Open Access Article
Open Access ArticleRhodium(I) complexes with proton-responsive anionic tethered N-heterocyclic carbene ligands: synthesis and application in alkyne dimerization
Mert Olgun
Karataş
 *ab,
Diego R.
Hinojosa
*ab,
Diego R.
Hinojosa
 a,
Vincenzo
Passarelli
a,
Vincenzo
Passarelli
 a,
Luis A.
Oro
a,
Luis A.
Oro
 a,
Jesús J.
Pérez-Torrente
a,
Jesús J.
Pérez-Torrente
 a and
Ricardo
Castarlenas
a and
Ricardo
Castarlenas
 *a
*a
aInstituto de Síntesis Química y Catálisis Homogénea (ISQCH)-Departamento de Química Inorgánica, CSIC-Universidad de Zaragoza, C/Pedro Cerbuna 12, CP. 50009, Zaragoza, Spain. E-mail: mkaratas@unizar.es; rcastar@unizar.es
bDepartment of Chemistry, Faculty of Sciences, Inönü University, 44280, Malatya, Turkey
First published on 19th September 2025
Abstract
A series of rhodium(I) complexes of formula Rh{κ2C,X-(IDippR)}(cod) and Rh{κ2C,X-(IDippR)}(CO)2 {IDippR = 1-(R)-3-(2,6-diisopropylphenyl)-imidazolin-2-carbene; X = N, O; R = (6-yl-2-pyridone), (CH2CH2NC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Ph), (CH2CH2COO); (cod = 1,5-cyclooctadiene)} containing anionic-tethered NHC-functionalized ligands, including pyridonato, amidato or carboxylato groups at the N-wingtip, has been prepared and their application to alkyne homocoupling has been investigated. The chelate coordination of the NHC-functionalized ligand generates 5, 6 and 7-membered metallacycles for pyridonato, amidato and carboxylato substituents, respectively. Preparation of the mixed bis-NHC derivative Rh{κ2C,O-(IDippCarbx)}(CO)(IPr) {IPr = 1,3-bis-(2,6-diisopropylphenyl)-imidazolin-2-carbene} is possible due to lability of one of the CO ligands in the bis-CO precursor. Reversible protonation is observed for pyridonato and carboxylato moieties but not for amidato counterpart. Alkyne dimerization catalytic activity is reported for N-based anionic groups whereas carboxylato counterparts are inefficient. A proton transfer mechanism is invoked for the gem-selectivity observed for cod-based catalysts whereas alkyne insertion is the regioselectivity-determining step for CO-containing analogues.
O)Ph), (CH2CH2COO); (cod = 1,5-cyclooctadiene)} containing anionic-tethered NHC-functionalized ligands, including pyridonato, amidato or carboxylato groups at the N-wingtip, has been prepared and their application to alkyne homocoupling has been investigated. The chelate coordination of the NHC-functionalized ligand generates 5, 6 and 7-membered metallacycles for pyridonato, amidato and carboxylato substituents, respectively. Preparation of the mixed bis-NHC derivative Rh{κ2C,O-(IDippCarbx)}(CO)(IPr) {IPr = 1,3-bis-(2,6-diisopropylphenyl)-imidazolin-2-carbene} is possible due to lability of one of the CO ligands in the bis-CO precursor. Reversible protonation is observed for pyridonato and carboxylato moieties but not for amidato counterpart. Alkyne dimerization catalytic activity is reported for N-based anionic groups whereas carboxylato counterparts are inefficient. A proton transfer mechanism is invoked for the gem-selectivity observed for cod-based catalysts whereas alkyne insertion is the regioselectivity-determining step for CO-containing analogues.
Introduction
N-Heterocyclic Carbenes (NHCs)1 are fascinating molecules with widespread applications ranging from biomedicine2 to materials science,3 but are particularly prominent in catalysis.4 Their unique structural framework allows for fairly unlimited design possibilities, driving the development of a myriad of mild-condition synthetic methods.5 Notably, the incorporation of a non-spectator functional group on the N-wingtip has enabled their participation in very efficient Metal–Ligand Cooperative (MLC) transformations.6 In this context, functional groups based on O,7 N,8 P,9 S,10 and Si11 heteroatoms, as well as carbon-based substituents,12 have been reported. Moreover, these types of bidentate or multidentate ligands could also play a crucial role in catalysis by stabilizing low-coordinated active species.13 In particular, NHCs decorated with deprotonatable moieties, such as pyridone,14 amide15 or carboxylic acids,16 have been successfully used to prepare anionic tethered NHC–metal catalysts (Chart 1a). The benefits of these pendant scaffolds are not limited to acting as internal bases that facilitate proton transfer processes, but also extend to modulating catalytic activity and selectivity through interactions with the metal center or substrates. Indeed, they prevent catalyst decomposition in acidic environments through imidazolium salt formation,17 where the pendant basic group act as a proton sponge that inhibits metal–NHC bond cleavage.18 | ||
| Chart 1 (a) Selected examples of catalysts with pyridonato- and carboxylato-tethered NHC ligands. (b) Rh–NHC–BHetA catalysts for alkyne functionalization. (c) Complexes prepared in this work. | ||
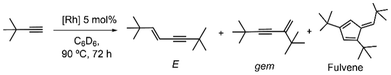
Alkyne dimerization is a straightforward and atom-economical route to 1,3-enynes, which are key structural motifs in a variety of biologically active molecules or functional materials.19 However, achieving high chemo-, regio- and stereoselectivity is a challenging task due to a multifaceted mechanistic scenario,20 which also includes competing pathways leading to cyclotrimers,21 polymers,22 butatrienes23 or other complex cyclic structures such as fulvenes,24 pentalenes,25 azulenes,26 or bicyclooctatrienes.27 While a few selective catalysts for E-,28Z-29 or gem-enynes12b,30 have been developed, further research is warranted not only to improve catalyst efficiency but also to gain deeper mechanistic insights that could be valuable for other C–H activation and C–C coupling transformations. In general, alkyne migratory insertion into metal–hydride or metal–alkynyl bonds is the rate- and regioselectivity-determining step.31 However, proton transfer processes from the terminal position of one alkyne to another have been demonstrated to be highly efficient alternative pathways.32 In this context, our group recently reported highly active Rh–NHC catalysts for the specific head-to-tail dimerization of alkynes to form 1,3-gem-enynes via a Ligand Assisted Proton Shuttle (LAPS) mechanism.20,33 We found that Bis-Heteroatomic Acidato ligands (BHetA), such as carboxylato, amidato, amidinato, and especially pyridonato, can act as efficient proton transfer agents, enhancing activity and selectivity (Chart 1b). Based on these findings, we envisage the potential of rhodium complexes bearing nitrogen- and oxygen-tethered NHC ligands as effective proton carriers for catalytic applications. Herein, we report on the synthesis of Rh(I) complexes with pyridonato-, amidato-, and carboxylato tethered-NHCs and their performance as alkyne dimerization catalysts.
Results and discussion
Synthesis and characterization of NHC-pyridonato Rh(I) complexes
The 1-(6-hydroxypyridin-2-yl)-3-(2,6-diisopropylphenyl)-imidazol-3-ium bromide salt [IDippOpyH2]Br (1) was prepared according to previously described procedures.34 In view of the tautomeric equilibrium35 of the heterocyclic tether between pyridone34a or hydroxypyridine34b isomer, the X-ray diffraction analysis of 1 was carried out (Fig. 1). As a matter of fact, the crystal structure of 1 revealed that the salt adopts the hydroxypyridine tautomeric form (Fig. 1a). In agreement with the presence of the OH group, the C8–O12 bond length [1.341(4) Å] is similar to that reported for Carom–O single bonds [1.362 Å, av.].36 The asymmetric unit of the crystal structure of 1 contains the ion pair IDippOpyH2/bromide. Within the IDippOpyH2 cation, the aromatic ring of the 2,6-iPr2C6H3 group lies almost perpendicular to the NHC core, whereas the hydroxypyridinyl fragment and the NHC core are nearly coplanar [O12–H12–Br 176.66(16)°]. Notably, O12–H12⋯Br and the C1–H1⋯Br intermolecular contacts are observed, thus rendering infinite chains growing along the b axis (Fig. 1b).Metalation of the ligand was achieved by treatment of 1 with [Rh(μ-Cl)(cod)]2 (cod = 1,5-cyclooctadiene) in the presence of 3 equivalents of tBuOK in THF for 30 min. The mononuclear chelate complex Rh{κ2C,N-(IDippOpy)}(cod) (2) was obtained as a result of the deprotonation of both the imidazolium and hydroxypyridine moieties (Scheme 1). In contrast to 1, the pyridonato tautomeric form of the tether was observed in 2. The use of an excess of base was deemed to be essential to incorporate the NHC-functionalized ligand into the metal center. In fact, several attempts to prepare the putative Rh(I) derivative RhX{κC-(IDippOpyH)}(cod) by selective deprotonation of the imidazolium fragment were unsuccessful. Moreover, the use of the monoolefin complex [Rh(μ-Cl)(η2-coe)2]2 (coe = cyclooctene) as a synthetic precursor resulted in a mixture of unidentified products. Bubbling carbon monoxide through a CH2Cl2 solution of 2 afforded the bis-carbonyl compound Rh{κ2C,N-(IDippOpy)}(CO)23, which was isolated as a yellow solid in 78% yield.
The solid-state structure of 2 was determined by X-ray diffraction analysis on single crystals obtained by slow diffusion of n-hexane into a concentrated CDCl3 solution of 2 (Fig. 2). The crystal structure of 2 shows a square planar coordination polyhedron at the metal centre given by the bidentate cod and IDippOpy ligands. As consequence of the short bite angles C1–Rh–N7 [78.37(12)°] both the NHC and the pyridine moieties of the IDippOpy ligand strongly deviate from the ideal arrangement with respect to the Rh–C1 and Rh–N7 bonds (NHC: pitch ϑ 1.2° yaw ψ 14.3°; py: pitch ϑ 7.0°; yaw ψ 7.0°).37 Notably, when comparing the C8–O12 bond lengths in 1 [1.341(4) Å] and 2 [1.247(4) Å], the short C8–O12 bond length of 2 is indicative of the fact that the IDippOpy moiety switches from a hydroxypyridine motif in 1 to a pyridonato scaffold in 2 upon deprotonation and coordination to rhodium.
The NMR spectra of 2 are in agreement with the structure observed in the solid state. The appearance of only one septuplet at δ 2.72 ppm for the two CH-isopropyl protons along with two multiplets at δ 6.18 and 3.50 ppm for the CH moieties of the cod ligand in the 1H NMR spectrum of 2, indicates an in-plane disposition of the NHC moiety relative to the metal coordination plane. This molecular topology defines a plane of symmetry that includes the imidazole and pyridone rings and bisects both the Dipp and cod groups. The assignment of the two doublets at δ 7.40 and 6.66 ppm, corresponding to the imidazolinyl protons, is inferred from their NOE correlations with the pyridonato and CH-isopropyl protons, respectively (Fig. 3a). The most characteristic signals in the 13C{1H}-APT spectrum are the three doublets observed for the carbon atoms coordinated to the rhodium center at δ 174.7 ppm (JC–Rh = 56.6 Hz) for the carbene, 105.0 ppm (JC–Rh = 6.7 Hz) for the trans-to-NHC cod, and 70.8 ppm (JC–Rh = 12.4 Hz) for the cis-to-NHC cod. Additionally, coordination of the nitrogen atom of the pyridonato tether to form a five-membered metallacycle is confirmed by the long range 1H–15N HSQC NMR experiment (Fig. 3b). A correlation peak is observed at δN 195.8 ppm, which falls within the typical range reported for Rh-κN-pyridonato species, but is approximately 20 ppm more shielded than that of uncoordinated pyridine-2-olato κO-derivatives.24 Concurrently, the carbene ligand of the bis-carbonyl complex 3 also adopt an in-plane disposition, as evidenced by the observation of only one resonance for the CH-isopropyl protons. Moreover, the 13C{1H}-APT spectrum of 3 confirms the presence of two carbonyl ligands resonating at δ 188.1 ppm (JC–Rh = 57.0 Hz) and 187.3 ppm (JC–Rh = 68.1 Hz).
Synthesis and characterization of NHC-amidato Rh(I) complexes
With the aim of introducing a basic amidato substituent into the NHC framework, the new 1-(2-benzamidoethyl)-3-(2,6-diisopropylphenyl)-imidazol-3-ium chloride salt (4) was synthesized via N-alkylation of 1-(2,6-diisopropylphenyl)-imidazole with N-(2-chloroethyl)benzamide in 74% yield (Scheme 2). Treatment of 4 with [Rh(μ-Cl)(cod)]2 and 2 equivalents of tBuOK in THF at room temperature for 16 h led to the formation of the six-membered metallacycle NHC-amidato complex Rh{κ2C,N-(IDippAmd)}(cod) (5). Exchange of cod by CO in 5 was not as straightforward as might be expected. Bubbling CO through a CH2Cl2 solution of 5 for 15 min afforded the bis-carbonyl complex Rh{κ2C,N-(IDippAmd)}(CO)2 (6), together with other unidentified products. In contrast, the transformation proceeds more cleanly upon bubbling CO in THF for only 5 min. It is noteworthy that previous attempts to exchange cod by CO within a related complex were unsuccessful.15cThe solid-state structure of 5 was determined by X-ray diffraction analysis on single crystals obtained by slow diffusion of n-hexane into a concentrated CH2Cl2 solution of 5 (Fig. 4). The crystal structure of 5 shows a distorted square planar geometry of the metal centre with a κ2C,N coordination of the ligand IDippAmd, thus confirming the deprotonation of both the imidazolium and the amido moieties. The resulting six-membered metallacycle Rh–C1–N2–C6–C7–N8 adopts a boat conformation with the Rh and C6 atoms in the out-of-plane position. Notably, the atom N8 exhibits a planar geometry [ ] and the C9–O10 [1.263(3) Å] and C9–N8 [C9–N8 1.327(3) Å] bond lengths are similar to those reported for the Csp2
] and the C9–O10 [1.263(3) Å] and C9–N8 [C9–N8 1.327(3) Å] bond lengths are similar to those reported for the Csp2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Csp2–N moieties.36 On the other hand, the bidentate coordination [C1–Rh–N8 85.21(8)°] of the ligand IDippAmd causes a significant deviation of the NHC core from the ideal arrangement with respect to the Rh–C1 bond (NHC: pitch ϑ 4.0°; yaw ψ 12.1°).37
O and Csp2–N moieties.36 On the other hand, the bidentate coordination [C1–Rh–N8 85.21(8)°] of the ligand IDippAmd causes a significant deviation of the NHC core from the ideal arrangement with respect to the Rh–C1 bond (NHC: pitch ϑ 4.0°; yaw ψ 12.1°).37
The NMR spectrum of 5 in CD2Cl2 at room temperature displays broad signals, showcasing the fluxional behaviour of the molecule, which is likely associated to the ring-flipping process of the six-membered metallacycle. However, well-defined resonances were observed in the 1H NMR spectrum at 263 K. In contrast to 2 and 3, an out-of-plane disposition of the NHC ring is observed for 5. Thus, as a consequence of the lack of symmetry, the methylene protons of the linker are diastereotopic and display two doublets of doublets of doublets at δ 5.25 and 4.38 ppm for NNHCCH2 protons, and a multiplet at 3.52 ppm for the NAmdCH2 protons. Also, the four olefin cod protons and the two CHDipp hydrogen atoms are inequivalent. The coordination of the carbene to the rhodium atom is supported by a doublet at δ 178.5 ppm (JC–Rh = 53.7) in the 13C{1H}-APT NMR spectrum. The substitution of cod by two carbonyl ligands in 6 results in a steric relief that allows the NHC ring to adopt an in-plane disposition resulting in Cs symmetry. This is reflected in the chemical equivalence of the protons of both methylene linkers of the NHC-amidato ligand. Moreover, the 13C{1H}-APT spectrum shows two doublets at 186.9 (JC–Rh = 63.0, COcis-NHC) and 184.9 ppm (JC–Rh = 58.2, COtrans-NHC) which confirms the presence of two carbonyl ligands in 6.
Synthesis and characterization of NHC-carboxylato Rh(I) complexes
Complex Rh{κ2C,O-(IDippCarbx)}(cod) (8), featuring a carboxylato-functionalized NHC ligand, was prepared in a similar way to that described for 2 and 5 from the known 1-(2-carboxyethyl)-3-(2,6-diisopropylphenyl)-imidazol-3-ium chloride salt (7)38 and isolated as a yellow solid in 85% yield. The proposed structure shows a seven-membered metallacycle resulting from the bidentate κ2C,O coordination of the ligand (Scheme 3). In fact, the difference between the asymmetric (1601 cm−1) and symmetric (1375 cm−1) carboxylato stretching bands in the infrared spectrum supports a κ1O-carboxylato coordination.39 An analogous chelate structure has been described for a Pd–NHC–carboxylato derivative.40 Similar to 5, complex 8 exhibits a fluxional process that is frozen at 213 K. However, the energy barrier for the inversion of the seven-membered NHC-carboxylato metallacycle of 8 is expected to be lower than that of the six-membered metalacyle of 5, for which well-resolved signals are observed upon cooling to 263 K. At 213 K, the 1H NMR spectrum of 8 displays two sets of signals for the diastereotopic methylene protons and the CH-isopropyl groups of the NHC wingtips. The 1H–1H NMR NOESY experiment at 213 K shows misleading cross-peaks due to a spin diffusion effect, therefore 1H–1H NMR ROESY experiment was recorded instead, confirming the proposed structure for 8 (see Fig. S30 in SI). Moreover, the carbene carbon atom is observed as a doublet at δ 178.4 ppm with a JC–Rh of 54.4 Hz in the 13C{1H}-APT spectrum.Interestingly, the NHC-carboxylato complex 8 exhibits a distinct reactivity compared to the NHC-pyridonato (2) and NHC-amidato (5) analogues upon exposure to carbon monoxide. Thus, in addition to the expected square planar bis-carbonyl complex Rh{κ2C,O-(IDippCarbx)}(CO)29a, a structural isomer 9b was detected in a 9a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9b 52
9b 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 equilibrium ratio in solution at 233 K, confirmed by the exchange peaks observed in the EXSY NMR experiment (Fig. S36 in SI). Compound 9a exhibits in the 13C{1H}-APT NMR spectrum the two expected low field doublets for the inequivalent carbonyl ligands at δ 186.8 and 186.7 ppm, with a JC–Rh of 56.0 and 58.2 Hz, respectively. However, the two CO ligands in 9b are equivalent, showing a single resonance at 186.4 ppm (JC–Rh = 69.0 Hz) (Fig. 5). A plausible explanation for this observation is the description of 9b as adopting a saw-horse or pseudo-tetrahedral structure.41 Nonetheless, at this point an Y-shaped three coordinated zwiterionic species, bearing a dangling carboxylate, or a dinuclear A-frame structure could not be ruled out. A transoid disposition for two carbonyl ligands within RhI derivatives is rare but not unprecedented.42
48 equilibrium ratio in solution at 233 K, confirmed by the exchange peaks observed in the EXSY NMR experiment (Fig. S36 in SI). Compound 9a exhibits in the 13C{1H}-APT NMR spectrum the two expected low field doublets for the inequivalent carbonyl ligands at δ 186.8 and 186.7 ppm, with a JC–Rh of 56.0 and 58.2 Hz, respectively. However, the two CO ligands in 9b are equivalent, showing a single resonance at 186.4 ppm (JC–Rh = 69.0 Hz) (Fig. 5). A plausible explanation for this observation is the description of 9b as adopting a saw-horse or pseudo-tetrahedral structure.41 Nonetheless, at this point an Y-shaped three coordinated zwiterionic species, bearing a dangling carboxylate, or a dinuclear A-frame structure could not be ruled out. A transoid disposition for two carbonyl ligands within RhI derivatives is rare but not unprecedented.42
 | ||
| Fig. 5 Selected region of the 13C{1H}-APT NMR spectrum at 233 K of the equilibrium mixture of 9a, 9b. | ||
Reaction of the mixture of 9a and 9b with IPr {IPr = 1,3-bis-(2,6-diisopropylphenyl)-imidazolin-2-carbene} in THF gave the mixed-NHC complex Rh{κ2C,O-(IDippCarbx)}(CO)(IPr) (10), which was isolated as a white solid in 92% yield. The 1H NMR spectrum revealed a single isomer displaying the characteristic signals of both IPr and IDippCarbx carbene ligands, and a single carbonyl ligand. Thus, three doublets at δ 191.2 (JC–Rh = 79.5 Hz, CO), 189.5 (JC–Rh = 43.3 Hz, Rh–CIPr), 185.7 ppm (JC–Rh = 43.1 Hz, Rh–CNHC–carbx) and one singlet at 174.3 ppm for the κ1O-carboxylato ligand appear in the low field region of the 13C{1H}-APT NMR spectrum of 10. The spectroscopic data are consistent with a structure in which both NHC moieties adopt a trans arrangement to minimize steric repulsion (Scheme 4).43
Protonation of NHC–BHetA ligands
In order to assess the ability of the anionic functionalized NHC ligands in proton transfer processes, we investigated the protonation and deprotonation of the cod complexes 2, 5 and 8. Protonation was carried out by adding 1.5 equivalents of triflic acid to 0.5 mL of CD2Cl2 (2 and 5) or CDCl3 (8) solutions of the BHetA complexes in an NMR tube at 223 K (Scheme 5). Formation of protonated species 11–13 was immediately observed by 1H NMR measurements at 223 K. No high-shielded signal attributable to the putative Rh(III)–hydride species, previously observed upon protonation of the IPr-chlorido analogue [Rh(μ-Cl)IPr(η2-coe)]2,44 was detected in the 1H NMR spectra. It is noteworthy that the complexes remain stable in solution for over 24 hours upon warming the reaction mixture to room temperature, with no evidence of imidazolium salt formation.17 Strong evidence for the cationic character of the complexes is provided by the detection of a single fluorine signal at δ −78.8 ppm in the 19F NMR spectra. Protonation of the pyridonato complex 2 occurs at the oxygen atom, leading to the formation of a hydroxypyridine moiety to give [Rh{κ2C,N-(IDippOpyH)}(cod)]OTf (11). In agreement with the proposed formation of the OH group, the broad signal at δ 11.46 ppm does not correlate with any nitrogen atom in the 1H–15N HMQC NMR spectrum. Moreover, the resonances corresponding to the protons of the heterocyclic ring (H10 and H12) shifted downfield upon protonation. In contrast to that observed for 2, protonation of the amidato derivative 5 takes place at the nitrogen atom resulting in the formation of the cationic complex [Rh{κ2C,O-(IDippAmdH)}(cod)]OTf (12). The new N–H signal appears as a broad triplet at δ 9.13 ppm and correlates with a nitrogen atom at 114.0 ppm in the 1H–15N J1-HMQC NMR spectrum. The observed nitrogen NMR chemical shift, falling in a similar range to that observed for the free ligand 4 (106.8 ppm), agrees with an uncoordinated nitrogen atom for 12. In addition, the presence of a free triflate anion inferred by 19F NMR suggests that the amide carbonyl group is likely coordinated given rise to an eight-membered metallacycle.45 In the case of the protonation of the carboxylato complex 8, the changes in the NMR signals were less pronounced than those observed for the pyridonato and amidato derivatives. Nevertheless, the observation of diastereotopic methylene protons supports the presence of a metallacycle motif in [Rh{κ2C,O-(IDippCarbxH)}(cod)]OTf (13).Next, the deprotonation of 11–13 was studied. Two equivalents of triethylamine were added to NMR tubes containing solutions of freshly formed 11–13 at room temperature. Within 10 minutes, the hydroxypyridine and carboxylic acid complexes were deprotonated, reverting to their respective parent complexes 2 and 8, respectively (Fig. 6). In contrast, the amide complex 12 remained unchanged for several days at room temperature, and heating the sample at 60 °C led to the decomposition of the complex. Interestingly, it was observed that in CDCl3 the amidato complex 5 transform into a new species RhCl{κ2C,O-(IDippAmdH)}(cod) (14),15c after 3 days at room temperature under day light conditions, likely induced by trace amounts of hydrochloric acid generated from CDCl3 over time (Scheme 6). In contrast, the pyridonato and carboxylato derivatives remain stable under CDCl3 solutions for a week. The distinct behaviour of the amidato complex can be explained by its higher basicity. Therefore, the susceptibility of the amidato moiety to protonation, along with its resistance to deprotonation in the presence of a base such as triethylamine, may represent a limitation in catalytic processes involving proton transfer.
 | ||
| Fig. 6 (a) Selected region of the 1H NMR spectrum of 2 in CD2Cl2. (b) treatment of 2 with HOTf to yield 11. (c) 11 after addition of NEt3. | ||
Catalytic reactions
Different mechanistic pathways can be invoked for alkyne dimerization, including oxidative addition, vinylidene, external nucleophilic attack, base mediated, or LAPS.20,33 In addition, alkyne oligomerization or polymerization can be competitive processes. In this context, we have recently disclosed that the presence of an internal base in Rh–NHC catalysts, such as a BHetA ligand, enhances the reactivity, but the catalytic outcome varies as a function of the ancillary ligand (Scheme 7). Thus, in the presence of a labile cyclooctene ligand the 1,3-gem-enynes are obtained via a LAPS process,33 but in the case of a CO ligand a (2 + 2 + 1) cyclotrimerization results in the formation of fulvenes.24 The effect of attaching a BHetA moiety at the wingtip of a NHC ligand has now been investigated. | ||
| Scheme 7 Proposed mechanistic pathways for alkyne functionalization. (a) LAPS, (b) base-mediated, (c) fulvene formation via interrupted dimerization. | ||
The alkyne tert-butylacetylene was chosen as a benchmark substrate because of the versatile chemoselectivity previously observed in function of the catalyst employed. Catalytic reactions were performed in NMR tubes in C6D6 (0.5 mL) using a 5 mol% of catalyst loading at 90 °C for 72 h (Table 1). Conversion and selectivity were monitored by 1H NMR spectroscopy. The cod derivatives with pyridonato (2) or amidato (5) ligands are less active than their CO counterparts 3 and 6, showing high gem-selectivity of 80 and 95%, respectively (entries 1 and 3), whereas NHC-carboxylato complex 8 is inactive (entry 5). In contrast, the CO-pyridonato compound 3 is the most active of the series but with reverse regioselectivity towards the E-enyne. Furthermore, small amounts of fulvene (4%) were also detected (entry 2). The fulvene quantity slightly increases when using the amidato-CO catalyst 6, but with a concomitant decrease in both activity and regioselectivity (entry 4). Carboxylato-CO catalyst 9 was unselective, whereas the bis-NHC compound 10 was completely inactive.
| Entry | Catalyst | Conversionb (%) | Selectivityb (%) E/gem/fulvene |
|---|---|---|---|
| a Experiments were carried out in C6D6 (0.5 mL) at 90 °C using an alkyne/catalyst ratio of 100/5, [catalyst] = 20 mM. b Conversion and selectivity were determined by 1H NMR. | |||
| 1 | 2 | 22 | 20/80/— |
| 2 | 3 | 83 | 88/8/4 |
| 3 | 5 | 14 | 5/95/— |
| 4 | 6 | 67 | 26/64/10 |
| 5 | 8 | — | —/—/— |
| 6 | 9 | 16 | 28/71/— |
| 7 | 10 | — | —/—/— |
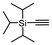
The most active catalyst 3 was subsequently evaluated for other alkynes (Table 2). Selectivity to E-enynes was maintained for the substrates studied although with slightly lower values ranging from 58–72%. In the case of triisopropylsilyl and aromatic alkynes also the Z-enyne was also formed.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Alkyne | Conversiona (%) | Selectivityb (%), E/Z/gem |
|---|---|---|---|
| a Experiments were carried out in C6D6 (0.5 mL) at 90 °C using an alkyne/catalyst ratio of 100/5, [3] = 20 mM. b Conversion and selectivity were determined by 1H NMR. | |||
| 1 |

|
83 | 88/—/8 |
| 2 |

|
48 | 67/—/33 |
| 3 |

|
63 | 68/—/32 |
| 4 |
|
78 | 64/27/9 |
| 5 |

|
77 | 68/21/11 |
| 6 |

|
93 | 58/14/28 |
| 7 |

|
53 | 72/17/11 |
| 8 |

|
82 | 60/14/26 |
Catalytic results can be analysed on the basis of our previous mechanistic studies on untethered NHC–BHetA analogues (Scheme 7).20,24,33 The predominant formation of the gem-enyne from cod derivatives suggests a proton-transfer mechanism via LAPS promoted by the BHetA-type pyridonato or amidato ligands (Scheme 6a), although alternative pathways including oxidative addition or base-mediated could not be excluded. However, the relatively low activity of these catalysts may be attributed to the strongly bound cod ligand, in contrast to the more labile monoolefin ligand present in untethered Rh(NHC)(BHetA)(coe) catalysts.33 At this point, the low activity associated to the carboxylato ligand is unexplained but is consistent with previous observations for related Rh–NHC-carboxylato catalysts.20 Conversely, the reversed selectivity towards E-enynes and trace formation of fulvenes observed for CO-bearing catalysts, suggests an insertion process as the regioselectivity-determining step (Scheme 7b and c).
Conclusion
A series of rhodium(I) complexes featuring anionic-tethered NHC-functionalized scaffolds, including pyridonato, amidato, and carboxylato functionalities, and bearing cod or CO as ancillary ligands, has been prepared and their application in alkyne homocoupling has been investigated. The chameleonic behavior of the pendant N-heterocyclic moiety is evidenced by its appearance as the hydroxypyridine tautomer in the salt, while it adopts a pyridonato form in the complexes. A coplanar arrangement of NHC and pyridonato rings within a five-membered metallacycle has been observed. In contrast, amidato-NHC derivatives feature a six-membered metallacycle which adopts a boat conformation. Moreover, NHC-carboxylato complexes display seven-membered metallacycle structures. The lability of one CO ligand in bis-CO NHC-carboxylato species enables the preparation of a mixed bis NHC complex. Protonation of the anionic ligand is reversible, except in the case of the NHC-amidato counterpart, for which the formation of an eight-membered metallacycle is proposed. Alkyne dimerization catalytic activity is observed for N-based anionic groups whereas their carboxylato counterparts are inactive. A proton transfer mechanism is proposed to account for the gem-selectivity observed with COD-based catalysts, whereas alkyne insertion constitutes the regioselectivity-determining step for CO-containing analogues. Further studies are currently underway in our laboratories to develop more efficient catalysts featuring anionic-tethered NHC motifs.Experimental
General considerations
All reactions were carried out with rigorous exclusion of air and moisture using Schlenk-tube techniques and a dry box when necessary. Reagents were purchased from commercial suppliers and used as received except for alkynes which were dried over molecular sieves. Particularly, phenylacetylene was first dried over molecular sieves and then distilled and stored over anhydrous CaCl2. Organic solvents were obtained oxygen- and water-free from a Solvent Purification System (Innovative Technologies). Deuterated solvents were dried and deoxygenated prior to use: C6D6 and toluene-d8 with sodium, CD2Cl2 with calcium hydride, and CDCl3 and CD3CN with molecular sieves. The organometallic precursor [Rh(μ-Cl)(cod)]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 and the imidazolium salts 1
46 and the imidazolium salts 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 and 7,38 were prepared as previously described in the literature. NMR chemical shifts (expressed in parts per million) are referenced to residual solvent peaks (1H and 13C) or external CFCl3 (19F) and NH3 (15N). Coupling constants, J, are given in hertz (Hz). Spectral assignments were achieved by combination of 1H, 13C{1H}-APT, 1H–1H COSY, and 1H–13C HSQC and HMBC experiments. The 2D 1H–15N HMQC long range experiments were recorded with a JN–H = 8 or 4 Hz. Attenuated total reflection infrared spectra (ATR-IR) of solid samples were recorded on a PerkinElmer Spectrum 100 FT-IR spectrometer. C, H, and N analyses were carried out in a PerkinElmer 2400 CHNS/O analyzer. High-resolution electrospray ionization mass spectra (HRMS-ESI) were recorded using a Bruker MicroToF-Q equipped with an API-ESI source and a Q-ToF mass analyzer, which leads to a maximum error in the measurement of 5 ppm, using sodium formate as a reference.
34 and 7,38 were prepared as previously described in the literature. NMR chemical shifts (expressed in parts per million) are referenced to residual solvent peaks (1H and 13C) or external CFCl3 (19F) and NH3 (15N). Coupling constants, J, are given in hertz (Hz). Spectral assignments were achieved by combination of 1H, 13C{1H}-APT, 1H–1H COSY, and 1H–13C HSQC and HMBC experiments. The 2D 1H–15N HMQC long range experiments were recorded with a JN–H = 8 or 4 Hz. Attenuated total reflection infrared spectra (ATR-IR) of solid samples were recorded on a PerkinElmer Spectrum 100 FT-IR spectrometer. C, H, and N analyses were carried out in a PerkinElmer 2400 CHNS/O analyzer. High-resolution electrospray ionization mass spectra (HRMS-ESI) were recorded using a Bruker MicroToF-Q equipped with an API-ESI source and a Q-ToF mass analyzer, which leads to a maximum error in the measurement of 5 ppm, using sodium formate as a reference.
Synthesis procedures and characterization data of Rh–NHC complexes
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.1 (C9), 145.6 (C4), 137.6 (C11), 134.5 (C3), 130.8 (C6), 124.9 (C2), 124.2 (C5), 119.4 (C12), 114.7 (C1), 105.0 (d, JC–Rh = 6.7, CHcod–trans-NHC), 89.8 (C10), 70.8 (d, JC–Rh = 12.4, CHcod–cis-NHC), 31.5 and 30.0 (CH2-cod), 28.5 (C7), 26.0 and 22.5 (C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CDCl3, 298 K): δ 214.0 (NNHC–Opy), 195.8 (NOpy), 185.9 (NNHC–Dipp).
O), 150.1 (C9), 145.6 (C4), 137.6 (C11), 134.5 (C3), 130.8 (C6), 124.9 (C2), 124.2 (C5), 119.4 (C12), 114.7 (C1), 105.0 (d, JC–Rh = 6.7, CHcod–trans-NHC), 89.8 (C10), 70.8 (d, JC–Rh = 12.4, CHcod–cis-NHC), 31.5 and 30.0 (CH2-cod), 28.5 (C7), 26.0 and 22.5 (C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CDCl3, 298 K): δ 214.0 (NNHC–Opy), 195.8 (NOpy), 185.9 (NNHC–Dipp).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 150.4 (C9), 146.5 (C4), 139.2 (C11), 135.2 (C3), 131.6 (C6), 125.0 (C5), 124.7 (C2), 117.2 (C12), 116.5 (C1), 90.3 (C10), 28.9 (C7), 24.7 and 23.9 (C8).
O), 150.4 (C9), 146.5 (C4), 139.2 (C11), 135.2 (C3), 131.6 (C6), 125.0 (C5), 124.7 (C2), 117.2 (C12), 116.5 (C1), 90.3 (C10), 28.9 (C7), 24.7 and 23.9 (C8).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 145.5 (C4), 138.9 (NCHN), 132.8 (C12), 132.0 (C6), 131.8 (C15), 130.1 (C3), 128.6 (C14), 128.0 (C13), 124.7 (C5), 124.3 (C2), 123.4 (C1), 49.2 (C9), 39.2 (C10), 28.7 (C7), 24.4 and 23.9 (C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CDCl3, 298 K): δ 183.0 (NNHC–Dipp), 179.8 (NNHC–Amd), 106.4 (NHAmd).
O), 145.5 (C4), 138.9 (NCHN), 132.8 (C12), 132.0 (C6), 131.8 (C15), 130.1 (C3), 128.6 (C14), 128.0 (C13), 124.7 (C5), 124.3 (C2), 123.4 (C1), 49.2 (C9), 39.2 (C10), 28.7 (C7), 24.4 and 23.9 (C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CDCl3, 298 K): δ 183.0 (NNHC–Dipp), 179.8 (NNHC–Amd), 106.4 (NHAmd).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 146.5 and 146.3 (C4), 142.5 (C12), 135.6 (C3), 130.4 (C6), 129.1 (C15), 128.1 (C13), 127.3 (C14), 123.9 (C2), 123.8 and 123.7 (C5), 120.4 (C1), 97.2 (d, JC–Rh = 8.8, CHcod–trans-NHC), 88.5 (d, JC–Rh = 7.3, CHcod–trans-NHC), 72.4 (d, JC–Rh = 11.9, CHcod–cis-NHC), 70.0 (d, JC–Rh = 12.3, CHcod–cis-NHC), 52.1 (C9), 46.6 (C10), 34.9, 32.4, 29.2, and 26.1 (CH2-cod), 28.7 and 28.2 (C7), 26.8, 26.3, 22.6, and 22.2 (all s, C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CDCl3, 263 K): δ 188.1 (NNHC–Dipp), 187.7 (NNHC–Amd).
O), 146.5 and 146.3 (C4), 142.5 (C12), 135.6 (C3), 130.4 (C6), 129.1 (C15), 128.1 (C13), 127.3 (C14), 123.9 (C2), 123.8 and 123.7 (C5), 120.4 (C1), 97.2 (d, JC–Rh = 8.8, CHcod–trans-NHC), 88.5 (d, JC–Rh = 7.3, CHcod–trans-NHC), 72.4 (d, JC–Rh = 11.9, CHcod–cis-NHC), 70.0 (d, JC–Rh = 12.3, CHcod–cis-NHC), 52.1 (C9), 46.6 (C10), 34.9, 32.4, 29.2, and 26.1 (CH2-cod), 28.7 and 28.2 (C7), 26.8, 26.3, 22.6, and 22.2 (all s, C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CDCl3, 263 K): δ 188.1 (NNHC–Dipp), 187.7 (NNHC–Amd).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 174.0 (d, JC–Rh = 49.1, Rh–CNHC), 146.5 (C4), 145.0 (C12), 137.5 (C3), 131.0 (C6), 129.0 (C15), 128.4 (C13), 127.4 (C14), 124.4 (C5), 124.0 (C2), 122.8 (C1), 51.9 (C10), 44.3 (C9), 28.9 (C7), 25.5 and 23.0 (C8).
O), 174.0 (d, JC–Rh = 49.1, Rh–CNHC), 146.5 (C4), 145.0 (C12), 137.5 (C3), 131.0 (C6), 129.0 (C15), 128.4 (C13), 127.4 (C14), 124.4 (C5), 124.0 (C2), 122.8 (C1), 51.9 (C10), 44.3 (C9), 28.9 (C7), 25.5 and 23.0 (C8).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 ratio. HRMS (ESI) m/z calcd for C20H24N2O4Rh [M + H]+: 459.0791, found: 459.0786.
48 ratio. HRMS (ESI) m/z calcd for C20H24N2O4Rh [M + H]+: 459.0791, found: 459.0786.
9a: 1H NMR (400.1 MHz, CD2Cl2, 233 K): δ 7.64 (d, JH–H = 2.0, 1H, H1), 7.49 (t, JH–H = 7.7, 1H, H6), 7.29 (d, JH–H = 7.7, 1H, H5), 6.96 (d, JH–H = 2.0, 1H, H2), 4.64 (t, JH–H = 5.1, 2H, H9), 2.70 (t, JH–H = 5.1, 2H, H10), 2.44 (sept, JH–H = 6.5, 2H, H7), 1.20 and 1.01 (both d, JH–H = 6.5, 12H, H8). 13C{1H}-APT NMR (100.1 MHz, CD2Cl2, 233 K): δ 186.8 (d, JC–Rh = 56.0, COtrans-NHC), 186.7 (d, JC–Rh = 58.2, COcis-NHC), 176.0 (d, JC–Rh = 47.8, Rh–CNHC), 175.8 (OCO), 146.1 (C4), 134.9 (C3), 130.2 (CH6), 124.7 (C2), 124.1 (CH5), 122.6 (C1), 48.5 (C9), 37.4 (C10), 28.4 (C7), 26.4 and 22.5 (C8).
9b: 1H NMR (400 MHz, CD2Cl2, 233 K): δ 7.49 (t, JH–H = 7.7, 1H, H6), 7.28 (d, JH–H = 7.7, 1H, H5), 7.21 (d, JH–H = 1.7, 1H, H1), 7.04 (d, JH–H = 1.7, 1H, H2), 4.49 (t, JH–H = 9.0, 2H, H9), 2.84 (t, JH–H = 9.0, 2H, H10), 2.45 (sept, JH–H = 6.5, 2H, H7), 1.14 and 1.01 (both d, JH–H = 6.5, 12H, H8). 13C{1H}-APT NMR (100 MHz, CDCl3, 233 K): δ 186.4 (d, JC–Rh = 69.0, 2C, CO), 176.6 (d, JC–Rh = 47.9, Rh–CNHC), 174.4 (s, OCO), 146.0 (s, C4), 134.9 (s, C3), 130.2 (s, CH6), 124.9 (C2), 124.1 (s, CH5), 121.2 (C1), 48.6 (s, C9), 38.0 (s, C10), 28.4 (C7), 26.3 and 22.7 (C8).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHNIPr), 6.27 (d, JH–H = 1.6, 1H, H1), 5.97 (d, JH–H = 1.6, 1H, H2), 3.71 (m, 2H, H9), 3.09 (sept, JH–H = 6.7, 4H, C
CHNIPr), 6.27 (d, JH–H = 1.6, 1H, H1), 5.97 (d, JH–H = 1.6, 1H, H2), 3.71 (m, 2H, H9), 3.09 (sept, JH–H = 6.7, 4H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) MeIPr), 2.51 (sept, JH–H = 6.7, 2H, H7a), 2.41 (m, 2H, H10), 1.43 and 1.06 (both d, JH–H = 6.7, 24H, MeIPr), 1.09 and 0.94 (both d, JH–H = 6.8, 12H, H8). 13C{1H}-APT NMR (75 MHz, C6D6, 298 K): δ 191. 2 (d, JC–Rh = 79.5, CO), 189.5 (d, JC–Rh = 43.3, Rh–CIPr), 185.7 (d, JC–Rh = 43.1, Rh–CNHC), 174.3 (OCO), 147.1 (Cq-IPr), 146.8 (C4), 137.1 (CqNIPr), 136.4 (C3), 129.8 (Cp-IPr), 129.4 (C6), 124.1 (
MeIPr), 2.51 (sept, JH–H = 6.7, 2H, H7a), 2.41 (m, 2H, H10), 1.43 and 1.06 (both d, JH–H = 6.7, 24H, MeIPr), 1.09 and 0.94 (both d, JH–H = 6.8, 12H, H8). 13C{1H}-APT NMR (75 MHz, C6D6, 298 K): δ 191. 2 (d, JC–Rh = 79.5, CO), 189.5 (d, JC–Rh = 43.3, Rh–CIPr), 185.7 (d, JC–Rh = 43.1, Rh–CNHC), 174.3 (OCO), 147.1 (Cq-IPr), 146.8 (C4), 137.1 (CqNIPr), 136.4 (C3), 129.8 (Cp-IPr), 129.4 (C6), 124.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHNIPr), 124.0 (Cm-IPr), 123.7 (C5), 123.5 (C2), 119.7 (C1), 47.9 (C9), 42.2 (C10), 28.8 (
CHNIPr), 124.0 (Cm-IPr), 123.7 (C5), 123.5 (C2), 119.7 (C1), 47.9 (C9), 42.2 (C10), 28.8 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) HMeIPr), 28.7 (C7), 26.4 and 23.1 (MeIPr), 25.9 and 23.9 (C8).
HMeIPr), 28.7 (C7), 26.4 and 23.1 (MeIPr), 25.9 and 23.9 (C8).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.9 (C9), 145.0 (C4), 144.3 (C11), 133.4 (C3), 131.2 (C6), 126.4 (C2), 124.4 (C5), 120.8 (q, JC–F = 320.1, CF3), 115.9 (C1), 111.2 (C12), 101.7 (C10), 104.4 (d, JC–Rh = 6.5, CHcod–trans-NHC), 72.9 (d, JC–Rh = 13.3, CHcod–cis-NHC), 31.4 and 29.4 (both s, CH2-cod), 28.5 (C7), 25.8 and 22.2 (both s, C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CD2Cl2, 243K): δ 209.6 (NNHC–OPy), 198.9 (NOPy), 189.8 (NNHC–Dipp). 19F NMR (282.3 MHz, CD2Cl2, 243 K): δ −78.8.
O), 149.9 (C9), 145.0 (C4), 144.3 (C11), 133.4 (C3), 131.2 (C6), 126.4 (C2), 124.4 (C5), 120.8 (q, JC–F = 320.1, CF3), 115.9 (C1), 111.2 (C12), 101.7 (C10), 104.4 (d, JC–Rh = 6.5, CHcod–trans-NHC), 72.9 (d, JC–Rh = 13.3, CHcod–cis-NHC), 31.4 and 29.4 (both s, CH2-cod), 28.5 (C7), 25.8 and 22.2 (both s, C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CD2Cl2, 243K): δ 209.6 (NNHC–OPy), 198.9 (NOPy), 189.8 (NNHC–Dipp). 19F NMR (282.3 MHz, CD2Cl2, 243 K): δ −78.8.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 146.2 (C4), 145.7 (C12), 135.7 (C3), 132.6 (C6), 130.2 (C15), 128.5 (C14), 127.3 (C13), 124.7 (C2), 124.6 (C1), 124.2 and 123.8 (C5), 120.1 (q, JC–F = 318.9, CF3), 99.4 and 95.1 (both d, JC–Rh = 7.5, CHcod–trans–NHC), 67.6 and 67.2 (both d, JC–Rh = 15.2, CHcod–cis–NHC), 51.3 (C9), 39.1 (C10), 34.2, 31.5, 30.4, and 27.4 (all s, CH2cod), 28.4 and 28.1 (C7), 25.9, 24.9, 22.7, and 22.5 (all s, C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CD2Cl2, 263 K): δ 192.8 (NNHC–Dipp), 187.9 (NNHC–Amd), 114.3 (NHamd). 19F NMR (282.3 MHz, CD2Cl2, 243 K): δ −78.9.
O), 146.2 (C4), 145.7 (C12), 135.7 (C3), 132.6 (C6), 130.2 (C15), 128.5 (C14), 127.3 (C13), 124.7 (C2), 124.6 (C1), 124.2 and 123.8 (C5), 120.1 (q, JC–F = 318.9, CF3), 99.4 and 95.1 (both d, JC–Rh = 7.5, CHcod–trans–NHC), 67.6 and 67.2 (both d, JC–Rh = 15.2, CHcod–cis–NHC), 51.3 (C9), 39.1 (C10), 34.2, 31.5, 30.4, and 27.4 (all s, CH2cod), 28.4 and 28.1 (C7), 25.9, 24.9, 22.7, and 22.5 (all s, C8). 1H–15N HMQC (long range) NMR (40.5 MHz, CD2Cl2, 263 K): δ 192.8 (NNHC–Dipp), 187.9 (NNHC–Amd), 114.3 (NHamd). 19F NMR (282.3 MHz, CD2Cl2, 243 K): δ −78.9.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 147.5 and 145.1 (C4), 135.4 (C3), 129.9 (C6), 124.6 (C2), 124.5 and 123.3 (C5), 121.5 (C1), 97.9 and 97.0 (both d, JC–Rh = 5.4, CHcod–trans-NHC), 68.7 and 68.1 (both d, JC–Rh = 14.0, CHcod–cis-NHC), 46.6 (C9), 35.8 (C10), 34.5, 30.9, 29.6, and 27.6 (all s, CH2cod), 28.4 and 28.3 (C7), 26.7, 26.2, 23.8, and 22.9 (all s, C8). 19F NMR (282.3 MHz, CDCl3, 243 K): δ −78.7.
O), 147.5 and 145.1 (C4), 135.4 (C3), 129.9 (C6), 124.6 (C2), 124.5 and 123.3 (C5), 121.5 (C1), 97.9 and 97.0 (both d, JC–Rh = 5.4, CHcod–trans-NHC), 68.7 and 68.1 (both d, JC–Rh = 14.0, CHcod–cis-NHC), 46.6 (C9), 35.8 (C10), 34.5, 30.9, 29.6, and 27.6 (all s, CH2cod), 28.4 and 28.3 (C7), 26.7, 26.2, 23.8, and 22.9 (all s, C8). 19F NMR (282.3 MHz, CDCl3, 243 K): δ −78.7.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 147.7 (C4), 145.0 (C12), 135.4 (C3), 131.2 (C6), 129.8 (C15), 128.2 (C14), 127.6 (C13), 125.4 (C2), 124.3 and 123.3 (C5), 118.9 (C1), 98.3 (d, JRh–C = 7.1, CHcod–trans-NHC), 97.0 (d, JRh–C = 7.3, CHcod–trans-NHC), 69.1 (d, JRh–C = 14.7, CHcod–cis-NHC), 67.2 (d, JRh–C = 14.7, CHcod–cis-NHC), 48.5 (C9), 37.8 (C10), 34.9, 30.5, 29.8, and 27.3 (all s, CH2cod), 28.4 and 28.3 (C7), 26.7, 25.4, 23.2, and 22.6 (all s, C8).
O), 147.7 (C4), 145.0 (C12), 135.4 (C3), 131.2 (C6), 129.8 (C15), 128.2 (C14), 127.6 (C13), 125.4 (C2), 124.3 and 123.3 (C5), 118.9 (C1), 98.3 (d, JRh–C = 7.1, CHcod–trans-NHC), 97.0 (d, JRh–C = 7.3, CHcod–trans-NHC), 69.1 (d, JRh–C = 14.7, CHcod–cis-NHC), 67.2 (d, JRh–C = 14.7, CHcod–cis-NHC), 48.5 (C9), 37.8 (C10), 34.9, 30.5, 29.8, and 27.3 (all s, CH2cod), 28.4 and 28.3 (C7), 26.7, 25.4, 23.2, and 22.6 (all s, C8).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 512/5318 [R(int) = 0.0486], Tmax/Tmin 0.7458/0.6765, data/restraints/parameters 5318/30/294, GoF(F2) = 1.061, R1 = 0.0481 [I > 2σ(I)], wR2 = 0.1240 (all data), largest diff. peak/hole 0.953/−1.017 e Å−3. CCDC deposit number 2464206.
512/5318 [R(int) = 0.0486], Tmax/Tmin 0.7458/0.6765, data/restraints/parameters 5318/30/294, GoF(F2) = 1.061, R1 = 0.0481 [I > 2σ(I)], wR2 = 0.1240 (all data), largest diff. peak/hole 0.953/−1.017 e Å−3. CCDC deposit number 2464206.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 483/7311 [R(int) = 0.0449], Tmax/Tmin 0.8132/0.7353, data/restraints/parameters 7311/0/303, GoF(F2) = 1.125, R1 = 0.0469 [I > 2σ(I)], wR2 = 0.1061 (all data), largest diff. peak/hole 1.066/−1.297 e Å−3. CCDC deposit number 2464205.
483/7311 [R(int) = 0.0449], Tmax/Tmin 0.8132/0.7353, data/restraints/parameters 7311/0/303, GoF(F2) = 1.125, R1 = 0.0469 [I > 2σ(I)], wR2 = 0.1061 (all data), largest diff. peak/hole 1.066/−1.297 e Å−3. CCDC deposit number 2464205.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 609/6223 [R(int) = 0.0614], Tmax/Tmin 0.9010/0.7685, data/restraints/parameters 6223/0/338, GoF(F2) = 1.038, R1 = 0.0302 [I > 2σ(I)], wR2 = 0.0753 (all data), largest diff. peak/hole 0.785/−0.720 e Å−3. CCDC deposit number 2464207.
609/6223 [R(int) = 0.0614], Tmax/Tmin 0.9010/0.7685, data/restraints/parameters 6223/0/338, GoF(F2) = 1.038, R1 = 0.0302 [I > 2σ(I)], wR2 = 0.0753 (all data), largest diff. peak/hole 0.785/−0.720 e Å−3. CCDC deposit number 2464207.
Conflicts of interest
There are no conflicts to declare.Data availability
Data supporting this article is including in SI. Supplementary information is available. See DOI: https://doi.org/10.1039/d5dt01516b.CCDC 2464205–2464207 (2, 1, and 5) contain the supplementary crystallographic data for this paper.54a–c
Acknowledgements
Financial support from the Spanish Ministerio de Ciencia, Innovación y Universidades MICIU/AEI/10.13039/501100011033, under the Projects PID2019-103965GB-I00 and PID2022-137208NB-I00, and the Departamento de Ciencia, Universidad y Sociedad del Conocimiento del Gobierno de Aragón (group E42_23R) is gratefully acknowledged. MOK thankfully acknowledge the University of Zaragoza for a contract under the program “Researchers Affected by Natural Disasters”. The use of the Servicio General de Apoyo a la Investigación-SAI of the University of Zaragoza, and the scientific-technical services of the ISQCH/CEQMA (CSIC) is gratefully acknowledged.References
- (a) A. J. Arduengo III and L. I. Iconaru, Dalton Trans., 2009, 6903–6914 RSC; (b) P. Belotti, M. Koy, M. N. Hopkinson and F. Glorius, Nat. Rev. Chem., 2021, 5, 711–725 CrossRef PubMed.
- (a) L. Oehninger, R. Rubbiani and I. Ott, Dalton Trans., 2013, 42, 3269–3284 RSC; (b) Y.-F. Zhang, Y.-K. Yin, H. Zhang and Y.-F. Han, Coord. Chem. Rev., 2024, 514, 215941 CrossRef CAS.
- (a) C. A. Smith, M. R. Narouz, P. A. Lummis, I. Singh, A. Nazemi, C.-H. Li and C. M. Crudden, Chem. Rev., 2019, 119, 4986–5056 CrossRef CAS PubMed; (b) H. Amouri, Chem. Rev., 2023, 123, 230–270 CrossRef CAS PubMed.
- (a) W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290–1309 CrossRef CAS; (b) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS PubMed; (c) Q. Zhao, G. Meng, S. P. Nolan and M. Szostak, Chem. Rev., 2020, 120, 1981–2048 CrossRef CAS PubMed; (d) Q. Liang and D. Song, Chem. Soc. Rev., 2020, 49, 1209–1232 RSC; (e) J. Thongpaen, R. Manguin and O. Baslé, Angew. Chem., Int. Ed., 2020, 59, 10242–10251 CrossRef CAS PubMed.
- L. Benhamou, E. Chardon, G. Lavigne, S. Bellemin-Laponnaz and V. César, Chem. Rev., 2011, 111, 2705–2733 CrossRef CAS PubMed.
- (a) H. Grützmacher, Angew. Chem., Int. Ed., 2008, 47, 1814–1818 CrossRef PubMed; (b) D. B. Grotjahn, Dalton Trans., 2008, 6497–6508 RSC; (c) S. Kuwata and T. Ikariya, Chem. Commun., 2014, 50, 14290–14300 RSC; (d) J. R. Khusnutdinova and D. Milstein, Angew. Chem., Int. Ed., 2015, 54, 12236–12273 CrossRef CAS PubMed; (e) B. Ramasamy and P. Ghosh, Eur. J. Inorg. Chem., 2016, 1448–1465 CrossRef CAS; (f) T. Higashi, S. Kusumoto and K. Nozaki, Chem. Rev., 2019, 119, 10393–10402 CrossRef CAS PubMed.
- (a) S. Hameury, P. de Fremont and P. Braunstein, Chem. Soc. Rev., 2017, 46, 632–733 RSC; (b) W. Stroek, N. A. V. Rowlinson, L. A. Hudson and M. Albrecht, Inorg. Chem., 2024, 63, 17134–17140 CrossRef CAS PubMed.
- (a) D. Pugh and A. A. Danopoulos, Coord. Chem. Rev., 2007, 251, 610–641 CrossRef CAS; (b) E. M. Richards, L. Casarrubios, J. M. D'Oyley, H. S. Rzepa, A. J. P. White, K. Goldberg, F. W. Goldberg, J. A. Bull and S. Diéz-González, Adv. Synth. Catal., 2025, 367, e202400909 CrossRef CAS.
- (a) S. Gaillard and J.-L. Renaud, Dalton Trans., 2013, 42, 7255–7270 RSC; (b) S. Bhattacharya, B. Atwi, K. Kundu, W. Frey and M. R. Buchmeiser, Organometallics, 2025, 44, 315–324 CrossRef CAS.
- (a) C. Fliedel and P. Braunstein, J. Organomet. Chem., 2014, 751, 286–300 CrossRef CAS; (b) M. Hruzd, S. L. Kleynemeyer, C. Michon, S. Bastin, E. Pollet, V. Ritleng and J.-B. Sortais, Chem. Commun., 2025, 61, 2969–2972 RSC.
- (a) G. Tan, S. Enthaler, S. Inoue, B. Blom and M. Driess, Angew. Chem., Int. Ed., 2015, 54, 2214–2218 CrossRef CAS PubMed; (b) T. Komuro, K. Hayasaka, K. Takahashi, N. Ishiwata, K. Yamauchi, H. Tobita and H. Hashimoto, Dalton Trans., 2024, 53, 4041–4047 RSC.
- (a) A. Zanardi, E. Peris and J. A. Mata, New J. Chem., 2008, 32, 120–126 RSC; (b) Q. Liang, K. M. Osten and D. Song, Angew. Chem., Int. Ed., 2017, 56, 6317–6320 CrossRef CAS PubMed; (c) B. Sánchez-Page, J. Munarriz, M. V. Jiménez, J. J. Pérez-Torrente, J. Blasco, G. Subias, V. Passarelli and P. Álvarez, ACS Catal., 2020, 10, 13334–13351 CrossRef; (d) M. O. Karatas, B. Alıcı, V. Passarelli, I. Ozdemir, J. J. Pérez-Torrente and R. Castarlenas, Dalton Trans., 2021, 50, 11206–11215 RSC.
- (a) S. T. Liddle, I. S. Edworthy and P. L. Arnold, Chem. Soc. Rev., 2007, 36, 1732–1744 RSC; (b) S. Bellemin-Laponnaz and S. Dagorne, Chem. Rev., 2014, 114, 8747–8774 CrossRef CAS PubMed; (c) E. Peris, Chem. Rev., 2018, 118, 9988–10031 CrossRef CAS PubMed.
- (a) K. Fujita, R. Tamura, Y. Tanaka, M. Yoshida, M. Onoda and R. Yamaguchi, ACS Catal., 2017, 7, 7226–7230 CrossRef CAS; (b) S. Siek, D. B. Burks, D. L. Gerlach, G. Liang, J. M. Tesh, C. R. Thompson, F. Qu, J. E. Shankwitz, R. M. Vasquez, N. Chambers, G. J. Szulczewski, D. B. Grotjahn, C. E. Webster and E. T. Papish, Organometallics, 2017, 36, 1091–1106 CrossRef CAS PubMed; (c) M. Huang, Y. Li, J. Liu, X.-B. Lan, Y. Liu, C. Zhao and Z. Ke, Green Chem., 2019, 21, 219–224 RSC; (d) P. Pandey, P. Daw, N. U. D. Reshi, K. R. Ehmann, M. Hölscher, W. Leitner and J. K. Bera, Organometallics, 2020, 39, 3849–3863 CrossRef CAS; (e) A. A. Kadam, M. Afandiyeva, W. Brennessel and C. R. Kennedy, Organometallics, 2024, 43, 2574–2580 CrossRef CAS PubMed; (f) H. A. G. Mayerstein and D. Song, ACS Catal., 2024, 14, 17489–17502 CrossRef.
- (a) C.-Y. Liao, K.-T. Chan, J.-Y. Zeng, C.-H. Hu, C.-Y. Tu and H. M. Lee, Organometallics, 2007, 26, 1692–1702 CrossRef CAS; (b) J. Berding, T. F. van Dijkman, M. Lutz, A. L. Spek and E. Bouwman, Dalton Trans., 2009, 6948–6955 RSC; (c) S. Warsink, J. A. Venter and A. Roodt, J. Organomet. Chem., 2015, 775, 195–201 CrossRef CAS; (d) Z. Huang, S. Wang, X. Zhu, Q. Yuan, Y. Wei, S. Zhou and X. Mu, Inorg. Chem., 2018, 57, 15069–15078 CrossRef CAS PubMed; (e) J. Cao, G. Xiong, Z. Luo, Q. Huang, W. Zhou, I. Dragutan, V. Dragutan, Y. Sun and F. Ding, Inorg. Chim. Acta, 2024, 568, 122076 CrossRef CAS.
- (a) C. Gandolfi, M. Heckenroth, A. Neels, G. Laourenczy and M. Albrecht, Organometallics, 2009, 28, 5112–5121 CrossRef CAS; (b) J. Thongpaen, T. E. Schmid, L. Toupet, V. Dorcet, M. Mauduit and O. Baslé, Chem. Commun., 2018, 54, 8202–8205 RSC; (c) Y. Hu and S. Guo, Appl. Organomet. Chem., 2019, 33, e4703 CrossRef; (d) R. Manguin, M. Galiana-Cameo, T. Kittikool, C. Barthes, J. Thongpaen, E. Bancal, S. Mallet-Ladeira, S. Yotphan, R. Castarlenas, M. Mauduit, J.-B. Sortais and O. Baslé, Chem. Commun., 2023, 59, 4193–4196 Search PubMed; (e) J. Sanz-Garrido, R. Andrés, C. González-Arellano and J. C. Flores, Adv. Synth. Catal., 2025, 367, e202401289 Search PubMed.
- (a) D. P. Allen, C. M. Crudden, L. A. Calhoun and R. Wang, J. Organomet. Chem., 2004, 689, 3203–3209 Search PubMed; (b) L. Palacios, A. Di Giuseppe, A. Opalinska, R. Castarlenas, J. J. Pérez-Torrente, F. J. Lahoz and L. A. Oro, Organometallics, 2013, 32, 2768–2774 CrossRef CAS; (c) V. M. Chernyshev, E. A. Denisova, D. B. Eremin and V. P. Ananikov, Chem. Sci., 2020, 11, 6957–6977 RSC.
- (a) R. Puerta-Oteo, M. V. Jiménez, F. J. Lahoz, F. J. Modrego, V. Passarelli and J. J. Pérez-Torrente, Inorg. Chem., 2018, 57, 5526–5543 CrossRef CAS PubMed; (b) J. Sanz-Garrido, A. Martin, C. González-Arellano and J. C. Flores, Dalton Trans., 2024, 53, 1460–1468 Search PubMed.
- (a) B. M. Trost and J. T. Masters, Chem. Soc. Rev., 2016, 45, 2212–2238 RSC; (b) Q. Liang, K. Hayashi and D. Song, ACS Catal., 2020, 10, 4895–4905 CrossRef CAS; (c) S. M. Weber and G. Hilt, Front. Chem., 2021, 9, 635826 CrossRef CAS PubMed.
- M. Galiana-Cameo, M. Borraz, Y. Zelenkova, V. Passarelli, F. J. Lahoz, J. J. Pérez-Torrente, L. A. Oro, A. Di Giuseppe and R. Castarlenas, Chem. – Eur. J., 2020, 26, 9598–9608 CrossRef CAS PubMed.
- A. Roglans, A. Pla-Quintana and M. Solà, Chem. Rev., 2021, 121, 1894–1979 CrossRef CAS PubMed.
- M. Angoy, M. V. Jiménez, P. García-Orduña, L. A. Oro, E. Vispe and J. J. Pérez-Torrente, Organometallics, 2019, 38, 1991–2006 Search PubMed.
- L. Leroyer, M. Maraval and R. Chauvin, Chem. Rev., 2012, 112, 1310–1343 Search PubMed.
- B. Español-Sánchez, J. Moradell, M. Galiana-Cameo, E. Barrenas, J. J. Pérez-Torrente, V. Passarelli and R. Castarlenas, Angew. Chem., Int. Ed., 2025, 64, e202507424 Search PubMed.
- Y. Shibata, K. Noguchi and K. Tanaka, Org. Lett., 2010, 12, 5596–5599 CrossRef CAS PubMed.
- V. Claus, M. Schukin, S. Harrer, M. Rudolph, F. Rominger, A. M. Asiri, J. Xie and S. K. Hashmi, Angew. Chem., Int. Ed., 2018, 57, 12966–12970 CrossRef CAS PubMed.
- C. M. Storey, A. Kalpokas, M. R. Gyton, T. Krämer and A. B. Chaplin, Chem. Sci., 2020, 11, 2051–2057 RSC.
- (a) M. Rubina and V. Gevorgyan, J. Am. Chem. Soc., 2001, 123, 11107–11108 CrossRef CAS PubMed; (b) S. Ventre, E. Derat, M. Amatore, C. Aubert and M. Petit, Adv. Synth. Catal., 2013, 355, 2584–2590 CrossRef CAS; (c) P. Żak, M. Bołt, J. Lorkowski, M. Kubicki and C. Pietraszuk, ChemCatChem, 2017, 9, 3627–3631 Search PubMed; (d) X. Zhuang, J.-Y. Chen, Z. Yang, M. Jia, C. Wu, R.-Z. Liao, C.-H. Tung and W. Wang, Organometallics, 2019, 38, 3752–3759 CrossRef CAS; (e) Y. Ueda, H. Tsurugi and K. Mashima, Angew. Chem., Int. Ed., 2020, 59, 1552–1556 CrossRef CAS PubMed.
- (a) M. Nishiura, Z. Hou, Y. Wakatsuki, T. Yamaki and T. Miyamoto, J. Am. Chem. Soc., 2003, 125, 1184–1185 CrossRef CAS PubMed; (b) R. H. Platel and L. L. Schafer, Chem. Commun., 2012, 48, 10609–10611 RSC; (c) O. Rivada-Wheelaghan, S. Chakraborty, L. J. W. Shimon, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2016, 55, 6942–6945 CrossRef CAS PubMed; (d) N. Gorgas, L. G. Alves, B. Stöger, A. M. Martins, L. F. Veiros and K. Kirchner, J. Am. Chem. Soc., 2017, 139, 8130–8133 CrossRef CAS PubMed; (e) N. Gorgas, B. Stöger, L. F. Veiros and K. Kirchner, ACS Catal., 2018, 8, 7973–7982 CrossRef CAS; (f) J. E. Stevens, J. D. Miller, M. C. Fitzsimmons, C. E. Moore and C. M. Thomas, Chem. Commun., 2024, 60, 5169–5172 RSC.
- (a) T. Chen, C. Guo, M. Goto and L.-B. Han, Chem. Commun., 2013, 49, 7498–7500 RSC; (b) G. Kleinhans, G. Guisado-Barrios, D. C. Liles, G. Bertrand and D. I. Bezuidenhout, Chem. Commun., 2016, 52, 3504–3507 RSC; (c) Q. Liang, K. Sheng, A. Salmon, V. Y. Zhou and D. Song, ACS Catal., 2019, 9, 810–818 CrossRef CAS; (d) J.-F. Chen and C. Li, ACS Catal., 2020, 10, 3881–3889 CrossRef CAS.
- (a) L. Rubio-Pérez, R. Azpíroz, A. Di Giuseppe, V. Polo, R. Castarlenas, J. J. Pérez-Torrente and L. A. Oro, Chem. – Eur. J., 2013, 19, 15304–15314 CrossRef PubMed; (b) O. V. Zatolochnaya, E. G. Gordeev, C. Jahier, V. P. Ananikov and V. Gevorgyan, Chem. – Eur. J., 2014, 20, 9578–9588 CrossRef CAS PubMed; (c) C. M. Storey, M. R. Gyton, R. E. Andrew and A. B. Chaplin, Chem. – Eur. J., 2020, 26, 14715–14723 CrossRef CAS PubMed; (d) C. Pfeffer, N. Wannenmacher, W. Frey and R. Peters, ACS Catal., 2021, 11, 5496–5505 CrossRef CAS; (e) Y. Sun, J. Zhang, Y. Zeng, L. Meng and X. Li, J. Org. Chem., 2024, 89, 605–616 CrossRef CAS PubMed.
- (a) H. Zhang and X. Bao, RSC Adv., 2015, 5, 84636–84642 RSC; (b) H. Chen, Z. Dang, X. Sha, Y. Wang, Z. Zhang, Y. Luo and Y. Lan, ACS Catal., 2024, 14, 16469–16478 CrossRef CAS; (c) X. Wu and L. Zhang, Org. Lett., 2024, 26, 5736–5740 CrossRef CAS PubMed.
- (a) M. Galiana-Cameo, A. Urriolabeitia, E. Barrenas, V. Passarelli, J. J. Pérez-Torrente, A. Di Giuseppe, V. Polo and R. Castarlenas, ACS Catal., 2021, 11, 7553–7567 CrossRef CAS; (b) B. Español-Sánchez, M. Galiana-Cameo, A. Urriolabeitia, V. Polo, V. Passarelli, J. J. Pérez-Torrente and R. Castarlenas, Organometallics, 2024, 43, 2951–2962 CrossRef.
- (a) A. Srinivasan, J. Campos, N. Giraud, M. Robert and O. Rivada-Wheelaghan, Dalton Trans., 2020, 49, 16623–16626 RSC; (b) M. Afandiyeva, A. A. Kadam, X. Wu, W. W. Brennessel and C. R. Kennedy, Organometallics, 2022, 41, 3014–3023 CrossRef CAS.
- M. Breugst and H. Mayr, J. Am. Chem. Soc., 2010, 132, 15380–15389 CrossRef CAS PubMed.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19 RSC.
- R. Azpíroz, L. Rubio-Pérez, A. Di Giuseppe, V. Passarelli, F. J. Lahoz, R. Castarlenas, J. J. Pérez-Torrente and L. A. Oro, ACS Catal., 2014, 4, 4244–4253 CrossRef.
- C. Lohre, T. Dröge, C. Wang and F. Glorius, Chem. – Eur. J., 2011, 17, 6052–6055 CrossRef CAS PubMed.
- G. B. Deacon and R. J. Phillis, Coord. Chem. Rev., 1980, 33, 227–250 CrossRef CAS.
- N. E. Nigglia and O. Baudoin, Helv. Chim. Acta, 2021, 104, e2100015 CrossRef.
- D. Ostendorf, C. Landis and H. Grützmacher, Angew. Chem., Int. Ed., 2006, 45, 5169–5173 CrossRef CAS PubMed.
- See for example: (a) L. H. Pignolet, D. H. Doughty, S. C. Nowicki, M. P. Anderson and A. L. Casalnuovo, J. Organomet. Chem., 1980, 202, 211–223 CrossRef CAS; (b) M. Montag, I. Efremenko, R. Cohen, G. Leitus, L. J. W. Shimon, Y. Diskin-Posner, Y. Ben-David, J. M. L. Martin and D. Milstein, Chem. – Eur. J., 2008, 14, 8183–8194 CrossRef CAS PubMed; (c) R. E. Andrew, D. W. Ferdani, C. A. Ohlin and A. B. Chaplin, Organometallics, 2015, 34, 913–917 CrossRef CAS.
- See: R. Azpíroz, M. O. Karataş, V. Passarelli, I. Özdemir, J. J. Pérez-Torrente and R. Castarlenas, Molecules, 2022, 27, 7002 CrossRef PubMed , and references therein.
- A. Di Giuseppe, R. Castarlenas, J. J. Pérez-Torrente, F. J. Lahoz and L. A. Oro, Chem. – Eur. J., 2014, 20, 8391–8403 CrossRef CAS PubMed.
- G. Storch, L. Deberle, J.-M. Menke, F. Rominger and O. Trapp, Chirality, 2016, 28, 744–748 CrossRef CAS PubMed.
- J. Chatt and L. M. Venanzi, J. Chem. Soc., 1957, 4735–4741 RSC.
- A. Coniglio, M. Bassetti, S. E. García-Garrido and J. Gimeno, Adv. Synth. Catal., 2012, 354, 148–158 CrossRef CAS.
- SAINT+: Area-Detector Integration Software, version 6.01, Bruker AXS, Madison, WI, 2001 Search PubMed.
- G. M. Sheldrick, SADABS program, University of Göttingen, Göttingen, Germany, 1999 Search PubMed.
- G. M. Sheldrick, SHELXS 97, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS.
- A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS PubMed.
- (a) M. O. Karataş, D. R. Hinojosa, V. Passarelli, L. A. Oro, J. J. Pérez-Torrente and R. Castarlenas, CCDC 2464205: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nq6h3; (b) M. O. Karataş, D. R. Hinojosa, V. Passarelli, L. A. Oro, J. J. Pérez-Torrente and R. Castarlenas, CCDC 2464206: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nq6j4; (c) M. O. Karataş, D. R. Hinojosa, V. Passarelli, L. A. Oro, J. J. Pérez-Torrente and R. Castarlenas, CCDC 2464207: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nq6k5.
| This journal is © The Royal Society of Chemistry 2025 |