 Open Access Article
Open Access ArticleStrategies for improving the design of porous fiber felt electrodes for all-vanadium redox flow batteries from macro and micro perspectives
Hengyuan
Hu
ab,
Meisheng
Han
*ab,
Jie
Liu
 ab,
Kunxiong
Zheng
ab,
Zhiyu
Zou
ab,
Yongbiao
Mu
ab,
Fenghua
Yu
ab,
Wenjia
Li
ab,
Lei
Wei
ab,
Kunxiong
Zheng
ab,
Zhiyu
Zou
ab,
Yongbiao
Mu
ab,
Fenghua
Yu
ab,
Wenjia
Li
ab,
Lei
Wei
 ab,
Lin
Zeng
ab,
Lin
Zeng
 ab and
Tianshou
Zhao
ab and
Tianshou
Zhao
 *ab
*ab
aShenzhen Key Laboratory of Advanced Energy Storage, Department of Mechanical and Energy Engineering, Southern University of Science and Technology, Shenzhen, 518055, China. E-mail: hanms@sustech.edu.cn; zhaots@sustech.edu.cn
bSUSTech Energy Institute for Carbon Neutrality, Southern University of Science and Technology, Shenzhen, 518055, China
First published on 19th February 2025
Abstract
All-vanadium redox flow batteries (VRFBs) have emerged as a research hotspot and a future direction of massive energy storage systems due to their advantages of intrinsic safety, long-duration energy storage, long cycle life, and no geographical limitations. However, the challenges around cost constrain the commercial development of flow batteries. Increasing the power density and energy efficiency of the flow batteries is key to breaking through the cost bottlenecks, which is closely related to porous fiber felt electrodes (PFFEs), in which redox reactions take place. Therefore, it is essential to summarize advanced strategies for improving the design of electrodes, which are conducive to the further expansion of low-cost and high-performing flow batteries. This paper reviews the growth rate and market size of the flow batteries, and summarizes the latest research progress in the improvement strategies of PFFEs from macro and micro perspectives, including structure design based on the data model, intrinsic treatment, and introduction of catalysts. Finally, this review summarizes the practicability of the above strategies and the prospective modification approaches, and looks forward to the future optimization directions of PFFEs, such as exploring the modification mechanisms using advanced in situ characterization techniques, introducing high-entropy catalysts, adopting new preparation technologies, and incorporating artificial intelligence. The review offers the optimization strategies of PFFEs for flow batteries and bridges the gap between the academic literature and industrial manufacturing.
Broader contextHigh-efficiency and long-duration energy storage technology is vital for stabilizing the grid and integrating renewable sources like solar and wind energy. All-vanadium redox flow batteries (VRFBs) are ideal for large-scale and long-duration energy storage due to their intrinsic safety, long life, and scalability. However, their high cost limits commercial adoption. Reducing the cost depends on improving power density and energy efficiency, with porous fiber felt electrodes (PFFEs) playing a key role. A systematic review of optimization strategies for the design of PFFEs is essential to bridge the gap between research and industrial application, accelerating the adoption of VRFB technology. In this context, this review explores various modification strategies for PFFEs from macroscopic and microscopic perspectives. It covers techniques such as multi-level structural designs, intrinsic treatments, heteroatom doping, and catalyst introduction. Macroscopic strategies aim to improve electrolyte flow and reaction efficiency, while microscopic optimization focuses on enhancing conductivity and activating reaction sites. The review also summarizes the practicability of the above strategies and the prospective modification approaches. Besides, the review proposes future research directions of PFFEs, including investigating electrode–electrolyte interfacial mechanisms using in situ characterization techniques, introducing high-entropy catalysts, adopting new preparation technologies, and incorporating artificial intelligence. This comprehensive review provides crucial insights that can drive VRFB technologies forward and is expected to have a profound impact on the large-scale deployment of energy storage solutions in the future. |
1. Introduction
Excessive reliance on fossil energy has led to irreversible environmental pollution and energy depletion, triggering a research frenzy in renewable energy such as solar energy, wind energy, etc. Despite the rapid development of photovoltaics and wind turbines over the past decade, and the rapid decline in the cost of photovoltaic and wind power, there is a huge difference between installed capacity and grid-connected electricity. This is because solar energy and wind energy have intermittent, decentralized, and unstable characteristics. The most effective way to overcome the shortcomings of renewable energy is to develop energy storage technology. As an important and the most mature energy storage device, lithium-ion batteries (LIBs) have the characteristics of high energy density and fast response, which make them very suitable for medium- and short-duration energy storage.1 Nonetheless, the potential safety hazards posed by LIBs during use and the inability to achieve long-duration energy storage prompt researchers to explore new, intrinsically safe, and long-duration energy storage technologies. Long-duration energy storage technology can not only effectively solve the intermittent problem of renewable energy power generation but also assist in building a new power system dominated by new energy, which is of great significance to the global energy layout. Notably, the Global Long-Duration Energy Storage Council released a report during the 29th United Nations Climate Change Conference, stating that long-duration energy storage is the key to achieving the net-zero emission target. By 2040, the global deployment scale of long-duration energy storage systems is expected to increase to 8 TW, which is 50 times the current scale. Therefore, it is crucial to develop intrinsic safe and long-duration energy storage devices.As a new type of long-duration energy storage device, water-based redox flow batteries have received favor from researchers due to their intrinsically safe, flexible duration, and wide application scenarios. Currently, the all-vanadium redox flow batteries (VRFBs) represent a type of the widely used flow batteries in business, which is attributed to their advantages such as no toxic by-products, environmental friendliness, high safety, and high energy efficiency (EE).2 Particularly, compared to rival LIBs, VRFBs have the core advantages of intrinsic safety, long cycling life, long-duration energy storage, and environmental friendliness. First, the use of water-based electrolytes in VRFBs determines their intrinsic safety. The safety of energy-storage batteries is an important guarantee for their rapid development. For LIBs, internal or external shorts would raise the internal temperature of the battery and thus accelerate exothermic reactions at both the anode and cathode, triggering combustion of organic electrolytes and thus leading to thermal runaway. Excitingly, these mechanisms do not occur in VRFBs due to the decoupling of capacity and power and the usage of water-based electrolytes, which can effectively prevent the occurrence of the troublesome thermal runaway seen in LIBs, thus enhancing safety and stability. Second, VRFBs have no phase change during charging and discharging and can be deeply charged and discharged, implying a long cycle life and low self-discharge probability. Third, compared to LIBs (a short- and medium-duration energy-storage technology), the decoupling of capacity and power endows VRFBs with the characteristic of long-duration energy storage, which is more effective at addressing the intermittent power output of solar and wind power, favoring increasing the proportion of new energy generation. Finally, the electrolytes of VRFBs can be recycled, significantly reducing resource waste and exhibiting high environmental protection. Despite their advantages, VRFBs continue to encounter cost-related challenges. Based on partial data, the cost of VRFBs is approximately 2.6 CNY per W h, whereas the average cost of LIBs is around 1.2 CNY per W h, only roughly 46% of VRFBs. More importantly, LIBs currently account for ∼97% of the battery energy storage market size, while VRFBs account for less than 0.6%, even lower than that of lead–acid batteries (∼1.0%, a low-cost and technologically mature aqueous battery for energy storage). The key factor limiting the large-scale development of VRFBs is their high cost. It can be predicted that once the cost bottleneck is broken through, VRFBs can achieve rapid and large-scale development in the next 10–20 years due to the characteristics of their intrinsic safety, long cycle life as well as long-duration energy storage, and their market share in the field of battery energy storage may reach 30–40% by 2040. Hence, decreasing VRFBs’ cost is urgently needed and is the top priority for future development.
Enhancing the EE and power density of VRFBs is crucial for overcoming the cost limitations. As shown in Fig. 1, VRFBs are primarily composed of battery stacks, positive/negative electrolytes, and battery management systems, accounting for 24%, 58%, and 18% of the VRFB cost, respectively, calculated based on a 4 h energy-storage duration.3 Among them, the battery stack provides a place for electrochemical reactions, and is the core component of the energy storage system to realize the mutual conversion of electrical energy and chemical energy.4 The battery stack is mainly composed of membranes, electrodes, bipolar plates, current collectors, and electrode frames, which have a great impact on the cost, power, cycle life, efficiency, and maintenance performance of the energy storage system. Specifically, the cost of VRFBs is closely related to their own EE and power density. On the one hand, increasing EE and power density can effectively reduce the usage amount of key components and volume of the battery stack. When the current density of the battery increases from 180 mA cm−2 to 400 mA cm−2, under the same battery capacity, the areas of the electrodes, membranes, and bipolar plates will all be reduced to 45% of the original. Taking a 16 kW stack as an example, when the rated current density increases from 180 to 400 mA cm−2 and the prerequisite is that EE is above 80%, the size of the stack can be reduced from 1026 × 650 × 450 mm to 640 × 560 × 510 mm (data from the research in our team). The obvious reduced volume can greatly decrease the battery stack cost. On the other hand, the improvement of EE and power density also helps to improve the electrolyte utilization, thus extending the energy-storage duration of VRFBs. The improvement of electrolyte utilization can reduce the amount of electrolyte used, thereby lowering costs. Besides, the extension of energy-storage duration also can decrease cost. For example, when energy-storage duration is extended from 4 to 10 h, the system cost of VRFBs will be reduced by 30–40%. After multiple cost compressions, the cost of VRFBs may reach 1–1.5 CNY per W h within the next 10 years.3 At that time, it will be on par with the cost of its competitor LIBs, which will greatly promote the large-scale development of VRFBs. Therefore, optimizing the performance of VRFBs is crucial. As an important component of the battery stack, porous fiber felt electrodes (PFFEs) are the key to determining EE and power density of the stack and even VRFBs. Electrodes mediate complex reaction processes involving macroscopic electrolyte flow, microscopic mass transport, and interfacial electrochemical reactions. At the microscopic level, ion transport is dependent on convection (determined by electrolyte flow rate), diffusion (driven by concentration gradients), and migration (driven by potential differences). At the electrode–electrolyte interfaces, ions undergo adsorption, electron transfer, and desorption. At the macroscopic level, owing to the optimal pore size distribution, the designed porous electrodes ensure the effective mass transfer of the electrolyte and the relatively uniform distribution of velocity and concentration, which in turn determine the polarization and mass transfer problems on the electrode surface.5 The above transport and reaction processes make PFFEs become the key components of VRFBs. Therefore, modifying the performances of PFFEs has become an important strategy to achieve cost reduction and efficiency increase in VRFBs.
At present, the market of the commercial PFFEs is huge, with a rapid development momentum. The newly installed capacity of VRFBs is increasing year by year, and the global flow battery market size exceeds 10 billion. It is worth noting that the market size of VRFBs in the Asia-Pacific region accounts for 32% of the global market share in 2021, with China's market size occupying a major portion. According to predictions from the Askci Consulting Co., Ltd and EVTank, the market size of VRFBs in China in 2025 will be approximately 8 billion CNY, with the market size of electrode materials being approximately 1.101 billion CNY. To meet the huge market demand for PFFEs, leading domestic and international PFFE enterprises such as Liaoning Jingu, Shenyang Fulai, Jiangyou Runsheng, SGL Group, and Toray Industries have established large-scale PFFE production lines, aiming to mass-produce low-cost and high-quality PFFEs for VRFBs to obtain significant profits. Notably, although commercial PFFEs have been produced on a large scale, their low catalytic activity, few active centers, and slow reaction kinetics greatly limit the EE and power density of VRFBs, preventing them from meeting the market's long-duration and efficient energy storage needs for flow batteries.6 Therefore, modifying and optimizing PFFEs are crucial. The main purpose of PFFE modification and optimization is to increase the specific surface area (SSA) of the materials, introduce active centers, and improve the hydrophilicity and catalytic efficiency of the materials, thereby enhancing the adsorption and catalytic performance of PFFEs for vanadium ions, reducing the polarization problem and improving the power density and EE of VRFBs.7 This paper provides an overview of the modification strategies for the design of PFFEs from two aspects: macrostructure designs based on electrolyte flow and microstructure designs based on mass transfer coupled with interfacial electrochemical reaction, as shown in Fig. 2. Finally, this review summarizes the valuable modification strategies of PFFEs, and puts forward practical suggestions for the long-term development of PFFEs. PFFEs can be divided into carbon felt (CF) and graphite felt (GF) due to different heat treatment temperatures, of which GF has a higher heat treatment temperature (>2000 °C) than CF (∼1000 °C). Consequently, GF has a higher degree of graphitization, higher electrical conductivity, and better mechanical properties than CF, endowing GF with a dominant demand in the current market. Nevertheless, GF and CF are both porous fiber felt products, which have similarities and versatility in modification strategies. Therefore, we do not give any additional distinction between CF and GF in subsequent discussions on the modification strategies.
2. Macroscopic design of the electrode structure
The macroscopic designs of the battery structure aim to design and optimize the deformation degree, external shape, and flow field structure of the electrode according to the specific needs and working conditions. These macroscopic designs are necessary, which are closely related to the polarization, mass transfer, and other problems existing in the flow batteries themselves. Specifically, there are usually three major types of polarization in VRFBs, namely ohmic polarization caused by the resistance of electrodes and electrolytes, concentration polarization caused by electrolyte diffusion and mass transfer, and electrochemical polarization caused by the electrochemical reactions occurring on the surface of PFFEs. These three types of polarization are closely related to a large difference in electrolyte flow rate, uneven concentration distribution, low mass transfer efficiency, and insufficient electrochemical reactions. The macroscopic designs of the electrode structure are an effective strategy to solve these bottlenecks. However, the macroscopic designs need to be carried out according to specific needs and working conditions, which determine that relying on the big data model to build the corresponding working conditions is the best way to design. Therefore, this section summarizes the macro designs of electrodes, such as compression ratio, shape, thickness, and flow field design, with the assistance of big data models, aiming to provide a reference for subsequent electrode research.2.1 The compression ratio of the electrode
Typically, when assembling the PFFEs, the entire PFFEs are stacked with the separator and the graphite bipolar plate in sequence, then pressurized as a whole. While ensuring sealing, the PFFEs are compressed to reduce the contact resistance between the PFFEs and the graphite bipolar plate, and improve the electrolyte fluidity. However, this compression process may have problems such as increased liquid-phase mass transfer resistance, excessive compression affecting electrolyte flow rate, inconsistent compression rates leading to overcharging, and leakage in a sealed high-pressure environment. Therefore, it is necessary to explore the compression ratio of PFFEs in combination with model predictions to optimize the performance of electrode materials.To explore the optimal compression ratio of PFFEs, Charvát et al. used a series of complex characterization techniques, including ex situ through-plane electrical conductivity measurement and comprehensive evaluation of single-cell performance, to regulate the compression effect of two different commercial CF electrodes, polyacrylonitrile-based electrodes and rayon electrodes.8 After testing, it was found that the area with a high electrode compression ratio has a small resistance, which can reduce the ohmic impedance and facilitate the rapid transfer of electrons. In contrast, the electrode with a low electrode compression ratio has high electrolyte permeability and a faster electrolyte flow rate, thereby reducing pump consumption. After weighing the charge–discharge load curve and pressure drop measurement data of the single cell, the researchers found that the optimal compression ratio of the polyacrylonitrile CF electrode is 30%, while the optimal compression ratio of the rayon CF electrode is 60%. At the optimal compression ratio, the total power loss caused by battery polarization and electrolyte pumping is evaluated as the minimum. It is worth noting that the optimal compression ratio of electrode materials can also be affected by the internal flow channels of the battery. Wang et al. tested the uneven electrode compression caused by the flow channel at different compression ratios and their impact on battery performance, and on this basis proposed an accurate model to study the influence of non-uniformly compressed electrodes on the flow and electrochemical performance of VRFBs with flow channels.9 The presence of the flow channel results in different porosity and permeability in different regions. The results showed that during the electrode compression process, the spatial distribution of the flow channel affects the electrolyte flow rate, the local current density, and the distribution of overpotential. With the flow rate remaining unchanged, the increase in the compression ratio causes an increase in the pressure difference between adjacent flow channels and the volumetric flow rate also increases, thus enhancing mass transfer. When the compression ratio is 55.7%, the distribution of active substances in the electrode is the most uniform, where the local current density and overpotential simulated by the model are the smallest, as shown in Fig. 3a and b. The non-uniform model proposed in this study can predict the influence of electrode compression and flow channels in VRFBs, providing an effective guidance for the design of VRFBs with compressed electrodes and flow channels.
 | ||
| Fig. 3 Distributions of (a) current density and (b) overpotential (i = 40 mA cm−2) under different electrode CRs.9 Copyright (2018). Elsevier. The simulated contour plots of rectangular electrodes, trapezoidal electrodes and sector electrodes. (c) Velocity and (d) V3+ concentration.10 Copyright (2019). Elsevier. | ||
2.2 The shape of the electrodes
Electrode shape is an often overlooked, but is an extremely important factor for improving the performance of flow batteries. The influence of the shape of the electrodes on the flow batteries is mainly reflected in the internal polarization and pumping consumption of the battery. The reason for this is that the change of electrode shape can significantly affect the electrolyte distribution, flow rate, and mass transfer correlation. However, it is difficult to study the concentration polarization and mass transfer problems through repeated experiments, and the establishment of data models for optimization has become an indispensable strategy. Building a data model and delving into the subtle link between electrode shape and battery performances is key to optimizing VRFB performance.Conventional rectangular electrodes can cause a large electrolyte concentration difference between the inlet and outlet of the electrolyte, which in turn affects mass transfer and produces concentration polarization, especially at high current densities. Zheng et al. proposed to build a circular VRFB assembled with circular electrodes, and gradually reduced the electrode cross-sectional area from inlet to outlet.11 Surprisingly, this circular electrode, with its decreasing cross-sectional area from inlet to outlet, improves the flow velocity and concentration difference of the electrolyte, thereby overcoming the disadvantages of concentration polarization and mass transfer of traditional rectangular electrodes. The assembled flow battery exhibits an EE of 83.31% at low flow rate, high current density, and low pump consumption. Not only circular electrodes, but also some trapezoidal and sector electrodes are also favored by researchers. Yue et al. proposed a trapezoidal electrode design to optimize the polarization and other problems in rectangular electrodes.12 Owing to the trapezoidal electrodes' gradual reduction of the cross-sectional area from inlet to outlet, the flow velocity in the electrolyte gradually increases from inlet to outlet and peaks at outlet, which significantly improves electrolyte mass transfer and distribution. Compared with the above-mentioned circular electrode, the EE of the trapezoidal electrode is as high as 85.64% under the same working conditions, which is better than the performance of the circular electrode. With the help of a 3D model, Gurieff et al. designed a radial sector electrode structure to promote the flow rate and concentration distribution of the electrolyte based on shape dynamics.10 Compared with the traditional rectangular and trapezoidal electrodes, the prepared sector electrode exhibits a higher electrolyte flow velocity and a more uniform concentration distribution, but there is a high pressure drop caused by rapid changes in the cross-sectional area, as shown in Fig. 3c and d.
Concentration polarization and limited mass transfer are headaches for flow batteries, which are closely related to the velocity and concentration distribution of the electrolyte. Designing electrodes with a decreasing inlet-to-outlet cross-section can effectively improve these problems because the design accelerates the flow rate of the electrolyte, promotes electrolyte distribution, and reduces pump consumption efficiency. However, some electrodes with higher curvature and greater design difficulty, such as circular electrodes and sector electrodes, are hard to quickly achieve the transition from laboratory research to industrial application. With the rapid development of 3D printing technology in terms of cost control and accuracy improvement, the related dilemma is expected to be significantly improved. Notably, the trapezoidal electrode is the most likely to be applied on a large scale in the future. Firstly, it can be processed and designed based on the existing rectangular electrodes, with low consumption of the process and cost. Secondly, compared with the traditional rectangular electrodes, the trapezoidal electrode has obvious advantages in mass transfer optimization and reduction of concentration polarization, which is an effective strategy to improve the performance of VRFBs. On the whole, trapezoidal electrodes are very promising electrode shapes, which can not only improve the disadvantages of traditional electrodes, but also reduce the problems of high voltage drop, which is in line with the needs of future commercial development.
2.3 The thickness of the electrode
Electrode thickness plays a crucial role in electrolyte distribution, cell polarization, conduction resistance, mass transfer efficiency, and power delivery in the power pump in VRFBs. On the one hand, a thinner electrode not only shortens the ion transport and electron transport distance, which is conducive to reducing ohmic polarization, but also reduces pumping power required, thereby improving overall efficiency.13 However, a thinner electrode is unable to provide a large number of active sites and a wide reaction contact area for redox reactions in VRFBs, which leads to a relatively large electrochemical reaction polarization and restricts the efficiency of VRFBs. During the preparation process of PFFEs, rationally designing the thickness of the electrode can maximize the performance of the electrode. On the other hand, a thicker electrode will produce high ohmic losses caused by ohmic polarization, especially when working in the high power/current region of the battery, which will seriously restrict the power density of VRFBs. Therefore, it is crucial to explore the electrode thickness through model prediction and trade-off of multiple external factors.Ali et al. established a three-dimensional (3D) numerical model for studying the electrode thickness size and porosity of VRFBs by combining the effects of electrode thickness and porosity, and electrolyte distribution and flow rate on VRFB performance.14 The predicted results show that an increase in electrode thickness can enhance the distribution uniformity of vanadium ion concentration, and the optimum thickness is 3 mm. At the same time, the decrease in electrode thickness and the increase in porosity can increase the pressure drop and pumping power, thereby providing the required electrolyte flow rate for VRFBs. In general, when the electrolyte flow rate is 10 ml min−1, the battery with higher porosity and a thickness of 1 mm achieves the highest power efficiency of 96.8%, as shown in Fig. 4a–d. The 3D model proposed in this study has high applicability to VRFBs under low electrolyte flow conditions, and provides a certain reference for designing electrode thickness.
 | ||
| Fig. 4 (a) Power efficiency for different electrode thicknesses and electrolyte flow rates; numerical calculation of cell voltage (b) based on the concentration and electrode thickness, (c) based on the electrode porosity and electrolyte flow rate, and (d) based on the electrode porosity and concentration.14 Copyright (2020). Elsevier. (e) Concentration polarization distribution on different felt designs at a current density of 200 mA cm−2. 32 kW stack efficiency at varied conversion fractions per pass: (f) constant current mode and (g) variable flow mode.15 Copyright (2022). Elsevier. | ||
The choice of electrode thickness depends on the specific requirements, and the optimal thickness of the electrode varies according to different needs. At present, there are two main types of electrodes on the market, one is a thick electrode with a thickness of 1–6 mm.16 Due to its high SSA, it is often used in traditional flow batteries. The second is a thin electrode with a thickness of 0.2–0.6 mm, which is often used in compact and miniaturized flow batteries.17 To balance the relationship between electrode thickness, battery performance, and economic benefits, Muñoz-Perales et al. used the stacking method to change the electrode thickness to explore the optimal electrode thickness under two types of flow fields: flow-through flow fields (FTFF) and interdigitated flow fields (IDFF).18 FFTF refers to a flow field with only one inlet and one outlet. IDFF refers to a flow field with multiple inlets and multiple outlets. Based on the established electrode material data model, it is found that the thicker electrode exhibits a lower voltage drop in FTFF, while the thinner electrode exhibits a higher mass transfer efficiency in IDFF. The electrochemical performance factors are comprehensively analyzed, and it is found that the electrodes with a thickness of 0.4–0.7 mm (stacked with 2 layers) may be the best configuration in terms of electrochemical performance under various flow field combinations. Furthermore, considering the electrochemical performance and related pressure loss, the thicker electrodes may be suitable for FTFF and the thinner electrodes may be suitable for IDFF.
Based on the data model, macroscopic control of electrode thickness is an efficient means to optimize the efficiency of VRFBs. Optimal electrode thickness is a combination of additional factors that need to be balanced. We believe that a thick electrode may be the best choice when there is a single-channel flow field and a full range of microscopic modifications to the electrode. However, thin electrodes may be the best choice when there is a multi-channel flow field, especially for stacks or flow batteries, which require miniaturization and low cost.
2.4 The flow field design of the electrode
Carefully designing PFFEs’ flow field is key to improving VRFBs' performance. A well-designed flow field has many benefits, which can significantly boost the electrolyte flow rate, enhance mass transfer, and make electrolyte distribution more even in the battery. Moreover, it can prevent vanadium species from piling up on the electrode surface due to slow redox reactions, ensuring stable battery performance. Optimized flow field of PFFEs works excellent in reducing polarization. It can cut down concentration polarization from uneven diffusion and mismatched kinetics, and also lessen extra ohmic and electrochemical reaction polarization caused by poor electrolyte–electrode contact and incomplete reactions.19Hao et al. used finite element analysis to adjust the flow field design of the CF electrode and achieved a significant increase in the power density of the vanadium flow battery. The researchers adopted parallel flow and cross-flow field designs for CF respectively, and revealed the positive effects of these two flow field designs in reducing CF pressure drop, in promoting the uniform distribution of reactants, and in increasing the flow rate, as shown in Fig. 4e–g.15 According to the measured local mass transfer coefficient, the parallel flow field has a better improvement effect on concentration polarization than the cross-flow field design at 200 mA cm−2. Further experimental verification shows that the CF with a parallel flow field has a VE of up to 78% at 200 mA cm−2 and the corresponding discharge capacity is also improved. To introduce the parallel flow field design into production, this experiment carried out dynamic modeling and simulation on a 32 kW industrial battery stack. The results show that the CF with parallel flow field design can also reach a system efficiency of 70% at 200 mA cm−2, which has a positive guiding significance for the subsequent application of CF flow field design to actual production. In addition, our team also proposed to predict the electrolyte flow in VRFBs using a deep neural network (U-Net) based on the flow field characteristics, so as to accurately identify the dead zones, further increase the under-rib convection, and improve the flow field design.20 Owing to the optimization of the flow field, the system efficiency of the battery at 200 mA cm−2 has been improved by 5.5%. Moreover, compared with the traditional numerical simulation, the U-Net-assisted prediction significantly reduces the computational time by 99.9%, greatly improving the efficiency. This new strategy of U-Net-assisted numerical simulation calculation is the key to optimizing the application of AI in flow field and even battery design. Therefore, we believe that applying some advanced machine learning or deep neural networks to assist in flow field design, especially the parallel flow field design, is a promising strategy at present.
3. Microscopic design of the electrode structure
In the energy conversion and storage of flow batteries, a large number of electrochemical reactions and microscopic mass transfer occur between the electrolyte and the electrode materials, which are mainly carried out through the contact, collision, and adsorption between the reactive ions and the electrode materials. Therefore, high-performance electrodes usually need to have a large number of active sites to provide a site for electrochemical reactions, which points out the directions for the microscopic design of electrode structures. This section summarizes the microscopic modification strategies of PFFEs, such as intrinsic treatment and introduction of catalysts, to provide new ideas for the subsequent research and development of electrodes.3.1 Intrinsic treatment
Intrinsic treatment is a commonly used method in PFFE modification strategies. Its characteristics include a simple process and ease of operation, without introducing additional impurities. After intrinsic treatment, PFFEs remain intact, thereby ensuring relatively stable performance. In addition, intrinsic treatment can introduce functional groups and defects into the PFFEs, increasing the SSA and improving the hydrophilicity, local electronegativity, electrochemical catalytic activity, and reversibility of the PFFEs.21 However, intrinsic treatment also has certain limitations, such as limited improvement of material properties.22 This section reviews the two strategies of intrinsic processing: heteroatom doping and design of pores. In addition, this section also lists the relevant material properties of the two strategies in Table 1.| Optimized strategies | Doped substances or improved topography | P max (mW cm−2) | EE | R CT (Ω) | R CT (Ω cm2) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Cycle number | Current density (mA cm−2) | R CT (Ω cm2) | ||||||
| a R CT for VO2+/VO2+. b R CT for V2+/V3+. | ||||||||
| Heteroatom doping | Dopamine and polyethyleneimine | N | 750.6 | 75.3% | 0.06 | — | 23 | |
| 600 | 300 | |||||||
| (NH4)2SO4 | N | 350.9 | 87.34% | — | 24 | |||
| 25 | 80 | |||||||
| CO2 | O | — | 83.28% | 3.30 | — | 25 | ||
| 30 | 50 | |||||||
| H3BO3 | B | — | 82.07 | — | 26 | |||
| 2000 | 240 | |||||||
| N2, O2 | N, O | — | ca. 78% | 26.7 | — | 27 | ||
| 30 | 50 | |||||||
| Silk protein | N, O | — | 86.8% | — | 28 | |||
| — | 40 | |||||||
| H3BO3, air | B, O | — | ca. 70% | — | 1.6 | 29 | ||
| 100 | 140 | 1.2 | ||||||
| Ethylenediamine tetra | N, P | — | 84% | — | 30 | |||
| 100 | 150 | |||||||
| LiCl, KCl, KClO3, NH4Cl | N, O | — | 75.9% | — | ca. 5 | 31 | ||
| 140 | 260 | ca. 5 | ||||||
| Br2 | Br | — | 86.8% | — | 32 | |||
| — | 50 | |||||||
| Design of pore | SSA and pore | 540 | ca. 75% | 20 | — | 33 | ||
| 200 | 120 | |||||||
| SSA and pore | — | ca. 78% | — | 0.068 | 34 | |||
| 500 | 320 | 0.068 | ||||||
Zhang et al. took the copolymer of dopamine and polyethyleneimine as the precursor and nitrogen source of the electrode materials, and innovatively utilized the in situ interfacial copolymerization technique to deposit villous N-doped carbon onto CF electrodes.23 Owing to the modification and optimization of N doping and the hierarchical interconnection structures of the materials, the assembled VRFBs exhibited a high EE of 73.6% at 300 mA cm−2, and still maintained good stability and extremely low EE attenuation (energy attenuation per cycle is 0.006) after 600 cycles at 200 mA cm−2. In-depth studies found that the improvement of the reaction kinetics of V2+/V3+ is mainly due to the enhanced adsorption of V2+/V3+ by the pyridine nitrogen formed by N doping, while the improvement of the reaction kinetics of VO2+/VO2+ is mainly due to the doped N atoms significantly reducing the activation energy barrier of the corresponding reaction. Jiang et al. fabricated and tested VRFBs with the B-doped GF electrodes.26 First-principles studies pointed out that the carbon surface of B doping has many active and stable reaction sites. After electrochemical testing, the VRFBs showed an EE of 87.40 and 82.52% at 160 and 240 mA cm−2, respectively, and even showed an EE of 77.97 and 73.63% at 320 and 400 mA cm−2, respectively, demonstrating their excellent performance based on the published literature. Even more excitingly, after 2000 cycles at 240 mA cm−2, the VRFBs have only 0.028% and 0.0002% capacity and efficiency decay per cycle, respectively. Moreover, the degradation of battery performance caused by long-cycle testing can be recovered by refreshing the electrolyte. These results indicate that the stable and high-performance B-doped GF is an exciting electrode for VRFBs.
Additionally, compared to single-atom doping, multi-atom co-doping has a synergistic effect, resulting in a more comprehensive modification and optimization of PFFEs. Huang et al. introduced N and O atoms into CF electrodes through plasma treatment technology, fabricating N, O dual-doped electrodes (O-N-CF).27 Compared to single-atom doping (O-CF and N-CF), the O-N-CF electrodes exhibit a better battery performance and a more outstanding VO2+/VO2+ redox activity. This is mainly attributed to the numerous functional groups introduced by N and O doping, which provide the active sites for vanadium ion reactions, improve the material's conductivity, and enhance the performance of O-N-CF. Ammonium persulfate (APS) is rich in N atoms and S atoms. Researchers introduced N-containing and S-containing groups on CF electrodes through heat treatment with APS for use in VRFBs.48 The CF electrodes treated with APS have a stable, oxygen-rich hydrophilic surface, significantly improving the overall performance of VRFBs. Zhang et al. prepared a N, P co-doped GF through a simple one-step activation method.30 The synergy between N atoms and P atoms introduces a large number of active sites in GF electrodes. The corresponding VRFBs maintain an EE of 84% at 150 mA cm−2. To enhance the doping efficiency and construct multi-dimensional defect engineering, a novel molten salt doping strategy is proposed. Jiang et al. used KClO3 as the oxygen source and NH4Cl as the nitrogen source, adopting a LiCl/KCl dual molten salt system to introduce N and O functional groups into GF electrodes, as shown in Fig. 5.31 The molten salt system doping strategy not only uniformly incorporates heteroatoms into GF electrodes but also builds a porous structure deep within the GF electrodes. The modified GF electrodes possess abundant N and O groups, and a large number of micropores, significantly enhancing the SSA, wettability, and electronic structure properties of GF electrodes. Notably, the synergistic effect of N and O functional groups enables the CF electrodes (GF/ON) to exhibit optimal electrocatalytic properties. The VRFBs assembled with GF/ON (GF/ON-PN) achieved an EE of 64.9% at 260 mA cm−2, and the obtained electrochemical performances, including discharge capacity, cycling stability, and circuit impedance, are superior to those of commercial brand-new GF electrodes. This strategy of N and O co-doping through the dual molten salt medium provides a practically valuable reference for subsequent preparations of high-performance VRFB electrodes.
 | ||
| Fig. 5 (a) Schematic diagram of the process of preparing N- and O-doped CF electrodes by the molten salt system. SEM images of (b) GF and (c) and (d) GF/ON. (e) The discharge capacity of pristine and GF/ON-PN at 260 mA cm−2, and (f) efficiency (CE and EE) and (g) system resistance of pristine and GF/ON-PN at 140 mA cm−2 for 200 cycles.31 Copyright (2024). Wiley-VCH. | ||
Surprisingly, some halogen atoms such as Br, Cl, and F can be doped into the edge of the carbon structures, thereby promoting charge transfer and electrocatalytic reactions.49 Park et al. prepared halogenated graphene nanoplatelets (F-, Cl-, and Br-GNP) by ball milling in the atmosphere of F2, Cl2, and Br2, respectively.32 The performances of these three groups of halogenated graphene nanoplatelets follow the order Br-GNP > Cl-GNP > F-GNP. The performance of the Br-GNP electrode is excellent, with a capacity retention rate of 86.8% at 50 mA cm−2. This new method shows a new perspective for preparing high-performance VRFB electrodes through heteroatom doping. Heteroatom doping is an efficient modification process that can accurately introduce specific heteroatoms to optimize material properties. However, there are some disadvantages in heteroatom doping, such as low doping efficiency, many side reactions, complex processes, and high costs. Currently, many new doping techniques are expected to solve the above problems. For example, the ion implantation method can precisely control the doping position and concentration of heteroatoms. The plasma treatment can help improve the doping efficiency, and the chemical vapor deposition (CVD) can uniformly dope heteroatoms on the surface of the material. The molten salt activation system can not only uniformly dope but also construct a multi-dimensional pore structure.50,51 Among these, the molten salt activation system treatment process is relatively simple, which can achieve the construction of pore structures and the uniform doping of heteroatoms. It is a very promising strategy among the new heteroatom doping methods in our opinion.
3.1.2.1 Pore size. In the structure of carbon materials, according to the standards of the International Union of Pure and Applied Chemistry, the pore size can be divided into macropores (>50 nm), mesopores (2–50 nm), and micropores (<2 nm).53 The presence of macropores will improve the permeability of the material, but will reduce the SSA of the materials. The presence of micropores increases the SSA of the materials and facilitates charge transfer, but leads to poor permeability. Mesopores are the bridge between macropores and micropores.54 There is a subtle relationship between the size of the pore, the SSA of the material, and permeability. Therefore, the trade-off between material permeability and SSA should be made when designing the pore size structures.
Specifically, in VRFBs, the migration processes of vanadium ions in the pore size are as follows. (i) Vanadium ions follow the electrolyte flow to the void regions of the reaction sites on the electrode material surface. (ii) Vanadium ions diffuse from the inside of the solution to the electrode–electrolyte interfaces. (iii) Redox reactions occur at the electrode–electrolyte interfaces. Notably, in process (iii), pore structures with a size of 50–100 nm can effectively eliminate the ion diffusion limitation caused by the space effect.55 Meantime, redox reactions usually occur in mesopores of 5–10 nm.56 Therefore, the pore structures of PFFEs should be multi-scale and multi-dimensional. Wu et al. used Zn metal compounds to construct CF electrodes with multi-dimensional pores (MPNCF-5), as shown in Fig. 6.34 MPNCF-5 can facilitate the swift infiltration of electrolytes, enhancing the diffusion efficiency and the redox reaction rate of vanadium ions within the mesopores/nanopores. After testing, the SSA of MPNCF-5 is as high as 4.586 cm2 g−1, i.e., 9 times that of ordinary CF electrodes, and the charge transfer resistance (RCT) of MPNCF-5 electrodes is 68.7 mΩ cm2. In addition, the VRFBs with MPNCF-5 electrodes can achieve an EE of 81.9% at 320 mA cm−2, which is 15.2% higher than the EE of the original CF electrodes (66.7%). Even at 320 mA cm−2, the MPNCF-5 electrodes can undergo 500 long-term cycles without significant attenuation of energy density.
 | ||
| Fig. 6 (a) Diagram of the preparation of porous CF electrodes. (b) SEM image of MPNCF-5. (c) Electrochemical impedance spectra of different batteries. (d) EE. (e) EE, VE, and Coulomb efficiency (CE) of the battery after 500 cycles at 320 mA cm−2.34 Copyright (2018). Royal Society of Chemistry. | ||
The combination of multi-scale and multi-dimensional pore structures is an effective strategy to balance the SSA and permeability of electrodes. In terms of designing pore structures, some novel strategies may help achieve the formation of multi-level pore structures. (i) Materials with multi-dimensional complex structures are selected as precursors for the preparation of electrodes. The complex structures of the precursor are expected to endow carbon materials with multi-dimensional structures.53 (ii) The preparation of materials is in combination with multi-dimensional pore-forming agents. For example, MgO and SiO2 contribute to the formation of micropores in materials, while some gases such as CO2 contribute to the formation of macropores in carbon materials.33,55,57 Therefore, we believe that using multi-dimensional complex materials as precursors and regulating the pore structure of materials with efficient multi-dimensional pore-forming agents and activators is a very promising strategy in pore structure design and formation.
3.1.2.2 Pore tortuosity. Pore tortuosity is a usually overlooked factor in the study of PFFEs. The influence of pore tortuosity on electrode materials is mainly reflected in the SSA of the material, and the flow rate and concentration distribution of the electrolyte. Combined with the relationship between tortuosity and velocity, the corresponding tortuosity formula can be constructed:
where τ is the tortuosity, u is the velocity, and x, y, z denote the corresponding directions.58 Typically, large tortuosity can impede the flow of the electrolyte and affect the permeability, resulting in a large transmission resistance. Based on 3D modeling, Wang et al. compared the velocity distribution, concentration distribution, and current distribution in cylindrical, slit, and spherical pores.59 The results show that the cylindrical pore structures have the maximum current density, the most uniform flow rate, and the smallest pore tortuosity at the same voltage. Although pore tortuosity is not a decisive factor in the performance of an electrode, it can still be used as a small strategy to optimize the electrodes. Some template strategies are expected to improve the tortuosity of materials, i.e., using long and straight nanorods as templates for constructing the pore structure of materials.60 In addition, it is also necessary to pay attention to the degree of compression of the materials, and a high degree of compression will increase the bending of the material.61
3.1.2.3 Electrode structure porosity. The structure porosity of electrodes can also significantly affect the performance of the electrodes. High porosity reduces the SSA of the material but promotes electrolyte delivery. In addition, the structure porosity can also affect factors such as electrolyte concentration, velocity, and current density. Trade-offs in porosity designs to achieve optimal electrode performance require big data to build the model.
A study compared three different structure porosity types of electrodes: uniform porosity, low structure porosity at the inlet of the electrolyte, and low structure porosity at the outlet of the electrolyte. The outcomes show that VRFBs with electrodes showing low structure porosity at the inlet of the electrolyte have the highest EE.62 Alphonse et al. numerically investigated the effect of increasing structure porosity on the potential of electrodes, overpotential, current density, and species concentration in VRFBs during the process of discharge by establishing the data models of equilibrium state. Four types of electrode structure porosity distributions were set up in the experiment: (i) the porosity of both the positive and negative electrodes is constant and the value is the same (set to 94%); (ii) the porosity of the negative electrode increases from 64% to 94% with the electrode thickness (increasing gradually by 10%), while the porosity of the positive electrode remains at 94%; (iii) the porosity of the positive electrode increases from 64% to 94% with the thickness of the electrode (increasing gradually by 10%), while the porosity of the negative electrode remains at 94%; and (iv) the porosity of both the positive and negative electrodes gradually increases from 64% to 94% with the electrode thickness (increasing gradually by 10%). It is found that the batteries in the fourth case exhibit the highest potential performance, lowest overpotential, and most uniform current distribution compared to the previous three cases. Therefore, hierarchical porous structures are indispensable for the high performance of materials.
Here, some advanced technical strategies are expected to solve the rational manufacturing of structure porosity, such as the use of AI-enabled 3D printing technology, which is expected to achieve precise control of porosity. Introducing some organic framework structures such as MOFs into materials is also an effective strategy for constructing precise porosity and pore structures.
3.2 Introduction of catalysts
The modification strategies of intrinsic treatment can improve the performance of PFFEs by increasing the number of active sites, SSA, etc.63 However, due to the limited increase in the number of active sites and SSA, there are also limitations to the performance optimization of intrinsic treatment, which cannot meet the needs of high-performance electrodes. Surprisingly, the introduction of catalysts can accelerate the speed of specific reactions and improve the catalytic activity of PFFEs, thus significantly improving the overall performance of PFFEs. Currently, the types of catalysts include carbon-based catalysts, metal catalysts, metal oxide catalysts, metal carbide catalysts, and other special catalysts. These catalyst materials each have their own advantages, and can effectively tune the performance of PFFEs.3.2.1.1 0D carbon-based catalysts. 0D carbon-based materials such as carbon quantum dots and fullerenes are the new generation of carbon-based materials. Their surface/interfacial effects, scale effects, and macroscopic quantum tunneling effects make them exhibit superior optoelectronic and mechanical properties, compared to conventional materials, and are widely used in electronics, catalysis, and other fields.65 Fullerene is a hollow molecule composed of carbon, whose nano-size and specific morphology endow it with a large SSA, high conductivity, good thermal conductivity, and special mechanical properties. In addition, the surface of fullerene has a rich electron cloud, which can interact with ions, thereby promoting the adsorption of ions.66,67 Meantime, the electron cloud can also coordinate with ions to form stable complexes, further enhancing the adsorption of ions. Diwany et al. first proposed the application of fullerene as a catalyst in VRFB electrodes and achieved efficient catalysis of VO2+/VO2+.68 Characterization test results showed that each fullerene has a spherical structure with a size of less than 1 nm. Notably, fullerene is mainly sp2 hybridized graphite carbon with some oxygen-containing functional groups, which make the material have abundant active sites and high conductivity. The high conductivity and special electron cloud structure of fullerene greatly improve the adsorption and catalytic performance of the material for vanadium ions. Furthermore, researchers found that, in a mixed acid electrolyte, the fullerene-modified materials have high stability and can prevent the precipitation of toxic chlorine in side reactions. This work opens up the way for the development and application of fullerene catalysts in VRFB electrode materials.
Similarly, as an important part of 0D carbon-based materials, carbon quantum dots have attracted the attention of researchers due to their ultra-small size, large SSA, and rich surface (edge) defects. Zhou et al. first reported the preparation of carbon dot-modified GF (CD/GF) electrodes by a one-pot solvothermal method.69 The doping mechanism of the one-pot solvothermal method is that during the solvothermal reaction process, carbon source molecules undergo reactions such as polymerization and carbonization in a high-temperature and high-pressure environment, thus forming carbon dots distributed on the GF surface. With a size of approximately 3–6 nm, these carbon dots are evenly distributed on the surface of GF, transforming it from a surface-ordered to a surface-disordered CD/GF structure. Moreover, the modification of CDs introduces a large number of defects into the electrode surface, significantly enhancing the hydrophilicity of CD/GF and increasing the adsorption of vanadium ions on the electrode. The high conductivity and stability of CDs also promote CD/GF to exhibit low RCT and high stability. Through electrochemical tests, the battery assembled with the CD/GF electrode was found to have an EE of 52.2% at a current density of 350 mA cm−2, and after 1000 long cycles at 150 mA cm−2, the EE still remained at 74%. In addition, this battery can adapt to work in a variety of temperature environments and has great application potential.
Although 0D carbon-based catalysts have been widely used in photocatalysis, biopharmaceutical catalysis, and other fields, there are still few studies on the modification of PFFEs in VRFBs. In the above studies, both fullerene and carbon quantum dots have a positive effect on the conductivity and stability of electrode materials, and also make a significant contribution to the catalytic performance. Therefore, it is necessary to study the application of 0D carbon-based catalysts in the modification of PFFEs. Further studies can focus on these materials and deeply analyze the contribution of size effects to the modification effect, which will be valuable work.
3.2.1.2 1D carbon-based catalysts. The traditional carbon-based electrodes have poor electrocatalytic activity, small SSA, and poor hydrophilicity. Therefore, at high current densities, they cannot meet the need for rapid transmission of a large number of charges and efficient catalysis. Notably, the unique tubular structures of 1D carbon-based materials shorten the transmission path of charges and improve the ion diffusion rate. Moreover, these materials also have a large SSA and excellent stability, which make them favored by researchers. 1D carbon-based materials can be divided into three categories according to diameter: carbon nanotubes (CNTs) with a diameter less than 50 nm, carbon nanofibers (CNFs) with a diameter between 50 and 200 nm, and carbon fibers with a diameter greater than 200 nm.70
CNTs possess a large number of sp2-hybridized and graphite-like hexagonal carbon ring network structures, which have a positive effect on the transmission of charges and the adsorption of ions. Zhang et al. using ZIF-67 arrays as precursors and melamine as the nitrogen and carbon source, and under the regulation of Co2+ ions, uniformly constructed N-doped CNTs (NCNT@CF) on CF through a bottom-up simple method (Fig. 7a and b).71 Specifically, ZIF-67 arrays are first in situ grown on the CF substrate through a hydrothermal reaction, with Co2+ attached thereon. Then, melamine is pyrolyzed into carbon and nitrogen substances at high temperature, attached to ZIF-67, and eventually evolved into N-doped CNTs. During this process, Co2+ is converted into the catalyst Co, which promotes the generation of CNTs. This doping method enables CNTs to be fully and uniformly attached to the electrode. Adjusting the microstructure of ZIF-67 and the amount of melamine can effectively regulate the micromorphology, N doping amount, and bonding strength of CNTs. Benefiting from the superiority of NCNTs, the assembled battery exhibits an astonishing EE of up to 76.6% at 300 mA cm−2 and maintains long-term stability over 550 charge/discharge cycles. Introducing functional groups into CNT catalysts is an effective and commonly used means to improve the activity of CNT catalysts. Dai et al. modified CNTs by high-temperature activation with KOH and applied them as cathode catalysts in VRFBs.72 Notably, the doping of CNTs mainly relies on the ultrasonic dispersion and impregnation drying method. Firstly, CNTs are ultrasonically dispersed in the solvent. Then, the GF is immersed in the solvent and dried to obtain CNT doped GF. Moreover, the activation of KOH introduces oxygen-containing functional groups and a large number of defects in CNTs. These oxygen-containing functional groups provide active centers for the electrode and optimize the hydrophilicity of the material, thereby enhancing the adsorption and catalysis of vanadium ions. Notably, some other functional groups, such as borate-based, phosphate-based, and sulfonic acid-based functional groups, also promote the adsorption and catalysis of vanadium ions by providing reaction sites for the electrode and enhancing the hydrophilicity of the electrode.73–76
 | ||
| Fig. 7 (a) Flow chart of the preparation process of NCNT@CF. (b) Catalytic mechanism of N-doping on vanadium ions.71 Copyright (2024). ACS Publications. (c) Flowchart of NBCNTs’ topography design and corresponding EE and VE performance; (d) TEM and (e) SEM image of NBCNTs.77 Copyright (2023). Elsevier. | ||
Unfortunately, in the application field of CNT catalysts, problems such as weak adhesion and morphology fragmentation are often encountered, which limit the application of CNT catalysts. Therefore, solving the problems of firmly and efficiently adhering CNTs to the PFFEs is an urgent task. Organic binders can fix CNTs on the CF relatively well, but the introduction of binders will hinder the transport of ions and affect the catalytic performance.78 To avoid the influence of foreign impurities on catalytic performance, researchers innovatively used in situ formed sucrose pyrolysis carbon as a binder to fix CNTs and GF.79 The composition of this binder mainly consists of carbon materials similar to CNTs and GF, therefore featuring high adhesiveness and low contact resistance. Recently, a new in situ growth strategy has also been used to introduce CNT catalysts, and this method can uniformly fix CNT catalysts without the need for binders. Liu et al. uniformly introduced N, B-doped CNTs (NBCNTs) on the surface of CF by using an in situ growth strategy, as shown in Fig. 7c–e.77 The mechanism of in situ growth of CNTs is to immerse CF in a growth solution prepared from urea, polyethylene glycol and boric acid, and then carbonize CF at high temperature. At high temperature, the growth solution will first generate B, N co-doped carbon nanosheets. During annealing, thermal stress appears in the carbon nanosheets due to the difference in the cooling rates of different parts. When the thermal stress is greater than the yield limit of the carbon nanosheets, they will curl to form NBCNTs. By adjusting the ratio of N and B, the morphology of CNTs can be designed, and they can be fixed on the surface of CF by in situ growth, avoiding the influence of binders. The EE of the VRFBs assembled with NBCNTs is 79.3% at 300 mA cm−2, and even remains at 68.7% at 500 mA cm−2. In addition, after 1000 long cycles of testing at 300 mA cm−2, the EE shows almost no attenuation.
Compared to CNTs, CNFs have larger sizes and are commonly used to prepare PFFEs, while less used as catalysts to modify PFFEs.80,81 However, some studies still explored the improvement effect of CNFs on the performance of VRFBs after compositing with PFFEs. Zhang et al. reported a strategy of wrapping carbon nanofiber networks around CF electrodes using the self-assembly process of polyaniline.82 This method does not require a binder and introduces N, S-doped porous carbon nanofiber networks to CF electrodes. Owing to the abundant active sites introduced by heteroatoms and the interconnected channels provided by the CNF network, the prepared electrodes exhibit an EE of 82.4% at a current density of 320 mA cm−2. Moreover, the electrodes also show excellent long-term stability over 1000 cycles. In addition to constructing the CNF network, Xu et al. proposed to construct a dual-gradient CNF/GF composite electrode structure to optimize the performance of VRFBs.83 The construction of the dual-gradient CNF/GF composite electrode involves introducing more CNFs on the side of the GF electrodes close to the membrane and less CNFs on the side close to the plate. The former relies on the high catalytic activity of CNFs to promote the redox reactions of vanadium ions in VRFBs, and the latter utilizes the high conductivity of CNFs to promote charge conduction. In addition to the macro-gradient distribution of CNFs on the GF, this work also radially micro-gradiently distributed CNFs along individual fibers. This macroscopic and microscopic gradient distribution strategy greatly reduces the resistance and improves the performance of VRFBs. After testing, these electrodes were found to show the lowest ohmic resistance, contact resistance, and RCT. The assembled battery exhibits an EE of 81.84% at a current density of 300 mA cm−2 and has a peak power density of 1109.5 mW cm−2. Notably, the modified electrodes also show far superior cycle stability and rate performance compared to commercial PFFEs.
The morphology and performance of 1D carbon-based catalysts play an important role in improving the performance of PFFEs. Currently, there are still shortcomings in the introduction of 1D carbon-based catalysts, such as the inability to firmly adhere the catalyst to the PFFE surface, the morphology of the catalyst hindering the flow of electrolytes, and complex preparation processes. Subsequent works can explore these directions in-depth, such as precisely regulating the morphology of 1D carbon-based catalysts with the help of the in situ characterization instruments and advanced preparation processes, introducing functional groups and defects into 1D carbon-based catalysts, and constructing high-performance 1D carbon-based catalyst/PFFE composite electrodes.
3.2.1.3 2D carbon-based catalysts. 2D carbon-based materials (graphene, graphite sheets, etc.) have received favor from researchers in recent years and are widely used in the fields of energy, catalysis, biology, etc. In the PFFE modification strategy, 2D carbon-based catalysts are highly desirable, which is closely related to their special structures, and excellent physical and chemical properties. The surface of 2D carbon-based materials has abundant covalent bonds, which facilitate electron transfer, making the PFFEs have high conductivity. Secondly, the large 2D plane provides a lot of space for the attachment of active sites and chemical reactions that optimize the overall catalytic performance of the PFFEs.84 Moreover, the strong acid–base inertness of these catalysts enables them to adapt to many electrolytes and expands their application range.
Graphene is a type of 2D carbon nanomaterial, which exists as single layer and multilayer graphene as well as graphene oxide (GO), reduced graphene oxide (rGO), etc.85 Graphene is composed of hexagonal honeycomb lattices of carbon atoms with sp2 hybrid orbitals, featuring a large SSA, high conductivity, and rich edge defects. Xia et al. deposited commercial graphene catalysts on the surface of CF electrodes through the solution coating method, and used them as the positive electrode (G/CF) of VRFBs to catalyze the VO2+/VO2+ redox reactions.86 The G/CF electrodes significantly enhance the catalytic performance of the VO2+/VO2+ redox reactions and reduce the ohmic polarization during the charge–discharge processes. The prepared VRFBs exhibit a peak power density of approximately 325 mW cm−2, which is 39 mW cm−2 higher than that of pure CF. Additionally, the VRFBs maintain the EE above 80% after 500 cycles at a current density of 80 mA cm−2. Notably, optimizing the graphene catalysts before introducing them to CF can significantly improve the modification effect. Fu et al. coated CF electrodes with Ar plasma-treated multi-layer graphene (Ar-GCF) via CVD, as shown in Fig. 8a–c.87 Specifically, methane was cracked in a mixture of methane and hydrogen at 1040 °C, depositing carbon atoms on CF to form carbon felt loaded with multilayer graphene (GCF). Then, GCF was treated with argon plasma to obtain the modified electrode material Ar-GCF. Ar-GCF has numerous defects and oxygen-containing functional groups, causing electrons on the material surface to tend to distribute near the O-containing functional groups, greatly enhancing the conductivity of the electrode materials. The power density, EE, and voltage efficiency (VE) of the VRFBs assembled with Ar-GCF are 1130.09 mW cm−2, 87.16%, and 89.11%, respectively, outperforming the commercial pristine CF electrodes. More importantly, the RCT of the Ar-GCF electrodes increases by only 15.79% after 800 cycles, while the RCT of the commercial pristine CF electrodes increases by 102.40% after 600 cycles, which strongly demonstrates the excellent stability of the Ar-GCF electrode in VRFBs. Some heteroatoms have also been used to modify the graphene catalysts. Li et al. introduced N-doped rGO (N-rGO) into GF electrodes (N-rGO/GF) by the freeze-drying method using urea as the nitrogen source.88 Notably, the rGO in this work was prepared as follows. First, GO was made by the Hummers' method using graphite powder, sodium nitrate and concentrated sulfuric acid, and a GO solution was obtained after the reactions. Then, the GF electrode was soaked in the urea–GO solution, followed by freeze-drying and pyrolysis in a nitrogen atmosphere tube furnace for the conversion of GO to rGO and nitrogen doping, getting the N-rGO/GF electrode. Owing to the synergistic effect of nitrogen atoms and graphene catalysts, N-rGO/GF exhibits a large SSA and a high density of active sites, significantly enhancing its catalytic performance for the VO2+VO2+ reactions.
 | ||
| Fig. 8 (a) Flowchart of Ar-GCF preparation. (b) SEM image of Ar-GCF. (c) O–graphene charge density difference (the yellow area indicates increased electron density, the cyan area indicates decreased electron density).87 Copyright (2023). Elsevier. (d) Schematic diagram of graphene modification. (e) CV of pure CF and G/CF. (f) Peak power density of pure CF and G/CF.89 Copyright (2021). Springer Nature. | ||
Although the modification effect of graphene catalysts on PFFEs is excellent, complex processes and high costs limit their development. To realize industrialized and large-scale production as soon as possible, many researchers have made great contributions. Long et al. proposed a method for large-scale preparation of G/CF electrodes by the CVD method, and reached a scale of 20 cm × 20 cm, as shown in Fig. 8d–f.89 The specific CVD method is as follows. H2 and CH4 are introduced into the tube furnace where CF is placed at a ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 under high temperature. CH4 decomposes into carbon atoms in the high temperature and H2 atmosphere and grows on the surface of CF to form graphene. Surprisingly, the EE of this electrode at a current density of 100 mA cm−2 is 86.37%, and the peak power density reaches 1189 mW cm−2. These achievements are encouraging. Unfortunately, in the field of graphene catalyst applications, the π–π bond stacking interaction and strong van der Waals force between the graphene layers cause these materials to easily suffer from problems such as agglomeration, thereby reducing the SSA and affecting performance.90 Advanced 3D fabrication techniques, such as the formation of vertical graphene on PFFE surfaces and the incorporation of porous graphene, are expected to address the issue of graphene agglomeration. These optimized 3D strategies are described in the next section.
3 under high temperature. CH4 decomposes into carbon atoms in the high temperature and H2 atmosphere and grows on the surface of CF to form graphene. Surprisingly, the EE of this electrode at a current density of 100 mA cm−2 is 86.37%, and the peak power density reaches 1189 mW cm−2. These achievements are encouraging. Unfortunately, in the field of graphene catalyst applications, the π–π bond stacking interaction and strong van der Waals force between the graphene layers cause these materials to easily suffer from problems such as agglomeration, thereby reducing the SSA and affecting performance.90 Advanced 3D fabrication techniques, such as the formation of vertical graphene on PFFE surfaces and the incorporation of porous graphene, are expected to address the issue of graphene agglomeration. These optimized 3D strategies are described in the next section.
3.2.1.4 3D carbon-based catalysts. 3D carbon materials are widely used in the fields of electrochemistry and electrocatalysis due to their high SSA and rich pore structures. Therefore, 3D carbon-based catalysts are also commonly used to modify PFFEs. These porous structures endow 3D carbon-based catalysts with large reaction contact area and fast ion transfer channels, which can effectively improve the catalytic efficiency. This section mainly elaborates and analyzes some highly concerned 3D carbon materials, such as 3D graphene catalysts, C–C composite catalysts composed of multi-dimensional carbon materials, and green and efficient bio-carbon-based catalysts.
(i) 3D graphene catalysts. The porous structures of the 3D carbon-based catalysts can not only effectively promote ion transfer but also improve the aggregation problem existing in the 2D graphene catalyst. Porous graphene is also called the graphene nanonetwork, which is characterized by a rich multi-dimensional pore structure inside. Compared with traditional graphene, porous graphene has a larger SSA, more active sites, and more edge defect structures.91 The performance improvement brought about by the porous structure of porous graphene can well counteract the negative effects brought about by graphene agglomeration and empower itself. Opar et al. introduced N-doped graphene onto CF electrodes (NMG-CF-7) through a simple hydrothermal process, and constructed an N-doped 3D porous graphene CF electrode (NMG-CF-7) under high-temperature pyrolysis, which was applied to VRFBs.92 Owing to the special structures of porous graphene, NMG-CF-7 exhibits a large SSA (19.45 m2 g−1) and a rich pore structure. The porous structures with a large SSA can increase the number of active sites and reduce the mass transfer resistance of vanadium ions and ohmic polarization, enabling the electrode to show improved electrochemical performances even at high current densities. Electrochemical tests show that NMG-CF-7 has an EE of 75.1% at 150 mA cm−2, with excellent rate performance and cycling stability. The emergence of porous graphene points out the direction for the high-performance commercial application of graphene.
In addition, the rational distribution of graphene catalysts can also offset the negative effects of graphene agglomeration. Guo et al. proposed a metal-free catalytic CVD strategy for in situ growth of vertical porous N-doped graphene on GF.93 The specific growth mechanism is as follows. Using CH4 as the carbon source and H2 as the reducing gas, 2D graphene will grow in situ on GF at a high temperature of 1100 °C. After the temperature drops to 900 °C, NH3 is introduced into the tube furnace. This will etch the grown graphene and introduce nitrogen atoms, forming N-doped 3D porous graphene. As shown in Fig. 9a–f, the introduced porous graphene catalysts are vertically, evenly, and tightly distributed on the GF materials. The reasonable distribution not only effectively improves the graphene agglomeration problem but also avoids the uneven coverage of active substances. In addition, the vertical multi-dimensional structures also provide channels for the large and rapid transport of charges, improving conductivity. Electrochemical tests show that the EE of the electrode material (NVG@GF) is 87.1 and 83.3% at 200 and 300 mA cm−2, respectively, with a peak power density of 1308.56 mW cm−2, which is superior to the modified electrode of carbon nanomaterials in the existing reports of VRFBs. More importantly, the decrease in EE caused by long-term cycling can be restored to the initial value by renewing the electrolyte. The many advantages of NVG@GF electrodes reveal their great value in the commercial field.
 | ||
| Fig. 9 (a) Schematic illustration of the preparation process, (b) and (c) SEM images, (d) structural advantages, (e) cycle stability and peak power density comparison with advanced nano carbon-modified electrodes, and (f) cycle performance at 300 mA cm−2 of the NVG@GF electrode.93 Copyright (2024). Wiley-VCH. (g) Flowchart of the synthesis of CNFs/CNTs grown on the surface of CF decomposed by C2H2 gas with the assistance of Ni nanoparticles. (h) HRTEM image of a CNF/CNT composite, with the inset showing the CNF and CNT sidewalls (white line: graphene layer orientation). (i) The coexisting structures in the CNF/CNT, respectively. (j) Schematic diagram of the advantages of CNF and CNT composite structures in catalyzing vanadium ions.94 Copyright (2013). ACS Publications. | ||
Notably, some high-performance graphene-modified PFFEs, such as porous graphene-modified PFFEs and vertical graphene-modified PFFEs, still have problems such as the complex preparation process. It is urgent to improve the current preparation methods such as template-assisted methods, self-assembly methods, and CVD methods, and apply them to actual production.95
(ii) C–C composite catalysts. C–C composite-based catalysts refer to composite catalysts composed of different types of 3D carbon-based materials. Different types of carbon-based materials have different advantages, and the combined carbon-based materials can play a synergistic role, making the C–C composite-based catalysts exhibit high performance.
Although CNFs and CNTs are both 1D carbon-based materials, they have different electrochemical characteristics. Due to the inclination of the graphene layer relative to the fiber axis of CNFs, CNFs expose edge planes with a large number of defects, which provide abundant active sites for ion adsorption and reaction on the outer surface of CNFs. Similarly, the graphene sheets of CNTs are composed of relatively inert basal planes, which endow CNTs with high conductivity and stability.96 The composite catalysts of CNFs and CNTs can give full play to the synergy and jointly improve the catalytic performance of VRFBs. Park et al. introduced a CNF/CNT composite catalyst on the surface of CF by acetylene gas pyrolysis and applied it as an electrode in VRFBs, as shown in Fig. 9g.94 The specific method for loading CNFs/CNTs on the CF substrate is as follows. The CF is nickelized to load reduced nickel and then calcined at high temperature in an atmosphere of C2H2 gas (carbon source). At 500 °C, the nucleation rate of acetylene is slow, and carbon atoms reach the entire nickel catalyst interface through diffusion and form CNFs without a hollow core. As the temperature rises to 700 °C, nucleation begins at the nickel metal/acetylene gas interface, resulting in the formation of CNTs, and thus the CNF/CNT composite catalyst is formed on the CF surface. The synergy between CNFs and CNTs lies in the efficient fit between the CNF outer wall with a large number of surface defects and the CNT surface for rapid electron transfer, which plays an important role in the vanadium redox reactions and significantly improves battery performance, as shown in Fig. 9h–j. Through electrochemical testing, the prepared electrodes show excellent rate performance and capacity retention at 100 mA cm−2, and the battery capacity can be restored at 40 mA cm−2.
Graphene is an efficient carbon-based catalyst, but it is limited by the problem of agglomeration. Other carbon-based materials combined with graphene to form C–C catalysts not only have the potential to overcome the agglomeration limitation of graphene, but also bring excellent performance improvement effects to VRFBs. Faraji et al. introduced functionalized CNTs (fCNTs) and GO on carbon materials by an electrochemical deposition method, and explored their catalytic activity in VO2+/VO2+ redox reactions.97 Specifically, the loading of GO-fCNTs is mainly achieved by using a solution rich in GO and fCNTs as the electrolyte and depositing GO and fCNTs on the graphite sheet through the galvanostatic method in a two-electrode system (the graphite sheet electrode is the working electrode and the platinum foil is the counter electrode). The introduction of GO can significantly improve the wettability of the electrode, mainly due to the large number of oxygen-containing functional groups on its surface. The introduction of fCNTs can construct a network structure on the electrode surface, which not only enhances the conductivity of the material, but also increases the SSA of the materials. In addition, the combination of fCNTs in GO also effectively improves the negative effects caused by agglomeration. Han et al. prepared GO and multi-walled CNT (MWCNT) composites by the electrostatic spraying method and used them to catalyze the VO2+/VO2+ redox reactions of VRFBs.98 The large SSA of GO combined with the complex conductive network of MWCNTs endow the modified electrode with excellent redox reversibility, ultra-low RCT, and high stability.
The C–C composite catalyst can give full play to the advantages of each component of the carbon-based catalysts, and maximize the performance of the catalysts under the enhancement of the synergistic effect, which provide certain guidance for the high-performance development of the carbon-based catalyst. However, C–C composite catalysts still have disadvantages such as difficult preparation, poor mechanical properties of the material, and weak bonding of each component material. Some new processes such as the self-template method and the use of metal–organic frameworks to construct morphologies are expected to improve the dilemma of such materials.
(iii) Bio-carbon-based catalysts. Introducing heteroatoms into carbon-based catalysts can effectively improve the catalytic performance, and their intrinsic special structures can also increase the rate of charge transfer, which prompt researchers to pay attention to biomass carbon materials with rich substances and contents, and special intrinsic structures.53 The rich substance contents of biomass enable it to have a large number of functional groups and defects without additional doping substances, which effectively simplifies the process. In addition, the special morphologies of biomass make it a natural template, which helps in the subsequent formation of multi-dimensional and multistage structures of the materials.99
Chen et al. used bamboo as a precursor to prepare an O, N co-doped hierarchical porous bamboo-based carbon modified CF (ABC-N-CF) through an acid treatment assisted plasma treatment approach, as shown in Fig. 10a–d.100 Acid treatment can selectively remove hemicellulose and lignin in bamboo to avoid the influence of impurities on material properties. O, N co-doping provides abundant functional groups and active centers for CF materials, while the 3D porous structures introduce defects and improve hydrophilicity for CF materials, which greatly improve the catalysis, adsorption, and other properties of CF electrode materials. Benefiting from the special structures of the material, the assembled battery shows a maximum power density of 1064.6 mW cm−2, which is better than the performance of the blank battery (741.9 mW cm−2). Meantime, compared with the blank battery, the assembled battery shows a higher EE and VE at each current density; especially at 250 mA cm−2, the EE is 20.9% higher than that of the blank battery, showing an excellent electrochemical performance. Hu et al. used lotus seed shells as raw materials to synthesize hard carbon materials by pyrolysis, and densely covered them on carbon fibers to prepare modified CF electrodes (Bio-CF).101 These electrodes have obvious catalytic effects on both V2+/V3+ and VO2+/VO2+ redox reactions. In addition, the Bio-CF electrodes can optimize the ion mass transfer and comprehensively optimize the performance of VRFBs. The assembled batteries provide a high EE (83.14%) and an excellent cycle stability at a current density of 100 mA cm−2.
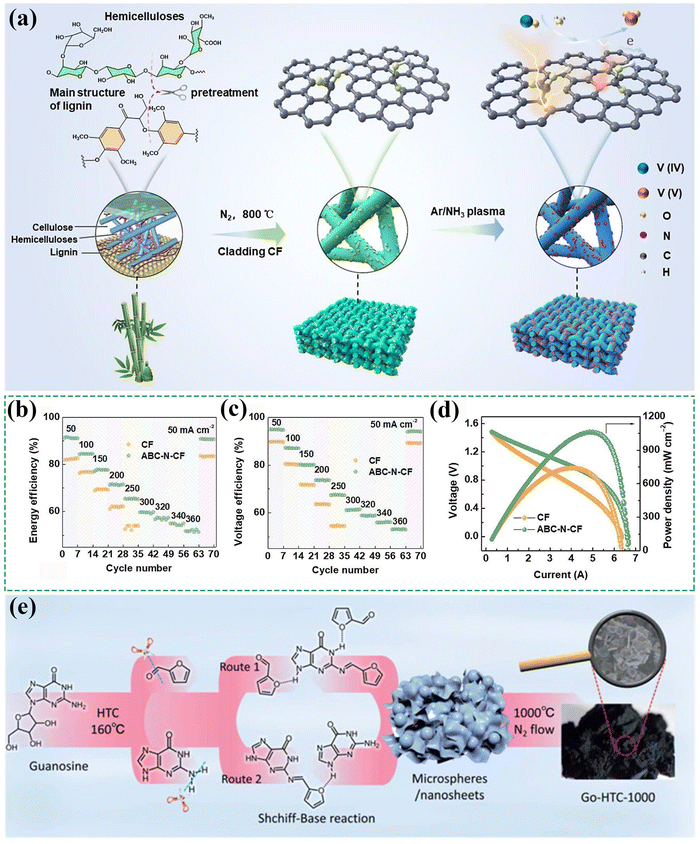 | ||
| Fig. 10 (a) Process flow diagram of ABC-N-CF electrode preparation. (b) EE and (c) VE of rate performance. (d) polarization curves and power density.100 Copyright (2024). ACS Publications. (e) Flow chart for the preparation of a unique carbon microsphere/nanosheet composite.102 Copyright (2020). Royal Society of Chemistry. | ||
In the previous section, this review outlines the existing difficulties of C–C composite-based catalysts, such as process difficulties and high costs. To improve these problems, a simple, green, and novel process for preparing C–C composites using the intrinsic structures of biomass as templates is proposed. Huang et al. used bio guanosine as a precursor to prepare N-doped carbon microspheres/nanosheets by a simple hydrothermal carbonization method and loaded them on GF to improve the electrocatalytic performance of VRFBs, as shown in Fig. 10e.102 The formation of the multi-dimensional morphologies of the carbon-based catalysts is the result of the synergistic effect of the intrinsic structures and molecules of bio guanosine under hydrothermal conditions. Among them, the construction of carbon microspheres is due to the cross-linking of a large amount of furfural and a small amount of the guanine component derived from the ribose of bio guanosine. Conversely, when the guanine component coexists with a small amount of furfural, the amino group of the guanine component undergoes a Schiff base reaction with the carbonyl group of furfural, thereby forming carbon nanosheets. Notably, this kind of carbon nanosheet shows a large SSA and a sponge-like morphology, which are closely related to the self-template effect of bio guanosine. Owing to the large SSA of the 2D carbon nanosheets and the abundant active sites provided by the carbon microspheres, the prepared electrode can still provide 66% EE at 500 mA cm−2, showing a strong rate performance. In addition, in the long-term cycling test, the prepared battery has almost no attenuation in EE after 100 cycles at 100 mA cm−2. This work opens up a new perspective for the application of biomass precursors in preparing C–C composites.
Bio-carbon-based catalysts have the characteristics of rich heteroatoms and special structures, which are good precursors for preparing carbon-based catalysts. However, biomass materials also have problems such as high impurity content, difficult regulation of the type and morphology of functional groups, and uneven material composition. Reasonable selection of biomass precursors, and understanding and analyzing the structures and characteristics of biomass will facilitate the application of such materials. Moreover, we summarize and compare the specific performance of the above carbon-based materials in Table 2, which has a certain reference value for the subsequent applications of carbon-based material catalysts. More importantly, we believe that depositing 3D porous graphene catalysts on PFFEs by the CVD method is a very promising modification strategy in the introduction of carbon-based catalysts.
| Characteristics | Doped substances | P max (mW cm−2) | EE | R CT (Ω) | R CT (Ω cm2) | Ref. | |
|---|---|---|---|---|---|---|---|
| Cycle number | Current density (mA cm−2) | R CT (Ω) | R CT (Ω cm2) | ||||
| a R CT for VO2+/VO2+. b R CT for V2+/V3+. | |||||||
| 0D | C76 | — | — | 4 | — | 68 | |
| — | — | ||||||
| CDs | — | 73% | ca. 7 | — | 69 | ||
| 1000 | 150 | ||||||
| 1D | CNTs | 803.9 | 82.4% | ca. 4 | — | 71 | |
| 550 | 200 | ca. 0.2 | |||||
| CNTs | — | 85.7% | — | 72 | |||
| 50 | 30 | ||||||
| CNTs | — | 80% | ca. 0.3 | — | 77 | ||
| 1000 | 300 | ca. 0.5 | |||||
| CNFs | — | 75.4% | — | 0.079 | 82 | ||
| 1000 | 320 | ||||||
| CNFs | 1109.5 | ca. 79% | — | 2.46 | 83 | ||
| 100 | 100 | ||||||
| 2D | Graphene | ca. 325 | ca. 81% | — | 86 | ||
| 500 | 80 | ||||||
| Graphene | 1130.09 | 87.16% | 0.66 | — | 87 | ||
| 800 | 100 | ||||||
| Graphene | 1189 | ca. 83% | 1.02 | — | 89 | ||
| 500 | 100 | — | |||||
| 3D | Mesoporous graphene | — | 80% | 0.94 | — | 92 | |
| 100 | 100 | 0.94 | |||||
| Vertical graphene | 1308.5 | 83.3% | — | ca. 3 | 93 | ||
| 750 | 300 | ||||||
| CNFs/CNTs | — | ca. 85% | — | 94 | |||
| — | 40 | ||||||
| Nanosheets/CNTs | — | — | 10.7 | — | 98 | ||
| — | — | 7.74 | |||||
| Derived from bamboo | 1064.6 | 85% | 0.45 | — | 100 | ||
| 1000 | 120 | ||||||
| Derived from lotus seed | 345 | 83% | 0.047 | — | 101 | ||
| 150 | 100 | ||||||
| Derived from guanosine | — | ca. 87% | ca. 4 | — | 102 | ||
| 100 | 100 | ca. 3 | |||||
Metal Bi has been proved to improve the catalysis of negative V3+/V2+ redox reactions for VRFBs.106 The catalytic mechanism of Bi is considered to be that Bi can generate BiHx intermediates in the vanadium salt environment, which can catalyze the V3+/V2+ redox reactions and inhibit the irreversible side reactions related to hydrogen, thereby improving the stability.107,108 Although metal Bi has good stability compared with other metals, hydrogen evolution and other side reactions are inevitable in the catalytic process. Using the deposition method to embed Bi deeply into the structural network of PFFEs not only avoids the dispersion of Bi by a liquid electrolyte, but also increases the overpotential of the hydrogen evolution reaction of the battery, thereby inhibiting hydrogen evolution and improving the efficiency of the battery.109 Li et al. introduced nano-Bi to the surface of GF electrodes by electrodeposition under the immersion of Bi3+ electrolyte ions.110 Nano-Bi can significantly improve the performance of VRFBs by suppressing the hydrogen evolution reaction and reducing the activation energy barrier of V2+/V3+. Through electrochemical tests, it was found that the introduction of nano-Bi not only accelerates the charge transfer rate at high current density, but also significantly improves the VE and EE of the battery. In addition to the hydrogen evolution problem of metal Bi, the loading amount of metal Bi is also worthy of exploration. Similarly, Yang et al. introduced Bi with different loadings on the CF surface by using a high-temperature hydrogen gas thermal reduction strategy.111 The loading method of Bi is as follows. First, CF is impregnated with the solution of Bi3+ and dried, and then high-temperature reduction is carried out under N2/H2 and Ar/H2 respectively to convert Bi3+ into Bi and anchor it on the CF surface. Moreover, this work innovatively uses heat treatment to enhance the adhesion between Bi and CF, and improve the stability of Bi. In addition, the researchers compared the effects of different Bi loadings on the electrochemical performance of the electrodes and found that the CF electrodes with 2% Bi loading (based on weight percentage) have the lowest RCT, and the assembled battery can reach 80.9% EE after cycling 300 times at 140 mA cm−2, showing good electrochemical performances. Too high Bi loading will increase the conductive resistance of the CF electrodes, while too low Bi loading cannot effectively improve the catalytic performance of the electrode material. Therefore, a reasonable catalyst loading is the key to optimizing the performance of the material.
To further enhance the modification effect of metallic Bi, researchers have also investigated the influence of the distribution and size of Bi on the surface of carbon electrodes on electrode performance. A uniform distribution of metallic Bi with a reasonable size on the surface of carbon materials can maximize the catalytic performance of Bi. Jiang et al. discovered that oxygen-containing functional groups on CF can regulate the size of Bi, promote its even distribution, and exhibit a synergistic catalytic effect with Bi.112 The synergistic mechanism between oxygen-containing functional groups and Bi is illustrated in Fig. 11a–f, where Fig. 11a–c represent the graphite structures without the introduction of oxygen-containing functional groups, and Fig. 11d–f represent the graphite structures with the introduction of oxygen-containing functional groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). As shown in Fig. 11d, the introduction of C
O). As shown in Fig. 11d, the introduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O causes C to protrude from the structural plane, effectively activating inert π electrons and enhancing the surface adsorption capacity. Additionally, as shown in Fig. 11f, a large number of electrons are transferred from Bi to the surface with CO groups, indicating that the CO groups have good adsorption properties for Bi. Simultaneously, the CO groups can promote the uniform distribution of electrons across Bi and the carbon substrate, which is beneficial for electrochemical reactions. Under the synergistic effect of oxygen-containing functional groups, small-sized Bi is uniformly distributed in the electrode material, endowing the electrode with excellent ion transport performance. The VRFBs assembled with these electrodes achieve an EE of 92.5% at 80 mA cm−2 and 88.4% at 160 mA cm−2, and even maintain an EE of 80.1% at 320 mA cm−2. For metal catalysts, the size effect is an interesting phenomenon, as the same metal catalyst may exhibit significantly different performances depending on its size.113
O causes C to protrude from the structural plane, effectively activating inert π electrons and enhancing the surface adsorption capacity. Additionally, as shown in Fig. 11f, a large number of electrons are transferred from Bi to the surface with CO groups, indicating that the CO groups have good adsorption properties for Bi. Simultaneously, the CO groups can promote the uniform distribution of electrons across Bi and the carbon substrate, which is beneficial for electrochemical reactions. Under the synergistic effect of oxygen-containing functional groups, small-sized Bi is uniformly distributed in the electrode material, endowing the electrode with excellent ion transport performance. The VRFBs assembled with these electrodes achieve an EE of 92.5% at 80 mA cm−2 and 88.4% at 160 mA cm−2, and even maintain an EE of 80.1% at 320 mA cm−2. For metal catalysts, the size effect is an interesting phenomenon, as the same metal catalyst may exhibit significantly different performances depending on its size.113
 | ||
| Fig. 11 (a) Original graphite surface. (b) Bi modification of the original graphite. (c) The charge difference plots of Bi modified graphite. (d) Graphite with carbonyl groups. (e) Graphite containing carbonyl groups after Bi modification. (f) The charge difference images of Bi modified graphite with carbonyl groups (the yellow areas indicate electron gain and the blue areas indicate electron loss).112 Copyright (2019). Elsevier. (g) Flow chart for the preparation of semi-embedded CF in Bi nanospheres. (h) SEM image of Bi-NS-CF. (i) TEM image of Bi-NS-CF. (j) Polarization curves. (k) The assembled battery of the side, peacekeeper.114 Copyright (2020). Wiley-VCH. | ||
Large-sized and sparse-distributed Bi can limit catalytic performance. Reducing the size of Bi and studying its impact on electrodes is an important research direction in the future. Zhou et al. achieved semi-embedding of nano-sized spherical Bi uniformly into CF through carbothermal reduction of bismuth oxide, and assembled it into a Bi-NS-CF electrode, as shown in Fig. 11g–k.114 This work controls the size of metallic Bi to 25 nm, and the Bi nanospheres are embedded inside the porous CF with high density and dispersion, significantly increasing the active sites on the CF surface. Moreover, the bismuth nanospheres are also bonded to the carbon structures, enabling them to maintain high stability in the flowing electrolyte. The peak power density of the Bi-NS-CF electrode reaches 1238 mW cm−2, with an EE of 78.6% and an EE retention rate of 98.2% after 1000 cycles. Liu et al. further reduced the size of Bi to 10 nm and fabricated a uniformly distributed Bi-modified electrode (Bi@TGF) through a polyvinylpyrrolidone-guided method.115 The precise Bi electrocatalyst not only plays a crucial role in catalyzing the V2+/V3+ redox reactions but also effectively suppresses the hydrogen evolution side reactions. The AVFBs assembled with Bi@TGF demonstrate a peak power density of 1023 mW cm−2 and an EE of 81.5% at 200 mA cm−2. Furthermore, the Bi@TGF electrodes exhibit an outstanding stability in a 2500-cycle ultra-long charging–discharging test at 300 mA cm−2, with an EE retention rate of 99.2%. Surprisingly, to further improve the modification effect of metal Bi on the electrodes, Xing et al. used N atoms to anchor the metal Bi on GF to form electrodes with Bi–N4 coordination (Bi SAs/NC@GF), which were applied to VRFBs (1.6 M V3+/VO2+ and 3 M H2SO4 as the electrolyte).116 The modification mechanisms are as follows. (i) Bi–N4 can promote the desolvation of vanadium ions in the electrolyte and reduce the reaction barrier of vanadium ions. The coordination bond of Bi–N4 promotes the deformation of the vanadium ion structure after solvation with water molecules ([V(H2O)6]3+/[V(H2O)6]2+) and forms *Bi[V(H2O)5]3+ with high catalytic activity and stability, thereby improving the performance of VRFBs. (ii) Relying on the inner-sphere reaction mechanism can facilitate charge transfer. During charge transfer, *Bi[V(H2O)5]3+ transforms to *Bi[V(H2O)5]2+, followed by desorption. Then, HSO4− and SO42− adsorb around V2+ to form coordination bonds, which contribute to facilitating the conversion of V3+ to V2+ and increase the charge transfer during the reaction. The assembled VRFBs exhibit a peak power density of 990 mW cm−2 at 240 mA cm−2 and achieve an EE of 80% after 1500 cycles. This work provides a new idea for metal Bi-modified electrodes.
Other metal nanoparticles also show outstanding performance on modified PFFEs. Mehboob et al. transformed Sn2+ in the electrolyte into Sn nanoparticles and deposited them on CF by in situ electrodeposition.117 Since the deposition potential of Sn2+/Sn is close to the potential of the V3+/V2+ reduction reactions, the catalytic efficiency of Sn is greatly improved. When the concentration of Sn2+ ions in the electrolyte is 0.01 M, the assembled VRFBs exhibit outstanding performance (77.3% EE at 150 mA cm−2). The addition of Sb3+ to the negative electrolyte and its deposition on the GF surface improve the catalytic performance.118 Under the optimal Sb3+ ion concentration of 5 mM, the EE shows a maximum (67.1%) at 120 mA cm−2. Notably, the above-mentioned metal nanoparticles mainly act on the negative electrode of VRFBs, while Ir is often used to improve the performance of the positive electrode of VRFBs because it can reduce the overpotential of the VO2 +/VO2+ redox reactions in CF electrodes. Cheng et al. prepared Ir-modified carbon electrode materials through electrospinning and pyrolysis processes.119 The VRFBs with these electrodes as the positive electrode show an EE of 75.4% at 150 mA cm−2.
Metal-based catalysts are a type of highly efficient catalyst with high commercial application value. However, side reactions such as hydrogen evolution and high prices still restrict the application of these catalysts. Currently, preparation processes such as deposition strategies and heat treatment are new ways to solve side reactions such as hydrogen evolution, which are expected to expand the application of metal-based catalysts. More importantly, regulating the size and distribution of metal particles, especially Bi, will have a significant improvement in performance, which brings new ideas for the research of high-performance electrodes.
 | ||
| Fig. 12 (a) Catalytic and adsorption mechanism of vanadium ions by metal oxides.120 Copyright (2021). ACS Publications. (b) Preparation steps of GF electrodes modified by TiO2 and defects.121 Copyright (2017). Chemistry Europe. (c) Flow chart of the preparation of the NiO-modified GF electrode.122 Copyright (2018). Elsevier. (d) Diagrammatic sketch of the catalytic effect of CeO2 on vanadium ions.123 Copyright (2014). Royal Society of Chemistry. | ||
TiO2 is a low-cost, highly hydrophilic, and stable metal oxide in acid solutions. Hsiao et al. prepared a TiO2-modified N-doped GF through a simple one-pot synthesis strategy.124 The specific TiO2 loading strategy is as follows. First, GF is impregnated with the Ti-containing solution to make Ti particles load on GF. Then, the product is annealed at high temperature in an N2 atmosphere for oxidation to form TiO2 and is stably loaded on GF. Owing to the etching of TiO2 and doped N atoms, the obtained electrodes exhibit a large SSA and high hydrophilicity, which significantly enhances adsorption and catalytic effects on VO2+/VO2+. The assembled battery shows an EE of 77.8% at 160 mA cm−2, which is significantly better than that of untreated GF. Although TiO2 is stable in acid solutions, a small amount of hydrogen evolution reaction still occurs. Researchers directly grew hydrogen-treated TiO2 nanorods on GF by a hydrothermal method and used them for VRFB electrodes, as shown in Fig. 12b.121 Hydrogen-treated TiO2 not only introduces defects and increases the SSA, but also completely inhibits the hydrogen evolution reaction. Meantime, the direct growth strategy avoids the negative effects such as increased resistance caused by the introduction of binders. The prepared electrodes can effectively catalyze the V3+/V2+ reactions, and exhibit high capacity retention and good stability. NiO is an important metal oxide, which can improve the electronic conductivity after combining with the carbon material structure. After the GF electrodes are combined with NiO, the hydrophilicity and SSA of the electrode are significantly improved, and the mass transfer function is also improved, as shown in Fig. 12c.122 In particular, the electrodes still show an EE of 74.52% at 125 mA cm−2 and long cycle stability (300 cycles at 100 mA cm−2). WO3 is also commonly used to modify CF electrodes to improve the adsorption of vanadium ions on the electrodes. This is mainly due to the multiple valence states of tungsten ions, which help the dissociation of water and the adsorption of a large number of OH−, thereby promoting the adsorption of vanadium ions on the electrode and improving the catalytic performance. Hosseini et al. introduced an N-doped WO3 catalyst on CF electrodes by hydrothermal reaction and used it for the redox reaction of VO2+/VO2+ in VRFB positive electrode catalysis.125 Owing to the synergy of N atom doping and WO3 catalyst, the prepared electrodes not only achieve high reversibility of the VO2+/VO2+ reactions at high current density, but also significantly reduce the RCT (from 76.18 to 13 Ω). In addition, the electrodes achieve 51% electrolyte utilization and 70% EE at 200 mA cm−2. PbO2 has the characteristics of cheap cost, high hydrogen evolution potential, and strong stability, which make it an efficient electrocatalyst. Wu et al. prepared PbO2 by pulsed electrodeposition and introduced it to GF for VRFBs.126 PbO2 is uniformly dispersed on the GF surface, which can catalyze the redox reaction of vanadium ions and significantly reduce the RCT on the electrode surface. The assembled battery shows an EE of 82% at a current density of 70 mA cm−2. However, PbO2 is a toxic substance, which to some extent limits the large-scale application of PbO2.
Mo has multiple states and oxides, which make MoO3 easily convertible to other oxidation states, thereby endowing MoO3 with high electrochemical activity. Xie et al. deposited MoO3 nanoparticles on GF by an impregnation process and used them for catalyzing the VO2+/VO2+ redox reactions at the positive electrodes of VRFBs.127 Due to the excellent electrochemical properties of MoO3, the prepared battery provided 80% electrolyte utilization at 100 mA cm−2 and exhibited 65.34% EE at ultra-high current density (250 mA cm−2). CeO2 is a low-cost and highly stable electrocatalyst with a fluorite cubic structure with a space group of Fm3m, where each Ce ion is coordinated with eight oxygens. The electronic structure of Ce ([Xe]4f15d16s2) makes it easier to achieve charge transfer between the 4-valent and 3-valent states, which helps to improve conductivity.128 Specifically, the electrocatalytic mechanisms of CeO2 are shown in Fig. 12d, accompanied by the reactions of Equations (1) and (2).123 Firstly, the conversion of Ce4+ and Ce3+ generates oxygen vacancies that are beneficial for the transport and binding of vanadium ions, as shown in Equation (1). Subsequently, at a low potential, the active sites of Ce3+ are occupied by OH− in the solution, resulting in the formation of H+, as shown in Equation (2). When VO2+ in the solution migrates towards the electrode, it undergoes a charge exchange with the electrode and is converted into VO2+. During this process, electron transfer occurs, and the generated VO2+ diffuses back into the solution. It is worth noting that the OH− groups on CeO2 can significantly enhance the electrocatalytic activity of CeO2. Zhou et al. loaded CeO2 nanoparticles on GF by a precipitation method and used them for catalyzing the VO2+/VO2+ reaction at the positive electrodes of VRFBs (CeO2/GF).123 The specific precipitation method is as follows. GF is impregnated with a Ce4+ solution for the adsorption of Ce4+. Then, ammonia water is added to adjust the pH to 8, thus forming the Ce(OH)3 precipitate. The GF loaded with the Ce(OH)3 precipitate is placed in a tube furnace and calcined at a high temperature to obtain CeO2/GF. The introduction of CeO2 significantly improves the electrocatalytic activity and hydrophilicity of the electrode. After balancing the electrocatalytic effect and electrical conductance loss, it was found that 0.2 wt% CeO2/GF had the optimal EE (64.7% at 200 mA cm−2) and excellent cycling stability. To further enhance the catalytic activity of CeO2, Bayeh et al. deposited hydrogen-annealed CeO2 nanowires on GF by a hydrothermal method and used them as electrodes for VRFBs.129 The hydrogen annealing process significantly enhanced the defects of CeO2 nanowires and improved their electrocatalytic performance. In addition, the uniform distribution of CeO2 nanowires on the GF surface also enhanced the cycling stability of the electrode. The assembled VRFBs showed the best EE of 64.75% at 160 mA cm−2, and there was no significant attenuation in EE after 100 cycles at 80 mA cm−2.
| CeO2 ↔ CeO2−x + (x/2)O2 (0 ≤ x ≤ 0.5) | (1) |
| CeO2−x + H2O → CeO2−x − OH + H+ + e− | (2) |
To improve the catalytic performance of metal oxide materials, a new type of metal–organic framework (MOF) method has been proposed. The MOF strategy is a preparation method of materials with porous structures formed by self-assembly of metal ions or metal clusters with organic ligands through coordination bonds. The materials prepared by the MOF method not only have a rich porous structure and a large SSA, but also a solid framework structure that can provide excellent stability for the material. Chen et al. first synthesized a MnO@C catalyst by the MOF method and used it to catalyze the redox reaction of the vanadium ion.130 Under the electron microscope, the prepared MnO@C has a small size and abundant oxygen-containing functional groups. The assembled battery shows great application potential in terms of both electrochemical performance and cost, being significantly superior to the battery assembled with the original CF electrode. Jiang et al. loaded the MOF-derived ZrO2@C catalyst on GF and used it to improve the catalytic performance of VRFBs, as shown in Fig. 13.131 The special MOF preparation strategy enables the ZrO2@C catalyst to grow uniformly in situ on GF, ensuring good dispersion of the catalyst and helping to improve the stability of VRFBs. Owing to the porous structures constructed by the MOF and the large SSA of the ZrO2@C catalyst, the battery can maintain 75.2 and 62.4% EE at 200 and 300 mA cm−2, respectively. In addition, after 500 cycles at 150 mA cm−2, the EE of the battery does not show obvious attenuation, demonstrating good cycling stability. The MOF strategy provides a new method for improving metal oxide catalysts and has positive reference significance.
 | ||
| Fig. 13 (a) Preparation flowchart, (b) and (c) SEM images, (d) HRTEM image, and (e) CE, VE and EE at 150 mA cm−2 for 500 cycles of ZrO2@C nanoparticle-modified GF electrodes.131 Copyright (2021). Elsevier. | ||
Metal oxide catalysts are widely used to modify CF electrodes due to low cost and good catalytic efficiency. However, metal oxide catalysts still have problems such as high resistance, strong toxicity and unstable binding with electrodes, which will affect the practical application of these catalysts.132,133 Some specific strategies are expected to improve these problems. For example, safety assessment systems have been introduced to control and reduce the environmental impact of such catalysts, while preparation processes have been improved, such as better immobilization of such catalysts to electrodes using in situ growth strategies, deep burial methods, or MOF methods to improve efficiency. In addition, among the above strategies, we believe that metal oxides improved by the MOF strategy, such as MnO2 and ZrO2, exhibit advantages such as good energy efficiency and excellent stability, and are highly promising catalysts. This is closely related to their large specific surface area, abundant defect sites, and stable porous structure.
 | ||
| Fig. 14 (a) Flow diagram of TiC in wormhole-like nanopores embedded in the surface of CF. (b) Schematic diagram of the battery electrode surface. (c) SEM image of TiC-WN-GF. (d) TEM image of TiC-WN-GF.135 Copyright (2023). Elsevier. | ||
The high stability of WC in acidic environments is the key to its popularity among researchers. Cheng et al. used ammonium tungstate as the tungsten source to introduce WC into CNFs through electrospinning technology and in situ carbothermal reaction, which was used to catalyze the redox reaction of vanadium ions.136 WC is firmly embedded in the CNFs, avoiding the introduction of binders and enhancing the bonding degree between WC and the materials. Benefiting from the high stability and high catalytic activity of WC, the prepared electrode has no obvious hydrogen evolution side reaction, and exhibits a high EE (61.1% at 150 mA cm−2) and electrochemical stability. The preparation process of WC often adopts high-temperature annealing processes that limit the performance of the material, thus restricting the application of WC catalysts. Wodaje et al. proposed a two-step strategy to introduce N-doped WC nanowires onto GF.137 The introduction of N doping changes the charge density on the surface of the material, promoting the adsorption and transport of vanadium ions. The nanowire structures of WC also provide a large SSA for the attachment of active sites, which promote the redox reaction of vanadium ions. Under the synergistic effect of N doping and the WC nanowire structures, the prepared battery exhibited an optimal EE of 74.8% at 100 mA cm−2 and excellent cycle stability.
Metal carbides are substances with acid resistance and high mechanical strength, and they have only gradually attracted the attention of researchers in recent years. However, the preparation process of metal carbides is highly dependent on extreme conditions such as high temperature and high pressure, which have extremely high equipment requirements, limiting the large-scale application of these catalysts. At the same time, some metal carbide raw materials are scarce or difficult to obtain, which also increases the preparation cost and difficulty. Exploring new carbide materials and simple processes is the key to promoting the future development of such metal carbides. In addition, among the metal carbides introduced above, we believe that TiC deposited in carbon nanopores is an outstanding catalyst. It has a relatively low cost compared to other metal carbides and can operate under various current densities, demonstrating excellent performance.
C3N4 is a typical 2D conjugated polymer material that is used for CF modification owing to its excellent physicochemical properties, high nitrogen content, and unique electronic structure. Huang et al. first used the new material C3N4 to modify CF electrodes and catalyze the redox reaction of vanadium ions in VRFBs.139 The way of loading C3N4 on CF is as follows. The CF is impregnated with a solution rich in melamine and dried, and then it is placed in an Ar atmosphere and calcined at 400 °C for 4 hours, thus obtaining the CF with C3N4 anchored. Moreover, C3N4 uniformly wraps around CF, and its wrinkled structure significantly increases the SSA of CF (from 3.088 to 6.835 m2 g−1), expanding the attachment of active sites. Moreover, in the electronic structure of C3N4, C atoms and N atoms possess lone electron pairs in their p orbitals, which can interact to form P-conjugated structures similar to six-membered rings, thereby forming a conjugated system. This system structure can significantly improve the reversibility of the redox reaction and the overall electrochemical performance. Owing to the introduction of the C3N4 catalyst, the prepared electrode maintained an EE of 85% at 50 mA cm−2, which is 9.1% higher than that of commercial CF. In addition, the electrode also had a large capacity and good cycle stability. The emergence of C3N4 materials provides new ideas for the development of high-performance VRFBs. In addition, some novel catalysts like TiB2, owing to their special electron-deficient site structures, can effectively alleviate the concentration polarization in the negative electrode region of VRFBs resulting from the sluggish kinetics of V2+/V3+. Huang et al. introduced the TiB2 catalyst with electron-deficient sites onto the electrodes of VRFBs to accelerate electron transfer.140 The oxidation reaction of V2+/V3+ has a high Tafel slope and a large overpotential during the oxidation process, which limits the performance of VRFBs to some extent. The TiB2 catalyst with an electron-deficient structure can promote the electron transfer in the oxidation reaction of V2+/V3+ and enhance the corresponding reaction kinetics. The faster reaction kinetics not only optimizes the performance of VRFBs to a certain extent but also promotes the conversion between V2+ and V3+, avoiding the concentration polarization caused by untimely conversion.
These special catalyst materials have gradually been applied in the field of VRFBs in recent years. Although these catalysts show attractive effects in modifying PFFEs, the demanding preparation process restricts their development. New and simple preparation processes are important driving forces for the development of such materials. Preparing and loading these catalysts at low cost, simply, and efficiently on CF/GF is an important research direction in the future. Among the other types of catalysts mentioned above, we consider the TiB2 catalyst to be highly interesting and valuable. This is mainly because the TiB2 catalyst utilizes its own electron-deficient structure characteristics to promote the conversion of vanadium species. It not only reduces the concentration polarization but also significantly enhances the catalytic performance of TiB2, which provides a novel perspective for subsequent catalyst design. Utilizing the unique structure of materials to promote the improvement of electrochemical performance represents a highly promising development path in the future. In addition, we summarize and compare the specific properties of the above catalysts in Table 3 to provide reference for subsequent studies.
| Optimized strategies | Doped substances | P max (mW cm−2) | EE | R CT (Ω) | R CT (Ω cm2) | Ref. | |
|---|---|---|---|---|---|---|---|
| Cycle number | Current density (mA cm−2) | R CT (Ω) | R CT (Ω cm2) | ||||
| a R CT for VO2+/VO2+. b R CT for V2+/V3+. | |||||||
| Metal-based catalysts | Bi | 1238 | 78.6% | — | — | 114 | |
| 1000 | 400 | 0.08 | |||||
| Bi | — | 80.9% | — | — | 111 | ||
| 300 | 140 | 20 | |||||
| Bi | — | 80.1% | — | 112 | |||
| 200 | 320 | ||||||
| Bi | 1023 | ca. 71.43% | — | 115 | |||
| 2500 | 300 | ||||||
| Bi | 990 | 80% | ca. 0.1 | 116 | |||
| 1500 | 240 | ||||||
| Sn | — | 77.3% | — | — | 117 | ||
| — | 150 | 14.6 | |||||
| Sb | — | ca. 78% | — | 118 | |||
| 53 | 60 | ||||||
| Ir | — | ca. 64% | 0.6 | — | 119 | ||
| — | 150 | — | |||||
| Metal oxide catalysts | TiO2 | — | 83.3% | 64 | — | 124 | |
| — | 100 | ||||||
| TiO2 | — | 66.1% | — | 121 | |||
| 100 | 150 | ||||||
| NiO | — | 78.5% | 5.4 | — | 122 | ||
| 300 | 100 | 2.8 | |||||
| WO3 | 350 | 65.4% | 13 | — | 125 | ||
| — | 200 | ||||||
| PbO2 | — | ca. 87.5% | 7.2 | — | 126 | ||
| 30 | 50 | ||||||
| MoO3 | — | 76.33% | 1.6 | — | 127 | ||
| 50 | 100 | — | |||||
| CeO2 | — | ca. 76% | 78.3 | — | 129 | ||
| 100 | 80 | — | |||||
| CeO2 | — | 74% | — | 123 | |||
| 100 | 100 | ||||||
| MnO (MOF) | — | ca. 73% | ca. 0.25 | — | 130 | ||
| 300 | 100 | — | |||||
| ZrO2 (MOF) | — | ca. 77% | 4.8 | — | 131 | ||
| 500 | 150 | 4.9 | |||||
| Metal carbide catalysts | TiC | — | ca. 84% | — | — | 134 | |
| 30 | 50 | 1.6 | |||||
| TiC | — | ca. 80% | — | — | 135 | ||
| 300 | 300 | ca. 5 | |||||
| WC | — | 82.5% | 15.7 | — | 136 | ||
| 50 | 50 | 23.7 | |||||
| WC | — | 74.5% | 6.3 | — | 137 | ||
| 100 | 100 | ||||||
| Other types of catalysts | B4C | — | ca. 69% | — | 2.14 | 138 | |
| 200 | 160 | ||||||
| C3N4 | — | ca. 84% | 23.1 | — | 139 | ||
| 25 | 50 | ||||||
| TiB2 | 74.2% | 4.66 | 140 | ||||
| 300 | 150 | ||||||
To significantly enhance the reference value of this work, after in-depth research on the above various strategies, we have carefully selected the key strategies that are highly promising to promote the successful transition of the PFFEs from laboratory research to industrial-scale applications. In the macroscopic design, the optimization of the electrode shape and thickness is crucial for industrial production and application. Among them, the trapezoidal electrode shape can effectively alleviate the concentration polarization phenomenon and is easy to implement in industrial production, having significant advantages. In terms of thickness selection, considering both cost and performance, a thick electrode is more suitable for a single-channel flow field, while a thin electrode is preferred for a multi-channel flow field. In terms of intrinsic treatment for microscopic design, the multi-atom co-doping strategy has attracted much attention. In particular, the preparation of multi-atom-doped hierarchical porous carbon materials using the molten salt system has outstanding advantages. This method has a simple process and can effectively enhance the synergistic effect among multiple atoms, thus greatly improving the material performance and showing great potential in the industrialization process. In the aspect of introducing catalysts, the 3D graphene-based carbon catalyst has achieved fruitful results in many laboratory studies and has become one of the most promising catalysts, which deserves special attention in future industrial applications. Among metal catalysts, Bi is a commonly used choice. Nano-sized bismuth metal uniformly and stably distributed on the electrode structure can endow the electrode material with excellent electrochemical properties, making it a highly promising catalyst material. Notably, the development of metal oxides is limited due to problems such as low conductivity and unstable binding. However, the low cost and ease of synthesis of metal oxides prompt researchers to continuously optimize their performance. Some efficient synthesis strategies such as the MOF strategy may be the key to breaking through the bottleneck of metal oxides. Moreover, for metal carbides, TiC is considered as an ideal choice due to its excellent performance in the preparation process and cost control. Some novel ideas for catalyst preparation are also highly valuable. For example, taking advantage of the electron-deficient structure characteristics of the TiB2 catalyst to promote the conversion of vanadium substances not only reduces the concentration polarization but also significantly enhances the catalytic performance of TiB2. In conclusion, these strategies are determined after a comprehensive consideration of cost and performance, and the detailed results are listed in Table 4. In future electrode structure design, it can be considered to combine the most effective path of each modification strategy, thus achieving better electrochemical performances. For example, 3D graphene doped with heterogeneous atoms is grown on the surface of a trapezoidal electrode, and then Bi nanoparticles are deposited on the graphene surface. Optimizing the growth and deposition process of 3D graphene and Bi nanoparticles is expected to endow the electrode with higher power density and EE.
| Optimized strategies | Doped substances or improved topography | P max (mW cm−2) | EE | R CT (Ω) | R CT (Ω cm2) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Cycle number | Current density (mA cm−2) | R CT (Ω cm2) | ||||||
| a R CT for VO2+/VO2+. b R CT for V2+/V3+. | ||||||||
| Trapezoidal electrode shape | — | — | — | 85.6% | Reduce concentration polarization | 12 | ||
| — | 80 | |||||||
| Heteroatom doping | LiCl, KCl, KClO3, NH4Cl | N, O | — | 75.9% | — | ca. 5 | 31 | |
| 140 | 260 | ca. 5 | ||||||
| Layered porous structure | ZnO etching | N, O | — | 81.9% | — | 0.068 | 34 | |
| 320 | 500 | 0.068 | ||||||
| Introduce catalyst | 3D Vertical graphene | N | 1308.5 | 83.3% | ca. 3 | — | 93 | |
| 750 | 300 | |||||||
| Bi | — | 1238 | 78.6% | — | — | 114 | ||
| 1000 | 400 | 0.08 | ||||||
| ZrO2 (MOF) | — | — | ca. 77% | 4.8 | — | 131 | ||
| 500 | 150 | 4.9 | ||||||
| TiC | — | — | ca. 80% | — | — | 135 | ||
| 300 | 300 | ca. 5 | ||||||
| TiB2 | — | 74.2 | 4.66 | — | 140 | |||
| 300 | 150 | |||||||
4. Summary and outlook
VRFBs, which possess characteristics such as intrinsic safety, long lifespan, flexible energy storage duration, and no geographical limitations, are considered as the most promising battery device for large-scale and long-duration energy storage. As a crucial component of the VRFBs, the performance of the PFFEs affects the EE and power density of the battery, thereby determining the overall performance of the battery. However, the traditional untreated PFFEs have low hydrophilicity and poor electrochemical activity, resulting in significant overpotentials during the operation of VRFBs, hindering the redox reactions, and restricting the EE and power density. Therefore, modifying and optimizing the PFFEs is essential to improve their adsorption and catalytic properties towards vanadium ions, thereby enhancing the performance of VRFBs and promoting their large-scale application. Therefore, based on the micro and macro perspectives, this review focuses on the advantages and disadvantages of strategies for improving the design of PFFEs and the corresponding mechanisms, and summarizes the promising modification strategies.However, in practical production applications, not all modification strategies are applicable to industrialized production. We believe that the prerequisites for the PFFE modification strategy to meet industrialized production are that the process is simple, the performance is excellent, and the cost performance is high. In microstructure design, intrinsic treatment is a simple and convenient process, which is a commonly used optimization strategy in industry, such as heat treatment and pickling, and the performance of optimized PFFEs is relatively excellent. Currently, common processes such as heat treatment and pickling used in actual production only introduce oxygen-containing functional groups into the PFFEs, and there are certain limitations on the optimization effect of PFFE performance. In subsequent production, not only diversified functional groups can be introduced, but also defects and vacancies can be constructed on the PFFE surface to improve the SSA of the material. These measures can significantly improve the cost performance of intrinsic treatment. In addition, the modification strategies of introducing catalysts have a more complex process, and the success rate and efficiency of the introduced catalyst are low. Although this modification strategy can greatly improve the performance of PFFEs, it is difficult to be widely applied in actual production and life due to its low-cost performance. Secondly, in the macrostructure design strategy, it is necessary to optimize the compression ratio, shape, thickness, and flow field of the electrode, with the help of the data model. With the support of big data models, these strategies can adjust PFFE parameters for different situations, which can greatly meet the different needs of the market for PFFEs. At the same time, these strategies are simple and effective, making them excellent modification options. It is worth noting that grooming strategies can be combined in a variety of ways, and the effect on improving the PFFE performance will also have a multiplier effect. Therefore, we believe that not only the structures of PFFEs should be controlled at the micro level, but also the PFFE parameters should be designed by combining the big data model at the macro level. Only such a diversified modification mode can maximize the modification effect and is expected to be applied in actual production. Battery stack costs account for about 24% of VRFBs' component costs based on the calculation of a 4 h energy storage duration. As an important battery stack component, the cost and performance of PFFEs directly determine the cost and performance of the stack. Therefore, adopting reasonable PFFE modification strategies can effectively reduce the cost of the design of PFFEs and improve their performance. This is critical for improving the cost performance of VRFBs and enhancing their value for business applications. In addition, based on the vigorous development of current technology, this review also looks forward to the future development trend of PFFEs.
(i) In-depth analysis of the structure–activity relationship and intrinsic mechanisms of PFFEs using novel in situ characterization methods. It is well-known that the electrochemical reaction mechanisms are different at the interfaces of the positive electrodes and the electrolyte and the negative electrodes and the electrolyte in flow batteries. Revealing the influence of electrode structural modification on the electrochemical reaction mechanisms at the interfaces of the positive electrodes and the electrolyte and the negative electrodes and the electrolyte is helpful for the differentiated structure design of PFFEs. This should be of concern in future research. Revealing the mechanisms requires advanced in situ characterization techniques that can monitor the electrochemical reaction process. Some advanced in situ characterization methods, such as in situ optical microscopy, in situ differential electrochemical mass spectrometry, in situ Raman spectra, and in situ infrared nano spectroscopy, can be utilized to monitor the dynamic evolution of microstructures, electronic structures, composition, and phase of the materials during the electrochemical reaction process, which can provide important information on the phase, composition transition process, and material failure mechanism of bulk electrode materials.141,142 These characterization techniques can be considered to reveal the reaction mechanisms at the electrode–electrolyte interfaces in the flow batteries.
(ii) Introducing high-entropy alloy catalysts on the PFFEs. In recent years, high-entropy alloys composed of five or more elements with equal or roughly equal atomic ratios have been favored by researchers, because of their large multi-element composition space and unique high-entropy hybrid structure, and have been widely used in the field of catalysts and batteries. The multi-element composition of high-entropy alloy catalysts with low cost, high activity, and high stability often brings unexpected cocktail effects. The presence of multiple elements will produce a synergistic effect, which not only provides more active sites for PFFEs and regulates the electronic structure of the materials, but also is expected to catalyze the redox reaction of the positive and negative electrodes and inhibit the side reactions.
(iii) Adopting new preparation technologies. In recent years, several advanced micro-nano fabrication techniques have the potential to bring breakthroughs in the field of PFFEs. These new preparation technologies include laser processing, 3D printing, and Joule heating-based ultra-fast synthesis with its derived methods such as high-temperature shock, extreme thermal treatment, and magnetic induction heating. They offer advantages on the micro and macro structural design of PFFEs. Laser processing can effectively and rapidly regulate the macro structure of PFFEs with high precision. 3D printing has the potential to prepare PFFEs in a fast way, and modulate their surface microstructure with the co-formation of catalysts. Joule heating-based ultra-fast synthesis with its derived methods possesses advantages in the preparation of catalysts such as rapid heating, simple processing, and low cost while also enabling precise control over the heating rate and environmental conditions to modulate the catalyst microstructure of the surface of PFFEs. Compared to traditional heating methods, the catalyst materials synthesized using Joule heating-based ultra-fast synthesis often exhibit remarkable electrochemical properties, including higher electrochemical catalytic activity and more excellent stability. The application of these novel techniques in the fabrication of PFFEs has potential to lead to cost reduction and performance enhancement, thus promoting the widespread use of PFFEs.
(iv) Empower PFFEs with artificial intelligence (AI). At present, AI is promoting the revolutionary upgrading of the industry, and using AI to empower electrodes is the core competitiveness of the next generation of electrodes. AI-empowered electrodes are to realize dataization, intelligence, and integration of electrodes. Dataization refers to the use of machine learning and database to establish the mapping relationship between material properties and characteristic process parameters, use this mapping relationship to predict the electrode performance, and then guide the forming process design. Intelligence refers to the integration of cross-scale calculation methods across atomic, microscopic and macroscopic scales, through the combination of computational simulations, theoretical models, and experimental tools at multiple levels, which is applied to independently design electrode materials. Integration refers to the fully automated and integrated design of electrodes, the integration of stacks, and the assembly of batteries to reduce errors between components. Empowering electrodes with AI is an important direction for future development.
In summary, the modification of PFFEs is an effective strategy to improve the performance of VRFBs and can significantly enhance the commercial value of VRFBs. Achieving the large-scale, low-cost, and high-performance development of PFFEs is an important direction for future research.
Author contributions
H. Hu collected the data and drafted the manuscript. M. Han provided the conception and guided H. Hu to complete the initial manuscript and revise it. J. Liu, K. Zheng, Z. Zou, Y. Mu, F. Yu, W. Li, L. Wei, and L. Zeng revised the manuscript. T. Zhao supervised the work, obtained the funds, and revised the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the Guangdong Major Project of Basic and Applied Basic Research (2023B0303000002), Shenzhen Key Laboratory of Advanced Energy Storage (no. ZDSYS20220401141000001), High level of special funds (G03034K001), and Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds). pdjh2025c10810, pdjh2025c10801.References
- G. Ji, L. He, T. Wu and G. Cui, Appl. Energy, 2025, 377, 124538 CrossRef.
- L. Pan, Z. Guo, H. Li, Y. Wang, H. Rao, Q. Jian, J. Sun, J. Ren, Z. Wang, B. Liu, M. Han, Y. Li, X. Fan, W. Li and L. Wei, ChemElectroChem, 2024, 11, e202400460 CrossRef CAS.
- H. Hu, M. Han, J. Liu, K. Zheng, Y. Mu, Z. Zou, F. Yu, W. Li and T. Zhao, Future Batteries, 2024, 4, 100008 Search PubMed.
- R.-Z. Zhang, M.-Y. Lu, W.-W. Yang, L.-X. Liang and Q. Xu, J. Energy Storage, 2024, 90, 111768 CrossRef.
- W. Jiang, F. Jiang, J. Zhang, F. Yang, L. Liu and M. Hu, J. Energy Storage, 2024, 80, 110274 CrossRef.
- X. Zhang, X. Ye, S. Huang and X. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 37111–37122 CAS.
- T. Cheng, S. Qi, Y. Jiang, L. Wang, Q. Zhu, J. Zhu, L. Dai and Z. He, Small, 2024, 20, 2400496 CAS.
- J. Charvát, P. Mazúr, J. Dundálek, J. Pocedič, J. Vrána, J. Mrlík, J. Kosek and S. Dinter, J. Energy Storage, 2020, 30, 101468 Search PubMed.
- Q. Wang, Z. G. Qu, Z. Y. Jiang and W. W. Yang, Appl. Energy, 2018, 220, 106–116 CAS.
- N. Gurieff, C. Y. Cheung, V. Timchenko and C. Menictas, J. Energy Storage, 2019, 22, 219–227 Search PubMed.
- Q. Zheng, F. Xing, X. Li, T. Liu, Q. Lai, G. Ning and H. Zhang, J. Power Sources, 2015, 277, 104–109 CrossRef CAS.
- M. Yue, Q. Zheng, F. Xing, H. Zhang, X. Li and X. Ma, AlChE J., 2018, 64, 782–795 CrossRef CAS.
- K.-Q. Zhu, Q. Ding, J.-H. Xu, Y.-R. Yang, C. Yang, J. Zhang, Y. Zhang, T.-M. Huang, W.-M. Yan, Z.-M. Wan and X.-D. Wang, Energy Convers. Manage., 2022, 267, 115915 CrossRef CAS.
- E. Ali, H. Kwon, J. Choi, J. Lee, J. Kim and H. Park, J. Energy Storage, 2020, 28, 101208 CrossRef.
- H. Hao, Q.-A. Zhang, Z. Feng and A. Tang, Chem. Eng. J., 2022, 450, 138170 CrossRef CAS.
- R. Banerjee, N. Bevilacqua, L. Eifert and R. Zeis, J. Energy Storage, 2019, 21, 163–171 CrossRef.
- S. Kumar and S. Jayanti, J. Power Sources, 2016, 307, 782–787 CrossRef CAS.
- V. Muñoz-Perales, M. van der Heijden, V. de Haas, J. Olinga, M. Vera and A. Forner-Cuenca, ChemElectroChem, 2024, 11, e202300380 CrossRef.
- J. Kim and H. Park, J. Power Sources, 2022, 545, 231904 CrossRef CAS.
- Z. Guo, J. Sun, S. Wan, Z. Wang, J. Ren, L. Pan, L. Wei, X. Fan and T. Zhao, Appl. Energy, 2025, 379, 124910 CrossRef CAS.
- Y. Jiang, M. Du, G. Cheng, P. Gao, T. Dong, J. Zhou, X. Feng, Z. He, Y. Li, L. Dai, W. Meng and L. Wang, J. Energy Chem., 2021, 59, 706–714 CrossRef CAS.
- Z. He, Y. Lv, T. Zhang, Y. Zhu, L. Dai, S. Yao, W. Zhu and L. Wang, Chem. Eng. J., 2022, 427, 131680 CrossRef CAS.
- K. Zhang, C. Yan and A. Tang, J. Mater. Chem. A, 2021, 9, 17300–17310 RSC.
- L. Qiao, M. Fang, J. Guo and X. Ma, ChemElectroChem, 2022, 9, e202200292 CrossRef CAS.
- Y.-C. Chang, J.-Y. Chen, D. M. Kabtamu, G.-Y. Lin, N.-Y. Hsu, Y.-S. Chou, H.-J. Wei and C.-H. Wang, J. Power Sources, 2017, 364, 1–8 CrossRef CAS.
- H. R. Jiang, W. Shyy, L. Zeng, R. H. Zhang and T. S. Zhao, J. Mater. Chem. A, 2018, 6, 13244–13253 RSC.
- Y. Huang, Q. Deng, X. Wu and S. Wang, Int. J. Hydrogen Energy, 2017, 42, 7177–7185 CrossRef CAS.
- M. E. Lee, D. Jang, S. Lee, J. Yoo, J. Choi, H.-J. Jin, S. Lee and S. Y. Cho, Appl. Surf. Sci., 2021, 567, 150810 CrossRef CAS.
- H. Kim, J. S. Yi and D. Lee, ACS Appl. Energy Mater., 2021, 4, 425–433 CrossRef CAS.
- Z. Jialin, L. Yiyang, L. Shanfu and X. Yan, Batteries, 2023, 9, 40 CrossRef.
- Y. Jiang, Y. Wang, G. Cheng, Y. Li, L. Dai, J. Zhu, W. Meng, J. Xi, L. Wang and Z. He, Carbon Energy, 2024, 6, e537 CrossRef CAS.
- M. Park, I.-Y. Jeon, J. Ryu, H. Jang, J.-B. Back and J. Cho, Nano Energy, 2016, 26, 233–240 CrossRef CAS.
- T. Liu, X. Li, C. Xu and H. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 4626–4633 CrossRef CAS PubMed.
- Q. Wu, X. Zhang, Y. Lv, L. Lin, Y. Liu and X. Zhou, J. Mater. Chem. A, 2018, 6, 20347–20355 RSC.
- J. Feng, Q. Guo, H. Liu, D. Chen, Z. Tian, F. Xia, S. Ma, L. Yu and L. Dong, Carbon, 2019, 155, 491–498 CrossRef CAS.
- X. Du, J. Huang, J. Zhang, Y. Yan, C. Wu, Y. Hu, C. Yan, T. Lei, W. Chen, C. Fan and J. Xiong, Angew. Chem., Int. Ed., 2019, 58, 4484–4502 CrossRef CAS PubMed.
- H. Xu, J. Yang, R. Ge, J. Zhang, Y. Li, M. Zhu, L. Dai, S. Li and W. Li, J. Energy Chem., 2022, 71, 234–265 CrossRef CAS.
- K. Xie, X. Liu, H. Li, L. Fang, K. Xia, D. Yang, Y. Zou and X. Zhang, Carbon Energy, 2024, 6, e427 CrossRef CAS.
- H. Radinger, A. Ghamlouche, H. Ehrenberg and F. Scheiba, J. Mater. Chem. A, 2021, 9, 18280–18293 RSC.
- R. M. Bachman, D. M. Hall and L. R. Radovic, Carbon, 2023, 201, 891–899 CrossRef CAS.
- S. McArdle, H. Fiedler, J. Leveneur, J. Kennedy and A. T. Marshall, J. Power Sources, 2024, 608, 234614 CrossRef CAS.
- S.-C. Kim, J. Paick, J. S. Yi and D. Lee, J. Power Sources, 2022, 520, 230813 CrossRef CAS.
- S. J. Park, M. J. Hong, Y. J. Ha, J.-I. Choi and K. J. Kim, Sci. Technol. Adv. Mater., 2024, 25, 2327274 CrossRef PubMed.
- Y. Gao, S. Zhang, X. Li, L. Li, L. Bao, N. Zhang, J. Peng and X. Li, Carbon, 2021, 181, 323–334 CrossRef CAS.
- W. Gao, Z. Lin, H. Chen, S. Yan, Y. Huang, X. Hu and S. Zhang, Fuel Process. Technol., 2022, 237, 107468 CrossRef CAS.
- G. Ma, G. Ning and Q. Wei, Carbon, 2022, 195, 328–340 CrossRef CAS.
- X. Cheng, F. Ran, Y. Huang, R. Zheng, H. Yu, J. Shu, Y. Xie and Y.-B. He, Adv. Funct. Mater., 2021, 31, 2100311 CrossRef CAS.
- A. B. Shah, Y. Wu and Y. L. Joo, Electrochim. Acta, 2019, 297, 905–915 CrossRef CAS.
- H. Song, J. Yu, Z. Tang, B. Yang and S. Lu, Adv. Energy Mater., 2022, 12, 2102573 CrossRef CAS.
- E. Kano, J. Uzuhashi, K. Kobayashi, K. Ishikawa, K. Sawabe, T. Narita, K. Sierakowski, M. Bockowski, T. Ohkubo, T. Kachi and N. Ikarashi, Phys. Status Solidi RRL, 2024, 18, 2400074 CrossRef CAS.
- S. Küspert, I. E. Campbell, Z. Zeng, S. E. Balaghi, N. Ortlieb, R. Thomann, M. Knäbbeler-Buß, C. S. Allen, S. E. Mohney and A. Fischer, Small, 2024, 2311260 CrossRef PubMed.
- K. J. Kim, M.-S. Park, Y.-J. Kim, J. H. Kim, S. X. Dou and M. Skyllas-Kazacos, J. Mater. Chem. A, 2015, 3, 16913–16933 RSC.
- H. Hu, M. Yan, J. Jiang, A. Huang, S. Cai, L. Lan, K. Ye, D. Chen, K. Tang, Q. Zuo, Y. Zeng, W. Tang, J. Fu, C. Jiang, Y. Wang, Z. Yan, X. He, L. Qiao and Y. Zhao, Sci. Total Environ., 2024, 912, 169141 CrossRef CAS PubMed.
- P. Wang, Y. Zhao, Y. Ban and M. Zheng, J. Energy Storage, 2024, 97, 112859 CrossRef.
- G. Nagarjuna, J. Hui, K. J. Cheng, T. Lichtenstein, M. Shen, J. S. Moore and J. Rodríguez-López, J. Am. Chem. Soc., 2014, 136, 16309–16316 Search PubMed.
- C. Wang, X. Li, X. Xi, W. Zhou, Q. Lai and H. Zhang, Nano Energy, 2016, 21, 217–227 Search PubMed.
- M. Jing, Z. Xu, D. Fang, X. Fan, J. Liu and C. Yan, J. Electrochem. Soc., 2021, 168, 030539 Search PubMed.
- A. Duda, Z. Koza and M. Matyka, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 036319 CrossRef PubMed.
- M. Wang, J. Du, J. Zhou, C. Ma, L. Bao, X. Li and X. Li, J. Power Sources, 2019, 424, 27–34 CrossRef CAS.
- S. Song, Y. Liang, Z. Li, Y. Wang, R. Fu, D. Wu and P. Tsiakaras, Appl. Catal., B, 2010, 98, 132–137 CrossRef CAS.
- R. Jervis, M. D. R. Kok, T. P. Neville, Q. Meyer, L. D. Brown, F. Iacoviello, J. T. Gostick, D. J. L. Brett and P. R. Shearing, J. Energy Chem., 2018, 27, 1353–1361 CrossRef.
- S. J. Yoon, S. Kim and D. K. Kim, Energy, 2019, 172, 26–35 CrossRef CAS.
- H. R. Jiang, W. Shyy, M. C. Wu, R. H. Zhang and T. S. Zhao, Appl. Energy, 2019, 233–234, 105–113 CrossRef CAS.
- A. Bayaguud, Y. Fu and C. Zhu, J. Energy Chem., 2022, 64, 246–262 CrossRef CAS.
- Z. Yang, T. Xu, H. Li, M. She, J. Chen, Z. Wang, S. Zhang and J. Li, Chem. Rev., 2023, 123, 11047–11136 CrossRef CAS PubMed.
- N. Mohamed and N. K. Allam, RSC Adv., 2020, 10, 21662–21685 RSC.
- J. A. Teprovich, Jr., J. A. Weeks, P. A. Ward, S. C. Tinkey, C. Huang, J. Zhou, R. Zidan and P. Jena, ACS Appl. Energy Mater., 2019, 2, 6453–6460 CrossRef.
- F. A. El Diwany, B. A. Ali, E. N. El Sawy and N. K. Allam, Chem. Commun., 2020, 56, 7569–7572 RSC.
- Y. Zhou, L. Liu, Y. Shen, L. Wu, L. Yu, F. Liang and J. Xi, Chem. Commun., 2017, 53, 7565–7568 RSC.
- M. U. Arshad, C. Wei, Y. Li, J. Li, M. Khakzad, C. Guo, C. Wu and M. Naraghi, Carbon, 2023, 204, 162–190 CrossRef CAS.
- K. Zhang, H. Wang, X. Zhang, L. Liu, B. Feng, Y. Wang and J. Liu, ACS Sustainable Chem. Eng., 2024, 12, 7318–7328 CrossRef CAS.
- L. Dai, Y. Jiang, W. Meng, H. Zhou, L. Wang and Z. He, Appl. Surf. Sci., 2017, 401, 106–113 CrossRef CAS.
- C. Noh, B. W. Kwon, Y. Chung and Y. Kwon, J. Power Sources, 2018, 406, 26–34 CrossRef CAS.
- Z. He, Y. Jiang, Y. Li, L. Wang and L. Dai, Int. J. Energy Res., 2018, 42, 1625–1634 CrossRef CAS.
- Y. Chung, C. Noh and Y. Kwon, J. Power Sources, 2019, 438, 227063 CrossRef CAS.
- Z. He, G. Cheng, Y. Jiang, L. Wang and L. Dai, J. Electrochem. Soc., 2018, 165, A932 CrossRef CAS.
- L. Liu, X. Zhang, D. Zhang, K. Zhang, S. Hou, S. Wang, Y. Zhang, H. Peng, J. Liu and C. Yan, Chem. Eng. J., 2023, 473, 145454 CrossRef CAS.
- Q. Jiang, Y. Ren, Y. Yang, L. Wang, L. Dai and Z. He, Composites, Part B, 2022, 242, 110094 CrossRef CAS.
- H. Yang, C. Fan and Q. Zhu, J. Energy Chem., 2018, 27, 451–454 CrossRef.
- Y. Jiang, G. Cheng, Y. Li, Z. He, J. Zhu, W. Meng, H. Zhou, L. Dai and L. Wang, Appl. Surf. Sci., 2020, 525, 146453 CrossRef CAS.
- J. Sun, M. C. Wu, X. Z. Fan, Y. H. Wan, C. Y. H. Chao and T. S. Zhao, Energy Storage Mater., 2021, 43, 30–41 CrossRef.
- X. Zhang, Q. Wu, Y. Lv, Y. Li and X. Zhou, J. Mater. Chem. A, 2019, 7, 25132–25141 RSC.
- Z. Xu, M. Jing, J. Liu, C. Yan and X. Fan, J. Mater. Sci. Technol., 2023, 136, 32–42 CrossRef CAS.
- C. Pan, C. Wang, Y. Fang, Y. Zhu, H. Deng and Y. Guo, Environ. Sci.: Nano, 2021, 8, 1863–1885 RSC.
- Y. Ren, F. Yu, X.-G. Li and J. Ma, Mater. Today Chem., 2021, 22, 100603 CrossRef CAS.
- L. Xia, Q. Zhang, C. Wu, Y. Liu, M. Ding, J. Ye, Y. Cheng and C. Jia, Surf. Coat. Technol., 2019, 358, 153–158 CrossRef CAS.
- H. Fu, X. Bao, M. He, J. Xu, Z. Miao, M. Ding, J. Liu and C. Jia, J. Power Sources, 2023, 556, 232443 CrossRef CAS.
- Q. Li, J. Liu, A. Bai, P. Li, J. Li, X. Zhang, M. Yu, J. Wang and H. Sun, J. Chem., 2019, 2019, 1–9 Search PubMed.
- T. Long, Y. Long, M. Ding, Z. Xu, J. Xu, Y. Zhang, M. Bai, Q. Sun, G. Chen and C. Jia, Nano Res., 2021, 14, 3538–3544 CrossRef CAS.
- J. L. Suter, R. C. Sinclair and P. V. Coveney, Adv. Mater., 2020, 32, 2003213 CrossRef CAS PubMed.
- J. Li, B. Ji, R. Jiang, P. Zhang, N. Chen, G. Zhang and L. Qu, Carbon, 2018, 129, 95–103 CrossRef CAS.
- D. O. Opar, R. Nankya, J. Lee and H. Jung, Appl. Surf. Sci., 2020, 531, 147391 CrossRef CAS.
- J. Guo, L. Pan, J. Sun, D. Wei, Q. Dai, J. Xu, Q. Li, M. Han, L. Wei and T. Zhao, Adv. Energy Mater., 2024, 14, 2302521 CrossRef CAS.
- M. Park, Y.-j Jung, J. Kim, H. i Lee and J. Cho, Nano Lett., 2013, 13, 4833–4839 CrossRef CAS PubMed.
- Y. Zhang, Q. Wan and N. Yang, Small, 2019, 15, 1903780 CrossRef CAS PubMed.
- J. Zhu, A. Holmen and D. Chen, ChemCatChem, 2013, 5, 378–401 CrossRef CAS.
- M. Faraji and M. Mohseni, Ionics, 2018, 24, 2753–2760 CrossRef CAS.
- P. Han, Y. Yue, Z. Liu, W. Xu, L. Zhang, H. Xu, S. Dong and G. Cui, Energy Environ. Sci., 2011, 4, 4710–4717 RSC.
- L. Ye, S. Qi, T. Cheng, Y. Jiang, Z. Feng, M. Wang, Y. Liu, L. Dai, L. Wang and Z. He, ACS Nano, 2024, 18, 18852–18869 CrossRef CAS PubMed.
- Y. Chen, H. Fu, W. Yang, L. Zhong, Q. Wang, B. Lu, N. Wang, Z. Chen, G. Shi, C. Jia, M. Ding, R. Xia, E. I. Iwuoha, U. Feleni, S. Admassie and X. Peng, ACS Sustainable Chem. Eng., 2024, 12, 10567–10576 CrossRef CAS.
- Z. Hu, Z. Miao, Z. Xu, X. Zhu, F. Zhong, M. Ding, J. Wang, X. Xie, C. Jia and J. Liu, Chem. Eng. J., 2022, 450, 138377 CrossRef CAS.
- B. Huang, Y. Liu, M. Xia, J. Qiu and Z. Xie, Sustainable Energy Fuels, 2020, 4, 559–570 RSC.
- K. Amini, J. Gostick and M. D. Pritzker, Adv. Funct. Mater., 2020, 30, 1910564 CrossRef CAS.
- M. H. Hossain, N. Abdullah, K. H. Tan, R. Saidur, M. A. Mohd Radzi and S. Shafie, The Chem. Record, 2024, 24, e202300092 CrossRef CAS PubMed.
- R. Zhang, K. Li, S. Ren, J. Chen, X. Feng, Y. Jiang, Z. He, L. Dai and L. Wang, Appl. Surf. Sci., 2020, 526, 146685 CrossRef CAS.
- Z. Yu, X. Jia, H. Gao, R. Su, X. Wang, T. Zhao and H. Jiang, Adv. Funct. Mater., 2024, 2420534 CrossRef.
- T. Liu, X. Li, H. Nie, C. Xu and H. Zhang, J. Power Sources, 2015, 286, 73–81 CrossRef CAS.
- D. J. Suárez, Z. González, C. Blanco, M. Granda, R. Menéndez and R. Santamaría, ChemSusChem, 2014, 7, 914–918 CrossRef PubMed.
- Y. Wen, T. P. Neville, A. Jorge Sobrido, P. R. Shearing, D. J. L. Brett and R. Jervis, J. Power Sources, 2023, 566, 232861 CrossRef CAS.
- B. Li, M. Gu, Z. Nie, Y. Shao, Q. Luo, X. Wei, X. Li, J. Xiao, C. Wang, V. Sprenkle and W. Wang, Nano Lett., 2013, 13, 1330–1335 CrossRef CAS PubMed.
- X. Yang, T. Liu, C. Xu, H. Zhang, X. Li and H. Zhang, J. Energy Chem., 2017, 26, 1–7 CrossRef.
- H. R. Jiang, Y. K. Zeng, M. C. Wu, W. Shyy and T. S. Zhao, Appl. Energy, 2019, 240, 226–235 CrossRef CAS.
- L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS PubMed.
- X. Zhou, X. Zhang, L. Mo, X. Zhou and Q. Wu, Small, 2020, 16, 1907333 CrossRef CAS PubMed.
- X. Liu, Y. Nie, L. Yu, L. Liu and J. Xi, J. Energy Storage, 2024, 91, 112035 CrossRef.
- F. Xing, Q. Fu, F. Xing, J. Zhao, H. Long, T. Liu and X. Li, J. Am. Chem. Soc., 2024, 146, 26024–26033 CrossRef CAS PubMed.
- S. Mehboob, A. Mehmood, J.-Y. Lee, H.-J. Shin, J. Hwang, S. Abbas and H. Y. Ha, J. Mater. Chem. A, 2017, 5, 17388–17400 RSC.
- J. Shen, S. Liu, Z. He and L. Shi, Electrochim. Acta, 2015, 151, 297–305 CrossRef CAS.
- D. Cheng, Y. Li, C. Han, Z. He, J. Zhu, L. Dai, W. Meng and L. Wang, Colloids Surf., A, 2020, 586, 124137 CrossRef CAS.
- J. Xu, Y. Zhang, Z. Huang, C. Jia and S. Wang, Energy Fuels, 2021, 35, 8617–8633 CrossRef CAS.
- J. Vázquez-Galván, C. Flox, C. Fàbrega, E. Ventosa, A. Parra, T. Andreu and J. R. Morante, ChemSusChem, 2017, 10, 2089–2098 CrossRef PubMed.
- N. Yun, J. J. Park, O. O. Park, K. B. Lee and J. H. Yang, Electrochim. Acta, 2018, 278, 226–235 CrossRef CAS.
- H. Zhou, J. Xi, Z. Li, Z. Zhang, L. Yu, L. Liu, X. Qiu and L. Chen, RSC Adv., 2014, 4, 61912–61918 RSC.
- Y.-S. Hsiao, J.-H. Huang, H.-Y. Lin, W. K. Pang, M.-T. Hung, T.-H. Cheng, S.-C. Hsu, H. C. Weng and Y.-C. Huang, Surf. Coat. Technol., 2024, 484, 130785 CrossRef CAS.
- M. G. Hosseini, S. Mousavihashemi, S. Murcia-López, C. Flox, T. Andreu and J. R. Morante, Carbon, 2018, 136, 444–453 CrossRef CAS.
- X. Wu, H. Xu, L. Lu, H. Zhao, J. Fu, Y. Shen, P. Xu and Y. Dong, J. Power Sources, 2014, 250, 274–278 CrossRef CAS.
- X. Xie, Y. Xiang and W. A. Daoud, ACS Appl. Energy Mater., 2020, 3, 10463–10476 CrossRef CAS.
- X. Huang, K. Zhang, B. Peng, G. Wang, M. Muhler and F. Wang, ACS Catal., 2021, 11, 9618–9678 CrossRef CAS.
- A. W. Bayeh, G.-Y. Lin, Y.-C. Chang, D. M. Kabtamu, G.-C. Chen, H.-Y. Chen, K.-C. Wang, Y.-M. Wang, T.-C. Chiang, H.-C. Huang and C.-H. Wang, ACS Sustainable Chem. Eng., 2020, 8, 16757–16765 CrossRef CAS.
- F. Chen, X. Cheng, L. Liu, L. Han, J. Liu, H. Chen, Q. Zhang and C. Yan, J. Power Sources, 2023, 580, 233421 CrossRef CAS.
- Y. Jiang, G. Cheng, Y. Li, Z. He, J. Zhu, W. Meng, L. Dai and L. Wang, Chem. Eng. J., 2021, 415, 129014 CrossRef CAS.
- D. Wang, Z. Lin, T. Wang, Z. Yao, M. Qin, S. Zheng and W. Lu, J. Hazard. Mater., 2016, 308, 328–334 CrossRef CAS PubMed.
- Y. Lv, C. Han, Y. Zhu, T. Zhang, S. Yao, Z. He, L. Dai and L. Wang, J. Mater. Sci. Technol., 2021, 75, 96–109 CrossRef.
- L. Wei, T. Zhao, L. Zeng, X. Zhou and Y. Zeng, Energy Technol., 2016, 4, 990–996 CrossRef CAS.
- L. Wei, L. Zeng, M. S. Han, W. J. Li, L. P. Chen, J. H. Xu and T. S. Zhao, J. Power Sources, 2023, 576, 233180 CrossRef CAS.
- G. Cheng, Y. Jiang, Y. Li, J. Chen, Z. He, W. Meng, L. Dai and L. Wang, Electrochim. Acta, 2020, 362, 137178 CrossRef CAS.
- A. W. Bayeh, D. M. Kabtamu, Y.-T. Ou, N.-Y. Hsu, H.-H. Ku, Y.-M. Wang, T.-C. Chiang, H.-C. Huang and C.-H. Wang, ACS Sustainable Chem. Eng., 2022, 10, 12271–12278 CrossRef CAS.
- H. R. Jiang, W. Shyy, M. C. Wu, L. Wei and T. S. Zhao, J. Power Sources, 2017, 365, 34–42 Search PubMed.
- Y. Huang, J. Huo, S. Dou, K. Hu and S. Wang, RSC Adv., 2016, 6, 66368–66372 Search PubMed.
- R. Huang, S. Liu, Z. He, W. Zhu, G. Ye, Y. Su, W. Deng and J. Wang, Adv. Funct. Mater., 2022, 32, 2111661 CrossRef CAS.
- Z. Huang, Z. Chen, M. Yang, M. Chu, Z. Li, S. Deng, L. He, L. Jin, R. E. Dunin-Borkowski, R. Wang, J. Wang, T. Yang and Y. Xiao, Energy Environ. Sci., 2024, 17, 5876–5891 RSC.
- X. He, J. M. Larson, H. A. Bechtel and R. Kostecki, Nat. Commun., 2022, 13, 1398 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |



