Open Access Article
Open Access ArticleSodium phosphaethynolate as P-source for the synthesis of molecular rhodium phosphides: an exploratory study
Zhongshu
Li†
 *a,
Jaap E.
Borger†
b,
Thomas L.
Gianetti
*a,
Jaap E.
Borger†
b,
Thomas L.
Gianetti
 c,
Fabian
Müller
b,
Bruno
Pribanic
b,
Peter
Coburger
c,
Fabian
Müller
b,
Bruno
Pribanic
b,
Peter
Coburger
 *d and
Hansjörg
Grützmacher
*d and
Hansjörg
Grützmacher
 *ab
*ab
aLIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou 510006, China. E-mail: lizhsh6@mail.sysu.edu.cn
bDepartment of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, Hönggerberg, 8093 Zürich, Switzerland. E-mail: hgruetzmacher@ethz.ch
cDepartment of Chemistry and Biochemistry, University of Arizona, Tucson, Arizona 85721, USA
dDepartment of Inorganic Chemistry, TU München, Lichtenbergstraße 4, 85747 Garching, Germany. E-mail: peter.coburger@tum.de
First published on 26th September 2025
Abstract
Molecular transition metal phosphides (TMPs) containing a Rh2P2 and unprecedented Rh4P4 core could be generated from Na[OCP] and rhodium(I) precursor complexes carrying the bischelating tropPPh2 ligand. These reaction proceed through a formal P-transfer step (trop = 5H-dibenzo[a,d]cyclohepten-5-yl), which involves migration of the CO unit to the Rh(I) center. With the related tetradentate ligand trop3P and tridentate ligand trop2PPh the first neutral and anionic [Rhx(L)n(PCO)y]z complexes containing up to two rhodium centers and three intact phosphaethynolate units. These complexes are inert against CO migration. The results demonstrate a large influence of the metal coordination sphere on product formation, and indicate that low-coordinate rhodium(I) precursors are most effective for the preparation of RhP complexes using Na[OCP] as P-source.
Introduction
Transition metal phosphide (TMP) nanomaterials have been identified as high-performing catalysts for electrocatalytic water splitting1 and deoxygenation of bio-oil,2 which may be of interest for the development of sustainable energy sources. Their variety in the structural composition and associated electronic structures is attractive as it provides for a handle on catalytic activity and material properties. Generally, TMPs can be divided into metal-rich phases containing M–M bonds and phosphide-rich phases with P–P bonds, which explains the broad range of structure types and consequently electronic properties. The synthesis of TMP bulk and nanomaterials mostly relies on the reaction of hazardous phosphorus sources such as PH3, P(SiMe3)3, or P4 with a suitable transition metal precursor under various reaction conditions, which frequently involve high temperatures.3 Molecular TMP complexes (featuring preformed M–P bonds) can be used as single-source molecular precursors for the synthesis of nanoparticles.2a,4 Their selective preparation, however, is challenging especially for phosphides of the late transition metals.5Binary rhodium phosphides of composition Rh2P, Rh3P2, Rh4P3, RhP2, and RhP3 have been reported (for a review see ref. 3c) and the structures of some of them are known since over 60 years.6 Only recently, the remarkable properties of especially Rh2P as component of catalytically active materials in electro-7 and photocatalytic8 water splitting, methanol oxidation,9 hydrogenation and hydrodesulfurization,10 hydroformulation,11 and amination12 has been discovered.
The development of large-scale syntheses of phosphaethynolate salts (M + [OCP]−; M = K, Na)13 has spurred the use of these salts as phosphorus containing small building blocks over the last decade.14 In analogy to the isovalence electronic azide anion, the [OCP]− anion may serve as “P−” transfer reagent under extrusion of CO (as suggested by the resonance structure [O![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C → P−]).14,15 Salt metathesis reactions of Na[OCP] with metal halides show the phosphaethynolate anion to coordinate to d- and f-block metal centers either through the P- or O-binding site, producing stable monomeric compounds of the type A (M
C → P−]).14,15 Salt metathesis reactions of Na[OCP] with metal halides show the phosphaethynolate anion to coordinate to d- and f-block metal centers either through the P- or O-binding site, producing stable monomeric compounds of the type A (M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Re,16 Co,17 Cu,17 Ag
Re,16 Co,17 Cu,17 Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 and Au
18 and Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17) and B (M
17) and B (M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) U,19 Th,19b Sc,20 Y,21 Nd
U,19 Th,19b Sc,20 Y,21 Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 and Sm
21 and Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21) or dinuclear complexes such as C (M = Fe)22 or D (M = Nd,21Scheme 1a). These type of complexes may indeed afford metal phosphides through CO migration or release.23 For example, the ligand-stabilized Ti2P2I,24 Ir2P2II,17 (for a related Pt2P2 complex see ref. 25), U2P2III,26 Ni2P2IV,27 Fe2P3V
21) or dinuclear complexes such as C (M = Fe)22 or D (M = Nd,21Scheme 1a). These type of complexes may indeed afford metal phosphides through CO migration or release.23 For example, the ligand-stabilized Ti2P2I,24 Ir2P2II,17 (for a related Pt2P2 complex see ref. 25), U2P2III,26 Ni2P2IV,27 Fe2P3V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4b and anionic [WP]−VI
4b and anionic [WP]−VI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 could be prepared (Scheme 1b). Note that V proved to be a suitable molecular precursor for the preparation FeP nanoparticles that were found to be especially active as electrocatalysts on the anodic and cathodic side for water-splitting.4b
28 could be prepared (Scheme 1b). Note that V proved to be a suitable molecular precursor for the preparation FeP nanoparticles that were found to be especially active as electrocatalysts on the anodic and cathodic side for water-splitting.4b
To the best of our knowledge, well-defined binary RhP phases or molecular rhodium phosphido complexes with a Rh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 1
P ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are unknown. Here we report the results of our attempts to prepare molecular Rh phosphide complexes using a variety of rhodium(I) precursor complexes and Na[OCP] as “P−” transfer reagent. These reactions afforded the first Rh(PCO) adducts of the type A and C. Furthermore, a new type of a dinuclear complex with a central μ2-P2(C
1 are unknown. Here we report the results of our attempts to prepare molecular Rh phosphide complexes using a variety of rhodium(I) precursor complexes and Na[OCP] as “P−” transfer reagent. These reactions afforded the first Rh(PCO) adducts of the type A and C. Furthermore, a new type of a dinuclear complex with a central μ2-P2(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2 heterocycle was discovered. Finally the formation of polynuclear Rh2P2 and unprecedented Rh4P4 complexes with Rh and P in a 1
O)2 heterocycle was discovered. Finally the formation of polynuclear Rh2P2 and unprecedented Rh4P4 complexes with Rh and P in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio is disclosed, whose electronic structures were analysed by DFT methods.
1 ratio is disclosed, whose electronic structures were analysed by DFT methods.
Results and discussion
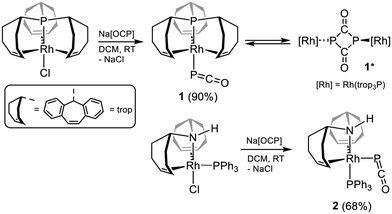
In order to evaluate the influence of different coordination environments around a rhodium(I) centre in reactions with Na[OCP], various Rh–X precursor complexes were tested. Commercially available [RhCl(PPh3)3] or [Rh2(μ2-Cl)2(cod)2] only gave black precipitates (likely Rh particles) and a variety of phosphorus compounds (detected by 31P NMR), which were not identified. However, Rh(I) complexes with trop-substituted (trop = 5H-dibenzo[a,d]cyclohepten-5-yl) tetra-, tri- and bidentate phosphine ligands, which were prepared according to known procedures (see the SI for details),29 reacted with Na[OCP] to give defined compounds.First the saturated 18-valence electron configured [RhCl(Ptrop3)] complex with a tetradentate tris(olefin) phosphine ligand, was reacted with Na[OCP] in dichloromethane (DCM) at room temperature. The instant formation of [Rh(PCO)(Ptrop3)] 1 resulted from a salt metathesis reaction (Scheme 2), and a yield of 90% was obtained after work-up. The intact P-bound PCO entity shows a 31P NMR resonance signal at δ = −346.9 ppm, (cf. δ31P Na[OCP] = −391 ppm), which is in the middle of the rather broad range of 31P NMR shifts observed for κ1P-PCO bound complexes (δ31P = −225.8 [Co(PDI)(PCO)] to −441.0 ppm ([W(CO)5(PCO)]−)).14a The resonance of the 31P nucleus of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ligand is split into a doublet of doublets by coupling with the 31P nucleus of the trop3P ligand in trans-position (2JP,P = 138.5 Hz) and the directly bound 103Rh nucleus (1JP,Rh = 36.7 Hz). The 31P nucleus of the trop3P ligand shows likewise a doublet of doublet resonance but the 1JP,Rh coupling (138.5 Hz) is significantly larger. The asymmetric stretching frequency of the PCO ligand appears at vasym(PCO) = 1839 cm−1 in the IR spectrum, which is in the range of κ1P-PCO bound ligands in other complexes.14a The related 18 valence electron complex [RhCl(PPh3)(HNtrop2)] also reacts cleanly in a salt metathesis reaction with Na[OCP] to give the corresponding complex [Rh(PCO)(PPh3)(HNtrop2)] 2. Note that in the precursor complex the chlorido ligand is located in axial position, while in 2 the PCO ligand is located in equatorial position of the trigonal bipyramidal structure (δ31P Rh(PCO) = −365.1 pm; vasym(PCO) = 1853 cm−1), which is indicated by the small 2JP,P coupling constant (10.0 Hz) typical of a cis-arrangement Ph3P–Rh–PCO (Scheme 2). This phenomenon was also observed when Cl− is replaced with other weakly binding ligands such as (O3SCF3)− (OTf−).29a Indeed, the Rh–PCO bond (2.493 Å) is remarkably long in comparison to the Rh–PPh3 bond (2.269 Å) (for details see S3 in the SI).
O ligand is split into a doublet of doublets by coupling with the 31P nucleus of the trop3P ligand in trans-position (2JP,P = 138.5 Hz) and the directly bound 103Rh nucleus (1JP,Rh = 36.7 Hz). The 31P nucleus of the trop3P ligand shows likewise a doublet of doublet resonance but the 1JP,Rh coupling (138.5 Hz) is significantly larger. The asymmetric stretching frequency of the PCO ligand appears at vasym(PCO) = 1839 cm−1 in the IR spectrum, which is in the range of κ1P-PCO bound ligands in other complexes.14a The related 18 valence electron complex [RhCl(PPh3)(HNtrop2)] also reacts cleanly in a salt metathesis reaction with Na[OCP] to give the corresponding complex [Rh(PCO)(PPh3)(HNtrop2)] 2. Note that in the precursor complex the chlorido ligand is located in axial position, while in 2 the PCO ligand is located in equatorial position of the trigonal bipyramidal structure (δ31P Rh(PCO) = −365.1 pm; vasym(PCO) = 1853 cm−1), which is indicated by the small 2JP,P coupling constant (10.0 Hz) typical of a cis-arrangement Ph3P–Rh–PCO (Scheme 2). This phenomenon was also observed when Cl− is replaced with other weakly binding ligands such as (O3SCF3)− (OTf−).29a Indeed, the Rh–PCO bond (2.493 Å) is remarkably long in comparison to the Rh–PPh3 bond (2.269 Å) (for details see S3 in the SI).
 | ||
| Scheme 2 Reaction of Na[OCP] with Rh(trop3P)Cl to afford 1, dimerization to 1* and related complex 2; trop = 5H-dibenzo[a,d]cyclohepten-5-yl. | ||
Compound 1 is soluble in tetrahydrofuran (THF) and 1,2-dichlorobenzene, in which it is stable for at least 24 h at room temperature. With the aim of extruding CO, solutions of 1 in both solvents were heated to 70 °C and analyzed using 31P NMR spectroscopy. Instead of decarbonylation, however, a dimerization towards [Rh2(Ptrop3)2[μ2-κP,κP′-(P2C2O2)]] 1* occurs. The formation of this complex with a bridging dianionic four-membered 1,3-diphosphacyclobutanyl-2,4-dione as-heterocycle, [P2(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2]2−, is indicated by the profound shift of the 31P resonance to higher frequencies in the 31P NMR spectrum (31Pδ = 183.0 ppm, Δδ = 530 ppm). A conversion of up to 30% can be reached after 18 h. UV irradiation of solutions of 1 gave the same result. It was not possible to achieve a higher conversion rate even upon prolonged heating, which indicates an equilibrium between 1 and 1*, with a sizable barrier for interconversion. This is confirmed by DFT calculations (ωB97X-D/Def2-SVP),30 which indicate that the process 2 1 ⇆ 1* is mildly exergonic by −7.0 kcal mol−1 with a barrier of ΔG# = 27.1 kcal mol−1 for the concerted [2 + 2]-dimerization.
O)2]2−, is indicated by the profound shift of the 31P resonance to higher frequencies in the 31P NMR spectrum (31Pδ = 183.0 ppm, Δδ = 530 ppm). A conversion of up to 30% can be reached after 18 h. UV irradiation of solutions of 1 gave the same result. It was not possible to achieve a higher conversion rate even upon prolonged heating, which indicates an equilibrium between 1 and 1*, with a sizable barrier for interconversion. This is confirmed by DFT calculations (ωB97X-D/Def2-SVP),30 which indicate that the process 2 1 ⇆ 1* is mildly exergonic by −7.0 kcal mol−1 with a barrier of ΔG# = 27.1 kcal mol−1 for the concerted [2 + 2]-dimerization.
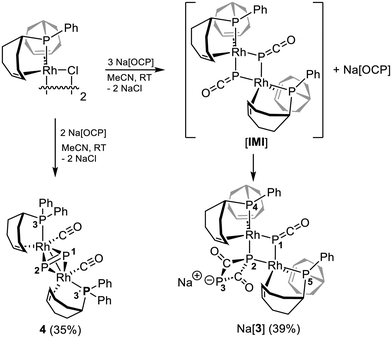
In the next step, the dinuclear complex [Rh2(μ2-Cl)2(trop2PPh)2]—featuring the tridentate trop2PPh ligand instead of tetradentate trop3P—was exposed to an excess of Na[OCP] in acetonitrile (MeCN) at room temperature (Scheme 3). Again no cleavage of the phosphorus-carbonyl bond could be observed. The reaction produced one major product within 24 h that directly precipitated as red powder from the reaction solution and was characterized as Na[3] (39% isolated yield) by multinuclear NMR spectroscopy, elemental analysis, and single crystal X-ray diffraction (XRD, Fig. 1bvide infra). Na[3] is sparingly soluble in THF and slowly decomposes in solution (see the SI). We propose that its formation proceeds via an Rh(PCO) adduct similar to 1, which may then dimerize through coordination of the available P-lone pair at the PCO ligand to a rhodium center of a second unsaturated 16e [Rh(PCO)(PhPtrop2)] fragment furnishing [IMI] with two μ2-κP-PCO moieties (Scheme 3; cf. E, Scheme 1). Subsequent nucleophilic attack of additional Na[OCP] at one of its carbonyl C-atoms then affords the final product. The 31P chemical shift found for P1 at δ = −471.6 ppm is the most low frequency chemical shift reported for a PCO ligand to date. The other 31P nuclei resonate at δ31P = 53.0 ppm (P2), 266.6 ppm (P3), and 120.2 ppm (P4/P5).
Heating or UV/Vis irradiation of Na[3] in THF does not promote the elimination of CO. Like for 1 and 2, we assume that the electronic saturation – all complexes discussed so far are 18 valence electron configured – may be the reason this inertness. We therefore chose the dinuclear complex [Rh2(μ2-Cl)2(tropPPh2)2] as precursor complex, which contains 16 valence electron configured d8-Rh(I) centers in a square planar coordination sphere. The stoichiometric reaction with Na[OCP] in acetonitrile at room temperature afforded [Rh2(CO)2(Ph2Ptrop)2(η2-P2)] 4, which precipitated from the reaction solution allowing isolation in 35% yield after filtration and several washing steps (Scheme 3).
The absence of any 31P NMR signals at low frequencies recorded for solutions of 4 show that no OCP unit remained intact. This is also indicated by the IR spectrum, which shows no absorptions in the region typical for coordinated OCP units (1841–1890 cm−1)14a but a strong absorption at vsym(CO) = 1966 cm−1 and a medium one at vasym(CO) = 1960 cm−1, which are assigned to Rh ← CO carbonyl stretching vibrations. The 31P nuclei P3 and P3′ of the Ph2Ptrop ligand give rise to one signal at δ = 70.8 ppm (in [D8]THF) indicating that these nuclei are magnetically equivalent and related by symmetry. Furthermore, two doublets are observed at 31P δ = 102.1 ppm and 124.6 ppm and the large 1JP,P coupling constant of 540.7 Hz indicates a direct P–P bond. These spectroscopic data are consistent with the formula given for 4 in Scheme 4 and is confirmed by a XRD analysis (vide infra).
In solution, 4 is not stable and over the course of 24 h in THF at room temperature, partly converted to a new complex featuring a set of 1H and 31P NMR resonance signals corresponding to a Rh-coordinated tropPPh2 ligand (δ31P ≈ 70 ppm). In addition, one broad 31P NMR signal emerged at δ = 119.8 ppm, which is in the same region as the signals originating from the P2 bridge in 4 (see above). No further evolution of the signal ratios was observed after 24 h, not even upon heating the sample to 65 °C. A variable temperature 31P NMR spectroscopy study in the range of T = −40–65 °C revealed a reversible process. At 65 °C a significant sharpening of the signals arising from both 4 and the newly formed product is observed, while at −40 °C multiple unidentified species are detected (see the SI for details). Although the exact nature of this process is as yet unclear, it is possible that these observations arise from an isomerization of syn-CO 4 to its C2 symmetric anti-CO conformer which may involve a number of intermediates. A DFT calculations indicates that the energy difference between these two isomers is very small, ΔGsyn-CO4→ anti-CO4 = 1.6 kcal·mol−1.
Encouraged by the result obtained with the dinuclear complex [Rh2(μ2-Cl)2(tropPPh2)2], we reacted the mononuclear Rh(I) complex [RhCl(Ph2Ptrop)2] with Na[OCP] in DME at room temperature. The result is shown in Scheme 4. The dark brown suspension was allowed to stir for 18 h and then filtered prior to removing the volatiles. The obtained black powder was washed with n-hexane and Et2O and subsequently analyzed by 31P NMR spectroscopy ([D6]benzene). The recorded spectrum showed the formation of 4 as the major product, uncoordinated tropPPh2 (δ = −14.1 ppm; see the SI), and a minor product that exhibits a broad doublet at δ = 64.2 ppm (J = 173.2 Hz) and a broad singlet at 41.0 ppm (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). Fortuitously, few black crystalline platelets of the latter could be isolated upon layering a solution of the crude product in DME with n-hexane and an XRD study allowed to determine also the structure of this minor product (vide infra) as a Rh4P4 cluster 5 shown in Scheme 4. Formally, 5 is the product of a dimerization reaction of 4 under loss of two equivalents of CO, 2 [4] → [5] + 2 CO. And although we failed to realize this process experimentally, DFT calculations show that it would be thermodynamically favored by ΔG = −16 kcal mol−1.
1 ratio). Fortuitously, few black crystalline platelets of the latter could be isolated upon layering a solution of the crude product in DME with n-hexane and an XRD study allowed to determine also the structure of this minor product (vide infra) as a Rh4P4 cluster 5 shown in Scheme 4. Formally, 5 is the product of a dimerization reaction of 4 under loss of two equivalents of CO, 2 [4] → [5] + 2 CO. And although we failed to realize this process experimentally, DFT calculations show that it would be thermodynamically favored by ΔG = −16 kcal mol−1.
Structure determinations of compounds 1*–5
Single crystals of 1* (orange), 2 (orange), 3 (red), 4 (orange), and 5 (dark brown) were grown and subjected to XRD analyses, which allowed to determine the structures of these compounds. The structural details of the PCO unit in 2 (P–C 1.532 Å, C–O 1.175 Å, P–C–O 176.9°) is in line with previous reports on related M–PCO complexes14a and not discussed in detail here (for a structure plot see S3 in the SI). The molecular structure of 1* (Fig. 1a) shows a central planar P2(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2 heterocycle, which is located on a crystallographic inversion center [P1–C1–P1′–C1′ dihedral = −180.0(3)°]. The averaged P–C bond lengths within the P2C2 cycle (1.840 Å) and the C
O)2 heterocycle, which is located on a crystallographic inversion center [P1–C1–P1′–C1′ dihedral = −180.0(3)°]. The averaged P–C bond lengths within the P2C2 cycle (1.840 Å) and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O distances (1.233 Å) indicate P–C single and C
O distances (1.233 Å) indicate P–C single and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond character.31 As expected the C1–P1–C1′ angle (80.3°) is significantly smaller than the P1–C1–P1′ angle (99.7°) (overall the bond metrics are comparable to related main group-13c,14,15,32 and transition metal-substituted22 1,3-diphosphacyclobutane-1,2-diones). The [Rh(Ptrop3)] fragments deviate significantly out-of-plane from the planar P2(C
O double bond character.31 As expected the C1–P1–C1′ angle (80.3°) is significantly smaller than the P1–C1–P1′ angle (99.7°) (overall the bond metrics are comparable to related main group-13c,14,15,32 and transition metal-substituted22 1,3-diphosphacyclobutane-1,2-diones). The [Rh(Ptrop3)] fragments deviate significantly out-of-plane from the planar P2(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2 ring (Rh–P1–P1′ 120.85°) such that one fragment is located above and the other below the central ring. The sum of bond angles at P1, P1′ amount to 306.48° indicating a free electron pair at each P center. Consequently, the bond lengths between the Rh(I) center and the three-valent phosphorus center of the formally dianionic [P2(
O)2 ring (Rh–P1–P1′ 120.85°) such that one fragment is located above and the other below the central ring. The sum of bond angles at P1, P1′ amount to 306.48° indicating a free electron pair at each P center. Consequently, the bond lengths between the Rh(I) center and the three-valent phosphorus center of the formally dianionic [P2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2]2− ring are significantly longer (Rh–P1 2.426 Å) than the Rh–P2 bonds (2.172 Å), in which the penta-valent P2 center of the Ptrop3 ligand is involved.
O)2]2− ring are significantly longer (Rh–P1 2.426 Å) than the Rh–P2 bonds (2.172 Å), in which the penta-valent P2 center of the Ptrop3 ligand is involved.
The mono-anionic part of the solid state structure of Na[3], and a plot of its unique Rh2[P2(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2](PCO) core, are depicted in Fig. 1b (the [Na(MeCN)4]+ counter cation has a contact to O3 [Na1⋯O3 2.289(2) Å but is not shown for clarity)]. The anion [3]− contains both a bridging μ2-κP-[P2(C
O)2](PCO) core, are depicted in Fig. 1b (the [Na(MeCN)4]+ counter cation has a contact to O3 [Na1⋯O3 2.289(2) Å but is not shown for clarity)]. The anion [3]− contains both a bridging μ2-κP-[P2(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2]2− heterocycle and a bridging μ2-κP-P
O)2]2− heterocycle and a bridging μ2-κP-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit, which complete the distorted trigonal bipyramidal coordination spheres of each Rh(I) center. Under the assumption that both bridging ligands act as 4 electron donors, the Rh(I) centers achieve a valence electron count of 18. To our knowledge, for both coordination modes there is no precedence. The averaged Rh1/2–P1 bond lengths (2.477 Å) to the PCO ligand and the averaged Rh1/2–P2 bond lengths (2.372 Å) to the P2(C
O unit, which complete the distorted trigonal bipyramidal coordination spheres of each Rh(I) center. Under the assumption that both bridging ligands act as 4 electron donors, the Rh(I) centers achieve a valence electron count of 18. To our knowledge, for both coordination modes there is no precedence. The averaged Rh1/2–P1 bond lengths (2.477 Å) to the PCO ligand and the averaged Rh1/2–P2 bond lengths (2.372 Å) to the P2(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2 heterocycle are again longer than the Rh1–P4 (2.278 Å) and Rh2–P5 (2.281 Å) bonds to the PhPtrop2 ligand. Within the central Rh2P2 cycle, the bond angles at the bridging P centers [Rh1–P1–Rh2 100.14(3)°; Rh1–P2–Rh2 106.58(3)°] are much larger than at the Rh(I) centers [P1–Rh1–P2: 74.71(2)°; P1–Rh2–P2: 75.55(3)°]. In the 1,3-diphosphacyclobutane-2,4-dione moiety, the averaged P3–C2/C3 distances (1.79 Å) are much shorter than the ones involving the bridging P2 center (P2–C2/C3 = 1.89 Å) indicating significant π-electron delocalization over the C2–P3–C3 fragment. The μ2-κP-P
O)2 heterocycle are again longer than the Rh1–P4 (2.278 Å) and Rh2–P5 (2.281 Å) bonds to the PhPtrop2 ligand. Within the central Rh2P2 cycle, the bond angles at the bridging P centers [Rh1–P1–Rh2 100.14(3)°; Rh1–P2–Rh2 106.58(3)°] are much larger than at the Rh(I) centers [P1–Rh1–P2: 74.71(2)°; P1–Rh2–P2: 75.55(3)°]. In the 1,3-diphosphacyclobutane-2,4-dione moiety, the averaged P3–C2/C3 distances (1.79 Å) are much shorter than the ones involving the bridging P2 center (P2–C2/C3 = 1.89 Å) indicating significant π-electron delocalization over the C2–P3–C3 fragment. The μ2-κP-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit has a P1–C1 bond length of 1.657(3) Å, which is significantly longer than the related one in 2 (1.522(4) Å; compare also P–C in [Na(DME)2]2[μ2-κO-OCP]2 = 1.589(3) Å)13b or other mononuclear κP-PCO complexes (<1.64 Å).14,15 This observation is in line with the assumption that the μ2- κP-bridging [P
O unit has a P1–C1 bond length of 1.657(3) Å, which is significantly longer than the related one in 2 (1.522(4) Å; compare also P–C in [Na(DME)2]2[μ2-κO-OCP]2 = 1.589(3) Å)13b or other mononuclear κP-PCO complexes (<1.64 Å).14,15 This observation is in line with the assumption that the μ2- κP-bridging [P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]− can act as 4 electron donor ligand (visualized by the resonance form (P2− ← C
O]− can act as 4 electron donor ligand (visualized by the resonance form (P2− ← C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O+)).
O+)).
The solid state structure of [Rh2(CO)2(Ph2Ptrop)2(η2-P2)] 4 is shown in Fig. 1c. As indicated by the spectroscopic data, the CO units are not bound as PCO anymore, but have migrated to the Rh(I) centers as terminal carbonyl ligands binding in cis-positions with respect to the diphosphorus moiety. The P1–P2 bond is 2.066(2) Å long, a value that is similar to that observed in the related complex [Rh2(CO)2(μ-dppm)2(μ,η2-P2)] (2.081(7) Å; dppm![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH2(PPh2)2),33 and close to the predicted length of a P
CH2(PPh2)2),33 and close to the predicted length of a P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P double bond (∑rcov[P
P double bond (∑rcov[P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P] = 2.04 Å).31c The P1–P2 axis is the mirror plane of the complex (in accord with the equivalence of the Ph2Ptrop ligands in the NMR spectra) and almost perpendicular to the Rh1–Rh1′ axis [Rh1–P2–P1–Rh1′ torsion angle = 79.60(4)°]. The short Rh1–Rh1′ distance of 2.7606(11) Å indicates a bonding interaction between both metal centers and in combination with the Rh–P distances (2.39 Å; average) allow to describe 4 best as a Rh2P2 tetrahedron.
P] = 2.04 Å).31c The P1–P2 axis is the mirror plane of the complex (in accord with the equivalence of the Ph2Ptrop ligands in the NMR spectra) and almost perpendicular to the Rh1–Rh1′ axis [Rh1–P2–P1–Rh1′ torsion angle = 79.60(4)°]. The short Rh1–Rh1′ distance of 2.7606(11) Å indicates a bonding interaction between both metal centers and in combination with the Rh–P distances (2.39 Å; average) allow to describe 4 best as a Rh2P2 tetrahedron.
Finally, the XRD analysis of single crystals of 5 allowed the identification of a new Rh4P4 metal phosphido cluster. This is encapsulated by four bidentate Ph2Ptrop ligands and by two bridging carbonyl groups (Fig. 1d). The Rh1–Rh2 and Rh3–Rh4 bonds are 2.772 Å (average) long and again correspond to single bonds. The Rh–P distances vary over a range from 2.394 to 2.471 Å and on the average (2.43 Å) are somewhat longer than in 4. Most remarkable in the structure of 5 are the P1–P2 [2.5369(9)], P2–P3 [2.5529(9)], P3–P4 [2.5724(9) Å], and P4–P1 [2.4759(9) Å] distances, which are similar and unusually long (average: 2.53 Å). This value is significantly longer than sum of covalent radii (∑rcov[P–P] = 2.22 Å)31c but much smaller than the sum of van der Waals radii (∑rvdW[P, P] = 3.80 Å)34 and in the same range as that reported for related [(η5-Cp)4Co4P4] (avg. P–P = 2.57 Å).35 The Rh–Rh, Rh–P and P–P distances fall with the range determined for the phosphorus poor and rich binary phases RhP2 and RhP3.36 Another interesting structural feature of 5 is that the distances between the Rh center and the coordinated olefinic units of the Ph2Ptrop ligand are comparatively short (given by the average distances of the Rh centers to the centroids of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ctrop units: Rh–ct = 2.13 Å). In contrast, the C
Ctrop units: Rh–ct = 2.13 Å). In contrast, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ctrop distances relatively long (average 1.41 Å) when compared to 4 (Rh–Ct 2.194; C
Ctrop distances relatively long (average 1.41 Å) when compared to 4 (Rh–Ct 2.194; C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ctrop = 1.39 Å; cf. uncoordinated Ph2Ptrop: 1.33 Å). These data reflect a high electron density within the Rh4P4 cluster leading on one side to long Rh–P and P–P bonds but on the other to significant electron donation from filled metal orbitals into the π* orbitals of the C
Ctrop = 1.39 Å; cf. uncoordinated Ph2Ptrop: 1.33 Å). These data reflect a high electron density within the Rh4P4 cluster leading on one side to long Rh–P and P–P bonds but on the other to significant electron donation from filled metal orbitals into the π* orbitals of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ctrop units.
Ctrop units.
Analyses of the electronic structure of 4 and 5 by DFT calculations
Calculations used for the elucidation of the electronic structures of 4 (Rh2P2 cluster core) and 5 (Rh4P4 cluster core) were carried out with the ORCA program package37 and details are given in the SI. The calculated bond lengths correspond well with the experimentally determined ones (Tables S1 and S2 in the SI). The Mayer-bond order (MBOs) for the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P bond (1.39) in the tetrahedral cluster 4 shows significant multiple bond character, while the Rh–P bonds (0.68, 0.70) correspond to single bonds. A significant bonding interaction between the rhodium centers is indicated by MBORh–Rh = 0.38 (see Table S1 for a listing of the MBOs of 4). Intrinsic bond orbitals (IBOs) have been successfully used to analyze the electronic structure of cluster compounds,38 and for 4 six fully occupied orbitals which account for bonding within the Rh2P2 unit are obtained (Fig. 2).
P bond (1.39) in the tetrahedral cluster 4 shows significant multiple bond character, while the Rh–P bonds (0.68, 0.70) correspond to single bonds. A significant bonding interaction between the rhodium centers is indicated by MBORh–Rh = 0.38 (see Table S1 for a listing of the MBOs of 4). Intrinsic bond orbitals (IBOs) have been successfully used to analyze the electronic structure of cluster compounds,38 and for 4 six fully occupied orbitals which account for bonding within the Rh2P2 unit are obtained (Fig. 2).
Orbital number 102 represents the σ-bond between the P–P atoms, while orbital no. 202 corresponds mainly to a π-bond between these atoms. This orbital also shows contributions from each rhodium center of 8%, thus accounting for Rh–P–P–Rh multicenter bonding. Orbitals no. 100 and 140 represent the backdonation from a filled d-orbital at Rh into an empty p orbital at one P center (composition: 68% Rh, 22% P). Finally, orbitals no. 194 and 195 describe the same Rh → P backdonation but this time towards the other P atom (main contributions: 57% Rh, 18% P). Additionally, both of these orbitals show some direct Rh–Rh bonding interactions. In summary, this analysis with IBOs allows to describe 4 as a cluster composed from a dicationic [P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P]2+ fragment coordinated to two Rh−1 centers, which strongly donate electron density into this unit.
P]2+ fragment coordinated to two Rh−1 centers, which strongly donate electron density into this unit.
A related analysis of the electronic structure of 5 with its Rh4P4 core of 5 shows that according to the MBOs (0.46, 0.50), all P atoms within the P4 subunit have substantial bonding interactions but a simple polyphosphide [P4]4− chain can be excluded (MBOs close to 1 would be expected). Likewise, the MBOs for the Rh–Rh and Rh–P interactions lie within a range of 0.43 to 0.68, which in summary indicate the presence of highly delocalized multi-center bonds between all atoms of the eight-membered cluster (see Tables S2–S4 for a listing of the MBOs).
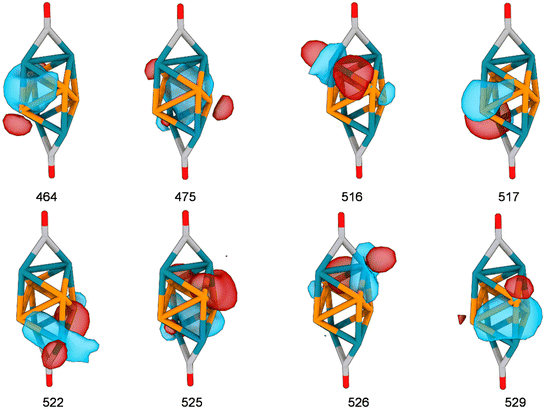
The number of doubly occupied bonding orbitals within the Rh4P4 unit was again determined by examining the intrinsic bond orbitals (IBO). In total, eight orbitals and thus 16 electrons were found which account for bonding within the Rh4P4 unit which are shown in Fig. 3.
For both, 4 and 5, the electronic structure was further studied using a QTAIM analysis as implemented in the program MultiWFN.39 In the case of the tetrahedral cluster 4, six bond critical points within the Rh2P2 unit were located, accounting for P–P, Rh–Rh and P–Rh bonding. Commonly analysed properties at those bond critical points are summarized in Table 1. According to established criteria (high electron density, negative Laplacian, magnitude of potential energy density significantly higher than kinetic energy density), the P–P interaction in 4 can be described as covalent bonding, whereas the properties at bond critical points of the Rh–P bonds indicate dative bonding (low electron density, positive Laplacian, magnitude of potential energy density similar to kinetic energy density). Thus, the QTAIM analysis supports the description of 4 as construct between a [P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P]2+ unit and two [Rh−1(CO)(Ph2Ptrop)] fragments as derived from the IBO analysis. In case of the eight vertices Rh4P4 cluster, 18 bond critical points were found: 4 accounting for P–P bonding, 12 accounting for Rh–P bonding and 2 accounting for Rh–Rh bonding. The descriptors given in Table 1 indicate that all bonding interactions are relatively weak and dative in nature, substantiating the description of the Rh4P4 core as a cluster with a highly delocalized electronic structure.
P]2+ unit and two [Rh−1(CO)(Ph2Ptrop)] fragments as derived from the IBO analysis. In case of the eight vertices Rh4P4 cluster, 18 bond critical points were found: 4 accounting for P–P bonding, 12 accounting for Rh–P bonding and 2 accounting for Rh–Rh bonding. The descriptors given in Table 1 indicate that all bonding interactions are relatively weak and dative in nature, substantiating the description of the Rh4P4 core as a cluster with a highly delocalized electronic structure.
| ρ | ∇ρ | G | V | H | |
|---|---|---|---|---|---|
| 4 (Rh2P2) | |||||
| P–P | 0.126 | −0.134 | 0.0428 | −0.119 | −0.0763 |
| Rh–Rh | 0.0483 | 0.0709 | 0.0311 | −0.0445 | −0.0134 |
| P–Rh | 0.080 | 0.0721 | 0.0467 | −0.0753 | −0.0286 |
| 5 (Rh4P4) | |||||
| P–P | 0.0538 | 0.0414 | 0.0202 | −0.0300 | −0.0098 |
| Rh–Rh | 0.0487 | 0.1100 | 0.0368 | −0.0461 | −0.0093 |
| P–Rh | 0.0766 | 0.0758 | 0.0446 | −0.0702 | −0.0256 |
Conclusions
In this paper we show that molecular transition metal phosphido (TMP) clusters can be obtained using sodium phosphaethynolate, Na[OCP], as “P−” transfer reagent. This approach may be especially interesting for the synthesis of clusters with late transition metal centers and metal to phosphorus ratios which are not easily accessible with protocols borrowed from solid state syntheses. The metal precursor complex is decisive for a successful TMP synthesis: (i) Results to date imply that precursor complexes, which are too strongly oxidizing must be avoided. Otherwise [OCP]− serves as reductant15 and not as nucleophile. (ii) The experiments with electronically saturated metal complexes – such as those with the fragments [Rh(Ptrop3)] or [Rh(PhPtrop2)] containing a tetradentate or tridentate ligand – suggest that these may lead to stable M(PCO) complexes but a migration of the CO unit requires an open coordination site at the metal. Bonding analyses of the small cluster 4 containing a tetrahedral Rh2P2 core and the unprecedented eight-vertices Rh4P4 cluster 5 show that the breadth of possible electronic structures observed for binary rhodium phosphor phases is reflected by molecular RhnPm clusters. The cluster [Rh2(CO)2(Ph2Ptrop)2(η2-P2)] 4 obeys the electron counting rules for electron precise clusters and hence 4 is an analogue of tetrahedran, C4H4 [that is b = ½(18m − 8n) − VEC with m = number of transition metal centers, n = number of main group element centers, VEC = valence electron count, b = number of bonds with the m + n cluster necessary to achieve an 18 valence electron count at each transition or 8 valence electron count at each main group element center. For 4 this results in: VEC = (2 × 9 Rh) + (2 × 2 CO) + (2 × 4 Ph2Ptrop) + (2 × 5 P) = 40; 18m + 8n = 52; b = ½(52–40) = 6]. On the other hand, cluster 5 with its Rh4P4 core held together by a total of eight bonding orbitals can be classified as a hypercloso cluster related to the archetype B8Cl8.40Author contributions
J. E. Borger carried out most of the experimental work. Z. Li and P. Coburger carried out the computational studies. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
Supplementary information: experimental details, NMR spectra, computational details, single-crystal X-ray structure determinations. See DOI: https://doi.org/10.1039/d5dt02274f.CCDC 1827127 (1*), 1827128 (2), 1827129 (4), 1827130 (3), and 1827133 (5) contain the supplementary crystallographic data for this paper.41a–e
Acknowledgements
This work was supported by the Swiss National Science Foundation (SNF 2-77199-18) and the ETH Zürich (ETH-36 17-2).References
- (a) B. Owens-Baird, Y. V. Kolen'ko and K. Kovnir, Chem. – Eur. J., 2018, 24, 7298–7311 CrossRef CAS PubMed; (b) P. W. Menezes, A. Indra, C. Das, C. Walter, C. Göbel, V. Gutkin, D. Schmeiβer and M. Driess, ACS Catal., 2017, 7, 103–109 CrossRef CAS; (c) M. Sun, H. Liu, J. Qu and J. Li, Adv. Energy Mater., 2016, 6, 1600087 CrossRef; (d) Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC; (e) S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6, 8069–8097 CrossRef CAS; (f) P. C. K. Vesborg, B. Seger and I. Chorkendorff, J. Phys. Chem. Lett., 2015, 6, 951–957 CrossRef CAS PubMed.
- (a) S. E. Habas, F. G. Baddour, D. A. Ruddy, C. P. Nash, J. Wang, M. Pan, J. E. Hensley and J. A. Schaidle, Chem. Mater., 2015, 27, 7580–7592 CrossRef CAS; (b) M. B. Griffin, F. G. Baddour, S. E. Habas, C. P. Nash, D. A. Ruddy and J. A. Schaidle, Catal. Sci. Technol., 2017, 7, 2954–2966 RSC.
- (a) S. Carenco, D. Portehault, C. Boissière, N. Mézailles and C. Sanchez, Chem. Rev., 2013, 113, 7981–8065 CrossRef CAS PubMed; (b) S. Carenco, D. Portehault, C. Boissière, N. Mézailles and C. Sanchez, Adv. Mater., 2014, 26, 371–390 CrossRef CAS PubMed; (c) J.-H. Chen and K. H. Whitmire, Coord. Chem. Rev., 2018, 355, 271–327 CrossRef CAS.
- (a) C. Panda, P. W. Menezes and M. Driess, Angew. Chem., Int. Ed., 2018, 57, 11130–11139 CrossRef CAS PubMed; (b) S. Yao, V. Forstner, P. W. Menezes, C. Panda, S. Mebs, E. M. Zolnhofer, M. E. Miehlich, T. Szilvási, N. Ashok Kumar, M. Haumann, K. Meyer, H. Grützmacher and M. Driess, Chem. Sci., 2018, 9, 8590–8597 RSC.
- (a) B. M. Cossairt, N. A. Piro and C. C. Cummins, Chem. Rev., 2010, 110, 4164–4177 CrossRef CAS PubMed; (b) M. Caporali, L. Gonsalvi, A. Rossin and M. Peruzzini, Chem. Rev., 2010, 110, 4178–4235 CrossRef CAS PubMed; (c) C. M. Hoidn, D. J. Scott and R. Wolf, Chem. – Eur. J., 2021, 27, 1886–1902 CrossRef CAS PubMed.
- (a) S. Rundqvist, Nature, 1960, 185, 31–32 CrossRef; (b) F.-E. Faller, E. F. Strotzer and W. Biltz, Z. Anorg. Allg. Chem., 1940, 244, 317–328 CrossRef CAS.
- (a) C. Xu, Q. Wang, R. Ding, Y. Wang, Y. Zhang and G. Fan, Appl. Surf. Sci., 2019, 489, 796–801 CrossRef CAS; (b) J. Kim, H. Kim and S. H. Ahn, ACS Sustainable Chem. Eng., 2019, 7, 14041–14050 CrossRef CAS; (c) F. Yang, X. Bao, D. Gong, L. Su, G. Cheng, S. Chen and W. Luo, ChemElectroChem, 2019, 6, 1990–1995 CrossRef CAS; (d) J.-Q. Chi, X.-J. Zeng, X. Shang, B. Dong, Y.-M. Chai, C.-G. Liu, M. Marin and Y. Yin, Adv. Funct. Mater., 2019, 29, 1901790 CrossRef; (e) H. Xin, L. Sun, Y. Zhao, Y. Lv, Q. Luo, S. Guo, D. Li, C. Mu, B. Huang and F. Ma, Chem. Eng. J., 2023, 466, 143277 CrossRef CAS; (f) B. Guo, J. Zhao, Y. Xu, X. Wen, X. Ren, X. Huang, S. Niu, Y. Dai, R. Gao, P. Xu and S. Li, ACS Appl. Mater. Interfaces, 2024, 16, 8939–8948 CrossRef CAS PubMed.
- Z. Chen, Y. Bu, L. Wang, X. Wang and J.-P. Ao, Appl. Catal., B, 2020, 274, 119117 CrossRef CAS.
- F. Luo, Y. Yu, X. Long, C. Li, T. Xiong and Z. Yang, J. Colloid Interface Sci., 2024, 656, 450–456 CrossRef CAS PubMed.
- (a) J. R. Hayes, R. H. Bowker, A. F. Gaudette, M. C. Smith, C. E. Moak, C. Y. Nam, T. K. Pratum and M. E. Bussell, J. Catal., 2010, 276, 249–258 CrossRef CAS; (b) Y. Kanda, K. Kawanishi, T. Tsujino, A. M. Al-otaibi and Y. Uemichi, Catalysts, 2018, 8, 160 CrossRef; (c) Y. Kanda, R. Saito, T. Ono, K. Kon, T. Toyao, S. Furukawa, Y. Obora and K.-i. Shimizu, J. Catal., 2022, 408, 294–302 CrossRef CAS.
- C. Galdeano-Ruano, C. W. Lopes, D. Motta Meira, A. Corma and P. Oña-Burgos, ACS Appl. Nano Mater., 2021, 4, 10743–10753 CrossRef CAS.
- C. Lin, J. Zhou, Z. Zheng and J. Chen, J. Catal., 2022, 410, 164–179 CrossRef CAS.
- (a) G. Becker, W. Schwarz, N. Seidler and M. Westerhausen, Z. Anorg. Allg. Chem., 1992, 612, 72–82 CrossRef CAS; (b) F. F. Puschmann, D. Stein, D. Heift, C. Hendriksen, Z. A. Gal, H.-F. Grützmacher and H. Grützmacher, Angew. Chem., Int. Ed., 2011, 50, 8420–8423 CrossRef CAS PubMed; (c) D. Heift, Z. Benkő and H. Grützmacher, Dalton Trans., 2014, 43, 831–840 RSC; (d) R. Suter, Z. Benkő, M. Bispinghoff and H. Grützmacher, Angew. Chem., Int. Ed., 2017, 56, 11226–11231 CrossRef CAS PubMed; (e) I. Krummenacher and C. C. Cummins, Polyhedron, 2012, 32, 10–13 CrossRef CAS; (f) A. R. Jupp and J. M. Goicoechea, Angew. Chem., Int. Ed., 2013, 52, 10064–10067 CrossRef CAS PubMed.
- (a) J. M. Goicoechea and H. Grützmacher, Angew. Chem., Int. Ed., 2018, 57, 16968–16994 CrossRef CAS PubMed; (b) L. Weber, Eur. J. Inorg. Chem., 2018, 2018, 2175–2227 CrossRef CAS.
- (a) D. Heift, Z. Benkő and H. Grützmacher, Chem. – Eur. J., 2014, 20, 11326–11330 CrossRef CAS PubMed; (b) A. M. Tondreau, Z. Benkő, J. R. Harmer and H. Grützmacher, Chem. Sci., 2014, 5, 1545–1554 RSC; (c) D. Heift, Z. Benkő, H. Grützmacher, A. R. Jupp and J. M. Goicoechea, Chem. Sci., 2015, 6, 4017–4024 RSC; (d) T. P. Robinson, M. J. Cowley, D. Scheschkewitz and J. M. Goicoechea, Angew. Chem., Int. Ed., 2015, 54, 683–686 CrossRef CAS PubMed; (e) N. Del Rio, A. Baceiredo, N. Saffon-Merceron, D. Hashizume, D. Lutters, T. Müller and T. Kato, Angew. Chem., Int. Ed., 2016, 55, 4753–4758 CrossRef CAS PubMed; (f) M. M. Hansmann and G. Bertrand, J. Am. Chem. Soc., 2016, 138, 15885–15888 CrossRef CAS PubMed; (g) Z. Li, X. Chen, Y. Li, C.-Y. Su and H. Grützmacher, Chem. Commun., 2016, 52, 11343–11346 RSC; (h) L. Liu, D. A. Ruiz, D. Munz and G. Bertrand, Chem, 2016, 1, 147–153 CrossRef CAS; (i) S. Yao, Y. Xiong, T. Szilvási, H. Grützmacher and M. Driess, Angew. Chem., Int. Ed., 2016, 55, 4781–4785 CrossRef CAS PubMed; (j) T. Krachko, A. W. Ehlers, M. Nieger, M. Lutz and J. C. Slootweg, Angew. Chem., Int. Ed., 2018, 57, 1683–1687 CrossRef CAS PubMed; (k) D. W. N. Wilson, A. Hinz and J. M. Goicoechea, Angew. Chem., Int. Ed., 2018, 57, 2188–2193 CrossRef CAS PubMed; (l) T. G. Saint-Denis, T. A. Wheeler, Q. Chen, G. Balázs, N. S. Settineri, M. Scheer and T. D. Tilley, J. Am. Chem. Soc., 2024, 146, 4369–4374 CrossRef CAS PubMed.
- S. Alidori, D. Heift, G. Santiso-Quinones, Z. Benkő, H. Grützmacher, M. Caporali, L. Gonsalvi, A. Rossin and M. Peruzzini, Chem. – Eur. J., 2012, 18, 14805–14811 CrossRef CAS PubMed.
- L. L. Liu, D. A. Ruiz, F. Dahcheh, G. Bertrand, R. Suter, A. M. Tondreau and H. Grützmacher, Chem. Sci., 2016, 7, 2335–2341 RSC.
- M. M. D. Roy, M. J. Ferguson, R. McDonald and E. Rivard, Chem. Commun., 2018, 54, 483–486 RSC.
- (a) C. J. Hoerger, F. W. Heinemann, E. Louyriac, L. Maron, H. Grützmacher and K. Meyer, Organometallics, 2017, 36, 4351–4354 CrossRef CAS; (b) C. Camp, N. Settineri, J. Lefèvre, A. R. Jupp, J. M. Goicoechea, L. Maron and J. Arnold, Chem. Sci., 2015, 6, 6379–6384 RSC.
- L. N. Grant, B. Pinter, B. C. Manor, H. Grützmacher and D. J. Mindiola, Angew. Chem., Int. Ed., 2018, 57, 1049–1052 CrossRef CAS PubMed.
- S. Bestgen, Q. Chen, N. H. Rees and J. M. Goicoechea, Dalton Trans., 2018, 47, 13016–13024 RSC.
- L. Weber, B. Torwiehe, G. Bassmann, H.-G. Stammler and B. Neumann, Organometallics, 1996, 15, 128–132 CrossRef CAS.
- W. Lü, C. Wang, Q. Luo, Q.-s. Li, Y. Xie, R. B. King and H. F. Schaefer III, New J. Chem., 2015, 39, 1390–1403 RSC.
- L. N. Grant, B. Pinter, B. C. Manor, R. Suter, H. Grützmacher and D. J. Mindiola, Chem. – Eur. J., 2017, 23, 6272–6276 CrossRef CAS PubMed.
- J. Sun, H. Verplancke, J. I. Schweizer, M. Diefenbach, C. Würtele, M. Otte, I. Tkach, C. Herwig, C. Limberg, S. Demeshko, M. C. Holthausen and S. Schneider, Chem, 2021, 7, 1952–1962 CAS.
- J. Du, D. Hunger, J. A. Seed, J. D. Cryer, D. M. King, A. J. Wooles, J. van Slageren and S. T. Liddle, J. Am. Chem. Soc., 2021, 143, 5343–5348 CrossRef CAS PubMed.
- G. Hierlmeier, A. Hinz, R. Wolf and J. M. Goicoechea, Angew. Chem., Int. Ed., 2018, 57, 431–436 CrossRef CAS PubMed.
- M. Joost, W. J. Transue and C. C. Cummins, Chem. Commun., 2017, 53, 10731–10733 RSC.
- (a) T. Zweifel, J.-V. Naubron, T. Büttner, T. Ott and H. Grützmacher, Angew. Chem., Int. Ed., 2008, 47, 3245–3249 CrossRef CAS PubMed; (b) U. Fischbach, H. Rüegger and H. Grützmacher, Eur. J. Inorg. Chem., 2007, 2007, 2654–2667 CrossRef; (c) F. F. Puschmann, J. Harmer, D. Stein, H. Rüegger, B. de Bruin and H. Grützmacher, Angew. Chem., Int. Ed., 2010, 49, 385–389 CrossRef CAS PubMed; (d) H. Schönberg, S. Boulmaâz, M. Wörle, L. Liesum, A. Schweiger and H. Grützmacher, Angew. Chem., Int. Ed., 1998, 37, 1423–1426 CrossRef.
- M. J. Frisch, et al., Gaussian 16, Revision C.01, 2016 Search PubMed.
- (a) F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19 RSC; (b) P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 186–197 CrossRef PubMed; (c) P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 12770–12779 CrossRef PubMed.
- K. M. Szkop, A. R. Jupp, R. Suter, H. Grützmacher and D. W. Stephan, Angew. Chem., Int. Ed., 2017, 56, 14174–14177 CrossRef CAS PubMed.
- M. Caporali, L. Gonsalvi, V. Mirabello, A. Ienco, G. Manca, F. Zanobini and M. Peruzzini, Eur. J. Inorg. Chem., 2014, 2014, 1652–1659 CrossRef CAS.
- S. Alvarez, Dalton Trans., 2013, 42, 8617–8636 RSC.
- G. L. Simon and L. F. Dahl, J. Am. Chem. Soc., 1973, 95, 2175–2183 CrossRef CAS.
- (a) M. Zumbusch, Z. Anorg. Allg. Chem., 1940, 243, 322–329 CrossRef CAS; (b) E. H. El Ghadraoui, R. Guerin and M. Sergent, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1983, 39, 1493–1494 CrossRef.
- (a) F. Neese, F. Wennmohs, U. Becker and C. Riplinger, J. Chem. Phys., 2020, 152, 224108 CrossRef CAS PubMed; (b) F. Neese, Wiley Interdiscip. Rev.:Comput. Mol. Sci., 2018, 8, e1327 Search PubMed.
- (a) G. Knizia, J. Chem. Theory Comput., 2013, 9, 4834–4843 CrossRef CAS PubMed; (b) W. Sun, X. Xia, C. Lu, X. Kuang and A. Hermann, Phys. Chem. Chem. Phys., 2018, 20, 23740–23746 RSC; (c) J. E. M. N. Klein, R. W. A. Havenith and G. Knizia, Chem. – Eur. J., 2018, 24, 12340–12345 CrossRef CAS PubMed; (d) M. T. Scharnhölz, P. Coburger, L. Gravogl, D. Klose, J. J. Gamboa-Carballo, G. Le Corre, J. Bösken, C. Schweinzer, D. Thöny, Z. Li, K. Meyer and H. Grützmacher, Angew. Chem., Int. Ed., 2022, 61, e202205371 CrossRef PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- W. Einholz, M. Hofmann, R. Schäfer, C. Wieloch, W. Keller, M. Ströbele and H.-J. Meyer, Eur. J. Inorg. Chem., 2021, 2021, 5037–5044 CrossRef CAS.
- (a) CCDC 1827127: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc1zb8l5; (b) CCDC 1827128: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc1zb8m6; (c) CCDC 1827129: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc1zb8n7; (d) CCDC 1827130: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc1zb8p8; (e) CCDC 1827133: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc1zb8sc.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |