Tailoring the dynamic nanocomposite hydrogels through surface-functionalized nanomaterials and interfacial crosslinking chemistry toward multifunctional biomedical and engineering applications
Yu-Chia
Su
,
Grace
Chen
 ,
Yi-Jhen
Lai
,
Guo-Zen
Song
,
Tai-Lin
Wu
and
Yi-Cheun
Yeh
,
Yi-Jhen
Lai
,
Guo-Zen
Song
,
Tai-Lin
Wu
and
Yi-Cheun
Yeh
 *
*
Institute of Polymer Science and Engineering, National Taiwan University, Taipei 10617, Taiwan. E-mail: yicheun@ntu.edu.tw
First published on 19th November 2025
Abstract
Dynamic nanocomposite hydrogels (DNCHs) represent a cutting-edge class of materials characterized by their tunable architecture and stimuli-responsive behavior, making them particularly well-suited for applications that require mimicking the adaptive functionality of biological systems. A wide range of chemical strategies and design methodologies have been explored to engineer their structure–property–function relationships. In this review, we present a comprehensive analysis of recent developments in DNCHs, systematically organized into six material-centric categories, including metal-, metal oxide-, carbon-, ceramic-, polymer-, and metal–organic framework (MOF)-based nanomaterials. We examine surface functionalization techniques and interfacial crosslinking mechanisms that underpin DNCH fabrication, supported by representative examples that highlight their composition, interfacial chemistry, and functional performance. We also critically evaluate current challenges and highlight key research opportunities to inform and inspire future interdisciplinary efforts. Taken together, this review presents a cohesive and forward-looking framework to support the rational design, functional implementation, and collaborative advancement of next-generation DNCHs.
1. Introduction
Hydrogels, as multifunctional soft matter, have rapidly become indispensable platforms in modern chemistry, materials science, and biomedicine. Their unique combination of high water content, biomimetic softness, and structural tunability enables them to function not only as tissue-mimicking scaffolds1,2 but also as versatile matrices for sensing,3,4 and drug delivery.5,6 For biomedical applications, they serve as injectable drug carriers,7,8 3D scaffolds for tissue regeneration,9,10 and protective dressings with antibacterial11,12 or antioxidant activity.13 In soft electronics, hydrogels act as ionic conductors,14,15 and stretchable sensors16,17 due to their transparency, ionic mobility, and mechanical compliance. In environmental engineering, hydrogel networks are used for pollutant adsorption.18,19 This wide range of functionality stems from the ability of hydrogels to integrate multiple responsibilities. Building upon these broad capabilities, recent advances in hydrogel-based delivery systems further highlight their versatility. By tailoring natural and synthetic matrices, researchers have achieved sustained release,20,21 targeted transport,22 and microenvironment-responsive behavior.23,24 Notably, gelatin-, collagen-, and zein-based hydrogels exemplify this adaptability, serving as biocompatible carriers that readily incorporate nanoparticulate components to enhance therapeutic efficacy.To further justify the selection of hydrogels as the core research platform, their unique thermodynamic adaptability and biocompatibility should be highlighted. Thermoresponsive hydrogels, particularly poly(N-isopropylacrylamide) (PNIPAM)-, gelatin-, and polysaccharide-based systems, exhibit reversible phase transitions and tunable permeability under physiological conditions.25 Incorporating sustainable solvents or biomolecular modulators further adjusts their LCST, mechanical stability, and self-assembly behavior, extending their use in green and bio-interactive materials.26,27 Moreover, recent thermodynamic analyses reveal entropy-driven swelling and osmotic regulation that grant these hydrogels exceptional resilience and responsiveness.28 Such characteristics make hydrogels an ideal framework for constructing dynamic nanocomposite systems with tunable physicochemical and biological functions.
Recent studies have shown that protein- and polysaccharide-based hydrogels, such as gelatin, collagen, and chitosan, can incorporate nanoscale fillers to improve mechanical strength and bioactivity.29 GelMA-based hybrids further exhibit excellent biocompatibility, osteogenic potential, and 3D-printability for tissue regeneration.30 Such integration bridges biopolymer matrices with nanotechnology, paving the way for nanocomposite and dynamic hydrogel systems. Moreover, hybrid designs combining carbon-based nanomaterials with chitosan matrices enable dual pH- and thermo-responsive release, broadening the functional versatility of polymeric hydrogels.31 These multifunctional hydrogels, integrating nanoscale design and dynamic architectures, enhance therapeutic efficacy and biocompatibility in advanced biomedical applications.32
The evolution of hydrogels from simple water-swollen polymeric networks to sophisticated multifunctional platforms has been significantly accelerated by the integration of nanomaterials into their three-dimensional network. Conventional polymeric hydrogels are characterized by their biomimetic softness, high water content, and easy chemical tunability, which makes them attractive for applications such as tissue engineering, wound healing, and controlled release. Nevertheless, their inherent limitations (e.g., low mechanical strength, limited functionality, and poor stimulability) hinder their wider applicability in demanding biomedical and engineering contexts. To address these shortcomings, nanocomposite (NC) hydrogels have emerged as versatile platforms that go beyond the traditional role of hydrogels. By uniformly embedding a variety of nanomaterials, this hybrid structure not only improves mechanical robustness but also confers tailored physicochemical properties while maintaining the hydrated and porous environment characteristic of traditional hydrogels.
The early-stage development of NC hydrogels focused on modulating the structures and properties by physically incorporating nanomaterials into the network. For example, graphene sheets dispersed in polyacrylamide (PAAm) hydrogels increased the elastic modulus by over 200% and the compressive strength by 2000%, due to uniform dispersion and hydrogen bonding between graphene and the polymeric network.33 Silica nanoparticles (NPs) not only increased the strength of the collagen/alginate hydrogel networks34 but also contributed to the alginate hydrogels with enhanced hydroxyapatite formation, cell proliferation, and osteoinductive bioactivity.35 Notably, some nanomaterials possess unique properties, such as iron oxide particles (IOPs) has magnetic capabilities,36,37 while gold and silver NPs provide plasmonic heat and intrinsic antimicrobial properties.38 While these systems emphasize the potential of NPs as reinforcing fillers and multifunctional additives, they lack the dynamic bonding between the polymeric network and the nanomaterials, which in turn limits their dynamic adaptability.
Dynamic NC hydrogels (DNCHs) utilize reversible chemical processes within the polymer backbone and/or at the nanomaterial–polymer interface to provide hydrogels with the ability to self-heal, undergo stress relaxation, and undergo stimulus-driven property changes. In the DNCH network, nanomaterials serve as interfacial crosslinkers, creating bonds or interactions between the polymer chains and the surface of the embedded nanomaterials. This enhances adhesion and stress transfer at the crucial boundary, improving the overall mechanical strength of the nanocomposite hydrogels. On the contrary, crosslinkers are generally defined as molecules that form bonds between the polymer chains themselves throughout the bulk material to construct the three-dimensional polymer network and determining fundamental properties. Therefore, the true power of DNCHs lies in the seamless integration of nanomaterial properties with reversible network behavior. For example, when gold nanomaterials were embedded in the network, near-infrared (NIR) irradiation resulted in localized heating that broke dynamic bonds to trigger drug release or shape modification on demand.39–41 For example, Chen et al. reported a NIR-II responsive nanocomposite hydrogel by incorporating gold nanoparticle-decorated graphene oxide (GOAu) into a thermoresponsive polymeric network made from polydextran aldehyde (PDA) and gelatin (Gel).39 Yang et al. reported that a dual-crosslinked hydrogel with gold nanorods (AuNRs), D-hydrogel@AuNR, which was constructed via ultraviolet light-initiated radical copolymerization of acrylamide (AM), isopropylacrylamide (NIPAAM), and dopamine methacrylamide (DMA) in a complex solution of AuNRs and cysteamine-grafted oxidized sodium alginate (OSA-SH).41
Building on these advances, DNCHs represent a qualitative leap in functionality: while traditional hydrogels mainly depend on permanent covalent or physical crosslinks, DNCHs utilize reversible, dynamic bonding at the polymer–nanomaterial interface. This distinction confers DNCHs unique properties, including autonomous self-healing, reversible stress relaxation, and multi–stimuli responsiveness. In practice, DNCHs can recover from mechanical damage, reconfigure their microstructure under external fields, and perform “on–off” functions such as light-triggered drug release or electrochemical sensing, which capabilities unattainable in conventional hydrogel systems.
Beyond conventional hydrogels, recent studies have highlighted DNCHs that integrate gelatin, collagen, or plant proteins (e.g., zein), providing hierarchical architectures with tunable viscoelasticity, self-healing ability, and on-demand responsiveness. These advanced DNCH systems have been used in nanotechnology-driven biomedical contexts, including drug delivery, regenerative scaffolds, and biosensing. Together, these developments of DNCHs as a rapidly expanding field that synergizes natural polymers with functional nanomaterials for healthcare and engineering applications.
Dynamic interactions between nanomaterials and polymeric networks can further be optimized through tailored surface functionalization of nanomaterials, introducing responsive crosslinking sites at the nanomaterial–polymer interface. Such surface engineering strategies significantly enhance nanomaterial dispersion stability, reinforce mechanical properties, and impart sophisticated stimulus-responsive functionalities to hydrogels. For instance, the surface of lanthanide-doped upconversion nanoparticles (UCNPs) can be engineered to establish dynamic hydrogen-bonding interactions within a hydrogel network, enabling the efficient conversion of deep-tissue-penetrating near-infrared (NIR) irradiation into visible luminescence for real-time imaging and concurrent photodynamic therapy.42 Similarly, graphene oxide can be functionalized with dynamic linker molecules that form reversible hydrogen bonds and π–π stacking interactions with polymers, enabling the rapid restoration of conductive percolation pathways after mechanical deformation.43 This functionality imparts hydrogels with self-healing electrical conductivity, making them well-suited for wearable strain and pressure sensing applications. Overall, these examples highlight the critical role of nanoparticle surface chemistry and dynamic interfacial crosslinking in dictating the multifunctional performance of DNCHs.
Recent research has demonstrated the significant potential of NC hydrogels in overcoming key challenges across various fields, and several excellent reviews have extensively examined these NC hydrogels,44–46 and even focusing on discussing their dynamic features.47 Nonetheless, existing reviews lack a systematic classification of DNCHs based on the type of incorporated nanomaterials, a gap hinders a comprehensive understanding of the structure–property relationships that underpin their functional performance. The performance of DNCHs depends on the dynamic bonds as well as on the nanomaterial class. Material identity dictates the interfacial anchors and dynamic bonds that are chemically accessible (e.g., Au–S/imine for Au; catechol coordination/silanes for oxides; π–π, H-bonding, or imine linkages for carbon materials; imine/boronate chemistry for selected MOFs), as well as the stimulus-responsiveness pathways within the hydrogel (e.g., plasmonic photothermal in Au/Ag; magnetic in Fe3O4; photocatalytic/UV shielding in TiO2/ZnO). It further defines class-specific constraints of stability, dispersion, mechanics, and long-term fate, which in turn shape characteristic application archetypes. A material-centric framework reveals design rules that span from surface anchors to dynamic bonds, stimuli, and ultimately, mechanics and safety.
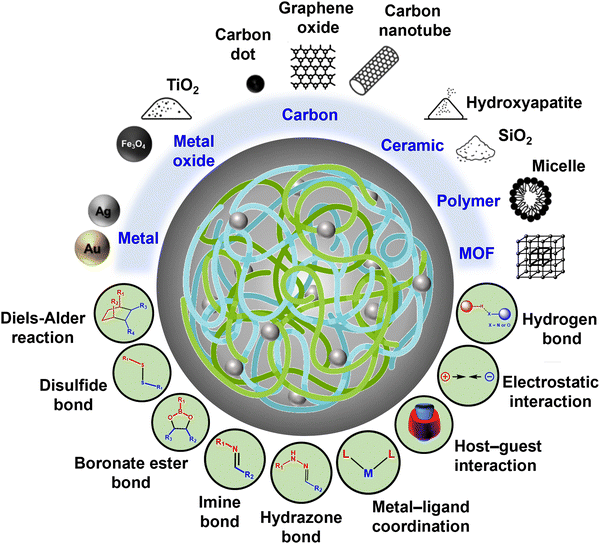
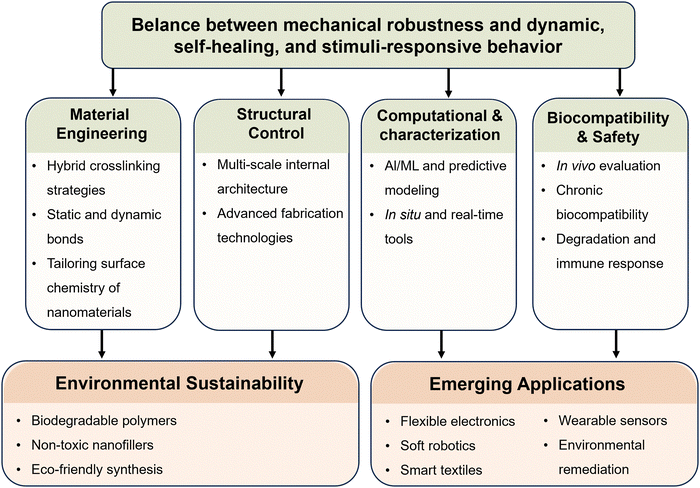
To address this limitation, this review provides a comprehensive overview of surface-functionalized nanomaterials and the interfacial crosslinking mechanisms employed in the fabrication of DNCHs. It further categorizes recent advancements in DNCH development into six material-based groups (i.e., metal-, metal oxide-, carbon-, ceramic-, polymer-, and MOF-based nanomaterials) and also highlights the commonly employed dynamic crosslinking interactions at the polymer–nanomaterial interface (e.g., disulfide bonds, boronate ester bonds, imine bonds, and host–guest interactions) (Fig. 1). The integration of these nanomaterials with dynamic interfacial crosslinks enables the design and fabrication of versatile DNCHs with tunable structures, properties, and functionalities. The dynamic features of DNCHs (e.g., stimuli responsiveness, self-healing behavior, and stress relaxation) are also shown in Fig. 2. A summary of recent DNCHs, highlighting their compositions, interfacial chemistry, and applications, is provided in Table 1, along with representative examples discussed in the subsequent sections.
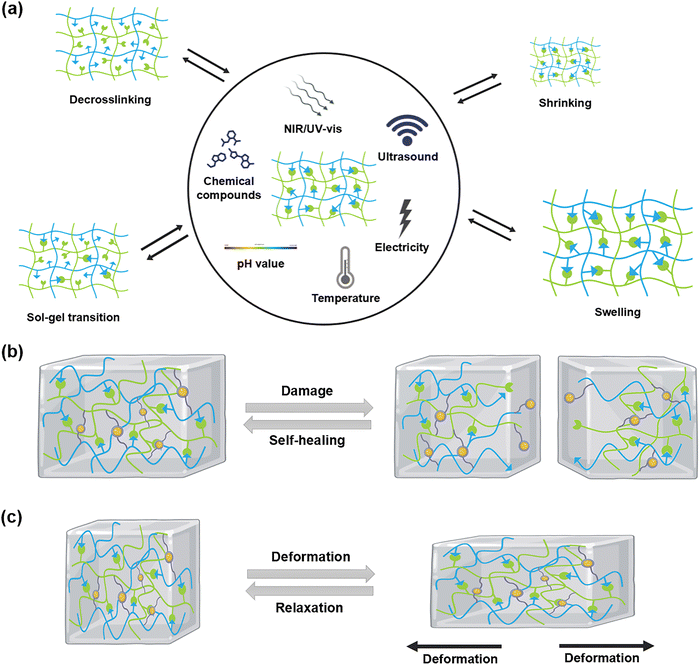 | ||
| Fig. 2 Dynamic features of DNCHs. (a) Stimuli responsiveness, (b) self-healing behavior, and (c) stress relaxation of the DNCHs during dynamic restructuring following stimulus-responsive changes. | ||
| Nanomaterial type | Nanomaterials | Functional groups on nanomaterials | Polymers | Functional groups on polymers | Dynamic crosslinking chemistry | Maximum mechanical strength | Applications | Ref. |
|---|---|---|---|---|---|---|---|---|
| Metal | Au nanoparticles (NPs) | –SH, –COOH | Poly(acrylamide) (PAAm) | –CONH2 | Au–S bond | 6.4 kPa | Skin motion sensing | 50 |
| Metal | Au NPs | –SH, –COOH | Poly(N-isopropylacrylamide) (PNIPAM) | –CONH2 | Au–S bond | 1.0 kPa | Drug release | 51 |
| Metal | Au NPs | –COOH, –NH2 | PAAm | –CONH2 | Au–N bond | 250 kPa | Drug delivery | 52 |
| Metal | Au NPs | –NH2 | Oxidized dextran (ODex), glycol chitosan (gC) | –CHO | –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (imine bond) N– (imine bond) |
0.1 kPa | Diabetic wound healing | 53 |
| Metal | Au NPs | Oxidized tannic acid (OTA) | O-Carboxymethyl chitosan (CMC) | –NH2 | –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (imine bond) N– (imine bond) |
0.2 kPa | Injectable brain implant for treating Parkinson's disease | 54 |
| Metal | Ag NPs | –OCH3, –COOH, –OH | Cellulose nanofiber (CNF), polyvinyl alcohol (PVA) | –COOH, –OH | Hydrogen bond, boronic ester bond, coordination bond | 1230 kPa | Wound healing, Drug release | 55 |
| Metal | Ag NPs | –OH, –NH2 | Polydopamine, PAAm | –CONH2, catechol | Hydrogen bond, thiol–quinone reaction | 263.1 kPa | Wearable sensor | 56 |
| Metal | Ag NPs | –OH | PVA, microfibrillated cellulose (MFC) | –OH | Hydrogen bond | 23.9 kPa | Antibacterial | 57 |
| Metal | Ag NPs | –OH | Bacterial cellulose (BC) | –OH | Hydrogen bond | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kPa 000 kPa |
Temporary cranioplasty with antibacterial and guided bone generation properties | 58 |
| Metal | Ag NPs | Maleimide groups | Gelatin, chondroitin sulfate | Furan groups | Diels–Alder cycloaddition | 19.9 kPa | Drug release | 59 |
| Metal | Pd NPs | –NH2, –OH | PAAm, hyaluronic acid (HA) | –CONH2, –COOH | Hydrogen bond, coordination bond | 7.4 kPa | Disc regeneration | 60 |
| Metal | Pt@PEG NPs | –OH | Methacrylate-anhydride gelatin (GelMA), ODex | –CHO | Imine bond | 25 kPa | Antimicrobial and diabetic wound healing | 61 |
| Metal | CuS NPs | –CONH2 | ODex, polyethylene glycol (PEG) | –CHO, –NH2 | Imine bond | 1.7 kPa | Antibacterial, anti-cancer, and wound healing | 62 |
| Metal | CuS NPs | –NH2 | PEG, Polycaprolactone (PCL) | –OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
Hydrogen bond | 1.0 kPa | Bone regeneration | 63 |
| Metal oxide | Iron oxide nanoparticles (IOPs) | Phosphonic acid groups | Difuran-functionalized poly(ethylene oxide) | Furan groups | Diels–Alder cycloaddition | 1.0 kPa | 64 | |
| Metal oxide | IOPs | –COOH | Linear monofunctionalized polyethylene glycol carboxylic acid (mPEG-COOH), 4-arm catechol-terminated polyethylene glycol (4cPEG) | Catechol groups | Metal–catechol coordination bond | 10 kPa | 65 | |
| Metal oxide | IOPs | –NH2, phenyl boronic groups | Dopamine-modified 4arm-PEG | Catechol groups | Boronate–catechol crosslinking | 2.5 kPa | 66 | |
| Metal oxide | IOPs | –NH2 | Gelatin, polydextran aldehyde (PDA) | –NH2, –CHO | Hydrogen bond, electrostatic interaction, imine bond | 0.5 kPa | Control release | 67 |
| Metal oxide | IOPs | –NH2 | Partially deacetylated chitin nanofibers (DEChNs) | –NH2, –OH | Hydrogen bond, electrostatic interaction | 5.5 kPa | Osteosarcoma therapy | 68 |
| Metal oxide | IOPs | –NH2 | Poly(acrylic acid-co-hydroxyethyl methacrylate) | –COOH, –OH | Hydrogen bond, ionic interaction | 1.0 kPa | Drug delivery | 69 |
| Metal oxide | IOPs | –NH2 | Methoxy poly(ethylene glycol) (MPEG), poly(lactic-co-glycolic acid) (PLGA) | –OH | Hydrogen bond | 4.0 kPa | Hyperthermia therapy and cancer therapy | 70 |
| Metal oxide | IOPs | –NH2, –OH | Polyacrylamide (PAAm), HA | Acrylamide groups, –COOH | Hydrogen bond | 7.4 kPa | Disc regeneration | 60 |
| Metal oxide | IOPs | Catechol groups | Gelatin methacryloyl (GelMA) | NH2, –OH, –COOH | Electrostatic interaction, hydrogen bond | 31.3 kPa | 71 | |
| Metal oxide | TiO2@CuO NPs | Chitosan (CS) | –NH2 | Coordination bond, van der Waals forces | 1.5 kPa | Sonodynamic therapy against breast cancer | 72 | |
| Metal oxide | TiO2 NPs | –OH | PAAm | –CONH2 | Hydrogen bond | 1460 kPa | Photodegradation of organic water pollutants | 73 |
| Metal oxide | ZnO NPs | –NH2, OH | Chitosan, poly(ethylene glycol) (PEG) | –NH2, –OH | Hydrogen bond, electrostatic interaction, ionic crosslinking | Wound healing | 74 | |
| Metal oxide | ZnO nanorods | –OH | Chitosan crosslinked with bioactive azelaic acid | –NH2, –OH, –COOH | Coordination bond | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kPa 000 kPa |
Transdermal drug delivery | 75 |
| Metal oxide | ZnO NPs | –OH | Modified silk fibroin (mSF), tannic acid (TA) | –NH2, –OH | Hydrogen bond, coordination bond | 10 kPa | Wound healing | 76 |
| Metal oxide | Hydrous zirconium oxide NPs | –OH | PVA | –OH | Hydrogen bond | Selective sequestration of phosphate from wastewater | 77 | |
| Metal oxide | (Zr(OH)4) NPs | –OH | Acrylic acid (AA), N,N-dimethylacrylamide (DMAA) | –COOH, –CONH2 | Coordination bond | 1200 kPa | Electro-responsive actuators | 78 |
| Metal oxide | (Zr(OH)4) NPs | –OH | 2-Acrylamido-2-methyl propane sulfonic acid (AMPS), acrylamide (AM) | –SO3H, –CONH2 | Hydrogen bond | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 kPa 600 kPa |
79 | |
| Carbon | Carbon dots | –NH2 | PDA | –CHO | Imine bond | 37.7 kPa | 3D printing, electrospinning | 80 |
| Carbon | Carbon nanotubes | –CHO | Oxidized dextran, catechol-modified chitosan | –NH2 | Imine bond | Drug-resistant biofilm elimination, and bacteria killing | 81 | |
| Carbon | Carbon nanotubes | –NH2, phenylboronic acid | Alginate dialdehyde (ADA), poly(N-isopropylacrylamide) (PNIPAM) | –CHO, –OH | Imine bond, boronate ester bond | 16.4 kPa | Drug release | 82 |
| Carbon | Carbon nanotubes | –OH | Poly(sulfobetaine methacrylate) (P(SBMA)), poly(acrylamide) (PAAm) | Dodecyl quaternary ammonium salt, –CONH2 | Ionic bond, hydrogen bond, cation–π interaction, π–π stacking | 211.5 kPa | Wearable strain sensor | 83 |
| Carbon | Graphene oxide | –COOH, –NH2 | Chondroitin sulfate multialdehyde (CSMA) | –CHO | Imine bond | 1.7 kPa | Cancer cell inhibition | 84 |
| Carbon | Graphene oxide | –COOH, –OH | PDA, gelatin | –CHO, –COOH, –NH2 | Coordination bond, imine bond | 84.0 kPa | Thermal-induced bactericidal activity and controlled drug release | 39 |
| Carbon | MXene | –O, –OH, –F, –Cl | Catechol-functionalized poly(vinyl alcohol) (PVA-CA) | –OH | Hydrogen bond | Wearable electronics and smart skin technologies | 85 | |
| Ceramic | LAPONITE® | –OH | PDA, polyethyleneimine-modified gelatin (PG) | –CHO, –NH2 | Imine bond, hydrogen bond, coordination bond, electrostatic interaction | 0.8 kPa | Copper ion sensing, electrospinning, and 3D printing | 86 |
| Ceramic | LAPONITE® | –OH | PDA, polyethyleneimine-modified gelatin (PG) | –CHO, –NH2 | Imine bond, hydrogen bond, coordination bond, electrostatic interaction | 7.1 kPa | Gas sensing | 87 |
| Ceramic | LAPONITE® | –OH | Poly(acrylic acid) (PAAc) | –COOH | Hydrogen bond | 1180 kPa | Adsorption of methylene blue | 88 |
| Ceramic | LAPONITE® | –OH | PAAc | –COOH | Hydrogen bond | 89 | ||
| Ceramic | LAPONITE® | –OH | ODex, polyaniline-grafted chitosan (CSP) | –CHO, –NH2 | Imine bond | Photothermal conversion, and capability hydrogel | 90 | |
| Ceramic | LAPONITE® | –OH | PVA, alginate | –OH | Hydrogen bond | 308 kPa | 91 | |
| Ceramic | LAPONITE® | –OH | Gelatin, PAAm | –COOH, –NH2, –CONH2 | Electrostatic interaction | 20.5 kPa | 92 | |
| Ceramic | LAPONITE® | –OH | Gelatin, alginate | –NH2, –COOH | Electrostatic interaction, hydrogen bond | Bone healing | 93 | |
| Ceramic | LAPONITE® | –OH | HA | –OH | Hydrogen bond | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.4 6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kPa kPa |
94 | |
| Ceramic | LAPONITE® | –OH | Hydrazide-functionalized gelatin (Gel-ADH), dibenzaldehyde-functionalized polyethylene glycol (diBA-PEG) | –NHNH2, –CHO | Hydrazone bond | 11.7 kPa | Cancer treatment | 95 |
| Ceramic | Polydopamine-functionalized LAPONITE® | –OH | Thiolated gelatin, methacrylate | –NH2 | Hydrogen bond | 153 kPa | 96 | |
| Ceramic | Hydroxyapatite (HAp) | –NH2 | PDA | –CHO | Imine bond | 8.6 kPa | Gas sensing | 97 |
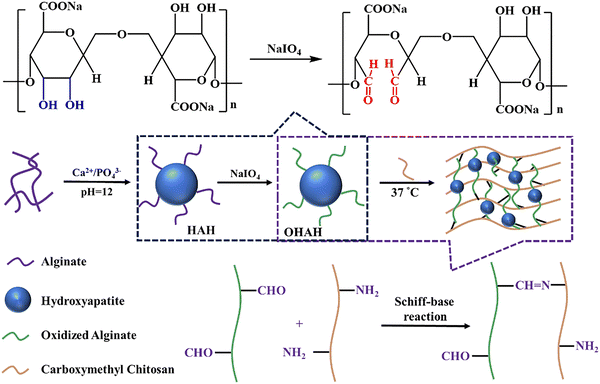
| Ceramic | HAp | –OH | Carboxymethyl chitosan (CMCS), oxidized alginate (OAlg) | –NH2, –CHO | Imine bond | 1.2 kPa | 98 | |
| Ceramic | HAp | –OH | CMCS, OAlg | –NH2, –CHO | Imine bond, hydrogen bond | 276.8 kPa | 99 | |
| Ceramic | HAp | –OH | PAAc | –COOH | Hydrogen bond | Bone regeneration | 100 | |
| Ceramic | HAp | –OH | Cellulose | –OH | Hydrogen bond | 101 | ||
| Ceramic | Silicates | –OH | Gelatin | –NH2 | Electrostatic interaction, hydrogen bond | Hemorrhage | 102 | |
| Ceramic | Silica (SiO2) | –SH | Thiol-modified polyethylene glycol (PEG-SH) | –SH | Disulfide bond | 30 kPa | 103 | |
| Ceramic | SiO2 | –NH2 | Poly(2-methacryloyloxyethyl phosphorylcholine-co-4-formylbenzoate ethyl methacrylate) (P(MPC-co-FBEMA)) | –CHO | Imine bond | Drug delivery | 104 | |
| Ceramic | SiO2 | –NH2 | PAAc | –COOH | Hydrogen bond | 105 | ||
| Ceramic | SiO2 | –OH | OAlg, gelatin | –CHO, –NH2 | Imine bond | 7.2 kPa | 106 | |
| Ceramic | SiO2 | –OH | Poly(hydrolytic polyacrylamide) (PHPAM) | –CONH2 | Hydrogen bond | 107 | ||
| Ceramic | SiO2 | –OH | Alg, chitosan, gelatin | –COOH, –NH2 | Hydrogen bond | 108 | ||
| Ceramic | SiO2, ZnO | –OH | Cellulose | –OH | Hydrogen bond | 5820 kPa | Photocatalytic degradation | 109 |
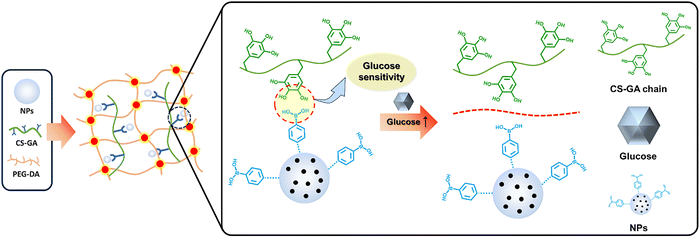
| Polymer | Polyethyleneimine–phenylboronic acid (PEI–PBA)/insulin polymeric NPs | Phenylboronic acid | Poly(ethylene glycol) diacrylate (PEG-DA), chitosan–gallic acid (CS–GA) | Polyphenol | Borate ester bond | 15 kPa | Diabetic wound repair | 110 |
| Polymer | Pluronic F127 (Micelle) | –CHO | Poly(ethylene oxide) (PEO) | Acylhydrazine | Acylhydrazone bond | 27.7 kPa | 111 | |
| Polymer | Pluronic F127 (Micelle) | –CHO | Dopamine-functionalized oxidized hyaluronic acid (OHA-Dop), adipic acid dihydrazide-modified hyaluronic acid (HA-ADH) | –CHO, hydrazide group (–NH–NH2) | Acylhydrazone bond | 5700 kPa | Wound healing | 112 |
| Polymer | Doxorubicin-loaded carboxymethyl chitosan nanoparticles (NP-DOX) and indocyanine green (ICG) encapsulated in aldehyde-terminated Pluronic F127 (AF127) (Micelle) | –CHO, –COOH, –NH2 | CMCS | –NH2, –COOH | Imine bond | Tumor injection and photothermal therapy (PTT) | 112 | |
| Polymer | PAA-b-PSt nanoparticles (Micelle) | –COOH | PAAc | –COOH | Hydrogen bond | 2.6 kPa | 113 | |
| Polymer | Poly(styrene-acrylic acid) (P(S-AA)) core–shell nanoparticle | –COOH | PAAm | –CONH2 | Hydrogen bond | 244 kPa | 114 | |
| Polymer | Poly (oxyethylene 20 oleyl ether acrylate) (O20AC) | –COO– | PVA | –OH | Hydrogen bond | 27.7 kPa | Flexible sensor | 115 |
| Polymer | Cetyltrimethylammonium bromide (CTAB) (Micelle) | Quaternary ammonium groups | CS, acrylic acid (AA), N-(2-hydroxyethyl) acrylamide (HEAA) | –COOH, –NH2, –NH2CO, –OH | Electrostatic interaction | 90 kPa | Wearable bioelectronics | 116 |
| Polymer | Hexadecyltrimethylammonium chloride (CTAC) micelles | Quaternary ammonium group | PAAc | –COOH | Electrostatic interaction | 120 kPa | Reconstructable 3D deformations | 117 |
| Polymer | Paeoniflorin (Pf)-encapsulated micelles (MIC@Pf) | –NH2 | Aminated gelatin (N-gel), PDA | –CHO, –NH2 | Imine bond | Hemostasis, antibacterial activity, and angiogenesis promotion | 24 | |
| Polymer | Chitosan NPs | –NH2 | OAlg, gelatin | –CHO, –NH2 | Electrostatic interaction, hydrogen bond | 2430 kPa | 118 | |
| Polymer | Polyaniline nanoparticles (PANI NPs) | –NH– | Chitosan, PAAc | –COOH, –NH2 | Hydrogen bond | 48.4 kPa | Early diagnosis of Parkinson's disease | 119 |
| Polymer | Diol-capped poly(N,N-dimethylacrylamide)-b-poly(N-isopropylacrylamide) (D-b-N ((OH)2-PDMA-b-PNIPAM)) (Micelle) | –S–S–, –OH | N,N-Dimethylacrylamide-stat-(2-acrylamidophenylboronic acid) copolymers | Boric acid | Boronate ester bond, disulfide bond | 5.3 kPa | 120 | |
| MOF | Ag-MOF | –NH2 | OAlg | –CHO, diol | Imine bond, boronic ester bond | — | Antibacterial | 121 |
| MOF | Cu-MOF | –NH2 | CMCS, oxidized sodium alginate (OSA) | –CHO, –NH2 | Imine bond | — | Antibacterial, anti-inflammatory properties, and infected wound repair | 122 |
| MOF | ZIF-8 MOF | Fluorescein (Flu) | PVA, carboxymethyl cellulose (CMC) | –COOH, –OH | Ionic bond, hydrogen bond | — | Multi-tiered data encryption | 123 |
It should be noted that some reported reviews serve as practical guides to the types of nanomaterials and crosslinking strategies employed in developing functional and dynamic hydrogels for medical use.48,49 Here, our manuscript adopts a more fundamental materials chemistry and engineering perspective, emphasizing the precise control of material properties through surface and interfacial chemistry to enable rational design across a broad spectrum of dynamic functions. The primary goal of our work is to present a cohesive, forward-looking framework that supports the rational design of next-generation DNCHs, offering guiding principles for future research. This represents a higher-level contribution than merely summarizing existing studies, as our manuscript also thoroughly discusses current challenges and identifies key research opportunities. Overall, we aim to deliver a timely and comprehensive scope by incorporating a broader range of nanomaterial types and examples, enhancing understanding and fostering inspiration within the community in different fields.
2. DNCHs fabricated with different surface-functionalized nanomaterials and interfacial crosslinking chemistries
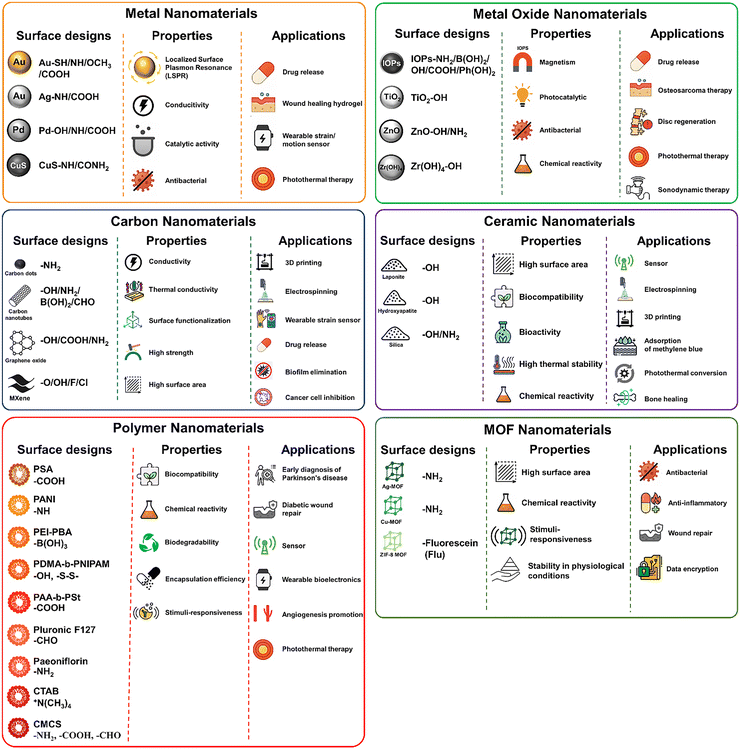
Nanomaterials represent a critical component influencing the properties and functionalities of DNCHs, with their performance governed by the intricate interplay between the characteristics of the nanomaterial fillers and the reversible crosslinking chemistries within the polymeric network. The surface design, properties, and applications of the representative nanomaterials (i.e., metal-, metal oxide-, carbon-, ceramic-, polymer-, and MOF-based nanomaterials) are illustrated in Fig. 3. Also, a summary of the nanomaterials commonly utilized in the fabrication of DNCHs is provided in Table 2, outlining their intrinsic benefits and limitations.| Nanomaterial types | Advantages | Limitations |
|---|---|---|
| Metal | • Strong localized surface plasmon resonance for optical/photothermal actuation. | • High cost (especially Au, Pt). |
| • Intrinsic antimicrobial activity (Ag). | • Potential cytotoxicity at elevated concentrations. | |
| • High electrical conductivity and catalytic functionality. | ||
| • Versatile surface functionalization. | ||
| Metal oxide | • Magnetic responsiveness (Fe3O4). | • Generate excessive ROS under light exposure. |
| • Photocatalytic activity and UV protection (TiO2, ZnO). | • Poor biodegradability and long-term fate unclear. | |
| • ROS generation for therapy. | • Often require silane or polymer grafting to stabilize dispersions and mitigate side reactions. | |
| • Good chemical stability and biocompatibility. | ||
| Carbon | • Exceptional mechanical reinforcement and large surface area. | • Hydrophobic agglomeration without extensive oxidation or grafting. |
| • Excellent electrical conductivity. | • Require polymer or ligand functionalization to ensure dispersion and reduce inflammatory responses. | |
| • High drug-loading capacity. | ||
| • Tunable optical properties (CQDs). | ||
| Ceramic | • High stiffness and wear resistance (silica, LAPONITE®). | • Intrinsically brittle and lacking dynamic responsiveness. |
| • Chemical inertness and thermal stability. | • Processing challenges and poor flexibility. | |
| • Bone-mimetic bioactivity (hydroxyapatite). | • Often need core–shell designs or co-crosslinking with softer polymers to avoid fracture. | |
| Polymer | • Excellent biodegradability and biocompatibility. | • Provide minimal mechanical reinforcement on their own. |
| • Tunable drug-release profiles (micelles, polymer dots). | • Must be co-incorporated with rigid fillers for strength. | |
| • Modular surface chemistry. | • Sometimes suffer from limited stimuli-responsiveness compared to inorganic counterparts. | |
| MOF | • High surface area and tunable pore size, enabling excellent molecular adsorption and storage capacity. | • Limited stability of some MOFs in aqueous or physiological environments. |
| • Hybrid MOF allows versatile surface functionalization and customized functionality. | • Synthesis may involve high cost and stringent conditions (e.g., temperature, solvent, and time). | |
| • Multiple stimuli-responsiveness (e.g., pH, light, and chemical species) enables intelligent controlled release and sensing. | • Biocompatibility and long-term safety require thorough evaluation. | |
| • Potential metal ion leaching from certain metal nodes (e.g., Cu, Co) may raise toxicity concerns. |
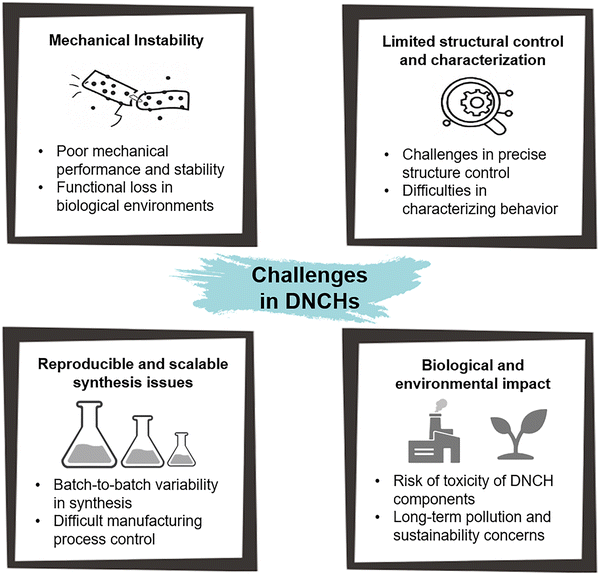
Metal-based nanomaterials (e.g., gold and silver) offer powerful surface plasmon resonance effects for optical and photothermal applications, and silver in particular provides intrinsic antimicrobial action. However, their high cost, propensity for aggregation under physiological salt conditions, and potential cytotoxicity at elevated concentrations impose significant constraints on long-term in vivo use. Similarly, metal-oxide fillers (e.g., iron oxide (Fe2O3/Fe3O4) and titanium dioxide (TiO2)) endow DNCHs with magnetic actuation or photocatalytic/UV-blocking capabilities. These materials also exhibit high chemical stability and biocompatibility. Nevertheless, the potential formation of reactive oxygen species (ROS) upon exposure to light, limited biodegradability, and uncertain toxicity necessitate careful surface design to stabilize dispersions and mitigate adverse reactions. Carbon-based nanomaterials (e.g., graphene oxide (GO), carbon nanotube (CNT), and carbon quantum dot (CQD)) significantly improve mechanical reinforcement and electrical conductivity. Their tunable optical properties further expand their applicability. However, challenges (e.g., hydrophobic agglomeration, inflammatory reactions, and the need for extensive oxidation or polymer functionalization) must be addressed to ensure biocompatibility and homogeneous distribution within the hydrogel network. Ceramic nanoparticles (e.g., silica and hydroxyapatite) are characterized by high stiffness, chemical inertness, and bioactivity. However, brittleness, processing difficulties, and limited dynamic reactivity pose significant challenges. Polymer nanoparticles (e.g., micelles and polymer dots) offer excellent biodegradability, biocompatibility, modular surface chemistry, and tunable drug release profiles. However, their mechanical contribution is limited without the simultaneous incorporation of rigid fillers, and their stimulability may be comparatively modest compared to inorganic nanomaterials, necessitating combination strategies for improved mechanical robustness and responsiveness. MOF nanoparticles exhibit a high surface area and tunable pore sizes, facilitating exceptional molecular adsorption and storage capabilities. Their hybrid architecture enables versatile surface functionalization and imparts responsiveness to multiple stimuli. Nevertheless, certain MOFs demonstrate limited stability in aqueous or physiological environments, and comprehensive evaluation of their biocompatibility and long-term safety remains essential.
Long-term in vivo studies reveal distinct toxicity thresholds and mechanisms of chronic adverse effects across different nanomaterials. Noble metals, such as Au and Pt, are generally biocompatible at low doses; however, high or repeated administration can lead to hepatic and renal dysfunction, depending on the dose and particle size. Smaller Au nanoclusters (<5 nm) undergo efficient renal clearance, reducing organ accumulation, whereas larger or uncapped AuNPs and sub-nanometer Pt species accumulate in the liver and kidneys, causing mitochondrial and redox perturbations.124–126 Silver-based systems release Ag ions from particulate reservoirs, resulting in continuous ROS formation, genotoxic stress, and immune dysregulation. Consequently, their effective exposure thresholds are lower than those of many inert materials unless the dissolution process is limited.127 Metal oxides such as Fe3O4 and TiO2 are considered moderately risky, as they tend to accumulate in the liver and spleen after being delivered systemically. Their slow core dissolution may release metal ions that catalyze ROS generation and provoke low-grade chronic inflammation.128,129 Carbon-based nanomaterials, particularly CNTs, persist in tissues and can induce asbestos-like inflammatory and fibrotic responses, which can be mitigated by oxidation or polymer grafting.130 Ceramic nanoparticles like silica are usually inert at low exposure levels but can cause granulomatous responses at high doses or if cleared poorly. Chronic exposure studies emphasize the significance of dose and clearance kinetics for long-term safety.131 In contrast, biodegradable polymeric nanoparticles are preferred for their low long-term organ burden due to their ability to degrade in vivo. However, this may reduce their residence time and therapeutic efficacy. Recent reviews emphasize the importance of balancing the degradation rate and therapeutic effectiveness in nanoparticle design.132 Finally, MOFs containing labile metals like Ag, Cu, or Zn can release toxic metal ions upon degradation. Ensuring the MOF framework stability and employing strategies like surface coating or encapsulation are essential to mitigate these risks.133
To address these concerns, surface functionalization has been utilized as a strategy for improving the biological safety of the above nanomaterials. Hydrophilic polymer coatings, such as PEG and its derivatives, provide steric stabilization that prevents aggregation and reduces non-specific protein adsorption. This leads to lower opsonization and improved biological stability, which often results in changes in biodistribution and decreases acute immunotoxicity.134 For metal and metal oxide nanoparticles, whose toxicity is often driven by ion dissolution, polymer shells act as diffusion barriers that suppress ion release. For instance, silver systems with polymer capping of PEG exhibit slower dissolution kinetics and significantly lower the release of Ag ions.135 Similarly, polymer grafting on carbon nanotubes reduces exposure of reactive surfaces, which in turn minimizes fibrotic and inflammatory responses in vivo.136 In biodegradable polymeric nanoparticles, PEGylation is widely employed to regulate degradation rate and circulation time, achieving a balance between therapeutic efficacy and minimal long-term accumulation.137 Whereas polymer functionalization on MOFs enhances structural stability against hydrolysis and curbs the uncontrolled release of metal ions. A demonstrated strategy involves an in situ polymerized coating on Zr-based MOFs, which significantly enhanced physiological stability by shielding against phosphate-driven decomposition, thereby reducing metal ion leakage and prolonging in vivo circulation.138
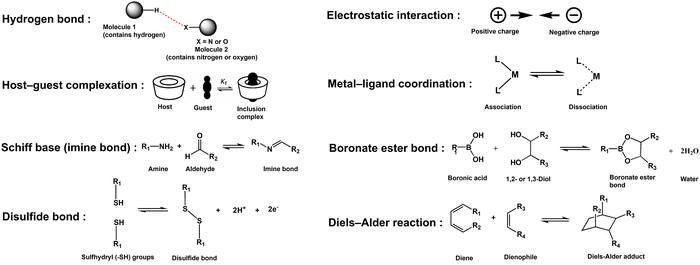
The selection of crosslinking chemistry at the material interface plays a critical role in determining the performance of DNCHs. Specifically, dynamic chemistries (i.e., non-covalent and dynamic covalent bonds) are commonly used to form reversible, stimuli-responsive crosslinks within the hydrogel network. The commonly employed dynamic chemistries have been summarized in Fig. 4 and Table 3, highlighting their inherent advantages and limitations.
| Crosslinking chemistry | Pros | Cons |
|---|---|---|
| Hydrogen bond | • Ultra-fast association/dissociation for shear-thinning and self-recovery. | • Individual bonds are weak with limited load-bearing capacity. |
| • Easily disrupted by water or competing H-bond donors/acceptors. | ||
| Electrostatic interaction | • Rapid and reversible crosslinking through electrostatic attractions. | • Instability under physiological ionic strength or in the presence of competing ions. |
| • Tunable crosslinking density by adjusting ionic concentrations or charge densities. | • Susceptibility to structural disruption under changes in environmental conditions (e.g., ionic strength and pH). | |
| • Good responsiveness to ionic strength and pH changes. | ||
| Host–guest complexation | • Rapid, reversible physical crosslinks (e.g., cyclodextrin–adamantane). | • Moderate binding affinities may lead to creep under load. |
| • Wide tunability of binding constants. | • Susceptible to competitive displacement by small molecules in complex media. | |
| • Highly biocompatible. | ||
| Metal–ligand coordination | • Multi–stimuli responsiveness (e.g., pH, redox, light, and magnetism). | • Potential metal-ion cytotoxicity or leaching. |
| • Strong crosslinking yields high modulus | • Coordination strength highly sensitive to local chemical environment. | |
| • Easy tuning via choice of metal ion/ligand. | ||
| Schiff base (imine bond) | • Rapid, catalyst-free self-healing under mild conditions. | • Hydrolytically unstable at low pH. |
| • Highly pH-responsive for on-demand gel–sol transitions. | • Unreacted aldehyde residues can be cytotoxic if not fully consumed | |
| • Good injectability and printability. | ||
| Schiff base (hydrazone bond) | • Stable at neutral pH yet acid-cleavable for targeted release in acidic microenvironments. | • Generally slower bond-exchange kinetics than simple imines. |
| • Potential residual hydrazide/hydrazone toxicity if not fully reacted. | ||
| Boronate ester bond | • Dual pH- and sugar-sensitivity for glucose or diol-triggered responses. | • Competitive binding by free diols (e.g. glucose and dopamine) can disrupt network. |
| • High reversibility under physiological conditions. | • Limited stability at physiological pH without careful ligand design. | |
| Disulfide (S–S) bond | • Redox-triggered cleavage/reformation for on-demand gel repair or cargo release. | • Susceptible to irreversible oxidation (sulfonic acids) under harsh oxidative stress. |
| • Light- or enzyme-mediated control possible. | • May exchange too slowly under mild reducing conditions. | |
| Diels–Alder adduct | • Thermally reversible covalent linkages with tunable transition temperatures. | • Requires elevated temperature (often >50 °C) to break/form bonds. |
| • Robust mechanical strength at ambient. | • Slower reaction rates at physiological temperature limit rapid self-healing. |
Non-covalent interactions (e.g., hydrogen bonds, electrostatic interactions, host–guest complexation, and metal–ligand coordination) deliver ultra-fast, reagent-free reversibility and generally low toxicity, but their inherently weak bond strengths and sensitivity to environmental factors (e.g., pH, ionic strength, and competing ligands) limit the degree of mechanical reinforcement they can impart on their own. Hydrogen bonds are reversible interactions between hydrogen and electronegative atoms (e.g., oxygen, nitrogen), offering biocompatibility and low toxicity. They respond to pH and temperature but have weak strength, limiting mechanical reinforcement. Electrostatic interactions occur between oppositely charged groups (e.g., amino and carboxylate), enabling fast, reversible crosslinking under mild conditions. Electrostatic interactions are biocompatible but sensitive to ionic strength and pH, reducing mechanical stability in physiological environments. Host–guest complexes involve reversible encapsulation (e.g., cyclodextrin–adamantane), enabling tunable, biocompatible crosslinks. However, moderate affinity makes them vulnerable to displacement by competing molecules. Metal–ligand coordination involves metal ions and ligands (e.g., imidazole), offering strong, tunable, and stimulus-responsive crosslinks. Nevertheless, potential toxicity and sensitivity to the local environment remain concerns.
Dynamic covalent bonds (e.g., imine, hydrazone, boronate ester, disulfide, and Diels–Alder bonds) can be reversibly formed, cleaved, or rearranged, enabling controlled reactivity and robust mechanical properties for advanced biomedical hydrogel applications. Schiff-base (e.g., imine and hydrazone) form via reversible aldehyde–amine or aldehyde–hydrazide condensation, enabling pH-sensitive, self-healing, and biocompatible crosslinks. However, they are hydrolytically unstable in acidic conditions and may pose cytotoxicity risks due to residual aldehydes. Boronate ester bonds form between boric acids and cis-diols, offering pH- and sugar-responsive behavior with fast exchange under neutral-basic pH. Nevertheless, they are unstable in acidic environments and prone to disruption by natural diols. Disulfide bonds result from thiol oxidation, enabling redox-responsive, reversible crosslinks suitable for drug delivery. They resist oxidation but may overoxidize irreversibly and exhibit slow exchange rates under physiological conditions. Diels–Alder bonds are thermally reversible covalent links formed via cycloaddition, offering strong, specific, and tunable bonding. However, high temperatures and slow kinetics at body temperature limit rapid biomedical self-healing applications.
Overall, dynamic bonds represent a versatile toolkit for the development of advanced biomedical hydrogels. However, their specific selection should be carefully tailored to the desired reactivity, biocompatibility, stability, and mechanical performance required for the targeted biomedical applications.
3.1 Metal-based nanomaterials in DNCHs
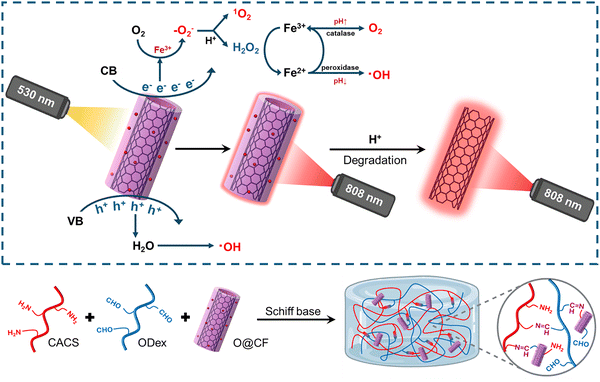
Metal-based nanomaterials (e.g., gold (Au), silver (Ag), palladium (Pd), and platinum (Pt)) are widely employed as dynamic crosslinkers in DNCHs due to their unique physicochemical properties. These nanomaterials exhibit a high surface-to-volume ratio, tunable surface plasmon resonance, excellent electrical conductivity, and inherent catalytic or antimicrobial properties. Their nanoscale dimensions and surface characteristics enable easy surface functionalization with various ligands, polymers, or biomolecules, facilitating precise integration within the hydrogel network. Across different metals, several universal functionalization strategies are commonly employed, including Au–S or Ag–thiolate coordination with thiol-containing ligands, amine–aldehyde Schiff base chemistry, carboxylate and amine coordination, and redox-mediated in situ NP growth within the polymer matrix. Once incorporated, functionalized NPs often serve recurring dynamic roles such as photothermal triggers, conductive fillers, antimicrobial agents, or catalytic/ROS-regulating centers. Thus, functionalized metal NPs act not only as crosslinking nodes but also as multifunctional enhancers, endowing DNCHs with dynamic properties such as self-healing, stimuli responsiveness, and electrical conductivity. Among these, Au and Ag NPs are especially favored in biomedical applications due to their biocompatibility and versatile functionalization potential, while Pd and Pt NPs are often utilized in catalytic or electronic domains. Here, we discuss recent advances in functionalization strategies and crosslinking mechanisms for these metal-based nanomaterials used in DNCH formation.The versatility of AuNP crosslinking strategies is exemplified by the work of Li et al. on diabetic wound healing.53 By engineering defect-rich molybdenum disulfide nanosheets loaded with bovine serum albumin-modified gold nanoparticle nanosheets (MoS2@Au@BSA), BSA-modified AuNPs were leveraged to introduce surface-exposed amine groups. These amine groups enabled Schiff base formation with the aldehyde groups on oxidized dextran (ODex), anchoring the nanosheets within the injectable hydrogel network (Fig. 5). Concurrently, glycol chitosan (gC), rich in primary amine groups, formed additional Schiff base linkages with ODex. The resulting hydrogel not only exhibited self-healing and tissue adhesion but also functioned as a glucose-powered cascade reaction system designed for the reconstruction of infected diabetic skin. Under acidic conditions, the MoS2@Au@BSA nanosheets exhibited peroxidase (POD)-like activity, converting hydrogen peroxide (H2O2) into hydroxyl radicals (˙OH) to eradicate bacteria and consume glucose. Conversely, in alkaline environments, catalase (CAT)- and superoxide dismutase (SOD)-like activities scavenged ROS and produced oxygen (O2), alleviating hypoxia to accelerate tissue regeneration. The hydrogel demonstrated antibacterial efficacy, as evidenced by significant reductions in E. coli and S. aureus survival. Furthermore, the hydrogel exhibited concentration-dependent bacterial eradication, demonstrating its dual role as a dynamic scaffold and therapeutic agent for infected diabetic wounds. AuNPs can also be synthesized in situ within hydrogels. For example, Gounden et al. reported the reduction of Au(III) within a chitosan (CS) hydrogel.142 Physical crosslinking between AuNPs and CS occurs through electrostatic interactions and steric stabilization. These interactions enhanced the mechanical strength and viscosity of the hydrogel network, presenting a porous, interconnected structure with reduced pore sizes due to additional crosslinking points. Functionally, this CS–Au hydrogel was investigated as an anticancer drug delivery system by encapsulating 5-fluorouracil (5-FU) with pH-responsive and sustained release behavior. Additionally, the hydrogel demonstrated self-healing properties and promoted cell migration. These features highlight its potential as a biocompatible scaffold for tissue engineering and targeted therapeutic applications.
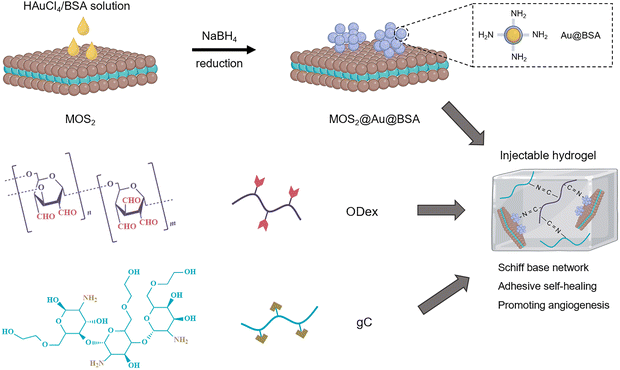 | ||
| Fig. 5 Schematic illustration of the synthesis of MoS2@Au@BSA nanosheets and the formation of an injectable hydrogel. | ||
Building on these advances, researchers have extended AuNP-crosslinked hydrogels to neurological applications. Xu et al. leveraged the conductive property of AuNPs to develop self-healing hydrogels for Parkinson's disease (PD) treatment.54 In this system, AuNPs were functionalized with oxidized tannic acid (OTA) and synthesized through enzymatic tannic acid oxidation using laccase (Fig. 6(a)). The quinone groups on OTA covalently interacted with the amine groups of O-carboxymethyl chitosan (CMC) via Schiff base formation, generating imine bonds that crosslinked OTA@Au NPs into the hydrogel network (Fig. 6(b)). The resulting CMC/OTA@Au (COA) hydrogels exhibited injectability, conductivity, and self-healing capabilities. In PD rat models, electrophysiological analyses demonstrated improvements in subthalamic nucleus (STN) activity, with normalized spike patterns and reduced spike rates, following treatment with COA hydrogels (Fig. 6(c) and (d)). Immunofluorescent analyses further revealed enhanced neuroprotection and reduced inflammation, including increased densities of tyrosine hydroxylase-positive (TH+) dopaminergic neurons and fibers as well as reduced glial fibrillary acidic protein–positive (GFAP+) astrocytes (Fig. 6(e)–(h)).
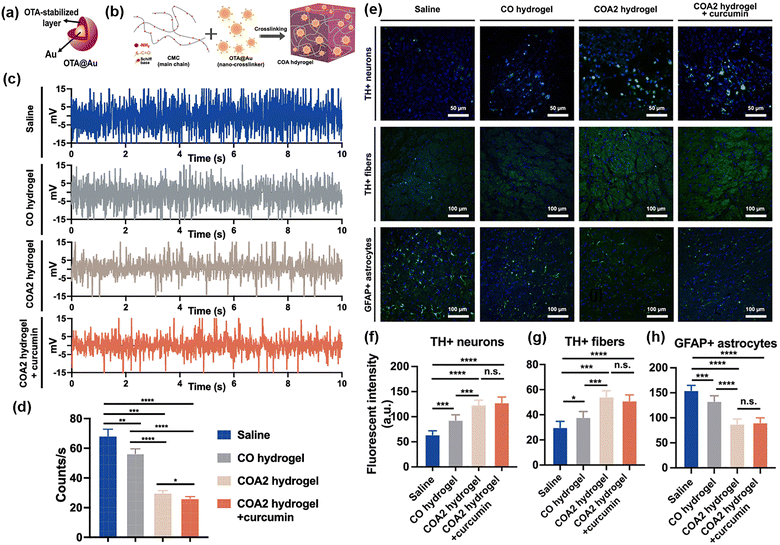 | ||
| Fig. 6 Schematic illustrations of (a) the structure of OTA@Au and (b) the proposed gelation mechanism of COA hydrogels. (c) Electrophysiological traces of STN spikes in different conditions treated PD rats within 10 s at 14 days. (d) Quantification of overall spike rates based on 30-second recordings from each group, excluding significant noise, at day 14. (e) Expression of TH+ dopaminergic neurons in the substantia nigra pars compacta (SNpc), TH+ dopaminergic fibers in the striatum, and GFAP+ astrocytes in the PD rat striatum was investigated at 14 days after surgery. Green fluorescence indicates marker expression, while blue fluorescence indicates cell nuclei. Quantification of fluorescence intensity is shown for (f) TH+ dopaminergic neurons in the SNpc, (g) TH+ dopaminergic fibers in the striatum, and (h) GFAP+ astrocytes in the striatum of each group. *p < 0.05, *p < 0.001, and p < 0.0001 between the indicated groups. Reproduced with permission from ref. 54 Copyright 2023, BioMed Central. | ||
The integration of conductivity and stimuli-responsiveness has further propelled AuNP-containing hydrogels into wearable technologies. A prime example is the work of Kościelniak et al., who synthesized DNCHs by crosslinking poly(acrylamide) (PAAm) with AuNPs modified with N,N′-diacryloylcystine salt (BISS) (Fig. 7(a)).50 Disulfide bonds in BISS were cleaved during synthesis, enabling sulfur atoms to covalently bond with AuNPs and form robust gold–sulfur (Au–S) linkages. This crucial step converted the inert Au surface into a platform decorated with polymerizable acryloyl groups. These surface-bound acryloyl groups then participated in the radical polymerization of acrylamide, covalently integrating the NPs into the (PAAm) backbone. Thus, these modified AuNPs acted as multi-crosslinkers, connecting multiple polymer chains to enhance mechanical performance and introduce electrical conductivity, with excess BISS providing secondary crosslinking. This dual crosslinking architecture markedly improved the tensile strength of hydrogel, from 31.8 to 93.6 kPa, and increased its strain limit to 650%, while preserving excellent conductivity and flexibility. Thus, AuNPs were transformed from passive fillers into multifunctional covalent crosslinkers that reinforced the network structure and enhanced dynamic sensing capabilities. The resulting hydrogels functioned as effective sensors, exhibiting resistance changes during bending and motion that correlated with the degree of deformation (Fig. 7(b)–(e)). Expanding this concept, Khodami et al. also functionalized AuNPs with BISS to introduce sulfur groups for strong Au–S bonds.51 These modified AuNPs served as multi-crosslinkers in PNIPAM hydrogel networks, with BISS also forming covalent crosslinks with the polymer chains. This dual-crosslinking mechanism enhanced the mechanical stability, self-healing capacity, and conductivity of the hydrogel, enabling applications such as NIR laser-activated drug release, skin adhesion, and tissue engineering. These studies demonstrate how dual-crosslinking architectures can synergize the electronic properties of AuNPs with smart material responsiveness.
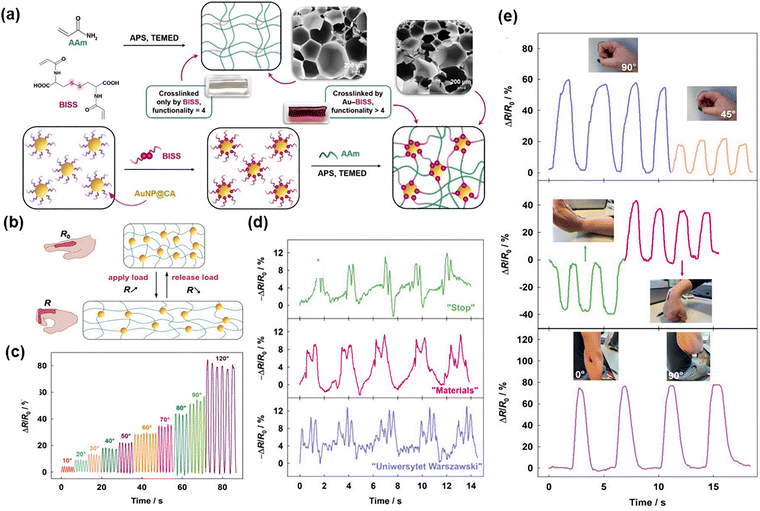 | ||
| Fig. 7 (a) Synthesis and scanning electron microscopy (SEM) images of p(AAm–BISS) gels and p(AAm–BISS)–Au gels. (b) Scheme of the operation principle of the hydrogel sensor. (c) Relative resistance changes (ΔR/R0) of the p(AAm–0.4%BISS)–Au (WG) gel during bending at different angles. (d) Speech recognition performance based on the detection of three words: “stop”, “materials”, and “Uniwersytet Warszawski” (“Warsaw University” in Polish), repeated five times. (e) Application of the hydrogel sensor for detecting finger bending to around 90° and 45°, up and down wrist movement, and elbow bending. Reproduced with permission from ref. 50 Copyright 2024, Elsevier. | ||
Zhang et al. exemplified the dual therapeutic functionality by synthesizing cellulose nanofibril/polyvinyl alcohol/curcumin–Ag (CNF/PVA/cAg) hydrogels.55 Curcumin was functionalized onto AgNPs through coordination bonds, forming cAg, to improve the stability and bioavailability of the NPs (Fig. 8(a)). The hydrogel network was formed through a combination of dynamic borate ester bonds between the cis-diol groups of CNFs and PVA with borate ions, intermolecular hydrogen bonds among CNFs, PVA, and cAg, and coordinate covalent bonds between the cAg and the polymeric network (Fig. 8(b)). This multifunctional design endowed the hydrogel with antimicrobial, antioxidant, and self-healing properties. In vivo studies demonstrated accelerated wound closure in diabetic rats over 14 days. Histological analyses further confirmed improved epidermis thickness, blood vessel regeneration, collagen deposition, and overall tissue repair in CNF/PB/cAg hydrogel-treated wounds.
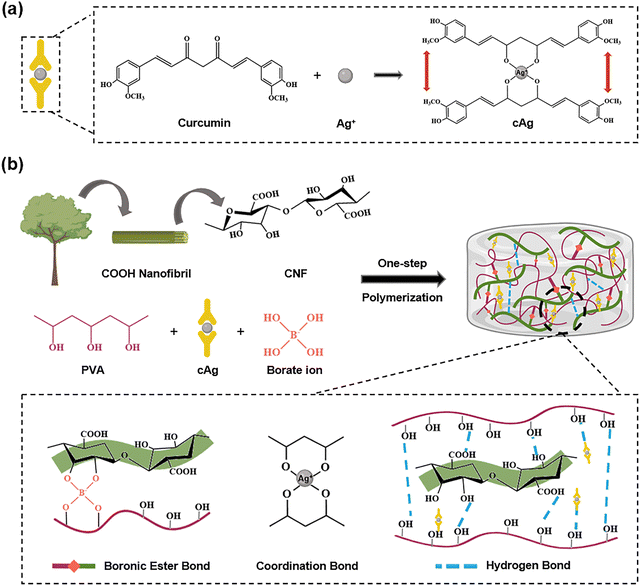 | ||
| Fig. 8 (a) Synthesis route of cAg. (b) Fabrication mechanism of the CNF/PB/cAg hydrogel and the proposed dynamic networks. | ||
While Zhang's work focused on therapeutic delivery, Shi et al. expanded the application of AgNPs into wearable sensing by incorporating silver-doped polydopamine NPs into poly(acrylamide) (PAAm) hydrogels.56 This work provided a clear illustration of interfacial engineering, detailing the transformation of silver into a dynamic crosslinker. Silver-doped polydopamine NPs were synthesized via an in situ redox reaction between silver nitrate (AgNO3) and polydopamine, where the polydopamine coating acted as a stabilizer and a versatile platform for further functionalization. This was followed by surface modification with N,N′-bis(acryloyl)cystamine (BA) through silver–thiolate coordination. This step involved the cleavage of the disulfide bond in BA, exposing thiol groups that formed dynamic Ag–thiolate coordination bonds with the silver surface. The acryloyl groups of BA then copolymerized with acrylamide, covalently embedding the NPs within the PAAm network. These NPs acted as multifunctional crosslinkers within the PAAm network, enabling dynamic crosslinking through both reversible Ag–thiolate coordination bonds and hydrogen bonds. This dual dynamic crosslinking strategy significantly enhanced the mechanical strength and electrical conductivity of the hydrogel. Consequently, the resulting hydrogels showed promising potential for wearable strain sensing. The hydrogel can detect strain from human motion, supporting applications in healthcare monitoring and human–machine interfaces. It captured various movements, including neck twisting, throat vibrations, finger bending at different amplitudes, and biceps contraction. Respiratory patterns during standing, running, and recovery were also monitored by attaching the sensor to the abdomen, demonstrating potential for real-time physiological sensing.
Beyond sensing applications, recent efforts have focused on optimizing AgNP synthesis and hydrogel stability through innovative strategies. For instance, Hasan et al. employed microfibrillated cellulose (MFC) as a green reducing and stabilizing agent to synthesize MFC-stabilized AgNPs (MFC-Ag).57 The resulting nanocomposites formed hydrogen bonds with PVA, yielding hydrogels with enhanced mechanical strength, thermal stability, and potent antimicrobial activity against multidrug-resistant bacteria. Taken together, these properties support their use for chronic wound management.
In parallel, PtNPs have also shown utility in regenerative applications, particularly in addressing chronic wounds. For example, Zhou et al. leveraged the catalytic properties of PtNPs to address diabetic wound regeneration.61 The PtNPs were functionalized with polyethylene glycol (PEG)–amine groups, which were adsorbed onto the surface of the NPs to provide colloidal stability and present reactive amine groups. These modified PtNPs were incorporated into a nanohybrid double network hydrogel, where the aldehyde groups of ODex formed dynamic Schiff base bonds with the amine groups on PtNPs and methacrylate-anhydride gelatin (GelMA). In this network, the PtNPs acted as crosslinking nodes precisely through the imine bonds formed at its functionalized surface, while also integrating its catalytic function. The result was a dual-network hydrogel that combined excellent mechanical strength with self-healing properties, all while maintaining the oxygen-generating enzymatic activity of the PtNPs. This ability to carry and release oxygen helped alleviate hypoxia in diabetic wounds. At the same time, the structural adaptability of the hydrogel accelerated tissue repair, offering a major step forward in treating chronic wounds.
3.2 Metal oxide-based nanomaterials in DNCHs
Metal oxide-based nanomaterials have attracted significant attention in the design of DNCHs due to their unique combination of chemical stability, biocompatibility, and tunable surface properties.49,157,158 Commonly employed metal oxide-based nanomaterials include iron oxide (Fe2O3/Fe3O4), TiO2, and zinc oxide (ZnO), with iron oxide being the most extensively explored. Common strategies for surface functionalization include silanization to introduce amine groups for dynamic covalent bonding, coordination with catechol or phenolic ligands for reversible crosslinking, and using ionic or hydrogen bonds for physical networks. These nanomaterials offer additional functionalities such as magnetic responsiveness,159–161 photocatalytic activity,162–164 ROS generation,151,165 and UV protection,164,166 enabling them to act as magnetic actuators, catalytic centers, or reinforcing fillers. By incorporating surface-functionalized metal oxide-based nanomaterials into polymer matrices, it is possible to construct hydrogels with enhanced mechanical properties, stimuli-responsiveness, therapeutic capabilities, and environmental adaptability. This section discusses how metal oxide-based nanomaterials serve as multifunctional crosslinkers in dynamic hydrogel systems, focusing on their surface chemistry, crosslinking mechanisms, and resulting biomedical and catalytic applications.To take advantage of these properties, Lu et al. incorporated amine-functionalized iron oxide nanoparticles (IOP_NH2) into a gelatin (Gel) and polydextran aldehyde (PDA) network to synthesize nanocomposite double-network hydrogels, Gel/PDA/IOP_NH2.67 The functionalization process begined with pristine IOPs, which were coated with (3-Aminopropyl)triethoxysilane (APTES). The ethoxyl groups of APTES were hydrolyzed and condensed with surface hydroxyl groups on the IOPs, creating a stable siloxane network and presenting reactive primary amine groups. The resulting IOP_NH2 nanoparticles enabled multiple interfacial bonding interactions (i.e., hydrogen bonding, electrostatic interactions, and dynamic imine bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)) with the aldehyde and carboxyl groups in the Gel/PDA matrix. These dynamic covalent and noncovalent linkages acted as multifunctional crosslinking centers, increasing the network density and energy dissipation capability, enhancing stability, mechanical strength, and structural integrity of hydrogel (Fig. 9(a)). As a result, the Gel/PDA/IOP_NH2 hydrogel exhibited a markedly improved storage modulus (G′ = 2330 Pa) compared to the Gel/PDA control (G′ = 471 Pa), and its compressive modulus increased from 13.63 ± 3.27 kPa to 16.19 ± 1.86 kPa. The tensile modulus and toughness also rose to 64.7 kPa and 168.3 kJ m−3, respectively, demonstrating that amine-functionalized magnetic nanoparticles effectively reinforced the hydrogel network through synergistic interfacial bonding and network reinforcement. When exposed to alternating magnetic fields (AMF), the hydrogels exhibited magnetothermal responsiveness, demonstrating their potential for smart actuation. Furthermore, the cumulative release behavior of bovine serum albumin–fluorescein isothiocyanate conjugate (BSA–FITC) under varying pH and temperature conditions (Fig. 9(b) and (c)) confirmed their capacity for controlled drug delivery, with accelerated release observed under acidic pH and elevated temperatures.
N)) with the aldehyde and carboxyl groups in the Gel/PDA matrix. These dynamic covalent and noncovalent linkages acted as multifunctional crosslinking centers, increasing the network density and energy dissipation capability, enhancing stability, mechanical strength, and structural integrity of hydrogel (Fig. 9(a)). As a result, the Gel/PDA/IOP_NH2 hydrogel exhibited a markedly improved storage modulus (G′ = 2330 Pa) compared to the Gel/PDA control (G′ = 471 Pa), and its compressive modulus increased from 13.63 ± 3.27 kPa to 16.19 ± 1.86 kPa. The tensile modulus and toughness also rose to 64.7 kPa and 168.3 kJ m−3, respectively, demonstrating that amine-functionalized magnetic nanoparticles effectively reinforced the hydrogel network through synergistic interfacial bonding and network reinforcement. When exposed to alternating magnetic fields (AMF), the hydrogels exhibited magnetothermal responsiveness, demonstrating their potential for smart actuation. Furthermore, the cumulative release behavior of bovine serum albumin–fluorescein isothiocyanate conjugate (BSA–FITC) under varying pH and temperature conditions (Fig. 9(b) and (c)) confirmed their capacity for controlled drug delivery, with accelerated release observed under acidic pH and elevated temperatures.
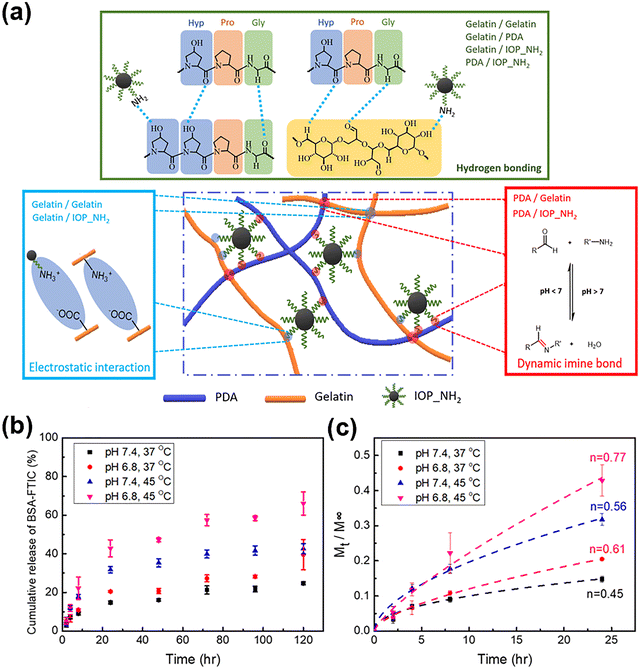 | ||
| Fig. 9 (a) Schematic illustration of Gel/PDA/IOP_NH2 nanocomposite double-network hydrogels constructed with gelatin, PDA, and IOP_NH2 through the formation of hydrogen bonding, electrostatic interactions, and dynamic imine bonds. (b) Cumulative release profiles of BSA-FITC from Gel/PDA/IOP_NH2 hydrogels immersed in the PBS solutions of different pH values at 37 or 45 °C, and (c) Korsmeyer–Peppas fitting of the BSA-FITC release data. Reproduced with permission from ref. 67 Copyright 2021, Wiley. | ||
Expanding on the dynamic capabilities of IOP-based hydrogels, Yu et al. engineered a boronate–catechol crosslinked hydrogel system by incorporating phenylboronic acid-polyethyleneimine-functionalized iron oxide nanoparticles (PP-IOPs) into dopamine-modified four-arm poly(ethylene glycol) (DA-4APEG) hydrogels.66 Boronate–catechol interactions at the PP-IOP/DA-4APEG interface served as dynamic crosslinking points, enhancing mechanical toughness, injectability, and self-healing properties of the hydrogel. These hydrogels responded to multiple stimuli, including pH, temperature, glucose, dopamine, H2O2, magnetic field, and NIR (Fig. 10(a)). Sol–gel transitions were observed with temperature changes between 30 °C and 80 °C (Fig. 10(b)) and pH shifts from 8 to 3 (Fig. 10(c)), alongside reversible transitions triggered by glucose, dopamine, and H2O2 (Fig. 10(d)). Magnetic responsiveness enabled hydrogel movement (Fig. 10(e)), and photothermal effects were confirmed via NIR-induced heating (Fig. 10(f)). These dynamic properties position DA-4APEG/PP-IOP hydrogels as promising candidates for advanced biomedical applications, including controlled drug delivery and responsive wound dressings.
 | ||
| Fig. 10 (a) Schematic illustration of the fabrication of DA-4APEG/PP-IOP NC hydrogels. (b) Temperature- and (c) pH-responsiveness of the hydrogel. (d) Glucose-, H2O2-, and dopamine-mediated gel–sol transition of the hydrogel. (e) Magnetic- and (f) NIR-responsiveness of the hydrogel. Reproduced with permission from ref. 66 Copyright 2024, Elsevier. | ||
Aside from covalent and supramolecular interactions, IOPs have also been incorporated into hydrogels through coordination chemistry, taking advantage of their natural affinity for oxygen-donor ligands. Blin et al. utilized the reversible Diels–Alder reaction to create a thermo-responsive polymeric network and achieved magneto-responsiveness by incorporating IOPs modified with difuran-functionalized, diphosphonic acid-terminated poly(ethylene oxide).64 The modified NPs act as multifunctional crosslinkers as the phosphonic acid groups form strong bidentate Fe–O–P bonds with the IOPs. A robust, thermally responsive hydrogel network can be constructed by combining with reversible Diels–Alder cycloadditions between furan and maleimide groups.
In another example, Ganguly et al. synthesized superparamagnetic, amine-functionalized maghemite (γ-Fe2O3) NPs using a room-temperature, layer-by-layer coating method, yielding NPs with surfaces enriched in amine groups.69 This functionalization process involved the sequential adsorption of polyelectrolytes, culminating in an outer layer presenting amine functionalities. These amine functionalities played a pivotal role in forming dynamic interactions, specifically hydrogen bonding and ionic interactions, with the carboxyl and hydroxyl groups present on poly(acrylic acid-co-hydroxyethyl methacrylate) (PAA-co-HEMA) chains during a redox-initiated in situ gelation process. These reversible, non-covalent interactions enabled the NPs to act as dynamic crosslinking nodes, facilitating the formation of a physically crosslinked, interpenetrating network with enhanced mechanical robustness and tunable swelling behavior. In addition to their role as dynamic crosslinkers, the intrinsic magnetic properties of the NPs endowed the hydrogel with dual stimuli-responsiveness. The system exhibited pH-sensitive swelling and magnetically controlled, diffusion-mediated drug release, demonstrating its promise as a remotely triggered drug delivery platform.
Xue et al. developed superparamagnetic hydrogels by incorporating polyethylene glycol/polyethyleneimine-functionalized superparamagnetic iron oxide nanoparticles (PEG/PEI-SPIONs) and aptamer-modified palladium-hydrogen nanozymes (PdH-Apt) into a double-network polyacrylamide/hyaluronic acid (PAAm/HA) hydrogel.60 The surface of the pristine SPIONs was modified through a covalent epoxide ring-opening reaction between their surface hydroxyl groups and the epoxy termini of PEG and PEI chains. This functionalization created a stable ether linkage (Fe–O–C) and presented a dense brush of reactive amine and hydroxyl groups on the NP surface. These newly introduced functional groups enabled dual covalent bonding mechanisms within the hydrogel matrix. Specifically, the amine groups of SPIONs formed imine bonds with aldehyde groups in PAAm and HA, while the hydroxyl groups participated in esterification reactions with carboxylic acid groups in the polymers (Fig. 11(a)). PdH-Apt NPs were synthesized by hydrogen adsorption onto Pd NPs, which enhanced their catalytic properties, and subsequently modified with aptamers for specific targeting capabilities. These PdH-Apt NPs interacted with the hydrogel network through weak physical crosslinking, including hydrogen bonding and electrostatic attraction with HA and PAAm polymers. The dual incorporation of PEG/PEI-SPIONs and PdH-Apt provided distinct advantages. The PEG/PEI-SPIONs conferred superparamagnetic properties, enabling precise control over hydrogel behavior using an external magnetic field. This allowed for dynamic mechanical stimulation, which facilitated cell recruitment, cytoskeletal remodeling, and differentiation. On the other hand, the PdH-Apt NPs provided catalytic activity for ROS scavenging and anti-inflammatory effects, promoting a favorable microenvironment for cell survival and disc regeneration (Fig. 11(b) and (c)).
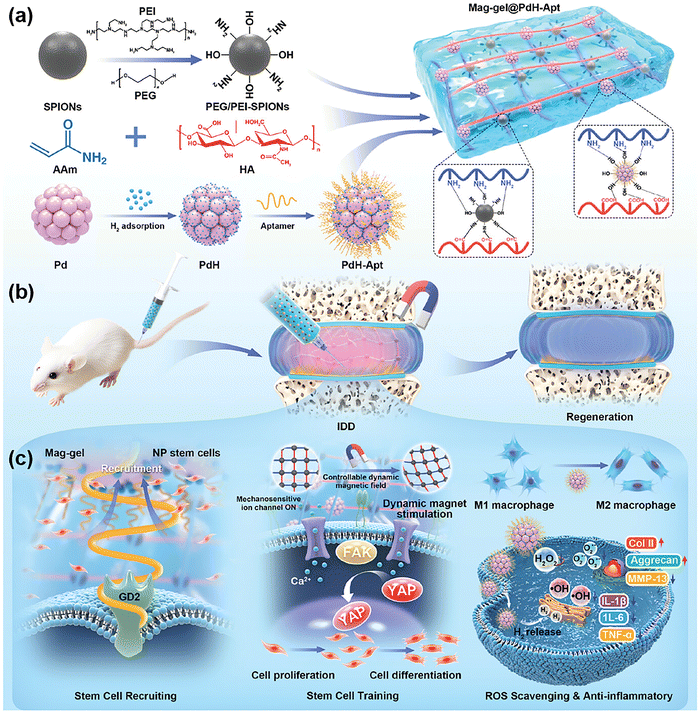 | ||
| Fig. 11 (a) Fabrication of superparamagnetic hydrogel. (b) Hydrogel is transplanted into the degenerated symptomatic intervertebral disc (IVD), and then stimulated with a magnetic field to promote NP regeneration. (c) Magnetotactic hydrogel promotes NP repair by recruiting endogenous stem cells, initiating stem cell training, and suppressing ROS and inflammation. Reproduced with permission from ref. 60 Copyright 2024, Wiley. | ||
Wu et al. introduced reactive amine groups to the surface of IOPs through the modification of PEI.70 These modified NPs became uniformly embedded within a hydrogel matrix formed through reversible inclusion complexation between PEG chains on nanocapsules and α-cyclodextrin, where they functioned as physical crosslinkers. This incorporation not only reinforced the mechanical integrity and thermo-responsiveness of the hydrogel during its temperature-triggered gel–sol transition but also imparted magnetic properties and peroxidase-like catalytic activity. Consequently, the resulting multifunctional magnetic hydrogel showed promise for synergistic tumor therapy by combining mild hyperthermia with ROS generation for effective cancer ablation.
In a complementary approach, Li et al. designed self-healing hydrogels using catechol-terminated 4-arm polyethylene glycol (4cPEG) and linear monofunctionalized polyethylene glycol carboxylic acid (mPEG-COOH)-stabilized IOPs.65 In this system, the catechol groups displace the linear mPEG-COOH on the IOP surfaces to form reversible metal–catechol coordination bonds, effectively serving as dynamic crosslinking nodes within the hydrogel matrix. This unique interfacial chemistry imparts the gel with reversible and self-healing mechanical properties while leveraging the inherent magnetic response of IOPs for remote control of its mechanics. Such multifunctional hydrogels are promising for soft tissue implants and other stimuli-responsive biomedical devices.
Maghsoudian et al. doped TiO2 NPs with copper to result in the formation of surface-exposed cupric oxide (CuO) moieties.72 These TiO2 NPs enhanced catalytic activity by inhibiting electron–hole recombination and boosting ROS production while reinforcing the hydrogel network. The CuO moieties form reversible coordination bonds with electron-donating groups (e.g., amino and hydroxyl groups) on chitosan. Complemented by hydrogen bonding and electrostatic forces, these interactions substantially strengthen the structure of the hydrogel. Consequently, the copper-doped TiO2 NPs acted as multifunctional crosslinkers within a chitosan/β-glycerophosphate hydrogel, enhancing its mechanical integrity and therapeutic efficacy in localized breast cancer treatment.
Yue et al. modified TiO2 NPs by dispersing them onto the surface of 2,2,6,6-tetramethylpiperidine-1-oxyl-oxidized chitin nanofibers (TOCN).73 This modification stabilized the TiO2 NPs in suspension and primed them for effective crosslinking within the hydrogel matrix. The interfacial chemistry involved a condensation reaction where the hydroxyl groups on the TiO2 surface reacted with the carboxyl groups on the TOCN, forming stable Ti–O–C covalent bonds. Upon incorporation into the PAAm network, these Ti–O–C bonds, supplemented by hydrogen bonding, integrated the NPs as covalent crosslinking points, and thus enhanced the mechanical strength and recyclability of the hydrogel for efficient photocatalytic degradation.
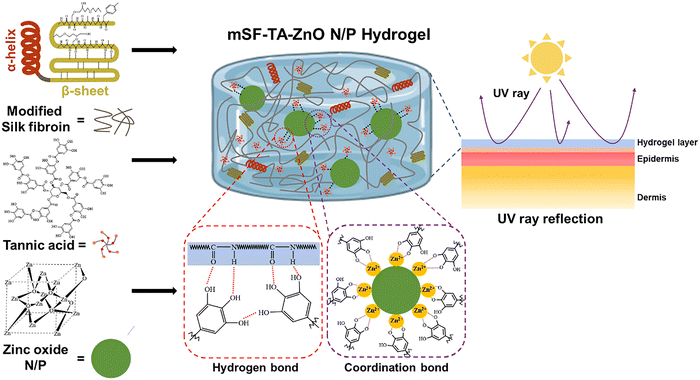
Yang et al. developed a multifunctional hydrogel sunscreen by incorporating modified silk fibroin (mSF), tannic acid (TA), and ZnO NPs into a composite matrix.76 The crosslinking mechanism involved coordination chemistry, where the oxygen atoms fromed the phenolic hydroxyl groups of TA donate electron pairs to the zinc cations on the nanoparticle surface. This process formed stable coordination bonds that enhanced the mechanical stability and UV-blocking capabilities of the hydrogel. The mSF–TA–ZnO hydrogels exhibited UV protection, antibacterial, and antioxidant properties. UV-Vis transmittance analysis confirmed superior UV-blocking performance in both UVA (350 nm) and UVB (300 nm) regions (Fig. 12), with high transmittance in the visible region, avoiding whitening effects commonly observed in commercial sunscreens. Skin irritation tests verified excellent biocompatibility, as the hydrogel exhibited minimal irritation across the tested time points.
Masud et al. demonstrated that ZnO NPs acted as effective crosslinkers in a chitosan/polyethylene glycol NC hydrogel.74 Due to their inherently negative surface charge, these NPs formed ionic bonds with the protonated amino groups of chitosan. Additionally, hydrogen bonding between PEG and chitosan provided further stabilization, resulting in a flexible, porous, and antimicrobial hydrogel structure. This bio-nanocomposite exhibited promising wound healing applications, combining controlled drug release and enhanced antibacterial activity.
3.3 Carbon-based nanomaterials in DNCHs
Carbon-based nanomaterials (e.g., graphene and its derivatives (graphene oxide (GO), reduced graphene oxide), carbon nanotubes (CNTs), carbon quantum dots (CQDs), and fullerenes) have proven to be highly versatile functional nanofillers for DNCHs. Their exceptionally high specific surface area and inherent mechanical robustness can be further tailored through surface functionalization (e.g., –NH2, –COOH, or –SH groups) to enhance compatibility with hydrogel matrices and promote strong interfacial crosslinking.176 In addition, the intrinsic electrical conductivity and unique optical properties of these carbon nanostructures enable multifunctional reactions, such as conductivity,177 biosensing,178 and photothermal conversion,179 within the hydrogel network. Grafting polymer segments or bioactive ligands onto the carbon surface using chemical or physical methods minimizes aggregation, improves dispersion, as well as introduces dynamic and reversible crosslinking motifs.180 This synergy not only strengthens the mechanical properties of the hydrogel but also imparts smart behaviors (i.e., self-healing, network reconfiguration, and stimulus-driven payload release), making carbon-based nanomaterials indispensable for developing high-performance, adaptive dynamic hydrogels.Lu et al. reported the microstructural and mechanical properties of dynamic covalently crosslinked polydextran aldehyde/carbon dot (PDA@CD) hydrogels synthesized via Schiff base reactions between amine-functionalized CDs and PDA with different degrees of oxidation (PDA10, PDA30, and PDA50) (Fig. 13(a)).80 In this system, dextran was first oxidized by sodium periodate to introduce aldehyde groups, yielding PDA chains with tunable oxidation levels. Amine-functionalized CDs, synthesized through silane-mediated surface modification, provided abundant NH2 terminal groups that could undergo Schiff base condensation with the aldehydes of PDA. The hydrogel networks were obtained simply by mixing aqueous PDA and CD solutions, where the degree of PDA oxidation dictated the density of dynamic crosslinking points. Such a design highlighted the critical role of nanoparticle functionalization in enabling CDs to act not only as nanofillers but also as dynamic covalent crosslinkers in DNCHs. The lyophilized hydrogels presented clearly differentiated porous architectures depending on the degree of PDA oxidation (Fig. 13(b)). The PDA10@CD hydrogel had the largest pores (62.30 ± 31.99 µm), while the PDA50@CD hydrogel had the smallest average pore diameter (35.07 ± 8.94 µm). It was noteworthy that the compressive modulus was related to the degree of oxidation, showing the PDA50@CD hydrogel exhibited a high modulus (37.65 ± 6.06 kPa), which exceeded the values of PDA30@CD (25.42 ± 5.50 kPa) and PDA10@CD (5.28 ± 2.56 kPa) (Fig. 13(c)). Moreover, representative images of three-dimensional printed PDA@CD hydrogels highlighted the versatile processing potential of these materials, clearly demonstrating their excellent self-supporting capability and stability during advanced biofabrication processes (Fig. 13(d)). Electrospinning of the composite hydrogels was performed with a polyethylene oxide (PEO)-based system containing CD and PDA30. The resulting microfibrous scaffolds exhibited a uniform morphology with an average fiber diameter of 4.47 ± 0.95 µm (Fig. 13(e)). Overall, these quantitative results clearly show that controlling the degree of oxidation of PDA effectively modulated both the microstructural properties and mechanical performance of dynamic, covalently cross-linked PDA@CD hydrogels.
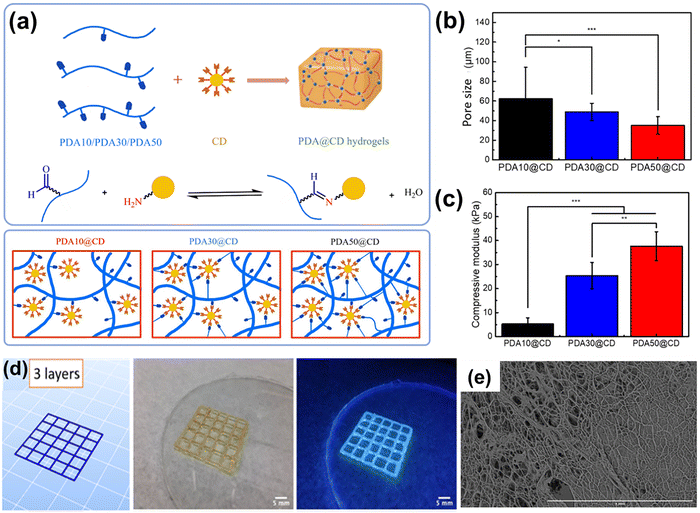 | ||
| Fig. 13 (a) Schematic illustrations of PDA@CD hydrogels with different oxidation degrees and molecular weights were constructed by forming dynamic covalent bonds. (b) Pore size and (c) compressive modulus of PDA@CD hydrogels. (d) 3D-printed structures using PDA30@CD hydrogels. (e) SEM image of PEO/PDA30@CD microfibrous scaffolds. Reproduced with permission from ref. 80 Copyright 2022, American Chemical Society. | ||
Liu et al. designed a multifunctional conductive hydrogel using polydopamine-coated carbon nanotubes (polydopamine@CNTs) to interact with the matrix of sulfobetaine methacrylate (SBMA), acrylamide (AM), and dodecyl quaternary ammonium salt (Q12).83 In this system, CNTs were first dispersed and functionalized by in situ polymerization of dopamine, which formed a uniform polydopamine coating on their graphitic surface. The polydopamine shell introduced catechol and secondary amine groups, improving CNT hydrophilicity and creating abundant interfacial binding sites. During free radical polymerization of SBMA, AM, and Q12 in aqueous solution, the polydopamine@CNTs served not only as conductive fillers but also as dynamic crosslinkers. They established multiple reversible interactions with the surrounding polymer chains, including hydrogen bonding between catechol/amide groups, ionic bonds, π–π stacking between polydopamine and CNT backbones, and cation–π interactions with Q12. This rich interfacial chemistry ensured homogeneous CNT dispersion, efficient load transfer, and continuous conductive pathways, thereby substantially enhancing the hydrogel's mechanical strength, stretchability, adhesion, and electrical conductivity. (Fig. 14). Upon NIR exposure, the hydrogel demonstrated potent antibacterial efficacy, achieving 98.47% and 94.32% reduction in viable Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) cells, respectively. In addition to antimicrobial function, the hydrogel exhibited excellent strain-sensing capability, with a highly repeatable and linear resistance response over a broad tensile strain range (10–300%). It could distinguish subtle versus large-scale deformation, with fast response time and minimal signal lag during cyclic loading. Real-time monitoring of biomechanical activities (i.e., finger bending, wrist motion, and elbow extension) was also demonstrated, highlighting its practical potential in wearable electronics. Taken together, the combination of dynamic bonding architecture, robust photothermal effects, and biosensing performance positions this hydrogel as a promising platform for wearable biomedical devices and smart wound care.
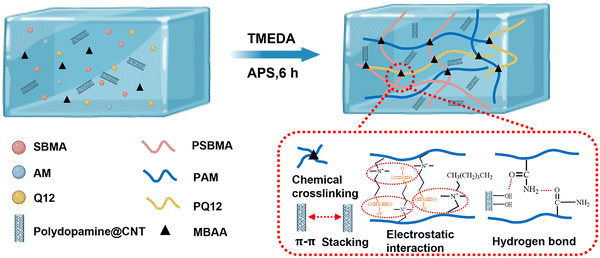 | ||
| Fig. 14 Illustration of the hydrogel formation process, detailing the multi-interaction mechanisms within the hydrogel matrix. | ||
Li et al. developed a multi-responsive hydrogel using alginate dialdehyde (ADA), PEI, PNIPAM, and phenylboronic acid-modified polyethyleneimine (PBA–PEI) functionalized multi-walled carbon nanotubes (PP–CNT) (Fig. 15(a)).82 In this system, multi-walled CNTs were first oxidized and subsequently functionalized with PBA–PEI, providing abundant amine and boronic acid groups on their surface. These functional groups enabled CNTs to engage in dual dynamic interactions: imine bonds with the aldehyde groups of ADA, and boronate ester linkages with diols in ADA. The presence of PNIPAM further introduced thermoresponsive behavior into the hydrogel network. Hydrogel formation was achieved by mixing ADA, PEI, PNIPAM, and PP–CNTs in aqueous solution, where the PP–CNTs acted as both crosslinking nodes and conductive nanofillers. In addition to these dynamic covalent linkages, Li et al. also engineered alginate/PNIPAM hydrogels reinforced with CNTs functionalized via amidation, forming amide (–CO–NH–) and hydrogen-bond interactions between the carboxyl groups of CNTs and the hydroxyls of alginate. These additional covalent and noncovalent linkages further enhanced interfacial load transfer and dissipative energy storage within the hydrogel network. As a result, the compressive modulus increased from 41.2 ± 3.5 kPa to 156.7 ± 7.2 kPa, while the fracture strain expanded by 1.8-fold, demonstrating the synergistic reinforcement effect of surface-functionalized CNTs. The study highlighted that the APN/PP–CNT (prepared with ADA, PEI, PNIPAM, and PP–CNT) hydrogel exhibited significant responsiveness to multiple stimuli, including pH, H2O2, temperature, and second NIR (NIR-II) light. The dynamic covalent bonds and the presence of CNTs provide the hydrogel with enhanced mechanical properties and stability, while also minimizing the leakage of nanomaterials from the hydrogel matrix. Under NIR-II laser irradiation (1064 nm, 1.0 W cm−2), the APN/PP–CNT hydrogel heated very rapidly, from ∼25 °C to ∼60 °C in 5 min (Fig. 15(b)). APN/PP–CNT gels were placed in solution and exposed to 5 min NIR-II pulses, producing clear stepwise increases in neomycin (Neo) release, where the cumulative release with repeated irradiation was much higher than without irradiation after ∼3 h (Fig. 15(c)). In vivo biocompatibility and antibacterial efficacy of the hydrogels were evaluated using a rat spinal implantation model. Hydrogels loaded with Neo were implanted at the lumbar spine and divided into groups with or without NIR-II irradiation. Histological analysis showed no significant inflammatory cell infiltration, confirming good biocompatibility. To assess antibacterial activity, tissues adjacent to the implants were homogenized and subjected to disc diffusion assays against E. coli and S. aureus. The AP/PP–CNT/Neo hydrogel exhibited consistent inhibition zones (∼13 mm) regardless of NIR-II exposure, indicating continuous drug release (Fig. 15(d) and (e)). In contrast, the APN/PP–CNT/Neo hydrogel only showed antibacterial effects after NIR-II irradiation, demonstrating its capability for on-demand, stimulus-triggered drug release. These results highlighted the potential of APN/PP–CNT hydrogel for controlled local therapy with excellent biocompatibility.
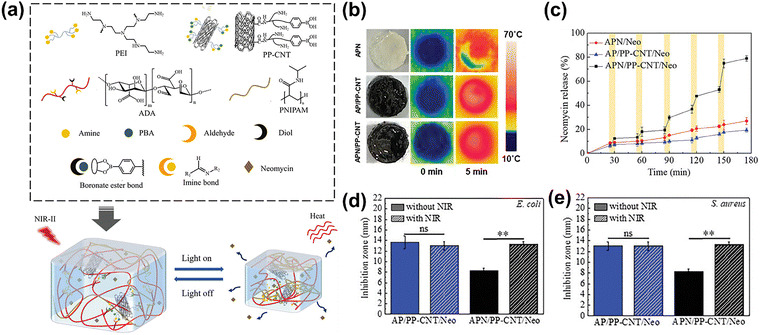 | ||
| Fig. 15 (a) Schematic representation of the APN/PP–CNT NC hydrogel, highlighting its constituent materials, dynamic covalent crosslinks, and NIR-II-triggered drug release mechanism. (b) Temperature elevation of hydrogels under 1064 nm NIR-II irradiation (1.0 W cm−2), demonstrating their photothermal responsiveness. (c) Neo release profiles from hydrogels in phosphate-buffered saline (PBS, pH 7.4), monitored during repeated NIR-II irradiation cycles (5 min on, 25 min off). Quantitative assessment of antibacterial efficacy via inhibition zone measurements against (d) E. coli and (e) S. aureus. Significance was set as p < 0.05, with ** indicating p < 0.01. Reproduced with permission from ref. 82 Copyright 2025, Wiley. | ||
Lin et al. designed a multifunctional antibacterial hydrogel (O@CF@G) synthesized from oxidized dextran (ODex) and caffeic acid-grafted chitosan (CACS), incorporating a tubular nanocomplex of oxidized carbon nanotubes (OCNT) coated with a photocatalytic covalent organic framework (COF) and doped with Fe3+ ions (O@CF).81 O@CF was uniformly embedded into the hydrogel through covalent crosslinking via Schiff base reaction, facilitated by the COF coating. In this system, OCNTs provided a tubular conductive backbone, while the COF shell introduced abundant aldehyde and amine-reactive sites that enabled Schiff base condensation with ODex and CACS. The Fe3+ ions coordinated with catechol groups of CACS, establishing reversible Fe–O and Fe–N coordination bonds that acted as dynamic crosslinking motifs. The hydrogel was fabricated by mixing ODex and CACS with O@CF nanocomplexes under aqueous conditions, where the combination of imine linkages and metal–ligand coordination ensured homogeneous distribution of nanofillers and robust interfacial bonding. The study highlighted that the COF coating on OCNT not only narrowed the energy band gap and increased light absorption, leading to improved ROS generation under light irradiation, but also enhanced the photothermal conversion efficiency, allowing O@CF to effectively heat up to 54.8 °C under 808 nm laser irradiation (Fig. 16). The O@CF@G hydrogel exhibited significant antibacterial activity against both Gram-positive and Gram-negative bacteria, effectively eliminating drug-resistant biofilms. Additionally, it demonstrated pH-dependent enzyme-like activities, contributing to hypoxia alleviation and promoting wound healing. In a diabetic wound model, O@CF@G-treated wounds demonstrated accelerated healing: by day 12, the wound closure rate exceeded 90%, compared to ∼65% in the control group and ∼75% in groups treated with hydrogel lacking O@CF. The adhesion ability of hydrogel to diabetic wounds while providing a protective barrier was particularly beneficial for treating irregularly shaped and large wounds, showing its potential for advanced biomedical applications.
Chen et al. developed a novel antibacterial hydrogel composed of polydextran aldehyde (PDA), gelatin, and gold nanoparticle-decorated graphene oxide (GOAu).7 The hydrogel formation involves dynamic covalent imine bonds between the aldehyde groups of PDA and the amine groups of gelatin, enhancing the structural integrity of the hydrogel (Fig. 17(a)). During hydrogel fabrication, PDA provided abundant aldehyde groups through sodium periodate oxidation of dextran, which reacted with the amine groups of gelatin to form reversible imine crosslinks. Meanwhile, GOAu nanosheets were embedded into the PDA–gelatin matrix, participating in additional noncovalent interactions such as hydrogen bonding and π–π stacking with polymer chains. The integration of GOAu not only reinforced the mechanical strength of the hydrogel but also served as a photothermal responsive agent for NIR-II triggered drug release. Upon 1064 nm NIR-II irradiation (0.75 W cm−2), the hydrogel exhibited a substantial increase in local temperature (up to ∼60 °C within 6 min), which enhanced the release of loaded drug Neo. Upon NIR-II irradiation, the PDA/gel/GOAu4/neo hydrogel with 4 wt% AuGO, exhibited a marked increase in drug release, achieving a cumulative release of 44.6% after three on/off cycles. This enhancement was attributed to the strong photothermal effect of GOAu, where the generated heat induced structural loosening of the gelatin matrix to facilitate Neo diffusion, demonstrating its potential as a remotely controllable drug delivery platform (Fig. 17(b)). The antibacterial efficacy of the hydrogels was quantitatively assessed through zone of inhibition (ZOI) and spotting assays under NIR-II irradiation. PDA/gel/neo hydrogels showed minimal activity, with ZOI diameters of 6.3 ± 0.2 and 6.1 ± 0.2 mm for S. aureus and E. coli, respectively. Incorporation of GO and GOAu significantly enhanced antibacterial performance, where PDA/gel/GO1/neo showed ZOIs of 17.5 ± 0.9 and 13.9 ± 1.0 mm for S. aureus and E. coli, respectively, while PDA/gel/GOAu4/neo reached 19.5 ± 1.2 and 16.5 ± 1.2 mm for S. aureus and E. coli, respectively. Spotting assays further confirmed the chemo–photothermal synergism, with GOAu-containing hydrogels achieving near-complete bacterial eradication under NIR-II exposure (Fig. 17(c)). These results emphasized the importance of photothermal-enhanced drug release in achieving potent antimicrobial effects.
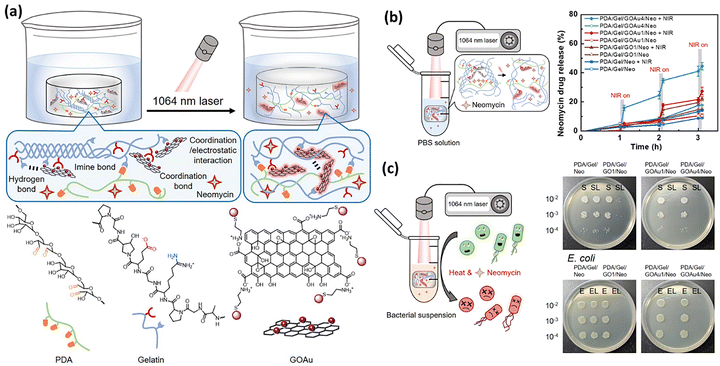 | ||
| Fig. 17 (a) Illustration of the NIR-II-responsive NC hydrogel assembled from PDA, gelatin, and GOAu via dynamic imine bonding and noncovalent interactions (coordination, electrostatic, and hydrogen bonding). (b) Schematic and quantitative data showing light-triggered drug release behavior under NIR-II irradiation (1064 nm, 0.75 W cm−2, 6 min), highlighting enhanced neomycin release. (c) Representation of antibacterial drug release under NIR-II light, with spotting assay results demonstrating the antimicrobial activity of neomycin-loaded hydrogels against S. aureus and E. coli, with and without irradiation (S: S. aureus; E: E. coli; SL/EL: under NIR-II). Reproduced with permission from ref. 39 Copyright 2024, American Chemical Society. | ||
Reza et al. designed a self-healing CSMA/BPEI/BPEI-GO hydrogel utilizing chondroitin sulfate multialdehyde (CSMA) as the polymer matrix and branched polyethylenimine (BPEI) conjugated graphene oxide (BPEI-GO) as the nanomaterial.84 The interactions between the BPEI-GO nanomaterials and the CSMA polymer were primarily facilitated through imine bonds formed via the reaction between the amines of BPEI and the aldehydes of CSMA. Mixing BPEI-GO with CSMA in aqueous conditions spontaneously led to hydrogel formation via dynamic Schiff base reactions between aldehydes and amines. This dynamic covalent chemistry not only enhanced the mechanical properties of the hydrogel but also endowed it with self-healing capabilities (Fig. 18(a)). The study highlights that the hydrogel effectively incorporated doxorubicin (DOX) for sustained drug delivery, achieving a loading efficiency of 60.1%. The release profile demonstrated a higher release rate at acidic pH 6.5 compared to neutral pH 7.4 and basic pH 10.0 conditions, indicating that the hydrogel was tailored for the tumor microenvironment (Fig. 18(b)). Upon NIR irradiation, the implanted hydrogel rapidly increased temperature to 44.5 °C within 1 min, peaking at 53.1 °C by 2 min and remaining stable thereafter (Fig. 18(c)), indicating a rapid and sustained photothermal response. Additionally, the hydrogel exhibited significant anticancer efficacy, with a reduced tumor recurrence rate of 33.3% in vivo when combined with photothermal therapy, compared to 66.7% for DOX-loaded hydrogels without NIR irradiation (Fig. 18(d)). Notably, free DOX induced weight loss in mice, while DOX-hydrogel mitigated this side effect (Fig. 18(e)). The results indicated that the unique structure and properties of hydrogel, derived from the interplay of dynamic covalent interactions and the photothermal effect of GO, position it as a promising candidate for advanced cancer treatment strategies.
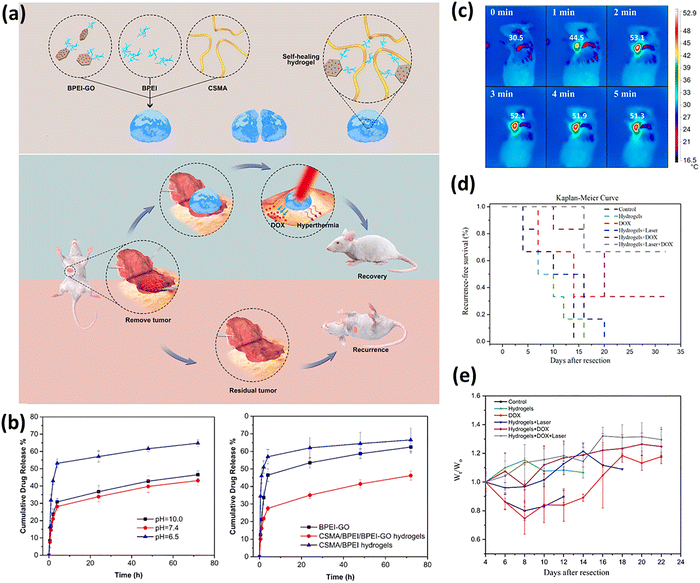 | ||
| Fig. 18 (a) Schematic illustration of CSMA/BPEI/BPEI hydrogel and its application in prevention of locoregional recurrence of breast cancer. (b) DOX release profile of BPEI-GO dispersion at pH of 6.5, 7.4, and 10.0., DOX release profile of BPEI-GO dispersion, CSMA/BPEI hydrogel and CSMA/BPEI/BPEI-GO hydrogel at pH of 6.5. Data are represented as mean ± SD (n = 3). (c) Thermal imaging confirmed rapid in vivo photothermal activation of CSMA/BPEI/BPEI-GO hydrogels under NIR laser exposure. (d) Kaplan–Meier plot showing recurrence-free survival across treatment groups. (e) Body weight during the treatment period. Reproduced with permission from ref. 84 Copyright 2019, American Chemical Society. | ||
Chae et al. developed a novel hydrogel, PVA–CA–MXene, designed for wearable strain sensors, utilizing poly(vinyl alcohol) (PVA) modified with catechol (CA) and Ti3C2Tx MXene as the nanomaterial.85 In this system, PVA was chemically modified with catechol groups to introduce adhesive and metal-coordinating functionalities, while Ti3C2Tx MXene nanosheets, known for their high conductivity but poor oxidative stability, were used as the conductive nanomaterial. The catechol units of PVA–CA formed coordination bonds with surface titanium atoms of MXene, while simultaneously engaging in hydrogen bonding with surface hydroxyl and fluorine groups. This interfacial chemistry effectively passivated MXene against oxidation and ensured homogeneous dispersion in the hydrogel matrix. The hydrogel was prepared by mixing aqueous dispersions of PVA–CA and exfoliated MXene, followed by a mild freezing–thawing process to induce physical crosslinking of PVA chains in combination with catechol–MXene coordination. The interaction The interaction between the catechol groups of the PVA–CA polymer and the multivalent titanium ions on the MXene surface facilitated strong coordination and hydrogen bonding, enhancing the stability and performance of the hydrogel. This study highlighted the significant improvements in oxidation resistance and self-healing capabilities of the PVA–CA–MXene hydrogel compared to traditional PVA–MXene formulations. The catechol modification not only protected the MXene from oxidation but also maintained its electrical and mechanical properties, crucial for effective strain sensing. Key findings indicated that the hydrogel exhibited excellent stretchability which up to 1100% and a gauge factor of 2.3, making it highly sensitive to subtle human motions such as finger bending and throat vibrations. These properties showed that the PVA–CA–MXene hydrogel was a promising candidate for applications in wearable electronics and smart skin technologies, particularly in monitoring real-time physiological signals.
Chiang et al. developed a gelatin/polydextran/LAPONITE® (PG/PDA/Tb3+@Lap) double-network hydrogel incorporating terbium ions (Tb3+) through dynamic covalent (imine bond) and non-covalent bond (coordination bond, hydrogen bond, and electrostatic interactions).86 Polydextran aldehyde (PDA) was obtained by oxidizing dextran, while polyethyleneimine-modified gelatin (PG) was prepared by grafting polyethyleneimine onto gelatin through amide coupling. The imine bond formation between the aldehyde groups of PDA and the amine groups of PG provided the primary crosslinking network. In addition, electrostatic interactions occurred between the negatively charged surface of LAPONITE® (Lap) and the positively charged groups on PG, further enhancing the network stability. Together with Tb3+ coordination, which imparted luminescence properties, these interactions endowed the hydrogel with both mechanical robustness and functional responsiveness. Incorporating terbium-loaded LAPONITE® (Tb3+@Lap) into the PG/PDA hydrogel matrix introduced multiple dynamic linkages (i.e., imine bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), Tb–O coordination, hydrogen bonds, and electrostatic interactions) that acted as multifunctional interfacial crosslinkers. These synergistic interactions significantly enhanced the mechanical performance, increasing the G′ from 408 Pa to 818 Pa and improving compressive stability under cyclic strain. The dynamic coordination and ionic bonding endowed the hydrogel with self-healing and shear-thinning behavior, enabling 3D printability, rapid mechanical recovery, and sustained flexibility. The resulting PG/PDA/Tb3+@Lap hydrogel demonstrated remarkable self-healing and shear-thinning properties, enabling efficient recovery after deformation and facile injectability. Mechanically, the integration of Tb3+@Lap doubled the network stiffness while preserving elasticity and processability, further improving toughness and viscoelastic recovery compared to single-network systems. Moreover, Tb3+ coordination endowed the hydrogel with luminescent sensing capabilities, allowing sensitive copper ion (Cu2+) detection with a limit of detection (LOD) of 1.12 µM. Beyond sensing, the hydrogel's tunable viscoelasticity and luminescence enable multifunctional applications. Electrospinning of the PG/PDA/Tb3+@Lap system yielded nanofibrous scaffolds with enhanced surface area and porosity, advantageous for cell adhesion and tissue regeneration. Furthermore, its shear-thinning property facilitates extrusion-based 3D printing, allowing the fabrication of customized hydrogel architectures for biomedical applications such as wound healing, tissue engineering, and drug delivery.
N), Tb–O coordination, hydrogen bonds, and electrostatic interactions) that acted as multifunctional interfacial crosslinkers. These synergistic interactions significantly enhanced the mechanical performance, increasing the G′ from 408 Pa to 818 Pa and improving compressive stability under cyclic strain. The dynamic coordination and ionic bonding endowed the hydrogel with self-healing and shear-thinning behavior, enabling 3D printability, rapid mechanical recovery, and sustained flexibility. The resulting PG/PDA/Tb3+@Lap hydrogel demonstrated remarkable self-healing and shear-thinning properties, enabling efficient recovery after deformation and facile injectability. Mechanically, the integration of Tb3+@Lap doubled the network stiffness while preserving elasticity and processability, further improving toughness and viscoelastic recovery compared to single-network systems. Moreover, Tb3+ coordination endowed the hydrogel with luminescent sensing capabilities, allowing sensitive copper ion (Cu2+) detection with a limit of detection (LOD) of 1.12 µM. Beyond sensing, the hydrogel's tunable viscoelasticity and luminescence enable multifunctional applications. Electrospinning of the PG/PDA/Tb3+@Lap system yielded nanofibrous scaffolds with enhanced surface area and porosity, advantageous for cell adhesion and tissue regeneration. Furthermore, its shear-thinning property facilitates extrusion-based 3D printing, allowing the fabrication of customized hydrogel architectures for biomedical applications such as wound healing, tissue engineering, and drug delivery.
Furthermore, Zeng et al. developed multifunctional lanthanide-based materials for sensing volatile organic compounds (VOCs).87 A dynamic supramolecular network was constructed by integrating PG, PDA, and europium-coordinated LAPONITE® modified with 2-thenoyltrifluoroacetone (TTA) (Eu3+(TTA)@Lap) (Fig. 19(a)). The resulting PG/PDA/Eu3+(TTA)@Lap hydrogel was also stabilized by multiple dynamic interactions (i.e., imine bonds, hydrogen bonding, electrostatic interactions, and coordination bonds) between the polymeric matrix and the Eu3+ luminophores. Upon excitation at 360 nm, the material exhibited strong red luminescence with sharp emission peaks corresponding to characteristic Eu3+ transitions (Fig. 19(b)). The observed color change from white under daylight to red under UV light confirmed efficient energy transfer from the TTA ligand to Eu3+ ions, demonstrating a pronounced antenna effect that enhances luminescence. The lyophilized hydrogel was exposed to various VOCs to evaluate gas sensing performance, each inducing distinct quenching in luminescence spectra (Fig. 19(c)). These spectral variations reflected selective interactions between VOC molecules and the luminescent matrix. Quantitative analysis of the relative intensity change ((I − I0)/I0) revealed compound-specific response patterns (Fig. 19(d)), with formaldehyde (FA) causing the most significant and distinguishable quenching effect. Linear discriminant analysis (LDA) was further performed on the luminescence data to assess selectivity (Fig. 19(e)). The canonical score plot generated from five luminescence peaks enabled clear separation of FA from the other tested VOCs. Additionally, the lyophilized form of the PG/PDA/Eu3+(TTA)@Lap hydrogel exhibited a remarkably low LOD of 39 ppb. The material maintained its structural integrity and sensing functionality even after physical damage and subsequent self-repair. This multifunctional platform exemplified how dynamic bonding chemistry, combined with lanthanide coordination, can be harnessed to develop injectable, recyclable, and highly sensitive sensors for environmental monitoring and potential biomedical applications.
 | ||
| Fig. 19 (a) A schematic diagram illustrating the components and interactions within the designed hydrogel system. (b) Luminescence spectra of the PG/PDA/Eu3+(TTA)@Lap lyophilized hydrogel. Inset: Photographs of the hydrogel under natural and UV light (365 nm). (c) Luminescence spectra of the PG/PDA/Eu3+(TTA)@Lap lyophilized hydrogel exposed to various gaseous VOCs (λex = 360 nm). (d) Signal patterns showing the relative luminescence intensity change ((I − I0)/I0) of the PG/PDA/Eu3+(TTA)@Lap lyophilized hydrogel in response to different VOCs. (e) Canonical score plot from LDA for gas sensing using the PG/PDA/Eu3+(TTA)@Lap lyophilized hydrogel (n = 6). Reproduced with permission from ref. 87 Copyright 2021, Wiley. | ||
Similarly, Li et al. developed a hydrogel made from polyacrylamide (PAAm), gelatin, and Lap, designed to combine strong mechanical properties with good biocompatibility.92 In this system, the carboxyl and amine groups of gelatin could interact with the amide groups of PAAm through hydrogen bonding and electrostatic interactions, while PAAm simultaneously formed the main polymeric network. The negatively charged LAPONITE® further acted as a physical crosslinker, reinforcing the structure via hydrogen bonding and electrostatic interactions with the polymer chains. Gelatin was also physically embedded in the network, helping to improve cell compatibility and reduce protein adsorption and hemolysis. As a result of this material design, the hydrogel exhibited impressive compressive strength (up to 208 kPa), stretchability, and thermal stability, along with a porous structure suitable for nutrient transport.
In addition, Cimen et al. developed an injectable, self-healing hydrogel designed for localized cancer treatment.95 The hydrogel was formed through reversible hydrazone bonds between gelatin modified with hydrazide groups (Gel-ADH) and PEG modified with aldehyde groups (diBA-PEG), giving it dynamic properties like pH-responsiveness, shear-thinning, and the ability to self-repair. To improve both drug delivery and mechanical strength, they incorporated Lap loaded with doxorubicin (Lap@Dox). Lap played a dual role as a drug carrier and reinforced the network through secondary interactions of hydrogen bonding and electrostatic forces with the gelatin chains. The final hydrogel set quickly (within ∼80 seconds), showed tunable stiffness (storage modulus around 6–12 kPa), and can release the drug in a controllable pH-dependent manner for over 10 days. These combined features made it a strong candidate for long-acting, minimally invasive cancer therapy.
Finally, Zhang et al. developed a chitosan/polyaniline/LAPONITE® (COL) hydrogel using imine bond formation and physical doping.90 Polyaniline (PAI) enhances NIR absorption and photothermal conversion, while oxidized dextran, obtained by oxidizing dextran, bears aldehyde groups that react with the amine groups of chitosan to form dynamic covalent imine bonds, enabling self-healing and degradability. LAPONITE® strengthened hydrogel and promoted osteogenesis. With a swelling ratio over 580%, 28-day degradation above 45%, and 23.7% photothermal efficiency, this hydrogel was promising for tumor photothermal therapy (PTT) and bone regeneration., thereby enabling self-healing and degradability. Lap strengthened the hydrogel and promoted osteogenesis. With a swelling ratio over 580%, 28-day degradation above 45%, and 23.7% photothermal efficiency, this hydrogel was promising for tumor photothermal therapy (PTT) and bone regeneration.
Ma et al. designed an injectable and self-healing hydrogel by crosslinking carboxymethyl chitosan (CMCS) and oxidized alginate–hydroxyapatite nanoparticles (OHAH) via dynamic imine bonds (Fig. 20).98 CMCS, derived from chitosan through carboxymethylation, provided amine groups that react with the aldehyde groups on OHAH. OHAH was prepared by oxidizing alginate in the HAp–alginate composite with NaIO4, introducing the aldehyde groups necessary for imine bond formation. These reversible covalent linkages endowed the hydrogel with excellent self-healing ability, allowing it to rapidly restore its structure after shear-induced disruption at 37 °C. In addition, the hydrogel maintained a porous internal architecture that supported nutrient diffusion and cell adhesion. In vitro culture with L929 fibroblasts demonstrated high cell viability and adhesion, highlighting the biocompatibility of the material and its potential as a scaffold for bone tissue engineering.
Ren et al. developed an injectable and biodegradable polysaccharide-based hydrogel incorporating HAp NPs and calcium carbonate microspheres (CMs) for bone tissue engineering and localized drug delivery.99 The amine groups on CMCS reacted with the aldehyde groups on oxidized alginate (OAlg) to form dynamic imine bonds, generating the hydrogel network with self-healing properties and tunable mechanical strength. HAp and CMs were further integrated into the polymeric network via hydrogen bonding interactions, contributing to the overall structural stability. The presence of HAp improved bioactivity, while CMs enabled the controlled release of tetracycline hydrochloride, providing effective antibacterial function.
Wang et al. developed a cellulose/HAp nanocomposite hydrogel (CHG) by uniformly dispersing HAp NPs within the cellulose hydrogel matrix.101 The hydroxyl groups on cellulose formed hydrogen bonds with the OH groups on HAp, enhancing the interaction between the two components and contributing to the overall structural stability. This incorporation significantly improved the thermal stability and mechanical strength of the hydrogel, with an increase in compressive strength from 100 kPa to 570 kPa. Moreover, the hydrogel demonstrated outstanding heavy metal ion adsorption capacity, with Cu2+ uptake increasing by over 300% compared to pure cellulose hydrogel, and retaining more than 78% of its recovery efficiency after 10 reuse cycles, indicating strong potential for water purification.
Kashimura et al. developed tough Hap-containing hydrogels inspired by the sacrificial bond formation mechanism observed in natural bone tissue.100 By integrating poly(acrylic acid) (PAAc) hydrogels with biomineralized HAp, the hydroxyl groups on HAp formed hydrogen bonds with the carboxyl groups on PAAc, strengthening the interaction between the mineral and polymer network. HAp also facilitated calcium ions (Ca2+) bridging to acidic polymers through five coupled equilibrium reactions, significantly enhancing the mechanical toughness and water stability of the hydrogel. These studies highlighted the versatile applications of HAp-containing hydrogels in bone tissue engineering, drug delivery, and environmental remediation, while also providing insights into novel design strategies for resilient biomaterials.
Zengin et al. developed a nanocomposite system using thiol-functionalized mesoporous silica nanoparticles (MSNs) as dynamic covalent crosslinkers within the PEG matrix to address the persistent challenge of combining mechanical strength with self-healing capabilities in injectable hydrogels.103 By leveraging thiol–disulfide exchange reactions, the authors created hydrogels with significantly enhanced stiffness up to 32 kPa in storage modulus, while maintaining rapid self-healing and injectability. Notably, the dynamic crosslinking strategy resulted in much greater mechanical reinforcement compared to systems where MSNs acted merely as passive fillers. These hydrogels also showed slow, glutathione-triggered degradation and tunable release of both small molecules and proteins, making them promising candidates for drug delivery and tissue regeneration. Furthermore, human mesenchymal stem cells (hMSCs) encapsulated within the hydrogels remained viable and displayed morphology indicative of cell–material interaction, particularly in stiffer gel variants. Overall, this work demonstrated a simple yet effective approach to engineering robust, multifunctional hydrogels suitable for applications in regenerative medicine.
Wu et al. synthesized amine-functionalized silica NPs as gelators to crosslink aldehyde-containing copolymers, which were prepared via free-radical polymerization of 2-methacryloyloxyethyl phosphorylcholine (MPC) and 4-formylbenzoate ethyl methacrylate (FBEMA) (Fig. 21(a)).104 These silica NPs enhanced the mechanical strength and also provided self-healing properties of the hydrogel through imine bond formation (Fig. 21(b) and (c)). The storage modulus of the hydrogels was tunable in a dose-dependent manner, with elasticity nearly doubling when NP concentration increased from 10% to 13%. In addition, the hydrogel demonstrated excellent injectability due to its shear-thinning behavior, allowing it to be smoothly extruded through a 23-gauge needle without clogging (Fig. 21(d)), an essential feature for minimally invasive biomedical applications. The hydrogels also exhibited pH-responsive behavior, where slight pH variations (6.4, 6.6, 6.8, and 7.4) significantly influenced mechanical strength, degradation, and drug release. Release profiles fitted to the Korsmeyer–Peppas model revealed that the drug release rate increases as pH decreases (Fig. 21(e)). At pH 6.4 and 6.6, the release followed near-zero-order kinetics (n ≈ 1), indicating hydrogel erosion-dominated release, while at higher pH (6.8 and 7.4), the release slowed and followed Fickian or anomalous diffusion (n < 0.75), reflecting more stable hydrogel structures. Congo red (Fig. 21(f) and (g)) and BSA-FITC (Fig. 21(h) and (i)) release studies further confirmed the pH-responsiveness and controlled drug delivery capability of the hydrogel, showing faster release rates at lower pH levels, highlighting its potential for self-healing and pH-sensitive drug delivery in tumor and wound microenvironments.
 | ||
| Fig. 21 (a) Schematic illustration of MSN-PEG NC hydrogels were formed via imine bond crosslinking between thiol-functionalized MSNs and PEG polymers. (b) and (c) Visual demonstration of self-healing by showing two hydrogel pieces adhering instantly upon contact. (d) PMF10-S 10–13 hydrogel demonstrated excellent injectability. (e) Drug release data fitted using the Korsmeyer–Peppas model, indicating different release mechanisms depending on pH. (f) Cumulative release profile of Congo red dye from the hydrogel under different pH conditions over 120 hours. (g) Photographs showing the start and end points of Congo red release at pH 6.4–7.4 over 5 days. (h) Cumulative release profile of BSA-FITC from the hydrogel across various pH values at 37 °C. (i) Representative images showing BSA release at the beginning and end of the 120-hour test. Reproduced with permission from ref. 104 Copyright 2020, American Chemical Society. | ||
3.4 Polymer-based nanostructures in DNCHs
Polymer-based nanostructures, such as self-assembled micelles210–212 and polymeric nanoparticles,213–215 are widely used in biomedical and engineering fields. Their small size and tunable polymer composition give rise to key features including core–shell morphology, large surface area, and controllable dimensions. These properties support efficient drug loading and controlled release. Many polymer nanostructures are also responsive to environmental stimuli like pH,216 temperature,217 or redox conditions,218 enabling structural changes or triggered release when needed. Surface functionalization further enhances their versatility by introducing ligands or reactive groups that enable specific targeting or reversible interactions with surrounding polymer matrices. As a result, these nanostructures are not only useful as delivery vehicles but also serve as dynamic components in larger polymer systems, where they can associate and dissociate in response to external cues.Xu et al. investigated a glucose-responsive hydrogel system for diabetic wound healing, focusing on integrating polymeric nanoparticles and dynamic borate bonds to achieve controlled drug release and enhanced tissue regeneration.110 In The materials used in the study include PEI–PBA/insulin polymeric nanoparticles, chitosan–gallic acid (CS–GA), polyethylene glycol diacrylate (PEG–DA), and boronic acid-functionalized components, which provide glucose sensitivity and structural stability (Fig. 22). The dynamic bond employed in this system is the borate ester bond, which allows reversible covalent interactions between boronic acid groups and hydroxyl-containing polymers, ensuring self-healing properties and responsiveness to glucose fluctuations. The hydrogel system offers multiple advantages, such as controlled drug release, improved mechanical stability, enhanced biocompatibility, and modulating ROS levels to promote vascular endothelial growth factor (VEGF) expression and accelerate wound healing.
The hydrogel exhibited a highly porous microarchitecture that facilitated oxygen exchange and cell infiltration, contributing to accelerated tissue regeneration. In diabetic wound models, treatment with the hydrogel resulted in rapid wound closure, with clear improvement observed by day 5 and nearly complete healing by day 20. Blood glucose measurements indicated a sharp reduction within 2 hours of application, with glucose levels maintained near normal for up to 12 hours. In parallel, the system effectively reduced oxidative stress and promoted a regenerative microenvironment by enhancing angiogenic factor expression and shifting cytokine levels toward an anti-inflammatory profile. These results highlight the capacity of hydrogel to integrate metabolic regulation, antioxidation, and immunomodulation into a single platform for improved diabetic wound healing.
Zhu et al. designed a reprogrammable hydrogel using coumarin acrylate (MAEMC) and acrylic acid (AAc) copolymerized within hexadecyltrimethylammonium chloride (CTAC) micelles, where ionic bonds between the negatively charged poly(acrylic acid) (PAAc) and positively charged CTAC surfactants formed robust polyelectrolyte/surfactant complexes (PESCs).117 These ionic crosslinks enhanced mechanical strength and localized coumarin units for efficient reversible photodimerization (365 nm) and photocleavage (254 nm). The team achieved spatially controlled gradient structures by leveraging ionic bonding and photolithography, enabling reversible 3D shape-morphing (e.g., rolls, helices) with pH-responsive reset capability (pH = 3). This dual crosslinking strategy offers a sustainable platform for adaptive soft robotics and biomedical devices. The electrostatic interactions between CTAC micelles and poly(acrylic acid) chains enabled the creation of a highly tunable hydrogel system with remarkable mechanical strength and photoresponsive properties. The system maintained excellent cycling stability and pH-responsive reset capability, with distinct fluorescent contrast between crosslinked domains, demonstrating an effective synergy between ionic and dynamic covalent chemistry for developing robust, reprogrammable soft materials. On the other hand, when micelles are integrated into hydrogels via dynamic covalent interactions, they act as reversible crosslinkers and nanocarriers, enabling simultaneous enhancement of the adaptability, self-healing capacity, and controlled degradability of hydrogels.
Guo et al. developed a multifunctional micelle–hydrogel platform by covalently integrating ROS-responsive polymeric micelles into a dynamic hydrogel matrix through reversible imine bonds.24 In the synthesis, oxidized dextran (ODex) was first prepared by partial periodate oxidation of dextran to introduce aldehyde groups. In parallel, gelatin was aminated to yield N-Gel with abundant free amine groups. Separately, polymeric micelles were fabricated by self-assembly of amphiphilic block copolymers bearing amine-functionalized PEG shells, which encapsulated paeoniflorin (Pf) as the therapeutic payload. These micelles were then mixed with the ODex and N-Gel precursors, where aldehyde–amine condensation spontaneously occurred under mild aqueous conditions, forming imine linkages.
The micelles were then combined with the ODex and N-Gel precursors, allowing spontaneous aldehyde–amine condensation under mild aqueous conditions to form imine linkages. In the gelatin–dextran-based hydrogel system, the incorporation of nano-sized ZnO (nZnO) particles and paeoniflorin-loaded micelles (MIC@Pf) introduced additional dynamic imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) bonds between the amino groups of N-Gel and the aldehyde groups of ODex. These reversible covalent bonds served as supplementary crosslinking sites, enhancing the mechanical stability and responsiveness of the network to the micro-environmental stimuli (e.g., pH and ROS). This one-pot process covalently tethered micelles into the hydrogel matrix, where they functioned both as drug carriers and dynamic crosslinkers. The resulting hydrogel exhibited a high swelling ratio of ∼1400%, indicating excellent water uptake suitable for wound applications (Fig. 23(a)). Under acidic (pH 5) or oxidative (1 mM hydrogen peroxide (H2O2)) conditions, cleavage of the imine bonds led to ∼70% degradation within 12 hours, enabling responsive disassembly and controlled drug release (Fig. 23(b)). The dynamic bonds also conferred rapid self-healing, with severed fragments rejoining within 30 minutes without external stimuli. The hydrogel demonstrated excellent self-healing ability, recovering its structure within seconds after undergoing a strain of up to 200%. Furthermore, the G′ of hydrogel increased significantly from approximately 400 Pa to 900 Pa, indicating enhanced elastic behavior. Moreover, the hydrogel maintained filament shape and structural integrity after extrusion, demonstrating shear-thinning injectability and printability, and the added nanomaterials contributed to mechanical reinforcement while preserving injectability and adaptability, making it suitable for irregular wound sites (Fig. 23(c) and (d)).
N) bonds between the amino groups of N-Gel and the aldehyde groups of ODex. These reversible covalent bonds served as supplementary crosslinking sites, enhancing the mechanical stability and responsiveness of the network to the micro-environmental stimuli (e.g., pH and ROS). This one-pot process covalently tethered micelles into the hydrogel matrix, where they functioned both as drug carriers and dynamic crosslinkers. The resulting hydrogel exhibited a high swelling ratio of ∼1400%, indicating excellent water uptake suitable for wound applications (Fig. 23(a)). Under acidic (pH 5) or oxidative (1 mM hydrogen peroxide (H2O2)) conditions, cleavage of the imine bonds led to ∼70% degradation within 12 hours, enabling responsive disassembly and controlled drug release (Fig. 23(b)). The dynamic bonds also conferred rapid self-healing, with severed fragments rejoining within 30 minutes without external stimuli. The hydrogel demonstrated excellent self-healing ability, recovering its structure within seconds after undergoing a strain of up to 200%. Furthermore, the G′ of hydrogel increased significantly from approximately 400 Pa to 900 Pa, indicating enhanced elastic behavior. Moreover, the hydrogel maintained filament shape and structural integrity after extrusion, demonstrating shear-thinning injectability and printability, and the added nanomaterials contributed to mechanical reinforcement while preserving injectability and adaptability, making it suitable for irregular wound sites (Fig. 23(c) and (d)).
 | ||
| Fig. 23 Concept for the fabrication of nZnO- and MIC@Pf-loaded hydrogels and the potential mechanisms of facilitating wound–healing processes. (a) Hydrogel fabrication strategy and (b) smart activities of the hydrogels in an inflammatory environment and corresponding tissue regeneration. (c) Self-healing, (d) injectability of hydrogels, and (e) in vivo evaluation of the healing effect of hydrogels. Reproduced with permission from ref. 24 Copyright 2022, American Chemical Society. | ||
In vivo wound healing studies showed that the hydrogel containing both nZnO and MIC@Pf achieved the most effective therapeutic outcome, with nearly complete re-epithelialization and hair regrowth by day 14. In contrast, hydrogels containing only nZnO or MIC@Pf alone resulted in slower recovery and less tissue regeneration, highlighting the synergistic effect of the dual-loaded system and the importance of dynamic micelle–hydrogel integration (Fig. 23(e)). This design underscored how imine-bonded polymeric micelles enhance not only controlled release and responsiveness but also significantly accelerate functional tissue repair.
Zhang et al. developed an injectable pH/thermal dual-responsive nano hydrogel composite for localized chemo-photothermal therapy, leveraging dynamic imine bonds formed between aldehyde-terminated Pluronic F127 (AF127) micelles and amino groups of carboxymethyl chitosan (CMCS) to enable reversible sol–gel transition and pH-sensitive drug release.112 The hydrogel incorporated indocyanine green (ICG)-loaded AF127 micelles for photothermal conversion and ROS generation under 808 nm laser irradiation, along with doxorubicin-encapsulated CMCS NPs (NP-DOX) (Fig. 24(a)). The composite demonstrated significant ROS production in tumor cells upon irradiation, amplifying oxidative stress to enhance therapeutic efficacy. The dynamic network between AF127 and CMCS demonstrated dual-responsive therapeutic precision through dynamic bond behavior. Photothermal stability was achieved via the robust covalent framework, which maintained structural integrity during repeated 808 nm laser exposure, enabling sustained ICG-mediated hyperthermia (Fig. 24(b)). Simultaneously, pH-triggered cleavage of imine bonds in acidic tumor microenvironments accelerated hydrogel degradation, releasing 93% of encapsulated DOX over 72 h compared to 48% at physiological pH (Fig. 24(c) and (d)). This pH-selective release mechanism ensured localized chemotherapy while minimizing systemic toxicity. In vivo validation demonstrated synergistic efficacy through reversible imine bonds, reducing tumor volume from 159 mm3 to 101 mm3via stable photothermal ablation and pH-responsive drug release (Fig. 24(e) and (f)).
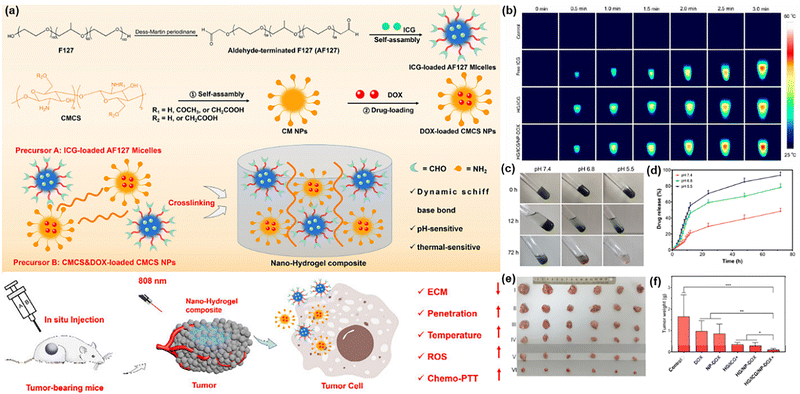 | ||
| Fig. 24 (a) Synthesis of precursor A and precursor B, as well as the preparation of nanoparticle–hydrogel composite. (b) Representative NIR thermal images of saline, free ICG, HG/ICG, and HG/ICG/NP-DOX under 808 nm laser irradiation (2 W cm−2). (c) Changes of the HG/ICG/NP-DOX (AF75/CM15) during drug release. (d) DOX release profiles from HG/ICG/NP-DOX (AF75/CM15) at different pH values. (e) Images of tumor tissues at the end of the treatment: control (I), DOX (II), NP-DOX (III), HG/ICG+ (IV), HG/NP-DOX (V), and HG/ICG/NP-DOX+ (VI). (f) Tumor weight at the end of the treatment, with * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001. Reproduced with permission from ref. 112 Copyright 2023, American Chemical Society. | ||
3.5 MOF in DNCHs
Metal–organic frameworks (MOFs), which are crystalline networks of metal nodes and organic linkers, are characterized by ultra-high surface areas and precisely tunable pore sizes and chemical properties.222,223 When incorporated into hydrogels, their rigid scaffolds serve as nanoscale reinforcements that significantly enhance stiffness, toughness, and fatigue resistance, while their porous channels accommodate and protect bioactive payloads or catalysts. The coordinative nature of the metal–ligand bonds enables MOFs to function as dynamic crosslinkers by forming reversible bonds with polymer side chains for self-healing and shear-free recovery. Collectively, these properties render MOF-enhanced hydrogels powerful platforms for smart drug delivery,224,225 biosensing,226,227 and catalytic microreactors.228,229Reza et al. reported a novel antibacterial spray-filming hydrogel (O-Alg/Ag-MOF/BX) synthesized from oxidized alginate (O-Alg), a silver-based MOF (Ag-MOF), and borax (BX) (Fig. 25(a)).121 In this study, imine bonds were generated by the reaction between the amines of Ag-MOF and the aldehydes of O-Alg, resulting in dynamic covalent bonds that contribute to the structural conformability of the hydrogel. In addition, boron ester bonds were formed between borax and the hydroxyl groups present in the alginate, which further increased the stability of the network. The hydrogel was prepared using an in situ process, in which amine-functionalized Ag-MOF precursors were sprayed together with O-Alg, enabling rapid formation of Schiff-base linkages and boronate ester crosslinks within 5–30 s. The Ag-MOF nanoparticles, with surface amine groups and porous frameworks, were crucial for promoting uniform dispersion and providing sustained silver ion release. The aldehyde and hydroxyl functionalities introduced on the O-Alg backbone provided multiple reactive sites, ensuring effective interfacial chemistry with both borax and Ag-MOF. This dual crosslinking strategy significantly improved the mechanical properties of hydrogel. The tensile strength increased from 0.47 ± 0.05 MPa in the Alg/BX system to 1.12 ± 0.08 MPa in the O-Alg/Ag-MOF/BX formulation, while the elongation at break improved from 58 ± 4% to 103 ± 6%, reflecting enhanced stretchability and ductility. SEM and atomic force microscope (AFM) analyses revealed a porous, wrinkled morphology, which enhanced adhesion and fluid absorption at the wound interface. The hydrogel demonstrated rapid film formation, significant antibacterial efficacy, and excellent biocompatibility, making it particularly suitable for treating irregular and large wounds. It effectively inhibited bacterial proliferation and provided a protective barrier for up to 12 hours (Fig. 25(b)).
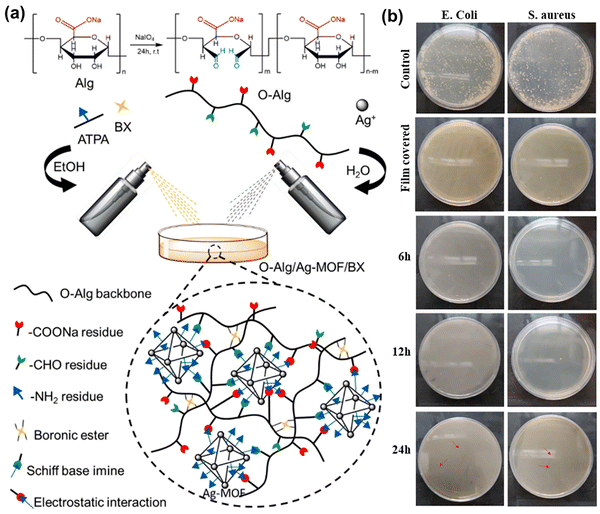 | ||
| Fig. 25 (a) Schematic of partially oxidized sodium alginate and the preparation of the O-Alg/Ag-MOF/BX hydrogel film through in situ forming Ag-MOF, electrostatic interaction, boronic ester, and Schiff base reactions. (b) Photographs of E. coli and S. aureus colonies formed on agar covered with O-Alg/Ag-MOF/BX hydrogel film after 6, 12, and 24 hours, and agar with no film covering was used as a control. Reproduced with permission from ref. 121 Copyright 2025, Elsevier. | ||
Fan et al. developed porous hydrogels based on MOF with self-healing, anti-inflammatory, and antibacterial properties for the repair of infected wounds.122 In their synthesis, the amino-functionalized UiO-66-NH2 MOF was first obtained by a solvothermal route and subsequently loaded with copper nanoparticles via ion exchange, followed by curcumin encapsulation through adsorption within the porous framework. The abundant amino groups on the MOF surface can stabilize Cu nanoparticle deposition and also provide reactive sites for imine bond formation with oxidized sodium alginate (OSA), ensuring homogeneous dispersion in the hydrogel matrix. This interfacial chemistry, involving dynamic Schiff base reactions between MOF–NH2/CMCS–NH2 and OSA–CHO groups, established reversible covalent crosslinks, reinforcing the hydrogel network while maintaining self-healing capacity. These hydrogels use dynamic imine bonds formed between the amines of carboxymethyl chitosan (CMCS) and the aldehydes of oxidized sodium alginate (OSA). In addition, the chemically stable MOF also forms imine bonds with OSA (Fig. 26(a)). Copper nanoparticles (Cu NPs) and curcumin are physically adsorbed within the MOF, enabling their controlled release (Fig. 26(b)). In addition, the viscosity of CMCS-OSA decreases with increasing shear rate, indicating a shear-thinning property (Fig. 26(c)). Consequently, the Cur@Cu-MOF20/CMCS-OSA hydrogel can be extruded smoothly and forms a stable gel again after the shear force is removed. It also adhered well to curved or twisted porcine skin (Fig. 26(e) and (f)). While Cur@Cu-MOF slightly lowered adhesion strength by consuming the aldehyde groups of OSA, the value remained comparable to previous reports, demonstrating reduced bacterial colonies in the CMCS-OSA group due to the positive charge of CMCS. Co-incubation with Cu-MOF/CMCS-OSA and Cur@Cu-MOF/CMCS-OSA hydrogels resulted in significantly fewer colonies (p < 0.001 for S. aureus, p < 0.05 for E. coli) (Fig. 26(i) and (j)). The inhibition rates against S. aureus and E. coli confirmed the improved antibacterial effect of Cur@Cu-MOF through the release of Cu2+ ions and positive charge effects. In addition, biofilm formation was effectively inhibited by the Cu-MOF/CMCS-OSA and Cur@Cu-MOF/CMCS-OSA hydrogels (Fig. 26(k) and (l)), highlighting their potential to prevent recurrent infections.
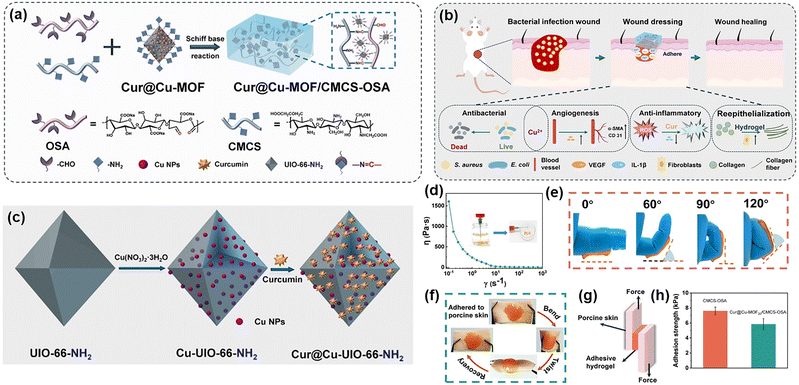 | ||
| Fig. 26 Schematic illustration of (a) the synthesis process of Cur@Cu-MOF/CMCS-OSA composite hydrogel and (b) its use for infected wound healing. (c) Synthetic route for Cur@Cu-UIO-66-NH2. (d) Shear-thinning behavior of the Cur@Cu-MOF20/CMCS-OSA hydrogel. Dynamic adhesion behaviors of the Cur@Cu-MOF20/CMCS-OSA hydrogel to (e) finger and (f) porcine skin. (g) Schematic illustration of the lap-shear measurement of hydrogels to porcine skin. (h) Adhesion strength of the Cur@Cu-MOF20/CMCS-OSA hydrogel. Reproduced with permission from ref. 122 Copyright 2024, Elsevier. | ||
Zheng et al. reported a multifunctional PVA/B/CMC/ZIF-8@Flu hydrogel consisting of fluorescein-functionalized ZIF-8, polyvinyl alcohol (PVA), carboxymethylcellulose (CMC), and borax (Fig. 27).123 ZIF-8@Flu was synthesized by a simple one-pot synthesis, during which fluorescein molecules became primarily anchored on the surface of ZIF-8 NPs through intermolecular forces and π–π interactions. The one-pot process involved mixing zinc nitrate, 2-methylimidazole, and fluorescein in aqueous solution, where rapid coordination between Zn2+ and 2-methylimidazole generated ZIF-8 crystals, while fluorescein molecules were simultaneously immobilized at the external surface through electrostatic attraction and aromatic π–π stacking. The resulting NPs were uniformly incorporated into the PVA/CMC matrix, where a combination of crosslinking mechanisms reinforced the three-dimensional hydrogel network. Hydrogen bonds between the hydroxyl groups of PVA and the carboxymethyl groups of CMC significantly improved the mechanical properties and structural stability of the hydrogel. In addition, borax-induced borate ester bonds created a reversible crosslinking network that was essential for self-healing. Moreover, the zinc ions from the ZIF-8 scaffold coordinated with the hydroxyl groups in PVA and CMC, further increasing mechanical strength and antibacterial activity. Notably, the surface-anchored fluorescein endowed ZIF-8 with additional functional groups (OH and COOH) that served as hydrogen-bond donors/acceptors, enhancing dispersion stability within the hydrogel matrix. These surface groups also participated in secondary interactions with polymeric hydroxyls and carboxylates, contributing to a more integrated interfacial network. This was consistent with findings from related studies on MOF polymer composites. As a result, the hydrogel exhibited stretchability, self-healing, multi-stimulus responsive fluorescence, shape memory, antibacterial activity, and water resistance. The ZIF-8@Flu component also acted as a physical spacer to prevent fluorescein aggregation, ensuring strong and stable fluorescence performance. The integration of fluorescent materials into the MOF structure enabled the hydrogel to emit fluorescence under UV light. In particular, the hydrogel reached a fluorescence maximum at 520 nm under 325 nm excitation and maintains an excitation-independent profile similar to that of ZIF-8@Flu. Immersion tests in solutions of different metal ions (0.01 M) showed that most ions have negligible effects, while Cu2+ significantly quenches the fluorescence. It was noteworthy that interfering ions have only a minimal effect on the quenching efficiency of Cu2+. Subsequent treatment with a L-cysteine (L-Cys) solution (0.1 M) restored fluorescence. These reversible changes were also illustrated by a CIE color chart from 1931, which showed that the untreated hydrogel emitted green light under 365 nm UV light, treatment with Cu2+ shifted the color, and treatment with L-Cys restored the original color, which was confirmed by photographic evidence.
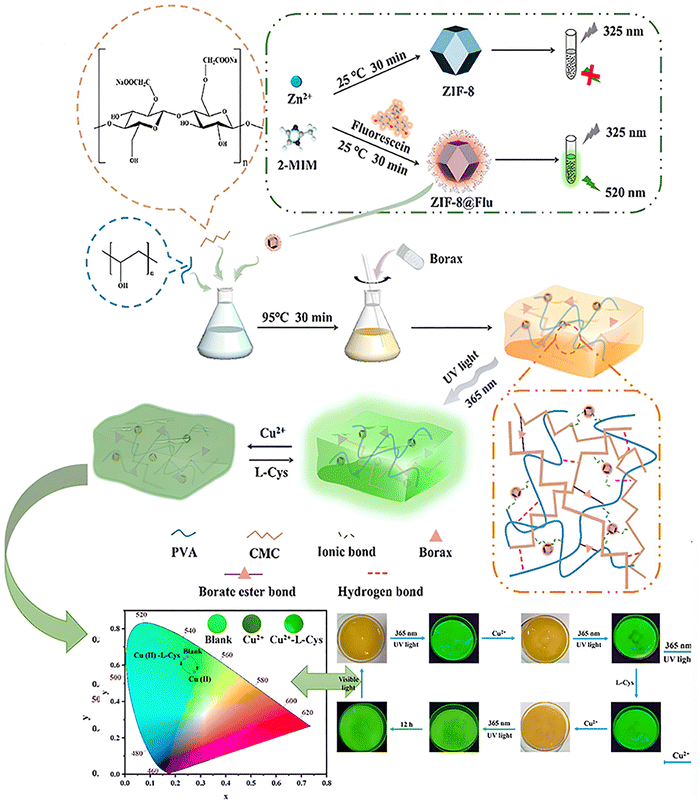 | ||
| Fig. 27 Schematic representation of the synthesis of ZIF-8@Flu and PVA/B/CMC/ZIF-8@Flu hydrogel. Fluorescence property of PVA/B/CMC/ZIF-8@Flu. Reproduced with permission from ref. 123 Copyright 2025, Elsevier. | ||
4. Biomedical applications of DNCHs
DNCHs have enabled a variety of biomedical and tissue engineering applications by combining the intrinsic bioactivity of nanomaterials with reversible crosslinking networks. Representative DNCH systems are summarized in Table 4, which covers Sections 3.1–3.6, and are specifically designed for drug delivery, antibacterial therapy, tissue repair (including wound healing), or biosensing. These examples illustrate how thoughtful choice of nanomaterial, polymer matrix, and interfacial chemistry yields multifunctional hydrogels with enhanced therapeutic performance. Overall, these DNCH systems demonstrate the versatility of nanomaterial–polymer interactions for biomedical applications. By tailoring interfacial chemistry and crosslinking dynamics, researchers have achieved smart hydrogels that actively participate in therapeutic processes while maintaining biocompatibility and mechanical integrity.| Nanomaterial types | Hydrogel formulation | Interfacial bonding | Biomedical application | Quantitative performance | Ref. |
|---|---|---|---|---|---|
| AuNPs (MoS2@Au@BSA) | ODex and glycol chitosan injectable hydrogel | Schiff base links; dual imine networks | Diabetic infected wound healing | Complete wound closure in ∼2 weeks; strong antibacterial activity | 53 |
| AgNPs (curcumin–Ag) | CNF/PVA borate-crosslinked hydrogel | Coordination bond, borate ester bond, and hydrogen bond | Diabetic wound healing (antibacterial) | ∼14-day wound closure; increased vascularization and collagen deposition | 55 |
| PtNPs (PEG–amine coated) | ODex and GelMA DN hydrogel | Schiff base (Pt–PEG–NH2 to ODex/GelMA) | Chronic wound therapy (diabetic) | >90% wound closure vs ∼65% control; O2 generation alleviated wound hypoxia | 61 |
| Fe3O4 (IOP–NH2) | Gel/PDA DN hydrogel | Imine bond and ionic interaction | Magnetothermal drug delivery | Accelerated BSA release at pH 5/45 °C; magnetic field responsive | 67 |
| CNTs (PBA–PEI MWCNT) | ADA/PEI/PNIPAM hydrogel | Dual dynamic (imine bond and borate ester bond) | NIR-triggered antibacterial drug delivery | ∼60 °C heating; 3× higher release with NIR vs without NIR | 82 |
| GOAu | PDA/gel hydrogel | Imine bond, π–π interaction, and hydrogen bond | NIR-II on-demand drug release (antibacterial) | ∼60 °C heating in 6 min; 44.6% release after 3 cycles; 19 mm ZOI (S. aureus) | 39 |
| BPEI–GO | CSMA hydrogel | Schiff base (BPEI–GO amine to CSMA aldehyde) | Combined chemo-photothermal therapy | DOX loading ∼60%; 53 °C in 2 min; tumor recurrence decreased to 33% | 84 |
| MXene (Ti3C2Tx) | Polymeric hydrogel (dynamic crosslinks) | Hydrogen bond and coordination bond | Wearable sensor & antimicrobial | 98.5% E. coli kill; linear sensing 10–300% strain | 87 |
| Polymeric NP (PEI–PBA insulin) | PEGDA and CS–GA hydrogel | Borate ester bond (PBA–diols) | Glucose-responsive insulin delivery (diabetic wounds) | Glucose-triggered release; near-complete healing by day 20 | 110 |
| Ag–MOF | O-Alg and borax spray hydrogel | Schiff base and borate ester bond | Sprayable antibacterial dressing | Gelation 5–30 s; protected against E. coli/S. aureus ≥12 h | 121 |
Among the various biomedical applications, DNCHs combine adaptive polymeric networks with nanoscale components to achieve synergistic antibacterial and wound–healing effects. DNCHs exhibit diverse therapeutic mechanisms depending on their composition, including photothermal activity, catalytic redox behavior, and self-healing functionality. Metal- and metal oxide-based DNCHs utilize nanoparticle-mediated antibacterial and regenerative catalysis, achieving complete bacterial eradication and rapid wound closure. Polymer-based systems provide biocompatible, self-healing matrices that promote hemostasis and diabetic wound repair. Carbon-based DNCHs offer conductive, photothermal antibacterial action, while MOF-based variants integrate porosity and multifunctionality for enhanced anti-inflammatory and regenerative performance. Taken together, DNCHs represent versatile platforms for efficient bacterial reduction and accelerated wound healing through integrated antimicrobial, antioxidant, and regenerative pathways. A comprehensive summary of DNCHs designed for antibacterial and wound–healing applications has been shown in Table 5, emphasizing key performance metrics (i.e., bacterial reduction efficiency and wound closure duration) across diverse DNCH systems.
| DNCH | Bacterial reduction | Wound closure time | Ref. |
|---|---|---|---|
| Au NP/ODex/gC | Survival of E. coli and S. aureus (%): | 8 days | 53 |
| Control: 100; experimental: 0 | |||
| Ag NP/CNF/PVA | S. aureus, E. coli, and C. albicans: | 14 days | 55 |
| Control: visible colonies; experimental: no colonies observed. | |||
| Ag NP/PAAm | Survival of bacterial (%) | 10 days | 56 |
| Control: 100; experimental: 0.21 (E. coli); 0.04 (S. aureus) | |||
| Ag NP/PVA/MFC | Zone of inhibition (mm) | 57 | |
Control: none; experimental: 4.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 (E. coli); 4.5 0.2 (E. coli); 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 (P. aeruginosa); 3.1 0.4 (P. aeruginosa); 3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 (S. aureus) 0.4 (S. aureus) |
|||
| Ag NP/BC | OD value for E. coli and S. aureus | 10 days | 58 |
| Control: 2.1; experimental: 1.5 | |||
| Pt@PFOB@PLGA/GelMA/ODex | Antimicrobial rate (%) for S. aureus | 9 days | 61 |
| Control: 0; experimental: > 99.9 | |||
| CuS/ODex/PEG | Antimicrobial rate (%) for E. coli and S. aureus | 14 days | 62 |
| Control: 0; experimental: 100 | |||
| CuS/PEG/PCL | Bacterial number (× 108 mL−1) for E. coli | 10 days | 63 |
| Control: 0.46; experimental: 0.12 | |||
| ZnO/CS/PEG | Zone of inhibition (mm) | 10 days | 74 |
| Control: 22 (E. coli); 19 (S. aureus); experimental: 30 (E. coli); 25 (S. aureus) | |||
| ZnO/mSF/TA | Zone of inhibition (mm) | 76 | |
| Control: none; experimental: 8 (E. coli); 9 (S. aureus) | |||
| OCNT/COF-Fe | Bactericidal efficiency: 100% (E. coli, S. aureus, and MRSA) | 12 days | 81 |
| PP–CNT/APN/Neo | Survival rates for pig skin infection: | 82 | |
| Control: none; experimental: 5% (S. aureus); 2% (E. coli) | |||
| PEG-DA/PEI-PBA/insulin/CS-GA | 20 days | 110 | |
| AF127/HA-ADH/OHA-Dop | 14 days | 112 | |
| Hydrogel@nZnO&MIC | Bactericidal efficiency: 96% (S. aureus and P. aeruginosa) | 14 days | 24 |
| O-Alg/Ag-MOF/BX sprayable hydrogel | Inhibition zones (cm): | 121 | |
| Experimental: 1.3 ± 0.1 (E. coli and S. aureus) | |||
| Cur@Cu-MOF/CMCS-OSA | Significant inhibition (S. aureus and E. coli) | 14 days | 122 |
| PVA/B/CMC/ZIF-8@Flu | Clear inhibition zones (S. aureus) | 123 | |
| CNTs/PDA/QCS | Bactericidal efficiency: 100% (E. coli and S. aureus) | 230 |
Dynamic crosslinking chemistries have been widely implemented in diverse biomedical contexts to realize multifunctional hydrogel systems. For instance, borate ester and coordination bonds have been used to design cellulose nanofibril/poly(vinyl alcohol) hydrogels incorporating curcumin-functionalized silver nanoparticles for diabetic wound healing,55 where the reversible network simultaneously enabled antimicrobial, antioxidant, and self-healing functions. Magnetically responsive double-network hydrogels containing superparamagnetic iron oxide nanoparticles and nanozymes have facilitated intervertebral disc regeneration through controlled mechanics and ROS scavenging,60 while imine-bonded iron oxide hydrogels have enabled magnetothermal-controlled drug release.67 Similarly, copper-doped TiO2 and CNT/covalent organic framework composite hydrogels have demonstrated remarkable photocatalytic and photothermal properties for localized cancer and chronic wound treatments.72,81 In addition, micelle–nanoparticle hybrid hydrogels integrating pH- and NIR-responsive units have shown strong potential for dual-mode chemo–photothermal therapy.112 Collectively, these academic examples highlight how dynamic interactions provide a versatile molecular toolbox for constructing self-healing, stimuli-responsive, and multifunctional biomaterials. Beyond laboratory research, dynamic crosslinked hydrogels have great potential to be translated into commercial products in the near future.
5. Challenges in DNCHs
Despite DNCHs holding considerable promise, their broad clinical adoption and translational application remain constrained by several critical challenges, such as mechanical instability, limited structural control and characterization, issues with reproducible and scalable synthesis, and concerns related to biological compatibility and environmental impact (Fig. 28).The heterogeneous crosslinked networks of DNCHs, especially the uniform dispersion of nanomaterials at high loading, often result in uneven stress distribution. Consequently, these hydrogels tend to exhibit inherently poor mechanical performance and limited structural stability, including low strength, brittleness, and a tendency to deform or fail under mechanical stress. In biological environments, such mechanical limitations can lead to uncontrolled degradation and functional loss, compromising the effectiveness of drug delivery systems, implantable hydrogel scaffolds, and hydrogel–tissue interfaces. These deficiencies significantly hinder their application in mechanically demanding contexts, such as load-bearing tissues (e.g., cartilage and bone), and reduce the operational lifespan of associated biomedical devices.
The multi-component composition of DNCHs presents significant challenges in achieving precise control and comprehensive structural characterization across multiple length scales. Although dynamic hydrogels are engineered to respond to external stimuli, fine-tuning their response kinetics and magnitude within complex biological environments remains difficult. This limitation is particularly critical for applications such as targeted drug delivery, where controlled and predictable release profiles are essential. Furthermore, the inherent complexity and dynamic behavior of these hydrogels complicate efforts to fully characterize and understand their structure–property relationships over time and across different spatial dimensions.
Achieving consistent and reproducible synthesis of DNCHs remains a significant barrier to their commercial-scale production and broader clinical adoption, despite their substantial scientific promise. Taking the nanomaterial synthesis for example, the final size and morphology of metal and metal oxide nanomaterials are strongly influenced by factors such as precursor concentration231 and reaction temperature.232 Similarly, the reproducibility of MOF synthesis is often challenged by their sensitive crystallization behavior, where even minor variations in conditions (e.g., reactant concentration, reaction time, or heating rate) can result in different crystal phases or impure products.233 The intrinsic complexity of these systems, arising from their multi-component makeup and dynamic, stimuli-responsive interactions, greatly contributes to these challenges. A pervasive issue is batch-to-batch variability, often attributed to poorly defined polymer precursors and inconsistently synthesized nanomaterials. This inconsistency propagates through the synthesis process, leading to variability in the final hydrogel properties, impeding regulatory approval and commercial translation. Moreover, the integration of dynamic bonding, while essential for imparting responsiveness, adds further complexity due to its sensitivity to environmental conditions, making it difficult to engineer hydrogels with stable performance across diverse applications. From a manufacturing perspective, numerous technical parameters (e.g., rheological behavior, nanomaterial dispersion, and mixing protocols) must be tightly controlled to ensure uniformity and reliability. These challenges emphasize the critical interdependence between materials science and process engineering, highlighting the urgent need for robust, standardized synthesis protocols and scalable fabrication strategies to bridge the gap between laboratory success and market readiness.
In biomedical applications, a central challenge in the development of DNCHs is achieving an optimal balance among biocompatibility, biodegradability, and mechanical stability. This often necessitates trade-offs, where enhancing mechanical strength or responsiveness through nanomaterial incorporation may inadvertently compromise the biological safety of materials. A primary concern is the potential toxicity of the embedded nanomaterials, which is influenced by various intrinsic factors, such as composition, size, surface area, morphology, surface functionalization, and propensity for agglomeration. For example, metal and metal oxide nanomaterials (e.g., silver, gold, and iron oxide) are particularly susceptible to inducing toxicity through the release of metal ions and the generation of oxidative stress. For instance, silver nanoparticles can liberate cytotoxic silver ions that interfere with essential cellular functions,234,235 while iron oxide nanoparticles may catalyze the production of ROS via Fenton-type reactions.236,237 Additionally, the degradation products of both the polymeric matrix and the nanomaterials may pose toxicological risks, particularly if they are not readily metabolized or excreted by the body. For example, MOFs may release their constituent metal ions and organic ligands upon degradation, with the resulting toxicity largely dependent on the intrinsic properties of these building blocks.238,239 Consequently, the stability of MOFs in biological environments is a critical determinant of their safety, as less stable structures are more prone to releasing toxic components. These considerations highlight the importance of carefully evaluating material choices to ensure both functional performance and long-term biocompatibility.
From an environmental perspective, DNCHs raise significant concerns throughout their life cycle, from synthesis to disposal. Nanofillers embedded within these hydrogels may leach into the environment during use or degradation, potentially entering ecosystems, accumulating in living organisms, and inducing toxic effects. The breakdown of DNCHs can release harmful byproducts, including unreacted monomers, crosslinkers, and nanomaterials. This issue is exacerbated in formulations that incorporate synthetic polymers or chemically robust crosslinkers, as these materials are typically non-biodegradable. The synthesis process itself may involve toxic solvents, chemical initiators, or crosslinkers, generating hazardous waste and posing risks of environmental contamination. In addition, some manufacturing methods are energy-intensive, increasing the carbon footprint. Critically, standardized protocols for the safe disposal, degradation, or recycling of DNCHs are currently insufficient, highlighting an urgent need for sustainable design and regulatory oversight in their development. Finally, comprehensive life cycle assessments (LCAs) for DNCHs are often lacking, resulting in a limited understanding of their long-term environmental sustainability and potential contribution to persistent pollution.
A significant challenge in the field of DNCHs is the high frequency of unexpected failures and negative results that often go unpublished, creating a gap in the collective knowledge. The provided sources detail numerous common pitfalls that can derail DNCH design, which helps explain this prevalence. For instance, nanoparticle aggregation can quench intended functionalities, rendering a material ineffective for sensing or catalysis. Similarly, a mismatch between the kinetics of dynamic bonds and the requirements of applications can lead to materials that either respond too slowly or fail to self-heal efficiently. Other frequent failure points include poor adhesion at the polymer–nanoparticle interface leading to mechanical weakness, unexpected cytotoxicity from material components, and the inability to scale lab-based synthesis methods for practical use. Future progress would greatly benefit from a culture that encourages the reporting of these “unsuccessful” outcomes, as this would help new researchers avoid repeating common mistakes and accelerate the development of robust, functional DNCHs.
Other than the current challenges, the future challenges of DNCHs extend beyond current limitations and are centered on translating laboratory successes into reliable, scalable, and commercially viable technologies. Critical hurdles include achieving long-term stability and durability under physiological and environmental conditions, preventing nanoparticle leaching, and minimizing mechanical fatigue during prolonged use. Another significant challenge lies in engineering advanced functionalized DNCHs capable of mimicking the anisotropic and hierarchical architectures of natural tissues while delivering precise, multi-responsive behavior to complex biological signals without cross-interference. Moreover, overcoming barriers in scalability and manufacturing by transitioning from small-batch laboratory synthesis to cost-effective, reproducible industrial-scale processes remains a pressing need. Finally, developing predictive models for complex material behaviors and establishing internationally accepted standards for testing, quality control, and regulatory approval are indispensable for accelerating innovation and enabling integration into next-generation applications such as soft robotics and bioelectronics.
6. Future research directions for DNCHs
Achieving a balance between mechanical robustness and dynamic, self-healing, and stimuli-responsive behavior is a key challenge in the design of DNCHs (Fig. 29). The integration of reversible dynamics with structural stability, particularly under physiologically harsh or mechanically demanding conditions, requires advanced and thoughtful material engineering. A fundamental design trade-off exists between adaptability and long-term mechanical integrity, necessitating careful selection of components and network architectures. This includes the development of novel hybrid crosslinking strategies, such as multi-network hydrogels capable of dissipating energy while preserving structural coherence, and the strategic incorporation of both static and dynamic bonds to optimize performance.Prioritizing the precise tuning of nanoparticle surface chemistry and interfacial interactions is a potential strategy to enhance the overall functionality of the hydrogel system. Functional group modification on nanoparticles will play a pivotal role in improving colloidal stability, achieving uniform dispersion, and sustaining bioactivity over extended periods. Additionally, the adoption of bioorthogonal and modular “plug-and-play” approaches (e.g., click chemistry and reversible ligand exchange) offers powerful tools for customizing interfacial bonding dynamics. These strategies could enable the fine control of hydrogel responsiveness and significantly enhance mechanical resilience, advancing the design of next-generation smart biomaterials.
Precisely controlling internal architecture and multi-scale structural design is essential for DNCHs to fulfill their intended functionalities and to effectively mimic the complex hierarchical organization of natural materials. The current limitations in controlling DNCH structure across multiple length scales constrain their potential in highly functional and biomimetic applications. Advanced fabrication technologies (e.g. multi-material 3D printing and high-throughput microfluidic platforms) are promising enablers in this context. These techniques provide increased production speed as well as offer an unprecedented level of spatial precision and structural complexity, which is fundamental for replicating the intricate architectures of biological tissues and developing next-generation, application-specific devices. This marks a transformative shift in the field, repositioning DNCHs from conventional bulk materials to finely engineered, multifunctional systems.
The integration of artificial intelligence (AI) and machine learning (ML) into DNCH development holds great potential for accelerating material discovery, predicting functional properties, and optimizing synthesis processes. By enabling intelligent and autonomous material design, these computational tools can significantly reduce experimental trial-and-error and facilitate rational, data-driven innovation. Concurrently, future research should focus on refining computational and modeling techniques to capture the complex interactions between dynamic nanoparticle behavior and hydrogel network mechanics. Such predictive models are essential for guiding material design and ensuring performance reliability. Equally important is the advancement of in situ and real-time characterization tools. Techniques (e.g., dynamic rheology, real-time spectroscopy, and high-resolution microscopy) need further development to uncover the mechanisms governing bond dynamics and network restructuring within DNCHs. These tools are critical for correlating microscale structural changes with macroscale functionality, especially under physiological or extreme environmental conditions. Bridging this gap between microscopic design and macroscopic performance through integrated computational and analytical approaches will be essential for moving beyond empirical methodologies and achieving reliable, high-performance materials for biomedical and environmental applications.
DNCHs represent a promising platform for next-generation applications in personalized medicine and wearable health monitoring. However, the transition from laboratory innovation to clinical and industrial implementation demands rigorous evaluation of key parameters including biocompatibility, toxicity, and biodegradability. Comprehensive in vivo studies are essential to address critical safety concerns, including nanomaterial accumulation, leaching, and long-term material stability in physiological environments. These studies must thoroughly assess chronic biocompatibility, elucidate degradation mechanisms, and evaluate the potential immunogenicity and systemic toxicity of both the hydrogel matrix and the embedded nanomaterials. Additionally, the development of advanced, real-time characterization tools to monitor material–biological interactions over extended durations is crucial for ensuring safe and predictable performance. In addition, designing DNCHs for biomedical use is inherently complex, requiring a careful balance between functional performance and long-term biological safety. This includes meticulous evaluation of degradation byproducts, immune responses, and overall systemic exposure. Integrating biological, chemical, and engineering insights to optimize material safety and efficacy would be appreciated, rather than focusing on isolated property enhancements.
DNCHs are poised to revolutionize soft robotics by developing bio-inspired actuators capable of complex, adaptive movements such as grasping, swimming, and walking. Future research emphasizes designing materials responsive to multiple stimuli (e.g., light, temperature, and pH) to achieve precise, programmable motion. A key opportunity lies in integrating sensing and actuation within a single material framework, creating systems with “embodied intelligence” that autonomously adapt to environmental changes. The incorporation of nanomaterials (e.g., carbon nanotubes and graphene oxide) addresses long-standing challenges by delivering high toughness and rapid response. Moreover, the intrinsic self-healing ability of DNCHs extends device durability, making robotic systems more reliable and resilient.
In environmental remediation, DNCHs are being engineered into selective, reusable, and highly efficient platforms for pollution control. Embedding functional nanofillers (e.g., MOFs or graphene oxide) enables the fabrication of smart adsorbents with high specificity for contaminants like heavy metals and organic dyes. Critically, DNCHs can be designed to release captured pollutants on demand under external stimuli, allowing for regeneration and repeated use, which supports sustainable and cost-effective remediation. Incorporating photocatalytic nanoparticles further advances the field by enabling in situ catalytic degradation of persistent organic pollutants, offering proactive and scalable water purification solutions.
Future opportunities for DNCHs in the energy sector present significant opportunities for next-generation storage and conversion devices. Their combination of flexibility, stretchability, and self-healing behavior makes them ideal candidates for solid-state electrolytes in flexible batteries and supercapacitors, extending the operational lifetime of wearable electronics. Incorporating plasmonic or carbon-based nanostructures enables efficient harvesting of solar and thermal energy for electrical conversion. Furthermore, advances in manufacturing fabrications facilitate the construction of tailored electrode architectures, paving the way for programmable, seamlessly integrated energy systems for wearable and implantable technologies.
The application of DNCHs in catalysis is advancing toward smart, stimuli-responsive reactor platforms that provide precise control over reaction kinetics and selectivity. By regulating access to embedded nanocatalysts, DNCHs prevent aggregation and enhance long-term catalytic activity for critical reactions such as CO2 reduction and hydrogen evolution. In biocatalysis, these hydrogels offer protective environments for enzymes, safeguarding them against denaturation while maintaining efficient diffusion for sustained activity. Structuring DNCHs into membranes and other geometries further expands their potential in continuous-flow microreactors, offering superior scalability, control, and efficiency compared to conventional batch processes.
Environmental sustainability must also be prioritized in the synthesis and functionalization of DNCHs. There is a growing need to shift toward greener and safer material development pathways. This can be achieved by utilizing biodegradable natural polymers, replacing toxic nanofillers with biocompatible alternatives, and designing closed-loop or solvent-free synthesis systems. Environmentally responsible manufacturing may include aqueous synthesis processes, safer crosslinkers and initiators, reduced energy consumption, and strategies for recycling or reusing synthesis byproducts. Furthermore, conducting robust ecotoxicity assessments and full life cycle analyses will be critical to validating the environmental impact of DNCHs.
Beyond established roles, DNCHs are poised to drive transformative innovation across multiple sectors. Their inherent adaptability and responsiveness make them ideal candidates for emerging applications such as flexible electronics, wearable sensors, regenerative agriculture, smart textiles, and next-generation sensing systems. Expanding research into these interdisciplinary domains will not only broaden the technological relevance of DNCHs but also unlock unforeseen societal and environmental benefits.
7. Conclusion
DNCHs represent a significant frontier in advanced materials science. These materials offer exceptional tunability and stimuli-responsiveness, making them highly promising for a wide array of applications, particularly within biomedicine. Their unique combination of adaptable properties, intrinsic responsiveness to external stimuli, and remarkable capacity to emulate the dynamic characteristics of biological systems position them as indispensable components for future innovations. Despite their immense potential, the full realization of DNCH capabilities is currently hindered by a complex interplay of scientific and engineering challenges. Chief among these are inherent mechanical weaknesses and issues concerning long-term stability within dynamic biological environments. Furthermore, the intricate nature of their synthesis pathways frequently leads to significant concerns regarding reproducibility, often stemming from poorly defined precursors and the delicate sensitivity of their dynamic bonds. To comprehensively address these challenges and unlock the transformative potential of DNCHs, intensified interdisciplinary collaboration is critically needed. A concerted effort involving chemists, materials scientists, biomedical engineers, clinicians, and industry partners is essential to accelerate DNCH development and ensure successful translation from fundamental research to practical application. In conclusion, DNCHs are poised at the threshold of a new era in materials science. Through focused, collaborative research aimed at overcoming the identified challenges, future research can not only push the boundaries of basic research but also pave the way for innovative, commercially viable applications to allow DNCHs to deliver profound impacts on human health, drive technological innovation, and contribute to environmental stewardship. Continued innovation and strategic interdisciplinary efforts will undoubtedly open new opportunities and drive the development of smarter, safer, and more effective DNCH systems.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included, and no new data were generated or analyzed as part of this review.Acknowledgements
Y.-C. Yeh appreciates the financial support from the National Science and Technology Council, Taiwan (NSTC 111-2113-M-002-008-, 112-2113-M-002-021-, 113-2113-M-002-001-, and 114-2113-M-002-007-MY3), and National Taiwan University (114L7877). Y.-C. Su appreciates the financial support from the Ministry of Science and Technology, Taiwan (MOST 111-2811-M-002-114) and the National Science and Technology Council, Taiwan (NSTC 112-2811-M-002-125 and 113-2811-M-002-127).References
- A. Tejo-Otero, F. Fenollosa-Artés, I. Achaerandio, S. Rey-Vinolas, I. Buj-Corral, M. Á. Mateos-Timoneda and E. Engel, Gels, 2022, 8, 40 CrossRef CAS PubMed
.
- R. Fatima and B. Almeida, J. Mater. Chem. B, 2024, 12, 8505–8522 RSC
.
- X. Sun, S. Agate, K. S. Salem, L. Lucia and L. Pal, ACS Appl. Bio Mater., 2021, 4, 140–162 CrossRef CAS PubMed
.
- J. Ma, J. Zhong, F. Sun, B. Liu, Z. Peng, J. Lian, X. Wu, L. Li, M. Hao and T. Zhang, Chem. Eng. J., 2024, 481, 148317 CrossRef CAS
.
- M. Vigata, C. Meinert, D. W. Hutmacher and N. Bock, Pharmaceutics, 2020, 12, 1188 CrossRef CAS
.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS
.
- A. Guo, Q. Cao, H. Fang and H. Tian, J. Controlled Release, 2025, 385, 114021 CrossRef CAS
.
- Y. Cheng, H. Zhang, H. Wei and C.-Y. Yu, Biomater. Sci., 2024, 12, 1151–1170 RSC
.
- I. Ventisette, F. Mattii, C. Dallari, C. Capitini, M. Calamai, B. Muzzi, F. S. Pavone, F. Carpi and C. Credi, ACS Appl. Bio Mater., 2024, 7, 4497–4509 CrossRef CAS PubMed
.
- Y. Luo, T. Zhang and X. Lin, Chem. Eng. J., 2022, 430, 132926 CrossRef CAS
.
- L. Zhang, N. K. A. Nasar, X. Huang, C. Hu, X. Pang, X. Chen, R. Qiao and T. P. Davis, Small Sci., 2024, 4, 2400097 CrossRef CAS PubMed
.
- Z. F. Shafeeq, F. Al-Saedi, E. S. Rajab, M. H. Al-Musawi, F. E. Ismaeel, S. Najafi, M. Vesal, S. N. Esfahani, F. Sharifianjazi, K. Tavamaishvili, M. Mirhaj and M. Tavakoli, Sci. Rep., 2025, 15, 32244 CrossRef CAS PubMed
.
- L. Maeso, P. E. Antezana, A. G. Hvozda Arana, P. A. Evelson, G. Orive and M. F. Desimone, Pharmaceutics, 2024, 16, 524 CrossRef CAS
.
- S. Wang, L. Yu, S. Wang, L. Zhang, L. Chen, X. Xu, Z. Song, H. Liu and C. Chen, Nat. Commun., 2022, 13, 3408 CrossRef CAS
.
- D. Chen, H. Bai, H. Zhu, S. Zhang, W. Wang and W. Dong, Chem. Eng. J., 2024, 480, 148192 CrossRef CAS
.
- D.-H. Ji, Y.-F. Ni, C.-Y. Lin and M.-Y. Yeh, ACS Appl. Electron. Mater., 2025, 7, 874–883 CrossRef CAS
.
- X. Peng, W. Wang, W. Yang, J. Chen, Q. Peng, T. Wang, D. Yang, J. Wang, H. Zhang and H. Zeng, J. Colloid Interface Sci., 2022, 618, 111–120 CrossRef CAS PubMed
.
- S. Ahmadi, S. Pourebrahimi, A. Malloum, M. Pirooz, C. Osagie, S. Ghosh, M. N. Zafar and M. H. Dehghani, Emerging Contam., 2024, 10, 100336 CrossRef CAS
.
- A. Pattnaik, P. Ghosh and A. K. Poonia, J. Mol. Liq., 2025, 424, 127120 CrossRef CAS
.
- T. Zheng and P. S. Doyle, RSC Pharm., 2025, 2, 186–196 RSC
.
- X. Jin, C.-x Wei, C.-w Wu and W. Zhang, ACS Omega, 2022, 7, 8493–8497 CrossRef CAS
.
- R. Narayanaswamy and V. P. Torchilin, Molecules, 2019, 24, 603 CrossRef PubMed
.
- X. Chen, Z. Hu, K. Zhao, X. Rao, C. Shen, Y. Chen, X. Ye, C. Fang, F. Zhou, Z. Ding and B. Zhu, Sci. Rep., 2024, 14, 22135 CrossRef CAS PubMed
.
- C. A. Guo, Y. Wu, W. L. Li, Y. Wang and Q. Q. Kong, ACS Appl. Mater. Interfaces, 2022, 14, 30480–30492 CrossRef CAS
.
- R. Umapathi, K. Kumar, S. Majid Ghoreishian, G. Mohana Rani, S. Young Park, Y. Suk Huh and P. Venkatesu, J. Mol. Liq., 2022, 360, 119404 CrossRef CAS
.
- R. Umapathi, S. M. Ghoreishian, K. Kumar, D. Dhiman, G. M. Rani, Y. S. Huh and P. Venkatesu, Phys. Chem. Chem. Phys., 2023, 25, 21131–21148 RSC
.
- K. Kumar, R. Umapathi, S. Mor, S. M. Ghoreishian, J. N. Tiwari, M. R. Farani, Y. S. Huh and P. Venkatesu, ACS Appl. Polym. Mater., 2023, 5, 3181–3200 CrossRef CAS
.
- A. Sepahvandi, J. Johnson, A. Arasan, R. Cataldo and S. M. Ghoreishian, Gels, 2025, 11, 342 CrossRef CAS
.
- N. Mamidi, F. F. De Silva, A. B. Vacas, J. A. Gutiérrez Gómez, N. Y. Montes Goo, D. R. Mendoza, R. L. Reis and S. C. Kundu, Adv. Healthcare Mater., 2024, 13, 2401195 CrossRef CAS
.
- N. Mamidi, F. Ijadi and M. H. Norahan, Biomacromolecules, 2024, 25, 2075–2113 CrossRef CAS
.
- N. Mamidi and R. M. V. Delgadillo, Colloids Surf., B, 2021, 204, 111819 CrossRef CAS
.
- N. Mamidi and A. O. Mahmoudsalehi, J. Mater. Chem. B, 2025, 13, 10440–10459 RSC
.
- A. Alam, H.-C. Kuan, Z. Zhao, J. Xu and J. Ma, Composites, Part A, 2017, 93, 1–9 CrossRef CAS
.
- H. Lee, Y. Kim, S. Kim and G. Kim, J. Mater. Chem. B, 2014, 2, 5785–5798 RSC
.
- H. Derakhshankhah, M. Eskandani, S. Akbari Nakhjavani, S. Tasoglu, S. Vandghanooni and M. Jaymand, Int. J. Polym. Mater. Polym. Biomater., 2024, 73, 266–278 CrossRef CAS
.
- G. Sharma, A. Verma, A. García-Peñas, A. Kumar, P. Dhiman, T. Wang and J. Amirian, Int. J. Biol. Macromol., 2024, 283, 137555 CrossRef CAS PubMed
.
- W. M. Seleka, E. Makhado, L. K. Kganyakgo, L. E. Mofokeng, D. Makwakwa and O. J. Botlhoko, Int. J. Hydrogen Energy, 2025, 142, 498–510 CrossRef CAS
.
- N. Hlapisi, S. P. Songca and P. A. Ajibade, Pharmaceutics, 2024, 16, 1268 CrossRef CAS PubMed
.
- X.-E. Chen, C.-J. Yan, C.-H. Lu and Y.-C. Yeh, ACS Appl. Polym. Mater., 2024, 6, 7340–7356 CrossRef CAS
.
- C. Zhao, B. Pan, T. Wang, H. Yang, D. Vance, X. Li, H. Zhao, X. Hu, T. Yang, Z. Chen, L. Hao, T. Liu and Y. Wang, Pharmaceutics, 2023, 15, 2729 CrossRef CAS PubMed
.
- K. Yang, X. Yin, Y. Yan, G. Luo, M. Xu, P. Pi, S. Xu and X. Wen, J. Appl. Polym. Sci., 2020, 137, 49309 CrossRef CAS
.
- S. Xie, B. Ren, G. Gong, D. Zhang, Y. Chen, L. Xu, C. Zhang, J. Xu and J. Zheng, ACS Appl. Nano Mater., 2020, 3, 2774–2786 CrossRef CAS
.
- L. Han, X. Lu, M. Wang, D. Gan, W. Deng, K. Wang, L. Fang, K. Liu, C. W. Chan, Y. Tang, L.-T. Weng and H. Yuan, Small, 2017, 13, 1601916 CrossRef
.
- A. Mellati, E. Hasanzadeh, M. Gholipourmalekabadi and S. E. Enderami, Mater. Sci. Eng., C, 2021, 131, 112489 CrossRef CAS PubMed
.
- M. J. F. Albao, J. R. F. Calsis, J. O. Dancel, L. M. De Juan-Corpuz and R. D. Corpuz, J. Chin. Chem. Soc., 2025, 72, 124–162 CrossRef CAS
.
- M.-H. Cai, X.-Y. Chen, L.-Q. Fu, W.-L. Du, X. Yang, X.-Z. Mou and P.-Y. Hu, Front. Bioeng. Biotechnol., 2021, 9, 630943 CrossRef PubMed
.
- C.-H. Lu, C.-H. Yu and Y.-C. Yeh, Acta Biomater., 2021, 130, 66–79 CrossRef CAS PubMed
.
- Y. Ma, X. Tian, L. Liu, J. Pan and G. Pan, Acc. Chem. Res., 2019, 52, 1611–1622 CrossRef CAS PubMed
.
- Q. Wang, Y. Zhang, Y. Ma, M. Wang and G. Pan, Mater. Today Bio, 2023, 20, 100640 CrossRef CAS
.
- P. Kościelniak, A. Więckowska, M. Karbarz and K. Kaniewska, J. Colloid Interface Sci., 2024, 674, 392–404 CrossRef
.
- S. Khodami, M. Gharakhloo, S. Dagdelen, P. Fita, J. Romanski, M. Karbarz, Z. Stojek and M. Mackiewicz, ACS Appl. Mater. Interfaces, 2024, 16, 57659–57671 CrossRef CAS
.
- B. Couturier, G. Kozak, J. Levering, A. Zini and M. B. Elinski, ACS Omega, 2024, 9, 42858–42867 CrossRef CAS PubMed
.
- Y. Li, R. Fu, Z. Duan, C. Zhu and D. Fan, Small, 2022, 18, 2200165 CrossRef CAS
.
- J. Xu, T.-Y. Chen, C.-H. Tai and S.-H. Hsu, Biomater. Res., 2023, 27, 8 CrossRef CAS PubMed
.
- S. Zhang, B. Gatsi, X. Yao, Y. Jin and H. Amhal, Carbohydr. Polym., 2025, 352, 123189 CrossRef CAS PubMed
.
- W. Shi, H. Li, J. Chen, Y. C. Ching, C. H. Chuah, C. Xu, M. Liu, J. Zhang, K. Y. Ching, Y. Liang, G. Li and W. Tang, Adv. Sci., 2024, 11, 2404451 CrossRef CAS
.
- M. S. Hasan, J. Al Foisal, G. M. A. Khan, R. Jahan, M. Hasanuzzaman, M. S. Alam, M. M. Karim, M. A. Gafur, M. A. Khan and M. A. Sabur, J. Polym. Environ., 2022, 30, 2875–2887 CrossRef CAS
.
- X. Yang, J. Huang, C. Chen, L. Zhou, H. Ren and D. Sun, ACS Appl. Mater. Interfaces, 2023, 15, 10506–10519 CrossRef CAS
.
- C. García-Astrain, C. Chen, M. Burón, T. Palomares, A. Eceiza, L. Fruk, M. Á. Corcuera and N. Gabilondo, Biomacromolecules, 2015, 16, 1301–1310 CrossRef
.
- B. Xue, Y. Peng, Y. Zhang, S. Yang, Y. Zheng, H. Hu, X. Gao, B. Yu, X. Gao, S. Li, H. Wu, T. Ma, Y. Hao, Y. Wei, L. Guo, Y. Yang, Z. Wang, T. Xue, J. Zhang, B. Luo, B. Xia and J. Huang, Adv. Sci., 2024, 11, 2408093 CrossRef CAS
.
- Z. Zhou, X. Mei, K. Hu, M. Ma and Y. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 17612–17626 CrossRef CAS
.
- L. Zhou, F. Chen, Z. Hou, Y. Chen and X. Luo, Chem. Eng. J., 2021, 409, 128224 CrossRef CAS
.
- X. Xue, H. Zhang, H. Liu, S. Wang, J. Li, Q. Zhou, X. Chen, X. Ren, Y. Jing, Y. Deng, Z. Geng, X. Wang and J. Su, Adv. Funct. Mater., 2022, 32, 2202470 CrossRef CAS
.
- T. Blin, A. Niederberger, L. Benyahia, J. Fresnais, V. Montembault and L. Fontaine, Polym. Chem., 2018, 9, 4642–4650 RSC
.
- Q. Li, D. G. Barrett, P. B. Messersmith and N. Holten-Andersen, ACS Nano, 2016, 10, 1317–1324 CrossRef CAS
.
- C.-C. Yu, Y.-C. Su and Y.-C. Yeh, Eur. Polym. J., 2024, 220, 113469 CrossRef CAS
.
- C.-H. Lu and Y.-C. Yeh, Small, 2021, 17, 2105997 CrossRef CAS PubMed
.
- J. Xu, P. Wang, Y. Zou, S. Zhang, C. Huang, L. Liu, J. Yu and Y. Fan, Biomacromolecules, 2022, 23, 1314–1325 CrossRef CAS
.
- S. Ganguly, P. Das, S. Srinivasan, A. R. Rajabzadeh, X. S. Tang and S. Margel, ACS Appl. Nano Mater., 2024, 7, 5272–5286 Search PubMed
.
- H. Wu, L. Liu, L. Song, M. Ma, N. Gu and Y. Zhang, ACS Nano, 2019, 13, 14013–14023 CrossRef CAS
.
- M. K. Jaiswal, J. R. Xavier, J. K. Carrow, P. Desai, D. Alge and A. K. Gaharwar, ACS Nano, 2016, 10, 246–256 CrossRef CAS
.
- S. Maghsoudian, M. P. Yektakasmaei, A. Shaabani, S. Perseh, Y. Fatahi, Z. Nouri, M. Gholami, N. Sayyari, H. A. Hoseinzadeh, H. Motasadizadeh and R. Dinarvand, Int. J. Biol. Macromol., 2025, 289, 138910 CrossRef CAS
.
- Y. Yue, X. Wang, Q. Wu, J. Han and J. Jiang, J. Colloid Interface Sci., 2020, 564, 99–112 CrossRef CAS
.
- R. A. Masud, M. S. Islam, P. Haque, M. N. I. Khan, M. Shahruzzaman, M. Khan, M. Takafuji and M. M. Rahman, Materialia, 2020, 12, 100785 CrossRef CAS
.
- J. Radwan-Pragłowska, Ł. Janus, M. Piątkowski, A. Sierakowska and D. Matysek, Colloids Surf., B, 2020, 194, 111170 CrossRef
.
- C. M. Yang, J. Lee, H. Lee and W. H. Park, Int. J. Biol. Macromol., 2022, 210, 1–10 CrossRef CAS PubMed
.
- H. Luo, M. Zhang, H. Rong, Z. Chen, X. Zeng, J. Wang, B. Liu and P. Liao, Environ. Sci.: Water Res. Technol., 2022, 8, 2614–2628 RSC
.
- H. Jiang and J. Tang, ACS Appl. Polym. Mater., 2020, 2, 3821–3827 CrossRef CAS
.
- H. Jiang, G. Zhang, X. Feng, H. Liu, F. Li, M. Wang and H. Li, Compos. Sci. Technol., 2017, 140, 54–62 CrossRef CAS
.
- C.-H. Lu and Y.-C. Yeh, ACS Biomater. Sci. Eng., 2022, 8, 4289–4300 CrossRef CAS
.
- X. Lin, M. Zhang, W. Lv, J. Li, R. Huang and Y. Wang, Adv. Funct. Mater., 2024, 34, 2310845 CrossRef CAS
.
- B.-Y. Li, T.-Y. Lin, Y.-J. Lai, T.-H. Chiu and Y.-C. Yeh, Small, 2025, 21, 2407420 CrossRef CAS
.
- J. Liu, X. Wang, K. Han, J. Liu, J. Yuan, M. Pan and Z. Pan, Polymer, 2024, 311, 127482 CrossRef CAS
.
- Q. Li, J. Wen, C. Liu, Y. Jia, Y. Wu, Y. Shan, Z. Qian and J. Liao, ACS Biomater. Sci. Eng., 2019, 5, 768–779 CrossRef CAS
.
- A. Chae, G. Murali, S.-Y. Lee, J. Gwak, S. J. Kim, Y. J. Jeong, H. Kang, S. Park, A. S. Lee, D.-Y. Koh, I. In and S.-J. Park, Adv. Funct. Mater., 2023, 33, 2213382 CrossRef CAS
.
- P.-Y. Chiang, P.-H. Zeng and Y.-C. Yeh, Int. J. Biol. Macromol., 2024, 260, 129359 CrossRef CAS
.
- P.-H. Zeng, G.-Z. Song and Y.-C. Yeh, Small, 2025, 21, 2501574 CrossRef CAS
.
- B. Gao, H. Yu, J. Wen, H. Zeng, T. Liang, F. Zuo and C. Cheng, J. Environ. Chem. Eng., 2021, 9, 106346 CrossRef CAS
.
- J. Du, J. Zhu, R. Wu, S. Xu, Y. Tan and J. Wang, RSC Adv., 2015, 5, 60152–60160 RSC
.
- L. Zhang, G. He, Y. Yu, Y. Zhang, X. Li and S. Wang, Biomolecules, 2022, 12, 1089 CrossRef CAS PubMed
.
- N. Golafshan, R. Rezahasani, M. Tarkesh Esfahani, M. Kharaziha and S. N. Khorasani, Carbohydr. Polym., 2017, 176, 392–401 CrossRef CAS
.
- C. Li, C. Mu, W. Lin and T. Ngai, ACS Appl. Mater. Interfaces, 2015, 7, 18732–18741 CrossRef CAS PubMed
.
- B. Liu, J. Li, X. Lei, S. Miao, S. Zhang, P. Cheng, Y. Song, H. Wu, Y. Gao, L. Bi and G. Pei, RSC Adv., 2020, 10, 25652–25661 RSC
.
- Y.-H. Kim, X. Yang, L. Shi, S. A. Lanham, J. Hilborn, R. O. C. Oreffo, D. Ossipov and J. I. Dawson, Nat. Commun., 2020, 11, 1365 CrossRef CAS
.
- Z. Cimen, S. Babadag, S. Odabas, S. Altuntas, G. Demirel and G. B. Demirel, ACS Appl. Polym. Mater., 2021, 3, 3504–3518 CrossRef CAS
.
- N. Rajabi, M. Kharaziha, R. Emadi, A. Zarrabi, H. Mokhtari and S. Salehi, J. Colloid Interface Sci., 2020, 564, 155–169 CrossRef CAS
.
- Y.-C. Su, L. C. Tseng, W.-T. Peng, C.-P. Hsu and Y.-C. Yeh, Inorg. Chem., 2025, 64, 8601–8619 CrossRef CAS PubMed
.
- L. Ma, W. Su, Y. Ran, X. Ma, Z. Yi, G. Chen, X. Chen, Z. Deng, Q. Tong, X. Wang and X. Li, Int. J. Biol. Macromol., 2020, 165, 1164–1174 CrossRef CAS PubMed
.
- B. Ren, X. Chen, S. Du, Y. Ma, H. Chen, G. Yuan, J. Li, D. Xiong, H. Tan, Z. Ling, Y. Chen, X. Hu and X. Niu, Int. J. Biol. Macromol., 2018, 118, 1257–1266 CrossRef CAS
.
- N. Kashimura, Y. Suzuki, T. Nonoyama and J. P. Gong, Chem. Mater., 2024, 36, 2944–2952 CrossRef CAS
.
- G. Wang, T. Lu, X. Zhang, M. Feng, C. Wang, W. Yao, S. Zhou, Z. Zhu, W. Ding and M. He, Int. J. Biol. Macromol., 2021, 186, 377–384 CrossRef CAS PubMed
.
- A. K. Gaharwar, R. K. Avery, A. Assmann, A. Paul, G. H. McKinley, A. Khademhosseini and B. D. Olsen, ACS Nano, 2014, 8, 9833–9842 CrossRef CAS PubMed
.
- A. Zengin, J. P. O. Castro, P. Habibovic and S. H. van Rijt, Nanoscale, 2021, 13, 1144–1154 RSC
.
- M. Wu, J. Chen, W. Huang, B. Yan, Q. Peng, J. Liu, L. Chen and H. Zeng, Biomacromolecules, 2020, 21, 2409–2420 CrossRef CAS
.
- A. A. Mohammed, N. G. Merrild, S. Li, A. Pinna and J. R. Jones, ACS Omega, 2022, 7, 43904–43914 CrossRef CAS
.
- M. Ghanbari, M. Salavati-Niasari, F. Mohandes, B. Dolatyar and B. Zeynali, RSC Adv., 2021, 11, 16688–16697 RSC
.
- P. Xu, Z. Shang, M. Yao and X. Li, J. Mol. Liq., 2022, 357, 119094 CrossRef CAS
.
- X. Chen, L. Sun, H. Wang, S. Cao, T. Shang, H. Yan and Q. Lin, Colloids Surf., B, 2023, 228, 113413 CrossRef CAS PubMed
.
- J.-X. Ren, J.-L. Zhu, S.-C. Shi, M.-Q. Yin, H.-D. Huang and Z.-M. Li, Carbohydr. Polym., 2022, 296, 119957 CrossRef CAS PubMed
.
- Z. Xu, G. Liu, Q. Li and J. Wu, Nano Res., 2022, 15, 5305–5315 CrossRef CAS
.
- P. Wang, G. H. Deng, L. Y. Zhou, Z. Y. Li and Y. M. Chen, ACS Macro Lett., 2017, 6, 881–886 CrossRef CAS
.
- C. Zhang, J. Ma, Q. Wang, Y. Wang, Z. Kang, Y. Chen, Z. Hui and X. Wang, ACS Appl. Nano Mater., 2023, 6, 7841–7854 CrossRef CAS
.
- Z. X. Chang, C. Y. Hong and W. J. Zhang, Macromol. Rapid Commun., 2025, 46, 2400681 CrossRef CAS
.
- J. Chen, R. An, L. L. Han, X. D. Wang, Y. L. Zhang, L. Y. Shi and R. Ran, Mater. Sci. Eng., C, 2019, 99, 460–467 CrossRef CAS
.
- G. Q. Cui, X. Zhao, L. Liu and G. F. Wu, Polymer, 2024, 300, 126990 CrossRef CAS
.
- P. Du, J. Wang, Y. Hsu and H. Uyama, Chem. Mater., 2024, 36, 1318–1332 CrossRef CAS
.
- C. N. Zhu, C. Y. Li, H. Wang, W. Hong, F. Huang, Q. Zheng and Z. L. Wu, Adv. Mater., 2021, 33, 2008057 CrossRef CAS
.
- L. T. Gao, Y. M. Chen, Y. Aziz, W. Wei, X. Y. Zhao, Y. He, J. Li, H. Li, H. Miyatake and Y. Ito, Carbohydr. Polym., 2024, 330, 121812 CrossRef CAS
.
- F. Wang, Y. Liu, H. Y. Cao, Y. Y. Zhao, Y. Q. Ge, X. F. Song, C. H. Li, X. T. Yang, X. B. Jiang and X. L. Gu, Compos. Sci. Technol., 2023, 241, 110118 CrossRef CAS
.
- Y. Gao, A. M. Deng, X. H. Wu, C. S. Sun and C. Z. Qi, React. Funct. Polym., 2021, 161, 104866 CrossRef CAS
.
- S. Javanbakht and R. Mohammadi, Carbohydr. Polym. Technol., 2025, 9, 100629 CAS
.
- Y.-L. Fan, H.-J. Liu, Z.-L. Wang, Z.-L. Wang, L.-L. Zhu, Y.-Z. Wang and F. Song, Chem. Eng. J., 2024, 497, 155037 CrossRef CAS
.
- W. Zeng, Q. Jiang, C. Ruan, W. Ni, C. Zhu, X. Zeng, X. Shi, R. You, N. Ma and F.-C. Tsai, Talanta, 2025, 283, 127088 CrossRef CAS PubMed
.
- X.-D. Zhang, D. Wu, X. Shen, P.-X. Liu, F.-Y. Fan and S.-J. Fan, Biomaterials, 2012, 33, 4628–4638 CrossRef CAS
.
- S. Mukherjee, V. S. Bollu, A. Roy, S. K. Nethi, K. Madhusudana, J. M. Kumar, R. Sistla and C. R. Patra, Adv. NanoBiomed Res., 2021, 1, 2000082 CrossRef CAS
.
- E. Alexander and K. W. Leong, Front. Nanotechnol., 2025, 7, 1512622 CrossRef
.
- A. Ivask, I. Kurvet, K. Kasemets, I. Blinova, V. Aruoja, S. Suppi, H. Vija, A. Käkinen, T. Titma, M. Heinlaan, M. Visnapuu, D. Koller, V. Kisand and A. Kahru, PLoS One, 2014, 9, e102108 CrossRef PubMed
.
- J. Bi, C. Mo, S. Li, M. Huang, Y. Lin, P. Yuan, Z. Liu, B. Jia and S. Xu, Biomater. Sci., 2023, 11, 4151–4183 RSC
.
- P. Kulich, S. Marvanová, R. Skoupý, M. Škorič, J. Vysloužil, O. Šerý, P. Mikuška, L. Alexa, P. Coufalík, K. Křůmal, P. Moravec, Z. Večeřa and M. Machala, J. Appl. Toxicol., 2025, 45, 1004–1018 CrossRef CAS
.
- M. S. P. Boyles, L. C. Stoehr, P. Schlinkert, M. Himly and A. Duschl, Fibers, 2014, 2, 45–74 CrossRef
.
- S. Murugadoss, D. Lison, L. Godderis, S. Van Den Brule, J. Mast, F. Brassinne, N. Sebaihi and P. H. Hoet, Arch. Toxicol., 2017, 91, 2967–3010 CrossRef CAS
.
- Y. Lesan and L. Xingde, Curr. Pharm. Biotechnol., 2016, 17, 227–236 Search PubMed
.
- A. Filiz, Ş. S. Bayazit and F. Baris Barlas, J. Drug Delivery Sci. Technol., 2025, 112, 107284 CrossRef CAS
.
- P.-D. Ly, K.-N. Ly, H.-L. Phan, H. H. Nguyen, V.-A. Duong and H. V. Nguyen, Front. Nanotechnol, 2024, 6, 1456939 CrossRef
.
- I. Pinzaru, D. Coricovac, C. Dehelean, E. A. Moacă, M. Mioc, F. Baderca, I. Sizemore, S. Brittle, D. Marti, C. D. Calina, A. M. Tsatsakis and C. Şoica, Food Chem. Toxicol., 2018, 111, 546–556 CrossRef CAS PubMed
.
- F. Ito, H. Hisashi and S. Toyokuni, Nagoya J. Med. Sci., 2018, 80, 597–604 CAS
.
- R. A. Petros and J. M. DeSimone, Nat. Rev. Drug Discovery, 2010, 9, 615–627 CrossRef CAS
.
- Y. Liu, C. S. Gong, Y. Dai, Z. Yang, G. Yu, Y. Liu, M. Zhang, L. Lin, W. Tang, Z. Zhou, G. Zhu, J. Chen, O. Jacobson, D. O. Kiesewetter, Z. Wang and X. Chen, Biomaterials, 2019, 218, 119365 CrossRef CAS
.
- F. Eker, E. Akdaşçi, H. Duman, M. Bechelany and S. Karav, Nanomaterials, 2024, 14, 1854 CrossRef CAS PubMed
.
- J. Zhang, L. Mou and X. Jiang, Chem. Sci., 2020, 11, 923–936 RSC
.
- S. A. Bansal, V. Kumar, J. Karimi, A. P. Singh and S. Kumar, Nanoscale Adv., 2020, 2, 3764–3787 RSC
.
- V. Gounden and M. Singh, Pharmaceutics, 2025, 17, 633 CrossRef CAS PubMed
.
- S. Sun and J. Chen, ACS Appl. Mater. Interfaces, 2024, 16, 46988–47002 CrossRef CAS PubMed
.
- Y. Wang, M. Zhang, Z. Yan, S. Ji, S. Xiao and J. Gao, Theranostics, 2024, 14, 1534–1560 CrossRef CAS
.
- M. Zakia, J. M. Koo, D. Kim, K. Ji, P. Huh, J. Yoon and S. I. Yoo, Green Chem. Lett. Rev., 2020, 13, 34–40 CrossRef CAS
.
- N. Ahmad, S. N. A. Bukhari, M. A. Hussain, H. Ejaz, M. U. Munir and M. W. Amjad, RSC Adv., 2024, 14, 13535–13564 RSC
.
- C.-H. Cheng, Y.-Y. Tu and J.-C. Lin, Int. J. Mol. Sci., 2022, 23, 14806 CrossRef CAS
.
- H. Pangli, S. Vatanpour, S. Hortamani, R. Jalili and A. Ghahary, J. Burn Care Res., 2021, 42, 785–793 CrossRef PubMed
.
- M. Rybka, Ł. Mazurek and M. Konop, Life, 2023, 13, 69 CrossRef CAS
.
- K. Nešović and V. Mišković-Stanković, Polym. Eng. Sci., 2020, 60, 1393–1419 CrossRef
.
- Z. He, Q. Xu, B. Newland, R. Foley, I. Lara-Sáez, J. F. Curtin and W. Wang, J. Mater. Chem. B, 2021, 9, 6326–6346 RSC
.
- M. Azharuddin, G. H. Zhu, D. Das, E. Ozgur, L. Uzun, A. P. F. Turner and H. K. Patra, Chem. Commun., 2019, 55, 6964–6996 RSC
.
- B. Klębowski, J. Depciuch, M. Parlińska-Wojtan and J. Baran, Int. J. Mol. Sci., 2018, 19, 4031 CrossRef PubMed
.
- X. Yin, T. Fan, N. Zheng, J. Yang, L. Yan, S. He, F. Ai and J. Hu, Nanoscale Adv., 2023, 5, 1729–1739 RSC
.
- J. Dadashi, M. A. Ghasemzadeh and M. Salavati-Niasari, RSC Adv., 2022, 12, 23481–23502 RSC
.
- A. G. Kurian, R. K. Singh, V. Sagar, J.-H. Lee and H.-W. Kim, Nano-Micro Lett., 2024, 16, 110 CrossRef CAS
.
- A. J. Clasky, J. D. Watchorn, P. Z. Chen and F. X. Gu, Acta Biomater., 2021, 122, 1–25 CrossRef CAS PubMed
.
- Z. Ahmadian, F. Kazeminava, M. Afrouz, M. Abbaszadeh, N. T. Mehr, J. A. Shiran, C. Gouda, M. Adeli and H. S. Kafil, Int. J. Biol. Macromol., 2023, 253, 126535 CrossRef CAS PubMed
.
- J. Zhang, Q. Huang and J. Du, Polym. Int., 2016, 65, 1365–1372 CrossRef CAS
.
- S. Ganguly and S. Margel, Polymers, 2021, 13, 4259 CrossRef CAS
.
- H.-V. Tran, N. M. Ngo, R. Medhi, P. Srinoi, T. Liu, S. Rittikulsittichai and T. R. Lee, Materials, 2022, 15, 503 CrossRef CAS
.
- F. Wahid, C. Zhong, H.-S. Wang, X.-H. Hu and L.-Q. Chu, Polymers, 2017, 9, 636 CrossRef PubMed
.
- J. Yang, D. Liu, X. Song, Y. Zhao, Y. Wang, L. Rao, L. Fu, Z. Wang, X. Yang, Y. Li and Y. Liu, Gels, 2022, 8, 270 CrossRef CAS
.
- J. Osuntokun, D. C. Onwudiwe and E. E. Ebenso, Green Chem. Lett. Rev., 2019, 12, 444–457 CrossRef CAS
.
- A. K. Gaharwar, N. A. Peppas and A. Khademhosseini, Biotechnol. Bioeng., 2014, 111, 441–453 CrossRef CAS PubMed
.
- E. L. Irede, R. F. Awoyemi, B. Owolabi, O. R. Aworinde, R. O. Kajola, A. Hazeez, A. A. Raji, L. O. Ganiyu, C. O. Onukwuli, A. P. Onivefu and I. H. Ifijen, RSC Adv., 2024, 14, 20992–21034 RSC
.
- Z. Li, Y. Li, C. Chen and Y. Cheng, J. Controlled Release, 2021, 335, 541–556 CrossRef CAS
.
- E. H. Fragal, V. H. Fragal, E. P. Silva, A. T. Paulino, E. C. da Silva Filho, M. R. Mauricio, R. Silva, A. F. Rubira and E. C. Muniz, Carbohydr. Polym., 2022, 292, 119665 CrossRef CAS
.
- J. Liao and H. Huang, Biomacromolecules, 2020, 21, 2574–2594 CrossRef CAS
.
- A. Pardo, M. Gómez-Florit, S. Barbosa, P. Taboada, R. M. A. Domingues and M. E. Gomes, ACS Nano, 2021, 15, 175–209 CrossRef CAS PubMed
.
- Z. Farzanegan and M. Tahmasbi, Appl. Radiat. Isot., 2023, 198, 110873 CrossRef CAS PubMed
.
- Y. He, J. Tang, Y. Hu, S. Yang, F. Xu, M. Zrínyi and Y. Mei Chen, Chem. Eng. J., 2023, 462, 142193 CrossRef CAS
.
- V. Verma, M. Al-Dossari, J. Singh, M. Rawat, M. G. M. Kordy and M. Shaban, Polymers, 2022, 14, 1444 CrossRef CAS PubMed
.
- L. M. Anaya-Esparza, Z. Villagrán-de la Mora, J. M. Ruvalcaba-Gómez, R. Romero-Toledo, T. Sandoval-Contreras, S. Aguilera-Aguirre, E. Montalvo-González and A. Pérez-Larios, Processes, 2020, 8, 1395 CrossRef CAS
.
- M. Sasani Ghamsari, S. Alamdari, W. Han and H.-H. Park, Int. J. Nanomed., 2017, 207–216 Search PubMed
.
- A. Madhusudhan, T. A. Suhagia, C. Sharma, S. K. Jaganathan and S. D. Purohit, Polymers, 2024, 16, 3318 CrossRef CAS PubMed
.
- Z. Cui, M. Zhou, P. J. Greensmith, W. Wang, J. A. Hoyland, I. A. Kinloch, T. Freemont and B. R. Saunders, Soft Matter, 2016, 12, 4142–4153 RSC
.
- H. Wang, Q. Chen and S. Zhou, Chem. Soc. Rev., 2018, 47, 4198–4232 RSC
.
- K. Naik, S. Tripathi, R. Ranjan, S. Agrawal, S. Singh, P. Dhar, K. Singh, V. Tiwari and A. S. Parmar, ACS Appl. Bio Mater., 2025, 8, 2229–2241 CrossRef CAS
.
- D. M. Alshangiti, T. K. El-damhougy, A. Zaher, M. Madani and M. Mohamady ghobashy, RSC Adv., 2023, 13, 35251–35291 RSC
.
- S. Mohammadi, S. Mohammadi and A. Salimi, Talanta, 2021, 224, 121895 CrossRef CAS
.
- X. Yang, P. Li, W. Tang, S. Du, M. Yu, H. Lu, H. Tan and X. Xing, Carbohydr. Polym., 2021, 251, 117040 CrossRef CAS
.
- J. Yue, P. Miao, L. Li, R. Yan, W.-F. Dong and Q. Mei, ACS Appl. Mater. Interfaces, 2022, 14, 49582–49591 CrossRef CAS
.
- A. K. Madikere Raghunatha
Reddy, A. Darwiche, M. V. Reddy and K. Zaghib, Batteries, 2025, 11, 71 CrossRef
.
- A. Ali, S. S. Rahimian Koloor, A. H. Alshehri and A. Arockiarajan, J. Mater. Res. Technol., 2023, 24, 6495–6521 CrossRef CAS
.
- Y. Lu, J. Li, H. Yu, W. Wang, L. Liu, K. Wang and L. Zhang, Polym. Test., 2018, 65, 21–28 CrossRef CAS
.
- R. Konnola and K. Joseph, RSC Adv., 2016, 6, 23887–23899 RSC
.
- H. Guo, T. Jiao, Q. Zhang, W. Guo, Q. Peng and X. Yan, Nanoscale Res. Lett., 2015, 10, 272 CrossRef
.
- J. L. Suter and P. V. Coveney, Sci. Rep., 2021, 11, 22460 CrossRef CAS
.
- W. Zhao, L. Qiang, C. Zhang, S. Li, Y. Liu, C. Wang, X. Ma, J. Wang and Y. Bao, ACS Appl. Mater. Interfaces, 2024, 16, 50175–50187 CrossRef CAS
.
- Y. Cao, Y. Cheng and G. Zhao, Langmuir, 2021, 37, 5522–5530 CrossRef CAS
.
- H. Zhang, X. Pang and Y. Qi, RSC Adv., 2015, 5, 89083–89091 RSC
.
- Y. Gao, Y. Dong, Y. Cao, W. Huang, C. Yu, S. Sui, A. Mo and Q. Peng, J. Biomed. Nanotechnol., 2021, 17, 1627–1634 CrossRef CAS
.
- B. Yang, Y. Wang, L. Xiao, X. Hu and G. Zhou, Macromol. Res., 2017, 25, 21–26 CrossRef CAS
.
- J. L. Hart, K. Hantanasirisakul, A. C. Lang, B. Anasori, Y. Gogotsi and M. L. Taheri, Microsc. Microanal., 2018, 24, 1606–1607 CrossRef
.
- I. C. Lee, Y.-C. E. Li, J. L. Thomas, M.-H. Lee and H.-Y. Lin, Mater. Horiz., 2024, 11, 876–902 RSC
.
- R. Chen, X. Jia, H. Zhou, S. Ren, D. Han, S. Li and Z. Gao, Mater. Today, 2024, 75, 359–385 CrossRef CAS
.
- I. Hussain, S. Sahoo, M. S. Javed, J. Lu and K. Zhang, Mater. Sci. Eng., R, 2024, 160, 100814 CrossRef
.
- S. Iravani, Soft Matter, 2023, 19, 6196–6212 RSC
.
- J.-N. Ma, Y.-L. Zhang, Y.-Q. Liu, D.-D. Han, J.-W. Mao, J.-R. Zhang, W.-C. Zhao and H.-B. Sun, Sci. Bull., 2022, 67, 501–511 CrossRef CAS
.
- X. Yang, C. Zhang, D. Deng, Y. Gu, H. Wang and Q. Zhong, Small, 2022, 18, 2104368 CrossRef CAS PubMed
.
- Z. Wu, J. Shi, P. Song, J. Li and S. Cao, Int. J. Biol. Macromol., 2021, 183, 870–879 CrossRef CAS
.
- H. Tomás, C. S. Alves and J. Rodrigues, Nanomedicine, 2018, 14, 2407–2420 CrossRef PubMed
.
- S. T. Stealey, A. K. Gaharwar and S. P. Zustiak, Pharmaceuticals, 2023, 16, 821 CrossRef CAS PubMed
.
- S. S. Das, K. Hussain, S. Singh, A. Hussain, A. Faruk and M. Tebyetekerwa, Curr. Pharm. Des., 2019, 25, 424–443 CrossRef CAS
.
- K. Khodaverdi, S. M. Naghib, M. R. Mozafari and M. Rahmanian, Carbohydr. Polym. Technol. Appl., 2025, 9, 100640 CAS
.
- M. Rivas, L. J. del Valle, C. Alemán and J. Puiggalí, Gels, 2019, 5, 14 CrossRef CAS
.
- Y. Y. Hsu and Y.-C. Yeh, Small, 2025, 21, e06085 CrossRef CAS
.
- J. L. Pablos, D. Lozano, M. Manzano and M. Vallet-Regí, Mater. Today Bio, 2024, 29, 101342 CrossRef CAS PubMed
.
- Y. T. Zheng, Y. Oz, Y. M. Gu, N. Ahamad, K. Shariati, J. Chevalier, D. Kapur and N. Annabi, Nano Today, 2024, 55, 102147 CrossRef CAS
.
- Z. E. Jassim and N. A. Rajab, Int. J. Pharm. Invest., 2025, 15, 849–857 CrossRef
.
- Z. L. Cao, X. L. Zuo, X. C. Liu, G. X. Xu and K. T. Yong, Adv. Colloid Interface Sci., 2024, 330, 103206 CrossRef CAS PubMed
.
- M. A. Beach, U. Nayanathara, Y. T. Gao, C. H. Zhang, Y. J. Xiong, Y. F. Wang and G. K. Such, Chem. Rev., 2024, 124, 5505–5616 CrossRef CAS PubMed
.
- Y. Y. Wang, H. Li, A. Rasool, H. B. Wang, R. Manzoor and G. L. Zhang, J. Nanobiotechnol., 2024, 22, 1 CrossRef PubMed
.
- W. C. Jia, Y. Wu, Y. J. Xie, M. H. Yu and Y. Chen, Adv. Mater., 2025, 37, 2413603 CrossRef CAS
.
- Y. O. Wu, S. S. Moonshi and H. T. Ta, ACS Appl. Bio Mater., 2025, 8, 4416–4431 CrossRef CAS
.
- A. H. Maboudi, M. H. Lotfipour, M. Rasouli, M. H. Azhdari, R. Macloughlin, S. Bekeschus and M. Doroudian, Nanotechnol. Rev., 2024, 13, 20230218 CrossRef CAS
.
- R. S. Othman, S. Zarei, H. R. Haghighat, A. A. Taromi and H. A. Khonakdar, Polym. Adv. Technol., 2025, 36, e70180 Search PubMed
.
- S. Kotta, H. M. Aldawsari, S. M. Badr-Eldin, A. B. Nair and K. Yt, Pharmaceutics, 2022, 14, 1636 CrossRef CAS
.
- S. Perumal, R. Atchudan and W. Lee, Polymers, 2022, 14, 2510 CrossRef CAS
.
- P. Verma, G. Gupta, T. S. Markandeywar and D. Singh, Assay Drug Dev. Technol., 2023, 21, 31–47 CrossRef CAS
.
- J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3–14 CrossRef CAS
.
- B. Mohan, D. Dhiman, Virender, Mehak, Priyanka, Q. Sun, M. Jan, G. Singh and N. Raghav, Microchem. J., 2023, 193, 108956 CrossRef CAS
.
- D. S. R. Khafaga, M. T. El-Morsy, H. Faried, A. H. Diab, S. Shehab, A. M. Saleh and G. A. M. Ali, RSC Adv., 2024, 14, 30201–30229 RSC
.
- M. Moharramnejad, A. Ehsani, M. Shahi, S. Gharanli, H. Saremi, R. E. Malekshah, Z. S. Basmenj, S. Salmani and M. Mohammadi, J. Drug Delivery Sci. Technol., 2023, 81, 104285 CrossRef CAS
.
- H. Sohrabi, S. Ghasemzadeh, S. Shakib, M. R. Majidi, A. Razmjou, Y. Yoon and A. Khataee, Ind. Eng. Chem. Res., 2023, 62, 4611–4627 CrossRef CAS
.
- B. Saboorizadeh, R. Zare-Dorabei, M. Safavi and V. Safarifard, Langmuir, 2024, 40, 22477–22503 CrossRef CAS PubMed
.
- D. Tian, R. Hao, X. Zhang, H. Shi, Y. Wang, L. Liang, H. Liu and H. Yang, Nat. Commun., 2023, 14, 3226 CrossRef CAS
.
- S. Bao, J. Li, B. Guan, M. Jia, O. Terasaki and J. Yu, Matter, 2020, 3, 498–508 CrossRef
.
- H. Ahmadi, M. Moussaei, V. Haddadi-Asl and H. Roghani-Mamaqani, Polymer, 2024, 311, 127489 CrossRef CAS
.
- H. Sharifi Dehsari, A. Halda Ribeiro, B. Ersöz, W. Tremel, G. Jakob and K. Asadi, CrystEngComm, 2017, 19, 6694–6702 RSC
.
- D. Zhang, Y. Chen, Y.-S. Huang, Q. Huang, K. Kwan Li and Y. Xia, Chem. – Eur. J., 2024, 30, e202400833 CrossRef CAS PubMed
.
- H. L. B. Boström, S. Emmerling, F. Heck, C. Koschnick, A. J. Jones, M. J. Cliffe, R. Al Natour, M. Bonneau, V. Guillerm, O. Shekhah, M. Eddaoudi, J. Lopez-Cabrelles, S. Furukawa, M. Romero-Angel, C. Martí-Gastaldo, M. Yan, A. J. Morris, I. Romero-Muñiz, Y. Xiong, A. E. Platero-Prats, J. Roth, W. L. Queen, K. S. Mertin, D. E. Schier, N. R. Champness, H. H.-M. Yeung and B. V. Lotsch, Adv. Mater., 2024, 36, 2304832 CrossRef
.
- B. Reidy, A. Haase, A. Luch, K. A. Dawson and I. Lynch, Materials, 2013, 6, 2295–2350 CrossRef CAS
.
- T. Jaswal and J. Gupta, Mater. Today: Proc., 2023, 81, 859–863 CAS
.
- S. Yu, H. Zhang, S. Zhang, M. Zhong and H. Fan, Front. Chem., 2021, 9, 651053 CrossRef CAS
.
- H. Wu, J.-J. Yin, W. G. Wamer, M. Zeng and Y. M. Lo, J. Food Drug Anal., 2014, 22, 86–94 CrossRef CAS
.
- P. Wiśniewska, J. Haponiuk, M. R. Saeb, N. Rabiee and S. A. Bencherif, Chem. Eng. J., 2023, 471, 144400 CrossRef
.
- P. Kumar, B. Anand, Y. F. Tsang, K.-H. Kim, S. Khullar and B. Wang, Environ. Res., 2019, 176, 108488 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2026 |