Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Physical properties and cytotoxicity of Cu(II) and Zn(II) complexes with a TMS-substituted indolo[2,3-c]quinoline-derived Schiff base†
Christopher
Wittmann
 a,
Iuliana
Besleaga
a,
Iuliana
Besleaga
 a,
Soheil
Mahmoudi
a,
Soheil
Mahmoudi
 ab,
Oleg
Palamarciuc
cd,
Mihaela
Balan-Porcarasu
ab,
Oleg
Palamarciuc
cd,
Mihaela
Balan-Porcarasu
 e,
Mihaela
Dascalu
c,
Sergiu
Shova
c,
Maria
Cazacu
e,
Mihaela
Dascalu
c,
Sergiu
Shova
c,
Maria
Cazacu
 c,
Mónika
Kiricsi
c,
Mónika
Kiricsi
 f,
Nóra
Igaz
f,
Nóra
Igaz
 f,
Orsolya
Dömötör
f,
Orsolya
Dömötör
 g,
Eva A.
Enyedy
g,
Eva A.
Enyedy
 *g,
Dana
Dvoranová
h,
Peter
Rapta
*g,
Dana
Dvoranová
h,
Peter
Rapta
 *h and
Vladimir B.
Arion
*h and
Vladimir B.
Arion
 *ac
*ac
aUniversity of Vienna, Institute of Inorganic Chemistry, Währinger Strasse 42, 1090 Vienna, Austria. E-mail: vladimir.arion@univie.ac.at
bUniversity of Vienna, Vienna Doctoral School in Chemistry (DoSChem), Währinger Strasse 42, 1090 Vienna, Austria
cInorganic Polymers Department, “Petru Poni” Institute of Macromolecular Chemistry, Aleea Gr. Ghica Voda 41 A, Iasi 700487, Romania
dPhysics of Semiconductors and Devices Laboratory, Faculty of Physics and Engineering and Institute of Applied Physics, Moldova State University, MD-2009 Chişinău, Republic of Moldova
eNMR Laboratory, “Petru Poni” Institute of Macromolecular Chemistry, Aleea Gr. Ghica Voda 41 A, Iasi 700487, Romania
fDepartment of Biochemistry and Molecular Biology, University of Szeged, Közép fasor 52, H-6726 Szeged, Hungary
gDepartment of Molecular and Analytical Chemistry, Interdisciplinary Excellence Centre, University of Szeged, Dóm tér 7-8, H-6720 Szeged, Hungary. E-mail: enyedy@chem.u-szeged.hu
hInstitute of Physical Chemistry and Chemical Physics, Faculty of Chemical and Food Technology, Slovak University of Technology in Bratislava, SK-81237 Bratislava, Slovakia. E-mail: peter.rapta@stuba.sk
First published on 2nd April 2025
Abstract
The incorporation of non-native chemical elements, such as silicon, into drug molecules has gained significant attention as a strategy to broaden the chemical space in medicinal chemistry and develop novel drug candidates. Traditionally, research has focused on the isosteric replacement of a carbon atom with silicon (“silicon switch”) in known drug structures or the attachment of a trimethylsilyl (TMS) group to biologically active scaffolds. In this study, a TMS-substituted indoloquinoline-based Schiff base (HLTMS) and its corresponding metal complexes, Cu(HLTMS)Cl2 (1) and Zn(HLTMS)Cl2 (2), were synthesized and comprehensively characterized using elemental analysis, spectroscopic techniques (IR, UV-vis, 1H and 13C NMR for HLTMS and 2), ESI mass spectrometry and single-crystal X-ray diffraction (SC-XRD) for 1 and electron diffraction (ED) for 2. The attachment of the TMS group enhanced the lipophilicity of HLTMS, while complex formation with Cu(II) substantially improved the antiproliferative activity. Exploitation of their intrinsic fluorescence to investigate cellular uptake and intracellular localization in cancer cells was impeded by limited solubility. Both HLTMS and 2 were found to generate reactive oxygen species under cell-free conditions in accord with their redox activity established by cyclic voltammetry. The photochemical activity of the indolo[2,3-c]quinoline-based proligand HLTMS and its complexes 1 and 2 has been disclosed. The compounds exhibited significant toxicity on various human cancer cells and disrupted the mitochondrial membrane potential, suggesting the contribution of mitochondrial dysfunction, triggered by HLTMS and its metal complexes, to their toxic effects. These findings highlight the potential of TMS-substituted Schiff bases as promising anticancer drug candidates.
Introduction
As carbon-based life forms, humans and living organisms in general are treated with medicines based on carbon skeletons and common elements such as nitrogen, oxygen, sulfur and halogens.1 The discovery and FDA approval of bortezomib, a boronic acid derivative, for multiple myeloma in 1995 and 2003, respectively,2 sparked interest in incorporation of non-native elements directly into the framework of organic compounds for drug design. This may be a promising strategy for the development of new drug candidates, thus allowing the expansion of the medicinal chemistry space. The isosteric exchange of carbon with silicon – known as the “carbon–silicon switch” – has emerged as one of the most frequently exploited pathways.3–6 The similarity of silicon to carbon makes it the ideal choice as a carbon bioisostere, while some differences between these elements lead to marked changes of the physico-chemical and pharmacological properties of the resulting sila-drugs.7 The differences are related to atomic size (covalent radii of 111 pm for Si and 67 pm for C), bond length (1.87 Å for the Si–C bond compared to 1.54 Å for the C–C bond), electronegativity (1.7 for Si vs. 2.5 for C) and silicon's ability to adopt a higher coordination number (up to 6) compared to carbon that can lead to changes in structural stability, bioavailability, interactions with biological targets, and ultimately, therapeutic efficacy.8,9 The increased lipophilicity, which is closely related to the specific molecular structure of silicon-containing compounds, enhances their cellular penetration capacity. Moreover, no “element-specific” intrinsic toxicity has been documented in the literature for small organosilicon molecules.10,11An easy strategy to include Si in the drug structure consists of the attachment of the trimethylsilyl (TMS) group to a biologically active scaffold.3 Some systematic studies to elucidate the impact of different silyl groups on the anticancer activity of mucobromic acid (MBA) bearing a furan-2(5H)-one core12 have shown that the attachment of Si-containing fragments can increase the stability of the compounds, improve their pharmacokinetic profile and decrease their toxicity.13 Similarly, the introduction of the TMS group into camptothecin, a DNA topoisomerase inhibitor, significantly enhanced its anticancer activity, even against multi-drug resistant cells.14
Recently, we reported on the antiproliferative activity and insights into the underlying mechanisms of action of a series of Schiff bases derived from four-ring heterocyclic scaffolds, namely indoloquinolines, indolobenzazepines, indolobenzazocines and indolobenzazonines, which differ by the size of the central N-containing ring, varying from 6-membered to 9-membered.15–25 In continuation of these studies, we aimed to perform (i) the synthesis of a TMS-substituted Schiff base proligand derived from a four-ring heterocyclic scaffold, (ii) the preparation of Cu(II) and Zn(II) complexes with these biologically active proligands and (iii) the investigation of physical and spectroscopic properties, solution stability, lipophilicity and anticancer potential of the compounds prepared.
Herein we report on the multistep synthesis of the 9-trimethylsilylindolo[2,3-c]quinoline-derived Schiff base HLTMS and on the preparation of copper(II) and zinc(II) complexes Cu(HLTMS)Cl2 (1) and Zn(HLTMS)Cl2 (2) (see Scheme 1). The compounds were characterized by elemental analysis, ESI mass spectrometry, IR, UV-vis, 1H and 13C NMR (for HLTMS and G) spectroscopy, single crystal X-ray diffraction for 1 and electron diffraction (ED) for 2 and stability in solution. In addition, their cytotoxic activity was screened in a panel of 60 cancer cell lines at the National Cancer Institute (NCI) of the National Institutes of Health (NIH), and also tested on A549 and MCF-7 tumor cells in one of our laboratories to determine their IC50 values and elucidate their effect on the mitochondrial membrane potential.
Results and discussion
Synthesis of HLTMS
The 9-trimethylsilylindolo[2,3-c]quinoline-derived Schiff base HLTMS was prepared by following the multistep synthetic pathway, as shown in Scheme 1, by adapting protocols reported previously.26–28 Species A was obtained in 88% yield by the reaction of acetanilide, Pd(OAc)2 and sodium dodecyl sulfate in water with t-butyl peroxide, 4-bromobenzaldehyde and trifluoroacetic acid at room temperature for 24 h. Silylation of species A using tris-(dibenzylideneacetone)dipalladium(0) (Pd2dba3) via a 2-(di-t-butylphosphino)biphenyl (JohnPhos)/KF catalyzed reaction in N,N′-dimethylpropyleneurea (DMPU) with small amounts of water and hexamethyldisilane at 100 °C for 2 h afforded species B in 40% yield. The TMS-substituted compound B was further deprotected with the liberation of compound Cvia reaction with 6 M HCl in acetone. 2-Amino-4′-trimethylsilylbenzophenone C was reacted further with chloroacetyl chloride to give the chloroacetylated species D in 58% yield. The chloro substituent in D was then replaced almost quantitatively by an azido group with the formation of species E. The base-catalyzed cyclization reaction of E resulted in azido-phenylquinolinone F in 70% yield. In a second cyclization step, species F, which was not stable at high temperature, was converted into the expected 9-trimethylsilylindolo[2,3-c]quinoline-2(1H)-one G with the release of nitrogen in 79% yield. Quantitative thionation of lactam species G in thiolactam H was realized by prolonged heating with Lawesson's reagent in toluene. Hydrazone I was obtained by reaction of H with hydrazine hydrate used as the reagent and solvent with 88% yield. Finally, Schiff base condensation of hydrazone I with 2-acetylpyridine in boiling methanol overnight afforded HLTMS in 77% yield. The NMR atom numbering scheme is shown in Fig. S1 in the ESI.† The 1H NMR spectra of species A, B, D, E, F and G are shown in Fig. S2–S7,† and the 13C and 29Si NMR spectra of species G are shown in Fig. S8 and S9.† The 1H and 13C NMR spectra of HLTMS, shown in Fig. S10 and S11,† were in accord with the expected structure. The 25 proton resonances registered in the spectrum are in accordance with the C1 molecular symmetry of HLTMS. There are no other isomeric species in solution. The presence of the TMS group is seen at 0.35 ppm, the methyl group at the Schiff base C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group at 2.68 ppm, while the two NH protons, one at the indole nitrogen and another at the nitrogen atom of the quinoline moiety resonate at 11.93 and 10.77 ppm, respectively. The ESI mass spectra of species B, C, D, F, G and HLTMS are shown in Fig. S12–S18.† The positive ion ESI mass spectrum of HLTMS showed two peaks with an m/z of 424.25 and 847.29, which could be assigned to [M + H]+ (calcd m/z 424.20) and to the dimeric associate [2M + H]+ (calcd m/z 847.38). In the negative ion ESI mass spectrum of HLTMS, a peak with an m/z of 422.08 was attributed to [M − H+]− (calcd m/z 422.18).
N group at 2.68 ppm, while the two NH protons, one at the indole nitrogen and another at the nitrogen atom of the quinoline moiety resonate at 11.93 and 10.77 ppm, respectively. The ESI mass spectra of species B, C, D, F, G and HLTMS are shown in Fig. S12–S18.† The positive ion ESI mass spectrum of HLTMS showed two peaks with an m/z of 424.25 and 847.29, which could be assigned to [M + H]+ (calcd m/z 424.20) and to the dimeric associate [2M + H]+ (calcd m/z 847.38). In the negative ion ESI mass spectrum of HLTMS, a peak with an m/z of 422.08 was attributed to [M − H+]− (calcd m/z 422.18).
Synthesis of copper(II) and zinc(II) complexes
The complexes Cu(HLTMS)Cl2 (1) and Zn(HLTMS)Cl2·2H2O (2) were prepared in quantitative yields by reactions of the corresponding metal chlorides with HLTMS in boiling methanol. The 1H and 13C NMR spectra of Zn(II) complex 2 became interpretable only after keeping for one week at room temperature in an NMR tube and were in agreement with its C1 molecular structure (Fig. S19 and S20†). The ESI(+) mass spectrum of 1 (Fig. S21†) showed a strong peak with an m/z of 521.13 and another one with an m/z of 1043.13, which were assigned to [M − Cl]+ (calcd m/z 521.10) and to a dimeric associate [(M − Cl)2 − H+]+ (calcd m/z 1043.18). A lower intensity peak with an m/z of 485.16 was attributed to [M − 2Cl − H]+. In the ESI(–) mass spectrum of 1 (Fig. S22†), the peak with an m/z of 519.05 was assigned to [M − Cl − 2H]− (calcd m/z 519.08). The ESI(+) mass spectrum of complex 2 (Fig. S23†) showed a peak with an m/z of 522.12 which was assigned to [M − Cl]+ (calcd m/z 522.09) and two remarkable peaks, one very strong with an m/z of 909.34 and the second with an m/z of 455.18. The first one could be assigned to [Zn(HLTMS)(LTMS)]+ (calcd m/z 909.30) and the second to the doubly charged ion [Zn(HLTMS)2]2+ (calcd m/z 455.15). Zn(II) to HLTMS species at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were formed in the mass spectrometer. The ESI(–) mass spectrum of 2 (Fig. S24†) contains two peaks with an m/z of 558.02 attributed to [Zn(LTMS)Cl2]− (calcd m/z 558.05) and an m/z of 1116.91 assigned to the dimeric associate [{Zn(HLTMS)Cl2}{Zn(LTMS)Cl2}]− (calcd m/z 1117.10). It should also be noted that all experimental isotopic patterns were in accordance with the calculated isotopic distributions for the assigned ions both in positive and negative ion modes.
2 were formed in the mass spectrometer. The ESI(–) mass spectrum of 2 (Fig. S24†) contains two peaks with an m/z of 558.02 attributed to [Zn(LTMS)Cl2]− (calcd m/z 558.05) and an m/z of 1116.91 assigned to the dimeric associate [{Zn(HLTMS)Cl2}{Zn(LTMS)Cl2}]− (calcd m/z 1117.10). It should also be noted that all experimental isotopic patterns were in accordance with the calculated isotopic distributions for the assigned ions both in positive and negative ion modes.
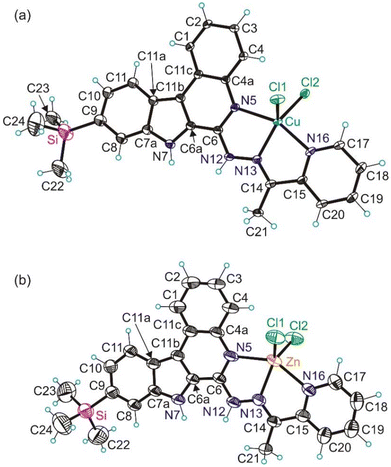
Molecular structures of complexes 1 and 2
The results of single crystal X-ray diffraction (SC-XRD) measurements of complex 1 and electron diffraction (ED) measurements of 2 are shown in Fig. 1. The details of data collection and refinement are summarized in Table S1 in the ESI.† Complex 1 crystallized in the monoclinic centrosymmetric space group P21/c, while 2 crystallized in the centrosymmetric triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Pertinent metric parameters for complexes 1 and 2 are shown in Table 1.
. Pertinent metric parameters for complexes 1 and 2 are shown in Table 1.
| Bond lengths (Å) | 1 | 2 |
|---|---|---|
| a τ 5 = 0 for square-pyramidal and 1 for trigonal–bipyramidal coordination geometry. b Two positions of N5 due to disorder were taken into account. | ||
| M–N5 | 2.020(14) | 2.240(8) |
| M–N13 | 1.984(5) | 2.144(7) |
| M–N16 | 2.026(5) | 2.258(10) |
| M–Cl1 | 2.4493(2) | 2.271(4) |
| M–Cl2 | 2.251(2) | 2.264(4) |
τ
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
0.34/0.35b | 0.35 |
Complexes 1 and 2 adopt a strongly distorted square-pyramidal coordination geometry (see the τ5-parameter in Table 1).29 The Schiff base HLTMS acts as a neutral tridentate ligand binding to copper(II) and zinc(II) via the quinoline moiety nitrogen atom N5, Schiff base nitrogen atom N13 and pyridine nitrogen atom N16, and together with the two chlorido co-ligands Cl1 and Cl2 forms the coordination polyhedron.
Stability, solubility and lipophilicity of HLTMS and complexes 1 and 2 in aqueous solution and their fluorescence properties
The aqueous stability of HLTMS and its metal complexes 1 and 2 was explored by UV-vis spectroscopy at pH 2.0, 7.4 and 11.7. The stability in DMSO was also studied since the stock solutions were prepared in this solvent due to their limited aqueous solubility. HLTMS was found to be stable in DMSO at least for 19 h (Fig. S25†). In aqueous solutions, slight changes of the UV-vis absorption spectra could be observed within a few hours, most probably due to the partial precipitation of HLTMS since the shape of the spectra did not change but only the absorption decreased with time. It is worth noting that no photostability issues were observed during measurements with a fiber optic equipped spectrophotometer operating with a Xe flash lamp. However, irradiation with other light sources, such as a diode array UV-vis photometer or a Xe lamp fluorimeter, induced remarkable spectral changes (Fig. S25†). To avoid photochemical reactions, the fiber optic UV-vis instrumental setup was used and particular attention was paid to the implementation of subsequent fluorometric measurements (see details in the Experimental section). As shown in Fig. 2, the UV-vis spectra recorded for HLTMS at pH 7.4 and 11.7 are practically the same, while this was not the case for the spectrum measured at pH 2. | ||
Fig. 2 UV-vis absorption spectra recorded for HLTMS and its complexes (1, 2) after dissolution (a) in DMSO and at pH (b) 2.0, (c) 7.4 and (d) 11.7 {c = 50 μM; ![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) = 1 cm; T = 25.0 °C}. = 1 cm; T = 25.0 °C}. | ||
Complexes 1 and 2 were also proved to be stable in DMSO for at least 19 h (Fig. S26 and S27†). In addition, the UV-vis spectra were recorded in tetrahydrofuran to confirm that DMSO, as the solvent, does not affect the inner coordination sphere of the metal complexes (Fig. S28†). Complex 1 was also stable in aqueous solution at pH 7.4 (10 mM HEPES) but showed spectral changes under acidic and basic conditions within 19.4 and 5.8 h, respectively. Schiff bases are sensitive to hydrolysis, especially in the presence of certain metal ions. We suppose that slow hydrolytic processes occur with 1. In contrast, complex 2 displayed only minor spectral changes in aqueous solutions (Fig. S27†). Taking a closer look at Fig. 2b, the UV-vis spectrum of the Zn(II) complex at pH 2 is shown to be practically the same as that of HLTMS, namely complex 2 dissociates completely under such acidic conditions. At pH 7.4, 1 and 2 retain their integrity and show no considerable dissociation even at pH 11.7. Thus, the predominant species at pH 7.4 for complex 1 is [Cu(LTMS)]+.
Due to the low aqueous solubility and high lipophilicity of the compounds, the partition constant (P) could not be determined using the traditional n-octanol/water partitioning shake-flask method. The SwissADME tool30 was applied to predict the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for HLTMS and its non-substituted analogue HLH, as well as for complexes 1 and 2 and Cu(II) and Zn(II) complexes with HLH (Table 2). The predictions suggest high log
P values for HLTMS and its non-substituted analogue HLH, as well as for complexes 1 and 2 and Cu(II) and Zn(II) complexes with HLH (Table 2). The predictions suggest high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for all compounds, and the TMS group enhances the lipophilicity of both the proligand and metal complexes.
P values for all compounds, and the TMS group enhances the lipophilicity of both the proligand and metal complexes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for proligands and metal complexes
P values for proligands and metal complexes
| Compound | HLTMS | HLH | Cu(LTMS)Cl2 | Zn(LTMS)Cl2 | Cu(LH)Cl2 | Zn(LH)Cl2 |
|---|---|---|---|---|---|---|
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
+3.51 | +2.94 | +2.95 | +2.96 | +2.40 | +2.40 |
The fluorescence properties of HLTMS and the two metal complexes 1 and 2 were studied in aqueous solution at pH 7.4 and pH 2.0. Due to the photosensitivity of HLTMS, special precautions listed in the Experimental section were taken in order to obtain reasonable spectral data. The fluorescence properties of the crystalline samples were also studied. Complex 1 is not fluorescent in both aqueous solution and in the solid state due to the quenching effect of the paramagnetic Cu(II) ion (d9, S = ½). HLTMS exhibits moderate fluorescence at both pH 7.4 and pH 2.0. The molecule can be excited in the visible wavelength range (λEX (max) = 400 nm (pH 7.4) and 525 nm (pH 2.0)), which is an advantageous feature for fluorescence microscopy. Interestingly, under acidic conditions, HLTMS behaves against the expectations that the same fluorescence emission spectrum is generally observed regardless of the excitation wavelength (Kasha's rule) (Fig. 3a).31 This is likely due to the presence of two isomeric fluorescent species in solution. We assume that these might be the exocyclic (C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N12) and endocyclic (C6
N12) and endocyclic (C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N5) tautomers.
N5) tautomers.
Also the excitation spectra were different for the two distinct emission bands and the characteristic excitation bands of the two species are recognisable in the absorption spectrum of HLTMS at the same pH. As expected, at pH 2, the fluorescence profile of 2 closely resembled that of HLTMS, as the complex dissociated under strong acidic conditions. At pH 7.4, the behaviors of both HLTMS and 2 are different (Fig. 3b). The excitation spectra of HLTMS and 2 are very similar to the UV-vis absorption spectra of the respective compound at the same pH. The emission maximum of 2 is red shifted compared to HLTMS, and both species emit light in the visible range. Both solid samples are also fluorescent. HLTMS shows two emission bands and this compound can be excited over a wide range (λEX = 250–450 nm), while 2 emits at somewhat shorter wavelengths and is excitable between 250–470 nm (Fig. 3c).
Cell-free ROS generation and redox activity
The ability of HLTMS, 1 and 2 to generate ROS in solution in the presence of H2O2 was studied by the indirect technique of electron paramagnetic resonance (EPR) spectroscopy and spin trapping technique. The investigated samples were dissolved in a 50% (v/v) mixture of DMSO/H2O and mixed with the spin trapping agent 5,5-dimethyl-1-pyrroline N-oxide (DMPO) in air. No EPR signal was observed for the DMSO/H2O/DMPO mixture as a reference (black trace in Fig. 4). First, the ability of 1 to generate ROS was investigated in the presence of ascorbate (Asc) as a natural reducing agent, which was added to the reaction mixture in the absence and presence of DMPO. The red trace in Fig. 4 represents the EPR spectrum measured 2 min after the addition of an excess of Asc to the solution of 1 in the absence of DMPO. This signal can be unambiguously attributed to the ascorbyl radical Asc˙ (aH = 0.177 mT, g = 2.0054) formed by the reduction of 1 with Asc. Changes in the UV-vis spectra of 1 were observed after the addition of Asc to Cu(II) complex 1 dissolved in the DMSO/H2O mixture, providing further evidence for the reaction of Asc with this complex (Fig. 5a).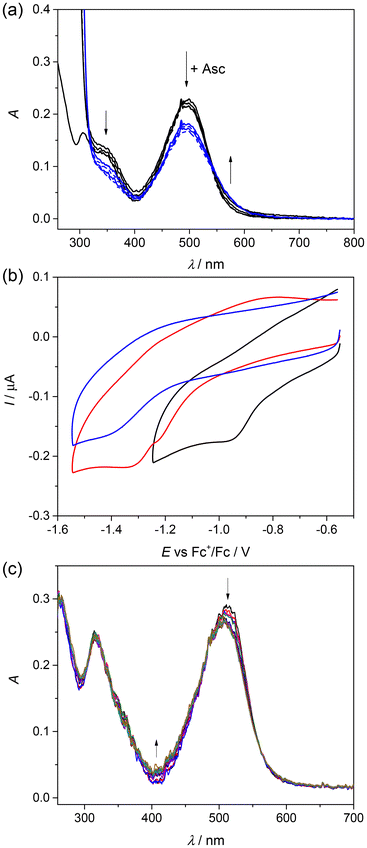 | ||
| Fig. 5 (a) Changes in the UV-vis spectra of 1 in a 50% (v/v) DMSO/H2O mixture measured in 10 s intervals after addition of 10 equiv. (black lines) and 30 equiv. (blue lines) of Asc; (b) cyclic voltammograms of 1 (black trace), 2 (red trace) and HLTMS (blue trace) in DMSO/0.1 M nBu4NPF6 on a Pt disc working electrode, scan rate 100 mV s−1; (c) a decrease of the absorption band at 516 nm was observed upon cathodic reduction of 1 in DMSO/0.2 M nBu4NPF6 at the first reduction peak when a honeycomb microstructured Pt working electrode was used. For the UV-vis spectra of HLTMS, 1 and 2, see Fig. 2. | ||
Since the formation of hydroxyl radicals via the Fenton-type reaction requires H2O2, the DMSO/H2O solutions of HLTMS, 1 and 2 were mixed with DMPO as the spin trapping agent under aerobic conditions and the EPR spectra were recorded after the addition of Asc and H2O2. The EPR spectra of the prepared reaction mixture 1/DMPO/Asc were recorded 2 min after the addition of H2O2 to the system. As seen in Fig. 4, complex 1 induced strong ROS generation along with the formation of reactive radical intermediates, as evidenced by the presence of the dominating six-line EPR signal (blue trace in Fig. 4) in the in vitro cell-free measurement. This EPR signal can be assigned to the spin-adduct ˙DMPO-CH3 (aN = 1.59 mT, aHβ = 2.28 mT; g = 2.0055), originating from the reactions of ROS with DMSO. The rapid reaction of hydroxyl radicals with DMSO produced methyl radicals ˙CH3, detectable via the reaction with DMPO as the corresponding carbon-centered spin-adduct. It should be noted that much smaller amounts of radicals were formed in the absence of Asc (see the magenta trace in Fig. 4) indicating the important role of the natural reducing agents to produce ROS.
No EPR signal was observed for the DMSO/H2O/DMPO/H2O2 mixture as a reference (not shown). For Zn(II) complex 2 and the corresponding ligand HLTMS, the formation of both ascorbyl radicals Asc˙ and ˙DMPO-CH3 spin adducts was observed in the presence of Asc and H2O2 (see the green and dark blue traces, respectively, in Fig. 4). However, the amount of spin adducts is substantially smaller, which indicates the important role of the metal redox-activity for ROS generation.
The redox activity of all samples was tested by cyclic voltammetry in DMSO (due to the low aqueous solubility). All three samples were redox active in the cathodic part, as shown in Fig. 5b. The easiest reduction was confirmed for Cu(II) complex 1 (Epc = –0.96 V vs. Fc+/Fc), while the most negative reduction potential was observed for the corresponding proligand HLTMS (Epc = –1.42 V vs. Fc+/Fc). The Zn(II) complex 2 also exhibited peaks at more negative reduction potentials (Epc = –1.23 V and −1.33 V vs. Fc+/Fc) compared to Cu(II) complex 1; however, this redox activity is most probably ligand-centered. A decrease of the absorption band at 516 nm was observed during the cathodic reduction of 1 in DMSO/0.2 M nBu4NPF6 (Fig. 5c) similar to that observed in the experiments with sodium ascorbate.
Photoinduced processes monitored by the EPR spin trapping technique
Molecules with a quinoline-based scaffold can behave as photosensitizers,32–34 so attention was also focused on the examination of the behavior of the compounds reported herein upon UV exposure (LED@365 nm) utilizing the EPR spin trapping technique. Fig. S34a–c† show the time-courses of the EPR spectra monitored upon LED@365 nm exposure for HLTMS, 1 and 2 in aerated DMSO solution in the presence of the DMPO spin trap. In all studied systems, the generation of EPR signals of the corresponding spin-adducts of various intensities upon exposure was detected. The photoactivation of the HLTMS/DMPO/DMSO/air system led to the generation of several spin-adducts, namely ˙DMPO-O2−/OOH (relative concentration, crel = 45%), ˙DMPO-OCH3 (crel = 34%), ˙DMPO-OR (crel = 15%) and ˙DMPO-CR* (crel = 7%) as shown in Fig. 6a. The presence of ˙DMPO-O2−/OOH in the system indicates the interaction of the excited states of HLTMS with molecular oxygen via the photoinduced electron transfer process (photooxidation I process), forming the superoxide radical anion. The second major spin-adduct, ˙DMPO-OCH3, is attributed to the methoxy radical ˙OCH3, added on the DMPO moiety, which may be formed via several pathways. The photoexcited ligand or superoxide radical anion interacts with the DMSO molecule affording the methyl radical ˙CH3, which immediately reacts with molecular oxygen to give the unstable ˙OOCH3 radical. This species is highly reactive, and quickly disproportionates to the methoxy radical with the consequent addition on the DMPO spin trap or reaction with DMPO with the formation of transient ˙DMPO-OOCH3, which rapidly transforms into ˙DMPO-OCH3. The two other spin-adducts ˙DMPO-OR and ˙DMPO-CR* are assigned to paramagnetic species added on DMPO and generated via interaction with DMSO.35,36The spin Hamiltonian parameters, the hyperfine coupling constants (hfcc) and g-values obtained from the simulation analysis are summarized in Table 3.
| Spin-adduct | a N (mT) | a H β (mT) | a H γ (mT) | g-Value |
|---|---|---|---|---|
| DMSO | ||||
| ˙DMPO-O2− | 1.283 ± 0.006 | 1.035 ± 0.010 | 0.140 ± 0.001 | 2.0058 |
| ˙DMPO-OCH3 | 1.321 ± 0.004 | 0.785 ± 0.005 | 0.166 ± 0.006 | 2.0058 |
| ˙DMPO-OR | 1.194 ± 0.062 | 1.465 ± 0.033 | — | 2.0058 |
| ˙DMPO-CR* | 1.483 ± 0.019 | 1.844 ± 0.054 | — | 2.0057 |
50% (v/v) DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) H
2
O H
2
O
|
||||
| ˙DMPO-O2− | 1.137 ± 0.005 | 1.086 ± 0.001 | 0.131 ± 0.004 | 2.0059 |
| ˙DMPO-OR | 1.412 ± 0.004 | 0.968 ± 0.020 | 0.152 ± 0.004 | 2.0059 |
| ˙DMPO-OH | 1.460 ± 0.005 | 1.359 ± 0.005 | 0.043 ± 0.005 | 2.0059 |
| ˙DMPO-CH3 | 1.570 ± 0.040 | 2.200 ± 0.011 | — | 2.0058 |
The photoexcitation of complex 1 in aerated DMPO/DMSO solution led to the generation of the same species (˙DMPO-O2−/OOH, crel = 65%; ˙DMPO-OCH3, crel = 27%; ˙DMPO-OR, crel = 3%; ˙DMPO-CR*, crel = 7%), but the intensity of the EPR signal is significantly lower. Here the concurrent reaction – reduction of Cu(II) via photoinduced electron – may occur, causing the decrease of the EPR signal.33 This is also supported by fluorescence experiments, where due to the quenching effect of the paramagnetic Cu(II) ion, complex 1 was found to be not fluorescent. The LED@365 nm exposure of the aerated reaction system 2/DMPO/DMSO resulted in the immediate formation of several spin-adducts (Fig. 6 and Fig. S29c†). The dominant species formed is ˙DMPO-OCH3 (crel = 61%), and the other species are attributed to ˙DMPO-O2−/OOH (crel = 30%) and ˙DMPO-CR* (crel = 9%). Here we suggest the main pathway via reactions of the photoexcited complex with DMSO to afford the methyl radical ˙CH3 (with the following cascade reaction to ˙DMPO-OCH3) and the dimsyl radical ˙CH2(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CH3, which is added to DMPO as ˙DMPO-CR*.36 This also correlates well with the relatively high fluorescence activity of 2 as was discussed above. The analogous experiments were performed also in the mixed solvent 50% (v/v) DMSO
O)CH3, which is added to DMPO as ˙DMPO-CR*.36 This also correlates well with the relatively high fluorescence activity of 2 as was discussed above. The analogous experiments were performed also in the mixed solvent 50% (v/v) DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) H2O. The intensity of EPR signals was significantly lower in comparison with the spectra obtained in pure DMSO (Fig. 6b) and this may be caused by the decomposition of the superoxide radical anion, as well as by the decreased stability of the spin-adduct ˙DMPO-O2−/OOH in the presence of water.33,35 In the HLTMS and complex 2 reaction system upon LED@365 nm exposure, the following radicals are present: ˙DMPO-O2−/OOH (crel ≈ 50%) and ˙DMPO-OCH3 (crel ≈ 47%) and the spin-adduct attributed to ˙DMPO-CH3 in a minor concentration (crel = 3%; the hfcc and g-values are presented in Table 3). The situation was different in the presence of complex 1, where in the reaction system the dominant species was ˙DMPO-OH (crel = 77%). The increased formation of the hydroxyl radical spin-adduct agrees well with the photodecomposition of hydrogen peroxide formed via superoxide radical anion dismutation as well as the complex reaction mechanism of Cu(II) with H2O2.34 Additionally, the reaction of DMSO with hydroxyl radicals generates methyl radicals, detected as the low-intensity six-line signal of the ˙DMPO-CH3 spin-adduct.33 These observations confirmed the photochemical activity of the indolo[2,3-c]quinoline ligand HLTMS and its metal complexes 1 and 2, indicating that they are potential candidates as photosensitizers for photodynamic therapy.37
H2O. The intensity of EPR signals was significantly lower in comparison with the spectra obtained in pure DMSO (Fig. 6b) and this may be caused by the decomposition of the superoxide radical anion, as well as by the decreased stability of the spin-adduct ˙DMPO-O2−/OOH in the presence of water.33,35 In the HLTMS and complex 2 reaction system upon LED@365 nm exposure, the following radicals are present: ˙DMPO-O2−/OOH (crel ≈ 50%) and ˙DMPO-OCH3 (crel ≈ 47%) and the spin-adduct attributed to ˙DMPO-CH3 in a minor concentration (crel = 3%; the hfcc and g-values are presented in Table 3). The situation was different in the presence of complex 1, where in the reaction system the dominant species was ˙DMPO-OH (crel = 77%). The increased formation of the hydroxyl radical spin-adduct agrees well with the photodecomposition of hydrogen peroxide formed via superoxide radical anion dismutation as well as the complex reaction mechanism of Cu(II) with H2O2.34 Additionally, the reaction of DMSO with hydroxyl radicals generates methyl radicals, detected as the low-intensity six-line signal of the ˙DMPO-CH3 spin-adduct.33 These observations confirmed the photochemical activity of the indolo[2,3-c]quinoline ligand HLTMS and its metal complexes 1 and 2, indicating that they are potential candidates as photosensitizers for photodynamic therapy.37
Cytotoxicity assays
The anticancer potential of the proligand HLTMS and metal complexes 1 and 2 was evaluated along with the selectivity towards specific types of cancers: leukemia, non-small cell lung (NSCLC), colon, central nervous system (CNS), melanoma, ovarian, renal, prostate and breast cancers, by the National Cancer Institute's Developmental Therapeutics Program. This study involved the NCI 60 human tumor cell line panel.38 One-dose (10 μM) assays indicate that HLTMS, as well as complexes 1 and 2, are highly cytotoxic (see Fig. S30–S32 in the ESI†). Five-dose (0.01 μM–100 μM) concentrations were applied for all three compounds and the data were used to calculate the 50% growth inhibition values of the tested cells (GI50), the total growth inhibition (TGI), and lethal dose concentration inducing 50% cell death (LC50). The GI50 data in μM are presented in Table 4. The 5-dose screen curves and mean graphs for HLTMS, 1 and 2 are shown in Fig. S33–S38 in the ESI.†The results indicate that all three compounds HLTMS, 1 and 2 exhibit strong inhibitory effects on cancer cell growth against the majority of cell lines within the panel. For the most active compound Cu(HLTMS)Cl2 (1), the average GI50, TGI, and LC50 concentrations across all cell lines in the panel were 0.181 μM, 3.07 μM, and 13.9 μM, respectively. For comparison, the Schiff base HLTMS was less active with the respective values of 0.518 μM, 12.1 μM and 63.9 μM, indicating that the two species might have distinct mechanisms of action.
A comparison of the GI50 values for different types of cancer cells indicates that the colon cancer cell lines, four of seven ovarian cancer cell lines, the non-small-cell lung cancer (NSCLC) cells and the central nervous system (CNS) tumor cells are the most sensitive to drug 1. In comparison, the renal cancer cell line A498 and the breast cancer cell line HS578T are the most resistant to all three compounds HLTMS, 1 and 2. In most of the cases, complex formation with Zn(II) decreased or maintained the cytotoxicity with some exceptions (RPMI-8226, HOP-92, NCI-H322M, SNB-75 and MALME-3M), while complex formation with Cu(II) enhanced or preserved the antiproliferative activity exhibited by HLTMS in all cell lines tested, except HT29 and KM12.
In order to select the appropriate concentration of the Schiff base HLTMS, its Cu(II) complex 1 and Zn(II) complex 2 for the subsequent biological experiments, first we measured the viability of A549 and MCF-7 human tumor cells using the (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)) assay upon treatments. Fig. 7 shows that HLTMS, Cu(II) complex 1, and Zn(II) complex 2 all exhibited high toxicity on both tumor cells at each tested concentration since they decreased significantly the viability of A549 and MCF-7 cells compared to the untreated control samples. At lower concentrations, HLTMS was more or similarly toxic to its metal complexes; however, at higher concentrations, complex 1 showed significantly elevated toxicity when compared to 2 or HLTMS in A549 and MCF-7 cell lines. According to our results, breast adenocarcinoma MCF-7 cells were more sensitive to the toxic effect of all compounds than A549 cells (Fig. 7). The determined IC50 values (μM ± SD) of HLTMS and its complexes 1 and 2 on both cell lines are displayed in Table 5. Cisplatin was used as the positive control, and according to our results, the synthesized Zn(II) and Cu(II) complexes are as cytotoxic to A549 and MCF-7 cells as this ‘gold standard’ drug.
 | ||
| Fig. 7 Cell viability of A549 and MCF-7 adenocarcinoma cells upon HLTMS, complex 1, and complex 2 treatments. Two-way ANOVA, Dunnett's multiple comparisons test, P value: # <0.0001. | ||
| IC50 values (μM) | ||||
|---|---|---|---|---|
| Cell lines | HLTMS | 1 | 2 | Cisplatin |
| A549 | 3.23 ± 1.28 | 6.33 ± 1.08 | 6.77 ± 1.07 | 5.39 ± 0.84 |
| MCF-7 | 1.43 ± 1.08 | 1.59 ± 1.37 | 2.57 ± 1.29 | 2.11 ± 0.50 |
| NIH-3T3 | >25 | 12.58 ± 1.04 | >25 | n.d. |
To evaluate the selectivity of HLTMS, 1 and 2 for cancer cells, their cytotoxicity on non-cancerous NIH-3T3 fibroblasts was also tested. The results summarized in Table 5 indicate that the healthy NIH-3T3 cells are less sensitive to HLTMS and 2, since the IC50 values were significantly higher than in the case of cancerous cells. Only compound 1 showed some toxicity in the NIH-3T3 cells, but the calculated IC50 value in this case again was one magnitude higher or twice as high as the IC50 values obtained on MCF-7 and A549 cells, respectively. So, the tested compounds, especially HLTMS and 2, demonstrate cancer cell-selectivity.
The mechanism of action behind the toxicity of compounds
Since the cell-free in vitro tests suggested the possibility of increased production of reactive oxygen species by HLTMS, 1 and 2, we aimed to evaluate whether the compounds induce disturbance in mitochondrial function.We monitored the changes in mitochondrial membrane potential by JC-1 staining following treatments with HLTMS, 1 and 2 (Fig. 8). The reduction of the red/green fluorescence ratio indicates a compromised mitochondrial membrane potential and thus mitochondrial function damage. As shown in Fig. 9, red/green fluorescence ratios were significantly decreased upon all the treatments compared to the control samples in both A549 and MCF-7 cells. The most significant mitochondrial dysfunction was found in MCF-7 cells exposed to Cu(HLTMS)Cl2 (1).
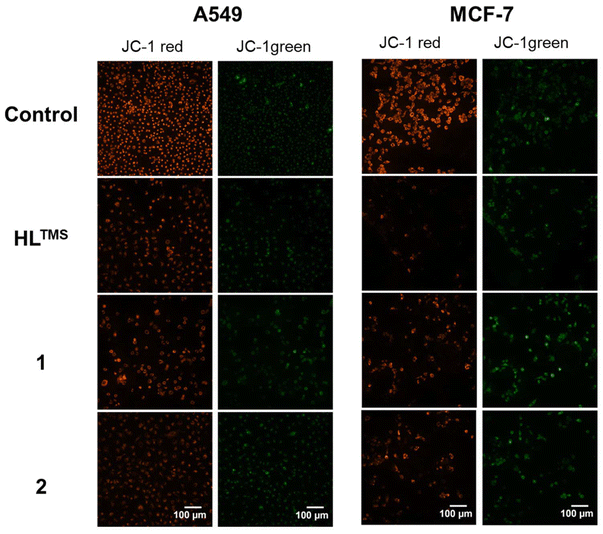 | ||
| Fig. 8 Representative fluorescence images of A549 and MCF-7 cells treated with HLTMS, 1 or 2 and stained with JC-1. | ||
Conclusion
We found out that the proligand HLTMS builds strongly distorted square-pyramidal Cu(II) and Zn(II) complexes, in which the organic ligands are tridentate, while the other two coordination sites are occupied by two chlorido co-ligands. Even though the SC-XRD and ED structures were of different quality, being in some cases affected by disorder, and measured at different temperatures, one can state that the coordination geometry is actually the same and independent of the metal.The Zn(II) complex with HLTMS (2) was found to transform into a complex of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry in the mass-spectrometer as evidenced by the presence of peaks attributed to singly charged and doubly charged ions, [Zn(HLTMS)(LTMS)]+ and [Zn(HLTMS)2]2+, respectively. This feature presumably affects also the solution behavior in DMSO, as equilibrium was achieved only in about one week and fully shifted to the complex of 1
2 stoichiometry in the mass-spectrometer as evidenced by the presence of peaks attributed to singly charged and doubly charged ions, [Zn(HLTMS)(LTMS)]+ and [Zn(HLTMS)2]2+, respectively. This feature presumably affects also the solution behavior in DMSO, as equilibrium was achieved only in about one week and fully shifted to the complex of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, and the 1H and 13C NMR spectra became interpretable.
1 stoichiometry, and the 1H and 13C NMR spectra became interpretable.
The attachment of TMS to the proligand backbone increased the lipophilicity. The protonation state of HLTMS corresponds to the neutral form at physiological pH, and metal complexes 1 and 2 were also stable at this pH. Both the proligand and complexes were stable in DMSO.
Both HLTMS and 2 were found to generate ROS under cell-free conditions and this was in accordance with their redox activity established by cyclic voltammetry. The disclosed photochemical activity of the indolo[2,3-c]quinoline-based proligand HLTMS and its Cu(II) and Zn(II) complexes 1 and 2 implies their potential as photosensitizers for photodynamic therapy.
The evaluation of the anticancer potential of HLTMS, 1 and 2 in the NCI-60 human tumor cell line panel revealed their strong inhibitory effects against the majority of cell lines. For the most active complex Cu(HLTMS)Cl2 (1), the average GI50, TGI and LC50 values across all cell lines in the panel (0.181 μM, 3.07 μM, and 13.9 μM, respectively) were superior to those of HLTMS, indicating their distinct mechanisms of action and the favorable effect of Cu(II). With several exceptions, complex formation with Zn(II) diminished or did not alter the cytotoxicity of HLTMS. JC-1 staining following treatments with HLTMS, 1 and 2 showed that all compounds induce mitochondrial disturbance of membrane potential contributing to their toxicity.
We can conclude that silylation in combination with the introduction of functional groups enhancing the solubility of both the proligand and metal complexes might allow full exploration of the potential of these kinds of compounds as photosensitizers in photodynamic therapy and investigate their cellular uptake and intracellular localization by fluorescence microscopy.
Experimental part
Chemicals
Pd2dba3, JohnPhos, hexamethyldisilane, DMPU, dimethyl sulfoxide, sodium dodecyl sulfate, CF3COOH, Pd(OAc)2, CuCl2·2H2O, and ZnCl2·2H2O were purchased from commercial suppliers and used as received.Synthesis of the Schiff base HLTMS and complexes 1 and 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 6
ethyl acetate 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 as the eluent to give a white solid, which was further re-crystallized in hexane
4 as the eluent to give a white solid, which was further re-crystallized in hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 1
ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (45 mL) due to the traces of benzaldehyde as impurity. Yield: 2.29 g, 88%. 1H NMR (500 MHz, DMSO-d6), δ, ppm: 10.07 (s, 1H), 7.91–7.82 (m, 1H), 7.71 (d, J = 8.6 Hz, 3H), 7.61–7.54 (m, 3H), 7.45 (d, J = 8.0 Hz, 1H), 7.36 (dd, J = 7.7, 1.4 Hz, 1H), 7.26 (td, J = 7.6, 1.0 Hz, 1H), 1.76 (s, 3H).
1 (45 mL) due to the traces of benzaldehyde as impurity. Yield: 2.29 g, 88%. 1H NMR (500 MHz, DMSO-d6), δ, ppm: 10.07 (s, 1H), 7.91–7.82 (m, 1H), 7.71 (d, J = 8.6 Hz, 3H), 7.61–7.54 (m, 3H), 7.45 (d, J = 8.0 Hz, 1H), 7.36 (dd, J = 7.7, 1.4 Hz, 1H), 7.26 (td, J = 7.6, 1.0 Hz, 1H), 1.76 (s, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 6
ethyl acetate 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 as the eluent to give a light yellow oil. Yield: 896 mg, 40%. 1H NMR (500 MHz, DMSO-d6) δ, ppm: 10.05 (s, 1H), 7.65 (d, J = 5.3 Hz, 4H), 7.59–7.50 (m, 2H), 7.38–7.34 (m, 1H), 7.27–7.21 (m, 1H), 1.76 (d, J = 8.5 Hz, 3H), 0.28 (s, 9H). ESI-MS (acetonitrile/methanol + 1% water), negative: m/z 309.95 [M–H]− (calcd m/z for [(C18H21NO2Si − H+)]− 310.13).
4 as the eluent to give a light yellow oil. Yield: 896 mg, 40%. 1H NMR (500 MHz, DMSO-d6) δ, ppm: 10.05 (s, 1H), 7.65 (d, J = 5.3 Hz, 4H), 7.59–7.50 (m, 2H), 7.38–7.34 (m, 1H), 7.27–7.21 (m, 1H), 1.76 (d, J = 8.5 Hz, 3H), 0.28 (s, 9H). ESI-MS (acetonitrile/methanol + 1% water), negative: m/z 309.95 [M–H]− (calcd m/z for [(C18H21NO2Si − H+)]− 310.13).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (30 mL). The organic phase was dried over magnesium sulfate and concentrated in vacuo to give a yellow solid. Yield: 180 mg, 99%. 1H NMR (500 MHz, DMSO-d6), δ, ppm: 13.72 (s, 1H), 12.05 (s, 1H), 8.63–8.51 (m, 2H), 7.96 (s, 1H), 7.89–7.83 (m, 1H), 7.53 (qd, J = 7.2, 5.6 Hz, 2H), 7.48 (d, J = 8.0 Hz, 1H), 0.34 (s, 9H).
1 (30 mL). The organic phase was dried over magnesium sulfate and concentrated in vacuo to give a yellow solid. Yield: 180 mg, 99%. 1H NMR (500 MHz, DMSO-d6), δ, ppm: 13.72 (s, 1H), 12.05 (s, 1H), 8.63–8.51 (m, 2H), 7.96 (s, 1H), 7.89–7.83 (m, 1H), 7.53 (qd, J = 7.2, 5.6 Hz, 2H), 7.48 (d, J = 8.0 Hz, 1H), 0.34 (s, 9H).
Physical measurements
Elemental analysis was carried out with a Carlo–Erba microanalyzer at the Microanalytical Laboratory at the Faculty of Chemistry, University of Vienna. The samples for electrospray ionization mass spectrometry (ESI-MS) were measured using an amaZon speed ETD Bruker instrument. The expected and experimental isotope distributions were compared. 1D (1H, 13C) and 2D (1H-1H COSY, 1H-13C HSQC, 1H-13C HMBC) NMR spectra were acquired on a Bruker AV NEO 500 or AV III 600 spectrometer in DMSO-d6 at 25 °C. The NMR spectra for Zn(II) complex 2 were recorded on a Bruker Avance NEO spectrometer operating at 400 MHz, with a 5 mm probe for direct detection of 1H, 13C, 19F, and 29Si. DMSO-d6 was used as the solvent and the spectra were calibrated on the residual peak of the solvent (δ 2.50 ppm for 1H and 39.50 ppm for 13C). The spectra were recorded at room temperature using the standard parameter sets provided by Bruker.Crystallographic structure determination
SC-XRD data for 1 were collected with a Stadivari Diffractometer (STOE & Cie GmbH, Germany) equipped with an EIGER2 R500 detector (Dectris Ltd, Switzerland). Data were processed and scaled with the STOE software suite X-Area (STOE & Cie GmbH). Crystal data, data collection parameters, and structure refinement details are given in Table S2.† The structure was solved by direct methods and refined by full-matrix least-squares techniques. Non-H atoms were refined with anisotropic displacement parameters. H atoms were inserted in calculated positions and refined with a riding model. The following computer programs and hardware were used: structure solution, SHELXT39 and refinement, SHELXL;40 molecular diagrams, ORTEP;41 computer, Intel CoreDuo. CCDC 2378782 (1).†Sample preparation and data collection for complex 2 by ED
Crystals were provided as a suspension in MeCN. The vial was sonicated for 10 min in order to generate a suspension of small, evenly sized crystals. A droplet of the resulting suspension was placed onto a TEM grid featuring a lacey carbon film (copper, Ted Pella, 200-mesh). The evaporation of acetonitrile was observed with a light microscope. The grid was then transferred to an ELSA698 tomography holder (Gatan INC) at room temperature and inserted into a JEM2100Plus TEM (200 keV LaB6, JEOL). The TEM was equipped with a 1024 × 1024 pixel SINGLA detector, featuring a 320 μm thick silicon sensor and a pixel size of 75 μm. Diffraction patterns were recorded by irradiating crystalline grains of ∼1–2 μm in length with the electron beam with a rotation from ∼−70° to 70°, i.e. the maximum possible rotation range of the goniometer of the JEM2100Plus. Data were processed with XDS.42 Five datasets measured with different crystal grains were merged to improve the completeness. The detector distance for each dataset was refined during the CORRECT step of the XDS program to improve the accuracy of the unit cell parameters. The structures were solved by employing SHELXT39 for ab initio phasing, followed by refinement and model building using SHELXL and ShelXle, respectively.40,43 Scattering factors were fitted in Cromer–Mann parametrisation f(s) = sum (i = 1…4) a_i exp(−b_i * s2) + c against the values tabulated in Table 4.2.6.8 of the International Tables of Crystallography, Volume C,44 starting from the parameters published by Peng 1999.45 Hydrogen atoms were placed in riding positions at inter-nuclear distances.46 CCDC 2378723 (2).†Spectrophotometric titrations and solubility tests
The UV-vis spectra for the proligand and complexes were recorded using an Agilent Cary 3500 8-path, fibre optic photometer equipped with a Xe-flash lamp light source. Additionally, an Agilent Cary 8454 diode array spectrophotometer was also applied for photostability assay. The path length was 1 cm. Stock solutions were prepared in DMSO (VWR International, Hungary) in ca. 5 mM concentration. Samples for spectroscopic measurement were diluted with aqueous HCl (pH 2.0); NaOH solution (pH 11.7 ) or 10 mM solution of 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) (pH 7.4). The final DMSO content was ≤1% (v/v) in the samples.Fluorescence measurements were implemented with a Fluoromax Plus fluorimeter (Horiba, Jobin Yvon). Since the aqueous solution of the ligand (and the Zn complex) showed photodegradation under the usual fluorometric conditions (excitation slit: 3–5 nm), we have therefore taken the following precautions: (i) every sample was scanned in the fluorimeter only once; for a new recording, a new portion was poured into the cuvette; (ii) a narrow circular window (diameter: 1 mm) and (iii) a neutral density filter (A = 1) were placed between the light source and the cuvette; and (iv) concomitantly the emission slit was opened wider (7–12 nm) in order to obtain reasonable spectra. This way, the loss of intensity was less than 15% during repeated measurements. Solid samples (fine crystalline compound) were measured in the solid sample holder, suitable for front-face luminescence assays, at 120° angle to the light source. The respective long pass filters (370, 400, 450 or 500 nm) were applied on the emission side in order to reduce light scattering.
Cell-free ROS generation and redox activity
EPR measurements were performed using an X-band Bruker EMX spectrometer (Germany). The spin trapping agent DMPO (Sigma-Aldrich) was distilled before use (70 Pa; 80–90 °C). Standard settings during EPR spin trapping experiments were microwave frequency ∼9.448 GHz; power of the microwave radiation 2 mW; sweep width 200 G; centre field 3350 G; modulation amplitude 2 G; time constant 20.5 ms; and sweep time 42 s, and a series of 10 spectra with 3 scans for each measurement. Optical spectra in dimethyl sulfoxide (DMSO, SeccoSolv max. 0.025% H2O, Merck) were collected on a Shimadzu 3600 UV-vis-NIR spectrometer (Japan). Experiments with sodium ascorbate and spectroelectrochemical studies were performed on a UV-vis-NIR Avantes spectrometer (Model AvaSpec-2048_14-USB2). The cyclic voltammograms (CV) of the investigated samples were recorded by using 0.1 M tetrabutylammonium hexafluorophosphate (nBu4NPF6, puriss quality from Fluka) as a supporting electrolyte and a single compartment electrochemical cell with a working Pt-disk electrode (from Ionode, Australia), a Pt wire as a counter electrode and an Ag wire as a pseudoreference electrode at a scan rate of 100 mV s−1. All potentials in voltammetric studies were quoted vs. ferricenium/ferrocene (Fc+/Fc) redox couple (purchased from Sigma-Aldrich). A Heka PG310USB (Lambrecht, Germany) potentiostat with the PotMaster 2.73 software package served as the potential control in voltammetric studies.Photoinduced processes monitored by EPR spin trapping
The paramagnetic intermediates generated upon UV-light exposure of the studied samples were obtained at room temperature (295 K) under air employing an EPR spectrometer EMXPlus (Bruker, Germany) operating at 100 kHz field modulation using the high sensitivity probe-head with a small quartz flat cell (WG 808-Q, Wilmad-LabGlass, optical cell length 0.045 cm). Standard settings during EPR spin trapping experiments were: microwave frequency ∼9.43 GHz; power of the microwave radiation 10.7 mW; magnetic field sweep width 10.0 mT; centre field 336.0 mT; modulation amplitude 0.1 mT; time constant 5.12 ms; sweep time 30 s, and number of scans NS = 10. The solutions of the studied samples, prepared in DMSO (anhydrous, ≥99% H2O, Merck) or mixed solvent with ultra-pure water, containing the spin trap DMPO were mixed directly before the EPR measurements, carefully aerated by a gentle air stream, and immediately transferred to the flat cell. The samples were irradiated directly in the EPR resonator (High Sensitivity Probe-head, Bruker), and the EPR spectra were recorded in situ. The g-values were determined using the built-in magnetometer. The irradiation source was a UV LED monochromatic radiator (LED@365 nm, Hönle, Germany). The value of the UVA irradiance (LED@365 nm), determined using a UVX radiometer (UVP, USA) within the EPR cavity, was 18 mW cm−2. All EPR experiments were performed at least in duplicate. EPR spectra acquisition started 2 min after experimental system mixing. The experimental EPR spectra were analyzed using WinEPR software (Bruker), while the calculations of spin-Hamiltonian parameters and relative concentrations of individual spin-adducts were performed with the EasySpin toolbox working on the MatLab® platform.47NCI-60 screening
The NCI-60 SRB assay was performed as described elsewhere.48 The GI50 values (the concentration of the metal complex causing 50% growth inhibition), TGI value (the concentration of the complex causing 0% cell growth), and LC50 (the concentration of the complex causing 50% cell death) were interpolated from dose–response curves that were plots of percentage cell growth vs. concentration of compounds assayed.Cell culture
The biological effects of the metal-free Schiff base HLTMS, and Cu(II) and Zn(II) complexes 1 and 2 were tested on two human adenocarcinoma cell lines, A549 lung and MCF-7 breast carcinoma cells. Both cell lines were obtained from ATCC and maintained in RPMI 1640 cell culture media (Biosera, Cholet, France) complemented with 10% fetal bovine serum (FBS, Biosera, Cholet, France), 2 mM L-glutamine (Biosera, Cholet, France), 0.01% streptomycin and 0.005% penicillin (Biosera, Cholet, France). They were cultured under standard conditions in a 37 °C incubator under 5% CO2 and 95% humidity. In addition, non-cancerous mouse fibroblast cells (NIH/3T3) were also used and maintained under similar conditions to the cancer cells.MTT assay
To test the effect of HLTMS, and complexes 1 and 2 on the viability of A549 and MCF-7 tumor cells and the NIH/3T3 non-cancerous cells, the MTT assay was performed. For this, 1 × 104 cells per well were seeded into 96-well plates and left to grow. On the following day, the cells were exposed to increasing concentrations (0; 1.56; 3.125; 6.25; 12.5, and 25 μM) of compounds for 24 h. After the treatments, cells were washed with PBS and then incubated with 0.5 mg mL−1 MTT reagent (Sigma-Aldrich, St Louis, Missouri, USA) at 37 °C for 1 h. After incubation, 100–100 μL of DMSO (Molar Chemicals, Halásztelek, Hungary) was added to the wells, and the absorbance of the samples was measured at 570 nm using a Synergy HTX plate reader (BioTek, Winooski, Vermont, USA). The viability measurements were repeated three times using 3 independent biological replicates.Analysis of mitochondrial membrane potential by JC-1 staining
To test whether the treatments cause mitochondrial damage and changes in mitochondrial membrane potential, JC-1 staining was performed. For the assay, 1 × 105 cells per well were seeded onto glass coverslips (VWR International, Radnor, Pennsylvania, USA) placed in 24-well plates. On the following day, cells were treated with 5 μM metal-free ligand HLTMS or metal complexes 1 or 2 for 24 h. After the treatments, the cells were washed twice in PBS, and JC-1 dye (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was added in 10 μg mL−1 final concentration diluted in cell culture media. After 15 min of incubation, the cells were washed with PBS three times, then the fluorescence of the samples was detected using an OLYMPUS BX51 microscope with an Olympus DP70 camera, and the red and green fluorescence intensity was quantified using ImageJ software. These experiments were also repeated three times with 3 biological replicates.Author contributions
Christopher Wittmann – data curation; formal analysis; investigation; and methodology. Iuliana Besleaga – data curation; formal analysis; and methodology. Soheil Mahmoudi – software; data curation; and investigation. Oleg Palamarciuc − investigation. Mihaela Balan-Porcarasu − formal analysis and investigation. Mihaela Dascalu − formal analysis and data curation. Sergiu Shova − visualization and investigation. Maria Cazacu − formal analysis and writing – original draft. Mónika Kiricsi − investigation and writing – original draft. Nóra Igaz − investigation. Orsolya Dömötör − investigation and writing – original draft. Éva A. Enyedy − writing – original draft; writing – review and editing; funding acquisition and resources. Dana Dvoranová − investigation and writing – original draft. Peter Rapta – investigation; methodology and writing – original draft. Vladimir B. Arion – conceptualization; funding acquisition; investigation; project administration and writing – review and editing.Data availability
The data supporting this article have been included as part of the ESI.†Crystallographic data for compounds 1 and 2 have been deposited at the CCDC under accession numbers 2378782 and 2378723.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a grant of the Ministry of Research, Innovation and Digitalization, project no. PNRR-III-C9-2023-I8-99/31.07.2023 within the National Recovery and Resilience Plan (Romania), by the Austrian Science Fund (FWF) via the grant no. P31293-N37 and was partially funded by the Slovak Research and Development Agency (SRDA) under the contract No. APVV-23-0195 and VEGA 1/0422/24. V. B. A. was supported by the Distinguished Guest Scientist Fellowship Program of the Hungarian Academy of Sciences. Project no TKP2021-EGA-32 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme. The authors also thank the Developmental Therapeutics Program of the National Cancer Institute for performing cytotoxicity studies with selected compounds in the 60 cancer cell line panel. We thank Prof. Tim Gruene for electron diffraction measurements.References
- https://www.scientificupdate.com/webinar_events/flipping-the-silicon-switch-and-healing-the-si-c-k-a-novel-strategy-in-medicinal-chemistry/20201201/ .
- J. Adams and M. Kauffman, Cancer Invest., 2004, 22, 304–311 CrossRef CAS PubMed.
- S. Fujii and Y. Hashimoto, Future Med. Chem., 2017, 9, 485–505 CrossRef CAS PubMed.
- W. Duan, Y. Sun, M. Wu, Z. Zhang, T. Zhang, H. Wang, F. Li, L. Yang, Y. Xu, Z.-J. Liu, T. Hua, H. Nie and J. Cheng, Eur. J. Med. Chem., 2021, 226, 113878 CrossRef CAS PubMed.
- Y. Yan, Q. Wei, Z. Su, N.-N. Hang, T. Hayashi and J. Ming, Nat. Commun., 2024, 15, 9915 CrossRef CAS PubMed.
- R. Tacke and S. Dörrich, in Atypical Elements in Drug Design, ed. J. Schwarz, Springer International Publishing, Cham, 2014, vol. 17, pp. 29–59 Search PubMed.
- https://news.wisc.edu/silicon-medicines-may-be-effective-in-humans/ .
- R. Tacke, B. Nguyen, C. Burschka, W. P. Lippert, A. Hamacher, C. Urban and M. U. Kassack, Organometallics, 2010, 29, 1652–1660 CrossRef CAS.
- M. Geyer, E. Wellner, U. Jurva, S. Saloman, D. Armstrong and R. Tacke, ChemMedChem, 2015, 10, 911–924 CrossRef CAS PubMed.
- R. Ramesh and D. S. Reddy, J. Med. Chem., 2018, 61, 3779–3798 CrossRef CAS PubMed.
- A. K. Franz and S. O. Wilson, J. Med. Chem., 2013, 56, 388–405 CrossRef CAS PubMed.
- R. Kitel, A. Byczek-Wyrostek, K. Hopko, A. Kasprzycka and K. Walczak, Pharmaceuticals, 2021, 14, 1079 CrossRef CAS PubMed.
- S. Fujii and Y. Hashimoto, Future Med. Chem., 2017, 9, 485–505 CrossRef CAS PubMed.
- A. Daud, N. Valkov, B. Centeno, J. Derderian, P. Sullivan, P. Munster, P. Urbas, R. C. DeConti, E. Berghorn, Z. Liu, F. Hausheer and D. Sullivan, Clin. Cancer Res., 2005, 11, 3009–3016 CrossRef CAS PubMed.
- V. B. Arion, A. Dobrov, S. Göschl, M. A. Jakupec, B. K. Keppler and P. Rapta, Chem. Commun., 2012, 48, 8559–8561 Search PubMed.
- A. Dobrov, S. Göschl, M. A. Jakupec, A. Popović-Bijelić, A. Gräslund, P. Rapta and V. B. Arion, Chem. Commun., 2013, 49, 10007–10009 RSC.
- L. K. Filak, S. Göschl, P. Heffeter, K. G. Samper, A. E. Egger, M. A. Jakupec, B. K. Keppler, W. Berger and V. B. Arion, Organometallics, 2013, 32, 903–914 Search PubMed.
- L. K. Filak, D. S. Kalinowski, T. J. Bauer, D. R. Richardson and V. B. Arion, Inorg. Chem., 2014, 53, 6934–6943 CrossRef CAS PubMed.
- M. F. Primik, S. Göschl, S. M. Meier, N. Eberherr, M. A. Jakupec, É.A Enyedy, G. Novitchi and V. B. Arion, Inorg. Chem., 2013, 52, 10137–10146 Search PubMed.
- F. Bacher, C. Wittmann, M. Nové, G. Spengler, M. A. Marć, E. A. Enyedy, D. Darvasiová, P. Rapta, T. Reiner and V. B. Arion, Dalton Trans., 2019, 48, 10464–10478 RSC.
- C. Wittmann, F. Bacher, E. A. Enyedy, O. Dömötör, G. Spengler, C. Madejski, J. Reynisson and V. B. Arion, J. Med. Chem., 2022, 65, 2238–2261 CrossRef CAS PubMed.
- C. Wittmann, A. S. Sivchenko, F. Bacher, K. K. H. Tong, N. Guru, T. Wilson, J. Gonzales, H. Rauch, S. Kossatz, T. Reiner, M. V. Babak and V. B. Arion, Inorg. Chem., 2022, 61, 1456–1470 CrossRef CAS PubMed.
- I. Kuznetcova, M. Ostojić, N. Gligorijević, S. Aranđelović and V. B. Arion, Inorg. Chem., 2022, 61, 20445–20460 Search PubMed.
- C. Wittmann, O. Dömötör, I. Kuznetcova, G. Spengler, J. Reynisson, L. Holder, G. J. Miller, E. A. Enyedy, R. Bai, E. Hamel and V. B. Arion, Dalton Trans., 2023, 52, 9964–9982 Search PubMed.
- I. Kuznetcova, F. Bacher, S. M. Alfadul, M. J. R. Tham, W. H. Ang, M. V. Babak, P. Rapta and V. B. Arion, Inorg. Chem., 2022, 61, 10167–10181 CrossRef CAS PubMed.
- J. B. Petersen, K. H. Lakowitz, C. Larsen, P. H. Nielsen, A. A. Lindberg, G. Jansen, B. Lamm and B. Samuelsson, Acta Chem. Scand., 1969, 23, 971–974 CrossRef CAS PubMed.
- L. J. Gooßen and A.-R. S. Ferwanah, Synlett, 2000, 1801–1803 Search PubMed.
- M. F. Primik, S. Göschl, M. A. Jakupec, A. Roller, B. K. Keppler and V. B. Arion, Inorg. Chem., 2010, 49, 11084–11095 CrossRef CAS PubMed.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- A. Daina, O. Michielin and V. Zoete, Sci. Rep., 2017, 7, 42717 CrossRef PubMed.
- Principles of Fluorescence Spectroscopy, ed. J. R. Lakowicz, Springer US, Boston, MA, 2006 Search PubMed.
- Z. Barbieriková, M. Bella, J. Kučerák, V. Milata, S. Jantová, D. Dvoranová, M. Veselá, A. Staško and V. Brezová, Photochem. Photobiol., 2011, 87, 32–44 CrossRef PubMed.
- M. Hricovíni, D. Dvoranová, Z. Barbieriková, S. Jantová, M. Bella, M. Šoral and V. Brezová, J. Photochem. Photobiol., A, 2017, 332, 112–121 CrossRef.
- M. Hricovíni, M. Mazúr, A. Sîrbu, O. Palamarciuc, V. Arion and V. Brezová, Molecules, 2018, 23, 721 CrossRef PubMed.
- D. Dvoranová, Z. Barbieriková and V. Brezová, Molecules, 2014, 19, 17279–17304 CrossRef PubMed.
- D. Dvoranová, K. Koci, L. Lajaunie, M. F. Edelmannova, M. P. Seabra, V. Brezová, R. Arenal, J. A. Labrincha and D. M. Tobaldi, ChemPhotoChem, 2025, 9, e202400254 CrossRef.
- S. Kwon, Y. Lee, Y. Jung, J. H. Kim, B. Baek, B. Lim, J. Lee, I. Kim and J. Lee, Eur. J. Med. Chem., 2018, 148, 116–127 CrossRef CAS PubMed.
- R. H. Shoemaker, Nat. Rev. Cancer, 2006, 6, 813–823 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A:Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C:Struct. Chem., 2015, 71, 3–8 Search PubMed.
- M. N. Burnett and C. K. Johnson, ORTEP-III: Oak Ridge Thermal Ellipsoid Plot Program for crystal structure illustrations, 1996 Search PubMed.
- W. Kabsch, Acta Crystallogr., Sect. D:Biol. Crystallogr., 2010, 66, 125–132 CrossRef CAS PubMed.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef PubMed.
- T. Janssen, A. Janner, A. Looijenga-Vosb and P. M. de Wolff, in International Tables for Crystallography: Mathematical, Physical and Chemical Tables, ed. T. Janssen, A. Janner, A. Looijenga-Vosb and P. M. de Wolff, International Union of Crystallography, Chester, UK, 1st edn, 2006, vol. C Search PubMed.
- L.-M. Peng, Micron, 1999, 30, 625–648 CrossRef CAS.
- T. Gruene, H. W. Hahn, A. V. Luebben, F. Meilleur and G. M. Sheldrick, J. Appl. Crystallogr., 2014, 47, 462–466 CrossRef CAS PubMed.
- S. Stoll and A. Schweiger, J. Magn. Reson., 2006, 178, 42–55 CrossRef CAS PubMed.
- S.-T. Von, H.-L. Seng, H.-B. Lee, S.-W. Ng, Y. Kitamura, M. Chikira and C.-H. Ng, J. Biol. Inorg. Chem., 2012, 17, 57–69 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. Figures with spectroscopic (NMR and UV-vis) and mass-spectrometric data, X-ray crystallographic data of complexes 1 and 2, NCI-60 one-dose screening data, and NCI-60 five-dose screening data. CCDC 2378782 (1) and 2378723 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00314h |
| This journal is © The Royal Society of Chemistry 2025 |