Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembled multidye-sensitized erbium single molecules for boosting energy transfer light upconversion in solution†
Filipe
Alves
a,
Inès
Taarit
a,
Laure
Guénée
b and
Claude
Piguet
 *a
*a
aDepartment of Inorganic and Analytical Chemistry, University of Geneva, 30 quai E. Ansermet, CH-1211 Geneva 4, Switzerland. E-mail: Claude.Piguet@unige.ch
bLaboratory of Crystallography, University of Geneva, 24 quai E. Ansermet, CH-1211 Geneva 4, Switzerland
First published on 29th May 2025
Abstract
Efficient near-infrared (NIR) to visible (VIS) light upconversion should combine large absorption coefficients εNIR with very large quantum yields ϕUC so that the overall brightness BUC = εNIR·ϕUC is maximum. Relying on linear optics, several photons are collected by strongly absorbing dyes, stored on long-lived intermediate excited states and finally piled up using mechanisms of simple or double operator natures. The miniaturization to implement detectable linear light upconversion in a single molecule is challenging because of the existence of the thermal vibrational bath, which increases non-radiative relaxation and limits quantum yields to 10−9 ≤ ϕUC ≤ 10−6. An acceptable brightness thus requires the connection of a maximum of cationic cyanine dyes around trivalent lanthanide luminophores. Taking advantage of the thermodynamic benefit brought by strict self-assembly processes, three cationic IR-780 dyes could be arranged around a single Er(III) cation in the trinuclear [ZnErZn(L5)3]10+ triple-stranded helicate. NIR excitation at 801 nm in acetonitrile at room temperature induces light upconversion via the energy transfer upconversion (ETU) mechanism. The final green Er(2H11/2,4S3/2 → 4I15/2) emission with ϕUC = 3.6 × 10−8 shows a record brightness of BUC = 2.8 × 10−2 M−1 cm−1 (Pexc = 25 W cm−2) for a molecular-based upconversion process.
Introduction
Among the different options to induce anti-Stokes processes,1–11 efficient light upconversion upon reasonable excitation intensities, often referred to as upconversion (UC), mainly relies on Bloembergen's strategy, which exploits linear optics.2,12,13 The existence of long-lived intermediate excited states is thus crucial for implementing the successive excitations required for piling several low-energy photons and finally reaching a high-energy excited state able to radiatively relax to states of lower energies (Fig. S1 and S2†). An obvious choice considers long-lived triplet excited states found in main group aromatic molecules as intermediate excited relays, followed by triplet–triplet annihilation upconversion involving the diffusion and collision of two independent molecules to reach the final emissive singlet excited state.3–5,7,8,10 However, this intermolecular strategy does not fit the criteria for being considered as a discrete molecular process, the subject of the present contribution.14,15 The second choice takes advantage of scales of electronic terms produced by interelectronic interactions in open-shell d-block (dn, n = 1–9) and f-block (fn, n = 1–13) metals, according to which these centers are deprived from vibrational-based non-radiative relaxation processes via their integration into low-phonon ionic solids or nanoparticles, thus providing long-lived intermediate excited relays.2,11,16–19 Again, miniaturization to reach single isolated (supra)molecules based on the latter ionic/polymeric materials is not trivial. Inspired by the excited-state absorption (ESA; Fig. S1a†) and the energy transfer upconversion (ETU; Fig. S1b†) implemented in doped solids, coordination chemists were, however, able to design some rare (supra)molecular complexes exhibiting UC processes in solution under reasonable incident excitation power (Pexc < 30 W cm−2) and using real intermediate excited states.20–29 Additionally, cooperative sensitization upconversion (CSU; Fig. S2a†)30–36 and cooperative luminescence (CL; Fig. S2b†),33,36,37 which consider quasi-virtual pair levels,38 have been recently described. These pioneer efforts aiming at implementing these four upconversion mechanisms for molecules in solution are regularly reviewed.14,15,39–42 Some reports of solid-state UC recorded for mixtures of co-crystallized coordination complexes, coordination polymers or clusters43–57 deserve mention for the sake of comprehensiveness of this introduction despite (i) the assignment of the UC mechanism to the single molecular nature is debatable and (ii) the statistically doped character of the multimetallic samples developed for ETU or CSU processes.Whatever the exact mechanism is, molecular-based near-infrared (NIR) to visible (VIS) UC in solution shows low quantum yields, of the order of 10−9 ≤ ϕUC ≤ 10−6 (normalized at P = 25 W cm−2), due to unavoidable large and penalizing non-radiative relaxation processes. Significant improvements should consider balancing the low UC quantum yield (ϕUC) by large NIR absorption cross-sections (εNIR) to finally provide acceptable UC brightness BUC = εNIR·ϕUCvia the antenna effect,58 a procedure successfully used in solid-state materials upon grafting polymethine dyes59 onto the surface of solid UC nanoparticles.60–64 At the molecular level, combining a cyanine dye, taken as a sensitizer for Ln-based UC via the ETU mechanism, has been first described for [IR-806]+[Er(ttfa)4]− ion pairs, which are assumed to be formed upon simple mixing in chloroform (Fig. 1a).65 As the UC quantum yield ϕUC is proportional to excitation rate constant kexcS,15 which in turn is proportional the absorption coefficient εm→nS (eqn (1)),66–68 the target brightness BUC = εNIR·ϕUC benefits twice from the large εNIR absorption coefficient of the cyanine dye (εNIR > 105 M−1 cm−1):
 | (1) |
 | ||
| Fig. 1 Dye-sensitized ETU implemented in (a) [IR-806]+[Er(ttfa)4]− ion pairs65 and (b and c) single molecular complexes [L2Er(hfac)3]+ and [L3Er(hfac)3]+ in solution (CCDC 2091959 for [L2Er(hfac)2(CF3CO2)]+ (ref. 28) and CCDC 2238348 for [L3Er(hfac)2(CF3CO2)]+ (ref. 29)). Color codes: C = grey, N = dark blue. O = red, F = light blue; S = yellow. The chemical structures of ligands [L2]+ and [L3]+ are shown in Scheme 1. | ||
In eqn (1), λP is the pump wavelength (in cm), P is the incident pump intensity (in W cm−2), σm→nA is the absorption cross section (in cm2) of the sensitizer-centered m → n transition related to the decadic molar absorption coefficient εm→n (in M−1 cm−1) according to σm→n = 3.8 × 10−21εm→n, h is the Planck constant (in J s) and c, the speed of light in vacuum (in cm s−1). The covalent connection of a cationic cyanine sensitizer to an erbium activator to give stable and characterized molecular complexes in solution has been reported for [L2Er(hfac)3]+ (Fig. 1b)28 and [L3Er(hfac)3]+ (Fig. 1c),29 which display record brightnesses for molecular UC in solution. The decrease of the sensitizer–activator distance in going from [L2Er(hfac)3]+ to [L3Er(hfac)3]+ boosts the UC quantum yield and associated brightness by a factor of three.
Further gain can be predicted upon increasing the number of sensitizers per activator in a single SnA (supra)molecular assembly (illustrated for n = 3 in Fig. 2). Beyond the predicted improvement by a factor n2 of brightness BUC (green pathway in Fig. 2), the possibility to accumulate excitations on the sensitizer provides a concomitant and supplementary mechanism, referred to as concerted-ETU (red pathway in Fig. 2),15 which may improve molecular UC when long-lived excited states are available on the sensitizers.26,27
Accordingly, it appears both trivial and appealing to increase the number of cyanine-bearing ligands per erbium activator in a target [Er(L3)n](3+n)+ (n = 2–3) molecular complex inspired by [L3Er(hfac)3]+.29 However, the stepwise increase of the positive charges brought by the connected dye-grafted ligands destabilizes the formation of the target complex in solution. Thus, we report below our effort to decipher the coulombic limits for the formation of multi-dye [Er(L3)n](3+n)+ assemblies in solution, while taking the less charged [Ln(L1)n]3+ analogues as references. Forcing three cationic dyes to approach a trivalent Er3+ activator for designing a stable complex thus appeared only possible with the help of additional favorable contributions arising from multi-component interactions implemented in thermodynamic self-assemblies.69–71 Connecting the cyanine dye to the segmental ligand L4 provided cationic [L5]+ (Scheme 1), which is explored for (i) the quantitative formation of the stable multi-dye triple-stranded helicate [ZnErZn(L5)3]10+ and (ii) the ultimate molecular-based NIR to green light upconverter provided by the latter assembly.
Results and discussion
The thermodynamic limits of successive intermolecular association reactions for [Ln(L3)n](3+n)+ (Ln = Eu, Y; n = 1–3) in solution
Three compact cyanine dye-grafted tridentate 2,6-bis(benzimidazole) cationic ligands [L3]+ (Scheme 1)29 were reacted with trivalent lanthanide Ln3+ according to equilibria (2)–(4):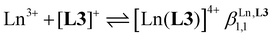 | (2) |
 | (3) |
 | (4) |
Spectrophotometric titrations of the ligand [L3]PF6 (2 × 10−5 M) with Eu(CF3SO3)3 (1.1 × 10−4 M) in dry acetonitrile exhibited stepwise changes in the UV part of the absorption spectra, which reflect the structural trans → cis reorganization of the benzimidazole–pyridine units (bzpy) upon complexation (Fig. S3†).72 An end point was detected for Eu/L3 = 1 ratio together with some inflexion around Eu/L3 = 0.5 (Fig. S4†). Evolving factor analysis73–75 confirmed the existence of only three UV-absorbing species corresponding to equilibria (2)–(3). Non-linear least-square fitting of the spectrophotometric data76–78 provided two rough stability constants  and
and  (Table 1, column 2) together with acceptable reconstructed absorption spectra (Fig. S5b†). Based on the latter thermodynamic association constants, only 80% of the ligand speciation exists in the form [Eu(L3)2]5+ at 1
(Table 1, column 2) together with acceptable reconstructed absorption spectra (Fig. S5b†). Based on the latter thermodynamic association constants, only 80% of the ligand speciation exists in the form [Eu(L3)2]5+ at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratio when the total ligand concentration amounts to 2 × 10−3 M (Fig. S6†). This explains the non-detection of the desired 1
2 stoichiometric ratio when the total ligand concentration amounts to 2 × 10−3 M (Fig. S6†). This explains the non-detection of the desired 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 complex [Eu(L3)3]6+ during the spectrophotometric titration conducted at 2 × 10−5 M. Higher concentrations can be investigated using 1H NMR techniques and titrations of [L3]+ (0.5 mM) upon stepwise additions of Eu(CF3SO3)3 (Appendix 1, Fig. A1-1 and A1-2†) or Y(CF3SO3)3 (Appendix 1, Fig. A1-3 and A1-4†) in CD3CN at 298 K confirmed the formation of [Ln(L3)]4+ and [Ln(L3)2]5+, together with the formation of traces of [Ln(L3)3]6+. Non-linear least-squares fits of the binding isotherms with the help of equilibria (2)–(4) provided the stability constants
3 complex [Eu(L3)3]6+ during the spectrophotometric titration conducted at 2 × 10−5 M. Higher concentrations can be investigated using 1H NMR techniques and titrations of [L3]+ (0.5 mM) upon stepwise additions of Eu(CF3SO3)3 (Appendix 1, Fig. A1-1 and A1-2†) or Y(CF3SO3)3 (Appendix 1, Fig. A1-3 and A1-4†) in CD3CN at 298 K confirmed the formation of [Ln(L3)]4+ and [Ln(L3)2]5+, together with the formation of traces of [Ln(L3)3]6+. Non-linear least-squares fits of the binding isotherms with the help of equilibria (2)–(4) provided the stability constants 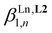 (n = 1–3) gathered in Table 1 (columns 3 and 4; see Appendix 1† for the detailed procedure). In agreement with the operation of the anti-electrostatic trend along the lanthanide series for L1,79,80 ligand [L3]+ also prefers mid-range metals with
(n = 1–3) gathered in Table 1 (columns 3 and 4; see Appendix 1† for the detailed procedure). In agreement with the operation of the anti-electrostatic trend along the lanthanide series for L1,79,80 ligand [L3]+ also prefers mid-range metals with  (Table 1, columns 3 and 4). Comparing the affinities of L1 and [L3]+ for trivalent lanthanides with similar ionic radii reveals a striking decrease in affinity with
(Table 1, columns 3 and 4). Comparing the affinities of L1 and [L3]+ for trivalent lanthanides with similar ionic radii reveals a striking decrease in affinity with  (Table 1, columns 4 and 5), culminating (eight orders of magnitude) for the balance between triple-helical [Er(L1)3]3+ and [Y(L3)3]6+ complexes.
(Table 1, columns 4 and 5), culminating (eight orders of magnitude) for the balance between triple-helical [Er(L1)3]3+ and [Y(L3)3]6+ complexes.
 of the tridentate ligands [L3]+ and L1 with Ln(CF3SO3)3 in CD3CN at 298 K
of the tridentate ligands [L3]+ and L1 with Ln(CF3SO3)3 in CD3CN at 298 K
| Method | Spectrophotometry | NMR | NMR | Spectrophotometry |
|---|---|---|---|---|
a Intermolecular ligand–metal affinity  (see Appendix 1†).
b Interligand interactions (see Appendix 1†).
b Interligand interactions  (see Appendix 1†). (see Appendix 1†).
|
||||
| Ligand | [L3]+ | [L3]+ | [L3]+ | L1 |
| Metal | Eu(CF3SO3)3 | Eu(CF3SO3)3 | Y(CF3SO3)3 | Er(CF3SO3)3 |

|
6.3(7) | 7.9(4) | 7.8(7) | 9.2(1) |

|
10.3(9) | 12.8(6) | 10.8(1.0) | 16.5(3) |

|
— | 14.8(7) | 12.7(1.1) | 20.9(3) |
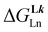 /kJ mol−1 /kJ mol−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
— | −40.8(1.5) | −36.9(3.9) | −49(1) |
 /kJ mol−1 /kJ mol−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
— | 14.9(1.1) | 15.3(3.2) | 11.6(1.5) |
| Ref. | This work | This work | This work | 72 |
A didactic way of comparing the thermodynamic behaviors of L1 and [L3]+ relies on the site binding model (Appendix 1 in the ESI†),80,81 from which a free energy of intermolecular ligand–metal affinity  modulated by an interligand interaction
modulated by an interligand interaction  fully characterizes the successive intermolecular ligand–metal binding association processes (Table 1, entries 6 and 7). The increase from
fully characterizes the successive intermolecular ligand–metal binding association processes (Table 1, entries 6 and 7). The increase from  to
to  quantifies a 25% reduction in affinity of Ln3+ for [L3]+, compared with that for L1, which is repeated each time a ligand is bound to the trivalent metallic center. Moreover, the concomitant 25% larger anti-cooperativity produced by repulsive interligand interactions estimated by
quantifies a 25% reduction in affinity of Ln3+ for [L3]+, compared with that for L1, which is repeated each time a ligand is bound to the trivalent metallic center. Moreover, the concomitant 25% larger anti-cooperativity produced by repulsive interligand interactions estimated by  further penalizes the successive binding of [L3]+ to Ln3+ (Table 1, entry 7). The cationic character of the latter ligand, which is responsible for these drastic destabilizing effects, prevents the formation of the target saturated triple-helical [Ln(L3)3]6+ as a major component in acetonitrile solution at millimolar concentrations and prevents its exploitation as a potential multi-dye molecular-based upconverter.
further penalizes the successive binding of [L3]+ to Ln3+ (Table 1, entry 7). The cationic character of the latter ligand, which is responsible for these drastic destabilizing effects, prevents the formation of the target saturated triple-helical [Ln(L3)3]6+ as a major component in acetonitrile solution at millimolar concentrations and prevents its exploitation as a potential multi-dye molecular-based upconverter.
Combining intra- and intermolecular association in self-assembly reactions to provide stable [ZnLnZn(L5)3]10+ (Ln = Eu, Er, Y) helicates in solution
The target segmental ligand [L5]+ combines the didentate–tridentate–didentate scaffold L4, which is programmed for the self-assembly of dimetallic trinuclear triple-stranded [ZnLnZn(L4)3]7+ helicates,82,83 with a cyanine [IR-780]I bound via a sulfur bridge at the 4-position of the central pyridine ring (Scheme 1). The multistep synthesis adapts previously published protocols23,29,82,84 to give 4, which was reacted with S-methylisothiourea85 and deprotected86 to yield thiol 6. Electrophilic attack with commercially available cyanine [IR-780]I (7) afforded the final ligand [L5]PF6 after metathesis (2% overall reaction yield for 13 steps; Scheme 2a, ESI Appendix 2†). Layering of tert-butyl methyl ether (C5H12O) on a propionitrile (C3H5N) solution of [L5]PF6 produced crystals of [L5]PF6·C3H5N·0.25(C5H12O) suitable for X-ray diffraction (Scheme 2b, Tables S1 and S2 and Fig. S7†). | ||
| Scheme 2 (a) Synthesis of the ligand [L5]PF6 and (b) molecular structure of [L5]+ in the crystal structure of [L5]PF6·C3H5N·0.25(C5H12O). Color codes: C = grey, N = dark blue, S = yellow. | ||
In solution, the 1H NMR spectrum of [L5]+ display 26 signals in agreement with an average C2v symmetry on the NMR time scale and the adoption of a symmetrical and delocalized form by the cyanine dye, a situation often referred to as the “cyanine limit” (Fig. 3, top).87 Notably, a significant signal broadening of the four H19 methyl groups of the cyanine backbone in [L5]+, which points to some hindered rotations around the C–S bonds due to the close distance between the cyanine and the segmental polyaromatic ligands. Among the possible d-block templating cations Mz+ (M = Cr2+, Zn2+, Cr3+, Ga3+) compatible with the quantitative self-assembly of triple-helical [MLnM(L4)]n+ complexes,26,82,83,88 closed-shell diamagnetic Zn2+ appeared to be the best suited for (i) making detailed NMR analysis easier (paramagnetic Cr2+ and, especially, Cr3+ are not compatible with high-resolution spectra),89 (ii) allowing sufficiently fast kinetics for reaching thermodynamic equilibria within hours (Ga3+ requires days)88 and (iii) not affecting energy transfers between the dye and the emissive lanthanide (ETU mechanism). Consequently, the stepwise additions of two equivalents of Zn(CF3SO3)2 and one equivalent of Ln(CF3SO3)3 (Ln = Y, Fig. S8;† Ln = Eu, Fig. S9†) selectively and quantitatively provide the target self-assembled triple-stranded [ZnLnZn(L5)3]10+ helicates within a few hours at 50 °C (Fig. 3, bottom). The 15 signals observed for the 14 groups of protons attached to the ligand strands (numbered 1–14 in Fig. 3) points to a threefold symmetry. The loss of symmetry plane upon helication makes the protons H9 of the methylene bridges diastereotopic (H9 and H9′ in the final complex; Fig. 3 bottom and Fig. S8†) and confirms a global D3 point group for [ZnLnZn(L5)3]10+ as previously detailed for [ZnLnZn(L4)3]7+.83 The systematic doubling of the proton signals of the dye in the final [ZnLnZn(L5)3]10+ helices (for instance two different signals for H18–H18′ and four different signals for the diastereotopic methyl groups H19–H19′) corresponds to a local loss in symmetry, generally referred to as “crossing the cyanine limit”, induced by ion-pairing effects.87,90 The substantial increase of the total charge resulting from the complexation of [L5]+ to Zn2+ and Ln3+ is probably at the origin of the subsequent charge localization induced by ion pairing in the [ZnLnZn(L5)3]10+ helicate (Fig. 3, bottom).
 | ||
| Fig. 3 1H NMR spectra recorded for the self-assembly of [ZnEuZn(L5)3](PF6)3(CF3SO3)7 (CD3CN, 298 K). | ||
In contrast to [Ln(L3)3]6+ (Ln = Eu, Y), which are quantitatively decomplexed at a total ligand concentration of 10−4 M (see previous section and Fig. A1-2 and A1-4†), the 1H NMR spectra of [ZnLnZn(L5)3]10+ recorded at total concentrations of 10−4–10−5 M show no change (Fig. S10 and S11†). This highlights the resistance to decomplexation boosted by the self-assembly process as the central ‘unstable’ [Ln(N∩N∩N)3]6+ unit is balanced by (i) the favorable formation of two stable [ZnN6]2+ scaffolds and (ii) the thermodynamic benefit70,80 of four preorganized intramolecular binding events to give the macrotetracyclic [ZnLnZn(L5)3]10+ helicate, which are lacking in the three successive anti-cooperative intermolecular binding processes leading to [Ln(L3)3]6+ (eqn (2)–(4)). Finally, whatever the order of addition of the metals to the solution of ligand [L5]+ is, the endpoint of the self-assembly remains invariant, which implies that all the possible kinetically accessible intermediates formed during the reaction will eventually fall into the thermodynamic minimum of the final helices (Fig. S12†). Evaporation of the solvent followed by (i) metathesis using an excess of KPF6 and (ii) size exclusion chromatography afforded [ZnYZn(L5)3](PF6)10·1.35H2O (yield 63%) and [ZnEuZn(L5)3](PF6)10·1.65H2O (yield 72%) (see Appendix 2 and Fig. S13†). The ESI-MS spectra display the expected series of multicharged adducts {[ZnLnZn(L5)3](PF6)n}(10−n)+ (n = 3–7; Fig. S14 and S15†), the isotopic distributions of which match the theoretical predictions (high-resolution mass spectroscopy time of flight, HR-MS ToF; Fig. S16 and S17†).
All attempts to obtain crystals suitable for X-ray diffraction studies failed in our hands, in a similar way to what was reported previously for the parent helicates [ZnEuZn(L4)3]X7 (X = ClO4−, CF3SO3−, PF6−).83 A conceivable molecular structure for [ZnEuZn(L5)3]10+ has been therefore built (Fig. 4) by combining the triple-helical platform reported for the DFT-optimized gas-phase structure of [ZnEuZn(L4)3]7+,83 which is isostructural with [CrEuCr(L4)3]9+ found in the X-ray crystal structure of [CrEuCr(L4)3](CF3SO3)7·(C3H5N)30 (CCDC 806425)25 or [GaErGa(L4)3]9+ found in the X-ray crystal structure of [GaErGa(L4)3](CF3SO3)9·(CH3CN)35.5 (CCDC 1003567),27 with the molecular structure of [L3Er(hfac)2(CF3CO2)]+ modelling the attached cyanine dyes (CCDC 2238348 in Fig. 1c).29
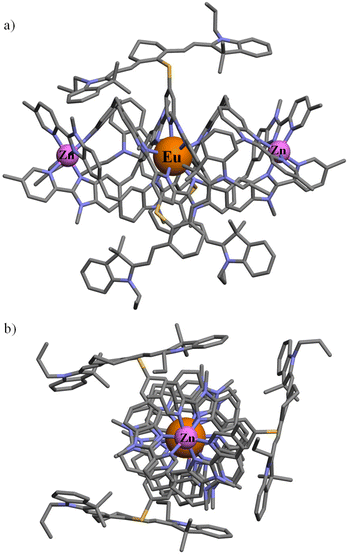 | ||
| Fig. 4 Proposed molecular structure of [ZnEuZn(L5)3]10+ built from the DFT-optimized gas-phase structure of [ZnEuZn(L4)3]7+ (ref. 83) and the molecular structure of [L3Er(hfac)2(CF3CO2)]+ (ref. 29) depicted (a) perpendicular to and (b) along the Zn⋯Eu⋯Zn axis (see text). | ||
Photophysical properties and ETU implemented in [ZnErZn(L5)3]10+ helicates in solution
The preparation of the erbium-containing triple-stranded [ZnErZn(L5)3]10+ helicate follows the same procedure used for its analogues [ZnLnZn(L5)3]10+ (Ln = Eu, Y; see above and Appendix 2†). It provides a diagnostic 1H NMR spectrum (Fig. S18†), in which the large paramagnetic moment and the slower electron relaxation rate associated with Er3+ ([Ar]4f11) result in broadened signals (Fig. S18†)89 and characteristic ESI-MS spectra (Fig. S19 and S20†). The absorption spectrum of the ligand [L5]+ is reminiscent of that of [L3]+, which combines the main NIR charge-transfer band of the cyanine dye around 780 nm (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 cm−1, Dye-π(S1 ← S0)) with several allowed intraligand π(Sn ← S0) transitions located on the polyaromatic backbone, and appearing in the UV part (300–250 nm; Fig. 5a).29 Upon complexation to the metallic cations to give [ZnLnZn(L5)3]10+ (Ln = Y, Er), the systematic transoid-to-cisoid rearrangements of the 2-benzimidazole-pyridine (bzpy) moieties are responsible for the reorganization of the polyaromatic scaffold, which results in a global splitting of the intraligand π(S1 ← S0) into two π(S1a,b ← S0) bands in the UV-Vis domain (400–330 nm), while the dye-based NIR absorption is slightly broadened (Fig. 5a).91 Interestingly, the splitting of the π(S1a,b ← S0) band can be exploited as a marker for the dissociation of the helical complexes in solution occurring at low concentrations. Its release indicates a stability limit in solution, which can be safely estimated at concentrations as low as 10−5 M in acetonitrile at 293 K (Fig. S21†). Moreover, the concomitant increase of the intensity of the Dye-π(S1 ← S0) transition upon dissociation of the complex (Fig. S21†) confirms the transformation of the localized polyene structure of the dye found in the highly charged [ZnLnZn(L5)3]10+ helicates (Fig. 3, bottom) into the delocalized cyanine limit in [L5]+ upon losing ion-pairing interactions (Fig. 3, top).87,90 The metal-centered Er(2S+1LJ ← 4I15/2) transitions are masked by intense ligand-based absorption covering the UV to NIR domain, and only the magnetic-allowed Er(4I13/2 ← 4I15/2) transition can be detected in the ‘free’ IR domain for [ZnErZn(L5)3]10+ (1510 nm; inset in Fig. 5a).
820 cm−1, Dye-π(S1 ← S0)) with several allowed intraligand π(Sn ← S0) transitions located on the polyaromatic backbone, and appearing in the UV part (300–250 nm; Fig. 5a).29 Upon complexation to the metallic cations to give [ZnLnZn(L5)3]10+ (Ln = Y, Er), the systematic transoid-to-cisoid rearrangements of the 2-benzimidazole-pyridine (bzpy) moieties are responsible for the reorganization of the polyaromatic scaffold, which results in a global splitting of the intraligand π(S1 ← S0) into two π(S1a,b ← S0) bands in the UV-Vis domain (400–330 nm), while the dye-based NIR absorption is slightly broadened (Fig. 5a).91 Interestingly, the splitting of the π(S1a,b ← S0) band can be exploited as a marker for the dissociation of the helical complexes in solution occurring at low concentrations. Its release indicates a stability limit in solution, which can be safely estimated at concentrations as low as 10−5 M in acetonitrile at 293 K (Fig. S21†). Moreover, the concomitant increase of the intensity of the Dye-π(S1 ← S0) transition upon dissociation of the complex (Fig. S21†) confirms the transformation of the localized polyene structure of the dye found in the highly charged [ZnLnZn(L5)3]10+ helicates (Fig. 3, bottom) into the delocalized cyanine limit in [L5]+ upon losing ion-pairing interactions (Fig. 3, top).87,90 The metal-centered Er(2S+1LJ ← 4I15/2) transitions are masked by intense ligand-based absorption covering the UV to NIR domain, and only the magnetic-allowed Er(4I13/2 ← 4I15/2) transition can be detected in the ‘free’ IR domain for [ZnErZn(L5)3]10+ (1510 nm; inset in Fig. 5a).
The emission spectra recorded upon ligand-centered excitation within the 280–320 nm range for both ligand [L5]+ and its complexes [ZnLnZn(L5)3]10+ (Ln = Y, Er) are dominated by the Stokes shifted NIR emission of the cyanine dye at 825–830 nm (Dye-π(S1 → S0)), together with some residual broad visible emission (400–450 nm) arising from the bound polyaromatic ligand strands π(S1 → S0) (Fig. S22, S24 and S26†). The associated excitation spectra (λem = 825 nm; Fig. S23, S25 and S27†) confirm the efficient communication between the appended cyanine dye and the polyaromatic scaffold, while the erbium-based IR Er(4I13/2 → 4I15/2) emissions at 1520 nm, induced upon either UV excitation (λexc = 325 nm; Fig. S28a†) or cyanine-based excitation (λexc = 805 nm; Fig. 5b and Fig. S28b†), provide the proof for the ultimate energy funneling toward the erbium emissive center and the operation of a linear one-photon downshifted emission mechanism (Fig. 5c, slope = 0.94).67,92
The associated Jablonski diagram established for [ZnErZn(L5)3]10+ (Fig. 6) appears to be similar to that previously found for [Er(L3)(hfac)3]+.29 Because of the thermodynamic requirement of using ≥10−5 M solution of complexes for avoiding any detectable dissociation, the emission spectra upon NIR dye-centered excitation of [ZnErZn(L5)3]10+ at 801 nm (ε = 778![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1, Fig. 5a) are recorded using the front face technique with a 1 mm cuvette to prevent primary and secondary inner filter effects due to the considerable absorptivity of the complexes.93
900 M−1 cm−1, Fig. 5a) are recorded using the front face technique with a 1 mm cuvette to prevent primary and secondary inner filter effects due to the considerable absorptivity of the complexes.93
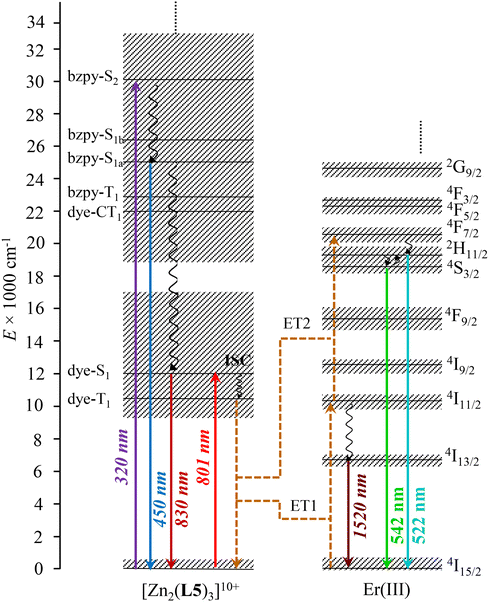 | ||
| Fig. 6 Jablonski diagram established for [ZnErZn(L5)3]10+, illustrating the mechanisms for inducing light downshifting and light UC through ligand-sensitized ETU. ET = intramolecular S → A energy transfer; ISC = intersystem crossing. The dye-centered triplet states are located according to ref. 62. | ||
NIR time-gated phosphorescence upon ligand-centered excitation of [ZnLnZn(L5)3]10+ (Ln = Y, Er) within the 280–320 nm range was attempted at low temperature (77 K) but no emitted signal could be detected. This demonstrates that no excited triplet state from either the polyaromatic scaffold or the cyanine dye in these complexes induces phosphorescence. They probably relax non-radiatively (via vibrational quenching and/or energy transfer) as demonstrated by Garfield et al. for related cyanine dyes.62 Focusing on the dye, it is worth recalling here that ultrafast lifetimes recorded previously for the residual singlet Dye-π(S1 → S0) emission detected at 825–830 nm for [L3]+ and [L3Ln(hfac)3]+ (Ln = Er, Y) in solution established unambiguously that the Dye-π(T1) state indeed plays a pivotal role for feeding the Er(4I11/2) in [L3Er(hfac)3]+via intramolecular energy transfer (ET1 in Fig. 5).38
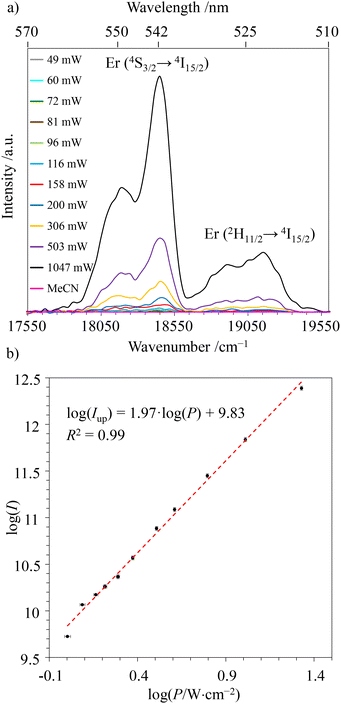
Switching now to NIR laser excitation at λexc = 801 nm of [ZnErZn(L5)3]10+ (10−5 M concentration), this produces a negligible metal-centered Er(4I9/2 ← 4I15/2) absorbance A = log(I0/I) = 2 × 10−7 (εEr ≈ 0.2 M−1 cm−1, 1 mm cell),15 which prevents the operation of detectable competitive ESA mechanism in these conditions. On the contrary, the latter NIR laser excitation beam at λexc = 801 nm is strongly absorbed by the dyes of [ZnErZn(L5)3]10+ at 10−5 M in acetonitrile at room temperature (A = log(I0/I) = 0.78 using ε = 78 × 104 M−1 cm−1 for the Dye-π(S1 ← S0) transition, 1 mm cell). This results in the detection of not only the standard downshifted Er(4I13/2 → 4I15/2) transition at 1520 nm (Fig. 5b) but also two well-resolved green upconverted Er(4S3/2 → 4I15/2) (542 nm) and Er(2H11/2 → 4I15/2) (525 nm) emission bands (Fig. 7a and S29†) following the ETU mechanism (ET1 and ET2 in Fig. 6). The log–log treatment of the UC signal gives a slope of 1.97 which confirms the piling up of two successive photons (Fig. 7b). The UC quantum yield (ϕUC) of [ZnErZn(L5)3]10+ in acetonitrile was determined using the relative method with parent [L3Er(hfac)3]+ as reference (Table 2; see Appendix 2† for details).29
Compared with those for the mono-dye model complex [L3Er(hfac)3]+, ϕUC increases by a factor of 3.3(7) and BUC by a factor of 14(2) for [ZnErZn(L5)3]10+. These boosting components align well with the predictions of a threefold increase in the quantum yield and a ninefold increase in brightness according to the ETU mechanism highlighted by a green pathway in Fig. 2. Any additional contribution originating from the concerted-ETU mechanism (red pathway in Fig. 2) appears to be small, if not negligible, as previously established for the [CrErCr(L4)3]9+ analogue.23 Theoretical modelling of the concerted-ETU mechanism26 applied to [SnA] chromophores highlighted the delicate balance between a favorable long excited-state lifetime located on the sensitizer (S) for accumulating the incident photons and fast sensitizer-to-activator (S → A) energy transfers, which contribute to reduce the sensitizer-based excited-state lifetime, for ultimately optimizing quantum yields. In the absence of accessible, reliable, and detailed kinetic analysis of the UC mechanism in [ZnErZn(L5)3]10+, no definitive rationalization is at hand, but the short lifetime of the feeding level of [L3]+, previously measured and reported for [L3Er(hfac)3]+,29 strongly suggests that a similar scenario operates for [L5]+ dye triplet state in [ZnErZn(L5)3]10+. This prevents sufficient accumulation of photons on the sensitizers prior to successive intramolecular energy transfers onto the activator.
Conclusion
The connection of a cationic cyanine dye to a tridentate 2,6-bis(benzimidazol-2-yl)pyridine ligand in [L3]+ limits the intermolecular affinity for binding Ln3+ to such an extent that the target triple-helical [Ln(L3)3]6+ complex is not accessible, and a maximum of two guests can be connected to give 80% of [Ln(L3)2]5+ at millimolar concentrations in acetonitrile. Taking advantage of self-assembly processes overcomes this thermodynamic restriction, and three cationic dyes can be successfully attached to a central trivalent lanthanide in triple-stranded [ZnErZn(L5)3]10+ helicate at 10−5 M in acetonitrile solution at room temperature. NIR laser excitation (λexc = 801 nm) of the Dye-π(S1 ← S0) transition in [ZnErZn(L5)3]10+ results in the detection of both downshifted Er(4I13/2 → 4I15/2) emission at 1520 nm (one-photon process) and green upconverted Er(4S3/2 → 4I15/2) (542 nm) and Er(2H11/2 → 4I15/2) (525 nm) emission bands (two-photon processes). As expected by ETU modeling (green pathway in Fig. 2), the UC quantum yield is boosted by a factor of ≈3 going from [L3Er(hfac)3]+ (one sensitizer)29 to [ZnErZn(L5)3]10+ (three sensitizers). The brightness of [ZnErZn(L5)3]10+, BUC = 2.8(3) × 10−2 M−1 cm−1, benefits a second time from a boosting factor of ≈three due to the presence of three dye sensitizers attached to the erbium emitter, which makes it finally one order of magnitude larger than the previous record held by [L3Er(hfac)3]+ (BUC = 2.0(1) × 10−3 M−1 cm−1, Table 2).Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is supported from the Swiss National Science Foundation (grant 200020_207313).References
- P. N. Prasad and D. J. Williams, Introduction to Nonlinear Optical Effects in Molecules & Polymers, John Wiley & Sons, Inc., New York – Chichester – Brisbane – Toronto – Singapore, 1991 Search PubMed.
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS PubMed.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- A. Monguzzi, R. Tubino, S. Hoseinkhani, M. Campioneand and F. Meinardi, Phys. Chem. Chem. Phys., 2012, 14, 4322–4332 RSC.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- X. Zhu, Q. Su, W. Feng and F. Li, Chem. Soc. Rev., 2017, 46, 1025–1039 RSC.
- V. Gray, K. Moth-Poulsen, B. Albinsson and M. Abrahamsson, Coord. Chem. Rev., 2018, 362, 54–71 CrossRef CAS.
- P. Bharmoria, H. Bildirir and K. Moth-Poulsen, Chem. Soc. Rev., 2020, 49, 6529–6554 RSC.
- B. S. Richards, D. Hudry, D. Busko, A. Turshatov and I. A. Howard, Chem. Rev., 2021, 121, 9165–9195 Search PubMed.
- C. Healy, L. Hermanspahn and P. E. Kruger, Coord. Chem. Rev., 2021, 432, 213756 CrossRef CAS.
- X. W. Cheng, J. Zhou, J. Y. Yue, Y. Wei, C. Gao, X. J. Xie and L. Huang, Chem. Rev., 2022, 122, 15998–16050 CrossRef CAS PubMed.
- N. Bloembergen, Phys. Rev. Lett., 1959, 2, 84–85 CrossRef CAS.
- F. Auzel, J. Lumin., 2020, 223, 116900 Search PubMed.
- L. J. Charbonnière, A. M. Nonat, R. C. Knighton and L. Godec, Chem. Sci., 2024, 15, 3048–3059 Search PubMed.
- H. Bolvin, A. Furstenberg, B. Golesorkhi, H. Nozary, I. Taarit and C. Piguet, Acc. Chem. Res., 2022, 55, 442–456 Search PubMed.
- B. M. van der Ende, L. Aarts and A. Meijerink, Phys. Chem. Chem. Phys., 2009, 11, 11081–11095 RSC.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 Search PubMed.
- J. Zhou, J. L. Leano Jr., Z. Liu, K.-L. Wong, R.-S. Liu and J.-C. G. Bünzli, Small, 2018, 1801882 CrossRef PubMed.
- H. Dong, L. D. Sun and C. H. Yan, J. Am. Chem. Soc., 2021, 143, 20546–20561 CrossRef CAS.
- A. Nonat, C. F. Chan, T. Liu, C. Platas-Iglesias, Z. Y. Liu, W. T. Wong, W. K. Wong, K. L. Wong and L. J. Charbonniere, Nat. Commun., 2016, 7, 11978 CrossRef CAS.
- B. Golesorkhi, H. Nozary, L. Guénée, A. Fürstenberg and C. Piguet, Angew. Chem., Int. Ed., 2018, 57, 15172–15176 CrossRef CAS.
- B. Golesorkhi, A. Fürstenberg, H. Nozary and C. Piguet, Chem. Sci., 2019, 10, 6876–6885 RSC.
- B. Golesorkhi, I. Taarit, H. Bolvin, H. Nozary, J. R. Jimenez, C. Besnard, L. Guénée, A. Fürstenberg and C. Piguet, Dalton Trans., 2021, 50, 7955–7968 RSC.
- S. Naseri, I. Taarit, H. Bolvin, J. C. Bünzli, A. Fürstenberg, L. Guénée, G. Le-Hoang, M. Mirzakhani, H. Nozary, A. Rosspeintner and C. Piguet, Angew. Chem., Int. Ed., 2023, 62, e202314503 CrossRef CAS PubMed.
- L. Aboshyan-Sorgho, C. Besnard, P. Pattison, K. R. Kittilstved, A. Aebischer, J.-C. G. Bünzli, A. Hauser and C. Piguet, Angew. Chem., Int. Ed., 2011, 50, 4108–4112 CrossRef CAS.
- Y. Suffren, D. Zare, S. V. Eliseeva, L. Guénée, H. Nozary, T. Lathion, L. Aboshyan-Sorgho, S. Petoud, A. Hauser and C. Piguet, J. Phys. Chem. C, 2013, 117, 26957–26963 CrossRef CAS.
- D. Zare, Y. Suffren, L. Guénée, S. V. Eliseeva, H. Nozary, L. Aboshyan-Sorgho, S. Petoud, A. Hauser and C. Piguet, Dalton Trans., 2015, 44, 2529–2540 RSC.
- B. Golesorkhi, S. Naseri, L. Guénée, I. Taarit, F. Alves, H. Nozary and C. Piguet, J. Am. Chem. Soc., 2021, 143, 15326–15334 CrossRef CAS PubMed.
- I. Taarit, F. Alves, A. Benchohra, L. Guénée, B. Golesorkhi, A. Rosspeintner, A. Fürstenberg and C. Piguet, J. Am. Chem. Soc., 2023, 145, 8621–8633 CrossRef CAS PubMed.
- N. Souri, P. Tian, C. Platas-Iglesias, K.-L. Wong, A. Nonat and L. J. Charbonnière, J. Am. Chem. Soc., 2017, 139, 1456–1459 CrossRef CAS PubMed.
- A. Nonat, S. Bahamyirou, A. Lecointre, F. Przybilla, Y. Mély, C. Platas-Iglesias, F. Camerel, O. Jeannin and L. J. Charbonnière, J. Am. Chem. Soc., 2019, 141, 1568–1576 CrossRef CAS PubMed.
- R. C. Knighton, L. K. Soro, A. Lecointre, G. Pilet, A. Fateeva, L. Pontille, L. Frances-Soriano, N. Hildebrandt and L. J. Charbonniere, Chem. Commun., 2021, 57, 53–56 RSC.
- R. C. Knighton, L. K. Soro, L. Frances-Soriano, A. Rodriguez-Rodriguez, G. Pilet, M. Lenertz, C. Platas-Iglesias, N. Hildebrandt and L. J. Charbonniere, Angew. Chem., Int. Ed., 2022, 61, e202113114 CrossRef CAS.
- R. C. Knighton, L. K. Soro, W. Thor, J. M. Strub, S. Cianferani, Y. Mely, M. Lenertz, K. L. Wong, C. Platas-Iglesias, F. Przybilla and L. J. Charbonniere, J. Am. Chem. Soc., 2022, 144, 13356–13365 CrossRef CAS.
- F. Pini, R. C. Knighton, L. K. Soro, L. J. Charbonnière, M. M. Natile and N. Hildebrandt, Adv. Opt. Mater., 2024, 2400423 CrossRef CAS.
- S. P. K. Panguluri, E. Jourdain, P. Chakraborty, S. Klyatskaya, M. M. Kappes, A. M. Nonat, L. J. Charbonnière and M. Ruben, J. Am. Chem. Soc., 2024, 146, 13083–13092 CrossRef CAS PubMed.
- L. K. Soro, R. C. Knighton, F. Avecilla, W. Thor, F. Przybilla, O. Jeannin, D. Esteban-Gomez, C. Platas-Iglesias and L. J. Charbonniere, Adv. Opt. Mater., 2023, 11, 2202307 CrossRef CAS.
- M. Stavola and D. L. Dexter, Phys. Rev. B: Condens. Matter Mater. Phys., 1979, 20, 1867–1885 CrossRef CAS.
- Y. Suffren, B. Golesorkhi, D. Zare, L. Guénée, H. Nozary, S. V. Eliseeva, S. Petoud, A. Hauser and C. Piguet, Inorg. Chem., 2016, 55, 9964–9972 CrossRef CAS PubMed.
- B. Golesorkhi, H. Nozary, A. Fürstenberg and C. Piguet, Mater. Horiz., 2020, 7, 1279–1296 RSC.
- A. M. Nonat and L. J. Charbonnière, Coord. Chem. Rev., 2020, 409, 213192 CrossRef CAS.
- L. J. Charbonnière, A. M. Nonat, R. C. Knighton and L. Godec, Chem. Sci., 2024, 15, 3048–3059 RSC.
- T. V. Balashova, A. P. Pushkarev, A. N. Yablonskiy, B. A. Andreev, I. D. Grishin, R. V. Rumyantcev, G. K. Fukin and M. N. Bochkarev, J. Lumin., 2017, 192, 208–210 CrossRef CAS.
- M. Li, S. Gui, D. Tian, E. Zhou, Y. Wang, Y.-F. Han, L. Yin and L. Huang, Dalton Trans., 2018, 47, 12868–12872 RSC.
- S. Dasari, S. Singh, P. Kumar, S. Sivakumar and A. K. Patra, Eur. J. Med. Chem., 2019, 163, 546–559 CrossRef CAS PubMed.
- J. Kalmbach, C. Wang, Y. You, C. Forster, H. Schubert, K. Heinze, U. Resch-Genger and M. Seitz, Angew. Chem., Int. Ed., 2020, 59, 18804–18808 Search PubMed.
- J. T. Mo, Z. Wang, P. Y. Fu, L. Y. Zhang, Y. N. Fan, M. Pan and C. Y. Su, CCS Chem., 2021, 3, 729–738 CrossRef CAS.
- J. Monteiro, E. A. Hiti, E. E. Hardy, G. R. Wilkinson, J. D. Gorden, A. E. V. Gorden and A. de Bettencourt-Dias, Chem. Commun., 2021, 57, 2551–2554 RSC.
- J. Wang, Y. Jiang, J. Y. Liu, H. B. Xu, Y. X. Zhang, X. Peng, M. Kurmoo, S. W. Ng and M. Zeng, Angew. Chem., Int. Ed., 2021, 60, 22368–22375 CrossRef CAS PubMed.
- D. A. Gálico, J. S. Ovens, F. A. Sigoli and M. Murugesu, ACS Nano, 2021, 15, 5580–5585 CrossRef PubMed.
- D. A. Galico, C. M. S. Calado and M. Murugesu, Chem. Sci., 2023, 14, 5827–5841 RSC.
- G. T. Sun, Y. Ren, Y. P. Song, Y. Xie, H. J. Zhang and L. N. Sun, J. Phys. Chem. Lett., 2022, 8509–8515 CrossRef CAS PubMed.
- Y. Gil, R. C. de Santana, A. S. S. de Camargo, L. G. Merizio, P. F. Carreno, P. Fuentealba, J. Manzur and E. Spodine, Dalton Trans., 2023, 52, 3158–3168 RSC.
- G. T. Sun, Y. Xie, Y. X. Wang, G. A. Mandl, S. L. Maurizio, H. J. Zhang, X. Ottenwaelder, J. A. Capobianco and L. N. Sun, Angew. Chem., Int. Ed., 2023, 62, e202304591 CrossRef CAS PubMed.
- G. T. Sun, Y. Xie, Y. X. Wang, H. J. Zhang and L. N. Sun, Angew. Chem., Int. Ed., 2023, 62, e202312308 CrossRef CAS PubMed.
- X. F. Duan, L. P. Zhou, H. R. Li, S. J. Hu, W. Zheng, X. Xu, R. L. Zhang, X. Y. Chen, X. Q. Guo and Q. F. Sun, J. Am. Chem. Soc., 2023, 145, 23121–23130 CrossRef CAS PubMed.
- Y. X. Wang, G. T. Sun, Q. C. Su, Y. Xie, F. F. Xing, H. J. Zhang and L. N. Sun, Chem. – Eur. J., 2024, 30, e202400911 CrossRef CAS PubMed.
- K. L. Wong, J. C. G. Bunzli and P. A. Tanner, J. Lumin., 2020, 224, 117256 CrossRef CAS.
- G. Qian and Z. Y. Wang, Chem. – Asian J., 2010, 5, 1006–1029 CrossRef CAS PubMed.
- W. Q. Zou, C. Visser, J. A. Maduro, M. S. Pshenichnikov and J. C. Hummelen, Nat. Photonics, 2012, 6, 560–564 Search PubMed.
- X.-Y. Wang, R. R. Valiev, T. Y. Ohulchanskyy, H. Agren, C. Yang and G. Chen, Chem. Soc. Rev., 2017, 46, 4150–4167 Search PubMed.
- D. J. Garfield, N. J. Borys, S. M. Hamed, N. A. Torquato, C. A. Tajon, B. Tian, B. Shevitski, E. S. Barnard, Y. D. Suh, S. Aloni, J. B. Neaton, E. M. Chan, B. E. Cohen and P. J. Schuck, Nat. Photonics, 2018, 12, 402–407 CrossRef CAS.
- G. C. Bao, S. H. Wen, G. G. Lin, J. L. Yuan, J. Lin, K. L. Wong, J. C. G. Bünzli and D. Y. Jin, Coord. Chem. Rev., 2021, 429, 213642 Search PubMed.
- P. Zhang, J. X. Ke, D. T. Tu, J. Y. Li, Y. F. Pei, L. Wang, X. Y. Shang, T. Y. Guan, S. Lu, Z. Chen and X. Y. Chen, Angew. Chem., Int. Ed., 2021, 61, e202112125 Search PubMed.
- I. Hyppänen, S. Lahtinen, T. Ääritalo, J. Mäkelä, J. Kankare and T. Soukka, ACS Photonics, 2014, 1, 394–397 CrossRef.
- R. C. Hilborn, Am. J. Phys., 1982, 50, 982–986 CrossRef CAS.
- M. Pollnau, D. R. Gamelin, S. R. Lüthi and H. U. Güdel, Phys. Rev., 2000, B61, 3337–3346 Search PubMed.
- T. W. Schmidt and F. N. Castellano, J. Phys. Chem. Lett., 2014, 5, 4062–4072 CrossRef CAS PubMed.
- J. D. Badjic, A. Nelson, S. J. Cantrill, W. B. Turnbull and J. F. Stoddart, Acc. Chem. Res., 2005, 38, 723–732 CrossRef CAS PubMed.
- C. Piguet, Chem. Commun., 2010, 46, 6209–6231 RSC.
- C.-A. Palma, M. Cecchini and P. Samori, Chem. Soc. Rev., 2012, 41, 3713–3730 RSC.
- B. Golesorkhi, L. Guénée, H. Nozary, A. Fürstenberg, Y. Suffren, S. V. Eliseeva, S. Petoud, A. Hauser and C. Piguet, Chem. – Eur. J., 2018, 24, 13158–13169 CrossRef CAS PubMed.
- E. R. Malinowski and D. G. Howery, Factor Analysis in Chemistry, Wiley, New York, Chichester, 1980 Search PubMed.
- H. Gampp, M. Maeder, C. J. Meyer and A. Zuberbühler, Talanta, 1986, 33, 943–951 CrossRef CAS PubMed.
- B. R. Hall, L. E. Manck, I. S. Tidmarsh, A. Stephenson, B. F. Taylor, E. J. Blaikie, D. A. Vander Griend and M. D. Ward, Dalton Trans., 2011, 40, 12132–12145 RSC.
- H. Gampp, M. Maeder, C. J. Meyer and A. Zuberbühler, Talanta, 1985, 32, 1133–1139 Search PubMed.
- M. Maeder and P. King, Analysis of Chemical Processes, Determination of the Reaction Mechanism and Fitting of Equilibrium and Rate Constants, in Chemometrics in Practical Applications, ed. K. Varmuza, 2012, InTech, DOI:10.5772/31896, ISBN: 978-953-51-0438-4.
- Specfit/32 from ReactLab Equilibria Pro: https://jplusconsulting.com/products/reactlab-equilibria/.
- S. Petoud, J.-C. G. Bünzli, F. Renaud, C. Piguet, K. J. Schenk and G. Hopfgartner, Inorg. Chem., 1997, 36, 5750–5760 CrossRef CAS PubMed.
- C. Piguet and J.-C. G. Bünzli, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschneidner Jr, J.-C. G. Bünzli and V. K. Pecharsky, Elsevier Science, Amsterdam, 2010, vol. 40, pp. 301–553 Search PubMed.
- G. Koper and M. Borkovec, J. Phys. Chem. B, 2001, 105, 6666–6674 CrossRef CAS.
- M. Cantuel, F. Gumy, J.-C. G. Bünzli and C. Piguet, Dalton Trans., 2006, 2647–2660 Search PubMed.
- L. Aboshyan-Sorgho, H. Nozary, A. Aebischer, J.-C. G. Bünzli, P.-Y. Morgantini, K. R. Kittilstved, A. Hauser, S. V. Eliseeva, S. Petoud and C. Piguet, J. Am. Chem. Soc., 2012, 134, 12675–12684 CrossRef CAS PubMed.
- C. Piguet, B. Bocquet and G. Hopfgartner, Helv. Chim. Acta, 1994, 77, 931–942 Search PubMed.
- C. Y. Zhang, Y. Zhou, J. T. Huang, C. H. Tu, X. A. Zhou and G. D. Yin, Org. Biomol. Chem., 2018, 16, 6316–6321 RSC.
- A. Pinchart, C. Dallaire, A. Van Bierbeek and M. Gingras, Tetrahedron Lett., 1999, 40, 5479–5482 Search PubMed.
- P. A. Bouit, C. Aronica, L. Toupet, B. Le Guennic, C. Andraud and O. Maury, J. Am. Chem. Soc., 2010, 132, 4328–4335 CrossRef CAS PubMed.
- D. Zare, Y. Suffren, H. Nozary, A. Hauser and C. Piguet, Angew. Chem., Int. Ed., 2017, 56, 14612–14617 Search PubMed.
- C. Piguet and C. F. G. C. Geraldes, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschneidner Jr, J.-C. G. Bünzli and V. K. Pecharsky, Elsevier Science, Amsterdam, 2003, vol. 33, pp. 353–463 Search PubMed.
- S. Pascal, S. H. Chi, J. W. Perry, C. Andraud and O. Maury, ChemPhysChem, 2020, 21, 2536–2542 CrossRef CAS PubMed.
- C. Piguet, J.-C. G. Bünzli, G. Bernardinelli, C. G. Bochet and P. Froidevaux, J. Chem. Soc., Dalton Trans., 1995, 83–97 RSC.
- P. S. May, A. Baride, M. Y. Hossan and M. Berry, Nanoscale, 2018, 10, 17212–17226 Search PubMed.
- A. V. Fonin, A. I. Sulatskaya, I. M. Kuznetsova and K. K. Turoverov, PLoS One, 2014, 9, e103878 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterizations of the new compounds. CCDC 2376478. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00438a |
| This journal is © The Royal Society of Chemistry 2025 |