Defective Ce–Mn solid solution loaded with Pd single atoms for an enhanced methane combustion reaction†
Haoyang
Liu
ab,
Huilin
Wang
*ab,
Xiang
Chu
ab,
Zijian
Wang
ab,
Yuou
Li
ab,
Shibo
Zhang
ab,
Xiao
Wang
*ab,
Zhenan
Qiao
 c,
Shuyan
Song
c,
Shuyan
Song
 *ab and
Hongjie
Zhang
*ab and
Hongjie
Zhang
 abd
abd
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China. E-mail: wanghl@ciac.ac.cn; wangxiao@ciac.ac.cn; songsy@ciac.ac.cn
bUniversity of Science and Technology of China, Hefei 230026, China
cState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, China
dDepartment of Chemistry, Tsinghua University, Beijing 100084, China
First published on 2nd May 2025
Abstract
Methane combustion reactions hold significant promise for addressing environmental issues, particularly the greenhouse effect attributed to anthropogenic emissions. However, current catalysts face challenges in simultaneously achieving low-temperature activity and high-temperature stability. In this work, a CeMn solid solution decorated with palladium single atoms (Pd/CeMn-AR) was synthesized via a one-pot auto-redox method. Experimental findings indicate that the Pd/CeMn-AR catalyst demonstrates favorable activity and stability in the methane oxidation reaction. Specifically, the T90 of Pd/CeMn-AR reaches 543 °C, significantly better than its Pd/CeO2 (627 °C), Pd/MnOx (634 °C) and Pd/CeMn-CP (557 °C) counterparts. Furthermore, after 10 hours of continuous reaction at elevated temperatures of 550 °C and 650 °C, the Pd/CeMn-AR catalyst retains more than 95% of its initial activity. Mechanistic investigations reveal that the autoxidative synthesis strategy enhances the exposure of oxygen vacancies, promotes the adsorption of substrates, and accelerates the oxidative conversion of intermediates, ultimately improving the catalytic efficiency.
Introduction
Mitigating climate change and ecological disruptions stemming from the greenhouse effect poses a formidable global challenge.1 Methane (CH4) is the second most prominent greenhouse gas after CO2, with a global warming potential of up to 25.2–4 Hence, reducing the emission of CH4 into the atmosphere is of great importance for sustainable human development.5–7 The methane combustion reaction (CH4 + O2 → CO2 + H2O) provides a simple but efficient way to handle this issue. Compared to traditional methane combustion utilizing an open flame, the catalytic combustion process facilitates lower operational temperatures and generates fewer toxic byproducts, thereby emerging as a research focus in recent years.8–12 Low-temperature catalytic activity and high-temperature thermal stability are the two most important performance indicators for catalytic methane combustion.3,13–17 Benefiting from abundant d-band electron states, noble metal-based catalysts are favourable for activating methane molecules through σ-d interactions, showing superior low-temperature activity.18 However, the harsh reaction conditions at high temperatures lead to agglomeration or decomposition of the loaded active centres, resulting in a significant loss of their initial activity.4,19–21 Therefore, designing noble metal active centres that can accommodate both low and high temperatures requires revolutionary updates to the catalyst structure.22,23Among the many precious metals, Pd-based catalysts are extensively researched and regarded as the most promising candidates for methane oxidation.24–26 Identifying highly stable and active palladium nanostructures has consistently been the focus of research endeavors.15 Wang's group reported the reversible transformation behaviour of Pd single atoms on CeO2 across various stages of automotive exhaust aftertreatment.27 The PdOx clusters reduced in a CO atmosphere exhibit enhanced low-temperature CH4 oxidation activity. However, the redispersion of PdOx clusters into Pd atoms leads to a decline in conversion efficiency, severely constraining their potential applications. Considering that atomically dispersed Pd atoms are thermodynamically stable at high temperature, constructing highly active Pd single atoms offers a viable solution to the inevitable deactivation issues. A major challenge lies in efficiently accomplishing methane and oxygen activation on an individual Pd atom.19 Unlike the direct supply of O by PdOx particles, the O2 activation process of a single-atom catalyst predominantly takes place on the support.16 Xie's group introduced Rh atoms onto Pd1/CeO2 and improved the oxygen vacancy formation ability of CeO2, while the CH4 conversion was still less than 10% at 400 °C.28 Wu's group synthesized a Pdδ+ (0 < δ < 2) single site with an under-coordinated local structure, which exhibited a weak interaction with the ceria support. This enhanced the oxygen supply capacity of the support and decreased T50 to 450 °C.24 Despite extensive efforts that have been made, there is still much room for improving the methane combustion performance of Pd/CeO2 catalysts.7
Herein, we synthesized a multicomponent methane combustion catalyst consisting of CeMn solid solutions loaded with Pd single atoms (Pd/CeMn-AR) based on a modified auto-redox reaction, exhibiting superior low-temperature activity and high-temperature stability. Specifically, the T90 of Pd/CeMn-AR reaches 543 °C, significantly better than its Pd/CeO2 (627 °C), Pd/MnOx (634 °C) and Pd/CeMn-CP (557 °C) counterparts. Notably, Pd/CeMn-AR can maintain more than 95% of its initial activity at elevated temperatures of 550 °C and 650 °C after 10 hours of continuous reaction. Mechanistic studies revealed the crucial role of the one-pot auto-redox reaction synthesis strategy in generating abundant surface oxygen vacancies, which facilitates substrate adsorption and accelerates the oxidative conversion of CH3* intermediates.
Results and discussion
Material characterization
According to previous studies, Pd/CeMn-AR was synthesized by a one-pot auto-redox reaction involving Ce3+, Pd2+, and MnO4−, as illustrated in Fig. 1a.29,30 We first study the morphology of the as-obtained catalyst by transmission electron microscopy (TEM). The low-magnification TEM image depicted in Fig. 1b reveals a nanosphere morphology composed of smaller nanoparticles. To identify the composition of the sample, aberration-corrected high-angle dark-field scanning transmission electron microscopy (HAADF-STEM) was conducted for a detailed structural observation. Fig. 1c displays measured lattice spacings of 0.32 nm and 0.28 nm, which correspond to the (111) and (200) planes of the CeMn solid solution, indicating that Ce and Mn are well fused. Meanwhile, some bright dots associated with Pd single atoms are observed, with no evidence of Pd nanoparticle formation. The corresponding energy dispersive X-ray spectroscopy (EDX) mapping images in Fig. 1d demonstrate a uniform distribution of Ce, Mn, Pd, and O elements, confirming the successful formation of nanosized solid solution and the atomically dispersed nature of Pd atoms. In addition, we have obtained a higher-resolution image of Pd/CeMn-AR by HAADF-STEM, as displayed in Fig. S1.† Enlargement of the yellow-boxed area in Fig. S1a† demonstrates bright spots corresponding to isolated Pd atoms, as confirmed by the strong peak at 400 pm from the X- to Y-axis in Fig. S1c.† It further demonstrates the atomic dispersion of Pd in Pd/CeMn-AR. We further prepared comparison samples with a similar Pd content via a conventional co-precipitation method (Pd/CeMn-CP), impregnation (Pd/CeO2) and a hydrothermal method (Pd/MnOx); see the detailed synthesis procedure in the ESI.†31 Fig. S2–S4† show the HAADF-STEM images and EDX mapping images of these three comparison samples, exhibiting a similar particle size and elemental distribution to those of Pd/CeMn-AR.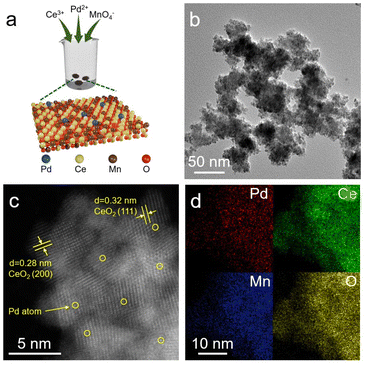 | ||
| Fig. 1 (a) Schematic illustration of the Pd/CeMn-AR synthesis process. (b) TEM image of Pd/CeMn-AR. (c) HAADF-STEM image of Pd/CeMn-AR. (d) EDX-mapping images of Pd/CeMn-AR. | ||
Next, powder X-ray diffraction (XRD) was employed to investigate the phase composition of various catalysts. In the XRD pattern in Fig. 2a, both Pd/CeMn-AR and Pd/CeMn-CP samples show broad diffraction peak positions at 2θ = 28.5°, 33.0°, 47.4°, 56.4°, 70° and 77.7°, which can be attributed to the cubic fluorite structure of CeMn solid solutions, consistent with previous studies.32–34 In the pattern of Pd/MnOx, the peaks at 12.8°, 19.9°, 32.7° and 56.45° correspond to the MnO2 phase (PDF #01-073-2509). The diffraction peaks situated at 2θ = 18.1°, 25.7°, 28.8°, 37.5°, 41.9°, 49.8°, 60.3°, 65.1° and 69.7° can be assigned to the MnO2 phase of MnOx (PDF #00-044-0141), and the diffraction peaks at 2θ = 14.3°, 21.7°, 26.1°, 28.9° and 43.9° can be assigned to the Mn3O4 phase of MnOx (PDF #03-065-2776). It is noteworthy that the absence of diffraction peaks associated with Pd nanoparticles or palladium oxides in all samples indicates a high dispersion of Pd element, aligning with our TEM observations.30
We further conducted X-ray photoelectron spectroscopy (XPS) to obtain detailed electronic information on each catalyst. Fig. 2b shows the fitting results of Pd 3d XPS spectra, where all samples show two sets of peaks at 338 eV and 338.9 eV corresponding to Pd2+ and Pd4+, suggesting that the Pd atoms are not aggregated to nanoparticles.35–39 This proves that Pd on the surface of various samples exists as single atoms. Fig. S5† shows the fitting results of the Mn 3d XPS spectra, where the peaks at 641 eV, 642.4 eV and 644.5 eV are attributed to Mn2+, Mn3+ and Mn4+.40–42 Fig. S6† shows the fitting results of Ce 3d XPS spectra, where the peaks from 882.5 eV to 898.4 eV represent the spin-coupled orbitals of Ce 3d3/2, and the peaks from 900.5 eV to 917 eV represent the spin-coupled orbitals of Ce 3d5/2. The peaks at 884.1 eV and 903.5 eV represent Ce3+.38 Previous studies indicate that elevated Ce3+ concentrations induce more oxygen vacancies on Ce–Mn solid solution surfaces, thereby improving methane combustion activity.35,43 The calculated Ce3+ ratios of Pd/CeMn-AR, Pd/CeMn-CP and Pd/CeO2 are 19.94%, 15.20%, and 14.97%. It shows that Pd/CeMn-AR prepared using an auto-redox method has more low-valent Ce species, which is favourable for the methane combustion reaction to proceed. Fig. S7† shows the fitting results of the O 1s XPS spectra of different catalysts, where the peaks at 529.7 eV, 531.9 eV and 533.2 eV represent lattice oxygen (Olatt), defective oxygen (Odef) and weakly bound oxygen (OOH). The concentration of oxygen vacancies can be calculated using Odef/(Olatt + Odef + OOH),44 and the order of the percentage of oxygen vacancies is Pd/CeMn-AR > Pd/CeMn-CP > Pd/CeO2. This suggests that more oxygen vacancies can be produced by the auto-redox method. Table S1† lists the proportion of different valence states of each element species for all catalysts, as determined by XPS. Then, Raman measurements were carried out to identify the oxygen vacancy content of various samples. Fig. S8† shows that the primary bonds observed at 450 cm−1 belong to the typical F2g mode of the CeO2 fluorite structure.38 The faint bands at 633 cm−1 are attributed to the defect-induced mode (D-mode), relating to the oxygen vacancies.45Fig. 2c shows the calculated results of these two Raman bond intensities (ID/IF2g) in each sample;37 the oxygen vacancy content follows the sequence: Pd/CeMn-AR > Pd/CeMn-CP > Pd/CeO2. We also used electron paramagnetic resonance (EPR) to measure the oxygen vacancy content of various samples. Fig. S9† shows the g = 2.003 signal corresponding to oxygen vacancy-trapped unpaired electrons in all catalysts, with intensity following Pd/CeMn-AR > Pd/CeMn-CP > Pd/CeO2, indicating the highest oxygen vacancy concentration in Pd/CeMn-AR. This is consistent with our Raman and XPS results, proving that the auto-redox synthesis strategy of Pd/CeMn-AR produces more oxygen vacancies, thereby enhancing methane combustion activity.
We then utilized in situ diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS) to investigate the presence of Pd species in all samples (Fig. 2d). Based on the strong interaction between d-orbital electrons of noble metals and carbon monoxide (CO) molecules, CO-DRIFTS can effectively probe the existing state of Pd centres. For Pd/CeMn-AR, the distinctive peak at 2150 cm−1 corresponds to CO adsorption on Ce3+,46 while a weak peak at 2120 cm−1 is attributed to the linear adsorption on Pd single atoms.37 A similar CO linear adsorption peak at 2132 cm−1 is also observed for Pd/CeMn-CP, confirming the atomically dispersed character of Pd species. However, the signals corresponding to CO bridge adsorption on Pd nanoparticles are present in both samples within the range of 1800 to 1970 cm−1. The signal for Pd/CeO2 exhibits a comparable trend to those of Pd/CeMn-AR and Pd/CeMn-CP.37,46 This observation aligns with previous studies on Pd single-atom catalysts, suggesting that it may be due to the mobility of Pd atoms in the CO environment during testing.46 Combined with TEM, EDX, XRD, XPS, and CO-DRIFTS results, we believe that Pd is dispersed on CeMn solid solutions in the form of single atoms. The nitrogen adsorption–desorption isotherms in Fig. S10† showed negligible differences in the surface areas of Pd/CeMn-AR and Pd/CeMn-CP (196 and 151 m2 g−1), indicating that the surface area is not a factor affecting the performance.
Catalytic performance evaluation
The methane combustion reaction was evaluated in a fixed-bed reactor with a weight hourly space velocity (WHSV) of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL gcat−1 h−1. As shown by the catalytic performance in Fig. 3a, the conversion of methane increases with the rise in reaction temperature. Among various samples, Pd/CeMn-AR shows the best low-temperature performance, with the T50 reaching 440 °C and the T90 reaching 543 °C, which were 82 °C and 84 °C lower than those of its typical Pd/CeO2 counterpart, respectively. The Pd/CeMn-CP catalyst shows the second best low-temperature performance, reaching a T50 of 452 °C and T90 of 557 °C. This observation not only indicates that CeMn solid solution supports are more advantageous than pure CeO2, but also highlights the significant influence of the preparation methodology on methane combustion performance. Additionally, to demonstrate the superiority of the one-pot synthesis approach, CeMn solid solution supports decorated with Pd single atoms were prepared via a similar redox reaction and impregnation procedure (namely, CeMn-AR-Pd), as shown in Fig. S11.† As shown in the catalytic results in Fig. S12,† the methane conversion of CeMn-AR-Pd reaches only 77% at 550 °C, significantly lower than that of Pd/CeMn-AR. We subsequently investigated the compositional effect in the ternary system by verifying the feeding ratio of Ce, Mn, and Pd precursors, as shown in Fig. S13.† At a constant Pd loading of 1 wt%, the optimal methane combustion performance of the catalyst was achieved when the Ce
000 mL gcat−1 h−1. As shown by the catalytic performance in Fig. 3a, the conversion of methane increases with the rise in reaction temperature. Among various samples, Pd/CeMn-AR shows the best low-temperature performance, with the T50 reaching 440 °C and the T90 reaching 543 °C, which were 82 °C and 84 °C lower than those of its typical Pd/CeO2 counterpart, respectively. The Pd/CeMn-CP catalyst shows the second best low-temperature performance, reaching a T50 of 452 °C and T90 of 557 °C. This observation not only indicates that CeMn solid solution supports are more advantageous than pure CeO2, but also highlights the significant influence of the preparation methodology on methane combustion performance. Additionally, to demonstrate the superiority of the one-pot synthesis approach, CeMn solid solution supports decorated with Pd single atoms were prepared via a similar redox reaction and impregnation procedure (namely, CeMn-AR-Pd), as shown in Fig. S11.† As shown in the catalytic results in Fig. S12,† the methane conversion of CeMn-AR-Pd reaches only 77% at 550 °C, significantly lower than that of Pd/CeMn-AR. We subsequently investigated the compositional effect in the ternary system by verifying the feeding ratio of Ce, Mn, and Pd precursors, as shown in Fig. S13.† At a constant Pd loading of 1 wt%, the optimal methane combustion performance of the catalyst was achieved when the Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn molar ratio was 2
Mn molar ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. On this basis, further variations in the Pd loading led to decreased catalytic performance. When evaluating the space velocities, the methane conversion rate gradually decreases, as shown in Fig. S14.†
1. On this basis, further variations in the Pd loading led to decreased catalytic performance. When evaluating the space velocities, the methane conversion rate gradually decreases, as shown in Fig. S14.†
We then carried out a kinetic study across all samples, while maintaining the methane conversion below 15%.47 The calculated apparent activation energies (Ea) in Fig. 3b reveal that Pd/CeMn-AR has the lowest energy barrier of 64.75 kJ mol−1, indicating a fast kinetic process. Considering that water resistance is also a key performance indicator for methane combustion catalysts, we incorporated 1.5% water vapor and evaluated the performance of Pd/CeMn-AR. As depicted in Fig. 3c, Pd/CeMn-AR maintained a high activity under wet conditions, with T50 at 464 °C and T90 at 562 °C, suggesting substantial application potential. Fig. 3d presents the long-term stability measurements of each sample. As expected, the stability of all single-atom samples is satisfactory. For Pd/CeMn-AR, nearly 97% of the original activity remained after 10 hours of continuous catalysis at 550 °C. At the same reaction time and temperature, as much as 98% of initial methane conversion remained under wet conditions. Furthermore, a comparative sample of CeO2-supported Pd particles was prepared (namely, PdNP/CeO2). As shown in Fig. S15 and S16,† although it exhibits better activity compared to PdNP/CeO2 in the low-temperature region, its conversion rapidly declines above 500 °C. Stability tests revealed that its activity decayed by 19% within the first 30 minutes and it retained only 74% of the initial activity after 2 hours. This result highlights the crucial role of stable single-atom Pd active centers in methane combustion at elevated temperatures. Fig. S17–S19 and Table S2† list the detailed structural characterization of the reacted samples. No significant Pd nanoparticle agglomeration was observed in the high-resolution TEM image, and the corresponding EDX mapping images showed that the Pd is uniformly distributed through the CeMn support. The XRD patterns of the used samples were consistent with those prior to the reaction. The above results confirm the catalyst's robust structural stability. Compared to the existing Pd-based single-atom catalysts for methane combustion, our Pd/CeMn-AR catalyst exhibits a decreased reaction temperature, faster kinetic process, enhanced structural stability, and improved atom utilization efficiency (Table S3†).
Mechanism investigation
To investigate the factors underlying the performance disparities among various samples, CH4 and O2 temperature programmed desorption (TPD) tests were carried out to examine the adsorption ability of various catalysts on both substrates. From the CH4-TPD profile in Fig. 4a, the peaks located at 200 °C are attributed to weak CH4 adsorption. The peaks located at 391 °C and 575 °C are attributed to medium CH4 adsorption. Compared to those of pure CeO2 and MnOx, the methane adsorption characteristics of both Pd/CeMn-AR and Pd/CeMn-CP exhibit an optimized state: the weak adsorption is enhanced, while the strong adsorption is weakened, and medium adsorption occurs. Previous studies have pointed out that medium methane adsorption is most efficient for methane activation, which explains the preference of CeMn solid solutions over CeO2 and MnOx. The O2-TPD results in Fig. 4b can be divided into three regions: weakly bound surface oxygen species (below 250 °C), active oxygen species adsorbed on oxygen vacancies (from 250 °C to 600 °C), and bulk oxygen species (above 600 °C).20,48 Compared to Pd/CeMn-CP, Pd/CeO2 and Pd/MnOx, Pd/CeMn-AR exhibits the strongest oxygen desorption ability within the second region and presents an additional desorption peak at 393 °C, indicating a higher concentration of oxygen vacancies, which is advantageous for methane combustion. Notably, such robust O2 desorption behavior was not found in the O2-TPD profiles of CeMn-AR-Pd in Fig. S20,† highlighting the significance of the one-pot synthesis method in preserving oxygen vacancies on the catalyst surface.To elucidate the methane conversion process, we recorded the in situ DRIFTS spectra in the reaction gas at 550 °C. As shown in Fig. 4c, the signals of the reaction substrates, intermediates and products appeared in the spectra, as the reaction gas was continuously passed. Bands corresponding to the OH stretching vibration (3736 cm−1 and 3536 cm−1), gaseous methane (3054 cm−1 and 2956 cm−1), and gaseous CO2 (2286 cm−1 and 2384 cm−1) appeared in the first minute, indicating a rapid combustion process.20,49,50 In line with prior investigations, both formate (1588 cm−1 and 1545 cm−1) and carbonate (1340 cm−1 and 1540 cm−1) species were detected throughout the measurement procedure, implying a sluggish intermediate-to-product conversion process.20,38,51 In contrast, the absorption bands belonging to the CH2O* intermediate (1225 cm−1) appeared only after 7 min, suggesting a fast dehydrogenation process of CH2O* to HCOO*.38 Therefore, a possible reaction mechanism of methane conversion on Pd/CeMn-AR is proposed in Fig. 4d. Initially, methane molecules are activated and form CHx* species at the Pd site. Subsequently, the surface lattice oxygen of the CeMn support participates in the reaction and oxidizes CH3* to form CH2O*. CH2O* species interact with OH* or the lattice oxygen on the surface to form carbonate species (CO3*) and formate species (HCOO*). These intermediates are further oxidized on the catalyst surface, yielding CO2 and H2O.18,51,52 Finally, oxygen molecules from the substrate are captured by oxygen vacancies on the support, completing the catalytic cycle.
Conclusions
In summary, we successfully synthesized a CeMn solid solution-loaded Pd single atom catalyst via a modified auto-redox reaction. Pd/CeMn-AR exhibited superior methane combustion performance, especially at elevated temperatures. The T90 of Pd/CeMn-AR is only 543 °C, significantly lower than those of Pd/CeO2 (627 °C), Pd/MnOx (634 °C) and Pd/CeMn-CP (557 °C). Furthermore, Pd/CeMn-AR showed only a 5% decrease of its initial activity after 10 hours of long-term stability testing. Systematic characterization and mechanism studies reveal that the abundant surface oxygen vacancies not only facilitate the adsorption of methane and oxygen molecules but also accelerate the oxidative conversion of CH3* intermediates. This work presents a convenient but efficient synthesis procedure for creating highly dispersed active centers and abundant defects, thereby enhancing catalytic activity. We anticipate that the proposed strategy based on the auto-redox reaction will find widespread application in various catalyst designs and contribute to other sustainable catalytic systems.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the financial aid from the National Science and Technology Major Project of China (2021YFB3500700), National Natural Science Foundation of China (22020102003, 22025506, 22271274, 22175071 and U23A20140), and Jilin Province Science and Technology Development Plan Project (20240402056GH). X. W. acknowledges funding from the National Natural Science Foundation of China Outstanding Youth Science Foundation of China (Overseas).References
- X. B. Feng, J. Lei, D. Y. Li, S. P. Tian, Z. Xing, W. Hua, C. He and K. Z. Li, J. Energy Chem., 2022, 75, 173–215 CrossRef CAS.
- H. G. Peng, C. Rao, N. Zhang, X. Wang, W. M. Liu, W. T. Mao, L. Han, P. F. Zhang and S. Dai, Angew. Chem., Int. Ed., 2018, 57, 8953–8957 CrossRef CAS PubMed.
- K. Murata, Y. Mahara, J. Ohyama, Y. Yamamoto, S. Arai and A. Satsuma, Angew. Chem., Int. Ed., 2017, 56, 15993–15997 CrossRef CAS PubMed.
- R. J. Farrauto, Science, 2012, 337, 659–660 CrossRef CAS PubMed.
- S. Yasumura, K. Nagai, S. Miyazaki, Y. C. Qian, D. T. Chen, T. Toyao, Y. Kamiya and K. I. Shimizu, J. Am. Chem. Soc., 2024, 146, 20982–20988 CrossRef CAS PubMed.
- E. D. Goodman, A. S. Asundi, A. S. Hoffman, K. C. Bustillo, J. F. Stebbins, S. R. Bare, S. F. Bent and M. Cargnello, Adv. Mater., 2021, 33, 2104533 CrossRef CAS PubMed.
- M. Cargnello, J. J. D. Jaén, J. C. H. Garrido, K. Bakhmutsky, T. Montini, J. J. C. Gámez, R. J. Gorte and P. Fornasiero, Science, 2012, 337, 713–717 CrossRef CAS PubMed.
- B. Yuan, T. Zhu, B. Nie, X. Zhang, Y. Han, M. Wang, C. Li and X. Zhang, J. Environ. Chem. Eng., 2024, 12, 113860 CrossRef CAS.
- X. Huang, W. Yang and J. Li, Processes, 2024, 12, 1630 CrossRef CAS.
- L. M. Toscani, P. A. Curyk, M. G. Zimicz, E. B. Halac, M. E. Saleta, D. G. Lamas and S. A. Larrondo, Appl. Catal., A, 2019, 587, 117235 CrossRef CAS.
- X. Y. Li, Y. X. Liu, J. G. Deng, S. H. Xie, X. T. Zhao, Y. Zhang, K. F. Zhang, H. Arandiyan, G. S. Guo and H. X. Dai, Appl. Surf. Sci., 2017, 403, 590–600 CrossRef CAS.
- Y. H. Xiao, W. T. Zhao, K. Zhang, Y. Y. Zhang, X. Y. Wang, T. H. Zhang, X. W. Wu, C. Q. Chen and L. L. Jiang, Dalton Trans., 2017, 46, 16967–16972 RSC.
- B. Yue, R. Zhou, X. Zheng and W. Lu, Fuel Process. Technol., 2008, 89, 728–735 CrossRef CAS.
- A. J. Zarur and J. Y. Ying, Nature, 2000, 403, 65–67 CrossRef CAS PubMed.
- Y. Wang, H. Arandiyan, H. A. Tahini, J. Scott, X. Tan, H. X. Dai, J. D. Gale, A. L. Rohl, S. C. Smith and R. Amal, Nat. Commun., 2017, 8, 15553 CrossRef CAS PubMed.
- X. L. Feng, D. P. Liu, B. L. Yan, M. Z. Shao, Z. M. Hao, G. B. Yuan, H. H. Yu and Y. Zhang, Angew. Chem., Int. Ed., 2021, 60, 18552–18556 CrossRef CAS PubMed.
- X. L. Feng, X. R. Gong, D. P. Liu, Y. Li, Y. Jiang and Y. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202313068 CrossRef CAS PubMed.
- Z. Y. Tang, T. Zhang, D. C. Luo, Y. J. Wang, Z. Hu and R. T. Yang, ACS Catal., 2022, 12, 13457–13474 CrossRef CAS.
- H. M. Duan, F. X. Kong, X. Z. Bi, L. Chen, H. T. Chen, D. J. Yang and W. X. Huang, ACS Catal., 2024, 14, 17972–17992 CrossRef CAS.
- J. Tao, H. Lin, J. Deng, Y. Liu, L. Jing, Z. Hou, L. Wei, Z. Wang and H. Dai, Appl. Catal., B, 2025, 360, 124554 CrossRef CAS.
- M. Cargnello, T. Montini, S. Polizzi, N. L. Wieder, R. J. Gorte, M. Graziani and P. Fornasiero, Dalton Trans., 2010, 39, 2122–2127 RSC.
- N. F. Wang, S. Q. Li, Y. C. Zong and Q. Yao, J. Aerosol Sci., 2017, 105, 64–72 CrossRef CAS.
- F. Niu, S. Q. Li, Y. C. Zong and Q. Yao, J. Phys. Chem. C, 2014, 118, 19165–19171 CrossRef CAS.
- W. W. Yang, F. Polo-Garzon, H. Zhou, Z. N. Huang, M. F. Chi, H. Meyer III, X. B. Yu, Y. Y. Li and Z. L. Wu, Angew. Chem., Int. Ed., 2023, 62, e202217323 CrossRef CAS PubMed.
- Y. Ozawa, Y. Tochihara, M. Nagai and S. Omi, Catal. Commun., 2003, 4, 87–90 CrossRef CAS.
- G. Groppi, C. Cristiani, L. Lietti, C. Ramella, M. Valentini and P. Forzatti, Catal. Today, 1999, 50, 399–412 CrossRef CAS.
- D. Jiang, G. Wan, J. H. Stenlid, C. E. García-Vargas, J. H. Zhang, C. J. Sun, J. R. Li, F. Abild-Pedersen, C. J. Tassone and Y. Wang, Nat. Catal., 2023, 6, 618–627 CrossRef CAS.
- T. C. Pu, J. Q. Ding, X. Tang, K. W. Yang, K. Wang, B. Huang, S. Dai, Y. He, Y. Shi and P. F. Xie, ACS Appl. Mater. Interfaces, 2022, 14, 43141–43150 CrossRef CAS PubMed.
- X. Wang, Y. B. Zhang, S. Y. Song, X. G. Yang, Z. Wang, R. C. Jin and H. J. Zhang, Angew. Chem., Int. Ed., 2016, 55, 4542–4546 CrossRef CAS PubMed.
- K. Qi, J. L. Xie, Z. Zhang, D. Fang, D. Han, X. Q. Liu, P. J. Gong, F. X. Li and F. He, Powder Technol., 2018, 338, 774–782 CrossRef CAS.
- M. Liu, S. Hu, Y. Zhang, C. Zhao, W. Jiang, C. Qi, X. Zhu, P. Qiu, Y. Sun, K. Kato, Y. Zhao, X. Li, M. Yamauchi and W. Luo, Adv. Mater. Interfaces, 2021, 8, 2002060 CrossRef CAS.
- L. M. Shi, W. Chu, F. F. Qu, J. Y. Hu and M. M. Li, J. Rare Earths, 2008, 26, 836–840 CrossRef.
- Y. J. Luo, D. F. Lin, Y. B. Zheng, X. S. Feng, Q. H. Chen, K. Zhang, X. Y. Wang and L. L. Jiang, Appl. Surf. Sci., 2020, 504, 144481 CrossRef CAS.
- E. Y. Liberman, B. S. Kleusov, E. A. Simakina, T. V. Kon'kova, V. N. Grunskii, A. D. Stoyanova and A. V. Denisenko, Russ. J. Appl. Chem., 2023, 96, 156–161 CrossRef CAS.
- L. X. Jia, L. L. Zhang, H. L. Wang, X. Wang, S. Y. Song and H. J. Zhang, ChemCatChem, 2024, 16, e202401251 CrossRef CAS.
- X. X. Han, L. L. Zhang, R. Zhang, K. Wang, X. Wang, B. Li, Z. P. Tao, S. Y. Song and H. J. Zhang, Dalton Trans., 2024, 53, 3290–3295 RSC.
- H. Zhao, Y. L. Tan, L. Li, Y. Su, A. Q. Wang, X. Y. Liu and T. Zhang, Chem. Eng. J., 2023, 451, 138937 CrossRef CAS.
- M. W. Wu, M. Miao, W. Z. Li, X. Zhang, L. L. Zhang, T. M. Zhen, Y. Fu, J. T. Jin and L. Yuan, Fuel, 2023, 331, 125575 CrossRef CAS.
- L. Li, S. T. Xue, M. J. Wei, H. Yao, Y. Dai and Z. Y. Fei, Appl. Surf. Sci., 2022, 599, 153909 CrossRef CAS.
- B. Chutia, N. Hussain, P. Puzari, D. Jampaiah, S. K. Bhargava, E. V. Matus, I. Z. Ismagilov, M. Kerzhentsev and P. Bharali, Energy Fuel, 2021, 35, 10756–10769 CrossRef CAS.
- J. Q. Yang, H. Deng, Y. Q. Lu, J. Z. Ma, W. P. Shan and H. He, Ind. Eng. Chem. Res., 2023, 62, 8665–8672 CrossRef CAS.
- G. Liu, L. Sun, J. H. Liu, F. Wang and C. J. Guild, Mol. Catal., 2017, 440, 148–157 CrossRef CAS.
- J. R. Wang, D. W. Liu, W. X. Zhang, B. Hou, Y. Deng, X. X. Ma and L. Xu, ChemCatChem, 2024, 16(9), e202301768 CrossRef CAS.
- J. K. Zhang, Y. Men, Y. M. Wang, L. N. Liao, S. Liu, J. G. Wang and W. An, Int. J. Hydrogen Energy, 2024, 51, 1185–1199 CrossRef CAS.
- H. Zhao, L. C. Bian, J. C. Du and Y. K. Zhao, Dalton Trans., 2022, 51, 18562–18571 RSC.
- L. A. Zhang, S. L. Wan, C. C. Du, Q. Wan, H. Pham, J. F. Zhao, X. Y. Ding, D. Y. Wei, W. Zhao, J. W. Li, Y. P. Zheng, H. Xie, H. Zhang, M. S. Chen, K. H. L. Zhang, S. Wang, J. D. Lin, J. Y. Huang, S. Lin, Y. Wang, A. K. Datye, Y. Wang and H. F. Xiong, Nat. Commun., 2024, 15, 1234 CrossRef CAS PubMed.
- H. L. Wang, M. S. Bootharaju, J. H. Kim, Y. Wang, K. Wang, M. Zhao, R. Zhang, J. Xu, T. Hyeon, X. Wang, S. Y. Song and H. J. Zhang, J. Am. Chem. Soc., 2023, 145, 2264–2270 CrossRef CAS PubMed.
- H. C. Yao and Y. F. Y. Yao, J. Catal., 1984, 86, 254–265 CrossRef CAS.
- P. F. Qu, S. L. Wang, W. Hu, Y. Wu, J. J. Chen, G. C. Zhang, P. Q. Shen, Y. Q. Chen and L. Zhong, Catal. Commun., 2020, 135, 105900 CrossRef CAS.
- J. Y. Cai, S. H. Wu, M. M. Liu, Y. Zhang, M. Wang, Y. Liu, Y. B. Yu and W. P. Shan, Appl. Catal., B, 2025, 361(13), 124559 CrossRef CAS.
- J. S. Tian, R. Kong, Z. Wang, L. Fang, T. Y. He, D. Jiang, H. G. Peng, T. L. Sun, Y. H. Zhu and Y. Wang, ACS Catal., 2023, 14, 183–191 CrossRef.
- S. Y. Chen, S. D. Li, R. Y. You, Z. Y. Guo, F. Wang, G. X. Li, W. T. Yuan, B. E. Zhu, Y. Gao, Z. Zhang, H. S. Yang and Y. Wang, ACS Catal., 2021, 11, 5666–5677 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Figures of the characterization of the as-prepared catalysts. See DOI: https://doi.org/10.1039/d5dt00409h |
| This journal is © The Royal Society of Chemistry 2025 |



