 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation of structurally related acyclic ligands OBETA, EHDTA, and EGTA for stable Mn2+ complex formation†
Elena
Grattoni
a,
Fabio
Travagin
 b,
Ferenc
Kálmán
b,
Ferenc
Kálmán
 c,
Zsolt
Baranyai
c,
Zsolt
Baranyai
 d,
Roberto
Negri
d,
Roberto
Negri
 be,
Fabio
Carniato
be,
Fabio
Carniato
 f,
Giovanni B.
Giovenzana
f,
Giovanni B.
Giovenzana
 b,
Carlos
Platas-Iglesias
b,
Carlos
Platas-Iglesias
 g and
Mauro
Botta
g and
Mauro
Botta
 *f
*f
aDip. di Scienze Chimiche e Farmaceutiche, Università di Trieste, Piazzale Europa 1, 34127 Trieste, TS, Italy
bDip. di Scienze del Farmaco, Università del Piemonte Orientale, Largo Donegani 2/3, 28100 Novara, Italy
cDep. of Inorganic and Analytical Chemistry, University of Debrecen, Egyetem tér 1, H-4010 Debrecen, Hungary
dBracco Imaging SpA, CRB Trieste, AREA Science Park, ed. Q – S.S. 14 Km, 163.5–34149 Basovizza, TS, Italy
eITIS “A. Volta”, Spalto Marengo 42, 15121 Alessandria, Italy
fDip. di Scienze e Innovazione Tecnologica, Università del Piemonte Orientale, Via T. Michel 11, 15121 Alessandria, Italy. E-mail: mauro.botta@uniupo.it
gUniversidade da Coruña, Centro de Interdisciplinar de Química e Bioloxía (CICA) and Departamento de Química, Facultade de Ciencias, 15071, A Coruña, Galicia, Spain
First published on 1st November 2024
Abstract
In recent years, significant research efforts have been dedicated to finding efficient and safe alternatives to the currently used gadolinium (Gd)-based MRI contrast agents. Among the most explored alternatives are paramagnetic chelates of the Earth-abundant Mn2+, which form a prominent class of metal complexes. The design of Mn2+ complexes with enhanced relaxation properties and improved safety profiles hinges on a delicate balance between thermodynamic and kinetic stability, as well as the presence of coordinated water molecules. In this study, we present a comprehensive investigation into the coordination chemistry of three structurally related polyetheraminocarboxylic chelating agents. Our aim is to elucidate the structural features, paramagnetic properties, and thermodynamic and kinetic inertness of the corresponding Mn2+ complexes. The most significant finding is the considerable difference in the dissociation rates of the complexes, with the octadentate EGTA complex being the most labile. The observed dissociation rates correlate well with the nitrogen inversion dynamics, as assessed through NMR spectral analysis of the analogous Zn2+ complexes.
Introduction
An intensive worldwide use of gadolinium-based chelates as MRI contrast agents (CAs) during the last 45 years led the scientific community to the awareness of specific problems and limitations of this fundamental class of metal complexes.1,2 Administration of hundreds of millions of doses of Gd-based contrast agents (GBCAs) brought to light some concerns for their in vivo applications.3–5 Moreover, GBCAs excreted by patients pass through wastewater treatments intact as evidenced by the presence of steadily increasing concentrations of anthropogenic Gd3+ in aquatic environments.6–10Recent years have seen significant research efforts aimed at finding efficient and safe alternatives to the currently used Gd-based MRI contrast agents. This includes the development of nanoparticles, stable organic radicals, and metal complexes with alternative paramagnetic metal ions.11,12 Among these, paramagnetic chelates of the Earth-abundant Mn2+ stand out as one of the most extensively studied families of metal complexes.13–15
The efficacy of MRI contrast agents is usually measured in terms of relaxivity, defined as the water proton relaxation rate variation observed in aqueous solution in the presence of a 1.0 mM concentration of the CA. The relaxivity of a paramagnetic metal complex depends on a complex interplay of different structural and dynamic parameters, such as the electronic relaxation rate, the rotational correlation time, and the presence of coordinated water molecules and their corresponding exchange lifetimes.16
The metal centre can accommodate coordinated water molecules depending on its coordination number and the steric hindrance created by the chelating agent. The coordination number of Mn2+ complexes is in the range of 6–8, and chelating agents with a denticity of 5–7 are usually employed to leave room for coordinated water molecules.13
However, maintaining the structural integrity of the metal complex under physiological conditions is crucial to prevent the release of free Mn2+ ions, which could disrupt delicate biochemical processes. High thermodynamic stability and kinetic inertness are critical prerequisites for resisting endogenous species that may compete with the ligand or metal ion. These properties are essential to prevent transchelation or transmetalation, processes where other biomolecules could displace the ligand or metal, potentially compromising the integrity and safety of the complex. The stability of metal complexes is generally associated with the denticity of the chelating agent: higher denticity provides better encapsulation of the metal ion, reducing its exposure to competing species.
The design of Mn2+-based contrast agents (MBCAs) involves balancing two opposing requirements: reducing the ligand denticity to increase the number of coordinated water molecules, which boosts relaxivity, while simultaneously increasing the denticity to ensure the complex's thermodynamic stability. Perhaps even more significant is the kinetic inertness, as demonstrated by Mn2+ complexes with bispidine ligands, whose kinetic stability far exceeds that of other Mn2+ complexes studied.17 It is important to highlight that kinetic inertness can be enhanced by increasing the stereochemical rigidity of the ligand's backbone, as observed with Mn-CDTA and Mn-PhDTA when compared to the parent Mn-EDTA complex.18,19 Another well-known example is Mn-PyC3A, where an increase in kinetic inertness is achieved by introducing a 2-picolyl donor group in place of an acetate group in CDTA.15 More recently, the introduction of a propylene bridge onto the cyclohexane ring of CDTA has resulted in the Mn-BCN-DTA complex, which exhibits a marked increase in kinetic inertness.20 Thus, even relatively minor structural modifications can lead to significant improvements in both the thermodynamic stability and/or kinetic inertness of these complexes.
Another feature of these complexes that shows significant dependence on small structural modifications of the chelating agents is the hydration state. In 2011, we were the first to highlight the presence of a mixture of coordination isomers in aqueous solution for Mn2+ complexes with the hexadentate ligand AAZ3A and its derivatives (Fig. 1). These isomers, seven-coordinate (q = 1) and six-coordinate (q = 0) species, differ in their hydration number q, with the population of each isomer appearing to correlate with the steric hindrance of the substituent.21 Subsequently, Mn2+ complexes with a homologous series of ligands based on cyclen, with denticity 5, 6, and 7 (DO3A, DO2A, DO1A; Fig. 1), were examined.22 In this case as well, the results did not show a clear predominance of the seven-coordinate species for Mn2+. Instead, equilibria between species with hydration numbers ranging from 0 to 1 were observed in aqueous media. Of particular interest is the significant difference in the hydration states between the isomeric complexes Mn(1,4-DO2A) and Mn(1,7-DO2A), for which q values were 0.87 and 0, respectively. All these examples highlight the often complex nature of Mn2+ chelates, where small modifications in the ligand backbone or the steric hindrance of the arms can significantly impact both the hydration state and the kinetic inertness of the complexes. Therefore, a more systematic and in-depth study is needed to understand how to modify the basic structure of a chelating agent to achieve a Mn2+ complex with a seven-coordinate ground state and one bound water molecule under fast exchange conditions.
Some years ago, we reported a systematic investigation of lanthanoid (Ln3+) complexes of OBETA (Fig. 1).23–25OBETA can be regarded as a structural analogue of EDTA, with an additional donor group provided by an ether oxygen, making it a potentially heptadentate chelating agent. It can also be considered a lower homologue of the more widely known octadentate ligand EGTA. While one would generally expect increased thermodynamic stability in Ln3+-OBETA complexes compared to their Ln3+-EDTA counterparts, it is quite surprising to observe that Ln3+-OBETA complexes not only exhibit greater stability than the corresponding Ln3+-EGTA complexes but also demonstrate significantly higher kinetic inertness.
Aiming at further improving the coordinating ability of OBETA, more recently we designed EHDTA, a rigidified derivative of OBETA, showing improved stability profiles while retaining the coordination behaviour observed with Ln3+-OBETA (Fig. 1).26
The structural characteristics of OBETA and EHDTA are particularly well-suited to achieve the ideal balance of low denticity and high stability required to enhance the performance of Mn2+ in MBCAs. In this study, we systematically investigate the Mn2+ complexes of these two heptadentate chelating agents, comparing their properties with those of the octadentate analogue EGTA (Fig. 1)27 to assess their potential as effective MBCAs.
Experimental
Materials
Solvents and chemicals were obtained from Carlo Erba (Milan, Italy) or BLD Pharm (Karlsruhe, Germany) and used as received. OBETA was prepared according to our previously reported procedure.23 Aqueous solutions were prepared from ultrapure laboratory grade water (18 MΩ cm) obtained from a Millipore/Milli-Q purification system. 1H and 13C NMR spectra were recorded on a Bruker Avance Neo 400 spectrometer operating at 9.4 T. Chemical shifts are reported in ppm with the protic impurities of the deuterated solvent as the internal reference. Mass spectra were obtained with a Thermo Finnigan LCQ-Deca XP-PLUS ion trap spectrometer equipped with an electrospray source. High resolution mass spectra were recorded on a Thermo Scientific Q-Exactive Plus spectrometer. TLC analyses were performed with silica gel (MN Kieselgel 60 F254) and visualized using UV light or sprayed with the Dragendorff reagent or alkaline KMnO4. Column chromatography was carried out on Macherey–Nagel silica gel 60 (0.063–0.200 mm).Solution thermodynamic studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (the concentration of the ligand was generally 0.002 M). For the pH measurements and titrations, a Metrohm 888 Titrando titration workstation Metrohm-6.0234.110 combined electrode was used. Equilibrium measurements were carried out at a constant ionic strength (0.1 M KCl) in 6 mL samples at 25 °C. The solutions were stirred, and N2 was bubbled through them. The titrations were made in the pH range of 1.7–12.0. KH-phthalate (pH = 4.005) and borax (pH = 9.177) buffers were used to calibrate the pH meter For the calculation of [H+] from the measured pH values, the method proposed by Irving et al. was used as follows.28 A 0.01 M HCl solution was titrated with standardized KOH solution at 0.1 M KCl ionic strength. The differences (A) between the measured (pHread) and calculated pH (−log[H+]) values were used to obtain the equilibrium H+ concentration from the pH values measured in the titration experiments (A = 0.05). For the equilibrium calculations, the stoichiometric water ionic product (pKw) was also needed to calculate [OH−] values under basic conditions. The VKOH–pHread data pairs of the HCl–KOH titration obtained in the pH range 10.5–12.0 were used to calculate the pKw value (pKw = 13.85). The protonation and stability constants were calculated with the PSEQUAD program.29
1 (the concentration of the ligand was generally 0.002 M). For the pH measurements and titrations, a Metrohm 888 Titrando titration workstation Metrohm-6.0234.110 combined electrode was used. Equilibrium measurements were carried out at a constant ionic strength (0.1 M KCl) in 6 mL samples at 25 °C. The solutions were stirred, and N2 was bubbled through them. The titrations were made in the pH range of 1.7–12.0. KH-phthalate (pH = 4.005) and borax (pH = 9.177) buffers were used to calibrate the pH meter For the calculation of [H+] from the measured pH values, the method proposed by Irving et al. was used as follows.28 A 0.01 M HCl solution was titrated with standardized KOH solution at 0.1 M KCl ionic strength. The differences (A) between the measured (pHread) and calculated pH (−log[H+]) values were used to obtain the equilibrium H+ concentration from the pH values measured in the titration experiments (A = 0.05). For the equilibrium calculations, the stoichiometric water ionic product (pKw) was also needed to calculate [OH−] values under basic conditions. The VKOH–pHread data pairs of the HCl–KOH titration obtained in the pH range 10.5–12.0 were used to calculate the pKw value (pKw = 13.85). The protonation and stability constants were calculated with the PSEQUAD program.29
Transmetallation studies
Rates of the metal exchange reactions of [Mn(EHDTA)]2− and [Mn(OBETA)]2− with Cu2+ were measured by spectrophotometry by following the formation of the related Cu2+ complexes at 295 nm with an Applied Photophysics DX-17MV stopped-flow instrument. The concentration of [Mn(EHDTA)]2− and [Mn(OBETA)]2− was 1.0 × 10−4 M, whereas the concentration of Cu2+ was 10–40 times higher in order to guarantee pseudo-first-order conditions. The temperature was maintained at 298 K and the ionic strength of the solutions was kept constant with 0.15 M NaCl. Rates of the exchange reactions with Cu2+ were studied in the pH range of ca. 3.3–4.9. To keep the pH values constant, monochloroacetic acid (pH range 2.0–3.3), 1,4-dimethylpiperazine (pH range 3.3–4.1) and N-methylpiperazine (pH range 4.1–5.0) buffers were used (0.01 M). The pseudo-first-order rate constants (kd) were calculated by fitting the absorbance data to eqn (1).| At = (A0 − Ap)e−kdt + Ap | (1) |
NMR experiments
1H and 13C NMR measurements were performed using a Bruker Avance III (9.4 T) spectrometer, equipped with a Bruker Variable Temperature Unit (BVT), a Bruker Cooling Unit (BCU) and a BB inverse z gradient probe (5 mm). VT-NMR spectra of [Zn(EHDTA)]2−, [Zn(OBETA)]2− and [Zn(EGTA)]2− were measured in 0.05 M samples prepared at pD = 7.40 in D2O. The 1H–1H (COSY), 1H–1H (EXSY) and 1H–13C (HSQC) correlation spectra were collected by using gradient pulses in the z direction with the standard Bruker pulse program. Band-shape analyses were performed by using the DNMR program included in the Bruker Topspin software package. Values of the chemical shift, the spin–spin coupling constants, intensity and LB without chemical exchange were fixed input parameters during the fitting procedure. Co-plotting the measured and calculated 1H and 13C NMR spectra obtained at different temperature shows a similarity >92% in all cases.Relaxometric measurements
The 1H 1/T1 NMRD profiles were obtained with a fast-field cycling Stelar SmartTracer relaxometer (Mede, Pavia, Italy) varying the magnetic-field strength from 0.00024 to 0.25 T (0.01–10 MHz range). The 1/T1 values are measured with an absolute uncertainty of ±1%. Temperature was controlled with a Stelar VTC-91 airflow heater equipped with a calibrated copper–constantan thermocouple (uncertainty of ±0.1 K). Data at higher magnetic field strength values (0.5–3 T, corresponding to 20–120 MHz proton Larmor frequency) were collected with a high field relaxometer (Stelar) equipped with the HTS-110 3T metrology cryogen-free superconducting magnet. The measurements were performed with a standard inversion recovery sequence (20 experiments, 2 scans) with a typical 90° pulse width of 3.5 μs, and the reproducibility of the data was within ±0.5%. The exact concentration of Mn2+ was determined by measurement of bulk magnetic susceptibility shifts of a tBuOH signal.2317O NMR data were recorded on a Bruker Avance III spectrometer (11.7 T) using a 5 mm probe under temperature control. An aqueous solution of the complex was enriched to achieve 2.0% of the 17O isotope (Cambridge Isotope Laboratories). The transverse relaxation rates were measured from the signal width at half-height as a function of temperature in the 278–350 K range.
DFT studies
The geometries of the Mn2+ complexes were optimized using the Gaussian 16 program package (version C.01).30 In these calculations we used the range-separated hybrid wB97XD31 functional (22% HF exchange) and the Def2-TZVPP32 basis set. All complexes were modelled in their sextet ground states using unrestricted wave functions. The stationary points obtained as a result of geometry optimization were characterised by frequency calculations. Solvent effects were incorporated using the integral equation formalism variant of the polarized continuum model (IEFPCM).33 Spin restricted calculations were conducted for the Zn2+ analogues using the same methodology. A superfine integration grid was used throughout.Results and discussion
Design and synthesis
The chelating agents OBETA and EHDTA share a common backbone represented by a 2,2′-oxybis(ethylamine) moiety, bearing the coordinating carboxymethyl arms on the distal nitrogen atoms. The backbone in EHDTA is embedded in a mesocyclic 5-membered tetrahydrofuran ring, with a cis-stereochemistry imperative to provide the correct convergent positioning of the whole donor atom set.Straightforward functionalisation of the commercially available 2,2′-oxydiethylamine allows the easy preparation of OBETA.24,34,35 Even if the parent diamine of EHDTA (cis-2,5-bis(amino-methyl)tetrahydrofuran, 6) is claimed to be a potential key biomass-derived starting material for the preparation of green polyamides and polyurethanes, large scale processes are still in their infancy and the commercial availability of this interesting diamine is limited to date to very small amounts.
The N–O–N donor set, endowed with a connectivity well suited for the formation of chelate rings and rigidified by the structural constraint imposed by the stereochemically defined oxolane ring, makes this diamine a preferred scaffold for coordination chemistry studies. This prompted us to explore in more detail alternative synthetic paths for this compound, along with the need for accessing multigram amounts for our purposes.
We recently reported a synthetic approach for the mesocyclic chelator EHDTA (Scheme 1, route A), starting from biomass-derived furfurylamine 1, initially protected as N-acetyl derivative 2. Tscherniak–Einhorn aminomethylation with N-hydroxymethylacetamide 3 generated the symmetrically difunctionalised furandiamide 4. Catalytic hydrogenation of the heterocyclic ring provided the stereochemically pure cis-diamide 5, which was then hydrolysed under acidic conditions to afford the desired diamine 6.
In this work, we report an alternative synthetic approach for diamine 6, outlined in Scheme 1 (route B), by starting from the well-known conversion of natural fructose to 5-hydroxymethylfurfural (HMF, 8). Catalytic hydrogenation of 8 selectively provides the cis-diol 9. Functional group interconversion was accomplished by O,O′-ditosylation (10), nucleophilic disubstitution (11), and hydrogenolysis, finally leading to the diamine 6.
For both routes, the synthesis of the chelating agent is completed by alkylating diamine 6 with t-butyl bromoacetate in acetonitrile, yielding tetraester 7, which is finally deprotected to give EHDTA.
The starting materials for both synthetic routes are commercially available in large amounts and are relatively cheap. Even if route A shows higher yield and a better atom economy with respect to route B, the availability of two distinct and proven synthetic approaches is extremely useful for the future preparation of lipophilic and bifunctional derivatives of EHDTA.
Solution thermodynamic studies
Protonation constants of EHDTA, OBETA and EGTA and stability constants of their [Mn(OBETA)]2− and [Mn(EGTA)]2− complexes were previously studied by our group.24,26 However, the stability and protonation constants of [Mn(EHDTA)]2− were not investigated before. Therefore, we decided to determine the stability and protonation constants of [Mn(EHDTA)]2− by pH-potentiometry. The protonation constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KiH) of the EHDTA ligand defined by eqn (2) and obtained by pH-potentiometry are listed in Table 1 (standard deviations are shown in parentheses),
KiH) of the EHDTA ligand defined by eqn (2) and obtained by pH-potentiometry are listed in Table 1 (standard deviations are shown in parentheses), | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnL, log
KMnL, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMn(HiL) and pMn values of the [Mn(EHDTA)]2− are listed and compared with those of [Mn(OBETA)]2− and [Mn(EGTA)]2− complexes in Table 1 (pMn = −log[Mn2+]free, [Mn2+]t = [L]t = 10 μM, pH = 7.4).
KMn(HiL) and pMn values of the [Mn(EHDTA)]2− are listed and compared with those of [Mn(OBETA)]2− and [Mn(EGTA)]2− complexes in Table 1 (pMn = −log[Mn2+]free, [Mn2+]t = [L]t = 10 μM, pH = 7.4). | (3) |
 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnL values of [Mn(EHDTA)]2− and [Mn(OBETA)]2− are quite similar and about 0.9log
KMnL values of [Mn(EHDTA)]2− and [Mn(OBETA)]2− are quite similar and about 0.9log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K units higher than that of [Mn(EGTA)]2−. Although the ∑log
K units higher than that of [Mn(EGTA)]2−. Although the ∑log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KiH values of EHDTA, OBETA and EGTA are very similar, the presence of an additional ether donor atom in EGTA decreases the thermodynamic stability as compared to OBETA, while the incorporation of a rigid tetrahydrofuran ring in the backbone of OBETA to give EHDTA does not have a significant impact on the stability constants of the Mn2+ complexes. The higher Mn2+ affinity of EHDTA and OBETA compared to EGTA is also confirmed by their conditional stability constants (pMn) calculated at pH = 7.4 and 25 °C in the presence of 10 μM Mn2+ and ligand. Since the basicity of the three ligands are comparable (∑log
KiH values of EHDTA, OBETA and EGTA are very similar, the presence of an additional ether donor atom in EGTA decreases the thermodynamic stability as compared to OBETA, while the incorporation of a rigid tetrahydrofuran ring in the backbone of OBETA to give EHDTA does not have a significant impact on the stability constants of the Mn2+ complexes. The higher Mn2+ affinity of EHDTA and OBETA compared to EGTA is also confirmed by their conditional stability constants (pMn) calculated at pH = 7.4 and 25 °C in the presence of 10 μM Mn2+ and ligand. Since the basicity of the three ligands are comparable (∑log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KiH, Table 1) it is not surprising that the pMn values of [Mn(EHDTA)]2− and [Mn(OBETA)]2− are quite similar and somewhat higher than that of [Mn(EGTA)]2−, because of their higher stability constant. By taking into account the protonation constants of the ligands and the stability and protonation constants of the Mn2+ complexes, the speciation of the Mn2+-EHDTA, Mn2+-OBETA and Mn2+-EGTA systems have been calculated (Fig. S12–S14†). The stability trend observed is in agreement with the negative contributions to both log
KiH, Table 1) it is not surprising that the pMn values of [Mn(EHDTA)]2− and [Mn(OBETA)]2− are quite similar and somewhat higher than that of [Mn(EGTA)]2−, because of their higher stability constant. By taking into account the protonation constants of the ligands and the stability and protonation constants of the Mn2+ complexes, the speciation of the Mn2+-EHDTA, Mn2+-OBETA and Mn2+-EGTA systems have been calculated (Fig. S12–S14†). The stability trend observed is in agreement with the negative contributions to both log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnL and pMn provided by the incorporation of ether donor atoms.36
KMnL and pMn provided by the incorporation of ether donor atoms.36
| EHDTA | OBETA | EGTA | ||||
|---|---|---|---|---|---|---|
| I | 0.1 M KCl | 0.1 M KCla | 0.1 M KClb,c | 0.1 M KNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
0.1 M KClb,c | 0.1 M KNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
| a Ref. 26. b Ref. 23. c Ref. 24. d Ref. 34. e Ref. 37. f pMn = −log[Mn2+]free, [Mn2+]t = [L]t = 10 μM, pH = 7.4. | ||||||
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K1H K1H |
9.37 (2) | 9.40 | 9.34 | 9.39 | 9.43 | 9.47 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K2H K2H |
8.75 (2) | 8.70 | 8.62 | 8.75 | 8.82 | 8.85 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K3H K3H |
3.25 (2) | 2.84 | 3.19 | 2.76 | 2.77 | 2.66 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K4H K4H |
2.21 (2) | 2.11 | 2.19 | 1.80 | 2.06 | 2.00 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K5H K5H |
2.01 (2) | 1.30 | 1.77 | — | 1.88 | — |
∑log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KiH KiH |
25.59 | 24.35 | 25.11 | 22.70 | 24.96 | 22.98 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnL KMnL |
13.17 (1) | 13.57 | 13.7e | 12.28 | 12.2e | |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnHL KMnHL |
3.98 (2) | 3.45 | — | 4.43 | — | |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnH2L KMnH2L |
3.73 (2) | — | — | — | — | |
| pMnf | 7.42 | 7.69 | 7.67 | 6.91 | 6.84 | |
Kinetic inertness of the Mn2+ complexes
To investigate the effect of substituting the oxyethylene bridge of EGTA with an ether oxygen atom or a five-membered tetrahydrofuran ring on the kinetic inertness of Mn2+ complexes, the transmetalation kinetics of [Mn(EHDTA)]2− and [Mn(OBETA)]2− were studied by monitoring their exchange reactions with Cu2+ in the pH range of 3.3–4.9. The mechanisms proposed for the transmetalation reactions of [Mn(EHDTA)]2− and [Mn(OBETA)]2− with Cu2+ are shown in Scheme 2. Experimental details and the equations used to evaluate the kinetic parameters are summarized in the ESI.† The rate and equilibrium constants that characterize the transmetalation reaction of [Mn(EHDTA)]2− and [Mn(OBETA)]2− are shown in Table 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/kd) values characterizing the transmetalation reactions of the Mn2+ complexes with EHDTA, OBETA and EGTA (25 °C)
2/kd) values characterizing the transmetalation reactions of the Mn2+ complexes with EHDTA, OBETA and EGTA (25 °C)
| [Mn(EHDTA)]2− | [Mn(OBETA)]2− | [Mn(EGTA)]2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
|
|---|---|---|---|
| I | 0.15 M NaCl | 0.15 M NaCl | |
a Ref. 27.
b
t
1/2 = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/kd at pH = 7.4 and 25 °C in the presence of 10 μM Cu2+. k1 = kMn(HL) × KMn(HL), k2 = kHMn(HL) × KMn(HL) and k3Cu = kMn(L)Cu × KMn(L)Cu. 2/kd at pH = 7.4 and 25 °C in the presence of 10 μM Cu2+. k1 = kMn(HL) × KMn(HL), k2 = kHMn(HL) × KMn(HL) and k3Cu = kMn(L)Cu × KMn(L)Cu.
|
|||
| k 0 (s−1) | — | — | — |
| k 1 (M−1 s−1) | (2.8 ± 0.3) × 105 | (1.3 ± 0.9) × 104 | 1.9 × 106 |
| k 2 (M−2 s−1) | — | (8 ± 3) × 108 | — |
| k 3 Cu (M−1 s−1) | (4.2 ± 0.9) × 103 | (2.5 ± 0.2) × 103 | 5 × 103 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnHL KMnHL |
3.4 ± 0.1 | 3.2 ± 0.2 | 4.55 |
| K MnLCu | — | 44 ± 20 | 317 |
| k d (s−1) at pH = 7.4b | 5.0 × 10−2 | 2.6 × 10−2 | 0.13 |
| t 1/2 (h) at pH = 7.4b | 3.8 × 10−3 | 7.4 × 10−3 | 1.5 × 10−3 |
The k0 rate constants obtained from the fitting procedure are very low and have a high error margin, confirming that the spontaneous dissociation pathway has no significant contribution to the dissociation rate of [Mn(EHDTA)]2− and [Mn(OBETA)]2− under our experimental conditions (pH 3.3–4.9). Comparing the k1 rate constants of the Mn2+ complexes reveals that the proton-assisted dissociation of [Mn(EHDTA)]2− and [Mn(OBETA)]2− is approximately 7 and 146 times slower, respectively, than that of [Mn(EGTA)]2−. On the other hand, the k3Cu rate constant characterizing the Cu2+-assisted dissociation of [Mn(EHDTA)]2− and [Mn(EGTA)]2− is rather similar and about two times higher than that of [Mn(OBETA)]2−. Systematic kinetic studies of Mn2+ complexes with various open-chain amino-polycarboxylate ligands have shown that a longer ligand backbone increases complex lability and reduces kinetic inertness.27 However, the kinetic inertness of the Mn2+ complexes can be improved by increasing the rigidity of the ligand backbone.20,38 Conversely, the preorganization of iminodiacetate groups imposed by cyclobutyl and cyclopentyl rings in the ligand backbone reduces the kinetic inertness of Mn2+ complexes. This is due to the creation of a suboptimal coordination environment around the Mn2+ ion.20,38 Based on the kinetic data summarized in Table 2, the most important pathway of the transmetalation reactions of [Mn(EHDTA)]2−, [Mn(OBETA)]2− and [Mn(EGTA)]2− is the proton assisted dissociation. Generally, the proton assisted dissociation of the Mn2+ complexes occurs through the formation of a labile protonated Mn(HL) intermediate. The protonation of the Mn2+ complexes takes place on the carboxylate group. The proton transfer from the carboxylate group to the nitrogen of the ligand backbone results in the dissociation of the Mn(HL) intermediate. The relatively fast proton-assisted dissociation of [Mn(EGTA)]2− can be attributed to the greater flexibility of the oxyethylene bridge, which facilitates proton transfer from the carboxylate group to the nitrogen of the iminodiacetate group. Replacing the oxyethylene group in [Mn(EGTA)]2− with an ether oxygen increases the rigidity of the complex, resulting in a slower proton-assisted dissociation of [Mn(OBETA)]2−. On the other hand, the incorporation of the ether oxygen in the tetrahydrofuran ring of OBETA leads to faster proton-assisted dissociation of [Mn(EHDTA)]2− due to the unfavourable coordination environment of the Mn2+ ion. The dissociation rate (kd) and half-life (t1/2) values of the Mn2+ complexes have been calculated at pH = 7.4 in the presence of 10 μM Cu2+ (Table 2). The comparison of the kd and t1/2 values also reveals that the dissociation rate of [Mn(OBETA)]2− is about 2 and 5 times lower than that of [Mn(EHDTA)]2− and [Mn(EGTA)]2−, respectively.
 | ||
| Fig. 2 Plot of the relaxivity (20 MHz, 25 °C) of selected Mn2+ complexes with q = 1 (◆) and q = 0 (◊) as a function of the molecular mass. The linear correlations were calculated without the value of [Mn-OBETA]2−. The chemical structures of all ligands shown in the figure are provided in Scheme S1 of the ESI.† | ||
Meanwhile, [Mn(OBETA)]2− exhibits an r1 value that is distinctly higher (ca. 25%), yet still below the level expected for a monohydrate complex. This suggests that [Mn(OBETA)]2− likely exists in solution as a mixture of two species with differing populations and hydration states: zero and one.22
pH dependency
The assessment of r1 as a function of pH reveals a characteristic pattern observed in small polyamine carboxyl complexes of Mn2+, akin to [Mn(EDTA)]2−. Across a broad spectrum of pH values, the relaxivity remains constant, with a notable increase observed within the acidic pH range (Fig. 3). | ||
| Fig. 3 The relaxivity of the three Mn2+ complexes investigated in this study, measured at 20 MHz and 25 °C, measured as a function of pH. | ||
This increase is likely due to a growing presence of the hexaaqua ions resulting from complex dissociation. Notably, complexes exhibiting greater stability display a wider pH range of constant r1 values.
 | ||
Fig. 4
1H NMRD profiles at 283 ( ), 288 ( ), 288 ( ), 298 (◆) and 310 K ( ), 298 (◆) and 310 K ( ) (pH = 7.5) for [Mn(EGTA)]2− (A), [Mn(EHDTA)]2− (B) and [Mn(OBETA)]2− (C). ) (pH = 7.5) for [Mn(EGTA)]2− (A), [Mn(EHDTA)]2− (B) and [Mn(OBETA)]2− (C). | ||
All the NMRD profiles exhibit a very similar pattern characteristic of low molecular weight and low hydration state complexes, where the OS contribution to r1 is predominant.13 A constant relaxivity region is observed between 0.01 and 1 MHz, followed by a broad dispersion in which r1 decreases with increasing frequency. As typical for q = 0 complexes, the r1 values decrease with increasing temperature following the trend of the solute–solvent diffusion coefficient (D) and the electronic relaxation times Tie (i = 1 and 2). This is especially true for [Mn(EGTA)]2− and [Mn(EHDTA)]2− where the data can be very well reproduced based on the classic Freed model for outer sphere relaxation, obtaining the parameters reported in Table 3.42,43 Given the very similar molecular sizes of the complexes, during the analysis we used standard values for the distance of the closest approach between the paramagnetic ion and the bulk water protons (a) and for the diffusion coefficient 298D. The small difference in relaxivity values observed at low frequencies is fully explainable based on a small difference in the values of the electronic relaxation parameters, Δ2 and τV. On the other hand, in the case of [Mn(OBETA)]2−, the experimental data can be satisfactorily fitted only by considering the presence in solution of a small population of a species with q = 1. To proceed with this type of analysis, it is necessary to consider a number of additional parameters, such as the rotational correlation time τR, the distance between the Mn2+ ion and the protons of the coordinated water molecule, and the average lifetime τM. We then proceeded with the best-fit procedure, also using q as a variable parameter.44–46 The result obtained is of excellent quality, as shown in Fig. 4, considering a population of 15% of the monohydrated species (q = 0.15 ± 0.01) characterized by a rotational correlation time of 71 ps (Table 3). An independent estimate of the hydration number of [Mn(OBETA)]2− can be obtained using the approximate method proposed by J. Peters and C.F.G.C. Geraldes, based on the low-field (0.01 MHz) r1 value and the molecular weight of the complexes.47 The results confirm that [Mn(OBETA)]2− is partially hydrated. The q value from this approach (q = 0.6 ± 0.4) is higher than the value obtained from the simultaneous analysis of 1H NMRD and 17O NMR data (q = 0.15 ± 0.01). This discrepancy arises from the limited accuracy of the model, estimated at ±0.4. Nevertheless, this result provides further confirmation that the q value of [Mn(OBETA)]2− is greater than that of [Mn(EGTA)]2− (q = 0.4) and [Mn(EHDTA)]2− (q = 0.4). For further verification of the plausibility of the approach and the results, we deemed it appropriate to proceed with a simultaneous fit of the 1H NMR data with the 17O NMR data, fixing q at the value of 0.15.
| Parameters | [Mn(OBETA)]2− | [Mn(EGTA)]2− | [Mn(EHDTA)]2− |
|---|---|---|---|
| a Fixed in the fitting procedure. a and 298D were fixed to 3.4 Å and 2.24 × 10−5 cm2 s−1, respectively. | |||
| 22 r 1 (298 K) (mM−1 s−1) | 2.0 | 1.7 | 1.7 |
| Δ 2/1019 s−2 | 4.4 ± 0.3 | 1.4 ± 0.2 | 2.6 ± 0.1 |
| τ V/ps | 20 ± 1 | 31 ± 2 | 30 ± 1 |
| τ M/ns | 6.7 ± 0.2 | — | — |
| ΔHM/kJ mol−1 | 31.5 ± 1.0 | — | — |
| τ R/ps | 71 ± 3 | — | — |
| A O/h/106 rad s−1 | 31.5 ± 1.0 | — | — |
| q | 0.15 ± 0.01 | 0a | 0a |
| r/Å | 2.83a | — | — |
17O NMR data
The optimal method for investigating the water exchange process in paramagnetic metal complexes involves measuring variable temperature 17O NMR transverse relaxation rates (R2) and shift values (Δω) of 17O-enriched water molecules.48 These measurements provide the exchange rate constant (kex = 1/τM) and the associated activation parameters. The resulting data can be analysed using the Swift–Connick equations.49 For very fast exchange, R2 decreases with increasing temperature, whereas for very slow exchange, R2 increases with temperature. Often, there is a maximum in the curve, indicating the transition from the “slow exchange” to the “fast exchange” regime.39 Finally, a global fitting of the shift and relaxation data is performed to obtain the kinetic parameters of the exchange. The R2 values for the three complexes were measured over the temperature range of 275–355 K (Fig. S19†). For [Mn(EGTA)]2− and [Mn(EHDTA)]2−, the data clearly indicate the absence of a water molecule in the inner coordination sphere of Mn2+, as the measured values deviate only slightly from those of pure solvent. In contrast, [Mn(OBETA)]2− exhibits a profile with a maximum at low temperatures, indicative of a rapid exchange regime. However, the profile's amplitude is significantly reduced compared to that of a complex with q = 1, supporting the hypothesis that a species with q = 1 is present in solution, albeit with a relatively small population. Experimental data are typically reported as reduced relaxation rates (R2r) and chemical shifts (Δωr) following normalization to the mole fraction of the bound water molecule (Fig. 5).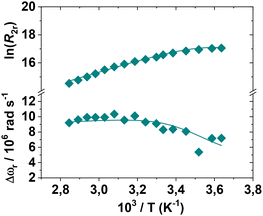 | ||
| Fig. 5 Reduced transverse relaxation rates and chemical shifts for an aqueous solution of [Mn-OBETA]2− at pH = 7.4 (11.7 T). | ||
The R2r values show a clear increase with decreasing temperature, a behaviour typical of complexes characterized by a fast exchange regime. The calculated τM value is approximately 7 ns, associated with a low activation enthalpy (ΔHM). Additionally, the presence of two species in equilibrium with different q values implies a small energy difference between the corresponding hydration states, resulting in a high water exchange rate. Thus, despite the implicit approximation that q is temperature-independent, the 1H and 17O NMR relaxometric data are well-reproduced using the parameters in Table 3, providing a detailed description of the behaviour of [Mn(OBETA)]2− in aqueous solution.
Solution structure and dynamics
The X-ray diffraction analysis of single crystals of Sr[Mn(EGTA)]·7H2O is reported in the literature, enabling detailed examination of the solid-state structure of the [Mn(EGTA)]2− complex.20 The Mn2+ ion (ionic radius 0.96 Å for CN 8)50 is directly bound to the eight donor atoms of the ligand: two ether (O5, O6), four carboxylate oxygen (O1, O3, O7, O9) and two nitrogen atoms (N1, N2). The Mn2+ ion is situated within a distorted dodecahedral coordination polyhedron. This structure is characterized by a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 arrangement, with the O1 atom at the top, followed by two nearly parallel pseudo-planes formed by atoms N1, N2, O6, and O7 in the first plane, and O3, O5, and O9 in the second (Fig. S39, ESI†). A similar coordination geometry was also identified around the larger Cd2+ ion (ionic radius: 1.10 Å for CN 8) in Sr[Cd(EGTA)]·7H2O.51 The analysis of the bond distances in [Mn(EGTA)]2− reveals that the Mn–O distances to ether oxygen atoms (Mn–O5: 2.495 Å, Mn–O6: 2.529 Å) are significantly longer than those involving the carboxylate oxygen donor atoms (2.21–2.29 Å). DFT calculations provide a geometry for the [Mn(EGTA)]2− very similar to the X-ray structure (Table S3, ESI†), although it overestimates significantly the Mn–O6 distance involving one of the ether oxygen atoms. The DFT structure obtained for the [Zn(EGTA)]2− complex shows a very similar wrapping of the ligand around the metal ion, with the Zn–O6 distance being further enlarged to 3.011 Å. Thus, the Mn2+ and Zn2+ complexes exhibit similar structures, with the metal ion weakening the interaction with the ether oxygen atoms as its ionic radius decreases. DFT calculations also provide very similar structures for the Mn2+ and Zn2+ complexes of OBETA and EHDTA (Fig. S43 and 44, ESI†). Thus, we investigated the dynamics of this family of Mn2+ complexes by performing multinuclear 1D and 2D NMR studies on the diamagnetic Zn2+ analogues.
3 arrangement, with the O1 atom at the top, followed by two nearly parallel pseudo-planes formed by atoms N1, N2, O6, and O7 in the first plane, and O3, O5, and O9 in the second (Fig. S39, ESI†). A similar coordination geometry was also identified around the larger Cd2+ ion (ionic radius: 1.10 Å for CN 8) in Sr[Cd(EGTA)]·7H2O.51 The analysis of the bond distances in [Mn(EGTA)]2− reveals that the Mn–O distances to ether oxygen atoms (Mn–O5: 2.495 Å, Mn–O6: 2.529 Å) are significantly longer than those involving the carboxylate oxygen donor atoms (2.21–2.29 Å). DFT calculations provide a geometry for the [Mn(EGTA)]2− very similar to the X-ray structure (Table S3, ESI†), although it overestimates significantly the Mn–O6 distance involving one of the ether oxygen atoms. The DFT structure obtained for the [Zn(EGTA)]2− complex shows a very similar wrapping of the ligand around the metal ion, with the Zn–O6 distance being further enlarged to 3.011 Å. Thus, the Mn2+ and Zn2+ complexes exhibit similar structures, with the metal ion weakening the interaction with the ether oxygen atoms as its ionic radius decreases. DFT calculations also provide very similar structures for the Mn2+ and Zn2+ complexes of OBETA and EHDTA (Fig. S43 and 44, ESI†). Thus, we investigated the dynamics of this family of Mn2+ complexes by performing multinuclear 1D and 2D NMR studies on the diamagnetic Zn2+ analogues.
The 1D (VT-1H and VT-13C NMR) and 2D (1H–1H COSY, 1H–1H EXSY and 1H–13C HSQC) NMR spectra of Zn2+ complexes with EHDTA, OBETA and EGTA measured in the temperature range 273–343 K are shown in Fig. 6 and 7, and S20–S34.† The chemical structure and the possible isomerization of [Zn(EHDTA)]2−, [Zn(OBETA)]2− and [Zn(EGTA)]2− with the assignment of the 1H and 13C NMR signals are shown in Scheme 3.
 | ||
| Fig. 6 1H NMR spectra of [Zn(EHDTA)]2− at 273 K and pH = 7.10 (A), [Zn(OBETA)]2− at 298 K and pH = 7.01 (B) and [Zn(EGTA)]2− at 343 K and pH = 7.04 (C) ([ZnL] = 50 mM, D2O, 9.4 T). | ||
The 1H NMR spectrum of [Zn(EGTA)]2− at 273 K displays four broad signals that indicate a fluxional behaviour in solution. Based on 2D NMR experiments (Fig. S32–S34†), the two broad multiplets and two singlets at 3.45, 3.95, 4.05 and 3.80 ppm in the 1H NMR spectra can be assigned to the 3H, 4H, 5H and 2,2′H methylene protons. The acetate methylene protons are magnetically equivalent due to the fast intramolecular acetate scrambling processes and the shortening of the lifetime of the Zn2+–O− coordination bond on the actual NMR time scale at 273 K (Scheme 3, and Fig. 6). However, the 2,2′H methylene protons are observed as an AB spin system for [Zn(OBETA)]2−, a situation that indicates that the acetate groups are undergoing fast rotation in the NMR timescale leading to a δ ↔ λ change in the configuration of the corresponding five-membered chelate rings. This process can follow a concerted mechanism and does not require de-coordination of the acetate groups. This pathway does not exchange the geminal protons of the acetate groups in [Zn(EHDTA)]2− due to the cis stereochemistry of the tetrahydrofuran ring, and thus the 1H NMR spectrum shows two AB spin systems for 2,2′H methylene protons (Fig. 6A).52,53
The 13C NMR spectra of [Zn(EGTA)]2− and [Zn(EHDTA)]2− recorded at 273 K (Fig. 7) show two signals for the magnetically non-equivalent carbons of the acetate groups (2,2′ and 1,1′, Fig. 7, S21 and S31†) bound to the same amine nitrogen atom. The VT-13C NMR spectra of [Zn(EGTA)]2− and [Zn(EHDTA)]2− reveal a progressive broadening and coalescence of the signals assigned to carbons of the acetate groups by increasing temperature, a situation that can be ascribed to intramolecular exchange between the acetate groups at the same nitrogen atom via inversion of the amine N atom, which requires de-coordination of the acetate group (Fig. S21 and S31,†Scheme 3). However, the 13C NMR spectra of [Zn(OBETA)]2− consist of four signals, which are practically unchanged in the temperature range of 273–343 K (Fig. 7 and S26†), indicating that the inversion of the amine N atom is fast within the timescale of this experiment.
In order to calculate the rate and the activation parameters characterizing the N inversion processes of [Zn(EGTA)]2− and [Zn(EHDTA)]2−, we performed a line-shape analysis of the 2,2′ 13C NMR signals in the temperature range 273–343 K. For [Zn(OBETA)]2−, this dynamic process was investigated using VT-1H NMR spectra, which reveal a broadening and coalescence of the AB system by increasing the temperature (Fig. S25†). The 1H and 13C NMR spectral parameters used for the calculations of the rate constants are summarized in Table S1.† Activation parameters for the N inversion process were estimated with the Eyring equation using the kex rate constant obtained in the line-shape analysis of the VT-1H and VT-13C NMR spectra (Fig. S38† and Table 4).
| [Zn(EHDTA)]2− | [Zn(OBETA)]2− | [Zn(EGTA)]2− | |
|---|---|---|---|
| ΔH‡/kJ mol−1 | 36.0 ± 0.5 | 71 ± 3 | 59 ± 1 |
| ΔS‡/J mol−1 K−1 | −90 ± 2 | −7 ± 3 | 14 ± 4 |
| ΔG‡298/kJ mol−1 | 62.8 | 72.8 | 55.5 |
| k 298ex/s−1 | 57 | 1.1 | 1150 |
The comparison of the activation parameters shown in Table 4 reveals that the N inversion process of [Zn(EGTA)]2− is characterized by a higher enthalpy (ΔH‡) than that in [Zn(EHDTA)]2−, with the entropy (ΔS‡) values having opposite signs. The relatively large negative value of the activation entropy for the N inversion in [Zn(EHDTA)]2− suggests that this process may be assisted by the coordination of a water molecule. For instance, a pseudo-heptacoordinated [Zn(CDTA)(H2O)]2− complex with a partially open acetate arm was observed in the X-ray structure of [{Zn(H2O)4}{Zn(CDTA)(H2O)}]·4H2O.54 The presence of the water molecule in the inner sphere of Zn2+ ion results in the weakening of the Zn2+–O− and Zn2+–N bonds, which may facilitate the de-coordination of the acetate arm and the inversion of the N donor atom in [Zn(EHDTA)]2−. However, the N inversion process of [Zn(EGTA)]2− cannot take place via the formation of a [Zn(EGTA)(H2O)]2− intermediate due to the presence of eight donor atoms in the flexible ligand backbone. Based on the relatively high enthalpy and small entropy values, it might be assumed that the N inversion process of [Zn(EGTA)]2− occurs by the substitution in the inner sphere of the Zn2+ ion of the carboxylate–O atom by an otherwise weakly coordinated ether O atom. Because of the large negative value of the activation entropy, the N inversion of [Zn(EHDTA)]2− is an entropy controlled process, which is characterized by significantly higher activation free energy (ΔG‡298) and lower exchange rate (k298ex) than in [Zn(EGTA)]2−.
According to the parameters summarized in Table 4, the coordination/decoordination processes of the carboxylate oxygen atoms in [Zn(OBETA)]2− is characterized by very high activation enthalpy and a slightly negative activation entropy, which is consistent with the formation of a temporarily ordered water shell in the transition state.
The dynamics of the N inversion in Zn2+ complexes correlate very well with the dissociation kinetics data of the Mn2+ analogues. In particular, complex dissociation following the acid-catalysed mechanism likely proceeds through the protonation of a negatively charged acetate group. This proton then triggers a cascade of processes involving carboxylate decoordination, transfer of the proton to an amine N atom and eventual complex dissociation. The NMR studies indicate that carboxylate decoordination is slower in [Zn(OBETA)]2− than in [Zn(EHDTA)]2−and [Zn(EGTA)]2−, which correlates nicely with the kinetics of the complex dissociation summarized in Table 2.
Conclusions
In this study, we investigated the relaxometric, thermodynamic, and kinetic properties of three Mn2+ complexes with structurally related polyetheraminocarboxylate ligands. The potentially octadentate EGTA ligand and the heptadentate EHDTA form complexes that lack inner-sphere water molecules. However, it is somewhat surprising that a fraction of the OBETA complex contains an inner-sphere water molecule in aqueous solution. It remains unclear whether this results from the formation of an eight-coordinate complex or partial de-coordination of the ligand, allowing water coordination.All three complexes exhibit comparable thermodynamic stability, although the inclusion of an additional ether donor atom in the ligand scaffold slightly reduces stability. The dissociation rates of these complexes differ significantly, with the EGTA complex being notably more labile. Interestingly, the observed dissociation rates correlate with the nitrogen inversion dynamics, as studied by NMR for their Zn2+ analogues. While studies on additional Mn2+/Zn2+ complexes are required to demonstrate this connection, the results reported here suggest that NMR studies on Zn2+ analogues are useful to assess the kinetic inertness of Mn2+ complexes.
The work reported here highlights how small structural changes may have a significant impact on the properties of Mn2+ complexes. The rigidification of the ligand scaffold of OBETA to give EHDTA does not result in an enhanced kinetic inertness, as it is generally observed. This structural modification does not hinder the dynamics of the complex, as shown by NMR studies on the Zn2+ analogue, and thus fails to slow down complex dissociation.
In summary, the lower stereochemical rigidity of OBETA not only promotes greater kinetic inertness, but also allows for the presence of a small population of monohydrated Mn2+ complexes (q = 1). This further confirms that, in the case of Mn2+ complexes, it is challenging to make accurate predictions based solely on the ligand's core structure. Significant variations in the hydration state and kinetic inertness can be induced by making slight modifications to the ligand backbone or altering the chemical nature of one or more pendant arms. We hope that by continuing in this direction, it will be possible to significantly increase the population of the q = 1 species without compromising—or even enhancing—kinetic inertness and thermodynamic stability by appropriately modifying [Mn(OBETA)]2−.
Author contributions
Conceptualization, project administration and supervision: M. B., G. B. G. and Z. B.; funding acquisition: M. B. and G. B. G.; preparation of the chelating agents and complexes: F. T., R. N. and F. C.; relaxometric studies: M. B. and F. C.; complexometric studies: F. K. and Z. B.; DFT studies: C.P.-I.; writing and finalization of the manuscript: all authors.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.† Raw data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is part of the project NODES which has received funding from the MUR – M4C2 1.5 of PNRR with grant agreement no. ECS00000036 (M. B., G. B. G., F. T.). C. P.-I. thanks Ministerio de Ciencia e Innovación (PID2022-138335NB-I00) and Xunta de Galicia (ED431C 2023/33) for generous financial support, as well as Centro de Supercomputación de Galicia (CESGA) for providing the computing facilities. The research was also partially funded by the Hungarian National Research, Development and Innovation Office (FK-134551) project, the New National Excellence Program ÚNKP-22-5 (F. K. K.). F. K. K. acknowledges financial support of the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.References
- J. Wahsner, E. M. Gale, A. Rodríguez-Rodríguez and P. Caravan, Chem. Rev., 2019, 119, 957–1057 CrossRef CAS PubMed.
- M. Le Fur and P. Caravan, Metallomics, 2019, 11, 240–254 CrossRef CAS PubMed.
- H. Malikova, Quant. Imaging Med. Surg., 2019, 9, 1470474–1471474 Search PubMed.
- K. A. Layne, D. M. Wood and P. I. Dargan, Clin. Toxicol., 2020, 58, 151–160 CrossRef CAS.
- L. Yang, I. Krefting, A. Gorovets, L. Marzella, J. Kaiser, R. Boucher and D. Rieves, Radiology, 2012, 265, 248–253 CrossRef PubMed.
- T. Wang, Q. Wu, Z. Wang, G. Dai, H. Jia and S. Gao, ACS Earth Space Chem., 2021, 5, 3130–3139 CrossRef CAS.
- V. Hatje, K. W. Bruland and A. R. Flegal, Environ. Sci. Technol., 2016, 50, 4159–4168 CrossRef CAS.
- R. Brünjes and T. Hofmann, Water Res., 2020, 182, 115966 CrossRef PubMed.
- J. Rogowska, E. Olkowska, W. Ratajczyk and L. Wolska, Environ. Toxicol. Chem., 2018, 37, 1523–1534 CrossRef CAS.
- E. Perrat, M. Parant, J.-S. Py, C. Rosin and C. Cossu-Leguille, Environ. Sci. Pollut. Res. Int., 2017, 24, 12405–12415 CrossRef CAS PubMed.
- E. M. Snyder, D. Asik, S. M. Abozeid, A. Burgio, G. Bateman, S. G. Turowski, J. A. Spernyak and J. R. Morrow, Angew. Chem., Int. Ed., 2020, 59, 2414–2419 CrossRef CAS.
- Z. Baranyai, F. Carniato, A. Nucera, D. Horváth, L. Tei, C. Platas-Iglesias and M. Botta, Chem. Sci., 2021, 12, 11138–11145 RSC.
- M. Botta, F. Carniato, D. Esteban-Gómez, C. Platas-Iglesias and L. Tei, Future Med. Chem., 2019, 11, 1461–1483 CrossRef CAS PubMed.
- A. Gupta, P. Caravan, W. S. Price, C. Platas-Iglesias and E. M. Gale, Inorg. Chem., 2020, 59, 6648–6678 CrossRef CAS.
- E. M. Gale, I. P. Atanasova, F. Blasi, I. Ay and P. Caravan, J. Am. Chem. Soc., 2015, 137, 15548–15557 CrossRef CAS PubMed.
- S. Aime, M. Botta, D. Esteban-Gómez and C. Platas-Iglesias, Mol. Phys., 2019, 117, 898–909 CrossRef CAS.
- D. Ndiaye, M. Sy, A. Pallier, S. Même, I. de Silva, S. Lacerda, A. M. Nonat, L. J. Charbonnière and É. Tóth, Angew. Chem., Int. Ed., 2020, 59, 11958–11963 CrossRef CAS PubMed.
- E. Molnár, B. Váradi, Z. Garda, R. Botár, F. K. Kálmán, É. Tóth, C. Platas-Iglesias, I. Tóth, E. Brücher and G. Tircsó, Inorg. Chim. Acta, 2018, 472, 254–263 CrossRef.
- K. Pota, Z. Garda, F. K. Kálmán, J. L. Barriada, D. Esteban-Gómez, C. Platas-Iglesias, I. Tóth, E. Brücher and G. Tircsó, New J. Chem., 2018, 42, 8001–8011 RSC.
- J. Martinelli, E. Romano, A. Laczovics, D. Horváth, E. Grattoni, Z. Baranyai and L. Tei, Chem. – Eur. J., 2024, 30, e202400570 CrossRef CAS PubMed.
- L. Tei, G. Gugliotta, M. Fekete, F. K. Kálmán and M. Botta, Dalton Trans., 2011, 40, 2025–2032 RSC.
- G. A. Rolla, C. Platas-Iglesias, M. Botta, L. Tei and L. Helm, Inorg. Chem., 2013, 52, 3268–3279 CrossRef CAS.
- Z. Baranyai, M. Botta, M. Fekete, G. B. Giovenzana, R. Negri, L. Tei and C. Platas-Iglesias, Chem. – Eur. J., 2012, 18, 7680–7685 CrossRef CAS.
- R. Negri, Z. Baranyai, L. Tei, G. B. Giovenzana, C. Platas-Iglesias, A. C. Bényei, J. Bodnár, A. Vágner and M. Botta, Inorg. Chem., 2014, 53, 12499–12511 CrossRef CAS PubMed.
- R. Negri, F. Carniato, M. Botta, G. B. Giovenzana and L. Tei, ChemPlusChem, 2016, 81, 235–241 CrossRef CAS PubMed.
- F. Travagin, M. L. Macchia, T. Grell, J. Bodnár, Z. Baranyai, F. Artizzu, M. Botta and G. B. Giovenzana, Dalton Trans., 2024, 53, 1779–1793 RSC.
- F. K. Kálmán and G. Tircsó, Inorg. Chem., 2012, 51, 10065–10067 CrossRef PubMed.
- H. M. Irving, M. G. Miles and L. D. Pettit, Anal. Chim. Acta, 1967, 38, 475–488 CrossRef CAS.
- L. Zekany and I. Nagypal, in Computational Methods for the Determination of Formation Constants, ed. D. J. Leggett, Springer US, Boston, MA, 1985, pp. 291–353 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Rev. C.01, Wallingford, CT, 2016 Search PubMed.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS.
- J. L. Mackey, M. A. Hiller and J. E. Powell, J. Phys. Chem., 1962, 66, 311–314 CrossRef CAS.
- J. R. Geigy AG, GB Pat., GB695346, 1953 Search PubMed.
- R. Uzal-Varela, F. Pérez-Fernández, L. Valencia, A. Rodríguez-Rodríguez, C. Platas-Iglesias, P. Caravan and D. Esteban-Gómez, Inorg. Chem., 2022, 61, 14173–14186 CrossRef CAS PubMed.
- G. Anderegg, Helv. Chim. Acta, 1964, 47, 1801–1814 CrossRef CAS.
- R. Uzal-Varela, D. Lalli, I. Brandariz, A. Rodríguez-Rodríguez, C. Platas-Iglesias, M. Botta and D. Esteban-Gómez, Dalton Trans., 2021, 50, 16290–16303 RSC.
- L. Helm, J. R. Morrow, C. J. Bond, F. Carniato, M. Botta, M. Braun, Z. Baranyai, R. Pujales-Paradel, M. Regueiro-Figuero, D. Esteban-Gómez, C. Platas-Iglesias and T. J. Scholl, in Contrast Agents for MRI: Experimental Methods, ed. V. C. Pierre and M. J. Allen, The Royal Society of Chemistry, 2017, ch. 2, pp. 121–242 Search PubMed.
- S. Aime, M. Botta and E. Terreno, in Advances in Inorganic Chemistry, Academic Press, 2005, vol. 57, pp. 173–237 Search PubMed.
- S. H. Koenig and R. D. Brown, Prog. Nucl. Magn. Reson. Spectrosc., 1990, 22, 487–567 CrossRef CAS.
- L. Hwang and J. H. Freed, J. Chem. Phys., 1975, 63, 4017–4025 CrossRef CAS.
- J. H. Freed, J. Chem. Phys., 1978, 68, 4034–4037 CrossRef CAS.
- I. Solomon, Phys. Rev., 1955, 99, 559–565 CrossRef CAS.
- N. Bloembergen, J. Chem. Phys., 1957, 27, 572–573 CrossRef CAS.
- N. Bloembergen and L. O. Morgan, J. Chem. Phys., 1961, 34, 842–850 CrossRef CAS.
- J. A. Peters and C. F. G. C. Geraldes, Inorganics, 2018, 6, 116 CrossRef CAS.
- D. H. Powell, O. M. N. Dhubhghaill, D. Pubanz, L. Helm, Y. S. Lebedev, W. Schlaepfer and A. E. Merbach, J. Am. Chem. Soc., 1996, 118, 9333–9346 CrossRef CAS.
- T. J. Swift and R. E. Connick, J. Chem. Phys., 1962, 37, 307–320 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- C. K. Schauer and O. P. Anderson, J. Am. Chem. Soc., 1987, 109, 3646–3656 CrossRef CAS.
- Y. Ba, R.-F. Song and Z.-W. Qiu, Magn. Reson. Chem., 1989, 27, 916–921 CrossRef CAS.
- J. Maigut, R. Meier, A. Zahl and R. van Eldik, Inorg. Chem., 2008, 47, 5702–5719 CrossRef CAS PubMed.
- A. Fuertes, C. Miravitlles, E. Escrivá, E. Coronado, D. Beltrán and L. Padel, J. Chem. Soc., Dalton Trans., 1989, 863–871 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4dt02761b |
| This journal is © The Royal Society of Chemistry 2025 |





