canada laboratories at the Canada Centre for Inland Waters (Burlington, ON). The SC and LC PFAS analysis involved extraction from aqueous matrices using weak-anion exchange (WAX) solid phase extraction (SPE).15 Final extracts were analysed by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS, Waters Acquity UHPLC and Waters Acquity TQS MS/MS). All mass spectrometry parameters including cone voltage and collision energies for precursor to product ion transitions were optimized using authentic standards and are available in previous publications.15,40 The analytes in this analysis changed slightly over time, as outlined in Table 1. The method detection limits (MDLs) varied based on extraction volumes used for each sample, but all were <1.4 ng L−1. Additional information are provided in ESI Appendix C.†A subset of samples was also analyzed for several ultra short-chain PFAS (Table 1) using a Thermo Scientific (Waltham, Massachusetts, USA) 5000 ion chromatography system coupled to a QTRAP 5500 (AB Sciex, Concord, ON, CAN) tandem mass-spectrometer (IC/MS/MS). This method has not been reported on previously; full details are provided in the ESI Appendix C.† Briefly, the method requires direct injection of 100 μL water from samples and standards, with quantitation against a 6-point minimum standard curve performed against prepared standards. The MDLs were approximately 0.2 and 0.5 ng L−1, for TFMS and PFPrS, respectively; PFPrA had background levels so that the calculation of a MDL was not possible. The analysis can also quantify PFBA, but this was not determined by this method in this study as PFBA is available in the SC PFAS analysis. If available, two multiple reaction monitoring (MRM) transitions were monitored for each analyte and one for each isotope labeled internal standard. A test for matrix effects using artificial groundwater determined percent recoveries ranging from 102.5% to 111.4% with an average of 107.3%. The artificial sweetener saccharin (along with several other compounds not presented here) were analyzed as previously reported with this same instrument and methodology (see more details in ESI Appendix C†).41
Additional analyses performed on water samples from the broader site studies included major anions, soluble reactive phosphorus, ammonium, alkalinity, major cations and trace metals, and volatile organic compounds (VOCs), with an analyte list in Table S1.† Details about these analyses are provided in ESI Appendix C.†
3. Results and discussion
3.1 Endobenthic zone exposure to PFAS in discharging groundwater
PFAS concentrations in samples of discharging groundwater collected from the shallow (<20 cm depth) sediments from both landfill sites represent aquatic exposure to sediment-dwelling endobenthic organisms, though not exposure from sediment-bound (sorbed) PFAS (e.g., (ref. 42)) via potential pathways such as direct contact and ingestion, meaning exposure is potentially under-represented here. The concentrations of SC- and LC-PFAS (largely PFAA and related compounds; Table 1) measured along two transects of the pond at the HB site (only August 2019) and along the streambank at the two study stretches of the DC site (August, November 2019 and March 2020) are shown in Fig. 2 and 3, respectively. Both sites had many groundwater plume samples distinguishable by high total SC + LC PFAS concentrations (∑SC + LC PFAS), reaching a maximum of 2640 ng L−1 at the HB site, while several samples from the DC site surpassed 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1, compared to those with low concentrations representing background groundwater. Note that differences in ∑SC + LC PFAS between samples run under PFAS analyses 1 and 2 (Table 1) are deemed minor, as those few PFAS analytes only measured in one of the analyses were at very low concentrations across comparable samples. Similar values were reported for the few samples collected at these sites by Propp et al.15 These PFAS concentrations also fall within the broad concentration range that previous studies have reported for landfill leachate or leachate-impacted groundwater plumes ((see Table 2 within ref. 15)) except for much higher values for some landfills in China (Yan et al. 2015).43 The total concentrations of the three USC PFAS (Table 1) for a subset of these locations and dates were >2500 ng L−1 and >500 ng L−1 for the HB (only two samples) and DC sites (Fig. 4), respectively.
000 ng L−1, compared to those with low concentrations representing background groundwater. Note that differences in ∑SC + LC PFAS between samples run under PFAS analyses 1 and 2 (Table 1) are deemed minor, as those few PFAS analytes only measured in one of the analyses were at very low concentrations across comparable samples. Similar values were reported for the few samples collected at these sites by Propp et al.15 These PFAS concentrations also fall within the broad concentration range that previous studies have reported for landfill leachate or leachate-impacted groundwater plumes ((see Table 2 within ref. 15)) except for much higher values for some landfills in China (Yan et al. 2015).43 The total concentrations of the three USC PFAS (Table 1) for a subset of these locations and dates were >2500 ng L−1 and >500 ng L−1 for the HB (only two samples) and DC sites (Fig. 4), respectively.
 | ||
| Fig. 2 Concentrations of SC and LC PFAS and specific conductance (SpC) in shallow groundwater along two transects of the HB site (Fig. 1a; 5 locations along Transect N–S and 11 locations along Transect E–W, positions in m; noting NS at +30 m and EW at 3 m are the same sample) from August 2019. A zone of higher groundwater discharge was noted for ∼20–30 m distance along Transect E–W.28 | ||
 | ||
| Fig. 3 Concentrations of SC and LC PFAS in shallow groundwater at five locations along each of the two study stretches of the DC site (Fig. 1b; south side with 2 to 5 m spacing for B stretch; north side with 10 m spacing for C stretch), each from sampling performed in August and November 2019, and March 2020 (left to right). Note the break in the y-axis, affecting only PFECHS. Higher groundwater discharge was noted for locations: B 15S and 20S; C 30N and 40N.27 | ||
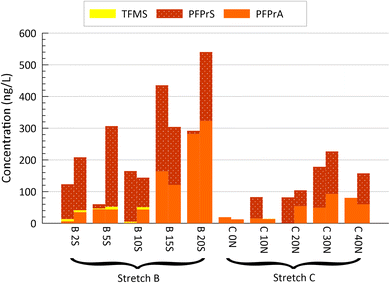 | ||
| Fig. 4 Concentrations of the USC PFAS: TFMS, PFPrA and PFPrS, in shallow groundwater at five locations along each of the two study stretches of the DC site (Fig. 1b; south side with 2 to 5 m spacing for B stretch; north side with 10 m spacing for C stretch), each from sampling performed in November 2019 (left) and March 2020 (right). Higher groundwater discharge was noted for locations: B 15S and 20S; C 30N and 40N.27 | ||
The spatial extent of the landfill plume footprint (i.e., the sediment area covered by the discharging landfill plume) represents the endobenthic (and epibenthic) exposure area. The plume footprint covers ∼25% of the HB site pond area, based on the detection of the high conductivity plume across the pond bottom by the previous electromagnetic geophysics survey.28 For the DC site, considering that at least 250 m of the stream borders landfill on one side and 250 m borders landfill on both sides, then potentially 750 m of streambank may experience some leachate-impacted groundwater discharge. These site assessments illustrate that groundwater plumes from historic landfills, even those closed 4–6 decades ago, can cause exposure for endobenthic ecosystems to PFAS concentrations in the μg L−1 range (i.e., similar to groundwater plume values; and likely higher given the limited set of PFAS analyzed) covering relatively large portions of the receiving surface waters.
3.2 Groundwater SC and LC PFAS composition
Given expected short groundwater travel times at both sites (i.e., with short separation distances (Fig. 1) and high permeability of the sand aquifers), the discharging plumes likely represent recent rather than decades-old leachate conditions. At both historic landfill sites, the measured PFAS are predominantly SC PFAA (C4–C7) or C8 PFAS rather than longer-chained PFCA (Fig. 2 and 3). This pattern has been commonly reported for landfill plumes11 and is likely due to the higher aqueous solubilities of SC PFAA43 and ubiquitous use of C8 PFAS. Furthermore, as was noted by Propp et al.15 based on a more limited set of samples, the HB site is dominated by PFCA (especially PFHxA; Fig. 2), as tends to be the case for modern municipal landfills,11 whereas the DC site is dominated by PFSA (Fig. 3). More specifically, the DC site groundwater samples were elevated in PFOS, PFBS, and PFHxS, which are PFAS congeners typically associated with aqueous film forming foam (AFFF) often used at fire-fighting training areas at airports and military bases (ref. 42 and references therein). The other major contributor here, PFECHS, has been linked to aircraft hydraulic fluid,44 motorsport vehicles,45 and possibly chrome plating.46 Marchiandi et al.47 reported PFECHs in surface waters affected by an industrial fire at a location that had stockpiled >100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 chemical and industrial drums. Interestingly, the PFCA concentrations at the DC site were similar to those of the HB site, as if the DC site had a “regular” landfill signature that was overwritten by the elevated PFSA concentrations. Though highly speculative, this composition might reflect dumping of hazardous materials associated with AFFF use.
000 chemical and industrial drums. Interestingly, the PFCA concentrations at the DC site were similar to those of the HB site, as if the DC site had a “regular” landfill signature that was overwritten by the elevated PFSA concentrations. Though highly speculative, this composition might reflect dumping of hazardous materials associated with AFFF use.
For the sulfonamides, FBSA and FOSA were both low (<5 ng L−1) for the HB site, with FHxSA and FDSA < MDL; whereas at the DC site, FBSA, FHxSA and FOSA had maximum concentrations of 670, 98, and 27 ng L−1, respectively, while FDSA < 1 ng L−1. Therefore, these less-frequently measured PFAS may contribute non-negligible concentrations to the total PFAS exposure. Considering the newer PFAS (Aug 2019 sampling only), most of the HB and DC samples were below detection for ADONA, HFPO-DA, 8ClPFOS, 9 Cl-PF3ONS F53B and 11Cl-PF3OUdS F53B, or else concentrations were low (<8 ng L−1). This isn't surprising given the decades-past closing dates of these landfills.
3.3 Groundwater ultra-short-chain PFAS
Data on USC PFAS sourced from landfills is scarce. A study by Björnsdotter et al.30 sampled three landfill sites in Sweden (active and closed, municipal and industrial landfills) and reported maximum concentrations for leachate-impacted samples of 800 and 90 ng L−1 for PFPrA and PFPrS, respectively, the latter from a hazardous waste landfill. In this study, PFPrA and PFPrS concentrations were slightly higher, reaching 2100 and 250 ng L−1 respectively, for the HB site, and 660 and 270 ng L−1 respectively, for the DC site. For the DC site, PFPrA and PFPrS are most highly correlated (Pearson, p < 0.05) with concentrations of SC-PFAS: PFPeA, PFPeS, PFHxA, PFHxS; thus suggesting a landfill source.The concentration of TFMS was low (<10 ng L−1) for all samples at both landfill sites. In contrast, TFMS reached 500 ng L−1 for landfill-related samples (though for hazardous waste) in the study of Björnsdotter et al.30 and it was a common chemical detected up to the μg L−1 range in groundwaters and surface waters in urban and industrial areas of Europe.48 Whether its relative absence here reflects a lack of TFMS use (in North America or at least in those specific locals) during the periods these landfills were open or if the TFMS has been flushed out or volatilized, isn't clear. An expanded investigation of other landfills with a range in closing dates would be needed to address this uncertainty.
These results for DC and HB site, though limited, suggest that USC PFAS can contribute notably to the PFAS profile measured in this study, supporting the general finding of Björnsdotter et al.30 for PFAS in leachate. This contribution would likely be greater if trifluoroacetic acid (TFA) were to be included. They also demonstrate for the first time that USC PFAS can be transported from landfills via groundwater plumes to nearby surface waters, where benthic organisms can be exposed to substantial concentrations.
3.4 Spatial and temporal variation in groundwater PFAS
Concentrations of leachate constituents are known to vary within individual landfills.49,50 However, few studies have investigated spatial and temporal variation in benthic zone exposure from a discharging landfill plume (but see (ref. 51)), with no reports on PFAS. The previous reports on these two landfill sites by Propp et al.27 and Hua et al.28 investigated this variability in detail, considering leachate indicators saccharin, ammonium, chloride, and specific conductance. Thus, here only some key observations related to PFAS are presented. Data from both sites illustrate that there can be substantial spatial variation in PFAS concentrations (by orders of magnitude) and composition over short distances (Fig. 2–4). These often match variation in other leachate indicators, such as SpC (Fig. 2), likely reflecting differences in the strength of the leachate as affected by such processes as chemical release to groundwater and groundwater residence time in the landfill, or influences of groundwater – surface water interactions. The discharging groundwater data is much more limited with respect to temporal patterns, especially for the HB site. However, there are a few examples of large changes in SC and LC PFAS concentrations between sampling events (several months) at the DC site (Fig. 3); this is particularly evident for location 10S of Stretch B, with an apparent flip from plume to background conditions. Propp et al.27 observed similar trends for other leachate compounds and suggested the cause was due to changes in groundwater – surface water interactions, including shifting plume direction and some transition from groundwater discharge to stream recharge conditions. A final interesting observation from the DC site is that Stretch B shows high PFECHS concentrations in comparison to Stretch C despite the two areas receiving their waste only one year apart, possibly illustrating differences due to the type of waste received. In addition to variation in leachate source and influences of groundwater-surface water interactions, sorption and transformation processes may also influence these patterns. However, this study was not designed to investigate these processes.3.5 Epibenthic exposure to PFAS in discharging groundwater
Exposure to epibenthic organisms (living on top of sediment) from contaminants of the landfill plumes will depend on the rate of groundwater discharge, the magnitude of contaminant concentrations, and surface water mixing processes, all of which can vary temporally and spatially. Temperature-based sediment-bed measurements (qualitative) indicate notable spatial variation in groundwater discharge at both sites. For the HB site, high groundwater discharge was measured along Transect E–W between 20 and 30 m from shore.28 While PFAS concentrations were higher near shore (3 and 8 m distances; Fig. 2), the contaminant flux, and thus potential epibenthic exposure, was likely higher for the 21 m distance location despite its ∼50% lower concentrations. For the DC site, groundwater discharge tended to be higher along the streambanks and varied along the stream length, influenced by meanders and streambed topography and debris.27 Higher fluxes were estimated for locations 15S and 20S for the B section and 30N and 40N for the C section, all locations with relatively high PFAS concentrations. Thus, potential epibenthic exposure was magnified at these locations. These findings illustrate the influence of spatially-variable groundwater discharge patterns on potential epibenthic exposure within the landfill plume footprints, and highlight that measured groundwater concentrations alone cannot determine areas of greater risk to epibenthic organisms.A proxy measure of landfill contaminant concentrations directly in the epibenthic zone was provided via SpC measurements at 1 cm above the sediment surface. No measurements found elevated epibenthic SpC in comparison to the overlying water in either study stretch at the DC site, which was likely due to the flowing stream conditions rapidly diluting the groundwater signature. A leachate impact might occur at times of low stream flow, inside eddy areas, etc., but these were not measured.
In contrast, continual SpC monitoring at the HB site pond (i.e., non-flowing conditions) revealed the epibenthic SpC was commonly elevated within the plume footprint (locations EC-E and EC-M; Fig. S8†). Noting the linear relationship between SpC and PFAS concentration in the shallow groundwater samples (linear regression R2 = 0.97, Fig. S16†), a continual estimate of the total PFAS (∑SC + LC + USC PFAS) concentration in the epibenthic zone at the EC-E location (Fig. 5) was derived by equating the range of SpC and total PFAS from background groundwater (700 μS cm−1 and 0 ng L−1, respectively) to the maximum in the plume groundwater (3500 μS cm−1 and 4800 ng L−1, respectively). This estimated measure reveals PFAS exposure occurred across the entire monitoring period but for a few occasions when the concentration dropped to near 0 ng L−1, likely reflecting some enhanced mixing in the pond during a time of rather low groundwater discharge (typically late summer). The total PFAS concentration varied substantially over several time scales, including (i) a diurnal pattern, (ii) spikes in concentration (to >2000 ng L−1) that appear influenced by recent rain events, and (iii) a potential seasonal pattern with prolonged higher concentrations to near-peak values (∼4000 ng L−1) in winter. The roles of changing groundwater discharge or altered mixing in the pond in controlling the SpC and predicted total PFAS concentrations aren't clear, though both factors are likely involved at times. For instance, Hua et al.28 noted that high winter exposure corresponded with ice development on the pond, which likely reduced wind-induced in-pond mixing, but also with elevated groundwater levels (not shown here) that likely created greater groundwater flux. These findings illustrate that epibenthic organisms in non-flowing water bodies may experience intermittent to long periods of exposure to high PFAS concentrations (potentially undiluted groundwater) within the footprint of discharging landfill plumes.
 | ||
| Fig. 5 Estimated concentration of total PFAS (∑SC + LC + USC) for epibenthic exposure within the HB pond plume footprint, based on continuous SpC measurements at ∼1 cm above the pond sediments. | ||
3.6 Stream PFAS concentrations and exposure to on-site pelagic organisms
Concentrations of the various PFAS in the HB pond outlet stream showed similar composition (Fig. S13†) and magnitude (Fig. 6a) over the monitoring period (five dates for USC – Oct 2019 – March 2020; and seven dates for SC + LC – July 2019 – March 2020). Only one sample had detectable TFMS. All these concentrations are ∼10 times lower than for the discharging plume groundwater, indicating dilution from the input of unaffected groundwater, precipitation (rain and snowmelt), and minor runoff and flow of the ephemeral inlet stream largely during the spring melt period. This similar dilution factor across the different PFAS (i.e., similar composition) suggests that most of the pond water PFAS was likely sourced from the landfill plume rather than from other sources (e.g., atmospheric deposition). | ||
| Fig. 6 Concentrations (a) and mass discharge (b) for various landfill indicators (chloride – Cl, saccharin – SAC) and PFAS groups (the sum of PFAA congeners analyzed with 4 to 9 carbon atoms (Table 1) – C4–C9, and PFPrA + PFPrS – C3 and TFMS (but for (b))), for the HB outlet stream on the given sampling dates. | ||
The outlet stream concentrations likely reflect the pond's open water concentrations in the east and south end of the pond, given observations suggest pond circulation is predominantly clockwise28 and the plume discharges to the north-east portion of the pond. Thus, exposure to organisms in the pond's overlying water (pelagic zone) extends beyond the plume footprint; epibenthic organisms outside the plume footprint may also experience some resulting exposure in low groundwater flux areas too. Recognizing the limited temporal data available, the outlet stream PFAS concentrations, supplemented by those of the conservative leachate indicators (Cl, saccharin), which show a similar temporal trend (Fig. 6a), suggest pelagic exposure to PFAS is likely occurring year-round. Unfortunately, there isn't sufficient data to assess any seasonality to the pattern.
At the DC stream site, the SC and LC PFAS stream concentrations increased from upstream to downstream by 50–144% for the four sampling dates (Fig. 7), all during base flows, indicating groundwater mass loading along the study reach. The increases were predominantly due to PFSAs, particularly PFOS and PFECHS, which makes sense given their dominance in the discharging groundwater samples (Fig. 3). PFPrS was below detection in all samples, and the other USC PFAS had a range of concentrations from 5 to 20 ng L−1. There was no clear increase downstream for any of the USC PFAS (not shown); likely the concentrations in groundwater were too low to make a clear change in the stream. The elevated PFAS concentrations in the upstream samples are suggestive of an unknown alternate source or sources upstream of these landfills (e.g., leaky sewers;27), though landfill C inputs upstream of the DC-U sampling location (Fig. 1b) may also be contributing.
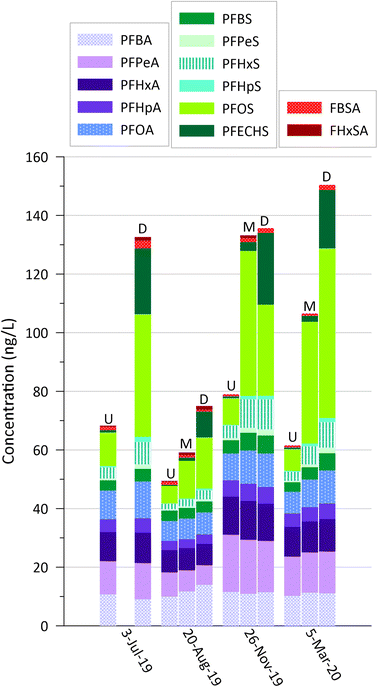 | ||
| Fig. 7 Concentrations of dominant SC and LC PFAS in surface water samples from the upstream (U), mid-stream (M; no sample for July 3, 2019), and downstream (D) sampling locations (Fig. 1). | ||
The magnitude of increase in the SC and LC PFAS concentrations from upstream to downstream at DC stream was similar for each sampling date (60–90 ng L−1 ∑12PFAAs) with the exception of August, which had a much smaller increase (20 ng L−1) as shown in Fig. 8b. This change in August is likely due to lower groundwater discharge to the stream during this drier month. Key leachate indicators saccharin (Fig. 8a) and ammonium (Fig. S14†) were sampled in stream water more frequently, and provide additional insight into the stream PFAS temporal patterns. On the days of PFAS sampling, the increases in saccharin and ammonium concentrations from upstream to downstream were modest (e.g., saccharin increase <25 ng L−1) compared to some other sampling dates (e.g., saccharin increases >80 ng L−1 in July 2019 and April 2020), particularly at times of higher base flows. This could mean that PFAS increases may have been higher at these and other unmeasured times of year. However, some sampling dates associated with higher and changing stream flows following rain events (e.g., Sept 10, 2019) showed saccharin and ammonium concentrations remained steady or decreased downstream; presumably PFAS concentrations did likewise. Future work should focus on clearly identifying these temporal patterns and their key drivers.
 | ||
| Fig. 8 Concentrations of saccharin (a) and the sum of 12 dominant SC and LC PFAS (b) from stream samples collected at the upstream (upright triangles) and downstream (downward triangles) locations along the DC stream (Fig. 1b). Samples were collected during base flow periods but for three at higher flows following a rain event indicated by arrows (at top). | ||
3.7 PFAS loading to downstream surface waters
The elevated PFAS concentrations in the outlet stream at the HB site (Fig. 6a) and the increase in concentrations along the DC site stream (Fig. 7 and 8) mean both landfill sites are providing PFAS loading to downstream receptors, potentially even reaching beyond their watersheds to larger water bodies (in this case, the Laurentian Great Lakes). Estimates of mass loading (measured stream discharge x stream concentrations) from the HB pond outlet for the ∑SC + LC PFAS, as well as for leachate indicators Cl and saccharin, are shown in Fig. 6b. PFAS mass loading is over 50 mg per day at times, with lower loading typically measured during the summer, when the outlet stream discharge was lower (Fig. S10†). Hua et al.28 noted that this coincides with a lower pond level (Fig. S11†), which is likely due to greater evaporation from the pond and reduced groundwater inputs with a lower water table. A crude estimate of annual loading from this single landfill site is 14.6 g per year for ∑SC + LC, based on an average daily load of 40 mg per day. Assuming a similar loading pattern with plume inputs starting in 1980 (12 years after the landfill opened), we can derive a crude estimate of the lifetime downstream loading (up to 2020) from this landfill to be 0.58 kg.For the DC site stream, mass discharge of all three landfill components measured – saccharin, ∑SC + LC PFAS (Fig. 9), and ammonium (Fig. S15†), increased notably (often doubling or more for PFAS) from the upstream to downstream location at nearly all sampling dates. This loading of up to ∼200 mg per day for ∑SC + LC PFAS reflects both the increased stream concentrations (Fig. 8) and typically higher flow downstream (Fig. S12†). One exception for which saccharin and ammonium mass discharge declined (but no PFAS data available), was a sampling date in early August 2019, when the stream at base flow was losing water to the ground across the study reach. In contrast, the mass discharge for saccharin and ammonium was higher following rain events (PFAS not measured then), which may reflect greater groundwater flux with an elevated water table from infiltration and following a decline in stream stage. Or it might also reflect some wash-off from groundwater seeps along the banks (often observed during non-peak flows). During those times, PFAS mass discharge contributed from the landfill was likely higher as well – potentially 5–10 times higher based on the difference in saccharin mass discharge observed between PFAS sampling dates and the rain dates. This variability over time makes estimating an annual load difficult. However, using the low flow August 2019 date as a minimum for the ∑SC/LC PFAS increase downstream (100 mg per day) gives a loading of ∼36 g per year and so a probable minimum of ∼2 kg over the lifetime of these DC landfills (assuming stable inputs over 55 years). These estimates could be many times too low.
 | ||
| Fig. 9 Calculated mass discharge for saccharin (a) and the sum of 12 dominant SC and LC PFAS (b) for the upstream (upright triangles) and downstream (downward triangles) locations along the DC stream (Fig. 1b). Samples were collected during base flow periods but for three at higher flows following a rain event indicated by arrows (at top). Note the uncollected upstream sample in March 2020. | ||
For comparison purposes, PFAS output from all U.S. landfills open between 1980 and 2014 to wastewater treatment plants in 2013 was estimated by Lang et al. as 563–638 kg per year (90th percentile range;52), based on 19 PFAS (predominantly being PFCAs and FTCAs, with lower releases of PFSAs and their precursors). The load is dominated by landfills in wet as opposed to temperate and arid climatic zones. A crude extrapolation of the HB and DC site mass loading data, though only considering SC and LC PFAA, could give some sense of the potential magnitude of the PFAS contribution to surface waters via plumes emanating from historic landfills (open after the 1950s). Assuming an average of the conservative loads of these two sites, 25 g per year of ∑SC + LC PFAS, applied to one tenth of the estimated “more than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 active and closed U.S. landfills”,19 gives at least 125 kg per year loading to local groundwater in the U.S., a value not that much lower than noted from the Lang et al. study.52 There are many factors that may render this estimate overly low, such as it does not capture many PFAS congeners common in landfills, or overly high, such as the proximity of these example sites to the receiving waters. Indeed, whether these sites are truly representative of average conditions, and what percentage of historic landfill sites may impact nearby surface waters is unknown. However, the order of magnitude result here suggests that cumulative inputs from historic landfills could be substantial. Further research is needed to provide a better estimate.
000 active and closed U.S. landfills”,19 gives at least 125 kg per year loading to local groundwater in the U.S., a value not that much lower than noted from the Lang et al. study.52 There are many factors that may render this estimate overly low, such as it does not capture many PFAS congeners common in landfills, or overly high, such as the proximity of these example sites to the receiving waters. Indeed, whether these sites are truly representative of average conditions, and what percentage of historic landfill sites may impact nearby surface waters is unknown. However, the order of magnitude result here suggests that cumulative inputs from historic landfills could be substantial. Further research is needed to provide a better estimate.
4. Conclusions
Observations from these two historic landfill sites illustrate the high PFAS concentrations, including USC PFAS, that can still exist in groundwater plumes discharging to surface waters many decades after landfill closure. This can lead to high (μg L−1 range) but spatially and temporally variable PFAS exposure to organisms living in the endobenthic and epibenthic zones within the discharging plume footprint. This type of exposure may favour more tolerant benthic organisms, but also those that are more mobile, being able to move out of the plume footprint even if it moves throughout the year. Concentrations in the receiving waters were substantially reduced via dilution (here by ∼10 times), suggesting a lower direct toxicity risk compared to the benthic zone species, though the impacted area for the pelagic zone exposure was greater (i.e., beyond the plume footprint). These two site investigations illustrate that PFAS exposures to all three ecological zones can extend year-round, though this may not hold for other sites, depending on the groundwater – surface water interactions.Both sites exhibited downstream PFAS mass loading in the g per year range. Considering the ubiquity of old landfills on the landscape and that these are rarely managed with off-site groundwater transport of PFAS in mind, their cumulative loading to downstream water bodies may be significant. Once in the surface water body (including its sediments) and transported downstream, PFAS components may work their way into and through the aquatic and terrestrial food webs53–56 and also pose a threat to fisheries.57 In addition, the impacted surface waters downstream may be used as a water supply for domestic use, livestock, or irrigation, posing a risk to human health.
Note that given the targeted analysis of largely (USC, SC, LC) PFCA and PFSA here, the findings may present an incomplete (minimum) picture of the exposure and loading of total PFAS at these sites, and thus the threat posed by PFAS of historic landfills. Further, whether the PFAS supplied by groundwater plumes at these two landfill sites were having an ecological impact, through direct exposure or through the food chain, is not known. This PFAS exposure is complicated by the mix of individual PFAS involved, most with limited information on aquatic toxicity,33 and the mixture of various other legacy and emerging contaminants (e.g., (ref. 15)). Further research is needed to assess the ecotoxicity of PFAS-containing leachate-impacted groundwater discharging to freshwater bodies, ideally through field studies and using multidisciplinary teams.33 Such studies must consider the potential spatial and temporal variability in contaminant concentrations across the different ecological zones and its links to groundwater – surface water interactions in the design of sampling and ecotoxicological protocols.
Data availability
Certain aspects of the data are confidential but some data can be provided upon request to the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding support from the Government of Ontario (Ministry of Environment, Conservation and Parks (MECP)). Such support does not indicate endorsement by the Government of Ontario of the contents of this material. Funding was also provided by Environment and Climate Change Canada (ECCC) and Natural Sciences and Engineering Research Council of Canada (V. Propp). Pam Collins, Ross Mackay, and Grant Hodgins (ECCC) provided laboratory and field assistance. Constructive comments from two anonymous reviews helped improve the final manuscript.References
- E. M. Sunderland, X. C. Hu, C. Dassuncao, A. K. Tokranov, C. C. Wagner and J. G. Allen, A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects, J. Exposure Sci. Environ. Epidemiol., 2019, 29(2), 131–147, DOI:10.1038/s41370-018-0094-1.
- A. O. De Silva, J. M. Armitage, T. A. Bruton, C. Dassuncao, W. Heiger-Bernays, X. C. Hu, A. Kärrman, B. Kelly, C. Ng, A. Robuck and M. Sun, PFAS exposure pathways for humans and wildlife: a synthesis of current knowledge and key gaps in understanding, Environ. Toxicol. Chem., 2021, 40(3), 631–657, DOI:10.1002/etc.4935.
- G. W. Olsen, D. C. Mair, C. C. Lange, L. M. Harrington, T. R. Church, C. L. Goldberg, R. M. Herron, H. Hanna, J. B. Nobiletti, J. A. Rios and W. K. Reagen, Per-and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000–2015, Environ. Res., 2017, 157, 87–95, DOI:10.1016/j.envres.2017.05.013.
- G. Zheng, S. M. Eick and A. Salamova, Elevated levels of ultrashort-and short-chain perfluoroalkyl acids in US homes and people, Environ. Sci. Technol., 2023, 57(42), 15782–15793, DOI:10.1021/acs.est.2c06715.
- M. Scheringer, X. Trier, I. T. Cousins, P. de Voogt, T. Fletcher, Z. Wang and T. F. Webster, Helsingør Statement on poly-and perfluorinated alkyl substances (PFASs), Chemosphere, 2014, 114, 337–339, DOI:10.1016/j.chemosphere.2014.05.044.
- S. Kurwadkar, J. Dane, S. R. Kanel, M. N. Nadagouda, R. W. Cawdrey, B. Ambade, G. C. Struckhoff and R. Wilkin, Per-and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution, Sci. Total Environ., 2022, 809, 151003, DOI:10.1016/j.scitotenv.2021.151003.
- B. D. Key, R. D. Howell and C. S. Criddle, Fluorinated organics in the biosphere, Environ. Sci. Technol., 1997, 31(9), 2445–2454, DOI:10.1021/es961007c.
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. De Voogt, A. A. Jensen, K. Kannan, S. A. Mabury and S. P. van Leeuwen, Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins, Integr. Environ. Assess. Manage., 2011, 7(4), 513–541, DOI:10.1002/ieam.258.
- J. Li, B. Xi, G. Zhu, Y. Yuan, W. Liu, Y. Gong and W. Tan, A critical review of the occurrence, fate and treatment of per-and polyfluoroalkyl substances (PFASs) in landfills, Environ. Res., 2023, 218, 114980, DOI:10.1016/j.envres.2022.114980.
- J. Glüge, M. Scheringer, I. T. Cousins, J. C. DeWitt, G. Goldenman, D. Herzke, R. Lohmann, C. A. Ng, X. Trier and Z. Wang, An overview of the uses of per-and polyfluoroalkyl substances (PFAS), Environ. Sci.: Processes Impacts, 2020, 22(12), 2345–2373, 10.1039/d0em00291g.
- H. Hamid, L. Y. Li and J. R. Grace, Review of the fate and transformation of per-and polyfluoroalkyl substances (PFASs) in landfills, Environ. Pollut., 2018, 235, 74–84, DOI:10.1016/j.envpol.2017.12.030.
- C. A. Huset, M. A. Barlaz, D. F. Barofsky and J. A. Field, Quantitative determination of fluorochemicals in municipal landfill leachates, Chemosphere, 2011, 82(10), 1380–1386, DOI:10.1016/j.chemosphere.2010.11.072.
- C. Gallen, D. Drage, S. Kaserzon, C. Baduel, M. Gallen, A. Banks, S. Broomhall and J. F. Mueller, Occurrence and distribution of brominated flame retardants and perfluoroalkyl substances in Australian landfill leachate and biosolids, J. Hazard. Mater., 2016, 312, 55–64, DOI:10.1016/j.jhazmat.2016.03.031.
- E. Hepburn, C. Madden, D. Szabo, T. L. Coggan, B. Clarke and M. Currell, “Contamination of Groundwater with Per- and Polyfluoroalkyl Substances (PFAS) from Legacy Landfills in an Urban Re-Development Precinct”, Environ. Pollut., 2019, 248, 101–113, DOI:10.1016/j.envpol.2019.02.018.
- V. R. Propp, A. O. De Silva, C. Spencer, S. J. Brown, S. D. Catingan, J. E. Smith and J. W. Roy, Organic contaminants of emerging concern in leachate of historic municipal landfills, Environ. Pollut., 2021, 276, 116474, DOI:10.1016/j.envpol.2021.116474.
- R. Li and J. MacDonald Gibson, Predicting the occurrence of short-chain PFAS in groundwater using machine-learned Bayesian networks, Front. Environ. Sci., 2022, 10, 958784 Search PubMed.
- E. L. McFarlan and L. D. Lemke, Per-and polyfluoroalkyl substances (PFAS) fate and transport across a groundwater-surface water interface, Sci. Total Environ., 2024, 951, 175672 Search PubMed.
- M. Sörengård, S. Bergström, P. McCleaf, K. Wiberg and L. Ahrens, Long-distance transport of per-and polyfluoroalkyl substances (PFAS) in a Swedish drinking water aquifer, Environ. Pollut., 2022, 311, 119981, DOI:10.1016/j.envpol.2022.119981.
- J. M. Suflita, C. P. Gerba, R. K. Ham, A. C. Palmisano, W. L. Rathje and J. A. Robinson, The world's largest landfill, Environ. Sci. Technol., 1992, 26(8), 1486–1495, DOI:10.1021/es00032a002.
- V. W. Lambou, J. M. Kuperberg, J. E. Moerlins, R. C. Herndon, and R. L. Gebhard, Proximity of sanitary landfills to wetlands and deepwater habitats: An evaluation and comparison of 1,153 sanitary landfills in 11 states. Environmental Protection Agency, Las Vegas, NV (USA), Environmental Monitoring Systems Lab, 1990 Search PubMed.
- B. Quan, J. Tang, X. Niu, P. Su, Z. Zhang and Y. Yang, Elaborating the Occurrence and Distribution of Per-and Polyfluoroalkyl Substances in Rivers and Sediment around a Typical Aging Landfill in China, Toxics, 2023, 11(10), 852, DOI:10.3390/toxics11100852.
- A. Walsh and C. G. Woods, Presence of perfluoroalkyl substances in landfill adjacent surface waters in North Carolina, Int. J. Environ. Res. Public Health, 2023, 20(15), 6524, DOI:10.3390/ijerph20156524.
- M. A. Briggs, A. K. Tokranov, R. B. Hull, D. R. LeBlanc, A. B. Haynes and J. W. Lane, Hillslope groundwater discharges provide localized stream ecosystem buffers from regional per- and polyfluoroalkyl substances contamination, Hydrol. Processes, 2020, 34(10), 2281–2291, DOI:10.1002/hyp.13752.
- A. K. Tokranov, D. R. LeBlanc, H. M. Pickard, B. J. Ruyle, L. B. Barber, R. B. Hull, E. M. Sunderland and C. D. Vecitis, Surface-water/groundwater boundaries affect seasonal PFAS concentrations and PFAA precursor transformations, Environ. Sci.: Processes Impacts, 2021, 23(12), 1893–1905, 10.1039/d1em00329a.
- M. A. Pétré, K. R. Salk, H. M. Stapleton, P. L. Ferguson, G. Tait, D. R. Obenour, D. R. Knappe and D. P. Genereux, Per-and polyfluoroalkyl substances (PFAS) in river discharge: Modeling loads upstream and downstream of a PFAS manufacturing plant in the Cape Fear watershed, North Carolina, Sci. Total Environ., 2022, 831, 154763, DOI:10.1016/j.scitotenv.2022.154763.
- C. Divine, A. Wadhawan, V. Pulikkal, P. Khambhammettu and J. Erickson, Polyfluoroalkyl substances requiring a renewed focus on groundwater-surface water interactions, Groundwater Monit. Rem., 2023, 43(1), 14–31, DOI:10.1111/gwmr.12569.
- V. R. Propp, S. J. Brown, P. Collins, J. E. Smith and J. W. Roy, Artificial sweeteners identify spatial patterns of historic landfill contaminated groundwater discharge in an urban stream, Groundwater Monit. Rem., 2022, 42(1), 50–64, DOI:10.1111/gwmr.12483.
- T. Hua, V. R. Propp, C. Power, S. J. Brown, P. Collins, J. E. Smith and J. W. Roy, Multizone aquatic ecological exposures to landfill contaminants from a groundwater plume discharging to a pond, Environ. Toxicol. Chem., 2023, 42(8), 1667–1684, DOI:10.1002/etc.5650.
- S. L. Capozzi, A. L. Leang, L. A. Rodenburg, B. Chandramouli, D. A. Delistraty and C. H. Carter, PFAS in municipal landfill leachate: Occurrence, transformation, and sources, Chemosphere, 2023, 334, 138924, DOI:10.1016/j.chemosphere.2023.138924.
- M. K. Björnsdotter, L. W. Yeung, A. Kärrman and I. E. Jogsten, Ultra-short-chain perfluoroalkyl acids including trifluoromethane sulfonic acid in water connected to known and suspected point sources in Sweden, Environ. Sci. Technol., 2019, 53(19), 11093–11101, DOI:10.1021/acs.est.9b02211.
- I. Fuertes, S. Gómez-Lavín, M. P. Elizalde and A. Urtiaga, Perfluorinated alkyl substances (PFASs) in northern Spain municipal solid waste landfill leachates, Chemosphere, 2017, 168, 399–407, DOI:10.1016/j.chemosphere.2016.10.072.
- S. Rehnstam, M. B. Czeschka and L. Ahrens, Suspect screening and total oxidizable precursor (TOP) assay as tools for characterization of per-and polyfluoroalkyl substance (PFAS)-contaminated groundwater and treated landfill leachate, Chemosphere, 2023, 334, 138925, DOI:10.1016/j.chemosphere.2023.138925.
- Z. Wang, J. C. DeWitt, C. P. Higgins and I. T. Cousins, A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?, Environ. Sci. Technol., 2017, 51(5), 2508–2518, DOI:10.1021/acs.est.6b04806.
- J. W. Roy, D. R. Van Stempvoort and G. Bickerton, Artificial sweeteners as potential tracers of municipal landfill leachate, Environ. Pollut., 2014, 184, 89–93, DOI:10.1016/j.envpol.2013.08.021.
- J. W. Roy and G. Bickerton, Toxic groundwater contaminants: an overlooked contributor to urban stream syndrome?, Environ. Sci. Technol., 2012, 46(2), 729–736, DOI:10.1021/es2034137.
- A. Fitzgerald, J. W. Roy and J. E. Smith, Calculating discharge of phosphorus and nitrogen with groundwater base flow to a small urban stream reach, J. Hydrol., 2015, 528, 138–151, DOI:10.1016/j.jhydrol.2015.06.038.
- J. W. Roy and G. Bickerton, Proactive screening approach for detecting groundwater contaminants along urban streams at the reach-scale, Environ. Sci. Technol., 2010, 44(16), 6088–6094, DOI:10.1021/es101492x.
- M. Hayashi, Temperature-Electrical Conductivity Relation of Water for Environmental Monitoring and Geophysical Data Inversion, Environ. Monit. Assess., 2004, 96(1–3), 119–128, DOI:10.1023/B:EMAS.0000031719.83065.68.
- D. P. Turnipseed, and V. B. Sauer, Discharge Measurements at Gaging Stations, Techniques of Water-Resources Investigations of the United States Geological Survey, Book 3 – Applications of Hydraulics, 2010 Search PubMed.
- J. J. MacInnis, I. Lehnherr, D. C. Muir, K. A. St. Pierre, V. L. St. Louis, C. Spencer and A. O. De Silva, Fate and transport of perfluoroalkyl substances from snowpacks into a lake in the High Arctic of Canada, Environ. Sci. Technol., 2019, 53(18), 10753–10762, DOI:10.1021/acs.est.9b03372.
- D. R. Van Stempvoort, J. W. Roy, S. J. Brown and G. Bickerton, Artificial sweeteners as potential tracers in groundwater in urban environments, J. Hydrol., 2011, 401(1–2), 126–133, DOI:10.1016/j.jhydrol.2011.02.013.
- R. H. Anderson, T. Thompson, H. F. Stroo and A. Leeson, Department of Defense–Funded Fate and Transport Research on Per-and Polyfluoroalkyl Substances at Aqueous Film–Forming Foam–Impacted Sites: US Department of Defense PFAS fate and transport research, Environmental Toxicology and Chemistry, 2021, 40(1), 37–43, DOI:10.1002/etc.4694.
- H. Yan, I. T. Cousins, C. Zhang and Q. Zhou, Perfluoroalkyl acids in municipal landfill leachates from China: Occurrence, fate during leachate treatment and potential impact on groundwater, Sci. Total Environ., 2015, 524, 23–31, DOI:10.1016/j.scitotenv.2015.03.111.
- A. O. De Silva, C. Spencer, B. F. Scott, S. Backus and D. C. Muir, Detection of a cyclic perfluorinated acid, perfluoroethylcyclohexane sulfonate, in the Great Lakes of North America, Environ. Sci. Technol., 2011, 45(19), 8060–8066, DOI:10.1021/es200135c.
- D. Szabo, D. Moodie, M. P. Green, R. A. Mulder and B. O. Clarke, Field-based distribution and bioaccumulation factors for cyclic and aliphatic per-and polyfluoroalkyl substances (PFASs) in an urban sedentary waterbird population, Environ. Sci. Technol., 2022, 56(12), 8231–8244, DOI:10.1021/acs.est.2c01965.
- Minnesota Pollution Control Agency, PFAS in the metal plating and finishing industry, 2022, document number: gp3-05, available at, https://www.pca.state.mn.us/.
- J. Marchiandi, D. Szabo, S. Dagnino, M. P. Green and B. O. Clarke, Occurrence and fate of legacy and novel per-and polyfluoroalkyl substances (PFASs) in freshwater after an industrial fire of unknown chemical stockpiles, Environ. Pollut., 2021, 278, 116839, DOI:10.1016/j.envpol.2021.116839.
- S. Schulze, D. Zahn, R. Montes, R. Rodil, J. B. Quintana, T. P. Knepper, T. Reemtsma and U. Berger, Occurrence of emerging persistent and mobile organic contaminants in European water samples, Water Res., 2019, 153, 80–90, DOI:10.1016/j.watres.2019.01.008.
- P. Kjeldsen, A. Grundtvig, P. Winther and J. S. Andersen, Characterization of an old municipal landfill (Grindsted, Denmark) as a groundwater pollution source: Landfill history and leachate composition, Waste Manage. Res., 1998, 16(1), 3–13, DOI:10.1177/0734242X9801600102.
- R. J. Slack, J. R. Gronow and N. Voulvoulis, Household hazardous waste in municipal landfills: contaminants in leachate, Sci. Total Environ., 2005, 337(1–3), 119–137, DOI:10.1016/j.scitotenv.2004.07.002.
- M. M. Lorah, I. M. Cozzarelli and J. K. Böhlke, Biogeochemistry at a wetland sediment–alluvial aquifer interface in a landfill leachate plume, J. Contam. Hydrol., 2009, 105(3–4), 99–117, DOI:10.1016/j.jconhyd.2008.11.008.
- J. R. Lang, B. M. Allred, J. A. Field, J. W. Levis and M. A. Barlaz, National estimate of per-and polyfluoroalkyl substance (PFAS) release to US municipal landfill leachate, Environ. Sci. Technol., 2017, 51(4), 2197–2205, DOI:10.1021/acs.est.6b05005.
- S. R. De Solla, A. O. De Silva and R. J. Letcher, Highly elevated levels of perfluorooctane sulfonate and other perfluorinated acids found in biota and surface water downstream of an international airport, Hamilton, Ontario, Canada, Environ. Int., 2012, 39(1), 19–26, DOI:10.1016/j.envint.2011.09.011.
- T. N. Penland, W. G. Cope, T. J. Kwak, M. J. Strynar, C. A. Grieshaber, R. J. Heise and F. W. Sessions, Trophodynamics of per-and polyfluoroalkyl substances in the food web of a large Atlantic slope river, Environ. Sci. Technol., 2020, 54(11), 6800–6811 CAS.
- A. Koch, M. Jonsson, L. W. Yeung, A. Karrman, L. Ahrens, A. Ekblad and T. Wang, Quantification of biodriven transfer of per-and polyfluoroalkyl substances from the aquatic to the terrestrial environment via emergent insects, Environ. Sci. Technol., 2021, 55(12), 7900–7909, DOI:10.1021/acs.est.0c07129.
- S. Mayakaduwage, A. Ekanayake, S. Kurwadkar, A. U. Rajapaksha and M. Vithanage, Phytoremediation prospects of per-and polyfluoroalkyl substances: a review, Environ. Res., 2022, 212, 113311, DOI:10.1016/j.envres.2022.113311.
- N. Barbo, T. Stoiber, O. V. Naidenko and D. Q. Andrews, Locally caught freshwater fish across the United States are likely a significant source of exposure to PFOS and other perfluorinated compounds, Environ. Res., 2023, 220, 115165, DOI:10.1016/j.envres.2022.115165.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00612g |
| This journal is © The Royal Society of Chemistry 2025 |
