Open Access Article
Open Access ArticleElectrolytic cement clinker precursor production sustained through orthogonalization of ion vectors†
Zishuai
Zhang
 a,
Aubry S. R.
Williams
a,
Aubry S. R.
Williams
 a,
Shaoxuan
Ren
a,
Shaoxuan
Ren
 a,
Benjamin A. W.
Mowbray
a,
Benjamin A. W.
Mowbray
 a,
Colin T. E.
Parkyn
a,
Yongwook
Kim
a,
Colin T. E.
Parkyn
a,
Yongwook
Kim
 a,
Tengxiao
Ji
a,
Tengxiao
Ji
 a and
Curtis P.
Berlinguette
a and
Curtis P.
Berlinguette
 *abcd
*abcd
aDepartment of Chemistry, The University of British Columbia, 2036 Main Mall, Vancouver, British Columbia V6T 1Z1, Canada. E-mail: cberling@chem.ubc.ca
bDepartment of Chemical and Biological Engineering, The University of British Columbia, 2360 East Mall, Vancouver, British Columbia V6T 1Z3, Canada
cStewart Blusson Quantum Matter Institute, The University of British Columbia, 2355 East Mall, Vancouver, British Columbia V6T 1Z4, Canada
dCanadian Institute for Advanced Research (CIFAR), 661 University Avenue, Toronto, M5G 1M1, Ontario, Canada
First published on 29th January 2025
Abstract
Electrochemical reactors can reduce the carbon intensity of cement production by using electricity to convert limestone (CaCO3) into Ca(OH)2, which can be converted into cement clinker by reacting with silica (SiO2) at high temperatures. A key challenge with electrochemical reactors is that the deposition of solid Ca(OH)2 at the membrane leads to unacceptably low energy efficiencies. To address this challenge, we connected the electrochemical reactor used for limestone calcination (“cement electrolyser”) to a distinctive chemical reactor (“calcium reactor”) so that Ca(OH)2 forms in the calcium reactor instead of within the electrochemical reactor. In this tandem system, the cement electrolyser generates H+ and OH− in the respective chemical and cathode compartments. The H+ then reacts with CaCO3 to release Ca2+, which is diverted into the calcium reactor to react with the OH− to form Ca(OH)2. We fabricated a composite membrane to selectively block the transport of Ca2+ into the cathode compartment. Charge balance in the cement electrolyser was enabled with monovalent ions (e.g., K+) as the positive charge carrier. This orthogonalized ion management was validated by operando imaging. The tandem reactor enabled the electrolysis process to operate for 50 hours at 100 mA cm−2 without any voltage increase, which represents a meaningful step forward for electrochemical cement clinker precursor production.
Broader contextCement production is a significant source of global carbon emissions, partly because of the energy intensity of thermally converting limestone (CaCO3) into cement clinker. To reduce this energy intensity, electricity can be used to convert limestone into calcium hydroxide (Ca(OH)2), which can then be processed into cement clinker. A significant challenge in using an electrolyser to decompose limestone is the deposition of Ca(OH)2 on the membrane that separates the reaction compartments. This deposition of Ca(OH)2 reduces the energy efficiency of the system. To address this issue, we developed a tandem unit that connects an electrochemical reactor (“cement electrolyser”) with a chemical reactor (“calcium reactor”). In this tandem unit, the cement electrolyser produces hydrogen ions (H+) and hydroxide ions (OH−) in separate compartments. The H+ ions react with CaCO3 to form calcium ions (Ca2+), which are diverted to the calcium reactor to form Ca(OH)2 with the OH− ions. A composite membrane that selectively blocks Ca2+ from entering the cathode compartment enabled the operation of this tandem reactor for 50 hours at 100 mA cm−2 without a voltage increase. This development represents a step forward in reducing the carbon footprint of cement production using electrochemical methods. |
Introduction
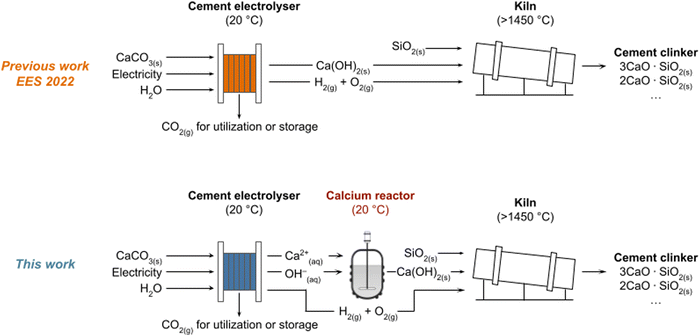
Cement production accounts for ∼8% of global CO2 emissions,1 primarily due to the production of cement clinker, the active component of cement.2 The commercial production of cement clinker involves the thermal decomposition of limestone (CaCO3) into CaO and CO2 (∼900 °C, eqn (1)). Heat generated from the combustion of fossil fuels drives this decomposition and releases significant amounts of CO2 into the atmosphere.1 Demonstrations of electrochemical reactors (“cement electrolysers”) have emerged that can convert CaCO3 into Ca(OH)2 (slaked lime; a cement clinker precursor) using renewable electricity to reduce CO2 emissions from cement production.1–4 The Ca(OH)2 can then be reacted with SiO2 to form cement clinker (>1450 °C, eqn (2)).1 While these cement electrolysers eliminate only one of two high-temperature steps (CaCO3 calcination, eqn (1)), they produce a pure stream of CO2 byproduct that can be stored or converted into value-added products.5–7 This electrochemical approach could reduce the carbon intensity of cement production by up to 75% (Fig. 1).1| CaCO3(s) ⇌ CaO(s) + CO2(g) | (1) |
| 3Ca(OH)2(s) + SiO2(s) → 3CaO·SiO2(s) + 3H2O(l) | (2) |
There are two key factors to consider when designing a cement electrolyser to decompose CaCO3. The first is that CaCO3 is not electrochemically active; it cannot be decomposed by electron transfer reactions at an electrode. However, CaCO3 reacts with acid to form soluble Ca2+ and gaseous CO2 (eqn (3)). A cement electrolyser must therefore continuously generate acid to drive the decomposition of CaCO3 and generate the reactive Ca2+ needed to form the Ca(OH)2 product. The second factor is that the two-step conversion of CaCO3 into Ca(OH)2 needs to occur at high production rates (e.g., current densities, j, above 100 mA cm−2). Consequently, a cement electrolyser must effectively manage the collective transport of the CaCO3 feedstock, reactive Ca2+, and Ca(OH)2 product.
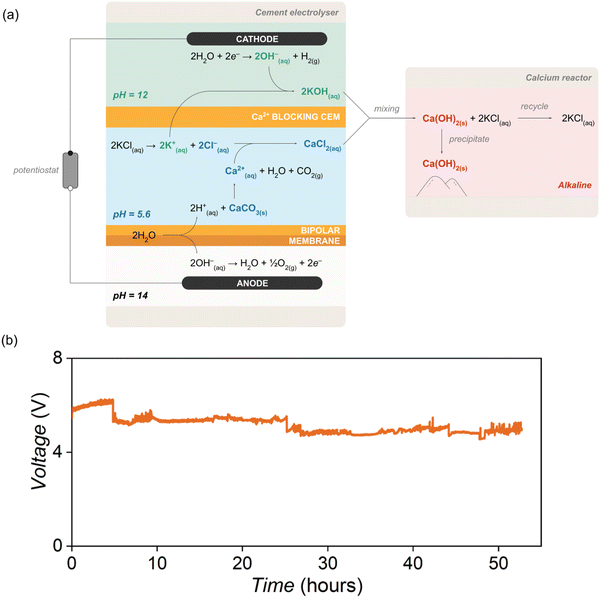
We addressed these points by designing an electrolyser that consisted of three compartments: an anode compartment for oxygen generation; a cathode compartment for hydroxide generation; and a chemical compartment situated between the anode and cathode compartments for CaCO3 decomposition with acid (Fig. 2).1 A bipolar membrane (BPM) separated the anode and chemical compartments, and a cation exchange membrane (CEM) separated the chemical and cathode compartments. The BPM delivered H+ into the chemical compartment to react with CaCO3 (eqn (3)).8–14 In the anode compartment, the oxygen evolution reaction (OER) consumed the generated OH−. In the cathode compartment, the hydrogen evolution reaction (HER) generated H2 and OH− to create an alkaline environment with a pH of ∼12 (eqn (4)).1 The working principle of the cement electrolyser is that Ca2+ generated in the chemical compartment passes through the CEM and enters the cathode compartment to react with OH− and form Ca(OH)2 (eqn (5) and Fig. 2). At the anode, the OER generates a pure O2 stream that can be combusted with the H2 generated at the cathode as a source of heat for thermal steps in cement production (Fig. 1).1
| CaCO3(s) + 2H+(aq) ⇌ Ca2+(aq) + CO2(g) + H2O | (3) |
| 2H2O → 2OH−(aq) + H2(g) + 2e− | (4) |
| Ca2+(aq) + 2OH−(aq) → Ca(OH)2(s) | (5) |
This cement electrolyser was capable of operating at a current density of 100 mA cm−2 with 100% current efficiency and enabled the highest rates of electrochemical Ca(OH)2 formation reported in the literature to date (486 mg h−1). However, this electrolyser was not able to sustain operation for extended time periods without incurring a massive increase in cell voltage (Fig. S1, ESI†). This drop in electrolyser performance occurred due to Ca(OH)2 deposition at the CEM (Nafion™), which stunted Ca2+ transport into the cathode compartment (Fig. S2, ESI†). This factor, as well as a decrease in membrane conductivity caused by strong Ca2+ binding and consequent membrane dehydration,15 led to a significant increase in cell voltage within hours (Fig. S1 and S2, ESI†). A robust cement electrolyser capable of sustained continuous operation (i.e., >100 hours) would manage the transport of Ca2+ more effectively to avoid Ca(OH)2 deposition on the membrane.
We report here a cement electrolyser that can sustain operation at 100 mA cm−2 for at least 50 hours. The electrolyser was designed to use potassium ions (K+) rather than calcium ions (Ca2+) as the positive charge carrier between the chemical and cathode compartments (Fig. 2). Unlike Ca2+, K+ does not form an insoluble deposit with OH− on the CEM or dehydrate the CEM. Moreover, the ionic mobility of K+ (∼30 mS cm−1)16 is higher than Ca2+ (∼14 mS cm−1),17 which increases the energy efficiency of the cement electrolyser.16,17 We designed a composite CEM to selectively block Ca2+ and accommodate the selective transport of K+ (Fig. 2). When K+ is forced as the main charge carrier across the membrane, Ca2+ and OH− accumulate in the chemical and cathode compartments, respectively, during electrolysis. We directed these ions out of the cement electrolyser and into a “calcium reactor” where Ca(OH)2 was formed (Fig. 1 and 2). This cement electrolyser and calcium reactor in tandem orthogonalized the transport of ions to enable the continuous conversion of CaCO3 into Ca(OH)2 at a current density of 100 mA cm−2 and voltage of ∼5 V over 50 hours. This report is the first demonstration of sustained cement clinker precursor production at high current densities.
Results
Design of electrochemical reactors for Ca(OH)2 production
This study was conducted using both a previously reported cement electrolyser design1 and a modified cement electrolyser with a calcium reactor. In the typical three-compartment cement electrolyser (Fig. S2, ESI†), the anode and cathode compartments were supplied with 1 M potassium hydroxide (KOH) and 1 M potassium chloride (KCl) solutions, respectively, and the chemical compartment was supplied with an aqueous slurry of CaCO3 microparticles in 1 M KCl at a flow rate of 300 mL min−1. For the 50-hour experiments, additional CaCO3 was added to the electrolyte reservoir after 24 h. A BPM (Fumasep FBM) separated the anode and chemical compartments, and a CEM (Nafion™ 117) separated the chemical and cathode compartments. The primary difference in the modified cement electrolyser was the use of a composite CEM in place of a pristine Nafion™ membrane. This composite membrane blocked Ca2+ transport and instead enabled K+ to be selectively transported across the CEM, which caused Ca2+ and OH− to accumulate in the chemical and cathode compartments, respectively. The Ca2+ and OH− enriched electrolytes from the chemical and cathode compartments were then manually mixed in a calcium reactor (specifically a 200 mL beaker) to form the Ca(OH)2 product (Fig. 2). The precipitated Ca(OH)2 was filtered from the mixed electrolyte so that the remaining KCl solution could be treated and reused. This stepwise CaCO3-to-Ca(OH)2 conversion process demonstrates the feasibility of the hybrid electrochemical and chemical method for Ca(OH)2 production, and future work could involve building a fully automated system.Design and fabrication of a composite membrane to block Ca2+ transport
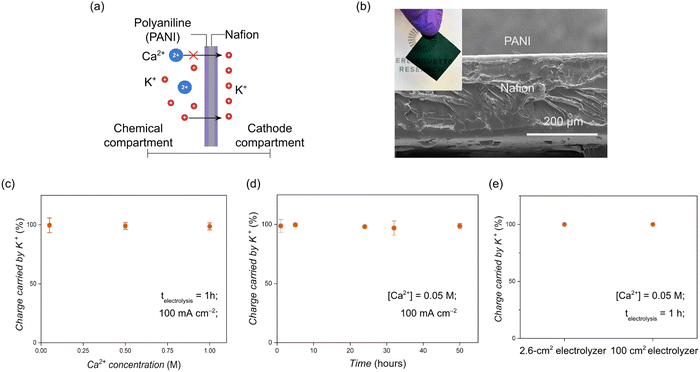
To establish a baseline for the durability of the cement electrolyser, we ran an electrolysis experiment using a pristine Nafion™ membrane (positioned between the chemical and cathode compartments) under constant current conditions. The cell voltage (Vcell) increased from 5 V to 8 V within 60 minutes of electrolysis at 100 mA cm−2 (Fig. S1, ESI†). After disassembling the cell, we observed white deposits on the cathode-facing side of the Nafion™ membrane. Scanning electron microscopy (SEM) and X-ray fluorescence (XRF) images indicated the deposits consisted of a calcium-containing species (Fig. S2, ESI†), and Fourier transform infrared spectroscopy (FTIR) confirmed the precipitates consisted primarily of Ca(OH)2 with trace amounts of CaCO3 (Fig. S3, ESI†). We also observed that the Nafion™ membrane became dehydrated and developed wrinkles and cracks. The pliable texture of the Nafion™ membrane also became rigid (Fig. S4, ESI†).To circumvent the deleterious effects of Ca(OH)2 deposition on the membrane, we set out to prevent Ca2+ from transporting through the CEM as the charge carrier. We fabricated a composite CEM that permitted only monovalent ion transport and blocked divalent ions such as Ca2+ (Fig. 3). We deposited layers of polyaniline (PANI) on both sides of a Nafion™ 117 membrane.18 The PANI-modified membrane was prepared by ion exchange of the protons in a pristine Nafion™ membrane with protonated aniline. Potassium persulfate (K2S2O8) was then used to polymerize aniline into PANI. The composite membrane was then stored in a hydrochloric acid (HCl) solution for 24 hours to ensure the PANI layer was fully protonated (i.e., positively charged). Following these steps, the composite membrane turned a translucent dark green colour in contrast to the translucent colourless appearance of pristine Nafion™. Scanning electron microscopy (SEM) images indicated the prepared PANI layers were roughly 5–10 μm thick and free of defects such as pinholes and large pores (Fig. 3).
Sustained electrochemical Ca(OH)2 production enabled by a Ca2+-blocking membrane
To evaluate the Ca2+-blocking capacity of the composite membrane, we modulated the CaCl2 concentration in the CaCO3 slurry fed to the chemical compartment of the cement electrolyser and monitored the amount of Ca2+ passing through the membrane during electrolysis. Excess CaCO3 was added to the slurry to consume H+ generated by the BPM and ensure the charge carrier across the composite membrane was only K+.1 We measured the concentration of calcium ions ([Ca2+]) in the cathode compartment over the course of each experiment using inductively coupled plasma optical emission spectrometry (ICP-OES) and used this data to calculate the charge carried by K+ across the CEM (details are shown in the Methods section and Tables S1 and S2, ESI†). We found the composite membrane blocked ∼100% of Ca2+ from crossing into the cathode compartment over a wide range of [Ca2+], specifically from 0.05 to 1.0 M (Fig. 3).We also measured the charge carried by K+ through the composite membrane over 50 hours of electrolysis. A constant [Ca2+] of 0.05 M was used to match the [Ca2+] measured in the chemical compartment after 120 minutes of electrolysis at 100 mA cm−2. Periodic deposition of Ca(OH)2 in the calcium reactor prevented the [Ca2+] from exceeding 0.05 M. We found the composite membrane demonstrated ∼100% Ca2+ blockage over the 50 hour test period (Fig. 3). We verified the robustness of the PANI layer by performing FTIR (Fig. S5, ESI†) and SEM (Fig. S6, ESI†) on the composite membrane before and after the 50-hour test. We observed no change in the FTIR spectra, and the PANI layer appeared intact after the 50-hour test. (Fig. S7, ESI†). We also fabricated a much larger composite membrane for testing in a zero-gap electrolyser with an active area of 100 cm2 to explore the scalability of the composite membrane. This experiment was again performed with a [Ca2+] of 0.05 M and a [K+] of 1 M in the 100 cm2 electrolyser, which we operated at 50 mA cm−2. The membrane maintained 100% charge carried by K+ when operating at a total current of 5 A (Fig. 3 and Fig. S8, ESI†).
When the composite membrane was used in the modified cement electrolyser, concentrations of Ca2+ and OH− in the chemical and cathode compartments, respectively, increased stoichiometrically during electrolysis. We manually mixed the Ca2+-rich and OH−-rich electrolytes in the calcium reactor after two hours of electrolysis at 100 mA cm−2. The Ca2+-blocking membrane in the cement electrolyser coupled with the calcium reactor enabled the cement electrolyser to operate for 50 hours with a stable cell voltage (Fig. 2). A “simulated scenario” was used for the durability test wherein [Ca2+] was maintained at 0.05 M (the [Ca2+] in the electrolyte after 120 minutes of electrolysis at 100 mA cm−2) and [K+] was set at 1 M. To maintain a constant [Ca2+], the electrolyte in the chemical compartment was refreshed every 6–8 hours and the catholyte was regularly monitored to confirm that no detectable Ca2+ had crossed the membrane. We observed a 0.4 V increase in cell voltage at 100 mA cm−2 compared with the pristine Nafion™ membrane, where the voltage increased by 2 V. This prolonged stability is a notable improvement on the previous electrolyser containing a pristine Nafion™ membrane. However, we initially observed CaCO3 impurity in the Ca(OH)2 product in the calcium reactor. We removed unreacted CaCO3 from the chemical compartment electrolyte by centrifuging the mixture at 3000 rpm for 10 minutes before mixing. We hypothesized that the HCO3− in the chemical compartment reacts with the OH− from the catholyte to form CO32− which then reacts with Ca2+ to form CaCO3. To mitigate this effect, we mixed the electrolytes in the calcium reactor only when the chemical compartment was below a pH of 5, which significantly decreased the CaCO3 impurities in Ca(OH)2.
We ran an additional experiment where we tested the pH in each compartment of the cement electrolyser containing the composite membrane every 30 minutes for two hours with a pH probe. We found that both the chemical and cathode compartments rapidly approached steady pH values of 5.6 and 13.5, respectively, with a significant pH change in both compartments within the first 30 minutes (Fig. S9, ESI†). The chemical compartment pH decreased from 9.9 to 6.1 in 30 minutes, and then to 5.6 by the end of the experiment (total experiment time of 2 hours). The cathode compartment became more basic with pH increasing from 8.8 to 12.9 in 30 minutes and then to 13.5 by the end of the 2-hour experiment. The anode compartment pH did not vary significantly from its starting value of 13.8.
Purity of the electrochemical Ca(OH)2
The American Society for Testing and Materials (ASTM) sets limits on the allowable amounts of potassium and chlorine in cement clinker because of their potential to adversely affect the finished product.19–21 In our study, we filtered out the solid Ca(OH)2 product after mixing the Ca2+-rich and OH−-rich electrolytes in the calcium reactor. The volume loss during filtration was consistently 2.5 ± 0.5 mL, independent of the total volume filtered, which ranged from 100–500 mL. This corresponds to KCl recovery efficiencies between 97.5–99.5%. After filtration, the solid product (∼1.5 g) was rinsed with 3 × 50 mL of water and then dried in an oven at 60 °C for at least 24 hours before analysis. The Ca(OH)2 particles had an average size of 1.45 ± 0.13 μm, as determined by SEM (Fig. S10, ESI†). This value closely matches the typical particle size of ∼4 μm found in commercial Ca(OH)2.22ICP-OES was used to quantify the amount of potassium retained in the Ca(OH)2 product from the supporting electrolyte. A dilution series of KCl in 2 wt% HNO3 was prepared to generate a calibration curve for potassium concentrations. The amount of residual potassium in the isolated product was determined using a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm solution of the electrochemically produced Ca(OH)2 prepared with 2 wt% HNO3. The results indicated a potassium concentration of 62.5 ± 4.2 ppm, equating to 0.16 ± 0.011 wt% potassium in the solid Ca(OH)2. This is less than the ASTM limit of alkali metals (K and Na) allowed in cement according of 0.60 wt%.19 To supplement the ICP-OES results, the Ca(OH)2 product was also characterized by X-ray diffraction (XRD; Fig. S11, ESI†). No KCl or CaCl2 was observed in the diffractogram, supporting the purity of the electrochemically produced Ca(OH)2.
000 ppm solution of the electrochemically produced Ca(OH)2 prepared with 2 wt% HNO3. The results indicated a potassium concentration of 62.5 ± 4.2 ppm, equating to 0.16 ± 0.011 wt% potassium in the solid Ca(OH)2. This is less than the ASTM limit of alkali metals (K and Na) allowed in cement according of 0.60 wt%.19 To supplement the ICP-OES results, the Ca(OH)2 product was also characterized by X-ray diffraction (XRD; Fig. S11, ESI†). No KCl or CaCl2 was observed in the diffractogram, supporting the purity of the electrochemically produced Ca(OH)2.
Conversion of Ca(OH)2 into calcium silicates
The electrochemical Ca(OH)2 was then converted into calcium silicates by thermal treatment with SiO2. We mixed Ca(OH)2 with SiO2 particles in a mortar and pestle and then transferred the mixture to a Pt crucible. The crucible was placed in a kiln and heated to a temperature of 1050 °C and held for 2 hours to form dicalcium silicate (2CaO·SiO2; Fig. S12, ESI†), and 1500 °C to form tricalcium silicate (3CaO·SiO2; Fig. S12, ESI†).Operando imaging system to diagnose membrane failure modes
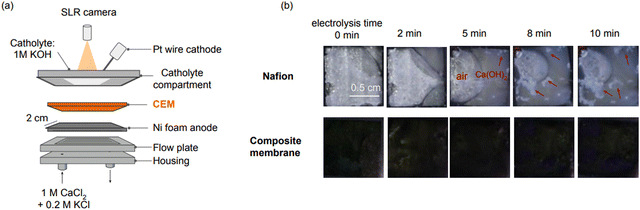
We designed an operando imaging system to directly visualize Ca(OH)2 deposition on the CEM and diagnose this membrane failure mode in real time (Fig. 4). The imaging system consisted of a modified two-compartment electrolyser with an open cathode compartment so we could image the CEM through the transparent catholyte with a high-resolution single-lens reflex (SLR) camera fixed above the electrolyser (Fig. 4 and Fig. S13, ESI†). A 1 M KOH solution was used as the catholyte to mimic the alkaline conditions of the chemical compartment in a cement electrolyser. A Pt wire was immersed into the catholyte to serve as the cathode. The anode on the opposite side of the CEM consisted of nickel (Ni) foam to facilitate the OER. Ni foam is an inexpensive, porous, and high surface area electrode material that is ideal for use as an OER electrocatalyst. The anode compartment was fed with an electrolyte containing 1 M CaCl2 and 0.2 M KCl through a serpentine flow plate pressed directly against the Ni foam. This mixed electrolyte solution created a scenario where Ca2+ and K+ could both serve as charge carriers across the CEM. We performed experiments using this setup with both pristine Nafion™ 117 and the PANI-modified composite membrane. With the pristine Nafion™ membrane, where Ca2+ was the charge carrier across the CEM, we observed the immediate formation of white Ca(OH)2 precipitates on the CEM surface that faced the cathode. These Ca(OH)2 precipitates (white spots in Fig. 4) formed on the Nafion™ membrane within five minutes of electrolysis at 100 mA cm−2 (Fig. 4) and grew in size over time. In contrast, no precipitate formed on the composite membrane. The high-resolution camera also detected the presence of bubbles trapped between the anode and CEM from the vertical orientation of the electrolyser, which was required to perform imaging.Energy and CO2 emission analysis
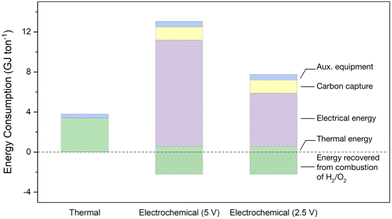
We calculated the energy required to convert CaCO3 into cement when using our cement electrolyser and compared it to the conventional thermal process (Fig. 5). We also calculated the net CO2 emissions per ton of cement for both methods (Fig. 6). The system boundary for the electrochemical pathway includes the electrolytic conversion of CaCO3 to Ca(OH)2, followed by its conversion to clinker in a kiln. In contrast, the system boundary for the thermal process includes the direct conversion of CaCO3 to clinker in a kiln. We modeled both processes based on a hypothetical production capacity of 3000 tons of cement per day, assuming 95 wt% of the product is clinker, and the clinker is 65 wt% CaO.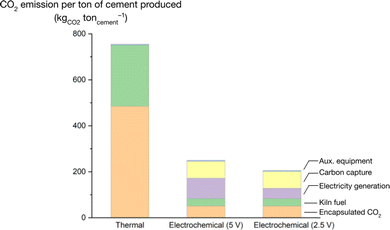 | ||
| Fig. 6 Comparison of net CO2 emissions for production of 1 ton of cement via conventional thermal pathway vs. electrochemical cement clinker precursor production at Vcell = 5 V (current performance) and 2.5 V (optimistic case) with carbon capture. To calculate the emissions from the electrochemical processes, we used a grid emissions intensity of 0.030 kg CO2 kWh−1, the same as that for the Province of Ontario,23 where the majority of Canada's cement is produced.24 | ||
For the thermal process, 3.4 GJ of thermal energy is required per ton of cement,25 provided by a fuel mix that would be used at a Canadian cement factory: 28% natural gas, 27% coal, 35% coke, 2% fuel oil, and 8% waste tires.26 The emissions intensity of the kiln fuel was calculated to be 87.3 kgCO2 GJ−1.27 Additional energy for auxiliary equipment (e.g., pumps, conveyors, crushers) adds approximately 0.4 GJ, bringing the total to 3.8 GJ ton−1 of cement.
In the electrochemical pathway, we considered two scenarios: one using the cell voltage reported in this study (5 V, Fig. 2); and another optimistic scenario where the cell voltage has been reduced by half to 2.5 V, which has been demonstrated to be feasible in prior work.28 In both cases, the electrical energy required to decompose limestone dominates the total energy input, with values of 10.6 GJ ton−1 and 5.3 GJ ton−1 of cement for the 5 V and 2.5 V cases, respectively. Both systems need an additional 2.8 GJ ton−1 of cement to convert Ca(OH)2 into clinker.2 However, the eletrochemical reactors also generate pure streams of H2 and O2, which can be combusted to supply most of the thermal energy of this step. If combusted with a thermal efficiency of 70%, the H2 and O2 streams supply 2.2 GJ ton−1 of cement. Retrofits will be required to safely handle and burn these gas streams due to the flame characteristics of H2 and its potential to embrittle stainless steel.29–31 Hydrogen blending has already been successfully demonstrated in several cement kilns.32–34
The electrochemical process also produces a stream of pure CO2 from the chemical compartment of the electrolyser when CaCO3 reacts with acid. Our model assumes 90% of this CO2 is captured by an amine scrubber with a reboiler duty of 3 MJ kg−1 of CO2.35 The reboiler and balance of the secondary kiln are fueled by pure natural gas for the electrochemical processes. Carbon capture is not considered for the thermal process as it remains very rare at established cement plants.35–38 The largest carbon capture unit currently operational at a cement plant, located at the Baimashan Cement Factory in Anhui, China,39 captures 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of CO2 annually, much less than 1% of the company's annual CO2 emissions of 183 million tons.40
000 tons of CO2 annually, much less than 1% of the company's annual CO2 emissions of 183 million tons.40
The low adoption of carbon capture at cement factories is due to sorbent degradation from O2, NOx, and SOx in flue gas,41–43 difficulty in retrofitting existing cement plants with large carbon capture units,44 and high capital and operating costs.45 In prior work we demonstrated that the CO2 emitted from the electrolyser chemical compartment can be fed directly into a CO2 electrolyser for conversion into valuable products.1
Discussion
Our previously reported cement electrolyser electrochemically generated acid in a chemical compartment to decompose limestone into Ca2+, which migrated across a CEM into a cathode compartment to react with OH− and form Ca(OH)2. The challenge with this setup was Ca(OH)2 deposited on the surface of the CEM during electrolysis, which impeded additional Ca2+ transport into the cathode compartment. This deposition caused the cement electrolyser to exhibit voltages much higher than 5 V.This study describes our effort to address the calcium deposition to achieve sustained cement electrolysis. To directly observe the Ca(OH)2 deposition on the membrane surface, our operando imaging system featured an electrolyser with an open cathode compartment. This cathode compartment resembled a batch-type cathode compartment without a flowing catholyte to improve visualization and provide favourable conditions for precipitate formation.
We observed that Ca(OH)2 deposited on the pristine Nafion™ membrane within five minutes of electrolysis. The spatial location of the deposits on the membrane appeared to have been influenced by the catholyte flow. Specifically, we observed that Ca(OH)2 deposits initially formed near the compartment walls or as static bubbles when the catholyte flow was stagnant (Fig. 4). The initial deposits served as “seeds” for subsequent Ca(OH)2 accumulation. These observations provide insight into how Ca(OH)2 deposition occurs and can inform alternate mitigation strategies.
In our first experiment, we attempted to remove the Ca(OH)2 deposits (when ΔV > 2 V) by periodically treating the CEM with acid (i.e., HCl). The acid treatment appeared to remove Ca(OH)2 deposits from the membrane surface, but not the Ca2+ bound within the membrane. This experiment proved that Vcell could be recovered when the Ca(OH)2 deposits were promptly removed (Fig. S14, ESI†), but would not recover after three hours of electrolysis. We attribute this irreversibility in voltage to the strong binding of Ca2+ to the sulfonic groups in Nafion™ membranes, which decreased H2O diffusion and membrane conductivity.15
We then set out to use membranes that would prevent the transport of Ca2+ across the CEM. Selective blocking of divalent cations (e.g., Ca2+, Mg2+) across CEMs has been reported in the fields of electrodialysis and water treatment, where chemical, mechanical, and electrochemical techniques have been used to modify ion-exchange membranes.46,47 Prior reports achieved this goal by applying a PANI coating to the surface of CEMs. This strategy increases K+ permselectivity by leveraging increased electrostatic repulsion between the modified cationic surface of Nafion™ (i.e., emeraldine-based PANI) and divalent cations in comparison to monovalent K+. We borrowed from these fields to chemically modify the surface of Nafion™ with thin PANI layers to design a composite membrane for exceptional selectivity for monovalent cation transport (i.e., K+).46 We observed high K+ selectivity over Ca2+ when using this composite membrane. We attribute this result to three reasons. First, the larger size of hydrated Ca2+ (effective radii = 0.412 nm) relative to K+ (effective radii = 0.331 nm) results in slower transport through the dense PANI layer.46 Second, the positively charged PANI layer leads to a large Donnan potential for divalent cations including the blocked Ca2+, since electrostatic repulsion effects are stronger for divalent cations.46 Finally, the much larger dehydration energy of Ca2+ (ΔGdehy = 1505 kJ mol−1) relative to K+ (ΔGdehy = 295 kJ mol−1) makes the transport of desolvated Ca2+ through the membrane less energetically favorable than K+.46
When designing our modified cement electrolyser, we used a similar configuration to our previous three-compartment cement electrolyser, which had a BPM to decompose CaCO3 and release Ca2+ and pure streams of CO2, O2, and H2. The CO2 could be utilized directly (e.g., with electrolysis) without further processing1 and the H2 could be used as fuel for a subsequent thermal kiln or be fed back to the anode for the hydrogen oxidation reaction (HOR) to reduce the electrolysis voltage.28 The key difference in the design was the composite CEM used to separate Ca2+ and OH− production and combining enriched electrolytes in a dedicated reactor to produce the target product, Ca(OH)2. This new design enabled us to generate the solid products in a separate chemical reactor without compromising the performance of the electrolyser and allowed us to optimize the electrochemical and chemical systems independently.
The electrolyser demonstrated in this study was operated as a batch process. However, for large-scale production, such as cement manufacturing, continuous operation is far more desirable. We hypothesize that our electrolyser can now be run continuously as Ca(OH)2 precipitation no longer limits its stability. Furthermore, we satisfied the two criteria required for continuous electrochemical production of a cement clinker precursor by maintaining a steady-state pH in the chemical compartment that is low enough to dissolve CaCO3, while simultaneously ensuring a high enough pH in the cathode compartment to precipitate Ca(OH)2. Our future work will explore continuous electrochemical cement clinker precursor production.
The supporting electrolyte in this study was 1 M KCl. The presence of potassium and chloride in cement clinker can negatively impact the final product. Elevated levels of alkalis (Na and K) can decrease the final strength of the cement,48 and promote the alkali-silica reaction, which can cause expansive stresses in concrete.49,50 Alkali concentrations in cement below 0.60 wt% are generally preferred, which is the limit for ordinary Portland cement (all types, I–V) when mixed with aggregates that are potentially reactive.19,51 Chloride ions pose another risk by accelerating the corrosion of the steel reinforcements in concrete.21,52 The resulting oxidation of the iron in the steel bar generates internal stresses in the concrete and weakens the bar itself,53,54 and is the most common mode of failure for reinforced concrete structures.52 Chloride content in cement is limited to between 0.06–1.00 wt%, depending on the end use.55
In this work, we demonstrated that the Ca(OH)2 produced by our electrolyser has concentrations of potassium and chloride well below the limits set on ordinary Portland cement. We also demonstrated the formation of calcium silicates by thermal clinkering of electrochemical Ca(OH)2 with SiO2 (Fig. S12, ESI†). This demonstrates that the Ca(OH)2 produced by our electrolyser is suitable for use as a clinker precursor free of harmful impurities, and is capable of forming dicalcium silicate at 1050 °C and tricalcium silicate at 1500 °C in a kiln.
The arrangement of an electrolyser and calcium reactor in tandem to produce Ca(OH)2 avoided the issues associated with Ca(OH)2 accumulation in the cement electrolyser. A similar and alternative approach could involve operating an independent electrolyser to generate acid and base by splitting water, then reacting CaCO3 with the acid and base in sequence to form Ca(OH)2 (Fig. S15, ESI†). However, a major shortcoming of this approach is the current efficiency of the electrolyser used to generate acid and base. Two-compartment electrolysers using CEMs or anion exchange membranes suffer from the significant crossover of H+ or OH−, respectively. Consequently, the majority (as much as 95%) of electrochemically generated H+ and OH− are neutralized rather than being consumed in Ca(OH)2 production. We fed CaCO3 to the three-compartment electrolyser to react with H+in situ to mitigate H+ crossover and avoid this issue. Our previously reported experiments established that 100% of electrochemically generated H+ (i.e., 100% current efficiency) can be utilized in reactions with CaCO3.1 In this work, we showed how to maintain this efficient utilization of electrochemically generated H+ while increasing the durability of the electrolyser with a calcium reactor where Ca2+ reacts with the electrochemically generated OH−.
In our system, we demonstrated a path to substantially reduce the capital expenditure (CAPEX) required for industrial operation of this process. By utilizing an integrated electrolyser to generate H+ and OH−in situ, we eliminated the need for the procurement, regeneration, and storage of large volumes of acids and bases. This minimizes the infrastructure investments, consequently reducing the overall CAPEX, a critical metric in the evaluation of new industrial processes (Fig. S16 and S17, ESI†).56
Our integrated electrolyser not only reduces the operational complexities, but also the risks associated with chlorine handling in traditional saltwater electrolysis.57 By bypassing these intricacies, we streamlined the production process and enhanced its economic feasibility. The avoidance of potential regulatory, safety, and environmental costs associated with chlorine further contributes to the financial viability of our approach, emphasizing the value of our integrated electrolyser system from a CAPEX perspective.
We compared the energy and CO2 emissions intensity of a plant employing the cement electrolyser against a conventional thermal cement plant. For the energy analysis, we considered two voltages for the cement electrolyser, current performance (5 V), and an optimistic future case (2.5 V). We found that the thermal cement plant consumes less energy, 3.8 GJ ton−1 of cement, than the cement electrolyser at its current performance (13.1 GJ ton−1 of cement) or in the future performance case (7.8 GJ ton−1 of cement, Fig. 5). However, the gas stream of pure CO2 exiting the chemical compartment of the cement electrolyser enabled direct integration of either carbon capture or utilization processes.1,58 When combined with an amine scrubber, the cement electrolyser reduced CO2 emissions by 67–73%, from 785 kgCO2 ton−1 of cement for the thermal process to 254 and 209 kgCO2 ton−1 of cement for the cement electrolyser with current (5 V) and future (2.5 V) performance, respectively (Fig. 6). This finding is consistent with our previous estimates.1
Conclusion
In summary, electrochemical methods for cement clinker precursor production have the potential to significantly reduce CO2 emissions from cement production. However, a major operational issue for cement electrolysers is the deposition of the Ca(OH)2 product on the CEM. This process increases cell voltages, damages the CEM, and limits electrolyser durability. To address this issue, we introduced a calcium reactor to form Ca(OH)2 outside the electrolyser. A Ca2+-blocking composite membrane, which we developed by chemically modifying a commercial CEM, decoupled electrochemistry from the chemistry. This design enabled the cement electrolyser to operate over 50 hours at 100 mA cm−2 with no notable increases in cell voltage. The effectiveness of the composite membrane was validated using an operando imaging system. This work represents an advance towards decarbonizing one of the highest-emitting manufacturing industries and describes a durable method for producing cement clinker precursors.Author contributions
C. P. B. supervised the project. Z. Z. conceived the study. Z. Z., T. J., S. R., and A. S. R. W. designed experiments. Z. Z., A. S. R. W., and C. T. E. P. performed the experiments and B. A. W. M. and A. S. R. W. performed the analysis and made figures. B. A. W. M., Z. Z. wrote the first draft and generated the figures. Y. W. designed the 100 cm2 electrolyser and made figures. All authors contributed to the final manuscript writing.Data availability
The data supporting the findings in this study are available either within the paper and its ESI.†Conflicts of interest
Berlinguette, C. P.; Zhang, Z.; Mowbray, B. A. W. “Methods and Apparatus for Converting Metal Carbonate Salts to Metal Hydroxides”. PCT International Application no. PCT/CA2023/050064, filed January 20, 2023. Priority date: January 20, 2022. The remaining authors declare no other competing interests.Acknowledgements
The authors are grateful to the New Frontiers in Research Fund (NFRFT-2022-00197), the Canadian Natural Science and Engineering Research Council (RGPIN-2018-06748), Canadian Foundation for Innovation (229288), Canadian Institute for Advanced Research (BSE-BERL-162173), and Canada Research Chairs for financial support. B.A.W.M. acknowledges funding from the Natural Sciences and Engineering Research Council of Canada doctoral fellowships. This research was undertaken thanks in part to funding from the Canada First Research Excellence Fund, Quantum Materials and Future Technologies Program.References
- Z. Zhang, B. A. W. Mowbray, C. T. E. Parkyn, C. T. E. Waizenegger, A. S. R. Williams, E. W. Lees, S. Ren, Y. Kim, R. P. Jansonius and C. P. Berlinguette, Energy Environ. Sci., 2022, 15, 5129–5136 RSC
.
- L. D. Ellis, A. F. Badel, M. L. Chiang, R. J.-Y. Park and Y.-M. Chiang, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 12584–12591 CrossRef CAS PubMed
.
- A. Perego, D. Kulkarni, H.-M. Chang, X. Wang, J. Wei, M. Li and I. V. Zenyuk, Meet. Abstr., 2021, MA2021-02, 840 CrossRef
.
- Q. Xie, L. Wan, Z. Zhang and J. Luo, iScience, 2023, 26, 106015 CrossRef CAS PubMed
.
- D. M. Weekes, D. A. Salvatore, A. Reyes, A. Huang and C. P. Berlinguette, Acc. Chem. Res., 2018, 51, 910–918 CrossRef CAS PubMed
.
- S. Ren, D. Joulié, D. Salvatore, K. Torbensen, M. Wang, M. Robert and C. P. Berlinguette, Science, 2019, 365, 367–369 CrossRef CAS PubMed
.
- E. W. Lees, B. A. W. Mowbray, F. G. L. Parlane and C. P. Berlinguette, Nat. Rev. Mater., 2021, 7, 55–64 CrossRef
.
- T. Li, E. W. Lees, M. Goldman, D. A. Salvatore, D. M. Weekes and C. P. Berlinguette, Joule, 2019, 3, 1487–1497 CrossRef CAS
.
- E. W. Lees, M. Goldman, A. G. Fink, D. J. Dvorak, D. A. Salvatore, Z. Zhang, N. W. X. Loo and C. P. Berlinguette, ACS Energy Lett., 2020, 2165–2173 CrossRef CAS
.
- T. Li, E. W. Lees, Z. Zhang and C. P. Berlinguette, ACS Energy Lett., 2020, 2624–2630 CrossRef CAS
.
- Z. Zhang, E. W. Lees, F. Habibzadeh, D. A. Salvatore, S. Ren, G. L. Simpson, D. G. Wheeler, A. Liu and C. P. Berlinguette, Energy Environ. Sci., 2022, 15, 705–713 RSC
.
- Z. Zhang, E. W. Lees, S. Ren, B. A. W. Mowbray, A. Huang and C. P. Berlinguette, ACS Cent. Sci., 2022, 8, 749–755 CrossRef CAS PubMed
.
- Z. Zhang, L. Melo, R. P. Jansonius, F. Habibzadeh, E. R. Grant and C. P. Berlinguette, ACS Energy Lett., 2020, 5, 3101–3107 CrossRef CAS
.
- Y. Kim, E. W. Lees and C. P. Berlinguette, ACS Energy Lett., 2022, 7, 2382–2387 CrossRef CAS
.
- G. Zhang, G. Yang, Q. Shen, S. Li, Z. Li, J. Liao, Z. Jiang, H. Wang, H. Zhang and W. Ye, J. Power Sources, 2022, 542, 231740 CrossRef CAS
.
- J. Peng, M. Tian, N. M. Cantillo and T. Zawodzinski, Electrochim. Acta, 2018, 282, 544–554 CrossRef CAS
.
- K. Hongsirikarn, J. G. Goodwin, S. Greenway and S. Creager, J. Power Sources, 2010, 195, 7213–7220 CrossRef CAS
.
- H. Farrokhzad, M. R. Moghbeli, T. Van Gerven and B. Van der Bruggen, React. Funct. Polym., 2015, 86, 161–167 CrossRef CAS
.
- ASTM, C150 Standard Specification for Portland Cement, ASTM International, Jul7 26 2022.
- J. L. Dalton, K. H. Gardner and B. J. Magee, Resour., Conserv. Recycl., 2004, 41, 227–241 CrossRef
.
-
H. F. W. Taylor, Cement Chemistry, 2nd edn, Thomas Telford Publishing, 1997 Search PubMed
.
- M. Fourmentin, G. Ovarlez, P. Faure, U. Peter, D. Lesueur, D. Daviller and P. Coussot, Rheol. Acta, 2015, 54, 647–656 CrossRef CAS
.
- Environment and Climate Change Canada, Emission Factors and Reference Values, https://www.canada.ca/en/environment-climate-change/services/climate-change/pricing-pollution-how-it-will-work/output-based-pricing-system/federal-greenhouse-gas-offset-system/emission-factors-reference-values.html, (accessed 1 February 2024).
- Canadian Industry Program for Energy Conservation, Canadian cement industry energy benchmarking, Natural Resources Canada, 2009.
- A. Mokhtar and M. Nasooti, Energy Strategy Rev., 2020, 28, 100458 CrossRef
.
- Canada's cement and concrete industry action plan to net-zero, Cement Association of Canada, 2023.
- M. E. Boesch and S. Hellweg, Environ. Sci. Technol., 2010, 44, 9143–9149 CrossRef CAS PubMed
.
- B. A. W. Mowbray, Z. B. Zhang, C. T. E. Parkyn and C. P. Berlinguette, ACS Energy Lett., 2023, 8, 1772–1778 CrossRef CAS
.
- F. B. Juangsa, A. S. Cezeliano, P. S. Darmanto and M. Aziz, S. Afr. J. Chem. Eng., 2022, 42, 23–31 Search PubMed
.
- Y. Wang, C. H. Sohn and J.-Y. Kim, Therm. Sci. Eng. Prog., 2024, 47, 102330 CrossRef CAS
.
- A. Ilyushechkin, L. Schoeman, L. Carter and S. S. Hla, Hydrogen, 2023, 4, 599–619 CrossRef CAS
.
- CEMEX successfully deploys hydrogen-based ground-breaking technology, https://www.cemex.com/w/cemex-successfully-deploys-hydrogen-based-ground-breaking-technology, (accessed 23 September 2024).
- HeidelbergCement produces cement with climate-neutral fuel mix using hydrogen technology, https://www.heidelbergmaterials.com/en/pr-01-10-2021, (accessed 23 September 2024).
- S. Betancur, Argos implements an innovative hydrogen injection process at its Honduran oven for a more sustainable operation – Cementos Argos: Empresa multinacional líder y sostenible, https://argos.co/argos-implements-an-innovative-hydrogen-injection-process-at-its-honduran-oven-for-a-more-sustainable-operation/, (accessed 23 September 2024).
- J. N. Knudsen, O. M. Bade, I. Askestad, O. Gorset and T. Mejdell, Energy Procedia, 2014, 63, 6464–6475 CrossRef CAS
.
- Global Status of CCS 2023, Global CCS Institute, 2023.
- Brevik CCS – World's first CO2-capture facility in the cement industry, https://www.brevikccs.com/en/brevik-ccs-worlds-first-co2-capture-facility-in-the-cement-industry, (accessed 20 September 2024).
- Facilities – Global CCS Institute, https://co2re.co/FacilityData, (accessed 20 September 2024).
- World's largest capture pilot plant for cement commissioned in China, https://www.globalccsinstitute.com/news-media/insights/worlds-largest-capture-pilot-plant-for-cement-commissioned-in-china/, (accessed 20 September 2024).
- 2023 Environmental, Social, and Governance Report, Anhui Conch Cement Company Ltd., 2024.
- I. J. Uyanga and R. O. Idem, Ind. Eng. Chem. Res., 2007, 46, 2558–2566 CrossRef CAS
.
- V. Buvik, K. K. Høisæter, S. J. Vevelstad and H. K. Knuutila, Int. J. Greenhouse Gas Control, 2021, 106, 103246 CrossRef CAS
.
- F. Vega, A. Sanna, B. Navarrete, M. M. Maroto-Valer and V. J. Cortés, Greenhouse Gases: Sci. Technol., 2014, 4, 707–733 CrossRef CAS
.
- T. Hills, D. Leeson, N. Florin and P. Fennell, Environ. Sci. Technol., 2016, 50, 368–377 CrossRef CAS PubMed
.
- A. G. Olabi, T. Wilberforce, K. Elsaid, E. T. Sayed, H. M. Maghrabie and M. A. Abdelkareem, J. Cleaner Prod., 2022, 362, 132300 CrossRef CAS
.
- C. Tang and M. L. Bruening, J. Polym. Sci., 2020, 58, 2831–2856 CrossRef CAS
.
-
M. Andreeva, Université Paris-Est, Kuban State agrarian university, 2017 Search PubMed
.
- I. Jawed and J. Skalny, Cem. Concr. Res., 1978, 8, 37–51 CrossRef CAS
.
- T. E. Stanton, Trans. Am. Soc. Civ. Eng., 1942, 107, 54–84 CrossRef
.
- S. Diamond, Cem. Concr. Compos., 1996, 18, 205–215 CrossRef CAS
.
- S. Diamond, Cem. Concr. Res., 1976, 6, 549–560 CrossRef CAS
.
- S. Robuschi, A. Tengattini, J. Dijkstra, I. Fernandez and K. Lundgren, Cem. Concr. Res., 2021, 144, 106439 CrossRef CAS
.
- Y. Tian, G. Zhang, H. Ye, Q. Zeng, Z. Zhang, Z. Tian, X. Jin, N. Jin, Z. Chen and J. Wang, Constr. Build. Mater., 2023, 369, 130504 CrossRef CAS
.
- I. Fernandez and C. G. Berrocal, Int. J. Concr. Struct. Mater., 2019, 13, 1–19 CrossRef
.
- American Concrete Institute, Building Code Requirements for Structural Concrete ACI 318-19(22), American Concrete Institute, Farmington Hills, MI, 2019.
- Kuldeep, W. D. Badenhorst, P. Kauranen, H. Pajari, R. Ruismäki, P. Mannela and L. Murtomäki, Membranes, 2021, 11, 718–731 CrossRef CAS PubMed
.
- S. Lokesh and R. Srivastava, Energy Fuels, 2022, 36, 13417–13450 CrossRef CAS
.
- D. J. D. Pimlott, A. Jewlal, B. A. W. Mowbray and C. P. Berlinguette, ACS Energy Lett., 2023, 8, 1779–1784 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ee04881d |
| This journal is © The Royal Society of Chemistry 2025 |