A disilane-bonded bis(methylpyridine) Cu(I) complex exhibiting reversible trigonal–tetrahedral switch with stimuli-responsive luminescence
Yoshinori
Yamanoi
 *a,
Yongjin
Zhao
b,
Toyotaka
Nakae
a,
Kaito
Segawa
cd,
Masaki
Yoshida
*a,
Yongjin
Zhao
b,
Toyotaka
Nakae
a,
Kaito
Segawa
cd,
Masaki
Yoshida
 ce,
Masako
Kato
ce,
Masako
Kato
 c,
Kazuma
Kikuchi
f,
Hiroaki
Imoto
c,
Kazuma
Kikuchi
f,
Hiroaki
Imoto
 f,
Kensuke
Naka
f,
Kensuke
Naka
 f,
Showa
Kitajima
g,
Hitoshi
Kasai
g,
Kouki
Oka
f,
Showa
Kitajima
g,
Hitoshi
Kasai
g,
Kouki
Oka
 g and
Suguru
Ito
g and
Suguru
Ito
 *b
*b
aDepartment of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: ymanoi@chem.s.u-tokyo.ac.jp
bDepartment of Chemistry and Life Science, Graduate School of Engineering Science, Yokohama National University, Yokohama, Kanagawa 240-8501, Japan. E-mail: suguru-ito@ynu.ac.jp
cDepartment of Applied Chemistry for Environment, School of Biological and Environmental Sciences, Kwansei Gakuin University, 1 Gakuen Uegahara, Sanda, Hyogo 669-1330, Japan
dDepartment of Chemistry, Faculty of Science, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
eDepartment of Chemistry, Graduate School of Science, The University of Osaka, 1-1 Machikaneyama, Toyonaka, Osaka 560-0043, Japan
fFaculty of Molecular Chemistry and Engineering, Graduate School of Science and Technology, Kyoto Institute of Technology, Goshokaido-cho, Matsugasaki, Sakyo-ku, Kyoto 606-8585, Japan
gInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai, Miyagi 980-8577, Japan
First published on 7th November 2025
Abstract
A stimuli-responsive tetranuclear copper(I) complex with 1,1,2,2-tetramethyl-1,2-bis(3-methylpyridin-2-yl)disilane (L1) showed reversible structural transformation between Cu4I4(L1)2·2CH3CN and Cu4I4(L1)2. The change altered copper coordination from a tetrahedral to trigonal type as confirmed by XRD. The solvated form showed stronger blue-green emission (λem = 493 nm, Φ = 0.56) than the desolvated form (λem = 471 nm, Φ = 0.06). The change in emission intensity in the crystalline state was attributed to the change in structural rigidity or flexibility induced by the coordination or non-coordination of CH3CN to the Cu(I) center.
Luminescent materials have attracted significant attention over the past decades due to their wide range of applications as smart materials.1 Among them, stimuli-responsive luminescent metal complexes have emerged as a research hotspot in recent years, owing to the structural modification of organic ligands and the high stability.2 In particular, copper(I) complexes are promising candidates for available crystalline-state emitters due to their diverse structural motifs and occasionally observed polymorphs.3 Extended π-conjugated ligands are commonly employed to construct the functional copper(I) complexes.
We have previously investigated stimuli-responsive σ–π conjugated aromatic disilane molecules,4 and reported crystalline polymorphs of copper(I) iodide complexes with 1,1,2,2-tetramethyl-1,2-di(pyridin-2-yl)disilane (L), which form an octanuclear complex at room temperature and a one-dimensional polymer at 77 K via a single-crystal-to-single-crystal phase transition.5 Additionally, we reported the optical properties of trigonal planar mononuclear Cu(I) complexes bearing disilane-bonded bis(methylpyridine) ligands.6–8 These complexes exhibited intense emission in the crystalline state and aggregation-induced emission (AIE) in THF–water mixtures.
Vapochromic luminescence of copper(I) complexes has been widely investigated.9 In many reported systems, the change in the emission colour in the crystalline state is due to the reversible exchange of different solvent molecules at the metal center.10 However, these changes are typically attributed to electronic effects rather than structural changes. In contrast, only a limited number of studies have addressed the direct impact of solvent coordination/uncoordination on the metal centre geometry, which can significantly affect emission behaviour.11 Coordination of solvent molecules to the metal ion generally induces structural distortions and activates high-energy vibrational modes associated with C–H bonds, which promote non-radiative decay pathways resulting in decreased emission intensity in the crystalline state.12
In this study, we investigated the reversible structural transformation (Fig. 1b) and luminescence changes in a tetranuclear copper(I) iodide complex synthesized using a flexible ligand L1 (Fig. 1a), in which a methyl group is introduced into the pyridine rings. We found that exposure to CH3CN vapor triggers coordination to the copper centres, transforming the flexible trigonal geometry into a more rigid tetrahedral arrangement. This structural change led to a significant enhancement in both luminescence efficiency and lifetime. These findings provide new insights into the relationship between the molecular structure and photophysical properties in the crystalline state and offer a novel strategy for designing stimuli-responsive luminescent materials.
 | ||
| Fig. 1 (a) Chemical structures of disilane-bonded L (previous work) and L1 (this work). (b) Chemical structures of stimuli-responsive copper complexes in this work. | ||
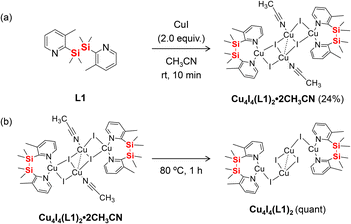
The tetrahedral copper(I) complex Cu4I4(L1)2·2CH3CN was synthesized by mixing CuI and L1 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in CH3CN at room temperature (Scheme 1a). The complex was isolated as air-stable crystals in 24% yield. The acetonitrile-free complex Cu4I4(L1)2 was quantitatively obtained by heating Cu4I4(L1)2·2CH3CN at 80 °C (Scheme 1b).
1 molar ratio in CH3CN at room temperature (Scheme 1a). The complex was isolated as air-stable crystals in 24% yield. The acetonitrile-free complex Cu4I4(L1)2 was quantitatively obtained by heating Cu4I4(L1)2·2CH3CN at 80 °C (Scheme 1b).
The structures of both complexes were confirmed by single-crystal X-ray diffraction.13 The molecular structure of Cu4I4(L1)2·2CH3CN is shown in Fig. 2a. The complex adopts a Cu4I4 core with distorted tetrahedral coordination geometries, in which acetonitrile molecules are coordinated to the copper centres. The iodide ligands exhibit both μ2- and μ3-bridging modes. The Cu6–N9 distance between CH3CN and Cu(I) is 2.015 Å,14 suggesting a relatively weak interaction and implying that CH3CN can be easily removed upon external stimuli.
 | ||
| Fig. 2 X-ray structures of (a) Cu4I4(L1)2·2CH3CN and (b) Cu4I4(L1)2 with thermal ellipsoids with 50% probability. Hydrogen atoms are omitted for clarity. | ||
The coordination environment around Cu3 and Cu3′ is significantly distorted from the ideal tetrahedral geometry, while Cu6 and Cu6′ adopt nearly regular tetrahedral geometries. The Cu6–Cu6′ distance is 2.850 Å, which closely matches twice the covalent radius of copper (1.32 Å), indicating the presence of intramolecular Cu(I)⋯Cu(I) cuprophilic interactions.15 In contrast, the Cu3–Cu6 distance is 3.4019 Å, suggesting no significant metal–metal interaction. The crystal packing (Fig. S1a) is stabilized by C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯π, C–H⋯π, and non-classical I⋯H–C hydrogen bonding interactions.16
N⋯π, C–H⋯π, and non-classical I⋯H–C hydrogen bonding interactions.16
The Cu–I distances were Cu3–I4 = 2.762 Å, Cu3–I5 = 2.949 Å, Cu6–I5 = 2.664 Å, and Cu6–I4 = 2.634 Å. The longer Cu3–I5 suggests that the linkage is readily cleaved upon CH3CN dissociation, leading to the formation of a three-coordinate complex.
Bourissou and co-workers reported that disilane-bonded diphosphine ligands can exhibit σ(Si–Si) coordination to copper centers.17 In their work, coordination of the Si–Si σ-bond to cationic Cu centres resulted in elongated Si–Si bonds (∼2.45 Å, compared to ∼2.36 Å in the free ligand) and Si–Cu bond lengths (∼2.72 Å), slightly exceeding the sum of van der Waals radii (2.43 Å). In contrast, in Cu4I4(L1)2·2CH3CN, the Si–Si bond length is 2.37 Å, close to that in the free ligand (ca. 2.33 Å), and the Si–Cu distance is 2.90 Å—significantly longer than the expected van der Waals contact. These results indicate that the disilane moiety in L1 does not coordinate to the Cu(I) centres in this complex.
The bond angles around Cu3 (Cu3′) are: N8–Cu3–N7 = 144.12°, N8–Cu3–I4 = 106.07°, N7–Cu3–I4 = 102.38°, and I5–Cu3–I4 = 97.32°, consistent with a distorted tetrahedral geometry. The bond angles around Cu6 (Cu6′) are: N9–Cu6–I4 = 106.41°, I5–Cu6–I4 = 108.14°, I5–Cu6–I5′ = 115.09°, and N9–Cu6–I5′ = 111.35°, closely resembling an ideal tetrahedral structure.
Upon heating Cu4I4(L1)2·2CH3CN at 80 °C for 1 h, the crystals transformed into the acetonitrile-free colourless complex Cu4I4(L1)2. The crystal structure of this phase is shown in Fig. 2b. It crystallizes in the monoclinic system (space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), and all Cu(I) centres adopt a trigonal planar geometry. The sum of bond angles around Cu3 (Cu3′) and Cu6 (Cu6′) are 358.72° and 359.99°, respectively, confirming near-planar coordination environments.
), and all Cu(I) centres adopt a trigonal planar geometry. The sum of bond angles around Cu3 (Cu3′) and Cu6 (Cu6′) are 358.72° and 359.99°, respectively, confirming near-planar coordination environments.
The Cu–I bond lengths are as follows: Cu3–I4 = 2.761 Å, Cu6–I4 = 2.535 Å, Cu6–I5 = 2.578 Å, and Cu6–I5′ = 2.541 Å. The Cu3–I5 (or Cu3′–I5′) distances are 3.602 Å, indicating negligible Cu⋯I interaction at these positions. The I–Cu–I bond angles are in the range of 97.3° to 115.1°. There is no significant Cu3–Cu6 interaction (3.001 Å), which exceeds the sum of van der Waals radii, while the Cu6–Cu6′ distance is 2.784 Å, shorter than that in Cu4I4(L1)2·2CH3CN, indicating stronger cuprophilic interactions in Cu4I4(L1)2.
No evidence of interaction between the Si–Si bond and the Cu(I) centres was observed in Cu4I4(L1)2 as well. The crystal packing of Cu4I4(L1)2 is stabilized by face-to-face CH⋯π interactions between methylpyridine units and non-classical I⋯H–C hydrogen bonds (Fig. S1b).
To gain deeper insights into the polymorphic behaviour of Cu4I4(L1)2·2CH3CN, powder X-ray diffraction (PXRD) analysis was carried out. The results are shown in Fig. S3. Upon heating the crystalline sample at 80 °C, the PXRD pattern underwent a clear transformation, corresponding to the loss of coordinated CH3CN and formation of Cu4I4(L1)2. Remarkably, the original pattern of Cu4I4(L1)2·2CH3CN was restored upon exposure to CH3CN vapor, indicating a reversible crystal-to-crystal phase transition. Simulated PXRD patterns based on single-crystal X-ray structures matched well with the experimental data for both phases, confirming the structural reversibility. Such reversible formation and cleavage of Cu–I bonds have also been observed in previous studies (copper(I) iodide complexes with 1,1,2,2-tetramethyl-1,2-di(pyridin-2-yl)disilane (L)) and represent an important example of the reversible stimulus-responsive behaviour of Cu(I) complexes.5
Thermogravimetric analysis (TGA) of Cu4I4(L1)2·2CH3CN (Fig. S2) further supported this transformation. A distinct weight loss of approximately 6% was observed at around 80 °C, corresponding to the loss of two CH3CN molecules per Cu4I4(L1)2 unit. No additional weight loss occurred up to 180 °C, demonstrating the thermal stability of the Cu4I4(L1)2 complex. Decomposition of the framework commenced beyond 180 °C.
Most of the metal complexes that show vapochromic emission have been studied in terms of coordination between different vapor molecules. However, there are a few examples that have examined in detail the solvent coordination/non-coordination crystalline complexes.18 In this study, a clear vapochromic response caused by the coordination and dissociation of CH3CN was observed between Cu4I4(L1)2·2CH3CN and Cu4I4(L1)2 in the crystalline state. As shown in Fig. 3, Cu4I4(L1)2·2CH3CN forms yellow-green cubic crystals under ambient light and displays green emission under UV irradiation. Upon heating to 80 °C, the coordinated CH3CN molecules are released, resulting in desolvated Cu4I4(L1)2 crystals, which are colourless under ambient light and emit blue luminescence under UV light. The emission maxima were 493 nm for Cu4I4(L1)2·2CH3CN and 471 nm for Cu4I4(L1)2, respectively. Exposure of the desolvated crystals to CH3CN vapor led to rapid recovery of the original emission and colour, indicating a reversible crystal-to-crystal phase transition mediated by CH3CN coordination. This process could be repeated multiple times without degradation.
Photoluminescence quantum yield (PLQY) and emission lifetime measurements further highlight the significant effect of CH3CN coordination. Cu4I4(L1)2·2CH3CN exhibited a PLQY of 0.56 and an emission lifetime of 5.91 μs, while Cu4I4(L1)2 displayed a much lower PLQY of 0.06 and a shorter lifetime of 0.80 μs. The higher PLQY and longer emission lifetime of Cu4I4(L1)2·2CH3CN are attributed to the enhanced radiative rate (kr) and reduced non-radiative rate (knr) resulting from the rigid structure (Table S2).19
Next, the emission behaviour of Cu4I4(L1)2 and Cu4I4(L1)2·2CH3CN was investigated at 77 K (Tables S1, S2 and Fig. S4–S6). Compared to measurements at 298 K, both complexes exhibited a red shift in their emission maxima (6–9 nm), significantly enhanced luminescence intensity, and longer emission lifetime at 77 K. It is reasonable to consider that the emission of these complexes originates from a metal–halide-to-ligand charge transfer (MXLCT) state. The HOMO of luminescent Cu(I) complexes is often mainly localized on the Cu–I cluster, while the LUMO is frequently localized on the ligands, resulting in a small overlap between the HOMO and LUMO.5,6 Consequently, the energy gap between the singlet and triplet states (ΔEST) becomes relatively small, which provides favourable conditions for the occurrence of TADF (thermally activated delayed fluorescence).20 These results indicate that the emission mechanism is delayed fluorescence dominant at 298 K,21 while phosphorescence is the predominant pathway at 77 K. Based on the spectroscopic data, ΔEST can be estimated to be approximately 0.05 eV, which is consistent with the TADF mechanism.
The two emissions of Cu4I4(L1)2·2CH3CN observed at 77 K (Fig. S6(a), 502 nm and 517 nm) are attributable to the vibronic structure of T1 → S0 (0–0 and 0–1). The energy difference of the two emission peaks (502 nm and 517 nm) is ca. 578 cm−1, which corresponds to the stretching vibration of the ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[u with combining low line]](https://www.rsc.org/images/entities/char_0075_0332.gif) –
–![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif)
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH3 moiety at approximately 400–600 cm−1. The two peaks share the same excitation spectra (Fig. S6(a), 417 nm) and exhibit almost identical lifetimes within experimental error (Fig. S5(b), λem: 502 nm: τ = 28.90 ± 0.74 μs and λem: 517 nm: τ = 28.70 ± 0.70 μs). The results strongly suggest that they represent vibronic sidebands of a single excited state rather than two distinct emissive states. Furthermore, the absence of a resolved vibrational structure in the Cu4I4(L1)2 complex can be attributed to the lack of coordinated acetonitrile, which prevents the vibrational modes associated with the
C–CH3 moiety at approximately 400–600 cm−1. The two peaks share the same excitation spectra (Fig. S6(a), 417 nm) and exhibit almost identical lifetimes within experimental error (Fig. S5(b), λem: 502 nm: τ = 28.90 ± 0.74 μs and λem: 517 nm: τ = 28.70 ± 0.70 μs). The results strongly suggest that they represent vibronic sidebands of a single excited state rather than two distinct emissive states. Furthermore, the absence of a resolved vibrational structure in the Cu4I4(L1)2 complex can be attributed to the lack of coordinated acetonitrile, which prevents the vibrational modes associated with the ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[u with combining low line]](https://www.rsc.org/images/entities/char_0075_0332.gif) –
–![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif)
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH3 moiety.
C–CH3 moiety.
Conflicts of interest
The authors declare no competing financial interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available: experimental section, crystal packing diagram, TGA, PXRD, emission lifetime measurement, excitation spectra and emission spectra. See DOI: https://doi.org/10.1039/d5dt01893e.CCDC 2477874 contains the supplementary crystallographic data for this paper.22
Acknowledgements
This work was financially supported by a Grant-in-Aid for Scientific Research (C) (no. JP25K08585) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This work was also performed in part under the Cooperative Research Program of Network Joint Research Centre for Materials and Devices (no. 20251109).References
- (a) A. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897–1091 CrossRef CAS; (b) L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993–1017 RSC; (c) Z. Yang, J. Cao, Y. He, J. H. Yang, T. Kim, X. Peng and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4563–4601 RSC; (d) M. Zhu and C. Yang, Chem. Soc. Rev., 2013, 42, 4963–4976 RSC.
- (a) A. Kobayashi and M. Kato, Chem. Lett., 2017, 46, 154–162 CrossRef CAS; (b) M. Kato, H. Ito, M. Hasegawa and K. Ishii, Chem. – Eur. J., 2019, 25, 5105–5112 CrossRef CAS PubMed; (c) P. Naumov, S. Chizhik, M. K. Panda, N. K. Nath and E. Boldyreva, Chem. Rev., 2015, 115, 12440–12490 CrossRef CAS; (d) Soft Crystals: Flexible Response Systems with High Structural Order, ed. M. Kato and K. Ishii, Springer, Singapore, 2023 Search PubMed.
- (a) D. Volz, M. Wallesch, C. Fléchon, M. Danz, A. Verma, J. M. Navarro, D. M. Zink, S. Bräse and T. Baumann, Green Chem., 2015, 17, 1988–2011 RSC; (b) P. C. Ford, E. Cariati and J. L. Bourassa, Chem. Rev., 1999, 99, 3625–3648 CrossRef; (c) M. Vitale and P. C. Ford, Coord. Chem. Rev., 2001, 219–221, 3–16 CrossRef; (d) A. Barbieri, G. Accorsi and N. Armaroli, Chem. Commun., 2008, 2185–2193 RSC; (e) E. Cariati, E. Lucenti, C. Botta, U. Giovanella, D. Marinotto and S. Righetto, Coord. Chem. Rev., 2016, 306, 566–614 CrossRef; (f) P. C. Ford, Coord. Chem. Rev., 1994, 132, 129–140 CrossRef; (g) R. Peng, M. Li and D. Li, Coord. Chem. Rev., 2010, 254, 1–18 CrossRef; (h) K. Tsuge, Y. Chishina, H. Hashiguchi, Y. Sasaki, M. Kato, S. Ishizaka and N. Kitamura, Coord. Chem. Rev., 2016, 306, 636–651 CrossRef; (i) A. Mensah, J.-J. Shao, J.-J. Ni, G.-J. Li, F.-M. Wang and L.-Z. Chen, Front. Chem., 2022, 9, 816363 CrossRef PubMed; (j) J. Beaudelot, S. Oger, S. Peruško, T.-A. Phan, T. Teunens, C. Moucheron and G. Evano, Chem. Rev., 2022, 122, 16365–16609 CrossRef; (k) Y. Liu, S.-C. Yiu, C.-L. Ho and W.-Y. Wong, Coord. Chem. Rev., 2018, 375, 514–557 CrossRef; (l) V. W.-W. Yam, V. K.-M. Au and S. Y.-L. Leung, Chem. Rev., 2015, 115, 7589–7728 CrossRef.
- (a) Y. Yamanoi, Acc. Chem. Res., 2023, 56, 3325–3341 CrossRef; (b) Y. Yamanoi, Phys. Chem. Chem. Phys., 2025, 27, 14517–14525 RSC.
- (a) T. Nakae, M. Nishio, T. Yamada and Y. Yamanoi, Molecules, 2021, 26, 6852 CrossRef; (b) Y. Zhao, T. Nakae, S. Takeya, M. Hattori, D. Saito, M. Kato, Y. Ohmasa, S. Sato, O. Yamamuro, T. Galica, E. Nishibori, S. Kobayashi, T. Seki, T. Yamada and Y. Yamanoi, Chem. – Eur. J., 2023, 29, e202204002 CrossRef CAS PubMed.
- Y. Zhao, T. Nakae, M. Yoshida, M. Kato, K. Omoto, S. Ito and Y. Yamanoi, Inorg. Chem., 2024, 63, 22361–22371 CrossRef CAS.
- As related studies, Hg(II) and Zn(II) complexes with 1,1,2,2-tetramethyl-1,2-dipyridin-3-yl)disilane have been reported. See: (a) E. Choi, H. Lee, T. H. Noh and O.-S. Jung, CrystEngComm, 2016, 18, 6997–7002 RSC; (b) E. Choi, M. Ryu, H. Lee and O.-S. Jung, Dalton Trans., 2017, 46, 4595–4601 RSC.
- N. Rani, G. K. Rao and A. K. Singh, J. Organomet. Chem., 2009, 694, 2442–2447 Search PubMed.
- (a) O. S. Wenger, Chem. Rev., 2013, 113, 3686–3733 CrossRef CAS PubMed; (b) X. Zhang, B. Li, Z.-H. Chen and Z.-N. Chen, J. Mater. Chem., 2012, 22, 11427–11441 RSC; (c) E. Li, K. Jie, M. Liu, X. Sheng, W. Zhu and F. Huang, Chem. Soc. Rev., 2020, 49, 1517–1544 RSC; (d) M. Kato, M. Yoshida, Y. Sun and A. Kobayashi, J. Photochem. Photobiol., C, 2022, 51, 100477 CrossRef CAS; (e) M. Kato, Chromic behaviors and color control of luminescent copper(I) complexes, in Photochemistry and Photophysics of Earth-Abundant Transition Metal Complexes, ed. R. V. Eldik and P. C. Ford, Elsevier, 2024, vol. 83, pp. 33–62 Search PubMed; (f) Q. Zhao, F. Li, C. Li, Z.-H. Chen and Z.-N. Chen, Chem. Soc. Rev., 2010, 39, 3007–3030 RSC; (g) X. Zhang, B. Li, Z.-H. Chen and Z.-N. Chen, J. Mater. Chem., 2012, 22, 11427–11441 RSC; (h) A. Kobayashi and M. Kato, Eur. J. Inorg. Chem., 2014, 4469–4483 Search PubMed.
- T. Hasegawa, A. Kobayashi, H. Ohara, M. Yoshida and M. Kato, Inorg. Chem., 2017, 56, 4928–4936 CrossRef CAS PubMed.
- S. Kondo, N. Yoshimura, A. Kobayashi, K. D. C. Kuruppu, W. M. C. Sameera, S. Fujii, M. Yoshida and M. Kato, J. Mater. Chem. C, 2024, 12, 1799–1808 Search PubMed.
- (a) W.-F. Fu, X. Gan, C.-M. Che, Q.-Y. Cao, Z.-Y. Zhou and N.-Y. Zhu, Chem. – Eur. J., 2004, 10, 2228–2236 CrossRef CAS; (b) A. Kobayashi, K. Komatsu, H. Ohara, W. Kamada, Y. Chishina, K. Tsuge, H.-C. Chang and M. Kato, Inorg. Chem., 2013, 52, 13188–13198 CrossRef CAS; (c) S. Ito, K. Tanaka and Y. Chujo, Dalton Trans., 2025, 54, 9219–9225 RSC.
- K. Sato, M. Hattori and Y. Yamanoi, RSC Adv., 2025, 15, 16968–16972 RSC.
- E. R. Barth, C. Golz, M. Knorr and C. Strohmann, Acta Crystallogr., Sect. E:Crystallogr. Commun., 2015, 71, m189–m190 CrossRef CAS PubMed.
- (a) B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832–2838 RSC; (b) A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- (a) L. Garzón-Tovar, Á. Duarte-Ruiz and K. Wurst, Inorg. Chem. Commun., 2013, 32, 64–67 CrossRef; (b) K.-T. Youm, J. Ko and M.-J. Jun, Polyhedron, 2006, 25, 2717–2720 CrossRef CAS.
- (a) N. T. Kim, H. Li, L. Venkataraman and J. L. Leighton, J. Am. Chem. Soc., 2016, 138, 11505–11508 CrossRef CAS PubMed; (b) P. Gualco, S. Ladeira, K. Miqueu, A. Amgoune and D. Bourissou, Angew. Chem., Int. Ed., 2011, 50, 8320–8324 CrossRef CAS; (c) P. Gualco, S. Ladeira, K. Miqueu, A. Amgoune and D. Bourissou, Organometallics, 2012, 31, 6001–6004 CrossRef CAS; (d) P. Gualco, A. Amgoune, K. Miqueu, S. Ladeira and D. Bourissou, J. Am. Chem. Soc., 2011, 133, 4257–4259 CrossRef CAS.
- (a) P. Kar, M. Yoshida, Y. Shigeta, A. Usui, A. Kobayashi, T. Minamidate, N. Matsunaga and M. Kato, Angew. Chem., Int. Ed., 2017, 56, 2345–2349 CrossRef CAS PubMed; (b) E. J. Fernández, J. M. López-de-Luzuriaga, M. Monge, M. E. Olmos, R. C. Puelles, A. Laguna, A. A. Mohamed and J. P. Fackler, Inorg. Chem., 2008, 47, 8069–8076 CrossRef; (c) J. Vallejo, E. Pardo, M. V. Chumillas, I. Castro, P. Amoros, M. Deniz, C. Ruiz-Perez, C. Y. Vivas, J. Krzystek, M. Julve, F. Lloret and J. Cano, Chem. Sci., 2017, 8, 3694–3702 RSC.
- D. E. Royzman, A. M. Noviello, K. M. Henline, R. D. Pike, J. P. Killarney, H. H. Patterson, C. Crawford and Z. Assefa, J. Inorg. Organomet. Polym., 2014, 24, 66–77 CrossRef CAS.
- (a) R. Ishimatsu, S. Matsunami, T. Kasahara, J. Mizuno, T. Edura, C. Adachi, K. Nakano and T. Imato, Angew. Chem., Int. Ed., 2014, 53, 6993–6996 CrossRef CAS; (b) H. Tanaka, K. Shizu, H. Miyazaki and C. Adachi, Chem. Commun., 2012, 48, 11392–11394 RSC.
- For the examples on metal complexes showing TADF, see: (a) E. Cariati, D. Roberto, R. Ugo, P. C. Ford, S. Galli and A. Siron, Inorg. Chem., 2005, 44, 4077–4085 CrossRef CAS PubMed; (b) H. V. R. Dias, H. V. K. Diyabalanage, M. G. Eldabaja, O. Elbjeirami, M. A. Rawashdeh-Omary and M. A. Omary, J. Am. Chem. Soc., 2005, 127, 7489–7501 CrossRef CAS; (c) A. Kobayashi, T. Ehara, M. Yoshida and M. Kato, Inorg. Chem., 2020, 59, 9511–9520 CrossRef CAS PubMed; (d) L. Kang, J. Chen, T. Teng, X.-L. Chen, R. Yu and C.-Z. Lu, Dalton Trans., 2015, 44, 11649–11659 RSC.
- CCDC 2477874: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p5ffr.
| This journal is © The Royal Society of Chemistry 2025 |