 Open Access Article
Open Access ArticleAir pollutant dynamics and behaviours in tobacco processing and storage environments: implications for air quality and health hazards
Anupam
Roy
a,
M. G.
Mostafa
 *b and
M. K.
Saha
c
*b and
M. K.
Saha
c
aInstitute of Environmental Science, University of Rajshahi, Rajshahi-6205, Bangladesh. E-mail: mgmostafa@ru.ac.bd
bInstitute of Environmental Science, University of Rajshahi, Rajshahi-6205, Bangladesh
cNational Environmental Specialist, Project Implementation Consultants, SASEC-2 Road Connectivity Project, Roads & Highways Department, Dhaka, Bangladesh
First published on 30th April 2025
Abstract
Tobacco curing poses serious environmental and health risks from elevated airborne pollutant emissions. This study aims to identify key air pollutants and associated behaviours during tobacco curing and storage operations, focusing on their impacts on air quality and potential health risks. This in situ analysis was conducted over 24 h at six tobacco curing houses (CHs) and three storage houses (SHs). Pollutant dynamics are influenced by ambient temperature and relative humidity, with higher temperatures and lower humidity amplifying emissions. Statistical analysis confirms that particulate matter (PM), total volatile organic compounds (TVOCs), HCHO, NO2, O3, CO, and SO2 for both environments exceed WHO standard limits, and most pollutants follow flat distributions with occasional spikes. Indoor–outdoor ratio (I/O) analysis shows that outdoor pollution stems from biomass combustion, while indoor levels result from both outdoor diffusion and indoor emissions. Pearson's correlation, Principal Component Analysis (PCA), and cluster analysis reveal a strong correlation among TVOCs, HCHO, NO2, and O3, suggesting similar sources and behaviours. Air quality indices (AQIs) indicate severe degradation, with CHs reaching unhealthy and SHs reaching very unhealthy levels, primarily driven by PM, NO2, and O3. These pollutants pose significant threats to human health, particularly for children sleeping in SHs, with TVOCs, HCHO, NO2, and PM primarily driving non-carcinogenic risks, and TVOCs are emerging as a major cancer risk. TVOCs, HCHO, and NO2 also impair plant health. This research highlights severe air pollution and associated health hazards in tobacco curing and storage environments, guiding policies to reduce exposure and promote sustainable tobacco production practices.
Environmental significanceThe investigation underscores the significant environmental impact of tobacco processing and storage, where critically high pollutant levels, such as PM2.5, PM10, TVOCs, HCHO, O3, SO2, and NO2, exceed national and international standards. The study highlights temperature and humidity as key drivers of emissions, with indoor air quality being influenced by both outdoor diffusion from tobacco curing and indoor emissions. These pollutants pose severe risks to human health, plant vitality, and crop yields. Implementing mitigation strategies is essential for protecting public health, supporting sustainable agricultural practices, and advancing progress toward Sustainable Development Goals (SDGs). |
1 Introduction
Clean air is fundamental to human health, yet air pollution remains a major global challenge.1 Alarmingly, 99% of the global population lives in areas where air quality exceeds the limits recommended by the World Health Organization's (WHO) Air Quality Guidelines.2,3 Socioeconomic factors are key drivers of air quality changes.4 On average, humans inhale approximately 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 liters of air each day.5,6 Dry air has a relatively stable composition by volume: N2 (78.084%), O2 (20.946%), Ar (0.934%), CO2 (0.033%), Ne (0.0018%), and trace gases (0.0012%), while water vapor can vary up to 4%.7 However, increasing emissions of particulate matter (dust, fumes, mists, and smoke), gaseous pollutants (gases and vapors), and odorous compounds (e.g., H2S) pose serious threats to human health, ecosystem security, climate stability, sustainable development, and plant vitality and human behavior.8–11 Implementing effective clean air policies can substantially reduce these detrimental effects on overall well-being.4 Key pollutants include criteria air pollutants (O3, CO, NO2, SO2, PM2.5, and PM10) and ambient air quality parameters (CO2, H2S, HCHO, and TVOCs).11,12 Air pollution originates from both natural and human sources but is predominantly driven by anthropogenic emissions and meteorological conditions.13 Human activities contribute to air pollution through stationary sources (agriculture, mining, industry, home heating, and waste incineration), mobile sources (vehicles, aircraft, and traffic dust), and indoor sources (tobacco smoke, biological allergens, combustion emissions, and volatile organic compounds). Additionally, natural sources include volcanic eruptions, soil erosion, and biological emissions (pollen, bacteria, spores, and viruses), which also contribute to air pollution.11 Outdoor air pollution is a major public health concern, increasing the risk of severe diseases, including cancer.14 However, the impact of air pollution is significantly greater in developing countries than in developed nations.15 Indoor air pollution, caused by physical, chemical, and biological contaminants, triggers toxicity mechanisms such as DNA methylation changes, oxidative stress, and gene activation.5,16 Biomass smoke exposure is linked to respiratory symptoms such as wheezing, coughing, and shortness of breath.17,18 Chronic exposure increases risks for respiratory infections, cardiovascular diseases, dementia, hypertension, and poor sleep quality.19–21 Indoor air pollution causes approximately 1.9 million deaths annually, particularly in rural areas, while ambient air pollution contributes to 0.5 million additional deaths. Air pollution caused approximately 7 million premature deaths worldwide in 2019,9,22 with 4–8% of global premature deaths and 20–30% of respiratory diseases linked to pollution, particularly from indoor exposure.23 Air pollution in Bangladesh caused 123
000 liters of air each day.5,6 Dry air has a relatively stable composition by volume: N2 (78.084%), O2 (20.946%), Ar (0.934%), CO2 (0.033%), Ne (0.0018%), and trace gases (0.0012%), while water vapor can vary up to 4%.7 However, increasing emissions of particulate matter (dust, fumes, mists, and smoke), gaseous pollutants (gases and vapors), and odorous compounds (e.g., H2S) pose serious threats to human health, ecosystem security, climate stability, sustainable development, and plant vitality and human behavior.8–11 Implementing effective clean air policies can substantially reduce these detrimental effects on overall well-being.4 Key pollutants include criteria air pollutants (O3, CO, NO2, SO2, PM2.5, and PM10) and ambient air quality parameters (CO2, H2S, HCHO, and TVOCs).11,12 Air pollution originates from both natural and human sources but is predominantly driven by anthropogenic emissions and meteorological conditions.13 Human activities contribute to air pollution through stationary sources (agriculture, mining, industry, home heating, and waste incineration), mobile sources (vehicles, aircraft, and traffic dust), and indoor sources (tobacco smoke, biological allergens, combustion emissions, and volatile organic compounds). Additionally, natural sources include volcanic eruptions, soil erosion, and biological emissions (pollen, bacteria, spores, and viruses), which also contribute to air pollution.11 Outdoor air pollution is a major public health concern, increasing the risk of severe diseases, including cancer.14 However, the impact of air pollution is significantly greater in developing countries than in developed nations.15 Indoor air pollution, caused by physical, chemical, and biological contaminants, triggers toxicity mechanisms such as DNA methylation changes, oxidative stress, and gene activation.5,16 Biomass smoke exposure is linked to respiratory symptoms such as wheezing, coughing, and shortness of breath.17,18 Chronic exposure increases risks for respiratory infections, cardiovascular diseases, dementia, hypertension, and poor sleep quality.19–21 Indoor air pollution causes approximately 1.9 million deaths annually, particularly in rural areas, while ambient air pollution contributes to 0.5 million additional deaths. Air pollution caused approximately 7 million premature deaths worldwide in 2019,9,22 with 4–8% of global premature deaths and 20–30% of respiratory diseases linked to pollution, particularly from indoor exposure.23 Air pollution in Bangladesh caused 123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths in 2017, increasing to 173
000 deaths in 2017, increasing to 173![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 in 2019.24 Health impacts vary with temperature, humidity, altitude, exposure levels, and indoor ventilation. Vulnerable groups include children, the elderly, and individuals with preexisting respiratory conditions.5 Temperature influences health and can confound pollutant effects, while humidity minimally affects gas toxicity but alters particle deposition through hygroscopic growth in the lungs. Sustainable Development Goals (SDGs 3, 7, 11, 13, and 15) emphasize reducing air pollution through improved energy efficiency, climate resilience, and ecosystem conservation.25 The Air Quality Index (AQI) tracks pollution levels using color codes and numerical values, guiding exposure limits and policy enforcement.26 Many countries, including the USA (1999), Hong Kong (2013), Canada (2017), the EU (2017), and South Korea (2018), have implemented AQI systems to manage air pollution. The USEPA-AQI remains the most widely used globally, including in Bangladesh,27 India,15 and China.2,4,8 Effective air quality management enhances public health, reduces mortality, and supports economic and social well-being. Strengthening regulations, promoting clean energy, and raising awareness are crucial in the fight against air pollution.
500 in 2019.24 Health impacts vary with temperature, humidity, altitude, exposure levels, and indoor ventilation. Vulnerable groups include children, the elderly, and individuals with preexisting respiratory conditions.5 Temperature influences health and can confound pollutant effects, while humidity minimally affects gas toxicity but alters particle deposition through hygroscopic growth in the lungs. Sustainable Development Goals (SDGs 3, 7, 11, 13, and 15) emphasize reducing air pollution through improved energy efficiency, climate resilience, and ecosystem conservation.25 The Air Quality Index (AQI) tracks pollution levels using color codes and numerical values, guiding exposure limits and policy enforcement.26 Many countries, including the USA (1999), Hong Kong (2013), Canada (2017), the EU (2017), and South Korea (2018), have implemented AQI systems to manage air pollution. The USEPA-AQI remains the most widely used globally, including in Bangladesh,27 India,15 and China.2,4,8 Effective air quality management enhances public health, reduces mortality, and supports economic and social well-being. Strengthening regulations, promoting clean energy, and raising awareness are crucial in the fight against air pollution.
Tobacco, Bangladesh's sixth-largest cash crop and second-highest export crop, ranks 14th globally in acreage and 12th in production, contributing 1.3% to global tobacco output. Major cultivation areas include Kushtia, Rangpur, Meherpur, Chuadanga, Jashore, Gazipur, and the Chattogram Hill Tracts, with extensions into Lalmonirhat, Nilphamari, Jhenaidah, and Rajshahi.28 In FY 2021–22, tobacco covered an area of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 ha, yielding 92
634 ha, yielding 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327 tons. The primary tobacco varieties cultivated include Virginia (69.85%), Joti (19.81%), and Motihari (10.34%).29 Kushtia was chosen as the study area due to its leading tobacco production, accounting for 29.58% of Bangladesh's total tobacco-cultivated land over the past five years.30 One of the critical processes in tobacco production is flue-curing, which is necessary for Virginia tobacco. This method involves drying tobacco leaves at controlled temperature and humidity by burning fuel wood for approximately 72 h.30 The process accelerates chlorophyll breakdown, imparts the characteristic yellow color to leaves and stems, converts starch into sugar, enhances aroma and flavor, increases nicotine concentration, and reduces moisture content.31 However, flue curing is highly resource-intensive, requiring approximately 15.56 tons of dry fuel wood per ha.30 This process releases a substantial amount of pollutants, many of which are toxic, carcinogenic, and mutagenic.11 The U.S. Environmental Protection Agency identified over 4000 chemical compounds in tobacco smoke in 1992, with approximately 60 known carcinogens.32 Nearly half of these compounds naturally exist in green tobacco leaves, while the remainder form during combustion. Key pollutants include nitrogen oxides (NOx), sulfur oxides (SOx), CO, PM, H2S, HCN, and various volatile and semi-volatile organic compounds. Additionally, UV-driven photochemical reactions transform NOx and VOCs into ground-level O3.32 Following the curing process, tobacco leaves are stored indoors for up to 45 days before being sold.30 Due to space limitations, many farmers store dried leaves inside their living quarters, often sleeping on them with their families, including women and children. The stored leaves undergo natural fermentation, primarily emitting NO2 due to the high nitrogen content (56–64%) in dried tobacco leaves. Additionally, VOCs and secondary O3 gas are released.31 Fine tobacco leaf particles, carried as particulate matter in the air, further degrade indoor air quality. Poor ventilation in storage rooms exacerbates exposure to these harmful emissions, posing severe health risks to tobacco-farming families. Although tobacco cultivation is economically profitable and supports local economies, it poses serious threats to food security, environmental sustainability, and public health. It contributes to deforestation, ecological disruption, and climate change. Tobacco plantations account for 3.5% of annual deforestation, with curing processes consuming an average of 23 m3 of fuel-wood per season, adding another 3%.33 According to the WHO,34 tobacco farming causes 5% of global deforestation and emits about 80 million tons of CO2 annually. In Bangladesh, the total GHG emissions from tobacco farming are estimated at (710
327 tons. The primary tobacco varieties cultivated include Virginia (69.85%), Joti (19.81%), and Motihari (10.34%).29 Kushtia was chosen as the study area due to its leading tobacco production, accounting for 29.58% of Bangladesh's total tobacco-cultivated land over the past five years.30 One of the critical processes in tobacco production is flue-curing, which is necessary for Virginia tobacco. This method involves drying tobacco leaves at controlled temperature and humidity by burning fuel wood for approximately 72 h.30 The process accelerates chlorophyll breakdown, imparts the characteristic yellow color to leaves and stems, converts starch into sugar, enhances aroma and flavor, increases nicotine concentration, and reduces moisture content.31 However, flue curing is highly resource-intensive, requiring approximately 15.56 tons of dry fuel wood per ha.30 This process releases a substantial amount of pollutants, many of which are toxic, carcinogenic, and mutagenic.11 The U.S. Environmental Protection Agency identified over 4000 chemical compounds in tobacco smoke in 1992, with approximately 60 known carcinogens.32 Nearly half of these compounds naturally exist in green tobacco leaves, while the remainder form during combustion. Key pollutants include nitrogen oxides (NOx), sulfur oxides (SOx), CO, PM, H2S, HCN, and various volatile and semi-volatile organic compounds. Additionally, UV-driven photochemical reactions transform NOx and VOCs into ground-level O3.32 Following the curing process, tobacco leaves are stored indoors for up to 45 days before being sold.30 Due to space limitations, many farmers store dried leaves inside their living quarters, often sleeping on them with their families, including women and children. The stored leaves undergo natural fermentation, primarily emitting NO2 due to the high nitrogen content (56–64%) in dried tobacco leaves. Additionally, VOCs and secondary O3 gas are released.31 Fine tobacco leaf particles, carried as particulate matter in the air, further degrade indoor air quality. Poor ventilation in storage rooms exacerbates exposure to these harmful emissions, posing severe health risks to tobacco-farming families. Although tobacco cultivation is economically profitable and supports local economies, it poses serious threats to food security, environmental sustainability, and public health. It contributes to deforestation, ecological disruption, and climate change. Tobacco plantations account for 3.5% of annual deforestation, with curing processes consuming an average of 23 m3 of fuel-wood per season, adding another 3%.33 According to the WHO,34 tobacco farming causes 5% of global deforestation and emits about 80 million tons of CO2 annually. In Bangladesh, the total GHG emissions from tobacco farming are estimated at (710![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 664 ± 19
664 ± 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 414) tCO2e, around 0.26% of the country's total annual emissions. Producing one kilogram of tobacco leaves releases approximately (7.7 ± 0.21) kg of CO2e.30 Despite these environmental impacts, farmers often remain unaware, focusing only on short-term economic gains. Notably, 65.28% of tobacco farmers acknowledged that tobacco curing severely pollutes the air.30 However, all previous assessments were based solely on questionnaire surveys. Given the growing global concern and evidence from countries such as the USA, Brazil, China, India, and Bangladesh, assessing real-time air pollution from tobacco curing is crucial. To address this, a portable OCEANUS AQM-9 (China) automatic air quality monitoring device was installed near CHs and inside SHs. Recent studies worldwide, including in Bangladesh, have extensively explored air pollution and its health effects from solid fuel cooking emissions5,10,23,35–37 and brick kiln emissions.12,38,39 Additionally, research has evaluated ambient air quality with seasonal variation in major cities2,4,8,9,13,26,40–45 and industrial areas.46 Numerous global studies also have focused on air pollution prediction and forecasting.1,3,22,47 Indoor and outdoor air quality studies have assessed air pollution-associated health risks15 in sleeping rooms and classrooms14,44,48–52 and the effects of tobacco smoke exposure.53 Research has also explored tobacco curing methods, fuel characteristics, and microbial changes during curing.54–56 Despite these extensive studies on air quality and health risks, no research either globally or in Bangladesh has specifically assessed the effects of tobacco leaf curing and storage on indoor and outdoor ambient air quality. Additionally, there are no estimations of the emissions of major toxic air pollutants from these processes, nor evaluations of the associated carcinogenic and non-carcinogenic health risks to surrounding communities and tobacco-growing families. This study aims to fill this critical research gap by assessing indoor and outdoor air quality in tobacco-processing communities, characterizing toxic pollutants, and evaluating the health risks associated with tobacco curing and storage. The findings of this research will provide essential insights into air pollution linked to tobacco processing, guiding policies and interventions to mitigate exposure, prevent long-term health effects, and promote sustainable tobacco production practices.
414) tCO2e, around 0.26% of the country's total annual emissions. Producing one kilogram of tobacco leaves releases approximately (7.7 ± 0.21) kg of CO2e.30 Despite these environmental impacts, farmers often remain unaware, focusing only on short-term economic gains. Notably, 65.28% of tobacco farmers acknowledged that tobacco curing severely pollutes the air.30 However, all previous assessments were based solely on questionnaire surveys. Given the growing global concern and evidence from countries such as the USA, Brazil, China, India, and Bangladesh, assessing real-time air pollution from tobacco curing is crucial. To address this, a portable OCEANUS AQM-9 (China) automatic air quality monitoring device was installed near CHs and inside SHs. Recent studies worldwide, including in Bangladesh, have extensively explored air pollution and its health effects from solid fuel cooking emissions5,10,23,35–37 and brick kiln emissions.12,38,39 Additionally, research has evaluated ambient air quality with seasonal variation in major cities2,4,8,9,13,26,40–45 and industrial areas.46 Numerous global studies also have focused on air pollution prediction and forecasting.1,3,22,47 Indoor and outdoor air quality studies have assessed air pollution-associated health risks15 in sleeping rooms and classrooms14,44,48–52 and the effects of tobacco smoke exposure.53 Research has also explored tobacco curing methods, fuel characteristics, and microbial changes during curing.54–56 Despite these extensive studies on air quality and health risks, no research either globally or in Bangladesh has specifically assessed the effects of tobacco leaf curing and storage on indoor and outdoor ambient air quality. Additionally, there are no estimations of the emissions of major toxic air pollutants from these processes, nor evaluations of the associated carcinogenic and non-carcinogenic health risks to surrounding communities and tobacco-growing families. This study aims to fill this critical research gap by assessing indoor and outdoor air quality in tobacco-processing communities, characterizing toxic pollutants, and evaluating the health risks associated with tobacco curing and storage. The findings of this research will provide essential insights into air pollution linked to tobacco processing, guiding policies and interventions to mitigate exposure, prevent long-term health effects, and promote sustainable tobacco production practices.
2 Materials and methods
2.1 Research location
Kushtia district, the study area, is located in the Khulna division of western Bangladesh and covers an area of 1621.15 km2. It lies between latitudes 23°42′ and 24°12′ north and longitudes 88°42′ and 89°22′ east, within Agro-Ecological Zone 11 (AEZ 11), known as the High Ganges River Floodplain. This study focused on six curing houses (CHs) and three tobacco storage houses (SHs) in Kazihata village (Table 1) (Dharmapur union, Bheramara upazila, Kushtia district), selected for their significant tobacco cultivation, which covers about 54.63% of the land in the Rabi season.29 The study village was also chosen for its minimal external pollution influences, being 10 km from the national highway and over 20 km from any industrial area.36| Code no. | Name of the place | Location | Weather | |
|---|---|---|---|---|
| Latitude | Longitude | |||
| a Note: CH indicates the community near the tobacco curing house; SH indicates inside the tobacco storage house. | ||||
| CH1 | In front of Shanto's house | 24.041376 | 88.923588 | Sunny |
| CH2 | In front of Idris's house | 24.030714 | 88.933962 | Sunny |
| CH3 | In front of Ziaur's house | 24.020412 | 88.862726 | Sunny |
| CH4 | In front of Kabir's house | 24.019428 | 88.889666 | Sunny |
| CH5 | In front of Monir's house | 24.024411 | 88.914216 | Sunny |
| CH6 | In front of Latif's house | 24.038072 | 88.947025 | Sunny |
| SH1 | Inside Idris's tobacco storage house | 24.030711 | 88.933852 | Sunny |
| SH2 | Inside Monir's tobacco storage house | 24.024540 | 88.914981 | Sunny |
| SH3 | Inside Latif's tobacco storage house | 24.037851 | 88.947197 | Sunny |
2.2 Measurement and instrumentation
Ambient air quality monitoring is an expensive process due to the sophisticated equipment and technology required. The analyzers used are highly sensitive and need precise calibration and maintenance for accurate measurements. Continuous monitoring also involves significant operational costs, including data processing, equipment upkeep, and technical expertise. Hence, the sample size is relatively limited. Tobacco curing, specifically for Virginia tobacco, is the most tedious stage of production, requiring around 72 h of continuous heating that releases significant air pollutants into the environment, which can also infiltrate nearby sleeping quarters. Additionally, stored tobacco leaves also emit substantial toxic pollutants. This study monitored air quality during the tobacco-curing season (March 16–25, 2024). A portable ambient air quality monitor (Brand: OCEANUS; Model: AQM-9; Country: China) was installed at a standard breathing height of 1.5 meters using an adjustable tripod.10,36 Continuous sampling was conducted using automatic sensors, employing light scattering for PM detection and a high-precision electrochemical sensor for gases, to provide time-averaged concentration data. At each location, the device operated for 24 h,8 starting at 09:00, recording two-hourly mean values, resulting in 12 data points per site.12 Monitored parameters included key air pollutants (O3, CO, NO2, SO2, PM2.5, and PM10), meteorological factors (AT and RH), and air quality indicators (CO2, H2S, HCHO, and TVOCs). The data stored in the device were subsequently retrieved via a computer for analysis.2.3 Data processing and analysis
The raw data were compiled into a master sheet and analyzed using Statistical Package for the Social Sciences (SPSS) software (Version 20) and Microsoft Excel 2016 for statistical evaluation.| Name of the pollutants | Measuring unit | Data processing time | Level to be truncated |
|---|---|---|---|
| a Source: USEPA.7 | |||
| O3 | ppm | 8 h average | 3 decimal places |
| CO | ppm | 8 h average | 1 decimal place |
| SO2 | ppb | 24 h average | Integer |
| NO2 | ppb | 24 h average | Integer |
| PM10 | μg m−3 | 24 h average | Integer |
| PM2.5 | μg m−3 | 24 h average | 1 decimal place |
| O3 (ppm) | CO (ppm) | SO2 (ppb) | NO2 (ppb) | PM2.5 (μg m−3) | PM10 (μg m−3) | AQI | Concern level with colour code |
|---|---|---|---|---|---|---|---|
| a Source: USEPA.7 | |||||||
| 0.000–0.054 | 0.0–4.4 | 0–35 | 0–53 | 0.0–12.0 | 0–54 | 0–50 | Good |
| 0.055–0.070 | 4.5–9.4 | 36–75 | 54–100 | 12.1–35.4 | 55–154 | 51–100 | Moderate |
| 0.071–0.085 | 9.5–12.4 | 76–185 | 101–360 | 35.5–55.4 | 155–254 | 101–150 | Unhealthy for sensitive groups |
| 0.086–0.105 | 12.5–15.4 | 186–304 | 361–649 | 55.5–150.4 | 255–354 | 151–200 | Unhealthy |
| 0.106–0.200 | 15.5–30.4 | 305–604 | 650–1249 | 150.5–250.4 | 355–424 | 201–300 | Very unhealthy |
| >0.200 | >30.5 | >605 | >1250 | >250.5 | >425 | >301 | Hazardous |
Step 3: calculation of the pollutant sub-index using eqn (1) (ref. 2, 4 and 41) to determine levels of concern.
 | (1) |
The highest sub-index AQI value is considered the site's overall USEPA AQI (eqn (2)):2,4,26
| USEPA AQI = maximum (AQIO3, AQICO, AQISO2, AQINO2, AQIPM2.5, AQIPM10) | (2) |
The Aggregated Air Quality Index (AAQI) is calculated using eqn (3):57
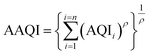 | (3) |
 | (4) |
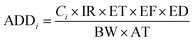 | (5) |
In step two, the hazard quotient (HQ) for NCR was calculated (eqn (6)), and the cumulative NCR, or hazard index (HI), was determined by summing HQs (eqn (7)):67
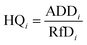 | (6) |
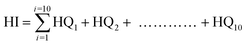 | (7) |
RfDi represents the reference dose (μg per kg-day) obtained from WHO-recommended values, with CO2 sourced from USEPA guidelines (Table 4).51 HQ or HI values are categorized as follows: no NCR hazard (<0.1), low NCR hazard (0.1–1.0), moderate NCR risk (1.1–10), and high NCR risk (>10).44
| Pollutants | AT | RH | CO2 | CO | O3 | NO2 | SO2 | H2S | HCHO | TVOCs | PM2.5 | PM10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average time | 24 h | 24 h | 24 h | 8 h | 8 h | 24 h | 24 h | 24 h | 24 h | 24 h | 24 h | 24 h | |
| Unit | °C | % | mg m−3 | mg m−3 | μg m−3 | μg m−3 | μg m−3 | mg m−3 | mg m−3 | mg m−3 | μg m−3 | μg m−3 | |
| a Note: CH is near the tobacco curing house, SH is the tobacco storage house, AT is ambient temperature, RH is relative humidity, TVOCs are total volatile organic compounds, PM is particulate matter, L and U are lower and upper limits, BAPCR is Bangladesh Air Pollution Control Rules, MPL is the maximum permissible limit, and NESREA is the National Environmental Standards and Regulations Enforcement Agency, Nigeria. | |||||||||||||
| Mean | CH | 39.38 | 45.51 | 958.09 | 5.21 | 137.24 | 354.22 | 234.08 | 0.29 | 2.47 | 12.53 | 97.87 | 282.29 |
| SH | 36.81 | 51.14 | 935.86 | 4.42 | 187.14 | 474.99 | 178.21 | 0.12 | 4.17 | 21.37 | 156.21 | 312.56 | |
| Median | CH | 39 | 45 | 973.61 | 4.62 | 143.20 | 352.40 | 240.15 | 0.30 | 2.59 | 12.71 | 90.66 | 283.62 |
| SH | 36 | 51 | 928.90 | 3.64 | 182.01 | 468.68 | 166.02 | 0.11 | 3.77 | 19.14 | 168.54 | 322.95 | |
| Standard deviation (SD) | CH | 2.22 | 2.28 | 256.50 | 3.01 | 39.62 | 104.83 | 73.60 | 0.12 | 1.11 | 5.61 | 32.25 | 103.69 |
| SH | 1.95 | 3.84 | 103.10 | 2.84 | 76.04 | 215.63 | 71.71 | 0.06 | 2.21 | 11.26 | 39.11 | 87.28 | |
| Standard error (SE) | CH | 0.26 | 0.27 | 30.23 | 0.35 | 4.67 | 12.35 | 8.67 | 0.01 | 0.13 | 0.66 | 3.80 | 12.22 |
| SH | 0.33 | 0.64 | 17.18 | 0.47 | 12.67 | 35.94 | 11.95 | 0.01 | 0.37 | 1.88 | 6.52 | 14.55 | |
| Kurtosis | CH | −1.35 | −0.68 | −0.84 | 0.26 | −0.61 | −0.30 | −0.46 | −0.48 | −0.18 | −0.05 | −0.13 | −0.36 |
| SH | −0.91 | −0.28 | 2.00 | 0.17 | −0.85 | −0.96 | −0.20 | −0.48 | −0.86 | −0.93 | 0.10 | 0.63 | |
| Skewness | CH | −0.08 | 0.21 | −0.10 | 0.69 | −0.44 | −0.15 | −0.14 | −0.21 | −0.03 | 0.09 | 0.35 | 0.18 |
| SH | 0.63 | −0.37 | 0.43 | 1.01 | 0.30 | 0.29 | 0.76 | 0.51 | 0.33 | 0.28 | −0.72 | −0.78 | |
| Minimum | CH | 36 | 41 | 452.30 | 0.40 | 41.72 | 99.14 | 86.00 | 0.03 | 0.15 | 0.86 | 38.50 | 86.32 |
| SH | 34 | 42 | 717.74 | 0.78 | 70.38 | 155.48 | 80.99 | 0.03 | 0.58 | 2.75 | 57.50 | 83.74 | |
| Maximum | CH | 43 | 51 | 1466.51 | 12.73 | 203.58 | 565.53 | 391.04 | 0.54 | 4.98 | 25.81 | 184.01 | 539.55 |
| SH | 41 | 58 | 1251.40 | 11.26 | 335.37 | 896.05 | 341.86 | 0.25 | 8.74 | 44.36 | 225.01 | 467.16 | |
| 95% confidence interval | CH (L) | 38.85 | 44.98 | 897.81 | 4.50 | 127.93 | 329.59 | 216.79 | 0.26 | 2.21 | 11.21 | 90.29 | 257.92 |
| CH (U) | 39.90 | 46.05 | 1018.36 | 5.92 | 146.55 | 378.86 | 251.38 | 0.32 | 2.73 | 13.84 | 105.45 | 306.65 | |
| SH (L) | 36.14 | 49.84 | 900.97 | 3.46 | 161.41 | 402.03 | 153.94 | 0.10 | 3.42 | 17.56 | 142.98 | 283.03 | |
| SH (U) | 37.47 | 52.44 | 970.74 | 5.38 | 212.87 | 547.95 | 202.47 | 0.14 | 4.91 | 25.18 | 169.44 | 342.09 | |
| MPL, BAPCR68 | NYS | NYS | NYS | 5 | 100 | 80 | 80 | 0.278 | 0.615 | NYS | 65 | 150 | |
| MPL, WHO62 | NYS | NYS | NYS | 4 | 100 | 25 | 40 | 0.15 | 0.1 | 0.3 | 15 | 45 | |
| MPL, USEPA61 | NYS | NYS | 1800 | 10 | 147 | 188 (1 h) | 196 (1 h) | 0.14 | NYS | NYS | 35 | 150 | |
| MPL, NESREA69 | 20–25.5 | 40–70 | 1440 | 1.89 | NYS | NYS | NYS | NYS | 0.03 | 0.2 | 15 | 20 | |
Incremental cancer risk (CR) represents the likelihood of developing cancer from lifetime exposure to a carcinogenic agent.52 Both CR and lifetime cancer risk (LCR) were determined using eqn (8) and (9):27,50
 | (8) |
 | (9) |
The correction factor  adjusts the Integrated Risk Information System (IRIS) risk measure, while ATc represents the average exposure time for CR determination, set at 70 years (25
adjusts the Integrated Risk Information System (IRIS) risk measure, while ATc represents the average exposure time for CR determination, set at 70 years (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 days).50,70 The cancer slope factor (CSFi) represents the risk per unit exposure (μg per kg-day) for parameter i, with values as follows: PM2.5 = 8 × 10−6,35 PM10 = 10−5,27,52 and TVOCs = 2.2 × 10−6.46,52 The acceptable CR threshold is 10−4.46 For regulatory assessment, this study classifies tolerable CR or LCR into five risk levels: very low (<10−6), low (10−6 to 10−5), medium (10−5 to 10−4), high (10−4 to 10−3), and very high (>10−3).71
550 days).50,70 The cancer slope factor (CSFi) represents the risk per unit exposure (μg per kg-day) for parameter i, with values as follows: PM2.5 = 8 × 10−6,35 PM10 = 10−5,27,52 and TVOCs = 2.2 × 10−6.46,52 The acceptable CR threshold is 10−4.46 For regulatory assessment, this study classifies tolerable CR or LCR into five risk levels: very low (<10−6), low (10−6 to 10−5), medium (10−5 to 10−4), high (10−4 to 10−3), and very high (>10−3).71
3 Results and discussion
3.1 Comprehensive descriptive analysis of key air quality parameters in tobacco processing and storage environments
Air pollution in tobacco processing and storage environments significantly impacts the health of communities, particularly tobacco-growing families, due to emissions from biomass combustion and the slow decomposition of dried tobacco leaves. The mean and median give insights into the average and typical values, while standard deviation (SD) and standard error (SE) measure spread and precision. Skewness, kurtosis, and range indicate the distribution and sharpness, and the 95% confidence interval (CI) estimates the reliability of the mean. These statistics offer a comprehensive understanding of central tendency, variability, distribution, and reliability.The mean concentrations of NO2 (474.99 μg m−3), O3 (187.14 μg m−3), HCHO (3.77 mg m−3), and TVOCs (3.77 mg m−3) were higher in SHs compared to CHs (354.22, 137.24, 2.47, and 2.47, respectively) (Table 4). This trend was consistent across median, range, and CI values, indicating elevated emissions in SHs. Variability was higher in SHs for NO2 (SD: 215.63 and SE: 35.94) than in CHs (SD: 104.83 and SE: 12.35), suggesting greater fluctuations. O3 showed moderate variability (SH: SD 215.63 and SE 4.67; CH: SD 104.83 and SE 12.67), while lower SD and SE for HCHO and TVOCs indicate more stable emissions for both environments. In SHs, NO2 accumulates indoors, with occasional outdoor airflow causing deposition and fluctuations, whereas CH, being open, allows more even dispersion. HCHO and TVOCs exhibit stability due to consistent sources and slower degradation rates, minimizing fluctuations in both environments. As O3 forms through photochemical reactions involving NO2 and TVOCs,52,63 its fluctuations reflect their variability, with high values for NO2 and low for TVOCs, resulting in moderate O3 fluctuations. NO2 in SHs (0.51) is mildly right-skewed, while in CHs (−0.44), it is slightly left-skewed. O3 has a near-normal distribution in SHs (0.29) but is left-skewed in CHs (−0.69). NO2 and O3 exhibit near-normal distributions, whereas HCHO and TVOCs show flatter distributions, indicating fewer extreme values. Ababio et al.5 reported NO2 levels of 70–360 μg m−3, while Ayeni et al.48 found TVOCs ranging from 0.32 to 10.00 mg m−3 in a printing room, both consistent with the research findings. The mean concentrations of O3, NO2, HCHO, and TVOCs in both SHs and CHs exceed national68 and international62 MPLs. These pollutants mainly originate from biomass combustion and heated tobacco leaves during curing and storage, sharing similar sources and behavior. Consequently, they can severely degrade ambient air quality and pose critical health risks to local communities and tobacco households.
This analysis highlights severe air pollution in tobacco processing and storage areas, with elevated levels of PM2.5, PM10, O3, NO2, SO2, HCHO, CO, and TVOCs, especially in SHs, degrading ambient air quality and posing significant health risks to local communities. Immediate intervention is essential to improve air quality and safeguard the health of tobacco-growing families.
3.2 Comparison of key air pollutants in tobacco processing and storage environments using a paired samples test
The indoor–outdoor (I/O) ratio helps assess pollutant variations and identify indoor sources.44 A paired t-test is commonly employed to evaluate differences in pollutant concentrations between indoor and outdoor environments.52 This test provides insights into significant variations in environmental parameters between the community near the tobacco curing house (CH or O) and the tobacco storage house (SH or I). However, the I/O ratio varies by site due to factors such as meteorological conditions (AT, RH, and wind speed/direction), indoor sources, ventilation patterns, household activities, penetration factors, particle deposition rates, and outdoor pollutant levels.52The mean differences (MDs) for AT, CO2, CO, SO2, and H2S were 2.57, 22.22, 0.79, 55.87, and 0.17, respectively (Table 5). Statistical analysis showed no significant differences (p > 0.05) in these air quality parameters between indoor and outdoor environments at t0.05, 35. The observed t-values (7.540, 0.666, 1.552, 4.559, and 10.434) exceeded the critical t-values (0, 0.507, 0.125, 0, and 0), confirming no statistically significant variation (Table 5). Data collection occurred in the summer (March) when biomass burning generated intense heat, significantly impacting the CH community but dissipating the SH. The summer AT range (22–35 °C) reported by Kaewrat et al.44 aligns with this study's findings. Biomass combustion during curing is the primary source of CO2, CO, SO2, and H2S,73 with additional emissions from the decomposition of the organic material of tobacco leaves due to heat exposure.10,31 This results in elevated outdoor pollution. Outdoor contaminants can infiltrate indoor spaces, either diluting or accumulating. Poor ventilation in SHs leads to indoor pollutant accumulation, supplemented by emissions from stored dry tobacco leaves, bringing concentrations close to but still lower than outdoor levels. RH was significantly higher in SHs but remained within the comfortable 70% range,44 likely due to lower AT in SHs compared to CHs. Table 5 shows statistically significant MD values for NO2 (120.76), O3 (49.90), HCHO (1.69), and TVOCs (8.84) at t0.05, 35. The observed t-values were lower than the critical t-values, indicating significant variation between indoor and outdoor environments. NO2 primarily forms during combustion, including tobacco curing. Indoors, it accumulates due to limited ventilation, with additional emissions from the slow fermentation of nitrogen-rich dry tobacco leaves (56–64% N),31 leading to higher NO2 levels in SHs compared to CHs. TVOCs are released from various sources, including heating from indoor dry tobacco leaves and small amounts from heating fuels.64 HCHO, a component of TVOCs, is a known byproduct of biomass combustion and tobacco smoke.48,63 O3 forms through NOx and VOC reactions in sunlight.41 In storage environments with limited ventilation, it can accumulate due to outdoor infiltration and indoor precursor emissions. These four pollutants are present indoors and outdoors, but open outdoor spaces lead to lower concentrations. In contrast, SH's enclosed environment traps pollutants from both indoor emissions and outdoor infiltration, significantly raising concentrations compared to CHs. PM2.5 levels are significantly higher in SHs due to accumulation from outdoor combustion sources74 and emissions from dry tobacco leaves in airtight spaces. Coarse PM10, linked to fly ash, tobacco leaf fragments, and dust from tobacco handling and firewood combustion, remains airborne briefly,51 with most particles settling before entering indoor spaces. Nevertheless, PM10 levels are slightly higher in SHs, mainly due to indoor emissions, with lower contributions from outdoor sources.
| Paired sample test | ||||||||
|---|---|---|---|---|---|---|---|---|
| Paired parameters (CH vs. SH) | Paired differences | Indoor/outdoor (I/O) ratio | Calculated t-value | df | Significance at the 5% level (2-tailed) | |||
| Mean | Standard deviation | Standard error | ||||||
| a Note: CH is the community near the tobacco curing house, SH indicates inside the tobacco storage house, AT is ambient temperature, RH is relative humidity, TVOCs are total volatile organic compounds, and PM is particulate matter. | ||||||||
| Pair 1 | AT–AT | 2.57 | 2.89 | 0.34 | 0.92 | 7.540 | 35 | 0.000 |
| Pair 2 | RH–RH | −5.63 | 4.83 | 0.57 | 1.11 | −9.880 | 35 | 0.000 |
| Pair 3 | CO2–CO2 | 22.22 | 282.94 | 33.35 | 0.97 | 0.666 | 35 | 0.507 |
| Pair 4 | CO–CO | 0.79 | 4.33 | 0.51 | 0.85 | 1.552 | 35 | 0.125 |
| Pair 5 | O3–O3 | −49.90 | 87.96 | 10.37 | 1.36 | −4.814 | 35 | 0.000 |
| Pair 6 | NO2–NO2 | −120.76 | 245.66 | 28.95 | 1.34 | −4.171 | 35 | 0.000 |
| Pair 7 | SO2–SO2 | 55.87 | 103.99 | 12.26 | 0.76 | 4.559 | 35 | 0.000 |
| Pair 8 | H2S–H2S | 0.17 | 0.14 | 0.02 | 0.42 | 10.434 | 35 | 0.000 |
| Pair 9 | HCHO–HCHO | −1.69 | 2.44 | 0.29 | 1.68 | −5.891 | 35 | 0.000 |
| Pair 10 | TVOCs–TVOCs | −8.84 | 12.27 | 1.45 | 1.70 | −6.112 | 35 | 0.000 |
| Pair 11 | PM2.5–PM2.5 | −58.35 | 51.25 | 6.04 | 1.58 | −9.660 | 35 | 0.000 |
| Pair 12 | PM10–PM10 | −30.32 | 139.56 | 16.45 | 1.10 | −1.843 | 35 | 0.069 |
The I/O ratio ranged from 0.42 to 1.70, with TVOCs (1.70) having the highest value, followed by HCHO (1.68), PM2.5 (1.58), O3 (1.36), and NO2 (1.34). These pollutants primarily originate in SHs due to the slow decomposition of organic matter under heat and moisture. The lowest I/O ratio was for H2S (0.42), followed by SO2, while AT, RH, CO2, CO, and PM10 had similar indoor and outdoor levels. Kaewrat et al.44 reported a typical I/O ratio range of 0.1 to 1.8, aligning with this study. Low SD and SE values for AT and RH indicate stable levels and precise mean estimation. A similar trend is observed for H2S, CO, HCHO, and TVOCs, as their lower concentrations and single-source emissions (either indoor or outdoor) lead to more stable MD fluctuations and reliable mean differences. In contrast, high SD values for CO2, O3, SO2, NO2, PM2.5, and PM10 indicate greater uncertainty in MD values, likely due to varying indoor and outdoor emission sources, environmental factors (AT, RH, and wind speed/direction), and their higher concentrations. Elevated SO2 and H2S levels in CHs suggest combustion-related emissions, while SH's enclosed conditions lead to the accumulation of NO2, O3, HCHO, TVOCs, and PM, raising air quality concerns and potential health risks in the storage environment.
3.3 Pearson's correlation coefficient for assessing potential correlations among the air quality parameters in tobacco processing and storage environments
Pearson's correlation coefficient helps analyze relationships between air pollutants and their effects, offering insights for air quality control, health risk mitigation, and worker safety in tobacco processing and storage environments. Table 6 shows that AT was positively correlated with all air pollutants. In CHs, it strongly correlated with CO2 (0.854), while in SHs, it was highly correlated with CO2, CO, ground-O3, NO2, HCHO, and TVOCs. Outdoors, CO2 and CO increased with increasing AT, while higher AT accelerated organic matter decomposition from dry tobacco leaves indoors, increasing ground-O3, NO2, HCHO, and TVOCs. SH's airtight conditions led to strong correlations with low fluctuation, whereas CHs, being open, showed only moderate correlations between AT and other pollutants. RH, in contrast, was negatively correlated with all pollutants, with particularly strong negative correlations with AT and CO2 in both environments. A strong AT-RH inverse correlation, also reported by Majumder et al.75 and Saha et al.,12 aligns with this study's findings. Table 6 also highlights a strong CO2–CO correlation in both houses, originating from biomass combustion. During curing, biomass fuel combustion releases CO2 from complete oxidation and CO from incomplete combustion due to limited oxygen supply.50 Similarly, SO2 and H2S showed a high correlation in both houses, as both originate from biomass combustion during tobacco curing. PM2.5 and PM10 followed the same pattern, driven by common sources such as fuel combustion, fine tobacco leaf fragments, dust, and wind dispersion.24 NO2 primarily originates from biomass combustion or the heating of organic compounds, showing a strong correlation with AT. HCHO, a component of TVOCs, also showed a positive correlation with AT. O3 is not directly emitted but forms when sunlight, especially UV light, interacts with TVOCs and NO2.52 As a result, NO2, TVOCs, HCHO, and O3 were strongly correlated with each other in both environments. The correlation matrix reveals that high AT and low RH drive maximum pollutant emissions. CO2–CO and SO2–H2S correlations suggest a common biomass combustion source, while NO2, TVOCs, HCHO, O3, and PM2.5–PM10 share similar origins, either biomass burning or tobacco leaf heating, or both. Understanding these relationships can guide targeted air quality control, improved ventilation strategies, and emission reduction efforts. Policymakers can use these insights to establish stricter air quality regulations in tobacco processing and storage areas, ensuring better worker safety.| a Black and violet digits indicate the correlation of parameters in the community near the tobacco curing house and inside the tobacco storage house, respectively; bold digits (>0.750) represent highly correlated values. |
|---|

|
3.4 Principal component analysis (PCA) of air quality parameters near the CH and inside the SH
Table 7 presents the Principal Component Analysis (PCA) results for air quality parameters in both CHs (open space) and SHs (airtight chamber), using the Varimax rotation method with Kaiser normalization.28 The analysis identifies key pollutant groups and their sources, explaining their contributions to air quality variation in both environments. Each column represents a Principal Component (PC), with PCs selected based on eigenvalues ≥1.0, accounting for at least 5% of the total variance.28,76 Eigenvalues reflect the variance captured by each PC, while variance percentages show individual contributions.| Factor | Principal component analysis | |||||
|---|---|---|---|---|---|---|
| Tobacco curing house (CH) | Tobacco storage house (SH) | |||||
| PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | |
| a Note: rotated component matrix, rotation method: Varimax with Kaiser normalization. | ||||||
| Eigenvalues | 3.586 | 3.249 | 2.158 | 2.020 | 6.627 | 3.570 |
| % of variance | 29.88 | 27.07 | 17.98 | 16.83 | 55.23 | 29.75 |
| Cumulative% | 29.88 | 56.96 | 74.94 | 91.77 | 55.23 | 84.98 |
| AT | 0.312 | 0.834 | 0.327 | 0.116 | 0.900 | 0.318 |
| RH | −0.294 | −0.805 | −0.353 | −0.145 | −0.736 | −0.367 |
| CO2 | 0.211 | 0.864 | 0.171 | 0.272 | 0.854 | 0.261 |
| CO | 0.099 | 0.877 | 0.007 | 0.162 | 0.695 | 0.531 |
| O3 | 0.821 | 0.242 | 0.082 | 0.383 | 0.892 | 0.336 |
| NO2 | 0.866 | 0.182 | 0.169 | 0.315 | 0.894 | 0.311 |
| SO2 | 0.247 | 0.273 | 0.125 | 0.893 | 0.580 | 0.676 |
| H2S | 0.269 | 0.193 | 0.165 | 0.885 | 0.562 | 0.711 |
| HCHO | 0.928 | 0.213 | 0.187 | 0.109 | 0.932 | 0.252 |
| TVOCs | 0.925 | 0.210 | 0.173 | 0.086 | 0.927 | 0.246 |
| PM2.5 | 0.190 | 0.218 | 0.939 | 0.132 | 0.183 | 0.922 |
| PM10 | 0.190 | 0.222 | 0.934 | 0.146 | 0.234 | 0.915 |
In CHs, four PCs were identified due to greater concentration fluctuations caused by wind. The PCs explained 29.88%, 27.07%, 17.98%, and 16.83% of the variance. In contrast, SHs had two PCs, which explained 55.23% and 29.75% of the variance, reflecting a more stable airflow in the airtight chamber. The factor loadings in the table indicate the correlation between pollutants and PCs. Higher absolute values suggest stronger correlations, indicating shared pollutant common sources.76 In total, the PCs captured 91.77% of the variance in CHs and 84.98% in SHs, effectively summarizing the air quality variation in both environments. In CHs, PC1 correlated with NO2, HCHO, TVOCs, and O3, linking them to green tobacco fermentation, photochemical reactions, and minimal fuel burning. PC2, associated with AT, CO2, and CO, pointed to fuel combustion. However, RH had a significant negative impact on AT, CO2, and CO in CHs. PC3, dominated by PM2.5 and PM10, was linked to particulate emissions from fuel burning, wind dust, and tobacco leaf heating, while PC4, correlated with SO2 and H2S, indicated biomass combustion during tobacco processing. In SHs, PC1 showed strong correlations with O3, NO2, HCHO, TVOCs, and AT, indicating pollutant accumulation from the storage of dry tobacco leaves in an airtight chamber. PC2, dominated by PM2.5 and PM10, suggested indoor buildup from outdoor fuel combustion. Wind fluctuations significantly impacted the pollutants, generating four PCs in CHs (open space) and only two PCs in SHs (airtight enclosed space). NO2, O3, HCHO, and TVOCs exhibited strong correlations (>0.75) in both environments. However, in SHs, they were significantly influenced by AT and CO2. Meanwhile, AT notably impacted CO2 and CO emissions, with RH playing a negative role. This section highlights that CO2, CO, SO2, and H2S are primarily generated outdoors, with significant dispersion indoors. PM originates in both environments, while NO2, O3, HCHO, and TVOCs are mainly sourced from airtight indoor storage, with minimal outdoor accumulation. These PCA results offer key insights into air pollutant sources and correlations in CHs and SHs, aiding stakeholders in focused air quality management and safety enhancements in tobacco curing and storage operations.
3.5 Cluster analysis of air quality parameters using the hierarchical dendrogram method in tobacco processing and storage environments
Fig. 1 presents hierarchical dendrograms comparing air quality parameters in CHs (Fig. 1a) and SHs (Fig. 1b). The dendrograms, generated using the Ward linkage method, display pollutant clustering patterns, with the vertical axis representing pollutants and the horizontal axis showing rescaled distances where clusters merge.75 Pollutants with smaller distances cluster together, indicating similar behavior and origins.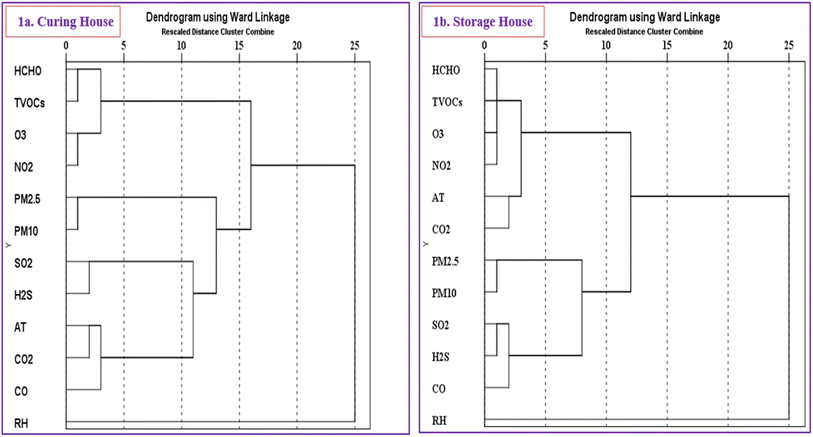 | ||
| Fig. 1 Hierarchical dendrogram showing the cluster analysis of air pollutant parameters in the community near the tobacco curing house (a) and inside the tobacco storage house (b). | ||
In both environments, all pollutants except RH formed a large cluster composed of several sub-clusters. RH remained separate, indicating that its origin and nature differ from those of the other pollutants. In Fig. 1a, three distinct sub-clusters were observed with the smallest distances: HCHO–TVOCs, O3–NO2, and PM2.5–PM10, indicating strong relationships within each cluster. HCHO is part of TVOCs,48 O3 is photo-chemically linked to NO2,41 and PM2.5/PM10 originates primarily from fuel burning.73 SO2 and H2S, both sulfur-based, formed a second sub-cluster, indicating similar sources of origin. Environmental parameters such as AT were highly correlated with CO2 and CO, forming another sub-cluster, as increased AT enhances CO2 production and CO emissions from incomplete combustion.50 The first two sub-clusters (HCHO–TVOCs and O3–NO2) formed a new sub-cluster, as their sources are similar, primarily from dry tobacco heating and occasional wood burning during curing. On the other hand, despite having a relatively larger distance, the (AT–CO2–CO) and (SO2–H2S) sub-clusters formed a new sub-cluster, as their main source is biomass combustion, and all are positively influenced by AT. They further merged with the (PM2.5–PM10) sub-cluster, as in CHs. PM is primarily derived from biomass combustion, with smaller contributions from tobacco heating, dust, and natural particles.44 As shown in Fig. 1b, pollutants were grouped into two sub-clusters with the smallest distances in SHs: HCHO–TVOCs–O3–NO2 and PM2.5–PM10, indicating strong correlations due to similar sources, mainly dry tobacco leaf heating and secondary accumulation from outdoors in an airtight chamber with poor ventilation. AT and CO2 formed a sub-cluster, with CO, SO2, and H2S forming a separate one, as these pollutants mainly accumulate from outdoor sources. Furthermore, at an even greater distance, the two sub-clusters (AT–CO2) and (HCHO–TVOCs–O3–NO2) merged to form a new sub-cluster, as HCHO, TVOCs, O3, and NO2 are primarily indoor pollutants, with AT playing a positive role in their emission increase. The dendrograms illustrate distinct environmental influences. The curing house clusters are more combustion-related, driven by biomass burning and tobacco leaf heating, with pollutant dispersion potentially influenced by wind speed and direction. In contrast, the storage house clusters reflect indoor pollutant accumulation, driven primarily by indoor sources and secondarily by outdoor infiltration, with AT playing a key role in emission dynamics. These hierarchical structures provide valuable insights into pollutant relationships, sources, and AQ in tobacco processing and storage environments. This dendrogram analysis provides valuable insights into pollutant sources in CHs and SHs, guiding policymakers in targeted air quality strategies and safety improvements for tobacco curing and storage operations.
3.6 Air quality of criteria air pollutants using USEPA-AQI and AAQI techniques in tobacco processing and storage environments
Air quality (AQ) monitoring is crucial for managing regional air pollution, guiding emission control efforts, and assessing suppression effectiveness.2,4,41 Reliable data, regular monitoring, and occupant education are key to mitigating health risks.50Fig. 2 presents a bar chart comparing Ambient Air Quality Index (AQI) values for key pollutants in two locations CHs (1st column for each cluster) and SHs (2nd column for each cluster). The measured parameters include six criteria pollutants (O3, CO, SO2, NO2, PM2.5, and PM10), along with the USEPA-AQI and the aggregated Air Quality Index (AAQI).26Fig. 2 illustrates that CO levels remained low in both locations, classified as “Good” (Q1) in SHs and “Moderate” (Q2) in CHs. Meanwhile, O3 reached an “Unhealthy” (Q4) level in CHs but was slightly lower in SHs, falling under “Unhealthy for sensitive groups” (Q3). SO2 was categorized as “USG” (Q3) in CHs and “Moderate” (Q2) in SHs, while NO2 remained relatively high in both locations, peaking at “USG” (Q3). Particulate matter (PM2.5 and PM10) showed the most severe pollution levels, ranging from “Unhealthy” (Q4) to “Very unhealthy” (Q5), with PM2.5 in SHs reaching the highest pollution level (Q5). The overall USEPA-AQI reflected these trends, reaching “Unhealthy” (Q4) in CHs and “Very unhealthy” (Q5) in SHs. The AAQI followed a similar pattern, with SHs exhibiting higher pollution levels, though both remained within the “USG” (Q3) category. The pollution intensity ranked as follows: PM2.5 > PM10 > NO2 > O3 > SO2 > CO for CHs and PM2.5 > O3 > PM10 > NO2 > SO2 > CO for SHs. Ababio et al.5 and Jung et al.46 reported significant air pollution in traditional biomass kitchens and industrial areas, respectively, aligning with this study's findings. However, air pollution is influenced by meteorological factors and is negatively correlated with RH, maximum wind speed, and precipitation.2,4 These findings highlight significant air quality concerns, particularly in enclosed spaces such as SHs, where poor ventilation exacerbates pollutant accumulation. High concentrations of PM, NO2, and O3 pose serious respiratory risks, especially for sensitive groups such as children, the elderly, and individuals with chronic conditions.41,62 To mitigate these risks, effective air quality control measures are essential. These include improving ventilation, installing advanced filters, and reducing emissions through better firing techniques such as LPG or electric ovens.35,36 Additionally, CHs should be located away from residential areas with improved exhaust systems to minimize pollution. This section suggests that tobacco curing and storage operations notably deteriorate air quality in both environments, with SHs being more severely affected. Special attention is needed for PM, O3, and NO2 in general for both houses, with SO2 being a concern only in CHs.
3.7 Evaluation of ecological and human health risks in tobacco processing and storage environments
Assessing ecological and health risks in tobacco processing and storage environments is crucial for understanding the impact of pollution. These processes emit harmful pollutants, endangering air quality, workers, and nearby residents. Effective evaluation informs policies and mitigation strategies to reduce tobacco-related pollution.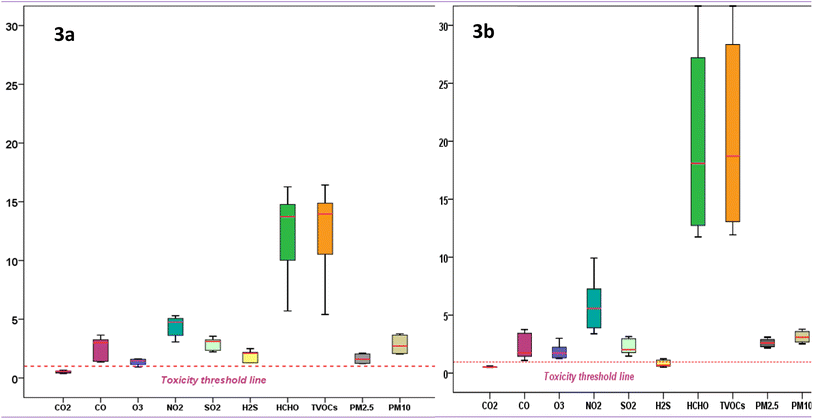 | ||
| Fig. 3 Box plot showing the Ecological Toxicity Potential (TP) status of air pollutants in the community near the tobacco curing house (3.3a) and inside the tobacco storage house (3.3b). | ||
The elevated levels of SO2, NO2, CO, and PM may damage chloroplasts and stomata, reducing photosynthesis and accelerating carbohydrate depletion, ultimately limiting plant growth and yield.38 High CO2 levels can alleviate stress by lowering stomatal conductance, whereas low CO2 levels promote root growth and stress resistance.77 Excess CO disrupts photosynthesis, causing leaf discoloration, wilting, and potentially plant death.38 The elevated SO2 level degrades chlorophyll, impairs photosynthesis, denatures proteins, and increases water loss by forcing stomata to open.78 High NO2 levels inhibit nitrogen assimilation, damage leaves, and alter the chloroplast structure, also contributing to acid rain.77 O3 exposure disrupts stomata signaling, and high levels cause chlorosis, pigmentation changes, premature senescence, and leaf damage, reduce photosynthesis, impair reproduction, and limit carbon transport to roots.41 Low levels of H2S enhance stress resistance, while high levels hinder growth, disrupt root development, damage photosynthesis, and induce oxidative stress, causing visible symptoms such as leaf discoloration and wilting.79 The impact of HCHO and VOCs on plants depends on the concentration, duration, and species. High levels cause oxidative stress, disrupting photosynthesis, and root growth, leading to stunted growth, discoloration, and wilting. Some plants close their stomata, impairing gas exchange and water uptake, while VOCs such as ethylene affect flower and fruit development, altering seed production.80 PM blocks stomata, reduces photosynthesis, and introduces toxins, weakening plant health and productivity. Over time, particle accumulation increases susceptibility to pests and pathogens, altering growth and yield without direct physical damage.38 He et al.3 reported earlier that ambient air pollution threatens ecosystems, hampers plant growth, accelerates climate change, disrupts food production, and undermines sustainable development. The ETP tool highlights the severe ecological risks posed by pollutants such as NO2, HCHO, TVOCs, and PM, which induce oxidative stress, impair photosynthesis, and weaken plant defenses. These impacts ultimately reduce crop yields and disrupt ecosystems, emphasizing the need for pollution control in tobacco processing environments.
Fig. 4 presents a column diagram illustrating the NCR across children, adult males, and adult females in two environments: near a tobacco curing house (CH) and inside a tobacco storage house (SH). The x-axis categorizes exposure groups, while the y-axis shows the hazard quotient (HQ) and hazard index (HI) values for ten air pollutants, with marked risk zones. HI values (green bars) ranged from moderate to high risk, with children experiencing the highest NCR, followed by adult females and adult males in both environments. This heightened risk for children is linked to their lower body weight.12 NCR was notably higher in the SH than in the CH, with children exceeding the high-risk zone (HI > 10), indicating a serious health concern, especially in sleeping areas. HQ values showed that TVOCs, HCHO, and NO2 were the main contributors to children's NCR risks in both environments, each posing moderate hazards. In the CH, TVOCs dominated NCR levels, while in the SH, both TVOCs and HCHO contributed significantly to adult males' and females' NCR, though all remained in the medium-risk zone. The pollutant risk ranking was as follows: TVOCs > HCHO > NO2 > PM2.5 > PM10 > SO2 for the CH and TVOCs > HCHO > NO2 > PM2.5 for the SH. Other pollutants contributed negligibly (<5%) to NCR. These elevated HQ and HI values underscore a significant threat to human health risks by increasing NCR. Ababio et al.5 identified significant NCR in traditional biomass kitchens, supporting this study's findings. Elevated CO2 levels increase airborne disease risks, with dry cough symptoms increasing notably above 1000 ppm.52 CO is highly hazardous due to its odorless, colorless, and lethal properties. It binds to hemoglobin 250 times more effectively than O2, impairs O2 transport, and leads to headache, dizziness, nausea, loss of consciousness, hypoxia, and even death.36,81 SO2 irritates the eyes, nose, and throat in the short term and exacerbates lung disease, asthma, and heart diseases with prolonged exposure, posing greater risks to children and the elderly.62 H2S, a toxic gas with a rotten-egg odor, causes eye, nose, and throat irritation, headaches, and nausea, with high concentrations leading to respiratory distress, unconsciousness, or death.82 NO2 exposure triggers respiratory issues, including throat swelling, breathing difficulties, conjunctivitis, itchy rashes, and asthma, while long-term exposure impairs lung function and O2 transport.41,52 While stratospheric O3 is harmless, ground-level O3 damages lung tissue, worsens asthma, causes heart disease, irritates eyes, causes wet cough, and causes nocturnal attacks of breathlessness.41 Acute HCHO exposure causes eye, nose, skin, and throat irritation, lacrimation, sneezing, coughing, nausea, and respiratory discomfort, with polyurethane emissions further contributing to asthma risks in children11,52 TVOCs irritate the eyes, skin, and respiratory system, causing throat dryness, allergies, and sensory irritation. Prolonged exposure can lead to neurotoxic, hepatotoxic, and genotoxic effects.62 High VOC levels contribute to respiratory issues and a 1.3-fold increase in asthma risk for every 10 μg m−3 rise.52 Elevated levels of PM2.5 and PM10 pose severe health risks, contributing to respiratory and cardiovascular diseases, asthma, and lung infections, with children, the elderly, and individuals with preexisting conditions being most vulnerable.41,62
 | ||
| Fig. 4 Column diagram showing non-carcinogenic hazard risks (NCRs) across community groups near the CH and inside the SH. | ||
Fig. 5 demonstrates a column diagram illustrating cancer risks (CRs) from TVOCs (green bars), PM2.5 (red bars), and PM10 (purple bars), along with lifetime cancer risks (LCRs, orange bars) across different community groups: children, adult females, and adult males in two environments, the CH and SH. Across all groups, LCR values were higher in SHs than in CHs. Among the groups, children exhibited the highest LCR in both environments, followed by adult females and then adult males. This pattern is attributed to lower body weight, as noted by Neumann et al.10 When categorizing LCR levels, all groups fell into the “Very high CR zone” (>10−3), except for adult females and males in CHs, who remained in the “High CR zone” (10−3–10−4). TVOCs were the dominant contributor to CR in both environments, with values ranging from the “High to very high CR zone” (10−4–10−3). In contrast, PM2.5 and PM10 played a minor role, with CR values fluctuating within the “Low to medium CR zone” (10−6–10−4). Neumann et al.10 reported significant CR in TVOCs, while Mbazima49 and Ayman et al.40 observed similar trends in PM2.5 and PM10, respectively, reinforcing this study's findings. TVOCs contain various harmful chemicals, with polycyclic aromatic hydrocarbons (PAHs), benzene, and HCHO being particularly carcinogenic.52 Prolonged exposure increases the risk of respiratory diseases, upper respiratory tract cancer, leukemia, and malignant brain tumors.41,46 Lung cancer, the leading cause of cancer-related deaths worldwide, accounted for 1.8 million deaths in 2018, with PM pollution ranked just behind tobacco smoking as a major contributor.14,52,66
 | ||
| Fig. 5 Column diagram showing lifetime cancer risks (LCRs) across community groups near the CH and inside the SH. Note: TVOCs are total volatile organic carbons and PM is particulate matter. | ||
Tobacco processing and storage expose workers and nearby residents to harmful air pollutants, raising cancer and non-carcinogenic risks. Children, especially those sleeping in storage houses, face the highest vulnerability. TVOCs, HCHO, NO2, and PM contribute most to NCR, while TVOCs drive cancer risk. To mitigate these risks, effective air quality controls are crucial. This includes reducing emissions through improved firing techniques, such as LPG or electric ovens.10,36 CHs should be located away from residential areas, with enhanced exhaust systems. Storage houses require better ventilation, and sleeping in these environments should be prohibited to safeguard public health.
4 Conclusions
The investigation confirms critically high air pollutant levels in tobacco processing and storage environments, with SHs exhibiting higher concentrations than CHs. Pollutant dynamics are strongly influenced by AT and RH, with higher temperatures driving increased emissions and low humidity amplifying these effects. Statistical analyses show that pollutants such as PM2.5, PM10, TVOCs, HCHO, NO2, O3, CO, and SO2 exceed national and international standards, with CO2, NO2, and PM10 showing high instability. Most pollutants follow normal distributions with occasional outliers and emission spikes, with clustering patterns mostly differing between SHs (skewed right) and CHs (skewed left). I/O analysis shows that outdoor conditions are mainly influenced by biomass combustion, while indoor pollutant levels result from both outdoor diffusion (CO2, CO, SO2, H2S, and PM) and indoor emissions (TVOCs, HCHO, NO2, O3, and PM). Pearson's correlation analysis reveals strong positive correlations among TVOCs, HCHO, NO2, and O3, while CO2 correlates with CO, SO2 with H2S, and PM2.5 with PM10, indicating shared sources and similar behaviours for each pair, with AT shaping emissions. PCA indicates greater pollutant variability in CHs, influenced by wind, with PC1 strongly correlating with TVOCs, HCHO, NO2, and O3; PC2 with AT, CO2, and CO; PC3 with PM2.5 and PM10; and PC4 with SO2 and H2S. In contrast, SH's airtight structure results in more stable conditions, with PC1 grouping AT, CO2, O3, NO2, TVOCs, and HCHO and PC2 dominated by PM2.5 and PM10. The hierarchical dendrogram reinforces the trends observed in the PCA and correlation matrix. Air quality indices (AQIs) display severe air quality degradation, with SHs reaching very unhealthy (Q5) and CHs unhealthy (Q4) levels. Pollution intensity ranks as PM2.5 > PM10 > NO2 > O3 > SO2 > CO in CHs and PM2.5 > O3 > PM10 > NO2 > SO2 > CO in SHs. These pollutants pose significant threats to human health, ecology, plant health, and crop yields, with TVOCs, HCHO, and NO2 particularly endangering plant health. Tobacco processing and storage expose workers and nearby residents to harmful pollutants, increasing CR and NCR, especially for children sleeping in storage houses. TVOCs, HCHO, NO2, and PM are the primary contributors to NCR, with TVOCs driving the CR, signaling urgent public health concerns. The study recommends targeted mitigation measures, including improving ventilation, using cleaner firing techniques such as LPG, positioning curing houses away from residential areas, and prohibiting sleeping in storage houses. These interventions are critical for mitigating immediate health risks and ensuring long-term human health and sustainable agricultural practices, advancing progress toward SDGs. This research offers key insights into the characterization and behavior of air pollutants from tobacco processing and storage, emphasizing their effects on air quality and health risks. These findings will support policymakers and stakeholders in decision-making, policy development, and promoting sustainable production practices. Moreover, this study utilizes a relatively small sample size, which may limit the applicability of the findings. To draw more precise conclusions and make well-informed decisions, future studies with larger sample sizes are recommended to enhance the accuracy and reliability of the results.Ethical statement
This article does not contain any experiment with any animal or human performed by any of the authors. The manuscript in part or in full has not been submitted or published anywhere.Data availability
All raw data are available from the corresponding author upon request.Author contributions
AR: research designing, data collection and analysis, table and graph making, and writing of the first original draft of the manuscript, reviewing and editing; MGM: helping with research designing, manuscript correction and editing, cover page preparation, and final manuscript sending; MKS: data collection and analysis and assisting with the first draft of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study has not received any funds from any organization. The authors thank Bheramara Upazila farmers for allowing air data collection and the Department of Agriculture Extension (DAE) for their support and cooperation.References
- Q. Guo, Z. He and Z. Wang, Aerosol Air Qual. Res., 2023, 23(6), 220448, DOI:10.4209/aaqr.220448.
- Q. Guo, Z. He and Z. Wang, Aerosol Air Qual. Res., 2024, 24(5), 230274, DOI:10.4209/aaqr.230274.
- Z. He, Q. Guo, Z. Wang and X. Li, Toxics, 2025, 13, 254, DOI:10.3390/toxics13040254.
- Q. Guo, Z. He and Z. Wang, Toxics, 2023, 11, 210, DOI:10.3390/toxics11030210.
- B. A. Ababio, M. A. Nkansaha, J. N. Hogarhb, T. P. Agyekumc and M. K. Commeh, Environ. Adv., 2024, 17, 100576, DOI:10.1016/j.envadv.2024.100576.
- M. S. More, G. A. Bodkhe, F. Singh, B. N. Dole, M. Tsai, T. Hianik and M. D. Shirsat, Synth. Met., 2023, 296, 117357, DOI:10.1016/j.synthmet.2023.117357.
- US EPA (United States Environmental Protection Agency), Technical Assistance Document for Reporting of Daily Air Quality-The Air Quality Index (AQI), EPA-454/B-09-001, North Carolina, 2009 Search PubMed.
- Q. Guo, Z. Wang, Z. He, X. Li, J. Meng, Z. Hou and J. Yang, Aerosol Air Qual. Res., 2021, 21(12), 210270, DOI:10.4209/aaqr.210270.
- S. N. Nautiyal, V. Joshi, A. S. Gautam, R. Kumar, S. Kumar, K. Singh and S. Gautam, J. Atmos. Chem., 2025, 82, 5, DOI:10.1007/s10874-025-09469-2.
- M. Neumann, W. N. Dlamini, R. Sallah-Ud-Din, A. K. Berekute, S. Siregar, M. E. Getnet, M. Maulana, W. Pan, S. C. Lung and K. Yu, Clean Technol. Environ. Policy, 2024, 26, 3003–3020 CrossRef CAS.
- WHO (World Health Organization), Guidelines for Air Quality, Geneva, Switzerland, 2000 Search PubMed.
- M. K. Saha, S. J. Ahmed, A. H. Sheikh, N. U. Ahsan and M. G. Mostafa, Int. J. Nat. Soc. Sci., 2020, 1(1), 60–70 Search PubMed.
- A. B. Chelani and S. Gautam, Water Air Soil Pollut., 2023, 234, 502, DOI:10.1007/s11270-023-06521-3.
- M. C. Turner, Z. J. Andersen, A. Baccarelli, W. R. Diver, S. M. Gapstur, C. A. Pope, D. Prada, J. Samet, G. Thurston and A. Cohen, Ca – Cancer J. Clin., 2020, 70(6), 460–479, DOI:10.3322/caac.21632.
- R. P. Kumar, S. J. Perumpully, C. Samuel and S. Gautam, Stoch. Environ. Res. Risk Assess., 2023, 37, 453–465, DOI:10.1007/s00477-022-02313-z.
- P. Kumar, S. Hama, R. A. Abbass, K. V. Abhijith, A. Tiwari, D. Grassie and C. Mitsakou, J. Build. Eng., 2024, 91, 109549, DOI:10.1016/j.jobe.2024.109549.
- I. Amadu, A. A. Seidu, A. Mohammed, E. Duku, M. K. Miyittah, E. K. Ameyaw, J. J. E. Hagan, M. H. Musah and B. O. Ahinkorah, Heliyon, 2023, 9(6), 16546, DOI:10.1016/j.heliyon.2023.e16546.
- P. Mitra, D. Chakraborty, S. Nayek, S. Kundu, D. Mishra, U. Dan and N. K. Mondal, Chemosphere, 2023, 311(1), 136995, DOI:10.1016/j.chemosphere.2022.136995.
- G. C. Kashyap, D. Rajendra and P. Puri, J. Public Health, 2023, 1–10, DOI:10.1007/s10389-023-02066-1.
- Y. Liu, N. Ning, T. Sun, H. Guan, Z. Liu, W. Yang and Y. Ma, Sci. Total Environ., 2023, 856(2), 159035, DOI:10.1016/j.scitotenv.2022.159035.
- X. Li, C. Duan, Q. Chen, J. Xiao and J. J. Zhang, Environ. Int., 2023, 175, 107953, DOI:10.1016/j.envint.2023.107953.
- Q. Guo, Z. He and Z. Wang, Toxics, 2023, 11, 51, DOI:10.3390/toxics11010051.
- M. Akteruzzamana, M. A. Rahmana, F. M. Rabbia, S. Asharofa, M. M. Rofia, M. K. Hasana, M. A. M. Islamb, M. A. R. Khanb, M. M. Rahmana and M. H. Rahaman, Heliyon, 2023, 9, e12852, DOI:10.1016/j.heliyon.2023.e12852.
- S. Khandker, A. S. M. Mohiuddin, S. A. Ahmad, A. McGushin and A. Abelsohn, Research Square, 1–14, DOI:10.21203/rs.3.rs-1184779/v1.
- B. A. Ababio, M. A. Nkansaha, J. N. Hogarhb, T. P. Agyekumc and M. K. Commeh, J. Hazard Mater. Adv., 2023, 11, 100358, DOI:10.1016/j.hazadv.2023.100358.
- A. S. Shihab, J. Ecol. Eng., 2023, 24(4), 110–116 CrossRef.
- US EPA (United States Environmental Protection Agency), Risk Assessment Guidance for Superfund, vol. 1, Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment), Washington DC, USA, 2009 Search PubMed.
- A. Roy and M. G. Mostafa, Asian J. Environ. Res., 2024, 1(3), 217–236, DOI:10.69930/ajer.v1i3.224.
- A. Roy, S. Naz and M. G. Mostafa, Journal of Sustainability and Environmental Management (JOSEM), 2024, 3(1), 9–18, DOI:10.3126/josem.v3i1.65224.
- A. Roy, S. Naz and M. G. Mostafa, International Journal of Plant and Environment, 2024, 10(4), 1–10, DOI:10.18811/ijpen.v10i04.01.
- EC (European Communities), Tobacco, Cigarettes and Cigarette Smoke An Overview, Institute for Health and Consumer Protection, European Environment Agency, EUR 22783 EN, Copenhagen, Denmark, 2007 Search PubMed.
- B. Deng, M. Chen, S. Wu, S. Liu, A. Liu, Q. Li and X. Duan, Thermochim. Acta, 2022, 717, 179348 CrossRef CAS.
- N. Lecours, G. E. G. Almeida, J. M. Abdallah and T. E. Novotny, Tob. Control, 2012, 21, 191–196, DOI:10.1136/tobaccocontrol-2011-050318.
- WHO (World Health Organization), A Report On Throughout Its Life Cycle, Tobacco Pollutes The Planet And Damages The Health Of All People. World Tobacco Day-2022, Rome, Italy, 2022, pp. , pp. 1–20, https://WNTD2022_Brochure_ENG-Web_0.pdf Search PubMed.
- M. A. Raheem, G. Jimoh and H. Abdulrahim, J. Environ. Public Health, 2022, 13, 7689141, DOI:10.1155/2022/7689141.
- S. Gautam, A. Pillarisetti, A. Yadav, D. Singh, N. Arora and K. Smith, Environ. Dev. Sustain., 2019, 21, 2567–2575, DOI:10.1007/s10668-018-0131-1.
- S. J. Perumpully, S. Gautam, J. J. Paul and M. Sreenath, Water Air Soil Pollut., 2024, 235, 54, DOI:10.1007/s11270-023-06854-z.
- M. Asif, S. Saleem, A. Tariq, M. Usman and R. A. U. Haq, ASEAN Journal of Science and Engineering, 2021, 1(2), 135–140 CrossRef.
- G. M. M. E. Rahman, A. R. Sany, A. H. Ahon and D. K. Paul, 2nd International Conference on Mechanical, Manufacturing and Process Engineering, retrieved from https://www.researchgate.net/publication/385347232 Search PubMed.
- N. Ayman, T. A. ElSeoud and S. Mostafa, J. Environ. Earth Sci., 2022, 1113, 012018, DOI:10.1088/1755-1315/1113/1/012018.
- DoE (Department of Environment), Ambient Air Quality in Bangladesh, September 2018, Clean Air and Sustainable Environment Project, Ministry of Environment, Government of the People’s Republic of Bangladesh, 2018 Search PubMed.
- O. Enkhjargal, M. Lamchin, X. Y. You, J. Chambers, D. Tuyagerel, R. Tovuudorj, Z. Khurelsukh, E. Sarangerel and N. Enkhtuya, Air Qual., Atmos. Health, 18, 615–629, DOI:10.1007/s11869-024-01678-0.
- A. Jubaer, M. K. Ali, S. M. T. Hassan, M. S. Islam, M. M. Alam, S. Islam, M. Z. I. Talukder and R. T. Sourav, Eur. J. Chem., 2024, 15(1), 79–86 CrossRef CAS.
- J. Kaewrat, R. Janta, S. Sichum, C. Rattikansukha, W. Tala and T. Kanabkaew, Toxics, 2022, 10, 520, DOI:10.3390/toxics10090520.
- J. Wang, Z. Chen, Y. Pang, Y. Zhao, Y. Mao, N. Mao and M. Xu, J. Geosci. Environ. Protect., 2022, 10, 122–136, DOI:10.4236/gep.2022.108009.
- J. Y. Jung, J. W. Kim, T. W. Koo, J. Y. Heo, Y. S. Jeong and C. M. Lee, Toxics, 2024, 12, 682, DOI:10.3390/toxics12090682.
- Q. Guo, Z. He, S. Li, X. Li, J. Meng, Z. Hou, J. Liu and Y. Chen, Aerosol Air Qual. Res., 2020, 20, 1429–1439, DOI:10.4209/aaqr.2020.03.0097.
- O. Ayeni, V. O. Agada, A. A. Mahamat, E. C. Ibrahim, A. M. Stanley and A. Salam, J. Environ. Sci. Technol., 2024, 15(1), 1–14, DOI:10.4314/etsj.v15i1.1.
- S. J. Mbazima, Health risk assessment of particulate matter 2.5 in an academic metallurgy workshop, Wiley, 2022, vol. 32, p. 13111, DOI:10.1111/ina.13111.
- V. V. Narayanan, R. R. Shaikh, A. Hashemi, H. Elsharkawy, D. Newport and L. G. Basaly, Environmental Science & Sustainable Development, 9(3), 47–57, DOI:10.21625/essd.v9i3.1072.
- S. Roy, S. U. Zaman and A. Salam, Environ. Res. Commun., 2023, 5, 025004, DOI:10.1088/2515-7620/acb90d.
- S. Sadrizadeha, R. Yaoc, F. Yuanc, H. Awbic, W. Bahnflethe, Y. Bif, G. Caof, C. Croitorug, R. Dearh and F. Haghighat, et al. , J. Build. Eng., 2022, 57, 104908, DOI:10.1016/j.jobe.2022.104908.
- G. Hurd-Kundeti, A. B. Petersen, K. Somsamouth and P. N. Singh, Int. J. Environ. Res. Publ. Health, 2019, 16, 3500, DOI:10.3390/ijerph16183500.
- H. Jin-rong, C. Yi, Y. Nan, J. Xin-wei, L. Hui-long, L. Ji-yuan, W. Yong and J. Yong-lei, J. South. Agric., 2024, 55(10), 2898–2907 Search PubMed.
- F. Pu, Y. Hu, C. Li, X. Cao, Z. Yang, Y. Liu, J. Zhang, X. Li, Y. Yang and W. Wang, et al. , Environ. Res. J., 2023, 218, 115022, DOI:10.1016/j.envres.2022.115022.
- L. Zhang, W. Li, Z. Peng and J. Zhang, BMC Microbiol., 2025, 25, 56, DOI:10.1186/s12866-025-03774-2.
- X. P. Zhang and M. H. Lin, J. Univ. Chinese Acad. Sci., 2020, 37(1), 39–50 CrossRef PubMed.
- J. Hu, Q. Ying, Y. Wang and H. Zhang, Environ. Int., 2015, 84, 17–25 CrossRef CAS PubMed.
- S. U. Zaman, M. Yesmin, M. R. S. Pavel, F. Jeba and A. Salam, Environ. Sci. Pollut. Res. Int., 2021, 28(8), 37727–37740, DOI:10.1007/s11356-021-13162-8.
- NAAQS (National Ambient Air Quality Standards-2009), Central Pollution Control Board, New Delhi, India, 2009, retrieved from https://cpcb.nic.in/uploads/National_Ambient_Air_Quality_Standards.pdf.
- US EPA (United States Environmental Protection Agency), Ambient Air Quality Standards, Chapter 33.1-15-02, Washington DC, USA, 2019 Search PubMed.
- WHO (World Health Organization), Air Quality Guidelines: Global Update 2021, Geneva, Switzerland, 2021 Search PubMed.
- E. David and V. Niculescu, Int. J. Environ. Res. Publ. Health, 2021, 18, 13147, DOI:10.3390/ijerph182413147.
- S. Mentese, D. Tasdibi and E. Orak, AIMS Environ. Sci., 2016, 3(4), 827–841, DOI:10.3934/environsci.2016.4.827.
- A. Bushra, H. M. Zakir, S. Sharmin, Q. F. Quadir, M. H. Rashid, M. S. Rahman and S. Mallick, Sci. Rep., 2022, 12, 14278, DOI:10.1038/s41598-022-17930-5022.
- P. D. Vaio, E. Magli, G. Caliendo, A. Corvino, F. Fiorino, F. Frecentese, I. Saccone, V. Santagada, B. Severino and G. Onorati, et al. , Atmosphere, 2018, 58(9), 1–15, DOI:10.3390/atmos9020058.
- US EPA (US Environmental Protection Agency), Baseline Human Health Risk Assessment, National Center for Environmental Assessment, Region VET 999, 18th Street, Suite 500, Denver CO 80202, 2001 Search PubMed.
- BAPCR (Bangladesh Air Pollution Control Rules 2022), Ministry of Environment, Forest and Climate Change, Government of the People's Republic of Bangladesh, 2022 Search PubMed.
- National Environmental (Air Quality Control) Regulations, Federal Republic of Nigeria Official Gazette, The Federal Government Printer, Lagos, Nigeria, Government Notice No. 43 S.l. No. 88, 2021, vol. 108(161), pp. B3345–B3377 Search PubMed.
- US EPA and IRIS (Integrated Risk Information System), Toxicological Review of Benzo[a]pyrene Farland WH, Tuxen LC (1997), new directions in cancer risk assessment: accuracy, precision, credibility, and uncertainty, Hum. Ecol. Risk Assess.: Int. J., 2017, 3, 667–671, DOI:10.1080/10807039709383726.
- M. Taghavi, M. Darvishiyan, M. Momeni, H. Eslami, R. Ali and A. Zarei, Research Square, 2022, 1–23 CAS.
- S. Hajat and T. Kosatky, J. Epidemiol Community Health, 2010, 64(9), 753–760 CrossRef PubMed.
- F. Reyes, S. Ahumada, F. Rojas, P. Oyola, Y. Vasquez, C. Aguilera, A. Henriquez, E. Gramsch, C. Kang and S. Saarikoski, et al. , Aerosol Air Qual. Res., 2021, 21(11), 210110, DOI:10.4209/aaqr.210110.
- A. Pandey, M. Brauer, M. Cropper, K. Balakrishnan, P. Mathur, S. Dey and P. Arokiasamy, Lancet Planet. Health, 2020, 5(1), 25–38, DOI:10.1016/S2542-5196(20)30298-9.
- A. K. Majumder, A. M. Kamruzzaman, M. Rahman and M. N. A. Patoary, GSC Adv. Res. Rev., 2024, 18(1), 201–212 CrossRef CAS.
- A. R. Uthappa, A. S. Devakumar, B. Das, G. R. Mahajan, S. B. Chavan, D. Jinger, P. K. Jha, P. Kumar, A. Kokila and R. Krishnamurthy, et al. , Frontiers, 2024, 1–12, DOI:10.3389//ffgc.2023.1322660.
- L. Shi, M. Zhang, B. Yang and L. Gao, Int. J. Sustain. Dev. World Ecol., 2018, 25(10), 1–11, DOI:10.1080/13504509.2017.1419390.
- K. Kareem, M. Rasheed, A. Liaquat, A. M. M. Hassan, M. I. Javed and M. Asif, ASEAN Journal of Science and Engineering, 2022, 2(2), 193–198 CrossRef.
- T. Ausma and D. Kok, Front. Plant Sci., 2019, 10, 743, DOI:10.3389/fpls.2019.00743.
- J. N. Cape, Environ. Pollut., 2003, 122(1), 145–157, DOI:10.1016/s0269-7491(02)00273-7.
- A. Ghorani-Azam, B. Riahi-Zanjani and M. Balali-Mood, J. Res. Med. Sci., 2016, 21, 65, DOI:10.4103/1735-1995.189646.
- OSHA (Occupational Safety and Health Administration), Hydrogen Sulfide-Hazards, U.S. Department of Labor, 2025, retrieved from https://www.osha.gov/hydrogen-sulfide/hazards Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

