Open Access Article
Open Access ArticleApproaching convergence in the electrochemical mechanism of aqueous Zn–MnO2 sustainable batteries†
Balaji
Sambandam
 *,
Vinod
Mathew
,
Muhammad H.
Alfaruqi
,
Sungjin
Kim
and
Jaekook
Kim
*,
Vinod
Mathew
,
Muhammad H.
Alfaruqi
,
Sungjin
Kim
and
Jaekook
Kim
 *
*
Department of Materials Science and Engineering, Chonnam National University, Gwangju 61186, South Korea. E-mail: balajisam@chonnam.ac.kr; jaekook@chonnam.ac.kr
First published on 17th April 2025
Abstract
Manganese oxide cathodes are quite appealing when considering moderate energy/power aqueous rechargeable zinc-ion batteries (ARZIBs) and long-term cycling. However, due to the variety of potential reaction pathways that are periodically proposed, the electrochemistry of manganese oxides with zinc is still unclear and fraught with dispute. Several mechanisms have been hypothesized/proposed over time for Zn–MnO2 in most common electrolyte of ZnSO4, including exclusive Zn2+ insertion, exclusive H+ conversion along with reversible layer hydroxide formation–dissolution, mixed insertion–conversion with and without layer formation–dissolution, exclusive MnO2/Mn2+ electrodeposition–dissolution with layer formation–dissolution, and mixed electrodeposition–dissolution and Zn2+/H+ insertion with layer formation–dissolution. To tackle this problem, we propose three potential roadmap approaches: selection of operando analyses, fine-tuned electrolyte pH, and electrolyte isotope labelling.
Broader contextIn developing efficient green-energy storage systems, the electrochemistry of manganese oxides for aqueous rechargeable zinc-ion batteries (Zn–MnO2) is controversial due to numerous proposed reactions. A specific manganese oxide polymorph under similar electrochemical conditions shows distinct behavior in the common ZnSO4 electrolyte, leading to misconceptions that hinder electrochemical performance and commercialization. While electrodeposition–dissolution is considered a major reaction, the ion intercalation mechanism should be verified in all polymorphs, except for δ-MnO2, which has already been confirmed. However, the nature of the inserting ion remains unclear. To address all these issues, we suggest real-time analyses, including neutron diffraction and electron paramagnetic resonance spectroscopy, to investigate Zn2+/H+ intercalation and MnO2/Mn2+ electrodeposition–dissolution reactions. Applying these techniques on a known MnO2 polymorph with fine-pH-tuned electrolytes would help clarify the mechanism. Other operando and non-operando analyses should also be explored. Manganese dissolution is critical in Zn–MnO2 batteries, and our initiation of AIMD simulations examines the manganese dissolution in detail. |
1. Introduction
In the pursuit of renewable and clean energy to meet future demands, aqueous batteries have garnered increasing attention for their safety and potential as advanced technologies in the future.1 Among aqueous energy storage technologies, aqueous Zn-metal-based batteries are particularly noteworthy because of their compatibility with water-based aqueous electrolytes and their high theoretical energy density (∼820 mA h g−1 and ∼5854 A h L−1) as anodes. These include alkaline Zn–MnO2 batteries, Zn–air batteries, mildly acidic aqueous rechargeable Zn-ion batteries (ARZIBs), and electrolytic Zn–MnO2 batteries with decoupled electrolytes.Among these Zn-metal aqueous rechargeable batteries, ARZIBs and Zn–MnO2 decoupled batteries, which have had a recent re-birth, are promising technologies aimed at greater utilization in stationary energy storage applications. The former has great potential due to high energy density, but there are many unaddressed factors, including the selection of a suitable membrane and cell design. On the other hand, ARZIBs typically operate in mildly acidic electrolytes with varied cathodes such as manganese oxides, making them a potential candidate for broadening suitable stationary energy storage systems (SESSs) through ease of cell assembling, high compatibility of the Zn anode in a mildly acidic medium (pH range 4–5) and environmental benignity.2 Despite these advantages, the fundamental electrochemical mechanism associated with Zn–MnO2 ARZIBs remains unaddressed, which impedes further developments.3 Taking this opportunity, the benchmark roadmap strategies on the electrochemical mechanism of Zn–MnO2 ARZIBs are provided here. This effort can remove the existing ambiguity related to the existing diverse theories and promote the realization of a unified conclusion on the electrochemical mechanism of ARZIBs.
2. Rapid emergence and controversial mechanism of mildly acidic aqueous Zn–MnO2 batteries
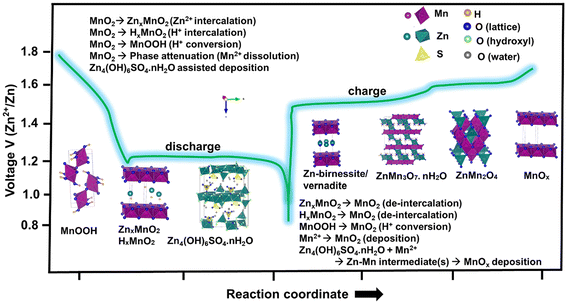
AZIBs have been a highly researched topic since the conception of a mildly acidic Zn–MnO2 battery with the ZnSO4 electrolyte, which is the electrolyte used in the majority of cases in this field, with reversible Zn-ion intercalation in 2012. The increasingly voluminous research publications (Fig. S1 in the ESI†) and technology transfers reported so far testify to the fact that zinc hydroxide sulphate precipitation resulting from Zn2+ and/or H+ insertion and Mn electrodissolution is a large contributor to the capacity and electrochemical results. This system is considered as a green, sustainable and renewable technology due to the low cost, safety, and environmental friendliness.2,4 Specifically, the Zn–MnO2 system with a considerably moderate voltage potential (∼1.3 V vs. Zn2+/Zn) and sufficient energy storage capability (100–140 W h L−1), reported from two start-ups,5,6 shows commercial prospects for stationary energy storage system applications.Despite these advantages, the dispute over the electrochemical reaction related to this aqueous electrolyte-based battery system with a MnO2 cathode has persisted owing to the diverse mechanisms proposed by different research groups. Over the past decade, paradoxical electrochemical reactions on MnO2, its polymorphs (α, β, δ, ε, and γ and a special structure), and related manganese oxides (MnO, Mn3O4, Mn2O3, and ZnMn2O4) have been continuously reported in ZnSO4 electrolytes. These reactions include exclusive Zn2+ insertion, exclusive H+ conversion, Zn2+/H+ co-insertion, mixed insertion–conversion, Zn2+/H+ insertion with Mn2+/MnO2 electrodissolution–deposition, Zn4(OH)6SO4·nH2O deposition/dissolution resulting from H+ contribution, exclusive Mn2+–MnO2 electrodissolution–deposition, and exclusive H+ insertion.7 Specifically, manganese oxides have grossly different crystal structures and permit insertion of varying amounts of Zn2+, H+ and co-intercalated solvents upon electrochemical charge/discharge. Most recent studies suggested that Zn4(OH)6SO4·nH2O precipitation–decomposition resulting from H+ (H3O+) and Mn2+–MnO2 electrodissolution–deposition is found to be major contributors to the capacity and resulting electrochemical mechanisms. However, all these manganese oxide-related cathode materials display almost identical galvanostatic discharge–charge profiles in the ZnSO4 electrolyte with and without Mn2+ additives, except the initial cycles, thus making the analysis of the reaction mechanism more complex than anticipated.8 Furthermore, the presence of Mn2+ additives, commonly MnSO4, in electrolytes also intensifies matters, leading to increased complexity to assess the mechanism. A few of the recent reports have mainly centered on settling the electrochemical mechanism debate by calling for convergence towards a unified electrochemical theory for Zn–MnO2 batteries.8,9 These reports conclude that the debate between Zn2+ and H+ (co-) intercalation mechanisms and the MnO2–Mn2+ electrodeposition–dissolution mechanism for Zn–MnO2 ARZIBs remains a close-call, needing urgent resolution via deeply investigating operando-assisted techniques.
For example, two separate studies, in recent times, presented conflicting findings regarding the impact of H+ intercalation on MnO2 cathodes.2,9 One study demonstrated its exclusive contribution, while the other indicated just a minimal contribution from H+ towards energy storage. In contrast, a very recent mechanism proposed involves Zn2+/H+ co-intercalation, accompanied by Mn2+ electrodissolution, in a MnO2 polymorph, as illustrated in Fig. 1, through various electrochemical analyses including operando and non-operando techniques. Notably, most of the reported MnO2 polymorphs and their related oxides do not provide solid evidence of Zn2+/H+ co-intercalation via operando X-ray diffraction analysis (XRD) probably due to their distinct crystal structures.7 This has led to a few groups dismissing intercalation feasibility and suggesting that the sole action of MnO2–Mn2+ electrodeposition–dissolution drives the capacity contribution in Zn–MnO2 batteries.8 These ongoing debates suggest that resolving the mechanism debate (listed in Table 1 in the ESI†) is intricate and remains fully unaddressed. The explored reaction mechanisms with possible intermediate/end products during discharge and charge are depicted in Fig. 2. The discharge reaction and the formation of intermediate/end products do not follow a precisely reversible path during the charge reaction and the formation of products.
A crucial study on the absence of Mn2+ additives in the ZnSO4 electrolyte suggested that the choice of MnO2 polymorphs is insignificant as the achieved capacities and charge–discharge curve profiles of different polymorphs remained almost identical after a few tens of cycles, while their initial discharge profiles varied.10 This might result from common phase transformation in the structure of MnO2 during cycling. In such a case, electrolyte pH regulation becomes critical. However, the initial variation in discharge profiles resulting from diversity in the crystal structures of these polymorphs requires an in-depth analysis. From detailed experimental studies along with in situ electrochemical quartz crystal microbalance (EQCM) analysis on the Zn–MnO2 and Zn–Csp (super P carbon) electrodes containing aqueous 2 M ZnSO4 + 0.2 M MnSO4 electrolytes, it was observed that the formation of ZHS is from an intermediate amphoteric complex of (Zn(H2O)x(OH)2), generally represented as Zn(OH)2. This complex results from the deprotonation of the solvated [Zn(H2O)6]2+ complex in the electrolyte which acts as a weak Brønsted acid. This confirms that the MnO2–Mn2+ electrodeposition–dissolution reaction follows H+ insertion into MnO2 and not through intercalation.11 Notably, irrespective of the type of positive cathode, with or without MnO2, the study found that the electrochemical features are fingerprinted once the few initial cycles are completed. We also observed that the electrochemical charge–discharge curves are identical, irrespective of the type of polymorph of MnO2, crossing few initial charge–discharge cycles, confirming that ZHS phase nucleation begins at ∼1.3 V due to the presence of hydroxide ions resulting from the participation of protons in the MnO2 and Mn dissolution. This slows down the pH variation below 1.3 V and the phase intensifies continuously until the discharge end. During charging, around 1.5 V, the ZHS phase starts dissolving reversibly (it can react with Mn2+ ions remaining in the electrolyte), and Mn2+–MnO2 deposition occurs as the charging progresses. The pH variation in the initial and subsequent cycles is quite complicated to understand. Different pH variation trends within region I and region II within the first cycle have been reported.12,13 This results in the nature of polymorphs, type of electrolyte with and without Mn2+ additives, aging time, and their initial pH values that can strongly affect the pH variation and resulting reaction during the electrochemical reaction.
Considering the above discussion, the present commentary article revives the call for this implicit controversial issue to converge towards an explicit mechanism that can be widely accepted. To achieve this anticipated goal, we provide a roadmap that recommends three critical strategies to be implemented as follows.
3. Roadmap strategies
(i) Operando experiments on selected crystalline MnO 2 polymorphs to lead the way in shedding more light on the reaction mechanism.Specifically, we propose δ-MnO2 (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) with a layered (2D) structure (interlayer spacing d ≈ 7 Å), α-MnO2 with 2 × 2 and 1 × 1 tunnel type (1D), β-MnO2 with 1 × 1 tunnel type (1D), and γ-MnO2 with 1 × 2 and 1 × 1 tunnel type (1D) as optimal choices for observing real-time ion storage (intercalation-based redox reactions) due to varied first cycle electrochemical charge–discharge traces. Among them, layered δ-MnO2, as evidenced by its lattice plane, shifts during the second discharge/charge reaction by operando analysis,14 which is helpful in understanding the intercalation mechanism, but still it is difficult to identify the type of intercalated ion and phase.15 Other MnO2 polymorphs have failed to reveal the intercalation phenomenon due to their distinct structures, making them incapable of elucidating the mechanism through operando XRD. It is advisable to rely on robust operando techniques to eliminate errors caused by unintentional blunders during sample handling and misinterpretation of the subsequent analysis in Zn–MnO2 batteries, which could cause delays in understanding the underlying mechanism. Recent concerns about conflict of interest regarding the proposed electrochemical reactions in Zn–Mn aqueous batteries have been addressed with supportive evidence from electrolyte pH and the implementation of adapted charging protocols.16,17 Reliable operando XRD analyses have aligned with the absence of intermediate or end products of MnOOH during discharge,9 neglecting a possible H+ conversion reaction that was previously proposed as an essential electrochemical mechanism through non-operando analyses.18 However, some reports argue that due to the amorphous characteristic of MnOOH, its confirmation through operando XRD analysis remains inconclusive and should be considered when addressing related mechanisms.
m) with a layered (2D) structure (interlayer spacing d ≈ 7 Å), α-MnO2 with 2 × 2 and 1 × 1 tunnel type (1D), β-MnO2 with 1 × 1 tunnel type (1D), and γ-MnO2 with 1 × 2 and 1 × 1 tunnel type (1D) as optimal choices for observing real-time ion storage (intercalation-based redox reactions) due to varied first cycle electrochemical charge–discharge traces. Among them, layered δ-MnO2, as evidenced by its lattice plane, shifts during the second discharge/charge reaction by operando analysis,14 which is helpful in understanding the intercalation mechanism, but still it is difficult to identify the type of intercalated ion and phase.15 Other MnO2 polymorphs have failed to reveal the intercalation phenomenon due to their distinct structures, making them incapable of elucidating the mechanism through operando XRD. It is advisable to rely on robust operando techniques to eliminate errors caused by unintentional blunders during sample handling and misinterpretation of the subsequent analysis in Zn–MnO2 batteries, which could cause delays in understanding the underlying mechanism. Recent concerns about conflict of interest regarding the proposed electrochemical reactions in Zn–Mn aqueous batteries have been addressed with supportive evidence from electrolyte pH and the implementation of adapted charging protocols.16,17 Reliable operando XRD analyses have aligned with the absence of intermediate or end products of MnOOH during discharge,9 neglecting a possible H+ conversion reaction that was previously proposed as an essential electrochemical mechanism through non-operando analyses.18 However, some reports argue that due to the amorphous characteristic of MnOOH, its confirmation through operando XRD analysis remains inconclusive and should be considered when addressing related mechanisms.
Significantly, irrespective of the suggested polymorphs and the nature of inserted ions, interconnected MnO2 (Mn4+–Mn2+ electrodeposition–dissolution) is observed through peak attenuation in operando XRD analysis, confirming its contribution arbitrarily.7 Operando extended X-ray absorption fine structure analysis (X-ray absorption spectroscopy, XAS)8 confirms multi-step Zn–Mn complex deposition–dissolution or deposition–conversion, as irreversible phases formed during the charge reaction, affirming its contribution to the reaction mechanism. This Zn–Mn multi-step reaction was proposed to be identical and independent of the polymorphs.19 Therefore, real-time analyses, including all polymorphs, particularly tunnel and layered MnO2, should be conducted under similar reaction conditions with known electrolyte pH to reveal these multi-step reactions. Utilizing operando XAS analysis, alongside electron paramagnetic resonance (EPR) without an Mn2+ additive in the electrolyte allows for cathode and electrolyte examinations, distinguishing Mn4+, Mn3+, and Mn2+ ions and thereby ease in understanding the electrochemical mechanism. This cathode study can identify potential Mn4+ ⇔ Mn3+/2+ conversions, offering a well-resolved hyperfine structure from diluted Mn2+ ions (electrodissolution to the electrolyte), impacting the MnO2 EPR signal distinctly. It can also aid in understanding the Mn4+ and Mn3+ intermediate and addresses the Zn–Mn multi-step reaction at the cathode during charge, considering altered Mn4+ dipole–dipole and exchange interactions (Mn4+–O2–Mn4+ and Mn4+–O2–Mn3+ sites in MnO2) with peak broadening. This study also aids in understanding the number of electrons transferred during MnO2 conversion.
(ii) Differentiating the magnitude of intercalation and electrodissolution reactions of selected MnO 2 polymorphs in tuned electrolytes with the pH varying between 4 and 5.
A pool of electrolytes with the pH varying between 4 and 5, resulting in varying H+ concentrations, can ultimately help in differentiating related reaction mechanisms for different polymorphs of MnO2. This action can be helpful to distinguish the degree of H+ intercalation and the Mn2+ depth of electrodissolution, both of which are strongly dependent on the electrolyte pH, during the electrochemical reaction. By directly comparing the results from using electrolytes with varying pH values, one can possibly differentiate the reaction mechanism as a function of H+. In a recent study, by varying the electrolyte pH within the range of 4.0–5.3, the galvanostatic discharge response tends to display a pH-dependent first discharge profile with altered solid-solution and plateau-type profiles.10 However, after a few cycles, the discharge trace tends to become identical, inferring a common electrochemical mechanism. Therefore, more research on this direction would be recommended.
(iii) Distinguishing the intercalating carrier ions through electrolyte isotope labelling.
Subsequently, a comprehensive analysis utilizing reliable operando techniques in conjunction with XRD analysis will determine the intercalation feasibility for driving the electrochemical mechanism. This assessment aims to ascertain, irrespective of any MnO2/Mn2+ electrodeposition–dissolution contribution, whether the driving force is exclusively Zn2+ or H+ or collectively Zn2+ and H+ ions? The proposed facile approach involves employing isotope labeling in the electrolyte (either in Zn2+ salts or water solvent) to trace the source of intercalation (Fig. 3).
For instance, a non-radioactive Zn2+ salt, such as 70ZnSO4·xH2O, can be an electrolyte agent to monitor intercalation phenomena in the MnO2 lattice. This approach aids in analyzing potential 70Zn–O binding effects through operando neutron-based techniques, including neutron powder diffraction, neutron imaging, and neutron depth profiling. The neutron scattering power varies for different isotopic substitutions, enhancing sensitivity to locate positions. Supplementary analyses, such as solid-state 70Zn-NMR, EQCM, operando-pH, and X-ray CT (refinement), further support these findings.20
Furthermore, a reported study suggested that the galvanostatic first discharge–charge voltage traces of electrochemically manganese dioxide and chemically manganese dioxide γ-MnO2 polymorphs are comparable with subsequent cycles, indicating H+ insertion into MnO2 rather than intercalation.11 We suggest that these candidates are optimal for understanding the electrochemical mechanism of Zn–MnO2 batteries for further development.
Manganese electrodeposition–dissolution: a key electrochemical reaction in a broad potential window
Since the Mn-electrodissolution/deposition reaction involves protons in an acidic environment, its electrodissolution/deposition potential can vary depending on the pH of the electrolyte.21 However, its working potential is much lower than 1.23 V vs. SHE for the following half-cell reaction: MnO2 + 4H+ + 2e− ↔ Mn2+ + 2H2O under standard conditions of pH = 0 and [aMn2+] = 1. In a full cell with a paired Zn anode, the expected electrodissolution/deposition potential would be predicted to be lower than ∼1.9 V vs. Zn2+/Zn, due to non-standard electrochemical conditions, with the electrolyte pH around 4.2. Similarly, in a mildly acidic aqueous electrolyte containing Zn salt, the availability of both H+ and Zn2+ ions is prominent. However, their intercalation mechanism into the MnO2 cathode is yet to be proven through standard analyses as different schools of thought remain intact.9,11,15 Based on the Nernst equation and available experimental results on α-MnO2, the calculated electrodissolution potential during discharge is varied from approximately 1.6 to 1.3 V, where the initial/final pH and dissolved [Mn2+] of the electrolyte are found to be 4.2/5.5 and 20/200 ppm, respectively (concentration has been converted into molarity as it is difficult to calculate the activity of dissolute Mn2+ in the electrolyte and hence the predicted potential region is not precise, but valued). This prediction reveals that pH variation is dominant until the voltage reaches 1.3 V. Below this threshold, due to the domination of the ZHS phase, pH variation is controlled.Notably, the process of manganese dissolution could be initiated before applying current in the electrochemical cell, i.e., at an open circuit. With this insight, we conducted ab initio molecular dynamics (AIMD) simulations to investigate the potential dissolution of Mn atoms from surface active materials into a ZnSO4 aqueous electrolyte. AIMD simulations are a well-established tool for exploring atomic-scale interactions and dynamics in condensed matter systems, particularly for studying the early stages of surface reactions and dissolution phenomena. In our work, AIMD was employed to probe the initial interactions between Mn-containing electrode surfaces and the ZnSO4/H2O electrolyte. We modelled three discharged electrode surface phases—Zn-intercalated ZnMn2O4, proton-converted MnOOH, and low-valence MnO—in the presence of Zn, SO4, and H2O molecules. These systems were designed to represent possible surface compositions during deep discharge. Through AIMD simulations, we monitored the evolution of atomic positions and analysed the displacement behaviour of Mn atoms as a proxy for their dissolution tendencies. Our results reveal distinct displacement trends for Mn atoms across different surface chemistries, providing molecular-level insights into the stability and interaction of each system with the electrolyte. The initial and final configurations of the simulation cells are presented for easy identification (Fig. 4). By analyzing the dynamics and displacement behavior of Mn atoms, distinct trends emerge, providing insight into the dissolution tendencies and chemical interactions of each system. In ZnMn2O4, the observed Mn displacements predominantly show inward movement in the z-direction, with minimal lateral adjustments (Fig. 4a). This suggests that the Mn atoms are stabilized either closer to the surface or within the crystal lattice, likely influenced by the presence of Zn and SO4 ions. The relatively stable or inward movement of Mn atoms in the z-direction might indicate a system that is less prone to dissolution under these conditions, with longer simulation times potentially needed to observe significant Mn ion leaching.22–24
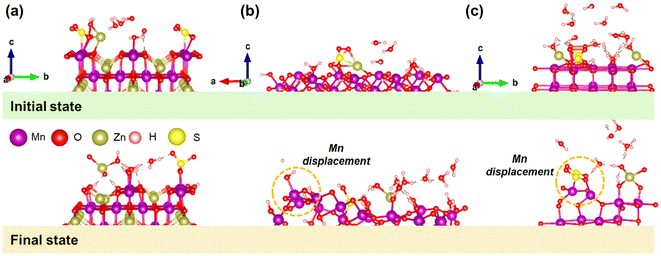 | ||
| Fig. 4 Initial and ab initio molecular dynamic simulated final states of (a) ZnMn2O4, (b) MnOOH, and (c) MnO surface slabs. | ||
In the MnOOH system, shown in Fig. 4b, the displacement behavior presents a more complex scenario. Most Mn atoms exhibit inward movement toward the surface, consistent with stabilization or surface relaxation. However, a subset of Mn atoms shows outward displacements. This is a strong indicator of these atoms moving outward into the surrounding space, signaling their detachment from the surface. The Mn disproportionation reaction of 2Mn3+ into Mn4+ and Mn2+ may play a key role in promoting these outward shifts, as it facilitates detachment of Mn atoms from their lattice sites.22 Compared to ZnMn2O4, MnOOH appears more prone to dissolution. MnO, on the other hand, shows the strongest evidence of dissolution among the three systems. Significant outward z-displacements of Mn atoms point to the detachment of Mn from the surface and potential formation of Mn2+ ions in solution, as depicted in Fig. 4c. The pronounced lateral displacements in MnO further highlight enhanced surface dynamics driven by interactions with Zn and SO4 ions. The presence of H2O in the system likely facilitates solvation and transport of Mn2+ ions, enhancing the dissolution process. These results indicate that MnO is the most dissolution-prone system under the given conditions, reflecting its chemical and structural susceptibility to ion leaching. The dissolution tendencies of Mn atoms vary significantly across the three systems influenced by their intrinsic structural properties and interactions with Zn, SO4, and H2O molecules. These simulation results provide valuable insights into the dissolution mechanisms of Mn-based materials in ZnSO4 electrolytes.
4. Conclusive remarks
Critical questions surrounding the electrochemical mechanism of aqueous Zn-ion batteries must be addressed promptly through essential electrochemical studies using operando techniques with in-depth non-operando/computational support. Using selected polymorphs of MnO2 to monitor structural evolution for the initial cycle and after a few cycles as a reference to address skeptical intercalation issues carefully is highly recommended in combination with operando neutron and electron paramagnetic analyses in different ZnSO4 electrolytes with subtle pH variations, within the limit of 4–5, supported by other relevant methods including operando XAS. Most research studies support electrodeposition–dissolution as a major reaction contribution, regardless of the type of polymorphs, leaving the ion intercalation reaction to be verified in all polymorphs of MnO2, except the layered one, whose confirmation has already been witnessed, without knowing the type of intercalating ion. Thus, it is imperative to develop more novel concepts and techniques that effectively support the primary electrochemical mechanism of Zn–MnO2 AZIBs, given their ongoing progression. Despite a few commercial challenges, a few recent academic publications and a few startups in the Zn–MnO2 batteries have successfully garnered increased public attention.Data availability
The simulation data that support the findings of this study are available at https://github.com/hilmyalfaruq/vaspsimulation.Conflicts of interest
The authors have declared that there are no competing interests.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MOE) (RS-2024-00463040). This work was also supported by the NRF grant funded by the Korean government (MSIT) (RS-2025-00520759).References
- D. Chao, W. Zhou, F. Xie, C. Ye, H. Li, M. Jaroniec and S.-Z. Qiao, Sci. Adv., 2020, 6, eaba4098 CrossRef CAS PubMed.
- S. W. D. Gourley, R. Brown, B. D. Adams and D. Higgins, Joule, 2023, 7, 1415–1436 CrossRef CAS.
- G. Li, L. Sun, S. Zhang, C. Zhang, H. Jin, K. Davey, G. Liang, S. Liu, J. Mao and Z. Guo, Adv. Funct. Mater., 2024, 34, 2301291 CrossRef CAS.
- Y. Zhang, Z. Chen, H. Qiu, W. Yang, Z. Zhao, J. Zhao and G. Cui, NPG Asia Mater., 2020, 12, 4 CrossRef CAS.
- https://www.enerpoly.com/technology#cell-pack .
- https://salientenergyinc.com/ .
- B. Sambandam, V. Mathew, S. Kim, S. Lee, S. Kim, J. Y. Hwang, H. J. Fan and J. Kim, Chem, 2022, 8, 924–946 CAS.
- D. Wu, L. M. Housel, S. T. King, Z. R. Mansley, N. Sadique, Y. Zhu, L. Ma, S. N. Ehrlich, H. Zhong, E. S. Takeuchi, A. C. Marschilok, D. C. Bock, L. Wang and K. J. Takeuchi, J. Am. Chem. Soc., 2022, 144, 23405–23420 CrossRef CAS PubMed.
- Y. Yuan, R. Sharpe, K. He, C. Li, M. T. Saray, T. Liu, W. Yao, M. Cheng, H. Jin, S. Wang, K. Amine, R. Shahbazian-Yassar, M. S. Islam and J. Lu, Nat. Sustain., 2022, 5, 890–898 CrossRef.
- U. Siamionau, Y. Aniskevich, A. Mazanik, O. Kokits, G. Ragoisha, J. H. Jo, S.-T. Myung and E. Streltsov, J. Power Sources, 2022, 523, 231023 CrossRef CAS.
- I. Aguilar, P. Lemaire, N. Ayouni, E. Bendadesse, A. V. Morozov, O. Sel, V. Balland, B. Limoges, A. M. Abakumov, E. Raymundo-Piñero, A. Slodczyk, A. Canizarès, D. Larcher and J.-M. Tarascon, Energy Storage Mater., 2022, 53, 238–253 CrossRef.
- C. F. Bischoff, O. S. Fitz, J. Burns, M. Bauer, H. Gentischer, K. P. Birke, H.-M. Henning and D. Biro, J. Electrochem. Soc., 2020, 167, 020545 CrossRef CAS.
- X. Xie, H. Fu, Y. Fang, B. Lu, J. Zhou and S. Liang, Adv. Energy Mater., 2022, 12, 2102393 CrossRef CAS.
- M. H. Alfaruqi, S. Islam, D. Y. Putro, V. Mathew, S. Kim, J. Jo, S. Kim, Y.-K. Sun, K. Kim and J. Kim, Electrochim. Acta, 2018, 276, 1–11 CrossRef CAS.
- N. J. Herrmann, H. Euchner, A. Groß and B. Horstmann, Adv. Energy Mater., 2024, 14, 2302553 CrossRef CAS.
- H. Yang, T. Zhang, D. Chen, Y. Tan, W. Zhou, L. Li, W. Li, G. Li, W. Han, H. J. Fan and D. Chao, Adv. Mater., 2023, 35, 2300053 CrossRef CAS PubMed.
- H. Wang, T. Wang, G. Stevenson, M. Chamoun and R. W. Lindström, Energy Storage Mater., 2023, 63, 103008 CrossRef.
- H. Pan, Y. Shao, P. Yan, Y. Cheng, K. S. Han, Z. Nie, C. Wang, J. Yang, X. Li, P. Bhattacharya, K. T. Mueller and J. Liu, Nat. Energy, 2016, 1, 16039 CrossRef CAS.
- V. R. Kankanallu, X. Zheng, D. Leschev, N. Zmich, C. Clark, C.-H. Lin, H. Zhong, S. Ghose, A. M. Kiss, D. Nykypanchuk, E. Stavitski, E. S. Takeuchi, A. C. Marschilok, K. J. Takeuchi, J. Bai, M. Ge and Y.-C. K. Chen-Wiegart, Energy Environ. Sci., 2023, 16, 2464–2482 RSC.
- Y. Zhang, Y. Liang, H. Dong, X. Wang and Y. Yao, J. Electrochem. Soc., 2020, 167, 070558 CrossRef.
- D. Chao, W. Zhou, C. Ye, Q. Zhang, Y. Chen, L. Gu, K. Davey and S.-Z. Qiao, Angew. Chem., Int. Ed., 2019, 58, 7823–7828 CrossRef CAS PubMed.
- R. Benedek, J. Phys. Chem. C, 2017, 121, 22049–22053 CrossRef CAS.
- K. Leung, Chem. Mater., 2017, 29, 2550–2562 CrossRef CAS.
- R. Benedek, M. M. Thackeray, J. Low and T. Bučko, J. Phys. Chem. C, 2012, 116, 4050–4059 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5eb00069f |
| This journal is © The Royal Society of Chemistry 2025 |