Open Access Article
Open Access ArticleIce nucleating activity of coastal seawater from the entrance to the Baltic Sea†
Eva R.
Kjærgaard
 a,
Amanda S.
Sejersen
a,
Max F.
Skov
a,
Amanda S.
Sejersen
a,
Max F.
Skov
 a,
Markus D.
Petters
a,
Markus D.
Petters
 bc and
Merete
Bilde
bc and
Merete
Bilde
 *a
*a
aDepartment of Chemistry, Aarhus University, DK-8000 Aarhus C, Denmark. E-mail: bilde@chem.au.dk
bDepartment of Chemical and Environmental Engineering, University of California Riverside, Riverside, CA, USA
cCenter for Environmental Research and Technology (CE-CERT), University of California Riverside, Riverside, CA, USA
First published on 8th July 2025
Abstract
Atmospheric ice nucleating particles (INPs) can affect cloud radiative properties and lifetimes and thus Earth's climate. Such particles may be emitted into the atmosphere from seawater via wave breaking processes. Here, we perform an exploratory investigation on the ice nucleating properties of seawater sampled on four days over a year (February, April, June, and November) from a coastal site near Aarhus in Denmark. We use a cold stage instrument (droplet size: 1 μL) to probe immersion mode freezing events. We find that bulk seawater contains INPs with T50 values around −20 °C independent of the month of sampling and INP concentrations ranging from 6 × 103 to 5 × 106 INP L−1 in a temperature range of −12 to −34 °C across all four samples. All samples displayed sensitivity to filtration (0.02 μm), as indicated by a decrease in INP concentration (lowering of freezing temperature). The filtered April and June samples froze at higher temperatures than the filtered November and February samples, which could indicate a variation in the population of INPs (>0.02 μm) over the year. Sea surface microlayer samples did not show enrichment of INPs compared to bulk seawater. Our results are discussed in the context of INP activity of seawater from other locations. While further studies are needed to understand the nature and potential seasonality of seawater INPs, we confirm the presence of INPs in coastal Baltic seawater that may contribute to atmospheric INP concentrations.
Environmental significanceIce nucleating particles (INPs) can initiate freezing in clouds and thereby influence cloud lifetime, radiative properties, precipitation patterns and ultimately Earth's radiative balance. Sea spray aerosols are a potential source of INPs. Understanding the presence and nature of INPs in sea spray aerosols is important for assessing their global significance. This study investigates the presence of INPs in coastal Baltic seawater. Our results show the presence of INPs in four seawater samples obtained over a year. Filtration experiments show that the main INPs in seawater samples from Ajstrup Beach are larger than 0.02 μm in size. In addition, the size and composition of INP samples differ between samples from November/February and April/June, highlighting the need for further research into seasonal variability of INPs in seawater. |
Introduction
The formation of ice in atmospheric clouds influences precipitation, hydrological patterns,3 cloud radiative properties and lifetimes and thus Earth's total radiation budget and climate.3,6 Ice crystals can form in the atmosphere from freezing of supercooled cloud droplets of pure water at −38 °C via homogeneous nucleation; however, the presence of ice nucleating particles (INPs) allows ice formation at warmer temperatures.3 Ice nucleation initiated by INPs can occur via deposition, contact, condensation, or immersion freezing.10,11 Immersion freezing, which is the focus of this study, is thought to dominate ice formation in mixed-phase clouds,11 which are responsible for most of the precipitation in mid-latitudes11,14 and are important in the marine atmosphere.16Around 71% of Earth's surface is covered by seawater, where wind-induced wave breaking represents a major source of aerosol particles, also known as sea spray, to the atmosphere.18 Sea spray aerosols (SSA) are a potential source of INPs in the atmosphere, as shown by, e.g., DeMott et al.20
There are several different approaches to investigating the potential of sea spray aerosols to act as INPs, as outlined by Ickes et al.22 Since the chemical composition of sea spray aerosols is coupled to that of the seawater it originates from,23 a first approach to investigating the ice nucleating activity of sea spray aerosols is to study INPs in seawater and sea surface microlayer (SML) samples. Irish et al.4,5 showed that INPs are present in both SML and bulk seawater samples from the Canadian Arctic Ocean using the droplet freezing assay technique. By performing heat and filtration treatments, they found that the INPs were likely heat-labile biological materials (0.02–0.2 μm in diameter). Wilson et al.24 found that the ice nucleating material in SML and bulk seawater samples from Arctic, Atlantic, North Pacific, and coastal British Columbia seawater was likely a biogenic material and <0.2 μm in diameter. Measurements from the Gulf of Mexico by Ladino et al.12 found moderate INP concentrations ranging from 6.0 × 101 to 1.1 × 105 L−1 at temperatures below −16.5 °C in SML and bulk seawater. Ladino et al. observed that the SML samples froze at colder temperatures compared to bulk surface water. Ladino et al.12 also found INPs in SML samples from the Saanich Inlet, off Vancouver Island, at concentrations higher than in the Gulf of Mexico and suggest that coastal water from higher latitudes freezes at higher temperatures than coastal water from more southern latitudes. Studies by Hartman et al.15 of Arctic seawater and SML reported enrichment factors of 1–10 for the majority of samples. Gong et al.7 measured INPs in SML and bulk seawater from Cabo Verde and found both enrichment and depletion of the concentration of INPs in SML compared to bulk water but with no clear trend in ice nucleation temperature. Similarly, Li et al.19 measured INPs in seawater sampled from the Barents, Kara and Laptev Seas, finding no clear trend in INP enrichment in SML compared to bulk seawater.
An additional way of studying INPs in sea spray is by performing laboratory experiments where seawater is aerosolized, and the aerosols emitted are tested for ice nucleating ability. Ickes et al.22 performed aerosolization of Arctic SML samples mixed with artificial seawater using a nebulizer and a sea spray tank and measured INPs present in the aerosols formed. Wang et al.25 and DeMott et al.20 performed mesocosm studies in a sea spray tank and a wave channel elucidating the complexity of water–air INP transfer in the presence of phytoplankton blooms. Wolf et al.26 also linked INPs in sea spray aerosols with the biological activity of seawater from the Florida Straits and the eastern tropical North Pacific.
Despite increasing evidence that seawater is a significant source of ice nucleating particles, our knowledge of the properties and concentrations of INPs in both the SML and bulk seawater remains limited. Several regions of the world are also understudied in this regard, for example with respect to spatial and seasonal trends. To the best of our knowledge, no studies have investigated INPs in seawater from the Baltic Sea. The Baltic Sea in Northern Europe is enclosed by land and linked to the North Sea via belts and straits. It is characterized by strong gradients in salinity.27 Recent work by Zinke et al.28 has parameterized sea spray aerosol fluxes from the Baltic Sea. In this study, we perform exploratory investigations to elucidate the ice nucleating activity of coastal seawater sampled close to Aarhus in Denmark, near the entrance to the Baltic Sea, where the salinity is around 20 g kg−1. Four samples were collected, one in each season, and analysed with the drop freezing assay technique.
Materials and methods
Sampling details
Coastal seawater samples were collected from Ajstrup Beach in Denmark (Fig. 1). Ajstrup Beach is a camping and vacation house area located at the Bay of Aarhus, ∼20 km south of the city of Aarhus. Salinity in the bay area varies between approx. 13 and 26 g kg−1 and the sea surface temperature has been found to vary from 0 to 22 °C over the year.29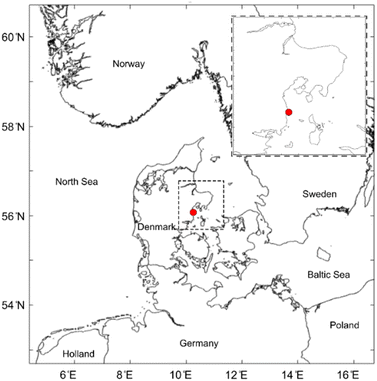 | ||
| Fig. 1 Location of the sampling site (red dot, coordinates: 56°02′25′′N, 10°16′16′′E), Ajstrup Beach, Denmark. The map is created with the MATLAB Mapping Toolbox (R2023b). | ||
The sampling took place on November 15th, 2022 (autumn), February 2nd, 2023 (winter), April 11th, 2023 (spring) and June 27th, 2023 (summer). Table S1† provides information from the Danish Meteorological Institute on weather and seawater conditions on sampling days.
All water samples were collected using a 250 ml BlueCap bottle (borosilicate glass, Simax®) from the end of a wooden pier, approx. 10 m from the coast. The bottle was lowered into the water with the lid closed, then the lid was unscrewed by hand at a depth of ∼30 cm and then closed again underwater before being recovered. These samples are referred to as bulk water samples. The bottle was handled during pre-cleaning and sampling with nitrile gloves. Equipment used for sampling seawater was cleaned with ethanol and then Milli-Q water prior to use and wrapped in tin foil. At the sampling site, the equipment was again rinsed with Milli-Q water and then rinsed with seawater 10 m downwind from the sampling site.
In November, surface water was collected with a BlueCap bottle, which was filled while holding it horizontally at the water–air interface. We refer to this sample as surface water. In April and June, the sea surface microlayer was sampled using the glass plate technique following the standard procedure of Harvey and Burzell.30 We used a glass plate (1600 cm2) with a plastic handle and a Teflon wiper (29 × 3.5 cm). Standing in the water parallel to the wind direction, the plate was dipped ∼29 cm into the water and slowly withdrawn (∼4 s duration). Then the water was allowed 2 s to drip off before the glass plate was wiped and the sample collected in a BlueCap bottle. In February, the weather did not allow for surface water sampling because the surface was not calm enough. All samples were stored at 4 °C until analysis, which took place on the day of sampling or up to two weeks after.
Seawater composition
We do not have measurements of seawater characteristics at the sampling site; however, Table 1 provides seawater characteristics obtained from the Danish Environmental Portal in the same month as the seawater samples collected for INP analysis (closest available site). In parentheses are seasonal average values from a total of six water samples per season. The dataset is from water samples collected at 1 m depth in the middle of Aarhus Bay (approx. coordinates 56°09′15.2′′N 10°18′36.1′′E), which is around 4 km from the harbor of Aarhus and 15 km from our sampling site at Ajstrup Beach. Fig. S1† shows seawater characteristics over the full year in Aarhus Bay.| Water temperature [°C] | Salinity [g kg−1] | pH | Chlorophyll a [μg L−1] | Total nitrogen [μg L−1] | Total phosphorus [μg L−1] | Oxygen content [mg L−1] | |
|---|---|---|---|---|---|---|---|
| Autumn | 11.3 (13.2 ± 4.1) | 25.7 (23.0 ± 2.4) | 7.9 (8.1 ± 0.1) | 3.7 (4.5 ± 2.2) | 230 (233 ± 21) | 20 (25 ± 4.4) | 9.06 (9.0 ± 0.5) |
| Winter | 4.5 (4.75 ± 0.5) | 27.4 (24.3 ± 4.2) | 7.9 (7.8 ± 0.03) | 1.5 (2.6 ± 1.4) | 270 (250 ± 10) | 25 (26 ± 1.8) | 10.2 (10.0 ± 1.2) |
| Spring | 8.0 (6.05 ± 1.9) | 17.1 (21.4 ± 3.5) | 8.0 (8.0 ± 0.04) | 1.4 (2.0 ± 0.7) | 170 (190 ± 22) | 9.8 (12.6 ± 3.5) | 10.5 (10.4 ± 0.7) |
| Summer | 20.6 (16.8 ± 2.2) | 19.1 (23.6 ± 2.9) | 8.2 (8.1 ± 0.06) | 1.0 (1.1 ± 0.3)) | 220 (185 ± 21) | 11 (12.8 ± 2.3) | 8.64 (8.6 ± 0.3) |
Salinity data in Table 1 are supported by a salinity measurement of the June sample of 18 g kg−1 measured using a portable refractometer (WZ-221) and water activity measurements of November and February samples from Ajstrup Beach. See ESI Section S1.3† for details. Following the parameterization by Tang et al.,31 salinity can be determined from water activity (average 0.9860 for November and 0.9855 for February), resulting in salinities of 24.9 g kg−1 and 25.5 g kg−1 for November and February, respectively. See Section S1.4 for details. On this basis, it seems reasonable to conclude that seawater salinities at the two sites (Ajstrup Beach and Aarhus Bay) are thus similar.
Cold stage
A new cold stage at Aarhus University was used for INP analysis. The cold stage is an implementation of the drop freezing assay technique.32 The cold stage is described in detail by Mahant et al.33 and has been developed as a low-cost portable experimental set-up similar to the NC State Cold Stage.34 Briefly, the cold stage is composed of an experimental chamber, a camera mounted on a stand above the chamber, an electronic control box and a computer for data acquisition and control. The experimental chamber is a 3D printed plastic box with ports on each side for the continuous flow of nitrogen gas. Inside the chamber is an aluminium plate placed on top of a Peltier element (thermoelectric module) and underneath is a water block (a plate with circulating cooling fluid). Between all three mentioned parts is thermal paste to ensure efficient heat transfer. A thermistor is placed into a small hole in the side of the aluminium plate and thermal paste is used to ensure contact between the thermistor and the metal surface.During an experiment, four hydrophobic glass slides (Hampton Research; HR3-215 22 mm siliconized) are placed on top of the aluminium plate. An electronic pipette (Eppendorf Xplorer®-4861000015) is used to place a minimum of 100 droplets (Vdrop = 1 μL) onto the glass slides. The droplets are cooled from 10 °C to −40 °C at a rate of 2 °C min−1. The temperature difference between the droplet and the temperature measured is estimated to be <1 °C for the given cooling rate.33,34 Droplets are monitored using a camera (IDA Imaging, 2592 × 1944 pixels) with a frame rate of 0.5 frames s−1. Two key parameters obtained from the experiments are: (1) the T50 value, which is the temperature at which half of the droplets are frozen and (2) the cumulative INP concentration (CINP in units of INPs L−1 water) at a given temperature, which is calculated using Vali's equation:35
Assuming that the seawater only consists of water and NaCl, freezing point depressions are −1.6, −1.7, −1.1 and −1.2 °C for salinities in Table 1 of the November, February, April, and June samples, respectively, following Schwidetzky et al.36 The deviation is due to the difference in salinity in the seawater sampled. The data shown from this work have not been corrected for freezing point depression.
Following Gong et al.7 we define enrichment factors between the sea surface microlayer (SML) and bulk seawater as
The enrichment of CINP in the SML is indicated when EF > 1, while depletion is indicated when EF < 1.
Test of water storage and handling
All bulk seawater samples were analysed for INP activity on the day of sampling. We tested (February and April) whether shaking the BlueCap bottle, by hand for 5 seconds, immediately before pipetting on the cold stage glass plate affected INP concentrations. This test was performed to investigate whether particles (e.g. larger suspended sediment) that would settle out of the sampling volume (over a 24 hour period) affected the INP activity of the sample. The seawater volume that was pipetted onto the cold stage was taken from the top 0.5 cm of the glass bottle. As can be seen in Table S3,† the shaken and unshaken values of T (5 × 105 L−1) fall within 0.7 °C of each other and are thus within an uncertainty of ±1 °C based on plate heterogeneity.33 We therefore do not differentiate between shaken and unshaken samples and use the combined dataset when available, if not otherwise stated. Furthermore, over a period of four consecutive days after sampling, three of the bulk seawater samples were analysed for INP activity every ∼24 hours. Across all four bulk seawater samples, no clear trend was observed in T (5 × 105 L−1) with time after sampling. The T (5 × 105 L−1) value was lower by up to ∼2 °C in one case (November) for day four compared to the day of sampling. A repeat measurement of the November sample after two weeks showed a T (5 × 105 L−1) value consistent with the value obtained on the day of sampling. We conclude from these tests that storage at 4 °C on a time scale of days does not systematically change the INP activity of the seawater to a degree that can be resolved in our system.Experimental details
Bulk seawater and surface or SML samples were divided into aliquots and treated in three different ways before analysis of INP activity using the cold stage: (a) untreated seawater samples (referred to as the fresh samples), (b) filtered through a 0.02 μm syringe filter (Whatman™ Anotop™, WHA68093022) (referred to as the filtered samples) and (c) seawater treated with pelleted activated charcoal and filtered twice by suction (filter with pore size of 100 μm), followed by filtration through a 0.02 μm syringe filter (referred to as the activated charcoal samples) following the procedure of Nielsen and Bilde.37 In some cases (February and April), the samples were heated to 70 °C while being treated with activated charcoal. Table S3† provides an overview of the experiments performed on the water samples collected from each season. For reference, an artificial seawater sample using Sigma Sea salt (Sigma-Aldrich, S9883, 55% Cl, 31% Na, 8% SO42−, 4% Mg, 1% K, 1% Ca, <1% other) and a homemade sea salt mixture37 (Cl−, Na+, SO42−, K+, Ca2+, Br−, Mg2+, and NO3− with mass contributions relative to Cl− of 1, 0.56, 0.14, 1.7 × 10−2, 2.2 × 10−2, 3.1 × 10−3, 6.8 × 10−2, and 2.6 × 10−3, respectively), both with a salinity of 20 g kg−1, were also analysed. As a pure water reference, HPLC grade water (Sigma-Aldrich 270733-1L, CAS-No: 7732-18-5) was filtered through a 0.02 μm syringe filter at the beginning and end of the period (13th October 2022 and 7th July 2023). Figures show the combined pure water dataset as a reference.Results and discussion
Seawater ice nucleating particles
We first consider the freezing behaviour of seawater sampled during February. Fig. 2a shows the frozen fractions of February bulk seawater (blue) as a function of temperature compared to an artificial sea salt solution with a salinity of 20 g kg−1 (light green) and the pure water reference (filtered HPLC water, grey). Fig. 2b depicts the corresponding cumulative INP concentrations versus temperature. Fig. 2 shows that the freezing temperatures of bulk winter seawater are significantly warmer than those of filtered HPLC water and artificial seawater, leading to the conclusion that the bulk seawater contains INPs that are active between −12 and −26 °C. As discussed above, freezing behaviour across the large set of measurements on different aliquots of the same bulk seawater sample (shaken, unshaken, repetitions on different days) agrees well (see Table S3†), demonstrating the reproducibility of our measurements.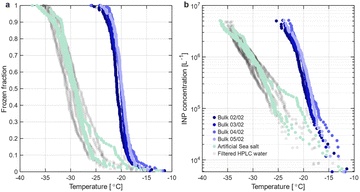 | ||
| Fig. 2 (a) The fraction of frozen droplets as a function of temperature for February seawater sampled on February 2nd, 2023 (not shaken, see Fig. S3† for all data), and analysed on four consecutive days (shades of blue), and for reference filtered HPLC water (grey) and artificial sea salt (light green). (b) Cumulative ice nucleating particle concentration as a function of temperature for the same samples. Data are not corrected for freezing point depression due to salt. | ||
Similar information for the other samples is given in Table S3.† No significant difference in INP concentrations was observed between the fresh seawater sampled on four days from different seasons in this study (see Fig. S10†). Fig. 3a shows a comparison of the INP concentrations at different temperatures for bulk seawater sampled in this study (all seasons) with bulk seawater samples from previous studies represented by dashed boxes. All data in this figure are measured in the immersion freezing mode with a cold stage instrument. While a comparison of variability in the performance or detection capabilities of the different freezing assays/instruments behind Fig. 3a is beyond the scope of this work, we refer the reader to the outcome of The Fifth International Workshop on ice nucleation measurements38 for considerations on intercomparability between results from different instruments for probing INPs. The concentration range detected in this study (Aarhus Bay) roughly agrees with concentrations from Canadian Arctic summer seawater reported by Irish et al.4 Compared to samples from the Gulf of California (Córdoba et al.13 and McCluskey et al.21) and the Gulf of Mexico (Ladino et al.12), we find significantly higher INP concentrations and warmer freezing temperatures. The lowest INP concentrations are observed in the Mediterranean Sea (Trueblood et al.8) and the largest range of INP concentrations, spanning more than four orders of magnitude, is from Cabo Verde (Gong et al.7). The world map, Fig. 3b, presents a comparison of studies investigating INPs in bulk seawater measured in immersion freezing mode with a cold stage instrument. The INP concentration measured at −20 °C (CINP (−20 °C)) is represented by colour and seasons by symbols. In the literature, some data are corrected for freezing point depression by salt and others are not and, in some cases, it is unclear from the text. Thus, the figure should be interpreted with care. Consistent with suggestions by Ladino et al.,12 there seems to be an increase in CINP (−20 °C) with increasing northern latitude, indicating a longitudinal gradient in INP concentrations in seawater. This is also observed in recent work targeting seawater in Baffin Bay.39 We notice that three sampling sites (including this study) are at the coasts and there is an overweight of summertime studies. Coastal sites can have higher INP concentrations compared to bulk seawater, likely due to terrestrial runoff, a pattern also observed in Arctic areas.19,39
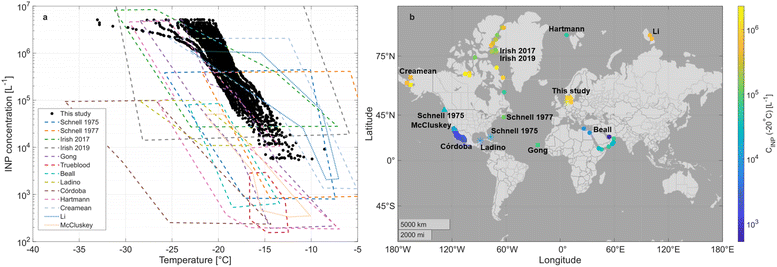 | ||
| Fig. 3 (a) Cumulative INP concentration vs. temperature for bulk seawater in this study (black circles). Previous field based data of CINP in seawater using droplet freezing assay techniques are represented by dashed (ship based seawater samples) or dotted (coastal seawater samples) boxes (main body of data) for the following studies: Schnell and Vali1 (blue), Schnell et al.2 (dark orange), Irish et al. 2017 (ref. 4) (green), Irish et al. 2019 (ref. 5) (grey), Gong et al.7 (purple), Trueblood et al.8 (red), Beall et al.9 (turquoise), Ladino et al.12 (olive green), Córdoba et al.13 (brown), Hartmann et al.15 (pink), Creamean et al.17 (sky blue), Li et al.19 (dark blue), and McCluskey et al.21 (light orange). (b) Locations of the current and previous studies of INPs (immersion mode measured with a cold stage instrument) in bulk seawater. The colour represents the INP concentration at −20 °C. The sampling season is represented by symbols: September, October, and November (square), December, January, and February (triangle), March, April, and May (asterisk), and June, July, and August (circle). Studies are represented by the first author's surname and correspond to the studies mentioned above for (a). | ||
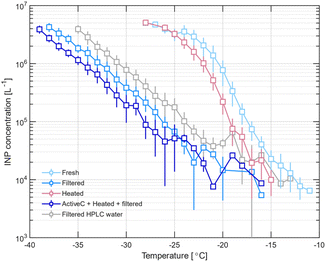
To investigate the physical size of the INPs, we filtered the samples through a 0.02 μm syringe filter to remove large particles. Fig. 4 presents INP concentration as a function of temperature for the February bulk sample before and after such filtration, showing a significant decrease in the freezing temperature after filtration: the CINP (−20 °C) value for bulk seawater decreases from 6.9 × 105 L−1 to 1.5 × 104 L−1 upon filtration. The uncertainties shown for the INP concentration in Fig. 4 represent 1 standard deviation. We estimate the uncertainty in the temperature by finding the temperature at which the INP concentration is equivalent to the highest and lowest uncertainty. For the data presented in Fig. 4, this results in an uncertainty ranging from 0.3 to 1.5 °C in the T50 value.
A decrease in INP concentration upon filtration of seawater was also observed by Irish et al.,4,5 who found a significant decrease in the freezing temperatures when Arctic summer seawater samples (bulk and SML) were filtered through a 0.02 μm filter but not when passed through a 0.2 μm filter, suggesting that the INPs were between 0.02 and 0.2 μm in size. Irish et al.4 suggested that the potential sources of INPs in their study include ultramicrobacteria, viruses, phytoplankton exudates, or bacteria exudates. Wilson et al.24 also concluded, upon filtration of Arctic, Atlantic, North Pacific and coastal British Columbia seawater through 0.02 and 0.2 μm filters.
To elucidate the chemical nature of the INPs present in the seawater, the seawater samples were treated with activated charcoal and/or heat treated. Activated charcoal is a hydrophobic material with an affinity for organic and non-polar compounds and is thus expected to remove such components from the seawater. Heat treatment can lead to denaturation of biological ice nucleation sites.40 A change in INP concentration at a given temperature after heating could thus be indicative of ice nuclei of biological origin.40 In this study, heat treatment was not done systematically across samples and only to 70 °C and in some cases in combination with active carbon treatment. Our data (bulk February, Fig. 4) potentially suggest a small effect of heating to 70 °C corresponding to a decrease in INP concentration. Typically, samples are heated to 90 °C or higher for such investigations.40 Thus, we cannot exclude that biological ice active sites sensitive to heat at higher temperatures than 70 °C were still present in the seawater.
Compared to filtering alone, our data indicate (although within uncertainties) a further small decrease in freezing temperature when the sample is exposed to activated charcoal and heating, followed by filtering (T50 decreases from −31.9 to −33.5 °C for the February sample). Since a similar effect was also observed in the absence of heating, specifically in November surface water and June bulk water (most pronounced), it suggests removal of INPs with affinity for activated charcoal in the size fraction below 0.02 μm. Such an effect seems hard to explain and warrants further study.
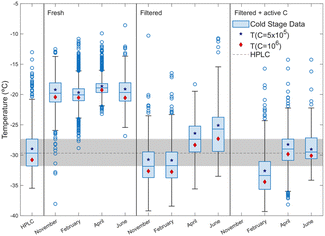
Fig. 5 shows results of cold stage experiments for fresh, filtered and activated charcoal treated bulk seawater from all four samples. The diamonds and stars represent the average freezing temperature of the sample at an INP concentration of 5 × 105 or 106 L−1, respectively. As previously noted, the freezing temperatures for the freshly sampled bulk seawater samples are very similar between the four samples in different seasons (see Fig. S10†). The data in Fig. 5 are not corrected for freezing point depression, but even after such correction, the results are similar (T50 within −1.3 °C for fresh samples). Recent experiments by Moore et al.41 measured similar ice nucleating activity in August 2022 for bulk coastal seawater samples from San Diego, consistent with previous measurements from the same location.7,9 They attribute changes in the airborne INP concentrations to changes in wind speed and wave-breaking rather than changes in seawater chemical and biological characteristics.
Interestingly, the filtration treatment (0.02 μm filter) results in a significant decrease in INP activity for all four samples, but in particular for November and February, despite the total INP concentrations being comparable. While single days cannot represent an entire season, this suggests that studies of seasonality could be interesting, as the seawater contains a larger fraction of INPs that are removed by filtration (0.02 μm) in the November and February samples compared to April and June.
During November, April and June, the surface water or SML was sampled. A box plot is shown in Fig. S11† for the surface and SML samples. In all cases, the T50 values of freshly sampled bulk and SML/surface water are similar within 2 °C. Enrichment factors are 0.98, 0.31, and 0.42 for November, April and June, respectively (see Table S4†). Enrichment factors below 1 indicate a depletion of INPs on the surface compared to the bulk seawater. However, given the proximity to the coast and the weather conditions (wind speed at or above 4 m s−1 and wind gusts in some cases above 8 m s−1 from model estimates from DMI, see Section S1.1†), the water mass was likely well-mixed during sampling with no difference between surface and bulk (30 cm below the surface). While we do not know the nature of our INPs, the low EFs found in this study could be consistent with studies by Rahlff et al.,42 who showed that enrichment of bacteria in the SML occurred only below wind speeds of 4 m s−1 in the Baltic Sea. Similarly, Sun et al.43 found that for wind speeds <6 m s−1 there is an accumulation of gel particles in the SML, which decreases at wind speeds >8 m s−1. Other studies found varying enrichment factors for INPs in SML samples relative to bulk seawater. Irish et al.4,5 reported that less than half of their SML samples from the Canadian Arctic had warmer freezing temperatures than bulk seawater for samples from both 2014 and 2016 and attributed this finding to yearly variation in the properties of SML related to oceanic conditions. Trueblood et al.8 found no significant enrichment of INPs in SML compared to bulk seawater samples from the Mediterranean Sea. Measurements from the Gulf of Mexico by Ladino et al.12 showed colder freezing temperatures for SML samples compared to bulk water. Studies by Wilson et al.24 measured INPs in bulk seawater and SML samples from the Arctic and Atlantic Oceans and found that SML samples consistently froze at higher temperatures than subsurface water. The SML was sampled using the rotating drum technique. Trueblood et al.,8 Irish et al.,4,5 and Ladino et al.12 used the glass plate technique to sample the SML as done in this study. It is possible that the technique used for sampling the SML could influence the collection efficiency of INPs: as suggested by Ickes et al.,22 it may be because the glass plate used for SML sampling does not have a strong affinity for INPs and this needs to be further investigated.
Exploratory statistical analysis was performed to test the association of bulk seawater freezing characteristics, including T (5 × 105 L−1), T (106 L−1), CINP (−20 °C) and T50 values, with chlorophyll a, phosphorus, nitrogen, and oxygen content in the same month (data obtained from https://miljoedata.miljoeportal.dk, see Table 1 and Fig. S1†) as well as the seawater temperature. Note that the data from https://miljoedata.miljoeportal.dk are based on water sampled at a nearby site at 1 m depth, while the INP samples were obtained at 30 cm depth. Selection criteria for inclusion of parameters in the analysis were that the seasonal variation of the parameter must be above 10%. Thus, for the fresh bulk seawater, only the CINP (−20 °C) parameter was included. A correlation matrix and corresponding correlation plots are shown in ESI Section S4.† This analysis shows a negative correlation (R in the range of −0.6 to −1) between INP freezing temperature for the filtered and activated charcoal treated seawater and concentration of nutrients, in particular phosphorus; i.e., the freezing temperature of filtered seawater is lower at high nutrient concentration. The fresh seawater CINP (−20 °C) values show similar negative correlation with the nutrient concentrations. The chlorophyll a concentration can be used as an indicator for the abundance of phytoplankton in water. The chlorophyll a concentration is higher in autumn and winter compared to spring and summer and we observe a potentially weak negative correlation (R in the range of −0.59 to −0.67) between the INP freezing temperature of filtered seawater and the concentration of chlorophyll a. In addition, a positive correlation is observed between the filtered seawater (all parameters) and the seawater temperature.
Conclusions
In this exploratory study, the ice nucleation activity of seawater was investigated for the first time from a coastal site near Aarhus, Denmark. The measurements were performed in immersion freezing mode with a cold stage instrument. The concentration of INPs ranged from 6 × 103 to 5 × 106 INP L−1 in a temperature range of −12 to −34 °C. The results showed that the measured INPs in fresh bulk seawater had T50 values of approx. −20 °C and there was little variation across samples from four different months (February, April, June and November). Filtration of the seawater samples (0.02 μm filter) resulted in a significant decrease in freezing temperature for all four samples and the decrease was largest for the November and February samples. These results are consistent with other studies also observing a decrease in freezing temperatures upon filtration with a 0.02 μm filter. Our data suggest that the main INPs in seawater samples from Ajstrup Beach are larger than 0.02 μm in size. Furthermore, the size and composition of INP samples differ between November/February and April/June, which could potentially indicate a seasonal trend. Exploratory investigations suggested that the freezing temperature decreased further when the seawater was treated with activated charcoal and in some cases heated to 70 °C. This effect was observed for all samples, both with and without simultaneous heating and warrants further investigation.No enrichment of INPs was observed in the sea surface microlayer or surface water samples compared to the bulk seawater.
Comparison with results obtained from the literature demonstrates interesting differences in immersion freezing INP concentrations in seawater, suggesting an increase in CINP (−20 °C) with increasing northern latitude, consistent with previous studies.
In this work, we have studied the INP activity of seawater collected at the coast, which might be affected by terrestrial runoff. In the future, it would be interesting to analyse seawater sampled in a gradient from the shore. The results of this study call for future work clarifying differences in INP concentrations in seawater at different locations around the world, at different distances from the shore and during different seasons.
Data availability
The data for ice nucleation experiments supporting this article have been included as an Excel file as part of the ESI.†Author contributions
Eva R. Kjærgaard: investigation, methodology, data curation, formal analysis, visualization, writing – original draft, and writing – review & editing. Amanda S. Sejersen: investigation, methodology, data curation, formal analysis, and writing – review & editing. Max Fobian Skov: formal analysis, visualization, data curation, and writing – review & editing. Markus D. Petters: conceptualization, methodology, and writing – review & editing. Merete Bilde: conceptualization, methodology, writing – review & editing, supervision, project administration, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Independent Research Fund Denmark (grant number 0217-00442B) and the Danish National Research Foundation (DNRF172) through the Center of Excellence for Chemistry of Clouds. MDP acknowledges funding from the U.S. National Science Foundation grant AGS 2112978. We thank Mette Kirstine Løchte Jørgensen for helping collect the June sample. We thank Lasse Z. Jensen for measuring the salinity (refractometer) of the June seawater sample. We thank Casper Fælled for help with acquiring data from the Miljødata website.References
- R. Schnell and G. Vali, Freezing nuclei in marine waters, Tellus, 1975, 27, 321–323 CrossRef.
- R. Schnell, Ice nuclei in seawater, fog water and marine air off the coast of Nova Scotia: Summer 1975, J. Atmos. Sci., 1977, 34, 1299–1305 CrossRef.
- P. J. DeMott, A. J. Prenni, X. Liu, S. M. Kreidenweis, M. D. Petters, C. H. Twohy, M. Richardson, T. Eidhammer and D. Rogers, Predicting global atmospheric ice nuclei distributions and their impacts on climate, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 11217–11222 CrossRef.
- V. E. Irish, P. Elizondo, J. Chen, C. Chou, J. Charette, M. Lizotte, L. A. Ladino, T. W. Wilson, M. Gosselin and B. J. Murray, Ice-nucleating particles in Canadian Arctic sea-surface microlayer and bulk seawater, Atmos. Chem. Phys., 2017, 17, 10583–10595 CrossRef.
- V. E. Irish, S. J. Hanna, Y. Xi, M. Boyer, E. Polishchuk, M. Ahmed, J. Chen, J. P. Abbatt, M. Gosselin and R. Chang, Revisiting properties and concentrations of ice-nucleating particles in the sea surface microlayer and bulk seawater in the Canadian Arctic during summer, Atmos. Chem. Phys., 2019, 19, 7775–7787 CrossRef CAS.
- Y. Yun and J. E. Penner, An evaluation of the potential radiative forcing and climatic impact of marine organic aerosols as heterogeneous ice nuclei, Geophys. Res. Lett., 2013, 40, 4121–4126 CrossRef.
- X. Gong, H. Wex, M. van Pinxteren, N. Triesch, K. W. Fomba, J. Lubitz, C. Stolle, T.-B. Robinson, T. Müller and H. Herrmann, Characterization of aerosol particles at Cabo Verde close to sea level and at the cloud level–Part 2: Ice-nucleating particles in air, cloud and seawater, Atmos. Chem. Phys., 2020, 20, 1451–1468 CrossRef.
- J. V. Trueblood, A. Nicosia, A. Engel, B. Zäncker, M. Rinaldi, E. Freney, M. Thyssen, I. Obernosterer, J. Dinasquet and F. Belosi, A two-component parameterization of marine ice-nucleating particles based on seawater biology and sea spray aerosol measurements in the Mediterranean Sea, Atmos. Chem. Phys., 2021, 21, 4659–4676 CrossRef.
- C. M. Beall, T. C. Hill, P. J. DeMott, T. Köneman, M. Pikridas, F. Drewnick, H. Harder, C. Pöhlker, J. Lelieveld and B. Weber, Ice-nucleating particles near two major dust source regions, Atmos. Chem. Phys., 2022, 22, 12607–12627 CrossRef.
- C. Hoose and O. Möhler, Heterogeneous ice nucleation on atmospheric aerosols: a review of results from laboratory experiments, Atmos. Chem. Phys., 2012, 12, 9817–9854 CrossRef.
- B. Murray, D. O'Sullivan, J. Atkinson and M. Webb, Ice nucleation by particles immersed in supercooled cloud droplets, Chem. Soc. Rev., 2012, 41, 6519–6554 RSC.
- L. A. Ladino, J. Juaréz-Pérez, Z. Ramírez-Díaz, L. A. Miller, J. Herrera, G. B. Raga, K. G. Simpson, G. Cruz, D. L. Pereira and F. Córdoba, The UNAM-droplet freezing assay: An evaluation of the ice nucleating capacity of the sea-surface microlayer and surface mixed layer in tropical and subpolar waters, Atmósfera, 2022, 35, 127–141 CrossRef.
- M. F. Córdoba, E. García-Mendoza, A. Olivos, G. B. Raga, M. de los Ángeles Horta and L. A. Ladino, Ice nucleating abilities of deep waters from the Mexican Pacific ocean, Atmos. Environ., 2023, 309, 119887 CrossRef.
- J. Mülmenstädt, O. Sourdeval, J. Delanoë and J. Quaas, Frequency of occurrence of rain from liquid-, mixed-, and ice-phase clouds derived from A-Train satellite retrievals, Geophys. Res. Lett., 2015, 42, 6502–6509 CrossRef.
- M. Hartmann, X. Gong, S. Kecorius, M. van Pinxteren, T. Vogl, A. Welti, H. Wex, S. Zeppenfeld, H. Herrmann and A. Wiedensohler, Terrestrial or marine?–Indications towards the origin of Ice Nucleating Particles during melt season in the European Arctic up to 83.7 N, Atmos. Chem. Phys., 2020, 2020, 1–35 Search PubMed.
- T. Raatikainen, M. Prank, J. Ahola, H. Kokkola, J. Tonttila and S. Romakkaniemi, The effect of marine ice-nucleating particles on mixed-phase clouds, Atmos. Chem. Phys., 2022, 22, 3763–3778 CrossRef.
- J. M. Creamean, J. N. Cross, R. Pickart, L. McRaven, P. Lin, A. Pacini, R. Hanlon, D. G. Schmale, J. Ceniceros, T. Aydell, N. Colombi, E. Bolger and P. J. DeMott, Ice Nucleating Particles Carried From Below a Phytoplankton Bloom to the Arctic Atmosphere, Geophys. Res. Lett., 2019, 46, 8572–8581 CrossRef.
- G. De Leeuw, E. L. Andreas, M. D. Anguelova, C. Fairall, E. R. Lewis, C. O’Dowd, M. Schulz and S. E. Schwartz, Production flux of sea spray aerosol, Rev. Geophys., 2011, 49, RG2001 CrossRef.
- G. Li, A. Welti, A. Rocchi, G. P. Fogwill, M. Dall’Osto and Z. A. Kanji, Terrestrial and Marine sources of ice nucleating particles in the Eurasian Arctic, Faraday Discuss., 2025, 258, 94–119 RSC.
- P. J. DeMott, T. C. Hill, C. S. McCluskey, K. A. Prather, D. B. Collins, R. C. Sullivan, M. J. Ruppel, R. H. Mason, V. E. Irish and T. Lee, Sea spray aerosol as a unique source of ice nucleating particles, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 5797–5803 CrossRef.
- C. S. McCluskey, T. C. J. Hill, F. Malfatti, C. M. Sultana, C. Lee, M. V. Santander, C. M. Beall, K. A. Moore, G. C. Cornwell, D. B. Collins, K. A. Prather, T. Jayarathne, E. A. Stone, F. Azam, S. M. Kreidenweis and P. J. DeMott, A Dynamic Link between Ice Nucleating Particles Released in Nascent Sea Spray Aerosol and Oceanic Biological Activity during Two Mesocosm Experiments, J. Atmos. Sci., 2017, 74, 151–166 Search PubMed.
- L. Ickes, G. C. Porter, R. Wagner, M. P. Adams, S. Bierbauer, A. K. Bertram, M. Bilde, S. Christiansen, A. M. Ekman and E. Gorokhova, The ice-nucleating activity of Arctic sea surface microlayer samples and marine algal cultures, Atmos. Chem. Phys., 2020, 20, 11089–11117 Search PubMed.
- C. J. Gaston, H. Furutani, S. A. Guazzotti, K. R. Coffee, T. S. Bates, P. K. Quinn, L. I. Aluwihare, B. G. Mitchell and K. A. Prather, Unique ocean-derived particles serve as a proxy for changes in ocean chemistry, J. Geophys. Res. Atmos., 2011, 116, D18310 CrossRef.
- T. W. Wilson, L. A. Ladino, P. A. Alpert, M. N. Breckels, I. M. Brooks, J. Browse, S. M. Burrows, K. S. Carslaw, J. A. Huffman and C. Judd, A marine biogenic source of atmospheric ice-nucleating particles, Nature, 2015, 525, 234–238 Search PubMed.
- X. Wang, C. M. Sultana, J. Trueblood, T. C. Hill, F. Malfatti, C. Lee, O. Laskina, K. A. Moore, C. M. Beall and C. S. McCluskey, Microbial control of sea spray aerosol composition: A tale of two blooms, ACS Cent. Sci., 2015, 1, 124–131 CrossRef PubMed.
- M. J. Wolf, M. Goodell, E. Dong, L. A. Dove, C. Zhang, L. J. Franco, C. Shen, E. G. Rutkowski, D. N. Narducci and S. Mullen, A link between the ice nucleation activity and the biogeochemistry of seawater, Atmos. Chem. Phys., 2020, 20, 15341–15356 CrossRef.
- A. Lehmann, K. Myrberg, P. Post, I. Chubarenko, I. Dailidiene, H. H. Hinrichsen, K. Hüssy, T. Liblik, H. E. M. Meier, U. Lips and T. Bukanova, Salinity dynamics of the Baltic Sea, Earth Syst. Dyn., 2022, 13, 373–392 CrossRef.
- J. Zinke, E. D. Nilsson, P. Markuszewski, P. Zieger, E. M. Mårtensson, A. Rutgersson, E. Nilsson and M. E. Salter, Sea spray emissions from the Baltic Sea: comparison of aerosol eddy covariance fluxes and chamber-simulated sea spray emissions, Atmos. Chem. Phys., 2024, 24, 1895–1918 Search PubMed.
- A. Bruhn, T. Janicek, D. Manns, M. M. Nielsen, T. J. S. Balsby, A. S. Meyer, M. B. Rasmussen, X. Hou, B. Saake and C. Göke, Crude fucoidan content in two North Atlantic kelp species, Saccharina latissima and Laminaria digitata—seasonal variation and impact of environmental factors, J. Appl. Phycol., 2017, 29, 3121–3137 CrossRef PubMed.
- G. W. Harvey and L. A. Burzell, A simple microlayer method for small samples, Limnol. Oceanogr., 1972, 17, 156–157 CrossRef.
- I. N. Tang, A. C. Tridico and K. H. Fung, Thermodynamic and optical properties of sea salt aerosols, J. Geophys. Res. Atmos., 1997, 102, 23269–23275 CrossRef.
- E. Bigg, The formation of atmospheric ice crystals by the freezing of droplets, Q. J. R. Meteorol. Soc., 1953, 79, 510–519 CrossRef.
- S. Mahant, S. Yadav, C. Gilbert, E. R. Kjærgaard, M. M. Jensen, T. Kessler, M. Bilde and M. D. Petters, An open-hardware community ice nucleation cold stage for research and teaching, HardwareX, 2023, 16, e00491 CrossRef.
- S. Yadav, R. Venezia, R. Paerl and M. Petters, Characterization of ice-nucleating particles over Northern India, J. Geophys. Res. Atmos., 2019, 124, 10467–10482 CrossRef.
- G. Vali, Quantitative evaluation of experimental results an the heterogeneous freezing nucleation of supercooled liquids, J. Atmos. Sci., 1971, 28, 402–409 CrossRef.
- R. Schwidetzky, M. Lukas, A. YazdanYar, A. T. Kunert, U. Pöschl, K. F. Domke, J. Fröhlich-Nowoisky, M. Bonn, T. Koop, Y. Nagata and K. Meister, Specific Ion–Protein Interactions Influence Bacterial Ice Nucleation, Chem.–Eur. J., 2021, 27, 7402–7407 CrossRef PubMed.
- L. S. Nielsen and M. Bilde, Exploring controlling factors for sea spray aerosol production: temperature, inorganic ions and organic surfactants, Tellus B, 2020, 72, 1–10 CrossRef.
- P. J. DeMott, O. Möhler, D. J. Cziczo, N. Hiranuma, M. D. Petters, S. S. Petters, F. Belosi, H. G. Bingemer, S. D. Brooks and C. Budke, The Fifth International Workshop on Ice Nucleation phase 2 (FIN-02): laboratory intercomparison of ice nucleation measurements, Atmos. Meas. Tech., 2018, 11, 6231–6257 CrossRef.
- C. M. Christian Ditlev Funder Castenschiold, S. Christiansen, M. Alsved, L. Ickes, S. Tesson, J. Löndahl, M. Bilde, T. Bataillon, K. Finster and T. Šantl-Temkiv, Atmospheric Biogenic Ice-Nucleating Particles Link to Microbial Communities in the Arctic Marine Environment in Western Greenland, Environ. Sci. Technol., 2025 Search PubMed , manuscript submitted.
- M. I. Daily, M. D. Tarn, T. F. Whale and B. J. Murray, An evaluation of the heat test for the ice-nucleating ability of minerals and biological material, Atmos. Meas. Tech., 2022, 15, 2635–2665 CrossRef.
- K. A. Moore, T. C. J. Hill, C. K. Madawala, R. J. Leibensperger Iii, S. Greeney, C. D. Cappa, M. D. Stokes, G. B. Deane, C. Lee, A. V. Tivanski, K. A. Prather and P. J. DeMott, Wind-driven emission of marine ice-nucleating particles in the Scripps Ocean-Atmosphere Research Simulator (SOARS), Atmos. Chem. Phys., 2025, 25, 3131–3159 CrossRef.
- J. Rahlff, C. Stolle, H.-A. Giebel, T. Brinkhoff, M. Ribas-Ribas, D. Hodapp and O. Wurl, High wind speeds prevent formation of a distinct bacterioneuston community in the sea-surface microlayer, FEMS Microbiol. Ecol., 2017, 93, fix041 CrossRef PubMed.
- C.-C. Sun, M. Sperling and A. Engel, Effect of wind speed on the size distribution of gel particles in the sea surface microlayer: insights from a wind–wave channel experiment, Biogeosciences, 2018, 15, 3577–3589 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Weather and ocean conditions from sampling, seawater characteristics from Aarhus Bay, water activity measurements, a table of INP data from experiments and additional figures, sea surface microlayer details, statistical analysis, and salt comparison. See DOI: https://doi.org/10.1039/d5ea00031a |
| This journal is © The Royal Society of Chemistry 2025 |