 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Alkylzinc-mediated transmetallation of a calcium hydride†
Kyle G.
Pearce
 *,
Mary F.
Mahon
and
Michael S.
Hill
*,
Mary F.
Mahon
and
Michael S.
Hill

Department of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: kgp29@bath.ac.uk
First published on 7th May 2025
Abstract
The dimeric β-diketiminato calcium hydride, [(BDI)CaH]2 (BDI = HC{(Me)CNDipp}2; Dipp = 2,6-i-Pr2C6H3), reacts with dialkylzinc reagents in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio through sequential dialkyl(hydrido)zincate formation, calcium alkyl extrusion followed by further irreversible transmetallation of both the organyl and spectator BDI ligands, demonstrating the generality of this transmetallation strategy to access calcium alkyls or ligated zinc alkylated species. When the reaction is performed with dimethyl zinc and an organocalcium dimer with a pre-installed kinetically stabilising aryl substituent, [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-H)Ca(BDI)], an organocalcium dimer comprised of differentiated alkyl and aryl functionalities can be accessed through hydride-for-methyl exchange, [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-Me)Ca(BDI)].
2 ratio through sequential dialkyl(hydrido)zincate formation, calcium alkyl extrusion followed by further irreversible transmetallation of both the organyl and spectator BDI ligands, demonstrating the generality of this transmetallation strategy to access calcium alkyls or ligated zinc alkylated species. When the reaction is performed with dimethyl zinc and an organocalcium dimer with a pre-installed kinetically stabilising aryl substituent, [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-H)Ca(BDI)], an organocalcium dimer comprised of differentiated alkyl and aryl functionalities can be accessed through hydride-for-methyl exchange, [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-Me)Ca(BDI)].
Introduction
Organocalcium chemistry has emerged as an area of significant study since Cloke and Lappert's crystallographic verification of the σ-alkyl calcium complex, [Ca{CH(SiMe3)2}2(diox)2] (1; diox = 1,4-dioxane), in 1991.1 Notwithstanding Anwander's remarkable report of dimethylcalcium,2 subsequent examples of diorganocalcium compounds have required the use of similarly bulky organic anions.3 In contrast, the synthetic viability of organocalcium species bearing less sterically demanding alkyl or aryl substituents has been reliant on heteroleptic systems and installation of a spectator ligand to afford the requisite levels of solubility and kinetic stability. Pre-eminent as a means to suppress calcium's proclivity for Schlenk-type equilibration to intractable homoleptic species has been the β-diketiminate class of anion.4 With regard to true organocalcium derivatives, the initial advance was provided by Harder and co-workers’ report of the β-diketiminato calcium hydride dimer, [(BDI)Ca(THF)H]2 (2; BDI = HC{(Me)CNDipp}2; Dipp = 2,6-i-Pr2C6H3).5 While this THF-adducted species provided the products of Ca–H insertion with a wide range of unsaturated small molecules that included cyclohexadiene and 1,1,-diphenylethylene, less conjugated alkenes were found to be unreactive.6 A significant broadening of the scope of this chemistry was enabled, however, through the synthesis of the base-free analogue of compound 2, [(BDI)CaH]2 (3),7 which reacts with a wide variety of unactivated terminal alkenes to afford the corresponding dimeric σ-n-alkyl derivatives, which are themselves sufficiently reactive to enable even the nucleophilic alkylation of benzene (Scheme 1).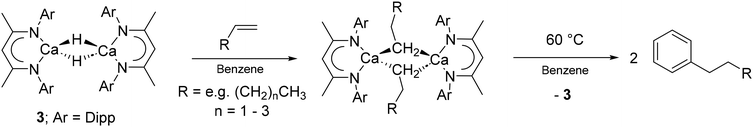 | ||
| Scheme 1 Preparation of calcium n-alkyl complexes from compound 3 and the nucleophilic alkylation of benzene. | ||
While this protocol is necessarily limited to the products of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C insertion (i.e. ethyl and higher homologues), we have recently developed a transmetallation-based approach to prepare further coordinatively unsaturated organocalcium complexes. Sequential reactions of 3 with arylmercuric reagents (Ar2Hg; Scheme 2) afford [(BDI)Ca(μ-H)(μ-Ar)Ca(BDI)] and [(BDI)Ca(μ-Ar)(μ-Ar′)Ca(BDI)] (Ar = C6H5, ortho-Me-C6H5, meta-Me-C6H5, para-Me-C6H5, 3,5-(tBu)2C6H3, Ar′ = C6H5, ortho-Me-C6H5, meta-Me-C6H5, para-Me-C6H5).8 These β-diketiminato arylcalcium derivatives describe a structural dependence between η1- and η6-aryl coordination and facilitate uncatalyzed access to biaryl molecules by direct SNAr displacement of halide from aryl bromides.8b
C insertion (i.e. ethyl and higher homologues), we have recently developed a transmetallation-based approach to prepare further coordinatively unsaturated organocalcium complexes. Sequential reactions of 3 with arylmercuric reagents (Ar2Hg; Scheme 2) afford [(BDI)Ca(μ-H)(μ-Ar)Ca(BDI)] and [(BDI)Ca(μ-Ar)(μ-Ar′)Ca(BDI)] (Ar = C6H5, ortho-Me-C6H5, meta-Me-C6H5, para-Me-C6H5, 3,5-(tBu)2C6H3, Ar′ = C6H5, ortho-Me-C6H5, meta-Me-C6H5, para-Me-C6H5).8 These β-diketiminato arylcalcium derivatives describe a structural dependence between η1- and η6-aryl coordination and facilitate uncatalyzed access to biaryl molecules by direct SNAr displacement of halide from aryl bromides.8b
 | ||
| Scheme 2 The use of compound 3 in the synthesis of arylcalcium compounds and further reactivity with aryl bromides to prepare biaryl molecules. | ||
Although the greater stability of zinc hydrides results in a more complex synthetic pathway in comparison to their mercuric analogues, we have extended this transmetallative approach to the preparation of a molecular calcium methyl complex.9 Employing the lighter group 12 congener to circumvent the severe toxicity of dimethyl mercury, the reaction between 3 and ZnMe2 under argon proceeds in an identifiable step-wise manner, first affording the pre-transmetallation dimethyl(hydrido)zincate intermediate (4). Compound 4 is subsequently prone to intramolecular equilibration to the desired β-diketiminato calcium methyl complex (5). Although 5 can be isolated in high yield, if retained in solution with [Zn(H)Me]n, further transmetallation affords the β-diketiminato zinc methyl (Scheme 3),10 the formation of which is irreversible due to the insoluble polymeric nature of [Ca(H)Me]∞.11
The notable reactivity of 3 and its related organocalcium derivatives is deduced to be a consequence of the high nucleophilicity of the Ca–H and Ca–C bonds and the relative coordinative unsaturation lent to the alkaline earth centre by the bulky yet only bidentate BDI ligand.7a,12 Motivated by a desire to broaden the scope of accessible calcium derivatives, we herein describe our further studies of hydride transmetallation with alkylzinc reagents, facilitating access to an organocalcium dimer comprising differentiated alkyl and aryl functionalities, [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-Me)Ca(BDI)].
Results and discussion
To assess the generality of the transmetallation strategy leading to compound 5 (Scheme 3), compound 3 was reacted with a further selection of dialkylzinc reagents. In a manner reminiscent of our previous study,11 addition of two equivalents of ZnEt2 to [(BDI)CaH]2 under argon provides an initial pre-transmetallation intermediate, the calcium diethyl(hydrido)zincate (6; Scheme 4, Fig. 1a; 92% isolated yield). Compound 6 exhibited a single BDI γ-methine singlet at δH 4.79 ppm in its 1H NMR spectrum, a chemical shift that is almost identical to that of 4 (4.77 ppm),11 and a Zn–H resonance at 3.06 ppm, which integrated against the triplet and quartet signals of the bridging ethyl units at δH 1.06 and −0.11 ppm in the requisite 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of intensities. Further study of the solution behaviour of compound 6 at ambient temperature revealed that it readily undergoes an onward, presumably intramolecular, reaction to afford [(BDI)CaEt]2 (Fig. S1†), which was clearly identified by its characteristic downfield Ca–CH2 resonance at −0.8 ppm in the 1H NMR spectrum.7a In an analogous manner to the chemistry depicted in Scheme 3, we suggest this latter species subsequently reacts with [Zn(Et)H]nvia BDI-for-hydride exchange giving rise to the known ethylzinc species, [(BDI)ZnEt], which was readily identified by its γ-BDI singlet at δH 4.98 ppm and ethyl triplet and quartet resonances at 0.89 and 0.24 ppm, respectively (Fig. S2;†Scheme 4; 86% isolated yield).13 An analogous reaction performed with ZniPr2 proceeds via a similar pathway, albeit the initially formed calcium di-iso-propyl(hydrido)zincate (7; Fig. 1b; 91% isolated yield) proved insoluble in common hydrocarbon solvents, precipitating from solution and perturbing any potential for further equilibration beyond this point.
4 ratio of intensities. Further study of the solution behaviour of compound 6 at ambient temperature revealed that it readily undergoes an onward, presumably intramolecular, reaction to afford [(BDI)CaEt]2 (Fig. S1†), which was clearly identified by its characteristic downfield Ca–CH2 resonance at −0.8 ppm in the 1H NMR spectrum.7a In an analogous manner to the chemistry depicted in Scheme 3, we suggest this latter species subsequently reacts with [Zn(Et)H]nvia BDI-for-hydride exchange giving rise to the known ethylzinc species, [(BDI)ZnEt], which was readily identified by its γ-BDI singlet at δH 4.98 ppm and ethyl triplet and quartet resonances at 0.89 and 0.24 ppm, respectively (Fig. S2;†Scheme 4; 86% isolated yield).13 An analogous reaction performed with ZniPr2 proceeds via a similar pathway, albeit the initially formed calcium di-iso-propyl(hydrido)zincate (7; Fig. 1b; 91% isolated yield) proved insoluble in common hydrocarbon solvents, precipitating from solution and perturbing any potential for further equilibration beyond this point.
Compounds 6 and 7 are broadly comparable and reminiscent of their methylzincate analogue, 4. Ca1 is connected to Zn1 through a three-centre alkyl-bridged bond, which elongates as a function of chain length: 4 < 6 < 7, exhibiting average Ca–C bond distances of ca. 2.62, 2.67 and 2.74 Å, respectively. While both Zn–H distances are commensurate with precedented organozinc species comprising bridging hydrides (ca. 1.6–1.8 Å),14 in a similar manner to compound 4, the hydrides located in the structures of 6 and 7 display significant asymmetry with respect to each calcium atom such that one Ca–H interaction is elongated by ca. 1 Å relative to the other [6: Ca1–H1 3.51(2) vs. Ca1–H11 2.35(2); 7: Ca1–H11 3.52(2) vs. Ca1–H1 2.37(2) Å].
We have previously observed that the reactivity of the two hydride ligands of 3 toward alkenes and the organomercurials depicted in Schemes 1 and 2 occurs sequentially and with retention of a dimeric structure throughout.7a,8b,15 Although no evidence for similar hydride discrimination could be identified during the current reactions of 3 with dialkylzinc reagents, we hypothesized that transmetallative access to organocalcium dimers comprising differentiated alkyl and aryl functions may be achievable through the pre-installation of a single kinetically stabilising aryl substituent. The previously reported [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-H)Ca(BDI)] (8)8b was, thus, reacted with half an equivalent of ZnMe2 at ambient temperature. Although [(BDI)CaMe]2 (5) was spectroscopically identified to form alongside multiple unidentified species under these conditions, performance of the reaction at −35 °C induced the overnight deposition of colourless single crystals (71% crystal yield), which were identified as [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-Me)Ca(BDI)] (9, Scheme 5) by X-ray diffraction analysis (Fig. 2). Although screening of multiple crystals by X-ray experiments identified 9 as the sole constituent of the solid-state sample, its instability to onward dismutation in solution was evidenced by its dissolution in d8-toluene at ambient temperature, which yielded a mixture of 5 and 9 in a respective 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at the first point of analysis (Fig. S7†).
1 ratio at the first point of analysis (Fig. S7†).
Each calcium centre in the structure of 9 presents a four-coordinate geometry furnished by two N–Ca contacts from the BDI ligand as well as two Ca–μ2–C–Ca interactions. Although both Ca–CH3 bond distances in 9 [Ca1–C30 2.5435(18), Ca2–C30 2.5031(17) Å] are commensurate with that of the centrosymmetric methylcalcium dimer, 5 [2.539(2) Å],11 they exhibit a slight asymmetry, presumably as a result of two C–H⋯Ca contacts, which were located and refined proximal to Ca2. In contrast, the two Ca–Caryl bond lengths are effectively identical [Ca1–C31 2.5400(15), Ca2–C31 2.5324(15) Å] and consistent with previous μ2-σ-aryl species.8,16 The aromatic ring also continues to subtend an angle of 37.5° relative to the plane defined by the Ca1–C30–Ca2–C31 unit, representing only a marginal deviation from the notably unusual orientation of the same substituent previously characterised in the solid-state structure of compound 8 (36.3°).8b
Conclusion
In summary, reactions of [(BDI)CaH]2 with dialkylzinc reagents occur in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio via a defined sequence of dialkyl(hydrido)zincate formation, calcium alkyl extrusion and further transmetallation of both the organyl and spectator BDI ligands to zinc. This work validates the generality of our transmetallative strategy to access calcium alkyls as an alternative to alkyl mercuric reagents or, as a consequence of the greater stability of zinc hydrides, ligated zinc alkyl complexes. Furthermore, this methodology can be utilised to access an organocalcium dimer comprised of differentiated alkyl and aryl functionalities, [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-Me)Ca(BDI)] (9), afforded from reacting dimethyl zinc with an organocalcium dimer with a pre-installed single kinetically stabilising aryl substituent, [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-H)Ca(BDI)].
2 ratio via a defined sequence of dialkyl(hydrido)zincate formation, calcium alkyl extrusion and further transmetallation of both the organyl and spectator BDI ligands to zinc. This work validates the generality of our transmetallative strategy to access calcium alkyls as an alternative to alkyl mercuric reagents or, as a consequence of the greater stability of zinc hydrides, ligated zinc alkyl complexes. Furthermore, this methodology can be utilised to access an organocalcium dimer comprised of differentiated alkyl and aryl functionalities, [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-Me)Ca(BDI)] (9), afforded from reacting dimethyl zinc with an organocalcium dimer with a pre-installed single kinetically stabilising aryl substituent, [(BDI)Ca(μ-3,5-tBu2C6H3)(μ-H)Ca(BDI)].
Data availability
Experimental details, NMR spectra and details of the X-ray crystallographic analysis can be found in the ESI.† Crystallographic data for all compounds have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications CCDC 2430071–2430073 for 6, 7 and 9, respectively.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge EPSRC (EP/X01181X/1, ‘Molecular s-block Assemblies for Redox-active Bond Activation and Catalysis: Repurposing the s-block as 3d-elements’).References
- F. G. N. Cloke, P. B. Hitchcock, M. F. Lappert, G. A. Lawless and B. Royo, J. Chem. Soc., Chem. Commun., 1991, 724–726 RSC.
- B. M. Wolf, C. Stuhl, C. Maichle-Mossmer and R. Anwander, J. Am. Chem. Soc., 2018, 140, 2373–2383 CrossRef PubMed.
- (a) A. Koch, M. Wirgenings, S. Krieck, H. Görls, G. Pohnert and M. Westerhausen, Organometallics, 2017, 36, 3981–3986 CrossRef; (b) M. S. Hill, M. F. Mahon and T. P. Robinson, Chem. Commun., 2010, 46, 2498–2500 RSC; (c) S. Harder, F. Feil and A. Weeber, Organometallics, 2001, 20, 1044–1046 CrossRef CAS; (d) F. Feil and S. Harder, Organometallics, 2000, 19, 5010–5015 CrossRef CAS; (e) M. R. Crimmin, A. G. M. Barrett, M. S. Hill, D. J. MacDougall, M. F. Mahon and P. A. Procopiou, Chem. – Eur. J., 2008, 14, 11292–11295 CrossRef CAS; (f) M. P. Coles, S. E. Sözeril, J. D. Smith, P. B. Hitchcock and I. J. Day, Organometallics, 2009, 28, 1579–1581 CrossRef CAS; (g) C. Eaborn, S. A. Hawkes, P. B. Hitchcock and J. D. Smith, Chem. Commun., 1997, 1961–1962 RSC.
- (a) L. Bourget-Merle, M. F. Lappert and J. R. Severn, Chem. Rev., 2002, 102, 3031–3065 CrossRef PubMed; (b) M. H. Chisholm, J. Gallucci and K. Phomphrai, Chem. Commun., 2003, 1, 48–49 RSC; (c) M. H. Chisholm, J. C. Gallucci and K. Phomphrai, Inorg. Chem., 2004, 43, 6717–6725 Search PubMed.
- S. Harder and J. Brettar, Angew. Chem., Int. Ed., 2006, 45, 3474–3478 CrossRef PubMed.
- J. Spielmann and S. Harder, Chem. – Eur. J., 2007, 13, 8928–8938 CrossRef.
- (a) A. S. S. Wilson, M. S. Hill, M. F. Mahon, C. Dinoi and L. Maron, Science, 2017, 358, 1168–1171 CrossRef; (b) A. S. S. Wilson, M. S. Hill and M. F. Mahon, Organometallics, 2019, 38, 351–360 CrossRef.
- (a) K. G. Pearce, C. Dinoi, M. S. Hill, M. F. Mahon, L. Maron, R. S. Schwamm and A. S. S. Wilson, Angew. Chem., Int. Ed., 2022, 61, e202200305 CrossRef; (b) K. G. Pearce, C. Dinoi, R. J. Schwamm, L. Maron, M. F. Mahon and M. S. Hill, Adv. Sci., 2023, 10, e2304765 CrossRef PubMed.
- R. W. Siegler, D. W. Nierenberg and W. F. Hickey, Hum. Pathol., 1999, 30, 720–723 CrossRef PubMed.
- J. Prust, A. Stasch, W. Zheng, H. W. Roesky, E. Alexopoulos, I. Usón, D. Böhler and T. Schuchardt, Organometallics, 2001, 20, 3825–3828 CrossRef.
- K. G. Pearce, S. E. Neale, C. L. McMullin, M. F. Mahon and M. S. Hill, Chem. Commun., 2024, 60, 7882–7885 RSC.
- S. Harder, B. Rösch, T. X. Gentner, H. Elsen, J. Langer and M. Wiesinger, Angew. Chem., Int. Ed., 2019, 58, 5396–5401 CrossRef PubMed.
- M. Cheng, D. R. Moore, J. J. Reczek, B. M. Chamberlain, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 8738–8749 CrossRef CAS PubMed.
- (a) A. Rit, T. P. Spaniol, L. Maron and J. Okuda, Organometallics, 2014, 33, 2039–2047 CrossRef CAS; (b) A. Lennartson, M. Hakansson and S. Jagner, Angew. Chem., Int. Ed., 2007, 46, 6678–6680 CrossRef CAS PubMed; (c) M. Chen, S. Jiang, L. Maron and X. Xu, Dalton Trans., 2019, 48, 1931–1935 RSC; (d) A. Hicken, A. J. P. White and M. R. Crimmin, Inorg. Chem., 2017, 56, 8669–8682 CrossRef CAS PubMed; (e) H. J. Hao, C. M. Cui, H. W. Roesky, G. C. Bai, H. G. Schmidt and M. Noltemeyer, Chem. Commun., 2001, 12, 1118–1119 RSC.
- (a) A. S. S. Wilson, C. Dinoi, M. S. Hill, M. F. Mahon and L. Maron, Angew. Chem., Int. Ed., 2018, 57, 15500–15504 CrossRef CAS; (b) A. S. S. Wilson, M. S. Hill and M. F. Mahon, Organometallics, 2018, 55, 5732–5735 Search PubMed; (c) M. S. Hill, M. F. Mahon, A. S. S. Wilson, C. Dinoi, L. Maron and E. Richards, Chem. Commun., 2019, 55, 5732–5735 RSC; (d) A. S. S. Wilson, C. Dinoi, M. S. Hill, M. F. Mahon, L. Maron and E. Richards, Angew. Chem., Int. Ed., 2020, 59, 1232–1237 CrossRef CAS PubMed; (e) A. S. S. Wilson, M. S. Hill, M. F. Mahon, C. Dinoi and L. Maron, Tetrahedron, 2021, 82, 131931 CrossRef CAS.
- (a) R. Fischer, H. Görls and M. Westerhausen, Inorg. Chem. Commun., 2005, 8, 1159–1161 CrossRef CAS; (b) R. Fischer, M. Gärtner, H. Görls and M. Westerhausen, Angew. Chem., Int. Ed., 2006, 45, 609–612 CrossRef CAS; (c) R. Fischer, M. Gärtner, H. Görls and M. Westerhausen, Organometallics, 2006, 25, 3496–3500 CrossRef CAS; (d) J. Langer, H. Görls and M. Westerhausen, Inorg. Chem. Commun., 2007, 10, 853–855 CrossRef CAS; (e) M. Westerhausen, M. Gärtner, R. Fischer, J. Langer, L. Yu and M. Reiher, Chem. – Eur. J., 2007, 13, 6292–6306 CrossRef CAS; (f) M. Westerhausen, Coord. Chem. Rev., 2008, 252, 1516–1531 CrossRef CAS; (g) J. Langer, S. Krieck, H. Görls and M. Westerhausen, Angew. Chem., Int. Ed., 2009, 48, 5741–5744 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2430071–2430073. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00958h |
| This journal is © The Royal Society of Chemistry 2025 |





