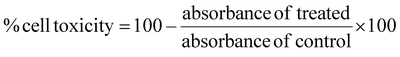Unveiling the multifaceted bioactivity of copper(II)–Schiff base complexes: a comprehensive study of antioxidant, anti-bacterial, anti-inflammatory, enzyme inhibition and cytotoxic potentials with DFT insights†
T. M.
Dhanya
a,
M. R.
Prathapachandra Kurup
a,
K. J.
Rajimon
b,
G.
Anjali Krishna
ac,
Jibin K.
Varughese
d,
K. G.
Raghu
e,
Sachin
Philip
a,
K. M.
Divya
af,
Maria
Augustine
ag and
P. V.
Mohanan
 *a
*a
aDepartment of Applied Chemistry, Cochin University of Science and Technology, Kochi 22, Kerala, India. E-mail: mdhanyat@gmail.com; mohan@cusat.ac.in
bDepartment of Chemistry, St Berchmans College, Changanacherry, Kerala, India. E-mail: rajimonkalambukattu@gmail.com
cDepartment of Science and Humanities, Mar Baselios Institute of Technology and Science, Nellimattom, Kothamangalam, Kerala, India. E-mail: anjalikrishna@mbits.ac.in
dJr HSST Chemistry, KMHSS Kuttoor, North Malappuram, India. E-mail: jibinkchem@gmail.com
eAgro-Processing and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology, Thiruvananthapuram, Kerala, India. E-mail: raghukgopal2009@gmail.com
fDepartment of Chemistry, NSS College, Cherthala, Kerala, India. E-mail: divyadevuty2008@gmail.com
gDepartment of Chemistry, St Paul's College, Kalamassery, Kerala, India. E-mail: mariamartinjoe@gmail.com
First published on 17th January 2025
Abstract
The rise of various diseases demands the development of new agents with antioxidant, antimicrobial, anti-inflammatory, enzyme-inhibiting, and cytotoxic properties. In this study, heterocyclic Schiff base complexes of Cu(II) featuring a benzo[b]thiophene moiety were synthesized and their biological activities evaluated. The complexes were characterized using FT-IR, UV-Vis, and EPR spectroscopy, TG–DTG analysis, magnetic moment measurements, molar conductivity measurements, and elemental analyses. Density functional theory (DFT) calculations were used to optimize the theoretical molecular orbital energies of the copper complexes. The complexes exhibited square pyramidal and square planar geometries. Biological assays demonstrated that these complexes generally outperformed the Schiff base ligands for various activities. The antioxidant capacity, measured via the DPPH assay in methanol, was comparable to those of the BHT and ascorbic acid standards, with 4BNPC showing the lowest IC50 value, which was attributed to the free OH group rather than coordination to the metal center. The anti-bacterial activity was assessed using the agar disc diffusion method against E. coli, P. aeruginosa, B. subtilis, and S. aureus, with BAC showing the largest inhibition zone compared to the others and ciprofloxacin as the reference. The anti-inflammatory activity, evaluated by the HRBC membrane stabilization method, showed that the 4BNPC Cu(II) complex had moderate activity similar to that of diclofenac. Enzyme inhibition studies against α-amylase revealed that the BAC complexes had the highest inhibition values, surpassing those of the Schiff base ligands. Cytotoxicity was assessed using Trypan blue exclusion for DLA and HepG2 cancer cell lines, and the MTT assay for H9c2 human cells. BMPC demonstrated superior cytotoxicity at both high and low concentrations against the normal H9c2 cell line. Among the tested compounds, BNPC showed moderate inhibition against HepG2 cells, while BMPC exhibited the greatest cytotoxicity at higher concentrations, particularly reaching nearly 100% cell death at 200 μg mL−1 in DLA cell lines. This suggests that BMPC is a promising candidate for further pharmacological research, particularly against DLA cells.
1. Introduction
Sulfur-containing heterocyclic compounds are noteworthy for their extensive pharmacological applications.1 The synthesis of functionalized heterocyclic molecules has become increasingly important in drug development. This is due to the growing prevalence of infectious diseases, which present major public health and economic challenges. Consequently, there is a pressing need for innovative and dynamic medications, particularly those involving Schiff base compounds. Schiff bases and their transition metal complexes are gaining attention in modern medicine due to their unique electronic and stereochemical properties, offering potential new mechanisms of drug action.2 The significance of transition metal complexes as medicinal agents is increasing as research in inorganic chemistry gains prominence. These complexes are beneficial for catalysis, the synthesis of materials, photochemistry, and biological systems.3 Metallodrugs present the possibility of novel mechanisms of action, influenced by the choice of metal, its oxidation state, the nature and number of coordinated ligands, and the coordination geometry.4 Copper is particularly promising due to its superior antioxidant, antimicrobial, anti-inflammatory, enzyme-inhibiting, and cytotoxic activities. It interacts with donor atoms on biological targets by forming coordinate bonds. This mechanism is more effective than relying on weaker intermolecular forces, such as hydrogen bonding.5 The coordination of the Schiff base ligands to Cu(II) ions is known to enhance their biological activity by utilizing copper's redox cycling ability, improved lipophilicity, and stabilization of the ligand framework. These properties enable the complexes to interact more effectively with biological targets, contributing to their potential therapeutic effects. Reviews in the literature indicate that the copper complexes have demonstrated significant antiviral, anticancer, antiproliferative, and antimitotic activities, along with generally lower host toxicity compared to platinum-based compounds.6 Benzo[b]thiophene moieties, in particular, have demonstrated significant biological activity, making them promising candidates for pharmaceutical applications.Recent studies on biological activity have demonstrated that antioxidants function by either donating protons or withdrawing electrons at the responsive site to neutralize free radicals.7 Modern drug development strategies include incorporating new compounds into existing ones or designing compounds with specific active sites to mitigate oxidative stress in cells. Notably, benzo[b]thiophene-substituted ligands and their potential complexes have exhibited significant antioxidant activity in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay through various mechanisms and synthesis methods. Antibiotics are essential in modern medicine. However, the emergence of drug-resistant bacteria has become a major concern. Additionally, the slowed discovery of new antimicrobial agents has contributed to a public health crisis.8–11 Infectious diseases caused by bacteria or fungi are becoming more common, and the mortality rate from these infections is increasing over time.12 The main reason for the impact of these diseases is the lack of effective treatments due to microbial resistance to existing drugs.13 These challenges necessitate the development of new antibacterial drug classes, and researchers have been focusing on creating novel antimicrobial agents to combat bacterial and fungal infections. Benzo[b]thiophene-coordinated drugs have shown potential for enhanced antimicrobial activity.14 Inflammation, an essential immune response, is linked to various immunological disorders and can be triggered by chemical agents, physical injuries, immune reactions, and pathogenic infections. Non-steroidal anti-inflammatory drugs (NSAIDs) have long been effective at reducing inflammation.15 Benzo[b]thiophene-containing ligands and their transition metal complexes also exhibit anti-inflammatory properties.16 The equilibrium of carbohydrate and lipid digestion in diabetes treatment is often disrupted by improper guidelines, with the most effective medications currently inhibit digestive enzymes. Research is ongoing into the inhibitory effects of benzo[b]thiophene-related Schiff bases and their complexes on the diabetes-related enzyme α-amylase. Cytotoxicity studies are crucial for the development of new pharmaceutical drugs, using in vitro tissue cells to monitor cell growth, reproduction, and morphological changes. For instance, Romero-Parra et al.17 reported the synthesis of benzothiophene derivatives that could inhibit or reduce cytotoxicity. Despite the strong biological activity of benzo[b]thiophene-related Schiff bases, few studies have been conducted. Consequently, there is significant interest among researchers in developing small chemical molecules with benzothiophene units and their associated transition metal complexes.
In this context, the current article outlines innovative strategies for the design, synthesis, and development of novel Schiff base–Cu(II) metal complexes with antioxidant, antimicrobial, anti-inflammatory, enzyme-inhibiting, and cytotoxic properties. Despite growing interest in Schiff base ligands and their metal complexes, as discussed above, there are limited reports on the potential of benzo[b]thiophene-derived Schiff base complexes as biological agents. We believe this article will serve as a valuable reference for specialists involved in the design and synthesis of transition metal complexes derived from benzo[b]thiophene Schiff base ligands. The ligands in this study were synthesized from benzo[b]thiophene-2-carboxaldehyde and phenolic amines to utilize the pharmacological potential of the benzo[b]thiophene scaffold. Substituents on the phenolic ring, such as methyl and nitro groups, were selected to introduce electronic and steric variations, enabling the exploration of the structure–activity relationships. These ligands were further coordinated with Cu(II) ions to form complexes, as copper's redox properties and biological relevance enhance the potential for antioxidant, antibacterial, anti-inflammatory, enzyme-inhibitory, and cytotoxic activities. The combination of these features makes the chosen ligands particularly suitable for current biological studies. In this study, we developed four novel heterocyclic Schiff base complexes of Cu(II) through a simple one-pot reaction method instead of the multi-step processes commonly reported. Specifically, we established a conventional method for synthesizing benzothiophene Schiff bases18 and their metal complexes, which could inhibit microbial growth and act as significant antioxidants to prevent damage caused by free radicals. The prepared complexes were thoroughly characterized using dedicated analytical and spectroscopic methods before their biological evaluation. While selecting appropriate targets for specific diseases can be challenging, we report that the newly synthesized Schiff base complexes exhibit potent antidiabetic activity, potentially leading to better modulation of diabetes through alpha-amylase inhibition.19–21 This work aims to provide a valuable reference material for researchers in the field, facilitating the design and synthesis of benzo[b]thiophene Schiff base ligands and their transition metal complexes.
2. Experimental
2.1 Materials
The chemicals used in this study were commercially obtained and used without further purification. Metal salt copper acetate monohydrate (Cu(CH3COO)2·H2O) and α-amylase were sourced from Sigma Aldrich. Solvents, namely, n-hexane, N,N-dimethyl formamide (DMF), ethyl acetate, acetone, methanol, petroleum ether, dimethyl sulfoxide (DMSO), ascorbic acid, and chloroform, were obtained from Merck and were either 99% pure or purified using standard research methods. Additionally, 1,1-diphenyl-2-picrylhydrazyl (DPPH) was procured from Sigma Aldrich. For the in vitro antibacterial activity tests of the synthesized compounds, two Gram-positive bacterial strains, Bacillus subtilis (MTCC 441) and Staphylococcus aureus (MTCC 2940), and two Gram-negative strains, Escherichia coli (MTCC 739) and Pseudomonas aeruginosa (MTCC 3541), were used. These strains were cultured in a nutrient broth medium overnight at 37 °C. All media, including Mueller Hinton agar (MHA), were procured from Himedia, India. Dalton's lymphoma ascites (DLA) cells were supplied by the Amala Cancer Research Centre, Thrissur, Kerala, India, and maintained in vivo in Swiss albino mice via intraperitoneal transplantation. H9c2 cells (embryonic BD1X rat heart tissue) were provided by the National Centre for Cell Sciences (NCCS), Pune, India, and cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS, penicillin (100 U mL−1), and streptomycin (100 mg mL−1) at 37 °C under a 5% CO2 atmosphere. Cells were subcultured to 80% confluence before experiments. The HepG2 human hepatocellular carcinoma cell line was also acquired from NCCS, Pune, India, and maintained in Eagle's minimum essential medium with 10% FBS, penicillin (100 U mL−1), and streptomycin (100 mg mL−1) under a 5% CO2 environment at 37 °C, with subculturing to 80% confluence before use. The MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was sourced from Sigma, St Louis, MO, USA.2.2 Instrumentation
The carbon, hydrogen, and nitrogen contents of the synthesized compounds were analyzed using a Vario EL III CHNS analyzer at the Sophisticated Test and Instrumentation Centre (SAIF), Cochin University of Science and Technology, Kochi, India. The electronic spectra of the compounds were recorded in DMF using a Thermo Scientific Evolution 201 UV-visible spectrophotometer (200–900 nm). Infrared spectra were obtained using a JASCO FT-IR-5300 spectrometer in the range of 4000–400 cm−1 as KBr pellets to predict structural information about the molecules. Molar conductivities of the synthesized complexes in DMSO solutions (10−3 M) at room temperature were measured using a Systronics model 303 direct reading conductivity meter. Magnetic susceptibility calculations were conducted at room temperature using a Magway MSB Mk 1 magnetic susceptibility balance. Thermogravimetric (TG) and derivative thermogravimetric (DTG) analyses were carried out with a PerkinElmer Pyris Diamond TG analyzer, heating the samples from 0 to 800 °C at a rate of 10 °C min−1 both in air and under nitrogen. The melting points of the synthesized complexes were determined using a 1102D model electro-thermal digital melting point apparatus. EPR spectra of the Cu complexes were captured in the polycrystalline solid state at 298 K, and in frozen DMF at 77 K using TCNE as the standard, 100 kHz modulation frequency, 2G modulation amplitude, and 9.1 GHz microwave frequency using an EOL model JES FA200 instrument from SAIF, IIT, Madras, India. Easy Spin was used to replicate some of the EPR spectra.2.3 Synthesis of Schiff base ligands (BA, BMP, BNP, 4BNP) using benzo[b]thiophene-2-carboxaldehyde
In our previous work, we reported the synthesis of Schiff bases by condensing benzo[b]thiophene-2-carboxaldehyde with 2-aminophenol, 2-amino-4-methylphenol, 2-amino-4-nitrophenol, and 4-amino-2-nitrophenol, resulting in the derivatives BA, BMP, BNP, and 4BNP. The compounds’ purity was confirmed using TLC, and their yields and melting points were recorded. Schemes S1–S4† illustrate the stages involved in the synthesis.2.4 Synthesis of benzo[b]thiophene-derived transition metal complexes (BAC, BMPC, BNPC, and 4BNPC)
Metal salts of copper acetate monohydrate (0.01 mmol) were dissolved in methanol (25 mL) and added to the solutions of the Schiff base ligands BA, BMP, BNP, and 4BNP (0.01 mol) in 40 mL methanol. This solution was then subjected to 12 h stirring at room temperature. The precipitated colored solution was subjected to slow evaporation and the Cu(II) complexes formed during slow evaporation. The final products were then washed multiple times with cold methanolic solution and petroleum ether before being dried in a desiccator containing phosphorus pentoxide. The Schiff base–Cu(II) metal complexes were designated as BAC, BMPC, BNPC, and 4BNPC, based on the Schiff bases used. The corresponding synthesis processes are detailed in Schemes S5 and S6.†2.5 Biological assays
The selection of reference agents (controls) in this study was carefully made based on their established roles and relevance to the respective biological activities being investigated. For the antioxidant activity, ascorbic acid was chosen due to its well-documented free radical scavenging properties, providing a benchmark for evaluating the radical-quenching potential of the synthesized compounds.22,23 BHT (butylated hydroxytoluene), a synthetic antioxidant commonly used in the food and pharmaceutical industries, was included to compare the synthetic compounds with industrially relevant standards. For antibacterial activity, ciprofloxacin, a broad-spectrum antibiotic, served as a reliable standard for evaluating the antibacterial activities of the synthesized compounds.24,25 In the anti-inflammatory assays, diclofenac, a clinically used anti-inflammatory drug, was employed to assess the ability of the synthesized compounds to stabilize the HRBC (human red blood cell) membrane under stress conditions. These controls were selected to ensure relevance, reliability, and comparability with the existing literature, providing a robust framework for assessing the biological efficacy of the synthesized complexes. The usage of these different controls was driven by the specific experimental needs of each assay, with antioxidant activity requiring free radical scavengers like ascorbic acid and BHT, antibacterial activity needing antibiotics like ciprofloxacin for reliable comparisons, and anti-inflammatory activity being assessed with clinically relevant anti-inflammatory drugs like diclofenac. The selection of these controls also offers a comparative advantage, as they are widely reported in similar studies, enabling direct comparison of the results with previously published findings and ensuring that the biological activities of the synthesized compounds are evaluated against appropriate, widely accepted benchmarks. This approach strengthens the scientific validity of the study.2.6 In vitro antioxidant activity using the DPPH assay
The DPPH method is widely regarded as the most reliable approach for assessing antioxidant activity. In this study, the antioxidant properties of the synthesized compounds were evaluated using a fixed reaction time and the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay. Minor modifications were made to the traditional DPPH assay to test the antioxidant activity of the newly developed compounds.26 The reaction between the colored free radical and the scavenger results in the formation of a colorless compound, 1,1-diphenyl-2-picrylhydrazine (DPPH-H). A stock solution was prepared by dissolving the ligands (1 mg mL−1) in methanol and their metal complexes in phosphate-buffered saline (PBS, pH = 7.4), with ascorbic acid serving as the standard solution. An appropriate amount of the sample stock solution was added to a fixed volume of DPPH (0.01 mmol) in methanol, and the total volume was adjusted to 3 mL in a methanol medium. The mixture was then incubated in the dark at room temperature for 30 min. A control experiment was conducted without the compound, using a solution containing only DPPH, methanol, or buffer. This control was tested at the same absorbance as the sample against BHT and vitamin C as standard references. The absorbance at 517 nm was measured using a UV spectrophotometer, with methanol as the blank and ascorbic acid as the standard. The spectrophotometer was auto-zeroed using methanol. The antioxidant activity was calculated by comparing the absorbance of the test samples to that of the control samples. The percentage inhibition was determined using the formula: % inhibition = (A0 − A1/A0) × 100, where A0 is the control absorbance and A1 is the sample absorbance. Additionally, the IC50 values, representing the concentration of the compounds required to scavenge 50% of the DPPH radicals (i.e., 50% of absorbance at 517 nm), were determined by plotting a graph of free radical scavenging versus compound concentration.2.7 Anti-bacterial study using the agar disc diffusion method
The anti-bacterial activity of the newly synthesized metal complexes was evaluated using the agar disc diffusion method, which assessed the effectiveness of the compounds against specific pathogens, particularly microorganisms. The process began with microbes being incubated on an agar plate, followed by the application of compound-filled paper disks. A single colony was aseptically transferred to 10 mL nutrient broth and then incubated at 37 °C. Sterile MHA plates were prepared, and bacterial strains, namely, Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Bacillus subtilis (B. subtilis), and Staphylococcus aureus (S. aureus), were evenly spread across each plate and approximately 20 μL of the compound was tested by placing the disk on top of the agar plates. The MHA plates were cultured at 37 °C for 18 h. After incubation, the inhibition zones around each disc were measured in centimeters. Methanol served as the control solvent, and ciprofloxacin was used as a reference compound. Each test was performed two to three times.27The bacterial strains E. coli, P. aeruginosa, B. subtilis, and S. aureus were selected to represent a diverse range of human pathogens with varying resistance profiles. E. coli and P. aeruginosa are Gram-negative bacteria known for their resistance mechanisms, such as outer membrane barriers and efflux pumps, while B. subtilis and S. aureus represent Gram-positive organisms.28 This selection ensures comprehensive evaluation of the Cu(II) complexes against both Gram-negative and Gram-positive bacteria with differing cell wall structures and resistance mechanisms, highlighting the potential broad-spectrum antibacterial efficacy of the synthesized compounds.
2.8 In vitro anti-inflammatory study using the human red blood cell stabilization (HRBS) method
The in vitro anti-inflammatory activity of the compounds was assessed using the HRBC (human red blood cell) membrane stabilization technique. For this procedure, 10 mL of fresh whole human blood was collected and transferred to heparinized centrifuge tubes. The blood was then centrifuged with isosaline (0.85% NaCl solution, prepared by dissolving 8.5 g of NaCl in water) and mixed with an equal volume of Alsever's solution (containing 2% dextrose, 0.8% sodium citrate, 0.05% citric acid, 0.42% sodium chloride, and 100 mL of distilled water). This mixture was autoclaved for 15 min at 121 °C and then allowed to cool to room temperature. An exact volume of the test compounds at four different concentrations (10, 20, 50, and 100 μg mL−1) was added to 1 mL of the HRBC solution. All test mixtures were incubated for 30 min at 37 °C, followed by centrifugation. The hemoglobin content in the supernatant was measured using a spectrophotometer at 560 nm.32 The percentage of hemolysis was calculated using the formula: % hemolysis = [(absorbance of control − absorbance of sample)/absorbance of control] × 100.2.9 Enzyme inhibition studies using α-amylase inhibition
The enzyme inhibition studies primarily focused on the α-amylase enzyme, with inhibition measured using the dinitrosalicylic acid (DNS) method, which was practical and straightforward.33 The assay involved preparing a mixture in which the sample, dissolved in DMSO at varying concentrations (25, 50, 100, and 150 μM), was combined with 500 μL of α-amylase enzyme (0.5 mg mL−1 in 0.05 M sodium phosphate buffer, pH 6.9) in separate test tubes. This mixture was incubated at 37 °C for 10 min. After incubation, 500 μL of a 1% starch solution (1 g in 100 mL of 0.05 M phosphate buffer) was added to each tube, followed by incubation for another 15 min at 37 °C. To stop the reaction, 500 μL of 96 mM DNS reagent (0.438 g in 20 mL of distilled water) was added to all the test tubes, which were then incubated for 15 min. The tubes were placed in a boiling water bath for 5 min, allowed to cool to room temperature, and then diluted with 10 mL of distilled water. A control sample containing only the enzyme (without the test compound) demonstrated 100% enzyme activity. A blank was prepared with 500 μL of starch and 500 μL of DNS reagent. Acarbose was used as the standard inhibitor to assess the activity of all tested samples. The optical density of the samples was measured at 540 nm. An α-amylase unit was defined as the amount of enzyme required to produce 1 mM maltose per minute at 37 °C under the specified conditions. The percentage of inhibition was calculated using the formula: % inhibition = [(absorbance of control − absorbance of the test)/absorbance of control] × 100. A graph was plotted to determine the IC50 values, by measuring the percentage of inhibition against the logarithm of the inhibitor concentration. The IC50 value was extrapolated from the non-linear graph as the mean of the inhibitory values. The assay was conducted in triplicate, and the final results were presented as the mean ± standard deviation.The Michaelis–Menten plot was constructed based on the initial velocity data, and the Lineweaver–Burk plot was obtained by plotting the double reciprocal (1/V versus 1/[S]). In these plots, ‘V’ represents the reaction velocity, and ‘[S]’ represents the substrate concentration, which is crucial for calculating Km (Michaelis constant), Vmax (maximum velocity), and the mode of inhibition for the α-amylase enzyme.34 For non-competitive inhibition, the equation  is used, where [I] is the inhibitor concentration and Vm+I and Vm are the Vmax in the presence and absence of the inhibitor, respectively.35
is used, where [I] is the inhibitor concentration and Vm+I and Vm are the Vmax in the presence and absence of the inhibitor, respectively.35
2.10 Cytotoxicity study
Similarly, the cytotoxicity of the compounds in HepG2 cell lines was also assessed using the MTT assay. The cells were seeded in 96-well plates at a density of 2 × 103 cells per well and grown to 80% confluence in MEM medium with 10% FBS, penicillin (100 U mL−1), and streptomycin (100 mg mL−1). The cells were cultured under a 5% CO2 environment at 37 °C. The test samples, at concentrations of 1, 5, 10, and 25 μg mL−1, were incubated with the cells for 24 h. After thorough washing, 100 μL of MTT solution (5 mg mL−1 in PBS) was added to each well. The plates were incubated for 4 h at 37 °C in a CO2 incubator, followed by the addition of 100 μL of DMSO to each well. The plates were shaken for 30 min, and the absorbance of the solubilized MTT formazan products was measured at 570 nm using a microplate reader (Bioteck Synergy). The viability of the control cells was considered to be 100%.
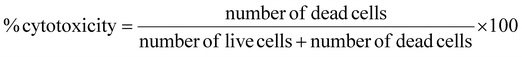 was used to compute the percentage of cytotoxicity.
was used to compute the percentage of cytotoxicity.
2.11 DFT optimization studies
DFT calculations were performed using the Gaussian 09 program.37 For the elements C, H, N, S, and O, we employed the 6-311G+(d,p) basis set along with the B3LYP functional, which combined Becke's three-parameter hybrid functional with Lee–Yang–Parr's correlation functional. For Cu, the Lanl2dz basis set was utilized. To account for long-range dispersion effects, both the Becke–Johnson damping (GD3BJ) and Grimme's third-order correction for dispersion were applied. The program's strict convergence criteria were used to fully optimize the ground state (S0) geometries in the gas phase. To ensure the global minimum, vibrational frequencies were calculated on the optimized geometries, confirming that all frequencies were positive. In addition, the frontier molecular orbital energies were used to calculate the quantum mechanical descriptors, such as the gap between MOs (E), ionization potential (IP), electron affinity (EA), electronegativity (χ), chemical hardness (η), mean energy (M), chemical potential (μ), chemical softness (σ), and electrophilicity index (ω). These computations are performed using the following formulae.| ΔE = ELUMO − EHOMO | (1) |
| IP = −EHOMO | (2) |
| EA = −ELUMO | (3) |
| χ = −μ = (IP + EA)/2 | (4) |
| η = ΔE/2 | (5) |
| σ = 1/η | (6) |
| ω = χ2/2η | (7) |
| M = −χ | (8) |
3. Results and discussion
3.1 Characterization of transition metal complexes
The transition metal complexes of Cu(II) incorporating benzo[b]thiophene-bound Schiff bases BA, BMP, BNP, and 4BNP were successfully synthesized and characterized. The parameters determining the structures of the complexes are well understood from their characterization methods. The methods involve elemental analysis mainly (Carbon, Hydrogen, Nitrogen, Sulfur, and metal fraction), UV-Vis spectroscopy, infrared spectral data (FT-IR), thermogravimetric analysis (TG–DTG), magnetic susceptibility, molar conductivity, and EPR spectroscopy. For predicting the geometry of metal complexes, it is essential to analyze the binding of ligands to metal acetates and their stoichiometry obeys a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Schemes S5 and S6† capture the conventional synthetic routes included in the synthesis of Schiff base–metal complexes. Most of these complexes are colored and insoluble in water but soluble in polar aprotic solvents like DMF and DMSO, and the yields are reasonable to high. The analytical statistics of these complexes (Table S1†) are in excellent agreement with the calculated values, thus confirming the proposed composition for all the Cu complexes. The complexes attained solubility in DMSO and the molar conductivities (10−3 M) of respective solutions were tested at ambient temperature. According to reports in the literature, the standard value of conductance for all complexes falls in the range of 3–20 Ω−1 cm2 mol−1 in DMSO, and this accounts for their non-electrolytic nature in this solvent.38 The metal composition was evaluated after digesting the sample with concentrated nitric acid.
1 ratio. Schemes S5 and S6† capture the conventional synthetic routes included in the synthesis of Schiff base–metal complexes. Most of these complexes are colored and insoluble in water but soluble in polar aprotic solvents like DMF and DMSO, and the yields are reasonable to high. The analytical statistics of these complexes (Table S1†) are in excellent agreement with the calculated values, thus confirming the proposed composition for all the Cu complexes. The complexes attained solubility in DMSO and the molar conductivities (10−3 M) of respective solutions were tested at ambient temperature. According to reports in the literature, the standard value of conductance for all complexes falls in the range of 3–20 Ω−1 cm2 mol−1 in DMSO, and this accounts for their non-electrolytic nature in this solvent.38 The metal composition was evaluated after digesting the sample with concentrated nitric acid.
Magnetic susceptibility measurements for the metal complexes containing the benzo[b]thiophene scaffold are made using a fundamental Gouy-type balance at room temperature. Whether these complexes are in square planar or square pyramidal geometry can be easily evaluated from their magnetic moment (μeff) values. The magnetic moment (μeff) values (1.7–1.9 B.M.) of the Cu(II) complexes exhibited one unpaired electron, indicating monomeric compounds with square pyramidal or square planar geometry.39 For the complexes BAC, BMPC, and BNPC, the magnetic moment values are found to be 1.88, 1.89, and 1.84 B.M., respectively, corresponding to square pyramidal geometry and for 4BNPC, the value obtained is 1.92 B.M., which indicates square planar geometry.40 The data regarding the magnetic moment values and molar conductance are also interpreted in Table S2.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–11
000–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 cm−1 (200–900 nm), as depicted in Figs. S1 and S2.† The bands were assigned to different transitions based on their positions and intensities, as listed in Table S3.† Apart from the n → π* transition seen in the ligands, the metal complexes also exhibit bands above 400 nm (25
111 cm−1 (200–900 nm), as depicted in Figs. S1 and S2.† The bands were assigned to different transitions based on their positions and intensities, as listed in Table S3.† Apart from the n → π* transition seen in the ligands, the metal complexes also exhibit bands above 400 nm (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1), which may be due to the charge transfer transitions.
000 cm−1), which may be due to the charge transfer transitions.
The electronic spectra of the Cu(II) complexes, [Cu(BA)2(H2O)]·H2O, [Cu(BMP)2H2O], [Cu(BNP)2(H2O)]·H2O and [Cu(4BNP)2(OAc)2]·H2O, exhibit significant absorption bands at around 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460–23
460–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 cm−1, which are caused by the π → π* intra-ligand transitions.41 Upon complexation with the metal, the band corresponding to azomethine showed a slight shift to a longer wavelength, showing the coordination of the Schiff base ligands to the metal through the azomethine moiety. The peak in the range of 27
410 cm−1, which are caused by the π → π* intra-ligand transitions.41 Upon complexation with the metal, the band corresponding to azomethine showed a slight shift to a longer wavelength, showing the coordination of the Schiff base ligands to the metal through the azomethine moiety. The peak in the range of 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930–21
930–21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1 could be due to ligand → metal charge transfer (MLCT) transitions. The band in the wavelength range of 21
500 cm−1 could be due to ligand → metal charge transfer (MLCT) transitions. The band in the wavelength range of 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–16
500–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 cm−1 corresponds to d–d transitions.42 The Jahn–Teller distortion observed in the Cu(II) complexes with a d9 configuration, resulting in the increased separation of the 2Eg and 2T2g energy levels, is expected to give rise to three permissible transitions: 2B1g → 2A1g (dx2−y2→ dz2), 2B1g → 2B2g (dx2−y2→ dxy), and 2B1g → 2Eg (dx2−y2→ dxz,dyz). These transitions occur within the wavelength ranges of approximately 850–550 nm, 850–640 nm, and 580–500 nm, respectively.43,44 Due to the proximity of the four lower orbital energies, these transitions are spin-permitted for square planar or square pyramidal copper complexes with a dx2−y2 ground state but are challenging to see. However, for all the copper complexes in the current analysis, these transitions are seen as shoulders in the range of 20
520 cm−1 corresponds to d–d transitions.42 The Jahn–Teller distortion observed in the Cu(II) complexes with a d9 configuration, resulting in the increased separation of the 2Eg and 2T2g energy levels, is expected to give rise to three permissible transitions: 2B1g → 2A1g (dx2−y2→ dz2), 2B1g → 2B2g (dx2−y2→ dxy), and 2B1g → 2Eg (dx2−y2→ dxz,dyz). These transitions occur within the wavelength ranges of approximately 850–550 nm, 850–640 nm, and 580–500 nm, respectively.43,44 Due to the proximity of the four lower orbital energies, these transitions are spin-permitted for square planar or square pyramidal copper complexes with a dx2−y2 ground state but are challenging to see. However, for all the copper complexes in the current analysis, these transitions are seen as shoulders in the range of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833–16
833–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 cm−1.45
520 cm−1.45
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), ν(C–O), ν(M–N), and ν(M–O). The coordination mechanism of the ligands with the metal ions was confirmed by comparing the diagnostic bands in the infrared spectra of the ligands and their metal complexes.46 Upon chelation, it was anticipated that these peak locations and their intensities would shift. Figs. S3 and S4† show the infrared spectra of the complexes, while Table S4† provides an overall idea about the assignment of these bands. The phenolic OH group was thought to be responsible for the bands in the 3450–3088 cm−1 range that were visible in the infrared spectra of the ligands (BA, BMP, BNP, and 4BNP).47 The shifting of the ν(C–O) stretching band to a lower frequency by 10–20 cm−1 served as confirmation that the deprotonated phenolic moiety was involved in the complexation process; this is not seen in 4BNP complexes.48 This change indicated the creation of a stronger M–O bond and weakening of the C–O bond. For the ligands BA, BMP, BNP, and 4BNP, a strong band seen at 1598, 1599, 1590, and 1619 cm−1, respectively, was attributed to the stretching vibration of azomethine (C
N), ν(C–O), ν(M–N), and ν(M–O). The coordination mechanism of the ligands with the metal ions was confirmed by comparing the diagnostic bands in the infrared spectra of the ligands and their metal complexes.46 Upon chelation, it was anticipated that these peak locations and their intensities would shift. Figs. S3 and S4† show the infrared spectra of the complexes, while Table S4† provides an overall idea about the assignment of these bands. The phenolic OH group was thought to be responsible for the bands in the 3450–3088 cm−1 range that were visible in the infrared spectra of the ligands (BA, BMP, BNP, and 4BNP).47 The shifting of the ν(C–O) stretching band to a lower frequency by 10–20 cm−1 served as confirmation that the deprotonated phenolic moiety was involved in the complexation process; this is not seen in 4BNP complexes.48 This change indicated the creation of a stronger M–O bond and weakening of the C–O bond. For the ligands BA, BMP, BNP, and 4BNP, a strong band seen at 1598, 1599, 1590, and 1619 cm−1, respectively, was attributed to the stretching vibration of azomethine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).49 The band corresponding to C
N).49 The band corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N migrated towards the lower side (ν value decreased to 20 cm−1), showing coordination through the azomethine nitrogen, as a result of complex formation.50 The bands in the 3418–3304 cm−1 range in the spectra of the Cu(II) complexes were identified as v(OH/H2O), indicating the existence of coordinated water molecules.51 The appearance of the extra spectral bands in the ranges of 1363–1475 cm−1 and 1311–1414 cm−1 for ν (OAc)asym (νas) and (OAc)sym (νs), respectively, provided supporting evidence that the complexes included an acetate ligand. However, it was challenging to draw inferences from the OH group of the ligands since it would overlap with the bands of the water molecules because coordinated water molecules were present in the spectra of the Cu(II) complexes.
N migrated towards the lower side (ν value decreased to 20 cm−1), showing coordination through the azomethine nitrogen, as a result of complex formation.50 The bands in the 3418–3304 cm−1 range in the spectra of the Cu(II) complexes were identified as v(OH/H2O), indicating the existence of coordinated water molecules.51 The appearance of the extra spectral bands in the ranges of 1363–1475 cm−1 and 1311–1414 cm−1 for ν (OAc)asym (νas) and (OAc)sym (νs), respectively, provided supporting evidence that the complexes included an acetate ligand. However, it was challenging to draw inferences from the OH group of the ligands since it would overlap with the bands of the water molecules because coordinated water molecules were present in the spectra of the Cu(II) complexes.
The parameter ‘G’ for the geometry is a value that denotes the exchange interaction between the copper centers in a polycrystalline compound. This value is determined individually for each complex using eqn (9), which is specific for axial spectra.53
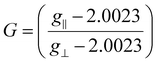 | (9) |
The presence or absence of the exchange interaction in solid complexes can be determined based on the value of G. When G is greater than 4, the exchange interaction is not significant. However, when G is less than 4, it suggests the existence of a considerable exchange interaction in the solid complex. In the case of the complexes BNPC and 4BNPC, the calculated G values were less than and equal to 4 (0.38, and 4.00, respectively), indicating considerable exchange interactions in their polycrystalline states. On the other hand, the G values for BAC and BMPC were 4.22 and 4.59, which were greater than 4, indicating the absence of any significant interactions.
The geometry of the Cu(II) complexes can be studied by analyzing their EPR spectra in the frozen state at 77 K. This information is presented in Figs. S8, S10, S12, and S14†. These EPR spectra provide additional insights into the structures of the complexes, as reported in the literature.54 The observed g∥ and g⊥ values for the synthesized novel Cu(II) complexes are g∥ = 2.23 and g⊥ = 2.053 for BAC, g∥ = 2.235 and g⊥ = 2.051 for BMPC, g∥ = 2.247 and g⊥ = 2.059 for BNPC, and g∥ = 2.333 and g⊥ = 2.063 for 4BNPC; four separate hyperfine lines are clearly visible in the parallel region due to the interaction between the electron spin and nuclear spin (more precisely, 63Cu with a spin value of I = 3/2). However, it is difficult to tell where these lines split in the perpendicular region. Normally, in certain Cu(II) complexes, there are three sets of resonances at low, mid, and high fields, which correspond to gx, gy, and gz, respectively; these are visible in the frozen DMF analysis. These ‘g’ values are assigned a particular value depending upon the peak positions, and the relative values of gx, gy, and gz can be used to calculate the Cu(II) ion's electronic ground state. If gz > gy > gx, the ‘R’ parameter is calculated using the expression R = (gy − gx)/(gz − gy), and if R is greater than unity, the ground state is dz2, while the ground state is dx2−y2, if R is less than unity, indicating a rhombic structure. These parameters are not observed in our complexes. The Cu(II) complexes’ EPR parameters are presented in Tables 1 and 2. To assess the covalency of the in-plane σ-bonds, in-plane π bonds, and out-of-plane π-bonds, the bonding parameters α2, β2, and γ2 were used. The investigation of the EPR parameters like g∥, g⊥gav, A∥, and the energies of the d–d transitions served as the foundation for this assessment. In particular, a mathematical expression (eqn (10))55 was used to estimate α2, the in-plane sigma bonding parameter.
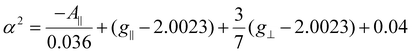 | (10) |
| Polycrystalline state at 298 K | ||||
|---|---|---|---|---|
| Complex | g ∥ | g ⊥ | g av | G |
| BAC | 2.233 | 2.057 | 2.145 | 4.22 |
| BMPC | 2.235 | 2.053 | 2.144 | 4.59 |
| BNPC | 2.064 | 2.163 | 2.114 | 0.38 |
| 4BNPC | 2.260 | 2.067 | 2.164 | 4.00 |
| Solvent state at 77 K (DMF) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complex | g ∥ | g ⊥ | α 2 | E d–d | K ∥ (Cu2+) | K ⊥ (Cu2+) | β 2 (Cu2+) | γ 2 (Cu2+) | A ∥ a | A ⊥ a | A av | g av |
| ‘A’ is expressed in ×10−3 cm. ‘a’ is expressed in units of cm−1 multiplied by a factor of 10−3. | ||||||||||||
| BAC | 2.230 | 2.053 | 0.289 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 920 |
0.616 | 0.549 | 1.313 | 1.041 | 0 | 0 | 0 | 2.142 |
| BMPC | 2.235 | 2.051 | 0.294 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 240 |
0.641 | 0.536 | 1.396 | 0.979 | 0 | 0 | 0 | 2.143 |
| BNPC | 2.247 | 2.059 | 0.260 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
0.610 | 0.566 | 1.432 | 1.230 | 20.5 | 1.40 | 10.95 | 2.153 |
| 4BNPC | 2.333 | 2.063 | 0.145 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730 |
0.885 | 0.650 | 5.401 | 2.912 | 19.5 | 1.25 | 10.38 | 2.198 |
To calculate the bonding parameters K∥2 = α2β2 and K⊥2 = α2γ2, the following expressions (eqn (11) and (12)) were used.
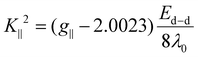 | (11) |
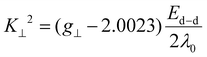 | (12) |
The orbital reduction factors K∥ and K⊥ are discussed here together with the one-electron spin–orbit coupling constant λ0, which is equal to −828 cm−1. According to Hathaway,56K∥ and K⊥ are almost identical for pure sigma bonding at 0.77 (K∥ ≈ K⊥ ≈ 0.77), but K∥ and K⊥ are different for in-plane π-bonding (K∥ < K⊥) and out-of-plane π-bonding (K⊥ < K∥). The fact that K⊥ is less than K∥ indicates that there is a lot of out-of-plane π-bonding in the BAC, BMPC, BNPC, and 4BNPC complexes. The parameter α2, which measures the strength of the in-plane sigma bonding, is used to evaluate the nature of the metal–ligand interaction. A value of α2 = 0.5 denotes a fully covalent M–L bond, while a value of α2 = 1 denotes an entirely ionic bond. The α2 values in the investigated complexes are less than 1, indicating a partial ionic and partial covalent nature to the metal–ligand bonds.
All these characterization methods finally led to the conclusion of metal complexes, and the possible structures of the metal complexes are given in Fig. S15.†
3.2 Biological applications of Schiff base ligand derived Cu(II) complexes
The results showed that the DPPH free radical scavenging capacities of the complexes were in accordance with the standard benchmarks (BHT and ascorbic acid). The Cu(II) complexes were thought to have a lower scavenging level than the reference, which was recorded in a methanolic medium as a solvent and demonstrated greater activity. A sample's higher antioxidant potential corresponds to a lower IC50 value. The effect on the scavenging activity of the synthesized compounds is very well denoted by their minimum inhibitory concentration (IC50) with reference to ascorbic acid and BHT as standards,59 as shown in Table 3. A typical antioxidant activity graph is obtained by plotting concentration against the percentage of inhibition, as shown in Fig. 1. The presence of the IC50 values for the complexes dissolved in methanol suggests the vital role of the hydroxyl group (OH) in these compounds. Lower IC50 values are obtained for the complexes of 4BNPC and BMPC in which the OH group exists in its free form. In all other complexes, there is an increase in the IC50 values suggesting the coordination of OH to the metal center.60 Overall the scavenging activity can be ranked as follows: 4BNPC > BMPC > BAC > BNPC. Among the synthesized metal complexes, 4BNPC exhibited the lowest IC50 value in which the OH group existed in its free form rather than being coordinated to the metal center.
| Metal complexes | IC50 (μg mL−1) (fixed reaction time 30 min) |
|---|---|
| Methanol | |
| BAC | 09.96 ± 0.02 |
| BMPC | 05.99 ± 0.01 |
| BNPC | 24.90 ± 0.02 |
| 4BNPC | 03.13 ± 0.01 |
| Standard (ascorbic acid) | 6.559 ± 0.03 |
| BHT | 12.23 ± 0.03 |
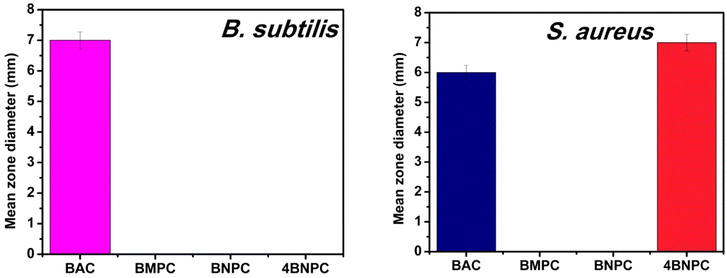 | ||
| Fig. 2 Graphical overview of the mean zone diameters for Gram-positive bacterial strains treated with Cu(II) complexes. | ||
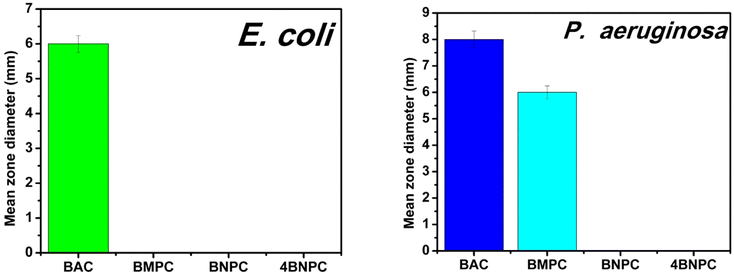 | ||
| Fig. 3 Graphical overview of the mean zone diameters for Gram-negative bacterial strains treated with Cu(II) complexes. | ||
| Compound | Mean zone diameter (mm) | |||
|---|---|---|---|---|
| E. coli | P. aeruginosa | B. subtilis | S. aureus | |
| BAC | 6 ± 0.2 | 8 ± 0.3 | 7 ± 0.2 | 6 ± 0.2 |
| BMPC | — | 6 ± 0.2 | — | — |
| BNPC | — | — | — | — |
| 4BNPC | — | — | — | 7 ± 0.2 |
| Ciprofloxacin | 19.0 ± 0.3 | 21.0 ± 0.2 | 21.0 ± 0.4 | 23.0 ± 0.3 |
This method provided preliminary insights into the efficacy of the compounds against various bacterial strains. The observed activity confirms their antibacterial potential and highlights the promising nature of these compounds as bioactive agents. To gain deeper insights into the molecular basis of their activity, future studies will focus on detailed mechanistic investigations. These will include exploring membrane disruption, reactive oxygen species (ROS) generation, and specific enzyme inhibition pathways. Such studies will provide deeper insights into the mechanisms of action and help in optimizing these compounds for enhanced antibacterial efficacy. These findings serve as an initial step, and further studies are needed to explore these mechanisms comprehensively. Future investigations will aim to uncover the molecular pathways targeted by these compounds, providing a deeper understanding of their antibacterial properties.
| % inhibition | ||||
|---|---|---|---|---|
| Concentration (μg mL−1) | ||||
| Compound | 25 | 50 | 100 | IC50 (μg mL−1) |
| BAC | 06.7 | 11.6 | 29.7 | — |
| BMPC | 27.2 | 36.7 | 48.1 | — |
| BNPC | 30.2 | 53.7 | 62.4 | 46.01 ± 0.30 |
| 4BNPC | 41.5 | 58.2 | 63.9 | 37.31 ± 0.30 |
| Diclofenac = 15.88 ± 0.20 μg mL−1 | ||||
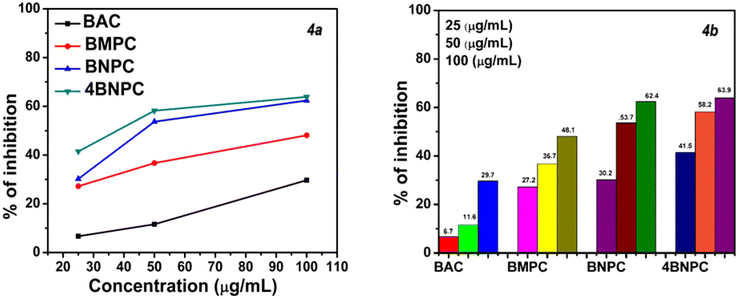
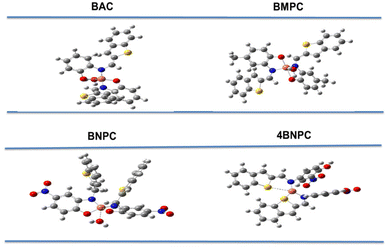
Following experimental investigations, we observed the anti-inflammatory effects of the Cu(II) complexes, even when present at a higher concentration of 100 μg mL−1. Remarkably, the metal complexes featuring a nitro group in the para and ortho positions exhibited considerably higher inhibition percentages than other substituted and non-substituted compounds. The increasing order of IC50 for the compounds was BNPC > 4BNPC, and for other compounds no IC50 was observed at a concentration of 100 μg mL−1. Moreover, the compound featuring a nitro substituent at the ortho position, 4BNPC, demonstrated a remarkably lower IC50 value, indicating greater activity among the synthesized compounds, exhibiting inhibition percentages of 41.5%, 58.2%, and 63.9% at concentrations of 25, 50, and 100 μg mL−1, respectively. These findings indicate that the compounds with electron-withdrawing groups at the ortho position and para position tend to exhibit moderate anti-inflammatory behavior compared to those with an electron-releasing group and compounds with no substitution. Our findings were benchmarked against the diclofenac standard drug for comparison. The graphical representation in Fig. 4 illustrates the percentage inhibition of Cu(II) complexes at various concentrations. When the IC50 values of the various compounds were compared to those of diclofenac, the 4BNPC complex displayed the lowest IC50 when compared to standard drugs.
The nitro group, as a strong electron-withdrawing substituent, enhances the electrophilicity of the Cu(II) center, facilitating stronger interactions with nucleophilic residues in enzyme active sites and improving inhibitory activity. Its polarity can also enhance cellular interactions by promoting better membrane permeability or binding to membrane-bound enzymes. Furthermore, the nitro group contributes to complex stability under physiological conditions, ensuring sustained activity. These electronic effects and structural optimizations enhance the interaction of the complex with biological targets, thereby increasing its anti-inflammatory efficacy.
The greatest inhibitory activity of the synthesized complexes, from the IC50 values, was obtained for BAC, BMPC, and 4BNPC, of which BAC exhibited a lower IC50 value than the other compounds displaying activity. The greater inhibitory potential is associated with lower IC50 values. The results revealed that the non-substituted complex displayed greater efficacy compared to the methyl- and nitro-substituted complexes. All of the studied compounds showed a significant correlation between their structure and activity, which was influenced by specific substituents on the phenyl rings. Furthermore, the non-substituted Cu(II) complexes demonstrated stronger inhibitory effects compared to the methyl- and nitro-substituted complexes. The increasing order of the IC50 value for the compounds is 4BNPC > BMPC > BAC; moreover, the compound featuring the non-substituted Cu(II) complex of BAC demonstrated a remarkably lower IC50 value indicating greater activity among the synthesized metal compounds. The variations in the inhibitory potential seen among these analogs are most likely explained by the varied positions of the substituents on the phenyl rings. The observations show that the Schiff base complexes of Cu(II) can favor α-amylase inhibition, even though their inhibitory potential is still less than that of the commonly used medicinal drug acarbose. As a result, these benzo[b]thiophene Schiff base ligands and their metal complexes exhibited varying degrees of inhibitory action and could be evaluated as the best candidates for α-amylase inhibition. The inhibition of α-amylase can therefore be accomplished by using these synthesized Cu(II) complexes as potential candidates.
3.2.4.1 Mode of inhibition using Michaelis–Menten and Lineweaver–Burk plots. Kinetic studies were conducted to determine how the synthesized Schiff base complexes inhibited the enzyme α-amylase. Researchers might examine a potential method to identify the kind of inhibition involved by examining the kinetics at the IC50 value with different substrate concentrations. The MM equation characterizes enzyme system rates as the ratio of the maximum reaction rate (V) to the substrate concentration ([S]), involving two key factors: the maximal reaction rate (Vmax) and the Michaelis constant (Km).64 The various tactics employed by these transitory inhibitors can be distinguished using steady-state enzyme kinetics. Here, we use the LB plots to investigate the kinetics of inhibition of the enzymes by the inhibitors. The inverse of the substrate concentration [1/S] vs. the inverse of the velocity [1/V] is used to plot the data. LB plots are unique for each of the three types of inhibition. Schiff base complexes are plotted as MM plots (Fig. S16†) and LB plots (Fig. S17†) in the presence and absence of the α-amylase inhibitors; the plots revealed that the synthesized compounds exhibited a non-competitive type of inhibition. This suggests that the active compounds exhibiting inhibition do not compete with the substrate for binding to the active site of the enzyme. They instead bind to another site on the enzyme, slowing down the conversion of the disaccharides to monosaccharides.
3.2.4.2 Measurement of kinetic parameters. The kinetic parameters, such as the Michaelis–Menten constant (Km), the maximum reaction velocity (Vmax), the dissociation constant (Ki), and the mode of inhibition, were determined and presented in Table S8.† It was observed that Vmax decreased for all the synthesized compounds, while Km remained unchanged. The secondary plot of 1/V vs. the inhibitor concentration yielded the EI dissociation constant, Ki.65 The Ki values can also be a helpful tool to assess the activity of the synthesized compounds in light of the compound's binding affinity for the enzyme. Lower Ki values signify a stronger binding affinity, which increases the possibility of inhibition. Upon complexation, the binding affinity is enlarged noticeably based on the Ki values. In any event, the increased inhibitory activity exhibited by the majority of produced compounds is shown by the lower Ki values compared to the α-amylase inhibition. The methyl- and nitro-substituted Cu(II) complexes demonstrated the best resistance to α-amylase when the Ki values were taken into account. The findings demonstrate that methyl groups, which have electron-releasing groups, are more powerful inhibitors than other groups. All compounds have a known structural activity connection, which depends on the various phenyl ring substituents while the Km values stay the same.
These findings underscore the biological relevance of the compounds and provide a foundation for future exploration of their therapeutic applications. While we present these initial findings, we recognize that mechanistic studies such as active site interactions, substrate-binding affinity, and enzyme–inhibitor complex formation would provide a deeper understanding of how the compounds exert their inhibitory effects. These studies will be pursued in future research to fully characterize the molecular mechanisms underlying their enzymatic inhibition. The addition of these kinetic studies has strengthened our understanding of the compounds’ activity, and further studies will be necessary to explore these mechanisms at the molecular level, contributing to our comprehensive understanding of their therapeutic potential.
Table S10† presents the cell viability percentages for the HepG2 cells. According to the findings, as the concentration of the samples increases, the cell viability decreases. This suggests the ability of the Schiff base–Cu(II) complexes to inhibit the growth of malignant cell lines. Since the HepG2 cells are cancer cells, we expect our compounds to exhibit moderate cell viability at lower concentrations. Among the compounds tested, the BNPC complex demonstrates a moderate inhibitory effect on the HepG2 cells, even at low concentrations. At higher concentrations, the lowest cell viability was observed for BMPC. In contrast, the other compounds do not exhibit much promising potential as anticancer agents.
Table S11† illustrates the correlation between the cytotoxicity and the concentration of the newly developed Schiff base ligands and their complexes. As the concentration of the developed compounds increased, the cytotoxicity also increased. Particularly, at a concentration of 200 μg mL−1, only BMPC displayed higher cytotoxicity, reaching approximately 100% against DLA cell lines. This result suggests that BMPC is a promising candidate for further pharmacological investigations against this specific cell line. In contrast, the other substances, however, showed noticeably less toxicity towards the DLA cells, suggesting that they might not be appropriate for extensive study against this cell type. Notably, based on the Trypan blue exclusion test, all compounds showed minimal cytotoxic effects against the DLA cell lines. Interestingly, BMPC exhibited moderate toxicity towards healthy cell lines but demonstrated significant toxicity against the malignant cell lines. Due to their minimal impact on healthy cells, the utilization of this compound at such concentrations could be considered suitable for therapeutic applications.
The anticancer activity of the metal complexes was evaluated using cytotoxicity assays, which served as a first-line screening tool to identify promising candidates. The compounds demonstrated significant cytotoxic activity, suggesting their potential as anticancer agents. However, the molecular mechanisms underlying their anticancer effects, such as apoptosis induction, reactive oxygen species (ROS) generation, and mitochondrial dysfunction, were not explored in this study. These aspects offer promising avenues for future investigations to better understand the full spectrum of their anticancer potential. Future investigations will focus on exploring these mechanisms in greater depth to uncover the specific pathways through which these compounds exert their anticancer effects. This initial screening lays the groundwork for further research to expand our knowledge of their therapeutic capabilities.
In light of the observed cytotoxicity to both cancer and normal cells, future research will focus on enhancing the selectivity of these compounds for cancer cells. This can be achieved through targeted delivery systems, such as conjugation with cancer-specific ligands or utilizing their distinct molecular mechanisms in cancer cells. The structure–activity relationship studies and in vivo testing will also be pivotal in optimizing the therapeutic index of these compounds, ensuring effective targeting of tumor cells while minimizing toxicity to normal tissues.
3.3 DFT optimization studies
The performance of DFT calculations on Schiff base–metal complexes is a valuable approach employed to gain insights into their electronic structure, which in turn helps to elucidate their conducting properties. This computational technique enhances researchers’ ability to predict and comprehend the conducting properties of these complexes, offering crucial insights into their energy levels, electron distribution, and electronic interactions, ultimately advancing our understanding of their electrical conducting behavior.66,67 The stability of the complexes can be evaluated by examining the bond lengths, with shorter bond lengths indicating greater stability. To investigate the Cu(II) complexes, quantum chemical calculations using density functional theory (DFT) were employed. The optimization process involved utilizing the B3LYP level and the 6-311+G(d,p) basis set for all the atoms other than Cu, and for copper we used Lanl2dz.37 These calculations were performed using the Gaussian 09 package.37 The molecular orbitals of all the analyzed structures were investigated (Fig. 5).The absence of imaginary frequencies during optimization indicates that a stable stationary point was achieved, confirming the reliability of the results obtained and relevant data, namely, the highest occupied molecular orbital (HOMO), the lowest unoccupied molecular orbital (LUMO), and energy gaps, were collected. The energy gap (Eg) is calculated as the difference between the HOMO and LUMO energies (EHOMO–ELUMO). The HOMOs and LUMOs of the Schiff base complexes in the gas phase appear to be delocalized throughout the entire molecule, according to the maps of the molecular orbitals. The molecular orbitals and their energy gaps provide insights into the energetic stability and chemical reactivity of a molecule. The obtained data were used to construct the frontier molecular orbital diagram (FMOD). This diagram provides a visual representation of the HOMO and LUMO energy levels and their relative positions within the electronic structure of metal complexes derived from Schiff base ligands. The band gap indicates significant chemical reactivity, biological characteristics, and polarizability. The FMOD of the metal complexes of Cu(II) are depicted in Fig. 6.
 | ||
| Fig. 6 The band gap diagram of the Cu(II) complexes of the benzo[b]thiophene scaffold along with the HOMO and LUMO surfaces. | ||
We also calculated the global descriptors of the Schiff base complexes of Cu(II) using the HOMO–LUMO energy values, including the chemical potential, chemical hardness, chemical softness, electronegativity nucleophilicity, and electrophilicity index to gain insights into the electronic properties and understand the quantum reactivity of the molecules. These descriptors enable a comprehensive understanding of the overall behavior of the molecule, whether it acts as an electrophile or a nucleophile. These parameters are calculated using eqn (S1)–(S6) (Table S12†) and the values for the Cu(II) complexes are listed in Tables S13–S16.† These variables relate to the number of electrons and the effect of an external potential change on the electron density.68 The synthesized compounds are stable, can take electrons from the environment to reduce their energy, and can give an idea about the chemical behavior of the compounds when the nucleophilicity or electrophilicity index is positive and the chemical potential is negative.69
The quantum chemical descriptors derived from DFT studies offer significant insights into the structure–activity relationships of these complexes. The identified biological activities can be linked to various important electronic parameters. The HOMO–LUMO energy gaps reveal insights into molecular stability and reactivity, with 4BNPC exhibiting the highest gap of 3.358 eV, indicating the greatest stability. This is followed by BNPC at 2.985 eV, BAC at 2.666 eV, and BMPC at 2.444 eV. The electrophilicity indices (ω) illustrate the capacity of the molecules to accept electrons from biological targets, with BNPC exhibiting the highest value (7.53), followed by 4BNPC (6.23) and the others (5.84), suggesting a greater potential for biological interactions. The electron-donating powers exhibit notable variation (4BNPC: 16.06, BNPC: 10.80, BAC: 10.60, BMPC: 9.11), indicating distinct abilities for electron transfer with biomolecules. The global softness values (S) span from 0.60 to 0.83, reflecting different levels of reactivity with biological substrates. The electronic parameters outlined here elucidate the complexes’ capacity to engage with biological targets via electron transfer mechanisms, their ability to penetrate membranes, and their general reactivity patterns, thus offering a theoretical basis for comprehending their documented biological activities.70
4. Conclusion
In this study, four novel Cu(II)–Schiff base complexes derived from the benzo[b]thiophene scaffold were successfully synthesized and analyzed, demonstrating their multifaceted biological potential. The synthesized complexes exhibited promising properties across various assays and their biological activities, including antioxidant, antibacterial, anti-inflammatory, enzyme inhibition, and cytotoxic effects, were evaluated. Results indicated that chelation or coordination with metals enhanced the biological activity, likely due to the increased lipophilicity of the metal ions, which reduced their reactivity. The Cu(II) complexes generally showed superior antioxidant, antimicrobial, anti-inflammatory, and enzyme-inhibiting activities. Notably, 4BNPC displayed excellent antioxidant performance, BAC showed significant antibacterial effects, and BAC complexes excelled at enzyme inhibition. BMPC was particularly effective, exhibiting high cytotoxicity against cancer cell lines, including nearly complete cell death at 200 μg mL−1 in DLA cells, highlighting its potential as a strong candidate for further cancer research. The anti-inflammatory effects of the complexes, especially 4BNPC, were only moderate compared to diclofenac, indicating that further optimization might be needed. While BMPC demonstrated high cytotoxicity, the effectiveness of other complexes varied, particularly in the HepG2 cell line where BNPC showed only moderate inhibition. Overall, while these Cu(II)–Schiff base complexes show considerable promise, especially BMPC, further studies are necessary to refine their anti-inflammatory properties and confirm their efficacy and safety in broader biological contexts. Future research should explore additional biological activities, such as antitumor or antiviral effects. Additionally, the cytotoxicity was tested on a single cell line (H9c2), which might not fully reflect the compounds’ effects on other cell types or in in vivo environments. Testing across a broader range of cell lines and in animal models could provide a more comprehensive understanding of their safety and efficacy. To address the potential therapeutic applications of the complexes, it is essential to evaluate their accumulation within cancer cells. Understanding the cellular take up and intracellular distribution of these complexes can provide valuable insights into their bioavailability, mode of action, and therapeutic efficacy. Although this study primarily focuses on the synthesis, characterization, and biological evaluation of the complexes, future investigations will include detailed studies on their accumulation within cancer cells to better assess their potential as anticancer agents. Moreover, mechanistic studies were not conducted to clarify how functional groups and metal coordination affected biological activity. Detailed mechanistic investigations could reveal specific pathways and interactions, aiding in the design of more effective compounds. Future research should also focus on optimizing the synthesis of the most promising compounds to enhance their potency and selectivity. Exploring different metal ions and ligand combinations, as well as varying substituents, may lead to compounds with better therapeutic potential. Additionally, evaluating pharmacokinetics and toxicity in more complex biological systems is essential for advancing these compounds from the laboratory to potential clinical applications.Author contributions
T. M. Dhanya – writing-original draft. M. R. Prathapachandra Kurup – formal analysis, methodology and visualization. K. J. Rajimon – investigation, software and conceptualization. G. Anjali Krishna – investigation and validation. Jibin K. Varughese – software and methodology. K. G. Raghu – methodology, conceptualization and investigation. Sachin Philip – software and formal analysis. K. M. Divya – data curation and validation. Maria Augustine – investigation and formal analysis. P. V. Mohanan – supervision, writing-review & editing.Data availability
The authors declare that the data are available on request.Conflicts of interest
The authors have no conflict of interest.Acknowledgements
The authors would like to acknowledge STIC CUSAT for spectral characterization, SAIF IIT MADRAS for EPR analysis, Unibiosys Research Lab for anti-bacterial studies and anti-inflammatory studies, CSIR-NIIST, Thiruvananthapuram and Amala Cancer Centre Thrissur for cytotoxicity studies.References
- N. Sreenivasachary, H. Kroth, P. Benderitter, A. Hamel, Y. Varisco, D. T. Hickman, W. Froestl, A. Pfeifer and A. Muhs, Discovery and characterization of novel indole and 7-azaindole derivatives as inhibitors of β-amyloid-42 aggregation for the treatment of Alzheimer's disease, Bioorg. Med. Chem. Lett., 2017, 27, 1405–1411 CrossRef CAS PubMed.
- K. L. Barry, C. D. Grimmer, O. Q. Munro and M. P. Akerman, Self-assembled supramolecular structures of O, N, N′ tridentate imidazole–phenol Schiff base compounds, RSC Adv., 2020, 10, 7867–7878 RSC.
- J. McGinley, M. McCann, K. Ni, T. Tallon, K. Kavanagh, M. Devereux, X. Ma and V. McKee, Imidazole Schiff base ligands: Synthesis, coordination complexes and biological activities, Polyhedron, 2013, 55, 169–178 CrossRef CAS.
- D. Rogolino, M. Carcelli, M. Sechi and N. Neamati, Viral enzymes containing magnesium: Metal binding as a successful strategy in drug design, Coord. Chem. Rev., 2012, 256, 3063–3086 CrossRef CAS.
- F. Siddique, O. Daoui, M. Ayoub, S. Elkhattabi, S. Chtita, S. Afzal, A. Mohyuddin, I. Kaukab, S. A. Ejaz and A. M. Salamatullah, Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery, Open Chem., 2024, 22, 20240064 CrossRef.
- R. Bansal and A. Suryan, A comprehensive review on steroidal bio conjugates as promising leads in drug discovery, ACS Bio Med Chem Au, 2024, 2, 340–369 CrossRef PubMed.
- A. A. Shanty and P. V. Mohanan, Heterocyclic Schiff bases as nontoxic antioxidants: Solvent effect, structure activity relationship and mechanism of action, Spectrochim. Acta, Part A, 2018, 192, 181–187, DOI:10.1016/j.saa.2017.11.019.
- J. M. Stokes, K. Yang, K. Swanson, W. Jin, A. Cubillos-Ruiz, N. M. Donghia, C. R. MacNair, S. French, L. A. Carfrae, Z. Bloom-Ackerman, V. M. Tran, A. Chiappino-Pepe, A. H. Badran, I. W. Andrews, E. J. Chory, G. M. Church, E. D. Brown, T. S. Jaakkola, R. Barzilay and J. J. Collins, A Deep Learning Approach to Antibiotic Discovery, Cell, 2020, 180, 688–702, DOI:10.1016/j.cell.2020.01.021.
- E. D. Brown and G. D. Wright, Antibacterial drug discovery in the resistance era, Nature, 2016, 529, 336–343, DOI:10.1038/nature17042.
- M. J. Culyba, C. Y. Mo and R. M. Kohli, Targets for combating the evolution of acquired antibiotic resistance, Biochemistry, 2015, 54, 3573–3582 CrossRef CAS PubMed.
- U. Hofer, The next step towards anticancer microbiota therapeutics, Nat. Rev. Microbiol., 2019, 17, 125, DOI:10.1038/s41579-019-0152-2.
- L. Chen, H. Deng, H. Cui, J. Fang, Z. Zuo, J. Deng, Y. Li, X. Wang and L. Zhao, Inflammatory responses and inflammation-associated diseases in organs, Oncotarget, 2018, 9, 7204–7218, DOI:10.18632/oncotarget.23208.
- A. D. Politano, K. T. Campbell, L. H. Rosenberger and R. G. Sawyer, Use of silver in the prevention and treatment of infections: Silver review, Surg. Infect., 2013, 14, 8–20, DOI:10.1089/sur.2011.097.
- T. M. Dhanya, G. Anjali Krishna, D. P. Savitha, A. A. Shanty, K. M. Divya, S. K. Priya and P. V. Mohanan, A review on the synthesis and biological relevance of benzo [b] thiophene derivatives, Phosphorus, Sulfur Silicon Relat. Elem., 2023, 198, 283–299 CrossRef CAS.
- C. Nathan and A. Ding, Non resolving inflammation, Cell, 2010, 140, 871–882 CrossRef CAS PubMed.
- L. M. Lichtenberger, Z. M. Wang, J. J. Romero, C. Ulloa, J. C. Perez, M. N. Giraud and J. C. Barreto, Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: Insight into the mechanism and reversal of NSAID-induced gastrointestinal injury, Nat. Med., 1995, 1, 154–158, DOI:10.1038/nm0295-154.
- J. Romero-Parra, J. Mella-Raipán, V. Palmieri, M. Allarà, M. J. Torres, H. Pessoa-Mahana, P. Iturriaga-Vásquez, R. Escobar, M. Faúndez, V. Di Marzo and C. D. Pessoa-Mahana, Synthesis, binding assays, cytotoxic activity and docking studies of benzimidazole and benzothiophene derivatives with selective affinity for the CB2 cannabinoid receptor, Eur. J. Med. Chem., 2016, 124, 17–35, DOI:10.1016/j.ejmech.2016.08.005.
- T. M. Dhanya, A. Krishnan, G. A. Krishna, S. Francis, P. V. Aswathy, M. Augustine, A. A. Shanty, K. M. Divya, D. P. Savitha and P. V. Mohanan, A novel benzothiophene incorporated Schiff base acting as a “turn-on” sensor for the selective detection of Serine in organic medium, Bioorg. Chem., 2023, 136, 106525 CrossRef CAS PubMed.
- A. Cavalli, M. L. Bolognesi, A. Minarini, M. Rosini, V. Tumiatti, M. Recanatini and C. Melchiorre, Multi-target-directed ligands to combat neurodegenerative diseases, J. Med. Chem., 2008, 51, 347–372 CrossRef CAS PubMed.
- R. Morphy and Z. Rankovic, Designed multiple ligands. An emerging drug discovery paradigm, J. Med. Chem., 2005, 48, 6523–6543 CrossRef CAS PubMed.
- J. Sterling, Y. Herzig, T. Goren, N. Finkelstein, D. Lerner, W. Goldenberg, I. Miskolczi, S. Molnar, F. Rantal and T. Tamas, Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer's disease, J. Med. Chem., 2002, 45, 5260–5279 CrossRef CAS PubMed.
- R. L. Prior, X. Wu and K. Schaich, Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements, J. Agric. Food Chem., 2005, 53, 4290–4302 CrossRef CAS PubMed.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, Use of a free radical method to evaluate antioxidant activity, LWT–Food Sci. Technol., 1995, 28, 25–30, DOI:10.1016/S0023-6438(95)80008-5.
- P. A. Wayne, Clinical and laboratory standards institute (CLSI), in Perform. Stand. Antimicrob. Susceptibility Test, 2015 Search PubMed.
- J. P. Michel, J. Chan and K. R. Noel, Microbiology–Concepts and Applications, 1993 Search PubMed.
- H. R. Afzal, N. U. H. Khan, K. Sultana, A. Mobashar, A. Lareb, A. Khan, A. Gull, H. Afzaal, M. T. Khan, M. Rizwan and M. Imran, Schiff Bases of Pioglitazone Provide Better Antidiabetic and Potent Antioxidant Effect in a Streptozotocin-Nicotinamide-Induced Diabetic Rodent Model, ACS Omega, 2021, 6, 4470–4479, DOI:10.1021/acsomega.0c06064.
- M. Balouiri, M. Sadiki and S. K. Ibnsouda, Methods for in vitro evaluating antimicrobial activity: A review, J. Pharm. Anal., 2016, 6, 71–79, DOI:10.1016/j.jpha.2015.11.005.
- A. Ragab, S. A. Fouad, Y. A. Ammar, D. S. Aboul-Magd and M. S. Abusaif, Antibiofilm and anti-quorum-sensing activities of novel pyrazole and pyrazolo [1, 5-a] pyrimidine derivatives as carbonic anhydrase I and II inhibitors: design, synthesis, radiosterilization, and molecular docking studies, Antibiotics, 2023, 12, 128 CrossRef CAS PubMed.
- P. S. Sundara Selvam, G. S. Chinnadurai, D. Ganesan and V. Kandan, Eggshell membrane-mediated V 2 O 5/ZnO nanocomposite: synthesis, characterization, antibacterial activity, minimum inhibitory concentration, and its mechanism, Appl. Phys. A, 2020, 126, 1–16 CrossRef.
- J. Hoque, M. M. Konai, S. Gonuguntla, G. B. Manjunath, S. Samaddar, V. Yarlagadda and J. Haldar, Membrane active small molecules show selective broad spectrum antibacterial activity with no detectable resistance and eradicate biofilms, J. Med. Chem., 2015, 58, 5486–5500 CrossRef CAS PubMed.
- S. A. Sadeek, M. S. El-Attar and S. M. Abd El-Hamid, Preparation and characterization of new tetradentate Schiff base metal complexes and biological activity evaluation, J. Mol. Struct., 2013, 1051, 30–40 CrossRef CAS.
- U. A. Shinde, A. S. Phadke, A. M. Nair, A. A. Mungantiwar, V. J. Dikshit and M. N. Saraf, Membrane stabilizing activity—a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil, Fitoterapia, 1999, 70, 251–257 CrossRef CAS.
- S. Giancarlo, L. M. Rosa, F. Nadjafi and M. Francesco, Hypoglycaemic activity of two spices extracts: Rhus coriaria L. and Bunium persicum Boiss, Nat. Prod. Res., 2006, 20, 882–886 CrossRef CAS PubMed.
- G. D. Brayer, Y. Luo and S. G. Withers, The structure of human pancreatic α-amylase at 1.8 Å resolution and comparisons with related enzymes, Protein Sci., 1995, 4, 1730–1742 CrossRef CAS PubMed.
- H. Lineweaver and D. Burk, The Determination of Enzyme Dissociation Constants, J. Am. Chem. Soc., 1934, 56, 658–666, DOI:10.1021/ja01318a036.
- L. Q. Ma, Y. Yu, H. Chen, M. Li, A. Ihsan, H.-Y. Tong, X.-J. Huang and Y. Gao, Sweroside alleviated aconitine-induced cardiac toxicity in H9c2 cardiomyoblast cell line, Front. Pharmacol., 2018, 9, 1138 CrossRef CAS PubMed.
- R. Bauernschmitt, M. Häser, O. Treutler and R. Ahlrichs, Calculation of excitation energies within time-dependent density functional theory using auxiliary basis set expansions, Chem. Phys. Lett., 1997, 264, 573–578 CrossRef CAS.
- T. Chandrasekar, A. Arunadevi and N. Raman, Synthesis, spectral characterization, DNA-binding and antimicrobial profile of biological active mixed ligand Schiff base metal (II) complexes incorporating 1, 8-diaminonaphthalene, J. Coord. Chem., 2021, 74, 804–822 CrossRef CAS.
- A. Gaur, W. Klysubun, N. N. Nair, B. D. Shrivastava, J. Prasad and K. Srivastava, XAFS study of copper(II) complexes with square planar and square pyramidal coordination geometries, J. Mol. Struct., 2016, 1118, 212–218 CrossRef CAS.
- A. D. Kulkarni, S. A. Patil and P. S. Badami, Electrochemical properties of some transition metal complexes: synthesis, characterization and in vitro antimicrobial studies of Co(II), Ni(II), Cu(II), Mn(II) and Fe(III) complexes, Int. J. Electrochem. Sci., 2009, 4, 717–729 CrossRef CAS.
- S. Jiang, H. Ni, F. Liu, S. Gu, P. Yu and Y. Gou, Binuclear Schiff base copper(II) complexes: Syntheses, crystal structures, HSA interaction and anti-cancer properties, Inorg. Chim. Acta, 2020, 499, 119186 CrossRef CAS.
- R. N. Patel, N. Singh and V. L. N. Gundla, Synthesis, structure and properties of ternary copper(II) complexes of ONO donor Schiff base, imidazole, 2, 2′-bipyridine and 1, 10-phenanthroline, Polyhedron, 2006, 25, 3312–3318 CrossRef CAS.
- E. Garribba and G. Micera, The determination of the geometry of Cu(II) complexes: an EPR spectroscopy experiment, J. Chem. Educ., 2006, 83, 1229 CrossRef CAS.
- E. R. Sevda and G. Dikmen, A New Dinuclear Copper(II)-Hydrazone Complex: Synthesis, Crystal Structure and Antibacterial Activity, Lett. Org. Chem., 2023, 20, 376–387 CrossRef.
- A. A. Alfares, L. A. Alnuaimy and A. G. M. Al-Daher, Synthesis and Characterization of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes with 2-Carboxy benzaldehyde Aroylhydrazones, Egypt. J. Chem., 2022, 65, 271–278 Search PubMed.
- M. A. El-Ghamry, F. M. Elzawawi, A. A. A. Aziz, K. M. Nassir and S. M. Abu-El-Wafa, New Schiff base ligand and its novel Cr(III), Mn(II), Co(II), Ni(II), Cu(II), Zn(II) complexes: Spectral investigation, biological applications, and semiconducting properties, Sci. Rep., 2022, 12, 17942 CrossRef CAS PubMed.
- M. M. Abo-Aly, A. M. Salem, M. A. Sayed and A. A. A. Aziz, Spectroscopic and structural studies of the Schiff base 3-methoxy-N-salicylidene-o-amino phenol complexes with some transition metal ions and their antibacterial, antifungal activities, Spectrochim. Acta, Part A, 2015, 136, 993–1000 CrossRef CAS PubMed.
- E. Funck and K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, John Wiley & Sons, New York–London, 1963, pp. 328 Search PubMed.
- K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds, part B: applications in coordination, organometallic, and bioinorganic chemistry, John Wiley & Sons, 2009 Search PubMed.
- I. Sheikhshoaie, Z. Tohidiyan and M. Khaleghi, Two new Cu(II) and Zn(II) Schiff base complexes: synthesis, characterization and their biological activity, 2016 Search PubMed.
- F. Sahan, M. Kose, C. Hepokur, D. Karakas and M. Kurtoglu, New azo–azomethine–based transition metal complexes: Synthesis, spectroscopy, solid–state structure, density functional theory calculations and anticancer studies, Appl. Organomet. Chem., 2019, 33, e4954 CrossRef.
- K. Jayakumar, M. Sithambaresan, N. Aiswarya and M. R. P. Kurup, Synthesis and spectral characterization of mono-and binuclear copper(II) complexes derived from 2-benzoylpyridine-N4-methyl-3-thiosemicarbazone: Crystal structure of a novel sulfur bridged copper(II) box-dimer, Spectrochim. Acta, Part A, 2015, 139, 28–36 CrossRef CAS PubMed.
- I. M. Procter, B. J. Hathaway and P. Nicholls, The electronic properties and stereochemistry of the copper(II) ion. Part I. Bis(ethylenediamine) copper(II) complexes, J. Chem. Soc. A, 1968, 1678–1684, 10.1039/J19680001678.
- N. Aiswarya, M. Sithambaresan, S. S. Sreejith, S. W. Ng and M. R. P. Kurup, Polymeric polymorphs and a monomer of pseudohalide incorporated Cu(II) complexes of 2, 4-dichlorido-6-((2-(dimethylamino) ethylimino) methyl) phenol.: Crystal structures and spectroscopic behavior, Inorg. Chim. Acta, 2016, 443, 251–266 CrossRef CAS.
- B. N. Figgis, Introduction to ligand fields, Interscience publishers, 1966 Search PubMed.
- B. J. Hathaway, G. Wilkinson, R. D. Gillard and J. A. McCleverty, Comprehensive coordination chemistry, Synth. React. Prop. Appl. Coord. Compd., 1987, 5, 533–774 Search PubMed.
- S. D. Pradeep, A. K. Gopalakrishnan, D. K. Manoharan, R. S. Soumya, R. K. Gopalan and P. V. Mohanan, Isatin derived novel Schiff bases: An efficient pharmacophore for versatile biological applications, J. Mol. Struct., 2023, 1271, 134121 CrossRef CAS.
- J. Priya and D. Madheswari, Biomolecular docking interactions, cytotoxicity and antioxidant property evaluations with novel Mn(II), Ni(II), Cd(II) and Pb(II) Schiff base ligand complexes: Synthesis and characterization, J. Biosci., 2022, 47, 29 CrossRef CAS PubMed.
- T. L. Yusuf, D. C. Akintayo, S. D. Oladipo, A. A. Adeleke, K. Olofinsan, B. Vatsha and N. Mabuba, The Effect of Structural Configuration On the DNA Binding and in vitro Antioxidant Properties of New Copper(II) N2O2 Schiff Base Complexes, New J. Chem., 2022, 46, 12968–12980, 10.1039/D2NJ01477G.
- A. Golcu, M. Tumer, H. Demirelli and R. A. Wheatley, Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: synthesis, characterization, properties and biological activity, Inorg. Chim. Acta, 2005, 358, 1785–1797 CrossRef CAS.
- C. J. Teal, S. P. Lu and M. S. Shoichet, Engineering hydrogels for affinity-based release of therapeutic proteins, Chem. Mater., 2024, 36, 614–641 CrossRef CAS.
- T. Samaraweera, T. Samaraweera, N. N. Senadeera and C. B. Ranaweera, In vitro Anti-Inflammatory Activity of Leaves of Jeffreycia zeylanica Using the Egg Albumin Denaturation Method and Human Red Blood Cell Stabilization Method, Asian Plant Res. J., 2023, 11, 56–64 CrossRef.
- B. Lee, W. Zhao, K.-C. Yang, Y. Y. Ahn and B. L. Perry, Systematic evaluation of state policy interventions targeting the US opioid epidemic, 2007–2018, JAMA Netw. Open, 2021, 4, e2036687–e2036687 CrossRef PubMed.
- T. M. Dhanya, K. J. Rajimon, N. K. Mohanan, A. Anilkumar, S. S. Pillai and P. V. Mohanan, Exploring the Enzyme Inhibition Potential of Schiff Base and Metal Complexes of Benzothiophene Derivatives, Discovery Chem., 2024, 1, 48 CrossRef.
- J. Ashraf, E. U. Mughal, R. I. Alsantali, A. Sadiq, R. S. Jassas, N. Naeem, Z. Ashraf, Y. Nazir, M. N. Zafar and A. Mumtaz, 2-Benzylidenebenzofuran-3 (2 H)-ones as a new class of alkaline phosphatase inhibitors: synthesis, SAR analysis, enzyme inhibitory kinetics and computational studies, RSC Adv., 2021, 11, 35077–35092 RSC.
- K. J. Rajimon, N. Sreelakshmi, D. S. R. Nair, N. F. Begum and R. Thomas, An in-depth study of the synthesis, electronic framework, and pharmacological profiling of 1-(anthracen-9-yl)-N-(4-nitrophenyl) methanimine: In vitro and in silico investigations on molecular docking, dynamics simulation, BSA/DNA binding and toxicity studies, Chem. Phys. Impact, 2024, 100462 CrossRef.
- K. J. Rajimon, A. Y. Alzahrani, E. S. Aazam, B. M. Abbas, P. Govindarajan and R. Thomas, Unveiling the multifaceted potential of (E)-N-(4-Chlorophenyl)-1-(thiophen-2-yl) methanimine with special reference to solution-state fluorescence, synthesis, electronic structure, antimicrobial, antibiofilm, larvicidal activities, toxicity, docking and dynamics, J. Mol. Struct., 2024, 137428 CrossRef CAS.
- M. Leopoldini, T. Marino, N. Russo and M. Toscano, Density functional computations of the energetic and spectroscopic parameters of quercetin and its radicals in the gas phase and in solvent, Theor. Chem. Acc., 2004, 111, 210–216 Search PubMed.
- A. C. Ekennia, D. C. Onwudiwe, L. O. Olasunkanmi, A. A. Osowole and E. E. Ebenso, Synthesis, DFT calculation, and antimicrobial studies of novel Zn(II), Co(II), Cu(II), and Mn(II) heteroleptic complexes containing benzoylacetone and dithiocarbamate, Bioinorg. Chem. Appl., 2015, 789063 Search PubMed.
- K. J. Rajimon, A. Y. Alzahrani, D. S. R. Nair, D. P. Venkatesh and R. Thomas, Exploring the structure and dynamics of a fluorescent schiff base (1E, 1′ E)-1, 1′-(1, 4-phenylene) bis (N-(4-chlorophenyl) methamine: Synthesis, spectroscopic analysis, thermal, electronic and crystallographic study with biological applications, J. Mol. Struct., 2024, 138546 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4dt02486a |
| This journal is © The Royal Society of Chemistry 2025 |