Open Access Article
Open Access ArticleChanging the properties of monodentate and P,C-chelating ferrocenyl-substituted 1,2,3-triazol-5-ylidene ligands through an inserted carbonyl moiety†
Věra
Varmužová
,
Ivana
Císařová
and
Petr
Štěpnička
 *
*
Department of Inorganic Chemistry, Faculty of Science, Charles University, Hlavova 2030, 128 40 Prague, Czech Republic. E-mail: stepnic@natur.cuni.cz
First published on 24th March 2025
Abstract
Triazolylidenes derived from readily accessible triazoles are useful ligands for coordination chemistry and catalysis. This work describes the synthesis of Group 11 metal complexes of new ferrocenyl-substituted triazolylidene ligands in which the ferrocene and triazolylidene moieties are separated by a carbonyl linker. In particular, complexes of types [MCl(FcC(O){CCN(Mes)NN(Me)}-κC5)] (M = Cu or Au; Fc = ferrocenyl) and [M(FcC(O){CCN(Mes)NN(Me)}-κC5)2][BF4] (M = Cu, Ag, or Au) were prepared from FcC(O)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH and characterised by spectroscopic methods, X-ray diffraction analysis and cyclic voltammetry. Using a similar strategy, the Pd(II) complex trans-[PdCl2(Ph2PfcC(O){CCN(Mes)NN(Me)}-κ2P,C5)] (fc = ferrocene-1,1′-diyl) was synthesised and analogously characterised. The phosphinocarbene ligand in this compound coordinates as a trans P,C-chelating ligand, unlike its analogues that lack the C
CH and characterised by spectroscopic methods, X-ray diffraction analysis and cyclic voltammetry. Using a similar strategy, the Pd(II) complex trans-[PdCl2(Ph2PfcC(O){CCN(Mes)NN(Me)}-κ2P,C5)] (fc = ferrocene-1,1′-diyl) was synthesised and analogously characterised. The phosphinocarbene ligand in this compound coordinates as a trans P,C-chelating ligand, unlike its analogues that lack the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O spacer and similar compounds that combine the phosphine and carbene donor groups on the ferrocene scaffold. The influence of the carbonyl spacer was evaluated in a pair of Pd(II) bis-carbene complexes, [PdBr2({C(Fc)CN(Mes)NN(Me)}-κC5)(iPr2-bimy)] and [PdBr2(FcC(O){CCN(Mes)NN(Me)}-κC5)(iPr2-bimy)] (iPr2-bimy = 1,3-diisopropyl-1,3-dihydro-2H-benzimidazol-2-ylidene), by Huynh's electronic parameters and the FeII/FeIII redox potential from cyclic voltammetry, which suggested an electron density decrease at the ferrocenyl group and decreased σ donor ability of the triazolylidene moiety upon introduction of the C
O spacer and similar compounds that combine the phosphine and carbene donor groups on the ferrocene scaffold. The influence of the carbonyl spacer was evaluated in a pair of Pd(II) bis-carbene complexes, [PdBr2({C(Fc)CN(Mes)NN(Me)}-κC5)(iPr2-bimy)] and [PdBr2(FcC(O){CCN(Mes)NN(Me)}-κC5)(iPr2-bimy)] (iPr2-bimy = 1,3-diisopropyl-1,3-dihydro-2H-benzimidazol-2-ylidene), by Huynh's electronic parameters and the FeII/FeIII redox potential from cyclic voltammetry, which suggested an electron density decrease at the ferrocenyl group and decreased σ donor ability of the triazolylidene moiety upon introduction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O linker. The Group 11 metal complexes were tested as catalysts for the metal-catalysed cyclisation of N-propargylbenzamide into 2-phenyl-5-methylene-4,5-dihydrooxazole. Among them, the chlorogold complex activated with a silver salt achieved the best results.
O linker. The Group 11 metal complexes were tested as catalysts for the metal-catalysed cyclisation of N-propargylbenzamide into 2-phenyl-5-methylene-4,5-dihydrooxazole. Among them, the chlorogold complex activated with a silver salt achieved the best results.
Introduction
During the decades that have passed since the early studies by Wanzlick1 and Öfele2 in the 1960s3 and the isolation of the first 1,3-dihydro-2H-imidazol-2-ylidene in 1991,4,5 heteroatom-stabilised carbenes have evolved into useful ligands for coordination chemistry and catalysis by transition metal complexes, efficient organocatalysts6 and versatile reagents.7 In parallel, the family of these compounds has been vastly expanded from imidazole-based carbene to analogues derived from other heterocycles.6Of particular interest in this area are 1,2,3-triazol-5-ylidenes, which were first reported in 2008,8 as examples of so-called abnormal or mesoionic carbenes9 (Scheme 1). Their attractiveness lies mainly in the facile synthesis of their precursor 1,2,3-triazoles10 and the possibility of nearly limitless modifications through the attached substituents that, in turn, allow for fine-tuning according to the purpose.11
 | ||
| Scheme 1 General synthesis of 1,2,3-triazol-5-ylidenes (only one of the triazole regioisomers is shown for clarity). | ||
The compounds of this type that are relevant to the present work are ferrocenyl-substituted 1,2,3-triazol-5-ylidenes (A and B in Scheme 2),12,13 which have been studied as redox-active and redox-switchable ligands14 and further utilised as ligands in asymmetric catalysis15 and Au(I) complexes with anticancer activity.16
In all these compounds, however, the ferrocenyl groups are attached directly to the triazolylidene ring; complexes with spacer groups between the triazolylidene and ferrocene moieties have not been synthesised thus far.17 Therefore, in continuation of our studies focused on the coordination chemistry of (phosphino)ferrocenyl carbene ligands,18 we aimed to synthesize triazolylidene complexes with ferrocenyl carbonyl substituents19 (Scheme 2). The inserted carbonyl group in the targeted compounds was expected to alter both the donor and stereochemical properties of the carbene ligands by masking the strong electron-donating ability of the ferrocene unit and increasing the overall molecular flexibility, respectively, as noted for ferrocene acylphosphines.20 A further impetus for the present work was a literature survey which showed that complexes with 4-acyltriazolylidene ligands have most likely not yet been reported.21
In addition to monodentate acyltriazolylidene ligands (E in Scheme 2), we have focused on related phosphinotriazolylidene F that expands the family of structurally attractive, P,C-chelating phosphinoferrocene carbene ligands22 and complements the previously studied type C and D donors (Scheme 2).18c
This work describes the synthesis and detailed structural characterisation of Group 11 metal complexes with ligand E and a Pd(II) complex with P,C-chelating ligand F. Additionally, the results of the catalytic evaluation of the former complexes in the model cyclisation of N-propargyl benzamide are presented.
Results and discussion
Synthesis and characterisation of complexes with monodentate acyltriazolylidene ligands
The triazolium salt required for the preparation of ferrocenylcarbonyl-substituted 1,2,3-triazol-5-ylidene complexes (E in Scheme 2) was obtained from alkynyl ketone 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 (Scheme 3). In the first step, the ketone was subjected to a Cu-catalysed reaction with mesityl azide to produce triazole 2. The cycloaddition reaction, which was performed in the presence of CuI (20 mol%) and diisopropylethylamine (2 equiv.)24 for 4 h, proceeded cleanly and provided the triazole in an 87% isolated yield. The use of only 1 equiv. of the amine and 10 mol% copper(I) salt resulted in incomplete conversion, whereas longer reaction times led to partial decomposition.
23 (Scheme 3). In the first step, the ketone was subjected to a Cu-catalysed reaction with mesityl azide to produce triazole 2. The cycloaddition reaction, which was performed in the presence of CuI (20 mol%) and diisopropylethylamine (2 equiv.)24 for 4 h, proceeded cleanly and provided the triazole in an 87% isolated yield. The use of only 1 equiv. of the amine and 10 mol% copper(I) salt resulted in incomplete conversion, whereas longer reaction times led to partial decomposition.
 | ||
| Scheme 3 Synthesis of triazolium salt 3 and its conversion into carbene complexes 4–8 (Mes = mesityl). | ||
Triazole 2 was subsequently methylated with Meerwein salt in dichloromethane to afford the desired triazolium salt 3 (82% yield); no alkylation was observed with methyl iodide in MeCN, even at an elevated temperature (3 equiv. of MeI, 60 °C, overnight).
Both intermediates were fully characterised by NMR and IR spectroscopy, electrospray ionisation (ESI) mass spectrometry and elemental analysis. The formation of the triazole ring was indicated by the characteristic signals due to the triazole C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH moiety (2:
CH moiety (2: ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, δH 8.23 and δC 128.58;
CH, δH 8.23 and δC 128.58; ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, δC 148.90). Upon alkylation, these signals shifted (3:
C, δC 148.90). Upon alkylation, these signals shifted (3: ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, δH 8.85 and δC 132.48;
CH, δH 8.85 and δC 132.48; ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, δC 138.54), and an additional signal due to the methyl substituent emerged in the spectra (δH 4.68 and δC 41.66). The presence of the tetrafluoroborate anion was corroborated by an intense band attributable to the ν3(BF4−) mode25 centred at ≈1054 cm−1 in the FTIR spectra and by the 19F NMR spectra showing a pair of resonances at δF ≈ −152 attributable to the 10BF4− and 11BF4− isotopomers in a 1
C, δC 138.54), and an additional signal due to the methyl substituent emerged in the spectra (δH 4.68 and δC 41.66). The presence of the tetrafluoroborate anion was corroborated by an intense band attributable to the ν3(BF4−) mode25 centred at ≈1054 cm−1 in the FTIR spectra and by the 19F NMR spectra showing a pair of resonances at δF ≈ −152 attributable to the 10BF4− and 11BF4− isotopomers in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio.
4 ratio.
The molecular structures of 2 and 3 are presented in Fig. 1. The latter compound crystallised as a hemihydrate 3·½H2O with two triazolium cations, two partially disordered anions and one water molecule in the asymmetric unit. The multiplication of the structurally independent “molecules”26 can be ascribed to the presence of adventitious water in a substoichiometric amount and its role in intermolecular interactions, as well as to the chirality of the crystal assembly (space group P21; see ESI†).
 | ||
| Fig. 1 Molecular structures of 2 (top) and cation 1 in the structure of 3·½H2O (bottom). For displacement ellipsoid plots and additional structure diagrams, see the ESI.† | ||
The bond lengths within the triazole ring of 2 (Table 1) compare well with parameters reported for the compound without the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O spacer, 4-ferrocenyl-1-mesityl-1H-1,2,3-triazole.14a The triazole ring is planar and oriented with its CH moiety towards the C
O spacer, 4-ferrocenyl-1-mesityl-1H-1,2,3-triazole.14a The triazole ring is planar and oriented with its CH moiety towards the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (torsion angle C1–C2–C3–O1: 18.2(2)°). The cyclopentadienyl ring and the triazole unit are mutually twisted by 26°, and the bulky mesityl substituent is nearly perpendicular to the triazole plane.
O bond (torsion angle C1–C2–C3–O1: 18.2(2)°). The cyclopentadienyl ring and the triazole unit are mutually twisted by 26°, and the bulky mesityl substituent is nearly perpendicular to the triazole plane.
| Parametera | 2 | 3·½H2O (mol 1)b | 3·½H2O (mol 2)b,c |
|---|---|---|---|
| a Definitions: Fe–C is the range of the ten Fe–C bonds in the ferrocene unit; tilt is the dihedral angle of the cyclopentadienyl ring planes; tz vs. Cp and tz vs. Mes are the interplanar angles between the triazole ring and the cyclopentadienyl C(4–8) ring and mesityl plane C(15–20), respectively. b Further data: N1–C14 1.473(3) in molecule 1 and 1.471(3) in molecule 2. c The atom numbering in molecule 2 is strictly analogous to that in molecule 1. | |||
| C1–C2 | 1.374(2) | 1.375(3) | 1.378(3) |
| C2–N1 | 1.369(1) | 1.371(3) | 1.366(3) |
| N1–N2 | 1.307(1) | 1.313(3) | 1.311(3) |
| N2–N3 | 1.364(1) | 1.330(3) | 1.328(3) |
| N3–C1 | 1.339(1) | 1.353(3) | 1.351(3) |
| Fe–C | 2.026(1)–2.068(1) | 2.026(2)–2.053(2) | 2.013(2)–2.075(2) |
| tilt | 4.84(7) | 1.7(2) | 1.8(2) |
| C3–O1 | 1.229(1) | 1.222(2) | 1.228(3) |
| C2–C3–C4 | 120.8(1) | 120.0(2) | 119.2(2) |
| tz vs. Cp | 26.18(7) | 23.0(1) | 21.6(1) |
| tz vs. Mes | 82.28(6) | 87.6(1) | 81.7(1) |
The two independent cations in the structure of 3·½H2O show only minor conformational differences. The changes in the bond lengths within the triazole ring upon alkylation are small, with the most pronounced differences being shortening of the N2–N3 bond and elongation of the N3–C1 bond; the bonds to N1, at which the methyl group was introduced, remain virtually unchanged. The mutual orientation of the triazole and the two adjacent rings C(4–8) and C(15–20) in 3 is similar to that in 2, but the central triazole ring in the salt is inverted so that its N1–C14 bond is directed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety (cf. the C1–C2–C3–O1 torsion angle: −165.5(1)° in molecule 1 and −156.2(2)° in molecule 2).
O moiety (cf. the C1–C2–C3–O1 torsion angle: −165.5(1)° in molecule 1 and −156.2(2)° in molecule 2).
Compound 3 reacted smoothly with excess silver(I) oxide and caesium carbonate14a,b,d in acetonitrile at ambient temperature to produce deep red bis-carbene complex 4 in a practically quantitative yield (94% isolated yield; Scheme 3). A similar reaction without27 carbonate (as an additional base) proceeded with incomplete conversion of the starting material. The reaction performed in the presence of KCl to obtain a chlorosilver(I) monocarbene complex also furnished 4 as the sole product.16
The formation of complex 4 was indicated by a low-field carbene resonance at δC 171.87 that was split into a pair of concentric doublets by 107Ag and 109Ag (both I = ½, ≈1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), with coupling constants (1JAgC = 171 and 197 Hz) proportional to the gyromagnetic ratios of the isotopes. A similar albeit smaller splitting was observed for the more distant triazolylidene CH signal (2JAgC = 14 and 16 Hz), whereas the C
1 ratio), with coupling constants (1JAgC = 171 and 197 Hz) proportional to the gyromagnetic ratios of the isotopes. A similar albeit smaller splitting was observed for the more distant triazolylidene CH signal (2JAgC = 14 and 16 Hz), whereas the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal shifted to a lower field to a position close to that determined for triazole 2 (cf. δC 189.04, 183.49 and 188.95 for 2, 3 and 4, respectively).
O signal shifted to a lower field to a position close to that determined for triazole 2 (cf. δC 189.04, 183.49 and 188.95 for 2, 3 and 4, respectively).
The addition of [AuCl(SMe2)] (1 equiv. relative to 3) to the in situ-generated 4 resulted in smooth transmetalation,28 which led to a separable mixture of the mono- and bis-carbene Au(I) complexes 5 and 6 as intensely violet solids in 48% and 34% yields, respectively (Scheme 3). As judged from the NMR spectra, a minor amount of another carbene complex (δC 174.19) was present in the crude mixture, which, unfortunately, could not be isolated.
Attempts to synthesise Cu(I) carbenes directly from salt 3 (by reacting 3 with either Cu2O, KCl and Cs2CO3 in MeCN at 80 °C or CuCl, K2CO3 and (PhCH2NEt3)Cl in acetone at 60 °C) were unsuccessful. Gratifyingly, transmetalation29 using 4 and freshly prepared CuCl produced the targeted compounds, again as a mixture of the mono- and bis-carbene complexes 7 and 8, with the latter dominating irrespective of the amount of CuCl applied (1 or 3 equiv.). The complexes were isolated by chromatography and obtained as deep violet solids in 16% and 66% yields, respectively (from the reaction with 3 equiv. of CuCl).
The NMR spectra of 5–8 were generally similar to those of 4 and consistent with the proposed structures. The characteristic carbene 13C NMR signals were detected at δC 162.12 and 174.27 for 5 and 6, respectively, and at δC 168.61 and 168.45 for 7 and 8, respectively.
All the carbene complexes were structurally authenticated by single-crystal X-ray diffraction analysis. The bis-carbene complexes 4 and 6 crystallised as isostructural solvates 4·CH2Cl2 and 6·CH2Cl2; the analogous copper(I) complex was isolated as acetone solvate 8·Me2CO with a very similar overall geometry. The molecular structure of the representative compound 4·CH2Cl2 is shown in Fig. 2; selected geometric data for all complexes are presented in Table 2 (further structural diagrams and geometric parameters are available in the ESI†).
 | ||
| Fig. 2 Complex cation in the structure of 4·CH2Cl2 (displacement ellipsoid plot is available in the ESI†). | ||
| Parametera | 8·Me2CO (M = Cu) | 4·CH2Cl2 (M = Ag) | 6·CH2Cl2 (M = Au) | |||
|---|---|---|---|---|---|---|
| a The parameters are defined as for precursors 2 and 3; see the footnote to Table 1. | ||||||
| M1–C1/31 | 1.898(1) | 1.899(1) | 2.074(1) | 2.075(1) | 2.014(2) | 2.014(2) |
| C1–M1–C31 | 175.74(6) | 174.42(5) | 176.25(7) | |||
| tz vs. tz | 34.87(8) | 35.67(8) | 35.3(1) | |||
| C1–C2/C31–C32 | 1.392(2) | 1.391(2) | 1.386(2) | 1.389(2) | 1.384(2) | 1.386(2) |
| C2–N1/C32–N31 | 1.369(2) | 1.366(2) | 1.370(2) | 1.368(2) | 1.367(2) | 1.365(2) |
| N1–N2/N31–N32 | 1.310(2) | 1.305(2) | 1.308(2) | 1.309(2) | 1.306(2) | 1.311(2) |
| N2–N3/N32–N33 | 1.336(2) | 1.334(2) | 1.335(2) | 1.340(2) | 1.333(2) | 1.337(2) |
| N3–C1/N33–C31 | 1.368(2) | 1.367(2) | 1.367(2) | 1.363(2) | 1.367(2) | 1.366(2) |
| Fe–C range | 2.036(2)–2.059(2) | 2.034(1)–2.055(2) | 2.032(1)–2.058(2) | 2.038(2)–2.059(2) | 2.031(2)–2.056(2) | 2.036(2)–2.057(2) |
| tilt | 2.8(1) | 2.32(8) | 1.93(8) | 2.41(9) | 2.3(1) | 2.8(1) |
| C3–O1/C33–O31 | 1.226(2) | 1.225(2) | 1.227(2) | 1.225(2) | 1.226(2) | 1.224(2) |
| C1–C2–C3–O1 | 143.5(2) | 140.4(2) | −141.7(2) | −142.2(1) | −139.6(2) | −140.7(2) |
| tz vs. Cp | 44.00(8) | 46.36(8) | 46.53(8) | 45.99(8) | 47.6(1) | 46.7(1) |
| tz vs. Mes | 81.19(7) | 88.83(7) | 86.14(7) | 77.34(7) | 86.49(9) | 78.01(9) |
The crystal structures of 4·CH2Cl2, 6·CH2Cl2 and 8·Me2CO reveal symmetrical linear coordination around the metal ions (C–M–C = 174–176°), with the M–C distances increasing from Cu to Au to Ag, in line with the trend in the covalent radii of the metals (albeit not linearly, see the ESI; Fig. S9†)30 and influenced by relativistic effects for gold.31 The two M–C distances in individual compounds are identical within the margins of experimental uncertainty and do not depart from the values reported for similar molecules.16,32 Compared with precursor 3, the triazolylidene rings present slightly elongated C1–C2 and C1–N3 distances (approximately 0.010–0.015 Å); the remaining in-ring distances are virtually unchanged.
Notably, the complex cations have the same arrangement, with the ferrocene units on one side and the mesityl substituents on the other. The mesityl groups are nearly perpendicular to the central triazolylidene plane, whereas the substituted cyclopentadienyl rings are twisted by approximately 44°. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–Me bonds point in the same direction and are oriented similarly to those in 3 (the C1–C2–C3–O1 dihedral angles were ≈140° in all the complexes).
O and N–Me bonds point in the same direction and are oriented similarly to those in 3 (the C1–C2–C3–O1 dihedral angles were ≈140° in all the complexes).
Monocarbene complexes 5 and 7 were isostructural (Fig. 3). An inspection of the geometric parameters (Table 3) reveals longer M–Cl and M–C bonds for the Au(I) complex, in agreement with the larger size of this metal ion and the trends observed for complexes [LMCl], where L = 1,4-bis(2,6-diisopropylphenyl)-3-methyl-1,2,3-triazol-5-ylidene and M = Cu or Au.33 The M–C bonds in 5 and 7 are shorter than those in the corresponding bis-carbene complexes, in line with a lower trans-influence of the chloride ligand.34 While the geometry of the carbene ligands does not differ from that of the bis-carbene complexes, 5 and 7 show smaller dihedral angles between the triazolylidene moiety and the adjacent rings (cyclopentadienyl and mesityl), which is indicative of reduced steric crowding around the metal centres ligated by only one bulky carbene donor; the parameters of the triazolylidene units are virtually the same, and even the substituents at the triazolylidene moiety are similarly oriented.
 | ||
| Fig. 3 Molecular structure of complex 5 (for additional structure diagrams and conventional displacement ellipsoid plots, see the ESI†). | ||
| Parametera | 5 (M = Cu) | 7 (M = Au) |
|---|---|---|
| a The parameters are defined as for precursors 2 and 3; see footnote to Table 1. | ||
| M1–Cl1 | 2.1003(6) | 2.2790(5) |
| M1–C1 | 1.876(2) | 1.985(2) |
| Cl1–M1–C1 | 174.48(6) | 177.30(5) |
| C1–C2 | 1.398(2) | 1.394(2) |
| C2–N1 | 1.367(2) | 1.365(2) |
| N1–N2 | 1.310(2) | 1.310(2) |
| N2–N3 | 1.336(2) | 1.338(2) |
| N3–C1 | 1.373(2) | 1.368(2) |
| Fe–C | 2.028(2)–2.053(2) | 2.031(2)–2.049(2) |
| tilt | 0.8(1) | 1.4(1) |
| C3–O1 | 1.225(2) | 1.226(2) |
| C1–C2–C3–O1 | 155.0(2) | 152.0(2) |
| tz vs. Cp | 35.5(1) | 41.1(1) |
| tz vs. Mes | 69.8(1) | 76.00(9) |
The carbene complexes and their common precursor 3 were studied by cyclic voltammetry at a glassy carbon disc electrode in dichloromethane that contained 0.1 M Bu4N[PF6] as the supporting electrolyte. In the accessible potential range, salt 3 displayed single reversible oxidation at 0.35 V vs. the ferrocene/ferrocenium reference,35 which was attributed to one-electron oxidation of the ferrocene unit (Fig. 4). In the cathodic region, the compound underwent reversible one-electron reduction at −1.56 V, followed by an additional irreversible redox event at approximately −2.1 V (the anodic peak potential at a 100 mV s−1 scan rate is given). A generally similar behaviour and redox potentials were reported for a compound in which the redox-active ferrocenyl group was attached directly to the triazolium ring.14a,e
 | ||
| Fig. 4 Cyclic voltammograms of triazolium salt 3 (recorded in 0.1 M Bu4N[PF6]/CH2Cl2 at a glassy carbon disc electrode and a 100 mV s−1 scan rate). | ||
Under similar conditions, monocarbene complex 5 showed reversible oxidation at 0.26 V and reversible reduction at −2.06 V (Fig. 5). The shift of the oxidative wave to a lower potential than that of 3 can be explained by the loss of positive charge, which makes the azole substituent less electron-withdrawing (maybe also due to backdonation from Au) and may lower the coulombic barrier for electron removal.
 | ||
| Fig. 5 Cyclic voltammogram of carbene complex 5 (recorded in 0.1 M Bu4N[PF6]/CH2Cl2 at a glassy carbon disc electrode and a 100 mV s−1 scan rate). | ||
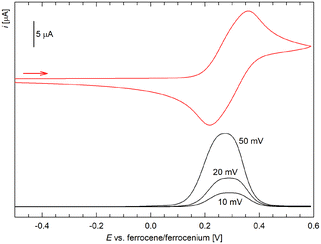
The redox responses of bis-carbene complexes 4, 6 and 8 were different: Cu(I) complex 8 displayed reversible oxidation centred at ≈0.30 V (Fig. 6). The redox wave was relatively broad (with a peak separation of ≈140 mV, which was significantly greater than the ≈80–85 mV for decamethylferrocene standard under the conditions applied) and consisted of two narrowly separated one-electron waves, as corroborated by differential pulse voltammetry, which revealed two unresolved peaks (Fig. 7; full resolution of the two peaks was not achieved even by changing the modulation amplitude).36 This behaviour can be explained by sequential oxidation of the two chemically equivalent ferrocene units, which probably do not communicate electronically but are differentiated by the first oxidation. In the cathodic region, complex 8 underwent two successive irreversible reductions at approximately −2.06 and −2.21 V (the peak potentials at a scan rate of 100 mV s−1 are given), which was attributable to redox changes localised at the triazolylidene units.
 | ||
| Fig. 6 Cyclic voltammogram of bis-carbene complex 8 (recorded in 0.1 M Bu4N[PF6]/CH2Cl2 at a glassy carbon disc electrode and a 100 mV s−1 scan rate). | ||
The redox behaviours of complexes 4 and 6 containing heavier metals were essentially similar except that their oxidation waves were less resolved (peak separation ≈105 mV), and for complex 6, the oxidation was associated with adsorption, which gave rise to an anodic prepeak and resulted in the anodic counterwave gaining the appearance of a stripping peak when the scan range was extended towards more positive potentials (see the ESI†).
The oxidations of 4 and 6 were detected at approximately 0.28 V, and their smaller separations can be explained by longer M–C distances (M = Ag and Au), which make the ferrocene units more distant and, hence, more independent. Cathodic waves were observed at approximately −2.05 and −2.22 V for the silver complex and at −2.02 V for its Au(I) congener (the second wave could not be unambiguously localised for 6).
Evaluation of the donor properties
The electronic properties of the acyltriazolylidene ligand E were evaluated from two directions: the Huynh electronic parameter (HEP)37 was used to probe the effect of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O linker on the carbene donor ability, and its influence on the ferrocene unit was monitored by cyclic voltammetry. For this purpose, a pair of Pd(II) bis-carbene complexes 10 and 11 were prepared from salt 3 and its “nonspaced” analogue 9, respectively. Compound 9 was obtained from ethynylferrocene and mesityl azide under modified conditions (see the ESI†) and subsequently alkylated according to the literature.14a Both salts were treated with Ag2O in the presence of Cs2CO3 (in MeCN), and the presumed Ag(I)-carbene intermediate was directly transmetalated37a,b with [PdBr2(iPr2-bimy)]2
O linker on the carbene donor ability, and its influence on the ferrocene unit was monitored by cyclic voltammetry. For this purpose, a pair of Pd(II) bis-carbene complexes 10 and 11 were prepared from salt 3 and its “nonspaced” analogue 9, respectively. Compound 9 was obtained from ethynylferrocene and mesityl azide under modified conditions (see the ESI†) and subsequently alkylated according to the literature.14a Both salts were treated with Ag2O in the presence of Cs2CO3 (in MeCN), and the presumed Ag(I)-carbene intermediate was directly transmetalated37a,b with [PdBr2(iPr2-bimy)]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 (in CH2Cl2; iPr2-bimy = 1,3-diisopropyl-1,3-dihydro-2H-benzimidazol-2-ylidene) to produce the targeted Pd(II) bis-carbene complexes 10 and 11 in good isolated yields (≈65%; Scheme 4).
38 (in CH2Cl2; iPr2-bimy = 1,3-diisopropyl-1,3-dihydro-2H-benzimidazol-2-ylidene) to produce the targeted Pd(II) bis-carbene complexes 10 and 11 in good isolated yields (≈65%; Scheme 4).
Complex 11 was orange as typical for simple ferrocene derivatives,39 whereas compound 10, which possesses a conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O chromophore, was intensely burgundy red. This difference was clearly manifested in the UV-vis spectra (λmax = 503 nm for 10 and 450 nm for 11; see the ESI†). In their NMR spectra, complexes 10 and 11 displayed all the expected resonances, including the set of signals due to a monosubstituted ferrocene unit and the iPr2-bimy ligand; the signal of the C
O chromophore, was intensely burgundy red. This difference was clearly manifested in the UV-vis spectra (λmax = 503 nm for 10 and 450 nm for 11; see the ESI†). In their NMR spectra, complexes 10 and 11 displayed all the expected resonances, including the set of signals due to a monosubstituted ferrocene unit and the iPr2-bimy ligand; the signal of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in 10 was observed at δC 189.16. In addition, two carbene 13C NMR signals were detected and clearly distinguished via 2D NMR spectra (the 13C NMR signal due to iPr2-bimy was correlated with the CHMe2 proton signals). For both compounds, the triazolylidene signal was found at a higher field than the resonance of the iPr2-bimy reporter group. The HEP values (the chemical shifts quoted here are relative to the solvent signal (CDCl3) at δC 77.7 per the recommendation) were 177.88 ppm for 10 and 179.97 ppm for 11, which correspond to the data obtained for similar compounds40,14c and suggest that the acyltriazolylidene ligand in 10 is a weaker σ donor than its analogue without the C
O group in 10 was observed at δC 189.16. In addition, two carbene 13C NMR signals were detected and clearly distinguished via 2D NMR spectra (the 13C NMR signal due to iPr2-bimy was correlated with the CHMe2 proton signals). For both compounds, the triazolylidene signal was found at a higher field than the resonance of the iPr2-bimy reporter group. The HEP values (the chemical shifts quoted here are relative to the solvent signal (CDCl3) at δC 77.7 per the recommendation) were 177.88 ppm for 10 and 179.97 ppm for 11, which correspond to the data obtained for similar compounds40,14c and suggest that the acyltriazolylidene ligand in 10 is a weaker σ donor than its analogue without the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O spacer in complex 11.
O spacer in complex 11.
This observation was consistent with the information inferred from cyclic voltammetry. Complexes 10 and 11 displayed single reversible oxidations at E°′ 0.26 and 0.12 V vs. ferrocene/ferrocenium, respectively (Fig. 8). The positions of the waves, which were ascribed to a ferrocene-centred, one-electron redox transition, indicated easier oxidation of 11, even though the difference in the redox potentials was smaller than, e.g., that between the redox potentials of ferrocene and benzoylferrocene (ΔE = 0.21 V in Bu4N[PF6]/MeCN).41 In addition, the complexes underwent simple (11: E ≈ −2.3 V) or multistep (10) irreversible reduction, similar to bis-carbene complexes 4, 6, and 8 (see the ESI†).
 | ||
| Fig. 8 Cyclic voltammograms (anodic branches) of complexes 10 and 11 (recorded in 0.1 M Bu4N[PF6]/CH2Cl2 at a glassy carbon disc electrode and a 100 mV s−1 scan rate). | ||
Overall, these results indicate that the carbonyl linker lowers the electron density at both parts of the ligand moiety (ferrocene and triazolylidene) and, consequently, makes the carbene ligand a weaker σ donor. The strong electron-donating effect of the ferrocenyl substituent (cf. Hammett constant σp for the ferrocenyl group: −0.18)42 is apparently unable to efficiently cancel out the electron-withdrawing effect of the introduced carbonyl linker (cf. σp for C6H5 and C6H5C(O): −0.01 and 0.43, respectively).
Catalytic experiments
Carbene complexes 4, 5, 6 and 8 were evaluated as catalysts for the metal-mediated cyclisation of N-propargylbenzamide (12) to 5-methyleneoxazoline 13 (Scheme 5).43 The reactions were performed at 25 °C in CD2Cl2 with 3 mol% catalyst and monitored by 1H NMR spectroscopy.Under these conditions, no appreciable reaction was observed when bis-carbene complexes 4, 6 and 8 were used (no product was detected after 24 h), whereas chlorogold(I) complex 5 reached only 1% conversion after a 6 h reaction time. However, when this complex was “activated” by one molar equivalent of silver bis(trifluoromethanesulfonyl)imide (AgNTf2) dissolved in MeCN, a smooth reaction occurred (Fig. 9). The NMR yield of product 13 after 6 h was 97%, and the reaction followed (pseudo)first-order kinetics, as expected for a simple catalytic process. However, the performance of the 5/AgNTf2 catalyst was worse than that of the archetypal [AuCl(PPh3)]/AgNTf2 system, which achieved full conversion within 1 h under otherwise identical conditions (Fig. 9). No reaction was observed in the presence of AgNTf2 only,44 and the silver salt did not oxidize the ferrocene moiety in 5 (the addition of AgNTf2 to 5 resulted in the separation of insoluble AgCl),45 as indicated by the practically identical UV-vis spectra recorded for complex 5 and a 5-AgNTf2 (5 equiv.) mixture in dichloromethane with a little MeCN added to mimic reaction conditions (see the ESI, Fig. S64†).
Complexes with a P,C-chelating phosphinocarbene ligand
The synthesis of analogous P,C-chelating phosphinocarbene complexes has proven more challenging. Although generally similar, it required a carefully designed sequence of synthetic steps that circumvented problems with functional group tolerance (e.g., avoiding unwanted Staudinger reaction46 between an azide and ferrocene-bound phosphine moiety or alkylation of the latter). The synthesis (Scheme 6) started from P-protected aldehyde 14,47 which was converted in two steps to alkynyl ketone 16 (72% yield from 14), as described for 1.23 To achieve good conversion, 3 equiv. of ethynylmagnesium bromide were needed in the first step (when only 1.5 equiv. were applied, only half of the starting aldehyde reacted), and the following oxidation was performed with 20 equiv. of MnO2. Cyclisation with mesityl azide in the presence of 20 mol% CuI and 2 equiv. of i-Pr2NEt produced triazole 17, which was alkylated with Meerwein salt (1.2 equiv., 2 h) to afford 18 (the isolated yields of these two steps were 90% or higher).Attempts to remove the borane protection group48 with methanol49 or morpholine50 failed (N.B. the reaction with methanol produced methyl 1′-(diphenylphosphino)ferrocene-1-carboxylate51 as the product of C(O)-triazole bond cleavage and concomitant phosphine deprotection). Eventually, the deprotection was achieved with 1,4-diazabicyclo[2.2.2]octane52 (dabco; 2 equiv.) in anhydrous THF overnight. Free phosphine 19 was obtained in good yield (78%) and acceptable purity by carefully optimised column chromatography and was directly converted to Pd(II) complex 20. For this transformation, freshly prepared 19 was treated with Ag2O and carefully dried Cs2CO3 in anhydrous acetonitrile, and the presumed Ag(I)-carbene intermediate was reacted without isolation with [PdCl2(MeCN)2] in dry dichloromethane. The product was isolated by column chromatography as a deep red solid in 14% yield. Although this yield may seem disappointingly low, it corresponds to the complexity of the last reaction step.
All the compounds along the reaction sequence were fully characterised using multinuclear NMR, high-resolution mass spectrometry (MS) and elemental analysis. The collected data corroborated the proposed structures and were consistent with the data obtained for the nonphosphinylated compounds discussed above. In addition, the structures of 15 and 17 were determined via single-crystal X-ray diffraction analysis.
Compound 15 (Fig. 10) crystallised with the symmetry of the monoclinic space group P21/n, forming a supramolecular assembly based on ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯O interactions and O–H⋯HB dihydrogen bonds that involve polarised BH hydrogens as acceptors (see ESI†);53 O–H⋯O interactions operating in the structures of ferrocenylmethanol54,55 and its phosphinylated derivatives56 were not detected.
C–H⋯O interactions and O–H⋯HB dihydrogen bonds that involve polarised BH hydrogens as acceptors (see ESI†);53 O–H⋯O interactions operating in the structures of ferrocenylmethanol54,55 and its phosphinylated derivatives56 were not detected.
 | ||
| Fig. 10 Crystal structure of 15. Selected distances and angles (in Å and °): Fe–C (range) 2.035(3)–2.057(3), P1–B1 1.927(3), P1–C6 1.789(3), P1–C11 1.823(2) P1–C17 1.811(3), C1–C23 1.497(4), C23–O1 1.441(3), C1–C23–O1 110.8(2), C23–C24 1.478(4), C24–C25 1.189(4), and C23–C24–C25 178.4(3). Displacement ellipsoid plot is available in the ESI.† | ||
The molecule of 15 comprises a regular ferrocene unit (tilt angle: 1.6(2)°) whose substituents assume an intermediate conformation (τ = −97.6(2)°; τ is the torsion angle C1–Cg1–Cg2–C6, where Cg1 and Cg2 are the centroids of cyclopentadienyl rings C(1–5) and C(6–10), respectively); the individual geometric parameters match those determined for Ph2PfcCH2OH·BH3 (fc = ferrocene-1,1′-diyl).53
Triazole 17 (Fig. 11) crystallised with two molecules in the asymmetric unit (space group P21/c), which show negligible differences, e.g., in the orientations of the “terminal” phenyl rings (see the ESI†). The substituted ferrocene units are tilted by 3.1(1)° in molecule 1 [3.2(1)° in molecule 2] and adopt a 1,3′ conformation57 characterised by τ58 angles of −145.5(1)° [147.3(1)°]. The dihedral angle between the planes of the triazole and carbonyl-substituted cyclopentadienyl rings is 24.5(1)° [27.3(1)°]. Otherwise, the geometries of the acyltriazole and –PPh2·BH3 groups are unexceptional considering the data for the compounds discussed above.
 | ||
| Fig. 11 Molecule 1 in the crystal structure of 17. Selected distances and angles for molecule 1 [molecule 2] (in Å and °): Fe–C (range) 2.016(2)–2.058(2) [2.024(2)–2.065(2)], P1–B1 1.930(2) [1.919(2)], P1–C9 1.794(2) [1.798(2)], P1–C24 1.814(2) [1.812(2)], P1–C30 1.812(2) [1.811(2)], C3–O1 1.228(2) [1.225(2)], C1–C2 1.373(3) [1.371(2)], C2–N1 1.368(3) [1.364(2)], N1–N2 1.302(2) [1.305(2)], N2–N3 1.364(2) [1.357(2)], and N3–C1 1.339(3) [1.339(2)]. A displacement ellipsoid plot is available in the ESI.† | ||
Compound 20, which represents the ultimate synthetic goal, was characterised as a structurally unique Pd(II) complex that features a phosphinocarbene ligand coordinating as a trans P,C-chelating donor. Notably, typical ligands capable of traversing trans positions in the coordination sphere of soft transition metals are symmetrical diphosphines with rigid organic backbones,59 whereas their donor-unsymmetric counterparts remain rare.60
A hint for the particular arrangement, which was later corroborated by structure determination, was initially provided by the NMR spectra showing an unusually large 13C–31P scalar coupling constant for the carbene resonance (δC 157.68, 2JPC = 195 Hz), in the range reported for complexes trans-[PdCl2(PR3)(IPr)] (R = Ph, o-tolyl, or cyclohexyl; IPr = 1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene-κC2; 2JPC = 180–200 Hz).61 Analogous complexes that contain cis-chelating ferrocene phosphinocarbene ligands of types C and D (Scheme 2)18c and their diaminocarbene analogues18b presented 2JPC coupling constants below 10 Hz.
Furthermore, the fixed geometry rendered the ferrocene CH groups in 20 diastereotopic and, hence, eight resonances due to these groups were observed in both the 1H and 13C{1H} NMR spectra as relatively broad signals due to structural dynamics. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group resonated at δC 191.34, and the signal of the bonding triazole carbon was detected at δC 142.87 as a 31P-coupled doublet (3JPC = 7 Hz). The 31P{1H} NMR signal was observed at δP 11.6, shifted downfield with respect to triazolium salt 19 (δP −19.8).
O group resonated at δC 191.34, and the signal of the bonding triazole carbon was detected at δC 142.87 as a 31P-coupled doublet (3JPC = 7 Hz). The 31P{1H} NMR signal was observed at δP 11.6, shifted downfield with respect to triazolium salt 19 (δP −19.8).
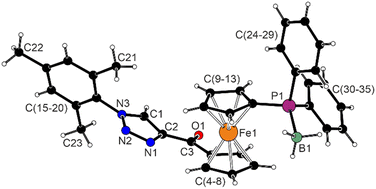
The molecular structure of complex 20·C6H14 is presented in Fig. 12. The compound is indeed a “square planar” Pd(II) complex, albeit distorted due to spatial constraints imposed by the trans-chelating phosphinocarbene ligand. The coordination environment is bent along both diagonals (P1–Pd1–C1 = 160.08(4)°, Cl1–Pd1–Cl2 = 169.18(2)°), which is reflected in the τ4 index62 of 0.22 that suggests angular distortion towards the tetrahedral geometry (ideal square and tetrahedron would yield τ4 values of 0 and 1, respectively). Among the interligand angles, the P1–Pd1–Cl2 angle is opened to 98°, whereas the three remaining angles are approximately 88° (Table 4). The Pd-donor distances compare well with the parameters reported for trans-[PdCl2{P(o-tolyl)3}(L)] (L = 3-methyl-1,4-diphenyltriazol-5-ylidene), which is a complex with a similar geometry and donor set.63
 | ||
| Fig. 12 Complex in the structure of 20·C6H14. The selected distances and angles (in Å and °): Fe–C (range) 2.041(2)–2.075(2), P1–C9 1.813(2), P1–C24 1.816(2), P1–C30 1.824(1), C3–O1 1.224(2), C1–C2 1.386(2), C2–N1 1.366(2), N1–N2 1.312(2), N2–N3 1.337(2), and N3–C1 1.360(2). A displacement ellipsoid plot is available in the ESI.† | ||
Compared with triazole 17, the ferrocene unit in 20·C6H14 is slightly more tilted (dihedral angle 6.49(9)°) and, mainly, less opened (τ = −68.5(1)°), approaching the ideal synclinal eclipsed conformation (τ = 72°)64 to enable chelate coordination. The triazolylidene ring is twisted by 52.39(9)° from the plane of the cyclopentadienyl ring C(5–9), and the terminal mesityl ring is oriented perpendicularly to both the C2N3 ring and the mean coordination plane {Pd1,P1,Cl1,Cl2,C1} (interplanar angles: 84.11(8)° and 84.59(5)°, respectively).
The cyclic voltammogram of 20 (Fig. 13) revealed one reversible oxidation at 0.41 V, which was attributed to the ferrocene-based redox event. The oxidation occurs at more positive potentials than for the nonchelating carbene complexes (vide supra), which corresponds to the electron-withdrawing nature of the additional substituent (PPh2) at the ferrocene unit, which is further enhanced by its coordination. In the cathodic region, complex 20 underwent two successive irreversible reductions at approximately −1.92 and −2.32 V, similar to those observed for the Group 11 metal complexes discussed above.
 | ||
| Fig. 13 Cyclic voltammograms (anodic branches) of complex 20 (recorded in 0.1 M Bu4N[PF6]/CH2Cl2 at a glassy carbon disc electrode and a 100 mV s−1 scan rate). | ||
Conclusions
We describe the synthesis of acyltriazolylidene complexes, which most likely represent the first compounds of this type. The proligand for these complexes, 4-acyltriazolium salt 3, was obtained smoothly via a Cu-catalysed [3 + 2]-cycloaddition reaction from mesityl azide and ferrocenyl ethynyl ketone (FcC(O)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) followed by alkylation of the formed triazole with Meerwein salt. The triazolium salt was converted to a series of Group 11 metal complexes, either homoleptic [ML2][BF4] (M = Cu, Ag, or Au) or with an auxiliary chloride ligand, [LMCl] (M = Cu or Au). These compounds were structurally characterised by a combination of spectroscopic methods and single-crystal X-ray diffraction analysis, and their catalytic properties were probed for the cyclisation of N-propargylbenzamide to the respective methylenoxazoline. Considering the presence of the redox-active ferrocenyl group, the compounds were further studied by voltammetric techniques, which revealed reversible ferrocene/ferrocenium transitions and irreversible reductions presumably localised at the carbene fragment. The 13C NMR and electrochemical data obtained for a pair of Pd(II) bis-carbene complexes with or without the C
CH) followed by alkylation of the formed triazole with Meerwein salt. The triazolium salt was converted to a series of Group 11 metal complexes, either homoleptic [ML2][BF4] (M = Cu, Ag, or Au) or with an auxiliary chloride ligand, [LMCl] (M = Cu or Au). These compounds were structurally characterised by a combination of spectroscopic methods and single-crystal X-ray diffraction analysis, and their catalytic properties were probed for the cyclisation of N-propargylbenzamide to the respective methylenoxazoline. Considering the presence of the redox-active ferrocenyl group, the compounds were further studied by voltammetric techniques, which revealed reversible ferrocene/ferrocenium transitions and irreversible reductions presumably localised at the carbene fragment. The 13C NMR and electrochemical data obtained for a pair of Pd(II) bis-carbene complexes with or without the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O linker and 1,3-diisopropyl-1,3-dihydro-2H-benzimidazol-2-ylidene ligand as the reporter moiety indicated that the carbonyl groups render both ligand parts (i.e., the ferrocene unit and the triazolylidene unit) less electron rich, thereby decreasing the ligand's σ donation ability.
O linker and 1,3-diisopropyl-1,3-dihydro-2H-benzimidazol-2-ylidene ligand as the reporter moiety indicated that the carbonyl groups render both ligand parts (i.e., the ferrocene unit and the triazolylidene unit) less electron rich, thereby decreasing the ligand's σ donation ability.
Finally, a similar synthetic strategy (albeit with additional protection/deprotection steps required to preserve the reactive phosphine moiety) and direct Ag-to-Pd transmetalation were used to prepare Pd(II) complex 20, which is a rare example of a complex that features a trans-spanning P,C-ligand.
Data availability
The data supporting this article have been included as part of the extensive ESI.† The crystallographic data for all the structures have been deposited with the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2427432–2427441.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Czech Science Foundation (project no. 23-06718S).References
- (a) H.-W. Wanzlick and E. Schikora, Angew. Chem., 1960, 72, 494 CrossRef CAS; (b) H.-W. Wanzlick and E. Schikora, Chem. Ber., 1961, 94, 2389 CrossRef CAS; (c) H.-W. Wanzlick, Angew. Chem., Int. Ed. Engl., 1962, 1, 75 Search PubMed.
- (a) K. Öfele, J. Organomet. Chem., 1968, 12, P42 CrossRef See also: (b) D. J. Cardin, B. Cetinkaya and M. F. Lappert, Chem. Rev., 1972, 72, 545 CrossRef CAS; (c) M. F. Lappert, J. Organomet. Chem., 1988, 358, 185 Search PubMed.
- W. Kirmse, Angew. Chem., Int. Ed., 2010, 49, 8798 CrossRef CAS PubMed.
- (a) A. J. Arduengo, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361 CrossRef CAS; (b) A. J. Arduengo, H. V. R. Dias, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1992, 114, 5530 CrossRef CAS; (c) A. J. Arduengo, J. R. Goerlich and W. J. Marshall, J. Am. Chem. Soc., 1995, 117, 11027 CrossRef CAS.
- The first isolable carbene, R2PCSiMe3, was reported earlier in: A. Igau, H. Grützmacher, A. Baceiredo and G. Bertrand, J. Am. Chem. Soc., 1988, 110, 6463 CrossRef CAS.
- (a) F. E. Hahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122 CrossRef CAS PubMed; (b) P. de Frémont, N. Marion and S. P. Nolan, Coord. Chem. Rev., 2009, 253, 862 CrossRef; (c) D. J. Nelson and S. P. Nolan, Chem. Soc. Rev., 2013, 42, 6723 RSC; (d) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS PubMed; (e) H. V. Huynh, Chem. Rev., 2018, 118, 9457 CrossRef CAS PubMed; (f) P. Bellotti, M. Koy, M. N. Hopkinson and F. Glorius, Nat. Rev. Chem., 2021, 5, 711 CAS.
- V. Nair, S. Bindu and V. Sreekumar, Angew. Chem., Int. Ed., 2004, 43, 5130 CAS.
- (a) P. Mathew, A. Neels and M. Albrecht, J. Am. Chem. Soc., 2008, 130, 13534 CAS For reviews, see: (b) O. Schuster, L. Yang, H. G. Raubenheimer and M. Albrecht, Chem. Rev., 2009, 109, 3445 CAS; (c) R. H. Crabtree, Coord. Chem. Rev., 2013, 257, 755 CAS; (d) G. Guisado-Barrios, M. Soleilhavoup and G. Bertrand, Acc. Chem. Res., 2018, 51, 3236 CAS; (e) Á. Vivancos, C. Segarra and M. Albrecht, Chem. Rev., 2018, 118, 9493 Search PubMed; (f) R. Maity and B. Sarkar, JACS Au, 2022, 2, 22 CAS.
- R. H. Crabtree, Coord. Chem. Rev., 2013, 257, 755 CAS.
- 1,2,3-Triazoles are readily accessible by Cu-catalysed Huisgen cycloaddition from azides and alkynes: (a) M. Meldal and C. Wenzel Tornøe, Chem. Rev., 2008, 108, 2952 CAS; (b) M. Breugst and H.-U. Reissig, Angew. Chem., Int. Ed., 2020, 59, 12293 CAS.
- (a) J. D. Crowley, A.-L. Lee and K. J. Kilpin, Aust. J. Chem., 2011, 64, 1118 CAS; (b) K. F. Donnelly, A. Petronilho and M. Albrecht, Chem. Commun., 2013, 49, 1145 CAS; (c) S. A. Patil, H. M. Heras-Martinez, A. M. Lewis, S. A. Patil and A. Bugarin, Polyhedron, 2021, 194, 114935 CAS; (d) G. Guisado-Barrios, M. Soleilhavoup and G. Bertrand, Acc. Chem. Res., 2018, 51, 3236 CAS; (e) W. Stroek and M. Albrecht, Chem. Soc. Rev., 2024, 53, 6322 RSC and ref. 8e and f.
- (a) B. Bildstein, J. Organomet. Chem., 2001, 617–618, 28 CrossRef CAS; (b) U. Siemeling, Eur. J. Inorg. Chem., 2012, 3523 CrossRef CAS.
- P. Štěpnička, Dalton Trans., 2022, 51, 8085 RSC.
- (a) L. Hettmanczyk, S. Manck, C. Hoyer, S. Hohloch and B. Sarkar, Chem. Commun., 2015, 51, 10949 RSC; (b) L. Hettmanczyk, L. Suntrup, S. Klenk, C. Hoyer and B. Sarkar, Chem. – Eur. J., 2017, 23, 576 CrossRef CAS PubMed; (c) D. Aucamp, T. Witteler, F. Dielmann, S. Siangwata, D. C. Liles, G. S. Smith and D. I. Bezuidenhout, Eur. J. Inorg. Chem., 2017, 1227 CrossRef CAS; (d) S. Klenk, S. Rupf, L. Suntrup, M. van der Meer and B. Sarkar, Organometallics, 2017, 36, 2026 CrossRef CAS; (e) C. Hoyer, P. Schwerk, L. Suntrup, J. Beerhues, M. Nössler, U. Albold, J. Dernedde, K. Tedin and B. Sarkar, Eur. J. Inorg. Chem., 2021, 1373 CrossRef CAS For similar compounds containing ferrocenyl and/or cobaltocenyl subsituents, see: (f) S. Vanicek, M. Podewitz, J. Stubbe, D. Schulze, H. Kopacka, K. Wurst, T. Müller, P. Lippmann, S. Haslinger, H. Schottenberger, K. R. Liedl, I. Ott, B. Sarkar and B. Bildstein, Chem. – Eur. J., 2018, 24, 3742 CrossRef CAS PubMed.
- (a) R. Haraguchi, S. Hoshino, T. Yamazaki and S. Fukuzawa, Chem. Commun., 2018, 54, 2110 RSC; (b) R. Haraguchi, T. Yamazaki, K. Torita, T. Ito and S. Fukuzawa, Dalton Trans., 2020, 49, 17578 RSC.
- D. Aucamp, S. V. Kumar, D. C. Liles, M. A. Fernandes, L. Harmse and D. I. Bezuidenhout, Dalton Trans., 2018, 47, 16072 RSC.
- This contrasts with the situation encountered among imidazol-2-ylidene and related ligands, where compounds with ferrocenylmethyl substituents dominate due to their facile synthesis; see ref. 12b. Selected recent examples: (a) K. Arumugam, J. Chang, V. M. Lynch and C. W. Bielawski, Organometallics, 2013, 32, 4334 CrossRef CAS; (b) K. Arumugam, C. D. Varnado, S. Sproules, V. M. Lynch and C. W. Bielawski, Chem. – Eur. J., 2013, 19, 10866 CrossRef CAS PubMed; (c) P. He, Y. Du, S. Wang, C. Cao, X. Wang, G. Pang and Y. Shi, Z. Anorg. Allg. Chem., 2013, 639, 1004 CrossRef CAS; (d) H. A. Özbek, P. S. Aktaş, J.-C. Daran, M. Oskay, F. Demirhan and B. Çetinkaya, Inorg. Chim. Acta, 2014, 423, 435 CrossRef; (e) J. F. Arambula, R. McCall, K. J. Sidoran, D. Magda, N. A. Mitchell, C. W. Bielawski, V. M. Lynch, J. L. Sesslerg and K. Arumugam, Chem. Sci., 2016, 7, 1245 CAS; (f) G. L. Reinhard, S. Jayaraman, J. W. Prybil, J. F. Arambula and K. Arumugam, Dalton Trans., 2022, 51, 1533 CAS.
- (a) K. Škoch, I. Císařová, F. Uhlík and P. Štěpnička, Dalton Trans., 2018, 47, 16082 Search PubMed; (b) K. Škoch, J. Schulz, I. Císařová and P. Štěpnička, Organometallics, 2019, 38, 3060 Search PubMed; (c) K. Škoch, P. Vosáhlo, I. Císařová and P. Štěpnička, Dalton Trans., 2020, 49, 1011 RSC; (d) M. Franc, J. Schulz and P. Štěpnička, Dalton Trans., 2024, 53, 11445 RSC.
- (Ferrocenylcarbonyl)triazoles are also rare and have been studied as redox-active organometalic tags in the preparation of new biologically active compounds and modification of self-assembled surfaces: (a) K. Kowalczyk, A. Błauż, W. M. Ciszewski, A. Wieczorek, B. Rychlik and D. Plażuk, Dalton Trans., 2017, 46, 17041 RSC; (b) K. Chrabąszcz, A. Błauż, M. Gruchała, M. Wachulec, B. Rychlik and D. Plażuk, Chem. – Eur. J., 2021, 27, 6254 CrossRef PubMed; (c) J. P. Collman, N. K. Devaraj and C. E. D. Chidsey, Langmuir, 2004, 20, 1051 CrossRef CAS PubMed.
- (a) P. Vosáhlo, J. Schulz, I. Císařová and P. Štěpnička, Dalton Trans., 2021, 50, 6232 RSC; (b) P. Vosáhlo, I. Císařová and P. Štěpnička, New J. Chem., 2022, 46, 21536 RSC; (c) P. Vosáhlo and P. Štěpnička, New J. Chem., 2023, 47, 4510 RSC.
- Correspondingly, only a handful of the related compounds featuring the more common imidazole-2-ylidene with N-acyl and similar substituents (e.g., ester or carbamoyl) have been reported: (a) F. Bonati, A. Burini, B. R. Pietroni and B. Bovio, J. Organomet. Chem., 1991, 408, 271 CrossRef CAS; (b) B. Bovio, A. Burini and B. R. Pietroni, J. Organomet. Chem., 1993, 452, 287 CrossRef CAS; (c) G. E. Dobereiner, C. A. Chamberlin, N. D. Schley and R. H. Crabtree, Organometallics, 2010, 29, 5728 CrossRef CAS; (d) M. Jonek, A. Makhloufi, P. Rech, W. Frank and C. Ganter, J. Organomet. Chem., 2014, 750, 140 CrossRef CAS.
- Selected examples: (a) H. Seo, H. Park, B. Y. Kim, J. H. Lee, S. U. Son and Y. K. Chang, Organometallics, 2003, 22, 618 CrossRef CAS; (b) S. Gischig and A. Togni, Organometallics, 2004, 23, 2479 CAS; (c) S. Gischig and A. Togni, Organometallics, 2005, 24, 203 CAS; (d) F. Visentin and A. Togni, Organometallics, 2007, 26, 3746 CAS; (e) A. Labande, J.-C. Daran, E. Manoury and R. Poli, Eur. J. Inorg. Chem., 2007, 1205 CAS; (f) J. Shi, P. Yang, Q. Tong and L. Jia, Dalton Trans., 2008, 938 RSC; (g) S. Gülcemal, A. Labande, J.-C. Daran, B. Çetinkaya and R. Poli, Eur. J. Inorg. Chem., 2009, 1806 Search PubMed; (h) N. Debono, A. Labande, E. Manoury, J.-C. Daran and R. Poli, Organometallics, 2010, 29, 1879 CAS; (i) J. Csizmadiová, M. Mečiarová, A. Almássy, B. Horváth and R. Šebesta, J. Organomet. Chem., 2013, 737, 47 Search PubMed; (j) A. Labande, N. Debono, A. Sournia-Saquet, J.-C. Daran and R. Poli, Dalton Trans., 2013, 42, 6531 CAS; (k) P. Loxq, N. Debono, S. Gülcemal, J.-C. Daran, E. Manoury, R. Poli, B. Çetinkaya and A. Labande, New J. Chem., 2014, 38, 338 RSC; (l) P. Loxq, J.-C. Daran, E. Manoury, R. Poli and A. Labande, Eur. J. Inorg. Chem., 2015, 609 CrossRef CAS; (m) Y. D. Lahneche, A. Lachguar, C. Mouton, J.-C. Daran, E. Manoury, R. Poli, M. Benslimane, A. Labande and E. Deydier, Inorg. Chim. Acta, 2019, 492, 91 CrossRef CAS.
- S. Barriga, C. F. Marcos, O. Riant and T. Torroba, Tetrahedron, 2002, 58, 9785 CrossRef CAS.
- (a) D. Font, C. Jimeno and M. A. Pericàs, Org. Lett., 2006, 8, 4653 CrossRef CAS PubMed; (b) D. Giguère, R. Patnam, M.-A. Bellefleur, C. St-Pierre, S. Sato and R. Roy, Chem. Commun., 2006, 2379 RSC.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part A: Theory and Applications in Inorganic Chemistry, Wiley, Hoboken, 6th edn, 2009, ch. 2.6.1, pp. 192–204 Search PubMed.
- K. M. Steed and J. W. Steed, Chem. Rev., 2015, 115, 2895 CrossRef CAS PubMed.
- For examples of reactions of azolium salts with Ag2O leading to carbene complexes, see: (a) H. M. J. Wang and I. J. B. Lin, Organometallics, 1998, 17, 972 CrossRef CAS; (b) R. Heath, H. Müller-Bunz and M. Albrecht, Chem. Commun., 2015, 51, 8699 RSC.
- Selected examples: (a) C. Mejuto, G. Guisado-Barrios, D. Gusev and E. Peris, Chem. Commun., 2015, 51, 13914 RSC; (b) R. Pretorius, M. R. Fructos, H. Müller-Bunz, R. A. Gossage, P. J. Pérez and M. Albrecht, Dalton Trans., 2016, 45, 14591 RSC; (c) J. R. Wright, P. C. Young, N. T. Lucas, A.-L. Lee and J. D. Crowley, Organometallics, 2013, 32, 7065 CrossRef CAS PubMed and ref. 32b and 33b.
- For examples of Ag-to-Cu transmetalation in similar systems, see: (a) H. Iwasaki, Y. Teshima, Y. Yamada, R. Ishikawa, Y. Koga and K. Matsubara, Dalton Trans., 2016, 45, 5713 RSC; (b) S. Hohloch, L. Suntrup and B. Sarkar, Inorg. Chem. Front., 2016, 3, 67 RSC; (c) T. Nakamura, T. Tareshima, K. Ogata and S. Fukuzawa, Org. Lett., 2011, 13, 620 CrossRef CAS PubMed; (d) A. Petronilho, H. Müller-Bunz and M. Albrecht, Chem. Commun., 2012, 48, 6499 RSC.
- B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832 RSC.
- P. Pyykkö, Chem. Rev., 1988, 88, 563 CrossRef.
- (a) S. Hohloch, D. Scheiffele and B. Sarkar, Eur. J. Inorg. Chem., 2013, 3956 CrossRef CAS; (b) L. Hettmanczyk, S. J. P. Spall, S. Klenk, M. van der Meer, S. Hohloch, J. A. Weinstein and B. Sarkar, Eur. J. Inorg. Chem., 2017, 2112 CrossRef CAS; (c) F. Lazreg, M. Vasseur, A. M. Z. Slawin and C. S. J. Cazin, Beilstein J. Org. Chem., 2020, 16, 482 CAS; (d) J. F. Schlagintweit, C. H. G. Jakob, N. L. Wilke, M. Ahrweiler, C. Frias, J. Frias, M. König, E.-M. H. J. Esslinger, F. Marques, J. F. Machado, R. M. Reich, T. S. Morais, J. D. G. Correia, A. Prokop and F. E. Kühn, J. Med. Chem., 2021, 64, 15747 CAS.
- (a) H. Inomata, K. Ogata, S. Fukuzawa and Z. Hou, Org. Lett., 2012, 14, 3986 CAS; (b) L. Hettmanczyk, D. Schulze, L. Suntrup and B. Sarkar, Organometallics, 2016, 35, 3828 CAS.
- (a) T. G. Appleton, H. C. Clark and L. E. Manzer, Coord. Chem. Rev., 1973, 10, 335 CrossRef CAS; (b) F. R. Hartley, Chem. Soc. Rev., 1973, 2, 163 RSC; (c) S. Fuertes, A. J. Chueca and V. Sicilia, Inorg. Chem., 2015, 54, 9885 CrossRef CAS PubMed.
- (a) G. Gritzner and J. Kůta, Pure Appl. Chem., 1984, 56, 461 CrossRef; (b) R. R. Gagné, C. A. Koval and G. C. Lisensky, Inorg. Chem., 1980, 19, 2854 CrossRef.
- P. Zanello, Inorganic Electrochemistry, Theory, Practice and Application, The Royal Society of Chemistry, Cambridge, 2003, ch. 2, pp. 110–115 Search PubMed.
- (a) H. V. Huynh, Y. Han, R. Jothibasu and J. A. Yang, Organometallics, 2009, 28, 5395 CrossRef CAS; (b) Q. Teng and H. V. Huynh, Dalton Trans., 2017, 46, 614 RSC; (c) H. V. Huynh, Chem. Rev., 2018, 118, 9457 CrossRef CAS PubMed.
- H. V. Huynh, Y. Han, J. H. H. Ho and G. K. Tan, Organometallics, 2006, 25, 3267 CrossRef CAS.
- Ferrocene displays an absorption band at 440 nm (in EtOH and gas phase): (a) D. R. Scott and R. S. Becker, J. Chem. Phys., 1961, 35, 516 CrossRef CAS; (b) A. T. Armstrong, F. Smith, E. Elder and S. P. McGlynn, J. Chem. Phys., 1967, 46, 4321 CrossRef CAS.
- (a) D. Yuan and H. V. Huynh, Organometallics, 2012, 31, 405 CrossRef CAS; (b) J. R. Wright, P. C. Young, N. T. Lucas, A.-L. Lee and J. D. Crowley, Organometallics, 2013, 32, 7065 CrossRef CAS PubMed.
- D. A. Khobragade, S. G. Mahamulkar, L. Pospíšil, I. Císařová, L. Rulíšek and U. Jahn, Chem. – Eur. J., 2012, 18, 12267 CrossRef CAS PubMed.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165 CrossRef CAS.
- (a) A. S. K. Hashmi, J. P. Weyrauch, W. Frey and J. W. Bats, Org. Lett., 2004, 6, 4391 CrossRef CAS PubMed; (b) A. S. K. Hashmi, M. Rudolph, S. Schymura, J. Visus and W. Frey, Eur. J. Org. Chem., 2006, 4905 CrossRef CAS; (c) A. S. K. Hashmi, A. M. Schuster and F. Rominger, Angew. Chem., Int. Ed., 2009, 48, 8247 CrossRef CAS PubMed; (d) J. P. Weyrauch, A. S. K. Hashmi, A. Schuster, T. Hengst, S. Schetter, A. Littmann, M. Rudolph, M. Hamzic, J. Visus, F. Rominger, W. Frey and J. W. Bats, Chem. – Eur. J., 2010, 16, 956 CrossRef CAS PubMed; (e) O. Bárta, I. Císařová, J. Schulz and P. Štěpnička, New J. Chem., 2019, 43, 11258 RSC.
- Although insoluble AgCl separated upon mixing 5 with AgNTf2, a possible Au–Ag cooperativity cannot be ruled out entirely. For examples, see: (a) D. Wang, R. Cai, S. Sharma, J. Jirak, S. K. Thummanapelli, N. G. Akhmedov, H. Zhang, X. Liu, J. L. Petersen and X. Shi, J. Am. Chem. Soc., 2012, 134, 9012 CrossRef CAS PubMed; (b) Z. Lu, J. Han, G. B. Hammon and B. Xu, Org. Lett., 2015, 17, 4534 CrossRef CAS PubMed.
- Changing the redox state of the ferrocene unit in [AuCl(L)] complexes with type B ligands (but with a soluble ferrocenium salt as an oxidant) was shown to influence catalytic activity in this particular cyclisation reaction (see ref. 14d).
- (a) Y. G. Gololobov, I. N. Zhmurova and L. F. Kasukhin, Tetrahedron, 1981, 37, 437 CrossRef CAS; (b) Y. G. Gololobov and L. F. Kasukhin, Tetrahedron, 1992, 48, 1353 CrossRef CAS.
- K. Škoch, I. Císařová, J. Schulz, U. Siemeling and P. Štěpnička, Dalton Trans., 2017, 46, 10339 RSC.
- J. M. Brunel, B. Faure and M. Maffei, Coord. Chem. Rev., 1998, 178–180, 665 CrossRef CAS.
- M. Van Overschelde, E. Vervecken, S. G. Modha, S. Cogen, E. Van der Eycken and J. Van der Eycken, Tetrahedron, 2009, 65, 6410 CrossRef CAS.
- (a) T. R. Ward, L. M. Venanzi, A. Albinati, F. Lianza, T. Gerfin, V. Gramlich and G. M. R. Tombo, Helv. Chim. Acta, 1991, 74, 983 CrossRef CAS; (b) T. Imamoto, M. Matsuo, T. Nonomura, K. Kishikawa and M. Yanagawa, Heteroat. Chem., 1993, 4, 475 CrossRef CAS.
- J. Podlaha, P. Štěpnička, J. Ludvík and I. Císařová, Organometallics, 1996, 15, 543 CrossRef CAS.
- H. Brisset, Y. Gourdel, P. Pellon and M. Le Corre, Tetrahedron Lett., 1993, 34, 4523 CrossRef CAS.
- P. Štěpnička and I. Císařová, Dalton Trans., 2013, 42, 3373 RSC.
- G. Gasser, A. J. Fischmann, C. M. Forsyth and L. Spiccia, J. Organomet. Chem., 2007, 692, 3835 CrossRef CAS.
- For related examples, see: (a) G. Ferguson, J. F. Gallagher, C. Glidewell and C. M. Zakaria, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1993, 49, 967 CrossRef; (b) G. Ferguson, J. F. Gallagher, C. Glidewell and C. M. Zakaria, J. Organomet. Chem., 1994, 464, 95 CrossRef CAS; (c) J. F. Gallagher, G. Ferguson, C. Glidewell and C. M. Zakaria, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1994, 50, 18 CrossRef; (d) Y. Li, G. Ferguson, C. Glidewell and C. M. Zakaria, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1994, 50, 857 CrossRef.
- (a) P. Štěpnička and T. Baše, Inorg. Chem. Commun., 2001, 4, 682 CrossRef; (b) P. Štěpnička and I. Císařová, New J. Chem., 2002, 26, 1389 RSC.
- S. I. Kirin, H.-B. Kraatz and N. Metzler-Nolte, Chem. Soc. Rev., 2006, 35, 348 RSC.
- The torsion angle C4–Cg1–Cg2–C9 is given, where Cg1 and Cg2 are the centroids of the cyclopentadienyl rings C(4–8) and C(9–13), respectively (analogously for molecule 2).
- (a) Z. Freixa and P. W. N. M. van Leeuwen, Coord. Chem. Rev., 2008, 252, 1755 CrossRef CAS; (b) C. A. Bessel, P. Aggarwal, A. C. Marschilok and K. J. Takeuchi, Chem. Rev., 2001, 101, 1031 CrossRef CAS PubMed.
- Representative examples: (a) I. R. Butler, M. Kalaji, L. Nehrlich, M. Hursthouse, A. I. Karaulov and K. M. A. Malik, J. Chem. Soc., Chem. Commun., 1995, 459 RSC; (b) K. Tani, M. Yabuta, S. Nakamura and T. Yamagata, J. Chem. Soc., Dalton Trans., 1993, 2781 RSC; (c) J. Kühnert, M. Dušek, J. Demel, H. Lang and P. Štěpnička, Dalton Trans., 2007, 2802 RSC; (d) P. Štěpnička, B. Schneiderová, J. Schulz and I. Císařová, Organometallics, 2013, 32, 5754 CrossRef; (e) J. Tauchman, I. Císařová and P. Štěpnička, Dalton Trans., 2014, 43, 1599 RSC; (f) L. Wang, M. Chen, P. Zhang, W. Li and J. Zhang, J. Am. Chem. Soc., 2018, 140, 3467 CrossRef CAS PubMed.
- T. E. Schmid, D. C. Jones, O. Songis, O. Diebolt, M. R. L. Furst, A. M. Z. Slawin and C. S. J. Cazin, Dalton Trans., 2013, 42, 7345 RSC.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955 RSC.
- A. Dasgupta, V. Ramkumar and S. Sankararaman, Eur. J. Org. Chem., 2016, 4817 CAS . The parameters were retrieved from the Cambridge Structural Database, refcode: IWUFAJ. The major difference between the compounds is the Pd–P bond length measuring 2.356 Å in the reference compound.
- K.-S. Gan and T. S. A. Hor, in Ferrocenes: Homogeneous Catalysis, Organic Synthesis Materials Science, ed. A. Togni and T. Hayashi, Wiley-VCH, Weinheim, Germany, 1995, ch. 1, pp. 3–104 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Complete experimental details, crystallographic data and structure diagrams, additional cyclic voltammograms, and copies of the NMR spectra. CCDC 2427432–2427441. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00545k |
| This journal is © The Royal Society of Chemistry 2025 |