 Open Access Article
Open Access ArticleThermal properties and ultra-low thermal conductivity of Zn2GeSe4
Oluwagbemiga P.
Ojo
 a,
Wilarachchige D. C. B.
Gunatilleke
a,
Wilarachchige D. C. B.
Gunatilleke
 a,
Adam J.
Biacchi
a,
Adam J.
Biacchi
 b,
Hsin
Wang
c and
George S.
Nolas
b,
Hsin
Wang
c and
George S.
Nolas
 *a
*a
aDepartment of Physics, University of South Florida, Tampa, FL 33620, USA. E-mail: gnolas@usf.edu
bNanoscale Device Characterization Division, National Institute of Standards and Technology (NIST), Gaithersburg, MD 20899, USA
cMaterials Science and Technology Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
First published on 11th March 2025
Abstract
Material-related discovery continues to drive advancements in technologically significant fields of interest. Moreover, an understanding of the thermal properties of materials is essential for any application of interest. Here we report on the structural, optical and thermal properties of Zn2GeSe4, which forms in the sphalerite crystal structure with an indirect 1.85 eV band gap, as obtained from our optical spectroscopy measurements. Analyses and modeling of the temperature-dependent thermal properties reveal a low speed of sound and Debye temperature, with ultra-low thermal conductivity over a large temperature range due to low-frequency soft modes as well as strong lattice anharmonicity. Our findings are presented and discussed in the context of the current intense interest in metal chalcogenides and will facilitate the development of these and similar materials for applications where low thermal conductivity is of interest.
Introduction
Material discoveries are integral in advancing technological applications of interest, and investigations that relate structure and stoichiometry with specific physical properties are key to technological advancements in addition to enhancing our fundamental knowledgebase. Multinary chalcogenides encompass one general class of materials that have recently generated strong interest. They have diverse physical properties that arise from varied structural, bonding and chemical features as well as stoichiometry and lattice disorder. Disorder in particular can play a key role in governing the physical properties of materials, and in certain cases can also provide tunability beyond typical property modifications such as doping and stoichiometric variations.1–5Multinary chalcogenides with compositions I–II2–III–VI4, where I is Cu or Ag, II is Cd, Zn, Co or Fe, III is In, Ga or Al and VI is S, Se or Te, form in the sphalerite-type crystal structure and have only recently been investigated.6–11 As compared to I2–II–IV–VI4 quaternary chalcogenides, which have been studied extensively,12–15 I–II2–III–VI4 are much less investigated. The I–II2–III–VI4 class of materials is a family of compounds that can be thought of as being derived as a result of atomic cross substitutions accompanied by lattice mutations, in which the atomic valence states are preserved and charge neutrality of the periodic lattice is maintained.16,17 This structure type can accommodate extreme off-stoichiometric variations.18 Zn2GeSe4 represents a particularly unique example within this family of compounds, combining a sphalerite-derived structure with compositional features that introduce cation-site vacancies. Such structural features provide an interesting pretext for exploring their effects on the thermal properties. Moreover, only a few ternary compositions with sphalerite-like crystal structures have been reported to date.19–21 To the best of our knowledge, the thermal properties of Zn2GeSe4 with a sphalerite-like crystal structure have not been previously investigated. In this work, we investigated and modeled the thermal properties, including temperature-dependent heat capacity and thermal conductivity over a large temperature range, as well as the optical and structural properties in order to develop an understanding of the effect of the compositional features on the thermal properties of Zn2GeSe4.
Experimental
Polycrystalline Zn2GeSe4 was synthesized via mechanical alloying and densified by hot pressing. High-purity Zn (99.9%, Alfa Aesar), Ge (99.9999%, Alfa Aesar) and Se (99.999%, Alfa Aesar) powders were weighed in a stoichiometric ratio into a stainless-steel jar together with stainless steel balls in a ball-to-powder ratio of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The jar was evacuated before being sealed under high-purity argon and subsequently ball milled at 425 rpm for 2 hours. Employing a TA Instruments Q600, differential thermal analyses were conducted under a nitrogen gas flow revealing thermal stability until 573 K. This information was important for densification via hot-pressing (Thermal Technology model HP20-4560-20) into a dense polycrystalline material for physical property measurements. The powder was loaded into a custom graphite die and molybdenum punch assembly and densified at 573 K and 160 MPa for 2 hours under a nitrogen atmosphere. The resulting pellet had a density of 4.66 g cm−3 (97% of theoretical density) as determined from its mass and dimensions. The Atomic Force Microscopy (AFM) microstructure analyses of a cross section of the densified pellet revealed grain sizes in the range of a few micrometers.
1. The jar was evacuated before being sealed under high-purity argon and subsequently ball milled at 425 rpm for 2 hours. Employing a TA Instruments Q600, differential thermal analyses were conducted under a nitrogen gas flow revealing thermal stability until 573 K. This information was important for densification via hot-pressing (Thermal Technology model HP20-4560-20) into a dense polycrystalline material for physical property measurements. The powder was loaded into a custom graphite die and molybdenum punch assembly and densified at 573 K and 160 MPa for 2 hours under a nitrogen atmosphere. The resulting pellet had a density of 4.66 g cm−3 (97% of theoretical density) as determined from its mass and dimensions. The Atomic Force Microscopy (AFM) microstructure analyses of a cross section of the densified pellet revealed grain sizes in the range of a few micrometers.
X-ray diffraction (XRD) data were collected employing a Bruker AXS D8 FOCUS X-ray diffractometer with Cu Kα radiation. The Rietveld refinement was performed using GSAS II software.22 Optical spectra were acquired using UV–vis–NIR spectrophotometry in the wavelength range of 400 nm to 1200 nm using a Shimadzu, Inc. 3600i Plus spectrophotometer equipped with a 60 mm integrating sphere and PMT/InGaAs detectors. The optical band gap was determined from the Tauc plot of the Kubelka–Munk function.
The thick densified 12.8 mm diameter pellet was cut into a parallelepiped of dimensions 2 × 2 × 5 mm3 for low-temperature thermal conductivity, κ, measurements, and a 1 mm thick disk for high temperature thermal diffusivity measurements. Thermal diffusivity, αth, was measured in a temperature range of 300 K to 573 K using a NETZSCH LFA457 system employing the laser flash method in a flowing argon environment, with an estimated uncertainty of ±5%. Temperature-dependent κ was determined using the formula κ = DαthCv. Here D denotes the measured density of the specimen and Cv, the isochoric specific heat capacity, was estimated from the Dulong–Petit limit, Cv = 3naR, where na is the number of atoms per formula unit and R is the gas constant.23 For the low-temperature κ measurements, the thermal transport option (TTO) module of a Quantum Design Physical Property Measurement System (PPMS) was used. The specimen was mounted onto the TTO module using Au coated manganin leads for κ measurements where Ag epoxy (H20E) was used to establish electrical and thermal contact between the specimen and the leads. A maximum experimental uncertainty of ±3% was calculated for the κ measurements. Low-temperature κ measurements of standards employing a custom designed instrument24,25 are used to calibrate PPMS data. Isobaric heat capacity, Cp, measurements were performed using the heat capacity (HC) module of PPMS in a temperature range from 1.8 K to 303 K accompanied by an appropriate addendum measurement. The specimen was attached to the mounting stage using thermal N-grease. The measurements were conducted employing a two-tau model with a maximum 2% sample temperature rise. A maximum experimental uncertainty of ±1% was calculated for the Cp measurements.
Results and discussion
Fig. 1(a) shows the experimental powder XRD data, the profile fit and the profile difference from Rietveld refinement. The results from the Rietveld refinement are given in Tables 1 and 2. The crystal structure can be described as a derivative of the zinc blende structure, where the cation site partially occupied by Zn and Ge atoms forms tetrahedral coordinated environments with the Se atoms (see Fig. 1(b)). The UV–vis–NIR reflectance spectra are shown in Fig. 2. Zn2GeSe4 exhibits a broad absorption onset, and an indirect optical band gap of 1.85 eV was obtained from a linear fit to the steepest gradient of a Tauc plot of the Kubelka–Munk function26,27 constructed by plotting (αhν)1/2 as a function of photon energy hν.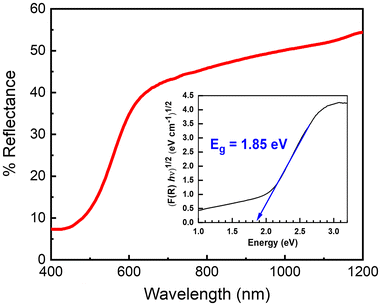 | ||
| Fig. 2 UV–vis–NIR reflectance spectrum, with the corresponding Tauc plot of the Kubelka–Munk function indicating an indirect optical band gap of 1.85 eV. | ||
| Compound | Zn2GeSe4 |
|---|---|
| Space group |
F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m (#216) 3m (#216) |
| a (Å) | 5.6710(21) |
| V (Å3) | 182.38(20) |
| d calc. (g cm−3) | 4.7271 |
| Zn/Ge–Se (Å) | 2.4556(3) |
| Se–Zn/Ge–S (°) | 109.47(1) |
| Radiation | Graphite monochromated |
| Cu Kα (1.5406 Å) | |
| 2θ range (°) | 20–100 |
| wRp, Rp | 0.06535, 0.04998 |
| Atom | Site | x | y | z | Occ. |
|---|---|---|---|---|---|
| Zn | 4a | 0 | 0 | 0 | 0.5 |
| Ge | 4a | 0 | 0 | 0 | 0.25 |
| Se | 4c | 1/4 | 1/4 | 1/4 | 1 |
Fig. 3 shows temperature-dependent κ data revealing very low values across the entire measured temperature range. The excellent agreement between the high and low κ data is an indication of the quality and homogeneity of the polycrystalline specimens used in this study. Above 100 K, the relatively flat temperature dependence of the data implies deviation from the standard phonon gas model. Such behavior has been reported in other materials, and can stem from a large atomic mass difference, such as partial vacancies, and lattice anharmonicity,28–38 prompting low-T temperature dependences that are atypical of crystalline materials. Moreover, as compared to structurally similar chalcogenides such as Cu3SbSe4,39 CuInSe2,40 CuInSnSe4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 and Cu2ZnSnSe4,42 as well as sphalerite CuZn2InS4
41 and Cu2ZnSnSe4,42 as well as sphalerite CuZn2InS4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (see Table 3), the κ values for Zn2GeSe4 are significantly lower. In order to evaluate the measured values for κ, we calculated the minimum thermal conductivity, κmin, for Zn2GeSe4 employing the modified Cahill relation43
7 (see Table 3), the κ values for Zn2GeSe4 are significantly lower. In order to evaluate the measured values for κ, we calculated the minimum thermal conductivity, κmin, for Zn2GeSe4 employing the modified Cahill relation43
 | (1) |
 and υs = θD(kB/ℏ)(6π2n)1/3, where R is the molar gas constant, n is the number of atoms per formula unit and ℏ is the reduced Planck constant. The values for θD and vs are 141 K and 1412 m s−1, respectively. These θD and vs values are low, significantly lower than those of analogous chalcogenides,41,42,49 and support the ultralow κ for Zn2GeSe4 described above. Using these results we can also estimate the Grüneisen parameter, γ, employing the relation
and υs = θD(kB/ℏ)(6π2n)1/3, where R is the molar gas constant, n is the number of atoms per formula unit and ℏ is the reduced Planck constant. The values for θD and vs are 141 K and 1412 m s−1, respectively. These θD and vs values are low, significantly lower than those of analogous chalcogenides,41,42,49 and support the ultralow κ for Zn2GeSe4 described above. Using these results we can also estimate the Grüneisen parameter, γ, employing the relation 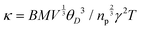 , where B = 2.43 × 10−8/(1–0.514/γ + 0.228/γ2), M is the average mass of an atom in the crystal (amu), V is the volume per atom and np is the number of atoms per primitive cell.50 The Grüneisen parameter (γ = 2) thus obtained indicates relatively large lattice anharmonicity further supporting the fact that Zn2GeSe4 possesses intrinsically very low κ.
, where B = 2.43 × 10−8/(1–0.514/γ + 0.228/γ2), M is the average mass of an atom in the crystal (amu), V is the volume per atom and np is the number of atoms per primitive cell.50 The Grüneisen parameter (γ = 2) thus obtained indicates relatively large lattice anharmonicity further supporting the fact that Zn2GeSe4 possesses intrinsically very low κ.
| Compound | Structure/space group | Band gap (eV), T = 300 K | κ (W m−1 K−1), T = 300 K |
|---|---|---|---|
Cu3SbSe4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61,62 61,62 |
Tetragonal, I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m 2m |
0.20 | 1.32 |
CuInSe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40,63 40,63 |
Chalcopyrite, I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d 2d |
0.96 | 1.70 |
CuInSnSe4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
Defect chalcopyrite, I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d 2d |
0.75 | 1.50 |
Cu2ZnSnSe4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42,64 42,64 |
Stannite, I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m 2m |
1.20 | 1.25 |
CuZn2InS4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
Sphalerite, F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m 3m |
— | 2.75 |
| Zn2GeSe4 (this work) | Sphalerite, F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m 3m |
1.85 | 0.57 |
The thermal properties revealed by our data and analyses warrant additional analysis of the Cp data. Fig. 5 shows Cp/T3versus T and indicates a relatively large deviation from Debye-like behavior that can be associated with low-frequency optic modes, which have been shown to directly affect κ in other materials.31,51–54,55–60 We therefore employed a phenomenological model that includes both Debye-like contributions and Einstein contributions to analyze the data. The solid line in Fig. 5 is a fit of the experimental data of the form48
 | (2) |
 | ||
| Fig. 5 C p/T3versus T data illustrating deviations from the Debye behavior. The solid line represents a fit of the phenomenological model described in the text. | ||
Conclusions
In this work we synthesized phase-pure polycrystalline Zn2GeSe4 in order to investigate the previously unexplored thermal properties of this material. UV–vis–NIR measurements revealed an indirect optical band gap of 1.85 eV, and analyses of the temperature-dependent κ and Cp data revealed very low κ over a large temperature range, as well as low θD and υs, attributable to the lattice anharmonicity resulting from structural disorder and low-frequency optic modes. These findings advance our understanding of the structure and physical properties of multinary chalcogenides and should facilitate further research and exploration on this and similar chalcogenides for applications of interest where low κ values are desirable.Ethical statement
Certain commercial equipment, instrumentation, or materials are identified in this document to adequately specify the experimental procedures. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.Author contributions
Oluwagbemiga P. Ojo: data curation, investigation, formal analysis, visualization, and writing – original draft. Wilarachchige D. C. B. Gunatilleke: data curation, investigation, formal analysis and visualization. Adam J. Biacchi: data curation, investigation, and visualization. Hsin Wang: data curation and investigation. George S. Nolas: conceptualization, investigation, supervision, writing – review & editing, and project administration.Data availability
All data are provided and data are also available upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
H.W. acknowledges the support of the U.S. Department of Energy, Vehicle Technologies Office, Powertrain Materials Core Program and the International Energy Agency (IEA) Advanced Materials for Transportation (AMT) Technology Collaboration Programme (TCP). Oak Ridge National Laboratory is managed by UT-Battelle LLC under contract DE-AC05000OR22725.References
- R. Waser, R. Dittmann, M. Salinga and M. Wuttig, Int. J. Mater. Res., 2010, 101, 182–198 Search PubMed.
- J. M. Hodges, Y. Xia, C. D. Malliakas, G. C. B. Alexander, M. K. Y. Chan and M. G. Kanatzidis, Chem. Mater., 2018, 30, 7245–7254 CAS.
- K. Ye, S. C. Siah, P. T. Erslev, A. Akey, C. Settens, M. S. B. Hoque, J. Braun, P. Hopkins, G. Teeter, T. Buonassisi and R. Jaramillo, Chem. Mater., 2019, 31, 8402–8412 CAS.
- Y. Xu, X. Wang, W. Zhang, L. Schäfer, J. Reindl, F. vom Bruch, Y. Zhou, V. Evang, J.-J. Wang, V. L. Deringer, E. Ma, M. Wuttig and R. Mazzarello, Adv. Mater., 2021, 33, 2006221 Search PubMed.
- A. Giri, G. Park and U. Jeong, Chem. Rev., 2023, 123, 3329–3442 CAS.
- G. S. Nolas, M. S. Hassan, Y. Dong and J. Martin, J. Solid State Chem., 2016, 242, 50–54 CAS.
- O. P. Ojo, W. D. C. B. Gunatilleke, H. Poddig, H. Wang, J. Martin, D. J. Kirsch and G. S. Nolas, Dalton Trans., 2021, 50, 17611–17617 CAS.
- D. Hobbis, K. Wei, H. Wang and G. S. Nolas, J. Alloys Compd., 2018, 743, 543–546 CAS.
- D. Hobbis, W. Shi, A. Popescu, K. Wei, R. E. Baumbach, H. Wang, L. M. Woods and G. S. Nolas, Dalton Trans., 2020, 49, 2273–2279 CAS.
- K. D. Profita and E. M. Heppke, Z. Naturforsch., B:J. Chem. Sci., 2024, 79(8–9), 489–496 Search PubMed.
- X. Yang, Y. Li and J. Chen, Miner. Eng., 2022, 183, 107596 Search PubMed.
- M. Ibáñez, R. Zamani, A. LaLonde, D. Cadavid, W. Li, A. Shavel, J. Arbiol, J. R. Morante, S. Gorsse, G. J. Snyder and A. Cabot, J. Am. Chem. Soc., 2012, 134, 4060–4063 Search PubMed.
- K. Wei, L. Beauchemin, H. Wang, W. D. Porter, J. Martin and G. S. Nolas, J. Alloys Compd., 2015, 650, 844–847 CrossRef CAS.
- S. Chen, A. Walsh, Y. Luo, J.-H. Yang, X. G. Gong and S.-H. Wei, Phys. Rev. B:Condens. Matter Mater. Phys., 2010, 82, 195203 CrossRef.
- F.-J. Fan, L. Wu and S.-H. Yu, Energy Environ. Sci., 2014, 7, 190–208 Search PubMed.
- B. R. Pamplin, J. Phys. Chem. Solids, 1964, 25, 675–684 Search PubMed.
- C. H. L. Goodman, J. Phys. Chem. Solids, 1958, 6, 305–314 Search PubMed.
- G. S. Nolas, H. Poddig, W. Shi, L. M. Woods, J. Martin and H. Wang, J. Solid State Chem., 2021, 297, 122058 Search PubMed.
- C. K. Lowe-Ma and T. A. Vanderah, Acta Crystallogr., Sect. C:Cryst. Struct. Commun., 1991, 47, 919–924 Search PubMed.
- D. P. Kozlenko, V. V. Shchennikov, V. I. Voronin, V. P. Glazkov and B. N. Savenko, Phys. Solid State, 2002, 44, 1628–1631 CAS.
- A. Tengå, F. J. García-García, A. S. Mikhaylushkin, B. Espinosa-Arronte, M. Andersson and U. Häussermann, Chem. Mater., 2005, 17, 6080–6085 Search PubMed.
- B. H. Toby and R. B. Von Dreele, J. Appl. Crystallogr., 2013, 46, 544–549 CAS.
- H. Wang, W. D. Porter, H. Böttner, J. König, L. Chen, S. Bai, T. M. Tritt, A. Mayolet, J. Senawiratne, C. Smith, F. Harris, P. Gilbert, J. Sharp, J. Lo, H. Kleinke and L. Kiss, J. Electron. Mater., 2013, 42, 1073–1084 Search PubMed.
- J. Martin and G. S. Nolas, Rev. Sci. Instrum., 2016, 87, 015105 Search PubMed.
- J. Martin, S. Erickson, G. S. Nolas, P. Alboni, T. M. Tritt and J. Yang, J. Appl. Phys., 2006, 99, 044903 Search PubMed.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi B, 1966, 15, 627–637 Search PubMed.
- P. Makuła, M. Pacia and W. Macyk, J. Phys. Chem. Lett., 2018, 9, 6814–6817 Search PubMed.
- G. S. Nolas, J.-M. Ward, J. Gryko, L. Qiu and M. A. White, Phys. Rev. B:Condens. Matter Mater. Phys., 2001, 64, 153201 Search PubMed.
- G. A. Lamberton Jr., R. H. Tedstrom, T. M. Tritt and G. S. Nolas, J. Appl. Phys., 2005, 97, 113715 CrossRef.
- C. L. Condron, S. M. Kauzlarich and G. S. Nolas, Inorg. Chem., 2007, 46, 2556–2562 CAS.
- T. Mori, J. Martin and G. Nolas, J. Appl. Phys., 2007, 102, 073510 Search PubMed.
- S. Stefanoski, J. Martin and G. S. Nolas, J. Phys.: Condens. Matter, 2010, 22, 485404 CrossRef CAS PubMed.
- O. Delaire, J. Ma, K. Marty, A. F. May, M. A. McGuire, M.-H. Du, D. J. Singh, A. Podlesnyak, G. Ehlers, M. D. Lumsden and B. C. Sales, Nat. Mater., 2011, 10, 614–619 CrossRef CAS PubMed.
- S. Stefanoski, C. D. Malliakas, M. G. Kanatzidis and G. S. Nolas, Inorg. Chem., 2012, 51, 8686–8692 CrossRef CAS PubMed.
- Y. Dong, H. Wang and G. S. Nolas, Inorg. Chem., 2013, 52, 14364–14367 CrossRef CAS PubMed.
- H. Wang, G. Qin, G. Li, Q. Wang and M. Hu, 2D Mater., 2017, 5, 015022 Search PubMed.
- G. Qin, Z. Qin, H. Wang and M. Hu, Phys. Rev. B, 2017, 95, 195416 CrossRef.
- S. C. Rakesh Roshan, N. Yedukondalu, T. Pandey, L. Kunduru, R. Muthaiah, R. K. Rajaboina, L. Ehm and J. B. Parise, ACS Appl. Electron. Mater., 2023, 5, 5852–5863 CrossRef CAS.
- E. J. Skoug, J. D. Cain and D. T. Morelli, Appl. Phys. Lett., 2011, 98, 261911 Search PubMed.
- S. M. Wasim, Sol. Cells, 1986, 16, 289–316 CAS.
- O. P. Ojo, L. Ma, W. D. C. B. Gunatilleke, A. F. May, L. M. Woods and G. S. Nolas, Inorg. Chem., 2023, 62, 16114–16121 CrossRef CAS PubMed.
- Y. Dong, H. Wang and G. S. Nolas, Phys. Status Solidi RRL, 2014, 8, 61–64 CrossRef CAS.
- D. G. Cahill, S. K. Watson and R. O. Pohl, Phys. Rev. B:Condens. Matter Mater. Phys., 1992, 46, 6131 CrossRef CAS PubMed.
- W. Lai, Y. Wang, D. T. Morelli and X. Lu, Adv. Funct. Mater., 2015, 25, 3648–3657 CrossRef CAS.
- Z. Wang, S. Liu, X. Zhang, Y. Gao and D. Tang, Int. J. Heat Mass Transfer, 2024, 218, 124753 CrossRef CAS.
- Z. Liu, W. Zhang, W. Gao and T. Mori, Energy Environ. Sci., 2021, 14, 3579–3587 RSC.
- O. P. Ojo, W. D. C. B. Gunatilleke, H. Wang and G. S. Nolas, J. Alloys Compd., 2024, 1004, 175812 CrossRef CAS.
- C. Kittel, Introduction to solid state physics, John Wiley & Sons, Inc., 5th edn, 1976 Search PubMed.
- K. J. Bachmann, F. S. L. Hsu, F. A. Thiel and H. M. Kasper, J. Electron. Mater., 1977, 6, 431–448 CrossRef CAS.
- G. A. Slack, Solid State Phys., 1979, 34, 1–71 CAS.
- S. Paschen, W. Carrillo-Cabrera, A. Bentien, V. H. Tran, M. Baenitz, Yu. Grin and F. Steglich, Phys. Rev. B:Condens. Matter Mater. Phys., 2001, 64, 214404 CrossRef.
- B. C. Chakoumakos, B. C. Sales and D. G. Mandrus, J. Alloys Compd., 2001, 322, 127–134 Search PubMed.
- M. A. Avila, K. Suekuni, K. Umeo, H. Fukuoka, S. Yamanaka and T. Takabatake, Phys. Rev. B:Condens. Matter Mater. Phys., 2006, 74, 125109 Search PubMed.
- G. S. Nolas, G. Fowler and J. Yang, J. Appl. Phys., 2006, 100, 043705 Search PubMed.
- M. Beekman, W. Schnelle, H. Borrmann, M. Baitinger, Yu. Grin and G. S. Nolas, Phys. Rev. Lett., 2010, 104, 018301 Search PubMed.
- M. Beekman, R. P. Hermann, A. Möchel, F. Juranyi and G. S. Nolas, J. Phys.: Condens. Matter, 2010, 22, 355401 CrossRef CAS PubMed.
- W. D. C. B. Gunatilleke, A. F. May, H. Wang and G. S. Nolas, Appl. Phys. Lett., 2020, 117, 092101 CrossRef.
- W. D. C. B. Gunatilleke, R. Juneja, O. P. Ojo, A. F. May, H. Wang, L. Lindsay and G. S. Nolas, Phys. Rev. Mater., 2021, 5, 085002 CrossRef CAS.
- W. D. C. B. Gunatilleke, M. Zhang, W. Wong-Ng, P. Zavalij, Y.-S. Chen and G. S. Nolas, J. Appl. Phys., 2023, 133, 095108 Search PubMed.
- K. S. Rana, D. Sarkar, Nidhi, A. Singh, C. Bera, K. Biswas and A. Soni, Phys. Rev. B, 2024, 109, 115202 CrossRef CAS.
- K. Tyagi, B. Gahtori, S. Bathula, V. Toutam, S. Sharma, N. K. Singh and A. Dhar, Appl. Phys. Lett., 2014, 105, 261902 CrossRef.
- A. Moser, O. Yarema, G. Garcia, M. Luisier, F. Longo, E. Billeter, A. Borgschulte, M. Yarema and V. Wood, Chem. Mater., 2023, 35, 6323–6331 CrossRef CAS PubMed.
- J. L. Shay, B. Tell, H. M. Kasper and L. M. Schiavone, Phys. Rev. B, 1973, 7, 4485–4490 Search PubMed.
- K. Wei and G. S. Nolas, ACS Appl. Mater. Interfaces, 2015, 7, 9752–9757 CAS.
| This journal is © The Royal Society of Chemistry 2025 |



