Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Changing the reaction pathway of the CaAl2 oxidation using ball milling†
Elias C. J.
Gießelmann
 ,
Guido
Kickelbick
,
Guido
Kickelbick
 and
Oliver
Janka
and
Oliver
Janka
 *
*
Inorganic Solid State Chemistry, Saarland University, Campus C4.1, 66123 Saarbrücken, Germany. E-mail: oliver.janka@uni-saarland.de
First published on 13th November 2024
Abstract
As previously shown, CaAl2 can be oxidized using elemental O2 to form CaAl2O4. This reaction, however, proceeds via Ca12Al14O33 and elemental Al as intermediates which are subsequently transformed into the stoichiometric reaction product. High-energy ball milling is known to decrease the crystallite size of a material and to significantly produce defects enabling different reaction pathways compared to a highly crystalline bulk material. In this subsequent study, a different oxidizing agent (H2O) as well as the ball milling behavior of CaAl2 and the consecutive oxidation via elemental O2 were studied. While the use of H2O as the oxidizing agent showed only minor differences in the reaction products, ball milling of CaAl2 decreases, as expected, the crystallite size of the material and introduces defects. This is visible both in the powder X-ray diffraction patterns and in the 27Al solid-state MAS NMR spectra. In the subsequent steps, the ball milled material was oxidized in an STA system. Already 5 min of ball milling significantly changes the energy pattern of the reaction. Powder X-ray diffraction studies on the oxidized material clearly indicate that a different reaction pathway occurs. Samples ball milled for 180 min even get pyrophoric.
1. Introduction
Light weight alloys are of key importance for modern technical applications such as automotive and transportation applications,1–5 architecture,5–7 but also as corrosion resistant parts for air- and spacecrafts,8–12 for medical applications like implants or stents,10–15 or as non-sparking tools for gas stations, oil rigs or fire fighters.16–18 In some of these alloys, intermetallic compounds play an important role.1,5,19,20 The thermal and chemical resistance alongside astonishing mechanical properties of materials from the Ti–Al system21 led to their use in aircraft and gas turbines.22,23 However, the corrosion resistance of many materials is insufficient at elevated temperatures, which can be improved by alloying or special structuring.24–26 Therefore, oxidation studies are an important field of research.26–30 Alloys based on Ni and Al show supreme corrosion stability under extreme conditions. In AlNi for example, a protective layer of aluminum oxide Al2O3 (corundum, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) is formed when the material is exposed to air at elevated temperatures.31–33 Finally, aluminum alloys can be protected from oxidation by addition of small amounts of CaAl2.34
m) is formed when the material is exposed to air at elevated temperatures.31–33 Finally, aluminum alloys can be protected from oxidation by addition of small amounts of CaAl2.34
Since intermetallic compounds are, in contrast to ionic compounds, not restricted to charge neutrality with respect to their compositions, they exhibit a plethora of different compositions and crystal structures35 in their respective binary phase diagrams.36 This manifold could, in theory, be utilized to synthesize valence precise e.g. ternary chalcogenides. Hoppe and co-workers prepared different AMO2 phases (A = Li–K, M = In, Tl) starting from the Zintl phases NaTl, KTl, and LiIn37–39 and also CsAuO could be obtained by the reaction of CsAu with dry O2.40 The oxidation of BaCu and YCu as well as related compounds led to the high-temperature superconductor YBa2Cu3O7−δ.41–44 Jung and coworkers used different homo- and heterogeneous alloys for the synthesis of various different oxides,45–53 but also CaAl2S4 and SrAl2S4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 as well as Eu2GeS4,55 EuAl2S4
54 as well as Eu2GeS4,55 EuAl2S4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 and LiGaX2 (X = O, S)57 were synthesized by this route.
56 and LiGaX2 (X = O, S)57 were synthesized by this route.
Recently, we have shown that the cubic Laves phase CaAl2 (space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) can also be oxidized to the expected product CaAl2O4 (space group P21/c);58 however, the reaction is far away from being straightforward. When heat-treating CaAl2 under air, Ar/O2 mixtures or elemental oxygen, initially Ca12Al14O33 (mineral mayenite; space group I
m) can also be oxidized to the expected product CaAl2O4 (space group P21/c);58 however, the reaction is far away from being straightforward. When heat-treating CaAl2 under air, Ar/O2 mixtures or elemental oxygen, initially Ca12Al14O33 (mineral mayenite; space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d) is formed. This compound is significantly richer in calcium as the starting material, leaving unoxidized elemental aluminum behind. This was proven by X-ray diffraction and 27Al NMR experiments. Repetitive grinding and oxidation finally led to almost phase pure CaAl2O4 samples. In this paper, we report a follow-up study in which a different oxidizing agent, H2O, was used to investigate the differences between water and O2. Furthermore, it is known that ball milling can not only be used to reduce the particle and crystallite size of a material, but also to introduce defects, activating the material. This can lead to phase transitions,59 partial reduction59 or can be used to e.g. surface modify transition metal oxides60,61 or to intercalate Li into activated transition metal oxides.62,63 Therefore, the precursor CaAl2 was activated via high-energy ball milling. The different reaction pathways as well as their intermediates were investigated with the help of powder X-ray diffraction experiments, 27Al solid-state NMR studies, and thermal analyses.
3d) is formed. This compound is significantly richer in calcium as the starting material, leaving unoxidized elemental aluminum behind. This was proven by X-ray diffraction and 27Al NMR experiments. Repetitive grinding and oxidation finally led to almost phase pure CaAl2O4 samples. In this paper, we report a follow-up study in which a different oxidizing agent, H2O, was used to investigate the differences between water and O2. Furthermore, it is known that ball milling can not only be used to reduce the particle and crystallite size of a material, but also to introduce defects, activating the material. This can lead to phase transitions,59 partial reduction59 or can be used to e.g. surface modify transition metal oxides60,61 or to intercalate Li into activated transition metal oxides.62,63 Therefore, the precursor CaAl2 was activated via high-energy ball milling. The different reaction pathways as well as their intermediates were investigated with the help of powder X-ray diffraction experiments, 27Al solid-state NMR studies, and thermal analyses.
2. Experimental
Synthesis
Caution: Ball-milled metals and intermetallic compounds can get pyrophoric after milling. The handling of the samples under an inert atmosphere (e.g. inside an Ar filled glove box) is advised.
Thermal analysis/oxidation
Simultaneous thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were carried out with a TGA/DSC 1 Star HT/1600 system (Mettler Toledo, Columbus, OH, USA) under an Ar/O2 atmosphere with flow rates of 40 mL min−1 each. For all oxidation experiments heating and cooling rates of 20 K min−1 and dwelling periods of 60 min were applied. Samples were placed into alumina crucibles (∅ = 6 mm, h = 4.5 mm) for the STA measurements.Powder X-ray diffraction
Powder X-ray diffraction (PXRD) patterns of the pulverized samples were recorded at room temperature on a D8-A25-Advance diffractometer (Bruker, Karlsruhe, Germany) in Bragg Brentano θ–θ-geometry (goniometer radius 280 mm) with CuKα-radiation (λ = 154.0596 pm). A 12 μm Ni foil working as the Kβ filter and a variable divergence slit were mounted at the primary beam side. A LYNXEYE detector with 192 channels was used at the secondary beam side. Experiments were carried out in the 2θ range of 6–130° with a step size of 0.013° and a total scan time of 1 h. The recorded data was evaluated using Rietveld refinements65,66 with the Bruker TOPAS 5.0 software,67 the fundamental instrument parameters were determined beforehand. The crystallite sizes were determined from TOPAS using the LVol-IB values. For the ball-milled samples, the strain L parameter was refined additionally. To determine the amorphous part of a sample, ∼15 mass% of elemental Si were added to the sample and homogenized. During the Rietveld analysis, the contribution of the internal standard was fixed to the weighed value, enabling the determination of an amorphous fraction where present.SEM/EDX data
Semi-quantitative EDX analyses of one ball-milled sample of CaAl2 were conducted on a JEOL 7000F (JEOL, Freising, Germany) scanning electron microscope equipped with an EDAX Genesis 2000 EDX detector (EDAX, Unterschleissheim, Germany). The sample was sprinkled on conductive carbon tape and one area scans as well as three independent data points were measured.27Al solid-state MAS NMR
27Al solid-state MAS NMR spectra were recorded on an Avance III 400 WB (Bruker Biospin, Ettlingen, Germany) at 104.35 MHz using magic-angle spinning (MAS). The samples were used as fine powders. To reduce density and electrical conductivity, samples were mixed with dried sodium chloride in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (sample
9 (sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaCl) or higher. The diluted samples were loaded into a cylindrical ZrO2 rotor with a diameter of 4 mm and spun at the magic angle at a frequency of 13 kHz. All experiments conducted were single-pulse experiments with a typical pulse length of 0.83 μs (≈30° pulse) and a relaxation delay of 1 s. Resonance shifts were referenced to an aqueous 1 molar AlCl3 solution. The NMR spectra were recorded using the Bruker Topspin68 software; the analysis was performed with the help of the Dmfit software.69
NaCl) or higher. The diluted samples were loaded into a cylindrical ZrO2 rotor with a diameter of 4 mm and spun at the magic angle at a frequency of 13 kHz. All experiments conducted were single-pulse experiments with a typical pulse length of 0.83 μs (≈30° pulse) and a relaxation delay of 1 s. Resonance shifts were referenced to an aqueous 1 molar AlCl3 solution. The NMR spectra were recorded using the Bruker Topspin68 software; the analysis was performed with the help of the Dmfit software.69
3. Results and discussion
Precursor characterization
As the starting material, the cubic Laves phase CaAl2 (MgCu2 type, space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 804.02(1) pm)58,70 was used. It could be shown by XRD and NMR investigations that ∼5 mass% of the Al richer phase CaAl4 is present (CaGa4 type, space group C2/m, a = 616.91(1), b = 618.50(1) c = 634.34(1) pm, β = 118.03(1)°).70 When oxidizing this compound with air or pure O2 the structures of the main products are the ternary oxides Ca12Al14O33 (mineral mayenite, own type, space group I
m, a = 804.02(1) pm)58,70 was used. It could be shown by XRD and NMR investigations that ∼5 mass% of the Al richer phase CaAl4 is present (CaGa4 type, space group C2/m, a = 616.91(1), b = 618.50(1) c = 634.34(1) pm, β = 118.03(1)°).70 When oxidizing this compound with air or pure O2 the structures of the main products are the ternary oxides Ca12Al14O33 (mineral mayenite, own type, space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d, a = 1198.2 pm)71 and the expected product CaAl2O4 (own type, space group P21/c, a = 870, b = 809.2, c = 1746.9 pm, β = 119.589).72 Besides these two ternaries, the binary oxides CaO (NaCl type, space group Fm
3d, a = 1198.2 pm)71 and the expected product CaAl2O4 (own type, space group P21/c, a = 870, b = 809.2, c = 1746.9 pm, β = 119.589).72 Besides these two ternaries, the binary oxides CaO (NaCl type, space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 474 pm)73 and Al2O3 (own type, space group R
m, a = 474 pm)73 and Al2O3 (own type, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, a = 486, c = 1324 pm)73 as well as two other ternary oxides Ca3Al2O6 (own type, space group Pa
c, a = 486, c = 1324 pm)73 as well as two other ternary oxides Ca3Al2O6 (own type, space group Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , a = 1526.2 pm)74 and CaAl4O7 (own type, space group C2/c, a = 1289, b = 888, c = 545 pm, β = 107.05°)75 have to be mentioned as important compounds that are observed during oxidation. A detailed discussion of the chemical structures of these can be found in the original literature cited above or in the previously published work.58Fig. 1 depicts a comparative schematic of the previously published results58 in comparison to the outcome of this work based on the crystal structures involved.
, a = 1526.2 pm)74 and CaAl4O7 (own type, space group C2/c, a = 1289, b = 888, c = 545 pm, β = 107.05°)75 have to be mentioned as important compounds that are observed during oxidation. A detailed discussion of the chemical structures of these can be found in the original literature cited above or in the previously published work.58Fig. 1 depicts a comparative schematic of the previously published results58 in comparison to the outcome of this work based on the crystal structures involved.
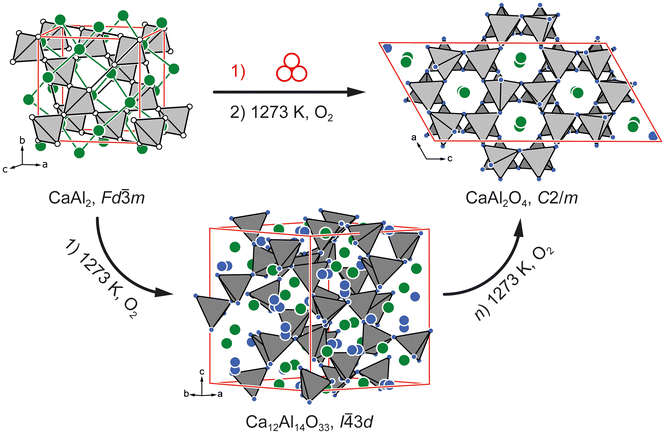 | ||
| Fig. 1 Two reaction pathways for the oxidation of cubic CaAl2 at 1273 K with elemental O2. A direct formation of monoclinic CaAl2O4 is observed when activating the material by ball milling. Without ball milling, cubic Ca12Al14O33 is formed initially as previously reported.58 | ||
Oxidation of CaAl2 using wet argon
In a first approach of changing the outcome of the oxidation reaction of CaAl2 the idea of using different oxygen sources came up. One possibility is to use water instead of oxygen in the home-built tube furnace setup. However, instead of using pure water, an argon stream was bubbled through degassed, demineralized water and fed into the furnace. The result does not show any noticeable difference to the outcome of the O2 oxidation, with mayenite (Ca12Al14O33) still being the main product. One exception might be that in contrast to small amounts of cubic Ca3Al2O6, approximately 5 mass% of the monoclinic oxide CaAl4O7 could be identified in the powder diffraction pattern (Fig. 2; Table 1).| Ca12Al14O33 | CaAl2O4 | CaAl4O7 | CaO | Al2O3 | Al | Fig. |
|---|---|---|---|---|---|---|
| 63 | 13 | 10 | 1 | 8 | 5 | S1 |
Ball milling experiments
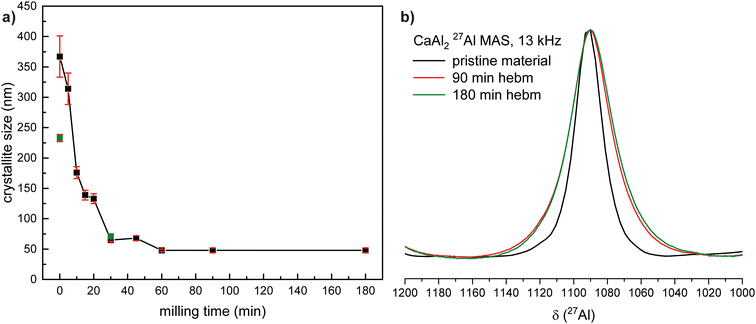
In the second approach, instead of changing the oxidizing agent, milling of the powders was employed. This can be used to reduce the average crystallite size, increase the surface area of the starting material and introduce defects and amorphous regions or even induce phase transitions.76–78 At first, intermetallic CaAl2 was treated in a vibrating ball mill for up to three hours. A significant decrease of the crystallite size during milling could be observed using powder X-ray diffraction. The broadening of the reflections is already visible in the comparison of the powder diffractograms shown in Fig. 3. From the diffraction data it can also be seen that no change in the crystal structures or the phase composition is observed. Fig. 4a illustrates the decrease of the crystallite size over time; for a second batch the crystallite sizes of the starting material and the 30 min milled material are shown. The crystallite size decreases with an almost exponential decay over the milling intervals starting from >350 nm down to roughly 50 nm after three hours (Table 2). A second batch that was milled for 30 min straight shows comparable results. The decrease in crystallite size could also be proven by 27Al solid state NMR experiments. The spectra for the species milled for 90 and 180 minutes show almost identical shifts and signal shapes; only a broadening of the central transition and spinning sidebands is visible as can be seen in Fig. 4b. However, a significant effect is visible when compared to pristine CaAl2.70,79 This clearly shows that the crystallite size is reduced alongside the formation of defects leading to broadened NMR resonances. Detailed simulations of the spectra can be found in the ESI.† To verify this even further, powder X-ray diffraction experiments with known amounts of Si as the internal standard were conducted on the 30 and 180 min ball milled samples. In this way, the amorphous phase contribution can be determined. For the 30 min milled sample the amorphous phase amounts to 5(1) mass% while at 180 min 20(1) mass% are observed. Fig. S37 and S38† show the respective diffraction data along with the Rietveld fits. The sample milled for 30 min was additionally examined by SEM/EDX investigations. No abrasion of the milling jar can be observed as no Fe can be found in the EDX results.| Milling time/min | Crystallite size/nm | Amorphous phase/mass% | Fig. |
|---|---|---|---|
| 0 | 367(34) | 0 | S2 |
| 5 | 314(26) | S3 | |
| 10 | 176(10) | S4 | |
| 15 | 139(8) | S5 | |
| 20 | 133(8) | S6 | |
| 30 | 65(2) | 5(1) | S7/S37 |
| 45 | 68(4) | S8 | |
| 60 | 48(2) | S9 | |
| 90 | 48(4) | S10 | |
| 180 | 48(4) | 20(1) | S11/S38 |
Oxidation experiments after ball milling
To study the impact of the milling on the oxidation behavior the milled samples were transferred to an STA system. Since the transfer of the samples into the system was not possible fully under argon, the measurement was started as fast as possible. For all samples except for the one milled for 180 min, no obvious oxidation during the transfer was observed. However, for the latter, a pyrophoric behavior was observed. Powder X-ray diffraction did not show a decomposition of the starting material after 180 min of ball milling (Fig. S11†). However, since a significant amorphous contribution was deduced (vide supra), it is likely that the mechanochemical treatment produced a material with an increased surface area and introduced defects near/at the surface. As known for nanoparticles of ignoble metals, these are highly pyrophoric.76–78,80–83 A similar situation could be assumed here.Fig. 5 shows a comparison of the obtained STA data for the starting material and the milled materials after 90 and 180 minutes. For pristine CaAl2, a highly exothermic reaction (Fig. 5a, red trace) at 1130 K can be observed, raising the temperature inside the STA system significantly (Fig. 5a, green trace). Furthermore, a multi-step reaction seems to take place as multiple effects are visible in the heat flux curve. In addition, a significant mass increase can be observed (Fig. 5a, black trace). The powder X-ray diffraction pattern also significantly changes. After oxidation, (Fig. 5b), several oxides can be observed, in line with what was reported previously.58 When oxidizing the material that was ball milled for 5 min in the STA system (Fig. S14†), an increase of CaAl2O4 can be observed right away based on powder X-ray diffraction. Fig. 5c depicts the STA results of CaAl2 ball milled for 90 min. In the heat flux (red trace) it becomes evident that the heat signature becomes smeared and less intense (note that the scale bar for the heat flux is the same in all STA graphs). The overall area remains almost the same, indicating an earlier onset and an overall less pronounced reaction onset. The mass increase is a little steeper than before; however, the overall mass gain (+53 mass%) is the same for pristine and 90 min ball milled CaAl2. The corresponding powder diffraction pattern is shown in Fig. 5d, indicating a different phase composition compared to the oxidized pristine material (Fig. 5b). Fig. 5e finally shows the STA results from the pyrophoric CaAl2 that was ball milled for 180 min and Fig. 5f shows the corresponding powder X-ray diffraction pattern. When the sample was transferred to the STA crucible, an initial reaction was observed that stopped by itself after some seconds. It is assumed that this initial oxidation only happens on the surface and does not proceed throughout the sample, similar to what was shown for e.g. AlNi.84 This assumption is strengthened by the STA results. There is still a mass increase of +42 mass%, which is, however, smaller than the one for the pristine and 90 min milled material. Finally, the amorphous phase contributions of the 30 and 180 min milled oxidized materials were determined. In both cases, the amorphous phase contribution amounts to <1 mass% (Fig. S39 and S40†).
Overall, it can be seen that with prolonged milling times, the amount of Ca12Al14O33 steadily decreases while at the same time the amount of CaAl2O4 increases (Table 3). Fig. 6 depicts the evolution of the ratio between the two interesting main oxides; a plot of all phases present is shown in Fig. S36† and is given in Table 3. This observation underlines our previous assumption: Ca diffuses to the surface of the particle, enabling the formation of Ca12Al14O33 and leaving elemental Al behind.58 When ball milling CaAl2, the crystallite size gets significantly reduced, shortening the Ca diffusion path length, hampering the formation of the mayenite type structure and enabling the direct formation of CaAl2O4. This is furthermore in line with our previous quantum-chemical calculations,58 rendering both competing oxides similar in formation energy with CaAl2O4 being the thermodynamically slightly more stable compound.
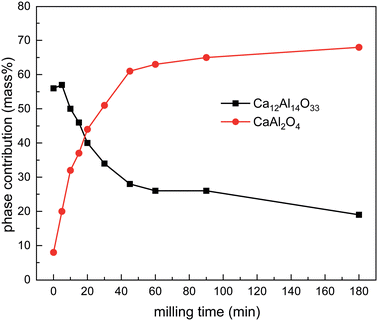 | ||
| Fig. 6 Results of the Rietveld refinements of the oxidation reactions after different milling times (selected phases only). For all observed phases see Fig. S36.† | ||
| Milling time (min) | Phase contributions obtained by PXRD (mass%) | Fig. | ||||||
|---|---|---|---|---|---|---|---|---|
| Ca12Al14O33 | CaAl2O4 | Ca3Al2O6 | CaAl4O7 | CaO | Al2O3 | Al | ||
| 0 | 56 | 8 | 10 | 0 | 3 | 13 | 10 | S13 |
| 5 | 57 | 20 | 4 | 0 | 0 | 11 | 8 | S14 |
| 10 | 50 | 32 | 0 | 7 | 0 | 8 | 3 | S15 |
| 15 | 46 | 37 | 0 | 7 | 0 | 7 | 3 | S16 |
| 20 | 40 | 44 | 0 | 8 | 0 | 6 | 2 | S17 |
| 30 | 34 | 50 | 0 | 7 | 0 | 7 | 2 | S18 |
| 45 | 28 | 61 | 0 | 6 | 0 | 4 | 1 | S19 |
| 60 | 26 | 63 | 0 | 8 | 0 | 3 | 0 | S20 |
| 90 | 26 | 65 | 0 | 7 | 0 | 2 | 0 | S21 |
| 180 | 19 | 69 | 0 | 12 | 0 | 0 | 0 | S22 |
Finally, we want to address the crystallite sizes determined for the oxidized material. As shown in Fig. 4a, the crystallite size of the ball milled CaAl2 steadily decreases with increasing milling time. When analyzing the crystallite sizes of the oxidized material from the STA investigations, the sample after 5 min of ball milling shows 36(3) nm for CaAl2O4 and 84(2) nm for Ca12Al14O33. The crystallite sizes of CaAl2O4 roughly stay the same regardless of the applied milling time; for Ca12Al14O33 an increase to ∼120 nm for 10–30 min is visible followed by a decrease to ∼50 nm for the 60 and 90 min milled samples. However, there is no clear trend visible in these data (Table S1†).
4. Conclusion
The intermetallic Laves phase CaAl2 was oxidized in two ways: using water (in Ar) and pure O2 after ball milling. While in the first case no difference between elemental oxygen and the un-ball milled material can be observed, the ball milled CaAl2 is oxidized in a significantly different way. This can be attributed to a reduced crystallite size of CaAl2 which is ∼50 nm after 180 min of ball milling. Interestingly, 27Al solid-state MAS NMR investigations on the milled material clearly showed that alongside the drastically reduced crystallite size also strain is observed in the material as the NMR signals broaden. STA investigations finally confirmed that ball milling has an influence on the oxidation behavior. While the pristine un-milled sample shows an intense sharp exothermic reaction, the milled samples show a significantly broadened effect while the area of the signal is approximately the same as for pristine CaAl2. Up to 90 minutes of milling all samples exhibit a similar mass gain of ∼150 mass%. When CaAl2 is milled for 180 min, the sample becomes pyrophoric; however, the reaction is not self-sustaining. This leads to the assumption that only the surface is oxidized as an additional mass gain is still observed in the STA experiments. The main product of the oxidation reaction of pristine CaAl2 is Ca12Al14O33 which crystallizes in the so called mayenite type structure. For the milled samples, the respective amount of Ca12Al14O33 drops in almost an exponential fashion while instead the stochiometric oxidation product CaAl2O4 is formed in higher amounts. The presented results clearly show that already short ball milling times can significantly influence the reaction pathway and therefore drastically shift the product scope.Author contributions
Conceptualization by GK and OJ; synthetic work by ECJG; initial draft of the manuscript by ECJG. ECJG performed the XRD, STA and 27Al NMR investigations; the entire work was supervised, guided, and revised by GK and OJ. The manuscript was corrected by all authors and finalized by GK and OJ.Data availability
Details on the Rietveld refinements, crystallite sizes, STA curves, and 27Al NMR spectroscopic data can be found in the ESI.†The data supporting this article have been included as part of the ESI.†
Conflicts of interest
The authors declare no competing interests.Acknowledgements
We thank Stefan Engel for the SEM/EDX investigations. Special thanks to Sylvia Beetz from the MWWT/Inorganic Chemistry workshop of Saarland University and to Britta Schreiber from the central glassblowing workshop for supplying parts and installing the furnace for the oxidation experiments. Instrumentation and technical assistance for this work were provided by the Service Center X-ray Diffraction, with financial support from Saarland University and German Science Foundation (project number INST 256/349-1) and by the Service Center NMR with financial support from Saarland University and German Research Foundation DFG (INST 256/384-1).References
- F. Ostermann, Anwendungstechnologie Aluminium, Springer Vieweg, Berlin, Heidelberg, Germany, 2014 Search PubMed.
- C. Kammer, Aluminium-Taschenbuch 1: Grundlagen und Werkstoffe, Aluminium-Zentrale, Düsseldorf, Germany, 1995 Search PubMed.
- C. Jaschik, H. Haferkamp and M. Niemeyer, in Magnesium Alloys and their Applications, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2000, pp. 41–46 Search PubMed.
- M. K. Kulekci, Int. J. Adv. Des. Manuf. Technol., 2008, 39, 851–865 CrossRef.
- O. Janka, in Applied Inorganic Chemistry, ed. R. Pöttgen, T. Jüstel and C. A. Strassert, De Gruyter, Berlin, Germany, 2023, pp. 158–173 Search PubMed.
- Empire State Realty Trust, Empire State Building Fact Sheet, https://www.esbnyc.com/sites/default/files/esb_fact_sheet_4_9_14_14.pdf.
- FMGB Guggenheim Bilbao Museoa, https://www.guggenheim-bilbao.eus/de/, (accessed 28.06.2023).
- C. Veiga, J. P. Davim and A. J. R. Loureiro, Rev. Adv. Mater. Sci., 2012, 32, 14–34 Search PubMed.
- M. V. Ribeiro, M. R. V. Moreira and J. R. Ferreira, J. Mater. Process. Technol., 2003, 143–144, 458–463 CrossRef CAS.
- M. Peters, J. Hemptenmacher, J. Kumpfert and C. Leyens, in Titanium and Titanium Alloys, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2003, pp. 1–36 Search PubMed.
- J. Barksdale, in The encyclopedia of the chemical elements, ed. C. A. Hampel, Reinhold Book Corp., New York, USA, 1968, pp. 732–738 Search PubMed.
- P. Enghag, in Encyclopedia of the Elements: Technical Data – History – Processing – Applications, ed. P. Enghag, Wiley VCH Verlag GmbH, Weinheim, Germany, 2004, pp. 493–509 Search PubMed.
- M. Peters and C. Leyens, Titan und Titanlegierungen, Wiley-VCH, Weinheim, Germany, 2002 Search PubMed.
- S. Engel and O. Janka, in Applied Inorganic Chemistry, ed. R. Pöttgen, T. Jüstel and C. A. Strassert, De Gruyter, 2023, pp. 255–264 Search PubMed.
- S. Engel and O. Janka, in Applied Inorganic Chemistry, ed. R. Pöttgen, T. Jüstel and C. A. Strassert, De Gruyter, 2023, pp. 265–270 Search PubMed.
- E. Gillam, H. P. Rooksby and L. D. Brownlee, Acta Crystallogr., 1964, 17, 762–763 CrossRef CAS.
- O. Janka and R. Pöttgen, Z. Naturforsch., B: Chem. Sci., 2020, 75, 421–439 CrossRef CAS.
- M. Buchner and O. Janka, in Applied Inorganic Chemistry, ed. R. Pöttgen, T. Jüstel and C. A. Strassert, De Gruyter, 2023, pp. 221–228 Search PubMed.
- J. R. Davis, ASM Specialty Handbook: Aluminum and Aluminum Alloys, ASM International, Materials Park, OH, USA, 1993 Search PubMed.
- D. Altenpohl, Aluminium und Aluminiumlegierungen, Springer, Berlin, Heidelberg, Germany, 1965 Search PubMed.
- P. K. Datta, H. L. Du, J. S. Burnell-Gray and R. E. Ricker, in Corrosion: Materials, ASM International, Materials Park, OH, USA, 2005, vol. 13B Search PubMed.
- R. K. Gupta and B. Pant, in Intermetallic Matrix Composites, ed. R. Mitra, Woodhead Publishing, Sawston, Cambridge, England, 2018, pp. 71–93 Search PubMed.
- F. Appel, U. Brossmann, U. Christoph, S. Eggert, P. Janschek, U. Lorenz, J. Müllauer, M. Oehring and J. D. H. Paul, Adv. Eng. Mater., 2000, 2, 699–720 CrossRef.
- C. Kenel, A. Lis, K. Dawson, M. Stiefel, C. Pecnik, J. Barras, A. Colella, C. Hauser, G. J. Tatlock, C. Leinenbach and K. Wegener, Intermetallics, 2017, 91, 169–180 CrossRef CAS.
- K. Meng, K. Guo, Q. Yu, D. Miao, C. Yao, Q. Wang and T. Wang, Corros. Sci., 2021, 183, 109320 CrossRef CAS.
- P. A. Loginov, G. M. Markov, N. V. Shvyndina, G. V. Smirnov and E. A. Levashov, Ceramics, 2022, 5, 389–403 CrossRef CAS.
- S. A. Kekare and P. B. Aswath, J. Mater. Sci., 1997, 32, 2485–2499 CrossRef CAS.
- Z. Li, W. Gao, Y. He and S. Li, High Temp. Mater. Processes, 2002, 21, 35–46 CAS.
- R. Pflumm, S. Friedle and M. Schütze, Intermetallics, 2015, 56, 1–14 CrossRef CAS.
- A. Rahmel, M. Schütze and W. J. Quadakkers, Mater. Corros., 1995, 46, 271–285 CrossRef CAS.
- G. Geramifard, C. Gombola, P. Franke and H. J. Seifert, Corros. Sci., 2020, 177, 108956 CrossRef CAS.
- H. J. Grabke, M. W. Brumm and B. Wagemann, Mater. Corros., 1996, 47, 675–677 CrossRef CAS.
- H. Qin, X. Chen, L. Li, P. W. Sutter and G. Zhou, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, E103–E109 CAS.
- Y.-O. Yoon, S.-H. Ha, G.-Y. Yeom, H. K. Lim and S. K. Kim, in Light Metals 2013, Springer, Cham, Germany, 2013, pp. 323–326 Search PubMed.
- P. Villars and K. Cenzual, Pearson's Crystal Data: Crystal Structure Database for Inorganic Compounds, ASM International®, Materials Park, Ohio, USA, 2023 Search PubMed.
- T. B. Massalski, H. Okamoto, P. R. Subramanian and L. Kacprzak, Binary alloy phase diagrams, ASM International, Ohio, U.S.A., 2nd edn, 1990 Search PubMed.
- R. Hoppe and H.-J. Röhrborn, Naturwissenschaften, 1961, 48, 453–454 CrossRef CAS.
- R. Hoppe and H.-J. Röhrborn, Z. Anorg. Allg. Chem., 1964, 327, 199–206 CrossRef CAS.
- R. Hoppe, J. Solid State Chem., 1986, 65, 127–144 CrossRef CAS.
- H. D. Wasel-Nielen and R. Hoppe, Z. Anorg. Allg. Chem., 1968, 359, 36–40 CrossRef CAS.
- P. J. Yvon, R. B. Schwarz, C. B. Pierce, L. Bernardez, A. Conners and R. Meisenheimer, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 39, 6690–6693 CrossRef CAS PubMed.
- W. Schauerte, H. U. Schuster, N. Knauf and R. Müller, Z. Anorg. Allg. Chem., 1992, 616, 186–190 CrossRef CAS.
- B. Cogel and H. U. Schuster, Z. Anorg. Allg. Chem., 1993, 619, 1765–1770 CrossRef CAS.
- H. Schuster and J. Wittrock, J. Therm. Anal. Calorim., 1993, 39, 1397–1401 CAS.
- A. Panahandeh and W. Jung, Z. Anorg. Allg. Chem., 2003, 629, 1651–1660 CrossRef CAS.
- P. Moser, W. Jung and H. U. Schuster, Z. Anorg. Allg. Chem., 1997, 623, 1781–1785 CrossRef CAS.
- P. Moser and W. Jung, Z. Anorg. Allg. Chem., 1998, 624, 1251–1255 CrossRef CAS.
- P. Moser, H. M. Schwunck and W. Jung, Z. Anorg. Allg. Chem., 1998, 624, 1256–1261 CrossRef CAS.
- H. M. Schwunck, P. Moser and W. Jung, Z. Anorg. Allg. Chem., 1998, 624, 1262–1266 CrossRef CAS.
- H. M. Schwunck, P. Moser and W. Jung, Z. Anorg. Allg. Chem., 1999, 625, 407–410 CrossRef CAS.
- H. M. Schwunck, P. Moser and W. Jung, Z. Anorg. Allg. Chem., 1999, 625, 463–466 CrossRef CAS.
- P. Moser, V. Cirpus and W. Jung, Z. Anorg. Allg. Chem., 1999, 625, 714–718 CrossRef CAS.
- P. Moser and W. Jung, Z. Anorg. Allg. Chem., 2000, 626, 1421–1425 CrossRef CAS.
- B. Eisenmann, M. Jakowski, W. Klee and H. Schäfer, Rev. Chim. Miner., 1983, 20, 255–263 CAS.
- M. Tampier, D. Johrendt, R. Pöttgen, G. Kotzyba, H. Trill and B. D. Mosel, Z. Anorg. Allg. Chem., 2002, 628, 1243–1245 CrossRef CAS.
- E. C. J. Gießelmann, S. Engel, S. Pohl, M. Briesenick, L. P. Rüthing, C. Kloos, A. Koldemir, L. Schumacher, J. Schmedt auf der Günne, G. Kickelbick and O. Janka, Chem. Mater., 2024 DOI:10.1021/acs.chemmater.4c02093.
- J. Sappl, F. Jung and C. Hoch, Chem. Mater., 2020, 32, 866–873 CrossRef CAS.
- E. C. J. Gießelmann, S. Engel, J. G. Volpini, H. Huppertz, G. Kickelbick and O. Janka, Inorg. Chem. Front., 2024, 11, 286–297 RSC.
- A. Betke and G. Kickelbick, New J. Chem., 2014, 38, 1264–1270 RSC.
- A. Fischer, C. Ney and G. Kickelbick, Eur. J. Inorg. Chem., 2013, 2013, 5701–5707 CrossRef CAS.
- A. Betke and G. Kickelbick, Inorganics, 2014, 2, 410–423 CrossRef CAS.
- D. Becker, M. Klos and G. Kickelbick, Inorg. Chem., 2019, 58, 15021–15024 CrossRef CAS PubMed.
- D. Becker, R. Haberkorn and G. Kickelbick, Eur. J. Inorg. Chem., 2019, 2019, 4835–4845 CrossRef CAS.
- R. Pöttgen, T. Gulden and A. Simon, GIT Labor-Fachz., 1999, 43, 133–136 Search PubMed.
- H. M. Rietveld, Acta Crystallogr., 1967, 22, 151–152 CrossRef CAS.
- H. M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65–71 CrossRef CAS.
- Bruker AXS Inc., Topas, Version 5, Karlsruhe, Germany, 2014 Search PubMed.
- Bruker Corp., Topspin, Karlsruhe, 2008 Search PubMed.
- D. Massiot, F. Fayon, M. Capron, I. King, S. Le Calvé, B. Alonso, J.-O. Durand, B. Bujoli, Z. Gan and G. Hoatson, Magn. Reson. Chem., 2002, 40, 70–76 CrossRef CAS.
- S. Engel, E. C. J. Gießelmann, L. E. Schank, G. Heymann, K. Brix, R. Kautenburger, H. P. Beck and O. Janka, Inorg. Chem., 2023, 62, 4260–4271 CrossRef CAS.
- W. Büssem and A. Eitel, Z. Kristallogr., 1936, 95, 175–188 Search PubMed.
- W. Hörkner and H. Müller-Buschbaum, J. Inorg. Nucl. Chem., 1976, 38, 983–984 CrossRef.
- W. P. Davey and E. O. Hoffman, Phys. Rev., 1920, 15, 333 CAS.
- H. E. Swanson, N. T. Gilfrich and G. M. Ugrinic, Natl. Bur. Stand. Circ., 1955, 5, 10–13 Search PubMed.
- E. Boyko and L. G. Wisnyl, Acta Crystallogr., 1958, 11, 444–445 CrossRef.
- H. Yang and H. Bakker, Mater. Sci. Eng., A, 1994, 181–182, 1207–1211 CrossRef.
- P. Baláž, M. Achimovičová, M. Baláž, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC.
- D. Tan and F. García, Chem. Soc. Rev., 2019, 48, 2274–2292 RSC.
- C. Benndorf, H. Eckert and O. Janka, Acc. Chem. Res., 2017, 50, 1459–1467 CrossRef CAS.
- A. Egeberg, T. Block, O. Janka, O. Wenzel, D. Gerthsen, R. Pöttgen and C. Feldmann, Small, 2019, 15, 1970200 CrossRef.
- T. Klein, C. Pauly, F. Mücklich and G. Kickelbick, Intermetallics, 2020, 124, 106851 CrossRef CAS.
- T. Klein and G. Kickelbick, Nanotechnology, 2020, 31, 265605 CrossRef CAS PubMed.
- S. Riegsinger, R. Popescu, D. Gerthsen and C. Feldmann, Chem. Commun., 2022, 58, 7499–7502 RSC.
- G. Kresse, M. Schmid, E. Napetschnig, M. Shishkin, L. Köhler and P. Varga, Science, 2005, 308, 1440–1442 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4dt02459a |
| This journal is © The Royal Society of Chemistry 2025 |