 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterobimetallic unsaturated silicon clusters (siliconoids) with transition metal-expanded scaffolds†
Luisa
Giarrana
 a,
Nadine E.
Poitiers
a,
Alida
Stürmer
a,
Michael
Zimmer
a,
Volker
Huch
a,
Bernd
Morgenstern
b and
David
Scheschkewitz
a,
Nadine E.
Poitiers
a,
Alida
Stürmer
a,
Michael
Zimmer
a,
Volker
Huch
a,
Bernd
Morgenstern
b and
David
Scheschkewitz
 *a
*a
aKrupp-Chair in Inorganic and General Chemistry, Saarland University, Campus C4.1 Saarbrücken, 66123 Saarbrücken, Germany. E-mail: david.scheschkewitz@uni-saarland.de
bService Center X-Ray Diffraction, Saarland University Campus Saarbrücken C4.1, 66123 Saarbrücken, Germany
First published on 13th June 2025
Abstract
We report a heterobimetallical unsaturated silicon cluster (siliconoid) with a formally anionic group 9 metal vertex (Ir) in close contact to the lithium counter-cation, thus constituting a rare example of transition metal–lithium interactions. The anionic cluster is obtained by reductive chloride elimination from the corresponding neutral siliconoid complex of iridium(I) chloride with lithium/naphthalene. The previously exohedral transition metal center is fully incorporated into the siliconoid cluster scaffold giving rise to an irida-heterosiliconoid reminiscent of the corresponding homonuclear Si7 species. Despite the formal negative charge at the iridium center, the nucleophilic site is on one of the adjacent silicon vertices judging from the reactivity toward group 4 metallocene dichlorides, Cp2MCl2 (M = Zr, Hf). Under elimination of LiCl, the Cp2MCl moieties in the heterobimetallic products are installed as pending functionalities under retention of the literally uncompromised iridasiliconoid core.
Introduction
Heterobimetallic species have been receiving growing attention over the last decades due to their unique electronic and catalytic properties. Mixed metallic compounds are frequently used as catalysts, for instance in the production of H2 in the context of energy storage,1a as enzyme surrogates in water oxidation1b or in (m)ethanol fuel cells.1c,d In Fischer–Tropsch chemistry, bimetallic phases1e and – increasingly – heterobimetallic nanoparticles are competent heterogenous catalysts.1f Numerous bimetallic main group-transition metal complexes have been reported for their activity in homogenous catalysis,2 among them neutral compounds such as I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a (N2 reduction) and anionic derivatives such as II (CO2 derivatization).3b Late first and second row transition metals readily form metal-element bonds to En ligands (e.g. E = group 13 to 15 atom), but the controlled implementation of a second or third transition metal center remains a challenge, especially if different to the first.4
3a (N2 reduction) and anionic derivatives such as II (CO2 derivatization).3b Late first and second row transition metals readily form metal-element bonds to En ligands (e.g. E = group 13 to 15 atom), but the controlled implementation of a second or third transition metal center remains a challenge, especially if different to the first.4
Due to the low electronegativity of the constituting elements, main group metalloid clusters, such as Zintl anions,5 represent competent ligand systems for late transition metals and thus considerable potential in homogenous catalysis.5 Especially germanium and tin-based Zintl anions6 and – more recently – their silicon congeners have been employed as extraordinarily electron-rich ligands towards transition metal centers.7 More specifically, Zintl clusters with either metal vertices (e.g.III, Fig. 1)8 or endohedral metal centers (e.g.IV)9 have been described, along with dimeric clusters such as V.7a Even multimetallic cluster species have been reported by the groups of Goicoechea and Sevov: the straightforward addition of a second transition metal to a rhodium-germanium cluster VI leads to the formation of VII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Fig. 1) or a Rh–Ir intermetallic cluster VIII.11 With the neutral rhodium species VI, the first example of a homogeneous catalyst derived from Zintl anions was disclosed with considerable activity in alkene hydrogenation and H/D exchange.10
10 (Fig. 1) or a Rh–Ir intermetallic cluster VIII.11 With the neutral rhodium species VI, the first example of a homogeneous catalyst derived from Zintl anions was disclosed with considerable activity in alkene hydrogenation and H/D exchange.10
As we proposed based on the isolation of lithiated derivatives, unsaturated silicon clusters (siliconoids)12 as otherwise neutral molecular species are conceptually related to Zintl anions through the formal replacement of negative charges by covalently bonded substituents.13 Accordingly, the availability of nucleophilic anionic siliconoids provided access to the first transition metal-substituted derivatives: a ligato-lithiated Si6 siliconoid served as a precursor for X-type ligation toward M(Cp)2Cl (M = Zr, Hf).12g In contrast, the realization of L-type coordination required the grafting of a tetrylene side-arm in the ligato position. The resulting globally neutral metallasiliconoid 1 (Scheme 1),13g,h however, exhibits only partial incorporation of the group 9 metal into the cluster scaffold due to the cage-opening chlorine transfer from the transition metal. Nonetheless, 1 proved to be competitively active and selective as the catalyst of the isomerization of 1-hexene to 2-hexene.
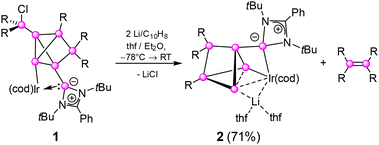 | ||
Scheme 1 Synthesis of Si6Ir-Li 2 by reduction of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12h with Li/C10H8 ( 12h with Li/C10H8 ( = Si; R = 2,4,6-triisopropylphenyl, cod = 1,5-cyclooctadiene). = Si; R = 2,4,6-triisopropylphenyl, cod = 1,5-cyclooctadiene). | ||
Herein, we report the first complete inclusion reactions of any transition metal as hetero-vertices of siliconoids using a reductive elimination approach. The reaction of 1 gives rise to a 7-vertex iridasiliconoid reminiscent of the neutral Si7 derivative. The product shows close contacts between the transition metal centers and the lithium counter-cation, further highlighting the conceptual relationship between siliconoids and Zintl anions. Finally, we demonstrate that heterobimetallic complexes with group 4 metals are readily obtained by reaction of the anionic clusters with the appropriate metallocene dichlorides.
Results and discussion
We anticipated that the reductive elimination of chloride from the incompletely metal-incorporated siliconoid 1 may either prompt the reintegration of its pending chlorosilyl group into the cluster framework or its complete cleavage. Both eventualities could in principle result in additional metal-cluster interactions and hence prompt the desired complete incorporation of the metal center into the cluster core. Indeed, reaction of 1 with lithium/naphthalene in thf led to the complete cleavage of the exohedral Tip2SiCl-group to yield a new product 2 in 71% isolated yield (Scheme 1). The lithium salt 2 was fully characterized by X-ray diffraction on single crystals, UV/vis spectroscopy and multinuclear NMR spectroscopy.The product mixture shows the characteristic 29Si NMR signal of tetrakis(2,4,6-triisopropylphenyl)disilene at 52.8 ppm,14 which is apparently formed as a systematic by-product that can however be separated by crystallization. The distribution of the remaining 29Si NMR chemical shifts of 2 is akin to that of Si6- and Si7 siliconoids.12a,c,e,g According to the 2D 29Si/1H correlation of the isolated product, the deshielded signal at 152.8 ppm is assigned to the SiTip2 unit and the most shielded resonances at −184.0 and −226.3 ppm to the Ir-bonded unsubstituted silicon vertices. One further highfield signal at −103.2 ppm can be attributed to the vertex carrying the amidinato silylene, which in turn gives rise to a signal at 36.0 ppm, slightly downfield-shifted compared to the precursor (1: δ29Si 32.9 ppm).12h The SiTip vertex resonates at 108.2 ppm and thus at unusually low field. Most notably, the major 7Li NMR signal of 2 is significantly downfield-shifted to 5.99 ppm. A minor broad signal at 1.12 ppm likely belongs to a different coordination mode of the Li counter-ion that however, does not affect the coordination and the chemical shifts of the remaining nuclei.
Single crystals of 2 were obtained from hexane in 71% yield and the molecular structure in the solid state was confirmed by X-ray diffraction (Fig. 2a). The unit cell contains two molecules of 2 in the asymmetric unit with slightly differing bonding values. In the following, the arithmetic mean values will be discussed (see ESI Table S2† for exact values). The cleavage of the exohedral silyl group in 1 indeed resulted in the full incorporation of the Ir(cod) center into a distorted benzpolarene motif in 2. The term benzpolarene was recently introduced by our group to refer to the highly polarized, tetracyclic global minimum isomer of benzene, in analogy to benzvalene as another prominent isomer of benzene.12e The amidinato silylene sidearm in 2 completes the coordination sphere at Ir1, while Li1 assumes a bridging position across the Si2–Ir1 bond.
The anionic iridasiliconoid 2 is therefore best described as a heteroanalogue of the doubly bridged propellane motif of the Si7 scaffolds IX and X, although with some noteworthy differences (Fig. 2b).12d While in IX and X, the “upper” three silicon atoms are almost linear (IX: 173.8°, X: 174.0°) giving rise for a seesaw coordination environment, the apical silicon atom Si1 of the central triangular motif of 2 is canted away from the Si5–Si6 vector towards Si2, the basal silicon atom connected to the lithium counter-cation (Si5–Si1–Si6 149.9(7)°). This is presumably a consequence of the tetravalency of this vertex (whereas Si3 is formally trivalent), which implies a more electron-precise bonding situation with considerably shorter bonds. Concomitantly, Si1 acquires a much more pronounced hemispheroidal coordination environment (2: ϕ = 0.5542, IX: ϕ = −0.0390, X: ϕ = −0.0994).13k For a tetracoordinate atom, the hemispheroidality parameter ϕ describes the deviation of the “naked” silicon vertex from a reference plane, defined by the three bonded atoms, for which the sum of bond angles is closest to 360°, in comparison to a fourth substituent, which is set to be a negative value as per convention.13k If ϕ results in a negative value, a tetrahedral coordination is suggested, whereas a positive value indicates a hemispheroidal coordination environment of the vertex in question.
The distance between the unsubstituted vertices in 2 (Si2–Si3 2.475(2) Å) is remarkably shortened compared to homonuclear Si6 and Si7 siliconoids.12a,c,e,g The metal-silicon bond due to the L-type coordination by the silylene side arm (Si6–Ir1 2.344(1) Å) is significantly shorter than the X-type interaction with the nudo-vertex Si3 (Si3–Ir1 2.411(1) Å). These values are in line with previously reported silicon-iridium single bonds (2.245 Å–2.574 Å).15 The pronounced lengthening of the third silicon-iridium contact (Ir1–Si2 2.641(1) Å) can be attributed to the bridging by the lithium counteraction in the sense of an unprecedented agostic 3c2e Ir–Si–Li interaction. In fact, only very few Ir–M (M = alkali metal) contact ion pairs have been crystallographically characterized.16 Despite its bridging nature, the Ir1–Li1 distance of 2.880(8) Å in 2 is in good agreement with the reported Ir⋯Li distances in a diphenyliridate (2.882/2.886 Å).17 The distance between Si2⋯Li1 (2.611(8) Å) in 2 is well within the range of further reported Si⋯Li siliconoid distances (2.56–2.77 Å).12c–e
The longest wavelength absorption in the UV/vis spectrum of 2 at λmax = 543 nm is slightly blue-shifted compared to the starting material 1 (λmax = 576 nm) but red-shifted in contrast to previously reported ligato-substituted siliconoids (λmax = 364 to 521 nm).12c,e,g The TD-DFT calculated value at λmax,calc = 528 nm (PBE0/DEF2-TZVPP level of theory)18 confirms the assignment to the HOMO → LUMO transition (78% contribution, see ESI† for further details). Besides, an additional intense absorption band at λ = 425 nm agrees well with calculated transitions at 418 nm (HOMO−3 → LUMO 35%, HOMO−2 → LUMO 39%) and 430 nm (HOMO → LUMO + 3 78%) (ESI Table S7†).
We probed the reactivity of Si6Ir-Li 2 towards Me3SiCl and Me3SnCl, as representative common electrophiles. The components of the reaction mixture, however, could not be isolated nor identified with certainty. To explore the application of anionic Si6Ir-Li 2 as a precursor for the grafting of a second transition metal center, we then investigated the reaction with group 4 metallocene dichlorides as electrophilic substrates (Scheme 2) since ZrCp2Cl2 and HfCp2Cl2 were shown to readily undergo clean salt metathesis reactions in the case of homonuclear siliconoids and as well as electron-precise silicon species.12g,20 The reactions of 2 with Cp2MCl2 (M = Zr, Hf) in benzene at room temperature yielded the corresponding substituted siliconoids 3a,b in isolated yields of 14% and 32%, respectively. Interestingly, 3a proved to be much less stable than 3b leading to as yet unidentified decomposition products during work-up. The yields are furthermore compromised by the competing formation of an unidentified side product, which could be due to the elimination of CpLi according to residual 1H and 13C NMR signals in the spectra of 3a/3b at 5.869/5.810 ppm and 114.32/112.85 ppm, respectively (ESI†).19 Attempts to detect CpLi by 7Li NMR, however, remained unsuccessful.
 | ||
Scheme 2 Synthesis of group 4 metallocenyl-substituted iridasiliconoids 3a,b from 2 and Cl2MCp2 (M = Zr (3a), Hf (3b);  = Si; R = 2,4,6-triisopropylphenyl; cod = 1,5-cyclooctadiene). = Si; R = 2,4,6-triisopropylphenyl; cod = 1,5-cyclooctadiene). | ||
The NMR spectra of 3a,b show very similar signal patterns. The 29Si NMR signals were assigned based on 2D 29Si/1H NMR correlation spectra. The signals of the SiTip2 unit (3a: 73.8 ppm, 3b: 75.9 ppm) are significantly upfield-shifted with a Δδ of about 75 ppm compared to the starting material (2: 149.3 ppm). Similar highfield-shifts are observed for the SiTip units, assigned based on the cross-peaks in the aromatic region (2: 108.2 ppm, 3a: 73.5 ppm, 3b: 68.1 ppm). The resonance attributed to the Si atom of the pending N-heterocyclic silylene (3a: 30.5 ppm, 3b: 29.9 ppm) is found in the same range as the corresponding signals of the precursors (1: 32.9 ppm, 2: 36.0 ppm) suggesting that the environment of the coordinated Ir-cod moiety is mostly preserved. The most downfield-shifted resonance compared to 2 is the signal assigned to the Si–M atom (2: −226.3 ppm, 3a: −40.9 ppm, 3b: −36.6 ppm). The two remaining highfield signals (3a: −77.9/−131.9 ppm, 3b: −90.4/−131.1 ppm) correlate to the unsubstituted silicon atoms (Si1, Si3) but are somewhat more deshielded than in the starting material (2: −103.2/−184.0 ppm).
Compared to the starting material 2, the longest wavelength absorptions in the UV/vis spectra of 3a,b are considerably red-shifted from λmax = 551 nm (2) to 601 nm (3a, Zr) and 647 nm (3b, Hf) indicating a significantly smaller HOMO–LUMO gap.
Single crystals of both 3a (14%) and 3b (32%) were obtained overnight from concentrated hexane solutions at −26 °C (3a) and room temperature (3b) (Fig. 3). X-ray diffraction analysis confirmed the molecular structures in the solid state with formation of bonds between the group 4 metal M (M = Zr, Hf) and Si2 rather than Ir1, possibly because of the higher stability of Si–M bonds21 compared to bimetallic transition metal bonds as well as the larger differences in electronegativity (Pauling values Si 1.90, Zr 1.33, Hf 1.3, Ir 2.20).22 The elongated distance between Si2 and Ir1 (Fig. 3, 3a: 2.726(1) Å, 3b: 2.748(1) Å) compared to 2 (2. 641(1) Å) suggests a somewhat weaker interaction in line with the presence of the additional Si2–M bond. Consequently, the Si2–Si3 bond (2: 2.475(2) Å) is shortened to the length of a typical Si–Si single bond (2.30–2.40 Å, 3a: 2.388(1) Å, 3b: 2.390(2) Å).21b The Si2–M bond lengths (3a: 2.744(9) Å, 3b: 2.723(1) Å) are on the shorter end of reported Si-M (M = Zr, Hf) single bonds (Si–Zr = 2.7429–2.924 Å, Si–Hf = 2.729–2.939 Å).12g,21 Comparable bond distances were reported for tris(trimethylsilyl) hafnium derivatives by Rheingold, Geib and Tilley, (dSi–Hf = 2.729 Å/2.748 Å)21a,b and for a zirconocenecyclosilane by Marschner and coworkers (2.743 Å).21cTable 1 compares pertinent structural and spectroscopic data of heterobimetallic siliconoids 2, 3a and 3b.
| Compound | δ 29Si NHSi [ppm] | δ 29Si unsubstituted Si [ppm] | d (Si–Si, unsubstituted) [Å] | d(Si2–M) [Å] | ∢ Si5–Si1–Si6 [°] |
|---|---|---|---|---|---|
| Si7Ir 1 | 32.9 | −125.6/−128.8 | 2.305(2)/2.548(2) | 2.305(2) | — |
| Si6Ir-Li 2 | 36.0 | −184.0/−226.3 | 2.475(2) | 2.658(1) | 149.9(7) |
| Si6Ir-Zr 3a | 73.5 | −77.9/−131.9 | 2.388(12)/2.495(1) | Si2⋯Ir1 2.726(1)/Si2–Zr1 2.744(9) | 136.4(2) |
| Si6Ir-Hf 3b | 68.1 | −90.4/−131.1 | 2.390(2)/2.494(2) | Si2⋯Ir1 2.748(1)/Si2–Hf1 2.723(1) | 135.9(7) |
As in the case of 2, the seesaw coordination at Si1 is strongly distorted to a hemispheroidal environment (3a: ϕ = 0.7148, 3b: ϕ = 0.7206). The more covalent bonding of the Si–M bond in 3a and 3b gives rise to even more acute Si5–Si1–Si6 angles than in 2 (3a: 136.4(2)°, 3b: 135.9(7)°, 2: 149.9(7)°) and the Si7 siliconoids IX and X (Fig. 2, IX: 173.8°, X: 174.0°).12a Although the Cl1–M1–Si2 angle (3a: 103.0(3), 3b: 100.8(4)°) is more acute than expected, an initially suspected Ir–Cl interaction is unlikely given the rather long distances (Ir⋯Cl: 4.5206(9) Å (3a), 4.478(2) Å (3b)).
Conclusions
In conclusion, we report on the isolation and full characterization of the first heterobimetallic siliconoid with fully incorporated transition metal vertex. The contact ion pair 2 comprising the anionic iridasiliconoid and the lithium counter-cation resembles the corresponding Si7 siliconoid species. Reactions of 2 with Cp2MCl2 (M = Zr and Hf) results in the heterobimetallic siliconoids 3a and 3b in which the group 4 metal moiety is exohedrally attached to the silicon vertex in α-position to iridium while the irida-incorporated heterosiliconoid scaffold is preserved in principle.Author contributions
L. Giarrana, N. E. Poitiers and A. Stürmer performed the synthetic work and data analysis, L. Giarrana, N. E. Poitiers and D. Scheschkewitz designed the study, D. Scheschkewitz acquired the funding; V. Huch and B. Morgenstern carried out the X-ray diffraction studies, M. Zimmer did the solid state and VT NMR measurements, L. Giarrana performed the DFT calculations, L. Giarrana and D. Scheschkewitz wrote the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Data availability
All data associated with this manuscript are available in the ESI.† Crystallographic data for 2, 3a and 3b have been deposited at the CCDC (2447272, 2447286 and 2447266†) and can be obtained from https://www.ccdc.cam.ac.uk/structures.Acknowledgements
Funding by the Deutsche Forschungsgemeinschaft (DFG SCHE 906/4-4) is gratefully acknowledged. Instrumentation and technical assistance for this work were provided by the Service Center X-ray Diffraction, with financial support from Saarland University and German Science Foundation (project number INST 256/506-1). The authors thank Dr. Diego Andrada and Prof. Stella Stopkowicz for access to their computational clusters.References
- (a) M. R. DuBois and D. L. DuBois, The roles of the first and second coordination spheres in the design of molecular catalysts for H2 production and oxidation, Chem. Soc. Rev., 2009, 38, 62 RSC; (b) R. Brimblecombe, G. F. Swiegers, G. C. Dismukes and L. Spiccia, Sustained water oxidation photocatalysis by a bioinspired manganese cluster, Angew. Chem., Int. Ed., 2008, 47, 7335 CrossRef CAS PubMed; (c) E. Antolini, Catalysts for direct ethanol fuel cells, J. Power Sources, 2007, 170, 1 CrossRef CAS; (d) S. Song, W. Zhou, Z. Liang, R. Cai, G. Sun, Q. Xin, V. Stergiopoulos and P. Tsiakaras, The effect of methanol and ethanol cross-over on the performance of PtRu/C-based anode DAFCs, Appl. Catal., B, 2005, 55, 65 CrossRef CAS; (e) B. H. Davis, Fischer–Tropsch synthesis: Overview of reactor development and future potentialities, Top. Catal., 2005, 32, 143 CrossRef CAS; (f) U. Pal, J. F. Sanchez Ramirez, H. B. Liu, A. Medina and J. A. Ascencio, Synthesis and structure determination of bimetallic Au/Cu nanoparticles, Appl. Phys. A, 2004, 79, 79 CrossRef CAS.
- Selection of reviews on heterometallic complexes in homogeneous catalysis: (a) P. Buchwalter, J. Rosé and P. Braunstein, Multimetallic catalysis based on heterometallic complexes and clusters, Chem. Rev., 2015, 115, 28 CrossRef CAS PubMed; (b) W. Xu, M. Li, L. Qiao and J. Xie, Recent advances of dinuclear nickel-and palladium-complexes in homogeneous catalysis, Chem. Commun., 2020, 56, 8524 RSC; (c) S. A. Laneman and G. G. Stanley, Homogeneous Bimetallic Hydroformylation Catalysis - Two Metals Are Better Than One, in Adv. in Chem, Amercian Chemical Society, 1992, ch. 24, vol. 230, pp. 349–366 Search PubMed; (d) R. G. Fernando, C. D. Gasery, M. D. Moulis, G. G. Stanley, M. Iglesias, E. Sola, L. A. Oro, I. Dutta, G. Sengupta, J. K. Bera, M. J. Page, D. B. Walker, B. A. Messerle, E. Bodio, M. Picquet, P. Le Gendre, M. Garland, L. Gan, D. Jennings, J. Laureanti and A. K. Jones, in Homo- and heterobimetallic complexes in catalysis, ed. P. Kalck, Springer International Publishing, Switzerland, 1st edn, 2016 Search PubMed; (e) R. M. Haak, S. J. Wezenberg and A. W. Kleij, Cooperative multimetallic catalysis using metallosalens, Chem. Commun., 2010, 46, 2713 RSC.
- (a) M. J. Dorantes, J. T. Moore, E. Bill, B. Mienert and C. C. Lu, Bimetallic iron–tin catalyst for N2 to NH3 and a silyldiazenido model intermediate, Chem. Commun., 2020, 56, 11030 RSC; (b) M. V. Vollmer, R. C. Cammarota and C. C. Lu, Reductive Disproportionation of CO2 Mediated by Bimetallic Nickelate(–I)/Group 13 Complexes, Eur. J. Inorg. Chem., 2019, 2140 CrossRef CAS.
- (a) E. Zintl and A. Harder, Polyplumbide, Polystannide und ihr Übergang in Metallphasen, Z. Phys. Chem. Abt. A, 1931, 154a, 47 CrossRef; (b) T. F. Fässler, in Zintl Ions: Principles and Recent Developments, Struct. Bonding, Springer-Verlag, Berlin, Heidelberg, 2011 CrossRef; (c) S. Scharfe, F. Kraus, S. Stegmeier, A. Schier and T. F. Fässler, Zintl ions, cage compounds, and intermetalloid clusters of group 14 and group 15 elements, Angew. Chem., Int. Ed., 2011, 50, 3630 CrossRef CAS PubMed; (d) R. J. Wilson, D. Weinert and S. Dehnen, Recent developments in Zintl cluster chemistry, Dalton Trans., 2018, 47, 14861 RSC.
- (a) M. T. Whited, Pincer-supported metal/main-group bonds as platforms for cooperative transformations, Dalton Trans., 2021, 50, 16443 RSC; (b) M. T. Whited, Metal–ligand multiple bonds as frustrated Lewis pairs for C–H functionalization, Beilstein J. Org. Chem., 2012, 8, 1554 CrossRef CAS PubMed; (c) M. T. Whited and B. L. H. Taylor, Metal/organosilicon complexes: structure, reactivity, and considerations for catalysis, Comments Inorg. Chem., 2020, 40, 217 CrossRef CAS.
- (a) W. T. Pennington, R. C. Haushalter and B. W. Eichhorn, Synthesis and structure of closo-Sn9Cr (CO)34−: The first member in a new class of polyhedral clusters, J. Am. Chem. Soc., 1988, 110, 8704 CrossRef; (b) J. M. Goicoechea and S. C. Sevov, Organozinc Derivatives of Deltahedral Zintl Ions: Synthesis and Characterization of closo-[E9Zn(C6H5)]3− (E = Si, Ge, Sn, Pb), Organometallics, 2006, 25, 4530 CrossRef CAS; (c) E. N. Esenturk, J. Fettinger and B. Eichhorn, Synthesis and characterization of the [Ni6Ge13(CO)5] 4− and [Ge9Ni2(PPh3)]2− Zintl ion clusters, Polyhedron, 2006, 25, 521 CrossRef CAS; (d) S. Scharfe, T. F. Fässler, S. Stegmaier, S. D. Hoffmann and K. Ruhland, [Cu@Sn9]3− and [Cu@Pb9]3−: Intermetalloid Clusters with Endohedral Cu Atoms in Spherical Environments, Chem. – Eur. J., 2008, 14, 4479 CrossRef CAS PubMed; (e) N. S. Willeit, V. Hlukhyy and T. F. Fässler, Synthesis, Structure and Catalytic Properties of Hyp3[Ge9Rh]PPh3, Z. Anorg. Allg. Chem., 2024, 24, e202400171 CrossRef.
- (a) S. Joseph, M. Hamberger, F. Mutzbaurer, O. Härtl, M. Meier and N. Korber, Chemistry with Bare Silicon Clusters in Solution: A Transition–Metal Complex of a Polysilicide Anion, Angew. Chem., Int. Ed., 2009, 48, 8770 CrossRef CAS PubMed; (b) M. Waibel, F. Kraus, S. Scharfe, B. Wahl and T. F. Fässler, [(MesCu)2(η 3-Si4)]4: A mesitylcopper-stabilized tetrasilicide tetraanion, Angew. Chem., Int. Ed., 2010, 49, 6611 CrossRef CAS PubMed; (c) D. O. Downing, P. Zavalij and B. W. Eichhorn, The closo–[Sn9Ir(cod)]3− and [Pb9Ir(cod)]3− Zintl Ions: Isostructural IrI Derivatives of the nido,–E94− Anions (E = Sn, Pb), Eur. J. Inorg. Chem., 2010, 890 CrossRef CAS; (d) F. S. Geitner and T. F. Fässler, Low oxidation state silicon clusters–synthesis and structure of [NHC Dipp Cu (η4-Si9)]3−, Chem. Commun., 2017, 53, 12974 RSC.
- J. M. Goicoechea and S. C. Sevov, Deltahedral germanium clusters: insertion of transition-metal atoms and addition of organometallic fragments, J. Am. Chem. Soc., 2006, 128, 4155 CrossRef CAS PubMed.
- B. Zhou, M. S. Denning, D. L. Kays and J. M. Goicoechea, Synthesis and Isolation of [Fe@Ge10]3−: A Pentagonal Prismatic Zintl Ion Cage Encapsulating an Interstitial Iron Atom, J. Am. Chem. Soc., 2009, 131, 2802 CrossRef CAS PubMed.
- O. P. E. Townrow, C. Chung, S. A. Macgregor, A. S. Weller and J. M. Goicoechea, A neutral heteroatomic zintl cluster for the catalytic hydrogenation of cyclic alkenes, J. Am. Chem. Soc., 2020, 142, 18330 CrossRef CAS PubMed.
- O. P. E. Townrow, A. S. Weller and J. M. Goicoechea, Controlled cluster expansion at a Zintl cluster surface, Angew. Chem., Int. Ed., 2024, 63, e202316120 CrossRef CAS PubMed.
- (a) K. Abersfelder, A. J. P. White, R. J. F. Berger, H. S. Rzepa and D. Scheschkewitz, A Stable Derivative of the Global Minimum on the Si6H6 Potential Energy Surface, Angew. Chem., Int. Ed., 2011, 50, 7936 CrossRef CAS PubMed; (b) A. Jana, V. Huch, M. Repisky, R. J. F. Berger and D. Scheschkewitz, Dismutational and global–minimum isomers of heavier 1,4−dimetallatetrasilabenzenes of group 14, Angew. Chem., Int. Ed., 2014, 53, 3514 CrossRef CAS PubMed; (c) P. Willmes, K. I. Leszczyńska, Y. Heider, K. Abersfelder, M. Zimmer, V. Huch and D. Scheschkewitz, Isolation and versatile derivatization of an unsaturated anionic silicon cluster (siliconoid), Angew. Chem., Int. Ed., 2016, 55, 2907 CrossRef CAS PubMed; (d) K. I. Leszczyńska, V. Huch, C. Präsang, J. Schwabedissen, R. J. F. Berger and D. Scheschkewitz, Atomically precise expansion of unsaturated silicon clusters, Angew. Chem., Int. Ed., 2019, 58, 5124 CrossRef PubMed; (e) Y. Heider, N. E. Poitiers, P. Willmes, K. I. Leszczyńska, V. Huch and D. Scheschkewitz, Site-selective functionalization of Si6R6 siliconoids, Chem. Sci., 2019, 10, 4523 RSC; (f) L. Klemmer, V. Huch, A. Jana and D. Scheschkewitz, An anionic heterosiliconoid with two germanium vertices, Chem. Commun., 2019, 55, 10100 RSC; (g) N. E. Poitiers, L. Giarrana, K. I. Leszczyńska, V. Huch, M. Zimmer and D. Scheschkewitz, Indirect and Direct Grafting of Transition Metals to Siliconoids, Angew. Chem., Int. Ed., 2020, 59, 8532 CrossRef CAS PubMed; (h) N. E. Poitiers, L. Giarrana, V. Huch, M. Zimmer and D. Scheschkewitz, Exohedral functionalization vs. core expansion of siliconoids with Group 9 metals: catalytic activity in alkene isomerization, Chem. Sci., 2020, 11, 7782 RSC; (i) N. E. Poitiers, V. Huch, M. Zimmer and D. Scheschkewitz, Chalcogen-Expanded Unsaturated Silicon Clusters: Thia-, Selena-, and Tellurasiliconoids, Chem. – Eur. J., 2020, 26, 16599 CrossRef CAS PubMed; (j) N. E. Poitiers, V. Huch, M. Zimmer and D. Scheschkewitz, Siliconoid Expansion by a Single Germanium Atom through Isolated Intermediates, Angew. Chem., Int. Ed., 2022, 61, e202205399 CrossRef CAS PubMed; (k) Y. Heider, P. Willmes, V. Huch, M. Zimmer and D. Scheschkewitz, Boron and phosphorus containing heterosiliconoids: stable p-and n-doped unsaturated silicon clusters, J. Am. Chem. Soc., 2019, 141, 19498 CrossRef CAS PubMed.
- (a) D. Scheschkewitz, A molecular silicon cluster with a “naked” vertex atom, Angew. Chem., Int. Ed., 2005, 44, 2954 CrossRef CAS PubMed; (b) M. Moteki, S. Maeda and K. Ohno, Systematic search for isomerization pathways of hexasilabenzene for finding its kinetic stability, Organometallics, 2009, 28, 2218 CrossRef CAS; (c) D. Nied, R. Köppe, W. Klopper, H. Schnöckel and F. Breher, J. Am. Chem. Soc., 2010, 132, 10264 CrossRef CAS PubMed; (d) K. Abersfelder, A. J. P. White, H. S. Rzepa and D. Scheschkewitz, Synthesis of a Pentasilapropellane. Exploring the Nature of a Stretched Silicon− Silicon Bond in a Nonclassical Molecule, Science, 2010, 327, 564 CrossRef CAS PubMed; (e) S. Ishida, K. Otsuka, Y. Toma and S. Kyushin, An organosilicon cluster with an octasilacuneane core: a missing silicon cage motif, Angew. Chem., Int. Ed., 2013, 52, 2507 CrossRef CAS PubMed; (f) A. Tshurusaki, C. Iizuka, K. Otsuka and S. Kyushin, Cyclopentasilane-fused hexasilabenzvalene, J. Am. Chem. Soc., 2013, 135, 16340 CrossRef PubMed; (g) A. Tsurusaki, K. Kamiyama and S. Kyushin, Tetrasilane-Bridged Bicyclo [4.1. 0] heptasil-1(6)-ene, J. Am. Chem. Soc., 2014, 136, 12896 CrossRef CAS PubMed; (h) F. Breher, Stretching bonds in main group element compounds—Borderlines between biradicals and closed-shell species, Coord. Chem. Rev., 2007, 251, 1007 CrossRef CAS; (i) T. Iwamoto and S. Ishida, Silicon compounds with inverted geometry around silicon atoms, Chem. Lett., 2014, 43, 164 CrossRef CAS; (j) S. Kyushin, in Organosilicon Compounds: Theory and Experiment (Synthesis), ed. V. Y. Lee, Academic Press (Elsevier), 2017, vol. 1, ch. 3, pp. 69–144 Search PubMed; (k) Y. Heider and D. Scheschkewitz, Stable unsaturated silicon clusters (siliconoids), Dalton Trans., 2018, 47, 7104 RSC; (l) Y. Heider and D. Scheschkewitz, Molecular silicon clusters, Chem. Rev., 2021, 121, 9674 CrossRef CAS PubMed.
- (a) H. Watanabe, K. Takeuchi, N. Fukawa, M. Kato, M. Goto and Y. Nagai, Air-stable tetrakis (2, 4, 6-triisopropylphenyl) disilene. Direct synthesis of disilene from dihalomonosilane, Chem. Lett., 1987, 16, 1341 CrossRef; (b) H. Watanabe, K. Takeuchi, K. Nakajima, Y. Nagai and M. Goto, The First Preparation of Disilene via Reductive DehaIogenation of 1,2-DichIorodisiIane. The Formation of an Unusual Air-Oxidation Product, 1-Oxa-2-silacyclopent-3-ene Derivative, Chem. Lett., 1988, 17, 1343 CrossRef.
- (a) S. Kaufmann, S. Schäfer, M. T. Gamer and P. W. Roesky, Reactivity studies of silylene [PhC(NtBu)2](C5Me5) Si–reactions with [M (COD) Cl]2 (M = Rh (i), Ir (i)), S, Se, Te, and BH3, Dalton Trans., 2017, 46, 8861 RSC; (b) M. Stoelzel, C. Präsang, B. Blom and M. Driess, N-heterocyclic silylene (NHSi) rhodium and iridium complexes: synthesis, structure, reactivity, and catalytic ability, Aust. J. Chem., 2013, 66, 1163 CrossRef CAS; (c) M. Aizenberg, J. Ott, C. J. Elsevier and D. Milstein, Rh(I) and Rh(III) silyl PMe3 complexes. Syntheses, reactions and 103Rh NMR spectroscopy, J. Organomet. Chem., 1998, 551, 81 CrossRef CAS.
- (a) M. Karni, J. Kapp, P. von Ragué Schleyer, Y. Apeloig and Z. Rappoport, in The Chemistry of Organic Silicon Compounds, ed. Z. Rappoport and Y. Apeloig, John Wiley & Sons, Chichester, 2001, ch. 1, vol. 3, pp. 1–183; M. Weidenbruch, Chapter 3, pp. 391–428 Search PubMed; (b) M. Kraftory, M. Kapon and M. Botoshansky, The Chemistry of Organic Silicon Compounds, ed. Z. Rappoport and Y. Apeloig, John Wiley & Sons, Chichester, 1998, ch. 5, vol. 2, pp. 181–264 Search PubMed.
- T. Iwasaki, T. Akaiwa, Y. Hirooka, S. Pal, K. Nozaki and N. Kambe, Synthesis of and structural insights into contact ion pair and solvent-separated ion pair diphenyliridate complexes, Organometallics, 2020, 39, 3077 CrossRef CAS.
- PBE0: (a) J. P. Perdew, M. Ernzerhof and K. Burke, Rationale for mixing exact exchange with density functional approximations, J. Chem. Phys., 1996, 105, 9982 CrossRef CAS; (b) C. Adamo and V. Barone, Toward reliable density functional methods without adjustable parameters: The PBE0 model, J. Chem. Phys., 1999, 110, 6158 CrossRef CAS; (c) J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- S. Bachmann, B. Gernert and D. Stalke, Solution structures of alkali metal cyclopentadienides in THF estimated by ECC-DOSY NMR-spectroscopy (incl. software), Chem. Commun., 2016, 52, 12861 RSC.
- T.-L. Nguyen and D. Scheschkewitz, Activation of a Si=Si Bond by η1-Coordination to a Transition Metal, J. Am. Chem. Soc., 2005, 127, 10174 CrossRef CAS PubMed.
- (a) H.-G. Woo, R. H. Heyn and T. D. Tilley, σ-Bond metathesis reactions for d0 metal-silicon bonds that produce zirconocene and hafnocene hydrosilyl complexes, J. Am. Chem. Soc., 1992, 114, 5698 CrossRef CAS; (b) J. Arnold, D. M. Roddick, T. D. Tilley, A. L. Rheingold and S. J. Geib, Preparation and characterization of tris (trimethylsilyl) silyl and tris (trimethylsilyl) germyl derivatives of zirconium and hafnium. X-ray crystal structures of (η5-C5Me5)Cl2HfSi(SiMe3)3 and (η5-C5Me5)Cl2HfGe(SiMe3)3, Inorg. Chem., 1988, 27, 3510 CrossRef CAS; (c) R. Fischer, D. Frank, W. Gaderbauer, C. Kayser, C. Mechtler, J. Baumgartner and C. Marschner, α, ω-Oligosilyl Dianions and Their Application in the Synthesis of Homo- and Heterocyclosilanes, Organometallics, 2003, 22, 3723 CrossRef CAS; (d) C. Kayser, D. Frank, J. Baumgartner and C. Marschner, Reactions of oligosilyl potassium compounds with Group 4 metallocene dichlorides, J. Organomet. Chem., 2003, 667, 149 CrossRef CAS; (e) Z. Dong, C. R. W. Reinhold, M. Schmidtmann and T. Müller, A Stable Silylene with a σ2, π- Butadiene Ligand, J. Am. Chem. Soc., 2017, 139, 7117 CrossRef CAS PubMed; (f) T.-I. Nguyen and D. Scheschkewitz, Activation of a Si Si Bond by η-Coordination to a Transition Metal, J. Am. Chem. Soc., 2005, 127, 10174 CrossRef CAS PubMed; (g) Z. Wu, J. B. Diminnie and Z. Xue, Synthesis and Characterization of Group 4 Amido Silyl Complexes Free of Anionic π-Ligands, Inorg. Chem., 1998, 37, 6366 CrossRef CAS; (h) A. Sauermoser, T. Lainer, G. Glotz, F. Czerny, B. Schweda, R. C. Fischer and M. Haas, Synthesis and Characterization of Methoxylated Oligosilyl Group 4 Metallocenes, Inorg. Chem., 2022, 61, 14742 CrossRef CAS PubMed; (i) B. L. L. Reant, D. De Alwis Jayasinghe, A. J. Wooles, S. T. Liddle and D. P. Mills, Comparison of group 4 and thorium M(IV) substituted cyclopentadienyl silanide complexes, Dalton Trans., 2023, 52, 7635 RSC.
- L. Pauling, J. Am. Chem. Soc., 1947, 69, 542 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2447272, 2447286 and 2447266. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt01135c |
| This journal is © The Royal Society of Chemistry 2025 |







