An enhanced Bi/nZVI activated molecular oxygen process for the degradation of sulfonamide antibiotics in a citrate buffering system†
Xiaoming
Su
ab,
You
Li
a,
Ziqi
Chen
a,
Shan
Jiang
a and
Jianyu
Gong
 *a
*a
aHubei Key Laboratory of Multi-Media Pollution Cooperative Control in Yangtze Basin, School of Environmental Science & Engineering, Huazhong University of Science and Technology (HUST), 1037 Luoyu Road, Wuhan, Hubei 430074, China. E-mail: jygong@hust.edu.cn
bYancheng Academy of Environmental Protection Technology and Engineering, Nanjing University, Yancheng 224000, China
First published on 1st November 2024
Abstract
Citric acid (CA) and sodium citrate (NaCA) have been effectively employed to synergize with bismuth-doped nanoscale zero-valent iron (Bi/nZVI) to degrade sulfonamide antibiotics (SAs) without the need for additional H2O2. In the integrated Bi/nZVI-CA/NaCA system, excellent oxidation activity of sulfamethazine (SM2), sulfadiazine (SD) and sulfamethoxazole (SMX) in the mixed solution was obtained. The bimetallic enhancement alongside ligand complexation significantly promoted Bi/nZVI to catalyze molecular oxygen and was conducive to the spontaneous generation of H2O2. Fe(II)[Cit]− was formed in the CA/NaCA system, and then underwent a Fenton-like reaction with spontaneously produced H2O2 to achieve the oxidation of SAs. Long service life was confirmed by the results of characterization, electrochemical analysis, utilization rate (UR), electronic efficiency (EE) and cycling degradation experiments. In the Bi/nZVI-CA/NaCA system, two comparable degradation pathways (hydroxylation and SO2 extrusion) for SM2, SMX and SD were obtained, while another degradation pathway for SMX was reflected in the opening of the N–O bond on the benzene ring. Additionally, post-reactive solution toxicity was assessed to ensure environmental safety. Overall, our findings provide a theoretical research basis for the effective elimination of SAs from contaminated environments.
1. Introduction
Sulfonamide antibiotics (SAs), a class of persistent organic pollutants, have been widely used in the medicine, animal husbandry and skin care product industries. Although the emission concentration of SAs in surface water or soil is quite low (ng L−1), it largely leads to the emergence of antibiotic resistance genes (ARGs), thereby posing potential risks to both human health and the ecological environment.1,2 Current wastewater treatment practices in treatment facilities primarily target the reduction of nitrogen, phosphorus, and readily biodegradable organics. However, these methods fall short when addressing the removal of emerging organic contaminants such as SAs.3,4 Consequently, it is imperative to investigate and develop novel treatment technologies, including physical,5 biological,6 and chemical approaches,7 to effectively degrade SAs.In the last two decades, nanoscale zero-valent iron (nZVI, Fe0) has been demonstrated to be an effective and promising iron-based material for the removal of organic compounds due to its strong reductive capability, eco-friendliness, and cost-effectiveness.8 Usually, there are two major ways by which nZVI removes organic compounds: direct reduction and the activation of molecular oxygen. Halogenated hydrocarbon organics, characterized by their low reduction potential (E0 = −0.44 V), are readily reduced by nZVI. These halogenated compounds undergo electron uptake from nZVI, leading to dehalogenation as demonstrated in eqn (1).9
| Fe0 + RCl + H+ → RH + Cl− + Fe2+ | (1) |
However, the activation of molecular oxygen by nZVI has garnered greater attention than direct reduction due to its more rapid degradation rate, fewer byproducts, and enhanced mineralization capability. O2 that received one electron would be reduced to ˙O2− (E0 = −0.33 V), while O2 that received two electrons would be reduced to H2O2 (E0 = −0.695 V). Both processes can promote the Fenton reaction involving Fe2+ and H2O2, resulting in the oxidative breakdown of the organic contaminants.10 The process of nZVI to activate molecular oxygen can be described as follows:
| Fe0 + O2 + 2H+ → Fe2+ + H2O2 | (2) |
| Fe2+ + O2 → Fe3+ + ˙O2− | (3) |
| Fe2+ + ˙O2− + 2H+ → Fe3+ + H2O2 | (4) |
| Fe2+ + H2O2 → Fe3+ + ˙OH + OH− | (5) |
It has been reported that the oxidation of nZVI by O2 can lead to the formation of Fe2+ and Fe3+, subsequently resulting in the precipitation of iron oxides (e.g. Fe2O3 and Fe3O4) and iron hydroxides (e.g. FeOOH and Fe(OH)2) on or near the nZVI surface.11 The oxide layer that forms on the iron surface is likely to hinder the formation of reactive oxygen species (ROS).12 A variety of approaches has been explored to enhance the activity of ZVI. Generally, bimetallic modification of nZVI and ligand complexation are considered to be common and effective ways.
Bimetallic modification of nZVI involves depositing metallic materials onto the surface of ZVI via physical or chemical methods. It has been studied that the prepared copper/zero-valent iron nanoparticles can achieve complete degradation when combined with H2O2 to degrade sulfadimethylpyrimidine.13 Nickel modified nano-zero-valent iron also had better degradation performance for tetracycline than nano-zero-valent iron.14 The bimetallic modification significantly boosts the reactivity of ZVI and promotes the removal of pollutants. However, the application of certain bimetallic ZVIs is constrained by various challenges. For instance, bimetallic systems such as Pd/nZVI15,16 and Ag/nZVI17 are cost-prohibitive, while others, like Cu/nZVI18 and Ni/nZVI,19 pose risks of introducing new heavy metals into the environment, which seriously limits their practical deployment. Bismuth (Bi), as a metal element widely existing in nature, has the characteristics of high hydrogen overvoltage and green friendliness.20 Bismuth bimetallics have been demonstrated to play an important role in organic matter removal.21 When nanoscale zero-valent iron activates molecular oxygen, it produces H2O2. Concurrently, bismuth present on the surface of nZVI reacts with this H2O2 to generate ˙OH (as shown in eqn (1)–(10)), thereby underscoring the effectiveness of bismuth in enhancing the environmental remediation capabilities of nZVI.22
| Bi0 + H2O2 → Bi3+ + ˙OH + OH− | (6) |
In addition, the addition of ligands to the reaction systems could decelerate iron deposition onto the nZVI surface, resulting in the production of reactive oxygen species (ROS).23 Commonly utilized ligands include oxalates,24 humic acid25 and ethylenediamine tetraacetic acid (EDTA).26 It has been reported that EDTA can form complexes with some toxic metals present in the environment, potentially causing toxicity to the environment or the human body. Additionally, the use of humic acid or oxalic acid as ligands in pollutant degradation processes is often associated with a low efficiency of degradation. Citric acid (CA), a naturally occurring complexing agent, is ubiquitous in nature and commonly used as an additive in the food industry, being benign to both the environment and human health.27 CA can create a buffer solution with sodium citrate (NaCA) to broaden the range of pH applied.28 Our previous studies have reported that the removal of atrazine was accelerated in various systems such as the Bi/Fe0 + CA system, Bi/Fe0 + NaCA system and Bi/Fe0 + CA + NaCA system, among which the Bi/Fe0 + CA + NaCA system was the most effective system.29 However, the mechanism and pathway by which the Bi/Fe0 + CA + NaCA system activates molecular oxygen to accelerate the degradation of pollutants are still unclear. Therefore, the degradation pathways and mechanisms underlying the use of the CA/NaCA buffer solution in ZVI-mediated synergistic oxidation of antibiotic organic compounds remain to be elucidated.
In this study, we conducted a thorough investigation with several key components: (i) evaluating the degradation efficacy of Bi/nZVI on three SAs using citric acid and sodium citrate as complexing agents, and analyzing the critical factors influencing the degradation process; (ii) appraising the longevity of Bi/nZVI via cycling experiments and material characterization studies; and (iii) postulating the degradation pathways of SAs in the Bi/nZVI-CA/NaCA system, with the goal of elucidating the oxidative degradation mechanism.
2. Materials and methods
nZVI and Bi/nZVI were synthesized following a previously reported method.29 The oxidative degradation experiments of SAs in different systems were carried out, and SAs used in this experiment included SM2 (0.2 mM), SD (0.1 mM), SMX (0.2 mM) and their mixed solution (containing 0.2 mM SM2, 0.1 mM SD and 0.2 mM SMX). 50 mg of dried particles (1 mg L−1), a certain amount of CA (0.1 mM) and NaCA (0.1 mM) were added into brown vials containing 50 mL of contaminant solution without adjusting the initial pH. For more information on materials and methods, see the ESI.†3. Results and discussion
3.1. Oxidative degradation of SAs
4% Bi/nZVI was selected for all other degradation experiments unless specified otherwise (Fig. S1†). Fig. 1a and b show the degradation curves of SM2 and SD in different systems. Both SM2 and SD showed negligible removal when exposed to nZVI or Bi/nZVI in the absence of CA and NaCA. Conversely, in the presence of CA and NaCA, the degradation efficiencies of SM2 and SD increased remarkably within the first 10 min due to the rapid formation of ˙OH. The degradation efficiencies of SM2 and SD in the Bi/nZVI-CA/NaCA system achieved were 74% and 63%, respectively, within 2 h, suggesting that bimetallic modification of nZVI and ligand complexation were favorable to accelerate the Fenton-like reaction rate.Interestingly, the degradation curves of SMX in different systems were also obtained. As seen in Fig. 1c, complete removal of SMX (100%) was achieved both in the nZVI and Bi/nZVI systems, which can be attributed to the direct reduction of nZVI or Bi/nZVI with SMX.30,31 SMX as a polar molecule generally possesses two pKa, i.e., pKa1 ∼ 1.85 and pKa2 ∼ 5.6, arising from the protonation of aniline N at pH 1–3 and deprotonation of sulfonamide NH at pH 4–11.32–34 Thus, SMX can exist in the form of cation (SMX(+)), neutral (SMX(0)), or anion molecules (SMX(−)) under different pH conditions. The initial pH of the SMX solution was 5.62, with the solution predominantly containing the species SMX(0) and SMX(−). The zeta potentials (ζ) of the two materials under different pH conditions are consistently positive (Fig. 1c). At a pH of 5.62, the zeta potentials of nZVI and Bi/nZVI are approximately +11 mV, indicating strong positive charges. The presence of SMX(−) at this pH facilitates the removal of SMX through electrostatic forces, resulting in a 100% degradation efficiency in both nZVI and Bi/nZVI systems. In contrast, the nZVI-CA/NaCA system achieves only 69% degradation efficiency within 2 h. The removal of SMX depends on both the direct reduction of SMX by nZVI and the oxidative degradation of SMX by active oxygen species. In the presence of CA and NaCA, the initial pH of the SMX solution is 4.58, with the solution predominantly containing SMX(0) and a minor presence of SMX(−). The positive charge on the surface of nZVI at a pH of 4.58 is only 7.12 mV, which is significantly lower compared to its charge at a pH of 5.62. In the nZVI system, the presence of less SMX(−) and a lower zeta potential significantly reduces of the ability of nZVI to reduce SMX, relying mainly on the oxidative degradation in the nZVI-CA/NaCA system. However, the Bi/nZVI-CA/NaCA system achieved 100% degradation efficiency within 2 h. The positive charge obtained on the surface of Bi/nZVI was ζ = 12.27 mV at pH 4.58, which was higher than that of nZVI (ζ = 7.12 mV), which enhanced the reducibility of Bi/nZVI to SMX. The presence of bismuth not only promoted the reduction but also the oxidation of SMX in the Bi/nZVI-CA/NaCA system.
Overall comparison shows that the Bi/nZVI-CA/NaCA system is one of the several reaction systems that has the best degradation effect on three sulfonamide antibiotics. Thus, the degradation of the mixed liquid in the Bi/nZVI-CA/NaCA system was carried out (Fig. 1d). The degradation efficiencies of SM2, SD and SMX in the mixed solution reached 31%, 57% and 100% respectively in the Bi/nZVI-CA/NaCA system within 2 h. The Bi/nZVI-CA/NaCA system exhibits superior performance towards SMX compared to other methods (Table S1†). The degradation efficiencies of SD and SMX in the mixed solution showed no significant change compared to the degradation efficiencies of SD or SMX alone. However, the degradation efficiency of SM2 in the mixed solution showed a significant decrease compared to the degradation efficiency of SM2 alone, which was largely attributed to the limited ability of the Bi/nZVI-CA/NaCA system to activate molecular oxygen. Then, the limited amount of ˙OH and ˙O2− preferentially attacked SD and SMX with simpler chemical structures compared to SM2, leading to a decrease in the degradation efficiency of SM2. The kinetic analysis of the SAs degradation followed a pseudo first-order reaction rate (Fig. S2†).
Then, the oxidizing capabilities of SM2, SD and SMX in a mixed solution across varying initial DO concentrations were investigated in the Bi/nZVI-CA/NaCA system, as depicted in Fig. S3a–c.† The initial DO concentrations were set at 1.91 mg L−1, 7.89 mg L−1 and 19.38 mg L−1, achieved by saturating the starting solution with N2, no aeration and O2 for 10 min, respectively. As expected, elevated oxygen levels corresponded with increased degradation efficiencies across all oxidative procedures. This enhancement is likely attributable to the facilitation of Fenton-like reactions through the activation of dissolved oxygen. The optimal degradation efficiencies in the mixed solution for SM2 (45%), SD (64%) and SMX (100%) were obtained following a 10 min exposure to bubbled O2. Meanwhile, the variation trend of DO concentration during the degradation of mixed liquid was further explored, as shown in Fig. S3d.† It was found that, regardless of the aeration conditions, the DO concentrations in the mixed solution were nearly fully depleted to approximately 0.5 mg L−1 within 10 min, after which they stabilized over time. This suggests that DO may be converted into H2O2 through either a double electron transfer or a single electron transfer mechanism.11,35 While it has been documented that contaminant removal can be enhanced by elevating the concentration of DO, it is important to acknowledge that excessive O2 could also induce the oxidation of nZVI.36 In the Bi/nZVI-CA/NaCA system, the mixed solution underwent aeration-free degradation for 2 h, after which air was introduced into the solution for an additional 10 min to extend the degradation process, as depicted in Fig. 2a. Interestingly, the concentration of DO surged from 0.5 mg L−1 to 6.7 mg L−1 after aerating the solution for 10 min, but then swiftly decreased to 0.6 mg L−1 within the subsequent 20 min, which implies that oxygen was effectively activated by the Bi/nZVI-CA/NaCA system. Concurrently, the degradation efficiencies for SM2 and SD in the mixed solution were significantly enhanced, achieving 57% and 75% respectively at 3 h, accompanied by a 15% TOC removal rate in the mixed solution (Fig. 2). These results underscore the pivotal role of DO in facilitating the oxidative degradation process and bolstering the accruement of efficiency in the treatment of the mixed solution.
 | ||
| Fig. 2 (a) Sequential degradation efficiency and (b) TOC removal efficiency of the mixed liquid under bubbled air for 10 minutes after the reaction reached stability in the Bi/nZVI-CA/NaCA system. | ||
Fe0 can react with O2 in solution to generate H2O2 under various reaction systems (eqn (2)–(4)), and then generate ˙OH via the traditional Fenton reaction (eqn (5)). To better understand the activation process of molecular oxygen by zero-valent iron, the concentrations of H2O2 in different systems were examined. As shown in Fig. S4,† a relatively high concentration of H2O2 was detected in both the nZVI and Bi/nZVI systems. Conversely, a low concentration or even no H2O2 was detected in the nZVI-CA/NaCA and Bi/nZVI-CA/NaCA systems, indicating that the existence of ligands promoted the production rate of ˙OH, resulting in a reduced H2O2 content in these two buffered systems. Furthermore, the differences of the ROS species produced by nZVI and Bi/nZVI in the presence of ligands were explored. As shown in Fig. 3a and b, there was a progressive increase in the intensities of ˙OH and ˙O2− over the course of the reaction. The concentrations of ˙OH and ˙O2− were observed to be in the descending order with the Bi/nZVI-CA/NaCA system exhibiting the highest level, followed by the nZVI-CA/NaCA system. The bismuth modified nZVI, in synergy with CA and NaCA, was found to be more effective at stimulating the generation of ROS compared to its non-bismuth modified ZVI, thereby promoting the degradation of SAs more efficiently. In the Bi/nZVI-CA/NaCA system, the generation rates of ROS in the initial mixed solution exposed to N2 and that exposed to O2 for 10 min were compared. The yields of ˙OH (Fig. 3c) and ˙O2− (Fig. 3d) in the initial mixed liquid exposed to O2 were significantly higher than that in the solution exposed to N2. This finding aligns with the observed degradation efficiencies of the mixed liquid under varying initial oxygen conditions within the Bi/nZVI-CA/NaCA system, with the sequence being O2 > no aeration > N2. These observations substantiate the critical influence of DO concentration on the generation of ROS by nZVI through both double and single electron transfer mechanisms.37
In order to explore the buffer effect of ligands on the reaction systems, trends in pH variation for SM2 (Fig. S5a†), SD (Fig. S5b†) and SMX (Fig. S5c†) in different systems were detected. A modest consumption of H+ was noted in both the nZVI and Bi/nZVI systems (eqn (2)), resulting in a slight elevation in pH values, ranging between 6 and 7. However, the pH levels in the solutions plummeted sharply from 5–7 to 4–5 with the addition of CA/NaCA, and then maintained at a weakly alkaline level (pH = 8–9) for a short time. The presence of these ligands facilitated the oxidative degradation of pollutants, a process that concurrently produced by-products (eqn (7)) which may impede the generation pathway.38,39 According to the previous analysis of the excellent oxidation activity in the nZVI-CA/NaCA and Bi/nZVI-CA/NaCA systems (Fig. 1), the addition of CA/NaCA is more favorable for the removal of pollutants. Additionally, the pH value of the mixed solution in the Bi/nZVI-NaCA/CA system was further measured, as shown in Fig. S5d.† As expected, a pH trend akin to that within the Bi/nZVI-NaCA/CA system was discerned, bolstering the assertion that the presence of ligands expands the operational pH spectrum for nZVI and Bi/nZVI while enhancing pollutant degradation efficiency.
| Fe0 + 2H+ → Fe2+ + H2 | (7) |
The changes in the concentrations of Fe[total] and Fe(II) were monitored during the degradation of the mixed solution (Fig. S6a†). The amount of iron dissolution was found to be negligible in the Bi/nZVI system. But the presence of CA and NaCA significantly facilitated the dissolution of soluble iron in the Bi/nZVI-CA/NaCA system. The concentration of Fe[total] sharply increased to 2157 μM within the initial 10 min, and subsequently stabilized as the pH transitioned to a weakly alkaline range. This behavior can be largely attributed to the acidic milieu created by CA and NaCA, which is favorable for the release of soluble iron from the Bi/nZVI matrix. In the early stages of the reaction within the Bi/nZVI-CA/NaCA system, soluble iron predominantly existed in the aqueous solution as Fe(II). The concentration of Fe(II) escalated swiftly to 2111 μM within just 10 min, followed by a decrease to 1160 μM at 60 min, after which it remained stable. Meanwhile, the concentration of Fe(III) was gradually increased to 1125 μM within 60 min, after which it remained constant. The occurrence of the Fenton-like reaction led to an elevated Fe(III) concentration accompanied by a decline in the Fe(II) levels in the Bi/nZVI-CA/NaCA system.
The possible loss of initial N and S contents in various sulfonamide antibiotics in the mixed liquid during the degradation process was investigated. As shown in Fig. S6b,† the content of NO3− in the Bi/nZVI-CA /NaCA system (0.85 mg L−1) is slightly higher than that in the Bi/nZVI system (0.64 mg L−1) within 2 h, and the concentrations are both low, indicating that NO3− is not the predominant product of SA degradation. But it is obvious that the concentration of NH4+ (2.13 mg L−1) is significantly higher than that of NO3− in the Bi/nZVI-CA/NaCA system, suggesting that N contained in SAs is predominantly released in the form of NH4+. Meanwhile, the concentrations of SO42− in different systems were investigated (Fig. S6c†). In deionized water, the concentrations of SO42− released by the Bi/nZVI and Bi/nZVI-CA/NaCA system after 2 hours were 10.81 mg L−1 and 16.27 mg L−1, respectively, derived from the iron source reagent (i.e. FeSO4·7H2O) used in the synthesis of Bi/nZVI. In the mixed liquid, the sole Bi/nZVI configuration exhibited minimal oxidative capacity towards SAs, indicating that the primary source of SO42− is FeSO4·7H2O used in the preparation of Bi/nZVI. The concentration of SO42− in the mixed solution of the Bi/nZVI-CA/NaCA system was 1.49 mg L−1 higher than that in deionized water. This increase is attributed to the presence of CA and NaCA, which promote the oxidation of the sulfonamide groups on SAs, leading to the production of SO42−. Therefore, SO42− can be identified as the primary degradation product of SAs in the Bi/nZVI-CA/NaCA system.
In order to investigate the specific role of iron citrate complexes in promoting the degradation of SAs, a complexation system experiment was designed. In the presence of CA and NaCA, Fe2+ or Fe3+ was used as the iron source instead of ZVI. As shown in Fig. S7,† a mixture of three sulfonamide antibiotics was degraded under different reaction systems. The initial concentrations of Fe2+ or Fe3+ was 2 mmol L−1, which was similar to the iron leaching amount of Bi/nZVI in the presence of CA and NaCA, and the initial concentrations of CA and NaCA were both 1 mmol L−1. The initial concentration of H2O2 was 400 μmol L−1. It was found that the four types of reaction systems rapidly degraded the three pollutants in the mixed solution within 1 minute, and then degraded the three pollutants in the mixed solution at a relatively slow rate. The degradation of SAs in the Fe3+ + H2O2 system is mainly due to the catalytic effect of Fe3+. Hydrogen peroxide can decompose and produce reactive oxygen species, reducing the concentration of SAs. In the Fe2+ + CA + NaCA system, the ferrous citrate complex formed by the complexation of Fe2+ with CA and NaCA (eqn (8)) activates molecular oxygen to undergo a series of reactions to generate hydrogen peroxide (eqn (9) and (10)), and ultimately undergoes a Fenton like reaction to produce ˙OH (eqn (11)) for oxidative degradation of SAs. When H2O2 is added, the overall degradation efficiency of the Fe2+ + CA + NaCA + H2O2 system for the three sulfonamide antibiotics is better than that of the traditional Fenton reaction (Fe2+ + H2O2) system, indicating that the ferrous citrate complex formed by the addition of CA and NaCA can degrade SAs more effectively than Fe2+ to some extent, promoting the removal of pollutants.
| Fe2+ + Cit3− → Fe(II)[Cit]− | (8) |
| Fe(II)[Cit]− + O2 → Fe(III)[Cit] + ˙O2− | (9) |
| ˙O2− + Fe(II)[Cit]− + 2H+ → Fe(III)[Cit] + H2O2 | (10) |
| H2O2 + Fe(II)[Cit]− → Fe(III)[Cit] + OH− + ˙OH | (11) |
The service life and reusability of Bi/nZVI in Bi/nZVI-CA/NaCA system were evaluated by conducting three consecutive degradation cycles. Obviously, degradation efficiencies of SM2, SD, SMX consistently demonstrated impressive performance throughout the cycling experiments, with negligible differences in the rate of degradation rate for each cycle, as depicted in Fig. 4a. After the third usage cycle, Bi/nZVI demonstrated an extended service life. This can be attributed to the protective complexes formed on the Bi/nZVI surface, which decelerate its direct oxidation by water and oxygen. Besides, the recyclability of the solution within the Bi/nZVI-CA/NaCA system was also thoroughly investigated (Fig. 4b). The results revealed a consistent trend during the repeated tests of the mixed liquid, thereby affirming the superior reusability of Bi/nZVI. Furthermore, utilization rate (UR) and electronic efficiency (EE) were introduced to evaluate the utilization, selectivity and reusability of Bi/nZVI to demineralize the mixed solution in the presence of CA/NaCA. UR is defined as the ratio of the amount of Bi/ZVI involved in the reaction of the target contaminant during a given reaction time to the total amount of Bi/ZVI initially added to the system, and EE describes the ratio of electrons delivered to the target contaminant to the amount of electrons supplied by Bi/ZVI, which is expressed as follows:
 | (12) |
 | (13) |
3.2. Characterization
The SEM images of nZVI, Bi/nZVI and 3rd Bi/nZVI are shown in Fig. S8.† The 3rd Bi/nZVI represents the Bi/nZVI collected following the completion of three consecutive degradation cycles within the Bi/nZVI-CA/NaCA system. Obviously, nZVI displays a spherical structure, with multiple spheres interconnected via magnetic and electrostatic forces, resulting in the formation of chain-like agglomerates as seen in Fig. S8a.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 The Bi/nZVI system exhibits enlarged spheres compared to nZVI, and a number of spheres are aggregated together to form a block (Fig. S8b†). The morphology of the 3rd Bi/nZVI (Fig. S8c†) is similar to that of Bi/nZVI, suggesting that the oxidation resistance of Bi/nZVI remains robust even after undergoing three degradation cycles. In addition, the SEM-EDS elemental spectrum only reveals the presence of Fe and O in nZVI (Fig. S9a†), while Bi is clearly observed in the samples of both Bi/nZVI (Fig. S9b†) and 3rd Bi/nZVI (Fig. S9c†), indicating not only the successful synthesis of the Bi/nZVI composite but also its remarkable durability through multiple usage cycles. The detailed element content of each sample is listed in Table S2.† In essence, the content of O follows the descending order: nZVI > 3rd Bi/nZVI > Bi/nZVI. Compared with ordinary nZVI, stronger anti-oxidation ability was obtained by Bi/nZVI after bimetallic modification. The O content in the 3rd Bi/nZVI system was slightly higher than that of fresh Bi/nZVI yet remained lower than that of the nZVI system. The observed trend in elemental composition can be rationalized by two key factors: (i) the incorporation of bismuth into the iron matrix impedes the oxidation process of the nanoparticles, effectively diminishing the rate at which they are subject to corrosion. (ii) The formation of complexes, notably ferrous citrate and ferric citrate, which envelop the nanoparticle surfaces, likely serve as a protective layer that discourages the deposition of iron oxides, thereby further retarding the oxidative degradation of Bi/nZVI.
40 The Bi/nZVI system exhibits enlarged spheres compared to nZVI, and a number of spheres are aggregated together to form a block (Fig. S8b†). The morphology of the 3rd Bi/nZVI (Fig. S8c†) is similar to that of Bi/nZVI, suggesting that the oxidation resistance of Bi/nZVI remains robust even after undergoing three degradation cycles. In addition, the SEM-EDS elemental spectrum only reveals the presence of Fe and O in nZVI (Fig. S9a†), while Bi is clearly observed in the samples of both Bi/nZVI (Fig. S9b†) and 3rd Bi/nZVI (Fig. S9c†), indicating not only the successful synthesis of the Bi/nZVI composite but also its remarkable durability through multiple usage cycles. The detailed element content of each sample is listed in Table S2.† In essence, the content of O follows the descending order: nZVI > 3rd Bi/nZVI > Bi/nZVI. Compared with ordinary nZVI, stronger anti-oxidation ability was obtained by Bi/nZVI after bimetallic modification. The O content in the 3rd Bi/nZVI system was slightly higher than that of fresh Bi/nZVI yet remained lower than that of the nZVI system. The observed trend in elemental composition can be rationalized by two key factors: (i) the incorporation of bismuth into the iron matrix impedes the oxidation process of the nanoparticles, effectively diminishing the rate at which they are subject to corrosion. (ii) The formation of complexes, notably ferrous citrate and ferric citrate, which envelop the nanoparticle surfaces, likely serve as a protective layer that discourages the deposition of iron oxides, thereby further retarding the oxidative degradation of Bi/nZVI.
XPS was employed to analyze the elemental composition of the various samples. The survey XPS spectra (Fig. S10a†) show the presence of Fe and O in nZVI, and the existence of Fe, O and Bi in Bi/nZVI and 3rd Bi/nZVI. Typically, the high resolution of the Fe 2p XPS spectra for nZVI, Bi/nZVI and 3rd Bi/nZVI are shown in Fig. S10b.† Fe(0), Fe(II) and Fe(III) exist on the surface of nZVI and Bi/nZVI.40 Two distinct peaks occurring at the binding energies of 707.1 eV and 719.4 eV are attributed to Fe(0), indicating the successful preparation of both nZVI and Bi/nZVI. It was observed that the Fe(0) proportion in nZVI was lower than that of Bi/nZVI, which would be attributed to the absence of a protective bismuth layer on the surface of nZVI, rendering it more susceptible to oxidation. The analysis was further confirmed by the observation of Fe(II) (710.4 eV and 723.8 eV) and Fe(III) (712.2 eV and 726 eV) in Bi/nZVI and the 3rd Bi/nZVI. It was observed that the intensity of the Fe(0) peak for the 3rd Bi/nZVI did not decrease significantly. This may be due to the protective effect of the complexes formed in the cycling degradation experiments encasing the surface of Bi/nZVI, which slowed down the oxidation of Bi/nZVI. Consequently, Bi/nZVI was able to retain high catalytic activity even after three usage cycles. In addition, two distinct peaks at 529.7 eV and 531.2 eV were resolved in the O 1s spectrum (Fig. S10c†), which are assigned to lattice oxygen (O2−) from metallic oxides and adsorbed oxygen (OH−) from α-FeOOH.41,42 The content of OH− in the pristine Bi/nZVI sample surpasses that of O2−, suggesting a predominance of α-FeOOH in the material composition of Bi/nZVI. There was no significant difference between the peaks of O2− and OH− for nZVI and Bi/nZVI, indicating that both the synthesized nZVI and Bi/nZVI exhibit robust stability. Nevertheless, a slight increase in the content of O2− was identified in the Bi/nZVI after the third cycle, thereby implying that 3rd Bi/nZVI experienced some degree of oxidation compared to the initial Bi/nZVI. It nonetheless retained commendable catalytic reactivity. Fig. S10d† shows the high resolution of the Bi 4f XPS spectra of Bi/nZVI and 3rd Bi/nZVI. The peaks of Bi(0) (156.8 and 162.1 eV) and Bi(III) (159.0 and 164.3 eV) were identified. The results showed that Bi0 existed on the surface of fresh Bi/nZVI, indicating that Bi3+ could be reduced to Bi0 by sodium borohydride, and the content of Bi0 in 3rd Bi/nZVI decreased slightly with the progress of the cycling experiment, which could be attributed to the falling off of Bi0 or the self-oxidation of Bi0 during the reaction.
The crystal structures of nZVI, Bi/nZVI and 3rd Bi/nZVI were demonstrated by the XRD (Fig. 5a) results. The distinctive broad peak at 2θ = 44.8° corresponds to the (110) crystallographic plane characteristic of a typical nZVI material (JCPD no. 01-1252).43 The Fe crystalline phase was slightly observed in the diffraction pattern of Bi/nZVI, but the Bi crystal phase was prominently detected at additional diffraction peaks (JCPD no. 96-500-0216). A large amount of Bi was enshrouded on the surface of nZVI, resulting in the covering of the Fe crystal phase in Bi/nZVI. The depletion of bismuth upon repeated use resulted in a more pronounced Fe diffraction peak in 3rd Bi/nZVI compared to the pristine Bi/nZVI, thereby gradually uncovering the Fe crystalline phase. No significant change in the nanoparticles’ structure was observed before and after usage, indicating that the Bi/nZVI retains its functionality over multiple cycles in the Bi/nZVI-CA/NaCA system, indicative of its prolonged service life.
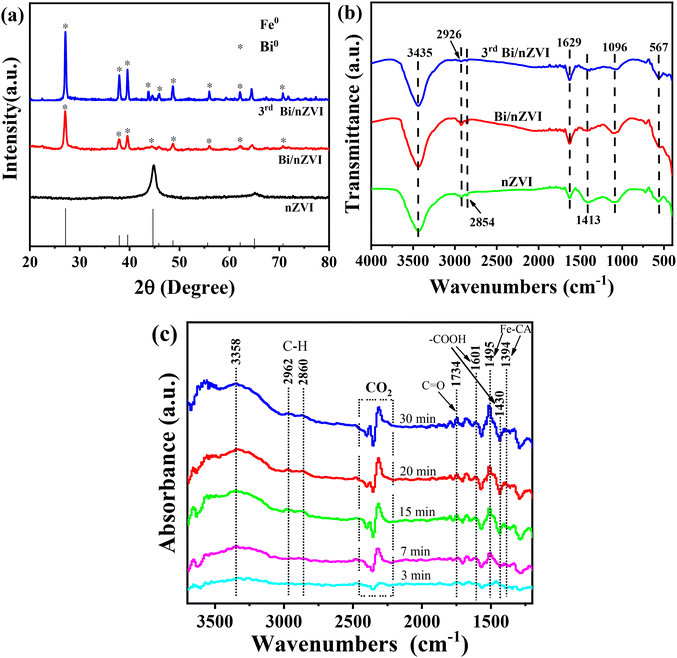 | ||
| Fig. 5 (a) XRD and (b) FT-IR of different samples; (c) in situ DRIFTS spectra of mixed liquid degraded in the Bi/nZVI-CA/NaCA system. | ||
The surface functional groups of each sample were investigated using FT-IR, as shown in Fig. 5b. The stretching and bending vibration of –OH are located at 3435 and 1629 cm−1, respectively. These vibrations are likely due to water molecules and hydroxyl groups attached to the sample surfaces.44 The peaks observed at approximately 2926 and 2854 cm−1 are indicative of C–H stretching vibrations, and the peaks near 1425 cm−1 are caused by C–O stretching vibrations.45 Absorption peaks corresponding to the lattice vibrations of Fe–O bonds, signifying the formation of Fe2O3 and Fe3O4 on the particle surface, were observed in all samples near 567 cm−1, confirming the existence of nZVI.3 Comparative FT-IR analyses of Bi/nZVI before and after the reaction reveal that Bi/nZVI after recycling continues to demonstrate a prolonged operational lifespan. Furthermore, in situ DRIFTS measurement was employed to identify the presence of citric acid complexes on the Bi/nZVI surface, as shown in Fig. 5c. The bands observed in the range of 2200–2400 cm−1 were attributed to CO2 in the environment,46 while the band near 3358 cm−1 was ascribed to the vibrational mode of the water molecules. The peak at 2860 cm−1 corresponds to the asymmetric stretching vibration of methylene (–CH2).47 A weaker intensity band at 2962 cm−1 was associated with the symmetric vibration of the methyl (–CH3) groups present in the citric acid complex adsorbed on the surface of Bi/nZVI.48 In particular, the absorption peak located at 1730 cm−1 was attributable to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond in the free carboxyl (–COOH) group in the system. The –COO groups were evidenced by the characteristic bands at 1601 and 1430 cm−1, which are the typical bands of citrate or its salts.49,50 The citric acid complex adsorbed on the Bi/nZVI surface exhibits a broad symmetric carboxyl stretching vibration at 1394 cm−1. The absorption peak located at 1495 cm−1 is attributed to Lewis acid sites, which arise from the bonding between citric acid and iron atoms.46 With increasing reaction time, this absorption peak intensifies due to the incremental binding of Fe2+and Fe3+ with citric acid adhering to the Bi/nZVI surface.
O double bond in the free carboxyl (–COOH) group in the system. The –COO groups were evidenced by the characteristic bands at 1601 and 1430 cm−1, which are the typical bands of citrate or its salts.49,50 The citric acid complex adsorbed on the Bi/nZVI surface exhibits a broad symmetric carboxyl stretching vibration at 1394 cm−1. The absorption peak located at 1495 cm−1 is attributed to Lewis acid sites, which arise from the bonding between citric acid and iron atoms.46 With increasing reaction time, this absorption peak intensifies due to the incremental binding of Fe2+and Fe3+ with citric acid adhering to the Bi/nZVI surface.
EIS experiments (Fig. S11a†) and Tafel polarization (Fig. S11b†) were conducted to gain insight into the oxidation corrosion of various materials. An important parameter determined from the EIS curve is the charge-transfer resistance (Rct), which can be obtained by fitting the semicircle diameter in the Nyquist plot.51 Additionally, the free corrosion potentials (Ecorr) derived from the Tafel curve is a pivotal parameter, indicative of the electron transfer rate and providing valuable insights into corrosion kinetics. Obviously, the Rct and Ecorr values for nZVI, Bi/nZVI and 3rd Bi/nZVI display a comparable trend, adhering to the sequence: nZVI > 3rd Bi/nZVI > Bi/nZVI. Notably, Bi/nZVI exhibits a smaller Rct and a more negative Ecorr compared to nZVI, suggesting that nZVI when modified with bismuth facilitates electron donation to O2 more readily. This enhancement bolsters the generation of ROS and finally leads to the effective removal of SAs.52 In addition, comparing the oxidation and corrosion of Bi/nZVI before and after the degradation reaction, the values of Rct and Ecorr for 3rd Bi/nZVI were found to be larger, indicating that the surface of 3rd Bi/nZVI became more oxidized, while Bi/nZVI exhibited enhanced reductive capability. Interestingly, the complexes generated during the degradation process encapsulated the surface of 3rd Bi/nZVI, resulting in its Rct and Ecorr being lower than that of nZVI. Therefore, this points to the superior electron-transfer proficiency and extended operational lifespan of 3rd Bi/nZVI.
3.3. Evolution of degradation pathways
The degradation studies were complemented by the identification of various organic intermediates and inorganic ions. A reduced concentration of organic intermediates was noted, attributable to the high reactivity and non-selectivity of ˙OH and ˙O2−. The mass spectrum of intermediate products produced by the degradation of SAs in the Bi/nZVI-CA/NaCA system is shown in Fig. S12.† Fifteen organic intermediates were identified by HR-LC-MS analysis.As shown in Fig. 6a, two degradation pathways for SM2 are proposed: (i) pathway I involves a hydroxylation addition process, where SM2 is initially hydroxylated to form product P1 with m/z = 295. Then, the compounds P2 (m/z = 110) and P3 (m/z = 124) are generated by the cleavage of the S–N bond in P1. (ii) In degradation pathway II, the formation of compound P4 (m/z = 215) is observed via the expulsion of SO2. Subsequently, P4 undergoes reactions with ˙OH and ˙O2−, resulting in the generation of P3 and a new product of P5 (m/z = 110). As expected, two degradation pathways for SD were obtained (i.e. hydroxylation addition and extrusion of SO2), which were similar to the degradation pathways of SM2. The extremely similar molecular structures of SM2 and SD have given rise to comparable pathways of molecular transformation in the Bi/nZVI-CA/NaCA system. Fig. 6b shows the proposed degradation pathways for SD. Initially, the product P6 (m/z = 267) would be formed by the hydroxylation addition reaction of SD (m/z = 251). P6 is subsequently attacked by ˙OH and ˙O2− to form products P7 (m/z = 96) and P2 (m/z = 110). Concurrently, extrusion of SO2 from SD yields the compound P8 (m/z = 187), which undergoes further oxidation to produce P5 and P7. Fig. 6c delineates three degradation pathways for SMX: (i) hydroxyl radicals can easily add or replace aromatic compounds.53,54 Within this pathway, SMX (m/z = 254) is hydroxylated to form P9 (m/z = 270). Subsequent processes include the cleavage of S–N bond and oxidation, which lead to the formation of P2 (m/z = 110) and P10 (m/z = 101). (ii) Product P11 (m/z = 190), resulting from the extrusion of SO2 from SD, underwent further oxidation to yield compounds P5 (m/z = 110) and P12 (m/z = 99). The N–O bond on the five membered ring of P12 broke to form the product P10. (iii) Pathway III is characterized as the direct ring-opening transformation of the five membered ring in SMX, which constitutes the principal degradation mechanism for SMX. The N–O bond in the five membered ring exhibits low bond strength, rendering it susceptible to cleavage, thus resulting in the creation of compound P13 (m/z = 256). Product P14 (m/z = 272) arose from the hydroxylation of a fraction of P13 and subsequently underwent oxidation to form products P2 (m/z = 110) and P10 (m/z = 101). At the same time, another part of P13 led to the creation of P15 via SO2 extrusion, which was further oxidized to produce products P5 (m/z = 110) and P10 (m/z = 101). Ultimately, the organic intermediates generated from the degradation of SAs within the Bi/nZVI-CA/NaCA system, including P2, P3, P5, P7 and P10, underwent successive reactions leading to their conversion into inorganic molecules such as CO2, H2O, NH4+ and SO42−. This process is in agreement with the analyses in Fig. S6.†
The main structure of SAs is a p-aminobenzene sulfonamide, in which a variable substituent (R group) is connected with the N atom on the sulfonamide group (–SO2–NH–). Five-membered or six-membered rings are considered as common substituent groups, and the difference of substituent groups greatly affects their transformation mechanisms and reaction kinetics significantly.55 Specifically, the substituents of SM2 and SD are characterized by six-membered heterocyclic substituents, in contrast to SMX, which features a five-membered oxazole ring as its substituent. The N atom within the five-membered oxazole ring of SMX acts as an electron donor group, indicating that the degradation pathways of SMX may diverge subtly from those of SM2 and SD. The analysis of intermediates formed during the degradation of SAs within the Bi/nZVI-CA/NaCA system reveals that SM2, SD and SMX share two similar degradation pathways: hydroxylation addition and SO2 extrusion. This convergence in transformation routes results in identical intermediates, specifically those with m/z value of 110, identified as P2 or P5. However, SMX exhibits an additional possible degradation pathway in the Bi/nZVI-CA/NaCA system compared to SM2 and SD, mainly characterized by the cleavage of the N–O bond in its five-membered oxazole ring. This mechanism is likely to be the predominant route for SMX degradation. Therefore, it can be speculated that the degradation efficacy of SMX in the Bi/nZVI-CA/NaCA system may surpass that of SM2 or SD. Corroborating this hypothesis, the above-mentioned degradation experiment results illustrated in Fig. 1 further confirmed this conjecture, with SMX achieving the best degradation efficiency of 100%, which exceeds the efficiencies recorded for SM2 and SD.
The degradation pathway of SMX in the Bi/nZVI system was further explored, leading to the detection of two intermediates. The mass spectrum of the intermediate products and detailed degradation pathway of SMX in the Bi/nZVI system are shown Fig. S13 and S14.† In the Bi/nZVI system, the N–O bond in the five-membered ring of SMX is highly susceptible to a ring-opening reaction owing to its relatively low bond energy, resulting in the formation of intermediate P13. Subsequently, P13 undergoes SO2 extrusion to yield product P15. This sequence of transformations provides an explanation for the observed 100% degradation efficiency of SMX in the Bi/nZVI system (Fig. 1). In the Bi/nZVI-CA/NaCA system, the presence of product P15 was confirmed, and it would be further oxidized to inorganic molecules by the attack of ˙OH and ˙O2−, indicating that CA and NaCA may reduce the production of intermediate products that potentially possess greater environmental toxicity. Indeed, Bi/nZVI is capable of degrading SMX through direct electron transfer and the activation of molecular oxygen in the presence of ligating agents. Compared to SM2 and SD, SMX indeed possesses a unique degradation pathway involving the opening of the five-membered ring. The superior degradation efficiency of SMX relative to SM2 and SD substantiates the validity of the proposed degradation pathways for SMX in either the Bi/nZVI or the Bi/nZVI-CA/NaCA system.
3.4. Proposed mechanism
The proposed degradation mechanism of SAs by the Bi/nZVI system in the presence of CA and NaCA is illustrated in Scheme 1. Initially, the acidic environment of the solution was significantly enhanced by ligands, leading to the gradual corrosion of the iron oxide shell layer on the Bi/nZVI surface. This process facilitated the exposure of nZVI, enhancing the reactivity of the system.56 Upon exposure, the metallic iron facilitates the release of Fe2+, which then rapidly coordinates with citrate ions to form ferrous citrate complexes (Fe(II)[Cit]−) as described in eqn (8). This interaction is accompanied by the activation of dissolved molecular oxygen, resulting in the formation of ferric citrate (Fe(III)[Cit]) and ˙O2− (eqn (9)). Subsequent reactions involve ˙O2− engaging with Fe(II)[Cit]− to generate Fe(III)[Cit] and H2O2, as outlined in eqn (10). Finally, Fe(II)[Cit]− reacts with H2O2 in a Fenton-like process that yields ˙OH and leads to the oxidation of contaminants (eqn (11)). Besides, Bi0 can also react with H2O2 to generate ˙OH (eqn (6)). Noticeably, the pollutants could be effectively removed in the Bi/nZVI-CA/NaCA system, mainly due to the presence of citric acid ligands. Compared to the traditional Fenton reaction, the Fenton-like reaction produced by Bi/nZVI-CA/NaCA system reduced the usage of hazardous oxidants such as H2O2, thereby lowering both the environmental impact and economic costs.4. Conclusions
In this work, Bi/nZVI was synthesized mainly using the sodium borohydride reduction method, and the Bi/nZVI-CA/NaCA system was established utilizing CA and NaCA as ligand donors. This system demonstrated remarkable oxidative performance on various sulfonamides and their combined solutions. In the presence of CA and NaCA, the doping of Bi improved the reactivity of zero-valent iron nanoparticles, which have a better degradation effect on pollutants than unmodified zero-valent iron nanoparticles. Concurrently, the incorporation of CA and NaCA provides an acidic environment to the solution, which fosters iron dissolution, subsequently amplifying the capacity of Bi/nZVI to generate ˙OH and ˙O2−. On comparing the degradation pathways of SM2, SD and SMX in the Bi/nZVI-CA/NaCA system, it has been found that the main degradation pathways for these antibiotics include hydroxylation addition and the extrusion of SO2. Additionally, the degradation of SMX is characterized by a unique route involving the cleavage of its five-membered ring structure. Ultimately, all intermediates are converted into small molecular inorganic substances such as CO2, H2O, NH4+ and SO42−. The recognition that molecular oxygen activation by Bi/nZVI serves as the primary mechanism for the degradation of SAs when CA and NaCA are present paves the way for devising strategies for the remediation of other organic pollutants in aqueous environments.Author contributions
Xiaoming Su: conceptualization, methodology, data curation, and writing – original draft. You Li: data curation, writing – original draft, and investigation. Ziqi Chen: data curation, investigation, validation, and visualization. Shan Jiang: validation and visualization. Jianyu Gong: supervision, project administration, and funding acquisition.Data availability
All information regarding the synthesis and degradation performance of iron materials is included in the manuscript or the ESI.†Conflicts of interest
The authors state that they have no known competing financial interests or personal relationships that could influence the work reported in this article.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 22276067). The authors thank the Analytical and Testing Center of HUST for the use of HR-LC-MS and also thank the Public Service Platform of Environmental Research Facilities within the School of Environmental Science and Engineering at Huazhong University of Science and Technology (HUST) for the use of XRD and SEM.References
- Z. Meng, X. Mo, Q. Xue, Z. Wang, X. Lu, J. Liu, Q. Ma, J. P. Sparks and M. He, Distribution, source apportionment, and ecological risk assessment of soil antibiotic resistance genes in urban green spaces, Environ. Res., 2024, 251, 118601 CrossRef CAS PubMed.
- J. Ding, W. Yang, X. Liu, J. Zhao, X. Fu, F. Zhang and H. Liu, Hydraulic conditions control the abundance of antibiotic resistance genes and their potential host microorganisms in a frequently regulated river-lake system, Sci. Total Environ., 2024, 946, 174143–174143 CrossRef CAS PubMed.
- S. Rončević, I. Nemet, T. Z. Ferri and D. Matković-Čalogović, Characterization of nZVI nanoparticles functionalized by EDTA and dipicolinic acid: A comparative study of metal ion removal from aqueous solutions, RSC Adv., 2019, 9, 31043–31051 RSC.
- Y. Zhang, Y. Hu, X. Li, L. Gao, S. Wang, S. Jia, P. Shi and A. Li, Prevalence of antibiotics, antibiotic resistance genes, and their associations in municipal wastewater treatment plants along the Yangtze River basin, China, Environ. Pollut., 2024, 348, 123800 CrossRef CAS.
- Z. Xu, M. Sun, X. Xu, X. Cao, J. A. Ippolito, S. K. Mohanty, B. J. Ni, S. Xu and D. C. W. Tsang, Electron donation of Fe-Mn biochar for chromium(VI) immobilization: Key roles of embedded zero-valent iron clusters within iron-manganese oxide, J. Hazard. Mater., 2023, 456, 131632 CrossRef CAS PubMed.
- L. Leng, L. Wei, Q. Xiong, S. Xu, W. Li, S. Lv, Q. Lu, L. Wan, Z. Wen and W. Zhou, Use of microalgae based technology for the removal of antibiotics from wastewater: A review, Chemosphere, 2020, 238, 124680 CrossRef CAS.
- M. Francoeur, C. Yacou, E. Petit, D. Granier, V. Flaud, S. Gaspard and A. Ayral, Removal of antibiotics by adsorption and catalytic ozonation using magnetic activated carbons prepared from Sargassum sp, J. Water Process Eng., 2023, 53, 103602 CrossRef.
- Z. Tang, Y. F. Kong, Y. Qin, X. Q. Chen, M. Liu, L. Shen, Y. M. Kang and P. Gao, Performance and degradation pathway of florfenicol antibiotic by nitrogen-doped biochar supported zero-valent iron and zero-valent copper: A combined experimental and DFT study, J. Hazard. Mater., 2023, 459, 13 Search PubMed.
- Y. Sun, K. Zheng, X. Du, H. Qin and X. Guan, Insights into the contrasting effects of sulfidation on dechlorination of chlorinated aliphatic hydrocarbons by zero-valent iron, Water Res., 2024, 255, 121494 CrossRef CAS.
- Q. Wang, Y. W. Pan, W. Y. Fu, H. Z. Wu, M. H. Zhou and Y. Zhang, Aminopolycarboxylic acids modified oxygen reduction by zero valent iron: Proton-coupled electron transfer, role of iron ion and reactive oxidant generation, J. Hazard. Mater., 2022, 430, 13 Search PubMed.
- B. Chen, N. Lv, W. F. Xu, L. Gong, T. Y. Sun, L. Y. Liang, B. Gao and F. He, Transport of nanoscale zero-valent iron in saturated porous media: Effects of grain size, surface metal oxides, and sulfidation, Chemosphere, 2023, 313, 8 Search PubMed.
- Z. Wang, L. Qiu, Y. Huang, M. Zhang, X. Cai, F. Wang, Y. Lin, Y. Shi and X. Liu, Alcohothermal synthesis of sulfidated zero-valent iron for enhanced Cr(VI) removal, Chin. Chem. Lett., 2023, 35, 109195 CrossRef.
- A. Adel, M. G. Alalm, H. K. El-Etriby and D. C. Boffito, Optimization and mechanism insights into the sulfamethazine degradation by bimetallic ZVI/Cu nanoparticles coupled with H2O2, J. Environ. Chem. Eng., 2020, 8, 104341 CrossRef CAS.
- H. Dong, Z. Jiang, C. Zhang, J. Deng, K. Hou, Y. Cheng, L. Zhang and G. Zeng, Removal of tetracycline by Fe/Ni bimetallic nanoparticles in aqueous solution, J. Colloid Interface Sci., 2018, 513, 117–125 CrossRef CAS PubMed.
- X. Shang, L. Yang, D. Ouyang, B. Zhang, W. Zhang, M. Gu, J. Li, M. Chen, L. Huang and L. Qian, Enhanced removal of 1,2,4-trichlorobenzene by modified biochar supported nanoscale zero-valent iron and palladium, Chemosphere, 2020, 249, 126518 CrossRef CAS.
- G. N. Zhou, J. Q. Chen, W. Q. Li, C. S. He, L. Gong, X. C. Liu, Y. R. Wang, D. Huang and Y. Mu, Enhanced retention of surface-adsorbed atomic hydrogen through sulfidation of nano zerovalent iron for water decontamination, Chem. Eng. J., 2023, 474, 146248 CrossRef CAS.
- L. Ling, C. Tang and W. Zhang, Visualization of silver nanoparticle formation on nanoscale zero-valent iron, Environ. Sci. Technol. Lett., 2018, 5, 520–525 CrossRef CAS.
- W. Yang, D. Xi, C. Li, Z. Yang, Z. Lin and M. Si, “In situ synthesized” iron-based bimetal promotes efficient removal of Cr(VI) in by zero-valent iron-loaded hydroxyapatite, J. Hazard. Mater., 2021, 420, 126540 CrossRef CAS.
- F. Tian, J. Tang, J. Zeng, Z. Luo, L. Zhang, F. Tang, Z. Han and X. Yang, Degradation of atrazine by Ni-doped sulfidated microscale zero-valent iron: Mechanistic insights for enhanced reactivity and selectivity, Chem. Eng. J., 2022, 435, 135120 CrossRef CAS.
- C. Chang, S. Yang, J. Dai, J. Li, C. Fu, J. Cui, W. Zeng, H. Liu, J. Qi, W. Jin and Y. Chen, Clean and efficient preparation of metallic bismuth from methane-sulfonate electrolyte in the membrane electrolysis cell, J. Environ. Chem. Eng., 2024, 12, 112798 CrossRef CAS.
- X. Li, M. Liu, N. Wu, V. K. Sharma and R. Qu, Enhanced removal of phenolic compounds by ferrate(VI): Unveiling the Bi(III)-Bi(V) valence cycle with in situ formed bismuth hydroxide as catalyst, Water Res., 2024, 248, 120827 CrossRef CAS.
- J. Gong, C.-S. Lee, Y.-Y. Chang and Y.-S. Chang, Novel self-assembled bimetallic structure of Bi/Fe0: The oxidative and reductive degradation of hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine (RDX), J. Hazard. Mater., 2015, 286, 107–117 CrossRef CAS.
- C. R. Keenan and D. L. Sedlak, Ligand-enhanced reactive oxidant generation by nanoparticulate zero-valent iron and oxygen, Environ. Sci. Technol., 2008, 42, 6936–6941 CrossRef CAS PubMed.
- X. Song, C. Zhang, B. Wu, X. Wang, Z. Chen and S. Zhang, Ligand effects on arsenite removal by zero-valent iron/O2: Dissolution, corrosion, oxidation and coprecipitation, J. Environ. Sci., 2019, 86, 131–140 CrossRef CAS PubMed.
- B. Yao, Y. Liu and D. Zou, Removal of chloramphenicol in aqueous solutions by modified humic acid loaded with nanoscale zero-valent iron particles, Chemosphere, 2019, 226, 298–306 CrossRef CAS PubMed.
- M. Liao, S. Zhao, K. Wei, H. Sun and L. Zhang, Precise decomplexation of Cr(III)-EDTA and in situ Cr(III) removal with oxalated zero-valent iron, Appl. Catal., B, 2023, 330, 122619 CrossRef CAS.
- A. Martínez, R. Vargas and A. Galano, Citric acid: A promising copper scavenger, Comput. Theor. Chem., 2018, 1133, 47–50 CrossRef.
- J. Hauptfleisch and K. Payne, An oral sodium citrate-citric acid non-particulate buffer in humans, Br. J. Anaesth., 1996, 77, 642–644 CrossRef CAS.
- H. Zhang, X. Su, B. Sun, Y. Xu and J. Gong, Citrate iron complex induced dramatically enhanced oxidation of atrazine with bimetallic Bi/Fe0: Reactivity, oxidation and mechanism, Chemosphere, 2021, 282, 131100 CrossRef CAS.
- A. Shanableh, S. Bhattacharjee, S. Alani, N. Darwish, M. Abdallah, M. Mousa and M. Semreen, Assessment of sulfamethoxazole removal by nanoscale zerovalent iron, Sci. Total Environ., 2021, 761, 143307 CrossRef CAS PubMed.
- X. Su, H. Lv, J. Gong and M. Zhou, Bi/mZVI combined with citric acid and sodium citrate to mineralize multiple sulfa antibiotics: performance and mechanism, Antibiotics, 2022, 11, 51 CrossRef CAS.
- M. Kobayashi, S. Kurosu, R. Yamaguchi and Y. Kawase, Removal of antibiotic sulfamethoxazole by zero-valent iron under oxic and anoxic conditions: removal mechanisms in acidic, neutral and alkaline solutions, J. Environ. Manage., 2017, 200, 88–96 CrossRef CAS PubMed.
- N. P. Xekoukoulotakis, C. Drosou, C. Brebou, E. Chatzisymeon, E. Hapeshi and D. Fatta-Kassinos, Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices, Catal. Today, 2011, 161, 163–168 CrossRef CAS.
- P. Sukul and M. Spiteller, Sulfonamides in the environment as veterinary drugs, Rev. Environ. Contam. Toxicol., 2006, 187, 67–101 CAS.
- Y. Xu, L. Zeng, L. Li, Y.-S. Chang and J. Gong, Enhanced oxidative activity of zero-valent iron by citric acid complexation, Chem. Eng. J., 2019, 373, 891–901 CrossRef CAS.
- R. Peng, J. Shao, Y. Xie, A. Chen, L. Peng, Q. Zeng and S. Luo, Oxalate-enhanced reactivity of nanoscale zero-valent iron under different conditions of O2, N2 or without aeration, Chem. Eng. J., 2017, 330, 398–406 CrossRef CAS.
- C. Zhang, D. Wang, Q. Liu and J. Tang, Ligand-citric acid enhanced in situ ROS generation by GBC@nZVI to promote the aerobic degradation of adsorbed 2,4-dichlorophenol, Chem. Eng. J., 2023, 147126 CrossRef CAS.
- H. Yoshino, S. Kurosu, R. Yamaguchi and Y. Kawase, A phenomenological reaction kinetic model for Cu removal from aqueous solutions by zero-valent iron (ZVI), Chemosphere, 2018, 200, 542–553 CrossRef CAS PubMed.
- X. Zou, Y. Zhang, K. Wei, J. Dai, C. Mao, L. Hu, H. Li, Y. Yao and L. Zhang, One-stop rapid decomplexation and copper capture of Cu(II)-EDTA with nanoscale zero valent iron, J. Hazard. Mater., 2023, 446, 130752 CrossRef CAS.
- Z. Xu, W. Huang, S. Wang, H. Song, J. Xu, G. Mailhot and Z. Li, Co-adsorption characteristics of antibiotics with different functional groups and cadmium combined contamination on activated carbon, J. Environ. Chem. Eng., 2023, 11, 110070 CrossRef CAS.
- X. Tian, X. Wang, Y. Nie, C. Yang and D. D. Dionysiou, Hydroxyl radical-involving p-nitrophenol oxidation during its reduction by nanoscale sulfidated zerovalent iron under anaerobic conditions, Environ. Sci. Technol., 2021, 55, 2403–2410 CrossRef CAS PubMed.
- J. Li, X. Dou, H. Qin, Y. Sun, D. Yin and X. Guan, Characterization methods of zerovalent iron for water treatment and remediation, Water Res., 2019, 148, 70–85 CrossRef CAS.
- H. Zhou, M. Ma, Y. Zhao, S. A. Baig, S. Hu, M. Ye and J. Wang, Integrated green complexing agent and biochar modified nano zero-valent iron for hexavalent chromium removal: A characterisation and performance study, Sci. Total Environ., 2022, 834, 155080 CrossRef CAS.
- B. Li, M. Li, J. Zhang, Y. Pan, Z. Huang and H. Xiao, Adsorption of Hg(II) ions from aqueous solution by diethylenetriaminepentaacetic acid-modified cellulose, Int. J. Biol. Macromol., 2019, 122, 149–156 CrossRef CAS PubMed.
- N. Horzum, M. M. Demir, M. Nairat and T. Shahwan, Chitosan fiber-supported zero-valent iron nanoparticles as a novel sorbent for sequestration of inorganic arsenic, RSC Adv., 2013, 3, 7828–7837 RSC.
- H. Liu, Y. Yi, Z. Qin, Y. Wu, L. Li, B. Chu, G. Jin, R. Li, Z. Tong, L. Dong and B. Li, In Situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy Study of NO + CO Reaction on La0.8Ce0.2Mn1−xFexO3 Perovskites: Changes in Catalytic Properties Caused by Fe Incorporation, Ind. Eng. Chem. Res., 2019, 58, 9065–9074 CrossRef CAS.
- J. Yao, W. Yin and E. Gong, Depressing effect of fine hydrophilic particles on magnesite reverse flotation, Int. J. Miner. Process., 2016, 149, 84–93 CrossRef CAS.
- J. Yao, H. Sun, F. Han, W. Yin, J. Hong, Y. Wang, C. Won and L. Du, Enhancing selectivity of modifier on magnesite and dolomite surfaces by pH control, Powder Technol., 2020, 362, 698–706 CrossRef CAS.
- S. Tsimbler, L. Shevchenko and V. Grigor'eva, The IR absorption spectra of the tartrate and citrate complexes of nickel, cobalt, and iron, J. Appl. Spectrosc., 1969, 11, 1096–1101 CrossRef.
- L. C. Bichara, H. E. Lanús, E. G. Ferrer, M. B. Gramajo and S. A. Brandán, Vibrational study and force field of the citric acid dimer based on the SQM methodology, Adv. Chem. Phys., 2011, 2011, 1–10 CrossRef.
- X. Dong, L. He, Y. Liu and Y. Piao, Preparation of highly conductive biochar nanoparticles for rapid and sensitive detection of 17 β-estradiol in water, Electrochim. Acta, 2018, 292, 55–62 CrossRef CAS.
- W. Shen, H. Kang and Z. Ai, Comparison of aerobic atrazine degradation with zero valent aluminum and zero valent iron, J. Hazard. Mater., 2018, 357, 408–414 CrossRef CAS PubMed.
- J. Du, W. Guo, H. Wang, R. Yin, H. Zheng, X. Feng, D. Che and N. Ren, Hydroxyl radical dominated degradation of aquatic sulfamethoxazole by Fe0/bisulfite/O2: Kinetics, mechanisms, and pathways, Water Res., 2018, 138, 323–332 CrossRef CAS.
- Y. Xie, J. Wan, Z. Yan, Y. Wang, T. Xiao, J. Hou and H. Chen, Targeted degradation of sulfamethoxazole in wastewater by molecularly imprinted MOFs in advanced oxidation processes: Degradation pathways and mechanism, Chem. Eng. J., 2022, 429, 132237 CrossRef CAS.
- J. Li, L. Zhao, M. Feng, C.-H. Huang and P. Sun, Abiotic transformation and ecotoxicity change of sulfonamide antibiotics in environmental and water treatment processes: A critical review, Water Res., 2021, 202, 117463 CrossRef CAS.
- A. Agrawal and P. G. Tratnyek, Reduction of nitro aromatic compounds by zero-valent iron metal, Environ. Sci. Technol., 1995, 30, 153–160 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4dt02556c |
| This journal is © The Royal Society of Chemistry 2025 |





