Open Access Article
Open Access ArticleUnlocking high-current-density nitrate reduction and formaldehyde oxidation synergy for scalable ammonia production and fixation†
Linjie
Zhang‡
 a,
Yimeng
Cai‡
a,
Yanghua
Li
a,
Chen
Sun
a,
Yi
Xiao
a,
Yibing
Yang
a,
Dechao
Chen
a,
Dongdong
Xiao
b,
Chi-Feng
Lee
a,
Yimeng
Cai‡
a,
Yanghua
Li
a,
Chen
Sun
a,
Yi
Xiao
a,
Yibing
Yang
a,
Dechao
Chen
a,
Dongdong
Xiao
b,
Chi-Feng
Lee
 c,
Yunjian
Wang
a,
Shiqiang
Feng
a,
Hsiao-Tsu
Wang
c,
Yunjian
Wang
a,
Shiqiang
Feng
a,
Hsiao-Tsu
Wang
 c,
Yu-Cheng
Shao
d,
Ting-Shan
Chan
d,
Hirofumi
Ishii
d,
Nozomu
Hiraoka
d,
Xiuyun
Wang
e,
Jun
Luo
c,
Yu-Cheng
Shao
d,
Ting-Shan
Chan
d,
Hirofumi
Ishii
d,
Nozomu
Hiraoka
d,
Xiuyun
Wang
e,
Jun
Luo
 f and
Lili
Han
f and
Lili
Han
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: llhan@fjirsm.ac.cn
bBeijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China
cDepartment of Physics, Tamkang University, New Taipei City 251301, Taiwan
dNational Synchrotron Radiation Research Center, Hsinchu 300092, Taiwan
eNational Engineering Research Center of Chemical Fertilizer Catalyst, Fuzhou University, Fuzhou 350002, China
fShenSi Lab, Shenzhen Institute for Advanced Study, University of Electronic Science and Technology of China, Longhua District, Shenzhen 518110, China
First published on 21st January 2025
Abstract
Nitrate electroreduction to ammonia holds great promise in sustainable green ammonia synthesis, yet faces a dearth of competent electrocatalysts adapted to varying nitrate concentration, and inadequate ammonia fixation. Herein, we present a high-performance Ag single-atom-decorated Cu2O nanowire catalyst (Ag1@Cu2O) that exhibits concentration-universal high-rate nitrate reduction, achieving >90% to near-unity ammonia faradaic efficiency (FE) across nitrate concentrations from 0.01 to 0.5 M. Notably, at 0.5 M nitrate concentration, it attains a two-ampere-level current density (2.3 A cm−2) at −1 V vs. RHE, resulting in a leading ammonia yield rate of 184.4 mgNH3 h−1 cm−2. In situ studies combined with theoretical calculations elucidate an Ag–Cu inter-site synergistic catalytic mechanism, in which single-atom Ag serves as an accelerator for active hydrogen generation and stabilization on Cu sites to boost the hydrogenation kinetics of N-containing intermediates, thus smoothing the energy barriers for ammonia production via the favorable *NHO pathway. Additionally, Ag1@Cu2O demonstrates near-unity formate FE for formaldehyde oxidation, reaching a 300 mA cm−2 current density at a mere 0.31 V vs. RHE. Motivated by this exceptional bifunctionality, we demonstrate an innovative tandem electrochemical–chemical strategy for upgrading ammonia into high-value ammonium formate by coupled electrolysis of nitrate reduction and formaldehyde oxidation, followed by straightforward chemical combination and isolation. In practice, membrane electrode assembly (MEA) electrolysis at 1.6 V for 100 h successfully outputs 10.7 g of high-purity ammonium formate. Furthermore, the commonality of this strategy is validated by application to various nitrate/aldehyde pairs. This work blazes a new trail for scalable, cost- and energy-efficient green ammonia production and fixation from nitrate reduction.
Broader contextAmmonia electrosynthesis from nitrate reduction presents a promising alternative to the energy-intensive and stringent Haber–Bosch process. However, it necessitates high-performance electrocatalysts, and the efficient extraction and storage of ammonia from the electrolyte remain critical challenges. In this study, we developed an Ag single-atom-decorated Cu2O nanowire catalyst capable of ammonia production at a current density exceeding 2 A cm−2, and showcased a tandem electrochemical–chemical route to upgrade ammonia into high-purity ammonium formate on a 10 g-scale by coupling cathodic nitrate reduction and anodic formaldehyde oxidation in a membrane assembly electrolyzer. Moreover, we elucidated the Ag–Cu inter-site synergistic catalytic mechanism for the high-rate nitrate reduction, and verified the broad adaptability of the route for upgrading ammonia into various other ammonium salts. |
Introduction
Ammonia (NH3) stands as a cornerstone of the N-fertilizer industry and a promising energy vector, yet the industrial Haber–Bosch process used for its synthesis is characterized by high energy consumption and carbon emissions.1–3 A promising alternative is the electrosynthesis of green NH3via the nitrate (NO3−) reduction reaction (NO3−RR) using renewable electricity under ambient conditions.4–6 NO3−, often found in industrial and domestic sewage, can cause severe nitrogen pollution to the environment if not disposed of properly.7,8 Converting this “NO3− trash” into “NH3 treasure” offers an economic and sustainable solution, also aiding in maintaining the nitrogen cycle. However, the efficiency of the electrochemical NO3−RR is plagued by keen competition with the hydrogen evolution reaction (HER) and varying NO3− levels in diverse sewage sources.9,10 Hence, developing highly active and concentration-universal electrocatalysts capable of high-rate NO3−-to-NH3 conversion is crucial.Besides the rational architecture of high-performance electrocatalysts, the efficient fixation of the produced NH3 is equally important but has been largely overlooked.11,12 As with hydrogen energy storage, the direct storage of liquid NH3 is both inconvenient and energy intensive. A more viable approach is to transform liquid NH3 into solid ammonium salts for its chemical storage and value-added conversion. Currently, very limited NH3 fixation routes for electrochemical NO3−RR have been developed, generally including (1) Ar- or air-assisted stripping of gaseous NH3 from the electrolyte post-electrolysis, followed by acid or CO2 trapping and subsequent transformation into solid NH4Cl, (NH4)2SO4 or NH4HCO3 through rotary evaporation;2,13–15 (2) direct treatment of the electrolyte post-electrolysis with external chemicals to convert NH3 into MgNH4PO4 fertilizer.16,17 Despite these advances, existing routes primarily produce basic ammonium fertilizers, which lack sufficient value-added benefits, and the involved purification and isolation processes are cumbersome and inefficient, necessitating considerable external feedstock input and increasing production costs. Furthermore, their low current densities of NO3−RR hamper scalable NH3 production and fixation. More importantly, the paired anodic oxygen evolution reaction (OER) is both thermodynamically unfavorable and kinetically sluggish, which not only pulls down the energy efficiency for NH3 electrosynthesis but also yields relatively valueless O2 byproduct.18,19 These challenges highlight the urgent need for new strategies to address the inefficiencies in NH3 production and fixation from routine NO3−RR.
Herein, we present an innovative tandem electrochemical–chemical synthetic strategy for scalable, energy-efficient and cost-effective upgrading of NH3 from NO3−RR into high-value pure ammonium formate (HCOONH4) solid. This approach aimfully pairs the formaldehyde (HCHO) oxidation reaction (FOR) to NO3−RR in a coupled electrolysis, followed by a set of straightforward chemical processes to realize NH3 fixation (Fig. 1(a)). HCHO, the main downstream product of methanol and a basic chemical raw material in industry, is also a notorious pollutant prevalent in various industrial sewage effluents and off-gases. However, it is found that the thermodynamic equilibrium potential of FOR (2HCHO + 4OH− − 2e− → 2HCOO− + H2 + 2H2O, E° = −0.22 V vs. RHE) is significantly lower than that of OER (1.23 VRHE).20 Consequently, the pairing with anodic FOR not only facilitates the concurrent elimination of HCHO contaminant but also significantly enhances the energy efficiency for NH3 production. Additionally, FOR produces valuable formate and H2 products in the anodic chamber, motivating us to conceive of the further combination of anodic formate with cathodic NH3, along with subsequent chemical steps, including acid neutralization, distillation and rotary evaporation, to give the desired HCOONH4 product. Notably, HCOONH4 is not just an N-fertilizer and NH3 carrier, it also finds extensive applications in fuel cells, hydrogen storage, chemical synthesis (e.g., formamide synthesis upon heating), pharmaceuticals, etc (Fig. 1(b)).21–23 In comparison to those previously obtained inorganic ammonium salts from the routine NO3−RR process, HCOONH4 commands a considerably higher market value, reaching approximately $800 per ton (Fig. 1(c) and Table S1, ESI†). Moreover, the isolation of HCOONH4 allows for the simultaneous recovery of a pure K2SO4 solid byproduct, thus further enhancing the economic efficiency of this route for NH3 fixation.
To implement this proof-of-concept, we have synthesized Ag single-atom-decorated Cu2O nanowires (Ag1@Cu2O NWs) densely grown on Cu foam, which serve as a high-performance bifunctional electrocatalyst for both NO3−RR and FOR. This catalyst design is motivated primarily by the demonstrated high activity of Cu alloys for both reactions, combined with the benefits of atomic-level electronic structure modulation by single-atom incorporation.24–26 Remarkably, Ag1@Cu2O NWs showcase concentration-universal high NO3−RR electrocatalytic activity, demonstrating a fast reaction rate and near-unity NH3 faradaic efficiency (FE) at low overpotential in low NO3−-level electrolytes, and a two-ampere-level current density of 2.3 A cm−2 coupled with an ultrahigh NH3 yield rate of 184.4 mgNH3 h−1 cm−2 in high NO3−-level electrolyte (0.5 M). The results of a series of in situ experiments and density functional theory (DFT) calculations reveal the pivotal role of single-atom Ag in inhibiting HER while facilitating the hydrogenation of N-containing intermediates adsorbed on Cu sites, leading to the high rate of NH3 production. Meanwhile, in the FOR electrocatalysis, it secures near-100% FE for both formate and H2 production with a large current density of 300 mA cm−2 at 0.31 V (vs. RHE, hereafter the same potential scale is used without iR-compensation). Leveraging the high rate, high FE and high current density afforded by Ag1@Cu2O NWs, membrane electrode assembly (MEA)-based paired NO3−RR/FOR electrolysis at 1.6 Vcell for 100 h successfully achieves the gram-scale synthesis of high-purity HCOONH4 solid (10.7 g), with a high collection efficiency of 84.8% and a low production cost of $237.4 t−1, based on a techno-economic analysis. Moreover, this route can be further extended to pairs of NO3−RR with anodic oxidation reactions of two other representative heterocyclic aldehydes of 4-pyridinecarboxaldehyde (4-PCA) and 5-hydroxymethylfurfural (HMF).
Results and discussion
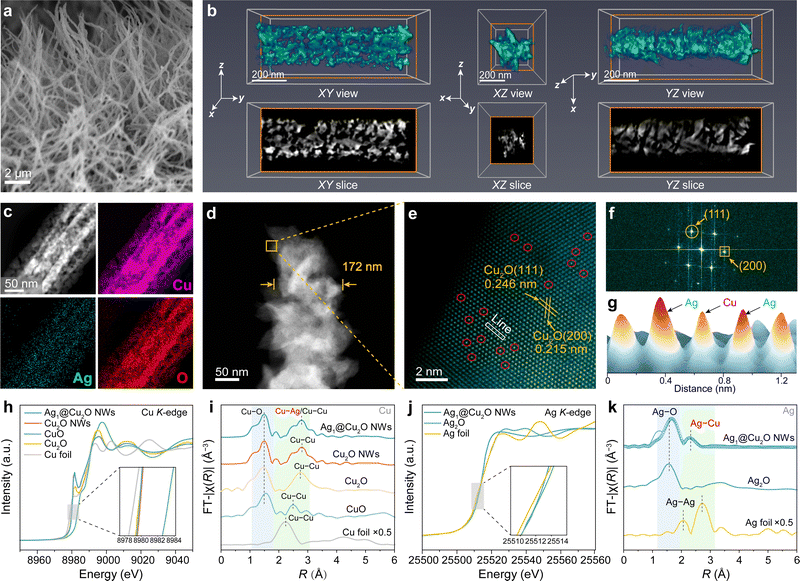
Synthesis and structural analyses of Ag1@Cu2O NWs
The Ag1@Cu2O NWs were synthesized by densely growing Cu(OH)2 nanowires on a low-cost Cu foam substrate, followed by immersing it into an AgNO3 solution and a subsequent low-temperature reduction process using H2/Ar at 200 °C (for experimental details, see Materials and methods and Fig. S1 and S2, ESI†). Scanning electron microscopy (SEM) analysis confirmed the dense and uniform nanowire morphology of the Ag1@Cu2O NWs, with an approximate length of 4.5 μm and a width of 172 nm (Fig. 2(a)). The interwoven nanowires construct a highly conductive network, facilitating the rapid charge transfer essential for catalytic activity. Compared to smooth pristine Cu2O NWs, the integration of Ag single-atoms significantly increases the surface roughness of Ag1@Cu2O NWs (Fig. S3, ESI†), thereby enhancing the exposure of active sites for catalysis. To scrutinize the surface details of Ag1@Cu2O NWs, a three-dimensional (3D) electron tomographic reconstruction was performed using a series of two-dimensional (2D) transmission electron microscopy (TEM) images of a single nanowire (Movie S1, ESI†). The volume-rendering images reveal a distinct pentagon-like star-shaped cross-section with extensive porosity in Ag1@Cu2O NWs (Fig. 2(b)). The internal pore volume within a single nanowire exceeds 35%, conferring upon Ag1@Cu2O NWs an intricate porous configuration that offers abundant active sites and efficient mass transport channels, thereby favoring enhanced catalytic reaction kinetics (Fig. S4, ESI†).27X-ray diffraction (XRD) analysis revealed a compositional transition from Cu(OH)2 NWs to Cu2O NWs following H2/Ar reduction (Fig. S5, ESI†). Furthermore, the absence of metallic Ag or its corresponding oxide in Ag1@Cu2O NWs was confirmed, indicating the presence of Ag single-atoms. This observation was further substantiated by energy-dispersive spectroscopy (EDS) mapping, which shows an even distribution of Ag atoms across the entire surface of Ag1@Cu2O NWs (Fig. 2(c)). The Ag mass loading in Ag1@Cu2O NWs was determined at 0.25 wt% using inductively coupled plasma optical emission spectroscopy (ICP-OES) (Table S2, ESI†). Such notably low Ag content greatly contributes to the cost-effective synthesis of Ag1@Cu2O NWs (Table S3, ESI†). The high-angle annular dark-field scanning TEM (HAADF-STEM) imaging revealed that the topography of Ag1@Cu2O NWs closely resembles that reconstructed by 3D electron tomography (Fig. 2(d) and Fig. S6, ESI†). Furthermore, lattice analysis of Ag1@Cu2O NWs using the aberration-corrected-HAADF-STEM (AC-HAADF-STEM) image and the corresponding fast Fourier transform (FFT) unveiled lattice spacings of 0.215 and 0.246 nm, attributed to the (200) and (111) planes of an fcc-type Cu2O (Fig. 2(e) and (f)), in line with the XRD results. Additionally, a multitude of distinct bright spots was recognized on the surface of Ag1@Cu2O NWs, indicating an isolated dispersion of Ag single-atoms rather than clusters, a result of substituting Ag atoms for Cu atoms within the Cu2O lattice.13 This observation was further supported by the line intensity profile in the region of interest, revealing a significant variation in Z-lining between atoms, correlating directly with the disparity in atomic numbers between Ag and Cu elements (Fig. 2(g)).28
The fine local electronic and coordination structure of Ag1@Cu2O NWs was further probed by synchrotron X-ray absorption near-edge structure (XANES) spectra at both the Cu K-edge and Ag K-edge. The Cu K-edge profiles of Ag1@Cu2O NWs, Cu2O NWs and reference Cu2O exhibit close proximity, manifesting a similar rising-edge shoulder at 8981 eV, indicating comparable Cu valences with the same tetrahedral coordination geometry (Fig. 2(h)).29 In contrast, the shoulder for reference CuO appears at 8986 eV, reflecting a distinct square planar coordination geometry. Compared to Cu2O NWs, the slight low-energy shift of the Cu K-edge of Ag1@Cu2O NWs suggests increased electron density donated from Ag single-atoms to the Cu2O support due to their strong electronic interaction, which was further confirmed by XPS analysis (Fig. S7–S9, ESI†).30 In the R-space of the Fourier-transformed (FT) k3-weighted extended X-ray absorption fine structure (EXAFS) spectra at the Cu K-edge (Fig. 2(i)), Ag1@Cu2O NWs display two pronounced peaks at approximately 1.50 and 2.78 Å, corresponding to Cu–O and Cu–Ag/Cu–Cu bonding in the first coordination shell, respectively. Correspondingly, the Ag K-edge XANES spectrum of Ag1@Cu2O NWs emerges between those of Ag foil and Ag2O (Fig. 2(j)). Additionally, the Ag K-edge FT-EXAFS spectrum of Ag1@Cu2O NWs exhibits a major peak around 1.5 Å and a minor peak at approximately 2.3 Å, which are assigned to Ag–O and Ag–Cu scattering, respectively (Fig. 2(k)). This result was further confirmed by the wavelet transform EXAFS (WT-EXAFS) spectrum of Ag1@Cu2O NWs (Fig. S10, ESI†). Corresponding EXAFS fitting analysis revealed coordination numbers of 1.69 for Ag–O and 1 for Ag–Cu shells (Table S4, ESI†). Importantly, the absence of a peak for Ag–Ag bonding indicates that Ag atoms are integrated into the Cu2O lattice as isolated single-atoms via ion exchange (Fig. S11, ESI†), effectively precluding the formation of clusters or nanoparticles.
Electrocatalytic NO3−RR performance
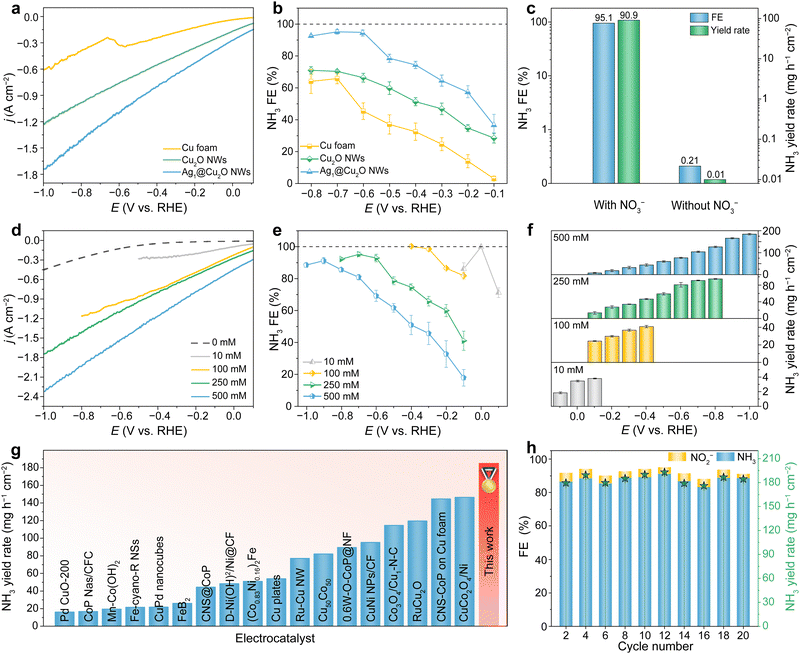
The electrocatalytic NO3−RR performance of Ag1@Cu2O NWs, and the control samples of Cu2O NWs and Cu foam were initially assessed in an electrolyte composed of 1 M KOH with 250 mM KNO3 using a three-electrode testing system. The linear sweep voltammetry (LSV) results depicted in Fig. 3(a) revealed a significant disparity in current densities among the samples. Notably, Ag1@Cu2O NWs exhibited the highest current density across all potentials, reaching an ampere-level 1.74 A cm−2 at −1 V, followed by the inferior Cu2O NWs, while Cu foam lagged far behind. Subsequently, variations in the concentrations of NO3− reactant, NO2− intermediate, and NH3 product during the NO3−RR at multiple potentials in chronoamperometry tests were analyzed by UV-vis absorption spectroscopy (Fig. S12–S14, ESI†). Accordingly, the calculated NH3 faradaic efficiency (FE) on Ag1@Cu2O NWs showed a progressive increase from −0.1 to −0.6 V, followed by a stable plateau up to −0.8 V, with a maximum of 95.1% at −0.7 V (Fig. 3(b)). Thereby, the NH3 yield rate on Ag1@Cu2O NWs achieved a maximum value of 90.88 mg h−1 cm−2 at −0.8 V, significantly surpassing those of Cu2O NWs (55.12 mg h−1 cm−2) and Cu foam (26.69 mg h−1 cm−2) by 1.9 and 4.6 folds, respectively (Fig. S15, ESI†). Noticeably, Cu foam predominantly produced intermediate NO2− with FEs ranging from 50% to 90% between −0.1 and −0.6 V, and only achieves a mediocre NH3 FE around 60% at more negative potentials (Fig. S16, ESI†). This is likely to be due to the insufficient *H adsorption capacity of Cu alone, which retards the hydrogenation of intermediates, such as *NO3, *NO2, and *NHO.31,32 While Cu2O NWs showed much improved NH3 FEs compared to Cu foam, they remained below 70%. Comparatively, the remarkably enhanced NO3−RR activity of Ag1@Cu2O NWs can be primarily attributed to the integration of Ag single-atoms within the Cu2O lattice, which facilitates rapid hydrogenation of the NO2− intermediate for efficient NH3 conversion. This boost in reaction kinetics was corroborated using electrochemical impedance spectroscopy (EIS), which provided evidence for the lowest charge transfer resistance for Ag1@Cu2O NWs among the samples (Fig. S17, ESI†). Notably, the superiority of Ag1@Cu2O NWs was confirmed by a series of catalyst screenings, including control over the growth density of Cu2O NWs, adjusting Ag loadings, incorporating Ag into other metal foams, incorporating Co nanoparticles onto Cu2O NWs, and comparing with planar Ag (Fig. S18–S22, ESI†).In addition, the authenticity of NH3 originating from the NO3−RR process was confirmed by a comparative experiment in 1 M KOH solution without NO3− at −0.7 V, which showed negligible NH3 production (Fig. 3(c)). This evidence was further reinforced by an 15NO3− isotopic labeling experiment, coupled with both qualitative and quantitative NH3 analysis using 1H nuclear magnetic resonance (NMR) spectroscopy (Fig. S23–S26, ESI†).
In practical industrial sewage, NO3− levels may vary by source. Hence, the adaptability of Ag1@Cu2O NWs to different NO3− concentrations (10, 100 and 500 mM) was further examined. As illustrated in Fig. 3(d), LSV results indicate that the current density increases with rising NO3− concentration. Notably, at 500 mM, the current density at −1 V reaches an ultrahigh two-ampere-level of 2.3 A cm−2, a magnitude rarely observed in the NO3−RR domain. Further quantitative analysis revealed prevailing NH3 production post −0.3 V, reaching a peak NH3 FE of 91.2% at −0.9 V (Fig. 3(e) and Fig. S27, S28, ESI†). As a result of the high current density and FE, Ag1@Cu2O NWs afford an excellent NH3 yield rate of 184.4 mg h−1 cm−2 at −1 V, far higher than the vast majority of previously reported values (Fig. 3(f), (g) and Table S5, ESI†). Moreover, at lower 10 mM and 100 mM NO3− concentrations, both near-unity (100 ± 1%) NH3 FEs could be obtained on Ag1@Cu2O NWs at low potentials of 0 V and −0.4 V (Fig. 3(e)), respectively, with corresponding NH3 yield rates of 3.45 and 40.75 mg h−1 cm−2 (Fig. 3(f)). At a more negative potential of −0.1 V with the 10 mM NO3− concentration, the NH3 FE abruptly decreases to 86.1% (Fig. 3(e)), indicating the occurrence of HER due to fast depletion of NO3− that cannot sustain long-term electrolysis. Consequently, nearly 100% NO3−-to-NH3 conversion can be achieved within 1 h (Fig. S29, ESI†), reducing NO3− levels to below 50 ppm in these two electrolytes, thereby meeting the World Health Organization's prescribed threshold for drinking water.33 Notably, compared to the low NO3− concentrations, the slightly declined NH3 FE at the 500 mM high NO3− concentration might be attributed to the crowded NO3− adsorption on active sites, which impedes subsequent hydrogenation and desorption of intermediates.34 Nevertheless, the above results strongly demonstrate the exceptional performance of Ag1@Cu2O NWs for concentration-universal high-rate NO3−-to-NH3 conversion.
Furthermore, the stability of Ag1@Cu2O NWs was rigorously examined in 1 M KOH and 500 mM NO3− at −1 V. As shown in Fig. 3(h), during 20 repeated cycles with each lasting for 1 h, both the NH3 FEs and yield rates exhibited only slight fluctuations, indicating the high reusability and durability of Ag1@Cu2O NWs. TEM and EDS mapping confirmed that the spent Ag1@Cu2O NWs retained their intact nanowire structure with atomically dispersed Ag atoms (Fig. S30, ESI†). Electron energy loss spectroscopy (EELS) mappings coupled with HRTEM images revealed that the nanowire surfaces were partially reduced to Cu0, leading to in situ restructuring into a tandem Cu0/Cu1+ heterostructure (Fig. S31, ESI†). Previous studies have shown that electron transfer at the Cu/Cu2O interface favors the formation of the key *NHO intermediate and meanwhile suppresses *H dimerization to increase the proton supply during the NO3−RR process.35,36 Thus, it is hypothesized that the superior NO3−-to-NH3 conversion performance of Ag1@Cu2O NWs across diverse concentrations might be attributed to the synergistic contribution of the Ag single-atoms and the unique Cu/Cu2O tandem structure, together with the high abundance of active sites and electrical conductivity offered by the advantageous elongated nanowire structure seamlessly planted onto the Cu foam.
Mechanistic study of NO3−RR
To elucidate the origin of the activity underlying such outstanding performance of Ag1@Cu2O NWs, a series of in situ experiments were conducted to probe the dynamic valence and structural evolution of the Cu and Ag sites, as well as the N-containing reaction intermediates during NO3−RR. Initially, in situ high-energy resolution fluorescence detected (HERFD) synchrotron XANES spectra were analyzed (Fig. S32, ESI†). As shown in Fig. 4(a), as the potential progressively decreases from open circuit potential (OCP) to −0.2 V and −0.4 V, the Cu K-edge exhibits a gradual shift to lower energies, indicating a reduction in Cu valence state, with some Cu1+ being reduced to Cu0. Additionally, we conducted linear fitting of normalized XANES spectra at different potentials using Cu foil and Cu2O as references to determine the potential-dependent apparent oxidation states of Ag1@Cu2O catalysts (Fig. S33, ESI†). The fitting results indicate that as the potential shifts negatively, Ag1@Cu2O NWs exhibit a gradual decrease in oxidation valence, suggesting that some Cu2O is reduced to Cu, thereby forming a tandem Cu/Cu2O heterostructure. This finding echoes the above TEM/EELS mapping analysis. Conversely, the Ag K-edge continuously shifts to higher energies from OCP to −0.2 V and then −0.4 V (Fig. 4(b)), indicating the gradually increased oxidation state of Ag single-atoms with decreasing potential. This indicates that there exists continuous electron transfer from Ag single-atoms to neighboring Cu atoms as the potential decreases, suggesting that both Cu atoms and Ag single-atoms might serve as active sites during the NO3−RR processes. Subsequent quasi-in situ electron paramagnetic resonance (EPR) analysis revealed that Ag1@Cu2O NWs exhibited a pronounced *H signal in the absence of NO3−, which was then markedly attenuated upon the addition of NO3− (Fig. 4(c)). This indicates that Ag1@Cu2O NWs could speed the formation of active *H, which are then rapidly consumed during the hydrogenation steps in the NO3−RR process. In contrast, the indigenous active *H formation on Cu2O NWs hampers further hydrogenation, thereby explaining its high NO2− FE.14,37 Combining the results from ex situ/in situ XANES and quasi-in situ EPR analyses, it can be reasonably inferred that Ag single-atoms act as an accelerator to facilitate H2O dissociation and subsequent active *H generation and stabilization on neighboring Cu0 atoms, due to their significant electronic interactions and a theoretical consideration of the relatively greater *H binding strength of metallic Cu compared to Ag. Whereas cationic Cu1+ atoms function as the primary active sites for the adsorption of anionic NO3− and the subsequently evolved N-containing intermediates. During NO3−RR, the active *H formed on Cu0 sites flow continuously across the Cu/Cu2O heterointerface to the *NO3− adsorbed on Cu1+ sites for their stepwise hydrogenation. This cooperative inter-site synergy facilitates the further conversion of *NO2− intermediates that is stagnant on Cu2O alone, thereby improving the reactivity and NH3 FE for NO3−RR.To gain insights into the NO3−RR pathway on Ag1@Cu2O NWs, in situ attenuated total reflectance Fourier transform infrared (ATR-FTIR) and differential electrochemical mass spectrometry (DEMS) techniques were employed to identify the N-containing intermediates during the NO3−RR process from OCP to −0.4 V. The ATR-FTIR analysis shown in Fig. 4(d) identifies distinct absorption peaks for *NO3 at approximately 1337 cm−1 and for NH4+ around 1440 cm−1, alongside the deoxygenated intermediates (*NO2 at ∼1201 cm−1 and *NO at ∼1541 cm−1) observed in the positive band and hydrogenated intermediates (*NH2 at ∼1126 cm−1 and *NH at ∼1299 cm−1) in the negative band.3,38 An increase in peak intensities of these deoxygenation/hydrogenation intermediates with decreasing potential was observed. Furthermore, online DEMS corroborated the presence of these intermediates by detecting characteristic m/z signals, including *NO2 (46), *NO (30), *NHO (31), *NH2O (32), *NH2OH (33), *NH2 (16) and NH3 (17) (Fig. 4(e) and Fig. S34, S35a, ESI†).3,39 Notably, the intensity of the H2 signal is two orders of magnitude lower than that of the NH3 signal at the same potential, indicating a minimal HER side reaction (Fig. S35b, ESI†). Upon cessation of the applied potential, all signals dropped immediately, and the NH3 signal was virtually invisible when using an NO3−-free electrolyte, confirming that all detected N-containing intermediates were generated in situ during the NO3−RR process rather than from external contaminants. The presence of the crucial intermediates of *NH2O and *NH2OH supports the NO3−RR process on Ag1@Cu2O NWs predominantly adhering to the *NHO pathway for NH3 synthesis (Fig. S36, ESI†).40
Further DFT calculations were performed to underpin the proposed Ag single-atom-intensified NO3−RR activity of Ag1@Cu2O NWs. The charge-density difference analysis upon NO3− adsorption revealed significant electron localization at the Ag1@Cu2O–*NO3 interface compared to the Cu2O–*NO3 interface, suggesting more favorable charge transfer between *NO3 and Ag1@Cu2O (Fig. S37, ESI†).9,41 Moreover, projected density of states (PDOS) analysis showed a downshift in the d-band center of Ag1@Cu2O (−2.07 eV) relative to that of Cu2O (−1.99 eV) (Fig. S38, ESI†). This shift results in an optimized interaction between Ag1@Cu2O and *NO3, facilitating the effective destabilization of *NO3 on Ag1@Cu2O, and thereby lowering the energy barrier for the initial hydrogenation of *NO3 (Fig. S39, ESI†).42 Additionally, the Gibbs free energy calculations for *H adsorption indicated that the Cu site in Ag1@Cu2O exhibits a stronger affinity for *H compared to Cu2O, albeit weaker than metallic Cu (Fig. 4(f) and Table S6, ESI†). This finding aligns with experimental observations indicating that the incorporation of Ag single-atoms into Cu2O significantly facilitates the generation of active *H. It also supports the hypothesis that the in situ evolved Cu0 in Ag1@Cu2O serves as the H2O dissociation site for the generation and stabilization of the active *H for subsequent hydrogenation reactions, rather than primarily promoting *H desorption for HER, as observed in Cu foam during the NO3−RR.43,44Fig. 4(g) illustrates the Gibbs free energy profiles for NO3−RR on Cu sites for Cu2O and Ag1@Cu2O via the *NHO pathway (Table S7, ESI†), with detailed adsorption configurations shown in Fig. S40 and S41 (ESI†). The results indicate that the initial *NO3 → *NO2 transformation with an energy barrier of 1.39 eV is considered the rate-determining step (RDS) on Cu2O. Conversely, the energy barrier of RDS on Ag1@Cu2O, characterized as *NH2O → *NH2OH, is notably diminished to 0.48 eV. This reduction indicates that the enhanced active *H generation and stabilization capability of Ag1@Cu2O not only remarkably lowers the energy barrier for the initial *NO3 deoxygenation process but also optimizes the following overall hydrogenation processes, thus enhancing the NO3−RR activity. This matched timely *H supply and smoothed hydrogenation of N-containing intermediates finally gives rise to the high-rate of NO3−-to-NH3 conversion achieved on Ag1@Cu2O (Fig. 4(h)).
Electrocatalytic FOR performance
Motivated by the high performance of Ag1@Cu2O NWs in NO3−RR, their potential for catalyzing the formaldehyde (HCHO) oxidation reaction (FOR) was subsequently investigated. This exploration seeks to supplant the thermodynamically unfavorable and value-less anodic OER with FOR to pair with cathodic NO3−RR. This pair aims to reduce the overall energy input and simultaneously acquire the valuable formate (HCOOH) product. The electrocatalytic FOR performance of Ag1@Cu2O NWs and control samples were assessed in an optimum electrolyte containing 1 M KOH and 0.2 M HCHO (Fig. S42, ESI†). As depicted by the LSVs in Fig. 5(a), Ag1@Cu2O NWs display a pronounced FOR current density, reaching 100 mA cm−2 at a mere 0.04 V and 300 mA cm−2 at 0.31 V, far surpassing the performance of Cu2O NWs and the negligible activity observed on Cu foam. This exceptional performance of Ag1@Cu2O NWs also exceeds those of the majority of previously reported FOR catalysts (Table S8, ESI†). Besides the liquid formate product, significant H2 production was concurrently observed at the electrode, as evidenced by intense bubbling and confirmed by gas chromatography (GC). The H2 peak intensity escalated with decreasing potential (Fig. S43, ESI†), highlighting the superiority of electrocatalytic FOR in affording dual high-value fuels of formate and H2. Further evidence of the superior electrocatalytic FOR activity of Ag1@Cu2O NWs was provided by a chronoamperometry test at 0.3 V, which revealed more rapid current attenuation compared to Cu2O NWs, with the current dropping to nearly 0 within 1 h, indicating the fast and complete oxidative depletion of HCHO (Fig. 5(b)).To exclude the current contribution from the oxidation of formate product or methanol additive (a stabilizer in commercial 37 wt% HCHO solution) during FOR, their oxidation current responses were examined via chronoamperometry at 0.3 V. As shown in Fig. 5(c), the sequential addition of 0.2 M methanol and 0.2 M formate to 1 M KOH electrolyte both led to negligible current changes. Conversely, the injection of 0.2 M HCHO induced an immediate sharp rise in current up to 251.7 mA cm−2, consistent with the LSV current density for FOR (Fig. S44, ESI†). These findings suggest that Ag1@Cu2O NWs possess high FOR selectivity and strong tolerance to other electrolyte impurities. This specificity may arise from the relatively low dissociation energy of the C–H bond in HCHO, which facilitates its preferential cleavage over methanol or formate, thereby ensuring superior selectivity and effectively preventing the over-oxidation of formate product to CO2.24 In addition, to rule out a non-electrochemical contribution to formate generation during FOR, 1H NMR analysis was performed for the same 0.2 M HCHO-laden 1 M KOH electrolyte after standing for 2 days without an applied bias. The result reveals negligible formate generation, confirming that the formate product is attributable solely to the electrochemical process and not the intermolecular redox disproportionation of HCHO (Fig. S45, ESI†).45
The ability to produce formate and H2via FOR on Ag1@Cu2O NWs was further quantitatively analyzed using 1H NMR and a drainage method, respectively (Fig. S46, ESI†). As shown in Fig. 5(d), Ag1@Cu2O NWs demonstrate excellent formate FEs exceeding 98.5% across a broad range of potentials from 0 to 0.3 V. Remarkably, the formate yield rate reaches 4.75 mmol h−1 cm−2 at 0.3 V, culminating in an HCHO conversion rate of approximately 95%. Concomitantly, the actual H2 amounts exhibit a close correspondence with the theoretical values, indicating a near-100% H2 FE, with a calculated H2 yield rate of 2.3 mmol h−1 cm−2 (Fig. 5(e) and Table S9, ESI†). Given that the theoretical stoichiometric yields of formate and H2 in FOR are in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (2HCHO + 4OH− − 2e− → 2HCOO− + H2 + 2H2O), these results not only substantiate the accuracy of both measured yield rates but also confirm that the formate is exclusively produced via FOR. Furthermore, 20 repeated testing cycles with each lasting for 1 h for chronoamperometric FOR at 0.3 V demonstrate both stable formate FE and yield rate throughout, underscoring the excellent catalytic stability of Ag1@Cu2O NWs (Fig. 5(f)). This superior sustainability can be attributed to the potent reducing properties of HCHO, which spontaneously reduces the electrooxidized Cu2+ back to Cu1+. This regeneration likely helps prevent the deactivation of Ag1@Cu2O NWs, thereby maintaining robust FOR activity.46
1 molar ratio (2HCHO + 4OH− − 2e− → 2HCOO− + H2 + 2H2O), these results not only substantiate the accuracy of both measured yield rates but also confirm that the formate is exclusively produced via FOR. Furthermore, 20 repeated testing cycles with each lasting for 1 h for chronoamperometric FOR at 0.3 V demonstrate both stable formate FE and yield rate throughout, underscoring the excellent catalytic stability of Ag1@Cu2O NWs (Fig. 5(f)). This superior sustainability can be attributed to the potent reducing properties of HCHO, which spontaneously reduces the electrooxidized Cu2+ back to Cu1+. This regeneration likely helps prevent the deactivation of Ag1@Cu2O NWs, thereby maintaining robust FOR activity.46
Paired NO3−RR/FOR electrolysis for scalable NH3 production and fixation
To examine the NH3 production and fixation capabilities in two-electrode paired NO3−RR/FOR electrolysis, an MEA electrolyzer was used (Fig. 6(a) and Fig. S47, ESI†), and 1 M KOH + 0.1 M KNO3 and 1 M KOH + 0.8 M HCHO were employed as the catholyte and anolyte, respectively. As shown in Fig. 6(b), the LSV curve for the coupled NO3−RR/FOR electrolysis on Ag1@Cu2O NWs reveals an ultralow cell voltage of 0.01 V to reach a current density of 100 mA cm−2, which is 1.69 V and 0.64 V lower than that required for the NO3−RR/OER pair with HCHO-free 1 M KOH anolyte and the HER/FOR pair with NO3−-free 1 M KOH catholyte, respectively, highlighting the energy-saving merit of the NO3−RR/FOR couple. More impressively, it requires only 1.6 V to attain a large current density of 600 mA cm−2, representing the best reported cell performance for NH3 electrosynthesis (Table S10, ESI†). Comprehensive analyses of the cathodic and anodic products from the NO3−RR/FOR electrolysis within the potential range of 0.8 to 1.8 V were then conducted (Fig. 6(c) and Fig. S48, ESI†). At the cathode, the maximal NH3 FE reaches 96% at 1.6 V, corresponding to an NH3 yield rate of 37.6 mg h−1 cm−2. At the anode, the formate FE remains above 97% across all cell voltages, achieving the highest formate yield rate of 206.8 mM h−1 cm−2 at 1.8 V. Moreover, after a chronoamperometry test at 1.6 V for 5 h, the cumulative formate yield approaches 97.4% (Fig. 6(d)). Throughout the coupled electrolysis, no evident H2 bubbles were observed at the cathode during NO3−RR, while distinct H2 bubble formation was noted at the anode during FOR (Movie S2, ESI†). This observation indicates that both HCHO and NO3− in the electrolytes are essentially transformed, thus laying a solid foundation for subsequent isolation and purification endeavors for upgrading NH3 into HCOONH4. It should be noted that the paired NO3−RR/FOR electrolysis can spontaneously occur without external electricity input due to its inherent primary cell nature (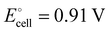 ). Therefore, the performance of this primary cell was compared with that of the MEA-based electrolytic cell. As shown in Fig. S49, ESI,† this primary cell exhibits a limited open circuit voltage (OCV) of 0.52 V and a maximum discharge current density of 80.3 mA cm−2, resulting in a peak power density of only 6.6 mW cm−2. Moreover, it could sustain a discharge current density of 50 mA cm−2 for just 1 h, with an NH3 yield rate of 3.5 mg h−1 cm−2, which is significantly lower than that achieved by the MEA-based electrolytic cell. Therefore, this primary cell is unsuitable for implementation in scalable NH3 production.
). Therefore, the performance of this primary cell was compared with that of the MEA-based electrolytic cell. As shown in Fig. S49, ESI,† this primary cell exhibits a limited open circuit voltage (OCV) of 0.52 V and a maximum discharge current density of 80.3 mA cm−2, resulting in a peak power density of only 6.6 mW cm−2. Moreover, it could sustain a discharge current density of 50 mA cm−2 for just 1 h, with an NH3 yield rate of 3.5 mg h−1 cm−2, which is significantly lower than that achieved by the MEA-based electrolytic cell. Therefore, this primary cell is unsuitable for implementation in scalable NH3 production.
To achieve the tandem electrochemical–chemical synthesis of high-value HCOONH4, MEA-based paired NO3−RR/FOR electrolysis was performed at 1.6 V, yielding NH3 and formate products in the cathodic and anodic chambers, respectively. Both the FEs and yield rates for the NH3 and formate remained stable over prolonged electrolysis of 100 h (Fig. S50, ESI†). Following electrolysis, the anolyte and catholyte were thoroughly mixed and subjected to chemical processes of acid neutralization, distillation, and rotary evaporation (see Materials and methods for details). This process results in the successful separation and recovery of pure solid HCOONH4 product and K2SO4 byproduct. Remarkably, a total treatment of 0.2 mol NO3− and 1.6 mol HCHO, enabled the retrieval of 10.7 g of HCOONH4 solid with a collection efficiency of 84.8% (Fig. 6(e)). The high purity of the HCOONH4 product was confirmed by the 1H NMR spectrum, which indicates complete transformation of NO3− and HCHO with no detectable impurity peaks. Additionally, 17.02 g of high-purity solid K2SO4 byproduct was simultaneously harvested, as confirmed by XRD analysis (Fig. S51, ESI†). Notably, this synthetic route does not produce any additional pollutants, and all feedstocks are recovered, ensuring complete recycling. Moreover, preliminary cost–benefit analysis estimates the production cost of HCOONH4 could potentially be as low as $237.4 ton−1, approximately 30% of its commercial commodity price (Note S1, ESI†). This highlights the significant potential of this route for efficient NO3−/HCHO sewage purification and economically viable large-scale NH3 production and upgrading into high-value HCOONH4.
To evaluate the applicability of this tandem electrochemical–chemical synthetic strategy for upgrading NH3 into other ammonium acid salts, we further investigated the coupling of NO3−RR with oxidation of higher aldehydes. Specifically, two other representative five- and six-membered heterocyclic aldehydic compounds, 5-hydroxymethylfurfural (HMF) and 4-pyridinecarboxaldehyde (4-PCA), were selected as anodic substrates for coupling electrolysis. As shown in Fig. 6(b), LSV curves for the NO3−RR/HMFOR and NO3−RR/4-PCAOR electrolysis exhibit comparable current densities, both superior to that for NO3−RR/OER, demonstrating the consistent high performance of Ag1@Cu2O NWs across these pairs. At the same voltage of 1.6 V for 2 h and 5 h, the measured cathodic NH3 FEs are 95.2% for NO3−RR/4-PCAOR and 91% for NO3−RR/HMFOR, respectively, indicating sustained catalytic activity despite the variation in anodic aldehydes (Fig. 6(f)). The corresponding anodic products, isonicotinic acid (INA) from 4-PCAOR and furan-2,5-dicarboxylic acid (FDCA) from HMFOR, were quantitatively analyzed by 1H NMR spectroscopy (Fig. S52 and S53, ESI†). The calculated FEs are 95.3% for INA and 98.9% for FDCA, with corresponding yields of 98.7% and 97.4% (Fig. S54, ESI†). The absence of impurity peaks in the 1H NMR spectra underscores the remarkable activity and selectivity of Ag1@Cu2O NWs for the electrooxidation of these heterocyclic aldehydes. Finally, the cathodic NH3 product was fixed using pure INA or FDCA powders isolated from the corresponding anolyte, leading to aqueous solutions of ammonium isonicotinic acid (0.23 M) and ammonium furandicarboxylic acid (0.44 M), with high collection efficiencies of 70.2% and 88.5%, respectively, and their high purity was verified by 1H NMR spectroscopy (Fig. 6(g) and (h)). These expansions successfully showcase the adaptability of this route for scalable NH3 fixation and upgrading into high-value chemicals from the valorization of nitrate and various industrial pollutants, demonstrating its broad applicability and both potential industrial and environmental benefits.
Conclusions
A high-performance bifunctional single-atom electrocatalyst, Ag1@Cu2O NWs, has been successfully developed for both nitrate reduction and formaldehyde oxidation. This catalyst exhibits high-rate nitrate-to-ammonia conversion across a wide range of nitrate concentrations, achieving a two-ampere-level current density of 2.3 A cm−2 along with a notable NH3 yield rate of 184.4 mg h−1 cm−2 at 0.5 M nitrate concentration. Using a combination of in situ techniques and DFT calculations, it has been elucidated that the Ag–Cu inter-site electronic synergy plays a pivotal role in accelerating active *H supply, which in turn facilitates the hydrogenation of N-containing intermediates occurring on the in situ evolved Cu/Cu2O heterostructure via the *NHO reaction pathway. This ultimately leads to significantly enhanced hydrogenation kinetics of NO3−RR on Ag1@Cu2O NWs, and hence accelerated ammonia production. For formaldehyde oxidation, Ag1@Cu2O NWs achieve a large current density of 300 mA cm−2 at 0.31 V vs. RHE, concurrently producing formate and H2 with near-100% FE. Motivated by this superb bifunctionality of Ag1@Cu2O NWs, a tandem electrochemical–chemical route for the scalable fixation of the produced ammonia into 10.7 g of HCOONH4 solid has been demonstrated by coupling NO3−RR and FOR in MEA-based electrolysis. The adaptability of this strategy has been validated by its successful extension to two more nitrate/aldehyde pairs. This study not only provides a versatile high-current-density electrocatalyst but also potentially inspires broader future efforts towards more diverse and scalable green ammonia fixation and valorization from nitrate electroreduction.Author contributions
L. Z. designed this study and, along with Y. C., was responsible for most of the investigations, methodology development, data collection and analysis, visualization and writing of the original manuscript. Y. L. and D. C. assisted with data collection and analysis. H.-T. W., C.-F. L. and T.-S. C. performed the ex situ XAFS measurements. H. I., N. H. and Y.-C. S. helped with the in situ XAFS measurements. C. S. performed the electron tomography for 3D reconstruction. D. X. conducted the AC-STEM characterizations. S. F. and Y. W. performed the EELS mapping. Y. X. and Y. Y. executed the DFT calculations. X. W. and J. L. contributed valuable discussions on the data analysis. L. H. supervised the project, directed the study, and revised the manuscript. All authors approve the final manuscript.Data availability
All data supporting this article have been included in the paper and the ESI.† Additional data related to this paper can be provided upon request from the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFA1505700), National Natural Science Foundation of China (22475214, 22205232, 22222801 and 21601187), Talent Plan of Shanghai Branch, Chinese Academy of Sciences (CASSHB-QNPD-2023-020), Natural Science Foundation of Fujian Province (2023J06044 and 2023J01213), and the Self-deployment Project of Haixi Institutes, Chinese Academy of Sciences (CXZX-2022-JQ06 and CXZX-2022-GH03). The XAFS study on beamline TLS 01C1 at National Synchrotron Radiation Research Center (NSRRC) and beamline BL12XU at SPring-8 (Japan) is highly acknowledged. The authors also greatly appreciate support by Transmission Electron Microscope Platform and High-performance Computing Platform of Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China.Notes and references
- C. Q. Yu, X. Huang, H. Chen, H. C. J. Godfray, J. S. Wright, J. W. Hall, P. Gong, S. Q. Ni, S. C. Qiao, G. R. Huang, Y. C. Xiao, J. Zhang, Z. Feng, X. T. Ju, P. Ciais, N. C. Stenseth, D. O. Hessen, Z. L. Sun, L. Yu, W. J. Cai, H. H. Fu, X. M. Huang, C. Zhang, H. B. Liu and J. Taylor, Nature, 2019, 567, 516–520 CrossRef CAS PubMed.
- K. Wang, R. Mao, R. Liu, J. Zhang, H. Zhao, W. Ran and X. Zhao, Nat. Water, 2023, 1, 1068–1078 CrossRef CAS.
- S. Han, H. Li, T. Li, F. Chen, R. Yang, Y. Yu and B. Zhang, Nat. Catal., 2023, 6, 402–414 CrossRef CAS.
- J. Y. Fang, Q. Z. Zheng, Y. Y. Lou, K. M. Zhao, S. N. Hu, G. Li, O. Akdim, X. Y. Huang and S. G. Sun, Nat. Commun., 2022, 13, 7899 CrossRef CAS PubMed.
- Z. Chang, G. Meng, Y. Chen, C. Chen, S. Han, P. Wu, L. Zhu, H. Tian, F. Kong, M. Wang, X. Cui and J. Shi, Adv. Mater., 2023, 35, e2304508 CrossRef PubMed.
- Y.-M. Cai, Y.-H. Li, Y. Xiao, Q. Meyer, Q. Sun, W.-J. Lai, S.-W. Zhao, J. Li, L.-J. Zhang, H. Wang, Z. Lin, J. Luo and L.-L. Han, Rare Met., 2024, 43, 5792–5801 CrossRef CAS.
- Z. Song, Y. Liu, Y. Zhong, Q. Guo, J. Zeng and Z. Geng, Adv. Mater., 2022, 34, e2204306 CrossRef PubMed.
- W. He, J. Zhang, S. Dieckhofer, S. Varhade, A. C. Brix, A. Lielpetere, S. Seisel, J. R. C. Junqueira and W. Schuhmann, Nat. Commun., 2022, 13, 1129 CrossRef CAS PubMed.
- J. Dai, Y. Tong, L. Zhao, Z. Hu, C. T. Chen, C. Y. Kuo, G. Zhan, J. Wang, X. Zou, Q. Zheng, W. Hou, R. Wang, K. Wang, R. Zhao, X. K. Gu, Y. Yao and L. Zhang, Nat. Commun., 2024, 15, 88 CrossRef PubMed.
- J. Sun, H. Yang, W. Gao, T. Cao and G. Zhao, Angew. Chem., Int. Ed., 2022, 61, e202211373 CrossRef CAS PubMed.
- M. Xie, S. Tang, Z. Li, M. Wang, Z. Jin, P. Li, X. Zhan, H. Zhou and G. Yu, J. Am. Chem. Soc., 2023, 145, 13957–13967 CrossRef CAS PubMed.
- F. Xie, X. Cui, X. Zhi, D. Yao, B. Johannessen, T. Lin, J. Tang, T. B. F. Woodfield, L. Gu and S.-Z. Qiao, Nat. Synth., 2023, 2, 129–139 CrossRef CAS.
- F. Y. Chen, Z. Y. Wu, S. Gupta, D. J. Rivera, S. V. Lambeets, S. Pecaut, J. Y. T. Kim, P. Zhu, Y. Z. Finfrock, D. M. Meira, G. King, G. Gao, W. Xu, D. A. Cullen, H. Zhou, Y. Han, D. E. Perea, C. L. Muhich and H. Wang, Nat. Nanotechnol., 2022, 17, 759–767 CrossRef CAS PubMed.
- J. Li, H. Li, K. Fan, J. Y. Lee, W. Xie and M. Shao, Chem. Catal., 2023, 3, 100638 CrossRef CAS.
- W. Guo, S. Zhang, J. Zhang, H. Wu, Y. Ma, Y. Song, L. Cheng, L. Chang, G. Li, Y. Liu, G. Wei, L. Gan, M. Zhu, S. Xi, X. Wang, B. I. Yakobson, B. Z. Tang and R. Ye, Nat. Commun., 2023, 14, 7383 CrossRef PubMed.
- R. Gao, T. Y. Dai, Z. Meng, X. F. Sun, D. X. Liu, M. M. Shi, H. R. Li, X. Kang, B. Bi, Y. T. Zhang, T. W. Xu, J. M. Yan and Q. Jiang, Adv. Mater., 2023, 35, e2303455 CrossRef PubMed.
- W. Zhu, F. Yao, Q. Wu, Q. Jiang, J. Wang, Z. Wang and H. Liang, Energy Environ. Sci., 2023, 16, 2483–2493 RSC.
- S. Verma, S. Lu and P. J. A. Kenis, Nat. Energy, 2019, 4, 466–474 CrossRef CAS.
- Q. Qian, X. He, Z. Li, Y. Chen, Y. Feng, M. Cheng, H. Zhang, W. Wang, C. Xiao, G. Zhang and Y. Xie, Adv. Mater., 2023, 35, e2300935 CrossRef PubMed.
- G. Han, G. Li and Y. Sun, Nat. Catal., 2023, 6, 224–233 CrossRef CAS.
- Z. J. Schiffer, S. Biswas and K. Manthiram, ACS Energy Lett., 2022, 7, 3260–3267 CrossRef CAS PubMed.
- Z. Pan, Z. Zhang, W. Li, X. Huo, Y. Liu, O. C. Esan, Q. Wu and L. An, ACS Energy Lett., 2023, 8, 3742–3749 CrossRef CAS.
- R. Siya and E. E. Richard, Synthesis, 1988, 91–95 Search PubMed.
- G. Li, G. Han, L. Wang, X. Cui, N. K. Moehring, P. R. Kidambi, D. E. Jiang and Y. Sun, Nat. Commun., 2023, 14, 525 CrossRef CAS PubMed.
- Y. Liu, Z. Zhuang, Y. Liu, N. Liu, Y. Li, Y. Cheng, J. Yu, R. Yu, D. Wang and H. Li, Angew. Chem., Int. Ed., 2024, 63, e202411396 CAS.
- L. J. Zhang, N. Jin, Y. B. Yang, X. Y. Miao, H. Wang, J. Luo and L. L. Han, Nano-Micro Lett., 2023, 15, 228 CrossRef CAS PubMed.
- H. Yadegari, A. Ozden, T. Alkayyali, V. Soni, A. Thevenon, A. Rosas-Hernandez, T. Agapie, J. C. Peters, E. H. Sargent and D. Sinton, ACS Energy Lett., 2021, 6, 3538–3544 CrossRef CAS.
- C. Du, J. P. Mills, A. G. Yohannes, W. Wei, L. Wang, S. Lu, J. X. Lian, M. Wang, T. Guo, X. Wang, H. Zhou, C. J. Sun, J. Z. Wen, B. Kendall, M. Couillard, H. Guo, Z. Tan, S. Siahrostami and Y. A. Wu, Nat. Commun., 2023, 14, 6142 CrossRef CAS PubMed.
- A. A. Guda, S. A. Guda, A. Martini, A. N. Kravtsova, A. Algasov, A. Bugaev, S. P. Kubrin, L. V. Guda, P. Šot, J. A. van Bokhoven, C. Copéret and A. V. Soldatov, npj Comput. Mater., 2021, 7, 203 CrossRef CAS.
- L. Cao, Q. Luo, W. Liu, Y. Lin, X. Liu, Y. Cao, W. Zhang, Y. Wu, J. Yang, T. Yao and S. Wei, Nat. Catal., 2018, 2, 134–141 CrossRef.
- W. Yu, J. Yu, M. Huang, Y. Wang, Y. Wang, J. Li, H. Liu and W. Zhou, Energy Environ. Sci., 2023, 16, 2991–3001 RSC.
- H. Xu, Y. Y. Ma, J. Chen, W. X. Zhang and J. P. Yang, Chem. Soc. Rev., 2022, 51, 2710–2758 RSC.
- S. Y. Xiang, Y. H. Liu, G. M. Zhang, R. Ruan, Y. P. Wang, X. D. Wu, H. L. Zheng, Q. Zhang and L. P. Cao, World J. Microbiol. Biotechnol., 2020, 36, 144 CrossRef CAS PubMed.
- Q. Hu, K. Yang, O. Peng, M. Li, L. Ma, S. Huang, Y. Du, Z. X. Xu, Q. Wang, Z. Chen, M. Yang and K. P. Loh, J. Am. Chem. Soc., 2024, 146, 668–676 CrossRef CAS PubMed.
- Y. Wang, W. Zhou, R. Jia, Y. Yu and B. Zhang, Angew. Chem., Int. Ed., 2020, 59, 5350–5354 CrossRef CAS PubMed.
- Y. Bu, C. Wang, W. Zhang, X. Yang, J. Ding and G. Gao, Angew. Chem., Int. Ed., 2023, 62, e202217337 CrossRef CAS PubMed.
- G. Zhang, X. Li, K. Chen, Y. Guo, D. Ma and K. Chu, Angew. Chem., Int. Ed., 2023, 62, e202300054 CrossRef CAS PubMed.
- H. Liu, X. Lang, C. Zhu, J. Timoshenko, M. Ruscher, L. Bai, N. Guijarro, H. Yin, Y. Peng, J. Li, Z. Liu, W. Wang, B. R. Cuenya and J. Luo, Angew. Chem., Int. Ed., 2022, 61, e202202556 CrossRef CAS PubMed.
- L. Wu, J. Feng, L. Zhang, S. Jia, X. Song, Q. Zhu, X. Kang, X. Xing, X. Sun and B. Han, Angew. Chem., Int. Ed., 2023, 62, e202307952 CrossRef CAS PubMed.
- J. Wang, C. Cai, Y. A. Wang, X. M. Yang, D. J. Wu, Y. M. Zhu, M. H. Li, M. Gu and M. H. Shao, ACS Catal., 2021, 11, 15135–15140 CrossRef CAS.
- L. J. Zhang, T. T. Gu, K. L. Lu, L. J. Zhou, D. S. Li and R. H. Wang, Adv. Funct. Mater., 2021, 31, 2103187 CrossRef CAS.
- K. Chen, Z. Ma, X. Li, J. Kang, D. Ma and K. Chu, Adv. Funct. Mater., 2023, 33, 2209890 CrossRef CAS.
- P. Li, J. Bi, J. Liu, Q. Zhu, C. Chen, X. Sun, J. Zhang and B. Han, Nat. Commun., 2022, 13, 1965 CrossRef CAS PubMed.
- L. Zhang, H. Hu, C. Sun, D. Xiao, H.-T. Wang, Y. Xiao, S. Zhao, K. H. Chen, W.-X. Lin, Y.-C. Shao, X. Wang, C.-W. Pao and L. Han, Nat. Commun., 2024, 15, 7179 CrossRef CAS PubMed.
- Y. Pan, Y. Li, C.-L. Dong, Y.-C. Huang, J. Wu, J. Shi, Y. Lu, M. Yang, S. Wang and Y. Zou, Chem, 2023, 9, 963–977 CAS.
- R. Cai, M. Sun, F. Yang, M. Ju, Y. Chen, M. D. Gu, B. Huang and S. Yang, Chem, 2024, 10, 211–233 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ee04382k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |