 Open Access Article
Open Access ArticleSimple and easy functionalization of para-hydroxy POCOP-M (M = Ni(II), Pd(II)) pincer complexes: synthesis of multimetallic species†
Juan S.
Serrano-García
 a,
Marco Antonio
García-Eleno
a,
Marco Antonio
García-Eleno
 *b,
Antonino
Arenaza-Corona
*b,
Antonino
Arenaza-Corona
 a,
Ernesto
Rufino-Felipe
a,
Ernesto
Rufino-Felipe
 a,
Hugo
Valdés
a,
Hugo
Valdés
 c,
Simon
Hernandez-Ortega
c,
Simon
Hernandez-Ortega
 a and
David
Morales-Morales
a and
David
Morales-Morales
 *a
*a
aInstituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad de México, CP 04510, Mexico. E-mail: damor@unam.mx
bCentro Conjunto de Investigación en Química Sustentable UAEM-UNAM, Universidad Autónoma del Estado de México, Carretera Toluca-Atlacomulco Km 14.5, Toluca, Estado de México CP 50200, Mexico. E-mail: qmargarcia@gmail.com
cDepartamento de Química Orgánica y Química Inorgánica, Universidad de Alcalá, Alcalá de Henares 28805, Madrid, Spain
First published on 14th November 2024
Abstract
A facile and efficient method for synthesizing mono- and bi-metallic pincer complexes (3-Ni/Ni and 3-Ni/Pd) has been developed. The procedure involves a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric reaction of a para-hydroxy POCOP pincer complex with cyanuric chloride in the presence of [NaB(OMe)4], which selectively affords the mono-substituted pincer complexes (2-Ni and 2-Pd). These complexes are then further reacted with another equivalent of the para-hydroxy POCOP pincer complex to produce the desired multimetallic species (3-Ni/Ni and 3-Ni/Pd). The complexes can be easily isolated in moderate to good yields using chromatographic column purification. This straightforward synthesis procedure offers an attractive option for the preparation of multimetallic complexes. Furthermore, the molecular structures of complexes 2-Ni, 2-Pd, 3-Ni/Pd and 3-Ni/S were determined. The crystal packing of the structures was stabilized by various interactions including C–H⋯X (X = N, Cl, and F), π⋯π, and C–F⋯F interactions, contributing to their unique topologies. While 2-Ni and 2-Pd complexes exhibit similar molecular structures, they display distinct arrangements in their crystalline packing, such as monomeric chains in 1D arrangement along the c-axis for 2-Ni, and a symmetric dimeric species creating a 2D arrangement in the direction of the bc-axes for 2-Pd. The latter was corroborated by Hirshfeld surface analysis and 2D dimensional fingerprint plots of each crystal structure.
1 stoichiometric reaction of a para-hydroxy POCOP pincer complex with cyanuric chloride in the presence of [NaB(OMe)4], which selectively affords the mono-substituted pincer complexes (2-Ni and 2-Pd). These complexes are then further reacted with another equivalent of the para-hydroxy POCOP pincer complex to produce the desired multimetallic species (3-Ni/Ni and 3-Ni/Pd). The complexes can be easily isolated in moderate to good yields using chromatographic column purification. This straightforward synthesis procedure offers an attractive option for the preparation of multimetallic complexes. Furthermore, the molecular structures of complexes 2-Ni, 2-Pd, 3-Ni/Pd and 3-Ni/S were determined. The crystal packing of the structures was stabilized by various interactions including C–H⋯X (X = N, Cl, and F), π⋯π, and C–F⋯F interactions, contributing to their unique topologies. While 2-Ni and 2-Pd complexes exhibit similar molecular structures, they display distinct arrangements in their crystalline packing, such as monomeric chains in 1D arrangement along the c-axis for 2-Ni, and a symmetric dimeric species creating a 2D arrangement in the direction of the bc-axes for 2-Pd. The latter was corroborated by Hirshfeld surface analysis and 2D dimensional fingerprint plots of each crystal structure.
Introduction
Pincer complexes serve as excellent platforms for synthesizing highly active catalysts and advanced materials. They have the ability to catalyze several reactions that are vital in many chemical industries, including (de)hydrogenation and cross-coupling, among others, making them essential for many applications.1 However, in most cases, pincer complexes are used as homogeneous catalysts, which limits their use in large scale applications due to several factors. Homogeneous catalysts are difficult to recover and reuse, and the isolation and purification of the products are often laborious.Metallodendrimers are a promising way to support homogeneous catalysts.2 This approach has several advantages over using traditional homogeneous catalysts. For example, metallodendrimers are soluble in many organic solvents, have well-defined and regular structures, and can be easily recovered by filtration due to their nanometric size. These features make them attractive for designing catalysts, as they allow for the recovery and purification of catalytic products. Therefore, various efforts have been made to develop procedures for the synthesis of pincer-dendrimers.
One of the most common strategies to functionalize a pincer complex is to introduce an easily functionalizing group or anchoring group, such as –OH, –NH2, halogen, alkyne, etc., into the pincer backbone.3 This approach was pioneered by van Koten and co-workers who reported the reaction of a para-hydroxy pincer complex with a dendrimer precursor derived from acyl chlorides (Scheme 1a),4 while Veggel and Reinhoudt described the synthesis of metallodendrimers of zero order (G0) by reacting a multi-pincer ligand precursor with a metal source (Scheme 1b).5 However, both methods were limited to the generation of homometallic species. Despite this limitation, both methodologies were easily carried out in the laboratory.
In our previous work, we successfully synthesized a series of para-hydroxy POCOP-pincer complexes and investigated their reactivity towards acyl chlorides to form ester derivatives.6 Based on these results, we hypothesized that by using these para-hydroxy pincers and cyanuric chloride as building blocks, we could control the generation of dendrimers G0, which would enable the incorporation of different pincer complexes, and ultimately allow for the synthesis of heterometallic species (Scheme 1c). The ability to synthesize heterometallic species has garnered significant attention due to their exceptional catalytic properties, particularly their capacity to perform tandem processes.7 The incorporation of different pincer complexes into these heterometallic species would also allow for fine-tuning of their catalytic properties for specific applications (Fig. 1).
Results and discussion
To conduct our investigation, we initially carried out the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric reaction of a para-hydroxy POCOP pincer complex with cyanuric chloride in the presence of [NaB(OMe)4] at 0 °C, as depicted in Reaction A in Scheme 1. We were able to isolate the functionalized pincer complexes 2-Ni and 2-Pd in yields of 80% and 47%, respectively, after workup. The 1H NMR spectra of both complexes showed the absence of the –OH signal, while the 13C NMR spectra exhibited the corresponding signals of the cyanuric chloride at 171 and 173 ppm, respectively. The mass spectra provided us with additional structural information, revealing the molecular ion [M]+ at 655 and 703 m/z, respectively, confirming the mono-metallic nature of 2-Ni and 2-Pd.
1 stoichiometric reaction of a para-hydroxy POCOP pincer complex with cyanuric chloride in the presence of [NaB(OMe)4] at 0 °C, as depicted in Reaction A in Scheme 1. We were able to isolate the functionalized pincer complexes 2-Ni and 2-Pd in yields of 80% and 47%, respectively, after workup. The 1H NMR spectra of both complexes showed the absence of the –OH signal, while the 13C NMR spectra exhibited the corresponding signals of the cyanuric chloride at 171 and 173 ppm, respectively. The mass spectra provided us with additional structural information, revealing the molecular ion [M]+ at 655 and 703 m/z, respectively, confirming the mono-metallic nature of 2-Ni and 2-Pd.
With this information, we then explored the reactivity of 2-Ni and 2-Pd towards another equivalent of pincer complex 1-Ni using similar reaction conditions, triazine derivative, 1-Ni, and [NaB(OMe)4] in THF at 0 °C, as shown in Reaction B in Scheme 1. We obtained the dimetallic species 3-Ni/Ni and 3-Ni/Pd in 53% and 69% yield, respectively. The 1H, 13C, and 31P NMR spectra of complex 3-Ni/Ni showed a highly symmetrical molecule, whereas complex 3-Ni/Pd exhibited the presence of two different pincer fragments. The 1H NMR spectrum showed the aromatic proton of the pincer backbone at 6.21 ppm for the Ni fragment and at 6.37 ppm for the Pd moiety. In the 31P NMR spectrum, two signals were observed at 195.1 and 190.4 ppm for the Pd- and Ni-pincer fragment, respectively. Furthermore, the mass spectra of complexes 3-Ni/Ni and 3-Ni/Pd showed the molecular ion [M]+ at 1125 and 1173 m/z, respectively, confirming the proposed structures.
Additionally, we investigated the reactivity of the triazine pincer (2-Ni) towards a fluorinated-thiolate (Reaction C, Scheme 1). The high affinity of the thiolate for the metal center led to a ligand exchange, with the chlorine being replaced by the thiolate ligand. The substitution of the chlorine atoms at the triazine moiety by the fluorinated-thiolate also occurred, resulting in the formation of complex 4-Ni/S in 48% yield. The 1H NMR spectrum of 4-Ni/S showed the signal of the pincer ligand backbone at 1.34 and 6.18 ppm, while the corresponding signals of the fluorinated-thiolate were observed at 6.60 and 7.10 ppm. The signals of the fluorinated-thiolate in the 13C NMR spectra appeared as multiplets with low intensity due to the 19F–13C coupling. The 31P NMR spectrum displayed only one signal at 190.0 ppm, consistent with the proposed structure. The mass spectrum of 4-Ni/S showed the molecular ion [M]+ at 1091 m/z, confirming the successful synthesis of the complex.
To determine the molecular structures of the complexes, we conducted single-crystal X-ray diffraction analyses. We obtained suitable crystals by slow evaporation of a solution of each complex in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate/hexane mixture. The crystal data and refinement parameters for each complex are presented in ESI.† Complexes 2-Ni and 2-Pd are isostructural and crystallized in a monoclinic system with a space group of P21/n, while complexes 2-Ni/Pd and 4-Ni/S crystallized in a triclinic system with a P
1 ethyl acetate/hexane mixture. The crystal data and refinement parameters for each complex are presented in ESI.† Complexes 2-Ni and 2-Pd are isostructural and crystallized in a monoclinic system with a space group of P21/n, while complexes 2-Ni/Pd and 4-Ni/S crystallized in a triclinic system with a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The X-ray diffraction analyses allowed us to confirm the proposed molecular structures of each complex.
space group. The X-ray diffraction analyses allowed us to confirm the proposed molecular structures of each complex.
The molecular structures of complexes 2-Ni, 2-Pd, 3-Ni/Pd, and 4-Ni/S are shown in Fig. 2. Consistent with our expectations, the metal centers in all complexes are tetracoordinated to two phosphine ligands, one carbon ligand, and one anionic ligand (chlorine or sulfur) and adopt a distorted square-planar geometry. The most significant angles and bond lengths are presented in Table S5 (see ESI†). Notably, the lengths of the Ni–P, Ni–Cl, and Ni–C bonds in all complexes were similar, ranging from 2.18 to 2.24 Å, 2.18 to 2.26 Å, and 1.88 to 1.93 Å, respectively. In contrast, the distances in the Pd pincer fragments were slightly higher, ranging from 2.24 to 2.27 Å, 2.30 Å, and 1.97 to 2.00 Å, respectively. Finally, the Ni–S bond distance was 2.23 Å. All of these measurements were consistent with previous reports.6,8
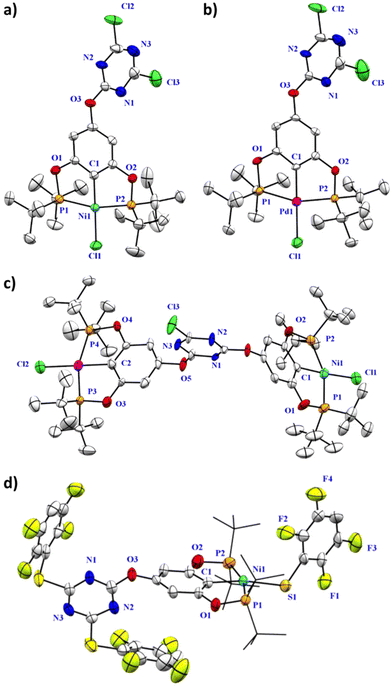 | ||
| Fig. 2 Molecular structures of (a) 2-Ni, (b) 2-Pd, (c) 3-Ni/Pd and (d) 4-Ni/S. Hydrogen atoms and disordered counter parts were omitted for clarity. The ellipsoids are represented at 40% probability. | ||
A more detailed study of the X-ray molecular structures revealed that the crystal packing is mainly stabilized by various interactions such as C–H⋯X (X = N, Cl, and F), π⋯π and C–F⋯F (Fig. 3). These interactions play a crucial role in the linkage and stability of the crystal lattice, giving rise to unique topologies in each structure. For example, while 2-Ni and 2-Pd complexes have similar molecular structures, their crystal packings are different. Compound 2-Ni forms monomeric chains in a 1D arrangement along the c-axis through C–H⋯Cl–Ni intermolecular interactions between neighboring molecules with a H⋯Cl distance of around 2.87 Å (Fig. 3a). In contrast, 2-Pd shows two C–H⋯Cl–Pd intermolecular interactions, one between the hydrogen of the phenyl moiety and Cl(1)–Pd(1), and the other between the tert-butyl moiety and Cl(1)–Pd(1), with a distance of 2.83 Å for both cases. Additionally, a π⋯π intermolecular interaction was observed through the (C3N3) aromatic rings of (C3N3) triazine fragments, which are found almost eclipsed face-to-face in an antiparallel disposition with a distance between centroid–centroid of 3.68 Å (Fig. 3b). This results in the formation of a symmetric dimeric species and a 2D arrangement in the direction of the bc-axes (Fig. 3b).
Compound 3-Ni/Pd also exhibits a dimeric structure that is generated by two intermolecular hydrogen bonds and a π⋯π interaction (Fig. 3c). The two C–H⋯N hydrogen bonds are formed between the hydrogen H4 of the phenyl moiety and a nitrogen atom of the triazine fragment, with a distance of 2.54 Å. In addition, a π⋯π interaction is observed between the (C3N3) triazine fragments, which are almost eclipsed face-to-face in an antiparallel π-stacking, with a distance between the centroids of 3.81 Å. These dimeric species form columns that run along the a-axis.
Compound 4-Ni/S is stabilized primarily by intermolecular interactions such as C–H⋯F–C, C–H⋯S–C, and C–F⋯F–C (Fig. 3d, e and S21†). The F⋯F non-bonded distances are found in the range of 2.86–2.93 Å with angles ranging from 107–110° (Fig. 3d), and these distances are slightly shorter than the sum of van der Waals radii (F⋯Frvdw = 2.94 Å). The pincer molecules are linked by intermolecular C–H⋯S–C hydrogen bonds with an H⋯S distance of 2.90 Å, resulting in a 1D chain structure along the c-axis (Fig. S21†). The fluorinated thiol fragments also participate in C–F⋯F–C interactions, which help to stabilize the 1D arrangement. Additionally, C–H⋯F and C–H⋯N (Fig. S22†) hydrogen bonds were observed with distances of 2.53 and 2.72 Å, respectively.
We employed the CrystalExplorer program9 to generate Hirshfeld surfaces for 2-Ni, 2-Pd, 3-Ni/Pd, and 4-Ni/S. In Fig. S23,† the Hirshfeld surface of all complexes is depicted, highlighting regions of close contact (red dots) analyzed via the dnorm function. Complexes 2-Ni and 2-Pd exhibited similar crystalline packaging, with similar dnorm and shape-index function maps (Fig. S23a–S23d†). Analysis of compound 3-Ni/Pd confirmed the presence of CH⋯N and π⋯π interactions (Fig. S23e and S23f†). Additionally, the dnorm mapping for 4-Ni/S indicated strong hydrogen bond interactions (Fig. S23g†), particularly the C–H⋯C hydrogen bond between a C–H of the pincer ring and the fluorinated ring.
Furthermore, fingerprints of the Hirshfeld surfaces were obtained for 2-Ni, 2-Pd, 3-Ni/Pd, and 4-Ni/S (Table S7†). Despite having different metal centers, compounds 2-Ni and 2-Pd displayed similar percentage contributions due to the maintenance of similar contact types. The most significant contributions were attributed to N⋯H/H⋯N and Cl⋯H/H⋯Cl interactions, depicted as symmetrical points across the diagonal in Table S7.† Similarly, compounds 3-Ni/Pd and 4-Ni/S displayed comparable patterns. However, in 4-Ni/S, the absence of chlorine atoms precluded Cl⋯H/H⋯Cl interactions, replaced instead by F⋯H/H⋯F contacts, observed at a higher percentage due to the increased number of fluorine atoms. Additionally, compound 4-Ni/S exhibited fluorine F⋯F (6.3%) and S⋯H/H⋯S (5.0%) contacts (Chart 1).
Conclusions
In summary, the solid-state structures of the complexes 2-Ni, 2-Pd, 3-Ni/Pd, and 4-Ni/S were investigated using single-crystal X-ray diffraction. The molecular structures indicate that the Ni(II) and Pd(II) centers exhibit a distorted square-planar environment and the POCOP pincer ligand coordinates to the metallic atom in a typical meridional fashion. Complexes 2-Ni and 3-Ni/Pd are monomeric structures, whereas the complexes 2-Ni and 3-Ni/Pd adopt a dimeric structure. In the crystal lattice, complexes 2-Ni and 4-Ni/S exhibit a 1D arrangement through C–H⋯X (X = Cl, S), while the 3-Ni/Pd complex displays a 2D arrangement due to C–H⋯Cl and π⋯π interactions. The crystal packing of 4-Ni/S is particularly intriguing due to the presence of C–F⋯F and C–F⋯H interactions. The interactions were supported by the determinations of Hirshfeld surface plots. Overall, these findings provide insights into the structural properties of these complexes and may facilitate the design and synthesis of novel materials with desirable properties.The presented methodology for the functionalization of para-hydroxy pincer complexes offers a straightforward approach for the synthesis of multimetallic species. Also, this methodology has the potential to be extended to other pincer complexes, opening up the possibility of creating a diverse range of dendritic architectures with various functional groups. These dendritic architectures could have a broad range of applications beyond catalysis, including drug delivery, sensors, and molecular recognition. The simplicity and efficiency of the methodology make it an attractive option for the scalable synthesis of these complex materials. Thus, we believe that this methodology will have a significant impact on the fields of materials science and catalysis, and it holds promise for the development of new and innovative technologies.
Author contributions
J. S. S.-G and M. A. G.-E. performed the experiments. A. A.-C. and E. R.-F. performed the supramolecular analyses. All authors were involved in the conceptual design of the study, discussed the results and wrote the manuscript.Data availability
The data supporting this article has been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
E. R. F. would like to thank Programa de Becas Posdoctorales-DGAPA-UNAM for postdoctoral scholarships (Oficio: CJIC/CTIC/1139/2020). A. A.-C. is grateful to CONAHCyT for the postdoctoral fellowship (Estancias posdoctorales por México 2022(1)). H. V. would like to thank the Generalitat de Catalunya for a Beatriu de Pinós contract (2019-BP-0080). D. M-M gratefully acknowledges CONAHCYT (A1-S-033933) and DGAPA-UNAM (PAPIIT IN223323) for generous financial support. And Dr. Ruben A. Toscano for its helpful support on the crystallographic part.References
- (a) H. Valdés, E. Rufino-Felipe, G. van Koten and D. Morales-Morales, Eur. J. Inorg. Chem., 2020, 2020, 4418 CrossRef; (b) H. Valdés, M. A. García-Eleno, D. Canseco-Gonzalez and D. Morales-Morales, ChemCatChem, 2018, 10, 3136 CrossRef; (c) H. Valdes, J. M. German-Acacio, G. van Koten and D. Morales-Morales, Dalton Trans., 2022, 51, 1724 RSC; (d) L. Alig, M. Fritz and S. Schneider, Chem. Rev., 2019, 119, 2681 CrossRef CAS; (e) A. Mukherjee and D. Milstein, ACS Catal., 2018, 8, 11435 CrossRef CAS; (f) Pincer Compounds Chemistry and Applications, ed. D. Morales-Morales, Elsevier, 2018 Search PubMed; (g) A. Kumar, T. M. Bhatti and A. S. Goldman, Chem. Rev., 2017, 117, 12357 CrossRef CAS PubMed; (h) M. Asay and D. Morales-Morales, Dalton Trans., 2015, 44, 17432 RSC; (i) M. Asay and D. Morales-Morales, Top. Organomet. Chem., 2016, 54, 239 CrossRef CAS; (j) C. Gunanathan and D. Milstein, Chem. Rev., 2014, 114, 12024 CrossRef CAS; (k) K. J. Szabó and O. F. Wendt, Pincer and Pincer–Type Complexes: Applications in Organic Synthesis and Catalysis, Wiley-VHC, 2014 CrossRef; (l) K. J. Szabó, Top. Organomet. Chem., 2013, 40, 203 CrossRef; (m) G. van Koten, in Organometallic Pincer Chemistry, ed. G. van Koten and D. Milstein, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, p. 1 Search PubMed; (n) N. Selander and K. J. Szabó, Chem. Rev., 2011, 111, 2048 CrossRef CAS PubMed; (o) J. Choi, A. H. R. MacArthur, M. Brookhart and A. S. Goldman, Chem. Rev., 2011, 111, 1761 CrossRef CAS PubMed; (p) D. Morales-Morales, Rev. Soc. Quim. Mex., 2004, 48, 338 CAS; (q) M. E. Van Der Boom and D. Milstein, Chem. Rev., 2003, 103, 1759 CrossRef CAS PubMed; (r) M. Albrecht and G. Van Koten, Angew. Chem., Int. Ed., 2001, 40, 3750 CrossRef CAS.
- (a) N. J. M. Pijnenburg, T. J. Korstanje, G. van Koten and R. J. M. K. Gebbink, in Palladacycles, 2008, pp. 361 Search PubMed; (b) R. A. Gossage, L. A. van de Kuil and G. van Koten, Acc. Chem. Res., 1998, 31, 423 CrossRef CAS; (c) H. P. Dijkstra, N. Ronde, G. P. M. van Klink, D. Vogt and G. van Koten, Adv. Synth. Catal., 2003, 345, 364 CrossRef CAS; (d) H. P. Dijkstra, M. Albrecht, S. Medici, G. P. M. van Klink and G. van Koten, Adv. Synth. Catal., 2002, 344, 1135 CrossRef CAS; (e) A. W. Kleij, R. A. Gossage, R. J. M. Klein Gebbink, N. Brinkmann, E. J. Reijerse, U. Kragl, M. Lutz, A. L. Spek and G. van Koten, J. Am. Chem. Soc., 2000, 122, 12112 CrossRef CAS; (f) A. W. Kleij, R. A. Gossage, J. T. B. H. Jastrzebski, J. Boersma and G. van Koten, Angew. Chem., Int. Ed., 2000, 39, 176 CrossRef CAS; (g) C. Schlenk, A. W. Kleij, H. Frey and G. van Koten, Angew. Chem., Int. Ed., 2000, 39, 3445 CrossRef CAS.
- (a) H. Valdés, L. González-Sebastián and D. Morales-Morales, J. Organomet. Chem., 2017, 845, 229 CrossRef; (b) H. Valdés, J. M. Germán-Acacio and D. Morales-Morales, in Organic Materials as Smart Nanocarriers for Drug Delivery, ed. A. M. Grumezescu, Elsevier – William Andrew, 2018, pp. 245 Search PubMed; (c) M. Q. Slagt, D. A. P. V. Zwieten, A. J. C. M. Moerkerk, R. J. M. K. Gebbink and G. V. Koten, Coord. Chem. Rev., 2004, 248, 2275 CrossRef CAS.
- P. J. Davies, D. M. Grove and G. van Koten, Organometallics, 1997, 16, 800 CrossRef CAS.
- W. T. S. Huck, B. Snellink-Ruël, F. C. J. M. van Veggel and D. N. Reinhoudt, Organometallics, 1997, 16, 4287 CrossRef CAS.
- (a) M. A. García-Eleno, E. Padilla-Mata, F. Estudiante-Negrete, F. Pichal-Cerda, S. Hernández-Ortega, R. A. Toscano and D. Morales-Morales, New J. Chem., 2015, 39, 3361 RSC; (b) M. K. Salomón-Flores, I. J. Bazany-Rodríguez, D. Martínez-Otero, M. A. García-Eleno, J. J. Guerra-García, D. Morales-Morales and A. Dorazco-González, Dalton Trans., 2017, 46, 4950 RSC.
- (a) J. A. Mata, F. E. Hahn and E. Peris, Chem. Sci., 2014, 5, 1723 RSC; (b) S. Patra and N. Maity, Coord. Chem. Rev., 2021, 434, 213803 CrossRef CAS; (c) R. Clauss, S. Baweja, D. Gelman and E. Hey-Hawkins, Dalton Trans., 2022, 51, 1344 RSC; (d) Z. Wen, E. Maisonhaute, Y. Zhang, S. Roland and M. Sollogoub, Chem. Commun., 2022, 58, 4516 RSC; (e) Y. Li, C. Wang, Q. Chen, H. Li, Y. Su, T. Cheng, G. Liu and C. Tan, Chem. – Asian J., 2021, 16, 2338 CrossRef CAS PubMed.
- (a) E. G. Morales-Espinoza, R. Coronel-García, H. Valdés, R. Reyes-Martínez, J. M. German-Acacio, B. A. Aguilar-Castillo, R. A. Toscano, N. Ortiz-Pastrana and D. Morales-Morales, J. Organomet. Chem., 2018, 867, 155 CrossRef CAS; (b) M. Joksch, A. Spannenberg and T. Beweries, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2019, 75, 1011 CrossRef CAS PubMed; (c) M. Joksch, H. Agarwala, J. Haak, A. Spannenberg and T. Beweries, Polyhedron, 2018, 143, 118 CrossRef CAS; (d) M. Joksch, J. Haak, A. Spannenberg and T. Beweries, Eur. J. Inorg. Chem., 2017, 2017, 3815 CrossRef CAS; (e) M. A. Garcia-Eleno, M. Quezada-Miriel, R. Reyes-Martinez, S. Hernandez-Ortega and D. Morales-Morales, Acta Crystallogr., Sect. C: Struct. Chem., 2016, 72, 393 CrossRef CAS; (f) S. Lapointe and D. Zargarian, Dalton Trans., 2016, 45, 15800 RSC; (g) V. Pandarus and D. Zargarian, Organometallics, 2007, 26, 4321 CrossRef CAS.
- P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka and M. A. Spackman, J. Appl. Crystallogr., 2021, 54, 1006 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2078439–2078442. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02841d |
| This journal is © The Royal Society of Chemistry 2025 |




