Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A tetradentate benzannulated P^N^N^P ligand and its Fe(II) complex: synthesis, characterization, and electrocatalytic hydrogen evolution
Baldeep K.
Sidhu
 ,
Amelia
Kacperkiewicz
,
Amelia
Kacperkiewicz
 ,
Jason D.
Braun
,
Jason D.
Braun
 and
David E.
Herbert
and
David E.
Herbert
 *
*
Department of Chemistry and the Manitoba Institute for Materials, University of Manitoba, 144 Dysart Road, Winnipeg, Manitoba, R3T 2N2, Canada. E-mail: david.herbert@umanitoba.ca
First published on 30th October 2025
Abstract
Iron complexes supported by phosphine and pyridine-based ligands are widely seen as efficient molecular catalysts for catalytic transformations, but examples of these complexes as molecular electrocatalysts for the hydrogen evolution reaction (HER) are limited. In this work, we present a ligand design based on a 6,6′-fused dimer of a 2,4-substituted (phosphino)phenanthridine (benzo[c]quinoline). Starting from the monomeric P^N precursor, we show how the P^N^N^P dimer can be selectively formed and report the synthesis and characterization of an Fe(II) complex of this unique scaffold. We demonstrate the potential of such frameworks in the electrocatalytic reduction of Brønsted acids in acetonitrile solution.
Introduction
Hybrid P^N ligands containing both soft phosphorus and hard nitrogen donors are widespread in organometallic chemistry, finding utility in catalytic hydrogenation,1,2 asymmetric transformations,3 cross-coupling reactions,4,5 and more. Higher denticity analogs are also gaining in popularity. Examples include tridentate P^N^P ‘pincer’ ligands6 and tetradentate P2N2 ligands based on salen7 or more flexible8–10 scaffolds (Fig. 1). The latter category is dominated by 1,5-diaza-3,7-diphosphacyclooctanes (Fig. 1a), first popularized for their use in bio-inspired electrocatalysis10 and now expanding into areas including acceptorless dehydrogenation11 and cross-coupling.12 Coordination complexes of 1,5-diaza-3,7-diphosphacyclooctanes largely present κ2-diphosphine coordination, with the P2N2 ligand binding solely through the phosphine donor sites. This allows for the uncoordinated tertiary amino groups to act as biomimetic proton relays in electrochemical hydrogen evolution reactivity (HER), similar to the proposed role of the bridgehead nitrogen present in the bridging dithiolate of [FeFe]-hydrogenase.13 Pendant bases are not, however, required for efficient electrocatalysis. Monometallic 3d metal complexes with polypyridine and/or phosphine ligands also efficiently catalyze the reduction of protons to H2.14 Porphyrins,15 amine-bis(phenolates),16 fluorinated diglyoxime ligands,17 and multidentate polypyridyl frameworks18–20 are all capable of supporting HER-active Fe centres. | ||
| Fig. 1 Selected examples of P2N2 ligands: (a) flexible, macrocyclic P^P chelating diphosphine ligands with pendant amines;10 (b) rigid salen-type P^N^N^P chelates;7 (c) flexible P^N^N^P scaffolds;8,9 (d) the benzannulated tetradentate P^N^N^P framework reported in this work. | ||
Our longstanding interest in ligands bearing benzannulated N-heterocylic donors based on phenanthridine (benzo[c]quinoline) moieties21 has recently extended to bipyridine analogs such as 6,6′-biphenanthridine (biphe)22 and its planar, doubly-fused congener 6,6′,7,7′-biphenanthridine (p-biphe).23 These motifs combine the benzannulation seen in 2,2′-biquinoline with that of 1,1′-biisoquinoline. In this work, we present a strategy for the synthesis of a substituted phosphine-decorated 6,6′-biphenanthridine, starting from its monomeric precursor.24 We strategized that the large π-extended biphenanthridine core might stabilize a reduced state and lower the overpotential for HER.25 We use this tetradentate P^N^N^P ligand, with two phosphine donor arms and two phenanthridine donor arms, to form a six-coordinate Fe(II) coordination complex bearing two axial acetonitrile units. The utility of this species in electrocatalytic HER activity is described.
Results and discussion
We previously described the synthesis of biphe via nickel(0)-mediated homocoupling of (6-trifluoromethanesulfonato)phenanthridine.22 Biphe can chelate transition metal ions via κ2-N^N coordination. To augment the binding ability of biphe we envisioned accessing a phosphine-substituted derivative capable of tetradentate P^N^N^P coordination via reductive dimerization of (4-diphenylphosphino-2-methyl)phenanthridine (L, Fig. 2). We have reported the preparation of the parent (4-diphenylphosphino)phenanthridine24 but find in general installing a substituent at the 2-position smooths the preparation of the (4-bromo)phenanthridine precursor needed for preparation of the phosphine.26 (4-Diphenylphosphino-2-methyl)phenanthridine has been used to support orange emissive halide-bridged Cu(I) dimers,27,28 tetrahedral and trigonal bipyramidal Fe(II) as well as pseudo-octahedral Fe(II) and Ru(II) complexes,29 along with a Ru(II)-based catalyst useful for acceptorless dehydrogenative couplings30–32 and a unique tandem hydrogen-borrowing dehydrogenative coupling/hydrodefluorination system.33Phenanthridine can be electrochemically hydrogenated to dihydrophenanthridine using an applied potential in the presence of weak Brønsted acids.34,35 Accordingly, we reasoned that with a strong enough reductant, we might be able to form a radical at the 6-position of L (see Fig. 2a for the IUPAC numbering scheme for phenanthridines) and facilitate reductive dimerization. The LUMOs of phenanthridines, in general, are localized at the π*(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) sub-unit.21 Should dimerization occur, addition of water to the reaction mixture was anticipated to lead to the formation of 5,5′,6,6′-tetrahydro-6,6′-bis((4-diphenylphosphino-2-methyl)phenanthridinyl) ([L^L]-2H2) which might then be oxidized to L^L. In our first successful attempt, L was reacted with an excess of sodium-mercury amalgam (5% Na in Hg) at 110 °C in toluene for 48 h. The reaction mixture initially turned from yellow to dark brown, then gradually to dark blue, and finally to olive-green. These vibrant colours are consistent with the expected radical mechanism. Quenching the reaction mixture with excess water, however, largely produced L^L with no evidence of [L^L]-2H2. 31P{1H} NMR spectroscopic analysis showed 44% conversion to L^L, minor amounts of the hydrogenated monomer 5,6-dihydro(4-diphenylphosphino-2-methyl)phenanthridine (L-H2), 5,6-dihydro-bis((4-diphenylphosphino-2-methyl)phenanthridyl) ([L^L]-H2), and some unreacted starting material (Fig. S1). In searching for optimal conditions for the formation of L^L, we varied the nature and equivalents of the reductant, reaction duration, and amount of water added to quench the reaction (Table S1). In the end, heating L in the presence of two equivalents of elemental sodium in refluxing toluene over 4 d yielded appreciable amounts of the target compound (Fig. 2a). The results of these trials led us to propose an alternate mechanism wherein direct elimination of sodium hydride from the dimerized disodium salt intermediate at high temperature directly forms L^L (Fig. 2b).36
N) sub-unit.21 Should dimerization occur, addition of water to the reaction mixture was anticipated to lead to the formation of 5,5′,6,6′-tetrahydro-6,6′-bis((4-diphenylphosphino-2-methyl)phenanthridinyl) ([L^L]-2H2) which might then be oxidized to L^L. In our first successful attempt, L was reacted with an excess of sodium-mercury amalgam (5% Na in Hg) at 110 °C in toluene for 48 h. The reaction mixture initially turned from yellow to dark brown, then gradually to dark blue, and finally to olive-green. These vibrant colours are consistent with the expected radical mechanism. Quenching the reaction mixture with excess water, however, largely produced L^L with no evidence of [L^L]-2H2. 31P{1H} NMR spectroscopic analysis showed 44% conversion to L^L, minor amounts of the hydrogenated monomer 5,6-dihydro(4-diphenylphosphino-2-methyl)phenanthridine (L-H2), 5,6-dihydro-bis((4-diphenylphosphino-2-methyl)phenanthridyl) ([L^L]-H2), and some unreacted starting material (Fig. S1). In searching for optimal conditions for the formation of L^L, we varied the nature and equivalents of the reductant, reaction duration, and amount of water added to quench the reaction (Table S1). In the end, heating L in the presence of two equivalents of elemental sodium in refluxing toluene over 4 d yielded appreciable amounts of the target compound (Fig. 2a). The results of these trials led us to propose an alternate mechanism wherein direct elimination of sodium hydride from the dimerized disodium salt intermediate at high temperature directly forms L^L (Fig. 2b).36
The formation of the hydrogenated monomer L-H2 and partially hydrogenated [L^L]-H2 likely arises from the presence of NaH in the reaction mixture, which can react with water to produce H2 gas or directly with L in the presence of water as a proton source.37 Indeed, higher amounts of L-H2 and [L^L]-H2 were observed when excess water was used to quench the unreacted sodium. Elimination of sodium hydride to make L^L is more favourable than formation of [L^L]-2H2 as NaH elimination restores aromaticity, which provides a driving force for the elimination. Accordingly, we hypothesized that filtering the reaction mixture before adding water would separate insoluble, solid NaH and prevent hydrogenation of the unreacted monomer or the dimer (Run 8, Table S1). Gratifyingly, adding a filtration step avoided all formation of hydrogenated side products. Our optimal workup excludes the water addition step altogether: the precipitate formed in the reaction mixture (presumed to be NaH) and any unreacted Na are separated by filtration and quenched with water separately, with no water added to the filtrate. In this way, L^L can be isolated in 70% yield as a light yellow solid following washing with minimal amounts of toluene to remove unreacted starting material.
1H NMR analysis showed the two phenanthridine units to be chemically equivalent, with loss of the diagnostic imine proton at the 6-position which typically appears up as a downfield singlet at δ > 9 ppm. Compared with the monomer L, signals of the two aromatic hydrogen nuclei at the 7- and 8-positions are significantly shifted upfield. Single crystals of quality suitable for X-ray diffraction experiments could be obtained by layering pentane over a concentrated dichloromethane solution of L^L (Fig. 3). The bond distances and bond angles in the dimer are generally similar to the P^N precursor itself.24 The newly formed C1–C27 bond has a bond distance of 1.500(4) Å, typical of a N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N sub-unit. The C1–N1 [1.303(4) Å] and C27–N2 [1.306(4) Å] distances are consistent with the C
N sub-unit. The C1–N1 [1.303(4) Å] and C27–N2 [1.306(4) Å] distances are consistent with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N character, in line with the ‘imine-bridged biphenyl’ character of phenanthridines in general.21 These distances, and the C9–N1–C1 [119.4(3)°] and C27–N2–C35 [118.9(2)°] bond angles, further confirm the L^L structural assignment and help rule out the formation of a hydrogenated variant. The N1–C1–C27–N2 (122.23°) torsion angle indicates a break in conjugation between the two phenanthridine units.
N character, in line with the ‘imine-bridged biphenyl’ character of phenanthridines in general.21 These distances, and the C9–N1–C1 [119.4(3)°] and C27–N2–C35 [118.9(2)°] bond angles, further confirm the L^L structural assignment and help rule out the formation of a hydrogenated variant. The N1–C1–C27–N2 (122.23°) torsion angle indicates a break in conjugation between the two phenanthridine units.
Mixing a CH2Cl2 solution of L^L with an acetonitrile solution of Fe(OTf)2 produced a deep green product (Fig. 4a). Note: it is important to keep the reaction mixture in the dark to avoid premature product decomposition. Multinuclear NMR spectroscopy and single crystal X-ray diffraction analysis of crystals grown via slow mixing of a layer of diethyl ether with a concentrated acetonitrile solution at −25 °C revealed the formation of a pseudo-octahedral Fe(II) complex with the imine and phosphine arms of L^L occupying equatorial positions, two acetonitrile units bound axially, and two outer-sphere trifluoromethanesulfonate (triflate) counterions (Fig. 4b). To relieve the steric clashing between C3–H and C29–H, the coordinated ligand bends such that the phenanthridinyl units lie at a 35° angle. This steric clashing is the likely origin of the limited photostability of this compound (vide infra).
To gain insight into the electronic structure of the complex, a UV-vis absorption spectrum in acetonitrile was collected and TD-DFT calculations were performed on the optimized solution-state geometry. An overlay of the experimental and TD-DFT simulated spectra is shown in Fig. 5, with the absorption spectrum in units of molar extinction coefficients shown in Fig. S2. The absorption spectrum shows multiple overlapping bands in the UV region, a strong absorption feature at 420 nm with a broad tail extending up to 500 nm, and a broad absorption centered at 600 nm. TD-DFT computed vertical excitations reveal that the broad lowest energy absorption band at 600 nm (ε = 1600 M−1 cm−1) has metal-to-ligand charge transfer (MLCT) character, with electron density transiting from an Fe localized t2g-type orbital to the vacant, localized C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N π* orbitals of both phenanthridinyl units of the ligand, consistent with general acceptor character of phenanthridine-based ligands.21 The higher energy transitions at 420–460 nm (ε ∼7000 M−1 cm−1) present partial MLCT character with significant contributions from L^L localized, intra-ligand charge-transfer (ILCT) character. As we progress into the UV region, metal contribution to the vertical excitations decreases and the transitions are predominantly ILCT. Compound 1 is light sensitive and slowly degrades over days when consistently exposed to visible light but is stable when stored in the dark, even in air-saturated solutions (see Fig. S3 for details).
N π* orbitals of both phenanthridinyl units of the ligand, consistent with general acceptor character of phenanthridine-based ligands.21 The higher energy transitions at 420–460 nm (ε ∼7000 M−1 cm−1) present partial MLCT character with significant contributions from L^L localized, intra-ligand charge-transfer (ILCT) character. As we progress into the UV region, metal contribution to the vertical excitations decreases and the transitions are predominantly ILCT. Compound 1 is light sensitive and slowly degrades over days when consistently exposed to visible light but is stable when stored in the dark, even in air-saturated solutions (see Fig. S3 for details).
Cyclic voltammograms of 1 show two reversible reduction events at −1.04 V and −1.33 V, as well as an irreversible reduction at −2.15 V vs. ferrocene/ferrocenium (FcH0/+). We attribute the first reduction event to the FeII/FeI redox couple. The second reduction likely corresponds to the FeI/Fe0 redox couple, with the possibility of ligand participation. Reduced metal complexes of electron-deficient bipyridine-based ligands have been seen to present ligand-reduced character.38 Based on previously studied complexes of the monomeric ligand L29 and related phenanthridine-containing ligands,39,40 we assign the irreversible reduction at much more negative potentials to a wholly ligand-centered reduction. On the oxidative side, the FeIII/FeII redox couple at −0.35 V and another quasi-reversible oxidation at 0.99 V are also observed. Cyclic voltammograms in the presence of trifluoroacetic acid (TFA) show a current enhancement at the second reduction event (−1.33 V). Both the irreversibility of the anodic wave and the cathodic current response grow with increasing concentration of acid, typical of a catalytic wave. In comparison, there are no changes to the first reduction event (Fig. S4). The direct reduction of trifluoroacetic acid on glassy carbon electrodes is known to occur at −1.81 V vs. FcH0/+.41 The current enhancement observed here can therefore be attributed to catalytic hydrogen evolution by 1.
Since the axial ligand positions in 1 are occupied by what we assume to be labile acetonitrile solvent molecules, these coordination sites can likely be made available for the binding of substrates such as H+. The mechanism of catalytic HER by transition metal complexes generally involves formation of metal-hydride intermediates.14 M–H formation can occur via one-electron reduction of the catalyst followed by protonation, or via two-electron reduction and protonation. H2 evolution can then either occur homolytically, involving two [M–H] equivalents, or a two-electron reduced M–H complex could be protonated to yield dihydrogen via a heterolytic mechanism. In the case of 1, since HER-associated current enhancement is only observed at the second reduction event, we assign the one-electron reduced species (labelled as [Fe]+ in Scheme 1) as the catalytically active species. Return anodic scans following the electrocatalytic wave show that, in the presence of acid, the second reduction is irreversible. As the anodic wave for the first reduction (FeII/I couple) is still observable in the presence of acid (Fig. S4), the first reduction is not accompanied by protonation. The [Fe]+ species may not be nucleophilic enough to be protonated. We therefore propose that, under electrocatalytic conditions, the dicationic Fe(II) complex undergoes sequential one-electron reductions followed by protonation to form an [Fe–H]+ species, with a hydride occupying the place of an axial acetonitrile molecule (Scheme 1). Subsequent protonation then yields H2 and regenerates the dicationic Fe(II) species, likely stabilized by recoordination of solvent unit to saturate the metal's coordination sphere.
 | ||
| Scheme 1 Proposed heterolytic pathway for the reduction of protons from 1via in situ generated [Fe]+. | ||
Considering the observed HER might originate from deposition of a catalytically active species on the surface of the electrode rather than from homogeneous 1, we subjected a solution of 1 to 50 cycles of electrocatalysis scanning from −0.1 to −1.7 V vs. FcH0/+. Removing the electrode to a fresh solution of electrolyte and acid which did not contain added 1, we do not see features in subsequent CV cycles consistent with electrocatalysis (Fig. S5). This confirmed that no electrocatalytically active material deposits on the electrode after at least 50 cycles of electrocatalysis, indicating that compound 1 behaves as the true homogeneous catalyst.
Using the half-wave potential of the catalytic wave observed under HER conditions (−1.37 V) and taking −0.779 V as the thermodynamic potential for the reduction of TFA in acetonitrile,41 we estimate an overpotential of 591 mV (see SI for the calculation). To determine the order of the reaction with respect to acid, ic/ip was plotted versus the concentration of acid with ic corresponding to the peak current of the catalytic wave and ip the current of the wave in the absence of substrate. Fig. 6b shows that the peak current increases with increasing concentration of acid. Fig. 7a reveals a linear relationship between ic/ip and the concentration of acid, with an R2 value of 0.96. The catalytic reaction is thus second-order with respect to acid, with a maximum ic/ip observed of 13.6. The order of the reaction with respect to the catalyst concentration can also be determined by plotting peak current vs. catalyst concentration (Fig. 7b). The linear relationship observed between peak current and concentration of the electrocatalyst shows a first-order reaction respect to 1. This is consistent with the proposed heterolytic pathway for H2 generation.
From Fig. 6b, it is evident that even at high substrate concentrations, a catalytic plateau current is not achieved and the S-shaped curve that is expected for a catalytic reaction that is not limited by the diffusion of the substrate is not obtained. This is indicative of competing side phenomenon such as substrate consumption, catalyst deactivation, or product inhibition.42 In such cases, foot-of-the-wave analysis (FOWA) can be used to gain insights on the turnover frequency (TOF) and kobs of the reaction by focusing our analysis on the foot of the catalytic wave; i.e., the initial portion of the wave where the CV response is linear and it can be assumed that competing side phenomenon are not occurring.43,44 The following equation can then be used to estimate the observed rate constant:
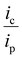 was plotted versus
was plotted versus for the linear region near the foot of the catalytic wave. That is, at potentials ranging between −1.25 V and −1.33 V. Assuming no competing side phenomenon occurring at the foot of the wave, this plot should yield a straight line with a slope equal to
for the linear region near the foot of the catalytic wave. That is, at potentials ranging between −1.25 V and −1.33 V. Assuming no competing side phenomenon occurring at the foot of the wave, this plot should yield a straight line with a slope equal to  . It is worth highlighting that in our case, we still expect there to be slight deviations from the ideal scenario for FOWA because we have a redox event (the FeII/I couple) occurring near the foot of the catalytic wave, which could give some diffusional contributions to the current observed at the foot of the wave. Nevertheless, FOWA should still give us an estimate of the rate constant with which catalytic HER occurs. Fig. 8 shows the plot obtained, with a slope of 19.698. Using the relationship
. It is worth highlighting that in our case, we still expect there to be slight deviations from the ideal scenario for FOWA because we have a redox event (the FeII/I couple) occurring near the foot of the catalytic wave, which could give some diffusional contributions to the current observed at the foot of the wave. Nevertheless, FOWA should still give us an estimate of the rate constant with which catalytic HER occurs. Fig. 8 shows the plot obtained, with a slope of 19.698. Using the relationship  , kobs is calculated at 602 s−1.
, kobs is calculated at 602 s−1.
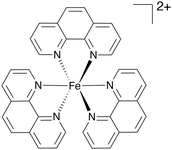
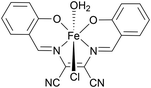
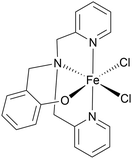
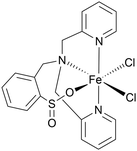
Comparing this to related Fe(II) and Fe(III) complexes shown in Table 1, complex 1 operates at a lower overpotential than a recently reported Fe(II) complex of phenanthroline (A)45 and an Fe(III) complex of a tetradentate Schiff base ligand (B),46 but has a slightly higher overpotential than the closely related Fe(II) complex C with a tetradentate N2P2 ligand.47 Complex 1 does however show a significantly improved kobs compared with all three of A–C. Some of the most active Fe(III)NNOCl2 HER catalysts (complexes D–F) have overpotentials ranging between 300–800 mV and kobs of 550–3300 s−1.18,48,49 Complex 1 seems to be approaching the activity of these species. This showcases the potential of π-extended tetradentate P^N-based ligands for boosting the electrocatalytic activity of Fe(II) for homogeneous HER and opens up the possibility of deploying benzannulated multidentate ligands in homogeneous HER electrocatalysis more widely.
Conclusions
In summary, we have shown how a substituted 6,6′-biphenanthridine (biphe) can be selectively formed from its monomer via reductive dimerization using sodium as the reducing agent. This protocol gives access to a tetradentate benzannulated P^N^N^P ligand L^L with two phosphine donor arms, extending the binding ability of biphe.22,23 This ligand can bind to Fe(II), forming a pseudo-octahedral Fe(II) complex (1) that presents two accessible, reversible reduction events, highlighting the molecule's ability to store electrons. The two-electron reduced complex can efficiently catalyze the reduction of protons in an acetonitrile solution at a low overpotential of 591 mV and with moderately high kobs of 602 s−1. This corresponds to a high electrocatalytic activity, approaching the efficiency of some of the most active Fe(III) homogeneous HER electrocatalysts. Analysis of the electrochemical behavior shows the HER process to be first-order with respect to the catalyst and second-order with respect to the acid, indicating a heterolytic pathway. We ascribe the high activity of this electrocatalyst to the stabilization of the reduced complex by increased π-extension of the ligand. Given the low photostability of this complex, efforts to planarize the substituted biphe are currently underway, with the goal of eliminating ligand photodissociation and catalyst degradation.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
Supplementary information (SI): multi-nuclear NMR and HR-MS spectra of all new compounds and other supporting data and figures, as well as crystallographic information files containing all X-ray data. See DOI: https://doi.org/10.1039/d5dt02202a.CCDC 2487856 and 2487857 contain the supplementary crystallographic data for this paper.50a,b
Acknowledgements
The Natural Sciences and Engineering Research Council is thanked for a Discovery Grant to D. E. H. (RGPIN-2022-04501) CGS-D Scholarships to B. K. S. and A. K. Purchase of an X-Ray diffractometer was possible through support of the Canada Foundation for Innovation and Research Manitoba (#32146). The Digital Research Alliance of Canada is thanked for computational support.References
- H. Wang, J. Wen and X. Zhang, Chiral Tridentate Ligands in Transition Metal-Catalyzed Asymmetric Hydrogenation, Chem. Rev., 2021, 121, 7530–7567 CrossRef CAS PubMed.
- D.-H. Liang, C.-J. Hou, Q. Li, H. Qin, L. Li and X.-P. Hu, Chiral P,N,N-Ligands for Asymmetric Hydrogenation, Adv. Synth. Catal., 2024, 366, 2165–2185 CrossRef CAS.
- M. P. Carroll and P. J. Guiry, P,N Ligands in Asymmetric Catalysis, Chem. Soc. Rev., 2014, 43, 819–833 RSC.
- A. Rajmane and A. Kumbhar, Polydentate P,N-Based, Ligands for Palladium-Catalyzed Cross-Coupling Reactions, Mol. Catal., 2022, 532, 112699 CAS.
- J. Bae and E. J. Cho, P,N Ligand in Ni-Catalyzed Cross-Coupling Reactions: A Promising Tool for π-Functionalization, ACS Catal., 2023, 13, 13540–13560 CrossRef CAS.
- L. S. Merz, J. Ballmann and L. H. Gade, Phosphines and N-Heterocycles Joining Forces: An Emerging Structural Motif in PNP-Pincer Chemistry, Eur. J. Inorg. Chem., 2020, 2020, 2023–2042 CrossRef CAS.
- A. Mezzetti, Ruthenium Complexes with Chiral Tetradentate PNNP Ligands: Asymmetric Catalysis from the Viewpoint of Inorganic Chemistry, Dalton Trans., 2010, 39, 7851–7869 RSC.
- W. Zuo, A. J. Lough, Y. F. Li and R. H. Morris, Amine(Imine)Diphosphine Iron Catalysts for Asymmetric Transfer Hydrogenation of Ketones and Imines, Science, 2013, 342, 1080–1083 CrossRef CAS PubMed.
- R. H. Morris, Exploiting Metal–Ligand Bifunctional Reactions in the Design of Iron Asymmetric Hydrogenation Catalysts, Acc. Chem. Res., 2015, 48, 1494–1502 CrossRef CAS PubMed.
- E. S. Wiedner, A. M. Appel, S. Raugei, W. J. Shaw and R. M. Bullock, Molecular Catalysts with Diphosphine Ligands Containing Pendant Amines, Chem. Rev., 2022, 122, 12427–12474 CrossRef CAS PubMed.
- A. S. Nanuwa, M. D. Hoffman, K. Nandi and J. M. Blacquiere, Selectivity of Ru and Fe PR2NR'2 Catalysts Toward Acceptorless Dehydrogenation of Benzylamine, Organometallics, 2024, 43, 2342–2348 CrossRef CAS.
- E. S. Isbrandt, D. E. Chapple, N. T. P. Tu, V. Dimakos, A. M. M. Beardall, P. D. Boyle, C. N. Rowley, J. M. Blacquiere and S. G. Newman, Controlling Reactivity and Selectivity in the Mizoroki–Heck Reaction: High Throughput Evaluation of 1,5-Diaza-3,7-Diphosphacyclooctane Ligands, J. Am. Chem. Soc., 2024, 146, 5650–5660 CrossRef CAS PubMed.
- H. Land, M. Senger, G. Berggren and S. T. Stripp, Current State of [FeFe]-Hydrogenase Research: Biodiversity and Spectroscopic Investigations, ACS Catal., 2020, 10, 7069–7086 CrossRef CAS.
- L. Tong, L. Duan, A. Zhou and R. P. Thummel, First-Row Transition Metal Polypyridine Complexes That Catalyze Proton to Hydrogen Reduction, Coord. Chem. Rev., 2020, 402, 213079 CrossRef CAS.
- I. Bhugun, D. Lexa and J.-M. Savéant, Homogeneous Catalysis of Electrochemical Hydrogen Evolution by Iron(0) Porphyrins, J. Am. Chem. Soc., 1996, 118, 3982–3983 CrossRef CAS.
- L.-L. Zhou, L.-Z. Tang, Y.-X. Zhang and S.-Z. Zhan, Synthesis of a Molecular Electrocatalyst Based on an Iron(III) Complex Supported by Amine-Bis(Phenolate) Ligand for Water Reduction, Polyhedron, 2015, 92, 124–129 CrossRef CAS.
- M. J. Rose, H. B. Gray and J. R. Winkler, Hydrogen Generation Catalyzed by Fluorinated Diglyoxime–Iron Complexes at Low Overpotentials, J. Am. Chem. Soc., 2012, 134, 8310–8313 CrossRef CAS PubMed.
- G. P. Connor, K. J. Mayer, C. S. Tribble and W. R. McNamara, Hydrogen Evolution Catalyzed by an Iron Polypyridyl Complex in Aqueous Solutions, Inorg. Chem., 2014, 53, 5408–5410 CrossRef CAS PubMed.
- A. Call, Z. Codolà, F. Acuña-Parés and J. Lloret-Fillol, Photo- and Electrocatalytic H2 Production by New First-Row Transition-Metal Complexes Based on an Aminopyridine Pentadentate Ligand, Chem. – Eur. J., 2014, 20, 6171–6183 CrossRef CAS PubMed.
- N. A. C. dos Santos, M. Natali, E. Badetti, K. Wurst, G. Licini and C. Zonta, Cobalt, Nickel, and Iron Complexes of 8-Hydroxyquinoline-Di(2-Picolyl)Amine for Light-Driven Hydrogen Evolution, Dalton Trans., 2017, 46, 16455–16464 RSC.
- D. E. Herbert, When Is a Pyridine Not a Pyridine? Benzannulated N-Heterocyclic Ligands in Molecular Materials Chemistry, Can. J. Chem., 2023, 101, 892–902 CrossRef CAS.
- D. B. Nemez, I. B. Lozada, J. D. Braun, J. A. G. Williams and D. E. Herbert, Synthesis and Coordination Chemistry of a Benzannulated Bipyridine: 6,6′-Biphenanthridine, Inorg. Chem., 2022, 61, 13386–13398 CrossRef CAS PubMed.
- D. B. Nemez, R. J. Ortiz, K. A. Veilleux, J. A. G. Williams and D. E. Herbert, In Pursuit of Low Energy Phosphorescence: Late Metal Coordination Complexes of the Planar, π-Extended Bipyridyl Ligand 6,6′,7,7′-Biphenanthridine, Chem. – Eur. J., 2025, e01802 CrossRef PubMed.
- R. Mondal, P. K. Giesbrecht and D. E. Herbert, Nickel(II), Copper(I) and Zinc(II) Complexes Supported by a (4-Diphenylphosphino)Phenanthridine Ligand, Polyhedron, 2016, 108, 156–162 CrossRef CAS.
- M. Drosou, F. Kamatsos and C. A. Mitsopoulou, Recent Advances in the Mechanisms of the Hydrogen Evolution Reaction by Non-Innocent Sulfur-Coordinating Metal Complexes, Inorg. Chem. Front., 2020, 7, 37–71 RSC.
- P. Mandapati, P. K. Giesbrecht, R. L. Davis and D. E. Herbert, Phenanthridine-Containing Pincer-like Amido Complexes of Nickel, Palladium, and Platinum, Inorg. Chem., 2017, 56, 3674–3685 CrossRef CAS PubMed.
- R. Mondal, I. B. Lozada, R. L. Davis, J. A. G. Williams and D. E. Herbert, Site-Selective Benzannulation of N-Heterocycles in Bidentate Ligands Leads to Blue-Shifted Emission from [(P^N)Cu]2(μ-X)2 Dimers, Inorg. Chem., 2018, 57, 4966–4978 CrossRef CAS PubMed.
- R. Mondal, I. B. Lozada, R. L. Davis, J. A. G. Williams and D. E. Herbert, Exploiting Synergy between Ligand Design and Counterion Interactions to Boost Room Temperature Phosphorescence from Cu(I) Compounds, J. Mater. Chem. C, 2019, 7, 3772–3778 RSC.
- R. Mondal, J. D. Braun, I. B. Lozada, R. Nickel, J. van Lierop and D. E. Herbert, Group VIII Coordination Complexes of Bidentate P^N Ligands Bearing π-Extended Quinoline or Phenanthridine N-Heterocycles, New J. Chem., 2021, 45, 4427–4436 RSC.
- R. Mondal and D. E. Herbert, Synthesis of Pyridines, Quinolines, and Pyrimidines via Acceptorless Dehydrogenative Coupling Catalyzed by a Simple Bidentate P^N Ligand Supported Ru Complex, Organometallics, 2020, 39, 1310–1317 CrossRef CAS.
- R. Mondal, I. B. Lozada, O. Stotska and D. E. Herbert, Catalytic Synthesis of Luminescent Pyrimidines via Acceptor-Less Dehydrogenative Coupling, J. Org. Chem., 2020, 85, 13747–13756 CrossRef CAS PubMed.
- R. Mondal, J. D. Braun, B. K. Sidhu, D. E. Nevonen, V. N. Nemykin and D. E. Herbert, Catalytic Synthesis of Donor-Acceptor-Donor (D-A-D) and Donor-Acceptor-Acceptor (D-A-A) Pyrimidine-Ferrocenes via Acceptorless Dehydrogenative Coupling: Synthesis, Structures, and Electronic Communication, Organometallics, 2021, 40, 1765–1775 CrossRef CAS.
- B. K. Sidhu, R. Mondal and D. E. Herbert, Tandem Hydrogen-Borrowing Dehydrogenative Coupling and Hydrodefluorination, Adv. Synth. Catal., 2024, 366(8), 1809–1819 CrossRef CAS.
- P. K. Giesbrecht, D. B. Nemez and D. E. Herbert, Electrochemical Hydrogenation of a Benzannulated Pyridine to a Dihydropyridine in Acidic Solution, Chem. Commun., 2018, 54, 338–341 RSC.
- E. Garcia-Torres and D. E. Herbert, Electrochemical Hydrogenation of N-Heterocycles and Related Substrates: A Mini-Review, Electrochem. Sci. Adv., 2025, 5, e00019 CrossRef CAS.
- J. J. Eisch and R. M. Thompson, Chemistry of Alkali Metal-Unsaturated Hydrocarbon Adducts. II. Radical-Anion Intermediates in the Metal Reductions of Aza-Aromatic Heterocycles, J. Org. Chem., 1962, 27, 4171–4179 CrossRef CAS.
- D. D. Tanner and C. M. Yang, On the Structure and Mechanism of Formation of the Lansbury Reagent, Lithium Tetrakis(N-Dihydropyridyl)Aluminate, J. Org. Chem., 1993, 58, 1840–1846 CrossRef CAS.
- J. M. Smieja, E. E. Benson, B. Kumar, K. A. Grice, C. S. Seu, A. J. M. Miller, J. M. Mayer and C. P. Kubiak, Kinetic and Structural Studies, Origins of Selectivity, and Interfacial Charge Transfer in the Artificial Photosynthesis of CO, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15646–15650 CrossRef CAS PubMed.
- J. D. Braun, I. B. Lozada, C. Kolodziej, C. Burda, K. M. E. Newman, J. van Lierop, R. L. Davis and D. E. Herbert, Iron(II) Coordination Complexes with Panchromatic Absorption and Nanosecond Charge-Transfer Excited State Lifetimes, Nat. Chem., 2019, 11, 1144–1150 CrossRef CAS PubMed.
- C. B. Larsen, J. D. Braun, I. B. Lozada, K. Kunnus, E. Biasin, C. Kolodziej, C. Burda, A. A. Cordones, K. J. Gaffney and D. E. Herbert, Reduction of Electron Repulsion in Highly Covalent Fe-Amido Complexes Counteracts the Impact of a Weak Ligand Field on Excited-State Ordering, J. Am. Chem. Soc., 2021, 143, 20645–20656 CrossRef CAS PubMed.
- B. D. McCarthy, D. J. Martin, E. S. Rountree, A. C. Ullman and J. L. Dempsey, Electrochemical Reduction of Brønsted Acids by Glassy Carbon in Acetonitrile—Implications for Electrocatalytic Hydrogen Evolution, Inorg. Chem., 2014, 53, 8350–8361 CrossRef CAS PubMed.
- E. S. Rountree, B. D. McCarthy, T. T. Eisenhart and J. L. Dempsey, Evaluation of Homogeneous Electrocatalysts by Cyclic Voltammetry, Inorg. Chem., 2014, 53, 9983–10002 CrossRef CAS PubMed.
- C. Costentin, S. Drouet, M. Robert and J.-M. Savéant, Turnover Numbers, Turnover Frequencies, and, Overpotential in Molecular Catalysis of Electrochemical Reactions. Cyclic Voltammetry and Preparative-Scale Electrolysis, J. Am. Chem. Soc., 2012, 134, 11235–11242 CrossRef CAS PubMed.
- C. Costentin and J.-M. Savéant, Multielectron, Multistep Molecular Catalysis of Electrochemical Reactions: Benchmarking of Homogeneous Catalysts, ChemElectroChem, 2014, 1, 1226–1236 CrossRef CAS.
- S.-P. Luo, Q.-X. Peng, J. Liu and S.-Z. Zhan, Effect of Metal Centers on Electrocatalytic Hydrogen Generation Catalyzed by Coordinatively Saturated Metal-1,10-Phenanthroline Complexes, Polyhedron, 2018, 139, 44–49 CrossRef CAS.
- L.-Z. Fu, L.-L. Zhou and S.-Z. Zhan, Electrochemical-Driven Water Reduction and Oxidation Catalyzed by an Iron(III) Complex Supported by 2,3-Bis(2-Hydroxybenzylideneimino)-2,3-Butenedinitrile, RSC Adv., 2015, 5, 42287–42293 RSC.
- D. Huang, B. He, Y. Zhao, M. Liu and L. Wang, Homogeneous Electrocatalytic Hydrogen Evolution by a N2P2Fe(II) Complex: Structural Characterization of Iron-Hydride Intermediate, J. Mol. Struct., 2025, 1325, 141021 CrossRef CAS.
- A. C. Cavell, C. L. Hartley, D. Liu, C. S. Tribble and W. R. McNamara, Sulfinato Iron(III) Complex for Electrocatalytic Proton Reduction, Inorg. Chem., 2015, 54, 3325–3330 CrossRef CAS PubMed.
- C. L. Hartley, R. J. DiRisio, T. Y. Chang, W. Zhang and W. R. McNamara, Electrocatalytic Hydrogen Evolution by an Iron Complex Containing a Nitro-Functionalized Polypyridyl Ligand, Polyhedron, 2016, 114, 133–137 CrossRef CAS.
- (a) CCDC 2487856: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phtfg; (b) CCDC 2487857: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phtgh.
| This journal is © The Royal Society of Chemistry 2025 |