 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymeric membranes in carbon capture, utilization, and storage: current trends and future directions in decarbonization of industrial flue gas and climate change mitigation
Arash
Mollahosseini
 *ab,
Mostafa
Nikkhah Dafchahi
c,
Saeed
Khoshhal Salestan
*ab,
Mostafa
Nikkhah Dafchahi
c,
Saeed
Khoshhal Salestan
 de,
Jia Wei
Chew
de,
Jia Wei
Chew
 d,
Mohammad
Mozafari
f,
Masoud
Soroush
fg,
Sabahudin
Hrapovic
h,
Usha D.
Hemraz
i,
Ronaldo
Giro
j,
Mathias B.
Steiner
j,
Young-Hye
La
k,
Seyed Fatemeh
Seyedpour Taji
e,
Khalid
Azyat
a,
Muhammad Amirul
Islam
a,
Sajjad
Kavyani
l,
Xinyu
Wang
ae,
Jae-Young
Cho
*ae and
Mohtada
Sadrzadeh
d,
Mohammad
Mozafari
f,
Masoud
Soroush
fg,
Sabahudin
Hrapovic
h,
Usha D.
Hemraz
i,
Ronaldo
Giro
j,
Mathias B.
Steiner
j,
Young-Hye
La
k,
Seyed Fatemeh
Seyedpour Taji
e,
Khalid
Azyat
a,
Muhammad Amirul
Islam
a,
Sajjad
Kavyani
l,
Xinyu
Wang
ae,
Jae-Young
Cho
*ae and
Mohtada
Sadrzadeh
 *e
*e
aQuantum and Nanotechnologies Research Centre, National Research Council Canada, 11421 Saskatchewan Drive, Edmonton, AB T6G 2M9, Canada. E-mail: arash.mollahosseini@gmail.com
bPhysical Sciences Department, MacEwan University, Edmonton, AB T5H 0K9, Canada
cDepartment of Chemical and Biological Engineering, University of Saskatchewan, 57 Campus Drive, Saskatoon, SK S7N 5A9, Canada
dChemical Engineering, Chalmers University of Technology, 412 96, Gothenburg, Sweden
eDepartment of Mechanical Engineering, 10-241 Donadeo Innovation Center for Engineering, University of Alberta, Edmonton, AB T6G 1H9, Canada
fDepartment of Chemical and Biological Engineering, Drexel University, Philadelphia, PA 19104, USA
gDepartment of Material Science and Engineering, Drexel University, Philadelphia, PA 19104, USA
hAquatic and Crop Resource Development, National Research Council of Canada, 6100 Royalmount Avenue, Montreal, H4P 2R2, Quebec, Canada
iHuman Health Therapeutics, National Research Council of Canada, 6100 Royalmount Avenue, Montreal, Quebec H4P 2R2, Canada
jIBM Research, Av. República do Chile, 330, CEP 20031-170, Rio de Janeiro, Brazil
kIBM Almaden Research Center, 650 Harry Rd, San Jose, California 95120, USA
lDepartment of Chemical and Materials Engineering, University of Alberta, Edmonton, Alberta, T6G 1H9, Canada
First published on 4th April 2025
Abstract
The urgency to mitigate global warming and climate change has catalyzed advancements in decarbonization technologies, with membrane separation emerging as a key area of interest. Noted for its compact design, high separation efficiency, scalability, and versatility, membrane technologies offer promising solutions for carbon capture, utilization, and storage (CCUS). In particular, polymeric membranes are attractive due to their cost-effectiveness, ease of fabrication, and mechanical flexibility. This review examines the latest developments in polymeric membranes for CCUS, emphasizing material properties, durability, stability, and process optimization. A thorough analysis of membrane-based separation processes is provided, covering various feedstocks and capturing mechanisms, including pre-combustion, post-combustion, oxy-fuel combustion, and chemical looping, with steam methane reforming processes as an integral part of major emission-intensive industries producing products such as petrochemicals and fertilizers together with non-green hydrogen. The review also explores complementary CCUS processes—absorption–stripping, adsorption, cryogenic, and biological technologies—and details the challenges faced by gas separation membranes, such as permeability-selectivity tradeoff, plasticization, and physical aging. The role of computational approaches, particularly artificial intelligence, in driving innovations through polymer and membrane modifier design is also highlighted. By addressing process simulation, design challenges, carbon utilization, economic feasibility, and technology readiness levels, this comprehensive review offers valuable insights into the current state and future potential of membrane-assisted decarbonization for CCUS applications.
Broader contextCarbon-neutral technologies are vital to protecting the environment and preserving planetary health. Carbon capture, utilization and storage (CCUS) technologies are the major focal points of scientific and industrial efforts to combat climate change. Membrane separation technologies are perfect candidates for CCUS applications in the energy, cement, and chemical industries. These technologies could be applied to the existing infrastructures with minimum environmental footprint. The advancement in polymeric membranes for CCUS has led to low-cost and efficient separation units with higher efficiency. A holistic overview of the technologies is offered to highlight global warming challenges and membrane's contributions to collaborative ecosystems. |
1. Introduction
To limit global annual temperature rise to 1.5 to 2 °C, greenhouse gas (GHG) emissions must be reduced by 2025, with a net-zero plan executed by 2070.1 Decarbonization strategies have been central in combating global warming over the past two decades, involving modifications to existing processes and adopting low-carbon technologies.2 While most decarbonization efforts focus on the transportation and energy sectors, there is also growing interest in developing new fuels and energy sources.3 However, the ongoing reliance on GHG-intensive industries is crucial for economic growth, as fossil fuel consumption and economic development are deeply connected.4 To address the environmental impacts of using fossil fuels, increasing attention has been given to improving process efficiency and minimizing emissions.5 Despite these efforts, GHG emissions are projected to grow at 1% annually until 2040, with CO2 emissions in the energy sector expected to increase from 36 billion metric tons in 2020 to 43.2 billion metric tons by 2040.6Given these projections, various mitigation strategies are urgently needed to prevent severe environmental changes. Some of the proposed pathways include enhancing energy efficiency, shifting to low-carbon or zero-carbon energy sources, and employing carbon capture utilization and storage (CCUS).6–8 CCUS is vital for separating CO2 from industrial and energy-related sources, transporting it for storage or utilization, and permanently removing it from the atmosphere.9 The primary CO2 sources are fossil fuel power plants, and industrial sectors like iron, steel, cement, and chemical production. Other sectors, including agriculture, livestock, and land-use changes, also contribute to rising GHG levels.10,11 Removing CO2 directly from its primary stationary sources has been identified as the most effective method for emission reduction, steering researchers toward CCUS processes.
CCUS mainly involves separating CO2 from exhaust or turbine streams in industrial and urban sectors, followed by storage. While the future role of CCUS technologies in achieving net-zero emissions remains uncertain, their application is necessary for current industrial sectors.12,13 Existing CCUS technologies, such as physical/chemical absorption, adsorption, bioremediation, and cryogenic separation, are energy-intensive and can increase the energy demand of power plants by 10–40%.14,15 Therefore, there is a pressing need for energy-efficient CO2 separation methods. Membrane separation has emerged as a promising candidate due to its energy-conserving nature and high separation efficiency.16–18
This literature review aims to (i) provide an overview of current CCUS scenarios, (ii) introduce various membrane-based materials for carbon capture, utilization, and storage, (iii) offer an overview of the products with more commercialization chances, (iv) discuss the technology readiness level (TRL) of membranes and compare it with the other CCUS technologies and take a look at impact assessment studies, (v) review the most recent efforts focused on the process simulation, computational, machine learning and artificial intelligence-related research for membrane-based gas separation processes, (vi) and compare the cost-effectiveness of these membrane-based solutions with existing conventional technologies. Many previous reviews focus on either the traditional CCUS technologies or specific aspects of membrane separation, such as material types or separation mechanisms.19–28 However, this review takes a broader approach by first examining the characteristics of various CO2-rich streams. Understanding the diversity and specific properties of these streams allows for a stronger foundation when discussing both conventional and advanced CCUS scenarios, addressing their distinct requirements and operational challenges. In addition to offering detailed insights into membrane materials, separation mechanisms, and performance metrics in CCUS applications, the review extends beyond traditional technologies, such as absorption–stripping, adsorption, cryogenic separation, and bioprocesses, by exploring membrane-based hybrid methods. This broader perspective enables a more complete analysis of how these emerging membrane technologies can integrate with existing systems to enhance efficiency and sustainability. It also addresses recent advancements in membrane technology, including modifications and applications, thereby filling a gap in the literature where these technological nuances are often overlooked.
The paper also differentiates itself from prior studies by discussing computational efforts, artificial intelligence, and machine learning for membrane design and optimization. Focusing on the economic feasibility and technology readiness levels (TRLs), it provides a pragmatic perspective on the future implementation of membrane-based CCUS. Ultimately, this review consolidates current techno-economic insights while offering a comprehensive roadmap for the future development of membrane-based CCUS technologies. It distinguishes itself from previous studies by adopting a holistic approach, addressing the entire spectrum of CCUS processes, from material science and separation mechanisms to economic feasibility and advanced computational methods. By integrating these diverse aspects into one framework, this review provides a more unified perspective on the potential and challenges of membrane-based CCUS, setting it apart from more narrowly focused works.
2. Emission-producing industries and their effluents
Understanding the target stream is essential before exploring various CCUS methods. Flue gas, also known as exhaust or stack gas, is the outlet stream of the combustion process, carrying the products of the fuel and air reaction. The composition of these streams can vary significantly depending on factors such as the pollution source, the nature of the plant, and operational conditions.29 Power plants generate flue gas that contains dust particles, sulfur oxides, and nitrogen oxides. Standard power plant emissions typically need to meet the following conditions: Impure carbon particles (soot) < 10 mg m−3, SO2 < 35 mg m−3, NOx < 50 mg m−3.30 World Health Organization (WHO), established Air Quality Guidelines (AQG, version 2021) with 24-hour concentration limit of SO2 < 40 μg m−3, NO2 < 25 μg m−3, fine particle matters with diameter equal or less than 2.5 μm (PM2.5) < 15 μg m−3, (PM10) < 45 μg m−3, and CO < 4 μg m−3.31 WHO does not recognize CO2 as a direct air pollutant for outdoor environments. Industries could create CO2 as a side product in a reaction or through combustion. Major producers are power plants (through furnaces, turbines, boilers), cement industry (through precalciners), refineries (through process heaters, catalytic cracker), iron and steel production industries (through blast furnaces, oxygen furnaces), petrochemical production (through steam cracking process), fertilizer production (through reforming processes for ammonia production, urea production), and alcohol production (through fermentation).32 Indirect generation of CO2 must also be considered through supply chain, feedstock and utility production, etc.33 The contribution of each industry to CO2 emissions varies by region due to differences in social and industrial activity profiles. As an example, the two major emission producers in the world, US is mainly producing CO2 (more than 50% of overall emission production) through consumption of coal and natural gas in power plants,32 while China creating CO2 through the manufacturing industries.34Pre-treating flue gas before the CCUS process can enhance CCUS efficiency and improve the maintenance of downstream equipment.35 When dealing with fuel sources such as municipal waste incineration, coal, sludge from water treatment plants, other products used as fuel in the cement plants, and biogas, the exhaust may contain other components, including hydrogen chloride, hydrogen fluoride, and heavy metal derivatives. The flue gas composition also depends on the air stream's characteristics fed into the combustor and the air/fuel ratio, as air pollutants can impact combustion efficiency and the exhaust stream quality. Combustion conditions are another crucial factor; for instance, a typical oxygen and hydrocarbon-fueled combustor converts most sulfur content to sulfur dioxide. However, high temperatures and excess oxygen favor the formation of sulfur trioxide. Conversely, low oxygen content in the combustion reaction can result in fuel derivatives in the exhaust.36
The sensitivity of each CCUS process needs to be considered in the design parameters, making it essential to understand the differences between various sources. Different filter materials and separation mechanisms react uniquely to contaminants and impurities during membrane separation. For example, moisture has a counterintuitive effect: while it can facilitate CO2 transport through amine-containing membrane materials, excessive water vapor may form a water film on the membrane, hindering the process.37Table 1 compares flue gas composition from various sources, while Table 2 illustrates the typical output composition after the CCUS process.
| Source | CO2 (vol%) | N2a1 (vol%) | O2 (vol%) | H2O (vol%) | Ar (vol%) | CH4 (vol%) | SO2 (ppm) | NOx (ppm) | H2S (ppm) |
|---|---|---|---|---|---|---|---|---|---|
| a CO: 23.45%. | |||||||||
| Natural gas combined cycle | 7 | 66 | 14 | 6 | 1 | N/A | N/A | 10–300 | N/A |
| Integrated gasification combined cycle | 3 | 76 | 12 | 14 | 1 | N/A | 10–200 | 10–100 | N/A |
| Coal-based power plants | 11 | 76 | 6 | 6 | 1 | N/A | 300–5000 | 500–800 | N/A |
| Municipal waste incineration power plant | 6–12 | Balance | 7–14 | 10–18 | 1 | N/A | 200–1500 | 200–500 | N/A |
| Cement industry resources | 19 | 59 | 7 | 13 | 1 | N/A | 5–1200 | 100–1500 | N/A |
| Household resources | 34–38 | 0–5 | 0–1 | 6 | N/A | 50–60 | N/A | N/A | 100–900 |
| Agriculture resources | 19–33 | 0–1 | Less than 0.5 | 6 | N/A | 60–75 | N/A | N/A | 3000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Agrifood resources | 26 | N/A | N/A | 6 | N/A | 68 | N/A | N/A | 400 |
| Refinery | 12.3 | 71.8 | 4.4 | 10.3 | 1.2 | N/A | N/A | N/A | N/A |
| Iron and steel industry (basic oxygen furnace) | 34.5 | 60.4 | N/A | 4.5 | 0.6 | N/A | N/A | N/A | N/A |
| Iron and steel industry (blast furnace) | 21.5 | 46.5 | 3.7 | 4.2 | 0.6 | N/A | N/A | N/A | N/A |
| CCUS process | Impurities |
|---|---|
| CO2 captured from natural gas sweetening | CH4, amines, H2O |
| CO2 captured from heavy oil production and upgrading | H2S, N2, O2, CO, H2O, H2, COS, Ar, SOx, NOx |
| CO2 captured from power plants using post-combustion capture | N2, amines, H2O, O2, NH3, SOx, NOx |
| CO2 captured from power plants using oxy-combustion capture | N2, O2, SO2, H2S, Ar |
| CO2 captured from power plants using pre-combustion capture | H2, CO, N2, H2S, CH4 |
Components in the CO2-rich stream can significantly alter its thermophysical properties. These changes may include a higher critical point pressure, increased likelihood of a two-phase stream within certain pressure and temperature ranges, and variations in density and compressibility. Additionally, transport properties that affect heat, mass, and momentum transfer can also change, impacting the stream's behavior.40–42
3. The CCUS perspective in different scenarios
CCUS technologies can be adopted for various scenarios, including pre-combustion, post-combustion, oxy-fuel combustion, and chemical looping combustion.14,43–45 Among the most common technologies for fuel processing, hydrogen production, and fertilizer manufacturing are steam methane reforming (SMR) and auto thermal reforming (ATR), which produce CO2 as a byproduct. Removing CO2 from SMR and ATR discharges may lessen the load and optimize the product's ultimate cost.46–49 It can also be used to directly remove CO2 from the air rather than targeting specific emission streams. The selection of the appropriate CCUS scenario and the related processes depends on several factors. These include the operational characteristics of the plant generating emissions, the economic feasibility and efficiency of the CCUS process, and the environmental regulations that dictate the permissible levels of emissions. Each scenario offers distinct advantages and challenges, with the choice largely driven by the specific needs and conditions of the industrial application.3.1. Pre-combustion
During fuel preparation for power generation, such as in coal gasification plants or integrated gasification power plants (IGCC), hydrocarbons react with water and oxygen, forming CO, CO2, and hydrogen. Subsequently, the water-gas shift (WGS) reaction converts CO into CO2, reducing its content in the syngas. This syngas is then utilized for power generation, but the high concentration of CO2 can adversely impact combustion efficiency. Therefore, removing excess CO2 is essential to optimize overall process efficiency. This CO2 removal step is also referred to as hydrogen or fuel purification, commonly known as upgrading. Fig. 1 illustrates the pre-combustion CO2 removal scenario in power generation, where the separation element is represented using a membrane unit, reflecting the focus of this review on membrane-based separation technologies.Removing CO2 before using the fuel is crucial, particularly in natural gas purification after extraction from wells. This process reduces the burden on downstream operations, minimizes pipeline corrosion, and enhances extraction efficiency when the separated CO2 stream is reinjected into the wells. Furthermore, CO2 removal increases the energy content of natural gas, ensuring compliance with market and regulatory standards. It also optimizes the performance of gas processing equipment and significantly boosts the economic value of the gas. A schematic of this process is shown in Fig. 2, highlighting its role in improving overall system efficiency.
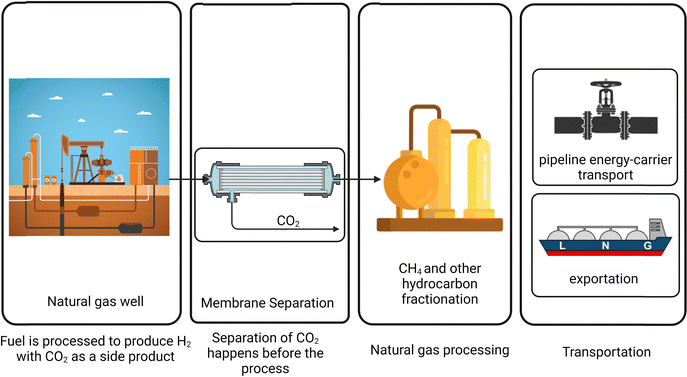 | ||
| Fig. 2 Schematic of pre-combustion CO2 removal from natural gas before conversion, consumption or exportation. | ||
3.2. Post-combustion
Post-combustion strategies focus on removing CO2 from exhaust streams after combustion, where hydrocarbon fuel is mixed with air. In conventional combustion streams at ambient pressure, CO2 concentrations range between 3–15%.50,51 The main challenge in these processes arises from the low concentration of CO2 and the high temperature of the outlet stream, both of which complicate the sizing and design of separation systems. Low concentrations drive up separation costs, leading to an 8 to 12% decrease in process efficiency.52,53 Despite these challenges, a key advantage of post-combustion CCUS strategies is their compatibility with existing infrastructure, requiring no significant changes to upstream processes. This adaptability is one reason why post-combustion remains the only fully commercialized CCUS strategy to date.54Fig. 3 provides a schematic representation of this scenario. | ||
| Fig. 3 Schematic of post-combustion CO2 removal from the flue gas after emission production in different industries. | ||
3.3. Oxy-fuel combustion
Oxy-fuel combustion refers to using pure oxygen for combustion instead of air. This process increases the CO2 concentration in the exhaust to over 80% and reduces NOx emissions by eliminating nitrogen from the combustion process. This higher concentration of CO2 makes it easier to capture and remove from the flue gas compared to post-combustion CCUS methods.55,56 Conventional furnaces, where 79% N2 enables steady propagation because of CO2's lower thermal diffusivity, stronger radiation, dissociation, and cooling effects. Stable oxy-fuel flames usually include up to 70% CO2 in the CO2/O2 combination since higher concentrations lead to instability.57,58 Oxy-fuel combustion has a few challenges to overcome, including high temperature (close to 3500 °C), and instability of the flame due to the pure O2 usage instead of air.Recirculating the flue gas (in wet or dry state) back to the burner is a common practice to help regulate the flame temperature during combustion, allowing the process to stay within the metallurgical constraints.59 Wet flue gas could trigger corrosion and erosion. Dry flue gas recirculation is therefore advised, in which flue gas is recycled downstream of the operation of gas cleanup units, including moisture condensers, particulate filters, and flue gas desulfurization units.60
Techno-economic evaluations identify oxy-fuel combustion as one of the most cost-effective and energy-efficient CCUS solutions available.61–63 However, the requirement for pure oxygen, which is usually produced via energy-intensive cryogenic processes, represents a significant drawback. The oxygen supply process can lead to an approximate 10% reduction in power plant efficiency, although this impact varies depending on the plant's baseline characteristics.64 These challenges, particularly the high energy demand for oxygen production, continue to pose significant obstacles to the large-scale implementation of oxy-fuel combustion.65
3.4. Chemical looping combustion
Chemical looping combustion (CLC) is a relatively new process to which CCUS strategies can be applied. It divides the combustion process into two reactors. In the first reactor (air reactor), an oxygen carrier, typically a metal such as nickel, iron, or copper66,67) reacts with air to oxidize the metal. The metal oxide is then transferred to a second reactor (fuel reactor) where it reacts with a hydrocarbon fuel, producing pure CO2 while reducing the metal oxide back to its metallic form. The metal is then cycled back to the air reactor for reuse.68Since the idea's inception in 1983, CLC has gained attention for its potential in carbon capture, leading to significant developments, including a 1 MWth pilot plant established in Germany in 2015.66,69 Research efforts are currently focused on improving various aspects of the process, such as enhancing the performance and durability of the oxygen carriers70–72 and refining process integration and intensification techniques to optimize efficiency.73–75 CLC shows promise for increasing CCUS efficiency while lowering energy penalties compared to traditional methods.
3.5. Direct air capture
Direct air capture (DAC) focuses on removing CO2 directly from the atmosphere, distinguishing itself among CCUS technologies by targeting non-stationary and widely distributed emission sources, which collectively contribute nearly 50% of human-made CO2 emissions. Originally proposed by Lackner in the 1990s as a method to combat climate change, DAC has gained considerable attention from researchers focused on improving its efficiency and reducing costs.76 Unlike traditional CCUS methods that concentrate on emissions from specific stationary sources, DAC relies on adsorption and absorption processes to capture CO2 from the air. However, one of its key challenges lies in the costly regeneration of sorbents, which limits the technology's economic viability. Additional concerns include DAC's high energy and material demands and complexities surrounding proper CO2 storage. Despite its promising potential, these issues continue to raise doubts about DAC's large-scale implementation.76,77Table 3 provides a comparison of different CCUS strategies. The ocean absorbs approximately 27% of atmospheric CO2, converting it into carbonate and bicarbonate ions while maintaining climate equilibrium. The rising atmospheric CO2 concentration, alongside the decreasing ocean pH, suggests a weakened capacity of the ocean as a natural carbon sink, prompting interest in direct air capture from the ocean (DOC).78 DOC, a less-explored subdivision of DAC, reverses the acidity of the ocean water with the controlled impact on the environment and sea life. Using alternative renewable energies and novel technologies with low emissions to produce alkaline solutions for pH adjustment has been a major topic of focus for academic and technology development teams.79 Utilization of DOC technologies will likely require the development of advanced solvents and adsorbent materials with improved capture capacity, selectivity, and lower regeneration costs.| CCUS scenario | Removal efficiency (vol% CO2) | CO2 separation cost (USD per tone CO2) | Energy consumption (GJ per tone CO2) | Pros | Cons |
|---|---|---|---|---|---|
| a The scenario covers technologies in autothermal reforming (ATR) and steam methane reforming (SMR).46,48 b The scenario covers direct ocean capture (DOC).83 | |||||
| Pre-combustiona | 90 | 34 to 63 | 3.35 | Proper for high concentration and partial pressure of CO2, easy separation, suitable for most of the existing plants, developed/matured technology | Temperature and efficiency complications in case of H2-rich streams, high Capex and Opex, |
| Applicable new IGCCs only | |||||
| Post-combustion | 90 | 46 to 74 | 4.14 | Matured process and already in use | Low CO2 removal efficiency in low CO2 concentrations |
| High parasitic power requirement | |||||
| Oxyfuel combustion | Higher than 90 | 52 | 4.05 | Proper for high CO2 levels, applicable to current plants through retrofitting and repowering | High cost of oxygen supply, energy-intensive, |
| Chemical looping | 96 to 99 | Less than 59.20 | 0.95 | Works with low-cost oxygen-carrying metals, proper for high CO2 levels, | Immature and under development |
| Direct air captureb | 85 to 93 | 140 to 340 | 5.25 | Proper for non-stationary sources | Low CO2 partial pressures in the air make the process cost and energy-deficient |
4. Current processes and technologies for CCUS
Several processes have been developed for carbon capture, utilization, and storage (CCUS), spanning a range from laboratory-scale research efforts to more commercialized applications. Each process exhibits unique characteristics in terms of scale, application scope, retrofit potential, and cost-effectiveness. Fig. 4 provides a general classification of these available processes, highlighting key distinctions across different approaches. In this subsection, an in-depth overview of these CCUS processes is presented to provide a comprehensive understanding of the current technological landscape and its implications.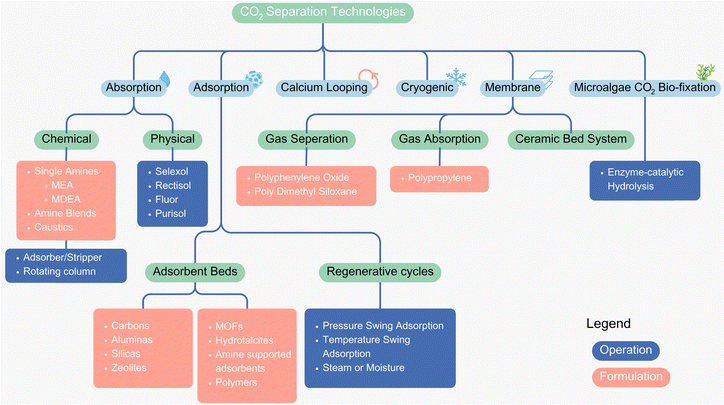 | ||
| Fig. 4 Classification of different CCUS technologies.14,45 | ||
4.1. Absorption–stripping
The only fully commercialized CCUS technology is absorption. Fig. 5 shows a typical amine-based absorption–stripping flow sheet for post-combustion carbon capture. In this process, CO2-laden flue gas enters a separator to remove trapped particles before passing through an absorption column, where it comes into contact a lean amine solution, absorbing the CO2. The resulting “rich” amine solution is heated via a heat exchanger before entering a stripper column, where steam removes the CO2. The CO2-laden vapor is condensed at the top of the column, and recycled vapor returns as reflux. The lean amine is then reheated in the heat exchanger and recirculated back to the absorption column. A significant drawback of this process is the high energy demand for regenerating the rich amine, which can account for up to 50% of the annual process costs, significantly increasing overall plant expenses.84,85 | ||
| Fig. 5 Typical post-combustion carbon capture process flow sheet.54 | ||
The selection of solvent is a crucial and continually evolving element in the CO2 absorption process. An ideal solvent should have high CO2 solubility, low energy requirements for regeneration, and fast reaction kinetics with CO2.86 Amines, particularly monoethanolamine (MEA), are the most recognized and cost-effective solvents, with other common options including diethanolamine (DEA), methyl diethanolamine (MDEA), and triethanolamine (TEA).85 Inorganic solvents, such as potassium carbonate and sodium carbonate mixed with ammonia, are also used, with potassium carbonate being the most popular. Inorganic solvents offer advantages like greater stability, reduced environmental impact, and lower energy demands for regeneration compared to primary and secondary amines.87 However, amines are preferred in coal-fired flue gas applications due to their superior CO2 selectivity.88
Ongoing research focuses on improving the efficiency of these absorbents by focusing on enhancing kinetics, solubility, energy efficiency, and key operational characteristics like foaming, viscosity, surface tension, and thermal stability, all while reducing environmental impact. Numerous studies have examined the properties of amine-based solutions for CCUS, including vapor–liquid equilibrium (VLE) data that are critical for process optimization.89–96
Post-combustion adsorption processes are often preferred over alternative technologies for several reasons: (i) they have a long history of practical use, providing extensive industry experience in handling and maintenance; (ii) they typically require minimal modifications to existing infrastructure; and (iii) maintenance of the CCUS system can be performed without disrupting upstream operations.14
In addition to solvent selection, the absorption–stripping process can be optimized through several advanced techniques and process integrations. Methods such as absorber inter-cooling, multi-solvent feeding, employing a semi-lean solvent stream in the stripper, and solvent splitting in the rich phase have shown potential to enhance efficiency.97 These modifications are aimed at addressing the main challenges of reducing energy penalties and achieving capture costs below $20 per ton of CO2.98 Another promising strategy is increasing CO2 concentration in the flue gas, which typically ranges from 3% to 15%, depending on the source. Utilizing membrane-based technologies for initial CO2 concentration can significantly improve the overall CCUS process efficiency.99,100
Despite their widespread use, absorption–desorption methods for CO2 capture present several challenges, including high energy requirements for solvent regeneration and producing harmful byproducts from oxidative degradation. Other issues, such as equilibrium limitations, amine degradation, and equipment corrosion due to the aqueous phase, further complicate the process.101 Continued research is focused on refining solvent performance and advancing process improvements to address these challenges. Comprehensive reviews of recent developments in absorption-based post-combustion CCUS technologies can be found in the literature.87,97,102–105
4.2. Adsorption
Adsorption-based CCUS technologies take advantage of CO2's stronger binding affinity for certain adsorbents compared to other flue gas components.106 Physical adsorption uses van der Waals forces to bind CO2 molecules to the adsorbent's surface, offering an easier regeneration process than absorption, which requires chemical bonds. The ease of regenerating adsorbents, either thermally or by pressure modulation, significantly reduces the energy consumption in the CCUS process, making adsorption a more energy-efficient option.Key performance metrics for adsorption-based CCUS technologies include adsorbent durability, CO2 selectivity, adsorption capacity, and the stability of the adsorbent after multiple adsorption/desorption cycles.107 In the temperature swing adsorption (TSA), the adsorbent is regenerated by increasing the adsorption bed's temperature, often using hot gas or steam. In contrast, pressure swing adsorption (PSA) and vacuum swing adsorption (VSA) use pressurized flue gas to adsorb CO2, followed by a reduction in column pressure to release the captured CO2.
While PSA and VSA are more energy-efficient under certain operational conditions,14 TSA may be a more practical solution for large-scale applications, as flue gases are often at atmospheric pressure, making it costly to compress high volumes of gas continuously.84 Therefore, TSA might provide a more feasible option in scenarios where cost control is critical despite its energy demands.108
4.3. Cryogenic technologies
Cryogenic CCUS involves separating CO2 from a gas stream by cooling it to the point where CO2 transitions to a liquid or solid phase, making it easier to extract. This phase-change-based technique relies on the differences in the boiling points or desublimation characteristics of the stream components. Fig. 6 categorizes the various cryogenic CCUS technologies, and Fig. 7 illustrates the decarbonization process of flue gas using cryogenic methods.When cryogenic separation is based on boiling point differences, it is classified as conventional vapor–liquid separation, commonly used in natural gas purification to liquefy and remove CO2. However, significant drawbacks include the high energy requirements for high-pressure equipment and the risk of solid formation leading to blockages. Additionally, water content in the gas stream must be meticulously removed to prevent ice formation, which can disrupt pressure profiles.109 The solidification of CO2 can be further avoided by the Ryan/Holmes extractive technology, which uses a heavier hydrocarbon for enhanced solubility of the liquified CO2, as well as a few other parameters that facilitate the separation process.110 The separation parameters, i.e., recovery ratio and purity of the streams, are adjusted by tuning operational pressure and temperature and using flash separation units and stripping columns, which eliminate O2/N2/Ar components (gases with lower boiling points).111,112
Cryogenic processes, while energy-intensive, offer high CO2 purity without toxic chemicals and can be applied to streams with varying CO2 concentrations. The unconventional cryogenic process, which uses CO2 desublimation (solid–vapor equilibrium), may reduce energy intensity at higher CO2 concentrations.113 Available technologies include:
(i) Normal pressure cooling process of the flue gas, requiring temperatures below −100 °C.
(ii) Direct multistep compression above the critical pressure (about 73 atm), where liquefied CO2 can be stored in the seabed—though highly energy-intensive.
(iii) Hybrid approaches, where pressurized streams are cooled to liquefy CO2 or pressurized liquid CO2 is solidified through throttling and temperature-pressure adjustments.
These methods must avoid air or nitrogen dilution to improve energy efficiency and could benefit from cold energy recovery.35,114,115
4.4. Biological processes
Biological carbon mitigation processes leverage bioreactions within living organisms to naturally consume CO2. Through photosynthesis, solar energy drives the conversion of CO2 into organic carbon, a process known as bio-sequestration. Practices such as agroforestry, cropland extension, and pasture development are examples of biological carbon mitigation, though their overall impact on atmospheric CO2 removal is considered minimal. Nonetheless, these practices persist as interim measures until more practical, large-scale solutions are developed.116One promising approach in biological carbon mitigation is the bioconversion of CO2 using microalgae bioreactors, which offer high photosynthetic efficiency (3% to 8%) and robust biomass productivity.117 Flue gases rich in CO2 provide an ideal environment for algal cultivation,118 with the potential for economic advantages in biorefineries.119 However, pollutants like SOx and NOx in flue gases can acidify the culture medium, limiting the growth of certain algal species. To mitigate these challenges, technical solutions such as feed stream pretreatment and selecting suitable algae (thermotolerant, pH-tolerant, with enhanced photosynthetic efficiency) have been proposed.118,120 Additionally, using enzymes can enhance CO2 consumption, enabling more sustainable reactors that utilize environmentally friendly solvents.121Fig. 8 illustrates various bioreactor systems for CO2 bio-sequestration. Table 4 also compares the technologies reviewed in Section 4.
 | ||
| Fig. 8 Photoreactors for microalgae culture to reduce carbon emission.122 | ||
| Technology | Energy consumption | Key challenges | Advantages | Ref. |
|---|---|---|---|---|
| Absorption–stripping | 3–6.5 GJ per ton CO2 | High energy for solvent regeneration, corrosion, solvent degradation | Mature and widely used, high CO2 selectivity | 123 and 124 |
| Adsorption (TSA) | 3.5–5.6 GJ per ton CO2 | High heating requirement, slower cycle times | Lower energy demand than absorption, reusable adsorbents | 125 and 126 |
| Adsorption (PSA/VSA) | 0.4–4 GJ per ton CO2 | Pressure dependency, moderate scalability | More energy-efficient than TSA, fast cycle times | 127 and 128 |
| Lower than TSA, varies with pressure | ||||
| Cryogenic separation | 2.3–4.4 GJ per ton CO2 | Extreme cooling needed, risk of ice/solid CO2‚ formation | High CO2 purity, no chemical waste | 129 |
| Biological processes (Microalgae) | Low, dependent on sunlight & reactor conditions | Slow process, requires large space and sunlight | Sustainable, can integrate with biofuel production | 130 and 131 |
| Membrane separation | 1 GJ per ton CO2 | Not commercialized | Retrofittable for different industries, lower capital and operation costs | 132 |
5. Membrane-based CCUS technologies
The concept of gas separation membranes was initially proposed in 1866.133 Asymmetric membranes were practically put in use into the 1960s by Loeb and Sourirajan's pioneering work.134,135 Membrane separation techniques rely on differences in diffusivity, solubility, absorption, and adsorption properties of gases across various materials.136 Membranes are particularly effective for CO2 separation due to the significant size difference between CO2 and other gases in flue gas mixtures.137 The advantages of membrane technology in CCUS applications include avoiding phase changes in gas streams, scalability, adaptability to both post-combustion and pre-combustion techniques, and the potential for process intensification. Additionally, membranes are low-maintenance, occupy a small footprint, and can function under harsh conditions depending on the material.138 For example, a membrane unit designed for a 600 MW power plant was estimated to require only 0.004 km2 area, significantly less than amine-based units.139The driving force for transport through the membrane is the chemical potential difference, which can manifest as pressure, concentration, temperature, or electrical gradient, depending on the specific process.140 In the gas separation process, the driving force is the transmembrane pressure. Gas molecules vary in size, represented by their kinetic diameter, along with other characteristics, such as activation energy and shape factors, which influence the separation process. Table 5 summarizes the molecular characteristics relevant to pre- and post-combustion processes.
| Molecule | Kinetic diameter (A) | Polarizability (A3) | Dipole moment (D) | Quadrupole moment (D A) |
|---|---|---|---|---|
| H2O | 2.65 | 1.50 | 1.85 | 2.30 |
| H2 | 2.89 | 0.78 | 0 | 0.66 |
| CO2 | 3.30 | 2.50 | 0 | 4.30 |
| O2 | 3.46 | 1.58 | 0 | 0.039 |
| NO | 3.49 | 1.70 | 0.15 | N/A |
| H2S | 3.60 | 3.78 | 0.97 | N/A |
| N2 | 3.64 | 1.71 | 0 | 1.54 |
| CO | 3.76 | 1.95 | 0.11 | 2.50 |
| CH4 | 3.80 | 2.44 | 0 | 0.02 |
Depending on the gas stream composition and the membrane characteristics, the transport mechanism may involve Knudsen diffusion, molecular sieving, solution diffusion, or a combination of these. In porous membranes, molecular sieving (with pore sizes between 0.5 to 2 nm) and Knudsen diffusion (with pore sizes from 5 to 10 nm) are the dominant mechanisms.142 Molecular sieving occurs when the membrane pore size is nearly equivalent to the size of the gas molecules, allowing smaller molecules to pass while blocking larger ones. In Knudsen diffusion, the permeation rate is proportional to the velocity of the gas molecules and inversely proportional to the square root of their molecular weight, provided the pore size is smaller than the gas molecules' mean free path.143 On the other hand, dense membranes separate gases through solution diffusion, where target gas molecules adsorb onto the membrane surface, diffuse through the membrane, and then desorb on the opposite side. Catalytic reactions can further facilitate this process, particularly in hydrogen purification using palladium membranes.144,145Fig. 9 illustrates the pore size ranges and corresponding transport mechanisms.
5.1. CCUS membranes from the material point of view
Gas separation membranes can be categorized into four main types: inorganic (ceramic), organic (polymeric), metallic, and hybrid membranes (also known as mixed matrix membranes, which contain both organic and inorganic components). Ceramic membranes, which emerged in the 1960s for applications such as gas separation and beer extraction, are particularly well-suited for use in harsh operating conditions due to their durability and thermal stability.146,147 Common ceramic membrane materials include alumina, zirconia, silicon nitride, and perovskites like calcium titanium oxide. These materials are prized for their robustness in extreme conditions. For example, dense perovskite-based ceramic membranes are well-suited for high-temperature oxygen separation in integrated gasification combined cycle (IGCC) plants equipped with CCUS technologies.148,149 Therefore, despite their high production costs, ceramic membranes remain a viable option for pre-combustion CCUS applications.149 Ceramic membranes are generally three times as expensive as polymeric filters,150 with ceramic materials costing around $500–$2000 per m2 compared to $50–$400 per m2 for polymeric membranes.150–155 These cost differences push many industries toward using polymeric membranes, with additional benefits such as defect-free large-scale production further encouraging this trend.156On the other hand, polymeric membranes offer a lower-cost alternative to ceramic ones. Various polymeric materials, such as cellulose-based polymers, polysulfone (PSF), polyether sulfone (PES), polyimide (PI), polyamide (PA), and polybenzimidazole (PBS), have been introduced for gas separation. While easier to fabricate, polymeric membranes have limited resistance to mechanical, thermal, and chemical stress. For example, high-temperature resistant polymers like PBS may struggle under extreme conditions, such as those found in IGCC plants, which can reach pressures of 20 bar and temperatures between 700–900 °C.136,157Table 6 offers a classification of gas separation membranes based on their materials.
| Membrane material | Working criteria | Target gas | Details | |
|---|---|---|---|---|
| Organic | Porous polymers (standalone and composite, rubbery/glassy) | Molecular sieving/solution-diffusion | CO2 or H2 | Low resistance to temperature and harsh operating conditions, low production cost |
| Inorganic | Dense metal (Palladium, Palladium composites) | Solution-diffusion | H2 | Highly selective to H2 |
| Moderate to high resistance to temperature | ||||
| Sensitive to impurities | ||||
| Dense ceramic (Molten carbonates, composite metal-ceramics, composite metal–metal) | Solution-diffusion/chemical reaction | CO2 or H2 | Moderate to high-temperature resistance, excellent corrosion resistance (towards organic solvents and a wide pH range), suitable for cleaning and steam sterilization, and long lifetime. Brittle (requires careful handling), typically disc or tubular shaped with a low surface area/volume ratio and high investment cost. | |
| Porous ceramics (mesoporous 2–50 nm) or microporous (less than 2 nm): amine-functionalized silica, zeolites, metal–organic frameworks | Molecular sieving/diffusion | CO2 or H2 |
Another way to classify membranes is based on their symmetry. Porous ceramic membranes are typically asymmetric, consisting of one or more mesoporous sub-layers or intermediate layers, topped with a dense (microporous) selective layer. Membranes made entirely of the same material across all layers are classified as “integral”.159 If different materials are used for the various layers (e.g., combinations of ceramics and organics), they are referred to as composite membranes.
5.2. Separation mechanism in CCUS membranes
As mentioned earlier, the separation performance of the membrane is assessed by several factors, among which selectivity and permeability are more important. Gas selectivity itself depends on two key mechanisms: diffusivity selectivity and solubility selectivity. Diffusivity selectivity occurs when the membrane discriminates gases based on molecular size, often referred to as size sieving, where smaller molecules permeate faster than larger ones. On the other hand, solubility selectivity is driven by the ability of certain gases to dissolve more readily in the membrane's polymer matrix, favoring the transport of more condensable gases, like CO2, over less condensable ones, such as N2 or O2. Both mechanisms can operate concurrently, with the relative dominance depending on the specific membrane material and the feed gas composition. Membrane materials are tailored to enhance either or both selectivity types to achieve the desired separation performance in CCUS applications. Advances in material science, including mixed matrix membranes, aim to optimize these factors, providing a balance between permeability and selectivity to improve process efficiency. This section explores the CCUS membrane-based process, focusing on how these two factors influence separation efficiency.| αij = βijPi−λij | (1) |
 | (2) |
Glassy polymer-based membranes exhibit a higher Robeson upper bound compared to rubbery membranes due to the increased gas solubility in their nonequilibrium excess volume.161,162 Glassy polymers, which are rigid below their glass transition temperature (Tg), tend to show better selectivity and mechanical strength. On the other hand, when the temperature exceeds Tg, polymers become flexible and rubbery, leading to significant changes in density, specific heat, dielectric coefficient, conductivity, and transport properties.163
Over the past few decades, glassy polymer-based membranes have gained attention due to their superior mechanical strength, reproducibility, and adaptability across a variety of applications.164
In contrast, solubility-selectivity favors larger, more soluble molecules, which may penetrate more easily due to their chemical affinity for the membrane material. For example, CO2, with its significantly higher quadrupole moment compared to other common flue gas components, exhibits better solubility in membranes functionalized with polar groups.166
Gas solubility is influenced by several factors, including the gas' characteristics, operating conditions, and the membrane material properties. Compressible gases like CO2, especially those with high polarity, tend to have greater solubility at higher pressures, and stronger interactions with the membrane's polar functional groups can further enhance this solubility. In polymeric membranes, gas sorption generally occurs in two distinct phases.
 | (3) |
However, the complex reaction mechanisms within FTMs complicate direct flux calculation using this equation due to factors such as: (i) CO2 partial pressure being dependent on both physisorption and chemisorption, and (ii) mass transfer resistance caused by interfacial adsorption/desorption, which is independent of membrane thickness. As a result, CO2 transport properties in FTMs are often measured similarly to solution-diffusion membranes. Still, caution is needed when interpreting CO2 permeability data, as high permeability in thick films doesn’t always translate to high permeance in thin-film composite membranes.168
Although the Robeson upper bound was initially developed for homogeneous polymeric membranes, it continues to serve as a baseline for evaluating improvements in membrane selectivity and permeability.169 Advances in materials, such as mixed matrix membranes (MMM), carbon molecular sieves (CMS), polymers with intrinsic microporosity (PIM), and thermally rearranged polymers (TR), have led to breakthroughs beyond the Robeson bound, particularly through approaches focusing on solubility-selectivity.170–173
5.3. Performance measurement
The performance of the gas separation membrane is commonly evaluated using constant pressure/variable volume (CP/VV), or the isobaric method, as depicted schematically in Fig. 10(a). Membrane performance is assessed through several key factors: solubility, diffusivity, permeability, and selectivity. The diffusion of a single gas through a porous membrane can be described by Fick's first law: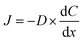 | (4) |
 is the concentration gradient across the membrane. Diffusivity, as a kinetic parameter, reflects the ability of gas molecules to move through the membrane. The steady-state flux of a single gas can be expressed as:
is the concentration gradient across the membrane. Diffusivity, as a kinetic parameter, reflects the ability of gas molecules to move through the membrane. The steady-state flux of a single gas can be expressed as: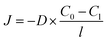 | (5) |
| C = S × p | (6) |
 | (7) |
| P = S × D | (8) |
 | (9) |
 .
.
Another important metric is permeance, expressed in gas permeation unit  :174
:174
 | (10) |
Selectivity is the membrane's ability to differentiate gases, defined for a binary mixture as the ratio of their permeabilities:
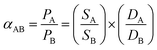 | (11) |
 is the solubility selectivity and
is the solubility selectivity and  is the diffusivity selectivity.175 A more recent approach to assessing membrane performance focuses on four aspects of CO2 separation:
is the diffusivity selectivity.175 A more recent approach to assessing membrane performance focuses on four aspects of CO2 separation: | (12) |
 | (13) |
 | (14) |
 | (15) |
To optimize a membrane-based gas separation process, both selectivity and permeability must be considered. The choice between diffusivity selectivity and solubility selectivity depends on the gas composition and the desired separation. When separating gases with similar molecular structures (e.g., N2 and O2), diffusivity plays a larger role, while solubility-based separation is more critical for gases with different chemical properties, including polarity, charge, etc. (e.g., CO2 and CH4).176 Additionally, the concept of fractional free volume (FFV) can explain membrane permeability:177
 | (16) |
The constant volume/variable pressure (CV/VP), also known as the time-lag or isochoric method, is a widely used technique for determining diffusion coefficients and assessing permeability in steady-state single or mixed gas streams due to its independence from specific gas types.181,182Fig. 10(b) illustrates the process. In this method, the gas permeates through the membrane and is collected in a downstream reservoir with a constant volume. A pressure transducer or sensor records the pressure in the storage tank over time, corresponding to the permeation test. The permeability of the membrane is calculated using the following equation:183
 | (17) |
 is the pressure change over time (mmHg s−1). The method can accurately evaluate the transport properties of the membranes within different humidity percentages.184 CV/VP is the simplified version of the time-lag method, which is used to measure the diffusivity gasses through the membrane.
is the pressure change over time (mmHg s−1). The method can accurately evaluate the transport properties of the membranes within different humidity percentages.184 CV/VP is the simplified version of the time-lag method, which is used to measure the diffusivity gasses through the membrane.
The time-lag parameter is calculated when the gas permeates from the constant pressure feed side into the constant volume permeate reservoir. Diffusivity coefficient, D is calculated using:185
 | (18) |
By measuring permeability from eqn (8), the solubility parameter can be derived using eqn (5). Typically, these performance measurements (CP/VV and CV/VP) are used for single gas permeability and ideal selectivity. However, a more realistic approach involves mixed gas feeds, adjusting the upstream gas concentrations using mass flow meters, and measuring the permeate composition with gas chromatography. This setup provides insight into the real selectivity of the membrane, accounting for the effects of gas mixtures on membrane performance. Selectivity, or the selectivity factor, is calculated using:186
 | (19) |
Operating conditions like temperature and pressure significantly influence gas solubility and diffusivity. The van’t Hoff–Arrhenius model and dual-mode sorption model (considering both Henry's law and Langmuir modes) describe these relationships.187,188 Given that gas sorption enthalpy is typically negative, an increase in temperature reduces gas solubility in the polymer matrix. However, this effect depends on the specific gas–polymer interactions. Likewise, pressure effects on solubility and diffusivity vary based on the gas type, pressure range, and membrane porosity. Further details on these correlations are discussed in the literature.188–190
5.4. Membrane design for gas separation: sublayers, intermediate layers, and selective coatings
Flat sheet gas separation thin-film composite membranes typically consist of a support layer for mechanical strength. Separation occurs via a top selective layer, employing either sieving (size exclusion) or selective solution of specific components. Occasionally, an intermediate or gutter layer between the support and selective layers adjusts pore structures and controls diffusion. Each layer will be discussed separately.On the lab scale, membranes are fabricated using a machine-driven or a handheld casting applicator. The wet film is then (immediately or after a measured time) moved to an immersion non-solvent coagulation bath to complete the phase inversion process. For large-scale production, roll-to-roll methods have been explored, allowing controlled sublayer thickness and smooth surface properties. Consumption rates of raw materials and solvents largely depend on the production methods. However, as an estimate, preparation of a single asymmetric porous sublayer by NIPS method requires approximately 50 g m−2.193 The process includes a casting system with adjustable gap and tension for controlling the thickness of the wet film, as well as a coagulation bath with a controlled dose of chemicals and unwinding and rewinding rollers. A schematic of this process is shown in Fig. 11.194,195 After production, flat sheets are commonly converted into spiral wound modules for pilot testing.196–198
 | ||
| Fig. 11 Schematic for pilot production of gas separation membrane sublayer (reproduced from ref. 194 and 195 with permission from Elsevier, copyright 2025). | ||
Sublayers are typically made of various polymeric materials selected for their chemical, thermal, and mechanical properties. The most common materials used for these sublayers include polysulfone (PSF), polyethersulfone (PES), polyacrylonitrile (PAN), polypropylene (PP), polyvinylidene fluoride (PVDF), polytetrafluoroethylene (PTFE), and cellulose acetate (CA).193 Each material offers specific benefits depending on the application. For example, PSF and PES are widely used due to their excellent thermal resistance and chemical stability, particularly when fabricated using the phase inversion method. PES is often preferred over PSF for sublayers because it has a higher hydrophilicity, which enhances adhesion to the selective layer during interfacial polymerization.199,200
The selection of sublayer material is essential for balancing properties like permeability, mechanical strength, and adhesion. Porosity and pore size are controlled through the addition of hydrophilic additives such as polyvinyl pyrrolidone (PVP) and polyethylene glycol (PEG) in the casting solution,17,201,202 which improve membrane performance by influencing the structure and properties of the support layer.
In the case of sublayers acting purely as mechanical supports, their selectivity should be close to 1, meaning they contribute minimally to gas separation.194 However, the pore size and distribution in the sublayer can impact the overall membrane performance. Larger pores in the sublayer, even with identical top layer thickness, may slightly increase permeability, necessitating careful design to ensure an even, smooth surface that allows for uniform coating of the selective layer. Fig. 12 offers a comparison of PES sublayer's pore size effect on the gas transport and separation performance of CO2 separation composite membrane.194 Thus, a sublayer membrane needs to be designed and tailormade based on the specific thin film top layer, feed gas, and operating conditions. Reducing surface roughness by adjusting fabrication parameters, along with achieving an even distribution of pores, can enhance the formation of a uniform selective layer.
 | ||
| Fig. 12 Increasing PES sublayer pore size with the same PVA selective layer thickness (a) thin film composite with PES average porosity of 42.2 nm, (b) thin film composite with PES average porosity of 69 nm, and (c) thin film composite with PES average porosity of 77.5 nm from ref. 194 with permission from Elsevier, copyright 2025). | ||
The permeability of the gutter layer should ideally be five to ten times higher than that of the selective layer to minimize any loss in selectivity, ensuring effective mass transfer.210 Materials like AF2400, a Teflon-based material, offer high gas permeance and don't require additional cross-linking, making them useful for forming homogeneous films. However, AF2400 is hydrophobic, necessitating the use of a more hydrophilic material in some cases.
Two commonly used materials for gutter layers are polydimethylsiloxane (PDMS) and poly(1-trimethylsilyl-1-propyne) (PTMSP). While PTMSP demonstrates higher CO2 permeability, it suffers from a substantial decrease in permeability (up to 80%) over time.191,193 In contrast, PDMS, which exhibits only a 5% permeability decline over similar periods, is a more durable and effective option for CO2 capture membranes. PDMS can be coated using techniques like dip coating or casting, and the casting solution is typically prepared using a standard ratio of PDMS, crosslinker, and catalyst.211
Nanomaterials such as covalent organic frameworks (COFs) are gaining attention as intermediate layers due to their tunable pore sizes. For example, a Pebax 1657 membrane modified with a COF intermediate layer showed a CO2/N2 selectivity of 28 and a permeance of 1840 GPU.212 Metal–organic frameworks (MOFs) are also being explored, particularly plate-like two-dimensional (2D) MOFs that offer a smoother surface than traditional three-dimensional (3D) MOFs, making them more suitable as gutter layers. An ultra-thin zinc(II) tetrakis(4-carboxy-phenyl)porphyrin) (ZnTCPP) MOF layer, when combined with Pebax 1657, achieved impressive performance, with CO2/N2 selectivity reaching 34 and a permeance of 1710 GPU,203 while the same thin film material (with the thickness of 910 nm) on PTMSP gutter layer resulted in GPU of 1160 and CO2/N2 selectivity of 20.213 Several fabrication techniques, including vacuum filtration, spin coating, dip coating, and casting, can be employed to form the gutter layer, depending on the desired membrane characteristics.203,211,212 The choice of method and material significantly influences the overall membrane performance and its ability to achieve efficient gas separation. Fig. 13 illustrates the practical applications of polymer- and nanomaterial-based gutter layers in enhancing decarbonization efficiency in gas separation membranes.
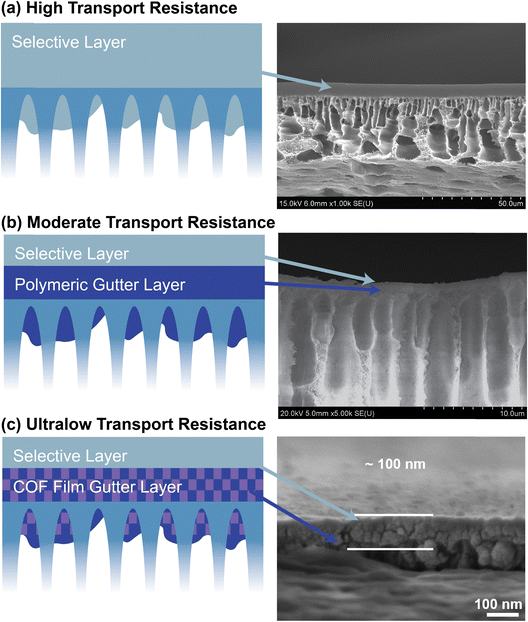 | ||
| Fig. 13 Using an intermediate or gutter layer to enhance the decarbonization capacity of the polymeric thin film composite membranes212 (with permission from Elsevier). | ||
Changing the nature of the membrane backbone chemically can shift the solubility-selectivity to diffusivity-selectivity, assuming the pore size and distribution remain constant. This creates the concept of CO2-affinity membranes, which are constructed from materials rich in oxygen, nitrogen, or sulfur functional groups. These functional groups, including acetate, nitrile, and ether, significantly enhance CO2 solubility.214 Various approaches, such as monomer synthesis and impregnating base membranes with functionalized materials like MOFs and COFs, have been used to develop these membranes.215–217
Polymeric materials from the ethylene oxide (EO) family, including PEG and PEO, are recognized for their high CO2 permeability due to their polar oxygen content. However, increased crystallinity in these materials reduces their permeability.135,218,219 To address this, methods such as copolymerization with materials like polyamides (Pebax) and polyimides have been explored to reduce crystallinity and improve mechanical stability. Pebax membranes, particularly Pebax 1657 and Pebax 2533, are widely studied for CO2 separation.220 Pebax has been shown to benefit from adding hard segments, like polyamide, to provide mechanical strength while retaining CO2 solubility.220 However, neat Pebax exhibits limited permeability, prompting research into solvent effects on its microstructure and crystallinity. Modifying the casting solution composition has been shown to alter permeability and selectivity, with solvents of higher boiling points increasing crystallinity and, therefore, permeability loss.
For FTMs, functional groups that can react with CO2 are introduced to the membrane structure to increase both permeability and selectivity.221–223 FTMs rely on reversible reactions between CO2 and functional groups like amines, and they exhibit higher performance in flue gas decarbonization due to their ability to operate at moderate feed pressures. PVAm membranes, with high amine content, are a leading candidate for FTMs, particularly when coupled with mobile amine carriers.
PEO and related materials, along with nanofillers, have been intensively researched as MMMs to enhance membrane performance. The addition of nanomaterials like MOFs, COFs, and carbon-based materials has improved both permeability and selectivity, with promising results in pushing beyond the Robeson upper bound. These developments, coupled with solvent and casting techniques, have improved the overall performance of gas separation membranes.
Recent advances in fabricating membranes for CCUS application along with the full technical details and performance results, including the effects of molecular weight, casting solvents, and different modifications, are discussed in the following subsection.
6. Advances in membranes for CCUS applications
Advancements in membrane fabrication for CCUS have been achieved through various functional modifications, including new monomer synthesis, altering the membrane backbone's chemistry,224 or impregnating the base membrane with functionalized MOFs,216 zeolitic imidazolate framework (ZIF),212,225 COFs,226 2D and 3D carbon-based structures (graphene oxide (GO),227–229 carbon nanotubes (CNT))230,231). For example, increasing the oxygen-to-carbon (O/C) ratio from 0 to 0.5 by using polyethylene oxide instead of polyethylene has been shown to enhance solubility-selectivity from 13 to 50.214,232 These membranes are developed with diverse design approaches to meet the specific challenges of CCUS applications. This section will cover the latest research works performed on different CCUS membranes.6.1. Polyethylene glycol and similar materials
The ethylene oxide (EO) family, which includes both low-molecular-weight polyethylene glycol (PEG) and high-molecular-weight polyethylene oxide (PEO), represents a common class of membrane materials with acceptable CO2 separation performance. The high content of polar oxygen functional groups in PEO grants it a higher affinity for CO2 permeation. However, these polar structures also increase the crystallinity of the PEO matrix, which reduces overall permeability. For example, amorphous PEO exhibits a permeability of 140 Barrers, while semicrystalline PEO has a permeability of only 13 Barrers. The crystalline structures obstruct free pathways within the membrane, reducing the FFV and, consequently, the permeability.233To address the crystallinity-related permeability loss, enhancing the molecular weight of the ethylene oxide segment has been employed as a strategy to improve membrane-forming capability, reduce crystallinity, and enhance mechanical stability in low-molecular-weight PEO. The micro-domains of the polymeric backbone can also be fine-tuned to further mitigate the permeability loss.
The selectivity of modified-PEO membranes is determined by the EO soft segments. For CO2/N2 binary mixtures, the selectivity performance remains comparable to that of neat PEO. However, the permeability of these membranes is heavily influenced by factors such as the length of both hard and soft segments, their spatial arrangement, and the copolymerization approach. By incorporating hard segments via co- or block-polymerization with materials such as polyamides, polyimides, polyether block amides (Pebax), and aryl sulfones, PEO-based membranes can achieve better control over crystallinity, maintaining both permeability and selectivity.135,218,219
Adding a hard segment to the EO membrane family is an effective method to maintain CO2 solubility while enhancing the mechanical stability of the membranes. This modification is commonly achieved through transesterification or polycondensation reactions involving aliphatic diols, diamines, and aromatic diacids. The resulting copolymer structure may undergo interactions at the interface between the hard and soft segments, necessitating an optimal design that minimizes disruptions. An ideal PEO-based membrane for CCUS should exhibit weak interpolymer interactions.234
One prominent example of an EO-containing copolymer is polyether block amide (PEBA), commonly known as Pebax. In this copolymer, the ether-containing soft segment enhances solubility through strong dipole-quadrupole interactions with polar components in the feed gas, while the crystalline polyamide (PA) segment provides mechanical strength and higher solubility selectivity due to its polar content. The PA segment also directly controls gas diffusivity by regulating the FFV and intersegmental polymer spaces. Pebax's affordability and favorable characteristics have made it a popular material for CO2 separation and other polar/non-polar gas mixtures such as CO2/CH4, CO2/N2, CO2/H2, H2S/CH4, CH4/N2, O2/N2, NH3/N2, NH3/H2, ethylbenzene/N2, and hydrofluorocarbons (HFCs)/hydroflurolefin (HFO).235
Given its popularity, Pebax is now considered a distinct class of gas separation membrane material with various production and modification methods. Among the different grades of Pebax, Pebax 1657 is commonly used due to its superior CO2 selectivity, while Pebax 2533 has the highest soft segment content, resulting in higher permeability.220Table 7 provides further details on various Pebax membrane materials and their separation performance.
| Pebax | Soft segment (polyether, wt%) | Hard segment (polyamide, wt%) |
|---|---|---|
| 1657 | 40 | 60 |
| 1074 | 55 | 45 |
| 5513 | 60 | 40 |
| 2533 | 80 | 20 |
While Pebax is one of the most widely studied materials for post-combustion CCUS applications, its low permeability limits its performance, prompting various modification strategies to enhance its efficiency. Although there is significant interest in Pebax-based CO2 separation membranes, only a few studies have focused on how fabrication parameters affect their structure and performance. For instance, Isanejad et al. examined the influence of organic solvents on the microstructure and performance of Pebax 1657.237 Their study demonstrated that even though the chemical structure of the membranes remained identical, the boiling points of the solvents used during fabrication played a crucial role in altering the crystallinity and free volume (d-spacing) of the membranes, as shown by X-ray diffraction (XRD) measurements. Dimethylacetamide (DMAC), for example, produced a membrane with the highest crystallinity. Initially, the d-spacing increased with crystallinity, but excessive crystallinity led to reduced free volume due to slower solvent evaporation, resulting in a more interconnected membrane matrix.
Solvent characteristics, such as specific volume, also impact membrane structure. Solvents with higher specific volumes create larger d-spacing by reducing van der Waals interactions, leading to membranes with higher FFV and, consequently, increased permeability.238 Karamouz et al. studied the effect of drying temperature on the structure and performance of Pebax 1074 membranes and found that drying temperatures of 60–80 °C resulted in denser membranes with better permeability and selectivity. However, temperatures above 80 °C caused the formation of non-selective micro-voids, which reduced selectivity due to rapid solvent evaporation.239
Modifying Pebax membranes can be done by blending Pebax with a base polymer such as polyethersulfone (PES) or by coating a thin Pebax film onto a nanoporous sublayer. Solvent compatibility is essential for successful casting solutions, with a 70/30 wt% ethanol/water mixture proving to be an effective solvent for minimizing structural impacts. Since the highly polar structure of Pebax requires a solvent with a high dielectric constant, several solvents have been proposed.240,241 Formic acid has also been shown to be a highly effective solvent for dissolving Pebax and preventing gelation at low temperatures, although its large-scale viability remains a concern.242 After dissolving Pebax and its modifiers, the mixture is typically refluxed at 70–80 °C for 2 hours, followed by post-treatment drying to remove residual solvents.
Crosslinking has emerged as another effective strategy for modifying Pebax membranes. Reported examples include Pebax/PVDF crosslinked with 2,4-toluylene diisocyanate (TDI),243 Pebax/PAN crosslinked with polydimethysiloxane (PDMS),244 PES/Pebax composite membrane crosslinked with poly ethylene glycol diacrylate (PEGDA),245 and Pebax/chitosan crosslinked with glutaraldehyde.246 The characteristics of the crosslinker and its impact on the membrane's final structure are crucial factors to consider when designing such modifications. Silane coupling agents containing amine groups can be incorporated for polymer crosslinking while enhancing polar interactions of CO2. Sanaeepour et al. conducted such amino-silane modification by enhancing the selectivity of Pebax 2533 using (3-aminopropyl(diethoxy)methyl silane (APDEMS)).222 They highlighted the benefits of R-(CH2)n-Si-X3 crosslinkers (where R and X represent amino and hydrolyzable groups), which reduce the gas diffusion energy barrier due to Si–O local mobility. These crosslinking modifications are designed to increase selectivity without significantly sacrificing permeability.246
The incorporation of nanofillers into Pebax membranes has been intensively studied, and various classes of nanofillers for CCUS applications have been comprehensively reviewed. The performance changes of the membranes were reported in Fig. 14.247 Among all the fillers, ranging from the novel MXene structures to more established fillers like graphene and carbon nanotubes, the bimetal oxide nanosheet ZnCo2O4 demonstrated the most significant improvements, enhancing permeability and selectivity by 166% and 76%, respectively. The better enhancement ratios were linked to the generation of oxygen vacancies (O−δ), which ultimately create more CO2 adsorption sites (C−δ).248 Another significant additive with a 628% selectivity enhancement ratio was NaY zeolite due to creating a diffusional path by micro-sized voids.249 Permeability-oriented enhancement strategies are particularly prominent among Pebax modification approaches, aiming to surpass the binary gas Robeson's trade-off.235
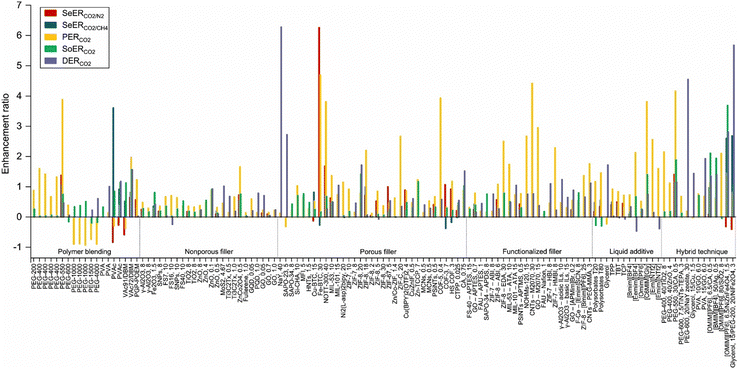 | ||
| Fig. 14 Selectivity, permeability, diffusivity, and solubility enhancement ratios for mixed matrix Pebax membranes (reproduced from ref. 247 with permission from Elsevier, copyright 2025). | ||
Copolymerization of polyesters and EO derivatives represents another family of CCUS membranes with improved inner microregions. A commercialized copolymer in this family is poly(butylene terephthalate) (PBT), known as polyactive. The ease of copolymerization for this combination enables systematic studies on the effects of segment length, molecular weight, and weight percentage of each block on transport and mechanical properties.250 Experiments suggest that the chain length, molecular weight, and thermal characteristics of polyactive segments have similar influences on membrane performance as those in Pebax. In combination with ester segments, PEO with a molecular weight within the range of 2000 to 2500 g mol−1 has been found to achieve the highest permeability.251 Imide copolymers are reported to outperform amides and esters when the PEO segment is sufficiently long to form a continuous phase. The enhanced performance of polyethylene imide is attributed to the limited hydrogen bonding interactions between the segments, resulting in complete phase separation.252
6.2. Facilitated transport membranes (FTMs)
The addition of the functionalized selective layer to the gas separation membranes bearing amine carriers can lead to the fabrication of FTMs. FTMs are well-suited candidates for post-combustion CCUS scenarios where the main flue gas elements are nitrogen and CO2.253 Current FTMs exhibit moderate selectivity (50–100) with high CO2 permeability (exceeding 1000 GPU).221–223 Compared to traditional solution-diffusion membranes, FTMs offer superior performance at moderate feed pressures, making them more cost-effective for flue gas decarbonization by reducing the need for high compression costs.254FTMs take advantage of CO2-philic structures and functional groups to create more CO2 adsorption sites on and within the selective top layer of the membrane, resulting in higher permeability and selectivity of the membrane. CO2 reacts reversibly with the target functional group on the surface of the selective layer and turns into an alternative species. It then diffuses through the membrane body due to the chemical potential difference driving force, originating from the partial pressure or concentration difference of CO2, and dissociates on the opposite side of the membrane in the form of CO2 (Fig. 15(a)). While the transport of the polar gases happens by reaction and diffusion, the inert gases like methane and nitrogen pass through only by diffusion. Thus, FTMs can selectively separate CO2. Several structures have been investigated for their CO2-philicity, with polymers containing a high content of amines reported as the most effective carriers for FTMs. The functional group can either be integrated into membrane's polymeric backbone (fixed-site amine carriers) or incorporated into the membrane matrix through modification strategies (mobile carriers).
 | ||
Fig. 15 (a) CO2 transport through amine-containing facilitated transport membrane,255 (b) reaction pathways for CO2 passage through facilitated transport membranes, and (c) chemical structure of PVAm or polyvinyl formamide-co-vinylamine (PVNF-co-Vam); m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o = 85 o = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 at pH of 11.7256 (reproduced from ref. 255 and 256 with permission from Elsevier, copyright 2025). 3 at pH of 11.7256 (reproduced from ref. 255 and 256 with permission from Elsevier, copyright 2025). | ||
Fig. 15(b) illustrates two main pathways for amine-facilitated transport in FTMs.255 Primary and secondary amines possess an unshared electron pair on the nitrogen atom, allowing them to act as nucleophiles and engage with the electrophilic carbonyl group of CO2, forming a zwitterion. The zwitterion undergoes a quick deprotonation process facilitated by another amine, yielding a more stable carbamate ion. In this pathway, converting one mole of CO2 requires two moles of amines. When a tertiary amine is used in FTM, acting as a Brønsted base only, bicarbonates are formed instead of carbamates. Carbonic acid is neutralized after the reaction of CO2 and water. This pathway requires one mole of amine for each mole of CO2. Although the second pathway is more efficient, the slow formation of carbonic acid hinders the reaction rate.257
An ideal example of FTM is the polyvinyl amine (PVAm) membrane, which is highly valued for its high content of primary amine groups.209,258 Compared to other polymers, including amine fixed-site carrier-containing polymers such as polyallylamine (PAAm),259 chitosan,246 and polyethyleneimine (PEI), PVAm has the highest amine content,260 making it a leading candidate for CO2 separation applications. Its compatibility with porous PES UF sublayers has increased its commercial potential as a composite membrane material.261,262
PVAm membranes are typically synthesized using N-vinyl formamide (NVF), a water-soluble isomer of acrylamide, in a solution polymerization process that involves free radical polymerization under nitrogen ambient in an aqueous solvent containing reactive initiators like α,α′-azodiisobutyramidine dihydrochloride (AIBA).209 The resulting poly(N-vinylformamide) (PVNF) undergoes a partial acid hydrolysis step using aqueous HCl, followed by a strong base anion-exchange process to adjust the pH to 10. This approach produces PVAm with a molecular weight between 0.8 to 1 kDa. An alternative synthesis method, inverse emulsion polymerization (IEP), offers higher viscosity263 and better control over the polymerization process. The aqueous monomer solution is distributed in an organic phase throughout the IEP, and the polymerization takes place in multiple dispersed polymer phases encircled by an emulsifier. Instead of the hydrophobic continuous phase, polymerization occurs inside the separated micelles. The reaction system can benefit from facilitated heat and mass movement, and the likelihood of developing gels greatly decreases. A recently less practiced approach for PVAm production is polyacrylamide conversion through the Hoffman reaction.260 Polyacrylamide is readily available and reasonably priced, making this strategy promising. However, the Hofmann process requires sodium hypochlorite treatment at a high pH, which might cause adverse effects, including chain scission.
Much research has focused on enhancing the molecular weight (MW) of PVAm. Increasing the MW of the casting solution improves the density of the selective layer, reducing the diffusion of non-polar gases while increasing chain mobility for better gas separation performance.264 An effort toward enhancing the MW of PVAm (from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 80
000 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was reported to significantly reduce the permeance of CH4 and increase the CO2/CH4 selectivity (10-fold growth).264 However, maintaining consistent reaction conditions during MW growth is challenging,264 and alternative methods, such as synthesizing sterically hindered PVAm to favor the bicarbonate pathway, are being explored.265 Attaching a bulky structure, such as a methyl group, to the amine hinders the carbamate pathway, promoting the chemisorption of CO2 as bicarbonate. As this requires fewer amine sites, more CO2 can pass through the membrane with the constant amine functional group content.
000) was reported to significantly reduce the permeance of CH4 and increase the CO2/CH4 selectivity (10-fold growth).264 However, maintaining consistent reaction conditions during MW growth is challenging,264 and alternative methods, such as synthesizing sterically hindered PVAm to favor the bicarbonate pathway, are being explored.265 Attaching a bulky structure, such as a methyl group, to the amine hinders the carbamate pathway, promoting the chemisorption of CO2 as bicarbonate. As this requires fewer amine sites, more CO2 can pass through the membrane with the constant amine functional group content.
Crosslinking substances bearing CO2-philic carriers to a fixed carrier membrane may improve CO2 transport, CO2/N2 selectivity, and mechanical integrity of the PVAm membranes.266 Crosslinking introduces hydrogen bonding into the polymer matrix, further enhancing amine carrier effectiveness.266,267 Several studies have investigated improving the performance of PVAm membranes through the incorporation of mobile amine carriers such as piperazine.261,268 Piperazine-modified PVAm membranes have outperformed those modified with ethylenediamine (EDA),266 showing a more than twofold improvement in selectivity. A team from Ohio State University, led by Winston Ho, has further advanced this field by optimizing PSF membranes with PVAm, piperazine, and glycinate carriers.261 Adding 0.1 wt% polyvinylpyrrolidone (PVP) improved hydrophilicity and pore size, achieving a CO2 permeance of 843 GPU and a CO2/N2 selectivity of 160. However, excessive crosslinking may lead to carrier depletion or polymer matrix densification, negatively affecting membrane performance.269,270
A significant portion of PVAm membranes is sourced from commercial aqueous Lupamin solutions or similar products, such as Polymin® VX271,272 (Fig. 15(c)). In such cases, PVAm is precipitated from the concentrated, viscous solution using ethanol. After separation and drying, the white precipitate is either exposed to another round of dissolution and precipitation or Soxhlet extraction for further purification. A final ion exchange-assisted pH adjustment is performed to transform the functional groups.273,274 pH adjustment is identified as a crucial step due to the improvement of free amine groups without protonation (elimination of ammonium salts), which eventually improves the CO2 reactivity of PVAm membranes (discussed and approved at different pHs by Kim et al.275). Casting solutions for wet or Petri dish casting, with an approximate concentration of 2 wt% PVAm, are commonly used with mobile amine carriers or inorganic modifiers. Undiluted PVAm membranes derived from commercial solutions often result in fragile, uneven films.271
In a recent pilot-scale study conducted in Wilsonville, Alabama, PVAm-based spiral-wound modules were tested on real coal-based flue gas (Fig. 16(a)).197 The membranes demonstrated a CO2 permeance of 1450 GPU and a CO2/N2 selectivity of 185. The study also examined the effects of feed flow rate, pressure, and temperature, as well as long-term stability under varying CO2 concentrations and exposure to heavy metal deposition. These findings demonstrate the potential of PVAm membranes for large-scale carbon capture and separation applications.
 | ||
| Fig. 16 Spiral wound module test rig for facilitated transport membrane assisted decarbonization; (a) schematic of the module's housing, (b) test setup (reproduced from ref. 197 with permission from Elsevier, copyright 2025). | ||
FTMs face several challenges, particularly in their mechanical characteristics and performance under varying operating conditions. Most FTMs operate at near-zero differential pressure, making it difficult to assess their mechanical integrity under such conditions. Additionally, different processes lead to varying CO2 concentrations and partial pressures in the exhaust, which can impact the efficiency of the separation process. Lower partial pressures are generally unfavorable because they reduce the driving force for separation. On the other hand, excessively high partial pressures of CO2 can cause amine carrier saturation, leading to decreased efficiency in CO2 transport and reduced overall performance.276
Flue gas decarbonization using FTMs typically occurs at moderate temperatures. However, if the gas stream is compressed, an inevitable rise in temperature can influence membrane performance. While FTMs can still perform well under such conditions, temperature optimization becomes essential to maintain efficiency. Humidity is another key factor in the performance of FTMs.256 Competitive sorption between water and nitrogen can hinder N2 passage and enhance CO2/N2 selectivity. Therefore, proper humidity control is crucial to ensure that FTMs operate effectively in gas separation processes, as water vapor content can significantly affect the selectivity and permeance of CO2.47
6.3. Polymers of intrinsic microporosity
PIMs represent a significant class of materials for CO2 separation, due to their unique combination of high permeability and selectivity derived from their intrinsic microporous structures. PIMs share certain similarities with COFs, as both possess highly porous structures that promote CO2 removal from gas streams. However, unlike COFs, PIMs do not require a covalent bond network to achieve their microporous architecture.277 Instead, their high permeability arises from their exceptionally high FFV, resulting from the incorporation of large, rigid, contorted polymer chains that disrupt efficient chain packing. This disruption creates a “ladder-like” structure that contributes to their microporous nature and enhances gas permeability.The primary polymerization reaction responsible for the formation of PIMs involves double-aromatic nucleophilic substitution, which allows for the simultaneous creation of two covalent bonds, establishing the polymer backbone.278 This process results in a highly tortuous structure made up of interconnected ring systems that restrict rotational motion along the polymer chain. The restricted rotational mobility prevents macromolecular sections from realigning, thereby maintaining the open, porous nature of the polymer. The bimodal narrow pore distribution within PIMs, typically ranging from 7 to 20 Å, provides selective molecular sieving, which is crucial for CO2 separation.279–281 These factors make PIMs highly effective for CO2 separation, pushing the performance of gas separation membranes beyond the traditional Robeson upper bound, which limits the trade-off between permeability and selectivity (Fig. 17). The enhanced internal molecular free volume (IMFV), as shown in Fig. 18, combined with the ability to finely tune their structure, contributes to the superior gas transport properties of PIMs, making them as a prominent material for CO2 separation.
 | ||
| Fig. 17 Selectivity-permeability log–log plots for (b) H2/N2, (a) O2/N2, (c) CO2/N2, and (d) CO2/CH4 and their evolution through time by different membrane materials introduction to the market; solid black lines (1991), solid blue lines (2008), and red dot lines (2015) upper bounds (reproduced through RCS open access policy from ref. 282). | ||
 | ||
| Fig. 18 Polymer of intrinsic microporosity (PIM); (a) sample structure with internal molecular free volume (IMFV), (b) chemical structure of a linker in a PIM, (c) PIM, (d) organic molecule of intrinsic microporosity (OMIM), (e) dendrimer of intrinsic microporosity (DIM), and (f) chemical structure of Triptycene PIM (reproduced from ref. 283 with permission from Elsevier, copyright 2025). | ||
In FTMs, high pressures lead to ‘carrier saturation,’ where the ability of CO2 carriers to facilitate gas transport diminishes due to the overwhelming influx of CO2. As a result, the transport mechanism shifts from facilitated transport to solution diffusion, reducing efficiency. Furthermore, high pressures compress swollen membranes, leading to water loss, reduced polymer flexibility, and decreased free volume for gas diffusion. This issue is further exacerbated by the fact that, at high pressures, water vapor permeates more readily than CO2, reducing the water vapor content essential for FTM function and leading to lower permeance and selectivity. Despite these advancements, PIMs face several limitations. One major drawback is their susceptibility to physical aging, where the polymer structure collapses over time, reducing its gas transport efficiency.284 Additionally, PIMs exhibit moderate selectivity compared to other advanced gas separation materials. Their chemical stability, while superior to some other high-FFV polymers such as poly(trimethylsilyl propyne), still presents a challenge, particularly under harsh industrial conditions. Efforts are ongoing to enhance the chemical robustness of PIMs through copolymerization and blending with more stable materials to mitigate aging and improve their long-term performance.
6.4. Biomaterial-modified membranes for decarbonization
The polymeric membranes used for CO2 separation are primarily made of synthetic materials. The growing interest in synthesizing biodegradable and sustainable membranes has spurred researchers to investigate the potential of renewable and biodegradable polymers as alternatives in membrane production. Due to their biocompatibility, biodegradability, and environmental sustainability, biopolymers are considered viable alternatives to conventional fossil-based/synthetic polymers for developing CO2 membranes. Below, we explore key biopolymers utilized in membrane fabrication for CO2 separation and capture.Cellulose acetate (CA), the most well-known derivative of cellulose, is produced by the acetylation of cellulose hydroxyl groups.290 It is widely used in membrane production due to its good solubility in a range of organic solvents and its ability to form membranes with controlled pore structures. The hydroxyl groups within CA are readily available for various modifications, including oxidation, etherification, hydrolysis, esterification, grafting, crosslinking, and copolymerization.291 CA membranes exhibit properties such as uniform pore structure, natural hydrophilicity, thermal stability, and suitability for gas separation, such as CO2 and CH4.292 The degree of acetylation is a key factor affecting the gas separation efficiency of CA membranes. CA is partially crystalline and exhibits different substitution levels (DS = 1–3), indicating the extent to which hydroxyl (–OH) groups per repeating cellulose unit are acetylated. Based on the DS values, CAs display CO2 permeability ranging from 1.8 to 6.6 Barrer at 35 °C.293 The CO2 gas permeability of membranes can be improved by employing cellulose acetate with higher DS without severe change in the gas selectivity because of the less internal hydrogen bonding among cellulose chains, providing a more porous structure.294 Studies evaluating the influence of the degree of acetylation (1.75–2.84) on the gas separation properties of cellulose acetate indicate that the gas permeability coefficient increases with a higher degree of acetylation.295 However, limited CO2 permeability poses a challenge when using CA as a membrane material in applications involving CO2-containing streams. Nikolaeva et al. improved CA separation efficiency by integrating ionic liquid-like functionalities, namely 1-methylimidazole, 1-methyl pyrrolidine, and 2-hydroxyethyl dimethylamine (HEDMA), onto the CA structure.296 Experimental evaluation of CO2/N2 mixed-gas permeation demonstrated a reduction in both CO2 and N2 permeability, with an initial decline in CO2/N2 selectivity followed by a gradual increase as the HEDMA content increased. CA membranes face drawbacks such as structural compression under high pressure, narrow pH tolerance (4.5–7.5), and temperature limits (up to 30 °C).297,298 To optimize CA membranes, various solvents and additives are used during preparation, including N-methyl pyrrolidone, N,N-dimethylacetamide, and mixed solvents like N,N-dimethylformamide with acetone or 2-propanol.299,300
Among cellulose products, nanocellulose stands out for its exceptional surface area and mechanical properties, which make it highly effective in carbon capture.301,302 Nanocellulose is classified into cellulose nanocrystals (CNC), cellulose nanofibers (CNF), and bacterial cellulose (BC). CNF is typically produced through a two-step process involving chemical or enzymatic pre-treatment followed by mechanical processing. This pre-treatment step not only enhances processability and uniform size distribution but also allows for tailoring the properties of the nanocelluloses for different gas separation applications, such as introducing CO2 reactive groups. CNC and CNF exhibit differences in length and crystallinity, with CNC being predominantly crystalline and CNF often described as having amorphous regions with crystalline segments. CNC offers advantages such as uniform size with nanometric dimensions in both length and width. TEMPO-mediated oxidation is a key reaction for nanocellulose synthesis, where cellulose is converted into polyglucuronic acid due to the oxidation of C6 alcohol groups in the anhydroglucose unit.303 CNFs with higher aspect ratios (5–50 nm diameter and several micrometers in length) and entangled networks are utilized as reinforcement agents or viscosity controllers in papermaking and polymer composites.304 In contrast, CNCs, due to their higher crystallinity and shorter length (< 100 nm), represent better dispersibility, improving the strain at failure of composites.305,306 A PVAm/nanocellulose hybrid membrane was developed for carbon capture applications.271 The developed films with nanocellulose (30–70%) were analyzed through water vapor sorption experiments and humid gas permeation tests. Improvements in gas permeability and selectivity were achieved by increasing water vapor and the PVAm content in the films. The highest selectivity (135 for CO2/CH4 and 218 for CO2/N2 separation) was observed in blends containing nanofibrillated cellulose (CNF) with 70 wt% PVAm at 60% RH, while the maximum permeability of approximately 187 Barrer was achieved at 80% RH. Modifying CNFs with amine and aminosilane is a practical strategy to increase their CO2 sorption capabilities. Chemical bonding of the aminosilanols from an aminosilane with cellulose hydroxyl groups occurs during the aminosilane functionalization of cellulose. In addition, the simultaneous self-attachment of amino silanols, due to an undesired side reaction, leads to the formation of siloxane bridges (Si–O–Si).307 Regarding amino silane modification of CNFs, ethanol-water suspension, and toluene are two of the most common media used for cellulose modifications. N-(2-aminoethyl)-3 aminopropyl methyl dimethoxysilane (APMDS) is mainly attached to hydroxyl groups of C6 position in nanocellulose structure because of space structure of atoms in cellulose molecule during chemical modification of cellulose nanofibers for CO2 adsorption. Amine loading of the modified CNF aerogels by APMDS is affected by the process parameters, such as the reaction time, the reaction temperature, silane proportion, and the kind of solvents. Tertiary butanol has been recommended as a highly efficient solvent, resulting in an amine loading of 9.02 mmol g−1 with 6% APMDS.307
Increasing the gas feed pressure reduces both the permeance and selectivity of the membranes. This is attributed to the stacking of polymer chains, which leads to membrane densification at higher pressures and restricts gas permeation. This effect becomes more pronounced at extremely high pressures, leading to membrane “plasticization,” where the polymer structure is permanently altered due to CO2 swelling in the spaces between polymer chains. Plasticization can cause a loss of membrane performance as gas transport pathways become obstructed. Several strategies have been explored to mitigate high-pressure plasticization and membrane compaction, including crosslinking membranes and reinforcing polymers with inorganic or organic nanofillers to enhance mechanical strength.309 To address these issues, maintaining high water vapor content in the feed gas is essential for preventing membrane drying in high-pressure applications. Combining NC with hydrophilic polymers has been suggested to enhance permeance significantly. For instance, in membranes combining CNF and PVAm, permeability increased over 200-fold, with relative humidity (RH) levels up to 85%. Selectivity also improved by up to 65% RH but declined at higher RH levels due to excessive water activity, which caused membrane swelling. Optimization studies recommend a membrane composition of 70% PVAm and 30% CNF, achieving maximum permeance and selectivity at 85% RH in NC-based FTMs.271,309
6.5. Mixed matrix membranes
The performance of polymeric membranes are inherently constrained by their permeability-selectivity trade-off, commonly represented by the Robeson upper bound. Strategies have been proposed to improve gas solubility—by creating chemical interactions between gas molecules and polymer chains—and increase gas diffusivity—primarily by enhancing the polymer's void fraction while minimizing the formation of non-selective voids.313 These studies have resulted in the fabrication of MMMs that incorporate inorganic fillers into the polymeric matrices, simultaneously enhancing permeance and selectivity and thus lowering operating pressure, energy consumption, and the overall footprint of separation processes.Embedding inorganic fillers facilitates the preferential transport of target gas molecules while obstructing the pathways of other molecules, thereby improving separation performance. An inorganic filler that is well-dispersed within a polymeric phase can substantially modify the FFV due to changes in the conformation, dynamics, or packing of polymer chains. This modification can effectively discriminate between smaller gas molecules and larger ones, leading to enhanced gas selectivity, such as H2/CO2.314 Moreover, the interfacial interactions between inorganic fillers and polymer chains play a crucial role in directing the transport pathways of gas molecules, thereby enhancing selective transport and improving gas permeabilities.315,316 Additionally, nanofillers in MMMs help prevent membrane plasticization by acting as crosslinking agents. Examples of these modifiers include CNT, GO, cellulose nanofibers (CNF), cellulose nanocrystals (CNC), MOFs, COFs, layered double hydroxides (LDHs), transition metal dichalcogenides (TMDs), and MXenes. Although these fillers have the potential to enhance both permeability and selectivity, the extent of these improvements depends on several key factors. Simply blending non-homogeneous phases does not always guarantee optimal membrane performance.
While MMMs are promising, they have several challenges that must be addressed. These challenges include inconsistencies at the phase interfaces, uneven distribution of fillers, and reduced stability compared to homogeneous systems.236 Larger fillers are prone to agglomeration, forming clusters that disrupt the membrane's homogeneity. As a result, the mechanical strength of the membrane deteriorates, leading to undesirable performance under high pressure. Thus, a modifier/filler should remain in the nanometric size range at its highest loading and should allow for controlling size distribution and preventing aggregation. Moreover, the interaction between the surface of nanomaterials and polymers plays a critical role in maintaining the membrane's mechanical integrity, requiring careful optimization. Fig. 19 depicts perfect and imperfect interactions (e.g., polymer rigidification, pore blockage, and interfacial defect) along with their possible impact on the selectivity-permeability trade-off, where losses in permeability and selectivity may occur depending on the nature of the filler and the polymer matrix.317
 | ||
| Fig. 19 Various nanomaterial-polymer interactions and their related consequences (reproduced through RSC open access policy from ref. 317). | ||
One major strategy to enhance nanomaterial-polymer interfacial compatibility is surface functionalization of the nanofiller using CO2-philic moieties (NH2, OH, COOH, and SO3), attaching polymeric chains, or connecting it with other nanomaterials. Modifications on the polymer backbone also promote electrostatic and hydrogen bonding interactions, improving compatibility.318 Drying and redispersion of nanomaterials induce agglomeration to reduce surface energy. To resolve the challenges of aggregation, multistep nanomaterial synthesis and size control, as well as one-pot in situ growth, have been suggested. Synthesizing nanomaterials using polymer chains as a scaffold limits size growth, promoting a high load of evenly distributed nanosized fillers throughout the membrane.319 However, selecting the right solvents for in situ growth is crucial, as they must be compatible with both the nanomaterial synthesis procedure and membrane fabrication. Another approach to reducing agglomeration is using wet nanomaterials, which excludes the drying step by exchanging the solvent in which the polymers will be dissolved.320
Nanostructured fillers, when properly applied, can significantly improve various membrane properties. Key factors include particle size, porosity, even distribution, and their affinity for CO2 molecules—often called “CO2-philicity.” Correct particle sizing is crucial to avoid clustering, which could result in a non-selective, heterogeneous top layer. Moreover, the interaction between the surface of nanomaterials and polymers plays a critical role in maintaining the membrane's mechanical integrity, requiring careful optimization.
A major challenge in MMM fabrication is the compatibility of nanofillers with the polymer matrix and solvents. Poor dispersion of nanofillers can lead to phase separation, uneven film formation, and defects that degrade membrane performance. Inhomogeneities in thermal behavior and elastic modulus between the phases may also cause mechanical delamination.321 Proper solvent selection during synthesis and fabrication is essential to maintain the chemical stability of nanomaterials and prevent phase inversion. Controlling the nanomaterial load and adjusting the viscosity of the polymer solution can mitigate phase separation and improve nanofiller distribution.
The porosity and functional groups of the fillers can further enhance both the performance and mechanical stability of the membrane. Non-porous fillers increase diffusion-path tortuosity, which typically reduces permeability. However, the presence of functional sites can enable selective diffusion, potentially enhancing effective permeability for targeted species. In contrast, porous fillers act as molecular sieves, facilitating gas transport based on kinetic size and shape. The connectivity of the filler network also plays a crucial role in optimizing gas diffusion pathways, significantly improving membrane performance. A schematic illustration of filler impact on gas transport in MMMs is shown in Fig. 20.
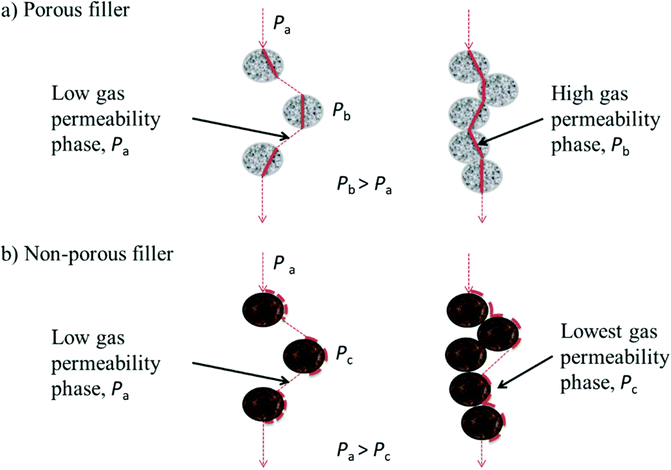 | ||
| Fig. 20 Gas diffusion in mixed-matrix membranes with (a) porous and (b) non-porous fillers (reproduced from ref. 280 with permission from RCS, copyright 2025). | ||
Several inorganic modifiers have so far been introduced to polymeric membranes with impermeable, surface-functionalized nanomaterials, porous, and non-3D (one, 2D) enhancers. Examples of these modifiers include CNT, GO, cellulose nanofibers (CNF), cellulose nanocrystals (CNC), MOFs, COFs, layered double hydroxides (LDHs), transition metal dichalcogenides (TMDs), and MXenes. Among these, MOFs and MXenes have gained particular attention due to their high surface area, tunable pore structures, and unique chemical functionalities, which enhance molecular selectivity and improve separation performance in polymeric membranes. MOFs represent a unique class of porous nanostructured compounds that have gained prominence as alternatives to conventional inorganic microporous materials like zeolites. MOFs consist of a metal core and an organic linker (ligand), and their hybrid organic/inorganic composition provides high surface area and tunable pore sizes, making them suitable for diverse separation applications.322–326 A key advantage of MOFs is their highly customizable molecular structure, allowing for precise control by selecting specific metal cores and organic ligands. Stabilized by chemical bonds, these metal centers resemble those found in metal oxide nanoparticles (NPs). The bonds within the MOF structure are strong enough to ensure material robustness while maintaining the activity of the metal centers.327 The active metal sites in MOFs are uniformly distributed throughout the entire structure, enhancing their affinity for CO2 molecules if properly selected. Additionally, the organic ligands used in MOFs often carry polar functional groups like –NH2 or –SO3, which synergistically improve CO2 adsorption and increase the dispersion of the MOF within polymer matrices compared to inorganic NPs.
In recent years, continuous advancements in MOF-based MMMs have been driven by the development of new organic linkers paired with different metals to improve decarbonization performance.328 These newly designed ligands not only protect the metal core from nucleophilic attacks but also support the stability and functionality of the framework. The chemistry behind MOF synthesis is crucial, as factors such as pore volume, aperture size, particle size, and filler distribution all influence the membrane's CO2 separation performance. Specifically, the aperture size determines molecular sieving capabilities, while other properties, such as pore shape and size, impact the overall separation efficiency.
Despite significant progress, several challenges remain in the commercialization of MOFs. Only MOFs with effective heat and mass transfer properties, such as Universitetet i Oslo (UiO)-66, are suitable for continuous flow reaction production.329 One key consideration for researchers is the development of environmentally friendly synthesis methods that use green solvents and moderate processing conditions.330 However, achieving repeatability in MOF synthesis remains difficult, particularly under intense operational conditions. These challenges can lead to poor dispersity, low reactivity, and hindered mass transfer, resulting in issues like undesired size distribution, material collapse, aggregation, and pulverization of MOFs.329
Although MOFs can potentially achieve satisfactory separation performance, their tendency to agglomerate and form non-selective voids restricts their full potential for gas separation. One practical method to overcome agglomeration is immobilizing or decorating MOFs on larger support structures, creating MOF-based templates. This hybridization significantly reduces surface energy and the tendency to agglomerate, forming a more stable structure for MOF deposition and growth. Such templates enhance the composite's multifunctional features, including increased adsorption capacity, enhanced porosity, improved permeability, and greater mechanical strength than standalone MOFs. Promising materials for MOF nucleation and growth include CNTs,331 GO,332,333 reduced GO (rGO),334 CNCs,335 and halloysite nanotubes (HNT).336 The synergy between the MOFs and these support materials offers significant performance improvements by combining adsorption and molecular sieving capabilities.
Furthermore, achieving homogeneous dispersion within the matrix remains a challenge. Synthesizing fillers with well-defined physical and chemical properties and leveraging their synergistic effects with 2D fillers holds promise. Carbon-based nanomaterials such as CNTs and GO have gained significant attention as promising membrane materials.337 However, their separation performance is heavily influenced by the degree of dispersion and chemical modifications. Functionalizing the surface of these carbon-based nanomaterials enhances their overall performance, leading to improved separation and durability in membrane applications.
Generally, GO exhibits a higher tendency for dispersion and is easier to functionalize compared to CNTs, primarily due to the presence of multiple functional groups on its surface. GO, an allotrope of carbon, consists of sp2-bonded carbon atoms arranged in a hexagonal honeycomb lattice.338 It forms 2D nanosheets with a high specific surface area and an atomically thin laminar structure, presenting a new class of highly permeable and selective nanomaterials for membrane-based separations.339,340 The physicochemical properties of GO nanosheets, such as morphology, size distribution, density of oxygen-containing functional groups, electronic mobility, and carbon radicals, significantly influence their potential for further modifications. Several oxygen-containing functional groups exist on GO, including hydroxyl and epoxide groups on the basal plane, and carboxylate groups primarily at the edges.341,342 The presence of both ionic groups and aromatic sp2 species enables GO to serve as a nucleation site for metal cations and further growth when organic linkers interact. Metal cations deposit on GO nanosheets through π–π interactions, hydrogen bonding, and Ag–O coordination.343,344 Due to these superior characteristics, GO is a promising template for developing MOFs-based hybrids. The use of GO-based hybrids in developing efficient MMMs for CO2 separation has been regarded as one of the promising solutions. By decorating MOFs on GO, it is possible to control the interlayer structure, improving permeability and separation performance due to the molecular sieving properties of the hybrid material.334
For example, different types of MOF nanosheets (such as ZIF-7, ZIF-8, CuBTC, and MIL-100) have been systematically integrated into the interlayers of reduced GO (rGO), benefiting from its polar oxygen groups, increased interlayer spacing, and high electronegativity.334 These properties facilitated strong anchoring of rGO and created a porous structure with uniform nanochannels, enhancing separation performance. In one study, ZIF-8@GO hybrids incorporated into a Pebax matrix improved CO2 separation by increasing both CO2 permeability (191%) and CO2/N2 selectivity (174%).333 Two main functions enhanced membrane performance: (i) the high-aspect ratio of GO nanosheets augmented the tortuous path length for gas diffusion within the polymer matrix, thereby limiting the diffusion of larger molecules while facilitating the passage of smaller ones, which improved diffusivity selectivity; and (ii) the intrinsic high permeability and ultra-microporosity of similarly, ZIF-8/GO hybrid composites incorporated into a polysulfone (PSF) matrix achieved a 7-fold increase in CO2/CH4 selectivity and an 87% increase in CO2 permeability compared to pristine membranes.332 Additionally, bimetallic ZIFs with different Co/Zn ratios were incorporated into the Pebax matrix, leading to a significant 250.37% enhancement in selectivity, surpassing the Robeson upper bound, due to finely tuned pores of the bimetallic Co60Zn40ZIF hybrid.345
CNTs have also attracted considerable research attention across various fields due to their unique structural, electronic, thermal, chemical, and mechanical properties, all of which can improve permeability, selectivity, and long-term stability.346,347 However, challenges remain in dispersing CNTs uniformly within the polymer matrix and eliminating interfacial defects, which can hinder the development of CNT-based MMMs with high gas selectivity.
To address these challenges, hybridizing CNTs with MOFs by growing MOFs on the surface of CNTs has been explored. For instance, NH2-MIL-101(Al) was deposited on CNT surfaces to introduce polar amino groups, improving interfacial adhesion. Polyimide-based MMMs incorporating MOF/CNT hybrids showed improved CO2 permeability and CO2/CH4 selectivity, surpassing the Robeson upper bound.331Fig. 21 shows SEM images of MOF particle growth on the outer surfaces of CNTs, with particle sizes around 50 nm. It also illustrates the separation performance of MOF/CNT MMMs compared to previously reported MMMs for the CO2/CH4 gas pair relative to the Robeson trade-off line and a schematic of MOF/CNT composite dispersion within 6FDA-durene polyimide. This strategy of growing MOFs on CNTs was also applied to decorate UiO-66 on halloysite nanotubes (UiO-66@HNT), which enhanced the CO2/N2 separation performance of Pebax-1657 MMMs due to the fast transport pathways for CO2 diffusion provided by the HNT lumen and the CO2 affinity of UiO-66. This also conferred good long-term stability and excellent interfacial compatibility with the MMMs.336
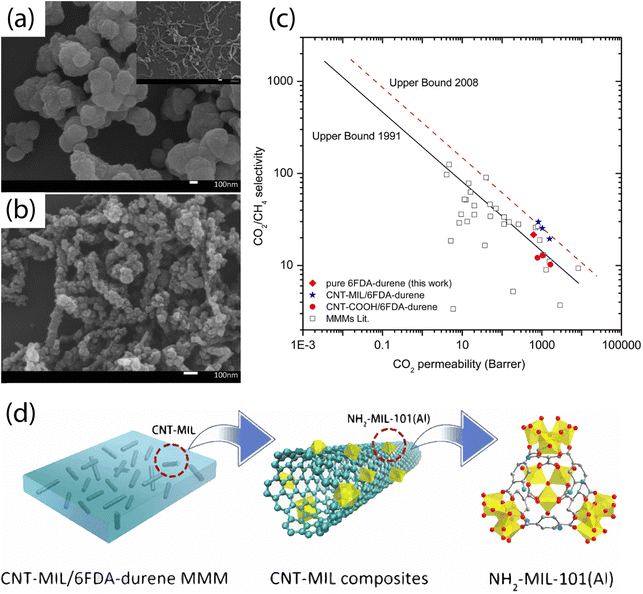 | ||
| Fig. 21 SEM images of (a) NH2-MIL-101(Al), with CNT-COOH (inset), and (b) CNT-MIL composite. (c) Gas separation performance of the CNT-MIL MMMs for the CO2/CH4 pair with respect to Robeson trade-off line in comparison with single MOF- or CNT-based MMMs reported in the literature. (d) Schematic of 6FDA-durene MMM containing NH2-MIL-101(Al)-decorated CNTs (Al, yellow; C, gray; O, red; and N, blue) (reproduced from ref. 331 with permission from Elsevier, copyright 2025). | ||
Another class of 2D nanomaterials for membrane-assisted decarbonization is crystalline COF, a porous structure formed by covalent bonds between light elements (carbon, hydrogen, nitrogen, oxygen, etc.). COFs possess a high surface area, tunable pore sizes, and excellent structural stability due to their covalent bonds, offering an advantage over MOFs, which rely on coordination bonds between metal clusters and ligands. Recent reviews on COF-based membrane gas separation highlight their potential for flue gas decarbonization.348 Notable examples include a PVAm-functionalized COF-based MMM with a permeability of 1738 Barrer and a CO2/N2 selectivity of 89349 and a COF-5-based Pebax 1657 membrane synthesized from 4-benzene boronic acid and 2,3,6,7,10,11-hexahydroxytriphenylene, achieving permeability of 493 Barrer and CO2/N2 selectivity of 49.3.350 Bilayer membranes having imine- and azine-based COF have recently been reported to have superior performance due to the interlaced pore network.351 Merging the capabilities of the two engineered materials has led to a hybrid membrane with MOF grown on the COF layer.352 For example, MMMs were fabricated through attaching UiO-66-NH2 to TpPa-1 COF353 (Fig. 22(a)). However, permeability values of these MMMs were not as high compared to other studies, as this hybrid was incorporated into a polysulfone (PSF) membrane rather than a more selective layer. A recent innovation introduced the concept of MOF-in-COF, where MOFs are grown as strings through the 1D channels of COFs, addressing the trade-off concerns typical in conventional membranes354,355 (Fig. 22(b)). The molecular sieving effect of these MOF-in-COF membranes has been particularly effective for hydrogen (H2) purification from gas mixtures. For instance, a membrane designed for biogas green hydrogen purification (H2/CO2) achieved a separation efficiency of 34.9. Although this selectivity may seem modest compared to flue gas decarbonization membranes, it is important to recognize that this process involves extracting hydrogen from a CO2-rich stream. A selectivity value of 34 is quite significant in this context compared to other membranes used for H2 purification and CO2 capture. The MOF-in-COF concept shows great promise, offering the potential to tailor pore sizes for specific gas separation applications.
 | ||
| Fig. 22 (a) Attaching UiO-66-NH2 to COF as a modifier of PSF membrane for gas separation membranes353 (with permission from Elsevier), and (b) Growing ZIF MOFs in COF porous structure as the selective membrane material for gas separation purposes.354 | ||
Graphene analogs, including exfoliated hexagonal boron nitrides (h-BNs), graphitic carbon nitride (g-C3N4), transition metal dichalcogenides (TMDs), and MXenes (metal carbides, nitrides, or carbonitrides), are emerging as promising 2D materials for membrane-assisted decarbonization.356 MXenes, in particular, stand out due to their distinct physicochemical properties, rich surface chemistry, and versatility for post-synthesis functionalization, making them a highly flexible superfamily of nanostructures that offer unprecedented design potential for gas separation membranes.357
MXenes are derived from MAX phases, represented by the formula Mn+1AXn, where M is an early transition metal such as Ti, Cr, Mo, and V, A is an element from groups 13–16 (e.g., Al, Ga, or Si), and X represents carbon and/or nitrogen. The resulting MXene structure, Mn+1XnTx, where Tx refers to surface terminations such as –OH, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and –F, is usually produced through selective etching of the A element using acids such as hydrofluoric and hydrochloric acid.358 MXene nanosheets can be incorporated into MMMs, where they act as molecular sieves, enhancing both the permeability and selectivity of polymeric membranes.359 MXenes alter the tortuosity and solubility of gases in these composite membranes, enhancing the solution-diffusion mechanism for gas transport by leveraging their interlayer nanogalleries and surface terminations.360 MXene, as a filler within the polymer matrix, alters the tortuosity and solubility of gases in composite membranes compared to pristine polymeric membranes.361,362 Additionally, surface terminations on MXenes enhance interfacial interactions with the polymer, increasing the affinity of composite membranes for condensable gases (e.g., CO2).363
O, and –F, is usually produced through selective etching of the A element using acids such as hydrofluoric and hydrochloric acid.358 MXene nanosheets can be incorporated into MMMs, where they act as molecular sieves, enhancing both the permeability and selectivity of polymeric membranes.359 MXenes alter the tortuosity and solubility of gases in these composite membranes, enhancing the solution-diffusion mechanism for gas transport by leveraging their interlayer nanogalleries and surface terminations.360 MXene, as a filler within the polymer matrix, alters the tortuosity and solubility of gases in composite membranes compared to pristine polymeric membranes.361,362 Additionally, surface terminations on MXenes enhance interfacial interactions with the polymer, increasing the affinity of composite membranes for condensable gases (e.g., CO2).363
For example, Shamsabadi et al. reported remarkable advancements in CO2 separation technology by incorporating Ti3C2Tx MXene nanosheets within Pebax-1657.363 With just 0.1 wt% Ti3C2Tx loading, CO2 permeability increased by 43%, while CO2/N2 selectivity doubled compared to pure Pebax membranes. This enhanced performance was attributed to strong interactions between the Ti3C2Tx nanosheets and the polymer matrix (Fig. 23(a)), as confirmed by characterizations and molecular dynamics simulations, facilitating higher CO2 solubility and selectivity. The nanochannels between the MXene layers also contributed to improved CO2 diffusivity, while the molecular sieving effect efficiently blocked N2 molecules. The high CO2 adsorption capacity of the hydroxyl groups on Ti3C2Tx and the altered morphology and phase separation within the Pebax matrix contributed to the improved performance (Fig. 23(b)). However, at loadings above 0.1 wt%, permeability decreased due to nanosheet agglomeration, which created nonselective voids at the MXene–polymer interface. Liu et al. showed similar improvements in CO2 permeance and CO2/N2 selectivity for Pebax MMMs containing 0.15 wt% Ti3C2Tx (Fig. 23(c)).364 Hu et al. took this approach further by synthesizing a Ti3C2Tx-carboxylated nanocellulose composite to improve interfacial compatibility and prevent nonselective void formation. Their composite membrane, containing 15.4 wt% Ti3C2Tx, achieved a CO2 permeability of 156.7 Barrer and a CO2/N2 selectivity of 47.8 (Fig. 23d).365 With the incorporation of 23.1 wt% Ti3C2Tx MXenes, the CO2 permeability increased, while the selectivity decreased due to MXene agglomeration. In another study, the structure and CO2 separation performance of Pebax-GO and Pebax-MXene membranes were systematically compared.366 Pebax-MXene membranes were able to accommodate up to 20 wt% MXene due to improved dispersion and interfacial interactions, whereas Pebax-GO membranes reached a maximum loading of only 5 wt%. However, for both membranes, optimal performance was achieved at a 1 wt% filler content under dry conditions. Under humidified conditions, Pebax-MXene membranes with higher loadings exhibited significantly enhanced separation performance. This improvement is attributed to water molecules trapped within the MXene nanogalleries, which facilitate the transport of CO2 molecules through the membranes.367
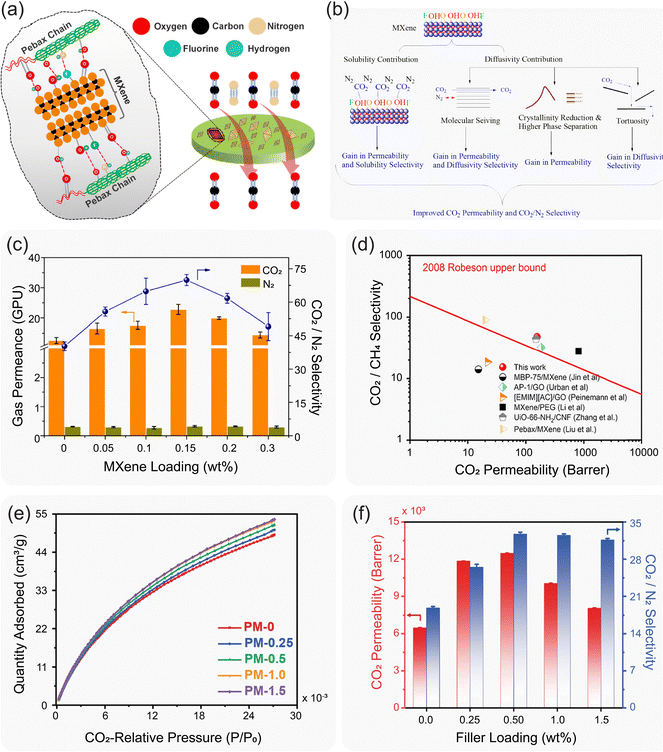 | ||
| Fig. 23 (a) Schematic illustration of the formation of hydrogen bonds between Ti3C2Tx surface terminations and Pebex chains. (b) Proposed CO2/N2 separation mechanisms in Ti3C2Tx-Pebax membranes.363 (c) Single gas separation properties of Ti3C2Tx-Pebax membranes as a function of the MXene loadings.364 (d) CO2 separation performance of carboxylated nanocellulose membranes containing 15.4 wt% Ti3C2Tx MXene compared to Robeson upper bound.365 (e) CO2 adsorption isotherms of the pristine PIMs and Ti3C2Tx-PIM MMMs. (f) CO2/N2 separation performance of Ti3C2Tx-PIM MMMs with a constant feed flow rate of 300 Nml min−1 at 3 bar and 25 °C.368 | ||
Despite the numerous advantages of PIMs, including low density, high specific surface area, and favorable physicochemical properties, unloaded PIM membranes often exhibit low CO2 selectivity compared to CH4 and N2.369,370 To address this limitation, Wang et al. fabricated MMMs by integrating Ti3C2Tx MXenes into the continuous phase of PIM-1.368 This innovative approach yielded significant enhancements in CO2 separation performance. The resulting MMM achieved a CO2 permeability of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 475.3 Barrer (Fig. 23(e)), marking an impressive 92.7% increase, and a CO2/N2 selectivity of 32.7, a notable improvement of 73.9%. These enhancements were attributed to a 46.1% increase in diffusion selectivity, facilitated by the ∼0.35 nm interlayer spacing between MXene layers within the PIM-1 matrix. Furthermore, sorption selectivity improved by 37.9% due to the incorporation of MXene sheets with polar functional groups (–OH, –O, –F), which enhanced the affinity for CO2 molecules and modified the pore size distribution and volume within the membrane (Fig. 23(f)). These advancements, driven by the synergistic effects of solution-diffusion and molecular sieving mechanisms, have led to a remarkable enhancement in both CO2/N2 selectivity and CO2 permeability.
475.3 Barrer (Fig. 23(e)), marking an impressive 92.7% increase, and a CO2/N2 selectivity of 32.7, a notable improvement of 73.9%. These enhancements were attributed to a 46.1% increase in diffusion selectivity, facilitated by the ∼0.35 nm interlayer spacing between MXene layers within the PIM-1 matrix. Furthermore, sorption selectivity improved by 37.9% due to the incorporation of MXene sheets with polar functional groups (–OH, –O, –F), which enhanced the affinity for CO2 molecules and modified the pore size distribution and volume within the membrane (Fig. 23(f)). These advancements, driven by the synergistic effects of solution-diffusion and molecular sieving mechanisms, have led to a remarkable enhancement in both CO2/N2 selectivity and CO2 permeability.
Research on MXene-based membranes for gas separation is in its early stages and requires further study. Thus far, most published papers have focused on using Ti3C2Tx for the fabrication of mixed matrix membranes. However, with over 30 types of MXenes reported, their potential separation performance remains largely unexplored. Additionally, several critical aspects require clarification: the orientation of MXenes within the continuous phase, the effects of high MXene loading, the physical aging of MXene-based membranes, and the impact of MXenes on membrane plasticization. Addressing these areas is crucial for advancing the application of MXene-based membranes in gas separation technologies. Table 8 reports some of the selected performance results for the polymeric membranes used for decarbonization purposes, along with their operational test condition. Table 9 offers more details on the membrane units that have been used on pilot scale for the same purpose.
| Selective layer | Support layer | Selective layer thickness (μm) | Operating condition | CO2 transport factor | CO2/N2 Selectivity (or CO2/CH4) selectivity | Ref. |
|---|---|---|---|---|---|---|
| Pebax@1657 | PES | 1.25 | 25 °C, 4 bar | CO2 permeability of 22 | 20 | 331 |
| Pebax@1657 | PMP | 25 | 25 °C, 7 bar | CO2 permeability of 240 | (15) | 332 |
| PEO | N/A | N/A | 25 °C | CO2 permeability of 204.3 and N2 permeability of 3.8 | 53.7 | 209 |
| PEO-PBT | PAN | 0.05 | 25 °C, 10 bar, mixed gas CO2/N2 feed composition of 28/72 vol% | CO2 permeability of 150 | 51 | 173 |
| PEO-PBT (64–36 v%) | N/A | N/A | 25 °C | CO2 permeability of 100 and N2 permeability of 2 | 50 | 209 |
| PEO-PBT (77–23 wt%) | N/A | 60 to 80 | 25 °C, 0.3 bar | CO2 permeability of 115 | 45.6 | 210 |
| PVAm (from commercial source) with piperazine glycinate as mobile amine carrier | PSF with PVP as an additive (pore size 38 nm, porosity 13.4%) | 30 | 57 °C, 17% relative humidity, 1.5 psig | 843 GPU | 160 | 221 |
| PVAm (made from NVF monomer) with piperazine as a mobile amine carrier | PSF with a molecular weight cut-off of 6000 | 0.78 | 50 °C, 0.11 MPa | 2.17 (μmol m−2 s−1 Pa−1) | 277 | 236 |
| PVAm (from a commercial source) with 2-(1-piperazinyl)ethylamine sarcosine modified with a multiwalled carbon nanotube as a mobile amine carrier | PSF with PVP as an additive (pore size 30.6 nm, porosity 12.9%) | 0.17 | Mixed feed pressure of 1 atm, CO2 partial pressure of 0.166 atm | 975 GPU | 165 | 235 |
| PIM-1@NUS-8-NH2 (10 wt%) | N/A | N/A | Room temperature, 2 bar | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Barrer 000 Barrer |
30 | 333 |
| PIM-1 with g-C3N4 (1 wt%) | N/A | 60–90 | 25 °C with a transmembrane pressure of 1 bar | 5785 Barrer | 16.3 | 334 |
| PIM-1 with MOF-801 (5 wt%) | N/A | 45 | 0.4 Mpa, 35 °C | 9686 Barrer | 27 | 335 |
| PIM-1 with Ag+/UiO-66-NH2 (30 wt%) | N/A | 120–140 | 25 °C, 1 bar | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Barrer 000 Barrer |
30 | 336 |
| Pebax@2533 with ZIF-8 decorated graphene oxide | Non | 55–65 | 25 °C, Mixed feed of CO2/N2 (15/85 vol%) at pressure of 0.1 MPa | CO2 permeability of 249 Barrer | 47.6 | 289 |
| PSF with ZIF-8 decorated graphene oxide | Non | 90 | 25 °C, CO2, CH4, and N2 at pressure of 250 kPa | CO2 permeability of 1.76 Barrer | CO2/CH4 selectivity of 6.3 | 288 |
| Pebax@1657 with bimetallic (Zr and Co) ZIF-decorated graphene oxide | Non | 50–70 | 30 °C, CO2 and N2 at pressure of 12 bar | CO2 permeability of 95.06 Barrer | 57.53 | 301 |
| 6FDA-durene polyimide with MIL-101 (Al) decorated carbon nanotube | Non | 20–40 | 25 °C, CO2/CH4 (50/50%) mixture at 2 atm | CO2 permeability of 818 Barrer | CO2/CH4 selectivity of 29.7 | 287 |
| MMM | Pebax | 75–90 | 25 °C, CO2/CH4 (50/50%) mixture at 5 bars | CO2 permeability of 119.08 Barrer | 76.26 | 292 |
| PEO@Zn-TCPP MMM (2.5 wt%) | N/A | 100–150 | 35 °C and 12 atm | 198 Barrer | 81 | 337 |
| Cu-TCPP-Pebax MMM (0.1 wt%) | N/A | 160 | Room temperature, 1 bar | 1183 Barrer | 57.6 | 338 |
| Ti3C2Tx-Pebax MMM (0.1 wt%) | N/A | 60–70 | 25 °C, 4 bar | 126 Barrer | 96 | 319 |
| Ti3C2Tx-Pebax thin-film composite containing 0.05 wt% of Ti3C2Tx | poly[1-(trimethylsilyl)-1-propyne as the gutter layer on a polyvinylidene fluoride support | 0.07 | 25 °C, 4 bar | 1986.5 GPU | 41.8 | 319 |
| Membrane | Team | Membrane geometry/module type | Active area | Size | Gas source | Ref. |
|---|---|---|---|---|---|---|
| a Megawatt electrical. b Ton of CO2 per day. | ||||||
| PVAm-2-(1-piperazinyl)ethylamine-Sarcosine modified with multiwalled carbon nanotube; no brand has been mentioned yet | Ohio State University, US | Spiral wound module with 4 envelopes of flat sheet | 1.4 m2 | 880 MWea | Coal-based flue gas | 197 |
| Polaris | Membrane technology and research (MTR) Inc., US | Spiral wound | 1 TPDb | Coal-fired power plant | 371 | |
| Polaris | Membrane technology and research (MTR) Inc., US | Spiral wound | 20 TPDb | Natural gas power plant | 372 | |
| PVAm | Norwegian University of Science and Technology (NTNU), Norway | Hollow fiber | 4 m2 to 20 m2 | Propane burner, cement factory | 254, 373 and 374 | |
| Polyactive | Helmholtz-Zentrum Geesthacht (HZG), Germany | 10 m2 | Coal-fired power plant | 375 | ||
| Chilled PI | Air Liquide, US | Hollow fiber | 0.3 MWe | Coal-fired power plant | 376 | |
Despite their superior separation performance in the lab scale, MMMs may not show the same performance real-world operating conditions in the industrial scale. Before commercialization, they must meet certain minimum requirements.377Table 10 provides a summary of membrane-based gas separation performance requirements for various commercial applications, including pre-combustion, post-combustion, air separation, and air dehumidification. Additionally, the long-term stability of MMMs in practical applications is a very important.378 The stability depends on the type of materials used, operating conditions, and the specific application. Harsh environmental conditions, including exposure to aggressive chemicals, high pressure, and high temperatures, can impair the separation performance of MMMs. Both the filler and polymer components govern their thermal and chemical stability. Considering the typical longevity of current polymeric membranes (3–5 years) and the challenges in MMM commercialization, MMMs demonstrate enhanced long-term stability compared to conventional polymeric membranes.379 This can be attributed to inorganic fillers, which not only improve resistance to plasticization by condensable gases at high pressure but also prevent the reduction in FFV because of physical aging.380 Therefore, strong interfacial interactions between the polymer and fillers can enhance the longevity of MMMs in practical applications.
| Application | Market size (USD/year) | Gas pair | Operation condition | Required permeance/permeability | Required selectivity | Ref. |
|---|---|---|---|---|---|---|
| Pre-combustion | 1.8 B | H2/CO2 | Feed pressure of 20 bar | 200–1000 GPU | >10 | 381 and 382 |
| 250–400 °C | ||||||
| Post-combustion | 700 M | CO2/N2 | 5% CO2 | 1000–5000 GPU | 30–50 | 381–383 |
| Low CO2 partial pressure | ||||||
| Air separation | 800 M | O2/N2 | 79% N2, 21% O2 | >0.8 Barrer | >8 | 384 and 385 |
| Feed pressure of ∼10 bar | ||||||
| Air-dehumidification | 900 M | H2O/N2 | ∼ 60–80% RH | >11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 GPU 900 GPU |
>1500 | 386 |
| 22–30 °C | ||||||
| Natural gas upgrading | 300 M | CO2/CH4 | Feed pressure of 70 bar | >100 GPU | 20–35 | 387 |
| 50 °C | ||||||
| 10% CO2 | ||||||
| Hydrogen recovery from ammonia purge gas | 200 M | H2/N2 | 40 bar | >1000 GPU | >290 | 388–390 |
| 20% N2, 60% H2 | ||||||
| Olefin production from steam cracking | 37 B | C2H4/C2H6 | Feed pressure of 6 bar | >30 GPU | >30 | 391 |
| C3H6/C3H8 | >1 Barrer | >3 | ||||
| Hydrogen production by gas steam reforming | 120 M | H2/CH4 | 4 bar | >85 GPU | >37 | 378 |
| 40 °C |
7. Challenges of gas separation membranes
Physical aging in glassy polymers occurs due to lattice contraction and chain rearrangement after solvent removal, particularly at temperatures below the glass transition temperature (Tg).392 This aging process leads to a reduction in the FFV within the polymer matrix, decreasing membrane permeability and overall separation performance over time.393 Both thin film selective layers and intermediate gutter layers are susceptible to aging, which poses a significant challenge to long-term membrane stability. For instance, gutter layers with a glassy nature, such as poly(trimethylsilyl propyne) (PTMSP), can lose up to 90% of their permeability due to physical aging.191 The rate of physical aging, r, can be quantified using the following equation394: | (20) |
Plasticization is another issue that negatively impacts polymer membranes. The polymer matrix loses its size-sieving capability, leading to diminished selectivity. Plasticization typically occurs when membranes are exposed to polarizable gases (like CO2) and heavy hydrocarbons under intense operating conditions. The phenomenon involves solvation effects that interfere with polymer interchain interactions, allowing non-plasticizing gases such as N2 and CH4 to permeate more easily.224 On a microscopic level, plasticization increases polymer chain mobility, disrupts the free volume within the polymer, and enlarges membrane pores. Macroscopically, the membrane becomes softer, exhibits increased ductility, and shifts toward a rubbery state with a lower Tg.395 The presence of widened hysteresis patterns during pressurization-depressurization cycles with CO2-rich streams further indicates plasticization.396
Plasticization is typically measured by observing the increase in CO2 permeability as feed pressure rises. The plasticization pressure is defined as the minimum pressure at which this increase is observed. Yuan et al. studied the effect of wet thickness on PVAm membrane performance and noted that thinner membranes are more prone to accelerated plasticization.266 Thinner selective layers, achievable through intermediate/gutter layer usage, wet coating thickness reduction, and coating parameter adjustments, are crucial for higher CO2 permeability. However, reduced thickness leads to accelerated plasticization due to a decrease in glass transition temperature.397 To mitigate this, crosslinking approaches using ethylenediamine have been proposed, as crosslinked membranes exhibit improved resistance to plasticization. Consequently, thinner crosslinked films can be produced without compromising CO2 separation performance.266
Wessling and his team conducted an in-depth assessment of plasticization across various membrane materials (polysulfone, polyethersulfone, cellulose acetate, cellulose triacetate, polyetherimide, copolyimide, Matrimid 5218, poly(2,6-dimethyl-p-phenylene oxide), bisphenol A polycarbonate, bisphenol Z polycarbonate, and tetramethyl bisphenol A polycarbonate). Their study aimed to correlate the critical plasticization pressure, CO2 concentration, FFV, and functional group density. Interestingly, they found that plasticization is not solely influenced by CO2 polarity-segment interactions, as even non-polar gases like argon can induce plasticization at high pressures.398 Their findings suggest that plasticization is more closely related to the absorbed CO2 content than to gas polarity. Plasticization depends on both pressure and a relatively constant critical CO2 concentration of approximately 38 ± 7 cm3(STP) cm−3. Therefore, the key factor is the sorption of CO2 rather than its polar characteristics. Table 11 provides plasticization data, permeability, and testing conditions for various membrane materials.399
| Membrane | Plasticization pressure (bar) | Permeability at plasticization pressure (Barrer) | Permeability at zero pressure (Barrer) | CO2 equilibrium plasticization concentration (cm3(STP) cm−3) | Operating temperature |
|---|---|---|---|---|---|
| Polysulfone | 34 | 3.6 | 5 | 47 | 23 |
| Polyethersulfone | 27 | 2.6 | 3.7 | 43 | 21 |
| Polyetherimide | 28 | 0.84 | 1.1 | 37 | 21 |
| Bisphenol A polycarbonate | 31 | 4.7 | 7.5 | 33 | 25 |
| Bis13phenolZ polycarbonate | 24 | 1.0 | 1.4 | 32 | 23 |
| Tetramethyl bisphenol A polycarbonate | 13 | 13 | 16 | 36 | 25 |
| poly(2,6dimethyl-p-phenylene)oxide | 14 | 8.2 | 99 | 34 | 25 |
| Polyimide matrimid 5218 | 12 | 4.8 | 5.7 | 47 | 22 |
| CopolyimideP84 | 22 | 0.92 | 1.1 | 48 | 23 |
| Cellulose acetate | 11 | 6.0 | 6.7 | 31 | 27 |
| Cellulosetriacetate | 10 | 7.3 | 9 | 31 | 24 |
Crosslinking enhances the mechanical stability of the membrane by forming covalent bonds between polymer chains, reducing the polymer matrix's flexibility and thereby limiting swelling under high-pressure conditions. This makes it a viable approach not only for preventing plasticization but also for addressing physical aging issues, where polymer membranes tend to densify and lose performance over time.222 However, crosslinking can negatively impact the FFV within the membrane. The reduction in FFV results in fewer free spaces for gas molecules to diffuse through, leading to a decrease in permeance. While the mechanical integrity and selectivity are improved through crosslinking, the trade-off often comes at the cost of gas permeability. To balance this, careful tuning of crosslinking density is required to ensure that the membrane maintains sufficient FFV to allow for gas transport while mitigating plasticization and aging effects. Advanced crosslinking strategies, such as the use of flexible crosslinkers or partial crosslinking, are being explored to minimize the adverse impact on permeability while maintaining stability and selectivity.
While the topic is generally tailored toward looking at the phenomena around CO2 filtration, the effect of other components and impurities in the target stream must not be ignored. Flue gas might contain NOx, SOx, humidity, H2S, CO, NH3, or even heavy metals depending on the source of the fuel in combustion processes.400 The presence of impurities could reduce the adsorption capacity of the membranes, reduce the driving forces of the permeation or negatively affect the structure of the membrane leading to reduced performance of the membrane separation unit.
SO2, a more studied element from the SOx family, has larger kinetic diameter compared to CO2 and deterioration of the performance is not mainly due to the diffusion of the gas molecule.401 The increased permeability of SO2 can be attributed to its higher critical temperature, which results in a greater affinity constant and higher adsorption in Langmuir free volume sites. MMMs with higher share of rubbery polymers have higher affinity toward SO2 and higher loading of Langmuir free volume sites. Due to its more condensable nature, SO2 has a higher plasticization effect. While the SOx components’ concentration is much smaller than CO2, it is important to note that each decarbonization process must be separately assessed with regards to the flue gas characteristics. On the other hand, the cogenerative effect of the impurities deteriorates the performance much more when humidity plays a major role. The presence of humidity triggers the conversion of SO2 to sulfuric acid, within the free volume of the MMM and degradation of the MMM's structure. Similar effects are identified with the formation of nitric acid because of NO2 and humidity reaction. From the NOx family, NO is more frequently observed in flue gas streams with concentrations not exceeding 500 ppm. NO has lower kinetic diameter in comparison with CO2 yet its lower adsorption affinity results in lower permeability. H2S, present in natural gas, and fermentation-generated biogas could reduce the performance of polymeric membranes. As an instance, in the case of PDMS membrane, CO2 permeability was reduced by 8% due to the diffusion competition of impurities.402 More importantly, N2's mass transport resistance was reduced due to the swelling of the polymeric matrix, explained by the Flory–Huggins theory.402 Nanoparticle's structure could also be sensitive to interaction with H2S. Metals such as copper could react with sour gas. This could jeopardize the performance of MMMs with Cu-based MOFs such as ZIF-8.400 On the other hand, porous organic polymers and carbon based nanomaterials could resist acid gases with due to their less exposed nature.400 This could highlight the opportunity for PIM-like structures and graphitic carbon nitrates (GCN) as acid-gas resistant decarbonization membranes. Nevertheless, the concern of sour gas is more attributed to the natural gas decarbonization or biogas purification, rather than the flue gas decarbonization.
8. Computational studies, artificial intelligence and machine learning contribution to membrane-based CCUS
8.1. Computational effort in membrane-based CCS technologies
As discussed earlier, the transport of gas molecules through the membrane matrix is controlled by the adsorption and diffusion characteristics of the penetrating molecules. If the desired molecules adsorb too strongly or too weakly, it can hinder membrane performance, causing slow diffusion or a low concentration of the targeted molecules.403 Additionally, separation performance can be significantly influenced by multicomponent phenomena, particularly when highly sorbing gases, such as water vapor and CO2, are present. Experimental data have revealed that multicomponent phenomena can decrease the solubility and increase the diffusivity of less soluble species in gas mixtures.189,404–406 This suggests that the intermolecular interactions between polymer chains and gas molecules, as well as the competition among gas molecules in a mixture, are not straightforward to interpret. Therefore, optimizing membrane properties is crucial to creating ideal interactions between the membrane and the desired molecule for effective adsorption and diffusion, ensuring optimal separation performance.Membrane modeling involves various methods to estimate membrane properties and consider multicomponent effects, primarily focusing on two fundamental performance metrics: gas permeability and membrane selectivity.403,407
Extensive efforts have been made to explain experimental results by considering various assumptions at the molecular scale. One of the most widely used theories is the dual-mode sorption model, developed for glassy polymers, which assumes that each gas molecule can be adsorbed either directly on the polymer chains (Henry's sorption law) or in the non-equilibrium voids between the chains of a glassy polymer (Langmuir sorption).408,409 Saberi et al. applied this theory to develop their model to explain gas permeation and CO2-induced plasticization in glassy polymers.410 Additionally, the dual-mode sorption model was extended using artificial intelligence methods to model mixed-gas sorption in PIM-1 and TZ-PIM.411 However, the need for mixed-gas sorption data may limit the applicability of this model to specific polymers and operating conditions.
Alternatively, thermodynamic methodologies such as non-equilibrium thermodynamics for glassy polymers (NET-GP) have been effectively applied, leveraging the inherent non-equilibrium characteristics of glassy polymers. Within this framework, equation of state (EoS) models, expanded to account for non-equilibrium conditions, are used to compute gas sorption and describe the non-equilibrium states in glassy polymers. This is achieved by introducing polymer density as an internal state variable to explain the system's degree of non-equilibrium.406,407 The advantage of this method is that it can be applied to multi-component gas mixtures using sorption data acquired from experiments with pure gas or binary mixtures. Subsequently, the diffusion coefficient can be represented as the product of a kinetic factor (mobility) and a thermodynamic factor, calculated using the NET-GP methodology to compute the permeability of the penetrating species.412,413
Classical molecular simulation is another approach used to study membrane properties and the transport phenomena of gas species. The accuracy of these interactions strongly depends on factors such as the gas models used and the methods for assigning partial charges. The accuracy of these interactions strongly depends on several factors, such as gas models and methods used for assigning partial charges. The importance of this type of simulation becomes more apparent when studying composite membranes.414,415
In computational studies involving MMMs, various molecular techniques are employed, including grand-canonical Monte Carlo (GCMC), equilibrium molecular dynamics (EMD), nonequilibrium molecular dynamics (NEMD), transition-state theory (TST), and even density functional theory (DFT), a quantum-based simulation. GCMC simulations are widely used to determine the gas adsorption properties of membranes (e.g., gas uptake or affinity). In contrast, gas diffusion properties within membranes are explored using MD simulations or the TST approach.416,417 After calculating gas adsorption and diffusion, permeability and membrane selectivity are determined based on the solution-diffusion model. This approach integrates adsorption and diffusion data to determine the membrane's effectiveness in a specific separation process.418
A distinct advantage of molecular simulation is its ability to approximate intermolecular interactions to ensure an intimate interface between the species that make up the composite membrane. This feature can pave the way for making compatible polymeric composites where emerging materials with unique properties can be embedded in the membrane matrices to promote separation performance. Thus, it provides guidance for selecting filler/polymer pairs by identifying noncovalent and covalent bonds between fillers and polymer chains. Typically, functional groups (e.g., –NH2 or –CN) on the surface of fillers or structural defects intentionally created in the crystalline structure of fillers can lead to a favorable interface and reduce non-selective voids, maintaining the level of selectivity.403 For instance, simulations showed that MXene nanosheets could form an intimate interface with the Pebax membrane matrix, supporting the cost-effective separation performance of the resulting membrane.363 In another study, Sadeghi and Howe used DFT simulations to examine how polymer fragments (specifically, Kapton and 6FDA-Durene) interact with ZIF-8 and Co-BDC surfaces.419 Their investigation uncovered that the presence of unsaturated sites can promote strong compatibility between the MOF and polymer. Conversely, when there was a deficiency of undercoordinated surface species, the adhesion between the MOF and polymer was weaker, particularly in cases where dispersion forces played a dominant role. It is noteworthy that molecular simulations can also calculate various structural properties of polymer membranes, including density, glass transition temperature (Tg), FFV, polymer solubility, and mechanical properties, which can reveal whether a polymer is suitable for a specific separation.420–422
8.2. A data-driven approach for membrane-based CCS studies
As previously mentioned, MMMs stand out as a highly advantageous membrane type due to their cost-effectiveness, ease of processing, and superior performance. The unique properties of fillers can also be imparted to the membrane when they are dispersed within the membrane matrix. However, choosing compatible polymers and fillers to prepare a defect-free composite remains a significant challenge. As a result, researchers have focused on applying cutting-edge computational approaches, such as data-driven methods, to make optimal choices.403,417The highly detailed atomistic simulations offer precise outcomes but come with substantial computational expenses.403 Hence, typically, the permeabilities of MMMs are calculated utilizing permeation models like Maxwell,423 Bruggeman,424 and Felske.425 These models integrate the gas permeabilities of fillers obtained through atomic simulations with experimental data on gas permeability in polymers. Consequently, once the gas adsorption and diffusion properties of fillers are known, the permeabilities of MMMs can be estimated without further simulations.403,426
In this regard, numerous studies on both real and hypothetical materials, including MOFs, COFs, and 2D materials, have examined their potential for diverse applications and objectives.427–434 Budhathoki et al. performed high-throughput atomistic simulations on 112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 888 real and hypothetical MOFs to obtain CO2 permeability and CO2/N2 selectivity.435 Afterward, using experimental data attributed to nine polymers and applying the Maxwell model, they estimated the separation performance of over one million possible hypothetical MMMs resulting from combining those MOFs and polymers. The results were then used for a techno-economic evaluation of membrane-based carbon capture, showing that many potential MMMs are predicted to have a carbon capture cost of less than $50 per ton of CO2 removed. Altintas and Keskin also performed high-throughput computational screening (HTCS) to evaluate a MOF database for membrane-based CO2/CH4 separation.436 They applied GCMC and MD simulations to identify the separation performance of those MOFs. They then calculated the permeability and selectivities of possible MMMs, where the best MOF candidates were embedded as fillers. This revealed a significantly improved CO2 separation performance compared to pristine membranes.
888 real and hypothetical MOFs to obtain CO2 permeability and CO2/N2 selectivity.435 Afterward, using experimental data attributed to nine polymers and applying the Maxwell model, they estimated the separation performance of over one million possible hypothetical MMMs resulting from combining those MOFs and polymers. The results were then used for a techno-economic evaluation of membrane-based carbon capture, showing that many potential MMMs are predicted to have a carbon capture cost of less than $50 per ton of CO2 removed. Altintas and Keskin also performed high-throughput computational screening (HTCS) to evaluate a MOF database for membrane-based CO2/CH4 separation.436 They applied GCMC and MD simulations to identify the separation performance of those MOFs. They then calculated the permeability and selectivities of possible MMMs, where the best MOF candidates were embedded as fillers. This revealed a significantly improved CO2 separation performance compared to pristine membranes.
Meanwhile, Yuan and Sarkisov proposed an efficient approach using lattice models and dynamic mean field theory to estimate gas permeability in MMMs.437 This model considers interfacial effects, suggesting potential gas transport hindrances due to unfavorable interface interactions. Although these models lack the resolution of molecular simulations due to the absence of long-range interactions, they serve as effective initial screening tools for probing diffusion in various MOF-based MMMs. The idea was that the identified candidates could then undergo detailed molecular simulations for a more comprehensive analysis.
The rapid advancement of high-performing MMMs can be achieved by integrating diverse modeling, simulation tools, and data science techniques, offering valuable insights to experimentalists. Transitioning MMMs from laboratory research to practical application requires significant effort and time. However, this transformation can be efficiently accomplished through the cohesive integration of experimental knowledge, theoretical knowledge, and big-data science methodologies. At this point, the conversation pivots toward exploring the application of artificial intelligence (AI) techniques for material classification or membrane performance regression.437
Apart from the studies that AI was implemented to find a correlation between parameters or build a model to predict the output versus inputs, AI-assisted methods have widely been used to optimize the operational parameters of carbon capture processes such as temperature, operating pressure, flow rates of species, and geometry of the reactors to increase the overall efficiency of the process.438,439 However, AI-assisted methods are often applied for polymeric membranes to select or discover the optimal polymeric structure or the best combination of polymers and additives to achieve high-efficiency separation performance. Data-driven analysis (including AI-assisted methods) aids in identifying the pros and cons of different materials to overcome certain drawbacks, such as weak intermolecular interactions between continuous and dispersed phases in a polymer composite, non-selective voids at the interface, or chain rigidity.279,440
The emergence of machine learning (ML) techniques has led to precise predictions for diverse material properties. Simultaneously, the availability of vast repositories containing both experimental and simulation data has facilitated the use of machine learning to uncover new materials through data analysis.441Fig. 24 displays a typical ML model workflow that utilizes data sources to ultimately accelerate the identification of high-performance materials.
Resources, including crystallographic data and molecular simulations, can provide chemical, structural, or energetic properties of substances. However, the first step in utilizing these large data sets is converting the information into formats, such as scalars or vectors, that are readable by ML models and accurately describe the properties of materials. Once material representations are acquired from experimental or computational data, ML models can be implemented for two purposes: regression and classification. The regression task, often applied to predict separation performance metrics, may lack accuracy due to factors such as limited data or the absence of physically relevant features. In such cases, classification methods are useful, categorizing materials as stable or unstable, or high performance or low performance, instead of providing precise numerical values. This approach can expedite identifying potential materials for use in MMMs for CO2 separation.442,443
Zhang et al. combined HTCS and ML models to evaluate the potential of ionic liquid-incorporated MOFs (IL@MOF) as fillers to overcome the trade-off limitation in membrane separation.390 They prepared a dataset of 8167 IL@MOF composites by considering the [NH2-Pmim][Tf2N] molecule and using the CoRE MOF 2019 database.444 All IL@MOF composites were assessed for CO2/N2 (15/85) separation under ambient conditions. To obtain a better understanding of the structure-performance relations, some chemical characteristics (e.g., unsaturation degree, metallic percentage, and oxygen/metal ratio), as well as textural properties (e.g., crystal density, pore-limited diameter, and surface area), were considered to train an ML model based on the random forest (RF) regression algorithm. The ML outputs demonstrated that the most effective descriptors for CO2/N2 selectivity and CO2 permeability are accessible pore volume and mass-accessible surface area. [NH2-Pmim][Tf2N]@ZIF-67 was eventually selected as the best filler due to its promising CO2/N2 separation performance observed in molecular simulations. The selected filler was then integrated into PIM-1 to fabricate a high-performing MMM. The experimental results for the MMM exhibited superior CO2/N2 selectivity and CO2 permeability compared to both the pristine PIM-1 membrane and the ZIF-67/PIM-1 MMM, surpassing the redefined Robeson upper bound for CO2/N2 separation in 2019.282
8.3. Future directions of ML/AI modeling: inclusion of degradation and ageing effects
Despite the recent advances, we still observe that membrane degradation and ageing are not explicitly addressed in AI/ML models, specifically in the case of polymer membranes. One approach recently presented by Giro et al.440 has implicitly accounted for degradation effects through inclusion of the half-decomposition temperature as a target figure-of-merit. The main reason for the methodological gap is the lack of high-quality training data. Experimental data on degradation are scarce and, if available, they are often qualitative. Moreover, there is a lack of standardization with regards to how these data are obtained in the lab. One potential route to address this gap could be the generation and use of synthetic data. Nevertheless, nowadays the problem complexity limits the AI/ML model efficiency, model accuracy and predictive power.Going forward, an important challenge will be to include into the ML models the physical degradation effects observed in polymer membranes, such as plasticization, competitive sorption, and aging.445 Plasticization in polymer membranes occurs at high pressure, due to CO2 related swelling. The effect increases the segment mobility of polymer chains, the free volume and the interchain spacing. This leads to an increasing permeability446 and a loss of selectivity.447 Competitive sorption is an effect that tends to reduce the solubility of gases due to competition for the adsorption of the more soluble gas in the mixture.448 Physical aging occurs in glassy polymers due to the relaxation of the nonequilibrium chain conformation towards an equilibrium state, below the glass transition temperature. Glassy polymer chains gradually relax into their favored higher packing density (densification), which decreases membrane permeability.445 A potential pathway for mitigating these degradation effects is the addition of polymer crosslinking.449
In the case of nanoporous membranes, such as MOF membranes, some advancements towards inclusion of degradation effects have emerged. In recent works, a natural language processing (NLP)-based approach was used to extract information with regards to MOF solvent removal and thermal stability from the literature.450,451 The data was then used to train ML models for predicting the stability of new MOFs with quantified uncertainties. In a similar approach, Terrones et al. enlarged a training data set for predicting MOF stability against water.452 Inclusion of the additional data improved the ML model performance in the prediction of both stability against water and stability under acidic conditions. As an extension of previous work and an example of generative design including degradation effects, Nandy et al. employed ML models to identify MOFs that are stable against heating and solvent removal.453 Nevertheless, ML models do not yet capture degradation caused by corrosive and acid substances. For example, substances such as H2S, SOX, and NOX can disrupt weak ligand-metal linkages in MOF OMS.454,455 In addition, MOF stability could be further improved by exploring structural changes and functional modifications.454
We conclude that future ML approaches to membranes should explicitly include degradation effects. In generative design, the inclusion of suitable figures-of-merits in the design workflow could lead to improved, higher-stability membranes.
9. Process simulation and design challenges
The process of upscaling membrane samples from the lab to larger scales facilitates the transition of the technology from its initial developmental stages to pilot- and industrial-scale applications. Process modeling and simulations allow the identification of several technological and economic aspects of a technology before implementation beyond the lab. Simulations can effectively analyze the performance of membrane filters and membrane contactors. It is generally believed that membrane systems are significantly simpler from an all-encompassing standpoint, and more succinct, methodical comparisons are expected in the literature. In contrast, the carbon capture process is influenced by various factors such as industry type, geographic location, seasonal fluctuations in market demand, etc., all of which impact the final costs and design criteria.Industries emit streams with varying CO2 concentrations, flow rates, pressure, temperature, and impurity levels. Scenarios from simulations help to understand the multitude of steps required and strike a balance between fixed/operating costs and the total quantity of CO2 captured.456 Small and medium-sized CCUS processes can benefit from the low cost of membrane separation. However, once a critical point is reached, as determined by process simulations, amine-based capture technologies become more technically and economically viable.457 One major process challenge is optimizing both the number of steps and the purity and quantity of CO2 removed from the flue gas. The primary goal for a single-stage membrane-assisted decarbonization system is to achieve an energy requirement of less than 2 GJ per ton of CO2 recovered. Simulations indicate that such targets are achievable only when a vacuum pump is used on the downstream side or when the CO2 concentration in the feed stream is high.458 Membrane selectivity plays a crucial role in the system’s feasibility; moderate selectivity values (∼50) may be sufficient under optimized conditions. More cost-effective approaches, such as increasing the CO2 concentration through partial recovery of the exhaust or coupling the membrane separation system with a cryogenic unit, have been proposed.253,458 Two-stage membrane separation is more common and toward cost-function minimization by including/excluding/optimizing the process options of vacuum pump, partial recycle, step-vise pressure difference, purity and recovery ratio adjustment, energy recovery, humidification adjustment, and impurity removal.
Initial simulations were conducted based on fixed permeability, constant pressure change, and non-reactive systems. The next generation of simulations is now available based on variable permeability, variable pressure difference, and reactive FTM systems.459,460 A major challenge for membrane-based decarbonization processes is the unique working specifications of each membrane or module. As the new generations of membranes integrate both reactive and molecular sieving properties, their permeability/selectivity coefficients, along with their behavior in modulated form, including concentration polarization, need to be evaluated individually. Accordingly, generic simulations may not be able to cover the broad spectrum of membranes currently available in the market.458
Jomekian et al. offered a perfect instance of a tailor-made simulation of precise process modeling using a specific MMM membrane.461 ZIF-8 modified Pebax 1657 membranes containing up to 60% of the nanofillers were modeled by connecting an Excel sheet performance database to Aspen software. While this simulation approach is not the most optimum one, promising results were reported in terms of using simulation tools. Using an experimental mixed gas setup, they reported the permeance and selectivity of their lab-made MMMs. The flux for the CO2 and the other gas, in their case, CH4, was calculated using the generic solution-diffusion formula (eqn (23) and (24)), and the flux for the membrane unit was solved by rearranging the formulas (eqn (25) and (26)):
 | (21) |
 | (22) |
 | (23) |
 | (24) |
| JCO2total = nJCO2-single stage | (25) |
 | (26) |
Another valuable report presented the simulation of two-stage membrane-based decarbonization of a 400 MW natural gas combined cycle (NGCC) power plant, along with the optimization of the carbon-to-electricity relative price (Fig. 25).462 The objective was to maximize the total net present value (NPV) of the power plant, considering no constraints on production and demand, through the simultaneous optimization of design and operational parameters. The NGCC was modeled with part loads varying from 0.66 to 1, fixed fuel, and air flow rates to avoid fluctuations in the flue gas composition, maintaining a fixed CO2 concentration of 3.9 mol%.
 | ||
| Fig. 25 (a) Schematic of two-stage membrane filtration model used for decarbonization of a 400 MW NGCC power plant, (b) Comparison of NPV for the power generation without carbon capture, with base membrane-decarbonization unit, and with advance membrane units (reproduced from ref. 462 with permission from Elsevier, copyright 2025). | ||
The two-stage membrane filtration was modeled under the assumptions of no pressure drop and no temperature change within the membranes. The feed and operating temperatures were set at 45 °C, and 30 °C, respectively. The CO2 concentration was 95 mol%, and the CO2 outlet pressure at the end of the second filtration was 137.9 bar (6-stage compression). The compressor efficiency was 0.8, and the vacuum pump and expander efficiency were 0.7, with adiabatic expansion or compression considered in the calculations of the pumps and compressors. Time step of one hour was used for power cost estimation, based on twice the power price in California in 2015, with a natural gas price of $3.13/GJ and a membrane cost of $50/m2.
A simplifying assumption was made that only CO2 and N2 permeated the membranes, and a crossflow model was applied for the simulation. A simplified crossflow symmetric membrane model was used, which is practical and easier to run compared to a more comprehensive asymmetric membrane model. Details of the membrane modeling for gas separation can be found elsewhere.463
The selectivity and permeance of the membrane were set to 50 and 1000 GPU, respectively, while four other design parameters, namely, the membrane surface area and the compressor size in each of the stages, were changed to create different simulation scenarios. Another scenario of improved membrane separation property was also developed (selectivity of 200 and permeance of 3500 GPU) for comparison purposes. Cycle load, each stage's feed pressure, each stage's permeate pressure, and the capture rate parameters were selected as the operation variables for the optimization. Interestingly, the comparison of the highly selective membrane and the base membrane in the lower carbon pricing range showed closer NPV values, while at a higher carbon price of $200/ton, the difference was significant. While the study provides a good example of real scenarios, several aspects are not yet covered. For example, the NPV can be affected by the possible selling scenarios of the carbon captured for oil recovery purposes. A more comprehensive approach would consider avoided risks or regulatory compliance to prevent fines. A lower NPV with CCUS does not necessarily reflect less profit for the plant because of emitted carbon. Given the specific economic and regulatory context, the costs associated with capturing and storing carbon currently outweigh the financial benefits derived from such activities. According to Yuan et al., decarbonizing power plants is inherently context-sensitive.462 Power demand, energy prices, and carbon regulations within a region are examples of market circumstances that impact the model's sensitivity and optimal design and operation.
Simulation and modeling approaches can also target more detailed information on membrane properties with the possibility of altering the techno-economic aspect of the carbon capture process. As an instance, Budhathoki et al. considered a three-stage membrane separation design to investigate the TEA of the process for twelve hypothetical membranes with CO2 permeance equal to 34, 1170 or 8000 GPU and CO2/N2 selectivity of 18, 35, 68 or 250.435 This simulation setting, coupled with optimizing the operating parameters using the framework for optimization, quantifying uncertainty, and sensitivity (FOQUS) through Aspen Custom Modeler Software (ACM), highlighted the influence of membrane characteristics on the TEA. An interpolation of the cost of carbon capture was made as a function of permeability and selectivity, and it was further extended to a database on MMMs with different performance data. The TEA was assessed using the cost of CO2 capture and cost of electricity production (COE), which is a function of total overnight cost (TOC), carbon capture operating variable cost (OCCC), capital cost factor (CCF) and capacity factor (CF), and the parasitic load (MW hparasitic load). The suffix “ref” indicates the same parameter for the plant without carbon capture, and the suffix “cc” refers to the parameters of the plant with carbon capture. Within the simulation framework described above, the cost of the CO2 capture factor was calculated for each permeance/selectivity pair (Fig. 26(a)). Interpolation of the cost of CO2 capture for several MMMs with different polymers and various nanofillers was then conducted to generate a cost sensitivity for MMMs-based carbon capture (Fig. 26(b)). Despite common assumptions, enhancing the permeability and selectivity of a membrane does not necessarily result in a reduced CO2 capture cost. Interestingly, the lowest CO2 capture cost does not result from the best MOF with the most suitable adjustments to pore and chemical characteristics. For a MMM to exhibit improved gas selectivity, the selectivity ratio between the MOF and the polymer should be at least ten times higher than their permeability ratio. This means that MOFs with the lowest cost of capture capacity (CCC) are not those with the highest permeability and selectivity. Instead, the optimal MOFs are those where the selectivity ratio surpasses the permeability ratio by at least an order of magnitude, provided that they also have higher permeability and selectivity than the polymer alone.
 | (27) |
 | (28) |
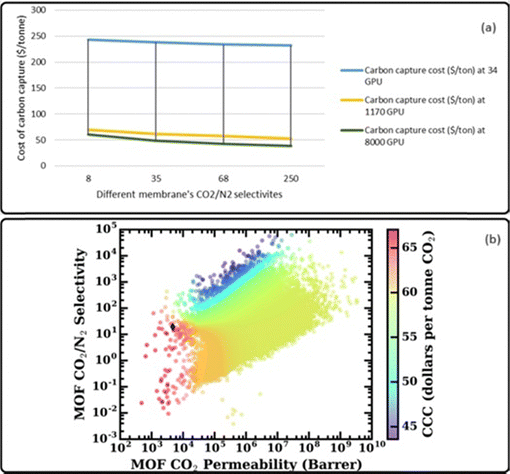 | ||
| Fig. 26 (a) Carbon capture cost calculation using the simulated process for hypothetical membranes (3 different permeances and 4 different selectivities), and (b) carbon capture cost estimation using the interpolation driven from a 650 MWe power plant-carbon capture simulation and using it for hypothetical MMMs assessment (reproduced from ref. 253,435 with permission from RSC, copyright 2025). | ||
9.1. Number of stages and its correlation with process efficiency and economics
Having CO2 separated in a single-stage process could impose many limitations to the process.464,465 Two-stage filtrations were studied and optimized by a few research teams. For instance, energy recovery from the CO2-depleted stream and post-capture CO2 liquefaction were taken into consideration when Shao et al. investigated a two-stage membrane technique for CO2 capture from coal-fired power stations.466 Vacuum pressure on the permeate side was found to be the preferred driving force after feed and permeate pressures were optimized. According to a cost analysis, the first-stage membrane accounted for the majority of overall expenses. For assumed parameters (1000 GPU permeance; CO2/N2 selectivity of 30, 50, and 200), the results showed that membrane technology is more economical than PSA and amine absorption for CO2 capture in coal-fired flue gas.Zhai et al. studied polymer membranes for capturing CO2 after combustion in coal-fired power plants.467 With a CO2/N2 selectivity of 50, a membrane permeance of 1000 GPU, and a flue gas flow rate of 500 m3 s−1 (STP) with 13.0% CO2, they discovered that the best way to minimize CO2 avoidance costs is to combine compressors and vacuum pumps. A two-stage membrane system cost $45.6/mt CO2 to capture, but it recovered 90% of the CO2 and was 95% pure. The cost was reduced to €31/t CO2 by recycling CO2 using a two-stage, two-step air sweep arrangement, which is in line with Kotowicz et al.468 A parametric study on two-stage membrane designs for CO2 collection in a 600 MW coal-fired power plant in North Rhine-Westphalia, Germany, was carried out by Zhao et al.469 To find the best CO2/N2 selectivity and capture costs, they conducted a sensitivity analysis using PEBAX polymer membranes and PRO/II software. A two-stage cascade system that achieved 70% CO2 recovery and 95% purity for a feed gas containing 14% CO2 had a capture cost of €31/t CO2 (∼32.2 $/t CO2, assuming an exchange rate of 1 € = 1.04 USD), according to the data, making it a feasible retrofit option. Correlations between membrane characteristics and system performance were also discovered by the study. According to these investigations, two-stage membrane systems can achieve 90% CO2 recovery and 90–95% purity, making them competitive with traditional amine-based CO2 capture methods. Further research is necessary to determine if membrane-based CO2 capture can remain competitive if recovery and purity standards rise from 90% to 98%. Further process optimization also requires analyzing the effects of these higher targets on the ideal number of membrane stages, membrane area, operating conditions, and overall cost.
The primary methods for determining the best membrane-based CCUS system configurations -taking into account the number of filtration steps, membrane size, and operating conditions- are process optimization techniques. One important factor to keep in mind is that the permeability and selectivity of the membrane are inextricably tied to the final product purity, membrane size, and operating conditions. Therefore, if future developments result in the creation and commercialization of membranes with greater permselectivity, any process optimization based on projected membrane performance could become outdated. Arias et al. used a mixed integer nonlinear mathematical programming (MINLP) modeling approach to find the optimal number of membrane stages, membrane areas, and operating conditions that minimize the total annual cost of CO2 capture from flue gas470 (Fig. 27). The number of membrane stages is highly influenced by the targeted CO2 purity (Table 12). A two-stage system with one recycle stream was shown to be ideal for purity levels between 90% and 93%, however three stages and two recycle streams were needed to achieve 94% to 96% purity. Four membrane phases were required to maintain efficiency for higher purity standards of 97% and 98%. This approach shows the trade-offs between increasing CO2 purity and its associated expenses. Higher purity requires a greater membrane area and higher energy usage due to increased pressure and compression requirements. Furthermore, Arias et al. showed that these enhanced multi-stage designs are competitive in terms of affordability and power consumption not only with traditional absorption-based CO2 collection techniques but also with other membrane-based separation procedures. In order to guarantee the economic viability of membrane-based CCUS technologies, these findings highlight the significance of carefully choosing the number of separation steps based on the intended CO2 recovery and purity.
 | ||
| Fig. 27 Process simulation and optimization for membrane separation-based CCUS with different stages; (a) 4 stages, (b) 3 stages, and (c) 2 stages (reproduced from ref. 470 with permission from Elsevier, copyright 2025). | ||
| Variable | Optimal configuration (4 stages, Fig. 27(a)) | Suboptimal configuration (3 stages, Fig. 27(b)) | Suboptimal configuration (2 stages, Fig. 27(c)) |
|---|---|---|---|
| TAC (M $ per year) | 123.54 | 134.22 | 136.93 |
| Total investment (M $ per year) | 66.99 | 66.61 | 67.56 |
| Total operating cost (M $ per year) | 56.55 | 67.61 | 69.37 |
| Total power (MW) | 278.31 | 320.246 | 325.974 |
| Power recovered in expander (MW) | 102.272 | 113.8 | 114.55 |
| Total net power (MW) | 176.035 | 206.626 | 211.416 |
| Total membrane area (m2) | 2082![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 164.65 164.65 |
1389![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645.09 645.09 |
1415![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 254.71 254.71 |
| Total heat transfer area (m2) | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523.82 523.82 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382.26 382.26 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001.73 001.73 |
| Total compressed permeate flow rate (mol s−1) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828.82 828.82 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 769.37 769.37 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 169.51 169.51 |
| High operating pressure (MPa) | 0.586 | 0.706 | 0.715 |
| Number of iterations | 38 | 7 | 5 |
Accordingly, process optimization plays a crucial role in enhancing the efficiency and cost-effectiveness of membrane-based CCUS systems. The studies reviewed demonstrate that multi-stage membrane configurations can achieve competitive CO2 recovery and purity levels compared to conventional amine-based methods, with the optimal number of stages being highly dependent on the target purity. As CO2 recovery and purity demands increase, additional membrane stages and higher membrane areas are required, leading to higher energy consumption and costs. However, advancements in membrane materials and further optimization of system configurations can help mitigate these challenges. Future research should focus on refining membrane properties, exploring alternative driving force mechanisms, and integrating novel separation strategies to improve the economic and environmental viability of CCUS technologies.
10. Comparison of membranes and conventional processes for CCUS
The right CCUS approach is determined based on the different CO2 streams that industries emit, which vary in concentration, flow rate, pressure, temperature, and impurity levels. Different strategies are needed for small, medium, and large industries to maximize technological efficiency and economic viability. While this paper insists on the benefits of membranes as one of the pioneer candidates for CCUS application, there are cons and pros when it is compared to the more conventional processes such as amine-based absorption. Because amine-based absorption has a high capture efficiency (>90%), it is the preferable method for large-scale businesses (such as cement and power plants) that produce high-volume, low-CO2-concentration emissions (∼3–15%). Hybrid amine-membrane systems, in which membranes pre-concentrate CO2 to lower the regeneration energy of amine solvents, can be advantageous for medium-sized companies (such as chemical plants and ammonia production). High-purity CO2 streams are produced by small-scale enterprises (such as small hydrogen plants and biogas upgrading), which makes membrane separation more feasible because of lower startup and operating costs. Nevertheless, until large scale membrane-based plants with higher efficiency would not be in practice a real comparison would be irrelevant. To offer a current comparative vision between the absorption and membrane processes, Table 13 brings different aspects of the technologies together.| Feature | Amine absorption | Membrane separation |
|---|---|---|
| CO2 capture efficiency | 85–95% | 50–90% (depends on membrane type & stages) |
| Energy consumption | 3–6.5 GJ per ton CO2 | ∼1 GJ per ton CO2 |
| Selectivity | High for CO2/N2 | Moderate, depends on membrane type |
| Operating pressure | Near ambient | Varies (vacuum-assisted options available) |
| Sensitivity to impurities | Highly sensitive (SO2, NOx cause degradation) | Plasticization and aging concerns but can be mitigated |
| Capital & operating costs | High due to solvent handling & regeneration | Lower due to modular design & no solvent regeneration |
| Scalability | Preferred for large-scale (>1 M ton CO2 per year) | Best suited for small to medium industries |
| Commercial readiness | TRL 9 (Fully commercial) | TRL 6–7 (Pilot studies ongoing) |
The scale of the industry and operating costs determine whether CO2 capture is economically viable. 50% of yearly running costs are related to amines' high energy requirements for solvent regeneration. Membranes, on the other hand, are appropriate for small-to-medium applications due to their lower energy consumption and versatility. Large-scale companies where the high capture efficiency outweighs the high energy and maintenance costs favour absorption-based CCUS. Small and medium-sized businesses can save money by using membrane-based separation, especially in decentralized environments like hydrogen manufacturing and biogas upgrading. One approach that shows promise is process integration. By pre-concentrating CO2 prior to solvent regeneration, hybrid membrane-amine systems can increase energy efficiency. Additionally, to increase CO2 purity and lower operating costs, membrane-based separation in conjunction with cryogenic procedures is being investigated.
Developing membrane separation and improving hybrid strategies to get around present constraints are key to the future of CCUS technologies. High-performance polymeric and MMMs will be the focus of material advances to improve durability, permeability, and selectivity. It is also crucial to conduct research on FTMs that provide better CO2 separation in industrial settings.
To decrease solvent regeneration energy and increase membrane longevity, process improvement will entail a hybrid integration of amine absorption and membrane separation. While the investigation of cryogenic-membrane hybrids can enhance separation efficiency in high-volume applications, the development of two-stage membrane systems will enable improved CO2 purity and optimized energy consumption. When determining the cost break-even points at which membrane separation outperforms amine absorption, techno-economic analyses will be essential. It is necessary to assess long-term operating costs while taking membrane deterioration, replacement cycles, and scalability into account. It is also necessary to look at the viability of modular membrane modules designed for small-scale and decentralized industries.
Membrane separation and amine absorption each have unique benefits and drawbacks. Because of its high capture efficiency and commercial maturity, amine-based CCUS continues to be the industry standard for large-scale applications. Membrane-based systems, on the other hand, give small and medium-sized businesses a competitive edge by lowering energy and capital expenditure. The gap can be filled with additional hybridization and material developments.
11. Utilization of the captured carbon and the contribution of polymeric membranes
Utilization of captured carbon (CCU) is viewed as a promising pathway to address the limitations of conventional short-term solutions for carbon capture and storage (CCS). However, there is strong reasoning that CCS is the mature, well-understood, established, and only practical technique for meeting CO2 emission reduction goals by 2050 and beyond, allowing ample time for a transition away from fossil fuels, thereby rendering CCU a highly unrealistic alternative to CCS.471 The best approach to addressing conflicting reasoning among CO2 emission reduction strategies is to view each process in its appropriate context. CCU should never be considered as the viable alternative to CCS for the mitigation of CO2 emission challenge to meet net-zero to net-negative CO2 emission milestones. It can rather be implemented in places where CO2 is the only feedstock or process driver (e.g., urea production or CO2-enhanced oil recovery), CO2 is the cheaper feedstock, or the CO2-derived product can viably replace the alternative product, or the net CO2 emission is not higher.471 The fertilizer industry is the largest consumer, using approximately 130 Mt of CO2 annually for urea manufacturing, while the oil sector follows closely, consuming 70 to 80 Mt of CO2 for enhanced oil recovery (EOR).472Polymeric membranes contribute to CCU in two major processes: (i) gas separation units, which are employed in membrane modules for the selective capture of CO2 from flue gases, biogas, or natural gas streams, and (ii) membrane reactors, which enhance process efficiency and selectivity by integrating reaction and separation. The following discussion will focus on membrane reactors, as gas separation units were covered in previous sections.
11.1. Membrane reactors
Membrane reactors combine reaction and separation processes in a single unit, allowing for enhanced process efficiency, higher reaction rates, selectivity, and control.473 The membrane component selectively separates one or more reaction mixture components, either by size, charge, or affinity, while allowing other components to pass through. This selective separation enables the continuous removal of reaction products or byproducts, shifting the reaction equilibrium towards the desired products and enhancing overall reaction rates. Membrane reactors find applications in CO2 conversion processes, including hydrogenation of CO2 to methanol, dry reforming of methane (DRM), reverse water gas shift (RWGS) reaction, CO2 hydrogenation to formic acid, and CO2 methanation, among which the conversion to methanol and RWGS for fuel production is viewed as the most important CO2 utilization pathway, presumably from a business perspective.A membrane in a membrane reactor provides four basic functions: extractor to separate the desired products from the reaction mixture, distributor to introduce the required ratio of reactants into the reaction zone, contactor to enhance the surface contact of the reactants with the catalysts immobilized on the surface or embedded into the membrane layer, and extender of catalyst lifetime to enhance reaction rates by removing water (following Le Chatelier's principle), which acts as a reaction byproduct.474 The two most common configurations for membrane reactors are the packed-bed membrane reactor (PBMR), where the membrane only separates products, and the catalytic membrane reactor (CMR), where the membrane acts as the catalyst support and separates products. There are four primary categories of membrane reactors: (i) electrochemical, (ii) thermocatalytic, (iii) photocatalytic, and (iv) biocatalytic. Among these, electrochemical membrane reactors typically do not utilize membranes for CO2 or product separation.
Among these, electrochemical membrane reactors typically do not utilize membranes for CO2 or product separation, nor do they serve as catalyst supports. Instead, the membrane acts as a barrier between the cathode and anode chambers, preventing the mixing of components while selectively conducting protons. This function is quite different from the concept of membrane reactors, where the membrane primarily serves as a means for selective reagent introduction, product separation (purification), and catalyst support. In membrane reactors, the membrane helps ensure the homogeneous distribution of catalysts and provides a large surface area for catalytic reactions. Consequently, the discussion will be confined to the remaining three categories, where membranes both support the catalyst and facilitate separation. Interested readers are encouraged to explore recent reviews on well-studied and industrially viable electrochemical CO2 reduction technologies.475–478
Zou et al. developed a WGS membrane reactor featuring a CO2-selective polymeric membrane and a commercial Cu/ZnO/Al2O3 catalyst for hydrogen production suitable for use in proton-exchange membrane fuel cells (PEMFCs).484 The membrane, made from cross-linked poly(vinyl alcohol) with fixed and mobile carriers that demonstrated good CO2 selectivity and permeability at 110–170 °C, effectively removed CO2 during the WGS, shifting the equilibrium towards more hydrogen production and reducing CO levels to below 10 ppm, meeting PEMFC hydrogen purity requirements. Lee et al. explored the use of polyimides (PI) membrane with 4,4′-(hexafluoroisopropylidene) diphthalic anhydride (6FDA) and hydroxyl aromatic diamines (2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane (APAF) and 3,3′-dihydroxy-4,4′-diamino-biphenyl (HAB) copolymer) in a membrane reactor for CO2 hydrogenation by the reverse WGS (Fig. 28(a)).489 Integrating the polyimide membrane into the reactor enhances the yield of carbon monoxide (CO) by 2–3 times compared to reactors without the membrane by selectively removing the byproduct water. Additionally, the membrane exhibits high H2O permselectivity at elevated temperatures due to bulky perfluoro moieties and local hydrophilicity provided by hydroxyl groups. The exceptionally high H2O permselectivity at high temperatures is governed by the favorable solution-diffusion model, which is opposite to inorganic membranes, where adsorptive transport is the main mechanism for H2O separation. These findings suggest that the use of polyimide hollow fiber membrane reactors can improve the efficiency of CO2 hydrogenation reactions, particularly at low temperatures where equilibrium limitations typically hinder product yield.
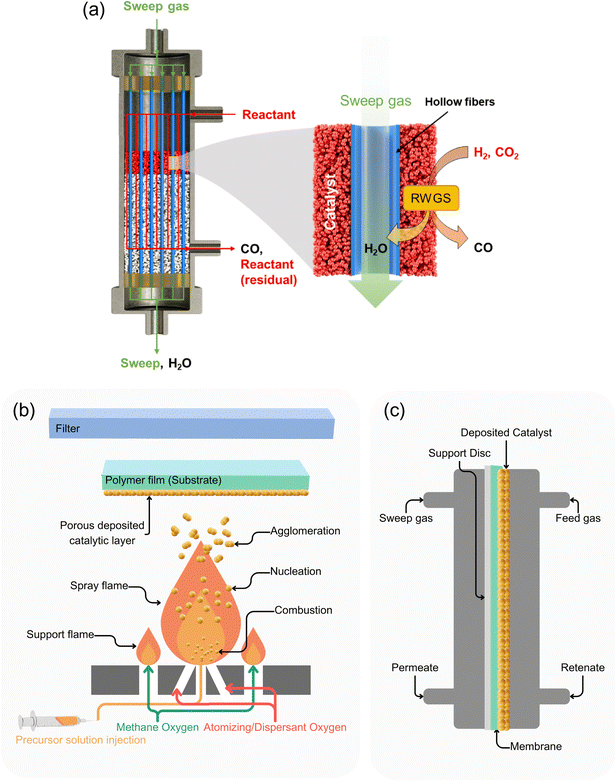 | ||
| Fig. 28 Schematic illustration of: (a) the hollow fiber membrane reactor with membrane/catalyst and transport of gases (reproduced from ref. 489 with permission from Elsevier, copyright 2025), (b) flame spray pyrolysis-based deposition on the membrane, and (c) the resulting membrane reactor.488 | ||
The above studies involved membranes only for separation; the next studies will cover membranes acting mainly as catalysts or supports for catalysts in addition to separation. Considering the thermal stability concerns of polymeric membranes, exploring low-temperature CO2 conversion pathways is deemed rational. Therefore, converting CO2 to cyclic carbonate is a logical choice, as it is a 100% green reaction.487 Liu et al. explored various compositions of ionic liquid monomers for preparing crosslinked block copolymer membranes for the conversion of CO2 and propylene oxide (PO) to propyl carbonate (PC).486 The variables considered for the monomer composition were the type of functional groups used for the quaternization of the tertiary amine groups of the 2-(dimethylamino)ethyl methacrylate (DMAEMA) block and the type of counterion for the positively charged quaternary ammonium ions. The best polymeric ionic liquid membrane (PILM) with a [DMAEMAEtOH]Br-quaternized block resulted in the highest yield of PC (98%). The gas-phase PO conversion for PILMs was 28 times that of pure polymeric ionic liquids (PILs). The high catalytic activity of the PILM was attributed to the high density of catalyst active sites and the easy access of these sites to PO and CO2 due to PO adsorption-induced swelling of the polymeric network, providing a microenvironment for the close contact of reagents. Despite a significant improvement in catalytic activity, this enhancement is brought about by membrane swelling, which can ultimately destroy the membrane under agitation; therefore, the stability of the membrane needs improvement. Process engineering, such as sandwiching the active membrane into a stable polymeric support, can play a crucial role in this case.
Xu et al. developed quaternized poly(4-vinylpyridine) (P4VP) membranes for selective CO2 separation, followed by cycloaddition to epichlorohydrin to produce cyclic carbonates.487 The (P4VP-C2-HCO3) membrane, made of quaternized poly(4-vinyl pyridine) (P4VP) followed by anion exchange of bromide (Br− with bicarbonate (HCO3−), integrated both CO2 capture from a dilute condition (similar to the concentration in air, 0.1 kPa of CO2) and catalytic conversion to cyclic carbonate in a single platform under mild temperature (57 °C) and atmospheric pressure. The high catalytic activity of the (P4VP-C2-HCO3) membrane may be due to the favorable catalytic activity of HCO3− in the initiation step of ring-opening of epichlorohydrin and the final step of cyclic product release and HCO3− regeneration. Although the catalytic activity of the membrane was promising, the cyclic carbonate production rate decreased dramatically within 30 hours. The blockage of catalyst sites by strong adsorption of byproducts, such as glycidol or 3-chloro-1,2-propanediol, and the dissolution of the membrane in epichlorohydrin were associated with the decrease in production rate. Interestingly, the decline in rate over time was not considered. The reaction involves catalysis by HCO3−, which is generated by any quaternized polymeric membrane during the facilitated CO2 transport process.490,491 Therefore, any quaternized membrane will lead to a certain conversion rate, as in the quaternized membrane (P4VP-C2-Br) before the anion exchange used in this study. Therefore, it can be assumed that the initial reaction rate was higher due to the preexisting HCO3− in the P4VP-C2-HCO3 membrane, which depleted over time by reproducing CO2, and the rate became similar to that of the quaternized membrane (P4VP-C2-Br) within 30 hours.492 A remarkable advancement in catalytic membrane reactors for a commercially important product (methanol) was recently achieved by Pham et al. (Fig. 28(b and c)).488 The authors integrated strategies to enable high-temperature reactions (>200 °C) and enhance the reaction rate using high-temperature stable and highly water-permeable polyimide (PI) and polybenzimidazole (PBI) membranes, flame spray pyrolysis-based direct deposition of nanosized, highly porous, and active CuO/ZrO2 thin layers on the membranes, and post-deposition reduction of CuO to Cu at a relatively low temperature (300 °C) under 5% H2 in Ar. These strategies enabled the membrane to operate stably at 200 °C and 20 bar, with a 113% increase in CO2 conversion and a 106% increase in methanol production compared to conventional reactors.
More interest and investment should be directed towards carefully selecting CO2 utilization pathways that have no alternatives, are cost-effective, scalable, and incorporate efficient process design and integration. Efforts should also focus on improving polymeric membrane performance as both support and separator, and on ongoing research for low-temperature catalyst development. Polymeric membranes can play a vital role in enhancing the catalytic activity of encapsulated catalysts through coordination, in addition to their separation function. Birdja et al. used polymeric membranes to encapsulate the Indium(III) Protoporphyrin catalyst within a polymer matrix, improving the overall catalytic performance for CO2 reduction.493 The polymeric membranes examined were didodecyldimethylammonium bromide (DDAB), Nafion, poly(4-vinylpyridine) (P4VP), and poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT). They assessed the influence of different substrates and polymer encapsulation on catalytic efficiency. The study demonstrated that both the substrate and polymer encapsulation significantly impacted the efficiency and selectivity of CO2 reduction to CO. The enhanced electrocatalytic CO2 reduction performance by P4VP has been previously reported for catalysts like cobalt phthalocyanine (CoPc). This improvement is attributed to the pyridine residues in the polymer, which influence coordination with the catalyst,433,434 P4VP can form strong interactions with the cobalt centers in the CoPc catalyst, improving the stability and distribution of the catalyst within the polymer matrix and enhancing overall catalytic activity and selectivity for CO2 reduction494
Thermocatalytic membrane reactors offer key advantages over standard industrial thermocatalytic reactors by combining reaction and separation in one step, reducing the need for external purification. The membrane enables selective product removal, enhancing reaction efficiency and potentially increasing productivity by maintaining optimal thermodynamic conditions. These reactors can achieve high conversion rates, particularly for thermodynamically favorable reactions, though they require significant energy input to sustain necessary conditions.
 | ||
| Fig. 29 Illustration of fabrication of polymeric photocatalytic membrane (reproduced from ref. 496 with permission from Elsevier, copyright 2025). | ||
A large fraction of works on CO2 conversion using membrane reactors are based on photocatalysis, which has been reviewed recently.497,498 Only a few examples with high efficiency, feasibility, and process intensification will be discussed here. Pomilla et al. investigated the conversion of CO2 to liquid fuels using photocatalytic methods within a continuous membrane reactor, where C3N4 nanosheets were embedded in a Nafion membrane.499 Their setup achieved successful CO2 conversion to liquid fuels at a rate of 32.8 μmol g−1 cat h−1, with selectivity towards methanol (54.6%) and ethanol (45.4%). The continuous membrane reactor demonstrated a total carbon conversion rate more than 10 times higher compared to a batch reactor (Fig. 30). This enhanced performance is attributed to two key factors: (i) the dispersion of nanosheets within the Nafion polymer matrix provides greater exposure of active sites to light and reactants (ii) the continuous removal of products by the membrane promotes the forward reaction and frees active sites for further conversion. However, this method exhibited a low selectivity for methanol. Brunetti et al. improved both the alcohol production rate (48.8 μmol g−1 cat h−1) and the selectivity for methanol (83.2%) by incorporating a TiO2–C3N4 composite into the Nafion membrane instead of using C3N4 alone.500 The enhanced catalytic activity and selectivity for methanol were attributed to better charge separation at the heterojunction formed by the TiO2–C3N4 interfaces.
 | ||
| Fig. 30 Schematic for the continuous flow photocatalytic membrane reactor for the conversion of CO2 to liquid fuels methanol and ethanol (reproduced from ref. 499 with permission from ACS, copyright 2025) | ||
In the quest to explore more efficient and selective photocatalysts, significant effort has been devoted to developing photocatalysts with tunable charge separation performance. This includes exploring MOF-based photocatalysts, as their photocatalytic activity and charge separation properties can be tuned by selecting photoactive organic ligands, doping with ions, or integrating photoactive materials.497 Zhao et al. applied a comprehensive design strategy by incorporating CdS semiconductor nanorods and UiO-66-NH2 MOF into a chitosan-based membrane to enhance conversion efficiency and selectivity.501 They selected CdS nanorods and UiO-66-NH2 MOF for efficient, broader light absorption and charge separation, thereby improving catalytic activity and selectivity. UiO-66-NH2 MOF served not only as a photoactive material but also as a highly selective CO2 adsorption material, enhancing the reaction rate and selective reduction of CO2 over other species in the reaction mixture. Chitosan was chosen as the membrane material for its highly selective adsorption of CO2 and improved proton transport due to its abundant –NH2 and –OH groups, which are critical for enhancing conversion rates and selectivity towards CO2 reduction. These groups also interact favorably with the CdS nanorods and UiO-66-NH2, providing uniform dispersion and suppressing agglomeration-induced photocatalytic deactivation. Consequently, the CdS/UiO-66-NH2 membrane reactor demonstrated higher CO production (313.2 μmol g−1 cat) and selectivity (99%) than the mixed powder form (521.9 μmol g−1 cat, 95%) after 6 hours of irradiation.
Although incorporating photocatalysts into membrane matrices addresses many issues associated with bulk catalyst dispersion—such as aggregation, reduced active site availability, light-scattering, poor proton transfer, and catalyst recovery—the need for a pure CO2 gas feed limits their applicability for selective product generation from crude gas mixtures.497 Integrating a CO2-selective gas separation membrane with a photocatalyst in a membrane reactor, where the membrane acts as a support for the catalyst and separates CO2 from gas mixtures, can effectively resolve this issue.497,502,503 Baniamer et al. designed a two-layer photocatalytic membrane reactor using Pebax 1657 as the CO2-selective gas separation layer and BiFeO3@ZnS as the photocatalyst layer for simultaneous CO2 separation and photoreduction to methanol.445 Their reactor successfully demonstrated simultaneous CO2 separation and photoreduction to methanol, with a methanol production yield of 5100 and 3360 μmol g−1 cat h−1 under UV and visible irradiation, respectively. This enhancement in methanol yield was attributed to the purified CO2 feed provided by the Pebax membrane, the broader light absorption by the BiFeO3@ZnS photocatalyst, and efficient charge separation at the localized p–n junction between BiFeO3 and ZnS interfaces.
Most photocatalytic membrane reactors utilize high-concentration CO2 feed gas, which requires costly separation and transportation steps. Direct air capture and conversion is the ideal scenario for addressing atmospheric CO2 removal effectively and providing renewable resources for synthesizing value-added products. However, the low concentration of CO2 in the air limits the rate of photocatalytic conversion, and other gases present in the air can adsorb onto the catalyst site, reducing efficiency and selectivity. To overcome this, Hu et al. developed a two-layered “Janus membrane” structure consisting of a polyimide (PI) selective layer for CO2 separation and enrichment from air, and a porous PI catalyst support layer embedded with Cu-doped TiO2 particles.504 The dense PI layer separated and enriched CO2 into the membrane, while the porous PI support layer allowed longer residence time for effective contact with the Cu-doped TiO2 photocatalyst (Fig. 31). This approach was highly successful, achieving an optimum CO2-to-CO conversion yield of 2.21 μmol g−1 cat h−1.
 | ||
| Fig. 31 Schematic for the direct capture and photocatalytic reduction of CO2 from air using Janus Polyimide/Cu-doped TiO2 membranes (reproduced from ref. 504 with permission from Elsevier, copyright 2025). | ||
Among various photocatalytic membrane reactors, direct air capture and conversion presents an economically viable renewable approach for producing CO2-derived products and a reliable method for reducing atmospheric CO2 levels. However, this process often has low efficiency, and the primary product is gaseous CO, which incurs additional isolation and storage costs. To address these challenges, a Janus membrane with broader solar light absorption, particularly visible light utilization (e.g., BiFeO3@ZnS), should be developed. The selective layer of the membrane could be composed of CO2-adsorbing and enriching materials like polyimide (PI), Pebax (PEBAX), or polybenzimidazole (PBI). The porous catalyst support layer could be fabricated from a blend of CO2-philic and hygroscopic polymers or a block copolymer containing segments of both types to facilitate the enrichment and contact of CO2 and H2O with the catalyst. This would enhance the production of liquid fuels such as methanol, which is easier to isolate, store, and serves as a valuable solvent and feedstock for various chemical processes.
Further process intensification strategies could be employed to improve CO2 conversion efficiency and methanol storage. One such strategy involves placing the photocatalytic membrane on the surface of natural water bodies (e.g., ponds, lakes, and rivers in urban areas) where sunlight is abundant. In these areas, CO2 levels are generally higher due to human activities, infrastructure, and reduced vegetation. Additionally, elevated temperatures can facilitate humid air and CO2-to-methanol conversion. The methanol collector could be submerged in the water, condensing the methanol, enhancing production rates, and maintaining a lower temperature for methanol storage.
Photocatalytic membrane reactors operate at lower temperatures and pressures than industrial thermocatalytic CO2 conversion methods, making them more sustainable. However, their productivity is generally lower, which makes them more suited for small-scale or specialty applications focused on environmental sustainability. The membrane in these reactors offers the benefit of separating reactants and products, enhancing selectivity and efficiency by preventing undesired side reactions. Despite this, the slower reaction kinetics and limited light penetration hinder productivity compared to thermocatalytic or electrochemical systems, particularly at large scales. Solar-driven photocatalytic CO2 reduction is appealing for its sustainability but faces challenges like low light utilization, scalability issues, and the need for new infrastructure. Large land coverage for solar light absorption and variability in sunlight intensity further impact its industrial feasibility. While not yet viable or profitable for large-scale industrial CO2 conversion, solar-driven photocatalytic membrane reactors could serve as a long-term CO2 removal strategy, potentially generating profit from conversion products in the future. In addition to advancing efficient photocatalysts, particularly in the visible light region, it is crucial to design reactors that optimize solar light use. This can be achieved by incorporating solar concentrators and strategically locating industries in regions with optimal solar light availability to enhance efficiency.
Díaz et al. employed a hollow-fiber membrane for sparging H2 into the bioreactor, which enhanced H2 mass transfer into the liquid phase and improved the conversion of CO2 and H2 to CH4. However, in this case, the membrane did not serve as a catalyst support or a medium for product separation.505 On the other hand, Luo et al. pioneered the use of a membrane as support for biocatalyst immobilization by co-immobilizing or sequentially immobilizing three enzymes—formate dehydrogenase (FDH), formaldehyde dehydrogenase (FaldDH), and alcohol dehydrogenase (ADH)—within the porous structure of a membrane to promote sequential conversion of CO2 to methanol.506 Although this approach was innovative, the immobilization did not enhance the conversion of CO2 to methanol. The complete conversion pathway from CO2 to methanol involves three steps: (1) FDH-catalyzed conversion of CO2 to formic acid, (2) FaldDH-catalyzed conversion of formic acid to formaldehyde, and (3) ADH-catalyzed conversion of formaldehyde to methanol. The primary bottleneck identified was the reversible step catalyzed by FaldDH, which converts formic acid to formaldehyde. Additionally, the slow conversion of CO2 to formic acid by FDH produced insufficient substrate to activate FaldDH effectively in the second step. To overcome these limitations, future strategies could include engineering mutations in FaldDH, identifying alternative enzymes or cofactors for efficient formic acid to formaldehyde conversion, or designing layered membrane structures with supports optimized for each enzyme's catalytic activity. For example, embedding FDH into a membrane that maintains a slightly alkaline environment could facilitate formic acid to formate transformation, given that FaldDH and ADH efficiently convert formate to methanol.507
Interestingly, contrasting results were reported when the three enzymes were co-immobilized into siliceous mesostructured cellular foams, achieving a 4.5-fold increase in CO2 conversion to methanol.508 In this study, enzyme immobilization was performed through incubation, as opposed to the pressurized filtration method used for membrane pore immobilization, which may have led to enzyme agglomeration or over-compaction, hiding their catalytic active sites. Therefore, adopting ambient pressure conditions for enzyme immobilization and conducting conversion reactions under low pressure may help realize the full benefits of enzyme immobilization.
Biocatalytic membrane reactors operate under milder conditions, making them more sustainable than traditional reactors. The membrane helps with catalyst separation, reusability, and product separation, which facilitates purification. However, their scalability is limited by reaction rates and the stability of biocatalysts. While they excel in selectivity and sustainability, enzyme deactivation or microbial growth can reduce productivity. In contrast, traditional thermocatalytic reactors achieve higher productivity and are better suited for large-scale CO2 conversion, although they require high temperatures and pressures. Biocatalytic systems offer moderate productivity in controlled environments but do not match the throughput of thermocatalytic or electrochemical systems. More focus should be placed on developing low-temperature, high-efficiency, and robust thermocatalytic membrane reactors for large-scale, profitable CO2 conversion technologies.
11.2. Enhanced oil recovery
Enhanced oil recovery (EOR) is one of the most prominent and established methods of Carbon Capture and Utilization (CCU), playing a crucial role in both improving oil extraction efficiency and mitigating CO2 emissions. The process involves injecting captured CO2 into existing oil reservoirs to enhance crude oil recovery, typically following primary and secondary recovery stages.509 Through EOR, CO2 acts as a solvent that reduces the viscosity of the trapped oil and increases its mobility, enabling the extraction of otherwise inaccessible oil. In this process, a significant portion of the injected CO2 remains sequestered underground, contributing to carbon storage while simultaneously increasing oil yield.510,511The concept of EOR is grounded in the principle that the injection of CO2 can improve oil displacement efficiency within the reservoir. CO2-EOR is categorized into two main types:
(i) Miscible CO2-EOR: this occurs when CO2 fully dissolves in the crude oil, reducing its viscosity and increasing the oil's mobility. Miscibility typically occurs under high-pressure conditions. The injected CO2 mixes with the oil, lowering its interfacial tension and causing the oil to swell, thus improving its flow toward the production wells.512
(ii) Immiscible CO2-EOR: in cases where reservoir conditions do not allow full miscibility, CO2 can still enhance oil recovery by displacing oil through its sheer pressure and causing the oil to move toward production wells. Although less efficient than miscible EOR, this method still improves recovery compared to conventional methods.512,513
EOR projects have been implemented in numerous regions worldwide, including North America, the Middle East, and Southeast Asia. In the United States, the Permian Basin is a leading example of CO2-EOR deployment, where captured CO2 from industrial sources is injected into mature oil fields. Approximately 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 barrels of oil per day were produced through CO2-EOR in the U.S. as of 2019.514 With the rising demand for carbon management solutions, the application of CO2-EOR is expected to expand globally, particularly in regions with declining conventional oil reserves. It is worth noting that a major share of the injected CO2 remains permanently sequestered underground, contributing to carbon storage while simultaneously increasing oil yield. However, the focus on EOR as a justification for CO2 sequestration requires a broader perspective, as many industrial carbon capture applications prioritize sequestration over oil recovery. For instance, facilities like the waste-to-energy plant at Klemetsrud in Norway and petrochemical plants in Europe focus primarily on capturing CO2 for permanent sequestration rather than utilizing it for EOR. The facility at Oslo, is set to become the world's first waste-to-energy plant with full-scale CCS by 2026, targeting the capture of 400
000 barrels of oil per day were produced through CO2-EOR in the U.S. as of 2019.514 With the rising demand for carbon management solutions, the application of CO2-EOR is expected to expand globally, particularly in regions with declining conventional oil reserves. It is worth noting that a major share of the injected CO2 remains permanently sequestered underground, contributing to carbon storage while simultaneously increasing oil yield. However, the focus on EOR as a justification for CO2 sequestration requires a broader perspective, as many industrial carbon capture applications prioritize sequestration over oil recovery. For instance, facilities like the waste-to-energy plant at Klemetsrud in Norway and petrochemical plants in Europe focus primarily on capturing CO2 for permanent sequestration rather than utilizing it for EOR. The facility at Oslo, is set to become the world's first waste-to-energy plant with full-scale CCS by 2026, targeting the capture of 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of CO2 annually. The project is part of Norway's ‘Longship’ initiative and demonstrates the potential for significant emission reductions in waste incineration through CCS.515,516 On the other hand, projects such as the Port Arthur, Texas Carbon Capture Project demonstrate how CO2 is captured, transported, and injected into geologic formations for long-term storage, with no connection to oil recovery. These cases demonstrate that the industrial demand for carbon capture extends beyond EOR and into permanent sequestration strategies to address climate change. In the Port Arthur Carbon Capture Project, Air Products and Chemicals retrofitted two steam methane reformers at their hydrogen production facility in Port Arthur to capture over 90% of CO2 emissions. Since 2013, the project has captured approximately 1 million tonnes of CO2 annually, which is transported via pipeline for use in EOR operations.517,518
000 tonnes of CO2 annually. The project is part of Norway's ‘Longship’ initiative and demonstrates the potential for significant emission reductions in waste incineration through CCS.515,516 On the other hand, projects such as the Port Arthur, Texas Carbon Capture Project demonstrate how CO2 is captured, transported, and injected into geologic formations for long-term storage, with no connection to oil recovery. These cases demonstrate that the industrial demand for carbon capture extends beyond EOR and into permanent sequestration strategies to address climate change. In the Port Arthur Carbon Capture Project, Air Products and Chemicals retrofitted two steam methane reformers at their hydrogen production facility in Port Arthur to capture over 90% of CO2 emissions. Since 2013, the project has captured approximately 1 million tonnes of CO2 annually, which is transported via pipeline for use in EOR operations.517,518
Increased oil recovery: CO2-EOR can increase the amount of recoverable oil from a reservoir by 10–20% beyond conventional methods. This represents a substantial economic benefit for oil producers.
Carbon storage: a major advantage of CO2-EOR is its dual role in both enhancing oil recovery and sequestering CO2 underground. Estimates suggest that for every ton of CO2 injected, 0.5–0.7 tons can remain permanently stored.
CO2 recycling: during the EOR process, a portion of the injected CO2 is produced along with the oil, but it can be captured, separated, and re-injected back into the reservoir, further improving the CO2 utilization efficiency.
Despite the high promise, the following challenges remain:521,522
Reservoir suitability: the success of CO2-EOR depends heavily on the characteristics of the reservoir, including pressure, temperature, and rock properties. Not all oil fields are suitable for CO2 injection, and achieving miscibility may require very high pressures, making the process energy-intensive.
CO2 availability and infrastructure: a reliable supply of captured CO2 is essential for large-scale CO2-EOR operations. Establishing pipelines and storage facilities to transport CO2 from industrial sources to oil fields requires substantial upfront investment.
Economic viability: the financial benefits of EOR depend on oil prices and the cost of CO2 capture and transportation. While CO2-EOR can be profitable under favorable economic conditions, fluctuating oil prices threaten its long-term viability.
Furthermore, while CO2-EOR offers a temporary solution for utilizing captured CO2, it does not eliminate the need to transition away from fossil fuel dependence in the long term. The process, by increasing oil production, paradoxically contributes to higher overall carbon emissions from the combustion of the additional oil produced. As a result, EOR must be seen as part of a broader strategy for carbon management, in conjunction with other forms of storage and utilization technologies aimed at achieving net-zero emissions.525,526
12. Technology readiness level of membrane technologies for CCUS and impact measurement
CCUS (Carbon Capture, Utilization, and Storage) processes can be evaluated from multiple perspectives: environmental impact, feasibility and scalability, economic viability, and technology readiness. The Technology Readiness Level (TRL) serves as a classification tool to determine a technology's maturity. In general, the earlier TRL stages raise questions regarding a technology's likelihood of success, while higher TRL levels indicate commercial viability and practical scalability. However, it is crucial to note that a higher TRL does not necessarily equate to an optimal process or a flawless solution. For example, amine-based absorption, with a TRL of 9, is widely adopted in various industrial sectors but remains energy-intensive and economically unfeasible for smaller-scale applications.99,529 Advances in the technical aspects of any field, process, or technology will influence both its TRL level and the overall costs associated with CCUS. Table 14 highlights the progress made in CCUS TRL levels since 2014 and their current status.| Technology | TRL 2014 | TRL (current) | Examples of current practices | Comments | |
|---|---|---|---|---|---|
| Common amine solvents | 9 | 9 | Widely used in fertilizer, soda ash, natural gas processing plants, e.g. Sleipner, Snøhvit, and used in Boundary Dam since 2014 | Commonly practiced in different industries, perfect for large-scale applications, energy extensive solvent regeneration, sensitivity of chemicals to impurities and oxygen, | |
| Physical solvent (Selexol, Rectisol) | 9 | 9 | Widely used in natural gas processing, coal gasification plants, e.g. Val Verde, Shute Creek, Century Plant, Coffeyville Gasification, Great Plains Synfuels Plant, Lost Cabin Gas plant | CAPEX and OPEX reductions are still a concern, | |
| Sterically hindered amine | 6–8 | 6–9 | Demonstration to commercial plants depending on technology providers, e.g. Petra Nova carbon capture | The environmental impact of the harmful chemicals is still a barrier | |
| Amino acid-based solvent*/Precipitating solvents | 4–5 | 4–5 | Lab test to conceptual studies | ||
| Ionic liquids | 1 | 2–3 | Lab tests | ||
| Solid adsorbents | Pressure swing | 3 | 9 | Air Products Port Arthur SMR CCS | |
| Adsorption/vacuum | |||||
| Swing adsorption | |||||
| Temperature swing | 1 | 5–7 | Large pilot tests to FEED studies for commercial plants | ||
| Adsorption (TSA) | |||||
| Electrochemically | 1 | 1 | Lab tests only | ||
| Mediated | |||||
| Adsorption | |||||
| Chemical looping | Calcium looping | 6 | 6–7 | Feasibility/cost studies for commercial scale | The technology uses a very cheap and abundant sorbent. The sorbent is susceptible to chemical deactivation due to competing reactions and deterioration in capture capacity |
| Chemical looping combustion | 2 | 5–6 | Pilot tests | ||
| Bioprocesses | Carbon biofixation | — | 4–6 | Microalgae cultivation and biomass co-firing for power generation | |
| Cryogenic | Cryogenic packed bed/antisublimation system | — | 3–4 | Well-developed for natural gas decarbonization, uncertain to apply for post-combustion flue gas | |
| Membranes | Gas separation membranes for natural gas processing | — | 9 | Petrobras santos basin pre-salt oil field CCS | Well-developed lab studies and a few pilot scale plants with variable CAPEX and OPEX depending on the scale |
| Polymeric | 6 | 7 | FEED studies for large pilots | ||
| Membranes | |||||
| Electrochemical membrane integrated with MCFCs | — | 7 | Large pilots at plant barry | ||
| Polymeric membranes/cryogenic separation hybrid | 6 | 6 | Pilot studies | ||
| Polymeric membranes/solvent hybrid | — | 4 | Conceptual studies | ||
| Room temperature | 2 | 2 | Lab test | ||
| Ionic liquid (RTIL) | |||||
| Membranes | |||||
| Inorganic membranes | — | 3 | Lab test | ||
| Facilitated transport membranes | — | 6–7 | The pre-pilot field testing was implemented at the cement industry | ||
A closer examination of technologies within different sectors of the CCUS industry provides a clearer perspective on where each separation or utilization technology stands in terms of TRL. However, a region-specific experience can yield a more realistic evaluation of these technologies since cases are more practically assessed in local contexts. For instance, a report from the Government of Alberta presents the TRL status of existing technologies for CO2 separation and utilization. Polymeric membranes are currently at the pilot study stage, but the operational scale may be smaller for high-performance FTM materials compared to other membrane types (Fig. 32). This underscores the significance of simultaneously exploring several promising polymeric membrane candidates to leverage a larger operational scale.
 | ||
| Fig. 32 Technology readiness level of (a) carbon capture technologies, (b) utilization technologies, reported by Alberta Innovates531 | ||
An essential insight from the Canadian Government's comprehensive study (carried out by Emission Reduction Alberta (ERA) and Alberta Innovates (AI)) is that the success of CO2 utilization is strongly linked to the effectiveness of large-scale carbon capture technologies. Consequently, the scalability of membrane separation technologies for small- and medium-sized processes is a significant advantage. The utilization of captured carbon is projected to account for around 10% of the CCUS market, indicating that further technological and economic advancements in membrane-based utilization technologies are necessary to develop more practical applications.
The recent surge in interest in various carbon capture processes stems from climate change mitigation policies and the push towards sustainable development goals (SDGs) established both internationally and locally. Different approaches exist to measure various aspects of sustainability, with the most well-known being the triple bottom line (TBL) approach. TBL encompasses environmental, economic, and social dimensions of sustainability. Environmental sustainability focuses on measuring and reducing carbon and ecological footprints, preventing resource depletion, conserving biodiversity, and monitoring air, water, and soil pollution. Social sustainability addresses quality of life, equity, community well-being, and income distribution. Economic sustainability targets gross domestic product (GDP) and its adjustments to provide a holistic measure of economic progress, investment in renewable resources and efficiency, and the assessment of public and private debt relative to economic output.
Another important approach is the Environmental Life Cycle Impact (LCA), which evaluates the full process of a technology or product. A recent study has compared CCUS technologies for power plant decarbonization, including a membrane hybrid process.532 Most LCA studies use the “cradle to grave” perspective, covering the entire lifecycle from raw material extraction to final disposal. Table 15 provides summarized data to offer comparative insights into the scale and environmental impacts of these projects. While this review emphasizes the importance of case sensitivity in CCUS processes and cautions against drawing broad conclusions, it is crucial to note that the use of membranes-either as standalone systems or in hybrid processes—can significantly reduce environmental impact. Membrane-based technologies demonstrate high capacity, suggesting their practicality for industries producing substantial emissions. However, focusing solely on power generation emissions is only part of the picture; a comprehensive assessment of other industries and processes is necessary, as suggested by the framework from Cuéllar-Franca et al.532
| Case | Process | Storage or utilization | Functional unita | Impact | Ref. |
|---|---|---|---|---|---|
| a A unit of electricity generated, expressed either in kW h, MW h or TW h. | |||||
| Cradle-to-grave LCA for coal-fired power plant | Chemical absorption, membrane and cryogenic separation, and pressure swing adsorption | Storage: geological and ocean | 1 MW h | Global warming potential | 533 |
| Acidification potential | |||||
| Cradle-to-grave LCA for pulverized coal-fired power plant | Chemical absorption | Storage: geological | 1 kW h | Abiotic depletion potential | 534 |
| Acidification potential | |||||
| Eutrophication potential | |||||
| Fresh water aquatic ecotoxicity potential | |||||
| Global warming potential | |||||
| Human toxicity potential | |||||
| Marine aquatic ecotoxicity potential | |||||
| Ozone depletion potential | |||||
| Photochemical ozone creation potential | |||||
| Terrestrial ecotoxicity potential | |||||
| Cradle-to-grave dynamic LCA of different power plants | Chemical absorption and oxy-fuel combustion | Storage: Ocean | 1 kW h | Acidification potential | 535 |
| Global warming potential | |||||
| Human toxicity potential | |||||
| Fresh water aquatic ecotoxicity potential | |||||
| Marine aquatic ecotoxicity potential | |||||
| Terrestrial ecotoxicity potential | |||||
| Cradle-to-grave dynamic LCA of different power plants | Chemical absorption and oxy-fuel combustion | Storage: Ocean | 1 MW h | Abiotic depletion potential | 536 |
| Acidification potential | |||||
| Eutrophication potential | |||||
| Global warming potential | |||||
| Human toxicity potential | |||||
| Marine aquatic ecotoxicity potential | |||||
| Ozone depletion potential | |||||
| Photochemical ozone creation potential | |||||
13. Outlook and concluding remarks
This review has provided an in-depth analysis of membrane technologies for CCUS applications, with a particular focus on polymeric, mixed-matrix, and emerging materials embedded with fillers such as MOFs and MXenes. Due to their compact design, high performance, and ease of scalability, membrance technologies are promising for carbon capture and separation in various industrial settings. Despite these advantages, several persistent challenges—such as plasticization, physical aging, and the inherent permeability-selectivity trade-off—must be addressed to fully realize the potential of membrane-based CCUS at scale.While conventional CCUS technologies like absorption–stripping, adsorption, and cryogenic methods continue to play a vital role, membrane-based approaches provide unique benefits that could make them the next frontier in CO2 capture. The development of advanced materials and hybrid membrane systems, combined with ongoing innovations in separation mechanisms and membrane design, signals significant progress. However, achieving industrial adoption will require further research to enhance selectivity, durability, and economic feasibility.
Future efforts should focus on translating laboratory-scale breakthroughs into commercial applications, with a strong emphasis on collaboration across academia, industry, and policy. Such partnerships are crucial for addressing current limitations, optimizing hybrid solutions, and advancing technology readiness. With sustained innovation and strategic investment, membrane-based CCUS technologies have the potential to significantly contribute to global decarbonization efforts, helping to curb greenhouse gas emissions and support long-term climate sustainability goals.
13.1. Critical challenges in membrane technology for CCUS
A more in-depth discussion on how different impurities could affect the performance of the polymeric membranes was offered previously. Looking at the concept from the point of membrane structure itself, highlights the importance of “degradation-resistant” membrane material design. The ideal gas separation membrane should be resistant to other degradation risks depending on the process in which it is going to be applied. As an instance, FTMs are prone to degradation due to reaction with NH3 and H2S.401 Acidic degradation of the polymeric membranes alters the free volume and changes the performance of the membrane. Chemical stability must be a major focus in the polymeric structure design for the membrane fabrication, as any undesired reaction between the functional sites of the polymer and the process stream could intensify the degradation.158,537 Age-induced degradation is also a concept described earlier in “Physical aging” section. Temperature-induced degradation is also a concern in precombustion CCS processes suppressing the lifetime of the membranes.537
Degradation affects the lifetime, efficiency, and overall cost-effectiveness of membrane-based CCUS processes. The cost of polymeric membranes ranges from $50 to $400 per m2, depending on material composition and fabrication complexity.150–155 MMMs could cost similarly depending on the nanomaterial loading and synthesis expenses.435 Compared to conventional amine-based absorption processes, membrane technology presents a lower operating cost due to its energy efficiency (2 to 5.5 GJ per ton CO2 less energy consumption (Table 4)) and reduced solvent handling. To be able to push the CCUS membrane technologies toward commercialization, degradation—whether thermal, chemical, or mechanical— as a major challenge influencing maintenance and replacement frequency must be considered. To address assess this challenge, Table 16 summarizes the economic implications of degradation in different membrane types, including projected replacement cycles and cost per ton of CO2 captured. It is worth mentioning that membrane-based technologies are highly process-dependent, i.e. the degradation profile and its effect on the scalability of the process highly depends on the operating conditions, harshness of the streams and the nature of the impurities. Optimizing materials and incorporating predictive AI/ML models for degradation forecasting can support material stability and boost economic feasibility. Nevertheless, more research should focus on developing stable, high-performance polymeric membranes with improved resistance to acidic gases (SO2, NOx) and thermal aging, which currently limit industrial scalability.
| Membrane type | Cost ($/m2) | Degradation rate (% per year) | Lifespan (years) | CO2 capture cost ($ per ton) | Key Challenges |
|---|---|---|---|---|---|
| Polymeric (e.g., Polyimide, PEBAX) | 50 | 10 | 2 | 30 | Prone to plasticization; lower thermal stability, short life time |
| Mixed matrix membrane (MMMs) | 120 | 6 | 3 | 30 | Dispersion issues; interface compatibility, reactivity of the nanomaterials with the impurities |
| Inorganic (e.g., Zeolite, MOF) | 300 | 3 | 4 | 40 | High fabrication cost; scalability challenges |
| Hybrid (polymeric + inorganic) | 200 | 4 | 4 | 30 | Optimization of polymer–inorganic interactions |
13.2. Potential solutions
13.3. Future prospects and research directions
The process design criteria, such as the number of separation stages and membrane performance, impacts on how cost-effective CO2 capture is. According to research, multi-stage membrane topologies offer a competitive recovery rate and purity levels while maximizing energy utilization, making them a strong substitute for conventional amine-based techniques. Process integration, operating pressure, and membrane selectivity are some of the variables that affect these systems' economic viability. It has been shown that two-stage membrane systems can recover up to 90% of CO2 with purity levels of 90–95% at capture costs between $32 and $45 per ton CO2. Further sophisticated multi-stage procedures can improve purity even further, but they come with higher energy and operational complexity costs. For example, surpassing 95% CO2 purity frequently calls for more separation processes, bigger membrane surfaces, and higher compression energy, all of which raise expenses. According to optimization models, two-stage and three-stage systems can offer a more realistic balance between cost and performance, even though four-stage systems offer greater separation efficiency.
Advances in membrane materials, better process integration, and the use of energy recovery techniques are likely to keep the CO2 capture prices on the decline going forward. Higher purity requirements (97–98%) are still difficult to meet, nevertheless, and necessitate careful balances between energy use and financial feasibility. To further lower costs and increase scalability, future research should concentrate on improving membrane materials, increasing system efficiency, and investigating hybrid capture systems.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. Sadrzadeh acknowledges the financial support for this work by the Natural Science and Engineering Research Council of Canada (NSERC) and Canada's Oil Sands Innovation Alliance (COSIA) under NSERC ALLRP 556293-20. J.-Y. Cho acknowledges the financial support by NRCan's Office of Energy Research and Development, National Research Council of Canada and Government of Canada. M. Soroush and M. Mozafari would like to acknowledge financial support from the U.S. National Science Foundation under Grant No. CMMI-2134607. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.References
- C. Bataille, N. Melton, S. Stiebert and P. Eng, The potential to decarbonize Canadian heavy industry Search PubMed.
- P. Warrian and A. Homeira, Innovation and de-carbonization in the Canadian steel industry, 2021 Search PubMed.
- T. Vass and M. Jaccard, Energy and Materials Research Group, Simon Fraser University, 2017 Search PubMed.
- K. Zaman and M. Abd-el Moemen, Renewable Sustainable Energy Rev., 2017, 74, 1119–1130 CrossRef.
- T. Wilberforce, A. G. Olabi, E. T. Sayed, K. Elsaid and M. A. Abdelkareem, Sci. Total Environ., 2021, 761, 143203 CrossRef CAS PubMed.
- R. P. Bhatt, Renewable hydropower technologies, 2017, 1, 75–98 Search PubMed.
- M. Aneke and M. Wang, Appl. Energy, 2016, 179, 350–377 CrossRef.
- S. Yu, L. Agbemabiese and J. Zhang, Appl. Energy, 2016, 165, 107–118 CrossRef.
- B. Metz, O. Davidson, H. De Coninck, M. Loos and L. Meyer, IPCC special report on carbon dioxide capture and storage, Cambridge University Press, Cambridge, 2005 Search PubMed.
- X. Xu, Y. Xu, J. Li, Y. Lu, A. Jenkins, R. C. Ferrier, H. Li, N. C. Stenseth, D. O. Hessen and L. Zhang, iScience, 2023, 26(6), 106798 CrossRef CAS PubMed.
- N. R. Kabange, Y. Kwon, S.-M. Lee, J.-W. Kang, J.-K. Cha, H. Park, G. D. Dzorkpe, D. Shin, K.-W. Oh and J.-H. Lee, Sustainability, 2023, 15, 15889 CrossRef CAS.
- L. Cameron and A. Carter, Why Carbon Capture and Storage Is Not a Net-Zero Solution for Canada’s Oil and Gas Sector, International Institute for Sustainable Development, 2023.
- T. Wilberforce, A. Baroutaji, B. Soudan, A. H. Al-Alami and A. G. Olabi, Sci. Total Environ., 2019, 657, 56–72 CrossRef CAS PubMed.
- R. Ben-Mansour, M. A. Habib, O. E. Bamidele, M. Basha, N. A. A. Qasem, A. Peedikakkal, T. Laoui and M. Ali, Appl. Energy, 2016, 161, 225–255 CrossRef CAS.
- M. G. Plaza, A. S. González, C. Pevida, J. J. Pis and F. Rubiera, Appl. Energy, 2012, 99, 272–279 CrossRef CAS.
- M.-B. Hägg and A. Lindbråthen, Ind. Eng. Chem. Res., 2005, 44, 7668–7675 CrossRef.
- A. Mousavinejad, A. Rahimpour, M. R. Shirzad Kebria, S. Khoshhal Salestan, M. Sadrzadeh and N. Tavajohi Hassan Kiadeh, Ind. Eng. Chem. Res., 2020, 59, 12834–12844 CrossRef CAS.
- F. Pazani, M. S. Maleh, M. Shariatifar, M. Jalaly, M. Sadrzadeh and M. Rezakazemi, Renewable Sustainable Energy Rev., 2022, 160, 112294 CrossRef CAS.
- R. S. K. Valappil, N. Ghasem and M. Al-Marzouqi, J. Ind. Eng. Chem., 2021, 98, 103–129 CrossRef.
- C. Z. Liang, T.-S. Chung and J.-Y. Lai, Prog. Polym. Sci., 2019, 97, 101141 CrossRef CAS.
- M. Rezakazemi, M. Sadrzadeh and T. Matsuura, Prog. Energy Combust. Sci., 2018, 66, 1–41 CrossRef.
- A. R. Kamble, C. M. Patel and Z. Murthy, Renewable Sustainable Energy Rev., 2021, 145, 111062 CrossRef CAS.
- K. Xie, Q. Fu, G. G. Qiao and P. A. Webley, J. Membr. Sci., 2019, 572, 38–60 CrossRef CAS.
- Y. Alqaheem, A. Alomair, M. Vinoba and A. Pérez, Int. J. Polym. Sci., 2017, 2017, 4250927 Search PubMed.
- B. Sasikumar, G. Arthanareeswaran and A. Ismail, J. Mol. Liq., 2018, 266, 330–341 CrossRef CAS.
- W. F. Yong and H. Zhang, Prog. Mater. Sci., 2021, 116, 100713 CrossRef CAS.
- G. Li, W. Kujawski, R. Válek and S. Koter, Int. J. Greenhouse Gas Control, 2021, 104, 103195 CrossRef CAS.
- G. Genduso, W. Ogieglo, Y. Wang and I. Pinnau, J. Membr. Sci., 2024, 122533 CrossRef CAS.
- H. Li and J. Yan, Appl. Energy, 2009, 86, 2760–2770 CrossRef CAS.
- Y. Zhang, T. Wang, W.-P. Pan and C. E. Romero, Advances in ultra-low emission control technologies for coal-fired power plants, Woodhead Publishing, 2019 Search PubMed.
- W. H. Organization, WHO global air quality guidelines: particulate matter (PM2. 5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide, World Health Organization, 2021 Search PubMed.
- P. Bains, P. Psarras and J. Wilcox, Prog. Energy Combust. Sci., 2017, 63, 146–172 CrossRef.
- M. Ouikhalfan, O. Lakbita, A. Delhali, A. H. Assen and Y. Belmabkhout, Energy Fuels, 2022, 36, 4198–4223 CrossRef CAS.
- R. Xu and B. Lin, J. Cleaner Prod., 2017, 140, 1330–1343 CrossRef CAS.
- M. Shen, L. Tong, S. Yin, C. Liu, L. Wang, W. Feng and Y. Ding, Sep. Purif. Technol., 2022, 121734 CrossRef CAS.
- J. G. Speight, Nat. Gas, 2019, 59–98 Search PubMed.
- Q. Yang, Q. Lin, S. Sammarchi, J. Li, S. Li and D. Wang, Greenhouse Gases: Sci. Technol., 2021, 11, 52–68 CrossRef CAS.
- Á. A. Ramírez-Santos, C. Castel and E. Favre, Sep. Purif. Technol., 2018, 194, 425–442 CrossRef.
- Á. A. Ramírez-Santos, C. Castel and E. Favre, J. Membr. Sci., 2017, 526, 191–204 CrossRef.
- H. Li, Ø. Wilhelmsen and J. Yan, Handbook of clean energy systems, 2015, pp. 1–17 Search PubMed.
- C. Zhao, J. Lv, G. Li, Q. Zhang, Y. Zhang, S. Liu and Y. Chi, Thermochim. Acta, 2019, 676, 20–26 CrossRef CAS.
- E. Luna-Ortiz, K. Szklarczyk-Marshall, M. Winter and E. McAllister-Fognini, Impact of hydrogen as impurity in the physical and transport properties of CO2 streams in CCS/CCUS transport systems: A technical discussion, in Proceedings of the 15th Greenhouse Gas Control Technologies Conference, 2021, pp. 15–18 Search PubMed.
- P. C. Chen and Y.-L. Lai, Energies, 2019, 12, 2202 CrossRef CAS.
- Z. Liang, K. Fu, R. Idem and P. Tontiwachwuthikul, Chin. J. Chem. Eng., 2016, 24, 278–288 CrossRef CAS.
- M. Babar, M. A. Bustam, A. Ali, A. S. Maulud, U. Shafiq, A. Mukhtar, S. N. Shah, K. Maqsood, N. Mellon and A. M. Shariff, Cryogenics, 2019, 102, 85–104 CrossRef CAS.
- R. Soltani, M. Rosen and I. Dincer, Int. J. Hydrogen Energy, 2014, 39, 20266–20275 CrossRef CAS.
- X. Wu, C. Wu and S. Wu, Chem. Eng. Res. Des., 2015, 96, 150–157 CrossRef CAS.
- S. Cloete, M. N. Khan and S. Amini, Int. J. Hydrogen Energy, 2019, 44, 3492–3510 CrossRef CAS.
- U. Zahid, S. S. Khalafalla, H. A. Alibrahim, U. Ahmed and A. G. A. Jameel, Energy Convers. Manage., 2023, 296, 117681 CrossRef CAS.
- A. A. Olajire, Energy, 2010, 35, 2610–2628 CrossRef CAS.
- T. C. Drage, C. E. Snape, L. A. Stevens, J. Wood, J. Wang, A. I. Cooper, R. Dawson, X. Guo, C. Satterley and R. Irons, J. Mater. Chem., 2012, 22, 2815–2823 RSC.
- D. Y. C. Leung, G. Caramanna and M. M. Maroto-Valer, Renewable Sustainable Energy Rev., 2014, 39, 426–443 CrossRef CAS.
- P. Fennell and B. Anthony, Calcium and chemical looping technology for power generation and carbon dioxide (CO2) capture, Elsevier, 2015 Search PubMed.
- Z. H. Liang, W. Rongwong, H. Liu, K. Fu, H. Gao, F. Cao, R. Zhang, T. Sema, A. Henni and K. Sumon, Int. J. Greenhouse Gas Control, 2015, 40, 26–54 CrossRef CAS.
- R. Stanger, T. Wall, R. Spörl, M. Paneru, S. Grathwohl, M. Weidmann, G. Scheffknecht, D. McDonald, K. Myöhänen and J. Ritvanen, Int. J. Greenhouse Gas Control, 2015, 40, 55–125 CrossRef CAS.
- H. I. Mathekga, B. O. Oboirien and B. C. North, Int. J. Energy Res., 2016, 40, 878–902 CrossRef CAS.
- M. Ditaranto and J. Hals, Combust. Flame, 2006, 146, 493–512 CrossRef CAS.
- H. Liu, R. Zailani and B. M. Gibbs, Fuel, 2005, 84, 833–840 CrossRef CAS.
- E. Koohestanian and F. Shahraki, J. Environ. Chem. Eng., 2021, 9, 105777 CrossRef CAS.
- A. Gopan, P. Verma, Z. Yang and R. L. Axelbaum, Int. J. Greenhouse Gas Control, 2020, 95, 102936 CrossRef CAS.
- Y. Tan, K. V. Thambimuthu, M. A. Douglas and R. Mortazavi.
- M. Simmonds, I. Miracca and K. Gerdes, in Greenhouse Gas Control Technologies 7, Elsevier, 2005, pp. 1125–1130 Search PubMed.
- F. Châtel-Pélage, R. Varagani, P. Pranda, N. Perrin, H. Farzan, S. J. Vecci, L. U. Yongqi, S. Chen, M. Rostam-Abadi and A. C. Bose, Therm. Sci., 2006, 10, 119–142 CrossRef.
- M. B. Toftegaard, J. Brix, P. A. Jensen, P. Glarborg and A. D. Jensen, Prog. Energy Combust. Sci., 2010, 36, 581–625 CrossRef CAS.
- S. Seddighi, P. T. Clough, E. J. Anthony, R. W. Hughes and P. Lu, Appl. Energy, 2018, 232, 527–542 CrossRef CAS.
- M. M. Hossain and H. I. de Lasa, Chem. Eng. Sci., 2008, 63, 4433–4451 CrossRef CAS.
- C.-C. Cormos, Int. J. Hydrogen Energy, 2012, 37, 13371–13386 CrossRef CAS.
- L. Zhu, Y. He, L. Li and P. Wu, Energy, 2018, 144, 915–927 CrossRef CAS.
- H. J. Richter and K. F. Knoche, Reversibility of combustion processes, ACS Publications, 1983 Search PubMed.
- J. Hu, V. V. Galvita, H. Poelman and G. B. Marin, Materials, 2018, 11, 1187 CrossRef PubMed.
- M. Qasim, M. Ayoub, N. A. Ghazali, A. Aqsha and M. Ameen, Ind. Eng. Chem. Res., 2021, 60, 8621–8641 CrossRef CAS.
- Z. Cheng, L. Qin, J. A. Fan and L.-S. Fan, Engineering, 2018, 4, 343–351 CrossRef CAS.
- J. Dai and K. J. Whitty, Chem. Eng. Process., 2022, 174, 108902 CrossRef CAS.
- R. D. Solunke and G. t Veser, Ind. Eng. Chem. Res., 2010, 49, 11037–11044 CrossRef CAS.
- N. Ruthwik, D. Kavya, A. Shadab, N. Lingaiah and C. Sumana, Chem. Eng. Process., 2020, 153, 107959 CrossRef CAS.
- M. Erans, E. S. Sanz-Pérez, D. P. Hanak, Z. Clulow, D. M. Reiner and G. A. Mutch, Energy Environ. Sci., 2022, 15, 1360–1405 RSC.
- C. Intervention, Committee on Geo engineering Climate: Technical Evaluation and Discussion of Impacts, 2015.
- O. Al Yafiee, F. Mumtaz, P. Kumari, G. N. Karanikolos, A. Decarlis and L. F. Dumée, Chem. Eng. J., 2024, 154421 CrossRef.
- Z. Li, X. Qin, Y. Li, H. Su, W. Zhang, G. Xu, Q. Ma, L. Hua and Q. Xu, Future Batteries, 2024, 100020 Search PubMed.
- P. Yuan, Z. Qiu and J. Liu Recent enlightening strategies for CO2 capture: a review, in IOP Conference Series: Earth and Environmental Science, IOP Publishing., 2017, vol. 64, no. 1, p. 012046 Search PubMed.
- X. Wang and C. Song, Front. Energy Res., 2020, 8, 560849 CrossRef.
- W. Y. Hong, Carbon Capture Sci. Technol., 2022, 3, 100044 CrossRef CAS.
- Z. Abubakar, E. M. Mokheimer and M. M. Kamal, Int. J. Energy Res., 2021, 45, 17461–17479 CrossRef CAS.
- L. M. Romeo, S. Espatolero and I. Bolea, Int. J. Greenhouse Gas Control, 2008, 2, 563–570 CrossRef CAS.
- B. Aghel, S. Sahraie and E. Heidaryan, Energy, 2020, 201, 117618 CrossRef CAS.
- M. Sharif, T. Zhang, X. Wu, Y. Yu and Z. Zhang, Int. J. Greenhouse Gas Control, 2020, 97, 103059 CrossRef CAS.
- B. Aghel, S. Janati, S. Wongwises and M. S. Shadloo, Int. J. Greenhouse Gas Control, 2022, 119, 103715 CrossRef CAS.
- N. J. M. C. Penders-van Elk, E. S. Hamborg, P. J. G. Huttenhuis, S. Fradette, J. A. Carley and G. F. Versteeg, Int. J. Greenhouse Gas Control, 2013, 12, 259–268 CrossRef CAS.
- F. A. Chowdhury, H. Okabe, S. Shimizu, M. Onoda and Y. Fujioka, Energy Procedia, 2009, 1, 1241–1248 CrossRef CAS.
- F. A. Chowdhury, H. Okabe, H. Yamada, M. Onoda and Y. Fujioka, Energy Procedia, 2011, 4, 201–208 CrossRef CAS.
- F. A. Chowdhury, H. Yamada, T. Higashii, K. Goto and M. Onoda, Ind. Eng. Chem. Res., 2013, 52, 8323–8331 CrossRef CAS.
- F. Porcheron, A. Gibert, M. Jacquin, P. Mougin, A. Faraj, A. Goulon, P.-A. Bouillon, B. Delfort, D. Le Pennec and L. Raynal, Energy Procedia, 2011, 4, 15–22 CrossRef CAS.
- F. Porcheron, A. Gibert, P. Mougin and A. Wender, Environ. Sci. Technol., 2011, 45, 2486–2492 CrossRef CAS PubMed.
- G. Puxty, R. Rowland, A. Allport, Q. Yang, M. Bown, R. Burns, M. Maeder and M. Attalla, Environ. Sci. Technol., 2009, 43, 6427–6433 CrossRef CAS PubMed.
- P. Singh, J. P. M. Niederer and G. F. Versteeg, Chem. Eng. Res. Des., 2009, 87, 135–144 CrossRef CAS.
- S. Park, H.-J. Song, M.-G. Lee and J. Park, Korean J. Chem. Eng., 2014, 31, 125–131 CrossRef CAS.
- S.-Y. Oh, S. Yun and J.-K. Kim, Appl. Energy, 2018, 216, 311–322 CrossRef CAS.
- J. D. Figueroa, T. Fout, S. Plasynski, H. McIlvried and R. D. Srivastava, Int. J. Greenhouse Gas Control, 2008, 2, 9–20 CrossRef CAS.
- N. MacDowell, N. Florin, A. Buchard, J. Hallett, A. Galindo, G. Jackson, C. S. Adjiman, C. K. Williams, N. Shah and P. Fennell, Energy Environ. Sci., 2010, 3, 1645–1669 RSC.
- H. Zhai and E. S. Rubin, Environ. Sci. Technol., 2013, 47, 3006–3014 CrossRef CAS PubMed.
- I. Iliuta and M. C. Iliuta, AIChE J., 2017, 63, 2996–3007 CrossRef CAS.
- P. Brandl, M. Bui, J. P. Hallett and N. Mac Dowell, Int. J. Greenhouse Gas Control, 2022, 120, 103771 CrossRef CAS.
- C. C. Chatziasteriou, E. S. Kikkinides and M. C. Georgiadis, Comput. Chem. Eng., 2022, 107938 CrossRef CAS.
- I. P. Koronaki, L. Prentza and V. Papaefthimiou, Renewable Sustainable Energy Rev., 2015, 50, 547–566 CrossRef CAS.
- F. de Meyer and S. Jouenne, Curr. Opin. Chem. Eng., 2022, 38, 100868 CrossRef.
- R. Zhao, L. Liu, L. Zhao, S. Deng, S. Li and Y. Zhang, Renewable Sustainable Energy Rev., 2019, 114, 109285 CrossRef CAS.
- M. Songolzadeh, M. T. Ravanchi and M. Soleimani, Int. J. Chem. Biomol. Eng., 2012, 6, 906–913 Search PubMed.
- N. Hedin, L. Andersson, L. Bergström and J. Yan, Appl. Energy, 2013, 104, 418–433 CrossRef CAS.
- M. K. Mondal, H. K. Balsora and P. Varshney, Energy, 2012, 46, 431–441 CrossRef CAS.
- A. S. Holmes, B. C. Price, J. M. Ryan and R. E. Styring, Oil Gas J., 1983, 81(26), 85–86 CAS.
- M. T. Besong, M. M. Maroto-Valer and A. J. Finn, Int. J. Greenhouse Gas Control, 2013, 12, 441–449 CrossRef CAS.
- C. Font-Palma, O. Errey, C. Corden, H. Chalmers, M. Lucquiaud, M. S. del Rio, S. Jackson, D. Medcalf, B. Livesey and J. Gibbins, Process Saf. Environ. Prot., 2016, 103, 455–465 CrossRef CAS.
- A. Ali, K. Maqsood, N. Syahera, A. B. M. Shariff and S. Ganguly, Chem. Eng. Technol., 2014, 37, 1675–1685 CrossRef CAS.
- C. Font-Palma, D. Cann and C. Udemu, C, 2021, 7, 58 CAS.
- C. Song, Q. Liu, S. Deng, H. Li and Y. Kitamura, Renewable Sustainable Energy Rev., 2019, 101, 265–278 CrossRef CAS.
- C. Ampelli, S. Perathoner and G. Centi, Philos. Trans. R. Soc., A, 2015, 373, 20140177 CrossRef PubMed.
- A. Alaswad, M. Dassisti, T. Prescott and A. G. Olabi, Renewable Sustainable Energy Rev., 2015, 51, 1446–1460 CrossRef CAS.
- W. Y. Cheah, P. L. Show, J.-S. Chang, T. C. Ling and J. C. Juan, Bioresour. Technol., 2015, 184, 190–201 CrossRef CAS PubMed.
- S.-H. Ho, W.-M. Chen and J.-S. Chang, Bioresour. Technol., 2010, 101, 8725–8730 CrossRef CAS PubMed.
- S.-H. Ho, C.-Y. Chen, D.-J. Lee and J.-S. Chang, Biotechnol. Adv., 2011, 29, 189–198 CrossRef CAS PubMed.
- G. Hu, N. J. Nicholas, K. H. Smith, K. A. Mumford, S. E. Kentish and G. W. Stevens, Int. J. Greenhouse Gas Control, 2016, 53, 28–40 CrossRef CAS.
- X. Xu, X. Gu, Z. Wang, W. Shatner and Z. Wang, Renewable Sustainable Energy Rev., 2019, 110, 65–82 CrossRef CAS.
- S. S. Warudkar, K. R. Cox, M. S. Wong and G. J. Hirasaki, Int. J. Greenhouse Gas Control, 2013, 16, 342–350 CrossRef CAS.
- J. Liu, D. S.-H. Wong, S.-S. Jang and Y.-T. Shen, J. Taiwan Inst. Chem. Eng., 2017, 73, 12–19 CrossRef CAS.
- J. Pirklbauer, G. Schöny, F. Zerobin, T. Pröll and H. Hofbauer, Energy Procedia, 2017, 114, 2173–2181 CrossRef CAS.
- D. Bahamón García, A. Díaz-Márquez, P. Gamallo Belmonte and L. F. Vega, Chem. Eng. J., 2018, 342, 458–473 CrossRef.
- R. Haghpanah, R. Nilam, A. Rajendran, S. Farooq and I. A. Karimi, AIChE J., 2013, 59, 4735–4748 CrossRef CAS.
- B. Wu, X. Zhang, Y. Xu, D. Bao and S. Zhang, J. Cleaner Prod., 2015, 101, 251–261 CrossRef CAS.
- M. Shen, L. Tong, S. Yin, C. Liu, L. Wang, W. Feng and Y. Ding, Sep. Purif. Technol., 2022, 299, 121734 CrossRef CAS.
- K. Nguyen, I. Iliuta, L.-C. Pasquier, F. Bougie and M. C. Iliuta, Appl. Energy, 2024, 376, 124207 CrossRef CAS.
- J. Pires, M. Alvim-Ferraz, F. Martins and M. Simões, Renewable Sustainable Energy Rev., 2012, 16, 3043–3053 CrossRef CAS.
- J. Fu, N. R. Ahmad, C. P. Leo, J. M. Aberilla, I. D. Cruz, B. Alamani and S. P. Koh, Gas Sci. Eng., 2024, 205401 CrossRef CAS.
- T. Graham, London, Edinburgh Dublin Philos. Mag. J. Sci., 1866, 32, 401–420 CrossRef.
- S. Sourirajan, Nature, 1964, 203, 1348–1349 CrossRef CAS.
- N. Du, H. B. Park, M. M. Dal-Cin and M. D. Guiver, Energy Environ. Sci., 2012, 5, 7306–7322 RSC.
- I. Sreedhar, R. Vaidhiswaran, B. M. Kamani and A. Venugopal, Renewable Sustainable Energy Rev., 2017, 68, 659–684 CrossRef CAS.
- P. Bernardo, E. Drioli and G. Golemme, Ind. Eng. Chem. Res., 2009, 48, 4638–4663 CrossRef CAS.
- M. Kanniche, R. Gros-Bonnivard, P. Jaud, J. Valle-Marcos, J.-M. Amann and C. Bouallou, Appl. Therm. Eng., 2010, 30, 53–62 CrossRef CAS.
- A. S. Bhown and B. C. Freeman, Environ. Sci. Technol., 2011, 45, 8624–8632 CrossRef CAS PubMed.
- V. Kulshrestha, K. Awasthi, N. K. Acharya, M. Singh, D. K. Avasthi and Y. K. Vijay, Desalination, 2006, 195, 273–280 CrossRef CAS.
- M. Pera-Titus, Chem. Rev., 2014, 114, 1413–1492 CrossRef CAS PubMed.
- K. Keizer, A. F. M. Leenaars and A. J. Burggraaf.
- N. Abdullah, M. A. Rahman, M. H. D. Othman, J. Jaafar and A. F. Ismail, Current Trends and Future Developments on (Bio-) Membranes, Elsevier, 2018, pp. 45–70 Search PubMed.
- M. R. Rahimpour, F. Samimi, A. Babapoor, T. Tohidian and S. Mohebi, Chem. Eng. Process., 2017, 121, 24–49 CrossRef CAS.
- S. Yun and S. T. Oyama, J. Membr. Sci., 2011, 375, 28–45 CrossRef CAS.
- H. Hanley, Trans. Faraday Soc., 1966, 62, 2395–2402 RSC.
- T. Finnigan and P. Skudder, Filtr. Sep., 1989, 26, 198–200 Search PubMed.
- S. Maley, Development of Ion Transport Membrane (ITM) Oxygen Technology for Integration in IGCC and Other Advanced Power Generation Systems, 2013.
- S. Smart, C. X. C. Lin, L. Ding, K. Thambimuthu and J. C. D. Da Costa, Energy Environ. Sci., 2010, 3, 268–278 RSC.
- V. Gitis and G. Rothenberg, Ceramic membranes: new opportunities and practical applications, John Wiley & Sons, 2016 Search PubMed.
- V. Singh, N. Meena, A. Golder and C. Das, Int. J. Coal Sci. Technol., 2016, 3, 226–234 CrossRef CAS.
- W. J. Koros and R. Mahajan, J. Membr. Sci., 2000, 175, 181–196 CrossRef CAS.
- B. Bhide and S. Stern, J. Membr. Sci., 1991, 62, 13–35 CrossRef CAS.
- S. Jana, M. Purkait and K. Mohanty, Appl. Clay Sci., 2010, 47, 317–324 CrossRef CAS.
- B. Nandi, R. Uppaluri and M. Purkait, Appl. Clay Sci., 2008, 42, 102–110 CrossRef CAS.
- N. Norahim, P. Yaisanga, K. Faungnawakij, T. Charinpanitkul and C. Klaysom, Chem. Eng. Technol., 2018, 41, 211–223 CrossRef CAS.
- J. Sánchez-Laínez, B. Zornoza, S. Friebe, J. Caro, S. Cao, A. Sabetghadam, B. Seoane, J. Gascon, F. Kapteijn and C. Le Guillouzer, J. Membr. Sci., 2016, 515, 45–53 CrossRef.
- K. Scott, Handbook of industrial membranes, Elsevier, 1995 Search PubMed.
- S. M. Samaei, S. Gato-Trinidad and A. Altaee, Sep. Purif. Technol., 2018, 200, 198–220 CrossRef CAS.
- R. Rautenbach and W. Dahm, J. Membr. Sci., 1986, 28, 319–327 CrossRef CAS.
- D. F. Sanders, Z. P. Smith, R. Guo, L. M. Robeson, J. E. McGrath, D. R. Paul and B. D. Freeman, Polymer, 2013, 54, 4729–4761 CrossRef CAS.
- L. M. Robeson, Q. Liu, B. D. Freeman and D. R. Paul, J. Membr. Sci., 2015, 476, 421–431 CrossRef CAS.
- J. C.-Y. Chen, University of Waterloo, 2002.
- M. Farnam, H. Mukhtar and A. Mohd Shariff, Appl. Mech. Mater., 2014, 625, 701–703 Search PubMed.
- B. Freeman and I. Pinnau, Trends Polym. Sci., 1997, 5, 167–173 CAS.
- X. Kong and J. Liu, J. Phys. Chem. C, 2019, 123, 15113–15121 CrossRef CAS.
- S. R. Wickramasinghe, M. J. Semmens and E. L. Cussler, Hollow fiber modules made with hollow fiber fabric, J. Membr. Sci., 1993, 84(1–2), 1–14 CrossRef CAS.
- Y. Han and W. W. Ho, J. Polym. Eng., 2020, 40, 529–542 CrossRef CAS.
- H. B. Park, J. Kamcev, L. M. Robeson, M. Elimelech and B. D. Freeman, Science, 2017, 356, eaab0530 CrossRef PubMed.
- M. G. Buonomenna, W. Yave and G. Golemme, RSC Adv., 2012, 2, 10745–10773 RSC.
- Z. Yang, W. Guo, S. M. Mahurin, S. Wang, H. Chen, L. Cheng, K. Jie, H. M. Meyer, D.-E. Jiang and G. Liu, Chem, 2020, 6, 631–645 CAS.
- P. M. Budd, N. B. McKeown and D. Fritsch, J. Mater. Chem., 2005, 15, 1977–1986 RSC.
- H. B. Park, C. H. Jung, Y. M. Lee, A. J. Hill, S. J. Pas, S. T. Mudie, E. Van Wagner, B. D. Freeman and D. J. Cookson, Science, 2007, 318, 254–258 CrossRef CAS PubMed.
- S. Sridhar, B. Smitha and T. Aminabhavi, Sep. Purif. Rev., 2007, 36, 113–174 CrossRef CAS.
- H. Lin, E. Van Wagner, B. D. Freeman, L. G. Toy and R. P. Gupta, Science, 2006, 311, 639–642 CrossRef CAS PubMed.
- A. Javaid, Chem. Eng. J., 2005, 112, 219–226 CrossRef CAS.
- J. Y. Park and D. R. Paul, J. Membr. Sci., 1997, 125, 23–39 CrossRef CAS.
- W. M. Lee, Polym. Eng. Sci., 1980, 20, 65–69 CrossRef CAS.
- A. v Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- D. W. Van Krevelen and K. Te Nijenhuis, Properties of polymers: their correlation with chemical structure; their numerical estimation and prediction from additive group contributions, Elsevier, 2009 Search PubMed.
- A. Singh, B. D. Freeman and I. Pinnau, J. Polym. Sci., Part B: Polym. Phys., 1998, 36, 289–301 CrossRef CAS.
- S. Deveci, Y. Oksuz, T. Birtane and M. Oner, Polym. Test., 2016, 55, 287–296 CrossRef CAS.
- R. Abedini, A. Mosayebi and M. Mokhtari, Process Saf. Environ. Prot., 2018, 114, 229–239 CrossRef CAS.
- P. J. Roman, F. Detlev, K. Thomas and P. Klaus-Viktor, J. Membr. Sci., 2012, 389, 343–348 CrossRef.
- R. Wang, C. Cao and T.-S. Chung, J. Membr. Sci., 2002, 198, 259–271 CrossRef CAS.
- M. Wang, Z. Wang, S. Zhao, J. Wang and S. Wang, Chin. J. Chem. Eng., 2017, 25, 1581–1597 CrossRef.
- C. Yeom, S. Lee and J. Lee, J. Appl. Polym. Sci., 2000, 78, 179–189 CrossRef CAS.
- S. Maghami, A. Mehrabani-Zeinabad, M. Sadeghi, J. Sánchez-Laínez, B. Zornoza, C. Téllez and J. Coronas, Chem. Eng. Sci., 2019, 205, 58–73 CrossRef CAS.
- S. R. Reijerkerk, K. Nijmeijer, C. P. Ribeiro Jr, B. D. Freeman and M. Wessling, J. Membr. Sci., 2011, 367, 33–44 CrossRef CAS.
- A. E. Gemeda, M. G. De Angelis, N. Du, N. Li, M. D. Guiver and G. C. Sarti, J. Membr. Sci., 2017, 524, 746–757 CrossRef CAS.
- G. Zhang and H. Lin, Green Energy Environ., 2023, 9(8), 1220–1238 CrossRef.
- A. T. Bridge, B. J. Pedretti, J. F. Brennecke and B. D. Freeman, J. Membr. Sci., 2022, 644, 120173 CrossRef CAS.
- Z. Dai, L. Ansaloni and L. Deng, Green Energy Environ., 2016, 1, 102–128 CrossRef.
- D. Wu, L. Zhao, V. K. Vakharia, W. Salim and W. W. Ho, J. Membr. Sci., 2016, 510, 58–71 CrossRef CAS.
- V. Vakharia, W. Salim, D. Wu, Y. Han, Y. Chen, L. Zhao and W. W. Ho, J. Membr. Sci., 2018, 555, 379–387 CrossRef CAS.
- A. Van Der Pluijm, N. Miyagishima, E. Van Der Burg and Y. Itami, US Pat., 9962660, 2018 Search PubMed.
- Y. Han, W. Salim, K. K. Chen, D. Wu and W. W. Ho, J. Membr. Sci., 2019, 575, 242–251 CrossRef CAS.
- W. Salim, V. Vakharia, Y. Chen, D. Wu, Y. Han and W. W. Ho, J. Membr. Sci., 2018, 556, 126–137 CrossRef CAS.
- P. Li, H. Z. Chen and T.-S. Chung, J. Membr. Sci., 2013, 434, 18–25 CrossRef CAS.
- J.-J. Shieh and T. S. Chung, J. Membr. Sci., 2000, 166, 259–269 CrossRef CAS.
- M. Sadrzadeh, E. Saljoughi, K. Shahidi and T. Mohammadi, Polym. Adv. Technol., 2010, 21, 568–577 CrossRef CAS.
- M. Sadrzadeh, M. Amirilargani, K. Shahidi and T. Mohammadi, J. Membr. Sci., 2009, 342, 236–250 CrossRef CAS.
- M. Liu, K. Xie, M. D. Nothling, P. A. Gurr, S. S. L. Tan, Q. Fu, P. A. Webley and G. G. Qiao, ACS Nano, 2018, 12, 11591–11599 CrossRef CAS PubMed.
- J. Zhao, G. He, G. Liu, F. Pan, H. Wu, W. Jin and Z. Jiang, Prog. Polym. Sci., 2018, 80, 125–152 CrossRef CAS.
- S. Dong, Z. Wang, M. Sheng, Z. Qiao and J. Wang, J. Membr. Sci., 2020, 610, 118221 CrossRef CAS.
- W. Yave, H. Huth, A. Car and C. Schick, Energy Environ. Sci., 2011, 4, 4656–4661 RSC.
- W. Yave, A. Car, S. S. Funari, S. P. Nunes and K.-V. Peinemann, Macromolecules, 2010, 43, 326–333 CrossRef CAS.
- Y. Chen, B. Wang, L. Zhao, P. Dutta and W. W. Ho, J. Membr. Sci., 2015, 495, 415–423 CrossRef CAS.
- Y. Chen, L. Zhao, B. Wang, P. Dutta and W. W. Ho, J. Membr. Sci., 2016, 497, 21–28 CrossRef CAS.
- M. J. Yoo, K. H. Kim, J. H. Lee, T. W. Kim, C. W. Chung, Y. H. Cho and H. B. Park, J. Membr. Sci., 2018, 566, 336–345 CrossRef CAS.
- P. Li, Z. Wang, W. Li, Y. Liu, J. Wang and S. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 15481–15493 CrossRef CAS PubMed.
- Y. Ying, Z. Yang, D. Shi, S. B. Peh, Y. Wang, X. Yu, H. Yang, K. Chai and D. Zhao, J. Membr. Sci., 2021, 632, 119384 CrossRef CAS.
- T. Li, Y. Pan, K.-V. Peinemann and Z. Lai, J. Membr. Sci., 2013, 425, 235–242 CrossRef.
- H. Lin and B. D. Freeman, J. Mol. Struct., 2005, 739, 57–74 CrossRef CAS.
- M. Liu, M. D. Nothling, S. Zhang, Q. Fu and G. G. Qiao, Prog. Polym. Sci., 2022, 126, 101504 CrossRef CAS.
- C. Gu, Y. Liu, W. Wang, J. Liu and J. Hu, Front. Chem. Sci. Eng., 2021, 15, 437–449 CrossRef CAS.
- B. Chen, H. Xie, L. Shen, Y. Xu, M. Zhang, M. Zhou, B. Li, R. Li and H. Lin, Small, 2023, 19, 2207313 CrossRef CAS PubMed.
- C. Wu, T. Yamagishi, Y. Nakamoto, S. Ishida, K. Nitta and S. Kubota, J. Polym. Sci., Part B:Polym. Phys., 2000, 38(17), 2285–2295 CrossRef CAS.
- H. W. Kim and H. B. Park, J. Membr. Sci., 2011, 372, 116–124 CrossRef CAS.
- P. Bernardo, J. C. Jansen, F. Bazzarelli, F. Tasselli, A. Fuoco, K. Friess, P. Izák, V. Jarmarová, M. Kačírková and G. Clarizia, Sep. Purif. Technol., 2012, 97, 73–82 CrossRef CAS.
- Y. Han, W. Salim, K. K. Chen, D. Wu and W. S. W. Ho, J. Membr. Sci., 2019, 575, 242–251 CrossRef CAS.
- H. Sanaeepur, S. Mashhadikhan, G. Mardassi, A. Ebadi Amooghin, B. Van der Bruggen and A. Moghadassi, Korean J. Chem. Eng., 2019, 36, 1339–1349 CrossRef CAS.
- V. Vakharia, W. Salim, D. Wu, Y. Han, Y. Chen, L. Zhao and W. S. W. Ho, J. Membr. Sci., 2018, 555, 379–387 CrossRef CAS.
- M. Liu, M. D. Nothling, S. Zhang, Q. Fu and G. G. Qiao, Prog. Polym. Sci., 2022, 101504 CrossRef CAS.
- M. Etxeberria-Benavides, O. David, T. Johnson, M. M. Łozińska, A. Orsi, P. A. Wright, S. Mastel, R. Hillenbrand, F. Kapteijn and J. Gascon, J. Membr. Sci., 2018, 550, 198–207 CrossRef CAS.
- B. Chen, H. Xie, L. Shen, Y. Xu, M. Zhang, M. Zhou, B. Li, R. Li and H. Lin, Small, 2023, 2207313 CrossRef CAS PubMed.
- T. Wang, C. Cheng, L.-G. Wu, J.-N. Shen, B. Van der Bruggen, Q. Chen, D. Chen and C.-Y. Dong, Environ. Sci. Technol., 2017, 51, 6202–6210 CrossRef CAS PubMed.
- H. W. Yoon, T. H. Lee, C. M. Doherty, T. H. Choi, J. S. Roh, H. W. Kim, Y. H. Cho, S.-H. Do, B. D. Freeman and H. B. Park, J. Phys. Chem. Lett., 2020, 11, 2356–2362 CrossRef CAS PubMed.
- N. Nidamanuri, Y. Li, Q. Li and M. Dong, Eng. Sci., 2020, 9, 3–16 CAS.
- A. D. Kiadehi, A. Rahimpour, M. Jahanshahi and A. A. Ghoreyshi, J. Ind. Eng. Chem., 2015, 22, 199–207 CrossRef.
- A. D. Kiadehi, M. Jahanshahi, A. Rahimpour and S. A. A. Ghoreyshi, Chem. Eng. Process., 2015, 90, 41–48 CrossRef CAS.
- H. Lin and B. D. Freeman, Macromolecules, 2006, 39, 3568–3580 CrossRef CAS.
- H. Lin, PhD dissertation, University of Texas, Austin, 2005.
- S. L. Liu, L. Shao, M. L. Chua, C. H. Lau, H. Wang and S. Quan, Prog. Polym. Sci., 2013, 38, 1089–1120 CrossRef CAS.
- W. K. Setiawan and K.-Y. Chiang, Chemosphere, 2023, 139478 CrossRef PubMed.
- G. Clarizia and P. Bernardo, Polymers, 2021, 14, 10 CrossRef PubMed.
- M. Isanejad, N. Azizi and T. Mohammadi, J. Appl. Polym. Sci., 2017, 134(9) DOI:10.1002/app.44531.
- R. Kesting, J. Polym. Sci., Part C: Polym. Lett., 1989, 27, 187–190 CrossRef CAS.
- F. Karamouz, H. Maghsoudi and R. Yegani, J. Nat. Gas Sci. Eng., 2016, 35, 980–985 CrossRef CAS.
- S. Wang, Y. Liu, S. Huang, H. Wu, Y. Li, Z. Tian and Z. Jiang, J. Membr. Sci., 2014, 460, 62–70 CrossRef CAS.
- M. M. Rahman, S. Shishatskiy, C. Abetz, P. Georgopanos, S. Neumann, M. M. Khan, V. Filiz and V. Abetz, J. Membr. Sci., 2014, 469, 344–354 CrossRef CAS.
- M. A. Wahab and A. Sunarti, Int. J. Membr. Sci. Technol., 2015, 2, 78–84 CrossRef.
- S. Sridhar, R. Suryamurali, B. Smitha and T. Aminabhavi, Colloids Surf., A, 2007, 297, 267–274 CrossRef CAS.
- L. Wang, Y. Li, S. Li, P. Ji and C. Jiang, J. Energy Chem., 2014, 23, 717–725 CrossRef.
- P. Taheri, M. S. Maleh and A. Raisi, J. Environ. Chem. Eng., 2021, 9, 105877 CrossRef CAS.
- Y. Liu, S. Yu, H. Wu, Y. Li, S. Wang, Z. Tian and Z. Jiang, J. Membr. Sci., 2014, 469, 198–208 CrossRef CAS.
- W. K. Setiawan and K.-Y. Chiang, Chemosphere, 2023, 338, 139478 CrossRef PubMed.
- W. Zhu, F. Liu, M. Gou, R. Guo and X. Li, Green Chem. Eng., 2021, 2, 132–143 CrossRef.
- Y. Zheng, Y. Wu, B. Zhang and Z. Wang, J. Appl. Polym. Sci., 2020, 137, 48398 CrossRef CAS.
- S. Metz, M. Mulder and M. Wessling, Macromolecules, 2004, 37, 4590–4597 CrossRef CAS.
- A. Car, C. Stropnik, W. Yave and K. V. Peinemann, Adv. Funct. Mater., 2008, 18, 2815–2823 CrossRef CAS.
- B. Zhu, X. Jiang, S. He, X. Yang, J. Long, Y. Zhang and L. Shao, J. Mater. Chem. A, 2020, 8, 24233–24252 RSC.
- T. C. Merkel, H. Lin, X. Wei and R. Baker, J. Membr. Sci., 2010, 359, 126–139 CrossRef CAS.
- X. He, A. Lindbråthen, T.-J. Kim and M.-B. Hägg, Int. J. Greenhouse Gas Control, 2017, 64, 323–332 CrossRef CAS.
- Y. Han and W. W. Ho, Chin. J. Chem. Eng., 2018, 26, 2238–2254 CrossRef CAS.
- Y. Han, D. Wu and W. W. Ho, J. Membr. Sci., 2019, 573, 476–484 CrossRef CAS.
- P. V. Kortunov, M. Siskin, L. S. Baugh and D. C. Calabro, Energy Fuels, 2015, 29, 5919–5939 CrossRef CAS.
- Y. Chen and W. W. Ho, J. Membr. Sci., 2016, 514, 376–384 CrossRef CAS.
- Y. Zhao and W. W. Ho, J. Membr. Sci., 2012, 415, 132–138 CrossRef.
- R. Pelton, Langmuir, 2014, 30, 15373–15382 CrossRef CAS PubMed.
- D. Wu, Y. Han, W. Salim, K. K. Chen, J. Li and W. W. Ho, J. Membr. Sci., 2018, 565, 439–449 CrossRef CAS.
- R. Pang, K. K. Chen, Y. Han and W. W. Ho, J. Membr. Sci., 2020, 612, 118443 CrossRef CAS.
- K. K. Chen, Y. Han, Z. Zhang and W. W. Ho, J. Membr. Sci., 2021, 628, 119215 CrossRef CAS.
- T. J. Kim, B. Li and M. B. Hägg, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 4326–4336 CrossRef CAS.
- T.-Y. Chen, X. Deng, L.-C. Lin and W. W. Ho, J. Membr. Sci., 2022, 645, 120195 CrossRef CAS.
- S. Yuan, Z. Wang, Z. Qiao, M. Wang, J. Wang and S. Wang, J. Membr. Sci., 2011, 378, 425–437 CrossRef CAS.
- L. Deng and M.-B. Hagg, Ind. Eng. Chem. Res., 2015, 54, 11139–11150 CrossRef CAS.
- Z. Qiao, Z. Wang, C. Zhang, S. Yuan, Y. Zhu, J. Wang and S. Wang, AIChE J., 2013, 59, 215–228 CrossRef CAS.
- C. Dong, Z. Wang, C. Yi and S. Wang, J. Appl. Polym. Sci., 2006, 101, 1885–1891 CrossRef CAS.
- J. Zhao, Z. Wang, J. Wang and S. Wang, J. Membr. Sci., 2006, 283, 346–356 CrossRef CAS.
- D. Venturi, D. Grupkovic, L. Sisti and M. G. Baschetti, J. Membr. Sci., 2018, 548, 263–274 CrossRef CAS.
- R. Casadei, E. Firouznia and M. G. Baschetti, Membranes, 2021, 11, 442 CrossRef CAS PubMed.
- Y. Han, D. Wu and W. W. Ho, J. Membr. Sci., 2018, 567, 261–271 CrossRef CAS.
- R. Casadei, D. Venturi, M. Giacinti Baschetti, L. Giorgini, E. Maccaferri and S. Ligi, Membranes, 2019, 9, 119 CrossRef CAS PubMed.
- T.-J. Kim, H. Vrålstad, M. Sandru and M.-B. Hägg, Energy Procedia, 2013, 37, 986–992 CrossRef CAS.
- C. Zhang, Z. Wang, Y. Cai, C. Yi, D. Yang and S. Yuan, Chem. Eng. J., 2013, 225, 744–751 CrossRef CAS.
- N. B. McKeown, Int. Scholarly Res. Not., 2012, 2012(1), 513986 Search PubMed.
- U. Scherf, J. Mater. Chem., 1999, 9, 1853–1864 RSC.
- A. A. Shamsabadi, M. Rezakazemi, F. Seidi, H. Riazi, T. Aminabhavi and M. Soroush, Prog. Energy Combust. Sci., 2021, 84, 100903 CrossRef.
- M. Z. Ahmad, R. Castro-Muñoz and P. M. Budd, Nanoscale, 2020, 12, 23333–23370 RSC.
- Y. Wang, B. S. Ghanem, Z. Ali, K. Hazazi, Y. Han and I. Pinnau, Small Struct., 2021, 2, 2100049 CrossRef CAS.
- B. Comesaña-Gándara, J. Chen, C. G. Bezzu, M. Carta, I. Rose, M.-C. Ferrari, E. Esposito, A. Fuoco, J. C. Jansen and N. B. McKeown, Energy Environ. Sci., 2019, 12, 2733–2740 RSC.
- N. B. McKeown, Polymer, 2020, 202, 122736 CrossRef CAS.
- H. Zhou and W. Jin, Membranes, 2018, 9, 3 CrossRef PubMed.
- S. Sepahvand, M. Bahmani, A. Ashori, H. Pirayesh, Q. Yu and M. N. Dafchahi, Int. J. Biol. Macromol., 2021, 182, 1392–1398 CrossRef CAS PubMed.
- M. Nikkhah Dafchahi, H. Resalati, A. R. Saraeyan, A. Ghasemian and A. R. Shakeri, Cellulose, 2018, 25, 4783–4790 CrossRef CAS.
- Z. Dai, V. Ottesen, J. Deng, R. M. L. Helberg and L. Deng, Fibers, 2019, 7, 40 CrossRef CAS.
- M. Zhang, T. Xu, Q. Zhao, K. Liu, D. Liang and C. Si, Carbon Capture Sci. Technol., 2024, 10, 100157 CrossRef CAS.
- X.-L. Wei, S. Liang, Y.-Y. Xu, Y.-L. Sun, J.-F. An and Z.-S. Chao, J. Membr. Sci., 2017, 530, 240–249 CrossRef CAS.
- J. Chen, J. Xu, K. Wang, X. Cao and R. Sun, Carbohydr. Polym., 2016, 137, 685–692 CrossRef CAS PubMed.
- Y. Liu, H. Huang, P. Huo and J. Gu, Carbohydr. Polym., 2017, 165, 266–275 CrossRef CAS PubMed.
- V. Vatanpour, A. Dehqan, S. Paziresh, S. Zinadini, A. A. Zinatizadeh and I. Koyuncu, Sep. Purif. Technol., 2022, 296, 121433 CrossRef CAS.
- H. Nguyen, M. Wang, M.-Y. Hsiao, K. Nagai, Y. Ding and H. Lin, J. Membr. Sci., 2019, 586, 7–14 CrossRef CAS.
- A. F. Ismail, K. Chandra Khulbe, T. Matsuura, A. F. Ismail, K. C. Khulbe and T. Matsuura, Gas Separation Membranes: Polymeric and Inorganic, 2015, pp. 37–192 Search PubMed.
- A. Puleo, D. R. Paul and S. Kelley, J. Membr. Sci., 1989, 47, 301–332 CrossRef CAS.
- D. Nikolaeva, K. Verachtert, I. Azcune, J. C. Jansen and I. F. Vankelecom, Carbohydr. Polym., 2021, 255, 117375 CrossRef CAS PubMed.
- A. Rehman, Z. Jahan, F. Sher, T. Noor, M. B. K. Niazi, M. A. Akram and E. K. Sher, Chemosphere, 2022, 307, 135736 CrossRef CAS PubMed.
- M. A. Silva, E. Belmonte-Reche and M. P. de Amorim, Carbohydr. Polym., 2021, 254, 117407 CrossRef CAS PubMed.
- A. Nagendran, S. Vidya and D. Mohan, Soft Mater., 2008, 6, 45–64 CrossRef CAS.
- L. Liu, C. M. Doherty, E. Ricci, G. Q. Chen, M. G. De Angelis and S. E. Kentish, J. Membr. Sci., 2021, 638, 119677 CrossRef CAS.
- Z. Dai, V. Ottesen, J. Deng, R. M. L. Helberg and L. Deng, Fibers, 2019, 7(5), 40.
- M. N. Dafchahi and B. Acharya, Biomass Convers. Biorefin., 2023, 1–15 Search PubMed.
- D. Pawcenis, E. Twardowska, M. Leśniak, R. J. Jędrzejczyk, M. Sitarz and J. Profic-Paczkowska, Int. J. Biol. Macromol., 2022, 213, 738–750 CrossRef CAS PubMed.
- T. Yi, H. Zhao, Q. Mo, D. Pan, Y. Liu, L. Huang, H. Xu, B. Hu and H. Song, Materials, 2020, 13, 5062 CrossRef CAS PubMed.
- N. A. D. Ho and C. Leo, Environ. Res., 2021, 197, 111100 CrossRef CAS PubMed.
- A. Pokharel, K. J. Falua, A. Babaei-Ghazvini, M. Nikkhah Dafchahi, L. G. Tabil, V. Meda and B. Acharya, Polymers, 2024, 16, 996 CrossRef CAS PubMed.
- Y. Li, P. Jia, J. Xu, Y. Wu, H. Jiang and Z. Li, Ind. Eng. Chem. Res., 2020, 59, 2874–2882 CrossRef CAS.
- J. Ø. Torstensen, R. M. Helberg, L. Deng, Ø. W. Gregersen and K. Syverud, Int. J. Greenhouse Gas Control, 2019, 81, 93–102 CrossRef CAS.
- S. N. Mithra and S. Ahankari, Mater. Today Sustainability, 2022, 19, 100191 CrossRef.
- R. Borgohain, U. Pattnaik, B. Prasad and B. Mandal, Carbohydr. Polym., 2021, 267, 118178 CrossRef CAS PubMed.
- F. Russo, F. Galiano, A. Iulianelli, A. Basile and A. Figoli, Fuel Process. Technol., 2021, 213, 106643 CrossRef CAS.
- A. Iulianelli, F. Russo, F. Galiano, M. Manisco and A. Figoli, Int. J. Greenhouse Gas Control, 2022, 117, 103657 CrossRef CAS.
- A. Tena, L. Fernández, M. Sánchez, L. Palacio, A. Lozano, A. Hernández and P. Prádanos, Chem. Eng. Sci., 2010, 65, 2227–2235 CrossRef CAS.
- T.-S. Chung, S. S. Chan, R. Wang, Z. Lu and C. He, J. Membr. Sci., 2003, 211, 91–99 CrossRef CAS.
- C. M. Zimmerman, A. Singh and W. J. Koros, J. Membr. Sci., 1997, 137, 145–154 CrossRef CAS.
- M. Mozafari, S. Khoshhal Salestan, A. Arabi Shamsabadi, K. Jha, M. Tanwar, H. Kim, Z. Fakhraai and M. Soroush, ACS Appl. Mater. Interfaces, 2025, 17(2), 3897–3910 CrossRef CAS PubMed.
- Y. Cheng, S. J. Datta, S. Zhou, J. Jia, O. Shekhah and M. Eddaoudi, Chem. Soc. Rev., 2022, 51, 8300–8350 RSC.
- M. L. Jue and R. P. Lively, React. Funct. Polym., 2015, 86, 88–110 CrossRef CAS.
- B. Seoane, V. Sebastián, C. Téllez and J. Coronas, CrystEngComm, 2013, 15, 9483–9490 RSC.
- Y. H. Deng, J. T. Chen, C. H. Chang, K. S. Liao, K. L. Tung, W. E. Price, Y. Yamauchi and K. C. W. Wu, Angew. Chem., 2016, 128, 12985–12988 CrossRef.
- K. C. Wong, P. S. Goh, A. F. Ismail, H. S. Kang, Q. Guo, X. Jiang and J. Ma, Membranes, 2022, 12, 186 CrossRef CAS PubMed.
- O. Shekhah, L. Fu, R. Sougrat, Y. Belmabkhout, A. J. Cairns, E. P. Giannelis and M. Eddaoudi, Chem. Commun., 2012, 48, 11434–11436 RSC.
- M. C. So, S. Jin, H.-J. Son, G. P. Wiederrecht, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 15698–15701 CrossRef CAS PubMed.
- O. Shekhah, J. Liu, R. Fischer and C. Wöll, Chem. Soc. Rev., 2011, 40, 1081–1106 RSC.
- S. Khoshhal, A. A. Ghoreyshi, M. Jahanshahi and M. Mohammadi, RSC Adv., 2015, 5, 24758–24768 RSC.
- X. Q. Cheng, Z. X. Wang, X. Jiang, T. Li, C. H. Lau, Z. Guo, J. Ma and L. Shao, Prog. Mater. Sci., 2018, 92, 258–283 CrossRef CAS.
- G. Wyszogrodzka, B. Marszałek, B. Gil and P. Dorożyński, Drug Discovery Today, 2016, 21, 1009–1018 CrossRef CAS PubMed.
- V. I. Isaeva, K. E. Papathanasiou and L. M. Kustov, Crystals, 2020, 10, 617 CrossRef CAS.
- S. Tai, W. Zhang, J. Zhang, G. Luo, Y. Jia, M. Deng and Y. Ling, Microporous Mesoporous Mater., 2016, 220, 148–154 CrossRef CAS.
- Z. Hu, Y. Peng, Z. Kang, Y. Qian and D. Zhao, Inorg. Chem., 2015, 54, 4862–4868 CrossRef CAS PubMed.
- R. Lin, L. Ge, S. Liu, V. Rudolph and Z. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 14750–14757 CrossRef CAS PubMed.
- S. Anastasiou, N. Bhoria, J. Pokhrel, K. S. K. Reddy, C. Srinivasakannan, K. Wang and G. N. Karanikolos, Mater. Chem. Phys., 2018, 212, 513–522 CrossRef CAS.
- L. Dong, M. Chen, J. Li, D. Shi, W. Dong, X. Li and Y. Bai, J. Membr. Sci., 2016, 520, 801–811 CrossRef CAS.
- W. Li, Y. Zhang, P. Su, Z. Xu, G. Zhang, C. Shen and Q. Meng, J. Mater. Chem. A, 2016, 4, 18747–18752 RSC.
- K. Cho, L. J. Andrew and M. J. MacLachlan, Angew. Chem., Int. Ed., 2023, 62, e202300960 CrossRef CAS PubMed.
- F. Guo, B. Li, R. Ding, D. Li, X. Jiang, G. He and W. Xiao, Membranes, 2021, 11, 693 CrossRef CAS PubMed.
- V. T. Do, C. Y. Tang, M. Reinhard and J. O. Leckie, Environ. Sci. Technol., 2012, 46, 13184–13192 CrossRef CAS PubMed.
- W. Sun, J. Shi, C. Chen, N. Li, Z. Xu, J. Li, H. Lv, X. Qian and L. Zhao, RSC Adv., 2018, 8, 10040–10056 RSC.
- M. Hu and B. Mi, Environ. Sci. Technol., 2013, 47, 3715–3723 CrossRef CAS PubMed.
- Z. Xu, Y. Zhang, X. Qian, J. Shi, L. Chen, B. Li, J. Niu and L. Liu, Appl. Surf. Sci., 2014, 316, 308–314 CrossRef CAS.
- C. Petit and T. J. Bandosz, Adv. Mater., 2009, 21, 4753–4757 CrossRef CAS.
- Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC.
- M. Tanhaei, A. R. Mahjoub and V. Safarifard, Ultrason. Sonochem., 2018, 41, 189–195 CrossRef CAS PubMed.
- K.-Y. A. Lin, F.-K. Hsu and W.-D. Lee, J. Mater. Chem. A, 2015, 3, 9480–9490 RSC.
- M. Ghadiri, A. Aroujalian, F. Pazani and P. Salimi, Sep. Purif. Technol., 2024, 330, 125315 CrossRef CAS.
- Q. Yang and B. Mi, Adv. Chronic Kidney Dis., 2013, 20, 536–555 CrossRef PubMed.
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- S. Xiong, L. Li, L. Dong, J. Tang, G. Yu and C. Pan, J. CO2 Util., 2020, 41, 101224 CrossRef CAS.
- X. Cao, Z. Wang, Z. Qiao, S. Zhao and J. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 5306–5315 CrossRef CAS PubMed.
- K. Duan, J. Wang, Y. Zhang and J. Liu, J. Membr. Sci., 2019, 572, 588–595 CrossRef CAS.
- H. Fan, A. Mundstock, A. Feldhoff, A. Knebel, J. Gu, H. Meng and J. R. Caro, J. Am. Chem. Soc., 2018, 140, 10094–10098 CrossRef CAS PubMed.
- J. Fu, S. Das, G. Xing, T. Ben, V. Valtchev and S. Qiu, J. Am. Chem. Soc., 2016, 138, 7673–7680 CrossRef CAS PubMed.
- Y. Cheng, Y. Ying, L. Zhai, G. Liu, J. Dong, Y. Wang, M. P. Christopher, S. Long, Y. Wang and D. Zhao, J. Membr. Sci., 2019, 573, 97–106 CrossRef CAS.
- H. Fan, M. Peng, I. Strauss, A. Mundstock, H. Meng and J. Caro, Nat. Commun., 2021, 12, 38 CrossRef CAS PubMed.
- A. Knebel and J. Caro, Nat. Nanotechnol., 2022, 17, 911–923 CrossRef CAS PubMed.
- G. Liu, W. Jin and N. Xu, Angew. Chem., Int. Ed., 2016, 55, 13384–13397 CrossRef CAS PubMed.
- H. E. Karahan, K. Goh, C. Zhang, E. Yang, C. Yıldırım, C. Y. Chuah, M. G. Ahunbay, J. Lee, Ş. B. Tantekin-Ersolmaz and Y. Chen, Adv. Mater., 2020, 32, 1906697 CrossRef CAS PubMed.
- N. Hemanth and B. Kandasubramanian, Chem. Eng. J., 2020, 392, 123678 CrossRef CAS.
- A. P. Isfahani, A. Arabi Shamsabadi and M. Soroush, Ind. Eng. Chem. Res., 2022, 62, 2309–2328 CrossRef.
- J. Shen, G. Liu, Y. Ji, Q. Liu, L. Cheng, K. Guan, M. Zhang, G. Liu, J. Xiong and J. Yang, Adv. Funct. Mater., 2018, 28, 1801511 CrossRef.
- C. Y. Chuah, K. Goh, Y. Yang, H. Gong, W. Li, H. E. Karahan, M. D. Guiver, R. Wang and T.-H. Bae, Chem. Rev., 2018, 118, 8655–8769 CrossRef CAS PubMed.
- J. G. Wijmans and R. W. Baker, J. Membr. Sci., 1995, 107, 1–21 CrossRef CAS.
- A. A. Shamsabadi, A. P. Isfahani, S. K. Salestan, A. Rahimpour, B. Ghalei, E. Sivaniah and M. Soroush, ACS Appl. Mater. Interfaces, 2019, 12, 3984–3992 CrossRef PubMed.
- G. Liu, L. Cheng, G. Chen, F. Liang, G. Liu and W. Jin, Chem. – Asian J., 2020, 15, 2364–2370 CrossRef CAS PubMed.
- Z. Hu, Y. Yang, X.-F. Zhang, C. Xu and J. Yao, Sep. Purif. Technol., 2023, 326, 124704 CrossRef CAS.
- F. Shi, J. Sun, J. Wang, M. Liu, Z. Yan, B. Zhu, Y. Li and X. Cao, J. Membr. Sci., 2021, 620, 118850 CrossRef CAS.
- H. W. Kim, H. W. Yoon, S.-M. Yoon, B. M. Yoo, B. K. Ahn, Y. H. Cho, H. J. Shin, H. Yang, U. Paik and S. Kwon, Science, 2013, 342, 91–95 CrossRef CAS PubMed.
- K. Wang, D. Chen, J. Tang, Z. Hong, Z. Zhu, Z. Yuan, Z. Lin, Y. Liu, R. Semiat and X. He, Chem. Eng. J., 2024, 483, 149305 CrossRef CAS.
- Z.-X. Low, P. M. Budd, N. B. McKeown and D. A. Patterson, Chem. Rev., 2018, 118, 5871–5911 CrossRef CAS PubMed.
- S. Kim and Y. M. Lee, Prog. Polym. Sci., 2015, 43, 1–32 CrossRef CAS.
- L. S. White, X. Wei, S. Pande, T. Wu and T. C. Merkel, J. Membr. Sci., 2015, 496, 48–57 CrossRef CAS.
- Y. Hua, S. Park and H. K. Jeong, J. Environ. Chem. Eng., 2024, 113753 CrossRef CAS.
- C. Astorino, E. De Nardo, S. Lettieri, G. Ferraro, C. F. Pirri and S. Bocchini, Membranes, 2023, 13(12), 903 CrossRef CAS PubMed.
- M. Sandru, T.-J. Kim, W. Capala, M. Huijbers and M.-B. Hägg, Energy Procedia, 2013, 37, 6473–6480 CrossRef CAS.
- J. Pohlmann, M. Bram, K. Wilkner and T. Brinkmann, Int. J. Greenhouse Gas Control, 2016, 53, 56–64 CrossRef CAS.
- S. Fu, D. Hasse and S. Kulkarni, Bench Scale Testing of Next Generation Hollow Fiber Membrane Modules, American Air Liquide Inc., Newark, DE (United States), 2020 Search PubMed.
- Y. Ding, Ind. Eng. Chem. Res., 2019, 59, 556–568 CrossRef.
- Y. Hua, S. Park and H.-K. Jeong, J. Environ. Chem. Eng., 2024, 113753 CrossRef CAS.
- C. Astorino, E. De Nardo, S. Lettieri, G. Ferraro, C. F. Pirri and S. Bocchini, Membranes, 2023, 13, 903 CrossRef CAS PubMed.
- H. An, S. Park, H. T. Kwon, H.-K. Jeong and J. S. Lee, J. Membr. Sci., 2017, 526, 367–376 CrossRef CAS.
- N. Prasetya, N. F. Himma, P. D. Sutrisna, I. G. Wenten and B. P. Ladewig, Chem. Eng. J., 2020, 391, 123575 CrossRef CAS.
- A. Y. Ku, P. Kulkarni, R. Shisler and W. Wei, J. Membr. Sci., 2011, 367, 233–239 CrossRef CAS.
- O. C. David, D. Gorri, A. Urtiaga and I. Ortiz, J. Membr. Sci., 2011, 378, 359–368 CrossRef CAS.
- R. W. Baker, Ind. Eng. Chem. Res., 2002, 41, 1393–1411 CrossRef CAS.
- R. Mahajan and W. J. Koros, Ind. Eng. Chem. Res., 2000, 39, 2692–2696 CrossRef CAS.
- T. Bui, Y. Wong, M. Islam and K. Chua, J. Membr. Sci., 2017, 539, 76–87 CrossRef CAS.
- L. Li, R. Xu, C. Song, B. Zhang, Q. Liu and T. Wang, Membranes, 2018, 8, 134 CrossRef PubMed.
- A. W. Thornton, D. Dubbeldam, M. S. Liu, B. P. Ladewig, A. J. Hill and M. R. Hill, Energy Environ. Sci., 2012, 5, 7637–7646 RSC.
- R. J. Gardner, R. A. Crane and J. F. Hannan, Chem. Eng. Prog., 1977, 73, 76–78 CAS.
- R. Spillman, in Membrane science and technology, Elsevier, 1995, vol. 2, pp. 589–667 Search PubMed.
- C. W. Colling, G. A. Huff Jr and J. V. Bartels, US Pat., 6830691, 2004 Search PubMed.
- C. H. Lau, P. T. Nguyen, M. R. Hill, A. W. Thornton, K. Konstas, C. M. Doherty, R. J. Mulder, L. Bourgeois, A. C. Y. Liu and D. J. Sprouster, Angew. Chem., Int. Ed., 2014, 53, 5322–5326 CrossRef CAS PubMed.
- Y. Huang and D. R. Paul, Polymer, 2004, 45, 8377–8393 CrossRef CAS.
- M. Yavari, S. Maruf, Y. Ding and H. Lin, J. Membr. Sci., 2017, 525, 399–408 CrossRef CAS.
- A. F. Ismail and W. Lorna, Sep. Purif. Technol., 2002, 27, 173–194 CrossRef CAS.
- A. Bos, I. Pünt, M. Wessling and H. Strathmann, J. Membr. Sci., 1999, 155, 67–78 CrossRef CAS.
- C. Zhou, T.-S. Chung, R. Wang, Y. Liu and S. H. Goh, J. Membr. Sci., 2003, 225, 125–134 CrossRef CAS.
- Y. Kamiya, K. Mizoguchi and Y. Naito, J. Polym. Sci., Part B:Polym. Phys., 1992, 30, 1177–1181 CrossRef.
- F. Moghadam and H. B. Park, Curr. Opin. Chem. Eng., 2018, 20, 28–38 CrossRef.
- S. Kanehashi, A. Aguiar, H. T. Lu, G. Q. Chen and S. E. Kentish, J. Membr. Sci., 2018, 549, 686–692 CrossRef CAS.
- C. A. Scholes, S. E. Kentish and G. W. Stevens, Sep. Purif. Rev., 2009, 38, 1–44 CrossRef CAS.
- C. A. Scholes, G. W. Stevens and S. E. Kentish, J. Membr. Sci., 2010, 350, 189–199 CrossRef CAS.
- H. Demir and S. Keskin, Macromol. Mater. Eng., 2024, 309, 2300225 CrossRef CAS.
- O. Vopička, M. G. De Angelis and G. C. Sarti, J. Membr. Sci., 2014, 449, 97–108 CrossRef.
- O. Vopička, M. G. De Angelis, N. Du, N. Li, M. D. Guiver and G. C. Sarti, J. Membr. Sci., 2014, 459, 264–276 CrossRef.
- E. Ricci, A. E. Gemeda, N. Du, N. Li, M. G. De Angelis, M. D. Guiver and G. C. Sarti, J. Membr. Sci., 2019, 585, 136–149 CrossRef CAS.
- E. Ricci, E. Di Maio, M. Degli Esposti, L. Liu, G. Mensitieri, P. Fabbri, S. E. Kentish and M. G. De Angelis, J. Membr. Sci., 2021, 628, 119226 CrossRef CAS.
- W. Koros, J. Polym. Sci., Polym. Phys. Ed., 1980, 18, 981–992 CrossRef CAS.
- O. Vopička and K. Friess, J. Polym. Sci., Part B: Polym. Phys., 2014, 52, 1490–1495 CrossRef.
- M. Saberi, A. Dadkhah and S. Hashemifard, J. Membr. Sci., 2016, 499, 164–171 CrossRef CAS.
- S. Khoshhal Salestan, A. Rahimpour, R. Abedini, M. A. Soleimanzade and M. Sadrzadeh, J. Polym. Sci., 2022, 60, 1392–1406 CrossRef CAS.
- M. Minelli and G. C. Sarti, J. Membr. Sci., 2013, 435, 176–185 CrossRef CAS.
- M. Minelli, S. Campagnoli, M. G. De Angelis, F. Doghieri and G. C. Sarti, Macromolecules, 2011, 44, 4852–4862 CrossRef CAS.
- P. K. Roy, K. Kumar, F. M. Thakkar, A. D. Pathak, K. G. Ayappa and P. K. Maiti, J. Membr. Sci., 2020, 613, 118377 CrossRef CAS.
- E. Tocci, A. Gugliuzza, L. De Lorenzo, M. Macchione, G. De Luca and E. Drioli, J. Membr. Sci., 2008, 323, 316–327 CrossRef CAS.
- H. Daglar, I. Erucar and S. Keskin, Mater. Adv., 2021, 2, 5300–5317 RSC.
- I. Erucar, G. Yilmaz and S. Keskin, Chem. – Asian J., 2013, 8, 1692–1704 CrossRef CAS PubMed.
- J. G. Wijmans and R. W. Baker, J. Membr. Sci., 1995, 107, 1–21 CrossRef CAS.
- S. Sadeghi and J. D. Howe, J. Phys. Chem. C, 2023, 127, 3715–3725 CrossRef CAS.
- E. Ghasemnejad-Afshar, S. Amjad-Iranagh, M. Zarif and H. Modarress, Polym. Test., 2020, 83, 106339 CrossRef CAS.
- K. q Yu, Z. s Li and J. Sun, Macromol. Theory Simul., 2001, 10, 624–633 CrossRef CAS.
- Q. Xu, J. Gao, F. Feng, T.-S. Chung and J. Jiang, J. Membr. Sci., 2023, 678, 121678 CrossRef CAS.
- J. C. Maxwell, A Treatise on Electricity and Magnetism, Oxford, Clarendon Press, 1873 Search PubMed.
- V. D. Bruggeman, Ann. Phys., 1935, 416, 636–791 CrossRef.
- J. Felske, Int. J. Heat Mass Transfer, 2004, 47, 3453–3461 CrossRef.
- S. Keskin and S. Alsoy Altinkaya, Computation, 2019, 7, 36 CrossRef CAS.
- H. Daglar and S. Keskin, Coord. Chem. Rev., 2020, 422, 213470 CrossRef CAS.
- O. F. Altundal, C. Altintas and S. Keskin, J. Mater. Chem. A, 2020, 8, 14609–14623 RSC.
- Y. J. Colón and R. Q. Snurr, Chem. Soc. Rev., 2014, 43, 5735–5749 RSC.
- C. Altintas, I. Erucar and S. Keskin, ACS Appl. Mater. Interfaces, 2018, 10, 3668–3679 CrossRef CAS PubMed.
- Z. Qiao, C. Peng, J. Zhou and J. Jiang, J. Mater. Chem. A, 2016, 4, 15904–15912 RSC.
- D. Torelli, H. Moustafa, K. W. Jacobsen and T. Olsen, npj Comput. Mater., 2020, 6, 158 CrossRef.
- E. Ren, P. Guilbaud and F.-X. Coudert, Digital Discovery, 2022, 1, 355–374 RSC.
- M. Li, W. Cai, C. Wang and X. Wu, Phys. Chem. Chem. Phys., 2022, 24, 18764–18776 RSC.
- S. Budhathoki, O. Ajayi, J. A. Steckel and C. E. Wilmer, Energy Environ. Sci., 2019, 12, 1255–1264 RSC.
- C. Altintas and S. Keskin, ACS Sustainable Chem. Eng., 2018, 7, 2739–2750 CrossRef PubMed.
- T. Yuan and L. Sarkisov, Adv. Theory Simul., 2022, 5, 2200159 CrossRef CAS.
- S. A. Abdollahi and S. F. Ranjbar, Sci. Rep., 2023, 13, 8812 CrossRef CAS PubMed.
- A. Priya, B. Devarajan, A. Alagumalai and H. Song, Sci. Total Environ., 2023, 163913 CrossRef CAS PubMed.
- R. Giro, H. Hsu, A. Kishimoto, T. Hama, R. F. Neumann, B. Luan, S. Takeda, L. Hamada and M. B. Steiner, npj Comput. Mater., 2023, 9, 133 CrossRef.
- Z. Zhang, X. Cao, C. Geng, Y. Sun, Y. He, Z. Qiao and C. Zhong, J. Membr. Sci., 2022, 650, 120399 CrossRef CAS.
- E. Ren and F.-X. Coudert, Chem. Sci., 2023, 14, 1797–1807 RSC.
- K. M. Jablonka, D. Ongari, S. M. Moosavi and B. Smit, Chem. Rev., 2020, 120, 8066–8129 CrossRef CAS PubMed.
- Y. G. Chung, E. Haldoupis, B. J. Bucior, M. Haranczyk, S. Lee, H. Zhang, K. D. Vogiatzis, M. Milisavljevic, S. Ling and J. S. Camp, J. Chem. Eng. Data, 2019, 64, 5985–5998 CrossRef CAS.
- F. Kadirkhan, P. S. Goh, A. F. Ismail, W. N. F. Wan Mustapa, M. H. M. Halim, W. K. Soh and S. Y. Yeo, Membranes, 2022, 12, 71 CrossRef CAS PubMed.
- A. Wypych, Databook of plasticizers, Elsevier, 2023 Search PubMed.
- M. Zhang, L. Deng, D. Xiang, B. Cao, S. S. Hosseini and P. Li, Processes, 2019, 7, 51 CrossRef CAS.
- S. Sridhar, S. Bee and S. Bhargava, Chem. Eng. Dig., 2014, 1, 1–25 Search PubMed.
- D. S. Bakhtin, S. E. Sokolov, I. L. Borisov, V. V. Volkov, A. V. Volkov and V. O. Samoilov, Membranes, 2023, 13, 519 CrossRef CAS PubMed.
- A. Nandy, G. Terrones, N. Arunachalam, C. Duan, D. W. Kastner and H. J. Kulik, Sci. Data, 2022, 9, 74 CrossRef CAS PubMed.
- A. Nandy, C. Duan and H. J. Kulik, J. Am. Chem. Soc., 2021, 143, 17535–17547 CrossRef CAS PubMed.
- G. G. Terrones, S.-P. Huang, M. P. Rivera, S. Yue, A. Hernandez and H. J. Kulik, J. Am. Chem. Soc., 2024, 146, 20333–20348 CrossRef CAS PubMed.
- A. Nandy, S. Yue, C. Oh, C. Duan, G. G. Terrones, Y. G. Chung and H. J. Kulik, Matter, 2023, 6, 1585–1603 CrossRef CAS.
- Y. Duan, L. Li, Z. Shen, J. Cheng and K. He, Membranes, 2023, 13, 480 CrossRef CAS PubMed.
- Q. Qian, P. A. Asinger, M. J. Lee, G. Han, K. Mizrahi Rodriguez, S. Lin, F. M. Benedetti, A. X. Wu, W. S. Chi and Z. P. Smith, Chem. Rev., 2020, 120, 8161–8266 CrossRef CAS PubMed.
- N. A. O. Sciences, D. E. Earth, L. Studies, B. O. C. Sciences and C. O. A. R. A. F. A. N. E. I. S. Science, 2019.
- Z. Dai and L. Deng, Sep. Purif. Technol., 2023, 126022 Search PubMed.
- E. Favre, Membranes, 2022, 12, 884 CrossRef CAS PubMed.
- M. Pfister, B. Belaissaoui and E. Favre, Ind. Eng. Chem. Res., 2017, 56, 591–602 CrossRef CAS.
- R. Bounaceur, E. Berger, M. Pfister, A. A. R. Santos and E. Favre, J. Membr. Sci., 2017, 523, 77–91 CrossRef CAS.
- A. Jomekian and R. M. Behbahani, J. Membr. Sci. Res., 2021, 7, 209–223 CAS.
- M. Yuan, H. Teichgraeber, J. Wilcox and A. R. Brandt, Int. J. Greenhouse Gas Control, 2019, 84, 154–163 CrossRef CAS.
- C. J. Geankoplis, Separation Process Principles, 2003 Search PubMed.
- R. Bounaceur, N. Lape, D. Roizard, C. Vallieres and E. Favre, Energy, 2006, 31, 2556–2570 CrossRef CAS.
- B. Belaissaoui, D. Willson and E. Favre, Chem. Eng. J., 2012, 211, 122–132 CrossRef.
- P. Shao, M. M. Dal-Cin, M. D. Guiver and A. Kumar, J. Membr. Sci., 2013, 427, 451–459 CrossRef CAS.
- H. Zhai, E. S. Rubin and P. L. Versteeg, Environ. Sci. Technol., 2011, 45(6), 2479–2485 CrossRef CAS PubMed.
- J. Kotowicz and Ł. Bartela, Energy, 2012, 38, 118–127 CrossRef CAS.
- L. Zhao, E. Riensche, L. Blum and D. Stolten, J. Membr. Sci., 2010, 359, 160–172 CrossRef CAS.
- A. M. Arias, M. C. Mussati, P. L. Mores, N. J. Scenna, J. A. Caballero and S. F. Mussati, Int. J. Greenhouse Gas Control, 2016, 53, 371–390 CrossRef CAS.
- N. Mac Dowell, P. S. Fennell, N. Shah and G. C. Maitland, Nat. Clim. Change, 2017, 7, 243–249 CrossRef CAS.
- L. Rizzo, W. Gernjak, P. Krzeminski, S. Malato, C. S. McArdell, J. A. S. Perez, H. Schaar and D. Fatta-Kassinos, Sci. Total Environ., 2020, 710, 136312 CrossRef CAS PubMed.
- N. Lu and D. Xie, Int. J. Chem. React. Eng., 2016, 14, 1–31 CrossRef CAS.
- T. Westermann and T. Melin, Chem. Eng. Process., 2009, 48, 17–28 CrossRef CAS.
- B. Kumar, B. Muchharla, M. Dikshit, S. Dongare, K. Kumar, B. Gurkan and J. M. Spurgeon, Environ. Sci. Technol. Lett., 2024, 11, 1161–1174 CrossRef CAS PubMed.
- M. Namdari, Y. Kim, D. J. Pimlott, A. M. Jewlal and C. P. Berlinguette, Chem. Soc. Rev., 2025, 54, 590–600 RSC.
- D. Segets, C. Andronescu and U.-P. Apfel, Nat. Commun., 2023, 14, 7950 CrossRef CAS PubMed.
- Z. Liu, J. Qian, G. Zhang, B. Zhang and Y. He, Sep. Purif. Technol., 2024, 330, 125177 CrossRef CAS.
- J. Kampen and M. S. Annaland, J. Mater. Chem. A, 2021, 9, 14627–14629 RSC.
- Z.-Y. Zhang, H. Tian, L. Bian, S.-Z. Liu, Y. Liu and Z.-L. Wang, J. Energy Chem., 2023, 83, 90–97 CrossRef CAS.
- R. P. W. J. Struis, S. Stucki and M. Wiedorn, J. Membr. Sci., 1996, 113, 93–100 CrossRef CAS.
- W.-L. Tan, H.-F. Tan, A. Ahmad and C. Leo, J. CO2 Util., 2021, 48, 101533 CrossRef CAS.
- S. Escorihuela, C. Cerdá-Moreno, F. Weigelt, S. Remiro-Buenamañana, S. Escolástico, A. Tena, S. Shishatskiy, T. Brinkmann, A. Chica and J. M. Serra, J. CO2 Util., 2022, 55, 101813 CrossRef CAS.
- J. Zou, J. Huang and W. W. Ho, Ind. Eng. Chem. Res., 2007, 46, 2272–2279 CrossRef CAS.
- H. Wang, L. Shan, W. Shi, M. Wang, G. Quan, Z. Wang, L. Cui and J. Yan, J. Environ. Chem. Eng., 2023, 11, 110218 CrossRef CAS.
- Q. Zhao, M. Fu, Z. Xu, L. Deng, Y. Li, X. Meng, Q. Su and W. Cheng, Mol. Catal., 2023, 551, 113651 CrossRef CAS.
- H. Xu, R. Jin and C. P. O’Brien, ACS Appl. Mater. Interfaces, 2023, 15, 56305–56313 CrossRef CAS PubMed.
- Q. H. Pham, E. Goudeli and C. A. Scholes, Chem. Eng. J., 2024, 489, 151442 CrossRef CAS.
- J. Lee, H.-G. Park, M.-H. Hyeon, B.-G. Kim, S. K. Kim and S.-Y. Moon, Chem. Eng. J., 2021, 403, 126457 CrossRef CAS.
- T. Wang, K. Ge, K. Chen, C. Hou and M. Fang, Phys. Chem. Chem. Phys., 2016, 18, 13084–13091 RSC.
- S. Shishatskiy, J. R. Pauls, S. P. Nunes and K.-V. Peinemann, J. Membr. Sci., 2010, 359, 44–53 CrossRef CAS.
- L. E. Hatch, J. M. Creamean, A. P. Ault, J. D. Surratt, M. N. Chan, J. H. Seinfeld, E. S. Edgerton, Y. Su and K. A. Prather, Environ. Sci. Technol., 2011, 45, 5105–5111 CrossRef CAS PubMed.
- Y. Y. Birdja, R. l E. Vos, T. A. Wezendonk, L. Jiang, F. Kapteijn and M. T. Koper, ACS Catal., 2018, 8, 4420–4428 CrossRef CAS PubMed.
- T. L. Soucy, Y. Liu, J. B. Eisenberg and C. C. McCrory, ACS Appl. Energy Mater., 2021, 5, 159–169 CrossRef.
- G. K. Dutta and N. Karak, J. Cleaner Prod., 2021, 285, 124906 CrossRef CAS.
- S. Kundu and N. Karak, Chem. Eng. J., 2022, 438, 135575 CrossRef CAS.
- A. H. Behroozi and R. Xu, Chem. Catal., 2023, 3(3), 100550 CrossRef CAS.
- J. J. Chen, P. C. Oh and S. B. M. Saleh, Korean J. Chem. Eng., 2024, 41, 609–637 CrossRef CAS.
- F. R. Pomilla, A. Brunetti, G. Marcì, E. I. García-López, E. Fontananova, L. Palmisano and G. Barbieri, ACS Sustainable Chem. Eng., 2018, 6, 8743–8753 CrossRef CAS.
- A. Brunetti, F. R. Pomilla, G. Marcì, E. I. Garcia-Lopez, E. Fontananova, L. Palmisano and G. Barbieri, Appl. Catal., B, 2019, 255, 117779 CrossRef CAS.
- W. Chen, G.-B. Huang, H. Song and J. Zhang, J. Mater. Chem. A, 2020, 8, 20963–20969 RSC.
- A. Nishimura, Y. Okano, M. Hirota and E. Hu, Int. J. Photoenergy, 2011, 2011, 305650 CrossRef.
- S. K. Movahed, P. Jafari and S. Mallakpour, J. Environ. Chem. Eng., 2023, 11, 110426 CrossRef CAS.
- C.-C. Hu, C.-Y. Wang, M.-C. Tsai, R. L. G. Lecaros, W.-S. Hung, H.-A. Tsai, K.-R. Lee and J.-Y. Lai, Chem. Eng. J., 2022, 450, 138008 CrossRef CAS.
- I. Díaz, C. Pérez, N. Alfaro and F. Fdz-Polanco, Bioresour. Technol., 2015, 185, 246–253 CrossRef PubMed.
- J. Luo, A. S. Meyer, R. V. Mateiu and M. Pinelo, New Biotechnol., 2015, 32, 319–327 CrossRef CAS PubMed.
- R. Cazelles, J. Drone, F. Fajula, O. Ersen, S. Moldovan and A. Galarneau, New J. Chem., 2013, 37, 3721–3730 RSC.
- M. Z. do Valle Gomes, G. Masdeu, P. Eiring, A. Kuhlemann, M. Sauer, B. Åkerman and A. E. Palmqvist, Catal. Sci. Technol., 2021, 11, 6952–6959 RSC.
- J. J. Sheng, J. Nat. Gas Sci. Eng., 2015, 22, 252–259 CrossRef.
- N. Kumar, M. A. Sampaio, K. Ojha, H. Hoteit and A. Mandal, Fuel, 2022, 330, 125633 CrossRef CAS.
- B. Jia, J.-S. Tsau and R. Barati, Fuel, 2019, 236, 404–427 CrossRef CAS.
- A. Abedini, F. Torabi and N. Mosavat, Int. J. Oil, Gas Coal Technol., 2015, 9, 265–279 CrossRef CAS.
- N. Zhang, M. Wei and B. Bai, Fuel, 2018, 220, 89–100 CrossRef CAS.
- B. F. Snyder, M. Layne and D. E. Dismukes, Int. J. Greenhouse Gas Control, 2020, 93, 102885 CrossRef CAS.
- A. Midttun, E. Enger, A. Lind, M. Lia, J. Meyer, M. V. Storaas, J. Lereim and P. Nygaard, Carbon capture—from waste to energy: a stylized case from a pioneering initiative at Klemetsrud, Oslo. Report to the CLIMIT–demo project 618215: Potential for financing and pricing Carbon Capture in Waste-to Energy Installations in cities, 2019.
- E. N. Kalogirou, in Waste-to-Energy Technologies and Global Applications, CRC Press, 2024, pp. 80–138 Search PubMed.
- C. Preston, The carbon capture project at air products’ port Arthur hydrogen production facility, in 14th greenhouse gas control technologies conference Melbourne, 2018, pp. 21–26.
- T. A. Meckel, A. P. Bump, S. D. Hovorka and R. H. Trevino, Greenhouse Gases: Sci. Technol., 2021, 11, 619–632 CrossRef.
- Y. Shiyi, M. Desheng, L. Junshi, Z. Tiyao, J. Zemin and H. Haishui, Pet. Explor. Dev., 2022, 49, 955–962 CrossRef.
- X. Zhang, Q. Liao, Q. Wang, L. Wang, R. Qiu, Y. Liang and H. Zhang, Energy, 2021, 225, 120297 CrossRef CAS.
- E. Adu, Y. Zhang and D. Liu, Can. J. Chem. Eng., 2019, 97, 1048–1076 CrossRef CAS.
- D. Lirong, S. Longde, L. Weifeng, W. Mingyuan, G. Feng, G. Ming and H. Jiang, Pet. Explor. Dev., 2023, 50, 1246–1260 CrossRef.
- A. Peltz, S. Anderson, N. Saunders, J. Koka, J. Graham and B. Portela, Strategies for attaining CO2 sequestration with environmental integrity, in Abu Dhabi International Petroleum Exhibition and Conference (p. D021S035R002), SPE, 2022 Search PubMed.
- R. J. Pawar, G. S. Bromhal, J. W. Carey, W. Foxall, A. Korre, P. S. Ringrose, O. Tucker, M. N. Watson and J. A. White, Int. J. Greenhouse Gas Control, 2015, 40, 292–311 CrossRef CAS.
- M. J. Regufe, A. Pereira, A. F. Ferreira, A. M. Ribeiro and A. E. Rodrigues, Energies, 2021, 14, 2406 CrossRef CAS.
- A. Hastings and P. Smith, Front. Clim., 2020, 2, 601778 CrossRef.
- G. Thakur, S. Bose and A. Selveindran, Carbon Storage Focused Reservoir Management: A Practical Example to Respond to Climate Change, in Proceedings of the Future Technologies Conference, Springer Nature Switzerland, Cham, 2023, pp. 578–592 Search PubMed.
- V. Nuñez-Lopez, R. Gil-Egui, P. Hosseininoosheri, S. D. Hovorka and L. W. Lake, Carbon life cycle analysis of CO2-EOR for net carbon negative oil (NCNO) classification, Univ. of Texas, Austin, TX (United States), 2019 Search PubMed.
- A. Kamolov, Z. Turakulov, S. Rejabov, G. Díaz-Sainz, L. Gómez-Coma, A. Norkobilov, M. Fallanza and A. Irabien, Membranes, 2023, 13, 130 CrossRef CAS PubMed.
- D. Kearns, H. Liu and C. Consoli, Technology readiness and costs of CCS, Global CCS institute, 2021, vol. 3.
- D. V. D. A. John Zhou, R. Chalaturnyk, G. Meikle, M. Gray and B. W. Sanah Dar, Bonnie Drozdowski, and Heather Campbell, Carbon Capture, Utilization, and Storage (CCUS) Technology Innovation to Accelerate Broad Deployment in Alberta, Alberta Innovates, Albertainnovates.ca, 2022.
- R. M. Cuéllar-Franca and A. Azapagic, J. CO2 Util., 2015, 9, 82–102 CrossRef.
- H. H. Khoo and R. B. Tan, Environ. Sci. Technol., 2006, 40, 4016–4024 CrossRef CAS PubMed.
- J. Koornneef, T. van Keulen, A. Faaij and W. Turkenburg, Int. J. Greenhouse Gas Control, 2008, 2, 448–467 CrossRef CAS.
- B. Singh, A. H. Strømman and E. G. Hertwich, Int. J. Greenhouse Gas Control, 2011, 5, 911–921 CrossRef CAS.
- Z. Nie, A. Korre and S. Durucan, Energy Procedia, 2011, 4, 2510–2517 CrossRef CAS.
- C. A. Scholes, K. H. Smith, S. E. Kentish and G. W. Stevens, Int. J. Greenhouse Gas Control, 2010, 4, 739–755 CrossRef CAS.
- S. C. Kumbharkar, P. B. Karadkar and U. K. Kharul, J. Membr. Sci., 2006, 286, 161–169 CrossRef CAS.
- M. A. Carreon and S. R. Venna, Metal-Organic Framework Membranes for Molecular Gas Separations, World Scientific, 2020 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |






